Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Corindus Vascular Robotics, Inc. | cvrs-8k_110917.htm |
Corindus Vascular Robotics, Inc. 8-K
Exhibit 99.1

1 Precision Vascular Robotics Corindus Vascular Robotics (CVRS) November 2017

Forward Looking Statements This presentation contains “forward - looking statements” (as such term is defined in section 27 a of the securities act of 1933 , as amended, and section 21 e of the securities exchange act of 1934 , as amended), and information relating to the company, that are based on the current beliefs of, and assumptions made by our management and the information currently available to our management . Forward - looking statements relate to expectations concerning matters that are not historical facts . Words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "opinion," "will" and similar expressions and their variants, are intended to identify forward - looking statements . These forward - looking statements include, but are not limited to statements related to our expected business, products, adoption of robotic medical procedures, results of operations, future financial condition, ability to increase our revenues, and similar matters . These forward - looking statements should be considered in light of various important factors, including, without limitation, the rate of adoption of our CorPath system and the rate of use of our cassettes ; risks associated with market acceptance, including pricing and reimbursement ; our ability to enforce our intellectual property rights ; our need for additional funds to support our operations ; our ability to manage expenses and cash flow ; factors relating to engineering, regulatory, manufacturing, sales and customer service challenges ; potential safety and regulatory issues that could slow or suspend our sales ; the effect of credit, financial and economic conditions on capital spending by our potential customers ; the impact of global and regional economic and credit market conditions on health care spending ; health care reform legislation in the united states and its impact on hospital spending, reimbursement and fees which will be levied on certain medical device revenues, decreases in hospital admissions and actions by payers to limit or manage surgical procedures timing and success of product development and market acceptance of developed products, procedure counts ; regulatory approvals, clearances and restrictions ; guidelines and recommendations in the health care and patient communities, intellectual property