Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - MARINUS PHARMACEUTICALS INC | a17-21553_1ex99d2.htm |
| 8-K - 8-K - MARINUS PHARMACEUTICALS INC | a17-21553_18k.htm |
Mindful Innovation Phase 2 Clinical Study Evaluating Ganaxolone in Children with CDKL5 Disorder September 11, 2017 Follow @MarinusPharma on Twitter!

Safe Harbor Statement To the extent that statements contained in this press release are not descriptions of historical facts regarding Marinus, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “may”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “believe”, and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements contained in this press release include, among others, statements regarding our interpretation of preclinical studies, development plans for our product candidate, including the development of dose forms, the clinical trial testing schedule and milestones, the ability to complete enrollment in our clinical trials, interpretation of scientific basis for ganaxolone use, timing for availability and release of data, the safety, potential efficacy and therapeutic potential of our product candidate and our expectation regarding the sufficiency of our working capital. Forward-looking statements in this release involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the conduct of future clinical trials, the timing of the clinical trials, enrollment in clinical trials, availability of data from ongoing clinical trials, expectations for regulatory approvals, the attainment of clinical trial results that will be supportive of regulatory approvals, and other matters, including the development of formulations of ganaxolone, and the availability or potential availability of alternative products or treatments for conditions targeted by the company that could affect the availability or commercial potential of our drug candidates. Marinus undertakes no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see Marinus' 10-K dated March 13, 2017 and other filings by the company with the U.S. Securities and Exchange Commission. You may access these documents for free by visiting EDGAR on the SEC web site at www.sec.gov. 1

Agenda Introduction Christopher M. Cashman, CEO Phase 2 clinical trial design & data Dr. Lorianne Masuoka, CMO Case summaries of patients w/ CDKL5 disorder Dr. Nicola Specchio, Principal Investigator, Ospedale Pediatrico Bambino Gesù, Rome Closing Remarks Christopher M. Cashman, CEO 2
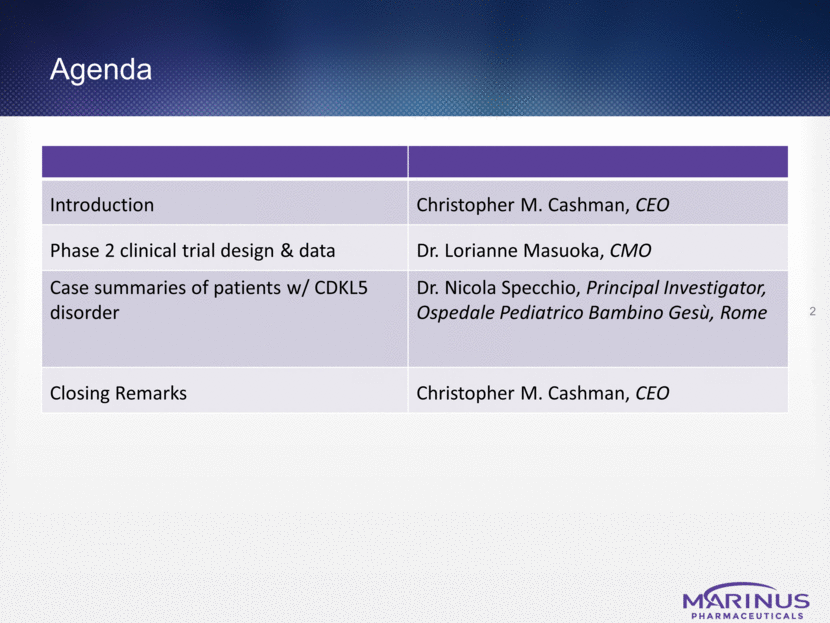
Marinus’s Development of Ganaxolone 3 Clear efficacy signal in refractory, CDKL5 disorder Need for mechanistically novel, efficacious & safe treatment options Committed to improving lives of patients with refractory epilepsies and psychiatric disorders
Ganaxolone in children with CDKL5 Disorder Dr. Lorianne Masuoka, CMO

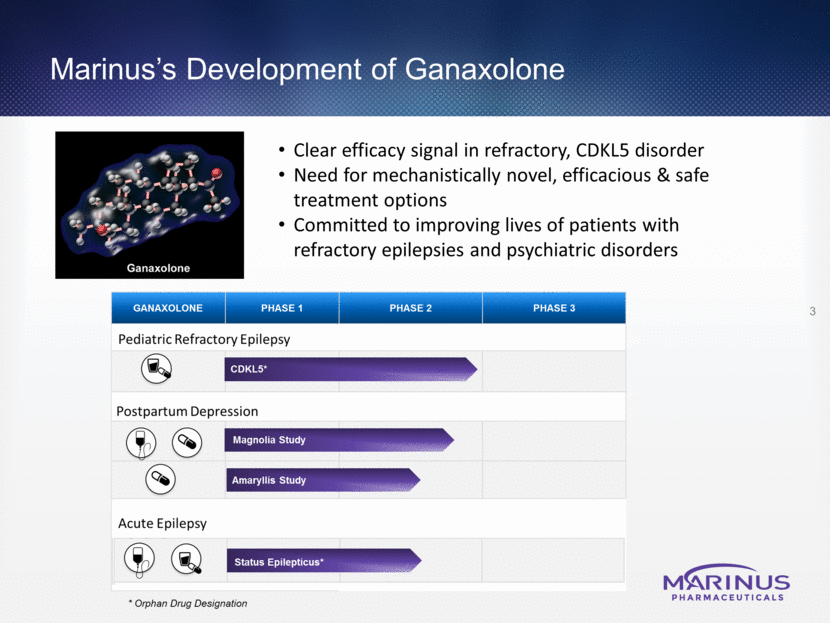
5 Ganaxolone (GNX) Targets Synaptic and Extrasynaptic GABAA Receptors GNX is a synthetic analog of allopregnanolone GNX is designed to modulate both synaptic and extrasynaptic GABAA receptors to calm over-excited neurons
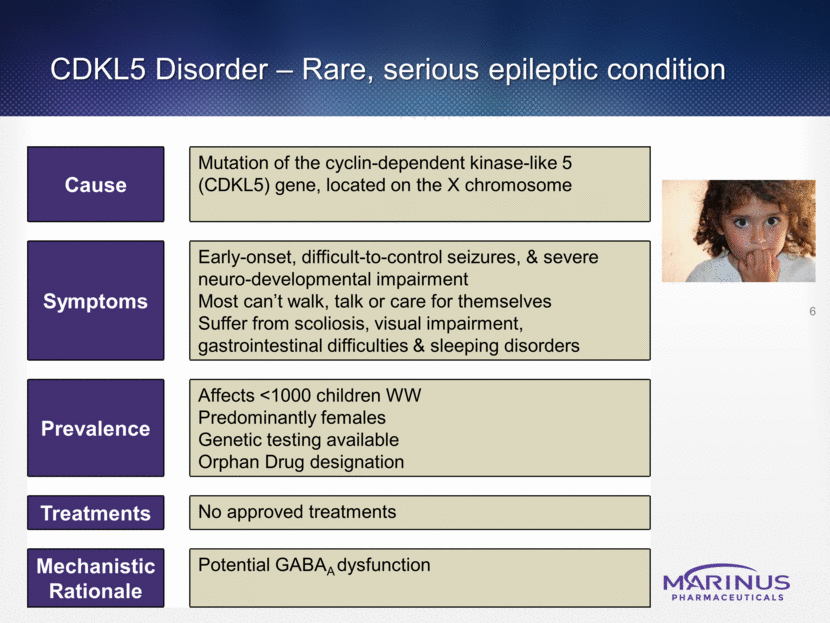
CDKL5 Disorder – Rare, serious epileptic condition 6 Cause Mutation of the cyclin-dependent kinase-like 5 (CDKL5) gene, located on the X chromosome Symptoms Early-onset, difficult-to-control seizures, & severe neuro-developmental impairment Most can’t walk, talk or care for themselves Suffer from scoliosis, visual impairment, gastrointestinal difficulties & sleeping disorders Prevalence Affects <1000 children WW Predominantly females Genetic testing available Orphan Drug designation Treatments No approved treatments Mechanistic Rationale Potential GABAA dysfunction
