Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - IMMUNE PHARMACEUTICALS INC | v476379_8k.htm |
Exhibit 99.1

IMMUNE PHARMACEUTICALS Rebuilding with Bertilimumab October 2017

2 This presentation and oral statements made by representatives of the Company may contain projections or other forward - looking statements regarding future events or the future financial performance of the Company . Actual events or results may differ materially from those in the projections or other forward - looking statements . There can be no assurance that the Company will ever successfully complete its anticipated corporate restructuring, including the proposed spin - off of Cytovia Inc . , or that the Company will be able to reduce expenses, capitalize on strategic alternatives, develop its assets, and generate value for shareholders . The Company may, at any time and for any reason until the proposed spin - off is complete, abandon the spin - off or modify its terms and conditions, or consider competing, alternate or complimentary transactions or offers by third parties at the discretion of Immune’s board of directors . Please see Immune’s filings with the Securities and Exchange Commission for a discussion of important risk factors that could cause actual events or results to differ materially from those in the projections or other forward - looking statements . Forward Looking Statement

3 We are focused on immunologic and inflammatory diseases Two active programs generating multiple shots on goal Bertilimumab is a fully human, anti - eotaxin - 1 mAb with applications in a variety of inflammatory diseases Currently running phase 2 trials in bullous pemphigoid (open - label single arm) and ulcerative colitis (randomized, double - blind, placebo - controlled) Strong efficacy signal from BP trial – first six subjects had a significant reduction in disease activity despite an aggressive prednisone taper Well - established safety profile – substantial human exposure (n>100 total; >60 IV); no drug associated SAE Multiple additional clinical applications including atopic dermatitis, asthma, other eosinophil - driven diseases, glioblastoma, and others Nanocyclo is a nano - encapsulated topical formulation of cyclosporine for atopic dermatitis and psoriasis New management team is singularly committed to execution Executive Summary

4 Focused Pipeline With Multiple Shots on Goal Program Indication Preclinical Phase 1 Phase 2 Phase 3 Bertilimumab Bullous Pemphigoid Ongoing Ulcerative Colitis Ongoing Atopic Dermatitis Phase 2 - ready Other inflammatory conditions Phase 2 - ready Nanocyclo Atopic Dermatitis Ongoing Psoriasis Ongoing

5 Berilimumab is a First - in - Class Antibody Eotaxin - 1 Implicated in Multiple Diseases • Ulcerative Colitis • Crohn ’ s Disease • Inflammatory hepatitis (including NASH) • Gastroenteritis • Asthma • Churg - Strauss Syndrome • Hypereosinophilic syndrome • Bone resorption in rheumatoid arthritis • Glioblastoma • Bullous pemphigoid • Severe atopic dermatitis DERMATOLOGY GASTROENTEROLOGY OTHER DISEASES Bertilimumab The first antibody to specifically target and neutralize eotaxin - 1 Eotaxin - 1 A drug target implicated in the etiology of a variety of inflammatory disorders
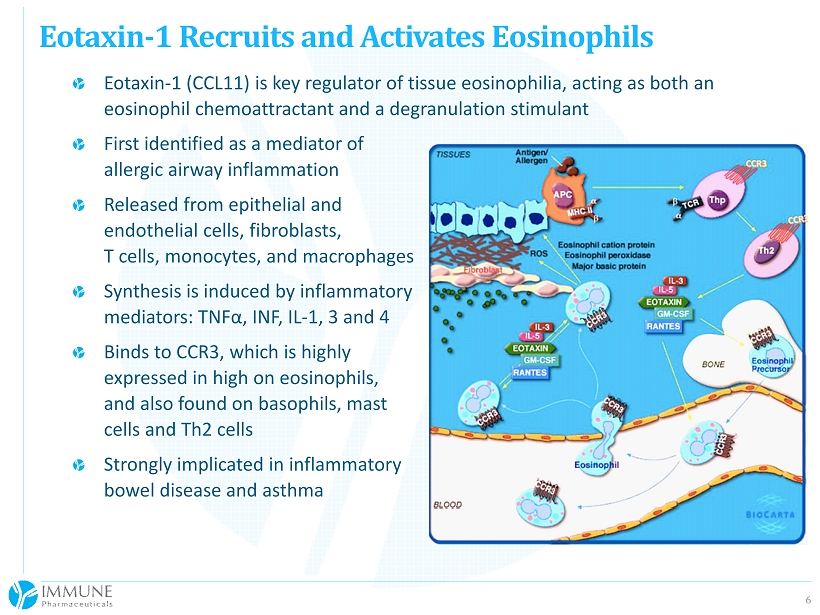
6 Eotaxin - 1 (CCL11) is key regulator of tissue eosinophilia, acting as both an eosinophil chemoattractant and a degranulation stimulant First identified as a mediator of allergic airway inflammation Released from epithelial and endothelial cells, fibroblasts, T cells, monocytes, and macrophages Synthesis is induced by inflammatory mediators: TNF α, INF, IL - 1, 3 and 4 Binds to CCR3, which is highly expressed in high on eosinophils, and also found on basophils, mast cells and Th2 cells Strongly implicated in inflammatory bowel disease and asthma Eotaxin - 1 Recruits and Activates Eosinophils

