Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Protea Biosciences Group, Inc. | v476204_8k.htm |
Exhibit 99.1

A Revolution In Oncology Medicine
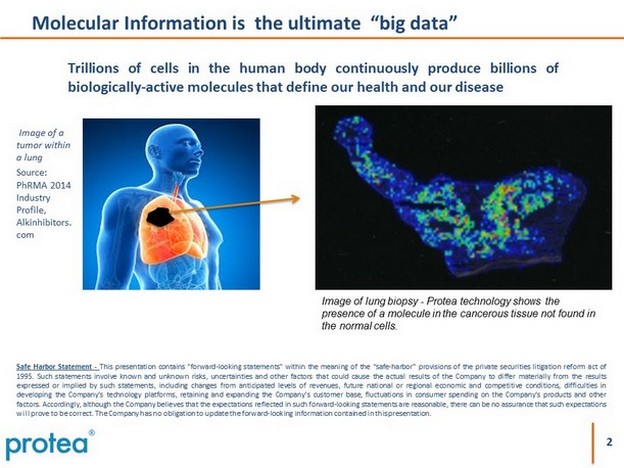
2 Molecular Information is the ultimate “big data” Trillions of cells in the human body continuously produce billions of biologically - active molecules that define our health and our disease Image of a tumor within a lung Source: PhRMA 2014 Industry Profile, Alkinhibitors. com Safe Harbor Statement - This presentation contains "forward - looking statements" within the meaning of the “safe - harbor” provisions of the private securities litigation reform act of 1995 . Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of the Company to differ materially from the results expressed or implied by such statements, including changes from anticipated levels of revenues, future national or regional economic and competitive conditions, difficulties in developing the Company’s technology platforms, retaining and expanding the Company’s customer base, fluctuations in consumer spending on the Company’s products and other factors . Accordingly, although the Company believes that the expectations reflected in such forward - looking statements are reasonable, there can be no assurance that such expectations will prove to be correct . The Company has no obligation to update the forward - looking information contained in this presentation . Image of lung biopsy - Protea technology shows the presence of a molecule in the cancerous tissue not found in the normal cells.

3 Overview We develop and commercialize proprietary technology that can rapidly identify the molecules produced by living cells: Our technology is used to improve new drug development and has been adopted by pharma industry leaders. Our clients include Fortune 500 & pharma/biotech companies. We are applying our technology to create a new class of oncology diagnostics: We can rapidly define the molecular fingerprint of normal and cancer cells Not the traditional “single molecule” approach, but a molecular fingerprint, where over 8,000 molecules can be reviewed in a full spectrum analysis in a matter of minutes.

4 Pharma Needs Better Molecular Information Current state of the art does not effectively address pharma needs, e.g.: “Is my drug reaching the target” “How long does it remain biologically active?” “What other molecular changes are occurring in the target cells?” We apply our technology to offer proprietary molecular information services: We can identify both small and large molecules Rapid - Data available in minutes We can analyze living cells There is little or no sample preparation Molecules can be imaged in tissue sections

5 The Management Team Name & Title Background Stephen Turner Chairman & CEO • Founder OncorMed (Gene Logic) - cancer genomics • Founder Quorum Sciences (Sold to Vertex) • Founder Bethesda Research Laboratories (BRL - Life Technologies) David Halverson President & COO • Exec. Director, Key Accounts for MPI Research • Head, U.S. & European Sales, Huntingdon Life Sciences • Dir. North America Bus. Dev, Quintiles Matthew Powell, Ph.D. V.P. & CSO • Technology Co - developer, Analytical Chemistry (WVU) Haddon Goodman V.P., CBO • Product Manager, Thermo Fisher Erin Seeley, PhD. Principal Investigator • Caprioli Laboratory. Vanderbilt • Expert , Oncology mass spectrometry imaging Science Advisory Board Mark Poznansky , MD, PhD Akos Vertes , Ph.D. Rossitza Lazova , MD • KOL Immunotherapy, Head, MGH/Harvard Cancer Vaccine Division • LAESI Technology Inventor, Prof. of Chemistry, GWU • KOL Dermato - pathologist

6 Advantages of Protea Technology for Oncology Diagnostics Proteins Peptides Lipids Metabolites • Diagnosis – Accurate and objective, not subjective – Resolves ambiguous or indeterminate biopsies – Fits with current pathology routine • Management – Disease Prognosis – Treatment selection and monitoring progress – Identification of tumor edge in surgery and metastases Cancer is molecular disease. It needs to be defined and managed by the use of molecular information. We provide more precise and comprehensive data, rapidly:

