Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - IMMUNE PHARMACEUTICALS INC | v446302_ex32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - IMMUNE PHARMACEUTICALS INC | v446302_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - IMMUNE PHARMACEUTICALS INC | v446302_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - IMMUNE PHARMACEUTICALS INC | v446302_ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2016
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number 001-36602
IMMUNE PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
| Delaware | 52-1841431 | |
| (State or other jurisdiction of | (IRS Employer Id. No.) | |
| incorporation or organization) |
430 East 29th Street, Suite 940
New York, NY 10016
(Address of principal executive offices)
Registrant’s telephone number, including area code: (646) 440-9310
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ | |
| Non-accelerated filer ¨ | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of August 11, 2016, 79,178,791 shares of the registrant’s common stock, par value $0.0001 per share, were outstanding.
TABLE OF CONTENTS
2
Immune Pharmaceuticals Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
($ in thousands, except share and per share amounts)
June 30, 2016 (Unaudited) | December 31, 2015 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 406 | $ | 4,543 | ||||
| Restricted cash | 43 | 31 | ||||||
| Other current assets | 283 | 258 | ||||||
| Total current assets | 732 | 4,832 | ||||||
| Property and equipment, net of accumulated depreciation of $153 and $77 | 344 | 371 | ||||||
| In-process research and development acquired | 27,500 | 27,500 | ||||||
| Intangible assets, net | 2,958 | 3,111 | ||||||
| Other assets | 339 | 370 | ||||||
| Total assets | $ | 31,873 | $ | 36,184 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 3,602 | $ | 2,439 | ||||
| Accrued expenses | 1,980 | 2,660 | ||||||
| Derivative financial instruments, warrants | - | 84 | ||||||
| Obligations under capital lease, current portion | 71 | 106 | ||||||
| Advances from related parties | 356 | - | ||||||
| Notes and loans payable, current portion, net of debt discount | 1,583 | 997 | ||||||
| Total current liabilities | 7,592 | 6,286 | ||||||
| Notes and loans payable, net of current portion and debt discount | 2,222 | 2,886 | ||||||
| Obligations under capital lease, net of current portion | 91 | 91 | ||||||
| Series D Preferred Stock derivative liability | 4,805 | 6,529 | ||||||
| Deferred tax liability | 10,870 | 10,870 | ||||||
| Total liabilities | 25,580 | 26,662 | ||||||
| Series D Preferred Stock, net of discount, par value $0.0001, 12,000 shares authorized, 1,263 shares issued and 616 shares outstanding as of June 30, 2016 and 1,263 shares issued and 963 outstanding as of December 31, 2015 | 1,103 | 1,659 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ Equity | ||||||||
| Common stock, $0.0001 par value; authorized 225,000,000 shares; 54,730,318 and 32,434,942 shares issued and outstanding at June 30, 2016 and December 31, 2015, respectively | 5 | 3 | ||||||
| Additional paid-in capital | 76,959 | 70,846 | ||||||
| Accumulated deficit | (71,774 | ) | (62,986 | ) | ||||
| Total stockholders’ equity | 5,190 | 7,863 | ||||||
| Total liabilities and stockholders’ equity | $ | 31,873 | $ | 36,184 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
3
Immune Pharmaceuticals Inc. and Subsidiaries
Condensed Consolidated Statements of Operations
($ in thousands, except share and per share amounts)
(Unaudited)
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2016 | 2015 | 2016 | 2015 | |||||||||||||
| Revenue: | ||||||||||||||||
| Licensing and other revenue | $ | - | $ | - | - | - | ||||||||||
| Costs and expenses: | ||||||||||||||||
| Research and development | 1,945 | 1,126 | 3,956 | 2,277 | ||||||||||||
| General and administrative | 923 | 1,682 | 3,336 | 3,984 | ||||||||||||
| Total costs and expenses | 2,868 | 2,808 | 7,292 | 6,261 | ||||||||||||
| Loss from operations | (2,868 | ) | (2,808 | ) | (7,292 | ) | (6,261 | ) | ||||||||
| Non-operating income (expense): | ||||||||||||||||
| Interest expense | (334 | ) | (65 | ) | (711 | ) | (166 | ) | ||||||||
| Change in fair value of derivative liability instrument | (374 | ) | - | (692 | ) | - | ||||||||||
| Other expense, net | 1 | (3 | ) | (12 | ) | (6 | ) | |||||||||
| Total non-operating expense | (707 | ) | (68 | ) | (1,415 | ) | (172 | ) | ||||||||
| Net loss before income taxes | (3,575 | ) | (2,876 | ) | (8,707 | ) | (6,433 | ) | ||||||||
| Income tax expense | 81 | - | 81 | — | ||||||||||||
| Net loss | $ | (3,656 | ) | $ | (2,876 | ) | (8,788 | ) | (6,433 | ) | ||||||
| Series C Preferred dividend | - | (55 | ) | - | (114 | ) | ||||||||||
| Deemed dividend | (2,028 | ) | - | (2,914 | ) | (1,701 | ) | |||||||||
| Net loss attributable to common stockholders | $ | (5,684 | ) | $ | (2,931 | ) | (11,702 | ) | (8,248 | ) | ||||||
| Basic and diluted net loss per common share | $ | (0.13 | ) | $ | (0.12 | ) | (0.30 | ) | (0.34 | ) | ||||||
| Weighted average common shares outstanding – basic and diluted: | 42,757,932 | 24,722,980 | 38,642,016 | 24,522,788 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
4
Immune Pharmaceuticals Inc. and Subsidiaries
Condensed Consolidated Statement of Changes in Stockholders’ Equity
($ in thousands, except share and per share amounts)
(Unaudited)
| Common Stock | Additional | |||||||||||||||||||
| Shares | Amount | Paid-In Capital | Accumulated Deficit | Total | ||||||||||||||||
| Balance at December 31, 2015 | 32,434,942 | $ | 3 | $ | 70,846 | $ | (62,986 | ) | $ | 7,863 | ||||||||||
| Conversion of Series D Preferred Stock to common stock and accretion of deemed dividend | 16,417,880 | 2 | 3,009 | - | 3,011 | |||||||||||||||
| Capital Access Agreements | 4,700,000 | - | 1,575 | - | 1,575 | |||||||||||||||
| Share Purchase Agreements | 411,111 | - | 348 | - | 348 | |||||||||||||||
| Costs related to equity financing | - | - | (206 | ) | - | (206 | ) | |||||||||||||
| Reclassification of Hercules warrants derivative liability to additional paid-in capital | - | - | 46 | - | 46 | |||||||||||||||
| Common stock issued to settle liabilities | 200,000 | - | 60 | - | 60 | |||||||||||||||
| Exercise of stock options | 216,385 | - | 8 | - | 8 | |||||||||||||||
| Share-based compensation | 350,000 | - | 1,273 | - | 1,273 | |||||||||||||||
| Net loss | - | - | - | (8,788 | ) | (8,788 | ) | |||||||||||||
| Balance at June 30, 2016 | 54,730,318 | $ | 5 | $ | 76,959 | $ | (71,774 | ) | $ | 5,190 | ||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
5
Immune Pharmaceuticals Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
($ in thousands)
(Unaudited)
| Six Months Ended June 30, | ||||||||
| 2016 | 2015 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (8,788 | ) | $ | (6,433 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 197 | 159 | ||||||
| Share-based compensation | 1,273 | 1,733 | ||||||
| Common shares issued for services | - | 280 | ||||||
| Derivative liability loss | 692 | - | ||||||
| Amortization of debt issuance costs | 321 | 9 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Increase (decrease) in other assets | 6 | 39 | ||||||
| Increase in security deposits | - | (110 | ) | |||||
| Increase in accounts payable | 1,064 | 1,461 | ||||||
| Decrease in accrued expenses | (680 | ) | (1,560 | ) | ||||
| Net cash used in operating activities | (5,915 | ) | (4,422 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Decrease in restricted cash | (12 | ) | (40 | ) | ||||
| Purchase of property and equipment | (52 | ) | (27 | ) | ||||
| Net cash used in investing activities | (64 | ) | (67 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds received from exercise of options | 8 | 95 | ||||||
| Proceeds received from sale of common stock | 1,923 | - | ||||||
| Proceeds from advances from related parties | 356 | - | ||||||
| Payments of transaction costs related to sale of common stock | (47 | ) | - | |||||
| Principal repayment of loans | (398 | ) | (976 | ) | ||||
| Net cash (used in) provided by financing activities | 1,842 | (881 | ) | |||||
| Decrease in cash | (4,137 | ) | (5,370 | ) | ||||
| Cash at beginning of period | 4,543 | 6,767 | ||||||
| Cash at end of period | $ | 406 | $ | 1,397 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid for interest | $ | 225 | $ | 166 | ||||
| Cash paid for income taxes | 11 | - | ||||||
| Supplemental disclosure of non-cash financing activities: | ||||||||
| Conversion of Series D Preferred Stock to common stock and accretion of deemed dividend | 3,009 | - | ||||||
| Reclassification of Hercules warrants derivative liability to additional paid-in-capital | 46 | - | ||||||
| Common stock issued to settle liabilities | 60 | - | ||||||
| Accrued dividends on Series C Preferred Stock | - | 114 | ||||||
| Dividends settled in common stock | - | 55 | ||||||
| Conversion of Series C Preferred Stock into common stock | - | 149 | ||||||
| Services expensed and accrued in 2014 and settled in common stock in 2015 | - | 258 | ||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
6
Immune Pharmaceuticals Inc. and Subsidiaries
Notes to Unaudited Condensed Consolidated Financial Statements
NOTE 1. DESCRIPTION OF BUSINESS
Organization and Description of Business
Immune Pharmaceuticals Inc., together with its subsidiaries (“Immune” or the “Company”), is a clinical stage biopharmaceutical company specializing in the development and commercialization of novel targeted therapeutics in the fields of immuno-inflammation and immuno-oncology. The Company focuses on a precision medicine approach to treatment of diseases by incorporating methods for better patient selection in its clinical trials and the potential for development of companion diagnostics. The Company’s Immuno-inflammation product pipeline includes: bertilimumab, a clinical-stage first-in-class fully human antibody, targeting eotaxin-1, a key regulator of immuno-inflammation, a portfolio of clinical-stage immune oncology products and nanocyclo, a topical nanocapsule formulation of cyclosporine A, for the treatment of atopic dermatitis and psoriasis.
NOTE 2. GOING CONCERN UNCERTAINTY, FINANCIAL CONDITION AND MANAGEMENT’S PLANS
The Company believes that its available cash as of the date of this filing will not be sufficient to fund its anticipated level of operations for at least the next 12 months. The Company’s ability to continue as a “going concern” is dependent on a combination of several of the following factors: the Company’s ability to raise capital, the Company’s ability to monetize assets and the receipt of grants. The Company has limited capital resources and its operations have been funded by the proceeds of equity and debt offerings. The Company has devoted substantially all of its cash resources to research and development (“R&D”) programs and incurred significant general and administrative expenses to enable it to finance and grow its business and operations. To date, the Company has not generated any significant revenue and may not generate any revenue for a number of years, if at all. If the Company is unable to raise additional funds in the future on acceptable terms, or at all, it may be forced to curtail its development activities. In addition, the Company could be forced to delay or discontinue certain or all product developments, and forego attractive business opportunities.
At June 30, 2016, the Company had a working capital deficit of approximately $6.9 million. Accumulated deficit amounted to $71.8 million and $63.0 million at June 30, 2016 and December 31, 2015 respectively. Net loss for the three and six months ended June 30, 2016 was $3.7 million and $8.8 million, respectively. Net loss for the three and six months ended June 30, 2015 was $2.9 million and $6.4 million, respectively. Net cash used in operating activities was $5.9 million and $4.4 million for the six months ended June 30, 2016 and 2015, respectively.
The Company has historically funded its operations primarily through the sale of equity and/or debt securities, including the sale of common stock, convertible notes, preferred stock and warrants. On April 19, 2016, the Company entered into a Capital Access Agreement with Regatta Select Healthcare, LLC (“Regatta”), which provided for Regatta to purchase up to an aggregate of 3,500,000 shares of the Company’s common stock, par value $0.0001 per share at the Company’s discretion. Gross proceeds from this agreement were $0.8 million. In addition, on June 10, 2016, the Company entered into a second Capital Access Agreement with Regatta, which also provided for Regatta to purchase up to an aggregate of 3,700,000 shares of the Company’s common stock, par value $0.0001 at the Company’s discretion. Gross proceeds from this agreement were $1.1 million. During the second quarter of 2016, the Company also executed share purchase agreements for the sale of 966,666 shares of the Company’s common stock for gross proceeds of approximately $0.3 million (see Note 10). On July 29, 2016, Company entered into a securities purchase agreement with certain institutional investors for the sale of an aggregate of 3,174,603 shares of the Company’s common stock, for aggregate gross proceeds of $1.0 million. Under the agreement, the Company also issued to the investors warrants to purchase 500,000 shares of its common stock.
The Company will require additional financing for the remainder of 2016 in order to continue at its expected level of operations. If the Company fails to obtain the needed capital, it will be forced to delay, scale back, partner out or eliminate some or all of its R&D programs, which could result in an impairment of the Company’s intangible assets and have a material adverse impact on its financial condition and results of operation. There is no assurance that the Company will be successful in any capital-raising efforts that it may undertake to fund operations during 2016. The Company anticipates that it will continue to issue equity and/or debt securities as a source of liquidity, when needed, until it is able to generate positive cash flow to support its operations. The Company cannot give any assurance that the necessary capital will be raised or that, if funds are raised, it will be on favorable terms. Any future sales of securities to finance the Company will dilute existing stockholders' ownership. The Company cannot guarantee when or if it will ever generate positive cash flow. In addition, the Company may partner or license the Company’s assets, which may allow for additional non-dilutive financing in the future. The Company has previously received grants in Israel and may receive additional grant funding in 2016. The Independent Registered Public Accounting Firms’ Reports issued in connection with the Company’s audited consolidated financial statements for the year ended December 31, 2015 stated that there is “substantial doubt about the Company’s ability to continue as a going concern.”
