Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - DAVITA INC. | dva-123117xex322.htm |
| EX-32.1 - EXHIBIT 32.1 - DAVITA INC. | dva-123117xex321.htm |
| EX-31.2 - EXHIBIT 31.2 - DAVITA INC. | dva-123117xex312.htm |
| EX-31.1 - EXHIBIT 31.1 - DAVITA INC. | dva-123117xex311.htm |
| EX-23.1 - EXHIBIT 23.1 - DAVITA INC. | dva-123117xex231.htm |
| EX-21.1 - EXHIBIT 21.1 - DAVITA INC. | dva-123117xex211.htm |
| EX-12.1 - EXHIBIT 12.1 - DAVITA INC. | dva-123117xex121.htm |
| EX-10.27 - EXHIBIT 10.27 - DAVITA INC. | dva-123117xex1027.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2017
Commission File Number: 1-14106

DAVITA INC.
(Exact name of registrant as specified in charter)
Delaware | 51-0354549 | |
(State of incorporation) | (I.R.S. Employer Identification No.) | |
2000 16th Street
Denver, CO 80202
Telephone number (303) 405-2100
Securities registered pursuant to Section 12(b) of the Act:
Title of each class: | Name of each exchange on which registered: | |
Common Stock, $0.001 par value | New York Stock Exchange | |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☒ | Accelerated filer | ☐ | ||
Non-accelerated filer | ☐ | (Do not check if a smaller reporting company) | Smaller reporting company | ☐ | |
Emerging growth company | ☐ | ||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of June 30, 2017, the aggregate market value of the Registrant's common stock outstanding held by non-affiliates based upon the closing price on the New York Stock Exchange was approximately $12.4 billion.
As of January 31, 2018, the number of shares of the Registrant’s common stock outstanding was approximately 182.0 million shares.
Documents incorporated by reference
Portions of the Registrant’s proxy statement for its 2018 annual meeting of stockholders are incorporated by reference in Part III of this Form 10-K.
PART I
Item 1. Business
We were incorporated as a Delaware corporation in 1994. Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to section 13(a) or 15(d) of the Exchange Act are made available free of charge through our website, located at http://www.davita.com, as soon as reasonably practicable after the reports are filed with or furnished to the Securities and Exchange Commission (SEC). The SEC also maintains a website at http://www.sec.gov where these reports and other information about us can be obtained. The contents of our website are not incorporated by reference into this report.
Overview of DaVita Inc.
The Company has consisted of two major divisions, DaVita Kidney Care (Kidney Care) and DaVita Medical Group (DMG). Kidney Care is comprised of our U.S. dialysis and related lab services, our ancillary services and strategic initiatives, including our international operations, and our corporate administrative support. Our U.S. dialysis and related lab services business is our largest line of business and is a leading provider of kidney dialysis services in the U.S. for patients suffering from chronic kidney failure, also known as end stage renal disease (ESRD). DMG is a patient- and physician-focused integrated healthcare delivery and management company with over two decades of providing coordinated, outcomes-based medical care in a cost-effective manner.
On December 5, 2017, we entered into an equity purchase agreement to sell our DMG division to Collaborative Care Holdings, LLC (Optum), a subsidiary of UnitedHealth Group Inc. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions. As a result of this pending transaction, the DMG business has been reclassified as held for sale and its results of operations are reported as discontinued operations for all periods presented in our consolidated financial statements included in this report.
For financial information about our DMG business see Note 21 to the consolidated financial statements included in this report.
Kidney Care Division
U.S. dialysis and related lab services business overview
Our U.S. dialysis and related lab services business is a leading provider of kidney dialysis services for patients suffering from ESRD. As of December 31, 2017, we provided dialysis and administrative services in the U.S. through a network of 2,510 outpatient dialysis centers in 46 states and the District of Columbia, serving a total of approximately 197,800 patients. We also provide acute inpatient dialysis services in approximately 900 hospitals and related laboratory services throughout the U.S.
The loss of kidney function is normally irreversible. Kidney failure is typically caused by Type I and Type II diabetes, high blood pressure, polycystic kidney disease, long-term autoimmune attack on the kidney and prolonged urinary tract obstruction. ESRD is the stage of advanced kidney impairment that requires continued dialysis treatments or a kidney transplant to sustain life. Dialysis is the removal of toxins, fluids and salt from the blood of patients by artificial means. Patients suffering from ESRD generally require dialysis at least three times a week for the rest of their lives.
According to the United States Renal Data System, there were over 495,000 ESRD dialysis patients in the U.S. in 2015. The underlying ESRD dialysis patient population has grown at an approximate compound rate of 3.8% from 2000 to 2015, the latest period for which such data is available. The growth rate is attributable to the aging of the U.S. population, increased incidence rates for diseases that cause kidney failure such as diabetes and hypertension, lower mortality rates for dialysis patients and growth rates of minority populations with higher than average incidence rates of ESRD.
Since 1972, the federal government has provided healthcare coverage for ESRD patients under the Medicare ESRD program regardless of age or financial circumstances. ESRD is the first and only disease state eligible for Medicare coverage both for dialysis and dialysis-related services and for all benefits available under the Medicare program. For patients with Medicare coverage, all ESRD payments for dialysis treatments are made under a single bundled payment rate. See page 6 for further details.
Although Medicare reimbursement limits the allowable charge per treatment, it provides industry participants with a relatively predictable and recurring revenue stream for dialysis services provided to patients without commercial insurance. For the year ended December 31, 2017, approximately 89.5% of our total dialysis patients were covered under some form of
2
government-based programs, with approximately 74.9% of our dialysis patients covered under Medicare and Medicare-assigned plans.
Treatment options for ESRD
Treatment options for ESRD are dialysis and kidney transplantation.
Dialysis options
• | Hemodialysis |
Hemodialysis, the most common form of ESRD treatment, is usually performed at a freestanding outpatient dialysis center, at a hospital-based outpatient center, or at the patient’s home. The hemodialysis machine uses an artificial kidney, called a dialyzer, to remove toxins, fluids and salt from the patient’s blood. The dialysis process occurs across a semi-permeable membrane that divides the dialyzer into two distinct chambers. While blood is circulated through one chamber, a pre-mixed fluid is circulated through the other chamber. The toxins, salt and excess fluids from the blood cross the membrane into the fluid, allowing cleansed blood to return back into the patient’s body. Each hemodialysis treatment that occurs in the outpatient dialysis centers typically lasts approximately three and one-half hours and is usually performed three times per week.
Hospital inpatient hemodialysis services are required for patients with acute kidney failure primarily resulting from trauma, patients in early stages of ESRD and ESRD patients who require hospitalization for other reasons. Hospital inpatient hemodialysis is generally performed at the patient’s bedside or in a dedicated treatment room in the hospital, as needed.
Some ESRD patients who are healthier and more independent may perform home-based hemodialysis in their home or residence through the use of a hemodialysis machine designed specifically for home therapy that is portable, smaller and easier to use. Patients receive training, support and monitoring from registered nurses, usually in our outpatient dialysis centers, in connection with their dialysis treatment. Home-based hemodialysis is typically performed with greater frequency than dialysis treatments performed in outpatient dialysis centers and on varying schedules.
• | Peritoneal dialysis |
Peritoneal dialysis uses the patient’s peritoneal or abdominal cavity to eliminate fluid and toxins and is typically performed at home. The most common methods of peritoneal dialysis are continuous ambulatory peritoneal dialysis (CAPD), and continuous cycling peritoneal dialysis (CCPD). Because it does not involve going to an outpatient dialysis center three times a week for treatment, peritoneal dialysis is an alternative to hemodialysis for patients who are healthier, more independent and desire more flexibility in their lifestyle.
CAPD introduces dialysis solution into the patient’s peritoneal cavity through a surgically placed catheter. Toxins in the blood continuously cross the peritoneal membrane into the dialysis solution. After several hours, the patient drains the used dialysis solution and replaces it with fresh solution. This procedure is usually repeated four times per day.
CCPD is performed in a manner similar to CAPD, but uses a mechanical device to cycle dialysis solution through the patient’s peritoneal cavity while the patient is sleeping or at rest.
Kidney transplantation
Although kidney transplantation, when successful, is generally the most desirable form of therapeutic intervention, the shortage of suitable donors, side effects of immunosuppressive pharmaceuticals given to transplant recipients and dangers associated with transplant surgery for some patient populations limit the use of this treatment option.
U.S. Dialysis and related lab services we provide
Outpatient hemodialysis services
As of December 31, 2017, we operated or provided administrative services through a network of 2,510 outpatient dialysis centers in the U.S. that are designed specifically for outpatient hemodialysis. In 2017, our overall network of U.S. outpatient dialysis centers increased by 160 primarily as a result of the opening of new dialysis centers, net of center closures, divestitures, and acquisitions, representing a total increase of approximately 6.8% from 2016.
As a condition of our enrollment in Medicare for the provision of dialysis services, we contract with a nephrologist or a group of associated nephrologists to provide medical director services at each of our dialysis centers. In addition, other
3
nephrologists may apply for practice privileges to treat their patients at our centers. Each center has an administrator, typically a registered nurse, who supervises the day-to-day operations of the center and its staff. The staff of each center typically consists of registered nurses, licensed practical or vocational nurses, patient care technicians, a social worker, a registered dietician, biomedical technician support and other administrative and support personnel.
Under Medicare regulations, we cannot promote, develop or maintain any kind of contractual relationship with our patients that would directly or indirectly obligate a patient to use or continue to use our dialysis services, or that would give us any preferential rights other than those related to collecting payments for our dialysis services. Our total patient turnover, which is based upon all causes, averaged approximately 26% in 2017 and 25% in 2016. However, in 2017, the overall number of patients to whom we provided services in the U.S. increased by approximately 5.4% from 2016, primarily from the opening of new dialysis centers and acquisitions, and continued growth within the industry.
Hospital inpatient hemodialysis services
As of December 31, 2017, we provided hospital inpatient hemodialysis services, excluding physician services, to patients in approximately 900 hospitals throughout the U.S. We render these services based on a contracted per-treatment fee that is individually negotiated with each hospital. When a hospital requests our services, we typically administer the dialysis treatment at the patient’s bedside or in a dedicated treatment room in the hospital, as needed. In 2017, hospital inpatient hemodialysis services accounted for approximately 5.0% of our U.S. dialysis revenues and 4.0% of our total U.S. dialysis treatments.
Home-based hemodialysis services
Many of our outpatient dialysis centers offer certain support services for dialysis patients who prefer and are able to perform either home-based hemodialysis or peritoneal dialysis in their homes. Home-based hemodialysis support services consist of providing equipment and supplies, training, patient monitoring, on-call support services and follow-up assistance. Registered nurses train patients and their families or other caregivers to perform either home-based hemodialysis or peritoneal dialysis.
ESRD laboratory services
We own two separately incorporated, licensed, clinical laboratories which specialize in ESRD patient testing. These specialized laboratories provide routine laboratory tests for dialysis and other physician-prescribed laboratory tests for ESRD patients and are an integral component of overall dialysis services that we provide. Our laboratories provide these tests predominantly for our network of ESRD patients throughout the U.S. These tests are performed to monitor a patient’s ESRD condition, including the adequacy of dialysis, as well as other medical conditions of the patient. Our laboratories utilize information systems which provide information to certain members of the dialysis centers’ staff and medical directors regarding critical outcome indicators.
Management services
We currently operate or provide management and administrative services pursuant to management and administrative services agreements to 39 outpatient dialysis centers located in the U.S. in which we either own a noncontrolling interest or are wholly-owned by third parties. Management fees are established by contract and are recognized as earned typically based on a percentage of revenues or cash collections generated by the outpatient dialysis centers.
Quality care
Centers for Medicare and Medicaid Services (CMS) promotes high quality services in outpatient dialysis facilities treating patients with ESRD through its Quality Incentive Program (QIP). QIP associates a portion of payment directly with a facility’s performance on quality of care measures. Payment reductions result when a facility’s overall score on applicable measures does not meet established standards. For the fifth year in a row, we are an industry leader in QIP standards. We are industry leaders for catheter rates and also lead the industry for the total number of patients in our peritoneal dialysis program.
In addition, CMS' Five-Star Quality Rating system, is a rating system that assigns one to five stars to rate the quality of outcomes for dialysis facilities. The rating system provides patients reported information about any given dialysis facility and identifies differences in quality between facilities so that patients can make more informed decisions about where to receive treatment. For the last three years in which data is available, we have been a leader in the industry under the CMS Five-Star Quality Rating system.
4
Our facilities employ registered nurses, licensed practical or vocational nurses, patient care technicians, social workers, registered dieticians, biomedical technicians and other administrative and support teammates who aim to achieve superior clinical outcomes at our centers.
Our physician leadership in the Office of the Chief Medical Officer (OCMO) for our U.S. dialysis and related lab services business includes 16 senior nephrologists, led by our Chief Medical Officer, with a variety of academic, clinical practice, and clinical research backgrounds. Our Physician Council is an advisory body to senior management composed of eight physicians with extensive experience in clinical practice. In addition, we currently have nine Group Medical Directors.
Sources of revenue—concentrations and risks
Our U.S. dialysis and related lab services business net revenues represent approximately 86% of our consolidated net revenues for the year ended December 31, 2017. Our U.S. dialysis and related lab services revenues are derived primarily from our core business of providing dialysis services and related laboratory services and, to a lesser extent, the administration of pharmaceuticals and management fees generated from providing management and administrative services to certain outpatient dialysis centers, as discussed above.
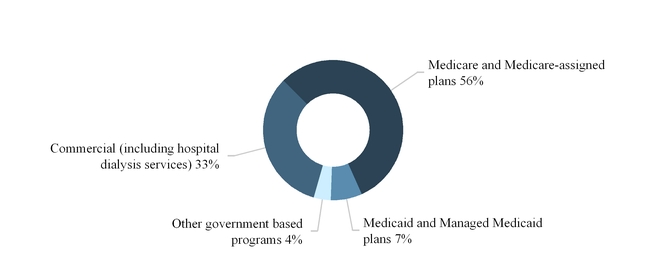
The sources of our U.S. dialysis and related lab services revenues are principally from government-based programs, including Medicare and Medicare-assigned plans, Medicaid and Managed Medicaid plans and commercial insurance plans.
The following graph summarizes our U.S. dialysis services revenues by source for the year ended December 31, 2017:
The following graph summarizes our U.S. dialysis services revenues by modality for the year ended December 31, 2017:
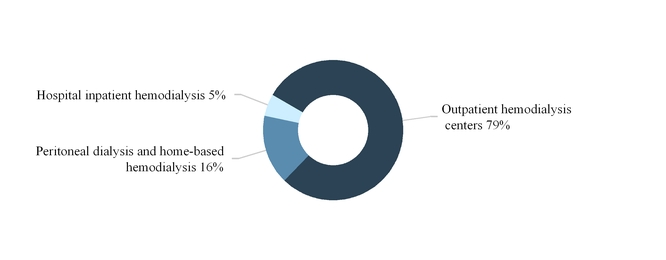
5
Medicare revenue
Government dialysis related payment rates in the U.S. are principally determined by federal Medicare and state Medicaid policy. For patients with Medicare coverage, all ESRD payments for dialysis treatments are made under a single bundled payment rate which provides a fixed payment rate to encompass all goods and services provided during the dialysis treatment, including certain pharmaceuticals, such as Epogen® (EPO), vitamin D analogs and iron supplements, irrespective of the level of pharmaceuticals administered to the patient or additional services performed. Most lab services are also included in the bundled payment. Under the ESRD Prospective Payment System (PPS), the bundled payments to a dialysis facility may be reduced by as much as 2% based on the facility’s performance in specified quality measures set annually by CMS through QIP, which was established by the Medicare Improvements for Patients and Providers Act of 2008. The bundled payment rate is also adjusted for certain patient characteristics, a geographic usage index and certain other factors.
Uncertainty about future payment rates remains a material risk to our business, as well as the potential implementation of or changes in coverage determinations or other rules or regulations by CMS or Medicare Administrative Contractors (MACs) that may impact reimbursement. An important provision in the law is an annual adjustment, or market basket update, to the ESRD PPS base rate. Absent action by Congress, the ESRD PPS base rate is automatically updated annually by a formulaic inflation adjustment.
In December 2013, CMS issued the 2014 final rule for the ESRD PPS, which phases in the payment reductions mandated by the American Taxpayer Relief Act of 2012 (ATRA), as modified by the Protecting Access to Medicare Act of 2014, which reduced our market basket inflation adjustment by 1.25% in both 2016 and 2017, and by 1% in 2018. In November 2017, CMS published the 2018 final rule for the ESRD PPS, which increased dialysis facilities’ bundled payment rate for 2018 relative to prior years. In particular, CMS projects that the 2018 final rule for the ESRD PPS will (i) increase the total payments to all ESRD facilities by 0.5% in 2018 compared to 2017; (ii) increase total payments to hospital-based ESRD facilities by 0.7% in 2018 compared to 2017; and (iii) increase total payments for freestanding facilities by 0.5% in 2018 compared to 2017. The 2018 final rule for the ESRD PPS also implements changes to the ESRD PPS outlier policy, broadening the pricing methodologies used to determine the cost of certain service drugs and biologicals in computing outlier payments when average sales price data is not available.
As a result of the Budget Control Act of 2011 (BCA) and subsequent activity in Congress, a $1.2 trillion sequester (across-the-board spending cuts) in discretionary programs took effect in 2013 reducing Medicare payments by 2%, which was subsequently extended through fiscal year 2027. These across-the-board spending cuts have affected and will continue to adversely affect our business, results of operations and financial condition. Although the Bipartisan Budget Act (BBA) of 2018 passed in February 2018 enacts a two-year federal spending agreement and raises the federal spending cap on non-defense spending for fiscal years 2018 and 2019, the Medicare program is frequently mentioned as a target for spending cuts.
The CMS Center for Medicare & Medicaid Innovation Center (Innovation Center) is working with various healthcare providers to develop, refine and implement Accountable Care Organizations (ACOs) and other innovative models of care for Medicare and Medicaid beneficiaries. We are uncertain of the extent to which the long-term operation and evolution of these models of care, including ACOs, Bundled Payments for Care Improvement Initiative, Comprehensive ESRD Care (CEC) Model (which includes the development of ESRD Seamless Care Organizations (ESCOs)), the Comprehensive Primary Care Initiative, the Duals Demonstration, or other models, will impact the healthcare market over time. Our U.S. dialysis business may choose to participate in one or several of these models either as a partner with other providers or independently. We currently participate in the CEC Model with the Innovation Center, including the ESCO organizations in the Arizona, Florida, and adjacent New Jersey and Pennsylvania markets. In areas where our U.S. dialysis business is not directly participating in this or other Innovation Center models, some of our patients may be assigned to an ACO, another ESRD Care Model, or another program, in which case the quality and cost of care that we furnish will be included in an ACO’s, another ESRD Care Model’s or other program’s calculations.
The Department of Health and Human Services (HHS) has also pledged to tie 50% of Medicare payments to quality or alternate payment models by the end of 2018. As new models of care emerge and evolve, we may be at risk for losing our Medicare patient base, which would have a materially adverse effect on our revenues, earnings and cash flows. Other initiatives in the government or private sector may also arise, including the development of models similar to ACOs, independent practice associations (IPAs) and integrated delivery systems or evolutions of those concepts which could adversely impact our business.
We anticipate that we will continue to experience increases in our operating costs in 2018 that will outpace any net Medicare rate increases that we may receive, which could significantly impact our operating results. In addition, we expect to continue experiencing increases in operating costs that are subject to inflation, such as labor and supply costs, including increases in maintenance costs and capital expenditures to improve, renovate and maintain our facilities, equipment and
6
information technology to meet changing regulatory requirements, regardless of whether there is a compensating inflation-based increase in Medicare payment rates or in payments under the bundled payment rate system.
ESRD patients receiving dialysis services become eligible for primary Medicare coverage at various times, depending on their age or disability status, as well as whether they are covered by a commercial insurance plan. Generally, for a patient not covered by a commercial insurance plan, Medicare becomes the primary payor for ESRD patients receiving dialysis services either immediately or after a three-month waiting period. For a patient covered by a commercial insurance plan, Medicare generally becomes the primary payor after 33 months, which includes the three-month waiting period, or earlier if the patient’s commercial insurance plan coverage terminates. When Medicare becomes the primary payor, the payment rates we receive for that patient shift from the commercial insurance plan rates to Medicare payment rates, which are on average significantly lower than commercial insurance rates.
Medicare pays 80% of the amount set by the Medicare system for each covered dialysis treatment. The patient is responsible for the remaining 20%. In most cases, a secondary payor, such as Medicare supplemental insurance, a state Medicaid program or a commercial health plan, covers all or part of these balances. Some patients who do not qualify for Medicaid, but otherwise cannot afford secondary insurance in the form of a Medicare Supplement Plan, can apply for premium payment assistance from charitable organizations to obtain secondary coverage. If a patient does not have secondary insurance coverage, we are generally unsuccessful in our efforts to collect from the patient the remaining 20% portion of the ESRD composite rate that Medicare does not pay. However, we are able to recover some portion of this unpaid patient balance from Medicare through an established cost reporting process by identifying these Medicare bad debts on each center’s Medicare cost report.
The 21st Century Cures Act, enacted in December 2016, included a provision that will allow Medicare beneficiaries with ESRD to choose a Medicare Advantage plan. Until the effective date of this law, this choice is available only to Medicare beneficiaries without ESRD. The ESRD related provisions of the 21st Century Cures Act are scheduled to take effect in 2021.
Medicaid revenue
Medicaid programs are state-administered programs partially funded by the federal government. These programs are intended to provide health coverage for patients whose income and assets fall below state-defined levels and who are otherwise uninsured. These programs also serve as supplemental insurance programs for co-insurance payments due from Medicaid-eligible patients with primary coverage under the Medicare program. Some Medicaid programs also pay for additional services, including some oral medications that are not covered by Medicare. We are enrolled in the Medicaid programs in the states in which we conduct our business.
Commercial revenue
Before a patient becomes eligible to elect to have Medicare as their primary payor for dialysis services, a patient’s commercial insurance plan, if any, is generally responsible for payment of such dialysis services for up to the first 33 months, as discussed above. Although commercial payment rates vary, average commercial payment rates established under commercial contracts are generally significantly higher than Medicare rates. The payments we receive from commercial payors generate nearly all of our profits. Payment methods from commercial payors can include a single lump-sum per treatment, referred to as bundled rates, or in other cases separate payments for dialysis treatments and pharmaceuticals, if used as part of the treatment, referred to as Fee-for-Service (FFS) rates. Commercial payment rates are the result of negotiations between us and insurers or third-party administrators. Our out-of-network payment rates are on average higher than in-network commercial contract payment rates. We continue to enter into some commercial contracts, covering certain patients that will primarily pay us under a single bundled payment rate for all dialysis services provided to these patients. However, some contracts will pay us for certain other services and pharmaceuticals in addition to the bundled payment. These contracts typically contain annual price escalator provisions. We are continuously in the process of negotiating agreements with our commercial payors and if our negotiations result in overall commercial contract payment rate reductions in excess of our commercial contract payment rate increases, or if commercial payors implement plans that restrict access to coverage or the duration or breadth of benefits or impose restrictions or limitations on patient access to commercial plans on non-contracted or out-of-network providers, it could have a material adverse effect on our business, results of operations and financial condition. In addition, if there is an increase in job losses in the U.S., or depending upon changes to the healthcare regulatory system by CMS and/or the impact of healthcare insurance exchanges, we could experience a decrease in the number of patients covered under commercial insurance plans and/or an increase in uninsured or underinsured patients. Patients with commercial insurance who cannot otherwise maintain coverage frequently rely on financial assistance from charitable organizations, such as the American Kidney Fund. If these patients are unable to obtain or continue to receive or receive for a limited duration such financial assistance, or if our assumptions about how patients will respond to any change in such financial assistance are incorrect, it could have a material adverse effect on our business, results of operations and financial condition.
7
Approximately 28% of our dialysis services revenues and approximately 10.5% of our dialysis patients are associated with non-acute commercial payors for the year ended December 31, 2017. Non-acute commercial patients as a percentage of our total dialysis patients for 2017 decreased 1.4% as compared to 2016. Less than 1% of our U.S. dialysis and related lab services revenues are due directly from patients. There is no single commercial payor that accounted for more than 10% of total U.S. dialysis and related lab services revenues for the year ended December 31, 2017. See Note 23 to the consolidated financial statements included in this report for disclosure on our concentration related to our commercial payors on a total consolidated net revenue basis.
The healthcare reform legislation enacted in 2010 introduced healthcare insurance exchanges which provide a marketplace for eligible individuals and small employers to purchase healthcare insurance. The business and regulatory environment continues to evolve as the exchanges mature, and regulations are challenged, changed and enforced. If commercial payor participation in the exchanges continues to decrease, it could have a material adverse effect on our business, results of operations and financial condition. Although we cannot predict the short- or long-term effects of these factors, we believe the healthcare insurance exchanges could result in a reduction in ESRD patients covered by traditional commercial insurance policies and an increase in the number of patients covered through the exchanges under more restrictive commercial plans with lower reimbursement rates or higher deductibles and co-payments that patients may not be able to pay. To the extent that the ongoing implementation of such exchanges or changes in statutes or regulations, or enforcement of statutes or regulations regarding the exchanges results in a reduction in reimbursement rates for our services from commercial and/or government payors, it could have a material adverse effect on our business, results of operations and financial condition.
In addition, in December 2016, CMS published an interim final rule that questioned the use of charitable premium assistance for ESRD patients and would have established new conditions for coverage standards for dialysis facilities. In January 2017, a federal court issued a preliminary injunction on CMS’ interim final rule and in June 2017, at the request of CMS, the court stayed the proceedings while CMS pursues new rulemaking options. In November 2017, when CMS published the 2018 final rule that updates payment policies and rates under the ESRD PPS, and the 2019 proposed Notice of Benefit and Payment Parameters, it did not pursue further discussion or rule making related to charitable premium assistance or propose changes to historical charitable premium assistance guidelines. This does not preclude CMS or another regulatory agency or legislative authority from issuing a new rule or guidance that challenges charitable premium assistance. Additionally, any other law, rule, or guidance issued by CMS or other regulatory or legislative authorities restricting or prohibiting the ability of patients with access to alternative coverage from selecting a marketplace plan on or off exchange, and/or otherwise restricting or prohibiting the use of charitable premium assistance, could adversely impact dialysis centers across the U.S. making certain centers economically unviable, restrict the ability of dialysis patients to obtain and maintain optimal insurance coverage, and have a material adverse effect on our business, results of operations, and financial condition.
Revenue from other pharmaceuticals and EPO
The impact of physician-prescribed pharmaceuticals on our overall revenues that are separately billable has significantly decreased since Medicare’s single bundled payment system went into effect beginning in January 2011, as well as some additional commercial contracts that pay us a single bundled payment rate. Approximately 2% of our total U.S. dialysis and related lab services net patient services revenues for the years ended December 31, 2017 and 2016, are associated with the administration of separately-billable physician-prescribed pharmaceuticals. Of this, the administration of EPO that was separately billable, accounted for approximately half of our separately billable pharmaceuticals of our U.S. dialysis and related lab services business for both years. EPO is produced by a single manufacturer, Amgen USA Inc. (Amgen). In 2017, we entered into a Sourcing and Supply Agreement with Amgen that expires on December 31, 2022. Under the terms of the agreement, we will purchase EPO in amounts necessary to meet no less than 90% of our requirements for erythropoiesis-stimulating agents (ESAs) through the expiration of the contract. The actual amount of EPO that we will purchase from Amgen will depend upon the amount of EPO administered during dialysis treatments as prescribed by physicians and the overall number of patients that we serve. Any interruption in the supply of EPO or product cost increases that we are unable to mitigate could materially impact our operations.
In addition to EPO, other drugs are included in and, in the future, other drugs will be added to the ESRD PPS. On January 1, 2018, calcimimetics, a drug class taken by many ESRD patients to treat mineral bone disease, became part of the ESRD PPS. The drug has both an oral form (Sensipar) and IV form (Parsabiv). Because the IV form is a new injectable for which there is no current functional category, neither Parsabiv nor Sensipar are considered accounted for in the ESRD PPS base rate and are reimbursed through a Transitional Drug Add-on Payment Adjustment (TDAPA). The TDAPA period is expected to continue for a period of two years. Currently, the oral and IV forms of the drug are produced and sold by a single manufacturer, Amgen. In December 2017, we entered into a Sourcing and Supply Agreement with Amgen for both the oral and IV versions of calcimimetics. Our operating results could be materially impacted by certain factors, including physician prescribing patterns, vendor contracts with Amgen and other suppliers, the timing of the entry into the market of a generic oral equivalent, whether
8
the drug enters into the ESRD PPS and becomes part of its bundled payment following TDAPA and, if so, at what rate, and how commercial payors will treat reimbursement of the drug.
Physician relationships
An ESRD patient generally seeks treatment at an outpatient dialysis center near his or her home where his or her treating nephrologist has practice privileges. Our relationships with local nephrologists and our ability to meet their needs and the needs of their patients are key factors in the success of our dialysis operations. Approximately 5,300 nephrologists currently refer patients to our outpatient dialysis centers. As is typical in the dialysis industry, one or a few physicians, including the outpatient dialysis center’s medical director, usually account for all or a significant portion of an outpatient dialysis center’s patient base.
Participation in the Medicare ESRD program requires that dialysis services at an outpatient dialysis center be under the general supervision of a medical director who is a licensed physician. We have engaged physicians or groups of physicians to serve as medical directors for each of our outpatient dialysis centers. At some outpatient dialysis centers, we also separately contract with one or more other physicians to serve as assistant or associate medical directors or to direct specific programs, such as home dialysis training programs. We have approximately 1,000 individual physicians and physician groups under contract to provide medical director services.
Medical directors for our dialysis centers enter into written contracts with us that specify their duties and fix their compensation generally for periods of ten years. The compensation of our medical directors is the result of arm’s length negotiations and generally depends upon an analysis of various factors such as the physician’s duties, responsibilities, professional qualifications and experience, among others.
Our medical director contracts for our dialysis centers generally include covenants not to compete. Also, except as described below, when we acquire an outpatient dialysis center from one or more physicians or where one or more physicians own minority interests in our outpatient dialysis centers, these physicians have agreed to refrain from owning interests in other competing outpatient dialysis centers within a defined geographic area for various time periods. These non-compete agreements restrict the physicians from owning or providing medical director services to other outpatient dialysis centers, but do not prohibit the physicians from referring patients to any outpatient dialysis center, including competing centers. Many of these non-compete agreements continue for a period of time beyond expiration of the corresponding medical director agreements, although some expire at the same time as the medical director agreement. Occasionally, we experience competition from a new outpatient dialysis center established by a former medical director following the termination of his or her relationship with us. As part of our Corporate Integrity Agreement (CIA), as described below, we also have agreed not to enforce investment non-compete restrictions relating to dialysis clinics or programs that were established pursuant to a partial divestiture joint venture transaction. Therefore, to the extent a joint venture partner or medical director has a contract(s) with us covering dialysis clinics or programs that were established pursuant to a partial divestiture, we will not enforce the investment non-compete provision relating to those clinics and/or programs.
If a significant number of physicians, including an outpatient dialysis center’s medical directors, were to cease referring patients to our outpatient dialysis centers, it would have a material adverse effect on our business, results of operations and financial condition.
Government regulation
Our dialysis operations are subject to extensive federal, state and local governmental laws and regulations. These laws and regulations require us to meet various standards relating to, among other things, government payment programs, dialysis facilities and equipment, management of centers, personnel qualifications, maintenance of proper records, and quality assurance programs and patient care.
If any of our operations are found to violate applicable laws or regulations, we could suffer severe consequences that could have a material adverse effect on our business, results of operations, financial condition and stock price, including:
• | Suspension or termination of our participation in government payment programs; |
• | Refund amounts received in violation of law or applicable payment program requirements; |
• | Loss of required government certifications or exclusion from government payment programs; |
• | Loss of licenses required to operate healthcare facilities or administer pharmaceuticals in some of the states in which we operate or elsewhere; |
9
• | Reductions in payment rates or coverage for dialysis and ancillary services and related pharmaceuticals; |
• | Civil or criminal liability, fines, damages and monetary penalties for violations of healthcare fraud and abuse laws, including the federal Anti-Kickback Statute contained in the Social Security Act of 1935, as amended (Anti-Kickback Statute), Stark Law and False Claims Act (FCA), and other failures to meet regulatory requirements; |
• | Enforcement actions by governmental agencies and/or claims for monetary damages from patients who believe their protected health information (PHI) has been used, disclosed or not properly safeguarded in violation of federal or state patient privacy laws including the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the Privacy Act of 1974; |
• | Mandated changes to our practices or procedures that significantly increase operating expenses; |
• | Imposition of and compliance with corporate integrity agreements that could subject us to ongoing audits and reporting requirements as well as increased scrutiny of our billing and business practices and potential fines; |
• | Termination of relationships with medical directors; and |
• | Harm to our reputation which could impact our business relationships, affect our ability to obtain financing and decrease access to new business opportunities, among other things. |
We expect that our industry will continue to be subject to substantial regulation, the scope and effect of which are difficult to predict. Our activities could be reviewed or challenged by regulatory authorities at any time in the future. This regulation and scrutiny could have a material adverse impact on us.
Licensure and certification
Our dialysis centers are certified by CMS, as is required for the receipt of Medicare payments. In some states, our outpatient dialysis centers also are required to secure additional state licenses and permits. Governmental authorities, primarily state departments of health, periodically inspect our centers to determine if we satisfy applicable federal and state standards and requirements, including the conditions of participation in the Medicare ESRD program.
To date, we have experienced some delays in obtaining Medicare certifications from CMS. Recent legislation will allow private entities to perform initial dialysis facilities certifications beginning in 2019. We may choose to use these private companies in the future, although the number of companies who will enter the market and the cost of surveys they might perform has yet to be determined.
Federal Anti-Kickback Statute
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or kind, to induce or reward either the referral of an individual for, or the purchase, or order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid.
Federal criminal penalties for the violation of the federal Anti-Kickback Statute include imprisonment, fines and exclusion of the provider from future participation in the federal healthcare programs, including Medicare and Medicaid. Violations of the federal Anti-Kickback Statute are punishable by imprisonment for up to ten years and fines of up to $100,000 or both. Larger fines can be imposed upon corporations under the provisions of the U.S. Sentencing Guidelines and the Alternate Fines Statute. Individuals and entities convicted of violating the federal Anti-Kickback Statute are subject to mandatory exclusion from participation in Medicare, Medicaid and other federal healthcare programs for a minimum of five years. Civil penalties for violation of this law include up to $100,000 in monetary penalties per violation, repayments of up to three times the total payments between the parties and suspension from future participation in Medicare and Medicaid. Court decisions have held that the statute may be violated even if only one purpose of remuneration is to induce referrals. The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (Affordable Care Act (ACA)) amended the federal Anti-Kickback Statute to clarify the intent that is required to prove a violation. Under the statute as amended, the defendant does not need to have actual knowledge of the federal Anti-Kickback Statute or have the specific intent to violate it. In addition, the ACA amended the federal Anti-Kickback Statute to provide that any claims for items or services resulting from a violation of the federal Anti-Kickback Statute are considered false or fraudulent for purposes of the FCA.
10
The federal Anti-Kickback Statute includes statutory exceptions and regulatory safe harbors that protect certain arrangements. Business transactions and arrangements that are structured to comply fully with an applicable safe harbor do not violate the federal Anti-Kickback Statute. However, transactions and arrangements that do not satisfy all elements of a relevant safe harbor do not necessarily violate the law. When an arrangement does not satisfy a safe harbor, the arrangement must be evaluated on a case-by-case basis in light of the parties’ intent and the arrangement’s potential for abuse. Arrangements that do not satisfy a safe harbor may be subject to greater scrutiny by enforcement agencies.
We enter into several arrangements with physicians that potentially implicate the Anti-Kickback Statute, such as:
Medical Director Agreements. Because our medical directors refer patients to our dialysis centers, our arrangements with these physicians are designed to substantially comply with the safe harbor for personal service arrangements. Although the Medical Director Agreements we enter into with physicians substantially comply with the safe harbor for personal service arrangements, including the requirement that compensation be consistent with fair market value, the safe harbor requires that when services are provided on a part-time basis, the agreement must specify the schedule of intervals of services, and their precise length and the exact charge for such services. Because of the nature of our medical directors’ duties, it is impossible to fully satisfy this technical element of the safe harbor. We believe that our fair market value arrangements with physicians who serve as medical directors do not violate the federal Anti-Kickback Statute; however, these arrangements could be subject to scrutiny since they do not expressly describe the schedule of part-time services to be provided under the arrangement.
Joint Ventures. We own a controlling interest in numerous U.S. dialysis related joint ventures. For the year ended December 31, 2017, these joint ventures represented approximately 24% of our net U.S. dialysis and related lab services revenues. We may continue to increase the number of our joint ventures. Our relationships with physicians and other referral sources relating to these joint ventures do not fully satisfy the safe harbor for investments in small entities. Although failure to comply with a safe harbor does not render an arrangement illegal under the federal Anti-Kickback Statute, an arrangement that does not operate within a safe harbor may be subject to scrutiny and the Department of Health and Human Services’ Office of Inspector General (OIG) has warned in the past that certain joint venture relationships have a potential for abuse. Based upon the foregoing, physician joint ventures that fall outside the safe harbors are not, by definition, prohibited by law. Instead, such joint ventures require case-by-case evaluation under the federal Anti-Kickback Statute.
In this regard, we have structured our joint ventures to satisfy as many elements of the safe harbor for investments in small entities as we believe are commercially reasonable. For example, we believe that these investments are offered and made by us on a fair market value basis and provide returns to the investors in proportion to their actual investment in the venture. We believe that our joint venture arrangements do not violate the federal Anti-Kickback Statute; however, since the arrangements do not satisfy all of the requirements of an applicable safe harbor, these arrangements could be subject to challenge on the ground that they are intended to induce patient referrals. In that regard, we were subject to investigation by the United States Attorney’s Office for the District of Colorado, the Civil Division of the United States Department of Justice (DOJ) and the OIG related to our relationships with physicians, including our joint ventures, and whether those relationships and joint ventures comply with the federal Anti-Kickback Statute and the FCA. In October 2014, we entered into a Settlement Agreement with the United States and relator David Barbetta to resolve the then pending 2010 and 2011 U.S. Attorney physician relationship investigations. In connection with the resolution of this matter, and in exchange for the OIG’s agreement not to exclude us from participating in the federal healthcare programs, we have entered into a five-year CIA with the OIG. The CIA (i) requires that we maintain certain elements of our compliance programs; (ii) imposes certain expanded compliance-related requirements during the term of the CIA; (iii) requires ongoing monitoring and reporting by an independent monitor, imposes certain reporting, certification, records retention and training obligations, allocates certain oversight responsibility to the Board’s Compliance Committee, and necessitates the creation of a Management Compliance Committee and the retention of an independent compliance advisor to the Board; and (iv) contains certain business restrictions related to a subset of our joint venture arrangement. The costs associated with compliance with the CIA could be substantial and may be greater than we currently anticipate. In addition, in the event of a breach of the CIA, we could become liable for payment of certain stipulated penalties, and could be excluded from participation in federal healthcare programs.
Lease Arrangements. We lease space for numerous dialysis centers from entities in which physicians, hospitals or medical groups hold ownership interests, and we sublease space to referring physicians at approximately 250 of our dialysis centers as of December 31, 2017. We believe that these arrangements comply with the federal Anti-Kickback Statute safe harbor for space rentals in all material respects. Therefore, we believe that these lease arrangements should not be subject to challenge under the federal Anti-Kickback Statute.
Common Stock. Some medical directors and other referring physicians may own our common stock. We believe that these interests materially satisfy the requirements of the Anti-Kickback Statute safe harbor for investments in large publicly traded companies. Therefore, we believe that these investments should not be subject to challenge under the federal Anti-Kickback Statute.
11
Discounts. Our dialysis centers sometimes acquire certain items and services at a discount that may be reimbursed by a federal healthcare program. We believe that our vendor contracts that include discount or rebate provisions are in compliance with the federal Anti-Kickback Statute safe harbor for discounts, and accordingly, we believe that our discounted vendor contracts should not be subject to challenge under the federal Anti-Kickback Statute.
If any of our business transactions or arrangements, including those described above, were found to violate the federal Anti-Kickback Statute, we, among other things, could face criminal, civil or administrative sanctions, including possible exclusion from participation in Medicare, Medicaid and other state and federal healthcare programs. Any findings that we have violated these laws could have a material adverse impact on our business, results of operations, financial condition and stock price.
Stark Law
The Stark Law prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities providing Designated Health Services (DHS), from referring Medicare and Medicaid patients to such entities for the furnishing of DHS, unless an exception applies. DHS is defined to mean any of the following enumerated items or services; clinical laboratory services; physical therapy services; occupational therapy services; radiology services, including magnetic resonance imaging, computerized axial tomography scans, and ultrasound services; radiation therapy services and supplies; durable medical equipment and supplies; parenteral and enteral nutrients, equipment, and supplies; prosthetics, orthotics and prosthetic devices and supplies; home health services; outpatient prescription drugs; inpatient and outpatient hospital services; and outpatient speech-language pathology services. The types of financial arrangements between a physician and a DHS entity that trigger the self-referral prohibitions of the Stark Law are broad and include direct and indirect ownership and investment interests and compensation arrangements. The Stark Law also prohibits the DHS entity receiving a prohibited referral from presenting, or causing to be presented, a claim or billing for the services arising out of the prohibited referral. The prohibition applies regardless of the reasons for the financial relationship and the referral; unlike the federal Anti-Kickback Statute, intent to induce referrals is not required. If the Stark Law is implicated, the financial relationship must fully satisfy a Stark Law exception. If an exception is not satisfied, then the arrangement could be subject to sanctions. Sanctions for violation of the Stark Law include denial of payment for claims for services provided in violation of the prohibition, refunds of amounts collected in violation of the prohibition, a civil penalty of up to $15,000 for each service arising out of the prohibited referral, a civil penalty of up to $100,000 against parties that enter into a scheme to circumvent the Stark Law prohibition, civil assessment of up to three times the amount claimed, and potential exclusion from the federal healthcare programs, including Medicare and Medicaid. Amounts collected for prohibited claims must be reported and refunded generally within 60 days after the date on which the overpayment was identified. Furthermore, Stark Law violations and failure to return overpayments timely can form the basis for FCA liability as discussed below.
The definition of DHS under the Stark Law excludes services paid under a composite rate, even if some of the components bundled in the composite rate are DHS. Although the ESRD bundled payment system is no longer titled a composite rate, we believe that the former composite rate payment system and the current bundled system are both composite systems excluded from the Stark Law. Since most services furnished to Medicare beneficiaries provided in our dialysis centers are reimbursed through a bundled rate, the services performed in our facilities generally are not DHS, and the Stark Law referral prohibition does not apply to those services. Certain separately billable drugs (drugs furnished to an ESRD patient that are not for the treatment of ESRD that CMS allows our centers to bill for using the so-called AY modifier) may be considered DHS. However, for compliance with the law we have implemented certain billing controls to limit DHS being billed out of our dialysis clinics. Likewise, the definition of inpatient hospital services, for purposes of the Stark Law, also excludes inpatient dialysis performed in hospitals that are not certified to provide ESRD services. Consequently, our arrangements with such hospitals for the provision of dialysis services to hospital inpatients do not trigger the Stark Law referral prohibition.
In addition, although prescription drugs are DHS, there is an exception in the Stark Law for EPO and other specifically enumerated dialysis drugs when furnished in or by an ESRD facility such that the arrangement for the furnishing of the drugs does not violate the federal Anti-Kickback Statute, and all billing and claims submission for the drugs does not violate any laws or regulations governing billing or claims submission. The exception is available only for drugs included on a list of Current Procedural Terminology/Healthcare Common Procedure Coding System (CPT/HCPCS) codes published by CMS, and for EPO, Aranesp® and equivalent drugs dispensed by the ESRD facility for use at home. While we believe that most drugs furnished by our dialysis centers are covered by the exception, dialysis centers may administer drugs that are not on the list of CPT/HCPCS codes and therefore do not meet this exception. In order for a physician who has a financial relationship with a dialysis center to order one of these drugs from the center and for the center to obtain Medicare reimbursement, another exception must apply.
We have entered into several types of financial relationships with referring physicians, including compensation arrangements. If our dialysis centers were to bill for a non-exempted drug and the financial relationships with the referring
12
physician did not satisfy an exception, we could in the future be required to change our practices, face civil penalties, pay substantial fines, return certain payments received from Medicare and beneficiaries or otherwise experience a material adverse effect as a result of a challenge to payments made pursuant to referrals from these physicians under the Stark Law.
Medical Director Agreements. We believe that our medical director agreements satisfy the personal services arrangement exception to the Stark Law. While we believe that the compensation provisions included in our medical director agreements are the result of arm’s length negotiations and result in fair market value payments for medical director services, an enforcement agency could nevertheless challenge the level of compensation that we pay our medical directors.
Lease Agreements. Some of our dialysis centers are leased from entities in which referring physicians hold interests and we sublease space to referring physicians at some of our dialysis centers. The Stark Law provides an exception for lease arrangements if specific requirements are met. We endeavor to structure our leases and subleases with referring physicians to satisfy the requirements for this exception.
Common Stock. Some medical directors and other referring physicians may own our common stock. We believe that these interests satisfy the Stark Law exception for investments in large publicly traded companies.
Joint Ventures. Some of our referring physicians also own equity interests in entities that operate our dialysis centers. None of the Stark Law exceptions applicable to physician ownership interests in entities to which they make DHS referrals apply to the kinds of ownership arrangements that referring physicians hold in several of our subsidiaries that operate dialysis centers. Accordingly, these dialysis centers do not bill Medicare for DHS referrals from physician owners. If the dialysis centers bill for DHS referred by physician owners, the dialysis center would be subject to the Stark Law penalties described above.
Other Operations. The operations of our ancillary and subsidiary businesses are also subject to compliance with the Stark Law, and any failure to comply with these requirements, particularly in light of the strict liability nature of the Stark Law, could subject these operations to the Stark Law penalties and sanctions described above.
While we believe that most of our operations do not implicate the Stark Law, particularly under the ESRD bundled payment system, and that to the extent that our dialysis centers furnish DHS, they either meet an exception or do not bill for services that do not meet a Stark Law exception, if CMS determined that we have submitted claims in violation of the Stark Law, or otherwise violated the Stark Law, we would be subject to the penalties described above. In addition, it might be necessary to restructure existing compensation agreements with our medical directors and to repurchase or to request the sale of ownership interests in subsidiaries and partnerships held by referring physicians or, alternatively, to refuse to accept referrals for DHS from these physicians, or take other actions to modify our operations. Any such penalties and restructuring or other required actions could have a material adverse effect on our business, results of operations and financial condition.
Fraud and abuse under state law
Many states in which we operate dialysis centers have statutes prohibiting physicians from holding financial interests in various types of medical facilities to which they refer patients. Some of these statutes could potentially be interpreted broadly as prohibiting physicians who hold shares of our publicly traded stock from referring patients to our dialysis centers if the centers use our laboratory subsidiary to perform laboratory services for their patients. States also have laws similar to or stricter than the federal Anti-Kickback Statute that may affect our ability to receive referrals from physicians with whom we have financial relationships, such as our medical directors. Some state anti-kickback statutes also include civil and criminal penalties. Some of these statutes include exemptions that may be applicable to our medical directors and other physician relationships or for financial interests limited to shares of publicly traded stock. Some, however, include no explicit exemption for medical director services or other services for which we contract with and compensate referring physicians or for joint ownership interests of the type held by some of our referring physicians or for financial interests limited to shares of publicly traded stock. If these statutes are interpreted to apply to referring physicians with whom we contract for medical director and similar services, to referring physicians with whom we hold joint ownership interests or to physicians who hold interests in DaVita Inc. limited solely to our publicly traded stock, we may be required to terminate or restructure some or all of our relationships with or refuse referrals from these referring physicians and could be subject to criminal, civil and administrative sanctions, refund requirements and exclusions from government healthcare programs, including Medicare and Medicaid. Such events could negatively affect the decision of referring physicians to refer patients to our centers.
13
The False Claims Act
The federal FCA is a means of policing false bills or false requests for payment in the healthcare delivery system. In part, the FCA authorizes the imposition of up to three times the government’s damages and civil penalties on any person who, among other acts:
• | Knowingly presents or causes to be presented to the federal government, a false or fraudulent claim for payment or approval; |
• | Knowingly makes, uses or causes to be made or used, a false record or statement material to a false or fraudulent claim; |
• | Knowingly makes, uses, or causes to be made or used, a false record or statement material to an obligation to pay the government, or knowingly conceals or knowingly and improperly, avoids or decreases an obligation to pay or transmit money or property to the federal government; or |
• | Conspires to commit the above acts. |
In addition, amendments to the FCA impose severe penalties for the knowing and improper retention of overpayments collected from government payors. Under these provisions, within 60 days of identifying an overpayment, a provider is required to notify CMS or the Medicare Administrative Contractor of the overpayment and the reason for it and return the overpayment. An overpayment impermissibly retained could subject us to liability under the FCA, exclusion, and penalties under the federal Civil Monetary Penalty statute. As a result of these provisions, our procedures for identifying and processing overpayments may be subject to greater scrutiny. We have made significant investments to accelerate the time it takes us to identify and process overpayments and we may be required to make additional investments in the future. Acceleration in our ability to identify and process overpayments could result in us refunding overpayments to government or other payors sooner than we have in the past. A significant acceleration of these refunds could have a material adverse effect on our operating cash flows.
The penalties for a violation of the FCA range from $5,500 to $11,000 (adjusted for inflation) for each false claim, plus up to three times the amount of damages caused by each false claim, which can be as much as the amounts received directly or indirectly from the government for each such false claim. On February 3, 2017, the DOJ issued a final rule announcing adjustments to FCA penalties, under which the per claim penalty range increases to $10,957 to $21,916 for penalties assessed after February 3, 2017, so long as the underlying conduct occurred after November 2, 2015. The federal government has used the FCA to prosecute a wide variety of alleged false claims and fraud allegedly perpetrated against Medicare and state healthcare programs, including coding errors, billing for services not rendered, the submission of false cost reports, billing for services at a higher payment rate than appropriate, billing under a comprehensive code as well as under one or more component codes included in the comprehensive code and billing for care that is not considered medically necessary. The ACA provides that claims tainted by a violation of the federal Anti-Kickback Statute are false for purposes of the FCA. Some courts have held that filing claims or failing to refund amounts collected in violation of the Stark Law can form the basis for liability under the FCA. In addition to the provisions of the FCA, which provide for civil enforcement, the federal government can use several criminal statutes to prosecute persons who are alleged to have submitted false or fraudulent claims for payment to the federal government.
Privacy and Security
The Health Insurance Portability and Accountability Act of 1996 and its implementing privacy and security regulations, as amended by the federal Health Information Technology for Economic and Clinical Health Act (HITECH Act), (collectively referred to as HIPAA), require us to provide certain protections to patients and their health information. The HIPAA privacy and security regulations extensively regulate the use and disclosure of PHI and require covered entities, which include healthcare providers, to implement and maintain administrative, physical and technical safeguards to protect the security of such information. Additional security requirements apply to electronic PHI. These regulations also provide patients with substantive rights with respect to their health information.
The HIPAA privacy and security regulations also require us to enter into written agreements with certain contractors, known as business associates, to whom we disclose PHI. Covered entities may be subject to penalties for, among other activities, failing to enter into a business associate agreement where required by law or as a result of a business associate violating HIPAA if the business associate is found to be an agent of the covered entity and acting within the scope of the agency. Business associates are also directly subject to liability under the HIPAA privacy and security regulations. In instances
14
where we act as a business associate to a covered entity, there is the potential for additional liability beyond our status as a covered entity.
Covered entities must report breaches of unsecured PHI to affected individuals without unreasonable delay but not to exceed 60 days of discovery of the breach by a covered entity or its agents. Notification must also be made to the HHS, and, for breaches of unsecured PHI involving more than 500 residents of a state or jurisdiction, to the media. All non-permitted uses or disclosures of unsecured PHI are presumed to be breaches unless the covered entity or business associate establishes that there is a low probability the information has been compromised. Various state laws and regulations may also require us to notify affected individuals in the event of a data breach involving individually identifiable information without regard to whether there is a low probability of the information being compromised.
Penalties for impermissible use or disclosure of PHI were increased by the HITECH Act by imposing tiered penalties of more than $50,000 per violation and up to $1.5 million per year for identical violations. In addition, HIPAA provides for criminal penalties of up to $250,000 and ten years in prison, with the severest penalties for obtaining and disclosing PHI with the intent to sell, transfer or use such information for commercial advantage, personal gain or malicious harm. Further, state attorneys general may bring civil actions seeking either injunction or damages in response to violations of the HIPAA privacy and security regulations that threaten the privacy of state residents. We believe our HIPAA Privacy and Security Program sufficiently addresses HIPAA and state privacy law requirements.
Healthcare reform
In March 2010, broad healthcare reform legislation was enacted in the U.S. through the ACA. Although many of the provisions of the ACA did not take effect immediately and continue to be implemented, and some have been and may be modified before or during their implementation, the reforms could have an impact on our business in a number of ways. We cannot predict how employers, private payors or persons buying insurance might react to federal and state healthcare reform legislation or what form many of these regulations will take before implementation.
The ACA introduced healthcare insurance exchanges, which provide a marketplace for eligible individuals and small employers to purchase healthcare insurance. The business and regulatory environment continues to evolve as the exchanges mature, and statutes and regulations are challenged, changed and enforced.
The ACA also requires that all non-grandfathered individual and small group health plans sold in a state, including plans sold through the state-based exchanges created pursuant to the healthcare reform laws, cover essential health benefits (EHBs) in ten general categories. The scope of the benefits is intended to equal the scope of benefits under a typical employer plan.
On February 25, 2013, HHS issued the final rule governing the standards applicable to EHB benchmark plans, new definitions, actuarial value requirements and methodology, and published a list of plan benchmark options that states can use to develop EHBs. The rule describes specific coverage requirements that (i) prohibit discrimination against individuals because of pre-existing or chronic conditions on health plans applicable to EHBs, (ii) ensure network adequacy of essential health providers, and (iii) prohibit benefit designs that limit enrollment and that prohibit access to care for enrollees. Subsequent regulations relevant to the EHB have continued the benchmark plan approach for 2016 and future years and have implemented clarifications and modifications to the existing EHB regulations, including the prohibition on discrimination, network adequacy standards and other requirements. In recent years, CMS has issued an annual Notice of Benefit and Payment Parameters rulemaking and related guidance setting forth standards for insurance plans provided through the exchanges.
Other aspects of the 2010 healthcare reform laws may affect our business as well, including changes affecting the Medicare and Medicaid programs. We note, however, that the 2016 Presidential and Congressional elections and subsequent developments have caused the future state of the exchanges and other ACA reforms to be very unclear. The Republicans, who now control the Administration and Congress, have repeatedly expressed a desire to repeal and replace the ACA. Further, in October 2017, the federal government announced that cost-sharing reduction payments to insurers would end, effective immediately, unless Congress appropriated the funds, and, in December 2017, Congress passed the Tax Cuts and Jobs Act, which includes a provision that eliminates the penalty under the ACA’s individual mandate and could impact the future state of the exchanges. Moreover, in February 2018, Congress passed the BBA which, among other things, repealed the Independent Payment Advisory Board that was established by the ACA and intended to reduce the rate of growth in Medicare spending. While certain provisions of the BBA may increase the scope of benefits available for certain chronically ill Federal health care program beneficiaries beginning in 2020, the ultimate impact of such changes cannot be predicted. While there may be significant changes to the healthcare environment in the future, the specific changes and their timing are not yet apparent. As a result, there is considerable uncertainty regarding the future with respect to the exchanges, and, indeed, many core aspects of the current health care marketplace. While specific changes and their timing are not yet apparent, the enacted reforms as well as
15
future legislative, regulatory, and executive changes could have a material adverse effect on our results of operations, including lowering our reimbursement rates and/or increasing our expenses.
Other regulations
Our U.S. dialysis and related lab services operations are subject to various state hazardous waste and non-hazardous medical waste disposal laws. These laws do not classify as hazardous most of the waste produced from dialysis services. Occupational Safety and Health Administration regulations require employers to provide workers who are occupationally subject to blood or other potentially infectious materials with prescribed protections. These regulatory requirements apply to all healthcare facilities, including dialysis centers, and require employers to make a determination as to which employees may be exposed to blood or other potentially infectious materials and to have in effect a written exposure control plan. In addition, employers are required to provide or employ hepatitis B vaccinations, personal protective equipment and other safety devices, infection control training, post-exposure evaluation and follow-up, waste disposal techniques and procedures and work practice controls. Employers are also required to comply with various record-keeping requirements. We believe that we are in material compliance with these laws and regulations.
A few states have certificate of need programs regulating the establishment or expansion of healthcare facilities, including dialysis centers. We believe that we are in material compliance with all applicable state certificate of need laws.
Capacity and location of our U.S. dialysis centers
Typically we are able to increase our capacity by extending hours at our existing dialysis centers, expanding our existing dialysis centers, relocating our dialysis centers, developing new dialysis centers and by acquiring dialysis centers. The development of a typical outpatient dialysis center by us generally requires approximately $2.1 million for leasehold improvements and other capital expenditures. Based on our experience, a new outpatient dialysis center typically opens within a year after the property lease is signed, normally achieves operating profitability in the second year after Medicare certification and normally reaches maturity within three to five years. Acquiring an existing outpatient dialysis center requires a substantially greater initial investment, but profitability and cash flows are generally accelerated and more predictable. To a limited extent, we enter into agreements to provide management and administrative services to outpatient dialysis centers in which we either own a noncontrolling equity investment, or are wholly-owned by third parties in return for management fees, which are typically based on a percentage of revenues or cash collections of the managed center’s operations.
The table below shows the growth of our U.S. dialysis operations by number of dialysis centers.
2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||
Number of centers at beginning of year | 2,350 | 2,251 | 2,179 | 2,074 | 1,954 | |||||||||
Acquired centers | 66 | 8 | 6 | 18 | 26 | |||||||||
Developed centers | 121 | 100 | 72 | 105 | 98 | |||||||||
Net change in centers with management and administrative services agreements(1) | (2 | ) | — | 2 | — | 4 | ||||||||
Sold and closed centers(2)(3) | (15 | ) | (4 | ) | (3 | ) | (2 | ) | (5 | ) | ||||
Closed centers(4) | (10 | ) | (5 | ) | (5 | ) | (16 | ) | (3 | ) | ||||
Number of centers at end of year | 2,510 | 2,350 | 2,251 | 2,179 | 2,074 | |||||||||
(1) | Represents dialysis centers in which we either own a noncontrolling equity investment, or are wholly-owned by third parties, and also includes dialysis centers we deconsolidated and transferred to management services agreements. |
(2) | Includes centers that were divested as a part of our Renal Ventures acquisition. |
(3) | Represents dialysis centers that were sold and/or closed for which patients were not retained. |
(4) | Represents dialysis centers that were closed for which the majority of patients were retained and transferred to one of our other existing outpatient dialysis centers. |
16
As of December 31, 2017, we operated or provided administrative services to a total of 2,510 U.S. outpatient dialysis centers. A total of 2,471 of such centers are consolidated in our financial statements. Of the remaining 39 unconsolidated U.S. outpatient dialysis centers, we own a noncontrolling interest in 34 centers and provide management and administrative services to five centers that are wholly-owned by third parties. The locations of the 2,471 U.S. outpatient dialysis centers consolidated in our financial statements at December 31, 2017 were as follows:
Ancillary services and strategic initiatives businesses, including our international operations
As of December 31, 2017, our ancillary services and strategic initiatives consisted primarily of pharmacy services, disease management services, vascular access services, clinical research programs, physician services, direct primary care, ESRD seamless care organizations, comprehensive care, and our international operations and relate primarily to our core business of providing kidney care services.
Ancillary services and strategic initiatives consist primarily of the following:
• | Pharmacy services. DaVita Rx is a pharmacy that specializes in providing oral medications and medication management services to patients with ESRD. The main objective of the pharmacy is to improve clinical outcomes and reduce total healthcare costs by facilitating increased patient compliance and to provide our patients a convenient way to fill their prescription needs. Revenues are recognized as prescriptions are filled and shipped to patients or when services are completed. |
• | Disease management services. VillageHealth provides advanced integrated care management services to health plans and government programs for members/beneficiaries diagnosed with ESRD, chronic kidney failure, and/or poly-comorbid conditions. Through a combination of clinical coordination, innovative interventions, medical claims analysis and information technology, we endeavor to assist our customers and patients in obtaining superior renal healthcare and improved clinical outcomes, as well as helping to reduce overall medical costs. Integrated care management revenues are typically based upon an established contract fee and are recognized as earned over the contract period and can include additional fees for cost savings recognized by certain customers. VillageHealth also operates Medicare Advantage ESRD Special Needs Plans in partnership with payors that work with CMS to provide ESRD patients full service healthcare. We are at risk for all medical costs of the program in excess of the capitation payments. Furthermore, in October 2015, VillageHealth entered into management service agreements to support three ESCO joint ventures in which we are an investor through certain wholly- or majority-owned dialysis clinics. |
17
• | Vascular access services. Lifeline provides management and administrative services to physician-owned vascular access clinics that provide vascular services for dialysis and other patients. Lifeline is also the majority-owner of nine vascular access clinics. Management fees generated from providing management and administrative services are recognized as earned typically based on a percentage of revenues or cash collections generated by the clinics. Revenues associated with the vascular access clinics that are majority-owned are recognized in the period when the services are provided. |
• | Clinical research programs. DaVita Clinical Research (DCR) is a provider-based specialty clinical research organization with a full spectrum of services for clinical drug research and device development. DCR uses its extensive, applied database and real-world healthcare experience to assist in the design, recruitment and completion of retrospective, prospective pragmatic and clinical trials. Revenues are based upon an established fee per study, as determined by contract with drug companies and other sponsors and are recognized as earned according to the contract terms. |
• | Physician services. Nephrology Practice Solutions (NPS) is an independent business that partners with physicians committed to providing outstanding clinical and integrated care to patients. NPS provides nephrologist employment opportunities in select markets and offers physician practice management services to nephrologists under administrative services agreements. These services include physician practice management, billing and collections, credentialing, coding, and other support services that enable physician practices to increase efficiency and manage their administrative needs. Fees generated from these services are recognized as earned typically based upon flat fees or cash collections generated by the physician practice. NPS also provides leading nephrology recruitment and staffing services which are billed on a per search basis. |
• | Direct primary care. Paladina Health is a healthcare services organization that operates membership-based primary care clinics mainly through employer-based on-site and near-site clinics. The clinics offer patients more personalized and improved access to primary care physicians, including unlimited visits and same-day or next-day appointments. Physicians focus on clinical outcomes and patient satisfaction. Revenues are recognized over the membership period. |
• | ESRD Seamless Care Organization joint ventures (ESCO JVs). In October 2015, certain of our dialysis clinics entered into partnerships with various nephrology practices, DaVita Rx and health systems to establish three ESCO JVs in Phoenix-Tucson Arizona, South Florida, and Philadelphia Pennsylvania-Camden, New Jersey. The ESCO JVs were formed under the CMS Innovation Center’s Comprehensive ESRD Care (CEC) Model, a demonstration to assess the impact of care coordination for ESRD patients in a dialysis-center oriented ACO setting. Each ESCO JV has a shared risk arrangement with CMS and the programs are evaluated on a performance year basis. The delivery of improved quality outcomes for patients and program savings depend on the contributions of the dialysis center teammates, nephrologists, health system and hospital partners, pharmacy providers including DaVita Rx, other primary care and specialty care providers and facilities, and integrated care management support from VillageHealth, which is also the manager of the ESCO JVs. In October 2017, CMS published the results for the first performance year, covering the period from October 2015 to December 2016, and all three ESCO JVs earned shared savings payments. |
• | Comprehensive care. DaVita Health Solutions provides high-quality, comprehensive medical care for high-risk patients when and where they need it most - at home, in a post-acute care facility or within the dialysis center. DaVita Health Solutions offers a broad suite of home- and outpatient-based care programs, including primary care, behavioral health, palliative care, comprehensive health assessments and other clinical services through 24/7 house calls at home, at skilled nursing facilities and at dialysis centers. |
International dialysis operations
As of December 31, 2017, we operated or provided administrative services to a total of 237 outpatient dialysis centers, which includes consolidated and nonconsolidated centers located in 11 countries outside of the U.S., serving approximately 22,900 patients. Our international dialysis operations have continued to grow steadily and expand as a result of developing and acquiring outpatient dialysis centers in various strategic markets. Our international operations are included as a component of our ancillary services and strategic initiatives. The table below summarizes the number and locations of our international
18
outpatient dialysis centers.
2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||
Number of centers at beginning of year | 154 | 118 | 91 | 73 | 36 | |||||||||
Acquired centers | 68 | 21 | 21 | 9 | 38 | |||||||||
Developed and hospital operated centers | 8 | 12 | 7 | 11 | 2 | |||||||||
Managed centers, net | — | — | (1 | ) | — | — | ||||||||
Closed centers | (1 | ) | — | — | (2 | ) | (3 | ) | ||||||
Net change in Asia Pacific Joint Venture (APAC JV) operated centers | 8 | 66 | — | — | — | |||||||||
Deconsolidated centers due to formation of APAC JV | — | (63 | ) | — | — | — | ||||||||
Number of centers at end of year | 237 | 154 | 118 | 91 | 73 | |||||||||
The locations of our international outpatient dialysis centers are as follows:
Poland | 51 | |
Germany | 44 | |
Malaysia(1) | 38 | |
India(1) | 24 | |
Saudi Arabia | 22 | |
Colombia | 20 | |
Brazil | 18 | |
Portugal | 8 | |
Taiwan(1) | 7 | |
China(1) | 4 | |
Singapore(1) | 1 | |
237 | ||
(1) | Includes centers that are operated or managed by our APAC JV. |
Corporate Administrative Support
Corporate administrative support consists primarily of labor, benefits and long-term incentive compensation costs for departments which provide support to all of our different operating lines of business. These expenses are included in our consolidated general and administrative expenses and are partially offset by the allocation of management fees.
DaVita Medical Group (DMG) Division
On December 5, 2017, we entered into an equity purchase agreement to sell our DMG division to Optum, a subsidiary of UnitedHealth Group Inc. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions. As a result of this pending transaction, the DMG business is classified as held for sale and its results of operations are reported as discontinued operations. In addition, prior periods' presentation has been revised to conform to current year presentation.
DMG business overview
DMG is a patient- and physician-focused integrated healthcare delivery and management company with over two decades of experience providing coordinated, outcomes-based medical care in a cost-effective manner. As of December 31, 2017, DMG served approximately 763,000 members under its care in southern California, central and south Florida, southern Nevada and central New Mexico through capitation contracts with some of the nation’s leading health plans. Of these members, approximately 319,900 individuals were patients enrolled in Medicare and Medicare Advantage, and the remaining approximately 443,100 individuals were managed care members whose health coverage is provided through their employer or who have individually acquired health coverage directly from a health plan or as a result of their eligibility for Medicaid benefits. In addition to its managed care business, during the year ended December 31, 2017, DMG provided care across all markets to approximately 966,600 patients whose health coverage is structured on a FFS basis, including patients enrolled through traditional Medicare and Medicaid programs, preferred provider organizations and other third party payors.
19
DMG patients as well as the patients of DMG’s associated physicians, physician groups and IPAs benefit from an integrated approach to medical care that places the physician at the center of patient care. As of December 31, 2017, DMG delivered services to its members via a network of over 750 primary care physicians, over 3,500 associated group and other network primary care physicians, approximately 180 network hospitals, and several thousand associated group and network specialists. Together with hundreds of case managers, registered nurses and other care coordinators, these medical professionals utilize a comprehensive information technology system, sophisticated risk management techniques and clinical protocols to provide high-quality, cost-effective care to DMG’s members.
U.S. healthcare spending has increased steadily over the past twenty years. These increases have been driven, in part, by the aging of the baby boomer generation, unhealthy behavioral and lifestyle choices in terms of exercise and diet, rapidly increasing costs in medical technology and pharmaceutical research, and provider reimbursement structures that may promote volume over quality in a FFS environment. These factors, as well as the steady growth of the U.S. population, have made the healthcare industry a growing market. In 2016, CMS reported that healthcare accounted for 17.9% of the U.S. gross domestic product and that healthcare spending increased 4.3% to reach $3.3 trillion. Medicare spending grew 3.6% to $672 billion in 2016 or 20% of National Health Expenditures, according to CMS. Medicare’s share of the federal budget was approximately 15.0% in 2017 according to the Congressional Budget Office (CBO). Medicare is frequently the focus of discussions on how to moderate the growth of both federal spending and healthcare spending in the U.S.