7 Bertilimumab (formerly CAT - 213) is a fully human IgG4 antibody High affinity (KD 8.8pM) and specificity for human eotaxin - 1 Does not bind to eotaxin - 2 and - 3 Prevents eotaxin - 1 - induced chemotaxis and shape change of human eosinophils both in vitro and in vivo Good pharmacokinetic profile Single IV dose at 0.01, 0.1, 1.0, 5.0 and 10 mg/kg Elimination T ½ of 14 days Clean safety profile Leveraging 3 CAT - sponsored studies Human exposure is over 100 subjects, including >60 receiving IV, 8 receiving intranasal, and 46 receiving intraocular Has been well - tolerated by multiple routes of administration Only one SAE, unrelated to drug (subject with pre - existing peripheral disease hospitalized for stent placement during the study) Bertilimumab Blocks Eotaxin - 1
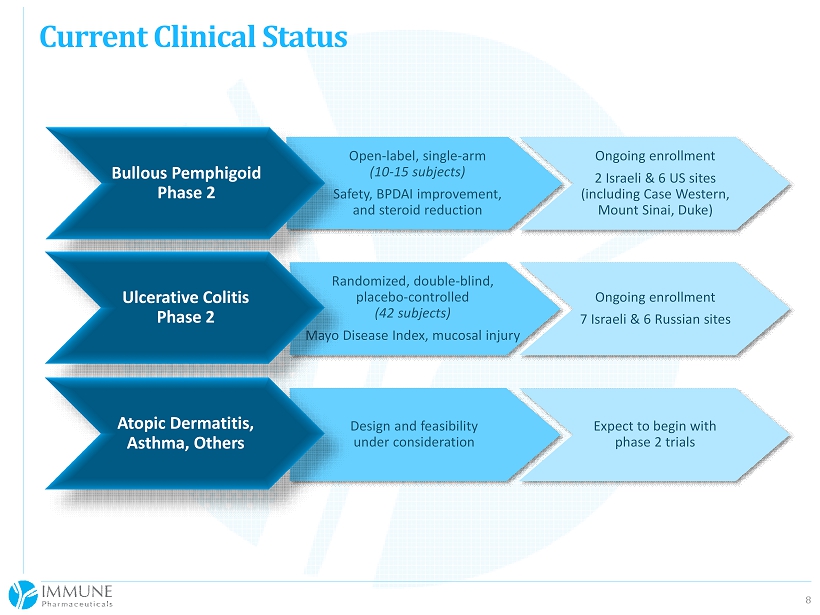
8 Current Clinical Status Randomized, double - blind, placebo - controlled (42 subjects) Mayo Disease Index, mucosal injury Ongoing enrollment 7 Israeli & 6 Russian sites Open - label, single - arm (10 - 15 subjects) Safety, BPDAI improvement, and steroid reduction Ongoing enrollment 2 Israeli & 6 US sites (including Case Western, Mount Sinai, Duke) Design and feasibility under consideration Expect to begin with phase 2 trials Bullous Pemphigoid Phase 2 Ulcerative Colitis Phase 2 Atopic Dermatitis, Asthma, Others