7
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation
The accompanying condensed consolidated financial statements include the accounts of Immune and its wholly-owned subsidiaries: Immune Pharmaceuticals Ltd., Immune Pharmaceuticals USA Corp., Maxim Pharmaceuticals, Inc. and Cytovia, Inc. All inter-company transactions and balances have been eliminated.
The accompanying unaudited condensed consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and instructions to Form 10-Q and do not include all disclosures necessary for a complete presentation of financial position, results of operations, and cash flows in conformity with U.S. GAAP. These financial statements should be read in conjunction with the consolidated financial statements and related notes for the year ended December 31, 2015 filed on March 30, 2016. The results of operations for the three and six months ended June 30, 2016 and 2015 are not necessarily indicative of the results that may be expected for the entire fiscal year or for any other interim period. In the opinion of management, the accompanying unaudited interim condensed consolidated financial statements contain all material adjustments consisting of normal and recurring accruals necessary to present fairly the Company's consolidated financial position as of June 30, 2016, the results of operations for the three and six months ended June 30, 2016 and 2015 and cash flows for the six months ended June 30, 2016 and 2015.
Use of Estimates
In preparing condensed consolidated financial statements in conformity with U.S. GAAP, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and revenues and expenses during the reported periods. Significant estimates include impairment and amortization of long-lived assets (including intangible assets and in-process research and development (“IPR&D”)), valuation of options, warrants and derivative liabilities and valuation of uncertain tax positions. Actual results could differ from those estimates.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board ("FASB") or other standard setting bodies.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. The ASU enhances the reporting model for financial instruments, which includes amendments to address aspects of recognition, measurement, presentation and disclosure. The update to the standard is effective for public companies for interim and annual periods beginning after December 15, 2017. The Company is currently evaluating the impact that the standard will have on its condensed consolidated financial statements and will adopt this standard as of January 1, 2018.
In February 2016, the FASB issued Accounting Standards Update No. 2016-02, Leases. The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The Company is currently evaluating the impact of the standard on its condensed consolidated financial statements.
8
In March 2016, the FASB issued ASU 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share Based Payment Accounting ("ASU 2016-09") as part of the FASB simplification initiative. The new standard provides for changes to accounting for stock compensation including 1) excess tax benefits and tax deficiencies related to share based payment awards will be recognized as income tax expense in the reporting period in which they occur; 2) excess tax benefits will be classified as an operating activity in the statement of cash flows; 3) the option to elect to estimate forfeitures or account for them when they occur; and 4) increase tax withholding requirements threshold to qualify for equity classification. The ASU is effective for public companies for annual periods, and interim periods within those annual periods, beginning after December 15, 2016, and early adoption is permitted. The Company early adopted the guidance during the first quarter of 2016 and did not have a material impact on its condensed consolidated financial statements.
NOTE 4. DERIVATIVE FINANCIAL INSTRUMENTS
The Company accounts for derivative financial instruments in accordance with ASC 815-40, “Derivative and Hedging – Contracts in Entity’s Own Equity” (“ASC 815-40”) for instruments which do not have fixed settlement provisions are deemed to be derivative instruments.
Hercules Warrants
On July 29, 2015, the Company and Immune Pharmaceuticals USA Corp. entered into a Loan and Security Agreement with Hercules Capital (“Hercules”) to borrow $4.5 million with a Company option to borrow an additional $5.0 million prior to June 15, 2016, subject to the achievement of certain clinical milestones and satisfaction of certain other conditions. As of June 15, 2016, the Company had not met certain of the milestones as defined in the Hercules agreement in order to draw down upon the additional $5.0 million and as a result the option expired. In connection with the Hercules Loan, the Company has issued to Hercules a five-year warrant to purchase an aggregate of 214,853 shares of its common stock at an exercise price of $1.70 per share, subject to certain adjustments, including, if lower, the effective price of any financing occurring six months after the issuance date (the “Hercules Warrants”).
The Company determined the fair value of the Hercules Warrants of $0.3 million on July 29, 2015 using the Binomial Lattice pricing model and recorded that amount as part of debt discount in the condensed consolidated balance sheets since it was considered part of the cost of the financing and is being amortized over the life of the loan using the effective interest method. The Hercules Warrants were re-measured at each balance sheet date until the expiration of the anti-dilution provision on January 29, 2016. For the three and six months ended June 30, 2016, the Company recorded a gain on the change in the estimated fair value of the Hercules Warrants of approximately $0 and $38,000, respectively, which was recorded as non-operating expense in the condensed consolidated statements of operations. Upon the expiration of the anti-dilution provision on January 29, 2016, the remaining balance of $46,000 of the derivative liability was reclassified to additional paid-in-capital in the condensed consolidated balance sheets (see Note 5).
Discover Series D Convertible Preferred Stock
During 2015, the Company issued Series D Redeemable Convertible Preferred Stock (“Series D Preferred Stock”) to Discover Growth Fund (“Discover”), with a conversion price of $2.50 per share. Discover can convert at any time and at conversion Discover will receive a conversion premium equal to the amount of dividends it would have received if the Series D Preferred Stock had been held to the term of agreement of 6.5 years. The Series D Preferred Stock dividend rate includes an adjustment feature that fluctuates inversely to the changes in the value of the Company’s common stock price. The conversion premium and dividends are redeemed upon conversion of the Series D Preferred Stock. The Company determined that the conversion premium and dividends with the noted features required liability accounting. Accordingly, the conversion premium and the dividend feature have been bifurcated from the convertible preferred stock and are carried as a derivative liability at fair value. Changes in the fair value of this derivative liability are recognized in earnings for each reporting period. For the three and six months ended June 30, 2016, the Company recorded a loss of $0.4 million and $0.7 million, respectively, on the change in the estimated fair value of the Discover derivative liability, which was recorded as a non-operating expense in the condensed consolidated statements of operations. The fair value of the Discover derivative liability as of June 30, 2016 and December 31, 2015 was $4.8 million and $6.5 million, respectively (see Notes 5 and 11).
9
The Series D Preferred Stock derivative liability was valued using the Binomial Lattice options pricing model. The assumptions used at June 30, 2016 and December 31, 2015 were as follows:
| June 30, | December 31, | |||||||
| 2016 | 2015 | |||||||
| Face value of Series D Preferred Stock | $ | 10,000 | $ | 10,000 | ||||
| Conversion price | $ | 2.50 | $ | 2.50 | ||||
| Dividend period (in years) | 6.50 | 6.50 | ||||||
| Dividend yield | 15.00 | % | 15.00 | % | ||||
| Implied discount | 38.0 | % | 42.0 | % | ||||
| Share price | $ | 0.40 | $ | 0.73 | ||||
NOTE 5. FAIR VALUE INSTRUMENTS
Financial Instruments and Fair Value
The Company accounts for financial instruments in accordance with ASC 820, “Fair Value Measurements and Disclosures” (“ASC 820”). ASC 820 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy under ASC 820 are described below:
Level 1 – Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2 – Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly; and
Level 3 – Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable.
The financial instruments recorded in the Company’s condensed consolidated balance sheets consist primarily of cash, restricted cash, debt and accounts payable. The carrying amounts of the Company’s cash and accounts payable approximate fair value due to their short-term nature. The fair value of the Company’s debt approximates its gross carrying value of approximately $4.5 million (which has been presented net of issuance costs), due to its variable interest rate. In estimating the fair value of the Company’s Hercules and Discover derivative liabilities, the Company used the Binomial Lattice options pricing model at inception and on each subsequent valuation date. Based on the fair value hierarchy, the Company classified the Hercules and Discover derivative liability within Level 3.
The following table presents the Company’s liabilities that are measured and recognized at fair value on a recurring basis classified under the appropriate level of the fair value hierarchy as of June 30, 2016 and December 31, 2015 ($ in thousands).
| As of June 30, 2016 | Fair Value Hierarchy at June 30, 2016 | |||||||||||||||
| Total carrying and estimated fair value | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | |||||||||||||
| Liabilities: | ||||||||||||||||
| Series D Preferred Stock Derivative Liability | $ | 4,805 | $ | - | $ | - | $ | 4,805 | ||||||||
| As of December 31, 2015 |
Fair Value Hierarchy at December 31, 2015 | |||||||||||||||
| Total carrying and estimated fair value |
Quoted prices in active markets (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
|||||||||||||
| Liabilities: | ||||||||||||||||
| Series D Preferred Stock Derivative Liability | $ | 6,529 | $ | - | $ | $ | 6,529 | |||||||||
| Derivative liability related to Hercules Warrants | $ | 84 | $ | - | $ | - | $ | 84 | ||||||||
10
Hercules Warrants
The following table sets forth a summary of changes in the estimated fair value of the Company’s Hercules Warrant derivative liability for the period from January 1, 2016 through June 30, 2016 ($ in thousands):
| Fair Value Measurements of Hercules Common Stock Warrants Using Significant Unobservable Inputs (Level 3) | ||||
| Balance at January 1, 2016 | 84 | |||
| Change in estimated fair value of liability classified warrants | (38 | ) | ||
| Reclassification from liability to additional paid-in capital | (46 | ) | ||
| Balance at June 30, 2016 | $ | - | ||
Series D Preferred Stock
The following table sets forth a summary of changes in the estimated fair value of the Company’s Series D Preferred Stock derivative liability for the period from January 1, 2016 through June 30, 2016 ($ in thousands):
| Fair Value Measurements of Series D Preferred Stock Derivative Liability Using Significant Unobservable Inputs (Level 3) |
||||
| Balance at January 1, 2016 | $ | 6,529 | ||
| Series D Preferred Stock conversions | (2,455 | ) | ||
| Change in estimate fair value of Series D Preferred Stock derivative liability | 731 | |||
| Balance at June 30, 2016 | $ | 4,805 | ||
NOTE 6. INTANGIBLE ASSETS
The Company’s intangible assets consist of licenses and patents relating to the Company’s bertilimumab, Nanonabs and AMB8LK technologies and were determined by management to have a useful life between 7 and 15 years.
The value of the Company’s intangible assets as of June 30, 2016 is summarized below ($ in thousands):
Bertilimumab iCo | NanomAbs Yissum | Human Antibodies Kadouche | Anti-ferritin Antibody MabLife | Total | ||||||||||||||||
| Balance as of December 31, 2015 | $ | 1,753 | $ | 475 | $ | 475 | $ | 408 | $ | 3,111 | ||||||||||
| Amortization | (84 | ) | (23 | ) | (23 | ) | (23 | ) | (153 | ) | ||||||||||
| Balance, June 30, 2016 | $ | 1,669 | $ | 452 | $ | 452 | $ | 385 | $ | 2,958 | ||||||||||
| Gross asset value | $ | 2,509 | $ | 694 | $ | 700 | $ | 547 | $ | 4,450 | ||||||||||
| Accumulated Amortization | (840 | ) | (242 | ) | (248 | ) | (162 | ) | (1,492 | ) | ||||||||||
| Balance, June 30, 2016 | $ | 1,669 | $ | 452 | $ | 452 | $ | 385 | $ | 2,958 | ||||||||||
Amortization expense amounted to $0.1 million and $0.2 million for the three and six months ended June 30, 2016, respectively. Amortization expense amounted to $0.1 million and $0.2 million for the three and six months ended June 30, 2015, respectively.
11
Estimated amortization expense for each of the five succeeding years, based upon intangible assets at June 30, 2016 is as follows ($ in thousands):
| Period Ending December 31, | Amount | |||
| 2016 (6 months) | $ | 152 | ||
| 2017 | 305 | |||
| 2018 | 305 | |||
| 2019 | 305 | |||
| 2020 | 305 | |||
| Thereafter | 1,586 | |||
| Total | $ | 2,958 | ||
NOTE 7. ACCRUED EXPENSES
Accrued expenses consist of the following ($ in thousands):
June 30, 2016 | December 31, 2015 | |||||||
| Salaries and employee benefits | $ | 766 | $ | 545 | ||||
| Rent | 65 | 691 | ||||||
| Provision for a claim (see Note 13) | 300 | 300 | ||||||
| Financing costs | 268 | 88 | ||||||
| Professional fees | 170 | 549 | ||||||
| Severance | 50 | 180 | ||||||
| Other | 361 | 307 | ||||||
| Total | $ | 1,980 | $ | 2,660 | ||||
NOTE 8. NOTES AND LOANS PAYABLE
The Company is party to loan agreements as follows ($ in thousands):
| June 30, | December 31, | |||||||
| 2016 | 2015 | |||||||
| Loan and security agreement, net of original issue discount of $0.7 million and $1.0 million, respectively (1) | $ | 3,418 | $ | 3,496 | ||||
| Note payable (2) (3) | 387 | 387 | ||||||
| Total notes and loans payable | $ | 3,805 | $ | 3,883 | ||||
| Notes and loans payable, current portion | $ | 1,583 | $ | 997 | ||||
| Notes and loans payable, long-term | 2,222 | 2,886 | ||||||
| Total notes and loans payable, net of original issue discount of $0.7 million and $1.0 million, respectively | $ | 3,805 | $ | 3,883 | ||||
Repayments under the Company’s existing debt agreements consist of the following ($ in thousands):
| Period Ending June 30, | Amount | |||
| 2016 | $ | 1,161 | ||
| 2017 | 1,810 | |||
| 2018 | 1,496 | |||
| 2019 | 21 | |||
| Total | $ | 4,488 | ||
12
Loan and Security Agreement (1)
On July 29, 2015, the Company and Immune Pharmaceuticals USA Corp. entered into a Loan and Security Agreement (the “Loan Agreement”) with Hercules to borrow $4.5 million with a Company option to borrow an additional $5.0 million prior to June 15, 2016, subject to the achievement of certain clinical milestones and other conditions. As of June 15, 2016, the Company had not met certain of the milestones as defined in the Hercules agreement in order to draw down upon the additional $5.0 million and as a result the option had expired. The Loan Agreement is collateralized by a first priority perfected security interest in all tangible and intangible assets of the Company and its subsidiaries, senior to all indebtedness. The interest rate on the Hercules Loan is calculated at the greater of 10% or the prime rate plus 5.25%. The Company may prepay the Hercules Loan at any time, subject to certain prepayment penalties. Hercules may convert up to $1.0 million of the loan in any subsequent institutionally led Company financing on the same terms, conditions and pricing of such subsequent financing. This option to convert would be at the then fair value, as it will be at the same term and pricing as the new investors, therefore it is deemed to have minimal value. The Hercules Loan’s matures on September 1, 2018 and included a nine-month interest-only payment period following the closing, after which escalating principal payments of $0.1 million per month began on April 1, 2016. Interest expense for the three and six months ended June 30, 2016 was $0.1 million and $0.2 million, respectively. As of June 30, 2016, the Company had made $0.4 million in principal payments.