Growth in Medicare spending is expected to continue due to demographic changes. According to the U.S. Census Bureau, the U.S. population aged 65 and over is expected to be 83.7 million in 2050 — almost double its estimated population of 43.1 million in 2012.
Medicare Advantage is an alternative to the traditional FFS Medicare program, which permits Medicare beneficiaries to receive benefits from a managed care health plan. Medicare Advantage plans contract with CMS to provide benefits that are at least comparable to those offered under the traditional FFS Medicare program in exchange for a fixed per-member monthly premium payment from CMS. The monthly premium varies based on the county in which the member resides, further adjusted to reflect the plan members’ expected medical cost risk. Individuals who elect to participate in the Medicare Advantage program typically receive greater benefits than traditional FFS Medicare Part B beneficiaries, including additional preventive services, vision, dental and prescription drug benefits, and often have lower deductibles and co-payments than traditional FFS Medicare.
CMS pays Medicare Advantage health plans under a bidding process. Plans bid against county-level benchmarks. If a plan’s bid is higher than the benchmark, enrollees pay the difference in the form of a monthly premium. If the bid is lower than the benchmark, the plan receives the difference between its payment amount and its bid as a rebate, which must be returned to enrollees in the form of additional benefits, reduced premiums, or lower cost sharing.
Managed care health plans were developed, primarily during the 1980s, in an attempt to mitigate the rising cost of providing healthcare benefits to populations covered by traditional health insurance. These managed care health plans often enroll members through their employers. As a result of the prevalence of these health plans, many seniors now becoming eligible for Medicare have been interacting with managed care companies through their employers for the last 30 years. Individuals turning 65 now are likely to be far more familiar with the managed care setting than previous Medicare populations. According to Kaiser Family Foundation, in 2017, Medicare Advantage represented 33% of total Medicare members, creating a significant opportunity for additional Medicare Advantage penetration of newly eligible seniors.
In an effort to reduce the number of uninsured and to begin to control healthcare expenditures, President Obama signed the ACA into law in March 2010, which was affirmed, in substantial part, by the U.S. Supreme Court in June 2012. As of the end of 2016, the number of uninsured nonelderly Americans was 27.6 million, a decrease of over 16 million since 2013. These previously uninsured Americans and potentially newly eligible Medicaid beneficiaries represent a significant new market opportunity for health plans. We believe that health plans looking to cover these newly eligible individuals under fixed premium arrangements will seek provider arrangements that can effectively manage the cost and quality of the care being provided to these newly eligible individuals, although the 2016 Presidential and Congressional elections and subsequent developments, including recent federal tax reform legislation, have caused the future state of the ACA to become less clear.
One of the primary ways in which the ACA funded expanded health insurance coverage is through cuts in Medicare Advantage reimbursement. County benchmarks have transitioned to a system in which each county’s benchmark is a certain percentage (ranging from 95% to 115%) of FFS Medicare. In a March 2017 report to Congress, the Medicare Payment Advisory Commission (MedPAC) estimated that 2017 Medicare Advantage benchmarks (including the average 4% for quality bonuses), bids, and payments would average 106%, 90%, and 100% of FFS spending, respectively.
20
Despite the fact that the plan bids average less than FFS spending, payments for enrollees in these plans usually exceed FFS spending because the benchmarks are high relative to FFS spending. For example, health maintenance organizations (HMOs) as a group bid an average of 88% of FFS spending, yet 2017 payments for HMO enrollees are estimated to average 99% of FFS spending (including the quality bonuses).
Nonetheless, changes in benchmarks and/or bids that lower payments to Medicare Advantage plans could adversely affect DMG’s operating results.
Many health plans recognize both the opportunity for growth from senior members as well as the potential risks and costs associated with managing additional senior members. In regions operated by DMG and numerous other markets, many health plans subcontract a significant portion of the responsibility for managing patient care to integrated medical networks such as DMG. These integrated healthcare networks, whether medical groups or IPAs, offer a comprehensive medical delivery system and sophisticated care management knowledge and infrastructure to more efficiently provide for the healthcare needs of the population enrolled with that health plan. While reimbursement models for these arrangements vary around the country, health plans in California, Florida, Nevada and New Mexico often prospectively pay the integrated healthcare network a fixed Per Member Per Month (PMPM) amount, or capitation payment, which is often based on a percentage of the amount received by the health plan. The capitation payment is for much-and sometimes virtually all-of the care needs of the applicable membership. Capitation payments to integrated healthcare networks, in aggregate, represent a prospective budget from which the network manages care-related expenses on behalf of the population enrolled with that network. To the extent that these networks manage care-related expenses below the capitated levels, the network realizes an operating profit. On the other hand, if care-related expenses exceed projected levels, the network will realize an operating deficit. Since premiums paid represent a significant amount per person, there is a significant revenue opportunity for an integrated medical network like DMG that is able to effectively manage its costs under a capitated arrangement.
Integrated medical networks, such as DMG, that have scale are positioned to spread an individual member’s cost exposure across a wider population and realize the benefits of pooling medical risk among large numbers of patients. In addition, integrated medical networks with years of managed care experience can utilize their sizeable medical experience data to identify specific medical care and quality management strategies and interventions for potential high cost cases and aggressively manage them to improve the health of its population base and, thus, lower cost. Many integrated medical networks, like DMG, also have established physician performance metrics that allow them to monitor quality and service outcomes achieved by participating physicians in order to reward efficient, high quality care delivered to members and initiate improvement efforts for physicians whose results can be enhanced.
Healthcare reform
The U.S. healthcare system, including the Medicare Advantage program, is subject to a broad array of new laws and regulations as a result of the ACA. This legislation made significant changes to the Medicare program and to the health insurance market overall. The ACA is considered by some to be the most dramatic change to the U.S. healthcare system in decades. The U.S. Supreme Court found that the individual mandate to obtain health insurance coverage under this legislation is constitutional and also found that the expanded Medicaid benefit included in the legislation is constitutional if states can opt out of the expanded Medicaid benefit without losing their funding under the pre-reform Medicaid program. In a separate, subsequent case, the U.S. Supreme Court also upheld the use of subsidies to individuals in federally-facilitated healthcare exchanges, rejecting an argument that such subsidies would apply only in the state-run healthcare exchanges.
The ACA reflects sweeping legislation that, if fully implemented, may have a significant impact on the U.S. healthcare system generally and the operations of DMG’s business. There are numerous steps required to implement the ACA, and implementation remains ongoing and uncertain. Congress also has enacted, and may continue to seek, legislative changes that alter, delay, or eliminate some of their provisions. For example, under the 2016 Omnibus budget agreement, Congress voted to delay certain new taxes that the ACA had enacted, including the excise tax on certain high-cost health plans, the medical device tax, and the tax on health insurers. In addition, the 2016 Presidential and Congressional elections and subsequent developments have caused the future state of the ACA to be unclear. In October 2017, the federal government announced that cost-sharing reduction payments to insurers would end, effective immediately, unless Congress appropriated the funds, and, in December 2017, Congress passed the Tax Cuts and Jobs Act, which includes a provision that eliminates the penalty under the ACA’s individual mandate and could impact the future state of the exchanges. Further, in February 2018, Congress passed the BBA, which, among other things, repealed the Independent Payment Advisory Board that was established by the ACA and intended to reduce the rate of growth in Medicare spending. While certain provisions of the BBA may increase the scope of benefits available for certain chronically ill federal health care program beneficiaries beginning in 2020, the ultimate impact of such changes cannot be predicted. While specific changes and their timing are not yet apparent, the enacted reforms as well as future legislative, regulatory, or executive changes could have a material adverse effect on our results of operations, including lowering our reimbursement rates and increasing our expenses.
21
One provision of the ACA required CMS to establish a Medicare Shared Savings Program (MSSP) that promotes accountability and coordination of care through the creation of ACOs. The program allows certain providers and suppliers (including hospitals, physicians and other designated professionals) to voluntarily form ACOs and work together along with other ACO participants to invest in infrastructure and redesign delivery processes to achieve high quality and efficient delivery of services. In 2014, DMG entered into an agreement with CMS to participate in the MSSP in California, Florida and Nevada. Under this program, which ran through 2016, DMG strove to attain improved clinical outcomes to its Medicare FFS patients in a more cost-effective manner, and had the opportunity to share with CMS in any financial savings created. For the 2016 MSSP program, DMG achieved approximately $3 million in savings however was not able to benefit in these savings as the minimum savings rate was not reached. In 2017, DMG participated in the CMS Innovation Center’s Next Generation ACO and will continue to participate through 2018. Results for 2017 participation will be available in the third quarter of 2018.
Payor environment
Government programs
DMG derives a significant portion of its revenues from services rendered to beneficiaries of Medicare (including Medicare Advantage), Medicaid, and other governmental healthcare programs.
Medicare. The Medicare program was established in 1965 and became effective in 1967 as a federally funded U.S. health insurance program for persons aged 65 and older, and it was later expanded to include individuals with ESRD and certain disabled persons, regardless of income or age. Since its formation, Medicare has grown to an approximately $672 billion program in 2016, covering approximately 57 million Americans and, based on the growing number of eligible beneficiaries and increases in the cost of healthcare, CBO projects that net Medicare spending will increase from $595 billion in 2017 to $1.2 trillion in 2027.
Initially, Medicare was offered only on a FFS basis. Under the Medicare FFS payment system, an individual can choose any licensed physician enrolled in Medicare and use the services of any hospital, healthcare provider or facility certified by Medicare. CMS reimburses providers for covered services if CMS considers them medically necessary.
FFS Medicare pays for physician services according to a physician fee schedule (PFS) set each year by CMS in accordance with formulas mandated by Congress. Historically, CMS annually adjusted the Medicare Physician Fee Schedule (Medicare PFS) payment rates based on an updated formula that included application of the Sustainable Growth Rate (SGR). On April 16, 2015, President Obama signed and enacted into law H.R. 2, the Medicare Access and CHIP Reauthorization Act of 2015, which, among other things, repealed the SGR and instituted a 0% update to the single conversion factor under the Medicare PFS from January 1 through June 30, 2015, a 0.5% update for July 2015 through the end of 2019, and a 0% update for 2020 through 2025. For 2026 and subsequent years, the update will be either 0.75% or 0.25%, depending on the Alternate Payment Model (APM) in which the physician participates. On October 14, 2016, CMS released a final rule implementing, among other changes, the Advanced APM incentive applicable to the physician fee schedule, under which physicians may receive bonus payments for participating in an Advanced APM. Among other things, the final rule identifies the criteria an APM must satisfy to be considered an Advanced APM, which could include some MSSP ACOs or providers participating in the CEC Model. Whether DMG’s subsidiary ACO or dialysis providers participating in CEC are considered to be Advanced APMs could potentially affect physicians’ willingness to participate in such entities, which may indirectly impact the operations of DMG’s subsidiary ACO or its providers participating in the CEC Model. In addition, under the final rule, DMG’s subsidiary ACO may also be required to submit certain quality data to CMS on behalf of its Merit-Based Incentive Payment System MIPS-eligible clinicians, which could result in an increase in operational costs. Given that the payment updates for APMs have yet to take effect, we cannot determine the impact of such payment models on our business at this time.
In addition, in recent years, Congress has enacted various laws seeking to reduce the federal debt level and contain healthcare expenditures. For example, the BCA called for the establishment of a Joint Select Committee (the Committee) on Deficit Reduction, tasked with reducing the federal debt level. However, because the Committee did not draft a proposal by the BCA’s deadline, President Obama issued an initial sequestration order that imposed automatic spending cuts on various federal programs. In particular, a 2% reduction to Medicare payments took effect on April 1, 2013, which was subsequently extended through 2027.
The instability of the federal budget may lead to legislation that could result in further cuts in Medicare and Medicaid payments to providers. In recent years, the government has enacted a patchwork of appropriations legislation to temporarily suspend the debt ceiling and continue government operations. Although the BBA passed in February 2018 enacts a two-year federal spending agreement and raises the federal spending cap on non-defense spending for fiscal years 2018 and 2019, the
22
Medicare program is frequently mentioned as a target for spending cuts. Spending cuts to the Medicare program could adversely affect our operating results.
Medicare Advantage. Medicare Advantage is a Medicare health plan program developed and administered by CMS as an alternative to the original FFS Medicare program. Under the Medicare Advantage program, Medicare beneficiaries may choose to receive benefits under a managed care health plan that provides benefits at least comparable to those offered under the original Medicare FFS payment system in exchange for which the health plan receives a monthly per patient premium payment from CMS. The Medicare Advantage monthly premium varies based on the county in which the member resides, and is adjusted to reflect the demographics and estimated risk profile of the members that enroll. Once a person is authorized by CMS to participate in Medicare Advantage, health plans compete for enrollment based on benefit design differences such as co-payments or deductibles, availability of preventive care, attractiveness of and access to a network of hospitals, physicians and ancillary providers and premium contribution or, most often in Medicare Advantage plans, the absence of any monthly premium. In certain parts of the country, many health plans that provide Medicare Advantage benefits subcontract with integrated medical networks such as DMG to transfer the responsibility for managing patient care.
In 2004, CMS adopted a risk adjustment payment system for Medicare Advantage health plans in which the participating health plans’ premiums are adjusted based on the actual illness burden of the members that enroll. The model bases a portion of the total CMS reimbursement payments on various clinical and demographic factors, including hospital inpatient diagnoses, additional diagnosis data from ambulatory treatment settings, hospital outpatient department and physician visits, gender, age and Medicaid eligibility. CMS requires that all managed care companies capture, collect and submit the necessary diagnosis code information to CMS twice a year for reconciliation with CMS’s internal database. Medical providers, such as DMG, provide this diagnosis code information to health plan customers for submission to CMS. Under this system, the risk-adjusted portion of the total CMS payment to the Medicare Advantage plans will equal the local rate set forth in the traditional demographic rate book, adjusted to reflect the plan members’ gender, age and morbidity.
Most Medicare beneficiaries have the option to enroll in private health insurance plans that contract with Medicare under the Medicare Advantage program. According to the Kaiser Family Foundation, the share of Medicare beneficiaries in such plans has risen rapidly in recent years; it reached approximately 33% in 2017 from approximately 13% in 2004. Plan costs for the standard benefit package can be significantly lower or higher than the corresponding cost for beneficiaries in the traditional Medicare FFS payment program, but prior to the ACA, private plans were generally paid a higher average amount, and they used the additional payments to reduce enrollee cost-sharing requirements, provide extra benefits, and/or reduce Medicare premiums. These enhancements were valuable to enrollees, but also resulted in higher Medicare costs overall and higher premiums for all Medicare Part B beneficiaries and not just those enrolled in Medicare Advantage plans. The ACA requires that future payments to plans be based on benchmarks in a range of 95% to 115% of local FFS Medicare costs, with bonus amounts payable to plans meeting high quality-of-care standards. In addition, health plans offering Medicare Advantage are required to spend at least 85% of their premium dollars on medical care, the so-called medical loss ratio (MLR). Since DMG is not a health plan, except for DaVita Health Plan of California, Inc. (DHPC), it is not subject to the 85% MLR requirement. See “DaVita Medical Group Division (DMG)—Knox-Keene” below. However, payments that health plans make to DMG will apply in full towards the health plans’ 85% MLR requirement. If a health plan does not meet the 85% MLR requirement, it must provide a rebate to its customers. Any such shortfalls would not impact amounts paid by health plans to DMG.
Medicaid. Medicaid is a federal entitlement program administered by the states that provides healthcare and long-term care services and support to low-income Americans. Medicaid is funded jointly by the states and the federal government. The federal government guarantees matching funds to states for qualifying Medicaid expenditures based on each state’s federal medical assistance percentage, which is calculated annually and varies inversely with average personal income in the state. Subject to federal rules, each state establishes its own eligibility standards, benefit packages, payment rates and program administration within broad federal statutory and regulatory guidelines. Every state Medicaid program must balance a number of potentially competing demands, including the need for quality care, adequate provider access, and cost-effectiveness. In an effort to improve quality and provide more uniform and cost-effective care, many states have implemented Medicaid managed care programs to improve access to coordinated healthcare services, including preventative care, and to control healthcare costs. Under Medicaid managed care programs, a health plan receives capitation payments from the state. The health plan, in turn, arranges for the provision of healthcare services by contracting with a network of medical providers, such as DMG. DMG has entered into capitation agreements with health plans to manage approximately 94,800 Medicaid managed care members in its southern California market.
Commercial payors
According to the 2017 Annual Survey conducted by the Kaiser Family Foundation, approximately 151 million non-elderly people in the U.S. received their health insurance through their employers, which contracted with health plans to
23
administer these healthcare benefits. Patients enrolled in health plans offered through an employment setting are generally referred to as commercial members. According to the survey, the percentage of workers covered was 55% in 2017 and 2016. Under the ACA, many uninsured individuals and many individuals who receive their health insurance benefits through small employers may purchase their healthcare benefits through insurance exchanges in which health plans compete directly for individual or small group members’ enrollment. DMG derives a significant amount of its enrollment from commercial members; however, these members represent a disproportionately small share of DMG’s operating profits.
Whether in the Medicare Advantage, commercial or Medicaid market, managed care health plans seek to provide a coordinated and efficient approach to managing the healthcare needs of their enrolled populations. By negotiating with providers, such as pharmacies, hospitals and physicians, and implementing various quality programs, managed care companies attempt to enhance their profitability by limiting their medical costs. These health plans have shown success in mitigating certain components of medical cost, but we believe they are limited by their indirect relationship with physicians, who in the aggregate direct most of their patients’ healthcare costs. We believe that physician-led and professionally-managed integrated medical networks such as DMG’s have a greater opportunity to influence cost and improve quality due to the close coordination of care at the most effective point of contact with the patient—the primary care physician.
Capitation and FFS revenue
There are a number of different models under which an integrated medical network receives payment for managing and providing healthcare services to its members.
Fee-for-service structure. Under traditional FFS reimbursement, physicians are paid a specified amount for each service or procedure that they provide during a patient visit. Under this structure, physician compensation is based on the volume of patient visits and procedures performed, thus offering limited financial incentive to focus on cost containment and preventative care. FFS revenues are derived primarily from DMG’s physician services.
Capitation structure. Under capitation, payors pay a fixed amount per enrolled member, thereby subcontracting a significant portion of the responsibility and risks for managing patient care to physicians. Global capitation represents a prospective budget from which the provider network then manages care-related expenses including payments to associated providers outside the group, such as hospitals and specialists. Compared to traditional FFS models, we believe that capitation arrangements better align provider incentives with both quality and efficiency of care. We believe that this approach improves the quality of the experience for patients and the potential profitability for efficient care providers.
Since premiums paid represent a significant amount per person, the revenue and, when costs are effectively managed, profit opportunity available to an integrated medical network under a capitated arrangement can be significant. This is particularly the case for senior members and members with multiple diseases. We believe that the advantages, savings and efficiencies made possible by the capitated model are most pronounced when the care demands of the population are the most severe and require the most coordination, such as for the senior population or patients with chronic, complex and follow-on diseases. While organized coordination of care is central to the capitated model, it is also well suited to the implementation of preventative care and disease management over the long-term since physicians have a financial incentive to improve the overall health of their patient population.
The inherent risk in assumption of global care risk relates to potential losses if a number of individual patients’ medical costs exceed the expected amount. This risk is especially significant to individual practitioners or smaller physician groups who lack the scale required to spread the risk over a broad population. DMG has the scale, comprehensive medical delivery resources, significant infrastructure to support practicing physicians, and demonstrated care management knowledge to spread the risk of losses over a large patient population.
Global model. In Florida, DMG may contract directly with health plans under global capitation arrangements that include hospital services, because state law permits DMG to assume financial responsibility for both professional and institutional services. In New Mexico, DMG assumed financial responsibility for professional services only.
24
In Nevada, DMG enters into global capitation arrangements to assume financial responsibility for both professional and institutional services. However, the Nevada Division of Insurance (NDI) has not opined on whether it is appropriate for an entity like DMG to enter into global capitation arrangements and assume financial responsibility for the provision of both professional and institutional services to either Medicare Advantage enrollees or enrollees of commercial health plans. In order to avoid an adverse finding by the NDI with respect to DMG’s global capitation arrangements in Nevada, DMG applied for an insurance license from the NDI and obtained the license in 2015. DMG is currently evaluating its ability to assign any of its existing contracts to the NDI license holder. Because of the current global capitation to DMG, and DMG’s assumption of nearly the entire professional and institutional risk in Nevada and Florida, DMG’s health plan customers function primarily to support DMG in undertaking marketing and sales efforts to enroll members and processing claims in these states.
In California, entities that maintain full or restricted licenses under the California Knox-Keene Health Care Service Plan Act of 1975 (Knox-Keene) are permitted to assume financial responsibility for both professional and institutional services. As described below, in December 2013, DMG obtained a restricted Knox-Keene license and therefore may enter into global capitation arrangements with health plans through which DMG will assume financial responsibility for both professional and institutional services.
Risk-sharing model. In California, DMG currently utilizes a capitation model in several different forms. While there are variations specific to each arrangement, HealthCare Partners Affiliates Medical Group and DaVita Medical Group Associates California, Inc. (collectively AMG), which are medical groups that have entered into management services agreements with DMG, have historically contracted with health plans to receive a PMPM or percentage of premium (POP) capitation payment for professional (physician) services and assumed the financial responsibility for professional services. In some cases, the health plans separately enter into capitation contracts with third parties (typically hospitals) who directly receive a capitation payment and assume contractual financial responsibility for institutional (hospital) services. In other cases, the health plan does not pay a capitation payment to the hospital, but rather administers and pays fee-for-service claims for hospital expenses. In both cases, AMG has been responsible under its health plan agreements for managing the care dollars associated with both the professional and institutional services provided for in the AMG capitation payment. In the case of institutional services and as a result of its managed care-related administrative services agreements with hospitals, AMG has recognized a percentage of the surplus of institutional revenues less institutional expense as AMG net revenues and has also been responsible for some percentage of any short-fall in the event that institutional expenses exceed institutional revenues. In connection with DMG’s obtaining a restricted Knox-Keene license in California, substantially all of the California health plan contracts, along with the revenues received under such contracts, have been assigned from AMG to DHPC. In addition, DMG now has the legal authority to transition these health plan contracts to global capitation arrangements in which DMG is responsible for arranging professional and institutional services in exchange for a single capitation payment. DMG has evaluated its various risk sharing arrangements, and is working with the Department of Managed Health Care and several health plans to accept global capitation. DMG converted three separate contracts to global risk in 2016, and converted two additional contracts in 2017. In total, approximately 28% of DMG’s membership is now covered under global risk plans. DMG is in the approval and implementation process to convert additional contracts to global risk in 2018. Completion of evaluation of possible additional conversions is expected to continue over time.
Government regulation
In addition to the laws and regulations to which our U.S. dialysis and related lab services business are subject to, the internal operations of DMG and its contractual relationships with healthcare providers such as hospitals, other healthcare facilities, and healthcare professionals are subject to extensive and increasing regulation by numerous federal, state, and local government entities. These laws and regulations often are interpreted broadly and enforced aggressively by multiple government agencies, including the OIG, the DOJ, and various state authorities. Many of these laws and regulations are the same as those that impact our U.S. dialysis and related lab services business. For example:
• | DMG’s financial relationships with healthcare providers including physicians and hospitals could subject DMG to criminal and civil sanctions and penalties under the federal Anti-Kickback Statute; |
• | The referral of Medicare patients by DMG-associated physicians for the provision of DHS may subject the parties to sanctions and penalties under the Stark Law; |
• | DMG’s financial relationships and those of its associated physicians may subject the parties to penalties and sanctions under state fraud and abuse laws; |
• | DMG’s submission of claims to governmental payors such as the Medicare and Medicaid programs for services provided by its associated physicians and clinical personnel may subject DMG to sanction and penalties under the FCA; and |
25
• | DMG’s handling of PHI may subject DMG to sanctions and penalties under HIPAA and its implementing privacy and security regulations, as amended by the HITECH Act, and state medical privacy laws which can include penalties and restrictions that are more severe than those which arise under HIPAA. |
A finding that claims for services were not covered or not payable, or the imposition of sanctions associated with a violation of any of these healthcare laws and regulations, could result in criminal and/or civil penalties and exclusion from participation in Medicare, Medicaid and other federal and state healthcare programs and could have a material adverse effect on DMG’s business, financial condition and results of operations. We cannot guarantee that the arrangements or business practices of DMG will not be subject to government scrutiny or be found to violate certain healthcare laws. Government audits, investigations and prosecutions, even if we are ultimately found to be without fault, can be costly and disruptive to DMG’s business. Moreover, changes in healthcare legislation or government regulation may restrict DMG’s existing operations, limit their expansion or impose additional compliance requirements and costs, any of which could have a material adverse effect on DMG’s business, financial condition and results of operations.
The following includes brief descriptions of some, but not all, of the laws and regulations that, in addition to those described in relation to our U.S. dialysis and related lab services business, affect DMG. DMG is also subject to the laws and regulations that apply to our U.S. dialysis and related lab services business. See “Kidney Care Division—Government regulation” above.
Licensing, certification, accreditation and related laws and guidelines. DMG clinical personnel are subject to numerous federal, state and local laws and regulations, relating to, among other things, licensing, professional credentialing and professional ethics. Since DMG clinical personnel perform services in medical office settings, hospitals and other types of healthcare facilities, DMG may indirectly be subject to laws applicable to those entities as well as ethical guidelines and operating standards of professional trade associations and private accreditation commissions, such as the American Medical Association and the Joint Commission. There are penalties for non-compliance with these laws, including discipline or loss of professional license, civil and/or criminal fines and penalties, loss of hospital admitting privileges, federal healthcare program disenrollment, loss of billing privileges, and exclusion from participation in various governmental and other third-party healthcare programs.
Professional licensing requirements. DMG’s clinical personnel, including physicians, must satisfy and maintain their professional licensing in the states where they practice medicine. Activities that qualify as professional misconduct under state law may subject them to sanctions, including the loss of their licenses and could subject DMG to sanctions as well. Many state boards of medicine impose reciprocal discipline, that is, if a physician is disciplined for having committed professional misconduct in one state where he or she is licensed, another state where he or she is also licensed may impose the same discipline even though the conduct did not occur in that state. Therefore, if a DMG-associated physician is licensed in multiple states, sanctions or loss of licensure in one state may result in sanction or the loss of licensure in other states. Professional licensing sanctions may also result in exclusion from participation in governmental healthcare programs, such as Medicare and Medicaid, as well as other third-party programs.
Corporate practice of medicine and fee splitting. California, Colorado, Nevada, and Washington are states in which DMG operates that have laws that prohibit business entities, such as our Company and our subsidiaries, from practicing medicine, employing physicians to practice medicine or exercising control over medical decisions by physicians (known collectively as the corporate practice of medicine). These states also prohibit entities from engaging in certain financial arrangements, such as fee-splitting, with physicians. In some states these prohibitions are expressly stated in a statute or regulation, while in other states the prohibition is a matter of judicial or regulatory interpretation.
Violations of the corporate practice of medicine vary by state and may result in physicians being subject to disciplinary action, as well as to forfeiture of revenues from payors for services rendered. For lay entities, violations may also bring both civil and, in more extreme cases, criminal liability for engaging in medical practice without a license.
In California, a violation of the corporate practice of medicine prohibition constitutes the unlawful practice of medicine, which is a public offense punishable by fines and other criminal penalties. In addition, any person who conspires with or aids and abets another in the unlawful practice of medicine is similarly guilty of a public offense and may be subject to comparable fines and criminal penalties. In Nevada, engaging in the corporate practice of medicine where not provided by a specific statute may also constitute the unlawful practice of medicine. This violation is a felony punishable by fines and other civil and criminal penalties. Physicians in Nevada can similarly be punished for aiding or assisting in the unlicensed practice of medicine.
26
In Colorado, any physician found to have abetted or assisted or conspired to engage in unprofessional conduct with respect to the practice of medicine is subject to disciplinary action, including the loss of licensure. Corporate entities or lay persons who are found to have engaged in the unauthorized practice of medicine may be subject to injunctive action and other criminal penalties. In Washington, the Secretary of Health is responsible for investigating complaints concerning the unlicensed practice of medicine and violations may be subject to a cease and desist order, civil fines, injunctive action, and other criminal penalties.
In our markets where the corporate practice of medicine is prohibited, DMG has historically operated by maintaining long-term management contracts with multiple associated professional organizations which, in turn, employ or contract with physicians to provide those professional medical services required by the enrollees of the payors with which the professional organizations contract. Under these management agreements, DMG performs only non-medical administrative services, does not represent that it offers medical services, and does not exercise influence or control over the practice of medicine by the physicians or the associated physician groups with which it contracts. For example, in California, DMG has full-service management contracts with AMG. The AMG entities are owned by California-licensed physicians and professional medical corporations and contract with physicians to provide professional medical services. In Nevada and Washington, DMG’s Nevada and Washington subsidiaries have similar management agreements with Nevada and Washington professional corporations, as applicable, that employ and contract with physicians to provide professional medical services. In Colorado, the physician groups contract through a provider network to include a pharmacy and ambulatory surgery center.
Some of the relevant laws, regulations, and agency interpretations in states with corporate practice of medicine restrictions have been subject to limited judicial and regulatory interpretation. Moreover, state laws are subject to change. Regulatory authorities and other parties, including DMG’s associated physicians, may assert that, despite the management agreements and other arrangements through which DMG operates, we are engaged in the prohibited corporate practice of medicine or that DMG’s arrangements constitute unlawful fee-splitting. If this were to occur, we could be subject to civil and/or criminal penalties, DMG’s agreements could be found legally invalid and unenforceable (in whole or in part), or we could be required to restructure its contractual arrangements.
If we were required to restructure DMG’s operating structures in our markets due to determination that a corporate practice of medicine violation existed, such a restructuring might include revisions of the California, Colorado, Nevada or Washington management services agreements, which might include a modification of the management fee, and/or establishing an alternative structure. For example, our subsidiaries in those states might have to obtain the equivalent of a California Knox-Keene license in such state in order to comply with the corporate practice of medicine rules while contracting directly with payors and, in turn, physicians, to provide physician services to the payors’ enrollees. In California, DMG’s restricted Knox-Keene license has created potential flexibility for DMG in the event regulatory authorities seek to enforce corporate practice of medicine or fee splitting laws based upon current management services relationships with AMG. DMG’s restricted Knox-Keene license allows DHPC to contract with or employ physicians as a result of an exemption from California’s corporate practice of medicine laws applicable to Knox-Keene licensees.
Knox-Keene. The California Department of Managed Health Care (DMHC) licenses and regulates Health Care Service Plans (HCSPs) pursuant to Knox-Keene, as amended. In addition to regulating Knox-Keene’s various patient’s rights protections for HCSP-enrolled individuals, the DMHC is responsible for ensuring the financial sustainability over time of licensed HCSPs and other regulated entities. As such, the DMHC is charged with continually monitoring the financial health of regulated entities. The DMHC’s Division of Financial Oversight monitors and evaluates the financial viability of health plans to ensure continued access to health care services. Financial examination reviews include examinations of financial statements and financial arrangements, both by routine and non-routine examinations. The examination also ensures that there is adequate tangible net equity (TNE), as determined according to calculations included in Knox-Keene. The TNE regulations for organizations holding a Knox-Keene license, like DHPC, vary depending on circumstances, but generally require any licensee to have on hand in cash or cash equivalents a minimum of the greater of (i) $1 million, (ii) the sum of 2% of the first $150 million of annualized premium revenues plus 1% of annualized premium revenues in excess of $150 million, or (iii) the sum of 8% of the first $150 million of annualized healthcare expenditures (except those paid on a capitated basis or managed hospital payment basis) plus 4% of the annualized healthcare expenditures (except those paid on a capitated basis or managed hospital payment basis) which are in excess of $150 million; plus 4% of annualized hospital expenditures paid on a managed hospital payment basis. In its sole discretion, the DMHC may require, as a condition to obtaining or maintaining an HCSP license, that a licensee accept certain contractual undertakings such that the licensee is obligated to maintain TNE in amounts greater than the minimum amount described above. Additionally, a licensed HCSP is subject to additional DMHC reporting requirements and financial oversight if the HCSP fails to maintain at least 130% of its required minimum TNE. During the 2016 financial examination, DHPC was required to provide evidence of exclusive fidelity bond coverage in the amount of at least $2 million, with a deductible amount not in excess of $100,000 with a requirement to notify the Director of DMHC 30 days prior to cancellation.
27
The DMHC interprets Knox-Keene HCSP licensing requirements to apply to both full-service HCSPs and downstream restricted HCSP contracting entities, including provider groups that enter into global risk contracts with licensed HCSPs. A global risk contract is a healthcare services contract in which a downstream contracting entity agrees to provide both professional (physician) services and institutional (hospital) services subject to an at-risk or capitated reimbursement methodology. According to the DMHC, entities that accept global risk must obtain a restricted Knox-Keene license. Under a restricted Knox-Keene license, entities may enter into global risk contracts with other licensed HCSPs. Holders of restricted Knox-Keene licenses must comply with the same financial requirements as HCSPs with full licenses, including demonstrating specific levels of TNE, but are not required to meet Knox-Keene requirements for functions they are not delegated such as marketing. The consequences of operating without a license include civil penalties, criminal penalties and the issuance of cease and desist orders.
DHPC holds a restricted Knox-Keene license, which allows DHPC to contract directly with full service HCSPs to simplify DMG's historic contractual and financial structure and to facilitate expansion into new markets in California. However, this also subjects DMG and DHPC to additional regulatory obligations, including (i) regulatory oversight of operations, (ii) the need to seek approval for all material business changes, (iii) significant requirements to maintain certain TNE levels, and (iv) other operating limitations imposed by Knox-Keene and its regulations. Under its restricted Knox-Keene license, DHPC is prohibited from declaring or paying any dividends or making any distribution of cash or property to its parent, affiliates, or shareholders, if such a distribution would cause it to fail to maintain the minimum applicable TNE, have insufficient working capital or cash flow as required by DMHC regulation or otherwise be unable to provide or arrange healthcare services. In addition, DHPC is subject to DMHC oversight and must seek approval before incurring any debt or guaranteeing any debt relating to its parent, affiliates, or shareholders. DHPC must also submit proposed global capitation contracts to the DMHC for approval.
DMG services
Approximately 83% of DMG’s operating revenues for the year ended December 31, 2017 were derived from multi-year capitation contracts with health plans. Under these contracts, DMG’s health plan customers delegate full responsibility for member care to physicians and healthcare facilities that are part of DMG’s provider network. In return, DMG receives a PMPM fee for each DMG member. As a result, DMG has financial and clinical accountability for a population of members. In California, DMG does not assume direct financial risk for institutional (hospital) services in some cases, but is responsible for managing the care dollars associated with both the professional (physician) and institutional services being provided for the PMPM fee attributable to both professional and institutional services. In those cases and as a result of its managed care-related administrative services agreements with hospitals, DMG recognizes the surplus of institutional revenues less institutional expense as DMG net revenues and is also responsible for any short-fall in the event that institutional expenses exceed institutional revenues.
DMG provides comprehensive and quality medical care through a network of participating physicians and other healthcare professionals. Through its group model, DMG employs, directly (where permitted by state law) and through its associated physician groups, over 750 primary care physicians. Through its IPA model, DMG contracts with a network of approximately 3,500 associated groups and other network primary care physicians who provide care for DMG’s members in an independent office setting. These physicians are complemented by several thousand network specialists and approximately 180 network hospitals that provide specialty or institutional care to the patients of DMG’s associated physicians, physician groups and IPAs.
In order to comply with local regulations prohibiting the corporate practice of medicine, many of DMG’s group physicians are employed by associated medical groups with which DMG has entered into long-term management agreements. The largest of these DMG managed medical groups is AMG, which employs, directly or indirectly, over 750 primary care physicians, specialists and hospitalists. See “Government Regulation—Corporate practice of medicine and fee splitting” above.
DMG does not own hospitals, although hospitals are an essential part of its provider network. In most cases, DMG contracts or otherwise aligns with hospitals to manage the utilization, readmission and cost of hospital services. Most DMG patients receive specialty care through DMG’s network based on referrals made by their primary care physician. These specialists may be reimbursed based on capitation, case rates or on a discounted FFS rate.
DMG group physicians typically see 18 to 22 patients per day, which we believe is an appropriate benchmark to ensure there is sufficient time to understand all of the patients’ clinical needs. DMG care teams, including nurses, engage in outreach to patients in order to help monitor fragile and high risk patients, and help improve adherence to physicians’ care plans. During these visits, DMG’s physicians, nurses and educators use the time to educate patients and manage their healthcare needs. The goal of this preventative care delivery model is to keep patients healthy. Education improves self-management and compliance which allows the patient to recognize early signs of their disease and seek appropriate care. We believe this translates into
28
earlier intervention, which in turn leads to fewer emergency room visits, fewer hospital admissions and fewer hospital bed days (the most expensive location for healthcare). This clinical model seeks to provide early diagnosis of disease or deterioration in a chronic and complex condition and provide preventive care to maintain optimal health and avert unnecessary hospitalization. Clinic-based case managers and hospitalists coordinate with the primary care physicians to ensure that patients are receiving proper care whether they are in the clinic, in the hospital or are not regularly accessing healthcare. Physicians and case managers encourage patients to regularly visit the clinics in order to enhance their day-to-day health and diagnose any illness or deterioration in condition as early as possible.
DMG’s information technology system, including DMG’s electronic health record and data warehouse, is designed to support the DMG delivery model with data-driven opportunities to improve the quality and cost effectiveness of the care received by its members. Using informatics technology, DMG has created disease registries that track large numbers of patients with defined medical conditions. DMG applies the data from these registries to manage the care for patients with similar medical conditions which we believe leads to a better medical outcome. We believe this approach to using data is effective because the information is communicated by the patient’s physician rather than the health plan or disease management companies.
DMG employs a wide variety of other information applications to service IPA and network providers using web connectivity. The HCP Connect! on-line portal provides web-based eligibility, referrals, electronic claims submission and explanation of benefits, and other communication vehicles for individual physician offices. The success of this suite of applications has enhanced DMG’s ability to manage its IPA networks, and has resulted in significant back-office efficiencies for DMG and its associated physician groups. DMG has further expanded its ability to share key utilization and clinical data with its internal and contracted physicians and specialists through the Physician Information Portal and the Clinical Viewer. Through these secure web portals, a physician is able to obtain web-based, point of care information regarding a patient, including diagnosis history, provide quality indicators, historical risk-adjustment coding information, pharmacy medication history, and other key information. In addition to its web-portals geared towards physicians, DMG has recently introduced a patient on-line portal to enable DMG’s patients to securely view their own clinical information, schedule physician appointments and interact electronically with their physicians. DMG believes these tools help lead to high quality clinical outcomes, create internal efficiencies, and enhance the satisfaction of its associated physicians and patients.
In addition, DMG uses its data to carefully track high utilizing patients through robust data warehousing and data mining technologies. DMG filters the data warehouse to identify and reach out to patients with high-utilization patterns who are inefficiently using resources, such as visiting an emergency room when either a same-day appointment or urgent care center would be more appropriate and satisfactory for the member. High utilizing patients are identified and tracked as part of DMG’s electronic health record by their physician and DMG’s care management staff. Specific care plans are attached to each of these patients and tracked carefully for full compliance. The objective is to proactively manage their care at times when these patients are either not compliant with the care plan or when changing circumstances require care managers to develop new and more suitable care plans. By using these resources, DMG has achieved improvements in quality of care, satisfaction and cost.
We believe DMG is well positioned to effectively leverage marketplace demands for greater provider accountability, measurable quality results and cost efficient medical care. We believe that DMG’s business model is likely to continue to be an attractive alternative for health plans looking for high quality, cost effective delivery networks, physicians seeking an attractive practice environment and patients interested in a highly integrated approach to managing their medical care. Additionally, we believe that the scale of DMG’s business allows it to spread capitation risk over a large population of members, invest in comprehensive analytic and healthcare information tools as well as clinical and quality measurement infrastructure, and recognize administrative and operating efficiencies. For these reasons, we believe that DMG offers patients, physicians and health plans a proven platform for addressing many of the most pressing challenges facing the U.S. healthcare system, including rising medical costs.
We also believe DMG has the ability to demonstrably improve medical outcomes and patient satisfaction while effectively managing costs through the following unique competitive strategies and internal progress and systems:
• | DMG’s clinical leadership and associated group and network physicians devote significant efforts to ensure that DMG’s members receive the most appropriate care in the most appropriate manner. |
• | DMG is committed to maximizing its patients’ satisfaction levels. |
• | DMG has the scale which, combined with its strong reputation and high quality patient care, makes it an attractive partner for health plans, compared to smaller provider groups that may have a higher risk of default and may not have the same resources to devote and develop the same level of patient care. |
29
• | DMG has over two decades of experience in managing complex disease cases for its population of patients. As a result, DMG has developed a rich dataset of patient care experiences and outcomes which permits DMG to proactively monitor and intervene in improving the care of its members. |
• | DMG’s senior management team possesses substantial experience with the healthcare industry with average experience of approximately 19 years, as of December 31, 2017. |
Locations of DMG clinics
As of December 31, 2017, DMG managed a total of 280 medical clinics, of which 68 clinics were located in California, 27 clinics were located in Colorado, 87 clinics were located in Florida, 60 clinics were located in Nevada, 14 clinics were located in New Mexico, and 24 clinics were located in Washington.
Competition
U.S. and International dialysis competition
The U.S. dialysis industry has consolidated significantly over time but still remains highly competitive, particularly in terms of acquiring existing outpatient dialysis centers. We continue to face a high degree of competition in the U.S. dialysis industry from large and medium-sized providers who compete directly with us for the acquisition of dialysis businesses, relationships with physicians to act as medical directors and skilled clinical personnel, as well as for individual patients. In addition, as we continue our international dialysis expansion into various international markets, we face competition from large and medium-sized providers for acquisition targets as well as physician relationships. Because of the ease of entry into the dialysis business and the ability of physicians to own dialysis centers and/or also be medical directors for their own centers, competition for growth in existing and expanding markets is not limited to large competitors with substantial financial resources. Acquisitions, developing new outpatient dialysis centers, patient retention and physician relationships are a critical component of our growth strategy and our business could be adversely affected if we are not able to continue to make dialysis acquisitions on reasonable and acceptable terms, continue to develop new outpatient dialysis centers, maintain or establish new relationships with physicians or if we experience significant patient attrition to our competitors. Competition for qualified physicians to act as medical directors and for inpatient dialysis services agreements with hospitals is also intense. Occasionally, we have also experienced competition from former medical directors or referring physicians who have opened their own outpatient dialysis centers. We also experience competitive pressures from other dialysis providers in connection with negotiating contracts with commercial healthcare payors and in recruiting and retaining qualified skilled clinical personnel.
Together with Fresenius Medical Care (FMC), we account for approximately 73% of outpatient dialysis patients in the U.S. with our Company serving approximately 37% of the total outpatient dialysis patients. Approximately 45% of the centers not owned by us or FMC are owned or controlled by hospitals or non-profit organizations. Hospital-based and non-profit dialysis units typically are more difficult to acquire than physician-owned dialysis centers.
FMC also manufactures a full line of dialysis supplies and equipment in addition to owning and operating outpatient dialysis centers worldwide. This may give FMC cost advantages over us because of its ability to manufacture its own products. Additionally, FMC has been one of our largest suppliers of dialysis products and equipment over the last several years. In 2018, we entered into and subsequently extended an agreement with FMC to purchase a certain amount of dialysis equipment, parts and supplies from FMC through December 31, 2020. The amount of purchases in future years from FMC will depend upon a number of factors, including the operating requirements of our centers, the number of centers we acquire, and growth of our existing centers.
DMG’s competition
DMG’s business is highly competitive. DMG competes with managed care organizations, hospitals, medical groups and individual physicians in its markets. DMG competes with other primary care physician groups or physicians who contract with health plans for membership. Health plans contract with care providers on the basis of costs, reputation, scope, efficiency and stability. Individual members select a primary care physician at the time of membership with the health plan. Location, name recognition, quality indicators and other factors go into that decision. For example, in California, DMG's competitors include Permanente Medical Group, which is the exclusive provider for Kaiser, and Heritage Provider Network. However, DMG’s principal competitors for members and health plan contracts vary considerably in type and identity by region.
Corporate compliance program
Our businesses are subject to extensive federal, state and local government regulations. Management has designed and implemented a corporate compliance program as part of our commitment to comply fully with all criminal, civil or
30
administrative laws or regulations applicable to any Federal health care program for which penalties and exclusions may be authorized and anti-corruption laws to maintain the high standards of conduct we expect from all of our teammates. We continuously review this program and enhance it as necessary. The primary purposes of the program include:
• | Assessing and identifying risks for existing and new businesses; |
• | Increasing, through training and education, the awareness of our teammates and affiliated professionals of the necessity of complying with all these laws; |
• | Developing and implementing compliance policies and procedures and creating controls to support compliance with these laws and such policies and procedures; |
• | Auditing and monitoring the activities of our operating units and business support functions on a regular basis to identify potential instances of noncompliance in a timely manner; and |
• | Ensuring that we take steps to resolve instances of noncompliance or to address areas of weakness or potential noncompliance as promptly as we become aware of them. |
We have a code of conduct that each of our teammates and affiliated professionals must follow and we have a confidential toll-free hotline for teammates and patients to report potential instances of noncompliance. Our Chief Compliance Officer administers the compliance program. The Chief Compliance Officer reports directly to our Chief Executive Officer, our Chief Executive Officer of Kidney Care and Chair of the Compliance Committee of our Board of Directors (Board Compliance Committee). On October 22, 2014, DaVita signed a CIA with HHS and the OIG. The CIA:
• | requires that we maintain certain elements of our compliance programs; |
• | imposes certain expanded compliance-related requirements during the term of the CIA, including increased training for teammates, physician partners and board members, implementing a series of procedures prior to entering into arrangements with referrals sources, execution of annual certifications by senior executives that evidence compliance with federal healthcare laws and regulations, internal compliance policies and the CIA, imposition of an executive recoupment program and quarterly and annual reports to the OIG; |
• | requires the formal allocation of certain oversight responsibility to the Board Compliance Committee and a resolution from that committee that it has made reasonable inquiry into the operations of the compliance program and the retention of an independent compliance advisor in year three of the CIA; |
• | contains certain business restrictions related to a subset of our joint venture arrangements, including our agreeing to not enter into certain types of partial divestiture joint venture transactions with nephrologists during the term of the CIA, among other restrictions; and |
• | requires that we engage an Independent Monitor who will provide additional oversight and reporting to the OIG for the term of the CIA. |
The costs associated with compliance with the CIA could be substantial and may be greater than we currently anticipate. In addition, in the event of a breach of the CIA, we may become liable for payment of certain stipulated penalties, and/or be excluded from participation on federal healthcare programs. In April 2015, the OIG notified us that it considered us to be in breach of the CIA because of three implementation deficiencies. We have remediated the deficiencies and have paid certain stipulated penalties. If we fail to comply with our CIA, we could be subject to substantial penalties and exclusion from participation in federal healthcare programs that could have a material adverse effect on our business, results of operations and financial condition.
Insurance
We are predominantly self-insured with respect to professional and general liability and workers' compensation risks through wholly-owned captive insurance companies. The Company is also predominantly self-insured with respect to employee medical and other health benefits. We also maintain insurance, excess coverage, or reinsurance for property and general liability, professional liability, directors’ and officers’ liability, workers' compensation and other coverage in amounts and on terms deemed adequate by management, based on our actual claims experience and expectations for future claims. Future claims could, however, exceed our applicable insurance coverage. Physicians practicing at our dialysis centers are required to maintain their own malpractice insurance, and our medical directors are required to maintain coverage for their individual private medical practices. Our liability policies cover our medical directors for the performance of their duties as
31
medical directors at our outpatient dialysis centers. DMG also maintains general and professional liability insurance through various independent and related parties. DMG has purchased its primary general and professional liability insurance from California Medical Group Insurance (CMGI) in which DMG owns a 67% equity interest.
Teammates
As of December 31, 2017, we employed approximately 74,500 teammates, including our international teammates:
● | Licensed professional staff (physicians, nurses and other healthcare professionals) | 25,800 | |
● | Other patient care and center support staff and laboratory personnel | 28,100 | |
● | Corporate, billing and regional administrative staff | 8,200 | |
● | DMG | 12,400 | |
Our businesses require skilled healthcare professionals with specialized training for treating patients with complex care needs. Recruitment and retention of nurses are continuing concerns for healthcare providers due to short supply. We have an active program of investing in our professional healthcare teammates to help ensure we meet our recruitment and retention targets, including expanded training opportunities, tuition reimbursements and other incentives.
32
Item 1A. Risk Factors
This Annual Report on Form 10-K contains statements that are forward-looking statements within the meaning of the federal securities laws. These statements involve known and unknown risks and uncertainties including those discussed below. The risks and uncertainties discussed below are not the only ones facing our business. In addition, please read the cautionary notice regarding forward-looking statements in Item 7 of this Part 1 under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Risk factors related to our overall business:
If we fail to adhere to all of the complex government laws and regulations that apply to our business, we could suffer severe consequences that could have a material adverse effect on our business, results of operations, financial condition and stock price.
Our operations are subject to extensive federal, state and local government laws and regulations, such as Medicare and Medicaid payment rules and regulations, federal and state anti-kickback laws, the Stark Law and analogous state self-referral prohibition statutes, the 21st Century Cures Act, Federal Acquisition Regulations, the False Claims Act (FCA), the Civil Monetary Penalty statute, the Foreign Corrupt Practices Act (FCPA) and federal and state laws regarding the collection, use and disclosure of patient health information (e.g., Health Insurance Portability and Accountability Act of 1996 (HIPAA)) and the storage, handling, shipment, disposal and/or dispensing of pharmaceuticals and blood products and other biological materials. The Medicare and Medicaid reimbursement rules impose complex and extensive requirements upon healthcare providers as well. Moreover, additional laws and regulations potentially affecting providers continue to be promulgated that may impact us. A violation or departure from any of these legal requirements may result in government audits, lower reimbursements, significant fines and penalties, the potential loss of certification, recoupment efforts or voluntary repayments, among other things.
We endeavor to comply with all legal requirements; however, there is no guarantee that we will be able to adhere to all of the complex government regulations that apply to our business. We further endeavor to structure all of our relationships with physicians and providers to comply with state and federal anti-kickback and physician self-referral laws. We utilize considerable resources to monitor laws and regulations and implement necessary changes. However, the laws and regulations in these areas are complex, changing and often subject to varying interpretations. For example, if an enforcement agency were to challenge the level of compensation that we pay our medical directors or the number of medical directors whom we engage, we could be required to change our practices, face criminal or civil penalties, pay substantial fines or otherwise experience a material adverse effect on our business, results of operations and financial condition as a result of a challenge to these arrangements.
In addition, failure to report and return overpayments within 60 days of when the overpayment is identified can lead to a violation of the FCA and associated penalties, as described in further detail below, and exclusion and penalties under the federal Civil Monetary Penalty statute, including civil monetary penalties of up to $20,000 (adjusted for inflation) for each item or service for which a person received an identified overpayment and failed to report and return such overpayment. These obligations to report and return overpayments could subject our procedures for identifying and processing overpayments to greater scrutiny. We have made investments in resources to decrease the time it takes to identify, quantify and process overpayments, and we may be required to make additional investments in the future. From time to time we may conduct internal compliance reviews, the results of which may involve the identification of overpayments or other liabilities. An acceleration in our ability to identify and process overpayments could result in us refunding overpayments to government and other payors more rapidly than we have in the past which could have a material adverse effect on our operating cash flows. Overpayments subject us to refunds and related damages and potential liabilities.
Additionally, the federal government has used the FCA to prosecute a wide variety of alleged false claims and fraud allegedly perpetrated against Medicare and state health care programs. Moreover, amendments to the federal Anti-Kickback Statute in the 2010 Affordable Care Act (ACA) make claims tainted by anti-kickback violations potentially subject to liability under the FCA, including qui tam or whistleblower suits. The penalties for a violation of the FCA range from $5,500 to $11,000 (adjusted for inflation) for each false claim plus three times the amount of damages caused by each such claim which generally means the amount received directly or indirectly from the government. On February 3, 2017, the Department of Justice (DOJ) issued a final rule announcing adjustments to FCA penalties, under which the per claim penalty range increases to a range from $10,957 to $21,916 for penalties assessed after February 3, 2017, so long as the underlying conduct occurred after November 2, 2015. Given the high volume of claims processed by our various operating units, the potential is high for substantial penalties in connection with any alleged FCA violations.
33
In addition to the provisions of the FCA, which provide for civil enforcement, the federal government can use several criminal statutes to prosecute persons who are alleged to have submitted false or fraudulent claims for payment to the federal government.
Certain civil investigative demands received by us or our subsidiaries specifically reference that they are in connection with FCA investigations alleging, among other things, that we or our subsidiaries presented or caused to be presented false claims for payment to the government. See "Item 3. Legal Proceedings" in Part I of this report and Note 16 to the consolidated financial statements included in this report for further details.
We are subject to a Corporate Integrity Agreement (CIA) which, for our domestic dialysis business, requires us to report probable violations of criminal, civil or administrative laws applicable to any federal health care program for which penalties or exclusions may be authorized under applicable healthcare laws and regulations. See "If we fail to comply with our Corporate Integrity Agreement, we could be subject to substantial penalties and exclusion from participation in federal healthcare programs that could have a material adverse effect on our business, results of operations and financial condition."
If any of our operations are found to violate these or other government laws or regulations, we could suffer severe consequences that would have a material adverse effect on our business, results of operations, financial condition and stock price, including:
• | Suspension or termination of our participation in government payment programs; |
• | Refunds of amounts received in violation of law or applicable payment program requirements; |
• | Loss of required government certifications or exclusion from government payment programs; |
• | Loss of licenses required to operate healthcare facilities or administer pharmaceuticals in some of the states in which we operate; |
• | Reductions in payment rates or coverage for dialysis and ancillary services and related pharmaceuticals; |
• | Criminal or civil liability, fines, damages or monetary penalties for violations of healthcare fraud and abuse laws, including the federal Anti-Kickback Statute, Stark Law violations, FCA or other failures to meet regulatory requirements; |
• | Enforcement actions by governmental agencies and/or state claims for monetary damages by patients who believe their protected health information (PHI) has been used, disclosed or not properly safeguarded in violation of federal or state patient privacy laws, including HIPAA and the Privacy Act of 1974; |
• | Mandated changes to our practices or procedures that significantly increase operating expenses; |
• | Imposition of and compliance with corporate integrity agreements that could subject us to ongoing audits and reporting requirements as well as increased scrutiny of our billing and business practices which could lead to potential fines; |
• | Termination of relationships with medical directors; and |
• | Harm to our reputation which could impact our business relationships, affect our ability to obtain financing and decrease access to new business opportunities, among other things. |
We are, and may in the future be, a party to various lawsuits, demands, claims, qui tam suits, governmental investigations and audits (including investigations resulting from our obligation to self-report suspected violations of law) and other legal proceedings, any of which could result in, among other things, substantial financial penalties or awards against us, substantial payments made by us, required changes to our business practices, exclusion from future participation in the Medicare, Medicaid and other federal healthcare programs and possible criminal penalties, any of which could have a material adverse effect on our business, results of operations and financial condition and materially harm our reputation.
We are the subject of a number of investigations and audits by the federal government, as further described in Note 16 to the consolidated financial statements included in this report. We may be subject to other investigations and audits by state or federal government agencies and/or private civil qui tam complaints filed by relators and other lawsuits, demands, claims and legal proceedings.
34
Responding to subpoenas, investigations and other lawsuits, claims and legal proceedings as well as defending ourselves in such matters will continue to require management’s attention and cause us to incur significant legal expense. Negative findings or terms and conditions that we might agree to accept as part of a negotiated resolution of pending or future government inquiries or relator proceedings could result in, among other things, substantial financial penalties or awards against us, substantial payments made by us, harm to our reputation, required changes to our business practices, exclusion from future participation in the Medicare, Medicaid and other federal healthcare programs and, in certain cases, criminal penalties, any of which could have a material adverse effect on us. It is possible that criminal proceedings may be initiated against us and/or individuals in our business in connection with investigations by the federal government. Other than as described in "Item 3. Legal Proceedings" in Part I of this report and Note 16 to the consolidated financial statements included in this report, we cannot predict the ultimate outcomes of the various legal proceedings and regulatory matters to which we are or may be subject from time to time, including those described in the aforementioned sections of this report, or the timing of their resolution or the ultimate losses or impact of developments in those matters, which could have a material adverse effect on our business results of operations and financial condition. See "Item 3. Legal Proceedings" in Part I of this report and Note 16 to the consolidated financial statements included in this report for further details regarding these and other matters.
Disruptions in federal government operations and funding create uncertainty in our industry and could have a material adverse effect on our business, results of operations and financial condition.
A substantial portion of our revenues is dependent on federal healthcare program reimbursement, and any disruptions in federal government operations could have a material adverse effect on our business, results of operations and financial condition. If the U.S. government defaults on its debt, there could be broad macroeconomic effects that could raise our cost of borrowing funds, and delay or prevent our future growth and expansion. Any future federal government shutdown, U.S. government default on its debt and/or failure of the U.S. government to enact annual appropriations could have a material adverse effect on our business, results of operations and financial condition. Additionally, disruptions in federal government operations may negatively impact regulatory approvals and guidance that are important to our operations, and create uncertainty about the pace of upcoming healthcare regulatory developments.
Healthcare reform could have a material adverse effect on our business, financial condition and results of operations.
We cannot predict how employers, private payors or persons buying insurance might react to the changes brought on by federal and state healthcare reform legislation, including the ACA and any subsequent legislation, or what form many of these regulations will take before implementation.
The ACA introduced healthcare insurance exchanges, which provide a marketplace for eligible individuals and small employers to purchase healthcare insurance. The business and regulatory environment continues to evolve as the exchanges mature, and statutes and regulations are challenged, changed and enforced. If commercial payor participation in the exchanges continues to decrease, it could have a material adverse effect on our business, results of operations and financial condition. Although we cannot predict the short- or long-term effects of these factors, we believe the healthcare insurance exchanges could result in a reduction in ESRD patients covered by traditional commercial insurance policies and an increase in the number of patients covered through the exchanges under more restrictive commercial plans with lower reimbursement rates or higher deductibles and co-payments that patients may not be able to pay. To the extent that the ongoing implementation of such exchanges or changes in statutes or regulations, or enforcement of statutes or regulations regarding the exchanges results in a reduction in reimbursement rates for our services from commercial and/or government payors, it could have a material adverse effect on our business, results of operations and financial condition.
The ACA also added several new tax provisions that, among other things, impose various fees and excise taxes, and limit compensation deductions for health insurance providers and their affiliates. These rules could negatively impact our cash flow and tax liabilities. In addition, the ACA broadened the potential for penalties under the FCA for the knowing and improper retention of overpayments collected from government payors and reduced the timeline to file Medicare claims. As a result, we made significant investments in new resources to accelerate the time it takes us to identify, quantify and process overpayments and we deployed significant resources to reduce our timeline and improve our claims processing methods to ensure that our Medicare claims are filed in a timely fashion. However, we may be required to make additional investments in the future. Failure to timely identify and return overpayments may result in significant penalties, which could have a material adverse effect on our business, results of operations and financial condition. Failure to file a claim within the one year window could result in payment denials, adversely affecting our business, results of operations and financial condition.
With the ACA, new models of care emerge and evolve and other initiatives in the government or private sector may arise, which could adversely impact our business. For example, the CMS Innovation Center (Innovation Center) is currently working with various healthcare providers to develop, refine and implement Accountable Care Organizations (ACOs) and other innovative models of care for Medicare and Medicaid beneficiaries, including Bundled Payments for Care Improvement
35
Initiative, CEC Model (which includes the development of ESRD Seamless Care Organizations), the Duals Demonstration, and other models. We are currently participating in the CEC Model with the Innovation Center, including with organizations in Arizona, Florida, and adjacent markets in New Jersey and Pennsylvania. Our U.S. dialysis business may choose to participate in additional models either as a partner with other providers or independently. Even in areas where we are not directly participating in these or other Innovation Center models, some of our patients may be assigned to an ACO, another ESRD Care Model, or another program, in which case the quality and cost of care that we furnish will be included in an ACO’s, another ESRD Care Model’s, or other program’s calculations. Additionally, CMS instituted new screening procedures, as required by the ACA, which we expect will delay the Medicare contractor approval process, potentially causing a delay in reimbursement. We anticipate the new screening and enrollment requirements will require additional personnel and financial resources and will potentially delay the enrollment and revalidation of our centers which in turn will delay payment. These delays could adversely affect our business, results of operations and financial condition. The BBA revised the manner in which beneficiaries are assigned to an ACO, specifically giving ACOs the choice to have beneficiaries assigned prospectively at the beginning of a performance year and giving beneficiaries the option to voluntarily align to the ACO in which the beneficiary’s main primary care provider participates. While prospective assignment may allow ACOs to identify beneficiaries for whom they will be held accountable and proactively take steps to ensure appropriate care, the ultimate impact of such changes on our business, results of operations and financial condition is not yet known.
Other ACA reform measures allow CMS to place a moratorium on new enrollment of providers and to suspend payment to providers upon a credible allegation of fraud from any source. These types of reform measures, as well as other measures, could adversely affect our business, results of operations, and financial condition, depending on the scope and breadth of the implementing regulations.
There is also a considerable amount of uncertainty as to the prospective implementation of the ACA and what similar measures or other changes might be enacted at the federal and/or state level. There have been multiple attempts through legislative action and legal challenges to repeal or amend the ACA. In addition, the 2016 Presidential and Congressional elections and subsequent developments in 2017 have caused the future state of the exchanges and other ACA reforms to be unclear. For example, in October 2017, the federal government announced that cost-sharing reduction payments to insurers would end, effective immediately, unless Congress appropriated the funds, and, in December 2017, Congress passed the Tax Cuts and Jobs Act, which includes a provision that eliminates the penalty under the ACA’s individual mandate and could impact the future state of the exchanges. Further, in February 2018, Congress passed the BBA which, among other things, repealed the Independent Payment Advisory Board that was established by the ACA and intended to reduce the rate of growth in Medicare spending. While certain provisions of the BBA may increase the scope of benefits available for certain chronically ill Federal health care program beneficiaries beginning in 2020, the ultimate impact of such changes cannot be predicted. While there may be significant changes to the healthcare environment in the future, the specific changes and their timing are not yet apparent. As a result, there is considerable uncertainty surrounding the ACA including the exchanges, and, indeed, many core aspects of the current health care marketplace. Previously enacted reforms and future changes could have a material adverse effect on our business, financial condition and results of operations, including, for example, by limiting the scope of coverage or the number of patients who are able to obtain coverage through the exchanges and other health insurance programs, lowering or eliminating the cost-sharing reduction subsidies under the ACA, lowering our reimbursement rates, and/or increasing our expenses.
In addition, in December 2016, CMS published an interim final rule that questioned the use of charitable premium assistance for ESRD patients and would have established new conditions for coverage standards for dialysis facilities. In January 2017, a federal district court in Texas issued a preliminary injunction on CMS’ interim final rule and in June 2017, at the request of CMS, the court stayed the proceedings while CMS pursues new rulemaking options. In November 2017, when CMS published the 2018 final rule that updates payment policies and rates under the ESRD PPS, and the 2019 proposed Notice of Benefit and Payment Parameters, it did not pursue further discussion or rule making related to charitable premium assistance or propose changes to historical charitable premium assistance guidelines. This does not preclude CMS or another regulatory agency or legislative authority from issuing a new rule or guidance that challenges charitable premium assistance. Additionally, any other law, rule, or guidance issued by CMS or other regulatory or legislative authorities restricting or prohibiting the ability of patients with access to alternative coverage from selecting a marketplace plan on or off exchange, and/or otherwise restricting or prohibiting the use of charitable premium assistance, could adversely impact dialysis centers across the U.S. making certain centers economically unviable, restrict the ability of dialysis patients to obtain and maintain optimal insurance coverage, and have a material adverse effect on our business, results of operations, and financial condition.
36
Privacy and information security laws are complex, and if we fail to comply with applicable laws, regulations and standards, including with respect to third-party service providers that utilize sensitive personal information on our behalf, or if we fail to properly maintain the integrity of our data, protect our proprietary rights to our systems or defend against cybersecurity attacks, we may be subject to government or private actions due to privacy and security breaches, any of which could have a material adverse effect on our business, financial condition and results of operations or harm our reputation.
We must comply with numerous federal and state laws and regulations in both the U.S. and the foreign jurisdictions in which we operate governing the collection, dissemination, access, use, security and privacy of PHI, including HIPAA and its implementing privacy, security, and related regulations, as amended by the federal Health Information Technology for Economic and Clinical Health Act (HITECH) and collectively referred to as HIPAA. We are also required to report known breaches of PHI consistent with applicable breach reporting requirements set forth in applicable laws and regulations. From time to time, we may be subject to both federal and state inquiries or audits related to HIPAA, HITECH and related state laws associated with complaints, desk audits, and self-reported breaches. If we fail to comply with applicable privacy and security laws, regulations and standards, including with respect to third-party service providers that utilize sensitive personal information, including PHI, on our behalf, properly maintain the integrity of our data, protect our proprietary rights, or defend against cybersecurity attacks, it could harm our reputation or have a material adverse effect on our business, results of operations and financial condition.
Information security risks have significantly increased in recent years in part because of the proliferation of new technologies, the use of the Internet and telecommunications technologies to conduct our operations, and the increased sophistication and activities of organized crime, hackers, terrorists and other external parties, including foreign state agents. Our business and operations rely on the secure processing, transmission and storage of confidential, proprietary and other information in our computer systems and networks, including sensitive personal information, including PHI, social security numbers, and credit card information of our patients, teammates, physicians, business partners and others.
We are continuously implementing multiple layers of security measures through technology, processes, and our people. We utilize security technologies to protect and maintain the integrity of our information systems and data and our defenses are monitored and routinely tested internally and by external parties. Despite these efforts, our facilities and systems and those of our third-party service providers may be vulnerable to privacy and security incidents; security attacks and breaches; acts of vandalism or theft; computer viruses and other malicious code; coordinated attacks by activist entities; emerging cybersecurity risks; misplaced or lost data; programming and/or human errors; or other similar events that could impact the security, reliability, and availability of our systems. Internal or external parties may attempt to circumvent our security systems, and we have in the past, and expect that we will in the future, experience external attacks on our network including reconnaissance probes, denial of service attempts, malicious software attacks including attacks intended to render our internal operating systems unavailable, and phishing attacks. Cybersecurity requires ongoing investment and diligence against evolving threats. Emerging and advanced security threats, including coordinated attacks, require additional layers of security which may disrupt or impact efficiency of operations. As with any security program, there always exists the risk that employees will violate our policies despite our compliance efforts or that certain attacks may be beyond the ability of our security and other systems to detect. There can be no assurance that investments and diligence will be sufficient to prevent or timely discover an attack.
Any security breach involving the misappropriation, loss or other unauthorized disclosure or use of confidential information, including PHI, financial data, competitively sensitive information, or other proprietary data, whether by us or a third party, could have a material adverse effect on our business, financial condition, and results of operations and materially harm our reputation. We may be required to expend significant additional resources to modify our protective measures, to investigate and remediate vulnerabilities or other exposures, or to make required notifications. The occurrence of any of these events could, among other things, result in interruptions, delays, the loss or corruption of data, cessations in the availability of systems and liability under privacy and security laws, all of which could have a material adverse effect on our business, financial condition or results of operations, materially harm our reputation and trigger regulatory actions and private party litigation. If we are unable to protect the physical and electronic security and privacy of our databases and transactions, we could be subject to potential liability and regulatory action, our reputation and relationships with our patients and vendors would be harmed, and our business, results of operations and financial condition could be materially and adversely affected. Failure to adequately protect and maintain the integrity of our information systems (including our networks) and data, or to defend against cybersecurity attacks, could subject us to monetary fines, civil suits, civil penalties or criminal sanctions and requirements to disclose the breach publicly, and could further result in a material adverse effect on our business, results of operations and financial condition or harm our reputation. As malicious cyber activity escalates, including activity that originates outside of the United States, the risks we face relating to transmission of data and our use of service providers outside of our network, as well as the storing or processing of data within our network, intensify. There have been increased federal and state HIPAA and other privacy and security enforcement efforts and we expect this trend to continue. While we
37
maintain cyber liability insurance, this insurance may not cover us for all types of losses and may not be sufficient to protect us against the amount of all losses.
