Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF RBSM, LLP, INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM - Icagen, Inc. | f10k2017ex23-1_icageninc.htm |
| EX-32.2 - CERTIFICATION - Icagen, Inc. | f10k2017ex32-2_icageninc.htm |
| EX-32.1 - CERTIFICATION - Icagen, Inc. | f10k2017ex32-1_icageninc.htm |
| EX-31.2 - CERTIFICATION - Icagen, Inc. | f10k2017ex31-2_icageninc.htm |
| EX-31.1 - CERTIFICATION - Icagen, Inc. | f10k2017ex31-1_icageninc.htm |
| EX-21.1 - LIST OF SUBSIDIARIES - Icagen, Inc. | f10k2017ex21-1_icageninc.htm |
| EX-4.19 - FORM OF AMENDED AND RESTATED WARRANT DATED MAY 15, 2017 ISSUED BY ICAGEN, INC. - Icagen, Inc. | f10k2017ex4-19_icageninc.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 000-54748
ICAGEN, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 20-0982060 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
4222 Emperor Blvd., Suite 350
Durham, North Carolina 27703
(Address of principal executive offices) (Zip Code)
(919) 941- 5206
(Registrant’s telephone number, including area code)
| Securities registered pursuant to Section 12(b) of the Act: | Name of each exchange on which registered | |
| (Title of Class) | None |
Securities registered pursuant to Section 12 (g) of the Act: Common Stock, $0.001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the issuer: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every interactive data file required to be submitted and posted pursuant to Rule 405 of Regulation S-T (section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of issuer’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated file, a non-accelerated file, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer, “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ |
| Emerging growth company | ☐ | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant as of June 30, 2017, was approximately $22,375,875 based on $3.50, the price at which the registrant’s common stock was last sold, which was January 7, 2015. The registrant has provided this information as of January 7, 2015 because its common stock was not publicly traded as of the last business day of its most recent completed second quarter.
As of April 13, 2018, the issuer had 6,393,107 shares of common stock outstanding.
Documents incorporated by reference: None
FORM 10-K
TABLE OF CONTENTS
Special Note Regarding Forward-Looking Statements
Many of the matters discussed within this Annual Report on Form 10-K (“Annual Report”) contain forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) on our current expectations and projections about future events. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “potential,” “continue,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” and similar expressions. These statements are based on our current beliefs, expectations, and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and generally beyond our control, that could cause actual results to differ materially from those expressed, projected or implied in or by the forward-looking statements. Such risks and uncertainties include the risks noted under Part 1. “Business,” Part 1A “Risk Factors” and Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” but are also contained elsewhere. We do not undertake any obligation to update any forward-looking statements. Unless the context requires otherwise, references to “we,” “us,” “our,” and “Icagen,” refer to Icagen, Inc. and its subsidiaries.
You should refer to Item 1A. “Risk Factors” section of this Annual Report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We do not undertake any obligation to update any forward-looking statements.
Company Overview
We are engaged in the advancement of early drug discovery. Our business model is divided into three sources of revenue: our integrated drug discovery services that we have been providing to third parties since our inception; partnerships and collaborations with third parties that should allow us to receive revenue for services provided as well as future milestone and royalty payments and potential licensing fees and other related fees paid for the licensing of our technology.
For the past two years, a significant portion of our revenue has been derived from our operations as a partner research organization providing integrated drug discovery services with unique expertise in the field of ion channel, transporter, neuroscience, muscle biology and rare disease targets while also covering many other classes of drug discovery targets and therapeutic areas. Our customers are pharmaceutical and biotechnology companies to whom we offer our industry-leading scientific expertise and technologies to aid in their determination of which molecules to advance into late stage preclinical studies and ultimately clinical trials. The core of our offering is the discovery of pre-clinical drug candidates (PDC’s), which are lead molecules (Leads) that are selected to enter into in-vivo studies during the pre-clinical phase of drug discovery. We offer a full complement of pre-clinical drug discovery services which include; assay development technologies (including high throughput fluorescence, manual and automated electrophysiology and radiotracer flux assays), cell line generation, high-throughput and ultra-high-throughput screening, medicinal chemistry, computational chemistry and custom assay services to our customers. Our capabilities also include molecular biology and the use of complex functional assays, electrophysiology, bioanalytics and pharmacology. We believe that this integrated set of capabilities enhances our ability to help our customers identify drug candidates.
More recently, we have begun to focus on partnership and collaboration opportunities with third parties, and we are currently in active negotiations regarding our first such collaboration. This collaboration is expected to provide us with an opportunity to derive revenue not only from our standard fees for integrated early discovery services but also from future milestone and royalty revenue from product candidates that may be developed and commercialized with our aid. We have developed in house a portfolio of multiple assets targeting different indications that we believe would be ideal candidates for partnership opportunities.
Lastly, we are regularly pursuing opportunities for the licensing of our proprietary XRPro technology, our legacy technology, which has unique capabilities in the transporter target class. For any licensing transactions that we may engage in, we anticipate receiving an up-front license fee, as well as fees for the equipment we provide and our services in aiding the licensee with the use of the technology.
1
We utilize a target class approach to drug discovery where we leverage our deep expertise in specific areas to more rapidly move drug discovery projects forward. Whereas traditional drug discovery tends to take a more linear approach to compound advancement, our depth of both technical assets, including our XRPro technology, and area experts allows us to use more parallel approaches to aid in eliminating problematic molecules early and identifying high quality leads in the drug discovery process. This saves time, money and increases the probability of success in human clinical studies. We believe that our deep understanding of the ion channel genome, neuroscience, muscle biology, and rare disease targets, in particular, and our ability to apply this knowledge in a target class approach to drug discovery facilitates our identification of small molecule drug candidates with novel mechanisms of action and enhanced selectivity and specificity profiles. Moreover, because our drug discovery and development process screens for potential side effects at an earlier stage than some alternative approaches, we believe that this process enables us to identify small molecule drug candidates that may have a reduced risk of clinical failure and may shorten clinical development timelines.

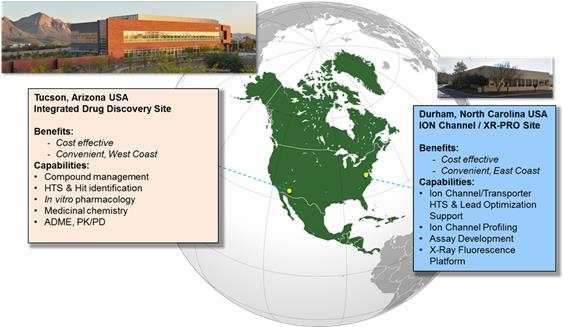
We currently operate out of two sites, one in Durham, North Carolina and the other in Tucson, Arizona (the “Tucson Facility”). The teams in North Carolina and Arizona have extensive experience over the last 20 plus years performing early drug discovery within Pfizer, Inc. (“Pfizer”) and Sanofi US Inc. (“Sanofi”), respectively, delivering Leads from the pre-clinical stage to the clinical stage of drug discovery. At the North Carolina site (“Icagen NC”), which we began to operate in July 2015, we have a leading biology expertise focused on ion channels which are important targets in Neuroscience. The North Carolina site also houses the XRpro® technology. The XRPro technology is an x-ray fluorescence technology that delivers ion channel screening, transporter screening, ion channel kinetics and custom screening services to our customers to detect and quantitatively analyze the x-ray signature of each element with an atomic number greater than 12. More specifically, our capabilities in North Carolina include a focus on ion channels and transporters, HTS and Lead optimization, ion channel profiling, assay development and x-ray fluorescence-based assays. At the Arizona site, which we acquired in July 2016, we have leading biology expertise and platform capabilities in rare diseases, muscle biology and integrated drug discovery. The Arizona site provides capacity in cell models, human biomarkers, muscle biology expertise and stem cells-based assays. In addition, the Arizona site provides compound management services, HTS and Hit identification, in vitro pharmacology, medicinal chemistry, computational chemistry and ADME. The Arizona facility also features high volume biology with a flexible robotic infrastructure capable of performing high throughput screening in ultra high 1536 format, enhancing our depth of expertise as a specialized pharmaceutical services company. This enables us to offer a broad range of integrated drug discovery services in a growing market. The extensive integrated drug discovery platform and technologies at the Arizona site enable us to utilize our biology expertise in both the North Carolina and Arizona sites to accelerate the drug discovery and identify quality leads faster.
2
Subsequent to our acquisition of certain assets of Pfizer and Sanofi, our customer base has expanded and a significant portion of our revenue has been derived from several commercial customers. For the year ended December 31, 2017, 56.2% of our revenue was derived from services sales, 42.4% was derived from deferred subsidy revenue and 1.4% was generated from Government revenue. For the year ended December 31, 2016, 59.5% (2015 - 84%) of our revenue was derived from services sales; 36.7% was derived from subsidies (2015 0%) and the remaining 3.8% was generated from Government revenues (2015 - 16%). Prior to our acquisition of certain assets from Pfizer and Sanofi, substantially all of our revenue was derived from government grants related to services provided by our XRpro technology.
To date, we have been granted twenty-one grants and contracts from United States governmental agencies; of which nine were granted from the Department of Defense and twelve were granted from the National Institutes of Health. Of such contracts, twenty have been completed and we received payment in full for all twenty completed contracts. There is one contract from the National Institutes of Health under which we performed services during the year ended December 31, 2017, this contract was for $600,000 and has been fully invoiced as of December 31, 2017. The NIH invited us to submit a Phase II SBIR application for the completed contract of which we are awaiting the outcome.
3
Strategy
Our goal is to become a leader in the discovery, development and commercialization of drugs in the early phase of drug discovery that address disease areas with significant unmet medical need and commercial potential. We intend to diversify our business and increase awareness about our company through the execution of our strategy, key elements of which are as follows:
| ● | Maximize and Strengthen and expand our core drug discovery technologies and development capabilities. All of our drug candidates and research programs have resulted from our core drug discovery technologies. We have steadily built these technologies, which span the key disciplines of biology, chemistry and pharmacology, over a number of years. We intend to continue to invest in these core technologies, as the key to our future research programs and drug candidates. We are focusing a significant portion of our business efforts on increasing our customers that utilize our scientific expertise and technologies to aid in their determination of which molecules to advance to into late stage preclinical and clinical trials. |
| ● | Establish strategic alliances with leading pharmaceutical and biotechnology companies. We plan to selectively enter into new strategic alliances with leading pharmaceutical and biotechnology companies to assist us in advancing our drug discovery and development programs. We expect that these alliances will provide us with access to the therapeutic area expertise and research, development and commercialization resources of our collaborators as well as augment our financial resources. We expect that in some of these alliances we will seek to maintain rights in the development of drug candidates and the commercialization of drugs as part of our effort to build our internal clinical development and sales and marketing capabilities. |
| ● | Build and advance our product candidate pipeline. Through our ion channel drug discovery and development programs, we have created a pipeline of drug candidates that address diseases with significant unmet medical need and commercial potential across a range of therapeutic areas. We plan to aggressively pursue the development and commercialization of these drug candidates. We believe that the breadth of our capabilities in ion channel drug discovery technology and rare diseases will enable us to continue to identify and develop additional drug candidates on an efficient and rapid basis. In addition to developing drug candidates internally, we continue to evaluate opportunities to in-license promising compounds and technologies. |
| ● | Enter into licensing relationships for our XRPro Technology. We are regularly pursuing opportunities for the licensing of our proprietary XRPro technology, our legacy technology, which has unique capabilities in the transporter target class. For any licensing transactions that we may engage in, we anticipate receiving an up-front license fee, as well as fees for the equipment we provide and our services in aiding the licensee with the use of the technology. |
Company History
The Company, formerly known as XRpro Sciences and Caldera, was founded in 2003 at the request of the then director of Los Alamos National Laboratory (“LANL”) for the purpose of commercializing the use of x-ray fluorescence to measure the chemical composition of pharmaceuticals. In March 2015, we effected a 1-for -2 reverse stock split of our common stock. In July 2015, we expanded our services and capabilities and entered into an asset purchase agreement with Icagen, Inc., an indirect subsidiary of Pfizer (“Icagen NC”), whereby certain assets were acquired from Icagen NC, including the name Icagen. We moved our headquarters to Research Triangle Park, Durham, North Carolina, where the Pfizer subsidiary’s operations were conducted and on August 28, 2015 changed our name to Icagen, Inc.
Icagen NC was founded in 1992 as a start-up biotech to discover, develop and commercialize small molecules targeting ion channels. Icagen NC sent its first molecule into the clinic for sickle cell anemia in 1999. Over the years Icagen NC also provided access to its innovative discovery platform. In 2007, Icagen NC entered into a collaborative agreement with Pfizer to identify novel compounds targeting voltage-gated sodium channels for the treatment of pain. Due to the success of the programs, Icagen NC was acquired in 2011 by Pfizer. Pfizer integrated Icagen NC into Neusentis which was a biotech-like unit within Pfizer combining research in pain, sensory disorders, and regenerative medicine for the next 4 years. In an effort to move to a more variable (outsourced) R&D model Pfizer in July 2015 divested Icagen NC to us and we re-launched the Icagen NC team and capabilities under the Icagen name.
Since the Icagen NC spinout from Pfizer, we have experienced accelerated growth and market acceptance as a leader in the area of ion channel and transporter targets with major large pharma clients and biotechs. We then began to look for ways to leverage that success and did so in July 2016 with the newly acquired Icagen Tucson business from Sanofi which added a complete integrated drug discovery capability beyond ion channels and transporters covering most classes of drug discovery targets.
4
The Tucson Research Center, a two-story laboratory and office building with approximately 113,950 square feet of space located in the Town of Oro Valley, Pima County, Arizona (the “Facility”), which we acquired from Sanofi in July 2016, enhances our depth of expertise as a specialized pharmaceutical services company, enabling us to offer a broad range of integrated drug discovery services in a growing market. The Facility provides capacity in cell models, human biomarkers, muscle biology expertise and stem cells-based assays. In addition, the site provides compound management services, HTS and Hit identification, in vitro pharmacology, medicinal chemistry, computational chemistry and ADME, as well as high volume biology with a flexible robotic infrastructure capable of performing high throughput screening in ultra high 1536 format.
Corporate Information
We were incorporated in the State of Delaware on November 12, 2003 under the name Caldera Pharmaceuticals, Inc. On December 4, 2014 we changed our name to XRpro Sciences, Inc. and on August 28, 2015, after our acquisition of certain assets of Icagen, Inc., we changed our name to Icagen, Inc. Our principal executive offices are located at 4222 Emperor Blvd., Suite 350 Durham, North Carolina 27703 our telephone number is (919) 941-5206.
Discussions with respect to our operations included herein include the operations of our operating subsidiary, Icagen Corp (formerly known as XRpro Corp), formed on July 10, 2010 and Icagen-T, Inc, formed on June 16, 2016, a subsidiary formed to acquire the assets of Sanofi’s ultra high-throughput biology, screening and chemistry capabilities and research facility in Oro Valley, Arizona. We have two other subsidiaries, XRpro Sciences Inc., formed on December 10, 2015 and Caldera Discovery Inc., formed on March 26, 2015, which have always been dormant.
Recent Developments
We have offered on a best efforts basis up to a maximum of forty (40) units and a minimum of ten (10) units, at a purchase price of $100,000 per unit (the “Offering’), each unit consisting of 28,571 shares of our Series C Convertible Preferred Stock, par value $0.001 per share (the “Series C Preferred Stock”) and a seven year warrant (the “Warrants”) to acquire 28,571 shares of our common stock, par value, $0.001 per share, at an exercise price of $3.50 per share. On April 4, 2018, we closed the first tranche of the Offering and entered into a securities purchase agreement (the “Purchase Agreements”) with one accredited investor that is a trust of which a member of our Board of Directors if the trustee, pursuant to which we offered and sold an aggregate of twenty (20) Units. The sale of the twenty (20) Units resulted in gross offering proceeds of $2,000,000. See “Item 7-Management’s Discussion and Analysis of Financial Condition and Results of Operations” for additional information with respect to the Offering.
5
Source and Availability of Raw Materials
In general, most of the materials we use for our research operation are readily available from multiple suppliers including specialty chemicals for certain types of assays. We do, however, conduct high throughput electrophysiology experiments on specific pieces of equipment from multiple vendors. In these cases, the consumables (i.e. chips or plates) are manufactured and sold by the same vendor, Bruker Nano GmbH, who manufactures the equipment. Should these vendors fail to deliver the consumables in a timely manner this could adversely affect our assay services operation.
Research and Development
For the years ended December 31, 2017 and 2016, we spent approximately $3,328,843 and $1,200,223, on research and development salaries. For more information regarding our research and development expenses, please see “Critical Accounting Policies” in “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Intellectual Property
Patents and Trade Secrets
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
6
We are maintaining and building our patent portfolio by filing new patent applications and prosecuting existing applications. In total, we hold approximately 75 patents, both U.S. and foreign, and approximately 10 pending patent applications, both U.S. and foreign, all related to our X-ray fluorescence-based technologies. As shown below, these patents and patent applications are spread across roughly 9 technology families.
Our patent estate as of April 13, 2018 is summarized below:
| ● | Method for Detecting Binding Events Using Micro X-Ray Fluorescence Spectrometry, which includes an issued U.S. patent that is expected to expire in about 2021; |
| ● | Flow Method and Apparatus for Screening Chemicals Using Micro X-Ray Fluorescence, which includes issued patents in the U.S., Europe, Japan and Singapore, such patents are expected to expire in 2022; |
| ● | Method and Apparatus for Detecting Chemical Binding, which includes about 10 issued patents in the U.S., Europe, Japan and Singapore; such patents are expected to expire in 2023; |
| ● | Drug Development and Manufacturing, which includes an issued U.S. patent that is expected to expire in about 2021; |
| ● | Advanced Drug Development and Manufacturing, which includes about 20 issued foreign patents, in Europe, Japan, and Hong Kong, expected to expire in about 2026, and a pending application in the U.S. which, if issued, is expected to expire between 2021-2026; |
| ● | Well Plate/Apparatus for Preparing Samples for Measurement by X-Ray Fluorescence Spectrometry, which includes issued over 15 issued patents in the U.S. Europe, and Japan, which are expected to expire in about 2028, and a pending application in the U.S., which, if issued, is also expected to expire in 2028; |
| ● | Method and Apparatus for Measuring Protein Post Translational Modification, which includes a patent issued in Japan, which is expected to expire in about 2028 and pending applications in U.S. and Japan, which, if issued, are also expected to expire in about 2028; |
| ● | Method and Apparatus for Measuring Analyte Transport Across Barriers, which includes 3 issued U.S. patents and issued patents in China and Hong Kong, which are expected to expire in about 2030/2031, and pending applications in U.S., Europe, and China, which, if issued, are also expected to expire in about 2030; and |
| ● | Method for Analysis Using X-Ray Fluorescence, which includes 4 issued U.S. patents, which is expected to expire in 2031, and a pending U.S. patent application which, if issued, is expected to expire in 2031. |
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Our issued patents and those that may issue in the future, may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related products or the length of term of patent protection that we may have for our products. In addition, our competitors may independently develop similar technologies or duplicate any technology developed by us, and the rights granted under any issued patents may not provide us with any meaningful competitive advantages against these competitors.
We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets are difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants, scientific advisors and other contractors, as well as physical security of our premises and our information technology systems. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition from many different sources and organizational sizes, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions. Many of the major multi-national contract research organizations offer some similar assay services to those we provide. These include companies with operations in the US, China, Europe and Asia. None of these companies, however, are exclusively focused and dedicated to ion channel services. There are also a small number of private companies of similar size to us that provide ion channel-related services. In addition, we also compete in the pre-clinical drug discovery space with in-house groups of pharmaceutical and biotechnology companies as well as universities.
7
Many of our competitors have significantly greater financial resources and expertise in research and development. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop screening services that are more effective, faster or are less expensive than any products that we may develop. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, as well as customers.
Government and Environmental Regulation and Laws
We currently operate two laboratories, one in our principal headquarters in Durham, North Carolina and the other in our facility located in Tucson, Arizona. Our laboratory services are subject to various regulatory requirements and our standard operating procedures are written in accordance with appropriate regulations and guidelines for operations.
There are certain licensing and regulations under federal, state and local laws relating to hazard communication and employee right-to- know regulations, our use and handling and disposal of bio-medical specimens and hazardous waste. In addition, there are regulations related to ensuring the health and safety of laboratory employees. Our laboratories are subject to applicable laws and regulations as appropriate from the Nuclear Regulatory Commission, Environmental Protection Agency, the Department of Transportation, and the National Fire Protection Agency and the Resource Conservation and Recovery Act. In addition, the Nuclear Regulatory Commission has rules and regulations regarding the use of x-ray devices and radioactive materials. To the best of our knowledge we are currently in compliance in all material respects with such laws and continual endeavors to maintain compliance. Lack of compliance with such laws could subject us to denial of the right to conduct business, fines, criminal penalties and other enforcement actions.
The Occupational Safety and Health Administration have also established extensive requirements relating to workplace safety for healthcare employers whose workers may be exposed to blood-borne pathogens. Our employees receive initial and periodic training focusing on lab safety including blood-borne pathogens.
The use of controlled substances in testing for drugs with a potential for abuse is regulated in the United States by the U.S. Drug Enforcement Administration. Our laboratories have all necessary licenses from the U.S. Drug Enforcement Administration for the use of controlled substances.
The United States has addressed the disclosure of confidential personal data with increased regulation. In the United States, various federal and state laws address the security and privacy of health and other personal information. We will continue to monitor our compliance with applicable regulations.
The regulations of the U.S. Department of Transportation, the U.S. Public Health Service and the U.S. Postal Service apply to the surface and air transportation of laboratory specimens. Our laboratory also must comply with the applicable International Air Transport Association regulations, which govern international shipments of laboratory specimens.
Employees
As of April 13, 2018, we employed seventy-two full time employees. A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. None of our employees are covered by collective bargaining agreements, and management considers relations with our employees to be good.
Available Information
Additional information about Icagen is contained at our website, www.icagen.com. Information on our website is not incorporated by reference into and does not form ant part of this Annual Report. We have included our website address as a factual reference and do not intend it to be an active link to our website. We make available on our website, www.icagen.com, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, all of which are available free of charge through the investor relations page of our internet website as soon as reasonably practicable after those reports are filed with the SEC. The following Corporate Governance documents are also posted on our website: Code of Business Conduct and Ethics and the Charters for the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee of the Board of Directors. Our phone number is (919) 433-5206 and our facsimile number is (919) 941- 0813.
8
Investing in our securities involves a high degree of risk. In addition to the risks related to our business set forth in this Form 10-K and the other information included and incorporated by reference in this Form 10-K, you should carefully consider the risks described below before purchasing our securities. Additional risks, uncertainties and other factors not presently known to us or that we currently deem immaterial may also impair our business operations.
We have a history of losses and there can be no assurance that we will generate or sustain positive earnings.
For the year ended December 31, 2017, we had a net loss of $(6,110,434) and for the year ended December 31, 2016 we had a net loss of $(5,504,412). The only year that we had net income was the year ended December 31, 2014 when we received proceeds from the settlement of litigation with LANS. We cannot be certain that our business strategy will ever be successful. Future revenues and profits, if any, will depend upon various factors, including the success, if any, of our expansion plans and our services to biotechnical and pharmaceutical customers, marketability of our instruments and services, our ability to maintain favorable relations with manufacturers and customers, and general economic conditions. There is no assurance that we can operate profitably or that we will successfully implement our plans. There can be no assurance that we will ever generate positive earnings.
A significant portion of our net revenue has been generated from services provided to five customers.
The termination of our relationship with either Pfizer and Sanofi would adversely affect our business. For the year ended December 31, 2017 we derived 56.2% of our revenue from commercial contracts (of which 78.8% of our services revenue was for services provided to five large pharmaceutical customers and one biotech company); 42.4% of our revenue was from subsidy revenue and the remaining 1.4% was derived from government contracts. For the year ended December 31, 2016, we derived 59.5% of our revenues from commercial contracts of which 79.4% of our revenue was for services provided to three large pharmaceutical customers; 36.7% of our revenue was from subsidy revenue and the remaining 3.8% was derived from government contracts. Prior to the acquisition of certain of the assets of Icagen NC we derived substantially all of our revenue from services we performed for two governmental agencies. Our business model which now concentrates on commercial customers is relatively new and there can be no assurance that we will be able to increase the revenue derived from commercial customers to a significant amount. As of the date hereof we have completed all contracts with the National Institutes of Health (“NIH”). Our Sanofi MSA provided that Sanofi make payments to Icagen-T of $8.75 million over the next forty-two (42) months in consideration of Icagen-T providing services to Sanofi, so long as Icagen-T complies with the covenants set forth in the Sanofi MSA. Our MSA with Pfizer which terminated in July 2017 guaranteed $1,000,000 of revenue to us for the twelve-month period ended June 30, 2017. Our MSA with Sanofi guaranteed $32 million over a five-year period of which: (i) $23.25 million has been received; ii) a further $2.75 million is expected to be paid in 2018; (iii) $3 million is expected to be paid over twelve months from July 2018 to June 2019; (iv) $2 million is expected to be paid over twelve months from July 2019 to June 2020; and (v) $1 million is expected to be paid over twelve months from July 2020 to June 2021, all subject to us meeting certain terms and conditions. There can be no assurance that we will attract a sufficient number of other pharmaceutical companies to provide our services to or that Pfizer will continue to use our services or that Sanofi will increase the scope of the services required. We do not have enough information regarding our new business model to assess its success.
During the past two years, a significant portion of our net revenue has been generated from services to be provided to Sanofi.
The termination of our relationship with Sanofi would adversely affect our business. Our Sanofi MSA required Sanofi to make significant payments to us during the last two years in consideration of Icagen-T providing services to Sanofi, so long as Icagen-T complies with the covenants set forth in the Sanofi MSA. The Sanofi MSA requires that Sanofi make payments to us of an aggregate $8,750,000 over the next three and a half years. Inasmuch as prior to the acquisition of the Tucson Facility, the Tucson Facility was used solely to service Sanofi and had no third-party customers, Sanofi continues to be a significant customer of Icagen-T. We cannot guarantee when, or if ever, our dependence upon Sanofi as a major customer at the Tucson Facility will end. There can be no assurance that we will attract a sufficient number of other pharmaceutical companies to provide our services to or that Sanofi will increase the scope of the services required. We do not have enough information regarding our new business model to assess its success.
Our business is dependent upon our ability to attract new commercial customers.
Our future success is dependent upon us attracting new commercial customers and increasing the services that we provide to existing customers, including Sanofi and Pfizer. The $1,000,000 guaranteed payment that we were to receive from Pfizer under the Pfizer MSA terminated on June 30, 2017. The payments that we are to receive from Sanofi under the terms of the Sanofi MSA are subject to termination in the event that we do not comply with certain covenants contained in the Sanofi MSA that are unrelated to our performance of services under the Sanofi MSA. In addition, the guaranteed payments from Sanofi in this year and years three through five of the Sanofi MSA are significantly less than those to be paid in year one and will not be sufficient to cover the costs of the operations at the Tucson Facility. We do not have enough information regarding our new business model which concentrates on commercial customers to assess its success. Our future success is dependent upon us attracting new customers and increasing the services that we provide to existing customers, including Sanofi and Pfizer. There can be no assurance that we will be able to attract new commercial customers or increase the services that we provide to existing customers, including Pfizer and Sanofi.
9
Our consolidated financial statements have been prepared assuming that we will continue as a going concern.
Although we have generated revenue, our operating losses, negative cash flows from operations and limited alternative sources of revenue raise substantial doubt about our ability to continue as a going concern. During the year ended December 31, 2017 and the years ended December 31, 2016, we did not generate enough revenue from operations to sustain our operations. We will be required to increase our revenue from customers and/or obtain additional financing in order to pay existing contractual obligations (which include the guaranteed payments to employees and amounts required to maintain the facility in Tucson and the amounts owed under the convertible notes that we issued to GPB Debt Holdings II, LLC in May 2017, the “Convertible Notes”) and to continue to cover operating losses and working capital needs. We cannot assure you that our revenue generated from operations or any future funds we raise will be sufficient to support our continued operations.
The audit report of RBSM LLP for the fiscal year ended December 31, 2017 contained a paragraph that emphasizes the substantial doubt as to our continuance as a going concern. If we cannot raise adequate capital on acceptable terms we will need to revise our business plans.
If we cannot establish profitable operations, we will need to raise additional capital to fully implement our business plan and to meet our current obligations, which may not be available on commercially reasonable terms, or at all, and which may dilute your investment.
We incurred a net loss of $(6,110,434) for the year ended December 31, 2017, a net loss of $(5,504,412) for the year ended December 31, 2016. Achieving and sustaining profitability will require us to increase our revenues and manage our product, operating and administrative expenses. We cannot guarantee that we will be successful in achieving profitability. Pursuant to the terms of the Pfizer APA, as amended July 15, 2016, we are required to pay Pfizer, commencing May 2017, minimum quarterly payments of $50,000 each for the period May 2017 to March 31, 2019, including interest on the difference between the unpaid deferred purchase consideration and the $50,000, a lump sum of unpaid deferred purchase consideration due for the period January 1, 2017 to December 31, 2018, the deferred portion of the quarterly payments from March 2017 until December 31, 2018 on March 31, 2019 and thereafter a minimum payment of $250,000 each quarter up to a maximum of $10,000,000. Pursuant to the terms of the Sanofi APA, Icagen-T agreed to retain 46 employees at an estimated remaining cost to Icagen-T of $4,459,000 and to maintain and pay the maintenance costs of the Sanofi chemical libraries that remain at the Tucson Facility. We are also required to make significant payments under the terms of the Convertible Notes. If we are unable to generate sufficient revenues to pay our expenses and our existing sources of cash and cash flows are otherwise insufficient to fund our activities, we will need to raise additional funds to continue our operations at their current level and in order to fully implement our business plan. We do not have any commitments in place for additional funds. If needed, additional funds may not be available on favorable terms, or at all. As of the date hereof, we expect that our current cash and revenues generated from services, our private placement financings will provide us with enough funds to continue our operations at our current level for the next four months. Unless we raise additional funds or increase revenues we will be forced to curtail our operations and limit our marketing expenditures. Furthermore, if we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or debt securities, such as senior secured notes, may have rights, preferences and privileges senior to those of our existing stockholders. If we are unsuccessful in achieving profitability and we cannot obtain additional funds on commercially reasonable terms or at all, we may be required to curtail significantly or cease our operations significantly, it could result in the loss of all of your investment in our stock.
We have claims against us that may result in material adverse outcomes.
On April 22, 2014, Dentons’ US LLP (Dentons) filed a complaint against us seeking to confess a judgment in the amount of $3,050,000.00 based upon a settlement agreement we entered into with Dentons, dated July 5, 2013. In May 2017, we entered into a settlement agreement with Dentons that requires that we pay Dentons an aggregate of $1,400,000 over a fourteen (14) month period of which $1,200,000 has been paid to date. In addition, to secure its obligations under the agreement, we executed and delivered to Dentons a Confession of Judgment Affidavit in Support of Confession of Judgment in the amount of $3,891,549.32, representing the amount of the Judgment had obtained plus the costs of suit and interest accrued through May 15, 2017. The Confession of Judgment is not to be filed unless we default on our obligations under the agreement and the Confession of Judgment will be returned to us upon payment in full under the agreement. If the Confession of Judgment were to be enforced against Icagen, Inc. by Dentons it could result in, among other things, our cash balances being depleted and/or extinguished, or the seizure of assets, which would have material adverse effect on us and our ability to continue to operate our business.
10
We depend significantly on our relationship with our two third party collaborators.
A termination or expiration of our current collaboration with Sanofi or any potential future collaborations would adversely affect us financially and could harm our business reputation. The Pfizer MSA provided that we will perform ion channel screening and other contract research for Pfizer Inc. Pfizer Inc. guaranteed revenue of $1,000,000 for each of the first two years of the agreement (which two-year period ended July 2017), which represented a substantial portion of our revenue for the year ended December 31, 2016. The Sanofi MSA provides that Icagen-T will perform services for Sanofi at our Tucson Facility for the next three years for payments from Sanofi to Icagen-T of $8.75 million, so long as Icagen-T complies with the covenants set forth in the Sanofi MSA. Our collaboration with Pfizer and/ or Sanofi or future collaborations we may enter into may not be scientifically or commercially successful.
Collaborations with pharmaceutical companies and other third parties often are terminated or allowed to expire by the other party. A termination or expiration of our current collaboration with Pfizer, Sanofi or any potential future collaborations would adversely affect us financially and could harm our business reputation.
If we do not comply with certain of the covenants under the Sanofi MSA, Sanofi has the right to terminate the Sanofi MSA and foreclose on its lien on the Tucson Facility.
The Sanofi MSA has several affirmative and negative covenants as well as certain maintenance covenants with which Icagen-T must comply. Under the Sanofi MSA, the failure to comply with the maintenance covenants and certain responsibilities with respect to maintenance of the chemical libraries results in the automatic termination of the Sanofi MSA which would result in termination of the subsidy payments to us as well as the right of Sanofi to exercise its rights under the Deed of Trust and foreclose on its $5,000,000 lien on the Tucson Facility.
Our business is difficult to evaluate because we have recently changed our business model to offering a full complement of screening services to the broader pharmaceutical sector. There can be no guarantee that we will be able to effectively integrate the Icagen and Sanofi business.
Since our acquisition of the Icagen NC assets, we have shifted our business model from offering only our XRpro screening services to governmental agencies as we did in the past to now offer a full complement of screening services to the broader pharmaceutical sector. With the addition of the assets acquired from Sanofi, we now offer ultra high-throughput biology, screening and chemical capabilities. There is a risk that we will be unable to successfully conduct our business or be able to successfully integrate the assets acquired with our management and structure. Our estimates of capital, personnel and equipment required for our expanded business model are based on the experience of management and businesses they are familiar with. We are subject to the risks such as our ability to implement our business plan, market acceptance of our services, under-capitalization, cash shortages, limitations with respect to personnel, financing and other resources, competition from better funded and experienced companies, and uncertainty of our ability to generate revenues. There is no assurance that our activities will be successful or will result in any revenues or profit, and the likelihood of our success must be considered in light of the stage of our development. Even if we generate revenue, there can be no assurance that we will be profitable. In addition, no assurance can be given that we will be able to consummate our business strategy and plans, as described herein, or that financial, technological, market, or other limitations may force us to modify, alter, significantly delay, or significantly impede the implementation of such plans. We have insufficient results for investors to use to identify historical trends or even to make quarter to quarter comparisons of our operating results. You should consider our prospects in light of the risk, expenses and difficulties we will encounter as an early stage company. Our revenue and income potential is unproven and our business model is continually evolving. We are subject to the risks inherent to the operation of a new business enterprise, and cannot assure you that we will be able to successfully address these risks.
To date we have not established any partnerships or collaborations or entered into any licenses for our technology.
Although we expect to generate future revenue from partnerships and collaborations as well as license revenue for the use of our technology, to date, we have not derived any revenue from such sources. To date, our sole source of revenue has been our provision of services. We are currently in active negotiations with a collaborator that if consummated will provide us with an opportunity to receive revenue for providing early drug discovery services as well as clinical milestone payments and royalty payments. However, there can be no assurance that a collaboration will be entered into or if entered into that the milestones will be met and that we will receive the milestone payments or royalty payments.
We may not be able to utilize our tax net operating loss carry-forwards to offset future taxable income.
At December 31, 2017 we had approximately $20,624,000 in tax net operating loss carry-forwards available to offset future taxable income, thereby potentially reducing our future tax expense/liabilities. However, these tax net operating loss carry-forwards may be limited in accordance with IRC Section 382 following a more than 50 percentage point change in ownership, in aggregate during any three year look-back period. This potential limitation on our ability to use our tax net operating loss carry-forwards to offset future taxable income could result in increased tax expense/liabilities and decreased net earnings. These loss carry-forwards expire through 2034 if unused.
11
We must expend a significant amount of time and resources to develop new products, and if these products do not achieve commercial acceptance, our operating results may suffer.
We expect to spend a significant amount of time and resources to develop new products and refine existing products and have spent significant time and money developing our XRpro® instruments. We commenced development of our XRpro® instruments in the year 2006 and since then have developed four enhanced versions of our original instrument; each enhancement was developed over an approximate two-year period of time. We enhance our XRpro® instruments on a regular basis, including recent improvements to the throughput capabilities of the instrument, increasing production efficiency. We may also be required to make modifications or enhancements at the request of our customers. Our expense for research and development salaries for the year ended December 31, 2017 was $3,328,843 and for the year ended December 31, 2016 was $1,200,223, most of which was used to develop assays for commercial applications. In light of the long product development cycles inherent in our industry, any developmental expenditure will typically be made well in advance of the prospect of deriving revenues from the sale of new products. Our ability to commercially introduce and successfully market new products will be subject to a wide variety of challenges during this development cycle that could delay introduction of these products. In addition, since our potential customers are not expected to be obligated by long-term contracts to purchase our products, our anticipated product orders may not materialize, or orders that do materialize may be canceled. As a result, if we do not achieve market acceptance of new products, our operating results will suffer. Our products may also be priced higher than competitive products, which may impair commercial acceptance. We cannot predict whether new products that we expect to introduce will achieve commercial acceptance.
