Attached files
| file | filename |
|---|---|
| EX-32.2 - E-Qure Corp. | ex32-2.htm |
| EX-32.1 - E-Qure Corp. | ex32-1.htm |
| EX-31.2 - E-Qure Corp. | ex31-2.htm |
| EX-31.1 - E-Qure Corp. | ex31-1.htm |
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
Commission file number 0-54862
E-QURE CORP.
(Exact Name Of Registrant As Specified In Its Charter)
| Delaware | 47-1691054 | |
| (State of Incorporation) | (I.R.S. Employer Identification No.) | |
| 20 West 64th Street, Suite 39G, New York, NY | 10023 | |
| (Address of Principal Executive Offices) | (ZIP Code) |
Registrant’s
Telephone Number, Including Area Code: (972) 8 916-7333
Securities Registered Pursuant to Section 12(g) of The Act: Common Stock, $0.00001
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in the definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
On June 30, 2017, the aggregate market value of the 6,295,624 common stock held by non-affiliates of the Registrant was approximately $3,113,284 based $0.14 per share, the last trade of the Registrants common stock on June 30, 2017. On April 16, 2018, the Registrant had 22,237,562 shares of common stock outstanding.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer (as defined in Rule 12b-2 of the Exchange Act) or a smaller reporting company .
| Large accelerated filer [ ] | Accelerated filer [ ] | Non-Accelerated filer [ ] | Smaller reporting company [X] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).Yes [ ] No [X]
TABLE OF CONTENTS
| 2 |
Cautionary Statement regarding Forward-Looking Statements
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The Registrant has based these forward-looking statements on its current expectations and projections about future events. These forward-looking statements are subject to known and unknown risks, uncertainties and assumptions about the Registrant that may cause its actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “continue,” or the negative of such terms or other similar expressions. Factors that might cause or contribute to such a discrepancy include, but are not limited to, those described in this Annual Report on Form 10-K and in the Registrant’s other Securities and Exchange Commission filings.
ITEM 1. DESCRIPTION OF BUSINESS
Overview
The Company was incorporated under the name Creative Learning Products, Inc. in the State of New Jersey on August 31, 1988 and changed its name to Creative Gaming, Inc. in May 1997. The Company changed its name to Management Services, Inc. in October 2006. In August 2008, the Company changed its name to Centriforce Technology Corp. In May 2010, the Company changed its name to ADB International Group, Inc. The Company changed its name to its current name, E-Qure Corp., on August 27, 2014.
On May 12, 2014, the board of directors approved a plan to redomicile from the State of New Jersey to the State of Delaware. In conjunction with the change in domicile the board of directors also approve a 1:100 reverse split of our Common Stock. The redomicile and reverse split were approved by FINRA and became effective on August 4, 2014.
Our Business
In January 2014, Mr. Weissberg negotiated with Lifewave Ltd., a public company organized under the laws of the State of Israel, for the purpose of acquiring certain of Lifewave’s IP assets pertaining to a wound healing device. The Registrant signed a patent purchase agreement with Lifewave on January 6, 2014 (the “Agreement”), the closing of which was subject to several material conditions, including our ability of raising equity capital sufficient to develop and commercially exploit the technology.
On June 4, 2014, we completed the purchase of all right, title and interest to certain IP assets, including rights to a wound treatment device. The IP assets, including the wound healing device, acquired by the Registrant are designed for wound treatment incorporating Bioelectrical Signal Therapy (“BST Device”). The BST Device implements patented and proprietary electrical stimulation technologies to treat hard-to-cure wounds and ulcers up to complete closure and/or cure.
Pursuant to the Agreement, the Registrant has agreed to pay Lifewave a royalty of from 10% to 20% of the profits (as defined in the Agreement) generated from the BST Device.
In June 2014, the Registrant entered into an agreement with the Austen BioInnovation Institute in Akron (“ABIA” or the “Institute”), for the purpose of bringing our BST Device to the U.S. market.
The Company’s management selected ABIA’s Product Innovation and Commercialization Division, which has significant expertise in wound healing, clinical trial development, and regulatory operations, to spearhead its pre-market clinical trial program, which is necessary to apply for regulatory approval from the United States Food and Drug Administration (“FDA”) to distribute the BST Device in the United States. As part of the Institute’s fully integrated regulatory and device development service Offerings, ABIA will prepare on behalf of the Company an application to obtain FDA approval. The initial trial will include 70 patients in a double-arm, randomized, multi-center study to assess the safety and efficacy of the BST Device in patients with Stage II and III pressure and venous stasis ulcers; and submit data to the FDA to obtain approval.
On December 18, 2015, the Registrant confirmed certain information that it had received from ABIA that, while ABIA still anticipated that it would be able to provide the Registrant with a final draft of the IDE application, ABIA had sustained financial difficulties and key personnel losses that would likely adversely effect its ability to perform under the Agreement on a timely basis, if at all. As a result, the Registrant requested that ABIA fully refund the monies paid to ABIA under the Agreement. In addition, the Registrant agreed to engage a professional regulatory consultant, who was a former member of ABIA’s regulatory staff, to serve as the Registrant’s FDA regulatory consultant on an interim basis, subject to the execution of a separate services agreement. The Registrant is also evaluating the advisability of engaging another firm to replace ABIA, which process may be expected to delay the IDE approval process for the BST Device.
| 3 |
The Company’s success is dependent upon the successful FDA clinical trial of its BST Device. The Device may need additional development and may never achieve safety or efficacy. The Company believes that its design and procedure show promise, but the path to commercial success, even if development milestones are met, may take more time and might be more costly.
There are a number of potential obstacles the Company might face, including the following:
●
We may not be able to raise sufficient additional funds that we may need to complete the all requisite clinical trials.
● Competitors may develop alternatives that render BST Device redundant or unnecessary.
● We may not have a sufficient and/or sustainable intellectual property position.
● Our device may be shown to have harmful side effects/characteristics that indicate it is unlikely to be safe and effective.
● Our device may not receive FDA regulatory approval.
● Even if our device receives regulatory approval, it may not be accepted by patients, the medical community or third-party payers.
Effective October 15, 2014, through our wholly-owned Israeli subsidiary, ESQURE, we entered into an Asset Purchase Agreement with Michael Cohen. We purchased all of Mr. Cohan’s assets (the “Seller’s Assets”) related to our BST Device for 875,000 restricted shares of common stock valued at $350,000. The Seller’s Assets settled a subscription receivable under a previous subscription agreement for the same number of shares.
Pursuant to the terms of the Asset Purchase Agreement, we purchased all of Seller’s assets (the “Seller’s Assets”) related to our BST Device as follows:
(i) medical research data held by Seller’s research partners, distributors and marketing advisors in Israel and elsewhere;
(ii) manufacturing and design files related to the BST device, including mechanical and electronics schemes drawings, printed circuit boards graphics, BST electrodes including rechargeable electrodes; and related files, as well as files related to all BST electrodes;
(iii) testing equipment and devices for BST manufacturing;
(iv) PowerLab devices including equipment of Lifewave Ltd (the entity from whom Registrant acquired the BST Device and technology);
(v) BST devices and electrodes under the control of BST distributors, physicians, hospitals, clinics worldwide;
(vi) the Main Server of Lifewave which holds all manufacturing, marketing and other material related to the Registrant’s BST Device; and
(vii) Injection molds for the manufacture of the Registrant’s BST Device plastic parts.
On December 28, 2014, the Registrant entered into a preliminary distribution agreement with Rubifarm S.A., an entity organized under the laws of Argentina (“Rubifarm”), which agreement is subject to approval by the regulatory authorities of Argentina. At the date of regulatory approval, which is anticipated during the 4th quarter of 2015, a definitive agreement will be executed and filed with the SEC. The agreement contemplates that Rubifarm will be granted exclusive distribution rights for the BST Device™ in Argentina for an initial term of 5 years subject to Rubifarm meeting a minimum purchase quota of $1.5 million during the initial 5-year term in order to retain its exclusivity.
On July 30, 2015, the Company reported that it entered into an exclusive distribution agreement (the “Distribution Agreement”) with Chemipal Ltd, a closely-held Tel-Aviv Stock Exchange listed company organized under the laws of the State of Israel (“Chemipal”). Chemipal has been actively engaged in the distribution of medical products in Israel since 1941. Under the Distribution Agreement, the Registrant has granted Chemipal exclusive distribution rights to the Registrant’s medical device for the treatment of chronic wounds (the “BST Device™”) and the accompanying disposable electrodes (sometimes collectively, the “Products”) in Israel for an initial 5 year term, subject to Chemipal satisfying a minimum purchase quota of $3 million during the term. The Chemipal agreement further provides as follows:
| 4 |
(i) the Registrant will supply the Distributor with an initial inventory;
(ii) the Registrant will re-supply the Distributor with Products from time-to-time, based upon payment by the Distributor to the Registrant;
(iii) based upon demand for the Products, the parties will reevaluate and increase the size of the inventory as sales of the Products increase; and
(iv) the Distributor will use the BST Device™ for treatment of patients in hospitals, long-term care facilities, medical centers and clinics and other potential users.
On December 18, 2015, the Registrant confirmed certain information that it had received from ABIA stating that it had sustained financial difficulties and key personnel losses that would adversely a ffect its ability to perform under the Agreement on a timely basis, if at all. As a result, the Registrant requested that ABIA fully refund the monies paid to ABIA under the Agreement.
In May 2016, the Company commenced legal action against ABIA in the Supreme Court of the State of New York, New York County alleging the breach of contract against ABIA of the Clinical Trial Agreement dated June 5, 2014 (the “CTA”) based upon, among other reasons: (i) the failure of ABIA to commit sufficient personnel to the Company’s BST device project; (ii) misrepresenting the ability of its staff to perform its obligations under the CTA; (iii) failing to provide the FDA with adequate evidence to support the IDE applications and providing incorrect responses to the FDA; and (v) misappropriating the Company’s funds for use on other ABIA projects and expenses rather than in fulfillment of its contract obligations. The Lawsuit seeks approximately $475,000 in actual damages, representing the fees paid by the Company to ABIA, loss of profits in an amount not less than $3 million and reasonable attorneys’ fees and costs and expenses. During October 2016, the Company signed settlement agreement with ABIA in the amount of $300,000 and subsequently received $281,400.
The Company engaged IMARC Research Inc. to provide a broad range of services related to its BST Device and the FDA application process. On October 14, 2016, the Company received notification from FDA that it has granted conditional approval to the IDE application, authorizing us to commence a clinical investigation of our BST Device for wound healing. We are dependent upon the success of our FDA application for us to be able to market our BST Device in the U.S.
On July 18, 2016, the Company received the CE Certificate of Conformity and the ISO 13485 Certification. The CE Certification for our BST Wound Healing Device is a declaration that it complies with the requirements of the EU related to health, safety and environmental protections and acknowledges that the BST Device may be legally marketed in the EU. As a result, we are prepared to commence manufacturing and marketing for our BST Device in Europe as well as other non-European countries that accept the CE Certification. The ISO is the International Organization for Standardization, and represents that the company’s quality systems and procedures satisfies the requirements for a comprehensive quality management for the design and manufacture of medical devices.
On October 14, 2016, the Registrant received notification from FDA that it has granted conditional approval to the IDE application, authorizing us to commence a clinical investigation of our BST Device for wound healing. The main condition set forth is that the trial shall begin initially with 10 patients, after which we will file a safety report with the FDA before proceeding with the trial, which contemplates testing the BST Device with 90 patients altogether.
On January 8, 2017, the Registrant entered into a five-year distribution agreement (the “Distribution Agreement”) with TekMedica SAS, organized under the laws of Colombia (“TekMedica” or the “Distributor”). Pursuant to the Distribution Agreement, the Registrant granted TekMedica the exclusive rights to distribute the Registrant’s medical device for the treatment of chronic wounds (the “BST Device™”) and the accompanying disposable electrodes (sometimes collectively, the “Products”) in Colombia (the “Territory”). The Distribution Agreement provides that Registrant will provide Distributor with supplies of the BST Devicee and disposable electrode for treatment of patients in hospitals, long-term care facilities, medical centers and out-patient clinics. The Distributor will make an initial advance payment to be applied against the first year’s quota together with an initial order supported by a Letter of Credit with subsequent orders as part of the quota, as set forth in the Distribution Agreement, with minimum annual quota’s during the five-year term. The Distributor will be responsible for securing any product certification, permit, license or approval that may be required in the Territory for the marketing, sale, sublicensing and delivery and use of the BST Devise and Products in the Territory.
| 5 |
On February 20, 2017, the Registrant received the official certification from the Israeli Ministry of Health authorizing the use of the Registrant’s BST Device in Israel. The BST Device implements patented and proprietary electrical stimulation technologies to treat hard-to-cure wounds and ulcers down to complete closure and/or cure.
We are a start-up company and we may require additional capital to implement our business and fund our operations. See “Management’s Discussion and Analysis” on page 60.
The Company’s principal executive office and mailing address is 20 West 64th Street, Suite 39G, New York, NY 10023. Our telephone number is (972) 54 427777.
Electrical Stimulation To Accelerate Wound Healing
The human cell is, in essence, an electrical unit. Human cells are enveloped by a plasma membrane that operates on the electrochemical physiology principle (the principal of the electrical properties of biological cells and tissues) of direct current exchange of ions. Injury to the outermost layer or epithelial layer of the human skin disrupts the body’s naturally occurring electrical current therefore creating an electrical field. This electrical field guides the epithelial cell migration during wound healing. Electrical stimulation has shown enhanced movement of epithelial cells through the application of electrical fields. Movement of epithelial cells does not occur in a linear fashion; rather, the cells migrate approximately along the electrical field. Epithelial cells have the ability to change direction in response to electrical fields. If the polarity of the electrical field is changed, a reversal of movement of the epithelial cell can be observed. Epithelial cells cultured in the presence of an electrical field demonstrate an increase in the extent of cell movement. Under the impact of direct electrical current, endothelial cell orientation may be observed as early as 4 hours after the onset of an electrical field. Electrical stimulation is believed to restart or accelerate chronic wound healing by imitating the natural electrical current that occurs in injured skin and which is absent around chronic wounds.
Our BST Device
The BST Device, using electrodes affixed around the opposing sides of the wound, emits an electrical field around the wound and therefore mimics the electrochemical physiology principle and induces local cell regeneration. Wound treatment with the BST Device is non-invasive, painless and efficient. The procedure calls for electric stimulation to be performed 3 times a day for a duration of 30 minutes. The patient is connected to the BST Device only for the duration of the treatment. Our BST Device is positioned to treat severe stage II, as well as stage III and IV wounds including pressure ulcers, diabetic ulcers and venous ulcers.
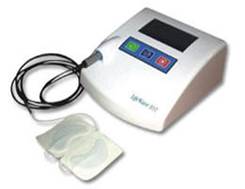
The BST Device is a standalone and easy-to-operate Device for adjunctive therapy in wound healing. The Device is able to automatically adjust the electric pulse amplitude based on a patient’s impedance reading. It has a built-in, pre-programmed timer to stop treatment after a 30 minute session. We believe that our BST Device represents a cost-effective wound healing method that can reduce hospitalization time and therefore reduce treatment costs.
| 6 |
The Global Wound Care Market
Based upon the knowledge and professional experience of members of our management in the wound care industry, we believe that the global market for wound care, which includes an array of surgical, chronic and acute wound care products reached around $24 billion in 2013 and we expect it to grow by approximately 7% annually.
