Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - SenesTech, Inc. | v462393_ex32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - SenesTech, Inc. | v462393_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - SenesTech, Inc. | v462393_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - SenesTech, Inc. | v462393_ex31-1.htm |
| EX-23 - EXHIBIT 23.1 - SenesTech, Inc. | v462393_ex23-1.htm |
| EX-21.1 - EXHIBIT 21.1 - SenesTech, Inc. | v462393_ex21-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-37941
SENESTECH, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 20-2079805 |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
3140 N. Caden Court, Suite 1, Flagstaff, AZ 86004
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: (928) 779-4143
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered |
| Common Stock, $0.001 par value | The NASDAQ Stock Market LLC (NASDAQ Capital Market) |
Securities registered pursuant to Section 12(g) of the Act:
NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of common stock held by non-affiliates of the registrant’s common stock on December 8, 2016 as reported by the Nasdaq Capital Market on such date was approximately $51,362,120. The registrant has elected to use December 8, 2016 (which was the initial trading date on the Nasdaq Capital Market of the registrant’s common stock) as the calculation date because on June 30, 2016 (the last business day of the registrant’s most recently completed second fiscal quarter), the registrant was a privately-held company. Shares of the registrant’s common stock held by directors, officers and stockholders whose beneficial ownership exceeded 5% of the registrant’s common stock outstanding have been excluded in that such persons may be deemed to be affiliates. The number of shares owned by such persons was determined based upon information supplied by such persons. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant, that such person is controlled by or under common control with the registrant, or that such person is an affiliate for any other purpose. There were 10,128,586 shares of the registrant’s common stock outstanding on December 8, 2016.
The number of shares of common stock outstanding as of March 29, 2017: 10,161,042
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive proxy statement to be delivered to shareholders in connection with the 2017 annual meeting of shareholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
SENESTECH, INC.
FORM 10-K
TABLE OF CONTENTS
| Item 1. | Business. |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains "forward-looking statements" within the meaning of the safe-harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can often be identified by words such as: "expect," "believe," "estimate," "plan," "strategy," "future," "potential," "continue," "may," "should," "will," and similar references to future periods. Examples include, among others, statements about:
| • | The likelihood of regulatory approvals for our product candidates; |
| • | The potential market opportunities for commercializing our product candidates; |
| • | The anticipated results and effects of our product candidates; |
| • | Our expectations regarding the potential market size for our products candidates, if approved for commercial use; |
| • | Estimates of our expenses, capital requirements and need for additional financing; |
| • | Our ability to enter into strategic partnership agreements and to achieve the expected results from such arrangements; |
| • | The initiation, timing, progress and results of future laboratory and field studies and our research and development programs; |
| • | Our ability to manufacture our product candidates in a commercially efficient manner; |
| • | The scope of protection we are able to obtain and maintain for our intellectual property rights covering our product candidates; |
| • | Our financial performance; |
| • | Developments and projections relating to our competitors and our industry; and |
| • | Our ability to sell our products at commercially reasonable values. |
Forward-looking statements are neither historical facts nor assurances about future performance. Instead, they are only predictions, based on current beliefs, expectations and assumptions about the future of our business and other future conditions. Forward-looking statements are subject to known and unknown risks, uncertainties and changes in circumstances that are difficult to predict and many of which are outside of our control. Actual events and results may differ materially. Therefore, you should not rely on any of these forward-looking statements.
Any forward-looking statement made by us in this report is based only on information available to us on the date of this report. Except as may be required by law, we undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise.
Our forward-looking statements can be affected by inaccurate assumptions we might make or by known or unknown risks, uncertainties and other factors. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form 10-K in greater detail under the Item 1A—“Risk Factors.” We caution readers that our business and financial performance are subject to substantial risks and uncertainties.
BUSINESS
Overview
We have developed and are seeking to commercialize globally a proprietary technology for managing animal pest populations through fertility control. We believe our innovative non-lethal approach, targeting reproduction, is more humane, less harmful to the environment, and more effective in providing a sustainable solution to pest infestations than traditional lethal pest management methods. Our approach is designed to promote food security and reduce infrastructure damage, disease outbreaks, environmental contamination and other costs associated with rodent infestations. Our first fertility control product, ContraPest®, will be marketed for use in controlling rat populations. We are pursuing regulatory approvals and amendments to existing registration in the US for ContraPest in various jurisdictions, including the U.S., India, Argentina and the EU. We submitted ContraPest for registration with the EPA on August 23, 2015, and the EPA granted registration approval for ContraPest effective August 2, 2016. We believe ContraPest is the first non-lethal fertility control product approved by the EPA for the management of rodent populations. However, before we can begin selling ContraPest in the U.S., we must obtain registration from the various state regulatory agencies. To date, we have received registration for ContraPest in 45 states and the District of Columbia, with additional applications pending. Other business initiatives include expanding our technology to other species and applications, and developing bio-synthetic sources of triptolide, an active ingredient in ContraPest that also has pharmaceutical applications. This initiative may produce a less expensive source of triptolide for our own use, and provide us with the potential opportunity to earn revenue from the sale of such product to other potential consumers of triptolide.
| 1 |
Current Problem
Rodent populations cause significant harm by:
• Decreasing the worldwide food supply. Rodents destroy crops through consumption and contamination, and the magazine Quality Assurance and Food Safety estimated that in 2014, 20% of stored food was lost due to rodent activity, of which a 5% reduction would be enough to feed an estimated 380 million people.
• Damaging public infrastructure. Rodents cause significant damage to public infrastructure by undermining foundations with burrowing, gnawing on electrical wiring and insulation, fireproofing systems and electronic and computer equipment. A study conducted in 2007 estimated that the cost of destruction to infrastructure by rats in the U.S. is $27 billion.
• Transmitting disease. Rodents transmit disease and deadly pathogens to humans and other species, such as E. coli, salmonella, leptospirosis, infectious jaundice, Weil’s disease, plague, murine typhus and Hantavirus. Rats caught during an independent study in New York City were found to be carrying more than 20 pathogens that cause disease in humans.
Current efforts to control rodent populations include the use of lethal chemical agents, also referred to as rodenticides, the sale of which constituted an estimated $900 million market worldwide in 2013 or more. In the United States, there are currently 193 such products registered by the EPA. Unfortunately, rodenticides have a number of serious shortcomings, as outlined below.
Rodenticides are not a long-term solution
Rodenticides do not target the rapid reproductive rates in rodents, to the contrary they accelerate rates by creating voids with plentiful food and harborage. The initial decline in rodent populations exposed to rodenticides is typically followed by a “population rebound” as surviving rodents quickly reproduce and rodents from surrounding areas migrate into the affected area. Studies have indicated that rat population rebounds can occur in as little as four months, while many populations rebound within six to nine months of being exposed to rodenticides. Moreover, even when rodenticides kill all of the rats in a designated area, populations have been observed to fully recover within 4-5 months.
Rats are prolific breeders; even a single pair of rats can result in a rapidly rebounding population. For example, a single female rat typically has a litter of ten to twelve pups or more every three weeks when food is plentiful. As a result, one pair of rodents can contribute over 15,000 progeny in 8 months, and four pairs of breeding adult rats and their progeny can produce up to 15 million rats in one year as a result of a geometric progression. In addition, rats are territorial and will protect their food source from immigrating rats. If a rat population is substantially decreased through poisoning by rodenticides, other rats will migrate to the unguarded food source and establish a new and expanding population.
Due to rebounding rodent populations exposed to rodenticides, property owners and pest management operators must continuously apply rodenticides of different active ingredients on a rotating basis in an effort to control these populations without favoring resistance to a particular poison. This creates an ongoing cycle of rodenticide treatment, with the costs of rodenticides remaining constant. Moreover, rodenticides are often distributed indiscriminately, on a non-targeted basis, which likely decreases their effectiveness in controlling rat infestations. The following chart, which is based on data derived from our population models, primary scientific literature, and current market pricing for rodenticides, demonstrates that the use of rodenticides does not sustainably reduce rat populations in the long term, while the cost of using rodenticides remains relatively constant over that same period.
| 2 |
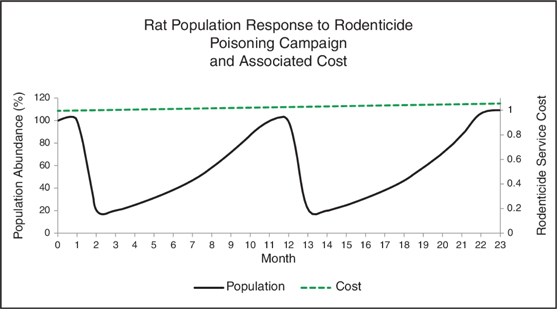
Rodenticides are an ineffective delivery method for rodents
Due to their understanding of cause and effect, studies have shown that rodents will generally not consume food that they have seen adversely affect other rodents; this is known as “bait shyness”. When the adverse effects of rodenticides are displayed by treated rodents, other rodents in the vicinity typically avoid the areas where the rodenticides were located.
Rodenticides are unsafe
Rodenticides contain lethal chemicals that can be toxic to humans and other animals. The EPA has observed that between 1993 and 2008, the American Association of Poison Control Centers logged between 12,000 and 15,000 reports of rat and mouse poison exposures each year in children under the age of six. These numbers and other concerns about pet and non-target wildlife exposures have spurred the EPA and similar authorities to renew its efforts to establish better protections for children and the environment. For example, the EPA and similar authorities in other jurisdictions have established stronger restrictions on the sale and use of ten active ingredients found in various registered rodenticide products. These restrictions prohibit the sale of “loose” rodenticide bait, such as pellets, powders, and liquids and require all such consumer-use baits be sold with protective bait stations. They also prohibited the use of second-generation anticoagulants, or SGARs, in any consumer-use product. In May 2014, the EPA and Reckitt Benckiser Group plc (a large manufacturer of rodenticides, including d-CON) reached an agreement to cancel 12 of their mouse and rat poisons that do not comply with the new EPA safety standards.
Rodenticides are harmful to the environment
Rodenticides are designed to cause the rodent pest to die over five or more days, permitting the target animal to continue to consume the rodenticide during that period. Since the target animal may consume significantly higher doses than are needed to be lethal, the lethal chemicals can accumulate in the target animal. This process is known as bio-accumulation. If the target animal is consumed by other animals (such as predators), such other animals can become sick or die from the lethal chemicals remaining in the deceased animal. In addition, deceased animals contaminated by rodenticides can spread the lethal chemicals to the surrounding area.
Rodenticides are inhumane
The most common type of rodenticide prevents blood from clotting. Once the rodent has consumed the rodenticide, lethal chemicals cause the rodent to bleed internally and through its eyes, nose and ears, gradually culminating in death over 10 to 14 days from exposure. Therefore, rodenticides result in a long, painful death which can be transmitted to any predator such as dogs, cats, and birds. This raises moral concerns, particularly in regions such as India, among people who do not want to cause unnecessary pain in animals.
| 3 |
Our Solution — Fertility Control
Our first fertility control product, ContraPest, targets the reproductive capabilities of rodents by inducing the gradual loss of their limited supply of eggs in female rodents and disruption of sperm in male rodents, resulting in contraception that can progress to sterility in both females and males. By targeting rodent fertility, our solution is sustainably effective, directed, safe, environmentally friendly and humane.
Our solution is sustainable over the long term
ContraPest, when used as directed, causes rodent populations to remain at a sustained low level. A third-party laboratory study in partnership with the United States Department of Agriculture National Wildlife Research Center, or NWRC, completed in December 2014 observed that 50 wild-caught rats treated with ContraPest in the presence of regular food and water resulted in a 95% reduction in litter sizes. A follow-up USDA study completed in June 2015 involving 50 wild-caught rats demonstrated a 96% reduction in litter size in female and male rats treated with ContraPest in an open arena study. We have also conducted open population studies, including in trash rooms in the New York City subway completed August 2013 and with the largest hog producer in the United States completed in March 2015, in which we have observed decreases in wild rodent populations of more than 40% over a 12-week period. We believe this decrease in population will continue and, based on studies conducted by third parties, will stabilize at an approximately 70% reduction in 12 months without rebound (based on an initial population of approximately 10,000 rats). Also, we have observed that the contraceptive effect of ContraPest in reducing rat population is present regardless of the amount consumed by any particular rat in that population.
Consequently, rat populations treated with ContraPest do not experience the same “population rebound” effect as those treated with rodenticides because the non-reproductive rodents continue to defend their territory from invasion by other rodents. Further, this low level of rodents contain a mixed gene pool that does not favor the development of resistance to the active ingredients. As a result, property owners and pest management operators may be able to substantially decrease the amount of ContraPest used over time, thus reducing the total cost of rodent population control in the long term. The following chart, which is based on data derived from our population models and field studies, predicts that as the use of ContraPest sustainably reduces rat populations over time and on an ongoing basis, the cost of ContraPest would be lower in the long term as compared to rodenticides.
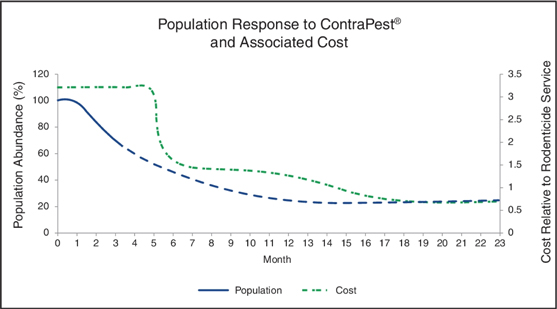
| 4 |
Our solution involves a targeted delivery
Our proprietary formulation appears to be attractive to rodents. To maintain proper hydration, rats drink 10% of their body weight in water each day; our product is a liquid bait and comprised mostly of water. Moreover, studies show that rats prefer and develop an addiction to eating sweet and fatty foods. ContraPest incorporates these elements, and our studies demonstrate that rats prefer ContraPest even when other familiar food and water sources were abundant, such as in indoor garbage collection areas of urban, industrial facilities. Once consumed, our product is designed to avoid a first pass through the liver effect and to deliver active ingredients directly to targeted reproductive organs, thus increasing its effectiveness and minimizing harm to the animal.
In addition, our solution utilizes a unique delivery system in the field that not only allows us to evaluate the effectiveness of our fertility control products, but also enables us to observe rodent pest behavior and determine the optimal locations for our proprietary bait stations. We also are able to customize the specific concentration to address the target pest.
Our solution is safe
Studies of ContraPest have demonstrated that doses of ContraPest are not lethal to rodents or harmful to people or other animals. The active ingredients in ContraPest are included at very low concentrations (together totaling less than 0.1% of the formulation) and have short half-lives of less than 20 minutes in the blood of rodents. Therefore, the active ingredients in ContraPest do not accumulate in tissues or organs of the rat (as poisons do) and thus do not sicken or kill predators or scavengers that eat a rat that has consumed ContraPest.
In addition, at the concentrations of active ingredients in our product, there is no potential for reproductive disruption in humans. A human would have to consume impossibly large amounts of the active ingredients in our product to have any effect. Further, the man-made chemical that is one of our active ingredients (4-vinylcyclohexene diepoxide, or VCD) has been used in manufacturing settings, and no toxic effects have been demonstrated in humans. The other active ingredient (triptolide) is a plant-derived ingredient used in traditional Chinese medicine to treat symptoms of rheumatoid arthritis.
Moreover, ContraPest is delivered in a ready-to-use and pre-packaged plastic container which is inserted into a tamper-resistant rodent bait station. As the container is inserted into the station, a spike punctures the foil-covered opening, allowing the liquid bait to flow into a tray within the bait station. The tank is a non-refillable container, which is recapped and disposed of when empty. Thus, as a “closed system,” there is little opportunity for handler exposure and virtually no opportunity for bystander exposure.
Our solution is environmentally friendly
ContraPest does not contain poisons, and the ingredients in ContraPest target the reproductive organs of the rodent and do not accumulate in other tissues or organs of the rat. As a result, the ingredients in ContraPest do not cause illness or death in other animals that come into contact with or eat the rat that has consumed ContraPest, and there is no risk of secondary exposure expected from the use of ContraPest. Also, the active ingredients in ContraPest are present in our product in very low concentrations, and break down into inactive, or inert, ingredients when they come into contact with soil or water in the environment. Moreover, ContraPest is packaged in a delivery system that dispenses the liquid bait directly into a tamper-resistant rodent bait station. These bait stations are limited to use only indoors and in the immediate perimeter of structures (no more than one foot from the exterior walls). As a result, ecological exposure of the liquid product will be limited to animals that directly contact or consume the bait, and there is little risk of exposure to non-target species or to the surrounding environment.
Our solution is humane
ContraPest does not cause rodent death and there have been no observations of any physical suffering in rodents exposed to ContraPest. ContraPest allows rodent pests to live out the course of their natural lives, while defending their territory and keeping out other invading rodents which have the potential of being disease vectors. By reducing rodents’ ability to reproduce, but keeping them alive, our product has the effect of humanely reducing rodent populations in the long-term.
| 5 |
Recent Research Regarding the Effectiveness of ContraPest
The majority of our research efforts have been focused on developing our lead product, ContraPest. We have completed studies regarding the effectiveness of our product, which were funded by and in cooperation with the NIH, the United States Department of Agriculture, or USDA, the NWRC, and the New York Metropolitan Transit Authority, or MTA, and other third parties. The following summarizes the results of these recent studies:
• A NWRC study involving approximately 50 rats completed in June 2015 demonstrated a 96% reduction in litter size in female and male rats treated with ContraPest in a laboratory setting;
• A January 2015 study in Rose Hill, North Carolina resulted in a 33% reduction in rodent activity over 12 weeks after being exposed to ContraPest, as compared to the use of rodenticide alone;
• A NIH-funded study in February 2013 in the subway trash rooms of the MTA in New York City observed that there was a 43% reduction in the rodent population in the trash rooms that were baited with ContraPest; and
• Internal laboratory studies involving 32 rats have shown zero pups born to any rat groups provided with ContraPest along with food and water, while rats given the control bait with no active ingredients had on average 11 pups per litter.
In September 2015 we initiated a research study with the Chicago Transit Authority, or CTA, to begin a field trial of our bait station. That study is now complete. While the observations and results are subject to a confidentiality agreement, the performance of the bait stations met expectations. We have additional field trials underway in Hawaii and Massachusetts (Somerville), and are contemplating further research trials in a variety of applications.
We have also begun exploring diverse applications with a variety of collaborators. We have conducted proof of concept studies with feral dogs on the Navajo Reservation in New Mexico with a grant from the USDA, and we have collected rabies and geographic data on stray dogs in the Tibetan refugee camps of Mainpat, India. We are currently collaborating with Texas A&M University to test the potential of our product candidates to manage feral pigs. Studies have also been conducted for proof of concept in Australia with wallaby, rat, and mouse populations and in New Zealand with rats and brushtail possums. We have also conducted early trials with cats in collaboration with the University of Florida. These diverse studies seek to provide evidence of the potential for ContraPest and the continued development of fertility control technology in general.
Business Strategy
Our goal is to become a leader in fertility control technology designed to promote food security and reduce infrastructure damage, disease outbreaks, environmental contamination and other costs associated with pest infestations and poor animal health. Key elements of our strategy are:
• Obtain regulatory approval for our lead product, ContraPest, throughout the U.S. and in Argentina, India, the EU and other parts of the world.
• Continue to develop and establish third party relationships with manufacturing, marketing and distribution partners in the U.S. and internationally.
• Educate our target markets on the long-term benefits our fertility control solution provides over lethal approaches.
• Establish a secure supply of active ingredients, including triptolide, by cultivating a diverse base of traditional agricultural suppliers and developing bio-synthetic sources of triptolide.
• Leverage our scientific research and core technologies to develop and commercialize a broad suite of products.
| 6 |
Manufacturing, Marketing and Distribution
Third-Party Relationships
We intend to continue to establish and develop relationships in the U.S. and internationally. To date, we have entered into the following arrangements:
NeoVenta — In September 2015, we entered into a sales and marketing agreement with NeoVenta Solutions, a sales and marketing company, for the sales of ContraPest in India and certain surrounding Southeast Asian countries. Under the agreement, NeoVenta will be responsible for seeking applicable regulatory approval to market ContraPest in these countries on our behalf. After such regulatory approval has been obtained, we have granted NeoVenta an exclusive license for 10 years to represent us in marketing, sales and distribution of ContraPest in these countries. We have retained all manufacturing responsibilities for ContraPest in these markets. NeoVenta has agreed to minimum sales commitments of rodent control products in the countries covered by the agreement, which total $23 million over the first five years. However, we believe that this understates the market potential for a non-lethal, effective and humane product in these countries.
Bioceres — In January 2016, we signed an agency agreement with INMET, the research and development subsidiary of Bioceres, Inc., a leading agricultural biotechnology company in Argentina, to seek regulatory approval for and conduct pre-sales marketing of ContraPest in Argentina. Under the agreement, INMET, which specializes in bacterial fermentation solutions, will act as our exclusive agent to obtain necessary governmental approvals to sell and market ContraPest in agricultural, residential and public transport applications throughout the country of Argentina. The parties intend to create a joint venture entity which we will control. Sales in Argentina will occur only after regulatory approval is obtained and the joint venture entity is formed. We have also entered into a services agreement with Bioceres and INMET to provide research and development services to develop an efficient production method for a bio-synthetic version of triptolide, one of the two active ingredients in ContraPest that also has pharmaceutical applications. The parties intend to create a second legal entity to pursue this triptolide research and development.
Subject to obtaining necessary regulatory approvals, we plan to market ContraPest in additional international jurisdictions. The expectation is that we will stage these market launches based on the length of time required to complete each country’s regulatory process, the market potential, identification and agreements with appropriate parties and the safety of our intellectual property.
We are currently exploring a potential relationship in Europe for the registration of ContraPest with EU regulatory agencies, the development of manufacturing in the EU, research and development of new products using our fertility control technology, and the granting of distribution rights in the EU. However, we have not yet entered into any binding agreements related to these matters.
Commercialization Plans
To date, we have generated minimal revenue from product sales, but we currently expect to fully commercialize ContraPest and begin to generate revenue from the sale of products the second quarter of 2017 on a limited basis.Subject to obtaining necessary regulatory approvals, we also intend to market ContraPest in international jurisdictions. Target segments for ContraPest include government (e.g., subways, transit systems and public housing agencies); healthcare; agriculture (e.g., farms, storage facilities and protein production facilities (including cattle, sheep, pig and poultry facilities)); food production (e.g., factories, meat-packing facilities, dairy production plants and vegetable and fruit preparation facilities); and commercial (e.g., major restaurant chains, retail locations, casinos and hotels). Since EPA approval, we have received calls or emails of interest from the following types of potential customers: zoos, animal research facilities, waste and recycling centers, parks, transit agencies, natural resource managers, island conservation groups, botanical gardens, animal sanctuaries, children’s gardens, healthcare providers, property managers, and food production facilities. In addition, we intend to approach large pest management companies to pursue potential partnerships for the distribution and sale of ContraPest.
Pricing and Value
We intend to value price our product, ContraPest, such that our pricing strategy will take into account not only the cost of goods sold, but an understanding of the cost of competitive products and the value of our product to the end user. We believe ContraPest will be perceived as a significant improvement over current products for managing rat infestations and, as such, should command a premium price. Our experience is that potential customers understand the advantages of ContraPest and become enthusiastic about its use. We plan to use promotional efforts to support the value message and to justify our product’s increased value and premium price, built around the following proposed advantages:
| • | ContraPest is sustainably effective; |
| 7 |
| • | ContraPest involves targeted delivery; |
| • | ContraPest is safe; |
| • | ContraPest is environmentally friendly; and |
| • | ContraPest is humane. |
Also, we will focus on specific advantages for the individual customer and expect to position our product as having the following additional general advantages:
| • | Savings in personnel costs to clean up dead rodents; |
| • | Reduction in disease vectors because there are no contaminated rat carcasses |
| • | Savings in eliminating the use of rodenticides during ContraPest baiting; |
| • | Public relations advantages (sustainably effective, safe, environmentally friendly and humane); |
| • | Savings by reducing loss or contamination of food inventories; and |
| • | Savings by reducing damage to infrastructure. |
We believe that the addressable markets for ContraPest will evolve from first adopters to those potential customers that have zero tolerance for poisons, to those potential customers that focus on efficacy and value pricing, to those potential customers and regulators that prefer an alternative to poisons, and finally to bulk agricultural and retail markets. We believe that the expansion into these markets will be aided by a progression of potential cost and pricing reductions, starting with raw material costs, packaging costs, scale economies, and finally delivery and distribution improvements.
Sales Approach
Because of the unique nature of our technology and the market demand for a non-lethal approach to rodent pest control, large pest management companies who we have approached about our product have expressed interest in learning more about ContraPest. Consequently, we plan to continue to foster these discussions, to exchange data, and may negotiate agreements with carefully selected partners to maximize their appropriate deployment of our product. The advantages to selling through such a third party include:
| • | Immediate availability of a field sales force experienced in selling rodent control products; |
| • | Familiarity with our target customers and the challenges they face; |
| • | Our field personnel, customer service, account receivable, and shipping and handling teams would be smaller, thus reducing start-up costs; and |
| • | Less need to substantially expand the sales force as our product gains traction with new customers. |
We plan to be deeply involved in the initial product launch and assist with in-depth product training, business development, co-travel with sales representatives and the creation of sales and marketing tools.
| 8 |
Raw Materials and Manufacturing Process
ContraPest contains two active ingredients, VCD, an industrial chemical, and triptolide, a plant derived chemical from the Thunder God Vine, Tripterygium wilfordii. ContraPest also contains several other inactive ingredients. Currently, we source VCD from a standard industrial chemical supply provider. However, in the near future we will be qualifying additional suppliers for VCD. Triptolide is derived from the Thunder God Vine, which is commonly cultivated and harvested wild in southeastern China and other Asian countries, and is available from a variety of sources. Currently, we have one EPA registered source of purified triptolide, and have validated a second source. However, the process to purify triptolide for use in ContraPest is expensive, and we are seeking other, less costly methods of triptolide production, including bio-synthetic methods. See the discussion under the heading “Manufacturing, Marketing and Distribution” for more information about our agreement with Bioceres and INMET to provide research and development services to develop an efficient production method for a bio-synthetic version of triptolide. This initiative may produce a less expensive source of triptolide for our own use, and provide us with the potential opportunity to earn revenue from the sale of such product to our licensees and other potential consumers of triptolide. The inactive ingredients in ContraPest are sourced from standard chemical supply providers.
