Attached files
| file | filename |
|---|---|
| EX-21.1 - EX-21.1 - Sientra, Inc. | sien-ex211_205.htm |
| EX-32.2 - EX-32.2 - Sientra, Inc. | sien-ex322_147.htm |
| EX-32.1 - EX-32.1 - Sientra, Inc. | sien-ex321_148.htm |
| EX-31.2 - EX-31.2 - Sientra, Inc. | sien-ex312_149.htm |
| EX-31.1 - EX-31.1 - Sientra, Inc. | sien-ex311_150.htm |
| EX-23.1 - EX-23.1 - Sientra, Inc. | sien-ex231_375.htm |
| EX-10.33 - EX-10.33 - Sientra, Inc. | sien-ex1033_446.htm |
| EX-10.32 - EX-10.32 - Sientra, Inc. | sien-ex1032_376.htm |
| EX-10.31 - EX-10.31 - Sientra, Inc. | sien-ex1031_377.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 001-36709
SIENTRA, INC.
(Exact Name of Registrant as Specified in its Charter)
|
Delaware |
|
20-5551000 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
(I.R.S. Employer Identification No.) |
|
420 South Fairview Avenue, Suite 200, Santa Barbara, California |
|
93117 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
(805) 562-3500
(Registrant’s Telephone Number, Including Area Code)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☒ |
|
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
☐ |
|
Smaller reporting company |
|
☐ |
|
(Do not check if a smaller reporting company) |
Emerging growth company |
|
☒ |
|||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of registrant's common stock held by non-affiliates of the registrant, based upon the closing price of a share of the registrant's common stock on June 30, 2017 as reported by NASDAQ Global Select Market on such date was approximately $108,772,000. The determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 9, 2018, there were 19,643,517 shares of the registrant’s common stock, par value $0.01 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement relating to its 2018 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. Such Proxy Statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
“Sientra”, “Allox”, “Allox2”, “BIOCORNEUM”, “Dermaspan”, “Softspan”, “Silishield”, “miraDry”, “Miramar Labs”, “miraDry and Design”, “miraDry Fresh”, “The Sweat Stops Here”, “Drop Design”, “miraWave”, “miraSmooth”, “miraFresh”, and “ML Stylized mark” are trademarks of our company. Our logo and our other trade names, trademarks and service marks appearing in this document are our property. Other trade names, trademarks and service marks appearing in this document are the property of their respective owners. Solely for convenience, our trademarks and trade names referred to in the document, appear without the TM or the (R) symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the rights of the applicable licensor to these trademarks and trade names.
1
SPECIAL NOTE REGARDING FORWARD‑LOOKING STATEMENTS
This Annual Report on Form 10‑K, or Annual Report contains forward‑looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Forward‑looking statements are often identified by the use of words such as, “anticipate,” “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes to identify these forward‑looking statements. These statements are based on the beliefs and assumptions of our management based on information currently available to management. Forward‑looking statements in this Annual Report on Form 10‑K include, but are not limited to, statements about:
|
|
• |
the timing and availability of alternative manufacturing sources and our ability to supply our silicone gel breast implants, tissue expanders and other products to our customers; |
|
|
• |
the success of our market reentry plan in light of limited inventory; |
|
|
• |
our ability to achieve profitability; |
|
|
• |
our ability to generate significant net sales through the sale of our silicone gel breast implants and other products; |
|
|
• |
the ability of our products to achieve and maintain market acceptance; |
|
|
• |
our ability to successfully commercialize our products; |
|
|
• |
our ability to comply with the applicable governmental regulations to which our products and operations are subject; |
|
|
• |
our ability to successfully integrate new products into our portfolio; |
|
|
• |
our ability to retain a high percentage of our customer base; |
|
|
• |
plans regarding the expansion of our sales force and marketing programs; |
|
|
• |
the productivity of our sales representatives and ability to achieve expected growth; |
|
|
• |
our assumptions about the breast implant market; |
|
|
• |
our ability to protect our intellectual property; |
|
|
• |
our ability to successfully defend against lawsuits filed against us and our officers; and |
|
|
• |
our estimates regarding expenses, net sales, capital requirements and needs for additional financing. |
These forward‑looking statements involve risks and uncertainties as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially from those expressed or implied by such forward‑looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section titled “Risk Factors” included under Part I, Item 1A below. You should read these factors and the other cautionary statements made in this Annual Report as being applicable to all related forward-looking statements wherever they appear in this Annual Report. We caution you that the risks, uncertainties and other factors referenced above may not contain all of the risks, uncertainties and other factors that may impact the results and timing of certain events to differ materially from those expressed or implied in forward-looking statements. In addition, we cannot guarantee future results, level of activity, performance or achievements. Any forward‑looking statement made by us in this Annual Report speaks only as of the date of this Annual Report. Except as required by law, we undertake no obligation to update any forward‑looking statements, whether as a result of new information, future events or otherwise, after the date of such statements.
2
Overview
Sientra, Inc. (“Sientra”, the “Company,” “we,” “our” or “us”) is a medical aesthetics company committed to making a difference in patients’ lives by enhancing their body image, growing their self‑esteem and restoring their confidence. We were founded to provide greater choices to board‑certified plastic surgeons and patients in need of medical aesthetics products. We have developed a broad portfolio of products with technologically differentiated characteristics, supported by independent laboratory testing and strong clinical trial outcomes. We sell our breast implants and tissue expanders exclusively to board‑certified and board‑admissible plastic surgeons and tailor our customer service offerings to their specific needs, which we believe helps secure their loyalty and confidence. We began selling BIOCORNEUM, an advanced silicone scar treatment directly to physicians after we acquired BIOCORNEUM from Enaltus LLC, or Enaltus, in March 2016. Additionally, we began selling the AlloX2, and Dermaspan lines of breast tissue expanders, as well as the Softspan line of general tissue expanders, after we acquired these product lines from Specialty Surgical Products, Inc., or SSP, in November 2016.
On June 11, 2017, we entered into a Merger Agreement with miraDry (formerly Miramar Labs), pursuant to which we commenced a tender offer to purchase all of the outstanding shares of miraDry’s common stock. Pursuant to the transaction, which closed on July 25, 2017 we added the miraDry System, the only FDA cleared device to reduce underarm sweat, odor and hair of all colors to our aesthetics portfolio. Following our acquisition of miraDry in July 2017, we began selling the miraDry System and bioTips. As a result of the miraDry acquisition, we determined that we will conduct our business in two operating segments: the Breast Products segment, which is comprised of our breast implants, tissue expanders and scar management products, and the miraDry segment, which is comprised of our recently acquired miraDry System.
The global market for aesthetic procedures is significant. The American Society of Aesthetic Plastic Surgery, or ASAPS, estimates that U.S. consumers spent approximately $15 billion on approximately ten million aesthetic procedures in 2016, including both surgical and non‑invasive cosmetic treatments. Breast augmentation surgery remains the leading aesthetic surgical procedure by dollars and number of procedures in the United States. According to ASAPS, over 310,000 primary breast augmentation procedures were performed in the United States in 2016. For breast reconstruction, American Society of Plastic Surgeons, or ASPS, estimates that approximately 109,000 procedures were performed in the United States in 2016. According to the ASAPS, cosmetic procedures have increased by 35% over the past five years with nonsurgical procedures up 39%. We believe several factors are contributing to the ongoing growth in aesthetic procedures, including continuing focus on body image and appearance, broader availability of safe non-invasive aesthetic procedures, and increased physician focus on aesthetic procedures.
We sell both our Breast Products and miraDry products in the U.S. through a direct sales organization, which as of December 31, 2017, consisted of 83 employees, including 68 sales representatives and 15 sales managers. Additionally, we also sell our miraDry System in several international markets where we leverage a combination of distributor relationships and direct sales efforts. As of December 31, 2017, our international operations were supported by 7 employees, including 6 sales representatives and 1 sales manager.
We commenced sales of our breast implants in the United States in the second quarter of 2012. Our Breast Products segment net sales were $31.5 million, $20.7 million and $38.1 million for the years ended December 31, 2017, 2016 and 2015, respectively. We generate revenues from sales of our miraDry System and from the sales of bioTips which are required for use for each miraDry procedure performed. We generated net sales of $5.1 million for the year ended December 31, 2017 from the acquisition date on July 25, 2017. With the acquisition of miraDry, we expect net sales, cost of goods sold, sales and marketing, general and administrative, and research and development expenses to increase in 2018 when compared to 2017 and prior periods. As of December 31, 2017, over 100,000 miraDry procedures have been sold worldwide.
3
The global market for aesthetic procedures is significant. The ASAPS estimates that U.S. consumers spent approximately $15 billion on approximately ten million aesthetic procedures in 2016, including both surgical and non‑invasive cosmetic treatments.
Breast Products
Breast augmentation surgery remains the leading aesthetic surgical procedure by dollars and number of procedures in the United States. According to ASAPS, over 310,000 primary breast augmentation procedures were performed in the United States in 2016. These procedures provide cosmetic solutions generally to enhance breast size and shape, correct breast asymmetries or help restore fullness after breastfeeding. For breast reconstruction, American Society of Plastic Surgeons, or ASPS, estimates that approximately 109,000 procedures were performed in the United States in 2016. These procedures are a surgical solution generally used to restore a breast to near normal shape and appearance following a mastectomy and typically utilize a breast tissue expander prior to implantation of a breast implant. Based on the number of procedures reported by ASAPS and by ASPS, and our estimates of average selling price, implant mix and implants per procedure, we estimate that the U.S. market for breast implants and breast tissue expanders exceeded $652 million in 2016.
We sell our breast implants and tissue expanders exclusively to board‑certified and board‑admissible plastic surgeons, as determined by the American Board of Plastic Surgery, who we refer to as Plastic Surgeons. These surgeons have completed the extensive multi‑year plastic surgery residency training required by the American Board of Plastic Surgery. While aesthetic procedures are performed by a wide range of medical professionals, including dermatologists, otolaryngologists, obstetricians, gynecologists, dentists and other specialists, the majority of aesthetic surgical procedures are performed by Plastic Surgeons. Plastic Surgeons are thought leaders in the medical aesthetics industry. According to the American Board of Plastic Surgery, there are approximately 6,500 board‑certified plastic surgeons in the United States.
miraDry
According to the ASAPS, cosmetic procedures have increased by 35% over the past five years with nonsurgical procedures up 39%. Laser and light-based hair removal continues to be the largest volume among non-invasive and non-injectable procedures. As an emerging market, energy-based procedures for sweat and odor reduction are not currently tracked by ASAPS data. No one treatment procedure is offered by all physicians, and treatments vary in terms of the treatment goal and desired effect. As a result, the total aesthetic market as reported by the ASAPS does not represent the market potential for miraDry or any other single product or treatment, but illustrates that each year patients elect to have millions of procedures to enhance their appearance. We believe several factors are contributing to the ongoing growth in aesthetic procedures, including:
|
|
• |
Broader availability of safe non-invasive aesthetic procedures. Technological developments have resulted in the introduction of a broader range of safe, non-invasive aesthetic procedures. According to the ASAPS, non-invasive aesthetic treatments are growing faster than invasive surgical procedures. |
|
|
• |
Increased physician focus on aesthetic procedures. We believe increased restrictions imposed by managed care and government agencies on reimbursement for medical treatments are motivating our customers to establish or expand their elective aesthetic practices, which generally consist of procedures paid for directly by patients. We expect this trend to continue as our customers look for ways to expand their practices and improve profitability. |
Hyperhidrosis is a medical condition of varying degree in which a person sweats excessively. The prevalence of hyperhidrosis in the United States is significant. A study published by Strutton et al. in the June 2004 issue of the Journal of the American Academy of Dermatology, or AAD, titled “US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey,” estimated that 2.8% of the general population has hyperhidrosis (in this study defined as excessive or abnormal sweating) with 50.8% thereof having axillary hyperhidrosis. Additionally, the general consensus of medical practitioners is that the definition of
4
hyperhidrosis includes anyone who is bothered by their sweat. As such, the definition of axillary hyperhidrosis is broad in scope and the condition depends upon whether patients have determined that their sweating is excessive or abnormal. Because this assessment is subjectively determined by the patients themselves, there is no quantifiable standard that medical practitioners use to determine whether a patient is suffering from axillary hyperhidrosis. If patients subjectively determine that their sweating is excessive and as such are bothered by their sweating, such patients are considered to be suffering from axillary hyperhidrosis.
In 2017, we commissioned a survey of over 2,000 consumers, evaluating several criteria including sweat bothered, dissatisfaction with current treatment, interest in a non-surgical long-term solution, and interest in the miraDry product description. Based on this survey, we believe the market for miraDry in the U.S. alone is approximately 37 million people.
Our Opportunity
Breast Products
We believe a significant opportunity exists in the U.S. marketplace due to the high barriers to entry in the U.S. breast implant market and the historical lack of product and service innovation for Plastic Surgeons.
For more than 20 years prior to the FDA approval of our breast implants in 2012, only two companies manufactured and distributed breast implants in the United States. We believe that this market concentration is largely a result of the considerable costs and risks associated with the lengthy regulatory approval process required by the FDA, which has created a significant barrier to entry in the U.S. breast implant market. All new breast implants require PMA approval from the FDA before they may be marketed in the United States. The PMA application process is lengthy and uncertain, and it must be supported by valid scientific evidence, which typically requires long‑term follow‑up of a large number of enrolled patients, as well as extensive pre‑clinical, clinical and other product data to demonstrate safety and effectiveness. We believe that in the near term, it is likely that the companies currently providing silicone gel breast implants in the United States will continue to be the only companies servicing the U.S. silicone breast implant market.
We believe the rigorous FDA approval process and the existence of only two competitors in the U.S. market have historically contributed to a lack of technological innovation in the U.S. breast implant industry resulting in limited product choices. Until the FDA approval of our breast implants in 2012, surgeons in the United States were only able to purchase basic round breast implants from our two U.S. competitors, while surgeons outside of the United States were able to purchase technologically‑advanced round and anatomically‑shaped breast implants.
miraDry
The miraDry procedure addresses a large underpenetrated market in the non-invasive, lifestyle aesthetics category. The miraDry treatment is the first and only FDA cleared solution to reduce underarm sweat, odor and hair of all colors with as little as one 60-minute treatment, allowing most patients to achieve immediately noticeable and durable results without the pain, expense, downtime, or repeat visits associated with surgical and minimally-invasive procedures. The sweat glands in the treated area are destroyed through targeted heating of the tissue, and because the body does not regenerate sweat glands, we believe the results will be lasting, although some patients may need to repeat the miraDry procedure to achieve the lasting results. Due to these advantages, we believe that the miraDry treatment is appealing to a wide range of individuals seeking a lasting solution to underarm sweat.
The miraDry System has been cleared by the FDA as indicated for use in the treatment of primary axillary hyperhidrosis, or a condition characterized by abnormal sweating in excess of that required for regulation of body temperature, plus unwanted underarm hair removal, and reduction of underarm hair. When used for the treatment of primary axillary hyperhidrosis, the miraDry System may reduce underarm odor. In addition, the miraDry System received CE mark approval for the treatment of primary axillary hyperhidrosis and approval in several other countries. We are approved to sell the miraDry System in over 40 international markets outside of North America, including countries in Asia, Europe, the Middle East and South America.
5
We believe that we are well positioned to take advantage of opportunities afforded by current market dynamics. By focusing on products with technologically differentiated characteristics, demonstrating strong clinical data, offering more product choices and providing services tailored specifically to the needs of physicians, we believe we can enhance our position in the market. Our competitive strengths include:
Proven and experienced leadership team. We have a highly experienced management team at both the corporate and operational levels with significant experience in the medical aesthetics industry. Members of our senior management team have extensive experience in the medical aesthetics industry.
Breast Products
Differentiated silicone gel and texturing technologies. We incorporate differentiated technologies into our proprietary breast implants to distinguish ourselves from our competitors, including our silicone shell, High‑Strength Cohesive silicone gel and a textured surface. Our breast implants offer a desired balance between strength, shape retention and softness due to the High-Strength Cohesive silicone gel used in our products. In addition, the texturing on Sientra’s implant shell is designed to reduce the incidence of malposition, rotation and capsular contracture.
Strong clinical trial outcomes. Our clinical trial results demonstrate the safety and effectiveness of our breast implants. Our breast implants were approved by the FDA based on data we collected from our ongoing, long‑term clinical trial of our breast implants in 1,788 women across 36 investigational sites in the United States. The clinical data we collected over a ten-year follow-up period demonstrated rupture rates, capsular contracture rates and reoperation rates that were comparable to or better than those of our competitors, based on our competitors’ published ten-year data.
Innovative services that deliver an improved customer experience. Our customer service offerings are intended to accommodate and anticipate the needs of our Plastic Surgeons so they can focus on providing better services to their patients. We provide an industry-leading ten‑year limited warranty that provides patients with a cash reimbursement for certain out‑of‑pocket costs related to revision surgeries in a covered event; a lifetime no‑charge implant replacement program for covered ruptures; and our industry‑first C3 Program through which we offer no‑charge replacement implants to breast augmentation patients who experience capsular contracture within the first five years after implantation with our smooth or textured breast implants. We also offer specialized educational initiatives and a streamlined ordering, shipping and billing process.
Board‑certified plastic surgeon focus. We sell our breast implants and tissue expanders exclusively to board‑certified and board‑admissible plastic surgeons who are thought leaders in the medical aesthetics industry. We address the specific needs of Plastic Surgeons through continued product innovation, expansion of our product portfolio and enhanced customer service offerings. We believe that securing the loyalty and confidence of Plastic Surgeons is essential to our success and that our association with Plastic Surgeons enhances our credibility and aligns with our focus on making a difference in patients’ lives.
miraDry
Strong clinical trial outcomes. The miraDry System is the only FDA cleared device to reduce underarm sweat, odor and hair of all colors. Clinical studies involving more than 150 patients demonstrate that one or two miraDry procedures can noticeably and measurably reduce the amount of sweat from the axilla, or underarm. In our study involving 120 subjects, 89% of patients that received treatment experienced reduction in their sweat with no reported deaths, injuries requiring immediate medical attention to prevent death, or permanent impairment. In a second study involving 31 patients, patients reported an average of 82% sweat reduction at 12 months and 100% of patients reported as being no longer bothered by their hyperhidrosis at 24 months. Because sweat glands do not regenerate after the procedure, we believe the results are lasting.
Patient satisfaction. miraDry allows most patients to achieve noticeable and measurable aesthetic results without the pain, expense, downtime, and risks associated with invasive and minimally-invasive procedures for sweat, odor and
6
hair reduction. In addition, unlike many other non-invasive procedures, patients are not required to undergo multiple treatment procedures to obtain aesthetic results. According to RealSelf.com, as of January 16, 2018, the leading online community helping people make confident choices in elective cosmetic procedures, the miraDry procedure received a 90% “worth it” rating of the participating patients.
Reproducible results. The miraDry procedure requires limited training and skill to obtain successful aesthetic results. The miraDry System was designed to be easy to operate and largely automated, resulting in a more consistent application and reproducible results.
Differentiated, high-value product for physician practices. Our selective distribution strategy was designed to enable our customers to market miraDry as a highly differentiated, non-invasive sweat, odor, and hair reduction procedure. Based on our commercial data and customer experiences, we have seen attractive economic benefits for our customers.
Our Strategy
Our objective is to become a leading global provider of differentiated medical aesthetic products and services tailored to meet the needs of physicians, allowing us to deliver on our commitment to enhance and make a difference in patients’ lives. To achieve our objective, we are pursuing the following business strategies:
Create awareness of our differentiated technologies, products and services with Plastic Surgeons and consumers. Since we commenced commercial operations, we have focused most of our marketing efforts on Plastic Surgeons to promote and create awareness of the benefits of our products. Among other marketing programs targeted at Plastic Surgeons, we offer educational initiatives exclusively to Plastic Surgeons through our Sientra Education Forums, and we have continued our consumer-directed efforts, including an exclusive collaboration with RealSelf.com. We believe that continuing to invest in expanding marketing initiatives will have a positive impact on our business.
Selectively pursue acquisitions and expand into new markets. We may continue to selectively pursue domestic and international acquisitions of businesses or technologies that may allow us to leverage our relationships with Plastic Surgeons and our existing commercial infrastructure to provide us with new or complementary products or technologies, and allow us to compete in new geographic markets or market segments or to increase our market share. For example, we began selling BIOCORNEUM directly to physicians after we acquired BIOCORNEUM from Enaltus in March 2016. We began selling the AlloX2 and Dermaspan lines of breast tissue expanders, and the Softspan line of general tissue expanders, after we acquired these product lines from SSP in November 2016. We began selling the miraDry System and bioTips after the acquisition in July 2017.
Broaden our product portfolio and launch new products and services. We plan to continue to develop products that address the unmet needs of physicians and patients by leveraging our innovative technologies in combination with our regulatory and product development expertise. We have a number of new breast implants and tissue expanders under development with different characteristics and configurations. We believe these expanded product choices will allow Plastic Surgeons to potentially achieve better outcomes for their patients.
Highly optimized, experienced and fully trained sales force. We maintain separate North American sales forces within our Breast Products and miraDry segments. Our Breast Products sales force primarily consists of Plastic Surgery Consultants, or PSCs, focused on selling all breast products exclusively to board‑certified and board‑admissible plastic surgeons. Additionally, our Breast Products segment is also supported by Multi Specialty Consultants, or MSCs, that sell scar management products directly to physicians. Our miraDry sales force is a bifurcated organization that has produced stronger focus and results on system sales and high-margin consumable sales. This organization is split between Area Sales Managers, or ASMs, who focus on system sales, and Practice Development Managers, or PDMs, who focus on high margin consumable sales, assisting practices to market miraDry to patients, product training and driving system utilization. We have continued to hire high quality, experienced sales representatives and sales management personnel in all categories and train the sales organization to optimize performance in their respective roles. We believe our sales force will continue to generate increased customer adoption and patient awareness momentum in the marketplace.
7
Invest in clinical studies and peer reviewed articles with key opinion leaders. We intend to continue to invest in clinical studies in order to provide published peer reviewed articles that support the clinical benefits of our products and technologies over those of our competitors. We believe our relationship with Plastic Surgeons and our continued focus on providing differentiated products and services will allow us to leverage our existing capabilities to increase our share of the breast implant market specifically and the medical aesthetics market generally.
Increase our international presence. There is strong global demand for aesthetic procedures outside of North America. We intend to increase our market penetration outside of North America and build global brand recognition. We have received regulatory approval or are otherwise free to market miraDry in numerous international markets. We intend to seek regulatory approval to market miraDry in additional international markets, as well as grow our international sales and marketing organization to focus on increasing sales and strengthening our customer relationships. As part of this strategy, we are and intend to continue to opportunistically deploy a direct sales force in select international markets.
Our Products
Our portfolio of products has been specifically tailored to meet the needs of the physicians we serve. We believe that our broad portfolio of products with technologically differentiated characteristics enable physicians to deliver better outcomes for their patients.
Breast Products
Our primary breast products are silicone gel breast implants for use in breast augmentation and breast reconstruction procedures, which we offer in approximately 400 variations of shapes, sizes, fill volumes and textures. Our breast implants are primarily used in elective procedures that are generally performed on a cash‑pay basis. Many of our proprietary breast implants incorporate one or more technologies that differentiate us from our competitors, including a High‑Strength Cohesive, or HSC, silicone gel and shell texturing. Our breast implants offer a desired balance between strength, shape retention and softness due to the integration of our silicone implant shell and High‑Strength Cohesive silicone gel used in our implants. The texturing on Sientra’s implant shell is designed to reduce the incidence of malposition, rotation and capsular contracture.
Breast Augmentation and Breast Reconstruction Products
Breast Implants. We offer the following breast implants:
|
|
• |
Anatomically‑shaped textured. A full line of textured, anatomically‑shaped HSC+ breast implants, all of which incorporate our High‑Strength Cohesive silicone gel and a textured surface. Our anatomically‑shaped implants are engineered for shape retention and feature a gradual upper‑pole slope and distributed volume that mimics the characteristics of a natural breast. They also provide a desired balance between strength, shape retention and softness and are designed to enhance tissue adherence to reduce malposition and capsular contracture. Due to the unique relationship between our implant gel and our implant shells, our anatomically‑shaped implants have enhanced ability to retain their shape without sacrificing the desired softness. We offer these anatomically‑shaped implants in three base configurations: Round Base, Classic Base and Oval Base. Our Round Base implants are available in one projection profile and eight volumes, our Classic Base implants are available in one projection profile and eight volumes and our Oval Base implants are available in three projection profiles and 25 volumes. Additionally, in the fourth quarter of 2016, we received FDA approval of 84 new anatomically-shaped devices, a 205% increase of our anatomically-shaped portfolio. Our Round Base implants were approved with an additional projection and 28 new sizes, our Classic Base implants were approved with an additional projection and 32 new sizes and our Oval Base implants were approved with an additional 24 sizes. |
8
|
|
projection implants are available in 15 volumes, our Moderate Plus projection implants are available in 22 volumes and our High projection implants are available in 16 volumes. Additionally, in the fourth quarter of 2016, we received approval of an additional projection and 52 more sizes. |
|
|
• |
Round smooth. A full line of smooth, round HSC breast implants, all of which incorporate our High‑Strength Cohesive silicone gel. Our smooth, round implants are designed to deliver full upper‑pole aesthetic results without compromising softness. We offer these smooth, round implants in five projection profiles: Low, Moderate, Moderate Plus, Moderate High and High. Additionally, in the fourth quarter of 2016, we received FDA approval of 8 more sizes. |
|
|
• |
Breast Tissue Expanders. We offer a full line of breast tissue expanders, marketed as AlloX2 and Dermaspan in 52 different shapes and sizes. Our AlloX2 is the first and only breast tissue expander with access to the periprosthetic space, with its patented technology, addressing fluid accumulation that can lead to postoperative complications. Our breast tissue expanders are temporary devices used in breast reconstruction and implanted during or after the completion of a mastectomy and intended to aid in the process of recreating tissue coverage to allow for the placement of the final implant to reconstruct the breast. |
Scar Management Products
We offer BIOCORNEUM, the only advanced scar treatment with a patented crosslinking silicone technology, Silishield, plus the protection of SPF 30. BIOCORNEUM acts as a quick drying, silicone gel that creates an invisible, breathable and flexible silicone sheet over scars. It is a silicone scar treatment, supported by clinical data, that prevents and minimizes the formation of hypertrophic and keloid scars, decreases the appearance of old scars, and helps to restore the function of healthy skin. The SPF 30 provides protection from sun exposure’s darkening effects. The patented gel helps to safeguard against chemical, microbial, and physical detriments while improving the cosmetic appearance of scar tissue by binding with the stratum corneum (the outer layer of skin cells). BIOCORNEUM decreases transepidermal water loss and increases the production of fibroblast growth factor to heal skin and prevent abnormal scarring.
Other Products
We also offer a range of other aesthetic products that have received 510(k) clearance from the FDA, including:
|
|
• |
temporary, single‑use, saline‑filled breast implant sizers that can be used to help identify the correct style and size implant for an individual patient; and |
|
|
• |
Softspan non‑breast tissue expanders, which are temporary devices intended to aid in the process of expanding tissue and skin surface area for burn care and other reconstructive use. |
miraDry
The miraDry Experience
The miraDry System provides patients with a precise and non-invasive and durable procedure to selectively destroy underarm sweat glands for both severely hyperhidrotic patients and those that are bothered by their underarm sweat. The miraDry System is clinically proven to reduce sweat in one or more procedures of approximately one hour, allowing most patients to achieve immediately noticeable and durable results without the pain, expense, downtime, or repeat visits associated with surgical and minimally-invasive procedures. The first step of the miraDry process is a patient consultation. We train our physician customers to properly explain to their patients the results they should expect from a miraDry procedure. The patient’s underarm is anesthetized for maximum comfort. Then the underarm is sized using a sizing template and an appropriately sized temporary treatment grid is then selected and applied to the underarm to guide treatment. The miraDry handpiece is applied step-by-step using the grid markings as guides to treat the entire axilla. During each application of microwave energy, the skin is first cooled, energy is applied, and then more cooling is applied to the skin’s surface providing constant temperature control of the tissue for the patient’s comfort. Following treatment, the patient is given post-treatment instructions.
9
Our surveys indicate that most patients find the miraDry procedure easy to tolerate. Due to the underarm being fully anesthetized prior to treatment, patients typically only report feeling a tugging sensation from the suction created when the handpiece is placed on the treatment area but otherwise report no sensation.
Launch of New Protocol and Software Upgrade: miraDry Fresh
On February 16, 2018, the company launched “miraDry Fresh”, a new and improved treatment protocol for the miraDry System. The miraDry fresh protocol and software upgrade reduces overall procedure time by up to 35% and includes a revision to the anesthesia process resulting in a treatment that can be administered with greater ease and is more delegateable.
miraDry System
The miraDry System consists of the miraDry console and the miraDry handpiece. The miraDry console contains a simple user interface with touchscreen software, power management and control functions, and a chiller unit that is responsible for the hydro-ceramic constant cooling. Our miraDry System also contains software that tracks and collects data on each procedure performed and any error messages that may be generated during the procedure. We collect and analyze this information to help physicians better understand their usage patterns and improve their marketing plans, utilization, and profitability.
|
|
• |
The color touch screen on the miraDry console provides operators with clear step-by-step visual instructions that guide the user through a miraDry procedure, providing continuous status updates and easy to follow notifications or corrective actions in the rare event of a procedure interruption. |
|
|
• |
The miraDry handpiece is used to apply the microwave energy while maintaining constant contact cooling of the skin during treatment. The handpiece also displays the heating and cooling cycles during each pulse. The handpiece is detachable to enable future product upgrades. |
|
|
• |
The unit is mobile, allowing a physician to easily transfer the miraDry System between treatment rooms. |
|
|
• |
Vents are built into the miraDry System control unit to provide airflow and reduce heat build-up. Our miraDry System can be used in a standard physician treatment room without any special ventilation requirements or room modifications. |
miraDry single-use bioTips
Our miraDry bioTips facilitate the proper suctioning of the skin to maintain constant contact of the skin with the handpiece during the treatment. Also, the bioTips facilitate the pay-per-procedure feature of our miraDry System. Our bioTips are typically shipped with branded gel packs for patients to apply after treatment.
Our proprietary consumable, the bioTip, is designed such that each bioTip is encoded to be used only with our proprietary system and expires within a set time and cannot be reused. Each bioTip is preprogrammed with enabling software that permits the miraDry System to perform a single patient treatment for a fixed duration of time and an encrypted security certificate that prevents the performance of a miraDry procedure unless the bioTip is recognized and authenticated by the specific miraDry System. The security certificate is designed to ensure that physicians pay for each patient treated and prevent the use of counterfeit bioTips.
Our Technology
Breast Products
Our current portfolio of breast implants utilizes what we believe are the most advanced technologies currently available on the market. These technologies are supported by rigorous product testing, analytics and clinical data. The advanced technologies in our products include:
10
High‑Strength Cohesive silicone gel. Our HSC and HSC+ breast implants offer a desired balance between strength, shape retention and softness due to the High‑Strength Cohesive silicone gel used in our products. The use of High‑Strength Cohesive silicone gel in our HSC and HSC+ breast implants in conjunction with our silicone shell allows the breast implants to hold a controlled shape while maintaining a soft feel.
The silicone material used in our breast implants has been designed to provide the characteristics desired by Plastic Surgeons for breast implants. At present, we are the only company in the United States that has received FDA approval to use High Strength Cohesive silicone gel in breast implants.
We have completed a number of studies conducted by independent laboratories to demonstrate the competitive advantages of using High‑Strength Cohesive silicone gel in our breast implants. We believe this technology differentiates our breast implants for the following reasons:
|
|
• |
our implant gel is stronger, which is evidenced by its resistance to gel fracture; |
|
|
• |
due to the unique relationship between our implant gel and our implant shells, our implants have an enhanced ability to retain their shape without sacrificing the desired softness; and |
|
|
• |
our shaped implants are softer and more elastic than our competitors’ shaped implants. |
We believe the beneficial properties of our implants arise from the characteristics of the gel, as well as the integration of the gel with our implant shell. Inside each of our implants, the gel adheres to the shell, creating additional structural strength and shape retention in the implant. This results in the ability to deliver strength and shaping capability without a stiffer gel or implant and without sacrificing the desired softness. We typically evaluate these characteristics using the following metrics:
|
|
• |
Peel‑force. Peel‑force is measured by the amount of force, measured in pound‑force, or lbf, necessary to separate the outer shell of the implant from the internal gel filling. A greater peel‑force measurement indicates greater gel‑shell integration. In the case of anatomically‑shaped implants, greater peel‑force can also be an indication of the ability of the implant to retain its shape, particularly the upper portions of the implant, also referred to as the upper pole. Upper pole stability is of particular importance in preserving the desired anatomical shape of an implant over time. |
|
|
• |
Gel strength. Gel strength is measured by the amount of force, measured in lbf, required to cause permanent fractures in the gel. A larger value indicates greater strength. |
|
|
• |
Gel elasticity and implant elasticity. Gel elasticity and implant elasticity can be measured by the level of resistance, measured in millimeters, or mm, to an applied constant force. A higher value represents greater softness and a lower deformation value represents greater firmness. |
Sientra’s Implant Texture. We sell breast implants that are available with a smooth outer surface or a textured outer surface. We believe our textured breast implants offer us clinical advantages over our competitors’ textured products, including:
|
|
• |
better tissue adherence to reduce the incidence of malposition and rotation; and |
|
|
• |
reduction in the rate of capsular contracture, a complication in which the patient’s body creates a scar‑tissue capsule around the implant that can tighten and squeeze the implant potentially causing discomfort, pain and even dislocation of the implant. While we have neither sought nor obtained FDA approval to state that our breast implants reduces the incidence of capsular contracture, we believe it may significantly reduce this risk, as evidenced by the lower rates of capsular contraction reported over a nine‑year follow‑up period in our ongoing clinical trial. |
On a breast implant, the desired texture should have a proportionate amount of surface disruption, as overly aggressive texture can result in double‑capsule formation while not enough texturing can result in a lack of
11
adherence resulting in malposition or rotation. We believe that our textured implants have the right combination of surface disruption without overly aggressive texturing.
By incorporating High‑Strength Cohesive silicone gel and our texturing into our breast implants, we believe we have a competitive advantage in marketing and differentiating our products to Plastic Surgeons.
miraDry
miraWave Technology. Our miraWave technology platform utilizes microwave energy to create heat within the skin or subcutaneous locations to create a therapeutic effect. Microwave energy has been used in various medical specialties for heating tissue for decades. In the dermatologic field, it is important that heating is confined to a very precise location, which the miraWave technology platform is designed to do. Due to its proprietary handpiece designs, when used with appropriate energy parameters, the miraDry System can heat dermatologic tissue in a precise and controlled manner.
Our miraDry System utilizes microwave energy to deliver heat to the location of the skin where most underarm sweat glands reside – at or just below the skin-fat interface. We designed a proprietary handpiece that automatically focuses the energy at the skin-fat interface, regardless of skin thickness. When the physician or medical professional places the handpiece to a specific area of the underarm as instructed by the graphic user interface, the energy is delivered automatically to the target tissue. The heat generated in the tissue exceeds the threshold for cellular necrosis, thereby ablating the sweat glands where the energy is focused. Surface cooling prevents the heat from damaging the superficial tissue above the skin-fat interface. In the underarm, many of the hair follicles are in the same relative location as the sweat glands. Therefore, the heating will also cause destruction and elimination of the hair follicles in those areas.
Our miraDry treatment has been clinically demonstrated to reduce sweat and hair from the underarm without causing injury to critical surrounding structures. The surface cooling protects the epidermis and the majority of the dermis from damaging heat. The deeper underlying structures are protected by two mechanisms. First, our anesthesia protocol calls for creating a distance barrier between the underlying structures and the surface of the skin where the handpiece is positioned. A significant volume of anesthesia fluid is administered between the skin (and target tissue) and the underlying structures, which causes a separation of the target tissue from the underlying structure. As the handpiece is positioned just outside the skin, the underlying structures are further away from the handpiece, keeping them safe from damaging heat. Second, we employ a vacuum suction system in the handpiece where the skin is pulled up into a vacuum chamber within the handpiece. Typically, the underlying structures either remain stationary or move slightly with the vacuum action, thereby creating further distance between the handpiece and the underlying structures.
Our Clinical Data
Breast Products
In 2012, our breast implants were approved by the FDA based on data we collected from our ongoing, long‑term clinical trial of our breast implants in 1,788 women across 36 investigational sites in the United States, which included 3,506 implants (approximately 53% of which were smooth and 47% of which were textured). Our clinical trial results demonstrate the safety and effectiveness of our breast implants and provide Plastic Surgeons and their patients the security and confidence to choose our products.
Our breast implant clinical trial is the largest prospective, long‑term safety and effectiveness pivotal study of breast implants in the United States and included the largest magnetic resonance imaging, or MRI, cohort with 571 patients. The MRI cohort is a subset of study patients that underwent regular MRI screenings in addition to the other aspects of the clinical trial protocol prior to FDA approval. Post-approval, all patients in the long-term clinical trial are subject to serial MRI screening as part of the clinical protocol. The clinical data we collected over a ten‑year follow‑up period demonstrated rupture rates, capsular contracture rates and reoperation rates that were comparable to or better than those of our competitors, at similar time points. In addition to our pivotal study, our clinical data is supported by our Continued Access Study of 2,497 women in the United States. We have also commissioned a
12
number of bench trials run by independent laboratories that we believe further demonstrate the advantages of our breast implants over those of our competitors.
We and our two competitors were required to run independent ten‑year clinical studies to obtain PMA approval from the FDA. Our clinical study was not designed to facilitate head‑to‑head comparisons. However, our clinical data and our competitors’ clinical data are publicly available to both surgeons and patients who are able to use such data to compare and contrast competing implants.
miraDry
Our DRI-UP clinical trial, conducted as an FDA-approved Investigational Device Exemption study, involved 120 subjects. The results of the study indicated that subjects with axillary hyperhidrosis receiving treatment for the reduction of axillary sweat using the miraDry System had a success rate of 89% as compared to the control group success rate of 54%, with no serious adverse events or unanticipated adverse device effects reported.
A second study on the long-term effect of the miraDry System showed all patients who participated in this study reported being no longer bothered by their hyperhidrosis at 24 months, with no serious adverse events or unanticipated adverse device effects.
A third, single center study designed to quantify the amount of odor reduction in the axillae after treatment(s) with the miraDry System treated 36 subjects with a miraDry treatment with follow-up visits at 1 month, 3 month and 6 month intervals after treatment. The study data did not show a statistically significant majority of treated subjects having at least a two point lower malodor score (scale of 0 to 10) but did show a statistically significant average malodor score difference between the treated and untreated axilla using both quantitative odor judges’ scores as well as patients’ subjective self-reported odor severity score (scale of 1 to 10).