positions and litigation, competition in the medical device industry and in the specific markets of surgery in which we operate, the inability to meet demand for products, the results of legal proceedings to which we are or may become a party, product liability and other litigation claims, adverse publicity regarding our company and safety of our products and the adequacy of training ; our ability to expand in foreign markets ; and other risk factors . Readers are cautioned not to place undue reliance on these forward - looking statements, which are based on current expectation and are subject to risks, uncertainties ; and assumptions that are difficult to predict, including those risk factors described in the company’s annual report on form 10 - K for the fiscal year ended on December 31 , 2016 . Our actual results may differ materially and adversely from those expressed in any forward - looking statements . We undertake no obligation to publicly update or release any revisions to these forward - looking statements except as required by law . 2

Corindus Today A leader in vascular robotics LARGE Market Opportunity with Long GROWTH Runway $4.5B 1 market opportunity in 2018 driven by over 2.5 million coronary and 3 million non - coronary procedures performed per year DIFFERENTIATED Technology ONLY FDA cleared robotic platform for percutaneous coronary intervention (“PCI”) and peripheral interventions 2 Proving BENEFIT to Patient, Physician, and Hospital Robotic precision reduces stent utilization to improve clinical outcomes Reducing radiation exposure for patients, physicians & lab staff Leading INNOVATION in Vascular Robotics Strong product development pipeline backed by robust IP portfolio Open architecture leverages hospital ecosystem & enables partnerships 1 Market opportunity assessment based on market research reports and Corindus estimate 2 Only the CorPath 200 System is indicated for use in peripheral vascular interventions 3 Weisz, G. et al. Safety and Feasibility of Robotic Percutaneous Coronary Intervention: PRECISE Study. J Am Coll Cardiol. 20 13; 61(15):1596 - 1600. PRECISE Trial was conducted with the CorPath 200 System. 3
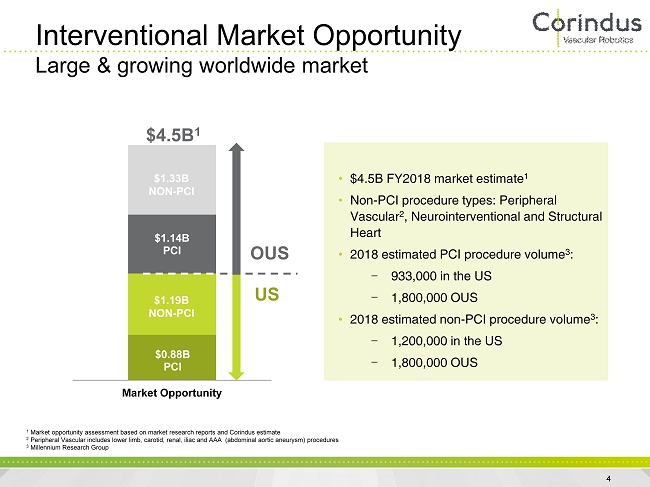