The advanced wound care market, which includes drug treatment, negative pressure devices, enzymatic debridement, electrical treatment and tissue engineered skin substitutes, represents the segment of the overall wound care market, which we believe has the greatest growth potential.
Chronic Wounds - Our Target Market
Our BST Device is designed for chronic wound treatments in hospitals, nursing homes and home care.
Chronic wound care represents a critical issue for healthcare providers. Unlike an acute wound, a chronic wound is one that shows no signs of significant healing within a period of three months. We believe that chronic wounds constitute an expensive and debilitating healthcare problem, with significant clinical and social implications. Without effective treatment, a chronic wound can lead to a more severe medical condition, such as infection, gangrene or amputation, which further impedes the patient’s quality of life and even death.
Patients can suffer from chronic wounds for months, or even years, before healing is achieved, if at all. The treatment of a patient suffering from a chronic wound – including nursing time, wound care products, pressure-relieving devices, special beds, surgical procedures, and hospitalization in the event of more severe complications – can possibly surpass $100,000 - with no guarantee of healing. Treatment usually requires a joint effort conducted by an interdisciplinary team of nurses, doctors and surgeons.
There are four types of chronic wounds (also known as trophic or skin ulcers): (i) Pressure ulcers; (ii) Venous insufficiency ulcers (also known as venous stasis ulcers); (iii) Diabetic or Neuropathic ulcers; and (iv) Arterial insufficiency ulcers.
Chronic wounds are classified by four stages according to their degree of severity, with Stages III and IV being the two most severe, requiring, in most cases, hospitalization and special treatment.
Chronic wounds primarily occur in patients over the age of 60 and those with restricted mobility such as being confined to a wheelchair or bed. The wounds are often large and/or deep, prone to infection, and debilitating due to the extent of tissue damage incurred, resulting a compromised state of the patient’s health. Sepsis can result in extremely severe cases, which in turn, may lead to amputation and even death.
A range of traditional passive therapy and advanced wound therapy products are used to prevent further wound deterioration. Passive therapy products include synthetic dressings and gels that offer early-stage treatment, by enabling the wound to remain clean and moist so as to prevent infection. Advanced wound therapy includes specialty beds, mattress overlays, compression devices, negative pressure therapy and mattress replacements used to accelerate blood flow to the wound. All advanced wound therapy treatments are expensive and require professional assistance.
In recent years, an array of modern, interactive wound management products has been developed to provide a controlled local environment to stimulate the healing process. These sophisticated products, based on growth factors, tissue bioengineering, and hyperbaric oxygen technology, not only are very expensive, but also require a high level of patient compliance.
There is a large number of patients suffering from chronic wounds. We believe, based upon the knowledge and experience of our management in the wound care industry that current treatments available may not be sufficient to manage the various chronic wound conditions. As a result, we believe that our BST device offers an additional, cost-effective and curative treatment method.
| 7 |
Our Wound Healing Strategy
The objective of our BST Device is to reduce treatment time and therefore costs while delivering optimal therapeutic results, which eventually will lead to lower wound treatment costs.
The cost of an unhealed wound goes far beyond the costs of the physician, hospital and medical equipment, particularly when there are subsequent complications that require additional medical treatment.
The healing time of chronic wounds usually ranges from a few weeks to a few months depending on the size and type of the wound. Wound treatment typically can involve many direct and indirect costs. Wound dressings comprise only 10-15% of the total direct treatment cost according to the International Committee on Wound Management. In contrast, a significant percentage of the total cost is attributable to care providers’ salaries and staff expenses. As a result, treatment methods that reduce healing time to closure of the wound inevitably lead to lower cost of care.
The majority of severe chronic wounds are treated in hospitals. Treatment options often have limited efficacy and typically are very expensive. In addition, they are mostly only partially reimbursed by most insurance, primarily due to their poor clinical viability. As a result, hospitals lose revenues due to long and possibly unsuccessful treatment cycles.
We believe that our BST Device is a very effective and cost-saving method for the chronic wound treatment market. Our belief is based on the knowledge and experience of our management in the wound care industry that our BST Device is designed to be able to significantly lower treatment cost compared to other wound therapies due to the fact that our wound heal method requires shorter healing times and therefore lower per day treatment costs.
Marketing and Sales
The Company intends to launch marketing efforts in the US upon FDA approval has been granted, of which there can be no assurance. Until such time, most of our efforts will be related to internally preparing for the launch of our BST Device in the US and launching a few European territories.
Assuming FDA approval, which we believe will be achieved, in part because of approval that has been received in Europe, the Company plans to indirectly sell its wound treatment solution by entering into distribution agreements with distributors specializing in medical devices complementary to BST Device or by creating its own sales and marketing force. The distributors or the company will then sell the treatment to hospitals, nursing homes, geriatric institutions, and private clinics.
In addition to indirect sales through distribution agreements, the Company considers the outright sale of its treatment to each institution such as hospitals, nursing homes, geriatric institutions, and private clinics, which would rent the Device to their patients on a monthly basis. Upon completion of the wound healing treatment, the patient would return the Device, which would then re-enter the medical device rental market.
Competition
Our principal competition in the chronic wound care market are expected to be the wound care products manufactured by Kinetic Concepts, Inc. (“KCI”) and Smith & Nephew. KCI is considered market leader and, we believe, commands a market share of approximately 21% in the United States. Smith & Nephew’s U.S. market share is believed to be approximately 19% based on revenues. Other significant competitors are ConvaTec, Johnson & Johnson and 3M, al manufacturers of wound healing devices.
In addition to the above-mentioned device manufacturers, we expect to face competition from specialty clinics that have been established by hospitals or physicians. Additionally, there are a number of private companies that provide wound care services. We may also face competition from general health care facilities and service providers, biopharmaceutical companies and pharmaceutical companies.
| 8 |
We also face indirect competition from numerous companies that offer a variety of wound healing methods including traditional wound care dressings, advanced wound care dressings, such as hydrogels, hydrocolloids, and alginates, skin substitutes, and products containing growth factors. While many of these methods compete with our BST Device, such methods can also be utilized as complementary therapies to our BST Device and vis versa.
We believe that the following treatments or therapies represent alternatives or complementary treatments and/or therapies to our BST device:
Hyperbaric oxygen therapy (“HBOT”):Hyperbaric oxygen therapy is a medical treatment that utilizes pressurized oxygen to heal wounds. The treatment is administered by placing a patient in a comfortable pressure chamber that circulates oxygen at two to three times the atmospheric pressure rate. The HBOT method is not specifically designed for chronic wounds and the treatment process is both very long and very expensive. HBOT treatments typically last 90-120 minutes and are administered 1-2 times a daily for a period of 5 to 6 days a week. The length of treatment depends on the wound’s severity, with some patients requiring 20-40 treatments. There are many companies that offer HBOT treatment in clinics as a direct service to patients.
Vacuum-assisted closure (“V.A.C.”) method: The V.A.C. method is designed for the treatment of acute care setting, serious trauma wounds, failed surgical closures, amputations, and serious pressure ulcers. The leading V.A.C. product was launched in 1995 by KCI, a major global medical technology company. We believe that the V.A.C. method is very expensive, painful and inconvenient, since the patient needs to be connected to the device 22 hours a day.
EZCARE (Smith & Nephew) - Following the acquisition of BlueSky Medical in May 2007, Smith & Nephew provide negative pressure wound therapy (NPWT). NPWT is a technology used to treat chronic wounds such as diabetic ulcers, pressure ulcers, as well as post-operative and hard-to-heal wounds. It aids in the healing of open wounds by the application of sub-atmospheric pressure (Similar technology to KCI).
In addition, recently developed technologies, or technologies that may be developed in the future, are or may be the basis for products which compete with our BST Device. There can be given no assurance that we will be able to successfully enter into the chronic wound heal market with our BST Device or that we will be able to compete effectively against such companies in the future.
Many of these companies have substantially greater capital and marketing resources, and greater experience in commercializing products and services than we have.
Patents, Trademarks, and Copyrights
We have a list of all pending and granted patents to date attached as exhibit 10.9 to our S-1 Registration Statement as filed with the Company’s S-1 on September 6, 2014. To the extent that we determine that additional intellectual property (“IP”) protection may be necessary, we plan to secure such protection by applying for additional patents, trademarks or copyrights related to our business and IP, as we deem necessary.
Government Regulation
Our operations and the marketing of our BST Device is subject to extensive regulation by numerous federal and state governmental authorities in the United States, foremost of which is the FDA. There can be no assurance that the FDA, other governmental agencies or a third party will not contend that certain aspects of our business and our BST Device is either subject to or is not in compliance with applicable laws, regulations or rules or that the state or federal regulatory agencies or courts would interpret such laws, regulations and rules in our favor. The sanctions for failure to comply with such laws, regulations or rules could include denial of the right to conduct business, significant fines and criminal and civil penalties. Additionally, any increase in the complexity or substantive requirements of such laws, regulations or rules could have a material adverse effect on our business.
| 9 |
Any change in current regulatory requirements or related interpretations by or positions of, state officials where we operate could adversely affect our operations within those states. State regulatory requirements could adversely affect our ability to establish operations in such other states.
Various state and federal laws apply to the operations of medical device providers including, but are not limited to, the following:
Licensure: Certain medical device providers are required to be licensed by various state regulatory bodies. However, if we are found to not be in compliance, we could be subject to fines and penalties or ordered to cease operations which could have an adverse effect on our business.
False Claims Act: The Federal False Claims Act and some state laws impose requirements in connection with the submission of claims for payment for health care services and products, including prohibiting the knowing submission of false or fraudulent claims and submission of false records or statements for reimbursement and payment to the United States government or state government. Such requirements would apply to the hospitals to which we provide our Device related to wound care treatment services. Not only are government agencies active in investigating and enforcing actions with respect to applicable health laws, but also health care providers are often subject to actions brought by individuals on behalf of the government. As such, “whistleblower” lawsuits, also known as “qui tam” actions, are generally filed under seal with a court to allow the government adequate time to investigate and determine whether it will intervene in the action. As a result, health care providers subject to qui tam actions are often unaware of the lawsuit until the government has made its determination whether to intervene, or not, at which time the seal is lifted. The Federal False Claims Act provides for penalties equal to three (3) times the actual amount of any overpayments plus $11,000 per claim. Under legislation passed in 2009, those who bill third parties are now obligated to discover and disclose any overpayments received or be subject to False Claims Act penalties as well
Fraud and Abuse Laws: Since a significant portion of reimbursement for healthcare products and services are currently paid through reimbursements under Medicare, Medicaid or similar programs, the federal government and many states have adopted statutes and regulations that address fraudulent and/or abusive behavior in connection with such programs.
As part of this regulatory scheme, the federal government believes that an “inducement” to refer a Medicare or Medicaid patient is likely to result in fraud or abuse on the Medicare or Medicaid programs. Therefore, the federal government adopted a number of laws and regulations to recoup funds and assess penalties which it believes were paid inappropriately. In cases of criminal fraud, the individuals responsible for the fraudulent activity can be subject to imprisonment.
One of the principal federal statutes regulating fraud and abuse is the Anti-Kickback Statute. The Anti-Kickback statute prohibits the solicitation, payment, receipt or offering of any direct or indirect remuneration in exchange for the referral of Medicare and Medicaid patients or for the purchasing, arranging for or recommending the purchasing, leasing or ordering of Medicare or Medicaid covered services, items or equipment. To be convicted of a violation of the Anti-Kickback Statute, the party must have had specific intent to induce the referral of Medicare or Medicaid patients or the purchase, lease or ordering of a good, item or service reimbursable by Medicare or Medicaid. Some of the federal courts have broadly construed the Anti-Kickback Statute and held that the “intent” required to support a criminal conviction will exist if only one purpose of the referral is to induce a prohibited referral.
| 10 |
To clarify some of the issues created by the Anti-Kickback Statute, the Center for Medicare and Medicaid Services issued “safe harbor” regulations identifying actions which will not be deemed to violate the Anti-Kickback Statute. Some of these “safe harbors” are in the area of joint ventures, personal services, and other arrangements. Conducting an activity that falls within a “safe harbor” regulation provides comfort that such activity will not be prosecuted. Compliance with each element of a particular “safe harbor” is required in order to assured of the protection provided by such “safe harbor.”Even though a transaction that does not fall within a “safe harbor” may be perfectly appropriate, the arrangement will be evaluated based on its facts and circumstances to determine if the parties intended to induce the referral of Medicare or Medicaid patients or the purchase, lease or ordering of a good, item or service reimbursable under Medicare or Medicaid.
An allegation of violation and/or a conviction for violation of the Anti-Kickback Statute and parallel state laws could have a significant impact on our ability to conduct our business. As noted earlier, significant fines, penalties, exclusion from Medicare and Medicaid programs and imprisonment of individuals can result. Because the burden to prove specific intent under the Anti-Kickback Statute can sometimes be difficult, the government has been pursuing enforcement under statutes that do not require specific intent such as the False Claims Act. In fact, in recent legislation the Congress has required that those submitting claims for third party reimbursement are required to discover and repay any overpayments, or they are subject to additional penalties.
The Stark Law: Federal and some state laws prohibit physician referrals to an entity in which the physician or his or her immediate family members have a financial interest for provision of certain designated health services that are reimbursed by Medicare or Medicaid. We cannot assure you that the federal government, or other states in which we operate, will not enact similar or more restrictive legislation or restrictions or interpret existing laws and regulations in a manner that could harm our business.
Health Care Reform: There are currently a number of legislative proposals that have been proposed as health care reform in the United States Congress. At this time, it is not clear which, if any, of these proposals will be enacted. Therefore, although one or more of these proposals, if enacted, could have an impact on our business, we cannot predict at this time what that impact will be until there is legislation that becomes law.
Ongoing Investigations: Federal and state investigations and enforcement actions continue to focus on the health care industry, scrutinizing a wide range of items such as joint venture arrangements and referral and billing practices. We believe planned activities will be substantially in compliance with applicable legal requirements. We cannot assure you, however, that a governmental agency or a third party will not contend that certain aspects of our business are subject to, or are not in compliance with, such laws, regulations or rules, or that state or federal regulatory agencies or courts would interpret such laws, regulations and rules in our favor, or that future interpretations of such laws will not require structural or organizational modifications of our business or have a negative impact on our business. Applicable laws and regulations are very broad and complex, and, in many cases, the courts interpret them differently, making compliance difficult. Although we try to comply with such laws, regulations and rules, a violation could result in denial of the right to conduct business, significant fines and criminal penalties. Additionally, an increase in the complexity or substantive requirements of such laws, regulations or rules, or reform of the structure of health care delivery systems and payment methods, could have a material adverse effect on our business.
Employees
We presently have one full-time employee, which is our CEO, Ohad Goren. Our CFO, Gal Paleg, dedicates 25% of his professional time to our business and Itsik Ben Yesha, our CTO, dedicates 50% of his professional time to our business. Our CEO, CTO and CFO are employed under a service agreement with the company.
