Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - PDS Biotechnology Corp | ex32_2.htm |
| EX-32.1 - EXHIBIT 32.1 - PDS Biotechnology Corp | ex32_1.htm |
| EX-31.2 - EXHIBIT 31.2 - PDS Biotechnology Corp | ex31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - PDS Biotechnology Corp | ex31_1.htm |
| EX-23.1 - EXHIBIT 23.1 - PDS Biotechnology Corp | ex23_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒
|
ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended December 31, 2017
|
☐
|
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from ________to ________
Commission file number 001-37568
|
Edge Therapeutics, Inc.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
26-4231384
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(IRS Employer Identification No.)
|
|
300 Connell Drive, Suite 4000, Berkeley Heights, NJ 07922
|
|
(Address of principal executive offices)
|
|
(800) 208-3343
|
|
(Registrant’s telephone number)
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulations S-K (§ 229.405 of this chapter) is not contained herein and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☑
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
Accelerated filer ☑
|
Non-accelerated filer ☐
|
Smaller Reporting Company ☐
|
|
Emerging growth company ☑
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. Yes ☑ No ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☑
The aggregate market value of the voting and non-voting common equity held by non-affiliates (without admitting that any person whose shares are not included in such calculation is an affiliate) of the registrant on June 30, 2017, was $316.3 million (based on the closing price for shares of the registrant's common stock as reported on the Nasdaq Global Select Market on that date).
The number of shares of the registrant’s Common Stock, par value $0.00033 per share, outstanding as of February 22, 2018 was 30,869,205.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive Proxy Statement for its 2018 Annual Meeting of Shareholders to be filed with the Securities and Exchange Commission no later than April 28, 2018 and to be delivered to shareholders in connection with the 2018 Annual Meeting of Shareholders, are herein incorporated by reference in Part III of this Annual Report on Form 10-K.
Page | 2
Edge Therapeutics, Inc.
FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2017
|
PAGE
|
|||
|
PART I
|
|||
|
Item 1
|
5
|
||
|
Item 1A
|
34
|
||
|
Item 1B
|
61
|
||
|
Item 2
|
61
|
||
|
Item 3
|
61
|
||
|
Item 4
|
61
|
||
|
PART II
|
|||
|
Item 5
|
61
|
||
|
Item 6
|
64 | ||
|
Item 7
|
65
|
||
|
Item 7A
|
75
|
||
|
Item 8
|
75
|
||
|
Item 9
|
75
|
||
|
Item 9A
|
75
|
||
|
Item 9B
|
75
|
||
|
PART III
|
|||
|
Item 10
|
76
|
||
|
Item 11
|
76
|
||
|
Item 12
|
76
|
||
|
Item 13
|
76
|
||
|
Item 14
|
76
|
||
|
PART IV
|
|||
|
Item 15
|
77
|
||
|
Item 16
|
77
|
||
|
80
|
|||
Cautionary Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K (this “Annual Report”) contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this Annual Report, including statements regarding our future results of operations and financial position, strategy and plans, and our expectations for future operations, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” contained in Item 1A of this Annual Report. In light of these risks, uncertainties and assumptions, actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements in this Annual Report and you should not place undue reliance on these forward-looking statements.
These forward-looking statements include, but are not limited to, statements about:
| ● |
our plans to manufacture, develop and commercialize our product candidates;
|
| ● |
our ability to complete our ongoing clinical studies and to advance our product candidates into additional clinical studies, including pivotal clinical studies, and successfully complete such clinical studies;
|
| ● |
the inherent risks in conducting clinical studies for our products;
|
| ● |
regulatory developments in the United States and foreign countries;
|
| ● |
our ability to obtain and maintain intellectual property protection for our proprietary assets and to overcome any intellectual property held by third parties that might block our ability to exploit our proprietary assets;
|
| ● |
the size of the potential markets for our product candidates and our ability to serve those markets;
|
| ● |
the rate and degree of market acceptance of our product candidates for any indication once approved;
|
| ● |
the performance of our third party contract manufacturers and contract research organizations;
|
| ● |
the success of competing products that are or become available for the indications that we are pursuing;
|
| ● |
the loss of key scientific or management personnel;
|
| ● |
our ability to obtain additional financing;
|
| ● |
the accuracy of our estimates regarding expenses, future revenues and capital requirements;
|
| ● |
our use of the net proceeds from our initial public offering of common stock and future financings, if any;
|
| ● |
our expectations regarding the time during which we will be an emerging growth company under the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”); and
|
| ● |
other risks and uncertainties, including those listed under Item 1A. Risk Factors.
|
Any forward-looking statements in this Annual Report reflect our current views with respect to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
In this Annual Report, unless otherwise stated or the context otherwise indicates, references to “Edge,” “Edge Therapeutics,” “the Company,” “we,” “us,” “our” and similar references refer to Edge Therapeutics, Inc., a Delaware corporation.
PART 1.
Overview
We are a clinical-stage biotechnology company that discovers, develops and seeks to commercialize novel, hospital-based therapies capable of transforming treatment paradigms in the management of acute, life-threatening neurological and other conditions. Our initial product candidates target rare, acute, life-threatening neurological and other conditions for which we believe the approved existing therapies, if any, are inadequate.
We believe EG-1962, our lead product candidate, can fundamentally improve patient outcomes and transform the management of aneurysmal subarachnoid hemorrhage, or aSAH, which is bleeding around the brain due to a ruptured brain aneurysm. A single dose of EG-1962 delivers high concentrations of nimodipine, the current standard of care, directly to the brain with sustained drug exposure over 21 days. EG-1962 utilizes our proprietary, programmable, biodegradable polymer-based development platform, or our PrecisaTM development platform, through a novel delivery mechanism that enables targeted and sustained drug exposure while potentially avoiding dose-limiting side effects associated with currently available formulations of nimodipine. EG-1962 has been granted orphan drug designation and Fast Track designation by the U.S. Food and Drug Administration, or FDA, for the treatment of patients with a subarachnoid hemorrhage, or SAH. The European Commission has granted orphan drug designation to EG-1962 for treatment of aSAH.
In July 2016 we commenced the Phase 3 NEWTON 2 study for EG-1962. NEWTON 2 is a multi-center, multi-national, randomized, double-blind, placebo-controlled, parallel-group study comparing the efficacy and safety of EG-1962 to standard of care oral nimodipine in adults with an aSAH. The primary endpoint of the NEWTON 2 study is the proportion of subjects with a favorable clinical outcome (a score of 6 – 8 on the extended Glasgow Outcome Scale, or GOSE) at day 90. The secondary endpoint is the subject’s score on the Montreal Cognitive Assessment scale, or MoCA. On December 29, 2017, we announced that an independent Data Monitoring Committee, or DMC, recommended that the Phase 3 NEWTON 2 study of EG-1962 continue as planned, based on the completion of a pre-planned futility analysis. The DMC made this recommendation after evaluating data from the Day 90 follow-up visit of the first 150 patients randomized and treated, as well as the available safety data in the study. A pre-planned interim analysis will be performed following the 90 day outcome assessment of the 210th patient. We expect to complete the interim analysis by the end of April 2018 with an announcement of the results to follow. Unless the results of the interim analysis are overwhelmingly positive, which we believe will be hard to achieve, the NEWTON 2 study is expected to continue to full data readout, in which case we expect the results of the study to be available in late 2018. The final results of the NEWTON 2 study, if positive, could form the basis for a marketing application to the FDA and other global health regulatory authorities for the approval of EG-1962 for the treatment of aSAH. In the United States, we plan to use the regulatory pathway set forth in Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act, or FD&C Act.
Our Phase 1/2 clinical study of EG-1962, which we refer to as our NEWTON study, met its primary and secondary endpoints of safety, tolerability, defining the maximum tolerated dose, or MTD, and pharmacokinetics. The results of the principal exploratory efficacy endpoint from the 90-day follow-up demonstrated that 59% (27 of 46) of patients treated with EG-1962 experienced a favorable clinical outcome (a GOSE score of 6-8) versus 28% (5 of 18) of patients treated with the standard of care oral nimodipine. At the final assessment, of the 46 patients treated with EG-1962, 28% (13 of 46) of patients achieved the highest clinical outcome score (GOSE=8, Upper Good Recovery) versus 6% (1 of 18) patients treated with the standard of care oral nimodipine.
A Phase 1 study of the safety, pharmacokinetics and clinical outcomes of EG-1962 administered intracisternally, or directly into the basal cisterns of the brain, is open for enrollment for patients with aSAH who do not receive an external ventricular drain, or EVD, but remain at risk for delayed neurological complications following surgical repair of a ruptured aneurysm. This study is a multicenter, randomized, controlled, open-label study in which nine patients are expected to receive EG-1962 via intracisternal administration and three patients are expected to receive standard of care oral nimodipine.
In the United States, approximately 35,000 patients with an average age of 52 experience an SAH each year. Of these, approximately 20,000 experience an aSAH and are hospitalized in the intensive care unit, or ICU, and approximately 75% of these patients suffer permanent brain damage or die within 30 days. Care for these patients results in overall inpatient charges of more than $5.0 billion per year in the United States. After the ruptured aneurysm is repaired, these patients remain at risk for multiple complications that are managed over the course of the patient’s treatment to optimize clinical outcomes. The most common and severe complications, which are thought to be at least in part due to the influx of calcium, include cerebral vasospasm, or narrowing of the brain arteries, and delayed cerebral ischemia, or DCI, a catastrophic delayed complication that occurs secondary to processes including cerebral vasospasm. DCI occurs in up to 30% of patients who survive the initial hemorrhage. DCI often leads to the death of brain tissue due to insufficient blood flow to certain areas of the brain and results in a significant economic burden to the health care system. The direct cost to hospitals in patients experiencing DCI is estimated to be approximately $50,000 in additional direct in-hospital per patient costs, but can increase to $70,000 based on the additional comorbidities associated with treating an aSAH patient and the extent of their disability. An analysis of the 2013 National Inpatient Sample Database showed that the aneurysmal subarachnoid hemorrhage patients who require an EVD, and undergo either neurosurgical micro clipping or endovascular coiling for repair of their aneurysm, had the highest per-patient hospital charges, exceeding $400,000 per patient, and experienced the longest hospitalizations of all aneurysmal subarachnoid hemorrhage patients. Not included in this analysis are the additional costs of rehabilitation, the long-term lifetime cost of illness related to an aSAH, the frequent inability to re-enter the work force, and the potential need for additional care at home from family members or long term care givers associated with chronically disabled patients, which present additional significant economic burden to the healthcare system and the families of those afflicted with aSAH.
In most countries, including the United States, current treatment guidelines recommend that all aSAH patients receive the L-type calcium channel blocker nimodipine over a 21-day period orally every four hours. In certain geographies outside the United States, the product can also be received via IV administration. As part of their routine course of treatment, we believe nearly half of aSAH patients have an EVD inserted into the brain to monitor and relieve intracranial pressure by draining out cerebrospinal fluid, or CSF. The EVD can also serve as a pathway to administer drugs. Nimodipine has been the standard of care for over 25 years and has a well-understood safety profile at its approved dose and route of administration. Nimodipine has been shown to improve outcomes in aSAH patients, and its mechanism of action is believed to modify several calcium channel mediated pathways that contribute to unfavorable outcomes.
However, the ability to administer nimodipine systemically at optimal therapeutic levels may be limited by side effects, primarily its potential to cause hypotension, or low blood pressure, which can exacerbate the complications of aSAH. EG-1962 is being investigated to deliver high and sustained concentrations of nimodipine directly to the site of injury in the brain in a single administration through an existing EVD, while potentially avoiding the dose-limiting side effects of oral nimodipine. Since current treatment guidelines recommend that all patients receive nimodipine prophylactically after aSAH, we believe that EG-1962, if approved, can become the new standard of care for all patients who receive an EVD after aSAH.
Our NEWTON study was a multicenter, randomized, controlled, open-label Phase 1/2 clinical study of EG-1962. It was designed to assess the safety, tolerability, MTD and pharmacokinetics of EG-1962 compared to the standard of care oral nimodipine in up to 96 patients with aSAH. Six different dose cohorts (72 patients) were evaluated in North America at escalating doses of 100 mg, 200 mg, 400 mg, 600 mg, 800 mg and 1200 mg, and one patient, who received EG-1962, was evaluated outside of North America. Twelve patients in each cohort were randomized in a ratio of 3 to 1 to receive either single dose EG-1962 or standard of care oral nimodipine for 21 days. After 90 days, multiple exploratory endpoints were evaluated, the principal exploratory endpoint of which was patient clinical outcomes; other exploratory endpoints included occurrence of DCI, use of rescue therapies and ICU and hospital length of stay, or LOS, all of which we believe are indicative of the potential efficacy of EG-1962. Seventeen medical centers in North America and one medical center in Finland randomized patients into the study.
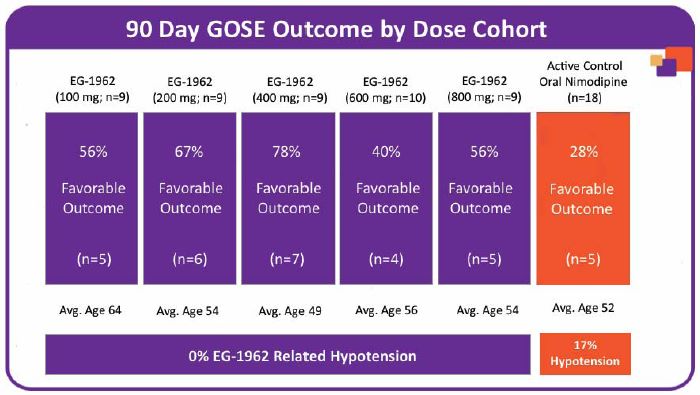
The NEWTON results showed that the primary and secondary study endpoints were met and all of the exploratory endpoints favored the EG-1962 groups when compared to the standard of care oral nimodipine. Safety and tolerability data were reported for all cohorts, while efficacy results were reported only for subjects treated with doses of EG-1962 up to and including 800 mg, as 1200 mg was not a tolerable dose. There were no safety concerns identified after administration of EG-1962 that precluded dose escalation. The number of deaths and serious adverse events in the EG-1962 treated group were comparable to those in the standard of care oral nimodipine group. Further, 0% of patients (0 of 55) experienced EG-1962-related hypotension in the treated group, while 17% of patients (3 of 18) experienced standard of care oral nimodipine related hypotension. The MTD was determined to be 800 mg, with the 600 mg dose considered to be the optimal dose to evaluate in the Phase 3 NEWTON 2 study.
Since at least 50% of aSAH patients may not require an EVD, market expansion opportunities exist. We intend to explore alternative routes of administration of EG-1962, as evidenced by the commencement of our study of intracisternal administration of EG-1962 in patients with aSAH who do not receive an EVD. Additionally, physicians have expressed interest in the use of an EVD in patients who do not routinely receive one as standard of care solely to administer EG-1962 in patients at high risk of delayed neurological complications.
In addition to EG-1962, we are using our Precisa PlatformTM to seek to develop additional product candidates targeting other acute, serious conditions where limited or no current approved therapies exist. We are developing EG-1964 as a prophylactic treatment in the management of chronic subdural hematoma, or cSDH, to prevent recurrent bleeding on the surface of the brain. A cSDH is a liquefied hematoma that has accumulated on the surface of the brain in an area referred to as the subdural space and is often caused by minor head trauma. We are also developing EG-1965, for the prevention of post-operative atrial fibrillation, or POAF. By way of a single administration at the time of the surgical intervention, we believe EG-1965 can deliver an effective concentration of drug directly to the atrial myocardium with sustained drug exposure for up to seven days.
Our current product development strategy involves identifying hospital-based products for other acute, life-threatening conditions where limited or no current therapies exist. We then apply our scientific, clinical, and technical expertise to design targeted, small or large molecule therapeutics. Our Precisa platform allows us to create polymer-based therapeutics, designed to be capable of delivering medicines directly to the site of injury to potentially avoid serious systemic side effects often associated with oral or intravenous delivery approaches and to potentially enable high and sustained drug exposure with only a single dose at the initial time of procedural or surgical intervention. Our approach is not expected to require a material change in physician behavior and may result in significant pharmacoeconomic advantages, as it seeks to avoid recurrent invasive procedures, mitigate clinical complications that often result in prolonged and expensive acute hospital care, and improve patient outcomes, which could potentially decrease the likelihood of costly long-term, skilled nursing care. While we have initially applied our approach to acute neurological and cardiological conditions, we intend to apply our Precisa development platform to seek to develop treatments for other acute, serious conditions by targeting other organs across additional therapeutic areas, including possibly trauma and other diseases.
We currently retain worldwide rights to all of our product candidates. If we receive marketing authorization for our products, we intend to establish targeted commercialization and marketing capabilities for our products in the United States, Canada and possibly the EU. In the United States and Canada, approximately 300 top academic medical centers treat approximately 90% of all aSAH patients. As such, we believe a small, targeted sales force could effectively cover these institutions and successfully commercialize our products, if approved. In the United States, we intend to commercialize by developing a hospital sales force of up to 50 representatives that would focus on academic medical centers and other major medical centers treating acute neurological conditions. We believe a similar sized sales force would be appropriate for the EU, should we decide to commercialize EG-1962 there using an Edge sales force. For commercialization of our products, if approved, outside of the United States and Canada, we may enter into collaborations or license agreements with strategic partners.
We are led by a team of executives and directors with significant experience in drug discovery, development and commercialization. Our co-founder and Chief Executive Officer, or CEO, Brian A. Leuthner, has been responsible for developing, launching and selling products in the hospital market for GlaxoWellcome plc, Johnson & Johnson, ESP Pharma, Inc. and The Medicines Company. Our other co- founder and Chief Scientific Officer, Dr. R. Loch Macdonald, is a world-renowned neurosurgeon and expert in the research and management of acute neurological and neurosurgical conditions. Other members of our management team have held senior positions at Abbott Laboratories, Alpharma, Inc., Auxilium Pharmaceuticals, Celgene Corporation, Eli Lilly and Company, Endo International plc, ESP Pharma, Inc., Johnson & Johnson, Lundbeck, Medarex, Inc., MedPointe, Inc., NPS Pharmaceuticals, Otsuka Pharmaceuticals, PTC Therapeutics, Purdue Pharma L.P., Schering Plough Corporation, Shire Pharmaceuticals, and Valeant Pharmaceuticals International, Inc. In addition, our Chairman of the Board, Dr. Sol Barer, is the former Chairman and CEO of Celgene Corporation and the current Chairman of Teva Pharmaceutical Industries Ltd., and the Chairman of our Scientific Advisory Board, Dr. Robert Langer, is a world-renowned scientist and pioneer in drug-delivery-related inventions.
Our Strategy
Our vision is to become a leading global biotechnology company that discovers, develops and commercializes novel, hospital-based therapies capable of transforming treatment paradigms in the management of acute, life-threatening medical conditions. We intend to utilize our product development and commercial execution strategies to achieve this vision. Key elements of our execution strategy are as follows:
|
•
|
Rapidly develop our lead product candidate, EG-1962 to improve clinical outcomes following an aSAH. We completed the NEWTON study, enrolling 72 patients in six different dose cohorts in North America with doses ranging from 100 mg to 1200 mg, and one patient outside of North America who received EG-1962 600 mg. During 2016, we initiated NEWTON 2, our pivotal Phase 3 study in the United States, Canada and Israel. In 2017, we expanded the study globally to additional centers across North America, Europe, and Australasia.
|
|
•
|
Expand the development of EG-1962 for alternative routes of administration to improve clinical outcomes following an aSAH. In order to deliver EG-1962 to aSAH patients that do not receive an EVD as standard of care, we have an ongoing Phase 1 study of EG-1962 administered directly into the basal cisterns. We are also exploring a lumbar route of administration for EG-1962.
|
|
•
|
Evaluate other indications for EG-1962 in therapeutic areas inside and outside of the brain. Published literature indicates that L-type calcium channel blockers have a broad range of potential uses in other therapeutic areas. By using our proprietary Precisa platform to enable site specific sustained delivery of nimodipine to a target organ, we believe that EG-1962 may demonstrate improvements in safety and/or efficacy profiles in these other therapeutic areas over existing standards of care.
|
|
•
|
Continue development of EG-1965 for the prevention of POAF. In 2018, we intend to complete formulation development and initiate pharmacology and toxicology studies required for filing an Investigational New Drug Application, or IND. Pending results of those studies, we may submit an IND in 2019.
|
|
•
|
Commercialize our product candidates, including EG-1962, if approved, through a targeted sales force in the United States and Canada and possibly Europe, and with potential strategic partnerships outside of the regions where we field our own sales force. We have retained the worldwide rights to all of our product candidates and intend to build a hospital-focused sales organization to market our approved products. We intend to establish targeted sales forces in the United States and Canada, and possibly Europe, for EG-1962, if approved, to sell into medical centers capable of treating acute neurological conditions.
|
|
•
|
Leverage our proprietary, programmable, polymer-based Precisa platform to seek to develop life-saving therapies in acute care areas. We intend to expand the use of our Precisa development platform in other therapeutic areas, such as cardiac surgery or neuro-oncology. Depending on the specific needs of the targeted therapy, these initiatives may focus on applying our Precisa development platform to previously approved medicines, or may result from the collaboration on, or in-licensing and development of, new chemical entities.
|
|
•
|
Identify business development or product acquisition opportunities that leverage our core expertise. We intend to identify assets that we might in-license or acquire that utilize our expertise in developing and manufacturing drugs for acute, hospital-based or other conditions, complement our efforts and activities in potentially commercializing EG-1962 in the hospital setting, and leverage the infrastructure developed to potentially bring EG-1962 and other products into the clinic and to the marketplace.
|
|
•
|
Continue to seek to maintain high barriers to entry around our product candidates and the markets in which they are utilized by using a multi-layered approach. One layer of defense relates to obtaining regulatory exclusivity, such as orphan drug protection, when and where available. An additional layer relates to patent rights. We currently have five issued U.S. patents, including composition of matter related to EG-1962, 35 granted foreign patents, four notices of acceptance/eligibility for grant/allowance of foreign applications, and more than 70 U.S. and foreign pending patent applications. Another layer of defense involves technical operations and manufacturing know-how and trade secrets relating to the design and manufacture of products using our Precisa development platform. An additional layer involves the difficulty a competitor may experience in proving bioequivalence. If a competitor were to attempt to prove bioequivalence, we believe such competitor would likely be required to conduct human clinical studies.
|
Our Product Candidates
EG-1962 for aSAH
EG-1962, a polymer-based microparticle containing nimodipine suspended in a diluent of sodium hyaluronate was developed using our Precisa development platform to improve patient outcomes following aSAH. Nimodipine, delivered via oral or intravenous routes of administration, is currently the standard of care for the management of aSAH and is believed to work by modifying several pathways contributing to unfavorable outcomes. Current U.S. treatment guidelines recommend that all aSAH patients, commencing within 96 hours after aneurysm rupture, receive nimodipine orally every four hours over a 21-day period. However, the ability to achieve optimal therapeutic levels of nimodipine when administered orally or intravenously may be limited by serious side effects, primarily hypotension, which can exacerbate the complications of aSAH. We believe that a single intraventricular dose of EG-1962 has the potential to improve patient outcomes by delivering high and sustained concentrations of nimodipine over a 21-day period directly to the brain, while avoiding these dose-limiting side effects.
Background on aSAH
An aSAH is a brain hemorrhage after which blood from a ruptured aneurysm enters the subarachnoid space, the area between the middle and deepest protective layers of the brain. The World Health Organization and published medical literature estimates that approximately 600,000 individuals worldwide suffer an aSAH each year. In the United States, approximately 35,000 patients with an average age of 52 experience an SAH each year. Of these, approximately 20,000 experience an aSAH and are hospitalized in ICU, and approximately 75% of these patients suffer permanent brain damage and die within 30 days. Patients with an aSAH typically present with a characteristic intense, unrelenting and overwhelming headache of sudden onset. After a computed tomography, or CT, scan is performed upon arrival at the hospital, the aneurysm is repaired to prevent rebleeding, and the patient is sent to the ICU for close monitoring over a 14 to 21 day period. An EVD is placed into the fluid chambers of the brain to measure and manage intracranial pressure in what we believe to be approximately 50% of all aSAH patients. These EVDs may also serve as routes of administration for drugs, including EG-1962, if approved.
After the ruptured aneurysm is repaired, patients remain at risk for multiple complications that are managed over the course of the patient’s treatment to optimize clinical outcomes. DCI is a common and serious complication following aSAH that typically occurs within three to 14 days after aneurysm rupture and is believed to be caused by cerebral vasospasm and other mechanisms. DCI occurs in up to 30% of the patients that survive the initial hemorrhage. Clinical features of DCI after aSAH mostly consist of focal neurological deficits, or a decrease in the level of consciousness, the time of onset and severity of which vary. DCI is sometimes reversible, but may also progress to death of brain tissue resulting in severe disability or death. It is thought that DCI and vasospasm are at least in part caused by the influx of calcium. When vasospasm and DCI occur after aSAH, rescue therapy is initiated and is comprised of hemodynamic management, which typically involves giving medicine to increase a patient’s blood pressure to try to push more blood and oxygen into the brain, and performing angioplasty, which involves injecting drugs directly into brain arteries to dilate such arteries or inflating small balloons into the narrowed arteries to open them. To date, the L-type calcium channel blocker nimodipine is the only drug approved in North America and Europe shown to have efficacy in reducing unfavorable clinical outcomes and neurological deficits after aSAH. However, given the limitations of oral nimodipine, better pharmacological treatments are needed to improve patient outcomes.
The pictures below illustrate the typical sequence of events for the up to 30% of aSAH patients who experience DCI.
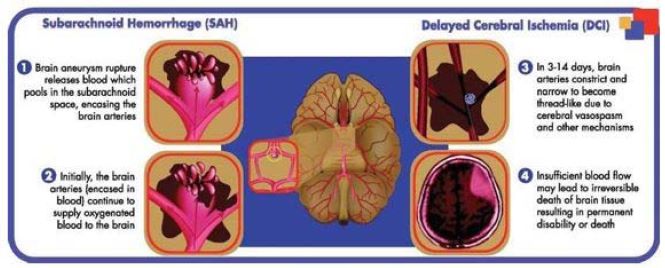
If these complications cannot be reversed during rescue therapy and DCI is not prevented, death of brain tissue results and can lead to permanent brain damage resulting in disability or death.
Current Standard of Care for aSAH
Current treatment guidelines recommend that all patients with an aSAH should be administered oral or, in Europe, intravenous nimodipine, an L-type calcium channel blocker which is believed to block several pathways that contribute to unfavorable outcomes. The FDA approved nimodipine in the oral form of gelatin capsules, or gelcaps as Nimotop® in 1988. In 2013, the FDA approved Nymalize®, an oral solution of nimodipine, and granted it marketing exclusivity as a result of its orphan drug designation. Nymalize has received regulatory approval only in the United States. While not approved for intravenous administration in the United States, nimodipine is currently available in oral gelcaps, oral tablets and/or intravenous forms in almost every country. Due to a lack of alternative therapies, nimodipine rapidly became standard of care and was incorporated into treatment guidelines for aSAH. Nimodipine is indicated to improve neurological outcomes by reducing the incidence and severity of ischemic deficits following aSAH. In a meta-analysis of clinical studies, oral nimodipine demonstrated an approximately 11% absolute risk reduction in unfavorable outcomes as compared to placebo.
After an aSAH, nimodipine is prescribed to be given over a 21-day course of treatment administered orally every four hours or intravenously for about seven days followed by 14 days of oral nimodipine. However, dosages of nimodipine delivered orally or intravenously may be limited because nimodipine is non-selective and dilates not only brain arteries, but other arteries throughout the rest of the body. Arterial dilation can cause adverse effects, such as hypotension, and by reducing blood flow to the already-injured brain, can exacerbate the complications of aSAH. Therefore, we believe a significant need remains for a better, commercially viable method of providing higher and more sustained concentrations of nimodipine at the site of brain injury without the potential to cause serious systemic side effects, primarily hypotension, associated with current formulations of nimodipine.
Our Solution: EG-1962
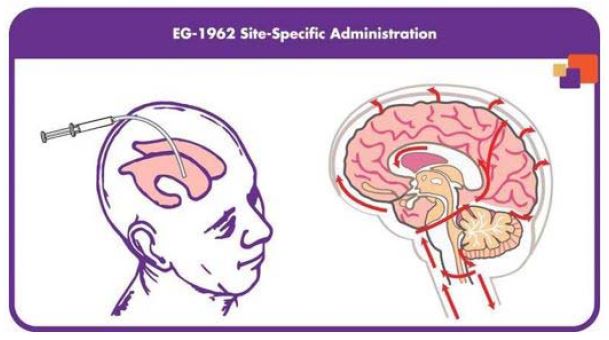
EG-1962 is a novel, proprietary formulation consisting of nimodipine encapsulated within a bioresorbable poly-D,L-lactide-co-glycolide, or PLGA, matrix and reconstituted with a sodium hyaluronate based buffer making a suspension that releases nimodipine over at least 21 days. The drug is administered directly into a cerebral ventricle in patients who have suffered in aSAH via an EVD that is in place as standard of care.
We believe this targeted approach enables EG-1962 to deliver high and sustained therapeutic concentrations of nimodipine directly to the brain while maintaining relatively low systemic nimodipine levels, thus providing for the potential to improve patient outcomes and avoid the serious side effects, primarily hypotension, associated with oral or intravenous nimodipine. If approved, it is anticipated that EG-1962 will be administered in the ICU typically by the neurosurgeon or neurointensivist via an EVD after the patient has been stabilized following an aSAH. EG-1962 is designed to release an initial dose of the nimodipine to quickly increase concentrations at the site of brain injury prior to the onset of any complications and then to release a steady dose of nimodipine over 21 days. If approved, we believe EG-1962 has the potential to address a significant unmet medical need and can become the standard of care for treatment of aSAH in patients who receive an EVD.
Clinical Studies
NEWTON 2
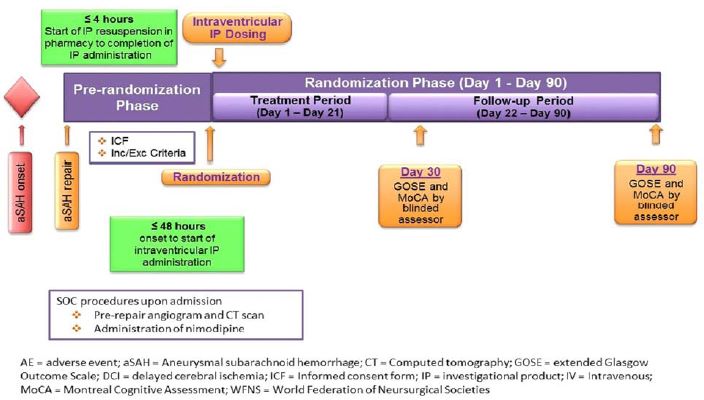
In July 2016, we initiated the NEWTON 2 study, a pivotal Phase 3 multi-center, multi-national, randomized, double-blind, placebo-controlled, parallel-group study comparing the efficacy and safety of EG-1962 to standard of care oral nimodipine in adults with an aSAH. Patients in the EG-1962 treatment arm receive a single 600 mg dose of EG-1962 plus placebo gelcaps or tablets administered for up to 21 days. Patients in the control arm of the study receive a single dose of intraventricular normal saline and up to 21 days of oral nimodipine gelcaps or tablets. The primary outcome measure is the proportion of patients with a favorable outcome of 6 – 8 as measured on the GOSE 90 days after aSAH. Additional outcome measures are neurocognitive outcome at day 90 measured by MoCA, safety (including delayed cerebral infarction at day 30) and health economic endpoints.
The NEWTON 2 study will assess up to 374 patients who will be randomized on a one to one basis within 48 hours of their aSAH to either the EG-1962 arm, or the control arm. On December 29, 2017, we announced that the DMC recommended that the NEWTON 2 study of EG-1962 continue as planned based on the completion of a pre-planned futility analysis. The DMC made this recommendation after evaluating data from the 90 day follow-up visit of the first 150 patients randomized and treated, as well as the available safety data in the study. We expect to complete the NEWTON 2 interim analysis by the end of April 2018 with an announcement of the results of this analysis to follow. Upon completion of this interim analysis, the DMC may recommend continuing enrollment of the study up to 374 completed patients; ending the study early, as efficacy will have been established at a highly statistically significant level at the time of such interim analysis; or discontinuing enrollment of additional patients, but analyzing all currently enrolled and completed patients. We believe the likelihood that the DMC will recommend that we end the study early based on highly positive statistical significance is extremely low. There is no formal futility analysis built into the interim analysis. However, as per the DMC charter, the DMC may recommend amending or terminating the NEWTON 2 study for safety concerns or based on a benefit : risk assessment that does not justify additional subject enrollment.
The figure below provides an overview of the study design for each patient in the NEWTON 2 study:
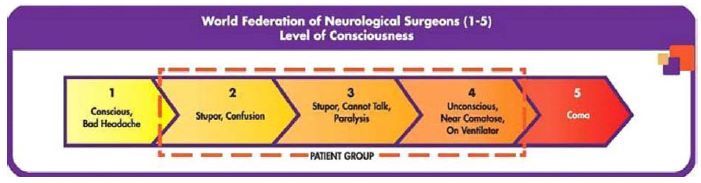
The primary enrollment criteria for the NEWTON 2 study are similar to the ones used in the NEWTON study and are based primarily on the patients’ level of consciousness as measured by the World Federation of Neurological Surgeon’s scale, or WFNS scale. The WFNS scale stratifies patients into grades 1 – 5 with a score of 1 being the highest (or normal) level of consciousness, and a score of 5 being the least conscious. It is believed the level of consciousness is the best prognostic indicator of clinical outcome after aSAH. Patients in the NEWTON 2 study are enrolled if their WFNS score is between 2 and 4. The table below illustrates the WFNS scale:
The primary clinical outcome endpoint used to assess patients at 90 days in the NEWTON 2 study is the GOSE. The GOSE is a widely used scale accepted by regulatory agencies as a study endpoint that was developed from the Glasgow Outcome Scale, or GOS, to define broad outcome categories for people who sustain traumatic and non-traumatic brain injuries. GOS is a global scale that rates patient status into one of five categories: Dead, Vegetative State, Severe Disability, Moderate Disability and Good Recovery. The scale focuses on how the injury has affected functioning in major areas of life rather than on specific neurological deficits and symptoms caused by the injury. The GOSE is an expansion of the GOS that refines the clinical outcome assessments for severe disability, moderate disability and good recovery into upper and lower categories thereby creating an eight-point scale as follows:
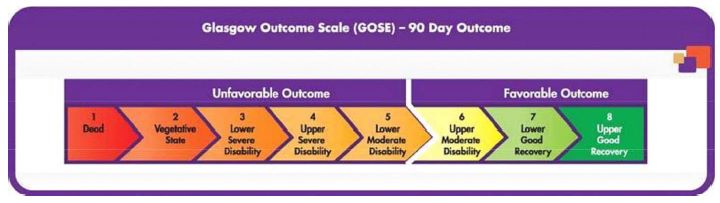
In the NEWTON 2 study, the GOSE is rated by an independent assessor who conducts an interview with the patient and/or caregiver from a standardized questionnaire. The NEWTON 2 protocol defines a “favorable outcome” for the clinical outcome as a GOSE score of 6-8 measured at 90 days.
