Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AveXis, Inc. | a18-7219_1ex99d1.htm |
| 8-K - 8-K - AveXis, Inc. | a18-7219_18k.htm |
2 This presentation contains forward-looking statements, including statements about: the timing, progress and results of preclinical studies for AVXS-201 and AVXS-301, including statements regarding the timing of preclinical studies and regulatory submissions, and our manufacturing strategy and developments. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward-looking statements include the risks described in the “Risk Factors” section of the preliminary prospectus supplement for the offering and the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2017, and the information included in AveXis’ Current Report on Form 8-K filed with the SEC on January 16, 2018, as well as those set forth from time to time in the Company’s other SEC filings, available at http://www.sec.gov. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Disclaimers

Executing on Our Strategy: Expanding Beyond SMA 3 Rett syndrome (MECP2) and ALS (SOD1) are rare, life-threatening, neurological monogenic diseases that have significant unmet need and limited treatment options – none addressing the root cause Licensed preclinical data from NCH generated by Chief Scientific Officer, Dr. Brian Kaspar, that demonstrate promising efficacy and safety AveXis intends to submit IND applications for both indications in late 2018/early 2019 Licensed preclinical data from NCH generated by Chief Scientific Officer, Dr. Brian Kaspar, that demonstrate promising efficacy and safety Obtained exclusive worldwide rights to AAV9 for Rett syndrome (MECP2) and a genetic form of amyotrophic lateral sclerosis caused by mutations in the superoxide dismutase 1 (SOD1) gene AveXis intends to submit IND applications for both indications in late 2018/early 2019 AveXis will leverage its scalable manufacturing platform for these programs