We may engage in acquisitions, mergers, joint ventures or dispositions, which may affect our results of operations, debt-to-capital ratio, capital expenditures or other aspects of our business, and if businesses we acquire have liabilities we are not aware of, we could suffer severe consequences that would have a material adverse effect on our business, results of operations and financial condition.
Our business strategy includes growth through acquisitions of dialysis centers and other businesses, as well as entry into joint ventures. We may engage in acquisitions, mergers, joint ventures or dispositions or expand into new business models, which may affect our results of operations, debt-to-capital ratio, capital expenditures or other aspects of our business. There can be no assurance that we will be able to identify suitable acquisition targets or merger partners or buyers for dispositions or that, if identified, we will be able to agree to terms with merger partners, acquire these targets or make these dispositions on acceptable terms or on the desired timetable. There can also be no assurance that we will be successful in completing any acquisitions, mergers or dispositions that we announce, executing new business models or integrating any acquired business into our overall operations. There is no guarantee that we will be able to operate acquired businesses successfully as stand-alone businesses, or that any such acquired business will operate profitably or will not otherwise have a material adverse effect on our business, results of operations and financial condition. Further, we cannot be certain that key talented individuals at the business being acquired will continue to work for us after the acquisition or that they will be able to continue to successfully manage or have adequate resources to successfully operate any acquired business. In addition, certain of our newly and previously acquired dialysis centers and facilities have been in service for many years, which may result in a higher level of maintenance costs. Further, our facilities, equipment and information technology may need to be improved or renovated to maintain or increase operational efficiency, compete for patients and medical directors, or meet changing regulatory requirements. Increases in maintenance costs and capital expenditures could have a material adverse effect on our financial condition, results of operations and cash flows.
Businesses we acquire may have unknown or contingent liabilities or liabilities that are in excess of the amounts that we originally estimated, and may have other issues, including those related to internal controls over financial reporting or issues that could affect our ability to comply with healthcare laws and regulations and other laws applicable to our expanded business. As a result, we cannot make any assurances that the acquisitions we consummate will be successful. Although we generally seek indemnification from the sellers of businesses we acquire for matters that are not properly disclosed to us, we are not always successful. In addition, even in cases where we are able to obtain indemnification, we may discover liabilities greater than the contractual limits, the amounts held in escrow for our benefit (if any), or the financial resources of the indemnifying party. In the event that we are responsible for liabilities substantially in excess of any amounts recovered through rights to indemnification or alternative remedies that might be available to us, or any applicable insurance, we could suffer severe consequences that could have a material adverse effect on our business, results of operations and financial condition.
Additionally, joint ventures, including our Asia Pacific Joint Venture (APAC JV), and minority investments inherently involve a lesser degree of control over business operations, thereby potentially increasing the financial, legal, operational and/or compliance risks associated with the joint venture or minority investment. In addition, we may be dependent on joint venture partners, controlling shareholders or management who may have business interests, strategies or goals that are inconsistent with ours. Business decisions or other actions or omissions of the joint venture partner, controlling shareholders or management may adversely affect the value of our investment, result in litigation or regulatory action against us, result in reputational harm to us or adversely affect the value of our investment or partnership.
If we are not able to continue to make acquisitions at the desired pace or at all, or maintain an acceptable level of non-acquired growth, or if we face significant patient attrition to our competitors or we are not able to retain or contract with an adequate number of medical directors or associated physicians, it could adversely affect our business, results of operations and financial condition.
Acquisitions, patient retention and medical director and physician retention are an important part of our growth strategy. We face intense competition from other companies for acquisition targets. In our U.S. dialysis business, we continue to face increased competition from large and medium-sized providers, which compete directly with us for the limited acquisition targets as well as for individual patients and medical directors. In addition, we compete for individual patients and medical directors based in part on the quality of our facilities. Moreover, as we continue our international expansion into various international markets, we will face competition from large and medium-sized providers for these acquisition targets as well. As we and our competitors continue to grow and open new dialysis centers, each center is required by applicable regulations to have a medical director, and we may not be able to retain an adequate number of nephrologists to serve as medical directors. Because of the ease of entry into the dialysis business and the ability of physicians to be medical directors for their own centers, competition for growth in existing and expanding markets is not limited to large competitors with substantial financial
38
resources. Individual nephrologists have opened their own dialysis units or facilities. In addition, Fresenius USA, our largest competitor, manufactures a full line of dialysis supplies and equipment in addition to owning and operating dialysis centers. This may give it cost advantages over us because of its ability to manufacture its own products. If we are not able to continue to make acquisitions at the desired pace or at all, continue to maintain acceptable levels of non-acquired growth, or if we face significant patient attrition to our competitors or if a physician chooses not to refer to DaVita, it could adversely affect our business, results of operations and financial condition.
If certain of our suppliers do not meet our needs, if there are material price increases, if we are not reimbursed or adequately reimbursed for drugs we purchase or if we are unable to effectively access new technology or superior products, it could negatively impact our ability to effectively provide the services we offer and could have a material adverse effect on our business, results of operations and financial condition.
We have significant suppliers that may be the sole or primary source of products critical to the services we provide, or to which we have committed obligations to make purchases, sometimes at particular prices. If any of these suppliers do not meet our needs for the products they supply, including in the event of a product recall, shortage or dispute, and we are not able to find adequate alternative sources, if we experience material price increases from these suppliers that we are unable to mitigate, or if some of the drugs that we purchase are not reimbursed or not adequately reimbursed by commercial or government payors, it could have a material adverse impact on our business, results of operations and financial condition. In addition, the technology related to the products critical to the services we provide is subject to new developments which may result in superior products. If we are not able to access superior products on a cost-effective basis or if suppliers are not able to fulfill our requirements for such products, we could face patient attrition which could have a material adverse effect on our business, results of operations and financial condition.
DMG operates in a different line of business from our historical business, and we face challenges managing DMG and may not realize anticipated benefits.
DMG operates in a different line of business from our historical business. We may not have the expertise, experience and resources to pursue all of our businesses at once, and we may be unable to successfully operate all businesses in the combined company. The administration of DMG requires implementation of appropriate operations, management, forecasting, and financial reporting systems and controls. We have experienced difficulties in effectively implementing these and other systems. The management of DMG requires and will continue to require the focused attention of our management team, including a significant commitment of its time and resources. The need for management to focus on these matters could have a material adverse effect on our business, results of operations and financial condition. If the DMG operations continue to be less profitable than we currently anticipate or we do not have the experience, the appropriate expertise or the resources to pursue all businesses in the combined company, our results of operations and financial condition may be materially and adversely affected. In that regard, we have taken goodwill impairment charges of $1.093 billion in total and may continue incurring additional impairment charges.
Laws regulating the corporate practice of medicine could restrict the manner in which DMG and other subsidiaries of ours are permitted to conduct their respective business, and the failure to comply with such laws could subject these entities to penalties or require a restructuring of these businesses.
Some states have laws that prohibit business entities, such as DMG and other subsidiaries of ours, including but not limited to, Nephrology Practice Solutions, Paladina Health, DaVita Health Solutions, VillageHealth, and Lifeline, from practicing medicine, employing physicians to practice medicine, exercising control over medical decisions by physicians (also known collectively as the corporate practice of medicine) or engaging in certain arrangements, such as fee-splitting, with physicians. In some states these prohibitions are expressly stated in a statute or regulation, while in other states the prohibition is a matter of judicial or regulatory interpretation. Of the states in which DMG currently operates, California, Colorado, Nevada and Washington generally prohibit the corporate practice of medicine, and other states may as well.
DMG and other DaVita entities operate by maintaining long-term contracts with their associated physician groups which are each owned and operated by physicians and which employ or contract with additional physicians to provide physician services. Under these arrangements, DMG and such other DaVita entities provide management services and receive a management fee for providing non-medical management services; however, DMG and such other DaVita entities do not represent that they offer medical services, and do not exercise influence or control over the practice of medicine by the physicians or the associated physician groups.
In addition to the above management arrangements, DMG has certain contractual rights relating to the orderly transfer of equity interests in certain of its physician groups through succession agreements and other arrangements with their physician equity holders. However, such equity interests cannot be transferred to or held by DMG or by any non-professional
39
organization. Accordingly, neither DMG nor DMG’s subsidiaries directly own any equity interests in any physician groups in California, Colorado, Nevada and Washington. The other DaVita entities operating in these and multiple other states have similar agreements and arrangements. In the event that any of these associated physician groups fail to comply with the management arrangement or any management arrangement is terminated and/or DMG or any of the other DaVita entities is unable to enforce its contractual rights over the orderly transfer of equity interests in its associated physician groups, such events could have a material adverse effect on the business, results of operations and financial condition of DMG and such other DaVita entities.
It is possible that a state regulatory agency or a court could determine that DMG’s agreements with physician equity holders of certain managed California, Colorado, Nevada and Washington associated physician groups and the way DMG carries out these arrangements as described above, either independently or coupled with the management services agreements with such associated physician groups, are in violation of the corporate practice of medicine doctrine. As a result, these arrangements could be deemed invalid, potentially resulting in a loss of revenues and an adverse effect on results of operations derived from such associated physician groups. Such a determination could force a restructuring of DMG’s management arrangements with associated physician groups in California, Colorado, Nevada and/or Washington, which might include revisions of the management services agreements, including a modification of the management fee and/or establishing an alternative structure that would permit DMG to contract with a physician network without violating the corporate practice of medicine prohibition. There can be no assurance that such a restructuring would be feasible, or that it could be accomplished within a reasonable time frame without a material adverse effect on DMG’s business, results of operations and financial condition. These same risks exist for the other DaVita entities utilizing similar structures.
In December 2013, DHPC obtained a restricted Knox-Keene license in California, which permits DHPC to contract with health plans in California to accept global risk without violating the corporate practice of medicine prohibition. However, DMG and DMG’s Colorado, Nevada and Washington associated physician groups, as well as those physician equity holders of associated physician groups who are subject to succession agreements with DMG, could be subject to criminal or civil penalties or an injunction for practicing medicine without a license or aiding and abetting the unlicensed practice of medicine.
The level of our current and future debt could have an adverse impact on our business and our ability to generate cash to service our indebtedness and for other intended purposes depends on many factors beyond our control.
We have substantial debt outstanding, we incurred a substantial amount of additional debt in connection with the acquisition of DMG and we may incur additional indebtedness in the future, including in anticipation of receiving the cash proceeds from the sale of DMG. For additional details regarding specific risks we face regarding the sale of DMG, see the discussion in the risk factors under the heading "Risk factors related to the sale of DMG." Our inability to generate sufficient cash to service our substantial indebtedness and for other intended purposes could have important consequences to you, for example, it could:
• | make it difficult for us to make payments on our debt securities; |
• | increase our vulnerability to general adverse economic and industry conditions; |
• | require us to dedicate a substantial portion of our cash flows from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, acquisitions and investments, repurchases of stock at the levels intended or announced, or at all, and other general corporate purposes; |
• | limit our flexibility in planning for, or reacting to, changes in our business and the markets in which we operate; |
• | expose us to interest rate volatility that could adversely affect our business, results of operations and financial condition, and our ability to service our indebtedness; |
• | place us at a competitive disadvantage compared to our competitors that have less debt; and |
• | limit our ability to borrow additional funds, or to refinance existing debt on favorable terms when otherwise available. |
In addition, we expect to continue to incur additional indebtedness in the future, and the amount of that additional indebtedness may be substantial. Although the indentures governing our senior notes and the agreement governing our senior secured credit facilities include covenants that could limit our indebtedness, we currently have the ability to incur substantial additional debt. If new debt is added to current debt levels, the related risks described above could intensify, in particular, if we
40
were to borrow new debt in anticipation of receiving the cash proceeds from the pending sale of DMG and if there is a delay in closing the sale of DMG or the sale of DMG does not close.
Our ability to make payments on our indebtedness, to fund planned capital expenditures and expansion efforts, including any strategic acquisitions we may make in the future, to repurchase our stock at the levels intended or announced and to meet our other liquidity needs, will depend on our ability to generate cash. This, to a certain extent, is subject to general economic, financial, competitive, regulatory and other factors that are beyond our control.
After the pending sale of DMG closes, our cash flows will be reduced accordingly. We cannot provide assurances that our business will generate sufficient cash flows from operations in the future or that future borrowings will be available to us in an amount sufficient to enable us to service our indebtedness or to fund other liquidity needs, including those described above. If we are unable to generate sufficient funds to service our outstanding indebtedness or to meet our other liquidity needs, including the intended purposes described above, we may be required to refinance, restructure, or otherwise amend some or all of such obligations, sell assets, change our intended or announced uses or strategy for capital deployment, including for stock repurchases, reduce capital expenditures or planned expansions or raise additional cash through the sale of our equity. We cannot make any assurances that any such refinancing, restructurings, sales of assets, or issuances of equity can be accomplished or, if accomplished, can be accomplished on favorable terms or that if accomplished that they would raise sufficient funds to meet these obligations or our other liquidity needs.
The borrowings under our senior secured credit facilities are guaranteed by a substantial portion of our direct and indirect wholly owned domestic subsidiaries, including certain of DMG’s subsidiaries, and are secured by a substantial portion of our and our subsidiaries’ assets, including those of certain of DMG’s subsidiaries. After the sale of DMG closes, we will have fewer assets with which to secure future debt or refinance or restructure existing debt. This will likely reduce the total amount of secured debt that we will be able to incur and may increase the interest rate we are required to pay on our existing secured debt and any secured debt we issue in the future. In addition, by reducing the amount of assets available to meet the claims of our secured creditors, it may also adversely affect the interest rates on our existing unsecured debt and any unsecured debt we issue in the future.
We may be subject to liability claims for damages and other expenses that are not covered by insurance or exceed our existing insurance coverage that could have a material adverse effect on our business, results of operations, financial condition and reputation.
Our operations and how we manage our Company may subject us, as well as our officers and directors to whom we owe certain defense and indemnity obligations, to litigation and liability for damages. Our business, profitability and growth prospects could suffer if we face negative publicity or we pay damages or defense costs in connection with a claim that is outside the scope or limits of coverage of any applicable insurance coverage, including claims related to adverse patient events, contractual disputes, professional and general liability and directors’ and officers’ duties. In addition, we have received notices of claims from commercial payors and other third parties, as well as subpoenas and CIDs from the federal government, related to our business practices, including our historical billing practices and the historical billing practices of acquired businesses. Although the ultimate outcome of these claims cannot be predicted, an adverse result with respect to one or more of these claims could have a material adverse effect on our business, results of operations and financial condition. We maintain insurance coverage for those risks we deem are appropriate to insure against and make determinations about whether to self-insure as to other risks or layers of coverage. However, a successful claim, including a professional liability, malpractice or negligence claim which is in excess of any applicable insurance coverage, or that is subject to our self-insurance retentions, could have a material adverse effect on our business, results of operations, financial condition and reputation. Additionally, as a result of the broad scope of our DMG division’s medical practice, we are exposed to medical malpractice claims, as well as claims for damages and other expenses, that may not be covered by insurance or for which adequate limits of insurance coverage may not be available.
In addition, if our costs of insurance and claims increase, then our earnings could decline. Market rates for insurance premiums and deductibles have been steadily increasing. Our business, results of operations and financial condition could be materially and adversely affected by any of the following:
• | the collapse or insolvency of our insurance carriers; |
• | further increases in premiums and deductibles; |
• | increases in the number of liability claims against us or the cost of settling or trying cases related to those claims; or |
• | an inability to obtain one or more types of insurance on acceptable terms, if at all. |
41
If we fail to successfully maintain an effective internal control over financial reporting, the integrity of our financial reporting could be compromised, which could have a material adverse effect on our ability to accurately report our financial results and the market’s perception of our business and our stock price.
The integration of acquisitions and addition of new business lines into our internal control over financial reporting has required and will continue to require significant time and resources from our management and other personnel and has increased, and will continue to, increase our compliance costs. Failure to maintain an effective internal control environment could have a material adverse effect on our ability to accurately report our financial results and the market’s perception of our business and our stock price. In addition, we could be required to restate our financial results in the event of a significant failure of our internal control over financial reporting or in the event of inappropriate application of accounting principles.
Deterioration in economic conditions and further disruptions in the financial markets could have a material adverse effect on our business, results of operations and financial condition.
Deterioration in economic conditions could have a material adverse effect on our business, results of operations and financial condition. Among other things, the potential decline in federal and state revenues that may result from such conditions may create additional pressures to contain or reduce reimbursements for our services from Medicare, Medicaid and other government sponsored programs. Increases in job losses in the U.S. as a result of adverse economic conditions has and may continue to result in a smaller percentage of our patients being covered by an employer group health plan and a larger percentage being covered by lower paying Medicare and Medicaid programs. Employers may also select more restrictive commercial plans with lower reimbursement rates. To the extent that payors are negatively impacted by a decline in the economy, we may experience further pressure on commercial rates, a further slowdown in collections and a reduction in the amounts we expect to collect. In addition, uncertainty in the financial markets could adversely affect the variable interest rates payable under our credit facilities or could make it more difficult to obtain or renew such facilities or to obtain other forms of financing in the future, if at all. Any or all of these factors, as well as other consequences of a deterioration in economic conditions which cannot currently be anticipated, could have a material adverse effect on our business, results of operations and financial condition.
We could be subject to adverse changes in tax laws, regulations and interpretations or challenges to our tax positions.
We are subject to tax laws and regulations of the U.S. federal, state and local governments as well as various foreign jurisdictions. We compute our income tax provision based on enacted tax rates in the jurisdictions in which we operate. As the tax rates vary among jurisdictions, a change in earnings attributable to the various jurisdictions in which we operate could result in an unfavorable change in our overall tax provision.
From time to time, changes in tax laws or regulations may be proposed or enacted that could adversely affect our overall tax liability. For example, the recent U.S. tax legislation enacted on December 22, 2017 represents a significant overhaul of the U.S. federal tax code. This tax legislation significantly reduced the U.S. statutory corporate tax rate and made other changes that we expect will reduce our effective U.S. federal tax rate in future periods. However, the tax legislation also included a number of provisions, including, but not limited to, the limitation or elimination of various deductions or credits (including for interest expense and for performance-based compensation under Section 162(m)), the imposition of taxes on certain cross-border payments or transfers, the changing of the timing of the recognition of certain income and deductions or their character, and the limitation of asset basis under certain circumstances, any of which could significantly and adversely affect our U.S. federal income tax position. The legislation also made significant changes to the tax rules applicable to insurance companies and other entities with which we do business. The estimated impact of the new law is based on management’s current knowledge and assumptions. We are continuing to evaluate the overall impact of this tax legislation on our operations and U.S. federal and state income tax position. The actual impact of the new law could be materially different from our current estimates based on our actual results and our further analysis of the new law. There can be no assurance that changes in tax laws or regulations, both within the U.S. and the other jurisdictions in which we operate, will not materially and adversely affect our effective tax rate, tax payments, financial condition and results of operations. Similarly, changes in tax laws and regulations that impact our patients, business partners and counterparties or the economy generally may also impact our financial condition and results of operations.
In addition, tax laws and regulations are complex and subject to varying interpretations, and any significant failure to comply with applicable tax laws and regulations in all relevant jurisdictions could give rise to substantial penalties and liabilities. We are regularly subject to audits by tax authorities and, although we believe our tax estimates are appropriate, the final determination of tax audits and any related litigation could be materially different from our historical income tax provisions and accruals. Any changes in enacted tax laws (such as the recent U.S. tax legislation), rules or regulatory or judicial interpretations; any adverse outcome in connection with tax audits in any jurisdiction; or any change in the pronouncements
42
relating to accounting for income taxes could materially and adversely impact our effective tax rate, tax payments, financial condition and results of operations.
Expansion of our operations to and offering our services in markets outside of the U.S. subjects us to political, economic, legal, operational and other risks that could have a material adverse effect on our business, results of operations and financial condition.
We are continuing to expand our operations by offering our services and entering new lines of business in certain markets outside of the U.S., which increases our exposure to the inherent risks of doing business in international markets. Depending on the market, these risks include those relating to:
• | changes in the local economic environment; |
• | political instability, armed conflicts or terrorism; |
• | social changes; |
• | intellectual property legal protections and remedies; |
• | trade regulations; |
• | procedures and actions affecting approval, production, pricing, reimbursement and marketing of products and services; |
• | foreign currency; |
• | repatriating or moving to other countries cash generated or held abroad, including considerations relating to tax-efficiencies and changes in tax laws; |
• | export controls; |
• | lack of reliable legal systems which may affect our ability to enforce contractual rights; |
• | changes in local laws or regulations; |
• | potentially longer ramp-up times for starting up new operations and for payment and collection cycles; |
• | financial and operational, and information technology systems integration; |
• | failure to comply with U.S. laws, such as the FCPA, or local laws that prohibit us, our partners, or our partners’ or our intermediaries from making improper payments to foreign officials for the purpose of obtaining or retaining business; and |
• | data and privacy restrictions. |
Issues relating to the failure to comply with any of the above may also impact our domestic business and/or raise scrutiny on our domestic practices.
Additionally, some factors that will be critical to the success of our international business and operations will be different than those affecting our domestic business and operations. For example, conducting international operations requires us to devote significant management resources to implement our controls and systems in new markets, to comply with local laws and regulations and to overcome the numerous new challenges inherent in managing international operations, including those based on differing languages, cultures and regulatory environments, and those related to the timely hiring, integration and retention of a sufficient number of skilled personnel to carry out operations in an environment with which we are not familiar.
Any expansion of our international operations through acquisitions or through organic growth could increase these risks. Additionally, while we may invest material amounts of capital and incur significant costs in connection with the growth and development of our international operations, including to start up or acquire new operations, we may not be able to operate them profitably on the anticipated timeline, or at all.
These risks could have a material adverse effect on our business, results of operations and financial condition.
43
Risk factors related to the sale of DMG:
The announcement and pendency of the sale of DMG may adversely affect our business, results of operations and financial condition.
The announcement and pending sale of DMG may be disruptive to our business and may adversely affect our relationships with current and prospective teammates, patients, physicians, payors, suppliers and other business partners. Uncertainties related to the pending sale of DMG may impair our ability to attract, retain and motivate key personnel and could cause suppliers and other business partners to defer entering into contracts with us or seek to change existing business relationships with us. The loss or deterioration of significant business and operational relationships could have an adverse effect on our business, results of operations and financial condition. In addition, activities relating to the pending sale and related uncertainties could divert the attention of our management and other teammates from our day-to-day business or disrupt our operations in preparation for and during the post-closing separation of DMG. It is also possible that we could have stranded costs following the closing of the pending sale, which could be material. If we are unable to effectively manage these risks, our business, results of operations and financial condition may be adversely affected.
If we fail to complete the proposed sale of DMG, or if there is a significant delay in completing the sale, our business, results of operations, financial condition and stock price may be materially adversely affected.
The completion of the proposed sale of DMG is subject to customary closing conditions, including the expiration or termination of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and the approval of a notice of material modification by the California Department of Managed Health Care. If any condition to the closing of the sale of DMG is neither satisfied nor, where permissible, waived, the sale of DMG will not be completed. In addition, satisfying the closing conditions to the sale of DMG may take longer than expected. Regulators may impose material conditions, terms, obligations, costs or restrictions in connection with their approval of or consent to the sale of DMG, which could delay completion of the transaction, or if such approvals or consents are not obtained, could prevent completion of the transaction. There can be no assurance that all of the closing conditions will be satisfied or waived or that other events will not intervene to delay, or result in a failure to close, the sale of DMG. In addition, either we or Optum may terminate the equity purchase agreement if, among other things, the sale has not been consummated by June 4, 2018 (subject to two three-month extensions that can be exercised by either party unilaterally). If the equity purchase agreement is terminated and our Board of Directors seeks an alternative transaction or another acquiror for the sale of the DMG business, we may not be able to negotiate a transaction with another party on terms comparable to, or better than, the terms of the equity purchase agreement with Optum.
If the sale of DMG is not completed for any reason, investor confidence could decline. A failed transaction may result in negative publicity and may affect our relationships with teammates, patients, physicians, payors, suppliers, regulators and other business partners. In addition, in the event of a failed transaction, we will have expended significant management resources in an effort to complete the sale, we have incurred additional debt in anticipation of receiving the sale proceeds but not have received the sale proceeds to repay such debt, and we will have incurred significant transaction costs, including legal fees, financial advisor fees and other related costs, without any commensurate benefit. Accordingly, if the proposed sale of DMG is not completed, or if there is a significant delay in completing the sale, our business, results of operations, financial condition and stock price may be materially adversely affected.
We may not be able to use the proceeds from the sale of DMG as planned or we may spend or invest the proceeds in ways that may not improve our results of operations or enhance the value of our common stock.
The purchase price for the sale of the DMG business is subject to customary adjustments, both upward and downward, which could be significant. We plan to use the proceeds from the sale of DMG for significant stock repurchases, to repay debt and for general corporate purposes, including growth investments. A number of factors may impact our ability to repurchase stock and the timing of any such stock repurchases, including market conditions, the price of our common stock, our cash flow position, leverage ratios, and legal, regulatory and contractual requirements and restrictions.
In addition, we may identify investments or other uses for the proceeds from the sale of DMG that we believe are more attractive than our current intended uses. Further, there can be no assurance that any investment of the proceeds from the sale of DMG will yield a favorable return.
44
Under the terms of the equity purchase agreement, we are subject to certain contractual restrictions while the sale of DMG is pending, and certain post-closing contractual obligations that, in some cases, could have a material adverse effect on our business, results of operations and financial condition.
Under the terms of the equity purchase agreement, we are subject to certain restrictions on the conduct of the DMG business prior to completing the sale of DMG, which may adversely affect our ability to execute certain of our business strategies, including the ability in certain cases to enter into or amend contracts, acquire or dispose of assets, incur indebtedness or incur capital expenditures. Such limitations could negatively affect our business and operations prior to the completion of the sale of DMG. Each of these risks may be exacerbated by delays or other adverse developments with respect to the completion of the sale of DMG.
In addition, we agreed to retain certain liabilities of the DMG business for which we have certain indemnification rights against the original 2012 HealthCare Partners (“HCP”) sellers. An escrow was established in connection with our acquisition of the DMG business from the HCP sellers as security for these indemnification rights, including with respect to the OIG investigation into certain patient diagnosis coding practices. We have submitted an indemnification claim against the sellers secured by the escrow for any and all liabilities incurred relating to these matters and intend to pursue recovery from the escrow. However, we can make no assurances that the indemnification and escrow will cover the full amount of our potential losses related to these matters, which could have a material adverse effect on our business, results of operations and financial condition.
Risk factors related to our U.S. dialysis and related lab services, ancillary services and strategic initiatives:
If patients in commercial plans are subject to restriction in plan designs or the average rates that commercial payors pay us decline significantly, it would have a material adverse effect on our business, results of operations and financial condition.
Approximately 33% of our dialysis services revenues for the year ended December 31, 2017 were generated from patients who have commercial payors (including hospital dialysis services) as their primary payor. The majority of these patients have insurance policies that pay us on terms and at rates that are generally significantly higher than Medicare rates. The payments we receive from commercial payors generate nearly all of our profit and all of our nonacute dialysis profits come from commercial payors. We continue to experience downward pressure on some of our commercial payment rates as a result of general conditions in the market, including as employers shift to less expensive options for medical services, recent and future consolidations among commercial payors, increased focus on dialysis services and other factors. In addition, many commercial payors that sell individual plans both on and off exchange have publicly announced losses in the marketplace. These payors may seek discounts on rates for marketplace plans on and off exchange. Commercial payment rates could be materially lower in the future.
We continuously are in the process of negotiating existing and potential new agreements with commercial payors who aggressively negotiate terms with us. Sometimes many significant agreements are being renegotiated at the same time. In the event that our continual negotiations result in overall commercial rate reductions in excess of overall commercial rate increases, the cumulative effect could have a material adverse effect on our business, results of operations and financial condition. Consolidations have significantly increased the negotiating leverage of commercial payors. Our negotiations with payors are also influenced by competitive pressures, and we may experience decreased contracted rates with commercial payors or experience decreases in patient volume as our negotiations with commercial payors continue. In addition to downward pressure on contracted commercial payor rates, payors have been attempting to design and implement plans to restrict access to coverage, and the duration and/or the breadth of benefits, which may result in decreased payments. In addition, payors have been attempting to impose restrictions and limitations on patient access to commercial exchange plans and non-contracted or out-of-network providers, and in some circumstances designate our centers as out-of-network providers. Rates for commercial exchange products and out-of-network providers are on average higher than rates for government products and in-network providers, respectively.
A number of commercial payors have incorporated policies into their provider manuals limiting or refusing to accept charitable premium assistance from non-profit organizations, such as the American Kidney Fund, which may impact the number of patients who are able to afford commercial exchange plans. Paying for coverage is a significant financial burden for many patients, and ESRD disproportionately affects the low-income population. Charitable premium assistance supports continuity of coverage and access to care for patients, many of whom are unable to continue working full-time as a result of their severe condition. A material restriction in patients' ability to access charitable premium assistance may restrict the ability of dialysis patients to obtain and maintain optimal insurance coverage, and may adversely impact a large number of dialysis centers across the U.S. by making certain centers economically unviable, and may have a material adverse effect on our business, results of operations and financial condition.
45
We also believe commercial payors have or will begin to restructure their benefits to create disincentives for patients to stay with commercial insurance or to select or remain with out-of-network providers. In addition, payors may seek to decrease payment rates for out-of-network providers. Decreases in the number of patients with commercial plans, decreases in out-of-network rates and restrictions on out-of-network access, our turning away new patients in instances where we are unable to come to agreement on rates, or decreases in contracted rates could result in a significant decrease in our overall revenues derived from commercial payors. If the average rates that commercial payors pay us decline significantly, or if we see a decline in commercial patients, it would have a material adverse effect on our business, results of operations and financial condition. For additional details regarding specific risks we face regarding regulatory changes that could result in fewer patients covered under commercial plans or an increase of patients covered under more restrictive commercial plans with lower reimbursement rates, see the discussion in the risk factor under the heading "Healthcare reform could have a material adverse effect on our business, financial condition and results of operations."
If the number of patients with higher-paying commercial insurance declines, it could have a material adverse effect on our business, results of operations and financial condition.
Our revenue levels are sensitive to the percentage of our patients with higher-paying commercial insurance coverage. A patient’s insurance coverage may change for a number of reasons, including changes in the patient’s or a family member’s employment status. Any changes impacting our highest paying commercial payors will have a disproportionate impact on us. In addition, many patients with commercial and government insurance rely on financial assistance from charitable organizations, such as the American Kidney Fund. Certain payors have challenged our patients’ and other providers’ patients' ability to utilize assistance from charitable organizations for the payment of premiums, including through litigation and other legal proceedings. Regulators have also questioned the use of charitable premium assistance for ESRD patients. CMS or another regulatory agency or legislative authority may issue a new rule or guidance that challenges charitable premium assistance. If any of these challenges to kidney patients’ use of premium assistance are successful or restrictions are imposed on the use of financial assistance from such charitable organizations such that kidney patients are unable to obtain, or continue to receive or receive for a limited duration, such financial assistance, it could have a material adverse effect on our business, results of operations and financial condition. In addition, if our assumptions about how kidney patients will respond to any change in financial assistance from charitable organizations are incorrect, it could have a material adverse effect on our business, results of operations and financial condition.
When Medicare becomes the primary payor, the payment rate we receive for that patient decreases from the employer group health plan or commercial plan rate to the lower Medicare payment rate. The number of our patients who have government-based programs as their primary payors could increase and the percentage of our patients covered under commercial insurance plans could be negatively impacted as a result of improved mortality or declining macroeconomic conditions. To the extent there are sustained or increased job losses in the U.S., independent of whether general economic conditions improve, we could experience a decrease in the number of patients covered under commercial plans and/or an increase in uninsured and underinsured patients. We could also experience a further decrease in the payments we receive for services if changes to the healthcare regulatory system result in fewer patients covered under commercial plans or an increase of patients covered under more restrictive commercial plans with lower reimbursement rates. In addition, our continual negotiations with commercial payors under existing and potential new agreements could result in a decrease in the number of our patients covered by commercial plans to the extent that we cannot reach agreement with commercial payors on rates and other terms, resulting in termination or non-renewals of existing agreements and our inability to enter into new agreements. Commercial payors have taken and may continue to take steps to control the cost of and/or the eligibility for access to healthcare services, including relative to products on and off the healthcare exchanges. These efforts could impact the number of our patients who are eligible to enroll in commercial insurance plans, and remain on the plans, including plans offered through healthcare exchanges. Additionally, we continue to experience higher amounts of write-offs due to uninsured and underinsured patients, which has resulted in an increase in uncollectible accounts. Commercial payors could also cease paying in the primary position after providing 30 months of coverage resulting in a material reduction in payment as the patient moves to Medicare primary. If there is a significant reduction in the number of patients under higher-paying commercial plans relative to government-based programs that pay at lower rates or a significant increase in the number of patients that are uninsured and underinsured, it would have a material adverse effect on our business, results of operations and financial condition.
Changes in the structure of and payment rates under the Medicare ESRD program could have a material adverse effect on our business, results of operations and financial condition.
Approximately 42% of our dialysis services revenues for the year ended December 31, 2017 were generated from patients who have Medicare as their primary payor. For patients with Medicare coverage, all ESRD payments for dialysis treatments are made under a single bundled payment rate which provides a fixed payment rate to encompass all goods and services provided during the dialysis treatment, including pharmaceuticals that were historically separately reimbursed to the
46
dialysis providers, such as EPO, vitamin D analogs and iron supplements, irrespective of the level of pharmaceuticals administered or additional services performed. Most lab services are also included in the bundled payment. Under the ESRD PPS, the bundled payments to a dialysis facility may be reduced by as much as 2% based on the facility’s performance in specified quality measures set annually by CMS through the ESRD Quality Incentive Program, which was established by the Medicare Improvements for Patients and Providers Act of 2008. The bundled payment rate is also adjusted for certain patient characteristics, a geographic usage index and certain other factors. In addition, the ESRD PPS is subject to rebasing, which can have a positive financial effect, or a negative one if the government fails to rebase in a manner that adequately addresses the costs borne by dialysis facilities. Similarly, as new drugs, services or labs are added to the ESRD bundle, CMS’ failure to adequately calculate the costs associated with the drugs, services or labs could have a material adverse effect on our business, results of operations and financial condition.
The current bundled payment system presents certain operating, clinical and financial risks, which include:
• | Risk that our rates are reduced by CMS. Uncertainty about future payment rates remains a material risk to our business. Each year, CMS publishes a final rule for the PPS, which has been phasing in reductions to the PPS base rate mandated by the American Taxpayer Relief Act of 2012 as modified by the Protecting Access to Medicare Act of 2014. |
• | Risk that CMS, through its contracted MACs or otherwise, implements Local Coverage Determinations (LCDs) or other decisions that limit the frequency a provider can bill Medicare for home dialysis treatments or other rules that may impact reimbursement. Such coverage determinations could have an adverse impact on our revenue. There is also risk commercial insurers could incorporate the requirements or limitations associated with such LCDs into their contracted terms with dialysis providers, which could have an adverse impact on our revenue. |
• | Risk that a MAC, or multiple MACs, change their interpretations of existing regulations, manual provisions and/or guidance; or seek to implement or enforce new interpretations that are inconsistent with how we have interpreted existing regulations, manual provisions and/or guidance. |
• | Risk that increases in our operating costs will outpace the Medicare rate increases we receive. We expect operating costs to continue to increase due to inflationary factors, such as increases in labor and supply costs, including increases in maintenance costs and capital expenditures to improve, renovate and maintain our facilities, equipment and information technology to meet changing regulatory requirements, regardless of whether there is a compensating inflation-based increase in Medicare payment rates or in payments under the bundled payment rate system. |
• | Risk of federal budget sequestration cuts. As a result of the Budget Control Act of 2011 and the BBA, an annual 2% reduction to Medicare payments took effect on April 1, 2013 and has been extended through 2027. These across-the-board spending cuts have affected and will continue to adversely affect our business, results of operations and financial condition. |
• | Risk that, if our clinical systems fail to accurately capture the data we report to CMS in connection with claims for which at least part of the government’s payments to us is based on clinical performance or patient outcomes or co-morbidities, we might be over-reimbursed by the government, which could subject us to certain liability. For example, CMS published a final rule that implemented a provision of the ACA, requiring providers to report and return Medicare and Medicaid overpayments within the later of (a) 60 days after the overpayment is identified, or (b) the date any corresponding cost report is due, if applicable. An overpayment impermissibly retained under this statute could subject us to liability under the FCA, exclusion, and penalties under the federal Civil Monetary Penalty statute. |
For additional details regarding the risks we face for failing to adhere to our Medicare and Medicaid regulatory compliance obligations, see the risk factor above under the heading "If we fail to adhere to all of the complex government laws and regulations that apply to our business, we could suffer severe consequences that could have a material adverse effect on our business, results of operations, financial condition and stock price."
Changes in state Medicaid or other non-Medicare government-based programs or payment rates could have a material adverse effect on our business, results of operations and financial condition.
Approximately 25% of our dialysis services revenues for the year ended December 31, 2017 were generated from patients who have state Medicaid or other non-Medicare government-based programs, such as coverage through the Department of Veterans Affairs (VA), as their primary coverage. As state governments and other governmental organizations
47
face increasing budgetary pressure, we may in turn face reductions in payment rates, delays in the receipt of payments, limitations on enrollee eligibility or other changes to the applicable programs. For example, certain state Medicaid programs and the VA have recently considered, proposed or implemented payment rate reductions.
The VA adopted Medicare’s bundled PPS pricing methodology for any veterans receiving treatment from non-VA providers under a national contracting initiative. Since we are a non-VA provider, these reimbursements are tied to a percentage of Medicare reimbursement, and we have exposure to any dialysis reimbursement changes made by CMS. Approximately 3% of our dialysis services revenues for the year ended December 31, 2017 were generated by the VA.
In 2013, we entered into a five-year Nationwide Dialysis Services contract with the VA which is subject to one-year renewal periods, consistent with all provider agreements with the VA under this contract. During the length of the contract, the VA has elected not to make adjustments to reimbursement percentages that are tied to a percentage of Medicare reimbursement rates. These agreements provide the VA with the right to terminate the agreements without cause on short notice. Should the VA renegotiate, or not renew or cancel these agreements for any reason, we may cease accepting patients under this program and may be forced to close centers or experience lower reimbursement rates, which could have a material adverse effect on our business, results of operations and financial condition.
State Medicaid programs are increasingly adopting Medicare-like bundled payment systems, but sometimes these payment systems are poorly defined and are implemented without any claims processing infrastructure, or patient or facility adjusters. If these payment systems are implemented without any adjusters and claims processing changes, Medicaid payments will be substantially reduced and the costs to submit such claims may increase, which will have a negative impact on our business, results of operations and financial condition. In addition, some state Medicaid program eligibility requirements mandate that citizen enrollees in such programs provide documented proof of citizenship. If our patients cannot meet these proof of citizenship documentation requirements, they may be denied coverage under these programs, resulting in decreased patient volumes and revenue. These Medicaid payment and enrollment changes, along with similar changes to other non-Medicare government programs could reduce the rates paid by these programs for dialysis and related services, delay the receipt of payment for services provided and further limit eligibility for coverage which could have a material adverse effect on our business, results of operations and financial condition.
Changes in clinical practices, payment rates or regulations impacting EPO and other pharmaceuticals could have a material adverse effect on our business, results of operations and financial condition and negatively impact our ability to care for patients.
Medicare bundles EPO into the PPS such that dosing variations do not change the amount paid to a dialysis facility. Although some Medicaid programs and other payors suggest movement towards a bundled payment system inclusive of EPO, some non-Medicare payors continue to pay for EPO separately from the treatment rate.
Additionally, evaluations on the utilization and reimbursement for ESAs, which have occurred in the past and may occur in the future, and related actions by the U.S. Congress and federal agencies, could result in further restrictions on the utilization and reimbursement for ESAs. Commercial payors have increasingly examined their administration policies for EPO and, in some cases, have modified those policies. Changes in labeling of EPO and other pharmaceuticals in a manner that alters physician practice patterns, including their independent determinations as to appropriate EPO dosing, or accepted clinical practices, and/or changes in private and governmental payment criteria, including the introduction of EPO administration policies could have a material adverse effect on our business, results of operations and financial condition. Further increased utilization of EPO for patients for whom the cost of EPO is included in a bundled reimbursement rate, or further decreases in reimbursement for EPO and other pharmaceuticals that are not included in a bundled reimbursement rate, could also have a material adverse effect on our business, results of operations and financial condition.
Additionally, as of January 1, 2018, calcimimetics entered the Medicare ESRD bundle. We implemented processes to provide the drug as required under the regulations and prescribed by physicians and have entered into agreements to provide for access to and distribution of the drug. If Medicare Advantage plans and/or Medicaid do not pay as required or the processes we have implemented to provide the drug do not perform as anticipated, then we could be subject to both financial and operational risk.
We may be subject to increased inquiries or audits from a variety of governmental bodies or claims by third parties. Although we believe our anemia management practices and other pharmaceutical administration practices have been compliant with existing laws and regulations, increased inquiries or audits from governmental bodies or claims by third parties would require management’s attention, and could result in significant legal expense. Any negative findings could result in substantial financial penalties or repayment obligations, the imposition of certain obligations on and changes to our practices and procedures as well as the attendant financial burden on us to comply with the obligations, or exclusion from future participation
48
in the Medicare and Medicaid programs, and could have a material adverse effect on our business, results of operations and financial condition.
If we fail to comply with our Corporate Integrity Agreement, we could be subject to substantial penalties and exclusion from participation in federal healthcare programs that could have a material adverse effect on our business, results of operations and financial condition.
In October 2014, we entered into a Settlement Agreement with the United States and relator David Barbetta to resolve the then pending 2010 and 2011 U.S. Attorney physician relationship investigations and paid $406 million in settlement amounts, civil forfeiture, and interest to the United States and certain states. In connection with the resolution of these matters, and in exchange for the OIG’s agreement not to exclude us from participating in the federal healthcare programs, we have entered into a five-year CIA with the OIG. The CIA (i) requires that we maintain certain elements of our compliance programs; (ii) imposes certain expanded compliance-related requirements during the term of the CIA; (iii) requires ongoing monitoring and reporting by an independent monitor, imposes certain reporting, certification, records retention and training obligations, allocates certain oversight responsibility to the Board’s Compliance Committee, and necessitates the creation of a Management Compliance Committee and the retention of an independent compliance advisor to the Board; and (iv) contains certain business restrictions related to a subset of our joint venture arrangements, including our agreeing to (1) unwind 11 joint venture transactions that were created through partial divestitures to, or partial acquisitions from, nephrologists, and that cover 26 of our 2,119 clinics that existed at the time we entered into the Settlement Agreement, all of which have been completed, (2) not enter into certain types of partial divestiture joint venture transactions with nephrologists during the term of the CIA, (3) non-enforcement of certain patient-related non-solicitation restrictions, and (4) certain other restrictions. The costs associated with compliance with the CIA could be substantial and may be greater than we currently anticipate. In addition, in the event of a breach of the CIA, we could become liable for payment of certain stipulated penalties, and could be excluded from participation in federal healthcare programs. The OIG notified us that it considered us to be previously in breach of the CIA because of three implementation deficiencies. While we have remediated the deficiencies and have paid certain stipulated penalties, we cannot provide any assurances that we may not be found in breach of the CIA in the future. In general, the costs associated with compliance with the CIA, or any liability or consequences associated with a breach, could have a material adverse effect on our business, results of operations and financial condition. For our domestic dialysis business, we are required under the CIA to report to the OIG (i) probable violations of criminal, civil or administrative laws applicable to any federal health care program for which penalties or exclusions may be authorized under applicable laws and regulations; (ii) substantial overpayments of amounts of money we have received in excess of the amounts due and payable under the federal healthcare program requirements; and (iii) employment of or contracting with individuals ineligible from participating in the federal healthcare programs (we refer to these collectively as Reportable Events). We have provided the OIG notice of Reportable Events, and we may identify and report additional events in the future. If any of our operations are found to violate government laws and regulations, we could suffer severe consequences that could have a material adverse effect on our business, results of operations, financial condition and stock price, including those consequences described under the risk factor "If we fail to adhere to all of the complex government laws and regulations that apply to our business, we could suffer severe consequences that could have a material adverse effect on our business, results of operations, financial condition and stock price."
Delays in state Medicare and Medicaid certification or other licensing and/or anything impacting the licensing of our dialysis centers could adversely affect our business, results of operations and financial condition.
Before we can begin billing for patients treated in our outpatient dialysis centers who are enrolled in government-based programs, we are required to obtain state and federal certification for participation in the Medicare and Medicaid programs. As state agencies responsible for surveying dialysis centers on behalf of the state and Medicare program face increasing budgetary pressure, certain states are having difficulty keeping up with certifying dialysis centers in the normal course resulting in significant delays in certification. If state governments continue to have difficulty keeping up with certifying new centers in the normal course and we continue to experience significant delays in our ability to treat and bill for services provided to patients covered under government programs, it could cause us to incur write-offs of investments or accelerate the recognition of lease obligations in the event we have to close centers or our centers’ operating performance deteriorates, and it could have an adverse effect on our business, results of operations and financial condition. Although the BBA passed in February 2018 allows for organizations approved by the Department of Health and Human Services (HHS) to accredit dialysis facilities and imposes certain timing requirements regarding the initiation of initial surveys to determine if certain conditions and requirements for payment have been satisfied, the ultimate impact of these changes cannot be predicted. In addition to certifications for Medicare and Medicaid, some states have licensing requirements for ESRD facilities. Delays in licensure, denials of licensure, or withdrawal of licensure could also adversely affect our business, results of operations and financial condition.
49
If our joint ventures were found to violate the law, we could suffer severe consequences that would have a material adverse effect on our business, results of operations and financial condition.
As of December 31, 2017, we owned a controlling interest in numerous dialysis-related joint ventures, which represented approximately 24% of our net U.S. dialysis and related lab services revenues for the year ended December 31, 2017. In addition, we also owned noncontrolling equity investments in several other dialysis related joint ventures. We may continue to increase the number of our joint ventures. Many of our joint ventures with physicians or physician groups also have certain physician owners providing medical director services to centers we own and operate. Because our relationships with physicians are governed by the federal and state anti-kickback statutes, we have sought to structure our joint venture arrangements to satisfy as many federal safe harbor requirements as we believe are commercially reasonable. However, although our joint venture arrangements do not satisfy all of the elements of any safe harbor under the federal Anti-Kickback Statute, they are not automatically prohibited under the federal Anti-Kickback Statute but are susceptible to government scrutiny. For example, in October 2014, we entered into a Settlement Agreement with the United States and relator David Barbetta to resolve the then pending 2010 and 2011 U.S. Attorney physician relationship investigations regarding certain of our joint ventures and paid $406 million in settlement amounts, civil forfeiture, and interest to the United States and certain states. For further details, see "If we fail to comply with our Corporate Integrity Agreement, we could be subject to substantial penalties and exclusion from participation in federal healthcare programs that could have a material adverse effect on our business, results of operations and financial condition".
There are significant risks associated with estimating the amount of dialysis revenues and related refund liabilities that we recognize, and if our estimates of revenues and related refund liabilities are materially inaccurate, it could impact the timing and the amount of our revenues recognition or have a material adverse effect on our business, results of operations and financial condition.
There are significant risks associated with estimating the amount of U.S. dialysis and related lab services revenues and related refund liabilities that we recognize in a reporting period. The billing and collection process is complex due to ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage and other payor issues. Determining applicable primary and secondary coverage for approximately 197,800 U.S. patients at any point in time, together with the changes in patient coverage that occur each month, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. Revenues associated with Medicare and Medicaid programs are also subject to estimating risk related to the amounts not paid by the primary government payor that will ultimately be collectible from other government programs paying secondary coverage, the patient’s commercial health plan secondary coverage or the patient. Collections, refunds and payor retractions typically continue to occur for up to three years and longer after services are provided. We generally expect our range of U.S. dialysis and related lab services revenues estimating risk to be within 1% of net revenues for the segment. If our estimates of U.S. dialysis and related lab services revenues and related refund liabilities are materially inaccurate, it could impact the timing and the amount of our revenues recognition and have a material adverse impact on our business, results of operations and financial condition.
Our ancillary services and strategic initiatives, including our pharmacy services and our international operations, that we invest in now or in the future may generate losses and may ultimately be unsuccessful. In the event that one or more of these activities is unsuccessful, our business, results of operations and financial condition may be negatively impacted and we may have to write off our investment and incur other exit costs.
Our ancillary services and strategic initiatives are subject to many of the same risks, regulations and laws, as described in the risk factors related to our dialysis business set forth in this Part I, Item 1A, and are also subject to additional risks, regulations and laws specific to the nature of the particular strategic initiative. We expect to add additional service offerings to our business and pursue additional strategic initiatives in the future as circumstances warrant, which could include healthcare services not related to dialysis. Many of these initiatives require or would require investments of both management and financial resources and can generate significant losses for a substantial period of time and may not become profitable in the expected timeframe or at all. There can be no assurance that any such strategic initiative will ultimately be successful. Any significant change in market conditions, or business performance, or in the political, legislative or regulatory environment, may impact the economic viability of any of these strategic initiatives.
If any of our ancillary services or strategic initiatives, including our pharmacy services and our international operations, are unsuccessful, it would have a negative impact on our business, results of operations and financial condition, and we may determine to exit that line of business. We could incur significant termination costs if we were to exit certain of these lines of business. In addition, we may incur a material write-off or an impairment of our investment, including goodwill, in one or more of our ancillary services or strategic initiatives. In that regard, we have taken, and may in the future take, impairment charges related to our ancillary services and strategic initiatives, including in our international and pharmacy businesses.
50
If a significant number of physicians were to cease referring patients to our dialysis centers, whether due to regulatory or other reasons, it would have a material adverse effect on our business, results of operations and financial condition.
Physicians, including medical directors, choose where they refer their patients. We believe that physicians prefer to have their patients treated at dialysis centers where they or other members of their practice supervise the overall care provided as medical director of the center. As a result, the primary referral source for most of our centers is often the physician or physician group providing medical director services to the center.
Our medical director contracts are for fixed periods, generally ten years, and at any given time a large number of them could be up for renewal at the same time. Medical directors have no obligation to extend their agreements with us and if we are unable to enforce noncompetition provisions contained in terminated medical director agreements, our former medical directors may choose to provide medical director services for competing providers or establish their own dialysis centers in competition with ours. Neither our current nor former medical directors have an obligation to refer their patients to our centers.
The aging of the nephrologist population and opportunities presented by our competitors may negatively impact a medical director’s decision to enter into or extend his or her agreement with us. Moreover, different affiliation models in the changing healthcare environment that limit a nephrologist’s choice in where he or she can refer patients, such as an increase in the number of physicians becoming employed by hospitals or a perceived decrease in the quality of service levels at our centers, may limit a nephrologist’s ability or desire to refer patients to our centers or otherwise negatively impact treatment volumes.
In addition, we may take actions to restructure existing relationships or take positions in negotiating extensions of relationships to assure compliance with the federal Anti-Kickback Statute, Stark Law and other similar laws. If the terms of any existing agreement are found to violate applicable laws, we may not be successful in restructuring the relationship, which could lead to the early termination of the agreement. These actions, in an effort to comply with applicable laws and regulations, could negatively impact the decision of physicians to extend their medical director agreements with us. If a significant number of physicians were to cease referring patients to our dialysis centers, it would have a material adverse effect on our business, results of operations and financial condition.
If there are shortages of skilled clinical personnel, or if changes to state staffing ratios are implemented with which we are required to comply, we may experience disruptions in our business operations and increases in operating expenses, among other things, which could have a material adverse effect on our business, results of operations and financial condition.
We face increasing labor costs generally, and in particular, face increased labor costs and difficulties in hiring nurses due to a nationwide shortage of skilled clinical personnel. We compete for nurses with hospitals and other healthcare providers. This nursing shortage may limit our ability to expand our operations. Furthermore, changes in certification requirements can impact our ability to maintain sufficient staff levels, including to the extent our teammates are not able to meet new requirements, among other things. In addition, if we experience a higher than normal turnover rate for our skilled clinical personnel, our operations and treatment growth may be negatively impacted, which could adversely affect our business, results of operations and financial condition.
In addition, currently pending and future proposed ballot initiatives or referendums, legislation or policy changes could cause us to incur substantial costs to challenge and, if implemented, impose additional requirements on our operations, including increases in the required staffing levels or staffing ratios for clinical personnel, minimum transition times between treatments, limits on how much patients may be charged for care, limitations as to the amount that can be spent on certain medical costs, and a ceiling on the percent of profit for such care. Changes such as these mandated by currently pending and future ballot initiatives or referendums, legislation or policy changes would likely materially reduce our revenues and increase our operating expense and impact our ability to staff our clinics to the new, elevated staffing levels, in particular given the ongoing nationwide shortage of healthcare workers, especially nurses. Any of these events or circumstances could materially reduce our revenues and increase our operating and other costs, require us to close dialysis centers or reduce shifts, and could have a material adverse effect on our employee relations, treatment growth, productivity, business, results of operations and financial condition.
Our business is labor intensive and could be materially adversely affected if we are unable to maintain satisfactory relations with our employees or if union organizing activities or legislative changes result in significant increases in our operating costs or decreases in productivity.
Our business is labor intensive, and our financial and operating results have been and continue to be subject to variations in labor-related costs, productivity and the number of pending or potential claims against us related to labor and employment
51
practices. Political efforts at the national or local level could result in actions or proposals that increase the likelihood or success of union organizing activities at our facilities and union organizing activities could increase for other reasons. Labor and employment claims, including the filing of class action suits, or work stoppages, wages and benefits or adverse outcomes of these types of claims could trend upwards. Any of these events or circumstances could have a material adverse effect on our employee relations, treatment growth, productivity, business, results of operations and financial condition.
Complications associated with our billing and collections system could materially adversely affect our business, results of operations and financial condition.
Our billing system is critical to our billing operations. If there are defects in the billing system, we may experience difficulties in our ability to successfully bill and collect for services rendered, including a delay in collections, a reduction in the amounts collected, increased risk of retractions from and refunds to commercial and government payors, an increase in our provision for uncollectible accounts receivable and noncompliance with reimbursement regulations, any or all of which could materially adversely affect our results of operations.
Risk factors primarily related to DMG:
DMG is subject to many of the same risks to which our dialysis business is subject.
As a participant in the healthcare industry, DMG is subject to many of the same risks as our dialysis business is, as described in the risk factors set forth above in this Part I, Item 1A, any of which could have a material adverse effect on DMG’s business, results of operations and financial condition.
Under most of DMG’s agreements with health plans, DMG assumes some or all of the risk that the cost of providing services will exceed its compensation.
Approximately 83% of DMG’s revenue for the year ended December 31, 2017 is derived from fixed per member per month (PMPM) fees paid by health plans under capitation agreements with DMG or its associated physician groups. While there are variations specific to each arrangement, DMG, through DaVita Health Plan of California, Inc. (DHPC), a subsidiary of HealthCare Partners Holdings, LLC and a restricted Knox-Keene licensed entity, and, in certain instances, DMG’s associated physician groups generally contract with health plans to receive a PMPM fee for professional services and assume the financial responsibility for professional services only. In some cases, the health plans separately enter into capitation contracts with third parties (typically hospitals) who receive directly a PMPM fee and assume contractual financial responsibility for hospital services. In other cases, the health plan does not pay any portion of the PMPM fee to the hospital, but rather administers claims for hospital expenses itself. In both scenarios, DMG enters into managed care-related administrative services agreements or similar arrangements with those third parties (typically hospitals) under which DMG agrees to be responsible for utilization review, quality assurance, and other managed care-related administrative functions and claim payments. As compensation for such administrative services, DMG is entitled to receive a percentage of the amount by which the institutional capitation revenue received from health plans exceeds institutional expenses; any such risk-share amount to which DMG is entitled is recorded as medical revenues, and DMG is also responsible for a percentage of any short-fall in the event that institutional expenses exceed institutional revenues. To the extent that members require more care than is anticipated and/or the cost of care increases, aggregate fixed PMPM amounts, or capitation payments, may be insufficient to cover the costs associated with treatment. If medical costs and expenses exceed estimates, except in very limited circumstances, DMG will not be able to increase the PMPM fee received under these risk agreements during their then-current terms and could, directly or indirectly through its contracts with its associated physician groups, suffer losses with respect to such agreements.
Changes in DMG’s or its associated physician groups’ anticipated ratio of medical expense to revenue can significantly impact DMG’s financial results. Accordingly, the failure to adequately predict and control medical costs and expenses and to make reasonable estimates and maintain adequate accruals for incurred but not reported claims, could have a material adverse effect on DMG’s business, results of operations and financial condition.
Historically, DMG’s and its associated physician groups’ medical costs and expenses as a percentage of revenue have fluctuated. Factors that may cause medical expenses to exceed estimates include:
• | the health status of members; |
• | higher than expected utilization of new or existing healthcare services or technologies; |
• | an increase in the cost of healthcare services and supplies, including pharmaceuticals, whether as a result of inflation or otherwise; |
52
• | changes to mandated benefits or other changes in healthcare laws, regulations and practices; |
• | periodic renegotiation of provider contracts with specialist physicians, hospitals and ancillary providers; |
• | periodic renegotiation of contracts with DMG’s affiliated primary care physicians and specialists; |
• | changes in the demographics of the participating members and medical trends; |
• | contractual or claims disputes with providers, hospitals or other service providers within a health plan’s network; |
• | the occurrence of catastrophes, major epidemics or acts of terrorism; and |
• | the reduction of health plan premiums. |
Risk-sharing arrangements that DMG and its associated physician groups have with health plans and hospitals could result in their costs exceeding the corresponding revenues, which could reduce or eliminate any shared risk profitability.
Most of the agreements between health plans and DMG and its associated physician groups contain risk-sharing arrangements under which the physician groups can earn additional compensation from the health plans by coordinating the provision of quality, cost-effective healthcare to members. However, such arrangements may require the physician group to assume a portion of any loss sustained from these arrangements, thereby reducing DMG’s net income. Under these risk-sharing arrangements, DMG and its associated physician groups are responsible for a portion of the cost of hospital services or other services that are not capitated. The terms of the particular risk-sharing arrangement allocate responsibility to the respective parties when the cost of services exceeds the related revenue, which results in a deficit, or permit the parties to share in any surplus amounts when actual costs are less than the related revenue. The amount of non-capitated medical and hospital costs in any period could be affected by factors beyond the control of DMG, such as changes in treatment protocols, new technologies, longer lengths of stay by the patient and inflation. Certain of DMG’s agreements with health plans stipulate that risk-sharing pool deficit amounts are carried forward to offset any future years’ surplus amounts DMG would otherwise be entitled to receive. DMG accrues for any such risk-sharing deficits. To the extent that such non-capitated medical and hospital costs are higher than anticipated, revenue may not be sufficient to cover the risk-sharing deficits the health plans and DMG are responsible for, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
Renegotiation, renewal or termination of capitation agreements with health plans could have a material adverse effect on DMG’s business, results operations and financial condition.
Under most of DMG’s and its associated physician groups’ capitation agreements with health plans, the health plan is generally permitted to modify the benefit and risk obligations and compensation rights from time to time during the terms of the agreements. If a health plan exercises its right to amend its benefit and risk obligations and compensation rights, DMG and its associated physician groups are generally allowed a period of time to object to such amendment. If DMG or its associated physician group so objects, under some of the risk agreements, the relevant health plan may terminate the applicable agreement upon 90 to 180 days written notice. If DMG or its associated physician groups enter into capitation contracts or other risk sharing arrangements with unfavorable economic terms, or a capitation contract is amended to include unfavorable terms, DMG could, directly or indirectly through its contracts with its associated physician groups, suffer losses with respect to such contract. Since DMG does not negotiate with CMS or any health plan regarding the benefits to be provided under their Medicare Advantage plans, DMG often has just a few months to familiarize itself with each new annual package of benefits it is expected to offer. Depending on the health plan at issue and the amount of revenue associated with the health plan’s risk agreement, the renegotiated terms or termination could have a material adverse effect on DMG’s business, results of operations and financial condition.
If DMG’s agreements or arrangements with any physician equity holder(s) of associated physicians, physician groups or IPAs are deemed invalid under state law, including laws against the corporate practice of medicine, or federal law, or are terminated as a result of changes in state law, or if there is a change in accounting standards by the Financial Accounting Standards Board (FASB) or the interpretation thereof affecting consolidation of entities, it could have a material adverse effect on DMG’s consolidation of total revenues derived from such associated physician groups.
DMG’s financial statements are consolidated in accordance with applicable accounting standards and include the accounts of its majority-owned subsidiaries and certain non-owned DMG-associated and managed physician groups. Such consolidation for accounting and/or tax purposes does not, is not intended to, and should not be deemed to, imply or provide to DMG any control over the medical or clinical affairs of such physician groups. In the event of a change in accounting standards promulgated by FASB or in interpretation of its standards, or if there is an adverse determination by a regulatory agency or a court, or a change in state or federal law relating to the ability to maintain present agreements or arrangements with such
53
physician groups, DMG may not be permitted to continue to consolidate the total revenues of such organizations. A change in accounting for consolidation with respect to DMG’s present agreement or arrangements would diminish DMG’s reported revenues but would not be expected to materially and adversely affect its reported results of operations, while regulatory or legal rulings or changes in law interfering with DMG’s ability to maintain its present agreements or arrangements could materially diminish both revenues and results of operations.
If DHPC is not able to satisfy financial solvency or other regulatory requirements, we could become subject to sanctions and its license to do business in California could be limited, suspended or terminated, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
Knox-Keene requires healthcare service plans operating in California to comply with financial solvency and other requirements overseen by the California Department of Managed HealthCare (DMHC). Under Knox-Keene, DHPC is required to, among other things:
• | Maintain, at all times, a minimum tangible net equity (TNE); |
• | Submit periodic financial solvency reports to the DMHC containing various data regarding performance and financial solvency; |
• | Comply with extensive regulatory requirements; and |
• | Submit to periodic regulatory audits and reviews concerning DHPC operations and compliance with Knox-Keene. |
In the event that DHPC is not in compliance with the provisions of Knox-Keene, we could be subject to sanctions, or limitations on, or suspension of its license to do business in California, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
If DMG’s associated physician group is not able to satisfy the California DMHC’s financial solvency requirements, DMG’s associated physician group could become subject to sanctions and DMG’s ability to do business in California could be limited or terminated, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
The California DMHC has instituted financial solvency regulations to monitor the financial solvency of capitated physician groups. Under these regulations, DMG’s associated physician group is required to, among other things:
• | Maintain, at all times, a minimum cash-to-claims ratio (where cash-to-claims ratio means the organization’s cash, marketable securities and certain qualified receivables, divided by the organization’s total unpaid claims liability). The regulation currently requires a cash-to-claims ratio of 0.75. |
• | Submit periodic reports to the California DMHC containing various data and attestations regarding performance and financial solvency, including incurred but not reported calculations and documentation, and attestations as to whether or not the organization was in compliance with Knox-Keene requirements related to claims payment timeliness, had maintained positive TNE (i.e., at least $1.00) and had maintained positive working capital (i.e., at least $1.00). |
In the event that DMG’s associated physician group is not in compliance with any of the above criteria, DMG’s associated physician group could be subject to sanctions, or limitations on, or removal of, its ability to do business in California, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
Reductions in Medicare Advantage health plan reimbursement rates stemming from recent healthcare reforms and any future related regulations could have a material adverse effect on DMG’s business, results of operations and financial condition.
A significant portion of DMG’s revenue is directly or indirectly derived from the monthly premium payments paid by CMS to health plans for medical services provided to Medicare Advantage enrollees. As a result, DMG’s results of operations are, in part, dependent on government funding levels for Medicare Advantage programs. Any changes that limit or reduce Medicare Advantage reimbursement levels, such as reductions in or limitations of reimbursement amounts or rates under programs, reductions in funding of programs, expansion of benefits without adequate funding, elimination of coverage for certain benefits, or elimination of coverage for certain individuals or treatments under programs, could have a material adverse effect on DMG’s business, results of operations and financial condition.
54
Each year, CMS issues a final rule to establish the Medicare Advantage benchmark payment rates for the following calendar year. Any reduction to Medicare Advantage rates impacting DMG that is greater compared to the industry average rate may have material adverse effect on DMG’s business, results of operations and financial condition. The final impact of the Medicare Advantage rates can vary from any estimate we may have and may be further impacted by the relative growth of DMG’s Medicare Advantage patient volumes across markets as well as by the benefit plan designs submitted. It is possible that we may underestimate the impact of the Medicare Advantage rates on our business, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
We took impairment charges against the goodwill of several of our DMG reporting units in five of the nine quarters since the fourth quarter of 2015 based on continuing developments in our DMG business, including recent annual updates to Medicare Advantage benchmark reimbursement rates, changes in our expectations concerning future government reimbursement rates and our expected ability to mitigate them, medical cost and utilization trends, commercial pricing pressures, commercial membership rates, underperformance of certain at-risk reporting units and other market factors. We may also need to take additional impairment charges against earnings in a future period, depending on the impact of continuing developments on the value of our DMG business. Specifically, if DMG’s fair value less the costs incurred in the sale of DMG falls below its carrying amount, we may need to recognize additional impairment charges on this business, and the amount of such charges, if any, could be significant. Our estimates of the fair value of this business rely on certain estimates and assumptions, including the terms and pricing agreed for the sale of this business, as well as applicable market multiples, discount and long-term growth rates, market data and future reimbursement rates, as applicable. Our estimates of the fair value of the DMG business could differ from the actual value that a market participant would pay for this business.
DMG’s Medicare Advantage revenues may continue to be volatile in the future, which could have a material adverse impact on DMG’s business, results of operations and financial condition.
The ACA contains a number of provisions that negatively impact Medicare Advantage plans, each of which could have a material adverse effect on DMG’s business, results of operations and financial condition. These provisions include the following:
• | Medicare Advantage benchmarks for 2011 were frozen at 2010 levels. From 2012 through 2016, Medicare Advantage benchmark rates were phased down from prior levels. The new benchmarks were fully phased-in in 2017 and range between 95% and 115% of the Medicare FFS costs, depending on a plan’s geographic area. If our costs escalate faster than can be absorbed by the level of revenues implied by these benchmark rates, then it could have a material adverse effect on DMG’s business and results of operations. |
• | Rebates received by Medicare Advantage plans that were reduced, with larger reductions for plans failing to receive certain quality ratings. |
• | The Secretary of the HHS has been granted the explicit authority to deny Medicare Advantage plan bids that propose significant increases in cost sharing or decreases in benefits. If the bids submitted by plans contracted with DMG are denied, this could have a material adverse effect on DMG’s business and results of operations. |
• | Medicare Advantage plans with medical loss ratios below 85% are required to pay a rebate to the Secretary of HHS. The rebate amount is the total revenue under the contract year multiplied by the difference between 85% and the plan’s actual medical loss ratio. The Secretary of HHS will halt enrollment in any plan failing to meet this ratio for three consecutive years, and terminate any plan failing to meet the ratio for five consecutive years. If a DMG-contracting Medicare Advantage plan experiences a limitation on enrollment or is otherwise terminated from the Medicare Advantage program, it could have a material adverse effect on DMG’s business and results of operations. |
• | Prescription drug plans are required to provide coverage of certain drug categories on a list developed by the Secretary of HHS, which could increase the cost of providing care to Medicare Advantage enrollees, and thereby reduce DMG’s revenues and earnings. The Medicare Part D premium amount subsidized for high-income beneficiaries has been reduced, which could lower the number of Medicare Advantage enrollees, which would have a negative impact on DMG’s business and results of operations. |
• | CMS increased coding intensity adjustments for Medicare Advantage plans beginning in 2014 and continuing through 2018, which reduces CMS payments to Medicare Advantage plans, which in turn will likely reduce the amounts payable to DMG and its associated physicians, physician groups, and IPAs under its capitation agreements. |
55
Recent legislative and executive efforts to enact further healthcare reform legislation have caused the future state of the exchanges, other ACA reforms, and many core aspects of the current U.S. health care system to be unclear. For example, in October 2017, the federal government announced that cost-sharing reduction payments to insurers would end, effective immediately, unless Congress appropriated the funds, and, in December 2017, Congress passed the Tax Cuts and Jobs Act, which includes a provision that eliminates the penalty under the ACA’s individual mandate and could impact the future state of the exchanges. Further, in February 2018, Congress passed the BBA which, among other things, repealed the Independent Payment Advisory Board that was established by the ACA and intended to reduce the rate of growth in Medicare spending. While certain provisions of the BBA may increase the scope of benefits available for certain chronically ill Federal health care program beneficiaries beginning in 2020, the ultimate impact of such changes cannot be predicted. While specific changes and their timing are not yet apparent, enacted reforms and future legislative, regulatory, or executive changes could have a material adverse effect on DMG’s business, results of operations and financial condition.