Our Chairman of the Board beneficially owns a substantial portion of our outstanding common stock and as well as a significant percentage of our voting power, which may limit your ability and the ability of our other stockholders, whether acting alone or together, to propose or direct the management or overall direction of our company.
The concentration of ownership of our stock could discourage or prevent a potential takeover of our company that might otherwise result in an investor receiving a premium over the market price for his shares. Our Chairman of the Board owns 164,284 shares of our common stock, 571,428 shares of the Series C Preferred Stock, which shares have the right to 1,714,284 votes and exercisable options, representing beneficial ownership of 13.3% of our outstanding shares of common stock and 23.2% of our voting power. In addition, the holders of the Series C Preferred Stock have the right to elect one director to our Board of Directors and have certain consent rights. In addition, the directors as a group beneficially own 2,586,240 shares of our common stock, 571,428 shares of the Series C Preferred Stock, which shares have the right to 1,714,284 votes and exercisable warrants and options, representing beneficial ownership of 31.6% of our outstanding shares of common stock and 31% of our voting power. Accordingly, our Chairman of the Board and our Board members alone and together with our directors would have significant influence over the election of our directors and the approval of actions for which the approval of our stockholders is required. If you acquire shares of our securities, you may have no effective voice in the management of our company. Such significant influence over control of our company may adversely affect the price of our common stock. Our Chairman of the Board as well as our board of directors may be able to significantly influence matters requiring approval by our stockholders, including the election of directors, as well as mergers or other business combinations which require the vote of a majority of our outstanding shares. Such significant influence may also make it difficult for our stockholders to receive a premium for their shares of our common stock in the event we merge with a third party or enter into different transactions which require stockholder approval. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock.
If we deliver products or services with defects, our credibility will be harmed and the sales and market acceptance of our products will decrease.
Our products and services are complex and may at times contain errors, defects and bugs when introduced. If in the future, we deliver products or services with errors, defects or bugs, our credibility and the market acceptance and sales of our products would be harmed. Further, if our products or services contain errors, defects or bugs, we may be required to expend significant capital and resources to alleviate such problems. Defects could also lead to product liability as a result of product liability lawsuits against us or against our customers. We may agree to indemnify our customers in some circumstances against liability arising from defects in our products or services. In the event of a successful product liability claim, we could be obligated to pay significant damages.
Most of our potential customers are from the pharmaceutical and biotechnology sector and are subject to risks faced by those industries.
We expect to derive a significant portion of our future revenues from sales to customers in the pharmaceutical and biotechnology sector, which includes governments and private companies. We expect a substantial part of our future revenue to be derived from pharmaceutical companies, including Pfizer, GSK and Sanofi. As a result, we will be subject to risks and uncertainties that affect the pharmaceutical and biotechnology industries, such as availability of capital and reduction and delays in research and development expenditures by companies in these industries, pricing pressures as third-party payers continue challenging the pricing of medical products and services, government regulation, and the uncertainty resulting from technological change.
12
In addition, our future revenues may be adversely affected by the ongoing consolidation in the pharmaceutical and biotechnology industries, as well as decisions of pharmaceutical companies to conduct the services we provide in house, which would reduce the number of our potential customers. Furthermore, we cannot assure you that the pharmaceutical and biotechnology companies that may be our customers will not develop their own competing products or capabilities, or choose our competitors’ technology instead of our technology.
Many of our current and potential competitors have significantly greater resources than we do, and increased competition could impair sales of our products and services.
We operate in a highly competitive industry and face competition from companies that design, manufacture and market instruments for use in the life sciences research industry, from genomic, pharmaceutical, biotechnology and diagnostic companies and from academic and research institutions and government or other publicly-funded agencies, both in the United States and elsewhere. We may not be able to compete effectively with all of these competitors. Many of these companies and institutions have greater financial, engineering, manufacturing, marketing and customer support resources than we do. As a result, our competitors may be able to respond more quickly to new or emerging technologies or market developments by devoting greater resources to the development, promotion and sale of products, which could impair sales of our products. Moreover, there has been significant merger and acquisition activity among our competitors and potential competitors. These transactions by our competitors and potential competitors may provide them with a competitive advantage over us by enabling them to rapidly expand their product offerings and service capabilities to meet a broader range of customer needs. Many of our potential customers are large companies that require global support and service, which may be easier for our larger competitors to provide. Many of the large pharmaceuticals companies have the financial resources to conduct the services we provide internally.
We believe that competition within the markets we serve is primarily driven by the need for innovative products that address the needs of customers. We attempt to counter competition by seeking to develop new products and provide quality, cost-effective products and services that meet customers’ needs. We cannot assure you, however, that we will be able to successfully develop new products or that our existing or new products and services will adequately meet our potential customers’ needs.
Rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition and frequent new product and service introductions characterize the markets for our products. To remain competitive, we may be required to develop new products and periodically enhance our existing products in a timely manner. We may face increased competition as new companies enter the market with new technologies that compete with our products and future products, and our services and future services. We cannot assure you that one or more of our competitors will not succeed in developing or marketing technologies products or services that are more effective or commercially attractive than our products or future products, or our services or future services, or that would render our technologies and products obsolete or uneconomical. Our future success will depend in large part on our ability to maintain a competitive position with respect to our current and future technologies, which we may not be able to do. In addition, delays in the launch of our new products or the provision of our services may result in loss of market share due to our customers’ purchases of competitors’ products or services during any delay.
We are dependent upon one vendor for consumables.
The consumables (i.e., chips or plates) used in our equipment upon which we conduct high throughput electrophysiology experiments are manufactured and sold by the same vendor who manufactures the equipment. Should this vendor fail to deliver the consumables in a timely manner this could adversely affect our assay services operations.
We depend on our key personnel, the loss of whom would impair our ability to compete.
We are highly dependent on the employment services of key management, engineering and scientific staff. The loss of the service of any of these persons could seriously harm our product development and commercialization efforts. In addition, research, product development and commercialization will require additional skilled personnel in areas such as chemistry and biology, and software and electronic engineering and recruitment and retention of personnel, particularly for employees with technical expertise, is uncertain. If we are unable to hire, train and retain a sufficient number of qualified employees, our ability to conduct and expand our business could be seriously reduced. Since our facilities are located in two specific cities, it may be difficult for us to attract employees in the cities in which our facilities are located. The inability to retain and hire qualified personnel could also hinder the planned expansion of our business and may result in us relocating some or all of our operations.
We have initiated and may in the future need to initiate lawsuits to protect or enforce our patents and other proprietary rights, which would be expensive and, if we lose, may cause us to lose some of our intellectual property rights, which would reduce our ability to compete in the market.
Our success will depend in part upon protecting our technology from infringement, misappropriation, duplication and discovery, and avoiding infringement and misappropriation of third party rights. We intend to rely, in part, on a combination of patent and contract law to protect our technology in the United States and abroad.
13
The risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| ● | the pending patent applications we have filed or to which we have exclusive rights may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | the claims of any patents which are issued may not provide meaningful protection; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | the patents licensed or issued to us or our customers may not provide a competitive advantage; |
| ● | other companies may challenge patents licensed or issued to us or our customers; |
| ● | patents issued to other companies may harm our ability to do business; |
| ● | other companies may independently develop similar or alternative technologies or duplicate our technologies; and |
| ● | other companies may design around the technologies we have licensed or developed. |
There can be no assurance that any of our patent applications or licensed patent applications will issue or that any patents that may issue will be valid and enforceable. We may not be successful in securing or maintaining proprietary patent protection for our products and technologies that we develop or license. In addition, our competitors may develop products similar to ours using methods and technologies that are beyond the scope of our intellectual property protection, which could reduce our anticipated sales. While some of our products have proprietary patent protection, a challenge to these patents can subject us to expensive litigation. Litigation concerning patents, other forms of intellectual property, and proprietary technology is becoming more widespread and can be protracted and expensive and distract management and other personnel from performing their duties.
We also rely upon trade secrets, unpatented proprietary know-how, and continuing technological innovation to develop a competitive position. If these measures do not protect our rights, third parties could use our technology, and our ability to compete in the market would be reduced. In addition, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries and our trade secrets may become known through other means not currently foreseen by us. We cannot assure you that others will not independently develop substantially equivalent proprietary technology and techniques or otherwise gain access to our trade secrets and technology, or that we can adequately protect our trade secrets and technology.
There can be no assurance that third parties will not assert infringement or other claims against us with respect to rights to any of our products. Litigation to protect and defend the rights to our licensed technology or to determine the validity of any third-party claims could result in significant expense to us and divert the efforts of our technical and management personnel, whether or not such litigation is determined in our favor. If we determine that additional rights are necessary for the development of our product(s) and further determine that a license to additional third-party rights is needed, there can be no assurance that we can obtain a license from the relevant party or parties on commercially reasonable terms, if at all. We could be sued for infringing patents or other intellectual property that purportedly cover products and/or methods of using such products held by persons other than us. Litigation arising from an alleged infringement could result in removal from the market, or a substantial delay in, or prevention of, the introduction of our products, any of which could have a material adverse effect on our business, financial condition, results of operations, and cash flows.
Additionally, in order to protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. Litigation may be necessary to:
| ● | assert claims of infringement; |
| ● | enforce our patents; |
| ● | protect our trade secrets or know-how; or |
| ● | determine the enforceability, scope and validity of the proprietary rights of others. |
Lawsuits could be expensive, take significant time and divert management’s attention from other business concerns. They would put our licensed patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing. We may also provoke third parties to assert claims against us. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in our industry are generally uncertain. If initiated, we cannot assure you that we would prevail in any of these suits or that the damages or other remedies awarded, if any, would be commercially valuable. During the course of these suits, there could be public announcements of the results of hearings, motions and other interim proceedings or developments in the litigation. If securities analysts or investors were to perceive any of these results to be negative, our stock price could decline.
14
We may incur substantial liabilities and may be required to limit commercialization of our products in response to product liability lawsuits.
We could be the subject of complaints or litigation from customers alleging product quality or operational concerns. Litigation or adverse publicity resulting from these allegations could materially and adversely affect our business, regardless of whether the allegations are valid or whether we are liable. We currently do not have product liability insurance coverage, and even if there was such coverage, there would be no assurance that such coverage would be sufficient to properly protect us. Further, claims of this type, whether substantiated or not, may divert our financial and management resources from revenue generating activities and the business operation.
We are subject to the risks of doing business internationally.
We currently offer our services to companies located outside of the United States, and therefore our business is subject to risks associated with doing business internationally, including:
| ● | trade restrictions and changes in tariffs; |
| ● | the impact of business cycles and downturns in economies outside of the United States; |
| ● | unexpected changes in regulatory requirements that may limit our ability to export our products or sell into particular jurisdictions; |
| ● | import and export license requirements and restrictions; |
| ● | difficulties in maintaining effective communications with employees and customers due to distance, language and cultural barriers; |
| ● | disruptions in international transport or delivery; |
| ● | difficulties in protecting our intellectual property rights, particularly in countries where the laws and practices do not protect proprietary rights to as great an extent as do the laws and practices of the United States; |
| ● | difficulties in enforcing agreements through non-U.S. legal systems; |
| ● | longer payment cycles and difficulties in collecting receivables; and |
| ● | potentially adverse tax consequences. |
If any of these risks materialize, our international sales could decrease and our foreign operations could suffer.
We may acquire other businesses or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of businesses and assets. To date we have made two acquisitions, the acquisition of certain of the assets of Icagen NC and the acquisition of the Tucson Facility from Sanofi. We also may pursue strategic alliances and joint ventures that leverage our technology and industry experience to expand our offerings or other capabilities. Though certain company personnel have business development and corporate transaction experience, including with licensing, and acquisitions, and strategic partnering, as a company we have limited experience with forming strategic alliances and joint ventures. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume known liabilities as well as unknown or contingent liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have a material adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a material negative effect on our results of operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance or joint venture.
To finance any acquisitions or joint ventures, we may choose to issue shares of our Common Stock as consideration, which would dilute the ownership of our stockholders. If the price of our Common Stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
15
The issuance of shares of common stock upon conversion of the Series C Preferred Stock would dilute the ownership of such holders and may adversely affect the market price of our common stock.
The conversion of the Series C Preferred Stock to common stock would dilute the ownership interest of existing holders of our common stock, and any sales in the public market of the common stock issuable upon conversion of the Series C Preferred Stock could adversely affect prevailing market prices of our common stock. Sales by such holders of a substantial number of shares of our common stock in the public market, or the perception that such sales might occur, could have a material adverse effect on the price of our common stock.
The holders of shares of the Series C Preferred Stock may exercise significant influence over us.
The holders of the Series C Preferred Stock will own approximately 15% of our shares of common stock on a fully diluted as-converted basis based on the number of shares of common stock outstanding as of the date hereof and have the right to votes or 31% of our outstanding voting securities.
In addition, under the terms of the Certificate of Designation that governs the Series C Preferred Stock, the Series C Preferred Stock generally ranks, with respect to liquidation, dividends and redemption, senior to other securities and, so long as any shares of Series C Preferred Stock remain outstanding, the approval of the holders of 75% of the Series C Preferred Stock outstanding at the time of approval is required in order for us to, among other things, (i) alter or change adversely the powers, preferences or rights given to the Series C Preferred Stock or alter or amend the Certificate of Designation; (ii) amend our Articles of Incorporation or bylaws in any manner that adversely affects any powers, preferences or rights of the Series C Preferred Stock; (iii) authorize or create any series or class of stock ranking as to redemption, distribution of assets upon a Liquidation Event (as defined in the Certificate of Designation) or dividends senior to, or otherwise pari passu with, the Series C Preferred Stock; (iv) declare or make any dividends other than dividend payments on the Series C Preferred Stock or other distributions payable solely in common stock; (v) authorize any increase in the number of shares of Series C Preferred Stock or issue any additional shares of Series C Preferred Stock; or (vi) enter into any agreement with respect to any of the foregoing.
16
The holders of Series C Preferred Stock will have rights, preferences and privileges that are not held by, and are preferential to, the rights of our common stockholders.
Upon our liquidation, dissolution or winding up, the holders of the Series C Preferred Stock will be entitled to receive out of our assets, in preference to the holders of the common stock and any junior preferred stock, an amount per share equal to the greater of (i) the sum of the Accreted Value (as defined in the Certificate of Designation) plus an amount equal to all accrued or declared and unpaid dividends on the Series C Preferred Stock that have not previously been added to the Accrued Value, or (ii) the amount that such shares would have been entitled to receive if they had converted into common stock immediately prior to such liquidation, dissolution or winding up. In addition, upon consummation of a specified change of control transaction, each holder of Series C Preferred Stock will be entitled to have us redeem the Series C Preferred Stock at a price specified in the Certificate of Designation. These provisions may make it more costly for a potential acquirer to engage in a business combination transaction with us. Provisions that have the effect of discouraging, delaying or preventing a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock. If there are insufficient assets to pay in full such amounts, then the available assets will be ratably distributed to the holders of the Series C Preferred Stock in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full. This will reduce the remaining amount of our assets, if any, available to distribute to holders of our common stock. The holders of Series C Preferred Stock also have a preferential right to receive cumulative dividends on the Accreted Value of each share of Series C Preferred Stock at an initial rate of 2% per annum, compounded quarterly.
In addition, the holders of the Series C Preferred Stock also have certain voting and conversion rights.
Our obligations to the holders of Series C Preferred Stock could limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our financial condition. These preferential rights could also result in divergent interests between the holders of shares of the Series C Preferred Stock and holders of our common stock.
It is difficult for us to determine the number of shares of common stock that we will be required to issue upon conversion of the Preferred Stock and exercise of our outstanding Warrants issued with the Preferred Stock.
Since the conversion price of our Preferred Stock issued in the March 2018 financing and the exercise price of the Purchaser Warrants issued in the March 2018 financing is subject to reduction if we issue certain future securities at prices that are lower than the conversion price of the Preferred Stock or exercise price of the Purchaser Warrants, we cannot at this time determine the number of shares of common stock that we will be required to issue upon conversion of the Preferred Stock or exercise of the Purchaser Warrants.
Our failure to fulfill all of our registration requirements may cause us to suffer damages, which may be very costly.
Pursuant to the terms of the registration rights agreement that we entered into with investors in our recent private placement offering, we are required to file a registration statement with respect to securities issued to them within a certain time period and maintain the effectiveness of such registration statement. The failure to do so could result in the payment of damages by us. There can be no assurance as to when this registration statement will be declared effective or that we will be able to maintain the effectiveness of any registration statement, and therefore there can be no assurance that we will not incur damages with respect to such agreements.
RISKS RELATED TO OUR DEBT OBLIGATIONS
The failure to comply with the terms of the Convertible Notes could result in a default under the terms of the notes and, if uncured, it could potentially result in action against our pledged assets.
In the May 2017 debt financing, we issued a note in the aggregate principal amount of $2,000,000 (the “Company Note”) together with a purchase warrant to purchase initially up to 857,143 shares of our common stock (the “Purchaser Warrants”), and Icagen-T issued a convertible note in the aggregate principal amount of $8,000,000 (the “Icagen-T Note”), which Convertible Notes are secured by a security interest in all of our existing and future assets, subject to existing security interests and exceptions. The Convertible Notes require us and Icagen-T, respectively, among other things, to maintain the security interest, make monthly installment payments, and meet various negative and affirmative covenants. If we or Icagen-T fails to comply with the terms of the Convertible Notes and/or the related agreements, the senior note holder could declare a note default and if the default were to remain uncured, the secured creditor would have the right to proceed against any or all of the collateral securing their Convertible Note, subject to the first priority of our secured creditors. Any action by our secured or unsecured creditors to proceed against our assets would likely have a serious disruptive effect on our business operations.
17
Servicing our debt requires a significant amount of cash. Our ability to generate sufficient cash to service our debt depends on many factors beyond our control.
Our ability to make payments on and to refinance our debt, to fund planned capital expenditures and to maintain sufficient working capital depends on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. We cannot assure you that our business will generate sufficient cash flow from operations or from other sources in an amount sufficient to enable us to service our debt or to fund our other liquidity needs. If our cash flow and capital resources are insufficient to allow us to make scheduled payments on our debt, we may need seek additional capital or restructure or refinance all or a portion of our debt on or before the maturity thereof, any of which could have a material adverse effect on our business, financial condition or results of operations. We cannot assure you that we will be able to refinance any of our debt on commercially reasonable terms or at all, or that the terms of that debt will allow any of the above alternative measures or that these measures would satisfy our scheduled debt service obligations. If we are unable to generate sufficient cash flow to repay or refinance our debt on favorable terms, it could significantly adversely affect our financial condition and the value of our outstanding debt. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. There can be no assurance that we will be able to obtain any financing when needed.
Our substantial leverage may impair our financial condition and prevent us from fulfilling our obligations under the notes.
We have a substantial amount of indebtedness. As of December 31, 2017, our total debt owed under outstanding notes was $10.1 million. Our substantial leverage could have important consequences to investors, including:
| ● | making it more difficult for us to satisfy our obligations with respect to the Convertible Notes; |
| ● | increasing our vulnerability to general adverse economic and industry conditions by making it more difficult for us to react quickly to changing conditions; |
| ● | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, acquisitions and other general corporate requirements; |
| ● | requiring a substantial portion of our cash flow from operations for the payment of interest on our indebtedness and reducing our ability to use our cash flow to fund working capital, capital expenditures, acquisitions and general corporate requirements; |
| ● | limiting our flexibility in planning for, or reacting to, changes in our business, and the industry in which we operate; and |
| ● | placing us at a competitive disadvantage compared with our competitors that have less indebtedness. |
Covenant restrictions under our indebtedness may limit our ability to operate our business.
The Convertible Notes contain, and our future indebtedness agreements may contain covenants that may restrict our ability to finance future operations or capital needs or to engage in other business activities. The Convertible Notes restrict our ability and the ability of our restricted subsidiaries to:
| ● | incur, assume or guarantee additional Indebtedness (as defined in the Convertible Notes); |
| ● | repurchase capital stock; |
| ● | make other restricted payments including, without limitation, paying dividends and making investments; |
| ● | create liens; |
| ● | sell or otherwise dispose of assets, including capital stock of subsidiaries; |
| ● | enter into agreements that restrict dividends from subsidiaries; |
| ● | enter into mergers or consolidations; and |
| ● | enter into transactions with affiliates |
A breach of any of these covenants would result in a default under our Convertible Notes. If an event of default under our Convertible Notes occurs, the lenders could elect to declare all amounts outstanding thereunder, together with accrued interest, to be immediately due and payable. If we were unable to pay such amounts, the lenders could proceed against the collateral pledged to them. We have pledged our inventory, accounts receivable, cash, securities, other general intangibles and the capital stock of certain subsidiaries to the lenders. In addition, Icagen-T has pledged all of its assets, including the Facility, subject to the Sanofi lien. In such an event, we cannot assure you that we would have sufficient assets to pay amounts due on the Convertible Notes.
18
It may be difficult to realize the value of the collateral securing the Convertible Notes.
The collateral securing the Convertible Notes is subject to any and all exceptions, defects, encumbrances, liens and other imperfections as may be accepted by the collateral agent for the notes and any other creditors that also have the benefit of liens on the collateral securing the Convertible Notes from time to time, whether on or after the date the Convertible Notes are issued. The existence of any such exceptions, defects, encumbrances, liens or other imperfections could adversely affect the value of the collateral securing the notes as well as the ability of the collateral agent to realize or foreclose on such collateral.
No appraisals of any collateral were prepared in connection with the Convertible Notes and appraisals relied upon were not currently obtained. The value of the collateral at any time will depend on market and other economic conditions, including the availability of suitable buyers. By their nature, some or all of the pledged assets may be illiquid and may have no readily ascertainable market value. Although we believe that the fair market value of the collateral exceeds the principal amount of the indebtedness secured thereby and the prior lien thereon, we cannot assure you that the fair market value of the collateral exceeds the principal amount of the indebtedness secured thereby and the prior lien thereon. The value of the assets pledged as collateral for the notes could be impaired in the future as a result of changing economic conditions, our failure to implement our business strategy, competition or other future trends.
It is difficult for us to determine the number of shares of common stock that we will be required to issue upon conversion of the Convertible Notes, and exercise of our outstanding Warrants.
Since the conversion price of our Convertible Notes issued in the May 2017 debt financing and the exercise price of the Purchaser Warrants issued in the May 2017 debt financing is subject to reduction if we issue certain future securities at prices that are lower than the conversion price of the Convertible Notes or exercise price of the Purchaser Warrants, we cannot at this time determine the number of shares of common stock that we will be required to issue upon conversion of the Convertible Notes or exercise of the Purchaser Warrants.
We may not be able to make the redemption payments required by the Convertible Notes.
Upon a Fundamental Transaction (as defined in the Convertible Notes) or an Event of Default, we are required to offer to repurchase all outstanding Convertible Notes at various prices. The source of funds for that purchase of Convertible Notes will be our available cash or cash generated from our subsidiaries’ operations or other potential sources, including borrowings, sales of assets or sales of equity. We cannot assure you that sufficient funds from such sources will be available at the time of any Fundamental Transaction or Event of Default to make required repurchases of Convertible Notes tendered. If the holders of the Convertible Notes exercise their right to require us to repurchase all of the notes upon a Fundamental Transaction, the financial effect of this repurchase could cause a default under our other indebtedness, even if the Fundamental Transaction itself would not cause a default. Accordingly, it is possible that we will not have sufficient funds at the time of a Fundamental Transaction or Event of Default to make the required repurchase of the Convertible Notes.
Conversion of our outstanding Convertible Notes and exercise of the outstanding warrants will dilute the ownership interest of existing stockholders.
Any issuance by us of our common stock upon conversion of the outstanding Convertible Notes or exercise of the outstanding warrants will dilute the equity ownership interest of existing stockholders, including holders who have received shares of our common stock upon prior conversion of Convertible Notes or exercise of Purchaser Warrants. Additionally, the Convertible Notes and Purchaser Warrants include anti-dilution and “make whole” premium provisions that, if triggered, would result in an increase in the number of shares of our common stock issuable upon conversion of the Convertible Notes or exercise of the warrants. Conversion of Convertible Notes or exercise of warrants in circumstances where these provisions have operated could have a significantly greater dilutive effect.
Our failure to fulfill all of our registration requirements may cause us to suffer liquidated damages, which may be very costly.
Pursuant to the terms of the registration rights agreement that we entered into with investors in our recent private placement offering, we are required to file a registration statement with respect to securities issued to them within a certain time period and maintain the effectiveness of such registration statement. The failure to do so could result in the payment of damages by us. There can be no assurance as to when this registration statement will be declared effective or that we will be able to maintain the effectiveness of any registration statement, and therefore there can be no assurance that we will not incur damages with respect to such agreements.
19
RISKS ASSOCIATED WITH INVESTING IN OUR COMMON STOCK
Our financial statements may not be comparable to companies that comply with public company effective dates.
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2) of the Jobs Act, that allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
As a result of our being a public company, we are subject to additional reporting and corporate governance requirements that require additional management time, resources and expense.
We are obligated to file with the SEC annual and quarterly information and other reports that are specified in the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We are also subject to other reporting and corporate governance requirements under the Sarbanes-Oxley Act of 2002, as amended, and the rules and regulations promulgated thereunder, all of which impose significant compliance and reporting obligations upon us. The costs of preparing and filing annual and quarterly reports and other information with the SEC has and will continue to cause our expenses to be higher than they would be if we were a privately-held company.
Our internal controls over financial reporting are not effective which could have a significant and adverse effect on our business and reputation.
We have identified a material weakness in our internal controls and can’t provide assurances that the weakness will be effectively remediated. As a public reporting company, we are in a continuing process of developing, establishing, and maintaining internal controls and procedures that allow our management to report on, and when applicable our independent registered public accounting firm to attest to, our internal controls over financial reporting if and when required to do so under Section 404 of the Sarbanes-Oxley Act of 2002. Our management is required to report on our internal controls over financial reporting under Section 404. If we fail to achieve and maintain the adequacy of our internal controls, we would not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. Our management has determined that the adequacy of our internal controls is not effective and is therefore unable to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. Moreover, our testing, or the subsequent testing by our independent registered public accounting firm, that must be performed may reveal other material weaknesses or that the material weaknesses described above have not been fully remediated. If we do not remediate any material weaknesses identified, or if other material weaknesses are identified or we are not able to comply with the requirements of Section 404 in a timely manner, our reported financial results could be materially misstated or could subsequently require restatement, we could receive an adverse opinion regarding our internal controls over financial reporting from our independent registered public accounting firm and we could be subject to investigations or sanctions by regulatory authorities, which would require additional financial and management resources, and the market price of our stock could decline.
Future sales of our common stock and other securities by our existing shareholders after a public market is established could cause our stock price to decline.
We currently have 6,393,107 shares of our common stock outstanding and 571,428 shares of the Series C Preferred Stock outstanding that are convertible into 571,428 shares of our common stock. All of the common shares are eligible for resale under Rule 144 and the shares issuable upon conversion of the Series C Preferred Stock will be eligible for resale under Rule 144 six (6) months after their issuance date; however, 792,762 shares of common stock and 571,428 shares currently issuable upon conversion of the Series C Preferred Stock are held by affiliates and are subject to certain volume limitations and secondly, Rule 144 requires that the shares be sold in “broker’s transactions” which is not possible before a public market for the common stock is established. If our shareholders were to sell substantial amounts of our common stock in the public market at the same time, the market price of the common stock could decrease significantly due to an imbalance in the supply and demand of our common stock. Even if they do not actually sell the stock, the perception in the public market that our shareholders might sell significant shares of the common stock could also depress the market price of the common stock.
A decline in the future publicly traded price of the shares of common stock might impede our ability to raise capital through the issuance of additional shares of our common stock or other equity securities and may cause you to lose part or all of your investment in our shares of common stock.
We do not expect to pay dividends on our common stock in the foreseeable future.
We do not expect to pay dividends on our common stock for the foreseeable future, and we may never pay dividends. In addition, the covenants contained in the Convertible Notes prohibit the payment of any dividends. Consequently, the only opportunity for common stockholders to achieve a return on their investment may be if a trading market develops and common stockholders are able to sell their shares for a profit or if our business is sold at a price that enables Common stockholders to recognize a profit. We currently intend to retain any future earnings to support the development and expansion of our business and do not anticipate paying cash dividends for the foreseeable future. Our payment of any future dividends will be at the discretion of our board of directors after taking into account various factors, including but not limited to our financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. In addition, our ability to pay dividends on our common stock may be limited by state law. Accordingly, we cannot assure investors any return on their investment, other than in connection with a sale of their shares or a sale of our business. At the present time, there is no trading market for our shares. Therefore, holders of our securities may be unable to sell them. We cannot assure investors that an active trading market will develop or that any third party will offer to purchase our business on acceptable terms and at a price that would enable our investors to recognize a profit.
20
Limitations on director and officer liability and indemnification of our Company’s officers and directors by us may discourage stockholders from bringing suit against an officer or director.
Our certificate of incorporation and bylaws provide, with certain exceptions as permitted by governing state law, that a director or officer shall not be personally liable to us or our stockholders for breach of fiduciary duty as a director or officer, except for acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or unlawful payments of dividends. These provisions may discourage stockholders from bringing suit against a director or officer for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director or officer.
We are responsible for the indemnification of our officers and directors.
Should our officers and/or directors require us to contribute to their defense, we may be required to spend significant amounts of our capital. Our certificate of incorporation and bylaws also provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on behalf of our Company. This indemnification policy could result in substantial expenditures, which we may be unable to recoup. If these expenditures are significant or involve issues which result in significant liability for our key personnel, we may be unable to continue operating as a going concern.
Our common stock is not currently traded on any market and there can be no assurance that an active trading market for the common stock will ever be established
The common stock is not currently traded on any market and therefore no public market for our common stock exists. In addition, no assurance can be given that an active trading market for the common stock will ever be established. Even if a public market for our common stock is established on a securities exchange, we cannot predict the extent to which investors’ interests will lead to an active trading market for our common stock or whether the market price of our common stock will be volatile or exceed the price paid by investors for the common stock or the exercise price of our warrants outstanding. If an active trading market does not develop, investors will continue to have difficulty selling any of our common stock. There may be limited market activity in our stock and we are likely to be too small to attract the interest of many brokerage firms and analysts. If we trade on OTC markets, the trading volume we will develop may be limited by the fact that many major institutional investment funds, including mutual funds as well as individual investors, follow a policy of not investing in OTC stocks and certain major brokerage firms restrict their brokers from recommending OTC stocks because they are considered speculative, volatile, thinly traded and the market price of the common stock may not accurately reflect the underlying value of our company. The market price of our common stock could be subject to wide fluctuations in response to quarterly variations in our revenues and operating expenses, announcements of new products or services by us or competitors, significant sales of our common stock, including “short” sales, the operating and stock price performance of other companies that investors may deem comparable to us, and news reports relating to trends in our markets or general economic conditions.
We may seek to raise additional funds in the future, which may be dilutive to stockholders or impose operational restrictions.
If our revenue from operations together with the proceeds of our recent debt financing and this offering are not sufficient to cover our operating expenses, we will need to raise additional capital. If we raise additional capital through the issuance of equity or of debt securities, the percentage ownership of our current stockholders will be reduced. We may also enter into strategic transactions, issue equity as part of license issue fees to our licensors, compensate consultants or settle outstanding payables using equity that may be dilutive. Our stockholders may experience additional dilution in net book value per share and any additional equity securities may have rights, preferences and privileges senior to those of the holders of our common stock.
The application of the “penny stock” rules to our common stock could limit the trading and liquidity of the common stock, adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
The SEC has adopted regulations which generally define a “penny stock” to be any equity security that has a market price, as therein defined, of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. Additionally, if the equity security is not registered or authorized on a national securities exchange that makes certain reports available, the equity security may also constitute a “penny stock.” If our common stock becomes traded on a securities market or exchange, as long as the trading price of our common stock is below $5 per share, the open-market trading of our common stock will be subject to the “penny stock” rules, unless we otherwise qualify for an exemption from the “penny stock” definition. The “penny stock” rules impose additional sales practice requirements on certain broker- dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). These regulations, if they apply, require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the associated risks. Under these regulations, certain brokers who recommend such securities to persons other than established customers or certain accredited investors must make a special written suitability determination regarding such a purchaser and receive such purchaser’s written agreement to a transaction prior to sale. These regulations may have the effect of limiting the trading activity of our common stock, reducing the liquidity of an investment in our common stock and increasing the transaction costs for sales and purchases of our common stock as compared to other securities. The stock market in general and the market prices for penny stock companies in particular, have experienced volatility that often has been unrelated to the operating performance of such companies. These broad market and industry fluctuations may adversely affect the price of our stock, regardless of our operating performance. Stockholders should be aware that, according to SEC Release No. 34-29093, the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include: (i) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (ii) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (iii) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (iv) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (v) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. The occurrence of these patterns or practices could increase the volatility of our share price.
21
We may not be able to attract the attention of major brokerage firms, which could have a material adverse impact on the market value of our common stock.
If a trading market develops for our common stock, it will rely in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts. However, security analysts of major brokerage firms may not provide coverage of our common stock since there is no incentive to brokerage firms to recommend the purchase of our common stock, which may adversely affect the market price of our common stock. If equity research analysts do provide research coverage of our common stock, the price of our common stock could decline if one or more of these analysts downgrade our common stock or if they issue other unfavorable commentary about us or our business. If one or more of these analysts’ ceases coverage of our company, we could lose visibility in the market, which in turn could cause our stock price to decline.
Item 1B. Unresolved Staff Comments
None.
Durham, North Carolina
The Company entered into an asset purchase agreement with Icagen, Inc., a subsidiary of Pfizer, Inc., whereby certain assets were acquired from Icagen, Inc., the agreement included the sub-letting of premises located at Research Triangle Park, Durham, North Carolina with annual calendar year escalations of 3.5%. The lease terminates on April 30, 2019. The rental expense for the year ended December 31, 2017 amounted to $198,820. We believe that we have adequate space for our anticipated needs.
Tucson, Arizona
The Company entered into an Asset Purchase Agreement with Sanofi, pursuant to which Icagen-T, a wholly owned subsidiary of the Company, acquired certain assets of Sanofi that include the Tucson Research Center, a two-story laboratory and office building with approximately 113,950 square feet of space located in the Town of Oro Valley, Pima County, Arizona and the land on which the Facility is built. We believe that we have adequate space for our anticipated needs.
Upon the closing of the Asset Purchase Agreement, on July 15, 2016, Icagen-T executed a Deed of Trust providing Sanofi with a five year, $5 million lien on the Tucson Facility, securing performance of Icagen-T’s obligations under the MSA and the Asset Purchase Agreement. The lien is subject to termination upon the payment by Icagen-T of $5 million to Sanofi. The parties have also agreed to a Deed of Sale that will revest in Sanofi all rights in the Tucson Facility in the event that Icagen-T sells the Tucson Facility at any time within the next five years and upon certain other events related to the leasing of space at the Tucson Facility. The reversion rights of Sanofi under the Deed of Sale will terminate after five years as well as upon payment of the $5 million to extinguish the lien created by the Deed of Trust.
Dentons Dispute
On May 11, 2017, the Company entered into a Settlement and Release Agreement with Dentons US LLP (“Dentons”) relating to disputes arising between them under a Settlement and Release Agreement, dated July 5, 2013 (the “2013 Settlement Agreement”), a judgment thereafter obtained by Dentons on May 7, 2014 in the Circuit Court of Cook County, Illinois Lawsuit based upon the 2013 Settlement in the amount of $3,050,000, and a lawsuit filed by the Company in San Francisco Superior Court in or about April 2014 against Dentons. In connection with the Agreement, the Company has agreed to pay Dentons the sum of $1,400,000 over a fourteen month period of which $1,200,000 has been paid to date. In addition, to secure its obligations under the Agreement, the Company executed and delivered to Dentons a Confession of Judgment Affidavit in Support of Confession of Judgment in the amount of $3,891,549.32, representing the amount of the Judgment had obtained plus the costs of suit and interest accrued through May 15, 2017. The Confession of Judgment is not to be filed unless the Company defaults on its obligations under the Agreement and it will be returned to the Company upon payment in full under the Agreement. The Agreement included mutual releases of claims each party had against the other and the parties also agreed to dismiss the litigation between them with prejudice; provided, that Dentons’ obligations commence after it has received $500,000 of the payments from the Company described above.
