Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2017
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from to
Commission File Number: 001-38318
Odonate Therapeutics, Inc.
(Exact name of Registrant as specified in its charter)
|
Delaware |
|
|
|
82-2493065 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
|
|
(I.R.S. Employer Identification Number) |
4747 Executive Drive, Suite 510
San Diego, CA 92121
(858) 731-8180
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.01 per share |
|
Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to the filing requirements for the past 90 days. Yes ☐ No ☒
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☐ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
|
Non-accelerated filer |
☒ |
(Do not check if a smaller reporting company) |
Smaller reporting company |
☐ |
|
|
|
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.) Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Company as of February 1, 2018 was approximately $173,411,152, based on the closing price on the Nasdaq Global Select Market reported for such date. Shares of common stock held by each officer and director and by each person who is known to own 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes. The Company has elected to use February 1, 2018 as the calculation date, as on the last business day of the Company’s most recently completed second fiscal quarter there was no public market for the Company’s common stock.
As of February 1, 2018, there were 27,331,429 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2018 Annual Meeting of Stockholders, which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission not later than 120 days after the registrant’s fiscal year ended December 31, 2017, are incorporated by reference into Part III of this Annual Report on Form 10-K.
|
|
|
|
|
Page |
|
|
|
|
1 |
|
|
|||
|
|
||||||
|
|
||||||
|
Item 1. |
|
|
2 |
|
|
|
|
Item 1A. |
|
|
43 |
|
|
|
|
Item 1B. |
|
|
63 |
|
|
|
|
Item 2. |
|
|
63 |
|
|
|
|
Item 3. |
|
|
63 |
|
|
|
|
Item 4. |
|
|
63 |
|
|
|
|
|
||||||
|
|
||||||
|
Item 5. |
|
|
64 |
|
|
|
|
Item 6. |
|
|
65 |
|
|
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
67 |
|
|
|
Item 7A. |
|
|
73 |
|
|
|
|
Item 8. |
|
|
74 |
|
|
|
|
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
74 |
|
|
|
Item 9A. |
|
|
74 |
|
|
|
|
Item 9B. |
|
|
74 |
|
|
|
|
|
||||||
|
|
||||||
|
Item 10. |
|
|
75 |
|
|
|
|
Item 11. |
|
|
75 |
|
|
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
75 |
|
|
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
75 |
|
|
|
Item 14. |
|
|
75 |
|
|
|
|
|
||||||
|
|
||||||
|
Item 15. |
|
|
76 |
|
|
|
|
Item 16. |
|
|
76 |
|
|
|
|
|
|
|
77 |
|
|
|
i
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of the federal securities laws, which statements involve substantial risks and uncertainties. All statements, other than statements of historical facts included in this Annual Report on Form 10-K, including statements concerning our plans, objectives, goals, strategies, future events, future revenues or performance, financing needs, plans or intentions relating to acquisitions, business trends and other information referred to under “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” are forward-looking statements. Forward-looking statements generally relate to future events or our future financial or operating performance. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Forward-looking statements are not historical facts and reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
There are a number of risks, uncertainties and other important factors that could cause our actual results to differ materially from the forward-looking statements contained in this Annual Report on Form 10-K. Such risks, uncertainties and other important factors include, among others, the risks, uncertainties and factors set forth above under “Risk Factors,” and the following risks, uncertainties and factors:
• our plans to develop and commercialize tesetaxel and any other product candidates;
• our ongoing and planned clinical studies;
• the timing of and our ability to obtain regulatory approvals for tesetaxel and any other product candidates;
• our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
• our ability to identify additional products or product candidates with significant commercial potential that are consistent with our commercial objectives;
• the rate and degree of market acceptance and clinical utility of tesetaxel and any other product candidates, if approved;
• our commercialization, marketing and manufacturing capabilities and strategy;
• significant competition in our industry;
• our intellectual property position;
• loss or retirement of key members of management;
• failure to successfully execute our growth strategy, including any delays in our planned future growth; and
• our failure to maintain effective internal controls.
There may be other factors that may cause our actual results to differ materially from the forward-looking statements, including factors disclosed in “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” You should evaluate all forward-looking statements made in this Annual Report on Form 10-K in the context of these risks and uncertainties.
We caution you that the risks, uncertainties and other factors referred to above may not contain all of the risks, uncertainties and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. All forward-looking statements in this Annual Report on Form 10-K apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this Annual Report on Form 10-K. We undertake no obligation to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances.
1
In this Annual Report on Form 10-K, unless context requires otherwise, references to “we,” “us,” “our,” “Odonate” or “the Company” refer to: (i) Odonate Therapeutics, Inc. as of and following the completion of the Conversion described below; and (ii) Odonate Therapeutics, LLC before the completion of the Conversion. All common stock and per share amounts for all periods presented in this Annual Report on Form 10-K have been adjusted retroactively, where applicable, to reflect the Conversion.
Company Overview
We are a pharmaceutical company dedicated to the development of best-in-class therapeutics that improve and extend the lives of patients with cancer. Our initial focus is on the development of tesetaxel, an investigational, orally administered chemotherapy agent that belongs to a class of drugs known as taxanes, which are widely used in the treatment of cancer. Tesetaxel has several potential therapeutic advantages over currently available taxanes, including: oral administration with a low pill burden and a patient-friendly dosing regimen; a formulation that does not contain solubilizing agents that are known to cause hypersensitivity (allergic) reactions; and improved activity against chemotherapy-resistant tumors. Tesetaxel has been generally well tolerated in clinical studies and has demonstrated robust single-agent antitumor activity in two multicenter, Phase 2 studies in patients with locally advanced or metastatic breast cancer (“MBC”). We are conducting a multinational, multicenter, randomized, Phase 3 study in MBC, known as CONTESSA, and we expect to report top-line results from this study in 2020. Our goal for tesetaxel is to develop an effective chemotherapy choice for patients that provides quality-of-life advantages over current alternatives.
Breast Cancer and Its Treatment
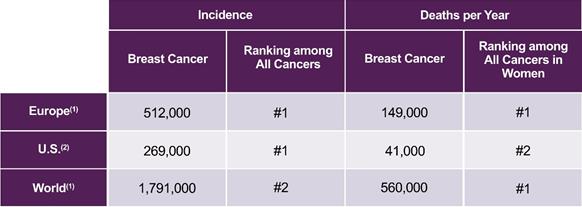
Breast cancer is the second-most common cancer worldwide, with an estimated 1.8 million new cases diagnosed per year (World Health Organization). In Europe, an estimated 512,000 new cases are diagnosed and approximately 149,000 women will die of the disease each year, making it the leading cause of cancer death in women (World Health Organization). In the U.S., an estimated 269,000 new cases are diagnosed and approximately 41,000 women will die of the disease each year, making it the second-leading cause of cancer death in women (American Cancer Society). The 5-year survival rate for patients with metastatic breast cancer is approximately 22% (American Cancer Society). Estimated breast cancer incidence and deaths per year in Europe, the U.S. and worldwide are shown in the following table.
Estimated Breast Cancer Incidence and Deaths per Year in Europe, the U.S. and Worldwide
|
|
|
(1) World Health Organization (2) American Cancer Society |
2
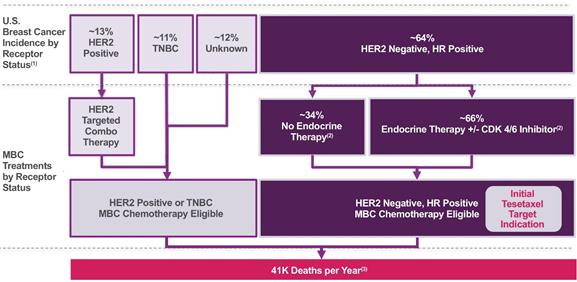
Breast cancer is a heterogeneous disease comprised of several molecular subtypes, which are commonly grouped into clinical subtypes based on receptor status. Receptors that are assessed in standard clinical practice include the estrogen receptor (“ER”) and progesterone receptor (“PgR”), collectively the hormone receptors (“HR”), and human epidermal growth factor receptor 2 (“HER2”). Breast cancers generally are categorized by the presence or absence of these receptors. The most common type of breast cancer is HER2 negative and HR positive, accounting for approximately 64% of newly diagnosed cases (Howlader et al, Journal of the National Cancer Institute 2014;106(5):1-8). HER2 positive breast cancer and triple-negative breast cancer (“TNBC”), the latter of which lacks all three receptors, are less common, accounting for approximately 13% and 11% of breast cancers, respectively (Howlader et al, Journal of the National Cancer Institute 2014;106(5):1-8). Estimated U.S. breast cancer incidence by receptor status and MBC treatments by receptor status are shown in the following figure.
Estimated U.S. Breast Cancer Incidence by Receptor Status and MBC Treatments
by Receptor Status
|
|
|
(1) Howlader et al, Journal of the National Cancer Institute 2014;106(5):1-8 (2) Caldeira et al, Oncology and Therapy 2016;4:189-197 (3) National Cancer Institute |
Current Treatments for HER2 Negative, HR Positive MBC
HER2 negative, HR positive disease, which represents the majority of all MBC cases, remains an area of high unmet medical need. Over the past two decades, only modest survival benefits have been achieved in this patient population; hence, treatment goals emphasize controlling disease-related symptoms, minimizing toxicity and maximizing quality-of-life. Patients with HER2 negative, HR positive disease are typically treated with endocrine therapy (with or without targeted agents such as a cyclin-dependent kinase (“CDK”) 4/6 inhibitor), chemotherapy or both.
3
Endocrine agents, which target certain hormone receptors inside and on the surface of tumor cells with the goal of slowing tumor growth, are preferred as initial treatment prior to chemotherapy for most patients with HER2 negative, HR positive MBC. These agents, which typically are used sequentially with or without targeted agents such as a CDK 4/6 inhibitor, include aromatase inhibitors (e.g., anastrozole, exemestane and letrozole), selective estrogen receptor modulators (“SERMs”; e.g., tamoxifen) and estrogen receptor downregulators (“ERDs”; e.g., fulvestrant).
The recently approved CDK 4/6 inhibitor, palbociclib, an orally administered therapy, has significantly improved outcomes for patients with MBC when used in combination with endocrine agents. In a multicenter, randomized, Phase 3 study, letrozole plus palbociclib given as initial therapy in post-menopausal women with HER2 negative, ER positive MBC resulted in median progression-free survival (“PFS”) of 24.8 months, compared to 14.5 months with letrozole alone (Finn et al, New England Journal of Medicine 2016;375(20):1925-1936). And, in a different multicenter, randomized, Phase 3 study, fulvestrant plus palbociclib given as second-line endocrine therapy in women with HER2 negative, HR positive MBC resulted in median PFS of 9.5 months, compared to 4.6 months with fulvestrant alone (Cristofanilli et al, The Lancet 2016;17(4):425-439). Despite these recent advances in endocrine therapy, virtually all MBC patients will eventually progress and require subsequent treatment with chemotherapy.
Chemotherapy
In HER2 negative, HR positive MBC, chemotherapy generally is used following disease progression on endocrine therapy. However, there is also a significant percentage of patients who receive chemotherapy as their first treatment for advanced disease because endocrine therapy is not indicated. This includes patients with: (i) a short relapse-free interval while on adjuvant (immediately following surgery) endocrine therapy (endocrine resistance); (ii) rapidly progressive disease/visceral crisis; and/or (iii) endocrine intolerance. In a recent analysis of a several-thousand-patient record database in Europe and the U.S., chemotherapy-only regimens were given in the first-line setting 33% to 35% of the time in Europe and 34% to 42% of the time in the U.S. (Caldeira et al, Oncology and Therapy 2016;4:189-197).
Chemotherapy agents used in the treatment of MBC generally are considered to be associated with significant side effects and a negative impact on quality-of-life. The approved chemotherapy agents for the treatment of HER2 negative MBC include: paclitaxel, nab-paclitaxel and docetaxel (taxanes); capecitabine (a fluoropyrimidine); doxorubicin and epirubicin (anthracyclines); gemcitabine (a nucleoside inhibitor); ixabepilone (an epothilone approved in the U.S.); and eribulin (a non-taxane microtubule dynamics inhibitor). The taxanes and eribulin are approved as monotherapy; capecitabine is approved as both monotherapy and combination therapy (with docetaxel); gemcitabine is approved as combination therapy only (with paclitaxel); and ixabepilone is approved in the U.S. as both monotherapy and combination therapy (with capecitabine).
4
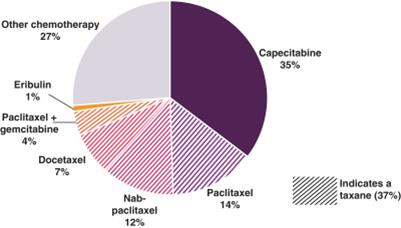
The choice and sequencing of chemotherapy regimens depend on a number of factors, including physician preference, previous therapies, pre-existing medical conditions, tumor burden and patient symptoms. As shown in the following chart, capecitabine, an oral chemotherapy and taxanes are the preferred first-line chemotherapy agents in HER2 negative, HR positive MBC.
Physician-reported Preferences for First-line Chemotherapy for Patients with HER2 Negative,
HR Positive MBC from Recent Survey of 201 U.S. Community-based Oncologists(1)
|
|
|
(1) Lin et al, Cancer Medicine 2016;5(2):209-220 |
Taxanes
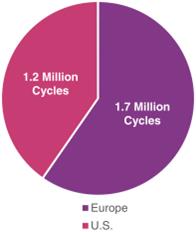
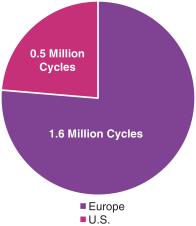
Taxanes are an established class of anticancer agents that are broadly used in various cancers, including breast cancer. Taxanes destroy cancer cells by preventing them from entering mitosis, a process of cell division, and thereby leading to apoptosis, or cell death. As shown in the figure below, taxanes are one of the most widely used classes of chemotherapy agents in both Europe and the U.S., with more than 2.8 million cycles administered in 2016 (Symphony Health Solutions 2016; IMS Health 2016).
>2.8 Million Cycles of Paclitaxel, Nab-paclitaxel and Docetaxel
Administered in 2016 in Europe and the U.S.(1)
|
|
|
(1) Symphony Health Solutions 2016; IMS Health 2016 |
5
While paclitaxel and docetaxel, the first two taxanes approved for the treatment of breast cancer, possess robust antitumor activity, they have low oral bioavailability and low solubility. Therefore, these pharmaceutical agents must be delivered intravenously, typically at an infusion center, and also are formulated with solubilizing agents that are known to cause hypersensitivity reactions. Nab-paclitaxel, a different formulation of paclitaxel that also is approved for the treatment of breast cancer, has a greatly reduced risk of hypersensitivity reactions, but must still be delivered intravenously.
Therapies given intravenously at an infusion center often are associated with:
|
• Fear of needles and complications associated with venous access; • Anxiety, including institutional-triggered side effects such as nausea and vomiting; • Heightened awareness of life-threatening disease presence; and • Disruption of daily activities. |
|
|
Source: Gornas et al, European Journal of Cancer Care 2010;19(1):131-136; Schott et al, BMC Cancer 2011;11:129 |
|
Capecitabine
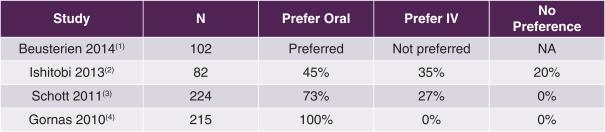
As the only orally administered chemotherapy routinely used for MBC in the U.S., capecitabine offers quality-of-life advantages over intravenous (“IV”) chemotherapy alternatives. In each of the 4 following recently published studies in which breast cancer patients were surveyed as to their preference of treatment modality, the authors concluded that patients preferred oral over IV chemotherapy.
Studies of Breast Cancer Patients’ Preference of Oral vs. IV Chemotherapy
|
|
|
(1) Beusterien et al, The Oncologist 2014;19(2):127-134 (2) Ishitobi et al, Patient Preference and Adherence 2013;7:1201-1206 (3) Schott et al, BMC Cancer 2011;11:129 (4) Gornas et al, European Journal of Cancer Care 2010;19(1):131-136 |
6
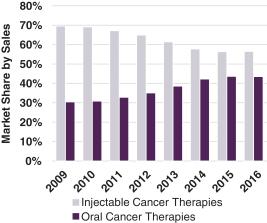
Overall, as shown in the figures that follow, the sales of oral therapies in the treatment of cancer have grown at a higher rate than the sales of injectable therapies over the past 7 years, and capecitabine is one of the most widely used chemotherapy agents in both Europe and the U.S., with more than 2.0 million cycles administered in 2016 (Symphony Health Solutions 2016; IMS Health 2016).
|
U.S. Market Share of Oral vs. Injectable Cancer Therapies(1) |
>2.0 Million Cycles of Capecitabine, an Oral Chemotherapy Agent, Administered in 2016 in Europe and the U.S.(1) |
|
|
|
|
|
|
|
(1) Symphony Health Solutions 2016; IMS Health 2016 |
|
7
Tesetaxel: A Chemotherapy with Potential Best-in-Class Properties
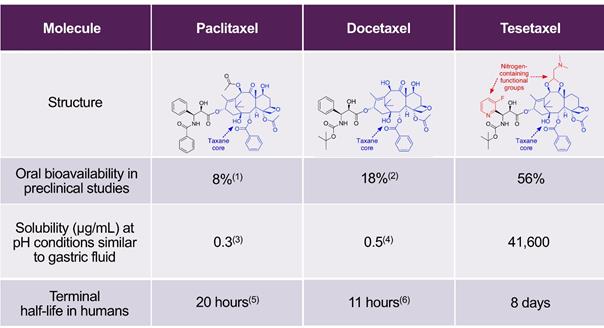
Tesetaxel, which we believe will qualify as a New Chemical Entity (“NCE”) if and when a New Drug Application (“NDA”) is submitted, retains the same taxane core as the approved taxanes, but includes the addition of two novel, nitrogen-containing functional groups. Tesetaxel is chemically designed to have high oral bioavailability, high solubility and a long terminal half-life and to not be expelled by the P-glycoprotein (“P-gp”) efflux pump, with the intent of retaining activity against chemotherapy-resistant tumor cells. The table below compares some of the chemical and pharmacologic properties of paclitaxel, docetaxel and tesetaxel.
Chemical and Pharmacologic Properties of Paclitaxel, Docetaxel and Tesetaxel
|
|
|
(1) Shanmugam et al, Drug Development and Industrial Pharmacy 2015;41(11):1864-1876 (2) McEntee et al, Veterinary and Comparative Oncology 2003;1(2):105-112 (3) Montaseri, Taxol: Solubility, Stability and Bioavailability 1997 (4) Bharate et al, Bioorganic & Medicinal Chemistry Letters 2015;25(7):1561-1567 (5) Taxol (paclitaxel) prescribing label (6) Taxotere (docetaxel) prescribing label Disclaimer: Table is provided for illustrative purposes and not for a direct comparison. |
8
We believe that tesetaxel’s unique properties may translate into significant benefits for patients. These may include:
|
|
• |
A formulation that does not contain polyoxyethylated castor oil or polysorbate 80, solubilizing agents contained in other taxane formulations known to cause hypersensitivity reactions; and |
|
|
• |
Durable antitumor activity. |
Preclinical Studies
Tesetaxel has exhibited potent antitumor activity in both in vitro (in a test tube) and in vivo (in a live organism) preclinical studies (Shionoya et al, Cancer Science 2003;94(5):459-466). Unique among taxanes, tesetaxel retains potent antitumor activity against chemotherapy-resistant tumor cells, including tumor cells over-expressing the P-gp efflux pump. A defense mechanism of tumor cells, this efflux pump functions to expel toxins, including many chemotherapy agents.
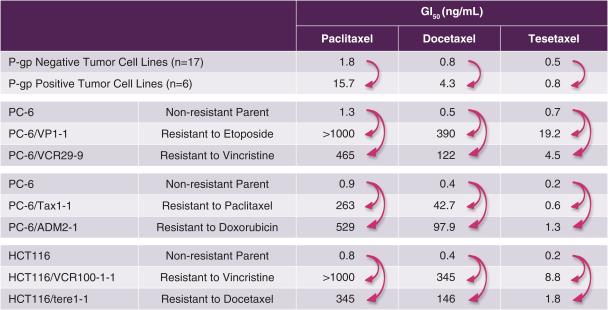
In Vitro Antitumor Activity
Tesetaxel has exhibited potent antitumor activity in in vitro preclinical studies, with an overall GI50 (the concentration of drug required to inhibit growth by 50%) of less than 1 nM. GI50 is a commonly used preclinical measurement of antitumor potency; lower GI50 numbers connote higher potency (1 nM is 1/1,000th of 1 µM). Of particular note, tesetaxel largely retains antitumor cytotoxic (cell-killing) potency against taxane-resistant (P-gp positive) and other chemotherapy-resistant tumors, while paclitaxel and docetaxel lose considerable antitumor potency (Shionoya et al, Cancer Science 2003;94(5):459-466). The relative loss of cytotoxic potency between P-gp negative and P-gp positive tumor cells and between non-drug-resistant and drug-resistant tumor cells for paclitaxel, docetaxel and tesetaxel is shown in the following table (low numbers connote high potency, and high numbers connote low potency).
In Vitro Cytotoxic Potency of Paclitaxel, Docetaxel and Tesetaxel in
P-gp Negative, P-gp Positive, Non-drug-resistant and Drug-resistant Tumors(1)
|
|
|
(1) Shionoya et al, Cancer Science 2003;94(5):459-466 |
9
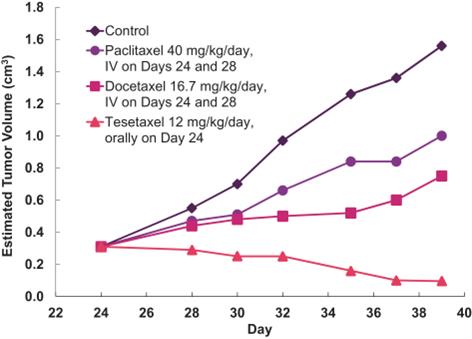
Tesetaxel administered orally exhibited significantly greater growth-inhibitory effects (inhibition rate (“IR”) > 90%) than paclitaxel and docetaxel administered IV (IR values of 26%-58%) in a mouse model in which P-gp positive, human breast tumors (DU4475) were implanted in mice (Shionoya et al, Cancer Science 2003;94(5):459-466). The relative in vivo antitumor activity for a control, paclitaxel, docetaxel and tesetaxel is shown in the following graph.
In Vivo Antitumor Activity of Paclitaxel, Docetaxel and Tesetaxel in
P-gp Positive Breast Tumors in Mice(1)
|
|
|
(1) Shionoya et al, Cancer Science 2003;94(5):459-466 |
10
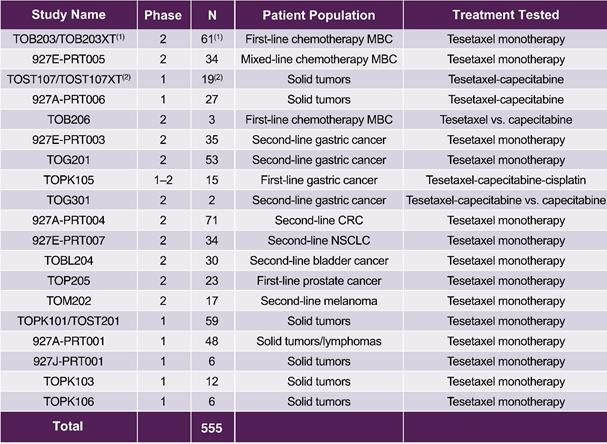
More than 500 patients have been treated with tesetaxel across 22 clinical studies. Tesetaxel was administered as monotherapy in 17 studies and in combination with other agents in 5 studies. Studies have been completed in MBC, gastric cancer, colorectal cancer, non-small cell lung cancer and other cancers as first-line, second-line or salvage therapy. Clinical studies that have been conducted with tesetaxel are shown in the following table.