7 Cancer Outlook and Prevalence – Clinical Need All data was taken from NIH National Cancer Institute Surveillance, Epidemiology, and End Results Program https://seer.cancer.gov/statfacts/ Cancer type Est. number of new diagnoses per year (2017) Est. number of deaths per year (2017) Percent surviving 5 years Number of people living with cancer (2014) Breast 252,710 40,610 89.70% 3,327,552 Lung 222,500 155,870 18.1% 527,228 Prostate 161,360 26,730 98.60% 3,085,209 Melanoma 87,110 9,730 91.70% 1,169,351 Pancreatic 53,670 43,090 8.2% 64,668 Ovarian 22,280 14,240 46.20% 222,060 Esophageal 16,940 15,690 18.80% 45,547 Protea’s clinical testing program will address the need for more accurate prognostic and diagnostic results

8 Protea : Creating A New Class of Molecular Oncology Tests Development Partnerships with Top Tier Cancer Research Centers TEST PARTNER MARKET (USA) Malignant Melanoma Differential diagnosis Yale Medicine R. Lazova , M.D. (CA Skin Inst.) 87k new dx/year Esophageal Cancer Prognostic Breast Cancer Treatment selection & monitoring M.D. Anderson S.H. Lin, M.D., Ph.D. 17k new dx/year 252k new dx/year Ovarian Cancer Treatment selection & monitoring Dana Farber Cancer Institute E. Stover, M.D., Ph.D. 22k new dx/year Immunotherapy Treatment selection & monitoring MGH/Harvard M. Poznansky , M.D. Ph.D. $16B in 2016 Pending: Pancreatic, Cervical, Rectal

9 • There are 2m biopsies per year in the US to rule out melanoma, of these, 25% or ~500k skin biopsies are labeled as indeterminate. • 41% of metastatic melanomas were missed by experts in a recent study of borderline melanocytic lesions (1) • One American dies every hour of melanoma Case Study: the need for an unambiguous melanoma test 12 separate dermatopathology KOLs misclassified this lesion as benign 1 The 2 year old child had distant metastases and died without being treated 1.. Gerami , et al. Histomorphologic Assessment and Interobserver Diagnostic Reproducibility of Atypical Spitzoid Melanocytic Neoplasms With Long - term Follow - up. Am J Surg Pathol 2014;38:934 – 940

10 Biopsy sectioned, is stained, and annotated by pathologist for specific cell regions for molecular fingerprinting Mass spectra data compared to A.I. classification algorithm Protea : Molecular Oncology Test Workflow Malignant 79 assays (98.75%) Benign 1 assay (4.76%) Data from a single sample - unparalleled diagnostic clarity Biopsy taken by clinician Mass spectra data collected

11 Validation Set Number of Patients Validation Patient Accuracy Malignant Melanoma 46 94% Benign Nevi 50 92% Malignant Benign Molecular Diagnosis of Melanoma • Molecular spectra from hundreds of skin biopsies provide Big Data • Data is mined and analyzed using Artificial Intelligence and Machine Learning • Melanoma Spectral Signature identified and Algorithm constructed • Algorithm identifies skin biopsies as benign or malignant objectively and accurately 1196 1197 1198 1199 1200 1201 1202 0 5 10 15 20 25 30 Intensity m/z Benign Malignant Example of a significant peak Classification performed using a Linear Discriminant Analysis machine learning algorithm. This type of analysis uses a full mass spectrum fingerprint for classification.

12 Protea : Oncology Diagnostics for Immunotherapy Management “ Novel System for Immunotherapy Assessment and Prognosis in Cancer” A Collaborative Project of the Vaccine and Immunotherapy Center (VIC) Massachusetts General Hospital, Harvard Medical School and Protea Biosciences “The goal of this project is to enhance the information rapidly available to patients and their physicians from tumor samples, which could be used as the basis for decisions regarding both the initiation of and modification of immunotherapy for solid tumors . “ Improved outcomes, but expensive with significant adverse events There is little or no tumor - based diagnostic data to evaluate treatment

13 Diagnostic Assays - Pipeline Assay Partner Launch Target Melanoma Yale 2018 Esophageal Cancer MD Anderson 2019 Breast Cancer MD Anderson 2020 Ovarian Cancer DFCI 2021 Pancreatic Cancer MD Anderson 2021 Cervical Cancer in - house 2022 Rectal Cancer MD Anderson 2022 Immunotherapy MGH 2023 3 additional oncology assays Undisclosed Pending finalization of research collaboration agreement in Q3, 2017 Diagnostic Assay • 12 - 18 months for sample acquisition/analysis, algorithm development, assay validation, and launch

14 Diagnostic Assays - Key Development Milestones 2017 2018 2019 2020 CLIA Lab Build - out Accreditation Melanoma Assay Sample acquisition/analysis Algorithm development Assay validation Assay launch Esophageal Cancer Assay Sample acquisition/analysis Algorithm development Assay validation Assay launch Breast Cancer Assay Sample acquisition/analysis Algorithm development Assay validation Assay launch

