Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Theravance Biopharma, Inc. | v466501_ex99-1.htm |
| 8-K - FORM 8-K - Theravance Biopharma, Inc. | v466501_8k.htm |

© 2017 Theravance Biopharma. All rights reserved. THERAVANCE ® , the Cross/Star logo, VIBATIV ® and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks, and TOUR TM is a trademark, of the Theravance Biopharma group of companies. 1Q 2017 Financial Results and Business Update May 9, 2017 Theravance Biopharma, Inc. (NASDAQ: TBPH)

2 Cautionary Statement Regarding Forward - Looking Statements Under the safe harbor provisions of the U . S . Private Securities Litigation Reform Act of 1995 , the company cautions investors that any forward - looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward - looking statements or projections . Examples of forward - looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies) . The company’s forward - looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non - clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities . Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10 - K filed with the Securities and Exchange Commission (SEC) on March 1 , 2017 , and other periodic reports filed with the SEC .
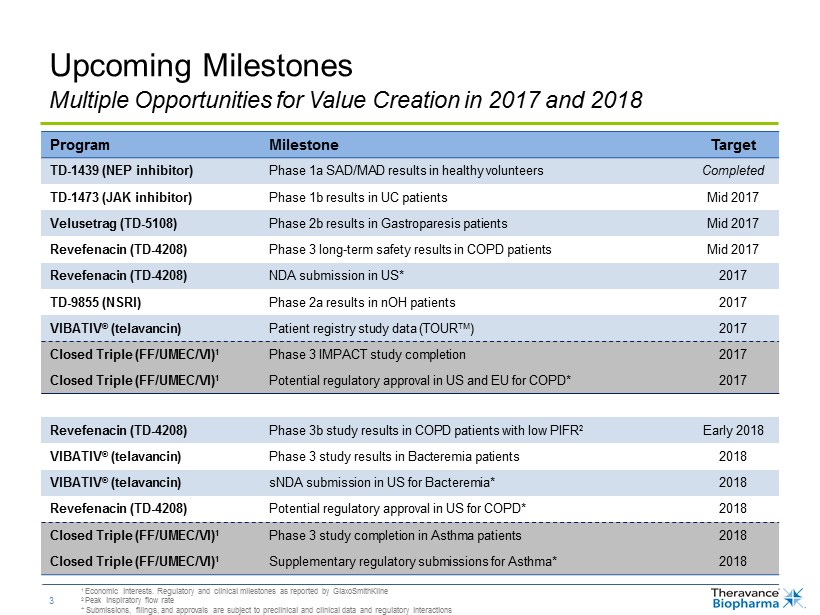
3 Upcoming Milestones Program Milestone Target TD - 1439 (NEP inhibitor) Phase 1a SAD/MAD results in healthy volunteers Completed TD - 1473 (JAK inhibitor) Phase 1b results in UC patients Mid 2017 Velusetrag (TD - 5108) Phase 2b results in Gastroparesis patients Mid 2017 Revefenacin (TD - 4208) Phase 3 long - term safety results in COPD patients Mid 2017 Revefenacin (TD - 4208) NDA submission in US* 2017 TD - 9855 (NSRI) Phase 2a results in nOH patients 2017 VIBATIV ® ( telavancin ) Patient registry study data (TOUR TM ) 2017 Closed Triple (FF/UMEC/VI) 1 Phase 3 IMPACT study completion 2017 Closed Triple (FF/UMEC/VI) 1 Potential regulatory approval in US and EU for COPD* 2017 Revefenacin (TD - 4208) Phase 3b study results in COPD patients with low PIFR 2 Early 2018 VIBATIV ® ( telavancin ) Phase 3 study results in Bacteremia patients 2018 VIBATIV ® ( telavancin ) sNDA submission in US for Bacteremia* 2018 Revefenacin (TD - 4208) Potential regulatory approval in US for COPD* 2018 Closed Triple (FF/UMEC/VI) 1 Phase 3 study completion in Asthma patients 2018 Closed Triple (FF/UMEC/VI) 1 Supplementary r egulatory submissions for Asthma* 2018 Multiple Opportunities for Value Creation in 2017 and 2018 1 Economic interests. Regulatory and clinical milestones as reported by GlaxoSmithKline 2 Peak inspiratory flow rate * Submissions, filings, and approvals are subject to preclinical and clinical data and regulatory interactions