Our manufacturing process involves the incorporation of our two active ingredients, in low concentrations, into several inert ingredients. Once incorporated, the entire product goes through a micro-encapsulation process in order to stabilize the final formulation. Stabilizing the product in this manner allows it to be delivered to rodents in a safe and effective manner. After production, the manufacturing line is cleaned using environmentally safe methods and products.
Currently, we have production scale capability in our facilities in Arizona to manufacture and launch ContraPest. Our internal production capabilities allow us to meet our current and anticipated demand during 2017 for ContraPest. We have secured equipment to double our manufacturing capacity and expect it to be online in the third quarter of 2017. Our manufacturing process has been designed in a modular, scalable and transportable fashion. This allows us to quickly respond to production requirements anywhere in the world.
In addition to our domestic manufacturing facility, we have also entered into an agreement with Bioceres and Inmet to jointly develop our manufacturing capacity in Argentina, and intend to enter into additional manufacturing agreements in the future on an opportunistic basis.
Scientific Background Regarding our Product
ContraPest is a liquid bait containing the active ingredients VCD and triptolide. When consumed, ContraPest causes contraception that can progress to sterility in male and female rats beginning with the first breeding cycle following consumption.
The female rat is born with a finite number of eggs, also called oocytes, and she remains fertile and will reproduce until the day she dies. Within the ovary, eggs are contained in structures called follicles. The non-regenerating and most immature stage of follicles is called primordial. The primordial follicles mature through several stages from primary to secondary to antral follicles and ultimately ovulate. Once the primordial follicles have become depleted, ovarian failure occurs, which terminates reproductive capability.
VCD has been well studied and causes specific loss of ovarian small follicles (both primordial and primary); because oocytes do not regenerate, loss of these follicles leads to ovarian failure. Following repeated dosing, VCD causes ovarian failure in rats. However, daily dosing of mice and rats with VCD does not produce generalized toxicity nor does it affect other tissues. A VCD-dosed rat will continue to reproduce until the pool of secondary and antral follicles are depleted through ovulation or atresia, which is the natural death of the follicle, which can take up to three months.
The second active ingredient, triptolide, targets growing follicles and exerts a significant suppression of male fertility by disrupting sperm maturation and stopping the movement of sperm. Female rats treated with triptolide ovulate fewer eggs because the follicles stop growing. Triptolide does not affect primordial follicles, but when used in combination with VCD, the result is contraception that can progress to sterility in female rats.
Both VCD and triptolide are supported by evidence regarding their safety and mechanism of action. Additionally, recent studies, both in the lab and in the field, have documented their effect in fertility reduction and therefore reduction in rat populations. The graph below displays the total numbers of pups after two breeding rounds in one study.
| 9 |
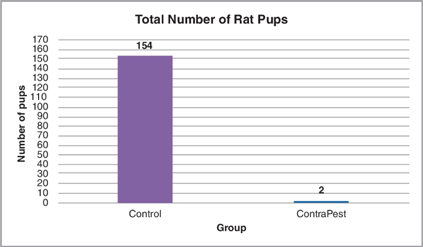
Figure: Total number of rat pups born after consumption of ContraPest. Sixteen female rats (n=8 control and n=8 treatment) were provided ContraPest or inactive bait for 15 days and bred with proven male breeders. After two breeding rounds, the number of pups was totaled. The bar on the left shows the number of pups born to control females while the bar on the right shows the number of pups born to females that consumed ContraPest.
Other Potential Products
We have developed a pipeline of potential additional fertility control and animal health products, with diverse applications, as outlined in the following chart and in more detail below.
| Product Candidate/Area |
Development Status | Segment | Primary Target | |||
| ContraPest | Environmental Protection Agency (EPA) granted registration approval for ContraPest effective August 2, 2016 | Population management | Rodents | |||
| Plant-based fertility control | Pilot studies have been completed; additional testing required for the use of this product to manage pest populations in select sites such as schools and hospitals | Population management | Rodents | |||
| Feral animal fertility control | Pilot studies are in process to show efficacy of this product candidate; to complete larger pivotal studies and regulatory submission | Population management | Feral dogs and hogs | |||
| Non-surgical spay and neutering | Pilot studies completed show encouraging signs of efficacy; to complete additional studies and regulatory submission | Companion animal health | Companion dogs and cats | |||
| Boar taint | Additional scientific and field studies and regulatory submission required | Food production and safety | Boars | |||
| Animal cancer treatment | Proof of concept study to be performed to determine whether proprietary formulation may provide effective delivery of triptolide to dogs for cancer therapy | Companion animal health | Companion dogs |
Boar Taint Product Candidate
Boar taint is the offensive odor or taste that can be evident during the cooking or eating of pork or pork products caused by hormones, called pheromones, present in non-castrated boars once they reach puberty. Castration without anesthesia shortly after birth is currently the standard procedure used to eliminate boar taint, but it results in lower meat production due to decreased weight gain, which is an effect of castration. This process also introduces a surgical risk of infection and can raise safety issues for workers.
| 10 |
If we are successful at developing a boar taint product candidate, we expect that it will target testosterone production and will be easily administered to feedlots and will have none of the safety issues associated with castration. The next step will be continued scientific and field studies followed by submission to and approval by the appropriate regulatory agencies. This process is expected to take approximately two years.
Feral Animal Fertility Control Product Candidate
Feral dogs and hogs present problems both in the United States and internationally. The negative impacts of feral dogs include threats to human health and safety, agriculture, natural resources and property. A 2005 study estimated monetary losses by feral dogs within the U.S. at $620 million annually. Feral pigs are can also be aggressive and are known for damaging crops and transmitting diseases to humans, livestock and other wildlife. Feral pigs are present across more than three quarters of the U.S. and are responsible for an estimated $1.5 billion in damages each year.
Current strategies for controlling feral animal populations are often ineffective, difficult to conduct and costly. Studies have shown that our fertility control technology is effective in both these species. Accordingly, we are currently conducting pilot studies to show efficacy of our approach prior to proceeding to larger pivotal studies and regulatory submission. We are currently completing specific development plans for this product candidate.
Companion Animal Product Candidates
We plan to develop the following products for use in companion animals such as domestic dogs and cats. However, applications for companion animals require FDA approval, which is a much longer and more expensive regulatory process. Our expectation is that we will pursue these technologies or through research and development partnerships with larger companies.
• Non-Surgical Spay and Neutering Product Candidate. Based on a low average of $100 for each spay or neuter procedure, the spay and neutering of companion animals constitutes a $1.9 billion market in the United States alone, with few effective non-surgical alternatives. We are developing a product that can be easily administered to the companion animal orally or by injection in combination with vaccinations. No surgery is required and the surgical risks of infection and pain could be eliminated. This product candidate targets the ovaries and testes and is delivered through a proprietary drug delivery methodology. Early field studies with feral dogs showed encouraging signs of efficacy.
• Animal Cancer Treatment Product Candidate. Cancer therapy for companion animals is often not a viable option since chemotherapy can be a long, painful and expensive process. However, we have developed a manufacturing technology that allows the chemotherapeutics to be encapsulated and delivered directly to the affected tissues without causing the side effects to the immune, hypothalamic systems or neuro pathways.
Competition
Currently, there are no non-lethal fertility control products that target rodents. Products that are used for managing rodent infestations include rodenticides, kill devices and traps.
Rodenticides
Rodenticides are poisons that use anticoagulants or phosphides to cause rodent death.
Anticoagulants can be single dose (i.e., second generation) or multiple dose (i.e., first generation) rodenticides. Generally, death occurs within one to two weeks after ingestion of lethal amounts. These poisons work by blocking the rodent’s blood clotting ability. In addition, they include chemicals that cause damage to tiny blood vessels, or capillaries, resulting in diffuse internal bleeding. These effects are gradual, developing over several days. In the end, the animal dies calmly, but leading up to death the rodent is likely to experience discomfort and pain. As a result, we believe that the use of anticoagulants is inhumane.
| 11 |
First generation anticoagulants are generally less toxic than second generation products, so they have shorter elimination half-lives, but they also require higher concentrations and consecutive intake over days to be lethal. First generation anticoagulants are marketed under a variety of brands such as Ramik, Rodex, Tomcat and Rozol and contain active ingredients such as warfarin, chlorophacinone, diphacinone or coumatetralyl. Second generation anticoagulant rodenticides, or SGARs, known as “superwarfarins,” are far more toxic than first generation rodenticides so they are applied in lower concentrations. Most are lethal after a single ingestion of bait. SGARs are also available under a variety of different brand names, including d-CON, Havoc, Di-Kill, Jaguar, Hawk, Boot Hill and Hombre. These products contain active ingredients such as difenacoum, brodifacoum, difethialone, flocoumafen, and bromadiolone. Companies that manufacture anticoagulants include Reckitt Benckiser Group plc, Syngenta, Bayer CropScience, BASF, Neogen and Liphatech.
Metal phosphides are considered single-dose fast acting rodenticides; death occurs commonly within one to three days after single bait ingestion. Death is caused by an acid in the digestive system of the rodent that reacts with the phosphide to generate the toxic phosphine gas. Metal phosphides have possible use in places where rodents are resistant to some of the anticoagulants. Zinc phosphide baits are also cheaper than most second-generation anticoagulants. They are marketed under brands that include Prozap, Eraze, and Ridall-Zinc by Neogen, MotomCo and Liphatech, respectively.
Rodenticide manufacturers compete by introducing new products to meet the changing demand of consumers, expansions and investments, acquisitions, and entering into strategic alliances with distributors and companies that have expertise in the rodenticide market. As a result, we believe the degree of competition in the rodenticide market is high. The market is highly concentrated among a few large rodenticide manufacturers, such as Syngenta, Bayer CropScience, BASF, Neogen and Liphatech. Also, there are high barriers to entry into the rodenticide market due to extensive capital investment and regulatory approval requirements.
Traps
Trapping is an option for those looking for a non-lethal way to manage a rodent infestation. There are several types of traps including spring, or snap traps, cage traps, glue traps and electronic traps. Often traps merely injure and trap the rodent still alive. Trapped rodents will do anything to free itself, including chewing off its limbs. Also, rats are relatively intelligent animals and can learn to avoid traps. Further, the use of traps is less popular in urban centers and among pest control companies. Therefore, traps are a less common alternative to rodenticides as a form of rodent control. Companies that manufacture traps include Victor, Havahart, Rat Zapper, Real-Kill, J.T. Eaton and others.
Kill Devices
Goodnature self-resetting kill traps, from New Zealand, are becoming widely used in conservation and island areas. Rats are attracted by a long-life lure that triggers a powerful impact that kills animals instantly and more humanely than other products. These traps are easy to install and maintain and can kill up to 24 rats per CO2 canister, thus reducing the amount of man hours needed to maintain the traps.
Animal Fertility Control
Animal fertility control has been in research and development for almost 30 years. GonaCon (GnRH) is the current product for fertility control approved by the USDA. GonaCon is injected into an animal and the animal must receive a booster after two years to maintain efficacy. The formula is typically provided through a dart gun and the animals should be marked so that the booster can be given at a later date. This is an extremely challenging delivery method for any wild animal in a natural environment. ZonaStat-H (PZP), a fertility product used since the late 1980’s for wild horses and burros, is delivered in the same manner as Gonacon. The only oral fertility product on the market is an avian product, developed by Innolytics, LLC in collaboration with the USDA Animal and Plant Health Inspection Service, and is made specifically for pigeons.
Government Regulation and Product Approval
Federal, state and local government authorities in the United States regulate, among other things, the testing, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, distribution and marketing of the products we develop. Our wildlife and pest fertility control products must be approved by the EPA Office of Pesticide Programs, or OPP, before they can be legally marketed and sold in the United States. The process for obtaining regulatory approval and compliance with appropriate federal, state and local regulations is rigorous and requires the expenditure of substantial time and financial resources.
| 12 |
Additional product candidates in our pipeline may require approval from other government agencies, namely the USDA and FDA. In 2015, the FDA and EPA entered into a “data sharing” agreement to streamline data review and speed the regulatory process avoiding redundancy where possible.
United States Review and Approval Processes
In the United States, the EPA regulates the sale, distribution and use of any pesticide under the Federal Insecticide, Fungicide and Rodenticide Act, or FIFRA. The EPA defines a pesticide as “any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pest.” FIFRA defines a pest as “any insect, rodent, nematode, fungus, or weed.” To register a new product, all active ingredients within the product must be registered with the EPA.
On August 23, 2015, we submitted a set of registration applications for two active ingredients and a formulated product – ContraPest - for EPA review and approval. Our application for ContraPest was submitted as a restricted use, indoor only, application. A restricted use product can only be handled by a certified pest control operator. The requirements for an application are specified in detail by EPA regulations. These include requirements for data on which the EPA can evaluate the environmental effects, health effects, and safety of the product. The environmental effects data is reviewed by the Environmental Fate and Effects Division, or EFED. The health effects data is reviewed by the Health Effects Division, or HED.
Prior to submission, our active ingredients and product information were reviewed by the Hazard and Science Policy Council, or HASPOC, which includes HED members, and data waiver requests were granted for all of the required toxicology studies. EFED also reviewed the applications, and provided certain recommendations for testing, which were incorporated into our filing.
Upon filing an application with the OPP Registration Division (RD), there was a preliminary screen, where the application was initially reviewed for all required sections regarding chemistry, toxicity and environmental fate. As per the registration process once the preliminary screen is finished, the review period begins. Under the timelines set forth by the Pesticide Registration Improvement Extension Act (PRIA 3), RD has 20 months to review the application and reach a registration decision. Once the review period begins, RD will disperse the application to all the applicable science departments for an in depth review. For an application with an official review period greater than six months, OPP has 90 days in which it may identify any substantive science omissions and may reject the application unless the applicant can correct the omissions within 10 business days. This technical screening review period passed without EPA raising any issue. After completing the science reviews, each department will make its recommendations to RD. Based on these science recommendations, RD may decide it needs additional data and will contact the applicant with options for proceeding, which may include extending the review period until requisite data or other information can be developed and submitted for review. If RD has sufficient information, it will develop its initial risk and registration decisions, which it will articulate and publish in a document for public comment. RD decided that it had sufficient data from our application and published the document for public comment on June 24, 2016. After the 30-day public comment period, OPP reviewed the public comments to address any additional concerns. This is considered one of the final steps prior to approval of a registration.
The EPA also requires that the following be submitted for review, in addition to data: a complete copy of the label proposed for the product, instructions for use and any claims that will be made by us, as well as, the complete formulation. We are also required to submit documentation describing the chemistry, manufacturing process and quality control parameters. This is done to ensure the product can be produced consistently. The entire submission must be reviewed and approved prior to any legal sales and distribution of the product. The EPA granted registration approval for ContraPest effective August 2, 2016. This EPA approval was granted on a restricted-use basis, including indoor and limited outdoor use, and is based on a liquid formation. We intend to diligently pursue additional related regulatory approvals from the EPA to support our product evolution, including seeking approval for full outdoor use, removal of the restricted-use status, alternative formulations and for additional species (utilizing already approved active ingredients). In addition, we believe that the EPA will support us in facilitating regulatory reviews outside of the U.S., and we are exploring a relationship with the Danish Environmental Protection Agency to assist us with obtaining regulatory approvals in the EU.
| 13 |
We have submitted for registration in each state, since product registration is required for every state in which the product will be distributed or sold. Each state has its own registration filing requirements. These registration programs are managed by state agricultural and/or environmental regulatory agencies. For some state registration applications, all that is required is the EPA stamped-approved label, a completed application form, and a fee payment. Other states, notably California, New York, and several others, require more robust registration applications and may require data not required by EPA. ContraPest has received registration from the regulatory agencies of Alabama, Alaska, Arizona, Arkansas, Colorado, Delaware, District of Columbia, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nevada, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, Washington, W. Virginia, Wisconsin, and Wyoming . Registrations in additional states are currently pending. States vary in the expected timing of approval, from a few weeks to several months.
International Review and Approval Processes
Canada — Canada also has a product registration program similar to the U.S. program. Canada has entered into a data and review agreement with EPA intended to expedite the approval process. We intend to submit an application to PMRA during 2017.
European Union — The European Chemicals Agency (ECHA) is a decentralized agency of the European Union, or EU. The agency is responsible for the scientific evaluation of chemicals intended for use in human health and the environment developed by companies for use in the EU. The agency has a biocidal product committee that is responsible for the review of any biocidal product and active substances. A biocidal product is used to control unwanted organisms that are harmful to human or animal health, or that cause damage to human activities. These harmful organisms include pests (e.g. insects, rats or mice) and microorganisms (e.g. molds or bacteria). Biocidal products include: insecticides, insect repellents, disinfectants, preservatives for materials, and anti-fouling paints. The biocidal product committee has similar but more stringent requirements to those of the EPA in the United States, requiring that evidence of purity, safety, efficacy, and consistency of manufacturing processes all be demonstrated.
The member state where the biocidal product will be placed on the market is responsible for authorizing the product. This is referred to as the ‘National authorization’. The process of national authorization relies however on the process of mutual recognition. Once a biocidal product is authorized by a first EU country (the ‘Reference Member State’), the other EU countries must, if requested to do so, authorize the biocidal products under the same terms and conditions. Some products can also be authorized at EU level, allowing the companies to place these on the entire EU market. In these cases, it is the European Commission that authorizes the products. This is referred to as the ‘Union authorization’.
In March 2016, ECHA decided to move toward a comparative assessment for rodenticides registered as a biocide. A comparative assessment will evaluate the risks and benefits of each rodenticide and a standard will be set for rodenticides based on this information. When a new rodenticide files for registration, it must meet this standard or best this standard to be accepted. This comparative assessment will be carried out by the Reference Member State. Representatives from various member states met in Helsinki the second week of June 2016 to determine which products or active ingredients will be used to set the standard for rodenticides. ECHA has moved to support the 5-year renewal, not the traditional 10-year renewal, of eight anticoagulant rodenticides.
On July 4, 2016, we met with the Danish Environmental Protection Agency (DEPA) for a pre-meeting to discuss the registration of ContraPest in the EU. This meeting was to determine if DEPA was willing and capable to review and support a ContraPest dossier. DEPA agreed they would have the capacity to support a ContraPest dossier and they have executed their commitment as the competent authority to carry our EU registration application forward. We are engaged in ongoing discussions with DEPA to establish the application content.
United Kingdom — In addition to registration routes listed above, the UK has a data sharing agreement with the United States, Australia, and New Zealand for any wildlife fertility management product, allowing for a potentially expedited registration process.
Australia — The Australian Pesticides and Veterinary Medicines Authority, or APVMA, is an Australian government statutory authority established in 1993 to centralize the registration of all agricultural and veterinary products into the Australian marketplace. Previously each State and Territory government had its own system of registration. The APVMA assesses applications from companies and individuals seeking registration so they can supply their product to the marketplace. Applications undergo rigorous assessment using the expertise of the APVMA’s scientific staff and drawing on the technical knowledge of other relevant scientific organizations, Commonwealth government departments and state agriculture departments. If the product works as intended and the scientific data confirms that when used as directed on the product label it will have no harmful or unintended effects on people, animals, the environment or international trade, the APVMA will register the product. As well as registering new agricultural and veterinary products, the APVMA reviews older products that have been on the market for a substantial period of time to ensure they still do the job users expect and are safe to use. The APVMA also reviews registered products when particular concerns are raised about their safety and effectiveness. The review of a product may result in confirmation of its registration, or it may see registration continue with some changes to the way the product can be used. In some cases the review may result in the registration of a product being cancelled and the product taken off the market.
| 14 |
Rest of the World — Country-specific regulatory laws have provisions that include requirements for certain labeling, safety, efficacy and manufacturers’ quality control procedures to assure the consistency of the products, as well as company records and reports. With the exception of the EU, most other countries’ regulatory agencies will generally defer to the EPA or accept OECD compliant data in establishing standards and regulations for pest management products. Some specific in-country studies will be required for particular countries but some countries will generally accept an EPA or EU compliant dossier.
Import Permits — Field and laboratory proof of principle studies have been conducted in other countries. In each country, import regulations have been met for the shipment of active ingredients. To date we have received import permits from Australia, New Zealand, Indonesia, Laos, and the Philippines. We expect that additional import permits will be obtained for various field trials in countries prior to registration.
Personnel
As of December 31, 2016, we had 27 full-time, and two part-time employees including a total of four with Ph.D. degrees. Within our workforce, 16 employees are engaged in research and development and 13 in business development, finance, legal, human resources, facilities, information technology and general management and administration. None of our employees are represented by labor unions or covered by collective bargaining agreements.
Intellectual Property and Other Proprietary Rights
Maintaining a strong position in the rodenticide market requires constant innovation along with a healthy research program to evolve product lines to remain competitive and relevant to the needs of the changing global marketplace. We protect the intellectual property resulting from these efforts with the broadest international patent protections available. Our proprietary data and trade secrets are protected with vigilance and attention to data exchanges among employees, consultants, collaborators and research and trade partners. We further strengthen our market position employing international regulatory expertise.
Patent Filings
Our intellectual property portfolio supporting ContraPest consists of nine international patent filings (in the United States, Europe, Canada, Brazil, Russia, Japan, Mexico, South Korea, and Australia) addressing the ContraPest compound. Claims directed toward the compound include composition-of-matter involving a diterpenoid epoxide or salts thereof in combination with an organic diepoxide, use claims for inducing follicle depletion and for reducing the reproductive capability of a mammalian animal or non-human mammalian population. Issued claims will have a patent term extending to 2033 or longer based on patent term determinations in each of the filing countries. The novelty of ContraPest extends to its method of field distribution and has required innovation to perfect the dosing of our product to rodents. We have filed an international patent application covering our novel bait station device to effectively and efficiently deliver our rodent bait at individual bait sites that would, if issued, offer patent term protection through at least 2036.
License Agreements
We have an exclusive patent license with the University of Arizona for background intellectual property that we plan to employ for future product development in the domestic animal fertility control market. The patent claims in the United States, Australia and New Zealand cover the use of 4-vinylcyclohexene diepoxide to deplete ovarian follicles in individual mammals and mammal populations. The license agreement, signed in 2005, will terminate with the last-to-expire patent claims, which have a term extending to 2026.
| 15 |
Trade Secrets and Trademarks
Beyond our patent right holdings, we broaden our intellectual property position with trademark, trade secret, know-how and continuous scientific discovery to accompany our product development efforts. We protect these proprietary assets with a combination of confidentially terms in all partnership agreements or as stand-alone agreements along with rights-ownership agreements and structured information transfer understandings prior to beginning any collaborative projects. We maintain the ContraPest trademark and are registering new trademarks for products from our evolving rodenticide product line and for products for mammalian species beyond rodentia.
Data Sets
We have exclusive use status with the EPA for the data sets we have developed and submitted to the EPA as part of our application for ContraPest. The exclusive use status applies to new active ingredients and the final formulation of the ContraPest product for a period of 10 years. For five years after the 10-year period of exclusivity, if another applicant or the EPA Administrator chooses to rely on one or more data sets that we submitted in support of an application submitted by another applicant, the new applicant must make a binding offer to compensate us and certify to EPA that it has done so. If we and the offeror cannot reach agreement on the terms of the compensation for the use of such data sets, FIFRA requires resolution by binding arbitration. The EPA rules do not describe how the compensation should be determined, and there is publicly available information about some, but not all, binding arbitration decisions. See Item 1A, “Risk Factors,” for more information regarding our intellectual property and other proprietary rights.
Available Information
We electronically file with the Securities and Exchange Commission (“SEC”) our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at www.senestech.com, free of charge, copies of these reports, as soon as reasonably practicable after electronically filing such reports with, or furnishing them to, the Securities and Exchange Commission. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this Annual Report on Form 10-K.
Directors and Executive Officers of the Registrant
The following table sets forth certain information with respect to our directors and executive officers as of January 31, 2016:
| Name | Age | Position | ||
| Loretta P. Mayer, Ph.D. | 67 | Chair of the Board, Chief Executive Officer and Chief Scientific Officer | ||
| Cheryl A. Dyer, Ph.D. | 64 | President, Chief Research Officer and Director | ||
| Thomas C. Chesterman | 57 | Executive Vice President, Chief Financial Officer, Treasurer and Assistant Secretary | ||
| Kim Wolin | 61 | Executive Vice President, Operations, and Secretary | ||
| Grover Wickersham | 68 | Vice-Chair of the Board, Chair of Nominating and Corporate Governance Committee | ||
| Marc Dumont | 73 | Director | ||
| Bob Ramsey | 71 | Director | ||
| Matthew Szot | 42 | Director; Chair of Audit and Compensation Committees | ||
| Julia Williams, M.D. | 57 | Director |
| Item 1A. | Risk Factors |
As discussed under Item 1 of Part I, “Business—Cautionary Note Regarding Forward-Looking Statements,” our actual results could differ materially from those expressed in our forward-looking statements. Factors that might cause or contribute to such differences include, but are not limited to, those discussed below. Additional risks and uncertainties not presently known to us, or that we currently deem immaterial, may also impair our business operations. If any of the following risks occur, our business, financial condition, operating results, cash flows and the trading price of our common stock could be materially adversely affected.
| 16 |
Risks Relating to Commercialization of Our Product Candidates
ContraPest and our other product candidates, if approved, may not achieve adequate market acceptance necessary for commercial success.
Even following receipt of any regulatory approval for ContraPest or any of our other product candidates, such products may not gain market acceptance. Market acceptance of any of our product candidates for which we receive approval depends on a number of factors, including:
| • | The efficacy and safety of such product candidates as demonstrated in trials; |
| • | The uses, indications or limitations for which the product candidate is approved; |
| • | Acceptance of the product candidate as a safe and effective alternative; |
| • | The potential and perceived advantages of product candidates over alternative products; |
| • | Product labeling or product insert requirements of the EPA or other regulatory authorities; |
| • | The timing of market introduction of our products as well as future competitive products; |
| • | Relative convenience and ease of use; |
| • | The effectiveness of our sales and marketing efforts and those of our collaborators; and |
| • | Unfavorable publicity relating to the product. |
If any of our product candidates are approved but fail to achieve market acceptance, we will not be able to generate significant revenues, which would compromise our ability to become profitable. Furthermore, the commercial success of ContraPest will depend on a number of factors, including the following:
| • | The development of a commercial organization or establishment of a commercial partnership with a commercial infrastructure; |
| • | Establishment of a commercially viable pricing; |
| • | Our ability to manufacture quantities of ContraPest using commercially acceptable processes and at a scale sufficient to meet anticipated demand and enable us to reduce our cost of manufacturing; |
| • | Our success in educating end users about the benefits, administration, and use of ContraPest; |
| • | The effectiveness of our own or our potential strategic partners’ marketing, sales and distribution strategy, and operations; and |
| • | A continued acceptable safety profile of ContraPest following approval. |
Many of these factors are beyond our control. If we are unable to successfully commercialize ContraPest, we may not be able to earn sufficient revenues to continue our business.
| 17 |
We have never marketed a product before, and if we are unable to establish an effective sales force and marketing and distribution infrastructures, or enter into and rely upon acceptable third party relationships, we may be unable to generate any revenue.