9 Severe autoimmune skin blistering disease Driven by autoantibodies to BP180 (type XVII collagen) and BP230 ( dystonin ) 30,000 patients in the US and EU ( Orphanet ) Typically treated with prednisone (0.5 - 1.0 mg/kg) Will usually get the disease under control However high prednisone doses increase morbidity and mortality over difficult - to - use topical steroids Steroids typically tapered over 6 - 12 months Second - line immunosuppressants like azathioprine, methotrexate and Rituxan have toxicity issues Unquestionably an unmet medical need for a steroid - sparing adjunctive or alternative therapy Single - arm, open - label phase 2 study ongling 10 - 15 subjects with moderate - to - severe disease Sites in the US (6) and Israel (2) Bullous Pemphigoid

10 First 6 subjects of an intended 10 - 15 subjects 85% reduction in BPDAI Total Activity Index (p=0.0096) All subjects achieved a >50% improvement 4/6 with >90% improvement Mean initial prednisone dose was just 26 mg (0.3 mg/kg), tapered to a mean of 9 mg (0.1 mg/kg) by the last assessment (p=0.0145) A standard prednisone regimen would have begun at ~60 mg and would have been at ~30 mg by day 84 According to SAB Chair Dr. Neil Korman (Professor of Dermatology at Case Western), these patients should have fared quite poorly on the protocol - mandated prednisone dose and taper Safety and tolerability of 3 bertilimumab doses (10 mg/kg, q2 weeks) is excellent thus far. Positive Interim Look at Phase 2 Study
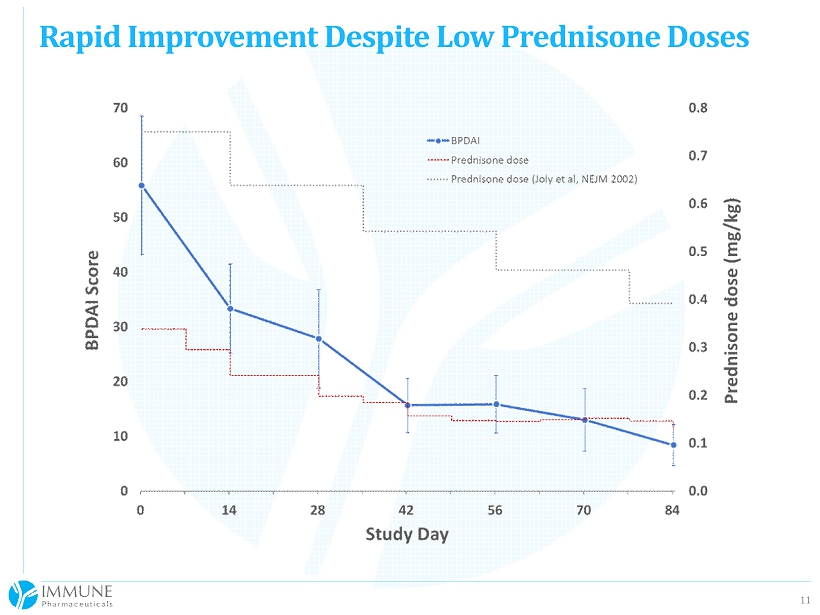
11 Rapid Improvement Despite Low Prednisone Doses
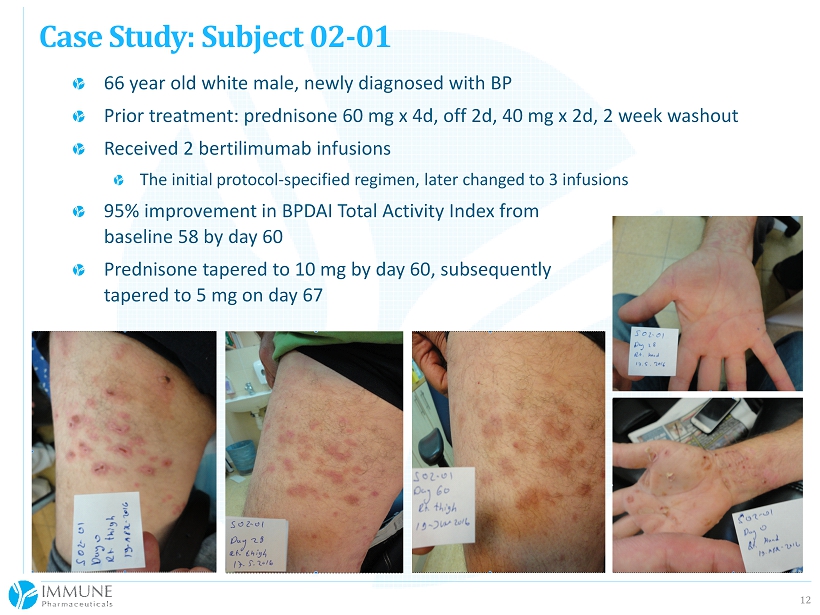
12 66 year old white male, newly diagnosed with BP Prior treatment: prednisone 60 mg x 4d, off 2d, 40 mg x 2d, 2 week washout Received 2 bertilimumab infusions The initial protocol - specified regimen, later changed to 3 infusions 95% improvement in BPDAI Total Activity Index from baseline 58 by day 60 Prednisone tapered to 10 mg by day 60, subsequently tapered to 5 mg on day 67 Case Study: Subject 02 - 01