Item 4. Mine Safety Disclosures
Not applicable.
22
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchase of Equity Securities
Our common stock is not currently trading on any established market and neither is the Series C Preferred Stock.
As of April 13, 2018, there were 266 holders of our common stock and 0 holders of our Preferred Stock.
Dividend Policy
We have never paid any cash dividends on our common stock to date, and do not anticipate paying such cash dividends in the foreseeable future. Whether we declare and pay dividends is determined by our Board of Directors at their discretion, subject to certain limitations imposed under Delaware corporate law. The timing, amount and form of dividends, if any, will depend on, among other things, our results of operations, financial condition, cash requirements and other factors deemed relevant by our Board of Directors.
Equity Compensation Plan Information
See “Item 11 – Executive Compensation” for equity compensation plan information.
Recent Sales of Unregistered Securities
We did not sell any equity securities during the fiscal year ended December 31, 2017 in transactions that were not registered under the Securities Act, other than as disclosed above or as previously disclosed in our filings with the Securities and Exchange Commission.
Issuer Purchases of Equity Securities
There were no issuer purchases of equity securities during the fiscal year ended December 31, 2017.
Item 6. Selected Financial Data
Not applicable because we are a smaller reporting company.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis is intended as a review of significant factors affecting our financial condition and results of operations for the periods indicated. The discussion should be read in conjunction with our consolidated financial statements and the notes presented herein. In addition to historical information, the following “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contains forward-looking statements that involve risks and uncertainties. Our actual results could differ significantly from those expressed, implied or anticipated in these forward-looking statements as a result of certain factors discussed herein and any other periodic reports filed and to be filed with the Securities and Exchange Commission.
Cautionary Note Regarding Forward-Looking Statements
This report and other documents that we file with the Securities and Exchange Commission contain forward-looking statements that are based on current expectations, estimates, forecasts and projections about our future performance, our business, our beliefs and our management’s assumptions. Statements that are not historical facts are forward-looking statements. Words such as “expect,” “outlook,” “forecast,” “would,” “could,” “should,” “project,” “intend,” “plan,” “continue,” “sustain”, “on track”, “believe,” “seek,” “estimate,” “anticipate,” “may,” “assume,” and variations of such words and similar expressions are often used to identify such forward-looking statements, which are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward- looking statements are not guarantees of future performance and involve risks, assumptions and uncertainties, including, but not limited to, those described in our reports that we file or furnish with the Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those indicated or anticipated by such forward-looking statements. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. Except to the extent required by law, we undertake no obligation to update publicly any forward-looking statements after the date they are made, whether as a result of new information, future events, changes in assumptions or otherwise.
23
Overview and Financial Condition
We are engaged in the advancement of early drug discovery. Our business model is divided into three sources of revenue: our integrated drug discovery services that we have been providing to third parties since our inception; partnerships and collaborations with third parties that should allow us to receive revenue for services provided as well as future milestone and royalty payments and potential licensing fees and other related fees paid for the licensing of our technology.
For the past two years, a significant portion of our revenue has been derived from our operations as a partner research organization providing integrated drug discovery services with unique expertise in the field of ion channel, transporter, neuroscience, muscle biology and rare disease targets while also covering many other classes of drug discovery targets and therapeutic areas. Our customers are pharmaceutical and biotechnology companies to whom we offer our industry-leading scientific expertise and technologies to aid in their determination of which molecules to advance into late stage preclinical studies and ultimately clinical trials. The core of our offering is the discovery of pre-clinical drug candidates (PDC’s), which are lead molecules (Leads) that are selected to enter into in-vivo studies during the pre-clinical phase of drug discovery. We offer a full complement of pre-clinical drug discovery services which include; assay development technologies (including high throughput fluorescence, manual and automated electrophysiology and radiotracer flux assays), cell line generation, high-throughput and ultra-high-throughput screening, medicinal chemistry, computational chemistry and custom assay services to our customers. Our capabilities also include molecular biology and the use of complex functional assays, electrophysiology, bioanalytics and pharmacology. We believe that this integrated set of capabilities enhances our ability to help our customers identify drug candidates.
More recently, we have begun to focus on partnership and collaboration opportunities with third parties, and we are currently in active negotiations regarding our first such collaboration. This collaboration is expected to provide us with an opportunity to derive revenue not only from our standard fees for integrated early discovery services but also from future milestone and royalty revenue from product candidates that may be developed and commercialized with our aid. We have developed in house a portfolio of nine assets targeting different indications that we believe would be ideal candidates for partnership opportunities.
Since inception, we have financed our operations primarily through private sales of our securities, settlement of legal matters. We expect to continue to seek to obtain our required capital through the private sale of securities and revenue derived for the services we provide.
Subsequent to our acquisition of certain assets from Pfizer and Sanofi, a substantial portion of our revenue has been derived from our operations as a partner research organization from two commercial customers. We have also entered into Master Services Agreements (“MSA”) with other various pharmaceutical companies where we have agreed to perform certain services for them.
For the year ended December 31, 2017, 56.2% of our revenue was derived from services sales, 42.4% was derived from deferred subsidy revenue and 1.4% was generated from Government revenue. For the year ended December 31, 2016, 59.5% (2015 – 84%) of our revenue was derived from commercial services; 36.7% was derived from subsidies (2015 – 0%) and the remaining 3.8% was generated from Government revenues (2015 – 16%). Despite generating funds from commercial customers and government grants, we continue to experience losses. We believe that our existing cash and cash equivalents, including the $2,000,000 that we raised from our recent sale of shares of Series C Preferred Stock and warrants to purchase common stock, will not be sufficient to meet our anticipated cash needs for the next four months. We will need to generate additional revenue from operations and/or obtain additional financing to pursue our business strategy, to respond to new competitive pressures or to take advantage of opportunities that may arise. These factors raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended December 31, 2017 with respect to this uncertainty. To meet our financing needs, we are considering multiple alternatives, including, but not limited to, additional equity financings, debt financings and/or funding from partnerships or collaborations. There can be no assurance that we will be able to complete any such transactions on acceptable terms or otherwise.
24
Prior to our acquisition of the Icagen assets, substantially all of our revenue was derived from government grants related to the use of our XRpro technology. To date, we have been granted twenty-one grants and contracts from United States governmental agencies; of which nine were granted from the Department of Defense and twelve were granted from the National Institutes of Health. Of such contracts, twenty have been completed and we received payment in full for all twenty completed contracts. There is one contract from the National Institutes of Health under which we performed services during the year ended December 31, 2017, this contract was for $600,000 and has been fully invoiced as of December 31, 2017. The NIH invited us to submit a Phase II SBIR application for the completed contract of which we are awaiting the outcome.
As a result of the agreements that we entered into with Pfizer and Sanofi, we have incurred significant obligations including the obligation ; (i) to continue to retain certain employees at our Icagen-T facility until July 15, 2018, which we estimate will require additional compensation of $4,459,000 at our Icagen-T facility; (ii) make additional payments in terms of the Asset Purchase and Collaboration Agreement that we entered into on June 26, 2015 with Pfizer including beginning in 2017, a quarterly earn out payment (the “Earn Out Payment”) of 10% of revenue earned during the quarter, with a minimum payment of $250,000 per quarter, up to a maximum aggregate payment of $10,000,000, such minimum being reduced to $50,000 for the quarters ending March 2017 to December 2018 and the difference between $250,000 or the quarterly amount paid and the actual calculation of deferred purchase consideration at 10% of gross revenue per quarter is being deferred and paid as one lump sum with the payment being made the quarter ended March 31, 2019, bearing interest at 12.5% per annum, which interest is payable quarterly; (iii) make minimum lease payments in terms of a sub-lease agreement entered into with Pfizer for the period July l, 2015 to April 30, 2019 with annual escalations of 3.5%, estimated to be $260,256.
We also have incurred obligations in connection with our litigations that resulted in significant payments by us during the year ended December 31, 2017. In terms of a Mutual Release and Assignment Agreement that we entered into with American Milling LP, we agreed to pay American Milling $800,000 of which $83,334 remains to be paid at December 31, 2017. In addition, we reached a Settlement and Release Agreement with Dentons pursuant to which we agreed to pay Dentons the sum of $1,400,000 over a period of fourteen months, of which $1,200,000 has been paid to date.
25
Discussions with respect to our operations included herein include the operations of our operating subsidiaries, Icagen Corp and Icagen-T, Inc. We have another two subsidiary companies, Caldera Discovery Inc. and XRpro Sciences Inc., which have always been dormant.
Results of Operations for the year ended December 31, 2017 and the year ended December 31, 2016
Revenues
For the year ended December 31, 2017, we had revenues totaling $22,656,610 ($12,735,867 of which was from commercial revenue, $ 9,600,000 was from subsidy revenue and $320,743 was from government grants), and for the year ended December 31, 2016, we had revenues totaling $11,995,377 ($7,139,394 of which was from commercial revenue, $4,400,000 was from subsidy revenue and $455,983 was from government grants), an increase of $10,661,233 or 88.9%. The increase in revenue is due to an increase in commercial revenues of $5,596,473; deferred subsidy revenue of $5,200,000; and a decrease in government revenue of $135,240. In the prior year, commercial revenues were $7,139,394 (representing 59.5% of our revenue); deferred subsidy revenue of $4,400,000 (representing 36.7% of our revenue) and government revenues were $455,983, (representing 3.8% of our revenue). The increase in revenue over the prior year is primarily attributable to the following; i) work performed for Sanofi under the MSA agreement that we entered into with them upon the acquisition of certain assets and personnel at the Tucson Facility, amounting to $6,900,000; ii) deferred subsidy revenue from Sanofi of $9,600,000 recognized during the current period to support our operations and maintain the facility and employees and iii) commercial revenue generated from our North Carolina site increased by $1,114,254 over the prior year, or by 31.2%. We continue to increase our customer base during the current year. Commercial revenue includes revenue from 36 (2016 – 32) customers, including four large pharma customers. The Government contract revenue was derived from one government contract which has terminated at December 31, 2017.
We continue to market our services to several pharmaceutical and biotechnology companies. We believe that we now have a comprehensive product offering and substantial credibility to offer a full range of products including the advantages and value propositions of the XRpro® technology. While we are optimistic about our prospects, there can be no assurance about whether or when our products will generate sufficient revenues with adequate margins in order for us to be profitable.
Cost of goods sold
Cost of goods sold totaled $11,175,692 and $6,371,873 for the years ended December 31, 2017 and 2016, respectively, an increase of $4,803,819 or 75.4%. Cost of goods sold is primarily comprised of direct expenses related to providing our services to our customers. These direct expenses include salary expenses directly related to our statements of work and research contracts including those of our scientific personnel expenses, recoverable expenses incurred on contracts, the cost of outside consultants, and direct materials used on our contracts.
| ● | The salary expense included in cost of sales for the year ended December 31, 2017 and 2016 respectively was $6,880,513 and $4,558,598, an increase of $2,321,915 or 50.9%. This is primarily due to the inclusion of the Tucson site for a full year during the current year, the site was acquired on July 15, 2016. For additional information regarding salary expense reference is made to the discussion of total salary expense in selling, general and administrative expenses below. |
| ● | The laboratory supplies and direct materials included in cost of sales for the year ended December 31, 2017 and 2016, respectively was $3,303,311 and $1,119,379, an increase of $2,183,932 or 195.1%, the increase is primarily due to the inclusion of the Tucson site for the full year and an increase in laboratory supply expense at our Durham site based on the type of activity being performed at that site. |
| ● | Outside contractors’ cost included in cost of sales for the year ended December 31, 2017 and 2016, respectively, amounted to $991,868 and $693,297, an increase of $298,571 or 43.1% is due to, (i) third party laboratory equipment maintenance contracts for the Tucson Facility and (ii) costs of four outside laboratory personnel at the North Carolina \facility who perform laboratory services for us on an ongoing basis. |
Gross profit
Gross profit was $11,480,918 and $5,623,504 for the years ended December 31, 2017 and 2016, respectively, an increase of $5,857,414, or 104.2%. The increase in gross profit is primarily due to the increase in the commercial revenue at the Tucson facility and the inclusion of this facility for the full fiscal year during the current year, the increase in commercial revenues at our North Carolina facility and the substantial deferred subsidy revenue generated by the Tucson facility.
26
Selling, general and administrative expenses
Selling, general and administrative expenses totaled $13,936,542 and $8,079,663 for the years ended December 31, 2017 and 2016, respectively, an increase of $5,856,879 or 72.5%. The increase is primarily due to the added expenses of the Tucson Facility for a full year in 2017 as opposed to the half year in 2016.
The major expenses making up selling, general and administrative expenses included the following:
| Year ended December 31, | Increase/ | Percentage | ||||||||||||||
| 2017 | 2016 | (decrease) | change | |||||||||||||
| Marketing and selling expenses | $ | 412,122 | $ | 256,078 | $ | 156,044 | 60.9 | % | ||||||||
| Payroll expense | 4,228,651 | 2,567,039 | 1,661,612 | 64.7 | % | |||||||||||
| Research and development salaries | 3,328,843 | 1,200,223 | 2,128,620 | 177.4 | % | |||||||||||
| Directors fees | 220,000 | 220,000 | - | - | % | |||||||||||
| Stock option compensation charge | 645,756 | 512,791 | 132,965 | 25.9 | % | |||||||||||
| Legal fees | 787,685 | 762,977 | 24,708 | 3.2 | % | |||||||||||
| Consulting fees | 551,138 | 397,469 | 153,669 | 38.7 | % | |||||||||||
| Professional fees | 109,707 | 77,500 | 32,207 | 41.6 | % | |||||||||||
| Facilities expense | 2,662,019 | 1,313,267 | 1,348,752 | 102.7 | % | |||||||||||
| Travel expenditure | 310,464 | 228,512 | 81,952 | 35.9 | % | |||||||||||
| Capital raising fee | 76,000 | 68,700 | 7,300 | 10.6 | % | |||||||||||
| Other | 604,157 | 475,107 | 129,050 | 27.2 | % | |||||||||||
| $ | 13,936,542 | $ | 8,079,663 | $ | 5,856,879 | 72.5 | % | |||||||||
The increase in marketing expenditure over the prior period is due to the employment of an outside marketing company to assist in our messaging to clients and to develop a marketing strategy. This has been discontinued and we expect marketing costs to decrease substantially.
Total salary expenses are allocated to the various expense categories detailed below depending on the level of activity of our employees on government and commercial projects, internal research and development expenses and administrative activities. An increase in activity on projects will result in an increase in salary expense charged to cost of sales with a corresponding decrease in salary expense charged to selling, general and administrative expenses. A comparison of salary expenses is presented below.
Total salary expenditure for the year ended December 31, 2017 and 2016, respectively is included in the following expense categories:
| Year ended December 31, | Increase/ | Percentage | ||||||||||||||
| 2017 | 2016 | (decrease) | change | |||||||||||||
| Cost of sales | $ | 6,880,513 | $ | 4,558,598 | $ | 2,321,915 | 50.9 | % | ||||||||
| Selling, general and administrative expenses | 4,228,651 | 2,567,039 | 1,661,612 | 64.7 | % | |||||||||||
| Research and development salaries | 3,328,843 | 1,200,223 | 2,128,620 | 177.4 | % | |||||||||||
| $ | 14,438,007 | $ | 8,325,860 | $ | 6,112,147 | 73.4 | % | |||||||||
The increase in total salary expenditure for the year ended December 31, 2017 of $6,112,417 or 73.4% is primarily due to the acquisition of the assets and employees of the Tucson Facility on July 15, 2016. An additional 46 employees, included in the 2017 results for a fully year, the employment of a VP of Business Development on March 1, 2016 and the employment of a Chief Business Development Officer in August 2016 as well as the employment of an additional two business development heads during the first half of 2017.
27
The payroll expense charged to Selling, general and administrative expenses for the year ended December 31, 2017 increased by $1,661,612. This increase is primarily due to the acquisition of the additional 7 administrative employees with the Tucson Facility, the employment of a VP of business development on March 1, 2016, a Chief Business Development Officer on August 22, 2016 and an additional two business development heads during the first half of 2017.
The research and development salaries increased by $2,128,620 primarily due to an average of 17 employees working on internal research projects at our Tucson facility. The level of research undertaken is dependent upon the commercial opportunities that are available at our Tucson and North Carolina sites. At times when we have more commercial activities, we have less employees available to research and development and therefore the research and development expenses are lower.
The stock option compensation charge increased by $132,965. The charge for each period is dependent upon the number of options issued, any new options issued, the value of the options and the vesting schedule of these options, during the current year, options were issued to certain key members of management at both the Tucson and North Carolina facilities, the increased compensation expense from these grants were offset by options previously granted which became fully vested and fully amortized.
Legal fees increased by $24,078 over the prior year. The slight increase is made up of a net reduction in litigation related expenditure of $349,356, offset by an increase in general corporate expenditure of $329,514 primarily related to the GPB debt funding as fully described in the notes to the financial statements.
The increase in consulting fees of $153,669 is primarily due to outside consultants assisting with the negotiation of a research project and additional administrative consulting undertaken.
Professional fees increased by $32,207, primarily due to an increase in payroll related fees based on the number of employees we have and the benefits provided and a slight increase in audit expense consistent with the increase in the size of the Group.
Facilities expense increased by $1,348,752 over the prior year, primarily due to the following movements; (i) an increase in cleaning and janitorial expenses of $120,049 due to the inclusion of the Tucson facility for a full year during the current period; (ii) an increase in facilities repairs and maintenance expenditure of $464,787, primarily due to repairs and maintenance contracts entered into at the Tucson Facility; (iii) an increase in Security services expenditure at our Tucson Facility of $212,821; (iv) an increase in utilities expenditure of $307,87 consisting primarily of electricity charges in Tucson to maintain the approximately 113,000 square foot Tucson Facility, all of these expenses are primarily due to operating the facility for a full year as compared to half a year in the prior year.
Travel expenditure increased by $81,952 due to increased travel incurred by the business development teams, the employment of an additional two business development heads and the travel expenditure related to the additional site and management thereof.
Depreciation and Amortization
We recognized depreciation expenses of $1,736,628 and $530,898 for the years ended December 31, 2017 and 2016, respectively, an increase of $1,205,730 or 227.1%, which is primarily due to our Tucson Facility having to renew software licensing in December 2016, with the bulk of the expenditure carried by Sanofi in the prior year. Software licenses are generally for a one year period and are amortized over the term of the license agreement. The balance of the depreciation expense is primarily made up of depreciation of our laboratory equipment and software licensing, which makes up the majority of our capital assets.
Amortization expense was $224,984 and $224,984 for the year ended December 31, 2017 and 2016. The amortization charge relates primarily to the intangibles acquired at our North Carolina site.
Other income
Other income is primarily made up of the reversal of deferred purchase consideration initially due on the acquisition of the North Carolina facility, due to our customer not meeting certain revenue milestones.
Other Expense
Other expense totaled $262,966 and $1,535,875 for the year ended December 31, 2017 and 2016, respectively, a decrease of $1,272,909 or 82.9%. Other expense in the current period represents expected severance costs to be paid on the reduction of our business development team by four heads to refocus our business strategy. In the prior year, other expense included estimated net legal settlement costs with respect to the Dentons dispute and the net legal settlement costs with respect to the Eisenschenk matter.
28
Interest expense
Interest expense totaled $2,282,046 and $755,067 for the year ended December 31, 2017 and 2016, respectively. The interest expense is primarily made up of the following; (i) Imputed interest cost of $308,252 and $497,643 for the years ended December 31, 2017 and 2016, respectively, a decrease of $189,391 or 38.1%, this is primarily due to the reassessment of the length of time to repay the Pfizer deferred purchase consideration based on our future revenue projections and minimum payments we are required to make; (ii) amortization of debt discount of $1,110,424 and $244,463 for the years ended December 31, 2017 and 2016, respectively, an increase of $865,961 or 354.2%, primarily due to the valuation of the conversion feature of the Convertible Notes and the warrants issued in connection with the Convertible Notes raise using a Black Scholes valuation model, which is amortized over the term of the note. The valuation of this discount amounted to $4,918,277 on a principal balance of $10,000,000; (iii) interest expense of $846,799 and $12,961 for the years ended December 31, 2017 and 2016, respectively, an increaser of $834,779, this is primarily due to the 13% coupon on the Convertible Notes, amounting to $812,500 during the current year; and (iv) other of $3,609 and $0 for the years ended December 31, 2017 and 2016, respectively, this consists primarily on foreign exchange movement on purchases and sales to foreign suppliers and customers.
Net loss
Net loss totaled $6,110,434 and $5,504,412 for the year ended December 31, 2017 and 2016, respectively. The increase in the net loss is primarily due to increased expenses resulting from the operation of the Tucson Facility and an increase in financing costs offset by a once off reduction in deferred purchase consideration during the current year.
Recent Financing
On April 4, 2018, we closed the first tranche of our best efforts offering of preferred stock and warrants for a maximum of forty (40) units and entered into a Securities Purchase Agreement with a trust of which one member of our Board of Directors is the trustee, pursuant to which we issued to the Purchaser twenty (20) units, at a purchase price of $100,000 per unit, each unit consisting of approximately 28,571 shares of our Series C Convertible Preferred Stock, and a seven year Warrant to acquire approximately 28,571 shares of our common stock, at an exercise price of $3.50 per share. An aggregate of 571,428 shares of Series C Preferred Stock and a warrant to purchase an aggregate of 571,428 shares of common stock were sold at the initial closing. The gross cash proceeds to us from the sale of the twenty (20) units was $2,000,000.
The Series C Preferred Stock ranks senior to the shares of our common stock and any other class or series of stock issued by us with respect to dividend rights, redemption rights and rights on the distribution of assets on any voluntary or involuntary liquidation, dissolution or winding up of our affairs. Holders of Series C Preferred Stock will be entitled to a cumulative dividend at the rate of 12.0% per annum, as set forth in the Certificate of Designation of Powers, Preferences and Rights of Series C Convertible Preferred Stock classifying the Series C Preferred Stock (the “Certificate of Designation”). The Series C Preferred Stock is convertible at the option of the holders at any time into such number of shares of common stock as shall be equal to $3.50 plus any accrued and unpaid dividends on such share of Series C Preferred Stock (the “Accreted Value”) divided by the conversion price, which initially shall be $3.50 per share, subject to certain customary anti-dilution adjustments. In addition, the Series C Preferred Stock automatically converts into shares of our common stock based upon the then effective conversion price upon the (i) closing of a sale of shares of common stock to the public in a Qualifying Public Offering (as defined below) or a reverse merger into a publicly reporting company that has its common stock listed or quoted and traded on a Trading Market (as such term is defined in the Certificate of Designation) or (ii) the date and time, or the occurrence of an event, specified by vote or written consent of the holders of at least seventy-five percent (75%) of the outstanding shares of Series C Preferred Stock (the “Requisite Holders”). A “Qualifying Public Offering” is defined as the first firm commitment underwritten public offering by us on or following the initial issuance date of the Series C Preferred Stock in which shares of common stock are sold for our account solely for cash to the public resulting in proceeds to it and/or our subsidiary, Icagen-T, Inc. of no less than $8,000,000 (after deduction only of underwriter discounts and commissions) and where the shares of common stock registered under the Securities Act, and sold in such public offering are simultaneously listed and commence trading on a Trading Market (as such term is defined in the Certificate of Designation).
In the event of our liquidation, dissolution or winding-up, holders of the Series C Preferred Stock are entitled to a preference on liquidation equal to $5.25 per share of Series C Preferred Stock plus all accrued and unpaid dividends.
Each holder of Series C Preferred Stock shall have the right to cast the number of votes equal to three times the number of shares into which the Series C Preferred Stock is convertible and the Series C holders as a group, shall have the right to elect one director on our Board of Directors. The Company cannot take the following actions without the approval of the Requisite Holders and the consent of our Board of Directors, including the Series C Preferred Stock director: (i) liquidate, dissolve or wind up our business, (ii) amend our Certificate of Incorporation or Bylaws, (iii) create any new class of stock unless it ranks junior to the Series C Preferred Stock with respect to dividends and liquidation, (iv) amend or alter any class of stock pari passu with the Series C Preferred Stock to make it senior with respect to dividends and liquidation, (v) purchase or redeem any other shares of our stock, or (vi) increase the size of our Board of Directors.
Upon the occurrence of a Cash Liquidity Event (as defined below), the holders of the Series C Preferred Stock can require us to redeem their shares of Series C Preferred Stock for a price per share equal to $5.25, subject to adjustments. In addition, we have the right to redeem the shares of Series C Preferred at any time for a price per share equal to $5.25 subject to adjustments. A “Cash Liquidity Event” is defined as the closing of any sale, lease or licensing transaction relating to a single asset or multiple assets other than in our ordinary course of business, including, but not limited to a sale of a building, sale of biological assets or other upfront payments, resulting in aggregate gross proceeds received by us at closing or closings in a transaction or transactions during any twelve (12) month period in excess of $40,000,000.
As part of the Units, we issued the Warrant to the Purchaser to purchase shares of our common stock at an initial exercise price of $3.50 per share (subject to applicable adjustments). The Warrants expire seven (7) years after the issuance date.
29
In addition, subject to limited exceptions, a holder of the Warrant will not have the right to exercise any portion of the Warrant if such holder, together with its affiliates, would beneficially own in excess of the Beneficial Ownership Limitation (as defined in the Warrant). A holder of the Warrant may adjust the Beneficial Ownership Limitation upon not less than sixty one (61) days’ prior notice to us, provided that such Beneficial Ownership Limitation in no event shall exceed 9.99%.
The Warrant also contains certain anti-dilution provisions that apply in connection with any stock split, stock dividend, stock combination, recapitalization and issuances of securities at prices below the conversion price or similar transactions.
If, at the time a holder exercises its Warrant, there is no effective registration statement registering for an issuance of the shares underlying the Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Warrant. If we fail to timely deliver the shares underlying the Warrant, it will be subject to certain buy-in provisions.
The Warrant also provides that we will not enter into or be party to a Fundamental Transaction unless (i) the Successor Entity (as defined in the Warrant) assumes in writing all of our obligations under the Warrant and the other Transaction Documents (as defined in the Securities Purchase Agreement) pursuant to written agreements in form and substance satisfactory to the Purchaser, including agreements to deliver to the Purchaser in exchange for the Warrants a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to the Warrant; (ii) we or the Successor Entity (as the case may be) agrees at our election or the Successor Entity (as the case may be) to purchase the Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value (as defined in the Warrant); or (iii) a Purchaser, at its election, requires us or the Successor Entity (as the case may be) to purchase the Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value.
Pursuant to the terms of the Purchase Agreement, we granted to the holders of the Series C Preferred Stock certain demand registration and piggyback registration rights, subject to certain rights of our lender.
Liquidity and Capital Resources
We have a history of annual losses from operations since inception and we have primarily funded our operations through sales of our unregistered equity securities and cash flows generated from government contracts and grants and more recently from commercial customers, subsidy income and the settlement of a lawsuit. We are generating substantially all of our revenues from commercial customers, and in the past from subsidy revenue, however, we continue to experience losses and will need to raise additional funds to meet our working capital requirements, despite the outcome of settlement agreements we have entered into, our lawsuits could have a significant impact on our financial position. We believe that our existing cash and cash equivalents will not be sufficient to meet our anticipated cash needs for the next twelve months. To date, we have never generated sufficient cash from operations to pay our operating expenses. To date, we have received $23.5 million from the MSA with Sanofi from services provided by Icagen-T for Sanofi and operating expense contributions paid by Sanofi. The remaining amount of $8.5 million we expect to receive from the Sanofi MSA is not sufficient to cover the operating expenses of the Tucson Facility. In addition, we expect our expenses to increase as our operations expand and our expenses may continue to exceed such revenue. We will need to generate additional revenue from operations and/or obtain additional financing to pursue our business strategy, to respond to new competitive pressures or to take advantage of opportunities that may arise. These factors raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended December 31, 2017 with respect to this uncertainty. To meet our financing needs, we are considering multiple alternatives, including, but not limited to, additional equity financings, debt financings and/or funding from partnerships or collaborations. There can be no assurance that we will be able to complete any such transactions on acceptable terms or otherwise.
On April 12, 2017, we sold to three (3) investors, which included two members of our Board of Directors, pursuant to the 2017 Purchase Agreement, 150 Units at a price of $10,000 per unit consisting of a 2017 note in the principal amount of $10,000 and a 2017 Bridge Warrant to acquire 1,500 shares of our common stock, par value, $0.001 per share, at an exercise price of $3.50 per share. The aggregate gross cash proceeds to us from the sale of the 150 Units was $1,500,000, these notes were repaid during May 2017, together with interest thereon.
On May 15, 2017, we and Icagen-T entered into a Securities Purchase Agreement with an institutional investor, pursuant to which (i) we issued to the investor the Company Note for cash proceeds of $1,920,000 after an original issue discount of 4% or $80,000, before deal related expenses; and (ii) Icagen-T issued to the investor the Icagen-T Note for cash proceeds of $7,680,000 after an original issue discount of 4% or $320,000, before deal related expenses. The Company Note and the Icagen-T Note are convertible into shares of common stock at a conversion price of $3.50 per share. On May 15, 2017, we also issued to the purchaser of the Convertible Notes, a warrant to purchase 857,143 shares of our common stock, which warrant was amended on April 13, 2018 in order to correct a typographical error in the definition of “Maximum Percentage” to make the beneficial ownership limitation in the warrant consistent with the beneficial ownership limitation in the Convertible Notes.
We used the proceeds from the Company Note to repay the $1,500,000 aggregate principal amount of the 8% bridge notes issued in April 2017 and all accrued but unpaid interest thereon and the $500,000 owed by us pursuant to the terms of the Dentons’ settlement agreement, and Icagen-T has used and intends to continue to use the net proceeds from the purchase price paid to Icagen-T for general corporate and working capital purposes of Icagen-T, provided, however, proceeds are not to be used for (a) the repayment of any Indebtedness other than Permitted Indebtedness (as defined in the Convertible Notes), (b) the redemption or repurchase of any securities of ours, Icagen-T and our Subsidiaries, or (c) except for the payments pursuant to the Settlement Agreement with Dentons, the settlement of any outstanding litigation; provided, further, Icagen-T will not use any of such proceeds in violation of its arrangements with Sanofi US Services, Inc.
30
On April 4, 2018, we closed the first tranche of our best efforts offering of preferred stock and warrants and entered into a Securities Purchase Agreement with a trust of which one member of our Board of Directors is the trustee, pursuant to which we issued to the Purchaser twenty (20) units, at a purchase price of $100,000 per unit. An aggregate of 571,428 shares of Series C Preferred Stock and a warrant to purchase an aggregate of 571,428 shares of common stock were sold at the initial closing. The gross cash proceeds to us from the sale of the twenty (20) units was $2,000,000.
As of December 31, 2017, we had cash totaling $2,763,596, other current assets totaling $2,027,147 and total assets of $14,638,554. We had total current liabilities of $4,971,100 and a net working capital deficit of $180,537, which includes deferred revenue of $219,828 primarily received from Sanofi, which will have no impact on cash flow. After eliminating these items, the working capital is $39,471. Total liabilities were $23,305,818, including deferred purchase consideration of $8,439,122. The deferred purchase consideration includes a net present value discount of $1,417,336 (made up of a gross present value discount of $2,468,700 less imputed interest expense of $(1,051,364)), the gross amount still due in terms of the acquisition agreement is $9,850,000 after the payment of $150,000 during the current period based on a potential earn out charge of the greater of (i)10% of gross revenues commencing in January 2017 per quarter and (ii) $250,000 per quarter, up to a maximum of $10,000,000 of which amounts in excess of $50,000 can be deferred and $200,000 was deferred for the quarters ended June 30, 2017 and September 30, 2017. The deferred amount bears interest at a rate of 12.5% per annum. Our stockholders’ deficit amounted to $8,667,264.
Should we not achieve our forecasted operating results or should strategic opportunities present themselves such that additional financial resources would present attractive investing opportunities for us, we may decide in the future to issue debt or sell our equity securities in order to raise additional cash. We cannot provide any assurances as to whether we will be able to secure any additional financing, or the terms of any such financing transaction if one were to occur.
The Los Alamos county loan was repaid on June 30, 2016.
| Year ended December 31, | Increase/ | Percentage | ||||||||||||||
| 2017 | 2016 | (decrease) | change | |||||||||||||
| Net cash (used in) provided by operating activities | $ | (9,998,561 | ) | $ | 4,367,547 | (14,366,108 | ) | (328.9 | )% | |||||||
| Net cash used in investing activities | (1,523,429 | ) | (1,986,014 | ) | 462,585 | (23.3 | )% | |||||||||
| Net cash provided by financing activities | 9,346,638 | 290,627 | 9,056,011 | 3,116.0 | % | |||||||||||
| Net (decrease) increase in cash and cash equivalents | $ | (2,175,352 | ) | $ | 2,672,160 | $ | (4,847,512 | ) | (181.4 | )% | ||||||
Net cash (used in) provided by operating activities was $(9,998,561) and $4,367,547 for the year ended December 31, 2017 and 2016, respectively. The decrease in cash provided by operating activities was primarily due to the following:
| Year ended December 31, | Increase/ | Percentage | ||||||||||||||
| 2017 | 2016 | (decrease) | change | |||||||||||||
| Net loss | $ | (6,110,434 | ) | $ | (5,504,413 | ) | (606,021 | ) | 11.0 | % | ||||||
| Adjustments for non-cash items | 3,183,350 | 3,490,849 | (307,499 | ) | (8.8 | )% | ||||||||||
| Changes in operating assets and liabilities | (7,071,477 | ) | 6,381,111 | (13,452,588 | ) | (210.8 | )% | |||||||||
| Net cash (used in) provided by operating activities | $ | (9,998,561 | ) | $ | 4,367,547 | $ | (14,366,108 | ) | (328.9 | )% | ||||||
The increase in net loss is discussed under net loss in the results of operations for the year ended December 31, 2017 and 2016, respectively and includes an increase in revenues, cost of goods sold and an increase in operating expenses offset by the subsidy revenues received.
The change in adjustments for non-cash of $307,499 is primarily due to the legal settlement accrual of $1,535,875 in the prior year, offset by the movement in depreciation expense of $1,205,730 over the prior year.
The change in operating assets and liabilities of $13,452,588 included i) the net movement in the subsidy received by Sanofi of $11,200,000, primarily due to the amortization of the remaining subsidy received in the prior year; ii) the increase in deferred revenue of $1,009,114; iii) the decrease in the movements of accounts payable of $1,018,007, primarily due to the cash settlement of legal settlement liabilities and iv) the decrease in the movements of prepaid expenses of $364,071, primarily due to the receipt of funds from Sanofi to cover certain agreed to expenses.
Net cash used in investing activities decreased by $462,585 primarily due to a decrease on the capital expenditure spend on licenses during the current year.
31
Net cash provided by financing activities increased by $9,056,011, primarily due to; i) the proceeds realized on the convertible debt issuance of $9,600,000; and ii) the net movement in other loans of $(543,989) over the prior period.
Capital Expenditures
Our current plan is to improve the efficiency of our laboratory operations by employing additional scientific personnel and equipment to further automate the processes required in our assay workflows to meet our customer requirements. We do not have a significant equipment budget for the 2018 year, however this is dependent on the volume of orders we receive from commercial customers and the laboratory throughput linked to those orders.
Critical Accounting Policies
Estimates
The preparation of these consolidated financial statements in accordance with US GAAP requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We continually evaluate our estimates, including those related to bad debts and recovery of long-lived assets. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Any future changes to these estimates and assumptions could cause a material change to our reported amounts of revenues, expenses, assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of the financial statements. Significant estimates include the allowance for doubtful accounts, the useful life of plant and equipment and intangible assets, the valuation of certain assets and intangibles acquired from Icagen, and assumptions used in assessing impairment of long-term assets and the assumptions used in determining percentage of completion on our long-term contracts.
Revenue recognition
Revenue sources consist of commercial contracts, deferred subsidy revenue, deferred revenue and government grants and contracts.
We enter into fixed fee commercial development contracts that are associated with the delivery of feasible research on drug candidates and the development of drug candidates. Revenue under such contracts is generally recognized upon delivery or as the development is performed.
We received certain deferred subsidy revenue which was utilized to support our operations, maintain the Tucson Facility that we operate and continue the employment of certain employees to provide, if needed, resources to certain of our customers. This deferred subsidy revenue was amortized over a straight-line basis to match the expected expenses to be incurred over the period July 15, 2016 to December 31, 2017.
We received and will receive certain revenue in advance of services delivered. This revenue is deferred and only recognized when services have been performed in terms of Master Services Agreements entered into with customers, together with their associated Statements of Work.