We are developing but do not currently have an infrastructure for the sales, marketing, and distribution of our products and the cost of establishing and maintaining such an infrastructure may exceed the cost-effectiveness of doing so. In order to market ContraPest and any other products that may be approved by the EPA and comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services for which we would incur substantial costs. If we are unable to establish adequate sales, marketing, and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not become profitable. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, we may be unable to compete successfully against more established companies.
Risks Related to our Financial Condition and Capital Requirements
We have incurred significant operating losses every quarter since our inception and anticipate that we will continue to incur significant operating losses in the future.
Investment in product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval, or become commercially viable. To date, we have financed our operations primarily through research grants as well as through the sale of equity securities and debt financings. Until August 2, 2016, we did not have any products approved by a regulatory authority for marketing or commercial sale, and we have not generated any revenue from product sales to date. We continue to incur significant research, development, and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses in every reporting period since our inception. For the years ended December 31, 2016 and 2015, we reported net losses of $11.0 million and $18.2 million, respectively. As of December 31, 2016, we had an accumulated deficit since inception of $61.3 million.
Since inception, we have dedicated a majority of our resources to the discovery and development of our proprietary product candidates. We expect to continue to incur significant expenses and operating losses for the foreseeable future. The size of our losses will depend, in part, on the rate of future expenditures and our ability to generate revenues. In particular, we expect to incur substantial and increased expenses as we:
| • | Continue the research and development of ContraPest and our other product candidates, including engaging in any necessary field studies; |
| • | Seek regulatory approvals for ContraPest in various jurisdictions and for our other product candidates; |
| • | Scale up manufacturing processes and quantities to prepare for the commercialization of ContraPest and any other product candidates for which we receive regulatory approval; |
| • | Establish an infrastructure for the sales, marketing and distribution of ContraPest and any other product candidates for which we may receive regulatory approval; |
| • | Attempt to achieve market acceptance for our products; |
| • | Expand our research and development activities and advance the discovery and development programs for other product candidates; |
| • | Maintain, expand and protect our intellectual property portfolio; and |
| • | Add operational, financial and management information systems and personnel, including personnel to support our clinical development and commercialization efforts and operations as a public company. |
We may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors that may adversely affect our financial condition. Our prior losses and expected future losses have had, and will continue to have, an adverse effect on our financial condition. If ContraPest or any other product candidate does not gain sufficient regulatory approval, or if approved, fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. A decline in the value of our company could cause you to lose all or part of your investment.
| 18 |
Depending on the commercial success of ContraPest, we may require additional capital to fund our operations. Failure to obtain this necessary capital if needed may force us to delay, limit, or terminate our product development efforts or other operations.
Developing product candidates, including conducting experiments and field studies, obtaining and maintaining regulatory approval and commercializing any products later approved for sale, is a time-consuming, expensive and uncertain process that takes years to complete. We expect our expenses to continue to increase in connection with our ongoing activities, particularly as we advance our commercialization activities.
Based upon our current operating plan, we expect that our cash and cash equivalents of approximately $11.8 million as of December 31, 2016, will be sufficient to fund our current operations for at least the next 12 months. However, we plan to substantially expand our operations, and as a result of many factors, some of which may be currently unknown to us, our expenses may be higher than expected.
Securing additional financing may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates, including ContraPest. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. If we are unable to raise additional capital when required or on acceptable terms, we may be required to:
| • | Significantly delay, scale back or discontinue the development or commercialization of our product candidates, including ContraPest; |
| • | Seek strategic partners for the manufacturing, sales and distribution of ContraPest or any of our other product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; and |
| • | Relinquish, or license on unfavorable terms, our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves. |
The occurrence of any of the events described above would have a material adverse effect on our business, operating results and prospects and on our ability to develop our product candidates.
If we are unable to continue as a going concern, our securities will have little or no value.
We have incurred operating losses since our inception, and we expect to continue to incur significant expenses and operating losses for the foreseeable future. If we encounter significant issues or delays in the launch of ContraPest, these prior losses and expected future losses could have an adverse effect on our financial condition, negatively impact our ability to fund continued operations, our ability to obtain additional financing in the future and our ability to continue as a going concern. There are no assurances that such financing, if necessary, will be available to us at all or will be available in sufficient amounts or on reasonable terms. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. Although we have raised additional capital since December 31, 2015 through private offerings of our equity securities and our initial public offering of 1,875,000 shares of our common stock in December, 2016, if we are unable to generate additional funds in the future through financings, sales of our products, licensing fees, royalty payments, or from other sources or transactions, we will exhaust our resources and will be unable to continue operations. If we cannot continue as a going concern, our stockholders would likely lose most or all of their investment in us.
| 19 |
Raising additional capital may cause dilution to our existing stockholders, restrict our operations, or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate sufficient product revenues, we expect to finance our cash needs primarily through the sale of equity securities, debt financings, credit facilities and government and foundation grants. We may also seek to raise capital through third-party collaborations, strategic alliances and similar arrangements. We currently do not have any committed external source of funds. Raising funds in the future may present additional challenges and future financing may not be available in sufficient amounts or on terms acceptable to us, if at all. The terms of any financing arrangements we enter into may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible debt securities would dilute all of our stockholders. The incurrence of indebtedness through credit facilities would result in increased fixed payment obligations and, potentially, the imposition of restrictive covenants. Those covenants may include limitations on our ability to incur additional debt, making capital expenditures or declaring dividends, and may impose limitations on our ability to acquire, sell, or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through collaborations, strategic alliances, or licensing arrangements or other marketing or distribution arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to expand our operations or otherwise capitalize on our business opportunities, our business, financial condition and results of operations could be materially adversely affected. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts, or grant others rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Risks Relating to the Development and Regulatory Approval of Our Product Candidates
Our future success is dependent on the regulatory approval and commercialization of ContraPest and any of our other product candidates.
The EPA granted registration approval for ContraPest effective August 2, 2016, but we must still obtain applicable state approval and will also seek regulatory approval in other jurisdictions. As a result, our near-term prospects, including our ability to finance our operations and generate revenue, are substantially dependent on our ability to obtain sufficient regulatory approval for ContraPest, and, if approved, to successfully commercialize ContraPest. We cannot commercialize our product candidates in the U.S. without first obtaining regulatory approval for each product and each use pattern from the EPA or, if applicable, the Food and Drug Administration, or FDA, and from any related applicable state authorities. Before obtaining regulatory approvals for the commercial sale of any product candidate for a target indication, the law requires that applicants demonstrate through laboratory and field studies and related data that the product candidate will perform its intended function without causing unreasonable adverse effects on the environment. The EPA or a comparable foreign regulatory authority may require more information, including additional data to support approval that may delay or prevent approval.
Regulatory approval processes of the EPA and comparable foreign regulatory authorities are lengthy, time-consuming and unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business may fail.
Although we obtained EPA approval for ContraPest in less than one year, the EPA review process for a product with one or more new active ingredients typically takes approximately two years to complete and approval is never guaranteed. Our other product candidates could fail to receive marketing approval from the EPA or, with respect to ContraPest or our other product candidates, from a comparable foreign regulatory authority for many reasons, including:
| • | Disagreement over the design or implementation of our trials; |
| • | Failure to demonstrate a product candidate that is safe; |
| • | Failure to demonstrate a product candidate’s benefits outweigh its risks; |
| • | Disagreement over our interpretation of data; |
| • | Disagreement over whether to accept efficacy results from trials; |
| • | The insufficiency of data collected from trials of ContraPest or our other product candidates to obtain regulatory approval; |
| • | Irreparable or critical compliance issues relating to our manufacturing process; or |
| 20 |
| • | Changes in the approval policies or regulations that render our data insufficient for approval. |
Any of these factors, some of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market any of our product candidates. Any such setback in our pursuit of regulatory approval would have a material adverse effect on our business and prospects.
Even following receipt of any regulatory approval for ContraPest and our other product candidates, we will continue to face extensive regulatory requirements and our products may face future development and regulatory difficulties.
Even following receipt of any regulatory approval for ContraPest or our product candidates, such products will be subject to ongoing requirements by the EPA and comparable state and foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping, and reporting of safety and other post-market information. The safety profile of any product will continue to be closely monitored by the EPA and comparable foreign regulatory authorities after approval. If the EPA or comparable foreign regulatory authorities become aware of new safety information after approval of ContraPest or any other product candidate, a number of potentially significant negative consequences could result, including:
| • | We may be forced to suspend marketing of such product; |
| • | Regulatory authorities may withdraw their approvals of such product after certain procedural requirements have been met; |
| • | Regulatory authorities may require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of such product; |
| • | The EPA or other regulatory bodies may issue safety alerts, press releases, or other communications containing warnings about such product; |
| • | The EPA may require the establishment or modification of restricted use or a comparable foreign regulatory authority may require the establishment or modification of a similar strategy that may, for instance, restrict distribution of our product and impose burdensome implementation requirements on us; |
| • | We may be required to change the way the product is administered or conduct additional trials; |
| • | We could be sued and held liable for harm caused; |
| • | We may be subject to litigation or product liability claims; and |
| • | Our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
Moreover, existing government regulations may change and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of ContraPest or any other product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and/or be subject to fines or enhanced government oversight and reporting obligations, which would adversely affect our business, prospects, and ability to achieve or sustain profitability.
Even following receipt of any regulatory approval for ContraPest and our other product candidates, we will continue to be subject to regulation of our manufacturing processes and advertising practices.
Manufacturers of pest control products are subject to continual government oversight and periodic inspections by the EPA and other regulatory authorities. If we or a regulatory agency discover problems with a facility where the product is manufactured, a regulatory agency may impose restrictions on the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing until certain procedural requirements have been met. The occurrence of any such event or penalty could limit our ability to market ContraPest or any other product candidates and generate revenue.
| 21 |
In addition, the EPA strictly regulates the advertising and promotion of pest control products, and these pest control products may only be marketed or promoted for their EPA approved uses, consistent with the product’s approved labeling. Advertising and promotion of any product candidate that obtains approval in the U.S. will be heavily scrutinized by the EPA, other applicable state regulatory agencies and the public. Violations, including promotion of our products for unapproved or off-label uses, are subject to enforcement actions, inquiries and investigations, and civil, criminal and/or administrative sanctions imposed by the EPA.
Failure to obtain regulatory approval in foreign jurisdictions would prevent ContraPest or any other product candidates from being marketed in those jurisdictions.
To market and sell our products globally, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain EPA approval. Obtaining foreign regulatory approvals and maintaining compliance with foreign regulatory requirements could result in significant delays, difficulties, and cost for us and could delay or prevent the introduction of our products in certain countries. Approval by the EPA does not ensure approval by regulatory authorities in other countries or jurisdictions, but EPA approval may influence decisions by the foreign regulatory authority. If we are unable to obtain approval of ContraPest or for any of our other product candidates by regulatory authorities in the world market, the commercial prospects of that product candidate may be significantly diminished and our business prospects could decline.
Risks Relating to Our Dependence on Third Parties
We do not currently have internal full-scale manufacturing capability and we must rely upon third parties to manufacture our products or develop our own full-scale manufacturing capability.
Our existing internal manufacturing platform is adequate for meeting our current demand for ContraPest, and is being expanded to meet further anticipated demand. We may be required to spend significant time and resources to expand these manufacturing facilities to fully meet demand. If we are unable to develop our own full-scale manufacturing capabilities, we may not be able to meet demand of our products, and our business plan could fail.
If a current or future strategic partner terminates or fails to perform its obligations under an agreement with us, the development and commercialization of our product candidates could be delayed or terminated.
We are currently party to various production, marketing and distribution arrangements, including strategic partnership agreements with Bioceres and NeoVenta. Partnership agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. If our partners do not devote sufficient time and resources to their strategic arrangement with us, we may not realize the potential commercial benefits of the arrangement, and our results of operations may be materially adversely affected.
Much of the potential revenue from our current and future strategic partnerships may consist of contingent payments, such as payments for achieving regulatory milestones or royalties payable on sales of our products. The milestone and royalty revenue that we may receive under these partnerships will depend upon our partners’ ability and willingness to successfully develop, introduce, market and sell ContraPest and any other product candidates for which we receive regulatory approval. Our partners may fail to develop or effectively commercialize products using our products or technologies because they:
| • | Decide not to devote the necessary resources due to internal constraints, such as limited personnel with the requisite expertise, limited cash resources or specialized equipment limitations, or the belief that other development programs may have a higher likelihood of obtaining marketing approval or may potentially generate a greater return on investment; |
| • | Decide to pursue other technologies or develop other product candidates, either on their own or in collaboration with others, including our competitors, to treat the same problems targeted by our own products; |
| 22 |
| • | Do not have sufficient resources necessary to carry the product candidate through development, marketing approval and commercialization; or |
| • | Cannot obtain the necessary regulatory approvals. |
Competition for our products and market forces in general may negatively impact any of our partners’ focus on and commitment to our relationship and, as a result, could delay or otherwise negatively affect the commercialization of our products, which would have a material adverse effect on our operating results and financial condition.
We face a number of challenges in seeking future strategic partnerships. Strategic partnerships are complex and any potential discussions may not result in a definitive agreement for many reasons. For example, whether we reach a definitive agreement for a future partnership will depend, among other things, upon our assessment of the potential partner’s resources and expertise, the terms and conditions of the proposed partnership, and the proposed partnership’s evaluation of a number of factors, such as the design or results of our field studies, the potential market for our product candidates, the costs and complexities of manufacturing and delivering our product candidates to customers, the potential of competing products, the existence of uncertainty with respect to ownership or the coverage of our intellectual property, and industry and market conditions generally. If we determine that additional partnerships for our product candidates are necessary and are unable to enter into such partnerships on acceptable terms, we might elect to delay or scale back the development or commercialization of our product candidates in order to preserve our financial resources or to allow us adequate time to develop the required physical resources and systems and expertise ourselves.
Risks Relating to Our Business Operations and Industry
We will need to expand our operations and grow the size of our organization, and we may experience difficulties in managing this growth.
As of December 31, 2016, we had 27 full-time and two part-time employees. As our development and commercialization plans and strategies develop, or as a result of any as yet unforeseen acquisitions, we will need additional managerial, operational, sales, marketing, scientific, financial headcount, and other resources. Our management, personnel, and systems currently in place may not be adequate to support this future growth. Future growth would impose significant added responsibilities on members of management, including:
| • | Managing our trials effectively, which we anticipate being conducted at numerous field study sites; |
| • | Identifying, recruiting, maintaining, motivating and integrating additional employees with the expertise and experience we will require; |
| • | Managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties; |
| • | Managing additional relationships with various strategic partners, suppliers, and other third parties; |
| • | Improving our managerial, development, operational, marketing, production, and finance reporting systems and procedures; and |
| • | Expanding our facilities. |
Our failure to accomplish any of these tasks could prevent us from successfully growing our business.
| 23 |
We depend on key personnel to operate our business. If we are unable to retain, attract, and integrate qualified personnel, our ability to develop and successfully grow our business could be harmed.
We believe that our future success is highly dependent on the contributions of our significant employees, as well as our ability to attract and retain highly skilled and experienced sales, research and development, and other personnel in the U.S. and abroad. All of our employees, including our co-founders (one of which is also our chief executive officer), are free to terminate their employment relationship with us at any time, subject to any applicable notice requirements, and their knowledge of our business and industry would be difficult to replace. If one or more of our co-founders, executive officers or significant employees terminates his or her employment or becomes disabled or experiences long-term illness, we may not be able to replace their expertise, fully integrate new personnel or replicate the prior working relationships, and the loss of their services might significantly delay or prevent the achievement of our research, development and business objectives. Qualified individuals with the breadth of skills and experience in our industry that we require are in high demand, and we may incur significant costs to attract them. Many of the other companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Additionally, our facilities are located in Arizona, which may make attracting and retaining qualified scientific and technical personnel from outside of Arizona difficult. Our failure to attract or retain key personnel could impede the achievement of our research, development, and commercialization objectives.
We have not fully assessed our internal control over financial reporting. We have previously identified and may in the future identify material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
In connection with the preparation of our consolidated financial statements as of and for the year ended December 31, 2015, we identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. We have addressed and resolved the issues identified in 2015.
We are in the process of implementing measures designed further to improve our internal control over financial reporting, including how to remediate the control deficiencies that led to our previously identified material weaknesses, including:
| · | the appointment of a Corporate Controller in May 2016; |
| · | the establishment of formalized accounting policies and procedures and internal controls; and |
| · | the implementation of manual and automated controls to support our overall control environment and the segregation of duties and procedures. |
We have not been required to and have not filed an annual report for fiscal year 2016, so pursuant to SEC regulations, we are not yet required to evaluate the effectiveness of our internal control over financial reporting. Similarly, because we are an emerging growth company, we are not required to include an auditor attestation report on our internal control over financial reporting in this annual report. As a result, we have not fully assessed our internal control over financial reporting and are unable to assure that the measures we have taken to date, together with any measures we may take in the future, will be sufficient to remediate the control deficiencies that led to our material weaknesses in our internal control over financial reporting, or to avoid potential future material weaknesses.
If we are unable to maintain an effective system of internal control over financial reporting, successfully remediate any existing or future material weaknesses in our internal control over financial reporting, or identify any additional material weaknesses, the accuracy and timing of our financial reporting may be adversely affected, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to Nasdaq listing requirements, investors may lose confidence in our financial reporting, and our stock price may decline as a result.
We may be subject to legal proceedings in the ordinary course of our business that could result in significant harm to our business, financial condition and operating results.
We could be subject to legal proceedings and claims from time to time in the ordinary course of our business, including actions arising from tort, contract or other claims. Litigation is expensive, time consuming, and could divert management’s attention away from running our business. The outcome of litigation or other proceedings is subject to significant uncertainty, and it is possible that an adverse resolution of one or more such proceedings could result in reputational harm and/or significant monetary damages, injunctive relief or settlement costs that could adversely affect our results of operations or financial condition as well as our ability to conduct our business as it is presently being conducted. Insurance might not cover such claims, might not provide sufficient payments to cover all the costs to resolve one or more such claims, and might not be available on terms acceptable to us. In addition, regardless of merit or outcome, claims brought against us that are uninsured or underinsured could result in unanticipated costs, which could harm our business, financial condition and operating results and reduce the trading price of our stock.
| 24 |
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the use of ContraPest and any of our other products. If we cannot successfully defend ourselves against claims from our product users, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | Decreased demand for any product that we may develop; |
| • | Termination of field studies or other research and development efforts; |
| • | Injury to our reputation and significant negative media attention; |
| • | Significant costs to defend the related litigation; |
| • | Substantial monetary awards to plaintiffs; |
| • | Loss of revenue; |
| • | Diversion of management and scientific resources from our business operations; and |
| • | The inability to commercialize our product candidates. |
We may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. Large judgments have been awarded in class action lawsuits based on products that had unanticipated side effects, including, without limitation, any potential adverse effects of our products on humans or other species. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
Business disruptions, including supply-chain disruptions, could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to a variety of potential business disruptions, including power shortages, telecommunications failures, water shortages, floods, fires, earthquakes, extreme weather conditions, medical epidemics and other natural or man-made disasters or other interruptions, for which we are predominantly self-insured. We do not carry insurance for all categories of risk that our business may encounter. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. Moreover, we rely on various third parties to supply various ingredients and other items which are critical for producing our product candidates. Our ability to produce our product candidates would be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. The ultimate impact on our operations from any business interruption impacting us or any of our significant suppliers is unknown, but our operations and financial condition would likely suffer adverse consequences. Further, any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our business, results of operations, financial condition, and cash flows from future prospects.
We are dependent on a key ingredient for ContraPest, triptolide, which has limited sources and must be in a very refined condition.
If we are unable to develop additional sources of triptolide, which is one of the key ingredients for ContraPest, the long term ability to produce ContraPest at a cost effective price could be in jeopardy; the limited sources could restrict our production if supplies were reduced; another use of the ingredient could cause the price to increase beyond our ability to market at a competitive price; and increased demand for the ingredient could cause the quality of the refined ingredient to be less than needed for our production.
| 25 |
A variety of risks associated with marketing our product candidates internationally could materially adversely affect our business.
We plan to seek regulatory approval of our product candidates outside of the U.S. and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
| • | Differing regulatory requirements in foreign countries; |
| • | Unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements; |
| • | Economic weakness, including inflation or political instability in particular foreign economies and markets; |
| • | Compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | Foreign taxes, including withholding of payroll taxes; |
| • | Foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| • | Difficulties staffing and managing foreign operations; |
| • | Workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | Potential liability under the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, or comparable foreign regulations; |
| • | Challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the United States; |
| • | Production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | Business interruptions resulting from geo-political actions, including war and terrorism. |
These and other risks associated with our international operations may materially adversely affect our ability to attain or maintain profitable operations.
We are subject to anti-corruption and anti-money laundering laws with respect to our operations and non-compliance with such laws can subject us to criminal and/or civil liability and harm our business.
We are subject to the FCPA, which is the U.S. domestic bribery statute contained in 18 U.S.C. §201, the U.S. Travel Act, the USA PATRIOT Act and possibly other anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees and third-party intermediaries from authorizing, offering or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. As we commercialize our product candidates and eventually commence international sales and business, we may engage with collaborators and third-party intermediaries to sell our products abroad and to obtain necessary permits, licenses and other regulatory approvals. We or our third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities. We can be held liable for the corrupt or other illegal activities of these third-party intermediaries, our employees, representatives, contractors, partners and agents, even if we do not explicitly authorize such activities.
Noncompliance with anti-corruption and anti-money laundering laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension and/or debarment from contracting with certain persons, the loss of export privileges, reputational harm, adverse media coverage and other collateral consequences. Responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees.
| 26 |
Risks Related to Protecting Our Intellectual Property
If we are unable to obtain or protect intellectual property rights, our competitive position could be harmed.
We depend on our ability to protect our proprietary technology. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing, and other agreements with employees and third parties, all of which offer only limited protection. Our commercial success will depend in part on our ability to obtain and maintain intellectual property protection in the United States and other countries with respect to our proprietary technology and products. Where we deem appropriate, we seek to protect our proprietary position by filing patent applications in the U.S. and abroad related to our novel technologies and products that are important to our business. Patent positions of companies generally are highly uncertain, involve complex legal and factual questions and have, in recent years, been the subject of much litigation. As a result, the issuance, scope, validity, enforceability, and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain.
The steps we have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the U.S. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we are unable to obtain and maintain protection for our technology and products, or if the scope of the protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights, we do not know whether any of our pending patent applications for any of our technologies or products will result in the issuance of patents that protect such technologies or products, or if our licensed patent will effectively prevent others from commercializing competitive technologies and products. Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Further, the examination process may require us to narrow the claims for our pending patent applications, which may limit the scope of patent protection that may be obtained if these applications issue. Because the issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, issued patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents, or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products. Protecting against the unauthorized use of our patented technology, trademarks, and other intellectual property rights, is expensive, difficult, and in some cases, may not be possible. In some cases, it may be difficult or impossible to detect third-party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
Intellectual property rights do not necessarily address all potential threats to any competitive advantage we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | Others may be able to make compounds that are the same as or similar to our future products but that are not covered by the claims of the patents that we own or have exclusively licensed; |
| • | We or any of our licensors or strategic partners might not have been the first to file patent applications covering certain of our inventions; |
| • | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing on our intellectual property rights; |
| • | Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; |
| 27 |
| • | Our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| • | We may not develop additional proprietary technologies that are patentable; and |
| • | The patents of others may have an adverse effect on our business. |
Our technology may be found to infringe third-party intellectual property rights.
Third parties may in the future assert claims or initiate litigation related to their patent, copyright, trademark and other intellectual property rights in technology that is important to us. The asserted claims and/or litigation could include claims against us, our licensors, or our suppliers alleging infringement of intellectual property rights with respect to our product candidates or components of those products. Regardless of the merit of the claims, they could be time consuming, resulting in costly litigation and diversion of technical and management personnel, or require us to develop non-infringing technology or enter into license agreements. We cannot assure you that licenses will be available on acceptable terms, if at all. Furthermore, because of the potential for significant damage awards, which are not necessarily predicable, it is not unusual to find even arguably unmeritorious claims resulting in large settlements. If any infringement or other intellectual property claim made against us by any third party is successful, or if we fail to develop non-infringing technology or license the proprietary rights on commercially reasonable terms and conditions, our business, operating results, and financial condition could be materially adversely affected.
If our product candidates, methods, processes, and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and we may have to:
| • | Obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| • | Redesign our product candidates or processes to avoid infringement; |
| • | Stop using the subject matter claimed in the patents held by others; |
| • | Pay damages; or |
| • | Defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
We may need to license intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights that are important or necessary to the development of our product candidates. It may be necessary for us to use the patented or proprietary technology of a third party to manufacture or otherwise commercialize our own technology or products, in which case we would be required to obtain a license from such third party. Licensing such intellectual property may not be available or may not be available on commercially reasonable terms, which could have a material adverse effect on our business and financial condition.
Risks Related to Owning Shares of Our Common Stock
Our share price may be volatile, which could subject us to securities class action litigation and prevent you from being able to sell your shares at or above the price at which you acquired it.
Our stock could be subject to wide fluctuation in response to many risk factors listed in this section, and others beyond our control, including:
| • | Results and timing of our submissions with the EPA and other comparable regulatory authorities; |
| 28 |
| • | Failure or discontinuation of any of our development programs; |
| • | Regulatory developments or enforcements in the U.S. and non-U.S. countries with respect to our products or our competitors’ products; |
| • | Failure to achieve pricing acceptable to the market; |
| • | Regulatory actions with respect to our products or our competitors’ products; |
| • | Actual or anticipated fluctuations in our financial condition and operating results; |
| • | Competition from existing products or new products that may emerge; |
| • | Announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations, or capital commitments; |
| • | Issuance of new or updated research or reports by securities analysts; |
| • | Fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | Share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | Additions or departures of key management or scientific personnel; |
| • | Disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; |
| • | Entry by us into any material litigation or other proceedings; |
| • | Announcement or expectation of additional financing efforts; |
| • | Sales of our common stock by us, our insiders, or our other stockholders; |
| • | Market conditions for stocks in general; and |
| • | General economic and market conditions unrelated to our performance. |
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions such as recessions, interest rate changes, or international currency fluctuations, may negatively impact the market price of shares of our common stock. In addition, such fluctuations could subject us to securities class action litigation, which could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business. You may not realize any return on your investment in us and may lose some or all of your investment.