13 Over 3.5 million patients with inflammatory bowel disease (IBD) in the US and EU (Kaplan, 2015) Patients with ulcerative colitis (UC) and Crohn’s disease have elevated eotaxin - 1 Half have elevated eotaxin - 1 Eotaxin - 1 strongly implicated as a target in IBD Tissue eotaxin - 1 levels correlated with Mayo Clinic Disease Activity Index, mucosal injury and histologic severity Greater eotaxin - 1 mRNA expression in areas of active vs. inactive disease Eotaxin - 1 correlates with increased tissue eosinophils Ulcerative Colitis Source: Ahrens et al , J Immunol 2008;181;7390 - 7399

14 Randomized, double - blind, placebo - controlled trial 42 subjects, 2:1 randomization Patients selected based on Mayo UC Score and eotaxin - 1 levels Sites in Israel (5) and Russia (6) Drug administration on Days 0, 14 and 28; 90 day follow - up Primary endpoint is clinical response (UC Mayo Clinic Index) at Day 42 Additional efficacy endpoints include mucosal injury, fecal calprotectin (validated inflammation marker), mucosal eotaxin - 1 and eosinophil levels, and clinical remission Bertilimumab Proof of Concept Trial in UC

15 In BP, we have potential to move directly into a pivotal phase 2/3 trial Will get FDA feedback from an end - of - phase 2 meeting and SAB input Could target steroid reduction endpoint or go head - to - head against steroids Expect to launch pivotal study in second half of 2018 Development path in UC will be determined by the results of the current study Several opportunities being vetted for next clinical indication (late 2018 target) Manufacturing Current 200L GS - NSO cell line process will not be used for future trials New CHO cell line and process more efficient and highly scalable Need to transfer process to a GMP facility for scale - up and validation Extent of comparability program unknown but human PK study may be required Intellectual Property/Market Exclusivity Current IP portfolio has several patents expiring in 2021 - 2022 that will be eligible for Patent Term Restoration of up to 5 years Pursuing Orphan exclusivity for BP 12 years of biologics exclusivity in the US and 10 years in the EU New initiatives to generate new IP around manufacturing and methods of use Bertilimumab Development Plan
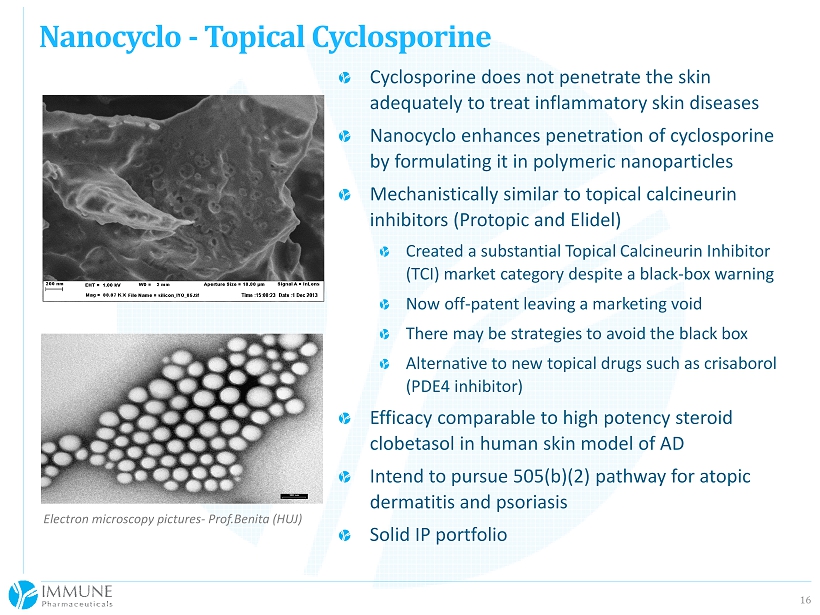
