Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a17-12182_18k.htm |
Exhibit 99.1
Transforming Oncology With Precision Cancer Therapeutics Company Overview may 2017 Company Overview January 2017

Forward-Looking Statements Certain statements in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Trovagene's expectations, strategy, plans or intentions. These forward-looking statements are based on Trovagene's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. While the list of factors presented in the 10-K is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Trovagene does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
About Trovagene Precision medicine biotechnology company developing therapeutics to improve cancer care by leveraging our proprietary Precision Cancer Monitoring® (PCM) technology in tumor genomics Industry leader in urine and blood based tumor genomic diagnostics Broad intellectual property and proprietary technology to measure, with high clinical sensitivities, circulating tumor DNA (ctDNA) in urine and blood for predicting response to cancer therapeutics CLIA/CAP-accredited laboratory for internal drug development programs First therapeutic candidate: PCM-075, a polo-like kinase 1 (PLK1) inhibitor Phase 1 completed with planned clinical development in AML NASDAQ listed: TROV
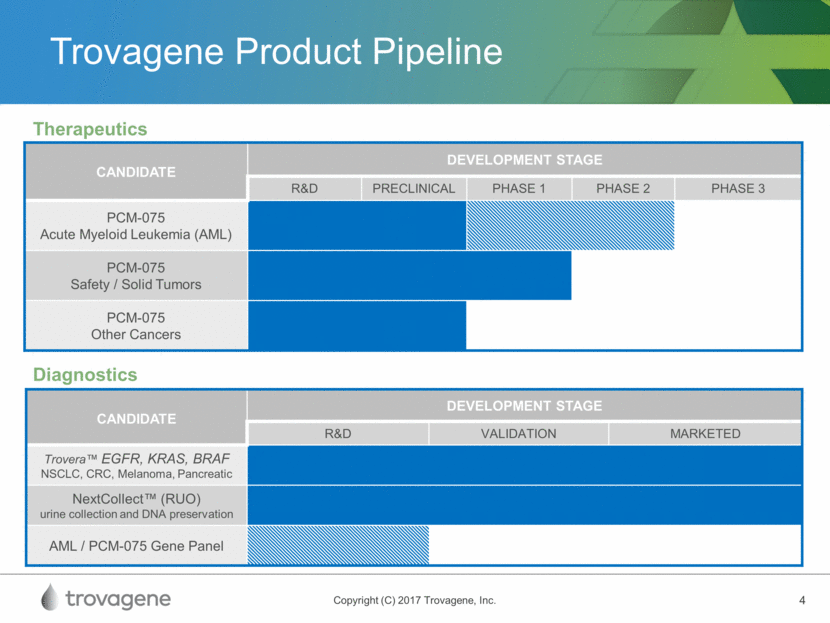
Trovagene Product Pipeline CANDIDATE DEVELOPMENT STAGE R&D PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PCM-075 Acute Myeloid Leukemia (AML) PCM-075 Safety / Solid Tumors PCM-075 Other Cancers Therapeutics CANDIDATE DEVELOPMENT STAGE R&D VALIDATION MARKETED Trovera™ EGFR, KRAS, BRAF NSCLC, CRC, Melanoma, Pancreatic NextCollect™ (RUO) urine collection and DNA preservation AML / PCM-075 Gene Panel Diagnostics