Our Services
Our services are designed to cater to the specific needs of physicians to enable them to maintain and grow their practices. We provide our customers with superior warranty programs, enhanced customer service offerings and specialized educational initiatives. We believe that tailoring our customer service offerings to physicians helps secure their loyalty and confidence.
Industry‑Leading Product Programs and Warranties
Through our C3 Program, we provide no‑charge replacement gel breast implants to patients who experience capsular contracture in the first five years following primary breast augmentation for every patient implanted with our smooth or textured breast implants. We also provide a ten‑year limited warranty that provides patients with the largest cash reimbursement for certain out‑of‑pocket costs related to revision surgeries in a covered event and a lifetime no‑charge implant replacement program for covered ruptures.
Enhanced Customer Service
Breast Products
Our Breast Products customer service policies have been specifically tailored to meet the needs of Plastic Surgeons, including:
|
|
• |
simplified account setup through our sales representatives with pre‑qualification and pre‑approved credit terms; |
|
|
• |
no‑charge shipping to and from accounts; |
|
|
• |
six‑month pre‑approved returns of unused products with no‑charge return shipping and no restocking fees; |
13
|
|
• |
individualized consignment inventory; and |
|
|
• |
order acceptance by phone, fax, email or through our sales representatives. |
miraDry
Our miraDry customer service policies have been designed to meet the needs of both physicians and distributors, including:
|
|
• |
In the event of a technical issue with a miraDry System in North America, one of our customer service personnel will call the physician and determine whether the technical issue may be resolved over the telephone or whether the issue requires an intervention. If the issue cannot be resolved by telephone, our customer service personnel will request our third-party logistics provider to visit the physician and provide on-site technical support. If the service provider determines that a replacement system is required, our logistics provider will deliver the replacement miraDry System or module into the physician’s office, set it up and ensure that the miraDry System is working properly. |
|
|
• |
In most markets outside of North America, our miraDry System is serviced and supported through our independent distributors and certified third-party service providers. We require our distributors to maintain adequate inventory of miraDry Systems and components to facilitate quick response time to service events and to maximize customer “up time.” |
|
|
• |
We provide a standard one year warranty on our miraDry Systems in the U.S. In addition to these product warranties, we offer extended service agreements to our customers to provide protection of their system and handpiece against breakage. However, we do not obtain a material portion of our revenue from our service contracts. |
Educational and Marketing Initiatives
Breast Products
We have implemented educational and marketing initiatives with a focus on both Plastic Surgeons and their patients considering breast augmentation or reconstruction.
Plastic Surgeons. In order to educate Plastic Surgeons about our product lines and, in particular, about the proper use of our anatomically‑shaped breast implants, we provide a variety of education programs for Plastic Surgeons under the banner of the Sientra Education Forum. To date:
|
|
• |
we have developed a tablet‑based mobile marketing tool for our sales representatives to use while calling on accounts that includes access to our patient and surgeon labeling, published clinical studies, marketing literature, details on our warranty and C3 programs, our educational iBooks and more. |
|
|
• |
we host symposia with one or more key‑note speakers who speak on topics ranging from our corporate identity and customer service offerings to surgical tips and suggestions from thought‑leading Plastic Surgeons. |
|
|
• |
we produce comprehensive guides for Plastic Surgeons via the Internet, referred to as iBooks, to provide them training and expertise on the implantation of anatomically‑shaped breast implants. |
14
|
|
United States, we engage them as consultant‑educators to conduct training sessions for other U.S.‑based Plastic Surgeons. |
|
|
• |
we periodically sponsor educational surgical preceptorships where a small group of Plastic Surgeons are able to observe a live surgery conducted by one of our trained preceptors and train with that preceptor. |
|
|
• |
We provide an educational series on Practice Management for Plastic Surgeons in the form of ENHANCE Webinars and Consulting, to provide them with insights and expertise on how to market and run their practices. |
Patients. We have been engaging directly with consumers who are considering breast augmentation or reconstruction. We initially focused our consumer educational and marketing activities on websites where consumers come to research their breast augmentation or reconstruction options, including:
|
|
• |
our own consumer website, branded with our “Feel So Good” campaign, that provides resources for consumers considering breast augmentation or reconstruction, including referrals and commentaries, product descriptions, patient planning guides and educational brochures and information regarding our rupture warranty and C3 programs; |
|
|
• |
our exclusive collaboration with RealSelf, the leading online community helping people make confident choices in elective cosmetic procedures. Together with RealSelf, we deliver fresh and meaningful content to the RealSelf community that answers common questions patients have regarding breast augmentation. This content is featured on a dedicated Sientra page on RealSelf’s website designed to build consumer engagement with the brand and open up the online conversation around breast augmentation directly with Plastic Surgeons; and |
|
|
• |
Our social media profiles, educating those interested in breast augmentation, breast reconstruction and scar treatment through Facebook, Instagram, LinkedIn and Twitter. We deliver four distinct content series to educate patients – Breast Implant Basics, Board-Certified Plastic Surgeons Basics, Scarring, and From Her Lips – as well as sharing applicable third party content about breast procedures and scarring. |
miraDry
We have implemented targeted marketing and practice support programs.
|
|
• |
In North America, we provide physicians and their staff product training and sales, marketing, and support services to help them make the miraDry treatment a key component of their practices. In other markets, we have our business development team work to train our distributors and their staff who in turn are responsible for training their customers. |
|
|
• |
We have hired a group of Practice Development Managers, or PDMs, who are focused on implementing our marketing programs in North America. Our PDMs provide all initial trainings for our miraDry System to our physician customers and their staff following the delivery of the system to the practice. Following this initial training, our PDMs, also educate our physician customers on current best practices and provide physicians and their staff with sales and marketing training and support to help them increase patient demand for the miraDry treatment. |
|
|
• |
In certain geographic regions, we provide customers with the option to qualify for marketing and advertising programs to help increase patient awareness and demand in their practice. |
|
|
• |
We also participate in industry tradeshows, clinical workshops, and conferences with expert panelists. |
We believe that our innovative services, including industry‑leading product programs and warranties, enhanced customer service offerings and educational and marketing initiatives, deliver an improved customer experience to our physicians and their patients.
15
We sell both our Breast Products and miraDry products in the U.S. through a direct sales organization, which as of December 31, 2017, consisted of 83 employees, including 68 sales representatives and 15 sales managers. Additionally, we also sell our miraDry System in several international markets where we leverage a combination of distributor relationships and direct sales efforts. As of December 31, 2017, our international operations were supported by 7 employees, including 6 sales representatives and 1 sales manager.
We continue to increase our penetration into the international markets in which we currently distribute the miraDry System, as well as expand into new markets through the identification and training of qualified distributors specializing in medical device distribution. We require our international distributors to provide ongoing training and support of their physician customers and invest in the marketing support of practices to expand the market and demand for the miraDry System for physicians and patients. Our distribution agreements generally provide the exclusive right to distribute our products within a designated territory.
In addition, our marketing team leads our efforts in brand development, trade show attendance, educational forums, product messaging, website development and advertising, among others.
Research and Development
We have incurred, and expect to continue to incur, significant research and development expenses. Our research and development expenses were approximately $9.8 million, $9.7 million and $7.2 million for the years ended December 31, 2017, 2016 and 2015, respectively. The addition of miraDry added $1.1 million for the year ended December 31, 2017 since the acquisition on July 25, 2017. Our Breast Products segment research and development is focused on enhancing and improving our breast products and tissue expanders, increasing our breast implant portfolio, product development related activities and expanding into synergistic markets. Our miraDry research and development is focused on products and procedure enhancements and development of products for new indications. Product and procedure enhancements include changes to improve efficacy of the therapy, the patient experience, and the physician/operator experience. As related to the miraDry System, for products for new indications, we will seek to leverage our miraWave microwave energy platform to develop products to serve additional needs in dermatology and plastic surgery. The goal is to be able to treat multiple indications with the existing miraDry console using different handpieces and custom software. Our miraDry research and development group is comprised of engineers, microwave scientists and technicians. We believe research and development is important to the success of the Company as we continue to develop and expand our product portfolio.
Manufacturing and Quality Assurance
Breast Products
We hold an FDA Medical Device Establishment Registration. All of our medical device products are listed under our Device Listing where it indicates we are the specification developer of our products, and except for our breast implant sizers, we are the owner of our products’ FDA approvals and clearances. This means that we are primarily responsible for the design, manufacturing and quality assurance of our products. However, we do not manufacture our products ourselves. Instead, we rely on our third-party manufacturers to manufacture and package our silicone gel breast implants, tissue expanders and other products to our specifications. When we receive our products from our third-party manufacturers, we inspect a representative sample of packaging and labeling prior to shipping them to our customers. We typically maintain strategic levels of inventory at our storage facilities located in Santa Barbara, California. As a result of the events with Silimed, currently all of the remaining inventory we received from Silimed is located at this storage facility. We have also recently started storing inventory manufactured by Vesta, pending FDA-approval, at this storage facility. This “at risk” inventory has been quarantined from the remaining Silimed inventories also located at this storage facility.
16
We, along with our third-party manufacturers are subject to the FDA’s Quality System Regulation, or QSR, reporting requirements and current Good Manufacturing Practices, or cGMP, audits by the FDA. Under the QSR and cGMP requirements, manufacturers, including third-party manufacturers, must follow stringent design, testing, production, control, supplier and contractor selection, complaint handling, documentation and other quality assurance procedures during all aspects of the manufacturing process. The FDA has regularly inspected both the Company and our suppliers. The Company has never been the subject of any 483 Observations or Warning Letters, or any other FDA assertions that we are in violation of the FDCA.
Prior to October 2015, all of our silicone gel breast implants were manufactured by Silimed. On October 9, 2015, we voluntarily placed a temporary hold on the sale of all Sientra devices manufactured by Silimed and recommended that plastic surgeons discontinue implanting the devices until further notice due to various international regulatory suspensions and inquiries of Silimed at that time. After ongoing discussions with the FDA, and our own review of the matter with the assistance of independent experts in quality management systems, cGMP, and data-based risk assessment, on March 1, 2016, we lifted the hold and informed our Plastic Surgeons of our controlled market re-entry plan designed to optimize our inventory supply, which continues to be limited. The events involving Silimed will likely continue to adversely impact our business. See “Risk Factors — Risks Relating to Our Business and Our Industry” for further detail.
On August 9, 2016, we announced our collaboration with Vesta, pursuant to which we are working with Vesta towards establishing a dedicated contract manufacturing facility for our breast implants. On March 14, 2017, we announced that we had executed a definitive manufacturing agreement with Vesta for the manufacture and supply of our breast implants. In addition, on March 14, 2017, we announced that we had submitted a PMA supplement to the FDA for the manufacturing of our PMA-approved breast implants by Vesta. On January 30, 2018, we announced the FDA has granted approval of the site-change PMA, supplement for our contract manufacturer, Vesta, to manufacture our silicone gel breast implants. In support of the move to the Vesta manufacturing facility, we also implemented new manufacturing process improvements which, in consultation with the FDA, required three (3) additional filings. In addition to approving the manufacturing site-change supplement, the FDA has approved two (2) of these three (3) process enhancement filings, while requesting additional information for the third submission. We continue to work closely with the FDA to address their information requests related to this third and final outstanding submission in order to resolve these matters in a timely manner.
miraDry
We occupy an approximately 29,000 square foot facility located in Santa Clara, California dedicated to the manufacture, distribution, and servicing of miraDry Systems and accessories.
All final assembly, calibration and testing of our miraDry Systems are performed at our Santa Clara facility. The consumable bioTip is manufactured by a contract manufacturer, Healthcare Technology International Limited (HTI), at their facility in Dongguan, China. Consumables are tested and packaged at our Santa Clara facility, then sent to Parter Sterilization Services in Carson, CA for ethylene oxide sterilization.
A critical component of our miraDry System is the custom microwave power amplifier contained in the miraDry console. The amplifier is manufactured by a single source manufacturer, Broadband Wireless, LLC, in Reno, Nevada (a subsidiary of United States Technologies, Inc.), or Broadband. We fully own the design and manufacturing process for this amplifier.
Manufacturing facilities that produce finished medical devices intended for distribution in the United States and internationally are subject to regulation and periodic unannounced inspection by the FDA and other domestic and international regulatory agencies. In the United States, we are required to manufacture our products in compliance with the FDA’s Quality System Regulation, or QSR, which cover the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage, and shipping of our products. The FDA most recently inspected our facility in August 2016 and at the conclusion of such routine audit, a Form 483 was issued with two observations. The FDA acknowledged receipt of periodic status reports documenting the completion of corrections and corrective actions taken by us to address each of the two observations. The FDA will verify acceptability of the actions taken during its next routine inspection. No further actions are required at this time. In international markets, we are required to obtain and maintain various quality management system certifications. We have obtained the following international certifications for the miraDry System: ISO 13485:2003
17
Quality Management Systems Requirements, in support of both our CE marking and Canadian Medical Devices Conformity Assessment System (CMDCAS) requirements. Our notified body, NSAI, most recently audited our facility in November 2017 and subsequently renewed our ISO 13485-2003 certification.
HTI, our disposables manufacturer, and Parton Sterilization Services, our sterilization service provider comply with the FDA’s QSR and are registered in good standing with the FDA. Additionally, we have procedures in place designed to ensure that all other purchased products and materials conform to specified requirements, including evaluation of suppliers, and where required, qualification of the components supplied.
Competition
Breast Products
The medical device industry is intensely competitive, subject to rapid change and highly sensitive to the introduction of new products or other market activities of industry participants. We primarily compete with two companies that manufacture and sell breast implants in the United States: Johnson & Johnson through its wholly owned subsidiary, Mentor Worldwide, LLC, or Mentor, and Allergan plc, or Allergan.
Both of our U.S. competitors are either publicly‑traded companies or divisions or subsidiaries of publicly‑traded companies with significantly more market share and resources than we have. These companies have greater financial resources for sales, marketing and product development, broader established relationships with healthcare providers and third‑party payors, and larger and more established distribution networks. In some instances, our competitors also offer products that include features that we do not currently offer. For example, Allergan sells temporary gel sizers for silicone gel implants and we sell only temporary saline filled sizers. In addition, our competitors may offer pricing programs with discounts across their non‑breast aesthetic product portfolios.
We also face potential future competition from a number of companies, medical researchers and existing medical device companies that may be pursuing new implant technologies, new material technologies and new methods of enhancing and reconstructing the breast.
We believe the primary competitive factors in our markets include:
|
|
• |
breadth of portfolio; |
|
|
• |
technological characteristics of products; |
|
|
• |
clinical evidence; |
|
|
• |
product price; |
|
|
• |
customer service; and |
|
|
• |
support by key opinion leaders. |
miraDry
The medical technology and aesthetic product markets are highly competitive and dynamic, and are characterized by rapid and substantial technological development and product innovations. Demand for the miraDry treatment could be limited by the products and technologies offered now or in the future by our competitors as well as the limited capital expenditure budgets of our physician customers. We designed the miraDry treatment to address the concerns of individuals who seek a durable solution to their axillary sweat. Therefore, we compete both directly and indirectly with those companies marketing botulinum toxin and other medical device companies. To a lesser extent, we indirectly compete with antiperspirants. We expect aesthetic medical device companies to pursue technological advances in the treatment of sweat, hair and odor removal that will continue to alter the competitive environment.
18
In the United States, our major competitor in the treatment of sweat is Allergan, which manufactures Botox; Botox is approved for the treatment of severe primary axillary hyperhidrosis. Cynosure also has recently received FDA clearance to market PrecisionTX for the treatment of primary axillary hyperhidrosis. These competitors have more resources than us and may prevent our miraDry System from gaining widespread market acceptance.
Due to less stringent regulatory requirements, there are many more aesthetic products and procedures available for use in international markets than are approved or cleared for use in the United States. There are also fewer limitations on the claims our competitors in international markets can make about the effectiveness of their products and the manner in which they can market them. As a result, we face more competition in these markets than in the United States.
Government Regulation
Our products are subject to extensive regulation by the FDA and other federal and state regulatory authorities, and other regulatory bodies in other countries.
Regulation by the FDA. The Federal Food, Drug and Cosmetic Act, or FDCA, and the FDA’s implementing regulations govern, among other things:
|
|
• |
product design and development; |
|
|
• |
pre‑clinical and clinical testing; |
|
|
• |
establishment registration and product listing; |
|
|
• |
product manufacturing; |
|
|
• |
product labeling and storage; |
|
|
• |
pre‑market clearance or approval; |
|
|
• |
post‑market studies; |
|
|
• |
advertising and promotion; |
|
|
• |
product sales and distribution; |
|
|
• |
record-keeping and device tracking; |
|
|
• |
complaint handling; |
|
|
• |
recalls and field safety corrective actions; and |
|
|
• |
post‑market surveillance and adverse event reporting, including reporting of deaths, serious injuries or device malfunctions. |
Unless an exemption applies, each new or significantly modified medical device we seek to commercially distribute in the United States will require either a pre‑market notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, or approval from the FDA of a PMA application. Both the 510(k) clearance and PMA approval processes can be expensive, lengthy and require payment of significant user fees, unless an exemption is available.
The FDA classifies medical devices into one of three classes. Unless specifically exempted from certain requirements, all three classes of devices are subject to general controls such as labeling, pre‑market notification and adherence to the FDA’s QSR, which cover manufacturers’ methods and documentation of the design, testing,
19
production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of products. Devices deemed to pose low to moderate risk are placed in Class I or II, which, absent an exemption, requires the applicant to obtain a 510(k) clearance. Class II devices are subject to special controls such as performance standards, post‑market surveillance, FDA guidelines, or particularized labeling requirements, as well as general controls. Some low risk devices are exempted by regulation from the 510(k) clearance requirement, and/or the requirement of compliance with substantially all of the QSR. A PMA application is required for devices deemed by the FDA to pose the greatest risk, such as life‑sustaining, life‑supporting or certain implantable devices, including all breast implants, or devices that are “not substantially equivalent” either to a device previously cleared through the 510(k) process or to a “preamendment” Class III device in commercial distribution in the United states before May 28, 1976 for which a regulation requiring a PMA application has not been issued by the FDA.
Our tissue expanders and our body contouring, facial and nasal implants received FDA clearance as Class II devices at various dates prior to approval of our breast implants in March 2012. Additionally, the miraDry System is currently regulated as a Class II device that requires 510(k) clearance.
To obtain 510(k) clearance, we must submit a pre‑market notification demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a preamendment device. The FDA’s 510(k) clearance pathway usually takes from three to 12 months from the date the application is completed, but it can take significantly longer and clearance is never assured. Although many 510(k) pre‑market notifications are cleared without clinical data, in some cases, the FDA requires significant clinical data to support substantial equivalence. In reviewing a pre‑market notification, the FDA may request additional information, including clinical data, which may significantly prolong the review process. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination regarding whether a new pre‑market submission is required for the modification of an existing device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA application is obtained. If the FDA requires us to seek 510(k) clearance or approval of a PMA application for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. In addition, in these circumstances, we may be subject to significant regulatory fines or penalties for failure to submit the requisite 510(k) clearance(s) or PMA application(s). In addition, the FDA is currently evaluating the 510(k) process and may make substantial changes to industry requirements.
Silicone gel breast implants are treated as Class III devices and a full PMA is required. A PMA for our breast implants was approved by the FDA in March 2012. The PMA application process is generally more costly and time consuming than the 510(k) process and requires proof of the safety and effectiveness of the device to the FDA’s satisfaction. Accordingly, a PMA application must be supported by valid scientific evidence that typically includes, but is not limited to, extensive information regarding the product, including pre‑clinical, clinical, and other product data to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. After a PMA application is submitted and found to be sufficiently complete, the FDA begins an in‑depth review of the submitted information. By statute, the FDA has 180 days to review the “accepted application,” although, generally, review of the application takes between one and three years, but may take significantly longer. During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA generally will conduct a pre‑approval inspection of the intended manufacturing facility to evaluate compliance with QSR, which requires manufacturers to implement and follow elaborate design, testing, control, documentation and other quality assurance procedures in the device design and manufacturing process.
The FDA may approve a PMA application with post‑approval conditions intended to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling, promotion, sale and distribution and collection of long‑term follow‑up data from patients in the clinical study that supported approval. Failure to comply with the conditions of approval can result in materially adverse enforcement action, including the loss or withdrawal of the approval. New PMA applications or PMA supplements are required for significant modifications
20
to the manufacturing process, labeling and design of a device that could affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application, and may not require as extensive clinical data or the convening of an advisory panel, depending on the nature of the proposed change.
Clinical Trials. A clinical trial is almost always required to support a PMA application and may be required for a 510(k) pre‑market notification. In the United States, absent certain limited exceptions, human clinical trials intended to support product clearance or approval require an Investigational Device Exemption, or IDE, application. Some types of studies deemed to present “non‑significant risk” are deemed to have an approved IDE once certain requirements are addressed and institutional review board, or IRB, approval is obtained. If the device presents a “significant risk” to human health, as defined by the FDA, the Sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to evaluate the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of subjects, unless the product is deemed a non‑significant risk device and eligible for more abbreviated IDE requirements. Clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the responsible institutional review boards at the clinical trial sites. There can be no assurance that submission of an IDE will result in the ability to commence clinical trials. Additionally, after a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to unacceptable health risks that outweigh the benefits of participation in the study. During a study, we are required to comply with the FDA’s IDE requirements for investigator selection, clinical trial monitoring, reporting, record keeping and prohibitions on the promotion of investigational devices or making safety or efficacy claims for them. We are also responsible for the appropriate labeling and distribution of investigational devices. Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy. The investigators must also obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of investigational devices and comply with all reporting and record-keeping requirements. The FDA’s grant of permission to proceed with clinical testing does not constitute a binding commitment that the FDA will consider the study design adequate to support clearance or approval. In addition, there can be no assurance that the data generated during a clinical study will meet chosen safety and effectiveness endpoints or otherwise produce results that will lead the FDA to grant marketing clearance or approval.
Other Regulatory Requirements. Even though our devices have been approved and commercialized, numerous regulatory requirements apply after a device is placed on the market, regardless of its classification or pre‑market pathway. These include, but are not limited to:
|
|
• |
establishment registration and device listing with the FDA; |
|
|
• |
QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, production, control, supplier and contractor selection, complaint handling, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
|
|
• |
labeling regulations that prohibit the promotion of products for uncleared or unapproved, or “off‑label,” uses, and impose other restrictions on labeling, advertising and promotion (in addition, the Federal Trade Commission has oversight of the advertising of medical devices other than “restricted” devices); |
|
|
• |
Medical Device Reporting, or MDR, regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; and |
21
|
|
mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death. |
The FDA requires us to conduct post‑market surveillance studies and to maintain a system for tracking our breast implants through the chain of distribution to the patient level. The FDA enforces regulatory requirements by conducting periodic, unannounced inspections and market surveillance. Inspections may include the manufacturing facilities of our subcontractors.
Failure by us or our manufacturer to comply with applicable regulatory requirements can result in enforcement actions by the FDA and other regulatory agencies. These may include, but may not be limited to, any of the following sanctions or consequences:
|
|
• |
warning letters or untitled letters that require corrective action; |
|
|
• |
fines and civil penalties; |
|
|
• |
unanticipated expenditures; |
|
|
• |
delays in or refusal to grant requests for 510(k) clearance or pre‑market approval of new products or modified products; |
|
|
• |
FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; |
|
|
• |
suspension or withdrawal of FDA clearance or approval; |
|
|
• |
product recall, detention or seizure; |
|
|
• |
operating restrictions, partial suspension or total shutdown of production; |
|
|
• |
injunctions and consent decrees; and |
|
|
• |
criminal prosecution. |
We and our contract manufacturers and some suppliers of components or device accessories also are required to manufacture our products in compliance with cGMP requirements set forth in the QSR. The QSR requires a quality system for the design, manufacture, packaging, labeling, storage, installation and servicing of marketed devices, and it includes extensive requirements with respect to quality management and organization, device design, buildings, equipment, purchase and handling of components or services, production and process controls, packaging and labeling controls, device evaluation, distribution, installation, complaint handling, servicing, and record keeping. The FDA evaluates compliance with the QSR through periodic, unannounced inspections that may include the manufacturing facilities of our subcontractors. If the FDA believes that we or any of our contract manufacturers or regulated suppliers are not in compliance with these requirements, it can shut down our manufacturing operations, require recall of our products, refuse to approve new marketing applications, institute legal proceedings to detain or seize products, enjoin future violations or assess civil and criminal penalties against us or our officers or other employees.
Healthcare Regulatory Laws. Our business activities, including but not limited to, research, sales, marketing, promotion, distribution, medical education and other activities may be subject to regulation under additional healthcare laws by numerous regulatory and enforcement authorities in the United States, in addition to the FDA. These laws include, without limitation, state and federal anti‑kickback, false claims, physician sunshine, and patient data privacy and security laws and regulations, including but not limited to those described below.
22
Additionally, our relationships with healthcare providers and other third parties are subject to scrutiny under these laws. Non‑compliance with the laws described below may generally result in the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations. Defending against any actions for non‑compliance of such laws can be costly, time‑consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
Federal Anti‑Kickback Law. The federal Anti‑Kickback Statute prohibits, among other things, knowingly or willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, to induce, or in return for, either the referral of an individual or the purchase, recommendation, order or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. The definition of “remuneration” has been broadly interpreted to include anything of value, including such items as improper payments, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, waiver of payments and providing anything at other than its fair market value. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the federal Anti‑Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case‑by‑case basis based on a cumulative review of all of its facts and circumstances.
The penalties for violating the federal Anti‑Kickback Statute include imprisonment for up to five years, fines of up to $25,000 per violation and possible exclusion from federal healthcare programs such as Medicare and Medicaid. Further, a person or entity does not need to have actual knowledge of this statute or specific intent to violate it in order to commit a violation. Rather, if “one purpose” of the remuneration is to induce referrals, the federal Anti-Kickback Statute is violated. In addition, a claim including items or services resulting from a violation of the federal Anti‑Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act, or FCA.
We have entered into consulting, speaker and other financial arrangements with physicians, including some who prescribe or recommend our products to patients. We engage such physicians as consultants, advisors and to educate other physicians. Noncompliance with the federal Anti‑Kickback Statute could result in the penalties set forth above.
Federal Civil False Claims Act. The FCA prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim to the federal government. The FCA has been used to prosecute persons submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. Manufacturers can be held liable under the FCA if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off‑label. Penalties for FCA violations include three times the actual damages sustained by the government, plus mandatory civil penalties of between $10,781.40 and $21,562.80 for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal FCA is a civil statute, FCA violations may also implicate various federal criminal statutes.
In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud, known as “qui tam”, or whistleblower, lawsuits. Because the complaint is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government intervenes and is ultimately successful in obtaining redress in the matter, or if the plaintiff succeeds in obtaining redress without the government’s involvement, then the plaintiff will receive a percentage of the recovery. Qui tam actions have increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a false claim action, pay fines or be excluded from Medicare, Medicaid or other federal or state healthcare programs as a result of an investigation arising out of such action.
23
Federal Criminal False Claims Laws. The federal criminal false claims laws prohibit, among other things, knowingly and willfully making, or causing to be made, a false statement or representation of a material fact for use in determining the right to any benefit or payment under a federal health care program. A violation of these laws may constitute a felony or misdemeanor and may result in fines or imprisonment.
Civil Monetary Penalties Law. The federal Civil Monetary Penalties Law prohibits, among other things, the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services. Noncompliance with such beneficiary inducement provision of the federal Civil Monetary Penalties Law can result in civil money penalties of up to $10,000 for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the federal healthcare programs.
Health Insurance Portability and Accountability Act of 1996. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, augmented two federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from governmental programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, including the Final HIPAA Omnibus Rule published on January 25, 2013, mandates, among other things, that certain types of entities and individuals adopts uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. Among other things, HITECH makes certain of HIPAA’s standards and requirements directly applicable to “business associates”—independent contractors or agents of covered entities that create, receive or obtain protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities and business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
Even when HIPAA does not apply, according to the U.S. Federal Trade Commission, or the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act, or the FTCA, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
In addition, certain state laws govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties.
Physician Payments Sunshine Act. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, PPACA, imposed, among other things, new annual reporting requirements for certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately, and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for
24
“knowing failures,” for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission. We are required to report detailed payment data and submit legal attestation to the accuracy of such data by March 31st of each calendar year.
In addition, there has been a recent trend of increased federal and state regulation of payments and other transfers of value provided to healthcare professionals and entities. Similar to the federal law, certain states also have adopted marketing and/or transparency laws relevant to device manufacturers, some of which are broader in scope. Certain states, such as California and Connecticut, also mandate that device manufacturers implement compliance programs. Other states, such as Massachusetts and Vermont, impose restrictions on device manufacturer marketing practices and require tracking and reporting of gifts, compensation, and other remuneration to healthcare professionals and entities. The need to build and maintain a robust compliance program with different compliance and/or reporting requirements increases the possibility that a healthcare company may violate one or more of the requirements, resulting in fines and penalties
Additional State Healthcare Laws. Many states have also adopted some form of each of the aforementioned laws, some of which may be broader in scope and may apply regardless of payor. Nevertheless, a determination of liability under such laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
Additionally, as some of these laws are still evolving, we lack definitive guidance as to the application of certain key aspects of these laws as they relate to our arrangements with providers with respect to patient training. We cannot predict the final form that these regulations will take or the effect that the final regulations will have on us. As a result, our provider and training arrangements may ultimately be found to be not in compliance with applicable laws.
United States Foreign Corrupt Practices Act. The United States Foreign Corrupt Practices Act, or FCPA, prohibits United States corporations and their representatives from offering, promising, authorizing or making corrupt payments, gifts or transfers to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA would include interactions with certain healthcare professionals in many countries.
International Regulation. We are approved to sell the miraDry System in over 40 international markets outside of North America. International sales of medical devices are subject to local government regulations, which may vary substantially from country to country. We may evaluate international expansion opportunities in the future for Breast Products. The time required to obtain approval in another country may be longer or shorter than that required for FDA approval, and the requirements may differ. There is a trend towards harmonization of quality system standards among the European Union, United States, Canada and various other industrialized countries.
The primary regulatory body in Europe is that of the European Union, which includes most of the major countries in Europe. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear the CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout Europe. The method of assessing conformity varies depending on the class of the product, but normally involves a combination of self‑assessment by the manufacturer and a third party assessment by a “Notified Body.” This third‑party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s product. An assessment by a Notified Body of one country within the European Union is required in order for a manufacturer to commercially distribute the product throughout the European Union. Additional local requirements may apply on a country‑by‑country basis. Outside of the European Union, regulatory approval would need to be sought on a country‑by‑country basis in order for us to market our Breast Products.
Coverage and Reimbursement. Sales of our products depend, in part, on the extent to which the procedures using our products will be covered by third‑party payors, such as government health care programs, commercial insurance and managed healthcare organizations. Breast augmentation and miraDry procedures are generally performed on a cash‑pay basis and are not covered by third‑party payors. In contrast, breast reconstruction procedures may be
25
covered by third‑party payors, but such third‑party payors are increasingly limiting coverage and reducing reimbursements for medical products and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost‑containment programs, including price controls, restrictions on coverage and reimbursement. Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical drug products and medical services, in addition to questioning their safety and efficacy. Adoption of price controls and cost‑containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net sales and results.
Moreover, the process for determining whether a third-party payor will provide coverage for a product or procedure may be separate from the process for establishing the reimbursement rate that such a payor will pay for the product or procedure. A payor’s decision to provide coverage for a product or procedure does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product or procedure does not assure that other payors will also provide coverage for the product or procedure. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to ensure profitability.
Health Reform. The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our business. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access.
There have been a number of legislative and regulatory proposals to change the healthcare system, reduce the costs of healthcare and change medical reimbursement policies. Doctors, clinics, hospitals and other users of our products may decline to purchase our products to the extent there is uncertainty regarding coverage from government or commercial payors. Further proposed legislation, regulation and policy changes affecting third-party reimbursement are likely. Among other things, Congress has in the past proposed changes to and the repeal of the Patient Protection and Affordable Care and Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the Affordable Care Act), and lawsuits have been brought challenging aspects of the law at various points. There have been repeated recent attempts by Congress to repeal or replace the Affordable Care Act. Some of the provisions of the ACA have yet to be implemented, and there have been legal and political challenges to certain aspects of the ACA. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress has considered legislation that would repeal and replace all or part of the ACA. While Congress has previously been successful at passing comprehensive repeal legislation through both Chambers of Congress, it had then been vetoed by former President Obama; however full repeal legislation is unlikely in the current political climate. Furthermore, the Tax Cuts and Jobs Act passed in December of 2017 included a provision that would repeal one of the primary pillars of the law, the ACA’s individual mandate penalty that essentially assessed a monetary penalty or fine on certain individuals who fail to maintain qualifying health coverage for all or part of a year. Congress may consider other legislation to repeal or replace elements of the ACA on a provision-by-provision basis.
The ACA had also imposed, among other things, a federal excise tax of 2.3% on certain entities that manufacture or import medical devices for sale in the United States. This tax had previously been suspended, and on January 22, 2018 both the House and Senate struck a short-term Continuing Resolution to fund the federal government, H.R. 195, which included a two-year suspension on the medical device excise tax. This two-year delay is retroactively applied starting December 31, 2017, and will officially extend through January 1, 2020. The future of this provision beyond that point is uncertain, as there may be additional attempt to fully repeal the provision.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.
26
Intellectual Property and Proprietary Rights
We believe that in order to maintain a competitive advantage in the marketplace, we must develop and maintain protection of the proprietary aspects of our product lines. We rely on a combination of trademarks, trade secrets, confidential information, copyrights, patent rights and other intellectual property rights to protect our intellectual property.
Our Breast Products trademark portfolio consists of seven registered U.S. trademarks. Our miraDry trademark portfolio consists of 97 worldwide registered trademarks and 30 pending trademark applications.
Our Breast Products patent portfolio consists of 1 pending U.S. patent application, as well as several in-licensed patent rights. Our miraDry patent portfolio is comprised of 21 granted or allowed U.S. patents, 80 granted or allowed foreign counterpart patents, 10 pending or published U.S. patent applications, and 34 pending or published foreign counterpart patent applications.
In addition, to protect our trade secrets, confidential information and other intellectual property rights, we have entered into confidentiality agreements with third parties, and confidential information and invention assignment agreements with employees, consultants and advisors.
There are risks related to our intellectual property rights. For further details on these risks, see Item 1A — “Risk Factors.”
Employees
As of December 31, 2017, we had 200 full‑time employees. None of our employees are represented by a collective bargaining agreement, and we have never experienced any work stoppage. We believe we have good relations with our employees.
Seasonality
We expect that, in the future, our net sales will fluctuate on a quarterly basis due to a variety of factors, including seasonality of breast augmentation procedures and purchase of miraDry procedures. We believe that aesthetic procedures are subject to seasonal fluctuation due to patients planning their procedures leading up to the summer season and in the period around the winter holiday season.
Corporate Information
We incorporated in Delaware on August 29, 2003 under the name Juliet Medical, Inc. and subsequently changed our name to Sientra, Inc. in April 2007. Our principal executive offices are located at 420 South Fairview Avenue, Suite 200, Santa Barbara, California, 93117, and our telephone number is (805) 562‑3500. Our website is located at www.sientra.com, and our investor relations website is located at http://investors.sientra.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, reports on Form 8-K and our Proxy Statements are available through our investor relations website, free of charge, as soon as reasonably possible after we file them with the SEC.
27
You should carefully consider the following risk factors, as well as the other information appearing elsewhere in this Annual Report on Form 10-K, including our financial statements and related notes, before deciding whether to purchase, hold or sell shares of our common stock. The occurrence of any of the following risks could harm our business, financial condition, results of operations and/or growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. You should consider all of the risk factors described when evaluating our business.
Risks Relating to Our Business and Our Industry
We will need to raise additional equity or debt capital, which may not be available on acceptable terms, or at all. If we are unable to raise additional funds, there may be substantial doubt in our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm included in this Annual Report contains an explanatory paragraph with respect to our liquidity.
As of December 31, 2017, we had cash and cash equivalents of approximately $26.6 million. We have incurred recurring losses from operations and cash outflows from operating activities that raise substantial doubt about our ability to continue as a going concern. In addition, while we were in compliance with the financial covenants in our credit agreement with MidCap Financial Trust at December 31, 2017, given the potential violations of those covenants during fiscal 2018, we have classified the debt as current in the consolidated balance sheet at December 31, 2017. To fund our ongoing operating and capital needs, we will need to raise additional equity or debt capital. We have taken steps to address our cash position. For example, we have the ability, upon receipt of FDA certifications of the manufacturing facility operated by Vesta by March 31, 2018, to borrow a $10.0 million term loan pursuant to the credit agreement, however, there can be no assurance that we will receive such approval by March 31, 2018. In addition, in February 2018, we entered into an At-The-Market Equity Offering Sales Agreement with Stifel, Nicolaus & Company, Incorporated as sales agent pursuant to which we may sell, from time to time, through Stifel shares of our common stock having an aggregate gross offering price of up to $50 million by any method deemed to be an “at-the-market offering” as defined in Rule 415 under the Securities Act. In addition to the foregoing actions, we will likely seek to raise additional equity or debt capital. There can be no assurance, however, that we will be successful in completing an equity or debt financing on a timeframe that coincides with our cash needs, on acceptable terms, or completing it at all.
As a result of the uncertainty surrounding our ability to raise additional capital and as to our future liquidity, the report of our independent registered public accounting firm included in this Annual Report on Form 10K includes an explanatory paragraph that refers to conditions that raise substantial doubt about our ability to continue as a going concern. The consolidated financial statements included in this Annual Report have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. However, if we are not successful in raising sufficient additional capital as needed, we may be compelled to reduce the scope of our operations and planned capital expenditures and/or sell or license certain assets at inopportune times, which could have a material and adverse effect on our ability to pursue our business strategy and our future financial condition.
We have incurred significant net operating losses since inception and cannot assure you that we will achieve profitability.
Since our inception, we have incurred significant net operating losses. As of December 31, 2017, we had an accumulated deficit of $279.5 million. To date, we have financed our operations primarily through sales of preferred stock, borrowings under our term loans, sales of our products since 2012, our initial public offering and follow-on public offerings of our common stock. We have devoted substantially all of our resources to the acquisition and clinical development of our products, the commercial launch of our products, the development of a sales and marketing team and the assembly of a management team to manage our business.
For the year ended December 31, 2017, our net loss was $64.0 million. The extent of our future operating losses and the timing of profitability are uncertain, especially in light of our inventory supply issues. We will need to generate significant sales to achieve profitability, and we might not be able to do so. Even if we do generate significant sales,
28
we might not be able to achieve, sustain or increase profitability on a quarterly or annual basis in the future. If our sales grow more slowly than we have forecasted, or if our operating expenses exceed our forecasts, our financial performance and results of operations will be adversely affected.
We may not successfully integrate newly acquired businesses into our business operations or realize the benefits of partnerships with other companies, acquisitions of complementary products or technologies or other strategic alternatives.