There is also uncertainty regarding both Medicare Advantage payment rates and beneficiary enrollment, which, if reduced, would reduce DMG’s overall revenues and net income. For example, although the Congressional Budget Office (CBO) predicted in 2010 that Medicare Advantage participation would drop substantially by 2020, the CBO has more recently predicted, without taking into account potential future reforms, that enrollment in Medicare Advantage (and other contracts covering Medicare Parts A and B) could reach 31 million by 2027. Although Medicare Advantage enrollment increased by approximately 5.6 million, or by 50%, between the enactment of the ACA in 2010 and 2015, there can be no assurance that this trend will continue. Further, fluctuation in Medicare Advantage payment rates are evidenced by CMS’s annual announcement of the expected average change in revenue from the prior year: for 2017, CMS announced an average increase of 0.85%; and for 2018, 0.45%. Uncertainty over Medicare Advantage enrollment and payment rates present a continuing risk to DMG’s business.
According to the Kaiser Family Foundation (KFF), Medicare Advantage enrollment continues to be highly concentrated among a few payors, both nationally and in local regions. In 2017, the KFF reported that three payors together accounted for more than half of Medicare Advantage enrollment and eight firms accounted for approximately 75% of the lives. In 441 counties in 2018, only one company will offer Medicare Advantage plans. Consolidation among Medicare Advantage plans in certain regions, or the Medicare program’s failure to attract additional plans to participate in the Medicare Advantage program, could have a material adverse effect on DMG’s business, results of operations and financial condition.
DMG’s operations are dependent on competing health plans and, at times, a health plan’s and DMG’s economic interests may diverge.
For the year ended December 31, 2017, 68% of DMG’s consolidated capitated medical revenues were earned through contracts with three health plans.
DMG expects that, going forward, substantially all of its revenue will continue to be derived from its contracts with health plans. Each health plan may immediately terminate any of DMG’s contracts and/or any individual credentialed physician upon the occurrence of certain events. They may also amend the material terms of the contracts under certain circumstances. Failure to maintain the contracts on favorable terms, for any reason, would materially and adversely affect DMG’s results of operations and financial condition. A material decline in the number of members could also have a material adverse effect on DMG’s results of operations.
Notwithstanding each health plan’s and DMG’s current shared interest in providing service to DMG’s members who are enrolled in the subject health plans, the health plans may have different and, at times, opposing economic interests from those of DMG. The health plans provide a wide range of health insurance services across a wide range of geographic regions, utilizing a vast network of providers. As a result, they and DMG may have different views regarding the proper pricing of services and/or the proper pricing of the various service providers in their provider networks, the cost of which DMG bears to the extent that the services of such service providers are utilized. These health plans may also have different views than DMG regarding the efforts and expenditures that they, DMG, and/or other service providers should make to achieve and/or maintain various quality ratings. In addition, several health plans have acquired or announced their intent to acquire provider organizations. If health plans with which DMG contracts acquire a significant number of provider organizations, they may not continue to contract with DMG or contract on less favorable terms or seek to prevent DMG from acquiring or entering into arrangements with certain providers. Similarly, as a result of changes in laws, regulations, consumer preferences, or other factors, the health plans may find it in their best interest to provide health insurance services pursuant to another payment or reimbursement structure. In the event DMG’s interests diverge from the interests of the health plans, DMG may have limited recourse or alternative options in light of its dependence on these health plans. There can be no assurances that DMG will continue to find it mutually beneficial to work with these health plans. As a result of various restrictive provisions that appear in some of the managed care agreements with health plans, DMG may at times have limitations on its ability to cancel an
56
agreement with a particular health plan and immediately thereafter contract with a competing health plan with respect to the same service area.
DMG and its associated physicians, physician groups and IPAs and other physicians may be required to continue providing services following termination or renegotiation of certain agreements with health plans.
There are circumstances under federal and state law pursuant to which DMG and its associated physician groups, IPAs and other physicians could be obligated to continue to provide medical services to DMG members in their care following a termination of their applicable risk agreement with health plans and termination of the receipt of payments thereunder. In certain cases, this obligation could require the physician group or IPA to provide care to such member following the bankruptcy or insolvency of a health plan. Accordingly, the obligations to provide medical services to DMG members (and the associated costs) may not terminate at the time the applicable agreement with the health plan terminates, and DMG may not be able to recover its cost of providing those services from the health plan, which could have a material adverse effect on DMG’s business, results of operations and financial condition.
DMG operates primarily in California, Florida, Nevada, New Mexico, Washington and Colorado and may not be able to successfully establish a presence in new geographic regions.
DMG derives substantially all of its revenue from operations in California, Florida, Nevada, New Mexico, Washington and Colorado (which we refer to as the Existing Geographic Regions). As a result, DMG’s exposure to many of the risks described herein is not mitigated by a greater diversification of geographic focus. Furthermore, due to the concentration of DMG’s operations in the Existing Geographic Regions, it may be adversely affected by economic conditions, natural disasters (such as earthquakes or hurricanes), or acts of war or terrorism that disproportionately affect the Existing Geographic Regions as compared to other states and geographic markets.
To expand the operations of its network outside of the Existing Geographic Regions, DMG must devote resources to identify and explore perceived opportunities. Thereafter, DMG must, among other things, recruit and retain qualified personnel, develop new offices, establish potential new relationships with one or more health plans, and establish new relationships with physicians and other healthcare providers. The ability to establish such new relationships may be significantly inhibited by competition for such relationships and personnel in the healthcare marketplace in the targeted new geographic regions. Additionally, DMG may face the risk that a substantial portion of the patients served in a new geographic area may be enrolled in a Medicare FFS program and will not desire to transition to a Medicare Advantage program, such as those offered through the health plans that DMG serves, or they may enroll with other health plans with whom DMG does not contract to receive services, which could reduce substantially DMG’s perceived opportunity in such geographic area. In addition, if DMG were to seek to expand outside of the Existing Geographic Regions, DMG would be required to comply with laws and regulations of states that may differ from the ones in which it currently operates, and could face competitors with greater knowledge of such local markets. DMG anticipates that any geographic expansion may require it to make a substantial investment of management time, capital and/or other resources. There can be no assurance that DMG will be able to establish profitable operations or relationships in any new geographic markets.
Reductions in the quality ratings of the health plans DMG serves could have a material adverse effect on its business, results of operations and financial condition.
As a result of the ACA, the level of reimbursement each health plan receives from CMS is dependent, in part, upon the quality rating of the Medicare plan. Such ratings impact the percentage of any cost savings rebate and any bonuses earned by such health plan. Since a significant portion of DMG’s revenue is expected to be calculated as a percentage of CMS reimbursements received by these health plans with respect to DMG members, reductions in the quality ratings of a health plan that DMG serves could have a material adverse effect on its business, results of operations and financial condition.
Given each health plan’s control of its plans and the many other providers that serve such plans, DMG believes that it will have limited ability to influence the overall quality rating of any such plan. The BBA passed in February 2018 implements certain changes to prevent artificial inflation of star ratings for Medicare Advantage plans offered by the same organization. In addition, CMS has terminated plans that have had a rating of less than three stars for three consecutive years, whereas Medicare Advantage plans with five stars are permitted to conduct enrollment throughout almost the entire year. Although CMS’ authority to terminate plans solely for failing to achieve the minimum quality star ratings has been suspended through the end of plan year 2018, low quality ratings can still potentially lead to the termination of a plan that DMG serves, DMG may not be able to prevent the potential termination of a contracting plan or a shift of patients to other plans based upon quality issues which could, in turn, have a material adverse effect on DMG’s business, results of operations and financial condition.
57
DMG’s records and submissions to a health plan may contain inaccurate or unsupportable information regarding risk adjustment scores of members, which could cause DMG to overstate or understate its revenue and subject it to various penalties.
DMG, on behalf of itself and its associated physicians, physician groups and IPAs, submits to health plans claims and encounter data that support the Medicare Risk Adjustment Factor (RAF) scores attributable to members. These RAF scores determine, in part, the revenue to which the health plans and, in turn, DMG is entitled for the provision of medical care to such members. The data submitted to CMS by each health plan is based, in part, on medical charts and diagnosis codes prepared and submitted by DMG. Each health plan generally relies on DMG and its employed or affiliated physicians to appropriately document and support such RAF data in DMG’s medical records. Each health plan also relies on DMG and its employed or affiliated physicians to appropriately code claims for medical services provided to members. Erroneous claims and erroneous encounter records and submissions could result in inaccurate PMPM fee revenue and risk adjustment payments, which may be subject to correction or retroactive adjustment in later periods. This corrected or adjusted information may be reflected in financial statements for periods subsequent to the period in which the revenue was recorded. DMG might also need to refund a portion of the revenue that it received, which refund, depending on its magnitude, could damage its relationship with the applicable health plan and could have a material adverse effect on DMG’s business, results of operations and financial condition.
In June 2015, we received a subpoena from the OIG requesting information relating to our and our subsidiaries’ (including DMG’s and its subsidiary JSA’s) provision of services to Medicare Advantage plans and related patient diagnosis coding and risk adjustment submissions and payments. See "Item 3. Legal Proceedings" in Part I of this report and Note 16 to the consolidated financial statements included in this report for further details and discussions of legal proceedings elsewhere in these Risk Factors.
Additionally, CMS audits Medicare Advantage plans for documentation to support RAF-related payments for members chosen at random. The Medicare Advantage plans ask providers to submit the underlying documentation for members that they serve. It is possible that claims associated with members with higher RAF scores could be subject to more scrutiny in a CMS or plan audit. There is a possibility that a Medicare Advantage plan may seek repayment from DMG should CMS make any payment adjustments to the Medicare Advantage plan as a result of its audits. The plans also may hold DMG liable for any penalties owed to CMS for inaccurate or unsupportable RAF scores provided by DMG. In addition, DMG could be liable for penalties to the government under the FCA that range from $5,500 to $11,000 (adjusted for inflation) for each false claim, plus up to three times the amount of damages caused by each false claim, which can be as much as the amounts received directly or indirectly from the government for each such false claim. On February 3, 2017, the DOJ issued a final rule announcing adjustments to FCA penalties, under which the per claim penalty range increases from $10,957 to $21,916 for penalties assessed after February 3, 2017, so long as the underlying conduct occurred after November 2, 2015.
CMS has indicated that payment adjustments will not be limited to RAF scores for the specific Medicare Advantage enrollees for which errors are found but may also be extrapolated to the entire Medicare Advantage plan subject to a particular CMS contract. CMS has described its audit process as plan-year specific and stated that it will not extrapolate audit results for plan years prior to 2011. Because CMS has not stated otherwise, there is a risk that payment adjustments made as a result of one plan year’s audit would be extrapolated to prior plan years after 2011.
There can be no assurance that a health plan will not be randomly selected or targeted for review by CMS or that the outcome of such a review will not result in a material adjustment in DMG’s revenue and profitability, even if the information DMG submitted to the plan is accurate and supportable.
Separately, as described in further detail in "Item 3. Legal Proceedings" in Part I of this report and Note 16 to the consolidated financial statements included in this report, on March 13, 2015, JSA, a subsidiary of DMG, received a subpoena from the OIG that relates, in part, to risk adjustment practices and data. See also discussions of legal proceedings elsewhere in these Risk Factors.
A failure to accurately estimate incurred but not reported medical expense could adversely affect DMG’s results of operations.
Patient care costs include estimates of future medical claims that have been incurred by the patient but for which the provider has not yet billed DMG. These claim estimates are made utilizing actuarial methods and are continually evaluated and adjusted by management, based upon DMG’s historical claims experience and other factors, including an independent assessment by a nationally recognized actuarial firm. Adjustments, if necessary, are made to medical claims expense and capitated revenues when the assumptions used to determine DMG’s claims liability changes and when actual claim costs are ultimately determined.
58
Due to the inherent uncertainties associated with the factors used in these estimates and changes in the patterns and rates of medical utilization, materially different amounts could be reported in DMG’s financial statements for a particular period under different conditions or using different, but still reasonable, assumptions. It is possible that DMG’s estimates of this type of claim may be inadequate in the future. In such event, DMG’s results of operations could be adversely impacted. Further, the inability to estimate these claims accurately may also affect DMG’s ability to take timely corrective actions, further exacerbating the extent of any adverse effect on DMG’s results of operations.
DMG faces certain competitive threats which could reduce DMG’s profitability and increase competition for patients.
DMG faces certain competitive threats based on certain features of the Medicare programs, including the following:
• | As a result of the direct and indirect impacts of the ACA, many Medicare beneficiaries may decide that an original Medicare FFS program is more attractive than a Medicare Advantage plan. As a result, enrollment in the health plans DMG serves may decrease. |
• | Managed care companies offer alternative products such as regional preferred provider organizations (PPOs) and private FFS plans. Medicare PPOs and private FFS plans allow their patients more flexibility in selecting physicians than Medicare Advantage health plans, which typically require patients to coordinate care with a primary care physician. The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 has encouraged the creation of regional PPOs through various incentives, including certain risk corridors, or cost reimbursement provisions, a stabilization fund for incentive payments, and special payments to hospitals not otherwise contracted with a Medicare Advantage plan that treat regional plan enrollees. The formation of regional Medicare PPOs and private FFS plans may affect DMG’s relative attractiveness to existing and potential Medicare patients in their service areas. |
• | The payments for the local and regional Medicare Advantage plans are based on a competitive bidding process that may indirectly cause a decrease in the amount of the PMPM fee or result in an increase in benefits offered. |
• | The annual enrollment process and subsequent lock-in provisions of the ACA may adversely affect DMG’s level of revenue growth as it will limit the ability of a health plan to market to and enroll new Medicare beneficiaries in its established service areas outside of the annual enrollment period. |
• | CMS allows Medicare beneficiaries who are enrolled in a Medicare Advantage plan with a quality rating of 4.5 stars or less to enroll in a 5-star rated Medicare Advantage plan at any time during the benefit year. Therefore, DMG may face a competitive disadvantage in recruiting and retaining Medicare beneficiaries. |
In addition to the competitive threats intrinsic to the Medicare programs, competition among health plans and among healthcare providers may also have a negative impact on DMG’s profitability. For example, due to the large population of Medicare beneficiaries, DMG’s Existing Geographic Regions have become increasingly attractive to health plans that may compete with DMG. DMG may not be able to continue to compete profitably in the healthcare industry if additional competitors enter the same market. If DMG cannot compete profitably, the ability of DMG to compete with other service providers that contract with competing health plans may be substantially impaired. Furthermore, if DMG is unable to obtain new members or experiences a loss of existing members to competitors during the open enrollment period for Medicare it could have a material adverse effect on DMG’s business, results of operations and financial condition.
DMG competes directly with various regional and local companies that provide similar services in DMG’s Existing Geographic Regions. DMG’s competitors vary in size and scope and in terms of products and services offered. DMG believes that some of its competitors and potential competitors may be significantly larger than DMG and have greater financial, sales, marketing and other resources. Furthermore, it is DMG’s belief that some of its competitors may make strategic acquisitions or establish cooperative relationships among themselves.
A disruption in DMG’s healthcare provider networks could have a material adverse effect on DMG’s operations and profitability.
In any particular service area, healthcare providers or provider networks could refuse to contract with DMG, demand higher payments, or take other actions that could result in higher healthcare costs, disruption of benefits to DMG’s members, or difficulty in meeting applicable regulatory or accreditation requirements. In some service areas, healthcare providers or provider networks may have significant market positions. If healthcare providers or provider networks refuse to contract with DMG, use their market position to negotiate favorable contracts, or place DMG at a competitive disadvantage, then DMG’s ability to market or to be profitable in those service areas could be adversely affected. DMG’s provider networks could also be
59
disrupted by the financial insolvency of a large provider group. Any disruption in DMG’s provider networks could result in a loss of members or higher healthcare costs.
DMG’s revenues and profits could be diminished if DMG fails to retain and attract the services of key primary care physicians.
Key primary care physicians with large patient enrollment could retire, become disabled, terminate their provider contracts, get lured away by a competing independent physician association or medical group, or otherwise become unable or unwilling to continue practicing medicine or contracting with DMG or its associated physicians, physician groups or IPAs. In addition, DMG’s associated physicians, physician groups and IPAs could view the business model as unfavorable or unattractive to such providers, which could cause such associated physicians, physician groups or IPAs to terminate their relationships with DMG. Moreover, given limitations relating to the enforcement of post-termination noncompetition covenants in California, it would be difficult to restrict a primary care physician from competing with DMG’s associated physicians, physician groups or IPAs. As a result, members who have been served by such physicians could choose to enroll with competitors’ physician organizations or could seek medical care elsewhere, which could reduce DMG’s revenues and profits. Moreover, DMG may not be able to attract new physicians to replace the services of terminating physicians or to service its growing membership.
Participation in ACO programs is subject to federal regulation, supervision, and evolving regulatory developments that may result in financial liability.
The ACA established the Medicare Shared Savings Program (MSSP) for ACOs, which took effect in January 2012. Under the MSSP, eligible organizations are accountable for the quality, cost and overall care of Medicare beneficiaries assigned to an ACO and may be eligible to share in any savings below a specified benchmark amount. The Secretary of HHS is also authorized, but not required, to use capitation payment models with ACOs. DMG has formed an MSSP ACO through a subsidiary, which operates in California, Florida, and Nevada and is evaluating whether to participate in more ACOs in the future. The continued development and expansion of ACOs will have an uncertain impact on DMG’s revenue and profitability. DaVita Kidney Care is also participating as a dialysis provider in Arizona, Florida, New Jersey, and Pennsylvania for the Innovation Center’s CEC Model.
The ACO programs are relatively new and therefore operational and regulatory guidance is limited. It is possible that the operations of DMG’s subsidiary ACO may not fully comply with current or future regulations and guidelines applicable to ACOs, may not achieve quality targets or cost savings, or may not attract or retain sufficient physicians or patients to allow DMG to meet its objectives. Additionally, poor performance could put the DMG ACO at financial risk with a potential obligation to CMS. Traditionally, other than fee-for-service billing by the medical clinics and healthcare facilities operated by DMG, DMG has not directly contracted with CMS and has not operated any health plans or provider sponsored networks. Therefore, DMG may not have the necessary experience, systems or compliance to successfully achieve a positive return on its investment in the ACO or to avoid financial or regulatory liability. DMG believes that its historical experience with fully delegated managed care will be applicable to operation of its subsidiary ACO, but there can be no such assurance.
California hospitals may terminate their agreements with HealthCare Partners Affiliates Medical Group and DaVita Health Plan of California, Inc. (formerly HealthCare Partners Plan, Inc., and, together with HealthCare Partners Affiliates Medical Group (AMG)) or reduce the fees they pay to DMG.
In California, AMG maintains significant hospital arrangements designed to facilitate the provision of coordinated hospital care with those services provided to members by AMG and its associated physicians, physician groups and IPAs. Through contractual arrangements with certain key hospitals, AMG provides utilization review, quality assurance and other management services related to the provision of patient care services to members by the contracted hospitals and downstream hospital contractors. In the event that any one of these key hospital agreements is amended in a financially unfavorable manner or is otherwise terminated, such events could have a material adverse effect on DMG’s business, results of operations and financial condition.
DMG’s professional liability and other insurance coverage may not be adequate to cover DMG’s potential liabilities.
DMG maintains primary professional liability insurance and other insurance coverage through California Medical Group Insurance Company, Risk Retention Group, an Arizona corporation in which DMG is the majority owner, and through excess coverage contracted through third-party insurers. DMG believes such insurance is adequate based on its review of what it believes to be all applicable factors, including industry standards. Nonetheless, potential liabilities may not be covered by insurance, insurers may dispute coverage or may be unable to meet their obligations, the amount of insurance coverage and/or related reserves may be inadequate, or the amount of any DMG self-insured retention may be substantial. There can be no
60
assurances that DMG will be able to obtain insurance coverage in the future, or that insurance will continue to be available on a cost-effective basis, if at all. Moreover, even if claims brought against DMG are unsuccessful or without merit, DMG would have to defend itself against such claims. The defense of any such actions may be time-consuming and costly and may distract DMG management’s attention. As a result, DMG may incur significant expenses and may be unable to effectively operate its business.
Changes in the rates or methods of third-party reimbursements may materially adversely affect DMG business, results of operations and financial condition.
Any negative changes in governmental capitation or FFS rates or methods of reimbursement for the services DMG provides could have a material adverse effect on DMG’s business, results of operations and financial condition. Since governmental healthcare programs generally reimburse on a fee schedule basis rather than on a charge-related basis, DMG generally cannot increase its revenues from these programs by increasing the amount it charges for its services. Moreover, if DMG’s costs increase, DMG may not be able to recover its increased costs from these programs. Government and private payors have taken and may continue to take steps to control the cost, eligibility for, use, and delivery of healthcare services due to budgetary constraints, and cost containment pressures as well as other financial issues. DMG believes that these trends in cost containment will continue. These cost containment measures, and other market changes in non-governmental insurance plans have generally restricted DMG’s ability to recover, or shift to non-governmental payors, any increased costs that DMG experiences. DMG’s business, results of operations and financial condition may be materially adversely affected by these cost containment measures, and other market changes.
DMG’s business model depends on numerous complex management information systems and any failure to successfully maintain these systems or implement new systems could materially harm DMG’s operations and result in potential violations of healthcare laws and regulations.
DMG depends on a complex, specialized, and integrated management information system and standardized procedures for operational and financial information, as well as for DMG’s billing operations. DMG may experience unanticipated delays, complications or expenses in implementing, integrating, and operating these integrated systems. Moreover, DMG may be unable to enhance its existing management information system or implement new management information systems where necessary. DMG’s management information system may require modifications, improvements or replacements that may require both substantial expenditures as well as interruptions in operations. DMG’s ability to implement and operate its integrated systems is subject to the availability of information technology and skilled personnel to assist DMG in creating and maintaining these systems.
DMG’s failure to successfully implement and maintain all of its systems could have a material adverse effect on its business, financial condition and results of operations. For example, DMG’s failure to successfully operate its billing systems could lead to potential violations of healthcare laws and regulations. If DMG is unable to handle its claims volume, or if DMG is unable to pay claims timely, DMG may become subject to a health plan’s corrective action plan or de-delegation until the problem is corrected, and/or termination of the health plan’s agreement with DMG. This could have a material adverse effect on DMG’s operations and profitability. In addition, if DMG’s claims processing system is unable to process claims accurately, the data DMG uses for its incurred but not reported (IBNR) estimates could be incomplete and DMG’s ability to accurately estimate claims liabilities and establish adequate reserves could be adversely affected. Finally, if DMG’s management information systems are unable to function in compliance with applicable state or federal rules and regulations, including medical information confidentiality laws such as HIPAA, possible penalties and fines due to this lack of compliance could have a material adverse effect on DMG’s financial condition, and results of operations.
DMG may be impacted by eligibility changes to government and private insurance programs.
Due to potential decreased availability of healthcare through private employers, the number of patients who are uninsured or participate in governmental programs may increase. The ACA has increased the participation of individuals in the Medicaid program in states that elected to participate in the expanded Medicaid coverage. A shift in payor mix from managed care and other private payors to government payors as well as an increase in the number of uninsured patients may result in a reduction in the rates of reimbursement to DMG or an increase in uncollectible receivables or uncompensated care, with a corresponding decrease in net revenue. Changes in the eligibility requirements for governmental programs such as the Medicaid program under the ACA and state decisions on whether to participate in the expansion of such programs also could increase the number of patients who participate in such programs and the number of uninsured patients. Even for those patients who remain in private insurance plans, changes to those plans could increase patient financial responsibility, resulting in a greater risk of uncollectible receivables. These factors and events could have a material adverse effect on DMG’s business, results of operations and financial condition.
61
Negative publicity regarding the managed healthcare industry generally or DMG in particular could adversely affect DMG’s results of operations or business.
Negative publicity regarding the managed healthcare industry generally, the Medicare Advantage program or DMG in particular, may result in increased regulation and legislative review of industry practices that further increase DMG’s costs of doing business and adversely affect DMG’s results of operations or business by:
• | requiring DMG to change its products and services; |
• | increasing the regulatory, including compliance, burdens under which DMG operates, which, in turn, may negatively impact the manner in which DMG provides services and increase DMG’s costs of providing services; |
• | adversely affecting DMG’s ability to market its products or services through the imposition of further regulatory restrictions regarding the manner in which plans and providers market to Medicare Advantage enrollees; or |
• | adversely affecting DMG’s ability to attract and retain members. |
Risk factors related to ownership of our common stock:
Provisions in our charter documents, compensation programs and Delaware law may deter a change of control that our stockholders would otherwise determine to be in their best interests.
Our charter documents include provisions that may deter hostile takeovers, delay or prevent changes of control or changes in our management, or limit the ability of our stockholders to approve transactions that they may otherwise determine to be in their best interests. These include provisions prohibiting our stockholders from acting by written consent; requiring 90 days advance notice of stockholder proposals or nominations to our Board of Directors (or 120 days for nominations made using proxy access); and granting our Board of Directors the authority to issue preferred stock and to determine the rights and preferences of the preferred stock without the need for further stockholder approval.
Most of our outstanding employee stock-based compensation awards include a provision accelerating the vesting of the awards in the event of a change of control. We also maintain a change of control protection program for our employees who do not have a significant number of stock awards, which has been in place since 2001, and which provides for cash bonuses to the employees in the event of a change of control. Based on the market price of our common stock and shares outstanding on December 31, 2017, these cash bonuses would total approximately $521 million if a change of control transaction occurred at that price and our Board of Directors did not modify this program. These change of control provisions may affect the price an acquirer would be willing to pay for our Company.
We are also subject to Section 203 of the Delaware General Corporation Law that, subject to exceptions, would prohibit us from engaging in any business combinations with any interested stockholder, as defined in that section, for a period of three years following the date on which that stockholder became an interested stockholder.
These provisions may discourage, delay or prevent an acquisition of our Company at a price that our stockholders may find attractive. These provisions could also make it more difficult for our stockholders to elect directors and take other corporate actions and could limit the price that investors might be willing to pay for shares of our common stock.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our corporate headquarters are located in Denver, Colorado, consisting of one owned 240,000 square foot building and two leased locations consisting of 164,800 square feet. Our headquarters are occupied by teammates engaged in management, finance, marketing, strategy, legal, compliance and other administrative functions. We lease six business offices located in California, Pennsylvania, Tennessee and Washington for our U.S. dialysis and related lab services business. For our DMG business we lease nine business offices located in California, Colorado, Nevada, New Mexico, Florida and Washington. Our laboratories are based in Florida where we operate our lab services out of five buildings, one owned and four leased. DaVita Rx leases three buildings located in California, Florida and Texas. We also own four administrative offices and lease administrative offices worldwide. Our leases on the properties listed above expire at various dates through the year 2036 for Kidney Care and through the year 2037 for DMG.
62
For our U.S. dialysis and related lab services business we own the land and buildings for 14 of our outpatient dialysis centers. We also own 14 separate land and buildings and seven land parcels for development. We lease a total of four owned properties to third-party tenants. Our remaining outpatient dialysis centers are located on premises that we lease.
For DMG, we own the land and buildings for 23 of our clinics. We also own one separate land parcel. Our remaining clinics are located on premises that we lease.
Our leases for our U.S. dialysis and related lab services and for DMG generally cover periods from five to 20 years and typically contain renewal options of five to ten years at the fair rental value at the time of renewal. Our leases are generally subject to periodic consumer price index increases, or contain fixed escalation clauses. Our outpatient dialysis centers range in size from approximately 700 to 33,000 square feet, with an average size of approximately 7,600 square feet. DMG’s clinics range in size from approximately 1,000 to 136,000 square feet, with an average size of approximately 10,200 square feet. Our international leases generally range from one to ten years.
Some of our outpatient dialysis centers are operating at or near capacity. However, we believe that we have adequate capacity within most of our existing dialysis centers to accommodate additional patient volume through increased hours and/or days of operation, or, if additional space is available within an existing facility, by adding dialysis stations. We can usually relocate existing centers to larger facilities or open new centers if existing centers reach capacity. With respect to relocating centers or building new centers, we believe that we can generally lease space at economically reasonable rates in the areas planned for each of these centers, although there can be no assurances in this regard. Expansion of existing centers or relocation of our dialysis centers is subject to review for compliance with conditions relating to participation in the Medicare ESRD program. In states that require a certificate of need or center license, additional approvals would generally be necessary for expansion or relocation.
Item 3. Legal Proceedings.
We operate in a highly regulated industry and are a party to various lawsuits, claims, qui tam suits, governmental investigations and audits (including investigations resulting from our obligation to self-report suspected violations of law) and other legal proceedings. We record accruals for certain legal proceedings and regulatory matters to the extent that we determine an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. As of December 31, 2017 and December 31, 2016, our total recorded accruals, including DMG, with respect to legal proceedings and regulatory matters, net of anticipated third party recoveries, were approximately $6 million and $69 million, respectively. While these accruals reflect our best estimate of the probable loss for those matters as of the dates of those accruals, the recorded amounts may differ materially from the actual amount of the losses for those matters, and any anticipated third party recoveries for any such losses may not ultimately be recoverable. Additionally, in some cases, no estimate of the possible loss or range of loss in excess of amounts accrued, if any, can be made because of the inherently unpredictable nature of legal proceedings and regulatory matters, which also may be impacted by various factors, including that they may involve indeterminate claims for monetary damages or may involve fines, penalties or non-monetary remedies; present novel legal theories or legal uncertainties; involve disputed facts; represent a shift in regulatory policy; are in the early stages of the proceedings; or result in a change of business practices. Further, there may be various levels of judicial review available to us in connection with any such proceeding.
The following is a description of certain lawsuits, claims, governmental investigations and audits and other legal proceedings to which we are subject.
Inquiries by the Federal Government and Certain Related Civil Proceedings
Swoben Private Civil Suit: On July 13, 2009, pursuant to the qui tam provisions of the federal FCA and the California False Claims Act, James M. Swoben, as relator, filed his initial qui tam action in the United States District Court for the Central District of California purportedly on behalf of the United States of America and the State of California against SCAN, and certain other defendants whose identities were under seal. In April 2013, HealthCare Partners (HCP), now known as our DMG subsidiary, was one of several defendants served with a civil complaint filed by a former employee of SCAN Health Plan (SCAN), an HMO. The allegations in the complaint relate to alleged overpayments received from government healthcare programs, including allegations of violations of the federal FCA and the California False Claims Act and allegations against HCP relating to patient diagnosis coding. The complaint sought monetary damages and civil penalties as well as costs and expenses. On October 18, 2017, the relator filed a Notice of Dismissal of the action as to HCP, and the government consented to the dismissal, as a result of which the suit is now dismissed, without prejudice.
2015 U.S. Office of Inspector General (OIG) Medicare Advantage Civil Investigation: In March 2015, JSA HealthCare Corporation (JSA), a subsidiary of DMG, received a subpoena from the Office of Inspector General (OIG) for the U.S. Department of Health and Human Services (HHS) requesting documents and information for the period from January 1, 2008
63
through December 31, 2013, for certain Medicare Advantage (MA) plans for which JSA provided services. It also requests information regarding JSA’s communications about patient diagnoses as they relate to certain MA plans generally, and more specifically as related to two Florida physicians with whom JSA previously contracted. We are producing the requested information and are cooperating with the government’s investigation.
In addition to the subpoena described above, in June 2015, we received a civil subpoena from the OIG covering the period from January 1, 2008 through the present and seeking production of a wide range of documents relating to our and our subsidiaries’ (including DMG’s and its subsidiary JSA’s) provision of services to MA plans and related patient diagnosis coding and risk adjustment submissions and payments. We believe that the request is part of a broader industry investigation into MA patient diagnosis coding and risk adjustment practices and potential overpayments by the government. The information requested includes information relating to patient diagnosis coding practices for a number of conditions, including potentially improper historical DMG coding for a particular condition. With respect to that condition, the guidance related to that coding issue was discontinued following our November 1, 2012 acquisition of HealthCare Partners (now known as our DMG business), and we notified CMS in April 2015 of the coding practice and potential overpayments. In that regard, we have identified certain additional coding practices which may have been problematic, some of which were the subject of the Swoben Private Civil Suit, and are in discussions with the DOJ relating to those practices. We are cooperating with the government. In addition, we are continuing to review other DMG coding practices to determine whether there were any improper coding issues. In connection with our acquisition of DMG in 2012, we have certain indemnification rights against the sellers and an escrow was established as security for the indemnification. We have submitted an indemnification claim against the sellers secured by the escrow for any and all liabilities incurred relating to these matters and intend to pursue recovery from the escrow. However, we can make no assurances that the indemnification and escrow will cover the full amount of our potential losses related to these matters.
2016 U.S. Attorney Prescription Drug Investigation: In early February 2016, we announced that our pharmacy services’ wholly-owned subsidiary, DaVita Rx, received a Civil Investigative Demand (CID) from the U.S. Attorney’s Office for the Northern District of Texas. The government is conducting an FCA investigation concerning allegations that DaVita Rx presented or caused to be presented false claims for payment to the government for prescription medications, as well as into our relationship with pharmaceutical manufacturers. The CID covers the period from January 1, 2006 through the present. In the spring of 2015, we initiated an internal compliance review of DaVita Rx during which we identified potential billing and operational issues, including potential write-offs and discounts of patient co-payment obligations, and credits to payors for returns of prescription drugs related to DaVita Rx. We notified the government in September 2015 that we were conducting this review of DaVita Rx and began providing regular updates of our review. Upon completion of our review, we filed a self-disclosure with the OIG in February 2016 and we have been working to address and update the practices we identified in the self-disclosure, some of which overlap with information requested by the U.S. Attorney’s Office. The OIG informed us in February 2016 that our submission was not accepted. They indicated that the OIG is not expressing an opinion regarding the conduct disclosed or our legal positions. In connection with our ongoing efforts working with the government we learned that a qui tam complaint had been filed covering some of the issues in the CID and our self-disclosure. In December 2017, we finalized and executed a settlement agreement with the government and relators in the qui tam matter that included total monetary consideration of $63.7 million, as previously announced, of which $41.5 million was an incremental cash payment and $21.2 million was for amounts previously refunded, and all of which was previously accrued. The government's investigation into our relationship with pharmaceutical manufacturers is ongoing and we are continuing to cooperate with the government in this investigation.
2017 U.S. Attorney American Kidney Fund Investigation: On January 4, 2017, we were served with an administrative subpoena for records by the United States Attorney’s Office, District of Massachusetts, relating to an investigation into possible federal health care offenses. The subpoena covers the period from January 1, 2007 through the present, and seeks documents relevant to charitable patient assistance organizations, particularly the American Kidney Fund, including documents related to efforts to provide patients with information concerning the availability of charitable assistance. We are cooperating with the government and are producing the requested information.
Although we cannot predict whether or when proceedings might be initiated or when these matters may be resolved (other than as described above), it is not unusual for inquiries such as these to continue for a considerable period of time through the various phases of document and witness requests and on-going discussions with regulators. In addition to the inquiries and proceedings specifically identified above, we are frequently subject to other inquiries by state or federal government agencies and/or private civil qui tam complaints filed by relators. Negative findings or terms and conditions that we might agree to accept as part of a negotiated resolution of pending or future government inquiries or relator proceedings could result in, among other things, substantial financial penalties or awards against us, substantial payments made by us, harm to our reputation, required changes to our business practices, exclusion from future participation in the Medicare, Medicaid and
64
other federal health care programs and, if criminal proceedings were initiated against us, possible criminal penalties, any of which could have a material adverse effect on us.
Shareholder Claims
Peace Officers’ Annuity and Benefit Fund of Georgia Securities Class Action Civil Suit: On February 1, 2017, the Peace Officers’ Annuity and Benefit Fund of Georgia filed a putative federal securities class action complaint in the U.S. District Court for the District of Colorado against us and certain executives. The complaint covers the time period of August 2015 to October 2016 and alleges, generally, that we and our executives violated federal securities laws concerning our financial results and revenue derived from patients who received charitable premium assistance from an industry-funded non-profit organization. The complaint further alleges that the process by which patients obtained commercial insurance and received charitable premium assistance was improper and "created a false impression of DaVita’s business and operational status and future growth prospects." In November 2017, the court appointed the lead plaintiff and an amended complaint was filed on January 12, 2018. Our response is due March 13, 2018. We dispute these allegations and intend to defend this action accordingly.
In re DaVita Inc. Stockholder Derivative Litigation: On August 15, 2017, the U.S. District Court for the District of Delaware consolidated the three previously disclosed shareholder derivative lawsuits: the Blackburn Shareholder action filed on February 10, 2017, the Gabilondo Shareholder action filed on May 30, 2017, and the City of Warren Police and Fire Retirement System Shareholder action filed on June 9, 2017. The complaint covers the time period from 2015 to present and alleges, generally, breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, corporate waste, and misrepresentations and/or failures to disclose certain information in violation of the federal securities laws in connection with an alleged practice to direct patients with government-subsidized health insurance into private health insurance plans to maximize our profits. An amended complaint was filed in September 2017, and on December 18, 2017 we filed a motion to dismiss and a motion to stay proceedings in the alternative. We dispute these allegations and intend to defend this action accordingly.
Other Proceedings
In addition to the foregoing, from time to time we are subject to other lawsuits, demands, claims, governmental investigations and audits and legal proceedings that arise due to the nature of our business, including contractual disputes, such as with payors, suppliers and others, employee-related matters and professional and general liability claims. From time to time, we also initiate litigation or other legal proceedings as a plaintiff arising out of contracts or other matters.
* * *
Other than as described above, we cannot predict the ultimate outcomes of the various legal proceedings and regulatory matters to which we are or may be subject from time to time, including those described in this "Item 3. Legal Proceedings" in Part I of this report or the timing of their resolution or the ultimate losses or impact of developments in those matters, which could have a material adverse effect on our revenues, earnings and cash flows. Further, any legal proceedings or regulatory matters we are involved in, whether meritorious or not, are time consuming, and often require management’s attention and result in significant legal expense, and may result in the diversion of significant operational resources, or otherwise harm our business, financial results or reputation.
Item 4. Mine Safety Disclosures.
Not applicable.
65
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock is traded on the New York Stock Exchange under the symbol DVA. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported by the New York Stock Exchange.
High | Low | ||||||
Year ended December 31, 2017: | |||||||
1st quarter | $ | 70.14 | $ | 62.24 | |||
2nd quarter | 70.16 | 61.48 | |||||
3rd quarter | 66.64 | 55.59 | |||||
4th quarter | 72.93 | 52.51 | |||||
Year ended December 31, 2016: | |||||||
1st quarter | $ | 74.18 | $ | 61.36 | |||
2nd quarter | 78.00 | 72.31 | |||||
3rd quarter | 78.77 | 62.76 | |||||
4th quarter | 67.44 | 54.50 | |||||
The closing price of our common stock on January 31, 2018 was $78.04 per share. According to Computershare, our registrar and transfer agent, as of January 31, 2018, there were 9,207 holders of record of our common stock. We have not declared or paid cash dividends to holders of our common stock since 1994. We have no current plans to pay cash dividends and we are restricted from paying dividends under the terms of our senior secured credit facilities and the indentures governing our senior notes. See “Liquidity and capital resources” under “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the notes to our consolidated financial statements.
Stock Repurchases
The following table summarizes our repurchases of our common stock during the fourth quarter of 2017:
Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs(1) | Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs (in millions) | |||||||||
October 1 - October 31, 2017 | 5,457,839 | $ | 59.90 | 5,457,839 | $ | 1,254.3 | |||||||
November 1 - November 30, 2017 | 431,645 | $ | 60.10 | 431,645 | $ | 1,228.4 | |||||||
December 1 - December 31, 2017 | 1,520,365 | $ | 71.87 | 1,520,365 | $ | 1,119.1 | |||||||
Total | 7,409,849 | $ | 62.37 | 7,409,849 | $ | 1,119.1 | |||||||
(1) | On October 10, 2017, our Board of Directors approved an additional share repurchase authorization in the amount of $1.3 billion. This share repurchase authorization was in addition to the $247 million remaining at that time under our Board of Directors’ prior share repurchase authorization announced in July 2016. We are authorized to make purchases from time to time in the open market or in privately negotiated transactions, including without limitations, through accelerated share repurchase transactions, derivative transactions, tender offers, Rule 10b5-1 plans or any combination of the foregoing, depending upon market conditions and other considerations. During the quarter ended December 31, 2017, we repurchased a total of 7,409,849 shares of our common stock for approximately $462 million at an average price of $62.37 per share. As of February 22, 2018, we have a total of $1.0 billion remaining in Board authorizations available for share repurchases under our repurchase programs. Although these share repurchase authorizations have no expiration dates, we are subject to share repurchase limitations under the terms of our senior secured credit facilities and the indentures governing our senior notes. |
66
Item 6. Selected Financial Data.
The following financial and operating data should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements filed as part of this report. The following table presents selected consolidated financial and operating data for the periods indicated.
Year ended December 31, | |||||||||||||||||||
2017 | 2016 | 2015 | 2014 | 2013 | |||||||||||||||
(in thousands, except share data) | |||||||||||||||||||
Income statement data: | |||||||||||||||||||
Net revenues | $ | 10,876,634 | $ | 10,707,467 | $ | 9,982,245 | $ | 9,312,049 | $ | 8,580,225 | |||||||||
Operating expenses and charges(2) | 9,063,879 | 8,677,757 | 8,845,479 | 7,711,891 | 7,464,599 | ||||||||||||||
Operating income | 1,812,755 | 2,029,710 | 1,136,766 | 1,600,158 | 1,115,626 | ||||||||||||||
Debt expense | (430,634 | ) | (414,116 | ) | (408,380 | ) | (410,223 | ) | (429,938 | ) | |||||||||
Debt refinancing and redemption charges | — | — | (48,072 | ) | (97,548 | ) | — | ||||||||||||
Other income, net | 17,665 | 7,511 | 8,073 | 1,935 | 6,750 | ||||||||||||||
Income from continuing operations before income taxes | 1,399,786 | 1,623,105 | 688,387 | 1,094,322 | 692,438 | ||||||||||||||
Income tax expense(3) | 323,859 | 431,761 | 207,510 | 366,894 | 246,795 | ||||||||||||||
Net income from continuing operations | 1,075,927 | 1,191,344 | 480,877 | 727,428 | 445,643 | ||||||||||||||
Net (loss) income from discontinued operations, net of tax(4) | (245,372 | ) | (158,262 | ) | (53,467 | ) | 135,902 | 298,182 | |||||||||||
Gain on disposal of discontinued operations, net of tax(4) | — | — | — | — | 13,375 | ||||||||||||||
Net income | 830,555 | 1,033,082 | 427,410 | 863,330 | 757,200 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | (166,937 | ) | (153,208 | ) | (157,678 | ) | (140,216 | ) | (123,755 | ) | |||||||||
Net income attributable to DaVita Inc. | $ | 663,618 | $ | 879,874 | $ | 269,732 | $ | 723,114 | $ | 633,445 | |||||||||
Basic income from continuing operations per share attributable to DaVita Inc.(5) | $ | 4.78 | $ | 5.12 | $ | 1.53 | $ | 2.77 | $ | 1.53 | |||||||||
Diluted income from continuing operations per share attributable to DaVita Inc.(5) | $ | 4.71 | $ | 5.04 | $ | 1.49 | $ | 2.71 | $ | 1.50 | |||||||||
Weighted average shares outstanding:(5) | |||||||||||||||||||
Basic | 188,626,000 | 201,641,000 | 211,868,000 | 212,302,000 | 209,939,000 | ||||||||||||||
Diluted | 191,349,000 | 204,905,000 | 216,252,000 | 216,928,000 | 214,764,000 | ||||||||||||||
Ratio of earnings to fixed charges(6) | 2.94:1 | 3.49:1 | 1.93:1 | 2.72:1 | 2.01:1 | ||||||||||||||
Balance sheet data: | |||||||||||||||||||
Working capital(1) | $ | 5,703,181 | $ | 1,283,784 | $ | 2,104,143 | $ | 1,547,518 | $ | 600,789 | |||||||||
Total assets(1) | $ | 18,948,193 | $ | 18,755,776 | $ | 18,524,224 | $ | 17,624,137 | $ | 16,614,893 | |||||||||
Long-term debt(1) | $ | 9,158,018 | $ | 8,944,676 | $ | 12,972,282 | $ | 8,298,624 | $ | 8,064,196 | |||||||||
Total DaVita Inc. shareholders' equity(5) | $ | 4,690,029 | $ | 4,648,047 | $ | 4,870,781 | $ | 5,170,513 | $ | 4,432,480 | |||||||||
(1) | In 2015, we retrospectively adopted ASU 2015-03 related to simplification of debt issuance costs as well as ASU 2015-17 related to classification of deferred taxes. All periods prior to 2015 have been recast to conform to the revised presentation. |
(2) | Operating expenses and charges in 2017 includes goodwill impairment charges of $34,696 related to our vascular access reporting unit, an equity investment loss of $6,293 for goodwill impairments at our APAC JV, an impairment on our investment in the APAC JV of $280,066, an asset impairment of $15,168 related to the restructuring of our pharmacy business, restructuring charges related to our international business of $2,700, a net gain on settlement of $529,504 and a gain adjustment on the 2016 ownership change of our APAC JV of $6,273. Operating expenses and charges in 2016 included goodwill impairment charges of $28,415 related to our vascular access reporting unit, an impairment of an investment of $14,993, an estimated gain on the ownership change of our APAC JV of $374,374, and an estimated accrual for certain legal matters of $15,770. Operating expenses and charges for 2015 included a settlement charge of $495,000 related to a private civil suit, goodwill impairment charges of $4,066 related to our international business, and an estimated accrual for certain legal matters of $22,530. Operating expenses and charges in 2014 and 2013 included an additional $17,000 and $397,000 loss contingency accrual related to the settlement of the 2010 and 2011 U.S. Attorney physician relationship investigations, respectively. |
(3) | Tax expense includes a net tax benefit of $251,510 related to U.S. tax legislation passed in December 2017. |
67
(4) | On December 5, 2017, we entered into an equity purchase agreement to sell our DMG division to Collaborative Care Holdings, LLC (Optum), a subsidiary of UnitedHealth Group Inc. As a result of this pending transaction, the DMG business has been reclassified as held for sale and its results of operations are reported as net (loss) income from discontinued operations, net of tax for all periods presented. Net (loss) income from discontinued operations, net of tax, also includes HomeChoice Partners Inc. (HomeChoice) which was divested on February 1, 2013. Net (loss) income from discontinued operations, net of tax, in 2017 includes estimated goodwill impairment charges of $651,659 related to certain DMG reporting units, a net tax benefit of $163,555 due to a remeasurement of deferred taxes resulting from DMG's reclassification to held for sale, a non-cash gain associated with our Magan acquisition of $17,129, restructuring charges of $9,569, and a reduction in estimated accruals for legal matters of $14,700. Net (loss) income from discontinued operations, net of tax, in 2016 included goodwill impairment charges of $253,000 related to certain DMG reporting units, a gain related to the partial sale of our interest in Tandigm of $40,280, a loss on the DMG Arizona sale of $10,489, an adjustment to reduce receivables associated with the DMG acquisition escrow provision relating to income tax items of $30,934, and estimated accruals for legal matters of $16,000. Net (loss) income from discontinued operations, net of tax, in 2015 included estimated goodwill and other intangible asset impairment charges of $206,169 related to certain DMG reporting units. Net (loss) income from discontinued operations, net of tax, in 2013 includes contingent earn-out obligation, a gain adjustment of $56,977 related to a decrease in DMG’s 2013 contingent earn-out obligation and an adjustment to reduce a tax asset associated with the DMG acquisition escrow provisions of $7,721. |
(5) | In the third quarter of 2013, the Board of Directors approved a two-for-one split of our common stock in the form of a stock dividend payable on September 6, 2013 to stockholders of record on August 23, 2013. Our common stock began trading on a post-split basis on September 9, 2013. Share repurchases consisted of 12,966,672 shares of common stock for $810,949 in 2017, 16,649,090 shares of common stock for $1,072,377 in 2016, and 7,779,958 shares of common stock for $575,380 in 2015. No repurchases of common stock were made in 2014 or 2013. Shares issued in connection with stock awards were 514,091 in 2017, 1,011,328 in 2016, 1,479,217 in 2015, 2,179,766 in 2014, and 1,928,137 in 2013. |
(6) | The ratio of earnings to fixed charges was computed by dividing earnings by fixed charges. Earnings for this purpose is defined as pretax income from continuing operations adjusted by adding back fixed charges expensed during the period, less noncontrolling interests. Fixed charges include debt expense (interest expense and the write-off and amortization of deferred financing costs), the estimated interest component of rental expense on operating leases and capitalized interest. |
68
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Forward-looking statements
This Annual Report on Form 10-K, including this Management’s Discussion and Analysis of Financial Condition and Results of Operations, contains statements that are forward-looking statements within the meaning of the federal securities laws. All statements that do not concern historical facts are forward-looking statements and include, among other things, statements about our expectations, beliefs, intentions and/or strategies for the future. These forward-looking statements may include statements regarding our future operations, financial condition and prospects, such as expectations for treatment growth rates, revenue per treatment, expense growth, levels of the provision for uncollectible accounts receivable, operating income, cash flow, operating cash flow, estimated tax rates, estimated charges and accruals, capital expenditures, the development of new dialysis centers and dialysis center acquisitions, government and commercial payment rates, revenue estimating risk and the impact of our level of indebtedness on our financial performance, and including earnings per share. These statements involve substantial known and unknown risks and uncertainties that could cause our actual results to differ materially from those described in the forward-looking statements, including risks resulting from the concentration of profits generated by higher-paying commercial payor plans for which there is continued downward pressure on average realized payment rates, and a reduction in the number of patients under such plans, including as a result of restrictions or prohibitions on the use and/or availability of charitable premium assistance, which may result in the loss of revenues or patients, or our making incorrect assumptions about how our patients will respond to any change in financial assistance from charitable organizations; the extent to which the ongoing implementation of healthcare exchanges or changes in or new legislation, regulations or guidance, or enforcement thereof, including among other things those regarding the exchanges, results in a reduction in reimbursement rates for our services from and/or the number of patients enrolled in higher-paying commercial plans; a reduction in government payment rates under the Medicare End Stage Renal Disease program or other government-based programs; the impact of the Medicare Advantage benchmark structure; risks arising from potential and proposed federal and/or state legislation or regulation, including healthcare-related and labor-related legislation or regulation, that could have a material adverse effect on our operations and profitability; the impact of the changing political environment and related developments on the current health care marketplace and on our business, including with respect to the future of the Affordable Care Act, the exchanges and many other core aspects of the current health care marketplace; uncertainties related to the impact of federal tax reform legislation; changes in pharmaceutical or anemia management practice patterns, payment policies, or pharmaceutical pricing; legal compliance risks, including our continued compliance with complex government regulations and the provisions of our current Corporate Integrity Agreement (CIA) and current or potential investigations by various government entities and related government or private-party proceedings, and restrictions on our business and operations required by our corporate integrity agreement and other current or potential settlement terms, and the financial impact thereof and our ability to recover any losses related to such legal matters from third parties; continued increased competition from large- and medium-sized dialysis providers that compete directly with us; our ability to reduce administrative expenses while maintaining targeted levels of service and operating performance, including our ability to achieve anticipated savings from our recent restructurings; our ability to maintain contracts with physician medical directors, changing affiliation models for physicians, and the emergence of new models of care introduced by the government or private sector, that may erode our patient base and reimbursement rates, such as accountable care organizations (ACOs), independent practice associations (IPAs) and integrated delivery systems; our ability to complete acquisitions, mergers or dispositions that we might announce or be considering, on terms favorable to us or at all, or to integrate and successfully operate any business we may acquire or have acquired, or to successfully expand our operations and services to markets outside the United States, or to businesses outside of dialysis; noncompliance by us or our business associates with any privacy laws or any security breach involving the misappropriation, loss or other unauthorized use or disclosure of confidential information; the variability of our cash flows; factors that may impact our ability to repurchase stock under our stock repurchase program and the timing of any such stock repurchases, including market conditions, the price of our common stock, our cash flow position and leverage ratios, and legal, regulatory and contractual requirements; the risk that we might invest material amounts of capital and incur significant costs in connection with the growth and development of our international operations, yet we might not be able to operate them profitably anytime soon, if at all; risks arising from the use of accounting estimates, judgments and interpretations in our financial statements; impairment of our goodwill, investments or other assets; the risks and uncertainties associated with the timing, conditions and receipt of regulatory approvals and satisfaction of other closing conditions of the DMG sale transaction, potential disruption in connection with the DMG sale transaction making it more difficult to maintain business and operational relationships, and uncertainties related to our use of proceeds from the DMG sale transaction, including our ability to repurchase stock; the risk that laws regulating the corporate practice of medicine could restrict the manner in which DMG conducts its business; the risk that the cost of providing services under DMG’s agreements may exceed our compensation; the risk that reductions in reimbursement rates, including Medicare Advantage rates, and future regulations may negatively impact DMG’s business, revenue and profitability; the risk that DMG may not be able to successfully establish a presence in new geographic regions or successfully address competitive threats that could reduce its profitability; the risk that a disruption in DMG’s healthcare provider networks could have an adverse effect on DMG’s business operations and profitability; the risk that
69
reductions in the quality ratings of health maintenance organization plan customers of DMG could have an adverse effect on DMG’s business; the risk that health plans that acquire health maintenance organizations may not be willing to contract with DMG or may be willing to contract only on less favorable terms; and the other risk factors set forth in Part I, Item 1A. of this Annual Report on Form 10-K. We base our forward-looking statements on information currently available to us, and we undertake no obligation to update or revise any forward-looking statements, whether as a result of changes in underlying factors, new information, future events or otherwise.
The following should be read in conjunction with our consolidated financial statements and “Item 1. Business”.
70
Company overview
The Company has consisted of two major divisions, DaVita Kidney Care (Kidney Care) and DaVita Medical Group (DMG). Kidney Care is comprised of our U.S. dialysis and related lab services, our ancillary services and strategic initiatives, including our international operations, and our corporate administrative support. Our U.S. dialysis and related lab services business is our largest line of business and is a leading provider of kidney dialysis services in the U.S. for patients suffering from chronic kidney failure, also known as end stage renal disease (ESRD). DMG is a patient- and physician-focused integrated healthcare delivery and management company with over two decades of providing coordinated, outcomes-based medical care in a cost-effective manner.
On December 5, 2017, we entered into an equity purchase agreement to sell our DMG division to Collaborative Care Holdings, LLC (Optum), a subsidiary of UnitedHealth Group Inc. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions. As a result of this pending transaction, the DMG business is classified as held for sale and its results of operations are reported as discontinued operations. In addition, prior periods' presentation has been revised to conform to current year presentation and DMG is not included in our Management's Discussion and Analysis below.
The overall financial performance of our U.S. dialysis and related lab services in 2017 benefited from increased treatment volume from acquired and non-acquired growth and cost control initiatives in our dialysis business. This was partially offset by an increase in labor costs and other center related costs.
Some of our major accomplishments and financial operating performance indicators in 2017 and year over year were as follows:
• | improved clinical outcomes in our U.S. dialysis operations, including the fifth consecutive year as a leader in CMS’ Quality Incentive Program; |
• | consolidated net revenue growth of 1.6%, which included 2.4% total net revenue growth in our U.S. dialysis segment, despite a decrease of $5 in average dialysis net patient service revenue per treatment; |
• | solid performance in our normalized non-acquired U.S. dialysis treatment growth of 3.5%, which contributed to an increase of approximately 4.1% in the overall number of U.S. dialysis treatments; |
• | a net increase of 160 U.S. dialysis centers, including dialysis centers from the Renal Ventures acquisition, and a net increase of 83 international dialysis centers; |
• | an increase in our overall number of patients we serve in the U.S. of approximately 5.4% in 2017; |
• | a decrease in U.S. dialysis and lab related services patient care costs of approximately $2 per treatment and a decrease in general and administrative expenses of approximately $1 per treatment; and |
• | consolidated operating cash flows of $1.9 billion, or $1.6 billion from continuing operations, which included the net VA settlement of $332 million. |
We believe 2018 will be challenging. We continue to expect clinical costs to increase due to inflation and a tight labor market and we do not foresee an opportunity to offset these pressures with productivity improvements. With labor cost inflation continuing to outpace Medicare reimbursement, we anticipate that margins on our Medicare business will continue to experience pressure. In addition, we will experience an increase in benefit costs as we transition to a 401(k) plan match program as our 2017 benefit costs did not include a comparable expense. In 2018 we also anticipate additional reimbursement pressure on our pharmacy business. We remain committed to our plans for international expansion in certain regions, which will continue to require investment. We anticipate that these challenges will be partially offset in 2018 by the expected reduction in income taxes as a result of recent U.S. tax reform legislation. In addition, in connection with our previously announced capital allocation strategy, in 2018 we plan to continue our evaluation of strategic alternatives for various assets in our portfolio.
71
Following is a summary of our consolidated operating results for reference in the discussion that follows.
Year ended December 31, | ||||||||||||||||||||
2017 | 2016 | 2015 | ||||||||||||||||||
(dollars in millions) | ||||||||||||||||||||
Net revenues: | ||||||||||||||||||||
Dialysis and related lab patient service revenues | $ | 10,094 | $ | 9,727 | $ | 9,155 | ||||||||||||||
Less: Provision for uncollectible accounts | (485 | ) | (431 | ) | (413 | ) | ||||||||||||||
Net dialysis and related lab patient service revenues | 9,608 | 9,296 | 8,743 | |||||||||||||||||
Other revenues | 1,268 | 1,411 | 1,240 | |||||||||||||||||
Total net consolidated revenues | 10,877 | 100 | % | 10,707 | 100 | % | 9,982 | 100 | % | |||||||||||
Operating expenses and charges: | ||||||||||||||||||||
Patient care costs | 7,640 | 70 | % | 7,432 | 69 | % | 6,856 | 69 | % | |||||||||||
General and administrative | 1,064 | 10 | % | 1,073 | 10 | % | 1,031 | 10 | % | |||||||||||
Depreciation and amortization | 560 | 5 | % | 509 | 5 | % | 464 | 5 | % | |||||||||||
Provision for uncollectible accounts | (7 | ) | — | % | 12 | — | % | 9 | — | % | ||||||||||
Equity investment loss (income) | 9 | — | % | (17 | ) | — | % | (14 | ) | — | % | |||||||||
Investment and other asset impairments | 295 | 3 | % | 15 | — | % | — | — | % | |||||||||||
Goodwill impairment charges | 36 | — | % | 28 | — | % | 4 | — | % | |||||||||||
Gain on changes in ownership interests | (6 | ) | — | % | (374 | ) | (3 | )% | — | — | % | |||||||||
Gain on settlement, net | (527 | ) | (5 | )% | — | — | % | — | — | % | ||||||||||
Settlement charge | — | — | % | — | — | % | 495 | 5 | % | |||||||||||
Total operating expenses and charges | 9,064 | 83 | % | 8,678 | 81 | % | 8,845 | 89 | % | |||||||||||
Operating income | $ | 1,813 | 17 | % | $ | 2,030 | 19 | % | $ | 1,137 | 11 | % | ||||||||
Certain columns, rows or percentages may not sum or recalculate due to the use of rounded numbers.
The following table summarizes our consolidated net revenues among our reportable segments:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(dollars in millions) | |||||||||||
Net revenues: | |||||||||||
U.S. dialysis and related lab patient service revenues | $ | 9,822 | $ | 9,551 | $ | 9,034 | |||||
Less: Provision for uncollectible accounts | (482 | ) | (430 | ) | (406 | ) | |||||
U.S. dialysis and related lab net patient service revenues | 9,340 | 9,121 | 8,628 | ||||||||
Other revenues | 20 | 17 | 14 | ||||||||
Total net U.S. dialysis and related lab services revenues | 9,360 | 9,138 | 8,642 | ||||||||
Other-ancillary services and strategic initiatives other revenues | 1,273 | 1,420 | 1,248 | ||||||||
Other-ancillary services and strategic initiatives net patient service revenues (less provision for uncollectible accounts) | 323 | 202 | 134 | ||||||||
Total net other-ancillary services and strategic initiatives revenues | 1,596 | 1,621 | 1,382 | ||||||||
Total net segment revenues | 10,956 | 10,759 | 10,024 | ||||||||
Elimination of intersegment revenues | (80 | ) | (52 | ) | (42 | ) | |||||
Consolidated net revenues | $ | 10,877 | $ | 10,707 | $ | 9,982 | |||||
Certain columns, rows or percentages may not sum or recalculate due to the use of rounded numbers.
72
The following table summarizes consolidated operating income and adjusted consolidated operating income:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(dollars in millions) | |||||||||||
U.S. dialysis and related lab services | $ | 2,297 | $ | 1,777 | $ | 1,260 | |||||
Other — ancillary services and strategic initiatives | (439 | ) | 267 | (104 | ) | ||||||
Total segment operating income | 1,858 | 2,044 | 1,156 | ||||||||
Reconciling corporate items: | |||||||||||
Corporate administrative support | (45 | ) | (14 | ) | (19 | ) | |||||
Consolidated operating income | $ | 1,813 | $ | 2,030 | $ | 1,137 | |||||
Reconciliation of non-GAAP measure: | |||||||||||
Goodwill impairment charges | 35 | 28 | 4 | ||||||||
Equity investment loss related to APAC JV goodwill impairment | 6 | — | — | ||||||||
Impairment of investment | 280 | 15 | — | ||||||||
Impairment of assets | 15 | — | — | ||||||||
Restructuring charges | 2 | — | — | ||||||||
Equity investment loss related to restructuring charges | 1 | — | — | ||||||||
Gain on settlement, net | (527 | ) | — | — | |||||||
Equity investment income related to gain on settlement | (3 | ) | — | — | |||||||
Gain on APAC JV ownership changes | (6 | ) | (374 | ) | — | ||||||
Accruals for legal matters | — | 16 | 22 | ||||||||
Settlement charge | — | — | 495 | ||||||||
Adjusted consolidated operating income(1) | $ | 1,616 | $ | 1,715 | $ | 1,658 | |||||
Certain columns, rows or percentages may not sum or recalculate due to the use of rounded numbers.
(1) | For the periods presented in the table above adjusted operating income is defined as operating income before certain items which we do not believe are indicative of ordinary results, including goodwill impairment charges, investment and other asset impairments, restructuring charges, a net settlement gain, gains on ownership changes, estimated accruals for certain legal matters and a settlement charge. Adjusted operating income as so defined is a non-GAAP measure and is not intended as a substitute for GAAP operating income. We have presented these adjusted amounts because management believes that these presentations enhance a user’s understanding of our normal consolidated operating income by excluding certain items which we do not believe are indicative of our ordinary results of operations. As a result, adjusting for these amounts allows for comparison to our normalized prior period results. |
Consolidated net revenues
Consolidated net revenues for 2017 increased by approximately $170 million, or 1.6%, from 2016. This increase in consolidated net revenues was due to an increase in U.S. dialysis and related lab services net revenues of approximately $222 million, principally as a result of solid volume growth from additional treatments, partially offset by a decrease of approximately $5 in average dialysis net patient service revenue per treatment and by one less treatment day in 2017, as discussed below. Revenue for 2017 was negatively impacted by a decrease of approximately $25 million from 2016 in our ancillary services and strategic initiatives driven primarily from decreases in revenue from our pharmaceutical business, partially offset by an increase in net revenues from expansion in our international business and increases in VillageHealth revenues, as described below.
Consolidated net revenues for 2016 increased by approximately $725 million, or 7.3%, from 2015. This increase in consolidated net revenues was due to an increase in U.S. dialysis and related lab services net revenues of approximately $496 million, principally resulted from solid volume growth from additional treatments, one additional treatment day in 2016, and an increase of $4 in the average dialysis net patient service revenue per treatment, as discussed below. In addition, revenue for 2016 increased by approximately $239 million from 2015 in our ancillary services and strategic initiatives driven primarily from growth in our pharmaceutical business and from expansion in our international business and other strategic initiatives.
73
Consolidated operating income
Consolidated operating income of $1.813 billion for 2017, which includes goodwill impairment charges of $35 million related to our vascular access reporting unit, an equity investment loss of $6 million for goodwill impairments at our APAC JV, an impairment of $280 million on our investment in the APAC JV, an asset impairment of $15 million related to the restructuring of our pharmacy business, restructuring charges in our international business of $3 million, a net gain on settlement of $530 million, and a gain adjustment on the 2016 ownership change of our APAC JV of $6 million, as discussed below, decreased by $217 million as compared to 2016, which included goodwill impairment charges of $28 million, an investment impairment of $15 million, an estimated gain on the ownership change of our APAC JV of $374 million and estimated accruals for legal matters of $16 million. Excluding these items from their respective periods, adjusted consolidated operating income for 2017 decreased by approximately $99 million due to an increase in adjusted operating losses in our ancillary and strategic initiatives of $59 million, an increase in expenses in our corporate administrative support of $31 million, and a decrease in adjusted operating income in U.S. dialysis and related lab services of $9 million, as described below.
Consolidated operating income of $2.030 billion for 2016, which included goodwill impairment charges of $28 million related to our vascular access reporting unit, an investment impairment of $15 million, an estimated gain on the ownership change of our APAC JV of $374 million and estimated accruals for legal matters of $16 million increased by approximately $893 million from 2015, which included estimated impairment charges of approximately $4 million, estimated accruals for legal matters of $22 million and a settlement charge of $495 million. Excluding these items from their respective periods, adjusted consolidated operating income for 2016 increased by approximately $57 million. Adjusted consolidated operating income increased primarily as a result of an increase in adjusted operating income in U.S. dialysis and related lab services of $22 million, a decrease in adjusted operating losses in our ancillary and strategic initiatives of $30 million, and a decrease in expenses in our corporate administrative support of $5 million, as described below.
U.S. dialysis and related lab services business
Our U.S. dialysis and related lab services business is a leading provider of kidney dialysis services through a network of 2,510 outpatient dialysis centers which we own and manage through management services agreements, in 46 states and the District of Columbia, serving a total of approximately 197,800 patients. We also provide acute inpatient dialysis services in approximately 900 hospitals. We estimate that we have approximately a 37% share of the U.S. dialysis market based upon the number of patients we serve. In 2017, our overall network of U.S. outpatient dialysis centers increased by 160 dialysis centers, primarily as a result of opening new dialysis centers and from acquisitions of existing dialysis centers. The overall number of patients that we serve in the U.S. increased by approximately 5.4% in 2017, including dialysis patients from the Renal Ventures acquisition, as compared to 2016.
The stated mission of our U.S. dialysis and related lab services is to be the provider, partner and employer of choice. We believe our attention to these three stakeholders—our patients, our business partners, and our teammates—represents a major driver of our long-term performance, although we are subject to the impact of external factors such as government policy, physician practice patterns, commercial payor payment rates and the mix of commercial and government patients, as further described in Item 1A Risk Factors. Two principal non-financial metrics we track are quality clinical outcomes and teammate turnover. We have developed our own composite index for measuring improvements in our clinical outcomes, which we refer to as the DaVita Quality Index (DQI). Our clinical outcomes as measured by DQI have improved over each of the past several years, which we believe directly decreases patient mortalities. Our patient mortality percentages have decreased from 19.0% in 2001 to 13.8% in 2016. For the fifth year in a row, we have been a leader in the industry in QIP standards and for the last three years for which data is available, we have been a leader in the industry under the CMS Five-Star Quality Rating systems. Over the last two years our clinical teammate turnover has increased slightly due to increased competition for skilled clinical personnel; however, despite this headwind, we have continued to improve our clinical performance. We will continue to focus on these three stakeholders and our clinical outcomes as we believe these are fundamental long-term value drivers.