PCM-075: A PLK1 Inhibitor PCM-075 is an oral, highly-selective polo-like kinase 1 (PLK1) inhibitor Licensed exclusive global development and commercialization rights from Nerviano Medical Sciences, S.r.l. Nerviano Medical Sciences: largest oncology-focused R&D company in Italy, highly regarded in Europe Other licensees include: Genentech (Roche), Pfizer, Array Pharmaceuticals, Servier, Ignyta, and other biotechnology companies Trovagene’s expertise in tumor genomic diagnostics, broad patents and technology uniquely enables our precision medicine approach in AML Many core genomic markers covered in ctDNA PCM technology, including NPM1, a founder genetic marker in AML, for diagnosis and monitoring of patient response Trovagene to optimize AML biomarker panel for patient selection and response to PCM-075
PCM-075 Licensing Terms Trovagene has exclusive global development and commercialization rights to PCM-075 Nerviano receives: Upfront payment of $2.0 million Milestones Development: Phase 3 Regulatory: NDA submission Commercial Launch Tiered royalty payments up to and over $1.0 billion Trovagene has all rights to manufacturing of bulk and finished goods Nerviano is the current manufacturer and has bulk material produced and available for clinical trials
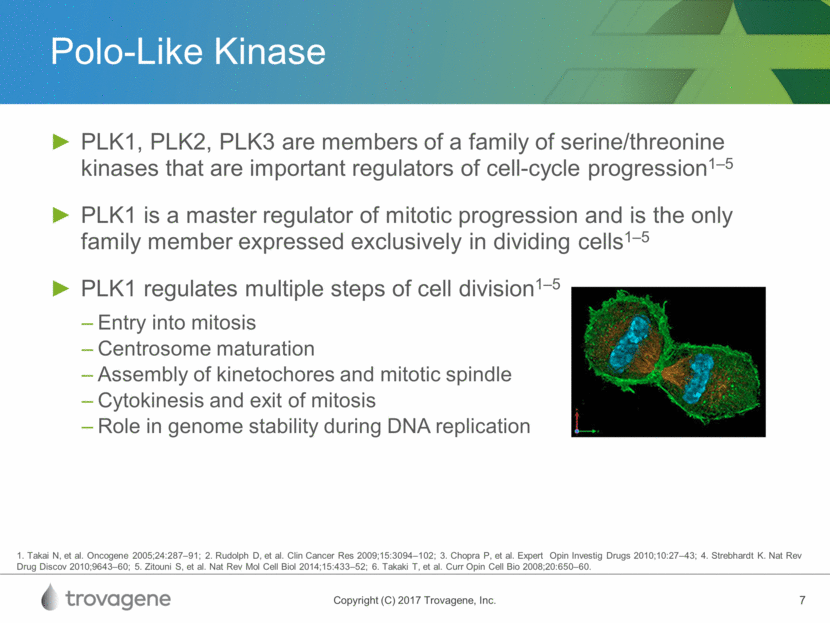
Polo-Like Kinase PLK1, PLK2, PLK3 are members of a family of serine/threonine kinases that are important regulators of cell-cycle progression1–5 PLK1 is a master regulator of mitotic progression and is the only family member expressed exclusively in dividing cells1–5 PLK1 regulates multiple steps of cell division1–5 Entry into mitosis Centrosome maturation Assembly of kinetochores and mitotic spindle Cytokinesis and exit of mitosis Role in genome stability during DNA replication 1. Takai N, et al. Oncogene 2005;24:287–91; 2. Rudolph D, et al. Clin Cancer Res 2009;15:3094–102; 3. Chopra P, et al. Expert Opin Investig Drugs 2010;10:27–43; 4. Strebhardt K. Nat Rev Drug Discov 2010;9643–60; 5. Zitouni S, et al. Nat Rev Mol Cell Biol 2014;15:433–52; 6. Takaki T, et al. Curr Opin Cell Bio 2008;20:650–60.