Clinical Studies of Tesetaxel
|
|
|
N = Number of patients treated CRC = Colorectal cancer NSCLC = Non-small cell lung cancer (1) Includes 46 patients who received tesetaxel once every 21 days. A cohort of 15 patients receiving tesetaxel weekly (days 1, 8 and 15 of a 28-day cycle) was discontinued early due to study termination. In this Annual Report on Form 10-K, Studies TOB203 and TOB203XT are referred to as Study TOB203. (2) The Phase 1 study in solid tumors was split into Study TOST107, which included data from the first two cycles, and TOST107XT, which included data from patients receiving three or more cycles. In this Annual Report on Form 10-K, Studies TOST107 and TOST107XT are referred to as Study TOST107. |
11
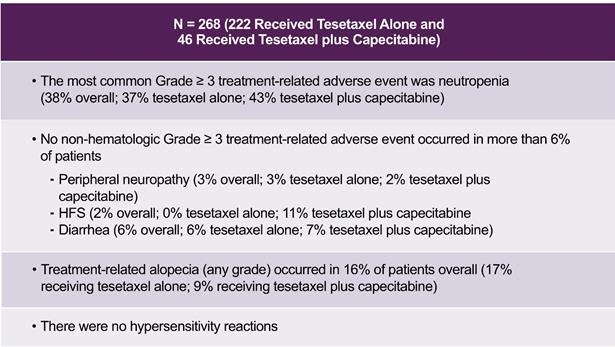
Tesetaxel, administered both alone and in combination with capecitabine, has been generally well tolerated. In the 8 studies (927A-PRT001, 927A-PRT004, 927E-PRT003, 927E-PRT005, 927E-PRT007, 927A-PRT006, TOST107 and TOST107XT) for which final study data are available, a total of 268 patients received tesetaxel either alone (222 patients from 5 studies) or in combination with capecitabine (46 patients from three studies). The most common Grade ≥ 3 (severe or serious) treatment-related adverse event (“AE”) was neutropenia (low level of neutrophils, a type of white blood cell), which occurred in 37% of patients receiving tesetaxel alone and 43% of patients receiving tesetaxel in combination with capecitabine and was generally reversible and manageable with supportive measures. Six percent (6%) of patients receiving tesetaxel alone and 11% of patients receiving tesetaxel in combination with capecitabine experienced treatment-related febrile neutropenia (fever coinciding with neutropenia).
Overall, there was no non-hematologic Grade ≥ 3 treatment-related AE that occurred in more than 6% of patients. Three percent (3%) of patients receiving tesetaxel alone and 2% of patients receiving tesetaxel in combination with capecitabine experienced Grade ≥ 3 treatment-related peripheral neuropathy (weakness, numbness and/or pain from damage to the nerves). No patients receiving tesetaxel alone and 11% of patients receiving tesetaxel in combination with capecitabine experienced Grade ≥ 3 treatment-related hand-foot syndrome (redness and swelling of the palms and soles, which may progress to dryness, scaling, pain, itching and sometimes blisters and ulceration). Six percent (6%) of patients receiving tesetaxel alone and 7% of patients receiving tesetaxel in combination with capecitabine experienced Grade ≥ 3 treatment-related diarrhea. Seventeen percent (17%) of patients receiving tesetaxel alone and 9% of patients receiving tesetaxel in combination with capecitabine experienced any grade of treatment-related alopecia (hair loss). Tesetaxel’s adverse event profile across all completed studies is shown in the following table.
Adverse Event Profile Across All Completed Studies(1)
|
|
|
(1) 927A-PRT001, 927A-PRT004, 927E-PRT003, 927E-PRT005, 927E-PRT007, 927A-PRT006, TOST107 and TOST107XT |
The results from the two Phase 2 clinical studies of tesetaxel monotherapy in MBC (TOB203 and 927E-PRT005) are summarized below.
12
Study TOB203: A Phase 2 Study of Tesetaxel as First-line Chemotherapy for MBC
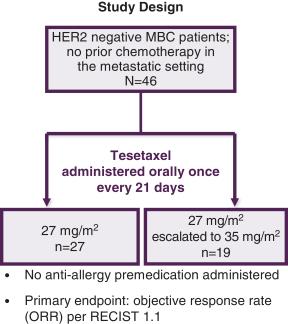
In Study TOB203, 46 patients with HER2 negative, HR positive or negative MBC were enrolled to receive, as first-line chemotherapy, tesetaxel administered orally at 27 mg/m2 (of body surface area: an average female cancer patient is approximately 1.8 m2) on the first day of a 21-day cycle, with escalation to 35 mg/m2 in subsequent cycles depending on tolerability, without anti-allergy premedication. Objective response rate (“ORR”) (complete response (disappearance of all target lesions) + partial response (at least a 30% decrease in the sum of the diameters of target lesions)) based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was the primary endpoint.
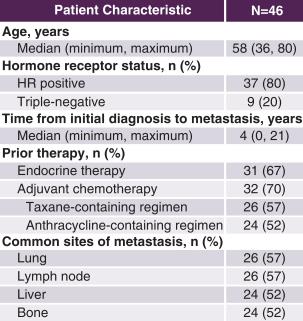
Median age was 58 years (range: 36-80 years). Twenty percent (20%) of patients had triple-negative disease, and the median time from initial diagnosis was 4 years (range: 0-21 years). Sixty-seven percent (67%) of patients received prior endocrine therapy, and 70% had received prior chemotherapy in the adjuvant setting, with 57% having received a taxane-containing regimen and 52% having received an anthracycline-containing regimen. Common metastatic sites included the lung and lymph nodes (57% of patients each) and the liver and bone (52% each). The figures that follow show the study design and patient characteristics.
|
|
|
13
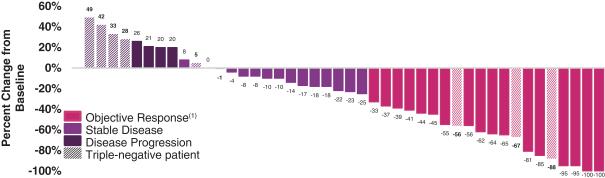
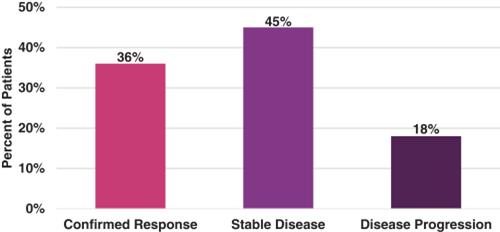
Forty-four (44) of 46 patients received at least one tumor scan and, therefore, were evaluable for response. The unconfirmed (based on a single scan) ORR was 45% (20 of 44 patients), and the confirmed (based on two tumor scans at least 4 weeks apart in time) ORR was 36% (16 of 44 patients). Median PFS was 5.8 months. Patient best response (confirmed) is shown in the following figure.
TOB203: Best Response
Antitumor activity by individual patient is shown in the following figure.
TOB203: Maximum Tumor Volume Change from Baseline in Target Lesions
|
|
|
(1) 16 confirmed; 4 unconfirmed |
14
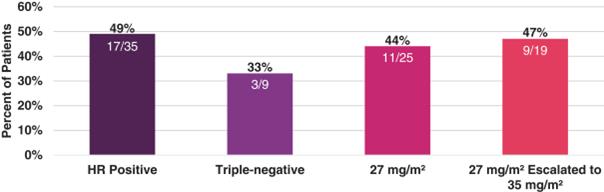
Unconfirmed ORR by patient subgroup is shown in the following figure.
TOB203: Unconfirmed ORR by Patient Subgroup
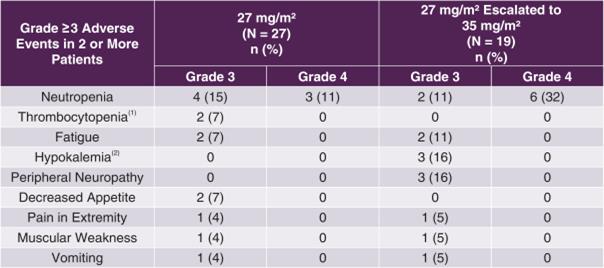
Tesetaxel was generally well tolerated. The starting tesetaxel dose (27 mg/m2) was escalated to 35 mg/m2 in 19 of 46 patients. CONTESSA, our Phase 3 study, is using a dose of 27 mg/m2. The most common Grade ≥ 3 AE was neutropenia, which was more common in the escalated dose (26% of patients in the non-escalated 27 mg/m2 dose group and 42% in the 27 mg/m2 escalated to 35 mg/m2 dose group). The incidence of Grade ≥ 3 febrile neutropenia was 4%. There was no Grade ≥ 3 peripheral neuropathy observed in the non-escalated 27 mg/m2 dose group. The incidence of Grade 2 alopecia (significant hair loss) was 15% and similar in both dose groups. There were no hypersensitivity reactions. A summary of Grade ≥ 3 AEs is shown in the following table.
TOB203: Grade ≥ 3 Adverse Events
|
|
|
(1) Low level of platelets, a type of blood cell (2) Low level of potassium |
15
Study 927E-PRT005: A Phase 2 Study of Tesetaxel as Mixed-line Chemotherapy for MBC
In Study 927E-PRT005, 34 patients with MBC were enrolled to receive, as first-, second- or third-line chemotherapy, tesetaxel administered orally at initial doses of 27 mg/m2 (79% of patients) or 35 mg/m2 (21% of patients) on the first day of a 21-day cycle. Median age was 52 years (range: 32-80 years), and median time from initial diagnosis of breast cancer was 3.5 years (range: 1-19 years).
Thirty-two percent (32%) of patients had no prior chemotherapy for advanced disease, 65% had one prior chemotherapy regimen for advanced disease, and 3% had two prior chemotherapy regimens for advanced disease. All patients received an anthracycline-based regimen in the adjuvant or metastatic setting. Common metastatic sites included the liver (59% of patients), bone (44%), lymph nodes (26%) and lung (21%).
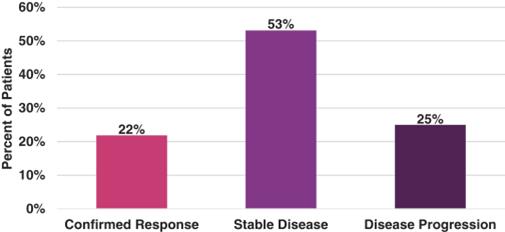
Thirty-two (32) patients completed at least one cycle of therapy and were included in the efficacy population. The unconfirmed ORR was 38% (12 of 32 patients), and the confirmed ORR was 22% (7 of 32 patients). Median time-to-progression was 3.4 months. Patient best response (confirmed) is shown in the following figure.
927E-PRT005: Best Response
16
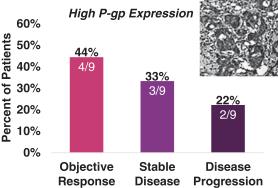
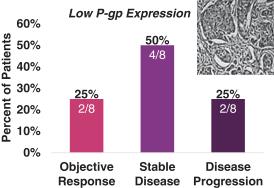
Seventeen (17) patients for which primary tumor biopsies were evaluated by immunohistochemistry staining were categorized according to their level of P-gp expression. Tumors with high levels of P-gp expression are generally associated with taxane resistance. Response (unconfirmed) by level of P-gp expression for these patients is shown in the following figure.
927E-PRT005: Response by Level of P-gp Expression
|
|
|
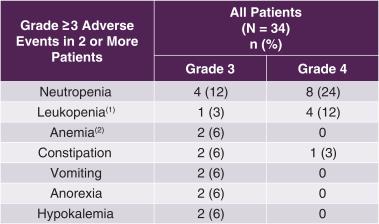
Tesetaxel was generally well tolerated. The most common Grade ≥ 3 AE was neutropenia (35% of patients). The incidence of Grade ≥ 3 febrile neutropenia was 3%, the incidence of Grade ≥ 3 peripheral sensory neuropathy (numbness and/or pain from damage to the nerves) was 3%, and the incidence of Grade 2 alopecia was 18%. There were no hypersensitivity reactions. A summary of Grade ≥ 3 AEs is shown in the following table.
927E-PRT005: Grade ≥ 3 Adverse Events
|
|
|
(1) Low level of leukocytes, a type of white blood cell (2) Low level of red blood cells |
17
Tesetaxel Efficacy, Tolerability and Dosing Regimen as Compared to Available Chemotherapies
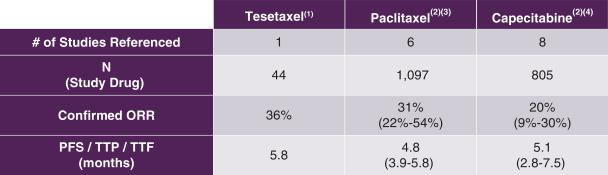
Tesetaxel as a single agent has demonstrated robust antitumor activity in MBC. The antitumor activity of tesetaxel in Study TOB203 as compared to that of paclitaxel and capecitabine as observed in first-line MBC studies is shown in the following table.
Efficacy Measures of Tesetaxel, Paclitaxel and Capecitabine in the
First-line Chemotherapy Treatment of MBC
|
|
|
ORR = Objective response rate; PFS = Progression-free survival; TTP = Time to progression; TTF = Time to treatment failure (1) Seidman et al, 2012 American Society of Clinical Oncology (ASCO) Annual Meeting (2) Randomized, multicenter studies in the first-line chemotherapy treatment of MBC (3) Albain et al, Journal of Clinical Oncology 2008;26(24):3950-3957; Bishop et al, Journal of Clinical Oncology 1999;17(8)2355-2364; Gradishar et al, European Journal of Cancer 2013;49(2):312-322; Gradishar et al, Journal of Clinical Oncology 2005;23(31)7794-7803; Gray et al, Journal of Clinical Oncology 2009;27(30):4966-4972; Paridaens et al, Journal of Clinical Oncology 2000;18(4):724-733 (4) Baselga et al, Journal of Clinical Oncology 2012;30(13):1484-1491; Goldstein et al, 2013 American Society of Clinical Oncology (ASCO) Annual Meeting; Harbeck et al, Breast Cancer Research and Treatment 2017;161:63-72; O’Shaughnessy et al, Annals of Oncology 2001;12:1247-1254; Robert et al, Journal of Clinical Oncology 2011;29(10):1252-1260; Stockler et al, Journal of Clinical Oncology 2011;29(34):4498-4504; Twelves et al, Breast Cancer: Basic and Clinical Research 2016;10:77-84; Vahdat et al, Cancer Research 2009;69(2):Supplement Disclaimer: Table is provided for illustrative purposes and not for a direct comparison. |
18
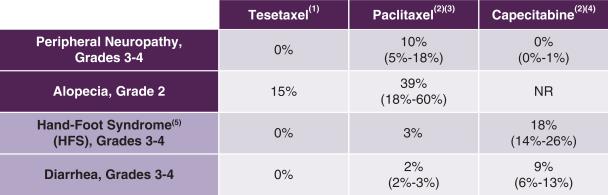
Tesetaxel has been generally well tolerated in clinical studies. The incidence of certain adverse events observed with tesetaxel as compared to those observed with paclitaxel and capecitabine in first-line MBC studies is shown in the following table.
Tolerability of Tesetaxel, Paclitaxel and Capecitabine in the
First-line Chemotherapy Treatment of MBC
|
|
|
NR = Not reported (1) Study TOB203 and, for HFS, an internal report of treatment-related AEs in 222 patients receiving tesetaxel monotherapy (2) Randomized, multicenter studies in the first-line chemotherapy treatment of MBC (3) Albain et al, Journal of Clinical Oncology 2008; Bishop et al, Journal of Clinical Oncology 1999; Gradishar et al, European Journal of Cancer 2013; Gray et al, Journal of Clinical Oncology 2009; Paridaens et al, Journal of Clinical Oncology 2000 (4) Harbeck et al, Breast Cancer Research and Treatment 2016; O’Shaughnessy et al, Annals of Oncology 2001; Robert et al, Journal of Clinical Oncology 2011; Stockler et al, Journal of Clinical Oncology 2011 (5) Redness and swelling of the palms and soles, which may progress to dryness, scaling, pain, itching and sometimes blisters and ulcerations Disclaimer: Table is provided for illustrative purposes and not for a direct comparison. |
19
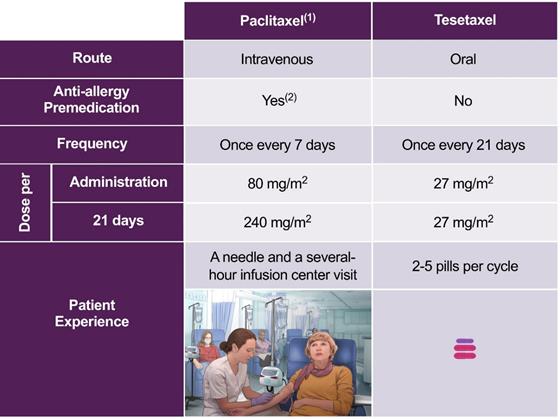
We believe that an all-oral regimen that may delay the need to receive intravenous chemotherapy in an infusion center will be preferred by patients. Paclitaxel, the most commonly used taxane in the treatment of MBC, generally requires weekly, several-hour visits to an infusion center, where patients must be pretreated with a corticosteroid, an antihistamine and an H2 antagonist prior to receiving their chemotherapy intravenously. By contrast, tesetaxel monotherapy or tesetaxel plus capecitabine are all-oral regimens that do not require administration in an infusion center.
The dosing regimens and patient experience for paclitaxel and tesetaxel are shown in the following table.
Dosing Regimens for Paclitaxel and Tesetaxel
|
|
|
(1) National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology 2017 (2) Corticosteroid plus antihistamine plus H2 antagonist as per prescribing label |
20
CONTESSA: A Multinational, Multicenter, Randomized, Phase 3 Study of Tesetaxel in MBC
We are conducting a 600-patient, multinational, multicenter, randomized, Phase 3 study, known as CONTESSA, that will compare tesetaxel (27 mg/m2 on the first day of a 21-day cycle) plus a reduced dose of capecitabine (1,650 mg/m2/day on days 1-14 of a 21-day cycle) to the approved dose of capecitabine alone (2,500 mg/m2/day on days 1-14 of a 21-day cycle) in patients with HER2 negative, HR positive MBC previously treated with a taxane in the neoadjuvant (prior to surgery) or adjuvant (immediately following surgery) setting. Where indicated, patients must have received endocrine therapy with or without a CDK 4/6 inhibitor. CONTESSA’s primary endpoint is PFS assessed by an Independent Radiologic Review Committee (“IRC”). The study is designed (with 90% statistical power) to detect a 42% improvement in PFS (hazard ratio (the ratio of the rates of occurrence of the endpoint events in the two groups of the study) = 0.71; median PFS 8.5 vs. 6.0 months). CONTESSA’s secondary endpoints are overall survival, ORR assessed by IRC, disease control rate (ORR + prolonged (≥24 weeks) stable disease) assessed by IRC and patient reported outcomes (“PROs”).
CONTESSA Study Design

In designing CONTESSA, we received non-binding advice from both the U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”). We believe CONTESSA may serve as a single pivotal study sufficient for product registration, provided that the study demonstrates a statistically significant and clinically meaningful improvement in the primary endpoint, PFS, for tesetaxel plus a reduced dose of capecitabine as compared to the approved dose of capecitabine alone as well as an overall favorable benefit-risk profile for the tesetaxel plus a reduced dose of capecitabine regimen. Generally, a single pivotal study can be sufficient for FDA approval only when the study provides highly reliable and statistically strong evidence of an important clinical benefit and in which confirmation of the result in a second clinical trial would be practically or ethically impossible. There can be no assurance that the outcome of CONTESSA will be sufficient for the approval of tesetaxel by the FDA, EMA or other regulatory agencies or that tesetaxel will be approved at all.
21
Rationale for CONTESSA Study Design
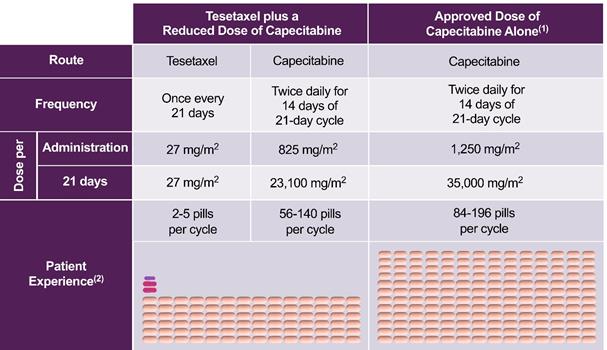
CONTESSA is designed to evaluate whether tesetaxel plus a reduced dose of capecitabine results in improved PFS with manageable toxicity and favorable quality-of-life compared to the approved dose of capecitabine alone. Tesetaxel plus a reduced dose of capecitabine incorporates two agents with synergistic mechanisms of action and is an all-oral regimen that offers a significant pill-burden reduction compared to the approved dose of capecitabine alone.
CONTESSA: Tesetaxel Arm Offers Significant Pill Burden Reduction
|
|
|
(1) Xeloda (capecitabine) prescribing label (2) Illustration for patients with body surface area of 1.78-1.90m2 |
22
Our rationale for the CONTESSA study design includes the following points:
|
|
• |
Capecitabine is a preferred agent as a first- or second-line chemotherapy treatment for patients with HER2 negative, HR positive MBC. Therefore, capecitabine, at the approved dose, is an appropriate control regimen for a registration-enabling Phase 3 study. |
|
|
• |
There is a high unmet medical need for combination chemotherapy regimens with improved benefit-risk profiles. |
|
|
• |
Combining the approved dose of capecitabine with currently available taxanes results in robust efficacy but with significant toxicity. |
|
|
• |
Preclinical and clinical studies support investigating whether reducing the dose of capecitabine in combination with a taxane will reduce toxicity without a reduction in efficacy. |
|
|
• |
Single-agent tesetaxel has demonstrated antitumor activity in two Phase 2 studies in MBC: TOB203 and 927E-PRT005. |
|
|
• |
In a Phase 1 study (TOST107), the combination of tesetaxel plus a reduced dose of capecitabine was associated with a tolerable AE profile, with minimal overlapping toxicity. |
Capecitabine as an Appropriate Control Regimen
Capecitabine is a preferred agent as a first- or second-line chemotherapy treatment for patients with HER2 negative, HR positive MBC, particularly those previously treated with a taxane in the neoadjuvant, adjuvant or advanced setting. Therefore, capecitabine, at the approved dose, is an appropriate control regimen for a registration-enabling Phase 3 study. The FDA- and EMA-approved capecitabine regimen is 2,500 mg/m2 on Days 1-14 of a 21-day cycle.
Medical Need for Combination Chemotherapy Regimens with Improved Benefit-risk Profiles
To date, combination chemotherapy regimens generally have not demonstrated superior benefit-risk profiles as compared to single-agent, sequential chemotherapy. Specifically, while currently available combination regimens have been associated with increased PFS and, in the case of docetaxel-capecitabine, increased overall survival, they also have been associated with significantly increased toxicity. As a result, single-agent, sequential chemotherapy remains the standard of care for many patients.
Nonetheless, there remains a large unmet need for chemotherapy regimens, including combination regimens, with improved benefit-risk profiles. In particular, newer combinations that preserve the response rates and PFS of currently available combination regimens, but are better tolerated and easier to take, are needed.