32
We account for our long-term Firm Fixed Price Government contracts and grants associated with the delivery of research on drug candidates and the development of drug candidates using the percentage-of-completion accounting method. Under this method, revenue is recognized based on the extent of progress towards completion of the long-term contract.
We generally use the cost-to-cost measure of progress for all our long-term contracts, unless we believe another measure will produce a more reliable result. We believe that the cost-to-cost measure is the best and most reliable performance indicator of progress on our long-term contracts as all our contract estimates are based on costs that we expect to incur in performing our long-term contracts and we have not experienced any significant variations on estimated to actual costs to date. Under the cost-to-cost measure of progress, the extent of progress towards completion is based on the ratio of costs incurred-to-date to the total estimated costs at the completion of the long-term contract. Revenues, including estimated fees or profits are recorded as costs are incurred.
When estimates of total costs to be incurred on a contract exceed total estimates of revenue to be earned, a provision for the entire loss on the contract is recorded in the period the loss is determined.
Research and Development
The remuneration of our research and development staff, materials used in internal research and development activities, and payments made to third parties in connection with collaborative research and development arrangements, are all expensed as incurred. Where we make a payment to a third party to acquire the right to use a product formula which has received regulatory approval, the payment is accounted for as the acquisition of a license or patent and is capitalized as an intangible asset and amortized over the shorter of the license period or the patent life.
Share-Based Compensation
ASC 718, “Compensation - Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Share-based compensation cost is measured at the grant date, based on the estimated fair value of the award and is recognized as expense over the employee’s requisite service period or vesting period on a straight-line basis. Share-based compensation expense recognized in the consolidated statements of operations for the year ended December 31, 2017 and 2016 is based on awards ultimately expected to vest and has been reduced for estimated forfeitures. This estimate will be revised in subsequent periods if actual forfeitures differ from those estimates. We have minimal awards with performance conditions and no awards dependent on market conditions.
The Company accounts for stock-based compensation issued to non-employees and consultants in accordance with the provisions of ASC 505-50, “Equity - Based Payments to Non-Employees.” Measurement of share-based payment transactions with nonemployees is based on the fair value of whichever is more reliably measurable: (a) the goods or services received; or (b) the equity instruments issued. The fair value of the share-based payment transaction is determined at the earlier of performance commitment date or performance completion date.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to us, but which will only be resolved when one or more future events occur or fail to occur. Our management assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against and by us or un-asserted claims that may result in such proceedings, our management evaluates the perceived merits of any legal proceedings or un-asserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed. Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Intangible assets
Certain of our intangible assets are subject to amortization. We evaluate the recoverability of intangible assets periodically by taking into account events or circumstances that may warrant revised estimates of useful lives or that indicate the asset may be impaired. Where intangibles are deemed to be impaired we recognize an impairment loss measured as the difference between the estimated fair value of the intangible and its book value.
| (a) | Cell lines |
Cell lines acquired by us are reported at acquisition value less any impairment. The useful life of cell lines is estimated to be indefinite.
33
| (b) | Discovery platform |
The discovery platform acquired by us is reported at acquisition value less accumulated amortization and any impairment. The estimated useful life of the discovery platforms acquired is estimated to be ten years.
| (c) | Trademarks and trade names |
The Trademarks and trade names acquired by us is reported at acquisition value less any impairments. The estimated useful life of trademarks and trade names is estimated to be indefinite.
| (d) | Patents |
Patents acquired by us are reported at acquisition value less accumulated amortization and impairments. The estimated useful life of patents is twenty years, the general useful life of patents.
| (e) | Assembled workforce |
Assembled workforce acquired by us is reported at acquisition value less amortization and impairments. The estimated useful life of the assembled workforce is ten years.
| (f) | Amortization |
Amortization is reported in the consolidated statement of operations on a straight-line basis over the estimated useful life of the intangible assets, unless the useful life is indefinite. Amortizable intangible assets are amortized from the date that they are available for use.
Plant and equipment
Plant and equipment is stated at cost less accumulated depreciation. Depreciation is computed using straight-line method over the estimated useful lives of the assets. The estimated useful lives of the assets are as follows:
| Leasehold improvements | 5 Years | |
| Laboratory equipment | 7 Years | |
| Furniture and fixtures | 10 Years | |
| Computer equipment | 3 Years |
The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition.
We examine the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable. We recognize an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. There was no impairment as of December 31, 2017.
Recent accounting pronouncements
For discussion of recently issued and adopted accounting pronouncements, please see Note 2 to the consolidated financial statements included herein.
Off Balance Sheet Arrangements
None.
Contractual Obligations
As a result of the agreements that we entered into with Pfizer and Sanofi, we are obligated; (i) to continue to retain certain employees at its Icagen-T facility until July 15, 2018, which we estimate will require additional compensation of $4,459,000 at the Tucson Facility; (ii) make additional payments in terms of the Asset Purchase and Collaboration Agreement that we entered into on June 26, 2015 with Pfizer including beginning in 2017, a quarterly earn out payment (the “Earn Out Payment”) of 10% of revenue earned during the quarter, with a minimum payment of $250,000 per quarter, up to a maximum aggregate payment of $10,000,000, such minimum being reduced to $50,000 for the quarters ending March 2017 to December 2018 and the difference between $250,000 or the quarterly amount paid and the actual calculation of deferred purchase consideration at 10% of gross revenue per quarter is being deferred and paid as one lump sum with the payment being made the quarter ended March 31, 2019, bearing interest at 12.5% per annum, which interest is payable quarterly; (iii) make minimum lease payments in terms of a sub-lease agreement entered into with Pfizer for the period July l, 2015 to April 30, 2019 with annual escalations of 3.5%, estimated to be $260,256.
34
In terms of a Mutual Release and Assignment Agreement entered into between American Milling LP and the Company, the Company agreed to pay American Milling $800,000 of which $83,334 remains to be paid at December 31, 2017.
We reached a Settlement and Release Agreement with Dentons and has agreed to pay Dentons the sum of $1,400,000 over a period of fourteen months of which $1,200,000 has been paid to date.
On May 15, 2017, we, and our wholly owned subsidiary, Icagen-T, entered into a Securities Purchase Agreement with an institutional investor (the “Purchaser”), pursuant to which (i) the Company issued to the Purchaser the Company Note which is a three year Senior Secured Convertible Note, maturing on May 15, 2020, bearing interest at the rate of 13% per annum (which interest rate increases to 18% per annum upon the occurrence of an event of default, as defined in the Note), in the aggregate principal amount of $2,000,000 for cash proceeds of $1,920,000 after an original issue discount of 4% or $80,000, before deal related expenses; and (ii) Icagen-T issued to the Purchaser the Icagen-T note which is a three year Senior Secured Convertible Note, maturing on May 15, 2020, bearing interest at the rate of 13% per annum, in the aggregate principal amount of $8,000,000 for cash proceeds of $7,680,000 after an original issue discount of 4% or $320,000, before deal related expenses. The Company Note and the Icagen-T Note are each convertible into shares of common stock at a conversion price of $3.50 per share.
On June 19, 2017, the Company entered into a four-year employment agreement with Douglas Krafte, Ph.D., pursuant to which Dr. Krafte is entitled to an annual base salary of $285,000 and will be eligible for annual discretionary performance bonus payments of up to 35% of his base salary payable in cash, which bonus, if any, will be awarded in the sole and absolute discretion of the Company’s board of directors and the compensation committee of the board of directors. Dr. Krafte continues to be engaged as the Company’s Chief Scientific Officer.
To date, we have not generated sufficient revenue to pay all of these commitments. If we do not increase our revenue, we may be required to raise additional capital to meet our obligations which could result in dilution to stockholders.
Inflation
The effect of inflation on the Company’s operating results was not significant.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not applicable because we are a smaller reporting company.
35
Item 8. Financial Statements and Supplemental Data
| 36 |
 | 805
Third Avenue New York, NY 10022 212.838.2676/ Fax www.rbsmllp.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
of
Icagen, Inc. and subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Icagen, Inc. and subsidiaries (the Company) as of December 31, 2017 and 2016, and the related statements of operations, stockholders’ equity (deficit), and cash flows for each of the years in the two year period ended December 31, 2017, and the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the two year period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 3 to the consolidated financial statements, the Company has incurred recurring operating losses which has resulted in an accumulated deficit of approximately $33.8 million at December 31, 2017. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments to reflect the possible effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
/s/ RBSM LLP We have served as the Company’s auditor since 2013. |
| New York, NY |
| April 17, 2018 |
F-1
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash | $ | 2,763,596 | $ | 4,938,948 | ||||
| Accounts receivable, net | 1,739,895 | 1,317,568 | ||||||
| Inventory | 73,885 | - | ||||||
| Prepaid expenses and other current assets | 213,367 | 467,807 | ||||||
| Assets held for resale | - | 27,000 | ||||||
| Total Current Assets | 4,790,743 | 6,751,323 | ||||||
| Non-Current Assets | ||||||||
| Intangibles, net | 7,427,071 | 7,498,890 | ||||||
| Plant and equipment, net | 2,181,753 | 2,677,734 | ||||||
| Deposits | 238,987 | 238,987 | ||||||
| Total Non-Current Assets | 9,847,811 | 10,415,611 | ||||||
| Total Assets | $ | 14,638,554 | $ | 17,166,934 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 1,471,645 | $ | 1,712,181 | ||||
| Other payables and accrued expenses | 2,332,109 | 1,633,801 | ||||||
| Legal settlement accrual | 493,333 | 1,426,667 | ||||||
| Loans payable | 139,394 | 442,109 | ||||||
| Accrued interest | 108,333 | - | ||||||
| Deferred revenue | 219,828 | 614,471 | ||||||
| Deferred subsidy | - | 5,600,000 | ||||||
| Deferred purchase consideration | 206,458 | 1,332,800 | ||||||
| Total Current Liabilities | 4,971,100 | 12,762,029 | ||||||
| Non-Current Liabilities | ||||||||
| Deferred purchase consideration, net | 8,232,664 | 7,454,511 | ||||||
| Loans payable | 71,296 | - | ||||||
| Convertible loans payable | 5,861,794 | - | ||||||
| Legal settlement accrual | - | 483,333 | ||||||
| Derivative liability | 4,168,964 | - | ||||||
| Total Non-Current Liabilities | 18,334,718 | 7,937,844 | ||||||
| Total Liabilities | 23,305,818 | 20,699,873 | ||||||
| Commitment and contingencies | - | - | ||||||
| Stockholders’ Deficit | ||||||||
| Preferred stock, $0.001 par value, 10,000,000 authorized, 400,000 shares designated as Series A Preferred Stock and unissued, 3,000,000 shares designated as Series B Preferred stock and unissued, 6,600,000 shares undesignated and unissued | - | - | ||||||
| Common stock, $0.001 par value; 50,000,000 shares authorized, 6,720,107 shares issued and 6,393,107 outstanding as of December 31, 2017 and 2016. | 6,392 | 6,392 | ||||||
| Additional paid-in-capital | 25,084,252 | 24,108,143 | ||||||
| Treasury stock, at cost (327,000 shares of common stock at December 31, 2017 and 2016. | (237 | ) | (237 | ) | ||||
| Accumulated deficit | (33,757,671 | ) | (27,647,237 | ) | ||||
| Total Stockholder’s Deficit | (8,667,264 | ) | (3,532,939 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 14,638,554 | $ | 17,166,934 | ||||
See notes to the consolidated financial statements
F-2
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| Sales | $ | 22,656,610 | $ | 11,995,377 | ||||
| Cost of sales | 11,175,692 | 6,371,873 | ||||||
| Gross profit | 11,480,918 | 5,623,504 | ||||||
| Operating expenses: | ||||||||
| Selling, general and administrative expenses | 13,936,542 | 8,079,663 | ||||||
| Depreciation | 1,736,628 | 530,898 | ||||||
| Amortization | 224,984 | 224,984 | ||||||
| Total Operating expenses | 15,898,154 | 8,835,545 | ||||||
| Operating loss | (4,417,236 | ) | (3,212,041 | ) | ||||
| Other income (expense) | ||||||||
| Other income | 502,494 | - | ||||||
| Other expense | (262,966 | ) | (1,535,875 | ) | ||||
| Interest income | 7 | 350 | ||||||
| Interest expense | (2,282,046 | ) | (755,067 | ) | ||||
| Derivative liability movement | 349,313 | - | ||||||
| Total other expense | (1,693,198 | ) | (2,290,592 | ) | ||||
| Net loss before income tax | (6,110,434 | ) | (5,502,633 | ) | ||||
| Income tax | - | (1,779 | ) | |||||
| Net loss | $ | (6,110,434 | ) | $ | (5,504,412 | ) | ||
| Net Loss Per Share - Basic and Diluted | $ | (0.96 | ) | $ | (0.85 | ) | ||
| Weighted Average Number of Shares Outstanding - Basic and Diluted | 6,393,107 | 6,472,158 | ||||||
See notes to the consolidated financial statements
F-3
CONSOLIDATED
STATEMENTS OF CHANGES IN STOCKHOLDERS’ (DEFICIT)
EQUITY FOR THE PERIOD JANUARY 1, 2016 TO DECEMBER 31, 2017
| Total | ||||||||||||||||||||||||
| Treasury | Additional | Stockholders’ | ||||||||||||||||||||||
| Common Stock | Stock | Paid-in | Accumulated | Equity | ||||||||||||||||||||
| Shares | Amount | Amount | Capital | Deficit | (deficit) | |||||||||||||||||||
| Balance as of January 1, 2016 | 6,481,857 | $ | 6,482 | $ | (237 | ) | $ | 23,711,824 | $ | (22,142,825 | ) | $ | 1,575,244 | |||||||||||
| Stock option based compensation | - | - | - | 512,791 | - | 512,791 | ||||||||||||||||||
| Common stock returned in legal settlement | (88,750 | ) | (90 | ) | - | (310,535 | ) | - | (310,625 | ) | ||||||||||||||
| Value of preferred stock in legal settlement | - | - | - | (50,400 | ) | - | (50,400 | ) | ||||||||||||||||
| Fair value of bridge note warrants issued | - | - | - | 244,463 | - | 244,463 | ||||||||||||||||||
| Net loss | - | - | - | - | (5,504,412 | ) | (5,504,412 | ) | ||||||||||||||||
| Balance as of December 31, 2016 | 6,393,107 | 6,392 | (237 | ) | 24,108,143 | (27,647,237 | ) | (3,532,939 | ) | |||||||||||||||
| Stock option based compensation | - | - | - | 645,756 | - | 645,756 | ||||||||||||||||||
| Fair value of bridge note warrants issued | 330,353 | 330,353 | ||||||||||||||||||||||
| Net loss | - | - | - | - | (6,110,434 | ) | (6,110,434 | ) | ||||||||||||||||
| Balance at December 31, 2017 | 6,393,107 | $ | 6,392 | $ | (237 | ) | $ | 25,084,252 | $ | (33,757,671 | ) | $ | (8,667,264 | ) | ||||||||||
See notes to the consolidated financial statements
F-4
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year ended | Year ended | |||||||
| December 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (6,110,434 | ) | $ | (5,504,412 | ) | ||
| Adjustment to reconcile net loss to net cash (used in) provided by operating activities: | ||||||||
| Depreciation expense | 1,736,628 | 530,898 | ||||||
| Amortization expense | 224,984 | 224,984 | ||||||
| Stock based compensation charge | 645,756 | 512,791 | ||||||
| Amortization of debt discount | 792,971 | - | ||||||
| Derivative liability movements | (349,313 | ) | - | |||||
| Deferred purchase consideration unearned by vendor | (500,000 | ) | - | |||||
| Imputed interest on acquisition of Icagen assets | 295,353 | 460,922 | ||||||
| Loss on disposal of assets held for resale | 6,619 | |||||||
| Discount on warrants issued | 330,353 | 244,463 | ||||||
| Movement in bad debt provision | - | (19,084 | ) | |||||
| Increase in legal settlement accrual | - | 1,535,875 | ||||||
| Changes in operating assets and liabilities | ||||||||
| Accounts receivable | (422,326 | ) | (331,314 | ) | ||||
| Inventory | (73,885 | ) | - | |||||
| Prepaid expenses and other current assets | 254,439 | (109,632 | ) | |||||
| Accounts payable | (240,536 | ) | 777,471 | |||||
| Deferred subsidy | (5,600,000 | ) | 5,600,000 | |||||
| Deferred revenues | (394,643 | ) | 614,471 | |||||
| Other payables and accrued expenses | (594,526 | ) | (169,887 | ) | ||||
| CASH USED IN OPERATING ACTIVITIES | (9,998,561 | ) | 4,367,547 | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Payment of deferred purchase consideration | (150,000 | ) | (125,000 | ) | ||||
| Purchase of plant and equipment | (1,240,646 | ) | (1,647,050 | ) | ||||
| Investment in deposits | - | (238,987 | ) | |||||
| Purchase of intangibles | (153,164 | ) | - | |||||
| Certificate of deposit refunded | - | 25,023 | ||||||
| Proceeds on assets held for resale | 20,381 | - | ||||||
| NET CASH USED IN INVESTING ACTIVITIES | (1,523,429 | ) | (1,986,014 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from convertible loan | 9,600,000 | - | ||||||
| Proceeds from bridge notes | 1,500,000 | 1,145,000 | ||||||
| Repayment of bridge notes | (1,500,000 | ) | (1,145,000 | ) | ||||
| Repayment of Los Alamos County loan | - | (142,502 | ) | |||||
| Proceeds from asset financing | 157,766 | 570,012 | ||||||
| Repayment of asset financing | (411,128 | ) | (136,883 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 9,346,638 | 290,627 | ||||||
| NET (DECREASE) INCREASE IN CASH | (2,175,352 | ) | 2,672,160 | |||||
| Cash at the beginning of the year | 4,938,948 | 2,266,788 | ||||||
| CASH AT END OF YEAR | $ | 2,763,596 | $ | 4,938,948 | ||||
| CASH PAID FOR INTEREST AND TAXES: | ||||||||
| Cash paid for income taxes | $ | - | $ | 1,779 | ||||
| Cash paid for interest | $ | 742,254 | $ | 13,152 | ||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES | ||||||||
| Value of Series A Stock redeemed and offset against stockholders’ equity | $ | - | $ | (183,750 | ) | |||
| Value of warrants issued concurrent with bridge notes | $ | 330,353 | $ | 203,214 | ||||
| Discount on convertible debt and warrants issued concurrent with debt | $ | 4,518,278 | $ | - | ||||
| Fair value of common stock returned in legal settlement | $ | - | $ | (310,625 | ) | |||
See notes to the consolidated financial statements
F-5
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 1. | GENERAL INFORMATION |
Icagen, Inc. (“the Company”, “we”, “us”, “our”) is a Delaware corporation. The principal office is located in Durham, North Carolina. The Company was incorporated in November 2003.
Icagen currently operates as a partner research organization providing integrated drug discovery services with unique expertise in the field of ion channel, transporter, neuroscience, muscle biology and rare disease targets while also covering many other classes of drug discovery targets and therapeutic areas. Our customers are pharmaceutical and biotechnology companies to whom we offer our industry- leading scientific expertise and technologies to aid in their determination of which molecules to advance into late stage preclinical studies and ultimately clinical trials. The core of our offering is the discovery of Pre-Clinical Drug Candidates (PDC’s), which are lead molecules (Leads) that are selected to enter into in-vivo studies during the Pre-Clinical Phase of drug discovery. We offer a full complement of pre-clinical drug discovery services which include; assay development technologies (including high throughput fluorescence, manual and automated electrophysiology and radiotracer flux assays), cell line generation, high-throughput and ultra-high- throughput screening, medicinal chemistry, computational chemistry and custom assay services to our customers. Our capabilities also include molecular biology and the use of complex functional assays, electrophysiology, bioanalytics and pharmacology. We believe that this integrated set of capabilities enhances our ability to help our customers identify drug candidates.
Icagen utilizes a target class approach to drug discovery where it leverages its deep expertise in specific areas to more rapidly move drug discovery projects forward. Whereas traditional drug discovery tends to take a more linear approach to compound advancement, the Company’s depth of both technical assets and area experts allows it to use more parallel approaches in eliminating problematic molecules early and identifying high quality leads in the drug discovery process. This saves time, money and increases the probability of success in human clinical studies. The Company believes that its deep understanding of the ion channel genome, neuroscience, muscle biology, and rare disease targets, in particular, and its ability to apply this knowledge in a target class approach to drug discovery facilitates its identification of small molecule drug candidates with novel mechanisms of action and enhanced selectivity and specificity profiles. Moreover, because the Company’s drug discovery and development process screens for potential side effects at an earlier stage than some alternative approaches, the Company believes that this process enables it to identify small molecule drug candidates that may have a reduced risk of clinical failure and may shorten clinical development timelines.
The Company operate out of two sites, one in Durham, North Carolina and the other in Tucson, Arizona. The teams in both North Carolina and Arizona have extensive experience over the last 20 plus years performing early drug discovery within Pfizer, Inc. (“Pfizer”) and Sanofi US Inc. (“Sanofi”), respectively, delivering leads from the pre-clinical stage to the clinical stage of drug discovery. We are now leveraging these capabilities to the broader market in the form of services, partnerships and collaborations with large pharmaceutical companies, biotech companies and foundations. At the North Carolina site, which we began to operate in July 2015, we have a leading biology expertise focused on ion channels which are important targets in Neuroscience. The North Carolina site also houses the XRpro® technology, our legacy technology, which has unique capabilities in the transporter target class. More specifically, our capabilities in North Carolina include a focus on ion channels & transporters, HTS and lead optimization, ion channel profiling, assay development and x-ray fluorescence-based assays. At the Arizona site, which we acquired in July 2016, we have leading biology expertise and platform capabilities in rare diseases, muscle biology and integrated drug discovery. The Arizona site provides capacity in cell models, human biomarkers, muscle biology expertise and stem cells-based assays. In addition, the Arizona site provides compound management services, HTS and Hit identification, in vitro pharmacology, medicinal chemistry, computational chemistry and ADME. The Arizona facility also features high volume biology with a flexible robotic infrastructure capable of performing high throughput screening in ultra-high 1536 format, enhancing our depth of expertise as a specialized pharmaceutical services company. This enables us to offer a broad range of integrated drug discovery services in a growing market. The extensive integrated drug discovery platform and technologies at the Arizona site enable us to utilize our biology expertise in both the North Carolina and Arizona sites to accelerate the drug discovery and identify quality leads faster.
F-6
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES |
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“US GAAP”).
All amounts referred to in the notes to the consolidated financial statements are in United States Dollars ($) unless stated otherwise.
Consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries in which it has at least a majority voting interest. All significant inter-company accounts and transactions have been eliminated in the consolidated financial statements. The entities included in these consolidated financial statements are as follows:
Icagen, Inc. - Parent Company
Icagen Corp (formerly XRpro Corp.) - Wholly owned subsidiary
Icagen-T, Inc. – wholly owned subsidiary
Caldera Discovery, Inc. - Wholly owned subsidiary
XRpro Sciences, Inc. – Wholly owned subsidiary
Estimates
The preparation of these consolidated financial statements in accordance with US GAAP requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We continually evaluate our estimates, including those related to bad debts and recovery of long-lived assets. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Any future changes to these estimates and assumptions could cause a material change to our reported amounts of revenues, expenses, assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of the financial statements. Significant estimates include the allowance for doubtful accounts, the useful life of plant and equipment and intangible assets, the valuation of certain assets and intangibles acquired from Icagen, and assumptions used in assessing impairment of long-term assets and the assumptions used in determining percentage of completion on our long-term contracts.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company’s management assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against and by the Company or un-asserted claims that may result in such proceedings, the Company’s management evaluates the perceived merits of any legal proceedings or un-asserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed. Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
F-7
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Fair value of financial instruments
The Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The carrying amounts reported in the balance sheets for cash, accounts receivable, loans payable, accounts payable and accrued expenses approximate their fair market value based on the short-term maturity of these instruments. The Company did not identify any assets or liabilities that are required to be presented on the balance sheets at fair value in accordance with the accounting guidance.
ASC 825-10 “Financial Instruments” allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding instruments.
Reporting by segment
No segmental information is presented as the Company operates in one segment and has changed its focus from Government contract revenue to revenues derived from commercial customers.
Concentrations of credit risk
The Company’s operations are carried out in the USA. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the USA and by the general state of the economy. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, and rates and methods of taxation, among other things.
The Company maintains cash with major financial institutions. The Federal Deposit Insurance Corporation (“FDIC”) provides insurance coverage for deposits of corporations, the current limit of coverage is $250,000. As a result of this coverage the Company cash balances of $2,328,049 are not covered by the FDIC as of December 31, 2017.
Concentration of major customers
The Company derives its revenues from commercial pharmaceutical and biotechnology companies as well as from Government research contracts and Government grants.
The commercial revenues are currently from several major pharmaceutical companies and smaller biotechnology and pharmaceutical companies.
The Company derived 89.4% of its services revenue from ten customers during the year ended December 31, 2017. During the year ended December 31, 2016, the Company derived 79.4% of its services revenue from three major customers. The Company continues to diversify its customer base.
The Government research contracts are from one government agency; the National Institutes of Health. The granting of research contracts from Government agencies is a competitive process and there is no certainty that we will be awarded future contracts, which may cause our revenue to fluctuate from year to year. Furthermore, Government grants are subject to audits by the granting agency. If such audits were to determine that expenditures of the grant funds did not meet the applicable criteria, these amounts could be subject to retroactive adjustment and refunded to the granting agency. The Company currently does not have any active Government grants.
F-8
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Total revenues by customer type are as follows:
| Year ended December 31, 2017 | Year ended December 31, 2016 | ||||||||
| Government grants | $ | 320,743 | $ | 455,983 | |||||
| Subsidy revenue | 9,600,000 | 4,400,000 | |||||||
| Services revenue | 12,735,867 | 7,139,394 | |||||||
| $ | 22,656,610 | $ | 11,995,377 | ||||||
Intangible assets
Certain of our intangible assets are subject to amortization. We evaluate the recoverability of intangible assets periodically by taking into account events or circumstances that may warrant revised estimates of useful lives or that indicate the asset may be impaired. Where intangibles are deemed to be impaired we recognize an impairment loss measured as the difference between the estimated fair value of the intangible and its book value.
| a) | Cell lines |
Cell lines acquired by the Company are reported at acquisition value less any impairment. The useful life of cell lines is estimated to be indefinite.
| b) | Discovery platform |
The discovery platform acquired by the Company is reported at acquisition value less accumulated amortization and any impairment. The estimated useful life of the discovery platforms acquired is estimated to be ten years.
| c) | Trademarks and trade names |
The Trademarks and trade names acquired by the Company is reported at acquisition value less any impairments. The estimated useful life of trademarks and trade names is estimated to be indefinite.
| d) | Patents |
Patents acquired by the Company are reported at acquisition value less accumulated amortization and impairments. The estimated useful life of patents is twenty years, the general useful life of patents.
| e) | Assembled workforce |
Assembled workforce acquired by the Company is reported at acquisition value less amortization and impairments. The estimated useful life of the assembled workforce is ten years.
| f) | Amortization |
Amortization is reported in the consolidated statement of operations on a straight-line basis over the estimated useful life of the intangible assets, unless the useful life is indefinite. Amortizable intangible assets are amortized from the date that they are available for use.
F-9
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Plant and equipment
Plant and equipment is stated at cost less accumulated depreciation. Depreciation is computed using straight-line method over the estimated useful lives of the assets. The estimated useful lives of the assets are as follows:
| Leasehold improvements | 5 Years | |
| Laboratory equipment | 7 Years | |
| Furniture and fixtures | 10 Years | |
| Computer equipment | 3 Years |
The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition.
We examine the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable. We recognize an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. There was no impairment as of December 31, 2017.
Accounts receivable and other receivables
We have a policy of reserving for uncollectible accounts based on our best estimate of the amount of probable credit losses in our existing accounts receivable. As a basis for accurately estimating the likelihood of collection of our accounts receivable, we consider a number of factors when determining reserves for uncollectable accounts. We believe that we use a reasonably reliable methodology to estimate the collectability of our accounts receivable. We review our allowances for doubtful accounts on a regular basis. We also consider whether the historical economic conditions are comparable to current economic conditions. If the financial condition of our customers or other parties that we have business relations with were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
The balance of the receivables provision as at December 31, 2017 and 2016 was $0. The amount charged to bad debt provision for the year ended December 31, 2017 and 2016 was $0 and $19,084, respectively.
Cash and cash equivalents
For purposes of the statements of cash flows, the Company considers all highly liquid instruments purchased with a maturity of three months or less and money market accounts to be cash equivalents. The Company maintains cash and cash equivalents with two financial institutions in the USA.
Revenue recognition
Revenue sources consist of commercial contracts, deferred subsidy revenue, deferred revenue and government grants and contracts.
We enter into fixed fee commercial development contracts that are associated with the delivery of feasible research on drug candidates and the development of drug candidates. Revenue under such contracts is generally recognized upon delivery or as the development is performed.
We received certain deferred subsidy revenue which was utilized to support our operations, maintain the facilities that we operate in and continue the employment of certain employees to provide, if needed, resources to certain of our customers. This deferred subsidy revenue was amortized over a straight-line basis to match the expected expenses to be incurred over the period July 15, 2016 to December 31, 2017.
We received and will receive certain revenue in advance of services delivered. This revenue is deferred and only recognized when services have been performed in terms of Master Services Agreements entered into with customers, together with their associated Statements of Work.
We account for our long-term Firm Fixed Price Government contracts and grants associated with the delivery of research on drug candidates and the development of drug candidates using the percentage-of-completion accounting method. Under this method, revenue is recognized based on the extent of progress towards completion of the long-term contract.
F-10
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Revenue recognition (continued)
We generally use the cost-to-cost measure of progress for all our long-term contracts, unless we believe another measure will produce a more reliable result. We believe that the cost-to-cost measure is the best and most reliable performance indicator of progress on our long-term contracts as all our contract estimates are based on costs that we expect to incur in performing our long-term contracts and we have not experienced any significant variations on estimated to actual costs to date. Under the cost-to-cost measure of progress, the extent of progress towards completion is based on the ratio of costs incurred-to-date to the total estimated costs at the completion of the long-term contract. Revenues, including estimated fees or profits are recorded as costs are incurred.
When estimates of total costs to be incurred on a contract exceed total estimates of revenue to be earned, a provision for the entire loss on the contract is recorded in the period the loss is determined.
Sales and Marketing
Sales and marketing expenses are expensed as incurred and included in Selling, general and administrative expenses. The Company expects to incur sales and marketing expenses in future periods to promote its services to drug discovery enterprises.
Research and Development
The remuneration of the Company’s research and development staff, materials used in internal research and development activities, and payments made to third parties in connection with collaborative research and development arrangements, are all expensed as incurred. Where the Company makes a payment to a third party to acquire the right to use a product formula which has received regulatory approval, the payment is accounted for as the acquisition of a license or patent and is capitalized as an intangible asset and amortized over the shorter of the license period or the patent life.
The amount expensed for unrecovered research costs, included in Selling, general and administrative expenses during the year ended December 31, 2017 and 2016 was $3,328,843 and $1,200,223, respectively.
Patent Costs
Legal costs in connection with approved patents and patent applications are expensed as incurred and classified as Selling, general and administrative expense in our consolidated statements of operations.
Share-Based Compensation
ASC 718, “Compensation - Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Share-based compensation cost is measured at the grant date, based on the estimated fair value of the award and is recognized as expense over the employee’s requisite service period or vesting period on a straight-line basis. Share-based compensation expense recognized in the consolidated statements of operations for the year ended December 31, 2017 and 2016 is based on awards ultimately expected to vest and has been reduced for estimated forfeitures. This estimate will be revised in subsequent periods if actual forfeitures differ from those estimates. We have minimal awards with performance conditions and no awards dependent on market conditions.
The Company accounts for stock-based compensation issued to non-employees and consultants in accordance with the provisions of ASC 505-50, “Equity - Based Payments to Non-Employees.” Measurement of share-based payment transactions with nonemployees is based on the fair value of whichever is more reliably measurable: (a) the goods or services received; or (b) the equity instruments issued. The fair value of the share-based payment transaction is determined at the earlier of performance commitment date or performance completion date.
Income Taxes
The Company utilizes ASC 740, Accounting for Income Taxes, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740, “Income Taxes”. Accounting guidance addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the consolidated financial statements, under which a company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position.
The tax benefits recognized in the consolidated financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. Accordingly, the Company would report a liability for unrecognized tax benefits resulting from uncertain tax positions taken or expected to be taken in a tax return. The Company elects to recognize any interest and penalties, if any, related to unrecognized tax benefits in tax expense.
F-11
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Net income (loss) per Share
Basic net income (loss) per share is computed on the basis of the weighted average number of common stock outstanding during the period.
Diluted net income (loss) per share is computed on the basis of the weighted average number of common stock and common stock equivalents outstanding. Dilutive securities having an anti-dilutive effect on diluted net income (loss) per share are excluded from the calculation.
Dilution is computed by applying the treasury stock method for options and warrants. Under this method, “in-the money” options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period. Dilution is computed by applying the if-converted method for convertible preferred stocks. Under this method, convertible preferred stock is assumed to be converted at the beginning of the period (or at the time of issuance, if later), and preferred dividends (if any) will be added back to determine income applicable to common stock. The shares issuable upon conversion will be added to weighted average number of common stock outstanding. Conversion will be assumed only if it reduces earnings per share (or increases loss per share).
Related parties
Parties are considered to be related to the Company if the parties that, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all related party transactions. All transactions are recorded at fair value of the goods or services exchanged.
Beneficial conversion feature of convertible notes payable
The Company accounts for convertible notes payable in accordance with guidelines established by the FASB ASC Topic 470-20, “Debt with Conversion and Other Options”. The beneficial conversion feature of a convertible note is normally characterized as the convertible portion or feature of certain notes payable that provide a rate of conversion that is below market value or in-the-money when issued. The Company records a beneficial conversion feature related to the issuance of a convertible note when issued and also records the estimated fair value of any warrants issued with those convertible notes. The beneficial conversion features that are contingent upon the occurrence of a future event are recorded when the contingency is resolved.
The beneficial conversion feature of a convertible note is measured by first allocating a portion of the note’s proceeds to any warrants, if applicable, as a discount on the carrying amount of the convertible on a relative fair value basis. The discounted face value is then used to measure the effective conversion price of the note. The effective conversion price and the market price of the Company’s common stock are used to calculate the intrinsic value of the conversion feature. The intrinsic value is recorded in the financial statements as a debt discount from the face amount of the note and such discount is amortized over the expected term of the convertible note (or to the conversion date of the note, if sooner) and is charged to amortization of debt discount on the Company’s consolidated statement of operations.
Derivative Liabilities
The Company has derivative financial instruments as of December 31, 2017.
ASC 815 generally provides three criteria that, if met, require companies to bifurcate conversion options from their host instruments and account for them as free standing derivative financial instruments. These three criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re- measured at fair value under otherwise applicable generally accepted accounting principles with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument subject to the requirements of ASC 815. ASC 815 also provides an exception to this rule when the host instrument is deemed to be conventional, as described.
The accounting treatment of derivative financial instruments requires that the Company record the embedded conversion option and warrants at their fair values as of the inception date of the agreement and at fair value as of each subsequent balance sheet date. Any change in fair value is recorded as non-operating, non-cash income or expense for each reporting period at each balance sheet date. The Company reassesses the classification of its derivative instruments at each balance sheet date. If the classification changes as a result of events during the period, the contract is reclassified as of the date of the event that caused the reclassification.
The Black-Scholes option valuation model was used to estimate the fair value of the conversion options. The model includes subjective input assumptions that can materially affect the fair value estimates. The expected volatility is estimated based on the most recent historical period of time, of other comparative securities, equal to the weighted average life of the options.
Conversion options are recorded as debt discount and are amortized as interest expense over the life of the underlying debt instrument using effective interest method.
Inventory
Inventory consists of consumables utilized in our research activities. These consumable inventories are valued at the lower of cost or net realizable value.
F-12
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Recent accounting pronouncements
In January 2017, the FASB issued Accounting Standards Update No. (“ASU”) 2017-02, an amendment to Topic 805, Business Combinations. The amendments in this Update clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The amendments in this Update affect all reporting entities that must determine whether they have acquired or sold a business. The amendments in this Update provide a more robust framework to use in determining when a set of assets and activities is a business. The amendments in this Update apply to annual periods beginning after December 15, 2017. The amendments in this Update should be applied prospectively on or after the effective date. No disclosures are required at transition. The Company does not expect this guidance to have a material impact on its financial statements.