Rett Syndrome 4 Rett syndrome (RTT) is a rare, neuro-developmental genetic disorder characterized by slowed growth; loss of normal movement and coordination; and loss of communication skills Median age at diagnosis for classic Rett syndrome is approximately 2.7 years Referrals to specialists are primarily from pediatricians, neurologists and geneticists Current treatments only address associated symptoms AveXis is the only company investigating a gene therapy that addresses the root cause of the disease Treatment Landscape Caused by an X-linked dominant mutation in the methyl CpG binding protein 2 (MECP2) gene in 90-95% of cases Monogenic Predominantly affects girls; incidence of approximately one in 10,000 female births in the U.S. Onset of signs and symptoms usually occurs between 6-18 months Hallmark symptoms include hand wringing or squeezing, clapping, rubbing, washing, or hand to mouth movements Disease is progressive with significant disability that can include autistic-like behaviors, breathing irregularities, feeding and swallowing difficulties, growth retardation, and seizures Overview (Tarquinio et al. 2017)

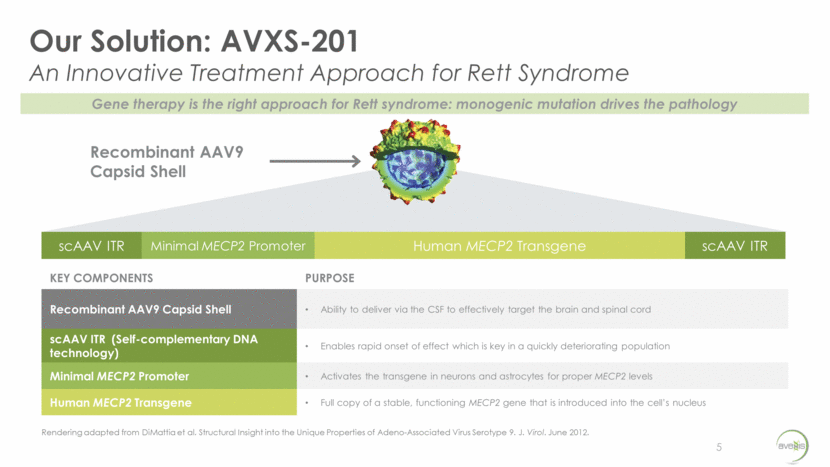
Our Solution: AVXS-201 An Innovative Treatment Approach for Rett Syndrome 5 scAAV ITR Minimal MECP2 Promoter Human MECP2 Transgene scAAV ITR KEY COMPONENTS PURPOSE Recombinant AAV9 Capsid Shell Ability to deliver via the CSF to effectively target the brain and spinal cord scAAV ITR (Self-complementary DNA technology) Enables rapid onset of effect which is key in a quickly deteriorating population Minimal MECP2 Promoter Activates the transgene in neurons and astrocytes for proper MECP2 levels Human MECP2 Transgene Full copy of a stable, functioning MECP2 gene that is introduced into the cell’s nucleus Recombinant AAV9 Capsid Shell Rendering adapted from DiMattia et al. Structural Insight into the Unique Properties of Adeno-Associated Virus Serotype 9. J. Virol. June 2012. Gene therapy is the right approach for Rett syndrome: monogenic mutation drives the pathology
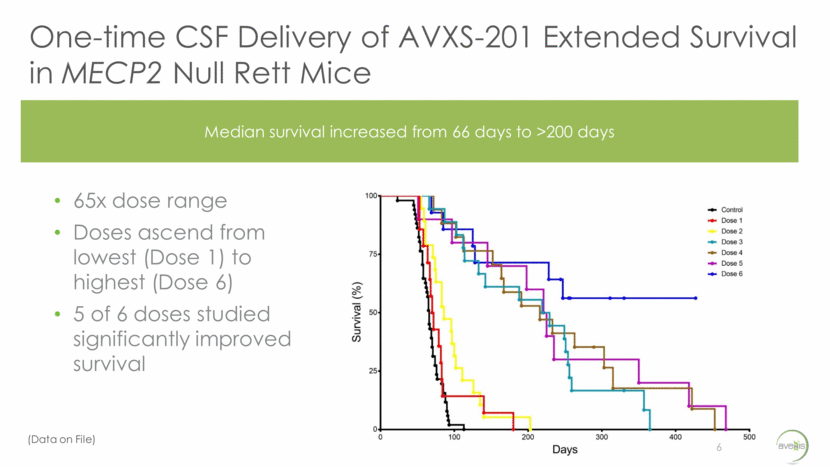
One-time CSF Delivery of AVXS-201 Extended Survival in MECP2 Null Rett Mice 6 65x dose range Doses ascend from lowest (Dose 1) to highest (Dose 6) 5 of 6 doses studied significantly improved survival Median survival increased from 66 days to >200 days (Data on File)