We believe our national scale, size and commitment to our patients, among other things, allows us to provide industry-leading quality care with superior clinical outcomes that attracts patients, referring physicians, and qualified medical directors to our network, which in turn provides our dialysis patient base with a large number of outpatient dialysis centers to choose from with convenient locations and access to a full range of other integrated services, which in turn provides us the ability to effectively and efficiently manage a patient’s care and certain costs while still maintaining strong legal and compliance programs.
74
The following graph summarizes our U.S. dialysis services revenues by modality for the year ended December 31, 2017:
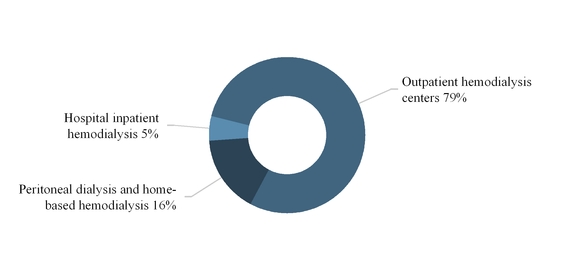
Approximately 86% of our 2017 consolidated net revenues were derived directly from our U.S. dialysis and related lab services business. Approximately 79% of our 2017 dialysis services revenues were derived from outpatient hemodialysis services in our 2,471 consolidated U.S. dialysis centers. Other dialysis services, which are operationally integrated with our dialysis operations, are peritoneal dialysis, home-based hemodialysis, hospital inpatient hemodialysis and management and administrative services provided to dialysis centers in which we own a noncontrolling interest or which are wholly owned by third parties. These services collectively accounted for the balance of our 2017 U.S. dialysis and related lab services revenues.
The principal drivers of our U.S. dialysis and related lab services revenues are:
• | the number of treatments, which is primarily a function of the number of chronic patients requiring approximately three treatments per week as well as, to a lesser extent, the number of treatments for peritoneal dialysis and home-based dialysis and hospital inpatient dialysis; and |
• | average dialysis net patient service revenue per treatment, including the mix of commercial and government patients. |
The total U.S. dialysis patient base is a relatively stable and growing factor, and is fundamentally influenced by a demographically growing need for dialysis services, as well as mortality rates that are common for patients with ESRD. The United States Renal Data System has reported an approximate compound annual growth rate of 3.8% from 2000 to 2015 for the U.S. dialysis patient population.
We believe our ability to maintain a stable or growing share of the U.S. dialysis patient base is influenced by the quality of our relationships with referring physicians and the quality of our clinical care, which can lead to reduced patient mortality rates, as described above, as well as our ability to open and acquire new dialysis centers.
Our average U.S. dialysis and related lab services net patient service revenue per treatment is driven by changes in our mix of commercial and government (principally Medicare and Medicaid) patients, commercial and government payment rates, and our billing and collecting operations performance.
On average, dialysis-related payment rates from contracted commercial payors are significantly higher than Medicare, Medicaid and other government program payment rates, and therefore the percentage of commercial patients in relation to total patients represents a major driver of our total average dialysis net patient service revenue per treatment. The percentage of commercial patients covered under contracted plans as compared to commercial patients with out-of-network providers has continued to increase, which can significantly affect our average dialysis net patient service revenue per treatment since commercial payment rates for patients with out-of-network providers are on average higher than in-network payment rates that are covered under commercial contracted plans.
In addition, growth of our government-based patients outpaced the growth of our commercial patients in 2017 due to a decrease in exchange patients. Government dialysis-related payment rates in the U.S. are principally determined by federal Medicare and state Medicaid policy. For patients with Medicare coverage, all ESRD payments for dialysis treatments are made under a single bundled payment rate which provides a fixed payment rate encompassing all goods and services provided during
75
the dialysis treatment, including certain pharmaceuticals such as Epogen® (EPO), vitamin D analogs and iron supplements, irrespective of the amount of pharmaceuticals administered to the patient or additional services performed. Most lab services are also included in the bundled payment. Under the ESRD PPS, the bundled payments to a dialysis facility may be reduced by as much as 2% based on the facility’s performance in specified quality measures set annually by CMS through QIP, which was established by the Medicare Improvements for Patients and Providers Act of 2008. The bundled payment rate is also adjusted for certain patient characteristics, a geographic usage index and certain other factors.
This bundled payment system presents certain operating, clinical and financial risks as further described in the risk factor in Item 1A Risk Factors under the heading "Changes in the structure of and payment rates under the Medicare ESRD program could have a material adverse effect on our business, results of operations and financial condition." For example, with regard to the expanded list of case-mix adjustors, there is a risk that our dialysis centers or billing and other systems may not accurately document and track the appropriate patient-specific characteristics, resulting in a reduction or overpayment in the amounts of the payments that we would otherwise be entitled to receive. In addition, as new drugs, services or labs are added to the ESRD bundle, CMS' failure to adequately calculate the costs associated with the drugs, services or labs could have a material adverse effect on our business, results of operations and financial condition.
Uncertainty about future payment rates remains a material risk to our business, as well as the potential implementation of or changes in coverage determinations or other rules or regulations by CMS or MACs that may impact reimbursement. An important provision in the law is an annual adjustment, or market basket inflation update, to the ESRD PPS base rate. Absent action by Congress, the PPS base rate is automatically updated annually by a formulaic inflation adjustment.
In December 2013, CMS issued the 2014 final rule for the ESRD PPS, which phases in the payment reductions mandated by ATRA, as modified by the Protecting Access to Medicare Act of 2014 which reduced our market basket inflation adjustment by 1.25% in 2016 and 2017, and by 1% in 2018. In November 2017, CMS published the 2018 final rule for the ESRD PPS, which increased dialysis facilities’ bundled payment rate for 2018 relative to prior years. In particular, CMS projects that the 2018 final rule for the ESRD PPS will (i) increase the total payments to all ESRD facilities by 0.5% in 2018 compared to 2017; (ii) increase total payments to hospital-based ESRD facilities by 0.7% in 2018 compared to 2017; and (iii) increase total payments for freestanding facilities by 0.5% in 2018 compared to 2017. The 2018 final rule for ESRD PPS also implements changes to the PPS outlier policy, broadening the pricing methodologies used to determine the cost of certain service drugs and biologicals in computing outlier payments when average sales price data is not available.
As a result of the BCA and subsequent activity in Congress, a $1.2 trillion sequester (across-the-board spending cuts) in discretionary programs took effect on April 1, 2013, reducing Medicare payments by 2% which was subsequently extended through fiscal year 2027. These across-the-board spending cuts have affected and will continue to adversely affect our business, results of operations and financial condition.
The CMS Innovation Center is working with various healthcare providers to develop, refine and implement ACOs and other innovative models of care for Medicare and Medicaid beneficiaries. We are uncertain of the extent to which the long-term operation and evolution of these models of care, including ACOs, Bundled Payments for Care Improvement Initiative, the CEC Model (which includes the development of ESCOs), the Comprehensive Primary Care Initiative, the Duals Demonstration, or other models, will impact the healthcare market over time. Our U.S. dialysis business may choose to participate in one or several of these models either as a partner with other providers or independently. We currently participate in the CEC Model with the Innovation Center, including with the ESCO organizations in the Arizona, Florida, and adjacent New Jersey and Pennsylvania markets. In areas where we are not directly participating in this or other Innovation Center models, some of our patients may be assigned to an ACO, another ESRD Care Model, or another program, in which case the quality and cost of care that we furnish will be included in an ACO’s, another ESRD Care Model’s or other programs’ calculations.
The Department of Health and Human Services (HHS) has also pledged to tie 50% of Medicare payments to quality or alternate payment models by the end of 2018. As new models of care emerge and evolve, we may be at risk for losing our Medicare patient base, which would have a material adverse effect on our revenues, earnings and cash flows. Other initiatives in the government or private sector may also arise, including the development of models similar to ACOs, independent practice associations (IPAs) and integrated delivery systems or evolutions of those concepts which could adversely impact our business.
We anticipate that we will continue to experience increases in our operating costs in 2018 that will outpace any net Medicare rate increases that we may receive, which could significantly impact our operating results. In particular, we expect to continue experiencing increases in operating costs that are subject to inflation, such as labor and supply costs, including increases in maintenance costs and capital expenditures to improve, renovate and maintain our facilities, equipment and information technology to meet changing regulatory requirements, regardless of whether there is a compensating inflation-based increase in Medicare payment rates or in payments under the bundled payment rate system.
76
Dialysis payment rates from commercial payors can vary and a major portion of our commercial rates are set at contracted amounts with payors and are subject to intense negotiation pressure. Our commercial payment rates also include payments for out-of-network patients that on average are higher than our in-network commercial contract rates. We continue to enter into some commercial contracts covering certain patients that will primarily pay us a single bundled payment rate for all dialysis services provided to these patients. However, some contracts will pay us for certain other services and pharmaceuticals in addition to the bundled payment. We are continuously in the process of negotiating agreements with our commercial payors, and if our negotiations result in overall commercial contract payment rate reductions in excess of our commercial contract payment rate increases, or if commercial payors implement plans that restrict access to coverage or the duration or breadth of benefits or impose restrictions or limitations on patient access to commercial plans on non-contracted or out-of-network providers, it could have a material adverse effect on our business, results of operations and financial condition. In addition, if there is an increase in job losses in the U.S., or depending upon changes to the healthcare regulatory system by CMS and/or the impact of healthcare insurance exchanges, we could experience a decrease in the number of patients covered under commercial insurance plans and/or an increase in uninsured or underinsured patients. Patients with commercial insurance who cannot otherwise maintain coverage frequently rely on financial assistance from charitable organizations, such as the American Kidney Fund. If these patients are unable to obtain or continue to receive or receive for a limited duration, such financial assistance, or if our assumptions about how patients will respond to any change in such financial assistance are incorrect, it could have a material adverse effect on our business, results of operations and financial condition. For further details, see the risk factor in Item 1A Risk Factors under the heading “If patients in commercial plans are subject to restriction in plan designs or the average rates that commercial payors pay us decline significantly, it would have a material adverse effect on our business, results of operations and financial condition.”
Our operating performance with respect to dialysis services billing and collection can also be a significant factor in the average U.S. dialysis and related lab services net patient service revenue per treatment we recognize and are able to collect. Over the past several years we have invested heavily in upgrades to our systems and internal processes that we believe have helped improve our operating performance and reduced our regulatory compliance risks, and we expect to continue to improve these systems and processes. We continue to upgrade our billing and other systems; however, as we continue to make upgrades to our systems and processes, or as payors change their systems and requirements, such as changes to what is included in the bundled payment from Medicare, we could experience a negative impact to our cash collection performance, which would affect our average U.S. dialysis and related lab services net patient service revenue per treatment.
Our U.S. dialysis and related lab services revenue recognition involves significant estimation risks. Our estimates are developed based on the best information available to us and our best judgment as to the reasonably assured collectability of our billings as of the reporting date based upon our actual historical collection experience. Changes in estimates are reflected in the then-current period financial statements based upon on-going actual experience and trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies.
Our average U.S. dialysis and related lab services net patient service revenue per treatment can be significantly impacted by several major factors, including our commercial payment rates; government payment policies regarding reimbursement amounts for dialysis treatments covered under Medicare’s bundled payment rate system, including our ability to capture certain patient characteristics; and changes in the mix of government and commercial patients and the number of commercial patients that are either covered under commercial contracts or are out of network.
Our annual average U.S. dialysis and related lab services net patient service revenue per treatment was approximately $330, $336 and $332 for 2017, 2016 and 2015, respectively. In 2017, our average U.S. dialysis and related lab services net patient service revenue per treatment decreased by approximately $5 per treatment due to a decrease in our commercial treatment volume, a decline in our commercial payor mix, including exchange patients, and an increase in our provision for uncollectible accounts. In 2016, our average U.S. dialysis and related lab services net patient service revenue per treatment increased by approximately $4 per treatment due to an increase in our average commercial payment rates and improvements in our commercial payor mix, partially offset by an increase in our provision for uncollectible accounts.
The principal drivers of our U.S. dialysis and related lab services patient care costs are clinical hours per treatment, labor rates, vendor pricing of pharmaceuticals, utilization levels of pharmaceuticals, business infrastructure costs, which include the operating costs of our dialysis centers, and certain professional fees. However, other cost categories can also present significant cost variability, such as employee benefit costs, payroll taxes, insurance costs and medical supply costs. In addition, currently pending and future proposed ballot initiatives or referendums, legislation or policy changes could cause us to incur substantial costs to challenge and, if implemented, impose additional requirements on our operations, including increases in the required staffing levels or staffing ratios for clinical personnel, minimum transition times between treatments, limits on how much patients may be charged for care, limitations as to the amount that can be spent on certain medical costs, and a ceiling on the percent of profit for such care. Changes such as these mandated by currently pending and future ballot initiatives or
77
referendums, legislation or policy changes would likely materially reduce our revenues and increase our operating expense and impact our ability to staff our clinics to the new, elevated staffing levels, in particular given the ongoing nationwide shortage of healthcare workers, especially nurses.
Our average clinical hours per treatment, or productivity levels, were flat in 2017 compared to 2016. We are always striving for improved productivity levels, however, changes in federal and state policies or regulatory billing requirements can lead to increased labor costs in order to implement these new requirements, which can adversely impact our ability to achieve optimal productivity levels. In addition, improvements in the U.S. economy have stimulated additional competition for skilled clinical personnel resulting in slightly higher teammate turnover in 2017, which we believe negatively affected productivity levels. In 2017 and 2016, we experienced an increase in our clinical labor rates of approximately 4.0% and 2.8%, respectively, consistent with general industry trends, mainly due to the high demand for and nationwide shortage of skilled clinical personnel, along with general inflation increases. In 2018, we will have a year-over-year accounting headwind of up to $100 million as we finish the transition from a profit sharing program to a 401(k) match program. With the old program, we accrued for the expense in the calendar year before payout; with the new program, we will accrue for the expense as we pay out. This accounting change created a one-year gap in 2017 when we did not need to accrue for any such payouts. We also continue to experience increases in the infrastructure and operating costs of our dialysis centers, primarily due to the number of new dialysis centers opened, and general increases in rent, utilities and repairs and maintenance. In 2017, we continued to implement certain cost control initiatives to manage our overall operating costs, including labor productivity.
Our U.S. dialysis and related lab services general and administrative expenses represented 8.1% and 8.2% of our U.S. dialysis and related lab services net revenues in 2017 and 2016, respectively. Although slightly down as a percent of net revenues, general and administrative expenses increased by $9 million, primarily due to an increase in labor and benefit costs and occupancy costs, partially offset by a decrease in long-term compensation, profit sharing and travel expenses. Increases in general and administrative expenses over the last several years were primarily related to strengthening our dialysis business, improving our regulatory compliance and other operational processes, responding to certain legal and compliance matters, and professional fees associated with enhancing our information technology systems. We expect that these levels of expenditures on our U.S. dialysis and related lab services general and administrative expenses will continue in 2018 and could possibly increase as we seek out new business opportunities within the dialysis industry and continue to invest in improving our information technology infrastructure and the level of support required for our regulatory compliance and legal matters.
78
Results of Operations
The following table reflects the results of operations for our U.S. dialysis and related lab services business:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(dollars in millions, except treatment data) | |||||||||||
U.S. dialysis and related lab patient service revenues | $ | 9,822 | $ | 9,551 | $ | 9,034 | |||||
Less: Provision for uncollectible accounts | (482 | ) | (430 | ) | (406 | ) | |||||
U.S. dialysis and related lab net patient service revenues | 9,340 | 9,121 | 8,628 | ||||||||
Other revenues | 20 | 17 | 14 | ||||||||
Total U.S. dialysis and related lab net services revenues | 9,360 | 9,138 | 8,642 | ||||||||
Operating expenses and charges: | |||||||||||
Patient care costs | 6,334 | 6,145 | 5,755 | ||||||||
General and administrative | 760 | 751 | 709 | ||||||||
Depreciation and amortization | 521 | 483 | 438 | ||||||||
Equity investment income | (25 | ) | (18 | ) | (15 | ) | |||||
Gain on settlement | (527 | ) | — | — | |||||||
Settlement charge and loss contingency accruals | — | — | 495 | ||||||||
Total operating expenses and charges | 7,063 | 7,361 | 7,382 | ||||||||
Operating income | $ | 2,297 | $ | 1,777 | $ | 1,260 | |||||
Reconciliation of non-GAAP measures: | |||||||||||
Gain on settlement, net | (527 | ) | — | — | |||||||
Equity investment income related to gain on settlement | (3 | ) | — | — | |||||||
Settlement charge | — | — | 495 | ||||||||
Adjusted operating income(1) | $ | 1,768 | $ | 1,777 | $ | 1,755 | |||||
Dialysis treatments | 28,271,113 | 27,162,545 | 25,986,719 | ||||||||
Average dialysis treatments per treatment day | 90,468 | 86,532 | 83,104 | ||||||||
Average U.S. dialysis and related lab services patient service revenue per treatment | $ | 347.43 | $ | 351.64 | $ | 347.64 | |||||
Less: Provision for uncollectible accounts per treatment | (17.05 | ) | (15.83 | ) | (15.64 | ) | |||||
Average U.S. dialysis and related lab services net patient service revenue per treatment | $ | 330.38 | $ | 335.81 | $ | 332.00 | |||||
Certain columns, rows or percentages may not sum or recalculate due to the use of rounded numbers.
(1) | For the periods presented in the table above adjusted operating income is defined as operating income before certain items which we do not believe are indicative of ordinary results, including a net settlement gain and a settlement charge related to a legal matter. Adjusted operating income as so defined is a non-GAAP measure and is not intended as a substitute for GAAP operating income. We have presented these adjusted amounts because management believes that these presentations enhance a user’s understanding of our normal consolidated operating income by excluding certain items which we do not believe are indicative of our ordinary results of operations. As a result, adjusting for these amounts allows for comparison to our normalized prior period results. |
Net revenues
U.S. dialysis and related lab services net revenues for 2017 increased by approximately $222 million, or 2.4%, from 2016. This increase in net revenues was primarily driven by solid volume growth from additional treatments of approximately 4.1% due to an increase in acquired and non-acquired treatments, including the acquisition of Renal Ventures. U.S. dialysis and related lab services’ net revenues was negatively impacted by approximately one less treatment day in 2017 as compared to 2016, a decrease in the average dialysis net patient service revenue per treatment of approximately $5, primarily due to a decrease in our commercial payor mix, including exchange patients. In addition, our provision for uncollectible accounts increased by $52 million in 2017.
79
U.S. dialysis and related lab services net revenues for 2016 increased by approximately $496 million, or 5.7%, from 2015. This increase in net revenues was primarily driven by solid volume growth from additional treatments of approximately 4.5% due to an increase in acquired and non-acquired treatment growth at existing and new dialysis centers, as well as one additional treatment day in 2016 as compared to 2015. U.S. dialysis and related lab services’ net revenues also benefited from an increase in the average dialysis net patient service revenue per treatment of approximately $4, primarily due to an increase in our average commercial payment rates and improvements in our commercial payor mix. In addition, our provision for uncollectible accounts increased by $24 million in 2016.
The following table summarizes our U.S. dialysis services revenues by source:
2017 | 2016 | 2015 | ||||||
Medicare and Medicare-assigned plans | 56 | % | 55 | % | 56 | % | ||
Medicaid and Managed Medicaid plans | 7 | 5 | 6 | |||||
Other government-based programs | 4 | 4 | 4 | |||||
Total government-based programs | 67 | 64 | 66 | |||||
Commercial (including hospital dialysis services) | 33 | 36 | 34 | |||||
Total U.S. dialysis and related lab services revenues | 100 | % | 100 | % | 100 | % | ||
Approximately 67% of our total U.S. dialysis services revenues for the year ended December 31, 2017 were from government-based programs, principally Medicare, Medicaid, Medicare-assigned and Managed Medicaid plans, representing approximately 89.5% of our total patients. Over the last year we have seen a decline in our commercial patients, which have been outpaced by the growth of our government-based patients. Less than 1% of our U.S. dialysis and related lab services revenues are due directly from patients. There is no single commercial payor that accounted for more than 10% of total U.S. dialysis and related lab services revenues for the year ended December 31, 2017.
On average, dialysis-related payment rates from contracted commercial payors are significantly higher than Medicare, Medicaid and other government program payment rates, and therefore the percentage of commercial patients as a relationship to total patients represents a major driver of our total average dialysis net patient service revenue per treatment. For a patient covered by a commercial insurance plan, Medicare generally becomes the primary payor after 33 months, which includes the three month waiting period, or earlier if the patient’s commercial insurance plan coverage terminates. When Medicare becomes the primary payor, the payment rates we receive for that patient shift from the commercial insurance plan rates to Medicare payment rates, which on average are significantly lower than commercial insurance rates. Medicare payment rates are insufficient to cover our costs associated with providing dialysis services, and we therefore lose money on each Medicare treatment that we provide.
Nearly all of our net earnings from our U.S. dialysis and related lab services are derived from commercial payors, some of which pay at established contract rates and others of which pay negotiated payment rates based on our usual and customary fee schedule for out-of-network patients, which are typically higher than commercial contracted rates. If we experience an overall net reduction in our contracted and non-contracted commercial payment rates as a result of negotiations, restrictions or changes to the healthcare regulatory system, including the potential impact of healthcare insurance exchanges, it could have a material adverse effect on our business, results of operations and financial condition.
Operating expenses and charges
Patient care costs. U.S. dialysis and related lab services patient care costs are those costs directly associated with operating and supporting our dialysis centers and consist principally of labor, benefits, pharmaceuticals, medical supplies and other operating costs of the dialysis centers. U.S. dialysis and related lab services patient care costs on a per treatment basis were $224 and $226 for 2017 and 2016, respectively. The $2 decrease in per treatment costs in 2017 as compared to 2016 was primarily attributable to a decrease in pharmaceutical unit costs due to a net price reduction as well as a decrease in profit sharing expense. These decreases were partially offset by an increase in labor and benefit costs due to an increase in teammates and clinical labor rates, and an increase in other direct operating expenses associated with our dialysis centers, including the impact of the hurricanes during the third quarter of 2017.
U.S. dialysis and related lab services patient care costs on a per treatment basis were $226 and $221 for 2016 and 2015, respectively. The $5 increase in per treatment costs in 2016 as compared to 2015 was primarily attributable to an increase in labor and benefit costs due to a decrease in productivity, increased turnover and clinical labor rates, an increase in other direct operating expenses associated with our dialysis centers and an increase in pharmaceutical unit costs. These increases were partially offset by a decrease in professional fees.
80
General and administrative expenses. U.S. dialysis and related lab services general and administrative expenses in 2017 increased by approximately $9 million as compared to 2016. This increase was primarily due to an increase in our labor and benefit costs, and occupancy costs, partially offset by a decrease in long-term incentive compensation, profit sharing and travel expenses.
U.S. dialysis and related lab services general and administrative expenses in 2016 increased by approximately $42 million as compared to 2015. This increase was primarily due to an increase in our labor and benefit costs, occupancy, and legal costs, partially offset by a decrease in long-term incentive compensation expense.
Depreciation and amortization. U.S. dialysis and related lab services depreciation and amortization expenses for 2017 increased by approximately $38 million as compared to 2016 and increased by $45 million in 2016 as compared to 2015. The increases were primarily due to both growth through new dialysis center developments and acquisitions as well as additional informational technology initiatives.
Gain on settlement, net. During the first quarter of 2017, we reached an agreement with the government for amounts owed to us for dialysis services provided from 2005 through 2011 to patients covered by the Department of Veterans Affairs (VA). As a result of this settlement we recognized a one-time net gain of $527 million as well as equity investment income of $3 million for our share of the settlement recognized by our nonconsolidated joint ventures. As such, the total effect of this settlement on our operating income was an increase of $530 million.
Provision for uncollectible accounts receivable. The provision for uncollectible accounts receivable for our U.S. dialysis and related lab services business was 4.9% for 2017 and 4.5% for both 2016 and 2015. We continue to experience higher amounts of accounts receivable write-offs due to uninsured and underinsured patients. We assess our level of provision for uncollectible accounts based upon our historical cash collection experience and trends, and have and will continue to adjust the provision as necessary as a result of changes in expectations based on our cash collections.
Equity investment income. Equity investment income was approximately $25 million, $18 million and $15 million in 2017, 2016 and 2015, respectively. The increases in equity investment income over the last three years were primarily due to the increase in the number of our nonconsolidated dialysis joint ventures and an increase in profitability at some of these joint ventures.
Segment operating income
U.S. dialysis and related lab services operating income for 2017, which includes a net gain on the VA settlement of $530 million, increased by approximately $520 million as compared to 2016. Excluding this item from 2017, U.S. dialysis and related lab services adjusted operating income decreased by approximately $9 million from 2016. This decrease in adjusted operating income was primarily due to a decrease in the average dialysis net patient service revenue per treatment of approximately $5, one less treatment day, partially offset by treatment growth, as described above. Adjusted operating income also decreased due to an increase in general and administrative expenses, partially offset by lower patient care costs, as described above.
U.S. dialysis and related lab services operating income for 2016 increased by approximately $517 million as compared to 2015, which included a settlement charge of $495 million. Excluding this item from 2015, U.S. dialysis and related lab services adjusted operating income increased by $22 million. This increase in adjusted operating income was primarily due to treatment growth as a result of additional dialysis treatments, one additional treatment day, and an increase in the average dialysis net patient service revenue per treatment of approximately $4, as described above. Adjusted operating income also increased due to a decrease in long-term incentive compensation expense, partially offset by higher patient care costs and an increase in general and administrative expenses, as described above.
Other—Ancillary services and strategic initiatives business
Our other operations include ancillary services and strategic initiatives which are primarily aligned with our core business of providing dialysis services to our network of patients. As of December 31, 2017, these consisted primarily of pharmacy services, disease management services, vascular access services, clinical research programs, physician services, direct primary care, ESRD seamless care organizations, and comprehensive care as well as our international operations.
Our ancillary services and strategic initiatives, including our pharmacy services and international operations among others, generated approximately $1.6 billion of net revenues in 2017, representing approximately 14% of our consolidated net revenues. We expect to add additional service offerings to our business and pursue additional strategic initiatives in the future as circumstances warrant, which could include healthcare services not related to dialysis. In addition, in connection with our
81
previously announced capital allocation strategy, in 2018 we plan to continue our evaluation of strategic alternatives for various assets in our portfolio. Any significant change in market conditions, or business performance, or in the political, legislative or regulatory environment, may impact the economic viability of any of our strategic initiatives. If any of our ancillary services or strategic initiatives, including our pharmacy services and our international operations, are unsuccessful, it would have a negative impact on our business, results of operations and financial condition, and we may determine to exit the line of business. We could incur significant termination costs if we were to exit certain of these lines of business. In addition, we may incur a material write-off or an impairment of our investment, including goodwill, in one or more of our ancillary services or strategic initiatives. In that regard, we have taken, and may in the future take, impairment charges related to our ancillary services and strategic initiatives, including in our international and pharmacy businesses.
As of December 31, 2017, our international dialysis operations provided dialysis and administrative services to a total of 237 outpatient dialysis centers located in 11 countries outside of the U.S. The total net revenues generated from our international operations, as reflected below, were approximately 3% of our 2017 consolidated net revenues.
82
The following table reflects the results of operations for the ancillary services and strategic initiatives:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(dollars in millions) | |||||||||||
U.S. revenues | |||||||||||
Other revenues | $ | 1,268 | $ | 1,413 | $ | 1,242 | |||||
Total | 1,268 | 1,413 | 1,242 | ||||||||
International revenues | |||||||||||
Net dialysis patient service revenues | 323 | 202 | 134 | ||||||||
Other revenues | 5 | 6 | 6 | ||||||||
Total | 328 | 208 | 140 | ||||||||
Total net revenues | $ | 1,596 | $ | 1,621 | $ | 1,382 | |||||
Operating expenses and charges: | |||||||||||
Operating and other general expenses | $ | 1,711 | $ | 1,686 | $ | 1,482 | |||||
Goodwill impairment | 36 | 28 | 4 | ||||||||
Impairment of investment | 295 | 15 | — | ||||||||
Gain from APAC JV ownership changes | (6 | ) | (374 | ) | — | ||||||
Total operating expenses and charges | 2,036 | 1,355 | 1,486 | ||||||||
Total ancillary services and strategic initiatives operating (loss) income | $ | (439 | ) | $ | 267 | $ | (104 | ) | |||
U.S. operating loss | $ | (110 | ) | $ | (65 | ) | $ | (45 | ) | ||
Reconciliation of non-GAAP: | |||||||||||
Goodwill impairment | 35 | 28 | — | ||||||||
Impairment of assets | 15 | — | — | ||||||||
Accruals for legal matters | — | 16 | 22 | ||||||||
Adjusted operating loss(1) | $ | (60 | ) | $ | (21 | ) | $ | (23 | ) | ||
International operating (loss) income | $ | (329 | ) | $ | 332 | $ | (59 | ) | |||
Reconciliation of non-GAAP: | |||||||||||
Goodwill impairment | — | — | 4 | ||||||||
Equity investment loss related to APAC JV goodwill impairment | 6 | — | — | ||||||||
Impairment of investment | 280 | 15 | — | ||||||||
Restructuring charges | 2 | — | — | ||||||||
Equity investment loss related to restructuring charges | 1 | — | — | ||||||||
Gain from APAC JV ownership changes | (6 | ) | (374 | ) | — | ||||||
Adjusted operating loss(1) | $ | (46 | ) | $ | (27 | ) | $ | (55 | ) | ||
Total adjusted ancillary services and strategic initiatives operating loss(1) | $ | (107 | ) | $ | (48 | ) | $ | (78 | ) | ||
Certain columns, rows or percentages may not sum or recalculate due to the use of rounded numbers.
(1) | For the periods presented in the table above adjusted operating loss is defined as operating loss before certain items which we do not believe are indicative of ordinary results, including goodwill impairment charges, investment and other asset impairments, restructuring charges, gains on ownership changes and accruals for legal matters. Adjusted operating loss as so defined is a non-GAAP measure and is not intended as a substitute for GAAP operating (loss) income. We have presented these adjusted amounts because management believes that these presentations enhance a user’s understanding of our normal consolidated operating (loss) income by excluding certain items which we do not believe are indicative of our ordinary results of operations. As a result, adjusting for these amounts allows for comparison to our normalized prior period results. |
83
Net revenues
Ancillary services and strategic initiatives net revenues for 2017 decreased by approximately $25 million, or 1.5%, as compared to 2016. This decrease was primarily related to a decrease in volume in our pharmaceutical business, partially offset by an increase in pharmaceutical rates, an increase in VillageHealth special needs plan revenues, an increase in shared savings revenue recognized by our ESCO joint ventures and an increase in net revenues from expansions in our international business and other strategic initiatives.
Ancillary services and strategic initiatives net revenues for 2016 increased by approximately $239 million, or 17.3%, as compared to 2015. This increase was primarily related to an increase in pharmaceutical rates, a decrease in reserves due to refunds of pharmacy reimbursements taken in 2015 that did not reoccur in 2016, an increase in VillageHealth special needs plan revenues and an increase in net revenues from expansions in our international business and other strategic initiatives. These increases were partially offset by a decrease in our pharmacy services volume.
Operating and general expenses
Ancillary services and strategic initiatives operating and general expenses for 2017, which includes restructuring charges related to our international business of $3 million, increased by approximately $25 million from 2016, which included an estimated accrual for certain legal matters of $16 million. Excluding these items from their respective periods, ancillary services and strategic initiatives adjusted operating expenses increased by $38 million. This increase in adjusted operating and general expenses was primarily related to an increase in medical costs at VillageHealth, an increase in labor and benefits costs and additional expenses associated with our international dialysis expansion, including losses from adverse changes in foreign exchange rates included in equity investment income, partially offset by a decrease in pharmaceutical costs due to decreased volume in our pharmacy services business.
Ancillary services and strategic initiatives operating and general expenses for 2016, which includes an estimated accrual for certain legal matters of $16 million, increased by approximately $203 million from 2015, which included an estimated accrual for certain legal matters of $22 million. Excluding these items from their respective periods, ancillary services and strategic initiatives adjusted operating expenses increased by $209 million. This increase in adjusted operating and general expenses was primarily due to an increase in pharmaceutical unit costs, labor and benefit costs, professional fees, other general and administration expenses, and additional expenses associated with our international dialysis expansion, partially offset by a decrease in prescription dispensing volume, long-term incentive compensation expense and foreign currency gains.
Investment and other asset impairments
During the year ended December 31, 2017, we recognized a non-cash other-than-temporary impairment charge of $280 million on our investment in the APAC JV. This charge resulted from changes in our expectations for the joint venture based on continuing market research and assessments by both us and the DaVita Care Pte. Ltd. (the APAC JV) concerning the size of the addressable market available to the joint venture at attractive risk-adjusted returns. We estimated the fair value of our retained interest in the APAC JV with the assistance of an independent third party valuation firm based on information available to management as of December 31, 2017. After this charge, our investment in the APAC JV was carried at $160 million as of December 31, 2017.
During the year ended December 31, 2017, we also recognized other asset impairment charges of $15 million related to a restructuring of our pharmacy business.
During the year ended December 31, 2016, we recognized an impairment of $15 million related to an investment in one of our international reporting units.
Goodwill impairment charges
During the year ended December 31, 2016, we recognized a goodwill impairment charge of $28 million related to our vascular access reporting unit as a result of changes in future governmental reimbursement rates for this business and our expected ability to mitigate them. Specifically, on November 2, 2016, CMS released the 2017 Physician Fee Schedule Final Rule and the Ambulatory Surgical Center Payment Final Rule which reflected significant changes in reimbursement structure for this business unit.
During the year ended December 31, 2017, we recognized an additional goodwill impairment charge of $35 million at our vascular access reporting unit. This charge resulted primarily from continuing changes in our outlook for this business unit as our partners and operators continued to evaluate and make decisions concerning changes in operations, including termination
84
of their management services agreements and center closures as a result of the changes in reimbursement structure discussed above. As of December 31, 2017, there was no goodwill remaining at our vascular access reporting unit.
We also recognized a goodwill impairment charge of $2 million at one of our international reporting units during the year ended December 31, 2017 and $4 million at another international reporting unit during the year ended December 31, 2015.
Restructuring charges
During the year ended December 31, 2017, we recognized total restructuring charges related to our international business of $2 million and recognized equity investment losses of $1 million related to restructuring charges at our APAC JV. These restructuring charges were related to a reorganization of our international general and administrative infrastructure at the global, regional and county levels in order to improve efficiency.
Gain on changes in ownership interests in APAC JV
As a result of our agreement with Khazanah Nasional Berhad (Khazanah) and Mitsui and Co., Ltd (Mitsui) concerning the APAC JV, we recorded an additional $6 million non-cash gain during the year ended December 31, 2017 related to a change in estimate of pending post-closing adjustments for the 2016 formation of this joint venture.
In 2016 we deconsolidated our Asia Pacific dialysis business and recognized an initial non-cash non-taxable estimated gain of $374 million on our retained investment in the APAC JV net of contingent obligations as a result of adjusting the carrying value of our retained interest in the APAC JV to our proportionate share of the estimated fair value of the business.
Segment operating (loss) income
Ancillary services and strategic initiatives operating results for 2017, which include goodwill impairment charges of $35 million at our vascular access reporting unit, an impairment of $280 million on our investment in the APAC JV, an asset impairment of $15 million related to the restructuring of our pharmacy business, equity investment losses of $6 million related to goodwill impairments at our APAC JV, restructuring charges related to our international business of $3 million and an adjustment to the gain on the 2016 ownership change of our APAC JV of $6 million, decreased by approximately $706 million from the same period in 2016, which included an estimated gain on the ownership change of our APAC JV of $374 million, a goodwill impairment charge of $28 million at our vascular access reporting unit, an estimated accrual for certain legal matters of $16 million and an investment impairment of $15 million. Excluding these items from their respective periods, adjusted operating losses increased by $59 million, primarily due to a decrease in revenues in our pharmacy services business, an increase in medical costs, higher labor and benefits costs, and additional expenses associated with our international operations, partially offset by an increase in VillageHealth special needs plan revenues, an increase in shared savings revenue recognized by our ESCO joint ventures, an increase in net revenues from expansion in our international business, and a decrease in pharmaceutical costs due to decreased volume in our pharmacy services business.
Ancillary services and strategic initiatives operating results for 2016, which includes an estimated gain on the ownership change of our APAC JV of $374 million, a goodwill impairment charge of $28 million at our vascular access reporting unit, an estimated accrual for certain legal matters of $16 million and an investment impairment of $15 million, increased by approximately $372 million from 2015, which included an estimated accrual for certain legal matters of $22 million, as well as a goodwill impairment charge of $4 million related to our international operations. Excluding these items from their respective periods, adjusted operating losses decreased by $30 million. This decrease in adjusted operating losses was primarily due to an increase in pharmaceutical rates, a decrease in reserves due to refunds of pharmacy reimbursements taken in 2015 that did not reoccur in 2016, an increase in VillageHealth special needs plan revenues and an increase in net revenues from our expansion in our international business and other strategic initiatives. The decrease in adjusted operating losses was partially offset by an increase in pharmaceutical unit costs, higher labor and benefits costs and additional expenses associated with our international dialysis expansion.
Corporate level charges
Debt expense. Debt expense for 2017, 2016, and 2015 consisted of interest expense of approximately $407 million, $394 million and $390 million, respectively, and amortization and accretion of debt discounts and premiums, amortization of deferred financing costs and amortization of interest rate cap agreements of approximately $24 million, $20 million, and $18 million, respectively. The increase in debt expense in 2017 as compared to 2016 was primarily due to an increase in our average interest rate, partially offset by a decrease in our average outstanding balance. Our overall weighted average effective interest rate in 2017 was 4.70% as compared to 4.43% in 2016.
85
The increase in debt expense in 2016 as compared to 2015 was primarily related to an increase in our weighted average outstanding principal balances as a result of a full year of interest on our 5.0% Senior Notes, which were issued in April 2015, and an increase in our interest rate on the amortization of our cap agreements in the fourth quarter of 2016. Our overall weighted average effective interest rate in 2016 was 4.43% as compared to 4.42% in 2015.
Corporate administrative support. Corporate administrative support consists primarily of labor, benefits and long-term incentive compensation expense, as well as professional fees for departments which provide support to all of our various operating lines of business. This is partially offset by internal management fees charged to our other lines of business for that support.
Corporate administrative support costs were approximately $45 million in 2017 and $14 million 2016. Corporate administrative support costs increased $31 million due to a decrease in internal management fees charged to our ancillary lines of business and increases in long-term incentive compensation and labor and benefits expenses, partially offset by decreases in professional fees and other general and administrative expenses.
Corporate administrative support costs were approximately $14 million in 2016 and $19 million in 2015. Corporate administrative support costs decreased $5 million primarily attributable to a decrease in long-term incentive compensation expense, primarily due to reductions in ultimate expected pay-outs as well as the departure of a senior executive, partially offset by increases in labor and benefits, professional fees, and other general and administrative expenses.
Other income. Other income was approximately $18 million in 2017 and $8 million in both 2016 and 2015, and consisted principally of interest income. Other income in 2017 as compared to 2016 increased approximately $10 million, primarily due to a decrease in foreign currency transaction losses. Other income in 2016 as compared to 2015 was flat, as short-term investment interest income increased but was offset by an increase in foreign currency transaction losses.
Provision for income taxes. The provision for income taxes for 2017, 2016 and 2015 represented an effective annualized tax rate of 23.1%, 26.6% and 30.1% of income from continuing operations, respectively. The effective tax rate in 2017 was lower primarily due to the enactment of new U.S. federal tax reform legislation known as the Tax Cuts and Jobs Act (the 2017 Tax Act) as signed into law on December 22, 2017. The 2017 Tax Act, among other changes, reduces the federal corporate income tax rate from 35% to 21%, effective January 1, 2018, resulting in a net income net tax benefit of $252 million in 2017 primarily related to a remeasurement of our net deferred tax liability. Excluding this item, our effective tax rate from continuing operations for 2017 was 41.1%. The effective tax rate in 2016 was lower primarily due to the gain on the APAC JV ownership changes, offset by goodwill impairment charges. See Note 12 to the consolidated financial statements for further information.
Noncontrolling interests
Net income attributable to noncontrolling interests for 2017, 2016 and 2015 was approximately $167 million, $153 million and $158 million, respectively. The increase in noncontrolling interests in 2017 was primarily due to additional income to noncontrolling interests related to the net gain on the settlement with the VA of $24 million, partially offset by the impairment of our vascular access reporting unit, which reduced income to noncontrolling interests by $2 million year over year.
The decrease in noncontrolling interests in 2016 was primarily due to the impairment of our vascular access reporting unit, which resulted in a decrease in income to noncontrolling interest of $8 million. The percentage of net U.S. dialysis and related lab services revenues generated from dialysis-related joint ventures was approximately 24% in 2017, and 23% in both 2016 and 2015.
Accounts receivable
Our consolidated accounts receivable balances at December 31, 2017 and December 31, 2016 were $1.715 billion and $1.504 billion, respectively, representing approximately 57 days and 52 days of revenue, respectively, net of the allowance for uncollectible accounts. The increase in consolidated DSO was primarily related to our U.S. dialysis and related lab services business and was due to changes we made in our collection policies and procedures to improve overall collections. We expect DSO to decline two to three days over the next few quarters as we continue to adjust and refine our collection operations for these new protocols. Our DSO calculation is based on the current quarter’s average revenues per day. There were no significant changes during 2017 from 2016 in the amount of unreserved accounts receivable over one year old or the amounts pending approval from third-party payors.
As of December 31, 2017 and 2016, our net patient services accounts receivable balances more than six months old represents approximately 21% and 16% of our dialysis accounts receivable balances, respectively. The increase was primarily
86
due to changes we made in our collection policies and procedures to improve overall collections. There were no significant unreserved balances over one year old. Approximately 1% of our revenues are classified as patient pay. Substantially all revenue realized is from government and commercial payors, as discussed above.
Amounts pending approval from third-party payors associated with Medicare bad debt claims as of December 31, 2017 and 2016, other than the standard monthly billing, consisted of approximately $104 million and $105 million, respectively, and are classified as other receivables. Currently, a significant portion of our Medicare bad debt claims are typically paid to us before the Medicare fiscal intermediary audits the claims. However, payments received from Medicare are subject to adjustment based upon the actual results of these audits. Such audits typically occur one to four years after the claims are filed.
Liquidity and capital resources
Available liquidity. As of December 31, 2017, our cash balance was $508 million and we also had approximately $44 million in short-term investments. We had $300 million drawn on our $1.0 billion revolving line of credit under our senior secured credit facilities, in addition to the approximately $14 million committed for outstanding letters of credit. We also have approximately $90 million of additional outstanding letters of credit related to Kidney Care and $0.2 million of committed outstanding letters of credit related to DMG, which is backed by a certificate of deposit. We believe that we will have sufficient liquidity, operating cash flows and access to borrowings to fund our scheduled debt service payments and other obligations for the foreseeable future. Our primary sources of liquidity are cash from operations and cash from borrowings.
Consolidated cash flows from operations during 2017 was $1.9 billion, of which $1.6 billion was from continuing operations, compared with consolidated cash flows from operations of $2.0 billion for 2016, of which $1.7 billion was from continuing operations. Consolidated cash flows declined due to an increase in DSO and the timing of other working capital items, partially offset by the payment received from the settlement with the VA, net of associated tax payments. Cash flows from operations in 2017 included cash interest payments of approximately $425 million and cash tax payments of $387 million. Cash flows from operations in 2016 included cash interest payments of approximately $407 million and cash tax payments of $339 million.
Non-operating cash outflows in 2017 included $905 million for capital asset expenditures, including $559 million for new center developments and relocations and $346 million for maintenance and information technology. We also spent an additional $804 million for acquisitions. In addition, during 2017 we received $21 million associated with stock award exercises and other share issuances. We also made distributions to noncontrolling interests of $211 million, which included $24 million related to the noncontrolling interest portion of the VA settlement gain, and received contributions from noncontrolling interests of $75 million associated with new or existing joint ventures. We also repurchased a total of 12,966,672 shares of our common stock for $811 million, or an average price of $62.54 per share, of which $8 million was unsettled at December 31, 2017.
Consolidated cash flows from operations during 2016 was $2.0 billion, of which $1.7 billion was from continuing operations, compared with cash flows from operations of $1.6 billion for 2015, of which $1.2 billion was from continuing operations. The increase in our operating cash flows in 2016 as compared to 2015 was primarily due to payments of $494 million, or $304 million after-tax, made in connection with the settlement of a private civil suit in 2015 and the timing of other working capital items, offset by an increase in our income tax payments and a slight increase in our cash interest payments. Cash flows from operations in 2016 included cash interest payments of approximately $407 million and cash tax payments of $339 million. Cash flows from operations in 2015 included cash interest payments of approximately $405 million and cash tax payments of $156 million.
Non-operating cash outflows in 2016 included $829 million for capital asset expenditures, including $470 million for new center developments and relocations and $359 million for maintenance and information technology. We also spent an additional $564 million for acquisitions. During 2016, we also received $1.3 billion from the maturity and sale of investments, however these proceeds were principally used to repurchase other investments or to fund distributions from our deferred compensation plans. In addition, during 2016 we received $37 million associated with stock award exercises and other share issuances and related excess tax benefits. We also made distributions to noncontrolling interests of $192 million, and received contributions from noncontrolling interests of $48 million associated with new or existing joint ventures. We also repurchased a total of 16,649,090 shares of our common stock for $1.1 billion, or an average price of $64.41 per share. In addition, we settled $25 million in share repurchases related to 2015.
During 2017, in the U.S. we opened 121 dialysis centers, acquired 66 dialysis centers, including dialysis centers from the Renal Ventures acquisition, closed and merged ten dialysis centers, closed nine dialysis centers, divested six dialysis centers, deconsolidated seven dialysis centers which we continue to operate under management services agreements, and terminated two management services agreements. In addition, our international dialysis operations acquired 68 dialysis centers,
87
opened eight dialysis centers, and closed one dialysis center. In addition, our APAC JV acquired two dialysis centers, opened nine dialysis centers and closed three dialysis centers.
During 2017, our DMG business acquired four primary care physician practices, including the acquisition of Magan, seven private medical practices, and one independent physician association.
On December 5, 2017, we entered into an equity purchase agreement to sell our DMG division to Optum, a subsidiary of UnitedHealth Group Inc., for $4.9 billion in cash, subject to net working capital and other customary adjustments. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions.
During 2016, in the U.S. we opened 100 new dialysis centers, acquired a total of eight dialysis centers, closed and merged five centers, added two centers which we operate under a management and administrative services agreement, terminated two management and administration services agreements, deconsolidated three centers which we now operate under management and administrative services agreements and closed four centers. Outside the U.S., we acquired 21 dialysis centers and opened 12 new dialysis and hospital operated centers. In addition, our APAC JV acquired three dialysis and hospital operated centers.
During 2016, our DMG business acquired three primary care physician practices including the acquisition of TEC, and four private medical practices.
During the year ended December 31, 2017, we made mandatory principal payments under our senior secured credit facilities totaling $88 million on Term Loan A and $35 million on Term Loan B. During the year ended December 31, 2016, we made mandatory principal payments under our senior secured credit facilities totaling $63 million on Term Loan A and $35 million on Term Loan B.
Interest rate cap agreements
As of December 31, 2017, we maintain several currently effective interest rate cap agreements that were entered into in November 2014 with notional amounts totaling $3.5 billion. These cap agreements became effective September 30, 2016 and have the economic effect of capping the LIBOR variable component of our interest rate at a maximum of 3.50% on an equivalent amount of our debt. These cap agreements expire on June 30, 2018. As of December 31, 2017, these cap agreements had an immaterial fair value. During the year ended December 31, 2017, we recognized debt expense of $8.3 million from these caps. During the year ended December 31, 2017, we recorded a loss of $0.1 million in other comprehensive income due to a decrease in the unrealized fair value of these cap agreements.
As of December 31, 2017, we also maintain several forward interest rate cap agreements that were entered into in October 2015 with notional amounts totaling $3.5 billion. These forward cap agreements will become effective June 29, 2018 and will have the economic effect of capping the LIBOR variable component of our interest rate at a maximum of 3.50% on an equivalent amount of its debt. These cap agreements expire on June 30, 2020. As of December 31, 2017, the total fair value of these cap agreements was an asset of approximately $1.0 million. During the year ended December 31, 2017, we recorded a loss of $8.8 million in other comprehensive income due to a decrease in the unrealized fair value of these cap agreements.
Other items
As of December 31, 2017, our Term Loan B debt bears interest at LIBOR plus an interest rate margin of 2.75%. Term Loan B is subject to interest rate caps if LIBOR should rise above 3.50%. Term Loan A bears interest at LIBOR plus an interest rate margin of 2.00%. The capped portion of Term Loan A is $122.5 million. In addition, the uncapped portion of Term Loan A, which is subject to the variability of LIBOR, is $652.5 million. Interest rates on our senior notes are fixed by their terms.
Our overall weighted average effective interest rate on the senior secured credit facilities was 4.45%, based on the current margins in effect of 2.00% for Term Loan A and the Revolver and 2.75% for Term Loan B, as of December 31, 2017.
As of December 31, 2017, our interest rates are fixed on approximately 52% of our total debt.
Our overall weighted average effective interest rate during the year ended December 31, 2017 was 4.70% and as of December 31, 2017 was 4.88%.
As of December 31, 2017, we had $300 million drawn on our $1.0 billion revolving line of credit under our senior secured credit facilities, in addition to approximately $14.4 million committed for outstanding letters of credit. We also have approximately $90.1 million of additional outstanding letters of credit related to Kidney Care and $0.2 million of committed outstanding letters of credit related to DMG, which is backed by a certificate of deposit.
88
We believe that we will generate significant operating cash flows and will have sufficient liquidity to fund our scheduled debt service and other obligations for the foreseeable future, including the next 12 months, under the terms of our debt agreements. However, our primary sources of liquidity are cash from operations and cash from borrowings, including general, economic, financial, competitive, regulatory and other factors that are beyond our control, as described in the risk factor in Item IA Risk Factors under the heading "The level of our current and future debt could have an adverse impact on our business and our ability to generate cash to service our indebtedness and for other intended purposes depends on many factors beyond our control."
Goodwill
We elected to early adopt ASU No. 2017-04, Intangibles-Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment, effective January 1, 2017. The amendments in this ASU simplify the test for goodwill impairment by eliminating the second step in the assessment. All goodwill impairment tests performed during 2017 have been performed under this new guidance.
During the year ended December 31, 2016, we recognized a goodwill impairment charge of $28 million related to our vascular access reporting unit as a result of changes in future governmental reimbursement rates for this business and our expected ability to mitigate them. Specifically, on November 2, 2016, CMS released the 2017 Physician Fee Schedule Final Rule and the Ambulatory Surgical Center Payment Final Rule which reflected significant changes in reimbursement structure for this business unit.
During the year ended December 31, 2017, we recognized an additional goodwill impairment charge of $35 million at our vascular access reporting unit. This charge resulted primarily from continuing changes in our outlook for this business unit as our partners and operators continued to evaluate and make decisions concerning changes in operations, including termination of their management services agreements and center closures, as a result of the changes in reimbursement structure discussed above. As of December 31, 2017, there was no goodwill remaining at our vascular access reporting unit.
During the year ended December 31, 2017, we also performed annual impairment assessments for various other reporting units. As a result of these assessments, we also recognized a goodwill impairment charge of $2 million at one of our international reporting units during the year ended December 31, 2017. During the year ended December 31, 2015, we recognized a goodwill impairment charge of $4 million in another international reporting unit.
Based on our most recent assessments, we determined that reductions in reimbursement rates, changes in actual or expected growth rates, or other significant adverse changes in expected future cash flows or valuation assumptions could result in goodwill impairment charges in the future for the following reporting units, which remain at risk of goodwill impairment as of December 31, 2017:
Reporting unit | Goodwill balance as of December 31, 2017 | Carrying amount coverage(1) | Sensitivities | |||||||
Operating income(2) | Discount rate(3) | |||||||||
(in millions) | ||||||||||
Kidney Care Germany | $ | 316 | 13.7% | (1.6)% | (11.1)% | |||||
Kidney Care Portugal | $ | 47 | 16.9% | (1.9)% | (6.0)% | |||||
Kidney Care Poland | $ | 47 | 11.8% | (1.9)% | (6.0)% | |||||
(1) | Excess of estimated fair value of the reporting unit over carrying amount as of the latest assessment date. |
(2) | Potential impact on estimated fair value of a sustained, long-term reduction of 3% in operating income as of the latest assessment date. |
(3) | Potential impact on estimated fair value of an increase in discount rates of 100 basis points as of the latest assessment date. |
There were no major changes in the business, prospects, or expected future results of these reporting units from their latest assessment date through December 31, 2017.
Except as described above, none of our various other reporting units was considered at risk of significant goodwill impairment as of December 31, 2017. Since the dates of our last annual goodwill impairment tests, there have been certain developments, events, changes in operating performance and other changes in key circumstances that have affected our businesses. However, except as further described above, these did not cause management to believe it is more likely than not that the fair values of any of our reporting units would be less than their respective carrying amounts as of December 31, 2017.
89
Long-term incentive compensation
Long-term incentive program (LTIP) compensation includes both stock-based awards (principally stock-settled stock appreciation rights, restricted stock units and performance stock units) as well as long-term performance-based cash awards. Long-term incentive compensation expense, which was primarily general and administrative in nature, was attributed among our U.S. dialysis and related lab services business, corporate administrative support, and the ancillary services and strategic initiatives.
Our stock-based compensation awards are measured at their estimated fair values on the date of grant if settled in shares or at their estimated fair values at the end of each reporting period if settled in cash. The value of stock-based awards so measured is recognized as compensation expense on a cumulative straight-line basis over the vesting terms of the awards, adjusted for expected forfeitures.
During 2017, we granted approximately 1,692,154 stock-settled stock appreciation rights (SSARs) with an aggregate grant-date fair value of $24.5 million and a weighted-average expected life of approximately 4.2 years and approximately 528,968 stock units with an aggregate grant-date fair value of $34.8 million and a weighted-average expected life of approximately 3.4 years. We also granted 15,000 cash-settled stock-based awards with an aggregate grant-date fair value of $0.3 million.
For the years ended December 31, 2017 and 2016, long-term incentive compensation expense of $62.0 million and $65.0 million decreased by approximately $3.0 million and $59.0 million as compared to 2016 and 2015, respectively. This decrease in long-term incentive compensation expense was primarily due to cumulative revaluation of liability-based awards for reductions in estimated ultimate payouts, as well as the final vesting of a prior broad grant that is no longer contributing expense.
As of December 31, 2017, there was $98.0 million in total estimated but unrecognized long-term incentive compensation expense for LTIP awards outstanding, including $61.2 million relating to stock-based awards under our equity compensation plans. We expect to recognize the performance-based cash component of these LTIP costs over a weighted average remaining period of 1.1 years and the stock-based component of these LTIP costs over a weighted average remaining period of 1.4 years.
For the years ended December 31, 2017, 2016 and 2015, we received $13.5 million, $28.4 million and $45.7 million, respectively, in actual tax benefits upon the exercise of stock awards. Since we issue stock-settled stock appreciation rights rather than stock options, we did not receive cash proceeds from stock option exercises during the years ended December 31, 2017, 2016 and 2015.
Stock repurchases
We repurchased a total of 12,966,672 shares for $811 million, or an average price of $62.54 during the year ended December 31, 2017. We also repurchased a total of 16,649,090 shares for $1.1 billion, or an average price of $64.41 during the year ended December 31, 2016 and a total of 7,779,958 shares for $575 million, or an average price of $73.96 during the year ended December 31, 2015. Subsequent to December 31, 2017, we have repurchased 1,237,800 additional shares of our common stock for $93 million, or an average price of $74.96 per share, through February 22, 2018.
On October 10, 2017, our Board of Directors approved an additional share repurchase authorization in the amount of $1.3 billion. This share repurchase authorization was in addition to the $247 million remaining at that time under our Board of Directors’ prior share repurchase authorization announced in July 2016. Accordingly, as of February 22, 2018, we have a total of $1.0 billion available under the current Board repurchase authorizations for additional share repurchases. Although these share repurchase authorizations do not have expiration dates, we remain subject to share repurchase limitations under the terms of our senior secured credit facilities and the indentures governing our senior notes.
Off-balance sheet arrangements and aggregate contractual obligations
In addition to the debt obligations reflected on our balance sheet, we have commitments associated with operating leases and letters of credit, as well as potential obligations associated with our equity investments in nonconsolidated businesses and to dialysis centers that are wholly-owned by third parties. Substantially all of our U.S. dialysis facilities are leased. We have potential obligations to purchase the noncontrolling interests held by third parties in several of our majority-owned joint ventures and other nonconsolidated entities. These obligations are in the form of put provisions that are exercisable at the third-party owners’ discretion within specified periods as outlined in each specific put provision. If these put provisions were exercised, we would be required to purchase the third-party owners’ equity interests at either the appraised fair market value or
90
a predetermined multiple of earnings or cash flows attributable to the equity interests put to us, which is intended to approximate fair value. The methodology we use to estimate the fair values of noncontrolling interests subject to put provisions assumes the higher of either a liquidation value of net assets or an average multiple of earnings, based on historical earnings, patient mix and other performance indicators that can affect future results, as well as other factors. The estimated fair values of noncontrolling interests subject to put provisions are a critical accounting estimate that involves significant judgments and assumptions and may not be indicative of the actual values at which the noncontrolling interests may ultimately be settled, which could vary significantly from our current estimates. The estimated fair values of noncontrolling interests subject to put provisions can fluctuate and the implicit multiple of earnings at which these noncontrolling interests obligations may be settled will vary significantly depending upon market conditions including potential purchasers’ access to the capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the third-party owners’ equity interests. The amount of noncontrolling interests subject to put provisions that employ a contractually predetermined multiple of earnings rather than fair value are immaterial. For additional information see Note 17 to the consolidated financial statements.
We also have certain other potential commitments to provide operating capital to several dialysis centers that are wholly-owned by third parties or centers in which we own a noncontrolling equity interest as well as to physician-owned vascular access clinics or medical practices that we operate under management and administrative services agreements.
The following is a summary of these contractual obligations and commitments as of December 31, 2017:
Less than 1 year | 1-3 years | 4-5 years | After 5 years | Total | |||||||||||||||
(dollars in millions) | |||||||||||||||||||
Scheduled payments under contractual obligations: | |||||||||||||||||||
Long-term debt | $ | 158 | $ | 1,078 | $ | 4,549 | $ | 3,318 | $ | 9,103 | |||||||||
Interest payments on the senior notes | 237 | 473 | 473 | 367 | 1,550 | ||||||||||||||
Interest payments on Term Loan B(1) | 148 | 290 | 71 | — | 509 | ||||||||||||||
Interest payments on Term Loan A(2) | 27 | 12 | — | — | 39 | ||||||||||||||
Kidney Care capital lease obligations | 20 | 44 | 43 | 190 | 297 | ||||||||||||||
Kidney Care operating leases | 447 | 807 | 665 | 1,304 | 3,223 | ||||||||||||||
DMG capital lease obligations | 37 | — | — | — | 37 | ||||||||||||||
DMG operating leases | 85 | 152 | 108 | 283 | 628 | ||||||||||||||
$ | 1,159 | $ | 2,856 | $ | 5,909 | $ | 5,462 | $ | 15,386 | ||||||||||
Potential cash requirements under other commitments: | |||||||||||||||||||
Letters of credit | 105 | $ | — | $ | — | $ | — | $ | 105 | ||||||||||
Noncontrolling interests subject to put provisions | 613 | 211 | 96 | 91 | 1,011 | ||||||||||||||
Non-owned and minority owned put provisions | 27 | — | 28 | — | 55 | ||||||||||||||
Operating capital advances | 1 | 1 | 1 | 2 | 5 | ||||||||||||||
Purchase commitments | 447 | 644 | 497 | — | 1,588 | ||||||||||||||
$ | 1,193 | $ | 856 | $ | 622 | $ | 93 | $ | 2,764 | ||||||||||
(1) | Based upon current LIBOR-based interest rates in effect at December 31, 2017 plus an interest rate margin of 2.75% for Term Loan B. |
(2) | Based upon current LIBOR-based interest rates in effect at December 31, 2017 plus an interest rate margin of 2.00% for Term Loan A. |
In 2010, we entered into and subsequently extended an agreement with FMC to purchase a certain amount of dialysis equipment, parts and supplies from FMC through December 31, 2017. In January 2018, we entered into a new agreement extending this agreement with FMC through December 31, 2020. The actual amount of purchases in future years from FMC will depend upon a number of factors, including the operating requirements of our centers, the number of centers we acquire, and growth of our existing centers.
We are party to agreements with Baxter Healthcare Corporation (Baxter) that commit us to purchase a certain amount of hemodialysis non-equipment product supplies, such as dialyzers, at fixed prices through 2018. In addition, in February 2018 we amended our agreement with Baxter related to certain peritoneal dialysis supplies. Under this new contract with Baxter we have committed to purchase a certain amount of peritoneal dialysis supplies at fixed prices (as set forth in the contract for each year) through 2022.
91
In January 2017, we entered into a Sourcing and Supply Agreement with Amgen USA Inc. (Amgen) that expires on December 31, 2022. Under the terms of the agreement, we will purchase EPO in amounts necessary to meet no less than 90% of our requirements for ESAs through the expiration of the contract. The actual amount of EPO that we will purchase will depend upon the amount of EPO administered during dialysis as prescribed by physicians and the overall number of patients that we serve.
Settlements of approximately $33 million of existing income tax liabilities for unrecognized tax benefits, including interest, penalties and other long-term tax liabilities, are excluded from the above table as reasonably reliable estimates of their timing cannot be made.
Supplemental information concerning certain Physician Groups and unrestricted subsidiaries
The following information is presented as supplemental data as required by the indentures governing our senior notes.
We provide services to certain physician groups, including those within our DMG business, which while consolidated in our financial statements for financial reporting purposes, are not subsidiaries of or owned by us, do not constitute “Subsidiaries” as defined in the indentures governing our outstanding senior notes, and do not guarantee those senior notes. In addition, we have entered into management agreements with these physician groups pursuant to which we receive management fees from the physician groups.
As of December 31, 2017, if these physician groups were not consolidated in our financial statements, our consolidated assets would have been approximately $18.522 billion and our consolidated other liabilities would have been approximately $3.342 billion. Our consolidated indebtedness would have remained approximately $9.400 billion due to these physician groups being classified as held for sale. For the year ended December 31, 2017, if these physician groups were not consolidated in our financial statements, our consolidated net income would have been reduced by approximately $21 million. Our consolidated total net revenues and consolidated operating income would have remained approximately $10.877 billion and $1.813 billion, respectively, due to these physician groups being reported as discontinued operations.
In addition, our DMG business owns a 67% equity interest in California Medical Group Insurance (CMGI), which is an Unrestricted Subsidiary as defined in the indentures governing our outstanding senior notes, and does not guarantee those senior notes. DMG's equity interest in CMGI is accounted for under the equity method of accounting, meaning that, although CMGI is not consolidated in our financial statements for financial reporting purposes, our consolidated income statement reflects our pro rata share of CMGI’s net income within net loss from discontinued operations.
For the year ended December 31, 2017, excluding DMG's equity investment income attributable to CMGI, our consolidated net income would be decreased by approximately $19 thousand. See Note 29 to the consolidated financial statements for further details.
Contingencies
The information in Note 16 to the consolidated financial statements of this report is incorporated by reference in response to this item.
Critical accounting policies, estimates and judgments
Our consolidated financial statements and accompanying notes are prepared in accordance with United States generally accepted accounting principles. These accounting principles require us to make estimates, judgments and assumptions that affect the reported amounts of revenues, expenses, assets, liabilities, contingencies and temporary equity. All significant estimates, judgments and assumptions are developed based on the best information available to us at the time made and are regularly reviewed and updated when necessary. Actual results will generally differ from these estimates. Changes in estimates are reflected in our financial statements in the period of change based upon on-going actual experience trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies. Interim changes in estimates are applied prospectively within annual periods. Certain accounting estimates, including those concerning revenue recognition and accounts receivable, impairments of goodwill and investments, accounting for income taxes, quarterly and annual variable compensation accruals, consolidation of variable interest entities, and fair value estimates are considered to be critical to evaluating and understanding our financial results because they involve inherently uncertain matters and their application requires the most difficult and complex judgments and estimates.
U.S. dialysis and related lab services revenue recognition and accounts receivable. There are significant estimating risks associated with the amount of U.S. dialysis and related lab services revenue that we recognize in a given reporting period. Payment rates are often subject to significant uncertainties related to wide variations in the coverage terms of the commercial
92
healthcare plans under which we receive payments. In addition, ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage, and other payor issues complicate the billing and collection process. Net revenue recognition and allowances for uncollectible billings require the use of estimates of the amounts that will ultimately be realized considering, among other items, retroactive adjustments that may be associated with regulatory reviews, audits, billing reviews and other matters.
Revenues associated with Medicare and Medicaid programs are recognized based on (a) the payment rates that are established by statute or regulation for the portion of the payment rates paid by the government payor (e.g., 80% for Medicare patients) and (b) for the portion not paid by the primary government payor, the estimated amounts that will ultimately be collectible from other government programs paying secondary coverage (e.g., Medicaid secondary coverage), the patient’s commercial health plan secondary coverage, or the patient. Our dialysis related reimbursements from Medicare are subject to certain variations under Medicare’s single bundled payment rate system whereby our reimbursements can be adjusted for certain patient characteristics and certain other factors. Our revenue recognition depends upon our ability to effectively capture, document and bill for Medicare’s base payment rate and these other factors. In addition, as a result of the potential range of variations that can occur in our dialysis-related reimbursements from Medicare under the single bundled payment rate system, our revenue recognition is subject to a greater degree of estimating risk.
Commercial healthcare plans, including contracted managed-care payors, are billed at our usual and customary rates; however, revenue is recognized based on estimated net realizable revenue for the services provided. Net realizable revenue is estimated based on contractual terms for the patients covered under commercial healthcare plans with which we have formal agreements, non-contracted commercial healthcare plan coverage terms if known, estimated secondary collections, historical collection experience, historical trends of refunds and payor payment adjustments (retractions), inefficiencies in our billing and collection processes that can result in denied claims for payments, a slowdown in collections, a reduction in the amounts that we expect to collect and regulatory compliance issues. Determining applicable primary and secondary coverage for our approximately 197,800 U.S. dialysis patients at any point in time, together with the changes in patient coverages that occur each month, requires complex, resource-intensive processes. Collections, refunds and payor retractions typically continue to occur for up to three years or longer after services are provided.
We generally expect the range of our U.S. dialysis and related lab services revenues estimating risk to be within 1% of its revenue, which can represent as much as approximately 5% of U.S. dialysis and related lab services’ adjusted operating income. Changes in estimates are reflected in the then-current financial statements based on on-going actual experience trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies. Changes in revenue estimates for prior periods are separately disclosed and reported if material to the current reporting period and longer term trend analyses, and have not been significant.
Laboratory service revenues for current period dates of services are recognized at the estimated net realizable amounts to be received.
Impairments of goodwill and investments. We account for impairments of goodwill and equity method and other investments in accordance with the provisions of applicable accounting guidance. Goodwill is not amortized, but is assessed for impairment when changes in circumstances warrant and at least annually. An impairment charge would be recorded to the extent that the carrying amount of a reporting unit’s goodwill exceeds its estimated fair value. Equity method and other investments are assessed for other-than-temporary impairment when changes in circumstances warrant. An other-than-temporary impairment charge is recorded when the fair value of an investment has fallen below its carrying amount and the shortfall is expected to be indefinitely or permanently unrecoverable.
Such changes can include, among others, changes in the legal environment, addressable market, business strategy, development or business plans, reimbursement structure, operating performance, future prospects, relationships with partners, and/or market value indications for the subject business. We use a variety of factors to assess changes in the financial condition, future prospects and other circumstances concerning the subject businesses and to estimate their fair value when applicable. Any change in the factors, assessments or assumptions involved could impact a determination of whether and when to assess goodwill or an investment for impairment as well as the outcome of such an assessment. These assessments and the related valuations can involve significant uncertainties and require significant judgment on various matters, some of which could be subject to reasonable disagreement.
Accounting for income taxes. Our income tax expense, deferred tax assets and liabilities, and liabilities for unrecognized tax benefits reflect management’s best assessment of estimated current and future taxes to be paid. We are subject to income taxes in the United States and numerous state and foreign jurisdictions, and changes in tax laws or regulations may be proposed or enacted that could adversely affect our overall tax liability. The actual impact of any such laws or regulations, including the 2017 Tax Act, could be materially different from our current estimates.