1 Market opportunity assessment based on market research reports and Corindus estimate 2 Peripheral Vascular includes lower limb, carotid, renal, iliac and AAA (abdominal aortic aneurysm) procedures 3 Millennium Research Group Interventional Market Opportunity Large & growing worldwide market • $4.5B FY2018 market estimate 1 • Non - PCI procedure types: Peripheral Vascular 2 , Neurointerventional and Structural Heart • 2018 estimated PCI procedure volume 3 : − 933,000 in the US − 1,800,000 OUS • 2018 estimated non - PCI procedure volume 3 : − 1,200,000 in the US − 1,800,000 OUS $0.88B PCI $1.14B PCI Market Opportunity OUS US $4.5B 1 $1.33B NON - PCI $1.19B NON - PCI 4
Market Drivers 5 Corindus poised for growth in PCI robotics market FAVORABLE PATIENT DEMOGRAPHICS SHORTAGE OF DOCTORS DISRUPTIVE TECHNOLOGY CONTINUED GROWTH OF PCI PROCEDURES WORLDWIDE
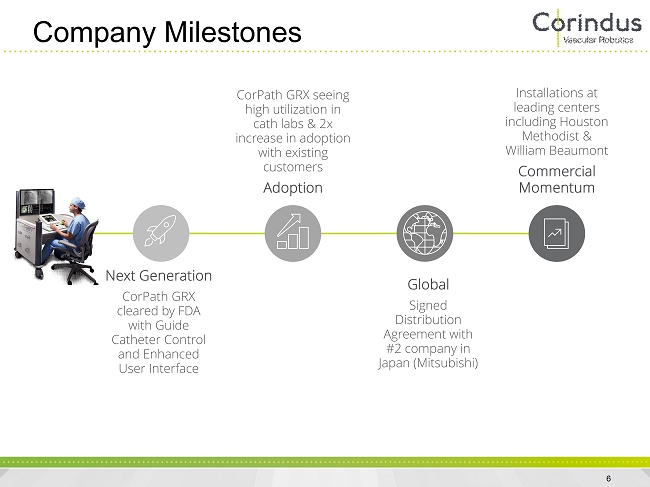
Company Milestones 6 Next Generation CorPath GRX cleared by FDA with Guide Catheter Control and Enhanced User Interface CorPath GRX seeing high utilization in cath labs & 2x increase in adoption with existing customers Adoption Global Signed Distribution Agreement with #2 company in Japan (Mitsubishi) Installations at leading centers including Houston Methodist & William Beaumont Commercial Momentum
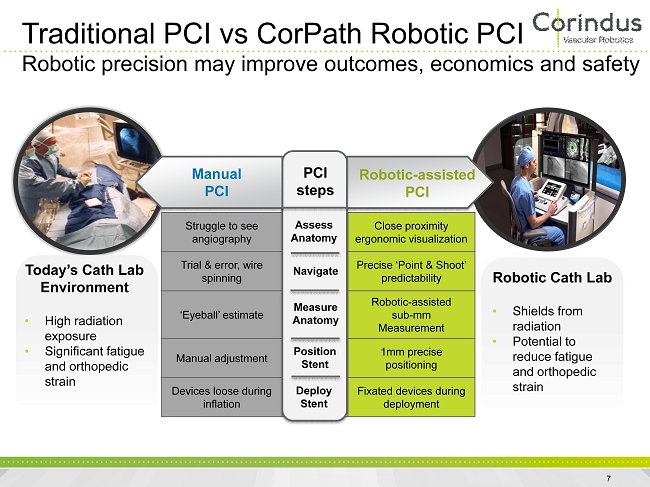
Struggle to see angiography Assess Anatomy Close proximity ergonomic visualization Trial & error, wire spinning Navigate Precise ‘Point & Shoot’ predictability ‘Eyeball’ estimate Measure Anatomy Robotic - assisted sub - mm Measurement Manual adjustment Position Stent 1mm precise positioning Devices loose during inflation Deploy Stent Fixated devices during deployment Manual PCI PCI steps Robotic - assisted PCI Assess Anatomy Navigate Measure Anatomy Position Stent Deploy Stent Traditional PCI vs CorPath Robotic PCI Robotic precision may improve outcomes, economics and safety Today’s Cath Lab Environment • High radiation exposure • Significant fatigue and orthopedic strain Robotic Cath Lab • Shields from radiation • Potential to reduce fatigue and orthopedic strain 7
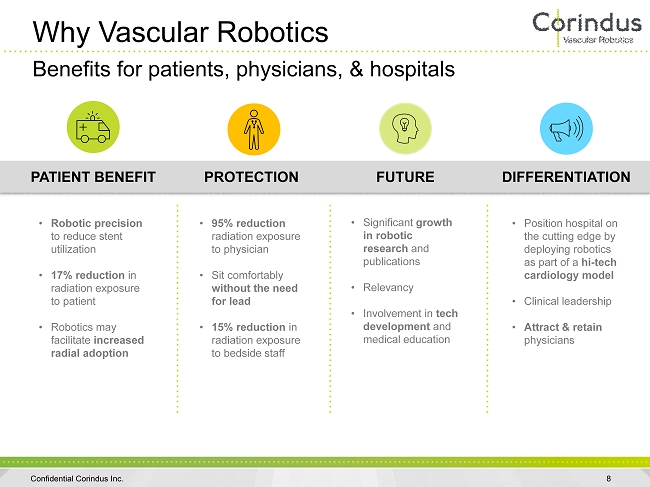
Why Vascular Robotics 8 Benefits for patients, physicians, & hospitals DIFFERENTIATION • Position hospital on the cutting edge by deploying robotics as part of a hi - tech cardiology model • Clinical leadership • Attract & retain physicians PATIENT BENEFIT • Robotic precision to reduce stent utilization • 17% reduction in radiation exposure to patient • Robotics may facilitate increased radial adoption PROTECTION • 95% reduction radiation exposure to physician • Sit comfortably without the need for lead • 15% reduction in radiation exposure to bedside staff FUTURE • Significant growth in robotic research and publications • Relevancy • Involvement in tech development and medical education Confidential Corindus Inc.