The Loan Agreement includes an end of term charge of $0.5 million payable on the earliest to occur of (i) the Term Loan Maturity Date, (ii) the date that Borrower prepays the outstanding Secured Obligations in full, or (iii) the date that the secured obligations become due and payable in full (as defined in the Loan Agreement). The Company accrues this charge for each reporting period and will accrue up to the $0.5 million charge over the 37-month term of the Hercules Loan as this charge is deemed a cost of the debt and the Company will be obligated to make this payment in the future. For the three and six months ended June 30, 2016, the Company recorded a charge of approximately $60,000 and $0.1 million, respectively, in interest expense in the condensed consolidated statements of operations.
The Company recorded $1.3 million in debt issuance costs relating to placement agent fees, legal fees, closing costs and the fair value of the placement agent warrants in its condensed consolidated balance sheet upon execution of the Loan Agreement. The Company early adopted ASU 2015-03 Simplifying the Presentation of Debt Issuance Costs, ASU 2015-03 requires the use of the effective interest method for the amortization of the debt discount. The Company will amortize the debt issuance costs over the term of the Loan, which expires on September 1, 2018. For the three and six months ended June 30, 2016, the Company recorded $0.2 million and $0.3 million, respectively, in interest expense related to the amortization of the debt issuance costs. At June 30, 2016 and December 31, 2015, the Company had approximately $0.7 million and $1.0 million, respectively; in debt issuance costs remaining to be amortized in its condensed consolidated balance sheets.
On July 21, 2016, Hercules, the Company and the holders of certain promissory notes (see Notes 14) entered into a Subordination Agreement to the Loan Agreement whereby the holders agreed to be subordinated to any security interest or lien that such creditors may have in any assets of the Company to Hercules.
MabLife Notes Payable (2)
In March 2012, the Company acquired from MabLife SAS (“MabLife”) through an assignment agreement, all rights, titles and interests in and to the patent rights, technology and deliverables related to the anti-Ferritin mAb, AMB8LK, including its nucleotide and protein sequences and its ability to recognize human acid and basic ferritins. The consideration was as follows: (i) $0.6 million payable in six annual installments (one of such installments being an upfront payment made upon execution of the agreement), and (ii) royalties of 0.6% of net sales of any product containing AMB8LK or the manufacture, use, sale, offering or importation of which would infringe on the patent rights with respect to AMB8LK. The Company is required to assign the foregoing rights back to MabLife, if it fails to make any of the required payments, is declared insolvent or bankrupt or terminates the agreement. In February 2014, the parties revised the payment arrangement for the purchase of the original assignment rights. Remaining payments of $0.1 million per year are due each year in 2016 and 2017.
In February 2014, the Company acquired from MabLife, through an irrevocable, exclusive, assignment of all rights, titles and interests in and to the secondary patent rights related to the use of anti-ferritin monoclonal antibodies in the treatment of some cancers, nucleotide and protein sequences of an antibody directed against an epitope common to human acidic and basic ferritins, monoclonal antibodies or antibody-like molecules comprising these sequences. As full consideration for the secondary patent rights, the Company will pay a total of $150,000 of which $15,000 and $25,000 was paid in 2014 and 2013 and $25,000 will be paid on the second through fourth anniversary of the agreement and an additional $35,000 on the fifth anniversary of the agreement.
13
During the first quarter of 2015, MabLife informed the Company that it has filed for bankruptcy. The Company is considering its options relating to the MabLife assignment agreement. For the three and six months ended June 30, 2016, the Company recorded $34,000 and $38,000, respectively, in interest expense. No interest expense was recorded for the three and six months ended June 30, 2015. No principal payments have been made as of June 30, 2016 subsequent to the announcement of the MabLife bankruptcy.
Revolving Line of Credit (3)
In April 2014, the Company entered into a three-year, $5.0 million revolving line of credit with Melini Capital Corp. (“Melini”), an existing stockholder who is related to Daniel Kazado, the Chairman of the Company’s Board of Directors. Borrowings under this revolving line of credit will incur interest at a rate of 12% per year, payable quarterly. The revolving line of credit is unsecured and subordinated to the Loan Agreement. Either party to the revolving line of credit has the right to terminate the revolving line of credit upon completion of a capital raise in excess of $5.0 million. To date, no amounts have been drawn under the revolving line of credit. Any amounts borrowed under the revolving line of credit must be repaid upon the maturity date of November 30, 2016 (see Note 14).
NOTE 9. INCOME TAXES
The Company has recognized a deferred tax liability of $10.9 million as of June 30, 2016 and December 31, 2015 related to the purchase of the AmiKet IPR&D. This deferred tax liability was recorded to account for the book vs. tax basis difference related to the IPR&D intangible asset, which was recorded in connection with the Merger. This deferred tax liability was excluded from sources of future taxable income, as the timing of its reversal cannot be predicted due to the indefinite life of the IPR&D. As such, this deferred tax liability cannot be used to offset the valuation allowance.
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company’s deferred tax assets relate primarily to its net operating loss carryforwards and other balance sheet basis differences. In accordance with ASC 740, “Income Taxes,” the Company recorded a valuation allowance to fully offset the gross deferred tax asset, because it is not more likely than not that the Company will realize future benefits associated with these deferred tax assets at June 30, 2016 and December 31, 2015.
During the second quarter of 2015, the Company received notices from New York State relating to audits of the 2011 to 2013 tax years. In June 2016, the Company paid $9,000 in full settlement of taxes and interest owed to New York State relating to the audits of the 2011 to 2013 tax years. During the third quarter of 2015, the Company received tax notices from the Israeli Tax Authority relating to the 2010 to 2014 tax years. During the second of 2016, the Company recorded a $70,000 liability relating to taxes due for the 2010 to 2014 tax years as a result of a settlement notice received from the Israeli Tax Authority.
NOTE 10. STOCKHOLDERS’ EQUITY
(a) Stock options and stock award activity
The following table illustrates the common stock options granted for the six months ended June 30, 2016:
| Title | Grant date |
No. of options |
Weighted price |
Weighted grant |
Vesting terms |
Assumptions used in Black-Scholes option pricing model | |||||||||
| Management, Directors and Employees | January - June 2016 | 2,890,000 | $ | 0.57 | $ | 0.36 |
Immediately -3 years |
Volatility Risk free interest rate Expected term, in years Dividend yield |
91.55%-92.15% 1.39%-2.06% 6-10 0.00% | ||||||
| Consultants | January - June 2016 | 335,000 | $ | 0.34 | $ | 0.24 | 1-3 years |
Volatility Risk free interest rate Expected term, in years Dividend yield |
91.55%-92.15% 1.24%-1.69% 5 0.00% | ||||||
14
The following table illustrates the stock awards for the six months ended June 30, 2016:
| Title | Grant date |
No. of stock awards |
Weighted average grant date fair value |
Vesting terms | |||||
| Consultants | January - June 2016 | 550,000 | $ | 0.50 | Immediately | ||||
The fair value of stock awards is determined using the share price on the date of grant.
The following table illustrates the common stock options granted for the six months ended June 30, 2015:
| Title | Grant date |
No. of options |
Weighted price |
Weighted grant |
Vesting terms |
Assumptions used in Black-Scholes option pricing model | |||||||||
| Management, Directors and Employees | January - June 2015 | 1,007,000 | $ | 2.02 | $ | 1.65 | 3 years |
Volatility Risk free interest rate Expected term, in years Dividend yield |
84.33%-91.55% 0.23%-2.17% 6-10 0.00% | ||||||
| Consultants | January - June 2015 | 160,000 | $ | 2.00 | $ | 1.46 | 1 years |
Volatility Risk free interest rate Expected term, in years Dividend yield |
91.55% 0.23%-0.27% 6 0.00% | ||||||
The following table illustrates the stock awards for the six months ended June 30, 2015:
| Title | Grant date | No. of stock awards | Weighted average grant date fair value | Vesting terms | |||||||
| Employees | January 2015 | 14,000 | $ | 2.47 | Immediately | ||||||
| Consultants | January - June 2015 | 489,469 | $ | 1.86 | Immediately | ||||||
The following table summarizes information about stock option activity for the six months ended June 30, 2016:
| Options | ||||||||||||||||||||
| No. of options | Weighted average exercise price | Exercise price range | Weighted average grant date fair value | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||
| Outstanding at December 31, 2015 | 4,988,988 | $ | 1.56 | $0.04 - $4.00 | $ | 1.97 | $ | 527 | ||||||||||||
| Granted | 3,225,000 | $ | 0.47 | $0.28-$0.73 | $ | 0.35 | $ | 31 | ||||||||||||
| Exercised | (291,221 | ) | $ | 0.04 | $ | 0.04 | $ | - | $ | 105 | ||||||||||
| Forfeited/Expired | (144,334 | ) | $ | 1.17 | $0.53-$2.38 | $ | 1.04 | $ | - | |||||||||||
| Outstanding at June 30, 2016 | 7,778,433 | $ | 1.17 | $0.04 - $4.00 | $ | 1.32 | $ | 198 | ||||||||||||
| Exercisable at June 30, 2016 | 4,135,394 | $ | 1.30 | $0.04 - $4.00 | $ | 1.67 | $ | 173 | ||||||||||||
At June 30, 2016, unamortized stock-based compensation for stock options was $1.8 million, with a weighted-average recognition period of approximately 1.8 years.
15
(b) Warrants
The following table illustrates warrants granted for the six months ended June 30, 2016:
| Title | Grant date |
No. of warrants |
Weighted average exercise price |
Weighted average grant date fair value |
Vesting terms |
Assumptions used in Black-Scholes option pricing model | |||||||||
| Consultants | January - June 2016 | 476,000 | $ | 0.62 | $ | 0.54 | Immediately |
Volatility Risk free interest rate Expected term, in years Dividend yield |
92.15% 1.24%-1.73% 5 0.00% | ||||||
The following table summarizes information about warrants outstanding at June 30, 2016:
| Number of warrants | Weighted average exercise price | Exercise price range | ||||||||||
| Warrants outstanding at December 31, 2015 | 10,692,138 | $ | 3.93 | $1.66-$65.60 | ||||||||
| Warrants issued to consultants | 476,000 | $ | 0.62 | $0.47-$0.91 | ||||||||
| Expired | (512,740 | ) | $ | 15.05 | $1.85-$65.60 | |||||||
| Warrants outstanding and exercisable at June 30, 2016 | 10,655,398 | $ | 3.25 | $0.47-$32.40 | ||||||||
Stock-based compensation expense for stock options and awards and warrants for the three and six months ended June 30, 2016 was $0.8 million and $1.3 million, respectively, which has not been tax-effected due to the recording of a full valuation allowance against net deferred tax assets. Stock-based compensation expense for stock options and awards and warrants for the three and six months ended June 30, 2015 was $0.9 million and $1.7 million, respectively, which has not been tax-effected due to the recording of a full valuation allowance against net deferred tax assets.
On June 1, 2016, the Company’s Board of Directors, pursuant to the recommendation of the Compensation Committee of the Board of Directors (the “Compensation Committee”) granted an option to purchase up to 360,000 shares at an exercise price of $0.40 per share to Dr. Daniel G. Teper, the Company’s Chief Executive Officer, of which 180,000 of the options shall vest over a three year period with one-third vesting after the one-year anniversary of the date of grant and the remaining two-thirds vesting in equal portions at the end of each three-month period over the course of the remaining two years. The grant date fair value of these options was $54,000. 180,000 options were granted as performance based bonus compensation. The options were granted at an exercise price of $0.40 per share and will vest upon the achievement of certain operational, financing and partnership objectives.
(c) Capital Access Agreements
April 19, 2016 Agreement
On April 19, 2016, the Company entered into a Capital Access Agreement (“April 19, 2016 Agreement”) with Regatta Select Healthcare, LLC (“Regatta”), which provided for Regatta to purchase up to an aggregate of 3,500,000 shares of the Company’s common stock, par value $0.0001 per share over the 12-month term of the April 19, 2016 Agreement.