93
Significant judgments and estimates are required in determining our consolidated income tax expense. Deferred income taxes arise from temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements, which will result in taxable or deductible amounts in the future. In evaluating our ability to recover our deferred tax assets within the jurisdiction from which they arise, we consider all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax-planning strategies, and results of recent operations, assumptions about the amount of future federal, state, and foreign pre-tax operating income adjusted for items that do not have tax consequences. The assumptions about future taxable income require significant judgments and are consistent with the plans and estimates we are using to manage the underlying businesses. To the extent that recovery is not likely, a valuation allowance is established. The allowance is regularly reviewed and updated for changes in circumstances that would cause a change in judgment about the realizability of the related deferred tax assets.
Variable compensation accruals. We estimate variable compensation accruals quarterly based upon the amounts expected to be earned and paid out resulting from the achievement of certain teammate-specific and/or corporate financial and operating goals. Our estimates, which include compensation incentives for bonuses and other awards, including long-term incentive programs, are updated periodically based on changes in our economic condition or cash flows that could ultimately impact the actual final payment amount. Actual results reflected in each fiscal quarter may vary due to the subjectivity involved in anticipating fulfillment of specific and/or corporate goals, as well as the final determination and approval of amounts by our Board of Directors, as applicable.
Consolidation of variable interest entities. We rely on the operating activities of certain entities that we do not directly own or control, but over which we have indirect influence and of which we are considered the primary beneficiary. Under accounting guidance applicable to variable interest entities, we have determined that these entities are to be included in our consolidated financial statements. The analyses upon which these determinations rest are complex, involve uncertainties, and require significant judgment on various matters, some of which could be subject to reasonable disagreement. While these determinations have a meaningful effect on the description and classification of various amounts in our consolidated financial statements, non-consolidation of these entities would not have had a material effect on our results of operations.
Fair value estimates. We rely on fair value measurements and estimates for purposes that require the recording, reassessment, or adjustment of the carrying amounts of certain assets, liabilities and noncontrolling interest subject to put provisions (temporary equity). These purposes can include the accounting for business combination transactions, impairment assessments for goodwill, investments, or other long-lived assets, and stock-based compensation, among others. The criticality of a particular fair value estimate to our consolidated financial statements depends upon the nature and size of the item being measured and the extent of uncertainties involved and the nature and magnitude or potential effect of assumptions and judgments required. Critical fair value estimates can involve significant uncertainties and require significant judgment on various matters, some of which could be subject to reasonable disagreement.
The FASB defines fair value as the amount at which an asset (or liability) could be bought (or incurred) or sold (or settled) between willing parties, that is, other than in a forced or liquidation sale. Critical fair value estimates can be required for measurement of goodwill and equity method and other investment impairments, as discussed previously. Fair value estimates can also be critical in accounting for major acquisitions or business combination transactions of significant size involving businesses or industries in which we and/or our professional valuation advisors do not have significant experience. In these cases, the nature and size of the item being measured and the extent of uncertainties involved, as well as the nature and magnitude or potential effect of assumptions and judgments required, can make the fair value estimate a critical accounting estimate.
Significant new accounting standards
See Note 1 to the consolidated financial statements included in this report for information regarding certain recent financial accounting standards that have been issued by the FASB.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
Interest rate sensitivity
The tables below provide information about our financial instruments that are sensitive to changes in interest rates. The table below presents principal repayments and current weighted average interest rates on our debt obligations as of December 31, 2017. The variable rates presented reflect the weighted average LIBOR rates in effect for all debt tranches plus interest rate margins in effect as of December 31, 2017. The Term Loan A margin in effect at December 31, 2017 is 2.00%, and along with the revolving line of credit, is subject to adjustment depending upon changes in certain of our financial ratios, including a leverage ratio. Term Loan B currently bears interest at LIBOR plus an interest rate margin of 2.75%.
94
Expected maturity date | Thereafter | Total | Average interest rate | Fair value | ||||||||||||||||||||||||||||||
2018 | 2019 | 2020 | 2021 | 2022 | ||||||||||||||||||||||||||||||
(dollars in millions) | ||||||||||||||||||||||||||||||||||
Long term debt: | ||||||||||||||||||||||||||||||||||
Fixed rate | $ | 36 | $ | 28 | $ | 27 | $ | 26 | $ | 1,276 | $ | 3,501 | $ | 4,894 | 5.28 | % | $ | 4,961 | ||||||||||||||||
Variable rate | $ | 142 | $ | 1,021 | $ | 46 | $ | 3,282 | $ | 8 | $ | 7 | $ | 4,506 | 4.45 | % | $ | 4,549 | ||||||||||||||||
Notional amount | Contract maturity date | Receive variable | Fair value | ||||||||||||||||||||||||||
2018 | 2019 | 2020 | 2021 | 2022 | |||||||||||||||||||||||||
(dollars in millions) | |||||||||||||||||||||||||||||
Cap agreements | $ | 7,000 | $ | 3,500 | $ | — | $ | 3,500 | $ | — | $ | — | LIBOR above 3.5% | $ | 1.0 | ||||||||||||||
Our senior secured credit facilities, which include Term Loan A and Term Loan B, consist of various individual tranches of debt that can range in maturity from one month to twelve months (currently, all tranches are one month in duration). For Term Loan A and Term Loan B, each tranche bears interest at a LIBOR rate that is determined by the duration of such tranche plus an interest rate margin. The LIBOR variable component of the interest rate for each tranche is reset as such tranche matures and a new tranche is established. LIBOR can fluctuate significantly depending upon conditions in the credit and capital markets.
As of December 31, 2017, our Term Loan A bears interest at LIBOR plus an interest rate margin of 2.00% and our Term Loan B debt bears interest at LIBOR plus an interest rate margin of 2.75%. LIBOR was greater than the 0.75% embedded LIBOR floor on Term Loan B, resulting in Term Loan B being subject to LIBOR-based interest rate volatility on the LIBOR variable component of our interest rate as of December 31, 2017. The LIBOR-based interest component is effectively limited to a maximum LIBOR rate of 3.50% on the outstanding principal debt on Term Loan B and on $122.5 million of Term Loan A as a result of the interest rate cap agreements, as described below. In addition, the uncapped portion of Term Loan A, which is subject to the variability of LIBOR, is $652.5 million. Interest rates on our senior notes are fixed by their terms.
As of December 31, 2017, we maintain several currently effective interest rate cap agreements that were entered into in November 2014 with notional amounts totaling $3.5 billion. These cap agreements became effective September 30, 2016 and have the economic effect of capping the LIBOR variable component of our interest rate at a maximum of 3.50% on an equivalent amount of our debt. These cap agreements expire on June 30, 2018. As of December 31, 2017, these cap agreements had an immaterial fair value. During the year ended December 31, 2017, we recognized debt expense of $8.3 million from these caps. During the year ended December 31, 2017, we recorded a loss of $0.1 million in other comprehensive income due to a decrease in the unrealized fair value of these cap agreements.
As of December 31, 2017, we also maintain several forward interest rate cap agreements that were entered into in October 2015 with notional amounts totaling $3.5 billion. These forward cap agreements will become effective June 29, 2018 and will have the economic effect of capping the LIBOR variable component of our interest rate at a maximum of 3.50% on an equivalent amount of its debt. These cap agreements expire on June 30, 2020. As of December 31, 2017, the total fair value of these cap agreements was an asset of approximately $1.0 million. During the year ended December 31, 2017, we recorded a loss of $8.8 million in other comprehensive income due to a decrease in the unrealized fair value of these cap agreements.
Our overall weighted average effective interest rate on the senior secured credit facilities was 4.45%, based on the current margins in effect of 2.00% for Term Loan A and the Revolver and 2.75% for Term Loan B, as of December 31, 2017.
Our overall weighted average effective interest rate during the year ended December 31, 2017 was 4.70% and as of December 31, 2017 was 4.88%.
As of December 31, 2017, we had $300 million drawn on our $1.0 billion revolving line of credit under our senior secured credit facilities, in addition to approximately $14.4 million committed for outstanding letters of credit. We also have approximately $90.1 million of additional outstanding letters of credit related to Kidney Care and $0.2 million of committed outstanding letters of credit related to DMG, which is backed by a certificate of deposit.
We believe that we will generate significant operating cash flows and will have sufficient liquidity to fund our scheduled debt service and other obligations and working capital needs for the foreseeable future, including the next 12 months, under the terms of our debt agreements. Our primary sources of liquidity are cash from operations and cash from borrowings.
95
One means of assessing exposure to debt-related interest rate changes is a duration-based analysis that measures the potential loss in net income resulting from a hypothetical increase in interest rates of 100 basis points across all variable rate maturities (referred to as a parallel shift in the yield curve). Under this model, with all else constant, it is estimated that such an increase would have reduced net income by approximately $27.6 million, $11.6 million, and $9.3 million, net of tax, for the years ended December 31, 2017, 2016, and 2015, respectively.
Exchange rate sensitivity
While our business is predominantly conducted in the U.S. we have developing operations in 11 other countries as well. For financial reporting purposes, the U.S. dollar is our reporting currency. However, the functional currencies of our operating businesses in other countries are typically those of the countries in which they operate. Therefore, changes in the rate of exchange between the U.S. dollar and the local currencies in which our international operations are conducted affect our results of operations and financial position as reported in our consolidated financial statements.
We have consolidated the balance sheets of our non-U.S. dollar denominated operations into U.S. dollars at the exchange rates prevailing at the balance sheet date and have translated their revenues and expense at average exchange rates during the period. Additionally, our individual subsidiaries are exposed to transactional risks mainly resulting from intercompany transactions between and among subsidiaries with different functional currencies. This exposes the subsidiaries to fluctuations in the rate of exchange between the invoicing or obligation currencies and the currency in which their local operations are conducted.
We evaluate our exposure to foreign exchange risk through the judgment of our regional and corporate management teams. Through 2017, our international operations remained fairly small relative to the size of our consolidated financial statements, constituting less than 6% of our consolidated assets as of December 31, 2017 and approximately 3% of our consolidated net revenues for the year ended December 31, 2017. In addition, our foreign currency translation gains (losses) were less than approximately 6%, (2)%, and (3)% of our consolidated operating income for the years ended December 31, 2017, 2016 and 2015.
Given the still small size of our international operations, management does not consider our exposure to foreign exchange risk to be significant to the consolidated enterprise. As such, through December 31, 2017 we have not engaged in transactions to hedge the exposure of our international transactions or net investments to foreign currency risk. However, we may do so in the future.
Item 8. Financial Statements and Supplementary Data.
See the Index to Financial Statements and Index to Financial Statement Schedules included at “Item 15. Exhibits, Financial Statement Schedules.”
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Management has established and maintains disclosure controls and procedures designed to ensure that information required to be disclosed in the reports that it files or submits pursuant to the Securities Exchange Act of 1934 (Exchange Act) as amended is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management including our Chief Executive Officer and Chief Financial Officer as appropriate to allow for timely decisions regarding required disclosures.
At the end of the period covered by this report, we carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures in accordance with the Exchange Act requirements. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective for timely identification and review of material information required to be included in our Exchange Act reports, including this report on Form 10-K. Management recognizes that these controls and procedures can provide only reasonable assurance of desired outcomes, and that estimates and judgments are still inherent in the process of maintaining effective controls and procedures.
There has not been any change in our internal control over financial reporting that was identified during the evaluation that occurred during the fourth fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
96
Item 9B. Other Information.
None.
97
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
We intend to disclose any amendments or waivers to the Code of Ethics applicable to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions, on our website. In 2002, we adopted a Corporate Governance Code of Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, and to all of our financial accounting and legal professionals who are directly or indirectly involved in the preparation, reporting and fair presentation of our financial statements and Exchange Act reports. The Code of Ethics is posted on our website, located at http://www.davita.com. We also maintain a Corporate Code of Conduct that applies to all of our employees, officers and directors, which is posted on our website.
Under our Corporate Governance Guidelines all Board Committees including the Audit Committee, Nominating and Governance Committee and the Compensation Committee, which are comprised solely of independent directors as defined within the listing standards of the New York Stock Exchange, have written charters that outline the committee’s purpose, goals, membership requirements and responsibilities. These charters are regularly reviewed and updated as necessary by our Board of Directors. All Board Committee charters as well as the Corporate Governance Guidelines are posted on our website located at http://www.davita.com.
The other information required to be disclosed by this item will appear in, and is incorporated by reference from, the sections entitled “Proposal No. 1. Election of Directors”, “Corporate Governance”, and “Security Ownership of Certain Beneficial Owners and Management” included in our definitive proxy statement relating to our 2018 annual stockholder meeting.
Item 11. Executive Compensation.
The information required by this item will appear in, and is incorporated by reference from, the sections entitled “Executive Compensation” and “Compensation Committee Interlocks and Insider Participations” included in our definitive proxy statement relating to our 2018 annual stockholder meeting. The information required by Item 407(e)(5) of Regulation S-K will appear in and is incorporated by reference from the section entitled “Compensation Committee Report” included in our definitive proxy statement relating to our 2018 annual stockholder meeting; however, this information shall not be deemed to be filed.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table provides information about our common stock that may be issued upon the exercise of stock-settled stock appreciation rights, restricted stock units and other rights under all of our existing equity compensation plans as of December 31, 2017, which consist of our 2011 Incentive Award Plan and our Employee Stock Purchase Plan. The material terms of these plans are described in Note 18 to the consolidated financial statements.
Plan category | Number of shares to be issued upon exercise of outstanding options, warrants and rights | Weighted average exercise price of outstanding options, warrants and rights | Number of shares remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | Total of shares reflected in columns (a) and (c) | ||||||||
(a) | (b) | (c) | (d) | |||||||||
Equity compensation plans approved by shareholders | 8,034,080(1) | 67.92(2) | 34,493,542 | 42,527,622 | ||||||||
Equity compensation plans not requiring shareholder approval | — | — | — | — | ||||||||
Total | 8,034,080 | $ | 67.92 | 34,493,542 | 42,527,622 | |||||||
(1) | Includes 752,029 shares of common stock reserved for issuance in connection with performance share units and performance stock appreciation rights at the maximum number of shares issuable thereunder. |
(2) | This weighted-average includes performance stock appreciation rights at 100% of target amount and excludes full value awards such as restricted stock units and performance share units. |
98
Other information required to be disclosed by Item 12 will appear in, and is incorporated by reference from, the section entitled “Security Ownership of Certain Beneficial Owners and Management” included in our definitive proxy statement relating to our 2018 annual stockholder meeting.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item will appear in, and is incorporated by reference from, the section entitled “Certain Relationships and Related Transactions” and the section entitled “Corporate Governance” included in our definitive proxy statement relating to our 2018 annual stockholder meeting.
Item 14. Principal Accounting Fees and Services.
The information required by this item will appear in, and is incorporated by reference from, the section entitled “Ratification of Appointment of Independent Registered Public Accounting Firm” included in our definitive proxy statement relating to our 2018 annual stockholder meeting.
99
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a) Documents filed as part of this Report:
(2) Index to Financial Statement Schedules:
(3) Exhibits
The information required by this Item is set forth in the Exhibit Index that precedes the signature pages of this Annual Report on Form 10-K.
Item 16. Form 10-K Summary.
None.
100
DAVITA INC.
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining an adequate system of internal control over financial reporting designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles and which includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
During the last fiscal year, the Company conducted an evaluation, under the oversight of the Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of the Company’s internal control over financial reporting. This evaluation was completed based on the criteria established in the report titled “Internal Control—Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Based upon our evaluation under the COSO framework, we have concluded that the Company’s internal control over financial reporting was effective as of December 31, 2017.
The Company’s independent registered public accounting firm, KPMG LLP, has issued an attestation report on the Company’s internal control over financial reporting, which report is included in this Annual Report.
F-1
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
DaVita Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of DaVita Inc. and subsidiaries (the Company) as of December 31, 2017 and 2016, the related consolidated statements of income, comprehensive income, equity, and cash flow for each of the years in the three‑year period ended December 31, 2017, and the related notes and financial statement Schedule II - Valuation and Qualifying Accounts (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three‑year period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 23, 2018 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KPMG LLP
We have served as the Company’s auditor since 2000.
Seattle, Washington
February 23, 2018
F-2
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
DaVita Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited DaVita Inc. and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2017 and 2016, the related consolidated statements of income, comprehensive income, equity, and cash flow for each of the years in the three-year period ended December 31, 2017, and the related notes and financial statement Schedule II - Valuation and Qualifying Accounts (collectively, the consolidated financial statements), and our report dated February 23, 2018 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Seattle, Washington
February 23, 2018
F-3
DAVITA INC.
CONSOLIDATED STATEMENTS OF INCOME
(dollars in thousands, except per share data)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Dialysis and related lab patient service revenues | $ | 10,093,670 | $ | 9,727,360 | $ | 9,155,447 | |||||
Less: Provision for uncollectible accounts | (485,398 | ) | (431,308 | ) | (412,905 | ) | |||||
Net dialysis and related lab patient service revenues | 9,608,272 | 9,296,052 | 8,742,542 | ||||||||
Other revenues | 1,268,362 | 1,411,415 | 1,239,703 | ||||||||
Total net revenues | 10,876,634 | 10,707,467 | 9,982,245 | ||||||||
Operating expenses and charges: | |||||||||||
Patient care costs and other costs | 7,640,005 | 7,431,582 | 6,856,062 | ||||||||
General and administrative | 1,064,026 | 1,072,841 | 1,031,125 | ||||||||
Depreciation and amortization | 559,911 | 509,497 | 463,905 | ||||||||
Provision for uncollectible accounts | (7,033 | ) | 11,677 | 9,240 | |||||||
Equity investment loss (income) | 8,640 | (16,874 | ) | (13,919 | ) | ||||||
Investment and other asset impairments | 295,234 | 14,993 | — | ||||||||
Goodwill impairment charges | 36,196 | 28,415 | 4,066 | ||||||||
Gain on changes in ownership interests | (6,273 | ) | (374,374 | ) | — | ||||||
(Gain) loss on settlements, net | (526,827 | ) | — | 495,000 | |||||||
Total operating expenses and charges | 9,063,879 | 8,677,757 | 8,845,479 | ||||||||
Operating income | 1,812,755 | 2,029,710 | 1,136,766 | ||||||||
Debt expense | (430,634 | ) | (414,116 | ) | (408,380 | ) | |||||
Debt redemption charges | — | — | (48,072 | ) | |||||||
Other income, net | 17,665 | 7,511 | 8,073 | ||||||||
Income from continuing operations before income taxes | 1,399,786 | 1,623,105 | 688,387 | ||||||||
Income tax expense | 323,859 | 431,761 | 207,510 | ||||||||
Net income from continuing operations | 1,075,927 | 1,191,344 | 480,877 | ||||||||
Net loss from discontinued operations, net of tax | (245,372 | ) | (158,262 | ) | (53,467 | ) | |||||
Net income | 830,555 | 1,033,082 | 427,410 | ||||||||
Less: Net income attributable to noncontrolling interests | (166,937 | ) | (153,208 | ) | (157,678 | ) | |||||
Net income attributable to DaVita Inc. | $ | 663,618 | $ | 879,874 | $ | 269,732 | |||||
Earnings per share: | |||||||||||
Basic net income from continuing operations per share attributable to DaVita Inc. | $ | 4.78 | $ | 5.12 | $ | 1.53 | |||||
Basic net income per share attributable to DaVita Inc. | $ | 3.52 | $ | 4.36 | $ | 1.27 | |||||
Diluted net income from continuing operations per share attributable to DaVita Inc. | $ | 4.71 | $ | 5.04 | $ | 1.49 | |||||
Diluted net income per share attributable to DaVita Inc. | $ | 3.47 | $ | 4.29 | $ | 1.25 | |||||
Weighted average shares for earnings per share: | |||||||||||
Basic | 188,625,559 | 201,641,173 | 211,867,714 | ||||||||
Diluted | 191,348,533 | 204,904,656 | 216,251,807 | ||||||||
Amounts attributable to DaVita Inc.: | |||||||||||
Net income from continuing operations | $ | 901,277 | $ | 1,032,373 | $ | 323,199 | |||||
Net loss from discontinued operations | (237,659 | ) | (152,499 | ) | (53,467 | ) | |||||
Net income attributable to DaVita Inc. | $ | 663,618 | $ | 879,874 | $ | 269,732 | |||||
See notes to consolidated financial statements.
F-4
DAVITA INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(dollars in thousands)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Net income | $ | 830,555 | $ | 1,033,082 | $ | 427,410 | |||||
Other comprehensive income (loss): | |||||||||||
Unrealized losses on interest rate cap and swap agreements: | |||||||||||
Unrealized losses on interest rate cap and swap agreements | (5,437 | ) | (3,670 | ) | (12,241 | ) | |||||
Reclassifications of net cap and swap agreements realized losses into net income | 5,058 | 2,566 | 3,111 | ||||||||
Unrealized gains (losses) on investments: | |||||||||||
Unrealized gains (losses) on investments | 3,705 | 1,427 | (1,413 | ) | |||||||
Reclassification of net investment realized losses (gains) into net income | (220 | ) | (423 | ) | (377 | ) | |||||
Foreign currency translation adjustments: | |||||||||||
Foreign currency translation adjustments | 99,770 | (39,614 | ) | (23,889 | ) | ||||||
Reclassification of foreign currency translation into net income | — | 10,087 | — | ||||||||
Other comprehensive income (loss) | 102,876 | (29,627 | ) | (34,809 | ) | ||||||
Total comprehensive income | 933,431 | 1,003,455 | 392,601 | ||||||||
Less: Comprehensive income attributable to noncontrolling interests | (166,935 | ) | (153,398 | ) | (157,678 | ) | |||||
Comprehensive income attributable to DaVita Inc. | $ | 766,496 | $ | 850,057 | $ | 234,923 | |||||
See notes to consolidated financial statements.
F-5
DAVITA INC.
CONSOLIDATED BALANCE SHEETS
(dollars in thousands, except per share data)
December 31, 2017 | December 31, 2016 | ||||||
ASSETS | |||||||
Cash and cash equivalents | $ | 508,234 | $ | 674,776 | |||
Short-term investments | 43,516 | 306,981 | |||||
Accounts receivable, less allowance of $218,399 and $238,897 | 1,714,750 | 1,503,950 | |||||
Inventories | 181,799 | 160,419 | |||||
Other receivables | 372,919 | 288,156 | |||||
Income tax receivable | 49,440 | — | |||||
Prepaid and other current assets | 112,058 | 99,510 | |||||
Current assets held for sale | 5,761,642 | 960,956 | |||||
Total current assets | 8,744,358 | 3,994,748 | |||||
Property and equipment, net | 3,149,213 | 2,864,121 | |||||
Intangible assets, net | 113,827 | 73,504 | |||||
Equity method and other investments | 245,534 | 492,039 | |||||
Long-term investments | 37,695 | 29,997 | |||||
Other long-term assets | 47,287 | 33,857 | |||||
Goodwill | 6,610,279 | 6,015,375 | |||||
Long-term assets held for sale | — | 5,252,135 | |||||
$ | 18,948,193 | $ | 18,755,776 | ||||
LIABILITIES AND EQUITY | |||||||
Accounts payable | $ | 509,116 | $ | 456,619 | |||
Other liabilities | 552,662 | 578,892 | |||||
Accrued compensation and benefits | 616,116 | 706,564 | |||||
Current portion of long-term debt | 178,213 | 160,262 | |||||
Income tax payable | — | 1,394 | |||||
Current liabilities held for sale | 1,185,070 | 807,233 | |||||
Total current liabilities | 3,041,177 | 2,710,964 | |||||
Long-term debt | 9,158,018 | 8,944,676 | |||||
Other long-term liabilities | 365,325 | 317,383 | |||||
Deferred income taxes | 486,247 | 530,869 | |||||
Long-term liabilities held for sale | — | 428,885 | |||||
Total liabilities | 13,050,767 | 12,932,777 | |||||
Commitments and contingencies | |||||||
Noncontrolling interests subject to put provisions | 1,011,360 | 973,258 | |||||
Equity: | |||||||
Preferred stock ($0.001 par value, 5,000,000 shares authorized; none issued) | |||||||
Common stock ($0.001 par value, 450,000,000 shares authorized; 182,462,278 and 194,554,491 shares issued and outstanding, respectively) | 182 | 195 | |||||
Additional paid-in capital | 1,042,899 | 1,027,182 | |||||
Retained earnings | 3,633,713 | 3,710,313 | |||||
Accumulated other comprehensive income (loss) | 13,235 | (89,643 | ) | ||||
Total DaVita Inc. shareholders' equity | 4,690,029 | 4,648,047 | |||||
Noncontrolling interests not subject to put provisions | 196,037 | 201,694 | |||||
Total equity | 4,886,066 | 4,849,741 | |||||
$ | 18,948,193 | $ | 18,755,776 | ||||
See notes to consolidated financial statements.
F-6
DAVITA INC.
CONSOLIDATED STATEMENTS OF CASH FLOW
(dollars in thousands)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash flows from operating activities: | |||||||||||
Net income | $ | 830,555 | $ | 1,033,082 | $ | 427,410 | |||||
Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
(Gain) loss on settlements, net | (526,827 | ) | — | 495,000 | |||||||
Depreciation and amortization | 777,485 | 720,252 | 638,024 | ||||||||
Impairment charges | 981,589 | 296,408 | 210,234 | ||||||||
Debt redemption charges | — | — | 48,072 | ||||||||
Stock-based compensation expense | 35,092 | 38,338 | 56,664 | ||||||||
Deferred income taxes | (395,217 | ) | 52,010 | 61,744 | |||||||
Equity investment income, net | 28,925 | 17,766 | 9,293 | ||||||||
Gain on sales of business interests, net | (23,402 | ) | (404,165 | ) | — | ||||||
Other non-cash charges, net | 66,925 | (7,338 | ) | 44,691 | |||||||
Changes in operating assets and liabilities, net of effect of acquisitions and divestitures: | |||||||||||
Accounts receivable | (156,305 | ) | (152,240 | ) | (202,867 | ) | |||||
Inventories | (18,625 | ) | 22,920 | (48,313 | ) | ||||||
Other receivables and other current assets | (117,154 | ) | (54,038 | ) | 32,761 | ||||||
Other long-term assets | (11,945 | ) | 35,893 | 3,723 | |||||||
Accounts payable | 26,876 | 11,897 | 30,998 | ||||||||
Accrued compensation and benefits | (78,239 | ) | 68,272 | 54,950 | |||||||
Other current liabilities | 1,908 | 176,494 | 113,470 | ||||||||
Settlement receipts (payments) | 526,827 | — | (493,775 | ) | |||||||
Income taxes | (52,176 | ) | 77,376 | 41,767 | |||||||
Other long-term liabilities | 11,157 | 30,517 | 33,354 | ||||||||
Net cash provided by operating activities | 1,907,449 | 1,963,444 | 1,557,200 | ||||||||
Cash flows from investing activities: | |||||||||||
Additions of property and equipment | (905,250 | ) | (829,095 | ) | (707,998 | ) | |||||
Acquisitions | (803,879 | ) | (563,856 | ) | (96,469 | ) | |||||
Proceeds from asset and business sales | 92,336 | 64,725 | 19,715 | ||||||||
Purchase of investments available for sale | (13,117 | ) | (13,539 | ) | (8,783 | ) | |||||
Purchase of investments held-to-maturity | (230,989 | ) | (1,133,192 | ) | (1,709,883 | ) | |||||
Proceeds from sale of investments available for sale | 6,408 | 18,963 | 2,058 | ||||||||
Proceeds from investments held-to-maturity | 492,470 | 1,240,502 | 1,637,358 | ||||||||
Purchase of equity investments | (4,816 | ) | (27,096 | ) | (17,911 | ) | |||||
Proceeds from sale of equity investments | — | 40,920 | — | ||||||||
Distributions received on equity investments | 106 | — | 129 | ||||||||
Net cash used in investing activities | (1,366,731 | ) | (1,201,668 | ) | (881,784 | ) | |||||
DAVITA INC.
CONSOLIDATED STATEMENTS OF CASH FLOW - continued
(dollars in thousands)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash flows from financing activities: | |||||||||||
Borrowings | 50,991,960 | 51,991,490 | 54,541,988 | ||||||||
Payments on long-term debt and other financing costs | (50,837,112 | ) | (52,116,120 | ) | (53,998,962 | ) | |||||
Purchase of treasury stock | (802,949 | ) | (1,097,822 | ) | (549,935 | ) | |||||
Distributions to noncontrolling interests | (211,467 | ) | (192,401 | ) | (174,635 | ) | |||||
Stock award exercises and other share issuances, net | 21,252 | 23,543 | 26,155 | ||||||||
Excess tax benefits from stock award exercises | — | 13,251 | 28,157 | ||||||||
Contributions from noncontrolling interests | 74,552 | 47,590 | 54,644 | ||||||||
Proceeds from sales of additional noncontrolling interests | 2,864 | — | — | ||||||||
Purchases of noncontrolling interests | (5,357 | ) | (21,512 | ) | (66,382 | ) | |||||
Net cash used in financing activities | (766,257 | ) | (1,351,981 | ) | (138,970 | ) | |||||
Effect of exchange rate changes on cash and cash equivalents | 254 | 4,276 | (2,571 | ) | |||||||
Net (decrease) increase in cash and cash equivalents | (225,285 | ) | (585,929 | ) | 533,875 | ||||||
Less: Net (decrease) increase in cash and cash equivalents from discontinued operations | (58,743 | ) | (15,788 | ) | 25,855 | ||||||
Net (decrease) increase in cash and cash equivalents from continuing operations | (166,542 | ) | (570,141 | ) | 508,020 | ||||||
Cash and cash equivalents of continuing operations at beginning of the year | 674,776 | 1,244,917 | 736,897 | ||||||||
Cash and cash equivalents of continuing operations at end of the year | $ | 508,234 | $ | 674,776 | $ | 1,244,917 | |||||
See notes to consolidated financial statements.
F-7
DAVITA INC.
CONSOLIDATED STATEMENTS OF EQUITY
(dollars and shares in thousands)
Non-controlling interests subject to put provisions | DaVita Inc. Shareholders' Equity | Non-controlling interests not subject to put provisions | ||||||||||||||||||||||||||||||||||||
Additional paid-in capital | Retained earnings | Accumulated other comprehensive income (loss) | ||||||||||||||||||||||||||||||||||||
Common stock | Treasury stock | |||||||||||||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | Total | ||||||||||||||||||||||||||||||||||
Balance at December 31, 2014 | $ | 829,965 | 215,641 | $ | 216 | $ | 1,108,211 | $ | 4,087,103 | — | $ | — | $ | (25,017 | ) | $ | 5,170,513 | $ | 189,798 | |||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||||||||||||||
Net income | 96,510 | 269,732 | 269,732 | 61,168 | ||||||||||||||||||||||||||||||||||
Other comprehensive loss | (34,809 | ) | (34,809 | ) | ||||||||||||||||||||||||||||||||||
Stock purchase shares issued | — | — | (6,079 | ) | 414 | 30,608 | 24,529 | |||||||||||||||||||||||||||||||
Stock unit shares issued | 348 | — | — | — | ||||||||||||||||||||||||||||||||||
Stock-settled SAR shares issued | 1,131 | 1 | (1 | ) | — | |||||||||||||||||||||||||||||||||
Stock-settled stock-based compensation expense | 56,899 | 56,899 | ||||||||||||||||||||||||||||||||||||
Excess tax benefits from stock awards exercised | 28,157 | 28,157 | ||||||||||||||||||||||||||||||||||||
Changes in non-controlling interests from: | ||||||||||||||||||||||||||||||||||||||
Distributions | (103,355 | ) | (71,280 | ) | ||||||||||||||||||||||||||||||||||
Contributions | 25,795 | 28,849 | ||||||||||||||||||||||||||||||||||||
Acquisitions and divestitures | 10,654 | 6,875 | ||||||||||||||||||||||||||||||||||||
Partial purchases | (8,538 | ) | (55,826 | ) | (55,826 | ) | (2,018 | ) | ||||||||||||||||||||||||||||||
Fair value remeasurement | 13,035 | (13,035 | ) | (13,035 | ) | |||||||||||||||||||||||||||||||||
Purchase of treasury stock | (7,780 | ) | (575,380 | ) | (575,380 | ) | ||||||||||||||||||||||||||||||||
Balance at December 31, 2015 | $ | 864,066 | 217,120 | $ | 217 | $ | 1,118,326 | $ | 4,356,835 | (7,366 | ) | $ | (544,772 | ) | $ | (59,826 | ) | $ | 4,870,780 | $ | 213,392 | |||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||||||||||||||
Net income | 99,834 | 879,874 | 879,874 | 53,374 | ||||||||||||||||||||||||||||||||||
Other comprehensive loss | (29,817 | ) | (29,817 | ) | 190 | |||||||||||||||||||||||||||||||||
Stock purchase shares issued | 438 | 1 | 23,902 | 23,903 | ||||||||||||||||||||||||||||||||||
Stock unit shares issued | 4 | — | (19,815 | ) | 276 | 19,815 | ||||||||||||||||||||||||||||||||
Stock-settled SAR shares issued | 218 | — | (36,685 | ) | 513 | 36,685 | ||||||||||||||||||||||||||||||||
Stock-settled stock-based compensation expense | 37,970 | 37,970 | ||||||||||||||||||||||||||||||||||||
Excess tax benefits from stock awards exercised | 13,251 | 13,251 | ||||||||||||||||||||||||||||||||||||
Changes in non-controlling interests from: | ||||||||||||||||||||||||||||||||||||||
Distributions | (111,092 | ) | (81,309 | ) | ||||||||||||||||||||||||||||||||||
Contributions | 33,517 | 14,073 | ||||||||||||||||||||||||||||||||||||
Acquisitions and divestitures | 28,874 | 3,423 | 3,423 | 2,585 | ||||||||||||||||||||||||||||||||||
Partial purchases | (6,660 | ) | (13,105 | ) | (13,105 | ) | (1,747 | ) | ||||||||||||||||||||||||||||||
Fair value remeasurement | 65,855 | (65,855 | ) | (65,855 | ) | |||||||||||||||||||||||||||||||||
Reclassifications and expirations of puts | (1,136 | ) | 1,136 | |||||||||||||||||||||||||||||||||||
Purchase of treasury stock | (16,649 | ) | (1,072,377 | ) | (1,072,377 | ) | ||||||||||||||||||||||||||||||||
Retirement of treasury stock | (23,226 | ) | (23 | ) | (34,230 | ) | (1,526,396 | ) | 23,226 | 1,560,649 | ||||||||||||||||||||||||||||
Balance at December 31, 2016 | $ | 973,258 | 194,554 | $ | 195 | $ | 1,027,182 | $ | 3,710,313 | — | $ | — | $ | (89,643 | ) | $ | 4,648,047 | $ | 201,694 | |||||||||||||||||||
DAVITA INC.
CONSOLIDATED STATEMENTS OF EQUITY - continued
(dollars and shares in thousands)
Non-controlling interests subject to put provisions | DaVita Inc. Shareholders' Equity | Non-controlling interests not subject to put provisions | ||||||||||||||||||||||||||||||||||||
Additional paid-in capital | Retained earnings | Accumulated other comprehensive income (loss) | ||||||||||||||||||||||||||||||||||||
Common stock | Treasury stock | |||||||||||||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | Total | ||||||||||||||||||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||||||||||||||
Net income | 103,641 | 663,618 | 663,618 | 63,296 | ||||||||||||||||||||||||||||||||||
Other comprehensive income | 102,878 | 102,878 | (2 | ) | ||||||||||||||||||||||||||||||||||
Stock purchase shares issued | 360 | 22,131 | 22,131 | |||||||||||||||||||||||||||||||||||
Stock unit shares issued | 117 | (101 | ) | (101 | ) | |||||||||||||||||||||||||||||||||
Stock-settled SAR shares issued | 398 | — | — | |||||||||||||||||||||||||||||||||||
Stock-settled stock-based compensation expense | 34,981 | 34,981 | ||||||||||||||||||||||||||||||||||||
Excess tax benefits from stock awards exercised | ||||||||||||||||||||||||||||||||||||||
Changes in noncontrolling interest from: | ||||||||||||||||||||||||||||||||||||||
Distributions | (128,853 | ) | (82,614 | ) | ||||||||||||||||||||||||||||||||||
Contributions | 52,911 | 21,641 | ||||||||||||||||||||||||||||||||||||
Acquisitions and divestitures | 43,799 | (823 | ) | (823 | ) | (5,770 | ) | |||||||||||||||||||||||||||||||
Partial purchases | (397 | ) | (2,752 | ) | (2,752 | ) | (2,208 | ) | ||||||||||||||||||||||||||||||
Fair value remeasurements | (32,999 | ) | 32,999 | 32,999 | ||||||||||||||||||||||||||||||||||
Purchase of treasury stock | (12,967 | ) | (810,949 | ) | (810,949 | ) | ||||||||||||||||||||||||||||||||
Retirement of treasury stock | (12,967 | ) | (13 | ) | (70,718 | ) | (740,218 | ) | 12,967 | 810,949 | ||||||||||||||||||||||||||||
Balance at December 31, 2017 | $ | 1,011,360 | 182,462 | $ | 182 | $ | 1,042,899 | $ | 3,633,713 | — | $ | — | $ | 13,235 | $ | 4,690,029 | $ | 196,037 | ||||||||||||||||||||
See notes to consolidated financial statements.
F-8
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
1. Organization and summary of significant accounting policies
Organization
DaVita Inc. (the Company) has consisted of two major divisions, DaVita Kidney Care (Kidney Care) and DaVita Medical Group (DMG). The Kidney Care division is comprised of the Company’s U.S. dialysis and related lab services, its ancillary services and strategic initiatives, including its international operations, and its corporate administrative support. The Company’s largest line of business is its U.S. dialysis and related lab services business, which operates kidney dialysis centers in the U.S. for patients suffering from chronic kidney failure also known as end stage renal disease (ESRD). As of December 31, 2017, the Company operated or provided administrative services through a network of 2,510 U.S. outpatient dialysis centers in 46 states and the District of Columbia, serving a total of approximately 197,800 patients. In addition, as of December 31, 2017, the Company operated or provided administrative services to a total of 237 outpatient dialysis centers serving approximately 22,900 patients located in 11 countries outside of the U.S.
The Company’s DMG division is a patient- and physician-focused integrated healthcare delivery and management company that provides medical services to members primarily through capitation contracts with some of the nation’s leading health plans. On December 5, 2017, the Company entered into an equity purchase agreement to sell its DMG division to Collaborative Care Holdings, LLC (Optum), a subsidiary of UnitedHealth Group Inc. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions. As a result of this pending transaction, the DMG business has been reclassified as held for sale and its results of operations are reported as discontinued operations for all periods presented in these consolidated financial statements. For financial information about the DMG business, see Note 21.
The Company’s U.S. dialysis and related lab services business qualifies as a separately reportable segment and the Company’s other ancillary services and strategic initiatives, including its international operations, have been combined and disclosed in the other segments category.
Basis of presentation
These consolidated financial statements are prepared in accordance with United States generally accepted accounting principles (U.S. GAAP). The financial statements include DaVita Inc. and its subsidiaries, partnerships and other entities in which it maintains a majority voting interest or other controlling financial interest (collectively, the Company). All significant intercompany transactions and balances have been eliminated. Non-marketable equity investments are recorded under the equity or cost method of accounting based upon whether the Company has significant influence over the investee. For the Company’s international subsidiaries, local currencies are considered their functional currencies. Translation adjustments result from translating the Company’s international subsidiaries’ financial statements from their functional currencies into the Company’s reporting currency (USD). Prior year balances and amounts have been reclassified to conform to the current year presentation.
The Company has evaluated subsequent events through the date these consolidated financial statements were issued and has included all necessary adjustments and disclosures.
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires the use of estimates and assumptions that affect the reported amounts of revenues, expenses, assets, liabilities, contingencies and noncontrolling interests subject to put provisions. Although actual results in subsequent periods will differ from these estimates, such estimates are developed based on the best information available to management and management’s best judgments at the time. All significant assumptions and estimates underlying the amounts reported in the financial statements and accompanying notes are regularly reviewed and updated when necessary. Changes in estimates are reflected in the financial statements based upon on-going actual experience trends, or subsequent settlements and realizations depending on the nature and predictability of the estimates and contingencies. Interim changes in estimates related to annual operating costs are applied prospectively within annual periods.
The most significant assumptions and estimates underlying these financial statements and accompanying notes involve revenue recognition and accounts receivable, contingencies, impairments of goodwill and investments, accounting for income taxes, long-term variable compensation accruals, consolidation of variable interest entities, and certain fair value estimates. Specific estimating risks and contingencies are further addressed within these notes to the consolidated financial statements.
F-9
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Patient service net revenues and accounts receivable
U.S. dialysis and related lab services
U.S. dialysis patient service net revenues are recognized in the period services are provided. Revenues consist primarily of payments from Medicare, Medicaid and commercial health plans for dialysis and ancillary services provided to patients. A usual and customary fee schedule is maintained for the Company’s dialysis treatments and other patient services; however, actual collectible revenue is normally recognized at a discount from the fee schedule.
Revenues associated with Medicare and Medicaid programs are recognized based on: (a) the payment rates that are established by statute or regulation for the portion of payment rates paid by the government payor (e.g., 80% for Medicare patients) and (b) for the portion not paid by the primary government payor, estimates of the amounts ultimately collectible from other government programs paying secondary coverage (e.g., Medicaid secondary coverage), the patient’s commercial health plan secondary coverage, or the patient. The Company’s reimbursements from Medicare are subject to certain variations under Medicare’s single bundled payment rate system, whereby reimbursements can be adjusted for certain patient characteristics and other factors. The Company’s revenue recognition will depend upon its ability to effectively capture, document and bill for Medicare’s base payment rate as well as these other variable factors.
Revenues associated with commercial health plans are estimated based on contractual terms for the patients under healthcare plans with which the Company has formal agreements, non-contracted health plan coverage terms if known, estimated secondary collections, historical collection experience, historical trends of refunds and payor payment adjustments (retractions), inefficiencies in the Company’s billing and collection processes that can result in denied claims for payments, and regulatory compliance matters.
Commercial revenue recognition also involves significant estimating risks. With many larger, commercial insurers the Company has several different contracts and payment arrangements, and these contracts often include only a subset of the Company’s centers. It is often not possible to determine which contract, if any, should be applied prior to billing. In addition, for services provided by non-contracted centers, final collection may require specific negotiation of a payment amount, typically at a significant discount from the Company’s usual and customary rates.
Under Medicare’s bundled payment rate system, services covered by Medicare are subject to estimating risk, whereby reimbursements from Medicare can vary significantly depending upon certain patient characteristics and other variable factors. Even with the bundled payment rate system, Medicare payments for bad debt claims as established by cost reports require evidence of collection efforts. As a result, billing and collection of Medicare bad debt claims can be delayed significantly and final payment is subject to audit.
Medicaid payments, when Medicaid coverage is secondary, can also be difficult to estimate. For many states, Medicaid payment terms and methods differ from Medicare, and may prevent accurate estimation of individual payment amounts prior to billing.
The Company’s range of revenue estimating risk for the U.S. dialysis and related lab services segment is generally expected to be within 1% of its revenue. Changes in revenue estimates for prior periods are not material.
Other revenues
Other revenues consist of the revenues associated with the ancillary services and strategic initiatives, management and administrative support services that are provided to outpatient dialysis centers that the Company does not own or in which the Company owns a noncontrolling interest, and administrative and management support services to certain other non-dialysis joint ventures in which the Company owns a noncontrolling interest. Revenues associated with pharmacy services are recognized as prescriptions are filled and shipped to patients. Revenues associated with disease management services, medical consulting services, clinical research programs, physician services, ESRD seamless care organizations, and comprehensive care are recognized in the period services are provided. Revenues associated with direct primary care are recognized over the membership period. Management fees are principally determined as a percentage of the managed operations’ revenues or cash collections and in some cases an additional component based upon a percentage of operating income. Management fees are included in net revenues when earned.
F-10
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Allowance for uncollectible accounts
Net revenue recognition and allowances for uncollectible billings require the use of estimates of the amounts that will ultimately be realized considering, among other items, retroactive adjustments that may be associated with regulatory reviews, audits, billing reviews and other matters. The Company’s policy is to write off any uncollectible accounts receivable balance only after all collection efforts have been exhausted or when write off is mandated by federal or state policies or required by certain payor contracts. It is also the Company’s policy to write off any accounts receivable balance associated with any payors or patients when the Company receives notification of a bankruptcy filing.
Other income
Other income includes interest income on cash and cash-equivalents and short- and long-term investments, other non-operating gains from investment transactions, and foreign currency transaction gains and losses.
Cash and cash equivalents
Cash equivalents are short-term highly liquid investments with maturities of three months or less at date of purchase.
Investments in debt and equity securities
The Company classifies certain debt securities as held-to-maturity and records them at amortized cost based on the Company’s intentions and strategies concerning those investments. Equity securities that have readily determinable fair values, and certain other financial instruments that have readily determinable fair values or redemption values, are classified as available for sale and recorded at estimated fair value.
Inventories
Inventories are stated at the lower of cost (first-in, first-out) or market and consist principally of pharmaceuticals and dialysis-related supplies. Rebates related to inventory purchases are recorded when earned and are based on certain qualification requirements which are dependent on a variety of factors including future pricing levels by the manufacturer and data submission.
Property and equipment
Property and equipment is stated at cost less accumulated depreciation and amortization and is further reduced by any impairments. Maintenance and repairs are charged to expense as incurred. Depreciation and amortization expenses are computed using the straight-line method over the useful lives of the assets estimated as follows: buildings, 20 to 40 years; leasehold improvements, the shorter of their economic useful life or the expected lease term; and equipment and information systems, principally three to eight years. Disposition gains and losses are included in current operating expenses.
Amortizable intangibles
Amortizable intangible assets and liabilities include non-competition and similar agreements, lease agreements and hospital acute services contracts, each of which have finite useful lives. Amortization expense is computed using the straight-line method over the useful lives of the assets estimated as follows: non-competition and similar agreements, two to ten years; and lease agreements and hospital acute service contracts, over the term of the lease or contract period, respectively.
Indefinite-lived intangibles
Indefinite-lived intangible assets include international licenses and accreditations that allow the Company to be reimbursed for providing dialysis services to patients, each of which has an indefinite useful life.
Equity method and other investments
Equity investments that do not have readily determinable fair values are carried on the cost or equity method, as applicable, net of any other-than-temporary impairment. The Company classifies its cost and equity method investments as “Equity method and other investments” on its balance sheet. See Note 9 to these consolidated financial statements for further details.
F-11
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Goodwill
Goodwill represents the difference between the fair value of businesses acquired and the fair value of the identifiable tangible and intangible net assets acquired. Goodwill is not amortized, but is assessed by individual reporting unit for impairment as circumstances warrant and at least annually. An impairment charge is recorded when a reporting unit's carrying amount is determined to exceed its fair value. The Company operates multiple reporting units. See Note 10 to these consolidated financial statements for further details.
Impairment of equity method and other investments
Equity method and other investments are assessed for other-than-temporary impairment when significant events or changes in circumstances indicate that an other-than-temporary impairment may have occurred. An other-than-temporary impairment charge is recorded when the fair value of an investment has fallen below its carrying amount and the shortfall is expected to be indefinitely or permanently unrecoverable.
Impairment of other long-lived assets
Other long-lived assets, including property and equipment and intangible assets, are reviewed for possible impairment whenever significant events or changes in circumstances indicate that an impairment may have occurred. Such changes can include changes in the Company’s business strategy and plans, changes in the quality or structure of its relationships with its partners or deteriorating performance of individual outpatient dialysis centers or other business units. An impairment of an amortizable or depreciable asset is indicated when the sum of the expected future undiscounted net cash flows identifiable to the related asset group is less than its carrying amount. Impairment losses are measured based on the difference between the estimated fair value and the carrying amount of the subject asset group and are included in operating expenses.
Indefinite-lived intangible assets are reviewed for possible impairment at least annually and whenever significant events or changes in circumstances indicate that an impairment may have occurred.
Self-insurance
The Company is predominantly self-insured with respect to professional and general liability and workers' compensation risks through wholly-owned captive insurance companies, with excess or reinsurance coverage for additional risk. The Company is also predominantly self-insured with respect to employee medical and other health benefits. The Company records insurance liabilities for the professional and general liability, workers’ compensation, and employee health benefit risks that it retains and estimates its liability for those risks using third party actuarial calculations that are based upon historical claims experience and expectations for future claims.
Income taxes
Federal and state income taxes are computed at currently enacted tax rates less tax credits using the asset and liability method. Deferred taxes are adjusted both for items that do not currently have tax consequences and for the cumulative effect of any changes in tax rates from those previously used to determine deferred tax assets or liabilities. Tax provisions include amounts that are currently payable, changes in deferred tax assets and liabilities that arise because of temporary differences between the timing of when items of income and expense are recognized for financial reporting and income tax purposes, changes in the recognition of tax positions and any changes in the valuation allowance caused by a change in judgment about the realizability of the related deferred tax assets. A valuation allowance is established when necessary to reduce deferred tax assets to amounts expected to be realized.
The Company uses a recognition threshold of more-likely-than-not and a measurement attribute on all tax positions taken or expected to be taken in a tax return in order to be recognized in the financial statements. Once the recognition threshold is met, the tax position is then measured to determine the actual amount of benefit to recognize in the financial statements.
F-12
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Stock-based compensation
The Company’s stock-based compensation awards are measured at their estimated fair values on the date of grant if settled in shares or at their estimated fair values at the end of each reporting period if settled in cash. The value of stock-based awards so measured is recognized as compensation expense on a cumulative straight-line basis over the vesting terms of the awards, adjusted for expected forfeitures. Stock-based compensation to be settled in shares is recorded to the Company’s shareholders’ equity, while stock-based compensation to be settled in cash is recorded to a liability.
Interest rate cap and swap agreements
The Company often carries a combination of currently effective interest rate caps, forward interest rate caps, or interest rate swaps on portions of its variable rate debt as a means of hedging its exposure to changes in LIBOR interest rates as part of its overall interest rate risk management strategy. These interest rate caps and swaps are not held for trading or speculative purposes and are typically designated as qualifying cash flow hedges. See Note 13 to these consolidated financial statements for further details.
Noncontrolling interests
Noncontrolling interests represent third-party equity ownership interests in entities which are consolidated by the Company for financial statement reporting purposes. As of December 31, 2017, third parties held noncontrolling equity interests in 589 consolidated legal entities, including 586 legal entities classified as continuing operations.
Fair value estimates
The Company relies on fair value measurements and estimates for purposes that require the recording, reassessment, or adjustment of the carrying amounts of certain assets, liabilities and noncontrolling interests subject to put provisions (temporary equity). These purposes can include the accounting for business combination transactions, impairment assessments for goodwill, investments, or other long-lived assets, and stock-based compensation, as well as recurring valuations of available for sale securities, noncontrolling interests in temporary equity, derivative instruments, and/or contingent consideration, as applicable. The Company has also classified its assets, liabilities and temporary equity into the appropriate fair value hierarchy levels as defined by the Financial Accounting Standards Board (FASB). See Note 24 to these consolidated financial statements for further details.
New accounting standards
On May 28, 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. In 2015 and 2016, the FASB issued ASU 2015-14, ASU2016-08, ASU 2016-10, ASU 2016-11, and ASU 2016-12, Revenue from Contracts with Customers (Topic 606), each of which amends the guidance in ASU 2014-09. These ASUs will replace most existing revenue recognition guidance in U.S. GAAP.
The Company will adopt these ASUs beginning January 1, 2018 using the cumulative effect method and will apply these ASUs only to those contracts that are not completed contracts as of that date with no cumulative effect adjustment. In preparation for the adoption of these ASUs, the Company has concluded that this guidance will result in a change to the presentation of its revenues, provision for uncollectible accounts and allowance for doubtful accounts, which will result in the Company’s provision for uncollectible accounts being recorded as a reduction to revenue. The guidance will also require additional disaggregated revenue disclosures. The guidance will not have a material impact on the Company’s consolidated financial position, results of operations, equity or cash flows. The Company expects to benefit from certain policy elections related to its adoption of these standards of approximately $30,000 in the first half of 2018.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. The amendments in this ASU revise accounting related to (i) the classification and measurement of investments in equity securities and (ii) the presentation of certain fair value changes for financial liabilities at fair value. The amendments in this ASU are effective for the Company beginning on January 1, 2018 and are to be applied through a cumulative effect adjustment to the statement of financial position. Early adoption is permitted under certain circumstances. The Company is still evaluating certain aspects of this ASU as well as the related impacts it may have on its consolidated financial statements when adopted on January 1, 2018.
F-13
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). The amendments in this ASU revise the accounting related to lessee accounting. Under the new guidance, lessees will be required to recognize a lease liability and a right-of-use asset for substantially all leases with lease terms in excess of twelve months. The new lease guidance also simplifies the accounting for sale and leaseback transactions primarily because lessees must recognize lease assets and lease liabilities. The amendments in this ASU are effective for the Company beginning on January 1, 2019 and are to be applied through a modified retrospective transition approach for leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements. Early adoption is permitted. The Company has assembled an internal lease task force that meets regularly to discuss and evaluate the overall impact of this guidance on its consolidated financial statements and related disclosures, as well as the expected timing of adoption. The Company is currently gathering information from its existing leases and believes that the new standard will have a material impact on its consolidated balance sheet but will not have a material impact on its results of operations or liquidity. The Company expects to adopt this ASU on January 1, 2019, and continues to evaluate the effect that the implementation of this ASU will have on its consolidated financial statements, related disclosures and controls.
In March 2016, the FASB issued ASU No. 2016-07, Investments - Equity Method and Joint Ventures (Topic 323): Simplifying the Transition to the Equity Method of Accounting. The amendments in this ASU eliminate the requirement that when an investment qualifies for use of the equity method as a result of an increase in the level of ownership interest or degree of influence, an investor must adjust the investment, results of operations, and retained earnings retroactively on a step-by-step basis as if the equity method had been in effect during all previous periods that the investment had been held. The amendments in this ASU were effective for the Company beginning on January 1, 2017 and were applied prospectively. The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. The changes required by this ASU involve several aspects of the accounting for share-based payment transactions, including income tax consequences, classification of awards as either equity or liabilities, classification on the statement of cash flow, and an election on estimating forfeitures. The amendments in this ASU were effective for the Company beginning January 1, 2017. The method of adoption differs for each of the topics covered by the ASU. The primary effect of this ASU for the Company is the presentation of excess tax benefits or deficiencies as a component of income tax expense within the Company's consolidated statements of income rather than within additional paid-in capital on its consolidated balance sheet. In addition, these excess tax benefits or deficiencies are presented as an operating activity rather than as a financing activity on the consolidated statements of cash flow.
The Company elected to apply the presentation requirements for cash flows related to excess tax benefits prospectively. Additionally, the Company has elected to continue to estimate forfeitures expected to occur in determining the amount of compensation expense to be recognized each period. While this new standard may cause volatility in the Company’s effective tax rates and diluted earnings per share due to tax effects of stock awards being recorded within the Company’s consolidated statements of operations, adoption of this standard did not have a material impact on the Company's consolidated financial statements for the year ended December 31, 2017.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments. The amendments in this ASU clarify how certain cash receipts and cash payments should be classified on the statement of cash flows. The new standard is effective for the Company beginning January 1, 2018 and is to be applied retrospectively to all periods presented. The adoption of this ASU is not expected to have a material impact on the Company’s consolidated financial statements when adopted on January 1, 2018.
In October 2016, the FASB issued ASU No. 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory. The amendments in this ASU allow entities to recognize the income tax consequences of an intra-entity transfer of an asset other than inventory when the transfer occurs. The current guidance does not allow recognition until the asset has been sold to an outside party. The amendments in this ASU are effective for the Company beginning on January 1, 2018 and are to be applied on a modified retrospective basis. The adoption of this ASU is not expected to have a material impact on the Company’s consolidated financial statements when adopted on January 1, 2018.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles-Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. The amendments in this ASU simplify the test for goodwill impairment by eliminating the second step in goodwill impairment assessments. The Company early adopted this ASU as of January 1, 2017.
In August 2017, the FASB issued ASU No. 2017-12, Derivatives and Hedging (Topic 815): Targeted Improvements to Accounting for Hedging Activities. The amendments in this ASU better align an entity’s risk management activities and
F-14
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
financial reporting for hedging relationships through changes to both the designation and measurement guidance for qualifying hedging relationships and the presentation of hedge results. The amendments in the new ASU are effective for the Company on January 1, 2019 and are to be applied prospectively. The adoption of this ASU is not expected to have a material impact on the Company’s consolidated financial statements when adopted on January 1, 2018.
In February 2018, the FASB issued ASU No. 2018-2, Income Statement - Reporting Comprehensive Income (Topic 220), Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income, which allows for the reclassification of certain income tax effects related to the Tax Cuts and Jobs Act between “Accumulated other comprehensive income” and “Retained earnings.” This ASU relates to the requirement that adjustments to deferred tax liabilities and assets related to a change in tax laws or rates to be included in “Income from continuing operations”, even in situations where the related items were originally recognized in “Other comprehensive income” (rather than in “Income from continuing operations”). The amendments in this ASU are effective for all entities for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years, with early adoption permitted. Adoption of this ASU is to be applied either in the period of adoption or retrospectively to each period in which the effect of the change in the tax laws or rates were recognized. The Company is still evaluating certain aspects of this ASU as well as the related impacts it may have on the Company's consolidated financial statements.
2. Earnings per share
Basic earnings per share is calculated by dividing net income attributable to the Company, adjusted for any change in noncontrolling interest redemption rights in excess of fair value, by the weighted average number of common shares and vested stock units outstanding, net of shares held in escrow that under certain circumstances may be returned to the Company.
Diluted earnings per share includes the dilutive effect of outstanding stock-settled stock appreciation rights (SSARs) and unvested stock units (under the treasury stock method) as well as shares held in escrow that the Company expects will remain outstanding.
The reconciliations of the numerators and denominators used to calculate basic and diluted net income per share are as follows:
F-15
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(shares in thousands) | |||||||||||
Numerators: | |||||||||||
Net income from continuing operations attributable to DaVita Inc. | $ | 901,277 | $ | 1,032,373 | $ | 323,199 | |||||
Net loss from discontinued operations attributable to DaVita Inc. | (237,659 | ) | (152,499 | ) | (53,467 | ) | |||||
Net income attributable to DaVita Inc. for basic earnings per share calculation | $ | 663,618 | $ | 879,874 | $ | 269,732 | |||||
Basic: | |||||||||||
Weighted average shares outstanding during the period | 190,820 | 203,835 | 214,062 | ||||||||
Contingently returnable shares held in escrow for the DaVita HealthCare Partners merger | (2,194 | ) | (2,194 | ) | (2,194 | ) | |||||
Weighted average shares for basic earnings per share calculation | 188,626 | 201,641 | 211,868 | ||||||||
Basic net income from continuing operations per share attributable to DaVita Inc. | $ | 4.78 | $ | 5.12 | $ | 1.53 | |||||
Basic net loss from discontinued operations per share attributable to DaVita Inc. | (1.26 | ) | (0.76 | ) | (0.26 | ) | |||||
Basic net income per share attributable to DaVita Inc. | $ | 3.52 | $ | 4.36 | $ | 1.27 | |||||
Diluted: | |||||||||||
Weighted average shares outstanding during the period | 190,820 | 203,835 | 214,062 | ||||||||
Assumed incremental shares from stock plans | 529 | 1,070 | 2,190 | ||||||||
Weighted average shares for diluted earnings per share calculation | 191,349 | 204,905 | 216,252 | ||||||||
Diluted net income from continuing operations per share attributable to DaVita Inc. | $ | 4.71 | $ | 5.04 | $ | 1.49 | |||||
Diluted net loss from discontinued operations per share attributable to DaVita Inc. | (1.24 | ) | (0.75 | ) | (0.24 | ) | |||||
Diluted net income per share attributable to DaVita Inc. | $ | 3.47 | $ | 4.29 | $ | 1.25 | |||||
Anti-dilutive stock-settled awards excluded from calculation(1) | 4,350 | 2,523 | 1,365 | ||||||||
(1) | Shares associated with stock-settled stock appreciation rights excluded from the diluted denominator calculation because they are anti-dilutive under the treasury stock method. |
3. Investments in debt and equity securities
The Company classifies certain debt securities as held-to-maturity and records them at amortized cost based on the Company’s intentions and strategies concerning those investments. Equity securities that have readily determinable fair values, and certain other financial instruments that have readily determinable fair values or redemption values, are classified as available for sale and recorded at estimated fair value.
The Company’s investments in these securities and certain other financial instruments consist of the following:
December 31, 2017 | December 31, 2016 | ||||||||||||||||||||||
Held to maturity | Available for sale | Total | Held to maturity | Available for sale | Total | ||||||||||||||||||
Certificates of deposit, commercial paper and money market funds due within one year | $ | 42,316 | $ | — | $ | 42,316 | $ | 255,781 | $ | — | $ | 255,781 | |||||||||||
Investments in mutual funds and common stock | — | 38,895 | 38,895 | 50,000 | 31,197 | 81,197 | |||||||||||||||||
$ | 42,316 | $ | 38,895 | $ | 81,211 | $ | 305,781 | $ | 31,197 | $ | 336,978 | ||||||||||||
Short-term investments | $ | 42,316 | $ | 1,200 | $ | 43,516 | $ | 305,781 | $ | 1,200 | $ | 306,981 | |||||||||||
Long-term investments | — | 37,695 | 37,695 | — | 29,997 | 29,997 | |||||||||||||||||
$ | 42,316 | $ | 38,895 | $ | 81,211 | $ | 305,781 | $ | 31,197 | $ | 336,978 | ||||||||||||
F-16
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The cost of certificates of deposit, commercial paper and money market funds at December 31, 2017 and 2016 approximate their fair value. As of December 31, 2017 and 2016, available for sale investments included $8,416 and $3,701, respectively, of gross pre-tax unrealized gains. During 2017 and 2016 the Company recorded gross pre-tax unrealized gains of $5,075 and $1,802, respectively, in other comprehensive income associated with changes in the fair value of these investments. During 2017, the Company sold investments in mutual funds and common stock for net proceeds of $6,408, and recognized a pre-tax gain of $360, or $220 after tax, that was previously recorded in other comprehensive income. During 2016, the Company sold investments in mutual funds and common stock for net proceeds of $14,971, and recognized a pre-tax gain of $690, or $423 after tax, that was previously recorded in other comprehensive income.
Investments in mutual funds classified as available for sale are held within trusts to fund existing obligations associated with several of the Company’s non-qualified deferred compensation plans.
4. Accounts receivable
Approximately 21% and 16% of the Company’s net patient services accounts receivable balances as of December 31, 2017 and 2016, respectively, were more than six months old. The increase was primarily due to changes the Company made in its collection policies and procedures to improve overall collections. There were no significant balances over one year old. Accounts receivable are principally from Medicare and Medicaid programs and commercial insurance plans.
Accounts receivable are reduced by an allowance for doubtful accounts. In evaluating the ultimate collectability of its accounts receivable, the Company analyzes its historical cash collection experience and trends for each payor to estimate the adequacy of the allowance for doubtful accounts and the amount of the provision for uncollectible accounts. Management regularly updates its analysis based upon the most recent information available to it to determine its current provision for uncollectible accounts and the adequacy of its allowance for doubtful accounts.
For receivables associated with U.S. dialysis and related lab services covered by government payors, like Medicare, the Company receives 80% of the payment directly from Medicare as established under the government’s bundled payment system and determines an appropriate allowance for doubtful accounts and provision for uncollectible accounts on the remaining balance due depending upon the Company’s estimate of the amounts ultimately collectible from other secondary coverage sources or from the patients. For receivables associated with services to patients covered by commercial payors that are either based upon contractual terms or for non-contracted health plan coverage, the Company provides an allowance for doubtful accounts by recording a provision for uncollectible accounts based upon its historical collection experience, potential inefficiencies in its billing processes and for which collectability is determined to be unlikely.
Approximately 1% of the Company’s U.S. dialysis and related lab services net accounts receivable are associated with patient pay and it is the Company’s policy to reserve 100% of the outstanding accounts receivable balances for dialysis services when those amounts due are outstanding for more than three months.
During the year ended December 31, 2017, the Company’s allowance for doubtful accounts decreased by $20,498. The decrease in 2017 was primarily due to an increase in write-offs of aged balances from an increase in uninsured and underinsured uncollectible patient balances related to the U.S. dialysis and related lab business. During the year ended December 31, 2016, the Company’s allowance for doubtful accounts decreased by $12,837. The decrease in 2016 was primarily due to an increase in the write-offs of patient pay billings in the Company's U.S. dialysis business. The decrease was also due to a reduction in accounts receivable older than six months.
5. Other receivables
Other receivables were comprised of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Supplier rebates and non-trade receivables | $ | 268,949 | $ | 183,498 | |||
Medicare bad debt claims | 103,970 | 104,658 | |||||
$ | 372,919 | $ | 288,156 | ||||
F-17
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
6. Prepaid and other current assets
Other current assets were comprised of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Prepaid expenses | $ | 104,727 | $ | 96,818 | |||
Other | 7,331 | 2,692 | |||||
$ | 112,058 | $ | 99,510 | ||||
7. Property and equipment
Property and equipment were comprised of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Land | $ | 33,814 | $ | 26,339 | |||
Buildings | 473,489 | 429,039 | |||||
Leasehold improvements | 2,816,675 | 2,495,070 | |||||
Equipment and information systems, including internally developed software | 2,352,246 | 2,182,912 | |||||
New center and capital asset projects in progress | 576,651 | 429,037 | |||||
6,252,875 | 5,562,397 | ||||||
Less accumulated depreciation | (3,103,662 | ) | (2,698,276 | ) | |||
$ | 3,149,213 | $ | 2,864,121 | ||||
Depreciation expense on property and equipment was $544,129, $494,945, and $444,720 for 2017, 2016 and 2015, respectively.
In addition, during the first quarter of 2017, the Company recognized an asset impairment charge of $15,168 related to the restructuring of its pharmacy business.
Interest on debt incurred during the development of new centers and other capital asset projects is capitalized as a component of the asset cost based on the respective in-process capital asset balances. Interest capitalized was $19,176, $12,990 and $9,723 for 2017, 2016 and 2015, respectively.
8. Intangibles
Intangible assets other than goodwill were comprised of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Noncompetition and other agreements | $ | 429,140 | $ | 407,220 | |||
Lease agreements | 7,623 | 7,244 | |||||
Indefinite-lived assets | 33,255 | 1,125 | |||||
Other | 583 | 583 | |||||
470,601 | 416,172 | ||||||
Less accumulated amortization | (356,774 | ) | (342,668 | ) | |||
$ | 113,827 | $ | 73,504 | ||||
Amortization expense from amortizable intangible assets, other than lease agreements, was $15,782, $14,552, and $19,185 for 2017, 2016 and 2015, respectively. Lease agreement intangible assets and liabilities were amortized to rent expense in the amounts of $(203), $(232) and $(331) for 2017, 2016 and 2015, respectively.
During the years ended December 31, 2017, 2016 and 2015, the Company recognized no impairment charges on any intangible assets other than goodwill.
F-18
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Amortizable intangible liabilities as of December 31, 2017 and 2016 were comprised of lease agreements of $5,447 and $6,011, respectively, which were net of accumulated amortization of $3,508 and $3,618, respectively.
There was no amortization benefit recognized from the alliance and product supply agreement in 2017 and 2016 as it expired in September 2015. Amortization benefit related to this agreement was $3,997 for 2015.
Lease agreement intangible liabilities are classified in other long-term liabilities and amortized to rent expense.
Scheduled amortization charges from amortizable intangible assets and liabilities as of December 31, 2017 were as follows:
Noncompetition and other agreements | Lease liabilities | Other | |||||||||
2018 | $ | 15,581 | $ | (849 | ) | $ | 102 | ||||
2019 | 14,051 | (658 | ) | 87 | |||||||
2020 | 12,629 | (628 | ) | 44 | |||||||
2021 | 9,929 | (602 | ) | — | |||||||
2022 | 6,808 | (553 | ) | — | |||||||
Thereafter | 21,341 | (2,157 | ) | — | |||||||
Total | $ | 80,339 | $ | (5,447 | ) | $ | 233 | ||||
9. Equity method and other investments
Equity investments that do not have readily determinable fair values are carried on the cost or equity method, as applicable. The Company maintains equity method investments in nonconsolidated investees as well as minor cost method investments in private securities of certain other healthcare businesses. The Company classifies its non-marketable cost- and equity method investments as "Equity method and other investments" on its balance sheet.