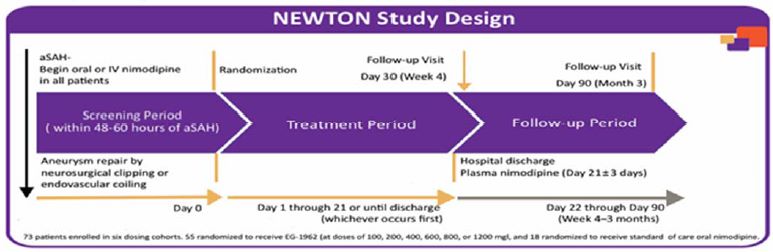
NEWTON Study
Our NEWTON study was a multicenter, randomized, controlled, open-label Phase 1/2 study of EG-1962 compared to the standard of care oral nimodipine. Of the total of 73 patients enrolled in the NEWTON study, 55 patients were randomized to EG-1962 and 18 patients were randomized to oral nimodipine.
The primary objectives of the study were to determine the MTD safety and tolerability of a single intraventricular administration of EG-1962. The key secondary objective was to determine pharmacokinetics of EG-1962. Clinical outcomes at day 90, including angiographic vasospasm, and DCI represented important exploratory clinical endpoints, along with ICU and hospital LOS. The primary clinical outcome scale utilized was the GOSE. A score of 6 – 8 on the GOSE scale was considered a favorable outcome. This requires that patients are able to look after themselves and return to work, among other criteria.
Subjects were randomized 3 to 1 to receive either a single dose EG-1962 or standard of care oral nimodipine. Six dose-levels (100 mg, 200 mg, 400 mg, 600 mg, 800 mg and 1200 mg) were evaluated. The active control was the standard of care, oral nimodipine. The active control group received 60 mg of oral nimodipine, which was administered every four hours for a total daily dose of 360 mg for 21 days in accordance with the currently approved dosing regimen. This dose of oral nimodipine remained unchanged except when it was medically warranted to reduce the dose due to adverse events consistent with the standard of care.
The figure below provides an overview of the study design for each patient:
NEWTON Clinical Results
The primary endpoints of the NEWTON study of safety, tolerability and MTD were all achieved, as were the secondary endpoint of characterizing the pharmacokinetics of EG-1962. Further, all exploratory endpoints favored EG-1962.
EG-1962 was well-tolerated with only one EG-1962-related serious adverse event reported, a possible allergic reaction in one patient following administration of EG-1962, which was immediately treated and resolved with no further clinical impact. This patient had a favorable clinical outcome. No patients (0 of 55) experienced EG-1962 related hypotension in the treated group, while 17% (3 of 18) of patients experienced oral nimodipine related hypotension.
The pharmacokinetic results demonstrated that a single, intraventricular administration of EG-1962 provides high CSF levels of nimodipine with plasma levels that do not exceed those associated with systemic hypotension. The mean maximum levels of nimodipine seen in the plasma ranged between 8.8 ng/mL (100 mg dose), and 25.4 ng/mL (800 mg dose), respectively. This compares to mean maximum levels of 46.2 ng/mL seen in the standard of care oral nimodipine patients. In a study published in the European Journal of Clinical Pharmacology, hypotension did not occur until plasma nimodipine concentration was greater than approximately 30 to 45 ng/mL.
We believe the pharmacokinetic data demonstrated that the high CSF concentrations of nimodipine achieved with EG-1962 allow for more effective inhibition of calcium channels located on the smooth muscle cells within the walls of blood vessels. Nimodipine circulating in the plasma inside the blood vessel is able to access the calcium channels by crossing the blood brain barrier into the smooth muscle cells, while nimodipine circulating in the CSF is able to access the smooth muscle cells from the outer wall of the blood vessel. Once on the smooth muscle cells, nimodipine is believed to act locally on harmful calcium-dependent mechanisms that may contribute to delayed neurological complications. We believe that because EG-1962 provides consistent steady-state plasma concentrations below those known to cause hypotension, the dual-access of EG-1962 on the calcium channels may contribute to favorable patient outcomes.
The pooled exploratory clinical results showed that 59% (27 of 46) of patients treated with EG-1962 achieved a favorable outcome (GOSE scores of 6-8) at 90 days compared to only 28% (5 of 18) in the active control standard of care oral nimodipine arm.
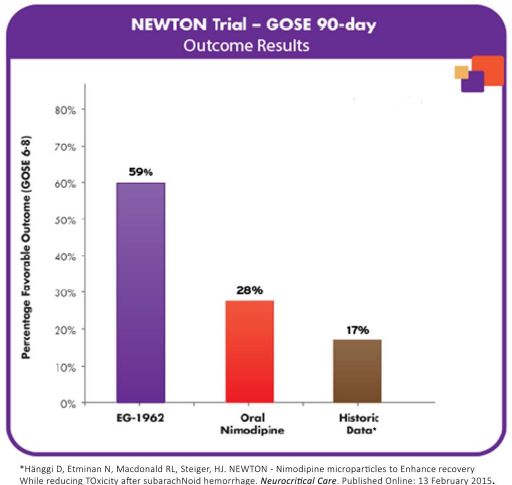
The EG-1962 results compared favorably to both those of the standard of care oral nimodipine arm and to published historical data from other contemporary studies of severe aSAH patients treated with the current standard of care, which showed that only 17% (26 of 151) of such patients had favorable outcomes after 90 days (defined as GOSE scores of 6-8). In addition, 28% (13 of 46) of patients treated with EG-1962 in the NEWTON study achieved a GOSE score of 8 (Upper Good Recovery), compared to only 6% (1 of 18) in the standard of care, oral nimodipine arm and less than 1% (1 of 151) in published historical data. This historical data, published in the journal “Neurocritical Care” on February 13, 2015, consisted of 151 patients who had WFNS scores of 2-4 with an EVD and outcomes measured by GOSE, which closely match the enrollment and evaluation criteria for the NEWTON study.
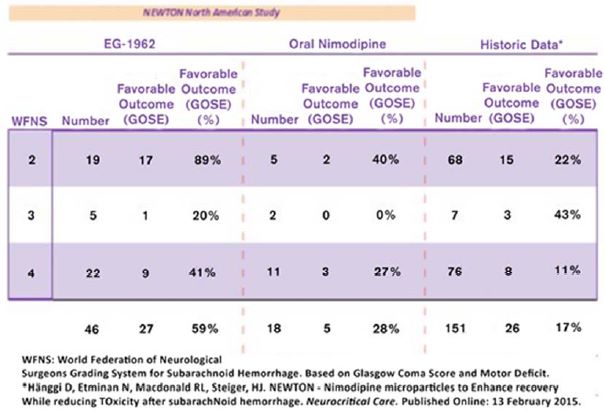
Additionally, the clinical outcome results were analyzed by WFNS score to compare rates of favorable outcome by WFNS grade to oral nimodipine standard of care controls. In the analysis by WFNS score, EG-1962 showed more than twice the rate of favorable outcome for WFNS grade 2 patients versus oral nimodipine (89% vs 40%). For WFNS 4 grade patients, the rate of favorable outcomes in patients treated with EG-1962 was 41% versus 27% for those patients treated with standard of care oral nimodipine. The data are presented in the table below:
There was no dose response with regards to outcome noted in the individual cohorts. All EG-1962 dose cohorts achieved favorable outcome proportions higher than oral nimodipine standard of care controls with proportions ranging from 40% to 78%. Differences in outcome rates were affected by the variability in WFNS scores and age among cohorts. The charts below show the key efficacy results for dose cohorts 1-5 (100 mg - 800 mg).
The study’s exploratory endpoints included rates of delayed complications such as angiographic vasospasm and DCI and the use of rescue therapy. The overall favorable outcome results seen in the EG-1962 arm were supported by the reduction in angiographic vasospasm, DCI, and rescue therapy. EG-1962 reduced the risk of angiographic vasospasm by 51% (30% EG-1962 vs. 61% oral nimodipine); reduced the risk of DCI by 55% (15% EG-1962 vs. 33% oral nimodipine); and reduced the risk of rescue therapy by 54% (26% EG-1962 vs. 56% oral nimodipine). The data for these exploratory endpoints are summarized in the table below:
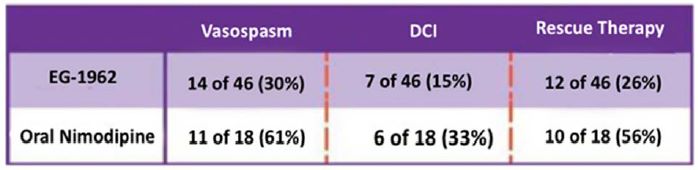
Further analysis in those patients suffering angiographic vasospasm and/or DCI noted nearly a threefold greater rate of favorable outcome (53% EG-1962 vs. 18% standard of care oral nimodipine), which we believe suggests a pleiotropic effect (meaning actions other than those for which the agent was specifically developed) of higher nimodipine levels in the CSF versus standard of care oral nimodipine.
Additionally, EG-1962 reduced ICU, and hospital LOS, which are supportive of the overall clinical outcome results seen with EG-1962 and suggest meaningful pharmacoeconomic benefits. EG-1962 demonstrated a 3.5-day reduction in ICU length of stay compared to oral nimodipine (median EG-1962 hospital LOS was 13.5 days, median oral nimodipine LOS was 17 days). In addition, EG-1962 demonstrated a 2.5 day reduction in hospital LOS compared to oral nimodipine (median EG-1962 hospital LOS was 22.5 days, median oral nimodipine hospital LOS was 25 days).
Regulatory Pathway
We expect to submit a New Drug Application, or NDA, under the FDA’s Section 505(b)(2) regulatory pathway. Section 505(b)(2) of the FD&C Act enables an applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing approved product, or published literature, in support of its NDA. In Europe, we expect to submit a marketing application to the European Medicines Agency, or EMA, using the Centralized Procedure for review and approval of EG-1962.
Intracisternal Study
A Phase 1 study of the safety, pharmacokinetics and clinical outcomes of EG-1962 administered intracisternally directly into the basal cisterns of the brain is open for enrollment for patients with an aSAH who do not receive an EVD, but remain at risk for delayed neurological complications following repair of a ruptured aneurysm. This study is a multicenter, randomized, controlled open-label study in which nine patients are expected to receive EG-1962 via intracisternal administration and three patients are expected to receive standard of care oral nimodipine. Based on our analysis of interim data from five patients, the four patients who received EG-1962 cisternally achieved steady state plasma concentrations of nimodipine over a 14-day period, with concentration levels below the levels associated with hypotension.
Commercial Strategy
Because an aSAH is a major medical emergency, patients are typically referred or immediately transported to academic or major medical centers where they are treated and monitored by a neurosurgeon or neurointensivist. If EG-1962 is approved, we plan to establish targeted commercialization and marketing capabilities for our products in the United States, Canada and possibly the European Union, or EU. In the United States and Canada, approximately 300 top academic medical centers treat approximately 90% of all aSAH patients. As such, we believe a small, targeted sales force could effectively cover these institutions and successfully commercialize our products, if approved. In the United States, we intend to commercialize by developing a hospital sales force of up to 50 representatives that would focus on academic medical centers and other major medical centers treating acute neurological conditions. We believe a similar sized sales force would be appropriate for the EU, should we decide to commercialize EG-1962 there using an Edge sales force. For commercialization of our products, if approved, outside of the United States and Canada, we may enter into collaborations or license agreements with strategic partners.
Reimbursement
Significant hospital resources are used to manage, treat and monitor patients with an aSAH. According to a study published in Neurosurgery in 2010, the average incremental direct hospital cost to treat an aSAH patient with DCI is approximately $50,000 compared to patients without DCI. In the same study, those patients suffering from DCI had an average hospital LOS of more than six days longer than those that did not experience severe complications. By contrast, patients in our NEWTON study treated with EG-1962 had a median reduction of ICU and hospital LOS of 3.5 and 2.5 days, respectively. Additionally, the lifetime cost of illness associated with chronically disabled patients presents a significant economic burden to the entire inpatient and long-term healthcare system. Further, we estimate that the average hospital charge in 2013 for a patient with an aSAH was about $300,000, and exceeds $400,000 for aSAH patients with an EVD. Accordingly, we believe the pharmacoeconomic benefits of EG-1962, if approved, can further support adoption by hospital administrators and payors and may help to justify premium pricing.
EG-1964
We are using our Precisa platform to seek to develop EG-1964 for the management of cSDH as a prophylactic treatment to prevent recurrent bleeding. EG-1964 contains synthetic aprotinin, a pancreatic trypsin inhibitor that, in its non-synthetic form, originally was obtained from bovine lung and initially marketed as Trasylol®. By way of a single administration at the time of the first neurosurgical intervention, EG-1964 may be able to deliver a high concentration of aprotinin directly to the subdural space with sustained drug exposure over 21 to 28 days. Since there are no effective ways to determine which patients are at risk for the recurrence of cSDH, we believe EG-1964, if approved, can be a prophylactic treatment for all patients with cSDH.
Background and Current Standard of Care
A cSDH is a liquefied hematoma that has accumulated on the surface of the brain in an area referred to as the subdural space. It is often caused by head trauma, most commonly in patients aged 60 or older. When the brain shrinks inside the skull over time, even minor head trauma can cause blood to leak into the subdural space. This results in a slow accumulation of blood over several days to weeks and, over time, the subdural hematoma expands by recurrent bleeding due to excess fibrinolysis, a mechanism that breaks down blood clots. Typically, the initial presentation of cSDH is managed with neurosurgical intervention during which small holes are drilled in the skull to drain the liquefied hematoma from the subdural space. Rebleeding in the subdural space occurs in up to 30% of cSDH patients, which requires a repeat neurosurgical intervention and is associated with risks of serious complications, including death. There are no therapeutic treatments currently available that reduce the risk of recurrence of cSDH. The only treatment available is to perform another neurosurgical operation that is often more extensive in order to drain the liquefied hematoma from the subdural space. If a therapeutic agent were approved to decrease the incidence of rebleeding, it could potentially be used in most patients as a prophylactic treatment.
Our Solution: EG-1964
Aprotinin, a serine protease inhibitor isolated from the lungs and pancreas which had been sold under the brand name Trasylol, was approved by the FDA in 1993 to reduce perioperative blood loss and the need for blood transfusion in patients undergoing cardiopulmonary bypass in the course of coronary artery bypass graft surgery who are at an increased risk for blood loss and blood transfusion. Plasmin, a naturally produced enzyme, breaks down blood clots. Aprotinin, by inhibiting plasmin, preserves or prevents the breakdown of the blood clot, thereby limiting recurrent bleeding. By 2000, aprotinin became the standard of care to prevent excessive bleeding in cardiac surgery. However, aprotinin is no longer used via intravenous administration as it has been withdrawn from the U.S. market due to the potential for serious side effects resulting from clotting outside of the targeted area, and the FDA has notified compounding pharmacies to cease making it available. We believe that delivery of synthetic aprotinin directly at the site of the brain injury would mitigate the potential increased risk of clotting and other systemic complications associated with the use of systemic aprotinin.
EG-1964 Commercial Strategy
Patients suffering from cSDH are typically treated in academic or major medical centers where they are monitored or managed by a neurosurgeon or neurointensivist. If both EG-1962 and EG-1964 are approved, we anticipate a large overlap in our sales force call points between EG-1962 and EG-1964. Under this scenario, we plan to increase the size of our sales force only modestly to commercialize EG-1964. We may selectively partner with third parties to commercialize our products in regions outside of the United States and Canada, or we may opt to commercialize our products in some of these regions on our own.
EG-1965
We are using our Precisa platform to seek to develop our product candidate EG-1965. EG-1965 contains amiodarone, a Vaughan Williams class III agent that (1) inhibits inward potassium current, (ii) has antisympathetic and calcium-blocking activity that leads to important effects on the sinoatrial, or SA, and atrial fibrillation, or AV, nodes, and (3) has sodium channel–inhibiting properties that increase the threshold for depolarization. EG-1965 is being developed using our Precisa development platform for the prevention of POAF. By way of a single administration at the time of the surgical intervention, we believe EG-1965 can deliver an effective concentration of amiodarone directly to the atrial myocardium with sustained drug exposure for up to seven days.
Background
POAF is the most frequent complication of cardiothoracic surgery, with an incidence varying between 20% and 60%, depending on the type of procedure and the criteria for diagnosis. It typically develops within the first five days following cardiothoracic surgery. POAF is independently associated with an increased risk of morbidity and mortality caused by stroke, heart failure, ventricular arrhythmias, thromboembolism and bleeding from anticoagulation. It significantly increases postoperative length of hospital stay, resource utilization and hospital readmission rate.
Current Standard of Care
No therapy is approved for prevention of POAF. Guidelines from the American College of Chest Physicians, the American College of Cardiology, the American Association of Thoracic Surgeons, the American Heart Association and the European Society of Cardiology recommend beta-blockers to reduce the likelihood of developing POAF or, for higher-risk patients, administration of amiodarone starting preoperatively. While amiodarone is generally regarded to be the most effective agent for preventing POAF, it is reserved as a second-line agent because of an associated risk for potentially severe systemic adverse reactions such as thyroid, pulmonary and hepatic toxicity. Although not specifically approved for this indication, amiodarone is commonly used to treat POAF and multiple studies employing systemic administration of amiodarone have demonstrated its effectiveness in preventing POAF.
Based on the lack of any approved therapies and the high morbidity and mortality and burden of illness associated with POAF, we believe this represents a major unmet medical need that requires new therapies to improve outcome of cardiothoracic surgery patients.
Our Solution: EG-1965
EG-1965, which utilizes our Precisa development platform containing amiodarone, is being developed for the potential prevention of POAF following cardiothoracic surgeries. EG-1965 is designed to be applied directly to the atrial myocardium and to release amiodarone to maintain therapeutic concentrations in the myocardium for up to seven days, sufficient to cover the period of highest risk for developing POAF.
The active pharmaceutical ingredient for EG-1965 is amiodarone. Amiodarone has been in clinical use in the US for over 30 years and in EU for over 50 years. The pharmacology of amiodarone has been exhaustively described.
We believe that sustained, local delivery of amiodarone directly to the target tissue (atrial myocardium) by EG-1965 provides a number of significant benefits versus systemic administration, including:
| ● |
Total dose of amiodarone in EG-1965 will be associated with very low circulating amiodarone concentrations near or below the lower limits of detection, thus virtually eliminating exposure of off-target tissues to potentially toxic concentrations of drug;
|
| ● |
Minimal or no interference with hepatic metabolism or transport of drugs cleared via CYP3A4, CYP2C8 or P-gp transporter mechanisms; and
|
| ● |
Effective local steady-state concentrations achieved with a single administration within hours and maintained over an appropriate/clinically relevant time period, without a loading dose, instead of the days required for systemic administration.
|
Clinical Development
The overall objective for EG-1965 is to establish it as an effective and safe treatment for preventing POAF in patients who have undergone cardiothoracic surgeries. In 2018, we intend to complete formulation development activities and commence pre-clinical studies of EG-1965. Based on the results of those studies, we may submit an IND for EG-1965 in 2019.
Commercial Strategy
Patients undergoing cardiothoracic surgery who are at risk for POAF are treated in the hospital by the cardiothoracic surgeon. Because both EG-1962 and EG-1965 are hospital-based products, we anticipate overlap in our sales force call points between the two, if both are approved. Since more hospitals perform cardiothoracic surgery than treat aneurysmal subarachnoid hemorrhage, we plan to increase the size of our sales force to commercialize EG-1965, if both it and EG-1962 are approved. We may selectively partner with third parties to commercialize our products in regions outside of the United States and Canada.
Our Precisa Development Platform
Overview
Precisa is our proprietary, programmable, biodegradable polymer-based development platform. Our Precisa platform’s proprietary nature stems from the use of our technology, know-how and trade secrets and those of our commercial partners. The Precisa platform is programmable in that it allows us to systematically vary the physical and chemical properties of formulations, such as size, shape and surface properties, as well as the type and mix of components of the formulation, in order to obtain the desired release kinetics of a specified therapeutic. For example, technology, know-how and trade secrets to which we have rights have resulted in the development of EG-1962, a formulation that is designed to utilize biodegradable PLGA microparticles to deliver a desired dose of nimodipine to the brain to improve patient outcomes following aSAH.
The Precisa development platform allows us to create polymer-based therapeutics that we believe are capable of delivering therapeutics directly to the site of injury to potentially avoid serious systemic side effects often associated with oral or intravenous delivery and to potentially enable high and sustained drug exposure with only a single dose at the initial time of procedural or surgical intervention.
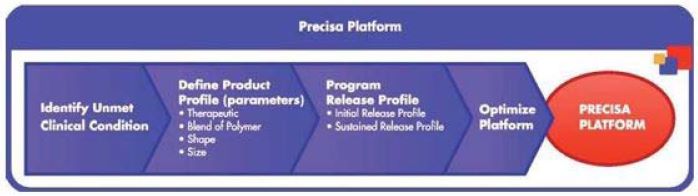
Rational Design. Once we have identified an unmet clinical condition and identified several therapeutics that may have activity against this condition, we intend to engineer multiple types of polymer-based formulations and systematically vary physical and chemical properties, such as particle size, surface properties, dose level and release profile, using an established process. We program our Precisa platform to achieve an initial and sustained release rate with effective targeting (based on form) for a particular administration given the organ or tissue target. We believe that this development platform allows us to advance a new product candidate from concept to preclinical testing in an expedited manner.
Targeted Delivery. We use our Precisa platform to design our product candidates based on specific physical and chemical properties (size, shape, surface area) that allow for one-time administration at or near the targeted injured organ or tissue. The diagram below depicts the specific form of Precisa microparticles containing nimodipine (EG-1962) that are approximately 65 microns in diameter, which is small enough to allow easy administration through an EVD, yet large enough to prevent macrophages from carrying the microparticles away from the site of injury.

Controlled and sustained drug exposure. We program our Precisa platform with a specific blend of one or more polymers in order to obtain the desired release profile of the selected therapeutic agent. This is accomplished by immersing the specified therapeutic agent in a matrix of one or more clinically-acceptable, biodegradable and biocompatible polymers. The polymeric foundation of EG-1962 is PLGA, the polymer in dissolvable sutures that has been used since the 1970’s. PLGA is biodegradable, has minimal toxicity in humans, even when used intracranially, and is one of the few matrix delivery systems where drug release can be sustained over weeks. EG-1962 is designed so that, upon administration, the therapeutic agent that is on the surface of the polymer is released to provide high early concentrations of such therapeutic agent. Subsequently, the therapeutic agent dispersed throughout the microparticle begins to diffuse through the polymer-based matrix and the polymer breaks down into lactic acid and glycolic acid, compounds naturally found in the body, in order to deliver the therapeutic with the desired release profile.
Selection of Therapeutic
We believe that we can use our Precisa development platform to incorporate therapeutics with a wide range of physicochemical properties such as small molecules and proteins. We have demonstrated in preclinical and clinical studies that nimodipine, an L-type calcium channel antagonist, manufactured into a polymer-based microparticle and suspended in diluent of sodium hyaluronate, provided differentiated pharmacokinetics. We are also using our Precisa development platform to formulate both EG-1964 and EG-1965.
Intellectual Property
The protection of our product candidates, our manufacturing methods, delivery systems and patient treatment protocols, and associated trade secrets and know-how are important to our business. We have sought patent protection in the United States and internationally relating to EG-1962, our lead product candidate, a microparticulate formulation of nimodipine; EG-1964, which utilizes our Precisa development platform containing aprotinin; and EG-1965, which utilizes our Precisa development platform containing amiodarone. Our policy is to seek, maintain and defend patent rights, whether developed internally or in-licensed, and to protect technologies, improvements and trade secrets that may be important to our business.
Our commercial success will depend in part upon obtaining and maintaining patent and trade secret protection for our current and future product candidates, including components of our proprietary formulations, methods of manufacturing our product candidates, delivery systems, and methods of treating patients with our product candidates, as well as successfully defending our patent rights against third party challenges. Our ability to prevent or stop third parties from making, using, selling, offering to sell or importing our product candidates will depend in part upon whether we have valid and enforceable patent rights that cover the activities of third parties.
Patent Rights
We have been building and plan to continue to build our patent portfolio. Where possible, we pursue multi-tiered patent protection for our product candidates and their manufacture, delivery and use. In addition to filing and prosecuting patent applications in the United States, we file counterpart patent applications in various countries and regions where we think such foreign filing is likely to be cost-effective.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing of a non-provisional patent application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the United States Patent and Trademark Office, or USPTO, in granting a patent. However, the term of a United States patent may be shortened, if the patent term for a patent is terminally disclaimed by its owner, over another patent.
Patent Rights Associated with EG-1962
We wholly-own one issued U.S. patent (expected to expire in 2029 if all maintenance fees are paid) directed to a method of treating a cerebral vasospasm in a human by administering a pharmaceutical composition via surgical injection into the subarachnoid space in a cistern closest to a cerebral artery at risk for vasospasm. We also have been granted patent protection for this invention in Australia (three patents granted), Canada, China, Israel, Japan, Korea (three patents granted), New Zealand (two patents granted) and Singapore. We are seeking patent protection in China, Europe and Hong Kong.
We have a wholly-owned U.S. patent application directed to a method of treating, and a microparticulate delivery system for treating, a delayed complication associated with brain injury where the brain injury includes interruption of at least one cerebral artery. Patent protection for this invention has been granted in Australia, Europe, Japan, New Zealand, Russia, Singapore, and the United Kingdom; a notice of allowance has issued in Israel. Patent protection is pending in Brazil, Canada, China (two applications), Hong Kong, and Korea.
We also have a wholly-owned U.S. patent (expected to expire in 2029 if all maintenance fees are paid) claiming a method of treating a cerebral artery in the subarachnoid space of a human at risk of interruption due to a brain injury by administering locally a microparticulate composition into a cerebral ventricle. A related patent application claiming the composition is pending in the United States. Patent protection for these inventions has been granted in China, Europe, New Zealand (two patents), Russia, and Singapore. We have received a notice of allowance in Australia. Further, we are seeking patent protection for these inventions in Brazil, Canada, Hong Kong, Israel, Japan (two applications pending), Korea and the United Kingdom.
We have two wholly-owned U.S. patent applications directed to (i) a sustained release microparticulate composition having particular release kinetics of the therapeutic agent dispersed in the composition that can be administered intracisterally, intraventricularly, or intrathecally and (ii) a method for treating a cerebral artery at risk of interruption due to a subarachnoid hemorrhage in a human by administering such microparticulate composition. This application has been granted in New Zealand. We are also seeking patent protection for these inventions in Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, Japan, Korea, Russia, and the United Kingdom.
We wholly own one pending U.S. application and one international PCT application directed to site-specific microparticulate pharmaceutical formulations comprising (a) a therapeutic amount of an L-type voltage-gated calcium channel antagonist; (b) a poly(DL-lactide-co-glycolide) (PLGA) polymer comprising from 25% to 50% glycolide; and (c) less than 5% hyaluronic acid, wherein the microparticles of the formulation are characterized by a particle size from about 20 μm to about 125 μm; (ii) a drug load of from about 50% to about 70%; (iii) sustained release; and (iv) at least 99% purity, and their use for treating a delayed complication of a brain injury associated with interruption of a cerebral artery comprising a delayed cerebral ischemia that is effective to reduce incidence of poor clinical outcome. The 30 month date for the PCT application to enter the national stage is October 7, 2018.
In addition to the foregoing, we have used our Precisa development platform, in collaboration with Evonik Industries, or Evonik, to seek to develop pharmaceutical compositions that contain particular polymorphic forms of nimodipine. Based on the collaboration, we co-own, together with Evonik, two issued U.S. patents claiming a process for producing microparticles encapsulating a particular polymorphic form of nimodipine, a semisolid delivery system containing microparticles comprising the particular polymorphic form of nimodipine, and a method of treating a cerebral artery in a subarachnoid space at risk of interruption due to a brain injury using such a delivery system. These patents are expected to expire in 2033 if all maintenance fees are paid. We also co-own, with Evonik, related patents granted in Australia and Canada, and a patent application that has received a notice of allowance in Korea, as well as patent applications pending in Brazil, China, Europe, Hong Kong, India, Israel, Japan, Russia, Singapore, and the United Kingdom relating to these technologies. The issued U.S. patents cover the microparticulate formulation used in the NEWTON study. Evonik, as successor to SurModics Pharmaceuticals, Inc., or SurModics, under our license agreement initially with SurModics, has granted us an exclusive, field-restricted, worldwide, royalty-bearing license under its patent rights together with enforcement rights against infringers, all pursuant to our license agreement with Evonik relating to the co-owned patent rights. The Evonik license agreement is discussed in more detail below.
We have two pending U.S. patent applications claiming a scalable process for producing microparticles encapsulating a particular polymorphic form of nimodipine (different from the form described in the applications co-owned with Evonik), a composition formulated for delivery by injection using this form of nimodipine, a micronized version of such composition, and a method of reducing the severity or incidence of delayed complications associated with a brain injury by administering the composition locally either intraventricularly, intracisternally, or intrathecally. An international patent application has been filed covering the inventions claimed.
We also have one U.S. and 11 foreign applications pending that claim a method for at least one adverse consequence of an eye disease comprising abnormal intraocular pressure, retinal vascular disease and retinal ganglion cell death in order to reduce visual loss in a human subject in need thereof comprising administering a therapeutic amount of a flowable microparticulate pharmaceutical composition intraocularly to a site of delivery selected from the group consisting of a vitreous humor of the eye, an aqueous humor of the eye, and or both.
Patent Rights Associated with EG-1964
With respect to EG-1964, we wholly-own one issued U.S. patent (scheduled to expire in 2031 if all maintenance fees are paid) directed to a method of treating hematoma expansion or recurrent bleeding resulting from a hemorrhagic condition (e.g., cSDH) by administering a pharmaceutical composition comprising an anti-fibrinolytic agent (e.g., aprotinin). We also have a pending U.S. patent application where we are pursuing claims to a pharmaceutical composition containing an anti-fibrinolytic agent. Patents have been granted in Australia (two patents, one notice of allowance), Japan, New Zealand, and Singapore. We are also seeking additional patent protection in Brazil, Canada, China, Europe, Hong Kong, Israel, Japan, Korea, Singapore, Russia, and the United Kingdom.
Patent Rights Associated with EG-1965
With respect to EG-1965, we have filed a U.S. non-provisional patent application directed to (i) a malleable drug delivery system comprising a particulate formulation, containing an anti-arrhythmic agent, that is characterized by its ability to contact, adhere to, and/or conform to the tissue surface susceptible to atrial fibrillation, and (ii) a method for reducing atrial fibrillation by using such delivery system. We expect to file additional patent applications in other countries and regions at the appropriate time.
Trade Secrets
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, for some aspects of our proprietary technology, trade secret protection is more appropriate than patent protection. However, trade secrets and know-how can be difficult to protect. We seek to protect our proprietary technologies, for example, our manufacturing processes, via, among other things, confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, and commercial partners. We also seek to preserve the confidentiality of our trade secrets and know-how by implementing and maintaining security of our premises and information, and limiting access to our trade secrets and know-how.
License Agreement with Evonik
In October 2010, we entered into a license agreement with SurModics, the predecessor to Evonik (the “Evonik Agreement”). Under the Evonik Agreement, we have an exclusive (even as to Evonik), worldwide, sublicensable and royalty-bearing license under certain Evonik patent rights (including patent rights jointly owned with Edge, the Evonik rights of which are exclusively licensed back to Edge) and know-how to develop, make, have made, use offer for sale, sell, export and import one or more specified active agents, including nimodipine, in a proprietary polymer-based formulation for intracranial delivery to prevent or treat delayed complications following intracranial hemorrhage. Depending on the manufacturing process we use, EG-1962 may be covered by Evonik patent rights (including patent rights jointly owned with Edge, the Evonik rights of which are exclusively licensed back to Edge) and/or covered by Evonik know-how.
Under the terms of the Evonik Agreement, in connection with EG-1962, we may be obligated to make milestone payments totaling $14.75 million. We made an initial upfront payment to Evonik upon execution of the Evonik Agreement in 2010, and made a second milestone payment in July 2016. We are obligated to make additional milestone payments upon achievement of certain development, regulatory and commercial milestone events for each product covered by the Evonik Agreement (an “Evonik Licensed Product”). In addition, we are obligated to pay a mid-single-digit percentage on net sales of Evonik Licensed Products, subject to reduction for certain specified circumstances. Our royalty obligations for each Evonik Licensed Product will continue, on a country by country basis, for the longer of twelve years from the first commercial sale of the Evonik Licensed Product if there is no valid claim that covers the manufacture, use or sale of the Product, or the period of time during which the manufacture, use or sale of the Evonik Licensed Product is covered by a valid claim of a licensed Evonik patent. In addition, we agreed to pay Evonik 15% of any consideration received from a sublicense of the licensed Evonik intellectual property rights, which does not include any royalties on sales, funds received for research and development or proceeds from any equity or debt investment.
We are obligated to use commercially reasonable efforts to develop and obtain regulatory approvals to market each Evonik Licensed Product in major markets throughout the world and to maximize net sales after receipt of such approvals, as well as to achieve certain specified development, regulatory and commercial milestones.
In September 2015, we and Evonik entered into Amendment No. 1 to the Evonik Agreement. This amendment clarified our obligations to pay Evonik certain royalty and milestone payments in respect of Evonik Licensed Products whether or not manufactured by Evonik and removed our obligation to negotiate exclusively with Evonik for Phase 3 and commercial supply of EG-1962.
The term of the Evonik Agreement, as amended, will continue until the expiration of our obligation to pay royalties to Evonik. Either party may terminate the Evonik Agreement due to material breach by the other party. Evonik may terminate the license agreement or convert it to a non-exclusive license, in either case upon giving us written notice, if we fail to use commercially reasonable efforts to hit certain specified development, regulatory and commercial milestones with respect to Evonik Licensed Products.