16
Beginning on the day following the date that certain closing conditions in the April 19, 2016 Agreement were satisfied, which conditions were satisfied on April 19, 2016, the Company had the right, but not the obligation, to direct Regatta via written notice (a “Put Notice”) to purchase up to a specific ceiling of Purchase Shares. The Company was permitted to deliver up to four Put Notices per month. The purchase price per a Purchase Share pursuant to such Put Notice (the “Purchase Price”) shall equal 83% of the lowest price of the Company’s Common Stock during the five consecutive trading days immediately following the date of such Put Notice (the “Put Date”). The number of Purchase Shares that may be purchased under each Put Notice was subject to a ceiling of the lesser of (a) $250,000 in Purchase Shares or (b) 200% of average daily volume of the shares traded on the market on which the Company’s common stock is traded, computed using the 10 business days prior to the Put Date multiplied by the average of the daily closing price for the 10 business days immediately preceding the Put Date. The Purchase Price was additionally subject to a floor price equal to 75% of the average closing bid price for the common stock for the 10 trading days prior to the Put Date.
During the term of the April 19, 2016 Agreement and for a period of one year thereafter, Regatta had a right of first offer to purchase any equity securities of the Company, as well as any rights, options, or warrants to purchase such equity securities, and any securities of any type whatsoever that are, or may become, convertible or exchangeable into or exercisable for such equity securities, which the Company has a bona fide proposal to sell or offers to sell, other than equity securities offered or sold in an underwritten public offering. On June 10, 2016, the Company entered into an amendment to the April 19, 2016 agreement with Regatta such that during the term of the April 19, 2016 Agreement and for a period of one year thereafter, should the Company execute an agreement to sell fixed price equity securities within a 15 day period after the settlement of a put notice to any third party at a price lower than that paid by Regatta in connection with the prior put, the Company would be required to pay Regatta an aggregate fee of approximately $30,000.
During the three months ended June 30, 2016, the Company sold all 3,500,000 shares of its common stock under the April 19, 2016 Agreement to Regatta for an aggregate gross proceeds of $0.8 million.
June 10, 2016 Agreement
On June 10, 2016, the Company entered into a Capital Access Agreement (“June 10, 2016 Agreement”) with Regatta Select Healthcare, LLC (“Regatta”), which provided for Regatta to purchase up to an aggregate of 3,700,000 shares of the Company’s common stock, par value $0.0001 per share over the 12-month term of the June 10, 2016 Agreement.
Beginning on the day following the date that certain closing conditions in the June 10, 2016 Agreement were satisfied (the “Commencement Date”), which conditions were satisfied on June 10, 2016, the Company had the right, but not the obligation, to direct Regatta via written notice (a “Put Notice”) to purchase up to a specific ceiling of Purchase Shares. The Company was permitted to deliver up to four Put Notices per month. The purchase price per a Purchase Share pursuant to such Put Notice (the “Purchase Price”) shall equal 83% of the lowest price of the Company’s Common Stock during the five consecutive trading days immediately following the date of such Put Notice (the “Put Date”). The number of Purchase Shares that may be purchased under each Put Notice was subject to a ceiling of the lesser of (a) $250,000 in Purchase Shares or (b) 200% of average daily volume of the shares traded on the market on which the Company’s Common Stock is traded, computed using the 10 business days prior to the Put Date multiplied by the average of the daily closing price for the 10 business days immediately preceding the Put Date. The Purchase Price was additionally subject to a floor price equal to 75% of the average closing bid price for the Common Stock for the 10 trading days prior to the Put Date.
During the term of the June 10, 2016 Agreement and for a period of one year thereafter, should the Company execute an agreement to sell fixed price equity securities to any third party at a price lower than that paid by Regatta within a 15 day period from the settlement of a put notice, then the Company would be required to pay Regatta an aggregate fee of approximately $30,000. As of June 30, 2016, the Company sold 2,500,000 shares of its common stock for aggregate gross proceeds of $0.8 million. In July 2016, the Company sold the remaining 1,200,000 shares of its common stock under the June 10, 2016 Agreement for an aggregate gross proceeds of $0.3 million.
(d) Share Purchase Agreements
During the second quarter of 2016, the Company entered into share purchase agreements with two accredited investors to sell 966,666 restricted shares of the Company’s common stock at a price of $0.36 per share for total proceeds of $0.3 million. These restricted shares may not be transferred or sold at least for a period of six months and unless they have been registered for sale pursuant to the Securities Act of 1933, as amended. As of June 30, 2016, 411,111 shares of the Company’s common stock had been issued with respect to these agreements and the Company recorded a stock subscription payable for the remaining shares, which were issued in July 2016.
17
NOTE 11. SERIES D PREFERRED STOCK
During 2015, the Company entered into Stock Purchase Agreements (the “Purchase Agreements”) with Discover Growth Fund (“Discover”) pursuant to which the Company agreed to issue and sell up to an aggregate of 1,263 shares of the Company’s newly designated Series D Redeemable Convertible Preferred Stock (“Series D Preferred Stock”) of the Company, par value $0.0001 per share, convertible into shares of the Company’s Common Stock, at a conversion price of $2.50 per share (the “Conversion Price”), at a purchase price of $10,000 per share, for total gross proceeds of $12.0 million after taking into account a 5% original issue discount, or the sale of approximately $12.6 million.
The Series D Preferred Stock is convertible at a price of $2.50 per share and has a six and a half year maturity term, at which time it will convert automatically into common stock at $2.50 per share. The Series D Preferred Stock bears an accrued annual dividend rate which may range from 0% to 15%, based on certain adjustments and conditions, including changes in the volume weighted average price of the Company’s common stock. Upon conversion, the Company shall pay the holders of the Series D Preferred Stock being converted a conversion premium equal to the amount of dividends that such shares would have otherwise been issued if they had been held through the 6.5-year term.
The dividends and conversion premium may be paid in cash or, at the Company’s option, shares of common stock. If the Company elects to pay the dividends or conversion premium amount in the form of common stock, the number of shares to be issued is calculated as follows: (i) if there is no triggering event (as such term is defined in the Certificate of Designations), 90.0% of the average of the five lowest individual daily volume weighted average prices during the applicable measurement period, which may be non-consecutive, less $0.05 per share of common stock, not to exceed 100% of the lowest sales price on the last day of such measurement period, less $0.05 per share of common stock, or (ii) following any triggering event, 80.0% of the lowest daily volume weighted average price during any measurement period, less $0.05 per share of common stock, not to exceed 80.0% of the lowest sales price on the last day of any measurement period, less $0.05 per share of common stock. In addition the dividend rate adjusts upward by 10%.
The Series D Preferred Stock has been accounted for as mezzanine equity in the Company’s condensed consolidated balance sheets at June 30, 2016 and December 31, 2015 in accordance with ASC 480 “Distinguishing Liabilities from Equity,” as upon liquidation, the Company will be required to redeem the outstanding Series D Preferred Stock for cash. The conversion premium and the dividends associated with the Series D Preferred Stock contain an anti-dilution feature within the dividend rate, which fluctuates inversely to the changes in the value of the Company’s stock price. The conversion premium and dividends with the noted features are redeemed upon conversion of the Series D Preferred Stock. The Company’s management has analyzed the conversion premium and dividends with the noted features and has determined that they require liability treatment. Accordingly, the conversion premium and the dividends have been bifurcated from the Series D Preferred Stock. Initial and subsequent measurement of this derivative liability will be at fair value, with changes in fair value recognized in earnings on a quarterly basis.
The fair value of the derivative liability at June 30, 2016 and December 31, 2015 was $4.8 million and $6.5 million, respectively. For the three and six months ended June 30, 2016, the Company recorded a loss on the change in the estimated fair value of the derivative liability of $0.4 million and $0.7 million, respectively, which was recorded in non-operating expense in the Company’s condensed consolidated statements of operations.
During the six months ended June 30, 2016, Discover converted 347 shares of Series D Preferred Stock into a total of 1,388,000 shares of the Company’s common stock. In addition, the Company issued an additional 15,029,880 shares of its common stock as payment of dividends and conversion premium. The Company also recorded a proportionate amount of the Series D Preferred Stock as a deemed dividend of approximately $2.9 million upon conversion, which was charged to additional paid-in capital. Subsequent to June 30, 2016 and through July 31, 2016, Discover converted an additional 216 shares of Series D Preferred Stock for a total of 864,000 shares of the Company’s common stock and an additional 14,622,370 shares of common stock as payment of dividends and conversion premium.
18
Due to the occurrence of a triggering event in July 2016, the dividend rate adjusted upward from 15% to 25% and the calculation of the dividend and conversion premium amount in the form of common stock was adjusted as per the terms of the agreement.
Below is the activity for the Company’s Series D Preferred Stock issuances for the periods presented ($ in thousands, except share amounts):
| Shares | Amount | |||||||
| Balance at December 31, 2015 | 963 | $ | 1,659 | |||||
| Conversion of Series D Preferred Stock | (347 | ) | (3,470 | ) | ||||
| Accretion of Series D Preferred Stock | - | 2,914 | ||||||
| Balance at June 30, 2016 | 616 | $ | 1,103 | |||||
NOTE 12. LOSS PER SHARE
Basic and diluted loss per share is computed by dividing loss attributable to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted weighted average shares outstanding for the three and six months ended June 30, 2016 and 2015 excludes shares underlying stock options and warrants and convertible preferred, since the effects would be anti-dilutive. Accordingly, basic and diluted loss per share is the same.
Such excluded shares are summarized as follows:
| Three month period | Six month period | |||||||||||||||
| ended June 30, | ended June 30, | |||||||||||||||
| 2016 | 2015 | 2016 | 2015 | |||||||||||||
| Common stock options | 7,778,433 | 3,394,029 | 7,778,433 | 3,394,029 | ||||||||||||
| Restricted stock units (unvested) | - | 25,000 | - | 25,000 | ||||||||||||
| Shares issuable upon conversion of Series D Preferred Stock (not including dividends and conversion premium paid in common stock) | 2,464,000 | - | 2,464,000 | - | ||||||||||||
| Common shares issuable upon conversion of Series C Preferred Stock | - | 1,742,453 | - | 1,742,453 | ||||||||||||
| Warrants | 10,655,398 | 9,086,385 | 10,655,398 | 9,086,385 | ||||||||||||
| Total shares excluded from calculation | 20,897,831 | 14,247,867 | 20,897,831 | 14,247,867 | ||||||||||||
NOTE 13. COMMITMENTS AND CONTINGENCIES
(a) Leases
In February 2015, the Company’s corporate headquarters was relocated to New York, NY under a lease agreement with Alexandria Real Estate, which expires in 2020. On August 31, 2015, the Company signed an amendment to the New York, NY lease agreement with Alexandria Real Estate for lab space and offices for an additional 1,674 square feet commencing on September 1, 2015 and ending in 2020. The total base rent for offices and lab space, as amended, is approximately $30,000 per month, subject to annual rent escalations. On January 15, 2016, the Company signed a one-year lease agreement with an option for an additional year for new office space in Israel. For the three and six months ended June 30, 2016, the Company recorded rent expense of $0.1 million and $0.2 million, respectively. For the three and six months ended June 30, 2015, the Company recorded rent expense of $0.1 million.
Future minimum lease payments under non-cancelable leases for office space, as of June 30, 2016, are as follows ($ in thousands):
| Period ending December 31, | Amount | |||
| 2016 (6 months) | $ | 234 | ||
| 2017 | 378 | |||
| 2018 | 392 | |||
| 2019 | 405 | |||
| 2020 | 139 | |||
| $ | 1,548 | |||
19
(b) Licensing Agreements
The Company is a party to a number of research and licensing agreements, including iCo Therapeutics Inc., MabLife, Yissum Research Development Company of The Hebrew University of Jerusalem, Ltd, Dalhousie University, Lonza Sales AG and Shire Biochem Inc., which may require the Company to make payments to the other party upon the other party attaining certain milestones as defined in the agreements. The Company may be required to make future milestone payments under these agreements.
On January 1, 2016, the Company, through its wholly owned subsidiary, Immune Pharmaceuticals Ltd. entered into a definitive research and license agreement (the “License”) with BioNanoSim Ltd., (“BNS”). The License was entered into pursuant to an existing binding MOU, dated June 10, 2015, by and between the Company and Yissum. Under the License, the Company obtained from BNS an exclusive, worldwide sublicense, with a right to further sublicense, for the development, manufacturing and commercialization of certain inventions and research results regarding Yissum’s patents in connection with nanoparticles for topical delivery of cyclosporine-A (“Nanocyclo”), for all topical skin indications. In consideration for the License, the Company will pay BNS the following payments throughout the term of the License:
| · | an annual maintenance fee of $30,000, commencing on January 1, 2021, which maintenance fee shall increase by 30% each year, up to a maximum annual maintenance fee of $0.1 million and may be credited against royalties or milestone payments payable in the same calendar year; |
| · | a license fee in the amount of $0.5 million, to be paid in four equal installments to be made between January 4, 2016 and October 1, 2016; and |
| · | royalties on net sales of products (as such term is defined in the License) by the Company in the amount of up to 5%, subject to certain possible reductions in certain jurisdictions; |
| · | sublicense fees in the amount of 18% of any non-sales related consideration received by the Company from a sublicense or an option to receive a sublicense for the products and/or the licensed technology (as such terms are defined in the license); and |
| · | milestones payments of up to approximately $4.5 million and 250,000 shares of the Company’s common stock upon the achievement of certain regulatory, clinical development and commercialization milestones, provided, however, that in the event that the Company receives consideration from a sublicensee for any such milestones, the Company will pay to BNS the higher of either (a) the amount of the particular milestone payment or (b) the amount of the sublicense fees that are due for such sublicensee consideration paid to the Company. |
In addition, the Company shall reimburse BNS within 60 days for expenses relating to patent fees and will sponsor a 12-month research program to prepare the program for investigational new drug (“IND”) submission. The Company recorded an expense of $0.5 million for the license fee of which $0.3 million was paid through the first half of 2016.