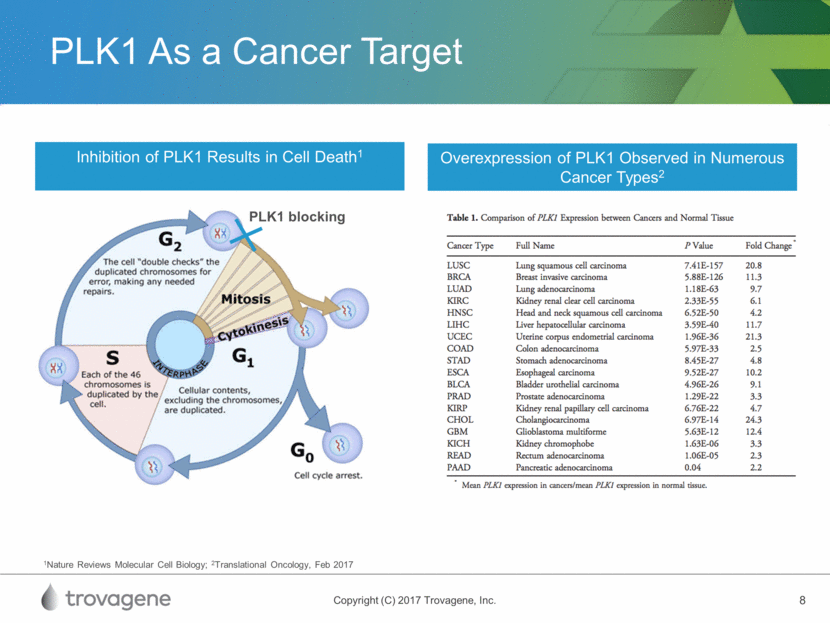
PLK1 As a Cancer Target 1Nature Reviews Molecular Cell Biology; 2Translational Oncology, Feb 2017 Inhibition of PLK1 Results in Cell Death1 Overexpression of PLK1 Observed in Numerous Cancer Types2 PLK1 blocking
PCM-075 Attributes Highly Selective Short Half-Life Acceptable Safety Profile Broad Applicability Orally Available
PCM-075 Biochemical Profile Selective, adenosine triphosphate (ATP) competitive PLK1 inhibitor Selectivity driven by polar interaction with the carboxyl side chain of Glutamate 140 position of PLK1 Polo-like kinase 1 (PLK1) inhibitor * IC50 is the half-maximal concentration at which PCM-075 inhibits enzymatic activity PLK Member PCM-075 IC50* (uM) PLK1 0.002 PLK2 > 10 PLK3 > 10
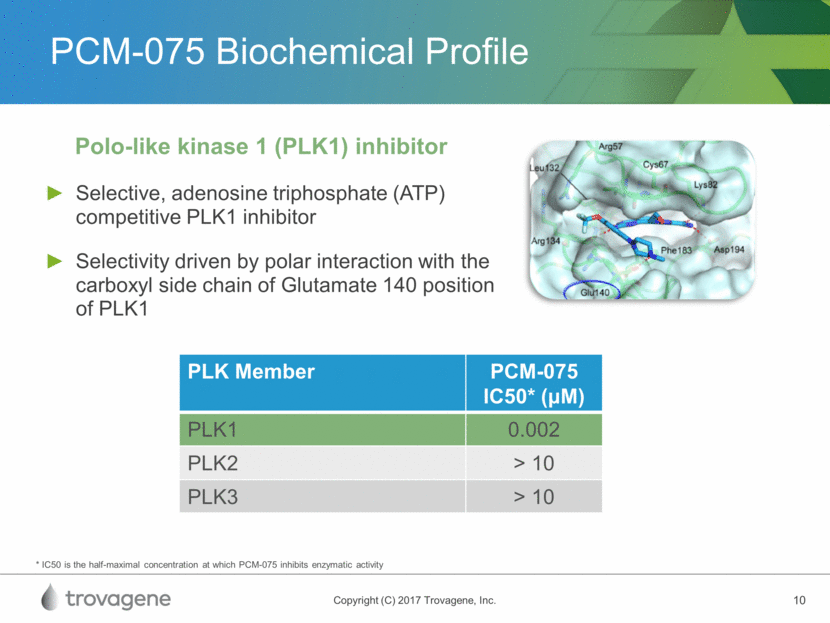
PCM-075 Mechanism of Action Induces a cellular phenotype of G2/M, leading to cell death Clear evidence of in-vitro pathway modulation in AML Mitotic inhibitor demonstrating anti-proliferative activity* AML-NS8 Patient-Derived Cells Treated with 200 nM PCM-075 for 24 Hrs1 * In numerous human cel lines; 1Casolaro et al (2013) PLOS One PCM-075 PCM-075
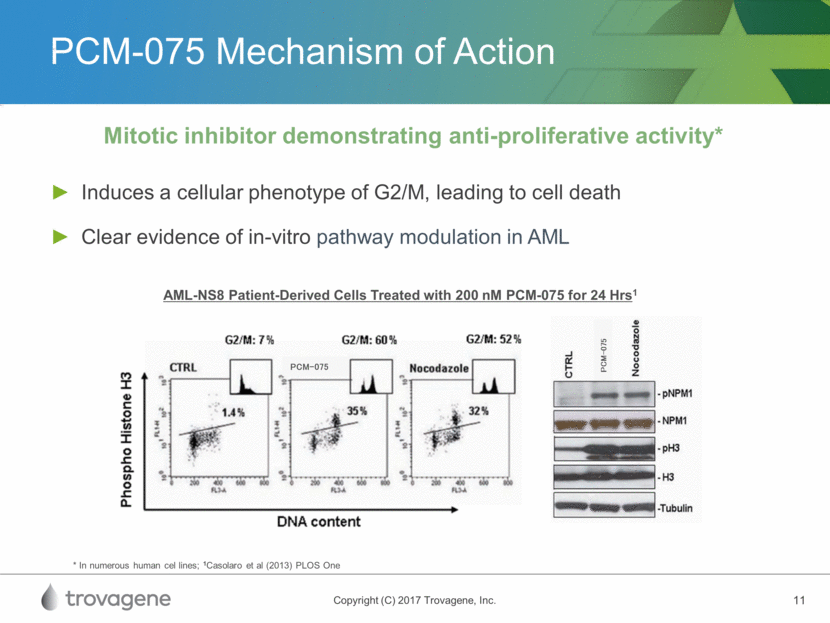