We also have certain manufacturing licenses from Oakwood Laboratories, L.L.C., as described below.
Manufacturing
Product candidates using our Precisa development platform are manufactured using a single-step emulsion process with well-defined and reproducible operations. We do not own or operate current Good Manufacturing Practice, or cGMP, compliant manufacturing facilities for the production of any of our product candidates and we do not have plans to develop our own manufacturing operations in the foreseeable future. We currently rely on third party contract manufacturing organizations, or CMOs, to produce the amounts of our product candidates necessary for our preclinical research, clinical studies, and planned commercial activities. As part of the manufacture and design process for our product candidates, we rely on internal, scientific and manufacturing know-how and trade secrets and the know-how and trade secrets of third party manufacturers. We currently employ highly qualified internal manufacturing and quality assurance resources to manage our manufacturing contractors.
Oakwood Amended and Restated Master Formulation Development Agreement
In June 2017, we entered into an Amended and Restated Master Formulation Development Agreement (the “Restated Development Agreement”) with Oakwood Laboratories, L.L.C. (“Oakwood”), pursuant to which Oakwood will continue to provide us with certain drug formulation development and non-commercial manufacturing services for EG-1962, in accordance with project plans to be entered into from time to time. Oakwood is currently performing process engineering, optimization and other scale up activities for us and is currently the sole manufacturer of EG-1962 used in Edge’s ongoing NEWTON 2 Phase 3 pivotal study for EG-1962.
Under the Restated Development Agreement, we agreed to pay Oakwood to perform services under agreed upon project plans and to pay Oakwood up to an aggregate of $4.5 million. In July 2017, we paid $1.5 million of such aggregate amount in connection with entering into the Restated Development Agreement. Of the remaining $3.0 million of such aggregate amount, $0.5 million is payable no later than April 1, 2018 and $2.5 million is payable no later than April 1, 2019. These remaining payments may be accelerated if: (i) we achieve various regulatory milestones, (ii) we close an equity or similar financing in excess of a predetermined amount, or (iii) there is an early termination, under certain circumstances, of the Restated Development Agreement or the Supply Agreement with Oakwood.
As additional consideration for performance under the Restated Development Agreement and the Supply Agreement (as defined below), we agreed to pay Oakwood a royalty, during the Royalty Term, in an amount equal to a low single digit percentage of net sales of EG-1962, regardless of the manufacturer or supplier thereof. The “Royalty Term” is the period commencing upon the commercial launch of EG-1962 by the Company and continuing until twelve (12) years following such launch.
The term of the Restated Development Agreement continues until the expiration or termination of the Supply Agreement, unless earlier terminated (the “Term”). We have the right to terminate project plans upon the occurrence of various circumstances described in the Restated Development Agreement. In the event that we terminate the most recent project plan prior to completion (including due to our decision to discontinue the development or commercialization of EG-1962), we must pay to Oakwood a termination fee. Either party has the right to terminate the Restated Development Agreement upon sixty (60) days written notice for failure by the other party to cure a material breach during the applicable cure period. We can terminate the Restated Development Agreement immediately upon notice to Oakwood if Oakwood’s annual financial audit report required to be provided to us contains any going concern or similar qualification or if Oakwood fails to maintain a pre-determined working capital ratio. Either party may also terminate the Restated Development Agreement immediately in the event of certain failures with regard to validation of the manufacturing process and other specified regulatory failures.
Oakwood Manufacturing and Supply Agreement
Concurrent with our entry into the Restated Development Agreement, in June 2017, we entered into a Manufacturing and Supply Agreement with Oakwood (the “Supply Agreement”), pursuant to which Oakwood will manufacture and supply, and we will purchase from Oakwood, EG-1962 in commercial quantities following the commercial launch of the product.
Pursuant to the Supply Agreement, the price per unit of EG-1962 to be purchased by us is based on the expected commercially usable units per batch. In addition, we have agreed to pay Oakwood milestone payments that could total up to an aggregate of $2.25 million upon the achievement of certain development and regulatory milestones.
We will have no minimum order requirement under the Supply Agreement until the third (3rd) year following commercial launch of EG-1962. Beginning in the third year following commercial launch and continuing until the fifth year following commercial launch, we will be required to (x) order, at a minimum, the greater of (a) five (5) batches and (b) fifty percent (50%) of the aggregate vials of EG-1962 purchased by us from all sources in such year (such greater amount being the “Minimum Order Commitment”) or (y) pay a catch-up price to Oakwood based on the amount of EG-1962 actually ordered by us during the applicable time period.
The term of the Supply Agreement will continue (unless earlier terminated) until three (3) years following commercial launch of EG-1962. Thereafter, the Supply Agreement will automatically renew for additional two (2)-year periods unless we provide notice of non-renewal at least twelve (12) months prior to the end of the then-current term. The Supply Agreement will also terminate automatically upon the termination of the Restated Development Agreement for any reason. Following the first anniversary of the commercial launch of EG-1962, either party may terminate upon two (2) years written notice. Further, either party may terminate the Supply Agreement upon a material breach by the other party that fails to be cured in the applicable cure period.
We may terminate the Supply Agreement immediately upon notice to Oakwood in the event that (a) any regulatory authority requires or causes the withdrawal of EG-1962 from the market or (b) we cease to develop or commercialize EG-1962; provided, that in the latter case of termination prior to completion of the most recent project plan attached to the Restated Development Agreement, we must pay to Oakwood a termination fee.
Other Third Party Manufacturers
EG-1962 is a polymer-based microparticle containing nimodipine suspended in a diluent of sodium hyaluronate. We have entered into long-term supply agreements with separate companies that provide polymer, nimodipine and sodium hyaluronate to ensure access to drug substance, diluent and EG-1962 microparticles for commercial supply purposes. We currently have access to sufficient drug substance of EG-1965 for formulation and our planned pre-clinical studies and are in the process of developing cGMP product for EG-1965 acceptable for use in future clinical studies.
Competition
Generally, our industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We may face competition from a number of sources, including pharmaceutical and biotechnology companies, drug delivery companies and academic and research institutions. Many of our potential competitors have substantially greater financial, technical and human resources than we do, as well as more experience in the development of product candidates, obtaining FDA and other regulatory approvals of products, and the commercialization of those products. Consequently, our competitors may develop products for the treatment of indications we are pursuing or may pursue in the future, and such competitors' products may be more effective, better tolerated or less costly than our product candidates. Our competitors may also be more successful in manufacturing and marketing their products than we are. We will also face competition in recruiting and retaining qualified personnel and establishing clinical study sites and patient enrollment in clinical studies.
To our knowledge, there is limited competition and are limited product candidates under development to improve patient outcomes after aSAH in the current marketplace. In the United States, only oral forms (gelcaps or solution) of nimodipine are approved for marketing for aSAH. We are aware that Grace Therapeutics, LLC, or Grace Therapeutics, has announced that it plans to submit an NDA for an intravenous infusion form of nimodipine in the first half of 2018 and that Idorsia Ltd. has announced that it expects to initiate a Phase 3 study evaluating the safety and efficacy of clazosentan in an aSAH population enriched for the risk of cerebral vasospasm in 2018. In addition, in the United States, sodium nitrite has been tested by Hope Pharmaceuticals, Inc., or Hope Pharmaceuticals, in a Phase 2 trial in 19 patients. In 2017, Hope Pharmaceuticals terminated an additional Phase 2 trial after enrolling 6 patients.
In Europe, both intravenous and oral forms of nimodipine are available. In Japan and some parts of Asia, fasudil (Eril ®) and ozagrel (a thromboxane synthetase inhibitor, known by various brand names) are available.
Government Regulation
Government authorities in the United States, at the federal, state and local levels, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug products. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific to each regulatory authority, submitted for review and approved by the regulatory authority.
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The FD&C Act and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, record-keeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical studies to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation, and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product. The conduct of certain preclinical tests must comply with federal regulations and requirements, including good laboratory practice, or GLP, and good manufacturing practice, or GMP, requirements. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither raised objections on nor placed the IND on hold within this 30-day period, the clinical study(ies) proposed in the IND may begin.
Clinical studies generally involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical studies must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice, or GCP, requirements, which are a collection of FDA and international standards meant to protect the rights, health and safety of patients, and to define the roles of sponsors, administrators, and monitors, as well as ensure data integrity; and (iii) under protocols detailing the objectives of the study, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
Satisfaction of FDA pre-market development requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease.
The submission of an NDA is required to introduce a new drug product into the U.S. market. It is the responsibility of the company seeking to market a drug to test it and submit evidence that it is safe and effective for the proposed labeled indication and population. The FDA and their scientists reviews the sponsor's NDA containing the data and proposed labeling.
The goals of the NDA are to provide enough information to permit an FDA reviewer to reach the following key decisions:
| ● |
Whether the drug is safe and effective in its intended use(s), and whether the benefits of the drug outweigh the risks.
|
| ● |
Whether the drug's proposed labeling (package insert) is appropriate, and what it should contain.
|
| ● |
Whether the methods used in manufacturing the drug and the controls used to maintain the drug's quality are adequate to preserve the drug's identity, strength, quality, and purity.
|
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the FDA’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of an NDA. Most such applications for standard review drug products are reviewed within ten to twelve months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to an application for a drug that treats a serious condition and that the FDA determines, if approved, would provide a significant improvement in safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions when compared to standard applications. The review process may be extended by FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
In assessing whether to approve an NDA, the FDA may request advice from an advisory committee. Advisory committees provide independent advice and recommendations to the FDA on scientific and technical matters related to the development and evaluation of products regulated by the FDA. Committee members are scientific experts such as physician-researchers and statisticians, and, on an ad hoc basis, may also include representatives of the public, including patients. Although the committees provide recommendations to the FDA, final decisions are made by FDA.
Section 505(b)(2) NDAs
Section 505(b)(2) of the FD&C Act enables the applicant to rely, in part, on FDA’s findings of safety and effectiveness in approving a similar product or published literature in support of its application. A Section 505(b)(2) NDA is one that contains full reports of investigations of safety and effectiveness but where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference.
Section 505(b)(2) NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. If the Section 505(b)(2) applicant can establish that reliance on FDA’s previous approval is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or assessments to support the change from the approved product. The 505(b)(2) pathway may be used to seek approval of the new product candidate for all, or some, of the label indications for which the referenced product has been approved, as well as for new indication(s) for which the referenced product is not approved. For the latter, FDA would likely require clinical studies to support approval of the product candidate for use in the new indication(s).
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book. The Orange Book identifies drug products approved on the basis of safety and effectiveness by the FDA under the FD&C Act. Thus, approval of a Section 505(b)(2) NDA can be delayed until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification that the listed patent is invalid or not infringed and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Expedited Review and Approval Programs
Several FDA programs are available that are intended to facilitate and expedite development and review of new drugs to address unmet medical need in the treatment of a serious or life-threatening condition. The four principal programs are Fast Track designation, breakthrough therapy designation, accelerated approval, and priority review designation. The Fast Track program is intended to expedite or facilitate the process for reviewing new drugs that meet certain criteria. Specifically, a new drug is eligible for Fast Track designation if (i) it is intended to treat a serious or life-threatening disease or condition for which there is no effective treatment and demonstrates the potential to address unmet medical needs for the condition, or (ii) it has been designated as a qualified infectious disease product. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. Potential benefits of Fast Track designation include, but are not limited to, more frequent meetings with FDA to discuss the drug’s development plan and ensure collection of appropriate data needed to support approval, and rolling review, which, in the case of Fast Track designation, means that if, based on preliminary evaluation of clinical data submitted by the sponsor, FDA determines that a Fast Track-designated drug may be effective, FDA may consider reviewing portions of the marketing application before the sponsor submits the complete application. Obtaining Fast Track designation does not, however, change the evidentiary standards for NDA approval.
Another program intended to expedite development and review of new drugs is the accelerated approval program. In 2012, Congress passed the Food and Drug Administration Safety Innovations Act, or FDASIA. Section 901 of FDASIA amends the FD&C Act to allow the FDA to base accelerated approval for drugs for serious or life-threatening conditions on whether the drug has an effect on a surrogate or an intermediate clinical endpoint.
A surrogate endpoint used for accelerated approval is a marker, such as a laboratory measurement, radiographic image, physical sign or other measure, that is thought to predict clinical benefit, but is not itself a measure of clinical benefit. Likewise, an intermediate clinical endpoint is a measure of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a drug, such as an effect on irreversible morbidity and mortality, or IMM.
The FDA bases its decision on whether to accept the proposed surrogate or intermediate clinical endpoint on the scientific support for that endpoint. Studies that demonstrate a drug’s effect on a surrogate or intermediate clinical endpoint must be “adequate and well controlled” as required by the FD&C Act.
Using surrogate or intermediate clinical endpoints can save valuable time in the drug approval process. The drug company will still need to conduct studies to confirm that the surrogate or intermediate endpoint actually predicts the clinical benefit. These studies are known as Phase 4 confirmatory trials.
Where confirmatory trials verify clinical benefit, FDA will generally terminate the requirement. Approval of a drug may be withdrawn or the labeled indication of the drug changed if trials fail to verify clinical benefit or do not demonstrate sufficient clinical benefit to justify the risks associated with the drug (e.g., show a significantly smaller magnitude or duration of benefit than was anticipated based on the observed effect on the surrogate).
Breakthrough therapy designation is designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint. For the purposes of this designation, clinically significant endpoint generally refers to an endpoint that measures an effect on irreversible morbidity or mortality or on symptoms that represent serious consequences of the disease. A drug that receives this designation is eligible for all Fast Track designation features, and intensive guidance on an efficient drug development program, including a commitment for FDA senior managers' involvement.
Additional measures intended to expedite drug product development and review were also included in the recently enacted 21st Century Cures Act, or the Cures Act. Signed into law on December 13, 2016, the Cures Act includes several provisions intended to enhance and accelerate the FDA’s processes for reviewing and approving new drugs and supplements to approved NDAs. These include, but are not limited to, provisions that (i) require FDA to establish a program to evaluate the potential use of real world evidence to help to support the approval of a new indication for an approved drug and to help to support or satisfy post-approval study requirements, (ii) require FDA to issue guidance for purposes of assisting sponsors in incorporating complex adaptive and other novel trial designs into proposed clinical protocols and applications for new drugs, and (iii) provide that FDA may rely upon qualified data summaries to support the approval of a supplemental application with respect to a qualified indication for an already approved drug.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, Risk Evaluation and Mitigation Strategies, or REMS, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. If at any time the FDA becomes aware of new information regarding the safety of an approved product, the FDA may issue an early public safety alert that makes initial recommendations in light of the new information until the FDA fully evaluates the information and makes final conclusions and recommendations. The FDA may also require manufacturers to change product labeling to address the new safety concerns.
In addition, quality-control, drug manufacture, packaging, and labeling procedures must continue to conform to cGMP after approval. Drug manufacturers and their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the FDA inspects manufacturing facilities to assess compliance with cGMP. Submission of a NDA would further expose our CMOs to a focused pre-approval inspection by the FDA or other regulatory health authority. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality assurance to maintain compliance with cGMP. Regulatory authorities may take regulatory action (e.g. Warning Letter, Consent Decree, Import Alert, etc.) if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
The Hatch-Waxman Amendments to the FD&C Act
Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application, or ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through testing to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing based on in vitro studies and/or human clinical studies, ANDA applicants are not required to conduct, or submit results of, additional pre-clinical or clinical tests to prove the safety or effectiveness of their drug product. Some ANDA applicants may apply for and be granted a waiver for the requirement to conduct in vivo bioequivalence studies. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug, and, subject to state laws, can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that: (i) a patent which claims the drug for which the applicant submitted the application or which claims a method of using such drug has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the ANDA applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the referenced product has expired.
Patent Term Extension and Marketing Exclusivity
Under the Hatch-Waxman Amendments, a portion of a product’s U.S. patent term that was lost during clinical development and regulatory review by the FDA may be restored as a patent term extension. The Hatch-Waxman Amendments also provide for a statutory protection, known as non-patent exclusivity, against the FDA’s acceptance or approval of certain competitor applications.
After NDA approval, owners of relevant drug patents may apply for up to a five-year patent extension. The allowable patent term extension is calculated as half of the drug’s testing phase - the time between IND application and NDA submission – and all of the review phase – the time between NDA submission and approval up to a maximum of five years. The time can be shortened if FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the patent term extension may not exceed 14 years.
For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the USPTO must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted. Only one patent applicable to an approved drug is eligible for the extension and the extension must be applied for prior to expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Market exclusivity provisions under the FD&C Act also can delay the submission or the approval of certain applications. The FD&C Act provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA, or a Section 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FD&C Act also provides three years of marketing exclusivity for an NDA, Section 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan Drugs
Under the Orphan Drug Act of 1983, or the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition – generally a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. The first NDA applicant to receive FDA approval for a particular drug to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market any drug considered the same drug as the drug with the orphan drug exclusivity for the same disease, except in limited circumstances, such as if the second applicant demonstrates the clinical superiority of its product to the product with orphan drug exclusivity through a demonstration of superior safety, superior efficacy, or a major contribution to patient care. In addition, if a company seeks orphan drug designation for a drug considered the same drug as a drug previously approved for the orphan indication at issue, the FDA will not designate the same drug as an orphan drug unless the company articulates a plausible hypothesis of the clinical superiority of its drug to the approved drug, and, following such designation, if the previously approved drug has unexpired orphan drug exclusivity, FDA will not approve the subsequent drug unless the sponsor demonstrates clinical superiority over the previously approved drug prior to approval.
In addition, as FDA has interpreted the Orphan Drug Act, even if a previously approved same drug does not have unexpired orphan exclusivity, while a demonstration of clinical superiority is not required for a subsequent orphan-designated drug to obtain marketing approval, a demonstration of clinical superiority is required for the subsequent orphan-designated same drug to be awarded a 7-year period of orphan exclusivity upon marketing approval. In recent years, there have been multiple legal challenges to this FDA interpretation, and in August 2017, Congress amended the orphan drug provisions of the FD&C Act through enactment of the FDA Reauthorization Act of 2017 to codify FDA’s longstanding interpretation. Section 527 of the FD&C Act now expressly provides that if a sponsor of a drug that is designated as an orphan drug and is otherwise the same as an already approved drug is seeking exclusive approval for the same rare disease or condition as the already approved drug, FDA shall require such sponsor, as a condition of such exclusive approval, to demonstrate that such drug is clinically superior to any already approved or licensed drug that is the same drug.
Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee. Edge has received orphan drug designation for EG-1962 for the treatment of subarachnoid hemorrhage.
Pediatric Information
Under the Pediatric Research Equity Act, or PREA, NDAs or supplements to NDAs must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. Recently, FDASIA amended the FD&C Act to require that a sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within 60 days of an end-of-Phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical studies, and/or other clinical development programs. Since EG-1962 has been granted orphan drug designation by the FDA Office of Orphan Product Development, or OOPD, it is exempt from the requirements of the PREA.
The Best Pharmaceuticals for Children Act amended the FD&C Act to provide NDA holders a six-month extension of any exclusivity–patent or non-patent–for a drug if certain conditions are met. Conditions for exclusivity include the FDA’s determination that information relating to the use of a new drug in the pediatric population may produce health benefits in that population, the FDA making a written request for pediatric studies as outlined in the FD&C Act, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated products, including drugs, are required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov. Similar requirements apply under the European Union Clinical Trials Directive. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. The European Union Clinical Trials Regulation which comes into force in 2018 will broaden the information that will be publicly available to include all information submitted in the clinical trial application and during the assessment procedure with the exception of information that falls within specific categories. In a rule issued on September 16, 2016 that went into effect on January 18, 2017, the Department of Health and Human Services, or HHS, clarified and expanded the clinical trial reporting requirements. The rule broadens the submission of additional registration and summary results information, and sets forth legal consequences for non-compliance. Notably, unless a waiver is granted, the rule requires submission of results information for studies not only where a drug is approved by the FDA, but also where a drug is unapproved, regardless of whether FDA approval is or will be sought. Although prior to enactment of the rule, disclosure of results for trials of unapproved drugs could be delayed until approval, the new rule generally limits the allowable delay period for results information submission for applicable clinical trials of unapproved drugs to two years after the submission of a certification (i.e., up to a total of three years after the primary completion date). Thus, for the first time, extensive results information must be posted even for drugs that do not make it to market. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs or to request detailed records from FDA under the Freedom of Information Act.
In January 2018, FDA initiated a pilot program to evaluate whether disclosing certain information included within clinical study reports (CSRs) for pivotal studies for approximately nine NDAs improves public access to drug approval information. As part of this pilot, FDA plans to post on their website the parts of the CSRs that were most important to the FDA’s assessment of the safety and efficacy of the drug. Once the pilot program is complete, FDA will seek public feedback on further steps.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we may obtain regulatory approval. In the United States, sales of any products for which we receive regulatory approval for commercial sale will depend in large part on the availability of coverage and reimbursement from third party payors. Third party payors include Federal and State healthcare programs, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the reimbursement rate that the payor will pay for the drug product and the patient or hospital cost-sharing amount. Third party payors or hospitals may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. Moreover, a payor’s decision to provide coverage for a drug product does not imply that adequate reimbursement will be provided for the product. In addition, we expect that our products may be used primarily in the inpatient hospital setting; products that are used in the inpatient hospital setting may be reimbursed by payors through a fixed payment for the hospital stay, or a similar type of “bundled” or “capitated” payment, that does not include a separate charge for drugs provided to the patient, which can discourage hospitals from furnishing higher-cost drugs to inpatients in some cases. While mechanisms such as stop loss payments, New Technology Add on Payments (NTAP), and outlier payments exist to allow hospitals to seek additional reimbursement from payers, it is not guaranteed that adequate third party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Third party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain adequate use, coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective. If third party payors do not consider a product to be cost-effective compared to other available therapies, they may cover the product after approval as a benefit under their plans or, if they do, may only provide coverage at a reduced price or under severe restrictions on the types of patients they choose to use the product.
The U.S. government and state legislatures have shown significant interest in implementing cost containment initiatives to limit the growth of Federal and State healthcare program costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. By way of example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the Affordable Care Act, contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries, annual fees based on pharmaceutical companies’ share of sales to federal health care programs, and measures that accelerate the testing and expansion of new payment methods that may increase healthcare providers’ incentives to make treatment decisions that contain costs. Adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third party payors fail to provide adequate coverage and reimbursement. In addition, the emphasis on cost containment measures in the United States has increased and we expect this trend will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates (including new payment methods that may discourage healthcare providers from using higher-cost products) may be implemented in the future.
Starting in 2014, the Affordable Care Act also expanded health insurance coverage to many previously uninsured Americans, through a combination of federal subsidies for lower-income people who enrolled in health plans through health insurance Exchanges and enabling States to expand Medicaid eligibility with the federal government paying a high share of the cost. President Trump and Congressional leaders have expressed interest in repealing these Affordable Care Act provisions and replacing them with alternatives that may be less costly and provide state Medicaid programs and private health plans more flexibility. There have been several U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. In December 2017, President Trump signed into law the Tax Cuts and Jobs Act of 2017, which includes a repeal of the individual mandate under the Affordable Care Act. Additionally, in October 2017, President Trump signed an Executive Order directing federal agencies to review regulations applicable to association health plans and short-term health insurance, and announced that the administration would halt federal subsidies to insurance plans under the Affordable Care Act. It is possible that other repeal and replacement initiatives, if enacted into law, could ultimately result in fewer individuals having health insurance coverage and/or in individuals having insurance coverage that provides less generous benefits.
Pharmaceutical companies are increasingly entering into agreements with US payers for "risk-based" or "outcomes-based" pricing contracts for novel, premium-priced pharmaceutical products. By the time EG-1962 is launched, these innovative pricing approaches may be expected as a means of obtaining use, coverage and reimbursement of the product, and may be incorporated into Edge's strategy to market the product to provide benefit to the greatest number of patients.
Other Healthcare Laws and Compliance Requirements
If we obtain regulatory approval of our products, we may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include, but are not limited to:
| ● |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs;
|
| ● |
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third party payers that are false or fraudulent;
|
| ● |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
|
| ● |
the federal transparency laws, including the federal Physician Payment Sunshine Act, that requires drug manufacturers to disclose payments and other transfers of value provided to physicians and teaching hospitals;
|
| ● |
HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information;
|
| ● |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; and
|
| ● |
state price transparency laws, such as the California state law requiring pharmaceutical manufacturers to report certain price increases, signed into law October 2017 by California Governor Jerry Brown.
|
The Affordable Care Act broadened the reach of the fraud and abuse laws by, among other things, amending the intent requirement of the federal Anti-Kickback Statute and the applicable criminal healthcare fraud statutes contained within 42 U.S.C. § 1320a-7b. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Patient Protection and Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act or the civil monetary penalties statute. Many states have adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The False Claims Act, or FCA, prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including biological products, that are false or fraudulent. Although we would not submit claims directly to payers, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state, and third party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, pharmaceutical companies have been prosecuted under the FCA in connection with their off-label promotion of drugs. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties of between $5,500 and $11,000 for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the FCA is a civil statute, conduct that results in a FCA violation may also implicate various federal criminal statutes. In addition, private individuals have the ability to bring actions under the FCA and certain states have enacted laws modeled after the FCA.
Patient Protection and Affordable Care Act
In March 2010, the Patient Protection and Affordable Care Act, or PPACA, was enacted, which includes measures that have or will significantly change the way health care is financed by both governmental and private insurers. Among the provisions of PPACA of greatest importance to the pharmaceutical industry are the following:
| ● |
The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of HHS as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Effective in 2010, PPACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and biologic agents from 15.1% of average manufacturer price, or AMP, to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. The PPACA also expanded the universe of Medicaid utilization subject to outpatient drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization as of 2010 and by expanding the population potentially eligible for Medicaid drug benefits, to be phased-in by 2014. The Centers for Medicare & Medicaid Services, or CMS, issued a regulation that would have expanded Medicaid rebate liability to the territories of the United States as well as of April 2017, but that decision has now been delayed. In addition, legislation predating the PPACA provides for the public availability of retail survey prices and, for multiple source drugs, certain weighted average AMPs under the Medicaid program. The implementation of this requirement by the CMS may also provide for the public availability of data on average pharmacy acquisition cost, which potentially could negatively impact our sales.
|
| ● |
In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs, the manufacturer must extend discounts on outpatient drugs to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. Effective in 2010, the PPACA expanded the types of entities eligible to receive discounted 340B pricing, although, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase.
|
| ● |
Effective in 2011, the PPACA imposed a requirement on manufacturers of branded drugs and biologic agents to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., “donut hole”).
|
| ● |
Effective in 2011, the PPACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications.
|
| ● |
The PPACA required pharmaceutical manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers were required to begin tracking this information in 2013 and to report this information to CMS on an annual basis beginning in 2014. The reported information was publicly available in a searchable format on a CMS website in September 2014 and will be made publicly available on an annual basis.
|
| ● |
As of 2010, a new Patient-Centered Outcomes Research Institute was established pursuant to PPACA to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient-Centered Outcomes Research Institute may affect the market for certain pharmaceutical products.
|
| ● |
The PPACA created the Independent Payment Advisory Board which, beginning in 2014, will have authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that supersedes the Payment Advisory Board’s recommendations and that will achieve the same or greater Medicare cost savings.
|
| ● |
The PPACA established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been appropriated to support the mission of the Center for Medicare and Medicaid Innovation in perpetuity.
|
European Union Regulatory Controls
In the EU, our future products may also be subject to extensive regulatory requirements. As in the United States, medicinal products can only be marketed if a marketing authorization from a competent regulatory agency has been obtained.
Similar to the United States, the various phases of non-clinical and clinical research in the EU are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the member state regimes. Under the current regime, before a clinical trial can be initiated it must be approved in each of the EU countries where the trial is to be conducted by two distinct bodies: the National Competent Authority, or NCA, and one or more Ethics Committees, or ECs. Under the current regime all suspected unexpected serious adverse reactions that occur during the clinical trial have to be reported to the NCA and ECs of the Member State where they occurred.
The EU clinical trials legislation is currently undergoing a revision process mainly aimed at harmonizing and streamlining the clinical trials authorization process, simplifying adverse event reporting procedures, improving the supervision of clinical trials, and increasing their transparency. The Clinical Trials Regulation EU No 536/2014 is estimated to enter into application during 2019 following determination of the full functionality of the EU clinical trials portal and database.
In the EU, pediatric data or an approved Pediatric Investigation Plan, or PIP, is required to have been approved by the EMA prior to submission of a marketing authorization application to the EMA. In most EU countries, we are also required to have an approved PIP before we can begin enrolling pediatric patients in a clinical trial. In early 2017, we submitted a PIP application in which we requested a waiver of conducting pediatric studies for EG-1962. On June 7, 2017, the European Medicines Agency granted a product specific waiver from conducting pediatric clinical studies for EG-1962 for the treatment of aSAH in all subsets of the pediatric population on the basis that the product does not represent a significant therapeutic benefit as clinical studies are not feasible for the pediatric population.
European Union Drug Review and Approval
In the European Economic Area, or EEA, (which is comprised of 28 Member States of the EU plus Norway, Iceland and Liechtenstein), medicinal products can only be commercialized after obtaining a Marketing Authorization, or MA. There are two types of marketing authorizations: the Community MA and National MA.
The Community MA is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use, or CHMP, of the EMA and is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products, such as medicinal products derived from biotechnology processes, orphan medicinal products, and medicinal products containing an active substance authorized in the EEA after May 20, 2004 and which are intended for the treatment of HIV/AIDS, cancer, neurodegenerative disorders, diabetes, or for autoimmune or viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA for indications other than those already stated before May 20, 2004, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. National MAs, which are issued by the competent authorities of the Member States of the EEA and cover marketing in their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member State through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State, or RMS. The competent authority of the RMS prepares a draft assessment report, a draft summary of the product characteristics, or SPC, and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Member States Concerned) for their approval. If the Member States Concerned raise no objections, based on a potential serious risk to public health, to the assessment, SPC, labeling, or packaging proposed by the RMS, the product is subsequently granted a national MA in all the Member States (i.e., in the RMS and the Member States Concerned).
Under the above described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy. For assessment of an MA submitted via the Centralized Procedure, the EMA is responsible for the validation and scientific evaluation of the application. The EMA's CHMP carries out a scientific assessment of the application and give a recommendation on whether the medicine should be authorized or not. A favorable opinion is accompanied by a draft summary of the product's characteristics, the package leaflet, and the proposed text for the packaging. The time limit for the evaluation procedure is 210 days, subject to extensions if additional questions need to be addressed. Within 15 days of the EMA adopting a positive opinion on the MA, the EMA will forward its opinion to the European Commission to start the decision-making phase. The adoption of the decision should take place within approximately 67 days of the opinion of the EMA. The decision with legally binding effect is subsequently published in the Community Register. An MA is initially valid for five years, subject to its renewal. Applications for renewal must be made to the EMA at least six months before the five-year period expires.
European Union New Chemical Entity Exclusivity
In the EU, new chemical entities, sometimes referred to as new active substances, qualify for eight years of data protection/exclusivity upon marketing authorization and an additional two years of market protection/exclusivity. This data exclusivity, if granted, prevents generic product applicants from referencing the innovator’s dossier in a generic application for eight years, after which a generic marketing authorization application can be submitted, and the innovator’s data may be referenced, but an approved generic product may not be placed on the market for a further two years. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
European Union Orphan Designation and Exclusivity
In the EU, the EMA’s Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the EU and for which no satisfactory method of diagnosis, prevention, or treatment has been authorized or the product is intended for the diagnosis, prevention, or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the EU would be sufficient to justify the necessary investment in developing the medicinal product. Additionally, the sponsor must establish that there exists no satisfactory authorized method of diagnosis, prevention, or treatment of the condition or even if such treatment exists, the product will be of significant benefit to those affected by that condition.
In the EU, orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity post-authorization. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Under EU law, before the marketing authorization is granted, the EMA’s Committee for Orphan Medicinal Products is required to re-assess whether the criteria for orphan designation are still met. Otherwise, the orphan designation can be removed from the Community Register. If the orphan designation is maintained, the orphan medicinal product will benefit from 10 years of orphan market exclusivity which can be extended to 12 years following completion of the agreed studies in a pediatric investigation plan.
In EU member states, nimodipine has been approved for administration orally in tablet form and intravenously by injection. EG-1962 has been granted an orphan designation in the EU for the treatment of aSAH.
The Potential U.K. Exit from the European Union as a Result of the June 2016 U.K. Referendum.
In June 2016, the U.K. affirmatively voted in a non-binding referendum advising the U.K. to exit from the European Union, commonly referred to as “Brexit”. In March 2017, the U.K. government formally notified the European Council of its intention to leave the EU. The formal notification triggered procedure set out in Article 50 of the Lisbon Treaty to begin the two-year negotiation process to conclude an agreement between the U.K. and the EU setting out the arrangements for the U.K.’s withdrawal, taking account of the framework for establishing the U.K.’s future relationship with the EU. The agreement must be concluded on behalf of the EU by the European Council acting by a qualified majority, after obtaining the consent of the European Parliament.
There is uncertainty about the terms of the final agreement and the framework for the future relationship between the U.K. and the EU. Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the U.K. determines which European Union laws to replace or replicate, and could have a material impact on its economy and the future growth of its various industries. In particular, there is uncertainty about whether the U.K. will continue to be within the single market and customs union for goods and services to be moved freely within the EU. This may impact whether goods including pharmaceutical products manufactured and/or imported into the U.K. from outside of the EU could continue to access the EU market. There is also uncertainty about whether marketing authorizations granted respectively in the U.K. and the EU would be mutually recognized following the U.K. leaves the EU.
Regulation Outside the EU
For other countries outside of the EU and the United States, such as Canada, or countries in Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials must be conducted in accordance with cGCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements applicable to a given country, we may not be able to obtain regulatory approval for our product candidates in such country if we choose to seek such approval, or we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Employees
As of December 31, 2017, we had 38 full-time employees, of whom six hold Ph.D. degrees, one holds an M.D. degree and one holds a D.O. degree. We have no collective bargaining agreements with our employees and have not experienced any work stoppages. We believe that relations with our employees are good.
Corporate and Available Information
We were incorporated in Delaware in 2009. We completed the initial public offering of our common stock in October 2015. Our common stock is currently listed on The NASDAQ Global Market under the symbol “EDGE.” We are an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012, and therefore we are currently subject to reduced public company reporting requirements.