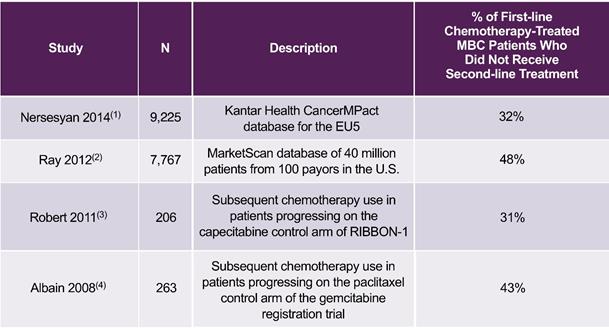
An important reason why there is a need for improved combination chemotherapy regimens is the fact that approximately one-third or more of MBC patients do not receive second-line chemotherapy after progressing on first-line chemotherapy, as shown in the following table. Since these patients, who are not necessarily identifiable when first-line chemotherapy is initiated, do not receive second- or later-line chemotherapy, they could benefit from first-line regimens that can significantly increase PFS with minimal increase in toxicity, including regimens that incorporate two therapeutic agents.
23
Percentage of MBC Patients Who Did Not Receive Additional Chemotherapy after
Progressing on First-line Chemotherapy Treatment
|
|
|
EU5 = European Union 5 (France, Germany, Italy, Spain and the United Kingdom) (1) Nersesyan et al, 2014 ISPOR 17th Annual European Congress (2) Ray et al, Journal of Clinical Oncology 2012;30(27):116 (3) Robert et al, Journal of Clinical Oncology 2011;29(10):1252-1260 (4) Albain et al, Journal of Clinical Oncology 2008;26(24):3950-3957 |
24
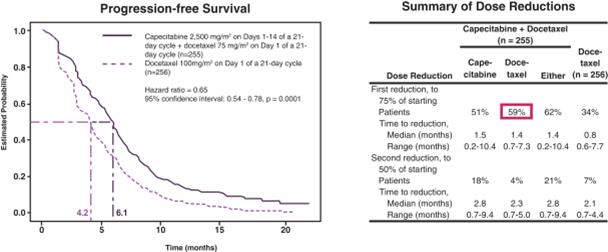
Combining the Approved Dose of Capecitabine with Docetaxel, an Approved Taxane
In a multicenter, randomized Phase 3 study in 511 patients receiving first-, second- or third-line chemotherapy that served as the basis for approval for capecitabine combined with docetaxel in the treatment of MBC, capecitabine at the approved dose of 2,500 mg/m2 (1,250 mg/m2 BID) on Days 1-14 of a 21-day cycle combined with docetaxel resulted in superior time to disease progression (“TTP”) (hazard ratio = 0.65, 95% confidence interval: 0.54-0.78, p=0.0001) and overall survival (hazard ratio = 0.78, 95% confidence interval: 0.63-0.95, p=0.01) as compared to docetaxel alone. However, there was significant drug-related toxicity on the combination regimen, resulting in a large percentage of dose reductions and discontinuations (59% of patients reduced their dose of docetaxel and 51% reduced their dose of capecitabine) (O'Shaughnessy et al, Journal of Clinical Oncology 2002;20(12):2812-2823). The results of this Phase 3 study are as follows.
A Phase 3 Study Evaluating the Combination of the Approved Dose of Capecitabine and
Docetaxel, an Approved Taxane, in the Treatment of MBC (1)
|
|
|
(1) O’Shaughnessy et al, Journal of Clinical Oncology 2002;20(12):2812-2823 |
25
Reducing the Dose of Capecitabine in Combination with a Taxane
Synergy when Combining a Taxane with Capecitabine in Preclinical Studies
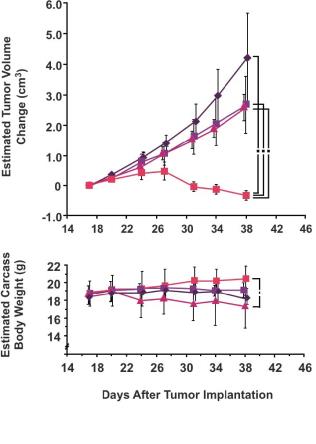
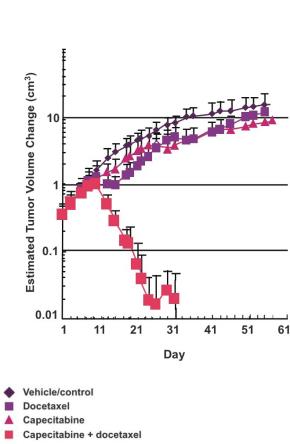
Preclinical studies have shown synergy when combining a taxane with capecitabine. Taxanes up-regulate tumor levels of thymidine phosphorylase, the enzyme essential for the activation of capecitabine. Specifically, in two in vivo preclinical studies of breast cancer, the combined administration of capecitabine and docetaxel resulted in antitumor efficacy significantly greater than the sum of the efficacy resulting from either agent administered as monotherapy (see the following figures). Furthermore, the synergy may be tumor-specific, as toxicity as measured by weight loss and effect on peripheral blood cells was minimal. These studies suggest the potential to reduce the dose of capecitabine without loss of efficacy.
Synergy with Taxane-Capecitabine Combinations
|
|
Capecitabine at 1/2 MTD + Docetaxel at 1/8 MTD(1)(2)
|
Capecitabine at 2/3 MTD + Docetaxel at 1/15 MTD(3)(4) |
|
|
|
|
|
|
MTD = Maximum tolerated dose (1) Sawada et al, Clinical Cancer Research 1998;4:1013-1019 (2) Capecitabine dosed 5 times every 7 days; docetaxel dosed once every 7 days (3) Fujimoto-Ouchi et al, Clinical Cancer Research 2001;7(4):1079-1086 (4) Capecitabine dosed on Days 1-14 and 22-36; docetaxel dosed on Days 8 and 29 |
|
26
Clinical Studies Evaluating Lower Doses of Capecitabine Combined with a Taxane
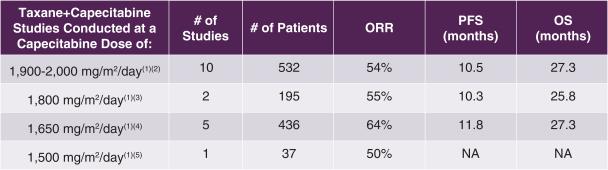
Consistent with preclinical findings of synergy between taxanes and capecitabine, clinical studies support investigating the combination of a taxane with a reduced dose of capecitabine such as 1,650 mg/m2/day on the first 14 days of a 21-day cycle, the dose of capecitabine chosen for combination with tesetaxel in CONTESSA. In a review of 18 first-line MBC studies of taxane plus capecitabine combinations shown in the following table, there was no apparent loss of efficacy when comparing capecitabine at 1,650 mg/m2/day to capecitabine at 2,000 mg/m2/day (on the first 14 days of a 21-day cycle). Among these studies, the capecitabine 1,650 mg/m2/day dose was the most studied dose less than 2,000 mg/m2/day (5/8 studies). According to Lortholary, the trend toward improved efficacy with lower doses of capecitabine may result from the significantly lower proportion of patients discontinuing study therapy prematurely because of toxicity, and highlights the importance of administering capecitabine using a schedule that optimizes dose intensity and tolerability (Lortholary et al, Breast Cancer Research and Treatment 2012;131:127-135).
Clinical Studies Evaluating the Combination of a Taxane with Different Doses of Capecitabine as
First-line Chemotherapy in the Treatment of MBC
|
|
|
PFS = Progression-free survival; ORR = Objective response rate; OS = Overall survival (1) Days 1-14 of a 21-day cycle (2) Bachelot et al, Oncology 2011;80(3-4):262-268; Campone et al, The Breast Journal 2013;19(3):240-249; Chitapanarux et al, Asia-Pacific Journal of Clinical Oncology 2012;8:76-82; Fan et al, Annals of Oncology 2013;24:1219-1225; Liao et al, Chemotherapy 2013;59:207-213; Michalaki et al, Anti-Cancer Drugs 2009;20(3):204-207; Michalaki et al, Anticancer Research 2010;30:3051-3054; Venturini et al, Cancer 2003;97(5):1174-1180; Wang et al, Cancer 2015;121:3412; Wardley et al, Journal of Clinical Oncology 2010;28(6):976-983 (3) Bisagni et al, Cancer Chemotherapy and Pharmacology 2013;71(4):1051-1057; Luck et al, Breast Cancer Research and Treatment 2015;149:141-149 (4) Hatschek et al, Breast Cancer Research and Treatment 2012;131(3):939-947; Lam et al, European Journal of Cancer 2014;50(18):3077-3088; Perez et al, Annals of Oncology 2010;21(2):269-274; Schwartzberg et al, Clinical Breast Cancer 2012;12(2):87-93; Tonyali et al, Journal of Cancer Research and Clinical Oncology 2013;139(6):981-986 (5) Silva et al, Clinical Breast Cancer 2008;8(2):162-167 |
Tesetaxel Plus a Reduced Dose of Capecitabine Generally Well Tolerated in Phase 1 Study (TOST107)
In a Phase 1 study (TOST107), the safety and tolerability of tesetaxel plus a reduced dose of capecitabine was evaluated in patients with advanced solid tumors. Eight (8) patients received tesetaxel at 27 mg/m2 orally on the first day of each 21-day cycle plus capecitabine at 1,750 mg/m2/day orally on days 1-14 of each 21-day cycle, and 9 patients received tesetaxel at 27 mg/m2 orally on the first day of each 21-day cycle plus capecitabine at 2,000 mg/m2/day orally on Days 1-14 of each 21-day cycle.
27
Tesetaxel in combination with capecitabine at either 1,750 mg/m2/day or 2,000 mg/m2/day was generally well tolerated with no indication of overlapping toxicity. The most common Grade ≥ 3 AE was neutropenia (47% of patients), which was reversible and manageable with supportive measures. There was a low rate of febrile neutropenia (6% of patients), which only occurred in the tesetaxel 27 mg/m2 plus capecitabine 2,000 mg/m2/day group. The incidence of Grade ≥ 3 peripheral neuropathy was 6%, Grade ≥ 3 hand-foot syndrome was 6%, and Grade ≥ 3 diarrhea was 6%. There was no Grade 2 alopecia, and there were no hypersensitivity reactions.
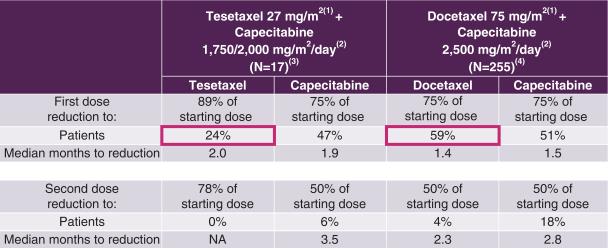
In response to drug-related side effects, physicians often reduce the dose of chemotherapy received by cancer patients. This reduction, while improving tolerability, can compromise the efficacy of the treatment. The frequency and extent of dose reductions in Study TOST107 as compared to those in the Phase 3 study that served as the basis for approval for capecitabine combined with docetaxel in the treatment of MBC are shown in the following table.
Dose Reductions for Tesetaxel plus Reduced Dose of Capecitabine
Compared to Those for Docetaxel plus the Approved Dose of Capecitabine
|
|
|
(1) Day 1 of a 21-day cycle (2) Days 1-14 of a 21-day cycle (3) TOST107/107XT (4) O’Shaughnessy et al, Journal of Clinical Oncology 2002;20(12):2812-2823 |
In summary, we believe that the data support the investigation of tesetaxel at 27 mg/m2 on the first day of a 21-day cycle plus capecitabine at 1,650 mg/m2/day on the first 14 days of a 21-day cycle as a novel, all-oral regimen with a potentially favorable benefit-risk profile for the treatment of patients with HER2 negative, HR positive MBC.
28
Studies in Patients with Other Forms of Cancer
In three studies between 2004 and 2006, tesetaxel as a single agent exhibited antitumor activity in gastric cancer, colorectal cancer (“CRC”) and non-small cell lung cancer (“NSCLC”).
|
|
• |
Study 927E-PRT003 was a Phase 2 study in which 35 patients with advanced or metastatic gastric cancer who had failed one previous chemotherapy regimen received single-agent tesetaxel. In this study, the confirmed ORR (as measured by RECIST 1.0), which was the primary endpoint, was 19%. |
|
|
• |
Study 927A-PRT004 was a Phase 2 study in which 71 patients with progressive, locally advanced or metastatic CRC received single-agent tesetaxel. In patients who had failed one previous chemotherapy regimen, the confirmed ORR (as measured by RECIST 1.0), which was the primary endpoint, was 10%. |
|
|
• |
Study 927E-PRT007 was a Phase 2 study in which 34 patients with locally advanced or metastatic NSCLC who had failed one previous chemotherapy regimen for advanced disease received single-agent tesetaxel. In this study, the confirmed ORR (as measured by RECIST 1.0), which was the primary endpoint, was 7%. |
Competition
The biotechnology and pharmaceutical industries are extremely competitive. Our potential competitors in the field are many in number and include major and mid-sized pharmaceutical and biotechnology companies. Many of our potential competitors have significantly more financial, technical and other resources than we do, which may give them a competitive advantage. In addition, they may have substantially more experience in effecting strategic combinations, in-licensing technology, developing drugs, obtaining regulatory approvals and manufacturing and marketing products. We cannot give any assurances that we can compete effectively with these other biotechnology and pharmaceutical companies. Any products that we may develop or discover will compete in highly competitive markets. Our potential competitors in these markets may succeed in developing products that could render our product candidates obsolete or non-competitive.
Tesetaxel faces significant competition. Multiple chemotherapies are currently available to physicians and patients for the treatment of HER2 negative, HR positive MBC. These include: paclitaxel, nab-paclitaxel and docetaxel (taxanes); capecitabine (a fluoropyrimidine); doxorubicin and epirubicin (anthracyclines); gemcitabine (a nucleoside inhibitor); ixabepilone (an epothilone that is approved in the U.S.); and eribulin (a non-taxane microtubule dynamics inhibitor). The taxanes and eribulin are approved as monotherapy; capecitabine is approved as both monotherapy and combination therapy (with docetaxel); gemcitabine is approved as combination therapy only (with paclitaxel); and ixabepilone is approved in the U.S. as both monotherapy and combination therapy (with capecitabine). In addition, there are novel chemotherapies in development, including new intravenous paclitaxel formulations, such as NantPharma’s Cynviloq and Sun Pharma’s Taclantis, and novel oral paclitaxel formulations, such as Athenex’s Oraxol and Daehwa Pharmaceutical’s DHP107. We believe that the extent to which tesetaxel is adopted by the marketplace, if it is approved, will depend on factors such as its safety and tolerability, efficacy, convenience, effect on quality-of-life and cost-effectiveness relative to other treatment alternatives.
Daiichi Sankyo License Agreement
In 2013, we licensed rights to tesetaxel in all major markets from Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), the original inventor of the product. Tesetaxel had previously been licensed to Genta Incorporated.
29
Under the Daiichi Sankyo license agreement, we currently hold exclusive rights to 15 issued patents covering tesetaxel. See “Business—Patents and Proprietary Rights.” We are obligated under the license agreement to use commercially reasonable efforts to develop and commercialize tesetaxel in the following countries: France, Germany, Italy, Spain, the United Kingdom and the U.S. We are required to make aggregate future milestone payments of up to $31.0 million, contingent on attainment of certain regulatory milestones, none of which have yet been achieved. Additionally, we will pay Daiichi Sankyo a tiered royalty that ranges from the low to high single digits, depending on annual net sales of tesetaxel. To date, no payments have been made to Daiichi Sankyo under the license agreement. The license agreement and accompanying royalty obligation terminate on a country-by-country basis on the last-to-expire patent in each such country, which we expect will be between 2026 and 2031 in the U.S., 2025 and 2030 in European countries and 2025 and 2030 in Japan, depending on the availability and application of patent term extensions.
NCE Exclusivity
We believe that tesetaxel will qualify as an NCE if and when an NDA is submitted. If tesetaxel qualifies as an NCE, we believe that NCE regulatory exclusivity, combined with our intellectual property, assuming the availability of 5 years of patent term restoration under the Hatch-Waxman Act, will provide exclusivity for tesetaxel in all major markets through at least 2031. Separate from patent protection, exclusivity refers to certain delays and prohibitions on approval of competitor drugs available under the statute that attach on approval of a drug.
Exclusivity in the U.S.
In the U.S., drugs approved by the FDA are eligible for regulatory exclusivity under the Federal Food, Drug, and Cosmetic Act (“FDCA”), which can delay the approval of generic competition by up to 7.5 years. Specifically, the FDCA provides a 5-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for an NCE. A drug is an NCE if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all of the data required for approval. However, an application may be submitted after 4 years if it contains a certification of patent invalidity or non-infringement. This certification will trigger an automatic 30-month stay in the approval of any generic competition, effectively extending the regulatory exclusivity period to 7.5 years.
NCE exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical studies necessary to demonstrate safety and effectiveness.
Exclusivity in Europe
In Europe, NCEs, sometimes referred to as new active substances, qualify for 8 years of data exclusivity upon marketing authorization and an additional two years of market exclusivity, for a total of 10 years of regulatory exclusivity. This exclusivity, if granted, prevents regulatory authorities in the EU from referring to the innovator’s data to assess a generic application for 8 years, after which generic marketing authorization can be submitted, and the innovator’s data may be referenced, but the generic product may not be approved for two years. This 10-year period can be extended to a maximum of 11 years if, during the first 8 years of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications that, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
30
However, even if a compound is considered to be an NCE and the sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the drug if such company can complete a full Marketing Authorization Application with a complete database of pharmaceutical tests, preclinical studies and clinical studies and obtain marketing approval of its product.
Exclusivity in Japan
In Japan, an NCE is eligible for at least 8 years of regulatory exclusivity. Specifically, under the Pharmaceutical Affairs Law, the regulatory authority re-examines the safety and efficacy of drugs after drug approval. The data submitted to the regulatory authority is not available to generic drug companies during the re-examination period. This effectively makes the re-examination system a regulatory exclusivity system in Japan. The re-examination period is 10 years following approval for an orphan drug and 8 years for an NCE. Innovators may also benefit from an additional 4- to 10-month waiting period for generic pricing approval. There may be an additional 4 years of market protection granted if a new indication for a drug is registered in the first 8 years of the re-examination period.
Patents and Proprietary Rights
The proprietary nature of, and protection for, our product candidates, processes and know-how are important to our business. Our success depends in part on our ability to protect the proprietary nature of our product candidates, technology and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing on our proprietary rights. We seek and maintain patent protection in the U.S. and internationally for our product candidates and other technology. We endeavor to patent or in-license technology, inventions and improvements that we consider important to the development of our business. In addition to patent protection, we intend to use other means to protect our proprietary rights, including pursuing terms of marketing or data exclusivity, orphan drug status (if applicable) and similar rights that are available under regulatory provisions in certain territories, including the U.S., Europe and Japan. We also rely on trade secrets, know-how and continuing innovation to develop and maintain our competitive position.
The intellectual property portfolio protecting our tesetaxel program includes 9 U.S., 4 European and 7 Japanese patents, as well as two pending U.S. patent applications and one pending European patent application. Of these, 5 U.S., 4 European and 6 Japanese patents are exclusively licensed to us by Daiichi Sankyo. The 20 issued patents consist of the following:
|
|
• |
Nine (9) U.S. patents, including: (i) two composition-of-matter patents expiring in 2020 and 2026; (ii) 4 method-of-manufacture patents expiring between 2023 and 2031; and (iii) three patents with composition-of-matter, method of manufacture and/or method of use claims expiring between 2031 and 2032, without taking into account any potential patent term restoration. |
|
|
• |
Four (4) European patents, including: (i) two composition-of-matter patents expiring in 2020 and 2022; and (ii) two method-of-manufacture patents expiring in 2022 and 2025, without taking into account any potential patent term restoration. |
|
|
• |
Seven (7) Japanese patents, including: (i) three composition-of-matter patents expiring between 2020 and 2022; (ii) three method-of-manufacture patents expiring between 2022 and 2025; and (iii) one patent with both composition-of-matter and method-of-manufacture claims expiring in 2031, without taking into account any potential patent term restoration. |
Among these patents, one issued U.S. composition-of-matter patent (U.S. Patent No. 7,410,980) covers the crystal form of tesetaxel used in our clinical formulation and will expire in 2026. If tesetaxel is approved by the FDA, we will be entitled to request patent term restoration that could extend the protection of this patent until 2031. The exact duration of the extension depends on the time we spend in clinical studies as well as the time the FDA spends reviewing our NDA. See “Business—Government Regulation—U.S. Patent Term Restoration.”
31
Our success depends on an intellectual property portfolio that supports our future revenue streams. We are maintaining and building our patent portfolio through filing new patent applications and prosecuting existing applications. We cannot be certain that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting our technology. Any of our intellectual property and proprietary rights could be challenged, invalidated, circumvented, infringed or misappropriated, or such intellectual property and proprietary rights may not be sufficient to permit us to take advantage of current market trends or otherwise to provide competitive advantages. For more information, please see “Risk Factors—Risks Relating to Intellectual Property.”
Manufacturing
We currently contract with third-party contract development and manufacturing organizations (“CDMOs”) for the manufacture of tesetaxel and intend to do so in the future. We do not own or operate manufacturing facilities and currently have no plans to build our own clinical- or commercial-scale manufacturing capabilities. Although we rely on CDMOs, we have personnel with extensive manufacturing experience to oversee these contract service providers.
To date, our third-party manufacturers have met our manufacturing requirements. We expect third-party manufacturers to be capable of providing sufficient quantities of tesetaxel to meet anticipated full-scale commercial demands. To meet our projected needs for commercial manufacturing, third parties with whom we currently work might need to increase their scale of production, or we will need to secure alternate suppliers. We believe that there are alternate sources of supply that can satisfy our clinical and commercial requirements, although we cannot be certain that identifying and establishing relationships with such sources, if necessary, would not result in significant delay or material additional costs.
Sales and Marketing
In order to commercialize tesetaxel, if approved, or any other product candidates that we may develop, we must build marketing, sales and distribution capabilities or make arrangements with third parties to perform these services. The commercial infrastructure for oncology products typically consists of a sales force that calls on oncologists, supported by sales management, medical liaisons, internal sales and marketing support and distribution support.
Additional capabilities important to the oncology marketplace include the management of key accounts such as managed care organizations, integrated delivery networks, group-purchasing organizations, specialty pharmacies and government accounts. To develop the appropriate commercial infrastructure, we will have to invest significant amounts of financial and management resources, some of which will be committed prior to any confirmation that any of our product candidates will be approved.
Where appropriate, we may elect in the future to utilize marketing partners, distributors or contract sales forces to assist in the commercialization of tesetaxel.
Government Regulation
Governmental authorities in the U.S., Europe, Japan and other countries where we may seek approval to commercialize tesetaxel extensively regulate the research, development, testing, manufacture, approval and marketing of pharmaceutical products. Our product candidates must be approved by these regulatory authorities before they may be legally marketed in the applicable jurisdictions. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Below is a general summary of applicable government regulations affecting our current and planned business activities in the U.S., Europe and Japan.