We have recently completed a series of business and product acquisitions including our acquisition of miraDry and our product acquisitions, including BIOCORNEUM and tissue expanders from SSP. As a result of these acquisitions, we have undergone substantial changes to our business and product offerings in a short period of time. In addition, in the future, we may consider other opportunities to partner with or acquire other businesses, products or technologies that may enhance our product platform or technology, expand the breadth of our markets or customer base or advance our business strategies.
Integrating the business practice and operations of a new business with that of our own is a complex, costly and time-consuming process, which requires significant management attention and resources. The integration process may disrupt our existing operations and, if implemented ineffectively, would preclude realization of the full benefits expected by us. Our failure to meet the challenges involved in successfully integrating our acquisitions in order to realize the anticipated benefits may cause an interruption of, or a loss of momentum in, our operating activities and could adversely affect our results of operations. Potential difficulties, costs and delays we may encounter as part of the integration process may include:
|
|
• |
distracting management from day‑to‑day operations; |
|
|
• |
potential incompatibility of corporate cultures; |
|
|
• |
an inability to achieve synergies as planned; |
|
|
• |
risks associated with the assumption of contingent or other liabilities of acquisition targets; |
|
|
• |
adverse effects on existing business relationships with suppliers or customers; |
|
|
• |
inheriting and uncovering previously unknown issues, problems and costs from the acquired company; |
|
|
• |
uncertainties associated with entering new markets in which we have limited or no experience; |
|
|
• |
increased legal and accounting costs relating to the partnership or acquisition or compliance with regulatory matters; |
|
|
• |
delays between our expenditures to acquire new products, technologies or businesses and the generation of revenues from those acquired products, technologies or businesses; |
|
|
• |
realization of assets and settlement of liabilities at amounts equal to estimated fair value as of the acquisition date of any acquisition or disposition; |
|
|
• |
costs and delays in implementing common systems and procedures (including technology, compliance programs, financial systems, distribution and general business operations, among others); and |
|
|
• |
increased difficulties in managing our business due to the addition of international locations. |
Any one or all of these factors may increase operating costs or lower anticipated financial performance. Many of these factors are also outside of our control. In addition, even if new business operations are integrated successfully, we may not realize the full benefits of the acquisition, including the synergies, cost savings or sales or growth
29
opportunities that we expect or within the anticipated time frame. Additional unanticipated costs may be incurred in the integration of the businesses. All of these factors could decrease or delay the expected accretive effect of the transaction, and negatively impact the price of our common stock. The failure to integrate the business operations of miraDry or any acquired business successfully would have a material adverse effect on our business, financial condition and results of operations.
In addition to integration related issues, the acquisition of miraDry has significantly increased the size of our business, augmenting a number of the risks included in these risk factors. Future success depends, in part, upon our ability to manage this expanded business, which will pose substantial challenges for management. There can be no assurance that we will be successful realizing the expected benefits from this acquisition.
We depend on a positive reaction from our Plastic Surgeons and their patients to successfully re-enter the Breast Product market and achieve profitability.
Our Breast Products segment has historically accounted for substantially all of our net sales and we expect our Breast Products to continue to be a substantial majority of our net sales.
We depend on a continued positive reception from our Plastic Surgeon customers and their patients to be able to reestablish the market position we had prior to the voluntary suspension of our Breast Products manufactured by Silimed. Additionally, our re-entry into the market has required us to effectively and responsibly educate accounts on the results of our testing and reconfirm our strong clinical data, while providing the same high levels of customer service to which our Plastic Surgeons are accustomed. Our plastic surgery consultants are working diligently to solidify the confidence and support of all our Plastic Surgeons; however, if we are not successful in re-establishing and maintaining these relationships or competing effectively in this market, our sales revenues, market share and financial performance will be affected negatively.
Our inability to manage our inventory supply issues, the inability to qualify Vesta or another third party as an alternate manufacturer, the potential loss of market acceptance of our Breast Products, or any adverse rulings by regulatory authorities, any adverse publicity or other adverse events relating to us or our Breast Products, or the introduction of competitive products by our competitors and other third parties, would adversely affect our business, financial condition and results of operations.
If the market acceptance for the miraDry System, which has a limited commercial history, fails to grow significantly, our business and future prospects will be harmed.
Commercial sales of the miraDry System commenced in the United States in 2012 and in Japan in 2011 for the treatment of primary axillary hyperhidrosis, or a condition characterized by abnormal sweating in excess of that required for regulation of body temperature, and which we refer to as being “sweat-bothered”, plus unwanted underarm hair removal, and permanent reduction of underarm hair of all colors for Fitzpatrick skin types I – IV. When used for the treatment of primary axillary hyperhidrosis, the miraDry System may also reduce underarm odor. We expect that the revenues we generate from sales of our miraDry System and bioTips will account for a substantial amount of our revenues for the next several years. Accordingly, our success depends on the acceptance among physicians and patients of the miraDry procedure as a preferred treatment for being sweat-bothered. Although we have received FDA clearance to market the miraDry procedure for the treatment of primary axillary hyperhidrosis in the United States and are approved or are otherwise free to market the miraDry procedure in over 40 international markets, the degree of market acceptance of the miraDry procedure by physicians and patients is unproven. We believe that market acceptance of the miraDry procedure will depend on many factors, including:
|
|
• |
the perceived advantages or disadvantages of the miraDry System compared to other products and procedures; |
|
|
• |
the safety and efficacy of the miraDry System relative to other products and alternative procedures; |
30
|
|
• |
the development and publication of long-term clinical data in peer-reviewed journals supporting the long term efficacy of the miraDry procedure; |
|
|
• |
our ability to obtain regulatory clearance to market miraDry for additional treatment indications in the United States and other international markets; |
|
|
• |
the success of patient education through direct-to-consumer marketing campaigns that utilize social media outlets and testimonials. |
We cannot guarantee that the miraDry System will achieve broad market acceptance among physicians and patients. Because we expect to derive a substantial portion of sales from the miraDry Systems and the sale of our consumable bioTip products, any failure of this product to achieve meaningful market acceptance will harm our business and future prospects.
We may not be able to procure and qualify a new manufacturer for our silicone gel breast implants and other products previously manufactured by Silimed, or obtain approval for all related manufacturing changes.
Our manufacturing contract with Silimed expired on its terms on April 1, 2017, and we did not renew it. Moreover, our existing inventory of breast implants that were previously manufactured by Silimed is limited.
Although we have entered into a definitive manufacturing agreement with Vesta, Vesta has not yet been qualified as a manufacturer to source our implants. In January 2018, the FDA granted approval of the site-change PMA supplement for Vesta to manufacture our silicone gel breast implants. In support of the move to the Vesta manufacturing facility, we also implemented new manufacturing process improvements which, in consultation with the FDA, required three (3) additional PMA supplements. In addition to approving the manufacturing site-change supplement, the FDA has approved two (2) of these three (3) process enhancement supplements, while requesting additional information for the third submission. We continue to work closely with the FDA to address their information requests related to this third and final outstanding submission in order to resolve these matters in a timely manner. Any delays or our inability to qualify Vesta or negotiate a manufacturing agreement and qualify another alternate manufacturer could result in a supply interruption, which would materially adversely affect our business, financial condition and results of operations.
Direct-to-consumer marketing and social media effort may expose us to additional regulatory scrutiny.
Our efforts to promote our products via direct-to-consumer marketing and social media initiatives may subject us to additional scrutiny of our practices of effective communication of risk informations, under the oversight of the FDA, FTC, or both.
Contracting with any third-party manufacturer and supplier involves inherent risks and various factors outside our direct control that may adversely affect the manufacturing and supply of our products.
Our reliance on any third-party manufacturer, including Vesta, Formulated Solutions, LLC, or Formulated Solutions, which supplies our BIOCORNEUM scar management products, SiMatrix, a Vesta subsidiary that supplies the tissue expanders we recently acquired from SSP, Healthcare Technology International which supplies bioTips for our miraDry System or any other third-party manufacturer we procure and qualify for the manufacture of our Breast Products or miraDry Products involves a number of risks. Changes that our manufacturers may make outside the purview of our direct control, or other mistakes and mishandling of our products, can have an impact on
31
our processes and quality, as well as the successful delivery of our products. Additionally, if any third-party manufacturer becomes unable or unwilling to supply our products, we may not be able to find an alternate supplier in a timely manner. For example, there are only a few suppliers of medical-grade silicone available, and if these suppliers become unable or unwilling to supply medical-grade silicone to Vesta, Formulated Solutions, SiMatrix or any other manufacturer that we may engage with, an alternate supply of medical-grade silicone may not be able to be found in a timely manner. Our existing manufacturing contracts will also expire, and there can be no assurance that our contracting counterparties will agree to continue to manufacture and supply our products or they may impose increased pricing terms if the contract is renegotiated or renewed.
Some of the additional risks with relying on third-party manufacturers and suppliers include:
|
|
• |
our products may not be manufactured in accordance with agreed upon specifications or in compliance with regulatory requirements or cGMP, or the manufacturing facilities may not be able to maintain compliance with regulatory requirements or cGMP, which could negatively affect the safety or efficacy of our products or cause delays in shipments of our products; |
|
|
• |
we may not be able to timely respond to unanticipated changes in customer orders, and if orders do not match forecasts, we may have excess or inadequate inventory of materials and components; |
|
|
• |
our products may be mishandled while in production or in preparation for transit; |
|
|
• |
we are subject to transportation and import and export risk, particularly given the global nature of our supply chain; |
|
|
• |
the third-party manufacturer may discontinue manufacturing and supplying products to us for risk management reasons; |
|
|
• |
the third-party manufacturer may lose access to critical services and components, resulting in an interruption in the manufacturing or shipment of our products; |
|
|
• |
the third-party manufacturer may encounter financial or other hardships unrelated to us and our demand for products, which could inhibit our ability to fulfill our orders; |
|
|
• |
there may be delays in analytical results or failure of analytical techniques that we depend on for quality control and release of products; |
|
|
• |
natural disasters, labor disputes, financial distress, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations affecting our manufacturer or its suppliers may occur; |
|
|
• |
latent defects may become apparent after products have been released and which may result in a recall of such products; and |
|
|
• |
there are inherent risks if we contract with manufacturers located outside of the United States, including the risks of economic change, recession, labor strikes or disruptions, political turmoil, new or changing tariffs or trade barriers, new or different restrictions on importing or exporting, civil unrest, infrastructure failure, cultural differences in doing business, lack of contract enforceability, lack of protection for intellectual property, war and terrorism. |
The materialization of any of these risks and limitations inherent in a third-party manufacturing contractual relationship could significantly increase our costs, impair our ability to generate net sales, and adversely affect market acceptance of our products and customers may instead purchase or use our competitors’ products, which could materially adversely and severely affect our business, financial condition and results of operations.
32
We have a limited operating history and may face difficulties encountered by companies early in their commercialization in competitive and rapidly evolving markets.
We commenced operations in 2007 and began commercializing silicone gel breast implants in the second quarter of 2012. Accordingly, we have a limited operating history upon which to evaluate our business and forecast our future net sales and operating results. In assessing our business prospects, you should consider the various risks and difficulties frequently encountered by companies early in their commercialization in competitive markets, particularly companies that develop and sell medical devices. These risks include our ability to:
|
|
• |
implement and execute our business strategy; |
|
|
• |
expand and improve the productivity of our sales force and marketing programs to grow sales of our existing and proposed products; |
|
|
• |
increase awareness of our brand and build loyalty among Plastic Surgeons; |
|
|
• |
manage expanding operations; |
|
|
• |
respond effectively to competitive pressures and developments; |
|
|
• |
enhance our existing products and develop new products; |
|
|
• |
obtain regulatory clearance or approval to enhance our existing products and commercialize new products; |
|
|
• |
perform clinical trials with respect to our existing products and any new products; and |
|
|
• |
attract, retain and motivate qualified personnel in various areas of our business. |
Due to our limited operating history, we may not have the institutional knowledge or experience to be able to effectively address these and other risks that we may face. In addition, we may not be able to develop insights into trends that could emerge and negatively affect our business and may fail to respond effectively to those trends. As a result of these or other risks, we may not be able to execute key components of our business strategy, and our business, financial condition and operating results may suffer.
If we fail to compete effectively against our competitors, some of which have significantly greater resources than we have, our net sales and operating results may be negatively affected.
Our industry is intensely competitive and subject to rapid change from the introduction of new products, technologies and other activities of industry participants. For example, our Breast Products competitors, Mentor, a wholly owned subsidiary of Johnson & Johnson, and Allergan are well-capitalized global pharmaceutical companies that have been the market leaders for many years and have the majority share of the breast implant market in the United States. These competitors also enjoy several competitive advantages over us, including:
|
|
• |
greater financial and human resources for sales, marketing and product development; |
|
|
• |
established relationships with health care providers and third-party payors; |
|
|
• |
established reputations and name recognition among health care providers and other key opinion leaders in the plastic surgery industry; |
|
|
• |
in some cases, an established base of long-time customers; |
|
|
• |
greater financial resources and economies-of-scale to put additional pricing pressure on competing products; |
33
|
|
• |
larger and more established distribution networks; |
|
|
• |
greater ability to cross-sell products; and |
|
|
• |
more experience in conducting research and development, manufacturing, performing clinical trials and obtaining regulatory approval or clearance. |
If we fail to compete effectively against our competitors, our net sales and operating results may be negatively affected.
The long-term safety of our Breast Products has not fully been established and our breast implants are currently under study in our PMA post-approval studies, which could reveal unanticipated complications.
We have been marketing our silicone gel breast implants in the United States with pre-market approval from the FDA since 2012. However, there could still be unanticipated complications or unforeseen health consequences of being implanted with our silicone gel breast implants over the long-term (defined as 10 years or more). Additionally, we rely on our clinical data to make favorable comparisons of our product to our competitive products, and our longer-term data may change over time. Further, future studies or clinical experience may indicate that treatment with our products is not differentiated to treatment with competitive products. Such results could slow the adoption of our products and significantly reduce our sales, which could prevent us from achieving our forecasted sales targets or achieving or sustaining profitability. Moreover, if long-term results and experience indicate that our products cause unexpected or serious complications, we could be subject to mandatory product recalls, suspension or withdrawal of clearance or approval by the FDA or other applicable regulatory bodies and significant legal liability.
Among the long-term health risks of breast implants which are being studied and followed, health regulators believe there is an association between breast implants and a rare form of lymphoma called anaplastic large-cell lymphoma.
In January 2011, the FDA issued a Safety Communication indicating that there was a possible association between saline and silicone gel breast implants and anaplastic large-cell lymphoma, or BIA-ALCL. Since our FDA approval in 2012, Sientra’s breast-implant product label, which is approved by the FDA, has been required to contain a description of BIA-ALCL as a possible, though “rare,” outcome. Since its report in January 2011, the FDA has continued to gather information about BIA-ALCL in women with breast implants through the review of medical device reports, review of medical literature, and collaboration with international regulators, scientific experts, ASPS, ASAPS, ISAPS, and other organizations.
As of August 23, 2017, the FDA updated its recommendations on BIA-ALCL and subsequently requested all breast implant manufacturers to revise their physician and patient labeling with the most up-to-date information. The FDA still describes BIA-ALCL as “rare” and states: “we have strengthened our understanding of this condition and concur with the World Health Organization designation of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) as a rare T-cell lymphoma that can develop following breast implants. The exact number of cases remains difficult to determine due to significant limitations in world-wide reporting and lack of global implant sales data. At this time, most data suggest that BIA-ALCL occurs more frequently following implantation of breast implants with textured surfaces rather than those with smooth surfaces.” The FDA noted it does not recommend prophylactic breast implant removal in a patient without symptoms or other abnormality.
Further studies or clinical experience may indicate that breast implants, including our products, expose individuals to a more substantial risk of developing BIA-ALCL or other unexpected complications than currently anticipated. As a result, we may be exposed to increased regulatory scrutiny, negative publicity and lawsuits from any individual who may develop BIA-ALCL after using our products, any of which could have a significant negative impact on our results of operations or financial condition. Moreover, if long-term results and clinical experience indicate that our products cause unexpected or serious complications, we could be subject to mandatory product recalls, suspension or withdrawal of regulatory clearances and approvals and significant legal liability.
34
If we are unable to train Plastic Surgeons on the safe and appropriate use of our products, we may be unable to achieve our expected growth.
An important part of our sales process includes the ability to educate Plastic Surgeons about the availability of anatomically-shaped breast implants and train Plastic Surgeons on the safe and appropriate use of our products. If we become unable to attract potential new Plastic Surgeon customers to our education and training programs, we may be unable to achieve our expected growth.
There is a learning process involved for Plastic Surgeons to become proficient in the use of our anatomically-shaped products. It is critical to the success of our commercialization efforts to train a sufficient number of Plastic Surgeons and provide them with adequate instruction in the appropriate use of our products via preceptorships and additional demonstration surgeries. This training process may take longer than expected and may therefore affect our ability to increase sales. Following completion of training, we rely on the trained Plastic Surgeons to advocate the benefits of our products in the marketplace. Convincing Plastic Surgeons to dedicate the time and energy necessary for adequate training is challenging, and we cannot assure you that we will be successful in these efforts. If Plastic Surgeons are not properly trained, they may misuse or ineffectively use our products. This may also result in, among other things, unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us, any of which could have an adverse effect on our business and reputation.
If we are unable to continue to enhance our existing product offerings and develop and market new products that respond to customer needs and preferences and achieve market acceptance, we may experience a decrease in demand for our products and our business could suffer.
We may not be able to compete effectively with our competitors, and ultimately satisfy the needs and preferences of our customers, unless we can continue to enhance existing products and develop and market new innovative products. Product development requires the investment of significant financial, technological and other resources. Product improvements and new product introductions also require significant planning, design, development and testing at the technological, product and manufacturing process levels and we may not be able to timely develop product improvements or new products. Our competitors’ new products may beat our products to market, be more effective with new features, obtain better market acceptance or render our products obsolete. Any new or modified products that we develop may not receive clearance or approval from the FDA, or achieve market acceptance or otherwise generate any meaningful sales or profits for us relative to our expectations based on, among other things, existing and anticipated investments in manufacturing capacity and commitments to fund advertising, marketing, promotional programs and research and development.
35
Laws impacting the U.S. healthcare system are subject to a great deal of uncertainty, which may result in adverse consequences to our business.
There have been a number of legislative and regulatory proposals to change the healthcare system, reduce the costs of healthcare and change medical reimbursement policies. Doctors, clinics, hospitals and other users of our products may decline to purchase our products to the extent there is uncertainty regarding coverage from government or commercial payors. Further proposed legislation, regulation and policy changes affecting third-party reimbursement are likely. Among other things, Congress has in the past proposed changes to and the repeal of the Patient Protection and Affordable Care and Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the Affordable Care Act), and lawsuits have been brought challenging aspects of the law at various points. There have been repeated recent attempts by Congress to repeal or replace the Affordable Care Act. Some of the provisions of the ACA have yet to be implemented, and there have been legal and political challenges to certain aspects of the ACA. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress has considered legislation that would repeal and replace all or part of the ACA. While Congress has previously been successful at passing comprehensive repeal legislation through both Chambers of Congress, it had then been vetoed by former President Obama; however full repeal legislation is unlikely in the current political climate. Furthermore, the Tax Cuts and Jobs Act passed in December of 2017 included a provision that would repeal one of the primary pillars of the law, the ACA’s individual mandate penalty that essentially assessed a monetary penalty or fine on certain individuals who fail to maintain qualifying health coverage for all or part of a year. Congress may consider other legislation to repeal or replace elements of the ACA on a provision-by-provision basis. We are unable to predict what legislation or regulation, if any, relating to the health care industry or third-party coverage and reimbursement may be enacted in the future at the state or federal level, or what effect such legislation or regulation may have on us. Denial of coverage and reimbursement of our products, or the revocation or changes to coverage and reimbursement policies, could have a material adverse effect on our business, results of operations and financial condition.
If changes in the economy and consumer spending reduce consumer demand for our products, our sales and profitability would suffer.
We are subject to the risks arising from adverse changes in general economic and market conditions. Certain elective procedures, such as breast augmentation, are typically not covered by insurance. Adverse changes in the economy may cause consumers to reassess their spending choices and reduce the demand for these surgeries and could have an adverse effect on consumer spending. This shift could have an adverse effect on our net sales. Furthermore, consumer preferences and trends may shift due to a variety of factors, including changes in demographic and social trends, public health initiatives and product innovations, which may reduce consumer demand for our products.
Any negative publicity concerning our products could harm our business and reputation and negatively impact our financial results.
The responses of potential patients, physicians, the news media, legislative and regulatory bodies and others to information about complications or alleged complications of our products could result in negative publicity and could materially reduce market acceptance of our products. These responses or any investigations and potential resulting negative publicity may have a material adverse effect on our business and reputation and negatively impact our financial condition, results of operations or the market price of our common stock. In addition, significant negative publicity could result in an increased number of product liability claims against us.
We are required to maintain high levels of inventory, which could consume a significant amount of our resources and reduce our cash flows.
We need to maintain substantial levels of inventory to protect ourselves from supply interruptions, provide our customers with a wide range of shapes and sizes of our breast implants, and account for the high return rates we experience as Plastic Surgeons typically order our products in multiple sizes for a single surgery and then return what they do not use. As a result of the substantial inventory levels we like to maintain, we are subject to the risk that a substantial portion of our inventory becomes obsolete. The materialization of any of these risks may have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory. Additionally, our ability to find an alternate
36
supplier in a timely manner, may affect our ability to maintain the level of inventory supply we require to protect ourselves from supply interruptions that could have an unfavorable impact on our net sales.
Any disruption at our facilities could adversely affect our business and operating results.
Our principal offices are located in Santa Barbara, California. Substantially all of our operations are conducted at this location, including customer service, development and management and administrative functions. Substantially all of our inventory of Breast Products is held at a second location in Santa Barbara, California, and we manufacture, distribute, and service our miraDry Systems at a third location in Santa Clara, California. Despite our efforts to safeguard our facilities, including acquiring insurance, adopting health and safety protocols and utilizing off-site storage of computer data, vandalism, terrorism or a natural or other disaster, such as an earthquake, fire or flood, could damage or destroy our inventory of finished goods, cause substantial delays in our operations, result in the loss of key information and cause us to incur additional expenses. Our insurance may not cover our losses in any particular case. In addition, regardless of the level of insurance coverage, damage to our facilities may have a material adverse effect on our business, financial condition and operating results.
If there are significant disruptions in our information technology systems, our business, financial condition and operating results could be adversely affected.
The efficient operation of our business depends on our information technology systems. We rely on our information technology systems to effectively manage sales and marketing data, accounting and financial functions, inventory, product development tasks, clinical data, and customer service and technical support functions. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, computer viruses or hackers, power losses, and computer system or data network failures. In addition, a variety of our software systems are cloud-based data management applications hosted by third-party service providers whose security and information technology systems are subject to similar risks.
The failure of our or our service providers’ information technology could disrupt our entire operation or result in decreased sales, increased overhead costs and product shortages, all of which could have a material adverse effect on our reputation, business, financial condition and operating results.
We may be adversely affected by earthquakes or other natural disasters and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Our corporate headquarters and certain other facilities are located in Santa Barbara, California, which in the past has experienced both severe earthquakes and wildfires. We do not carry earthquake insurance. Earthquakes, wildfires or other natural disasters could severely disrupt our operations, and have a material adverse effect on our business, results of operations, financial condition and prospects.
If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as our enterprise financial systems or manufacturing resource planning and enterprise quality systems, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place currently are limited and are unlikely to prove adequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which, particularly when taken together with our lack of earthquake insurance, could have a material adverse effect on our business.
Failure to obtain hospital or group purchasing organization contracts could have a material adverse effect on our financial condition and operating results.
A portion of our net sales is derived from sales to hospitals. Many hospital customers, through the contracting process, limit the number of breast implant suppliers that may sell to their institution. Hospitals may choose to contract with our competitors who have a broader range of products that can be used in a wider variety of procedures or our competitors may actively position their broader product portfolios against us during the hospital
37
contracting process. Any limitations on the number of hospitals to which we can sell our products may significantly restrict our ability to grow.
In addition, contracts with hospitals and group purchasing organizations, or GPOs, often have complex insurance and indemnification requirements, which may not be beneficial to us, or we may not be able to successfully negotiate contracts with a substantial number of hospitals and GPOs at all, which could adversely affect our business, financial condition and results of operations.
Our business could suffer if we lose the services of key personnel or are unable to attract and retain additional qualified personnel.
We are dependent upon the continued services of key personnel, including members of our executive management team who have extensive experience in our industry. The loss of any one of these individuals could disrupt our operations or our strategic plans. Additionally, our future success will depend on, among other things, our ability to continue to hire and retain the necessary qualified sales, marketing and managerial personnel, for whom we compete with numerous other companies, academic institutions and organizations. If we lose key employees, if we are unable to attract or retain other qualified personnel, or if our management team is not able to effectively manage us through these events, our business, financial condition, and results of operations may be adversely affected.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
As of December 31, 2017, we had approximately 200 full-time employees. Our management and personnel, and the systems and facilities we currently have in place, may not be adequate to support future growth. Effectively executing our growth strategy requires that we increase net sales through sales and marketing activities, recruit and retain additional employees and continue to improve our operational, financial and management controls, reporting systems and procedures. If we are not able to effectively expand our organization in these ways, we may not be able to successfully execute our growth strategy, and our business, financial condition and results of operations may suffer.
We are subject to political, economic and regulatory risks associated with doing business outside of the United States.
As a result of our acquisition of miraDry, we now face new risks inherent in conducting business internationally, including compliance with international and U.S. laws and regulations that apply to international operations. We are able to market and sell the miraDry System in over 40 international markets outside of North America, including countries in Asia, Europe, the Middle East and South America. In addition, we may seek to market and sell the miraDry System in additional countries, as well as seek approval to market and sell our breast products in international markets, in the future. These laws and regulations are complex, and there is a risk that some provisions may be breached by us, for example through fraudulent or negligent behavior of individual employees, our failure to comply with certain formal documentation requirements, or otherwise. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, requirements to obtain export licenses, implementation of compliance programs, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, our business and our operating results.
In addition to the foregoing, engaging in international business inherently involves a number of other difficulties and risks, including:
|
|
• |
longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
|
|
• |
political and economic instability or sanctions in areas in which we operate; |
38
|
|
• |
potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements and other trade barriers; |
|
|
• |
regulations related to customs and import/export matters; |
|
|
• |
tax issues, such as tax law changes and variations in tax laws; |
|
|
• |
challenges in collecting accounts receivable from customers in the jurisdictions in which we operate; |
|
|
• |
complying with laws, rules and regulations relating to the manufacturing, marketing, distribution and sale of products in the jurisdictions in which we do or will operate; |
|
|
• |
operating under regulations in jurisdictions related to obtaining eligibility for government or private payor reimbursement for our products at the wholesale/retail level; |
|
|
• |
difficulties and costs of staffing and managing foreign operations, including cultural and language differences and additional employment regulations, union workforce negotiations and potential disputes in the jurisdictions in which we operate; |
|
|
• |
difficulties associated with compliance with a variety of laws and regulations governing international trade, including the Foreign Corrupt Practices Act; |
|
|
• |
difficulties protecting or procuring intellectual property rights; and |
|
|
• |
fluctuations in foreign currency exchange rates. |
These factors or any combination of these factors could have a material adverse effect on our results of operations and financial condition.
Risks Related to Our Financial Results
Our debt obligations could impair our financial condition and limit our operating flexibility.
Our indebtedness under our credit agreements with MidCap Financial Trust and our other financial obligations could:
|
|
• |
impair our ability to obtain financing or additional debt in the future for working capital, capital expenditures, acquisitions or general corporate purposes; |
|
|
• |
impair our ability to access capital and credit markets on terms that are favorable to us; |
|
|
• |
have a material adverse effect on us if we fail to comply with financial and affirmative and restrictive covenants in our Credit Agreements and an event of default occurs as a result of a failure that is not cured or waived; |
|
|
• |
require us to dedicate a portion of our cash flow for interest payments on our indebtedness and other financial obligations, thereby reducing the availability of our cash flow to fund working capital and capital expenditures; and |
|
|
• |
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate. |
There is no guarantee that we will be able to pay the principal and interest under the Credit Agreements or that future working capital, borrowings or equity financing will be available to repay or refinance any amounts
39
outstanding under the Credit Agreements. In addition, we may enter into debt agreements in the future that may contain similar or more burdensome terms and covenants, including financial covenants. See Liquidity and Capital Resources for additional detailed discussion of our outstanding indebtedness.
Our quarterly net sales and operating results are unpredictable and may fluctuate significantly from quarter to quarter due to factors outside our control, which could adversely affect our business, results of operations and the trading price of our common stock.
Our net sales and operating results may vary significantly from quarter to quarter and year to year due to a number of factors, many of which are outside of our control and any of which may cause our stock price to fluctuate. Our net sales and results of operations will be affected by numerous factors, including:
|
|
• |
the timing to qualify Vesta with the FDA and the availability of any alternative manufacturing sources to supply our silicone gel breast implants and certain other products; |
|
|
• |
our ability to integrate and achieve the anticipated benefits of our acquisitions of the miraDry, BIOCORNEUM and the tissue expanders from SSP; |
|
|
• |
the impact of the buying patterns of patients and seasonal cycles in consumer spending; |
|
|
• |
our ability to drive increased sales of anatomically-shaped breast implants products; |
|
|
• |
our ability to establish and maintain an effective and dedicated sales organization; |
|
|
• |
pricing pressure applicable to our products, including adverse third-party coverage and reimbursement outcomes; |
|
|
• |
results of clinical research and trials on our existing products; |
|
|
• |
the impact of the past regulatory inquiries of Silimed on our brand and reputation; |
|
|
• |
timing of our research and development activities and initiatives; |
|
|
• |
the mix of our products sold due to different profit margins among our products; |
|
|
• |
timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors; |
|
|
• |
the ability of our suppliers to timely provide us with an adequate supply of products; |
|
|
• |
the evolving product offerings of our competitors; |
|
|
• |
regulatory approvals and legislative changes affecting the products we may offer or those of our competitors; |
|
|
• |
increased labor and related costs; |
|
|
• |
interruption in the manufacturing or distribution of our products; |
|
|
• |
the effect of competing technological, industry and market developments; |
|
|
• |
changes in our ability to obtain regulatory clearance or approval for our products; |
|
|
• |
our ability to expand the geographic reach of our sales and marketing efforts. |
40
Many of the products we may seek to develop and introduce in the future will require FDA approval or clearance before commercialization in the United States, and commercialization of such products outside of the United States would likely require additional regulatory approvals, CE Certificates of Conformity and export licenses. As a result, it will be difficult for us to forecast demand for these products with any degree of certainty. In addition, we will be increasing our operating expenses as we expand our commercial capabilities. Accordingly, we may experience significant, unanticipated quarterly losses. If our quarterly or annual operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly or annual fluctuations in our operating results may, in turn, cause the price of our common stock to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2017, we had federal net operating loss carryforwards, or NOLs, of approximately $239 million, which begin expiring in 2027, if not utilized to offset taxable income. In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOLs to offset future taxable income. In general, an “ownership change” occurs if there is a cumulative change in our ownership by “5% shareholders” that exceeds 50 percentage points over a rolling three-year period. Our existing NOLs may be subject to limitations arising from previous ownership changes, and if we undergo one or more ownership changes in connection with future transactions in our stock, our ability to utilize NOLs could be further limited by Section 382 of the Code. As a result of these limitations, we may not be able to utilize a material portion of the NOLs reflected on our consolidated balance sheet and for this reason, we have fully reserved against the value of our NOLs on our consolidated balance sheet. We have not completed a Section 382 analysis to determine if an ownership change has occurred. Until such analysis is completed, we cannot be sure that the full amount of the existing federal NOLs will be available to us, even if we do generate taxable income before their expiration.
Future changes in financial accounting standards may cause adverse unexpected net sales or expense fluctuations and affect our reported results of operations.
A change in accounting standards could have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. New pronouncements and varying interpretations of existing pronouncements have occurred and may occur in the future. Changes to existing rules or current practices may adversely affect our reported financial results of our business.
Risks Related to Our Intellectual Property and Potential Litigation
If our intellectual property rights do not adequately protect our products or technologies, others could compete against us more directly, which would hurt our profitability.
Our success depends in part on our ability to protect our intellectual property rights. We rely on a combination of trademarks, trade secrets, confidential information, copyrights, patent rights and other intellectual property rights to protect our intellectual property. In addition, to protect our trade secrets, confidential information and other intellectual property rights, we have entered into confidentiality agreements with third parties, and confidential information and invention assignment agreements with employees, consultants and advisors. However, these agreements may not provide sufficient protection or adequate remedies for violation of our rights in the event of unauthorized use or disclosure of confidential and proprietary information. Without additional protection under the patent or other intellectual property laws, such unauthorized use or disclosure may enable competitors to duplicate or surpass our technological achievements. Moreover, the laws of certain foreign countries do not recognize intellectual property rights or protect them to the same extent as do the laws of the United States. Failure to protect our proprietary rights could seriously impair our competitive position.
41
The medical device industry is characterized by patent and other intellectual property litigation and we have and could become subject to litigation that could be costly, result in the diversion of management’s time and efforts, require us to pay damages or prevent us from marketing our existing or future products.
Our commercial success will depend in part on not infringing the patents or violating the other proprietary rights of others. Significant litigation regarding patent rights occurs in our industry. Our competitors in both the United States and abroad, many of which have substantially greater resources and have made substantial investments in patent portfolios and competing technologies, may have applied for or obtained or may in the future apply for and obtain, patents that will prevent, limit or otherwise interfere with our ability to make, use and sell our products. Absent specific circumstances, we do not generally conduct independent reviews of patents issued to third parties. We may not be aware of whether our products do or will infringe existing or future patents. In addition, patent applications in the United States and elsewhere can be pending for many years, and may be confidential for 18 months or more after filing, and because pending patent claims can be revised before issuance, there may be applications of others now pending of which we are unaware that may later result in issued patents that will prevent, limit or otherwise interfere with our ability to make, use or sell our products. We may not be aware of patents that have already been issued that a third party might assert are infringed by our products. It is also possible that patents of which we are aware, but which we do not believe are relevant to our product candidates, could nevertheless be found to be infringed by our products. The large number of patents, the rapid rate of new patent applications and issuances, the complexities of the technology involved and the uncertainty of litigation increase the risk of business assets and management’s attention being diverted to patent litigation. In the future, we may receive communications from various industry participants alleging our infringement of their patents, trade secrets or other intellectual property rights and/or offering licenses to such intellectual property. Any lawsuits resulting from such allegations could subject us to significant liability for damages and invalidate our proprietary rights, even if they lack merit. Any existing or potential intellectual property litigation also could force us to do one or more of the following:
|
|
• |
stop making, selling or using products or technologies that allegedly infringe the asserted intellectual property; |
|
|
• |
lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others; |
|
|
• |
incur significant legal expenses; |
|
|
• |
pay substantial damages or royalties to the party whose intellectual property rights we may be found to be infringing; |
|
|
• |
pay the attorney fees and costs of litigation to the party whose intellectual property rights we may be found to be infringing; |
|
|
• |
redesign those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible; or |
|
|
• |
attempt to obtain a license to the relevant intellectual property from third parties, which may not be available on reasonable terms or at all. |
Any litigation or claim against us, even those without merit, may cause us to incur substantial costs, and could place a significant strain on our financial resources, divert the attention of management from our core business, negatively impact shareholder value and harm our reputation. If we are found to infringe the intellectual property rights of third parties, we could be required to pay substantial damages (which may be increased up to three times of awarded damages) and/or substantial royalties and could be prevented from selling our products unless we obtain a license or are able to redesign our products to avoid infringement. Any such license may not be available on reasonable terms, if at all, and there can be no assurance that we would be able to redesign our products in a way that would not infringe the intellectual property rights of others. If we fail to obtain any required licenses or make any necessary changes to our products or technologies, we may have to withdraw existing products from the market or may be
42
unable to commercialize one or more of our products, all of which could have a material adverse effect on our business, results of operations and financial condition.
In addition, we generally indemnify our customers with respect to infringement by our products of the proprietary rights of third parties. Third parties may assert infringement claims against our customers. These claims may require us to initiate or defend protracted and costly litigation on behalf of our customers, regardless of the merits of these claims. If any of these claims succeed, we may be forced to pay damages on behalf of our customers or may be required to obtain licenses for the products they use. If we cannot obtain all necessary licenses on commercially reasonable terms, our customers may be forced to stop using our products.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Many of our employees were previously employed at other medical device companies, including our competitors or potential competitors, in some cases until recently. We have been the subject of and may, in the future, be subject to claims that we, or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of these former employers or competitors. In addition, we have been and may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. If our defense to those claims fails, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Any litigation or the threat thereof may adversely affect our ability to hire employees. A loss of key personnel or their work product could hamper or prevent our ability to commercialize product candidates, which could have an adverse effect on our business, results of operations and financial condition.
We are and may be subject to warranty or product liability claims or other litigation in the ordinary course of business that may adversely affect our business, financial condition and operating results.
As a supplier of medical devices, we are and may be subject to warranty or product liability claims alleging that the use of our products has resulted in adverse health effects or other litigation in the ordinary course of business that may require us to make significant expenditures to defend these claims or pay damage awards. The breast implant industry has a particularly significant history of product liability litigation. The risks of litigation exist even with respect to products that have received or in the future may receive regulatory approval for commercial sale, such as our Breast Products. In addition, our silicone gel breast implants are sold with a warranty providing for no-charge replacement implants in the event of certain ruptures that occur any time during the life of the patient and this warranty also includes cash payments to offset surgical fees if the rupture occurs within 10 years of implantation.
We maintain product liability insurance, but this insurance is limited in amount and subject to significant deductibles. There is no guarantee that insurance will be available or adequate to protect against all claims. Our insurance policies are subject to annual renewal and we may not be able to obtain liability insurance in the future on acceptable terms or at all. In addition, our insurance premiums could be subject to increases in the future, which may be material. If the coverage limits are inadequate to cover our liabilities or our insurance costs continue to increase as a result of warranty or product liability claims or other litigation, then our business, financial condition and operating results may be adversely affected.
Fluctuations in insurance cost and availability could adversely affect our profitability or our risk management profile.
We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, general liability insurance, property insurance, employment practices, and workers’ compensation insurance. If the costs of maintaining adequate insurance coverage increase significantly in the future, our operating results could be materially adversely affected. Likewise, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers. If we operate our business without insurance, we could be
43
responsible for paying claims or judgments against us that would have otherwise been covered by insurance, which could adversely affect our results of operations or financial condition.
Risks Related to Our Legal and Regulatory Environment
We are subject to extensive federal and state healthcare regulation, and if we fail to comply with applicable regulations, we could suffer severe criminal or civil sanctions or be required to restructure our operations, any of which could adversely affect our business, financial condition and operating results.