16 Nanocyclo - Topical Cyclosporine Electron microscopy pictures - Prof.Benita (HUJ) Cyclosporine does not penetrate the skin adequately to treat inflammatory skin diseases Nanocyclo enhances penetration of cyclosporine by formulating it in polymeric nanoparticles Mechanistically similar to topical calcineurin inhibitors ( Protopic and Elidel ) Created a substantial Topical Calcineurin Inhibitor (TCI) market category despite a black - box warning Now off - patent leaving a marketing void There may be strategies to avoid the black box Alternative to new topical drugs such as crisaborol (PDE4 inhibitor) Efficacy comparable to high potency steroid clobetasol in human skin model of AD Intend to pursue 505(b)(2) pathway for atopic dermatitis and psoriasis Solid IP portfolio

17 New management Elliot Maza, JD, CPA, President and CEO – extensive and successful experience in rebuilding struggling biotech and drug manufacturing companies Tony Fiorino, MD, PhD, CMO/COO – extensive and successful experience in leading clinical stage biotech companies and a former fund manager well - versed in finance Adding critical team members (clinical ops, manufacturing, IR) post - financing Revised corporate strategy to focus on our core assets Bertilimumab Continue development in BP and UC with a plan to efficiently move into pivotal studies Assess potential additional indications and mechanisms for achieving clinical proof - of - concept and launch clinical study in one or more new indications Move to new GMP manufacturing process Nanocyclo Complete formation work and GMP scale up Fully develop 505(b)(2) strategy and move program into the clinic in 2018 Contemplate pipeline expansion via in - licensing only when these programs are stable Pursuing possible spin - off of Cytovia , Immune’s subsidiary focused on oncology drugs, into a separate, stand - alone entity Management Reboot and Corporate Restructuring
18 Developing a first - in - class, fully human mAb in phase 2 for two indications Targeting both Orphan and larger indications with unmet medical need Preliminary data from open - label BP trial demonstrated significant reduction in disease activity index (85%) despite aggressive steroid tapering Will generate additional data in 2018 and initiate phase 2/3 development UC trial will conclude in 2018 – next steps to be determined after unblinding Several candidates for next indication in the clinic Low - risk second asset poised to enter clinical development Clean cap structure and a low share count Actively traded, NASDAQ - listed stock with good daily volume New management team implementing a focused approach coupled with a prudent corporate restructuring to unlock value for shareholders Immune Pharmaceuticals Investment Highlights