In January 2017, the FASB issued ASU 2017-04, an amendment to Topic 350, Intangibles – Goodwill and Other, an entity no longer will determine goodwill impairment by calculating the implied fair value of goodwill by assigning the fair value of a reporting unit to all of its assets and liabilities as if that reporting unit had been acquired in a business combination. Because these amendments eliminate Step 3 from the goodwill impairment test, they should reduce the cost and complexity of evaluating goodwill for impairment. An entity should apply the amendments in this Update on a prospective basis. The amendments in this Update are effective for Goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We are currently evaluating the effect ASU 2017-04 will have on our consolidated financial statements.
In February 2017, the FASB issued ASU 2017-05, an amendment to Subtopic 610-20, Other Income—Gains and Losses from the Derecognition of Nonfinancial Assets. The amendments in this Update are required for public business entities and other entities that have goodwill reported in their financial statements, under the amendments in this Update, an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. The amendments in this Update modify the concept of impairment from the condition that exists when the carrying amount of goodwill exceeds its implied fair value to the condition that exists when the carrying amount of a reporting unit exceeds its fair value. An entity no longer will determine goodwill impairment by calculating the implied fair value of goodwill by assigning the fair value of a reporting unit to all of its assets and liabilities as if that reporting unit had been acquired in a business combination. An entity should apply the amendments in this Update on a prospective basis. The amendments in this Update are effective for fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We are currently evaluating the effect ASU 2017-05 will have on our consolidated financial statements.
In March 2017, the FASB issued ASU 2017-07, Compensation-Retirement Benefits (Topic 715). This Update is being issued primarily to improve the presentation of net periodic pension cost and net periodic postretirement benefit cost. This Update also includes amendments to the Overview and Background Sections of the FASB Accounting Standards Codification. Under generally accepted accounting principles (GAAP), defined benefit pension cost and postretirement benefit cost (net benefit cost) comprise several components that reflect different aspects of an employer’s financial arrangements as well as the cost of benefits provided to employees. Those components are aggregated for reporting in the financial statements. The amendments in this Update apply to all employers, including not-for-profit entities, that offer to their employees defined benefit pension plans, other postretirement benefit plans, or other types of benefits accounted for under Topic 715. The amendments in this Update require that an employer disaggregate the service cost component from the other components of net benefit cost. The amendments also provide explicit guidance on how to present the service cost component and the other components of net benefit cost in the income statement and allow only the service cost component of net benefit cost to be eligible for capitalization. The amendments in this Update are effective for public business entities for annual periods beginning after December 15, 2017, including interim periods within those 3 annual periods. For other entities, the amendments in this Update are effective for annual periods beginning after December 15, 2018, and interim periods within annual periods beginning after December 15, 2019. Early adoption is permitted as of the beginning of an annual period for which financial statements have not been issued or made available for issuance. The amendments in this Update should be applied retrospectively for the presentation of the service cost component and the other components of net periodic pension cost and net periodic postretirement benefit cost in the income statement and prospectively, on and after the effective date, for the capitalization of the service cost component of net periodic pension cost and net periodic postretirement benefit in assets. We are currently evaluating the effect ASU 2017-07 will have on our consolidated financial statements.
In March 2017, the FASB issued ASU 2017-08, Receivables—Nonrefundable Fees and Other Costs (Subtopic 310-20) Premium Amortization of Purchased Callable Debt Securities. The amendments in this Update affect all entities that hold investments in callable debt securities that have an amortized cost basis in excess of the amount that is repayable by the issuer at the earliest call date (that is, at a premium). The amendments in this Update shorten the amortization period for certain callable debt securities held at a premium. Specifically, the amendments require the premium to be amortized to the earliest call date. The amendments do not require an accounting change for securities held at a discount; the discount continues to be amortized to maturity. Under current GAAP, premiums and discounts on callable debt securities generally are amortized to the maturity date. The amendments in this Update more closely align the amortization period of premiums and discounts to expectations incorporated in market pricing on the underlying securities. As a result, the amendments more closely align interest income recorded on bonds held at a premium or a discount with the economics of the underlying instrument. For public business entities, the amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. Early adoption is permitted, including adoption in an interim period. If an entity early adopts the amendments in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period. An entity should apply the amendments in this Update on a modified retrospective basis through a cumulative effect adjustment directly to retained earnings as of the beginning of the period of adoption. Additionally, in the period of adoption, an entity should provide disclosures about a change in accounting principle. We are currently evaluating the effect ASU 2017-08 will have on our consolidated financial statements.
F-13
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Recent accounting pronouncements (continued)
In May 2017, the FASB issued Accounting Standards Update No. (“ASU’’) 2017-09, Compensation – Stock Compensation, an amendment to Topic 718. The amendments in this Update provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. 2. An entity should account for the effects of a modification unless all the following are met:
| 1. | The fair value (or calculated value or intrinsic value, if such an alternative measurement method is used) of the modified award is the same as the fair value (or calculated value or intrinsic value, if such an alternative measurement method is used) of the original award immediately before the original award is modified. If the modification does not affect any of the inputs to the valuation technique that the entity uses to value the award, the entity is not required to estimate the value immediately before and after the modification. |
| 2. | The vesting conditions of the modified award are the same as the vesting conditions of the original award immediately before the original award is modified. |
| 3. | The classification of the modified award as an equity instrument or a liability instrument is the same as the classification of the original award immediately before the original award is modified. |
The current disclosure requirements in Topic 718 apply regardless of whether an entity is required to apply modification accounting under the amendments in this Update. The amendments in this Update are effective for all entities for annual periods beginning after December 15, 2017. Early adoption is permitted and should be applied prospectively to an award modified on or after the adoption date. The amendments proposed in this ASU are not expected to have a material impact on our consolidated financial statements.
In May 2017, the FASB issued ASU 2017-10, service concession arrangements, an amendment to Topic 853. Topic 853 provides guidance for operating entities when they enter into a service concession arrangement with a public-sector grantor who both:
| 1. | Controls or has the ability to modify or approve the services that the operating entity must provide with the infrastructure, to whom it must provide them, and at what price |
| 2. | Controls, through ownership, beneficial entitlement, or otherwise, any residual interest in the infrastructure at the end of the term of the arrangement. |
In a service concession arrangement within the scope of Topic 853, the operating entity should not account for the infrastructure as a lease or as property, plant, and equipment. An operating entity should refer to other Topics to account for various aspects of a service concession arrangement. For example, an operating entity should account for revenue relating to construction, upgrade, or operation services in accordance with Topic 605, Revenue Recognition, or Topic 606, Revenue from Contracts with Customers.
The amendments in this Update apply to the accounting by operating entities for service concession arrangements within the scope of Topic 853. These updates are effective when the Company adopts the updates to Topic 606. The amendments proposed in this ASU are not expected to have an impact on our consolidated financial statements.
In July 2017, the FASB issued Accounting Standards Update No. (“ASU’’) 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480) and Derivatives and Hedging (Topic 815). The amendments in this Update provide guidance about:
| 1. | Accounting for certain financial instruments with down round features |
| 2. | Replacement of the indefinite deferral for mandatorily redeemable financial instruments of certain non-public entities and certain non-controlling interests |
The amendments in Part I of this Update change the classification analysis of certain equity-linked financial instruments (or embedded features) with down round features. When determining whether certain financial instruments should be classified as liabilities or equity instruments, a down round feature no longer precludes equity classification when assessing whether the instrument is indexed to an entity’s own stock. The amendments also clarify existing disclosure requirements for equity-classified instruments. As a result, a freestanding equity-linked financial instrument (or embedded conversion option) no longer would be accounted for as a derivative liability at fair value as a result of the existence of a down round feature. For freestanding equity classified financial instruments, the amendments require entities that present earnings per share (EPS) in accordance with Topic 260 to recognize the effect of the down round feature when it is triggered. That effect is treated as a dividend and as a reduction of income available to common shareholders in basic EPS. Convertible instruments with embedded conversion options that have down round features are now subject to the specialized guidance for contingent beneficial conversion features (in Subtopic 470-20, Debt—Debt with Conversion and Other Options), including related EPS guidance (in Topic 260).
F-14
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Recent accounting pronouncements (continued)
The amendments in Part II of this Update recharacterize the indefinite deferral of certain provisions of Topic 480 that now are presented as pending content in the Codification, to a scope exception. Those amendments do not have an accounting effect.
The amendments in Part I of this Update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. Early adoption is permitted for all entities, including adoption in an interim period. If an entity early adopts the amendments in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period. The amendments in Part 1 of this Update should be applied in either of the following ways: 1. Retrospectively to outstanding financial instruments with a down round feature by means of a cumulative-effect adjustment to the statement of financial position as of the beginning of the first fiscal year and interim period(s) in which the pending content that links to this paragraph is effective 2. Retrospectively to outstanding financial instruments with a down round feature for each prior reporting period presented in accordance with the guidance on accounting changes in paragraphs 250-10-45-5 through 45-10.
The amendments in Part II of this Update do not require any transition guidance because those amendments do not have an accounting effect.
The Company is currently evaluating the impact this ASU will have on its consolidated financial statements.
In August 2017, the FASB issued ASU 2017-12, Derivatives and Hedging, (Topic 815), Targeted Improvements to accounting for Hedging Activities. The amendments in this update provide guidance about:
The amendments in this Update better align an entity’s risk management activities and financial reporting for hedging relationships through changes to both the designation and measurement guidance for qualifying hedging relationships and the presentation of hedge results. To meet that objective, the amendments expand and refine hedge accounting for both nonfinancial and financial risk components and align the recognition and presentation of the effects of the hedging instrument and the hedged item in the financial statements.
For public business entities, the amendments in this Update are effective for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. Early application is permitted in any interim period after issuance of the Update. Transition Requirements For cash flow and net investment hedges existing at the date of adoption, an entity should apply a cumulative-effect adjustment related to eliminating the separate measurement of ineffectiveness to accumulated other comprehensive income with a corresponding adjustment to the opening balance of retained earnings as of the beginning of the fiscal year that an entity adopts the amendments in this Update. The amended presentation and disclosure guidance is required only prospectively.
The impact this ASU will have on the Company’s consolidated financial statements is expected to be immaterial.
In September 2017, the FASB issued ASU 2017-13, Revenue Recognition (Topic 605), Revenue from Contracts with Customers (Topic 606), Leases (Topic 840 and Leases (Topic 842). The amendments in this update provide guidance about:
The transition provisions in ASC Topic 606 require that a public business entity and certain other specified entities adopt ASC Topic 606 for annual reporting periods beginning after December 15, 2017, including interim reporting periods within that reporting period. All other entities are required to adopt ASC Topic 606 for annual reporting periods beginning after December 15, 2018, and interim reporting periods within annual reporting periods beginning after December 15, 2019.
The transition provisions in ASC Topic 842 require that a public business entity and certain other specified entities adopt ASC Topic 842 for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. FN3 All other entities are required to adopt ASC Topic 842 for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020.
The impact this ASU will have on the Company’s consolidated financial statements is expected to be immaterial.
In November 2017, the FASB issued ASU 2017-14, Income Statement – Reporting Comprehensive Income (Topic220), Revenue Recognition (Topic 605) and Revenue from Contracts with Customers (Topic 606). The amendments in this update provide guidance about:
Certain amendments made to SEC materials and staff guidance relating to Operating-Differential subsidiaries, and amendments to the wording and disclosure requirements of Topic 605, Revenue Recognition.
F-15
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Recent accounting pronouncements (continued)
In January 2018, the FASB issued ASU 2018-1, Leases (Topic 842), Land Easement practical expedient for Top 842. The amendments in this update provide guidance about:
The amendments in this Update permit an entity to elect an optional transition practical expedient to not evaluate under Topic 842 land easements that exist or expired before the entity’s adoption of Topic 842 and that were not previously accounted for as leases under Topic 840. An entity that elects this practical expedient should apply the practical expedient consistently to all of its existing or expired land easements that were not previously accounted for as leases under Topic 840. Once an entity adopts Topic 842, it should apply that Topic prospectively to all new (or modified) land easements to determine whether the arrangement should be accounted for as a lease. An entity that does not elect this practical expedient should evaluate all existing or expired land easements in connection with the adoption of the new lease requirements in Topic 842 to assess whether they meet the definition of a lease. The amendment in this Update clarifies that an entity should determine whether land easements are leases in accordance with Topic 842 before applying the guidance.
The impact this ASU will have on the Company’s consolidated financial statements is expected to be immaterial.
In February 2018, the FASB issued ASU 2018-2, Income Statement- Reporting Comprehensive Income (Topic 220), Reclassification of certain tax effects from accumulated other comprehensive income.
The amendments in this Update allow a reclassification from accumulated other comprehensive income to retained earnings for stranded tax effects resulting from the Tax Cuts and Jobs Act. Consequently, the amendments eliminate the stranded tax effects resulting from the Tax Cuts and Jobs Act and will improve the 2 usefulness of information reported to financial statement users. However, because the amendments only relate to the reclassification of the income tax effects of the Tax Cuts and Jobs Act, the underlying guidance that requires that the effect of a change in tax laws or rates be included in income from continuing operations is not affected. The amendments in this Update also require certain disclosures about stranded tax effects.
The amendments in this Update are effective for all entities for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption of the amendments in this Update is permitted, including adoption in any interim period, (1) for public business entities for reporting periods for which financial statements have not yet been issued and (2) for all other entities for reporting periods for which financial statements have not yet been made available for issuance. The amendments in this Update should be applied either in the period of adoption or retrospectively to each period (or periods) in which the effect of the change in the U.S. federal corporate income tax rate in the Tax Cuts and Jobs Act is recognized.
The impact this ASU will have on the Company’s consolidated financial statements will be a reduction in the tax effect of net operating losses carried forward.
In February 2018, the FASB issued ASU 2018-3 Technical Corrections and Improvements to Financial Instruments – Overall (Sub topic 825-10), Recognition and Measurement of Financial Assets and Financial Liabilities. The amendments in this update provide guidance about:
The amendment clarifies that an entity measuring an equity security using the measurement alternative may change its measurement approach to a fair value method in accordance with Topic 820, Fair Value Measurement, through an irrevocable election that would apply to that security and all identical or similar investments of the same issuer. Once an entity makes this election, the entity should measure all future purchases of identical or similar investments of the same issuer using a fair value method in accordance with Topic 820.
The amendment clarifies that the adjustments made under the measurement alternative are intended to reflect the fair value of the security as of the date that the observable transaction for a similar security took place.
The amendment clarifies that remeasuring the entire value of forward contracts and purchased options is required when observable transactions occur on the underlying equity securities.
The amendment clarifies that when the fair value option is elected for a financial liability, the guidance in paragraph 825-10- 45-5 should be applied, regardless of whether the fair value option was elected under either Subtopic 815-15, Derivatives and Hedging— Embedded Derivatives, or 825- 10, Financial Instruments— Overall.
The amendments clarify that for financial liabilities for which the fair value option is elected, the amount of change in fair value that relates to the instrument specific credit risk should first be measured in the currency of denomination when presented separately from the total change in fair value of the financial liability. Then, both components of the change in the fair value of the liability should be remeasured into the functional currency of the reporting entity using end-of-period spot rates.
F-16
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2. | ACCOUNTING POLICIES AND ESTIMATES (continued) |
Recent accounting pronouncements (continued)
The amendment clarifies that the prospective transition approach for equity securities without a readily determinable fair value in the amendments in Update 2016-01 is meant only for instances in which the measurement alternative is applied. An insurance entity subject to the guidance in Topic 944, Financial Services— Insurance, should apply a prospective transition method 4 Area for Correction or Improvement Summary of Amendments when applying the amendments related to equity securities without readily determinable fair values. An insurance entity should apply the selected prospective transition method consistently to the entity’s entire population of equity securities for which the measurement alternative is elected.
The amendments in this Update are effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years beginning after June 15, 2018. Public business entities with fiscal years beginning between December 15, 2017, and June 15, 2018, are not required to adopt these amendments until the interim period beginning after June 15, 2018, and public business entities with fiscal years beginning between June 15, 2018, and December 15, 2018, are not required to adopt these amendments before adopting the amendments in Update 2016-01. For all other entities, the effective date is the same as the effective date in Update 2016-01. All entities may early adopt these amendments for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, as long as they have adopted Update 2016-01.
The amendments in this update are not expected to have a material impact on the Company’s consolidated financial statements.
In March 2018, the FASB issued ASU 2018-4 Investments – Debt Securities (Topic 320) and Regulated Operations (Topic 980), Amendments to SEC Paragraphs pursuant to SEC Staff Accounting Bulletin no. 117 and SEC Release No. 33-9273. The amendments in this update provide guidance about:
Certain amendments made to SEC materials and staff guidance relating to Investments – Debt Securities (Topic 320) and Regulated Operations (Topic 980).
The amendments in this update are not expected to have a material impact on the Company’s consolidated financial statements.
In March 2018, the FASB issued ASU 2018-5, Income Taxes (Topic 740) Amendments to SEC paragraphs pursuant to SEC Staff Accounting Bulletin No. 118
These amendments affect the wording of SEC paragraphs in the accounting standard codification dealing with Income Taxes (Topic 740).
The amendments in this update are not expected to have a material impact on the Company’s consolidated financial statements.
Any new accounting standards, not disclosed above, that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the financial statements upon adoption.
| 3. | GOING CONCERN |
As shown in the accompanying consolidated financial statements, the Company incurred a net loss of $(6,110,434) and $(5,504,412) during the years ended December 31, 2017 and 2016, respectively. As of December 31, 2017, and 2016 the Company had accumulated deficits of $33,757,671 and $27,647,237, respectively. The Company’s working capital position improved from $(6,010,706), including deferred revenue and deferred subsidy of $219,828 for the year ended December 31, 2017 and $6,214,471 for the year ended December 31, 2016, related to the Sanofi acquisition to $(180,357). The Company’s working capital is insufficient to meet its short-term cash requirements and fund any future operating losses. These operating losses create an uncertainty about the Company’s ability to continue as a going concern. Our plan, through the acquisition of the assets of Sanofi and Icagen and the continued promotion of our services to existing and potential customers is to generate sufficient revenues to cover our anticipated expenses. The Company concluded a preferred stock equity raise on April 4, 2018, raising $2,000,000. Although no assurances can be given as to the Company’s ability to deliver on its revenue plans, or that unforeseen expenses may arise, the management of the Company believes that the revenue to be generated from operations together with additional issuances of equity or other potential financing will provide the necessary funding for the Company to continue as a going concern. The consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. The Company is economically dependent upon future capital or financing to fund ongoing operations.
F-17
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 4. | INVENTORY |
Inventory represents the value of certain consumables utilized in the Company’s biological screening processes. These consumables are purchased in bulk and expensed as they are utilized.
| 5. | PREPAID EXPENSES AND OTHER CURRENT ASSETS |
December 31, | December 31, 2016 | ||||||||
| Other receivables - Sanofi | $ | - | $ | 305,867 | |||||
| Prepaid insurance | 75,774 | 51,791 | |||||||
| Prepaid maintenance | 129,260 | 80,687 | |||||||
| Prepaid rent | 2,500 | 21,430 | |||||||
| Prepaid subscriptions | 5,833 | 7,243 | |||||||
| Other | - | 789 | |||||||
| $ | 213,367 | $ | 467,807 | ||||||
In the prior year, the Company entered into a Transitional services Agreement with Sanofi, in terms of which certain expenditure is to be paid by Sanofi. This expenditure relates to certain software purchases, an environmental insurance policy purchased and certain transitional property taxes.
| 6. | ASSETS HELD FOR RESALE |
The Company closed its Los Alamos and Cambridge sites during the fourth quarter of 2015 and consolidated its operations at the Icagen site in North Carolina. Excess laboratory equipment that was surplus to its requirements were consigned to a company that specializes in selling used laboratory equipment. The expected value the equipment is expected to realize was $27,000, of which $20,381 has been received to date, with some equipment remaining unsold of $6,619 written off during the year ended December 31, 2017.
| 7. | ACQUISITION OF ASSETS OF TUCSON FACILITY |
On July 15, 2016, Icagen-T, Inc. consummated the transactions with Sanofi (Sanofi Asset Purchase Agreement), pursuant to which Icagen-T acquired certain assets of Sanofi Tucson Facility, and the land on which the Tucson Facility is built; and (ii) certain machinery and equipment located at the Tucson Facility. The cash purchase price under the Sanofi Asset Purchase Agreement was $1. Icagen-T assumed certain liabilities, offered to continue the employment of up to 46 employees at the Facility for at least two years and maintain the Sanofi chemical libraries that will remain at the Tucson Facility and continue to be owned by Sanofi.
Upon the closing of the Sanofi Asset Purchase Agreement, on July 15, 2016, Icagen-T executed a Deed of Trust providing Sanofi with a five year, $5 million lien on the Tucson Facility, securing performance of Icagen-T’s obligations under the MSA and the Sanofi Asset Purchase Agreement. The lien is subject to termination upon the payment by Icagen-T of $5 million to Sanofi. The parties have also agreed to a Deed of Sale that will revest in Sanofi all rights in the Tucson Facility in the event that Icagen-T sells the Tucson Facility at any time within the next five years and upon certain other events related to the leasing of space at the Tucson Facility. The reversion rights of Sanofi under the Deed of Sale will terminate after five years as well as upon payment of the $5 million to extinguish the lien created by the Deed of Trust.
In terms of the agreement, Icagen-T, our wholly owned subsidiary is required to maintain the following covenants:
| ● | Minimum cash balance of $575,000 over five consecutive days at month end |
| ● | A current ratio of 1.05 times |
| ● | A net worth of $1,500,000 after adding back a value of $15,000,000 as a deemed property value. |
| ● | Certain affirmative covenants confirming we are in compliance with the agreement. |
The purchase price allocated to the acquisition of the assets of the Tucson Facility of $1 was allocated to fixed assets in the prior year.
F-18
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 8. | INTANGIBLE ASSETS |
| a. | Cell lines and discovery platform |
The Company has established a core set of technologies for the discovery of drugs that act upon ion channel targets. All of the assets acquired were developed internally and are based upon its ion channel platform and include the following acquired components:
| ● | Extensive cell line and plasmid repositories |
| ● | Technologies including High Throughput screening (HTS), electrophysiology, informatics, in vitro and in vivo ADME, animal efficacy and safety models. |
The value placed on these individual components is $5,000,500 for cell lines and $1,450,500 for the discovery platform, no initial value has been ascribed to plasmid repositories due to the commodity nature of these plasmids.
The useful life ascribed to the cell lines is indefinite due to the proprietary nature of these internally generated cell lines and will be tested for impairment on a regular basis and the useful life of the acquired discovery platform is expected to be ten years based on our internal experience on the usefulness of internally generated procedures and protocols used in ion channel drug discovery procedures. The cell lines and discovery platform will be considered for impairment on a regular basis.
| b. | Trade name and trademarks |
In terms of the purchase agreement entered into between the Company and Pfizer, the name and all rights to the name of Icagen were assigned to us. The use of this name, which was the original name of the publicly traded company acquired by Pfizer in 2011, has significant value and is a well-known industry name. The value placed on the trade name and trademarks acquired is $637,500. The useful life of the trade name and trademarks is indefinite and will be tested for impairment on a regular basis.
| c. | Assembled workforce |
In terms of the purchase agreement entered into between the Company and Pfizer, we agreed to retain the services of the scientific personnel who have extensive knowledge and experience in ion channel research and services. This workforce was originally acquired by Pfizer and prior to that had worked for the original Icagen company. The value placed in the assembled workforce acquired is $282,500, the useful life is expected to be ten years based on our estimate of the useful life of current knowledge and the rate of evolution within the industry.
| d. | Patents |
The patents the Company holds and pending patent applications consist of the following:
| ● | Method for Detecting Binding Events Using Micro X-Ray Fluorescence Spectrometry, which includes an issued U.S. patent that is expected to expire in about 2021; |
| ● | Flow Method and Apparatus for Screening Chemicals Using Micro X-Ray Fluorescence, which includes issued patents in the U.S., Europe, Japan and Singapore, such patents are expected to expire in 2022; |
| ● | Method and Apparatus for Detecting Chemical Binding, which includes about 10 issued patents in the U.S., Europe, Japan and Singapore; such patents are expected to expire in 2023; |
| ● | Drug Development and Manufacturing, which includes an issued U.S. patent that is expected to expire in about 2021; |
| ● | Advanced Drug Development and Manufacturing, which includes about 20 issued foreign patents, in Europe, Japan, and Hong Kong, expected to expire in about 2026, and a pending application in the U.S. which, if issued, is expected to expire between 2021-2026; |
| ● | Well Plate/Apparatus for Preparing Samples for Measurement by X-Ray Fluorescence Spectrometry, which includes issued over 15 issued patents in the U.S. Europe, and Japan, which are expected to expire in about 2028, and a pending application in the U.S. which, if issued, is also expected to expire in 2028; |
| ● | Method and Apparatus for Measuring Protein Post Translational Modification, which includes a patent issued in Japan, which is expected to expire in about 2028 and pending applications in U.S. and Japan, which, if issued, are also expected to expire in about 2028; |
F-19
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 8. | INTANGIBLE ASSETS (continued) |
| ● | Method and Apparatus for Measuring Analyte Transport Across Barriers, which includes 3 issued U.S. patents and issued patents in China and Hong Kong, which are expected to expire in about 2030/2031, and pending applications in U.S., Europe, and China, which, if issued, are also expected to expire in about 2030; and |
| ● | Method for Analysis Using X-Ray Fluorescence, which includes 4 issued U.S. patents, which is expected to expire in 2031, and a pending U.S. patent application which, if issued, is expected to expire in 2031. |
Intangible assets consist of the following:
| December 31, 2017 | December 31, 2016 | ||||||||||||||||
| Cost | Amortization and Impairment | Net book value | Net book value | ||||||||||||||
| Cell lines | $ | 5,153,664 | $ | - | $ | 5,153,664 | $ | 5,000,500 | |||||||||
| Discovery platform | 1,450,500 | (362,625 | ) | 1,087,875 | 1,232,925 | ||||||||||||
| Trade names and trademarks | 637,500 | - | 637,500 | 637,500 | |||||||||||||
| Assembled workforce | 282,500 | (70,625 | ) | 211,875 | 240,125 | ||||||||||||
| Patents | 972,000 | (635,843 | ) | 336,157 | 387,840 | ||||||||||||
| $ | 8,496,164 | $ | (1,069,093 | ) | $ | 7,427,071 | $ | 7,498,890 | |||||||||
The aggregate amortization expense charged to operations was $224,984 and $224,984 for the year ended December 31, 2017 and 2016, respectively. The amortization policies followed by the Company are described in Note 2.
Amortization expense for future periods is summarized as follows:
| Amount | |||||
| 2018 | $ | 224,984 | |||
| 2019 | 224,984 | ||||
| 2020 | 224,984 | ||||
| 2021 | 224,984 | ||||
| 2022 and thereafter | 735,971 | ||||
| Total | $ | 1,635,907 | |||
| 9. | PLANT AND EQUIPMENT |
Plant and equipment consists of the following:
| December 31, 2017 | December 31, 2016 | ||||||||||||||||
| Cost | Depreciation and Impairment | Net book value | Net book value | ||||||||||||||
| Laboratory equipment | $ | 2,449,748 | $ | (1,053,131 | ) | $ | 1,396,617 | $ | 1,613,987 | ||||||||
| Computer software | 1,321,788 | (604,928 | ) | 716,860 | 1,022,340 | ||||||||||||
| Computer equipment | 82,170 | (38,354 | ) | 43,816 | 38,768 | ||||||||||||
| Leasehold improvements | 38,974 | (14,514 | ) | 24,460 | 2,639 | ||||||||||||
| $ | 3,892,680 | $ | (1,710,927 | ) | $ | 2,181,753 | $ | 2,677,734 | |||||||||
The aggregate depreciation charge to operations was $1,736,628 and $530,898 for the years ended December 31, 2017 and 2016, respectively. The depreciation policies followed by the Company are described in Note 2.
F-20
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 10. | OTHER PAYABLES AND ACCRUED EXPENSES |
| December 31, 2017 | December 31, 2016 | ||||||||
| Bonus and vacation accrual | $ | 1,871,488 | $ | 1,125,119 | |||||
| Payroll liabilities | 44,858 | 32,312 | |||||||
| Sanofi transitional services expense | - | 268,189 | |||||||
| Credit card liability | - | 35,862 | |||||||
| Severance cost accrual | 262,966 | - | |||||||
| Other | 152,797 | 172,319 | |||||||
| $ | 2,332,109 | $ | 1,633,801 | ||||||
The Company accrues for vacation pay and bonus accruals in anticipation of making payments based on the achievement of pre- determined goals.
The Company refocused its operations and reduced the number of staff responsible for business development by four people. The Company provided severance packages to these staff members based on agreements entered into and company policy. We will, incur the severance costs over the following nine months.
In the prior year, the company entered into a Transitional services Agreement with Sanofi, in terms of which certain expenditure was paid by Sanofi and recoverable from Icagen-T, these expenses related to normal operating expenditure.
| 11. | LEGAL SETTLEMENT ACCRUAL |
The legal settlement liability is disclosed as follows:
| December 31, 2017 | December 31, 2016 | ||||||||
| Settlement liability accruals | |||||||||
| Dentons dispute | $ | 400,000 | $ | 1,400,000 | |||||
| Eisenschenk matter | 83,333 | 500,000 | |||||||
| Other | 10,000 | 10,000 | |||||||
| 493,333 | 1,910,000 | ||||||||
| Judgement liability | - | - | |||||||
| 493,333 | 1,910,000 | ||||||||
| Disclosed as follows: | |||||||||
| Short-term portion | 493,333 | 1,426,667 | |||||||
| Long-term portion | - | 483,333 | |||||||
| $ | 493,333 | $ | 1,910,000 | ||||||
In terms of a Mutual Release and Assignment Agreement entered into between American Milling LP and the Company, American Milling is a claimant in the Estate of Sigmund Eisenschenk matter. American Milling agreed to assign all its claims, both past and future against the Estate of Sigmund Eisenschenk to the Company for $800,000, to be paid by the Company in instalments of which $716,666 was paid as of December 31, 2017 and the remaining $83,334 was paid on March 30, 2018.
The Company agreed to settle the Dentons dispute by the payment of $1,400,000 over a 14 month period. As of December 31, 2017, the Company had paid $1,000,000 and a further $200,000 was paid on March 15, 2018. The remaining $200,000 will be paid during the next six months.
F-21
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 12. | DEFERRED REVENUE |
Deferred revenue primarily represents payments received in advance from Sanofi in terms of the MSA agreement entered into with them on July 15, 2016. Revenue is recognized on a monthly basis upon agreed rates for the number of employees assigned to certain Sanofi projects and is offset against the payments received from Sanofi in terms of the agreed upon payment schedule, the remaining excess payments received is deferred revenue and is expected to be realized within a 12 month period.
| 13. | DEFERRED SUBSIDY |
Deferred subsidy revenue represented a prepayment received from Sanofi to support our operations, maintain the facilities that we operate in and continue the employment of certain employees to provide, if needed, resources to Sanofi. This deferred subsidy revenue was amortized over a straight-line basis to match the expected expenses to be incurred over the period July 15, 2016 thru December 31, 2017.
| 14. | DEFERRED PURCHASE CONSIDERATION |
In terms of the Icagen asset purchase agreement entered into on July 1, 2015, the Company has the following deferred purchase price obligations:
| ● | commencing May 30, 2017, the Company is obligated to pay additional purchase price consideration calculated at the greater of (i) 10% (ten percent) of gross revenues per quarter (exclusive of revenue paid by Sanofi to Icagen-T and revenue generated by Icagen-T) and (ii) $250,000 per quarter up to an aggregate maximum of $10,000,000. These earn out payments are payable quarterly, 60 days after the completion of each calendar quarter. There are no indications that the Company will not meet the maximum earn out payment. |
The Company amended its agreement with Pfizer Research (NC), Inc. (the Second Amendment”), whereby the Company, at its option, may defer payment of any amount exceeding $50,000 of the minimum additional purchase price consideration of $250,000 per quarter until March 31, 2019 such that the Company is only required to pay $50,000 per quarter for the quarters ending March 2017 to December 2018. Deferred purchase consideration bears interest at a rate of 12.5% per annum, which interest is payable quarterly. The deferred purchase consideration in terms of this agreement is payable, together with the deferred purchase consideration for the quarter ended March 31, 2019, as one lump sum. The Second Amendment also provides that if there is an Insolvency Event (as such term is defined in the Second Amendment) prior to the time that Pfizer Research (NC), Inc. has received the Maximum Earn Out Payment, then upon such Insolvency Event, the full amount of any Earn Out Shortfall (the difference between the Maximum Earn Out Payment and the amount of all Earn Out Payments paid to date) shall be due and payable without further notice, demand or presentment for payment. The minimum deferred purchase consideration of $50,000 for the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017 was paid in June 2017 September 2017 and December 2017.
The $500,000 deferred purchase consideration due on July 1, 2017, was not earned by Pfizer due to Pfizer not meeting its $4,000,000 revenue target. This liability of $500,000 was reversed as other income during the Year ended December 31, 2017.
F-22
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 14. | DEFERRED PURCHASE CONSIDERATION (continued) |
Deferred purchase consideration is disclosed as follows:
| December 31, 2017 | December 31, 2016 | ||||||||
| Deferred purchase consideration | |||||||||
| Opening balance | $ | 10,500,000 | $ | 10,625,000 | |||||
| Reversal of unearned purchase consideration | (500,000 | ) | - | ||||||
| Interest due on deferred purchase consideration | 25,578 | - | |||||||
| Repayment | (169,120 | ) | (125,000 | ) | |||||
| Closing balance | 9,856,458 | 10,500,000 | |||||||
| Present value discount on future payments | |||||||||
| Opening balance | (1,712,689 | ) | (2,186,510 | ) | |||||
| Imputed interest expense | 300,511 | 576,180 | |||||||
| Fair value adjustments | (5,158 | ) | (102,359 | ) | |||||
| Closing balance | (1,417,336 | ) | (1,712,689 | ) | |||||
| Deferred purchase consideration, net | 8,439,122 | 8,787,311 | |||||||
| Disclosed as follows: | |||||||||
| Short-term portion | 206,458 | 1,332,800 | |||||||
| Long-term portion | 8,232,664 | 7,454,511 | |||||||
| Deferred purchase consideration, net | $ | 8,439,122 | $ | 8,787,311 | |||||
| 15. | INCOME TAXES |
The income tax provision/ (benefit) is different from that which would be obtained by applying the statutory Federal income tax rate of 35% to income before income tax expense. The items causing this difference for the years ended December 31, 2017 and 2016 are as follows:
| Year ended December 31, 2017 | Year ended December 31, 2016 | ||||||||
| Income tax benefit at federal rate | $ | (2,139,000 | ) | $ | (1,926,000 | ) | |||
| State tax, net of federal benefit | (305,000 | ) | (275,000 | ) | |||||
| Stock based compensation | 258,000 | 205,000 | |||||||
| Accrual to cash adjustments | 169,000 | 757,000 | |||||||
| Prior year underprovision | (548,000 | ) | (30,000 | ) | |||||
| Discount on notes | 444,000 | 98,000 | |||||||
| Income tax rate change | 3,672,000 | ||||||||
| Other | 51,000 | (101,000 | ) | ||||||
| (1,710,000 | ) | (1,272,000 | ) | ||||||
| Utilization of net operating loss carry-forwards | - | - | |||||||
| Valuation allowance | (1,710,000 | ) | 1,272,000 | ||||||
| $ | - | $ | - | ||||||
F-23
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 15. | INCOME TAXES (continued) |
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of deferred tax assets and liabilities at December 31, 2017 and 2016 are as follows:
| December 31, | December 31, | ||||||||
| 2017 | 2016 | ||||||||
| Deferred tax assets | |||||||||
| Accrual to cash adjustments | $ | 611,000 | $ | 1,401,000 | |||||
| options based compensation | 884,000 | 1,102,000 | |||||||
| Capital loss | 32,500 | 50,000 | |||||||
| Plant and equipment | 173,000 | - | |||||||
| Net operating loss | 5,492,000 | 6,499,000 | |||||||
| 7,912,500 | 9,052,000 | ||||||||
| Valuation allowance | (7,538,500 | ) | (8,519,000 | ) | |||||
| 374,000 | 533,000 | ||||||||
| Deferred tax liabilities | |||||||||
| Plant and equipment | - | (184,000 | ) | ||||||
| Amortization of intangibles | (374,000 | ) | (349,000 | ) | |||||
| $ | - | $ | - | ||||||
We have established a valuation allowance against our gross deferred tax assets sufficient to bring our net deferred tax assets to zero due to the uncertainty surrounding the realization of such assets. Management has determined it is more likely than not that the deferred tax assets are not realizable beyond our deferred tax liabilities due to our historical loss position. The valuation allowance decreased by $980,500 due to the net operating loss realized in the current year, including a true-up of prior year deferred taxes of $548,000, in addition to this our valuation allowances also decreased by 3,672,000 due to the federal tax rate change from 35% to 21%, in terms of the Tax Cuts and Jobs Act, discussed below.