As a device manufacturer, even though we do not control referrals or bill directly to Medicare, Medicaid or other third-party payors, we are subject to healthcare fraud and abuse regulation and enforcement by the federal government and the states in which we conduct our business, as well as other healthcare laws and regulations. The healthcare laws and regulations that may affect our ability to operate include:
|
|
• |
the federal Anti-Kickback Statute, which applies to our business activities, including our marketing practices, educational programs, pricing policies and relationships with healthcare providers, by prohibiting, among other things, knowingly and willfully soliciting, receiving, offering or providing any remuneration (including any bribe, kickback or rebate) directly or indirectly, overtly or covertly, in cash or in kind, intended to induce or in return for the purchase or recommendation of any good, facility, item or service reimbursable, in whole or in part, under a federal healthcare program, such as the Medicare or Medicaid programs. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to commit a violation. Rather, if “one purpose” of the remuneration is to induce referrals, the federal Anti-Kickback Statute is violated. In addition, following passage of the PPACA violations of the federal Anti-Kickback Statute became per se violations of the False Claims Act; |
|
|
• |
federal civil and criminal false claims laws and civil monetary penalty laws, including the FCA, that prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other government payors that are false or fraudulent, or making a false statement to decrease or conceal an obligation to pay or transmit money or property to the federal government, and which may apply to entities that provide coding and billing advice to customers; |
|
|
• |
HIPAA, and its implementing regulations, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
|
|
• |
and, HIPAA, as amended by HITECH, also imposes certain regulatory and contractual requirements on certain types of people and entities subject to the law and their business associates regarding the privacy, security and transmission of individually identifiable health information; |
44
|
|
• |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be provided to healthcare providers and entities; state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers and entities or marketing expenditures; and state laws governing the privacy and security of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Because of the breadth of these laws, it is possible that some of our business activities, including our relationships with physicians and other health care providers and entities, some of whom recommend, purchase and/or prescribe our products, could be subject to challenge under one or more of such laws. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and/or criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, exclusion from governmental health care programs, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
We recently settled a securities class action lawsuit and may be subject to additional claims and our insurance may not be sufficient to cover additional expenses incurred.
In May 2017, we settled a class action lawsuit which named the Company and certain of its officers as defendants for allegedly false and misleading statements concerning the Company’s business, operations, and prospects in connection with the Company’s September 2015 common stock offering. In connection with the settlement, we received $9.3 million in insurance proceeds to pay the settlement amount. We may, in the future, be subject to regulatory claims, including claims for violations of the federal securities laws, rules and regulations relating to our September 2015 common stock offering. If that occurs, we may be required to a pay a monetary settlement or judgment and we may not have sufficient insurance coverage remaining to cover the costs of any such claims or any related potential indemnification obligations to our current or former directors and officers. Moreover, even if these claims against us are not successful, the defense of such claims could result in substantial costs and significant adverse impact on our reputation and divert management’s attention and resources, which could have a material adverse effect on our business, operating results or financial condition.
45
Our medical device products and operations are subject to extensive governmental regulation both in the United States and abroad, and our failure to comply with applicable requirements could cause our business to suffer.
Our medical device products and operations are subject to extensive regulation by the FDA and various other federal, state and foreign governmental authorities, such as Health Canada. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:
|
|
• |
design, development and manufacturing; |
|
|
• |
testing, labeling, content and language of instructions for use and storage; |
|
|
• |
clinical trials; |
|
|
• |
product safety; |
|
|
• |
marketing, sales and distribution; |
|
|
• |
regulatory clearances and approvals including pre-market clearance and approval; |
|
|
• |
conformity assessment procedures; |
|
|
• |
product traceability and record keeping procedures; |
|
|
• |
advertising and promotion; |
|
|
• |
product complaints, complaint reporting, recalls and field safety corrective actions; |
|
|
• |
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
|
|
• |
post-market studies; and |
|
|
• |
product import and export. |
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
Before we can market or sell a new regulated product or a significant modification to an existing product in the United States, we must obtain either clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FDCA, or an approval of a PMA application unless the device is specifically exempt from pre-market review. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. In the PMA approval process, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on specific data, including, but not limited to, pre-clinical, clinical trial, and other product data. The PMA process is typically required for devices for which the 510(k) process cannot be used and that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices. Modifications to products that are approved through a PMA application generally need FDA approval. Similarly, some modifications made to products cleared through a 510(k) may require a new 510(k). The FDA’s 510(k) clearance process usually takes from three to 12 months, but may last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is submitted to the FDA until an approval is obtained.
46
In the United States, our silicone gel breast implants are marketed pursuant to a PMA order issued by the FDA in March 2012, and our tissue expanders are marketed pursuant to pre-market clearance under Section 510(k) of the FDCA. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, our product introductions or modifications could be delayed or canceled, which could cause our sales to decline. The FDA may demand that we obtain a PMA prior to marketing certain of our future products. In addition, if the FDA disagrees with our determination that a product we market is subject to an exemption from pre-market review, the FDA may require us to submit a 510(k) or PMA in order to continue marketing the product. Further, even with respect to those future products where a PMA is not required, we cannot assure you that we will be able to obtain the 510(k) clearances with respect to those products.
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
|
|
• |
we may not be able to demonstrate to the FDA’s satisfaction that our products are safe and effective for their intended uses; |
|
|
• |
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and |
|
|
• |
the manufacturing process or facilities we use may not meet applicable requirements. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions that may prevent or delay approval or clearance of our products under development or impact our ability to modify our currently approved or cleared products on a timely basis. Any change in the laws or regulations that govern the clearance and approval processes relating to our current and future products could make it more difficult and costly to obtain clearance or approval for new products, or to produce, market and distribute existing products.
The FDA could also reclassify some or all of our products that are currently classified as Class II to Class III requiring additional controls, clinical studies and submission of a PMA for us to continue marketing and selling those products. We cannot guarantee that the FDA will not reclassify any of our Class II devices into Class III and require us to submit a PMA for FDA review and approval of the safety and effectiveness of our product. Any delay in, or failure to receive or maintain clearance or approval for our products under development could prevent us from generating sales from these products or achieving profitability. Additionally, the FDA and other regulatory authorities have broad enforcement powers. Regulatory enforcement or inquiries, or other increased scrutiny on us, could dissuade some surgeons from using our products and adversely affect our reputation and the perceived safety and efficacy of our products.
In addition, even after we have obtained the proper regulatory clearance or approval to market a product, the FDA has the power to require us to conduct post-marketing studies. For example, we are required to continue to study and report clinical results to the FDA on our silicone gel breast implants. Failure to conduct this or other required studies in a timely manner could result in the revocation of the PMA approval or 510(k) clearance for the product that is subject to such a requirement and could also result in the recall or withdrawal of the product, which would prevent us from generating sales from that product in the United States.
Failure to comply with applicable laws and regulations could jeopardize our ability to sell our products and result in enforcement actions such as:
|
|
• |
warning letters; |
|
|
• |
fines; |
|
|
• |
injunctions; |
|
|
• |
civil penalties; |
47
|
|
• |
recalls or seizures of products; |
|
|
• |
delays in the introduction of products into the market; |
|
|
• |
total or partial suspension of production; |
|
|
• |
refusal of the FDA or other regulator to grant future clearances or approvals; |
|
|
• |
withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of our products; and/or |
|
|
• |
in the most serious cases, criminal penalties. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, results of operations and financial condition.
If we or if our third-party manufacturer fail to comply with the FDA’s good manufacturing practice regulations, it could impair our ability to market our products in a cost-effective and timely manner.
We and our third-party manufacturers are required to comply with the FDA’s QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. The FDA audits compliance with the QSR through periodic announced and unannounced inspections of manufacturing and other facilities. The FDA may conduct inspections or audits at any time. If we or our manufacturers fail to adhere to QSR requirements, have significant non-compliance issues or fail to timely and adequately respond to any adverse inspectional observations or product safety issues, or if any corrective action plan that we or our manufacturers propose in response to observed deficiencies is not sufficient, the FDA could take enforcement action against us, which could delay production of our products and may include:
|
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
• |
unanticipated expenditures to address or defend such actions; |
|
|
• |
customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
|
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
|
• |
refusing or delaying our requests for 510(k) clearance or pre-market approval of new products or modified products; |
|
|
• |
withdrawing 510(k) clearances or pre-market approvals that have already been granted; |
|
|
• |
refusal to grant export approval for our products; or |
|
|
• |
criminal prosecution. |
Any of the foregoing actions could have a material adverse effect on our reputation, business, financial condition and operating results. Furthermore, our manufacturer may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
48
Our ability to market the miraDry System in the United States is limited to the treatment of sweat, odor and hair in the underarm, and if we want to expand our marketing claims, we will need to obtain additional FDA clearances or approvals, which may not be granted.
We currently only have FDA clearance to market the miraDry System in the United States for the treatment of primary hyperhidrosis of the axilla, or the underarm, and for hair reduction procedures in the axilla. This clearance restricts our ability to market or advertise the miraDry System for other specific body areas, and other conditions such as underarm odor, which could limit physician adoption and patient demand for the miraDry System. We believe that future applications using the miraDry System could be used to treat other body areas, such as the feet and hands, where patients experience sweat-bothered symptoms. Developing and promoting these new treatment applications for our miraDry System is an element of our growth strategy, but we cannot predict when or if we will receive the clearances required to implement these additional products and applications. In addition, we will be required to conduct additional clinical trials or studies to support our applications, which may be time-consuming and expensive, and may produce results that do not result in submission of, or FDA clearances for, new treatment applications. In the event that we do not obtain additional FDA clearances, our ability to promote the miraDry System in the United States will be limited. Because we anticipate that sales in the United States will continue to be a significant portion of our business for the foreseeable future, ongoing restrictions on our ability to market the miraDry System in the United States could harm our business and limit our revenue growth.
There is no guarantee that the FDA will grant 510(k) clearance or PMA approval of our future products, and failure to obtain necessary clearances or approvals for our future products would adversely affect our ability to grow our business.
Some of our future products may require FDA clearance of a 510(k) or FDA approval of a PMA. The FDA may not approve or clear these products for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or pre-market approval of new products.
Significant delays in receiving clearance or approval, or the failure to receive clearance or approval for our new products would have an adverse effect on our ability to expand our business.
If we modify our FDA approved or cleared devices, we may need to seek additional clearances or approvals, which, if not granted, would prevent us from selling our modified products.
In the United States, our silicone gel breast implants are marketed pursuant to a PMA order issued by the FDA in March 2012, and our tissue expanders are marketed pursuant to pre-market clearance under Section 510(k) of the FDCA. Any modifications to a PMA-approved or 510(k)-cleared device that could significantly affect its safety or effectiveness, including significant design and manufacturing changes, or that would constitute a major change in its intended use, manufacture, design, components, or technology requires a new 510(k) clearance or, possibly, approval of a new PMA application or PMA supplement. For example, on March 14, 2017, we announced that we had submitted a PMA supplement to the FDA for the manufacturing of our PMA-approved breast implants by Vesta. Certain changes to a PMA-approved device do not require submission and approval of a new PMA or PMA supplement and may only require notice to FDA in a PMA 30-Day Notice, Special PMA Supplement – Changes Being Effected or PMA Annual Report. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. We have modified some of our 510(k) cleared products, and have determined based on our review of the applicable FDA guidance that in certain instances the changes did not require new 510(k) clearances or PMA approvals. If the FDA disagrees with our determination and requires us to seek new 510(k) clearances or PMA approvals for modifications to our previously cleared or approved products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Furthermore, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective or that appropriate regulatory submissions were not made. Delays in receipt or failure to receive approvals, the loss of previously received approvals, or the failure to comply with any other existing or future regulatory requirements, could reduce our sales, profitability and future growth prospects.
49
A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products that leads to corrective actions, could have a significant adverse impact on us.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. Manufacturers may also, under their own initiative, recall a product if any material deficiency in a device is found or withdraw a product to improve device performance or for other reasons. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. Similar regulatory agencies in other countries have similar authority to recall devices because of material deficiencies or defects in design or manufacture that could endanger health. Any recall would divert management attention and financial resources and could cause the price of our stock to decline, expose us to product liability or other claims and harm our reputation with customers. Such events could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. A recall involving our silicone gel breast implants could be particularly harmful to our business, financial and operating results. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or similar foreign governmental authorities. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA or foreign governmental authorities. If the FDA or foreign governmental authorities disagree with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA or a foreign governmental authority could take enforcement action for failing to report the recalls when they were conducted.
In addition, under the FDA’s medical device reporting regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Repeated product malfunctions may result in a voluntary or involuntary product recall. We are also required to follow detailed record-keeping requirements for all firm-initiated medical device corrections and removals, and to report such corrective and removal actions to FDA if they are carried out in response to a risk to health and have not otherwise been reported under the medical device reporting regulations. Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals or clearances for the device before we may market or distribute the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties, or civil or criminal fines. We may also be required to bear other costs or take other actions that may have a negative impact on our sales as well as face significant adverse publicity or regulatory consequences, which could harm our business, including our ability to market our products in the future.
Any adverse event involving our products, whether in the United States or abroad, could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
If the third parties on which we rely to conduct our clinical trials and to assist us with pre-clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval for or commercialize our products.
We often must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct our clinical trials and preclinical development activities. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is
50
compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
We may be subject to regulatory or enforcement actions if we engage in improper marketing or promotion of our products.
Our educational and promotional activities and training methods must comply with FDA and other applicable laws, including the prohibition of the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside of its cleared or approved indications is known as “off-label” use. Physicians may use our products off-label in their professional medical judgment, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our educational and promotional activities or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of warning letters, untitled letters, fines, penalties, injunctions, or seizures, which could have an adverse impact on our reputation and financial results. It is also possible that other federal, state or foreign enforcement authorities may take action if they consider our educational and promotional activities or training methods to constitute promotion of an off-label use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products could be impaired. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion. It is also possible that other federal, state or foreign enforcement authorities might take action, such as federal prosecution under the federal civil False Claims Act, if they consider our business activities constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention, result in substantial damage awards against us, and harm our reputation.
Changes in existing third-party coverage and reimbursement may impact our ability to sell our products when used in breast reconstruction procedures.
Maintaining and growing sales of our products when used in breast reconstruction procedures depends, in part, on the availability of coverage and adequate reimbursement from third-party payors, including government programs such as Medicare and Medicaid, private insurance plans and managed care programs. Breast augmentation procedures are generally performed on a cash‑pay basis and are not covered by third‑party payors. In contrast, breast reconstruction procedures may be covered by third‑party payors. Therefore, hospitals and other healthcare provider customers that purchase our products to use in breast reconstruction procedures typically bill various third-party payors to cover all or a portion of the costs and fees associated with the procedures in which our products are used, including the cost of the purchase of our products. Decreases in the amount third-party payors are willing to reimburse our customers for breast reconstruction procedures using our products could create pricing pressures for us. The process for determining whether a third-party payor will provide coverage for a product or procedure may be separate from the process for establishing the reimbursement rate that such a payor will pay for the product or procedure. A payor’s decision to provide coverage for a product or procedure does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product or procedure does not assure that other payors will also provide such coverage. Adequate third-party reimbursement may not be available to enable us to maintain our business in a profitable way. We may be unable to sell our products on a profitable basis if third-party payors deny coverage or reduce their current levels of payment, or if our costs of production increase faster than increases in reimbursement levels.
51
Furthermore, the healthcare industry in the United States has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by imposing lower payment rates and negotiating reduced contract rates with service providers. Therefore, we cannot be certain that the breast reconstruction procedures using our products will be reimbursed at a cost-effective level. Nor can we be certain that third-party payors using a methodology that sets amounts based on the type of procedure performed, such as those utilized by government programs and in many privately managed care systems, will view the cost of our products to be justified so as to incorporate such costs into the overall cost of the procedure. Moreover, we are unable to predict what changes will be made to the reimbursement methodologies used by third-party payors in the future.
To the extent we sell our products internationally, market acceptance may depend, in part, upon the availability of coverage and reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country, and include both government-sponsored healthcare and private insurance. We may not obtain international coverage and reimbursement approvals in a timely manner, if at all. Our failure to receive such approvals would negatively impact market acceptance of our products in the international markets in which those approvals are sought.
Legislative or regulatory health care reforms may make it more difficult and costly to produce, market and distribute our products after clearance or approval is obtained, or to do so profitably.
Recent political, economic and regulatory influences are subjecting the health care industry to fundamental changes. Both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation and regulations designed to contain or reduce the cost of health care, improve quality of care, and expand access to healthcare, among other purposes. Such legislation and regulations may result in decreased reimbursement for medical devices and/or the procedures in which they are used, which may further exacerbate industry-wide pressure to reduce the prices charged for medical devices. This could harm our ability to market and generate sales from our products.
In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations, revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products.
In the future there may continue to be additional proposals relating to the reform of the U.S. healthcare system. Certain of these proposals could limit the prices we are able to charge for our products or the amount of reimbursement available for our products, and could limit the acceptance and availability of our products, any of which could have a material adverse effect on our business, results of operations and financial condition.
Our customers and much of our industry are required to be compliant under the federal Health Insurance Portability and Accountability Act of 1996, the Health Information Technology for Economic and Clinical Health Act and implementing regulations (including the final Omnibus Rule published on January 25, 2013) affecting the transmission, security and privacy of health information, and failure to comply could result in significant penalties.
Numerous federal and state laws and regulations, including HIPAA, and HITECH, govern the collection, dissemination, security, use and confidentiality of health information that identifies specific patients. HIPAA and HITECH require our surgeon and hospital customers to comply with certain standards for the use and disclosure of health information within their companies and with third parties. The Privacy Standards and Security Standards under HIPAA establish a set of standards for the protection of individually identifiable health information by health plans, health care clearinghouses and certain health care providers, referred to as Covered Entities, and the Business Associates with whom Covered Entities enter into service relationships pursuant to which individually identifiable health information may be exchanged. Notably, whereas HIPAA previously directly regulated only these Covered Entities, HITECH, which was signed into law as part of the stimulus package in February 2009, makes certain of HIPAA’s privacy and security standards also directly applicable to Covered Entities’ Business Associates. As a result, both Covered Entities and Business Associates are now subject to significant civil and criminal penalties for failure to comply with Privacy Standards and Security Standards.
HIPAA requires Covered Entities (like our customers) and Business Associates to develop and maintain policies and procedures with respect to protected health information that is used or disclosed, including the adoption of
52
administrative, physical and technical safeguards to protect such information. HITECH expands the notification requirement for breaches of patient-identifiable health information, restricts certain disclosures and sales of patient-identifiable health information and provides for civil monetary penalties for HIPAA violations. HITECH also increased the civil and criminal penalties that may be imposed against Covered Entities and Business Associates and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney fees and costs associated with pursuing federal civil actions. Additionally, certain states have adopted comparable privacy and security laws and regulations, some of which may be more stringent than HIPAA.
We are not currently required to comply with HIPAA or HITECH because we are neither a Covered Entity nor a Business Associate (as that term is defined by HIPAA). However, in administering our warranties and complying with FDA-required device tracking, we do regularly handle confidential and personal information similar to that which these laws seek to protect. We also occasionally encounter hospital customers who pressure us to sign Business Associate Agreements, or BAAs, although, to date, we have refused, given that we do not believe we are business associates to such Covered Entities under HIPAA or HITECH. If the law or regulations were to change or if we were to agree to sign a BAA, the costs of complying with the HIPAA standards are burdensome and could have a material adverse effect on our business. In addition, under such situations there would be significant risks and financial penalties for us if we were then found to have violated the laws and regulations that pertain to Covered Entities and Business Associates.
We are unable to predict what changes to the HIPAA Privacy Standards and Security Standards might be made in the future or how those changes could affect our business. Any new legislation or regulation in the area of privacy and security of personal information, including personal health information, could also adversely affect our business operations. If we do not comply with existing or new applicable federal or state laws and regulations related to patient health information, we could be subject to criminal or civil sanctions and any resulting liability could adversely affect our financial condition.
In addition, even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the FTCA, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Medical data is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
In addition, certain state laws govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties.
An adverse outcome of a sales and use tax or value-added tax (VAT) audit could have a material adverse effect on our results of operations and financial condition.
We sell our products in all 50 states and each state (and some local governments) has its own sales tax laws and regulations. We charge each of our customers sales tax on each order and report and pay that tax to the appropriate state authority, unless we believe there is an applicable exception. In some states, there are no available exceptions; in some states, we believe our products can be sold tax-free. In other states, we believe we can sell our products tax-free only for customers who request tax-exempt treatment due to the nature of the devices we sell or due to the nature of the customer’s use of our device. We also sell internationally and some sales may be subject to value-added tax. We may be audited by the taxing authorities of one or more jurisdictions and there can be no assurance, however, that an audit will be resolved in our favor. Such an audit could be expensive and time-consuming and result in substantial management distraction. If the matter were to be resolved in a manner adverse to us, it could have a material adverse effect on our results of operations and financial condition.
53
Risks Related to Our Common Stock
Our stock price may be volatile, and you may not be able to resell shares of our common stock at or above the price you paid.
The market price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In recent years, the stock markets generally have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may significantly affect the market price of our common stock, regardless of our actual operating performance.
We do not anticipate paying any cash dividends in the foreseeable future, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We do not anticipate declaring any cash dividends to holders of our common stock in the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by our Credit Agreements. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
Our executive officers, directors and principal stockholders own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of March 9, 2018, our executive officers, directors and principal stockholders beneficially owned approximately 36% of our outstanding voting stock. As a result, these stockholders have the ability to influence us through their ownership position and may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.
We are an “emerging growth company” and intend to take advantage of reduced disclosure requirements applicable to emerging growth companies, which could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that apply to other public companies that are not “emerging growth companies.” As an emerging growth company:
|
|
• |
we are exempt from the requirement to obtain an attestation and report from our auditors on the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act; |
|
|
• |
we are permitted to provide less extensive disclosure about our executive compensation arrangements in our periodic reports, proxy statements and registration statements; and |
|
|
• |
we are not required to give our stockholders non-binding advisory votes on executive compensation or golden parachute arrangements. |
In addition, the JOBS Act provides that an emerging growth company may take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
54
We may remain an emerging growth company until December 31, 2019 (the last day of the fiscal year following the fifth anniversary of our initial public offering). However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenue equals or exceeds $1.0 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
As a public company, we are required to assess our internal control over financial reporting on an annual basis, and any future adverse results from such assessment could result in a loss of investor confidence in our financial reports and have an adverse effect on our stock price.
As a public company, we are required to comply with certain of the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, regarding internal control over financial reporting, including a report of management on the Company’s internal controls over financial reporting in their annual reports on Form 10-K.
In connection with the preparation and audit of our 2016 financial statements, we identified certain deficiencies in our internal controls over financial reporting that we concluded to be a material weakness and that our internal control over financial reporting was not effective as of December 31, 2016. The material weakness resulted from the inadequate design and operation of internal controls related to the accounting for significant unusual transactions. Management determined that this material weakness was remediated as of December 31, 2017. Refer to Part II, Item 9A, "Controls and Procedures" for additional information.
Additionally, for as long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to utilize the provision exempting us from the requirement that our independent registered public accounting firm provide an attestation on the effectiveness of our internal control over financial reporting. The process of becoming fully compliant with Section 404 may divert internal resources and will take a significant amount of time and effort to complete, and may result in additional deficiencies and material weaknesses being identified by us or our independent registered public accounting firm. We may experience higher than anticipated operating expenses, as well as increased independent registered public accounting firm fees during the implementation of any required changes and thereafter. Completing documentation of our internal control system and financial processes, remediation of control deficiencies and management testing of internal controls will require substantial effort by us. If our internal control over financial reporting or our related disclosure controls and procedures are not effective, we may not be able to accurately report our financial results or file our periodic reports in a timely manner, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to decline.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that our officers, directors or the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock.
Certain holders of shares of our common stock are entitled to certain rights, subject to some conditions, with respect to the registration of their shares under the Securities Act of 1933, as amended.
We cannot predict what effect, if any, sales of our shares in the public market or the availability of shares for sale will have on the market price of our common stock. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options or warrants, or the perception that such sales may occur, however, could adversely affect the market price of our common stock and also could adversely affect our future ability to raise capital through the sale of our common stock or other equity-related securities of ours at times and prices we believe appropriate.
55
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, commercialization efforts, expanded research and development activities and costs associated with operating a public company. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our common stock.
As of December 31, 2017, the number of shares of common stock reserved for issuance under our 2014 plan was 2,789,442. The number of shares of our common stock reserved for issuance under the 2014 Plan automatically increases on January 1 of each year, continuing through and including January 1, 2024, by 4% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares determined by our board of directors. Unless our board of directors elects not to increase the number of shares available for future grant each year, our stockholders may experience additional dilution, which could cause our stock price to fall. Pursuant to the foregoing provision, effective January 1, 2017, our board of directors increased the number of shares of common stock reserved for issuance under the 2014 Plan by 4% of the number of shares of our capital stock outstanding on December 31, 2017, or 776,079 shares.
As of December 31, 2017, the number of shares of common stock reserved for issuance under our ESPP was 770,549. The number of shares of our common stock reserved for issuance under the ESPP automatically increases on January 1 of each year, beginning on January 1, 2015 and continuing through and including January 1, 2024, by 1% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares determined by our board of directors. Unless our board of directors elects not to increase the number of shares available for future grant each year, our stockholders may experience additional dilution, which could cause our stock price to fall. Pursuant to the foregoing provision, effective January 1, 2017, our board of directors increased the number of shares of common stock reserved for issuance under the ESPP by 1% of the number of shares of our capital stock outstanding on December 31, 2017, or 194,020 shares.
Pursuant to the Inducement Plan approved by our board of directors, our compensation committee of the board of directors is authorized to grant stock options or restricted stock units which may be exercised or settled, as applicable, to new employees as inducements material to such new employees entering into employment with us in accordance with NASDAQ Marketplace Rule 5635(c)(4). As of December 31, 2017, a total of 366,000 RSUs and options had been awarded by the compensation committee and the number of shares available for future grant was 34,000 shares. In February 2018, the compensation committee increased the number of shares available under the Inducement Plan by 500,000 and granted options to purchase 257,235 shares.
The number of shares that may be granted under the Inducement Plan may be increased in the future by our board of directors.
56
Anti-takeover provisions in our organizational documents and under Delaware law may discourage or prevent a change of control, even if an acquisition would be beneficial to our stockholders, which could reduce our stock price and prevent our stockholders from replacing or removing our current management.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions include:
|
|
• |
a board of directors divided into three classes serving staggered three-year terms, such that not all members of the board will be elected at one time; |
|
|
• |
a prohibition on stockholder action through written consent, which requires that all stockholder actions be taken at a meeting of our stockholders; |
|
|
• |
a requirement that special meetings of stockholders be called only by the chairman of the board of directors, the chief executive officer, or by a majority of the total number of authorized directors; |
|
|
• |
advance notice requirements for stockholder proposals and nominations for election to our board of directors; |
|
|
• |
a requirement that no member of our board of directors may be removed from office by our stockholders except for cause and, in addition to any other vote required by law, upon the approval of not less than two-thirds of all outstanding shares of our voting stock then entitled to vote in the election of directors; |
|
|
• |
a requirement of approval of not less than two-thirds of all outstanding shares of our voting stock to amend any bylaws by stockholder action or to amend specific provisions of our certificate of incorporation; and |
|
|
• |
the authority of the board of directors to issue preferred stock on terms determined by the board of directors without stockholder approval and which preferred stock may include rights superior to the rights of the holders of common stock. |
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These and other provisions in our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including a merger, tender offer or proxy contest involving our Company. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
If securities or industry analysts issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our clinical trials and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us, or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Item 1B. Unresolved Staff Comments
Not applicable.
57
Our headquarters located in Santa Barbara, California is approximately 20,000 square feet. The term of the lease for our headquarters expires in February 2020. We also lease warehouse spaces located in Santa Barbara, California, which is approximately 10,000 square feet. The lease term expires in January 2019. We believe that our existing facilities are adequate for our current needs. As additional space is needed in the future, we believe that suitable space will be available in the required locations on commercially reasonable terms.
Our miraDry facilities are located in Santa Clara, California, where we lease and occupy approximately 29,000 square feet of office, manufacturing and research and development space. The current term of our Santa Clara lease expires on May 31, 2019, with no option to extend the term of the lease. We also maintain a small office in Hong Kong.
From time to time, we are involved in legal proceedings and regulatory proceedings arising out of our operations. We establish reserves for specific liabilities in connection with legal actions that we deem to be probable and estimable. The ability to predict the ultimate outcome of such matters involves judgments, estimates, and inherent uncertainties. The actual outcome of such matters could differ materially from management’s estimates.
Class Action Shareholder Litigation
On September 25, 2015, a lawsuit styled as a class action of the Company’s stockholders was filed in the United States District Court for the Central District of California naming the Company and certain of its officers as defendants for allegedly false and misleading statements concerning the Company’s business, operations, and prospects. On October 28, November 5, and November 19, 2015, three lawsuits styled as class actions of the Company’s stockholders were filed in the Superior Court of California for the County of San Mateo naming the Company, certain of its officers and directors, and the underwriters associated with the Company’s follow-on public offering that closed on September 23, 2015 as defendants for allegedly false and misleading statements in the Company’s offering documents associated with the follow-on offering concerning its business, operations, and prospects. On September 13, 2016, the parties to the actions pending in the San Mateo Superior Court and the United States District Court for the Central District of California signed a memorandum of understanding that sets forth the material deal points of a settlement that covers both actions and includes class-wide relief. Following a final fairness hearing in the federal court, on May 23, 2017, the federal court extended an order granting final approval of the settlement and dismissing the federal court action with prejudice. Following a final fairness hearing in the state court, on May 31, 2017, the state court entered an order granting final approval of the settlement and dismissing the state court action with prejudice.
As a result of these developments, the Company determined a probable loss had been incurred and recognized a net charge to earnings of approximately $1.6 million within general and administrative expense which was comprised of the loss contingency of approximately $10.9 million, net of expected insurance proceeds of approximately $9.4 million. In the first quarter of 2017, the Company received $9.3 million in insurance proceeds and paid the $10.9 million loss contingency. The remaining insurance proceeds receivable is classified as “insurance recovery receivable” on the accompanying consolidated balance sheets.
Silimed Litigation
On July 27, 2017, the Company entered into a settlement agreement, or the Settlement Agreement, with Silimed to settle outstanding litigations with Silimed. Pursuant to the Settlement Agreement, in exchange for a mutual release of claims and covenants not to sue or pursue certain litigation, Sientra paid Silimed a lump sum of $9.0 million on September 7, 2017 and agreed to further pay $1.0 million on or by July 1, 2018. In addition, should the Company enter into international markets using certain breast implant specifications, the Company has agreed to make royalty payments of $12.50 on each of its net sales of such products, up to a maximum royalty of $5.0 million. As a result of the settlement, the Company has recorded $10.0 million for the year ended December 31, 2017 in legal settlement expense.
58
It is possible that additional suits will be filed, or allegations made by stockholders, with respect to these same or other matters and also naming the Company and/or its officers and directors as defendants. The Company believes it has meritorious defenses and intends to defend these lawsuits vigorously.
miraDry Class Action Litigation
On August 3, 2017, a lawsuit styled as a verified class action on the part of the former stockholders of miraDry was filed in the Court of Chancery for the State of Delaware against the former board of directors of miraDry, or the Defendants, alleging breach of their fiduciary duties in connection with the Company’s acquisition of miraDry. On August 30, 2017, the Defendants moved to dismiss the verified class action complaint for failure to state a claim upon which relief can be granted. On November 11, 2017 the parties notified the Court that they had reached an agreement to settle the matter pending completion of confirmatory discovery regarding the fairness of the settlement and obtaining approval from the court. Under the terms of the proposed settlement, in exchange for a full and final settlement and release of all claims, the Defendants (and/or their indemnitors and/or insurers) agreed to a pay a settlement consideration of $0.4 million.
Item 4. Mine Safety Disclosures
Not applicable.
59
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock has been traded on the NASDAQ Global Select Market under the symbol “SIEN” since our initial public offering on October 29, 2014. Prior to this time, there was no public market for our common stock. The following table shows the high and low sale prices per share of our common stock as reported on the NASDAQ Global Select Market for the periods indicated:
|
|
|
High |
|
|
Low |
|
||
|
Year ended December 31, 2016 |
|
|
|
|||||
|
First Quarter |
|
$ |
10.45 |
|
|
$ |
5.61 |
|
|
Second Quarter |
|
|
8.68 |
|
|
|
5.60 |
|
|
Third Quarter |
|
|
9.26 |
|
|
|
6.57 |
|
|
Fourth Quarter |
|
|
10.22 |
|
|
|
6.92 |
|
|
Year ended December 31, 2017 |
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
10.37 |
|
|
$ |
7.81 |
|
|
Second Quarter |
|
|
10.02 |
|
|
|
7.20 |
|
|
Third Quarter |
|
|
16.49 |
|
|
|
9.46 |
|
|
Fourth Quarter |
|
|
16.68 |
|
|
|
12.53 |
|
On March 9, 2018, the last reported sale price for our common stock on the NASDAQ Global Select Market was $10.31 per share.
Stock Performance Graph
The graph set forth below compares the cumulative total stockholder return on our common stock between October 29, 2014 (the date of our initial public offering) and December 31, 2017, with the cumulative total return of (a) the NASDAQ Health Care Index and (b) the NASDAQ Composite Index, over the same period. This graph assumes the investment of $100 on October 29, 2014 in our common stock, the NASDAQ Health Care Index and the NASDAQ Composite Index and assumes the reinvestment of dividends, if any. The graph assumes our closing sales price on October 29, 2014 of $16.75 per share as the initial value of our common stock and not the initial offering price to the public of $15.00 per share.
The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock. Information used in the graph was obtained from the Nasdaq Stock Market LLC, a financial data provider and a source believed to be reliable. The Nasdaq Stock Market LLC is not responsible for any errors or omissions in such information.
60
CUMULATIVE TOTAL RETURN SUMMARY
December 2017
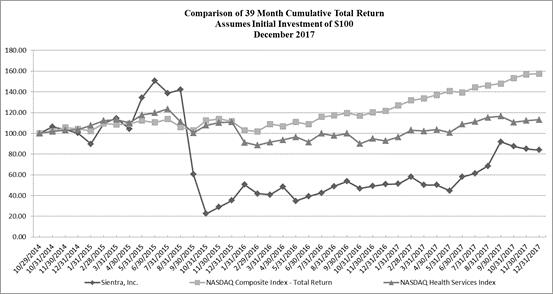
This performance graph shall not be deemed filed for purposes of Section 18 of the Exchange Act or otherwise subject to liabilities under that section and shall not be deemed to be incorporated by reference into any filing of Sientra, Inc. under the Securities Act, except as shall be expressly set forth by specific reference in such filing.
Holders of Record
As of March 9, 2018, there were approximately 117 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividends
We have not paid any cash dividends on our common stock since inception and do not anticipate paying cash dividends in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plans
Information about our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10‑K.
61
Sales of Unregistered Securities
None.
Use of Proceeds from Public Offering of Common Stock
None.
Purchases of Equity Securities by the Issuer or Affiliated Purchasers
There were no repurchases of shares of common stock made during the year ended December 31, 2017.
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, the financial statements and related notes, and other financial information included in this Annual Report on Form 10‑K.
We derived the financial data for the years ended December 31, 2017, 2016 and 2015 and as of December 31, 2017 and 2016 from our financial statements, which are included elsewhere in this Annual Report on Form 10‑K. The consolidated statement of operations data for the years ended December 31, 2014 and 2013, and the consolidated balance sheet data as of December 31, 2015, 2014 and 2013, were derived from the audited financial statements that are not included in this Annual Report on Form 10-K. Historical results are not necessarily indicative of the results to be expected in future periods.
|
|
|
Year Ended December 31, |
|
|||||||||||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||||
|
Statement of operations data |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales |
|
$ |
36,542 |
|
|
$ |
20,734 |
|
|
$ |
38,106 |
|
|
$ |
44,733 |
|
|
$ |
35,171 |
|
|
Gross profit |
|
|
22,371 |
|
|
|
13,854 |
|
|
|
27,452 |
|
|
|
33,233 |
|
|
|
26,579 |
|
|
Net loss |
|
|
(64,028 |
) |
|
|
(40,166 |
) |
|
|
(41,230 |
) |
|
|
(5,811 |
) |
|
|
(19,125 |
) |
|
Net loss per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(3.34 |
) |
|
$ |
(2.20 |
) |
|
$ |
(2.61 |
) |
|
$ |
(2.28 |
) |
|
$ |
(82.25 |
) |
|
Weighted average shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
19,159,057 |
|
|
|
18,233,177 |
|
|
|
15,770,972 |
|
|
|
2,545,371 |
|
|
|
232,512 |
|
|
|
|
As of December 31, |
|
|||||||||||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||||
|
Balance sheet data |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Working capital |
|
$ |
5,218 |
|
|
$ |
72,484 |
|
|
$ |
118,609 |
|
|
$ |
103,151 |
|
|
$ |
24,509 |
|
|
Total assets |
|
|
92,213 |
|
|
|
114,283 |
|
|
|
140,805 |
|
|
|
139,078 |
|
|
|
53,166 |
|
|
Long-term debt, excluding current position |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
21,671 |
|
|
|
15,092 |
|
|
Stockholders' equity (deficit) |
|
|
27,623 |
|
|
|
83,617 |
|
|
|
118,871 |
|
|
|
95,639 |
|
|
|
(126,673 |
) |
62
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes appearing elsewhere in this Annual Report on Form 10‑K. This discussion contains forward‑looking statements that reflect our plans, estimates and beliefs, and involve risks and uncertainties. Our actual results and the timing of certain events could differ materially from those anticipated in these forward‑looking statements as a result of several factors, including those discussed in the section titled “Risk Factors” included under Part I, Item 1A and elsewhere in this Annual Report. See “Special Note Regarding Forward‑Looking Statements” in this Annual Report.
Overview
We are a medical aesthetics company committed to making a difference in patients’ lives by enhancing their body image, growing their self‑esteem and restoring their confidence. We were founded to provide greater choices to board‑certified plastic surgeons and patients in need of medical aesthetics products. We have developed a broad portfolio of products with technologically differentiated characteristics, supported by independent laboratory testing and strong clinical trial outcomes. We sell our breast implants and breast tissue expanders, or Breast Products, exclusively to board‑certified and board‑admissible plastic surgeons and tailor our customer service offerings to their specific needs, which we believe helps secure their loyalty and confidence.
On June 11, 2017, we entered into a Merger Agreement with miraDry (formerly Miramar Labs) pursuant to which we commenced a tender offer to purchase all of the outstanding shares of miraDry’s common stock. Pursuant to the transaction, which closed on July 25, 2017 we added the miraDry System, the only FDA cleared device to reduce underarm sweat, odor and hair of all colors to our aesthetics portfolio. Following our acquisition of miraDry in July 2017, we began selling the miraDry System and bioTips. As a result of the miraDry acquisition, we determined that we will conduct our business in two operating segments. The Breast Products segment is comprised of our breast implants, tissue expanders and scar management products. The miraDry segment is comprised of our recently acquired miraDry System.