(c) Litigation
Immune Pharmaceuticals Inc. was the defendant in litigation involving a dispute with the plaintiffs Kenton L. Cowley and John A. Flores. The complaint alleges breach of contract, breach of covenant of good faith and fair dealing, fraud and rescission of contract with respect to the development of a topical cream containing ketamine and butamben, known as EpiCept NP-2. A summary judgment in Immune’s favor was granted in January 2012 and the plaintiffs filed an appeal in the United States Court of Appeals for the Ninth Circuit in September 2012. A hearing on the motion occurred in November 2013. In May 2014, the court scheduled the trial in November 2014 and a mandatory settlement conference in July 2014. In July 2014, the parties failed to reach a settlement at the mandatory settlement conference. The case was tried by a jury, which rendered a decision on March 23, 2015, in favor of the Company on all causes of action. In April 2015, the plaintiffs filed a motion for a new trial, which was heard by the Court on June 8, 2015. In October 2015, the court denied the plaintiff’s motion for a new trial and on October 9, 2015; the plaintiffs filed a notice of appeal to the court. The court on plaintiff’s motion has made no ruling. During the three and six months ended June 30, 2016, in connection with the trial, the Company incurred approximately $0 and $39,000, respectively, of legal costs. During the three and six months ended June 30, 2015, in connection with the trial, the Company incurred approximately $0.4 million of legal costs.
In October 2014, the Company received a written demand from a former lender (“Lender”), for $9.1 million, which was based on an agreement with Immune Ltd from 2011, relating to a loan of $0.3 million, which was repaid in full in 2011. The Lender demands to receive certain warrants to purchase shares of the Company’s common stock, to participate in a future public offering or merger of the Company, with certain discounted terms and cash damages. The Company currently estimates that its future loss, if any, would range between $0.3 million (as accrued for) to $1.0 million, which if determined to be payable to Lender. The Company intends to vigorously defend itself against those demands, if and when official legal proceedings relating to this matter are ever initiated.
20
From time to time the Company is involved in legal proceedings arising in the ordinary course of business. The Company believes there is no other litigation pending that could have, individually or in the aggregate, a material adverse effect on its results of operations or financial condition.
NOTE 14. RELATED PARTY TRANSACTIONS
Daniel Kazado and Melini Capital Corp (“Melini”)
Mr. Daniel Kazado is Chairman of Immune’s Board of Directors. In April 2014, the Company entered into a $5.0 million revolving line of credit with Melini, an existing stockholder, who is related to the Chairman of the Board of Directors. Borrowings under this revolving line of credit will incur interest at a rate of 12% per year, payable quarterly. The revolving line of credit is unsecured and subordinated to the Loan Agreement with Hercules. To date, no amounts have been drawn under the revolving line of credit. Any amounts borrowed by the Company under the revolving line of credit become due upon the maturity date of November 30, 2016.
Promissory Notes issued to Certain Related Parties
Daniel Kazado
On July 15, 2016, the Company’s Board of Directors approved and the Company issued a $0.3 million promissory note to Daniel Kazado. The note bears interest at a rate of 5% per year and matures one year from the date of issuance. The outstanding balance of the note may be paid in cash or, at the option of either party, converted into shares of the Company’s common stock at a conversion rate of $0.45 per share, the last bid price of the Company’s common stock on the date of approval. On August 4, 2016, the Company exercised its option to pay off the promissory note by issuing 666,667 restricted shares of the Company’s common stock. These restricted shares may not be transferred or sold at least for a period of six months and unless they have been registered for sale pursuant to the Securities Act of 1933, as amended.
Daniel Teper
On June 24, 2016, the Company’s Board of Directors approved and the Company issued a $0.4 million promissory note to Daniel G. Teper, the Company’s Chief Executive Officer and an Immune director. The note bears interest at a rate of 5.0% per year and matures one year from the date of issuance. The outstanding balance of the note may be paid in cash or, at the option of either party, converted into shares of the Company’s common stock at a conversion rate of $0.41 per share, the last bid price of the Company’s common stock on the date of approval. On August 4, 2016, the Company exercised its option to pay off the promissory note by issuing 868,902 restricted shares of the Company’s common stock. These restricted shares may not be transferred or sold at least for a period of six months and unless they have been registered for sale pursuant to the Securities Act of 1933, as amended.
Monica Luchi
On July 15, 2016, the Company’s Board of Directors approved and the Company issued a $0.4 million promissory note to Monica Luchi, the Company Chief Medical Officer. The note bears interest at a rate of 5.0% per year and matures one year from the date of issuance. The outstanding balance of the note may be paid in cash or, at the option of either party, converted into shares of the Company’s common stock at a conversion rate of $0.45 per share, the last bid price of the Company’s common stock on the date of approval. On August 4, 2016, the Company exercised its option to pay off the promissory note by issuing 777,778 restricted shares of the Company’s common stock. These restricted shares may not be transferred or sold at least for a period of six months and unless they have been registered for sale pursuant to the Securities Act of 1933, as amended.
21
NOTE 15. SUBSEQUENT EVENTS
On July 18, 2016, the Company entered into Amendment No. 1 to the License Option Agreement (the “Amendment”) with Novel Pain Therapeutics LLC, (“NPT”), a new company dedicated to the development and commercialization of novel formulations of painpharmaceuticals, an exclusive option for a worldwide license agreement for Amiket and Amiket Nano (the “Products”) for the treatment of peripheral neuropathic pain. The Amendment amends that certain License Option Agreement (the “Option Agreement”) between the Company and NPT dated May 15, 2016. Pursuant to the Option Agreement, the Company granted NPT a 60 day option to negotiate a definitive license agreement for the Company’s products AmiKet™ and Amiket™ Nano for the treatment of peripheral neuropathic pain (the “Products”). The Amendment provides that, as a condition for entry into definitive agreements relating to the Products, (i) the Company shall form a new subsidiary (“New Sub”) to own the Products and related intellectual property and assets and (ii) NPT or its designees shall reimburse the Company up to $0.3 million for amounts contributed to New Sub in research and development capital. NPT or its designees shall own the same percentage of New Sub as they would have owned of NPT had NPT issued membership interests to the Company and entered into a definitive license agreement with the Company as contemplated by the Option Agreement. The Amendment further provides that NPT or its designees shall invest up to $20.0 million in New Sub in tranches and on terms to be agreed upon by the parties, subject to entry into satisfactory definitive agreements relating to the ownership of New Sub and royalties related to the Products. The Amendment extends the term of the Option Agreement to September 15, 2016.
The Company and NPT had previously signed a License Option Agreement on May 15, 2016 whereby NPT will assume all research and development costs upon execution of the license agreement and Immune will be eligible to receive up to $160.0 million in milestone payments which is comprised of an upfront fee of at least $15.0 million in the form of equity in NPT, up to $25.0 million in development milestones, and up to $120.0 million in commercial milestones, as well as product sales royalties. Immune is also eligible to receive 25% and up to 50% of sublicense fees received by NPT.
On July 29, 2016, Company entered into a securities purchase agreement with certain institutional investors for the sale of an aggregate of 3,174,603 shares of the Company’s common stock, for aggregate gross proceeds of $1.0 million. Under the agreement, the Company also issued to the investors warrants to purchase 500,000 shares of its common stock. The warrants were sold concurrently with the sale of the shares of common stock, pursuant to the agreement, in a concurrent private placement. The warrants will be exercisable for a period of five years from the date of issuance at an exercise price equal to $1.00 per share, subject to adjustment as provided under the terms of the warrants. The shares of common stock were issued in a registered direct offering pursuant to a prospectus supplement filed with the Securities and Exchange Commission on July 29, 2016, in connection with a takedown from the Registration Statement on Form S-3 (File No. 333-333-198647), which was declared effective by the SEC on October 28, 2014. In connection with the sale of the warrants, the Company relied upon the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended, for transactions not involving a public offering. The Company paid to the investors a commitment fee of $0.1 million by issuing 350,000 restricted shares of its common stock. These restricted shares may not be transferred or sold at least for a period of six months and unless they have been registered for sale pursuant to the Securities Act of 1933, as amended.
22
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The interim financial statements and this Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the financial statements and notes thereto for the year ended December 31, 2015, and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations, both of which are contained in our Annual Report on Form 10-K for the year ended December 31, 2015. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties, and assumptions. The Company has based these forward-looking statements on its current expectations and projections of future events. Such statements reflect the Company’s current views with respect to future events and are subject to unknown risks, uncertainties and other factors that may cause results to differ materially from those contemplated in such forward looking statements. Statements made in this document related to, among other statements, the development, commercialization and market expectations of the Company’s drug candidates, to the establishment of corporate collaborations, and to the Company’s operational projections are forward-looking and are made pursuant to the safe harbor provisions of the Securities Litigation Reform Act of 1995. Among the factors that could result in a materially different outcome are the inherent uncertainties accompanying new product development, action of regulatory authorities and the results of further clinical trials. The Company’s actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including but not limited to those set forth under the caption “Risk Factors” in the Annual Report on Form 10-K for the year ended December 31, 2015.
Overview
Immune Pharmaceuticals Inc., together with its subsidiaries (“Immune” or the “Company”), is a clinical stage biopharmaceutical company specializing in the development and commercialization of novel targeted therapeutics in the fields of immuno-inflammation and immuno-oncology. The Company focuses on a precision medicine approach to treatment of diseases by incorporating methods for better patient selection in its clinical trials and the potential for development of companion diagnostics. The Company’s Immuno-inflammation product pipeline includes: bertilimumab, a clinical-stage first-in-class fully human antibody, targeting eotaxin-1, a key regulator of immuno-inflammation a portfolio of clinical-stage immune oncology products and NanoCyclo, a topical nanocapsule formulation of cyclosporine-A, for the treatment of atopic dermatitis and psoriasis. The Company’s immune-oncology pipeline includes ceplene, and early immune-oncology treatment effective for the maintenance of remission in patients with AML; ceplene is already approved in 30 countries outside the US, and the company plans to work towards US approval for the product beginning this year. Two vascular disrupting agents are Phase II ready, having generated encouraging preliminary proof of concept study results. In addition, there are two platform assets which provide an opportunity for broad application: a bispecific antibody platform and a nanotechnology combination platform.
The Company’s current product portfolio is summarized in the table below:
| Product(s)/ Product Candidate(s) |
Primary Indication(s) | Status | Commercialization Rights | |||
| BERTILIMUMAB | Bullous Pemphigoid | Phase II | Immune (Worldwide) | |||
| IBD (Crohn's and ulcerative colitis) | Phase II | Immune (Worldwide) | ||||
| Atopic Dermatitis | Phase I/II | Immune (Worldwide) | ||||
| NASH | Phase I/II | Immune (Worldwide) | ||||
| NANOCYCLO (cyclosporin-A) | Atopic Dermatitis, Psoriasis | Preclinical | Immune (Worldwide) | |||
| IMMUNO-ONCOLOGY | ||||||
| Crolibulin | Solid Tumors | Phase II | Immune (Worldwide) | |||
| Azixa | Glioblastoma multiforme | Phase II | Immune (Worldwide) | |||
| NanomAbs | Solid Tumors | Preclinical | Immune (Worldwide) | |||
| Bispecific Antibodies | Oncology | Preclinical | Immune (Worldwide) | |||
| Ceplene/IL-2 | Acute Myeloid Leukemia | Phase III/ (owned in the EU and RoW by Meda AB) | Immune (Americas, Israel), Meda (EU, RoW) | |||
| OTHER/ PAIN | ||||||
| AmiKet | Neuropathic Pain | Phase III (Ready) | Immune (Worldwide) | |||
| LidoPAIN | Pain | Phase II | Immune (Worldwide) |
23
The Company’s immuno-oncology pipeline includes ceplene, a first-in-class small molecule targeting the Histamine-2 Receptor to overcome immunosuppression in Acute Myeloid Leukemia (“AML”) and potentially other malignancies. Ceplene has completed Phase III clinical trials and is approved by the European Medicines Agency and in Israel and is marketed by Meda AB. Azixa and Crolibulin are Phase II clinical stage vascular disrupting agents “VDAs”). NanomAbs, is a technology platform that allows the targeted delivery of combinations of chemotherapeutics into cancer cells; and a novel technology platform for the construction of bispecific antibodies for immunotherapies has already generated encouraging pre-clinical data.
Products and Programs
Bertilimumab
The Company’s lead product candidate, bertilimumab, is a fully human monoclonal antibody that targets eotaxin-1, a chemokine involved in inflammation. Eotaxin-1 has been validated as a bio-marker of disease severity and a therapeutic target for several inflammatory diseases. In 2016, the Company continued its enrollment of patients in Phase II clinical trials of a placebo-controlled, double-blind study with bertilimumab for the treatment of ulcerative colitis (“UC”), a common form of inflammatory bowel disease (“IBD”), and an open label trial in newly diagnosed patients with a rare autoimmune skin disease, bullous pemphigoid (“BP”), a rare auto-immune disease. In November 2015, Immune obtained clearance of its Investigational New Drug application from the Food and Drug Administration (“FDA”) allowing for expansion in 2016 of its phase II clinical trial in BP to multiple sites in the United States. The Company is also currently planning to expand the bertilumimab clinical development program to include severe atopic dermatitis (“AD”) and a series of preclinical and pilot clinical studies in multiple indications including liver disease such as nonalcoholic steatohepatitis (“NASH”).