As described in Note 20, effective as of August 1, 2016, the Company deconsolidated its Asia Pacific dialysis business held by DaVita Care Pte. Ltd. (the APAC JV), adjusted its retained investment in the APAC JV to estimated fair value at that time, and has accounted for this retained investment on the equity method since that time.
During the year ended December 31, 2017, the Company recognized a non-cash other-than-temporary impairment charge of $280,066 on its investment in the APAC JV. This charge resulted from changes in its expectations for the joint venture based on continuing market research and assessments by both the Company and the APAC JV concerning the size of the addressable market available to the joint venture at attractive risk-adjusted returns. The Company estimated the fair value of its retained interest in the APAC JV with the assistance of an independent third party valuation firm based on information available to management as of December 31, 2017. After this charge, the Company’s investment in the APAC JV was carried at $160,481 as of December 31, 2017.
During the year ended December 31, 2016, the Company recorded an impairment of $14,993 related to an investment at one of its other international reporting units.
Total equity method and other investments in nonconsolidated businesses were $245,534 and $492,039 at December 31, 2017 and 2016, respectively. The decrease in these equity investments was primarily due to the impairment of the Company's investment in the APAC JV. During 2017, 2016 and 2015, the Company recognized equity investment (loss) income of $(8,640), $16,874 and $13,919, respectively, from its equity method investments in nonconsolidated businesses.
F-19
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
10. Goodwill
Changes in the carrying value of goodwill by reportable segments were as follows:
U.S. dialysis and related lab services | Other ancillary services and strategic initiatives | Consolidated total | |||||||||
Balance at January 1, 2016 | $ | 5,629,183 | $ | 267,032 | $ | 5,896,215 | |||||
Acquisitions | 75,295 | 123,632 | 198,927 | ||||||||
Divestitures | (12,891 | ) | (29,645 | ) | (42,536 | ) | |||||
Goodwill impairment charges | — | (28,415 | ) | (28,415 | ) | ||||||
Foreign currency and other adjustments | — | (8,816 | ) | (8,816 | ) | ||||||
Balance at December 31, 2016 | $ | 5,691,587 | $ | 323,788 | $ | 6,015,375 | |||||
Acquisitions | 485,434 | 131,598 | 617,032 | ||||||||
Divestitures | (32,260 | ) | (126 | ) | (32,386 | ) | |||||
Goodwill impairment charges | — | (36,196 | ) | (36,196 | ) | ||||||
Foreign currency and other adjustments | — | 46,454 | 46,454 | ||||||||
Balance at December 31, 2017 | $ | 6,144,761 | $ | 465,518 | $ | 6,610,279 | |||||
Goodwill | $ | 6,144,761 | $ | 536,038 | $ | 6,680,799 | |||||
Accumulated impairment charges | — | (70,520 | ) | (70,520 | ) | ||||||
$ | 6,144,761 | $ | 465,518 | $ | 6,610,279 | ||||||
The Company elected to early adopt ASU No. 2017-04, Intangibles-Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment effective January 1, 2017. The amendments in this ASU simplify the test for goodwill impairment by eliminating the second step in the assessment. All goodwill impairment tests performed during 2017 have been performed under this new guidance.
Each of the Company’s operating segments described in Note 25 to these consolidated financial statements represents an individual reporting unit for goodwill impairment testing purposes, except that each sovereign jurisdiction within the Company’s international operating segments is considered a separate reporting unit.
Within the U.S. dialysis and related lab services operating segment, the Company considers each of its dialysis centers to constitute an individual business for which discrete financial information is available. However, since these dialysis centers have similar operating and economic characteristics, and the allocation of resources and significant investment decisions concerning these businesses are highly centralized and the benefits broadly distributed, the Company has aggregated these centers and deemed them to constitute a single reporting unit.
The Company has applied a similar aggregation to the vascular access service centers in its vascular access services reporting unit, to the physician practices in its physician services and direct primary care reporting units, and to the dialysis centers within each international reporting unit. For the Company’s other operating segments, discrete business components below the operating segment level constitute individual reporting units.
During the year ended December 31, 2016, the Company recognized a goodwill impairment charge of $28,415 related to the Company’s vascular access reporting unit as a result of changes in future governmental reimbursement rates for this business and the Company’s expected ability to mitigate them. Specifically, on November 2, 2016, CMS released the 2017 Physician Fee Schedule Final Rule and the Ambulatory Surgical Center Payment Final Rule which reflected significant changes in reimbursement structure for this business unit.
During the year ended December 31, 2017, the Company recognized an additional goodwill impairment charge of $34,696 at its vascular access reporting unit. This charge resulted primarily from continuing changes in the Company’s outlook for this business unit as the Company’s partners and operators continued to evaluate and make decisions concerning changes in operations, including termination of their management services agreements and center closures, as a result of the changes in reimbursement structure discussed above. As of December 31, 2017, there was no goodwill remaining at the Company's vascular access reporting unit.
F-20
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
During the year ended December 31, 2017, the Company also performed annual impairment assessments for various other reporting units. As a result of these assessments, the Company also recognized a goodwill impairment charge of $1,500 at one of its international reporting units during the year ended December 31, 2017. During the year ended December 31, 2015, the Company also recognized a goodwill impairment charge of $4,066 at another international reporting unit.
Based on the most recent assessments, the Company determined that reductions in reimbursement rates, changes in actual or expected growth rates, or other significant adverse changes in expected future cash flows or valuation assumptions could result in goodwill impairment charges in the future for the following reporting units, which remain at risk of goodwill impairment as of December 31, 2017:
Reporting unit | Goodwill balance as of December 31, 2017 | Carrying amount coverage(1) | Sensitivities | |||||||
Operating income(2) | Discount rate(3) | |||||||||
Kidney Care Germany | $ | 316,369 | 13.7% | (1.6)% | (11.1)% | |||||
Kidney Care Portugal | $ | 46,713 | 16.9% | (1.9)% | (6.0)% | |||||
Kidney Care Poland | $ | 46,610 | 11.8% | (1.9)% | (6.0)% | |||||
(1) | Excess of estimated fair value of the reporting unit over carrying amount as of the latest assessment date. |
(2) | Potential impact on estimated fair value of a sustained, long-term reduction of 3% in operating income as of the latest assessment date. |
(3) | Potential impact on estimated fair value of an increase in discount rates of 100 basis points as of the latest assessment date. |
There were no major changes in the business, prospects, or expected future results of these reporting units from their latest assessment date through December 31, 2017.
Except as described above, none of the Company’s other reporting units were considered at risk of significant goodwill impairment as of December 31, 2017. Since the dates of the Company’s last annual goodwill impairment tests, there have been certain developments, events, changes in operating performance and other changes in key circumstances that have affected the Company’s businesses. However, except as further described above, these did not cause management to believe it is more likely than not that the fair values of any of the Company’s reporting units would be less than their respective carrying amounts as of December 31, 2017.
11. Other liabilities
Other liabilities were comprised of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Payor refunds and retractions | $ | 292,370 | $ | 270,298 | |||
Insurance and self-insurance accruals | 64,924 | 76,857 | |||||
Accrued interest | 83,362 | 82,234 | |||||
Accrued non-income tax liabilities | 28,317 | 23,643 | |||||
Other | 83,689 | 125,860 | |||||
$ | 552,662 | $ | 578,892 | ||||
12. Income taxes
The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.
F-21
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Income before income taxes from continuing operations consisted of the following:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Domestic | $ | 1,725,822 | $ | 1,278,754 | $ | 730,249 | |||||
International | (326,036 | ) | 344,351 | (41,862 | ) | ||||||
$ | 1,399,786 | $ | 1,623,105 | $ | 688,387 | ||||||
Income tax expense (benefit) for continuing operations consisted of the following:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Current: | |||||||||||
Federal | $ | 330,191 | $ | 322,940 | $ | 124,503 | |||||
State | 47,228 | 44,525 | 20,442 | ||||||||
International | 3,422 | 1,928 | 856 | ||||||||
Total current income tax | 380,841 | 369,393 | 145,801 | ||||||||
Deferred: | |||||||||||
Federal | (98,760 | ) | 88,412 | 71,016 | |||||||
State | 37,347 | (28,530 | ) | (9,737 | ) | ||||||
International | 4,431 | 2,486 | 430 | ||||||||
Total deferred income tax | (56,982 | ) | 62,368 | 61,709 | |||||||
$ | 323,859 | $ | 431,761 | $ | 207,510 | ||||||
Income taxes are allocated between continuing and discontinued operations as follows:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Continuing operations | $ | 323,859 | $ | 431,761 | $ | 207,510 | |||||
Discontinued operations | (364,856 | ) | 24,052 | 88,216 | |||||||
$ | (40,997 | ) | $ | 455,813 | $ | 295,726 | |||||
The reconciliation between the Company’s effective tax rate from continuing operations and the U.S. federal income tax rate is as follows:
Year ended December 31, | ||||||||
2017 | 2016 | 2015 | ||||||
Federal income tax rate | 35.0 | % | 35.0 | % | 35.0 | % | ||
State income taxes, net of federal benefit | 3.7 | 2.6 | 1.7 | |||||
Gain on APAC JV ownership changes | (0.2 | ) | (9.9 | ) | — | |||
APAC investment impairment | 6.4 | — | — | |||||
Impact of 2017 Tax Act | (20.5 | ) | — | — | ||||
Other | 2.0 | 1.8 | 2.3 | |||||
Impact of noncontrolling interests primarily attributable to non-tax paying entities | (3.3 | ) | (2.9 | ) | (8.9 | ) | ||
Effective tax rate | 23.1 | % | 26.6 | % | 30.1 | % | ||
On December 22, 2017, the President signed into law the tax legislation known as the Tax Cuts and Jobs Act (the 2017 Tax Act). The 2017 Tax Act includes a number of changes to existing U.S. tax laws that impact the Company, most notably a reduction in the U.S. corporate income tax rate from 35.0% to 21.0% effective January 1, 2018. The 2017 Tax Act also provides for full expensing of qualified assets placed into service after September 27, 2017, as well as prospective changes beginning in 2018, imposes a one-time transition tax on certain foreign subsidiaries, and changes how foreign earnings are subject to U.S. tax prospectively.
F-22
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The Company recognized the income tax effects of the 2017 Tax Act in its 2017 financial statements in accordance with Staff Accounting Bulletin No. 118, which provides SEC staff guidance for the application of ASC Topic 740, Income Taxes, in the reporting period in which the 2017 Tax Act was signed into law. As such, the Company’s financial results reflect the income tax effects of the 2017 Tax Act for which accounting under ASC Topic 740 is complete and provisional amounts, primarily as it relates to the full expensing provisions of the 2017 Tax Act, for those specific income tax effects for which the accounting is incomplete but a reasonable estimate could be determined.
The Company has completed the accounting for income taxes with respect to the mandatory one-time tax on accumulated earnings of its foreign subsidiaries and has determined that there is no mandatory repatriation and therefore no income tax liability associated with this one-time tax.
The Company measures deferred tax assets and liabilities using enacted tax rates that will apply in the years in which the temporary differences are expected to be recovered or paid. Accordingly, the Company’s deferred tax assets and liabilities were remeasured to reflect a reasonable estimate of the reduction in the U.S. corporate income tax rate from 35.0% to 21.0%, resulting in a provisional $251,510 net tax benefit.
While the Company has substantially completed its provisional analysis of the income tax effects of the 2017 Tax Act and recorded a reasonable estimate of such effects, the net one-time benefit related to the 2017 Tax Act may differ, possibly materially, due to, among other things, further refinement of the underlying calculations, changes in interpretations and assumptions that the Company has made, additional guidance that may be issued by the U.S. Government, and actions and related accounting policy decisions the Company may take as a result of the 2017 Tax Act. The Company will complete its analysis over a one-year measurement period ending December 22, 2018, and any adjustments during this measurement period will be included in net earnings from continuing operations as an adjustment to income tax expense in the reporting period in which such adjustments are determined.
Deferred tax assets and liabilities arising from temporary differences for continuing operations were as follows:
December 31, | |||||||
2017 | 2016 | ||||||
Receivables | $ | 19,705 | $ | 25,197 | |||
Accrued liabilities | 96,537 | 224,712 | |||||
Net operating loss carryforwards | 108,429 | 128,813 | |||||
Other | 37,794 | 73,525 | |||||
Deferred tax assets | 262,465 | 452,247 | |||||
Valuation allowance | (61,282 | ) | (56,016 | ) | |||
Net deferred tax assets | 201,183 | 396,231 | |||||
Intangible assets | (501,763 | ) | (676,781 | ) | |||
Property and equipment | (100,376 | ) | (141,919 | ) | |||
Investments in partnerships | (61,529 | ) | (95,936 | ) | |||
Other | (23,762 | ) | (12,464 | ) | |||
Deferred tax liabilities | (687,430 | ) | (927,100 | ) | |||
Net deferred tax liabilities | $ | (486,247 | ) | $ | (530,869 | ) | |
At December 31, 2017, the Company had federal net operating loss carryforwards of approximately $137,852 that expire through 2036, although a substantial amount expire by 2028. The Company also had state net operating loss carryforwards of $445,554 that expire through 2036 and international net operating loss carryforwards of $138,717, some of which have an indefinite life. The utilization of a portion of these losses may be limited in future years based on the profitability of certain entities. The net increase of $5,266 in the valuation allowance is primarily due to newly created net operating loss carryforwards in state and foreign jurisdictions that the Company does not anticipate being able to utilize.
The 2017 Tax Act includes a mandatory one-time tax on accumulated earnings of foreign subsidiaries, and as a result, all previously unremitted earnings for which no U.S. deferred tax liability had been accrued would now be subject to U.S. tax. Irrespective of the fact that the Company will not experience any one-time tax under this provision of the 2017 Tax Act, it
F-23
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
intends to continue to indefinitely reinvest these earnings, as well as capital invested and future earnings from its foreign subsidiaries to fund its international operations. In addition, the Company expects future U.S. cash generation will be sufficient to meet future U.S. cash needs. Determination of the amount of any applicable deferred taxes on the earnings is not practical since the computation would depend on a number of factors that cannot be known unless a decision is made to repatriate the earnings.
Unrecognized tax benefits
A reconciliation of the beginning and ending liability for unrecognized tax benefits that do not meet the more-likely-than-not threshold is as follows:
Year ended December 31, | |||||||
2017 | 2016 | ||||||
Beginning balance | $ | 24,066 | $ | 39,011 | |||
Additions for tax positions related to current year | 7,606 | 9,714 | |||||
Additions for tax positions related to prior years | 804 | — | |||||
Reductions related to lapse of applicable statute | (1,380 | ) | (1,277 | ) | |||
Impact of 2017 Tax Act | 3,731 | — | |||||
Reductions related to settlements with taxing authorities | (2,051 | ) | (23,382 | ) | |||
Ending balance | $ | 32,776 | $ | 24,066 | |||
As of December 31, 2017, the Company’s total liability for unrecognized tax benefits relating to tax positions that do not meet the more-likely-than-not threshold is $32,776, all of which would impact the Company’s effective tax rate if recognized. This balance represents an increase of $8,710 from the December 31, 2016 balance of $24,066, primarily due to additions for tax positions related to the current year.
The Company recognizes accrued interest and penalties related to unrecognized tax benefits in its income tax expense. At December 31, 2017 and 2016, the Company had approximately $4,195 and $2,595, respectively, accrued for interest and penalties related to unrecognized tax benefits, net of federal tax benefit.
The Company and its subsidiaries file U.S. federal and state income tax returns and various foreign income tax returns. The Company is no longer subject to U.S. federal and state examinations by tax authorities for years before 2013 and 2008, respectively.
13. Long-term debt
Long-term debt was comprised of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Senior Secured Credit Facilities: | |||||||
Term Loan A | $ | 775,000 | $ | 862,500 | |||
Term Loan B | 3,377,500 | 3,412,500 | |||||
Revolver | 300,000 | — | |||||
Senior notes | 4,500,000 | 4,500,000 | |||||
Acquisition obligations and other notes payable | 150,512 | 117,547 | |||||
Capital lease obligations | 297,170 | 292,252 | |||||
Total debt principal outstanding | 9,400,182 | 9,184,799 | |||||
Discount and deferred financing costs | (63,951 | ) | (79,861 | ) | |||
9,336,231 | 9,104,938 | ||||||
Less current portion | (178,213 | ) | (160,262 | ) | |||
$ | 9,158,018 | $ | 8,944,676 | ||||
F-24
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Scheduled maturities of long-term debt at December 31, 2017 were as follows:
2018 | 178,213 | |
2019 | 1,049,091 | |
2020 | 73,362 | |
2021 | 3,307,507 | |
2022 | 1,283,671 | |
Thereafter | 3,508,338 | |
Term Loans
Total outstanding borrowings under Term Loan A and Term Loan B can consist of various individual tranches that can range in maturity from one month to twelve months (currently all tranches are one month in duration). For Term Loan A and Term Loan B, each tranche bears interest at a London Interbank Offered Rate (LIBOR) that is determined by the duration of such tranche plus an interest rate margin. The LIBOR variable component of the interest rate for each tranche is reset as such tranche matures and a new tranche is established. At December 31, 2017, the overall weighted average interest rate for Term Loan A was determined based upon the LIBOR interest rates in effect for all of the individual tranches plus the interest rate margin of 2.00%. At December 31, 2017, Term Loan B bears interest at LIBOR (floor of 0.75%) plus a margin of 2.75%. The Company is subject to LIBOR-based interest rate volatility on Term Loan B as the LIBOR-based component of the interest rate exceeded the floor of 0.75% as of December 31, 2017. The overall weighted average interest rate for Term Loan B was determined based upon the LIBOR interest rates in effect for all individual tranches plus the interest rate margin.
The Company has several interest rate cap agreements that have the economic effect of capping the LIBOR variable component of the Company’s interest rate at a maximum of 3.50% on $3,500,000 of outstanding principal debt. The remaining $652,500 outstanding principal balance of Term Loan A would still be subject to LIBOR-based interest rate volatility. In addition, the Company maintains several forward interest rate cap agreements with notional amounts totaling $3,500,000, which will be effective June 29, 2018. The cap agreements will have the economic effect of capping the LIBOR variable component of the Company’s interest rate at a maximum of 3.50% on an equivalent amount of the Company’s debt. See below for further details. The Company is restricted from paying dividends under the terms of its senior secured credit facilities.
During the year ended December 31, 2017, the Company made mandatory principal payments under its senior secured credit facilities totaling $87,500 on Term Loan A and $35,000 on Term Loan B.
Revolving lines of credit
The Company has $300,000 drawn on its $1,000,000 revolving line of credit under its senior secured credit facilities, in addition to approximately $14,383 committed for outstanding letters of credit. The Company also has approximately $90,085 of additional outstanding letters of credit related to Kidney Care and $211 of committed outstanding letters of credit related to DMG, which is backed by a certificate of deposit.
Senior Notes
The Company’s senior notes as of December 31, 2017 consisted of $1,500,000 of 5.0% Senior Notes due 2025, $1,750,000 5 1/8% senior notes due 2024 and $1,250,000 of 5 3/4% senior notes due 2022 (collectively Senior Notes).
The Senior Notes are unsecured obligations, rank equally in right of payment with the Company’s existing and future unsecured senior indebtedness, and are guaranteed by substantially all of the Company’s direct and indirect wholly-owned domestic subsidiaries and require semi-annual interest payments. The Company may redeem some or all of the Senior Notes at any time on or after certain specific dates and at certain specific redemption prices as outlined in each senior note agreement. The Company is restricted from paying dividends under the indentures governing its Senior Notes.
Interest rate cap and swap agreements
During the year ended December 31, 2017 the Company had several currently effective and forward interest rate cap agreements as a means of hedging its exposure to and volatility from variable-based interest rate changes as part of its overall interest rate risk management strategy. These agreements were not held for trading or speculative purposes and had the economic effect of capping the Company’s maximum exposure to LIBOR variable interest rate changes on specific portions of the Company’s floating rate debt, as described below. These cap agreements are also designated as cash flow hedges and, as a
F-25
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
result, changes in the fair values of these cap agreements are reported in other comprehensive income. The amortization of the original cap premium is recognized as a component of debt expense on a straight-line basis over the term of the cap agreements. The cap agreements do not contain credit-risk contingent features.
As of December 31, 2017, the Company maintains several currently effective interest rate cap agreements that were entered into in November 2014 with notional amounts totaling $3,500,000. These cap agreements became effective September 30, 2016 and have the economic effect of capping the LIBOR variable component of the Company’s interest rate at a maximum of 3.50% on an equivalent amount of the Company’s debt. These cap agreements expire on June 30, 2018. As of December 31, 2017, these cap agreements had an immaterial fair value. During the year ended December 31, 2017, the Company recognized debt expense of $8,278 from these caps. During the year ended December 31, 2017, the Company recorded a loss of $115 in other comprehensive income due to a decrease in the unrealized fair value of these cap agreements.
As of December 31, 2017, the Company also maintains several forward interest rate cap agreements that were entered into in October 2015 with notional amounts totaling $3,500,000. These forward cap agreements will become effective June 29, 2018 and will have the economic effect of capping the LIBOR variable component of the Company’s interest rate at a maximum of 3.50% on an equivalent amount of its debt. These cap agreements expire on June 30, 2020. As of December 31, 2017, the total fair value of these cap agreements was an asset of approximately $1,032. During the year ended December 31, 2017, the Company recorded a loss of $8,782 in other comprehensive income due to a decrease in the unrealized fair value of these cap agreements.
The following table summarizes the Company’s derivative instruments as of December 31, 2017 and 2016:
Fair value | |||||||||
Derivatives designated as hedging instruments | Balance sheet location | December 31, 2017 | December 31, 2016 | ||||||
Interest rate cap agreements | Other long-term assets | $ | 1,032 | $ | 9,929 | ||||
The following table summarizes the effects of the Company’s interest rate cap and swap agreements for the years ended December 31, 2017, 2016 and 2015:
Amount of unrealized losses in OCI on interest rate cap and swap agreements | Location of losses reclassified from accumulated OCI into income | Amount of losses reclassified from accumulated OCI into income | ||||||||||||||||||||||||
Year ended December 31, | Year ended December 31, | |||||||||||||||||||||||||
Derivatives designated as cash flow hedges | 2017 | 2016 | 2015 | 2017 | 2016 | 2015 | ||||||||||||||||||||
Interest rate cap agreements | $ | (8,897 | ) | $ | (5,198 | ) | $ | (16,114 | ) | Debt expense | $ | 8,278 | $ | 3,899 | $ | 2,439 | ||||||||||
Interest rate swap agreements | — | (815 | ) | (3,971 | ) | Debt expense | — | 299 | 2,664 | |||||||||||||||||
Tax benefit | 3,460 | 2,343 | 7,844 | Tax expense | (3,220 | ) | (1,632 | ) | (1,992 | ) | ||||||||||||||||
Total | $ | (5,437 | ) | $ | (3,670 | ) | $ | (12,241 | ) | $ | 5,058 | $ | 2,566 | $ | 3,111 | |||||||||||
As of December 31, 2017, the Company’s Term Loan B debt bears interest at LIBOR plus an interest rate margin of 2.75%. Term Loan B is subject to an interest rate cap if LIBOR should rise above 3.50%. Term Loan A bears interest at LIBOR plus an interest rate margin of 2.00%. The capped portion of Term Loan A is $122,500. In addition, the uncapped portion of Term Loan A, which is subject to the variability of LIBOR, is $652,500. See above for further details. Interest rates on the Company’s Senior Notes are fixed by their terms.
The Company’s overall weighted average effective interest rate on the senior secured credit facilities was 4.45%, based upon the current margins in effect of 2.00% for Term Loan A and the Revolver and 2.75% for Term Loan B, as of December 31, 2017.
The Company’s overall weighted average effective interest rate during the year ended December 31, 2017 was 4.70% and as of December 31, 2017 was 4.88%.
F-26
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Debt expense
Debt expense consisted of interest expense of $406,341, $394,013 and $389,755 and the amortization and accretion of debt discounts and premiums, amortization of deferred financing costs and the amortization of interest rate cap agreements of $24,293, $20,103 and $18,625 for 2017, 2016 and 2015, respectively. The interest expense amounts are net of capitalized interest.
14. Leases
The majority of the Company’s facilities are leased under non-cancellable operating leases ranging in terms from five to fifteen years and which contain renewal options of five to ten years at the fair rental value at the time of renewal. The Company’s leases are generally subject to periodic consumer price index increases or contain fixed escalation clauses. The Company also leases certain facilities and equipment under capital leases.
Future minimum lease payments under non-cancellable operating and capital leases are as follows:
Operating leases | Capital leases | ||||||
2018 | $ | 446,935 | $ | 35,258 | |||
2019 | 422,245 | 36,038 | |||||
2020 | 384,764 | 36,689 | |||||
2021 | 351,962 | 32,578 | |||||
2022 | 313,005 | 33,004 | |||||
Thereafter | 1,303,594 | 234,094 | |||||
$ | 3,222,505 | 407,661 | |||||
Less portion representing interest | (110,491 | ) | |||||
Total capital lease obligations, including current portion | $ | 297,170 | |||||
Rent expense under all operating leases for 2017, 2016, and 2015 was $530,748, $478,531 and $440,601, respectively. Rent expense is recorded on a straight-line basis over the term of the lease for leases that contain fixed escalation clauses or include abatement provisions. Leasehold improvement incentives are deferred and amortized to rent expense over the term of the lease. The net book value of property and equipment under capital leases was $257,772 and $263,438 at December 31, 2017 and 2016, respectively. Capital lease obligations are included in long-term debt. See Note 13 to these consolidated financial statements.
15. Employee benefit plans
The Company has a savings plan for substantially all of its Kidney Care employees which has been established pursuant to the provisions of Section 401(k) of the Internal Revenue Code (IRC). The plan allows for employees to contribute a percentage of their base annual salaries on a tax-deferred basis not to exceed IRC limitations. The Company has not provided any matching contributions for its Kidney Care employees through December 31, 2017.
Beginning in 2018, the Company has implemented a 401(k) matching program under which the Company will match 50% of the employee's contribution up to 6% of the employee's salary, subject to certain limitations. The matching contributions will be subject to certain eligibility and vesting conditions.
The Company also maintains a voluntary compensation deferral plan, the DaVita Voluntary Deferral Plan. This plan is non-qualified and permits certain employees whose annualized base salary equals or exceeds a minimum annual threshold amount as set by the Company to elect to defer all or a portion of their annual bonus payment and up to 50% of their base salary into a deferral account maintained by the Company. Total contributions to this plan in 2017, 2016 and 2015 were $4,497, $5,344 and $4,234, respectively. Deferred amounts are generally paid out in cash at the participant’s election either in the first or second year following retirement or in a specified future period at least three to four years after the deferral election was effective. During 2017, 2016 and 2015 the Company distributed $1,731, $916 and $1,270, respectively, to participants in this plan. Participants are credited with their proportional amount of annual earnings from the plan. The assets of this plan are held in a rabbi trust and as such are subject to the claims of the Company’s general creditors in the event of its bankruptcy. As of December 31, 2017 and 2016, the total fair value of assets held in this plan’s trust were $38,816 and $30,192, respectively.
F-27
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The Company also maintains a legacy Executive Retirement Plan for certain members of management. This plan is non-qualified and contributions to the plan were made at the discretion of DVA Renal Healthcare based upon a pre-determined percentage of a participant’s base salary. Effective November 2005, all contributions to this plan were discontinued and the balance of the plan assets will be paid out upon termination or retirement of each individual participant. During 2017, 2016 and 2015 the Company distributed $1,058 and $149, $25 respectively, to participants in this plan. As of December 31, 2017 and 2016, the total fair value of assets held under this plan’s trust was $79 and $1,005, respectively.
The fair value of all of the assets held in plan trusts as of December 31, 2017, and 2016 totaled $38,895 and $31,197, respectively. The assets of these plans are available for sale and as such are recorded at fair value with changes in the fair market values being recorded in other comprehensive income. Any fair value changes to the corresponding liability balance are recorded as compensation expense. See Note 3 to these consolidated financial statements.
Most of the Company’s outstanding employee stock plan awards include a provision accelerating the vesting of the award in the event of a change of control. The Company also maintains a change of control protection program for its employees who do not have a significant number of stock awards, which has been in place since 2001, and which provides for cash bonuses to employees in the event of a change of control. Based on the market price of the Company’s common stock and shares outstanding on December 31, 2017, these cash bonuses would total approximately $520,778 if a change of control transaction occurred at that price and the Company’s Board of Directors did not modify the program. This amount has not been accrued at December 31, 2017, and would only be accrued upon a change of control. These change of control provisions may affect the price an acquirer would be willing to pay for the Company.
16. Contingencies
The majority of the Company’s revenues are from government programs and may be subject to adjustment as a result of: (i) examination by government agencies or contractors, for which the resolution of any matters raised may take extended periods of time to finalize; (ii) differing interpretations of government regulations by different Medicare contractors or regulatory authorities; (iii) differing opinions regarding a patient’s medical diagnosis or the medical necessity of services provided; and (iv) retroactive applications or interpretations of governmental requirements. In addition, the Company’s revenues from commercial payors may be subject to adjustment as a result of potential claims for refunds, as a result of government actions or as a result of other claims by commercial payors.
The Company operates in a highly regulated industry and is a party to various lawsuits, claims, qui tam suits, governmental investigations and audits (including investigations resulting from its obligation to self-report suspected violations of law) and other legal proceedings. The Company records accruals for certain legal proceedings and regulatory matters to the extent that the Company determines an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. As of December 31, 2017 and December 31, 2016, the Company’s total recorded accruals, including DMG, with respect to legal proceedings and regulatory matters, net of anticipated third party recoveries, were approximately $6,000 and $69,000, respectively. While these accruals reflect the Company’s best estimate of the probable loss for those matters as of the dates of those accruals, the recorded amounts may differ materially from the actual amount of the losses for those matters, and any anticipated third party recoveries for any such losses may not ultimately be recoverable. Additionally, in some cases, no estimate of the possible loss or range of loss in excess of amounts accrued, if any, can be made because of the inherently unpredictable nature of legal proceedings and regulatory matters, which also may be impacted by various factors, including that they may involve indeterminate claims for monetary damages or may involve fines, penalties or non-monetary remedies; present novel legal theories or legal uncertainties; involve disputed facts; represent a shift in regulatory policy; are in the early stages of the proceedings; or result in a change of business practices. Further, there may be various levels of judicial review available to the Company in connection with any such proceeding.
The following is a description of certain lawsuits, claims, governmental investigations and audits and other legal proceedings to which the Company is subject.
Inquiries by the Federal Government and Certain Related Civil Proceedings
2015 U.S. Office of Inspector General (OIG) Medicare Advantage Civil Investigation: In March 2015, JSA HealthCare Corporation (JSA), a subsidiary of DMG, received a subpoena from the Office of Inspector General (OIG) for the U.S. Department of Health and Human Services (HHS) requesting documents and information for the period from January 1, 2008 through December 31, 2013, for certain MA plans for which JSA provided services. It also requests information regarding JSA’s communications about patient diagnoses as they relate to certain MA plans generally, and more specifically as related to two
F-28
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Florida physicians with whom JSA previously contracted. The Company is producing the requested information and is cooperating with the government’s investigation.
In addition to the subpoena described above, in June 2015, the Company received a civil subpoena from the OIG covering the period from January 1, 2008 through the present and seeking production of a wide range of documents relating to the Company’s and its subsidiaries’ (including DMG’s and its subsidiary JSA’s) provision of services to MA plans and related patient diagnosis coding and risk adjustment submissions and payments. The Company believes that the request is part of a broader industry investigation into MA patient diagnosis coding and risk adjustment practices and potential overpayments by the government. The information requested includes information relating to patient diagnosis coding practices for a number of conditions, including potentially improper historical DMG coding for a particular condition. With respect to that condition, the guidance related to that coding issue was discontinued following the Company’s November 1, 2012 acquisition of HealthCare Partners (now known as the Company's DMG business), and the Company notified CMS in April 2015 of the coding practice and potential overpayments. In that regard, the Company has identified certain additional coding practices which may have been problematic, some of which were the subject of the Swoben Private Civil Suit, and is in discussions with the DOJ relating to those practices. The Company is cooperating with the government. In addition, the Company is continuing to review other DMG coding practices to determine whether there were any improper coding issues. In connection with the Company's acquisition of DMG in 2012, the Company has certain indemnification rights against the sellers and an escrow was established as security for the indemnification. The Company has submitted an indemnification claim against the sellers secured by the escrow for any and all liabilities incurred relating to these matters and intends to pursue recovery from the escrow. However, the Company can make no assurances that the indemnification and escrow will cover the full amount of the Company’s potential losses related to these matters.
2016 U.S. Attorney Prescription Drug Investigation: In early February 2016, the Company announced that its pharmacy services’ wholly-owned subsidiary, DaVita Rx, received a Civil Investigative Demand (CID) from the U.S. Attorney’s Office for the Northern District of Texas. The government is conducting a federal False Claims Act (FCA) investigation concerning allegations that DaVita Rx presented or caused to be presented false claims for payment to the government for prescription medications, as well as into the Company’s relationship with pharmaceutical manufacturers. The CID covers the period from January 1, 2006 through the present. In the spring of 2015, the Company initiated an internal compliance review of DaVita Rx during which it identified potential billing and operational issues, including potential write-offs and discounts of patient co-payment obligations, and credits to payors for returns of prescription drugs related to DaVita Rx. The Company notified the government in September 2015 that it was conducting this review of DaVita Rx and began providing regular updates of its review. Upon completion of its review, the Company filed a self-disclosure with the OIG in February 2016 and has been working to address and update the practices it identified in the self-disclosure, some of which overlap with information requested by the U.S. Attorney’s Office. The OIG informed the Company in February 2016 that its submission was not accepted. They indicated that the OIG is not expressing an opinion regarding the conduct disclosed or the Company’s legal positions. In connection with the Company’s ongoing efforts working with the government the Company learned that a qui tam complaint had been filed covering some of the issues in the CID and the Company’s self-disclosure. In December 2017, the Company finalized and executed a settlement agreement with the government and relators in the qui tam matter and that included total monetary consideration of $63,700, as previously announced, of which $41,500 was an incremental cash payment and $22,200 was for amounts previously refunded, and all of which was previously accrued. The government’s investigation into the Company's relationship with pharmaceutical manufacturers is ongoing and the Company is continuing to cooperate with the government in this investigation.
2017 U.S. Attorney American Kidney Fund Investigation: On January 4, 2017, the Company was served with an administrative subpoena for records by the United States Attorney’s Office, District of Massachusetts, relating to an investigation into possible federal health care offenses. The subpoena covers the period from January 1, 2007 through the present, and seeks documents relevant to charitable patient assistance organizations, particularly the American Kidney Fund, including documents related to efforts to provide patients with information concerning the availability of charitable assistance. The Company is cooperating with the government and is producing the requested information.
* * *
Although the Company cannot predict whether or when proceedings might be initiated or when these matters may be resolved (other than as described above), it is not unusual for inquiries such as these to continue for a considerable period of time through the various phases of document and witness requests and on-going discussions with regulators. In addition to the inquiries and proceedings specifically identified above, the Company is frequently subject to other inquiries by state or federal government agencies and/or private civil qui tam complaints filed by relators. Negative findings or terms and conditions that the Company might agree to accept as part of a negotiated resolution of pending or future government inquiries or relator
F-29
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
proceedings could result in, among other things, substantial financial penalties or awards against the Company, substantial payments made by the Company, harm to the Company’s reputation, required changes to the Company’s business practices, exclusion from future participation in the Medicare, Medicaid and other federal health care programs and, if criminal proceedings were initiated against the Company, possible criminal penalties, any of which could have a material adverse effect on the Company.
Shareholder Claims
Peace Officers’ Annuity and Benefit Fund of Georgia Securities Class Action Civil Suit: On February 1, 2017, the Peace Officers’ Annuity and Benefit Fund of Georgia filed a putative federal securities class action complaint in the U.S. District Court for the District of Colorado against the Company and certain executives. The complaint covers the time period of August 2015 to October 2016 and alleges, generally, that the Company and its executives violated federal securities laws concerning the Company’s financial results and revenue derived from patients who received charitable premium assistance from an industry-funded non-profit organization. The complaint further alleges that the process by which patients obtained commercial insurance and received charitable premium assistance was improper and "created a false impression of DaVita’s business and operational status and future growth prospects." In November 2017, the court appointed the lead plaintiff and an amended complaint was filed on January 12, 2018. The Company's response is due March 13, 2018. The Company disputes these allegations and intends to defend this action accordingly.
In re DaVita Inc. Stockholder Derivative Litigation: On August 15, 2017, the U.S. District Court for the District of Delaware consolidated the three previously disclosed shareholder derivative lawsuits: the Blackburn Shareholder action filed on February 10, 2017, the Gabilondo Shareholder action filed on May 30, 2017, and the City of Warren Police and Fire Retirement System Shareholder action filed on June 9, 2017. The complaint covers the time period from 2015 to present and alleges, generally, breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, corporate waste, and misrepresentations and/or failures to disclose certain information in violation of the federal securities laws in connection with an alleged practice to direct patients with government-subsidized health insurance into private health insurance plans to maximize the Company’s profits. An amended complaint was filed in September 2017, and on December 18, 2017 the Company filed a motion to dismiss and a motion to stay proceedings in the alternative. The Company disputes these allegations and intends to defend this action accordingly.
Other Proceedings
In addition to the foregoing, from time to time the Company is subject to other lawsuits, demands, claims, governmental investigations and audits and legal proceedings that arise due to the nature of its business, including contractual disputes, such as with payors, suppliers and others, employee-related matters and professional and general liability claims. From time to time, the Company also initiates litigation or other legal proceedings as a plaintiff arising out of contracts or other matters.
Resolved Matters
Swoben Private Civil Suit: On July 13, 2009, pursuant to the qui tam provisions of the FCA and the California False Claims Act, James M. Swoben, as relator, filed his initial qui tam action in the United States District Court for the Central District of California purportedly on behalf of the United States of America and the State of California against SCAN, and certain other defendants whose identities were under seal. In April 2013, HealthCare Partners (HCP), now known as the Company’s DMG subsidiary, was one of several defendants served with a civil complaint filed by a former employee of SCAN Health Plan (SCAN), an HMO. The allegations in the complaint relate to alleged overpayments received from government healthcare programs, including allegations of violations of the federal FCA and the California False Claims Act and allegations against HCP relating to patient diagnosis coding. The complaint sought monetary damages and civil penalties as well as costs and expenses. On October 18, 2017, the relator filed a Notice of Dismissal of the action as to HCP, and the government consented to the dismissal, as a result of which the suit is now dismissed, without prejudice.
Solari Post-Acquisition Matter: In 2016, HCP Nevada disclosed to the OIG for the HHS that proper procedures for clinical and eligibility determinations may not have been followed by Las Vegas Solari Hospice (Solari), which was acquired in March 2013 and sold in September 2016 by HCP Nevada. In June 2016, the Company was notified by the OIG that the disclosure submission had been accepted into the OIG’s Self Disclosure Protocol. HCP Nevada had previously made a disclosure and repayment of overpayments to National Government Services (NGS), the Medicare Administrative Contractor for HCP Nevada, for claims submitted by Solari to the federal government prior to DMG’s acquisition of Solari and claims made to the government post-acquisition for which the sellers had certain responsibilities pursuant to a management services
F-30
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
agreement. In October 2017, the Company finalized and executed a settlement agreement with the OIG including payment of an immaterial amount.
2011 Suit against the U.S. Department of Veterans Affairs: As previously disclosed, the Company had a pending lawsuit in the U.S. Court of Federal Claims against the federal government which was originally filed in May 2011. The lawsuit related to the U.S. Department of Veterans Affairs (VA) underpayment of dialysis services the Company provided from 2005 through 2011 to veterans pursuant to VA regulations. In the first quarter of 2017, the Company received a payment of $538,000 related to the settlement with the VA. The Company's consolidated entities recognized a net gain of $527,000 on this settlement. The Company's nonconsolidated and managed entities recognized a gain of $9,000, of which the Company's equity investment share was $3,000. The net effect was a net increase of $530,000 to the Company's operating income.
2015 U.S. Department of Justice Vascular Access Investigation and Related Qui Tam Litigation: In November 2015, the Company announced that RMS Lifeline, Inc., a wholly-owned subsidiary of the Company that operates under the name Lifeline Vascular Access (Lifeline), received a CID from the DOJ. The CID relates to two vascular access centers in Florida that are part of Lifeline’s vascular access business. The CID covers the period from January 1, 2008 through the present. The Company acquired these two centers in December 2012. Based on the language of the CID, the DOJ appeared to be looking at whether angiograms performed at the two centers were medically unnecessary and therefore whether related claims filed with federal healthcare programs possibly violated the FCA. Lifeline does not perform dialysis services but instead provides vascular access management services for dialysis patients. The Company cooperated with the government and produced the requested information. The DOJ investigation was initiated pursuant to a complaint brought under the qui tam provisions of the FCA (the Complaint). The Complaint was originally filed under seal in August 2014 in the U.S. District Court, Middle District of Florida, United States ex. rel James Spafford v. DaVita HealthCare Partners, Inc., et al., Case Number 6:14-cv-1251-Orl-41DAB, naming several doctors along with the Company as defendants. In December 2015, a First Amended Complaint was filed under seal. In May 2016, the First Amended Complaint was unsealed. The First Amended Complaint alleged violations of the FCA due to the submission of claims to the government for allegedly medically unnecessary angiograms and angiography procedures at the two vascular access centers as well as employment-related claims. The Complaint covers alleged conduct dating from July 2008, prior to the Company’s acquisition of the centers, to the present. The DOJ declined to intervene. In January 2017, the Company finalized and executed a settlement agreement with the relator and the government for an immaterial amount, and in April 2017, the court dismissed the case with prejudice.
Vainer Private Civil Suit: As previously disclosed, the Company received a subpoena for documents from the OIG relating to the pharmaceutical products Zemplar, Hectorol, Venofer, Ferrlecit and erythropoietin (EPO), as well as other related matters, covering the period from January 2003 to December 2008. The Company subsequently learned that the allegations underlying this inquiry were made as part of a civil complaint filed by relators, Daniel Barbir and Dr. Alon Vainer, pursuant to the qui tam provisions of the federal FCA. The relators also alleged that the Company’s drug administration practices for the Company’s dialysis operations for Vitamin D and iron agents from 2003 through 2010 fraudulently created unnecessary waste, which was billed to and paid for by the government. In June 2015, the Company finalized the terms of the settlement with plaintiffs, including a settlement amount of $450,000 and attorney fees and other costs of $45,000 which was paid in 2015.
* * *
Other than as described above, the Company cannot predict the ultimate outcomes of the various legal proceedings and regulatory matters to which the Company is or may be subject from time to time, including those described in this Note 16, or the timing of their resolution or the ultimate losses or impact of developments in those matters, which could have a material adverse effect on the Company’s revenues, earnings and cash flows. Further, any legal proceedings or regulatory matters involving the Company, whether meritorious or not, are time consuming, and often require management’s attention and result in significant legal expense, and may result in the diversion of significant operational resources, or otherwise harm the Company’s business, financial results or reputation.
17. Noncontrolling interests subject to put provisions and other commitments
Noncontrolling interests subject to put provisions
The Company has potential obligations to purchase the equity interests held by third parties in several of its majority-owned joint ventures and other nonconsolidated entities. These obligations are in the form of put provisions that are exercisable at the third-party owners’ discretion within specified periods as outlined in each specific put provision. If these put provisions were exercised, the Company would be required to purchase the third-party owners’ equity interests at either the appraised fair market value or a predetermined multiple of earnings or cash flows attributable to the equity interests put to the Company,
F-31
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
which is intended to approximate fair value. The methodology the Company uses to estimate the fair values of noncontrolling interests subject to put provisions assumes the higher of either a liquidation value of net assets or an average multiple of earnings, based on historical earnings, patient mix and other performance indicators that can affect future results, as well as other factors. The estimated fair values of noncontrolling interests subject to put provisions are a critical accounting estimate that involves significant judgments and assumptions and may not be indicative of the actual values at which the noncontrolling interests may ultimately be settled, which could vary significantly from the Company’s current estimates. The estimated fair values of noncontrolling interests subject to put provisions can fluctuate and the implicit multiple of earnings at which these noncontrolling interests obligations may be settled will vary significantly depending upon market conditions including potential purchasers’ access to the capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the third-party owners’ equity interests. The amount of noncontrolling interests subject to put provisions that employ a contractually predetermined multiple of earnings rather than fair value are immaterial.
The Company has certain other potential commitments to provide operating capital to a number of dialysis centers that are wholly-owned by third parties or businesses in which the Company owns a noncontrolling equity interest as well as to physician-owned vascular access clinics or medical practices that the Company operates under management and administrative service agreements of approximately $5,385.
Certain consolidated joint ventures are originally contractually scheduled to dissolve after terms ranging from 10 to 50 years. While noncontrolling interests in these limited life entities qualify as mandatorily redeemable financial instruments, they are subject to a classification and measurement scope exception from the accounting guidance generally applicable to other mandatorily redeemable financial instruments. Future distributions upon dissolution of these entities would be valued below the related noncontrolling interest carrying balances in the consolidated balance sheet.
Other commitments
In January 2017, the Company entered into a Sourcing and Supply Agreement with Amgen USA Inc. (Amgen) that expires on December 31, 2022, replacing the Company’s prior agreement that was to expire in 2018. Under the terms of the agreement, the Company will purchase EPO in amounts necessary to meet no less than 90% of its requirements for erythropoiesis-stimulating agents (ESAs) through the expiration of the contract from Amgen. The actual amount of EPO that the Company will purchase will depend upon the amount of EPO administered during dialysis as prescribed by physicians and the overall number of patients that the Company serves.
In 2010, the Company entered into an agreement with Fresenius Medical Care (FMC) which committed the Company to purchase a certain amount of dialysis equipment, parts and supplies from FMC through 2013. This agreement has been subsequently extended through December 31, 2020. During 2017, 2016 and 2015, the Company purchased $176,212, $164,766 and $154,566, respectively, of certain equipment, parts and supplies from FMC.
In 2014, the Company entered into an agreement with Baxter Healthcare (Baxter) which committed the Company to purchase a certain amount of its hemodialysis non-equipment product supplies, such as dialyzers, at fixed prices through 2018. During 2017, 2016 and 2015, the Company purchased $166,764, $162,109 and $112,931 of hemodialysis product supplies from Baxter under this agreement.
Other than operating leases disclosed in Note 14 to the consolidated financial statements, the letters of credit disclosed in Note 13 to the consolidated financial statements, and the arrangements as described above, the Company has no off balance sheet financing arrangements as of December 31, 2017.
18. Long-term incentive compensation and shareholders’ equity
Long-term incentive compensation
Long-term incentive program (LTIP) compensation includes both stock-based awards (principally stock-settled stock appreciation rights, restricted stock units and performance stock units) as well as long-term performance-based cash awards. Long-term incentive compensation expense, which was primarily general and administrative in nature, was attributed to the Company’s U.S. dialysis and related lab services business, corporate administrative support, and the ancillary services and strategic initiatives.
In March 2016, the FASB issued ASU No. 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. The changes required by this ASU involve several aspects of the accounting for
F-32
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
share-based payment transactions, including income tax consequences, classification of awards as either equity or liabilities, classification on the statement of cash flows, and an election on estimating forfeitures. The amendments in this ASU were effective for the Company beginning January 1, 2017. See the New accounting standards section in Note 1 for further details.
The Company’s stock-based compensation awards are measured at their estimated fair values on the date of grant if settled in shares or at their estimated fair values at the end of each reporting period if settled in cash. The value of stock-based awards so measured is recognized as compensation expense on a cumulative straight-line basis over the vesting terms of the awards, adjusted for expected forfeitures.
Stock-based compensation to be settled in shares is recorded to the Company’s shareholders’ equity, while stock-based compensation to be settled in cash is recorded to a liability. Shares issued upon exercise of stock awards have generally been issued from authorized but unissued shares.
Long-term incentive compensation plans
The Company’s 2011 Incentive Award Plan (the 2011 Plan) is the Company’s omnibus equity compensation plan and provides for grants of stock-based awards to employees, directors and other individuals providing services to the Company, except that incentive stock options may only be awarded to employees. The 2011 Plan authorizes the Company to award stock options, stock appreciation rights, restricted stock units, restricted stock, and other stock-based or performance-based awards, and is designed to enable the Company to grant equity and cash awards that qualified as performance-based compensation under Section 162(m) of the Internal Revenue Code for tax years 2017 and prior. The 2011 Plan mandates a maximum award term of five years and stipulates that stock appreciation rights and stock options be granted with prices not less than fair market value on the date of grant. The 2011 Plan also requires that full value share awards such as restricted stock units reduce shares available under the 2011 Plan at a ratio of 3.5:1. The Company’s nonqualified stock appreciation rights and stock units awarded under the 2011 Plan generally vest over 36 to 48 months from the date of grant. At December 31, 2017, there were 6,648,199 stock-settled stock appreciation rights, 1,075,572 stock-settled stock units, 23,000 cash-settled stock appreciation rights and 1,600 cash-settled stock units outstanding, and 27,369,515 shares available for future grants, under the 2011 Plan.
F-33
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
A combined summary of the status of the Company’s stock-settled awards under the 2011 Plan, including base shares for stock-settled stock appreciation rights (SSARs) and stock-settled stock unit awards is as follows:
Year ended December 31, 2017 | |||||||||||||||
Stock appreciation rights | Stock units | ||||||||||||||
Awards | Weighted average exercise price | Weighted average remaining contractual life | Awards | Weighted average remaining contractual life | |||||||||||
Outstanding at beginning of year | 7,337,266 | $ | 64.90 | 785,553 | |||||||||||
Granted | 1,692,154 | 65.29 | 528,968 | ||||||||||||
Exercised | (2,022,418 | ) | 54.27 | (119,000 | ) | ||||||||||
Canceled | (358,803 | ) | 70.61 | (119,949 | ) | ||||||||||
Outstanding at end of period | 6,648,199 | $ | 67.92 | 2.3 | 1,075,572 | 2.0 | |||||||||
Exercisable at end of period | 2,628,008 | $ | 62.78 | 0.6 | — | 0.0 | |||||||||
Weighted-average fair value of grants | |||||||||||||||
2017 | $ | 14.51 | $ | 65.73 | |||||||||||
2016 | $ | 13.74 | $ | 70.99 | |||||||||||
2015 | $ | 17.97 | $ | 80.25 | |||||||||||
Awards Outstanding | Weighted average exercise price | Awards exercisable | Weighted average exercise price | ||||||||||
Range of SSARs base prices | |||||||||||||
$50.01–$60.00 | 1,856,145 | 59.05 | 1,712,675 | 59.15 | |||||||||
$60.01–$70.00 | 2,715,542 | 66.70 | 632,849 | 67.47 | |||||||||
$70.01–$80.00 | 1,443,749 | 74.77 | 243,041 | 73.16 | |||||||||
$80.01–$90.00 | 632,763 | 83.59 | 39,443 | 81.51 | |||||||||
Total | 6,648,199 | $ | 67.92 | 2,628,008 | $ | 62.78 | |||||||
The Company granted 15,000 cash-settled stock-based awards during 2017. Liability-classified stock-based awards contributed $114, $376 and $(236) to stock-based compensation expense for the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017 the Company had 24,600 liability-classified stock-based awards outstanding, none of which were vested, and a total stock-based compensation liability balance of $99.
For the years ended December 31, 2017, 2016, and 2015, the aggregate intrinsic value of stock-based awards exercised was $34,895, $73,944 and $117,260, respectively. At December 31, 2017, the aggregate intrinsic value of stock-based awards outstanding was $117,722 and the aggregate intrinsic value of stock awards exercisable was $25,609.
Estimated fair value of stock-based compensation awards
The Company has estimated the grant-date fair value of stock-settled stock appreciation rights awards using the Black-Scholes-Merton valuation model and stock-settled stock unit awards at intrinsic value on the date of grant, except for portions of the Company’s performance stock unit awards for which a Monte Carlo simulation was used to estimate the grant-date fair value. The following assumptions were used in estimating these values and determining the related stock-based compensation expense attributable to the current period:
Expected term of the awards: The expected term of awards granted represents the period of time that they are expected to remain outstanding from the date of grant. The Company determines the expected term of its stock awards based on its historical experience with similar awards, considering the Company’s historical exercise and post-vesting termination patterns, and the terms expected by peer companies in near industries.
F-34
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Expected volatility: Expected volatility represents the volatility anticipated over the expected term of the award. The Company determines the expected volatility for its awards based on the volatility of the price of its common stock over the most recent retrospective period commensurate with the expected term of the award, considering the volatility expectations implied by the market price of its exchange-traded options and the volatilities expected by peer companies in near industries.
Expected dividend yield: The Company has not paid dividends on its common stock and does not currently expect to pay dividends during the term of stock awards granted.
Risk-free interest rate: The Company bases the expected risk-free interest rate on the implied yield currently available on stripped interest coupons of U.S. Treasury issues with a remaining term equivalent to the expected term of the award.
A summary of the weighted average valuation inputs described above used for estimating the grant-date fair value of stock-settled stock appreciation rights awards granted in the periods indicated is as follows:
Year ended December 31, | ||||||||
2017 | 2016 | 2015 | ||||||
Expected term | 4.2 | 4.2 | 4.1 | |||||
Expected volatility | 23.9 | % | 21.0 | % | 24.6 | % | ||
Expected dividend yield | — | % | — | % | — | % | ||
Risk-free interest rate | 1.7 | % | 1.0 | % | 1.5 | % | ||
The Company estimates expected forfeitures based upon historical experience with separate groups of employees that have exhibited similar forfeiture behavior in the past. Stock-based compensation expense is recorded only for awards that are expected to vest.
Employee stock purchase plan
The Employee Stock Purchase Plan entitles qualifying employees to purchase up to $25 of the Company’s common stock during each calendar year. The amounts used to purchase stock are accumulated through payroll withholdings or through optional lump sum payments made in advance of the first day of the purchase right period. This compensatory plan allows employees to purchase stock for the lesser of 100% of its fair market value on the first day of the purchase right period or 85% of its fair market value on the last day of the purchase right period. Purchase right periods begin on January 1 and July 1, and end on December 31. Contributions used to purchase the Company’s common stock under this plan for the 2017, 2016 and 2015 participation periods were $22,131, $23,902 and $24,523, respectively. Shares purchased pursuant to the plan’s 2017, 2016 and 2015 participation periods were 360,368, 438,002 and 413,859, respectively. At December 31, 2017, there were 7,124,027 shares remaining available for future grants under this plan, after an additional 7,500,000 shares were approved to the plan by stockholders on June 20, 2016.
The fair value of participants’ purchase rights was estimated as of the beginning dates of the purchase right periods using the Black-Scholes-Merton valuation model with the following weighted average assumptions for purchase right periods in 2017, 2016 and 2015, respectively: expected volatility of 23%, 22% and 26%; risk-free interest rate of 1.3%, 0.8% and 0.2%, and no dividends. Using these assumptions, the weighted average estimated fair value of these purchase rights was $15.19, $16.73 and $18.76 for 2017, 2016 and 2015, respectively.
Long-term incentive compensation expense and proceeds
For the years ended December 31, 2017, 2016 and 2015, the Company recognized $61,978, $64,956 and $123,957, respectively, in total long-term incentive program (LTIP) expense, of which $34,431, $34,530 and $52,665, respectively, was stock-based compensation expense for stock appreciation rights, stock units and discounted employee stock plan purchases, which are primarily included in general and administrative expenses. The estimated tax benefits recorded for stock-based compensation in 2017, 2016 and 2015 were $7,717, $12,731 and $19,689, respectively. As of December 31, 2017, there was $98,015 total estimated unrecognized compensation expense for outstanding LTIP awards, including $61,166 related to stock-based compensation arrangements under the Company’s equity compensation and stock purchase plans. The Company expects to recognize the performance-based cash component of this LTIP expense over a weighted average remaining period of 1.1 years and the stock-based component of this LTIP expense over a weighted average remaining period of 1.4 years.
For the years ended December 31, 2017, 2016 and 2015, the Company received $13,473, $28,397 and $45,749, respectively, in actual tax benefits upon the exercise of stock awards. Since the Company issues stock-settled stock appreciation
F-35
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
rights rather than stock options, there have been no cash proceeds from stock option exercises during the years ended December 31, 2017, 2016 and 2015.
Stock repurchases
During the years ended December 31, 2017 and 2016, the Company repurchased a total of 12,966,672 shares and 16,649,090 shares of its common stock for $810,949 and $1,072,377, or an average price of $62.54 and $64.41 per share, respectively, pursuant to previously announced authorizations by the Board of Directors. The Company also repurchased 1,237,800 shares of its common stock for $92,790, or an average price of $74.96 per share, subsequent to December 31, 2017 through February 22, 2018.
On October 10, 2017, the Company's Board of Directors approved an additional share repurchase authorization in the amount of $1,252,961. This share repurchase authorization was in addition to the $247,039 remaining at that time under the Company’s Board of Directors’ prior share repurchase authorization announced in July 2016. Accordingly, as of February 22, 2018, the Company has a total of $1,026,326 available under the current Board repurchase authorizations for additional share repurchases. Although these share repurchase authorizations do not have expiration dates, the Company remains subject to share repurchase limitations under the terms of its senior secured credit facilities and the indentures governing its senior notes.
The Company retired all shares held in its treasury effective as of December 31, 2017 and 2016.
Charter documents & Delaware law
The Company’s charter documents include provisions that may deter hostile takeovers, delay or prevent changes of control or changes in management, or limit the ability of stockholders to approve transactions that they may otherwise determine to be in their best interests. These include provisions prohibiting stockholders from acting by written consent, requiring 90 days advance notice of stockholder proposals or nominations to the Board of Directors and granting the Board of Directors the authority to issue up to five million shares of preferred stock and to determine the rights and preferences of the preferred stock without the need for further stockholder approval.
The Company is also subject to Section 203 of the Delaware General Corporation Law which, subject to exceptions, would prohibit the Company from engaging in any business combinations with any interested stockholder, as defined in that section, for a period of three years following the date on which that stockholder became an interested stockholder. These restrictions may discourage, delay or prevent a change in the control of the Company.
Changes in DaVita Inc.’s ownership interest in consolidated subsidiaries
The effects of changes in DaVita Inc.’s ownership interest in consolidated subsidiaries on the Company’s equity are as follows:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Net income attributable to DaVita Inc. | $ | 663,618 | $ | 879,874 | $ | 269,732 | |||||
Changes in paid-in-capital for: | |||||||||||
Sales of noncontrolling interest | (114 | ) | — | — | |||||||
Purchase of noncontrolling interests | (2,752 | ) | (13,105 | ) | (55,826 | ) | |||||
Net transfer in noncontrolling interests | (2,866 | ) | (13,105 | ) | (55,826 | ) | |||||
Net income attributable to DaVita Inc. net of transfers in noncontrolling interests | $ | 660,752 | $ | 866,769 | $ | 213,906 | |||||
The Company acquired additional ownership interests in several existing majority-owned joint ventures for $5,357, $21,512, and $66,382 in 2017, 2016, and 2015, respectively.
F-36
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
19. Other comprehensive (loss) income
Charges and credits to other comprehensive (loss) income have been as follows:
Interest rate cap and swap agreements | Investment securities | Foreign currency translation adjustments | Accumulated other comprehensive (loss) income | ||||||||||||
Balance at January 1, 2015 | $ | (1,795 | ) | $ | 3,151 | $ | (26,373 | ) | $ | (25,017 | ) | ||||
Unrealized losses | (20,085 | ) | (1,974 | ) | (23,889 | ) | (45,948 | ) | |||||||
Related income tax | 7,844 | 561 | — | 8,405 | |||||||||||
(12,241 | ) | (1,413 | ) | (23,889 | ) | (37,543 | ) | ||||||||
Reclassification from accumulated other comprehensive losses (income) into net income | 5,103 | (618 | ) | — | 4,485 | ||||||||||
Related income tax | (1,992 | ) | 241 | — | (1,751 | ) | |||||||||
3,111 | (377 | ) | — | 2,734 | |||||||||||
Balance at December 31, 2015 | $ | (10,925 | ) | $ | 1,361 | $ | (50,262 | ) | $ | (59,826 | ) | ||||
Unrealized (losses) gains | (6,013 | ) | 1,802 | (39,614 | ) | (43,825 | ) | ||||||||
Related income tax | 2,343 | (565 | ) | — | 1,778 | ||||||||||
(3,670 | ) | 1,237 | (39,614 | ) | (42,047 | ) | |||||||||
Reclassification from accumulated other comprehensive losses (income) into net income | 4,198 | (690 | ) | 10,087 | 13,595 | ||||||||||
Related income tax | (1,632 | ) | 267 | — | (1,365 | ) | |||||||||
2,566 | (423 | ) | 10,087 | 12,230 | |||||||||||
Balance at December 31, 2016 | $ | (12,029 | ) | $ | 2,175 | $ | (79,789 | ) | $ | (89,643 | ) | ||||
Unrealized (losses) gains | (8,897 | ) | 5,075 | 99,770 | 95,948 | ||||||||||
Related income tax | 3,460 | (1,368 | ) | — | 2,092 | ||||||||||
(5,437 | ) | 3,707 | 99,770 | 98,040 | |||||||||||
Reclassification from accumulated other comprehensive losses (income) into net income | 8,278 | (360 | ) | — | 7,918 | ||||||||||
Related income tax | (3,220 | ) | 140 | — | (3,080 | ) | |||||||||
5,058 | (220 | ) | — | 4,838 | |||||||||||
Balance at December 31, 2017 | $ | (12,408 | ) | $ | 5,662 | $ | 19,981 | $ | 13,235 | ||||||
The reclassification of net cap and swap realized losses into income are recorded as debt expense in the corresponding consolidated statements of income. See Note 13 to these consolidated financial statements for further details.
The reclassification of net investment realized gains into income are recorded in other income in the corresponding consolidated statements of income. See Note 3 to these consolidated financial statements for further details.
20. Acquisitions and divestitures
Acquisition of Renal Ventures
On May 1, 2017, the Company completed its acquisition of 100% of the equity of Colorado-based Renal Ventures Management, LLC (Renal Ventures) for approximately $359,913 in net cash. Renal Ventures operated 36 dialysis centers, one uncertified dialysis center and one home program, that provided services to approximately 2,600 patients in six states. As a part of this transaction, the Company was required to divest three Renal Ventures outpatient dialysis centers, and three outpatient dialysis centers and one uncertified dialysis center of the Company for approximately $21,219 in net cash. The Company also incurred approximately $11,950 in transaction and integration costs during the year ended December 31, 2017 associated with this acquisition that are included in general and administrative expenses.
The initial purchase price allocation for the Renal Ventures acquisition is recorded at estimated fair values based upon the best information available to management and will be finalized when certain information arranged to be obtained has been
F-37
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
received. In particular, certain working capital items, income tax amounts and the fair value of intangibles and fixed assets are pending final audit, issuance of final tax returns and valuation reports.
The following table summarizes the assets acquired and liabilities assumed in the transactions and recognized at the acquisition date at estimated fair values:
Current assets, net of cash acquired | $ | 22,739 | |
Property and equipment | 36,295 | ||
Amortizable intangible and other long-term assets | 11,547 | ||
Goodwill | 298,200 | ||
Current liabilities | (8,389 | ) | |
Long-term liabilities | (479 | ) | |
$ | 359,913 | ||
Amortizable intangible assets acquired, primarily related to non-compete agreements, had weighted-average estimated useful lives of five years. The total estimated amount of goodwill deductible for tax purposes associated with this acquisition was approximately $298,200.
Other routine acquisitions
During 2017, the Company also acquired 30 dialysis centers in the U.S. and 68 dialysis centers outside the U.S. for a total of $308,550 in net cash, earn-outs of $2,692, and deferred purchase price and liabilities assumed of $23,748. During 2016, the Company acquired eight dialysis centers in the U.S. and 21 dialysis centers outside the U.S. for a total of $165,108 in net cash, earn-outs of $1,511, and deferred purchase price of $17,963. During 2015, the Company acquired six dialysis centers in the U.S. and 21 dialysis centers outside the U.S. for a total of $54,551 in net cash and deferred purchase price of $7,452. The assets and liabilities for all acquisitions were recorded at their estimated fair values at the dates of the acquisitions and are included in the Company’s financial statements and operating results from the effective dates of the acquisitions. For several of the 2017 acquisitions, certain income tax amounts are pending final evaluation and quantification of any pre-acquisition tax contingencies. In addition, valuation of intangibles and certain other working capital items relating to several of these acquisitions are pending final quantification.
The following table summarizes the assets acquired and liabilities assumed in the above described transactions and recognized at their acquisition dates at estimated fair values, as well as the estimated fair value of noncontrolling interests assumed in these transactions:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Current assets | $ | 14,366 | $ | 3,996 | $ | 2,647 | |||||
Property and equipment | 18,192 | 8,840 | 4,466 | ||||||||
Amortizable intangible and other long-term assets | 11,663 | 5,876 | 8,924 | ||||||||
Non-amortizable intangibles | 32,296 | — | — | ||||||||
Goodwill | 318,832 | 198,927 | 67,183 | ||||||||
Deferred income taxes | (210 | ) | 597 | (717 | ) | ||||||
Noncontrolling interests assumed | (44,303 | ) | (30,337 | ) | (18,905 | ) | |||||
Liabilities assumed | (15,846 | ) | (3,317 | ) | (1,595 | ) | |||||
Aggregate purchase cost | $ | 334,990 | $ | 184,582 | $ | 62,003 | |||||
Amortizable intangible assets acquired, primarily related to non-compete agreements, during 2017, 2016 and 2015 had weighted-average estimated useful lives of seven, seven and eleven years, respectively. The total amount of goodwill deductible for tax purposes associated with these acquisitions for 2017, 2016, and 2015 was approximately $237,363, $169,379 and $43,823, respectively.
F-38
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Change in ownership interests in Asia Pacific joint venture
On August 1, 2016, the Company consummated an agreement with Khazanah Nasional Berhad (Khazanah) and Mitsui and Co., Ltd (Mitsui) whereby Khazanah and Mitsui subscribed to invest a total of $300,000 over three years in exchange for a 40% total equity interest in the Company’s APAC JV. Khazanah and Mitsui made initial investments of $50,000 each on August 1, 2016 as well as additional subscribed contributions of $50,000 each on August 1, 2017. Subsequent to those contributions, the Company now holds a 60% voting interest and a 73.3% current economic interest in the APAC JV.
Based on the governance structure and voting rights put in place upon the formation of the APAC JV, certain key decisions affecting the JV’s operations are no longer at the unilateral discretion of the Company, but rather are shared with the noncontrolling investors. As a result, the Company deconsolidated its Asia Pacific dialysis business in the third quarter of 2016 and recognized an initial non-cash non-taxable estimated gain of $374,374 on its retained investment, net of contingent obligations. This retained interest was adjusted to the Company’s proportionate share of the estimated fair value of the business, as implied by the Khazanah and Mitsui investment and adjusted for certain time value of money and uncertainty discounts. The Company then recognized an additional $6,293 gain in the first quarter of 2017 upon resolution of certain post-closing adjustments related to this transaction.
The Company’s non-cash gain on its retained investment in the APAC JV in the third quarter of 2016 was computed with the assistance of an independent third party valuation firm and was based upon the best information available to management at that time. Subsequent to its deconsolidation on August 1 2016, the Company’s retained interest in the APAC JV has been accounted for under the equity method. See Note 9 for further details on the accounting for this retained investment and a subsequent other-than-temporary impairment thereof recognized in 2017.
Pro forma financial information (unaudited)
The following summary, prepared on a pro forma basis, combines the results of operations as if all acquisitions within continuing operations in 2017 and 2016 had been consummated as of the beginning of 2016, including the impact of certain adjustments such as amortization of intangibles, interest expense on acquisition financing and income tax effects.
Year ended December 31, | |||||||
2017 | 2016 | ||||||
(unaudited) | |||||||
Pro forma net revenues | $ | 11,005,330 | $ | 11,076,750 | |||
Pro forma net income from continuing operations | 907,443 | 1,052,700 | |||||
Pro forma basic net income per share from continuing operations attributable to DaVita Inc. | 4.81 | 5.22 | |||||
Pro forma diluted net income per share from continuing operations attributable to DaVita Inc. | 4.74 | 5.14 | |||||
Contingent earn-out obligations
The Company has several contingent earn-out obligations associated with acquisitions that could result in the Company paying the former shareholders of acquired companies a total of up to approximately $11,466 if certain EBITDA, operating income performance targets or quality margins are met over the next two to six years.