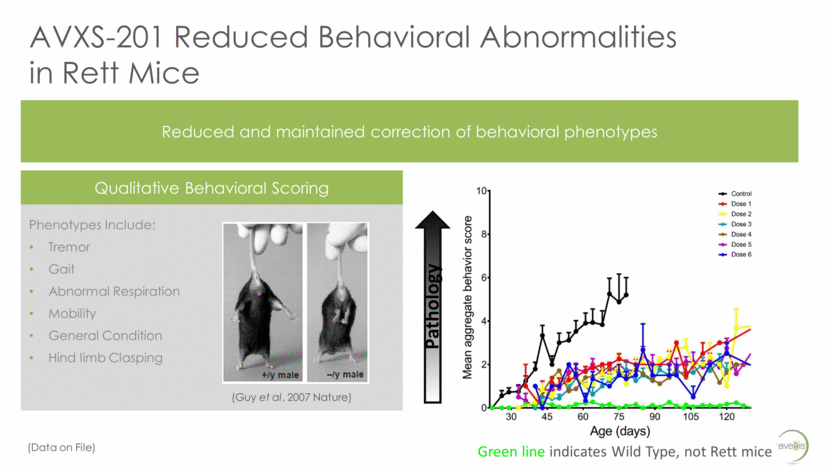
AVXS-201 Reduced Behavioral Abnormalities in Rett Mice Reduced and maintained correction of behavioral phenotypes Phenotypes Include: Tremor Gait Abnormal Respiration Mobility General Condition Hind limb Clasping Qualitative Behavioral Scoring Pathology (Guy et al. 2007 Nature) Green line indicates Wild Type, not Rett mice (Data on File)
Median age at diagnosis is 55 Referrals to specialists are primarily from general neurologists, with a very small number from primary care physicians Current treatment offers modest benefits but does not address the root genetic cause AveXis is investigating a gene therapy that addresses the root cause of the disease Treatment Landscape Genetic ALS with SOD1 Mutation 8 Amyotrophic lateral sclerosis (ALS) is a rare, neurodegenerative genetic disorder that affects nerve cells in the brain and the spinal cord and leads to progressive degeneration of motor neurons Caused by mutations in the gene that produces the copper zinc superoxide dismutase 1 (SOD1) enzyme Monogenic Genetic (or familial) ALS affects 1,000-2,000 in the U.S., 15 – 20% caused by mutations in SOD1 Onset usually occurs in people between 40-70 years of age Disease is progressive with significant disability including muscle weakness resulting in loss of the ability to speak, eat, move, and eventually breathe, typically resulting in death within 3-5 years of diagnosis Overview
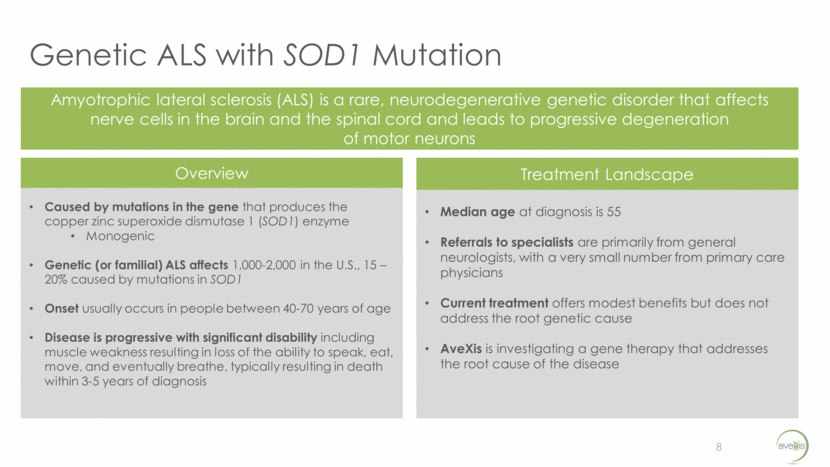
Our Solution: AVXS-301 An Innovative Treatment Approach for Amyotrophic Lateral Sclerosis 9 KEY COMPONENTS PURPOSE Recombinant AAV9 Capsid Shell Ability to deliver via the CSF to effectively target the brain and spinal cord scAAV ITR (Self-complementary DNA technology) Enables rapid onset of effect which is key in a quickly deteriorating population H1 promoter- shRNA SOD1 Polymerase III promoter to drive expression of shRNA to suppress SOD1 Stuffer Sequence Stuffer sequence to ensure optimal vector size for proper packaging Rendering adapted from DiMattia et al. Structural Insight into the Unique Properties of Adeno-Associated Virus Serotype 9. J. Virol. June 2012. Gene therapy is the right approach for ALS: monogenic mutation drives the pathology