Phase 2 Study in CDKL5 Disorder Trial Design: 2 sites in US; 1 site in Italy Oral ganaxolone - up to 1,800 mg/day + existing treatment regimen 6 females, 1 male; ages 2-16 Enrollment criteria: Confirmed CDKL5 mutation, stable background treatment, >4 seizures per 28-day period in baseline Endpoints: Primary: % change in seizure frequency per 28 days relative to baseline Secondary: % increase in seizure-free days from baseline, safety and tolerability, CGI 7 12 weeks Baseline Treatment Period 26 weeks 52 weeks 1-year extension
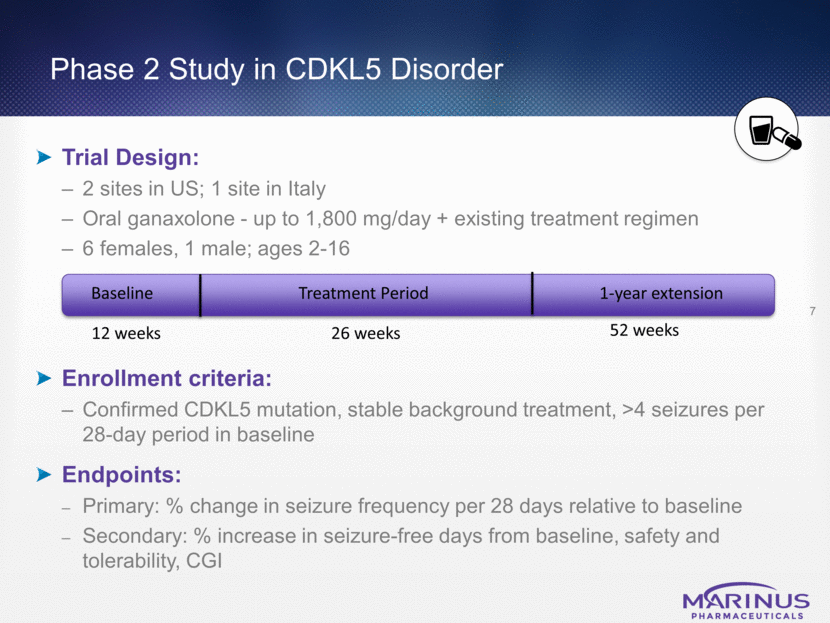
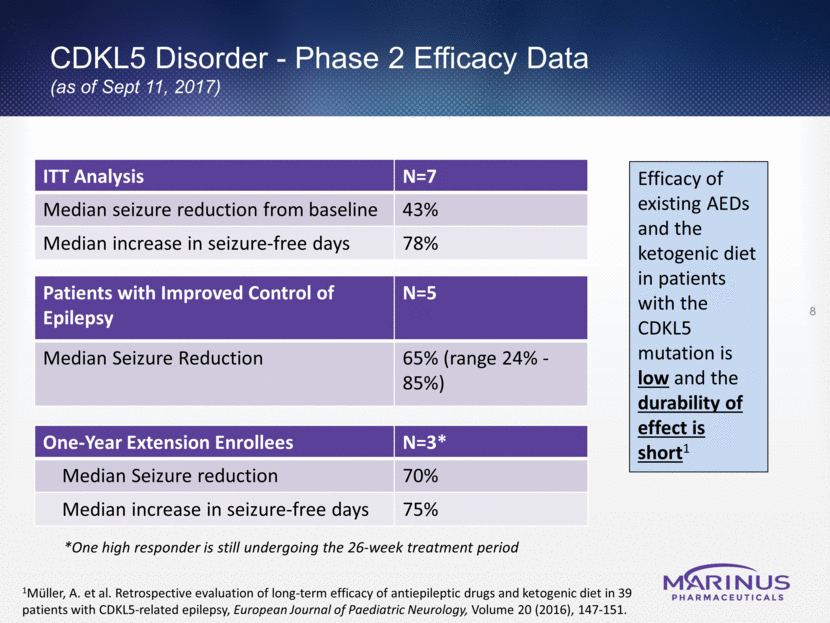
CDKL5 Disorder - Phase 2 Efficacy Data (as of Sept 11, 2017) 8 One-Year Extension Enrollees N=3* Median Seizure reduction 70% Median increase in seizure-free days 75% ITT Analysis N=7 Median seizure reduction from baseline 43% Median increase in seizure-free days 78% *One high responder is still undergoing the 26-week treatment period Efficacy of existing AEDs and the ketogenic diet in patients with the CDKL5 mutation is low and the durability of effect is short1 1Müller, A. et al. Retrospective evaluation of long-term efficacy of antiepileptic drugs and ketogenic diet in 39 patients with CDKL5-related epilepsy, European Journal of Paediatric Neurology, Volume 20 (2016), 147-151. Patients with Improved Control of Epilepsy N=5 Median Seizure Reduction 65% (range 24% - 85%)
CDKL5 Disorder - Phase 2 Safety Profile (as of Sept 11, 2017) Generally safe and well-tolerated No serious adverse events No reports of somnolence or dizziness (the most frequently reported AE in prior studies) Two patients discontinued prior to completing the 26-week treatment due to lack of efficacy 9