Our principal executive offices are located at 300 Connell Drive, Suite 4000, Berkeley Heights, NJ 07922, and our telephone number is (800) 208-3343.
You may find on our website (http://www.edgetherapeutics.com) electronic copies of our annual report on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K (and any amendments thereto) filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. Such filings are placed on our website as soon as reasonably possible after they are filed with the Securities and Exchange Commission, or SEC. Our current charters for our audit, compensation, and nominating and corporate governance committees and our Code of Ethics are available on our website as well. Any waiver of our Code of Ethics may be made only by our Board of Directors.
You can read our SEC filings over the internet at the SEC’s web site at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. Please call the SEC at (202) 551-8090 or (800) 732-0330 for further information on the operation of the public reference facilities.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with the other information contained in this Annual Report, including our financial statements and the related notes appearing elsewhere in this Annual Report, before making your decision to invest in shares of our common stock. We cannot assure you that any of the events discussed in the risk factors below will not occur. These risks could have a material and adverse impact on our business, results of operations, financial condition and cash flows and our future prospects would likely be materially and adversely affected. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to the Development and Regulatory Approval of Our Product Candidates
We depend almost entirely on the potential success of one product candidate, EG-1962, which is in Phase 3 clinical development. We cannot be certain that we will be able to successfully develop or obtain regulatory approval for EG-1962 or any other product candidate.
We currently have only one late stage product candidate, EG-1962, in clinical development, and our business depends almost entirely on its successful clinical development, regulatory approval and commercialization. We currently have no drug products for sale and may never be able to develop marketable drug products. EG-1962 will require substantial additional clinical development, testing, and regulatory approval before we are permitted to commence its commercialization. Our only other product candidates, EG-1964 and EG-1965, are still in pre-clinical development. The clinical studies of our product candidates are and will be, and the manufacturing and marketing of our product candidates are and will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to investigate and, if approved, market any product candidate. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must successfully meet a number of critical developmental milestones. For example, for EG-1962, these include:
| ● |
providing adequate and well-controlled data that the product candidate is safe and effective and shows a significant benefit over the active comparator in patients for the intended indication;
|
| ● |
demonstrating that the product candidate formulation is reproducible and can meet the relevant release specifications for each market we intend to commercialize in; and
|
| ● |
completing the development and scale-up to permit manufacture of our product candidates in commercial quantities and at acceptable prices.
|
The time necessary to achieve these developmental milestones for any individual product candidate is long and uncertain, and we may not successfully complete these milestones for EG-1962 or any other product candidates that we may develop.
We are continuing to test and develop our product candidates and may explore possible design or formulation changes to address safety, efficacy, manufacturing efficiency and performance issues. We may not be able to finalize the design or formulation of any product candidate. In addition, we may select components, solvents, excipients or other ingredients to include in our product candidates that have not previously been used in approved pharmaceutical products, which may require us to perform additional studies and may delay clinical testing and regulatory approval of our product candidates. We may not be able to complete development of any product candidates that will be safe and effective and that will have a commercially reasonable treatment and storage period. If we are unable to complete development of EG-1962, or any other product candidates that we may develop, we will not be able to commercialize and earn revenue from them.
We cannot be certain that our NEWTON 2 Phase 3 clinical study of EG-1962 will be sufficient to support the submission of a marketing application for this product candidate, and in any event we may be required to obtain additional clinical and non-clinical data before a complete marketing application may be submitted.
In general, the FDA requires two adequate and well-controlled trials to support approval of an NDA, but in certain circumstances, will approve an NDA based on one adequate and well-controlled trial and confirmatory evidence based on the proposed indication and robustness of data from the single pivotal study. If successful, we believe the results from our NEWTON 2 Phase 3 clinical study of EG-1962, together with safety and efficacy data from the EG-1962 development program, could form the basis of an NDA submission in the United States using the FDA 505(b)(2) pathway for EG-1962. However, depending upon the outcome of the current program, the FDA and/or other global health authorities may require that we provide additional data, including possibly additional clinical studies, before we can submit a marketing application for EG-1962.
The regulatory approval processes of the FDA and comparable foreign regulatory authorities are inherently unpredictable, and if our product candidates are subject to multiple cycles of review or we are ultimately unable to obtain regulatory approval for our product candidates, including EG-1962, our business will be substantially harmed. In addition, the regulatory approval processes can delay our clinical trials, which can jeopardize our ability to generate revenues from the sale of our products.
Of the large number of drugs in development in the United States, only a small percentage successfully complete the FDA regulatory approval process and are commercialized. We are not permitted to market any of our product candidates in the United States or in other global markets until we receive approval of an NDA from the FDA or the requisite approval from such other global regulatory authorities. The NEWTON 2 study may, if successful, support and form the basis of approval for the global marketing applications for EG-1962. Successfully completing a Phase 3 clinical study and obtaining approval of an NDA is complex, lengthy, and expensive. The FDA or a comparable foreign regulatory authority may delay, limit or deny approval of EG-1962 for many reasons, including, among others:
| ● |
disagreement with, or disapproval of, the design of, procedures for, or implementation of, our clinical trials;
|
| ● |
our inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial;
|
| ● |
our failure to obtain institutional review board, or IRB, approval or the approval of other reviewing entities, including FDA and comparable foreign regulatory authorities, to conduct a clinical trial at each site;
|
| ● |
disagreement with the sufficiency of the final content and data included in our marketing application;
|
| ● |
feedback from the FDA or a comparable foreign regulatory authority, an IRB or DMC, on results from earlier stage or concurrent preclinical and clinical studies, that might require modification to the protocol;
|
| ● |
decision by the FDA, a comparable foreign regulatory authority, an IRB, or recommendation by the DMC or us to suspend or terminate clinical trials at any time for safety issues or for any other reason;
|
| ● |
challenges in meeting regulatory requirements to commence clinical trials in countries outside the United States;
|
| ● |
failure to conduct the trial in accordance with regulatory requirements;
|
| ● |
failure to demonstrate that EG-1962 provides an overall benefit to risk or significant enough improvement over the comparator in the proposed indication;
|
| ● |
failure of EG-1962 to demonstrate efficacy at the level of statistical significance required for approval;
|
| ● |
a negative interpretation of the data from our preclinical studies or clinical trials;
|
| ● |
deficiencies in the manufacturing processes or failure of third party manufacturing facilities with whom we contract for clinical and commercial supplies to effectively and consistently manufacture product under cGMP;
|
| ● |
failure to demonstrate adequate and reproducible product stability to support product commercialization;
|
| ● |
failure of our CMOs to successfully pass a FDA preapproval inspection or similar regulatory inspection prior to commercial approval;
|
| ● |
failure of Edge and our CMOs to adequately develop and characterize our drug product and/or our active pharmaceutical ingredient;
|
| ● |
failure to adequately demonstrate process performance qualification prior to product commercialization;
|
| ● |
inability to validate analytical and microbiological methods consistent with industry and government agency expectations;
|
| ● |
failure of our CMOs to provide integral data used to compile our regulatory submissions;
|
| ● |
insufficient data collected from clinical studies of EG-1962, or changes in the approval policies or regulations that render our preclinical and clinical data insufficient to support the submission and filing of a marketing authorization application or to obtain regulatory approval;
|
| ● |
Failure to overcome the orphan exclusivity of (1) Nymalize, an oral nimodipine solution, for which the FDA granted market exclusivity in 2013 for the orphan-designated aSAH indication for which Nymalize obtained marketing approval or (2) any other drug, such as the orphan-designated nimodipine-based intravenous drug currently under development by Grace Therapeutics, should such drug be considered the same drug as EG-1962, obtain marketing approval for the treatment of aSAH before EG-1962, and obtain orphan exclusivity for that indication. The orphan drug marketing exclusivity for Nymalize expires in May 2020; or
|
| ● |
changes in governmental regulations or administrative actions.
|
The FDA or a comparable foreign regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or cause us to abandon the development program. Even if we obtain regulatory approval, our product candidates may be approved for fewer or more limited indications than we request, such approval may be contingent on the performance of costly post-approval clinical trials, or we may not be allowed to include the labeling claims necessary or desirable for the successful commercialization of such product candidate. For instance, it is possible that EG-1962 could be approved but fail to replace nimodipine as the new standard of care for treating patients with an aSAH. In addition, if EG-1962 produces undesirable side effects or safety issues, the FDA may require the establishment of a REMS, including Elements to Ensure Safe Use, which are the most extensive elements of a REMS plan. Additionally, a comparable foreign regulatory authority may require the establishment of a similar strategy that may, for instance, restrict distribution of our products and impose burdensome implementation requirements on us.
Further, if we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed or may not happen at all. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials and non-head-to-head analysis (e.g., historical comparisons) may not be predictive of future trial results.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials, including with respect to EG-1962. Moreover, an assessment by the DMC to continue to the study at the futility or interim analyses is no indication that the trial will be successful. Due to, among other things, the small sample size of the data we have to date for EG-1962, there can be no assurance that our initial positive results will be indicative of results in future clinical trials with a larger and more diverse patient population and with a double-blind, double dummy trial design, such as our NEWTON 2 Phase 3 study. Furthermore, there can be no assurance that non-head-to-head analyses (e.g., historical comparisons) will be predictive of future trial results. Many companies in the biotechnology industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy, failure by the study drug to demonstrate sufficiently improved efficacy over a comparator arm, or adverse safety profiles, notwithstanding promising results in earlier trials. Our future clinical trials may not be successful.
The results of clinical trials may not support our product candidate claims.
Even if our clinical studies are completed as planned, we cannot be certain that their results will support our proposed product candidate claims, that the FDA or government authorities in other countries will agree with our conclusions regarding such results, or that the FDA or governmental authorities in other countries will not require additional clinical trials. The clinical trial process may fail to demonstrate that our product candidates show sufficient improved efficacy compared to standard of care to support approval or commercialization of any product candidate, or that they are safe for humans for indicated uses. This failure could cause us to abandon a product candidate and may delay development of other product candidates. Any delay in, or termination of, our clinical trials will delay or prevent the filing of our marketing application with the FDA or other global health authorities and, ultimately, our ability to commercialize our product candidates and generate product revenues.
We may be required to suspend or discontinue clinical trials for a number of reasons, which could preclude approval of any of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. A clinical trial may be suspended or terminated by us, an IRB, the FDA or other regulatory authorities due to a failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, presentation of unforeseen safety issues, failure to demonstrate a benefit from using the investigational drug, changes in governmental regulations or administrative actions, lack of adequate funding to continue the clinical trial, or for other business-related reasons. In addition, clinical trials for our product candidates could be suspended due to adverse side effects. Although we are primarily concerned with hypotension in our clinical trials for EG-1962, we may also observe inflammation, infection, unacceptable elevated intracranial pressure or hydrocephalus or other unknown effects resulting from the delivery, in a single administration, of sustained concentrations of nimodipine directly to the site of injury in the brain. Other drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. We may also voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to patients. If we elect or are forced to suspend or terminate any clinical trial of any product candidates that we develop, the commercial prospects of such product candidates will be harmed and our ability to generate product revenues from any of these product candidates will be delayed or eliminated. Any of these occurrences may significantly harm our business, financial condition, results of operations and prospects.
Preliminary or interim results of a clinical trial are not necessarily predictive of future or final results.
On December 29, 2017, we announced that the DMC recommended that the NEWTON 2 study of EG-1962 continue as planned based on the completion of a pre-planned futility analysis. The DMC made this recommendation after evaluating data from the 90 day follow-up visit of the first 150 patients randomized and treated, as well as the available safety data in the study. A pre-planned interim analysis will be performed following the 90 day outcome assessment of the 210th patient. We expect to complete the NEWTON 2 interim analysis by the end of April 2018 with an announcement of the results to follow. Upon completion of this interim analysis, the DMC may recommend continuing enrollment of the study up to 374 completed patients; ending the study early, as efficacy will have been established at a highly statistically significant level at the time of such interim analysis; or discontinuing enrollment of additional patients, but analyzing all currently enrolled and completed patients. We believe the likelihood that the DMC will recommend that we end the study early is extremely low. There is no formal futility analysis built into the interim analysis. However, as per the DMC charter, the DMC may recommend amending or terminating the NEWTON 2 study for safety concerns or based on a benefit : risk assessment that does not justify additional subject enrollment. If the DMC does not recommend that we end the study early based on highly positive statistical significance, the study is expected to continue to full data readout, in which case we expect the results of the study to be available in late 2018.
Interim or preliminary data from clinical trials that we may conduct may not be indicative of the final results of the trial and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Interim or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the interim or preliminary data. As a result, interim or preliminary data should be viewed with caution until the final data are available. Even if our clinical trials are completed as planned, we cannot be certain that their results will support our proposed indications.
Approval of EG-1962 could be more costly and take longer than anticipated as a result of Nymalize’s existing orphan drug exclusivity or any other drug’s orphan drug exclusivity that could delay commercialization of EG-1962.
Regulatory authorities in some jurisdictions, including the US and EU, may designate drugs for the treatment or prevention of rare diseases or conditions with relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. In the EU, a drug may be granted orphan designation if the product is intended to treat a life-threatening or chronically debilitating condition affecting not more than five in 10,000 individuals in the EU or if the product is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the EU and that without incentives it is unlikely that the marketing of the medicinal product in the EU would generate sufficient return to justify the necessary investment. Furthermore, the sponsor must also establish that there exists no satisfactory authorized method of treatment of the condition or even if such treatment exists, the product will be of significant benefit to those affected by that condition.
In the United States, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a seven-year period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for the same indication for that time period absent a demonstration of clinical superiority. A similar provision in EU law allows ten years of market exclusivity attaching to the granted marketing authorization during which EEA regulators are not permitted to accept another application for a market approval or accept an application for line-extension for the same therapeutic indication in respect of a similar medicinal product. The ten-year orphan market exclusivity can be extended to a total of twelve years following satisfactory completion of the agreed studies set out in the pediatric investigation plan.
The market exclusivity period can be reduced to six years if at the end of the fifth year of that period, a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that maintenance of the orphan designation and accordingly the marketing exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or the EMA determines that the request for designation was materially defective or if the FDA subsequently finds that the drug in fact had not been eligible for orphan drug designation at the time of submission of the request. In the EU, the conditions for orphan designation must be confirmed by the EMA and its Committee for Orphan Medicinal Products before market approval is granted. The designated orphan medicinal product will be removed from the Community Registry of Orphan Medicinal Products if the criteria are no longer met.
If a drug is approved for an orphan indication, the FDA will not designate as an orphan another drug deemed the “same drug” for the same use as the approved orphan drug unless the sponsor of the new drug provides a plausible hypothesis that its new drug is clinically superior to the approved orphan drug. For small molecule drugs, FDA defines “same drug” to mean a drug that contains the same active moiety (meaning the molecule or ion of the molecule, responsible for the physiological or pharmacological action of the drug substance) as the previously approved drug, even if the particular ester or salt or other noncovalent derivative has not previously been approved. Clinical superiority means that the drug is shown to provide a significant therapeutic advantage over and above the approved same drug and can be established on the basis of greater safety, greater effectiveness, or, in unusual cases where neither greater safety nor greater effectiveness is shown, on the basis of a major contribution to patient care. Similarly, in the EU and the EEA, the orphan market exclusivity may be broken or otherwise derogated from in the following circumstances: (a) if the second product is not considered similar; (b) the second applicant can establish in the application that the second similar medicinal product is safer, more effective or otherwise clinically superior including a major contribution to patient care; or (c) the holder of the MA for the first authorized orphan drug is unable to supply sufficient quantities of the medicinal product.
Of relevance to Edge, oral nimodipine (in the form of gelcaps) was approved for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in patients with an aSAH in 1988 as Nimotop and subsequently was approved in a generic version in 2007. Nimotop has now been discontinued from marketing, and the FDA has determined that it was not discontinued for safety or effectiveness reasons. In September 2011, OOPD granted Nymalize, an oral nimodipine solution, orphan drug designation for the treatment of subarachnoid hemorrhage. Subsequently, in May 2013, the FDA approved Nymalize for the treatment of subarachnoid hemorrhage, thereby granting the oral nimodipine solution seven years of marketing exclusivity until May 2020. In order for us to obtain marketing approval for EG-1962 for the same aSAH indication as Nymalize during the period of marketing exclusivity for Nymalize (e.g., up until May 2020) and to obtain orphan drug exclusivity for EG-1962 for the treatment of aSAH at any time, we will need to demonstrate the clinical superiority of EG-1962 to the oral nimodipine solution or demonstrate that EG-1962 is otherwise not the “same drug” as Nymalize as that term is defined in FDA’s Orphan Drug regulations. We intend to demonstrate that EG-1962 is clinically superior to the oral nimodipine solution by demonstrating superiority over oral nimodipine gelcaps and tablets in a head-to-head comparison in our NEWTON 2 Phase 3 study. To the extent the OOPD disagrees that we can demonstrate the clinical superiority of EG-1962 to Nymalize by demonstrating the superiority of EG-1962 to oral nimodipine gelatin capsules (a Nimotop generic) and requires us to include a head-to-head comparison of EG-1962 and Nymalize, our Phase 3 program would be more costly and may take longer than is currently anticipated or we may be required to delay the commercial launch of EG-1962 in the United States until Nymalize’s orphan drug exclusivity expires in May 2020.
In addition, for EG-1962 to be awarded its own 7-year period of orphan drug exclusivity upon approval, FDA could require that Edge demonstrate clinical superiority over both Nimotop and Nymalize, as well as over any other nimodipine same drugs that might obtain marketing approval prior to EG-1962 obtaining marketing approval. Even if we obtain orphan drug exclusivity for a product such as EG-1962, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition or another sponsor’s nimodipine drug product may prove clinically superior to EG-1962.
We are aware that an intravenous drug containing nimodipine is under development by Grace Therapeutics and that Grace Therapeutics has announced it plans to file an NDA on such drug in the first half of 2018. Grace Therapeutics has received orphan drug designation, but not orphan drug exclusivity, for this drug for the treatment of SAH. Should Grace Therapeutics’ nimodipine drug be considered the same drug as EG-1962 and Grace Therapeutics obtains marketing approval and orphan drug exclusivity for its drug before EG-1962 is approved, EG-1962 may be delayed from entering the market until seven years after the Grace Therapeutics drug received its orphan drug exclusivity unless Edge is able to demonstrate that EG-1962 is clinically superior to Grace Therapeutics’ drug.
A Fast Track designation by the FDA may not actually lead to a faster development or regulatory review or approval process.
We received Fast Track designation for EG-1962 from the FDA in May 2016. We may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program.
Even if our product candidates receive regulatory approval, they may still face future development and regulatory challenges and any approved products will be subject to extensive post-approval regulatory requirements.
If we obtain regulatory approval for a product candidate, it would be subject to extensive ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post- market information. The safety profile of any product will continue to be closely monitored by the FDA and comparable foreign regulatory authorities after approval. If the FDA or comparable foreign regulatory authorities become aware of new safety information after approval of any of our product candidates, these regulatory authorities may require labeling changes or, depending on the nature of the safety information, establishment of a REMS or similar strategy, impose significant restrictions on a product’s indicated uses or marketing, impose ongoing requirements for potentially costly post-approval studies or post-market surveillance, cause a recall or even move to withdraw the marketing approval for the product.
In addition, manufacturers of therapeutic products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP regulations, including a focused pre-approval inspection in connection with any regulatory submission for approval that we might file. If we or a regulatory agency discover previously unknown problems with a product, such as problems with the facility where the product is manufactured, a regulatory agency may take regulatory actions against the manufacturing facility or us, leading to a product recall or withdrawal, or suspension of manufacturing.
If we, our product candidates or the manufacturing facilities for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may, among other things:
| ● |
issue warning letters or untitled letters;
|
| ● |
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners;
|
| ● |
require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance;
|
| ● |
seek an injunction or impose civil or criminal penalties or monetary fines;
|
| ● |
suspend or withdraw regulatory approval;
|
| ● |
suspend any ongoing clinical studies;
|
| ● |
challenge any pending applications or supplements to applications filed by us;
|
| ● |
suspend or impose restrictions on operations, including costly new manufacturing requirements; or
|
| ● |
seize or detain products or refuse to permit the import or export of products.
|
The occurrence of any event or penalty described above may inhibit our ability to commercialize our products and generate revenue.
Advertising and promotion of any product candidate that obtains approval in the United States may be heavily scrutinized by the FDA, including the Office of Prescription Drug Promotion, the Department of Justice, or the DOJ, HHS, Office of Inspector General, state attorneys general, members of Congress and the public. Violations, including promotion of our products for unapproved (or off-label) uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA. Additionally, advertising and promotion of any product candidate that obtains approval outside of the United States will be heavily scrutinized by comparable foreign regulatory authorities.
In the United States, engaging in impermissible promotion of our products for off-label uses can also subject us to false claims litigation under federal and state statutes, which can lead to civil and criminal penalties and fines and agreements that materially restrict the manner in which we promote or distribute those drug products. These false claims statutes include the FCA, which allows any individual to bring a lawsuit against a pharmaceutical company on behalf of the federal government alleging submission of false or fraudulent claims, or causing to present such false or fraudulent claims, for payment by a federal program such as Medicare or Medicaid. If the government prevails in the lawsuit, the individual will share in any fines or settlement funds. Since 2004, these FCA lawsuits against pharmaceutical companies have increased significantly in volume and breadth, leading to several substantial civil and criminal settlements based on certain sales practices promoting off-label drug uses. This growth in litigation has increased the risk that a pharmaceutical company will have to defend a false claim action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from the Medicare, Medicaid, and other federal and state healthcare programs. If we do not lawfully promote our approved products, we may become subject to such litigation and, if we are not successful in defending against such actions, those actions may have a material adverse effect on our business, financial condition and results of operations.
Failure to obtain regulatory approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell our products in the EU, Canada, Japan and other international jurisdictions, we must obtain separate and distinct marketing approvals and comply with the respective regulatory requirements of each of these jurisdictions. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval, but can involve additional testing or safety surveillance. In particular, nimodipine, the therapeutic agent used in EG-1962, has not previously been approved for use in Japan. As a result, the time required to obtain approval for EG-1962 in Japan may differ substantially from the time required to obtain approval for EG-1962 by the FDA. In the EU, an approved PIP, or a waiver therefrom, is required prior to marketing authorization. A delay in reaching an approved PIP or in obtaining a waiver or meeting the conditions could delay authorization in the EU. We may need to partner with third parties in order to obtain regulatory approvals outside the United States. Approval by the FDA does not necessarily guarantee approval by regulatory authorities in other countries or jurisdictions. Nor does the approval by one regulatory authority outside the United States ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of EG-1962 or any of our other product candidates by regulatory authorities in the EU, Canada, and other international jurisdictions, the commercial prospects of those product candidates may be significantly diminished and our business prospects could dramatically decline.
Risks Related to the Potential Commercial Manufacture and Selling and Marketing of Our Product Candidates
If we are unable to establish sales and marketing capabilities to market and sell our product candidates, we may be unable to generate any revenue.
In order to market and sell EG-1962 and our other product candidates in development, we currently intend to build and develop our own sales, marketing and distribution operations in the United States and Canada, and possibly the EU. Although our management team has previous experience with such efforts, there can be no assurance that we will be successful in building these operations. If we are unable to establish adequate sales, marketing and distribution capabilities, we may not be able to generate product revenue and may not become profitable. We may also be competing with many companies that currently have extensive and well-funded sales and marketing operations. Even if any of our product candidates are approved, we may be unable to compete successfully against these more established companies.
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among hospitals, physicians, patients/caregivers and healthcare payors.
Even if we obtain regulatory approval for any of our product candidates that we may develop or acquire in the future, the product may not gain market acceptance among hospitals, physicians, health care payors, patients and the medical community. Market acceptance of any of our product candidates for which we receive approval depends on a number of factors, including:
| ● |
the efficacy and safety of such product candidates as demonstrated in clinical trials;
|
| ● |
the clinical indications for which the product candidate is approved and any REMS that may be imposed as a condition of approval;
|
| ● |
acceptance by major operators of hospitals, physicians and patients of the product candidate as a safe and effective treatment, particularly the ability of EG-1962 and our other product candidates to establish themselves as the new standard of care for the indications that we are pursuing;
|
| ● |
the potential and perceived advantages of our product candidates over alternative treatments as compared to the relative costs of the product candidates and alternative treatments;
|
| ● |
the safety of our product candidates seen in a broader patient group, including its use outside the approved indications;
|
| ● |
the prevalence and severity of any side effects, such as hypotension with respect to EG-1962;
|
| ● |
product labeling or product insert requirements of the FDA or other regulatory authorities;
|
| ● |
the timing of market introduction of our products as well as competitive products;
|
| ● |
the availability of adequate reimbursement and pricing by third party payors and government authorities;
|
| ● |
relative convenience and ease of administration; and
|
| ● |
the effectiveness of our sales and marketing efforts and those of our future collaborators.
|
There may be delays in getting our drugs on hospitals’ local formularies or limitations on coverages which can occur in the early stages of commercialization for newly approved drugs. If any of our product candidates are approved but fail to achieve market acceptance among hospitals, physicians, patients or health care payors, we will not be able to generate significant revenues, which would have a material adverse effect on our business, prospects, financial condition and results of operations.
Even if we are able to commercialize our product candidates, the products may not receive coverage and adequate reimbursement from third party payors, which could harm our business.
Our ability to commercialize any products successfully will depend, in large part, on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from federal and state healthcare programs, private health insurers and other organizations. Payors, such as federal and state healthcare programs, private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors are critical to new product acceptance. A trend in the healthcare industry and elsewhere is cost containment. Government authorities and third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, by increasing patient cost-sharing, or by adopting new methods to pay physicians and hospitals that may discourage them from using higher-cost drugs in some instances. In addition, we expect that our products may be used chiefly in the inpatient hospital setting; products that are used in the inpatient hospital setting may be reimbursed by payors at a fixed payment for the hospital stay, or a similar payment method that does not include a separate charge for drugs provided to the patient, which can discourage hospitals from furnishing higher-cost drugs to inpatients in some cases. Increasingly, third party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Third party payors also may seek additional clinical evidence, beyond the data required to obtain marketing approval, demonstrating clinical benefits and value in specific patient populations before covering our products for those patients. In particular, even if EG-1962 or any other product candidates we develop are established as having superior efficacy compared to the current standard of care, payors may not adequately reimburse for such product candidates. Accordingly, we cannot be sure that coverage and adequate reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, and/or the price of, any product candidate for which we obtain marketing approval. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
Coverage decisions may depend upon clinical and economic standards that disfavor new drug products such as ours when more established or lower cost therapeutic alternatives are already available or subsequently become available. Decisions regarding the extent of coverage and amount of reimbursement to be provided for products and product candidates that we develop will be made on a plan-by-plan basis. As a result, the coverage determination process is often a time-consuming and costly process that may require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained. There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may only be temporary. Reimbursement rates may vary according to the use of the drug and the setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly or eventually obtain coverage and profitable reimbursement rates from both government-healthcare programs and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
In the EEA, even if the product is approved by the European Commission following a positive opinion by the EMA’s CHMP through the Centralized Procedure, national governments or independent organizations set up by them to make such decisions may not approve pricing and reimbursement for the product on grounds relating to cost-effectiveness under the respective national health service systems. This will have an effect of limiting or otherwise restricting access to the products by patients using public healthcare services in member states of the EEA.
Recently enacted and future legislation, including potentially unfavorable pricing regulations or other healthcare reform initiatives, may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities, impact or limit how the cost of our products are reimbursed by government programs, or affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the Medicare Modernization Act, established the Medicare Part D program, which covers outpatient drugs, and generally provided authority for the private plans that deliver Part D drug benefits to limit the number of drugs that will be covered in any therapeutic class thereunder. The Medicare Modernization Act does not permit CMS to interfere in negotiations over drug pricing and coverage between Part D plans and manufacturers, and Part D has generally been considered successful in containing costs without CMS interference in these negotiations; however, program costs have been increasing in recent years and interest in permitting CMS intervention, or otherwise taking additional steps to contain drug costs in Part D, is increasing. Efforts to reduce drug costs or coverage under Part D could decrease the coverage and reimbursement rate that we receive for any of our approved products that are outpatient drugs eligible for Part D coverage. Furthermore, private payors often follow Medicare coverage policies and payment limitations in setting their own reimbursement rates. Therefore, any general reduction in reimbursement rates paid by Medicare Part D plans or policies to increase the rebates that manufacturers pay to Part D plans or to give these plans more discretion to limit coverage may result in a similar reduction in payments from private payors.
The PPACA, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, the Affordable Care Act, among other things, imposes a significant annual fee on companies that manufacture or import branded prescription drug products (with an exception for certain orphan drugs). It also contains substantial new provisions intended to broaden access to health insurance, reduce or constrain the growth of health care spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, and impose additional health policy reforms (including accelerating the testing and expansion of new payment methods that contain healthcare costs), any of which could negatively impact our business. The Affordable Care Act is likely to continue the downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
We expect that the Affordable Care Act, as well as other healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product (including through promoting the adoption of methods to pay healthcare providers that can discourage them from furnishing higher-cost drugs to their patients in some cases), and could seriously harm our future revenues. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may compromise our ability to generate revenue, attain profitability or commercialize our products. Similar austerity measures to contain healthcare costs under national rules may be applied in various EU Member States to limit or restrict market access to the product.
The Affordable Care Act also improved the market for healthcare products in one respect, by expanding health insurance coverage. Starting in 2014, the Affordable Care Act expanded health insurance coverage to many previously uninsured Americans, through a combination of federal subsidies for lower-income individuals who enrolled in health plans through health insurance Exchanges and enabling States to expand Medicaid eligibility with the federal government paying a high share of the cost. Following the November 2016 U.S. elections, uncertainty exists about the future of this coverage expansion. In December 2017, President Trump signed into law the Tax Cuts and Jobs Act of 2017, which includes a repeal of the individual mandate under the Affordable Care Act. The President and congressional leaders have continued to express interest in repealing Affordable Care Act provisions and replacing them with alternatives that may be less costly and provide state Medicaid programs and private health plans more flexibility. It is possible that these repeal and replacement initiatives, if enacted into law, could ultimately result in fewer individuals having health insurance coverage and/or in individuals having insurance coverage with less generous benefits. Any reduction in the number of patients receiving healthcare coverage may impact the reimbursements to hospitals and impact the ability of hospitals and patients to pay for our products, including EG-1962. The scope of potential future legislation to repeal and replace Affordable Care Act provisions is highly uncertain in many respects, and it is possible that some of the Affordable Care Act provisions that generally hurt the research-based pharmaceutical industry (such as those discussed above) could also be repealed along with Affordable Care Act coverage expansion provisions; however, at this time the coverage expansion provisions of the Affordable Care Act appear most likely to be repealed and replaced.
Laws and regulations governing international operations may preclude us from developing, manufacturing and selling certain product candidates outside of the United States and require us to develop and implement costly compliance programs.
As we expand our operations outside of the United States, we must comply with applicable local laws and regulations relating to our business operations in each jurisdiction in which we plan to operate. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required.
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. The anti- bribery provisions of the FCPA are enforced primarily by the DOJ. The SEC is involved with enforcement of the books and records provisions of the FCPA.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical studies and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the United States, or the sharing with certain non-U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. If we expand our presence outside of the United States, it will require us to dedicate additional resources to compliance with these laws, and these laws may preclude us from developing, manufacturing, or selling certain products and product candidates outside of the United States, which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial penalties, including suspension or debarment from government contracting. Violation of the FCPA can result in significant civil and criminal penalties. Indictment alone under the FCPA can lead to suspension of the right to do business with the U.S. government until the pending claims are resolved. Conviction of a violation of the FCPA can result in long-term disqualification as a government contractor. The termination of a government contract or relationship as a result of our failure to satisfy any of our obligations under laws governing international business practices would have a negative impact on our operations and harm our reputation and ability to procure government contracts. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions.
Similar consequences may accrue from business practices that violate other applicable anti-bribery/anti-corruption statutes in other jurisdictions, such as the United Kingdom Bribery Act.
We intend that EG-1962 and our other product candidates will be sold outside of the United States, either by Edge or its affiliates, through one or more international partners or by license agreement, and if we do, we will be subject to the risks of doing business outside of the United States.
Because we intend that EG-1962 and our other product candidates, if approved, will be sold outside of the United States, either by Edge or its affiliates, through one or more international partners or by license agreement, our business is subject to risks associated with doing business outside of the United States. Accordingly, our business and financial results in the future could be adversely affected due to a variety of factors, including:
| ● |
failure to develop an international sales, marketing and distribution system for our products;
|
| ● |
changes in a specific country’s or region’s political and cultural climate or economic condition;
|
| ● |
unexpected changes in foreign laws and regulatory requirements;
|
| ● |
difficulty of effective enforcement of contractual provisions in local jurisdictions;
|
| ● |
inadequate intellectual property protection in foreign countries;
|
| ● |
inadequate data protection against unfair commercial use;
|
| ● |
trade-protection measures, import or export licensing requirements such as Export Administration Regulations promulgated by the United States Department of Commerce and fines, penalties or suspension or revocation of export privileges;
|
| ● |
the effects of applicable foreign tax structures and potentially adverse tax consequences;
|
| ● |
significant adverse changes in foreign currency exchange rates; and
|
| ● |
failure of third party international partners with whom we contract for commercialization outside the United States to effectively and consistently commercialize EG-1962 and/or other Edge products.
|
Risks Related to Our Dependence on Third Parties
We rely completely on third party suppliers to manufacture our clinical drug supplies for our product candidates, and we intend to rely on third parties to produce pre-clinical, clinical and commercial supplies of any future product candidate.
We do not currently have the infrastructure or capability, nor do we have plans to acquire the infrastructure or capability, to internally manufacture our clinical or commercial drug supply of our product candidates, or any future product candidates, for use in the conduct of our non-clinical studies and clinical trials, and we lack the internal resources and the capability to manufacture any product candidates on a clinical or commercial scale. For example, the active pharmaceutical ingredient, or API, for EG-1962 for our NEWTON 2 Phase 3 pivotal study was manufactured at a third party contract manufacturer’s site. Additionally, the diluent of sodium hyaluronate for EG-1962 for our NEWTON 2 Phase 3 pivotal study was manufactured at a third party contract manufacturer’s site and the polymer microparticle for EG-1962 as formulated for our NEWTON 2 Phase 3 pivotal study was manufactured at another third-party contract manufacturer’s site. Currently, Oakwood is the sole manufacturer and supplier of the EG-1962 used in the NEWTON 2 Phase 3 pivotal study and we do not expect to enter into additional manufacturing and supply agreements for such clinical supply.