An active market in the shares may not continue to develop in which investors can resell our common stock.
We cannot predict the extent to which an active market for our common stock will continue to develop or be sustained, or how the development of such a market might affect the market price for our common stock. Market conditions in effect at the time you acquire our stock may not be indicative of the price at which our common stock will trade in the future. Investors may not be able to sell their common stock at or above the price they acquired it.
| 29 |
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. We cannot assure that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our share price would likely decline. If one or more of these analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
Future sales, or the possibility of future sales, of a substantial number of our common shares could adversely affect the price of the shares and dilute stockholders.
Future sales of a substantial number of our common shares, or the perception that such sales will occur, could cause a decline in the market price of our common shares. As of March 29, 2017, we have 10,161,042 common shares outstanding. Each of our directors and executive officers and certain of our other security holders are subject to certain lock-up agreements which expire in June, 2017. If, after the end of such lock-up agreements, these stockholders sell substantial amounts of common shares in the public market, or the market perceives that such sales may occur, the market price of our common shares and our ability to raise capital through an issue of equity securities in the future could be adversely affected.
In addition, in the future, we may issue additional common shares or other equity or debt securities convertible into common shares in connection with a financing, acquisition, litigation settlement, employee arrangements, or otherwise. Any such issuance could result in substantial dilution to our existing stockholders and could cause our common share price to decline.
We are an “emerging growth company” as that term is used in the JOBS Act, and we intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors and adversely affect the market price of our common stock or make it more difficult to raise capital as and when we need it.
We are an “emerging growth company” as that term is used in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved, and exemptions from any rules that the Public Company Accounting Oversight Board may adopt requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. We currently intend to take advantage of some, but not all, of the reduced regulatory and reporting requirements that will be available to us under the JOBS Act, so long as we qualify as an “emerging growth company.” For example, so long as we qualify as an “emerging growth company,” we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would have otherwise been required to provide in filings we make with the SEC, which may make it more difficult for investors and securities analysts to evaluate us.
We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which in certain circumstances could be for up to five years.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company,” we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our business, results of operations, financial condition and cash flows, and future prospects may be materially and adversely affected.
| Item 1B. | Unresolved Staff Comments. |
Not applicable.
| 30 |
| Item 2. | Properties. |
As of December 31, 2016, our corporate headquarters are located in Flagstaff, Arizona, where we lease and occupy 17,797 square feet of office and industrial space pursuant to a lease that commenced on December 20, 2011 and expires on December 31, 2019. Our manufacturing facility is located within our corporate headquarters, occupying 4,865 square feet of the total space. On November 16, 2016, we leased an additional 25,000 square feet of research and development space, also in Flagstaff, This lease expires on November 15, 2018. We believe that our existing facilities are adequate and meet our current needs for business, manufacturing and research and development.
| Item 3. | Legal Proceedings. |
As previously disclosed in our Current Report on Form 8-K dated and filed January 23, 2017, on January 23, 2017 we entered into an agreement (the “Settlement Agreement”) with Neogen Corporation (“Neogen”). Pursuant to the Settlement Agreement, the parties agreed to (a) terminate the existing Exclusive License Agreement between us and Neogen dated May 15, 2014 (the “License Agreement”), with neither Neogen or us having any further obligations thereunder (other than certain confidentiality obligations); (b) dismiss with prejudice the court action filed by Neogen in the District Court for the District of Arizona on January 19, 2017 (the “Court Action”), as further described below; and (c) mutually release any and all existing or future claims between the parties and their affiliates related to or arising from the License Agreement or the Court Action. As part of the Settlement Agreement, we agreed to pay to Neogen upon the execution of the Settlement Agreement an aggregate of $1,000,000, which includes, in part, reimbursement of payments previously made to the Company by Neogen under the License Agreement.
In the Court Action, Neogen raised claims relating to, among other things, alleged breaches by us of the License Agreement, interference with Neogen’s business, indemnification and misrepresentation. As part of the Settlement Agreement, Neogen agreed to dismiss with prejudice the Court Action and release us and our affiliates from any and all existing or future claims relating to or arising from the License Agreement. Pursuant to the Settlement Agreement, the parties agreed that such agreement is a settlement of all disagreements that have arisen between Neogen and us and that the entry into the Settlement Agreement shall not be construed or considered to be an admission of any liability by either party or of the merits of any or claims that may have been raised between them, including in the Court Action.
| Item 4. | Mine Safety Disclosures. |
Not applicable.
| 31 |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information
Our common stock is traded on the NASDAQ Capital Market under the symbol “SNES.” The following table sets forth the high and low closing prices for our common stock for the periods indicated, as reported by the NASDAQ Capital Market. Our common stock was initially listed for trading on the NASDAQ Capital Market on December 8, 2016.
| High | Low | |||||||
| Year Ended December 31, 2016: | ||||||||
| Fourth Quarter (beginning December 8, 2016) | $ | 8.25 | $ | 8.00 | ||||
Holders
As of March 29, 2017, there were 867 holders of record of our common stock. Because many shares of our common stock are held by brokers and other institutions on behalf of shareholders, we are unable to determine the total number of shareholders represented by these holders of record.
Stock Performance Graph
Not applicable.
Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors may deem relevant.
| Item 6. | Selected Financial Data. |
Not applicable.
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with our consolidated financial statements and related notes. This Management’s Discussion and Analysis of Financial Condition and Results of Operations may contain some statements and information that are not historical facts but are forward-looking statements. For a discussion of these forward-looking statements, and of important factors that could cause results to differ materially from the forward-looking statements contained in this report, see Item 1 of Part I, “Business—Cautionary Note Regarding Forward-Looking Statements,” and Item 1A of Part I, “Risk Factors.”
Overview
Since our inception in 2004, we have devoted substantially all of our resources to organizing and staffing our company, conducting research and development activities for our product candidates, business planning, raising capital and acquiring and developing product and technology rights. Until August 2016, we did not have any products approved for sale, and we have not generated any revenue from product sales. We have funded our operations to date with proceeds from the sale of common stock and preferred stock, the issuance of convertible and other promissory notes and, to a lesser extent, payments received in connection with research grants and licensing fees. Through December 31, 2016, we had received net proceeds of $41.8 million from our sales of common stock, preferred stock and issuance of convertible and other promissory notes and an aggregate of $1.6 million from research grants and licensing fees. In December 2015, our outstanding convertible and certain other promissory notes and accrued interest thereon, aggregating $2.9 million, were exchanged for shares of Series B convertible preferred stock.
| 32 |
On December 8, 2016, immediately prior to our public offering of 1,875,000 shares of our common stock, 883,609 of our Series A and Series B convertible preferred shares were exchanged for 883,609 share of our common stock in connection with our public offering.
We have incurred significant operating losses every year since our inception. Our net losses was $11.0 million and $18.2 million for the years ended December 31, 2016 and 2015 respectively. As of December 31, 2016, we had an accumulated deficit of $61.3 million. We expect to continue to incur significant expenses and generate operating losses for at least the next 12 months.
We have historically utilized, and intend to continue to utilize, various forms of stock-based awards in order to hire, retain and motivate talented employees, consultants and directors and encourage them to devote their best efforts to our business and financial success. In addition, we believe that our ability to grant stock-based awards is a valuable and necessary compensation tool that aligns the long-term financial interests of our employees, consultants and directors with the financial interests of our stockholders.
As a result, a significant portion of our operating expenses includes stock-based compensation expense. Stock-based compensation expense has been, and will continue to be for the foreseeable future, a significant recurring expense in our business and an important part of our compensation strategy. Specifically, our stock-based compensation expense for the year ended December 31, 2016 and December 31, 2015 was $3.4 million and $11.3 million, respectively, which represented 31.4% and 70.9%, respectively, of our total operating expenses for those periods.
Components of our Results of Operations
Revenue
To date, we have generated minimal revenue, less than $1,000, from product sales, but we do expect to generate revenue from the sale of products or royalties beginning in the first quarter of 2017. Except for the minimal product sales noted above, all of our revenue to date has been derived from payments received in connection with research grants and licensing fees received as a result of our execution of the former license agreement with Neogen.
We recognized revenue of $132,000 and $55,000 for the years ended December 31, 2016 and 2015 respectively. In addition, under our former license agreement with Neogen, we recognized revenue of $186,000 for each of the years ended December 31, 2016 and 2015. We do not anticipate additional grant revenue under the NIH grants or additional revenue from our former license agreement with Neogen.
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with the discovery and development of our product candidates, which include:
• Employee-related expenses, including salaries, related benefits, travel and stock-based compensation expense for employees engaged in research and development functions;
• Expenses incurred in connection with the development of our product candidates; and
• Facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and supplies.
We expense research and development costs as incurred.
At this time, we cannot reasonably estimate the costs for completing the development of ContraPest or the cost associated with the development of any of our other product candidates.
| 33 |
We plan to continue to hire employees to support our research and development efforts and anticipate that we will continue to utilize various forms of stock-based compensation awards in order to attract and retain employees for our research and development efforts. As a result, we anticipate that stock-based compensation expense will continue to represent a significant portion of our research and development expenses for the foreseeable future.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs, including stock-based compensation, for personnel in executive, finance and administrative functions. General and administrative expenses also include direct and allocated facility-related costs as well as professional fees for legal, consulting, accounting and audit services.
We anticipate that our general and administrative expenses may increase in the future as we increase our headcount to support commercialization of any approved products and further development of our product candidates. We also anticipate that we will incur increased accounting, audit, legal, regulatory, compliance, director and officer insurance costs as well as investor and public relations expenses associated with being a public company.
We plan to continue to hire employees to support our commercialization of any approved products and further development of our product candidates, and anticipate that we will continue to utilize various forms of stock-based compensation awards in order to attract and retain qualified employees. As a result, we anticipate that stock-based compensation expense will continue to represent a significant portion of our general and administrative expenses for the foreseeable future.
Other Income (Expense), Net
Interest Income. Interest income consists primarily of interest income earned on cash and cash equivalents. Our interest income has not been significant due to nominal cash and investment balances and low interest earned on invested balances.
Interest Expense. Interest expense consists of interest accrued on $2.9 million in convertible and other promissory notes we issued during 2014 and 2015 that were exchanged for Series B convertible preferred stock in December 2015.
Other Income (Expense), Net. Other income (expense), net, consists primarily of net losses on extinguishment of convertible and non-convertible, secured and unsecured promissory notes.
Income Taxes
Since our inception, we have not recorded any U.S. federal or state income tax benefits for the net losses we have incurred in each year or for our earned research and development tax credits, due to our uncertainty of realizing a benefit from those items. As of December 31, 2016, we had federal and state net operating loss carryforwards of $34.0 million and $27.8 million, respectively, which begin to expire in 2021 and 2016, respectively, unless previously utilized.
| 34 |
Comparison of the Years December 31, 2016 to 2015
The following table summarizes our results of operations for the years ended December 31, 2016 and 2015:
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| (in thousands) | ||||||||
| Revenue | $ | 318 | $ | 241 | ||||
| Operating expenses: | ||||||||
| Research and development | 2,705 | 7,221 | ||||||
| General and administrative | 8,129 | 8,665 | ||||||
| Total operating expenses | 10,834 | 15,886 | ||||||
| Loss from operations | (10,516 | ) | (15,645 | ) | ||||
| Interest expense | (87 | ) | (855 | ) | ||||
| Loss on extinguishment debt | (161 | ) | (995 | ) | ||||
| Other income (expense), net | (31 | ) | (678 | ) | ||||
| Net loss | $ | (10,795 | ) | $ | (18,173 | ) | ||
Revenue
Revenue was $318,000 for the year ended December 31, 2016, compared to $241,000 for the year ended December 31, 2015.
We recognized revenue of $132,000 and $55,000 for the years ended December 31, 2016 and 2015, respectively, as a result of the services performed.
Under our former license agreement with Neogen, we recognized revenue of $186,000 for each year ended December 31, 2016 and 2015, respectively.
Research and Development Expenses
| Year Ended December 31, | Increase (Decrease) | |||||||||||
| 2016 | 2015 | |||||||||||
| (in thousands) | ||||||||||||
| Direct research and development expenses: | ||||||||||||
| Unallocated expenses: | ||||||||||||
| Personnel related (including stock-based compensation) | $ | 2,016 | $ | 5,965 | $ | (3,949 | ) | |||||
| Facility related | 218 | 190 | 28 | |||||||||
| Other | 471 | 1,066 | (595 | ) | ||||||||
| Total research and development expenses | $ | 2,705 | $ | 7,221 | $ | (4,516 | ) | |||||
| 35 |
Research and development expenses were $2.7 million for the year ended December 31, 2016, compared to $7.2 million for the year ended December 31, 2015. The $4.5 million decrease in research and development expenses was primarily due to an decrease of $4.0 million in personnel-related costs. This decrease in personnel-related costs resulted from a decrease in stock-based compensation expense of $4.5 million and increased research and development salaries of $500,000 due to five headcount additions in 2015. Manufacturing costs decreased from $310,000 for the year ended December 31, 2015 to $99,000 for the year ended December 31, 2016. This $210,000 decrease was primarily associated with decreased bait-box design and production costs during 2016. Further, other general research and development expenses for activities not directly associated with our principal research and development programs decreased by $240,000 during 2016 primarily as a result of reduced laboratory related expenses.
We continue to investigate other applications of our core technology to other product candidates, which includes laboratory tests and academic collaborations. We also continue to develop our supply chain, particularly identifying and improving our sourcing of triptolide, a key active ingredient for our product candidates.
General and Administrative Expenses
General and administrative expenses were $8.1 million for the year ended December 31, 2016, compared to $8.6 million for the year ended December 31, 2015. The decrease of $500,000 in general and administrative expenses was due to a decrease of $2.8 million in personnel-related costs, including a reduction of $3.3 million in stock-based compensation expense during 2016 offset by a $1.0 million for a contract cancellation settlement, $500,000 in additional salary costs, an increase of $400,000 in legal, accounting and audit-related fees, an increase of $270,000 in travel expenses and $140,000 in increased EPA registration fees.
Interest Expense
We recorded $87,000 of interest expense for the year ended December 31, 2016, compared to $855,000 for the year ended December 31, 2015. The decrease in interest expense of $768,000 was a result of a decrease of $2.9 million of convertible notes that were issued in 2014 and exchanged for Series B convertible preferred stock in December of 2016.
Other Income (Expense), Net
We recorded $31,000 of other expense, net, for the year ended December 31, 2016, compared to $678,000 for the year ended December 31, 2015. The $647,000 net decrease in other expense was primarily due to the expense related to the year-over-year fair market value adjustment of our convertible promissory notes.
Liquidity and Capital Resources
Since our inception, we have incurred significant operating losses. We have generated limited revenue to date from research grants and licensing fees received under our former license agreement with Neogen. We have not yet begun full scale marketing of our first product, ContraPest and we continue to develop other product candidates, which are in various phases of development. We have funded our operations to date primarily with proceeds from the sale of common stock and preferred stock, the issuance of convertible and other promissory notes and, to a lesser extent, payments received under research grants and pursuant to our former license agreement with Neogen. Through December 31, 2016, we had received net proceeds of $41.8 million from our sales of common stock and preferred stock and issuance of convertible and other promissory notes, and an aggregate of $1.6 million from licensing fees.
| 36 |
In the course of our research and development activities, we have sustained operating losses since our inception and expect such losses to continue for the foreseeable future. Our ultimate success depends upon the outcome of a combination of factors, including our ability to: (i) engage in successful research and development efforts; (ii) obtain regulatory approval of ContraPest and our other product candidates; (iii) achieve market acceptance and commercialization of ContraPest and our other products; (iv) successfully market our products and establish an effective sales force and marketing infrastructure to generate significant revenue; (v) retain and attract key personnel to develop, operate and grow our business; and (vi) successfully obtain additional financing as needed. As of December 31, 2016, we had an accumulated deficit of $61.3 million.
From time to time in 2014 and 2015, members of our management have provided financing to us in the form of promissory notes totaling $4.0 million, of which $0 remained outstanding as of December 31, 2016. In November 2015, we issued to NAU Ventures 400,000 shares of Series A convertible preferred stock (on a post-reverse split basis), valued at $4.4 million, and a warrant, valued at $330,000, in exchange for full cancellation of the outstanding principal and unpaid accrued interest on a promissory note, totaling $3.2 million. In December 2015, the principal amount under our convertible and other promissory notes and accrued interest (aggregating $2.9 million) were exchanged for shares of Series B convertible preferred stock.
Based upon our current operating plan, we expect that our cash and cash equivalents of approximately $11.8 million as of December 31, 2016, will be sufficient to fund our current operations for at least the next 12 months. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect.
Funding Requirements
We expect our expenses to increase substantially in connection with our ongoing activities, particularly as we advance field studies of our product candidates in development. In addition, we incur additional costs associated with operating as a public company.
In particular, we expect to incur substantial and increased expenses as we:
| • | Continue the research and development of ContraPest and our other product candidates, including engaging in any necessary field studies; |
| • | Seek regulatory approvals for ContraPest and our other product candidates; |
| • | Scale up manufacturing processes and quantities to prepare for the commercialization of ContraPest and any other product candidates for which we receive regulatory approval; |
| • | Establish an infrastructure for the sales, marketing and distribution of ContraPest and any other product candidates for which we may receive regulatory approval; |
| • | Attempt to achieve market acceptance for our products; |
| • | Expand our research and development activities and advance the discovery and development programs for other product candidates; |
| • | Maintain, expand and protect our intellectual property portfolio; and |
| • | Add operational, financial and management information systems and personnel, including personnel to support our product development and commercialization efforts and operations as a public company. |
| 37 |
Cash Flows
The following table summarizes our sources and uses of cash for each of the periods presented:
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Cash used in operating activities | $ | (6,696 | ) | $ | (3,666 | ) | ||
| Cash used in investing activities | (57 | ) | (130 | ) | ||||
| Cash provided by financing activities | 18,438 | 3,116 | ||||||
| Net increase (decrease) in cash and cash equivalents | $ | 11,685 | $ | (680 | ) | |||
Operating Activities.
During the year ended December 31, 2016, operating activities used $6.7 million of cash, primarily resulting from our net loss of $10.8 million, partially offset by non-cash charges of $3.8 million and by changes in our operating assets and liabilities of $342,000. Our net loss was primarily attributed to research and development activities and our general and administrative expenses, as we generated limited research grant and licensing revenue during the period. Net cash used by changes in our operating assets and liabilities for the year ended December 31, 2016 consisted primarily of an increase in prepaid expenses of $301,000, a $221,000 decrease in deferred revenue related to our former license agreement with Neogen, an increase in inventories of $57,000 and an increase in deposits of $3,000, partially offset by a $916,000 increase in accrued expenses and accounts payable, an increase in deferred rents and accounts receivable of $8,000. The decrease in accrued expenses and accounts payable was due to increased payments of accrued expenses and accounts payable as a result of the receipt of cash raised in financing activities.
During the year ended December 31, 2015, operating activities used $3.7 million of cash, primarily resulting from our net loss of $18.2 million, partially offset by non-cash charges of $13.9 million and by cash provided by changes in our operating assets and liabilities of $648,000. Our net loss was primarily attributed to research and development activities and our general and administrative expenses, as we generated limited research grant and licensing revenue during the year. Net cash provided by changes in our operating assets and liabilities for the year ended December 31, 2015 consisted primarily of a $151,000 decrease in deferred revenue related to our former license agreement with Neogen offset by a $779,000 increase in accounts payable and accrued expenses and a decrease of accounts receivable of $18,000. The increase in accounts payable and accrued expenses was due to increased spending associated with research and development programs as well as the timing of vendor invoicing and payments.
Investing Activities.
During the year ended December 31, 2016 and December 31, 2015, we used $57,000 and $130,000, respectively, of cash in investing activities, in each case consisting of purchases of property and equipment.
Financing Activities.
During the year ended December 31, 2016, net cash provided by financing activities was $18.4 million as a result of $18.8 million of proceeds from the issuance of shares of common stock in our initial public offering and the rights offering discussed elsewhere in this Annual Report on Form 10-K, $896,000 of proceeds received from the issuance of Series B convertible preferred stock, $521,000 of proceeds received from the exercise of stock options and warrants, $326,000 of proceeds received from our issuance of notes payable, all of which were partially offset by payments of $1.9 million related to the notes payable, notes payable related party and convertible notes payable, $2.2 of deferred offering costs, $176,000 of payments of preferred stock dividends and $21,000 in payments of capital lease obligations.
| 38 |
During the year ended December 31, 2015, net cash provided by financing activities was $3.1 million as a result of $3.1 million of proceeds received from our issuance of related party convertible and other promissory notes, $155,000 of proceeds received from the issuance of Series B convertible preferred stock, and $56,000 of proceeds received from the exercise of stock options, all of which were partially offset by payments of $132,000 related to the issuance costs for the convertible promissory notes and $100,000 in repayments of notes payable balances.
Recent Developments
We previously identified material weaknesses in our internal control over financial reporting for the year ended December 31, 2015 which we have addressed and resolved the issues identified for the year ended December 31, 2015. See “Risk Factors —We have not fully assessed our internal control over financial reporting. We have previously identified and may in the future identify material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock.” While we have not yet assessed our internal control over financial reporting, we are in the process of implementing measures designed to further improve our internal control over financial reporting, including how to remediate the control deficiencies that led to our previously identified material weaknesses, including:
| · | the appointment of a Corporate Controller in May 2016; |
| · | the establishment of formalized accounting policies and procedures and internal controls; and |
| · | the implementation of manual and automated controls to support our overall control environment and the segregation of duties and procedures. |
Critical Accounting Policies and Significant Judgments and Estimates
Our financial statements are prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of our financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2 to our audited financial statements included elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
We recognize revenue in accordance with the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”), Topic 605, Revenue Recognition. Accordingly, we recognize revenue from our licensing agreements and contracts to perform pilot studies when (1) persuasive evidence of an arrangement exists; (2) the performance of service has been rendered to a customer or delivery has occurred; (3) the amount of fee to be paid by a customer is fixed and determinable; and (4) the collectability of the fee is reasonably assured.
We have generated revenue from a license agreement with a strategic partner pursuant to which we have granted to such partner an exclusive license in North America to manufacture, distribute and sell commercial control products based on our intellectual property, which includes ContraPest, for the later of 10 years or the expiration of the patent for ContraPest (if issued).
| 39 |
When we receive non-refundable, upfront license fee payments for the exclusive rights to licensing our intellectual property, management determines if such license has stand-alone value. Since management determined that the license to our intellectual property did not have stand-alone value, we recognize revenue attributable to that license on a straight-line basis over the estimated related performance period. Any changes in the estimated period of performance will be accounted for prospectively as a change in estimate.
Our licensing agreement also provides for a future fixed amount of contingent milestone payments and contingent sales-based royalties to be received upon the achievement of milestone events. We recognize revenue that is contingent upon the achievement of a substantive milestone in its entirety in the period in which the milestone is achieved and the milestone payments are due and collectible. A milestone is considered substantive when the consideration payable to us for such milestone has all of the following characteristics: (1) there is substantive uncertainty at the date the arrangement is entered into that the event will be achieved; (2) the event can only be achieved based in whole or part on either our performance or a specific outcome resulting from our performance; and (3) if achieved, the event would result in additional payments being due to us. In making this assessment in the future, we will consider all facts and circumstances relevant to the arrangement, including whether any portion of the milestone consideration is related to future performance or deliverables. In addition, we will account for sales-based royalties as revenue upon achievement of certain sales milestones.
Stock-Based Compensation
We recognize compensation costs related to stock options granted to employees based on the estimated fair value of the awards on the date of grant, net of estimated forfeitures, in accordance with ASC Topic 718 — Stock Compensation (“ASC 718”). We estimate the grant date fair value of the awards, and the resulting stock-based compensation expense, using the Black-Scholes option-pricing model. The grant date fair value of stock-based awards is expensed on a straight-line basis over the vesting period of the respective award. We account for stock-based compensation arrangements with non-employees using a fair value approach. The fair value of these stock options is measured using the Black-Scholes option-pricing model reflecting the same assumptions as applied to employee options in each of the reported periods, other than the expected life, which is assumed to be the remaining contractual life of the option. The fair value of the stock options granted to non-employees is re-measured as the stock options vest and is recognized in the statements of operations and comprehensive loss during the period the related services are rendered.
We recorded stock-based compensation expense of approximately $3.4 million and $11.3 million for the years ended December 31, 2016 and 2015 respectively. We expect to continue to grant stock options and other equity-based awards in the future, and to the extent that we do, our stock-based compensation expense recognized in future periods will likely increase.
The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions, which determine the fair value of stock-based awards. If we had made different assumptions, our stock-based compensation expense, net loss and loss per share of common stock could have been significantly different. Our assumptions are as follows:
| • | Expected term. The expected term represents the period that the stock-based awards are expected to be outstanding. Our historical share option exercise experience does not provide a reasonable basis upon which to estimate an expected term because of a lack of sufficient data. Therefore we estimate the expected term by using the simplified method, which calculates the expected term as the average of the time-to-vesting and the contractual life of the options. |
| • | Expected volatility. Expected volatility is derived from the average historical volatilities of publicly traded companies within our industry that we consider to be comparable to our business over a period approximately equal to the expected term. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own common stock price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation. |
| • | Risk-free interest rate. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to the expected term. |
| • | Expected dividend. The expected dividend is assumed to be zero as we have never paid dividends and have no current plans to pay any dividends on our common stock. |
| 40 |
| • | Expected forfeitures. We use historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. To the extent actual forfeitures differ from the estimates, the difference will be recorded as a cumulative adjustment in the period that the estimates are revised. |
Significant Factors, Assumptions and Methodologies Used in Determining Fair Value of Our Common Stock
As noted above, we are required to estimate the fair value of the common stock underlying our stock-based awards when performing the fair value calculations using the Black-Scholes option-pricing model. In the absence of an active market for our common stock, we utilized methodologies in accordance with the framework of the American Institute of Certified Public Accountants’ Technical Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation , to estimate the fair value of our common stock. In addition, we have conducted periodic assessments of the valuation of our preferred stock and our common stock. Specifically, we obtained a report assessing the fair value of the common stock underlying our Series B convertible preferred stock as of December 31, 2015. This valuation performed a Monte Carlo option model to determine the fair value of the underlying common stock. A Monte Carlo option model is used to calculate the value of an asset with multiple sources of uncertainty or with complicated features. Based on this iterative analysis, and given that the Series B convertible preferred stock was issued at a fair value of $7.75 per share, the fair value of the common stock was determined to be $7.575 per share as of December 31, 2015, which valuation was used by us for purposes of our stock-based compensation calculations during 2015.