This Annual Report on Form 10-K contains forward-looking statements that are based on current expectations, estimates, forecasts and projections about us, our future performance, the market in which we operate, our beliefs and our management’s assumptions. In addition, other written or oral statements that constitute forward-looking statements may be made by us or on our behalf. Words such as “expects”, “anticipates”, “targets”, “goals”, “projects”, “intends”, “plans”, “believes”, “seeks”, “estimates”, variations of such words and similar expressions are intended to identify such forward-looking statements. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult to predict or assess. Therefore, actual outcomes and results may differ materially from what is expressed or forecast in such forward-looking statements.
| 11 |
Risks Related to Our Business
Our Independent Registered Public Accounting Firm Has Expressed Substantial Doubt As To Our Ability To Continue As A Going Concern.
The audited financial statements included in the Registration Statement have been prepared assuming that we will continue as a going concern and do not include any adjustments that might result if we cease to continue as a going concern. We believe that in order to continue as a going concern, including the costs of being a public company, we will need approximately $35,000 per year simply to cover the administrative, legal and accounting fees. We have incurred significant losses since our inception. We have funded these losses primarily through the sale of restricted shares of our Common Stock and the issuance of convertible notes, which have subsequently been converted into restricted shares of Common Stock.
Based on our financial history, our independent registered public accounting firm has expressed substantial doubt as to our ability to continue as a going concern. We are a development stage company that has generated no revenue.
Notwithstanding our success in funding our business from the sale of equity and debt securities to date, there can be no assurance that we will have adequate capital resources or be able to continue to raise equity and/or debt capital to fund planned operations or that any additional funds will be available to us when needed or at all, or, if available, will be available on favorable terms or in amounts required by us. If we are unable to obtain adequate capital resources to fund operations, we may be required to delay, scale back or eliminate some or all of our plan of operations, which may have a material adverse effect on our business, results of operations and ability to operate as a going concern.
We Have Limited Operating History And Face Many Of The Risks And Difficulties Frequently Encountered By Start-Up Companies.
In January 2014, the Company acquiring certain patents pertaining to a wound healing device. The Company’s new plan of operation has not yet generated any revenues. We have no operation history as a medical device company upon which an evaluation of the Company and its prospects could be based. There can be no assurance that management of the Company will be successful in commercially exploiting our wound healing device and implementing the corporate infrastructure to support its new operations so that the Company will generate sufficient revenues to meet its expenses or to achieve or maintain profitability.
If we are unable to raise sufficient capital as needed, we may be required to reduce the scope of our business development activities, which could harm our business plans, financial condition and operating results, or cease our operations entirely, in which case, you will lose all your investment.
If The Clinical Trial Studies Of Our Current Patented Device Does Not Produce Results Necessary To Support Regulatory Clearance Or Approval In The United States, We Will Be Unable To Commercialize Our Device.
We have engaged a third party to conduct clinical trials and we reasonably expect trial results in 2018. Clinical testing may be expected to take a significant amount of time, is expensive and carries uncertain outcomes. The initiation and completion of the trial study may be prevented, delayed, or halted for numerous reasons, including, but not limited to, the following:
| 12 |
● the FDA, institutional review boards or other regulatory authorities do not approve a clinical study protocol, force us to modify a previously approved protocol, or place a clinical study on hold;
● patients do not enroll in, or enroll at a lower rate than we expect, or do not complete a clinical study;
● patients or investigators do not comply with study protocols;
● patients do not return for post-treatment follow-up at the expected rate;
● patients experience serious or unexpected adverse side effects for a variety of reasons that may or may not be related to our product causing a clinical trial study to be put on hold;
●sites participating in an ongoing clinical study withdraw, requiring us to engage new sites;
● difficulties or delays associated with establishing additional clinical sites;
● third-party clinical investigators decline to participate in our clinical studies, do not perform the clinical studies on the anticipated schedule, or act in ways inconsistent with the investigator agreement, clinical study protocol, good clinical practices, and other FDA and Institutional Review Board requirements;
●third-party entities do not perform data collection and analysis in a timely or accurate manner;
● regulatory inspections of our clinical studies require us to undertake corrective action or suspend or terminate our clinical studies;
● changes in federal, state, or foreign governmental statutes, regulations or policies;
● interim results are inconclusive or unfavorable as to immediate and long-term safety or efficacy; or
● the study design is inadequate to demonstrate safety and efficacy.
Clinical trial failure can occur at any stage of the testing. Our clinical study may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and/or non-clinical testing in addition to those we have planned. Our failure to adequately demonstrate the safety and efficacy of our device would prevent receipt of regulatory clearance or approval and, ultimately, the commercialization of that device or indication for use. Any of these occurrences may harm our business, financial condition and prospects significantly.
Our Expected Revenue Will Be Generated From Our Sole Product, And Any Decline In The Future Sales Of This Product Or Failure To Gain Market Acceptance Of This Product Will Negatively Impact Our Business.
We expect our revenue to be derived entirely from sales of our wound healing Device for the foreseeable future. If we are unable to achieve and maintain market acceptance of our product and do not achieve sustained positive cash flow, we will be severely constrained in our ability to fund our operations, fulfill our business plan and be able to possibly develop and commercialize other potential products. In addition, if we are unable to market our product as a result of a quality problem, failure to maintain or obtain regulatory approvals, unexpected or serious complications or other unforeseen negative effects related to our product or the other factors discussed in these risk factors, we would lose our only potential source of revenue, and our business will be materially adversely affected.
If We Fail To Develop And Retain Our Sales Force, Our Business Could Suffer.
We plan to develop a direct sales force in the United States. As we launch our product, assuming that we are successful in securing FDA approval, any efforts to increase our marketing efforts and expand into new geographies will be dependent on our ability to hire and retain, as well as grow and develop our sales personnel. We intend to make a significant investment in recruiting and training sales representatives. There is significant competition for sales personnel experienced in relevant medical device sales. Once hired, the training process may be expected to be lengthy because it requires significant education for new sales representatives to achieve the level of competency with our product and meet the expectations of potential clients. Upon completion of the training, and any previous experience in marketing medical devices, generally, our sales representatives typically require lead time in the field to grow their network of accounts and achieve the productivity levels we expect them to reach in any individual territory. If we are unable to attract, motivate, develop and retain a sufficient number of qualified sales personnel, and if our sales representatives do not achieve the productivity levels we expect them to reach, our revenue will not grow at the rate we expect and our financial performance will suffer. Also, to the extent we hire personnel from our competitors, we may have to wait until applicable non-competition provisions have expired before deploying such personnel in restricted territories or incur costs to relocate personnel outside of such territories, and we may be subject to allegations that these new hires have been improperly solicited, or that they have divulged to us proprietary or other confidential information of their former employers.
| 13 |
If We Are Unable To Educate Physicians On The Safe And Effective Use Of Our Product, We May Be Unable To Achieve Our Expected Growth.
An important part of our sales process will include the education of physicians on the safe and effective use of our wound healing device. There is a learning process for physicians to become proficient in the use of our product and, based upon the use of our Device in Europe, it typically takes several procedures for a physician to become comfortable using the device. If a physician experiences difficulties during an initial procedure or otherwise, that physician may be less likely to continue to use our product, or to recommend it to other physicians. It is critical to the success of our commercialization efforts to educate physicians on the proper use of the device, and to provide them with adequate product support during training. It is important for our expected growth that these physicians advocate for the benefits of our product in the broader marketplace. If physicians are not properly trained, they may misuse or ineffectively use our product. This may also result in unsatisfactory patient outcomes, negative publicity or lawsuits against us, any of which could have a material adverse effect on our business.
There May Not Be A Wide Enough Client Base To Sustain Our Business.
The Company’s principal business is to engage in marketing and selling its wound healing treatments with our device. The Company hopes to sell its wound healing device treatments in numbers large enough to make its business model work for profitability, of which there can b no assurance.
If We Are Unable To Protect Our Intellectual Property, Our Business Will Be Negatively Affected.
The market for medical devices is subject to frequent litigation regarding patent and other intellectual property rights. It is possible that our device may not withstand challenges made by others or that our patents protect our rights adequately.
Our success depends in large part on our ability to secure and maintain effective patent protection for our product in the United States and internationally. We have acquired patents that have been granted as well as patents pending and expect to continue to file patent applications for various aspects of our device technology. However, we face the risks that:
● we may fail to secure necessary patents on our patents pending prior to or after obtaining regulatory clearances, thereby permitting competitors to market competing products; and
● our already-granted patents may be re-examined, invalidated or not extended.
If we are unable to protect our intellectual property adequately, our business and commercial prospects will suffer.
We May Be Accused Of Infringing Intellectual Property Rights Of Third Parties.
Other parties may claim that our Device infringes on their proprietary rights. We may be subject to claims and legal proceedings regarding alleged infringement by us of the intellectual property rights of third parties. Such claims, whether or not meritorious, may result in the expenditure of significant financial and managerial resources, legal fees, injunctions or the payment of damages. In the event that our patents do not fully protect us, we may need to obtain licenses from third parties who allege that we have infringed their rights, but such licenses may not be available on terms acceptable to us or at all. In addition, we may not be able to obtain or utilize on terms that are favorable to us, or at all, licenses or other rights with respect to intellectual property we do not own.
We May Face Product Liability Claims That Could Result In Costly Litigation And Significant Liabilities.
Marketing of our device and clinical testing of our product may expose us to product liability and other tort claims. Although we intend to purchase and maintain product liability insurance, the coverage limits of our policies may not be adequate and one or more successful claims brought against us may have a material adverse effect on our business and results of operations. Additionally, product liability claims could negatively affect our business reputation, adversely impacting continued product sales as well as our ability to obtain and maintain regulatory approval for our products.
| 14 |
Current Or Future Government Regulations May Add To Our Operating Costs.
We may face unanticipated increases in operating costs because of any changes in governmental regulations related to our wound healing Device, specifically, and/or medical devices, generally. We have no assurance that the independent clinical trials will result in favorable data that will be accepted by the FDA. Laws and regulations may be introduced and court decisions may be rendered that materially affect the demand for our product. Complying with new regulations and/or court decisions could increase our operating costs. Furthermore, we may be subject to the laws of various jurisdictions where we actually conduct business. Our failure to qualify to do business in a jurisdiction that requires us to do so could subject us to fines or penalties and could have a material adverse impact on our business and operations.
We Are Required To Comply With Medical Device Reporting, Or MDR, Requirements And Must Report Certain Malfunctions, Deaths And Serious Injuries Associated With Our Device Which Can Result In Voluntary Corrective Actions, Mandatory Recall Or Agency Enforcement Actions.
Under applicable FDA MDR regulations, medical device manufacturers are required to submit information to the FDA when they receive a report or become aware that a device has or may have caused or contributed to a death or serious injury or has or may have a malfunction that would likely cause or contribute to death or serious injury if the malfunction were to recur. All manufacturers placing medical devices on the market in the European Economic Area and the United States are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the regulatory agency, or Competent Authority, in whose jurisdiction the incident occurred.
Malfunction of our wound healing Device could result in future voluntary corrective actions, such as recalls, including corrections, or customer notifications, or agency action, such as inspection or enforcement actions. If malfunctions do occur, we may be unable to correct the malfunctions adequately or prevent further malfunctions, in which case we may need to cease manufacture and distribution of the affected products, initiate voluntary recalls, and redesign the products. Regulatory authorities may also take actions against us, such as ordering recalls, imposing fines, or seizing the affected products. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
We May Be Subject To Federal, State And Foreign Healthcare Laws And Regulations, And A Finding Of Failure To Comply With Such Laws And Regulations Could Have A Material Adverse Effect On Our Business.
Our operations are, and will continue to be, directly and indirectly affected by various federal, state or foreign healthcare laws, including, but not limited to, those described below. In particular, we are subject to the federal Anti-Kickback Statute, which prohibit, among other things, any person or entity from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, in cash or in kind, in return for or to induce the referring, ordering, leasing, purchasing or arranging for or recommending the referring, ordering, purchasing or leasing of any good, facility, item or service, for which payment may be made, in whole or in part, under federal healthcare programs, such as the Medicare and Medicaid programs.
We are also subject to the federal HIPAA statute, which, among other things, created federal criminal laws that prohibit knowingly and willfully executing, or attempting to execute, a scheme or artifice to defraud any health care benefit program or making any materially false, fictitious or fraudulent statements relating to health care matters.
We are also subject to the federal “sunshine” law, which requires us to track and report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to “payments or other transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals and to report annually to CMS ownership and investment interests held by physicians, as defined above, and their immediate family members in our company so long as it is privately held.
| 15 |
In addition, we are subject to the federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, persons or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim to, or the knowing use of false records or statements to obtain payment from, or approval by, the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers”, may share in any amounts paid by the entity to the government in fines or settlement. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim.
Many states have also adopted laws similar to each of the above federal laws, such as anti-kickback and false claims laws which may be broader in scope and apply to items or services reimbursed by any third-party payor, including commercial insurers, as well as laws that restrict our marketing activities with physicians, and require us to report consulting and other payments to physicians. Some states mandate implementation of compliance programs to ensure compliance with these laws. We also are subject to foreign fraud and abuse laws, which vary by country.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us now or in the future, we may be subject to penalties, including civil and criminal penalties, damages, fines, disgorgement, exclusion from governmental health care programs, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
Our Future Success Is Dependent, In Part, On The Performance And Continued Service Of Ohad Goren and Itsik Ben Yesha, Our Chief Executive Officer and Chief Technology Officer.
The Company will be dependent on its key executives, Ohad Goren and Itsik Ben Yesha, our CEO and CTO, respectively, for the foreseeable future. The loss of the services from either one could have a material adverse effect on the operations and prospects of the Company. They are expected to handle all aspects of our medical device business and manage our operations. Their responsibilities include developing business arrangements, directing the development of the Company’s technology and IP, overseeing technical aspects of our business, regulations and formulating strategies and materials to be used during our presentations and meetings. At this time, the Company does not have an employment agreement with either Mr. Goren or Ben Yesha, though the Company may enter into such an agreement with Mr. Goren, its CEO and Mr. Ben Yesha, its CTO, on terms and conditions usual and customary in our industry. The Company does not currently have “key man” life insurance on neither Mr. Goren, Mr. Ben Yesha nor on Mr. Ron Weissberg, our Chairman and control shareholder.
We Operate In A Highly Competitive Industry And Compete Against Several Large Companies Which Could Adversely Affect Our Ability to Succeed.
There are numerous established companies that offer wound healing products including products from Kinetic Concepts, Inc. and Smith & Nephew, which have far greater financial and other resources and far longer operating histories than we do. We are a new entry into this competitive market and may struggle to differentiate ourselves as a viable competitor whose wound healing Device provides more value and efficacy than the competition.
We Are An “Emerging Growth Company” Under The Recently Enacted Jobs Act And We Cannot Be Certain If The Reduced Disclosure Requirements Applicable To Emerging Growth Companies Will Make Our Common Stock Less Attractive To Investors.