As of December 31, 2017, the prior three years remain open for examination by the federal or state regulatory agencies for purposes of an audit for tax purposes.
At December 31, 2017, we had tax loss carry forwards of approximately $21,124,000. These net operating loss carry forwards expire in 2037, if unused. The Company files its tax returns on a cash basis.
Pursuant to the Internal Revenue Code of 1986, as amended (“IRC”), §382, our ability to use net operating loss carry forwards to offset future taxable income is limited if we experience a cumulative change in ownership of more than 50% within a three-year period.
The Tax Cuts and Jobs Act (the “Act”) was signed into law on December 22, 2017 and significantly changes tax law in the United States by, among other items, reducing the federal corporate income tax rate from a maximum of 35% to 21% (effective January 1, 2018). The Act embraces a territorial system for the taxation of future foreign earnings and modifies certain business deductions by, among other changes, repealing the domestic production activities deduction, further limiting the deductibility of certain executive compensation and increasing the limitation on the deductibility of certain meals and entertainment expenses. On the other hand, the Act permits 100% bonus depreciation on assets placed in service through 2022 (with a phase-out period through 2026). The full effects of these changes will be reflected for the first time in the determination of income tax expense for the year ending December 31, 2018. The Company determined that it had no liability as of December 31, 2018 for the one-time transition tax on deemed repatriated earnings of foreign subsidiaries imposed by the Act.
The Company will evaluate the impact of the Global Intangible Low-Taxed Income (“GILTI”) provision of the Act, beginning with the year ending December 31, 2018, the year for which it will first apply. The FASB has issued guidance stating that a company may elect to treat the additional taxes due in the United States as a result of GILTI inclusions as current period expenses when incurred or to include such amounts in the company’s determination of deferred taxes. The Company has not yet elected a method and will do so once the impact of GILTI has been evaluated.
| 16. | LOANS PAYABLE |
| December 31, 2017 | December 31, 2016 | ||||||||
| Asset purchase arrangements | $ | 210,690 | $ | 442,109 | |||||
| 210,690 | 442,109 | ||||||||
| Disclosed as follows: | |||||||||
| Short-term portion | 139,394 | 442,109 | |||||||
| Long-term portion | 71,296 | - | |||||||
| $ | 210,690 | $ | 442,109 | ||||||
| Amount | |||||
| Within 1 year | $ | 139,394 | |||
| Within 1 - 2 years | 71,296 | ||||
| $ | 210,690 | ||||
F-24
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 16. | LOANS PAYABLE (continued) |
Asset Purchase arrangements
The Company acquired laboratory equipment from Nanion Technologies on April 21, 2016 pursuant to the terms of a lease agreement. The lease consists of twelve equal monthly instalments of $28,751 each with a remaining balance due of $225,000 at the end of the twelve-month period. In terms of US GAAP, the total purchase consideration was discounted back to present value at the Company’s estimated weighted average cost of capital of 6.7%, resulting in an estimated present value of future payments of $533,290. The discount of $36,722 was expensed as an additional interest expense over the period in which the payments were made.
In July 2017, the Company agreed to purchase the equipment from Nanion for a purchase consideration of $225,000 for a total of 8 installments of $28,751 each, totaling $230,008, the installments bearing interest at an effective interest rate of 5.9% per annum. The Company owed $86,556 as of December 31, 2017.
The Company acquired additional laboratory equipment on August 11, 2017 for a purchase consideration of $59,320 in terms of a deferred purchase arrangement whereby a deposit of $5,932 was paid and twenty-four monthly instalments of $2,472 which commenced on September 11, 2017. The installments bearing interest at an effective rate of 10.33% per annum. The Company owed $45,502 as of December 31, 2017.
The Company acquired laboratory software during September 2017 for a purchase consideration of $98,446 in terms of a deferred purchase arrangement whereby a deposit of $10,546 was paid and a further 35 monthly instalments of $2,750 which commenced on September 30, 2017. The installments bear interest at an effective 6.15% per annum. The Company owed $78,632 as of December 31, 2017.
| 17. | BRIDGE NOTES |
On June 30, 2016, the Company sold in a private placement offering to 11 investors (the “Offering”) pursuant to a securities purchase agreement entered into with each investor, 104.5 units at a per unit price of $10,000, each unit (the “Units”) consisting of a note in the principal amount of $10,000 (the “Notes”) and a five-year warrant (the “Warrants”) to acquire 1,500 shares of the Company’s common stock, par value, $0.001 per share, at an exercise price of $3.50 per share for gross proceeds of $1,045,000. On July 7, 2016, the Company sold an additional 10 Units in the Offering to one investor for gross proceeds of $100,000. The aggregate cash proceeds to the Company from the sale of the 114.5 Units was $1,145,000.
The Notes bore interest at a rate of 8% per annum and were to mature on June 30, 2017. Pursuant to a Security and Pledge Agreement the Notes were secured by a lien on all of the current assets of the Company (excluding the equity of and assets of the Company’s wholly owned subsidiary, Icagen-T, Inc.). Amounts overdue bore interest at a rate of 1% per month.
The Warrants have an initial exercise price of $3.50 per share and are exercisable for a period of five years from the date of issuance. Each Warrant is exercisable for one share of Common Stock, which resulted in the issuance of Warrants exercisable to purchase an aggregate of 171,750 shares of Common Stock. The Warrants are subject to adjustment in the event of stock splits and other similar transactions. The Warrants were valued using a Black-Scholes valuation model and the proceeds received were allocated based on the percentage of the Warrants to the total value of the securities in this offering, resulting in a debt discount of $203,214 on the Warrants issued prior to June 30, 2016. A further Interest of $14,183 was recorded for Units issued on July 7, 2016. An additional $27,066 was allocated to the value of the Placement Agent Warrants described below.
The Company retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, the Company agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering ($1,145,000) and agreed to reimburse approximately $15,000 in respect of out of pocket expenses incurred by the placement agent in connection with the Offering. The Company also issued the placement agent the same warrant that the investors received exercisable for an aggregate amount of 28,625 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold) (the “Placement Agent Warrants”).
On August 8, 2016, the Notes amount to $1,145,000, together with interest thereon of $11,081 were redeemed in full. The remaining debt discount related to these Notes of $244,463 was expensed upon the redemption of the Notes.
On April 12, 2017, the Company sold in a private placement offering (the “Bridge Note Offering”) to three investors, which included two members of the Board of Directors, pursuant to a securities purchase agreement entered into with each investor (the “Purchase Agreements”), 150 units at a price of $10,000 per unit (the “Units”) each Unit consisting of a note (the ’‘Note”) in the principal amount of $10,000 and a five year warrant (the “Bridge Warrants”) to acquire 1,500 shares of the Company’s common stock, par value, $0.001 per share, at an exercise price of $3.50 per share. The aggregate gross cash proceeds to the Company from the sale of the 150 Units was $1,500,000.
F-25
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 17. | BRIDGE NOTES (continued) |
The Notes bore interest at a rate of 8% per annum and matured on the earlier of (i) the date that is thirty (30) days after the date of issuance or (ii) the closing of the Company’s next debt financing. Pursuant to a Security and Pledge Agreement the Notes were secured by a lien on all of the current assets of the Company (excluding the equity of and assets of the Company’s wholly owned subsidiary, Icagen-T, Inc.). Amounts overdue bore interest at a rate of 1% per month. The notes were repaid during May 2017 upon the closing of the convertible debt funding.
The Bridge Warrants have an initial exercise price of $3.50 per share and are exercisable for a period of five years from the date of issuance. Each Warrant is exercisable for one share of common stock, which resulted in the issuance of Bridge Warrants exercisable to purchase an aggregate of 225,000 shares of common stock. In addition, the Company also issued 25,000 warrants to the Placement Agent as compensation for the Bridge Note Offering.
The movement on bridge notes is as follows:
| December 31, 2017 | December 31, 2016 | ||||||||
| Bridge note liability | |||||||||
| Opening balance | $ | - | $ | - | |||||
| Bridge notes raised | 1,500,000 | 1,045,000 | |||||||
| Accrued interest | 9,753 | 11,081 | |||||||
| Repayment | (1,509,753 | ) | (1,056,081 | ) | |||||
| Closing balance | - | - | |||||||
| Discount on bridge notes | |||||||||
| Opening balance | - | - | |||||||
| Fair value of warrants issued | 330,353 | 244,463 | |||||||
| Amortization of bridge note discount | (330,353 | ) | (244,463 | ) | |||||
| Closing balance | - | - | |||||||
| Bridge notes, net | $ | - | $ | - | |||||
| 18. | CONVERTIBLE DEBT |
On May 15, 2017, the Company, and its wholly owned subsidiary, Icagen-T, Inc. (“Icagen-T”), entered into a Securities Purchase Agreement (“Securities Purchase Agreement”) with an institutional investor (the “Purchaser”), pursuant to which (i) the Company issued to the Purchaser a three year Senior Secured Convertible Note (“Company Note”), maturing on May 15, 2020, bearing interest at the rate of 13% per annum (which interest rate increases to 18% per annum upon the occurrence of an event of default, as defined in the Company Note), in the aggregate principal amount of $2,000,000 for cash proceeds of $1,920,000 after an original issue discount of 4% or $80,000, before deal related expenses; and (ii) Icagen-T issued to the Purchaser a three year Senior Secured Convertible Note (“Icagen-T Note”), maturing on May 15, 2020, bearing interest at the rate of 13% per annum, in the aggregate principal amount of $8,000,000 for cash proceeds of $7,680,000 after an original issue discount of 4% or $320,000, before transaction related expenses. The Company Note and the Icagen-T Note (collectively, the “Convertible Notes”) are each convertible into shares of common stock at a conversion price of $3.50 per share.
The Purchaser may elect to have the Company and/or Icagen-T redeem the Convertible Notes upon the occurrence of certain events, including upon a certain Events of Default (as defined in the Notes). The Convertible Notes contain customary Events of Default.
In addition, any time after issuance, so long as no Event of Default has occurred and/or is continuing, each of the Company and Icagen-T, has the right to redeem all or part of each Convertible Note then outstanding, with a minimum prepayment amount of $500,000, at any time upon five (5) business days’ notice to the Purchaser by paying an amount in cash equal to: a range between 101% and 103% of the Conversion Amount being redeemed if paid in full and if an Event of Default has occurred and is continuing the Purchaser has the right to require the Company to redeem the Conversion Amount for an amount of cash equal to a range between 116% and 118% of the Conversion Amount being redeemed. The “Conversion Amount” is defined as the sum of (a) the portion of the principal to be converted, redeemed or otherwise with respect to which this determination is being made, (b) all accrued and unpaid Interest with respect to such portion of such principal, (c) all accrued and unpaid late charges with respect to such portion of such principal and such Interest, if any, and (d) all other amounts due hereunder.
The Notes contain certain covenants, such as restrictions on the incurrence of indebtedness, the existence of liens, the payment of restricted payments, redemptions, the payment of cash dividends and the transfer of assets. If the Company fails to timely deliver the shares underlying the Notes, it will be subject to certain buy-in provisions.
F-26
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 18. | CONVERTIBLE DEBT (continued) |
In addition, pursuant to the Securities Purchase Agreement, the Company and Icagen-T have agreed to provide certain registration rights with respect to the Conversion Shares underlying the Icagen-T Note and, if Rule 144 under the Securities Act, is unavailable, for the Warrant Shares and Conversion Shares underlying the Company Note.
In addition, pursuant to the Convertible Notes, neither the Company nor Icagen-T shall enter into or be party to a Fundamental Transaction (as defined in the Convertible Notes) unless (i) the Successor Entity (as defined in the Convertible Notes) assumes in writing all of the obligations of the Company, Icagen-T and each Subsidiary under the Convertible Notes and the other Transaction Documents (as defined in the Securities Purchase Agreement) pursuant to written agreements in form and substance reasonably satisfactory to the Purchaser and approved by the Purchaser prior to such Fundamental Transaction, including agreements to deliver to the Purchaser in exchange for the Convertible Note and securities of the Successor Entity evidenced by a written instrument substantially similar in form and substance to the Notes, including, without limitation, having principal amounts, interest rates and late charges equal to the payment rights and amounts, principal amounts then outstanding, the interest rates and late charges in the Notes as well as having the conversion rights, redemption rights, rankings, Events of Default the same as in the Notes and satisfactory to the Purchaser, and (ii) the Successor Entity is a trading issuer whose common stock is registered under Section 12 of the Securities Exchange Act of 1934, as amended, and is quoted and/or listed for trading on a Qualifying Market.
The Convertible Notes also contain certain anti-dilution provisions that apply in connection with any stock split, stock dividend, stock combination, recapitalization and, sales of securities below the conversion price of the Notes.
In addition, subject to limited exceptions, a holder of the Company Note and Icagen-T Note will not have the right to convert any portion of such note if such holder, together with its affiliates, would beneficially own in excess of the Beneficial Ownership Limitation. A holder of the Company Note and Icagen-T Note may adjust the Beneficial Ownership Limitation upon not less than 61 days’ prior notice to the Company, provided that such Beneficial Ownership Limitation in no event shall exceed 9.99%.
The Company used the proceeds from the Company Note to repay the $1,500,000 aggregate principal amount of the 8% bridge notes issued in April 2017 and all accrued but unpaid interest thereon and to pay an amount of $500,000 owed by the Company pursuant to the terms of the Dentons settlement agreement, Icagen-T has been using the net proceeds from the purchase price paid to Icagen-T for its general corporate and working capital purposes; provided, however, neither the Company nor Icagen-T may use any of their respective net proceeds for (a) the repayment of any indebtedness other than Permitted Indebtedness (as defined in the Convertible Notes), (b) the redemption or repurchase of any securities of the Company, Icagen-T or their Subsidiaries, or (c) except for the payments pursuant to the Settlement Agreement, the settlement of any outstanding litigation; provided, further, Icagen-T will not use any of such proceeds in violation of its arrangements with Sanofi.
In connection with the Convertible Notes, the Company issued a warrant (the “Purchaser Warrant”) to purchase initially up to 857,143 shares of common stock at an initial exercise price of $3.50 per share, subject to applicable adjustments. The Purchaser Warrant expires on May 15, 2022.
In addition, subject to limited exceptions, a holder of the Purchaser Warrant will not have the right to exercise any portion of the Purchaser Warrant if such holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of the common stock outstanding immediately after giving effect to its conversion (the “Beneficial Ownership Limitation”). A holder of the Purchaser Warrant may adjust the Beneficial Ownership Limitation upon not less than 61 days’ prior notice to the Company, provided that such Beneficial Ownership Limitation in no event shall exceed 9.99%.
The Purchaser Warrant also contain certain anti-dilution provisions that apply in connection with any stock split, stock dividend, stock combination, recapitalization and, issuances of securities at prices below the conversion price or similar transactions.
If, at the time a holder exercises the Purchaser Warrant, there is no effective registration statement available for an issuance of the shares underlying the Purchaser Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of our common stock determined according to a formula set forth in the Purchaser Warrant. If the Company fails to timely deliver the shares underlying the Purchaser Warrants, it will be subject to certain buy-in provisions.
The Purchaser Warrant also provides that the Company will not enter into or be party to a Fundamental Transaction (as defined in the Purchaser Warrant) unless (i) the Successor Entity (as defined in the Purchaser Warrant) assumes in writing all of the obligations of the Company under the Purchaser Warrant and the other Transaction Documents (as defined in the Securities Purchase Agreement) pursuant to written agreements in form and substance satisfactory to the Purchaser, including agreements to deliver to the Purchaser in exchange for the Purchaser Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to the Purchaser Warrant; (ii) the Company or the Successor Entity (as the case may be) agrees at the election of the Company or the Successor Entity (as the case may be) to purchase the Purchaser Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value (as defined in the Purchaser Warrant); or (iii) the Purchaser, at its election, requires the Company or the Successor Entity (as the case may be) to purchase the Purchaser Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value.
F-27
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 18. | CONVERTIBLE DEBT (continued) |
The Company Note is secured by a security interest in all of the existing and future assets of the Company and the domestic subsidiaries, other than Icagen-T, including a pledge of all of the capital stock of each of the Domestic Subsidiaries, other than Icagen-T, subject to existing security interests, for the benefit of the Purchaser, to secure the Company obligations under the Company Note, as evidenced by (i) a security and pledge agreement, and (ii) a guaranty executed by each Domestic Subsidiary, other than Icagen-T, pursuant to which the domestic subsidiaries, other than Icagen-T, guaranteed all obligations of the Company under the Transaction Documents.
The Icagen-T Note is secured by a security interest in all of the existing and future assets of the Company, Icagen-T and the other Domestic Subsidiaries, including a pledge of all of the capital stock of each of the Domestic Subsidiaries, other than Icagen-T, subject to existing security interests, for the benefit of the Purchaser, to secure Icagen-T’s obligations under the Icagen-T Note, as evidenced by (i) a security and pledge agreement, and (ii) a guaranty executed by the Company and each Domestic Subsidiary, other than Icagen-T, pursuant to which the Company and the Domestic Subsidiaries, other than Icagen-T, guaranteed all of the obligations of Icagen-T under the Transaction Documents.
In addition, the Company and Icagen-T entered into a Subordinated Deed of Trust, Assignment of Rents, Fixture Filing and Security Agreement with the trustee named therein and the Purchaser as beneficiary, securing all of Icagen-T’s obligations to the Purchaser by a senior priority security interest in the Property/Facilities, which is subordinated only to a Deed of Trust entered into with Sanofi.
Upon an Event of Default, the Purchaser may, among other things, collect or take possession of the Company collateral or Icagen-T collateral, as the case may be, proceed with the foreclosure of the security interest in the collateral or sell, lease or dispose of the collateral. Each of the Subsidiaries has also guaranteed all of the Company’s obligations under the Company Note pursuant to the terms of the Company Guaranty and the Icagen-T Guaranty.
The transactions contemplated by the Securities Purchase Agreement closed and funded on May 15, 2017.
The movement on convertible debt is as follows:
| December 31, 2017 | December 31, 2016 | ||||||||
| Convertible debt | |||||||||
| Opening balance | $ | - | $ | - | |||||
| Convertible debt issued | 10,000,000 | - | |||||||
| Closing balance | 10,000,000 | - | |||||||
| Debt discount | |||||||||
| Opening balance | - | - | |||||||
| Original issue discount | (400,000 | ) | - | ||||||
| Fair value of warrants and beneficial conversion feature of notes | (4,518,277 | ) | - | ||||||
| Amortization of debt discount | 780,071 | - | |||||||
| Closing balance | (4,138,206 | ) | - | ||||||
| Convertible debt, net | 5,861,794 | - | |||||||
| Disclosed as follows: | |||||||||
| Short-term portion | - | - | |||||||
| Long-term portion | 5,861,794 | - | |||||||
| Convertible debt, net | $ | 5,861,794 | $ | - | |||||
F-28
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 19. | DERIVATIVE LIABILITY |
The Convertible Notes, together with the Purchaser Warrants issued to the note holders, disclosed in note 16 above, have variable priced conversion rights which may adjust whenever new securities are issued at prices lower than the current conversion and exercise price of the Convertible Notes and Purchaser Warrants issued to note holders. This gives rise to a derivative financial liability, which was initially valued upon the issue of the Convertible Notes and Purchaser Warrants using a Black-Scholes valuation model. The Beneficial conversion feature of the Convertible Notes was valued at $3,069,649 and the Purchaser Warrants issued in connection with the Convertible Notes were valued at $1,448,629.
The value of the derivative liability will be re-assessed periodically and a mark-to -market adjustment, if applicable will be recorded in the statement of operations. The value of the derivative liability was re-assessed on December 31, 2017 and a mark-to-market gain of $349,313 was credited to the statement of operations for the year ended December 31, 2017.
The following assumptions were used in the Black-Scholes valuation model.
| Year
ended December 31, 2017 | |||||
| Calculated stock price | $ | 3.50 | |||
| Risk free interest rate | 1.49 to 1.92 | % | |||
| Valuation period | 2.6 to 5 years | ||||
| expected volatility of underlying stock | 42.6 to 54.5 | % | |||
| Expected dividend rate | 0 | % | |||
The movement on derivative liabilities is as follows:
| December 31, 2017 | December 31, 2016 | ||||||||
| Opening balance | $ | - | $ | - | |||||
| Derivative liability on beneficial conversion feature of convertible debt and warrants issued to note holders | 4,518,277 | - | |||||||
| Mark-to-market adjustment | (349,313 | ) | - | ||||||
| Closing balance | $ | 4,168,964 | $ | - | |||||
| 20. | PREFERRED STOCK |
Preferred Stock consists of 10,000,000 authorized preferred shares of $0.001 par value each of which 400,000 are designated as Series A 8% convertible redeemable preferred shares of $0.001 each and 3,000,000 are designated as Series B convertible preferred shares of $0.001 each, with the remaining 6,600,000 preferred shares remaining undesignated.
Series A 8% Convertible, Redeemable Preferred Stock (“Series A Stock”)
Series A Stock consists of 400,000 designated shares of $0.001 par value each, 0 shares issued and outstanding as of December 31, 2017 and 2016.
Series B Convertible Preferred Stock (“Series B Stock”)
Series B Stock consist of 3,000,000 designated shares of $0.001 each, 0 shares outstanding as of December 31, 2017 and 2016.
| 21. | COMMON STOCK |
Common stock consists of 50,000,000 authorized shares of $0.001 each, 6,720,107 shares issued and 6,393,107 shares outstanding as of December 31, 2017 and 2016.
In terms of the Joint Stipulation to Vacate and Dismiss of the Estate of Sigmund Eisenschenk, dated December 5, 2016, the Estate returned 88,750 Common Shares valued at $310,625, to the Company. These shares were cancelled in 2017.
F-29
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 22. | WARRANTS |
Warrants exercisable for 75,000 shares of common stock at an average exercise price of $4.20 per share expired during the year ended December 31, 2017.
In terms of the Bridge Note Offering described in note 16 above, the Company sold in a private placement offering to 3 investors pursuant to a securities purchase agreement entered into with each investor, 150 Units at a per unit price of $10,000, each Unit consisting of a Note in the principal amount of $10,000 and a five-year Warrant to acquire 1,500 shares of the Company’s common stock, par value, $0.001 per share, at an exercise price of $3.50 per share for gross proceeds of $1,500,000.
The Bridge Warrants have an initial exercise price of $3.50 per share and are exercisable for a period of five years from the date of issuance. Each Bridge Warrant is exercisable for one share of common stock, which resulted in the issuance of Bridge Warrants exercisable to purchase an aggregate of 225,000 shares of common stock. In addition to this, the Company also issued warrants exercisable for 25,000 shares of common stock to the Placement Agent as compensation for the Bridge note funding discussed in note 16 above. The Bridge Warrants may be exchanged for warrants that are issued on any subsequent debt funding and are subject to adjustment in the event of stock splits and other similar transactions.
The Bridge Warrants were valued using a Black-Scholes valuation model and the proceeds received were allocated based on the percentage of the Bridge Warrants to the total value of the securities in this offering, resulting in a debt discount of $330,353.
In terms of the Convertible Notes described in note 17 above, the Company issued a Purchaser Warrant to purchase initially up to 857,143 shares of common stock at an initial exercise price of $3.50 per share, subject to applicable adjustments. The Purchaser Warrant expires on May 15, 2022.
In addition, subject to limited exceptions, a holder of the Purchaser Warrant will not have the right to exercise any portion of the Purchaser Warrant if such holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of the common stock outstanding immediately after giving effect to its conversion. A holder of the Purchaser Warrant may adjust the Beneficial Ownership Limitation upon not less than 61 days’ prior notice to the Company, provided that such Beneficial Ownership Limitation in no event shall exceed 9.99%.
The Purchaser Warrant also contain certain anti-dilution provisions that apply in connection with any stock split, stock dividend, stock combination, recapitalization and, issuances of securities at prices below the conversion price or similar transactions.
If, at the time a holder exercises the Purchaser Warrant, there is no effective registration statement available for an issuance of the shares underlying the Purchaser Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of our common stock determined according to a formula set forth in the Purchaser Warrant. If the Company fails to timely deliver the shares underlying the Purchaser Warrants, it will be subject to certain buy-in provisions.
The Purchaser Warrant also provides that the Company will not enter into or be party to a Fundamental Transaction unless (i) the Successor Entity (as defined in the Warrant) assumes in writing all of the obligations of the Company under the Purchaser Warrant and the other Transaction Documents (as defined in the Securities Purchase Agreement) pursuant to written agreements in form and substance satisfactory to the Purchaser, including agreements to deliver to the Purchaser in exchange for the Purchaser Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to the Purchaser Warrant; (ii) the Company or the Successor Entity (as the case may be) agrees at the election of the Company or the Successor Entity (as the case may be) to purchase the Purchaser Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value (as defined in the Purchaser Warrant); or (iii) the Purchaser, at its election, requires the Company or the Successor Entity (as the case may be) to purchase the Purchaser Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value.
The Purchaser Warrants were valued using a Black-Scholes valuation model and the proceeds received were allocated based on the percentage of the Purchaser Warrants to the total value of the securities in this offering, resulting in a debt discount of $1,448,629.
F-30
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 22. | WARRANTS (continued) |
The fair value of warrants issued was valued using the Black-Scholes pricing model and the following weighted average assumptions were used:
| Year ended December 31, 2017 | |||||
| Calculated stock price | $ | 3.50 | |||
| Risk free interest rate | 1.86 | % | |||
| Expected life of warrants (years) | 5 years | ||||
| expected volatility of underlying stock | 54.5 | % | |||
| Expected dividend rate | 0 | % | |||
The volatility of the common stock is estimated using historical data of companies similar in size and in the same industry as the Company. The risk-free interest rate used in the Black-Scholes pricing model is determined by reference to historical U.S. Treasury constant maturity rates with maturities approximate to the life of the warrants granted. An expected dividend yield of zero is used in the valuation model, because the Company does not expect to pay any cash dividends in the foreseeable future. As of December 31, 2017, the Company does not anticipate any awards will be forfeited in the valuation of the warrants.
A summary of all of the Company’s warrant activity during the period January 1, 2016 to December 31, 2017 is as follows:
| No. of shares | Exercise price per share | Weighted average exercise price | |||||||||||
| Outstanding January 1, 2016 | 2,146,970 | $3.50 to $11.40 | $ | 4.28 | |||||||||
| Granted | 200,375 | 3.50 | 3.50 | ||||||||||
| Forfeited/cancelled | (199,704 | ) | 4.00 to 11.40 | (11.12 | ) | ||||||||
| Exercised | - | - | - | ||||||||||
| Outstanding December 31, 2016 | 2,147,641 | $3.50 to $4.20 | 3.57 | ||||||||||
| Granted | 1,107,143 | 3.50 | 3.50 | ||||||||||
| Forfeited/cancelled | (75,000 | ) | 4.20 | 4.20 | |||||||||
| Exercised | - | - | - | ||||||||||
| Outstanding December 31, 2017 | 3,179,784 | $3.50 to $4.20 | $ | 3.5 | |||||||||
The following table summarizes warrants outstanding and exercisable as of December 31, 2017:
| Warrants outstanding | Warrants exercisable | ||||||||||||||||||||||
| Exercise price | No. of shares | Weighted average remaining years | Weighted average exercise price | No. of shares | Weighted average exercise price | ||||||||||||||||||
| $ | 3.50 | 2,961,383 | 3.05 | 2,961,383 | |||||||||||||||||||
| $ | 3.85 | 143,401 | 2.50 | 143,401 | |||||||||||||||||||
| $ | 4.20 | 75,000 | 0.24 | 75,000 | |||||||||||||||||||
| 3,179,784 | 2.96 | $ | 3.5 | 3,179,784 | $ | 3.5 | |||||||||||||||||
| 23. | STOCK OPTIONS |
In October 2005, the Company’s Board of Directors adopted the Caldera Pharmaceuticals, Inc. 2005 Stock Option Plan (the “Plan”), which permits awards of incentive and nonqualified stock options and other forms of incentive compensation to employees and non- employees such as directors and consultants. The Board has set aside 1,500,000 shares of common stock for issuance upon exercise of grants made under the Plan. Options granted under the Plan vest either immediately or over a period of up to two years, and expire 1 year to 10 years from the grant date. In terms of the Plan agreement, the plan expired during October 2015, ten years after its adoption, therefore there are no further options available under this plan for future grants.
On December 9, 2015, the Board of directors approved the 2015 Stock Incentive Plan which was approved by our stockholders exercising approximately 50.2% of our voting power. The plan became effective on March 26, 2016, 20 days following the mailing of an information statement to our stockholders.
F-31
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 23. | STOCK OPTIONS (continued) |
The 2015 Stock Incentive Plan (“the 2015 Plan”) provides the directors, officers, employees and consultants of the Company with appropriate incentives and rewards to encourage them to enter into and continue in the employ or service of the Company, to acquire a proprietary interest in the long-term success of the Company and to reward the performance of individuals in fulfilling long-term corporate objectives. The Board set aside 800,000 shares of common stock for issuance upon exercise of grants made under the Plan. Options granted under the Plan vest either immediately or over a period of time, determined at the grant date and will expire over a period of time, determined at the grant date.
Options exercisable for 33,332 and 177,479 shares of common stock for the years ended December 31, 2017 and 2016 were cancelled during the year.
The options exercisable over 33,332 shares of common stock related to an employee who had been severed and were not vested yet, these options were returned and are available for reissuance under the 2015 stock option plan. The options exercisable over 177,479 were held by employees and consultants under the 2005 Stock option plan were not exercised in terms of the option agreements entered into and have expired. The shares underlying such options were returned to and are no longer available for re-issuance under the 2005 Plan.
On March 15, 2017, the Company granted ten-year options to purchase an aggregate of 50,000 shares of common stock at an exercise price of $3.50 per share to the non-employee directors of the Company and granted ten-year options to purchase 20,000 shares of common stock at an exercise price of $3.50 per share to Richard Cunningham, the CEO of the Company. A further 50,000 options were reserved for issuance to employees.
The fair value of options issued were valued using the Black-Scholes pricing model and the following weighted average assumptions were used:
| Year ended December 31, 2017 | |||||
| Calculated stock price | $ | 3.50 | |||
| Risk free interest rate | 2.51 | % | |||
| Expected life of warrants (years) | 10 | ||||
| expected volatility of underlying stock | 70.9 | % | |||
| Expected dividend rate | 0 | % | |||
The volatility of the common stock is estimated using historical data of companies similar in size and in the same industry as the Company. The risk-free interest rate used in the Black-Scholes pricing model is determined by reference to historical U.S. Treasury constant maturity rates with maturities approximate to the life of the options granted. An expected dividend yield of zero is used in the valuation model, because the Company does not expect to pay any cash dividends in the foreseeable future. As of December 31, 2017, the Company does not anticipate any awards will be forfeited in the valuation of the options.
A summary of all of our option activity during the period January 1, 2015 to December 31, 2017 is as follows:
| No. of shares | Exercise price per share | Weighted average exercise price | |||||||||||
| Outstanding January 1, 2016 | 908,270 | $0.40 to $11.42 | $ | 3.60 | |||||||||
| Granted | 602,500 | 3.50 | 3.50 | ||||||||||
| Forfeited/cancelled | (177,479 | ) | 4.00 to 11.40 | (3.70 | ) | ||||||||
| Exercised | - | - | - | ||||||||||
| Outstanding December 31, 2016 | 1,333,291 | $0.40 to $11.42 | 3.59 | ||||||||||
| Granted | 120,000 | 3.50 | 3.50 | ||||||||||
| Forfeited/cancelled | (33,332 | ) | 3.50 | 3.50 | |||||||||
| Exercised | - | - | - | ||||||||||
| Outstanding December 31, 2017 | 1,419,959 | $0.40 to $11.42 | $ | 3.59 | |||||||||
F-32
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 23. | STOCK OPTIONS (continued) |
The following tables summarize information about stock options outstanding as of December 31, 2017:
| Options outstanding | Options exercisable | ||||||||||||||||||||||
| Exercise price | No. of shares | Weighted average remaining years | Weighted
average exercise price | No. of shares | Weighted average exercise price | ||||||||||||||||||
| $ | 0.40 | 15,000 | 4.33 | 15,000 | |||||||||||||||||||
| $ | 3.00 | 312,500 | 5.20 | 312,500 | |||||||||||||||||||
| $ | 3.50 | 939,168 | 8.04 | 424,989 | |||||||||||||||||||
| $ | 4.00 | 8,791 | 2.03 | 8,791 | |||||||||||||||||||
| $ | 5.00 | 128,500 | 2.98 | 128,500 | |||||||||||||||||||
| $ | 11.42 | 16,000 | 3.67 | 16,000 | |||||||||||||||||||
| 1,419,959 | 6.83 | $ | 3.59 | 905,780 | $ | 3.63 | |||||||||||||||||
The weighted-average grant-date fair values of options granted during the year ended December 31, 2017 was $323,161 ($2.69 per share) and for the year ended December 31, 2016 was $1,389,483 ($2.31 per share). As of December 31, 2017, there were unvested options to purchase 5145,179 shares of common stock. Total expected unrecognized compensation cost related to such unvested options is $1,171,088 which is expected to be recognized over a period of 39 months.
Stock option-based compensation expense totaled $645,756 and $492,841 for the years ended December 31, 2017 and 2016, respectively.
Stock options outstanding as of December 31, 2017 as disclosed in the above table, have an intrinsic value of $202,750.
| 24. | OTHER INCOME |
Other income consists of the following:
| Year ended | Year ended | ||||||||
| December 31, | December 31, | ||||||||
| 2017 | 2016 | ||||||||
| Reversal of deferred purchase consideration | $ | 500,000 | $ | - | |||||
| Other | 2,494 | - | |||||||
| $ | 502,494 | $ | - | ||||||
The $500,000 deferred purchase consideration due on July 1, 2017, was not earned by Pfizer due to Pfizer not meeting its $4,000,000 revenue target. This liability of $500,000 was reversed as other income during the Year ended December 31, 2017.
F-33
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 25. | OTHER EXPENSE |
Other expense consists of the following:
| Year
ended December 31, 2017 | Year ended December 31, 2016 | ||||||||
| Legal settlement expenses | $ | - | $ | (1,535,875 | ) | ||||
| Severance costs | (262,966 | ) | - | ||||||
| $ | (262,966 | ) | $ | (1,535,875 | ) | ||||
The Company refocused its operations and reduced the number of staff responsible for business development
by four people. The Company provided severance packages to these staff members based on agreements entered into and company policy.
We will, incur the severance costs over the following nine months.
In the prior year, the Company settled the Eisenschenk matter, an additional net expense of $135,875 was incurred.
In the current year a settlement was reached with Dentons for $1,400,000, which was accrued for in the prior year.
| 26. | INTEREST EXPENSE |
Interest expense consists of the following:
| Year ended December 31, 2017 | Year ended December 31, 2016 | ||||||||
| Imputed interest | $ | (308,252 | ) | $ | (497,643 | ) | |||
| Debt discount | (1,110,424 | ) | (244,463 | ) | |||||
| Interest expense | (859,760 | ) | (12,961 | ) | |||||
| Other | (3,610 | ) | - | ||||||
| $ | (2,282,046 | ) | $ | (755,067 | ) | ||||
| 27. | NET LOSS PER COMMON SHARE |
Basic loss per share is based on the weighted-average number of common shares outstanding during each period. Diluted loss per share is based on basic shares as determined above, plus the incremental shares that would be issued upon the assumed exercise of “in-the- money” stock options and warrants using the treasury stock method and the inclusion of all convertible securities, including preferred stock and convertible notes, assuming these securities were converted at the beginning of the period or at the time of issuance, if later. The computation of diluted net loss per share does not assume the issuance of common shares that have an anti-dilutive effect on net loss per share.
For the year ended December 31, 2017 and 2016, the following options, warrants and convertible notes were excluded from the computation of diluted loss per shares as the result of the computation was anti-dilutive:
| Year ended December 31, 2017 | Year ended December 31, 2016 | ||||||||
| Stock options | 1,419,959 | 1,333,291 | |||||||
| Warrants | 3,179,784 | 2,147,641 | |||||||
| Convertible notes | 2,857,143 | - | |||||||
| 7,456,886 | 3,480,932 | ||||||||
F-34
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 28. | RELATED PARTY TRANSACTIONS |
Timothy Tyson
On May 19, 2016, the Company issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Tyson as compensation for services rendered. These options vest equally over a period of 36 months.
On July 6, 2016, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $100,000 to Mr. Tyson in consideration of $100,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on July 6, 2016, Mr. Tyson was issued five year Warrants to acquire 15,000 shares of common stock exercisable at $3.50 per share.
On March 15, 2017, the Company issued Mr. Tyson ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
On April 13, 2017, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $500,000 to Mr. Tyson in consideration of $500,000. The Bridge Note matured 30 days from the date of issuance and was redeemed, together with accrued interest thereon during May 2017.