The assumptions underlying these valuations represent management’s best estimates, which involve inherent uncertainties and the application of management’s judgment. If we had made different assumptions than those used, the amount of our stock-based compensation expense, net income and net income per share amounts could have been significantly different. The fair value per share of our common stock for purposes of determining stock-based compensation expense is the closing price of our common stock as reported on the applicable grant date. The compensation cost that has been included in the statements of operations and comprehensive loss for all stock-based compensation arrangements is as follows:
| Years Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| (in thousands) | ||||||||
| General and administrative expenses | $ | 2,964 | $ | 6,331 | ||||
| Research and development expense | 403 | 4,931 | ||||||
| Total stock-based compensation expense | $ | 3,367 | $ | 11,262 | ||||
The intrinsic value of stock options outstanding as of December 31, 2016 is $9.7 million, of which $7.2 million and $2.5 million would have been related to stock options that were vested and unvested, respectively, at that date.
| 41 |
Emerging Growth Company Status
The Jumpstart Our Business Startups Act of 2012, or the JOBS Act, permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have irrevocably elected to “opt out” of this provision and, as a result, we intend to comply with new or revised accounting standards when they are required to be adopted by public companies that are not emerging growth companies.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk. |
Not applicable.
| 42 |
| Item 8. | Financial Statements and Supplementary Data. |
SENESTECH, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
and Stockholders of SenesTech, Inc.
We have audited the accompanying balance sheets of SenesTech, Inc. as of December 31, 2016 and 2015, and the related statements of income, comprehensive income, stockholders' equity, and cash flows for each of the years in the two-year period ended December 31, 2016. SenesTech's management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company's internal control over financial reporting. Accordingly, we express no such opin ion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of SenesTech, Inc. as of December 31, 2016 and 2015, and the results of its operations and its cash flows for each of the years in the two-year period ·ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
/s/ M&K CPAS, PLLC
www.mkacpas.com
Houston, Texas
March 31, 2017
| F-2 |
BALANCE SHEETS
(In thousands, except shares and per share data)
| December 31, | December 31, | |||||||
| 2016 | 2015 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 11,826 | $ | 141 | ||||
| Accounts receivable | 10 | 13 | ||||||
| Prepaid expenses | 337 | 36 | ||||||
| Inventory | 57 | - | ||||||
| Total current assets | 12,230 | 190 | ||||||
| Property and equipment, net | 631 | 613 | ||||||
| Deferred offering costs | 9 | 6 | ||||||
| Security deposits | - | 132 | ||||||
| Total assets | $ | 12,870 | $ | 941 | ||||
| LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||
| Current liabilities: | ||||||||
| Short-term debt | $ | 45 | $ | 27 | ||||
| Accounts payable | 351 | 544 | ||||||
| Accrued contract cancellation settlement | 1,000 | - | ||||||
| Accrued expenses | 371 | 758 | ||||||
| Notes payable, related parties | 30 | 462 | ||||||
| Convertible notes payable, related parties | - | 200 | ||||||
| Deferred revenue | - | 221 | ||||||
| Total current liabilities | 1,797 | 2,212 | ||||||
| Notes payable, related parties | 6 | 34 | ||||||
| Long-term debt, net | 138 | 450 | ||||||
| Common stock warrant liability | 69 | 63 | ||||||
| Deferred rent | 33 | 28 | ||||||
| Deferred compensation obligations | - | 2,000 | ||||||
| Total liabilities | 2,043 | 4,787 | ||||||
| Commitments and contingencies (See note 15) | - | - | ||||||
| Series A convertible preferred stock, $0.001 par value, authorized 2,000,000 | ||||||||
| shares; 400,000 shares issued and outstanding at December 31, 2015; | ||||||||
| liquidation preference of $2.017 at December 31, 2015 | - | 4,380 | ||||||
| Series B convertible preferred stock, $0.001 par value, authorized 7,515,000 | ||||||||
| shares; 399,512 shares issued and outstanding at December 31, 2015 | - | 3,096 | ||||||
| Stockholders' equity (deficit): | ||||||||
| Common stock, $0.001 par value, 100,000,000 shares authorized, 10,157,292 and | ||||||||
| 4,108,766 shares issued and outstanding at December 31, 2016 and 2015, respectively | 10 | 4 | ||||||
| Additional paid-in capital | 72,069 | 39,000 | ||||||
| Accumulated other comprehensive income, series A convertible preferred stock dividend | - | 17 | ||||||
| Stock subscribed but not issued | 59 | 14 | ||||||
| Accumulated deficit | (61,311 | ) | (50,357 | ) | ||||
| Total stockholders' equity (deficit) | 10,827 | (11,322 | ) | |||||
| Total liabilities and stockholders' equity (deficit) | $ | 12,870 | $ | 941 | ||||
The accompanying notes are an integral part of these financial statements.
| F-3 |
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except shares and per share data)
| For the Years | ||||||||
| Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Revenue: | ||||||||
| License revenue | $ | 186 | $ | 186 | ||||
| Other revenue | 132 | 55 | ||||||
| Total revenue | 318 | 241 | ||||||
| Operating expenses: | ||||||||
| Research and development | 2,705 | 7,221 | ||||||
| General and administrative | 8,129 | 8,665 | ||||||
| Total operating expenses | 10,834 | 15,886 | ||||||
| Net operating loss | (10,516 | ) | (15,645 | ) | ||||
| Other income (expense): | ||||||||
| Interest expense | (32 | ) | (418 | ) | ||||
| Interest expense, related parties | (55 | ) | (437 | ) | ||||
| (Loss) gain on extinguishment of notes and convertible notes, related parties | (161 | ) | 569 | |||||
| Loss on extinguishment of NAU promissory note | - | (1,530 | ) | |||||
| Loss on extinguishment of secured promissory note | - | (34 | ) | |||||
| Other income (expense) | (31 | ) | (678 | ) | ||||
| Total other income (expense) | (279 | ) | (2,528 | ) | ||||
| Net loss | (10,795 | ) | (18,173 | ) | ||||
| Series A convertible preferred stock dividends | (159 | ) | (17 | ) | ||||
| Net loss and comprehensive loss | $ | (10,954 | ) | $ | (18,190 | ) | ||
| Weighted average common shares outstanding - basic and fully diluted | 6,417,936 | 3,852,349 | ||||||
| Net loss per common share - basic and fully diluted | $ | (1.71 | ) | $ | (4.71 | ) | ||
The accompanying notes are an integral part of these financial statements.
| F-4 |
STATEMENT OF CHANGES IN CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT)
(In thousands, except shares and per share data)
| Accumulated | ||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Convertible | Series B Convertible | Additional | Stock Subscribed | Other | Total | |||||||||||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Common Stock | Paid-In | not Issued | Comprehensive | Accumulated | Stockholders' | |||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Shares | Amount | Loss | Deficit | Equity (Deficit) | |||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2014 | - | $ | - | - | $ | - | 3,629,921 | $ | 4 | $ | 26,281 | 10,496 | $ | 158 | $ | - | $ | (32,167 | ) | $ | (5,724 | ) | ||||||||||||||||||||||||||
| Issuance of series A convertible preferred stock for cancellation of note | 400,000 | 4,380 | - | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Issuance of series A convertible preferred stock for cash | - | - | 20,000 | 155 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Issuance of series B convertible preferred stock for cash | - | - | 379,512 | 2,941 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon conversion of notes | - | - | - | - | 80,417 | - | 610 | - | - | - | - | 610 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for services | - | - | - | - | 100 | - | 1 | 3,250 | 15 | - | - | 16 | ||||||||||||||||||||||||||||||||||||
| Shares of common stock forfeited | - | - | - | - | - | - | - | (3,041 | ) | (47 | ) | - | - | (47 | ) | |||||||||||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options and warrants | - | - | - | - | 390,873 | - | 78 | - | - | - | - | 78 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | 11,262 | - | - | - | - | 11,262 | ||||||||||||||||||||||||||||||||||||
| Warrant issued in connection with cancellation of NAU promissory note | - | - | - | - | - | - | 330 | - | - | - | - | 330 | ||||||||||||||||||||||||||||||||||||
| Recognition of warrants issued with 2014/2015 convertible notes | - | - | - | - | - | - | 97 | - | - | - | - | 97 | ||||||||||||||||||||||||||||||||||||
| Recognition of warrant issued with promissory notes | - | - | - | - | - | - | 229 | - | - | - | - | 229 | ||||||||||||||||||||||||||||||||||||
| Reclassification of subscribed shares to common stock | - | - | - | - | 7,455 | - | 112 | (7,455 | ) | (112 | ) | - | - | - | ||||||||||||||||||||||||||||||||||
| Dividends on preferred shares | - | - | - | - | - | - | - | - | - | 17 | (17 | ) | - | |||||||||||||||||||||||||||||||||||
| Net loss for the year ended December 31, 2015 | - | - | - | - | - | - | - | - | - | - | (18,173 | ) | (18,173 | ) | ||||||||||||||||||||||||||||||||||
| Balance, December 31, 2015 | 400,000 | $ | 4,380 | 399,512 | $ | 3,096 | 4,108,766 | $ | 4 | $ | 39,000 | 3,250 | $ | 14 | $ | 17 | $ | (50,357 | ) | $ | (11,322 | ) | ||||||||||||||||||||||||||
| Issuance of series B convertible preferred stock for cash | - | - | 115,668 | 896 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Issuance of series B convertible preferred stock for conversion of notes | - | - | 2,007 | 16 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon conversion of series A preferred stock | (400,000 | ) | (4,380 | ) | - | - | 400,000 | - | 4,380 | - | - | - | - | 4,380 | ||||||||||||||||||||||||||||||||||
| Issuance of common stock upon conversion of series B preferred stock | - | - | (517,187 | ) | (4,008 | ) | 517,187 | 1 | 4,007 | - | - | - | - | 4,008 | ||||||||||||||||||||||||||||||||||
| Issuance of common stock sold for cash | - | - | - | - | 4,353,486 | 4 | 18,828 | - | - | - | - | 18,832 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for services | - | - | - | - | 126,373 | - | 338 | 5,250 | 45 | - | - | 383 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for services, related parties | - | - | - | - | 13,320 | - | 295 | - | - | - | - | 295 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | 2,689 | - | - | - | - | 2,689 | ||||||||||||||||||||||||||||||||||||
| Forgiveness of accrued liabilities, related party | - | - | - | - | - | - | 2,003 | - | - | - | - | 2,003 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options and warrants | - | - | - | - | 630,935 | 1 | 520 | - | - | - | - | 521 | ||||||||||||||||||||||||||||||||||||
| Recognition of warrants issued with unsecured notes | - | - | - | - | - | - | 9 | - | - | - | - | 9 | ||||||||||||||||||||||||||||||||||||
| Cashless exercise of warrants | - | - | - | - | 7,225 | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Dividends paid on preferred shares | - | - | - | - | - | - | - | - | - | (17 | ) | (159 | ) | (176 | ) | |||||||||||||||||||||||||||||||||
| Net loss for the year ended December 31, 2016 | - | - | - | - | - | - | - | - | - | - | (10,795 | ) | (10,795 | ) | ||||||||||||||||||||||||||||||||||
| Balance, December 31, 2016 | - | $ | - | - | $ | - | 10,157,292 | $ | 10 | $ | 72,069 | 8,500 | $ | 59 | $ | - | $ | (61,311 | ) | $ | 10,827 | |||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
| F-5 |
STATEMENTS OF CASH FLOWS
(In thousands)
| For the Years | ||||||||
| Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (10,795 | ) | $ | (18,173 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 196 | 182 | ||||||
| Stock-based compensation | 2,689 | 11,262 | ||||||
| Shares of common stock issued for services, net of forfeitures | 678 | (10 | ) | |||||
| Common stock warrant issued as compensation | - | 53 | ||||||
| Non-cash interest expense from convertible notes and notes payable | - | 519 | ||||||
| Amortization of debt discount | 27 | 177 | ||||||
| Change in fair value of convertible notes payable, related parties | - | 671 | ||||||
| Loss on remeasurement of common stock warrant liability | 6 | 10 | ||||||
| Loss (gain) on extinguishment of secured convertible promissory note, related parties | - | (569 | ) | |||||
| (Gain) loss on extinguishment of debts, net | 161 | 1,564 | ||||||
| (Increase) decrease in current assets: | ||||||||
| Accounts receivable | 3 | 18 | ||||||
| Prepaid expenses | (301 | ) | (3 | ) | ||||
| Inventory | (57 | ) | - | |||||
| Deposits | (3 | ) | 3 | |||||
| Increase (decrease) in current liabilities: | ||||||||
| Accounts payable | (193 | ) | 393 | |||||
| Accrued expenses | 1,109 | 388 | ||||||
| Deferred rent | 5 | - | ||||||
| Deferred revenues | (221 | ) | (151 | ) | ||||
| Net cash used in operating activities | (6,696 | ) | (3,666 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of property and equipment | (57 | ) | (130 | ) | ||||
| Net cash used in investing activities | (57 | ) | (130 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Dividend payments on preferred stock | (176 | ) | - | |||||
| Proceeds from the issuance of series B convertible preferred stock | 896 | 155 | ||||||
| Proceeds from the issuance of common stock | 18,832 | - | ||||||
| Proceeds from the issuance of convertible notes payable, related parties | 310 | 1,915 | ||||||
| Repayments of convertible notes payable, related parties | (310 | ) | - | |||||
| Proceeds from the issuance of convertible notes payable | 16 | - | ||||||
| Repayments of convertible notes payable | (500 | ) | - | |||||
| Proceeds from notes payable, related parties | - | 222 | ||||||
| Repayments of notes payable, related parties | (1,101 | ) | (71 | ) | ||||
| Proceeds from notes payable | - | 1,000 | ||||||
| Repayments of notes payable | (29 | ) | (13 | ) | ||||
| Repayments of capital lease obligations | (21 | ) | (16 | ) | ||||
| Payment of deferred offering costs | - | (132 | ) | |||||
| Proceeds from exercise of stock options and warrants | 521 | 56 | ||||||
| Net cash provided by financing activities | 18,438 | 3,116 | ||||||
| NET CHANGE IN CASH | 11,685 | (680 | ) | |||||
| CASH AT BEGINNING OF PERIOD | 141 | 821 | ||||||
| CASH AT END OF PERIOD | $ | 11,826 | $ | 141 | ||||
| SUPPLEMENTAL INFORMATION: | ||||||||
| Interest paid | $ | 393 | $ | 16 | ||||
| Income taxes paid | $ | - | $ | - | ||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Issuance of series A convertible preferred stock and common stock warrant in connection with cancellation of debt | $ | - | $ | 4,380 | ||||
| Issuance of series B convertible preferred stock in connection with conversion of convertible notes and notes payable | $ | - | $ | 2,941 | ||||
| Issuance of shares of common stock upon conversion of convertible notes payable | $ | 8,387 | $ | 610 | ||||
| Issuance of capital lease obligations for purchase of equipment | $ | 157 | $ | 30 | ||||
| Debt discount on convertible notes | $ | 9 | $ | 229 | ||||
| Issuance of warrants with notes payable | $ | - | $ | 97 | ||||
| Original issue discount | $ | 147 | $ | - | ||||
| Contributed capital, debt forgiveness by related parties | $ | 2,003 | $ | - | ||||
| Related party convertible note extinguished for settlement payable | $ | 404 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
| F-6 |
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
1. Organization and Description of Business
SenesTech, Inc. (the “Company”) was formed in July 2004 and incorporated in the state of Nevada. The Company subsequently reincorporated in the state of Delaware in November 2015. The Company has its corporate headquarters in Flagstaff Arizona.
The Company has developed proprietary technology for managing animal pest populations through fertility control. The Company believes that its innovative non-lethal approach, targeting reproduction, is more humane, less harmful to the environment, and more effective in providing a sustainable solution to pest infestations than traditional lethal pest management methods. Its first fertility control product candidate, ContraPest, will be marketed for use in controlling the rat population. The innovative compound is consumed by rats and leaves them non-reproductive without other observable side effects. The Company is pursuing regulatory approvals for ContraPest in various jurisdictions, including the United States (“U.S.”), India, Argentina and the European Union (“EU”). On August 23, 2015, the Company submitted ContraPest for registration with the
U.S. Environmental Protection Agency (“EPA”), and the EPA granted registration approval for ContraPest effective August 2, 2016. Following regulatory approval for ContraPest, the Company plans to commercialize and distribute ContraPest by leveraging new and existing third party relationships with manufacturing, marketing and distribution partners in the U.S. and internationally.
Need for Additional Capital
In the course of its research and development activities, the Company has sustained operating losses since its inception and expects such losses to continue for the foreseeable future. The Company’s ultimate success depends upon the outcome of a combination of factors, including: (i) the success of its research and development; (ii) regulatory approval and commercialization of ContraPest and its other product candidates; (iii) market acceptance and commercial viability of ContraPest and other products if the Company obtains the necessary regulatory approvals; (iv) the ability to market its products and establish an effective sales force and marketing infrastructure to generate significant revenue; (v) the ability to retain and attract key personnel to develop, operate and grow its business; and (vi) the timely and successful completion of additional financing as needed. The Company has funded its operations to date through the sale of convertible preferred stock and common stock, including an initial public offering of 1,875,000 shares of its common stock on December 8, 2016, debt financing, consisting primarily of convertible notes and, to a lesser extent, payments received in connection with research grants and licensing fees. As of December 31, 2016, the Company had cash and cash equivalents of $11,826. Based upon its current operating plan, they expect that cash and cash equivalents at December 31, 2016, will be sufficient to fund its current operations for at least the next 12 months. However, for reasons detailed above, the Company may require additional capital and would have to continue to fund its operating losses and research and development activities in the near term by issuing additional debt and equity instruments. However, if such equity or debt financing is not available at adequate levels, the Company will need to reevaluate its plans.
All amounts shown in these financial statements are in thousands, except percentages and per share and share amounts. Per share and share amounts reflect post-reverse split values.
| F-7 |
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of the financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. The significant estimates in the Company’s financial statements include the valuation of preferred stock, common stock and related warrants, and other stock-based awards. Actual results could differ from such estimates.
Reclassifications
Certain prior year amounts have been reclassified to conform to the current period presentation. These reclassifications had no impact on net earnings, financial position or cash flows.
Deferred Offering Costs
Deferred offering costs consist primarily of legal, accounting and other direct and incremental fees and costs related to the Company’s initial public offering on December 8, 2016. Deferred offering costs of $2,234 were offset against the proceeds received from the initial public offering in December of 2016. At December 31, 2015, deferred offering costs of $132 were deferred in the accompanying balance sheet.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of ninety days or less at the time of deposit to be cash equivalents. The Company does not have any cash equivalents for the periods presented.
Accounts Receivable
Accounts receivable consist primarily of trade receivables. The Company provides an allowance for doubtful trade receivables equal to the estimated uncollectible amounts. That estimate is based on historical collection experience, current economic and market conditions and a review of the current status of each customer's trade accounts receivable. The allowance for doubtful trade receivables was $0 as of December 31, 2016 and 2015 as we believe all of our receivables are fully collectable.
Inventories
Inventories are stated at the lower of cost or market value, using the first-in, first-out convention. Inventories consist of raw materials and finished goods. As of December 31, 2016 and 2015, the Company had inventories of $57 and $0, respectively.
Prepaid Expenses
Prepaid expenses consist primarily of payments made for director compensation to be earned in the first 6 months of 2017 as well as payments made for director and officer insurance, rent and legal deposits to be expensed in the current year.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Equipment held under capital leases are stated at the present value of minimum lease payments less accumulated amortization.
Depreciation on property and equipment is computed using the straight-line method over the estimated useful lives of the respective assets. The cost of leasehold improvements is amortized over the life of the improvement or the term of the lease, whichever is shorter. Equipment held under capital leases are amortized over the shorter of the lease term or estimated useful life of the asset. The Company incurs maintenance costs on its major equipment. Repair and maintenance costs are expensed as incurred.
Impairment of Long-Lived Assets
Long-lived assets, such as property and equipment, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require long-lived assets or asset groups to be tested for possible impairment, the Company compares the undiscounted cash flows expected to be generated from the use of the asset or asset group to its carrying amount. If the carrying amount of the long-lived asset or asset group is not recoverable on an undiscounted cash flow basis, an impairment charge is recognized to the extent that the carrying amount exceeds its fair value. Fair value is determined through various valuation techniques, such as discounted cash flow models and the use of third- party independent appraisals. The Company has not recorded an impairment of long-lived assets since its inception.
Revenue Recognition
The Company recognizes revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies when (i) persuasive evidence of an arrangement exists; (ii) the performance of service has been rendered to a customer or delivery has occurred; (iii) the amount of fee to be paid by a customer is fixed and determinable; and (iv) the collectability of the fee is reasonably assured.
| F-8 |
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
2. Summary of Significant Accounting Policies – (continued)
The Company has generated revenue from a license agreement with a strategic partner, pursuant to which the Company had granted to such partner the exclusive right to manufacture and distribute its product, ContraPest, once the required regulatory approvals were received (See Note 16). This licensing agreement was subsequently terminated on January 23, 2017 (See Note 18). The terms of the licensing agreement contained multiple elements or deliverables, as discussed below. Management evaluates whether the arrangement involving the multiple deliverables contains more than one unit of accounting. To determine the units of accounting under a multiple-element arrangement, management evaluates certain separation criteria, including whether the deliverables have stand-alone value, based on the relevant facts and circumstances of the arrangement.
The Company determined that the license granted pursuant to the license agreement did not have stand-alone value and, therefore, the nonrefundable, upfront license fee payments received by the Company are recognized on a straight-line basis over the estimated related performance period (i.e. from the effective date of the agreement through the estimated completion date of the Company’s substantive performance obligations).
In accordance with the terms of the license agreement, the Company was also to receive a future fixed amount of contingent milestone payments (i.e. post-regulatory approval license fees) and contingent sales-based royalties to be received upon the achievement of certain milestone events. The milestone events under the agreement include regulatory approval, patent issuance or alternative intellectual property coverage, and sales-based events. The Company did not earn or receive any of the potential contingent milestone payments, as the milestone events to receive such post-approval license fees and sales-based royalties were not achieved. The Company recognizes revenue that is contingent upon the achievement of a substantive milestone event in its entirety in the period in which the milestone is achieved. A milestone is considered substantive when the consideration payable to the Company for such milestone has all of the following characteristics: (i) there is substantive uncertainty at the date the arrangement is entered into that the event will be achieved; (ii) the event can only be achieved based in whole or part on either the Company’s performance or a specific outcome resulting from the Company’s performance; and
(iii) if achieved, the event would result in additional payments being due to the Company. As the potential contingent consideration was to be received only upon the achievement of milestone events that are considered substantive, the Company would only recognize such revenue in the period the milestone is achieved and the milestone payments are due and collectible. In addition, the Company accounts for sales-based royalties as revenue upon achievement of certain sales milestones.
Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue on the balance sheet. Amounts expected to be recognized as revenue in the next twelve months following the balance sheet date are classified as a current liability.
The Company recognizes other revenue earned from pilot studies upon the performance of specific services under the respective service contract.
To date, the Company has generated minimal revenue from the commercial sales of products.
Research and Development
Research and development costs are expensed as incurred. Research and development expenses primarily consist of salaries and benefits for research and development employees, stock-based compensation, consulting fees, lab supplies, and costs incurred related to conducting scientific trials and field studies, and regulatory compliance costs. Also, included in research and development expenses is an allocation of facilities related costs, including depreciation of research and development equipment.
| F-9 |
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
2. Summary of Significant Accounting Policies – (continued)
Stock-based Compensation
Employee stock-based awards, consisting of stock options expected to be settled in shares of the Company’s common stock, are recorded as equity awards. The grant date fair value of these awards is measured using the Black-Scholes option pricing model. The Company expenses the grant date fair value of its stock options on a straight-line basis over their respective vesting periods. Performance-based awards are expensed over the performance period when the related performance goals are probable of being achieved.
For equity instruments issued to non-employees, the stock-based consideration is measured using a fair value method. The measurement of the stock-based compensation is subject to re-measurement as the underlying equity instruments vest.
Convertible Preferred Stock
With the closing of the Company’s initial public offering, in December, 2016, the Series A and Series B convertible preferred stock were converted into shares of common stock. The holder of all of the outstanding shares of Series A convertible preferred stock agreed to convert and has converted all of its shares of Series A convertible preferred stock into shares of common stock on a one-for-one basis immediately prior to the consummation of this offering. See Note 12.
The Series A convertible preferred stock and Series B convertible preferred stock were presented outside of permanent equity, in temporary or mezzanine equity, on the Company’s December 31, 2015 balance sheet. The Company initially records preferred stock that may be redeemed at the option of the holder based on the occurrence of an event outside of the Company’s control, at the value of the proceeds received. Subsequently, if it is probable that the preferred stock will become redeemable, the Company recognizes changes in the redemption value immediately as they occur and adjusts the carrying amount of the preferred stock to equal its redemption value at the end of each reporting period. If it is not probable that the preferred stock will become redeemable, the Company does not adjust its carrying amount. In the absence of retained earnings, these charges are recorded against additional paid-in capital, if any, and then to accumulated deficit.
Valuation of Common Stock
Due to the absence of an active market for the Company’s common stock, the Company utilized methodologies in accordance with the framework of the American Institute of Certified Public Accountants’ Technical Practice Aid, Valuation of Privately- Held Company Equity Securities issued as Compensation, to estimate the fair value of its common stock. The valuation methodology includes estimates and assumptions that require significant judgments made by the Company’s management. These estimates assumptions include a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector, and the likelihood of achieving a liquidity event, such as an initial public offering or sale.
Significant changes to the key assumptions used in the valuations could result in different fair values of the Company’s common stock at each valuation date.
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statements and tax bases of assets and liabilities and net operating loss carryforwards using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the period that includes the enactment date.