We qualify as an “emerging growth company” under the recently enacted JOBS Act. As a result, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. For so long as we are an emerging growth company, among other things, we will not be required to:
● have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act;
● submit certain executive compensation matters to shareholder advisory votes, such as “say-on-pay” and “say-on-frequency”;
● obtain shareholder approval of any golden parachute payments not previously approved; and
● disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive’s compensation to median employee compensation.
| 16 |
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “Emerging Growth Company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
We will remain an “Emerging Growth Company” for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our total annual gross revenues exceed $1 billion; (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, which would occur if the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three-year period.
Until such time, however, because the JOBS Act has only recently been enacted, we cannot predict whether investors will find our stock less attractive because of the more limited disclosure requirements that we may be entitled to follow and other exemptions on which we are relying while we are an “emerging growth company”. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
Our By-Laws Provide For Indemnification Of Our Directors And the Purchase Of D&O Insurance At Our Expense And Limit Their Liability Which May Result In A Major Cost To Us And Hurt The Interest Of Our Shareholders Because Corporate Resources May Be Expended For The Benefit Of Our Directors.
The Company’s By-Laws include provisions that eliminate the personal liability of the directors of the Company for monetary damages to the fullest extent possible under the laws of the State of Delaware or other applicable law. These provisions eliminate the liability of directors to the Company and its stockholders for monetary damages arising out of any violation of a director of his fiduciary duty of due care. Under Delaware law, however, such provisions do not eliminate the personal liability of a director for (i) breach of the director’s duty of loyalty, (ii) acts or omissions not in good faith or involving intentional misconduct or knowing violation of law, (iii) payment of dividends or repurchases of stock other than from lawfully available funds, or (iv) any transaction from which the director derived an improper benefit. These provisions do not affect a director’s liabilities under the federal securities laws or the recovery of damages by third parties.
Reporting Requirements Under The Exchange Act And Compliance With The Sarbanes-Oxley Act Of 2002, Including Establishing And Maintaining Acceptable Internal Controls Over Financial Reporting, Are Costly And May Increase Substantially.
The rules and regulations of the SEC require a public company to prepare and file periodic reports under the Exchange Act, which will require that the Company engage legal, accounting, auditing and other professional services. The engagement of such services is costly. Additionally, the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) requires, among other things, that we design, implement and maintain adequate internal controls and procedures over financial reporting. The costs of complying with the Sarbanes-Oxley Act and the limited technically qualified personnel we have may make it difficult for us to design, implement and maintain adequate internal controls over financial reporting. We expect these costs to be approximately $35,000 per year. In the event that we fail to maintain an effective system of internal controls or discover material weaknesses in our internal controls, we may not be able to produce reliable financial reports or report fraud, which may harm our overall financial condition and result in loss of investor confidence and a decline in our share price.
| 17 |
As a public company, we may be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Act of 2010 and other applicable securities rules and regulations. Despite recent reforms made possible by the JOBS Act, compliance with these rules and regulations will nonetheless increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “Emerging Growth Company.” The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and operating results.
We are working with our legal, independent accounting and financial advisors to identify those areas in which changes should be made to our financial and management control systems to manage our growth and our obligations as a public company. These areas include corporate governance, corporate control, disclosure controls and procedures and financial reporting and accounting systems. We have made, and will continue to make, changes in these and other areas. However, we anticipate that the expenses that will be required for being a public company could be material. We estimate that the aggregate cost of increased legal services; accounting and audit functions; personnel, such as a chief financial officer familiar with the obligations of public company reporting; consultants to design and implement internal controls could be material. In addition, if and when we retain independent directors and/or additional members of senior management, we may incur additional expenses related to director compensation and/or premiums for directors’ and officers’ liability insurance, the costs of which we cannot estimate at this time. We may also incur additional expenses associated with investor relations and similar functions, the cost of which we also cannot estimate at this time. However, these additional expenses individually, or in the aggregate, may also be material.
In addition, being a public company could make it more difficult or more costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
The increased costs associated with operating as a public company may decrease our operating performance, and may cause us increase the prices of our product to offset the effect of such increased costs. Additionally, if these requirements divert our management’s attention from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations.
Future Manufacturing Of Our Product May Be Interrupted Due To International Political Situations, Natural Disasters Or Other Causes.
Once FDA approval is granted, we plan to manufacture our BST Device principally in Israel. Domestic situations in Israel and surrounding countries could possibly result in production and delivery problems. We are subject to the risk that future manufacturing and delivery of our BST Device may be interrupted as a result of natural disasters or capacity constraints with our vendors’ or suppliers’ hardware. Any such interruptions may lead to a loss of customers or distributors and, accordingly, may adversely affect our business and results of operations.
Risks Related to Our Common Stock
There Is No Assurance Of A Liquid Public Market Of Our Common Stock Or That You May Be Able To Liquidate Your Investment In Our Common Stock.
At present, our Common Stock is subject to quotation of the OTCQB market. There is only a limited, liquid public trading marketing for our Common Stock and there can be no assurance that one will ever develop. Market liquidity will depend on the perception of our business and any steps that our management might take to bring us to the awareness of investors. There can be given no assurance that there will be any awareness generated. Consequently, investors may not be able to liquidate their investment or liquidate it at a price that reflects the value of the business or the value of their initial investment in our Common Stock. As a result, holders of our securities may not find purchasers for our securities should they to decide to sell securities held by them. Consequently, our securities should be purchased only by investors who have no need for liquidity in their investment and who can hold our securities for a prolonged period of time.
| 18 |
The Market Price Of Our Common Stock May Be Volatile.
In the event an active trading market develops for our Common Stock, the market price of our Common Stock may be highly volatile, as is the stock market in general, and the market for securities subject to quotation on OTC Markets in particular. Some of the factors that may materially affect the market price of our Common Stock are beyond our control, such as changes in conditions or trends in the industry in which we operate or sales of our Common Stock. These factors may materially adversely affect the market price of our Common Stock, regardless of our business performance. Public stock markets have experienced extreme price and trading volume volatility. This volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the operating performance of the specific companies. These market fluctuations may adversely affect the market price of our Common Stock.
A Large Number Of Additional Shares Will Be Available For Resale Into The Public Market Pursuant To This Offering As Well As In The Near Future, Which May Cause The Market Price Of Our Common Stock To Decline Significantly, Even If Our Business Performs As Expected.
Sales of a substantial number of shares of our Common Stock in the public market pursuant to our Offerings, or the perception that additional shares of Common Stock become available pursuant to Rule 144 promulgated by the SEC under the Act, could adversely affect the market price of our Common Stock. As of April 16, 2018, we have 22,237,562 shares of Common Stock outstanding of which 15,921,328 shares of Common Stock are restricted as a result of applicable securities laws. As restrictions on resale expire, the market price could drop significantly if the holders of these restricted shares sell them or are perceived by the market as intending to sell them. For a more detailed description, see “Shares Eligible for Future Sale.”
If holders of restricted securities sell a large number of shares pursuant to Rule 144 under the Act, they could adversely affect the market price for our Common Stock.
You Will Experience Dilution Of Your Ownership Interest Because Of The Future Issuance Of Additional Shares Of Our Common Stock Or Our Preferred Stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. We are authorized to issue an aggregate of 500,000,000 shares of Common Stock, par value $0.00001 per share, of which 22,237,562 are currently outstanding.
We may also issue additional shares of our Common Stock or other securities that are convertible into or exercisable for Common Stock in connection with hiring or retaining employees or consultants, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our Common Stock or other securities may have a negative impact on the market price of our Common Stock. There can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with hiring or retaining employees or consultants, future acquisitions, future sales of our securities for capital raising purposes or for other business purposes, including at a price (or exercise prices) below the price at which shares of our Common Stock will be quoted on the OTCQB Markets.
We May Never Pay Any Dividends To Our Shareholders.
We currently intend to retain any future earnings for use in the operation and expansion of our business. Accordingly, we do not expect to pay any dividends in the foreseeable future, but will review this policy as circumstances dictate. The declaration and payment of all future dividends, if any, will be at the sole discretion of our board of directors, which retains the right to change our dividend policy at any time. Consequently, stockholders must rely on sales of their Common Stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment.
| 19 |
Insider Will Continue To Have Substantial Control Over Us After This Offering and Will Be Able To Influence Corporate Matters.
Our directors and executive officers and stockholders holding more than 5% of our Common Stock and their affiliates will beneficially own, in the aggregate, approximately 30% of our outstanding Common Stock. As a result, if these stockholders were to choose to act together, they would be able to exercise significant influence over all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as a merger or other sale of our company or its assets. This concentration of ownership could limit your ability to influence corporate matters and may have the effect of delaying or preventing a third party from acquiring control over us. For information regarding the ownership of our outstanding stock by our executive officers and directors and their affiliates, see “Security Ownership of Certain Beneficial Owners and Management.”
We cannot assure you that the interests of our management team will coincide with the interests of the investors. So long as our management team collectively controls a significant portion of our Common Stock, these individuals, or entities controlled by them, will continue to collectively be able to strongly influence or effectively control our decisions.
Anti-Takeover Provisions Of The Delaware General Corporation Law May Discourage Or Prevent A Change Of Control, Even If An Acquisition Would Be Beneficial To Our Shareholders, Which Could Reduce Our Stock Price.
We are subject to the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These and other provisions in our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including a merger, tender offer or proxy contest involving our company. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our Common Stock to decline.
State Blue Sky Registration, Potential Limitations On Resale Of Our Common Stock.
The holders of our shares of Common Stock and those persons, who desire to purchase our Common Stock in any trading market that might develop, should be aware that there may be state blue-sky law restrictions upon the ability of investors to resell our securities. Accordingly, investors should consider the secondary market our securities to be a limited one.
It is the present intention of management after the active commencement of operations in to seek coverage and publication of information regarding the Company in an accepted publication manual, which permits a manual exemption. The manual exemption permits a security to be distributed in a particular state without being registered if the Registrant issuing the security has a listing for that security in a securities manual recognized by the state. However, it is not enough for the security to be listed in a recognized manual. The listing entry must contain (1) the names of issuer’s officers, and directors, (2) an issuer’s balance sheet, and (3) a profit and loss statement for either the fiscal year preceding the balance sheet or for the most recent fiscal year of operations. Furthermore, the manual exemption is a non-issuer exemption restricted to secondary trading transactions, making it unavailable for issuers selling newly issued securities.
Most of the accepted manuals are those published in Standard and Poor’s, Moody’s Investor Service, Fitch’s Investment Service, and Best’s Insurance Reports, and many states expressly recognize these manuals. A smaller number of states declare that they “recognize securities manuals” but do not specify the recognized manuals. The following states do not have any provisions and therefore do not expressly recognize the manual exemption: Alabama, Georgia, Illinois, Kentucky, Louisiana, Montana, South Dakota, Tennessee, Vermont and Wisconsin.
Our Common Stock Is Considered A Penny Stock, Which May Be Subject To Restrictions On Marketability, So You May Not Be Able To Sell Your Shares.
| 20 |
We may be subject now and in the future to the Penny Stock rules if our shares of Common Stock sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer’s confirmation.
In addition, the penny stock rules require that prior to a transaction, the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any Offerings and reduce the trading activity for shares of our Common Stock. As long as our shares of Common Stock are subject to the penny stock rules, the holders of such shares of Common Stock may find it more difficult to sell their securities.
The Control Deficiencies In Our Internal Control Over Financial Reporting May Until Remedied Cause Errors in Our Financial Statements Or Cause Our Filings With The SEC To Not Be Timely.
We have identified control deficiencies in our internal control over financial reporting as of the evaluation done by management as of December 31, 2017, including those related to (i) absent or inadequate segregation of duties within a significant account or process, (ii) inadequate documentation of the components of internal control, and (iii) inadequate design of information technology general and application controls that prevent the information system from providing complete and accurate information consistent with financial reporting objectives and current needs. If our internal control over financial reporting or disclosure controls and procedures are not effective, there may be errors in our financial statements that could require a restatement or our filings may not be timely made with the SEC. Based on the work undertaken and performed by us, however, we believe the financial statements contained in our reports filed with the SEC are fairly stated in all material respects in accordance with GAAP for each of the periods presented. We intend to implement additional corporate governance and control measures to strengthen our control environment as we are able, but we may not achieve our desired objectives. We may identify material weaknesses and control deficiencies in our internal control over financial reporting in the future that may require remediation and could lead investors losing confidence in our reported financial information, which could lead to a decline in our stock price.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. DESCRIPTION OF PROPERTIES
Our principal executive office is located at 20 West 64th Street, Suite 39G, New York, NY 10023. Our telephone number is (972) 54-427777, which is the telephone of the offices of our Chairman, Ron Weissberg, located in Israel. There is no lease on the premises the Company is occupying and the Company is not responsible for paying rent. We are not generating sufficient revenue at this time to justify a separate corporate office. Once our business grows and generates revenue, we will look for more office space in a separate corporate office.
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
| 21 |
ITEM 5. MARKET FOR REGISTRANT’S COMMON STOCK AND RELATED STOCKHOLDER MATTER
Market Information
The Company’s Common Stock is subject to quotation on the OTCQB Market under the symbol “EQUR”. There is currently no active trading market in the Common Stock on the OTC market. There can be no assurance that there will be an active trading market for the Common Stock once the Company becomes a reporting company under the Exchange Act. In the event that an active trading market commences, there can be no assurance as to the market price of the shares of Common Stock, whether any trading market will provide liquidity to investors, or whether any trading market will be sustained.
For the periods indicated, the following table sets forth the high and low bid prices per share of Common Stock. The below prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions but does reflect the price of our Common Stock adjusted for the reverse split.
| Fiscal 2017 | Fiscal 2016 | Fiscal 2015 | ||||||||||||||||||||||
| High | Low | High | Low | High | Low | |||||||||||||||||||
| First Quarter ended March 31 | $ | 0.51 | $ | 0.10 | $ | 0.10 | $ | 0.10 | $ | 1.75 | $ | 0.40 | ||||||||||||
| Second Quarter ended June 30 | $ | 0.23 | $ | 0.10 | $ | 0.10 | $ | 0.10 | $ | 0.46 | $ | 0.11 | ||||||||||||
| Third Quarter ended September 30 | $ | 0.15 | $ | 0.05 | $ | 0.12 | $ | 0.12 | $ | 0.51 | $ | 0.15 | ||||||||||||
| Fourth Quarter ended December 31 | $ | 0.08 | $ | 0.04 | $ | 0.13 | $ | 0.13 | $ | 0.32 | $ | 0.11 | ||||||||||||
Holders of Capital Stock
We have 223 holders of record of our Common Stock. This does not include an indeterminate number of persons who hold our Common Stock in brokerage accounts and otherwise in “street name.”
Rule 144 Shares
As of the date of this annual report, there were 477,762,255 shares of our Common Stock that are currently available for sale to the public in accordance with the volume and trading limitations of Rule 144.