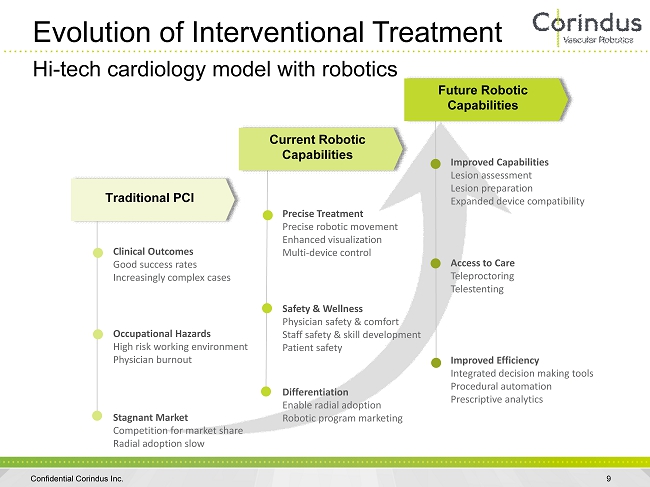
Evolution of Interventional Treatment 9 Hi - tech cardiology model with robotics Occupational Hazards High risk working environment Physician burnout Traditional PCI Stagnant Market Competition for market share Radial adoption slow Precise Treatment Precise robotic movement Enhanced visualization Multi - device control Safety & Wellness Physician safety & comfort Staff safety & skill development Patient safety Differentiation Enable radial adoption Robotic program marketing Access to Care Teleproctoring Telestenting Improved Capabilities Lesion assessment Lesion preparation Expanded device compatibility Improved Efficiency Integrated decision making tools Procedural automation Prescriptive analytics Clinical Outcomes Good success rates Increasingly complex cases Current Robotic Capabilities Future Robotic Capabilities Confidential Corindus Inc.

Telestenting First - in - Human Study Study showed 86.4% technical success and 95% procedural success 10 REMOTE - PCI REMOTE - PCI Study explored feasibility of remote telestenting using a robotic system Single - center prospective observational study performed at Spectrum Health, Grand Rapids, MI 20 patients treated via physician at remote cockpit leveraging telehealth technology Madder R, et al. “Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location from the patient: an early exploration into the feasibility of telestenting (the REMOTE - PCI study).” Eurointervention , 2017;12:1569 - 1576. This study, conducted under local IRB, may involve off - label usage and was not sponsored by Corindus.

Vascular Robotic Clinical Roadmap Demonstrating excellence in multiple lesion types and anatomies Product Evolution Robotic system capability Exploratory Feasibility Expand Use NEURO PERIPHERAL PCI 11 CorPath 200 and CorPath GRX Systems are indicated for PCI. Only the CorPath 200 System is indicated for use in peripheral vas cul ar interventions. CorPath Systems are not indicated for use in neuro or structural interventions. • Below the Knee • Ostial Stenting • Atherectomy • Drug Eluting Balloons • Left Main Intervention • Complex PCI & CTO • Ostial Lesion • Staff Radiation Protection • Outcomes • Remote PCI • Exploratory Work
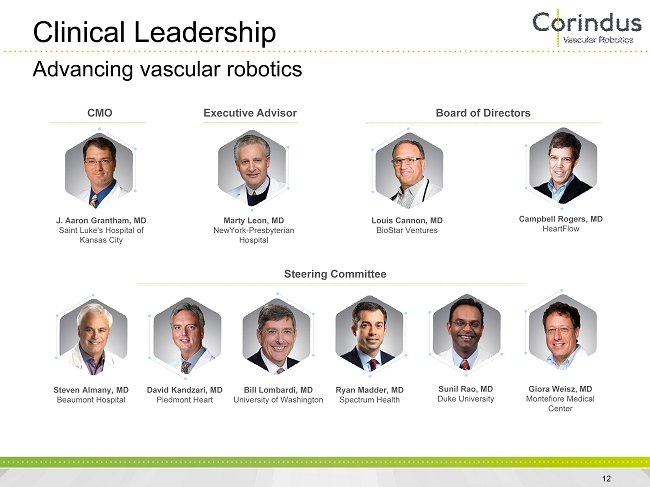