In connection with the Bridge Note, Mr. Tyson was issued five-year Bridge Warrants to acquire 75,000 shares of common stock exercisable at $3.50 per share. The Bridge Warrants are also exchangeable, at the option of the holder, for a like number of warrants issued to any lender in the Company’s next debt financing.
Richard Cunningham
On March 15, 2017, the Company issued Mr. Cunningham ten-year options exercisable for 20,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
Clive Kabatznik
On May 19, 2016, the Company issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Kabatznik as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $50,000 to Mr. Kabatznik in consideration of $50,000 in cash. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Kabatznik was issued five year Warrants to acquire 7,500 shares of common stock exercisable at $3.50 per share.
On March 15, 2017, the Company issued Mr. Kabatznik ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
Edward Roffman
On June 16, 2015, pursuant to the terms of a restricted stock award, 19,000 restricted shares were issued to the Chairman of our audit committee who is also a board director, as a once off compensation expense for his services as chairman of the audit committee. As of June 30, 2016, all of these shares were vested.
On May 19, 2016, the Company issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Roffman as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $25,000 to Mr. Roffman in consideration of $25,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Roffman was issued five-year Warrants to acquire 3,750 shares of common stock exercisable at $3.50 per share.
On March 15, 2017, the Company issued Mr. Roffman ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
F-35
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 28. | RELATED PARTY TRANSACTIONS (continued) |
Vincent Palmieri
On May 19, 2016, the Company issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Palmieri as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $50,000 to Mr. Palmieri in consideration of $50,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Palmieri was issued five year Warrants to acquire 7,500 shares of common stock exercisable at $3.50 per share.
As an employee of Taglich Brothers Inc., Mr. Palmieri was issued Placement Agent warrants in connection with the note referred to above to purchase 6,000 shares of common stock.
As an employee of Taglich Brothers Inc., Mr. Palmieri was issued 2017 Placement Agent Warrants to purchase 6,000 shares of common stock.
On March 15, 2017, the Company issued Mr. Palmieri ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
As an employee of Taglich Brothers Inc., Mr. Palmieri was issued 2017 Placement Agent Warrants to purchase 6,000 shares of common stock.
First South Africa Management
The Company incurred an expense of $180,000 for services provided by First South Africa Management for provision of CFO services by Mr. Korb and $42,000 for bookkeeping services for the year ended December 31, 2016. As of December 31, 2016, the Company owed First South Africa Management $18,600.
The Company incurred an expense of $180,000 for services provided by First South Africa Management for provision of CFO services by Mr. Korb for the year ended December 31, 2017 and $89,600 for bookkeeping services for the year ended December 31, 2017. As of December 31, 2017, the Company owed First South Africa Management $23,500.
Douglas Krafte
On February 25, 2016, upon the resignation of Dr. Benjamin Warner, as our Chief Scientific Officer, Douglas Krafte, age 56, the Chief Scientific Officer of our subsidiary Icagen Corp., was appointed to the position of Chief Scientific Officer for Icagen Inc. and its subsidiaries.
On May 19, 2016, the Company issued ten-year options exercisable for 100,000 shares of common stock at $3.50 per common shares to Mr. Krafte as compensation for services rendered. These options vested as to 25,000 options on June 30, 2016 and the remaining 75,000 equally over a period of 36 months, commencing on July 1, 2016.
On June 19, 2017, the Company entered into a four-year employment agreement with Douglas Krafte, Ph.D., pursuant to which Dr. Krafte is entitled to an annual base salary of $285,000 and will be eligible for annual discretionary performance bonus payments of up to 35% of his base salary payable in cash, which bonus, if any, will be awarded in the sole and absolute discretion of the Company’s board of directors and the compensation committee of the board of directors. Dr. Krafte continues to be engaged as the Company’s Chief Scientific Officer.
Michael Taglich
On May 19, 2016, the Company issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Taglich as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $250,000 to Mr. Taglich in consideration of $250,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Taglich was issued five year Warrants to acquire 37,500 shares of common stock exercisable at $3.50 per share.
F-36
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 28. | RELATED PARTY TRANSACTIONS (continued) |
The Company retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, the Company agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering ($1,145,000) and agreed to reimburse approximately $15,000 in respect of out of pocket expenses incurred by the placement agent in connection with the Offering. The Company also issued the placement agent the same warrant that the investors received exercisable for an aggregate amount of 28,625 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold) (the “Placement Agent Warrants”). Mr. Taglich was issued 2017 Placement Agent Warrants to purchase 7,820 shares of common stock.
On March 15, 2017, the Company issued Mr. Taglich ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
On April 12, 2017, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $500,000 to Mr. Taglich in consideration of $500,000. The Note matures 30 days from the date of issuance. In connection with the Note, Mr. Taglich was issued five year Warrants to acquire 75,000 shares of common stock exercisable at $3.50 per share. These warrants are also exchangeable, at the option of the holder, for a like number of warrants issued to any lender in the Company’s next debt financing.
The Company retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, the Company agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering (excluding $500,000 invested by the Company’s Chairman of the Board of Directors, Timothy Tyson) for a total commission of $60,000. The Company also issued the Placement Agent the same warrant that the investors received exercisable for an aggregate amount of 25,000 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold, excluding Notes sold to the Chairman) (the “2017 Placement Agent Warrants”). The Placement Agent has the right to exchange the Placement Agent Warrants for a like number of warrants to be issued to the lender in the Company’s next debt financing. As an employee and Principal of Taglich Brothers Inc. Mr. Taglich was issued 2017 Placement Agent Warrants to purchase 7,500 shares of common stock.
Robert Taglich
On April 12, 2017, the Company entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $500,000 to Mr. Robert Taglich in consideration of $500,000. The Bridge Note matured 30 days from the date of issuance and was redeemed, together with accrued interest thereon during May 2017.
In connection with the Bridge Note, Mr. Robert Taglich was issued five-year Bridge Warrants to acquire 75,000 shares of common stock exercisable at $3.50 per share. The Bridge Warrants are also exchangeable, at the option of the holder, for a like number of warrants issued to any lender in the Company’s next debt financing.
As an employee and Principal of Taglich Brothers Inc. Mr. Robert Taglich was issued 2017 Placement Agent Warrants to purchase 7,500 shares of Common stock.
Benjamin Warner
On July 7, 2017, the Employment Agreement between Dr. Benjamin Warner and the Company, dated March 15, 2013, as amended (the “Employment Agreement”) was terminated. In addition, on July 7, 2017, Dr. Benjamin Warner resigned from the Board of Directors of the Company and from all other positions with the Company. In connection with his resignation, the Company executed certain release agreements (the “Release Agreements”) with Dr. Warner. Pursuant to the Release Agreements, Dr. Warner’s Employment Agreement was terminated by mutual agreement, Dr. Warner and the Company exchanged mutual releases and the Company agreed to continue the payments currently due to Dr. Warner under the Employment Agreement, through the end of its stated term notwithstanding its termination.
| 29. | OPERATING LEASES |
The Company entered into an asset purchase agreement with Icagen, Inc., a subsidiary of Pfizer, Inc., whereby certain assets were acquired from Icagen, Inc., the agreement included the sub-letting of premises located at Research Triangle Park, Durham, North Carolina. The lease terminates on April 30, 2019. The rental expense for the year ended December 31, 2017 amounted to $194,820.
Future annual minimum payments required under operating lease obligations as of December 31, 2017, are as follows:
| Amount | |||||
| 2018 | $ | 193,306 | |||
| 2019 | 66,950 | ||||
| Total | $ | 260,256 | |||
F-37
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 30. | COMMITMENTS AND CONTINGENCIES |
As a result of the agreements that the Company entered into with Pfizer and Sanofi, is obligated; (i) to continue to retain certain employees at its Icagen-T facility until July 15, 2018, which it estimates will require additional compensation of $4,459,000 at its Icagen-T facility; (ii) make additional payments in terms of the Asset Purchase and Collaboration Agreement that it entered into on June 26, 2015 with Pfizer including beginning in 2017, a quarterly earn out payment (the “Earn Out Payment”) of 10% of revenue earned during the quarter, with a minimum payment of $250,000 per quarter, up to a maximum aggregate payment of $10,000,000, such minimum being reduced to $50,000 for the quarters ending March 2017 to December 2018 and the difference between $250,000 or the quarterly amount paid and the actual calculation of deferred purchase consideration at 10% of gross revenue per quarter is being deferred and paid as one lump sum with the payment being made the quarter ended March 31, 2019, bearing interest at 12.5% per annum, which interest is payable quarterly; (iii) make minimum lease payments in terms of a sub-lease agreement entered into with Pfizer for the period July l, 2015 to April 30, 2019 with annual escalations of 3.5%, estimated to be $260,256.
In terms of a Mutual Release and Assignment Agreement entered into between American Milling LP and the Company, the Company agreed to pay American Milling $800,000 of which $83,334 remains to be paid at December 31, 2017.
The Company reached a Settlement and Release Agreement with Dentons and has agreed to pay Dentons the sum of $1,400,000 over a period of fourteen months of which $1,200,000 has been paid to date.
On May 15, 2017, the Company, and its wholly owned subsidiary, Icagen-T, entered into a Securities Purchase Agreement with an institutional investor (the “Purchaser”), pursuant to which (i) the Company issued to the Purchaser the Company Note which is a three year Senior Secured Convertible Note, maturing on May 15, 2020, bearing interest at the rate of 13% per annum (which interest rate increases to 18% per annum upon the occurrence of an event of default, as defined in the Note), in the aggregate principal amount of $2,000,000 for cash proceeds of $1,920,000 after an original issue discount of 4% or $80,000, before deal related expenses; and (ii) Icagen-T issued to the Purchaser the Icagen-T note which is a three year Senior Secured Convertible Note, maturing on May 15, 2020, bearing interest at the rate of 13% per annum, in the aggregate principal amount of $8,000,000 for cash proceeds of $7,680,000 after an original issue discount of 4% or $320,000, before deal related expenses. The Company Note and the Icagen-T Note are each convertible into shares of common stock at a conversion price of $3.50 per share.
On June 19, 2017, the Company entered into a four-year employment agreement with Douglas Krafte, Ph.D., pursuant to which Dr. Krafte is entitled to an annual base salary of $285,000 and will be eligible for annual discretionary performance bonus payments of up to 35% of his base salary payable in cash, which bonus, if any, will be awarded in the sole and absolute discretion of the Company’s board of directors and the compensation committee of the board of directors. Dr. Krafte continues to be engaged as the Company’s Chief Scientific Officer.
| 31. | LITIGATION |
Set forth below is a factual description of the status of Company’s legal proceedings.
Dentons Dispute
On May 11, 2017, the Company entered into a Settlement and Release Agreement with Dentons US LLP (“Dentons”) relating to disputes arising between them under a Settlement and Release Agreement, dated July 5, 2013 (the “2013 Settlement Agreement”), a judgment thereafter obtained by Dentons on May 7, 2014 in the Circuit Court of Cook County, Illinois Lawsuit based upon the 2013 Settlement in the amount of $3,050,000, and a lawsuit filed by the Company in San Francisco Superior Court in or about April 2014 against Dentons. In connection with the Agreement, the Company has agreed to pay Dentons the sum of $1,400,000 over a fourteen month period of which $500,000 was paid on May 15, 2017, a further $500,000 was paid on December 15, 2018 and $200,000 was paid on March 15, 2018. In addition, to secure its obligations under the Agreement, the Company executed and delivered to Dentons a Confession of Judgment Affidavit in Support of Confession of Judgment in the amount of $3,891,549.32, representing the amount of the Judgment had obtained plus the costs of suit and interest accrued through May 15, 2017. The Confession of Judgment is not to be filed unless the Company defaults on its obligations under the Agreement and it will be returned to the Company upon payment in full under the Agreement. The Agreement included mutual releases of claims each party had against the other and the parties also agreed to dismiss the litigation between them with prejudice; provided, that Dentons’ obligations commence after it has received $500,000 of the payments from the Company described above.
F-38
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 32. | SUBSEQUENT EVENTS |
Private Placement
We have offered on a best efforts basis up to a maximum of forty (40) units and a minimum of ten (10) units, at a purchase price of $100,000 per unit, each unit consisting of 28,571 shares of Series C Convertible Preferred Stock, par value $0.001 per share and a seven year Warrant to acquire 28,571 shares of our common stock, par value, $0.001 per share, at an exercise price of $3.50 per share. On April 4, 2018, we closed the first tranche of the Offering and entered into a securities purchase agreement (the “Purchase Agreement”) with one accredited investor that is a trust of which a member of our Board of Directors is the trustee, pursuant to which we offered and sold an aggregate of twenty (20) Units. The sale of the twenty (20) Units resulted in gross offering proceeds of $2,000,000.
The Series C Preferred Stock ranks senior to the shares of our common stock, and any other class or series of stock issued by us with respect to dividend rights, redemption rights and rights on the distribution of assets on any voluntary or involuntary liquidation, dissolution or winding up of our affairs. Holders of Series C Preferred Stock are entitled to a cumulative dividend at the rate of 12.0% per annum, as set forth in the Certificate of Designation of Series C Convertible Preferred Stock classifying the Series C Preferred Stock, a form of which is attached as an annex to the Purchase Agreement (the “Certificate of Designation”). The Series C Preferred Stock will be convertible at the option of the holders at any time into such number of shares of common stock as shall be equal to the $3.50 plus any accrued and unpaid dividends on such share of Series C Preferred Stock (the “Accreted Value”) divided by the conversion price, which initially shall be $3.50 per share, subject to certain customary anti-dilution adjustments. In addition, the Series C Preferred Stock shall automatically convert into shares of our common stock based upon the then effective conversion price upon the (i) closing of a sale of shares of common stock to the public in a Qualifying Public Offering (as defined below) or a reverse merger into a publicly reporting company that has its common stock listed or quoted and traded on a Trading Market ( as such term is defined in the Certificate of Designation) or (ii) the date and time, or the occurrence of an event, specified by vote or written consent of the holders of at least seventy-five percent (75%) of the outstanding shares of Series C Preferred Stock (the “Requisite Holders”) (the time of such closing or the date and time specified of the event specified in such vote or written consent is referred to herein as the “Mandatory Conversion Date”). A “Qualifying Public Offering” is defined as the first firm commitment underwritten public offering by the Company on or following the initial issuance date of the Series C Preferred in which shares of common stock are sold for its account solely for cash to the public resulting in proceeds to it and/or its subsidiary, Icagen-T, Inc. of no less than $8,000,000 (after deduction only of underwriter discounts and commissions) and where the shares of common stock registered under the Securities Act of 1933, as amended, and sold in such public offering are simultaneously listed and commence trading on a Trading Market ( as such term is defined in the Certificate of Designation)
In the event of any liquidation, dissolution or winding-up of the Company, holders of the Series C Preferred Stock shall be entitled to a preference on liquidation equal to $5.25 per share of Series C Preferred Stock plus all accrued and unpaid dividends.
Each holder of Series C Preferred Stock shall have the right to cast the number of votes equal to three times the number of shares into which the Series C Preferred Stock is convertible and the Series C holders as a group, shall have the right to elect one director on our Board of Directors. The Company cannot take the following actions without the approval of the Requisite Holders and the consent of our Board of Directors, including the Series C Preferred Stock director: (i) liquidate, dissolve or wind up our business, (ii) amend our Certificate of Incorporation or Bylaws, (iii) create any new class of stock unless it ranks junior to the Series C Preferred Stock with respect to dividends and liquidation, (iv) amend or alter any class of stock pari passu with the Series C Preferred Stock to make it senior with respect to dividends and liquidation, (v) purchase or redeem any other shares of our stock, or (vi) increase the size of our Board of Directors.
Upon the occurrence of a Cash Liquidity Event, the holders of the Series C Preferred Stock can require the Company to redeem their shares for Series C Preferred Stock for a price per shares equal to $5.25 subject to adjustments. In addition, we have the right to redeem the shares of at any time for a price per shares equal to $5.25 subject to adjustments. A “Cash Liquidity Event” is defined as the closing of any sale, lease or licensing transaction relating to a single asset or multiple assets other than in our ordinary course of business, including, but not limited to a sale of a building, sale of biological assets or other upfront payments, resulting in aggregate gross proceeds received by us at closing or closings in a transaction or transactions during any twelve (12) month period in excess of $40,000,000.
F-39
ICAGEN, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 32. | SUBSEQUENT EVENTS (continued) |
As part of the Unit we issued the Warrant to the Purchaser at an initial exercise price of $3.50 per share (subject to applicable adjustments) (the “Exercise Price”). The Warrant expires seven years after the issuance date.
In addition, subject to limited exceptions, a holder of the Warrant will not have the right to exercise any portion of the Warrant if such holder, together with its affiliates, would beneficially own in excess of 9.99% of the shares of common stock outstanding immediately after giving effect to such exercise. A holder of the Warrant may adjust this limitation upon not less than 61 days’ prior notice to the Company, provided that such limitation in no event shall exceed 9.99%.
The Warrant also contain certain anti-dilution provisions that apply in connection with any stock split, stock dividend, stock combination, recapitalization and issuances of securities at prices below the conversion price or similar transactions.
If, at the time a holder exercises its Warrant, there is no effective registration statement registering available for an issuance of the shares underlying the Warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to the Company upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Warrant. If the Company fails to timely deliver the shares underlying the Warrant, it will be subject to certain buy-in provisions.
The Warrant also provides that the Company will not enter into or be party to a Fundamental Transaction unless (i) the Successor Entity (as defined in the Warrant) assumes in writing all of the obligations of the Parent under the Warrant and the other Transaction Documents (as defined in the Securities Purchase Agreement) pursuant to written agreements in form and substance satisfactory to the Purchaser, including agreements to deliver to the Purchaser in exchange for the Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to the Warrant; (ii) the Company or the Successor Entity (as the case may be) agrees at the election of the Company or the Successor Entity (as the case may be) to purchase the Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value (as defined in the Warrant); or (iii) the Purchaser, at its election, requires the Company or the Successor Entity (as the case may be) to purchase the Warrant from the Purchaser by paying to the Purchaser cash in an amount equal to the Black Scholes Value.
On April 3, 2018, the Company filed the Certificate of Designation with the Secretary of State of the State of Delaware establishing the Series C Convertible Preferred Stock which entitles each holder of Series C Preferred Stock to a cumulative dividend at the rate of 12.0% per annum, payable quarterly in arrears.
The Series C Preferred Stock ranks senior to the shares of the Common Stock and any other class or series of stock issued by the Company with respect to dividend rights, redemption rights and rights on the distribution of assets on any voluntary or involuntary liquidation, dissolution or winding up of the affairs of the Company.
Registration Rights
Pursuant to the terms of the Purchase Agreement, the Company granted to the holder of the Series C Preferred Stock certain demand registration and piggyback registration rights, subject to certain rights of our lender.
The Company has evaluated subsequent events through the date the consolidated financial statements were available to be issued and has concluded that no such events or transactions took place that would require disclosure herein.
F-40
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosures
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
The Company has adopted and maintains disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in the reports filed under the Exchange Act, such as this Annual Report on Form 10-K, is collected, recorded, processed, summarized and reported within the time periods specified in the rules of the SEC. The Company’s disclosure controls and procedures are also designed to ensure that such information is accumulated and communicated to management to allow timely decisions regarding required disclosure. As required under Exchange Act Rule 13a-15, the Company’s management, including the Chief Executive Officer and the Company’s Chief Financial Officer, after evaluating the effectiveness of disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and15d-15(e)) as of the end of the period covered by this Annual Report on Form 10-K, have concluded that due to a lack of segregation of duties that the Company’s disclosure controls and procedures are ineffective to ensure that information required to be disclosed by the Company in the reports that the Company files or submits under the Exchange Act, is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including the Company’s Chief Executive Officer and the Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. Subject to receipt of additional financing or revenue generated from operations, the Company intends to retain additional individuals to remedy the ineffective controls.
Management’s Annual Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act as a process designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements. Management conducted an assessment of the Company’s internal control over financial reporting as of December 31, 2017 based on the framework and criteria established by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework 2013 (“COSO”). The COSO framework requires rigid adherence to control principles that require sufficient and adequately trained personnel to operate the control system. The Company has insufficient accounting personnel for it to be able to segregate duties as required by COSO or to maintain all reference material required to ensure Company personnel are properly advised or trained to operate the control system. Based on the assessment, management concluded that, as of December 31, 2017, the Company’s internal control over financial reporting was ineffective based on those criteria.
The Company’s management, including its Chief Executive Officer and Chief Financial Officer, does not expect that the Company’s disclosure controls and procedures and its internal control processes will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of error or fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that the breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that occurred during our year ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the SEC that permit the Company to provide only management’s report in this annual report.
None.
37
Item 10. Directors, Executive Officers and Corporate Governance
Directors, Executive Officers and Other Key Employees
Below is certain information regarding our current directors and executive officers.
| Name | Age | Position | ||
| Richard Cunningham | 47 | President and Chief Executive Officer | ||
| Douglas Krafte | 60 | Chief Scientific Officer | ||
| Mark Korb | 50 | Chief Financial Officer | ||
| Timothy C. Tyson | 65 | Director, Non-Executive Chairman of the Board, member of the Compensation Committee, member of the Nominating Committee | ||
| Clive Kabatznik | 61 | Director, member of Audit Committee | ||
| Vincent Palmieri | 46 | Director, Chairman of the Compensation Committee, member of Audit Committee | ||
| Edward Roffman | 68 | Director, Chairman of the Audit Committee | ||
| Michael Taglich | 52 | Director, chairman of the nominating committee |
Richard Cunningham, President and Chief Executive Officer
Mr. Cunningham became our President and Chief Executive Officer on November 24, 2014. From April 2008 until November 2014, Mr. Cunningham has held various positions at Boehringer Ingelheim, a pharmaceutical company, which positions include, serving as Executive Director from January 2014 until November 2014, a Director from June 2010 until December 2013 and National Account Director from April 2008 until June 2010. Prior to working at Boehringer Ingelheim Mr. Cunningham was a senior executive in the commercial organization leading sales, marketing and contracting activities at Valeant Pharmaceuticals. Mr. Cunningham began his career in healthcare at Premier Inc. a healthcare company that served as a group purchasing and service organization for over 1700 hospitals throughout the nation. While at Premier he served as the Marketing Director at Premier Practice Management, a subsidiary and start-up company of Premier Inc.
Douglas Krafte, Ph.D., Chief Scientific Officer
Douglas Krafte was appointed as our Chief Scientific Officer on February 25, 2016. Dr. Krafte has held a variety of positions over 25 years within the pharma/biotech sectors across multiple therapeutic areas most recently until the acquisition in July 2015 of Icagen, Inc., the subsidiary of Pfizer, Inc., as Executive Director & Site Head for the US arm of Pfizer’s Pain & Sensory Disorders Research Unit, as well as positions at Aurora Biosciences, Boehringer Ingelheim and Sterling Winthrop. Over the years, he has gained extensive experience in managing and leading small molecule drug discovery teams that have successfully advanced multiple molecules to the clinic. Dr. Krafte is an expert in drug discovery targeting ion channel proteins. Two of the most recent projects identified clinical candidates that remain in clinical development with Pfizer. He was a member of the Leadership Team for Pain & Sensory Disorders Unit reporting to the Chief Scientific Officer and also served on the Emerging Science Fund which evaluates a wide range of asset and technology opportunities across all therapeutic and platform areas at Pfizer. Dr. Krafte has extensive experience in managing drug discovery projects and teams, technology evaluation, and due diligence from both the perspective of the buyer and seller. He is currently a member of the Biophysical Society, Society for Neuroscience, American Heart Association and Cardiac Electrophysiology Society. He serves as a mentor for the 4D Strategic Initiative which advises Principal Investigators from the University of North Carolina-Chapel Hill and affiliated partners in drug, device and diagnostic development and commercialization. Dr. Krafte did his post-doctoral training at the California Institute of Technology in Molecular Neurobiology and received his MS/PhD in Physiology from the University of Rochester.
38
Mark Korb, Chief Financial Officer
Mark Korb has served as our Chief Financial Officer since August 14, 2013. Mr. Korb has over 20-years’ experience with high-growth companies and experience taking startup operations to the next level. Since July 2011, First South Africa Management, a company for which Mr. Korb has served as the Chief Financial Officer since January 2010 has been providing consulting services to us, including the financial expertise required of public companies. First South Africa Management provides financial management and strategic management services to various companies.
From 2007 to 2009 Mr. Korb was the group chief financial officer and director of Foodcorp (Proprietary) Limited (“Foodcorp”), a multimillion dollar consumer goods company based in South Africa. In his role as chief financial officer, Mr. Korb delivered operational and strategic leadership for the full group financial function during a period of change including mergers, acquisitions and organic growth. As a board director he cultivated relationships with shareholders, bond holders, financial institutions, rating agencies, and auditors. Mr. Korb was also responsible for leading the group IT strategy and implementation and supervised 16 direct reports including 10 divisional financial directors. From 2001 to 2007 Mr. Korb was the group Chief Financial Officer of First Lifestyle, initially a publicly traded company on the Johannesburg Stock Exchange in South Africa which was then purchased by management which included Mr. Korb. He anchored the full group financial function with responsibility for mergers and acquisitions activity, successfully leading the process whereby the company was sold to Foodcorp mentioned above. Upon completion of the merger, Mr. Korb was appointed as the group Chief Financial Officer of Foodcorp. Mr. Korb is also the Chief Financial Officer to several other companies including, Qpagos, a company involved in the electronic payment technology in Mexico and MCW Energy Group Limited, a Canadian company engaged in the creation of technology for the environmentally- safe extraction of oil form oil sands and oil shale deposits.
Timothy C. Tyson, Director and Non-Executive Chairman of the Board
Mr. Tyson has served as our Non-Executive Chairman of the Board since April 1, 2014 and has been a director since October 1, 2012. Since 2008, Mr. Tyson has served as the Chairman of the Board of Directors of Aptuit LLC, a global, private equity owned, pharmaceutical services company, headquartered in Greenwich, CT and he served as the Chief Executive Officer of Aptuit LLC from 2008 until March 2012. Mr. Tyson also serves as the Chairman and CEO of Avara Pharmaceuticals Services, Inc., a company he founded in 2015 that is a CDMO serving the pharmaceutical industry. Mr. Tyson served as President and CEO of Valeant Pharmaceuticals International from 2003-2008. Prior to joining Valeant, Mr. Tyson ran multiple divisions for GlaxoSmithKline (“GSK”) and was a member of the Corporate Executive Team, reporting to the CEO. During his 14-year tenure at GSK, he was President, Global Manufacturing and Supply and ran Glaxo Dermatology and Cerenex Pharmaceuticals. Mr. Tyson was also responsible for managing all sales and marketing for GlaxoWellcome’s U.S. operations, where he launched 32 new products, eight of which reached sales of greater than $1 billion. From 1980-1988, Mr. Tyson held executive positions in technical operations and R & D, at Bristol-Myers. Prior to his tenure at Bristol-Myers, he was an operations manager for Procter & Gamble. Mr. Tyson is a 1974 graduate of the United States Military Academy at West Point. While on active duty at Ft. McClellan, AL, he earned a Masters of Public Administration, in 1976, and a Masters of Business Administration, in 1979, from Jacksonville State University. In 2002, Mr. Tyson received the Bicentennial Leadership Award from the United States Military Academy at West Point and was named 2007 Alumnus of the Year at Jacksonville State University. He was inducted into the Six Sigma Hall of Fame in 2011 and was honored in 2012 at West Point by the Thayer Hotel Room Dedication program. He was recognized as a President’s Club awardee for four years at GSK and his GSK organization was recognized as Marketer of the Year for two consecutive years by MedAdNews.
Mr. Tyson’s business experience in the pharmaceutical industry and his overall understanding of the industry in which we operate make him a desirable board candidate.
39
Clive Kabatznik, Director
Mr. Kabatznik, currently serves as the President of First South Africa Management, a company that he founded that has been engaged in management consultancy services since January 1996. From 2017 to the present Mr. Kabatznik has been Chairman of the Board of Datos Health, Inc. a Software provider of digital medical products primarily in the sphere of remote patient monitoring. From 2005 until the present, Mr. Kabatznik has served as a director of Strategy First, Inc.; a Montreal based digital publisher and distributor of video games. From 2009 until 2015, he was the operating manager of New Bedford Media LLC, a company he co-founded which focuses on the acquisition and operation of digital media companies. From 1995 until 2009, he served as Chief Executive Officer of Silverstar Holdings, a United States publicly listed company that he founded that was established to acquire, own and operate companies, with an emphasis on businesses which stand to benefit from new Internet and technology-based platforms. Prior to 1995, Mr. Kabatznik was engaged in investment banking.
Mr. Kabatznik has served as President of Colonial Capital, Inc., a Miami-based investment banking company that specializes in advising middle market companies in areas concerning mergers, acquisitions, private and public agency funding and debt placements.
Mr. Kabatznik’s business experience with small public companies, his achievements in the financial industry and his overall business understanding make him a desirable board candidate.
Vincent Palmieri, Director
Mr. Palmieri, is a Vice President of Taglich Brothers, Inc. and specializes in sourcing, evaluating, and executing new investments as well as monitoring existing investments in small public and private companies. Mr. Palmieri received a Bachelor of Science in Accounting from the Pennsylvania State University and an MBA from the Stern School of Business at New York University.
Mr. Palmieri’s expertise in financial and accounting matters, his overall business understanding, as well as his familiarity and knowledge regarding public companies and corporate governance issues that public companies face make him an ideal board candidate.
Edward Roffman, Director
Mr. Roffman has been a director since December 2011. Since April 2006, Mr. Roffman has consulted with various early-stage high technology companies. During this time, consulting projects have included the part-time Chief Financial Officer of LERNA, LLC (from April 2014 to August 2015). AdSource, LLC (since January 2014 to December 2016) and Emerge Digital, Inc. (from January 2012 to June 2014), all in online digital advertising, the part-time Chief Financial Officer of Public Media Works, Inc. (October 2010 to October 2011) (Public Media Works was in the video rental business) and from January 2008 to December 2009, Mr. Roffman was the part-time Chief Financial Officer of Cryptic Studios, a developer of massively multiplayer video games. Mr. Roffman has also been a principal of Creekside, LLC, a consulting firm which specializes in the software, internet and consumer products industries. Mr. Roffman is a CPA with over 40 years of experience in accounting and finance. Mr. Roffman earned a BBA in accounting from Temple University. Mr. Roffman also served as a director of Andalay Solar Inc. from August 2006 until June 2015.
Mr. Roffman’s achievements in financial and accounting matters, his overall business understanding, as well as his familiarity and knowledge regarding public companies and corporate governance issues that public companies face makes him an ideal board candidate.
Michael Taglich, Director
Mr. Taglich, is Chairman of the Board and President of Taglich Brothers, Inc., a New York City based securities firm. From 1987 to 1992, Mr. Taglich served as a Vice President at Weatherly Securities. He brings a broad depth and breadth of capital and business background to our Board of Directors, with extensive experience in exit strategies. Mr. Taglich is also currently Chairman of the Board of Air Industries, Inc., a manufacturer of precision aerospace components. He also serves as a Director of Bridgeline Digital, Inc. Mr. Taglich holds a B.S. degree in General and International Business from New York University and holds Series 27 and Series 7 security licenses.
Mr. Taglich’s capital and business background, his overall business understanding, as well as his familiarity and his service on public company boards provide him with the knowledge regarding public companies and corporate governance issues that public companies face makes him an attractive board candidate.
40
Corporate Governance
Term of Office
Our directors hold office until the next annual general meeting of our shareholders or until removed from office in accordance with our bylaws. Our officers are appointed by our board of directors and hold office until removed by the board.
Leadership Structure
We currently have two separate individuals serving as our Chairman of the Board and as our Chief Executive Officer and we do not have a formal policy on whether the same person should (or should not) serve as both the Chief Executive Officer and Chairman of the Board. Due to the size of our Company, we believe that this structure is appropriate in recognition of the time commitment and activities required to function effectively as a Chairman and as a Chief Executive Officer. Mr. Tyson was appointed as our Non-Executive Chairman of the Board in April 2014. Mr. Cunningham has served as our Chief Executive Officer since November 2014. Our Board of Directors has determined that this leadership structure is appropriate and effective for us given our stage of operations. In serving as Non-Executive Chairman of the Board, Mr. Tyson serves as a significant resource for our Chief Executive Officer, other members of management and the Board of Directors. We believe that the division of duties and additional avenues of communication between the Board and management with Mr. Tyson serving as Non-Executive Chairman of the Board provides a basis for the proper functioning of our Board and oversight of management.
Our Board of Directors has several independent directors. We do not have a separate lead independent director. We believe the combination of Mr. Tyson as our Non-Executive Chairman of the Board and Mr. Cunningham as our Chief Executive Officer is an effective structure for us. Our current structure is operating effectively to foster productive, timely and efficient communication among the independent directors and management. We do have active participation in our committees by our independent directors, who comprise all of the members of all of our committees. Each committee performs an active role in overseeing our management and there are complete and open lines of communication with the management and independent director.
Board Committees
We have an Audit Committee, Compensation Committee and Nominating Committee, each comprised primarily of independent directors.
Audit Committee
The Audit Committee is comprised of Mr. Roffman, Mr. Palmieri and Mr. Kabatznik. The Audit Committee is responsible for recommending our independent public accounting firm and reviewing management’s actions in matters relating to audit functions. The Audit Committee reviews with our independent public accountants the scope and results of the audit engagement and the system of internal controls and procedures. The Audit Committee also reviews the effectiveness of procedures intended to prevent violations of laws. The Audit Committee also reviews, prior to publication, our Annual and Quarterly Reports on Form 10-K and Form 10-Q. Our Board has determined that all Audit Committee members are independent under applicable SEC regulations. Our Board of Directors has determined that both Mr. Roffman and Mr. Kabatznik qualify as “audit committee financial experts” as that term is used in Section 407 of Regulation S-K.
Compensation Committee
Our Compensation Committee consists of Mr. Tyson and Mr. Palmieri. This committee performs several functions, including reviewing all forms of compensation provided to our executive officers, directors, consultants and employees, including stock compensation. Our Board has determined that the Compensation Committee members are independent under applicable SEC regulations.
Nominating Committee
Our Nominating Committee consists of Mr. Taglich and Mr. Tyson. This committee performs several functions, including (i) considering and recommending to the Board of Directors, individuals for appointment or election as directors; (ii) recommending to the Board of Directors individuals for appointment to vacancies on any committee of the Board of Directors; and (iii) recommending to the Board of Directors regarding any changes to the size of the Board of Directors or any committee. Our Board has determined that the Nominating Committee members are independent under applicable SEC regulations.
41
Director Independence
Although our common stock is not listed on any national securities exchange, for purposes of independence we use the definition of independence applied by The NASDAQ Stock Market. The Board has determined that Messrs. Roffman, Kabatznik, Palmieri, Taglich and Tyson are “independent” in accordance with such definition.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our executive officers, directors and persons who beneficially own more than 10 percent of a registered class of Icagen equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock. Such officers, directors and persons are required by SEC regulation to furnish us with copies of all Section 16(a) forms that they file with the SEC.
Based solely on a review of the copies of such forms that were received by us, or written representations from certain reporting persons that no Forms 5 were required for those persons, we are not aware of any failures to file reports or report transactions in a timely manner during the year ended December 31, 2017.
Code of Ethics
We maintain a Code of Business Conduct and Ethics which is applicable to all of our directors, officers and employees. We will send a copy of the Code of Code of Business Conduct and Ethics, free of charge, upon our receipt of a written request therefor addressed to us at 4222 Emperor Blvd, Suite 350, Durham, North Carolina 27703, Attention: Richard Cunningham.
Item 11. Executive Compensation
The following table sets forth all compensation awarded, earned or paid for services rendered by our principal executive officer, principal financial officer and each executive officer whose compensation exceeded $100,000 during each of the fiscal years ended December 31, 2017 and 2016.