We sell both our Breast Products and miraDry products in the U.S. through a direct sales organization, which as of December 31, 2017, consisted of 83 employees, including 68 sales representatives and 15 sales managers. Additionally, we also sell our miraDry System in several international markets where we leverage a combination of distributor relationships and direct sales efforts. As of December 31, 2017, our international operations were supported by 7 employees, including 6 sales representatives and 1 sales manager.
We have two reporting segments: Breast Products and miraDry. The Breast Products segment focuses on sales of our breast implants, tissue expanders and scar management products under the brands Sientra, AlloX2, Dermaspan, Softspan and BIOCORNEUM. The miraDry segment focuses on sales of the miraDry System, consisting of a console and a handheld device which uses consumable single-use bioTips.
Our primary products are silicone gel breast implants for use in breast augmentation and breast reconstruction procedures, which we offer in approximately 400 variations of shapes, sizes, fill volumes and textures. Our breast implants are primarily used in elective procedures that are generally performed on a cash‑pay basis. Many of our proprietary breast implants incorporate one or more technologies that differentiate us from our competitors, including High‑Strength Cohesive silicone gel and shell texturing. Our breast implants offer a desired balance between strength, shape retention and softness due to the silicone shell and High‑Strength Cohesive silicone gel used in our implants. The texturing on Sientra’s implant shell is designed to reduce the incidence of malposition, rotation and capsular contracture.
Our breast implants were approved by the FDA in 2012, based on data we collected from our ongoing, long‑term clinical trial of our breast implants in 1,788 women across 36 investigational sites in the United States, which included 3,506 implants (approximately 53% of which were smooth and 47% of which were textured). Our clinical trial is the largest prospective, long‑term safety and effectiveness pivotal study of breast implants in the United
63
States and includes the largest magnetic resonance imaging, or MRI, cohort with 571 patients. The MRI cohort is a subset of study patients that underwent regular MRI screenings in addition to the other aspects of the clinical trial protocol prior to FDA approval. Post-approval, all patients in the long-term clinical trial are subject to serial MRI screenings as part of the clinical protocol. The clinical data we collected over a ten‑year follow‑up period demonstrated rupture rates, capsular contracture rates and reoperation rates that were comparable to or better than those of our competitors, at similar time points. In addition to our pivotal study, our clinical data is supported by our Continued Access Study of 2,497 women in the United States. We have also commissioned a number of bench studies run by independent laboratories that we believe further demonstrate the advantages of our breast implants over those of our competitors.
In addition, we offer BIOCORNEUM, an advanced silicone scar treatment, directly to physicians and the AlloX2, and Dermaspan lines of breast tissue expanders, as well as the Softspan line of general tissue expanders.
We sell our silicone gel breast implants and tissue expanders exclusively to board-certified and board-admissible plastic surgeons, as determined by the American Board of Plastic Surgery, who we refer to as Plastic Surgeons. We seek to provide Plastic Surgeons with differentiated services, including enhanced customer service offerings and an industry-leading ten‑year limited warranty that provides patients with the largest cash reimbursement for certain out‑of‑pocket costs related to revision surgeries in a covered event; a lifetime no‑charge implant replacement program for covered ruptures; and our industry‑first CapCon Care Program, or C3 Program, through which we offer no‑charge replacement implants to breast augmentation patients who experience capsular contracture within the first five years after implantation with our smooth or textured breast implants.
We continue to focus our efforts on securing and qualifying an alternate manufacturing supplier. In July 2017, we entered into a Settlement Agreement with Silimed, our previous contract manufacturer. On August 9, 2016, we announced our collaboration with Vesta Intermediate Funding, Inc., or Vesta, a Lubrizol Lifesciences company, pursuant to which we are working with Vesta towards establishing a dedicated contract manufacturing facility for our breast implants. On March 14, 2017, we announced that we had executed a definitive manufacturing agreement with Vesta for the manufacture and supply of our breast implants and that we had submitted a PMA supplement to the FDA for the manufacturing of our PMA-approved breast implants by Vesta. On January 30, 2018, we announced the FDA has granted approval of the site-change pre-market approval, or PMA, supplement for our contract manufacturer, Vesta, to manufacture our silicone gel breast implants. In support of the move to the Vesta manufacturing facility, we also implemented new manufacturing process improvements which, in consultation with the FDA, required three (3) additional PMA supplements. In addition to approving the manufacturing site-change supplement, the FDA has approved two (2) of these three (3) process enhancement supplements, while requesting additional information for the third submission. We continue to work closely with the FDA to address their information requests related to this third and final outstanding submission in order to resolve these matters in a timely manner.
miraDry Segment
In July 2017, we completed our acquisition of miraDry, following which we began selling the miraDry System, the only FDA cleared device to reduce underarm sweat, odor and hair of all colors through the precise and non-invasive delivery of microwave energy to the region where sweat glands reside. The energy generates heat at the dermal-fat interface which results in destruction of the sweat glands. At the same time, a continuous hydro-ceramic cooling system protects the superficial dermis and keeps the heat focused at the dermal-fat interface where the sweat glands reside. Because sweat glands do not regenerate after the procedure, we believe the results are lasting. Microwaves are the ideal technology as the energy can be focused directly at the dermal-fat interface where the glands reside.
The miraDry System has been cleared by the FDA as indicated for use in the treatment of primary axillary hyperhidrosis, or a condition characterized by abnormal sweating in excess of that required for regulation of body temperature, plus unwanted underarm hair removal, and permanent reduction of underarm hair of all colors for Fitzpatrick skin types I – IV. Permanent hair reduction is defined as long-term, stable reduction in the number of hairs regrowing when measured at 6, 9 and 12 months after the completion of a treatment regime. When used for the treatment of primary axillary hyperhidrosis, the miraDry System may reduce underarm odor. In addition, the
64
miraDry System received CE mark approval for the treatment of primary axillary hyperhidrosis and approval in several other countries.
The miraDry System provides patients with a non-invasive and durable procedure to selectively destroy underarm sweat glands for both severely hyperhidrotic patients and those that are bothered by their underarm sweat. The miraDry System is clinically proven to reduce sweat in one or more procedures of approximately 60-minutes, allowing most patients to achieve immediately noticeable and durable results without the pain, expense, downtime, or repeat visits associated with surgical and minimally-invasive procedures. The sweat glands in the treated area are destroyed through targeted heating of the tissue, and because the body does not regenerate sweat glands, we believe the results will be lasting, although some patients may need to repeat the miraDry procedure to achieve the lasting results.
The miraDry System consists of a console and a handheld device which uses consumable single-use bioTips. The miraDry procedure is not technique-dependent, does not require significant training or skill for the treatment provider, and the user-interface guides the provider through each step of the procedure for each treatment. We sell our miraDry System and consumable single-use bioTips only to physicians, consisting of dermatologists, plastic surgeons, aesthetic specialists and physicians specializing in the treatment of hyperhidrosis. Aesthetic specialists are physicians who elect to offer aesthetic procedures as a significant part of their practices but are generally not board-certified dermatologists or plastic surgeons. Physicians can market the miraDry procedure as a premium, highly-differentiated, non-surgical sweat reduction procedure. We are approved to sell the miraDry System in over 40 international markets outside of North America, including countries in Asia, Europe, the Middle East and South America.
The miraDry segment generated net sales of $5.1 million for the year ended December 31, 2017 from the acquisition date on July 25, 2017. With the acquisition of miraDry, we expect net sales, cost of goods sold, sales and marketing, general and administrative, and research and development expenses to increase in 2018 when compared to 2017 and prior periods.
Components of Operating Results
Net Sales
We recognize revenue on breast implants and tissue expanders, net of sales discounts and estimated returns, as the customer has a standard six-month window to return purchased breast implants and tissue expanders. Our Breast Products segment net sales for the year ended December 31, 2017 and 2016 reflects the combined effect of the temporary hold on sales and implanting of breast products manufactured by Silimed until March 1, 2016, our controlled re-entry to market designed to optimize our supply of breast implant inventory, the commercial introductions of our scar management products as of March 2016 and our tissue expander portfolio as of November 2016. Net sales for our miraDry segment include net sales of the miraDry System and consumable bioTips as of the acquisition date of July 25, 2017.
We expect that, in the future, our net sales will fluctuate on a quarterly basis due to a variety of factors, including seasonality of breast augmentation procedures and purchase of miraDry procedures. We believe that aesthetic procedures are subject to seasonal fluctuation due to patients planning their procedures leading up to the summer season and in the period around the winter holiday season.
Cost of Goods Sold and Gross Margin
Cost of goods sold consists primarily of costs of finished products purchased from our third‑party manufacturers, reserve for product warranties, inventory fair market value adjustment, royalty costs, and warehouse and other related costs. With the acquisition of miraDry, cost of goods sold also consists of raw material, labor, overhead, and variable manufacturing costs associated with the manufacturing of the miraDry Systems and bioTips.
With respect to our supplier contracts, all our products and raw materials are manufactured under contracts with fixed unit costs.
65
We provide a commercial warranty on our silicone gel breast implants and a standard warranty on our miraDry Systems, handpieces and bioTips. The estimated warranty costs are recorded at the time of sale. In addition, the inventory fair market value associated with non-cash purchase accounting adjustments and royalty costs related to both the SSP and miraDry acquisitions are recorded at the time of sale.
We expect our overall gross margin, which is calculated as net sales less cost of goods sold for a given period divided by net sales, to fluctuate in future periods primarily as a result of quantity of units sold, manufacturing price increases, the changing mix of products sold with different gross margins, overhead costs and targeted pricing programs.
Sales and Marketing Expenses
Our sales and marketing expenses primarily consist of salaries, bonuses, benefits, incentive compensation and travel for our sales, marketing and customer support personnel. Our sales and marketing expenses also include expenses for trade shows, our no‑charge customer shipping program and no-charge product evaluation units, as well as educational, promotional and marketing activities, including direct and online marketing. We expect our sales and marketing expenses to fluctuate in future periods as a result of headcount and timing of our marketing programs. However, we generally expect these costs will increase in absolute dollars.
Research and Development Expenses
Our research and development, or R&D, expenses primarily consist of clinical expenses, product development costs, regulatory expenses, consulting services, outside research activities, quality control and other costs associated with the development of our products and compliance with Good Clinical Practices, or cGCP, requirements. R&D expenses also include related personnel and consultant compensation and stock‑based compensation expense. We expense R&D costs as they are incurred.
We expect our R&D expenses to vary as different development projects are initiated, including improvements to our existing products, expansions of our existing product lines, new product acquisitions and our FDA‑required PMA post‑approval studies of our breast implants. However, we generally expect these costs will increase in absolute terms over time as we continue to expand our product portfolio and add related personnel.
General and Administrative Expenses
Our general and administrative, or G&A, expenses primarily consist of salaries, bonuses, benefits, incentive compensation and stock-based compensation for our executive, financial, legal, business development and administrative functions. Other G&A expenses include outside legal counsel and litigation expenses, independent auditors and other outside consultants, corporate insurance, facilities, information technologies expenses and changes in the valuation of deferred and contingent consideration. In 2015, G&A expenses also include the federal excise tax on the sale of our medical devices in the United States. In 2016 and 2017, we did not have expense for the federal excise tax on the sale of our medical devices, as the tax was suspended for 2016 and 2017.
66
We expect future G&A expenses to increase as we continue to build our finance, legal, information technology, human resources and other general administration resources to continue to advance the commercialization of our products. In addition, we expect to continue to incur G&A expenses in connection with operating as a public company, which may increase further when we are no longer able to rely on the “emerging growth company” exemption we are afforded under the Jumpstart Our Business Startups Act, or the JOBS Act.
Other Income (Expense), net
Other income (expense), net primarily consists of interest income, interest expense, changes in the fair value of common stock warrants and amortization of issuance costs associated with our Credit Agreements.
Income tax expense consists of an estimate for income taxes based on the projected income tax expense for the period ended December 31, 2017. We operate in several tax jurisdictions and are subject to taxes in each jurisdiction in which we conduct business. To date, we have incurred cumulative net losses and maintain a full valuation allowance on our net deferred tax assets due to the uncertainty surrounding realization of such assets. However, as a result of the BIOCORNEUM and tissue expander portfolio acquisitions, we have deferred tax liabilities associated with indefinite-lived intangible assets that cannot be considered sources of income to support the realization of the deferred tax assets, and have provided for tax expense and a corresponding deferred tax liability associated with these indefinite-lived intangible assets.
Critical Accounting Policies and Significant Judgments and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, net sales and expenses and the disclosure of contingent assets and liabilities in our financial statements. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about our financial condition and results of operations that are not readily apparent from other sources. Actual results may differ from these estimates.
While our significant accounting policies are more fully described in Note 2 to our financial statements, we believe that the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
We recognize revenue related to sales of products directly to customers in markets where we have regulatory approval, net of trade discounts and allowances, provided that (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred and title and risk of loss have transferred, (iii) the price is fixed or determinable and (iv) collectability is reasonably assured. Sales prices are documented in the executed sales contract or purchase order prior to shipment. We evaluate the creditworthiness of customers to determine that appropriate credit limits are established prior to the acceptance of an order. Revenue for extended warranties and service agreements are recognized ratably over the term of the agreement.
A portion of our revenue is generated from the sale of consigned inventory of breast implants maintained at doctor, hospital, and clinic locations. For these products, revenue is recognized at the time we are notified by the customer that the product has been implanted, not when the consigned products are delivered to the customer’s warehouse.
We also sell the miraDry System internationally through a combination of distributor networks and direct sales efforts. We recognize revenue when title to the product and risk of loss transfer to customers, provided there are no remaining performance obligations required or any written matters requiring customer acceptance, which generally
67
occurs upon shipment. Standard terms in these agreements do not allow for trial periods, rights of return, refunds, payments contingent on obtaining financing or other terms that could impact the customer’s obligation.
Appropriate reserves are established for anticipated sales returns based on historical experience, recent gross sales and any notification of pending returns. For Breast Products specifically, we allow for the return of Breast Products from customers within six months after the original sale and record estimated sales returns as a reduction of sales in the same period revenue is recognized. Sales return provisions are calculated based upon historical experience with actual returns. Actual sales returns in any future period are inherently uncertain and thus may differ from the estimates. If actual sales returns differ significantly from the estimates, an adjustment to revenue in the current or subsequent period would be recorded. The Company has established an allowance for sales returns of $3.9 million for both December 31, 2017 and 2016, recorded net against accounts receivable in the consolidated balance sheet.
Warranty Reserve
We offer a limited warranty and a lifetime product replacement program for our silicone gel breast implants and a product warranty for our miraDry System. Under the breast implant limited warranty program, we will reimburse patients for certain out‑of‑pocket costs related to revision surgeries performed within ten years from the date of implantation in a covered event. Under the lifetime product replacement program, we provide no‑charge replacement breast implants under a covered event. The programs are available to all patients implanted with our silicone breast implants after April 1, 2012 and are subject to the terms, conditions, claim procedures, limitations and exclusions. Timely completion of a device tracking and warranty enrollment form by the patient’s Plastic Surgeon is required to activate the programs and for the patient to be able to receive benefits under either program. Under the miraDry warranty, we currently provide a standard product warranty for the consoles, handpieces and miraDry consumables, or bioTips. Additionally, an extended warranty may be purchased to provide additional protection of the miraDry System.
We recorded expense for the accrual of warranties in the amounts of $0.2 million, $0.1 million and $0.4 million, for the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017 and 2016, we held total warranty liabilities of $1.6 million and $1.4 million, respectively.
Stock‑Based Compensation
We recognize stock‑based compensation using a fair‑value based method for costs related to all employee share‑based payments, including stock options, restricted stock units, and the employee stock purchase plan. Stock-based compensation cost is measured at the date of grant based on the estimated fair value of the award.
We estimate the fair value of our stock‑based awards to employees and directors using the Black‑Scholes option pricing model. The grant date fair value of a stock‑based award is recognized as an expense over the requisite service period of the award on a straight‑line basis. In addition, we use the Monte-Carlo simulation option-pricing model to determine the fair value of market-based awards. The Monte-Carlo simulation option-pricing model uses the same input assumptions as the Black-Scholes model; however, it also further incorporates into the fair-value determination the possibility that the market condition may not be satisfied. Compensation costs related to these awards are recognized regardless of whether the market condition is satisfied, provided that the requisite service has been provided.
The Black‑Scholes and Monte-Carlo models require inputs of subjective assumptions, including the risk‑free interest rate, expected dividend yield, expected volatility and expected term, among other inputs. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, our stock‑based compensation expense could be materially different in the future.
68
We recorded total non‑cash stock‑based compensation expense of $6.8 million, $3.2 million and $2.4 million for the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017, we had total unrecognized compensation costs of $2.5 million related to unvested stock options. These costs are expected to be recognized over a weighted average period of 1.98 years. As of December 31, 2017, we had total unrecognized compensation costs of $5.7 million related to unvested restricted stock units, or RSUs. These costs are expected to be recognized over a weighted average period of 1.89 years.
The following table represents stock-based compensation expense included in operating expenses in the accompanying consolidated statement of operations for the years ended December 31, 2017, 2016 and 2015 (in thousands:
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
$ |
1,368 |
|
|
$ |
1,000 |
|
|
$ |
771 |
|
|
Research and development |
|
|
645 |
|
|
|
344 |
|
|
|
284 |
|
|
General and administrative |
|
|
4,753 |
|
|
|
1,892 |
|
|
|
1,327 |
|
|
Total |
|
$ |
6,766 |
|
|
$ |
3,236 |
|
|
$ |
2,382 |
|
Acquisitions
We account for acquired business combinations using the acquisition method of accounting, which requires that assets acquired and liabilities assumed be recorded at fair value, with limited exceptions. Valuations are generally completed for business acquisitions using a discounted cash flow analysis. The most significant estimates and assumptions inherent in a discounted cash flow analysis include the amount and timing of projected future cash flows, the discount rate used to measure the risks inherent in the future cash flows, the assessment of the asset’s life cycle, and the competitive and other trends impacting the asset, including consideration of technical, legal, regulatory, economic and other factors. Each of these factors and assumptions can significantly affect the value of the intangible asset. The excess of the fair value of consideration transferred over the fair value of the net assets acquired is recorded as goodwill.
We believe the fair values assigned to the assets acquired and liabilities assumed are based on reasonable assumptions. However, these assumptions may be incomplete or inaccurate, and unanticipated events and circumstances may occur. We will finalize these amounts as we obtain the information necessary to complete the measurement processes. Any changes resulting from facts and circumstances that existed as of the acquisition dates may result in adjustments to the provisional amounts recognized at the acquisition dates. We will finalize these amounts no later than one year from the respective acquisition dates.
Determining the useful life of an intangible asset also requires judgment, as different types of intangible assets will have different useful lives and certain assets may even be considered to have indefinite useful lives. Useful life is the period over which the intangible asset is expected to contribute directly or indirectly to our future cash flows. We determine the useful lives of intangible assets based on a number of factors, such as legal, regulatory, or contractual provisions that may limit the useful life, and the effects of obsolescence, anticipated demand, existence or absence of competition, and other economic factors on useful life.
Acquisition-related deferred and contingent consideration associated with a business combination is initially recognized at fair value and then remeasured each reporting period, with changes in fair value recorded in general and administrative expense. We use the Monte-Carlo Simulation model to estimate the fair value of deferred and contingent consideration, which requires input assumptions about the likelihood of achieving specified milestone criteria, projections of future financial performance, and assumed discount rates. Changes in the fair value of the acquisition-related deferred and contingent consideration obligations result from several factors including changes in discount periods and rates, changes in the timing and amount of revenue estimates and changes in probability assumptions with respect to the likelihood of achieving specified milestone criteria. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, our contingent consideration fair value expense could be materially different in the future.
69
Recent Accounting Pronouncements
Please refer to Note 2 in the notes to our financial statements included in this Annual Report on Form 10-K for information on recent accounting pronouncements and the expected impact on our financial statements.
Results of Operations
Comparison of the Years Ended December 31, 2017 and 2016
The following table sets forth our results of operations for the years ended December 31, 2017 and 2016:
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Statement of operations data |
|
|
|
|
|
|
|
|
|
Net sales |
|
$ |
36,542 |
|
|
$ |
20,734 |
|
|
Cost of goods sold |
|
|
14,171 |
|
|
|
6,880 |
|
|
Gross profit |
|
|
22,371 |
|
|
|
13,854 |
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
33,911 |
|
|
|
20,607 |
|
|
Research and development |
|
|
9,813 |
|
|
|
9,704 |
|
|
General and administrative |
|
|
31,537 |
|
|
|
21,959 |
|
|
Legal settlement |
|
|
10,000 |
|
|
|
1,618 |
|
|
Total operating expenses |
|
|
85,261 |
|
|
|
53,888 |
|
|
Loss from operations |
|
|
(62,890 |
) |
|
|
(40,034 |
) |
|
Other income (expense), net |
|
|
|
|
|
|
|
|
|
Interest income |
|
|
172 |
|
|
|
63 |
|
|
Interest expense |
|
|
(1,232 |
) |
|
|
(98 |
) |
|
Other income (expense), net |
|
|
(95 |
) |
|
|
(36 |
) |
|
Total other income (expense), net |
|
|
(1,155 |
) |
|
|
(71 |
) |
|
Loss before income taxes |
|
|
(64,045 |
) |
|
|
(40,105 |
) |
|
Income tax (benefit) expense |
|
|
(17 |
) |
|
|
61 |
|
|
Net loss |
|
$ |
(64,028 |
) |
|
$ |
(40,166 |
) |
Net Sales
Net sales increased $15.8 million, or 76%, to $36.5 million for the year ended December 31, 2017, as compared to $20.7 million for the year ended December 31, 2016. Net sales of our Breast Products segment increased $10.8 million to $31.5 million for the year ended December 31, 2017, as compared to $20.7 million for the year ended December 31, 2017. The increase was a result of the controlled re-entry to market designed to optimize our supply of breast implant inventory after the voluntary hold on the sale and implanting of all Sientra devices manufactured by Silimed between October 9, 2015 and March 1, 2016. Net sales of our Breast Products segment for the year ended December 31, 2017 also included sales of the tissue expander portfolio we acquired from SSP as of November 2016 and the integration of our scar management products after our acquisition of BIOCORNEUM in March 2016. The acquisition of miraDry contributed $5.1 million of net sales since the acquisition on July 25, 2017.
As of December 31, 2017, our sales organization included 90 employees, including 83 U.S. employees and 7 international employees, as compared to 43 employees as of December 31, 2016. The increase is primarily attributed to the acquisition of miraDry.
70
Cost of Goods Sold and Gross Margin
Cost of goods sold increased $7.3 million, or 106%, to $14.2 million for the year ended December 31, 2017, as compared to $6.9 million for the year ended December 31, 2016. This increase was due to an increase in sales volume of the Breast Products segment and a $2.3 million reserve of excess and obsolete inventory in 2017, mainly related to breast products that are not expected to be sold prior to expiration in 2018. In addition, the acquisition of miraDry increased cost of goods by $2.7 million since the acquisition on July 25, 2017.
The gross margins for the years ended December 31, 2017 and 2016 were 61.2% and 66.8%, respectively. The decrease for the year ended December 31, 2017 was primarily due to the inclusion of miraDry which carries a lower margin and an increase in excess and obsolete inventory reserves. The increase in inventory reserves resulted from the timing and recognition of products anticipated to expire prior to being sold and discontinuation of certain product lines.
Sales and Marketing Expenses
Sales and marketing expenses increased $13.3 million, or 64.6%, to $33.9 million for the year ended December 31, 2017, as compared to $20.6 million for the year ended December 31, 2016. The increase is primarily due to the increase in employee related costs as a result of increased sales and headcount. The acquisition of miraDry increased sales and marketing expenses by $5.4 million since the acquisition on July 25, 2017.
Research and Development Expenses
R&D expenses increased $0.1 million, or 1.1%, to $9.8 million for the year ended December 31, 2017, as compared to $9.7 million for the year ended December 31, 2016. The acquisition of miraDry increased research and development expenses by $0.9 million since the acquisition on July 25, 2017.
General and Administrative Expenses
G&A expenses increased $9.5 million, or 43.6%, to $31.5 million for the year ended December 31, 2017, as compared to $22.0 million for the year ended December 31, 2016. The increase consisted primarily of an increase in employee related costs, an increase in acquisition related costs, an increase in amortization and change in fair market value of contingent consideration related to acquisitions. The acquisition of miraDry increased general and administrative expenses by $2.2 million since the acquisition on July 25, 2017.
Legal Settlement Expenses
Legal settlement expenses were $10.0 million for the year ended December 31, 2017 for the settlement of the Silimed Litigation. Legal settlement expenses were $1.6 million for the year ended December 31, 2016 for the settlement of the Class Action Shareholder Litigation which was comprised of a loss contingency of approximately $10.9 million, net of expected insurance proceeds of approximately $9.4 million.
Other Income (Expense), net
Total other income (expense), net for the year ended December 31, 2017 was primarily associated with expenses related to the change in fair value of warrants, interest expense and amortization of issuance costs associated with our Credit Agreements. Total other income (expense), net for the year ended December 31, 2016 was primarily associated with interest income on cash held in a money market account, interest paid on inventory payable and expense recognized for the change in fair value of warrants.
Income Tax (Benefit) Expense
Income tax benefit was $17 thousand for the year ended December 31, 2017, as compared to expense of $0.1 million for the year ended December 31, 2016. Income tax expense is primarily associated with a deferred tax liability associated with indefinite lived intangibles from acquisitions that cannot offset the deferred tax asset. The income
71
tax benefit for 2017 is due to measuring the December 31, 2017 deferred tax liability using the future decreased federal corporate income tax rate under the Tax Cuts and Jobs Act.
Comparison of the Years Ended December 31, 2016 and 2015
The following table sets forth our results of operations for the years ended 2016 and 2015:
|
|
|
Year Ended |
|
|||||
|
|
|
December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Statement of operations data |
|
|
|
|
|
|
|
|
|
Net sales |
|
$ |
20,734 |
|
|
$ |
38,106 |
|
|
Cost of goods sold |
|
|
6,880 |
|
|
|
10,654 |
|
|
Gross profit |
|
|
13,854 |
|
|
|
27,452 |
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
20,607 |
|
|
|
25,762 |
|
|
Research and development |
|
|
9,704 |
|
|
|
7,199 |
|
|
General and administrative |
|
|
21,959 |
|
|
|
18,738 |
|
|
Legal settlement |
|
|
1,618 |
|
|
|
— |
|
|
Goodwill impairment |
|
|
— |
|
|
|
14,278 |
|
|
Total operating expenses |
|
|
53,888 |
|
|
|
65,977 |
|
|
Loss from operations |
|
|
(40,034 |
) |
|
|
(38,525 |
) |
|
Other income (expense), net |
|
|
|
|
|
|
|
|
|
Interest income |
|
|
63 |
|
|
|
32 |
|
|
Interest expense |
|
|
(98 |
) |
|
|
(3,097 |
) |
|
Other income (expense), net |
|
|
(36 |
) |
|
|
360 |
|
|
Total other income (expense), net |
|
|
(71 |
) |
|
|
(2,705 |
) |
|
Loss before income taxes |
|
|
(40,105 |
) |
|
|
(41,230 |
) |
|
Income tax expense |
|
|
61 |
|
|
|
— |
|
|
Net loss |
|
$ |
(40,166 |
) |
|
$ |
(41,230 |
) |
Net Sales
Net sales decreased $17.4 million, or 45.6%, to $20.7 million for the year ended December 31, 2016, as compared to $38.1 million for the year ended December 31, 2015. The decrease was a result of both our voluntary hold on the sale and implanting of all Sientra devices manufactured by Silimed between October 9, 2015 and March 1, 2016 and our controlled re-entry to market designed to optimize our supply of breast implant inventory. The decrease in breast implant net sales was offset by $3.8 million of scar management product net sales for the year ended December 31, 2016, following the acquisition of BIOCORNEUM in March 2016.
As of December 31, 2016, our sales organization included 43 employees, as compared to 51 employees as of December 31, 2015.
Cost of Goods Sold and Gross Margin
Cost of goods sold decreased $3.8 million, or 35.4%, to $6.9 million for the year ended December 31, 2016, as compared to $10.7 million for the year ended December 31, 2015. This decrease was due to a decrease in sales volume driven by both our voluntary hold on sales from October 9, 2015 to March 1, 2016 and our controlled re-entry into the marketplace, offset by a decrease to our allowance for sales returns.
The gross margins for the years ended December 31, 2016 and 2015 were 66.8% and 72.0%, respectively. The decrease for the year ended December 31, 2016 was primarily due to increased inventory reserves partially offset by increased sales of our scar management products, which generally have higher gross margins. The increase in
72
inventory reserves resulted from the timing and recognition of products anticipated to expire prior to being sold and discontinuation of certain product lines.
Sales and Marketing Expenses
Sales and marketing expenses decreased $5.2 million, or 20.0%, to $20.6 million for the year ended December 31, 2016, as compared to $25.8 million for the year ended December 31, 2015. This decrease consisted primarily of a $2.9 million decrease in employee–related costs as a result of decreased headcount and a $2.6 million decrease in marketing costs due to lower no-charge customer shipping costs and less direct marketing activities.
Research and Development Expenses
R&D expenses increased $2.5 million, or 34.8%, to $9.7 million for the year ended December 31, 2016, as compared to $7.2 million for the year ended December 31, 2015. This increase was primarily due to a $2.6 million increase in product development costs and related consulting fees, a $0.2 million increase of employee incentive compensation, offset by a $0.4 million decrease in clinical trial expenses.
General and Administrative Expenses
G&A expenses increased $3.3 million, or 17.2%, to $22.0 million for the year ended December 31, 2016, as compared to $18.7 million for the year ended December 31, 2015. This increase consisted primarily of a $2.9 million increase in outside legal counsel and litigation expenses and a $0.8 million increase in amortization expense related to intangible assets acquired in the fiscal year, offset by a $0.8 million decrease in medical device excise tax costs as a result of the suspension of the tax during calendar years 2016 and 2017.
Legal Settlement Expenses
Legal settlement expenses were $1.6 million for the year ended December 31, 2016 for the settlement of the Class Action Shareholder Litigation which was comprised of a loss contingency of approximately $10.9 million, net of expected insurance proceeds of approximately $9.4 million. There were no legal settlement expenses for the year ended December 31, 2015.
Goodwill Impairment
There were no goodwill impairment charges for the year ended December 31, 2016. Goodwill impairment charges for the year ended December 31, 2015 were $14.3 million. For additional information on the goodwill impairment for the year ended December 31, 2015, see Note 6 to our Financial Statements included herein.
Other Income (Expense), net
Total other income (expense), net for the year ended December 31, 2016 was primarily associated with interest income on cash held in a money market account, interest paid on inventory payable and expense recognized for the change in fair value of warrants. Total other income (expense), net for the year ended December 31, 2015 was primarily associated with interest income on cash held in a money market account, interest expense on our Oxford term loans, which were repaid in full in the fourth quarter of 2015 and income recognized for the change in fair value of warrants.
Income Tax Expense
Income tax expense was $0.1 million for the year ended December 31, 2016 . There was no income tax expense for the year ended December 31, 2015. Income tax expense for the year ended December 31, 2016 was associated with a deferred tax liability associated with indefinite lived intangibles from acquisitions that cannot offset the deferred tax asset.
73
Liquidity and Capital Resources
Since our inception, we have incurred significant net operating losses and anticipate that our losses will continue in the near term. We expect our operating expenses will continue to grow as we expand our operations. We will need to generate significant net sales to achieve profitability. To date, we have funded our operations primarily with proceeds from the sales of preferred stock, borrowings under our term loans, sales of our products since 2012, and the proceeds from the sale of our common stock in public offerings.
To fund our ongoing operating and capital needs, we will need to raise additional equity or debt capital. In February 2018, we entered into an At-The-Market Equity Offering Sales Agreement with Stifel, Nicolaus & Company, Incorporated as sales agent pursuant to which we may sell, from time to time, through Stifel shares of our common stock having an aggregate gross offering price of up to $50 million. In addition, upon receipt of FDA certifications of the manufacturing facility operated by Vesta by March 31, 2018, we may receive a $10.0 million term loan pursuant to our credit agreement with MidCap Financial Trust. We may also seek additional capital through the sale of equity securities or issuance of debt. There can be no assurance, however, that we will obtain FDA certifications or otherwise be successful in completing an equity or debt financing on a timeframe that coincides with our cash needs, on acceptable terms, or completing it at all. Our independent registered public accounting firm has included in its auditor’s report on our consolidated financial statements, included in this Annual Report on Form 10-K, a “going concern” explanatory paragraph, meaning that the recurring losses from operations and negative cash flows from operations raise substantial doubt regarding our ability to continue as a going concern. While there can be no assurances that the Company will be successful in obtaining the level of financing needed for its operations, the Company believes it is probable that the actions taken, together with any additional equity or debt financing the Company may pursue, will be sufficient to address liquidity needs and alleviate the substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time. If the Company is unsuccessful in raising capital, it may need to reduce activities, curtail or cease certain operations.
In November 2014, we completed our IPO, raising approximately $77.0 million in net proceeds. In September 2015, we completed a follow-on public offering of common stock raising approximately $61.4 million in net proceeds.
On March 13, 2017, we entered into the SVB Loan Agreement. Under the terms of the SVB Loan Agreement, SVB made available to us a $15.0 million Revolving Line of Credit and a $5.0 million term loan. On July 25, 2017, we repaid in full our outstanding indebtedness under the SVB Loan Agreement and the agreement was terminated and replaced with the Credit Agreements with Midcap.
On July 25, 2017, we borrowed $25.0 million pursuant to the Term Loan Credit Agreement with Midcap and the other lenders party thereto. We used the proceeds (i) to repay in full our then-existing indebtedness under the SVB Loan Agreement, which totaled approximately $5.0 million, (ii) to pay fees and expenses in connection with the foregoing and (iii) for general corporate purposes. The Term Loan Credit Agreement provides for (i) the Closing Date Term Loan, (ii) until March 31, 2018, an additional $10.0 million term loan facility subject to the satisfaction of certain conditions, including FDA certification of the manufacturing facility operated by Vesta and (iii) an additional $5.0 million term loan facility subject to the satisfaction of certain conditions, including evidence that the Company’s Net Revenue (as defined in the Term Loan Credit Agreement) for the past 12 months was greater than or equal to $75.0 million. In addition, on July 25, 2017, we also entered into a Revolving Credit Agreement with MidCap and the other lenders party thereto. The amount available to be drawn under the Revolving Credit Agreement is based on a Borrowing Base equal to 85% of the net collectible value of eligible accounts receivable plus 40% of eligible finished goods inventory, provided that availability from eligible finished goods inventory does not exceed 20% of the Borrowing Base. We may make (subject to the applicable borrowing base at the time) and repay borrowings from time to time under the Revolving Credit Agreement until the maturity of the facility on December 1, 2021.
See Note 5 to the financial statement for a full description of our debt and revolving line of credit.
As of December 31, 2017, we had $26.6 million in cash and cash equivalents. Our historical cash outflows have primarily been associated with research and development activities, activities relating to commercialization and increases in working capital, including the expansion of our sales force and marketing programs. In addition, we have used cash to fund the acquisitions of miraDry, BIOCORNEUM and the tissue expander portfolio from SSP.
74
The following table shows a summary of our cash flows (used in) provided by operating, investing and financing activities for the periods indicated:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(45,916 |
) |
|
$ |
(34,430 |
) |
|
$ |
(18,184 |
) |
|
Investing activities |
|
|
(20,319 |
) |
|
|
(12,835 |
) |
|
|
(1,128 |
) |
|
Financing activities |
|
|
25,611 |
|
|
|
1,676 |
|
|
|
35,384 |
|
|
Net change in cash and cash equivalents |
|
$ |
(40,624 |
) |
|
$ |
(45,589 |
) |
|
$ |
16,072 |
|
Cash used in operating activities
Net cash used in operating activities was $45.9 million, $34.4 million and $18.2 million during the years ended December 31, 2017, 2016 and 2015, respectively. The $11.5 million increase in cash used in operating activities between the years ended December 31, 2017 and 2016 was primarily associated with a $23.8 million increase in net loss, offset by the timing of the receipt of the $9.4 million Class Action Shareholder Litigation insurance receivable. The $16.2 million increase in cash used in operating activities between the years ended December 31, 2016 and 2015 was primarily associated with a $17.4 million decrease in net sales, a $2.1 million increase in operating expenses, excluding goodwill impairment for 2015, and an increase in cash outflows from operating assets and liabilities, primarily related to customer deposits and timing of accounts payable and accrued liability payments.
Cash used in investing activities
Net cash used in investing activities was $20.3 million, $12.8 million and $1.1 million during the years ended December 31, 2017, 2016 and 2015, respectively. The increase in cash used in investing activities of $7.5 million between the years ended December 31, 2017 and 2016 was primarily due to $18.4 million for the acquisition of miraDry in July 2017. The increase in cash used in investing activities of $11.7 million between the years ended December 31, 2016 and 2015 was primarily due to $6.9 million for the acquisition of BIOCORNEUM in March 2016 and $5.0 million for the acquisition of the AlloX2 and Dermaspan lines of breast tissue expanders, in addition to the Softspan line of general tissue expanders from SSP in November 2016.
Cash provided by financing activities
Net cash provided by financing activities was $25.6 million, $1.7 million and $35.4 million for the years ended December 31, 2017, 2016, and 2015, respectively. The increase in cash provided by financing activities of $23.9 million between the years ended December 31, 2017 and 2016 was primarily the result of borrowing under the Term Loan. The decrease in cash provided by financing activities of $33.7 million between the years ended December 31, 2016 and 2015 was primarily the result of a decrease in cash proceeds from the issuance of our common stock. For the year ended December 31, 2015, we received cash proceeds from the issuance of common stock, net of underwriters discount in a follow-on offering of $62.0 million, offset by the repayment of long-term debt of $26.6 million.
Our liquidity position and capital requirements are subject to a number of factors. For example, our cash inflow and outflow may be impacted by the following:
|
|
• |
the timing to qualify Vesta with the FDA and the timing and availability of any alternative manufacturing sources, and costs associated with procuring and qualifying such manufacturing capacity; |
|
|
• |
net sales generated by our Breast Products and miraDry segments, and any other future products that we may develop and commercialize; |
75
|
|
• |
cost associated with developing and commercializing our proposed products or technologies; |
|
|
• |
expenses we incur in connection with potential litigation or governmental investigations; |
|
|
• |
cost of obtaining and maintaining regulatory clearance or approval for our current or future products; |
|
|
• |
cost of ongoing compliance with regulatory requirements; |
|
|
• |
anticipated or unanticipated capital expenditures; and |
|
|
• |
unanticipated G&A expenses. |
Our primary short-term capital needs, which are subject to change, include expenditures related to:
|
|
• |
support of our sales and marketing efforts related to our current and future products; |
|
|
• |
new product acquisition and development efforts; |
|
|
• |
facilities expansion needs; |
|
|
• |
investment in inventory required to meet customer demands; and |
|
|
• |
expenses we incur in connection with defending against litigation. |
Although we believe the foregoing items reflect our most likely uses of cash in the short-term, we cannot predict with certainty all of our particular short-term cash uses or the timing or amount of cash used. If cash generated from operations is insufficient to satisfy our working capital and capital expenditure requirements, we may be required to sell additional equity or debt securities or obtain credit facilities. Additional capital, if needed, may not be available on satisfactory terms, if at all. Furthermore, any additional equity financing may be dilutive to stockholders, and debt financing, if available, may include restrictive covenants. For a discussion of other factors that may impact our future liquidity and capital funding requirements, see “Risk Factors — Risks Related to Our Financial Results.”