We have entered into a Manufacturing and Supply Agreement with Oakwood (the “Supply Agreement”), pursuant to which Oakwood will manufacture and supply, and we will purchase from Oakwood, EG-1962 in commercial quantities following the commercial launch of the product. There is no guarantee that Oakwood will have sufficient capacity to satisfy our manufacturing and supply needs of EG-1962. If EG-1962 gets approved but we are unable to do negotiate commercial supply agreements with other third parties, there may be significant shortfalls in the commercial quantities of EG-1962 available for sale if Oakwood cannot meet our demand. Similarly, we have single-source suppliers for the API, the sodium hyaluronate and the polymer microparticles that comprise EG-1962. If any of these suppliers are unable to supply us with our requirements, and we are unable to do negotiate commercial supply agreements with other third parties, there may be significant shortfalls in the commercial quantities of EG-1962 available for sale.
Reliance on third party suppliers and manufacturers entails risks to which we would not be subject if we manufactured product candidates or products ourselves. Although we are primarily responsible for the oversight of regulatory compliance with respect to the third party supply and manufacture of our products, we rely on the third party for regulatory compliance and quality assurance activities. The possibility exists of breach of the manufacturing and/or supply agreement by the third party because of factors beyond our control (including a failure to synthesize and manufacture our product candidates or any products we may eventually commercialize in accordance with our specifications), and the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that could be costly or damaging to us. In addition, although we are not in control of the day-to-day activities of our third party suppliers and manufacturers, we are nonetheless responsible for ensuring that our product candidates and any products that we release for clinical or commercial use are manufactured according to cGMP and similar foreign standards. Any failure by our third party suppliers or manufacturers to comply with cGMP or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates. In addition, such failure could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for product candidates previously granted to us, or take other regulatory or legal action, including recall or seizure, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, detention of product, refusal to permit the import or export of products, injunction, or imposing civil and criminal penalties.
Because of the complex nature of our compounds, our current suppliers and manufacturers and any future suppliers and manufacturers may not be able to supply and/or manufacture our compounds at a cost or in quantities or in a timely manner necessary to make commercially successful products. If we successfully commercialize any of our drugs, we may be required to establish large-scale commercial manufacturing capabilities, either internally or with another party, in addition to Oakwood. In addition, if our drug development pipeline increases and matures, we will have a greater need for clinical trial and commercial supply and manufacturing capacity. We have no prior experience manufacturing pharmaceutical products on a commercial scale and some of these suppliers and manufacturers will need to increase their scale of production to meet our projected needs for commercial supply and manufacturing, the satisfaction of which may not be met on a timely basis.
We rely on CROs to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for our products or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third party CROs to monitor and manage data for our ongoing preclinical and clinical programs. We rely on these parties to assist us in the execution of our preclinical and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on CROs does not relieve us of our regulatory responsibilities. We also rely on third parties to conduct our preclinical studies in accordance with GLP requirements and the Laboratory Animal Welfare Act of 1966 requirements (and the equivalent requirements outside the United States). We and our CROs are required to comply with regulations and current GCPs, which are enforced by the FDA, and EU law requirements governing GCPs to be applied by the Competent Authorities of the Member States of the EU and EEA, and comparable foreign regulatory authorities to ensure that the health, safety and rights of patients are protected in clinical development and clinical trials, and that trial data integrity as well as protection of personal data of the trial subjects is assured. Regulatory authorities ensure compliance with these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements. In addition, our clinical trials must be conducted with products produced under cGMP requirements. Failure to comply with these regulations may require us to repeat preclinical and clinical trials, which would delay the regulatory approval process.
Although we have certain mandated oversight responsibilities for our CROs, including with respect to quality management, trial management, data handling, record keeping and noncompliance, our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Because we have relied on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available to identify and monitor our third party providers. To the extent we are unable to identify and successfully manage the performance of third party service providers in the future, our business may be adversely affected. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
If we lose our relationships with CROs or other third party vendors, our drug development efforts could be delayed.
We rely on third party vendors and CROs for preclinical studies and clinical trials related to our drug development efforts. Switching or adding additional CROs or third party vendors involves additional cost and requires management time and focus. Our CROs and third party vendors have the right to terminate their agreements with us in the event of an uncured material breach. In addition, some of our CROs and third party vendors have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the patients participating in our clinical trials warrants such termination. Identifying, qualifying and managing performance of third party service providers can be difficult, time consuming and cause delays in our development programs. In addition, there is a natural transition period when a new CRO or third party vendor commences work, and the new CRO or third party vendor may not provide the same type or level of services as the original provider. Furthermore, one of our CROs may undergo a change in control or management, which may result in a different level of service from the CRO. If any of our relationships with our third party CROs or third party vendors terminate, we may not be able to enter into timely arrangements with alternative CROs or third party vendors or do so on commercially reasonable terms.
Disruptions in our supply chain could delay the commercial launch of our product candidates.
Any significant disruption in our supplier relationships could harm our business. We currently rely on a single source supplier for the API nimodipine, as well as a single supplier for the polymer microparticles and the diluent that comprise EG-1962. Further, Oakwood is the sole manufacturer of EG-1962 for clinical uses and may be for commercial uses, if EG-1962 is approved. If any of our suppliers is unable or unwilling to manufacture sufficient quantities of these key materials in a reasonable timeframe or on commercially acceptable terms, or suffers a major natural or man-made disaster at its manufacturing facility, we would not be able to manufacture EG-1962 until a qualified alternative supplier is identified. Although alternative sources of supply exist, the number of third party suppliers with the necessary manufacturing and regulatory expertise and facilities is limited, and it could be expensive and take a significant amount of time to arrange for alternative suppliers. Any significant delay in the supply of a product candidate or its key materials for an ongoing clinical trial could considerably delay completion of our clinical studies, product testing and potential regulatory approval of our product candidates and considerably delay the commencement of commercial production and sale of approved product. If our manufacturers or we are unable to purchase these key materials after regulatory approval has been obtained for our product candidates, either the commercial launch of our product candidates could be delayed or there could be a shortage in supply, which would impair our ability to generate revenues from the sale of our product candidates.
Our relationships with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. As a pharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that may affect our ability to operate include, but are not limited to, the following:
| ● |
the federal healthcare Anti-Kickback Statute constrains our marketing practices, educational programs, pricing policies, and relationships with healthcare providers or other entities, by prohibiting, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
|
| ● |
federal civil and criminal false claims laws and civil monetary penalty laws impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
| ● |
HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services;
|
| ● |
HIPAA, as amended by HITECH, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
| ● |
the federal physician sunshine requirements under the Affordable Care Act requires manufacturers of drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to physicians, certain other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and certain other healthcare providers and their immediate family members and applicable group purchasing organizations; and
|
| ● |
analogous state and foreign laws and regulations, such as measures relating to inducements designed to promote prescription, supply, sale or intake of drugs, including state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and certain other healthcare providers or marketing expenditures. Additionally, state and foreign laws govern the privacy or personal data and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
|
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, to provide accurate information to the FDA or comparable foreign regulatory authorities, to comply with manufacturing standards we have established, to comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted, implemented, and are enforcing a Code of Conduct and other compliance-based policies and procedures, and we intend to continue to adopt, implement, and enforce additional compliance-based policies and procedures as appropriate, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity, such as employee training on enforcement of the Code of Conduct and other policies and procedures, may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Risks Related to Our Business and Strategy
Our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel.
Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. We are highly dependent upon current management, especially our founder, Brian A. Leuthner, who is the driving force behind the operation and successful implementation of our business strategy. Although we have an employment agreement with Mr. Leuthner and other key employees, these agreements are at-will and do not prevent them from terminating their employment with us at any time and possibly joining one of our competitors. We do not maintain “key person” insurance for any of our executives or other employees. We intend to increase our technical and management staff as needs arise and supporting resources are available, but the loss of one or more of our senior executive officers, including their death or incapacitation, could be detrimental to us if we cannot recruit suitable replacements in a timely manner. The competition for qualified personnel in the pharmaceutical field is intense and, as a result, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
As of December 31, 2017, we had 38 full-time employees. As our development and commercialization plans and strategies develop, or as a result of any future acquisitions, we will likely need additional managerial, operational, sales, marketing, financial, legal and other resources. Our management, personnel and systems currently in place may not be adequate to support this future growth. Future growth would impose significant added responsibilities on members of management, including:
| ● |
managing our clinical studies and product development and commercialization processes effectively;
|
| ● |
identifying, recruiting, maintaining, motivating and integrating additional employees;
|
| ● |
managing our internal development efforts effectively while complying with our contractual obligations to licensors, contractors and other third parties;
|
| ● |
improving our managerial, development, operational and finance, human resources, and quality management systems, including automation; and
|
| ● |
expanding our facilities.
|
As our operations expand, we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical studies and potential commercialization effectively and hire, train and integrate additional management, administrative and sales and marketing personnel. We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing Edge.
The pharmaceutical industry is highly competitive and is subject to rapid and significant technological change, which could render our technologies and products obsolete or uncompetitive.
There is no assurance that our product candidates will be the most effective, the safest, the first to market, or the most economical to make or use. The introduction of competitive therapies as alternatives to our product candidates could dramatically reduce the value of those development projects or chances of successfully commercializing those product candidates, which could have a material adverse effect on our long-term financial success. Additionally, other technologies may become available that can monitor intracranial pressure without the need for an EVD, which would require physicians to put in place an EVD solely to administer EG-1962 and thereby substantially increase the additional level of invasiveness needed to deliver EG-1962 through our initial route of administration.
We plan to compete with companies in North America and internationally, including major pharmaceutical and chemical companies, specialized CROs, research and development firms, universities and other research institutions. In the United States, oral nimodipine gelcaps are manufactured by generic companies, and there is no brand name drug. In May 2013, the FDA approved Nymalize, an oral nimodipine solution, for the treatment of aSAH, and, at approval, granted it seven years of orphan drug marketing exclusivity for this indication because it had previously received orphan designation for the treatment of subarachnoid hemorrhage. We are aware that Grace Therapeutics has announced that they plan to submit an NDA for an intravenous infusion form of nimodipine in the first half of 2018, that Grace Therapeutics has received orphan drug designation for its product, and therefore that Grace Therapeutics could obtain orphan drug exclusivity on its product, thereby potentially blocking us from commercializing EG-1962 for seven years if Grace Therapeutics’ drug is considered the same drug as EG-1962. We are also aware that Idorsia Ltd. has announced that it expects to initiate a Phase 3 study evaluating the safety and efficacy of clazosentan in an aSAH population enriched for the risk of cerebral vasospasm in 2018. In Japan, there are two drugs that are used to treat aSAH patients, fasudil and sodium ozagrel. Many of our potential competitors have greater financial resources and selling and marketing capabilities, greater experience in clinical testing and human clinical trials of pharmaceutical products and greater experience in obtaining FDA and other regulatory approvals than we do. In addition, some of our potential competitors may have lower development and manufacturing costs.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, the servers of our cloud-based computing providers and other systems, and those of our CROs and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
Any future collaborators may compete with us or have interests which conflict with ours. This may restrict our research and development efforts and limit the areas of research in which we intend to expand.
Large pharmaceutical companies with whom we may seek to collaborate may have internal programs or enter into collaborations with our competitors for products addressing the same medical conditions targeted by our technologies. Thus, such collaborators may pursue alternative technologies or product candidates in order to develop treatments for the diseases or disorders targeted by our collaborative arrangements. Such collaborators may pursue these alternatives either on their own or in collaboration with others, including our competitors. Depending on how other product candidates advance, a corporate partner may slow down or abandon its work on our product candidates or terminate its collaborative arrangement with us in order to focus on these other prospects.
If any conflicts arise, our future collaborators may act in their own interests, which may be adverse to ours. In addition, in our future collaborations, we may be required to agree not to conduct any research that is competitive with the research conducted under our future collaborations. Our future collaborations may have the effect of limiting the areas of research that we may pursue. Our collaborators may be able to develop products in related fields that are competitive with the products or potential products that are the subject of these collaborations.
We may engage in future acquisitions that could disrupt our business, cause dilution to our stockholders and harm our financial condition and operating results.
While we currently have no specific plans to acquire any other businesses or products, we may, in the future, make acquisitions of, or investments in, companies that we believe have products or capabilities that are a strategic or commercial fit with our current product candidates and business or otherwise offer opportunities for our company. In connection with these acquisitions or investments, we may:
| ● |
issue stock that would dilute our stockholders’ percentage of ownership;
|
| ● |
expend cash;
|
| ● |
incur debt and assume liabilities; and
|
| ● |
incur amortization expenses related to intangible assets or incur large and immediate write-offs.
|
We also may be unable to find suitable acquisition candidates and we may not be able to complete acquisitions on favorable terms, if at all. If we do complete an acquisition, we cannot assure you that it will ultimately strengthen our competitive position or that it will not be viewed negatively by customers, financial markets or investors. Further, future acquisitions could also pose numerous additional risks to our operations, including:
| ● |
problems integrating the purchased business, products or technologies;
|
| ● |
increases to our expenses;
|
| ● |
the failure to have discovered undisclosed liabilities of the acquired asset or company;
|
| ● |
diversion of management’s attention from their day-to-day responsibilities;
|
| ● |
harm to our operating results or financial condition;
|
| ● |
entrance into markets in which we have limited or no prior experience; and
|
| ● |
potential loss of key employees, particularly those of the acquired entity.
|
We may not be able to complete one or more acquisitions or effectively integrate the operations, products or personnel gained through any such acquisition without a material adverse effect on our business, financial condition and results of operations.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations, or those of our vendors, could be subject to natural disasters, power shortages, telecommunications failures, water shortages, fires, medical epidemics and other manmade disasters or business interruptions, for which we or they are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third party manufacturers to produce our product candidates and supply the applicable therapeutic for our product candidates. Our ability to obtain clinical supplies of product candidates could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. Similar concerns will apply for commercial product supply. In addition, we rely on third party CROs and other third party vendors for the conduct of our clinical studies. Our ability to conduct such clinical studies could be disrupted, if the operations of these CROs and other third party vendors are affected by a manmade or natural disaster or other business interruption.
Our ability to use our net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2017, we had federal and state net operating loss carryforwards, or NOLs, of $101.5 million and $31.9 million, respectively, due to prior period losses. In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its NOLs to offset future taxable income. We believe that we may have already undergone one or more ownership changes.
In addition, future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Code. Although we have not completed an analysis under Section 382 of the Code, it is likely that the utilization of the NOLs will be limited. There is also a risk that due to regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, our existing NOLs could expire or otherwise be unavailable to offset future income tax liabilities. For these reasons, we may not be able to utilize a material portion of the NOLs reflected on our balance sheet, even if we attain profitability.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights, our competitive position could be harmed.
We depend on our ability to protect our proprietary technology. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with employees and third parties, all of which offer only limited protection. Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products. Where we have the right to do so under our license agreements, we seek to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business.
The patent positions of biotechnology and pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain.
The steps we have taken to police and protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the United States. The rights already granted under any of our currently issued/granted patents and those that may be granted under future issued/granted patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights, we do not know whether any of the pending patent applications for any of our product candidates will result in the issuance of patents that protect our technology or products, or will effectively prevent others from commercializing competitive technologies and products. Our pending applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Further, the examination process may require us, or for in-licensed technology, our licensors, to narrow the claims, which may limit the scope of patent protection that may be obtained. Although we have a number of issued/granted patents, the issuance/grant of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and issued/granted patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents, or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products.
Protecting against the unauthorized use of our patented technology, trademarks and other intellectual property rights is expensive, difficult and, may in some cases not be possible. In some cases, it may be difficult or impossible to detect third party infringement or misappropriation of our intellectual property rights, even in relation to issued/granted patent claims, and proving any such infringement may be even more difficult.
We could be required to incur significant expenses to obtain our intellectual property rights, and we cannot ensure that we will obtain meaningful patent protection for our products.
The patent prosecution process is expensive and time-consuming, and we, Evonik or any future licensors may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, it is also possible that we or our licensors will fail to identify patentable aspects of further inventions made in the course of our development and commercialization activities before they are publicly disclosed, making it too late to obtain patent protection on them. Further, given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms where these are available in any countries where we are prosecuting patents. This includes in the United States under the Drug Price Competition and Patent Term Restoration Act of 1984, which permits a patent term extension for regulatory delay of up to five years beyond the expiration date of a patent based only on its earliest filing date plus any patent term adjustments due to patent office delays during prosecution that covers an approved product where the permission for the commercial marketing or use of the product is the first permitted commercial marketing or use, and as long as the remaining term of the patent does not exceed fourteen years. We can likewise apply for Supplementary Protection Certificates in Europe to extend the statutory 20-year maximum patent term by up to five years. Similar legislation exists in Australia, Israel, Japan, and South Korea. However, the applicable authorities, including the FDA in the United States, and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States, and these foreign laws may also be subject to change. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
In March 2013, the United States transitioned to a ‘first to file’ system in which the first inventor to file a patent application that meets the requirements for patent eligibility is entitled to the patent. Third parties are allowed to submit prior art prior to the issuance of a patent by the USPTO, and may become involved in post-grant proceedings, for example, ex parte reexamination and inter partes review proceedings on patents granted from applications filed before or after March 16, 2013, post- grant review or derivation proceedings for patents granted from applications filed on or after March 16, 2013, or interference proceedings for applications filed before March 16, 2013, challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position with respect to third parties.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submissions, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued/granted patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patents and patent applications covering our product candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation may be necessary in the future to enforce or defend our intellectual property rights, to protect our trade secrets or to determine the validity and scope of our own intellectual property rights or the proprietary rights of others. This can be expensive and time consuming and results can be uncertain. Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend their intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property, particularly in certain parts of the world. Litigation could result in substantial costs and diversion of management resources, which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned by, or licensed to, us is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. If any of these occur, our business could be materially and adversely affected.
From time to time we may need to rely on licenses to proprietary technologies, which may be difficult, expensive or not possible to obtain or we may lose certain licenses which may be difficult or not possible to replace.
We may need to obtain licenses to patents and other proprietary rights held by third parties to develop, manufacture and market our product candidates. For example, EG-1962 requires a microparticulate delivery system to facilitate direct delivery to the brain. We have licensed certain patent rights and/or know-how from Evonik and Oakwood that may claim or cover EG-1962. It is possible that either or both of these licenses could be terminated. In that case, we may lose our ability to develop, manufacture or market certain products to the extent those products rely on Evonik and/or Oakwood patents and know-how. In such event or under other circumstances, we may have to obtain a new license from Evonik or Oakwood or some other third party, which licenses may not be available on commercially reasonable terms or at all. If we are unable to timely obtain these licenses on commercially reasonable terms and maintain these licenses, our ability to commercially market our product candidates may be inhibited or prevented, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates, and to use our proprietary technologies without infringing the proprietary rights of third parties. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference (for patents with an effective date before March 16, 2013) and various post grant proceedings before the USPTO, and opposition proceedings at other patent offices. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. In the event a third party were to assert an infringement claim against us and we were ultimately found to infringe the third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and commercializing our products and technology. However, we may not be able to obtain an appropriate license on commercially reasonable terms or at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, in any such proceeding or litigation, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Any claims by third parties that we have misappropriated their confidential information or trade secrets could have a similar negative impact on our business.
Our trade secrets are difficult to protect.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property.
Our success depends upon the skills, knowledge and experience of our scientific and technical personnel, our consultants and advisors as well as our partners, licensors and contractors. Because we operate in a highly competitive technical field of drug discovery, we rely in part on trade secrets to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We enter into confidentiality and invention assignment agreements with our employees and certain of our corporate partners, consultants, sponsored researchers and other advisors. These agreements generally require that the receiving party keep confidential and not disclose to third parties all confidential information developed by the receiving party or made known to the receiving party by us during the course of the receiving party’s relationship with us. These confidentiality and assignment agreements may be breached and may not effectively assign intellectual property rights to us.
Our trade secrets also could be independently discovered by competitors, in which case we would not be able to prevent use of such trade secrets by our competitors. The enforcement of a claim alleging that a party illegally obtained and was using our trade secrets could be difficult, expensive and time consuming and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. The failure to obtain or maintain meaningful trade secret protection could adversely affect our competitive position.
We may be subject to claims that our employees or consultants have wrongfully used or disclosed alleged trade secrets of their former employers or other third parties.
Many of our employees and consultants were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Some of these employees, including each member of our senior management, and consultants executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees and consultants have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s or consultant’s former employer. We are not aware of any threatened or pending claims related to these matters or concerning the agreements with our senior management, but in the future litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property disputes could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and/or management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates throughout the world could be prohibitively expensive.
Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued/granted patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business and will have uncertain outcomes.
Latest European General Court’s decisions on public access to clinical trial data held by EMA
On February 5, 2018, the European General Court delivered three judgments relating to Pari Pharma GmbH v EMA (Case T-235/1); MSD Animal Health Innovation and Intervet International v EMA (Case T-729/15) and PTC Therapeutics International v EMA (Case T-718/15). These judgments clarified the meaning of commercially confidential information, or CCI, and determined whether the EMA had failed to carry out balancing exercise, as required by EU law when deciding whether certain documents held by the EMA should be disclosed to third parties upon request. These European Court decisions are very significant in relation to the ability for third parties to access data contained in applications for marketing authorization which are held by the EMA.
The European General Court has ruled that the general presumption is subject to certain, narrowly construed criteria. Documents held by the EMA can be withheld from disclosure if they constitute CCI and it is incumbent upon the data owner to show specifically and actually how, once the documents have been disclosed, competitors would be able to enter the relevant market. The assembly of public and private data may constitute CCI if the assembly provides added value but there is no added value if the data were submitted in response to questions raised by the EMA. The fact that money or resources have been spent to obtain the information does not automatically make that information CCI. Data that have been obtained using study designs contained in publicly available guidelines are unlikely to be considered CCI. It is insufficient to refer to the documents as constituting a ‘road map’ for competitors to follow as any such assertion must be supported by unique and specific evidence. The Court has ruled that the unfair use of information by competitors is curtailed by the data exclusivity/protection principles; the fact that competitors have to run their own studies to obtain a marketing authorization; certain categories of information are already protected by the exceptions provided in the EU Public Access Regulation 1049/2001. The Court has ruled that misuse of the data by a competitor is not relevant to an assessment of CCI, and the argument that data exclusivity or protection may be lost due to use of the disclosed documents in third countries does not make the data in question confidential. As regards the requirement for a balancing exercise, the General Court has ruled that unless the documents are protected by an exception provided in the EU Public Access Regulation, the EMA is not under any obligation to show that there is an overriding public interest to justify disclosing the documents. When the EMA claims the existence of an overriding public interest, it must set out specific circumstances to justify the disclosure of CCI.
Risks Related to Our Financial Position and Capital Needs
We have incurred significant losses since our inception and anticipate that we will continue to incur losses for the foreseeable future.
We are a clinical-stage biotechnology company. Investment in biotechnology product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidate, such as EG-1962, our lead product candidate, will fail to gain regulatory approval or become commercially viable. We have not generated any revenue from product sales to date, and we continue to incur significant development and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses in each period since our inception in 2009. For the years ended December 31, 2017 and December 31, 2016, we reported a net loss of $50.9 million and $38.8 million, respectively.
We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates, and begin to commercialize any approved products. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If EG-1962 or any of our other product candidates fails in clinical studies or does not gain regulatory approval, or if approved, fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ (deficit) equity and working capital.
We have not generated any revenues since inception and may never become profitable.
We have not generated any revenues since our inception. Our ability to generate revenue and become profitable depends upon our ability to successfully obtain marketing approval and commercialize products, including EG-1962 or other product candidates that we may develop, in-license or acquire in the future. Even if we are able to successfully achieve regulatory approval for these product candidates, we do not know when any of these products will generate revenue for us, if at all. Our ability to generate revenue from EG-1962 or other product candidates also depends on a number of additional factors, including our ability to:
| ● |
successfully complete development activities, including the necessary clinical trials;
|
| ● |
complete and submit marketing authorization applications to the FDA and obtain regulatory approval for an indication for which there is a commercial market;
|
| ● |
complete and submit marketing authorization applications to, and obtain regulatory approval from, foreign regulatory authorities;
|
| ● |
set a commercially viable price for our products;
|
| ● |
develop and obtain commercial quantities of our products at acceptable cost levels;
|
| ● |
develop a commercial organization capable of sales, marketing and distribution for the products we intend to sell ourselves in the markets in which we have retained commercialization rights;
|
| ● |
find suitable partners to help us market, sell and distribute our approved products in other markets; and
|
| ● |
obtain coverage and adequate reimbursement from third party, including government, payors.
|
In addition, because of the numerous risks and uncertainties associated with product development, including that our product candidates may not advance through development or be shown to be safe and effective for their intended uses, the FDA or any other regulatory agency may require additional clinical trials or preclinical studies. We are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Even if we are able to complete the process described above, we anticipate incurring significant costs associated with commercializing these products.
Even if we are able to generate revenues from the sale of our products, we may not become profitable and may need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
We have a limited operating history, which may make it difficult for potential or current investors to evaluate the success of our business to date and to assess our future viability.
We were formed in January 2009. Our operations to date have been limited to organizing and staffing our company, acquiring or developing product and technology rights, and conducting product development activities for our product candidates, primarily EG- 1962. We have not yet obtained regulatory approval for any of our product candidates. Consequently, any predictions about our future success, performance or viability may not be as accurate as they could be if we had a longer operating history or approved products on the market.
We will require additional capital to fund our operations and if we fail to obtain necessary financing, we will not be able to complete the development and commercialization of our product candidates, including EG-1962.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to advance the clinical development of our product candidates and to launch and commercialize any product candidates for which we receive regulatory approval, including potentially building our own commercial organization to address the United States and certain other markets. As of December 31, 2017, we had cash and cash equivalents of $88.1 million. We may require additional capital for the further development of our product candidates and, if we conduct additional Phase 3 studies of EG-1962, we may need to raise additional funds sooner in order to accelerate development of our product candidates. Furthermore, we will need to raise additional funds to commercialize any of our product candidates.
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our products or product candidates or one or more of our other research and development initiatives. We also could be required to:
| ● |
seek collaborators or licensees for one or more of our current or future product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or
|
| ● |
relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves.
|
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in this “Risk Factors” section. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
| ● |
the progress, timing, costs and results of the clinical studies for our product candidates to obtain regulatory approval;
|
| ● |
the outcome of our efforts to demonstrate clinical superiority over Nymalize in order to obtain marketing approval prior to the expiration of Nymalize’s orphan drug exclusivity period, including whether FDA accepts data from a head-to-head study of EG-1962 against a Nimotop generic (gelcap) and nimodipine tablets to demonstrate superiority over Nymalize;
|
| ● |
the clinical development plans we establish for our product candidates;
|
| ● |
the number and characteristics of product candidates that we develop or may in-license;
|
| ● |
the outcome, timing and cost of meeting regulatory requirements established by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect;
|
| ● |
the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights;
|
| ● |
the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us or our product candidates;
|
| ● |
the effect of competing technological and market developments;
|
| ● |
the cost and timing of completion of commercial-scale outsourced manufacturing activities; and
|
| ● |
the cost of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own.
|
Our debt obligations expose us to risks that could adversely affect our business, operating results and financial condition and may result in further dilution to our shareholders.
We have amended our loan and security agreement with Hercules Capital, Inc., or Hercules (the “Amended Loan Agreement”), pursuant to which we may borrow up to $20.0 million from Hercules at an initial interest rate of 9.15% per annum, and for which we have paid Hercules an origination fee of $170,000. At closing, we borrowed $15.0 million of the amount available to draw under the Amended Loan Agreement (and received proceeds net of the amount then outstanding under the Original Loan Agreement (as defined below), fees and expenses). On May 23, 2017, we elected to draw down the second tranche of $5 million. We were required to repay the indebtedness on or before February 3, 2020, however, as a result of achieving certain milestones under the Amended Loan Agreement, the maturity date has been extended to August 3, 2020. Further, the loan originally required interest only payments through March 1, 2018, however as a result of achieving such milestones, the interest only payments have been extended to September 1, 2018, whereupon we must make 24 equal monthly payments of principal plus interest. To the extent we desire to prepay the indebtedness prior to maturity, we will be obligated to pay a prepayment penalty to Hercules ranging from 0.5% to 2% of the amounts being prepaid, depending on when such prepayment occurs. In addition, at the time that the loan is either due or prepaid, we must pay Hercules a fee equal to 4.5% of the total amounts funded at such time. Our ability to make payments on this indebtedness depends on our ability to generate cash in the future. We expect to experience negative cash flow for the foreseeable future as we fund our operations and capital expenditures. There can be no assurance that we will be in a position to repay this indebtedness when due or obtain extensions of the maturity date. We anticipate that we will need to secure additional funding in order for us to be able to satisfy our obligations when due. We cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. If that additional funding involves the sale of equity securities or convertible securities, it would result in the issuance of additional shares of our capital stock, which would result in dilution to our stockholders. The indebtedness is secured by substantially all of our assets other than intellectual property, on which we have given Hercules a negative pledge. In addition, under the Amended Loan Agreement, we are subject to certain customary covenants that limit or restrict our ability to, among other things, incur additional indebtedness, grant any security interests, pay cash dividends, repurchase our common stock, make loans, or enter into certain transactions without the prior consent of Hercules.
This level of debt could have important consequences to an investor in our securities. For example, it could:
| ● |
limit our flexibility in planning for the development, clinical testing, approval and marketing of our products;
|
| ● |
place us at a competitive disadvantage compared to any of our competitors that are less leveraged than we are;
|
| ● |
increase our vulnerability to both general and industry-specific adverse economic conditions; and
|
| ● |
limit our ability to obtain additional funds.
|
In addition, as part of a previous financing with Hercules, we issued a warrant to Hercules to purchase up to 78,596 shares of our Common Stock, which is exercisable until October of 2020. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” for a more detailed discussion of the transaction with Hercules.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements, although our existing loan with Hercules may limit the amount of additional debt we may issue. To the extent that we raise additional capital through the sale of equity or convertible debt securities, our then-existing stockholders’ ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of then-existing stockholders. Debt financings may be coupled with an equity component, such as warrants to purchase stock, which could also result in dilution of our then-existing stockholders’ ownership. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens being placed on our assets and intellectual property. If we were to default on such indebtedness, we could lose such assets and intellectual property. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us.
Risks Related to Ownership of Our Common Stock
The trading market in our common stock has been extremely limited and substantially less liquid than the average trading market for a stock quoted on the NASDAQ Global Market.
Prior to our initial public offering, or IPO, there was no market for shares of our common stock. Since our initial listing on the NASDAQ Global Market on October 1, 2015, the trading market in our common stock has been limited and substantially less liquid than the average trading market for companies quoted on the NASDAQ Global Market. The quotation of our common stock on the NASDAQ Global Market does not assure that a meaningful, consistent and liquid trading market currently exists. We cannot predict whether a more active market for our common stock will develop in the future. An absence of an active trading market could adversely affect our stockholders' ability to sell our common stock at current market prices in short time periods, or possibly at all. Additionally, market visibility for our common stock may be limited and such lack of visibility may have a depressive effect on the market price for our common stock. As of December 31, 2017, approximately 49% of our outstanding shares of common stock was held by our officers, directors, beneficial owners of 5% or more of our capital stock and their respective affiliates, which adversely affects the liquidity of the trading market for our common stock, inasmuch as federal securities laws restrict sales of our shares by these stockholders under certain circumstances. If our affiliates continue to hold their shares of common stock, there will be limited trading volume in our common stock, which may make it more difficult for investors to sell their shares or increase the volatility of our stock price.
Market volatility may affect our stock price and the value of our stockholders’ investment.
The trading price of our common stock, similar to other biotechnology companies, is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including, among others:
| ● |
announcement of the results of or any other information about our NEWTON 2 Phase 3 program for EG-1962 or any other future clinical studies of our product candidates, including any delays in enrollment rates or timing of these studies, as well as the lack of news about the status of our programs;
|
| ● |
regulatory actions with respect to our products or our competitors’ products;
|
| ● |
the recruitment or departure of key personnel;
|
| ● |
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments;
|
| ● |
results of clinical trials of our competitors;
|
| ● |
the success of competitive products or technologies;
|
| ● |
actual or anticipated changes in our growth rate relative to our competitors;
|
| ● |
regulatory or legal developments in the United States and other countries;
|
| ● |
developments or disputes concerning patent applications, issued/granted patents or other proprietary rights;
|
| ● |
the level of expenses related to any of our product candidates or clinical development programs;
|
| ● |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
| ● |
variations in our financial results or those of companies that are perceived to be similar to us;
|
| ● |
fluctuations in the valuation of companies perceived by investors to be comparable to us;
|
| ● |
share price and volume fluctuations attributable to inconsistent trading volume levels of our shares;
|
| ● |
announcement or expectation of additional financing efforts;
|
| ● |
sales of our common stock by us, our insiders or our other stockholders;
|
| ● |
changes in the structure of healthcare payment systems;
|
| ● |
market conditions in the pharmaceutical and biotechnology sectors; and
|
| ● |
general economic, industry and market conditions.
|
In addition, the stock market in general, and pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks, including those described in these “Risk Factors,” could have a dramatic and material adverse impact on the market price of our common stock.
If securities or industry analysts do not publish research, publish inaccurate or unfavorable research or cease publishing research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. The price of our common stock could decline if one or more equity research analysts downgrade our common stock or if analysts issue other unfavorable commentary or cease publishing reports about us or our business. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
Future sales of a substantial number of shares of our common stock in the public market or other issuances of our common stock or rights to purchase common stock, including pursuant to equity incentive plans could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
Our stock price could decline as a result of sales of a large number of shares of our common stock, including shares issuable upon exercise of stock options and warrants, or the perception that these sales could occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
As of December 31, 2017, the holders of up to 3,290,905 shares, or 11%, of our common stock outstanding, will have rights, subject to some conditions, to require us to file registration statements covering the sale of their shares or to include their shares in registration statements we may file for ourselves or other stockholders. Once we register the offer and sale of shares for the holders of registration rights, they can be freely sold in the public market.
In addition, in the future, we may issue additional shares of common stock or other equity or debt securities convertible into common stock in connection with a financing, acquisition, litigation settlement, employee arrangements, or otherwise. Any such issuance could result in substantial dilution to our then-existing stockholders and could cause our stock price to decline.