Case summaries Dr. Nicola Specchio Principal Investigator, Ospedale Pediatrico Bambino Gesù in Rome

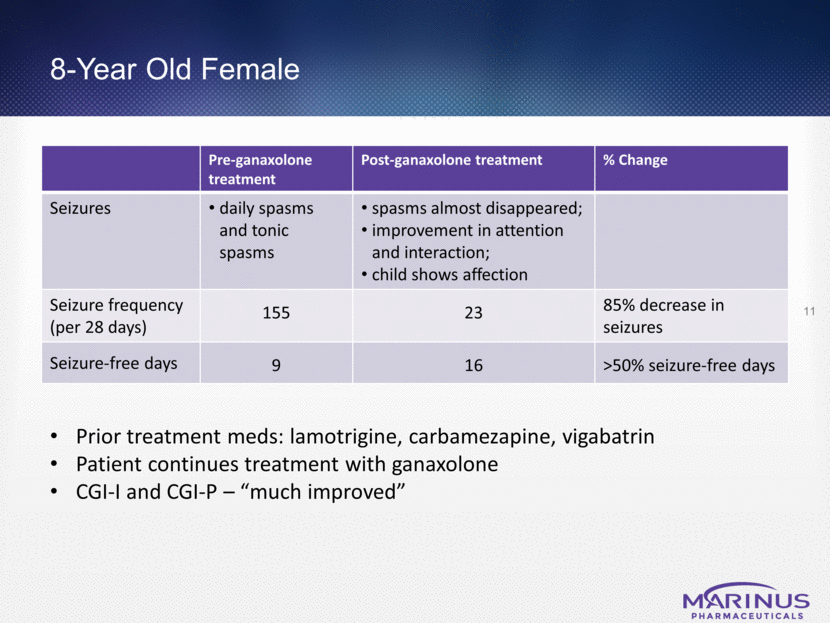
8-Year Old Female Pre-ganaxolone treatment Post-ganaxolone treatment % Change Seizures daily spasms and tonic spasms spasms almost disappeared; improvement in attention and interaction; child shows affection Seizure frequency (per 28 days) 155 23 85% decrease in seizures Seizure-free days 9 16 >50% seizure-free days 11 Prior treatment meds: lamotrigine, carbamezapine, vigabatrin Patient continues treatment with ganaxolone CGI-I and CGI-P – “much improved”
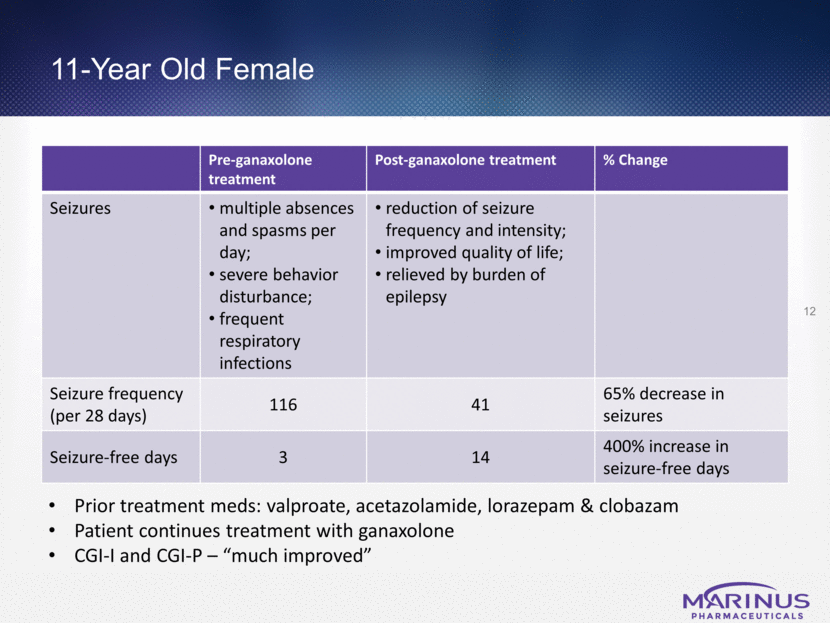
11-Year Old Female Pre-ganaxolone treatment Post-ganaxolone treatment % Change Seizures multiple absences and spasms per day; severe behavior disturbance; frequent respiratory infections reduction of seizure frequency and intensity; improved quality of life; relieved by burden of epilepsy Seizure frequency (per 28 days) 116 41 65% decrease in seizures Seizure-free days 3 14 400% increase in seizure-free days 12 Prior treatment meds: valproate, acetazolamide, lorazepam & clobazam Patient continues treatment with ganaxolone CGI-I and CGI-P – “much improved”
Why Ganaxolone in CDKL5 Disorder? 13 Remarkable response in efficacy, safety and behavior with ganaxolone in this extremely rare, refractory patient population that has little success in controlling their seizures with existing treatment regimens Magnitude of Effect The children with improved seizure control continued improvement beyond the 6 months of treatment Durability of Effect Ganaxolone has a clean safety profile; it was generally safe and well-tolerated with no SAEs Safety
Thank you Follow @MarinusPharma on Twitter!