32
In the U.S., the FDA regulates drugs under the FDCA and its implementing regulations. Failure to comply with the applicable U.S. requirements at any time during the product development or approval process, or after approval, may subject an applicant to administrative or judicial sanctions, any of which could have a material adverse effect on us. These sanctions could include:
|
|
• |
refusal to approve pending applications; |
|
|
• |
withdrawal of an approval; |
|
|
• |
imposition of a clinical hold; |
|
|
• |
warning or untitled letters; |
|
|
• |
seizures or administrative detention of product; |
|
|
• |
total or partial suspension of production or distribution; or |
|
|
• |
injunctions, fines, restitution, disgorgement, refusal of government contracts or civil or criminal penalties. |
U.S. Drug Approval Process
The process required by the FDA before a pharmaceutical product may be marketed in the U.S. generally involves the following:
|
|
• |
completion of extensive preclinical laboratory tests, in vivo preclinical studies and formulation studies conducted according to Good Laboratory Practices (“GLPs”) and other applicable regulations; |
|
|
• |
submission to the FDA of an investigational new drug (“IND”) application, which must become effective before human clinical studies may begin; |
|
|
• |
performance of adequate and well-controlled human clinical studies according to Good Clinical Practices (“GCPs”) and other applicable regulations to establish the safety and efficacy of the product candidate for its intended use; |
|
|
• |
submission to the FDA of a NDA or other applications for approval; |
|
|
• |
completion of an FDA pre-approval inspection of the manufacturing facility or facilities to assess compliance with current Good Manufacturing Practices (“cGMP”) and conformance with the manufacturing-related elements of the application to assure consistent production of the product within required specifications; |
|
|
• |
potential FDA audit of the study sites that generated the data in support of the NDA; and |
|
|
• |
FDA review and approval of the NDA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The IND will also include a protocol detailing the objectives of the clinical study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated.
33
All clinical studies must be conducted under the supervision of one or more qualified investigators in accordance with FDA requirements. An institutional review board (“IRB”) must review and approve the protocol and will monitor the study until completion. Clinical studies must be conducted under protocols detailing the objectives of the study, dosing procedures, research subject selection, inclusion and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol, and any material amendments to the protocol, must be submitted to the FDA as part of the IND, and sponsors must report to the FDA serious and unexpected adverse reactions in a timely manner. Sponsors also must make certain financial disclosures to the FDA regarding any financial relationships with study investigators.
Human clinical studies are typically conducted in three sequential phases that may overlap or be combined.
|
|
• |
Phase 1—The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and elimination. Initial human testing is often conducted in patients for product candidates intended to treat severe or life-threatening diseases, such as cancer, especially when the product candidate may be inherently too toxic to ethically administer to healthy volunteers. |
|
|
• |
Phase 2—Clinical studies are performed on a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
|
|
• |
Phase 3—Clinical studies are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide an adequate basis for product labeling. A pivotal study is a clinical study that is intended to meet regulatory authority requirements for the evaluation of a product candidate’s efficacy and safety such that it can be used to justify the approval of the product. |
Human clinical studies are inherently uncertain, and Phase 1, Phase 2 and Phase 3 testing may not be successfully completed or may not be completed at all. The FDA or the sponsor may suspend a clinical study at any time for a variety of reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study if the clinical study is not being conducted in accordance with the IRB’s requirements or if the product candidate has been associated with unexpected serious harm to patients.
The results of product development, preclinical studies and clinical studies, along with descriptions of the manufacturing process, analytical tests and other control mechanisms, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. Within 60 days following submission of the application, the FDA reviews the NDA to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to accept any NDA that it deems incomplete or not properly reviewable at the time of submission, and may request additional information. Once the submission is accepted for filing, the FDA begins an in-depth, substantive review of the NDA, which includes an assessment of the preclinical and clinical data, the product’s formulation and manufacturing, and whether the product is safe and effective for the proposed intended use. The review timeline for NDAs for new molecular entities is 10 months from the date the application is accepted for filing for a standard review; and 6 months from the date the application is accepted for filing for a priority review.
34
FDA Expedited Review and Approval
The FDA has various programs, including fast-track designation, breakthrough therapy designation, accelerated approval and priority review, which are intended to facilitate and expedite the development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening conditions. Different qualifying criteria apply to each program, and each program offers different mechanisms to expedite the development or approval process, such as additional opportunities to meet with the FDA regarding the product candidate’s clinical development program, the opportunity for rolling review of a marketing application, approval based on a surrogate endpoint or, in the case of priority review, a shorter timeline for reviewing a marketing application. We may seek to take advantage of one or more of these expedited programs for tesetaxel or other product candidates in the future. However, even if a product candidate qualifies for one or more of these programs, the development or approval of the product candidate may not be shortened. The FDA may also later determine that a product candidate no longer meets the criteria for designation, and designation does not guarantee that the FDA will ultimately approve the product.
U.S. Patent Term Restoration
Depending on the timing, duration and specifics of FDA approval of the use of our product candidates, one of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to 5 years as compensation for patent term lost during product development and the FDA regulatory review process, provided the patent term restoration does not extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. In the future, if available, we intend to apply for an extension of patent term for one of our currently owned patents beyond its current expiration date; however, there can be no assurance that any such extension will be granted to us.
U.S. Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval for various reasons, such as non-compliance with regulatory requirements or significant safety and performance problems with the product. Later discovery of previously unknown problems with a product may result in recalls or restrictions on the product or even complete withdrawal of the product from the market. Holders of an approved NDA are required to report certain adverse reactions to the FDA and maintain pharmacovigilance programs to proactively look for these adverse events. Manufacturers are also required to comply with restrictions on the advertising and promotion of their products, including restrictions on “off-label” promotion for uses outside those described in the approved product labeling.
In addition, after a product candidate has been approved, the FDA may require that certain additional post-approval requirements be satisfied, including the conduct of additional clinical studies. Certain changes to an approved product, such as adding new indications, making certain manufacturing changes or making certain additional labeling claims are subject to further FDA review and approval. Before a company can market products for additional indications, it must obtain approval from the FDA of a new NDA or NDA supplement, which generally requires that additional clinical studies be conducted. A company cannot be sure that any additional approval for new indications for any product candidate will be approved on a timely basis, or at all.
Changes to the manufacturing process for a given drug are strictly regulated and, depending on the significance of the change, may require FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements on us and any third-party manufacturers we use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance and be subject to periodic or for-cause inspection by the FDA and other regulatory authorities to ensure such compliance.
35
U.S. Reimbursement and Pricing
Significant uncertainty exists as to the coverage and reimbursement status of tesetaxel and any other products for which we may seek regulatory approval. Sales in the U.S. will depend in part on the availability of adequate financial coverage and reimbursement from third-party payors, which include government health programs such as Medicare, Medicaid, TRICARE and the Veterans Administration, as well as managed care organizations and private health insurers. Prices at which we or our customers seek reimbursement for our product candidates can be subject to challenge, reduction or denial by payors.
The process for determining whether a payor will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list or formulary, which might not include all of the FDA-approved products for a particular indication. Also, third-party payors may refuse to include a particular branded drug on their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is available. Medicare Part D, Medicare’s outpatient prescription drug benefit, contains protections to ensure coverage and reimbursement for oral oncology products, and all Part D prescription drug plans are required to cover substantially all oral anti-cancer agents. However, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be available.
Private payors often rely on the lead of the governmental payors in rendering coverage and reimbursement determinations. Sales of our product candidates will therefore depend substantially on the extent to which the costs of our products will be paid by third-party payors. Achieving favorable coverage and reimbursement from the Centers for Medicare and Medicaid Services (“CMS”) and/or the Medicare Administrative Contractors is typically a significant gating issue for successful introduction of a new product.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for marketing, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, which would be in addition to the costs expended to obtain regulatory approvals. Third-party payors may not consider our product candidates to be medically necessary or cost-effective compared to other available therapies, or the rebate percentages required to secure favorable coverage may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient to realize an appropriate return on our investment in drug development.
U.S. Healthcare Fraud and Abuse Laws and Compliance Requirements
We are subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales and marketing programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
36
|
|
under these laws in a variety of ways. These include: providing inaccurate billing or coding information to customers or improperly promoting a product’s off-label use; violating the Anti-Kickback Statute; or misreporting pricing information to government programs. |
|
|
• |
Provisions of the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items or services. |
|
|
• |
The federal Physician Payment Sunshine Act requirements, under the Patient Protection and Affordable Care Act, which require manufacturers of certain drugs and biologics to track and report to CMS payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer. |
|
|
• |
Provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information. |
|
|
• |
Section 1927 of the Social Security Act, which requires that manufacturers of drugs and biological products covered by Medicaid report pricing information to CMS on a monthly and quarterly basis, including the best price available to any customer of the manufacturer, with certain exceptions for government programs, and pay prescription rebates to state Medicaid programs based on a statutory formula derived from reported pricing information. State law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers, state transparency reporting and compliance laws; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and which may not have the same effect, thus complicating compliance efforts. |
European Government Regulation
In Europe, tesetaxel and any future products we may seek to develop and commercialize may also be subject to extensive regulatory requirements. As in the U.S., medicinal products can only be marketed if a marketing authorization from the competent regulatory authorities has been obtained.
Similar to the U.S., the various phases of preclinical and clinical research in Europe are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the member state regimes. Under the current regime, before a clinical study can be initiated, it must be approved in each of the EU countries where the study is to be conducted by two distinct bodies: The National Competent Authority (“NCA”) and one or more Ethics Committees (“EC”). Under the current regime, all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical study have to be reported to the NCA and ECs of the Member State where they occurred.
In 2014, a new Clinical Trials Regulation 536/2014, replacing the current Directive, was adopted. The new Regulation will become directly applicable in all EU Member States (without national implementation) once the EU Portal and Database are fully functional. It is expected that the Regulation will apply in 2019. The new Regulation seeks to simplify and streamline the approval of clinical studies in the EU. For example, the sponsor shall submit a single application for approval of a clinical study via the EU Portal. As part of the application process, the sponsor shall propose a reporting Member State, which will coordinate the validation and evaluation of the application. The reporting Member State shall consult and coordinate with the other concerned Member States. If an application is rejected, it can be amended and resubmitted through the EU Portal. If an approval is issued, the sponsor can start the clinical study in all concerned Member States. However, a concerned Member State can, in limited circumstances, declare an “opt-out” from an approval. In such a case, the clinical study cannot be conducted in that Member
37
State. The Regulation also aims to streamline and simplify the rules on safety reporting, and introduces enhanced transparency requirements such as mandatory submission of a summary of the clinical study results to the EU Database.
European Drug Approval Process
In the European Economic Area (“EEA”), which is comprised of the 28 Member States of the EU plus Norway, Iceland and Liechtenstein, medicinal products can only be commercialized after obtaining a Marketing Authorization (“MA”). There are two types of marketing authorizations:
|
|
• |
The Community MA, which is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use (“CHMP”), of the EMA and which is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of drugs, such as biotechnology medicinal drugs, orphan medicinal drugs and medicinal drugs containing a new active substance indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for drugs containing a new active substance not yet authorized in the EEA, or for drugs that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU. Under the Centralized Procedure in the EU, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, defined by three cumulative criteria: the seriousness of the disease (e.g., disabling or life-threatening diseases) to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days, excluding clock stops. |
|
|
• |
National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for drugs not falling within the mandatory scope of the Centralized Procedure. Where a drug has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member States through the Mutual Recognition Procedure. If the drug has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure, an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State (“RMS”). Within 210 days after receipt of a valid application, the competent authority of the RMS prepares a draft assessment report, a draft summary of the drug characteristics (“SmPC”) and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Member States Concerned) for their approval. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials. If the Member States Concerned raise no objections, based on a potential serious risk to public health, to the assessment, SmPC, labeling or packaging proposed by the RMS, the drug is subsequently granted a national MA in all the Member States (i.e., in the RMS and the Member States Concerned). |
Under the above described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the drug on the basis of scientific criteria concerning its quality, safety and efficacy.
38
Patent Term Extension in Europe
Similar to the patent term extensions available in the U.S., European patent law offers the possibility to apply for a supplementary protection certificate (“SPC”) in order to compensate patent holders for the regulatory delays caused by marketing authorization procedures for medicinal products. SPCs extend the patent term for a period that is equal to the time that elapsed between the filing date of the patent application and the date of the first marketing authorization in the EU, minus 5 years. The overall term of an SPC may not exceed 5 years. An SPC may afford a maximum patent duration of 25 years or, when calculated from the date of first marketing approval, an effective patent exclusivity period of 15 years after first marketing authorization. Applications for SPCs must be filed and approved on a country-by-country basis.
Pharmaceutical Coverage, Reimbursement and Pricing in Europe
In Europe, similar political, economic and regulatory developments may affect our ability to profitably commercialize tesetaxel or any other products, if approved. European countries vary significantly in their approach to coverage, reimbursement and pricing assessments. Some countries allow drug products to be marketed only after a reimbursement price has been agreed to. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies, or so-called health technology assessments, in order to obtain reimbursement or pricing approval. The considerations in each country can also vary with respect to the value placed on unmet need, generic availability, dosing, administration, level of innovation and many other dynamics. In certain cases, these decisions are made sequentially and are often interdependent. Many also have multi-layered, decision-making bodies at the country, regional, local and even hospital level, with various responsibilities within the process.
For example, the United Kingdom typically bases reimbursement decision on a determination of cost effectiveness as defined by cost per quality-adjusted life year (“QALY”), as assessed by National Institute for Health and Care Excellence (“NICE”). Funding by the National Health Service (“NHS”) is typically granted on an incremental cost-effectiveness ratio (“ICER”) of £30,000 or less per QALY. Unlike other countries, the United Kingdom does have a specific process for cancer therapies that do not gain NICE recommendation initially. Cancer therapies can enter into a conditional reimbursement agreement for no more than two years funded by the Cancer Drug Fund (“CDF”) while clinical value continues to be assessed in order to inform the final guidance. At the end of this two-year period, recommendation may be granted for permanent reimbursement on fulfillment of the evidence commitment by the manufacturer.
Similarly, Germany also makes reimbursement decisions based on the determination of additional benefit. Unlike the ICER approach, an efficiency frontier of the total cost and total benefit of all available agents is employed as a cost-benefit methodology. Newly approved agents are compared in terms of cost-benefit ratio either against alternatives or within a specific indication.
France has its own system of therapeutic index to assess medicines for reimbursement and pricing. Reimbursement is determined through a Service Médical Rendu (“SMR”) rating of clinical benefit (low, moderate, substantial), with the exception of hospital-only drugs that may be reimbursed at 100%. Pricing is negotiated based on an Amélioration du Service Médical Rendu (“ASMR”) rating of therapeutic improvement compared to available alternatives (negative (VI), no improvement (V), minor (IV), moderate (III), substantial (II), major (I)).
It is possible that we may be required to conduct cost-effectiveness studies of our product candidates relative to other available therapies in certain countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our financial results may suffer.
39
Japanese Government Regulation
Tesetaxel and any future products we may seek to develop and commercialize will also be subject to extensive regulatory requirements in Japan. As in the U.S., medicinal products can only be marketed if a marketing authorization from the competent regulatory authorities has been obtained. In Japan, the Ministry of Health, Labor, and Welfare (“MHLW”) oversees the regulation and safety of pharmaceuticals, medical devices, cosmetics and food and is the organizational body responsible for approving or rejecting an NDA. Within MHLW, the Pharmaceuticals and Medical Devices Agency (“PMDA”) oversees regulatory affairs for drugs and medical devices and is the body responsible for regulatory review of NDAs.
Japanese Drug Approval Process
The drug approval process in Japan involves a series of activities, including preclinical and clinical (Phase 1, 2 and 3) studies, bridging studies, submission of an NDA by the manufacturer, and review of the NDA by the PMDA. MHLW and PMDA are the main regulatory authorities regulating clinical studies. To conduct a clinical study, a pharmaceutical company must register a protocol with MHLW. Prior to submitting a protocol to the MHLW, an applicant usually submits a Clinical Trial Notification (“CTN”) to the PMDA. All documents must be translated into Japanese. The notification mainly consists of a description and product summary, preclinical data, the clinical study protocol, analysis plan, SOPs, contact person and the names of participating research institution(s). Also, compliance with GCP often requires an IRB to review the clinical study protocol, provide written informed consent forms for participants and report adverse events.
In order to obtain marketing approval, an applicant must submit an NDA for drug marketing authorization to the PMDA for review. Once the PMDA has received the NDA, a team of reviewers evaluates the application data, including quality, pharmacology, pharmacokinetics, toxicology, clinical implications, biostatistics and GCP on-site inspection. During the review process, the reviewers exchange opinions with external experts (expert meetings) to discuss important problems. A general review conference attended by team members, external experts and representatives of the applicant is held after the expert meeting. After the review, the PMDA makes a recommendation and sends the application to the MHLW. MHLW then obtains a recommendation from the Pharmaceutical Affairs and Food Sanitation Council (“PAFSC”) before making a decision regarding approval or rejection of the application.
When data from clinical studies performed in foreign countries are used for an NDA in Japan, the data are first checked to assure that it complies with legal requirements in Japan. Following the legal assessment, an evaluation to determine whether or not the drug is apt to be affected by ethnic factors (intrinsic or extrinsic factors) is conducted. When necessary, a bridging study in Japanese patients is performed, and, when it is concluded that the clinical study outcome in a foreign population can be extrapolated to the Japanese population, the foreign data can be accepted. It is mandatory to conduct pharmacokinetic studies in Japanese people.
Drug approval reviews are normally processed in the order in which the application forms are received. However, orphan drugs and other drugs considered to be especially important from a medical standpoint, such as new drugs to treat serious diseases, may be designated for priority review.
Products for priority review are given priority at each stage of the review process as much as possible. For example, for products designated for priority review at the development stage, it is possible to obtain priority interview advice on indications and other items concerning the designated product. The target review period for priority review is 9 months, compared to 12 months for standard reviews.
Patent-term Extension in Japan
The term of a patent that covers an approved drug may be extended for the shorter of 5 years or the period during which the patent could not be exploited due to obtaining regulatory approval. This period is calculated from the later of the patent registration date (grant date) or the clinical study start date to the regulatory approval date. Unlike in the U.S., patent-term extension in Japan can be applied to more than one patent.
40
Pharmaceutical Coverage, Reimbursement and Pricing in Japan
In Japan, there is significant uncertainty as to the reimbursement and pricing status of tesetaxel and any other products for which we may seek regulatory approval. Japanese pricing of prescription pharmaceuticals is subject to tight government control. Drug prices are set both according to standardized formulas and through negotiations between government officials and applicant companies on a product-by-product basis. In those negotiations, the level of innovation, usefulness and marketability are important determinants of price. With few exceptions, the Japanese MHLW sets the reimbursement prices for all newly launched prescription drugs in Japan.
There are currently three main pricing methodologies, which are dependent on the number of similar products available. The Cost Calculation Method applies to first-to-market products and is based on manufacturer cost inputs as well as international reference markets. Comparison Method I applies to products with less than three non-generic competitors and allows for premiums based on innovation, usefulness and marketability among others, plus an adjustment to international reference markets. Comparison Method II is reserved for products with three or more non-generic competitors and anchors price to the lowest competitor in the basket, with only downward adjustments possible to the international reference markets. Over 80% of products in Japan are priced through Comparison Method I.
In order to control the proportion of the country’s total healthcare expenditure that is devoted to drugs, the government mandates revisions in the prices of all prescription drugs every two years. However, pricing reform introduced in 2016 may have an impact on the commercial landscape in future years. There are three main changes in discussion, including annual pricing reviews, the addition of a cost-effectiveness analysis, and the “huge sellers” provision to reduce budget risk for expensive drugs with significant budget impact. These are expected to go into effect in 2018, with additional proposals under consideration for later roll out, including annual reference pricing, indication expansion discounts and optimal use guidelines. These changes may impact the timeline for pricing negotiations as well as the result of these negotiations. The adoption of more restrictive pricing and reimbursement policies along with existing controls could limit our commercial revenue in the Japanese market.
Rest of the World Regulation
For other countries outside of the U.S., EU and Japan, such as Eastern Europe, Latin America, Asia and emerging markets, the requirements governing the conduct of clinical studies, drug licensing, pricing and reimbursement vary from country to country. In all cases, clinical studies must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
Corporate Conversion
On December 6, 2017, we converted from a Delaware limited liability company to a Delaware corporation by filing a certificate of conversion with the Delaware Secretary of State, and we changed our name from “Odonate Therapeutics, LLC” to “Odonate Therapeutics, Inc.” (the “Conversion”). As a result of the Conversion, the membership interests of Odonate Therapeutics, LLC were converted into shares of Odonate Therapeutics, Inc. Prior to the Conversion, we formed a holding company named Odonate Holdings, LLC (“Odonate Holdings”) to own Odonate Therapeutics, LLC. Following the Conversion, Odonate Holdings distributed its interest in Odonate Therapeutics, Inc. to prior members of Odonate Therapeutics, LLC, but retained record title to the 2,931,402 shares of common stock underlying outstanding incentive units previously granted to employees, officers, directors and consultants by Odonate Management Holdings, LLC and 154,285 shares of common stock beneficially owned by Tang Capital Partners, LP.
41
Implications of Being an Emerging Growth Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). An emerging growth company may take advantage of specified reduced reporting and other regulatory requirements for up to 5 years that are otherwise applicable to public companies. These reduced reporting provisions include, among others:
|
|
• |
a requirement to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure; |
|
|
• |
an exemption from the auditor attestation requirement on the effectiveness of our system of internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002, as amended (“Sarbanes-Oxley Act”); |
|
|
• |
an exemption from the adoption of new or revised financial accounting standards until they would apply to private companies; |
|
|
• |
an exemption from compliance with any new requirements adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer; |
|
|
• |
an exemption from the requirement to seek non-binding advisory votes on executive compensation and golden parachute arrangements; and |
|
|
• |
reduced disclosure about executive compensation arrangements. |
We have elected to take advantage of the scaled disclosure requirements and other relief described above or elsewhere in this Annual Report on Form 10-K so long as we are an emerging growth company. We will remain an emerging growth company for 5 years unless, prior to that time, we: (i) have more than $1.07 billion in annual gross revenue; (ii) have a market value for our common stock held by non-affiliates of more than $700 million as of the last day of our second fiscal quarter of any fiscal year; or (iii) issue more than $1.0 billion of non-convertible debt over a three-year period. We have availed ourselves of the reduced reporting obligations with respect to audited financial statements and related Management’s Discussion and Analysis of Financial Condition and Results of Operations and executive compensation disclosure in this Annual Report on Form 10-K, and expect to continue to avail ourselves of the reduced reporting obligations available to emerging growth companies in future filings with the U.S. Securities and Exchange Commission (“SEC”).
Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the U.S. Securities Act of 1933 (“Securities Act”) for complying with new or revised accounting standards. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period and, as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
Employees
As of December 31, 2017, we had 60 employees. None of our employees are represented by labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good.
42
Corporate and Other Information
We are a Delaware corporation that was initially formed as a Delaware limited liability company in March 2013. On December 6, 2017, in anticipation of our initial public offering, we converted into a Delaware corporation. We currently operate in one segment.
Our principal executive offices are located at 4747 Executive Drive, Suite 510, San Diego, California, 92121, and our telephone number is (858) 731-8180. Our corporate website address is www.odonate.com. Information contained on or accessible through our website is not a part of this Annual Report on Form 10-K, and the inclusion of our website address in this Annual Report on Form 10-K is an inactive textual reference only.
We file electronically with the U.S. Securities and Exchange Commission (“SEC”) our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at www.odonate.com, under “Corporate Resources,” free of charge, copies of these reports as soon as reasonably practicable after filing or furnishing these reports with the SEC.
An investment in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below before deciding whether to purchase shares of our common stock. In assessing these risks, you should also refer to the other information contained in this Annual Report on Form 10-K, including our financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our business, financial condition, results of operations, cash flow and prospects could be materially and adversely affected by any of these risks or uncertainties. In any such case, the trading price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business
We are substantially dependent on our ability to successfully develop and commercialize tesetaxel.