Future issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, or in connection with an acquisition, litigation settlement, employee arrangements or otherwise, we may sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of common stock or common stock-related securities, together with the exercise of outstanding options and warrants to purchase 6,837,448 shares of common stock as of December 31, 2017 and any additional shares issued in connection with acquisitions, if any, may result in material dilution to our then-existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our common stock.
We may be at an increased risk of securities litigation, which is expensive and could divert management attention.
The market price of our common stock may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of December 31, 2017, our executive officers, directors, holders of 5% or more of our capital stock and their respective affiliates beneficially owned approximately 49% of our outstanding voting stock (assuming no exercise of outstanding stock options). These stockholders may be able to determine the outcome of all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that our then-existing stockholders’ may feel are in their best interest. The interests of this group of stockholders may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including seeking a premium value for their common stock, and might affect the prevailing market price for our common stock.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation, or certificate of incorporation, and amended and restated bylaws, or bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These provisions include:
| ● |
authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval;
|
| ● |
prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of stockholders to elect director candidates;
|
| ● |
prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders;
|
| ● |
eliminating the ability of stockholders to call a special meeting of stockholders;
|
| ● |
establishing a staggered board of directors; and
|
| ● |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings.
|
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Delaware law, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our amended and restated certificate of incorporation or amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be our stockholders’ sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of our outstanding indebtedness preclude, and any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be our stockholders’ sole source of gain for the foreseeable future.
We are an “emerging growth company” and we intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which could potentially be for up to five years after the date of our IPO, which occurred on October 1, 2015. If investors find our common stock less attractive as a result of our reduced reporting requirements, there may be a less active trading market for our common stock and our stock price may be more volatile. We may also be unable to raise additional capital as and when we need it.
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. This annual report on Form 10-K includes a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However, for as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirement.
Our compliance with Section 404 requires that we incur additional accounting expense and management efforts. We currently do not have an internal audit group. We may not be able to complete any required Section 404 evaluation, testing and remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure our stockholders that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by NASDAQ, the Securities and Exchange Commission, or the SEC, or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
None.
Our corporate headquarters consists of approximately 20,410 square feet of office space located at 300 Connell Drive, Berkeley Heights, New Jersey, that we occupy under a 63 month lease which ends in November of 2021. While we believe that our existing facilities are adequate for our near-term needs, we expect to lease additional space prior to expiration of our existing lease to meet the needs of the business. We believe that suitable additional or alternative space would be available if required in the future on commercially reasonable terms.
From time to time in the ordinary course of our business, we are subject to claims, legal proceedings and disputes. We are not currently subject to any material legal proceedings.
Not applicable.
PART II
| ITEM 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities Market Information
|
On October 1, 2015, our common stock began trading on the NASDAQ Global Market under the symbol “EDGE”. Prior to that time, there was no public market for our common stock. Shares sold in our initial public offering on October 1, 2015 were priced at $11.00 per share.
On February 22, 2018, the closing price for our common stock as reported on the NASDAQ Global Market was $15.50. The following table sets forth the high and low sales prices per share of our common stock as reported on the NASDAQ Global Market for the period indicated.
|
Year Ended December 31, 2017
|
High
|
Low
|
||||||
|
Fourth Quarter
|
$
|
11.16
|
$
|
9.07
|
||||
|
Third Quarter
|
$
|
11.51
|
$
|
9.20
|
||||
|
Second Quarter
|
$
|
10.72
|
$
|
8.81
|
||||
|
First Quarter
|
$
|
12.99
|
$
|
7.62
|
||||
|
Year Ended December 31, 2016
|
High
|
Low
|
||||||
|
Fourth Quarter
|
$
|
13.50
|
$
|
9.25
|
||||
|
Third Quarter
|
12.29
|
8.61
|
||||||
|
Second Quarter
|
10.64
|
7.43
|
||||||
|
First Quarter
|
$
|
13.86
|
$
|
6.23
|
||||
Stockholders
As of February 22, 2018, there were 40 stockholders of record, which excludes stockholders whose shares were held in nominee or street name by brokers.
Performance Graph
The following graph illustrates a comparison of the total cumulative stockholder return for our common stock since October 1, 2015, which is the date our shares began trading, to two indices: the NASDAQ Composite Index and the NASDAQ Biotechnology Index. The graph assumes an initial investment of $100 on October 1, 2015, in our common stock, the stocks comprising the NASDAQ Composite Index, and the stocks comprising the NASDAQ Biotechnology Index. Historical stockholder return is not necessarily indicative of the performance to be expected for any future periods.
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act or the Exchange Act, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed under such acts.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. In addition, the terms of our outstanding indebtedness restrict our ability to pay dividends, and any future indebtedness that we may incur could preclude us from paying dividends. Any future determination related to dividend policy will be made at the discretion of our board of directors and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report.
Issuer Purchases of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Annual Report.
Use of Proceeds from Registered Securities
On October 6, 2015, we closed the sale of 8,412,423 shares of our Common Stock, including 1,097,272 shares of our Common Stock sold pursuant to the underwriters’ full exercise of their option to purchase additional shares, for aggregate gross offering proceeds of approximately $92.5 million at a price to the public of $11.00 per share. All of the shares issued and sold in the IPO were registered under the Securities Act pursuant to a Registration Statement on Form S-1, as amended (File No. 333-206416), which was declared effective by the SEC on September 30, 2015 and a Registration Statement on Form S-1 (File No. 333-207217) filed pursuant to Rule 462(b) of the Securities Act. The IPO commenced on September 30, 2015 and did not terminate until the sale of all of the shares offered.
We received aggregate net proceeds from our IPO of approximately $82.8 million, after deducting underwriting discounts and commissions and other offering expenses payable by us.
We intend to use our net proceeds from the IPO for the overall development of our product candidates. We have invested the net proceeds of the IPO in short-term, investment-grade, interest-bearing cash equivalents. There has been no material change in our planned use of the net proceeds from the IPO described in the IPO prospectus.
On April 21, 2017, we completed a registered direct common stock offering for gross proceeds of $18.0 million. We received approximately $17.4 million in net proceeds after deducting the finder's fee and other offering costs. We have invested the net proceeds in short-term, investment-grade, interest-bearing cash equivalents.
The selected financial data presented below for, and as of the end of, each of the years in the five year period ended December 31, 2017, have been derived from our audited financial statements and are not indicative of our future operating results. The following selected financial data should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and the notes thereto included elsewhere in this report. The selected financial data in this section are not intended to replace our financial statements and the related notes.
|
Year Ended December 31,
|
||||||||||||||||||||
|
2017
|
2016
|
2015
|
2014
|
2013
|
||||||||||||||||
|
Statement of Operations Data:
|
||||||||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
$
|
34,311,650
|
$
|
24,825,379
|
$
|
17,839,951
|
$
|
8,473,522
|
$
|
4,484,367
|
||||||||||
|
General and administrative
|
17,654,970
|
14,686,767
|
8,658,867
|
4,720,661
|
2,003,992
|
|||||||||||||||
|
Total operating expenses
|
51,966,620
|
39,512,146
|
26,498,818
|
13,194,183
|
6,488,359
|
|||||||||||||||
|
Loss from operations
|
(51,966,620
|
)
|
(39,512,146
|
)
|
(26,498,818
|
)
|
(13,194,183
|
)
|
(6,488,359
|
)
|
||||||||||
|
Other income (expense), net
|
(1,479,240
|
)
|
(1,154,838
|
)
|
(2,687,233
|
)
|
402,122
|
(853,739
|
)
|
|||||||||||
|
Loss before income taxes
|
(53,445,860
|
)
|
(40,666,984
|
)
|
(29,186,051
|
)
|
(12,792,061
|
)
|
(7,342,098
|
)
|
||||||||||
|
Benefit (provision) for income taxes
|
2,586,057
|
1,845,986
|
1,107,405
|
590,675
|
459,018
|
|||||||||||||||
|
Net loss
|
(50,859,803
|
)
|
(38,820,998
|
)
|
(28,078,646
|
)
|
(12,201,386
|
)
|
(6,883,080
|
)
|
||||||||||
|
Cumulative dividend on Series C, C-1 and C-2 convertible preferred stock (2)
|
-
|
-
|
(4,356,408
|
)
|
(1,580,701
|
)
|
(1,076,256
|
)
|
||||||||||||
|
Net loss attributable to common stockholders
|
$
|
(50,859,803
|
)
|
$
|
(38,820,998
|
)
|
$
|
(32,435,054
|
)
|
$
|
(13,782,087
|
)
|
$
|
(7,959,336
|
)
|
|||||
|
Loss per share attributable to common stockholders basic and diluted (1)
|
$
|
(1.67
|
)
|
$
|
(1.34
|
)
|
$
|
(4.01
|
)
|
$
|
(8.16
|
)
|
$
|
(4.71
|
)
|
|||||
|
Weighted average common shares outstanding basic and diluted (1)
|
30,393,952
|
28,864,216
|
8,087,924
|
1,688,475
|
1,688,475
|
|||||||||||||||
|
Year Ended December 31,
|
||||||||||||||||||||
|
2017
|
2016
|
2015
|
2014
|
2013
|
||||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Cash (2)
|
$
|
88,067,647
|
$
|
106,398,919
|
$
|
130,189,421
|
$
|
13,728,972
|
$
|
7,858,169
|
||||||||||
|
Total Assets
|
92,621,077
|
110,914,447
|
134,092,658
|
16,846,492
|
8,733,792
|
|||||||||||||||
|
Long Term Debt
|
17,382,907
|
14,953,143
|
3,025,423
|
2,327,515
|
-
|
|||||||||||||||
|
Convertible preferred stock (2)
|
-
|
-
|
-
|
36,788,409
|
20,680,692
|
|||||||||||||||
|
Accumulated deficit
|
(151,948,359
|
)
|
(101,074,968
|
)
|
(62,253,970
|
)
|
(29,818,916
|
)
|
(16,036,829
|
)
|
||||||||||
|
Total stockholders' equity (deficit) (2)
|
63,419,030
|
89,276,557
|
122,477,527
|
(27,833,747
|
)
|
(15,349,645
|
)
|
|||||||||||||
| (1) |
See Note 2 (H) to our audited financial statements for an explanation of the method used to calculate net loss per share of common stock, basic and diluted per share amounts.
|
| (2) |
On October 6, 2015, pursuant to the closure of the IPO, the company raised net proceeds of approximately $82.8 million. All outstanding shares of convertible preferred stock were converted into common stock.
|
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes appearing elsewhere in this Annual Report. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors. We discuss factors that we believe could cause or contribute to these differences below and elsewhere in this Annual Report, including those set forth under Item 1A. “Risk Factors” and under “Forward-Looking Statements” in this Annual Report.
We are a clinical-stage biotechnology company that discovers, develops and seeks to commercialize novel, hospital-based therapies capable of transforming treatment paradigms in the management of acute, life-threatening neurological and other conditions. Our initial product candidates target rare, acute, life-threatening neurological and other conditions for which we believe the approved existing therapies, if any, are inadequate.
We believe EG-1962, our lead product candidate, can fundamentally improve patient outcomes and transform the management of aSAH, which is bleeding around the brain due to a ruptured brain aneurysm. A single dose of EG-1962 delivers high concentrations of nimodipine, the current standard of care, directly to the brain with sustained drug exposure over 21 days. EG-1962 utilizes our proprietary, programmable, biodegradable polymer-based development platform, or our Precisa development platform, through a novel delivery mechanism that enables targeted and sustained drug exposure while potentially avoiding dose-limiting side effects associated with currently available formulations of nimodipine. EG-1962 has been granted orphan drug designation and Fast Track designation by the FDA, for the treatment of patients with SAH. The European Commission has granted orphan drug designation to EG-1962 for treatment of aSAH.
In July 2016, we commenced the Phase 3 NEWTON 2 study for EG-1962. NEWTON 2 is a multi-center, multi-national, randomized, double-blind, placebo-controlled, parallel-group study comparing the efficacy and safety of EG-1962 to standard of care oral nimodipine in adults with an aSAH. The primary endpoint of the NEWTON 2 study is the proportion of subjects with a favorable clinical outcome (a GOSE score of 6 – 8) at day 90. The secondary endpoint is the subject’s score on the MoCA. On December 29, 2017, we announced that an independent DMC recommended that the Phase 3 NEWTON 2 study of EG-1962 continue as planned, based on the completion of a pre-planned futility analysis. The DMC made this recommendation after evaluating data from the Day 90 follow-up visit of the first 150 patients randomized and treated, as well as the available safety data in the study. A pre-planned interim analysis will be performed following the 90 day outcome assessment of the 210th patient. We expect to complete the interim analysis by the end of April 2018 with an announcement of the results to follow. Unless the results of the interim analysis are overwhelmingly positive, which we believe will be hard to achieve, the NEWTON 2 study is expected to continue to full data readout, in which case we expect the results of the study to be available in late 2018. The final results of the NEWTON 2 study, if positive, could form the basis for a marketing application to the FDA and other global health regulatory authorities for the approval of EG-1962 for the treatment of aSAH. In the United States, we plan to use the regulatory pathway set forth in Section 505(b)(2) of the FD&C Act.
Our Phase 1/2 clinical study of EG-1962, which we refer to as our NEWTON study, met its primary and secondary endpoints of safety, tolerability, defining the MTD, and pharmacokinetics. The results of the principal exploratory efficacy endpoint from the 90-day follow-up demonstrated that 59% (27 of 46) of patients treated with EG-1962 experienced a favorable clinical outcome (a GOSE score of 6-8) versus 28% (5 of 18) of patients treated with the standard of care oral nimodipine. At the final assessment, of the 46 patients treated with EG-1962, 28% (13 of 46) of patients achieved the highest clinical outcome score (GOSE=8, Upper Good Recovery) versus 6% (1 of 18) patients treated with the standard of care oral nimodipine.
A Phase 1 study of the safety, pharmacokinetics and clinical outcomes of EG-1962 administered intracisternally, or directly into the basal cisterns of the brain, is open for enrollment for patients with aSAH who do not receive an EVD but remain at risk for delayed neurological complications following surgical repair of a ruptured aneurysm. This study is a multicenter, randomized, controlled, open-label study in which nine patients are expected to receive EG-1962 via intracisternal administration and three patients are expected to receive standard of care oral nimodipine.
In the United States, approximately 35,000 patients with an average age of 52 experience an SAH each year. Of these, approximately 20,000 experience an aSAH and are hospitalized in the intensive care unit, or ICU, and approximately 75% of these patients suffer permanent brain damage or die within 30 days. Care for these patients results in overall inpatient charges of more than $5.0 billion per year in the United States. After the ruptured aneurysm is repaired, these patients remain at risk for multiple complications that are managed over the course of the patient’s treatment to optimize clinical outcomes. The most common and severe complications, which are thought to be at least in part due to the influx of calcium, include cerebral vasospasm, or narrowing of the brain arteries, and DCI, a catastrophic delayed complication that occurs secondary to processes including cerebral vasospasm. DCI occurs in up to 30% of patients who survive the initial hemorrhage. DCI often leads to the death of brain tissue due to insufficient blood flow to certain areas of the brain and results in a significant economic burden to the health care system. The direct cost to hospitals in patients experiencing DCI is estimated to be approximately $50,000 in additional direct in-hospital per patient costs, but can increase to $70,000 based on the additional comorbidities associated with treating an aSAH patient and the extent of their disability. An analysis of the 2013 National Inpatient Sample Database showed that the aneurysmal subarachnoid hemorrhage patients who require an EVD and undergo either neurosurgical micro clipping or endovascular coiling for repair of their aneurysm, had the highest per-patient hospital charges, exceeding $400,000 per patient, and experienced the longest hospitalizations of all aneurysmal subarachnoid hemorrhage patients. Not included in this analysis, are the additional costs of rehabilitation, the long-term lifetime cost of illness related to an aSAH, the frequent inability to re-enter the work force, and the potential need for additional care at home from family members or long-term care givers associated with chronically disabled patients, which present additional significant economic burden to the healthcare system and the families of those afflicted with aSAH.
We have never been profitable and have incurred net losses in each year since inception. Our net losses were $50.9 million, $38.8 million and $28.1 million for the years ended December 31, 2017, 2016 and 2015 respectively. As of December 31, 2017, we had an accumulated deficit of $151.9 million. Substantially all of our net losses resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. Our net losses may fluctuate significantly from quarter to quarter and year to year.
We do not expect to generate any revenues from product sales unless and until we successfully complete development and obtain regulatory approval for one or more of our product candidates, which we expect will take a number of years, or we enter into outbound licensing or future collaboration agreements. If we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution.
Furthermore, as a result of our IPO in 2015, we expect to incur additional costs associated with operating as a public company. Accordingly, at least until we can generate significant revenue from product sales, we will seek to fund our operations through public or private equity or debt financings or other sources. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all and could be forced to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us in strategic partnerships and alliances and licensing arrangements. Our failure to raise capital or enter into such other arrangements when needed would have a negative impact on our financial condition and ability to develop our product candidates.
As of December 31, 2017, we had $88.1 million in cash and cash equivalents.
Financial Operations Overview
Revenue
We have not generated any revenues from commercial product sales and do not expect to generate any such revenue in the near future. We may generate revenue in the future from a combination of research and development payments, license fees and other upfront payments or milestone payments.
Research and Development Expenses
Research and development expenses include employee-related expenses, licensing fees to use certain technology in our research and development projects, costs of acquiring, developing and manufacturing clinical trial materials, as well as fees paid to consultants and various entities that perform certain research and testing on our behalf. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations or information provided by vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued expenses. Costs incurred in connection with research and development activities are expensed as incurred.
The following table summarizes our research and development expenses incurred for the periods indicated (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
EG-1962 product candidate
|
$
|
22,075
|
$
|
15,911
|
$
|
10,962
|
||||||
|
EG-1964 product candidate
|
640
|
319
|
1,091
|
|||||||||
|
Pipeline
|
371
|
392
|
71
|
|||||||||
|
Internal Operating Expenses
|
11,226
|
8,203
|
5,716
|
|||||||||
|
Total
|
$
|
34,312
|
$
|
24,825
|
$
|
17,840
|
||||||
We expect our research and development expenses to increase for the foreseeable future as we advance our product candidates through preclinical studies and clinical trials. The process of conducting preclinical studies and clinical trials necessary to obtain regulatory approval is costly and time-consuming. Successful development of future product candidates from our research and development programs is highly uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each future product candidate and are difficult to predict. We anticipate we will make determinations as to which product candidates to pursue and how much funding to direct to each product candidate on an ongoing basis in response to the scientific and clinical success of each product candidate as well as ongoing assessments as to the commercial potential of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation, related to our executive, finance, legal, business development and support functions. Other general and administrative expenses include travel expenses, professional fees for auditing, tax and legal services and facility-related costs.
The following table summarizes our general and administrative expenses incurred for the periods indicated (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
General and administrative expenses
|
$
|
17,655
|
$
|
14,687
|
$
|
8,659
|
||||||
We expect that general and administrative expenses will increase in the future as we expand our operating activities and incur additional costs associated with being a publicly-traded company. These increases will likely include legal, accounting and filing fees, directors’ and officers’ liability insurance premiums and fees and other costs associated with investor relations.
Warrant Remeasurement
Warrant remeasurement reflects adjustments to fair value of our liability-classified warrants. As of December 31, 2015, we no longer had liability classified warrants.
Other Expense
Other expense reflects the loss on asset disposal representing the book value of leasehold improvements at our former location due to the Company’s relocation of its corporate office and debt issuance costs on our new loan in 2016.
Interest Income
Interest income consists of interest income earned on our cash and cash equivalents.
Interest Expense
Interest expense consists of interest expense on our borrowings under the Amended Loan Agreement with Hercules.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in the notes to our financial statements appearing in this Annual Report, we believe that the following critical accounting policies are most important to understanding and evaluating our reported financial results.
Income Taxes
We file U.S. federal income tax returns and New Jersey state tax returns. Our deferred tax assets are primarily comprised of federal and state tax net operating losses and tax credit carryforwards and are recorded using enacted tax rates expected to be in effect in the years in which these temporary differences are expected to be utilized. At December 31, 2017, we had federal net operating loss, or NOL, carryforwards of approximately $101.5 million, which expire at various dates between 2029 and 2037. At December 31, 2017, we had federal research and development credits carryforwards of approximately $1.9 million and Orphan Drug credit of approximately $22.1 million. We may be subject to the net operating loss utilization provisions of Section 382 of the Internal Revenue Code. The effect of an ownership change would be the imposition of an annual limitation on the use of NOL carryforwards attributable to periods before the change. The amount of the annual limitation depends upon our value immediately before the ownership change, changes to our capital during a specified period prior to the change, and the federal published interest rate. Although we have not completed an analysis under Section 382 of the Code, it is likely that the utilization of the NOLs will be limited.
On December 22, 2017, H.R. 1 (also, known as the Tax Cuts and Jobs Act (the “Act”)) was signed into law. Among its numerous changes to the Internal Revenue Code, the Act reduces U.S. federal corporate tax rate to 21%. As a result, the Company believes that the most significant impact on its consolidated financial statements will be reduction of approximately $13.6 million for the deferred tax assets related to net operating losses and other assets. Such reduction is offset by changes to the Company’s valuation allowance. The Company is also in the process of considering the impact under the Act of the disallowance of certain incentive based compensation tax deductibility under Internal Revenue Code Section 162(m). If an adjustment to the deferred tax asset is required, the impact will be offset by a corresponding adjustment to the valuation allowance.
Accrued Clinical Expenses
When preparing our financial statements, we are required to estimate our accrued clinical expenses. This process involves reviewing open contracts and communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. Payments under some of the contracts we have with parties depend on factors, such as successful enrollment of certain numbers of patients, site initiation and the completion of clinical trial milestones.
When accruing clinical expenses, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If possible, we obtain information regarding unbilled services directly from our service providers. However, we may be required to estimate the cost of these services based only on information available to us. If we underestimate or overestimate the cost associated with a trial or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, our estimated accrued clinical expenses have approximated actual expense incurred.
Stock-based Compensation
We estimate the fair value of our stock-based option awards to employees and non-employees using the Black-Scholes option-pricing model, which requires the input of assumptions, including: (1) the expected volatility of our stock, (2) the expected term of the award, (3) the risk-free interest rate and (4) expected dividends. In accordance with FASB ASC 505, we re-measure the fair value of non-employee stock-based compensation as the awards vest, and recognize the resulting value, if any, as expense during the period the related services are rendered. We believe that all stock options issued under our stock option plans meet the criteria of “plain vanilla” stock options. The expected term of the options outstanding was determined using the “simplified” method as prescribed by Staff Accounting Bulletin, No. 107, Share Based Payment. The risk free interest rate is based on U.S. Treasury notes with remaining terms similar to the expected term of the option. The volatility was based on a representative group of small publicly traded drug development companies. The dividend yield assumption is zero since we have never paid cash dividends and have no present intention to pay cash dividends.
The fair value of options granted for the periods indicated was estimated using the Black-Scholes option valuation model utilizing the following assumptions:
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Weighted
Average
|
Weighted
Average
|
Weighted
Average
|
||||||||||
|
Volatility
|
88.87
|
%
|
77.20
|
%
|
79.80
|
%
|
||||||
|
Risk-Free Interest Rate
|
1.88
|
%
|
1.39
|
%
|
1.74
|
%
|
||||||
|
Expected Term in Years
|
6.00
|
6.02
|
6.05
|
|||||||||
|
Dividend Rate
|
0.00
|
%
|
0.00
|
%
|
0.00
|
%
|
||||||
|
Fair Value of Option on Grant Date
|
$
|
6.93
|
$
|
5.39
|
$
|
5.42
|
||||||
Stock-based compensation expense amounted to $6.2 million, $5.3 million, and $2.9 million for the years ended December 31, 2017, 2016 and 2015, respectively. At December 31, 2017, there was approximately $13.0 million of unamortized stock compensation expense, which is expected to be recognized over a remaining average vesting period of 2.48 years.
We expect the impact of our stock-based compensation expense for stock options to employees to grow in future periods due to the potential increases in the value of our common stock and headcount.
Basic and Diluted Net Loss Per Share of Common Stock
We compute basic and diluted net loss per share of common stock by dividing net loss attributable to common stockholders by the weighted average common shares outstanding during the period. For all periods presented, Common Stock underlying the options and warrants have been excluded from the calculation because their effect would be anti-dilutive. Therefore, the weighted average shares outstanding used to calculate both basic and diluted loss per common share are the same.
Results of Operations
Comparison of the Years Ended December 31, 2017 and 2016
|
Year Ended December 31,
|
Increase (Decrease)
|
|||||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Operating expenses:
|
||||||||||||||||
|
Research and development expenses
|
$
|
34,312
|
$
|
24,825
|
$
|
9,487
|
38
|
%
|
||||||||
|
General and administrative expenses
|
17,655
|
14,687
|
2,968
|
20
|
%
|
|||||||||||
|
Total operating expenses
|
51,967
|
39,512
|
12,455
|
32
|
%
|
|||||||||||
|
Loss from operations
|
(51,967
|
)
|
(39,512
|
)
|
(12,455
|
)
|
32
|
%
|
||||||||
|
Other expense
|
-
|
(163
|
)
|
163
|
100
|
%
|
||||||||||
|
Interest income (expense), net
|
(1,479
|
)
|
(992
|
)
|
(487
|
)
|
49
|
%
|
||||||||
|
Loss before income taxes
|
(53,446
|
)
|
(40,667
|
)
|
(12,779
|
)
|
31
|
%
|
||||||||
|
Benefit for income taxes
|
2,586
|
1,846
|
740
|
40
|
%
|
|||||||||||
|
Net loss and comprehensive loss
|
$
|
(50,860
|
)
|
$
|
(38,821
|
)
|
$
|
(12,039
|
)
|
31
|
%
|
|||||
Research and Development Expenses
Research and development expenses increased to $33.3 million in the year ended December 31, 2017 from $24.8 million for the same period in 2016. The increase of $9.5 million was primarily attributable to an increase in external expenses for the EG-1962 and EG-1964 product of $6.1 million and $0.3 million, respectively and additional internal personnel costs of $3.0 million to support the growth in our R&D activities.
General and Administrative Expenses
General and administrative expenses increased to $17.7 million in the year ended December 31, 2017 from $14.7 million for the same period in 2016. The $3.0 million increase was due primarily to increases in personnel costs of $1.3 million, stock based compensation of $0.4 million, legal fees $0.3 million and investor relations of $0.7 million and professional fees of $0.2 million.
Other Expense
Other expense reflects the non-recurring loss on asset disposal in 2016 representing the book value of leasehold improvements at our former location due to the Company's relocation of its corporate office and debt issuance costs on our new loan.
Interest Income and Expense, net
Interest income and expense, net increased primarily due to interest expense for our loan offset by an increase in interest income from interest earned on our cash and cash equivalents.
Benefit for Income Taxes
Benefit for income taxes increased as a result of selling additional New Jersey Net Operating Losses in 2017 as compared to 2016.
Comparison of the Years Ended December 31, 2016 and 2015
The following table summarizes the results of our operations for the years ended December 31, 2016 and 2015:
|
Year Ended December 31,
|
Increase (Decrease)
|
|||||||||||||||
|
2016
|
2015
|
$
|
%
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Operating expenses:
|
||||||||||||||||
|
Research and development expenses
|
$
|
24,825
|
$
|
17,840
|
$
|
6,985
|
39
|
%
|
||||||||
|
General and administrative expenses
|
14,687
|
8,659
|
6,028
|
70
|
%
|
|||||||||||
|
Total operating expenses
|
39,512
|
26,499
|
13,013
|
49
|
%
|
|||||||||||
|
Loss from operations
|
(39,512
|
)
|
(26,499
|
)
|
(13,013
|
)
|
49
|
%
|
||||||||
|
Warrant remeasurement
|
-
|
(1,880
|
)
|
1,880
|
(100
|
)%
|
||||||||||
|
Other expense
|
(163
|
)
|
-
|
(163
|
)
|
100
|
%
|
|||||||||
|
Interest income (expense), net
|
(992
|
)
|
(807
|
)
|
(185
|
)
|
23
|
%
|
||||||||
|
Loss before income taxes
|
(40,667
|
)
|
(29,186
|
)
|
(11,481
|
)
|
39
|
%
|
||||||||
|
Benefit for income taxes
|
1,846
|
1,107
|
739
|
67
|
%
|
|||||||||||
|
Net loss and comprehensive loss
|
$
|
(38,821
|
)
|
$
|
(28,079
|
)
|
$
|
(10,742
|
)
|
38
|
%
|
|||||
Research and Development Expenses
Research and development expenses increased to $24.8 million in the year ended December 31, 2016 from $17.8 million for the same period in 2015. The increase of $7.0 million was primarily attributable to an increase in external expenses for the EG-1962 product of $5.0 million and additional internal personnel costs of $2.9 million to support the growth in our R&D activities, offset by a decrease in the EG-1964 study of $0.7 million.
General and Administrative Expenses
General and administrative expenses increased to $14.7 million in the year ended December 31, 2016 from $8.7 million for the same period in 2015. The $6.0 million increase was due primarily to increases in personnel costs of $1.1 million, stock based compensation of $1.4 million, facilities expense of $0.8 million, insurance costs of $0.7 million and professional fees of $1.9 million
Warrant Remeasurement
Warrant remeasurement reflects adjustments to fair value of our liability classified warrants. As of December 31, 2015, we no longer had liability classified warrants.
Other Expense
Other expense reflects the loss on asset disposal representing the book value of leasehold improvements at our former location due to the Company’s relocation of its corporate office and debt issuance costs on our new loan.
Interest Income and Expense, net
Interest income and expense, net increased primarily due to interest expense for our loan offset by an increase in interest income from interest earned on our cash and cash equivalents.
Benefit for Income Taxes
Benefit for income taxes increased as a result of selling additional New Jersey Net Operating Losses in 2016 as compared to 2015.
Liquidity and Capital Resources
Since our inception and through December 31, 2017, we have raised aggregate net proceeds of $207.9 million to fund our operations, primarily $82.8 million from the sale of Common Stock, $87.5 million from the sale of preferred stock, $17.4 million net proceeds from a registered direct common stock offering and $20.0 million from a loan. As of December 31, 2017, we had total cash and cash equivalents of $88.1 million as compared to $106.4 million as of December 31, 2016. The $18.3 million decrease in total cash was due primarily to increased funding of operations, which mainly consisted of research and development activities and general and administrative expenses offset by proceeds from a registered direct common stock offering and issuance of debt.
On October 6, 2015, we completed the IPO of our common stock for aggregate gross proceeds of $92.5 million. We received $82.8 million in net proceeds after deducting underwriting discounts and commissions and other offering costs of $9.7 million. In connection with the IPO, all preferred stock was converted into common stock. There is no preferred stock outstanding as of December 31, 2017.
On April 21, 2017, we completed a registered direct common stock offering for gross proceeds of $18.0 million. We received $17.4 million in net proceeds after deducting the finder's fee and other offering costs.
We have no committed sources of capital. Therefore, until such time, if ever, that we generate product revenue, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings and research collaboration and license agreements. We may be unable to raise capital or enter into such other arrangements when needed or on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed may have a negative impact on our financial condition and our ability to develop our product candidates.
Hercules Loan and Security Agreement
On August 28, 2014, we entered into a loan and security agreement with Hercules Technology Growth Capital, Inc. (the "Original Loan Agreement"). The Original Loan Agreement provided funding for an aggregate principal amount of up to $10.0 million in three separate term loans. The first term loan was funded on August 28, 2014 in the amount of $3.0 million. The second term loan of $3.0 million was funded on January 29, 2015. Both the first and second term loans were due to mature on March 1, 2018. We elected not to draw the third term loan of $4.0 million, the availability of which expired on June 30, 2015. Initially, the loan bore interest at a rate per annum equal to the greater of (i) 10.45% or (ii) the sum of (a) 10.45% plus (b) the prime rate (as reported in The Wall Street Journal) minus 4.50%. On April 6, 2015, the base interest rate on the loan was lowered to the greater of (i) 9.95% or (ii) the sum of (a) 9.95% plus (b) the prime rate (as reported in The Wall Street Journal) minus 4.50. We were required to make interest-only payments on the loan through September 2015.
Commencing in October 2015, the term loans began amortizing in equal monthly installments of principal and interest over 30 months. On the maturity date or the date the loan otherwise became due and payable, we were also required to make a payment equal to 1.5% of the total amounts funded under the Original Loan Agreement.
On August 1, 2016, we entered into an Amended and Restated Loan and Security Agreement (the “Amended Loan Agreement”) with Hercules Capital, Inc., formerly known as Hercules Technology Growth Capital, Inc. Pursuant to the Amended Loan Agreement, we may borrow up to $20.0 million. At closing, we borrowed $15.0 million of the amount available for draw under the Amended Loan Agreement (and received proceeds net of the amount then outstanding under the Original Loan Agreement, fees and expenses). On May 23, 2017, we elected to draw down the second tranche of $5 million. Amounts drawn under the Amended Loan Agreement bear interest at a rate per annum equal to the greater of either (i) the sum of (a) 9.15%, plus (b) the prime rate as reported in The Wall Street Journal minus 4.50% or (ii) 9.15%. The effective interest rate on the loan as of December 31, 2017 was 9.15%. The Amended Loan Agreement requires interest-only payments until March 1, 2018, and on that date we begin to repay the principal balance of the loan in 24 equal monthly payments of principal and interest through the scheduled maturity date of February 3, 2020. In January 2018, we satisfied a certain condition as described in the Amended Loan Agreement to extend the period of interest-only payments to September 1, 2018 and the maturity date was extended to August 3, 2020. The period only interest-only payments may be extended for an additional six months at the lender’s discretion.
Pursuant to the Amended Loan Agreement, in March 2018, we must make a payment of $90,000 which is equal to 1.5% of the total amounts funded under the Original Loan Agreement. On the maturity date or the date the loan otherwise becomes due and payable, under the Amended Loan Agreement we must also make a payment of $900,000, which is equal to 4.5% of the total amounts available under the Amended Loan Agreement. In addition, if we prepay the term loan (i) during the first year following the initial closing, we must pay a prepayment charge equal to 2% of the amount being prepaid, (ii) during the second year following the closing, we must pay a prepayment charge equal to 1% of the amount being prepaid, and (iii) after the second year following the closing, we must pay a prepayment charge equal to 0.5% of the amount being prepaid.