Contingent earn-out obligations are remeasured to fair value at each reporting date until the contingencies are resolved with changes in the liability due to the remeasurement recognized in earnings. See Note 24 to these consolidated financial statements for further details. As of December 31, 2017, the Company estimated the fair value of these contingent earn-out obligations to be $6,388, of which a total of $216 is included in other liabilities, and the remaining $6,172 is included in other long-term liabilities in the Company’s consolidated balance sheet.
F-39
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The following is a reconciliation of changes in the contingent earn-out obligations for the year ended December 31, 2017:
Beginning balance January 1, 2017 | $ | 2,950 | |
Contingent earn-out obligations associated with acquisitions | 2,692 | ||
Remeasurement of fair value | 746 | ||
$ | 6,388 | ||
21. Held for sale and discontinued operations
DaVita Medical Group (DMG)
On December 5, 2017, we entered into an equity purchase agreement to sell our DMG division to Optum, a subsidiary of UnitedHealth Group Inc., for $4,900,000 in cash, subject to net working capital and other customary adjustments. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions. As a result of this pending transaction, the DMG business has been reclassified as held for sale and its results of operations are reported as discontinued operations for all periods presented.
The following table presents the financial results of discontinued operations related to DMG:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Net revenues | $ | 4,676,213 | $ | 4,113,414 | $ | 3,837,260 | |||||
Expenses | 4,634,782 | 3,994,624 | 3,596,342 | ||||||||
Goodwill and other asset impairment charges | 651,659 | 253,000 | 206,169 | ||||||||
(Loss) income from discontinued operations before taxes | (610,228 | ) | (134,210 | ) | 34,749 | ||||||
Income tax benefit (expense) | 364,856 | (24,052 | ) | (88,216 | ) | ||||||
Net loss from discontinued operations, net of tax | $ | (245,372 | ) | $ | (158,262 | ) | $ | (53,467 | ) | ||
As previously disclosed, the Company’s DMG business has continued to experience declining operating results in recent years, and prior to being reclassified as held for sale the Company recorded goodwill and other asset impairment charges for the DMG business of $651,659, $253,000 and $206,169 in 2017, 2016 and 2015, respectively. These charges resulted from continuing developments in the Company’s DMG business, including recent annual updates to Medicare Advantage benchmark reimbursement rates, changes in expectations concerning future government reimbursement rates and the Company’s expected ability to mitigate them, medical cost and utilization trends, commercial pricing pressures, underperformance of certain DMG business units and other market factors.
F-40
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The following table presents the financial position of discontinued operations related to DMG:
December 31, 2017 | December 31, 2016 | ||||||
Assets | |||||||
Cash and cash equivalents | $ | 179,668 | $ | 238,411 | |||
Other current assets | 888,697 | 722,545 | |||||
Property and equipment, net | 379,945 | 311,246 | |||||
Intangible assets, net | 1,316,550 | 1,454,263 | |||||
Other long-term assets | 116,805 | 94,684 | |||||
Goodwill | 2,879,977 | 3,391,942 | |||||
Total assets held for sale | $ | 5,761,642 | $ | 6,213,091 | |||
Total current assets held for sale | $ | 5,761,642 | $ | 960,956 | |||
Total long-term assets held for sale | $ | — | $ | 5,252,135 | |||
Liabilities | |||||||
Other liabilities | $ | 505,734 | $ | 460,458 | |||
Medical payables | 457,040 | 349,506 | |||||
Current portion of long-term debt | 2,845 | 4,779 | |||||
Long-term debt | 35,003 | 2,652 | |||||
Other long-term liabilities | 184,448 | 418,723 | |||||
Total liabilities held for sale | $ | 1,185,070 | $ | 1,236,118 | |||
Total current liabilities held for sale | $ | 1,185,070 | $ | 807,233 | |||
Total long-term liabilities held for sale | $ | — | $ | 428,885 | |||
The following table presents cash flows of discontinued operations related to DMG:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Net cash provided by operating activities from discontinued operations | $ | 351,557 | $ | 287,049 | $ | 365,138 | |||||
Net cash used in investing activities from discontinued operations | $ | (232,329 | ) | $ | (430,917 | ) | $ | (121,893 | ) | ||
DMG acquisitions
During 2017, the Company's DMG business acquired other medical businesses for a total of $135,416 in net cash, deferred purchase price of $1,038, and liabilities assumed of $10,145. During 2016, the Company acquired other medical businesses for a total of $398,748 in net cash and deferred purchase price and liabilities assumed of $7,694. During 2015, the Company acquired other medical businesses for a total of $41,918 in net cash and deferred purchase price of $944. For several of the 2017 acquisitions, certain income tax amounts are pending final evaluation and quantification of any pre-acquisition tax contingencies. In addition, valuation of medical claims liabilities and certain other working capital items relating to several of these acquisitions are pending final quantification. The assets and liabilities for all acquisitions were recorded at their estimated fair values at the dates of the acquisitions and are included in the Company’s current held for sale assets and liabilities.
22. Variable interest entities
The Company relies on the operating activities of certain entities that it does not directly own or control, but over which it has indirect influence and of which it is considered the primary beneficiary. These entities are subject to the consolidation guidance applicable to variable interest entities (VIEs).
Under U.S. GAAP, VIEs typically include entities for which (i) the entity’s equity is not sufficient to finance its activities without additional subordinated financial support; (ii) the equity holders as a group lack the power to direct the activities that most significantly influence the entity’s economic performance, the obligation to absorb the entity’s expected losses, or the right to receive the entity’s expected returns; or (iii) the voting rights of some investors are not proportional to their obligations to absorb the entity’s losses.
F-41
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The Company has determined that substantially all of the entities it is associated with that qualify as VIEs must be included in its consolidated financial statements. A number of these VIEs are within the Company's DMG business, which has been reclassified as held for sale and as a discontinued operation in these financial statements. The Company manages these entities and provides operating and capital funding as necessary for the entities to accomplish their operational and strategic objectives. A number of these entities are subject to nominee share ownership or share transfer restriction agreements that effectively transfer the majority of the economic risks and rewards of their ownership to the Company. In other cases the Company’s management agreements with these entities include both financial terms and protective and participating rights to the entities’ operating, strategic and non-clinical governance decisions which transfer substantial powers over and economic responsibility for the entities to the Company. In some cases such entities are subject to broad exclusivity or noncompetition restrictions that benefit the Company. Further, in some cases the Company has contractual arrangements with its related party nominee owners that effectively indemnify these parties from the economic losses from, or entitle the Company to the economic benefits of, these entities.
The analyses upon which these consolidation determinations rest are complex, involve uncertainties, and require significant judgment on various matters, some of which could be subject to different interpretations. At December 31, 2017, these consolidated financial statements include total assets of VIEs of $870,314 and total liabilities and noncontrolling interests of VIEs to third parties of $475,143, including assets of $595,670 and liabilities and noncontrolling interests of $319,777 related to the Company's DMG business which is classified as held for sale.
The Company also sponsors certain deferred compensation plans whose trusts qualify as VIEs and the Company consolidates each of these plans as their primary beneficiary. The assets of these plans are recorded in short-term or long-term investments with related liabilities recorded in accrued compensation and benefits and other long-term liabilities. See Note 15 to these consolidated financial statements for disclosures on the assets of these consolidated non-qualified deferred compensation plans.
23. Concentrations
Approximately 67%, 64% and 66% of total U.S. dialysis services revenues in 2017, 2016 and 2015, respectively, are from government-based programs, principally Medicare and Medicaid. Related net accounts receivable and other receivables from Medicare, including Medicare-assigned plans, and Medicaid, including Managed Medicaid plans, were approximately $869,083 and $831,445, as of December 31, 2017 and 2016, respectively.
There is no single commercial payor that accounted for more than 10% of total consolidated accounts receivable or consolidated net revenues at December 31, 2017 and 2016.
24. Fair values of financial instruments
The Company measures the fair value of certain assets, liabilities and noncontrolling interests subject to put provisions (temporary equity) based upon certain valuation techniques that include observable or unobservable inputs and assumptions that market participants would use in pricing these assets, liabilities, temporary equity and commitments. The Company has also classified certain assets, liabilities and temporary equity that are measured at fair value into the appropriate fair value hierarchy levels as defined by the FASB.
F-42
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The following tables summarize the Company’s assets, liabilities and temporary equity measured at fair value on a recurring basis as of December 31, 2017 and 2016:
December 31, 2017 | Total | Quoted prices in active markets for identical assets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||
Assets | |||||||||||||||
Available for sale securities | $ | 38,895 | $ | 38,895 | $ | — | $ | — | |||||||
Interest rate cap agreements | $ | 1,032 | $ | — | $ | 1,032 | $ | — | |||||||
Liabilities | |||||||||||||||
Contingent earn-out obligations | $ | 6,388 | $ | — | $ | — | $ | 6,388 | |||||||
Temporary equity | |||||||||||||||
Noncontrolling interests subject to put provisions | $ | 1,011,360 | $ | — | $ | — | $ | 1,011,360 | |||||||
December 31, 2016 | |||||||||||||||
Assets | |||||||||||||||
Available for sale securities | $ | 31,197 | $ | 31,197 | $ | — | $ | — | |||||||
Interest rate cap agreements | $ | 9,929 | $ | — | $ | 9,929 | $ | — | |||||||
Liabilities | |||||||||||||||
Contingent earn-out obligations | $ | 2,950 | $ | — | $ | — | $ | 2,950 | |||||||
Temporary equity | |||||||||||||||
Noncontrolling interests subject to put provisions | $ | 973,258 | $ | — | $ | — | $ | 973,258 | |||||||
Available for sale securities represent investments in various open-ended registered investment companies, or mutual funds, and are recorded at fair value estimated based upon redemption prices reported by each mutual fund. See Note 3 to these consolidated financial statements for further discussion.
The interest rate cap agreements are recorded at fair value estimated from valuation models utilizing the income approach and commonly accepted valuation techniques that use inputs from closing prices for similar assets and liabilities in active markets as well as other relevant observable market inputs at quoted intervals such as current interest rates, forward yield curves, implied volatility and credit default swap pricing. The Company does not believe the ultimate amount that could be realized upon settlement of these interest rate cap agreements would be materially different from the fair value estimates currently reported. See Note 13 to these consolidated financial statements for further discussion.
The estimated fair value measurements of contingent earn-out obligations are primarily based on unobservable inputs, including projected EBITDA. The estimated fair value of these contingent earn-out obligations is remeasured as of each reporting date and could fluctuate based upon any significant changes in key assumptions, such as changes in the Company credit risk adjusted rate that is used to discount obligations to present value.
See Note 17 to these consolidated financial statements for a discussion of the Company’s methodology for estimating the fair values of noncontrolling interests subject to put obligations.
Other financial instruments consist primarily of cash, accounts receivable, accounts payable, other accrued liabilities and debt. The balances of non-debt financial instruments are presented in the consolidated financial statements at December 31, 2017 and 2016 at their approximate fair values due to the short-term nature of their settlements. The carrying amount of the Company’s senior secured credit facilities totaled $4,428,376 as of December 31, 2017, and their fair value was approximately $4,495,649 based upon quoted market prices. The carrying amount of the Company’s Senior Notes was approximately $4,460,176 at December 31, 2017 and their fair value was approximately $4,566,175 at December 31, 2017 based upon quoted market prices.
25. Segment reporting
The Company has consisted of two major divisions, DaVita Kidney Care (Kidney Care) and DaVita Medical Group (DMG). The Kidney Care division is comprised of the Company’s U.S. dialysis and related lab services business, various ancillary services and strategic initiatives, including its international operations, and the Company’s corporate administrative
F-43
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
support. The Company’s U.S. dialysis and related lab services business is its largest line of business and is a leading provider of kidney dialysis services in the U.S. for patients suffering from chronic kidney failure, also known as ESRD. The Company’s ancillary services and strategic initiatives consist primarily of pharmacy services, disease management services, vascular access services, clinical research programs, physician services, direct primary care, ESRD seamless care organizations and comprehensive care as well as the Company’s international operations.
The Company’s DMG division is a patient- and physician-focused integrated healthcare delivery and management company with over two decades of providing coordinated outcomes-based medical care in a cost-effective manner. On December 5, 2017, the Company entered into an equity purchase agreement to sell its DMG division to Optum, a subsidiary of UnitedHealth Group Inc. The transaction is expected to close in 2018 and is subject to regulatory approval and other customary closing conditions. As a result of this pending transaction, the DMG business has been reclassified as held for sale and its results of operations are reported as discontinued operations for all periods presented in these consolidated financial statements.
The Company’s operating segments have been defined based on the separate financial information that is regularly produced and reviewed by the Company’s chief operating decision maker in making decisions about allocating resources to and assessing the financial performance of the Company’s various operating lines of business. The chief operating decision maker for the Company is its Chief Executive Officer.
The Company’s separate operating segments include its U.S. dialysis and related lab services business, each of its ancillary services and strategic initiatives, its consolidated international kidney care operations in each country and under the Saudi Ministry of Health charter, its equity method investment in the Asia Pacific joint venture, and its other health operations in Europe. The U.S. dialysis and related lab services business qualifies as a separately reportable segment, and all other ancillary services and strategic initiatives operating segments, including the international operating segments, have been combined and disclosed in the other segments category.
The Company’s operating segment financial information included in this report is prepared on the internal management reporting basis that the chief operating decision maker uses to allocate resources and assess the financial performance of the Company's operating segments. For internal management reporting, segment operations include direct segment operating expenses but generally exclude corporate administrative support costs, which consist primarily of indirect labor, benefits and long-term incentive-based compensation expenses of certain departments which provide support to all of the Company’s various operating lines of business, except to the extent that such costs are charged to and borne by certain ancillary services and strategic initiatives via internal management fees. These corporate administrative support costs are reduced by internal management fees received from the Company’s ancillary lines of business.
F-44
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
The following is a summary of segment revenues, segment operating margin (loss), and a reconciliation of segment operating margin to consolidated income from continuing operations before income taxes:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Segment revenues: | |||||||||||
U.S. dialysis and related lab services | |||||||||||
Patient service revenues: | |||||||||||
External sources | $ | 9,767,123 | $ | 9,524,067 | $ | 9,014,577 | |||||
Intersegment revenues | 55,175 | 27,355 | 19,414 | ||||||||
Total U.S. dialysis and related lab services revenues | 9,822,298 | 9,551,422 | 9,033,991 | ||||||||
Less: Provision for uncollectible accounts | (482,007 | ) | (429,882 | ) | (406,530 | ) | |||||
Net U.S. dialysis and related lab services patient service revenues | 9,340,291 | 9,121,540 | 8,627,461 | ||||||||
Other revenues(1) | 19,774 | 16,649 | 13,971 | ||||||||
Total net U.S. dialysis and related lab services revenues | 9,360,065 | 9,138,189 | 8,641,432 | ||||||||
Other - Ancillary services and strategic initiatives | |||||||||||
Net patient service revenues | 323,156 | 201,867 | 134,496 | ||||||||
Other external sources | 1,248,588 | 1,394,766 | 1,225,731 | ||||||||
Intersegment revenues | 24,603 | 24,739 | 22,204 | ||||||||
Total ancillary services and strategic initiatives revenues | 1,596,347 | 1,621,372 | 1,382,431 | ||||||||
Total net segment revenues | 10,956,412 | 10,759,561 | 10,023,863 | ||||||||
Elimination of intersegment revenues | (79,778 | ) | (52,094 | ) | (41,618 | ) | |||||
Consolidated net revenues | $ | 10,876,634 | $ | 10,707,467 | $ | 9,982,245 | |||||
Segment operating margin (loss): | |||||||||||
U.S. dialysis and related lab services | $ | 2,297,198 | $ | 1,777,014 | $ | 1,259,632 | |||||
Other—Ancillary services and strategic initiatives | (439,477 | ) | 266,324 | (103,901 | ) | ||||||
Total segment margin | 1,857,721 | 2,043,338 | 1,155,731 | ||||||||
Reconciliation of segment operating margin to consolidated income from continuing operations before income taxes: | |||||||||||
Corporate administrative support | (44,966 | ) | (13,628 | ) | (18,965 | ) | |||||
Consolidated operating income | 1,812,755 | 2,029,710 | 1,136,766 | ||||||||
Debt expense | (430,634 | ) | (414,116 | ) | (408,380 | ) | |||||
Debt redemption charges | — | — | (48,072 | ) | |||||||
Other income | 17,665 | 7,511 | 8,073 | ||||||||
Consolidated income from continuing operations before income taxes | $ | 1,399,786 | $ | 1,623,105 | $ | 688,387 | |||||
(1) | Includes management fee revenues from providing management and administrative services to dialysis ventures in which the Company owns a noncontrolling interest or which are wholly-owned by third parties. |
Depreciation and amortization expense by segment is as follows:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
U.S. dialysis and related lab services | $ | 520,965 | $ | 482,768 | $ | 438,238 | |||||
Other - Ancillary services and strategic initiatives | 38,946 | 26,729 | 25,667 | ||||||||
$ | 559,911 | $ | 509,497 | $ | 463,905 | ||||||
F-45
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Subsequent to the issuance of the Company’s fiscal year 2016 consolidated financial statements and their inclusion in its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 24, 2017 (the 2016 10-K), the Company determined that it had misstated its disclosure of segment assets at December 31, 2016 in Note 25 to those consolidated financial statements. This misstatement resulted in an overstatement of "U.S. dialysis and related lab services" segment assets of $338,963 and a corresponding understatement of "Other - ancillary services and strategic initiatives" segment assets of the same amount. The Company performed an assessment of the materiality of this misstatement and concluded that this misstatement as originally disclosed was not materially misleading in its 2016 consolidated financial statements taken as a whole. The Company therefore has not amended its financial statements filed on its 2016 10-K to correct this misstatement, but has provided the corrected disclosure here.
Summary of assets by segment is as follows:
Year ended December 31, | |||||||
2017 | 2016 | ||||||
Segment assets | |||||||
U.S. dialysis and related lab services (including equity investments of $84,866 and $66,924, respectively) | $ | 11,776,042 | $ | 11,108,386 | |||
Other - Ancillary services and strategic initiatives(1) (including equity investments of $160,668 and $425,115, respectively) | 1,410,509 | 1,434,299 | |||||
DMG - Held for sale (including equity investments of $10,321 and $10,350, respectively) | 5,761,642 | 6,213,091 | |||||
Consolidated assets | $ | 18,948,193 | $ | 18,755,776 | |||
(1) | Includes approximately $125,932 and $96,396 in 2017 and 2016, respectively, of net property and equipment related to the Company’s international operations. |
Expenditures for property and equipment by segment is as follows:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
U.S. dialysis and related lab services | $ | 759,218 | $ | 675,994 | $ | 584,513 | |||||
Other - Ancillary services and strategic initiatives | 50,891 | 68,702 | 56,685 | ||||||||
DMG - Held for sale | 95,141 | 84,399 | 66,800 | ||||||||
$ | 905,250 | $ | 829,095 | $ | 707,998 | ||||||
26. Supplemental cash flow information
The table below provides supplemental cash flow information:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash paid: | |||||||||||
Income taxes | $ | 387,159 | $ | 339,411 | $ | 156,075 | |||||
Interest | 424,547 | 406,987 | 405,120 | ||||||||
Non-cash investing and financing activities: | |||||||||||
Fixed assets under capital lease obligations | 48,378 | 28,127 | 74,035 | ||||||||
F-46
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
27. Selected quarterly financial data (unaudited)
2017 | 2016 | ||||||||||||||||||||||||||||||
December 31 | September 30 | June 30 | March 31 | December 31 | September 30 | June 30 | March 31 | ||||||||||||||||||||||||
Net revenues | $ | 2,780,913 | $ | 2,765,071 | $ | 2,699,399 | $ | 2,631,251 | $ | 2,699,419 | $ | 2,725,407 | $ | 2,675,474 | $ | 2,607,167 | |||||||||||||||
Operating income | $ | 150,337 | $ | 395,294 | $ | 391,196 | $ | 875,928 | $ | 363,445 | $ | 813,103 | $ | 431,129 | $ | 422,033 | |||||||||||||||
Net income from continuing operations, before taxes | $ | 46,825 | $ | 289,384 | $ | 288,060 | $ | 775,517 | $ | 259,669 | $ | 710,246 | $ | 331,231 | $ | 321,959 | |||||||||||||||
Net income (loss) from discontinued operations, net of income taxes | $ | 143,587 | $ | (370,872 | ) | $ | (24,520 | ) | $ | 6,433 | $ | 11,772 | $ | 20,213 | $ | (118,443 | ) | $ | (71,804 | ) | |||||||||||
Net income (loss) attributable to DaVita Inc. | $ | 303,396 | $ | (214,476 | ) | $ | 127,001 | $ | 447,697 | $ | 157,726 | $ | 571,332 | $ | 53,382 | $ | 97,434 | ||||||||||||||
Basic net income from continuing operations per share attributable to DaVita Inc. | $ | 0.86 | $ | 0.81 | $ | 0.79 | $ | 2.29 | $ | 0.74 | $ | 2.69 | $ | 0.84 | $ | 0.83 | |||||||||||||||
Basic net income (loss) from discontinued operations per share attributable to DaVita Inc. | $ | 0.80 | $ | (1.95 | ) | $ | (0.13 | ) | $ | 0.04 | $ | 0.07 | $ | 0.11 | $ | (0.58 | ) | $ | (0.35 | ) | |||||||||||
Basic net income (loss) per share attributable to DaVita Inc. | $ | 1.66 | $ | (1.14 | ) | $ | 0.66 | $ | 2.33 | $ | 0.81 | $ | 2.80 | $ | 0.26 | $ | 0.48 | ||||||||||||||
Diluted net income from continuing operations per share attributable to DaVita Inc. | $ | 0.85 | $ | 0.80 | $ | 0.78 | $ | 2.26 | $ | 0.73 | $ | 2.65 | $ | 0.82 | $ | 0.81 | |||||||||||||||
Diluted net income (loss) from discontinued operations per share attributable to DaVita Inc. | $ | 0.79 | $ | (1.92 | ) | $ | (0.13 | ) | $ | 0.03 | $ | 0.07 | $ | 0.11 | $ | (0.56 | ) | $ | (0.34 | ) | |||||||||||
Diluted net income (loss) per share attributable to DaVita Inc. | $ | 1.64 | $ | (1.12 | ) | $ | 0.65 | $ | 2.29 | $ | 0.80 | $ | 2.76 | $ | 0.26 | $ | 0.47 | ||||||||||||||
28. Consolidating financial statements
The following information is presented in accordance with Rule 3-10 of Regulation S-X. The operating and investing activities of the separate legal entities included in the Company’s consolidated financial statements are fully interdependent and integrated. Revenues and operating expenses of the separate legal entities include intercompany charges for management and other services. The Company’s Senior Notes are guaranteed by substantially all of its domestic subsidiaries. Each of the guarantor subsidiaries has guaranteed the Senior Notes on a joint and several basis. However, the guarantor subsidiaries can be released from their obligations in the event of a sale or other disposition of all or substantially all of the assets of such subsidiary, including by merger or consolidation or the sale of all equity interests in such subsidiary owned by the Company, if such subsidiary guarantor is designated as an unrestricted subsidiary or otherwise ceases to be a restricted subsidiary, and if such subsidiary guarantor no longer guaranties any other indebtedness of the Company. Certain domestic subsidiaries, foreign subsidiaries, joint ventures, partnerships and third parties are not guarantors of the Senior Notes.
F-47
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Statements of Income
DaVita Inc. | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
For twelve months ended December 31, 2017 | |||||||||||||||||||
Dialysis and related lab patient service revenues | $ | — | $ | 6,884,750 | $ | 3,393,026 | $ | (184,106 | ) | $ | 10,093,670 | ||||||||
Less: Provision for uncollectible accounts | — | (340,586 | ) | (151,982 | ) | 7,170 | (485,398 | ) | |||||||||||
Net dialysis and related lab patient service revenues | — | 6,544,164 | 3,241,044 | (176,936 | ) | 9,608,272 | |||||||||||||
Other revenues | 793,751 | 1,204,501 | 68,322 | (798,212 | ) | 1,268,362 | |||||||||||||
Total net revenues | 793,751 | 7,748,665 | 3,309,366 | (975,148 | ) | 10,876,634 | |||||||||||||
Operating expenses and charges | 527,942 | 6,475,550 | 3,035,535 | (975,148 | ) | 9,063,879 | |||||||||||||
Operating income | 265,809 | 1,273,115 | 273,831 | — | 1,812,755 | ||||||||||||||
Debt expense | (426,149 | ) | (209,612 | ) | (34,831 | ) | 239,958 | (430,634 | ) | ||||||||||
Other income, net | 411,731 | 11,169 | 18,467 | (423,702 | ) | 17,665 | |||||||||||||
Income tax expense | 65,965 | 237,670 | 20,224 | — | 323,859 | ||||||||||||||
Equity earnings in subsidiaries | 478,192 | 74,375 | — | (552,567 | ) | — | |||||||||||||
Net income from continuing operations | 663,618 | 911,377 | 237,243 | (736,311 | ) | 1,075,927 | |||||||||||||
Net (loss) income from discontinued operations, net of tax | — | (433,185 | ) | 4,069 | 183,744 | (245,372 | ) | ||||||||||||
Net income | 663,618 | 478,192 | 241,312 | (552,567 | ) | 830,555 | |||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (166,937 | ) | (166,937 | ) | ||||||||||||
Net income attributable to DaVita Inc. | $ | 663,618 | $ | 478,192 | $ | 241,312 | $ | (719,504 | ) | $ | 663,618 | ||||||||
For twelve months ended December 31, 2016 | |||||||||||||||||||
Dialysis and related lab patient service revenues | $ | — | $ | 6,665,601 | $ | 3,215,085 | $ | (153,326 | ) | $ | 9,727,360 | ||||||||
Less: Provision for uncollectible accounts | — | (272,430 | ) | (158,878 | ) | — | (431,308 | ) | |||||||||||
Net dialysis and related lab patient service revenues | — | 6,393,171 | 3,056,207 | (153,326 | ) | 9,296,052 | |||||||||||||
Other revenues | 767,791 | 1,378,956 | 30,184 | (765,516 | ) | 1,411,415 | |||||||||||||
Total net revenues | 767,791 | 7,772,127 | 3,086,391 | (918,842 | ) | 10,707,467 | |||||||||||||
Operating expenses and charges | 493,175 | 6,907,469 | 2,195,955 | (918,842 | ) | 8,677,757 | |||||||||||||
Operating income | 274,616 | 864,658 | 890,436 | — | 2,029,710 | ||||||||||||||
Debt expense | (407,925 | ) | (191,083 | ) | (40,434 | ) | 225,326 | (414,116 | ) | ||||||||||
Other income, net | 396,797 | 3,726 | 7,694 | (400,706 | ) | 7,511 | |||||||||||||
Income tax expense | 77,334 | 238,446 | 115,981 | — | 431,761 | ||||||||||||||
Equity earnings in subsidiaries | 693,720 | 667,278 | — | (1,360,998 | ) | — | |||||||||||||
Net income from continuing operations | 879,874 | 1,106,133 | 741,715 | (1,536,378 | ) | 1,191,344 | |||||||||||||
Net (loss) income from discontinued operations, net of tax | — | (412,413 | ) | 78,771 | 175,380 | (158,262 | ) | ||||||||||||
Net income | 879,874 | 693,720 | 820,486 | (1,360,998 | ) | 1,033,082 | |||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (153,208 | ) | (153,208 | ) | ||||||||||||
Net income attributable to DaVita Inc. | $ | 879,874 | $ | 693,720 | $ | 820,486 | $ | (1,514,206 | ) | $ | 879,874 | ||||||||
F-48
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Statements of Income - (continued)
DaVita Inc. | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
For twelve months ended December 31, 2015 | |||||||||||||||||||
Dialysis and related lab patient service revenues | $ | — | $ | 6,471,702 | $ | 2,814,909 | $ | (131,164 | ) | $ | 9,155,447 | ||||||||
Less: Provision for uncollectible accounts | — | (281,976 | ) | (130,929 | ) | — | (412,905 | ) | |||||||||||
Net dialysis and related lab patient service revenues | — | 6,189,726 | 2,683,980 | (131,164 | ) | 8,742,542 | |||||||||||||
Other revenues | 727,887 | 1,208,607 | 24,013 | (720,804 | ) | 1,239,703 | |||||||||||||
Total net revenues | 727,887 | 7,398,333 | 2,707,993 | (851,968 | ) | 9,982,245 | |||||||||||||
Operating expenses and charges | 488,595 | 6,925,234 | 2,283,618 | (851,968 | ) | 8,845,479 | |||||||||||||
Operating income | 239,292 | 473,099 | 424,375 | — | 1,136,766 | ||||||||||||||
Debt (expense) and refinancing charges | (449,598 | ) | (178,389 | ) | (32,450 | ) | 203,985 | (456,452 | ) | ||||||||||
Other income, net | 365,752 | 1,261 | 6,921 | (365,861 | ) | 8,073 | |||||||||||||
Income tax expense (benefit) | 60,671 | 163,401 | (16,562 | ) | — | 207,510 | |||||||||||||
Equity earnings in subsidiaries | 174,957 | 322,022 | — | (496,979 | ) | — | |||||||||||||
Net income from continuing operations | 269,732 | 454,592 | 415,408 | (658,855 | ) | 480,877 | |||||||||||||
Net (loss) income from discontinued operations, net of tax | — | (279,635 | ) | 64,292 | 161,876 | (53,467 | ) | ||||||||||||
Net income | 269,732 | 174,957 | 479,700 | (496,979 | ) | 427,410 | |||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (157,678 | ) | (157,678 | ) | ||||||||||||
Net income attributable to DaVita Inc. | $ | 269,732 | $ | 174,957 | $ | 479,700 | $ | (654,657 | ) | $ | 269,732 | ||||||||
F-49
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Statements of Comprehensive Income
DaVita Inc. | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
For the year ended December 31, 2017 | |||||||||||||||||||
Net income | $ | 663,618 | $ | 478,192 | $ | 241,312 | $ | (552,567 | ) | $ | 830,555 | ||||||||
Other comprehensive income | 3,106 | — | 99,770 | — | 102,876 | ||||||||||||||
Total comprehensive income | 666,724 | 478,192 | 341,082 | (552,567 | ) | 933,431 | |||||||||||||
Less: Comprehensive income attributable to noncontrolling interest | — | — | — | (166,935 | ) | (166,935 | ) | ||||||||||||
Comprehensive income attributable to DaVita Inc. | $ | 666,724 | $ | 478,192 | $ | 341,082 | $ | (719,502 | ) | $ | 766,496 | ||||||||
For the year ended December 31, 2016 | |||||||||||||||||||
Net income | $ | 879,874 | $ | 693,720 | $ | 820,486 | $ | (1,360,998 | ) | $ | 1,033,082 | ||||||||
Other comprehensive loss | (290 | ) | — | (29,337 | ) | — | (29,627 | ) | |||||||||||
Total comprehensive income | 879,584 | 693,720 | 791,149 | (1,360,998 | ) | 1,003,455 | |||||||||||||
Less: Comprehensive income attributable to noncontrolling interest | — | — | — | (153,398 | ) | (153,398 | ) | ||||||||||||
Comprehensive income attributable to DaVita Inc. | $ | 879,584 | $ | 693,720 | $ | 791,149 | $ | (1,514,396 | ) | $ | 850,057 | ||||||||
For the year ended December 31, 2015 | |||||||||||||||||||
Net income | $ | 269,732 | $ | 174,957 | $ | 479,700 | $ | (496,979 | ) | $ | 427,410 | ||||||||
Other comprehensive loss | (10,920 | ) | — | (23,889 | ) | — | (34,809 | ) | |||||||||||
Total comprehensive income | 258,812 | 174,957 | 455,811 | (496,979 | ) | 392,601 | |||||||||||||
Less: Comprehensive income attributable to noncontrolling interest | — | — | — | (157,678 | ) | (157,678 | ) | ||||||||||||
Comprehensive income attributable to DaVita Inc. | $ | 258,812 | $ | 174,957 | $ | 455,811 | $ | (654,657 | ) | $ | 234,923 | ||||||||
F-50
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Balance Sheets
DaVita Inc. | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
As of December 31, 2017 | |||||||||||||||||||
Cash and cash equivalents | $ | 149,305 | $ | — | $ | 358,929 | $ | — | $ | 508,234 | |||||||||
Accounts receivable, net | — | 1,208,715 | 506,035 | — | 1,714,750 | ||||||||||||||
Other current assets | 68,027 | 604,450 | 87,255 | — | 759,732 | ||||||||||||||
Current assets held for sale | — | 4,992,067 | 769,575 | — | 5,761,642 | ||||||||||||||
Total current assets | 217,332 | 6,805,232 | 1,721,794 | — | 8,744,358 | ||||||||||||||
Property and equipment, net | 408,010 | 1,560,390 | 1,180,813 | — | 3,149,213 | ||||||||||||||
Intangible assets, net | 250 | 50,971 | 62,606 | — | 113,827 | ||||||||||||||
Investments in subsidiaries | 10,009,874 | 3,085,722 | — | (13,095,596 | ) | — | |||||||||||||
Intercompany receivables | 3,677,947 | — | 1,313,213 | (4,991,160 | ) | — | |||||||||||||
Other long-term assets and investments | 47,297 | 68,344 | 214,875 | — | 330,516 | ||||||||||||||
Goodwill | — | 4,732,320 | 1,877,959 | — | 6,610,279 | ||||||||||||||
Total assets | $ | 14,360,710 | $ | 16,302,979 | $ | 6,371,260 | $ | (18,086,756 | ) | $ | 18,948,193 | ||||||||
Current liabilities | $ | 238,706 | $ | 1,181,139 | $ | 436,262 | $ | — | $ | 1,856,107 | |||||||||
Current liabilities held for sale | — | 739,294 | 445,776 | — | 1,185,070 | ||||||||||||||
Total current liabilities | 238,706 | 1,920,433 | 882,038 | — | 3,041,177 | ||||||||||||||
Intercompany payables | — | 3,690,042 | 1,301,118 | (4,991,160 | ) | — | |||||||||||||
Long-term debt and other long-term liabilities | 8,857,373 | 682,630 | 469,587 | — | 10,009,590 | ||||||||||||||
Noncontrolling interests subject to put provisions | 574,602 | — | — | 436,758 | 1,011,360 | ||||||||||||||
Total DaVita Inc. shareholders' equity | 4,690,029 | 10,009,874 | 3,085,722 | (13,095,596 | ) | 4,690,029 | |||||||||||||
Noncontrolling interests not subject to put provisions | — | — | 632,795 | (436,758 | ) | 196,037 | |||||||||||||
Total equity | 4,690,029 | 10,009,874 | 3,718,517 | (13,532,354 | ) | 4,886,066 | |||||||||||||
Total liabilities and equity | $ | 14,360,710 | $ | 16,302,979 | $ | 6,371,260 | $ | (18,086,756 | ) | $ | 18,948,193 | ||||||||
F-51
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Balance Sheets - (continued)
DaVita Inc. | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
As of December 31, 2016 | |||||||||||||||||||
Cash and cash equivalents | $ | 549,921 | $ | — | $ | 124,855 | $ | — | $ | 674,776 | |||||||||
Accounts receivable, net | — | 1,048,580 | 455,370 | — | 1,503,950 | ||||||||||||||
Other current assets | 277,911 | 462,684 | 114,471 | — | 855,066 | ||||||||||||||
Current assets held for sale | — | 514,407 | 446,549 | — | 960,956 | ||||||||||||||
Total current assets | 827,832 | 2,025,671 | 1,141,245 | — | 3,994,748 | ||||||||||||||
Property and equipment, net | 337,200 | 1,444,248 | 1,082,673 | — | 2,864,121 | ||||||||||||||
Intangible assets, net | 487 | 42,037 | 30,980 | — | 73,504 | ||||||||||||||
Investments in subsidiaries | 9,717,728 | 2,021,062 | — | (11,738,790 | ) | — | |||||||||||||
Intercompany receivables | 3,250,692 | — | 866,955 | (4,117,647 | ) | — | |||||||||||||
Other long-term assets and investments | 39,994 | 73,466 | 442,433 | — | 555,893 | ||||||||||||||
Goodwill | — | 4,480,344 | 1,535,031 | — | 6,015,375 | ||||||||||||||
Long-term assets held for sale | — | 5,066,453 | 185,682 | — | 5,252,135 | ||||||||||||||
Total assets | $ | 14,173,933 | $ | 15,153,281 | $ | 5,284,999 | $ | (15,856,437 | ) | $ | 18,755,776 | ||||||||
Current liabilities | $ | 303,840 | $ | 1,343,748 | $ | 256,143 | $ | — | $ | 1,903,731 | |||||||||
Current liabilities held for sale | — | 533,250 | 273,983 | — | 807,233 | ||||||||||||||
Total current liabilities | 303,840 | 1,876,998 | 530,126 | — | 2,710,964 | ||||||||||||||
Intercompany payables | — | 2,382,428 | 1,735,219 | (4,117,647 | ) | — | |||||||||||||
Long-term debt and other long-term liabilities | 8,614,445 | 835,845 | 342,638 | — | 9,792,928 | ||||||||||||||
Long-term liabilities held for sale | — | 340,282 | 88,603 | — | 428,885 | ||||||||||||||
Noncontrolling interests subject to put provisions | 607,601 | — | — | 365,657 | 973,258 | ||||||||||||||
Total DaVita Inc. shareholders' equity | 4,648,047 | 9,717,728 | 2,021,062 | (11,738,790 | ) | 4,648,047 | |||||||||||||
Noncontrolling interests not subject to put provisions | — | — | 567,351 | (365,657 | ) | 201,694 | |||||||||||||
Total equity | 4,648,047 | 9,717,728 | 2,588,413 | (12,104,447 | ) | 4,849,741 | |||||||||||||
Total liabilities and equity | $ | 14,173,933 | $ | 15,153,281 | $ | 5,284,999 | $ | (15,856,437 | ) | $ | 18,755,776 | ||||||||
F-52
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Statements of Cash Flow
DaVita Inc. | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
For the year ended December 31, 2017 | |||||||||||||||||||
Cash flows from operating activities: | |||||||||||||||||||
Net income | $ | 663,618 | $ | 478,192 | $ | 241,312 | $ | (552,567 | ) | $ | 830,555 | ||||||||
Changes in operating assets and liabilities and non-cash items included in net income | (534,302 | ) | 366,947 | 691,682 | 552,567 | 1,076,894 | |||||||||||||
Net cash provided by operating activities | 129,316 | 845,139 | 932,994 | — | 1,907,449 | ||||||||||||||
Cash flows from investing activities: | |||||||||||||||||||
Additions of property and equipment, net | (155,972 | ) | (490,800 | ) | (258,478 | ) | — | (905,250 | ) | ||||||||||
Acquisitions | — | (693,522 | ) | (110,357 | ) | — | (803,879 | ) | |||||||||||
Proceeds from asset sales, net of cash divested | — | 90,340 | 1,996 | — | 92,336 | ||||||||||||||
Investments and other items | 211,619 | (9,003 | ) | 47,446 | — | 250,062 | |||||||||||||
Net cash provided by (used in) investing activities | 55,647 | (1,102,985 | ) | (319,393 | ) | — | (1,366,731 | ) | |||||||||||
Cash flows from financing activities: | |||||||||||||||||||
Long-term debt and related financing costs, net | 173,529 | (12,662 | ) | (6,019 | ) | — | 154,848 | ||||||||||||
Intercompany borrowing | 22,589 | 218,980 | (241,569 | ) | — | — | |||||||||||||
Other items | (781,697 | ) | (2,493 | ) | (136,915 | ) | — | (921,105 | ) | ||||||||||
Net cash (used in) provided by financing activities | (585,579 | ) | 203,825 | (384,503 | ) | — | (766,257 | ) | |||||||||||
Effect of exchange rate changes on cash | — | — | 254 | — | 254 | ||||||||||||||
Net (decrease) increase in cash and cash equivalents | (400,616 | ) | (54,021 | ) | 229,352 | — | (225,285 | ) | |||||||||||
Less: Net decrease in cash and cash equivalents from discontinued operations | — | (54,021 | ) | (4,722 | ) | — | (58,743 | ) | |||||||||||
Net (decrease) increase in cash and cash equivalents from continuing operations | (400,616 | ) | — | 234,074 | — | (166,542 | ) | ||||||||||||
Cash and cash equivalents of continuing operations at beginning of the year | 549,921 | — | 124,855 | — | 674,776 | ||||||||||||||
Cash and cash equivalents of continuing operations at end of the year | $ | 149,305 | $ | — | $ | 358,929 | $ | — | $ | 508,234 | |||||||||
F-53
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Statements of Cash Flow - (continued)
DaVita Inc. | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
For the year ended December 31, 2016 | |||||||||||||||||||
Cash flows from operating activities: | |||||||||||||||||||
Net income | $ | 879,874 | $ | 693,720 | $ | 820,486 | $ | (1,360,998 | ) | $ | 1,033,082 | ||||||||
Changes in operating assets and liabilities and non-cash items included in net income | (612,706 | ) | 350,684 | (168,614 | ) | 1,360,998 | 930,362 | ||||||||||||
Net cash provided by operating activities | 267,168 | 1,044,404 | 651,872 | — | 1,963,444 | ||||||||||||||
Cash flows from investing activities: | |||||||||||||||||||
Additions of property and equipment, net | (139,303 | ) | (382,305 | ) | (307,487 | ) | — | (829,095 | ) | ||||||||||
Acquisitions | — | (472,413 | ) | (91,443 | ) | — | (563,856 | ) | |||||||||||
Proceeds from asset and business sales, net of cash divested | — | 70,342 | (5,617 | ) | — | 64,725 | |||||||||||||
Investments and other items | 153,031 | (29,038 | ) | 2,565 | — | 126,558 | |||||||||||||
Net cash provided by (used in) investing activities | 13,728 | (813,414 | ) | (401,982 | ) | — | (1,201,668 | ) | |||||||||||
Cash flows from financing activities: | |||||||||||||||||||
Long-term debt and related financing costs, net | (92,460 | ) | (27,830 | ) | (4,152 | ) | — | (124,442 | ) | ||||||||||
Intercompany borrowing | 236,052 | (231,800 | ) | (4,252 | ) | — | — | ||||||||||||
Other items | (1,061,203 | ) | (21,525 | ) | (144,811 | ) | — | (1,227,539 | ) | ||||||||||
Net cash used in financing activities | (917,611 | ) | (281,155 | ) | (153,215 | ) | — | (1,351,981 | ) | ||||||||||
Effect of exchange rate changes on cash | — | — | 4,276 | — | 4,276 | ||||||||||||||
Net (decrease) increase in cash and cash equivalents | (636,715 | ) | (50,165 | ) | 100,951 | — | (585,929 | ) | |||||||||||
Less: Net (decrease) increase in cash and cash equivalents from discontinued operations | — | (50,165 | ) | 34,377 | — | (15,788 | ) | ||||||||||||
Net (decrease) increase in cash and cash equivalents from continuing operations | (636,715 | ) | — | 66,574 | — | (570,141 | ) | ||||||||||||
Cash and cash equivalents of continuing operations at beginning of the year | 1,186,636 | — | 58,281 | — | 1,244,917 | ||||||||||||||
Cash and cash equivalents of continuing operations at end of the year | $ | 549,921 | $ | — | $ | 124,855 | $ | — | $ | 674,776 | |||||||||
F-54
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Consolidating Statements of Cash Flow - (continued)
DaVita Inc. | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Consolidating Adjustments | Consolidated Total | |||||||||||||||
For the year ended December 31, 2015 | |||||||||||||||||||
Cash flows from operating activities: | |||||||||||||||||||
Net income | $ | 269,732 | $ | 174,957 | $ | 479,700 | $ | (496,979 | ) | $ | 427,410 | ||||||||
Changes in operating assets and liabilities and non-cash items included in net income | (125,981 | ) | 684,760 | 74,032 | 496,979 | 1,129,790 | |||||||||||||
Net cash provided by operating activities | 143,751 | 859,717 | 553,732 | — | 1,557,200 | ||||||||||||||
Cash flows from investing activities: | |||||||||||||||||||
Additions of property and equipment, net | (115,269 | ) | (319,695 | ) | (273,034 | ) | — | (707,998 | ) | ||||||||||
Acquisitions | — | (76,983 | ) | (19,486 | ) | — | (96,469 | ) | |||||||||||
Proceeds from asset sales | — | 19,715 | — | — | 19,715 | ||||||||||||||
Investments and other items | (74,474 | ) | (2,144 | ) | (20,414 | ) | — | (97,032 | ) | ||||||||||
Net cash used in investing activities | (189,743 | ) | (379,107 | ) | (312,934 | ) | — | (881,784 | ) | ||||||||||
Cash flows from financing activities: | |||||||||||||||||||
Long-term debt and related financing costs, net | 640,009 | (11,953 | ) | (8,358 | ) | — | 619,698 | ||||||||||||
Intercompany borrowing | 466,038 | (370,839 | ) | (95,199 | ) | — | — | ||||||||||||
Other items | (572,295 | ) | (66,382 | ) | (119,991 | ) | — | (758,668 | ) | ||||||||||
Net cash provided by (used in) financing activities | 533,752 | (449,174 | ) | (223,548 | ) | — | (138,970 | ) | |||||||||||
Effect of exchange rate changes on cash | — | — | (2,571 | ) | — | (2,571 | ) | ||||||||||||
Net increase in cash and cash equivalents | 487,760 | 31,436 | 14,679 | — | 533,875 | ||||||||||||||
Less: Net increase (decrease) in cash and cash equivalents from discontinued operations | — | 31,436 | (5,581 | ) | — | 25,855 | |||||||||||||
Net increase in cash and cash equivalents from continuing operations | 487,760 | — | 20,260 | — | 508,020 | ||||||||||||||
Cash and cash equivalents of continuing operations at beginning of the year | 698,876 | — | 38,021 | — | 736,897 | ||||||||||||||
Cash and cash equivalents of continuing operations at end of the year | $ | 1,186,636 | $ | — | $ | 58,281 | $ | — | $ | 1,244,917 | |||||||||
F-55
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
29. Supplemental data (unaudited)
The following information is presented as supplemental data as required by the indentures governing the Company’s Senior Notes.
Condensed Consolidating Statements of Income
Consolidated Total | Physician Groups | Unrestricted Subsidiaries | Company and Restricted Subsidiaries(1) | ||||||||||||
For the year ended December 31, 2017 | |||||||||||||||
Dialysis and related lab patient service revenues | $ | 10,093,670 | $ | — | $ | — | $ | 10,093,670 | |||||||
Less: Provision for uncollectible accounts | (485,398 | ) | — | — | (485,398 | ) | |||||||||
Net dialysis and related lab patient service revenues | 9,608,272 | — | — | 9,608,272 | |||||||||||
Other revenues | 1,268,362 | — | — | 1,268,362 | |||||||||||
Total net revenues | 10,876,634 | — | — | 10,876,634 | |||||||||||
Operating expenses and charges | 9,063,879 | — | — | 9,063,879 | |||||||||||
Operating income | 1,812,755 | — | — | 1,812,755 | |||||||||||
Debt expense | (430,634 | ) | — | — | (430,634 | ) | |||||||||
Other income, net | 17,665 | — | — | 17,665 | |||||||||||
Income tax expense | 323,859 | — | — | 323,859 | |||||||||||
Net income from continuing operations | 1,075,927 | — | — | 1,075,927 | |||||||||||
Net (loss) income from discontinued operations, net of tax | (245,372 | ) | 13,611 | 19 | (259,002 | ) | |||||||||
Net income | 830,555 | 13,611 | 19 | 816,925 | |||||||||||
Less: Net income attributable to noncontrolling interests | (166,937 | ) | 7,183 | — | (174,120 | ) | |||||||||
Net income attributable to DaVita Inc. | $ | 663,618 | $ | 20,794 | $ | 19 | $ | 642,805 | |||||||
Condensed Consolidating Statements of Comprehensive Income
Consolidated Total | Physician Groups | Unrestricted Subsidiaries | Company and Restricted Subsidiaries(1) | ||||||||||||
For the year ended December 31, 2017 | |||||||||||||||
Net income | $ | 830,555 | $ | 13,611 | $ | 19 | $ | 816,925 | |||||||
Other comprehensive income | 102,876 | — | — | 102,876 | |||||||||||
Total comprehensive income | 933,431 | 13,611 | 19 | 919,801 | |||||||||||
Less: Comprehensive income attributable to noncontrolling interest | (166,935 | ) | 7,183 | — | (174,118 | ) | |||||||||
Comprehensive income attributable to DaVita Inc. | $ | 766,496 | $ | 20,794 | $ | 19 | $ | 745,683 | |||||||
(1) | After the elimination of the unrestricted subsidiaries and the physician groups |
F-56
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Condensed Consolidating Balance Sheets
Consolidated Total | Physician Groups | Unrestricted Subsidiaries | Company and Restricted Subsidiaries(1) | ||||||||||||
As of December 31, 2017 | |||||||||||||||
Cash and cash equivalents | $ | 508,234 | $ | — | $ | — | $ | 508,234 | |||||||
Accounts receivable, net | 1,714,750 | — | — | 1,714,750 | |||||||||||
Other current assets | 759,732 | 3,033 | — | 756,699 | |||||||||||
Other current assets held for sale | 5,761,642 | 423,205 | 2,733 | 5,335,704 | |||||||||||
Total current assets | 8,744,358 | 426,238 | 2,733 | 8,315,387 | |||||||||||
Property and equipment, net | 3,149,213 | — | — | 3,149,213 | |||||||||||
Amortizable intangibles, net | 113,827 | — | — | 113,827 | |||||||||||
Other long-term assets | 330,516 | — | — | 330,516 | |||||||||||
Goodwill | 6,610,279 | — | — | 6,610,279 | |||||||||||
Total assets | $ | 18,948,193 | $ | 426,238 | $ | 2,733 | $ | 18,519,222 | |||||||
Current liabilities | $ | 1,856,107 | $ | — | $ | — | $ | 1,856,107 | |||||||
Current liabilities held for sale | 1,185,070 | 308,884 | — | 876,186 | |||||||||||
Total current liabilities | 3,041,177 | 308,884 | — | 2,732,293 | |||||||||||
Payables to parent | — | — | 2,733 | (2,733 | ) | ||||||||||
Long-term debt and other long-term liabilities | 10,009,590 | — | — | 10,009,590 | |||||||||||
Noncontrolling interests subject to put provisions | 1,011,360 | — | — | 1,011,360 | |||||||||||
Total DaVita Inc. shareholders' equity | 4,690,029 | 117,354 | — | 4,572,675 | |||||||||||
Noncontrolling interests not subject to put provisions | 196,037 | — | — | 196,037 | |||||||||||
Shareholders' equity | 4,886,066 | 117,354 | — | 4,768,712 | |||||||||||
Total liabilities and shareholders' equity | $ | 18,948,193 | $ | 426,238 | $ | 2,733 | $ | 18,519,222 | |||||||
F-57
DAVITA INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - (continued)
(dollars in thousands, except per share data)
Condensed Consolidating Statements of Cash Flow
Consolidated Total | Physician Groups | Unrestricted Subsidiaries | Company and Restricted Subsidiaries(1) | ||||||||||||
For the year ended December 31, 2017 | |||||||||||||||
Cash flows from operating activities: | |||||||||||||||
Net income | $ | 830,555 | $ | 13,611 | $ | 19 | $ | 816,925 | |||||||
Changes in operating and intercompany assets and liabilities and non-cash items included in net income | 1,076,894 | 27,312 | (19 | ) | 1,049,601 | ||||||||||
Net cash provided by operating activities | 1,907,449 | 40,923 | — | 1,866,526 | |||||||||||
Cash flows from investing activities: | |||||||||||||||
Additions of property and equipment | (905,250 | ) | (5,406 | ) | — | (899,844 | ) | ||||||||
Acquisitions and divestitures, net | (803,879 | ) | — | — | (803,879 | ) | |||||||||
Proceeds from asset sales | 92,336 | — | — | 92,336 | |||||||||||
Investments and other items, net | 250,062 | (3,800 | ) | — | 253,862 | ||||||||||
Net cash used in investing activities | (1,366,731 | ) | (9,206 | ) | — | (1,357,525 | ) | ||||||||
Cash flows from financing activities: | |||||||||||||||
Long-term debt and related financing costs, net | 154,848 | — | — | 154,848 | |||||||||||
Intercompany | — | (36,220 | ) | — | 36,220 | ||||||||||
Other items | (921,105 | ) | — | — | (921,105 | ) | |||||||||
Net cash used in financing activities | (766,257 | ) | (36,220 | ) | — | (730,037 | ) | ||||||||
Effect of exchange rate changes on cash | 254 | — | — | 254 | |||||||||||
Net decrease in cash and cash equivalents | (225,285 | ) | (4,503 | ) | — | (220,782 | ) | ||||||||
Less: Net decrease in cash and cash equivalents from discontinued operations | (58,743 | ) | (4,503 | ) | — | (54,240 | ) | ||||||||
Net decrease in cash and cash equivalents from continuing operations | (166,542 | ) | — | — | (166,542 | ) | |||||||||
Cash and cash equivalents of continuing operations at beginning of the year | 674,776 | — | — | 674,776 | |||||||||||
Cash and cash equivalents of continuing operations at end of the year | $ | 508,234 | $ | — | $ | — | $ | 508,234 | |||||||
(1) | After the elimination of the unrestricted subsidiaries and the physician groups |
F-58
EXHIBIT INDEX
Agreement and Plan of Merger, dated as of May 20, 2012, by and among DaVita Inc., Seismic Acquisition LLC, HealthCare Partners Holdings, LLC, and the Member Representative.(28) | ||
Amendment, dated as of July 6, 2012, to the Agreement and Plan of Merger, dated as of May 20, 2012, by and among DaVita Inc., Seismic Acquisition LLC, HealthCare Partners Holdings, LLC, and the Member Representative.(25) | ||
Restated Certificate of Incorporation of DaVita Inc., as filed with the Secretary of State of Delaware on November 1, 2016.(1) | ||
Amended and Restated Bylaws for DaVita Inc. dated as of September 7, 2016.(1) | ||
Indenture, dated August 28, 2012, by and among DaVita Inc., the guarantors named therein and The Bank of New York Mellon Trust Company, N.A., as Trustee.(4) | ||
Form of 5.750% Senior Notes due 2022 and related Guarantee (included in Exhibit 4.1).(4) | ||
Indenture, dated June 13, 2014, by and among DaVita Inc., the guarantors named therein and The Bank of New York Mellon Trust Company, N.A., as Trustee.(26) | ||
Form of 5.125% Senior Notes due 2024 and related Guarantee (included in Exhibit 4.3).(26) | ||
Second Supplemental Indenture for the 5.750% Senior Notes due 2022, dated June 13, 2014, by and among DaVita Inc., the guarantors named therein and The Bank of New York Mellon Trust Company, N.A., as Trustee.(21) | ||
Indenture for the 5.000% Senior Notes due 2025, dated April 17, 2015, by and among DaVita Inc., the guarantors named therein and The Bank of New York Mellon Trust Company, N.A., as Trustee.(22) | ||
Form of 5.000% Senior Notes due 2025 and related Guarantee (included in Exhibit 4.6).(22) | ||
Employment Agreement, effective September 22, 2005, by and between DaVita Inc. and James Hilger.(8)* | ||
Amendment to Mr. Hilger’s Employment Agreement, effective December 12, 2008.(18)* | ||
Second Amendment to Mr. Hilger’s Employment Agreement, effective December 27, 2012.(23)* | ||
Employment Agreement, effective July 25, 2008, between DaVita Inc. and Kent J. Thiry.(15)* | ||
Employment Agreement, effective August 1, 2008, between DaVita Inc. and Allen Nissenson.(16)* | ||
Employment Agreement, effective March 17, 2010, by and between DaVita Inc. and Javier Rodriguez.(20)* | ||
Employment Agreement, effective April 27, 2016, by and between DaVita HealthCare Partners Inc. and Kathleen A. Waters.(6)* | ||
Consulting Agreement, effective June 15, 2017, by and between DaVita Inc. and Roger J. Valine.(3)* | ||
Amendment to Stock Appreciation Rights Agreements, effective June 15, 2017, by and between DaVita Inc. and Roger J. Valine.(3)* | ||
Employment Agreement, effective November 1, 2016, by and between DaVita Inc. and Charles G. Berg.(9)* | ||
Page 1 of 5
Amendment to Employment Agreement, effective October 13, 2017, by and among DaVita Inc., Charles G. Berg and DaVita Medical Management, LLC.(3)* | ||
Employment Agreement, effective February 21, 2017, by and between DaVita Inc. and Joel Ackerman.(9)* | ||
Sourcing and Supply Agreement between DaVita Inc. and Amgen USA Inc. effective as of January 6, 2017.(6)** | ||
Equity Purchase Agreement, dated as of December 5, 2017, by and among DaVita Inc., Collaborative Care Holdings, LLC, and solely with respect to Section 9.3 and Section 9.18 thereto, UnitedHealth Group Incorporated.(2) | ||
Form of Indemnity Agreement.(12)* | ||
Form of Indemnity Agreement.(7)* | ||
DaVita Deferred Compensation Plan.(9)* | ||
Executive Incentive Plan (as Amended and Restated effective January 1, 2009).(19)* | ||
Executive Retirement Plan.(18)* | ||
DaVita Voluntary Deferral Plan.(5)* | ||
Deferred Bonus Plan (Prosperity Plan).(17)* | ||
Amendment No. 1 to Deferred Bonus Plan (Prosperity Plan).(18)* | ||
Amended and Restated Employee Stock Purchase Plan.(13)* | ||
Amended and Restated DaVita Inc. Severance Plan.(23)* | ||
Change in Control Bonus Program.(18)* | ||
DaVita Inc. Non-Employee Director Compensation Policy.(14)* | ||
DaVita Inc. Non-Employee Director Compensation Policy. *✓ | ||
Form of Restricted Stock Units Agreement—Board members (DaVita Inc. 2011 Incentive Award Plan). (24)* | ||
Form of Stock Appreciation Rights Agreement—Executives (DaVita Inc. 2011 Incentive Award Plan).(24)* | ||
Form of Restricted Stock Units Agreement—Executives (DaVita Inc. 2011 Incentive Award Plan).(24)* | ||
Form of Restricted Stock Units Agreement (DaVita Inc. 2011 Incentive Award Plan).(23)* | ||
Form of Stock Appreciation Rights Agreement (DaVita Inc. 2011 Incentive Award Plan).(23)* | ||
Form of Long-Term Incentive Program Award Agreement (For 162(m) designated teammates) (DaVita Inc. 2011 Incentive Award Plan).(23)* | ||
Form of Long-Term Incentive Program Award Agreement (DaVita Inc. 2011 Incentive Award Plan).(23)* | ||
Page 2 of 5
Credit Agreement, dated as of June 24, 2014, by and among DaVita Inc., the guarantors the guarantors party thereto, the lenders party thereto, JPMorgan Chase Bank, N.A., as Administrative Agent and Collateral Agent, Barclays Bank PLC, and Wells Fargo Bank, National Association as Co-Syndication Agents, Bank of America, N.A., Credit Suisse AG, Goldman Sachs Bank USA, JPMorgan Chase Bank, N.A., Morgan Stanley Senior Funding, Inc., and SunTrust Bank, as Co-Documentation Agents, Barclays Bank PLC, Wells Fargo Securities, LLC, Credit Suisse Securities (USA) LLC, Goldman Sachs Bank USA, J.P. Morgan Securities, LLC, Bank of America, N.A., Morgan Stanley Senior Funding, Inc., and SunTrust Robinson Humphrey, Inc. as Joint Lead Arrangers and Joint Bookrunners, The Bank of Nova Scotia, Credit Agricole Securities (USA) Inc., The Bank of Tokyo-Mitsubishi UFJ, Ltd., and Sumitomo Mitsui Banking Corporation, as Senior Managing Agents, HSBC Securities (USA) Inc., Fifth Third Bank, and Compass Bank as Managing Agents. (21) | ||
Amended and Restated DaVita Inc. 2011 Incentive Award Plan.(11)* | ||
Form of Non-Competition and Non-Solicitation Agreement, dated as of May 20, 2012, between DaVita Inc. and Dr. Robert Margolis, Dr. William Chin, Dr. Thomas Paulsen, Mr. Zan Calhoun, and Ms. Lori Glisson. (28) | ||
Form of Non-Competition and Non-Solicitation Agreement, dated as of May 20, 2012, between DaVita Inc. and Mr. Matthew Mazdyasni, Dr. Sherif Abdou, and Dr. Amir Bacchus.(28) | ||
Escrow Agreement, dated as of August 28, 2012, by and among DaVita Inc., The Bank of New York Mellon Trust Company, N.A., as trustee, The Bank of New York Mellon Trust Company, N.A., as escrow agent and The Bank of New York Mellon Trust Company, N.A., as bank and securities intermediary.(4) | ||
Form of 2014 Long Term Incentive Program Cash Performance Award Agreement under the DaVita Inc. 2011 Incentive Award Plan and Long-Term Incentive Program (for 162(m) designated teammates).(10) * ** | ||
Form of 2014 Long Term Incentive Program Cash Performance Award Agreement under the DaVita Inc. 2011 Incentive Award Plan and Long-Term Incentive Program.(10)* ** | ||
Form of 2014 Long Term Incentive Program Performance Stock Units Agreement under the DaVita Inc. 2011 Incentive Award Plan and Long-Term Incentive Program (for 162(m) designated teammates).(10) * ** | ||
Form of 2014 Long Term Incentive Program Restricted Stock Units Agreement under the DaVita Inc. 2011 Incentive Award Plan and Long-Term Incentive Program.(10)* | ||
Form of 2014 Long Term Incentive Program Stock Appreciation Rights Agreement under the DaVita Inc. 2011 Incentive Award Plan and Long-Term Incentive Program.(10)* | ||
Corporate Integrity Agreement, dated as of October 22, 2014, by and among the Office of Inspector General of The Department of Health and Human Services and DaVita Inc.(27) | ||
Computation of Ratio of Earnings to Fixed Charges.✓ | ||
List of our subsidiaries.✓ | ||
Consent of KPMG LLP, independent registered public accounting firm.✓ | ||
Powers of Attorney with respect to DaVita. (Included on Page S-1). | ||
Certification of the Chief Executive Officer, dated February 23, 2018, pursuant to Rule 13a-14(a) or 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.✓ | ||
Certification of the Chief Financial Officer, dated February 23, 2018, pursuant to Rule 13a-14(a) or 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.✓ | ||
Page 3 of 5
Certification of the Chief Executive Officer, dated February 23, 2018, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.✓ | ||
Certification of the Chief Financial Officer, dated February 23, 2018, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.✓ | ||
101.INS | XBRL Instance Document.✓ | |
101.SCH | XBRL Taxonomy Extension Schema Document.✓ | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document.✓ | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document.✓ | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document.✓ | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document.✓ | |
✓ | Included in this filing. |
* | Management contract or executive compensation plan or arrangement. |
** | Portions of this exhibit are subject to a request for confidential treatment and have been redacted and filed separately with the SEC. |
(1) | Filed on November 2, 2016 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2016. |
(2) | Filed on December 6, 2017 as an exhibit to the Company’s Current Report on Form 8-K. |
(3) | Filed on November 7, 2017 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2017. |
(4) | Filed on August 28, 2012 as an exhibit to the Company’s Current Report on Form 8-K. |
(5) | Filed on November 8, 2005 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. |
(6) | Filed on May 2, 2017 as an exhibit to the Company’s Quarterly Report on 10-Q for the quarter ended March 31, 2017. |
(7) | Filed on March 3, 2005 as an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004. |
(8) | Filed on August 7, 2006 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ending June 30, 2006. |
(9) | Filed on February 24, 2017 as an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2016. |
(10) | Filed on November 6, 2014 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014. |
(11) | Filed on April 28, 2014 as Appendix A to the Company's Definitive Proxy Statement on Schedule 14A. |
(12) | Filed on December 20, 2006 as an exhibit to the Company’s Current Report on Form 8-K. |
(13) | Filed on June 4, 2007 as an exhibit to the Company’s Current Report on Form 8-K. |
(14) | Filed on May 8, 2008 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2008. |
(15) | Filed on July 31, 2008 as an exhibit to the Company’s Current Report on Form 8-K. |
(16) | Filed on November 6, 2008 as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2008. |
(17) | Filed on February 29, 2008 as an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007. |
(18) | Filed on February 27, 2009 as an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008 |
(19) | Filed on June 18, 2009 as an exhibit to the Company’s Current Report on Form 8-K. |
(20) | Filed on April 14, 2010 as an exhibit to the Company’s Current Report on Form 8-K. |
Page 4 of 5
(21) | Filed on August 1, 2014 as an exhibit to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2014. |
(22) | Filed on April 17, 2015 as an exhibit to the Company’s Current Report on Form 8-K. |
(23) | Filed on March 1, 2013 as an exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012. |
(24) | Filed on August 4, 2011 as an exhibit to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2011. |
(25) | Filed on July 9, 2012 as an exhibit to the Company’s Current Report on Form 8-K. |
(26) | Filed on June 16, 2014 as an exhibit to the Company's current Report on Form 8-K. |
(27) | Filed on October 23, 2014 as an exhibit to the Company's current report on Form 8-K. |
(28) | Filed on May 21, 2012 as an exhibit to the Company's Current Report on Form 8-K. |
Page 5 of 5
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, we have duly caused this Annual Report on Form 10-K to be signed on our behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on February 23, 2018.
DAVITA INC. | ||
By: | /S/ KENT J. THIRY | |
Kent J. Thiry Chairman and Chief Executive Officer | ||
KNOW ALL MEN BY THESE PRESENT, that each person whose signature appears below constitutes and appoints Kent J. Thiry, Joel Ackerman, and Kathleen Waters, and each of them his or her true and lawful attorneys-in-fact and agents with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
S-1
Signature | Title | Date | ||
/S/ KENT J. THIRY | Chairman and Chief Executive Officer | February 23, 2018 | ||
Kent J. Thiry | (Principal Executive Officer) | |||
/S/ JOEL ACKERMAN | Chief Financial Officer | February 23, 2018 | ||
Joel Ackerman | (Principal Financial Officer) | |||
/S/ JAMES K. HILGER | Chief Accounting Officer | February 23, 2018 | ||
James K. Hilger | (Principal Accounting Officer) | |||
/S/ PAMELA M. ARWAY | Director | February 23, 2018 | ||
Pamela M. Arway | ||||
/S/ CHARLES G. BERG | Director | February 23, 2018 | ||
Charles G. Berg | ||||
/S/ CAROL A. DAVIDSON | Director | February 23, 2018 | ||
Carol A. Davidson | ||||
/S/ BARBARA J. DESOER | Director | February 23, 2018 | ||
Barbara J. Desoer | ||||
/S/ PASCAL DESROCHES | Director | February 23, 2018 | ||
Pascal Desroches | ||||
/S/ PAUL J. DIAZ | Director | February 23, 2018 | ||
Paul J. Diaz | ||||
/S/ PETER T. GRAUER | Director | February 23, 2018 | ||
Peter T. Grauer | ||||
/S/ JOHN M. NEHRA | Director | February 23, 2018 | ||
John M. Nehra | ||||
/S/ WILLIAM L. ROPER | Director | February 23, 2018 | ||
William L. Roper | ||||
/S/ PHYLLIS R. YALE | Director | February 23, 2018 | ||
Phyllis R. Yale | ||||
S-2
DAVITA INC.
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
Balance at beginning of year | Acquisitions | Amounts charged to income | Amounts written off | Balance at end of year | ||||||||||||||||
Description | ||||||||||||||||||||
(in thousands) | ||||||||||||||||||||
Allowance for uncollectible accounts: | ||||||||||||||||||||
Year ended December 31, 2017 | $ | 238,897 | $ | — | $ | 478,365 | $ | 498,863 | $ | 218,399 | ||||||||||
Year ended December 31, 2016 | $ | 251,734 | $ | — | $ | 442,985 | $ | 455,822 | $ | 238,897 | ||||||||||
Year ended December 31, 2015 | $ | 229,802 | $ | — | $ | 422,145 | $ | 400,213 | $ | 251,734 | ||||||||||
S-3