PCM-075 In-Vivo Activity Response observed in various xenograft models as a single agent and in combination Compound Median Survival Time (days) %TGI Placebo 28 Cytarabine 38 128.6 PCM-075 62 221.4 In Vivo Disseminated Leukemia Model (AML-NS8 Cells) Treatment with PCM-075 Started 20 Days Post-Inoculation 60 mg/kg BID (Days 1 and 2, 3 Cycles) Casolaro et al (2013) PLOS One Vehicle PCM-075 60 mg/kg BID Cytarabine 75 mg/kg Vehicle PCM-075 60 mg/kg BID Cytarabine 75 mg/kg
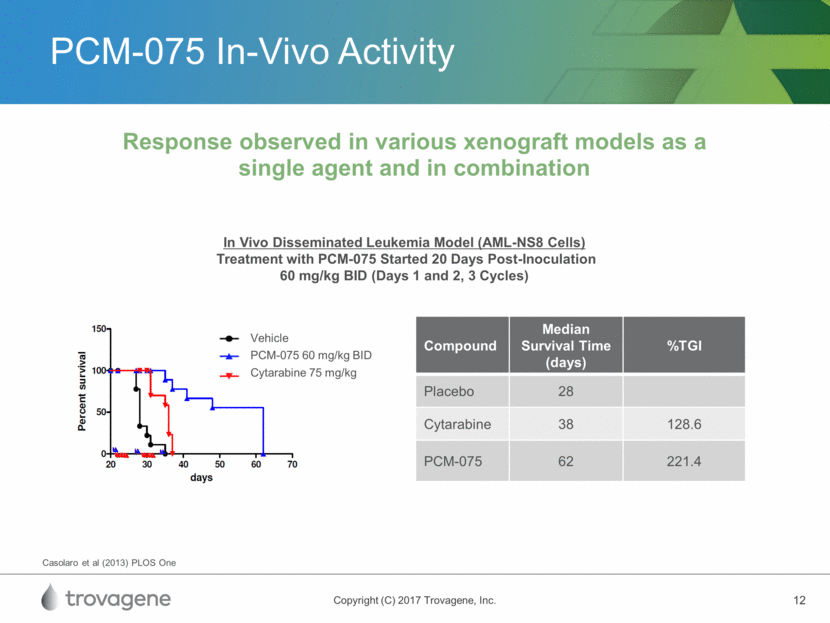
PLK Inhibition in AML Pan-PLK inhibitor demonstrated increased response rates, improvements in event-free survival, and overall survival benefit in combination with low-dose cytarabine (LDAC) Adverse events observed in Phase 3 study, potentially due to drug accumulation and pan-PLKi activity Opportunity for a more selective PLKi with less drug accumulation paired with a precision medicine diagnostic Clinical activity published on PLK inhibitors Compound Selectivity Half-Life (hrs) Dosing Clinical Activity in AML Volasertib pan-PLK 135 iv 25-30% CR+CRi (+LDAC)* PCM-075 PLK1 24 oral P1/2 * higher incidence of severe adverse events with volasertib plus LDAC in phase III study
AML Market Opportunity AML: aggressive hematologic malignancy Incidence: 20,000 new cases and 10,400 deaths annually in the U.S. Prognosis: 5 year survival rate is 25% Treatment options vary based on patient condition / age, but can