Since our inception, we have invested substantially all of our capital resources on the development of tesetaxel, which we initially are developing for the treatment of locally advanced or metastatic breast cancer (“MBC”). We are conducting a multinational, multicenter, randomized, Phase 3 study of tesetaxel in patients with human epidermal growth factor receptor 2 (“HER2”) negative, hormone receptor (“HR”) positive MBC who have received no more than one chemotherapy regimen for advanced disease and have received a taxane in the neoadjuvant (prior to surgery) or adjuvant (immediately following surgery) setting. If the results of this study, known as CONTESSA, are negative or inconclusive, we may be unable to obtain regulatory approval for tesetaxel. Further, even if the results of CONTESSA are positive, we cannot assure you that the U.S. Food and Drug Administration (“FDA”), the European Medicines Agency (“EMA”) or any other regulatory authority will approve tesetaxel for marketing.
Our ability to generate revenue and our future success depends in large part on the success of CONTESSA, the approval of tesetaxel, the nature of any potential requirements for post-approval studies and the successful commercialization of tesetaxel, if approved. Delays in obtaining regulatory approval for tesetaxel would, among other consequences, require further development expenditures, delay the launch of tesetaxel and impact our ability to raise additional capital, all of which would have a material adverse effect on our business and financial condition.
43
The commercial adoption of tesetaxel and any other product candidates we develop will depend on the degree of their market acceptance.
Even with the requisite approvals from the FDA, the EMA and other regulatory authorities, the commercial adoption of tesetaxel and any other product candidates we develop will depend on the degree of their acceptance by physicians, patients, third-party payors and others in the medical community. The degree of market acceptance will depend on a number of factors, including:
|
|
• |
the safety and efficacy of the product as demonstrated in clinical studies; |
|
|
• |
the perception of physicians, patients, third-party payors and others in the medical community of the relative safety, efficacy, convenience, effect on quality-of-life and cost-effectiveness of the product, compared to those of other available treatments; |
|
|
• |
the product’s prescribing label, including the description of the product’s approved indication(s), the description of its efficacy, including the endpoints in which it showed an improvement, and the prevalence and severity of any side effects, including any limitations or warnings arising therefrom; |
|
|
• |
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
|
|
• |
the strength of marketing and distribution support and timing of market introduction of competitive products; |
|
|
• |
the publicity concerning our products or competing products and treatments; |
|
|
• |
product liability litigation alleging injuries relating to our products or similar classes of drugs; |
|
|
• |
our ability to access third parties to manufacture or distribute our products on acceptable terms or at all; |
|
|
• |
any post-approval study requirements for our products and the results thereof; and |
|
|
• |
sufficient third-party insurance coverage and reimbursement. |
Even if a potential product such as tesetaxel displays a favorable efficacy and safety profile in preclinical and clinical studies, market acceptance of the product is not fully known until after its commercial launch. Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our product candidates may require significant resources and may never be successful. If tesetaxel or other product candidates are approved but fail to achieve an adequate level of acceptance by physicians, patients, third-party payors and others in the medical community, we will not be able to generate sufficient revenue to become or remain profitable.
We have only limited assets and will need to raise additional capital before we can expect to generate revenue or become profitable.
As of December 31, 2017, we had no revenue, an accumulated deficit of $39.3 million and cash of $198.1 million. We believe that our existing cash as of December 31, 2017 will be sufficient to meet our anticipated cash requirements through at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission (“SEC”). However, to fund future operations to the point at which we are able to generate positive cash flow from sales of tesetaxel or other potential product candidates, we will need to raise significant additional capital. The amount and timing of future funding requirements will depend on many factors, including the timing and results of our ongoing development efforts, the potential expansion of our current development programs, potential new development programs and related general and administrative support. We anticipate that we will seek to fund our operations through public and private equity and debt financings or other sources, such as potential licenses or other collaboration agreements. We cannot assure you that anticipated additional financing will be available to us on favorable terms, or at all. Although we have been successful in obtaining financing through the issuance of our equity securities, we cannot assure you that we will be able to do so in the future. If we are unable to raise additional capital to fund our clinical development and commercialization of tesetaxel, if approved, and other business activities, we could be forced to abandon one or more programs and curtail or cease our operations.
44
We have never generated any revenue and may never be profitable.
We have no products approved for marketing, have never generated any revenue from product sales and have incurred losses in each year since our inception. Our ability to generate revenue and achieve profitability depends on our ability, alone or with marketing partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, tesetaxel. We expect to continue to incur losses for the foreseeable future, and we anticipate these losses will increase as we continue to develop tesetaxel. Our ability to generate revenue from product sales depends heavily on our success in many areas, including but not limited to:
|
|
• |
successfully completing the development of tesetaxel; |
|
|
• |
obtaining regulatory approvals to market tesetaxel; |
|
|
• |
successfully managing third-party service providers involved in the manufacturing and development of tesetaxel; |
|
|
• |
successfully commercializing tesetaxel, either independently or with marketing partners; |
|
|
• |
obtaining market acceptance of tesetaxel, including garnering market share from existing and future treatment alternatives; |
|
|
• |
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; |
|
|
• |
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how; and |
|
|
• |
attracting, hiring and retaining qualified personnel. |
If tesetaxel or any other product candidates we may develop are approved for marketing, we anticipate incurring significant commercialization costs. Our expenses could increase beyond our current expectations if we are required by the FDA, the EMA or other regulatory authorities to change our manufacturing processes or quality procedures or perform additional or unanticipated preclinical, clinical or other studies. In cases where we are successful in obtaining regulatory approvals to market tesetaxel or other product candidates, our revenue will be dependent, in part, on the size of the markets in the territories for which we gain regulatory approval, the acceptance of the price of the product in those markets and the ability to obtain reimbursement at any price. If the number of our addressable patients is not as significant as we estimate, the indication approved by regulatory authorities is narrower than we expect or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of such products, even if approved. If we are not able to generate revenue from the sale of approved products, we may never become profitable.
We currently have no sales, marketing or distribution capabilities. If we elect to commercialize tesetaxel ourselves and we are unable to establish effective sales, marketing or distribution capabilities or if we are unable to enter into agreements with third parties to commercialize tesetaxel or other product candidates that we may develop, we may not be able to effectively generate product revenues.
We currently do not have sales, marketing or distribution capabilities. In order to commercialize tesetaxel, if approved, or any other product candidates that we may develop, we must build marketing, sales and distribution capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. If tesetaxel receives regulatory approval and we decide to commercialize tesetaxel ourselves, building the requisite sales, marketing or distribution capabilities will be expensive and time-consuming and will require significant attention of our leadership team to manage. Any failure or delay in the development of our sales, marketing or distribution capabilities would adversely impact the commercialization of any product. The competition for talented individuals experienced in selling and marketing pharmaceutical products is intense, and we cannot assure you that we can assemble an effective team. Additionally, we may choose to collaborate, either globally or on a territory-
45
by-territory basis, with third parties on the commercialization of tesetaxel or any other product candidates that we may develop. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval or any such commercialization may experience delays or limitations.
We may be subject to additional risks related to operating in foreign countries either ourselves or through a third-party, including:
|
|
• |
differing regulatory requirements in foreign countries; |
|
|
• |
unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements; |
|
|
• |
economic weakness, including inflation or political instability in particular foreign economies and markets; |
|
|
• |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
|
|
• |
foreign taxes, including withholding of payroll taxes; |
|
|
• |
foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
|
|
• |
difficulties staffing and managing foreign operations; |
|
|
• |
workforce uncertainty in countries where labor unrest is more common than in the U.S.; |
|
|
• |
potential liability under the Foreign Corrupt Practices Act of 1977 or comparable foreign regulations; |
|
|
• |
challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the U.S.; |
|
|
• |
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
|
|
• |
business interruptions resulting from geopolitical actions, including war and terrorism. |
If we are not successful in commercializing tesetaxel or other product candidates, our future product revenue will suffer and we may incur significant additional losses.
Because a number of companies compete with us, many of which have greater resources than we do, and because we face rapid changes in science in our industry, we cannot be certain that our products will be accepted in the marketplace or capture market share.
Competition from other biotechnology and pharmaceutical companies is intense and is expected to increase. A number of companies are pursuing the development of pharmaceuticals in oncology, our area of focus. Many of these companies are very large, and have financial, technical, sales and distribution and other resources substantially greater than ours. The greater resources of these competitors may enable them to develop, obtain regulatory approval for or market competing products more quickly or effectively, making it extremely difficult for us to capture a share of the market for our products. Additionally, the biotechnology and pharmaceutical industries are subject to rapid changes in science, and our competitors may develop and market products with improved therapeutic profiles relative to our product candidates that would render our product candidates noncompetitive.
46
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, reduce the commercial attractiveness of a prescribing label or result in significant negative consequences following regulatory approval, if approved.
Clinical studies of tesetaxel or other product candidates we may develop could reveal a high and unacceptable incidence and severity of undesirable side effects. Undesirable side effects could adversely affect patient enrollment in clinical studies, cause us or regulatory authorities to interrupt, delay or halt clinical studies or result in the delay, denial or withdrawal of regulatory approval by the FDA, the EMA or other regulatory authorities. For example, in 2007, tesetaxel was placed on clinical hold by the FDA while in development by the original sponsor due to the occurrence of several fatalities in the setting of severe neutropenia (a low level of neutrophils, a type of white blood cell) in patients with advanced cancer. While this clinical hold was lifted in 2008, and tesetaxel has since been evaluated in multiple clinical studies in 300 patients without any interruption due to safety issues, we cannot assure you that safety-related interruptions in tesetaxel’s clinical development will not occur again in the future. Any such recurrence could potentially delay or prevent the ultimate approval of the product candidate. Undesirable or adverse side effects also could result in regulatory authorities mandating a more restrictive prescribing label for the product, which, in turn, could limit the market acceptance of the product even if approved for marketing and commercialization.
Drug-related side effects could result in potential product liability claims. We carry product liability insurance in the amount of $10.0 million in the aggregate. We believe our product liability insurance coverage is sufficient in light of our clinical programs; however, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts or maintain coverage at all to protect us against losses due to liability. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations, business and financial condition. In addition, regardless of merit or eventual outcome, product liability claims may result in impairment of our business reputation, withdrawal of clinical study participants, costs due to related litigation, distraction of management’s attention from our primary business, initiation of investigations by regulators, substantial monetary awards to patients or other claimants, the inability to commercialize our product candidates and decreased demand for our product candidates, if approved for marketing.
Additionally, if one or more of our product candidates receives regulatory approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including but not limited to:
|
|
• |
the withdrawal of approvals by regulatory authorities; |
|
|
• |
the requirement of additional warnings on the prescribing label; |
|
|
• |
the requirement of a Risk Evaluation and Mitigation Strategy (“REMS”) plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers and/or other elements to assure safe use; |
|
|
• |
litigation and the potential to be held liable for harm caused to patients; and |
|
|
• |
an adverse effect on our reputation. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate and could significantly harm our business, results of operations, financial condition and prospects.
47
We may not be successful in our efforts to identify, in-license or acquire, discover, develop or commercialize additional product candidates.
Although a substantial amount of our effort will focus on the development and potential commercialization of tesetaxel, we also may seek to identify, in-license or acquire, discover, develop and commercialize additional product candidates in the oncology field. We cannot assure you that our efforts to in-license or acquire additional product candidates will be successful. Even if we are successful in in-licensing or acquiring additional product candidates, their requisite development activities may require substantial resources, and we cannot assure you that these development activities will result in regulatory approvals.
We will need to increase the size and capabilities of our organization, and we may experience difficulties in managing our growth.
Odonate was formed in 2013 and, as of December 31, 2017, had 60 employees. As we advance the development of tesetaxel, we must continue to grow the size of the organization. Future growth will impose significant added responsibilities on members of management, including:
|
|
• |
identifying, recruiting, integrating, retaining and motivating additional employees; |
|
|
• |
effectively managing our development efforts, including the clinical development and FDA, EMA or other regulatory authority review processes for our product candidates; |
|
|
• |
effectively managing our third-party service providers involved in the development and manufacture of our product candidates; and |
|
|
• |
improving our operational, financial and management controls, reporting systems and procedures. |
Our future financial performance and our ability to successfully develop and commercialize our product candidates will depend, in part, on our ability to effectively manage any future growth. Our management will have to dedicate a significant amount of its attention to managing these growth activities. In addition, we expect to incur additional costs in hiring, training and retaining such additional personnel.
If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully execute the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
Our future success depends on our ability to retain our key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the principal members of our management and scientific teams. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
To induce valuable employees to remain at our company, in addition to salary and cash incentives, we granted equity awards that vest over time. The value to employees of these equity awards that vest over time may be significantly affected by changes in the price of our common stock that are beyond our control, and may at any time be insufficient to retain employees who receive more lucrative offers from other companies. Any of our employees could leave our employment at any time, with or without notice.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel or consultants will also be critical to our success. We rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our discovery, development and commercialization strategies. The loss of the services of any of our executive officers, key employees or consultants could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy.
48
Replacing executive officers, key employees or consultants may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel or consultants on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel.
We may hire part-time employees or use consultants. As a result, certain of our employees, officers, directors or consultants may not devote all of their time to our business, and may from time to time serve as employees, officers, directors and consultants of other companies.
Risks Related to Our Industry
Drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies may not be predictive of future study results.
Clinical testing is expensive, can take many years to complete and its outcome is inherently uncertain. Failure can occur at any time during the clinical study process. The results of preclinical studies and early clinical studies of our product candidates may not be predictive of the results of later-stage clinical studies. Product candidates that have shown promising results in early-stage clinical studies may still suffer significant setbacks in subsequent clinical studies. For example, the safety or efficacy results generated to date in our clinical studies do not ensure that later clinical studies will demonstrate similar results. There is a high failure rate for pharmaceutical product candidates proceeding through clinical studies, and product candidates in later stages of clinical studies may fail to show the desired safety and efficacy, despite having progressed through preclinical studies and initial clinical studies.
A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical studies due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies. Moreover, preclinical and clinical data often are susceptible to varying interpretations and analyses. We do not know whether any clinical studies we may conduct will demonstrate consistent or adequate efficacy and safety sufficient to obtain regulatory approval to market our product candidates.
Results from CONTESSA and any future clinical studies we may undertake may not be sufficient to obtain regulatory approvals to market our product candidates on a timely basis, if at all.
Pharmaceutical product candidates are subject to extensive government regulations related to development, clinical studies, manufacturing and commercialization. In order to sell any product that is under development, we must first receive regulatory approval. To obtain regulatory approval, we must conduct preclinical and clinical studies that demonstrate that our product candidates are safe and effective. The process of obtaining FDA, EMA and other regulatory authority approvals is costly, time-consuming, uncertain and subject to unanticipated delays.
The FDA, EMA and other regulatory authorities have substantial discretion in the approval process and may not agree that we have demonstrated that our product candidates are safe and effective. If our product candidates are ultimately not found to be safe and effective, we would be unable to obtain regulatory approval to manufacture, market and sell them. We can provide no assurances that the FDA, EMA or other regulatory authorities will approve our product candidates or, if approved, what the scope of the approved indication might be.
CONTESSA and future clinical studies that we may undertake may be delayed or halted.
CONTESSA and any other clinical studies of our product candidates that we may conduct in the future may be delayed or halted for various reasons, including:
|
|
• |
insufficient financial resources; |
|
|
• |
insufficient supplies of drug product to treat the patients in the studies; |
49
|
|
• |
ineffectiveness of the product candidates; |
|
|
• |
patients experiencing unexpected side effects or other safety concerns being raised during treatment; |
|
|
• |
changes in governmental regulations or administrative actions; |
|
|
• |
failure to conduct studies in accordance with required clinical practices; |
|
|
• |
inspection of clinical study operations or study sites by the FDA or other regulatory authorities, resulting in a clinical hold; |
|
|
• |
political unrest at foreign clinical sites; or |
|
|
• |
natural disasters at any of our clinical sites. |
If studies are delayed or halted, we may incur significant additional expenses, and the potential approval of our product candidates may be delayed, which would have a material adverse effect on our business and financial condition.
Even if we obtain regulatory approval for tesetaxel or another product candidate, our products will remain subject to regulatory scrutiny.
If tesetaxel or other product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies and submission of safety, efficacy and other post-market information.
Manufacturers and manufacturers’ facilities are required to comply with extensive FDA, EMA and other regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices (“cGMP”) regulations. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMPs and adherence to commitments made in any New Drug Application (“NDA”), Market Authorization Application (“MAA”) or other marketing application. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals we receive for our product candidates may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical studies, which must comply with applicable Good Clinical Practice (“GCP”) regulations. We could also be asked to conduct post-marketing clinical studies to verify the safety and efficacy of our products in general or in specific patient subsets. If initial regulatory approval was obtained via the accelerated approval pathway, we could be required to conduct a successful post-marketing clinical study to confirm clinical benefit for our products. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of regulatory approval. We will be required to report certain adverse reactions and production problems, if any, to the FDA, EMA and other regulatory authorities. Any new legislation addressing drug safety or approval issues could result in delays in product development or commercialization, or increased costs to assure compliance. We will have to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote our products for indications or uses for which they do not have approval. The holder of an approved NDA or MAA must submit new or supplemental applications and obtain approval for certain changes to the approved product, product labeling or manufacturing process.
50
If a regulatory authority discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, such regulatory authority may impose restrictions on that product or us. If we fail to comply with applicable regulatory requirements, a regulatory or enforcement authority may, among other things:
|
|
• |
issue warning or untitled letters; |
|
|
• |
impose civil or criminal penalties; |
|
|
• |
suspend or withdraw regulatory approval; |
|
|
• |
suspend any of our ongoing clinical studies; |
|
|
• |
refuse to approve pending applications or supplements to approved applications submitted by us; |
|
|
• |
impose restrictions on our and our contract manufacturers’ operations, including closing manufacturers’ facilities; |
|
|
• |
seize or detain products; or |
|
|
• |
require a product recall. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, this would have a material adverse effect on our business and financial condition.
If we are unable to achieve and maintain coverage and adequate levels of reimbursement for tesetaxel and other product candidates, if approved, their commercial success may be severely hindered.
Successful sales of tesetaxel and any other product candidates that may receive regulatory approval depend on the availability of coverage and adequate reimbursement from third-party payors. Patients who are prescribed medications for the treatment of their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Coverage decisions may depend on clinical and economic standards that disfavor new products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Assuming we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate or may require co-payments that patients find unacceptably high. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products.
The market for tesetaxel and any other product candidates that we attempt to commercialize will depend significantly on access to third-party payors’ drug formularies, or lists of medications for which third-party payors provide coverage and reimbursement. The industry competition to be included in such formularies often leads to downward pricing pressures on pharmaceutical products. Also, third-party payors may refuse to include a particular branded drug in their formularies or otherwise restrict patient access through formulary controls or otherwise to a branded drug when a less costly generic equivalent or other alternative is available.
Third-party payors, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the U.S., no uniform policy requirement for coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
51
Third-party coverage and reimbursement for our product candidates for which we may receive regulatory approval may not be available or adequate in either the U.S. or international markets, which could have a material adverse effect on our business, results of operations, financial condition and prospects.
We will be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy, security laws and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Our operations may be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act and physician sunshine laws and regulations. These laws will impact, among other things, our clinical development, proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The U.S. laws that will affect our ability to operate include:
|
|
• |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
|
|
• |
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
|
|
• |
federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
|
|
• |
HIPAA, as amended by the federal Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
|
|
• |
the federal physician sunshine requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, (collectively, the “PPACA”), which require manufacturers of drugs, devices, biologics and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; and |
|
|
• |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available thereunder, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent healthcare reform legislation has strengthened these laws. For example, the PPACA, among other things, amended the previous intent requirement of the
52
federal Anti-Kickback Statute and criminal healthcare fraud statutes. Now, a person or entity does not have to have actual knowledge of the statutes or specific intent to violate them. The PPACA also provides that the federal government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of these laws or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if a person becomes subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Recently enacted and future legislation may increase the difficulty and cost of obtaining regulatory approval, and the subsequent commercialization, of our product candidates, if approved, and may affect the prices we may obtain.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay regulatory approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain regulatory approval.
For example, in 2010, President Obama signed into law the PPACA, which contains provisions, among others, that may impact our potential product candidates, including:
|
|
• |
an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; |
|
|
• |
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
|
|
• |
expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance; |
|
|
• |
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices; |
|
|
• |
extension of manufacturers’ Medicaid rebate liability; |
|
|
• |
expansion of eligibility criteria for Medicaid programs; |
|
|
• |
new requirements to report financial arrangements with physicians and teaching hospitals; |
|
|
• |
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; |
|
|
• |
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
|
|
• |
the Independent Payment Advisory Board, which, if created, would have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs; and |
|
|
• |
a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services (“CMS”) to test innovative payment and service delivery models to lower Medicare and Medicaid spending. |
53
Other legislative changes have been proposed and adopted since the PPACA was enacted. These changes included aggregate reductions of Medicare payments to providers of up to two percent per fiscal year. Additionally, in January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Further, while the healthcare reform agenda and policies of the new Trump administration are not fully known, it is possible that additional regulatory changes, as well as the repeal (in whole or in part) of the PPACA, could negatively affect insurance coverage and/or drug prices. These new laws also may result in additional reductions in Medicare and other healthcare funding as well as insurance coverage and payments.
We expect that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and additional downward pressure on the reimbursement received for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Moreover, there recently has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. Individual states in the U.S. have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. Additionally, legislation has been introduced to repeal the PPACA. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the regulatory approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent regulatory approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Governments outside the U.S. tend to impose strict price controls, which may adversely affect our revenues, if any.
In some countries, particularly certain countries of the European Union (“EU”), the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of regulatory approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical study that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
54
Risks Relating to Our Reliance on Third Parties
We rely on third parties to conduct our preclinical and clinical studies. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have agreements with third-party contract research organizations (“CROs”) to monitor and manage data for our preclinical and clinical programs. We rely heavily on these third parties for execution of our preclinical and clinical studies and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with cGCPs, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce these cGCPs through periodic inspections of study sponsors, principal investigators and study sites. If we, the investigators, the sites or any of these CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical studies may be deemed unreliable and the regulatory authorities may require us to perform additional clinical studies before approving our marketing applications. We cannot assure you that, upon inspection, such regulatory authorities will determine that any of our clinical studies comply with the cGCP regulations. In addition, our clinical studies must be conducted with product produced under cGMP regulations. Failure to comply with these regulations may require us to repeat clinical studies, which would delay or compromise the regulatory approval process.
If our relationships with any of these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. In addition, our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing preclinical and clinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical studies may be extended, delayed or terminated. As a result, we may not be able to obtain regulatory approval for, or successfully commercialize, our product candidates and may incur significant additional expenses. In addition, potential approval of our product candidates may be delayed, which would have a material adverse effect on our business, results of operations and financial condition.
If the third-party manufacturers on which we rely fail to produce our product candidates on a timely basis, or comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the studies, regulatory submissions, required approvals or commercialization of our product candidates.
We contract with third-party contract development and manufacturing organizations (“CDMOs”) to manufacture our product candidate, and we would expect to rely on these CDMOs to produce commercial quantities of tesetaxel. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, which include difficulties with production costs and yields, quality control and assurance and shortages of qualified personnel, as well as compliance with strictly enforced governmental regulations, including cGMPs. The CDMOs we contract with may not perform as agreed or may terminate their agreements with us.
In addition to product approval, any facilities in which our product candidates are manufactured or tested for their ability to meet required specifications must be inspected and approved by regulatory authorities before a commercial product can be manufactured. Failure of such a facility to be approved could delay the approval of one or more of our product candidates.