Stock Option Grants
On January 1, 2015, the board of director approved the 2015 Employee Incentive Plan. The total number of shares of Common Stock reserved for issuance by the Company either directly as Stock Awards or underlying Options granted under this Plan is 5,000,000 shares of Common Stock. On January 1, 2015, the Company granted options as follows under its 2015 Employee Incentive Plan: (i) Professor Ohry was granted options to purchase 250,000 shares of the Registrant’s common stock (“Option Shares”) at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Ohry SAB Agreement”). Provided the Ohry SAB Agreement remains in effect, 75,000 shares shall vest July 1, 2015, and the remaining 175,000 Option Shares shall vest at the rate of 25,000 Option Shares per quarter on the first day of each consecutive quarter; (ii) Dr. Ben Zion Weiner was granted options to purchase 350,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Weiner SAB Agreement”). Provided the Weiner SAB Agreement remains in effect, 105,000 Option Shares shall vest July 1, 2015 and the remaining 245,000 Option Shares shall vest at the rate of 35,000 Option Shares per quarter on the first day of each consecutive quarter; and (iii) Michel Sessler was granted options to purchase 150,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Sessler SAB Agreement”). Provided the Sessler SAB Agreement remains in effect, 45,000 Option Shares shall vest July 1, 2015 and the remaining 105,000 Option Shares shall vest at the rate of 15,000 Option Shares per quarter on the first day of each consecutive quarter.
| 22 |
Following is a table summarizing options still outstanding and exercisable along with exercise price and range of remaining term.
| Type | Quantity | Exercise Price | Term | |||||||||
| Avi Ohry | 250,000 | $ | 1.00 | 24 Months | ||||||||
| Dr. Ben Zion Weiner | 350,000 | $ | 1.00 | 24 Months | ||||||||
| Michael Sessler | 150,000 | $ | 1.00 | 24 Months | ||||||||
| Total | 750,000 | |||||||||||
Securities Authorized for Issuance Under Equity Compensation Plans
No equity compensation plan or agreements under which our Common Stock is authorized for issuance has been adopted during the fiscal years ended December 31, 2017 and 2016.
Sale of Unregistered Securities
Issuances of Common Stock and Common Stock Receivable in 2015:
During the three months ended March 31, 2015, we received $70,000 in stock receivable. During the three months ended June 30, 2015, we received $100,000 in stock receivable.
Issuances of Common Stock in 2016:
During the year ended December 31, 2016, we did not issue any restricted stock.
Issuances of Common Stock and Common Stock Receivable in 2017:
On April 20, 2017, we issued 225,000 shares valued at $39,128 to two consultants for services provided. During the three months ended June 30, 2017, we authorized the issuance of 75,000 additional shares to the same two consultants valued at $0.14 or $21,000, which was recorded as stock payable.
Penny Stock Considerations
Our Common Stock will be deemed to be “penny stock” as that term is generally defined in the Securities Exchange Act of 1934 to mean equity securities with a price of less than $5.00. Our shares thus will be subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in certain transactions involving a penny stock.
Under the penny stock regulations, a broker-dealer selling a penny stock to anyone other than an established customer or accredited investor must make a special suitability determination regarding the purchaser and must receive the purchaser’s written consent to the transaction prior to the sale, unless the broker-dealer is otherwise exempt. Generally, an individual with a net worth in excess of $1,000,000 or annual income exceeding $100,000 individually or $300,000 together with his or her spouse is considered an accredited investor. In addition, under the penny stock regulations the broker-dealer is required to:
| 23 |
● Deliver, prior to any transaction involving a penny stock, a disclosure schedule prepared by the SEC relating to the penny stock market, unless the broker-dealer or the transaction is otherwise exempt;
● Disclose commissions payable to the broker-dealer and our registered representatives and current bid and offer quotations for the securities;
● Send monthly statements disclosing recent price information pertaining to the penny stock held in a customer’s account, the account’s value and information regarding the limited market in penny stocks; and
● Make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction, prior to conducting any penny stock transaction in the customer’s account.
Because of these regulations, broker-dealers may encounter difficulties in their attempt to buy or sell shares of our Common Stock, which may affect the ability of Selling Shareholders or other holders to sell their shares in the secondary market and have the effect of reducing the level of trading activity in the secondary market. These additional sales practice and disclosure requirements could impede the sale of our Common Stock even if our Common Stock becomes publicly traded. In addition, the liquidity for our Common Stock may be decreased, with a corresponding decrease in the price of our Common Stock. Our shares are likely to be subject to such penny stock rules for the foreseeable future.
ITEM 6. SELECTED FINANCIAL DATA
None.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATION
The following plan of operation provides information which management believes is relevant to an assessment and understanding of our results of operations and financial condition. The discussion should be read along with our financial statements and notes thereto. This section includes a number of forward-looking statements that reflect our current views with respect to future events and financial performance. Forward-looking statements are often identified by words like believe, expect, estimate, anticipate, intend, project and similar expressions, or words which, by their nature, refer to future events. You should not place undue certainty on these forward-looking statements. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our predictions.
Plan of Operations
In January 2014, Mr. Weissberg negotiated with Lifewave Ltd., a public company organized under the laws of the State of Israel, for the purpose of acquiring certain of Lifewave’s IP assets pertaining to a wound healing device. The Registrant signed a patent purchase agreement with Lifewave on January 6, 2014 (the “Agreement”), the closing of which was subject to several material conditions, including our ability of raising equity capital sufficient to develop and commercially exploit the technology.
On June 4, 2014, we completed the purchase of all right, title and interest to certain IP assets, including rights to a wound treatment device. The IP assets, including the wound healing device, acquired by the Registrant are designed for wound treatment incorporating Bioelectrical Signal Therapy (“BST Device”). The BST Device implements patented and proprietary electrical stimulation technologies to treat hard-to-cure wounds and ulcers up to complete closure and/or cure.
Pursuant to the Agreement, the Registrant has agreed to pay Lifewave a royalty of from 10% to 20% of the profits (as defined in the Agreement) generated from the BST Device.
In June 2014, the Registrant entered into an agreement with the Austen BioInnovation Institute in Akron (“ABIA” or the “Institute”), for the purpose of bringing our BST Device to the U.S. market.
| 24 |
The Company’s management selected ABIA’s Product Innovation and Commercialization Division, which has significant expertise in wound healing, clinical trial development, and regulatory operations, to spearhead its pre-market clinical trial program, which is necessary to apply for regulatory approval from the United States Food and Drug Administration (“FDA”) to distribute the BST Device in the United States. As part of the Institute’s fully integrated regulatory and device development service Offerings, ABIA will prepare on behalf of the Company an application to obtain FDA approval. The initial trial will include 70 patients in a double-arm, randomized, multi-center study to assess the safety and efficacy of the BST Device in patients with Stage II and III pressure and venous stasis ulcers; and submit data to the FDA to obtain approval.
On December 18, 2015, the Registrant confirmed certain information that it had received from ABIA that, while ABIA still anticipated that it would be able to provide the Registrant with a final draft of the IDE application, ABIA had sustained financial difficulties and key personnel losses that would likely adversely effect its ability to perform under the Agreement on a timely basis, if at all. As a result, the Registrant requested that ABIA fully refund the monies paid to ABIA under the Agreement. In addition, the Registrant agreed to engage a professional regulatory consultant, who was a former member of ABIA’s regulatory staff, to serve as the Registrant’s FDA regulatory consultant on an interim basis, subject to the execution of a separate services agreement. The Registrant is also evaluating the advisability of engaging another firm to replace ABIA, which process may be expected to delay the IDE approval process for the BST Device.
The Company’s success is dependent upon the successful FDA clinical trial of its BST Device. The Device may need additional development and may never achieve safety or efficacy. The Company believes that its design and procedure show promise, but the path to commercial success, even if development milestones are met, may take more time and might be more costly.
There are a number of potential obstacles the Company might face, including the following:
● We may not be able to raise additional funds we may need to complete the clinical trials.
● Competitors may develop alternatives that render BST Device redundant or unnecessary.
● We may not have a sufficient and sustainable intellectual property position.
● Our device may be shown to have harmful side effects or other characteristics that indicate it is unlikely to be safe and effective.
● Our device may not receive regulatory approval.
● Even if our device receives regulatory approval, it may not be accepted by patients, the medical community or third-party payers.
During the year ended December 31, 2017, the Registrant did not raise any equity and debt capital.
Recent Developments
Effective October 15, 2014, through our wholly-owned Israeli subsidiary, ESQURE, we entered into an Asset Purchase Agreement with Michael Cohen. We purchased all of Mr. Cohan’s assets (the “Seller’s Assets”) related to our BST Device for 875,000 restricted shares of common stock valued at $350,000. The Seller’s Assets settled a subscription receivable under a previous subscription agreement for the same number of shares. Pursuant to the terms of the Asset Purchase Agreement, we purchased all of Seller’s Assets related to our BST Device.
On December 28, 2014, the Registrant entered into a preliminary distribution agreement with Rubifarm S.A., an entity organized under the laws of Argentina (“Rubifarm”), which agreement is subject to approval by the regulatory authorities of Argentina. At the date of regulatory approval, which is anticipated during the 4th quarter of 2015, a definitive agreement will be executed and filed with the SEC. The agreement contemplates that Rubifarm will be granted exclusive distribution rights for the BST Device™ in Argentina for an initial term of 5 years subject to Rubifarm meeting a minimum purchase quota of $1.5 million during the initial 5-year term in order to retain its exclusivity.
| 25 |
On July 30, 2015, the Company reported that it entered into an exclusive distribution agreement (the “Distribution Agreement”) with Chemipal Ltd, a closely-held Tel-Aviv Stock Exchange listed company organized under the laws of the State of Israel (“Chemipal”). Chemipal has been actively engaged in the distribution of medical products in srael since 1941. Under the Distribution Agreement, the Registrant has granted Chemipal exclusive distribution rights to the BST Device and the accompanying disposable electrodes (sometimes collectively, the “Products”) in Israel for an initial 5 year term, subject to Chemipal satisfying a minimum purchase quota of $3 million during the term.
On December 18, 2015, the Registrant confirmed certain information that it had received from ABIA that, while ABIA still anticipated that it would be able to provide the Registrant with a final draft of the IDE application, ABIA had sustained financial difficulties and key personnel losses that would likely adversely effect its ability to perform under the Agreement on a timely basis, if at all. As a result, the Registrant requested that ABIA fully refund the monies paid to ABIA under the Agreement. In addition, the Registrant agreed to engage a professional regulatory consultant, who was a former member of ABIA’s regulatory staff, to serve as the Registrant’s FDA regulatory consultant on an interim basis, subject to the execution of a separate services agreement. The Registrant is also evaluating the advisability of engaging another firm to replace ABIA, which process may be expected to delay the IDE approval process for the BST Device.
In May 2016, the Company commenced legal action against ABIA in the Supreme Court of the State of New York, New York County (the “Lawsuit”). The Lawsuit alleges the breach of contract against ABIA to a Clinical Trial Agreement between the Company and ABIA dated June 5, 2014 (the “CTA”) based upon, among other reasons: (i) the failure of ABIA to commit sufficient personnel to the Company’s BST device project; (ii) misrepresenting the ability of its staff to perform its obligations under the CTA; (iii) failing to provide the FDA with adequate evidence to support the IDE applications and providing incorrect responses to the FDA; and (v) misappropriating the Company’s funds for use on other ABIA projects and expenses rather than in fulfillment of its contract obligations. The Lawsuit seeks approximately $475,000 in actual damages, representing the fees paid by the Company to ABIA, loss of profits in an amount not less than $3 million and reasonable attorneys’ fees and costs and expenses. During October 2016, the Company signed settlement agreement with ABIA on the amount of $300,000.
On July 18, 2016, the Company received the CE Certificate of Conformity and the ISO 13485 Certification. The CE Certification for our BST Wound Healing Device is a declaration that it complies with the requirements of the EU related to health, safety and environmental protections and acknowledges that the BST Device may be legally marketed in the EU. As a result, we are prepared to commence manufacturing and marketing for our BST Device in Europe as well as other non-European countries that accept the CE Certification. The ISO is the International Organization for Standardization, and represents that the company’s quality systems and procedures satisfies the requirements for a comprehensive quality management for the design and manufacture of medical devices.
On October 14, 2016, the Registrant received notification from FDA that it has granted conditional approval to the IDE application, authorizing us to commence a clinical investigation of our BST Device for wound healing. The main condition set forth is that the trial shall begin initially with 10 patients, after which we will file a safety report with the FDA before proceeding with the trial, which contemplates testing the BST Device with 90 patients altogether.
On January 8, 2017, the Registrant entered into a five-year distribution agreement (the “Distribution Agreement”) with TekMedica SAS, organized under the laws of Colombia (“TekMedica” or the “Distributor”). Pursuant to the Distribution Agreement, the Registrant granted TekMedica the exclusive rights to distribute the Registrant’s medical device for the treatment of chronic wounds (the “BST Device™”) and the accompanying disposable electrodes (sometimes collectively, the “Products”) in Colombia (the “Territory”).
The Distribution Agreement provides that Registrant will provide Distributor with supplies of the BST Devicee and disposable electrode for treatment of patients in hospitals, long-term care facilities, medical centers and out-patient clinics. The Distributor will make an initial advance payment to be applied against the first year’s quota together with an initial order supported by a Letter of Credit with subsequent orders as part of the quota, as set forth in the Distribution Agreement, with minimum annual quota’s during the five-year term. The Distributor will be responsible for securing any product certification, permit, license or approval that may be required in the Territory for the marketing, sale, sublicensing and delivery and use of the BST Devise and Products in the Territory.
| 26 |
On February 20, 2017, the Registrant received the official certification from the Israeli Ministry of Health authorizing the use of the Registrant’s BST Device in Israel. The BST Device implements patented and proprietary electrical stimulation technologies to treat hard-to-cure wounds and ulcers up to complete closure and/or cure.
Results of Operations during the year ended December 31, 2017 as compared to the year ended December 31, 2016
We have not generated any revenues since inception. We had operating expenses mainly related to general and administrative expenses and research and development expenses. During the year ended December 31, 2017, we incurred $853,168 in net loss from operations due to general and administrative expenses of $628,717 and research and development expenses of $224,451 as compared to a net loss from operations of $973,829 due to general and administrative expenses of $912,627 and research and development expenses of $342,602 during the year ended December 31, 2016, and other income received of $281,400 due to a gain on legal settlement.
Our net loss from continuing operations during the years 2017 and 2016 were $853,168 and $973,829, respectively. We paid no income tax in 2017 and 2016.
Liquidity, Capital Resources and Strategy
On December 31, 2017, we had total assets of $95,344, consisting of $10,962 in cash, $21,000 in prepaid expenses and other assets of $63,382, as compared to total assets of $408,108 at December 31, 2016. consisting of $292,976 in cash, $62,816 in accounts receivable and $52,316 in prepaid expenses. We had total current liabilities of $367,765 as of December 31, 2017 consisting of $229,714 in accounts payable and accrued salaries to management and $138,051 in loans from shareholders. We had total current liabilities of $1,564 as of December 31, 2016 consisting of accounts payable. We had no long-term liabilities as of December 31, 2017 and 2016.
We used $420,065 in our operating activities during the year 2017, which was due to a net loss of $853,168 offset by stock-based compensation of $167,303, imputed interest of $6,900, an increase in accounts payable and accrued expenses of $228,150, a decrease in accounts receivable of $62,816, an increase in other assets of $63,382 and a decrease of $31,316 in prepaid expenses.
We used $587,663 in our operating activities during the year 2016, which was due to a net loss of $973,829 offset by stock-based compensation of $401,298, a loss on stock receivable of $100,000, an increase in accounts receivable of $62,816 and a decrease in prepaid expenses of $52,316.