The Company records net deferred tax assets to the extent it believes these assets will more likely than not be realized. These deferred tax assets are subject to periodic assessments as to recoverability and if it is determined that it is more likely than not that the benefits will not be realized, valuation allowances are recorded which would increase the provision for income taxes. In making such determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial operations.
| F-10 |
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
2. Summary of Significant Accounting Policies – (continued)
In November 2015, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update No. 2015-17, Balance Sheet Classification of Deferred Taxes, which eliminates the guidance in Topic 740, Income Taxes, that required an entity to separate deferred tax assets and liabilities between current and noncurrent amounts in a classified balance sheet. The amendments require that all deferred tax assets and liabilities of the same jurisdiction or a tax filing group, as well as any related valuation allowance, be offset and presented as a single noncurrent amount in a classified balance sheet. The standard became effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods, and may be applied on either prospectively to all deferred tax liabilities and assets or retrospectively to all periods presented. Early adoption was permitted and the Company early adopted this standard for the year ended December 31, 2015 and currently for December 31, 2016. The adoption of this standard did not have a material impact on the Company’s financial statements.
The Company applies a more-likely-than-not recognition threshold for all tax uncertainties. Only those benefits that have a greater than fifty percent likelihood of being sustained upon examination by the taxing authorities are recognized. Based on its evaluation, the Company has concluded there are no significant uncertain tax positions requiring recognition in its financial statements.
The Company recognizes interest and/or penalties related to uncertain tax positions in income tax expense. There are no uncertain tax positions as of December 31, 2016 or 2015 and as such, no interest or penalties were recorded in income tax expense.
Comprehensive Loss
Net loss and comprehensive loss were the same for all periods presented; therefore, a separate statement of comprehensive loss is not included in the accompanying financial statements.
Loss Per Share Attributable to Common Stockholders
Basic loss per share attributable to common stockholders is calculated by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted loss per share attributable to common stockholders is computed by dividing the loss attributable to common stockholders by the weighted average number of common shares and potentially dilutive securities outstanding for the period determined using the treasury stock and if-converted methods. For purposes of the computation of diluted loss per share attributable to common stockholders, the Series A convertible preferred stock, Series B convertible preferred stock, convertible promissory notes, common stock purchase warrants, and common stock options are considered to be potentially dilutive securities but have been excluded from the calculation of diluted loss per share attributable to common stockholders because their effect would be anti-dilutive given the net loss reported for the years ended December 31, 2016 and 2015. Therefore, basic and diluted loss per share attributable to common stockholders was the same for all periods presented.
The following table sets forth the outstanding potentially dilutive securities that have been excluded in the calculation of diluted loss per share attributable to common stockholders (in common stock equivalent shares):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Series A convertible preferred stock | — | 400,000 | ||||||
| Series B convertible preferred stock | — | 399,512 | ||||||
| Convertible promissory notes | — | 56,500 | ||||||
| Common stock purchase warrants | 829,285 | 610,487 | ||||||
| Restricted stock unit | 455,430 | — | ||||||
| Common stock options | 1,477,300 | 1,282,862 | ||||||
| Total | 2,762,015 | 2,749,361 | ||||||
| F-11 |
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
2. Summary of Significant Accounting Policies – (continued)
In August 2014, the FASB issued Accounting Standards Update No. 2014-15, Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern (“ASU 2014-15”). This standard requires management to perform an evaluation in each interim and annual reporting period whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity's ability to continue as a going concern within one year of the date the financial statements are issued. If such conditions or events exist, ASU 2014-14 also requires certain disclosures of management’s plans and evaluation, as well as the plans, if any, that are intended to mitigate those conditions or events that will alleviate the substantial doubt. ASU No. 2014- 15 is effective for the annual period ending after December 15, 2016 and for annual and interim periods thereafter. Early adoption is permitted for annual or interim reporting periods for which the financial statements have not been previously issued. The Company is evaluating the impact of the adoption of ASU No. 2014-15 on its financial statements and related disclosures.
In January 2016, the FASB issued Accounting Standards Update No. 2016-01, Financial Instruments — Overall: Recognition and Measurement of Financial Assets and Financial Liabilities (“ASU 2016-01”). This standard affects the accounting for equity instruments, financial liabilities under the fair value option and the presentation and disclosure requirements of financial instruments. ASU 2016-01 is effective in the first quarter of 2019. The Company is evaluating the impact of the adoption of ASU 2016-01 on its financial statements and related disclosures.
In February 2016, the FASB issued Accounting Standards Update No. 2016-02, Leases (“ASU 2016-02”). This standard amends various aspects of existing accounting guidance for leases, including the recognition of a right-of-use asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. This standard also introduces new disclosure requirements for leasing arrangements. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years for public business entities. Early adoption is permitted and the new standard must be adopted using a modified retrospective approach, and provides for certain practical expedients. The Company is evaluating the impact of the adoption of ASU 2016-02 on its financial statements and related disclosures.
In March 2016, the FASB issued Accounting Standards Update No. 2016-09, Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”). This standard involves several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities and classification on the statement of cash flows. ASU 2016-09 is effective for annual periods beginning after December 15, 2016 and interim periods within those annual periods for public business entities. The method of adoption is dependent on the specific aspect of accounting addressed in this new guidance. Early adoption is permitted in any interim or annual period. The Company is evaluating the impact of the adoption of ASU 2016-09 on its financial statements.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic230): Classification of Certain Cash Receipts and Cash Payments. The amendments in this ASU provide guidance on the following eight specific cash flow classification issues: (1) debt prepayment or debt extinguishment costs; (2) settlement of zero-coupon debt instruments or other debt instruments with coupon interest rates that are insignificant in relation to the effective interest rate of the borrowing; (3) contingent consideration payments made after a business combination; (4) proceeds from the settlement of insurance claims; (5) proceeds from the settlement of corporate-owned life insurance policies, including bank-owned life insurance policies; (6) distributions received from equity method investees; (7) beneficial interests in securitization transactions; and (8) separately identifiable cash flows and application of the predominance principle. Current GAAP does not include specific guidance on these eight cash flow classification issues. The amendments of this ASU are effective for reporting periods beginning after December 15, 2017, with early adoption permitted. The adoption of ASU No. 2016-15 is not expected to have a material impact on the consolidated financial statements and related disclosures.
F-12
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
3. Fair Value Measurements
The accounting guidance for fair value, among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The framework for measuring fair value consists of a three-level valuation hierarchy that prioritizes the inputs to valuation techniques used to measure fair value based upon whether such inputs are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions made by the reporting entity. The three-level hierarchy for the inputs to valuation techniques is briefly summarized as follows:
| • | Level 1 — Inputs are unadjusted, quoted prices in active markets for identical assets and liabilities at the measurement date; |
| • | Level 2 — Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
| • | Level 3 — Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
An asset’s or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs.
Assets and liabilities measured at fair value are based on one or more of the following three valuation techniques:
| A. | Market approach: Prices and other relevant information generated by market transactions involving identical or comparable assets or liabilities. |
| B. | Cost approach: Amount that would be required to replace the service capacity of an asset (replacement cost). |
| C. | Income approach: Techniques to convert future amounts to a single present amount based upon market expectations, including present value techniques, option-pricing and excess earnings models. |
Items Measured at Fair Value on a Recurring Basis
The following table sets forth the Company’s financial instruments that were measured at fair value on a recurring basis by level within the fair value hierarchy:
| December 31, | Valuation | |||||||||
| 2016 | 2015 | Technique | ||||||||
| Common stock warrant liability | $ | 69 | $ | 63 | C | |||||
| Convertible notes payable – current liability | $ | — | $ | — | C | |||||
F-13
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
3. Fair Value Measurements – (continued)
The following table sets forth a summary of the changes in the fair value of the Company’s Level 3 financial liabilities:
| Common | 2014/2015 | |||||||
| Stock | Convertible | |||||||
| Warrant | Notes | |||||||
| Liability | Payable | |||||||
| Balance at December 31, 2014 | — | 447 | ||||||
| Issuance of common stock warrant | 53 | — | ||||||
| Issuance of convertible notes | — | 1,818 | ||||||
| Change in fair value(1) | 10 | 728 | ||||||
| Extinguishment of convertible notes for Series B convertible preferred stock | — | (2,993 | ) | |||||
| Balance at December 31, 2015 | $ | 63 | $ | — | ||||
| Change in fair value | 6 | — | ||||||
| Balance at December 31, 2016 | $ | 69 | $ | — |
(1) The change in the fair value of the common stock warrant and convertible notes payable was recorded as an increase to other income (expense) and interest expense of $677 and $63, respectively, in the statements of operations and comprehensive loss
Financial Instruments Not Carried at Fair Value
The carrying amounts of the Company’s financial instruments, including accounts payable and accrued liabilities, approximate fair value due to their short maturities. The estimated fair value of the convertible notes and other notes, not recorded at fair value, are recorded at cost or amortized cost which was deemed to estimate fair value.
4. Prepaid expenses
Prepaid expenses consist of the following:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Director compensation | $ | 215 | $ | 6 | ||||
| Director and officer insurance | 70 | — | ||||||
| Legal retainer | 25 | — | ||||||
| Rent | 17 | 16 | ||||||
| Engineering, software licenses and other | 10 | 14 | ||||||
| Total accrued expenses | $ | 337 | $ | 36 | ||||
5. Property and Equipment
Property and equipment, net consist of the following:
| December 31, | ||||||||||
| Useful Life | 2016 | 2015 | ||||||||
| Research and development equipment | 5 years | $ | 989 | $ | 834 | |||||
| Office and computer equipment | 3 years | 235 | 181 | |||||||
| Furniture and fixtures | 7 years | 17 | 12 | |||||||
| Leasehold improvements | * | 189 | 189 | |||||||
| 1,430 | 1,216 | |||||||||
| Less accumulated depreciation and amortization | 799 | 603 | ||||||||
| Total | $ | 631 | $ | 613 | ||||||
* Shorter of lease term or estimated useful life
Depreciation and amortization expense was approximately $196 and $182 for the year ended December 31, 2016 and 2015, respectively.
F-14
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
6. Accrued Expenses
Accrued expenses consist of the following:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Compensation and related benefits | $ | 82 | $ | 425 | ||||
| Accrued interest | — | 4 | ||||||
| Accrued interest – related parties | — | 329 | ||||||
| Accrued Litigation | 286 | — | ||||||
| Other | 3 | — | ||||||
| Total accrued expenses | $ | 371 | $ | 758 | ||||
7. Accrued contract cancellation settlement
The accrued contract cancellation settlement of $1.0 million was a result of the Company entering into a settlement agreement with Neogen Corporation in which Neogen and the Company agreed to (a) terminate the existing Exclusive License Agreement between us and Neogen dated May 15, 2014 (the “License Agreement”), with neither Neogen or the Company having any further obligations thereunder (other than certain confidentiality obligations); (b) dismiss with prejudice the court action filed by Neogen in the District Court for the District of Arizona on January 19, 2017 (the “Court Action”), and (c) mutually release any and all existing or future claims between the parties and their affiliates related to or arising from the License Agreement or the Court Action. Under the terms of the agreement, the Company agreed to make a one-time payment in the amount of $1.0 million in settlement of all claims and termination of all existing contracts between the parties. See notes 17 and 18 for further details.
8. Borrowings
A summary of the Company’s borrowings, including capital lease obligations, is as follows:
| At December 31, | ||||||||
| Short-term debt: | 2016 | 2015 | ||||||
| NAU Promissory Note | $ | — | $ | — | ||||
| Current portion of long-term debt | 45 | 27 | ||||||
| Total short-term debt | $ | 45 | $ | 27 | ||||
| Long-term debt: | ||||||||
| Capital lease obligations | $ | 51 | $ | 72 | ||||
| Other unsecured promissory notes | 132 | 400 | ||||||
| Other promissory notes | — | 5 | ||||||
| Total | 183 | 477 | ||||||
| Less: current portion of long-term debt | 45 | 27 | ||||||
| Total long-term debt | $ | 138 | $ | 450 | ||||
Capital Lease Obligations
Capital lease obligations are for computer and lab equipment leased through Great American and Thermo Fisher. These capital leases expire at various dates through May of 2020.
Unsecured Promissory Note with Northern Arizona University
In July 2015, the Company and NAU Ventures entered into an Amended and Restated Letter Agreement (“NAU Agreement”) to issue to NAU Ventures 400,000 shares of Series A convertible preferred stock and a three-year warrant to purchase 210,526 shares of common stock at an exercise price of $15.00 per share (“NAU Warrant”), subject to standard anti-dilution provisions, in exchange for the cancellation of the NAU Promissory Note. The NAU Warrant agreement includes an early termination provision that states in the event of a public offering, consolidation, merger, sale or other disposition of all or substantially all of the assets, the NAU Warrant will terminate unless exercised prior to the occurrence of such above-mentioned events.
F-15
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
8. Borrowings – (continued)
The NAU Agreement replaced in its entirety a prior letter agreement, dated July 2, 2014, that was not consummated. This previous letter agreement provided for similar terms as the NAU Agreement.
Pursuant to the NAU Agreement, the cancellation of the NAU Promissory Note and issuance of Series A convertible preferred stock was contingent on reincorporation of the Company to a Delaware corporation. In November 2015, a certificate of conversion was filed to reincorporate the Company from a Nevada corporation to a Delaware corporation. Immediately following the reincorporation, the transactions contemplated by the NAU Agreement closed. The Company issued to NAU Ventures 2,000,000 shares of Series A convertible preferred stock, valued at $4,380, and the NAU Warrant, valued at $330, in exchange for full cancellation of the NAU Promissory Note. As a result, the outstanding balance on the NAU Promissory Note, including accrued interest of $1,313, of $3,180 was extinguished. The Company recorded a loss on the extinguishment of the NAU Promissory Note of $1,530 during the year ended December 31, 2015.
The fair value of the Series A convertible preferred stock was determined using a simulation model of discounted cash flows and included an assumption for the likelihood of a qualified financing or a change in control event, each as defined in the documents creating the Series A preferred stock. The Warrant was valued using the following inputs to a Monte Carlo model: underlying stock price of $7.50, term of three years, exercise price of $15.00, volatility of 70.6%, risk free rate of 1.27% and an estimate of the likelihood of an early termination. The estimated fair value of the NAU Warrant on the date of issuance was recorded in additional paid-in capital.
On August 1, 2016, the Company and NAU Ventures, LLC, the holder of the Series A convertible preferred stock, entered into a Conversion and Termination Agreement (the “Conversion Agreement”). Pursuant to the Conversion Agreement, the holder has agreed to convert all of its shares of Series A convertible preferred stock into 400,000 shares of common stock immediately prior to the consummation of the Company’s proposed initial public offering. In addition, the Company has agreed to make a cash payment of $175,890 to the holder of the Series A convertible preferred stock for its agreement to waive all accrued dividends on the Series A convertible preferred stock and convert all of its shares of Series A convertible preferred stock into common stock immediately prior to the consummation of this offering. In the event the Company’s initial public offering was not consummated on or before July 31, 2017, then the Conversion Agreement would have terminated in its entirety and the terms of the Series A convertible preferred stock would have reverted back to its original terms.
With the closing of the Company’s initial public offering, in December, 2016, NAU converted all of their shares of Series A convertible preferred stock into shares of common stock on a one-for-one basis immediately prior to the consummation of this offering. See Note 12.
Secured Promissory Note
In April 2015, the Company issued a secured promissory note to an investor with an aggregate principal amount of $500 (the “Secured Promissory Note”) together with common stock warrants to purchase 69,333 shares of common stock, for total proceeds of $500. The Secured Promissory Note required interest at 4% per annum with a maturity date in April 2016. The Secured Promissory Note was collateralized by all of the Company’s personal property. See Note 11 for a description of the features of the warrants.
The Secured Promissory Note exists independently from the detachable warrants and is accounted for separately. At the time of issuance, the proceeds received from the issuance of the Secured Promissory Note was allocated to the Secured Promissory Note and warrant based on the relative fair values of the Secured Promissory Note with the warrants and without the warrants. The Company determined the estimated fair value of the Secured Promissory Note using a lattice model and the estimated fair value of the warrants using a Monte Carlo model. The relative fair value of the warrants of $113 was recorded to additional paid-in- capital. The remainder of the proceeds was allocated to the Secured Promissory Note. The Company recorded the value of the warrant as a debt discount on the related note. The Secured Promissory Note is carried at amortized cost and the debt discount of $113 is amortized to interest expense, using the effective interest method, through the maturity date of the Secured Promissory Note. During the year ended December 31, 2015, the Company recorded accretion of debt discount of $79 within interest expense in the accompanying statements of operations and comprehensive loss.
On December 31, 2015, the Secured Promissory Note was cancelled as consideration for the purchase of shares of Series B convertible preferred stock at $7.75 per share, which was the same price as the shares sold to other investors in the Company’s Series B convertible preferred stock financing in December 2015. The Company issued 66,705 shares of Series B convertible preferred
F-16
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
8. Borrowings – (continued)
stock to the holder in exchange for the cancellation of the Secured Promissory Note with an aggregate principal amount of $500 plus unpaid accrued interest. As a result, the Company issued shares of Series B convertible preferred stock for $517 to reacquire the Secured Promissory Note (including unpaid accrued interest), with a net carrying amount of $483 which resulted in a loss on the date of extinguishment of $34, representing the elimination of the related unamortized debt discount on the Secured Promissory Note.
Other Promissory Notes
During September, October and December 2015, the Company issued unsecured promissory notes to an investor with an aggregate principal amount of $500 (the “2015 Unsecured Notes”) together with warrants to purchase 69,333 shares of common stock for total cash proceeds of $500. The 2015 Unsecured Notes required interest at 4% per annum with a maturity date in April 2017. The 2015 Unsecured Notes are convertible into common stock or Series B convertible preferred stock solely upon mutual agreement of both the Company and the holder at a conversion price of $7.75 per share. Also, the Company may prepay the 2015 Unsecured Notes at any time prior to their maturity date without the consent of the holder or penalty. See Note 11 for a description of the features of the warrants.
The 2015 Unsecured Notes notes exist independently from the detachable warrants and are accounted for separately. At the date of issuance, the proceeds received from the issuance of the 2015 Unsecured Notes was allocated to the 2015 Unsecured Notes and warrants based on the relative fair values of the 2015 Unsecured Notes with the warrants and without the warrants. The Company determined the estimated fair value of the 2015 Unsecured Notes using a lattice model and the estimated fair value of the warrants using a Monte Carlo model. The relative fair value of the warrants of $116 was recorded to additional paid-in- capital. The remainder of the proceeds was allocated to the 2015 Unsecured Notes. The Company recorded the value of the warrants as a debt discount on the related 2015 Unsecured Notes. The 2015 Unsecured Notes are carried at amortized cost and the debt discount of $116 is amortized to interest expense, using the effective interest method, through the maturity date of the 2015 Unsecured Notes. The outstanding carrying amount of the 2015 Unsecured Notes, net of $100 unamortized debt discount, was $0 and $400 at December 31, 2016 and 2015, respectively, and was classified on the balance sheet as long-term debt.
In May 2012, the Company entered into a promissory note for the purchase of equipment. The note is payable in monthly payments of $1 with an interest rate of 5% per annum. The note matured in May 2016.
At December 31, 2016 and 2015, the note had a balance outstanding of $0 and $5, respectively.
2016 Unsecured Notes
In February and March 2016, the Company issued to two investors unsecured, short-term promissory notes (the “2016 Unsecured Notes”) with an aggregate principal amount of $310 bearing interest at a rate of 4% per annum. In May 2016, these 2016 Unsecured Notes were surrendered as consideration for purchase of 124,000 shares of common stock in the Rights Offering at the subscription price of $2.50 per share. See Note 13.
In March 2016, the Company issued to two investors additional 2016 Unsecured Notes with an aggregate principal amount of $16, together with detachable warrants to purchase 9,032 shares of common stock, for total proceeds of $16. These 2016 Unsecured Notes were then immediately surrendered as consideration for the purchase of 10,036 shares of Series B convertible preferred stock at a price of $7.75 per share. At issuance, the Company allocated the proceeds of $7 and $9 to the debt and equity components, respectively. The Company recorded the equity component as a discount to these 2016 Unsecured Notes. The extinguishment of these 2016 Unsecured Notes, upon their cancellation and exchange into the Series B convertible preferred stock resulted in a write-off of the unamortized debt discount and the Company recorded a loss on extinguishment of $9 in the year ended December 31, 2016.
F-17
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
9. Notes Payable, Related Parties
A summary of the Company’s notes payable, related parties is as follows:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Unsecured promissory note, interest rate of 4.25% and 8% per annum | $ | 36 | $ | 73 | ||||
| Unsecured promissory note, interest rate of 8% per annum | — | 236 | ||||||
| Unsecured promissory note, interest rate of 6% per annum | — | 27 | ||||||
| Unsecured promissory notes, interest rate of 4% per annum | — | 160 | ||||||
| Total notes payable, related parties | 36 | 496 | ||||||
| Less: current portion of notes payable, related parties | 30 | 462 | ||||||
| Total notes payable, long-term | $ | 6 | $ | 34 | ||||
The Company issued a series of unsecured promissory notes to a previous executive employee for deferred salaries to be repaid in a future period. The notes accrue interest at a rate of 8% per annum. The outstanding balance on these notes, including accrued interest, totaled $380 at December 31, 2015. In March 2016, the Company issued an amended and restated promissory note (“Revised Note”) in the amount of $414, which includes accrued and unpaid interest and other settlement costs and replaced in their entirety the previous unsecured promissory notes. The Revised Note of $414 requires the Company to pay an initial payment of $25 upon the effective date of the note, and monthly and quarterly payments of $10 and $25, respectively, thereafter, with interest accruing at 12% per annum. Additionally, the Revised Note provides for an acceleration of the amount due in the event of (i) a merger, sale or acquisition of substantially of the Company’s assets; (ii) initial public offering; (iii) total equity raise of $2.5 million or more; and (iii) debt financing of $2.5 million with an unaffiliated lender within thirty days of such events.
In May 2016, the Company repaid the outstanding balance under the Revised Note, plus accrued and unpaid interest totaling $389, as the consummation of the Rights Offering triggered an acceleration of the amounts due under the Revised Note.
In April 2013, the Company and a previous employee entered into an agreement to settle all outstanding obligations consisting of a promissory note of $40, dated March 2009, and deferred salaries amounting to $72. The note and salary obligation continue to bear interest at 8% and 4.25%, respectively. The note requires monthly payments of $1 and matures in May 2018. The deferred salary obligation requires monthly payments of $1 and matures in June 2018.
Amounts outstanding on these obligations were $36 and $73 at December 31, 2016 and 2015, respectively.
F-18
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
9. Notes Payable, Related Parties – (continued)
In October 2011, the Company entered into an unsecured promissory note in the amount of $30 with its chief executive officer with interest accruing at 4%. At the same time, the Company entered into an unsecured promissory note in the amount of $30 with its Chief Research Officer with the same terms and conditions. Both of these notes were repaid in 2015.
In 2015, the Company entered into short-term unsecured promissory notes, totaling $195, with its previous chief executive officer. The notes accrued interest at 4% per annum and matured in December 2015. The funds were used to meet working capital requirements. The Company repaid $35 on the promissory notes during 2015 and repaid the balance in May 2016.
In August 2015, the Company entered into a short-term unsecured promissory note for $27 with a member of its advisory board. The note accrued interest at 6% per annum and was due in February 2016. Upon maturity, the holder transferred the note to a certain stockholder who acquired the note. The maturity date of the note was then extended for ninety days. On April 7, 2016, this note was settled with the issuance of 54,000 shares of common stock. See note 13.
Equity Participation Promissory Notes
In September 2016, the Company entered into a settlement agreement with the holder of equity participation promissory notes. The settlement agreement terminated the holder’s equity participation and conversion rights. The Company agreed to pay the promissory note holder principal, interest and expenses of $464 with $88 paid at the agreement execution and equal monthly payments of $14. Interest continues to accrue on unpaid balances at 1.25% per month with the total outstanding balance to be paid in full by the earlier of (i) May 25, 2017, (ii) the sale, merger or acquisition of the Company or substantially all of its assets, or (iii) 45 days after an initial public offering. This settlement was fully paid at December 31, 2016.
Interest expense on the notes payable, related parties, was $56 and $33 for the years ended December 31, 2016 and 2015, respectively.
F-19
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
10. Convertible Notes Payable, Related Parties
A summary of the Company’s convertible notes payable, related parties is as follows:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Convertible notes payable, short term: | ||||||||
| Equity Participation Notes | $ | — | $ | 200 | ||||
| 2014/2015 Convertible Notes | — | — | ||||||
| Other convertible notes payable | — | — | ||||||
| Total convertible notes payable, short term | $ | — | $ | 200 | ||||
Equity Participation Promissory Notes
In late 2009 and early 2010, the Company entered into unsecured promissory note agreements with certain of its existing stockholders for an aggregate principal amount of $675 with an original maturity date of May 31, 2010 (the “Equity Participation Notes”). The Company continues to accrue unpaid interest on the Equity Participation Notes at a rate 15% per annum, The terms of the Equity Participation Notes provide for the holder to convert, at any time, the outstanding principal and unpaid accrued interest into shares of common stock at an conversion rate of $10.00 per share provided, however, that in the event, that the Company’s common stock is registered in an initial public offering, prior to such time the Company achieves a net profit of $15 million, then the conversion rate shall be $5.00 per share. Additionally, the Equity Participation Notes provide the holder an equity participation right at the rates from .8330% to 4% on the first $15 million of net profit, which is defined as net profit before interest, taxes, depreciation, amortization (“EBITDA”) and compensation of officers, directors and any of their related parties. There were no amounts accrued for these contingent equity participation payments at December 31, 2016 and 2015.
F-20
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
10. Convertible Notes Payable, Related Parties – (continued)
2014 Convertible Promissory Notes
In the first quarter of 2015, the Company made an offer to the holders of the 2014 Notes to allow the holders to tender their convertible notes prior to maturity in exchange the Company would agree to include interest through the original maturity date in calculating the number of conversion shares. In May and June 2015, the Company converted the outstanding principal and accrued interest due on the 2014 Notes, totaling $452, into 71,446 shares of common stock at the following conversion rates: principal at $7.50 per share and interest at $5.00 per share. The discount on the 2014 Notes was amortized to interest expense through the conversion dates using the effective interest method. The Company recorded an expense of $231, including the charge of the unamortized discount of $142, for the inducement to convert the 2014 Notes as additional interest expense. The Company recorded interest expense on the 2014 Notes of $0 and $312 during the year ended December 31, 2016 and 2015, respectively.