Contractual Obligations and Commitments
The following table summarizes our contractual obligations and commitments as of December 31, 2017:
|
|
|
Payments Due by Period |
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
More than |
|
|
|
|
|
Total |
|
|
Less than 1 year |
|
|
1 - 3 years |
|
|
3 - 5 years |
|
|
5 years |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
Operating lease obligations |
|
$ |
2,370 |
|
|
$ |
1,164 |
|
|
$ |
1,206 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Purchase obligations (1) |
|
|
10,771 |
|
|
|
10,771 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Term loan principle obligations (2) |
|
|
25,000 |
|
|
|
25,000 |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Total contractual obligations |
|
$ |
38,141 |
|
|
$ |
36,935 |
|
|
$ |
1,206 |
|
|
$ |
— |
|
|
$ |
— |
|
|
(1) |
Purchase obligations include open purchase commitments with our contract manufacturers. We currently expect to fund these commitments with cash flows from operations, existing cash balances or borrowings under our term loan. |
|
(2) |
Term loan principle obligations do not include interest obligations related to the Term Loan Credit Agreement and Revolving Credit Agreement as the interest obligation cannot be reasonably estimated. The Term Loan Credit Agreement and Revolving Credit Agreement are discussed in Note 5. |
76
Off‑Balance Sheet Arrangements
During the periods presented we did not have, nor do we currently have, any off‑balance sheet arrangements as defined under SEC rules.
Item 7A. Quantitative and Qualitative Disclosures about Market Risks
As of December 31, 2017, we had $26.6 million in cash and cash equivalents. We generally hold our cash in checking accounts and interest-bearing money market accounts. Our exposure to market risk related to interest rate sensitivity is affected by changes in the general level of U.S. interest rates. Due to the short-term maturities of our cash equivalents and the low risk profile of our investments, an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our cash equivalents.
Item 8. Financial Statements and Supplementary Data
The financial statements required to be filed pursuant to this Item 8 are appended to this report beginning on page F‑1. An index of those financial statements is included in Part IV, Item 15 below.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10‑K. The term “disclosure controls and procedures,” as defined in Rules 13a‑15(e) and 15d‑15(e) under the Exchange Act means control and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Based on this evaluation, the Company’s principal executive officer and principal financial officer have concluded that, as of December 31, 2017, the Company’s disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America and includes policies and procedures that:
|
|
• |
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company; |
|
|
• |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
77
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
As of December 31, 2017, our management assessed the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control – Integrated Framework (2013), or the COSO 2013 Framework. Based on this assessment, management concluded that as of December 31, 2017, our internal control over financial reporting was effective based on those criteria.
This annual report does not include an attestation report of the company’s registered public accounting firm due to the established rules of the Securities and Exchange Commission.
Remediation of Previous Material Weakness
As described in Item 9A in our Annual Report on Form 10-K for the year ended December 31, 2016, we previously identified a material weakness in our internal control over financial reporting related to accounting for significant unusual transactions. During 2017, management implemented new internal controls relating to the recognition and measurement of significant unusual transactions, including those involving external service providers, as well as management review controls over business combinations, specifically key assumptions, financial data and calculations used to measure the fair value of acquired assets and liabilities, including contingent consideration prepared by its external service provider. As a result, management determined that the material weakness identified as of December 31, 2016 has been remediated as of December 31, 2017.
Changes in Internal Control over Financial Reporting
Except for the remediation of the material weakness described herein, there was no change in our internal control over financial reporting that occurred during the year ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
On March 13, 2018, the Company entered into a Second Amendment to Employment Agreement, or the Amendment, with Jeffrey M. Nugent, the Company’s Chief Executive Officer. The Amendment provides for, among other things, a special long-term performance bonus of up to $5,000,000 based on the achievement of certain market-based performance criteria established by the Compensation Committee over a five-year period commencing on March 15, 2018. In addition, the Amendment provides that Mr. Nugent’s annual discretionary bonus may be up to 100% of his base salary.
78
Item 10. Directors, Executive Officers, and Corporate Governance
Incorporated by reference from the information in our Proxy Statement for our 2018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10‑K relates.
Item 11. Executive Compensation
Incorporated by reference from the information in our Proxy Statement for our 2018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10‑K relates.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Incorporated by reference from the information in our Proxy Statement for our 2018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10‑K relates.
Item 13. Certain Relationships and Related Transactions and Director Independence
Incorporated by reference from the information in our Proxy Statement for our 2018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10‑K relates.
Item 14. Principal Accountant Fees and Services
Incorporated by reference from the information in our Proxy Statement for our 2018 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10‑K relates.
79
Item 15. Exhibits, Financial Statements and Schedule
|
(a)(1) |
Financial Statements. |
The response to this portion of Item 15 is set forth under Item 8 above.
|
(a)(2) |
Financial Statement Schedule. |
All schedules have been omitted because they are not required or because the required information is given in the Financial Statements or Notes thereto.
|
(a)(3) |
Exhibits. |
List of Exhibits required by Item 601 of Regulation S-K. See Item 15(b) below.
80
INDEX TO FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULE
|
|
|
Pages |
|
|
F-2 |
|
|
|
F-3 |
|
|
|
F-4 |
|
|
|
F-5 |
|
|
|
F-6 |
|
|
|
F-7 |
|
|
|
F-37 |
F-1
Report of Independent Registered Public Accounting Firm
The Stockholders and Board of Directors
Sientra, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Sientra, Inc. and subsidiaries (the Company) as of December 31, 2017 and 2016, the related consolidated statements of income, stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2017, and the related notes and financial statement schedule II (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company’s recurring losses from operations, insufficient cash flows generated from operations, potential violations of financial covenants and need to obtain additional capital raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
|
|
(signed) KPMG LLP |
|
We have served as the Company’s auditor since 2014.
|
|
|
|
Los Angeles, California |
|
|
|
March 13, 2018 |
|
|
F-2
(in thousands, except per share data)
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2017 |
|
|
2016 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
26,588 |
|
|
$ |
67,212 |
|
|
Accounts receivable, net of allowances of $4,816 and $4,329 at December 31, 2017 and December 31, 2016, respectively |
|
|
6,569 |
|
|
|
3,082 |
|
|
Inventories, net |
|
|
20,896 |
|
|
|
18,484 |
|
|
Insurance recovery receivable |
|
|
39 |
|
|
|
9,375 |
|
|
Prepaid expenses and other current assets |
|
|
1,473 |
|
|
|
1,852 |
|
|
Total current assets |
|
|
55,565 |
|
|
|
100,005 |
|
|
Property and equipment, net |
|
|
4,763 |
|
|
|
2,986 |
|
|
Goodwill |
|
|
12,507 |
|
|
|
4,878 |
|
|
Other intangible assets, net |
|
|
18,803 |
|
|
|
6,186 |
|
|
Other assets |
|
|
575 |
|
|
|
228 |
|
|
Total assets |
|
$ |
92,213 |
|
|
$ |
114,283 |
|
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Current portion of long-term debt |
|
$ |
24,639 |
|
|
$ |
— |
|
|
Accounts payable |
|
|
5,811 |
|
|
|
3,555 |
|
|
Accrued and other current liabilities |
|
|
13,474 |
|
|
|
6,507 |
|
|
Legal settlement payable |
|
|
1,000 |
|
|
|
10,900 |
|
|
Customer deposits |
|
|
5,423 |
|
|
|
6,559 |
|
|
Total current liabilities |
|
|
50,347 |
|
|
|
27,521 |
|
|
Deferred and contingent consideration |
|
|
12,597 |
|
|
|
1,637 |
|
|
Warranty reserve and other long-term liabilities |
|
|
1,646 |
|
|
|
1,508 |
|
|
Total liabilities |
|
|
64,590 |
|
|
|
30,666 |
|
|
Commitments and contingencies (Note 11) |
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.01 par value – Authorized 10,000,000 shares; none issued or outstanding |
|
|
— |
|
|
|
— |
|
|
Common stock, $0.01 par value — Authorized 200,000,000 shares; issued 19,474,702 and 18,671,409 and outstanding 19,401,975 and 18,598,682 shares at December 31, 2017 and December 31, 2016 respectively |
|
|
194 |
|
|
|
186 |
|
|
Additional paid-in capital |
|
|
307,159 |
|
|
|
299,133 |
|
|
Treasury stock, at cost (72,727 shares at December 31, 2017 and December 31, 2016) |
|
|
(260 |
) |
|
|
(260 |
) |
|
Accumulated deficit |
|
|
(279,470 |
) |
|
|
(215,442 |
) |
|
Total stockholders’ equity |
|
|
27,623 |
|
|
|
83,617 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
92,213 |
|
|
$ |
114,283 |
|
See accompanying notes to the consolidated financial statements.
F-3
Consolidated Statements of Operations
(in thousands, except per share data)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Net sales |
|
$ |
36,542 |
|
|
$ |
20,734 |
|
|
$ |
38,106 |
|
|
Cost of goods sold |
|
|
14,171 |
|
|
|
6,880 |
|
|
|
10,654 |
|
|
Gross profit |
|
|
22,371 |
|
|
|
13,854 |
|
|
|
27,452 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing |
|
|
33,911 |
|
|
|
20,607 |
|
|
|
25,762 |
|
|
Research and development |
|
|
9,813 |
|
|
|
9,704 |
|
|
|
7,199 |
|
|
General and administrative |
|
|
31,537 |
|
|
|
21,959 |
|
|
|
18,738 |
|
|
Legal settlement |
|
|
10,000 |
|
|
|
1,618 |
|
|
|
— |
|
|
Goodwill impairment |
|
|
— |
|
|
|
— |
|
|
|
14,278 |
|
|
Total operating expenses |
|
|
85,261 |
|
|
|
53,888 |
|
|
|
65,977 |
|
|
Loss from operations |
|
|
(62,890 |
) |
|
|
(40,034 |
) |
|
|
(38,525 |
) |
|
Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
172 |
|
|
|
63 |
|
|
|
32 |
|
|
Interest expense |
|
|
(1,232 |
) |
|
|
(98 |
) |
|
|
(3,097 |
) |
|
Other income (expense), net |
|
|
(95 |
) |
|
|
(36 |
) |
|
|
360 |
|
|
Total other income (expense), net |
|
|
(1,155 |
) |
|
|
(71 |
) |
|
|
(2,705 |
) |
|
Loss before income taxes |
|
|
(64,045 |
) |
|
|
(40,105 |
) |
|
|
(41,230 |
) |
|
Income tax (benefit) expense |
|
|
(17 |
) |
|
|
61 |
|
|
|
— |
|
|
Net loss |
|
$ |
(64,028 |
) |
|
$ |
(40,166 |
) |
|
$ |
(41,230 |
) |
|
Basic and diluted net loss per share attributable to common stockholders |
|
$ |
(3.34 |
) |
|
$ |
(2.20 |
) |
|
$ |
(2.61 |
) |
|
Weighted average outstanding common shares used for net loss per share attributable to common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
19,159,057 |
|
|
|
18,233,177 |
|
|
|
15,770,972 |
|
See accompanying notes to the consolidated financial statements.
F-4
Consolidated Statements of Stockholders’ Equity
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
Total |
|
|||||||
|
|
|
Preferred stock |
|
|
Common stock |
|
|
Treasury stock |
|
|
paid-in |
|
|
Accumulated |
|
|
stockholders’ |
|
||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
deficit |
|
|
equity |
|
|||||||||
|
Balances at December 31, 2014 |
|
|
— |
|
|
$ |
— |
|
|
|
14,985,704 |
|
|
$ |
150 |
|
|
|
72,727 |
|
|
$ |
(260 |
) |
|
|
229,795 |
|
|
$ |
(134,046 |
) |
|
$ |
95,639 |
|
|
Proceeds from follow-on offering, net of costs |
|
|
— |
|
|
|
— |
|
|
|
3,000,000 |
|
|
|
30 |
|
|
|
— |
|
|
|
— |
|
|
|
61,367 |
|
|
|
— |
|
|
|
61,397 |
|
|
Employee stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,382 |
|
|
|
— |
|
|
|
2,382 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
36,189 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
119 |
|
|
|
— |
|
|
|
119 |
|
|
Employee stock purchase program (ESPP) |
|
|
— |
|
|
|
— |
|
|
|
44,250 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
564 |
|
|
|
— |
|
|
|
564 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(41,230 |
) |
|
|
(41,230 |
) |
|
Balances at December 31, 2015 |
|
|
— |
|
|
$ |
— |
|
|
|
18,066,143 |
|
|
$ |
180 |
|
|
|
72,727 |
|
|
$ |
(260 |
) |
|
$ |
294,227 |
|
|
$ |
(175,276 |
) |
|
$ |
118,871 |
|
|
Employee stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,236 |
|
|
|
— |
|
|
|
3,236 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
478,099 |
|
|
|
5 |
|
|
|
— |
|
|
|
— |
|
|
|
918 |
|
|
|
— |
|
|
|
923 |
|
|
Employee stock purchase program (ESPP) |
|
|
— |
|
|
|
— |
|
|
|
122,667 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
752 |
|
|
|
— |
|
|
|
753 |
|
|
Vested restricted stock |
|
|
|
|
|
|
|
|
|
|
4,500 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(40,166 |
) |
|
|
(40,166 |
) |
|
Balances at December 31, 2016 |
|
|
— |
|
|
$ |
— |
|
|
|
18,671,409 |
|
|
$ |
186 |
|
|
|
72,727 |
|
|
$ |
(260 |
) |
|
$ |
299,133 |
|
|
$ |
(215,442 |
) |
|
$ |
83,617 |
|
|
Employee stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,766 |
|
|
|
— |
|
|
|
6,766 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
480,236 |
|
|
|
5 |
|
|
|
— |
|
|
|
— |
|
|
|
1,341 |
|
|
|
— |
|
|
|
1,346 |
|
|
Employee stock purchase program (ESPP) |
|
|
— |
|
|
|
— |
|
|
|
108,081 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
646 |
|
|
|
— |
|
|
|
647 |
|
|
Vested restricted stock |
|
|
— |
|
|
|
— |
|
|
|
293,910 |
|
|
|
3 |
|
|
|
— |
|
|
|
— |
|
|
|
(3 |
) |
|
|
— |
|
|
|
— |
|
|
Shares withheld for tax obligations on vested RSUs |
|
|
— |
|
|
|
— |
|
|
|
(78,934 |
) |
|
|
(1 |
) |
|
|
— |
|
|
|
— |
|
|
|
(724 |
) |
|
|
— |
|
|
|
(725 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(64,028 |
) |
|
|
(64,028 |
) |
|
Balances at December 31, 2017 |
|
|
— |
|
|
$ |
— |
|
|
|
19,474,702 |
|
|
$ |
194 |
|
|
|
72,727 |
|
|
$ |
(260 |
) |
|
$ |
307,159 |
|
|
$ |
(279,470 |
) |
|
$ |
27,623 |
|
See accompanying notes to the consolidated financial statements.
F-5
Consolidated Statements of Cash Flows
(in thousands)
|
|
|
Year Ended |
|
|||||||||
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(64,028 |
) |
|
$ |
(40,166 |
) |
|
$ |
(41,230 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill impairment |
|
|
— |
|
|
|
— |
|
|
|
14,278 |
|
|
Depreciation and amortization |
|
|
3,034 |
|
|
|
1,177 |
|
|
|
318 |
|
|
Provision for doubtful accounts |
|
|
493 |
|
|
|
437 |
|
|
|
233 |
|
|
Provision for warranties |
|
|
294 |
|
|
|
71 |
|
|
|
385 |
|
|
Provision for inventory |
|
|
3,125 |
|
|
|
1,323 |
|
|
|
469 |
|
|
Amortization of acquired inventory step-up |
|
|
999 |
|
|
|
61 |
|
|
|
— |
|
|
Change in fair value of warrants |
|
|
95 |
|
|
|
39 |
|
|
|
(360 |
) |
|
Change in fair value of deferred and contingent consideration |
|
|
1,025 |
|
|
|
37 |
|
|
|
— |
|
|
Non-cash portion of debt extinguishment loss |
|
|
17 |
|
|
|
— |
|
|
|
— |
|
|
Amortization of debt discount and issuance costs |
|
|
140 |
|
|
|
— |
|
|
|
— |
|
|
Non-cash interest expense |
|
|
1 |
|
|
|
3 |
|
|
|
1,386 |
|
|
Stock-based compensation expense |
|
|
6,766 |
|
|
|
3,236 |
|
|
|
2,382 |
|
|
Loss on disposal of property and equipment |
|
|
25 |
|
|
|
124 |
|
|
|
— |
|
|
Deferred income taxes |
|
|
(21 |
) |
|
|
61 |
|
|
|
— |
|
|
Changes in assets and liabilities, net of effects from acquisitions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(1,890 |
) |
|
|
927 |
|
|
|
715 |
|
|
Inventories |
|
|
527 |
|
|
|
2,390 |
|
|
|
(898 |
) |
|
Prepaid expenses, other current assets and other assets |
|
|
674 |
|
|
|
(529 |
) |
|
|
147 |
|
|
Insurance recovery receivable |
|
|
9,336 |
|
|
|
(9,375 |
) |
|
|
— |
|
|
Accounts payable |
|
|
1,290 |
|
|
|
(564 |
) |
|
|
1,546 |
|
|
Accrued and other liabilities |
|
|
3,218 |
|
|
|
(1,422 |
) |
|
|
1,571 |
|
|
Legal settlement payable |
|
|
(9,900 |
) |
|
|
10,900 |
|
|
|
— |
|
|
Customer deposits |
|
|
(1,136 |
) |
|
|
(3,160 |
) |
|
|
874 |
|
|
Net cash used in operating activities |
|
|
(45,916 |
) |
|
|
(34,430 |
) |
|
|
(18,184 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(1,864 |
) |
|
|
(1,126 |
) |
|
|
(1,128 |
) |
|
Business acquisitions, net of cash acquired |
|
|
(18,455 |
) |
|
|
(11,709 |
) |
|
|
— |
|
|
Net cash used in investing activities |
|
|
(20,319 |
) |
|
|
(12,835 |
) |
|
|
(1,128 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
1,346 |
|
|
|
923 |
|
|
|
119 |
|
|
Proceeds from issuance of common stock under ESPP |
|
|
647 |
|
|
|
753 |
|
|
|
564 |
|
|
Tax payments related to shares withheld for vested restricted stock units (RSUs) |
|
|
(725 |
) |
|
|
— |
|
|
|
— |
|
|
Proceeds from issuance of common stock, net of underwriters discount |
|
|
— |
|
|
|
— |
|
|
|
62,040 |
|
|
Deferred equity issuance costs, IPO |
|
|
— |
|
|
|
— |
|
|
|
(71 |
) |
|
Deferred equity issuance costs, follow-on offering |
|
|
— |
|
|
|
— |
|
|
|
(643 |
) |
|
Repayment of long-term debt |
|
|
— |
|
|
|
— |
|
|
|
(26,625 |
) |
|
Gross borrowings under the Term Loan |
|
|
25,000 |
|
|
|
— |
|
|
|
— |
|
|
Gross borrowings under the Revolving Line of Credit |
|
|
5,000 |
|
|
|
— |
|
|
|
— |
|
|
Repayment of the Revolving Line of Credit |
|
|
(5,000 |
) |
|
|
— |
|
|
|
— |
|
|
Deferred financing costs |
|
|
(657 |
) |
|
|
— |
|
|
|
|
|
|
Net cash provided by financing activities |
|
|
25,611 |
|
|
|
1,676 |
|
|
|
35,384 |
|
|
Net decrease in cash and cash equivalents |
|
|
(40,624 |
) |
|
|
(45,589 |
) |
|
|
16,072 |
|
|
Cash and cash equivalents at: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of period |
|
|
67,212 |
|
|
|
112,801 |
|
|
|
96,729 |
|
|
End of period |
|
$ |
26,588 |
|
|
$ |
67,212 |
|
|
$ |
112,801 |
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ |
870 |
|
|
$ |
96 |
|
|
$ |
1,884 |
|
|
Supplemental disclosure of non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment in accounts payable and accrued liabilities |
|
|
1,088 |
|
|
|
939 |
|
|
|
22 |
|
|
Acquisition of business, deferred and contingent consideration obligations at fair value |
|
|
10,912 |
|
|
|
1,600 |
|
|
|
— |
|
|
Forgiveness of SVB Loan commitment fee |
|
|
750 |
|
|
|
— |
|
|
|
— |
|
|
Accrued deferred financing costs |
|
|
6 |
|
|
|
— |
|
|
|
— |
|
See accompanying notes to the consolidated financial statements.
F-6
Notes to the Consolidated Financial Statements
(1) Formation and Business of the Company
|
(a) |
Formation |
Sientra, Inc. (“Sientra”, the “Company,” “we,” “our” or “us”), was incorporated in the State of Delaware on August 29, 2003 under the name Juliet Medical, Inc. and subsequently changed its name to Sientra, Inc. in April 2007. The Company acquired substantially all the assets of Silimed, Inc. on April 4, 2007. The purpose of the acquisition was to acquire the rights to the silicone breast implant clinical trials, related product specifications and pre-market approval, or PMA, assets. Following this acquisition, the Company focused on completing the clinical trials to gain FDA approval to offer its silicone gel breast implants in the United States.
In March 2012, the Company announced it had received approval from the FDA for its portfolio of silicone gel breast implants, and in the second quarter of 2012 the Company began commercialization efforts to sell its products in the United States. The Company, based in Santa Barbara, California, is a medical aesthetics company that focuses on serving board-certified plastic surgeons and offers a portfolio of silicone shaped and round breast implants, scar management, tissue expanders, and body contouring products.
In November 2014, the Company completed an initial public offering, or IPO, and its common stock is listed on the Nasdaq Stock Exchange under the symbol “SIEN.”
|
(b) |
Follow-on Offering |
On September 23, 2015, the Company closed a follow-on public offering, whereby it sold 3,000,000 shares of its common stock, at a price to the public of $22.00 per share. The Company received net proceeds from the follow-on offering of approximately $61.4 million, after deducting underwriting discounts and commissions of $4.0 million and offering expenses of approximately $0.6 million.
|
(c) |
Acquisition of miraDry |
On June 11, 2017, Sientra entered into an Agreement and Plan of Merger, or the Merger Agreement, with miraDry (formerly Miramar Labs), pursuant to which Sientra commenced a tender offer to purchase all of the outstanding shares of miraDry’s common stock for (i) $0.3149 per share, plus (ii) the contractual right to receive one or more contingent payments upon the achievement of certain future sales milestones. The total merger consideration was $18.7 million in upfront cash and the contractual rights represent potential contingent payments of up to $14 million. The transaction, which closed on July 25, 2017, added the miraDry® System to Sientra’s aesthetics portfolio.
|
(d) |
Regulatory Review of Vesta Manufacturing |
The Company has engaged Vesta Intermediate Funding, Inc., or Vesta, a Lubrizol Lifesciences company, for the manufacture and supply of the Company’s breast implants. On March 14, 2017, the Company announced it had submitted a PMA supplement to the FDA for the manufacturing of the Company’s PMA-approved breast implants by Vesta. On January 30, 2018, the Company announced the FDA has granted approval of the site-change pre-market approval, or PMA, supplement for the Company’s contract manufacturer, Vesta, to manufacture their silicone gel breast implants. In support of the move to the Vesta manufacturing facility, the Company also implemented new manufacturing process improvements which, in consultation with the FDA, required three (3) additional PMA supplements. In addition to approving the manufacturing site-change supplement, the FDA has approved two (2) of these three (3) process enhancement supplements, while requesting additional information for the third submission. The Company continues to work closely with the FDA to address their information requests related to this third and final outstanding submission in order to resolve these matters in a timely manner.
F-7
There have been regulatory inquiries related to medical devices manufactured by Silimed Indústria de Implantes Ltda. (formerly, Silimed-Silicone e Instrumental Medico-Cirugio e Hospitalar Ltda.), or Silimed, the Company’s former sole source contract manufacturer for its silicone gel breast implants. Following extensive independent, third-party testing and analyses of its devices manufactured by Silimed, which tests indicated no significant safety concerns with the use of Silimed’s products, the Company lifted the temporary hold on the sale of such devices. While the Company continues to sell its remaining inventory of devices manufactured by Silimed, its existing manufacturing contract with Silimed expired on its terms in April 2017 and the Company did not renew the contract.
(2) Summary of Significant Accounting Policies
|
(a) |
Basis of Presentation and Use of Estimates |
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America, or GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Assets and liabilities which are subject to significant judgment and use of estimates include the allowance for doubtful accounts, sales return reserves, provision for warranties, valuation of inventories, recoverability of long-lived assets, valuation allowances with respect to deferred tax assets, useful lives associated with property and equipment and finite lived intangible assets, and the valuation and assumptions underlying stock-based compensation and other equity instruments. On an ongoing basis, the Company evaluates its estimates compared to historical experience and trends, which form the basis for making judgments about the carrying value of assets and liabilities. In addition, the Company engages the assistance of valuation specialists in concluding on fair value measurements in connection with stock-based compensation and other equity instruments.
|
(b) |
Going Concern |
Since inception, the Company has incurred net losses. During the years ended December 31, 2017, 2016 and 2015 the Company incurred net losses of $64.0 million, $40.2 million and $41.2 million, respectively. The Company used $45.9 million of cash in operations for the year ended December 31, 2017, $34.4 million for the year ended December 31, 2016 and $18.2 million for the year ended December 31, 2015. At December 31, 2017 and 2016 the Company had an accumulated deficit of $279.5 million and $215.4 million, respectively. At December 31, 2017, the Company had cash and cash equivalents of $26.6 million.
These consolidated financial statements have been prepared assuming that the Company will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty. The Company has incurred recurring losses from operations and cash outflows from operating activities that raise substantial doubt about its ability to continue as a going concern. In addition, while the Company was in compliance with the financial covenants in its credit agreement with MidCap Financial Trust at December 31, 2017, given the potential violations of those covenants during fiscal year 2018, the Company has classified the debt as current in the consolidated balance sheet at December 31, 2017. Management has taken and is taking actions to improve our liquidity. For example, the Company has the ability to receive a $10.0 million term loan pursuant to our credit agreement with MidCap Financial Trust, upon receipt of FDA certifications of the manufacturing facility operated by Vesta by March 31, 2018. In addition, in February 2018, the Company entered into an At-The-Market Equity Offering Sales Agreement with Stifel, Nicolaus & Company, Incorporated as sales agent pursuant to which the Company may sell, from time to time, through Stifel, shares of our common stock having an aggregate gross offering price of up to $50 million. In addition, the Company may raise additional capital through the sale of equity securities and incremental debt financing. While there can be no assurances that the Company will be successful in obtaining the level of financing needed for its operations, the Company believes it is probable that the actions taken, together with any additional equity or debt financing the Company may pursue, will be sufficient to address liquidity needs and alleviate the substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time. If the Company is unsuccessful in raising capital, it may need to reduce activities, curtail or cease certain operations.
F-8
The Company considers all highly liquid investments purchased with an original maturity of three months or less when purchased to be cash equivalents. Cash and cash equivalents consist primarily of cash in checking accounts and interest-bearing money market accounts.
|
(d) |
Concentration of Credit and Supplier Risks |
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents. The Company’s cash and cash equivalents are deposited in demand accounts at a financial institution that management believes is creditworthy. The Company is exposed to credit risk in the event of default by this financial institution for cash and cash equivalents in excess of amounts insured by the Federal Deposit Insurance Corporation, or FDIC. Management believes that the Company’s investments in cash and cash equivalents are financially sound and have minimal credit risk and the Company has not experienced any losses on its deposits of cash and cash equivalents.
The Company relies on a limited number of third-party manufacturers for the manufacturing and supply of its products. This could result in the Company not being able to acquire the inventory needed to meet customer demand, which would result in possible loss of sales and affect operating results adversely.
|
(e) |
Fair Value of Financial Instruments |
The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, and customer deposits are reasonable estimates of their fair value because of the short maturity of these items. The fair value of the common stock warrant liability, deferred and contingent consideration are discussed in Note 2. The fair value of the debt is based on the amount of future cash flows associated with the instrument discounted using the Company’s market rate. As of December 31, 2017, the carrying value of the debt is presented as a current liability of $24.6 million, representing the principal obligations, net of debt issuance costs, owed under the term loan.
|
(f) |
Fair Value Measurements |
Certain assets and liabilities are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs.
Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
|
|
• |
Level 1 — Quoted prices in active markets for identical assets or liabilities. |
|
|
• |
Level 2 — Observable inputs (other than Level 1 quoted prices) such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. |
|
|
• |
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. |
The Company’s common stock warrant liabilities are carried at fair value determined according to the fair value hierarchy described above. The Company has utilized an option pricing valuation model to determine the fair value of its outstanding common stock warrant liabilities. The inputs to the model include fair value of the common stock related to the warrant, exercise price of the warrant, expected term, expected volatility, risk-free interest rate and
F-9
dividend yield. The warrants are valued using the fair value of common stock as of the measurement date. The Company historically has been a private company and lacks company-specific historical and implied volatility information of its stock. Therefore, it estimates its expected stock volatility based on the historical volatility of publicly traded peer companies for a term equal to the remaining contractual term of the warrants. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve for time periods approximately equal to the remaining contractual term of the warrants. The Company has estimated a 0% dividend yield based on the expected dividend yield and the fact that the Company has never paid or declared dividends. As several significant inputs are not observable, the overall fair value measurement of the warrants is classified as Level 3.
The Company assessed the fair value of the deferred consideration and contingent consideration for future royalty payments related to the acquisition of BIOCORNEUM, the contingent consideration for future milestone payments for the acquisition of the tissue expander portfolio from SSP and the deferred and contingent consideration for the future milestone payments related to the acquisition of miraDry using a Monte-Carlo simulation model. Significant assumptions used in the measurement include future net sales for a defined term and the risk-adjusted discount rate associated with the business. As the inputs are not observable, the overall fair value measurement of the deferred consideration and contingent consideration is classified as Level 3.
The following tables present information about the Company’s liabilities that are measured at fair value on a recurring basis as of December 31, 2017 and 2016 and indicate the level of the fair value hierarchy utilized to determine such fair value (in thousands):
|
|
|
Fair Value Measurements as of |
|
|||||||||||||
|
|
|
December 31, 2017 Using: |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liability for common stock warrants |
|
$ |
— |
|
|
|
— |
|
|
|
194 |
|
|
|
194 |
|
|
Liability for deferred consideration |
|
|
— |
|
|
|
— |
|
|
|
1,255 |
|
|
|
1,255 |
|
|
Liability for contingent consideration |
|
|
— |
|
|
|
— |
|
|
|
12,319 |
|
|
|
12,319 |
|
|
|
|
$ |
— |
|
|
|
— |
|
|
|
13,768 |
|
|
|
13,768 |
|
|
|
|
Fair Value Measurements as of |
|
|||||||||||||
|
|
|
December 31, 2016 Using: |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liability for common stock warrants |
|
$ |
— |
|
|
|
— |
|
|
|
99 |
|
|
|
99 |
|
|
Liability for deferred consideration |
|
|
— |
|
|
|
— |
|
|
|
395 |
|
|
|
395 |
|
|
Liability for contingent consideration |
|
|
— |
|
|
|
— |
|
|
|
1,242 |
|
|
|
1,242 |
|
|
|
|
$ |
— |
|
|
|
— |
|
|
|
1,736 |
|
|
|
1,736 |
|
F-10
The liability for common stock warrants and the current portion of deferred consideration is included in “accrued and other current liabilities” and the long-term liabilities for the deferred consideration and contingent consideration are included in “deferred and contingent consideration” in the consolidated balance sheet. The following table provides a rollforward of the aggregate fair values of the Company’s common stock warrants, deferred and contingent consideration for which fair value is determined by Level 3 inputs (in thousands):
|
Warrant Liability |
|
|
|
|
|
Balance, December 31, 2016 |
|
$ |
99 |
|
|
Fair value of warrants to be issued upon borrowing under the SVB Loan Agreement (Note 5) |
|
|
88 |
|
|
Warrants extinguished upon termination of SVB Loan Agreement |
|
|
(88 |
) |
|
Change in fair value through December 31, 2017 |
|
|
95 |
|
|
Balance, December 31, 2017 |
|
$ |
194 |
|
|
Deferred Consideration Liability |
|
|
|
|
|
Balance, December 31, 2016 |
|
$ |
395 |
|
|
Initial fair value of acquisition-related deferred consideration |
|
|
966 |
|
|
Change in fair value of deferred consideration |
|
|
(106 |
) |
|
Balance, December 31, 2017 |
|
$ |
1,255 |
|
|
Contingent Consideration Liability |
|
|
|
|
|
Balance, December 31, 2016 |
|
$ |
1,242 |
|
|
Initial fair value of acquisition-related contingent consideration |
|
|
9,946 |
|
|
Change in fair value of contingent consideration |
|
|
1,131 |
|
|
Balance, December 31, 2017 |
|
$ |
12,319 |
|
In connection with the acquisition of miraDry on July 25, 2017, contingent consideration of up to an aggregate of $14.0 million may be payable upon achieving certain future sales milestones and had a fair value of $10.4 million at December 31, 2017.
In connection with the acquisition of the tissue expander portfolio from SSP on November 2, 2016, contingent consideration of up to an aggregate of $2.0 million may be payable upon achieving certain future sales milestones and had a fair value of $1.8 million and $1.1 million at December 31, 2017 and 2016, respectively.
The Company recognizes changes in the fair value of the warrants in “other income (expense), net” in the consolidated statement of operations and changes in deferred consideration and contingent consideration are recognized in “general and administrative” expense in the consolidated statement of operations.
|
(g) |
Property and Equipment |
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is computed using the straight‑line method over the estimated useful life of the asset, generally three to five years. Leasehold improvements are depreciated over the shorter of the lease term or the estimated useful life of the related asset. Upon retirement or sale of an asset, the cost and related accumulated depreciation or amortization are removed from the consolidated balance sheet and any resulting gain or loss is reflected in operations in the period realized. Maintenance and repairs are charged to operations as incurred.
|
(h) |
Goodwill and Other Intangible Assets |
Goodwill represents the excess of the purchase price over the fair value of net assets of purchased businesses. Goodwill is not amortized, but instead is subject to impairment tests on at least an annual basis and whenever circumstances suggest that goodwill may be impaired. After the acquisition of miraDry, management began evaluating the Company as two reporting units, Breast Products and miraDry. The Company’s annual test for impairment is performed as of October 1 of each fiscal year. The Company makes a qualitative assessment of
F-11
whether it is more likely than not that a reporting unit’s fair value is less than its carrying amount. If the Company concludes that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company will recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value.
The Company tests indefinite-lived intangible assets for impairment on at least an annual basis and whenever circumstances suggest the assets may be impaired. The Company’s annual test for impairment is performed as of October 1 of each fiscal year. If indicators of impairment are present, the Company evaluates the carrying value of the intangible assets in relation to estimates of future undiscounted cash flows. If the carrying value of the intangible asset exceeds its fair value, an impairment loss is recognized in an amount equal to the difference. The Company also evaluates the remaining useful life of an indefinite-lived intangible asset to determine whether events and circumstances continue to support an indefinite useful life.
Judgments about the recoverability of purchased finite‑lived intangible assets are made whenever events or changes in circumstance indicate that impairment may exist. Each fiscal year the Company evaluates the estimated remaining useful lives of purchased intangible assets and whether events or changes in circumstance warrant a revision to the remaining periods of amortization. Recoverability of finite‑lived intangible assets is measured by comparison of the carrying amount of the asset to the future undiscounted cash flows the asset is expected to generate. The intangible asset is amortized to the consolidated statement of operations based on estimated cash flows generated from the intangible over its estimated life.
|
(i) |
Impairment of Long‑Lived Assets |
The Company’s management routinely considers whether indicators of impairment of long‑lived assets are present. If such indicators are present, management determines whether the sum of the estimated undiscounted cash flows attributable to the assets in question is less than their carrying value. If less, the Company will recognize an impairment loss based on the excess of the carrying amount of the assets over their respective fair values. Fair value is determined by discounted future cash flows, appraisals or other methods. If the assets determined to be impaired are to be held and used, the Company will recognize an impairment charge to the extent the present value of anticipated net cash flows attributable to the asset are less than the asset’s carrying value. The fair value of the asset will then become the asset’s new carrying value. There have been no impairments of long‑lived assets recorded during the years ended December 31, 2017, 2016 and 2015. The Company may record impairment losses in future periods if factors influencing its estimates change.
|
(j) |
Business Combinations |
Business combinations are accounted for using the acquisition method of accounting. Under the acquisition method, assets acquired and liabilities assumed are recorded at their respective fair values as of the acquisition date in our financial statements. The excess of the fair value of consideration transferred over the fair value of the net assets acquired is recorded as goodwill. Contingent consideration obligations incurred in connection with a business combination are recorded at their fair values on the acquisition date and remeasured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded in earnings.
|
(k) |
Segment Reporting |
Reportable segments represent components for which separate financial information is available that is utilized on a regular basis by the Chief Executive Officer, who has been identified as the Chief Operating Decision Maker, or CODM, as defined by authoritative guidance on segment reporting, in determining how to allocate resources and evaluate performance. The segments are determined based on several factors, including client base, homogeneity of products, technology, delivery channels and similar economic characteristics. Based on the financial information presented to and reviewed by the CODM, the Company has determined that it has two reportable segments: Breast Products and miraDry.
F-12
The Company recognizes revenue related to sales of products directly to customers in markets where it has regulatory approval, net of trade discounts and allowances, provided that (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred and title and risk of loss have transferred, (iii) the price is fixed or determinable and (iv) collectability is reasonably assured. Sales prices are documented in the executed sales contract or purchase order prior to shipment. The Company evaluates the creditworthiness of customers to determine that appropriate credit limits are established prior to the acceptance of an order. Revenue for extended warranties and service agreements are recognized ratably over the term of the agreement.
A portion of the Company’s revenue is generated from the sale of consigned inventory of breast implants maintained at doctor, hospital, and clinic locations. For these products, revenue is recognized at the time the Company is notified by the customer that the product has been implanted, not when the consigned products are delivered to the customer’s warehouse.
The Company also leverages a distributor network for selling the miraDry System internationally. The Company recognizes revenue when title to the product and risk of loss transfer to customers, provided there are no remaining performance obligations required of the Company or any written matters requiring customer acceptance, which generally occurs upon shipment. Standard terms in these agreements do not allow for trial periods, rights of return, refunds, payments contingent on obtaining financing or other terms that could impact the customer’s obligation.