We financed our negative cash flow from operations in 2017 through proceeds from borrowings on notes payable of $138,051. We financed our negative cash flow from operations in 2016 through available cash resources.
We had no investing activities during the years ended December 31, 2017 and 2016.
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America with an auditor’s going concern opinion for the years 2017 and 2016. This means that there is substantial doubt that we can continue as an on-going business for the next twelve months unless we obtain additional capital to pay our bills and meet our other financial obligations. This is because we have not generated any revenues and no revenues are anticipated.
The Company has reported a net loss of $853,168 for the year ended December 31, 2017 and $973,829 for the year ended December 31, 2016 and total accumulated deficits of $31,618,687 and $30,765,519 as of December 31, 2017 and 2016, respectively.
| 27 |
The Company had no revenues from operations during the years ended December 31, 2017 and 2016. As of December 31, 2017, the Company had $10,962 cash on hand and had negative working capital of $335,803.
We believe that our current cash on hand of $10,962, as of December 31, 2017, will not be sufficient to meet our operating requirements throughout the ensuing twelve month period. We require additional financing at satisfactory terms and conditions, of which there can be no assurance, in order to satisfy our ongoing capital requirements for the next twelve months in order to execute our plan of operation as presently constituted.
We do not expect to generate cash flow from operations unless we receive FDA approval for our BST Device.
Our management believes that our operations will generate revenues in the US beginning of 2019. We expect that FDA approval for our BST Device will improve our ability to generate revenues from sales in other geographic areas. Our future ability to generate cash flows from operations will depend on the demand for our BST Device, as well as general economic, financial, competitive and other factors, many of which are beyond our control.
If and when we receive FDA approval of our BST Device, of which there can be no assurance, our business might not generate sufficient future cash flow in an amount sufficient to enable us to fund our liquidity needs, including working capital, capital expenditures, investments and other general corporate requirements.
Availability of Additional Capital
We have no commitments or arrangements, formal or otherwise, from any person or entity to provide us with any additional capital. The Company may be unable to implement its present plan of operation and this could have a material adverse effect on our business, prospects, financial condition and results of operations.
Our future financing transactions may include the issuance of equity and/or debt securities. In the event that we seek to raise funds through additional private placements of equity or convertible debt, the trading price of our common stock could be adversely effected. Further, if we issue additional equity or debt securities, stockholders may experience dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock. We are not aware of any material trend, event or capital commitment, which would or could potentially adversely affect our liquidity. We do not have any arrangements with potential investors or lenders to provide us with any additional financing and there can be no assurance that any such additional financing will be available when required in order to proceed with the business plan.
Off-Balance Sheet Arrangements
As of December 31, 2017 and 2016, we did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Securities Exchange Act of 1934.
Critical Accounting Policies
Our significant accounting policies are described in the notes to our financial statements for the years ended December 31, 2017 and 2016, and are included elsewhere in this annual report.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
We have not entered into, and do not expect to enter into, financial instruments for trading or hedging purposes.
| 28 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| 29 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of E-Qure Corp.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of E-Qure Corp. (the Company) as of December 31, 2017 and 2016, and the related statements of income, comprehensive income, stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2017, and the related notes and schedules (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 8 to the financial statements, the Company suffered a net loss from operations and has a net capital deficiency, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 8. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
M&K CPAS, PLLC
We have served as the Company’s auditor since 2011.
Houston, TX
April 16, 2018
| 30 |
E-QURE CORP.
At December 31, 2017 and 2016
| December 31, 2017 | December 31, 2016 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash | $ | 10,962 | $ | 292,976 | ||||
| Receivables | - | 62,816 | ||||||
| Prepaid expenses | 21,000 | 52,316 | ||||||
| Total current assets | 31,962 | 408,108 | ||||||
| Other assets | 63,382 | - | ||||||
| Total Assets | $ | 95,344 | $ | 408,108 | ||||
| Liabilities and Stockholders’ Equity (Deficit) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable - trade | $ | 1,564 | $ | 1,564 | ||||
| Accrued salary | 228,150 | - | ||||||
| Loan from shareholder | 138,051 | - | ||||||
| Total current liabilities | 367,765 | 1,564 | ||||||
| Stockholders’ equity (deficit): | ||||||||
| Preferred stock, $0.00001 par value; 20,000,000 shares authorized; no shares issued and outstanding | - | |||||||
| Common stock, $0.00001 par value; 500,000,000 shares authorized; and 22,237,562 and 22,012,562 issued and outstanding at December 31, 2017 and 2016, respectively. | 222 | 220 | ||||||
| Additional paid in capital | 31,325,044 | 31,171,843 | ||||||
| Stock payable | 21,000 | - | ||||||
| Accumulated deficit | (31,618,687 | ) | (30,765,519 | ) | ||||
| Total stockholders’ equity (deficit) | (272,421 | ) | 406,544 | |||||
| Total Liabilities and Stockholders’ Equity (Deficit) | $ | 95,344 | $ | 408,108 | ||||
See Summary of Significant Accounting Policies and Notes to Financial Statements.
| 31 |
E-QURE CORP.
For the Years Ended December 31, 2017 and 2016
| December 31, 2017 | December 31, 2016 | |||||||
| Revenues | $ | - | $ | - | ||||
| Expenses | ||||||||
| General and administrative | (628,717 | ) | (912,627 | ) | ||||
| Research and development | (224,451 | ) | (342,602 | ) | ||||
| Total | (853,168 | ) | (1,255,229 | ) | ||||
| (Loss) from operations | (853,168 | ) | (1,255,229 | ) | ||||
| Other income (expense) | ||||||||
| Gain on legal settlement | - | 281,400 | ||||||
| Total other income (expense) | (853,168 | ) | 281,400 | |||||
| Total expenses | (853,168 | ) | (973,829 | ) | ||||
| Loss from continuing operations before income tax | (853,168 | ) | (973,829 | ) | ||||
| Income tax | - | - | ||||||
| Net loss | $ | (853,168 | ) | $ | (973,829 | ) | ||
| Basic and diluted per share amount: | ||||||||
| Basic and diluted net loss | $ | (0.04 | ) | $ | (0.04 | ) | ||
| Weighted average shares outstanding (basic and diluted) | 22,169,754 | 22,012,562 | ||||||
See Summary of Significant Accounting Policies and Notes to Financial Statements.
| 32 |
E-QURE CORP.
Statement of Changes in Stockholders’ Equity
For the Years Ended December 31, 2017 and 2016
| Common Stock | Additional | Total Stockholders’ | ||||||||||||||||||||||||||
| Common | Subscription | Paid-in | | Stock | Accumulated | Equity | ||||||||||||||||||||||
| Shares | Amount | Receivable | Deficit | Payable | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance at December 31, 2015 | 22,012,562 | $ | 220 | $ | (100,000 | ) | $ | 30,770,545 | $ | - | $ | (29,791,690 | ) | $ | 879,075 | |||||||||||||
| Stock-based compensation | - | - | - | 401,298 | - | 401,298 | ||||||||||||||||||||||
| Loss on stock receivable | - | - | 100,000 | - | - | 100,000 | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | (973,829 | ) | (973,829 | ) | |||||||||||||||||||
| Balance at December 31, 2016 | 22,012,562 | $ | 220 | $ | - | $ | 31,171,843 | $ | - | $ | (30,765,519 | ) | $ | 406,544 | ||||||||||||||
| Stock-based compensation | - | - | - | 107,176 | - | - | 107,176 | |||||||||||||||||||||
| Shares for services | 225,000 | 2 | - | 39,125 | 21,000 | - | 60,128 | |||||||||||||||||||||
| Imputed interest | - | - | - | 6,900 | - | - | 6,900 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (853,168 | ) | (853,168 | ) | |||||||||||||||||||
| Balance at December 31, 2017 | 22,237,562 | $ | 222 | $ | - | $ | 31,325,044 | $ | 21,000 | $ | (31,618,687 | ) | $ | (272,421 | ) | |||||||||||||
See Summary of Significant Accounting Policies and Notes to Financial Statements.
| 33 |
E-QURE CORP.
For the Years Ended December 31, 2017 and 2016
| December 31, 2017 | December 31, 2016 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (853,168 | ) | $ | (973,829 | ) | ||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||
| Stock-based compensation | 167,303 | 401,298 | ||||||
| Imputed interest | 6,900 | - | ||||||
| Loss on stock receivable | - | 100,000 | ||||||
| Changes in assets and liabilities: | ||||||||
| Increase (decrease) in accounts payable and accrued expenses | 228,150 | - | ||||||
| (Increase) decrease in accounts receivable | 62,618 | (62,816 | ) | |||||
| (Increase) decrease in other assets | (63,382 | ) | - | |||||
| (Increase) decrease in prepaid expenses | 31,316 | (52,316 | ) | |||||
| Cash used in operating activities | (420,065 | ) | (587,663 | ) | ||||
| Cash flow from financing activities: | ||||||||
| Proceeds from borrowings of debt | 138,051 | - | ||||||
| Cash provided by financing activities | 138,051 | - | ||||||
| Change in cash | (282,014 | ) | (587,663 | ) | ||||
| Cash - beginning of year | 292,976 | 880,639 | ||||||
| Cash - end of year | $ | 10,962 | $ | 292,976 | ||||
See Summary of Significant Accounting Policies and Notes to Financial Statements.
| 34 |
E-Qure Corp.
December 31, 2017
1. The Company
Organizational Background:
Organizational Background: E-Qure Corp., (“EQUR” or the “Company”) is a Delaware corporation with a mailing address in New York, NY and offices in Israel.
Basis of Presentation: The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. The Company has not established any source of revenue to cover its operating costs, and as such, has incurred an operating loss since inception. Further, as of December 31, 2016, the cash resources of the Company were insufficient to meet its current business plan, and the Company had negative working capital. These and other factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
Significant Accounting Policies
Use of Estimates:
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from the estimates.
Cash and Cash Equivalents:
For financial statement presentation purposes, the Company considers those short-term, highly liquid investments with original maturities of three months or less to be cash or cash equivalents. There were no cash equivalents as of December 31, 2017 or 2016.
Accounts Receivable:
Receivable consist primarily of other receivables, which is a balance due from a service provider to the Company. The Company does not need to provide an allowance for doubtful receivables. The allowance for doubtful other receivables was $0 as of December 31, 2017 and 2016.
Prepaid Expenses
Prepaid expenses consist primarily of payments made for legal expenses to be earned and expensed during the following fiscal year.
Stock Based Compensation:
Stock-based awards are accounted for using the fair value method in accordance with ASC 718, Share-Based Payments. Our primary type of share-based compensation consists of stock options. We use the Black-Scholes option pricing model in valuing options. The inputs for the valuation analysis of the options include the market value of the Company’s common stock, the estimated volatility of the Company’s common stock, the exercise price of the warrants and the risk free interest rate.
| 35 |
Accounting For Obligations And Instruments Potentially To Be Settled In The Company’s Own Stock:
We account for obligations and instruments potentially to be settled in the Company’s stock in accordance with FASB ASC 815, Accounting for Derivative Financial Instruments. This issue addresses the initial balance sheet classification and measurement of contracts that are indexed to, and potentially settled in, the Company’s own stock.
Fair Value of Financial Instruments:
FASB ASC 825, “Financial Instruments,” requires entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet, for which it is practicable to estimate fair value. FASB ASC 825 defines fair value of a financial instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties. At December 31, 2017 and 2016, the carrying value of certain financial instruments (cash and cash equivalents, accounts payable and accrued expenses.) approximates fair value due to the short-term nature of the instruments or interest rates, which are comparable with current rates.
Fair Value Measurements:
The
Company measures fair value under a framework that utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques
used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical
assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). The three
levels of inputs which prioritize the inputs used in measuring fair value are:
Level
1: Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that
the Company has the ability to access.
Level 2: Inputs to the valuation methodology include:
- Quoted prices for similar assets or liabilities in active markets;
- Quoted prices for identical or similar assets or liabilities in inactive markets;
- Inputs other than quoted prices that are observable for the asset or liability;
- Inputs that are derived principally from or corroborated by observable market data by correlation
or other means.
If the asset or liability has a specified (contractual) term, the level 2 input must be observable
for substantially the full term of the asset or liability.
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value
measurement.
The assets or liability’s fair value measurement level within the fair value hierarchy is
based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize
the use of observable inputs and minimize the use of unobservable inputs. The following table presents assets that were measured
and recognize at fair value on December 31, 2017 and 2016 and the year then ended on a recurring basis:
Fair Value Measurements at December 31, 2017
| Quoted Prices in Active | Significant | |||||||||||||||
| Markets for Identical Assets | Other Observable Inputs | Significant Unobservable Inputs | ||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| None | $ | - | $ | - | $ | - | $ | - | ||||||||
| Total assets and liabilities at fair value | $ | - | $ | - | $ | - | $ | - |
Fair Value Measurements at December 31, 2016
| Quoted Prices in Active | Significant | |||||||||||||||
| Markets for Identical Assets | Other Observable Inputs | Significant Unobservable Inputs | ||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| None | $ | - | $ | - | $ | - | $ | - | ||||||||
| Total assets and liabilities at fair value | $ | - | $ | - | $ | - | $ | - |
| 36 |
When the Company changes its valuation inputs for measuring financial assets and liabilities at fair value, either due to changes in current market conditions or other factors, it may need to transfer those assets or liabilities to another level in the hierarchy based on the new inputs used. The Company recognizes these transfers at the end of the reporting period that the transfers occur. For the fiscal periods ended December 31, 2017 and 2016, there were no significant transfers of financial assets or financial liabilities between the hierarchy levels.
Earnings per Common Share:
We compute net income (loss) per share in accordance with ASC 260, Earning per Share. ASC 260 requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period using the treasury stock method and convertible preferred stock using the if-converted method. In computing Diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. Diluted EPS excludes all dilutive potential shares if their effect is anti-dilutive. All per share disclosures retroactively reflect shares outstanding or issuable as though the reverse split had occurred January 1, 2011.
Income Taxes:
We have adopted ASC 740, Accounting for Income Taxes. Pursuant to ASC 740, we are required to compute tax asset benefits for net operating losses carried forward. The potential benefits of net operating losses have not been recognized in these financial statements because the Company cannot be assured it is more likely than not it will utilize the net operating losses carried forward in future years.
We must make certain estimates and judgments in determining income tax expense for financial statement purposes. These estimates and judgments occur in the calculation of certain tax assets and liabilities, which arise from differences in the timing of recognition of revenue and expense for tax and financial statement purposes.
Deferred tax assets and liabilities are determined based on the differences between financial reporting and the tax basis of assets and liabilities using the tax rates and laws in effect when the differences are expected to reverse. ASC 740 provides for the recognition of deferred tax assets if realization of such assets is more likely than not to occur. Realization of our net deferred tax assets is dependent upon our generating sufficient taxable income in future years in appropriate tax jurisdictions to realize benefit from the reversal of temporary differences and from net operating loss, or NOL, carryforwards. We have determined it more likely than not that these timing differences will not materialize and have provided a valuation allowance against substantially all of our net deferred tax asset.