2014/2015 Secured Convertible Promissory Notes
During the period from December 2014 through November 2015, the Company issued secured convertible promissory notes (the “2014/2015 Convertible Notes”) to certain existing stockholders which provided for borrowings of $2,365. The 2014/2015 Convertible Notes bore interest at a rate of 4% per annum, matured one year from their date of issuance and were collateralized by all of the Company’s personal property. The 2014/2015 Convertible Notes could not be repaid prior to their maturity date without the consent of the holders of at least a majority of the outstanding principal amount of the 2014/2015 Convertible Notes. The 2014/2015 Convertible Notes contained two conversion options contingent upon the occurrence of a future event, as follows: (i) all outstanding principal and unpaid accrued interest on the 2014/2015 Convertible Notes would automatically convert into equity securities upon the consummation of an equity financing with proceeds of at least $2 million (“Qualified Financing”) at conversion rates of 70% or 80%, as noted below; and (ii) if the Company was acquired, prior to a Qualified Financing, in a change in control transaction, the holder had an option to have all outstanding principal plus any unpaid accrued interest paid in cash or convert such amount into shares of common stock at a conversion price equal to $7.50 per share, subject to adjustment for such events as stock splits, combination, and reorganization.
The Company received proceeds of $875 from the 2014/2015 Convertible Notes issued from December 2014 through February 2015 with an aggregate principal amount of $875. In the event of a Qualified Financing, these 2014/2015 Convertible Notes provided automatic conversion of all outstanding principal and unpaid accrued interest thereon into the shares of the equity securities sold in the Qualified Financing at a conversion rate of 70% of the price per share paid by the other purchasers in the Qualified Financing.
From April 2015 through November 2015, the Company issued additional 2014/2015 Convertible Notes with an aggregate principal amount of $1,490 together with detachable common stock warrants for proceeds of $1,490. However, in the event of a Qualified Financing, these 2014/2015 Convertible Notes provided automatic conversion of all outstanding principal and unpaid accrued interest thereon into the shares of the equity securities sold in the Qualified Financing at a higher conversion rate of 80% of the price per share paid by the other purchasers in the Qualified Financing.
The Company determined that the conversion option upon a Qualified Financing is the predominant conversion option and is a conditional obligation that the Company would settle by issuing a variable number of its shares. In this manner, the value that the convertible note holder would receive upon a Qualified Financing conversion event is a fixed monetary amount known at inception. Although the two contingent conversion features are embedded derivative features, they did not require bifurcation to be accounted for
F-21
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
10. Convertible Notes Payable, Related Parties – (continued)
separately. Therefore, the 2014/2015 Convertible Notes are measured initially at fair value and subsequently with changes in fair value recognized in earnings.
There were a total of 131,733 detachable common stock warrants issued with the 2014/2015 Convertible Notes. The 2014/2015 Convertible Notes and warrants exist independently as separate securities. See Note 9 for a description of the warrants. As the 2014/2015 Convertible Notes are measured at fair value, such notes are allocated a portion of the proceeds equal to their fair value with the remaining proceeds being allocated to the detachable warrants. The estimated aggregate fair value of the 2014/2015 Convertible Notes issued was determined to be $2,268 with the remaining $97 of proceeds allocated to the detachable warrants. The Company determined the estimated fair value of the 2014/2015 Convertible Notes using a lattice model. For the year ended December 31, 2016 and 2015, the Company recognized the changes in fair value on the 2014/2015 Convertible Notes of $0 and $671, respectively, within other income (expense), in the accompanying statements of operations and comprehensive loss.
As part of a Series B convertible preferred stock financing in December 2015, the Company provided an offer to the holders of the 2014/2015 Convertible Notes to cancel and exchange their notes as consideration for their purchase of the Series B convertible preferred stock at the same price as the shares to be sold to other investors in the preferred stock financing. In consideration of the Company pricing the Series B convertible preferred stock at $7.75 per share in the preferred stock financing and securing the $7.75 conversion price, the holders of the 2014/2015 Convertible Notes waived the discount that provided for the automatic conversion of the convertible notes into the equity securities sold in a Qualified Financing of at least $2 million. With this offer, the holders of the 2014/2015 Convertible Notes received no additional consideration, there was no modification of terms in their notes, and it did not represent the exercise of a conversion right obtained in the terms of the 2014/2015 Convertible Notes at issuance. On December 31, 2015, all holders of the 2014/2015 Convertible Notes accepted the Company’s offer to cancel and exchange their notes as consideration for the purchase of the Series B convertible preferred stock. The Company determined that the offer made to the holders of the 2014/2015 Convertible Notes should be accounted for as an extinguishment of debt.
The Company issued 312,861 shares of Series B convertible preferred stock to the holders of the 2014/2015 Convertible Notes to cancel and exchange the aggregate $2,365 principal amount and unpaid accrued interest totaling $2,465 on their notes as consideration for their purchase of the preferred stock at the $7.75 per share price. In determining the reacquisition price in the extinguishment of the convertible notes, the value of the Series B convertible preferred stock was readily determinable as the preferred stock was sold separately to other investors as part of the preferred stock financing at $7.75 per share. As a result, the $2,425 amount to reacquire the convertible notes was less than the $2,993 fair value carrying amount of the 2014/2015 Convertible Notes on December 31, 2015, the date of extinguishment. As such, the Company recorded a gain on extinguishment of $569, which represents the elimination of the fair value accounting adjustments in the year ended December 31, 2015.
Other convertible notes
The Company entered into an unsecured promissory note with a stockholder in the amount of $60 with interest accruing at a rate of 10% per annum. The note provided for the holder to convert the outstanding principal and accrued and unpaid interest into shares of common stock at a conversion rate equal to $7.50 per share. In 2015, the holder converted the note balance, including accrued and unpaid interest, in the aggregate amount of $68, into 8,971 shares of common stock in accordance with the original conversion terms of the note.
F-22
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
11. Common Stock Warrants and Common Stock Warrant Liability
The table summarizes the common stock warrant activity as of December 31, 2016 as follows:
| Common Stock Warrants | Number of Warrants | Date
Issued | Term | Exercise Price | ||||||||||
| Secured Promissory Note | 69,333 | April 2015 | 5 years (1) | $ | 7.50 | |||||||||
| 2014/2015 Convertible Notes | 131,734 | April – November 2015 | 5 years (1) | $ | 7.50 | |||||||||
| Other Promissory Notes | 69,333 | September –December 2015 | 5 years (1) | $ | 7.50 | |||||||||
| 270,400 | ||||||||||||||
| University of Arizona | 15,000 | June 2015 | 5 years (2) | $ | 7.50 | |||||||||
| Consulting Agreement | 121,228 | July 2015 | 10 years (3) | $ | 7.50 | |||||||||
| Northern Arizona University | 210,526 | November 2015 | 3 years (4) | $ | 15.00 | |||||||||
| Warrants issued | 617,154 | |||||||||||||
| Warrants exercised | 6,667 | |||||||||||||
| Outstanding at December 31, 2015 | 610,487 | |||||||||||||
| Initial Public Offering Underwriter | 187,500 | December 2016 | 5 years | $ | 9.60 | |||||||||
| Marketing and Development Services | 100,000 | February 2016 | 5 years(1) | $ | 7.50 | |||||||||
| Other Advisory Services | 40,000 | August 2016 | 3 years(1) | $ | 7.50 | |||||||||
| Promissory Notes | 9,031 | March 2016 | 3 years(1) | $ | 7.50 | |||||||||
| Warrants issued | 336,531 | |||||||||||||
| Warrants exercised | 117,733 | |||||||||||||
| Outstanding at December 31, 2016 | 829,285 | |||||||||||||
| (1) | The warrants also terminate, if not exercised, upon the closing of (i) an initial public offering of common stock; or (ii) a liquidation, dissolution or winding up of the Company. |
| (2) | In the event of a terminating change of the Company, as defined in the warrant agreement, the warrant holder would be paid in cash the aggregate fair market value of the warrant shares immediately prior to the consummation of the terminating change event. |
| (3) | The warrant also terminates, if not exercised, (i) two years after the closing of an initial public offering of common stock; or (ii) the closing of a liquidation, dissolution or winding up of the Company. |
| (4) | The warrant also terminates, if not exercised, upon the closing of (i) an initial public offering of common stock; or (ii) a consolidation, merger, sale or other disposition of all or substantially all of the Company’s assets |
Secured Promissory Note, 2014/2015 Convertible Notes, other promissory notes Common Stock Warrants
In conjunction with the issuance of the Secured Promissory Note, 2014/2015 Convertible Notes, and certain other promissory notes, the Company issued detachable common stock warrants (“Warrants”) to purchase an aggregate 270,400 shares of common stock, with an exercise price of $7.50 per share. The Warrants are exercisable until the earlier of (i) 5 years from the date of grant; (ii) the closing of an initial public offering of common stock by the Company; and (iii) the closing of liquidation, dissolution or winding up of the Company.
The Warrants have a net share settlement (cashless exercise) provision. With this provision the holder may, in lieu of payment of the exercise price in cash, surrender the warrant and receive a net amount of shares based on the fair market value of the common stock at the time of exercise of the warrant after deduction of the aggregate exercise price. However, the Warrants would be exercised automatically in full pursuant to the net exercise provision, without any further action on behalf of the holder, immediately prior to the time the Warrants would otherwise terminate.
The Warrants are considered freestanding instruments as (i) they were transferred together with the notes issued but exist independently as a separate security; (ii) they may be exercised separately from the notes; and (iii) they are exercisable for a specific period (term) and do not impact the notes if and when exercised.
F-23
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
11. Common Stock Warrants and Common Stock Warrant Liability – (continued)
The Company estimated the fair value of the Warrants at issuance using a Monte Carlo option pricing model based on the following significant inputs: common stock price of $7.50 to $7.575; comparable company volatility of 58.0% to 76.7%; risk- free rates of 1.31% to 1.76%; and the probability of an equity event occurring. The Company reflected the amounts recorded for the Warrants issued within stockholders’ deficit, as additional paid-in-capital. Although the Warrants are a derivative that can be net share settled, the Warrants are considered indexed to the Company’s common stock and the Company has the ability to settle the warrant contract in common shares and met the conditions within the contract to classify the Warrants as an equity instrument.
Common Stock Warrant Issued for Marketing and Development Services
In February 2016, the Company issued to a stockholder a warrant to purchase 100,000 shares of common stock at an exercise price of $7.50 per share as consideration for providing marketing and development services in Southeast Asia. The warrant was fully vested and exercisable on the date of grant. The common stock warrant has the similar features as the Warrants disclosed in Note 11, except it is exercisable until the earlier of (i) five years from the date of grant; (ii) two years after the closing of an initial public offering of common stock by the Company; and (iii) the closing of a liquidation, dissolution or winding up of the Company. The Company estimated the fair value of the common stock warrant to be $431 on the date of grant using a Black- Scholes option pricing model based on the following significant inputs: common stock price of $7.57; comparable company volatility of 77.8%; remaining term 3.75 years; dividend yield of 0% and risk-free rate of 2.09%. The Company recorded the fair value of the warrant as stock-based compensation expense within general and administrative expense on the date of grant.
March 2016 Promissory Notes Common Stock Warrants
In March 2016, the Company issued certain 2016 Unsecured Notes with common stock warrants to purchase an aggregate of 9,032 shares of common stock at an exercise price of $7.50 per share. See Note 8. The common stock warrants are exercisable until the earlier of (i) 3 years from the date of grant; (ii) 2 years after the closing of an initial public offering of common stock; and (iii) the closing of a liquidation, dissolution or winding up of the Company. The Company estimated the fair value of the common stock warrants on the date of grant using a Monte Carlo pricing model based on the following significant inputs: common stock price of $7.575; comparable company volatility 79.6%; and risk-free rate of 1.49%.
August 2016 Other Advisory Services
On August 16, 2016, the Company issued to each of two advisors warrants to purchase 20,000 shares of common stock at an exercise price of $7.50 per share, in each case, on a post-reverse split basis, as consideration for providing advisory services to the Company. The warrants were fully vested and exercisable on the date of grant until the earlier of (i) three years from the date of grant; (ii) two years after the closing of an initial public offering of common stock; and (iii) the closing of a liquidation, dissolution or winding up of the Company. The Company recorded the fair value of the warrants as stock-based compensation expense within general and administrative expense on the date of grant.
Common Stock Warrant Issued To Initial Public Offering Underwriter
In December 2016, the Company issued to the underwriter of its IPO a warrant to purchase 100,000 shares of common stock at an exercise price of $9.60 per share as consideration for providing services in connection with our initial public offering. The warrant was fully vested and exercisable on the date of grant. The common stock warrant is exercisable until five years from the date of grant. The Company estimated the fair value of the common stock warrant to be $939 on the date of grant using a Black- Scholes option pricing model based on the following significant inputs: common stock price of 8.00; comparable company volatility of 82.1%; remaining term 5 years; dividend yield of 0% and risk-free rate of 1.92%.
University of Arizona Common Stock Warrant
In connection with the June 2015 amended and restated exclusive license agreement with the University of Arizona (“University”), the Company issued to the University a common stock warrant to purchase 15,000 shares of common stock at an exercise price of $7.50 per share. The warrant is exercisable immediately and expires, if not exercised, five years from the date of grant. In the event of a “terminating change” of the Company, as defined in the warrant agreement, the warrant holder would be paid in cash the aggregate fair market value of the underlying shares immediately prior to the consummation of the terminating change event. Due to the cash settlement provision, the derivative warrant liability was recorded at fair value and is revalued at the end of each reporting period. The changes in fair value are reported in other income (expense) in the statements of operations and comprehensive loss. The estimated fair value of the derivative warrant liability was $53 at the date of grant.
F-24
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
11. Common Stock Warrants and Common Stock Warrant Liability – (continued)
The estimated fair value of the derivative warrant liability was $63 at December 31, 2015. As this derivative warrant liability is revalued at the end of each reporting period, the fair values as determined at the date of grant and subsequent periods was based on the following significant inputs using a Monte Carlo option pricing model: common stock price of $7.50 to $7.575; comparable company volatility of 60.8% to 81.4% of the underlying common stock; risk-free rates of 1.21% to 1.76%; and dividend yield of 0%; including the probability assessment of a terminating change event occurring. The change in fair value of the derivative warrant liability was $10 for the year ended December 31, 2015 and was recorded in other income (expense) in the accompanying statements of operations and comprehensive loss.
July 2015 Consulting Agreement Common Stock Warrant
In July 2015, the Company issued a common stock warrant to purchase 121,227 shares of common stock, with an exercise price of $7.50 per share, as consideration for services under a consulting arrangement. The warrant was fully vested and exercisable on the date of grant. This common stock warrant has the similar features as the Warrants described above, except it is exercisable until the earlier of (i) 10 years from the date of grant; (ii) 2 years after the closing of an initial public offering of common stock by the Company; and (iii) the closing of a liquidation, dissolution or winding up of the Company. The estimated the fair value of the common stock warrant on the date of grant was $537 as determined by using a Black-Scholes option pricing model based on the following significant inputs: common stock price of $7.575; comparable company volatility of 60.9%; expected term of 6.25 years; risk-free rate of 2.09%; and dividend yield of 0%. The Company recorded the fair value of the, warrant as stock-based compensation expense within general and administrative expense in the accompanying statements of operations and comprehensive loss in 2015.
Northern Arizona University Common Stock Warrant
In November 2015, the Company issued a common stock warrant to purchase 210,526 shares of common stock at an exercise price of $15.00 per share to Northern Arizona University (“NAU”) as part of the consideration given with the Series A convertible preferred stock in exchange for the full cancellation of the NAU Promissory Note. See Note 8.
F-25
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
12. Convertible Preferred Stock
Series A Convertible Preferred Stock
In November 2015, the Company issued 400,000 shares of Series A convertible preferred stock, valued at $4,380, in exchange for cancellation of the NAU Promissory Note. See Note 8.
The Series A convertible preferred stock was recorded at the date of issuance at fair value. The Company’s Series A convertible preferred stock has been classified as temporary equity on its balance sheet at December 31, 2015. Upon certain liquidation events, as discussed below, that are not solely within the control of the Company, including liquidation, sale or transfer of control of the Company, holders of the Series A convertible preferred stock can cause the redemption of the Series A convertible preferred stock for cash highlighting the potential future cash obligation.
A general summary of the rights with respect to the Series A convertible preferred stock are provided below:
Dividends
The holders of the Series A convertible preferred stock are entitled to dividends at the rate of 6% of the original issue price ($5.00) per annum which accrues whether or not earned or declared by the Board of Directors, whether or not there are profits or funds legally available for the payment and are cumulative to the extent not paid. The Company is restricted to pay or declare any dividend or make any other distribution on the common stock, or purchase, redeem or acquire for value any shares of common stock as long as the Series A convertible preferred stock is outstanding.
Voting
Each holder of the Series A convertible preferred stock is entitled to the number of votes equal to the number of shares of common stock into which such shares of Series A convertible preferred stock could be converted. The preferred stockholders shall vote as a separate class to (i) approve amendments to the Certificate of Incorporation, (ii) authorization of any new classes of stock, and (iii) any asset transfers or acquisitions or any voluntary dissolution or liquidation of the Company.
For so long as any shares of preferred stock remain outstanding, the holders of the Series A convertible preferred stock may appoint one member of the Board in a nonvoting observer capacity.
Conversion Rights
Series A convertible preferred stock may, at the option of the holder, be converted at any time into shares of common stock at a conversion rate of $5.00 per share, subject to certain adjustments for stock splits, stock dividends, reclassifications and certain other events.
Each share of Series A convertible preferred stock will automatically be converted into shares of common stock on the then- effective Series A conversion price (i) at any time upon the affirmative election of the holders of the majority of the outstanding shares of the Series A convertible preferred stock or (ii) immediately upon the closing of a firmly underwritten public offering of common stock in which the gross cash proceeds to the Company are at least $20 million and the Company’s shares have been listed for trading on the New York Stock Exchange, NASDAQ Global Select Market or NASDAQ Global Market (“Qualified IPO”). Upon conversion, any declared and unpaid dividends would be paid.
Redemption Rights
Pursuant to the NAU Agreement, in connection with a Qualified IPO, the Company agreed to use the proceeds to redeem all shares of Series A convertible preferred stock (or common stock issued upon conversion) at a price per share equal to the greater of (i) the original issue price ($5.00) of Series A convertible preferred stock plus all unpaid accrued dividends and (ii) the then fair market value (“Redemption Price”).
In connection with a “change of control event,” the Company would use such proceeds to redeem all shares of Series A convertible preferred stock (or common stock issued upon conversion) at a price per share equal to the greater of (i) the original issue price ($5.00) of the Series A convertible preferred stock plus all unpaid accrued dividends and (ii) the then-current Redemption Price. A change of control event is defined as a liquidation, merger, stock sale or sale of substantially all of the assets of the Company.
F-26
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
12. Convertible Preferred Stock – (continued)
Liquidation Rights
The holders of the Series A convertible preferred stock have a liquidation preference that gives such holders first priority upon a change in control event whereby such holders shall be entitled to receive an amount in liquidation equal to the original issued price ($5.00) plus accrued unpaid dividends.
Series B Convertible Preferred Stock
In December 2015, the Company issued Series B convertible preferred stock at $7.75 per share as follows: (i) 312,861 shares to the holders of the 2014/2015 Convertible Notes in exchange for the cancellation of such notes; (ii) 66,651 shares to the holder of the Secured Promissory Note in exchange for the cancellation of such note; and (iii) 20,000 shares sold to a related party investor for cash in the Series B convertible preferred stock financing.
The Series B convertible preferred stock has been classified as temporary equity on the accompanying balance sheet at December 31, 2015. Although the Series B convertible preferred stock is not subject to mandatory redemption, upon certain change in control liquidation events that are outside of the Company’s control, the holders of the Series B convertible preferred stock can elect to receive, at their option, cash in amount equal to the liquidation value of such holder’s Series B convertible preferred stock.
As of December 31, 2015, the Company has 399,512 shares of Series B convertible preferred stock issued and outstanding with an aggregate carrying value of $3,096.
For year ended December 31, 2016, the Company issued an aggregate of 115,668 shares of Series B convertible preferred stock to investors at a per share price of $7.75 for total cash consideration of $896. In addition, in January 2016, a holder of 33,578 shares of Series B convertible preferred stock converted its shares into 33,578 shares of common stock.
In March 2016, certain 2016 Unsecured Notes were exchanged by the holders for 2,007 shares of Series B convertible preferred stock. See Note 8.
Upon the closing of the initial public offering in December of 2016, 483,609 shares of the Series B convertible preferred stock automatically converted into 483,609 shares of the Company’s common stock.
Significant provisions of the Series B convertible preferred stock are as follows:
Dividends
Dividends may be declared and paid on the Series B convertible preferred stock from funds legally available thereof as and when determined by the Board of Directors.
Liquidation Value
A change in control is treated as a liquidation event that entitles the holder to receive, at their option, cash in amount equal to the liquidation value of each holder’s Series B convertible preferred shares. The liquidation value for each share of Series B convertible preferred stock is an amount equal to $7.75 per share, subject to adjustment in the event of a stock split, stock dividend or similar event.
Conversion Rights
The holder of the Series B convertible preferred stock has the right to convert at any time all or part of the preferred shares into shares of common stock. Each share of Series B convertible preferred stock will automatically convert into shares of common stock on the closing of an underwritten public offering of the Company’s equity securities which results in gross proceeds of at least $5 million. The initial conversion price is $7.75 per share, subject to certain adjustments for stock splits, stock dividends, reclassification and certain other defined events.
Voting
Each holder of the Series B convertible preferred stock is entitled to the number of votes equal to the number of shares of common stock into which such shares of Series A convertible preferred stock could be converted. The holders of shares of Series B convertible preferred stock are entitled to vote on all matters submitted to the vote of the stockholders.
F-27
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
12. Convertible Preferred Stock – (continued)
Redemption Rights
The Series B convertible preferred stock is not subject to redemption.
13. Stockholders’ Deficit
Capital Stock
The Company was organized under the laws of the state of Nevada on July 27, 2004 and was subsequently reincorporated under the laws of the state of Delaware on November 10, 2015. In connection with the reincorporation, as approved by the stockholders, the Company changed its authorized capital stock to consist of (i) 100 million shares of common stock, $.001 par value, and (ii) 2 million shares of preferred stock, $0.001 par value, designated as Series A convertible preferred stock. In December 2015, the Company amended its Certificate of Incorporation to change its authorized capital stock to provide for 15 million authorized shares of preferred stock of which 7,515,000 was designated as Series B convertible preferred stock, par value $.001 per share.
Prior to November 10, 2015, the Company’s authorized capital stock consisted of 100 million shares of common stock, $.001 par value, and 10 million shares of preferred stock, $.001 par value.
Common Stock
The Company had 10,157,292 and 4,108,766 shares of common stock issued and outstanding as of December 31, 2016 and 2015, respectively. The holders of shares of common stock are entitled to one vote per share on all matters submitted to a vote of the stockholders. As long as shares of preferred stock are outstanding, the holders of outstanding shares of common stock are not entitled to receive any dividends. In the event of liquidation, dissolution or winding up of the Company, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preference of any then outstanding shares of preferred stock. The holders of common stock have no preemptive, conversion or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock.
In May and June 2015, the 2014 Notes with an outstanding principal and accrued interest of $452 were converted into 71,446 shares of common stock (conversion rate: principal – $7.50 per share; interest – $5.00 per share). The shares of common stock were recorded at their estimated fair value of $7.575 per share on the date of issuance. See Note 10.
In November 2015, the Company converted a note payable with a stockholder that had an outstanding principal balance, including accrued interest, totaling $68, into 8,971 shares of common stock in accordance with its original conversion terms. See Note 10.
On April 7, 2016, a short-term unsecured promissory note for $27 was settled with the issuance of 54,000 shares of common stock.
During 2015, the Company issued 390,873 shares of common stock upon the exercise of stock options for cash proceeds of $78.
On December 8, 2016, in connection with its initial public offering, the Company issued 1,875,000 shares of common stock for net consideration of $12.6 million.
In May, 2016, certain 2016 Unsecured Notes were surrendered as consideration for purchase of 124,000 shares of common stock in the Right’s offering at the subscription price of $2.50 per share.
For the year ended December 31, 2016, the Company issued 630,935 shares of common stock upon the exercise of stock options and warrants for cash proceeds of $521.
F-28
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
13. Stockholders’ Deficit – (continued)
During the year ended December 31, 2016, the Company issued an aggregate of 13,320 ordinary shares in net settlement of vested restricted stock units, valued at $295.
Rights Offering
In April 2016, the Company offered to the existing holders of shares of (i) its common stock and (ii) Series B convertible preferred stock, in each case, as of April 8, 2016 (the “Record Date”), at no charge, non-transferable subscription rights, on a pro rata basis, to purchase shares of common stock at a subscription price of $2.50 per share (the “Rights Offering”). In addition, the holders also had the right to purchase additional shares of common stock, if any shares remain unsubscribed. The Company offered subscription rights on 5,794,162 shares of its common stock. The Rights Offering was conducted as a private placement on a “best efforts” basis, with no minimum subscription required.
The subscription rights were initially exercisable beginning on April 8, 2016 and expiring on April 29, 2016 (the “Subscription Period”). However, the Company reserved the right to extend the Subscription Period for up to two additional weeks. The Company extended the Subscription Period for one additional week. The Rights Offering closed on May 6, 2016.
The Company issued 2,478,486 shares of common stock and received aggregate consideration of $6,199 in the Rights Offering. The aggregate consideration received consisted of: (i) $5,284 in cash; (ii) $821 in consideration paid through the cancellation of $821 in outstanding principal amount (and related unpaid interest) under certain 2016 Unsecured Notes (as defined below) and the 2015 Unsecured Notes; and (iii) the extinguishment of $94 in amounts owed by the Company for services and related miscellaneous expenses. Such cash proceeds will be used for working capital and general corporate purposes. As the Rights Offering was offered to certain existing holders of the Company’s stock, the shares sold are treated as outstanding from the date of their issuance in the computation of loss per share, basic and diluted in future periods.
14. Stock-based Compensation
Effective December 2008, the Company established the 2008 – 2009 Non-Qualified Stock Option Plan (the “2008 – 2009 Plan”) under which 20,000 stock options remain outstanding at December 31, 2016. The stock-based awards were issued with a price not less than $15.00 per share or 100% of the fair value of a share of common stock on the date of grant. After July 2015, no further awards were granted under the 2008 – 2009 Plan. Such outstanding awards will continue to be governed by their existing terms under the 2008 – 2009 Plan.