include: Chemotherapy Radiation Stem cell transplant Genetic landscape includes: NPM1, genetic marker in leukemia, accounting for ~ 30% of all AML patients Other significant markers such as FLT3, DNMT3A, NRAS, KIT and fusion markers National Cancer Institute SEER 2016
PCM-075 Clinical Development Plan Active IND for PCM-075 with clinical and regulatory organization engaged Phase 1/2 study planned with first patient to be dosed in 2017 Dose-escalation with PCM-075 as single agent and then in combination with chemotherapy agents to assess patient response and explore use of correlative biomarkers to select patients most likely to respond Inclusion criteria: adults with previously untreated AML, ineligible for intensive therapy, and ECOG 0-2 Clinical Advisory Board Dr. Jorge Cortes, MD Anderson Cancer Center (Deputy Chair Leukemia) Dr. Sandra Silberman, Duke Dr. Filip Janku, MD Anderson Cancer Center Dr. David Berz, Beverly Hills Cancer Center

Milestones Q2 Launch of NextCollect™ Q2 FDA filing of IND for Phase 1/2 AML trial Q2 Pharma services collaborations Q3 Phase 1 solid tumor trial published Q3 FDA acceptance of IND for Phase 1/2 trial Q4 PCM-075 AML biomarker panel Q4 ASH Presentations Q4 First patient dosed in Phase 1/2 AML trial
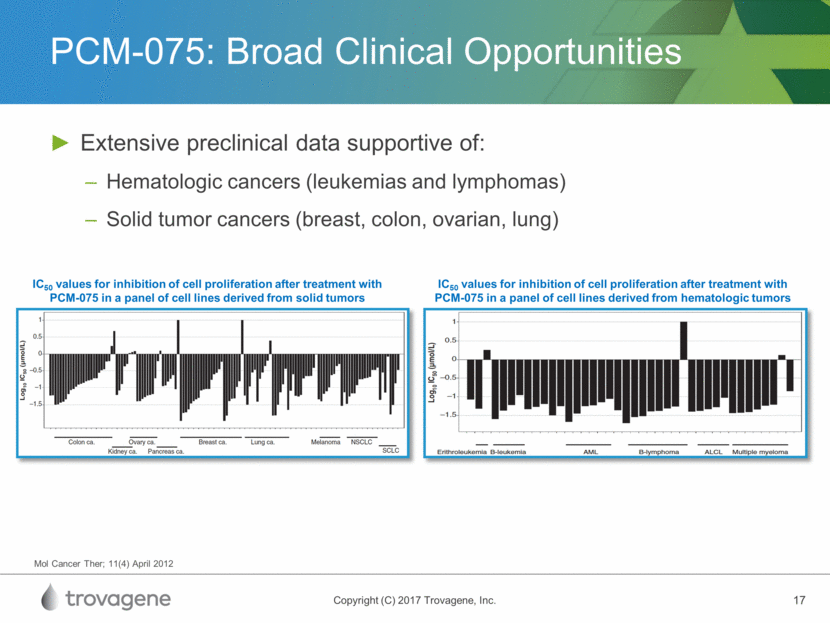