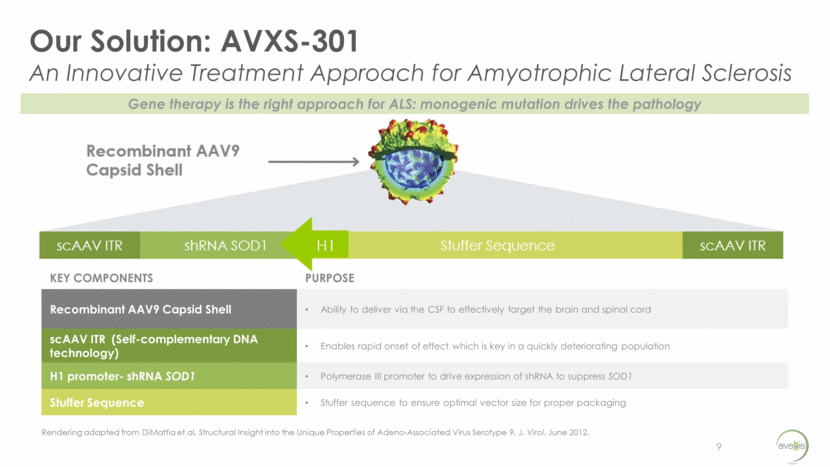
One-Time CSF Delivery of AVXS-301 Extended Survival in Male and Female ALS Model 10 Control Dose 1 Dose 2 Dose 3 Dose 4 Median survival increased from ~130 days to >200 days (Society of Neuroscience, 2017) Control Dose 4 Control Dose 4 Doses ascend from lowest (Dose 1) to highest (Dose 4)
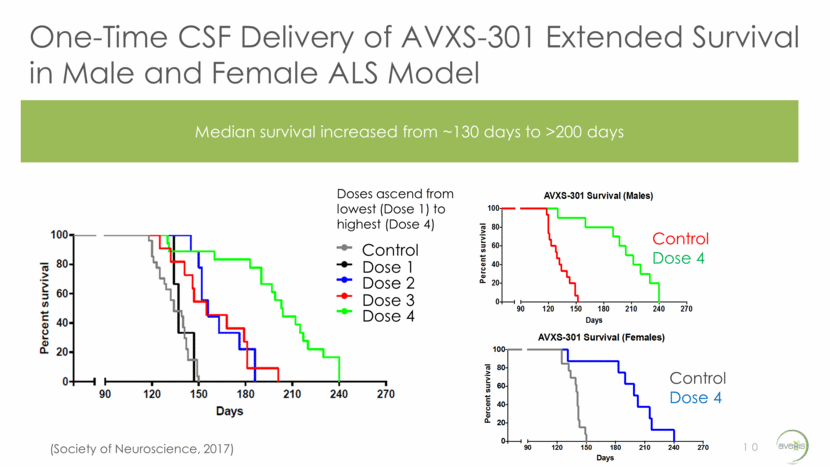
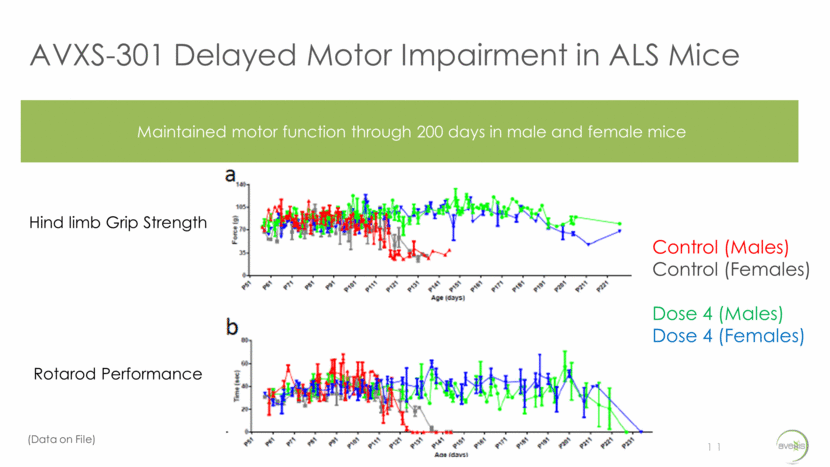
AVXS-301 Delayed Motor Impairment in ALS Mice 11 Maintained motor function through 200 days in male and female mice Hind limb Grip Strength Rotarod Performance Control (Males) Control (Females) Dose 4 (Males) Dose 4 (Females) (Data on File)
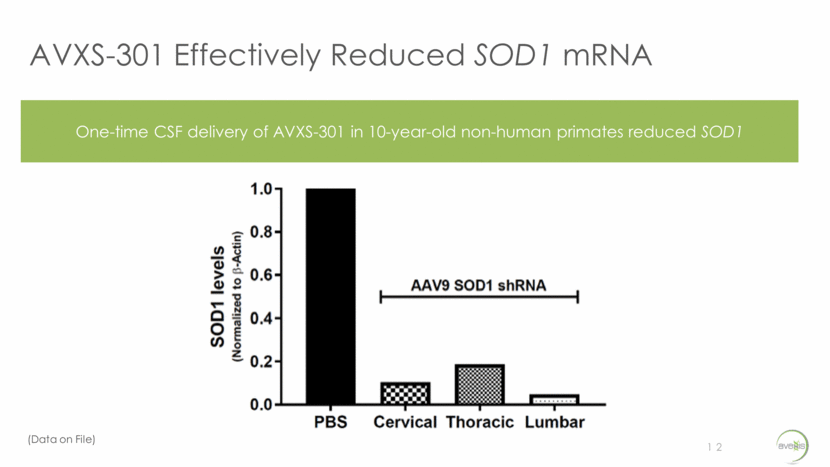
AVXS-301 Effectively Reduced SOD1 mRNA 12 One-time CSF delivery of AVXS-301 in 10-year-old non-human primates reduced SOD1 (Data on File)
Preclinical Summary of AAV9 Gene Therapy for Rett Syndrome and ALS 13 Gene therapy resulted in one of the longest living Rett syndrome and ALS mouse models reported to date, surpassing 200 days median survival Treated Rett syndrome mice displayed sustained reduction in behavioral abnormalities compared to untreated animals Treated ALS mice maintained motor function compared to untreated animals Safety profile in mice and non-human primates indicated gene therapy appeared to be safe and well-tolerated Preclinical data of one-time delivery of aav9 gene therapy in rett syndrome and genetic als demonstrated promising efficacy and safety
Next Steps 14 AVXS-201 and AVXS-301 leverage AveXis’ scalable manufacturing platform by interchanging 1 plasmid Engineering runs at scale completed for AVXS-201 and AVXS-301 GMP campaign ongoing for AVXS-201 and scheduled for AVXS-301 Complete remaining IND-enabling preclinical work KOL meetings for both AVXS-201 and AVXS-301 have occurred and clinical plans are forthcoming AveXis intends to submit IND applications for both indications in late 2018/early 2019 AVEXIS INTENDS TO SUBMIT IND APPLICATIONS FOR AVXS-201 and avxs-301 IN LATE 2018/EARLY 2019
Thank You