Appropriate reserves are established for anticipated sales returns based on historical experience, recent gross sales and any notification of pending returns. For Breast Products specifically, the Company allows for the return of Breast Products from customers within six months after the original sale and records estimated sales returns as a reduction of sales in the same period revenue is recognized. Sales return provisions are calculated based upon historical experience with actual returns. Actual sales returns in any future period are inherently uncertain and thus may differ from the estimates.
If actual sales returns differ significantly from the estimates, an adjustment to revenue in the current or subsequent period would be recorded. The Company has established an allowance for sales returns of $3.9 million as of both December 31, 2017 and 2016, recorded net against accounts receivable in the consolidated balance sheet.
Shipping and handling charges are largely provided to customers free of charge for breast products. The associated costs are viewed as part of the Company’s marketing programs and are recorded as a component of sales and marketing expense in the consolidated statement of operations as an accounting policy election. Shipping and handling charges are typically billed to the customer for sales of the miraDry Systems and are recorded as a component of cost of goods sold in the consolidated statement of operations. For the years ended 2017, 2016 and 2015 shipping costs amounted to $1.0 million, $0.6 million and $1.1 million, respectively.
|
(m) |
Accounts Receivable and Allowance for Doubtful Accounts |
Accounts receivable are recorded at the invoiced amount and do not bear interest. The Company maintains allowances for doubtful accounts for estimated losses resulting from the inability to collect from some of its customers. The allowances for doubtful accounts are based on the analysis of historical bad debts, customer credit‑worthiness, past transaction history with the customer, and current economic trends. If the financial condition of the Company’s customers were to deteriorate, adversely affecting their ability to make payments, additional allowances may be required. The Company has established an allowance for doubtful accounts of $0.9 million and $0.4 million as of December 31, 2017 and 2016, respectively.
|
(n) |
Inventories and Cost of Goods Sold |
Inventories represent raw materials, work in process and finished goods that are recorded at the lower of cost or market on a first‑in, first‑out basis, or FIFO. miraDry inventory costs are determined using a standard cost, which approximates actual cost on an average cost basis. The Company periodically assesses the recoverability of all inventories to determine whether adjustments for impairment or obsolescence are required. The Company evaluates the remaining shelf life and other general obsolescence and impairment criteria in assessing the recoverability of the Company’s inventory.
F-13
The Company recognizes the cost of inventory transferred to the customer in cost of goods sold when revenue is recognized.
At December 31, 2017 and 2016, approximately $1.6 million and $2.0 million, respectively, of the Company’s Breast Products segment inventory was held on consignment at doctors’ offices, clinics, and hospitals. The value and quantity at any one location is not significant.
|
(o) |
Income Taxes |
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company operates in several tax jurisdictions and is subject to taxes in each jurisdiction in which it conducts business. To date, the Company has incurred cumulative net losses and maintains a full valuation allowance on its net deferred tax assets due to the uncertainty surrounding realization of such assets. However, the Company has deferred tax liabilities associated with indefinite lived intangible assets that cannot be considered sources of income to support the realization of the deferred tax assets, and has provided for tax expense (or benefit) and a corresponding deferred tax liability associated with these indefinite lived intangible assets. Tax benefit for the year ended December 31, 2017 was $17 thousand and tax expense for the year ended December 31, 2016 was $0.1 million. There was no tax expense (or benefit) for the year ended December 31, 2015.
The Company accounts for uncertain tax position in accordance with ASC 740‑10, Accounting for Uncertainty in Income Taxes. The Company assesses all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position’s sustainability and is measured at the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and the Company will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate. The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of tax benefit might change as new information becomes available.
|
(p) |
Research and Development Expenditures |
Research and development costs are charged to operating expenses as incurred. Research and development, or R&D, primarily consist of clinical expenses, regulatory expenses, product development, consulting services, outside research activities, quality control and other costs associated with the development of the Company’s products and compliance with Good Clinical Practices, or GCP, requirements. R&D expenses also include related personnel and consultant compensation and stock-based compensation expense.
|
(q) |
Advertising |
Expenses related to advertising are charged to sales and marketing expense as incurred. Advertising costs were $1.8 million, $0.6 million and $1.0 million for the years ended December 31, 2017, 2016 and 2015, respectively.
F-14
The Company applies the fair value provisions of ASC 718, Compensation — Stock Compensation, or ASC 718. ASC 718 requires the recognition of compensation expense, using a fair‑value based method, for costs related to all employee share‑based payments, including stock options, restricted stock units, and the employee stock purchase plan. ASC 718 requires companies to estimate the fair value of share‑based payment awards on the date of grant using an option‑pricing model. We estimate the fair value of our stock‑based awards to employees and directors using the Black‑Scholes option pricing model. The grant date fair value of a stock‑based award is recognized as an expense over the requisite service period of the award on a straight‑line basis. In addition, we use the Monte-Carlo simulation option-pricing model to determine the fair value of market-based awards. The Monte-Carlo simulation option-pricing model uses the same input assumptions as the Black-Scholes model; however, it also further incorporates into the fair-value determination the possibility that the market condition may not be satisfied. Compensation costs related to these awards are recognized regardless of whether the market condition is satisfied, provided that the requisite service has been provided.
The option-pricing models require the input of subjective assumptions, including the risk‑free interest rate, expected dividend yield, expected volatility and expected term, among other inputs. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, our stock‑based compensation expense could be materially different in the future. These assumptions are estimated as follows:
|
|
• |
Risk‑free interest rate—The risk‑free interest rate is based on the yields of U.S. Treasury securities with maturities similar to the expected term of the options for each option group. |
|
|
• |
Dividend yield—We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero. |
|
|
• |
Expected volatility—As we do not have a significant trading history for our common stock, the expected stock price volatility for our common stock was estimated by taking the average of (i) the median historic price volatility and (ii) the median of the implied volatility averages, with a three‑month lookback from the valuation date, for any trading options of industry peers based on daily price observations over a period equivalent to the expected term of the time to a liquidity event. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own common stock share price becomes available. |
|
|
• |
Expected term—The expected term represents the period that our stock‑based awards are expected to be outstanding. |
During the year ended December 31, 2017, the Company adopted ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting and changed its policy from estimating forfeitures to recording forfeitures when they occur.
The following table presents the weighted‑average assumptions used to estimate the fair value of options granted during the periods presented:
|
|
|
Year Ended December 31, |
||||||||||||||||||||||||||||
|
Stock Options |
|
2017 |
|
2016 |
|
2015 |
||||||||||||||||||||||||
|
Expected term (in years) |
|
|
4.47 |
|
|
to |
|
6.07 |
|
|
|
|
5.47 |
|
|
to |
|
6.07 |
|
|
|
|
5.27 |
|
|
to |
|
6.08 |
|
|
|
Expected volatility |
|
|
45 |
|
% |
to |
|
56 |
|
% |
|
|
51 |
|
% |
to |
|
53 |
|
% |
|
|
45 |
|
% |
to |
|
52 |
|
% |
|
Risk-free interest rate |
|
|
1.24 |
|
% |
to |
|
2.45 |
|
% |
|
|
1.42 |
|
% |
to |
|
1.54 |
|
% |
|
|
1.48 |
|
% |
to |
|
1.92 |
|
% |
|
Dividend yield |
|
— |
|
— |
|
— |
||||||||||||||||||||||||
The following table presents the weighted-average assumptions used to estimate the fair value of the stock purchase rights granted under the employee stock purchase plan:
F-15
|
|
Year Ended December 31, |
|||||||||||||||||||||||||||||
|
ESPP |
|
2017 |
|
2016 |
|
2015 |
||||||||||||||||||||||||
|
Expected term (in years) |
|
|
0.50 |
|
|
to |
|
2.10 |
|
|
|
|
0.50 |
|
|
to |
|
2.10 |
|
|
|
|
0.50 |
|
|
to |
|
2.10 |
|
|
|
Expected volatility |
|
|
46 |
|
% |
to |
|
55 |
|
% |
|
|
42 |
|
% |
to |
|
58 |
|
% |
|
|
42 |
|
% |
to |
|
44 |
|
% |
|
Risk-free interest rate |
|
|
0.08 |
|
% |
to |
|
1.30 |
|
% |
|
|
0.08 |
|
% |
to |
|
0.85 |
|
% |
|
|
0.08 |
|
% |
to |
|
0.71 |
|
% |
|
Dividend yield |
|
— |
|
— |
|
— |
||||||||||||||||||||||||
|
(s) |
Product Warranties |
The Company offers a limited warranty and a lifetime product replacement program for the Company’s silicone gel breast implants and a product warranty for the Company’s miraDry Systems. Under the breast implant limited warranty, the Company will reimburse patients for certain out-of-pocket costs related to revision surgeries performed within ten years from the date of implantation in a covered event. Under the breast implant lifetime product replacement program, the Company provides no-charge replacement breast implants if a patient experiences a covered event. Under the miraDry warranty, the Company currently provides a standard product warranty for the consoles, handpieces and the miraDry consumables, or bioTips. Additionally, an extended miraDry warranty may be purchased to provide additional protection of the miraDry System. The portion of the warranty provision expected to be incurred within 12 months is classified as current within accrued liabilities and other, while the remaining amount is classified as long-term within warranty reserve and other long-term liabilities.
The following table provides a rollforward of the accrued warranties (in thousands):
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Beginning balance as of January 1 |
|
$ |
1,378 |
|
|
$ |
1,332 |
|
|
Assumed warranty liability from acquisition |
|
|
137 |
|
|
|
— |
|
|
Warranty costs incurred during the period |
|
|
(167 |
) |
|
|
(25 |
) |
|
Changes in accrual related to warranties issued during the period |
|
|
301 |
|
|
|
177 |
|
|
Changes in accrual related to pre-existing warranties |
|
|
(7 |
) |
|
|
(106 |
) |
|
Balance as of December 31 |
|
$ |
1,642 |
|
|
$ |
1,378 |
|
|
(t) |
Net Loss Per Share |
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Net loss (in thousands) |
|
$ |
(64,028 |
) |
|
$ |
(40,166 |
) |
|
$ |
(41,230 |
) |
|
Weighted average common shares outstanding, basic and diluted |
|
|
19,159,057 |
|
|
|
18,233,177 |
|
|
|
15,770,972 |
|
|
Net loss per share attributable to common stockholders |
|
$ |
(3.34 |
) |
|
$ |
(2.20 |
) |
|
$ |
(2.61 |
) |
The Company excluded the following potentially dilutive securities, outstanding as of December 31, 2017, 2016 and 2015 from the computation of diluted net loss per share attributable to common stockholders for the years ended December 31, 2017, 2016 and 2015 because they had an anti-dilutive impact due to the net loss attributable to common stockholders incurred for the periods.
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Stock options to purchase common stock |
|
|
1,867,627 |
|
|
|
2,057,296 |
|
|
|
1,967,906 |
|
|
Warrants for the purchase of common stock |
|
|
47,710 |
|
|
|
47,710 |
|
|
|
47,710 |
|
|
|
|
|
1,915,337 |
|
|
|
2,105,006 |
|
|
|
2,015,616 |
|
F-16
|
(u) |
Recent Accounting Pronouncements |
Recently Adopted Accounting Standards
In July 2015, the FASB issued ASU 2015-11, Inventory - Simplifying the Measurement of Inventory. The standard simplifies the subsequent measurement of inventory by requiring inventory to be measured at the lower of cost and net realizable value, thereby simplifying the previous guidance under which an entity must measure inventory at the lower of cost or market. The Company adopted ASU 2015-11 in the first quarter of 2017 on a prospective basis. The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation - Stock Compensation (Topic 718). The standard identifies areas for simplification involving several aspects of accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, an option to recognize gross stock compensation expense with actual forfeitures recognized as they occur, as well as certain classifications on the consolidated statement of cash flows. Under ASU 2016-09, differences between the tax deduction for share-based awards and the related compensation expenses recognized under ASC 718 are now accounted for as a component of the provision for income taxes. In addition, ASU 2016-09 eliminates the requirement that excess tax benefits from share-based compensation reduce income taxes payable prior to being recognized in the financial statements. The Company adopted ASU 2016-09 in the first quarter of 2017 on a modified retrospective basis. As of December 31, 2017, the Company had cumulative excess benefits related to share-based compensation of $0.1 million, which had previously not been reflected as a deferred tax asset. As a result of the adoption of ASU 2016-09, the excess benefits were reclassified to the Company’s net operating loss carryover, resulting in an increase to the deferred tax assets and valuation allowance of $0.1 million as of January 1, 2017. There was no impact to retained earnings as a result of the adoption of ASU 2016-09. Additionally, in accordance with ASU 2016-09, the Company has made an accounting policy election to account for forfeitures when they occur.
In January 2017, the FASB issued ASU 2017-04, Intangibles - Goodwill and Other (Topic 350) - Simplifying the Test for Goodwill Impairment. The standard update eliminates Step 2 from the goodwill impairment test. The guidance requires an entity to perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value. In addition, the guidance eliminates the requirements for any reporting unit with a zero or negative carrying amount to perform a qualitative assessment. The Company adopted ASU 2017-04 in the first quarter of 2017 on a prospective basis. The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
Recently Issued Accounting Standards
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers. The standard was issued to provide a single framework that replaces existing industry and transaction specific GAAP with a five step analysis of transactions to determine when and how revenue is recognized. The accounting standard update will replace most existing revenue recognition guidance in GAAP when it becomes effective. In August 2015, the FASB issued ASU 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, to defer the effective date of ASU 2014-09 by one year. Therefore, ASU 2014-09 will become effective for the Company beginning in fiscal year 2018. Early adoption would be permitted for the Company beginning in fiscal year 2017. The standard permits the use of either the retrospective or cumulative transition method. In December 2016, the FASB issued ASU 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers. ASU 2016-20 is intended to clarify and suggest improvements to the application of current standards under Topic 606 and other Topics amended by ASU 2014-09, Revenue from Contracts with Customers (Topic 606). The effective date of ASU 2016-20 is the same as the effective date for ASU 2014-09. In preparation for our adoption of the new standard in our fiscal year ending December 31, 2018, we are reviewing contracts and other forms of agreements with our customers and are evaluating the provisions contained therein in light of the five-step model specified by the new guidance. That five-step model includes: (1) determination of whether a contract—an agreement between two or more parties that creates legally enforceable rights and obligations—exists; (2) identification of the performance obligations in the contract; (3) determination of the transaction price; (4) allocation
F-17
of the transaction price to the performance obligations in the contract; and (5) recognition of revenue when (or as) the performance obligation is satisfied. We are also evaluating the impact of the new standard on certain common practices currently employed by us and by other medical device companies, such as allowance for sales returns, rebates and other pricing programs. We plan to adopt the new standard using the modified retrospective method at the beginning of our first quarter of fiscal year 2018. We are in process of evaluating the impact of the new standard, and do not anticipate there being a material impact to net sales. As a result of the transition to the new standard, we have identified certain prospective impacts to accounting policies, which include recording any amounts received or receivable for which the Company does not expect to be entitled as a refund liability, as well separately disclosing the balance of expected assets for recovery in a footnote to inventory beginning in FY2018. A complete list of the impacts from transition to 606 will be included in the first quarterly filing of fiscal 2018.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842) which supersedes FASB Accounting Standard Codification Leases (Topic 840). The standard is intended to increase the transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. This accounting standard update will be effective for the Company beginning in fiscal year 2019. The Company is currently evaluating the impact that adoption of the standard will have on the financial statements and related disclosures.
In January 2017, the FASB issued ASU 2017-01, Business Combinations (Topic 805) - Clarifying the Definition of a Business. The standard adds guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses by providing a more specific definition of a business. The updated accounting standard will be effective for the Company beginning in fiscal year 2018. The Company will evaluate the impact of this ASU on future acquisitions.
In May 2017, the FASB issued ASU 2017-09, Compensation—Stock Compensation (Topic 718): Scope of Modification Accounting. The standard provides clarification on when modification accounting should be used for changes to the terms or conditions of a share-based payment award to which an entity would be required to apply modification accounting under ASC 718. The ASU is effective for the Company beginning in fiscal year 2018. The Company does not expect the adoption of this guidance will have a material impact on its consolidated financial statements and related disclosures.
(v)Reclassifications
Certain reclassifications have been made to prior year amounts to conform to the current year presentation.
F-18
|
|
(a) |
Acquisition of miraDry |
On June 11, 2017, Sientra entered into the Merger Agreement with miraDry, pursuant to which Sientra commenced a tender offer to purchase all of the outstanding shares of miraDry’s common stock for (i) $0.3149 per share, plus (ii) the contractual right to receive one or more contingent payments upon the achievement of certain future sales milestones. The total merger consideration was $18.7 million in upfront cash and the contractual rights represent potential contingent payments of up to $14 million. The transaction, which closed on July 25, 2017, or the Acquisition Date, added the miraDry System, the only FDA cleared device to reduce underarm sweat, odor and permanently reduce hair of all colors, to Sientra’s aesthetics portfolio. In connection with the acquisition, the Company recorded $3.1 million of professional fees for the year ended December 31, 2017, which are included in general and administrative expense. The aggregate preliminary acquisition date fair value of the consideration transferred was approximately $29.6 million, consisting of the following (in thousands):
|
|
|
Fair Value |
|
|
|
Cash consideration at Acquisition Date (other than debt payoff) |
|
$ |
6,193 |
|
|
Cash consideration at Acquisition Date (debt payoff) |
|
|
12,467 |
|
|
Deferred consideration |
|
|
966 |
|
|
Contingent consideration |
|
|
9,946 |
|
|
Total purchase consideration |
|
$ |
29,572 |
|
The Company funded the cash consideration, including the debt payoff amount with cash on hand. The cash consideration included the payoff of miraDry’s existing term loan, or the Note Purchase Agreement dated January 27, 2017 and bridge loan, or the January 2017 Bridge Loan, including interest. The deferred consideration relates to cash held back to be used for either potential litigation-related expenses or for payments to certain former investors of miraDry, as defined in the Note Purchase Agreement dated January 27, 2017, one year following the Acquisition Date. Contingent consideration of future cash payments of a maximum of $14.0 million represents the contractual right of certain former miraDry shareholders to receive one or more contingent payments upon achievement of certain future sales milestones and includes certain amounts due to investors related to the remaining balances on the January 2017 Bridge Note and accrued royalty obligations, with certain amounts held back for potential litigation-related expenses. The fair value of the contingent consideration at the acquisition date was determined using a Monte-Carlo simulation model. The inputs include the estimated amount and timing of future net sales, and a risk-adjusted discount rate. The inputs are significant inputs not observable in the market, which are referred to as Level 3 inputs and are further discussed in Note 2. The contingent consideration component is subject to the recognition of subsequent changes in fair value through general and administrative expense in the consolidated statement of operations.
F-19
In accordance with ASC 805, the Company has recorded the acquired assets (including identifiable intangible assets) and liabilities assumed at their respective fair value. The preliminary allocation of the total purchase price is as follows (in thousands):
|
|
|
July 25, |
|
|
|
|
|
2017 |
|
|
|
Cash |
|
$ |
205 |
|
|
Accounts receivable, net |
|
|
2,091 |
|
|
Inventories, net |
|
|
7,064 |
|
|
Other current assets |
|
|
170 |
|
|
Property and equipment, net |
|
|
528 |
|
|
Goodwill |
|
|
7,629 |
|
|
Intangible assets |
|
|
14,800 |
|
|
Restricted cash |
|
|
305 |
|
|
Other assets |
|
|
12 |
|
|
Liabilities assumed: |
|
|
|
|
|
Accounts payable |
|
|
(908 |
) |
|
Accrued and other current liabilities |
|
|
(2,294 |
) |
|
Other current liabilities |
|
|
(30 |
) |
|
Net assets acquired |
|
$ |
29,572 |
|
Goodwill has been allocated to the miraDry reportable segment. The goodwill recognized is attributable primarily to the assembled workforce and additional market opportunities. Goodwill is not expected to be deductible for tax purposes.
A summary of the intangible assets acquired, estimated useful lives and amortization method is as follows (in thousands):
|
|
|
|
|
|
|
Estimated useful |
|
Amortization |
|
|
|
Amount |
|
|
life |
|
method |
|
|
Developed technology |
|
$ |
3,000 |
|
|
15 years |
|
Accelerated |
|
Customer relationships |
|
|
6,300 |
|
|
14 years |
|
Accelerated |
|
Distributor relationships |
|
|
500 |
|
|
9 years |
|
Accelerated |
|
Trade name |
|
|
5,000 |
|
|
15 years |
|
Accelerated |
|
|
|
$ |
14,800 |
|
|
|
|
|
The Company retained an independent third-party appraiser to assist management in its valuation; however, the purchase price allocation has not been finalized. This could result in adjustments to the carrying value of the assets acquired and liabilities assumed, the useful lives of intangible assets and residual amount allocated to goodwill. The preliminary allocation of the purchase price is based on the best estimates of management and is subject to revision based on the final valuations and estimates of useful lives.
F-20
Unaudited Pro Forma Information
The following unaudited pro forma financial information presents combined results of operations for each of the periods presented, as if miraDry had been acquired as of the beginning of fiscal year 2016. The pro forma information includes adjustments to amortization for intangible assets acquired, the purchase accounting effect on inventory acquired, interest expense for the additional indebtedness incurred to complete the acquisition, restructuring charges in connection with the acquisition and acquisition costs. The pro forma data are for informational purposes only and are not necessarily indicative of the consolidated results of operations of the combined business had the merger actually occurred at the beginning of fiscal year 2016 or of the results of future operations of the combined business. Consequently, actual results will differ from the unaudited pro forma information presented below (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
|
|
Pro Forma |
|
|||||
|
Net sales |
|
$ |
46,747 |
|
|
$ |
41,179 |
|
|
Net loss |
|
|
(69,545 |
) |
|
|
(69,226 |
) |
|
Pro forma loss per share attributable to ordinary shares - basic and diluted |
|
$ |
(3.62 |
) |
|
$ |
(3.78 |
) |
|
|
(b) |
Acquisition of BIOCORNEUM |
On March 9, 2016, the Company entered into an asset purchase agreement with Enaltus LLC, or Enaltus, to acquire exclusive U.S. rights to BIOCORNEUM, an advanced silicone scar treatment marketed exclusively to physicians. The acquisition of BIOCORNEUM aligns with the Company’s business development objectives and adds a complementary product that serves the needs of its customers. In connection with the acquisition, the Company recorded $0.2 million of professional fees for the year ended, December 31, 2016 which is included in general and administrative expense. The aggregate acquisition date fair value of the consideration transferred was approximately $7.4 million, which consisted of the following (in thousands):
|
|
|
Fair Value |
|
|
|
Cash |
|
$ |
6,859 |
|
|
Deferred consideration |
|
|
434 |
|
|
Contingent consideration |
|
|
116 |
|
|
|
|
$ |
7,409 |
|
The deferred consideration and contingent consideration consist of future royalty payments to be paid on a quarterly basis to Enaltus on future BIOCORNEUM sales for the 4.5 years beginning January 1, 2024. The Company determined the fair value of the deferred consideration and contingent consideration at the acquisition date using a Monte-Carlo simulation model. The fair value of the deferred consideration is based on the future minimum royalty payments using the risk-free U.S. Treasury yield curve discount rate. The minimum estimated future payments due under the deferred consideration are $0.5 million. The fair value of the contingent consideration is based on projected future BIOCORNEUM sales and a risk-adjusted discount rate. The terms of the agreement do not provide for a limitation on the maximum potential future payments. The inputs are significant inputs not observable in the market, which are referred to as Level 3 inputs and are further discussed in Note 2. The deferred consideration and contingent consideration components are subject to the recognition of subsequent changes in fair value through general and administrative expense in the consolidated statement of operations.
The Company allocated the total consideration transferred to the tangible and identifiable intangible assets acquired based on their respective fair values on the acquisition date, with the remaining unallocated amount recorded as goodwill. The goodwill arising from the transaction is primarily attributable to expected operational synergies, and all of goodwill will be deductible for income tax purposes. The consolidated financial statements for the years ended December 31, 2017 and 2016 include the results of operations of BIOCORNEUM from the date of acquisition.
F-21
The following table summarizes the allocation of the fair value of the consideration transferred by major class for the business combination completed on March 9, 2016 (in thousands):
|
|
|
March 9, |
|
|
|
|
|
2016 |
|
|
|
Inventories, net |
|
$ |
100 |
|
|
Prepaid expenses |
|
|
36 |
|
|
Goodwill |
|
|
3,273 |
|
|
Intangible assets |
|
|
4,000 |
|
|
|
|
$ |
7,409 |
|
A summary of the intangible assets acquired, estimated useful lives and amortization method is as follows (in thousands):
|
|
|
|
|
|
|
Estimated useful |
|
|
Amortization |
|
|
|
|
Amount |
|
|
life (in years) |
|
|
method |
||
|
Customer relationships |
|
$ |
3,200 |
|
|
|
10 |
|
|
Accelerated |
|
Trade name |
|
|
800 |
|
|
|
12 |
|
|
Straight-line |
|
|
|
$ |
4,000 |
|
|
|
|
|
|
|
The Company retained an independent third-party appraiser to assist management in its valuation and the purchase price has been finalized. Pro forma results of operations have not been presented because the effect of the acquisition was not material to the Company's results of operations.
|
(c) |
Acquisition of Tissue Expander Portfolio from Specialty Surgical Products, Inc. |
On November 2, 2016, the Company entered into an asset purchase agreement with Specialty Surgical Products, Inc., or SSP, to acquire certain assets, consisting of the Dermaspan, Softspan, and AlloX2 tissue expanders, from SSP. The acquisition adds a complete portfolio of premium, differentiated tissue expanders and aligns with the Company’s business development plans for growth in the breast reconstruction market. In connection with the acquisition, the Company recorded $0.1 million of professional fees for the year ended December 31, 2016, which is included in general and administrative expense. The aggregate acquisition date fair value of the consideration transferred was approximately $6.0 million, which consisted of the following (in thousands):
|
|
|
Fair Value |
|
|
|
Cash |
|
$ |
4,950 |
|
|
Contingent consideration |
|
|
1,050 |
|
|
|
|
$ |
6,000 |
|
The contingent consideration consists of future cash payments of a maximum of $2.0 million to be paid to SSP based upon the achievement of certain milestones of future net sales. The Company determined the fair value of the contingent consideration at the acquisition date using a Monte-Carlo simulation model. The inputs include the estimated amount and timing of future net sales, and a risk-adjusted discount rate. The inputs are significant inputs not observable in the market, which are referred to as Level 3 inputs and are further discussed in Note 2. The contingent consideration components are subject to the recognition of subsequent changes in fair value through general and administrative expense in the consolidated statement of operations.
The Company allocated the total consideration transferred to the tangible and identifiable intangible assets acquired and liabilities assumed based on their respective fair values on the acquisition date, with the remaining unallocated amount recorded as goodwill. The goodwill arising from the transaction is primarily attributable to expected operational synergies, and all of goodwill will be deductible for income tax purposes. The financial statements for the years ended December 31, 2016 and 2017 include the results of operations of the Dermaspan, Softspan, and AlloX2 tissue expanders from the date of acquisition.
F-22
The following table summarizes the allocation of the fair value of the consideration transferred by major class for the business combination completed on November 2, 2016 (in thousands):
|
|
|
November 2, |
|
|
|
|
|
2016 |
|
|
|
Accounts receivable, net |
|
$ |
196 |
|
|
Inventories, net |
|
|
1,555 |
|
|
Equipment |
|
|
34 |
|
|
Goodwill |
|
|
1,605 |
|
|
Intangible assets |
|
|
2,860 |
|
|
Liabilities assumed |
|
|
(250 |
) |
|
|
|
$ |
6,000 |
|
A summary of the intangible assets acquired, estimated useful lives and amortization method is as follows (in thousands):
|
|
|
|
|
|
|
Estimated useful |
|
Amortization |
|
|
|
Amount |
|
|
life |
|
method |
|
|
Customer relationships |
|
$ |
1,740 |
|
|
9 years |
|
Accelerated |
|
Regulatory approvals |
|
|
670 |
|
|
14 months |
|
Straight-line |
|
Trade names |
|
|
450 |
|
|
indefinite-lived |
|
|
|
|
|
$ |
2,860 |
|
|
|
|
|
The Company retained an independent third-party appraiser to assist management in its valuation and the purchase price has been finalized. Pro forma results of operations have not been presented because the effect of the acquisition was not material to the Company's results of operations.
(4) Balance Sheet Components
Inventories, net consist of the following (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Raw materials |
|
$ |
1,642 |
|
|
$ |
— |
|
|
Work in progress |
|
|
3,956 |
|
|
|
— |
|
|
Finished goods |
|
|
15,298 |
|
|
|
18,484 |
|
|
|
|
$ |
20,896 |
|
|
$ |
18,484 |
|
Property and equipment, net consist of the following (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Leasehold improvements |
|
$ |
402 |
|
|
$ |
86 |
|
|
Manufacturing equipment and toolings |
|
|
4,260 |
|
|
|
2,264 |
|
|
Computer equipment |
|
|
387 |
|
|
|
287 |
|
|
Software |
|
|
797 |
|
|
|
669 |
|
|
Office equipment |
|
|
142 |
|
|
|
129 |
|
|
Furniture and fixtures |
|
|
816 |
|
|
|
743 |
|
|
|
|
|
6,804 |
|
|
|
4,178 |
|
|
Less accumulated depreciation |
|
|
(2,041 |
) |
|
|
(1,192 |
) |
|
|
|
$ |
4,763 |
|
|
$ |
2,986 |
|
Depreciation expense for the years ended December 31, 2017, 2016 and 2015 was $0.9 million, $0.4 million and $0.3 million, respectively.
F-23
Accrued and other current liabilities consist of the following:
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Payroll and related expenses |
|
$ |
3,579 |
|
|
$ |
2,592 |
|
|
Accrued commissions |
|
|
3,297 |
|
|
|
1,222 |
|
|
Accrued equipment |
|
|
1,091 |
|
|
|
887 |
|
|
Deferred consideration, current portion |
|
|
977 |
|
|
|
— |
|
|
Audit, consulting and legal fees |
|
|
920 |
|
|
|
803 |
|
|
Accrued sales and marketing expenses |
|
|
794 |
|
|
|
39 |
|
|
Other |
|
|
2,816 |
|
|
|
964 |
|
|
|
|
$ |
13,474 |
|
|
$ |
6,507 |
|
(5) Debt and Revolving Loan
On July 25, 2017, the Company entered into a Credit and Security Agreement, or the Term Loan Credit Agreement, and a Credit and Security Agreement, or the Revolving Credit Agreement with Midcap, and, together with the Term Loan Credit Agreement, the Credit Agreements, which replaced the Company’s then-existing Silicon Valley Bank Loan Agreement, or the SVB Loan Agreement. Upon executing the Credit Agreements, the Company repaid in full its indebtedness under the SVB Loan Agreement, which totaled approximately $5.0 million related to a revolving line of credit, or the Revolving Line of Credit. In connection with the refinancing, the Company was forgiven a commitment fee payable of approximately $0.8 million, which was included in long term liabilities, net of the current portion included in accrued and other current liabilities in the consolidated balance sheet, and corresponding warrants of approximately $0.1 million related to the SVB Loan Agreement were extinguished. The Company also recorded an expense of approximately $16 thousand related to the write-off of related issuance costs, which has been included in interest expense in the consolidated statements of operations. Unamortized debt issuance costs from the SVB Loan Agreement related to the modification will be amortized over the term of the new Credit Agreements.
Under the terms of the Term Loan Credit Agreement, as of July 25, 2017, Midcap funded $25.0 million to the Company, the Closing Date Term Loan. Midcap also made available to the Company until March 31, 2018, a $10.0 million term loan, or the March 2018 Term Loan, subject to the satisfaction of certain conditions, including FDA certifications of the manufacturing facility operated by Vesta, and an additional $5.0 million term loan, subject to the satisfaction of certain conditions, including evidence that the Company’s Net Revenue for the past 12 months was greater than or equal to $75.0 million, as defined in the Term Loan Credit Agreement, collectively the Term Loans. Under the Revolving Credit Agreement, Midcap made available to the Company a revolving line of credit, or the Revolving Loan. The amount of loans available to be drawn is based on a borrowing base equal to 85% of the net collectible value of eligible (as defined by the agreement) accounts receivable plus 40% of eligible (as defined by the agreement) finished goods inventory, or the Borrowing Base, provided that availability from eligible finished goods inventory does not exceed 20% of the Borrowing Base. The Company used the proceeds to repay in full the Company’s then-existing indebtedness under its SVB Loan Agreement and to pay fees and expenses in connection with the foregoing and the Company intends to use the proceeds for general corporate purposes.
Any indebtedness under the Term Loan Credit Agreement bears interest at a floating per annum rate equal to the LIBOR as reported by Midcap with a floor of 1.00%, which as of December 31, 2017 was 1.36%, plus 7.50%. The Term Loans have a scheduled maturity date of December 1, 2021, or the Maturity Date. The Company must make monthly payments of accrued interest under the Term Loans from the funding date of the Term Loans, until December 31, 2018, followed by monthly installments of principal and interest through the Maturity Date. The Company may prepay all of the Term Loans prior to its maturity date provided the Company pays Midcap a prepayment fee. The Company paid an origination fee of 0.50% of the Term Loans total amount of $40.0 million on the closing date. As of December 31, 2017, there was $25.0 million outstanding related to the Term Loans. As of December 31, 2017, the unamortized debt issuance costs on the Term Loans was approximately $0.3 million and are included as a reduction to the current portion of long-term debt on the consolidated balance sheet.
F-24
Any indebtedness under the Revolving Credit Agreement bears interest at a floating per annum rate equal to the LIBOR as reported by Midcap with a floor of 1.00%, plus 4.50%. The Company may make and repay borrowings from time to time under the Revolving Credit Agreement until the maturity of the facility on December 1, 2021. The Company is required to pay an annual collateral management fee of 0.50% on the outstanding balance, and an annual unused line fee of 0.50% of the average unused portion. The Company may prepay all of the outstanding balance prior to the maturity date provided the Company pays Midcap a prepayment fee. The Company paid an origination fee of 0.50% of the Revolving Loan amount of $10.0 million on the closing date. As of December 31, 2017, there were no borrowings outstanding related to the Revolving Loan. As of December 31, 2017, the unamortized debt issuance costs related to the Revolving Loan was approximately $0.1 million and was included in prepaid expenses and other current assets on the consolidated balance sheet.
The amortization of debt issuance costs for the year ended December 31, 2017 was $0.1 million, relating to the Revolving Line of Credit, the Term Loans, and the Revolving Loan and were included in interest expense in the consolidated statement of operations.
The Credit Agreements includes customary affirmative and restrictive covenants and representations and warranties, including a financial covenant for minimum revenues, a financial covenant for minimum cash requirements, a covenant against the occurrence of a “change in control,” financial reporting obligations, and certain limitations on indebtedness, liens, investments, distributions, collateral, mergers or acquisitions, taxes, and deposit accounts. Upon the occurrence of an event of default, a default interest rate of an additional 5.0% may be applied to any outstanding principal balances, and Midcap may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Credit Agreements. The Company’s obligations under the Credit Agreements are secured by a security interest in substantially all of The Company’s assets, other than intellectual property.
While the Company was in compliance with the financial covenants in its credit agreement with MidCap at December 31, 2017, given the potential violations of those covenants during fiscal year 2018, as discussed in Note 2, the Company has classified the debt, net of unamortized debt issuance costs, as current in the consolidated balance sheet at December 31, 2017. On this basis, as of December 31, 2017, the future debt payments of $25.0 million are considered payable in 2018.
(6) Goodwill and Other Intangible Assets, net
|
(a) |
Goodwill |
The Company has determined that it has two reporting units, Breast Products and miraDry, and evaluates goodwill for impairment at least annually on October 1st and whenever circumstances suggest that goodwill may be impaired.
F-25
The changes in the carrying amount of goodwill during the years ended December 31, 2017 and 2016 were as follows (in thousands):
|
Balances as of December 31, 2015 |
|
Breast Product |
|
|
miraDry |
|
|
Total |
|
|||
|
Goodwill |
|
$ |
14,278 |
|
|
$ |
— |
|
|
$ |
14,278 |
|
|
Accumulated impairment losses |
|
|
(14,278 |
) |
|
|
— |
|
|
|
(14,278 |
) |
|
Goodwill, net |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill acquired |
|
|
4,878 |
|
|
|
— |
|
|
|
4,878 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances as of December 31, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill |
|
$ |
19,156 |
|
|
$ |
— |
|
|
$ |
19,156 |
|
|
Accumulated impairment losses |
|
|
(14,278 |
) |
|
|
— |
|
|
|
(14,278 |
) |
|
Goodwill, net |
|
$ |
4,878 |
|
|
$ |
— |
|
|
$ |
4,878 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill acquired (Note 3) |
|
|
— |
|
|
|
7,629 |
|
|
|
7,629 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances as of December 31, 2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill |
|
$ |
19,156 |
|
|
$ |
7,629 |
|
|
$ |
26,785 |
|
|
Accumulated impairment losses |
|
|
(14,278 |
) |
|
|
— |
|
|
|
(14,278 |
) |
|
Goodwill, net |
|
$ |
4,878 |
|
|
$ |
7,629 |
|
|
$ |
12,507 |
|
During 2015, the Company experienced a significant sustained decline in stock price, the impact of regulatory inquiries and the fire at Silimed’s facility for manufacturing Sientra’s breast implants. These factors were identified as indicators of impairment. As a result of the analysis performed, the Company recorded a goodwill impairment charge of $14.3 million for the year ended December 31, 2015.