Management will continue to evaluate the realizability of the deferred tax asset and its related valuation allowance. If our assessment of the deferred tax assets or the corresponding valuation allowance were to change, we would record the related adjustment to income during the period in which we make the determination. Our tax rate may also vary based on our results and the mix of income or loss in domestic and foreign tax jurisdictions in which we operate.
In addition, the calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations. We recognize liabilities for anticipated tax audit issues in the U.S. and other tax jurisdictions based on our estimate of whether, and to the extent to which, additional taxes will be due. If we ultimately determine that payment of these amounts is unnecessary, we will reverse the liability and recognize a tax benefit during the period in which we determine that the liability is no longer necessary. We will record an additional charge in our provision for taxes in the period in which we determine that the recorded tax liability is less than we expect the ultimate assessment to be.
| 37 |
ASC 740 which requires recognition of estimated income taxes payable or refundable on income tax returns for the current year and for the estimated future tax effect attributable to temporary differences and carry-forwards. Measurement of deferred income tax is based on enacted tax laws including tax rates, with the measurement of deferred income tax assets being reduced by available tax benefits not expected to be realized.
Uncertain Tax Positions:
The Financial Accounting Standards Board issued Interpretation No. 740, “Accounting for Uncertainty in Income Taxes – an interpretation of FASB Statement No. 109, Accounting for Income Taxes” (“FIN No. 740”) which was effective for the Company on January 1, 2007. ASC No. 740 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC No. 740, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The tax benefits recognized in the financial statements from such position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC No. 740 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods and disclosure requirements.
Our federal and state income tax returns are open for fiscal years ending on or after December 31, 2008. We are not under examination by any jurisdiction for any tax year. At December 31, 2017, we had no material unrecognized tax benefits and no adjustments to liabilities or operations were required under ASC 740.
Recently Issued Accounting Pronouncements
In May 2017, the FASB issued Update 2017-09 - Compensation - Stock Compensation (Topic 718): Effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period, for (1) public business entities for reporting periods for which financial statements have not yet been issued and (2) all other entities for reporting periods for which financial statements have not yet been made available for issuance. Early adoption is permitted. This adoption is not expected to have a material impact on our financial position or results of operations.
In February 2017, FASB issued Update 2017-06 - Plan Accounting: Defined Benefit Pension Plans (Topic 960), Defined Contribution Pension Plans (Topic 962), Health and Welfare Benefit Plans (Topic 965): Employee Benefit Plan Master Trust Reporting (a consensus of the Emerging Issues Task Force). Under Topic 960, investments in master trusts are presented in a single line item in the statement of net assets available for benefits. Similar guidance is not provided in Topic 962 or 965, which has resulted in diversity in practice. For each master trust in which a plan holds an interest, the amendments in this Update require a plan’s interest in that master trust and any change in that interest to be presented in separate line items in the statement of net assets available for benefits and in the statement of changes in net assets available for benefits, respectively. Topics 960 and 962 require plans to disclose their percentage interest in the master trust and a list of the investments held by the master trust, presented by general type, within the plan’s financial statements. Stakeholders said that the disclosure can be misleading when the plan has a divided interest in the individual investments of the master trust (that is, when the plan has a specific, rather than a proportionate, interest in the master trust). The amendments in this Update remove the requirement to disclose the percentage interest in the master trust for plans with divided interests and require that all plans disclose the dollar amount of their interest in each of those general types of investments, which supplements the existing requirement to disclose the master trust’s balances in each general type of investments. Early adoption is permitted. This adoption is not expected to have a material impact on our financial position or results of operations.
In the opinion of management, the information furnished in these interim financial statements reflects all adjustments necessary for a fair statement of the financial position and results of operations and cash flows as of and for the twelve-month periods ended December 31, 2017 and 2016. All such adjustments are of a normal recurring nature.
| 38 |
Management does not anticipate that the adoption of these standards will have a material impact on the financial statements.
2. Stockholders’ Equity
Common Stock
We are currently authorized to issue up to 500,000,000 shares of $0.00001 par value common stock. All issued shares of common stock are entitled to vote on a 1 share/1 vote basis. On May 12, 2014 the Board approved a 1 for 100 reverse split of the common stock. In conjunction with the reverse split the Company domiciled from New Jersey to Delaware.
Issuances of Common Stock and Common Stock Receivable in 2016:
During the year ended December 31, 2016, we did not issue any shares of Common Stock. The Company decided to write off its remaining outstanding balance of $100,000 in stock receivable because it was deemed to be uncollectible.
Issuances of Common Stock in 2017:
On April 20, 2017, we issued 225,000 shares valued at $39,128 to two consultants for services provided. During the three months ended June 30, 2017, we authorized the issuance of 75,000 additional shares to the same two consultants valued at $0.14 or $21,000, which was recorded as stock payable.
Preferred Stock
We are currently authorized to issue up to 25,000,000 shares of $0.00001 par value preferred stock. Effective December 31, 2007 the board of directors approved the cancellation of all previously issued preferred shares and approved the cancellation and extinguishment of all common and preferred share conversion rights of any kind, including without limitation, warrants, options, convertible debt instruments and convertible preferred stock of every series and accompanying conversion rights of any kind. There are no preferred shares outstanding as of December 31, 2017 and 2016.
Stock Options
On January 1, 2015, the Company authorized the adoption of the 2015 Employee Incentive Plan.
Stock Option Grants
On January 1, 2015, the board of director approved the 2015 Employee Incentive Plan. The total number of shares of Common Stock reserved for issuance by the Company either directly as Stock Awards or underlying Options granted under this Plan is 5,000,000 shares of Common Stock. On January 1, 2015, the Company granted options as follows under its 2015 Employee Incentive Plan: (i) Professor Ohry was granted options to purchase 250,000 shares of the Registrant’s common stock (“Option Shares”) at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Ohry SAB Agreement”). Provided the Ohry SAB Agreement remains in effect, 75,000 shares shall vest July 1, 2015, and the remaining 175,000 Option Shares shall vest at the rate of 25,000 Option Shares per quarter on the first day of each consecutive quarter; (ii) Dr. Ben Zion Weiner was granted options to purchase 350,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Weiner SAB Agreement”). Provided the Weiner SAB Agreement remains in effect, 105,000 Option Shares shall vest July 1, 2015 and the remaining 245,000 Option Shares shall vest at the rate of 35,000 Option Shares per quarter on the first day of each consecutive quarter; and (iii) Michel Sessler was granted options to purchase 150,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Sessler SAB Agreement”). Provided the Sessler SAB Agreement remains in effect, 45,000 Option Shares shall vest July 1, 2015 and the remaining 105,000 Option Shares shall vest at the rate of 15,000 Option Shares per quarter on the first day of each consecutive quarter.
| 39 |
Following is a table summarizing options still outstanding and exercisable along with exercise price and range of remaining term.
| Type | Quantity | Exercise Price | Term | |||||||
| Avi Ohry | 250,000 | $ | 1.00 | 24 Months | ||||||
| Dr. Ben Zion Weiner | 350,000 | $ | 1.00 | 24 Months | ||||||
| Michael Sessler | 150,000 | $ | 1.00 | 24 Months | ||||||
| Total | 750,000 | |||||||||
During the years ended December 31, 2017 and 2016, we expensed $107,176 and $401,298,respectively, in relation the options granted above.
3. Notes Payable
During the year ended December 31, 2017, the Company issued three notes for a total of $138,051, two of which were issued to related parties. The notes are due on demand and bear no interest rate. As such, the imputed interest is calculated and included under additional paid-in capital. As of December 31, 2017, the Company has recorded $6,900 in imputed interest.
| December 31, 2017 | December 31, 2016 | |||||||
| Roni Weisberg, Chairman | $ | 57,172 | - | |||||
| Itsik Ben Yesha, CTO | $ | 44,005 | - | |||||
| Michael Cohen | $ | 36,874 | - | |||||
4. Other Assets
As of December 31, 2017 and 2016, the Company recorded $63,382 and $0, respectively, as other assets representing securities compliance services to be repaid in cash or securities compliance services pursuant to an arrangement with the Company’s securities compliance consultant.
5. Related Party Transactions
On January 1, 2015, the board of director approved the 2015 Employee Incentive Plan. The total number of shares of Common Stock reserved for issuance by the Company either directly as Stock Awards or underlying Options granted under this Plan is 5,000,000 shares of Common Stock. On January 1, 2015, the Company granted options as follows under its 2015 Employee Incentive Plan: (i) Professor Ohry was granted options to purchase 250,000 shares of the Registrant’s common stock (“Option Shares”) at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Ohry SAB Agreement”). Provided the Ohry SAB Agreement remains in effect, 75,000 shares shall vest July 1, 2015, and the remaining 175,000 Option Shares shall vest at the rate of 25,000 Option Shares per quarter on the first day of each consecutive quarter; (ii) Dr. Ben Zion Weiner was granted options to purchase 350,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Weiner SAB Agreement”). Provided the Weiner SAB Agreement remains in effect, 105,000 Option Shares shall vest July 1, 2015 and the remaining 245,000 Option Shares shall vest at the rate of 35,000 Option Shares per quarter on the first day of each consecutive quarter; and (iii) Michel Sessler was granted options to purchase 150,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Sessler SAB Agreement”). Provided the Sessler SAB Agreement remains in effect, 45,000 Option Shares shall vest July 1, 2015 and the remaining 105,000 Option Shares shall vest at the rate of 15,000 Option Shares per quarter on the first day of each consecutive quarter. See also Note 2 above. We expensed $107,176 and $401,298, respectively, in relation to the option granted.
| 40 |
As of December 31, 2017 and 2016, we had accrued interest of $1,564 due to Mr. Weissberg, who is the Company’s Chairman of the audit committee. The principal underlying the note was converted in 2014. As of December 31, 2017 and 2016, we had accrued salaries of $228,150 and $0, respectively, due to three of our officers.
6. Prepaid Expenses
As of December 31, 2017 and 2016, the Company had $21,000 and $52,316 in prepaid expenses representing prepaid securities compliance services pursuant to an arrangement with the Company’s securities compliance services provider.
| December 31 | ||||||||
| 2017 | 2016 | |||||||
| Legal expenses | $ | 21,000 | $ | 52,316 | ||||
| Total prepaid expenses | $ | 21,000 | $ | 52,316 | ||||
7. Taxes
We have adopted ASC 740 which provides for the recognition of a deferred tax asset based upon the value the loss carry-forwards will have to reduce future income taxes and management’s estimate of the probability of the realization of these tax benefits. Our net operating loss carryovers incurred prior to 2008 considered available to reduce future income taxes were reduced or eliminated through our recent change of control (I.R.C. Section 382(a)) and the continuity of business limitation of I.R.C. Section 382(c).
We have a current operating loss carry-forward of $2,028,871 resulting in deferred tax assets of $710,105. We have determined it more likely than not that these timing differences will not materialize and have provided a valuation allowance against substantially all our net deferred tax asset.
Future utilization of currently generated federal and state NOL and tax credit carry forwards may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended and similar state provisions. The annual limitation may result in the expiration of NOL and tax credit carry forwards before full utilization.
| December 31 | ||||||||
| 2017 | 2016 | |||||||
| Individual components giving rise to the deferred tax assets are as follows: | $ | $ | ||||||
| Future tax benefit arising from net operating loss carryovers | 710,105 | 1,282,879 | ||||||
| Less valuation allowance | (710,105 | ) | (1,282,879 | ) | ||||
| Net deferred asset | $ | - | $ | - | ||||
The Company is not under examination by any jurisdiction for any tax year. Our federal and state income tax returns are open for fiscal years ending on or after December 31, 2008.
8. Going Concern
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. The Company has not established any source of revenue to cover its operating costs, and as such, has incurred an operating loss since inception. These and other factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
| 41 |
9. Commitments and Contingencies
During 2016, the Company had filed a lawsuit against one of its suppliers for lack of services performed. During October 2016, the Company signed with the supplier a confidential settlement agreement. Pursuant to the agreement, the supplier was obligated to pay the Company $281,400, which was paid during October 2016.
10. Subsequent Events
There were no subsequent events following the year ended December, 31, 2017 through the date the financial statements were issued that would materially affect the financial statements.
| 42 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of December 31, 2017, the Company’s chief executive officer and chief financial officer conducted an evaluation regarding the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) under the Exchange Act. Based upon the evaluation of these controls and procedures, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were ineffective as of the end of the fiscal year 2017 under the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013).
Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15. Internal control over financial reporting is defined in Rule 13a-15(f) and 15(d)-15(f) under the Exchange Act as a process designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements. Management conducted an assessment of the Company’s internal control over financial reporting as of December 31, 2017 based on the framework and criteria established by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Based on the assessment, management concluded that, as of December 31, 2017, the Company’s internal control over financial reporting was not effective based on those criteria. Management has identified corrective actions for the weakness and has begun implementation during 2018.
The Company recognizes the following weaknesses and deficiencies of the Company as of December 31, 2017:
| 43 |
We recognized the following deficiencies that we believe to be material weaknesses:
- The Company has not fully designed, implemented or assessed internal controls over financial reporting as a result of which management assessed and concluded that internal controls were ineffective this year.
We recognized the following deficiencies that we believe to be significant deficiencies:
-
The Company has no formal control process related to the identification and approval of related party transactions.
- No formal written policy for the approval, identification and authorization of related party transactions
currently exists.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
This annual report does not include an attestation report of the company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only Management’s report in this annual report.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting or in other factors identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during the fourth quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
ITEM 10. DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT AND CORPORATE GOVERNANCE
Our directors were elected to serve until the next annual meeting of shareholders and until his respective successors will have been elected and will have qualified. The following table sets forth the name, age and position held with respect to our present director and executive officer:
| Name | Age | Title | Executive Officer Since | |||
| Ohad Goren | 47 | Chief Executive Officer | 06/2014 | |||
| Gal Peleg | 40 | Chief Financial Officer | 08/2014 | |||
| Ron Weissberg | 59 | Chairman | 12/2013 | |||
| Itsik Ben Yesha | 65 | Chief Technology Officer | 06/2014 | |||
| Dr. Michael Sessler | 59 | Director | 08/2014 |
Ohad Goren, age 47, Chief Executive Officer. During the past five years, Mr. Goren has served in the following positions: from 2011 through 2013 Mr. Goren served as an advisor to several biot-ech and high-tech startup companies. During 2010 and 2011, Mr. Goren served as CEO of Pollogen Ltd., a private Israeli company engaged in the development, manufacture and marketing of medical aesthetic devices with sales in Europe, North and South America, and major Asian countries. Prior to joining Pollogen Ltd., during 2008 to 2009, Mr. Goren served as a consultant to start-up companies principally in the biotechnology and hi-tech industries. From 2005 through 2008, Mr. Goren was the CEO of Lifewave Ltd., a public Israeli company, where he had responsibility for Lifewave’s IPO, overseeing the development of its chronic wound treatment device and Lifewave’s regulatory compliance, among other duties. From 1997 to 2005 Mr. Goren served as the Marketing and Sales Manager of the Services Division of Oracle Corp., in Israel. From 1990 to 1997 Mr. Goren served as the Deputy Consul at the Israeli Embassy, Washington, D.C. Mr. Goren received his BA degree and his MBA degree from the University of Maryland, in the U.S.
| 44 |
Gal Peleg, age 40, Chief Financial Officer. On August 21, 2014, the Registrant appointed Mr. Gal Peleg as Chief Financial Officer, replacing Ron Weissberg, the Registrant’s Chairman and principal stockholder, who had served as interim CFO since December 27, 2013. Mr. Weissberg will continue to serve as Chairman of the Board of Directors.