PCM-075: Broad Clinical Opportunities Extensive preclinical data supportive of: Hematologic cancers (leukemias and lymphomas) Solid tumor cancers (breast, colon, ovarian, lung) Mol Cancer Ther; 11(4) April 2012 IC50 values for inhibition of cell proliferation after treatment with PCM-075 in a panel of cell lines derived from solid tumors IC50 values for inhibition of cell proliferation after treatment with PCM-075 in a panel of cell lines derived from hematologic tumors
Trovagene ctDNA PCM Technology Proprietary ctDNA technology allows us to uniquely measure, with high clinical sensitivity, circulating genetic fragments of cancer tumors Over 120 patents issued and 60 pending Only liquid biopsy company with ctDNA technology compatible in both urine and blood for predicting patient response to therapeutics Trovera® single gene assays: CLIA and clinically validated tests for lung, colorectal, pancreatic, melanoma, other cancer types, and histiocytic disorders ERTHOSTM Select multigene panel with >200 mutations for broad applications, under development
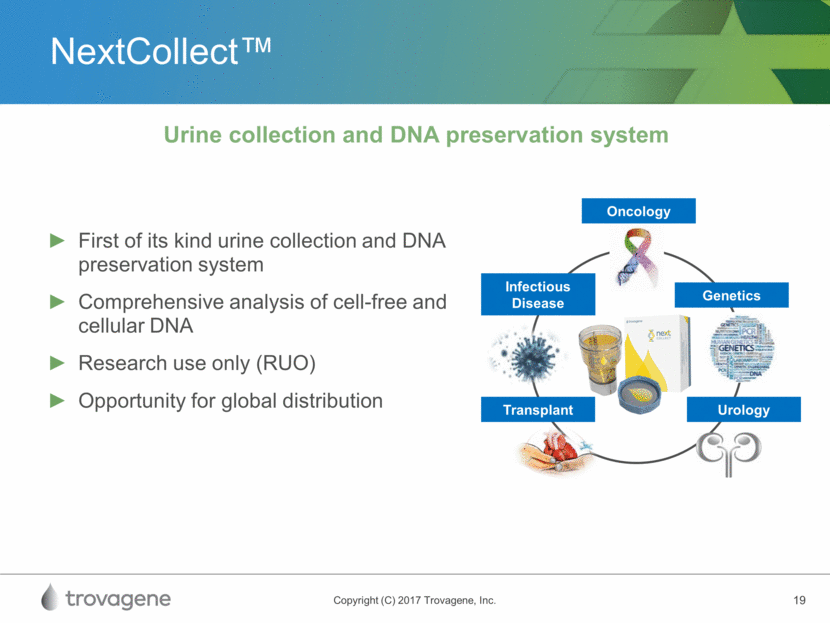
NextCollect™ Infectious Disease Transplant Urology Genetics Oncology First of its kind urine collection and DNA preservation system Comprehensive analysis of cell-free and cellular DNA Research use only (RUO) Opportunity for global distribution Urine collection and DNA preservation system