The loan is secured by substantially all of our assets, other than intellectual property, which is the subject of a negative pledge. Under the Amended Loan Agreement, we are subject to certain customary covenants that limit or restrict our ability to, among other things, incur additional indebtedness, investments, distributions, transfer assets, make acquisitions, grant any security interests, pay cash dividends, repurchase its Common Stock, make loans, or enter into certain transactions without prior consent. The Amended Loan Agreement contains several events of default, including, among others, payment defaults, breaches of covenants or representations, material impairment in the perfection of Hercules’ security interest or in the collateral and events related to bankruptcy or insolvency. Upon an event of default, Hercules may declare all outstanding obligations immediately due and payable (along with a prepayment charge), a default rate of an additional 5.0% may be applied to the outstanding loan balances, and Hercules may take such further actions as set forth in the Amended Loan Agreement, including collecting or taking such other action with respect to the collateral pledged in connection with the Amended Loan Agreement.
Cash flows
The following table shows a summary of our cash flows for each of the periods indicated (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Net cash used in operating activities
|
$
|
(40,697
|
)
|
$
|
(32,189
|
)
|
$
|
(21,753
|
)
|
|||
|
Net cash used in investing activities
|
(188
|
)
|
(687
|
)
|
(1,305
|
)
|
||||||
|
Net cash provided by financing activities
|
22,554
|
9,085
|
139,518
|
|||||||||
|
Net (decrease) increase in cash
|
$
|
(18,331
|
)
|
$
|
(23,791
|
)
|
$
|
116,460
|
||||
Net Cash Used in Operating Activities
Net cash used in operating activities was $40.7 million, $32.2 million and $21.8 million for the years ended December 31, 2017, 2016 and 2015, respectively. The increase in cash used in operating activities of $8.5 million in 2017 was primarily due to an increase in our research and development expenses of $9.4 million and general and administrative expenses of $3.0 million offset by an increase in the sale of New Jersey NOL of $0.7 million and increase in accounts payable and accrued expenses of $3.1 million. The increase in cash used in operating activities of $10.4 million in 2016 was primarily due to an increase in our research and development expenses of $6.9 million and general and administrative expenses of $5.9 million offset by an increase in the sale of New Jersey NOL and increase in accounts payable.
Net Cash Used in Investing Activities
Net cash used in investing activities was $0.2 million, $0.7 million and $1.3 million for the years ended December 31, 2017, 2016 and 2015, respectively, which in each period relates entirely to purchases of property and equipment.
Net Cash Provided by Financing Activities
Net cash provided by financing activities of $22.6 million for the year ended December 31, 2017 was primarily due to the receipt of net proceeds from the issuance of common stock of $17.4 million and debt of $5.0 million.
Net cash provided by financing activities of $9.1 million for the year ended December 31, 2016 was primarily due to the receipt of net proceeds from the issuance of debt of $10.8 million less payments of our existing debt obligations of $1.5 million and deferred offering costs of $0.5 million.
Net cash provided by financing activities of $139.5 million for the year ended December 31, 2015 was primarily due to the proceeds from the issuance of common stock of $82.8 million, preferred stock of $52.4 million and debt of $3.0 million.
Operating Capital Requirements
We expect that our primary uses of capital will continue to be third-party clinical research, development and manufacturing services, compensation and related expenses, laboratory and related supplies, legal and other regulatory expenses and general administrative costs. We believe that our existing cash and cash equivalents as of December 31, 2017, will be sufficient to meet our anticipated cash requirements for at least the next 12 months.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Moreover, if circumstances are favorable, we may seek to secure additional capital opportunistically. Our future capital requirements are difficult to forecast and will depend on many factors, including:
|
●
|
the initiation, progress, timing, costs and results of the clinical trials for our product candidates to meet regulatory approval, particularly whether the FDA requires us to complete a second Phase 3 trials for EG-1962 or requires changes to the anticipated design of our Phase 3 program for EG-1962, such as changes in the required control arm of any such trial;
|
|
●
|
the outcome of interactions with the FDA and other non-U.S. health authorities that may alter our proposed Phase 3 program for EG-1962 that is required to meet the standards of a marketing authorization approval in aSAH;
|
|
●
|
the clinical development plans we establish for our product candidates;
|
|
●
|
the number and characteristics of product candidates that we develop or may acquire or in-license;
|
|
●
|
the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights;
|
|
●
|
the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us or our product candidates;
|
|
●
|
the effect of competing technological and market developments;
|
|
●
|
the cost and timing of completion of both clinical and commercial-scale manufacturing activities, which may be outsourced; and
|
|
●
|
the cost of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own.
|
Please see the section titled "Risk Factors" elsewhere in this Annual Report for additional risks associated with our substantial capital requirements.
We will need to raise additional capital and may seek collaborations in the future in order to further advance our various product candidates and, if such product candidates are successful, in order to commercialize such product candidates. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates or one or more of our other research and development initiatives. We also could be required to seek collaborators for one or more of our current or future product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available or relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves. Our failure to raise capital as and when needed would have a material adverse effect on our financial condition and our ability to pursue our business strategy.
Contractual Obligations and Commitments
The following is a summary of our contractual obligations as of the date indicated:
|
As of December 31, 2017
|
Total
|
Less than
One Year
|
1-3 Years
|
3-5 Years
|
More than
5 Years
|
|||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Debt principal, interest and fees
|
$
|
24,221
|
$
|
4,986
|
$
|
19,235
|
$
|
-
|
$
|
-
|
||||||||||
|
Operating lease obligations
|
2,340
|
602
|
1,208
|
530
|
-
|
|||||||||||||||
|
Milestone payments
|
3,000
|
500
|
2,500
|
-
|
-
|
|||||||||||||||
|
Total contractual obligations
|
$
|
29,561
|
$
|
6,088
|
$
|
22,943
|
$
|
530
|
$
|
-
|
||||||||||
This table above does not include (a) any milestone payments related to contingent events which may become payable to third parties under our license agreements as the timing and likelihood of such payments are not known, or (b) contracts that are entered into in the ordinary course of business which are not material in the aggregate in any period presented above.
Purchase Commitments
We have no material non-cancelable purchase commitments with service providers as we have generally contracted on a cancelable, purchase order basis.
Milestone and Royalty-based Commitments
Pursuant to the Evonik Agreement, in exchange for the license, we agreed to make milestone payments totaling up to $14.75 million upon the achievement of certain development, regulatory and sales milestones detailed in the Evonik Agreement. We paid $0.25 million upon execution of the Evonik Agreement. In August 2016, we paid a milestone of $1.0 million after we dosed the first patient in the Phase 3 clinical trial of EG-1962. In addition, the Evonik Agreement calls for us to pay royalties on sales of certain products based on a mid-single digit percentage of net sales. The Evonik Agreement provides for the reduction of royalties in certain circumstances.
Under the Restated Development Agreement, we agreed to pay Oakwood to perform services under agreed upon project plans and to pay Oakwood up to an aggregate of $4.5 million. In July 2017, we paid $1.5 million of such aggregate amount in connection with entering into the Restated Development Agreement. Of the remaining $3.0 million of such aggregate amount, $0.5 million is payable no later than April 1, 2018 and $2.5 million is payable no later than April 1, 2019. These remaining payments may be accelerated if: (i) we achieve various regulatory milestones, (ii) we close an equity or similar financing in excess of a predetermined amount, or (iii) there is an early termination, under certain circumstances, of the Restated Development Agreement or the Supply Agreement with Oakwood. In addition, the Restated Development Agreement calls for us to pay royalties on sales of certain products based on a low single digit percentage of net sales of EG-1962, regardless of the manufacturer or supplier thereof.
Pursuant to the Oakwood Commercial Supply Agreement, the price per unit of EG-1962 to be purchased by us is based on the expected commercially usable units per batch. In addition, we have agreed to pay Oakwood milestone payments that could total up to an aggregate of $2.25 million upon the achievement of certain development and regulatory milestones.
JOBS Act
On April 5, 2012, the JOBS Act was enacted. Section 107 of the JOBS Act provides that an "emerging growth company" can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an "emerging growth company" can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
Subject to certain conditions set forth in the JOBS Act, as an "emerging growth company," we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor's attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding a supplement to the auditor's report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis.
Off-balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
The primary objectives of our investment activities are to ensure liquidity and to preserve principal, while at the same time maximizing the income we receive from our cash and cash equivalents without significantly increasing risk. As of December 31, 2017, we had cash and cash equivalents of $88.1 million that were held in a non-interest-bearing money operating account and an institutional U.S. Treasury money market fund. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the short-term maturities of our cash equivalents and the low risk profile of our investments, an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our cash equivalents. To minimize the risk in the future, we intend to maintain our portfolio of cash equivalents and short-term investments in institutional market funds that are comprised of U.S. Treasury and Treasury backed repurchase agreements.
The financial statements required to be filed pursuant to this Item 8 are appended to this report. An index of those financial statements is found in Item 15.
None.
Evaluation of Disclosure Controls and Procedures
An evaluation was carried out, under the supervision of and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15 (e)) under the Exchange Act of 1934 as of the end of the period covered by this report. Based on the evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures are effective to ensure that the information required to be disclosed by us in the reports we file or submit under the Exchange Act was recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rules 13a—15(f) and 15d-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed under the supervision of our principal executive officer and principal financial officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles.
Our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived, operated, tested and monitored, can provide only reasonable, not absolute, assurance that the objectives of the control system are met because of inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. As a result of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Management evaluated the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework (the 2013 Framework). Management, under the supervision and with the participation of the principal executive officer and principal financial officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2017 and concluded that it was effective.
Our independent registered public accounting firm has not performed an evaluation of our internal control over financial reporting during any period in accordance with the provisions of the Sarbanes-Oxley Act. For as long as we remain an "emerging growth company" as defined in the JOBS Act, we intend to take advantage of the exemption.
Changes in Internal Control over Financial Reporting
There were no changes to our internal control over financial reporting that occurred during the period covered by this Annual Report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Not applicable.
PART III
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders or an amendment to this Annual Report, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders or an amendment to this Annual Report, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
| ITEM 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders or an amendment to this Annual Report, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders or an amendment to this Annual Report, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders or an amendment to this Annual Report, which we intend to file with the SEC within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
PART IV
| (a) |
The following documents are filed as part of this report:
|
(1) Financial Statements:
Report of Independent Registered Public Accounting Firm
Balance Sheets
Statements of Operations and Comprehensive Loss
Statements of Convertible Preferred Stock and Changes in Stockholders' Equity (Deficit)
Statements of Cash Flows
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules:
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto.
(3) Exhibits. The exhibits filed as part of this Annual Report are set forth on the Exhibit Index immediately following our consolidated financial statements. The Exhibit Index is incorporated herein by reference.
None.
|
Exhibit
Number
|
Exhibit Description
|
|
|
Eighth Amended and Restated Certificate of Incorporation of Edge Therapeutics, Inc. (filed as exhibit 3.1 to the Company's Current Report on Form 8-K filed on October 6, 2015, and incorporated by reference herein).
|
||
|
Second Amended and Restated Bylaws of Edge Therapeutics, Inc. (filed as exhibit 3.2 to the Company's Current Report on Form 8-K filed on October 6, 2015, and incorporated by reference herein).
|
||
|
Form of Certificate of Common Stock. (filed as exhibit 4.1 to the Company's Pre-Effective Amendment No. 1 to the registration statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
||
|
Warrant to Purchase 16,667 Shares of Capital Stock Issued to New Jersey Economic Development Authority, dated as of May 3, 2010. (filed as exhibit 4.2 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
||
|
First Amendment to Warrant Issued to New Jersey Economic Development Authority, dated October 9, 2013 (filed as exhibit 4.3 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Form of Warrant Issued to Series B-1 Stockholders. (filed as exhibit 4.4 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Form of Warrant to Purchase Series C Preferred Stock issued to Maxim Group LLC. (filed as exhibit 4.5 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Warrant Agreement, dated as of August 28, 2014, by and between the Company and Hercules. (filed as exhibit 4.6 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Form of Warrant to Purchase Series C-1 Preferred Stock issued to Maxim Group LLC. (filed as exhibit 4.7 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Investors' Rights Agreement, dated as of April 6, 2015, by and among the Company and the Investors named therein. (filed as exhibit 4.8 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Warrant to Purchase 18,000 Shares of Common Stock issued to Maxim Partners LLC, dated as of October 6, 2015. (filed as exhibit 4.1 to the Company's Quarterly Report on Form 10-Q filed on November 6, 2015, and incorporated by reference herein).
|
||
|
Licensing Agreement by and between the Company and Evonik Industries (as successor in interest to SurModics Pharmaceuticals, Inc.), dated as of October 20, 2010. (filed as exhibit 10.1 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Amendment No. 1 to the License Agreement, effective as of September 21, 2015, by and between the Company and Evonik Corporation. (filed as exhibit 10.15 to the Company's Pre-Effective Amendment No. 1 to the registration statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
||
|
Amended and Restated Master Formulation Development Agreement, by and between the Company and Oakwood Laboratories LLC, dated as of June 30, 2017 (filed as exhibit 10.1 to the Company’s Form 10-Q for the quarter ended June 30, 2017 and incorporated by reference herein).
|
||
|
Manufacturing and Supply Agreement, by and between the Company and Oakwood Laboratories LLC, dated as of June 30, 2017 (filed as Exhibit 10.2 to the Company’s Form 10-Q for the quarter ended June 30, 2017 and incorporated by reference herein).
|
||
|
Edge Therapeutics, Inc. 2010 Equity Incentive Plan and forms of agreement thereunder. (filed as exhibit 10.2 to the Company's Pre-Effective Amendment No. 1 to the registration statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
|
Amendment to the Edge Therapeutics, Inc. 2010 Equity Incentive Plan, dated June 30, 2014 (filed as exhibit 10.11 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Edge Therapeutics, Inc. 2012 Equity Incentive Plan and forms of agreement thereunder. (filed as exhibit 10.3 to the Company's Pre-Effective Amendment No. 1 to the registration statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
||
|
Edge Therapeutics, Inc. 2014 Equity Incentive Plan and forms of agreement thereunder. (filed as exhibit 10.4 to the Company's Pre-Effective Amendment No. 1 to the registration statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
||
|
Second Amended and Restated Employment Agreement by and between Brian A. Leuthner and the Company dated as of June 10, 2015. (filed as exhibit 10.5 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Second Amended and Restated Employment Agreement by and between Albert N. Marchio, II and the Company dated as of June 8, 2015 (filed as exhibit 10.8 to the Company's Registration Statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Amended and Restated Employment Agreement by and between Herbert J. Faleck and the Company dated as of August 11, 2015 (filed as exhibit 10.13 to the Company's registration statement on Form S-1 (File No. 333- 206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
Second Amended and Restated Employment Agreement by and between Dr. R. Loch Macdonald and the Company dated September 21, 2015 (filed as exhibit 10.14 to the Company's Pre-Effective Amendment No. 1 to the registration statement on Form S-1 (File No. 333- 206416) filed on September 21, 2015, and incorporated by reference herein).
|
||
|
Executive Employment Agreement by and between W. Bradford Middlekauff and the Company dated as of October 30, 2015 (filed as exhibit 10.1 to the Company's Current Report on Form 8-K filed on November 5, 2015, and incorporated by reference herein).
|
||
|
Employment Agreement by and between Daniel Brennan and the Company, dated as of October 17, 2016 (filed as exhibit 10.1 to the Company's Current Report on Form 8-K filed on October 18, 2016, and incorporated by reference herein).
|
||
|
Employment Agreement by and between Alyssa Wyant and the Company, dated as of February 21, 2017.
|
||
|
Executive Employment Agreement by and between Andrew Saik and the Company dated as of October 31, 2017 (file as exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 1, 2017, and incorporated by reference herein).
|
||
|
Form of Indemnification Agreement for officers and directors (filed as exhibit 10.9 to the Company's Registration Statement on Form S-1 (File No. 333-206416) filed on August 14, 2015, and incorporated by reference herein).
|
||
|
10.18
|
Form of Executive Stock Option Agreement.
|
|
|
10.19
|
Form of Employee Stock Option Agreement.
|
|
|
Lease, dated February 18, 2016, between The Connell Company and Edge Therapeutics, Inc. (filed as exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on May 3, 2016, and incorporated by reference herein).
|
||
|
Amended and Restated Loan and Security Agreement, dated as of August 1, 2016, by and between the Company and Hercules Capital, Inc. (filed as exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on November 1, 2016, and incorporated by reference herein).
|
||
|
Consent of KPMG LLP (filed herewith).
|
||
|
Principal Executive Officer's Certifications Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
Principal Financial Officer's Certifications Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
32.1 (1)
|
Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.2 (1)
|
Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
| (1) |
This certification is deemed not filed for purpose of section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act.
|
| + |
Indicates management contract or compensatory plan.
|
| * |
Confidential Treatment has been granted with respect to certain portions of this Exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
|
| ** |
Confidential Treatment has been requested with respect to certain portions of this Exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
|
Pursuant to the requirements of the Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Edge Therapeutics, Inc.
|
|
|
March 1, 2018
|
By: /s/ Brian A. Leuthner
|
|
Brian A. Leuthner
|
|
|
President and Chief Executive Officer
|
|
|
(Principal Executive Officer)
|
|
|
March 1, 2018
|
By: /s/ Andrew Saik
|
|
Andrew Saik
|
|
|
Chief Financial Officer
|
|
|
(Principal Financial Officer)
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this Form 10-K has been signed by the following persons in the capacities indicated below and on the dates indicated:
|
Signature
|
Title
|
Date
|
|
/s/ Brian A. Leuthner
|
President and Chief Executive Officer and Director
(Principal Executive Officer)
|
March 1, 2018
|
|
Brian A. Leuthner
|
||
|
/s/ Andrew Saik
|
Chief Financial Officer
(Principal Financial Officer)
|
March 1, 2018
|
|
Andrew Saik
|
||
|
/s/ Albert N. Marchio, II
|
Chief Accounting Officer
(Principal Accounting Officer)
|
March 1, 2018
|
|
Albert N. Marchio, II
|
||
|
/s/ Sol Barer
|
Chairman, Board of Directors
|
March 1, 2018
|
|
Sol Barer, Ph.D.
|
|
/s/ Isaac Blech
|
Vice Chairman, Board of Directors
|
March 1, 2018
|
|
Isaac Blech
|
||
|
/s/ Kurt Conti
|
Director
|
March 1, 2018
|
|
Kurt Conti
|
||
|
/s/ Rosemary A. Crane
|
Director
|
March 1, 2018
|
|
Rosemary A. Crane
|
||
|
/s/ James I. Healy
|
Director
|
March 1, 2018
|
|
James I. Healy, M.D., Ph.D.
|
||
|
/s/ James Loughlin
|
Director
|
March 1, 2018
|
|
James Loughlin
|
||
|
/s/ R. Loch Macdonald
|
Chief Scientific Officer and Director
|
March 1, 2018
|
|
R. Loch Macdonald, M.D., Ph.D.
|
||
|
/s/ Liam Ratcliffe
|
Director
|
March 1, 2018
|
|
Liam Ratcliffe, M.D., Ph.D.
|
||
|
/s/ Robert Spiegel
|
Director
|
March 1, 2018
|
|
Robert Spiegel, M.D.
|
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Edge Therapeutics, Inc.:
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Edge Therapeutics, Inc. (the Company) as of December 31, 2017 and 2016, and the related statements of operations and comprehensive loss, convertible preferred stock and changes in stockholders’ equity (deficit), and cash flows for each of the years in the three-year period ended 2017 and the related notes (collectively, the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KPMG LLP
We have served as the Company’s auditor since 2014.
Short Hills, New Jersey
March 1, 2018
EDGE THERAPEUTICS, INC.
Balance Sheets
|
December 31,
2017
|
December 31,
2016
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
88,067,647
|
$
|
106,398,919
|
||||
|
Prepaid expenses and other current assets
|
986,680
|
954,581
|
||||||
|
Total current assets
|
89,054,327
|
107,353,500
|
||||||
|
Property and equipment, net
|
3,423,880
|
3,418,077
|
||||||
|
Other assets
|
142,870
|
142,870
|
||||||
|
Total assets
|
$
|
92,621,077
|
$
|
110,914,447
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
LIABILITIES
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
4,369,133
|
$
|
3,471,032
|
||||
|
Accrued expenses
|
5,422,205
|
3,213,715
|
||||||
|
Short term debt
|
3,075,421
|
-
|
||||||
|
Total current liabilities
|
12,866,759
|
6,684,747
|
||||||
|
Noncurrent liability:
|
||||||||
|
Long term debt
|
17,382,907
|
14,953,143
|
||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Preferred stock, 5,000,000 shares authorized at December 31, 2017 and 2016, zero outstanding
|
-
|
-
|
||||||
|
Common stock, $0.00033 par value, 75,000,000 shares authorized at December 31, 2017 and December 31, 2016, 30,869,205 shares and 28,918,516 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively
|
10,400
|
9,756
|
||||||
|
Additional paid-in capital
|
214,309,370
|
190,341,769
|
||||||
|
Accumulated deficit
|
(151,948,359
|
)
|
(101,074,968
|
)
|
||||
|
Total stockholders' equity
|
62,371,411
|
89,276,557
|
||||||
|
Total liabilities and stockholders' equity
|
$
|
92,621,077
|
$
|
110,914,447
|
||||
See accompanying notes to the financial statements.
EDGE THERAPEUTICS, INC.
Statements of Operations and Comprehensive Loss
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development expenses
|
$
|
34,311,650
|
$
|
24,825,379
|
$
|
17,839,951
|
||||||
|
General and administrative expenses
|
17,654,970
|
14,686,767
|
8,658,867
|
|||||||||
|
Total operating expenses
|
51,966,620
|
39,512,146
|
26,498,818
|
|||||||||
|
Loss from operations
|
(51,966,620
|
)
|
(39,512,146
|
)
|
(26,498,818
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Warrant remeasurement
|
-
|
-
|
(1,879,823
|
)
|
||||||||
|
Other expense
|
-
|
(163,463
|
)
|
-
|
||||||||
|
Interest income
|
700,903
|
212,299
|
9,084
|
|||||||||
|
Interest expense
|
(2,180,143
|
)
|
(1,203,674
|
)
|
(816,494
|
)
|
||||||
|
Loss before income taxes
|
(53,445,860
|
)
|
(40,666,984
|
)
|
(29,186,051
|
)
|
||||||
|
Benefit for income taxes
|
2,586,057
|
1,845,986
|
1,107,405
|
|||||||||
|
Net loss and comprehensive loss
|
(50,859,803
|
)
|
(38,820,998
|
)
|
(28,078,646
|
)
|
||||||
|
Cumulative dividend on Series C, C-1 and C-2 convertible preferred stock
|
-
|
-
|
(4,356,408
|
)
|
||||||||
|
Net loss attributable to common stockholders
|
$
|
(50,859,803
|
)
|
$
|
(38,820,998
|
)
|
$
|
(32,435,054
|
)
|
|||
|
Loss per share attributable to common stockholders basic and diluted
|
$
|
(1.67
|
)
|
$
|
(1.34
|
)
|
$
|
(4.01
|
)
|
|||
|
Weighted average common shares outstanding basic and diluted
|
30,393,952
|
28,864,216
|
8,087,924
|
|||||||||
See accompanying notes to the financial statements.
EDGE THERAPEUTICS, INC.
Statements of Convertible Preferred Stock and Changes in Stockholders' Equity (Deficit)
|
Convertible Preferred Stock
|
Common Stock
|
Additional
Paid-in
Capital
|
Deficit
Accumulated
|
Total
|
||||||||||||||||||||||||
|
Shares
Issued
|
Amount
|
Shares
Issued
|
Amount
|
|||||||||||||||||||||||||
|
Balance - January 1, 2015
|
11,895,755
|
$
|
36,788,409
|
1,688,475
|
$
|
770
|
$
|
1,984,399
|
$
|
(29,818,916
|
)
|
$
|
(27,833,747
|
)
|
||||||||||||||
|
Issuance of Series C-2 Preferred Stock, net of issuance costs of $3,782,650
|
12,043,006
|
52,217,328
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Other
|
-
|
2,130
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||
|
Dividend Series C Preferred Stock
|
-
|
1,101,926
|
-
|
-
|
-
|
(1,101,926
|
)
|
(1,101,926
|
)
|
|||||||||||||||||||
|
Dividend Series C-1 Preferred Stock
|
-
|
1,008,346
|
-
|
-
|
-
|
(1,008,346
|
)
|
(1,008,346
|
)
|
|||||||||||||||||||
|
Dividend Series C-2 Preferred Stock
|
-
|
2,246,136
|
-
|
-
|
-
|
(2,246,136
|
)
|
(2,246,136
|
)
|
|||||||||||||||||||
|
Conversion of Preferred Stock to Common Stock upon initial public offering
|
(23,938,761
|
)
|
(93,364,275
|
)
|
18,566,856
|
6,127
|
93,358,148
|
-
|
93,364,275
|
|||||||||||||||||||
|
Initial public offering of common stock, net of issuance costs
|
-
|
-
|
8,412,423
|
2,776
|
82,752,836
|
-
|
82,755,612
|
|||||||||||||||||||||
|
Conversion of Preferred Stock Warrant to Common Stock Warrant
|
-
|
-
|
-
|
-
|
3,726,043
|
-
|
3,726,043
|
|||||||||||||||||||||
|
Issuance of common stock from exercise of stock options
|
-
|
-
|
4,753
|
1
|
1,093
|
-
|
1,094
|
|||||||||||||||||||||
|
Issuance of common stock from exercise of warrants
|
-
|
-
|
138,338
|
46
|
(46
|
)
|
-
|
-
|
||||||||||||||||||||
|
Stock based compensation expense
|
-
|
-
|
-
|
-
|
2,899,304
|
-
|
2,899,304
|
|||||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
(28,078,646
|
)
|
(28,078,646
|
)
|
|||||||||||||||||||
|
Balance - December 31, 2015
|
-
|
-
|
28,810,845
|
9,720
|
184,721,777
|
(62,253,970
|
)
|
122,477,527
|
||||||||||||||||||||
|
Stock based compensation expense
|
-
|
-
|
-
|
-
|
5,305,070
|
-
|
5,305,070
|
|||||||||||||||||||||
|
Issuance of common stock from exercise of stock options
|
-
|
-
|
63,639
|
21
|
293,967
|
-
|
293,988
|
|||||||||||||||||||||
|
Issuance of common stock from exercise of warrants
|
-
|
-
|
44,032
|
15
|
20,955
|
-
|
20,970
|
|||||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
(38,820,998
|
)
|
(38,820,998
|
)
|
|||||||||||||||||||
|
Balance - December 31, 2016
|
-
|
-
|
28,918,516
|
9,756
|
190,341,769
|
(101,074,968
|
)
|
89,276,557
|
||||||||||||||||||||
|
Stock based compensation expense
|
-
|
-
|
-
|
-
|
6,182,841
|
-
|
6,182,841
|
|||||||||||||||||||||
|
Issuance of common stock, net of issuance costs
|
-
|
-
|
1,800,000
|
594
|
17,382,349
|
-
|
17,382,943
|
|||||||||||||||||||||
|
Issuance of common stock from exercise of stock options
|
-
|
-
|
35,366
|
12
|
118,176
|
-
|
118,188
|
|||||||||||||||||||||
|
Issuance of common stock from exercise of warrants
|
-
|
-
|
94,200
|
31
|
53,118
|
-
|
53,149
|
|||||||||||||||||||||
|
Issuance of common stock from 401K match
|
-
|
-
|
21,123
|
7
|
217,529
|
-
|
217,536
|
|||||||||||||||||||||
|
Cumulative-effect of new share-based compensation guidance
|
-
|
-
|
-
|
-
|
13,588
|
(13,588
|
)
|
-
|
||||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
(50,859,803
|
)
|
(50,859,803
|
)
|
|||||||||||||||||||
|
Balance - December 31, 2017
|
-
|
$
|
-
|
30,869,205
|
$
|
10,400
|
$
|
214,309,370
|
$
|
(151,948,359
|
)
|
$
|
62,371,411
|
|||||||||||||||
See accompanying notes to the financial statements.
EDGE THERAPEUTICS, INC.
Statements of Cash Flows
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$
|
(50,859,803
|
)
|
$
|
(38,820,998
|
)
|
$
|
(28,078,646
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Stock-based compensation expense
|
6,182,841
|
5,305,070
|
2,899,304
|
|||||||||
|
Stock-based 401K company common match
|
217,536
|
-
|
-
|
|||||||||
|
Warrant remeasurement
|
-
|
-
|
1,879,823
|
|||||||||
|
Depreciation expense
|
182,918
|
100,117
|
53,116
|
|||||||||
|
Loss on disposal of fixed assets
|
-
|
102,788
|
-
|
|||||||||
|
Amortization of debt discount
|
32,869
|
75,214
|
104,311
|
|||||||||
|
Amortization of debt issuance costs
|
108,407
|
90,800
|
94,648
|
|||||||||
|
Non-cash interest expense
|
363,909
|
175,909
|
38,521
|
|||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Prepaid expenses and other assets
|
(32,099
|
)
|
38,794
|
(814,156
|
)
|
|||||||
|
Accounts payable
|
898,101
|
1,420,556
|
(71,751
|
)
|
||||||||
|
Accrued expenses
|
2,208,490
|
(676,918
|
)
|
2,142,187
|
||||||||
|
Net cash used in operating activities
|
(40,696,831
|
)
|
(32,188,668
|
)
|
(21,752,643
|
)
|
||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchases of property and equipment
|
(188,721
|
)
|
(686,705
|
)
|
(1,305,086
|
)
|
||||||
|
Net cash used in investing activities
|
(188,721
|
)
|
(686,705
|
)
|
(1,305,086
|
)
|
||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Proceeds from issuance of debt
|
5,000,000
|
11,022,286
|
3,000,000
|
|||||||||
|
Proceeds from exercise of stock options
|
118,188
|
293,988
|
1,094
|
|||||||||
|
Proceeds from exercise of warrants
|
53,149
|
20,970
|
-
|
|||||||||
|
Payments for issuance costs
|
-
|
(544,773
|
)
|
(1,402,845
|
)
|
|||||||
|
Payments for debt issuance costs
|
-
|
(219,042
|
)
|
-
|
||||||||
|
Repayment of debt
|
-
|
(1,488,558
|
)
|
(533,729
|
)
|
|||||||
|
Proceeds from issuance of common stock, net of underwriting costs
|
17,382,943
|
-
|
86,059,087
|
|||||||||
|
Proceeds from issuance of preferred stock, net of issuance costs
|
-
|
-
|
52,394,571
|
|||||||||
|
Net cash provided by financing activities
|
22,554,280
|
9,084,871
|
139,518,178
|
|||||||||
|
Net (decrease) increase in cash
|
(18,331,272
|
)
|
(23,790,502
|
)
|
116,460,449
|
|||||||
|
Cash and cash equivalents at beginning of period
|
106,398,919
|
130,189,421
|
13,728,972
|
|||||||||
|
Cash and cash equivalents at end of period
|
$
|
88,067,647
|
$
|
106,398,919
|
$
|
130,189,421
|
||||||
|
Supplemental disclosure of cash flow information:
|
||||||||||||
|
Cash paid for:
|
||||||||||||
|
Interest
|
$
|
1,635,562
|
$
|
790,402
|
$
|
559,175
|
||||||
|
Supplemental cash flow information:
|
||||||||||||
|
Conversion of Preferred Stock to Common Stock
|
$
|
-
|
$
|
-
|
$
|
93,364,275
|
||||||
|
Conversion of Preferred Stock Warrants to Common Stock Warrants
|
$
|
-
|
$
|
-
|
$
|
3,726,043
|
||||||
|
Deferred issuance costs included in accrued expenses and accounts payable
|
$
|
-
|
$
|
-
|
$
|
549,178
|
||||||
|
Non-cash financing costs
|
$
|
-
|
$
|
-
|
$
|
175,114
|
||||||
|
Accrued capital expenditures included in accrued expenses and accounts payable
|
$
|
-
|
$
|
167,285
|
$
|
71,040
|
||||||
See accompanying notes to the financial statements.
Edge Therapeutics, Inc.
Notes to Financial Statements
Note 1 - Nature of operations:
Edge Therapeutics, Inc. (the "Company") is a clinical-stage biotechnology company that discovers, develops and seeks to commercialize novel, hospital-based therapies capable of transforming treatment paradigms in the management of acute, life-threatening neurological and other conditions. The Company's initial product candidates target rare, acute, life-threatening neurological and other conditions for which the Company believes the approved existing therapies, if any, are inadequate. The Company's product candidates utilize its proprietary, programmable, biodegradable polymer-based development platform (the Precisa Platform), a novel delivery mechanism that seeks to enable targeted and sustained drug exposure and avoid the dose-limiting side effects associated with the current standards of care.
From the Company's inception, it has devoted substantially all of its efforts to business planning, engaging regulatory, manufacturing and other technical consultants, acquiring operating assets, planning and executing clinical trials and raising capital. The Company's future operations are highly dependent on a combination of factors, including (i) the success of its research and development, (ii) the development of competitive therapies by other biotechnology and pharmaceutical companies, and, ultimately, (iii) regulatory approval and market acceptance of the Company's proposed future products.
Note 2 - Summary of Significant Accounting Policies
| (A) |
Use of estimates:
|
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
| (B) |
Significant risks and uncertainties:
|
The Company's operations are subject to a number of factors that may affect its operating results and financial condition. Such factors include, but are not limited to: the results of clinical testing and trial activities of the Company's product candidates, the Company's ability to obtain regulatory approval to market its products, the Company's intellectual property, competition from products manufactured and sold or being developed by other companies, the price of, and demand for, Company products if approved for sale, the Company's ability to negotiate favorable licensing or other manufacturing and marketing agreements for its products, and the Company's ability to raise capital.
The Company currently has no commercially approved products and there can be no assurance that the Company's research and development programs will be successfully commercialized. Developing and commercializing a product requires significant time and capital and is subject to regulatory review and approval as well as competition from other biotechnology and pharmaceutical companies. The Company operates in an environment of rapid change and is dependent upon the continued services of its employees and consultants and obtaining and protecting its intellectual property.
| (C) |
Cash equivalents and concentration of cash balance:
|
The Company considers all highly liquid securities with a maturity weighted average of less than three months to be cash equivalents. The Company's cash and cash equivalents in bank deposit accounts, at times, may exceed federally insured limits.
| (D) |
Property and equipment:
|
Property and equipment is recorded at cost. Depreciation is recorded for property and equipment using the straight-line method over the estimated useful lives of three to five years. Leasehold improvements are amortized over the shorter of the estimated useful life or term of the underlying lease. The Company reviews the recoverability of all long-lived assets, including the related useful lives, whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset might not be recoverable.
| (E) |
Research and development:
|
Costs incurred in connection with research and development activities are expensed as incurred. These costs include licensing fees to use certain technology in the Company's research and development projects as well as fees paid to consultants and various entities that perform certain research and testing on behalf of the Company.