Clinical Leadership 12 J. Aaron Grantham, MD Saint Luke's Hospital of Kansas City Marty Leon, MD NewYork - Presbyterian Hospital Louis Cannon, MD BioStar Ventures Campbell Rogers, MD HeartFlow Steven Almany, MD Beaumont Hospital David Kandzari, MD Piedmont Heart Bill Lombardi, MD University of Washington Ryan Madder, MD Spectrum Health Sunil Rao, MD Duke University Giora Weisz, MD Montefiore Medical Center CMO Executive Advisor Board of Directors Steering Committee Advancing vascular robotics
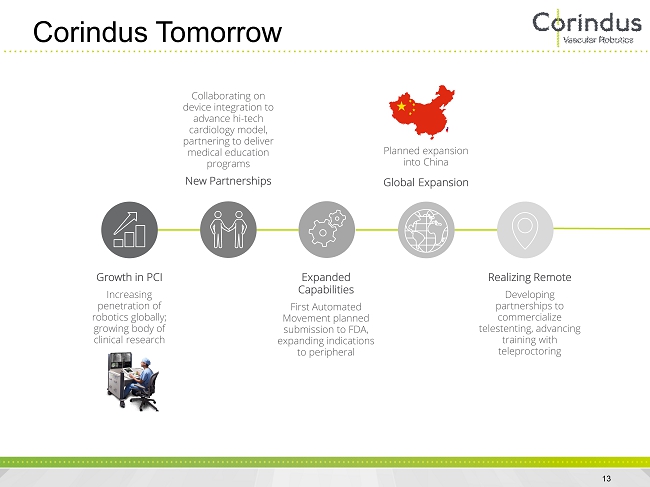
Corindus Tomorrow 13 Growth in PCI Increasing penetration of robotics globally; growing body of clinical research Collaborating on device integration to advance hi - tech cardiology model, partnering to deliver medical education programs New Partnerships Expanded Capabilities First Automated Movement planned submission to FDA, expanding indications to peripheral Planned expansion into China Global Expansion Realizing Remote Developing partnerships to commercialize telestenting , advancing training with teleproctoring

14 Corindus Vascular Robotics Strategic Objectives Near Term Mid to Long Term Mid to Long Term x Recurring revenue streams and NG3 system launch x Expansion into additional disease states (neurovascular and structural heart) x Global expansion and remote tele - proctoring x Establish at least 25 new robotic programs x Pursue co - development opportunities, add at least one additional collaboration x Drive system utilization x Prepare Japan distributor for launch x Ramp up educational and training opportunities for physicians x Further clinical trial development x Software enhancement – Gen 2.2
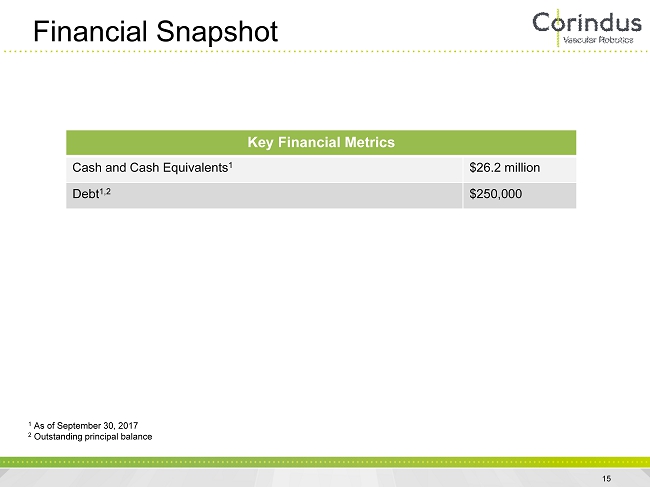
1 As of September 30, 2017 2 Outstanding principal balance Financial Snapshot Key Financial Metrics Cash and Cash Equivalents 1 $26.2 million Debt 1,2 $250,000 15
Corindus Vascular Robotics, Inc. is a global technology leader in robotic - assisted vascular interventions. The company's CorPath ® System is the first FDA - cleared medical device to bring robotic - assisted precision to percutaneous coronary interventions. With the CorPath System, Corindus Vascular Robotics brings robotic precision to interventional procedures to help optimize clinical outcomes and minimize the costs associated with complications of improper stent placement with manual procedures. Visit us at www.corindus.com About Corindus Vascular Robotics 16