Summary Compensation Table
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($) | Non-Equity Plan Compensation ($) | Non-Qualified Deferred Compensation Earnings ($) | All
Other Compensation ($) | Total ($) | ||||||||||||||||||||||||
| Richard Cunningham, | 2017 | 305,625 | 260,000 | 53,860 | - | - | 20,693 | 640,178 | ||||||||||||||||||||||||
| president and Chief Executive Officer (1)(2) | 2016 | 300,000 | 150,000 | - | - | - | 25,779 | 475,779 | ||||||||||||||||||||||||
| Douglas Krafte, | 2017 | 278,537 | 63,854 | - | - | - | 41,506 | 383,897 | ||||||||||||||||||||||||
| Chief Scientific Officer (3)(4) | 2016 | 215,328 | 36,168 | 243,376 | - | - | 32,491 | 527,363 | ||||||||||||||||||||||||
| Mark Korb, | 2017 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Chief Financial Officer (5) | 2016 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| (1) | All other compensation for Mr. Cunningham includes $19,105 (2016 - $24,382) for Company contributed health care and $1,588 (2016 - $1,397) for life, and disability benefits. |
| (2) | Mr. Cunningham was awarded 20,000 options on March 15, 2017 which vest equally over a 36 month period commencing on March 15, 2017. These options were valued using the Black-Scholes option pricing model. |
| (3) | All other compensation for Douglas Krafte includes $27,383 (2016 - $19,981) for company contributed health care; $12,534 (2016 - $11,317) for company contributions to his 401(k) plan and $1,588 (2016 - $1,193) for life and disability benefits. |
| (4) | Dr. Krafte was awarded 100,000 common stock options on May 3, 2016, which vested as to 25,000 immediately and 2,083 per month for a period of 36 months. The options were valued at $243,376 using a Black-Scholes valuation model. |
| (5) | Mr. Korb is not compensated directly for his services as our CFO, however he is compensated by First South Africa Management (“FSAM”). Clive Kabatznik, one of our directors, is the managing member of FSAM, which has a consulting agreement with the Company, for a fee of $15,000 per month for CFO services and a further $8,500 per month for bookkeeping services and operates on a month to month basis. |
42
Outstanding Equity Awards at Fiscal Year End
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each named executive officer as of December 31, 2017:
| OPTION AWARDS | STOCK AWARDS | |||||||||||||||||||||||||||||||||
| Number of securities underlying unexercised options Exercisable | Number of securities underlying unexercised options Unexercisable | Equity incentive plan awards: Number of securities underlying unearned options | Option exercise price | Option expiry | Number of shares or units of stock that have not vested | Market value of shares or units of stock that have not vested | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested | Equity
incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested | ||||||||||||||||||||||||||
| Name | (#) | (#) | (#) | ($) | Date | (#) | ($) | (#) | ($) | |||||||||||||||||||||||||
| Richard Cunningham(1) | 154,166 | 95,834 | - | 3.50 | 1/7/2025 | - | - | - | - | |||||||||||||||||||||||||
| 5,000 | 15,000 | - | 3.50 | 3/14/2027 | - | - | - | - | ||||||||||||||||||||||||||
| Douglas Krafte(2) | 62,500 | 37,500 | - | 3.50 | 5/18/2026 | - | - | - | - | |||||||||||||||||||||||||
| Mark Korb(3) | 37,500 | - | - | 3.00 | 3/14/2023 | - | - | - | - | |||||||||||||||||||||||||
| (1) | Mr. Cunningham was awarded 250,000 options on January 7, 2015 which vest as follows; 50,000 vested on November 24, 2015, 150,000 vest equally over a period of 36 months commencing on November 24, 2015 and 50,000 on November 24, 2018. This does not include an additional 20,000 options awarded to Mr. Cunningham on March 15, 2017 by the Compensation Committee. A further 20,000 options were awarded on March 15, 2017 which vest equally over a 36 month period. |
| (2) | Dr. Krafte was awarded 100,000 options on May 19, 2016 which vest as to 25,000 on May 19, 2016 and the remaining equally over a period of 36 months. |
| (3) | Mr. Korb was awarded 37,500 options on May 15, 2013 which are fully vested. |
Employment Agreements
Richard Cunningham
During November 2014, Mr. Richard Cunningham was appointed CEO and President of the Company. Effective November 24, 2014, the Company entered into an employment agreement with Mr. Cunningham for him to serve as the Chief Executive Officer and President of the Company. The employment agreement is for a term of four years, pursuant to which Mr. Cunningham is entitled to an annual base salary of $300,000, which was increased by 2.5% in March 2017 to $307,500 and is eligible for discretionary performance bonus payments of up to 100% of his base salary payable in cash. In addition, Mr. Cunningham was guaranteed a minimum bonus amount of $100,000 payable immediately after the first year of employment with the Company provided he remained employed by the Company on the one- year anniversary of his commencement of employment. The bonus was paid in 2015. Additionally, pursuant to the terms of his agreement, on January 7, 2015, Mr. Cunningham was granted options to purchase 250,000 shares of the Company’s common stock at an exercise price of $3.50 (post-stock split) per share. These options vest as follows: (i) Fifty Thousand shares vest on the one-year anniversary of the effective date of the Employment Agreement; (ii) One Hundred Fifty Thousand shares vest monthly on a pro rata basis commencing on the last day of months thirteen through forty-eight of the term of the Employment Agreement; and (iii) Fifty Thousand shares vest on the four year anniversary of the effective date of the Employment Agreement. Upon a change of control, as defined in the Company’s existing stock option plan, all unvested options issued to the Mr. Cunningham shall become fully vested immediately upon the change of control. In March 2017, Mr. Cunningham was awarded a cash bonus equal to seventy percent of his base salary plus ten year options to purchase twenty thousand shares of our common stock at an exercise price of $3.50 per share, vesting monthly on a pro-rata basis over three years.
43
The Employment Agreement also includes confidentiality obligations and inventions assignments by Mr. Cunningham.
If Mr. Cunningham’s employment is terminated for any reason, he or his estate as the case may be, will be entitled to receive the accrued base salary, bonus earned, vacation pay, expense reimbursement and any other entitlements accrued by him to the extent not previously paid (the “Accrued Obligations”); provided, however , that if his employment is terminated at any time by the Company without Just Cause (as defined in the Employment Agreement) or by Mr. Cunningham for Good Reason (as defined in the Employment Agreement) then in addition to paying the Accrued Obligations; the Company shall continue to pay the Executive his then-current base salary and continue to provide benefits to the Executive at least equal to those which he had at the time of termination for a period of nine months after termination. The right to receive any option which has not yet vested or been awarded shall terminate upon the termination of Executive’s employment for any reason. The period(s) to exercise the option following termination of employment, shall be according to the Corporation’s existing stock option plan and customary form of employee stock option agreement.
Douglas Krafte
On June 19, 2017, we entered into a four-year employment agreement with Douglas Krafte, Ph.D. (the “Krafte Employment Agreement”), pursuant to which Dr. Krafte is entitled to an annual base salary of $285,000 and will be eligible for an annual discretionary performance bonus payments of up to 35% of his base salary payable in cash, which bonus, if any, will be awarded in the sole and absolute discretion of our board of directors and the compensation committee of the board of directors. Dr. Krafte will continue to be engaged as our Chief Scientific Officer.
The Krafte Employment Agreement also includes confidentiality obligations, inventions assignments by Dr. Krafte and non-compete and non-solicitation provisions.
If Dr. Krafte’s employment is terminated for any reason, he or his estate as the case may be, will be entitled to receive the accrued base salary, vacation pay, expense reimbursement and any other entitlements accrued by him to the extent not previously paid (the “Krafte Accrued Obligations”); provided, however , that if his employment is terminated at any time after July 1, 2017 by us without Just Cause (as defined in the Krafte Employment Agreement) or by Dr. Krafte for Good Reason (as defined in the Krafte Employment Agreement) then in addition to paying the Krafte Accrued Obligations; we will be obligated to continue to pay Dr. Krafte his then-current base salary and continue to provide benefits to Dr. Krafte at least equal to those which he had at the time of termination for a period of nine months after termination.
44
Compensation of Directors
The table below summarizes all compensation for the year ended December 31, 2017 of our directors who are also not named executive officers:
| Name | Fees
earned or paid in cash ($) |
Stock
awards ($) |
Option
awards ($) |
Non-Equity
Plan Compensation ($) |
Non-Qualified Deferred Compensation Earnings ($) |
All
Other Compensation ($) |
Total ($) |
|||||||||||||||||||||
| Timothy Tyson (1)(3)(4) | 120,000 | 26,930 | - | - | - | 146,930 | ||||||||||||||||||||||
| Clive Kabatznik (2)(3)(4) | 25,000 | 26,930 | - | - | - | 51,930 | ||||||||||||||||||||||
| Vincent Palmieri (2)(3)(4) | 25,000 | 26,930 | - | - | - | 51,930 | ||||||||||||||||||||||
| Edward Roffman (2)(3)(4) | 25,000 | 26,930 | - | - | - | 51,930 | ||||||||||||||||||||||
| Michael Taglich (2)(3)(4) | 25,000 | 26,930 | - | - | - | 51,930 | ||||||||||||||||||||||
| (1) | Mr. Tyson earned $120,000 for his services as a non-executive chairman of the Company. |
| (2) | Messrs. Kabatznik, Palmieri, Roffman and Taglich were each compensated for their services as Board directors at a rate of $25,000 per annum. |
| (3) | Each of the directors of the Company were awarded 10,000 stock options on March 15, 2017, valued at $26,930 each using a Black-Scholes option valuation model utilizing the assumptions as disclosed under footnote 20 to the annual financial statements. |
| (4) | As of December 31, 2017, the following are the aggregate number of option and stock awards held by each of our directors who were not also named Executive Officers: |
| Option | Stock | |||||||
| awards | awards | |||||||
| Name | (Amount) | (Amount) | ||||||
| Timothy Tyson(a) | 98,500 | - | ||||||
| Clive Kabatznik(b) | 60,000 | - | ||||||
| Vincent Palmieri(c) | 32,500 | - | ||||||
| Edward Roffman(d) | 47,500 | 19,000 | ||||||
| Michael Taglich(e) | 32,500 | - | ||||||
| (a) | The options outstanding includes; (i) on October 1, 2013, Mr. Tyson was awarded options exercisable for 10,000 shares of common stock of which all are vested; (ii) on April 1, 2014, Mr. Tyson was awarded options exercisable for 66,000 shares of common stock, of which all are vested; (iii) on May 19, 2016 Mr. Tyson was awarded options exercisable for 12,500 shares of common stock of which 6,713 are vested as of December 31, 2017; and (iv) on March 15, 2017, Mr. Tyson was awarded options exercisable over 10,000 shares of common stock of which 2,500 are vested at December 31, 2017. |
| (b) | The options outstanding includes; (i) on March 14, 2013, Mr. Kabatznik was awarded options exercisable for 37,500 shares of common stock of which all are vested; (ii) on May 19, 2016, Mr. Kabatznik was awarded options exercisable for 12,500 shares of common stock, of which 6,713 are vested as of December 31, 2017; and (iii) on March 15, 2017, Mr. Kabatznik was awarded options exercisable over 10,000 shares of common stock of which 2,500 are vested at December 31, 2017. |
45
| (d) | The options outstanding includes; (i) on April 1, 2014, Mr. Palmieri was awarded options exercisable for 10,000 shares of common stock of which all are vested; (ii) on May 19, 2016, Mr. Palmieri was awarded options for 12,500 shares of common stock of which 6,713 are vested as of December 31, 2017; and on March 15, 2017, Mr. Palmieri was awarded options exercisable over 10,000 shares of common stock of which 2,500 are vested at December 31, 2017. |
| (e) | The restricted stock and options outstanding includes; (i) on June 16, 2015, Mr. Roffman was granted 19,000 shares of common stock for his services as Audit Committee Chair which are fully vested; (ii) On May 1, 2012, Mr. Roffman was awarded options exercisable for 15,000 shares of common stock of which all are vested; (iii) on April 1, 2014, Mr. Roffman was awarded options exercisable for 10,000 shares of common stock of which all are vested as of December 31, 2017; (iv) on May 19, 2016, Mr. Roffman was awarded options exercisable for 12,500 shares of common stock of which 6,713 are vested as of December 31, 2017; and (v) on March 15, 2017, Mr. Roffman was awarded options exercisable over 10,000 shares of common stock of which 2,500 are vested at December 31, 2017. |
| (f) | The options outstanding includes: (i) on April 1, 2014, Mr. Taglich was awarded options exercisable for 10,000 shares of common stock of which all are vested; (ii) on May 19, 2016, Mr. Taglich was awarded options exercisable for 12,500 shares of common stock of which 6,713 are vested as of December 31, 2017; on March 15, 2017, Mr. Taglich was awarded options exercisable over 10,000 shares of common stock of which 2,500 are vested at December 31, 2017. |
Each director is reimbursed for travel and other out-of-pocket expenses incurred in attending Board of Director and committee meetings.
Each non-employee director receives cash compensation for services provided as a director of $25,000 per annum, other than Tim Tyson, our Chairman of the Board who receives cash compensation of $120,000. In March 2017 each director was issued an option to purchase 10,000 shares of common stock at an exercise price of $3.50 per share vesting pro-rata on a monthly basis over three years.
Equity Compensation Plan Information
The following table sets forth information about the securities authorized for issuance under our equity compensation plans for the fiscal year ended December 31, 2017.
| Number of securities to be issued upon exercise of outstanding options | Weighted average exercise price of outstanding options | Number of securities remaining for future issuance under equity compensation plans | ||||||||||
| Equity Compensation plans approved by the stockholders | ||||||||||||
| 2005 Stock incentive plan | 730,791 | $ | 3.67 | - | ||||||||
| 2015 stock incentive plan | 689,168 | $ | 3.50 | 910,832 | ||||||||
| 1,419,959 | $ | 3.59 | 910,832 | |||||||||
In December 2015, the Board of Directors of Icagen adopted, ratified and approved the proposal to authorize the Plan and stockholders of Icagen holding in excess of a majority of the voting power on the record date approved the Plan.
46
Item 12. Security Ownership of Executive Officers, Directors and Five Percent Shareholders
The following table sets forth information, as of April 13, 2018, or as otherwise set forth below, with respect to the beneficial ownership of our common stock and shares of Series C Preferred Stock: (i) all persons known to us to be the beneficial owners of more than 5% of the outstanding shares of our common stock and shares of Series C Preferred Stock; (ii) each of our directors and our executive officers named in the Summary Compensation Table; and (iii) all of our directors and our executive officer as a group. The address of each beneficial owner is c/o Icagen, Inc., 4222 Emperor Boulevard, Suite 350, Durham, North Carolina 27703.
| Amount and | Amount
of and Nature |
Percent | ||||||||||||||||||
| Nature of | of Series C | of | Percent | |||||||||||||||||
| Beneficial | Percentage of | Preferred | Series C | of | ||||||||||||||||
| Ownership, | Common Stock | Stock | Preferred | Total | ||||||||||||||||
| Including | Beneficially | Beneficially | Stock | Voting | ||||||||||||||||
| Name of beneficial owner | Common Stock | Owned (1) | Owned(2) | Owned | Power(3) | |||||||||||||||
| Richard Cunningham | 210,118 | (4) | 3.2 | % | - | - | * | |||||||||||||
| Douglas Krafte | 72,917 | (5) | 1.1 | % | - | - | * | |||||||||||||
| Mark Korb | 37,500 | (6) | 1.0 | % | - | - | * | |||||||||||||
| Timothy Tyson | 955,121 | (7) | 13.3 | % | 571,428 | 100 | % | 23.2 | % | |||||||||||
| Clive Kabatznik | 122,337 | (8) | 1.9 | % | - | - | * | |||||||||||||
| Vincent Palmieri | 249,563 | (9) | 3.8 | % | - | - | 1.0 | % | ||||||||||||
| Edward Roffman | 70,087 | (10) | 1.1 | % | - | - | * | |||||||||||||
| Michael Taglich | 868,596 | (11) | 12.8 | % | - | - | 5.6 | % | ||||||||||||
| Benjamin Warner | 1,589,885 | (12) | 24.5 | % | - | - | 18.5 | % | ||||||||||||
| Joseph Abrams | 398,872 | (13) | 6.2 | % | - | - | 3.9 | % | ||||||||||||
| Robert Taglich | 645,996 | (14) | 9.6 | % | - | - | 3.9 | % | ||||||||||||
| All officers and directors as a group (8 persons) | 2,586,240 | 31.6 | % | 571,428 | 100 | % | 31.0 | % | ||||||||||||
| * | Less than 1% |
| (1) | Based on 6,393,107 shares of common stock outstanding as of April 13, 2018 and 571,428 shares of Series C Preferred Stock outstanding. Each share of Series C Preferred Stock converts into one share of common stock but has the right to three votes per share and will vote together with the Common Stock. |
| (2) | Based on 571,428 shares of Series C Preferred Stock outstanding as of April 13, 2018. |
| (3) | Based on voting rights attached to each share. Each share of Series C Preferred Stock exercises three votes per share of common stock that it converts into. On April 13, 2018, there were 6,393,107 shares of common stock outstanding representing 6,393,107 votes and 571,428 shares of Series C Preferred Stock outstanding representing 1,714,284 votes for Total Voting Power of 8,107,391. Percent of Total Voting Power for each beneficial owner is derived by dividing (i) the sum of the common stock votes (exclusive of options and warrants owned by such holder) and number of votes that the Series C Preferred Stock owned by such beneficial owner is entitled by (ii) the Total Voting Power. |
| (4) | The share ownership includes (i) 21,428 shares of common stock and warrants exercisable for 5,357 shares of common stock; (ii) Mr. Cunningham was awarded options exercisable for 250,000 shares of common stock on January 7, 2015, of which 50,000 shares vested on November 24, 2015, 150,000 vest monthly during months 13 through 48 of the term of his employment agreement, and 50,000 vests on the four-year anniversary of his employment agreement, of which 173,333 are vested and a further 8,333 will vest in the next 60 days. In addition, on March 15, 2017, Mr. Cunningham was awarded options exercisable for 20,000 shares of common stock, of which 6,667 are vested and a further 1,667 vest in the next 60 days. |
| (5) | On May 19, 2016, Dr. Krafte was awarded options exercisable for 100,000 shares of common stock, of which 68,750 are vested and a further 4,167 will vest in the next 60 days. |
| (6) | On May 15, 2013, Mr. Korb was awarded options exercisable for 37,500 shares of common stock, all of which are vested. |
| (7) | The share ownership includes: (i) 164,284 shares of common stock and warrants exercisable for 131,071 shares of common stock; (ii) 571,428 shares of Series C Preferred Stock initially convertible into 571,284 shares of common stock and entitled to 1,714,284 votes and warrants issued with the Series C Preferred Stock exercisable for 571,418 shares of common stock owned by a trust of which Mr. Tyson is the trustee; however, the warrant contains a provision limiting its exercise to such number of shares of common stock that would constitute 9.99% of our total number of shares of common stock outstanding when aggregate with other shares of common stock owned by Mr. Tyson and his affiliated entities; (iii) on October 1, 2013, Mr. Tyson was awarded options exercisable for 10,000 shares of common stock, all of which are vested; (iv) on April 1, 2014, Mr. Tyson was awarded options exercisable for 66,000 shares of common stock, all of which are vested; (v) on May 19, 2016, Mr. Tyson was awarded options exercisable for 12,500 shares of common stock, of which 7,755 are vested and a further 694 will vest in the next 60 days; and (vi) on March 15, 2017, Mr. Tyson was awarded options exercisable for 10,000 shares of common stock, of which 3,333 are vested and a further 556 vest in the next 60 days. |
| (8) | The share ownership includes: (i) 50,000 shares of common stock owned by First South Africa Management and warrants exercisable for 22,500 shares of common stock; (ii) on March 14, 2013, Mr. Kabatznik was awarded options exercisable for 37,500 shares of common stock, all of which are vested; (iii) on May 19, 2016, Mr. Kabatznik was awarded options exercisable for 12,500 shares of common stock, of which 7,755 are vested and a further 694 will vest in the next 60 days; (iv) on March 15, 2017, Mr. Kabatznik was awarded options exercisable for 10,000 shares of common stock, of which 3,333 are vested and a further 556 vest in the next 60 days. Mr. Kabatznik has the sole voting and dispositive power with respect to the securities held by First South Africa Management. |
| (9) | The share ownership includes: (i) 74,736 shares of common stock and warrants exercisable for 152,490 shares of common stock; (ii) Mr. Palmieri was awarded options exercisable for 10,000 shares of common stock, all of which are vested; (iii) on May 19, 2016, Mr. Palmieri was awarded options for 12,500 shares of common stock, of which 7,755 are vested and a further 694 will vest in the next 60 days; and (iv) on March 15, 2017, Mr. Palmieri was awarded options exercisable for 10,000 shares of common stock, of which 3,333 are vested and a further 556 vest in the next 60 days. |
47
| (10) | The share ownership includes: (i) 29,000 shares of common stock and warrants exercisable for 3,750 shares of common stock; (ii) on May 1, 2012, Mr. Roffman was awarded options exercisable for 15,000 shares of common stock, all of which are vested; (iii) on April 1, 2014, Mr. Roffman was awarded options exercisable for 10,000 shares of common stock of which all are vested; (iv) on May 19, 2016, Mr. Roffman was awarded options exercisable for 12,500 shares of common stock of which 7,755 are vested and a further 694 will vest in the next 60 days; and (v) on March 15, 2017 Mr. Roffman was awarded options exercisable for 10,000 shares of Common Stock, of which 3,333 are vested and a further 556 vest in the next 60 days. |
| (11) | The share ownership includes: (i) 453,314 shares of common stock and 392,945 warrants to purchase shares of common stock, which includes; (a) 285,714 shares of common stock and warrants to purchase 71,429 shares of common stock, held by Mr. Taglich’s Keogh account; (b) 65,084 shares of common stock and warrants to purchase 33,572 shares of common stock, held in the TAG/Kent Partnership, an entity controlled by Mr. Taglich; (c) 62,726 shares of common stock; (d) 16,933 shares of common stock and warrants to purchase 10,000 shares of common stock, that Mr. Taglich holds jointly with Claudia Taglich; and (e) 22,856 shares of common stock and warrants to purchase 5,716 shares of common stock, held by four custodial accounts for Mr. Taglich’s minor children; (ii) on April 1, 2014, Mr. Taglich was awarded options exercisable for 10,000 shares of common stock, all of which are vested; (iii) on May 19, 2016, Mr. Taglich was awarded options exercisable for 12,500 shares of common stock of which 7,755 are vested and a further 694 will vest in the next 60 days; (iv) on March 15, 2017, Mr. Taglich was awarded options exercisable for 10,000 shares of common stock, of which 556 vest in the next 60 days; and (v) [28,571] shares of Series C Preferred Stock and warrants issued with the Series C Preferred Stock exercisable for [28,571] shares of common stock. |
| (12) | The share ownership includes: (i) 1,497,385 shares of common stock, including 54,135 shares of common stock held jointly by Dr. Warner and his wife, Ellen McBee; and (ii) on March 15, 2013, Dr. Warner was awarded options exercisable for 92,500 shares of common stock, all of which are vested. |
| (13) | The share ownership includes: (i) 316,372 shares of common stock owned by the Joseph W & Patricia G Abrams Family Trust; and (ii) 30,000 warrants awarded to Mr. Abrams to purchase shares of common stock for bridge note funding; and (iii) on March 14, 2013, Mr. Abrams was awarded options exercisable for 52,500 shares of common stock, all of which are vested. Mr. Abrams has sole dispositive power of the Joseph W & Patricia G Abrams Family Trust. |
| (14) | The share ownership includes: 315,609 shares of common stock and warrants to purchase 330,387 shares of common stock, which includes (a) 285,714 shares of common stock and warrants exercisable for 71,429 shares of common stock held by Mr. Taglich’s IRA account, and (b) 50,696 shares of common stock and warrants exercisable for 10,000 shares of Common Stock. |
Item 13. Certain Relationships and Related Transactions, and Director
Independence Related-Party Transactions
Although not in a written policy, our Audit Committee reviews on an on-going basis potential conflicts of interest, and approves if appropriate, all our “Related Party Transactions” defined as those transactions required to be disclosed pursuant to Item 404 of Regulation S-K.
The following is a summary of transactions since January 1, 2016 to which we have been a party.
Timothy Tyson
On May 19, 2016, we issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Tyson as compensation for services rendered. These options vest equally over a period of 36 months.
On July 6, 2016, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $100,000 to Mr. Tyson in consideration of $100,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on July 6, 2016, Mr. Tyson was issued five year Warrants to acquire 15,000 shares of common stock exercisable at $3.50 per share.
On March 15, 2017, we issued Mr. Tyson ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
On April 13, 2017, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $500,000 to Mr. Tyson in consideration of $500,000. The Bridge Note matured 30 days from the date of issuance and was redeemed, together with accrued interest thereon during May 2017.
In connection with the Bridge Note, Mr. Tyson was issued five-year Bridge Warrants to acquire 75,000 shares of common stock exercisable at $3.50 per share. The Bridge Warrants are also exchangeable, at the option of the holder, for a like number of warrants issued to any lender in our next debt financing.
On April 4, 2018, we entered into a Securities Purchase Agreement with Mr. Tyson pursuant to which we issued to Mr. Tyson 20 Units of our preferred stock in a private placement for total offering proceeds of $2,000,000. Each Unit is comprised of 28,571 shares of Series C Preferred Stock initially convertible into 28,571 shares of our common stock and a warrant to purchase 28,571 shares of the common stock at an exercise price of $3.50 per share.
Richard Cunningham
On March 15, 2017, we issued Mr. Cunningham ten-year options exercisable for 20,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
48
Benjamin Warner
On July 7, 2017, our employment agreement between Dr. Benjamin Warner, dated March 15, 2013, as amended (the “Employment Agreement”) was terminated. In addition, on July 7, 2017, Dr. Benjamin Warner resigned from our Board of Directors and from all other positions with us. In connection with his resignation, the Company executed certain release agreements (the “Release Agreements”) with Dr. Warner. Pursuant to the Release Agreements, Dr. Warner’s Employment Agreement was terminated by mutual agreement, Dr. Warner and we exchanged mutual releases and we agreed to continue the payments currently due to Dr. Warner under the Employment Agreement, through the end of its stated term notwithstanding its termination.
Clive Kabatznik
On May 19, 2016, we issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Kabatznik as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $50,000 to Mr. Kabatznik in consideration of $50,000 in cash. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Kabatznik was issued five year Warrants to acquire 7,500 shares of common stock exercisable at $3.50 per share.
On March 15, 2017, we issued Mr. Kabatznik ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
Edward Roffman
On June 16, 2015, pursuant to the terms of a restricted stock award, 19,000 restricted shares were issued to the Chairman of our audit committee who is also a board director, as a once off compensation expense for his services as chairman of the audit committee. As of June 30, 2016, all of these shares were vested.
On May 19, 2016, we issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Roffman as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $25,000 to Mr. Roffman in consideration of $25,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Roffman was issued five year Warrants to acquire 3,750 shares of common stock exercisable at $3.50 per share.
On March 15, 2017, we issued Mr. Roffman ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
Vincent Palmieri
On May 19, 2016, we issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Palmieri as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $50,000 to Mr. Palmieri in consideration of $50,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Palmieri was issued five year Warrants to acquire 7,500 shares of common stock exercisable at $3.50 per share.
As an employee of Taglich Brothers Inc., Mr. Palmieri was issued Placement Agent warrants in connection with the note referred to above to purchase 6,000 shares of common stock.
As an employee of Taglich Brothers Inc., Mr. Palmieri was issued 2017 Placement Agent Warrants to purchase 6,000 shares of common stock.
On March 15, 2017, we issued Mr. Palmieri ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
As an employee of Taglich Brothers Inc., Mr. Palmieri was issued 2017 Placement Agent Warrants to purchase 6,000 shares of common stock.
49
First South Africa Management
We incurred an expense of $180,000 for services provided by First South Africa Management for provision of CFO services by Mr. Korb and $42,000 for bookkeeping services for the year ended December 31, 2016. As of December 31, 2016, we owed First South Africa Management $18,600.
We incurred an expense of $180,000 for services provided by First South Africa Management for provision of CFO services by Mr. Korb for the year ended December 31, 2017 and $89,600 for bookkeeping services for the year ended December 31, 2017. As of December 31, 2017, we owed First South Africa Management $23,500.
Michael Taglich
On May 19, 2016, we issued ten-year options exercisable for 12,500 shares of common stock at $3.50 per share to Mr. Taglich as compensation for services rendered. These options vest equally over a period of 36 months.
On June 30, 2016, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $250,000 to Mr. Taglich in consideration of $250,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Taglich was issued five year Warrants to acquire 37,500 shares of common stock exercisable at $3.50 per share.
We retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, the Company agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering ($1,145,000) and agreed to reimburse approximately $15,000 in respect of out of pocket expenses incurred by the placement agent in connection with the Offering. We also issued the placement agent the same warrant that the investors received exercisable for an aggregate amount of 28,625 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold) (the “Placement Agent Warrants”). Mr. Taglich was issued 2017 Placement Agent Warrants to purchase 7,820 shares of common stock.
On March 15, 2017, we issued Mr. Taglich ten-year options exercisable for 10,000 shares of common stock at an exercise price of $3.50 per share. These options vest equally over a 36-month period.
On April 12, 2017, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $500,000 to Mr. Taglich in consideration of $500,000. The Note matures 30 days from the date of issuance. In connection with the Note, Mr. Taglich was issued five year Warrants to acquire 75,000 shares of common stock exercisable at $3.50 per share. These warrants are also exchangeable, at the option of the holder, for a like number of warrants issued to any lender in the Company’s next debt financing.
We retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, we agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering (excluding $500,000 invested by our Chairman of the Board of Directors, Timothy Tyson) for a total commission of $60,000. We also issued the Placement Agent the same warrant that the investors received exercisable for an aggregate amount of 25,000 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold, excluding Notes sold to the Chairman) (the “2017 Placement Agent Warrants”). The Placement Agent has the right to exchange the Placement Agent Warrants for a like number of warrants to be issued to the lender in the Company’s next debt financing. As an employee and Principal of Taglich Brothers Inc. Mr. Taglich was issued 2017 Placement Agent Warrants to purchase 7,500 shares of common stock.
Douglas Krafte
On February 25, 2016, upon the resignation of Dr. Benjamin Warner, as our Chief Scientific Officer, Douglas Krafte, age 56, the Chief Scientific Officer of our subsidiary Icagen Corp., was appointed to the position of Chief Scientific Officer for Icagen Inc. and its subsidiaries.
On May 19, 2016, we issued ten-year options exercisable for 100,000 shares of common stock at $3.50 per common shares to Mr. Krafte as compensation for services rendered. These options vested as to 25,000 options on June 30, 2016 and the remaining 75,000 equally over a period of 36 months, commencing on July 1, 2016.
On June 19, 2017, we entered into a four-year employment agreement with Douglas Krafte, Ph.D., pursuant to which Dr. Krafte is entitled to an annual base salary of $285,000 and will be eligible for annual discretionary performance bonus payments of up to 35% of his base salary payable in cash, which bonus, if any, will be awarded in the sole and absolute discretion of our board of directors and the compensation committee of the board of directors. Dr. Krafte continues to be engaged as our Chief Scientific Officer.
Robert Taglich
On June 30, 2016, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $200,000 to Mr. Taglich in consideration of $200,000. The Note matures on June 30, 2017. The Note, together with interest thereon was repaid on August 8, 2016.
In connection with the Note above, on June 30, 2016, Mr. Taglich was issued five year Warrants to acquire 30,000 shares of common stock exercisable at $3.50 per share.
50
We retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, the Company agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering ($1,145,000) and agreed to reimburse approximately $15,000 in respect of out of pocket expenses incurred by the placement agent in connection with the Offering. We also issued the placement agent the same warrant that the investors received exercisable for an aggregate amount of 28,625 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold) (the “Placement Agent Warrants”). Mr. Taglich was issued 2017 Placement Agent Warrants to purchase 6,405 shares of common stock.
On April 12, 2017, we entered into a Securities Purchase Agreement and issued a secured 8% Bridge Note for $500,000 to Mr. Taglich in consideration of $500,000. The Note matures 30 days from the date of issuance. In connection with the Note, Mr. Taglich was issued five year Warrants to acquire 75,000 shares of common stock exercisable at $3.50 per share. These warrants are also exchangeable, at the option of the holder, for a like number of warrants issued to any lender in the Company’s next debt financing.
We retained Taglich Brothers, Inc. as the exclusive placement agent for the Offering. In connection therewith, we agreed to pay the placement agent a six percent (6%) commission from the gross proceeds of the Offering (excluding $500,000 invested by our Chairman of the Board of Directors, Timothy Tyson) for a total commission of $60,000. We also issued the Placement Agent the same warrant that the investors received exercisable for an aggregate amount of 25,000 shares of common stock at an exercise price of $3.50 per share (2,500 shares of common stock for each $100,000 in principal amount of Notes sold, excluding Notes sold to the Chairman) (the “2017 Placement Agent Warrants”). The Placement Agent has the right to exchange the Placement Agent Warrants for a like number of warrants to be issued to the lender in the Company’s next debt financing. As an employee and Principal of Taglich Brothers Inc. Mr. Taglich was issued 2017 Placement Agent Warrants to purchase 7,500 shares of common stock.
Item 14. Principal Accountant Fees and Services
RBSM LLP serves as our independent registered public accounting firm.
Independent Registered Public Accounting Firm Fees and Services
The following table sets forth the aggregate fees including expenses billed to us for the years ended December 31, 2017 and 2016 by our auditors:
| Year ended December 31, 2017 | Year ended December 31, 2016 | |||||||
| Audit fees and expenses | $ | 72,500 | $ | 45,000 | ||||
| Taxation preparation fees | 12,000 | 10,000 | ||||||
| Audit related fees | - | 2,500 | ||||||
| Other fees | - | - | ||||||
| $ | 84,500 | $ | 57,500 | |||||
| (1) | Audit fees and expenses were for professional services rendered for the audit and reviews of the consolidated financial statements of the Company, professional services rendered for issuance of consents and assistance with review of documents filed with the SEC. |
| (2) | Taxation preparation fees were fees for professional services rendered to file our federal and state tax returns. |
| (3) | We incurred fees to our independent auditors of $0 and $2,500 for audit related fees during the fiscal years ended December 31, 2016. |
| (4) | We incurred fees to our independent auditors of $0 for other fees during the fiscal years ended December 31, 2017 and 2016. |
Audit Committee’s Pre-Approval Practice.
Prior to our engagement of our independent auditor, such engagement was approved by our board of directors. The services provided under this engagement may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to one year and any pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget. Pursuant our requirements, the independent auditors and management are required to report to our board of directors at least quarterly regarding the extent of services provided by the independent auditors in accordance with this pre-approval, and the fees for the services performed to date. Our board of directors may also pre-approve particular services on a case-by-case basis. All audit-related fees, tax fees and other fees incurred by us for the year ended December 31, 2017, were approved by our board of directors.
51
Item 15. Exhibits and Financial Statement Schedules and Reports on Form 10-K
(a)(1) The following financial statements are included in this Annual Report on Form 10-K for the fiscal year ended December 31, 2016.
| 1. | Independent Auditor’s Report |
| 2. | Consolidated Balance Sheets as of December 31, 2017 and 2016 |
| 3. | Consolidated Statements of Operations for the years ended December 31, 2017 and 2016 |
| 4. | Consolidated Statements of changes in Stockholders’ (Deficit) Equity for the years ended December 31, 2017 and 2016 |
| 5. | Consolidated Statements of Cash Flows for the years ended December 31, 2017 and 2016 |
| 6. | Notes to Consolidated Financial Statements |
| (a)(2) | All financial statement schedules have been omitted as the required information is either inapplicable or included in the Consolidated Financial Statements or related notes. |
| (a)(3) | The following exhibits are either filed as part of this report or are incorporated herein by reference: |
52
53
| (1) | Filed herewith |
| (2) | Certain exhibits and schedules, to this agreement have been omitted in accordance with Item 601(b)(2) of Regulation S-K. A copy of any omitted exhibit and/or schedule will be furnished supplementally to the SEC upon request. |
| * | Management contract or compensatory plan or arrangement required to be identified pursuant to Item 15(a) (3) of this report. |
This item is not applicable.
54
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned.
ICAGEN, INC.
| Signature | Title | Date | ||
| /s/ Richard Cunningham | Chief Executive Officer and President | April 17, 2018 | ||
| Richard Cunningham | (Principal Executive Officer) | |||
| /s/ Mark Korb | Chief Financial Officer | April 17, 2018 | ||
| Mark Korb | (Principal Financial and Accounting Officer) |
Pursuant to the requirements of the Securities Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Date: April 17, 2018 | By: | /s/ Richard Cunningham |
| Chief Executive Officer and President | ||
| Date: April 17, 2018 | By: | /s/ Timothy Tyson |
| Timothy Tyson | ||
| Non-Executive Chairman | ||
| Date: April 17, 2018 | By: | /s/ Vincent Palmieri |
| Vincent Palmieri | ||
| Director | ||
| Date: April 17, 2018 | By: | /s/ Michael Taglich |
| Michael Taglich | ||
| Director | ||
| Date: April 17, 2018 | By: | /s/ Edward Roffman |
| Edward Roffman | ||
| Director | ||
| Date: April 17, 2018 | By: | /s/ Clive Kabatznik |
| Clive Kabatznik | ||
| Director |
55