55
Any of these factors could cause us to delay or suspend any clinical studies, regulatory submissions, required approvals or commercialization of one or more of our product candidates, entail higher costs and result in our being unable to effectively commercialize products.
Risks Relating to Intellectual Property
Our success in developing and marketing our product candidates depends significantly on our ability to obtain and maintain patent protection and operate without infringing on the rights of others.
We depend on patents and other intellectual property to prevent others from improperly benefiting from products or inventions that we developed or acquired. Our patents and patent applications cover our product candidates and inventions. The intellectual property portfolio protecting our tesetaxel program includes 9 U.S., 4 European and 7 Japanese patents, as well as two pending U.S. patent applications and one pending European patent application. Of those, 5 U.S., 4 European and 6 Japanese patents are exclusively licensed to us by Daiichi Sankyo. Among the licensed patents, one issued U.S. patent (U.S. Patent No. 7,410,980) covers the crystal form of tesetaxel used in our clinical formulation and will expire in 2026. If tesetaxel is approved by the FDA, we will be entitled to request patent term restoration that could extend the protection of this patent until 2031. The exact duration of the extension depends on the time we spend in clinical studies as well as the time the FDA spends reviewing our NDA. See “Business—Government Regulation—U.S. Patent Term Restoration.” The licensed portfolio includes 4 other issued U.S. patents that cover compositions of matter and various methods useful for preparing tesetaxel, as well as European and Japanese counterparts of these U.S. patents. We also own 4 U.S. patents that cover additional methods useful for preparing tesetaxel and certain salt and crystal forms of tesetaxel, as well as two pending U.S. patent applications related to tesetaxel. For one of those U.S. patent applications, if it results in an issued patent, that patent would expire in 2038.
The patent position of pharmaceutical firms like ours is highly uncertain and involves complex legal and factual questions. We intend to continue to file patent applications because we believe it is appropriate to obtain patents covering our products and their manufacture and use. There can be no assurance, however, that any additional patents will issue, that the scope of any patent that has issued or may issue will be sufficient to protect our product candidates, or that any current or future issued patent will be held not invalid if subsequently challenged. Additionally, we may have to incur significant expense and expend management time defending or enforcing our patents. If we cannot obtain and maintain effective patent rights and/or regulatory exclusivity for our product candidates, we may not be able to compete effectively, and our business and results of operations would be harmed.
The scope and terms of our patents may be insufficient to protect our product candidates for an adequate amount of time.
In the U.S., the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours.
Patents may be eligible for limited patent term extension in the U.S. under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. Similar patent extensions exist in the EU and Japan. The Hatch-Waxman Act permit a patent term extension of up to 5 years for a patent covering an approved product as compensation for patent term that elapsed during product development and the FDA regulatory review process, provided the extension does not extend the total patent term beyond 14 years from approval, and only one patent per approved product is extended. We may not receive an extension if we fail to apply within applicable deadlines or fail to apply prior to expiration of relevant patents. Moreover, the length of the extension could be less than we request. If we
56
are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that product will be shortened and our competitors may obtain approval to market competing products sooner, impacting our revenue.
If the FDA or foreign regulatory authorities approve generic versions of any of our products that receive marketing approval or such authorities do not grant our products appropriate periods of exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
NDA applicants are required to list with the FDA each patent with claims covering the applicant’s product or method of using the product for which approval is sought. Upon approval of a drug, each of the patents listed in the application for the drug that cover the drug or an approved use of the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic or 505(b)(2) NDA applicants in support of approval of an Abbreviated New Drug Application (“ANDA”) or a 505(b)(2) NDA. An ANDA is a streamlined way to seek approval for marketing a drug product that has the same active ingredient in the same strength and dosage form as the listed drug and has been shown to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct or submit results of preclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way can often be substituted by pharmacists under prescriptions written for the original listed drug.
Both ANDA and 505(b)(2) NDA applicants are required to make a certification to the FDA concerning any patents listed for the approved NDA product in the FDA’s Orange Book. Specifically, ANDA and 505(b)(2) NDA applicants must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a statement certifying that its proposed ANDA labeling does not contain (or carves out) any language regarding patented methods-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the submitted application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the proposed product will not infringe the already approved product’s listed patents, or that such patents are invalid or unenforceable, is called a Paragraph IV certification. If the applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA or 505(b)(2) NDA has been received by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving a submitted application until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant or 505(b)(2) applicant.
In addition to the protections from competitors that may be afforded by patents, pharmaceutical products may also be eligible for regulatory exclusivity, such as the exclusivity that may be granted to New Chemical Entities (“NCEs”). While we believe that tesetaxel will qualify as an NCE if and when an NDA is submitted for tesetaxel, such determination is only made at the time of approval. Accordingly, we do not have any agreement with the FDA, EMA or other regulatory body that tesetaxel will in fact be regarded as an NCE, and there can be no assurance that it will be treated as such at the time of approval (if approval is granted).
Competition that our products may face from generic versions of our products could materially and adversely impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates.
57
If we fail to comply with our obligations under our licenses, we may lose rights to critical patents that are important to the commercialization and revenue potential of tesetaxel.
We have licensed patent rights covering tesetaxel from Daiichi Sankyo. If, for any reason, our license agreement with Daiichi Sankyo is terminated or we otherwise lose those rights, it could adversely affect our business. Our license agreement with Daiichi Sankyo imposes, and any future collaboration agreements or license agreements we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement or other obligations on us. Failure to fulfill these obligations could pose a material risk to our patent protection for tesetaxel and any future product candidates.
If our product candidates infringe the rights of others, we could be subject to expensive litigation, become liable for substantial damages, be required to obtain licenses from others or be prohibited from selling our product candidates altogether.
Our competitors or others may have patent rights that they choose to assert against us or our licensors, licensees, suppliers, customers or potential marketing partners. Moreover, we may not know about patents or patent applications that our products would infringe. Because patent applications do not publish for at least 18 months, if at all, and can take many years to issue, there may be currently pending applications unknown to us that may later result in issued patents that our product candidates would infringe. In addition, if third parties file patent applications or obtain patents claiming inventions also claimed by us or our licensors in issued patents or pending applications, we may have to participate in interference proceedings in the U.S. Patent and Trademark Office (“USPTO”) to determine priority of invention. If third parties file oppositions in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of our foreign patent applications.
If a third party claims that we infringe its proprietary rights, any of the following may occur:
|
|
• |
we may become involved in time-consuming and expensive litigation, even if the claim is without merit; |
|
|
• |
we may become liable for substantial damages for past infringement if a court decides that our science infringes a competitor’s patent; |
|
|
• |
a court may prohibit us from selling or licensing our product without a license from the patent holder, which may not be available on commercially acceptable terms, if at all, or which may require us to pay substantial royalties or grant cross licenses to our patents; or |
|
|
• |
we may have to redesign our product candidates so that they do not infringe patent rights of others, which may not be possible or commercially feasible. |
Any of these events would have a material adverse effect on our business, results of operations and financial condition.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the U.S. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we or our licensors were the first to make the inventions claimed in our owned and licensed patents or pending applications, or that we or our licensor were the first to file for patent protection of such inventions.
58
Assuming the other requirements for patentability are met, in the U.S., prior to March 15, 2013, the first to make the claimed invention is entitled to the patent, while, outside the U.S., the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act (“Leahy-Smith Act”), enacted on September 16, 2011, the U.S. has moved to a first-to-file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. In general, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, results of operations and financial condition.
Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and provide new opportunities for third parties to challenge issued patents in the USPTO. We may be subject to the risk of third-party prior art submissions on pending applications or become a party to opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patents for tesetaxel. There is a lower standard of evidence necessary to invalidate a patent claim in a USPTO proceeding relative to the standard in U.S. district or federal court. This could lead third parties to challenge and successfully invalidate our patents that would not otherwise be invalidated if challenged through the court system.
In addition to patent protection, we will need to successfully preserve our trade secrets. If we are unable to maintain effective proprietary rights for tesetaxel or any future product candidates, we may not be able to compete effectively in our markets.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve information or know-how that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary science and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, there remains the possibility that agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. For instance, the FDA has introduced its Transparency Initiative and is currently considering whether to publicly disclose additional information from drug sponsors; in such case, we cannot guarantee that our trade secrets will not be disclosed.
Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, science or information to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed, that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating our trade secrets.
59
Risks Related to Ownership of Our Common Stock
The price of our common stock may be volatile, and you may lose all or part of your investment.
The market price of our common stock could fluctuate significantly, and you may not be able to resell your shares near, at or above the price that you purchased them. Those fluctuations could be based on various factors in addition to those otherwise described in this Annual Report on Form 10-K, including those described under “Risk Factors—Risks Related to Our Business,” “Risk Factors—Risks Related to Our Industry,” “Risk Factors—Risks Related to Our Reliance on Third Parties” and “Risk Factors—Risks Related to Intellectual Property,” and the following:
|
|
• |
unfavorable developments relating to the regulatory status of our product candidates, such as the FDA refusing to accept for filing our NDA or issuing a complete response letter, or a delay in the regulatory review process; |
|
|
• |
adverse actions taken by regulatory authorities with respect to our clinical studies, manufacturing supply chain or future sales and marketing activities; |
|
|
• |
unfavorable results from our clinical studies; |
|
|
• |
delays in the initiation or completion of our clinical studies; |
|
|
• |
adverse changes to our relationships with third-party service providers; |
|
|
• |
manufacture, supply or distribution shortages; |
|
|
• |
departures of our management; |
|
|
• |
a change in competitive landscape that is unfavorable to our product candidates; |
|
|
• |
actual or threatened intellectual property litigation that involves our product candidates; |
|
|
• |
adverse developments concerning the pharmaceutical industry in general; |
|
|
• |
higher-than-expected expenses related to our development programs or overall corporate operations; |
|
|
• |
financial results that are not in line with analyst expectations; |
|
|
• |
changes in analyst estimates, ratings and price targets; |
|
|
• |
press reports or other negative publicity, whether or not true, about our business; |
|
|
• |
release or expiry of lock-up or other transfer restrictions on our outstanding common stock; |
|
|
• |
sales or perceived potential sales of additional common stock; |
|
|
• |
sales of our common stock by us, our executive officers and directors or our stockholders in the future; |
|
|
• |
fluctuations in the stock prices of pharmaceutical and biotechnology stocks; and |
|
|
• |
general economic and market conditions and overall fluctuations in the U.S. equity markets. |
Any of these factors may result in large and sudden changes in the volume and trading price of our common stock. In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of management, result in negative press reports and, if adversely determined, have a material adverse effect on our results of operations and financial condition.
In addition, the Nasdaq Global Select Market, in general, and the stocks of small pharmaceutical and biotechnology companies, in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. Further, a decline in the financial markets and related factors beyond our control may cause our common stock price to decline rapidly and unexpectedly.
60
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended (“Exchange Act”). Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the U.S. SEC.
Disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Future sales of our common stock, or the perception that such sales may occur, could depress our common stock price.
We will need additional capital in the future to continue our planned operations. Sales of a substantial number of shares of our common stock in the public market, or the perception that such sales may occur, could depress the market price of our common stock. Our principal stockholders, executive officers and directors and certain other equity holders have agreed with the underwriters of our initial public offering not to offer, sell, dispose of or hedge any shares of common stock or securities convertible into or exchangeable for shares of common stock, subject to specified limited exceptions and extensions, during the period ending on June 4, 2018 (subject to extension), except with the prior written consent of Goldman Sachs & Co. LLC and Jefferies LLC. All of our outstanding shares will be freely tradable after the expiration date of the lock-up agreements, except for any shares held or acquired by persons who may be deemed to be our affiliates. Shares of our common stock held by our affiliates will continue to be subject to the volume and other restrictions of Rule 144 under the U.S. Securities Act of 1933 (“Securities Act”). Goldman Sachs & Co. LLC and Jefferies LLC may, in their sole discretion and at any time without notice, release all or any portion of the shares subject to the lock-up. Sales of a substantial number of such shares on expiration of the lock-up and market stand-off agreements, the perception that such sales may occur or early release of these agreements could cause our market price to fall or make it more difficult for you to sell your common stock at a time and price that you deem appropriate.
Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of our common stock for return on your investment.
We intend to retain most, if not all, of our available funds and earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our common stock as a source for any future dividend income.
Our board of directors (“Board”) has significant discretion as to whether to distribute dividends and in what amounts. Even if our Board decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our Board.
61
Our directors, executive officers and principal stockholders have substantial control over the Company, which could limit your ability to influence the outcome of key transactions, including a change of control.
Our current directors, officers and stockholders who own greater than 5% of our outstanding common stock, together with their affiliates, beneficially own, in the aggregate, approximately 73% of our outstanding common stock, based on the number of shares outstanding as of February 1, 2018. As a result, these current directors, officers and stockholders, if they act, will be able to influence or control matters requiring approval by our stockholders, including the election of directors and the approval of mergers, acquisitions or other extraordinary transactions. In addition, our current directors, officers and stockholders, acting together, would have the ability to control the management and affairs of our company. They may also have interests that differ from yours and may vote in a way with which you disagree and that may be adverse to your interests. This concentration of ownership may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company and could affect the market price of our common stock.
We are an emerging growth company, as defined in the Securities Act, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company, as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including relief from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, less extensive disclosure obligations regarding executive compensation in our registration statements, periodic reports and proxy statements, exemptions from the requirements to hold a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved and an extended transition period for complying with new or revised accounting standards. As a result, our stockholders may not have access to certain information that they may deem important. We could be an emerging growth company for up to 5 years, although circumstances could cause us to lose that status earlier, including if our total annual gross revenues exceed $1.07 billion, if we issue more than $1.0 billion in non-convertible debt during any three-year period, or if the market value of our common stock held by non-affiliates exceeds $700 million as of June 30 of any year.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and, as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
62
Item 1B. Unresolved Staff Comments
None.
Our principal executive offices are located at 4747 Executive Drive, Suite 510, San Diego, California 92121. We also maintain offices at 18 W. 18th St., New York, New York 10011. We lease approximately 8,300 square feet of office space in San Diego and approximately 1,000 square feet of office space in New York. These leases are on a month-to-month basis.
We are not currently a party to any material legal proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
63
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock began trading on the Nasdaq Global Select Market on December 7, 2017, under the symbol “ODT.” Prior to that time, there was no public market for our common stock. The following table sets forth the high and low sales prices per share of our common stock as reported on the Nasdaq Global Select Market for the period indicated.
|
Year Ended December 31, 2017 |
|
High |
|
|
Low |
|
||
|
Fourth Quarter (Beginning December 7, 2017) |
|
$ |
25.37 |
|
|
$ |
22.31 |
|
Comparative Stock Performance Graph
The following performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the U.S. Securities and Exchange Commission (“SEC”), nor shall such information be incorporated by reference into any future filing under the Securities Act or Exchange Act.
The following graph shows a comparison, from December 7, 2017 (the date our common stock began trading on the Nasdaq Global Select Market) through December 31, 2017, of the cumulative total return for our common stock, the Nasdaq Composite Index and the Nasdaq Biotechnology Index. The graph assumes an initial investment of $100 on December 7, 2017. The comparisons in the graph are not intended to forecast or be indicative of possible future performance of our common stock.
64
As of February 1, 2018, we had approximately 23 holders of record of our common stock. Certain shares are held in “street” name, and, accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never declared or paid any cash dividends on our common stock and do not currently intend to do so for the foreseeable future. Any determination to pay dividends to holders of our common stock will be at the discretion of our Board and will depend on many factors, including our financial condition, results of operations, projections, liquidity, earnings, legal requirements, restrictions in the agreements governing any indebtedness we may enter into and other factors that our Board deems relevant.
Use of Proceeds from Initial Public Offering
On December 11, 2017, we closed our initial public offering (“IPO”) of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters in the IPO purchased 441,073 shares of common stock in connection with the exercise of their option to purchase additional shares of common stock. The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs. The offer and sale of the shares of common stock in the IPO were registered pursuant to a registration statement on Form S-1 (File No. 333-221533), which the SEC declared effective on December 6, 2017. No offering expenses were paid directly or indirectly to any of our directors or officers (or their associates) or persons owning 10% or more of any class of our equity securities or to any other affiliates. The underwriters for our IPO were Goldman Sachs & Co. LLC, Jefferies LLC and Cowen and Company, LLC.
There has been no material change in the use of proceeds from our IPO as described in our final prospectus filed with the SEC pursuant to Rule 424(b)(4) on December 8, 2017.
Item 6. Selected Financial Data
The following table summarizes the historical financial and operating data for the periods indicated. The historical statement of operations data for the years ended December 31, 2017 and 2016 and the balance sheet data as of December 31, 2017 and 2016 are derived from the financial statements and related notes included in Item 8 of this Annual Report on Form 10-K.
65
The historical results presented below are not necessarily indicative of the results to be expected for any future period. This information should be read in conjunction with “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes included in Item 8 of this Annual Report on Form 10-K. Our financial statements are prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”).
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
(in thousands, except share and per share amounts) |
|
|||||||||
|
Statements of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
27,902 |
|
|
$ |
2,622 |
|
|
$ |
- |
|
|
General and administrative |
|
|
4,842 |
|
|
|
463 |
|
|
|
158 |
|
|
Total operating expenses |
|
|
32,744 |
|
|
|
3,085 |
|
|
|
158 |
|
|
Net loss |
|
$ |
(32,744 |
) |
|
$ |
(3,085 |
) |
|
$ |
(158 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(2.31 |
) |
|
$ |
(0.54 |
) |
|
$ |
(0.17 |
) |
|
Weighted average shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
14,169,464 |
|
|
|
5,762,419 |
|
|
|
922,706 |
|
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Selected Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
198,105 |
|
|
$ |
2,599 |
|
|
$ |
138 |
|
|
Working capital |
|
|
195,434 |
|
|
|
2,269 |
|
|
|
111 |
|
|
Total assets |
|
|
203,494 |
|
|
|
2,881 |
|
|
|
140 |
|
|
Total liabilities |
|
|
7,512 |
|
|
|
598 |
|
|
|
29 |
|
|
Accumulated deficit |
|
|
(39,292 |
) |
|
|
(6,548 |
) |
|
|
(3,463 |
) |
|
Total stockholders’ equity |
|
|
195,982 |
|
|
|
2,283 |
|
|
|
111 |
|
66
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our audited financial statements and the related notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, include forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” set forth in this Annual Report on Form 10-K for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a pharmaceutical company dedicated to the development of best-in-class therapeutics that improve and extend the lives of patients with cancer. Our initial focus is on the development of tesetaxel, an investigational, orally administered chemotherapy agent that belongs to a class of drugs known as taxanes, which are widely used in the treatment of cancer. Tesetaxel has several potential therapeutic advantages over currently available taxanes, including: oral administration with a low pill burden and a patient-friendly dosing regimen; a formulation that does not contain solubilizing agents that are known to cause hypersensitivity (allergic) reactions; and improved activity against chemotherapy-resistant tumors. Tesetaxel has been generally well tolerated in clinical studies and has demonstrated robust single-agent antitumor activity in two multicenter, Phase 2 studies in patients with locally advanced or metastatic breast cancer (“MBC”). We are conducting a multinational, multicenter, randomized, Phase 3 study in MBC, known as CONTESSA, and we expect to report top-line results from this study in 2020. Our goal for tesetaxel is to develop an effective chemotherapy choice for patients that provides quality-of-life advantages over current alternatives.
On December 6, 2017, we converted from a Delaware limited liability company to a Delaware corporation by filing a certificate of conversion with the Delaware Secretary of State, and we changed our name from “Odonate Therapeutics, LLC” to “Odonate Therapeutics, Inc.” (the “Conversion”). As a result of the Conversion, the membership interests of Odonate Therapeutics, LLC were converted into shares of Odonate Therapeutics, Inc. Prior to the Conversion, we formed a holding company named Odonate Holdings, LLC (“Odonate Holdings”) to own Odonate Therapeutics, LLC. Following the Conversion, Odonate Holdings distributed its interest in Odonate Therapeutics, Inc. to prior members of Odonate Therapeutics, LLC, but retained record title to the 2,931,402 shares of common stock underlying outstanding incentive units previously granted to employees, officers, directors and consultants by Odonate Management Holdings, LLC and 154,285 shares of common stock beneficially owned by Tang Capital Partners, LP. All common stock and per share amounts for all periods presented in this Annual Report on Form 10-K have been adjusted retroactively, where applicable, to reflect the Conversion.
On December 11, 2017, we closed our initial public offering (“IPO”) of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters in the IPO purchased 441,073 shares of common stock in connection with the exercise of their option to purchase additional shares of common stock. The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs.
Components of Our Results of Operations
Research and Development Expense
Research and development expenses consist primarily of costs associated with the development of our product candidates and include salaries, benefits, travel and other related costs, including equity-based compensation expenses, for personnel engaged in research and development functions; expenses incurred under agreements with contract research organizations (“CROs”), investigative sites and consultants that conduct our preclinical and clinical studies; manufacturing development and scale-up expenses and the cost of acquiring and manufacturing clinical study materials and commercial materials,
67
including manufacturing registration and validation batches; payments to consultants engaged in the development of our product candidates, including equity-based compensation, travel and other expenses; costs related to compliance with quality and regulatory requirements; and research and development facility-related expenses, which include direct and allocated expenses, and other related costs.
Research and development expenses are charged to operations as incurred when these expenditures relate to our research and development efforts and have no alternative future uses. Payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
All of our research and development expenses to date have been incurred in connection with tesetaxel. We expect our research and development expenses to increase for the foreseeable future as we advance tesetaxel through clinical development, including the conduct of our ongoing Phase 3 study, CONTESSA.
General and Administrative Expense
General and administrative expenses consist primarily of salaries, related benefits, travel, equity-based compensation expense and facility-related expenses for personnel in finance and administrative functions. General and administrative expenses also include professional fees for legal, patent, consulting, accounting and audit services and other related costs.
We anticipate that our general and administrative expenses will increase in the future as we build our infrastructure to support our continued research and development of tesetaxel. We also anticipate increased expenses related to accounting, legal and regulatory-related services associated with maintaining compliance with exchange listing and the U.S. Securities and Exchange Commission (“SEC”) requirements, director and officer insurance premiums and other costs associated with being a public company.
Results of Operations
The following table summarizes our results of operations for each of the periods set forth below:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
27,902 |
|
|
$ |
2,622 |
|
|
General and administrative |
|
|
4,842 |
|
|
|
463 |
|
|
Total operating expenses |
|
$ |
32,744 |
|
|
$ |
3,085 |
|
Research and Development Expense
The following table summarizes our research and development expense for each of the periods set forth below:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Clinical development costs |
|
$ |
18,129 |
|
|
$ |
1,223 |
|
|
Personnel and related costs |
|
|
6,957 |
|
|
|
1,295 |
|
|
Equity-based compensation expense |
|
|
2,633 |
|
|
|
51 |
|
|
Other research and development costs |
|
|
183 |
|
|
|
53 |
|
|
Total research and development expense |
|
$ |
27,902 |
|
|
$ |
2,622 |
|
68
Research and development expense was $27.9 million and $2.6 million for the years ended December 31, 2017 and 2016, respectively. The increase of $25.3 million was primarily due to increased activities in connection with our tesetaxel clinical development program, including the initiation of CONTESSA, resulting in increased clinical development costs of $16.9 million, increased personnel and related costs of $5.7 million and increased equity-based compensation expense of $2.6 million.