Eotaxin-1 is important for eosinophil transmigration across blood vessels into tissue. Eotaxin-1 is increased in the serum and skin lesions of BP patients. Eosinophils co-localize with basal keratinocytes that have increased eotaxin-1 and chemokine receptor type 3 (“CCR3”) expression; CCR3 is the main receptor on eosinophils and eotaxin-1 has the highest affinity for CCR3 amongst other chemokines that affect eosinophils. Eotaxin-1 is correlated with BP disease severity. Eotaxin-1 is expressed on type 2 T helper (“Th2”) cells and eosinophils in BP lesions and an increasing body of data documents the role of these cells in both the innate and the adaptive arm of the immune system.
Bertilimumab neutralizes eotaxin-1, as illustrated below. A Phase II clinical study in BP is currently underway.
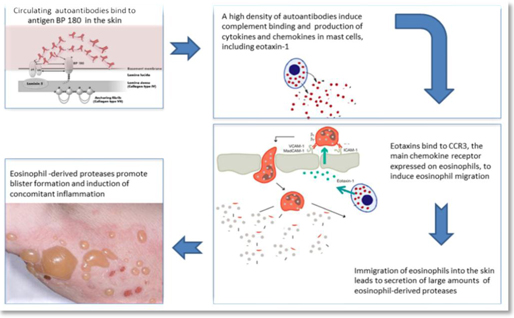
24
Recent nonclinical studies have demonstrated a link between eosinophils and the pathogenesis of IBD models in vivo, and studies have been performed assessing the molecular mechanisms of eosinophil recruitment and eosinophil function in Crohn’s disease models of colitis. Importantly, these nonclinical experimental analyses have been supported by studies in patients. Gene profiling on a cohort of adult and pediatric patients with UC at diagnosis identified significant upregulation of eotaxin-1 (Garcia-Zepeda et al., 1996; Ahrens et al., 2008). Eotaxin-1 levels correlated with tissue eosinophil numbers, which in turn correlated with the UC Histologic Index of Severity.
Coburn LA et al. (PLoS One, 2013; 8(12): e82300) demonstrated the correlation of high eotaxin-1 levels with disease severity. Tissue eotaxin-1 significantly increased based on Mayo Clinic Disease Activity Index, mucosal injury and histology severity. Increased eotaxin-1 correlates with tissue eosinophil counts. There is greater eotaxin-1 mRNA expression in areas of active vs. inactive disease. Patients on corticosteroids have lower serum eotaxin-1 but persistent high tissue eotaxin-1 levels. These data support patient selection and therapeutic targeting.
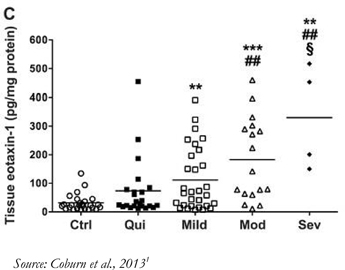
Furthermore, intestinal CD68+ macrophages were shown to express eotaxin-1 in colonic biopsy samples from pediatric patients with UC. Also, elevated levels of eosinophils have been observed in colonic biopsy samples from patients with UC. Increased levels of eosinophils and eosinophil-derived granular proteins including major basic protein (“MBP”), eosinophil cationic protein (“ECP”), eosinophil peroxidase (“EPO”), and eosinophil derived neurotoxin (“EDN”) have been shown to correlate with morphological changes to the GI tract, disease severity and GI dysfunction. As models of IBD as well as in human disease the “eotaxin-1: eosinophil” pathway appear to be of importance in the pathogenesis of UC; a phase 2 study is currently underway to test this hypothesis.
In AD, eosinophils are important effector cells that promote inflammation and tissue damage. Tissue eosinophilia has been correlated with eotaxin-1 and CCR-3 expression both at mRNA and protein level and a higher blood eosinophil count has been found in atopic subjects with a marked eotaxin-1 skin expression. There is experimental evidence that CCR3 expression is elevated also in non-lesional skin of atopic subjects suggesting that eotaxin-1 and other CCR3 binding chemokines may be involved in the very initial steps of AD inflammation mechanism. Also the expression of CCR3 on keratinocytes may suggest a role of eotaxin-1 not only in the triggering and maintenance of inflammation, but also in the epidermal changes typical of chronic AD.
Bertilimumab addresses large unsatisfied markets such as UC, with potential for use in Crohn’s disease, and severe AD, as well as an orphan indication and BP, with limited treatment alternatives. Upon successful development, regulatory approval and commercialization in multiple indications, bertilimumab has the potential to address the following markets based on research from GlobalData Plc (“GlobalData”) and multiple analysts’ reports:
| · | the $14.0 billion Crohn’s & colitis market; | |
| · | the $7.3 billion atopic dermatitis market; and | |
| · | the $1.6 billion bullous pemphigoid market. |
The Company’s strategy is to enter into a strategic partnership with a large pharmaceutical or biotechnology company in order to accelerate the development of bertilimumab and maximize its commercial potential.
25
Immuno Oncology
The Company's Immuno Oncology programs include the following:
Ceplene
Ceplene (histamine dihydrochloride), the Company’s lead oncology product candidate functions in AML by reducing the immunosuppressive effects of reactive oxygen species on T cells and Natural Killer (“NK”) cells, permitting their effective activation by Interleukin-2 (“IL-2”) in order to eliminate residual leukemic cells in patients who are in clinical complete remission. The Company’s current research is in delineating the value of combining ceplene with immune checkpoint inhibitors, which should pave the way for future trials and label expansion. celpene is already approved for marketing in Europe and Israel and has orphan drug status in the U.S and European Union.
The Company intends to leverage recent data on predictive bio-markers analysis of the recently completed Phase IV study and earlier studies to design and seek FDA guidance for a pivotal study in AML supporting a new drug application in the U.S. Ceplene’s tumor killing mechanism of action has recently been further elucidated and presented at the conference on “Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application" in Philadelphia, PA.
The diagram below depicts how Ceplene is able to suppress tumor growth by inhibiting NOX-2 in turn inhibiting macrophage and leukemic cell ROS production allowing IL-2 activation of NK cells and T cytotoxic cells with consequent leukemic cell death.
26
The European Phase IV study, recently completed and analysed, demonstrated Ceplene/IL-2 treatment to be highly efficacious in monocytic forms of AML (FAB M4/M5) as shown below.

Azixa
Azixa (verubulin) is a Phase II novel microtubular destabilizer that functions as a VDA. It evades multidrug resistance pumps, thus crossing the blood-brain-barrier and achieving high central nervous system concentrations. In Phase I and II clinical trials in glioblastoma multiforme (“GBM”), evidence of objective response was seen, including in patients who had failed previous bevacizumab (Avastin) therapy. Many scientists in the field of angiogenesis have suggested that the combination of anti-angiogenic and VDAs may be synergistic by both disrupting existing tumor vessels and inhibiting the formation of new vessels simultaneously. In addition, the Company is continuing preclinical work studying the potential advantages of combining Azixa with immune checkpoint inhibitors. If the results of these studies warrant further investigation, the Company may plan further clinical studies for GBM as well as other solid tumors. Azixa has orphan drug status for GBM in the U.S.
Crolibulin
Crolibulin is a novel small molecule VDA and apoptosis inducer for the treatment of patients with solid tumors and is a novel microtubule destabilizer that is selective for pathologic vasculature. Crolibulin has shown promising vascular targeting activity with potent anti-tumor activity in preclinical in vitro and in vivo studies and in a Phase I clinical trial conducted in part by the National Cancer Research Institute and the Company. The molecule has been shown to induce tumor cell apoptosis and selectively inhibit growth of proliferating cell lines, including multi-drug resistant cell lines. Murine models of human tumor xenografts demonstrated crolibulin inhibits growth of established tumors of a number of different cancer types. In preclinical animal tumor models, combination therapy has demonstrated synergistic activity with cytotoxic drugs as well as anti-angiogenic drugs. This may support further development of crolibulin by the Company or with a partner in a variety of cancers other than ATC, including but not limited to refractory ovarian cancer and neuro-endocrine tumors.
27
NanoCyclo
In January 2016, the Company entered into a worldwide exclusive licensing agreement with BioNanoSim Ltd., an Israeli company led by Professor Simon Benita, former Head of the Drug Research Institute at the Hebrew University of Jerusalem, for the development of a topical nanocapsule formulation of cyclosporine. NanoCyclo is the first stable formulation to leverage nanotechnology to ensure local dermal penetration of and minimize systemic exposure to cyclosporine, a drug used orally for the treatment of psoriasis and AD. NanoCylco has the possibility of development under a 505(b)2-regulatory submission, which is typically a faster process than the traditional 505(b)1 regulatory submission utilized by new chemical entities. Based on market research from GlobalData and multiple analysts’ reports, NanoCyclo could address the estimated $7.3 billion AD market and the $5.3 billion psoriasis market.
NanomAbs
The Company’s NanomAbs technology platform is an antibody-drug conjugate platform potentially capable of generating novel drugs with enhanced profiles, as compared to stand-alone antibodies. The technology conjugates mAbs to drug loaded nanoparticles to target drugs to specific cells. NanomAbs selectively accumulates in diseased tissues and cells, resulting in higher drug accumulation at the site of action with minimal off-target exposure. The Company is currently attempting to build a longer-term pipeline of NanomAbs for the treatment of cancer and may seek to enter into collaborative agreements with other companies to acquire complementary drugs or technologies to potentially accelerate the development of NanomAbs product candidates.
Bispecific Antibodies
In December 2015, the Company published data with a novel bispecific antibody in a poster presentation at the IBC Life Sciences Antibody Engineering & Therapeutics Conference in San Diego, CA. The Company’s poster presentation was titled "Design and Validation of a Novel Tetravalent IgG1-like BiSpecific Antibody Format". In this publication, the Company described positive study results with this novel platform for the production of tetravalent IgG1-like bispecific antibodies. The prototype bispecific antibody retained effector functions and mediated redirect killing of target cells by cytokine induced killer T cells demonstrating direct anti-cancer effects in vitro as well as anti-tumor activity and improved survival in a mouse xenograft model of disseminated leukemia. The Company believes that this newly developed platform may be further used to generate novel bispecific antibodies against immune-oncology targets. This work was developed by a collaborative European consortium and funded by a European grant.
Going forward, the Company is focusing on the development of bispecific antibodies targeting two immune check points, an immune check point and tumor specific target and undisclosed novel targets.
Other / Pain
AmiKet / AmiKet Nano
AmiKet is a prescription topical analgesic cream containing a formulation of two FDA approved drugs: amitriptyline, which is a widely-used antidepressant often used in chronic pain disorders; and ketamine, an N-methyl-D-aspartic acid (“NMDA”), antagonist that is used as an intravenous anesthetic. AmiKet has completed Phase I and II clinical trials involving 1,700 patients for the treatment of neuropathic pain. In 2010, the FDA granted AmiKet orphan drug status for the treatment of postherpetic neuralgia (“PHN”). The PHN Phase III clinical trial has been designed on the basis of two statistically significant Phase II clinical trials. The Company is currently developing a new improved formulation of the product utilizing nano-particle technology licensed from Yissum Research Development Company of The Hebrew University of Jerusalem, Ltd. (“Yissum”). AmiKet Nano is expected to provide patent protection until 2036 and allow for the development of additional indications including diabetic peripheral neuropathic pain, chemotherapy-induced peripheral neuropathic pain, as well as chronic back pain. In October 2015 Immune’s Pain Scientific Advisory Board met to discuss a Phase III clinical trial aimed at seeking a broad neuropathic pain label and on the benefits of its novel nano formulation. The Company believes that the Advisory Board recommendation could serve as the basis for further development and commercialization for AmiKet/AmiKet Nano with a broader peripheral neuropathic pain indication, addressing an $8.0 billion market according to GlobalData. The Company continues to review strategic opportunities by seeking investors or potential corporate partners to unlock the value of its pain franchise with a focus on Amiket Nano.
28
Recent Developments
On July 29, 2016, Company entered into a securities purchase agreement with certain institutional investors for the sale of an aggregate of 3,174,603 shares of the Company’s common stock, for aggregate gross proceeds of $1.0 million. Under the securities purchase agreement, the Company also issued to the investors warrants to purchase 500,000 shares of its common stock. The warrants were sold concurrently with the sale of the shares of common stock, pursuant to the agreement, in a concurrent private placement. The warrants will be exercisable for a period of five years from the date of issuance at an exercise price equal to $1.00 per share, subject to adjustment as provided under the terms of the warrants.
On June 28, 2016, the Company announced that recent preclinical experiments conducted by Dr. Boris Shor, Immune's Executive Director of R&D, in collaboration with an independent U.S. based Clinical Research Organization in a murine colon cancer model, demonstrated that the combination of azixa , a microtubule binding vascular disrupting agent , and immune checkpoint inhibitors such as anti-CTLA-4 antibody resulted in enhanced activity compared to the activity elicited by the single agents alone, independent of the dose of azixa. As a result, the Company has filed a provisional patent application with the United States Patent and Trademark Office relating to the combination of azixa in combination with the immune checkpoint inhibitors such as an anti-CTLA-4 antibody and anti-PD1 monoclonal antibodies in the treatment of cancer.