The Company conducted the annual goodwill impairment test in the fourth quarter of 2016 and 2017 and determined goodwill had not been impaired for the years ended December 31, 2017 and 2016.
|
(b) |
Other Intangible Assets |
The components of the Company’s other intangible assets consist of the following definite-lived and indefinite-lived assets (in thousands):
|
|
|
Average |
|
|
|
|
||||||||||
|
|
|
Amortization |
|
|
December 31, 2017 |
|
||||||||||
|
|
|
Period |
|
|
Gross Carrying |
|
|
Accumulated |
|
|
Intangible |
|
||||
|
|
|
(in years) |
|
|
Amount |
|
|
Amortization |
|
|
Assets, net |
|
||||
|
Intangibles with definite lives |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer relationships |
|
|
11 |
|
|
$ |
11,240 |
|
|
$ |
(1,859 |
) |
|
$ |
9,381 |
|
|
Trade names - finite life |
|
|
14 |
|
|
|
5,800 |
|
|
|
(216 |
) |
|
|
5,584 |
|
|
Developed technology |
|
|
15 |
|
|
|
3,000 |
|
|
|
(95 |
) |
|
|
2,905 |
|
|
Distributor relationships |
|
|
9 |
|
|
|
500 |
|
|
|
(40 |
) |
|
|
460 |
|
|
Non-compete agreement |
|
|
2 |
|
|
|
80 |
|
|
|
(57 |
) |
|
|
23 |
|
|
Regulatory approvals |
|
|
1 |
|
|
|
670 |
|
|
|
(670 |
) |
|
|
- |
|
|
Acquired FDA non-gel product approval |
|
|
11 |
|
|
|
1,713 |
|
|
|
(1,713 |
) |
|
|
- |
|
|
Total definite-lived intangible assets |
|
|
|
|
|
$ |
23,003 |
|
|
$ |
(4,650 |
) |
|
$ |
18,353 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangibles with indefinite lives |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names - indefinite life |
|
— |
|
|
|
450 |
|
|
|
— |
|
|
|
450 |
|
|
|
Total indefinite-lived intangible assets |
|
|
|
|
|
$ |
450 |
|
|
$ |
— |
|
|
$ |
450 |
|
F-26
|
|
|
|
|
|
|
December 31, 2016 |
|
|||||||||
|
|
|
Average Amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period |
|
|
Gross Carrying |
|
|
Accumulated |
|
|
Intangible |
|
||||
|
|
|
(in years) |
|
|
Amount |
|
|
Amortization |
|
|
Assets, net |
|
||||
|
Intangibles with definite lives |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer relationships |
|
|
10 |
|
|
$ |
4,940 |
|
|
$ |
(602 |
) |
|
$ |
4,338 |
|
|
Trade names - finite life |
|
|
12 |
|
|
|
800 |
|
|
|
(56 |
) |
|
|
744 |
|
|
Regulatory approvals |
|
|
1 |
|
|
|
670 |
|
|
|
(96 |
) |
|
|
574 |
|
|
Non-compete agreement |
|
|
2 |
|
|
|
80 |
|
|
|
(17 |
) |
|
|
63 |
|
|
Acquired FDA non-gel product approval |
|
|
11 |
|
|
|
1,713 |
|
|
|
(1,696 |
) |
|
|
17 |
|
|
Total definite-lived intangible assets |
|
|
|
|
|
$ |
8,203 |
|
|
$ |
(2,467 |
) |
|
$ |
5,736 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangibles with indefinite lives |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade names - indefinite life |
|
— |
|
|
|
450 |
|
|
|
— |
|
|
|
450 |
|
|
|
Total indefinite-lived intangible assets |
|
|
|
|
|
$ |
450 |
|
|
$ |
— |
|
|
$ |
450 |
|
Amortization expense for the year ended December 31, 2017, 2016 and 2015 was $2.2 million, $0.8 million and $0.1 million, respectively. The following table summarizes the estimated amortization expense relating to the Company's intangible assets as of December 31, 2017 (in thousands):
|
|
|
Amortization |
|
|
|
Period |
|
Expense |
|
|
|
2018 |
|
$ |
2,308 |
|
|
2019 |
|
|
2,321 |
|
|
2020 |
|
|
2,209 |
|
|
2021 |
|
|
1,996 |
|
|
2022 |
|
|
1,762 |
|
|
Thereafter |
|
|
7,757 |
|
|
|
|
$ |
18,353 |
|
(7) Income Taxes
The provision for income tax consists of the following:
|
|
|
Year Ended |
|
|||||||||
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Deferred tax |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
(38 |
) |
|
|
55 |
|
|
|
— |
|
|
State |
|
|
17 |
|
|
|
6 |
|
|
|
— |
|
|
|
|
$ |
(21 |
) |
|
$ |
61 |
|
|
$ |
— |
|
Current income tax expense for the year ended December 31, 2017 was $4 thousand and related to foreign jurisdictions. There was no current income tax expense for the years ended December 31, 2016 and 2015. Total income tax benefit for the year ended December 31, 2017 was $17 thousand. Total income tax expense for the year ended December 31, 2016 was $0.1 million. There was no current or deferred income tax (benefit) expense for the year ended December 2015.
F-27
Actual income tax expense differs from that obtained by applying the statutory federal income tax rate of 34% to income before income taxes as follows (in thousands):
|
|
|
Year Ended |
|
|||||||||
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Tax at federal statutory rate |
|
$ |
(21,776 |
) |
|
$ |
(13,636 |
) |
|
$ |
(14,018 |
) |
|
State, net of federal benefit |
|
|
(2,637 |
) |
|
|
(1,321 |
) |
|
|
(1,624 |
) |
|
Permanent items |
|
|
1,327 |
|
|
|
1,420 |
|
|
|
898 |
|
|
Research and development credits |
|
|
(53 |
) |
|
|
— |
|
|
|
— |
|
|
State rate change |
|
|
(56 |
) |
|
|
87 |
|
|
|
180 |
|
|
Other |
|
|
(103 |
) |
|
|
9 |
|
|
|
1 |
|
|
Change in federal statutory rate |
|
|
34,555 |
|
|
|
— |
|
|
|
— |
|
|
Change in valuation allowance |
|
|
(11,274 |
) |
|
|
13,502 |
|
|
|
14,563 |
|
|
|
|
$ |
(17 |
) |
|
$ |
61 |
|
|
$ |
— |
|
The tax effects of temporary differences and carryforwards that give rise to significant portions of the deferred tax assets are as follows (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Net operating loss carryforwards |
|
$ |
61,171 |
|
|
$ |
65,626 |
|
|
Research and development credits |
|
|
3,049 |
|
|
|
2,233 |
|
|
Accruals and reserves |
|
|
4,993 |
|
|
|
4,362 |
|
|
Intangibles |
|
|
5,605 |
|
|
|
8,309 |
|
|
|
|
|
74,818 |
|
|
|
80,530 |
|
|
Less valuation allowance |
|
|
(71,075 |
) |
|
|
(80,470 |
) |
|
Total deferred tax assets |
|
$ |
3,743 |
|
|
$ |
60 |
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
$ |
(18 |
) |
|
|
(60 |
) |
|
Intangibles - deferred tax liability |
|
|
(3,663 |
) |
|
|
— |
|
|
Indefinite-lived intangibles (naked credit) |
|
|
(102 |
) |
|
|
(61 |
) |
|
Total deferred tax liabilities |
|
|
(3,783 |
) |
|
|
(121 |
) |
|
Net deferred taxes |
|
$ |
(40 |
) |
|
$ |
(61 |
) |
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. Generally, the ultimate realization of deferred tax assets is dependent on the generation of future taxable income during the periods in which those temporary differences become deductible. Based on all the relevant factors, a valuation allowance of $71.1 million has been established against deferred tax assets as of December 31, 2017 as management determined that it is more likely than not that sufficient taxable income will not be generated to realize these temporary differences. Net deferred tax liabilities are recorded in warranties and other long-term liabilities in the consolidated balance sheet.
F-28
As of December 31, 2017, the Company had net operating loss carryforwards of approximately $239 million and $138 million available to reduce future taxable income, if any, for federal and state income tax purposes, respectively. The federal net operating loss carryforward begins expiring in 2027, and the state net operating loss carryforwards begin expiring in 2017. It is possible that the Company will not generate taxable income in time to use these NOLs before their expiration. In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an “ownership change”, the corporation's ability to use its pre-change NOL carryforwards and other pre-change tax attributes to offset its post-change income may be limited. In general, an “ownership change” occurs if there is a cumulative change in a loss corporation’s ownership by 5% shareholders that exceeds 50 percentage points over a rolling three-year period. The Company has not performed a detailed analysis to determine whether an ownership change under Section 382 of the Code has previously occurred. As a result, if the Company earns net taxable income, its ability to use their pre-change net operating loss carryforwards to offset U.S. federal taxable income may become subject to limitations, which could potentially result in increased future tax liability to the Company. Until such analysis is completed, the Company cannot be sure that the full amount of the existing federal NOLs will be available to them, even if taxable income is generated before their expiration.
At December 31, 2017 the Company had research and development credit carryforwards of approximately $1.9 million and $2.1 million available to reduce future taxable income, if any, for federal and California state income tax purposes, respectively. The federal credit carryforwards begin expiring in 2027 and the state credits carryforward indefinitely.
At December 31, 2017, the Company had unrecognized tax benefits of approximately $1.0 million associated with the research and development credits. All of the unrecognized tax benefits that, if recognized, would affect the annual effective rate. The Company does not anticipate that total unrecognized net tax benefits will significantly change over the next twelve months.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
|
Ending balance at December 31, 2015 |
|
$ |
732 |
|
|
Additions based on tax positions taken in the current year |
|
|
— |
|
|
Ending balance at December 31, 2016 |
|
|
732 |
|
|
Additions based on acquisitions during the current year |
|
|
186 |
|
|
Additions based on tax positions taken in the current year |
|
|
48 |
|
|
Ending balance at December 31, 2017 |
|
$ |
966 |
|
It is the Company’s policy to include penalties and interest expense related to income taxes as a component of other (income) expense and interest expense, respectively, as necessary. There was no interest expense or penalties related to unrecognized tax benefits recorded through December 31, 2017.
The Company files U.S. federal, state and foreign income tax returns in jurisdictions with varying statutes of limitations. The years that may be subject to examination will vary by jurisdiction. The Company’s tax years 2014 to 2017 will remain open for examination by the federal and state tax authorities.
The Company has adopted ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, on a modified prospective basis. Under ASU 2016-09, differences between the tax deduction for share based awards and the related compensation expenses recognized under ASC 718 are now accounted for as a component of the provision for income taxes. In addition, ASU 2016-09 eliminated the requirement that excess tax benefits from share based compensation reduce taxes payable prior to being recognized in the financial statements. As of December 31, 2017, we had cumulative excess benefits related to share based compensation of $0.1 million which had not been reflected as a deferred tax asset. As a result of the adoption of ASU 2016-09, the excess benefits were reclassified to our net operating loss carryover resulting in an increase in our deferred tax assets and valuation allowance of $0.1 million as of January 1, 2016. There was no impact to retained earnings as a result of the adoption of ASU 2016-09 on January 1, 2017.
F-29
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act makes broad and complex changes to the U.S. tax code, including, but not limited to, reducing the U.S. federal corporate tax rate to 21% and changing rules to allow an indefinite carryover period and a limitation of 80% of taxable income for net operating loss carryforwards created in tax years beginning after December 31, 2017. As a result and in accordance with Staff Accounting Bulletin No. 118, the Company has recorded a decrease related to its net deferred tax assets before valuation allowance of $34.6 million with a corresponding reduction in the valuation allowance.
(8) Employee Benefit Plans
In September 2016, the Company adopted a Section 401(k) Retirement Savings Plan for the benefit of eligible employees. All employees become eligible to participate in the plan the first of the month following their hire date and may contribute their pretax or after–tax salary, up to the Internal Revenue Service annual contribution limit. The Company makes contributions to the 401(k) plan under a safe harbor provision, whereby the Company contributes 3% of each participating employee’s annual compensation. The Company contributions vest immediately. The Company contributed and included in operating expense $0.6 million and $0.1 million for the years ended December 31, 2017 and 2016, respectively.
|
(a) |
Authorized Stock |
The Company’s Amended and Restated Certificate of Incorporation authorizes the Company to issue 210,000,000 shares of common and preferred stock, consisting of 200,000,000 shares of common stock with $0.01 par value and 10,000,000 shares of preferred stock with $0.01 par value. As of December 31, 2017, the Company had no preferred stock issued or outstanding.
|
(b) |
Common Stock Warrants |
On January 17, 2013, the Company entered into a Loan and Security Agreement, or the Original Term Loan Agreement, with Oxford Finance, LLC, or Oxford. On June 30, 2014, the Company entered into the Amended and Restated Loan and Security Agreement, or the Amended Term Loan Agreement, with Oxford. In connection with the Original Term Loan Agreement and the Amended Term Loan Agreement, the Company issued to Oxford (i) seven-year warrants in January 2013 to purchase shares of the Company’s common stock with a value equal to 3.0% of the tranche A, B and C term loan amounts and (ii) seven-year warrants in June 2014 to purchase shares of the Company’s common stock with a value equal to 2.5% of the tranche D term loan amount. The warrants have an exercise price per share of $14.671. As of December 31, 2017, there were warrants to purchase an aggregate of 47,710 shares of common stock outstanding.
|
(c) |
Stock Option Plans |
In April 2007, the Company adopted the 2007 Equity Incentive Plan, or 2007 Plan. The 2007 Plan provides for the granting of stock options to employees, directors and consultants of the Company. Options granted under the 2007 Plan may either be incentive stock options or nonstatutory stock options. Incentive stock options, or ISOs, may be granted only to Company employees. Nonstatutory stock options, or NSOs, may be granted to all eligible recipients. A total of 1,690,448 shares of the Company’s common stock were reserved for issuance under the 2007 Plan.
As of December 31, 2017, pursuant to the 2007 Plan, there were 493,943 options outstanding and no shares of common stock available for future grants.
The Company’s board of directors adopted the 2014 Equity Incentive Plan, or 2014 Plan, in July 2014, and the stockholders approved the 2014 Plan in October 2014. The 2014 Plan became effective upon completion of the IPO on November 3, 2014, at which time the Company ceased granting awards under the 2007 Plan. Under the 2014 Plan, the Company may issue ISOs, NSOs, stock appreciation rights, restricted stock awards, restricted stock unit awards and other forms of stock awards, or collectively, stock awards, all of which may be granted to employees,
F-30
including officers, non-employee directors and consultants of the Company and their affiliates. ISOs may be granted only to employees. A total of 1,027,500 shares of common stock were initially reserved for issuance under the 2014 Plan, subject to certain annual increases.
As of December 31, 2017, pursuant to the 2014 Plan, there were 2,789,442 shares of common stock reserved and 321,570 shares of common stock available for future grants.
Pursuant to a board-approved Inducement Plan, the Company may issue NSOs and restricted stock unit awards, or collectively, stock awards, all of which may only be granted to new employees of the Company and their affiliates in accordance with NASDAQ Stock Market Rule 5635(c)(4) as an inducement material to such individuals entering into employment with the Company. As of December 31, 2017, inducement grants for 330,000 shares of common stock have been awarded, and 34,000 shares of common stock were reserved for future issuance under the Inducement Plan.
Options under the 2007 Plan and the 2014 Plan may be granted for periods of up to ten years as determined by the Company’s board of directors, provided, however, that (i) the exercise price of an ISO shall not be less than 100% of the estimated fair value of the shares on the date of grant, and (ii) the exercise price of an ISO granted to a more than 10% shareholder shall not be less than 110% of the estimated fair value of the shares on the date of grant. An NSO has no such exercise price limitations. NSOs under the Inducement Plan may be granted for periods of up to ten years as determined by the board of directors, provided, the exercise price will be not less than 100% of the estimated fair value of the shares on the date of grant. Options generally vest with 25% of the grant vesting on the first anniversary and the balance vesting monthly on a straight-lined basis over the requisite service period of three additional years for the award. Additionally, options have been granted to certain key executives that vest upon achievement of performance conditions based on performance targets as defined by the board of directors, which have included net sales targets and defined corporate objectives over the performance period with possible payout ranging from 0% to 100% of the target award. Compensation expense is recognized on a straight-lined basis over the vesting term of one year based upon the probable performance target that will be met. The vesting provisions of individual options may vary but provide for vesting of at least 25% per year.
The following summarizes all option activity under the 2007 Plan, 2014 Plan and Inducement Plan:
|
|
|
|
|
|
|
Weighted |
|
|
Weighted average |
|
||
|
|
|
|
|
|
|
average |
|
|
remaining |
|
||
|
|
|
Option |
|
|
exercise |
|
|
contractual |
|
|||
|
|
|
Shares |
|
|
price |
|
|
term (year) |
|
|||
|
Balances at December 31, 2015 |
|
|
2,785,672 |
|
|
$ |
6.66 |
|
|
|
6.60 |
|
|
Granted |
|
|
571,753 |
|
|
|
7.08 |
|
|
|
|
|
|
Exercised |
|
|
(478,099 |
) |
|
|
1.93 |
|
|
|
|
|
|
Forfeited |
|
|
(92,349 |
) |
|
|
15.35 |
|
|
|
|
|
|
Balances at December 31, 2016 |
|
|
2,786,977 |
|
|
|
7.27 |
|
|
|
6.28 |
|
|
Granted |
|
|
180,000 |
|
|
|
9.12 |
|
|
|
|
|
|
Exercised |
|
|
(480,236 |
) |
|
|
2.80 |
|
|
|
|
|
|
Forfeited |
|
|
(306,954 |
) |
|
|
13.01 |
|
|
|
|
|
|
Balances at December 31, 2017 |
|
|
2,179,787 |
|
|
$ |
7.60 |
|
|
|
7.27 |
|
|
Vested and expected to vest at December 31, 2017 |
|
|
2,179,787 |
|
|
|
7.60 |
|
|
|
|
|
|
Vested and exercisable at December 31, 2017 |
|
|
1,336,308 |
|
|
$ |
7.85 |
|
|
|
6.66 |
|
The weighted average grant date fair value of stock options granted to employees and directors during the years ended December 31, 2017, 2016 and 2015 was $4.54, $3.97, and $4.60 per share, respectively. Stock-based compensation expense for stock options for the years ended December 31, 2017, 2016 and 2015 was $2.2 million, $1.7 million and $2.0 million, respectively. Tax benefits arising from the disposition of certain shares issued upon exercise of stock options within two years of the date of grant or within one year of the date of exercise by the option holder, or Disqualifying Dispositions, provide the Company with a tax deduction equal to the difference
F-31
between the exercise price and the fair market value of the stock on the date of exercise. As of December 31, 2017 there was $2.5 million of total unrecognized compensation cost related to stock options granted under the plans. The costs are expected to be recognized over a weighted average period of 1.98 years. The expense is recorded within the operating expense components in the consolidated statement of operations based on the employees receiving the awards.
The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s common stock for those stock options that had exercise prices lower than the fair value of the Company’s common stock. The aggregate intrinsic value of stock options exercised was $3.1 million, $3.0 million, and $0.6 million during the years ended December 31, 2017, 2016 and 2015, respectively.
The expected term of employee stock options, risk‑free interest rate and volatility represents the weighted average, based on grant date period, which the stock options are expected to remain outstanding. The Company utilized the simplified method to estimate the expected term of the options pursuant to ASC Subtopic 718‑10 for all option grants to employees. The expected volatility is based upon historical volatilities of an index of a peer group because it is not practicable to make a reasonable estimate of the Company’s volatility. The risk‑free interest rate is based on the U.S. Treasury yield curve in effect at the time of the grant for periods corresponding with the expected term of the option. The dividend yield assumption is based on the Company’s history and expectation of dividend payouts. The Company has never declared or paid any cash dividends on its common stock, and the Company does not anticipate paying any cash dividends in the foreseeable future.
During the year ended December 31, 2017, the Company adopted ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting and changed its policy from estimating forfeitures to recording forfeitures when they occur.
For purposes of financial accounting for stock‑based compensation, the Company has determined the fair values of its options based in part on the work of a third‑party valuation specialist. The determination of stock‑based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If the Company had made different assumptions, its stock‑based compensation expense, and its net loss could have been significantly different.
|
(d) |
Restricted Stock Units |
The Company has issued restricted stock unit awards, or RSUs, to employees and non-employees under the 2014 Plan and Inducement Plan. The RSUs issued to employees vest on a straight-line basis, either quarterly over a 4-year requisite service period or annually over a 3-year requisite service period. The RSUs issued to non-employees are generally for consulting services and vest over the contractual service period.
Activity related to RSUs is set forth below:
|
|
|
|
|
|
|
Weighted average |
|
|
|
|
|
Number |
|
|
grant date |
|
||
|
|
|
of shares |
|
|
fair value |
|
||
|
Balances at December 31, 2015 |
|
|
17,993 |
|
|
$ |
3.88 |
|
|
Granted |
|
|
557,240 |
|
|
|
8.21 |
|
|
Vested |
|
|
(4,500 |
) |
|
|
3.88 |
|
|
Forfeited |
|
|
(140,000 |
) |
|
|
8.44 |
|
|
Balances at December 31, 2016 |
|
|
430,733 |
|
|
|
7.99 |
|
|
Granted |
|
|
832,145 |
|
|
|
9.19 |
|
|
Vested |
|
|
(293,910 |
) |
|
|
7.75 |
|
|
Forfeited |
|
|
(40,416 |
) |
|
|
8.47 |
|
|
Balances at December 31, 2017 |
|
|
928,552 |
|
|
$ |
9.12 |
|
F-32
The weighted average grant date fair value of RSUs granted to employees and directors during the years ended December 31, 2017, 2016 and 2015 was $9.19, $8.21, and $3.88 per share, respectively. Stock-based compensation expense for RSUs for the years ended December 31, 2017, 2016 and 2015 was $4.1 million, $1.2 million and $2 thousand, respectively. As of December 31, 2017, there was $5.7 million total unrecognized compensation cost related to non-vested RSU awards. The cost is expected to be recognized over a weighted average period of 1.89 years.
|
(e) |
Employee Stock Purchase Plan |
The Company’s board of directors adopted the 2014 Employee Stock Purchase Plan, or ESPP, in July 2014, and the stockholders approved the ESPP in October 2014. The ESPP allows eligible employees to purchase shares of the Company’s common stock at a discount through payroll deductions of up to 15% of their eligible compensation, subject to any plan limitations. The ESPP provides offering periods not to exceed 27 months, and each offering period will include purchase periods, which will be the approximately six-month period commencing with one exercise date and ending with the next exercise date, except that the first offering period commenced on the first trading day following the effective date of the Company’s registration statement. Employees are able to purchase shares at 85% of the lower of the fair market value of the Company’s common stock on the first trading day of the offering period or on the exercise date. A total of 255,500 shares of common stock were initially reserved for issuance under the ESPP. The number of shares available for sale under the ESPP will be increased annually on the first day of each fiscal year, equal to the lesser of i) 1% of the total outstanding shares of the Company’s common stock as of the last day of the immediately preceding fiscal year; ii) 3,000,000 shares of common stock, or iii) such lesser amount as determined by the board of directors.
As of December 31, 2017, the number of shares of common stock reserved for issuance under the ESPP was 770,549. During the year ended December 31, 2017, employees purchased 108,081 shares under the ESPP at a weighted average exercise price of $5.98 per share. During the year ended December 31, 2016, employees purchased 122,667 shares under the ESPP at a weighted average exercise price of $6.14 per share. As of December 31, 2017, the number of shares of common stock available for future issuance under the ESPP was 495,551. Stock-based compensation related to the ESPP for the years ended December 31, 2017, 2016 and 2015 was $0.4 million, $0.3 million, and $0.4 million, respectively.
(10) Segment Reporting and Geographic Information
|
(a) |
Reportable Segments |
The Company has two reportable segments: Breast Products and miraDry. The Breast Products segment focuses on sales of silicone gel breast implants, tissue expanders and scar management products under the brands Sientra, AlloX2, Dermaspan, Softspan and BIOCORNEUM. The miraDry segment focuses on sales of the miraDry System, consisting of a console and a handheld device which uses consumable single-use bioTips. These segments align with the Company’s principal target markets. On July 25, 2017, the Company acquired miraDry. See Note 3a – Acquisitions for additional details. miraDry has been included in the consolidated results of operations as of the Acquisition Date and financial performance of the acquired business is reported in the miraDry segment.
The Company’s CODM assesses the performance of each segment and allocates resources to those segments based on net sales and operating income (loss). Operating income (loss) by segment includes items that are directly attributable to each segment, including sales and marketing functions, as well as finance, information technology, human resources, legal and related corporate infrastructure costs, along with certain benefit-related expenses. There are no unallocated expenses for the two segments.
The following tables present the net sales, net operating loss and net assets by reportable segment for the periods presented (in thousands):
F-33
|
|
December 31, |
|
||||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Net sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
Breast Product |
|
$ |
31,485 |
|
|
$ |
20,734 |
|
|
$ |
38,106 |
|
|
miraDry |
|
|
5,057 |
|
|
|
— |
|
|
|
— |
|
|
Total net sales |
|
$ |
36,542 |
|
|
$ |
20,734 |
|
|
$ |
38,106 |
|
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Loss from operations |
|
|
|
|
|
|
|
|
|
|
|
|
|
Breast Product |
|
$ |
(56,657 |
) |
|
$ |
(40,034 |
) |
|
$ |
(38,525 |
) |
|
miraDry |
|
|
(6,233 |
) |
|
|
— |
|
|
|
— |
|
|
Total loss from operations |
|
$ |
(62,890 |
) |
|
$ |
(40,034 |
) |
|
$ |
(38,525 |
) |
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Breast Product |
|
$ |
59,365 |
|
|
$ |
114,283 |
|
|
miraDry |
|
|
32,848 |
|
|
|
— |
|
|
Total assets |
|
$ |
92,213 |
|
|
$ |
114,283 |
|
|
(b) |
Geographic Information |
Net sales are attributed to geographic areas based on where the Company’s products are shipped. The following tables present the net sales by geographical region for the periods presented (in thousands):
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
United States |
|
$ |
33,473 |
|
|
$ |
20,734 |
|
|
$ |
38,106 |
|
|
International |
|
|
3,069 |
|
|
|
— |
|
|
|
— |
|
|
Total net sales |
|
$ |
36,542 |
|
|
$ |
20,734 |
|
|
$ |
38,106 |
|
(11) Commitments and Contingencies
|
(a) |
Operating Lease Commitment |
In August 2013, the Company entered into a four-month warehouse lease in Santa Barbara, California, commencing on September 1, 2013. This operating lease is used for additional general office, warehouse, and research and development. This lease has been renewed until January 2019.
In March 2014, the Company entered into a 68-month lease agreement in Santa Barbara, California. The operating lease is for general office use only and commenced on July 1, 2014. The terms of the lease provide for rental payments on a graduated scale.
In December 2013, the Company entered into a 62-month non-cancelable operating lease for its office building space in Santa Clara, California, commencing on March 1, 2014. In connection with the lease, the Company entered into a letter of credit, which is secured by a restricted cash balance of $0.3 million, included in other assets on the consolidated balance sheets.
The Company also has a one year operating lease for office space in Hong Kong which expires in November 2018.
F-34
The Company recognizes rent expense on a straight-line basis over the lease term in operating expenses within the consolidated statement of operations. Rent expense for the years ended December 31, 2017, 2016 and 2015 was $1.1 million, $0.5 million and $0.5 million, respectively.
As of December 31, 2017, future minimum lease payments under all non‑cancelable operating leases are as follows (in thousands):
|
Year Ended December 31: |
|
|
|
|
|
2018 |
|
$ |
1,164 |
|
|
2019 |
|
|
1,034 |
|
|
2020 |
|
|
172 |
|
|
2021 |
|
|
— |
|
|
2022 and thereafter |
|
|
— |
|
|
|
|
$ |
2,370 |
|
|
(b) |
Contingencies |
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company accrues a liability for such matters when it is probable that future expenditures will be made and such expenditures can be reasonably estimated.
Class Action Shareholder Litigation
On September 25, 2015, a lawsuit styled as a class action of the Company’s stockholders was filed in the United States District Court for the Central District of California naming the Company and certain of its officers as defendants for allegedly false and misleading statements concerning the Company’s business, operations, and prospects. On October 28, November 5, and November 19, 2015, three lawsuits styled as class actions of the Company’s stockholders were filed in the Superior Court of California for the County of San Mateo naming the Company, certain of its officers and directors, and the underwriters associated with the Company’s follow-on public offering that closed on September 23, 2015 as defendants for allegedly false and misleading statements in the Company’s offering documents associated with the follow-on offering concerning its business, operations, and prospects. On September 13, 2016, the parties to the actions pending in the San Mateo Superior Court and the United States District Court for the Central District of California signed a memorandum of understanding that sets forth the material deal points of a settlement that covers both actions and includes class-wide relief. Following a final fairness hearing in the federal court, on May 23, 2017, the federal court extended an order granting final approval of the settlement and dismissing the federal court action with prejudice. Following a final fairness hearing in the state court, on May 31, 2017, the state court entered an order granting final approval of the settlement and dismissing the state court action with prejudice.
As a result of these developments, the Company determined a probable loss had been incurred and recognized a net charge to earnings of approximately $1.6 million within general and administrative expense within the consolidated statement of operations which was comprised of the loss contingency of approximately $10.9 million, net of expected insurance proceeds of approximately $9.4 million. In the first quarter of 2017, the Company received $9.3 million in insurance proceeds and paid the $10.9 million loss contingency. The remaining insurance proceeds receivable is classified as insurance recovery receivable on the accompanying consolidated balance sheets.
Silimed Litigation
On July 27, 2017, the Company entered into a settlement agreement, or the Settlement Agreement, with Silimed to settle outstanding litigations with Silimed. Pursuant to the Settlement Agreement, in exchange for a mutual release of claims and covenants not to sue or pursue certain litigation, Sientra paid Silimed a lump sum of $9.0 million on September 7, 2017 and agreed to further pay $1.0 million on or by July 1, 2018. In addition, should the Company enter into international markets using certain breast implant specifications, the Company has agreed to make royalty payments of $12.50 on each of its net sales of such products, up to a maximum royalty of $5.0 million. As a result of
F-35
the settlement, the Company has recorded $10.0 million for the year ended December 31, 2017 in legal settlement expense.
It is possible that additional suits will be filed, or allegations made by stockholders, with respect to these same or other matters and also naming the Company and/or its officers and directors as defendants. The Company believes it has meritorious defenses and intends to defend these lawsuits vigorously.
(12) Subsequent Events
Shelf Registration Statement and At-The-Market Offering of Common Stock
On January 8, 2018, we filed a “shelf” registration statement on Form S-3 with the SEC, which was declared effective by the SEC on February 2, 2018. On February 20, 2018, we entered into an At-The-Market Equity Offering Sales Agreement, or the Sales Agreement, with Stifel, Nicolaus & Company, Incorporated, as sales agent, or Stifel, pursuant to which we may offer and sell, from time to time, through Stifel shares of our common stock having an aggregate gross offering price of up to $50 million. We are not obligated to sell any shares under the Sales Agreement. Subject to the terms and conditions of the Sales Agreement, Stifel will use commercially reasonable efforts consistent with its normal trading and sales practices, applicable state and federal law, rules and regulations, and the rules of the NASDAQ Global Select Market to sell shares from time to time based upon our instructions, including any price, time or size limits we specify. Under the Sales Agreement, Stifel may sell shares by any method deemed to be an “at-the-market offering” as defined in Rule 415 under the Securities Act or any other method permitted by law. Stifel’s obligations to sell shares under the Sales Agreement are subject to the satisfaction of certain conditions, including the continued effectiveness of the associated registration statement on Form S-3. We will pay Stifel a commission of 3.0% of the aggregate gross proceeds from each sale of shares occurring pursuant to the Sales Agreement, if any.
(13) Summary of Quarterly Financial Information (Unaudited)
The following tables set forth our unaudited quarterly statements of operations data and our key metrics for each of the eight quarters ended December 31, 2017. We have prepared the quarterly data on a consistent basis with the audited financial statements included in this report. In the opinion of management, the financial information reflects all necessary adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of this data. This information should be read in conjunction with the audited financial statements and related notes included elsewhere in this report. The results of historical periods are not necessarily indicative of the results of operations for a full year or any future period.
|
|
|
Quarter Ended |
|
|||||||||||||
|
2017 |
|
March 31 |
|
|
June 30 |
|
|
September 30 |
|
|
December 31 |
|
||||
|
|
|
(in thousands, except share data) |
|
|||||||||||||
|
Net sales |
|
$ |
7,489 |
|
|
$ |
8,169 |
|
|
$ |
9,819 |
|
|
$ |
11,065 |
|
|
Gross profit |
|
|
5,167 |
|
|
|
5,548 |
|
|
|
6,335 |
|
|
|
5,321 |
|
|
Net loss |
|
|
(11,422 |
) |
|
|
(20,391 |
) |
|
|
(14,381 |
) |
|
|
(17,834 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(0.61 |
) |
|
$ |
(1.07 |
) |
|
$ |
(0.74 |
) |
|
$ |
(0.92 |
) |
|
|
|
Quarter Ended |
|
|||||||||||||
|
2016 |
|
March 31 |
|
|
June 30 |
|
|
September 30 |
|
|
December 31 |
|
||||
|
|
|
(in thousands, except share data) |
|
|||||||||||||
|
Net sales |
|
$ |
1,471 |
|
|
$ |
6,244 |
|
|
$ |
6,531 |
|
|
$ |
6,488 |
|
|
Gross profit |
|
|
711 |
|
|
|
4,499 |
|
|
|
4,717 |
|
|
|
3,927 |
|
|
Net loss |
|
|
(11,937 |
) |
|
|
(10,193 |
) |
|
|
(9,963 |
) |
|
|
(8,073 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(0.66 |
) |
|
$ |
(0.56 |
) |
|
$ |
(0.55 |
) |
|
$ |
(0.43 |
) |
F-36
Schedule II — Valuation and Qualifying Accounts
December 31, 2017, 2016 and 2015
(In thousands)
|
|
|
|
|
|
|
Additions |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at |
|
|
charged to |
|
|
|
|
|
|
Balance at |
|
|||
|
|
|
beginning of |
|
|
costs and |
|
|
|
|
|
|
end of |
|
|||
|
|
|
period |
|
|
expenses |
|
|
Deductions(1) |
|
|
period |
|
||||
|
Year Ended December 31, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for sales returns |
|
$ |
10,018 |
|
|
$ |
85,429 |
|
|
$ |
(94,787 |
) |
|
$ |
660 |
|
|
Year Ended December 31, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for sales returns |
|
$ |
660 |
|
|
$ |
33,797 |
|
|
$ |
(30,549 |
) |
|
$ |
3,908 |
|
|
Year Ended December 31, 2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for sales returns |
|
$ |
3,908 |
|
|
$ |
48,098 |
|
|
$ |
(48,100 |
) |
|
$ |
3,906 |
|
|
(1) |
Amounts represent actual sales returns. |
F-37
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit |
|
|
|
|
|
SEC File |
|
|
|
|
|
Filed |
|
Number |
|
Exhibit Description |
|
Form |
|
No. |
|
Exhibit |
|
Filing |
|
Herewith |
|
|
2007 Equity Incentive Plan, as amended, and forms of award agreements thereunder. |
|
S-1 |
|
333-198837 |
|
10.2 |
|
September, 19 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3# |
|
2014 Equity Incentive Plan and forms of award agreements thereunder. |
|
S-1/A |
|
333-198837 |
|
10.3 |
|
October 20, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4# |
|
|
S-1 |
|
333-198837 |
|
10.4 |
|
September, 19 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5# |
|
|
S-1/A |
|
333-198837 |
|
10.5 |
|
October 20, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6 |
|
|
S-1 |
|
333-198837 |
|
10.6 |
|
September, 19 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7+ |
|
|
S-1/A |
|
333-198837 |
|
10.8 |
|
October, 20 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.8 |
|
|
S-1 |
|
333-198837 |
|
10.9 |
|
September, 19 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9 |
|
|
S-1 |
|
333-198837 |
|
10.10 |
|
September, 19 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit |
|
|
|
|
|
SEC File |
|
|
|
|
|
Filed |
|
Number |
|
Exhibit Description |
|
Form |
|
No. |
|
Exhibit |
|
Filing |
|
Herewith |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.11# |
|
|
10-Q |
|
001-36709 |
|
|
November 9, 2016 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12# |
|
|
10-Q |
|
001-36709 |
|
|
November 9, 2016 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.13# |
|
Employment Agreement by and between Sientra, Inc. and Patrick F. Williams, dated October 26, 2016. |
|
10-Q |
|
001-36709 |
|
|
November 9, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.14# |
|
Employment Agreement by and between Sientra, Inc. and Jeffrey Nugent, dated November 12, 2015. |
|
10-Q |
|
001-36709 |
|
10.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15# |
|
|
|
001-36709 |
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.16# |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.17# |
|
Sientra, Inc. Inducement Plan and forms of award agreements thereunder. |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit |
|
|
|
|
|
SEC File |
|
|
|
|
|
Filed |
|
Number |
|
Exhibit Description |
|
Form |
|
No. |
|
Exhibit |
|
Filing |
|
Herewith |
|
|
|
|
001-36709 |
|
|
|
|
|||||
|
10.20# |
|
|
10-Q |
|
001-36709 |
|
10.3 |
|
May 9, 2017 |
|
|
|
|
|
|
|
|
|
|
|
||||||
|
10.22 |
|
|
10-Q |
|
001-36709 |
|
10.2 |
|
August 9, 2017 |
|
|
|
|
10.23# |
|
|
10-Q |
|
001-36709 |
|
10.3 |
|
August 9, 2017 |
|
|
|
|
10.24 |
|
|
S-1 |
|
333-214121 |
|
10.8 |
|
October 14, 2016 |
|
|
|
|
10.25 |
|
|
S-1 |
|
333-214121 |
|
10.9 |
|
October 14, 2016 |
|
|
|
|
10.26 |
|
Asset Purchase Agreement, dated January 18, 2008, by and between Miramar Labs, Inc. and Jan Wallace. |
|
S-1 |
|
333-214121 |
|
10.10 |
|
October 14, 2016 |
|
|
|
10.27 |
|
|
S-1 |
|
333-214121 |
|
10.15 |
|
October 14, 2016 |
|
|
|
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit |
|
|
|
|
|
SEC File |
|
|
|
|
|
Filed |
|
Number |
|
Exhibit Description |
|
Form |
|
No. |
|
Exhibit |
|
Filing |
|
Herewith |
|
|
|
S-1 |
|
333-214121 |
|
10.23 |
|
October 14, 2016 |
|
|
||
|
10.29+ |
|
|
S-1 |
|
333-214121 |
|
10.24 |
|
October 14, 2016 |
|
|
|
|
10.30 |
|
|
8-K |
|
001-36709 |
|
10.1 |
|
February 20, 2018 |
|
|
|
|
10.31# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.32# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.33# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21.1 |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.1 |
|
Consent of KPMG LLP, an independent registered public accounting firm. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24.1 |
|
Power of Attorney (included in signature page to this Annual Report on Form 10-K). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit |
|
|
|
|
|
SEC File |
|
|
|
|
|
Filed |
|
Number |
|
Exhibit Description |
|
Form |
|
No. |
|
Exhibit |
|
Filing |
|
Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.2 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS* |
|
XBRL Instance Document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH* |
|
XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase Document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
|
|
|
|
|
|
+ |
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
|
# |
Indicates management contract or compensatory plan, contract, or agreement. |
|
* |
XBRL (Extensible Business Reporting Language) information is furnished and not filed herewith, is not a part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections. |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: March 13, 2018 |
SIENTRA, INC. |
||
|
|
|
|
|
|
|
By: |
|
/s/ Jeffrey Nugent |
|
|
|
|
Jeffrey Nugent |
|
|
|
|
Chairman and Chief Executive Officer |
|
|
|
|
|
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jeffrey Nugent and Patrick F. Williams, and each of them, as his true and lawful attorneys‑in‑fact and agents, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10‑K, and to file the same, with exhibits thereto and other documents in connection therewith, with the U.S. Securities and Exchange Commission, granting unto said attorneys‑in‑fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys‑in‑fact and agents, and either of them, his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