General and Administrative Expense
The following table summarizes our general and administrative expense for each of the periods set forth below:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
|
|
(in thousands) |
|
|||||
|
General and administrative costs |
|
$ |
4,291 |
|
|
$ |
463 |
|
|
Equity-based compensation expense |
|
|
551 |
|
|
|
- |
|
|
Total general and administrative expense |
|
$ |
4,842 |
|
|
$ |
463 |
|
General and administrative expense was $4.9 million and $0.5 million for the years ended December 31, 2017 and 2016, respectively. The increase of $4.4 million was due to increased administrative support costs in connection with our tesetaxel clinical development program, including increased personnel, professional and other general administrative costs of $3.8 million and increased equity-based compensation expense of $0.6 million.
Liquidity and Capital Resources
As of December 31, 2017 and 2016, we had cash in the amount of $198.1 million and $2.6 million, respectively. We believe that our existing cash as of December 31, 2017 will be sufficient to meet our anticipated cash requirements through at least one year from the date this Annual Report on Form 10-K is filed with the SEC.
We have incurred losses in each year since our inception. Our net loss was $32.7 million and $3.1 million for the years ended December 31, 2017 and 2016, respectively. As of December 31, 2017 and December 31, 2016, we had an accumulated deficit of $39.3 million and $6.5 million, respectively. Substantially all of our operating losses resulted from expenses incurred in connection with advancing tesetaxel through development activities and general and administrative costs associated with our operations.
To date, we have funded our operations through the sale of equity securities. Since our inception, we have raised $238.1 million in net proceeds from the sale of equity securities.
The following table sets forth a summary of the net cash flow activity for each of the periods set forth below:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(25,858 |
) |
|
$ |
(1,733 |
) |
|
Investing activities |
|
|
(83 |
) |
|
|
- |
|
|
Financing activities |
|
|
221,447 |
|
|
|
4,194 |
|
|
Net increase in cash |
|
$ |
195,506 |
|
|
$ |
2,461 |
|
69
Net cash used in operating activities was $25.9 million and $1.7 million for the years ended December 31, 2017 and 2016, respectively. Net cash used in operating activities was the result of our net loss and change in working capital, partially offset by non-cash contributions for expenses by an affiliate of a significant stockholder, equity-based compensation and depreciation expense.
Net cash used in investing activities was $0.1 million for the year ended December 31, 2017. Net cash used in investing activities was the result of purchases of property and equipment. No cash was used in investing activities for the year ended December 31, 2016.
Net cash provided by financing activities was $221.5 million and $4.2 million for the years ended December 31, 2017 and 2016, respectively. For the year ended December 31, 2017, net cash provided by financing activities was the result of our sale of common stock in our IPO for net proceeds of $137.5 million and, prior to our IPO, our sale of common stock in private financings for net proceeds of $84.0 million. For the year ended December 31, 2016, net cash provided by financing activities was the result of our sale of common stock, net of capital distributions of $0.8 million to our common stockholders.
Until such time as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic partnerships and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic partnerships or licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, associated intellectual property, our other technologies, future revenue streams or research programs or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our product candidate even if we would otherwise prefer to develop and market such product candidate ourselves.
Contractual Obligations and Commitments
We enter into contracts in the normal course of business with CROs, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancelable contracts and not included in the table of contractual obligations and commitments.
In 2013, we licensed rights to tesetaxel in all major markets from Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), the original inventor of the product. Under the Daiichi Sankyo license agreement, we are obligated to use commercially reasonable efforts to develop and commercialize tesetaxel in the following countries: France, Germany, Italy, Spain, the United Kingdom and the U.S. We are required to make aggregate future milestone payments of up to $31.0 million, contingent on attainment of certain regulatory milestones. Additionally, we will pay Daiichi Sankyo a tiered royalty that ranges from the low to high single digits, depending on annual net sales of tesetaxel.
70
A summary of our contractual obligations and commitments as of December 31, 2017 is set forth below (in thousands):
|
|
|
Payments Due by Period |
|
|||||||||||||||||
|
|
|
Less than 1 Year |
|
|
1 to 3 Years |
|
|
3 to 5 Years |
|
|
More than 5 Years |
|
|
Total Amounts Committed |
|
|||||
|
Milestone payments(1) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
31,000 |
|
|
(1) |
Represents potential aggregate future milestone payment amounts to Daiichi Sankyo. The actual amount and timing of these payments are uncertain, as the payments are contingent on future events. |
Off-Balance Sheet Arrangements
During the periods presented, we did not have, nor do we currently have, any off-balance sheet arrangements as defined under the rules of the SEC.
Jumpstart Our Business Startups Act
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under this act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. However, we also intend to rely on other exemptions provided by the JOBS Act, including without limitation, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, as amended.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses during the reporting periods. These items are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our audited financial statements elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies related to accrued expenses and equity-based compensation are most critical to understanding and evaluating our reported financial results.
Accrued Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses
71
as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued expenses include costs associated with conducting our development and regulatory activities, including fees paid to third-party professional consultants and service providers, and costs to develop and manufacture clinical study materials.
We base our accrued expenses on our estimates of the services received and efforts expended pursuant to our contractual arrangements. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our service providers will exceed the level of services provided and result in a prepayment of the expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepayment accordingly.
Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differs from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. To date, there have been no material differences from our estimates to the amount actually incurred.
Equity-based Compensation
Equity-based compensation expense represents the grant-date fair value of employee awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. We account for awards to non-employees using the fair value approach. Non-employee awards are subject to periodic revaluation over their vesting terms.
Following the Conversion, we adopted the Odonate Therapeutics, Inc. 2017 Stock Option Plan (the “2017 Plan”) in order to grant stock options to our employees, officers, directors and consultants. Recipients of stock options are eligible to purchase shares of our common stock at an exercise price equal to the fair market value of such stock on the date of grant. The maximum term of options granted under the 2017 Plan is 10 years. The options generally vest over a 4-year period from either the date of grant or the commencement of service.
Following the Conversion, we adopted the Odonate Therapeutics, Inc. 2017 Employee Stock Purchase Plan (the “ESPP”) in order to provide a means for eligible employees to accumulate shares of our common stock over time through regular payroll deductions. Under the ESPP, eligible employees may purchase shares of our common stock twice per month at a price equal to 85% of the closing price of our common stock on the date of each purchase. Eligible employees purchasing shares under the ESPP are subject to an annual cap equal to the lesser of $25,000 or 10% of the employee’s annual cash compensation. Shares purchased under the ESPP cannot be sold for a period of one year following the purchase date (or such shorter period of time if the participating employee’s employment terminates before this one-year anniversary). Enrollment under the ESPP had not commenced as of December 31, 2017.
Prior to the Conversion, we, through Odonate Management Holdings, LLC (“Management Holdings”), issued an aggregate of 2,931,402 incentive units under the Odonate Management Holdings Equity Incentive Plan (the “Management Plan”). The incentive units were issued to certain of our directors, officers, employees and consultants in consideration for bona fide services provided to us. Pursuant to the Management Plan, the incentive units are considered “profits interests” within the meaning of U.S. federal and state tax rules. Incentive units do not entitle their holders to receive distributions if we were to be liquidated immediately after the grant. Instead, the incentive unitholders are entitled to receive an allocation of a portion of our profits arising after the date of the grant and, subject to vesting conditions, distributions made out of a portion of our profits arising after the grant date of the incentive units. Accordingly, the financial benefits of incentive units to the awardee, and the costs to the issuing company, are substantially similar to a stock option grant. All incentive units generally vest over a 4-year period from either the date of grant or the commencement of service. Following the Conversion, we have not granted, and will no longer grant, incentive units under the Management Plan.
72
Following the Conversion, the outstanding incentive units of Management Holdings remain outstanding, but represent an indirect interest in the common stock of the Company on vesting of the awards. As part of the Conversion, the shares of common stock underlying the outstanding incentive units were issued to a newly formed entity, Odonate Holdings, LLC (“Odonate Holdings”). While Odonate Holdings holds the shares of common stock underlying outstanding incentive units, it has no other operations. In addition, Odonate Holdings granted us an irrevocable proxy directing us to vote all shares of common stock underlying outstanding incentive units held by Odonate Holdings in the same proportion as the votes cast by all other stockholders, which is sometimes called “mirrored voting.” In the event that any incentive units in Management Holdings are forfeited, the shares of common stock underlying such forfeited incentive units will be transferred from Odonate Holdings to us and cancelled.
As of December 31, 2017 and December 31, 2016, there were 2,931,402 and 1,934,716 incentive units outstanding, respectively, all of which are incentive units in Odonate Holdings and represent an indirect right, subject to vesting, in our common stock. As of December 31, 2017 and December 31, 2016, we had 183,699 and 0 stock options outstanding, respectively. The grant-date fair value of equity awards was estimated using a Black-Scholes option-pricing model. We used a volatility and risk-free rate of 73% to 79% and 1.3% to 2.5%, respectively, to estimate the fair value of the equity awards. The estimated volatility was based on the historical equity volatility of comparable companies.
Prior to our IPO, there was no public market for our common stock and incentive units at the time of grant. As such, the fair value of the common stock and incentive units were estimated on each grant date by our management. In order to determine the fair value of our common stock and incentive units, our management considered, among other things, recently available independent valuations and valuations derived from the sale of our equity securities to third parties in recent equity financings. These independent valuations were performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation.
Following the closing of our IPO, we determine the fair value of our common stock based on the closing price of our common stock as reported on the Nasdaq Global Select Market.
Recent Accounting Pronouncements
See Note 2 to the financial statements included in Item 8 of this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
We do not currently have any cash equivalents or investments, but we do maintain significant amounts of cash at one or more financial institutions that are in excess of federally insured limits.
Effects of Inflation
Inflation generally affects us by increasing our cost of labor and clinical study costs. We do not believe that inflation has had a material effect on our results of operations during the periods presented.
Effects of Foreign Currency Exchange Rates
We are exposed to market risk related to changes in foreign currency exchange rates. From time to time, we engage in contractual arrangements with service providers globally. We are therefore subject to fluctuations in foreign currency rates in connection with these arrangements. We do not currently hedge our foreign currency exchange rate risk. As of December 31, 2017, we had minimal or no liabilities denominated in foreign currencies.
73
Item 8. Financial Statements and Supplementary Data
Our financial statements, together with the report of our independent registered public accounting firm, appear in this Annual Report on Form 10-K beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Management’s Evaluation of our Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, evaluated, as of the end of the period covered by this Annual Report on Form 10-K, the effectiveness of our disclosure controls and procedures. Based on that evaluation of our disclosure controls and procedures as of December 31, 2017, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date are effective at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (“Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Management’s Annual Report on Internal Control Over Financial Reporting
This Annual Report on Form 10-K does not include a report of management’s assessment regarding our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) or an attestation report of our independent registered accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies. Additionally, our auditors will not be required to formally opine on the effectiveness of our internal control over financial reporting pursuant to Section 404 until we are no longer an “emerging growth company” as defined in the JOBS Act.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting that occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Effective February 12, 2018, Robert Millham, the Company’s Chief Operating Officer, resigned to pursue other interests. In connection with his departure, the Company agreed to continue to pay his base salary for a period of one year from the date of his resignation.
74
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item is incorporated by reference to the information set forth in the sections titled “Executive Officers” and “The Board of Directors and Its Committees” in our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the U.S. Securities and Exchange Commission (“SEC”) within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
We have adopted a Code of Conduct that applies to all of our directors, officers and employees, including our principal executive officer and principal financial officer. Our Code of Conduct is posted on our website located at www.odonate.com. We intend to disclose any material future amendments to provisions of the Code of Conduct, and waivers of the Code of Conduct granted to executive officers and directors, on the website within four business days following the date of the amendment or waiver.
Item 11. Executive Compensation
The information required by this item is incorporated by reference to the information set forth in the section titled “Executive Officer and Director Compensation” in our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is incorporated by reference to the information set forth in the section titled “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” in our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated by reference to the information set forth in the section titled “Certain Relationships and Related Party Transactions” in our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
Item 14. Principal Accountant Fees and Services
The information required by this item is incorporated by reference to the information set forth in the section titled “Independent Registered Public Accounting Firm Fees” in our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the SEC within 120 days of the end of the fiscal year covered by this Annual Report on Form 10-K.
75
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Financial Statements
The response to this portion of Item 15 is set forth under Item 8 hereof.
(a)(2) Financial Statement Schedules
No financial statement schedules are provided because the information called for is not required or is shown in the financial statements or the notes thereto.
(a)(3) Exhibits
|
Exhibit |
|
Description |
|
|
|
|
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
10.1+* |
|
Limited Liability Company Operating Agreement of Odonate Holdings, LLC |
|
|
|
|
|
10.2+* |
|
|
|
|
|
|
|
10.3+* |
|
|
|
|
|
|
|
10.4+* |
|
Odonate Therapeutics, Inc. 2017 Employee Stock Purchase Plan |
|
|
|
|
|
10.5† |
|
|
|
|
|
|
|
10.6+ |
|
|
|
|
|
|
|
24.1 |
|
|
|
|
|
|
|
31.1* |
|
|
|
|
|
|
|
31.2* |
|
|
|
|
|
|
|
32.1# |
|
|
|
* |
Filed herewith |
|
† |
Confidential treatment has been granted with respect to certain portions (indicated by asterisks) of this exhibit. Omitted portions have been filed separately with the SEC. |
|
+ |
Indicates a management contract or compensatory plan or arrangement |
|
# |
Furnished herewith and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act |
None.
76
Pursuant to the requirements of Section 13 of 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
ODONATE THERAPEUTICS, INC. |
||
|
|
|
|
|
|
Date: February 14, 2018 |
By: |
|
/s/ Kevin C. Tang |
|
|
|
|
Kevin C. Tang |
|
|
|
|
Chairman and Chief Executive Officer |
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below hereby constitutes and appoints Kevin C. Tang and John G. Lemkey, and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, to sign in any and all capacities (including, without limitation, the capacities listed below), with respect to this Annual Report on Form 10-K, and any and all amendments thereto, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done to enable Odonate Therapeutics, Inc. to comply with the provisions of the Securities Exchange Act and all the requirements of the Securities and Exchange Commission, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities set forth opposite their names and on the dates indicated below.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Kevin C. Tang Kevin C. Tang |
|
Chairman and Chief Executive Officer (principal executive officer) |
|
February 14, 2018 |
|
|
|
|
|
|
|
/s/ John G. Lemkey John G. Lemkey |
|
Chief Financial Officer (principal financial and accounting officer) |
|
February 14, 2018 |
|
|
|
|
|
|
|
/s/ Jeff L. Vacirca, M.D. Jeff L. Vacirca, M.D. |
|
Director, Vice Chairman |
|
February 14, 2018 |
|
|
|
|
|
|
|
/s/ Aaron I. Davis Aaron I. Davis |
|
Director |
|
February 14, 2018 |
|
|
|
|
|
|
|
/s/ Craig A. Johnson Craig A. Johnson |
|
Director |
|
February 14, 2018 |
|
|
|
|
|
|
|
/s/ Robert H. Rosen Robert H. Rosen |
|
Director |
|
February 14, 2018 |
|
|
|
|
|
|
|
/s/ George F. Tidmarsh George F. Tidmarsh, M.D., Ph.D. |
|
Director |
|
February 14, 2018 |
77
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
Odonate Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Odonate Therapeutics, Inc. (the Company) as of December 31, 2017 and 2016, the related statements of operations, stockholders’ equity and cash flows for the years then ended, and the related notes to the financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Squar Milner LLP
We have served as the Company's auditor since 2017.
San Diego, California
February 14, 2018
F-2
(in thousands, except par value and share amounts)
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
198,105 |
|
|
$ |
2,599 |
|
|
Prepaid expenses |
|
|
4,841 |
|
|
|
268 |
|
|
Total current assets |
|
|
202,946 |
|
|
|
2,867 |
|
|
Property and equipment, net |
|
|
165 |
|
|
|
14 |
|
|
Other |
|
|
383 |
|
|
|
- |
|
|
Total assets |
|
$ |
203,494 |
|
|
$ |
2,881 |
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
4,302 |
|
|
$ |
572 |
|
|
Accrued expenses |
|
|
3,210 |
|
|
|
26 |
|
|
Total current liabilities |
|
|
7,512 |
|
|
|
598 |
|
|
Commitments and contingencies (Note 4) |
|
|
|
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
|
|
|
Common stock, $0.01 par value—100,000,000 shares authorized; 26,890,356 and 12,082,514 shares issued and outstanding at December 31, 2017 and 2016, respectively |
|
|
240 |
|
|
|
102 |
|
|
Additional paid-in capital |
|
|
235,034 |
|
|
|
8,729 |
|
|
Accumulated deficit |
|
|
(39,292 |
) |
|
|
(6,548 |
) |
|
Total stockholders' equity |
|
|
195,982 |
|
|
|
2,283 |
|
|
Total liabilities and stockholders' equity |
|
$ |
203,494 |
|
|
$ |
2,881 |
|
See accompanying notes.
F-3
(in thousands, except share and per share amounts)
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
27,902 |
|
|
$ |
2,622 |
|
|
General and administrative |
|
|
4,842 |
|
|
|
463 |
|
|
Total operating expenses |
|
|
32,744 |
|
|
|
3,085 |
|
|
Net loss |
|
$ |
(32,744 |
) |
|
$ |
(3,085 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(2.31 |
) |
|
$ |
(0.54 |
) |
|
Weighted average shares outstanding: |
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
14,169,464 |
|
|
|
5,762,419 |
|
See accompanying notes.
F-4
Statements of Stockholders’ Equity
(in thousands, except share amounts)
|
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Total Stockholders' |
|
||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
|
Balance at December 31, 2015 |
|
|
922,706 |
|
|
|
9 |
|
|
$ |
3,565 |
|
|
$ |
(3,463 |
) |
|
$ |
111 |
|
|
Redemption of common stock |
|
|
(58,670 |
) |
|
|
- |
|
|
|
(32 |
) |
|
|
- |
|
|
|
(32 |
) |
|
Issuance of common stock, net of issuance costs |
|
|
9,283,762 |
|
|
|
93 |
|
|
|
4,924 |
|
|
|
- |
|
|
|
5,017 |
|
|
Issuance of common stock underlying incentive units |
|
|
1,934,716 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Non-cash contributions for expenses |
|
|
- |
|
|
|
- |
|
|
|
1,012 |
|
|
|
- |
|
|
|
1,012 |
|
|
Equity-based compensation |
|
|
- |
|
|
|
- |
|
|
|
51 |
|
|
|
- |
|
|
|
51 |
|
|
Distributions |
|
|
- |
|
|
|
- |
|
|
|
(791 |
) |
|
|
- |
|
|
|
(791 |
) |
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(3,085 |
) |
|
|
(3,085 |
) |
|
Balance at December 31, 2016 |
|
|
12,082,514 |
|
|
|
102 |
|
|
|
8,729 |
|
|
|
(6,548 |
) |
|
|
2,283 |
|
|
Issuance of common stock, net of issuance costs |
|
|
13,811,156 |
|
|
|
138 |
|
|
|
221,309 |
|
|
|
- |
|
|
|
221,447 |
|
|
Issuance of common stock underlying incentive units |
|
|
996,686 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Non-cash contributions for expenses |
|
|
- |
|
|
|
- |
|
|
|
1,812 |
|
|
|
- |
|
|
|
1,812 |
|
|
Equity-based compensation |
|
|
- |
|
|
|
- |
|
|
|
3,184 |
|
|
|
- |
|
|
|
3,184 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(32,744 |
) |
|
|
(32,744 |
) |
|
Balance at December 31, 2017 |
|
|
26,890,356 |
|
|
$ |
240 |
|
|
$ |
235,034 |
|
|
$ |
(39,292 |
) |
|
$ |
195,982 |
|
See accompanying notes.
F-5
(in thousands)
|
|
|
Year Ended December, 31 |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(32,744 |
) |
|
$ |
(3,085 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Equity-based compensation |
|
|
3,184 |
|
|
|
51 |
|
|
Non-cash contributions for expenses |
|
|
1,812 |
|
|
|
1,012 |
|
|
Depreciation |
|
|
20 |
|
|
|
- |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Prepaid expenses and other assets |
|
|
(4,956 |
) |
|
|
(266 |
) |
|
Accounts payable |
|
|
3,642 |
|
|
|
529 |
|
|
Accrued expenses |
|
|
3,184 |
|
|
|
26 |
|
|
Net cash used in operating activities |
|
|
(25,858 |
) |
|
|
(1,733 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(83 |
) |
|
|
- |
|
|
Net cash used in investing activities |
|
|
(83 |
) |
|
|
- |
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net of issuance costs |
|
|
221,447 |
|
|
|
5,017 |
|
|
Redemption of common stock |
|
|
- |
|
|
|
(32 |
) |
|
Distributions |
|
|
- |
|
|
|
(791 |
) |
|
Net cash provided by financing activities |
|
|
221,447 |
|
|
|
4,194 |
|
|
Net increase in cash |
|
|
195,506 |
|
|
|
2,461 |
|
|
Cash, beginning of period |
|
|
2,599 |
|
|
|
138 |
|
|
Cash, end of period |
|
$ |
198,105 |
|
|
$ |
2,599 |
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
Property and equipment purchases included in accounts payable |
|
$ |
88 |
|
|
$ |
14 |
|
See accompanying notes.
F-6
1. Business
Odonate Therapeutics, Inc. (“Odonate” or the “Company”) is a pharmaceutical company dedicated to the development of best-in-class therapeutics that improve and extend the lives of patients with cancer. The Company’s initial focus is on the development of tesetaxel, an investigational, orally administered chemotherapy agent that belongs to a class of drugs known as taxanes, which are widely used in the treatment of cancer. Tesetaxel has several potential therapeutic advantages over currently available taxanes, including: oral administration with a low pill burden and a patient-friendly dosing regimen; a formulation that does not contain solubilizing agents that are known to cause hypersensitivity (allergic) reactions; and improved activity against chemotherapy-resistant tumors. Tesetaxel has been generally well tolerated in clinical studies and has demonstrated robust single-agent antitumor activity in two multicenter, Phase 2 studies in patients with locally advanced or metastatic breast cancer (“MBC”). The Company is conducting a multinational, multicenter, randomized, Phase 3 study in MBC, known as CONTESSA. The Company’s goal for tesetaxel is to develop an effective chemotherapy choice for patients that provides quality-of-life advantages over current alternatives.
On December 6, 2017, the Company converted from a Delaware limited liability company to a Delaware corporation by filing a certificate of conversion with the Delaware Secretary of State, and the Company changed its name from “Odonate Therapeutics, LLC” to “Odonate Therapeutics, Inc.” (the “Conversion”). As a result of the Conversion, the membership interests of Odonate Therapeutics, LLC were converted into shares of Odonate Therapeutics, Inc. Prior to the Conversion, the Company formed a holding company named Odonate Holdings, LLC (“Odonate Holdings”) to own Odonate Therapeutics, LLC. Following the Conversion, Odonate Holdings distributed its interest in Odonate Therapeutics, Inc. to prior members of Odonate Therapeutics, LLC, but retained record title to the 2,931,402 shares of common stock underlying outstanding incentive units previously granted to employees, officers, directors and consultants by Odonate Management Holdings, LLC and 154,285 shares of common stock beneficially owned by Tang Capital Partners, LP. All common stock and per share amounts for all periods presented in the accompanying financial statements and notes thereto have been adjusted retroactively, where applicable, to reflect the Conversion.
On December 11, 2017, the Company closed its initial public offering (“IPO”) of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters in the IPO purchased 441,073 shares of common stock in connection with the exercise of their option to purchase additional shares of common stock (see Note 12). The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs.
As of December 31, 2017, the Company had $198.1 million in cash. The Company has incurred operating losses and negative cash flows from operations since inception. Management believes the Company’s existing cash as of December 31, 2017 will be sufficient to meet the Company’s anticipated cash requirements through at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission (“SEC”).