On June 20, 2016, the Company announced in a presentation at a conference on “Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application" in Philadelphia, PA., that ceplene suppresses tumor growth by inhibiting NOX-2, a type of immune check point. The data presented also suggests that ceplene significantly improves the anti-tumor efficacy of immune checkpoint inhibitors targeting PD-1 and PDL-1 in AML, lymphoma and breast cancer, forming the basis for the clinical development of ceplene and other immune checkpoint inhibitors in lymphomas and solid tumors.
On June 14, 2016, the Company announced the expansion of its bertilimumab Phase II clinical trial in bullous pemphigoid to six academic clinical sites in the U.S. The sites include University Hospital Cleveland Medical Center, Mount Sinai in New York, Duke University, the University of Iowa, the University of Buffalo, and the University of Utah.
On June 7, 2016, the Company announced that it had filed a provisional patent together with Hadasit, the Technology Transfer company of The Hadassah Medical Organization in Jerusalem, Israel, on the oral use of anti-eotaxin monoclonal antibodies, including Immune's bertilimumab, for the treatment of inflammatory gastro-intestinal and liver diseases.
On May 15, 2016, the Company signed with Novel Pain Therapeutics LLC, (“NPT”), a new company dedicated to the development and commercialization of novel formulations of pain pharmaceuticals, an exclusive option for a worldwide license agreement for Amiket and Amiket Nano (the “Products”) for the treatment of peripheral neuropathic pain. Upon execution of the license agreement, NPT will assume all research and development costs and Immune will be eligible to receive up to $160.0 million, comprised of an upfront fee of at least $15.0 million in the form of equity, up to $25.0 million in development milestones, and up to $120.0 million in commercial milestones, as well as product sales royalties. Immune will also be eligible to receive 25% and up to 50% of sublicense fees received by NPT. On July 18, 2016, the Company entered into Amendment No. 1 to the license option agreement (the “Amendment”). The Amendment provides that, as a condition for entry into definitive agreements relating to the Products, (i) the Company shall form a new subsidiary (“New Sub”) to own the Products and related intellectual property and assets and (ii) NPT or its designees shall reimburse the Company up to $300,000 for amounts contributed to New Sub in research and development capital. NPT or its designees shall own the same percentage of New Sub as they would have owned of NPT had NPT issued membership interests to the Company and entered into a definitive license agreement with the Company as contemplated by the original option agreement. The Amendment further provides that NPT or its designees shall invest up to $20 million in New Sub in tranches and on terms to be agreed upon by the parties, subject to entry into satisfactory definitive agreements relating to the ownership of New Sub and royalties related to the Products. The Amendment extends the term of the option agreement to September 15, 2016.
On April 19, 2016, the Company entered into a Capital Access Agreement with Regatta Select Healthcare, LLC (“Regatta”), which provided for Regatta to purchase up to an aggregate of 3,500,000 shares of the Company’s common stock, par value $0.0001 per share. Gross proceeds from this agreement were $0.8 million. On June 10, 2016, the Company entered into a second Capital Access Agreement with Regatta, which provided for Regatta to purchase up to an aggregate of 3,700,000 shares of the Company’s common stock, par value $0.0001 per share, at the Company’s discretion (see Note 10). Gross proceeds from this agreement were $1.1 million.
29
On April 19, 2016, the Company presented data at the American Association of Cancer Research annual conference in New Orleans on the results of a Phase IV European post-marketing clinical study. This study reported on an immune mechanism based biomarker which predicted the efficacy of treatment with Ceplene in all patients with AML and in particularly in the more difficult to treat older AML patient population. The study showed that immunotherapy with Ceplene and low-dose IL-2 led to a re-distribution of cytotoxic T-cell subsets in blood towards a tumor-killing phenotype. These immunological properties of Ceplene/IL-2 therapy significantly predicted clinical outcomes such as relapse and survival.
On March 22, 2016, the Company announced the publication of preclinical data in support of its bispecific antibody platform in the Journal of Immunology by Dr. Jean Kadouche, scientific co-founder of Immune, and other collaborators from a consortium of academic centers. The authors described the design and validation of a novel platform for the production of tetravalent IgG1-like bispecific antibodies, including final in-vivo proof-of-concept data for the benchmark molecule targeting human leukocyte antigen class II histocompatibility antigen and cluster of differentiation 5 (“HLA-DR/CD5”) protein. This work has been funded in part by an European grant to the collaborative European consortium.
On February 24, 2016, Immune announced publication of new data on the role of eotaxin-1 in UC and crohn's disease. The article, published in Digestive Disease Science, presented results of an observational clinical study intended to characterize serum and intestinal wall eotaxin-1 levels in IBD patients, and explored the effect of targeting eotaxin-1 by specific antibodies in an animal IBD model. These studies were conducted under the oversight of Professor Eran Goldin, Chairman of the Digestive Diseases Institute at Shaare Zedek Medical Center of the Hebrew University School of Medicine in Jerusalem, and supported in part by an unrestricted grant from the Company.
On February 10, 2016 the Company announced that it had filed a patent application directed to the treatment with Ceplene/IL-2 in solid tumors and other cancers. The new invention provides methods of treating cancer by administration of ceplene (histamine dihydrochloride) in combination with immune checkpoint inhibitors. The new invention also provides methods of predicting the efficacy of ceplene and IL-2 therapy in patients with AML.
On January 1, 2016, Immune Pharmaceuticals Inc., through its wholly owned subsidiary, Immune Pharmaceuticals Ltd. (“Immune Ltd.”) entered into a definitive research and license agreement with BioNanoSim Ltd., (“BNS”). The license was entered into pursuant to an existing binding MOU, dated June 10, 2015, by and between the Company and Yissum. Under the license, the Company obtained from BNS an exclusive, worldwide sublicense, with a right to further sublicense, for the development, manufacturing and commercialization of certain inventions and research results regarding Yissum’s patents in connection with nanoparticles for topical delivery of cyclosporine-A (Nanocyclo), for all topical skin indications. In consideration for the License, the Company will pay BNS the following payments throughout the term of the License:
| · | an annual maintenance fee of $30,000, commencing on January 1, 2021, which maintenance fee shall increase by 30% each year, up to a maximum annual maintenance fee of $0.1 million and may be credited against royalties or milestone payments payable in the same calendar year; | |
| · | a license fee in the amount of $0.5 million, to be paid in four equal installments to be made between January 4, 2016 and October 1, 2016; and | |
| · | royalties on net sales of products (as such term is defined in the License) by the Company in the amount of up to 5%, subject to certain possible reductions in certain jurisdictions; | |
| · | sublicense fees in the amount of 18% of any non-sales related consideration received by the Company from a sublicense or an option to receive a sublicense for the products and/or the licensed technology (as such terms are defined in the license); and | |
| · | milestones payments of up to approximately $4.5 million and 250,000 shares of the Company’s common stock upon the achievement of certain regulatory, clinical development and commercialization milestones, provided, however, that in the event that the Company receives consideration from a sublicensee for any such milestones, the Company will pay to BNS the higher of either (a) the amount of the particular milestone payment or (b) the amount of the sublicense fees that are due for such sublicensee consideration paid to the Company. |
30
In addition, the Company shall reimburse BNS within 60 days for expenses relating to patent fees and will sponsor a 12-month research program to prepare the program for investigational new drug (“IND”) submission.
Our Business
Results of Operations
Three months ended June 30, 2016 compared to the three months ended June 30, 2015
Revenues
| Three months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Revenue | $ | - | $ | - | $ | - | ||||||
The Company recorded no revenue for the three months ended June 30, 2016 and 2015. The Company has devoted substantially all of its financial resources and efforts to developing bertilimumab, the Company’s Phase II drug candidate, for the treatment of inflammatory diseases and NanomAbs, the Company’s platform for the targeted delivery of cancer drugs, manufacturing bertilimumab under cGMPs, conducting preclinical studies and clinical trials. The Company is still in the early stages of development of its product candidates, and has not completed the development of bertilimumab, NanomAbs or other drug candidates.
Research and development (“R&D”) expense ($ in thousands)
| Three months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Research and development | $ | 1,945 | $ | 1,126 | $ | 819 | ||||||
R&D expenses increased by $0.8 million or 73%, driven by higher salaries and employee benefits, share based compensation and clinical trial expenses. Salaries and employee benefits increased by $0.4 million while share based compensation expense increased by $0.2 million primarily due to higher R&D employee head count in 2016. Clinical trial expenses increased by $0.4 million as the Company continues to ramp up its clinical trials related to bertilimumab.
General and administrative (G&A) expense ($ in thousands)
| Three months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| General and administrative | $ | 923 | $ | 1,682 | $ | (759 | ) | |||||
G&A expenses decreased $0.8 million or 45%, driven by lower share based compensation and lower consulting and business development expenses. Share based compensation expense decreased by $0.4 million during the quarter ended June 30, 2016 as the fair value of stock options granted was lower compared to the fair value of stock options and warrants granted during the second quarter of 2015. Lower consulting and business development expense of $0.2 million was due to the Company’s reduction in the use of outside consultants as the Company had more employees in 2016 than in 2015.
Non-operating expense ($ in thousands)
| Three months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Non-operating expense | $ | 707 | $ | 68 | $ | 639 | ||||||
31
Non-operating expense was $0.7 million during the three months ended June 30, 2016 compared with non-operating expense of $0.1 million during the three months ended June 30, 2015, an increase of $0.6 million. Non-operating expense for the three months ended June 30, 2016 consisted of interest expense of $0.3 million primarily relating to cash interest expense and amortization of the debt issuance costs for the Company’s loan agreement with Hercules. In addition, non-operating expense included a $0.4 million loss on the change in fair value of derivative liability instrument. The loss on the change in fair value of derivative liability instrument of $0.4 million for the three months ended June 30, 2016 was related to the derivative liability associated with the Company’s Series D Preferred Stock. Non-operating expense for the three months ended June 30, 2015, was primarily comprised of interest expense of $0.1 million related to the MidCap Financial Trust (“Midcap”) senior secured term loan, which was repaid in July 2015.
Six months ended June 30, 2016 compared to the six months ended June 30, 2015
Revenues
| Six months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Revenue | $ | - | $ | - | $ | - | ||||||
The Company no revenue during the six months ended June 30, 2016 and 2015.
R&D expense
| Six months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Research and development | $ | 3,956 | $ | 2,277 | $ | 1,679 | ||||||
R&D expense increased by $1.7 million or 74% during the six-month period ended June 30, 2016 to $4.0 million compared with $2.3 million during the six-month period ended June 30, 2015. The increase was driven by higher salaries and employee benefits, share based compensation, clinical trial expenses and program license expenses. Salaries and employee benefits increased by $0.6 million, while share based compensation expense increased by $0.2 million due to higher R&D headcount. Clinical trial expenses increased by $0.4 million as the Company continues to ramp up its clinical trials related to bertulimumab in both BP and UC. Program license expense increased by $0.5 million driven by the licensed technology agreement with BNS Ltd.
G&A expense
| Six months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| General and administrative | $ | 3,336 | $ | 3,984 | $ | (648 | ) | |||||
G&A expense decreased by $0.6 million or 16%, during the six month period ended June 30, 2016 to $3.4 million, compared with $4.0 million during the six month period ended June 30, 2015 driven by lower share based compensation expense of $0.6 million and lower legal fees of $0.4 million. This was partially offset by higher investor relations expenses of $0.1 million and professional and consulting services including accounting services of $0.2 million.
Non-operating expense
| Six months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Non-operating expense | $ | 1,415 | $ | 172 | $ | 1,243 | ||||||
32
Non-operating expense was $1.4 million for the six months ended June 30, 2016 compared with non-operating expense of $0.2 million for the six months ended June 30, 2015, an increase of $1.2 million. Non-operating expense for the six months ended June 30, 2016 consisted of interest expense of $0.7 million primarily relating to cash interest expense, amortization of the debt issuance costs and early termination fees related to the Company’s loan agreement with Hercules. In addition, non-operating expense included a $0.7 million loss on the change in fair value of derivative liability instrument associated with the Company’s Series D Preferred Stock. Non-operating expense for the six months ended June 30, 2015, was primarily comprised of interest expense of $0.2 million related to the MidCap senior secured term loan which was repaid in July 2015
Deemed Dividend
The Company recorded approximately $2.0 million and $2.9 million in deemed dividend for the three and six months ended June 30, 2016, respectively, primarily as a result of dividend and conversion premium on the Company’s Series D Preferred Stock. For the three and six months ended June 30, 2015, the Company recorded accrued dividend as a deemed dividend of $55,000 and $1.8 million, respectively, related to its Series C Preferred Stock, which was exchanged in connection with the Company’s July 2015 Series C Exchange.
Liquidity and Capital Resources
The following table summarizes select balance sheet and working capital amounts as at June 30, 2016 and December 31, 2015 ($ in thousands):
| As of | As of | |||||||||||
| June 30, | December 31, | |||||||||||
| 2016 | 2015 | Change | ||||||||||
| Cash | $ | 406 | $ | 4,543 | $ | (4,137 | ) | |||||
| Working capital deficit | $ | (6,860 | ) | $ | (1,370 | ) | $ | (5,490 | ) | |||
| Notes and loans payable, current portion | $ | 1,583 | $ | 997 | $ | 586 | ||||||
The Company believes that its available cash as of the date of this filing will not be sufficient to fund its anticipated level of operations for at least the next 12 months. The Company’s ability to continue as a “going concern” is dependent on a combination of several of the following factors: the Company’s ability to raise capital, the Company’s ability to monetize assets and the receipt of grants. The Company has limited capital resources and its operations have been funded by the proceeds of equity and debt offerings. The Company has devoted substantially all of its cash resources to R&D programs and incurred significant G&A expenses to enable it to finance and grow its business and operations. To date, the Company has not generated any significant revenues and may not generate any such revenue for a number of years, if at all. If the Company is unable to raise additional funds in the future on acceptable terms, or at all, it may be forced to curtail its development activities. In addition the Company could be forced to delay or discontinue certain or all product developments, and forego attractive business opportunities.