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data, such as patient enrollment, clinical site activations or information provided by vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred.
| (F) |
Patent costs:
|
The Company expenses patent costs as incurred and classifies such costs as general and administrative expenses in the accompanying statements of operations and comprehensive loss.
| (G) |
Stock-based compensation:
|
The Company measures employee stock-based awards at grant-date fair value and recognizes employee compensation expense on a straight-line basis over the vesting period of the award.
Determining the appropriate fair value of stock-based awards requires the input of subjective assumptions, including, for stock options, the expected life of the option, and expected stock price volatility. The Company uses the Black-Scholes option pricing model to value its stock option awards. The assumptions used in calculating the fair value of stock-based awards represent management's best estimates and involve inherent uncertainties and the application of management's judgment. As a result, if factors change and management uses different assumptions, stock-based compensation expense could be materially different for future awards.
The expected life of stock options was estimated using the "simplified method," as the Company has limited historical information to develop reasonable expectations about future exercise patterns and employment duration for its stock options grants. The simplified method is based on the average of the vesting tranches and the contractual life of each grant. For stock price volatility, the Company uses comparable public companies as a basis for its expected volatility to calculate the fair value of options grants. The risk-free interest rate is based on U.S. Treasury notes with a term approximating the expected life of the option.
| (H) |
Net loss per common share:
|
Basic and diluted net loss per common share is determined by dividing net loss attributable to common stockholders by the weighted average common shares outstanding during the period. For all periods presented, the common shares underlying the preferred stock, common stock options and warrants have been excluded from the calculation because their effect would be anti-dilutive. Therefore, the weighted average shares outstanding used to calculate both basic and diluted loss per common share are the same.
The following potentially dilutive securities have been excluded from the computations of diluted weighted average shares outstanding as they would be anti-dilutive:
|
As of December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Stock options to purchase Common Stock
|
6,462,795
|
5,316,511
|
4,302,267
|
|||||||||
|
Warrants to purchase Common Stock
|
374,653
|
541,415
|
600,184
|
|||||||||
|
Total
|
6,837,448
|
5,857,926
|
4,902,451
|
|||||||||
|
(I)
|
Income taxes:
|
The Company provides for deferred income taxes under the asset and liability method, which requires deferred tax assets and liabilities to be recognized for the future tax consequences attributable to net operating loss carryforwards and for differences between the financial statement carrying amounts and the respective tax bases of assets and liabilities. Deferred tax assets are reduced if necessary by a valuation allowance if it is more likely than not that some portion or all of the deferred tax assets will not be realized.
| (J) |
Fair value of financial instruments:
|
Financial Accounting Standards Board ("FASB") guidance specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
|
●
|
Level 1 — Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities.
|
|
●
|
Level 2 — Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active). Level 2 includes financial instruments that are valued using models or other valuation methodologies.
|
|
●
|
Level 3 — Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable.
|
| (K) |
Subsequent events:
|
Subsequent events have been evaluated through the date these financial statements were issued.
| (L) |
New accounting standards:
|
In February 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2016-02, "Leases (Topic 842)." The new standard requires organizations that lease assets—referred to as "lessees"—to recognize on the balance sheet the assets and liabilities for the rights and obligations created by those leases (see Note 9). This standard is effective for annual reporting periods beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted. The Company is evaluating the impact of adoption.
In March 2016, the FASB issued ASU No. 2016-09 which simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. Public companies will be required to adopt this standard in annual reporting periods beginning after December 15, 2016, and interim periods within those annual periods. The Company adopted this ASU on January 1, 2017.
The impact of adopting ASU 2016-09 resulted in the following:
|
●
|
The Company recognized $84,786 of tax benefit along with a full valuation allowance as of the adoption date related to the historical excess tax benefits from historical option exercises related to employee equity award activity.
|
|
●
|
The Company elected to recognize forfeitures as they occur. The cumulative effect adjustment as a result of the adoption of this amendment on a modified retrospective basis was not material.
|
There were no other material impacts to our consolidated financial statements as a result of adopting this updated standard.
Note 3 – Fair Value of Financial Instruments
|
Fair Value Measurements at Reporting Date Using
|
||||||||||||||||
|
Total
|
Quoted Prices in
Active Markets
(Level 1)
|
Quoted Prices in
Inactive Markets
(Level 2)
|
Significant
Unobservable Inputs
(Level 3)
|
|||||||||||||
|
As of December 31, 2017:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
88,067,647
|
$
|
88,067,647
|
$
|
-
|
$
|
-
|
||||||||
|
As of December 31, 2016:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
106,398,919
|
$
|
106,398,919
|
$
|
-
|
$
|
-
|
||||||||
There were no transfers between Levels 1, 2, or 3 during 2017 or 2016.
Note 4 – Property and Equipment
Property and equipment is summarized as follows:
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Furniture and equipment
|
$
|
564,596
|
$
|
456,515
|
||||
|
Leasehold Improvements
|
438,996
|
358,356
|
||||||
|
Construction in Process
|
2,725,569
|
2,725,569
|
||||||
|
3,729,161
|
3,540,440
|
|||||||
|
Less accumulated depreciation
|
(305,281
|
)
|
(122,363
|
)
|
||||
|
Property and equipment, net
|
$
|
3,423,880
|
$
|
3,418,077
|
||||
Note 5 – Accrued Expenses
Accrued expenses and other liabilities consist of the following:
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Accrued research and development costs
|
$
|
2,857,025
|
$
|
654,795
|
||||
|
Accrued professional fees
|
267,646
|
366,394
|
||||||
|
Accrued compensation
|
1,886,638
|
1,866,255
|
||||||
|
Accrued other
|
385,896
|
319,434
|
||||||
|
Deferred rent
|
25,000
|
6,837
|
||||||
|
Total
|
$
|
5,422,205
|
$
|
3,213,715
|
||||
Note 6 - Convertible Preferred Stock
Immediately prior to the closing of the IPO on October 6, 2015, all of the outstanding shares of convertible preferred stock, including shares received for accrued dividends, automatically converted into 18,566,856 shares of common stock at the applicable conversion ratio then in effect. There were no shares of preferred stock outstanding as of December 31, 2017 and 2016.
Dividends
The holders of the Series C, Series C-1 and Series C-2 Convertible Preferred Stock were entitled to receive, when, as and if declared by the board, cumulative dividends at the rate of 8% of the original purchase price per annum. The Series C, Series C-1 and Series C-2 dividends accrued from the date of issuance and were payable semi-annually on January 1 and July 1 in cash or common stock at the Company's option. In accordance with accounting literature, Series C, Series C-1 and Series C-2 dividends since the date of issuance have been accrued in conjunction with the conversion of the Preferred Stock into Common.
The other series of Convertible Preferred Stock had no dividend requirement.
Stock Warrants
In connection with certain of our preferred stock sales and debt issuances we issued warrants to the placement agent and lender, for preferred stock. The warrants were recorded as liabilities with changes in fair value being recorded in the statement of operations and are calculated utilizing the Black-Scholes option pricing model. At the closing of the IPO date on October 6, 2015 these warrants become exercisable for shares of our common stock. These warrants were exercisable for 600,184 shares of common stock at exercise prices ranging from $5.79 to $12.10 and expire at various dates through 2020. During 2017 and 2016, 166,762 and 58,769 warrants were exercised resulting in the issuance of 94,200 and 44,032 shares of common stock, respectively. As of December 31, 2017, 374,653 warrants were exercisable.
Note 7 - Stock Options
The Company has three equity compensation plans: the 2010 Equity Incentive Plan, the 2012 Equity Incentive Plan and the 2014 Equity Incentive Plan (the "Plans"). Originally, the Company was able to grant up to 548,206 and 1,096,411 shares of Common Stock as both incentive stock options ("ISOs") and nonqualified stock options ("NQs") under the 2010 Equity Incentive Plan and the 2012 Equity Incentive Plan, respectively. In 2013, the Company's stockholders approved an increase to 1,279,146 shares authorized for issuance under the 2010 Equity Incentive Plan. In 2014, the Board of Directors of the Company (the "Board") approved an increase to 1,350,412 shares authorized for issuance under the 2010 Equity Incentive Plan.
In 2014, the Company's stockholders approved the 2014 Equity Incentive Plan pursuant to which the Company may grant up to 1,827,351 shares as both ISOs and NQs, subject to increases as hereafter described (the "Plan Limit"). However, on January 1, 2015 and each January 1 thereafter prior to the termination of the 2014 Equity Incentive Plan, pursuant to the terms of the 2014 Equity Incentive Plan, the Plan Limit was and shall be increased by the lesser of (x) 4% of the number of shares of Common Stock outstanding as of the immediately preceding December 31 and (y) such lesser number as the Board of Directors may determine in its discretion. On January 1, 2016 the Plan Limit was increased to 3,047,323 shares. As of January 1, 2017, the Plan Limit increased to 4,204,063.
Pursuant to the terms of the Plans, ISOs have a term of ten years from the date of grant or such shorter term as may be provided in the option agreement. Unless specified otherwise in an individual option agreement, ISOs generally vest over a four year term and NQs generally vest over a three or four year term. Unless terminated by the Board, the Plans shall continue to remain effective for a term of ten years or until such time as no further awards may be granted and all awards granted under the Plans are no longer outstanding.
The Company issued the following non-qualified options to purchase shares of common stock to its newly appointed executives. The awards were granted outside of the Company's 2014 Equity Incentive Plan and vests over four years with 25% vesting one year following the date of hire, and the remaining 75% vesting in 36 equal monthly installments thereafter, subject to continued service to the Company through each vesting date and subject to acceleration or forfeiture upon the occurrence of certain events as set forth in the applicable option agreement and employment agreement. The grant awards were made pursuant to the NASDAQ inducement grant exception as a material component of employment compensation.
|
Issue Date
|
25% Vesting Date
|
Executive
|
Number
of Options
|
|||
|
November 16, 2015
|
October 30, 2016
|
SVP, General Counsel and Secretary
|
80,000
|
|||
|
November 1, 2016
|
October 17, 2017
|
Chief Operating Officer
|
150,000
|
|||
|
March 1, 2017
|
February 28, 2018
|
SVP, Regulatory Affairs
|
80,000
|
|||
|
November 1, 2017
|
October 31, 2018
|
Chief Financial Officer
|
200,000
|
The Company's stock-based compensation expense was recognized in operating expense as follows:
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Stock-Based Compensation
|
||||||||||||
|
Research and development
|
$
|
2,687,975
|
$
|
2,177,643
|
$
|
1,129,556
|
||||||
|
General and administrative
|
3,494,866
|
3,127,427
|
1,769,748
|
|||||||||
|
Total
|
$
|
6,182,841
|
$
|
5,305,070
|
$
|
2,899,304
|
||||||
The fair value of options and warrants granted during the years ended December 31, 2017, 2016 and 2015 was estimated using the Black-Scholes option valuation model utilizing the following assumptions:
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Weighted
Average
|
Weighted
Average
|
Weighted
Average
|
||||||||||
|
Volatility
|
88.87
|
%
|
77.20
|
%
|
79.80
|
%
|
||||||
|
Risk-Free Interest Rate
|
1.88
|
%
|
1.39
|
%
|
1.74
|
%
|
||||||
|
Expected Term in Years
|
6.00
|
6.02
|
6.05
|
|||||||||
|
Dividend Rate
|
0.00
|
%
|
0.00
|
%
|
0.00
|
%
|
||||||
|
Fair Value of Option on Grant Date
|
$
|
6.93
|
$
|
5.39
|
$
|
5.42
|
||||||
The following table summarizes the number of options outstanding and the weighted average exercise price:
|
Number of
Shares
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contractual
Life in Years
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Options outstanding at January 1, 2015
|
2,445,711
|
3.13
|
||||||||||||||
|
Granted
|
1,902,609
|
7.87
|
||||||||||||||
|
Exercised
|
(4,753
|
)
|
0.23
|
|||||||||||||
|
Forfeited
|
(30,640
|
)
|
7.98
|
|||||||||||||
|
Expirations
|
(10,660
|
)
|
8.28
|
|||||||||||||
|
Options outstanding at December 31, 2015
|
4,302,267
|
$
|
5.19
|
8.14
|
$
|
31,659,550
|
||||||||||
|
Vested and expected to vest at December 31, 2015
|
4,213,091
|
$
|
5.14
|
8.12
|
$
|
31,202,132
|
||||||||||
|
Exercisable at December 31, 2015
|
1,857,077
|
$
|
2.83
|
7.05
|
$
|
17,952,965
|
||||||||||
|
Options outstanding at December 31, 2015
|
4,302,267
|
$
|
5.19
|
|||||||||||||
|
Granted
|
1,211,400
|
8.09
|
||||||||||||||
|
Exercised
|
(63,639
|
)
|
4.62
|
|||||||||||||
|
Forfeited
|
(133,517
|
)
|
5.79
|
|||||||||||||
|
Options outstanding at December 31, 2016
|
5,316,511
|
$
|
5.84
|
7.60
|
$
|
35,599,646
|
||||||||||
|
Vested and expected to vest at December 31, 2016
|
5,235,931
|
$
|
5.80
|
7.58
|
$
|
35,246,927
|
||||||||||
|
Exercisable at December 31, 2016
|
3,027,112
|
$
|
4.41
|
6.76
|
$
|
24,559,384
|
||||||||||
|
Options outstanding at December 31, 2016
|
5,316,511
|
$
|
5.84
|
|||||||||||||
|
Granted
|
1,365,400
|
9.39
|
||||||||||||||
|
Exercised
|
(35,366
|
)
|
3.34
|
|||||||||||||
|
Forfeited
|
(183,750
|
)
|
9.46
|
|||||||||||||
|
Options outstanding at December 31, 2017
|
6,462,795
|
$
|
6.50
|
7.13
|
$
|
20,467,335
|
||||||||||
|
Vested and expected to vest at December 31, 2017
|
6,462,795
|
$
|
6.50
|
7.13
|
$
|
20,467,335
|
||||||||||
|
Exercisable at December 31, 2017
|
4,066,066
|
$
|
5.14
|
6.21
|
$
|
18,100,589
|
||||||||||
At December 31, 2017 there was approximately $12,968,689 of unamortized stock compensation expense, which is expected to be recognized over a remaining average vesting period of 2.48 years.
Note 8 – Income Taxes
A reconciliation of the statutory U.S. federal income tax rate to the Company's effective tax rate is as follows:
|
Year Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Federal statutory rate
|
34.0
|
%
|
34.0
|
%
|
34.0
|
%
|
||||||
|
State taxes
|
1.1
|
%
|
1.2
|
%
|
2.9
|
%
|
||||||
|
Change in Statutory Rate
|
(25.6
|
)%
|
-
|
-
|
||||||||
|
Permanent differences
|
(11.0
|
)%
|
(10.6
|
)%
|
(5.8
|
)%
|
||||||
|
Research and development
|
21.2
|
%
|
16.0
|
%
|
14.3
|
%
|
||||||
|
State taxes/ sale of NOL
|
4.8
|
%
|
4.5
|
%
|
3.8
|
%
|
||||||
|
Valuation allowance
|
(19.7
|
)%
|
(40.6
|
)%
|
(45.8
|
)%
|
||||||
|
Other
|
-
|
-
|
0.4
|
%
|
||||||||
|
Effective tax rate
|
4.8
|
%
|
4.5
|
%
|
3.8
|
%
|
||||||
The tax effects of temporary differences that gave rise to significant portions of the deferred tax assets were as follows:
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Federal net operating losses
|
$
|
21,312,121
|
$
|
23,644,647
|
||||
|
State net operating losses
|
2,268,249
|
1,554,730
|
||||||
|
Stock options
|
1,608,750
|
1,419,494
|
||||||
|
Federal tax credit
|
24,060,243
|
12,709,438
|
||||||
|
State tax credits
|
384,740
|
353,684
|
||||||
|
Amortization
|
612,878
|
69,529
|
||||||
|
Accrued expense
|
7,027
|
2,731
|
||||||
|
Other
|
30,999
|
18,042
|
||||||
|
Total gross deferred tax assets
|
50,285,007
|
39,772,295
|
||||||
|
Less valuation allowance
|
(50,285,007
|
)
|
(39,772,295
|
)
|
||||
|
Net deferred tax assets
|
$
|
-
|
$
|
-
|
||||
In assessing the realizability of the net deferred tax assets, the Company considers all relevant positive and negative evidence to determine whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The realization of the gross deferred tax assets is dependent on several factors, including the generation of sufficient taxable income prior to the expiration of the net operating loss carryforwards. There was a full valuation allowance against the net deferred tax assets as of December 31, 2017 and December 31, 2016.
At December 31, 2017, the Company had federal net operating loss ("NOL") carryforwards of approximately $101.5 million which expire between 2029 and 2037. At December 31, 2017, the Company had federal research and development credits carryforwards of approximately $1.9 million and an orphan drug credit carryover of approximately $22.1 million. The Company may be subject to the net operating loss utilization provisions of Section 382 of the Internal Revenue Code. The effect of an ownership change would be the imposition of an annual limitation on the use of NOL carryforwards attributable to periods before the change. The amount of the annual limitation depends upon the value of the Company immediately before the change, changes to the Company's capital during a specified period prior to the change, and the federal published interest rate. Although we have not completed an analysis under Section 382 of the Code, it is likely that the utilization of the NOLs will be limited.
At December 31, 2017, the Company had approximately $31.9 million of State of New Jersey NOL's which expire between 2030 and 2037. At December 31, 2017, the Company had approximately $0.4 million of the State of New Jersey research development credits carryforwards. The State of New Jersey has enacted legislation permitting certain corporations located in New Jersey to sell state tax loss carryforwards and state research and development credits, or net loss carryforwards. In 2017, the Company sold $26,097,607 of State of New Jersey NOL's and $424,466 of State of New Jersey R&D Credits for $2,586,057. In 2016, the Company sold $19,196,765 of State of New Jersey NOL's and $257,222 of State of New Jersey R&D Credits for $1,845,986.
Entities are also required to evaluate, measure, recognize and disclose any uncertain income tax provisions taken on their income tax returns. The Company has analyzed its tax positions and has concluded that as of December 31, 2017, there were no uncertain positions. The Company's U.S. federal and state net operating losses have occurred since its inception in 2009 and as such, tax years subject to potential tax examination could apply from that date because the utilization of net operating losses from prior years opens the relevant year to audit by the IRS and/or state taxing authorities. In September 2017, the IRS concluded auditing the Company's 2015 tax year resulting in a no change letter. Interest and penalties, if any, as they relate to income taxes assessed, are included in the income tax provision. The Company did not have any unrecognized tax benefits and has not accrued any interest or penalties through 2017.
On December 22, 2017, H.R. 1 (also, known as the Tax Cuts and Jobs Act (the “Act”)) was signed into law. Among its numerous changes to the Internal Revenue Code, the Act reduces U.S. federal corporate tax rate to 21%. As a result, the Company believes that the most significant impact on its consolidated financial statements will be reduction of approximately $13.6 million for the deferred tax assets related to net operating losses and other assets. Such reduction is offset by changes to the Company’s valuation allowance. The Company is also in the process of considering the impact under the Act of the disallowance of certain incentive based compensation tax deductibility under Internal Revenue Code Section 162(m). If an adjustment to the deferred tax asset is required, the impact will be offset by a corresponding adjustment to the valuation allowance.
Note 9 – Commitments and Contingencies
Evonik
The Company entered into an agreement with SurModics Pharmaceuticals, Inc. ("SurModics") in October 2010 for the exclusive worldwide licensing of certain technology, patent rights and know-how rights related to the production of EG-1962, the Company's lead product candidate (the "Evonik Agreement"). This agreement was later transferred to Evonik Industries AG ("Evonik") when it purchased substantially all the assets of SurModics.
Pursuant to the Evonik Agreement, in exchange for the license, the Company agreed to make milestone payments totaling up to $14.75 million upon the achievement of certain development, regulatory and sales milestones detailed in the Evonik Agreement. The Company paid $0.25 million upon execution of the Evonik Agreement. In August 2016, the Company paid a milestone of $1.0 million after the first patient in the Phase 3 clinical trial of EG-1962 was dosed. In addition, the Evonik Agreement calls for the Company to pay royalties on sales of certain products based on a mid-single digit percentage of net sales. The Evonik Agreement provides for the reduction of royalties in certain limited circumstances.
The term of the Evonik Agreement will continue until the expiration of the Company’s obligation to pay royalties to Evonik. Either party may terminate the Evonik Agreement due to material breach by the other party. Evonik may terminate the Evonik Agreement or convert it to a non-exclusive license, in either case upon giving the Company written notice, if the Company fails to use commercially reasonable efforts to hit certain specified development, regulatory and commercial milestones.
Oakwood Amended and Restated Master Formulation Development Agreement
In June 2017, the Company entered into an Amended and Restated Master Formulation Development Agreement (the “Restated Development Agreement”) with Oakwood Laboratories, L.L.C. (“Oakwood”), pursuant to which Oakwood will continue to provide the Company with certain drug formulation development and non-commercial manufacturing services for EG-1962, in accordance with project plans to be entered into from time to time. Oakwood is currently performing process engineering, optimization and other scale up activities for the Company and is currently the sole manufacturer of EG-1962 used in Edge’s ongoing NEWTON 2 Phase 3 pivotal trial for EG-1962.
Under the Restated Development Agreement, we agreed to pay Oakwood to perform services under agreed upon project plans and to pay Oakwood up to an aggregate of $4.5 million. In July 2017, we paid $1.5 million of such aggregate amount in connection with entering into the Restated Development Agreement. Of the remaining $3.0 million of such aggregate amount, $0.5 million is payable no later than April 1, 2018 and $2.5 million is payable no later than April 1, 2019. These remaining payments may be accelerated if: (i) we achieve various regulatory milestones, (ii) we close an equity or similar financing in excess of a predetermined amount, or (iii) there is an early termination, under certain circumstances, of the Restated Development Agreement or the Supply Agreement with Oakwood.
As additional consideration for performance under the Restated Development Agreement and the Supply Agreement (as defined below), the Company agreed to pay Oakwood a royalty, during the Royalty Term, in an amount equal to a low single digit percentage of net sales of EG-1962, regardless of the manufacturer or supplier thereof. The “Royalty Term” is the period commencing upon the commercial launch of EG-1962 by the Company and continuing until twelve (12) years following such launch.
The term of the Restated Development Agreement continues until the expiration or termination of the Supply Agreement, unless earlier terminated (the “Term”). The Company has the right to terminate project plans upon the occurrence of various circumstances described in the Restated Development Agreement. In the event that the Company terminates the most recent project plan prior to completion (including due to the Company’s decision to discontinue the development or commercialization of EG-1962), the Company must pay to Oakwood a termination fee. Either party has the right to terminate the Restated Development Agreement upon sixty (60) days written notice for failure by the other party to cure a material breach during the applicable cure period. The Company can terminate the Restated Development Agreement immediately upon notice to Oakwood if Oakwood’s annual financial audit report required to be provided to the Company contains any going concern or similar qualification or if Oakwood fails to maintain a pre-determined working capital ratio. Either party may also terminate the Restated Development Agreement immediately in the event of certain failures with regard to validation of the manufacturing process and other specified regulatory failures.
Oakwood Manufacturing and Supply Agreement
Concurrent with its entry into the Restated Development Agreement, on June 30, 2017, the Company entered into a Manufacturing and Supply Agreement with Oakwood (the “Supply Agreement”), pursuant to which Oakwood will manufacture and supply, and the Company will purchase from Oakwood, EG-1962 in commercial quantities following the commercial launch of the product.
Pursuant to the Supply Agreement, the price per unit of EG-1962 to be purchased by the Company is based on the expected commercially usable units per batch. In addition, the Company has agreed to pay Oakwood milestone payments that could total up to an aggregate of $2.25 million upon the achievement of certain development and regulatory milestones.
The Company will have no minimum order requirement under the Supply Agreement until the third (3rd) year following commercial launch of EG-1962. Beginning in the third year following commercial launch and continuing until the fifth year following commercial launch, the Company will be required to (x) order, at a minimum, the greater of (a) five (5) batches and (b) fifty percent (50%) of the aggregate vials of EG-1962 purchased by the Company from all sources in such year (such greater amount being the “Minimum Order Commitment”) or (y) pay a catch-up price to Oakwood based on the amount of EG-1962 actually ordered by the Company during the applicable time period.
The term of the Supply Agreement will continue (unless earlier terminated) until three (3) years following commercial launch of EG-1962. Thereafter, the Supply Agreement will automatically renew for additional two (2)-year periods unless Edge provides notice of non-renewal at least twelve (12) months prior to the end of the then-current term. The Supply Agreement will also terminate automatically upon the termination of the Restated Development Agreement for any reason. Following the first anniversary of the commercial launch of EG-1962, either party may terminate upon two (2) years written notice. Further, either party may terminate the Supply Agreement upon a material breach by the other party that fails to be cured in the applicable cure period.
The Company may terminate the Supply Agreement immediately upon notice to Oakwood in the event that (a) any regulatory authority requires or causes the withdrawal of EG-1962 from the market or (b) the Company ceases to develop or commercialize EG-1962; provided, that in the latter case of termination prior to completion of the most recent project plan attached to the Restated Development Agreement, the Company must pay to Oakwood a termination fee.
Employment Agreements
The Company has entered into employment agreements with each of its executive officers. The agreements generally provide for, among other things, salary, bonus and severance payments. The employment agreements provide for between 12 months and 18 months of severance benefits to be paid to an executive (as well as certain potential bonus, COBRA and equity award benefits), subject to the effectiveness of a general release of claims, if the executive terminates his or her employment for good reason or if the Company terminates the executive's employment without cause. The continued provision of severance benefits is conditioned on each executive's compliance with the terms of the Company's confidentiality and invention and assignment agreement as well as his or her release of claims.
Leases
Effective December 13, 2013, the Company entered into a 63 month lease for approximately 8,000 square feet of office space in Berkeley Heights, New Jersey. On February 18, 2016, the Company entered into a new 63 month lease for approximately 20,410 square feet of office space within the same office complex in Berkeley Heights, New Jersey. The terms of the new lease were structured so that the termination date of the December 13, 2013 lease coincided with the commencement date of the new lease on August 13, 2016. As a result of the lease termination, the Company wrote off $67,118 of leasehold improvements.
Rent expense is recognized on a straight line basis where there are escalating payments, and was approximately $602,925, $344,341 and $207,541 for the years ended December 31, 2017, 2016 and 2015 , respectively.
The following is a schedule by years of future minimum rental payments required under operating leases that have initial or remaining non-cancelable lease terms in excess of one year as of December 31, 2017:
|
Year Ended December 31,
|
||||
|
2018
|
$
|
602,461
|
||
|
2019
|
604,541
|
|||
|
2020
|
603,371
|
|||
|
2021
|
530,385
|
|||
|
2022 and after
|
-
|
|||
|
Total minimum payments required
|
$
|
2,340,758
|
||
Note 10 - Debt
On August 28, 2014, the Company entered into a loan and security agreement with Hercules Technology Growth Capital, Inc. (the "Original Loan Agreement"). The Original Loan Agreement provided funding for an aggregate principal amount of up to $10,000,000 in three separate term loans. The first term loan was funded on August 28, 2014 in the amount of $3,000,000. The second term loan of $3,000,000 was funded on January 29, 2015. Both the first and second term loans were due to mature on March 1, 2018. The Company elected not to draw the third term loan of $4.0 million, the availability of which expired on June 30, 2015. Initially, the loan bore interest at a rate per annum equal to the greater of (i) 10.45% or (ii) the sum of (a) 10.45% plus (b) the prime rate (as reported in The Wall Street Journal) minus 4.50%. On April 6, 2015, the base rate on the loan was lowered to the greater of (i) 9.95% or (ii) the sum of (a) 9.95% plus (b) the prime rate (as reported in The Wall Street Journal) minus 4.50. The Company was required to make interest-only payments on the loan through September 2015.
Commencing in October 2015, the term loans began amortizing in equal monthly installments of principal and interest over 30 months. On the maturity date or the date the loan otherwise became due and payable, the Company was also required to make a payment equal to 1.5% of the total amounts funded under the Original Loan Agreement.
On August 1, 2016, the Company entered into an Amended and Restated Loan and Security Agreement (the “Amended Loan Agreement”) with Hercules Capital, Inc., formerly known as Hercules Technology Growth Capital, Inc. Pursuant to the Amended Loan Agreement, the Company may borrow up to $20,000,000. At closing, the Company borrowed $15,000,000 of the amount available for draw under the Amended Loan Agreement (and received proceeds net of the amount then outstanding under the Original Loan Agreement, fees and expenses). On May 23, 2017, the Company elected to draw down the second tranche of $5 million. Amounts drawn under the Amended Loan Agreement bear interest at a rate per annum equal to the greater of either (i) the sum of (a) 9.15%, plus (b) the prime rate as reported in The Wall Street Journal minus 4.50% or (ii) 9.15%. The effective interest rate on the loan as of December 31, 2017 was 9.15%. The Amended Loan Agreement requires interest-only payments until March 1, 2018, and on that date the Company will begin to repay the principal balance of the loan in 24 equal monthly payments of principal and interest through the scheduled maturity date of February 3, 2020. In January 2018, the Company satisfied a certain condition as described in the Amended Loan Agreement to extend the period of interest-only payments to September 1, 2018 and the maturity date was extended to August 3, 2020. The interest-only payment period may be extended for an additional six months at the lender’s discretion.
Pursuant to the Amended Loan Agreement, in March 2018, the Company must make a payment of $90,000, which is equal to 1.5% of the total amounts funded under the Original Loan Agreement. On the maturity date or the date the loan otherwise becomes due and payable, under the Amended Loan Agreement the Company must also make a payment of $900,000, which is equal to 4.5% of the total amounts available under the Amended Loan Agreement. In addition, if the Company prepays the term loan (i) during the first year following the initial closing, the Company must pay a prepayment charge equal to 2% of the amount being prepaid, (ii) during the second year following the closing, the Company must pay a prepayment charge equal to 1% of the amount being prepaid, and (iii) after the second year following the closing, the Company must pay a prepayment charge equal to 0.5% of the amount being prepaid.
The loan is secured by substantially all of the Company’s assets, other than intellectual property, which is the subject of a negative pledge. Under the Amended Loan Agreement, the Company is subject to certain customary covenants that limit or restrict the Company's ability to, among other things, incur additional indebtedness, investments, distributions, transfer assets, make acquisitions, grant any security interests, pay cash dividends, repurchase its Common Stock, make loans, or enter into certain transactions without prior consent. The Amended Loan Agreement contains several events of default, including, among others, payment defaults, breaches of covenants or representations, material impairment in the perfection of Hercules’ security interest or in the collateral and events related to bankruptcy or insolvency. Upon an event of default, Hercules may declare all outstanding obligations immediately due and payable (along with a prepayment charge), a default rate of an additional 5.0% may be applied to the outstanding loan balances, and Hercules may take such further actions as set forth in the Amended Loan Agreement, including collecting or taking such other action with respect to the collateral pledged in connection with the Amended Loan Agreement.
Future principal payments on the note as of December 31, 2017 were as follows:
|
Year Ended December 31,:
|
(000's)
|
|||
|
2018
|
$
|
3,075
|
||
|
2019
|
9,822
|
|||
|
2020
|
7,103
|
|||
|
$
|
20,000
|
|||
The estimated fair value of the debt (categorized as a Level 2 liability for fair value measurement purposes) is determined using current market factors and the ability of the Company to obtain debt at comparable terms to those that are currently in place. The Company believes the estimated fair value at December 31, 2017 approximates the carrying amount.
Note 11 – Retirement Plan
The Company has a 401(k) defined contribution plan for the benefit for all employees and permits voluntary contributions by employees subject to IRS-imposed limitations. The 401K employer contribution for the 2017, 2016 and 2015 plan years were $258,383, $16,979 and $15,253 respectively.
Note 12 – Selected Quarterly Financial Data (Unaudited)
The following table summarizes unaudited quarterly financial data for the years ended December 31, 2017 and 2016 (in thousands, except per share data).
|
2017
|
||||||||||||||||||||
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Total
|
||||||||||||||||
|
Total operating expenses
|
$
|
11,791
|
$
|
13,149
|
$
|
10,903
|
$
|
16,124
|
$
|
51,967
|
||||||||||
|
Loss from operations
|
$
|
(11,791
|
)
|
$
|
(13,149
|
)
|
$
|
(10,903
|
)
|
$
|
(16,124
|
)
|
$
|
(51,967
|
)
|
|||||
|
Net loss and comprehensive loss
|
$
|
(12,170
|
)
|
$
|
(13,504
|
)
|
$
|
(11,281
|
)
|
$
|
(13,905
|
)
|
$
|
(50,860
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(12,170
|
)
|
$
|
(13,504
|
)
|
$
|
(11,281
|
)
|
$
|
(13,905
|
)
|
$
|
(50,860
|
)
|
|||||
|
Loss per share attributable to common stockholders basic and diluted
|
$
|
(0.42
|
)
|
$
|
(0.44
|
)
|
$
|
(0.37
|
)
|
$
|
(0.45
|
)
|
$
|
(1.67
|
)
|
|||||
|
2016
|
||||||||||||||||||||
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Total
|
||||||||||||||||
|
Total operating expenses
|
$
|
9,032
|
$
|
9,264
|
$
|
10,277
|
$
|
10,939
|
$
|
39,512
|
||||||||||
|
Loss from operations
|
$
|
(9,032
|
)
|
$
|
(9,264
|
)
|
$
|
(10,277
|
)
|
$
|
(10,939
|
)
|
$
|
(39,512
|
)
|
|||||
|
Net loss and comprehensive loss
|
$
|
(9,170
|
)
|
$
|
(9,376
|
)
|
$
|
(10,759
|
)
|
$
|
(9,516
|
)
|
$
|
(38,821
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(9,170
|
)
|
$
|
(9,376
|
)
|
$
|
(10,759
|
)
|
$
|
(9,516
|
)
|
$
|
(38,821
|
)
|
|||||
|
Loss per share attributable to common stockholders basic and diluted
|
$
|
(0.32
|
)
|
$
|
(0.33
|
)
|
$
|
(0.37
|
)
|
$
|
(0.33
|
)
|
$
|
(1.34
|
)
|
|||||
Basic and diluted net loss per share amounts included in the above table were computed independently for each of the quarters presented. Accordingly, the sum of the quarterly basic and diluted net loss per share amounts may not agree to the total for the year.
Page | 96