Mr. Peleg is a Certified Public Accountant. From 2007 to the present, Mr. Peleg served as Chief Financial Officer of Medical Life-Wave, a medical device company that was listed on the Tel-Aviv Stock Exchange (“TASE”). He was responsible for quarterly, annual and other reports filed with TASE, compliance with regulatory protocol and the rules and regulations under the Israeli securities laws. From January 2006 through October 2007, Mr. Peleg served as controller of Tescom Software System Ltd, a TASE listed, software testing company with worldwide operations and offices in Israel, United States and Singaport. From 2004 to 2006, Mr. Peleg served as controller of Internet Gold, a majority stockholder of BCOM Ltd., an Israeli company listed on NASDAQ and TASE. From 2001 to 2004, Mr. Peleg worked at Ernst & Young as Senior, Bio-Tech/Medical Device Section, providing audit services in accordance with US GAAP and Israeli GAAP.
Ron Weissberg, age 59, Chairman of the Board since December 27, 2013. From December 27, 2013 until June 5, 2014, Mr. Weissberg served as the Registrant’s CEO, on which latter date the Board of Directors appointed Ohad Goren as the CEO. In addition, from December 27, 2013 until August 21, 2014, Mr. Weissberg also served as the Registrant’s CFO, on which latter date the Board of Directors appointed Mr. Gal Peleg as CFO.
From May 2011 to the present, Mr. Weissberg has served as an executive officer and director of Bio-Light Israeli Life Sciences Investments Ltd, a public company listed on the Tel-Aviv Stock Exchange, engaged in the business of biomed innovation. From May 2003 to the present, Mr. Weissberg has been a director of Midroog Ltd., an Israeli Credit Rating Agency, a company engaged in the business of credit rating for the domestic Israeli market. From February 1988 to the present, Mr. Weissberg has been a director of The Israel Land Development Company Ltd (“ILDC”), a public company listed on the TASE, engaged in the business of owning and managing commercial real estate in Israel, Europe and Canada.
Since 2011, Mr. Weissberg has been ILDC’s Vice-Chairman. From 1994 until 2006, he was chairman of ILD Insurance Company Ltd, a public company engaged in the business of underwriting property, casualty and life insurance with total portfolio of approximately US$1 billion and 500 employees. ILD Insurance is listed on the TASE. From 1996 to 2000, Mr. Weissberg also served as its CEO. From June 2008 until October 2010, he was the CEO and a director of Portfolio Green Ltd., a public company listed on the TASE engaged in the business of Real Estate Development in the U.S.
The Registrant believes that Mr. Weissberg’s many years of experience as a senior executive officer and director of several successful public companies in a variety in industries, all of which have had far greater resources and operating history than the Registrant, together with his material equity position in the Registrant, renders him highly qualified to serve on the Registrant’s Board of Directors.
Itsik Ben Yesha, age 65, Chief Technology Officer and was a founder and a principal and involved in the Registrant’s efforts leading to the negotiations and closing of the above-referenced Patent Purchase Agreement. From 1991 through 2013, Mr. Ben Yesha was the founder and a partner of Hisense Ltd., an Israeli medical device company for respiratory monitoring devices for infants. He previously served as Executive Vice President of Lifewave Ltd. From 1998 to 2003 Mr. Ben Yesha was the Executive Vice President and VPL Division President with Valor Computerized Systems Ltd., a CAD/CAM Software company listed on the Frankfurt Stock Exchange and later acquired by Mentor Graphics (NASDAQ: MENT). From 1979 to 1997, Mr. Ben Yesha served in Tadiran Telecom Group in various rolls, starting as a R&D engineer, designing computerized electronic exchanges (Tadex, Coral), and finally serving as the CFO of Tadiran Wireless Telecom division, bringing it from $0 to $50 million in annual sales within 3 years.
| 45 |
Dr. Michael Sessler, age 59, Director. Dr. During the last 22 years, Dr. Sessler has been actively engaged in business development. From 1992 through 2009, Dr. Sessler was the director of business development for the Electra Consumer Products subsidiary of Elco Group (ELCO), a listed public company on the Tel-Aviv Stock Exchange (“TASE”) with total assets in excess of $1.3 billion and net revenues in excess of $1.9 billion in 2013. During his tenure at ELCO, Dr. Sessler also established and managed new ELCO businesses in Europe, Asia, South America and Australia and also served as Senior Vice President of Electra Group until 2009.
Since 2009, Dr. Sessler has been a managing director of Allsons Ltd., an investment company with assets in Europe and Israel. During the past 5 years, Dr. Sessler has also been member of the board of directors of The Leser Group Ltd., a private New York-based real-estate development company with bonds traded on the TASE and over $130 million in assets.
Dr. Sessler is a qualified and experienced BOD member due to his experience in developing and managing major subsidiaries of public companies, working in challenging domestic and international markets, as well as his experience in introducing new products to a wide variety of markets, together with his ability to manage and oversee manufacturing control processes. In the opinion of the Company’s management and board of directors, Dr. Sessler is a highly qualified professional to serve as a member of the Company’s board of directors.
Dr. Sessler is a medical doctor, having received is MD from the Medical University of Rome, Italy under the guidance of Prof. Rita Levi Montalccini, Nobel Prize winner in Physiology/Medicine in 1986.
Director Independence. In determining whether or not our directors are considered independent, the Company used the definition of independence as defined in NASDAQ Rule 4200. Based on that definition we believe that Dr. Michael Sessler is independent.
NASDAQ Rule 4200. The NASDAQ Rule 4200, which sets forth several tests to determine whether a director of a listed company is independent. Rule 4200 provides that a director would not be considered independent if the director or an immediate family member accepted any compensation from the listed company in excess of $120,000 during any period of 12 consecutive months within the three years preceding the determination of independence (excluding compensation for board or board committee service, compensation paid to an immediate family member as a non-executive employee, benefits paid under a tax-qualified retirement plan and non-discretionary compensation).
Directors’ Term of Office. Our directors are elected to serve until the next annual meeting of shareholders and until their respective successors will have been elected and will have qualified.
Audit Committee and Financial Expert, Compensation Committee, Nominations Committee. We do not have any of the above mentioned standing committees because our corporate financial affairs and corporate governance are simple in nature at this stage of development and each financial transaction is approved by our sole officer or director.
Code of Ethics. We do not currently have a Code of Ethics applicable to our principal executive officers; however, the Company plans to implement such a code in the third quarter of 2018.
Potential Conflicts of Interest. Since we do not have an audit or compensation committee comprised of independent Directors, the functions that would have been performed by such committees are performed by our Board of Directors. Thus, there is a potential conflict of interest in that our Directors have the authority to determine issues concerning management compensation, in essence their own, and audit issues that may affect management decisions. We are not aware of any other conflicts of interest with any of our Executives or Directors.
| 46 |
Board’s Role in Risk Oversight. The Board assesses on an ongoing basis the risks faced by the Company. These risks include financial, technological, competitive, and operational risks. In addition, since the Company does not have an Audit Committee, the Board is also responsible for the assessment and oversight of the Company’s financial risk exposures.
Involvement in Certain Legal Proceedings. We are not aware of any material legal proceedings that have occurred within the past ten years concerning any Director or control person which involved a criminal conviction, a pending criminal proceeding, a pending or concluded administrative or civil proceeding limiting one’s participation in the securities or banking industries, or a finding of securities or commodities law violations.
Section 16(a) Compliance. Section 16(a) of the Securities and Exchange Act of 1934 requires the Registrant’s directors and executive officers, and persons who own beneficially more than ten percent (10%) of the Registrant’s Common Stock, to file reports of ownership and changes of ownership with the Securities and Exchange Commission. Copies of all filed reports are required to be furnished to the Registrant pursuant to Section 16(a). Based solely on the reports received by the Registrant and on written representations from reporting persons, the Registrant was informed that its executive officers, directors and ten percent (10%) shareholders have not filed reports required to be filed under Section 16(a).
ITEM 11. EXECUTIVE COMPENSATION
The following table sets forth information concerning the total compensation that we have paid or that has accrued on behalf of our chief executive officer and other executive officers during the fiscal years ending December 31, 2017, 2016 and 2015.
| Long Term | ||||||||||||||||||||||||||||
| Annual Compensation | Compensation Awards | |||||||||||||||||||||||||||
| Other | Restricted | Securities | ||||||||||||||||||||||||||
| Annual | Stock | Underlying | All Other | |||||||||||||||||||||||||
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Compensation ($) | Award(s) ($) | Options ($) | Compensation ($) | |||||||||||||||||||||
| Ohad Goren, CEO | 2017 | 140,400 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
| 2016 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| Gal Peleg, CFO | 2017 | 17,550 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
| 2016 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| Itsik Ben Yesha, CTO | 2017 | 72,500 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
| 2016 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
Option Grants
On January 1, 2015, the board of director approved the 2015 Employee Incentive Plan. The total number of shares of Common Stock reserved for issuance by the Company either directly as Stock Awards or underlying Options granted under this Plan is 5,000,000 shares of Common Stock. On January 1, 2015, the Company granted options as follows under its 2015 Employee Incentive Plan: (i) Professor Ohry was granted options to purchase 250,000 shares of the Registrant’s common stock (“Option Shares”) at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Ohry SAB Agreement”). Provided the Ohry SAB Agreement remains in effect, 75,000 shares shall vest July 1, 2015, and the remaining 175,000 Option Shares shall vest at the rate of 25,000 Option Shares per quarter on the first day of each consecutive quarter; (ii) Dr. Ben Zion Weiner was granted options to purchase 350,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Weiner SAB Agreement”). Provided the Weiner SAB Agreement remains in effect, 105,000 Option Shares shall vest July 1, 2015 and the remaining 245,000 Option Shares shall vest at the rate of 35,000 Option Shares per quarter on the first day of each consecutive quarter; and (iii) Michel Sessler was granted options to purchase 150,000 Option Shares at an exercise price equal to one dollar ($1.00) per Option Share. The Option Shares shall vest pursuant to the terms of a Scientific Advisory Board Agreement dated January 1, 2015 (the “Sessler SAB Agreement”). Provided the Sessler SAB Agreement remains in effect, 45,000 Option Shares shall vest July 1, 2015 and the remaining 105,000 Option Shares shall vest at the rate of 15,000 Option Shares per quarter on the first day of each consecutive quarter.
| 47 |
Following is a table summarizing options still outstanding and exercisable along with exercise price and range of remaining term.
| Type | Quantity | Exercise Price | Term | |||||||
| Avi Ohry | 250,000 | $ | 1.00 | 24 Months | ||||||
| Dr. Ben Zion Weiner | 350,000 | $ | 1.00 | 24 Months | ||||||
| Michael Sessler | 150,000 | $ | 1.00 | 24 Months | ||||||
| Total | 750,000 | |||||||||
Aggregated Option Exercises and Fiscal Year-End Option Value
There were no stock options exercised during the years ended December 31, 2017 and 2016 by the executive officers named in the Summary Compensation Table.
Long-Term Incentive Plan (“LTIP”) Awards
There were no awards made to a named executive officers in the last completed fiscal year under any LTIP.
Compensation of Directors
Directors are permitted to receive fixed fees and other compensation for their services as directors. The Board of Directors has the authority to fix the compensation of directors. No amounts have been paid to, or accrued to, directors in such capacity.
Employment Agreements
We have employment agreement in place with our CEO, CFO and CTO.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDER MATTERS
The table below discloses any person (including any “group”) who is known to the Registrant to be the beneficial owner of more than five (5%) percent of the Registrant’s Common Stock securities and the beneficial ownership of Registrant’s director and executive officer. As of April 16, 2018, the Registrant had 22,237,562 shares of Common Stock issued and outstanding.
| 48 |
| Name of Beneficial Owner | Common Stock Beneficially Owned | Percentage of Common Stock Owned | ||||||
| Ohad Goren, CEO | ||||||||
| 31 Nahal Ga’aton Street, Modiin, 71700, Israel | 1,150,000 | 5.17 | % | |||||
| Gal Peleg, CFO | ||||||||
| 11 Yosef Nakar Street, Petach Tikva, Israel | 0 | 0 | % | |||||
| Ron Weissberg, Chairman | ||||||||
| 7 Hamitnachalim Street, Savyon, Israel | 3,545,624 | 15.94 | % | |||||
| Itsik Ben Yesha, Chief Technology Officer | ||||||||
| 126 Alon Street, Shilat, 73188, Israel | 1,600,000 | 7.27 | % | |||||
| Dr. Michael Sessler, Director | ||||||||
| 50 Hagiva Street, Savyon, Israel | 0 | 0 | % | |||||
| Yochanan Korman, Shareholder | ||||||||
| 13 Haprahim Street, Ramat Hasharon, Israel | 1,523,541 | 7.19 | % | |||||
| Director and Officer (4 people) | 6,290,786 | 28.29 | % | |||||
(1) Applicable percentage ownership is based on 22,012,745 shares of Common Stock outstanding as of April 16, 2018. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of Common Stock that are currently exercisable or exercisable within 60 days of April 16, 2018 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTORS INDEPENDENCE
Certain Related Party Transactions During the Last Two Fiscal Years
There have been no related party transactions during the last two fiscal years.
Indebtedness of Management
No officer, director or security holder known to us to own of record or beneficially more than 5% of our Common Stock or any member of the immediate family or sharing the household (other than a tenant or employee) of any of the foregoing persons is indebted to us.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The Registrant’s Board of Directors has appointed M&K CPAS, PLLC (“MK”) as independent public accountant for the fiscal year ended December 31, 2017 and 2016.
Principal Accounting Fees
The following table presents the fees for professional audit services rendered by MK for the audit of the Registrant’s annual financial statements for the years ended December 31, 2017 and 2016, and fees billed for other services rendered by MK during those periods.
| Year Ended | Year Ended | |||||||
| December 31, 2017 | December 31, 2016 | |||||||
| Audit fees (1) | $ | 9,300 | $ | 8,800 | ||||
| Audit-related fees (2) | — | — | ||||||
| Tax fees (3) | — | — | ||||||
| All other fees | — | — | ||||||
(1) Audit fees consist of audit and review services, consents and review of documents filed with the SEC.
(2) Audit-related fees consist of assistance and discussion concerning financial accounting and reporting standards and other accounting issues.
(3) Tax fees consist of preparation of federal and state tax returns, review of quarterly estimated tax payments, and consultation concerning tax compliance issues.
| 49 |
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as exhibits to this report on Form 10-K or incorporated by reference herein. Any document incorporated by reference is identified by a parenthetical reference to the SEC filing that included such document.
| 50 |
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| E-Qure Corp. | ||
| Date: April 16, 2018 | By: | /s/ Ohad Goren |
| Name: | ||
| Title: | Chief Executive Officer, Principal Accounting and Financial Officer and Director | |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Date: April 16, 2018 | By: | /s/ Gal Paleg |
| Name: | ||
| Title: | Chief Executive Officer, Principal Accounting and Financial Officer and Director |
| 51 |