At June 30, 2016, the Company had working capital deficit of approximately $6.9 million. Accumulated deficit amounted to $71.8 million and $63.0 million at June 30, 2016 and December 31, 2015, respectively. Net loss for the three and six months ended June 30, 2016 was $3.7 million and $8.8 million, respectively. Net loss for the three and six months ended June 30, 2015 was $2.9 million and $6.4 million, respectively. Net cash used in operating activities was $5.9 million and $4.4 million for the six months ended June 30, 2016 and 2015, respectively. Operations since inception have been funded primarily with the proceeds from equity and debt offerings.
The Company has historically funded its operations primarily through the sale of equity and/or debt securities, including the sale of common stock, convertible notes, preferred stock and warrants. On April 19, 2016, the Company entered into a Capital Access Agreement with Regatta, which provided for Regatta to purchase up to an aggregate of 3,500,000 shares of the Company’s common stock, par value $0.0001 per share. The Company received approximately $0.8 million in proceeds from this agreement. In addition, on June 10, 2016, the Company entered into a second Capital Access Agreement with Regatta, which also provided for Regatta to purchase up to an aggregate of 3,700,000 shares of the Company’s common stock, par value $0.0001 per share. The Company received approximately $1.1 million in proceeds from this agreement (see Note 10). During the second quarter of 2016, the Company also executed share purchase agreements for the sale of 966,666 shares of the Company’s common stock which resulted in proceeds of approximately $0.3 million (See Note 10). In addition, on July 29, 2016, Company entered into a securities purchase agreement with certain institutional investors for the sale of an aggregate of 3,174,603 shares of the Company’s common stock, for aggregate gross proceeds of $1.0 million. Under the agreement, the Company also issued to the investors warrants to purchase 500,000 shares of its common stock.
33
The Company will require additional financing for the remainder of 2016 in order to continue at its expected level of operations. If the Company fails to obtain the needed capital, it will be forced to delay, scale back, partner out or eliminate some or all of its R&D programs, which could result in an impairment of the Company’s intangible assets and have a material adverse impact on its financial condition and results of operation. There is no assurance that the Company will be successful in any capital-raising efforts that it may undertake to fund operations during 2016. The Company anticipates that it will continue to issue equity and/or debt securities as a source of liquidity, when needed, until it is able to generate positive cash flow to support its operations. The Company cannot give any assurance that the necessary capital will be raised or that, if funds are raised, it will be on favorable terms. Any future sales of securities to finance the Company will dilute existing stockholders' ownership. The Company cannot guarantee when or if it will ever generate positive cash flow. In addition, the Company may partner or license the Company’s assets, which may allow for additional non-dilutive financing in the future. The Company has previously received grants in Israel and may receive additional grant funding in 2016. The Independent Registered Public Accounting Firms’ Reports issued in connection with the Company’s audited consolidated financial statements for the year ended December 31, 2015 stated that there is “substantial doubt about the Company’s ability to continue as a going concern.”
Funding Requirements
The Company’s future financing requirements will depend on many factors, some of which are beyond the Company’s control. Factors affecting the Company’s financing requirements include, but are not limited to:
| · | the rate of progress and cost of the Company’s clinical trials, preclinical studies and other discovery and research and development activities; | |
| · | the timing of, and costs involved in, seeking and obtaining marketing approvals for the Company’s products, and in maintaining quality systems standards for the Company’s products; | |
| · | The Company’s ability to manufacture sufficient quantities of its future products to meet expected demand; | |
| · | the costs of preparing, filing, prosecuting, maintaining and enforcing any patent claims and other intellectual property rights, litigation costs and the results of litigation; | |
| · | The Company’s ability to enter into collaboration, licensing or distribution arrangements and the terms and timing of these arrangements; | |
| · | the potential need to expand the Company’s business, resulting in additional payroll and other overhead expenses; | |
| · | the potential need to acquire, by acquisition or in-licensing, other products or technologies; and | |
| · | the emergence of competing technologies or other adverse market or technological developments. |
Future capital requirements will also depend on the extent to which the Company acquires or invests in additional complementary businesses, products and technologies. The Company’s strategy to fund its operations is to monetize its multiple asset groups either through partnerships, asset-centric subsidiaries or joint ventures, raise capital, and the potential exercise for cash of warrants and options
Cash Flow Activities
The following table summarizes the Company’s cash flows for the periods set forth below ($ in thousands):
| Six months ended June 30, | ||||||||||||
| 2016 | 2015 | Change | ||||||||||
| Net cash used in operating activities | $ | (5,915 | ) | $ | (4,422 | ) | $ | (1,493 | ) | |||
| Net cash used in investing activities | $ | (64 | ) | $ | (67 | ) | $ | 3 | ||||
| Net cash provided by (used in) financing activities | $ | 1,842 | $ | (881 | ) | $ | 2,723 | |||||
Operating Activities
Net cash used in operating activities for the six months ended June 30, 2016 was $5.9 million. Net cash used in operating activities in the six months ended June 30, 2016, exclusive of changes in operating assets and liabilities, was $6.3 million; the net loss of $8.8 million included non-cash charges for stock based compensation of $1.3 million, depreciation and amortization, including debt issuance costs of $0.5 million and changes in the fair value of derivative liability instrument of $0.7 million. Cash provided from changes in operating assets and liabilities in the six months ended June 30, 2016 was $0.4 million primarily due to an increase in accounts payable of $1.1 million partially offset by a decrease in accrued expenses of $0.7 million.
34
Investing Activities
For the six months ended June 30, 2016, our net cash used in investing activities was $64,000.
Financing Activities
For the six months ended June 30, 2016, net cash provided by financing activities was $1.8 million primarily driven by the receipt of $1.9 million from the issuance of the Company's common stock related to the Capital Access Agreements and the receipt of cash from share purchase agreements entered into during the second quarter of 2016. In addition the Company received $0.4 million in loans from related parties. This was partially offset by the payment of $0.4 million in principal payments related to the Hercules loan.
Recently Issued Accounting Pronouncements
See Note 3, Summary of Significant Accounting Policies, to the Consolidated Financial Statements for a discussion of recent accounting developments.
Off-Balance Sheet Arrangement
As of June 30, 2016, the Company had no off-balance sheet arrangements. Immune has no guarantees or obligations other than those, which arise out of the Company’s ordinary business operations.
Critical Accounting Policies and Significant Judgments and Estimates
A summary of the Company’s significant accounting policies is contained in the notes to the Company’s consolidated financial statements in its Annual Report on Form 10-K for the year ended December 31, 2015. There have been no material changes to those policies during the three and six months ended June 30, 2016.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As a smaller reporting company, as that term is defined in Item 10(f)(1) of Regulation S-K, the Company is not required to provide information required by this item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Management, with the participation of the Company’s Principal Executive Officer and Principal Financial Officer, carried out an evaluation of the effectiveness of the Company’s “disclosure controls and procedures”(as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of June 30, 2016, the end of the period covered by this Quarterly Report on Form 10-Q (the “Evaluation Date”). Based upon that evaluation, the Company’s Chief Executive Officer and Principal Financial Officer have concluded that as of the Evaluation Date, the Company’s disclosure controls are not effective.
Management’s assessment identified the following material weaknesses in the Company’s internal control over financial reporting: segregation of duties issues due to lack of sufficient accounting and finance personnel and lack of a sufficient technology infrastructure to support the financial reporting function. Management has begun to implement certain remedial measures, including the purchase of a license to a new accounting general ledger system, which is in the process of being implemented. In addition, the Company has hired consultants to help document the Company’s internal control environment. Remediation efforts will continue through the next several financial close cycles until such time as the Company’s management is able to conclude that its remediation efforts are effective.
35
Changes in Internal Control over Financial Reporting
During the six months ended June 30, 2016, the Company’s management has taken the following actions that materially affect, or are reasonably likely to materially affect, the Company’s internal control over financial reporting and to remediate the material weaknesses described in its 2015 Annual Report on Form 10-K.
| · | In March 2016, the Company hired a Senior Director of Finance and Assistant Controller with expertise in understanding complex accounting and financial reporting issues and disclosure rules required under GAAP and SEC reporting regulations. |
| · | During the first quarter of 2016, the Company began implementing a new general ledger accounting system. |
The Company is not involved in any legal proceedings which management believes may have a material adverse effect on our business, financial condition, operations, cash flows, or prospects. The Company from time to time is involved in legal proceedings in the ordinary course of our business, which can include employment claims, product claim, patent infringement, etc. The Company does not believe that any of these claims and proceedings against us as they arise are likely to have, individually or in the aggregate, a material adverse effect on our financial condition or results of operations.
Except as set forth below, there have been no changes to the risk factors set forth in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2015.
If we fail to comply with the continued listing requirements of the NASDAQ Capital Market LLC (“NASDAQ”), our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Our common stock is listed for trading on the NASDAQ. We must satisfy NASDAQ’s continued listing requirements, including, among other things, a minimum closing bid price requirement of $1.00 per share for 30 consecutive business days. If a company’s common stock trades for 30 consecutive business days below the $1.00 minimum closing bid price requirement, NASDAQ will send a deficiency notice to the company, advising that it has been afforded a "compliance period" of 180 calendar days to regain compliance with the applicable requirements. Thereafter, if such a company does not regain compliance with the bid price requirement, a second 180-day compliance period may be available.
A delisting of our common stock from NASDAQ could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, employees and fewer business development opportunities.
On July 6, 2016, Immune Pharmaceuticals Inc. (the “Company”) received a notification from the Listing Qualifications Department of The NASDAQ Stock Market LLC (“NASDAQ”) indicating that the Company had been granted an additional 180 calendar day extension, or until January 3, 2017 (the “Extension”), to regain compliance with the requirements under NASDAQ Listing Rule 5810(c)(3) (the “Rule”). The notification stated that Extension determination was based on the Company meeting the continued listing requirement for market value of publicly held shares and all other applicable requirements for initial listing on the Capital Market, with the exception of the bid price requirement. The notification has no immediate effect on the NASDAQ listing or trading of the Company’s common stock.
As previously reported on a Current Report on Form 8-K dated January 11, 2016, the Company had received a prior notification from NASDAQ, dated January 5, 2016, indicating that the Company was not in compliance with the Rule because the minimum bid price of the Company’s common stock on the NASDAQ Capital Market had closed below $1.00 per share for 30 consecutive business days.
36
In the event the Company does not regain compliance with the Rule by January 3, 2017, NASDAQ will notify the Company that its common stock will be delisted from the NASDAQ Capital Market, unless the Company requests a hearing before a NASDAQ Hearings Panel.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
During the second quarter of 2016, the Company entered into share purchase agreements with two accredited investors to sell 966,666 shares of the Company’s common stock at a price of $0.36 per share for total proceeds of $0.3 million. In connection with the sale of these shares, the Company relied upon the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended, for transactions not involving a public offering. As of June 30, 2016, 411,111 shares of the Company’s common stock had been issued with respect to these agreements and the Company issued the remaining 555,555 shares in July 2016.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
None.
37
| Exhibit No. |
Description of Exhibit | |
| 10.1 | License Option Agreement dated as of May 15, 2016 by and between the Company and Novel Pain Therapeutics LLC.** | |
| 10.2 | Capital Access Agreement, dated as of June 10, 2016, by and between the Company and Regatta Select Healthcare, LLC. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 13, 2016). ** | |
| 10.3 | Amendment No. 1 to Capital Access Agreement, dated as of June 10, 2016, by and between the Company and Regatta Select Healthcare, LLC. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on June 13, 2016).** | |
| 10.4 | Amendment No. 1 to License Option Agreement dated as of July 18, 2016 by and between the Company and Novel Pain Therapeutics, LLC. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 19, 2016). ** | |
| 10.5 | Form of Promissory Note (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 19, 2016). ** | |
| 10.6 | Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on August 3, 2016). ** | |
| 10.7 | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on August 3, 2016). ** | |
| 31.1 | Certification of Principal Executive Officer pursuant to Exchange Act Rules 13a- 14(a) and 15d-14(a), adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
| 31.2 | Certification of Principal Financial Officer pursuant to Exchange Act Rules 13a- 14(a) and 15d-14(a), adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
| 32.1 | Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* | |
| 32.2 | Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* | |
| 101.INS | XBRL Instance Document* | |
| 101.SCH | XBRL Taxonomy Extension Schema Document* | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document* | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document * | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document* | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document* | |
| * Filed herewith. | ||
| ** Previously filed with the SEC as indicated, and hereby incorporated herein by reference. |
38
SIGNATURE PAGE
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| IMMUNE PHARMACEUTICALS INC. | ||
| By: | /s/ Daniel G. Teper | |
| Daniel G. Teper | ||
|
Chief Executive Officer (Principal Executive Officer) | ||
| August 15, 2016 |
| By: | /s/ John C. Militello | |
| John C. Militello | ||
|
VP of Finance, Controller and Chief Accounting Officer (Principal Financial Officer and Principal Accounting Officer) | ||
| August 15, 2016 |
39