15 15 Projections 2017 2018 2019 2020 2021 Total Revenue 2,088,928 11,692,875 16,729,594 33,270,492 51,699,615 Cost of Revenue 1,362,827 2,325,595 4,101,688 4,991,172 5,640,093 Gross Profit 726,101 9,367,280 12,627,906.21 28,279,319.73 46,059,521.87 Profit Margin 35% 80% 75% 85% 89% Results from Operations (4,916,684.03) 2,043,131.96 3,417,526.78 15,291,273.14 29,265,863.33 Net Income (Loss) (14,102,831.47) 1,576,797.52 3,221,080.53 15,193,997.58 29,263,758.80 Working Capital (Incr)/Decr 17,298 (1,223,661) (839,453) (2,756,816) (3,071,521) CAP EX (177,000) - (2,500,000) (1,500,000) (1,500,000) Dx CAP EX (2,035,132) (1,500,000) (949,000) (1,312,132) (949,000) Debt & Cap Lease Payments (1,293,925) (1,154,990) (1,195,895) (1,184,071) (146,421) Cash Flow from Financing 2,589,000 Working Capital Requirement (7,055,534) (1,806,635) (1,133,445) 9,756,458 24,990,104 Safe Harbor Statement - This presentation contains "forward - looking statements" within the meaning of the “safe - harbor” provisions of the private securities litigation reform act of 1995 . Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of the Company to differ materially from the results expressed or implied by such statements, including changes from anticipated levels of revenues, future national or regional economic and competitive conditions, difficulties in developing the Company’s technology platforms, retaining and expanding the Company’s customer base, fluctuations in consumer spending on the Company’s products and other factors . Accordingly, although the Company believes that the expectations reflected in such forward - looking statements are reasonable, there can be no assurance that such expectations will prove to be correct . The Company has no obligation to update the forward - looking information contained in this presentation .

16 Protea Summary Capitalization following 1 for 50 reverse split Summary Current Capitalization : (assumes post 1 for 50 reverse split) Common Stock ~ 8 , 034 , 499 Warrants ( 1 ) ~ 7 , 205 , 666 Options ~ 333 , 000 Convert Debt ( 2 ) ~ 2 , 163 , 358 Fully Diluted Shares Out ~ 17 , 736 , 523 (1) Range Exercise price of the warrants is $ 3 . 75 to $ 110 . 00 (2) Conversion Price of the Current Debt is $ 3 . 75 to $ 25 . 00

17 Our Industry Collaborators and Partners Agilent Technologies (NYSE:A) – to co - develop new methods for molecular characterization of biotherapeutics ; Protein Metrics, Inc. – Software Development – to advance analytical capabilities for protein biotherapeutics MatTek Corporation – Tissue Models – to enable imaging of human based in vitro tissue models Proteos Inc . – Biotherapeutic Production - leader in the production of recombinant proteins and therapeutic antibodies DARPA Consortium with The George Washington University , GE Global Research and the Stanford Research Institute International - to co - develop new technology to accelerate understanding the mechanisms of action of new biological or chemical threat agents We are partnering with a growing list of key industry providers for co - marketing opportunities in the areas of mass spectrometry equipment, software, and joint customer collaborations

18 The Harmony of Molecular Services and Molecular Diagnostics Molecular Services Proteomics Metabolomics Biotherapeutics Imaging Molecular Diagnostics/Prognostics Melanoma (Proteomic/Metabolomic) Esophageal (Proteomic/Metabolomic) Ovarian (Proteomic/Biotherapeutic) Wound Healing (Proteomic/Biotherapeutic) Immunotherapy (Biotherapeutic) Commercial Molecular Services Division enables Molecular Diagnostics Division through in house expertise, industry partnerships, and market and commercial need. • In house technology expertise drives development of new diagnostic tests • Completion of commercial service projects for R&D and preclinical researchers provides market - need feedback for diagnostic testing • Diagnostic testing reveals opportunities for turnkey molecular services

19 Investment Thesis Protea is uniquely positioned as a leader in the emerging industry for large scale molecular information: Proprietary technology and infrastructure is in place for enterprise success Our melanoma diagnostic workflow, will complete validation this year, is disruptive and will enable an entire new class of tests for oncology medicine. Test development collaborations are already well underway with top tier medical research centers, including MGH, M.D. Anderson, and Yale We will impact oncology drug development, patient diagnosis, prognosis and treatment management and provide multiple high barriers to entry. Protea Biosciences Group, Inc. 1311 Pineview Drive, Morgantown, WV 26505 www.proteabio.com stephen.turner@proteabio.com