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The Company’s financial statements are prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”). The preparation of the Company’s financial statements requires it to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in the Company’s financial statements and accompanying notes. The most significant estimates in the Company’s financial statements relate to accrued expenses and equity-based compensation. These estimates and assumptions are based on current facts, historical experience and various other factors believed to be reasonable under the
F-7
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially and adversely from these estimates. To the extent there are material differences between the estimates and actual results, the Company’s future results of operations will be affected.
Common Stock Splits
In November 2017, the Company completed a 2-for-1 forward split of the Company’s outstanding common stock. The accompanying financial statements and notes to the financial statements give retroactive effect to the forward split for all periods presented.
In August 2016, the Company completed a 50-for-1 reverse split of the Company’s outstanding common stock. The accompanying financial statements and notes to the financial statements give retroactive effect to the reverse split for all periods presented.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment.
Fair Value Measurements
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or non-recurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
|
|
Level 1: |
Observable inputs such as quoted prices in active markets. |
|
|
Level 2: |
Inputs, other than the quoted prices in active markets that are observable either directly or indirectly. |
|
|
Level 3: |
Unobservable inputs for which there are little or no market data, which require the reporting entity to develop its own assumptions. |
The carrying amounts of the Company’s cash, prepaid expenses, accounts payable and accrued expenses are considered to be representative of their respective fair values because of the short-term nature of those instruments. As of December 31, 2017 and 2016, the Company had no financial assets or liabilities measured at fair value on a recurring basis.
Cash
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents. The Company maintains its cash in a checking account. As of December 31, 2017 and 2016, the Company held no cash equivalents.
F-8
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash. The Company maintains deposits at federally insured financial institutions in excess of federally insured limits. The Company has not experienced any losses in such accounts and management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held.
Property and Equipment
Property and equipment, generally consisting of office equipment and software, is stated at cost and depreciated on a straight-line basis over the estimated useful life of the related assets, which ranges from three to five years.
Research and Development Expense
Research and development expenses consist primarily of costs associated with the development of the Company’s product candidates and include: salaries, benefits, travel and other related costs, including equity-based compensation expenses, for personnel engaged in research and development functions; expenses incurred under agreements with contract research organizations (“CROs”), investigative sites and consultants that conduct the Company’s preclinical and clinical studies; manufacturing development and scale-up expenses and the cost of acquiring and manufacturing clinical study materials and commercial materials, including manufacturing registration and validation batches; payments to consultants engaged in the development of the Company’s product candidates, including equity-based compensation, travel and other expenses; costs related to compliance with quality and regulatory requirements; and research and development facility-related expenses, which include direct and allocated expenses and other related costs.
Research and development expenses are charged to operations as incurred when these expenditures relate to the Company’s research and development efforts and have no alternative future uses. Payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
Patent Costs
Costs related to filing and pursuing patent applications are recorded as general and administrative expenses and expensed as incurred since recoverability of such expenditures is uncertain.
Equity-based Compensation
The Company issues stock options and had historically issued incentive units, considered “profits interests” within the meaning of U.S. federal and state tax rules, to employees, consultants and certain directors. Equity-based compensation expense represents the cost of the grant-date fair value of employee awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. The Company accounts for awards to non-employees using the fair value approach. Non-employee awards are subject to periodic revaluation over their vesting terms.
Income Taxes
Income taxes are accounted for using the asset and liability method. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates applicable to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation
F-9
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
allowance against deferred tax assets is recorded if, based on the weight of all available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. For uncertain tax positions that meet a “more likely than not” threshold, the Company recognizes the benefit of uncertain tax positions in the financial statements. The Company’s policy is to record interest and penalties, if any, related to uncertain tax positions as a component of general and administrative expenses.
Comprehensive Loss
Comprehensive loss is defined as a change in equity during a period from transactions and other events and circumstances from non-owner sources. There have been no items qualifying as other comprehensive loss, and, therefore, for all periods presented, the Company’s comprehensive loss was the same as its reported net loss.
Net Loss per Share
Basic net loss per share is calculated by dividing net loss by the weighted average common shares outstanding during the period, without consideration of common stock equivalents. Basic net loss per share excludes 2,931,402 and 1,934,716 outstanding shares of common stock held by Odonate Holdings as of December 31, 2017 and December 31, 2016, respectively, to be used to settle incentive units previously issued under the Odonate Management Holdings Equity Incentive Plan (the “Management Plan”). These shares of common stock are subject to transfer to the Company and cancellation until such incentive units are vested and exercised, and, as such, are considered common stock equivalents. Therefore, the shares of common stock held by Odonate Holdings are excluded from the basic net loss per share calculation until the incentive units are exercised.
Diluted net loss per share is calculated by adjusting weighted average common stock outstanding for the dilutive effect of common stock equivalents outstanding for the period. Common stock equivalents were excluded from the calculation of diluted net loss per stock because they were anti-dilutive.
Recent Accounting Pronouncements
Recently Adopted
In November 2015, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2015-17, Income Taxes (Topic 740) – Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”). This guidance simplifies the presentation of deferred income taxes by requiring that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. ASU 2015-17 is effective for annual reporting periods beginning after December 15, 2016 and interim periods within those annual periods. The Company adopted this guidance following the Conversion with no material impact on its financial statements.
In May 2017, the FASB issued ASU No. 2017-09, Compensation – Stock Compensation (Topic 718): Scope of Modification Accounting (“ASU 2017-09”). The amendments in ASU 2017-09 provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. ASU 2017-09 is effective for annual reporting periods beginning after December 15, 2017 and interim periods thereafter. Early adoption is permitted. The Company adopted this guidance during the year ended December 31, 2017 with no material impact on its financial statements.
Not Yet Adopted
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”). This guidance requires recognition of lease assets and lease liabilities on the balance sheet and disclosure of key information about leasing arrangements. ASU 2016-02 is effective for annual reporting periods beginning after December 15, 2018 and interim periods thereafter. Early adoption is permitted. The guidance is required to be adopted at the earliest period presented using a modified retrospective approach. The Company is evaluating the impact the adoption of ASU 2016-02 will have on its financial statements and disclosures.
F-10
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Accrued expenses consist of the following (in thousands):
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Accrued clinical development costs |
|
$ |
3,033 |
|
|
$ |
- |
|
|
Accrued compensation and related expenses |
|
|
169 |
|
|
|
26 |
|
|
Other accrued expenses |
|
|
8 |
|
|
|
- |
|
|
Total accrued expenses |
|
$ |
3,210 |
|
|
$ |
26 |
|
4. Commitments and Contingencies
Commitments
The Company enters into contracts in the normal course of business with CROs, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancelable contracts and not considered contractual obligations and commitments.
Contingencies
From time to time, the Company may become subject to claims and litigation arising in the ordinary course of business. The Company is not a party to any material legal proceedings, nor is it aware of any pending or threatened litigation.
5. Stockholders’ Equity
Common Stock Sales
On December 11, 2017, the Company closed its IPO of 6,250,000 shares of common stock at a public offering price of $24.00 per share. On January 10, 2018, the underwriters in the IPO purchased 441,073 shares of common stock in connection with the exercise of their option to purchase additional shares of common stock (see Note 12). The aggregate gross proceeds from the IPO were $160.6 million, and the net proceeds were $147.3 million after deducting underwriting discounts and commissions and offering costs.
In September 2017, the Company raised $74.0 million in cash (net of $15,000 of issuance costs) through the sale of 4,968,132 common stock at a price of $14.90 per share to new and certain existing investors.
In March 2017, the Company raised $10.0 million in cash (net of $14,000 of issuance costs) through the sale of 2,593,024 common stock at a price of $3.86 per share to certain existing investors.
In June 2016, the Company raised $5.0 million in cash (net of $15,000 of issuance costs) through the sale of 9,283,762 common stock at a price of $0.54 per share to certain existing investors. During June 2016, the Company also redeemed 58,670 common stock for an aggregate of $32,000.
Non-cash Contributions for Expenses
For the years ended December 31, 2017 and 2016, the Company recorded non-cash contributions for expenses of $1.8 million and $1.0 million, respectively, related to certain costs incurred on behalf of the Company and recorded corresponding increases to additional paid-in capital (see Note 9).
F-11
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
The Company made no distributions for the year ended December 31, 2017. The Company made a one-time distribution to common stockholders of $0.8 million in December 2016.
6. Equity Incentive Plans
2017 Stock Option Plan
Following the Conversion, the Company adopted the Odonate Therapeutics, Inc. 2017 Stock Option Plan (the “2017 Plan”) in order to grant stock options to employees, officers, directors and consultants of the Company. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to the fair market value of such stock on the date of grant. The maximum term of options granted under the 2017 Plan is 10 years. The options generally vest over a 4-year period from either the date of grant or the commencement of service.
The Company reserved 4,800,000 shares of common stock for issuance under the 2017 Plan. As of December 31, 2017, 4,616,301 shares of common stock remained available for future grant under the 2017 Plan.
2017 Employee Stock Purchase Plan
Following the Conversion, the Company adopted the Odonate Therapeutics, Inc. 2017 Employee Stock Purchase Plan (the “ESPP”) in order to provide a means for eligible employees to accumulate shares of the Company’s common stock over time through regular payroll deductions. Under the ESPP, eligible employees of the Company may purchase shares of the Company’s common stock twice per month at a price equal to 85% of the closing price of the Company’s common stock on the date of each purchase. Eligible employees purchasing shares under the ESPP are subject to an annual cap equal to the lesser of $25,000 or 10% of the employee’s annual cash compensation. Shares purchased under the ESPP cannot be sold for a period of one year following the purchase date (or such shorter period of time if the participating employee’s employment terminates before this one-year anniversary).
A total of 500,000 shares of common stock were reserved for issuance under the ESPP, and no shares were issued under the ESPP as of December 31, 2017.
Management Plan
In August 2016, the Company adopted the Management Plan in order to allow for directors, officers, employees and consultants of Odonate (the “Management Plan Participants”) to share in the performance of the Company. The incentive units issued under the Management Plan were issued to Odonate Management Holdings, LLC, which issued incentive units to the Management Plan Participants on the same terms and conditions. The incentive units generally vest over a 4-year period from either the date of grant or the commencement of service and are subject to continued service requirements. Generally, on termination of services, unvested incentive units are forfeited. Following the IPO, the vested incentive units may be exercised by the Management Plan Participants, with the value received by the Management Plan Participants in the form of cash or shares of common stock equal to the fair market value on the date of exercise less the exercise price of the incentive unit. Following the Conversion, the Company has not granted, and will no longer grant, incentive units.
F-12
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
The activity related to equity awards, which are comprised of stock options and incentive units, during the year ended December 31, 2017 is summarized as follows:
|
|
|
Equity Awards |
|
|
Weighted Average Exercise Price per Share |
|
||
|
Outstanding at December 31, 2016 |
|
|
1,934,716 |
|
|
$ |
0.54 |
|
|
Granted |
|
|
1,854,441 |
|
|
$ |
8.88 |
|
|
Exercised |
|
|
- |
|
|
$ |
- |
|
|
Forfeited |
|
|
(674,056 |
) |
|
$ |
0.54 |
|
|
Outstanding at December 31, 2017 |
|
|
3,115,101 |
|
|
$ |
5.50 |
|
|
Exercisable at December 31, 2017 |
|
|
511,752 |
|
|
$ |
0.54 |
|
The average remaining contractual term of stock options outstanding as of December 31, 2017 was 10.0 years. The incentive units have no expiration.
As of December 31, 2017, the aggregate intrinsic value of vested equity awards was $12.5 million. For the year ended December 31, 2016, there was no intrinsic value of vested equity awards, as no equity awards had vested. Aggregate intrinsic value represents the product of the number of equity awards vested multiplied by the difference between the Company’s closing stock price per share on the last trading day of the fiscal period, which was $25.00 as of December 29, 2017, and the exercise price.
The total fair value of equity awards vested during the year ended December 31, 2017 was $0.3 million.
Equity-based Compensation Expense
For the years ended December 31, 2017 and 2016, the weighted average grant-date fair value per equity award was $17.47 and $0.25, respectively. The Company estimated the fair value of each equity award on the grant date using the Black-Scholes option-pricing model with the following assumptions:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Expected volatility |
|
73%-78% |
|
|
78%-79% |
|
||
|
Expected life |
|
6 years |
|
|
6-10 years |
|
||
|
Risk-free interest rate |
|
2.0%-2.3% |
|
|
1.3%-2.5% |
|
||
|
Expected dividend yield |
|
|
0 |
% |
|
|
0 |
% |
Expected Volatility. Due to the Company’s lack of Company-specific historical or implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar public companies in the life sciences industry. The Company selected the peer group based on comparable characteristics, including development stage, product pipeline and enterprise value. The Company computed historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the equity-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own share price becomes available.
Expected Life. The expected life represents the period that the equity awards are expected to be outstanding. It is based on the “simplified method” for developing the estimate of the expected life. Under this approach, the expected life is presumed to be the midpoint between the average vesting date and the end of the contractual term.
F-13
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
Risk-free Interest Rate. The Company bases the risk-free interest rate assumption on U.S. Treasury constant maturities with maturities similar to those of the expected term of the equity award being valued.
Expected Dividend Yield. The Company bases the expected dividend yield assumption on the fact that it has never paid dividends and does not expect to pay dividends in the foreseeable future.
In addition to assumptions used in the Black-Scholes option-pricing model, the Company estimates a forfeiture rate to calculate the equity-based compensation expense for equity awards. The forfeiture rate is based on an analysis of actual and estimated forfeitures.
The classification of equity-based compensation expense is summarized as follows (in thousands):
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Equity-based compensation expense: |
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
2,633 |
|
|
$ |
51 |
|
|
General and administrative |
|
|
551 |
|
|
|
- |
|
|
Total equity-based compensation expense |
|
$ |
3,184 |
|
|
$ |
51 |
|
As of December 31, 2017 and 2016, total compensation cost related to unvested equity awards was $16.9 million and $0.4 million, which will be recognized over a weighted average period of 3.3 years and 3.7 years, respectively.
7. Income Taxes
Prior to the Conversion, the Company operated as a limited liability company (“LLC”). An LLC combines corporate protection from personal liability for business debts along with the pass-through tax structure of a partnership or sole proprietorship. Business income is passed through the business to the LLC members, who report their share of profits or losses on their respective income tax returns. Net operating losses incurred by the Company through the date of the Conversion are passed through to the LLC members and are not able to be carried forward to the Company. Accordingly, no provision for income taxes is reflected in the Company’s financial statements for the year ended December 31, 2016 and through the Conversion.
From the Conversion through December 31, 2017, the Company did not record a provision for income taxes due to a full valuation allowance against its deferred tax assets.
In December 2017, the Tax Cuts and Jobs Act (the “2017 Tax Act”) was enacted. The 2017 Tax Act includes a number of changes to existing U.S. tax laws that impact the Company, most notably a reduction of the U.S. corporate income tax rate from 34% to 21% for tax years beginning after December 31, 2017. The 2017 Tax Act also provides for a one-time transition tax on certain foreign earnings, the acceleration of depreciation for certain assets placed into service after September 27, 2017 and other prospective changes beginning in 2018, including repeal of the domestic manufacturing deduction, acceleration of tax revenue recognition, capitalization of research and development expenditures, additional limitations on executive compensation and limitations on the deductibility of interest.
The Company recognized the income tax effects of the 2017 Tax Act in its 2017 financial statements in accordance with Staff Accounting Bulletin No. 118, which provides SEC staff guidance for the application of ASC Topic 740, Income Taxes, during the reporting period in which the 2017 Tax Act was signed into law. As such, the Company’s financial results reflect the income tax effects of the 2017 Tax Act for which the accounting under ASC Topic 740 is complete, and provisional amounts for those specific income tax effects of the 2017 Tax Act for which the accounting under ASC Topic 740 is incomplete but a reasonable estimate could be determined. The Company did not identify items for which the income tax effects of the 2017 Tax Act have not been completed and a reasonable estimate could not be determined as of December 31, 2017.
F-14
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
The difference between the provision for income taxes and income taxes computed using the U.S. federal income tax rate effective is as follows (in thousands):
|
|
|
Period Ended December 31, |
|
|
|
|
|
2017 |
|
|
|
Federal statutory rate of 34% |
|
$ |
(1,296 |
) |
|
State taxes, net of federal benefit |
|
|
(222 |
) |
|
Research and development credits |
|
|
(214 |
) |
|
Change in valuation allowance |
|
|
907 |
|
|
Impact of the 2017 Tax Act |
|
|
709 |
|
|
Other permanent differences |
|
|
116 |
|
|
Income tax provision |
|
$ |
- |
|
Deferred income tax assets arising from differences between accounting for financial statement purposes and tax purposes, less valuation allowance are as follows (in thousands):
|
|
|
December 31, |
|
|
|
|
|
2017 |
|
|
|
Deferred tax assets: |
|
|
|
|
|
Accrued expenses |
|
$ |
945 |
|
|
Depreciation and amortization |
|
|
786 |
|
|
Capitalized research and development |
|
|
306 |
|
|
Total gross deferred tax assets |
|
|
2,037 |
|
|
Less valuation allowance |
|
|
(2,037 |
) |
|
Total deferred tax assets |
|
$ |
- |
|
As of both December 31, 2017 and the Conversion, the Company established a full valuation allowance against its federal and state deferred tax assets due to the uncertainty surrounding the realization of such assets.
As of December 31, 2017, the Company had both federal and state net operating loss carryforwards of approximately $3.4 million. The federal and state net operating loss carryforwards begin to expire in 2037, unless utilized. As of December 31, 2017, the Company also has federal and state research and development credit carryforwards of approximately $0.2 million and $0.1 million, respectively. The federal research and development credit carryforwards will begin to expire in 2037, unless utilized. The state research and development credit carryforwards will carry forward indefinitely, unless utilized.
Pursuant to Section 382 and 383 of the Internal Revenue Code (“IRC”), utilization of the Company’s federal net operating loss carryforwards and research credits may be subject to annual limitations in the event of any significant future changes in its ownership structure. These annual limitations may result in the expiration of net operating loss carryforwards and research and development credit carryforwards prior to utilization. The Company has not completed an IRC Section 382 and 383 analysis regarding the limitation of net operating loss and research and development credit carryforwards.
As of December 31, 2017 and 2016 the Company had no unrecognized tax benefits. The Company does not anticipate there will be a significant change in unrecognized tax benefits within the next 12 months.
The Company had no accrual for interest or penalties on the balance sheets as of December 31, 2017 and 2016 and has not recognized interest or penalties in the statements of operations for the years ended December 31, 2017 and 2016.
F-15
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
The Company is subject to taxation in the U.S. and various state jurisdictions. The Company’s tax returns for the tax years 2014 through 2016 are open and are subject to examination by federal and state taxing authorities. If such examinations result in adjustments to the income or expense amounts, the amounts allocated to the LLC members could be adjusted accordingly. The Company is not currently undergoing a tax audit in any federal or state jurisdiction.
8. License Agreement
In 2013, the Company licensed rights to tesetaxel in all major markets from Daiichi Sankyo Company, Limited (“Daiichi Sankyo”), the original inventor of the product. Under the Daiichi Sankyo license agreement, the Company is obligated to use commercially reasonable efforts to develop and commercialize tesetaxel in the following countries: France, Germany, Italy, Spain, the United Kingdom and the U.S. The Company is required to make aggregate future milestone payments of up to $31.0 million, contingent on attainment of certain regulatory milestones. Additionally, the Company will pay Daiichi Sankyo a tiered royalty that ranges from the low to high single digits, depending on annual net sales of tesetaxel.
9. Related Party Transactions
Commencing in 2016, the Company received certain services and other benefits from an affiliate (the “Affiliate”) of the Chairman and Chief Executive Officer of the Company. The Company was not charged any fees for these services and other benefits, which included personnel costs for research and development and administrative functions, rent and facility costs, and other direct expenses. For the years ended December 31, 2017 and 2016, the Company recorded expenses of $1.8 million and $1.0 million, respectively, for services and other benefits provided without charge to the Company, which amounts were recorded as corresponding increases to additional paid-in capital. Personnel costs were based on actual costs incurred by the Affiliate, which were allocated based on the estimated percentage of time employees spent on Odonate on an employee-by-employee basis. Rent and facility costs were based on actual costs incurred by the Affiliate and allocated based on the Company’s use of shared space using headcount. Other direct expenses paid by the Affiliate were specifically identifiable to the Company and were allocated directly to the Company. The Chairman and Chief Executive Officer of the Company has elected to receive an annual salary of $1.00 and to not receive any bonuses, equity or other compensation.
Management believes that the method used to allocate costs is a fair and reasonable reflection of the utilization of the services provided to, or the benefit received by, the Company during the periods presented. The allocations may not, however, reflect the costs that the Company would have incurred if the Company had not received these services. Actual costs would depend on a number of factors, including strategic decisions in the areas of hiring, facility location and whether to outsource certain functions.
10. 401(k) Plan
During 2016, the Company adopted a defined contribution 401(k) plan available to eligible employees. Employee contributions are voluntary and are determined on an individual basis, limited to the maximum amount allowable under U.S. federal tax regulations. The Company makes a mandatory annual contribution of 3% of the eligible employees’ compensation to the 401(k) plan. In addition, the Company makes matching contributions of up to 6% of the eligible employees’ compensation to the 401(k) plan. For the years ended December 31, 2017 and 2016, the Company incurred costs of $0.2 million and $12,000, respectively, related to the 401(k) plan.
F-16
ODONATE THERAPEUTICS, INC.
Notes to Financial Statements
11. Selected Quarterly Financial Data (Unaudited)
The following is a summary of the quarterly results of the Company for the years ended December 31, 2017 and 2016 (in thousands, except share and per share amounts):
|
|
|
2017 |
|
|||||||||||||
|
|
|
First Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Fourth Quarter |
|
||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
2,454 |
|
|
$ |
3,282 |
|
|
$ |
9,126 |
|
|
$ |
13,040 |
|
|
General and administrative |
|
|
272 |
|
|
|
618 |
|
|
|
1,246 |
|
|
|
2,706 |
|
|
Total operating expenses |
|
|
2,726 |
|
|
|
3,900 |
|
|
|
10,372 |
|
|
|
15,746 |
|
|
Net loss |
|
$ |
(2,726 |
) |
|
$ |
(3,900 |
) |
|
$ |
(10,372 |
) |
|
$ |
(15,746 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(0.26 |
) |
|
$ |
(0.31 |
) |
|
$ |
(0.74 |
) |
|
$ |
(0.81 |
) |
|
Weighted average shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
10,464,723 |
|
|
|
12,740,822 |
|
|
|
14,036,856 |
|
|
|
19,339,389 |
|
|
|
|
2016 |
|
|||||||||||||
|
|
|
First Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Fourth Quarter |
|
||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
- |
|
|
$ |
60 |
|
|
$ |
796 |
|
|
$ |
1,766 |
|
|
General and administrative |
|
|
41 |
|
|
|
69 |
|
|
|
173 |
|
|
|
180 |
|
|
Total operating expenses |
|
|
41 |
|
|
|
129 |
|
|
|
969 |
|
|
|
1,946 |
|
|
Net loss |
|
$ |
(41 |
) |
|
$ |
(129 |
) |
|
$ |
(969 |
) |
|
$ |
(1,946 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
$ |
(0.04 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.19 |
) |
|
Weighted average shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
|
922,706 |
|
|
|
1,734,993 |
|
|
|
10,147,798 |
|
|
|
10,147,798 |
|
12. Subsequent Events
On January 10, 2018, the underwriters in the IPO purchased 441,073 shares of common stock in connection with the exercise of their option to purchase additional shares of common stock in exchange for gross proceeds of approximately $10.6 million and net proceeds of approximately $9.8 million after deducting underwriting discounts and commissions and offering costs.
F-17