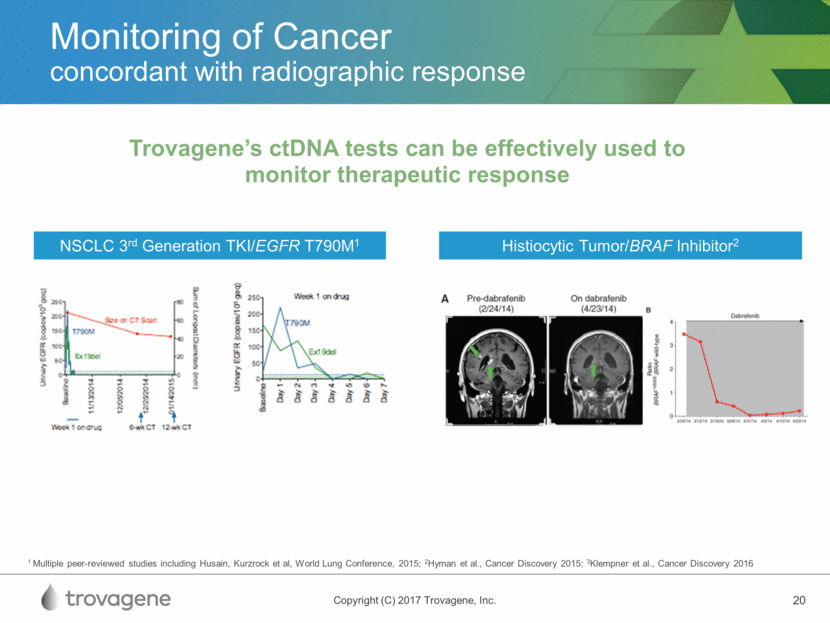
Monitoring of Cancer concordant with radiographic response Trovagene’s ctDNA tests can be effectively used to monitor therapeutic response NSCLC 3rd Generation TKI/EGFR T790M1 Histiocytic Tumor/BRAF Inhibitor2 1 Multiple peer-reviewed studies including Husain, Kurzrock et al, World Lung Conference, 2015; 2Hyman et al., Cancer Discovery 2015; 3Klempner et al., Cancer Discovery 2016
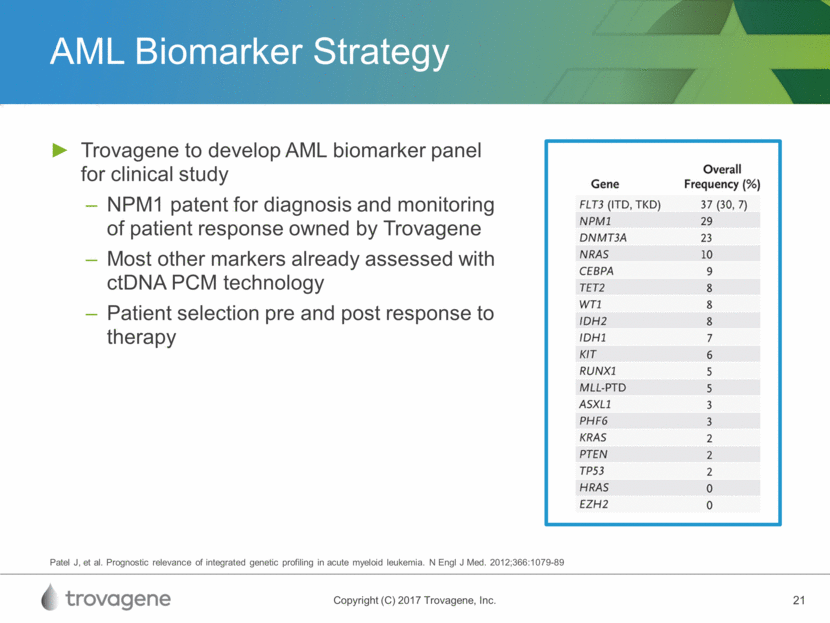
AML Biomarker Strategy Trovagene to develop AML biomarker panel for clinical study NPM1 patent for diagnosis and monitoring of patient response owned by Trovagene Most other markers already assessed with ctDNA PCM technology Patient selection pre and post response to therapy Patel J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079-89
Summary PCM-075 Oral and highly-selective PLK1 inhibitor Favorable 24-hour half-life for AML treatment therapy with reversible hematologic toxicities Phase 1/2 trial planned for PCM-075 with first patient to be dosed in 2017 PCM diagnostic technology Development of PCM-075 AML biomarker panel Launch NextCollect™ Pharma lab services collaborations
Thank you for more information, please email ir@trovagene.com