Effective July 2015, the Company’s stockholders approved the 2015 Equity Incentive Plan (the “2015 Plan”), which permits the issuance of up to 2,000,000 shares reserved for the grant of stock options, stock appreciation rights, restricted stock units and other stock-based awards for employees, directors or consultants of the Company. The Board of Directors approved an additional 1,000,000 shares of common stock for issuance under the 2015 Plan. The stock-based awards are generally issued with a price equal to no less than fair value at the date of grant. Options granted under the 2015 Plan generally vest immediately, or ratably over a two- to 36-month period coinciding with their respective service periods; however, participants may exercise their options prior to vesting as provided by the 2015 Plan. Unvested shares issued for option exercised early may be subject to a repurchase by the Company if the participant terminates at the original exercise price. Options under the 2015 Plan generally have a contractual term of five or ten years. Certain stock option awards provide for accelerated vesting upon a change in control or an initial public offering. As of December 31, 2016, the Company had 1,419,480 shares of common stock available for issuance under the 2015 Plan.
The Company measures the fair value of stock options with service-based and performance-based vesting criteria to employees, directors and consultants on the date of grant using the Black-Scholes option pricing model. The fair value of equity instruments issued to non-employees is re-measured as the award vests. The Black-Scholes valuation model requires the Company to make certain estimates and assumptions, including assumptions related to the expected price volatility of the Company’s stock, the period under which the options with be outstanding, the rate of return on risk-free investments, and the expected dividend yield for the Company’s stock.
During 2016, the Company issued a total of 126,373 shares of common stock, valued at $383, for services performed.
F-29
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
14. Stock-based Compensation – (continued)
The weighted-average assumptions used in the Black-Scholes option-pricing model used to calculate the fair value of options granted during the year ended December 31, 2015, were as follows:
| Employee | Non-Employee | |||||||
| Expected volatility | 66.4 | % | 76.6 | % | ||||
| Expected dividend yield | — | — | ||||||
| Expected term (in years) | 4.0 | 5.0 | ||||||
| Risk-free interest rate | 1.3 | % | 1.7 | % | ||||
The weighted-average assumptions used in the Black-Scholes option-pricing model used to calculate the fair value of options granted during the year ended December 31, 2016, were as follows:
| Employee | Non-Employee | |||||||
| Expected volatility | 82.1 | % | N/A | |||||
| Expected dividend yield | — | N/A | ||||||
| Expected term (in years) | 3.25 | N/A | ||||||
| Risk-free interest rate | 1.83 | % | N/A | |||||
Due to the Company’s limited operating history and lack of company-specific historical or implied volatility, the expected volatility assumption was determined based on historical volatilities from traded options of biotech companies of comparable in size and stability, whose share prices are publicly available. The expected term of options granted to employees is calculated based on the mid-point between the vesting date and the end of the contractual term according to the simplified method as described in SEC Staff Accounting Bulletin
F-30
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
14. Stock-based Compensation – (continued)
110 because the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term due to the limited period of time its awards have been outstanding. For non-employee options, the expected term of options granted is the contractual term of the options. The risk-free rate by reference to the implied yields of U.S. Treasury securities with a remaining term equal to the expected term assumed at the time of grant. The expected dividend assumption is based on the Company’s history and expectation of dividend payouts. The Company has not paid and does not intend to pay dividends.
The table summarizes the stock option activity, for both plans, for the periods indicated as follows:
| Number of Options | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (years) | Aggregate Intrinsic Value(1) | |||||||||||||
| Outstanding at December 31, 2014 | 255,509 | $ | 2.35 | 3.9 | $ | 3,230 | ||||||||||
| Granted | 2,477,255 | $ | 0.50 | 7.1 | ||||||||||||
| Exercised | 384,206 | $ | 0.05 | |||||||||||||
| Forfeited | 210,876 | $ | 0.50 | |||||||||||||
| Expired | 13,348 | $ | 15.00 | |||||||||||||
| Outstanding at December 31, 2015 | 2,124,334 | $ | 0.50 | 6.4 | $ | 4,249 | ||||||||||
| Granted | 200,000 | $ | 7.98 | 5.0 | $ | 34 | ||||||||||
| Exercised | 621,602 | $ | 0.73 | |||||||||||||
| Forfeited | 210,849 | $ | 0.50 | |||||||||||||
| Expired | 14,583 | $ | 15.00 | |||||||||||||
| Outstanding at December 31, 2016 | 1,477,300 | 1.61 | 5.8 | $ | 9,662 | |||||||||||
| Exercisable at December 31, 2016 | 999,310 | $ | 1.00 | 5.6 | $ | 7,145 | ||||||||||
| (1) | The aggregate intrinsic value on the table was calculated based on the difference between the estimated fair value of the Company’s stock and the exercise price of the underlying option. The estimated stock values used in the calculation was $8.15 and $2.50 per share for each of the years ended December 31, 2016 and 2015 respectively. |
The weighted average grant-date fair value of options granted to employees for the year ended December 31, 2016 was $7.98 per share.
The stock-based compensation expense was recorded as follows:
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Research and development | $ | 403 | $ | 4,931 | ||||
| General and administrative | 2,966 | 6,331 | ||||||
| Total stock-based compensation expense | $ | 3,369 | $ | 11,262 | ||||
The allocation between research and development and general and administrative expense was based on the department and services performed by the employee or non-employee.
Included in the table above, the Company recorded stock-based compensation expense of $207 and $338 for the years ended December 31, 2016 and 2015, respectively, for stock options granted to non-employees.
F-31
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
14. Stock-based Compensation – (continued)
In June 2016, the Company entered into an employment letter agreement with its chief executive officer which replaced the previous employment agreement dated October 16, 2013. At the same time, the Company entered into an employment letter agreement with its president and chief research officer that contained similar features and terms which replaced her previous employment agreement dated October 16, 2013. By entering into the employment letter agreements (the “2016 agreements”) and accepting the signing bonus, the chief executive officer and the president and chief research officer (collectively, the “executive officers”) waived all rights to receive any compensation amounts provided for in the previous employment agreements.
The 2013 agreements, among other provisions, provided
for a signing bonus of $1,000 on the acceptance and signing of such agreements. Upon the signing of the 2013 agreements, the Company
recorded a deferred compensation obligation as an undiscounted noncurrent liability, in the amount of an aggregate $2,000 for the
amount of the signing bonuses, since the payment of such obligation was not reliably determinable. The amount was classified as
a noncurrent liability as an event that makes the payment due and payable had not occurred.
In June 2016, the Company reversed the deferred compensation obligation of $2,000 to additional paid in capital which is in the period the terms of 2016 agreements were accepted and replaced the previous employment agreements. As such, the executive officers, whom are also principal stockholders, have forgiven the compensation that was previously earned and due under the 2013 agreements.
The 2016 agreements, among other things, provide for an annual base salary which may be adjusted periodically and, upon signing of the 2016 agreements, a signing bonus was payable within one business day after the signing the 2016 agreements. In addition, the 2016 agreements provide for an annual incentive bonus with a minimum target value equal to a certain stated percentage of annual base salary; however, any incentive bonus is determined at the discretion of the Board of Directors.
The 2016 agreements also provide for the grant of the award of restricted stock units (RSU) representing the right to receive 220,000 shares of the Company’s common stock. The RSU award will vest and be settled over a three-year period, with one- third of the units vesting on the twelve-month anniversary of the date of grant, and the remaining units vesting in equal quarterly tranches over the following twenty-four months of continuous service.
Upon the sale of the Company, the 2016 agreements provides for a bonus of (a) 1% of the amount of the net sales price of the Company that is $100,000 or less, plus (ii) an additional 0.5% of the amount of the net sales price of the Company that is more than $100,000, payable in cash or other proceeds payable to other stockholders in such Company. Under the terms of her agreement, the executives shall be entitled to this change of control bonus if the change of control transaction occurs within 12 months following the termination of the executive’s employment by the Company without cause (as such term is defined in the 2016 agreement excluding death or disability) or within 12 months following the executive’s resignation for good reason (as such term is defined in the 2016 agreement), provided that the executive remains in compliance with the confidentiality and other ongoing post-termination obligations under the 2016 agreement.
In the event of terminated without cause or resignation for good reason (as such terms are defined in the 2016 agreements) or upon death, all base salary and benefits for a period of twelve months following the effective date of such termination will be payable. Also, any earned but unpaid annual bonus, and all outstanding equity awards will accelerate immediately upon the date of termination.
In recognition of his continued support and cooperation, and to resolve a dispute regarding whether his options appropriately expired in the first quarter of 2016, in July 2016, the Company’s Board of Directors agreed to issue to its former chief executive officer 120,000 shares of the Company’s common stock. The expense of $300 associated with this full and final settlement was recorded in the year ended December 31, 2016.
At December 31, 2016, the total compensation cost related to non-vested options not yet recognized was $4,503, which will be recognized over a weighted average period of four years, assuming the employees complete their service period required for vesting.
Effective December 2008, the Company established the 2008-2009 Non-Qualified Stock Option Plan (the “2008 – 2009 Plan”) under which 20,000 stock options remain outstanding at September 30, 2016. The stock-based awards were issued with a price not less than $15.00 per share or 100% of the fair value of a share of common stock on the date of grant. After July 2015, no further awards were granted under the 2008 – 2009 Plan. Such outstanding awards will continue to be governed by their existing terms under the 2008 – 2009 Plan.
F-32
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
14. Stock-based Compensation – (continued)
Effective July 2015, the Company’s stockholders approved the 2015 Equity Incentive Plan (the “2015 Plan”), which permits the issuance of up to 2,000,000 shares reserved for the grant of stock options, stock appreciation rights, restricted stock units and other stock-based awards for employees, directors or consultants
Restricted Stock Units
The following table summarizes restricted stock unit activity for the years ended December 31, 2016 and 2015:
| Number of Units | Weighted Average Grant-Date Fair Value Per Units | |||||||
| Outstanding as of December 31, 2014 | — | $ | — | |||||
| Granted | — | $ | — | |||||
| Vested | — | $ | — | |||||
| Forfeited | — | $ | — | |||||
| Outstanding as of December 31, 2015 | — | $ | — | |||||
| Granted | 474,720 | (1) | $ | 1.57 | ||||
| Vested | (19,290 | ) | $ | 8.10 | ||||
| Forfeited | — | $ | — | |||||
| Outstanding as of December 31, 2016 | 455,430 | $ | 0.76 | |||||
| (1) | 440,000 restricted stock units were granted and issued on September 30th with a weighted average grant date fair value of $0.50 and vest 1/3 on the first anniversary of grant and the remaining balance quarterly over the subsequent 8 quarters. 19,290 restricted stock units were granted on December 19, 2016 with a weighted average grant date fair value of $8.10 and vested immediately. An additional 15,430 restricted stock units were granted on December 19, 2016 and fully vest on the first anniversary of grant. The Company accounts for the restricted stock units as equity-settled awards in accordance with ASC 718. The total fair value of restricted stock units vested during the years ended December 31, 2016 and 2015 was $156 and $0.00, respectively. |
15. Income Taxes
Tax Rate Reconciliation
The income tax benefit differed from the amounts computed by applying the federal statutory income tax rate of 34% to pretax income from operations as a result of the following:
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Income tax benefit at statutory federal rate | $ | (3,723 | ) | $ | (6,179 | ) | ||
| Increase (reduction) in income taxes resulting from: | ||||||||
| Nondeductible expenses | 7 | 69 | ||||||
| Fair value adjustment on convertible notes | 2 | 226 | ||||||
| State and local income taxes, net of federal income tax benefit | (505 | ) | (799 | ) | ||||
| Federal valuation allowance | 3,714 | 5,884 | ||||||
| State valuation allowance | 505 | 799 | ||||||
| $ | — | $ | — | |||||
F-33
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
15. Income Taxes – (continued)
Significant Components of Current and Deferred Taxes
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets are presented below:
| At December 31, | ||||||||
| 2016 | 2015 | |||||||
| Deferred tax assets: | ||||||||
| Deferred rent | $ | 25 | $ | 11 | ||||
| Deferred revenue | - | 85 | ||||||
| Federal and state net operating loss carryforwards | 10,818 | 10,727 | ||||||
| Stock-based compensation | 4,831 | 6,493 | ||||||
| Compensation accruals and other | 553 | 36 | ||||||
| Total deferred tax assets | 16,227 | 17,352 | ||||||
| Valuation allowance | (16,144 | ) | (17,277 | ) | ||||
| Net deferred tax assets | 83 | 75 | ||||||
| Deferred tax liabilities: | ||||||||
| Total deferred tax liabilities | (83 | ) | (75 | ) | ||||
| Net deferred tax asset (liability) | $ | - | $ | - | ||||
| Property and equipment | (83 | ) | (75 | ) | ||||
In assessing the realizability of deferred tax assets, management considers whether it is more-likely-than-not that some portion or all of the deferred taxes will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considered the scheduled reversal of the deferred tax liabilities including the impact of available carryback and carryforward periods and does not believe it is more-likely-than-not the Company will realize the benefits of the deferred tax assets. Accordingly, a valuation allowance has been recorded against the deferred tax assets. The valuation allowance decreased by approximately $1.1 million for the year ended December 31, 2016 and increased $6.7 million for the year ended December 31, 2015.
At December 31, 2016 and 2015, the Company had federal and state net operating loss carryforwards of $34.0 million and $27.8 million, respectively. The state loss carryforwards will begin expiring in 2016 and the federal loss carryforwards will begin expiring in 2021, unless previously utilized.
Utilization of the net operating loss carryforwards may be subject to a substantial annual limitation due to the ownership change limitations provided by Section 382 of the Internal Revenue Code and similar state provisions. Generally, in addition to certain entity reorganizations, the limitation applies when one or more 5% stockholders increase their ownership, in the aggregate, by more than 50% percentage points over a 36-month time period testing period, or the beginning the day after the most recent ownership change, if shorter. The annual limitation may result in the expiration of net operating losses and credit before utilization.
The Company files a federal income tax return. For taxable years ending before 2012, the Company is no longer subject to U.S. federal examination; however, the Internal Revenue Service has the ability to review years prior to 2012 to the extent the Company utilizes tax attributes carried forward from those prior years. The statute of limitations on the Company’s state filings is four years.
16. License and Other Agreements
Neogen Corporation
In May 2014, the Company entered into an exclusive license agreement with Neogen Corporation (“Neogen”). The Company granted an exclusive license to Neogen to (i) use the Company’s intellectual property (“IP”), consisting primarily of the ContraPest technology and (ii) manufacture, distribute and sell commercial rodent control products in the United States and certain U.S. territories, Canada and Mexico. Under the terms of the licensing agreement, the Company was required to submit an application to the United States Environmental Protection Agency (“EPA”) for approval of ContraPest, complete two agricultural field trials that support commercial feasibility for use of the product, and submit such studies and results to Neogen for their approval. The application to the EPA was submitted in August 2015, and the EPA granted registration approval for ContraPest effective August 2, 2016. The first field trial is complete, but has not yet been approved by Neogen. With respect to the second trial, the EPA indicated to the Company that it would be more efficient to wait until the product was approved rather than applying for an additional experimental use permit. Given that the EPA has granted registration approval, the Company is now preparing to commence the second field trial.
F-34
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
16. License and Other Agreements – (continued)
The Company has received nonrefundable, upfront license fee payments, totaling $488. The remaining license fee of $162 will be received when Neogen formally accepts the Company’s report on its study of the field trials. The Company has determined that the license does not have stand-alone value therefore, the license fees of $488 are deferred and recognized, on a straight-line basis, from May 2014, the effective date of the agreement, over the estimated related period of performance through December 2016, which includes the acceptance by Neogen of the Company’s study for the field trials that support commercial feasibility for use of the product.
For each of the years ended December 31, 2016 and 2015, the Company recognized revenue of $186 under the licensing agreement. As of December 31, 2016 and 2015, deferred revenue amounted to $0 and $186, respectively. Any changes in the estimated period of performance wound have been accounted for prospectively as a change in estimate.
In addition, Neogen was obligated under the licensing agreement to pay additional consideration to the Company consisting of future fixed-amount of contingent milestone payments (i.e. post-regulatory approval license fees) of up to an aggregate of $3 million, and sales-based royalties on the net sales of licensed products by Neogen, as well as its affiliates and sublicensees. The Company did not receive or earn these potential contingent consideration payments as the milestone events to receive such post-approval license fees and sales based royalties were not achieved prior to the termination of the agreement. The agreement was to expire upon the later of (i) the last expiration of last patent included in the licensed IP; or (ii) the tenth anniversary of the effective date of the agreement (i.e. May 2024).
As previously disclosed in our Current Report on Form 8-K dated and filed January 23, 2017, on January 23, 2017 we entered into a termination agreement (the “Settlement Agreement”) with Neogen Corporation (“Neogen”). Pursuant to the Settlement Agreement, the parties agreed to (a) terminate the existing Exclusive License Agreement between us and Neogen dated May 15, 2014 (the “License Agreement”), with neither Neogen or us having any further obligations thereunder (other than certain confidentiality obligations); (b) dismiss with prejudice the court action filed by Neogen in the District Court for the District of Arizona on January 19, 2017 (the “Court Action”), as further described below; and (c) mutually release any and all existing or future claims between the parties and their affiliates related to or arising from the License Agreement or the Court Action. As part of the Settlement Agreement, we agreed to pay to Neogen upon the execution of the Settlement Agreement an aggregate of $1.0 million in settlement of all claims.
NeoVenta Solutions, Inc.
In September 2015, the Company entered into a marketing, sales and distribution agreement with NeoVenta Solutions, Inc. (“NeoVenta”). The Company granted an exclusive license to NeoVenta to market, sell and distribute its product in certain granted foreign countries (“granted countries”), consisting of Bangladesh, India, Indonesia, Malaysia, Singapore, Sri Lanka and Thailand. Other granted countries can be added when approved and agreed upon by the parties. NeoVenta will be responsible for seeking applicable regulatory approvals to market ContraPest in the granted countries. After such regulatory approvals have been obtained, the Company has granted to NeoVenta an exclusive license to market, sell and distribute its product in these granted countries. NeoVenta will make purchases of product at predetermined prices from the Company. NeoVenta has agreed to minimum sales commitments of rodent control products in granted countries, which total $23 million over the first five years. NeoVenta will make purchases of product at predetermined prices from the Company.
The initial term of the agreement is for five years from the earlier of (i) obtaining the required certifications for ContraPest or any products saleable in any of the countries in the territory; or (ii) six months after approval of ContraPest or any other products by the EPA. The agreement will automatically renew for one additional five year period. Thereafter, the agreement will renew for successive one-year periods unless written notification of intent not to renew is provided by either party to the other not less than sixty days prior to the beginning of any one-year renewal period.
F-35
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
16. License and Other Agreements – (continued)
Somerville
In May 2014, the Company entered into a service contract with the City of Somerville, Massachusetts (“Somerville”) whereby the Company was providing services and/or supplies in connection with conducting a rodent population study through field trials for Somerville. The total contract amount was not to exceed $215 for the services rendered and/or supplies received. Through an amendment to the contract, the contract was extended to December 31, 2016. Services under the contract are now complete. The Company has recognized revenue for services rendered under this contract of $132 during the year ended December 31, 2016 in other revenue in the statements of operations and comprehensive loss.
Chicago Transit Authority
In September 2015 the Company entered into a services contract with the Chicago Transit Authority, or CTA, to begin a field trial of the Company’s bait station, which study is now complete. The total contract amount was not to exceed $58. The services contract ended in March 2016. The Company recognized revenue for services rendered under this contract of $42 during the year ended December 31, 2015, in other revenue in the statements of operations and comprehensive loss.
University of Arizona
In 2005, the Company entered into an exclusive license agreement with the Arizona Board of Regents of the University of Arizona (“University”) to in-license certain patents and other intellectual property to be used in the future product development in the domestic animal fertility control market. The patent claims in the United States, Australia and New Zealand cover the use of the vinyl cyclohexene Diep oxide to deplete ovarian follicles in individual mammals and reproduction of mammals. The license agreement gives the Company exclusive rights to commercialize products based on this intellectual property. The University owns the patent rights, but the agreement requires the Company to pay all costs incurred by the University in maintaining and perfecting the patent rights. In exchange for the intellectual property, the Company paid the University a nonrefundable fee of $5, agreed to reimburse the University for its patent costs, pay milestone payments totaling up to $75 upon the achievement of certain research, development and regulatory milestones. In addition, the University is entitled to royalty fees of 5% of net sales of the licensed product and sublicensing royalty income of licensed product.
In June 2015, the Company and University executed an amended and restated exclusive license agreement. The amendment reduced the milestone payments to totaling up to $50,000 upon the achievement of certain research, development and regulatory milestones and royalty fees from 5% to 2% of net sales of licensed product and 4% of any sublicensing royalty income of licensed product. As consideration for the amended terms, the Company entered into a warrant purchase agreement whereby the University was granted a warrant to purchase 15,000 shares of common stock, with an initial fair value of $53. The warrant is exercisable immediately for a term of five years from the effective date of the amendment. See Note 9.
The agreement will terminate with last-to-expire patent licensed under the agreement which extends to 2026. The Company may terminate the agreement or the grant of rights under the agreement, at any time, upon ninety days prior written notice to the University. Future milestone payments are considered to be contingent consideration and will be accrued when probable of being paid. At December 31, 2015 and 2016, no milestone payments were probable of being paid and it is not likely in the near future.
Bioceres/INMET S.A. Agreement
In January 2016, the Company entered into a services agreement with Bioceres, Inc. (“Bioceres”), a wholly-owned subsidiary of Bioceres S.A., a leading agricultural biotechnology company in Argentina, and its Argentinean subsidiary, Ingenieria Metabolica
S.A. (“INMET”) to develop a production method for synthetic triptolide, the main ingredient in ContraPest. The Company also entered into an agency agreement with INMET whereby the Company appointed INMET as its exclusive agent to seek regulatory approval for and conduct pre-sales and marketing of its product, ContraPest, in Argentina. The Company and INMET have also agreed to manufacture and distribute its product in Argentina and other countries, as mutually agreed, through a newly formed entity.
F-36
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
16. License and Other Agreements – (continued)
The term of the service agreement is for two years. The service agreement can be terminated at any time upon written notice by either party for any reason. The term of the agency agreement with INMET is the earlier of: (i) when the Company and INMET incorporate the joint venture entity in Argentina or (ii) January 2018.
17. Commitments and Contingencies
Legal Proceedings
The Company may be subject to legal proceedings and claims arising from contracts or other matters from time to time in the ordinary course of business. Management is not aware of any pending or threatened litigation where the ultimate disposition or resolution could have a material adverse effect on its financial position, results of operations or liquidity.
Neogen Settlement Agreement
As previously disclosed in the Company’s Current Report on Form 8-K dated and filed January 23, 2017, on January 23, 2017, the Company entered into an agreement (the “Settlement Agreement”) with Neogen Corporation (“Neogen”). Pursuant to the Settlement Agreement, the parties agreed to (a) terminate the existing Exclusive License Agreement between us and Neogen dated May 15, 2014 (the “License Agreement”), with neither Neogen or the Company having any further obligations thereunder (other than certain confidentiality obligations); (b) dismiss with prejudice the court action filed by Neogen in the District Court for the District of Arizona on January 19, 2017 (the “Court Action”), as further described below; and (c) mutually release any and all existing or future claims between the parties and their affiliates related to or arising from the License Agreement or the Court Action. Prior to the notice of filing received by SenesTech, the Company was unaware that any action was contemplated or actioned and was proceeding with all elements of the agreement in good faith. All communications prior to the complaint indicated that Neogen also was proceeding in good faith to execute on the agreement.
Under the terms of the agreement, the Company agreed to make a one-time payment in the amount of $1,000 in settlement of all claims and termination of all existing contracts between the parties. Both Neogen and the Company further agreed to drop any and all legal complaints, claims or threat of litigation for failure to perform under the previous contractual relationship.
Although notice of the legal action by Neogen and the subsequent agreement to terminate existing agreements with Neogen, occurred AFTER December 31, 2016, as per the provisions of Accounting Standards Codification Topic 450 Loss Contingencies, included in the financial statements of the Company at December 31, 2016 is a $1,000 charge to general and administrative expenses and a corresponding accrual of contract cancellation settlement agreement related to this agreement.
Employment Agreements
The Company entered into an employment agreement, dated October 2013, with the Chief Executive Officer which provides for an employment term of three years and will automatically renew for an additional three-year period unless terminated by either party by giving ninety days written notice. At the same time, the Company entered into an employment agreement with its Chief Research Officer that contained similar features and terms. The agreements, among other provisions, provides for an annual base salary which will be reviewed and may be adjusted periodically, and upon signing the agreement, a signing bonus of $1,000. The signing bonus was to be paid over three years in eleven quarterly installments of $91 per quarter, payable only from product revenue after providing for all operational expenses and such bonus was not to be paid from funds received from capital investment. The bonus would become immediately payable and due upon the employees’ termination, disability or death. Upon the signing of the employment agreements in 2013, the Company recorded a deferred compensation obligation, undiscounted non-current liability, in the amount of an aggregate $2,000 in signing bonuses, since the payment of such obligation was not reliably determinable. The amount was classified as a non-current liability until an event occurs that makes the payment due and payable.
In addition, upon the sale of the Company, the agreements provided for, at the employees’ election, a lump sum cash payment equal to the current value of shares of common stock held, or no less than $3.00 per share, whichever is greater. In addition, upon the sale of the Company, the agreement provided for a bonus of (a) 1% of the amount of the net sales price that is $100,000 or less and (ii) 0.5% of the amount of the net sales price that is more than $100,000. Under the terms of the agreement, this change of control bonus would be paid regardless of whether employed by the Company at the time of any sale of all or a portion of the stock or assets, and if deceased, such amount would be paid to the employees’ estate.
The agreements provided for cash payments pursuant to a phantom equity interest in all patents owned and/or leased by the Company, such that the payments under the phantom equity interest shall be determined as if the phantom patent interested represented a one half of one percent (0.5%) gross profits interest in the patents. Gross profit means the amount received from customers that license product that has registrations. The patent bonus is due in perpetuity for so long as the Company or any successor exists regardless of whether the employees continue to be employed at the time the profits are earned by the Company or any successor entity.
In June 2016, the Company entered into an employment letter agreement with its chief executive officer which replaced the previous employment agreement dated October 16, 2013. At the same time, the Company entered into an employment letter agreement with its president and chief research officer that contained similar features and terms which replaced her previous employment agreement dated October 16, 2013. By entering into the employment letter agreements (the “2016 agreements”) and accepting the signing bonus, the chief executive officer and the president and chief research officer (collectively, the “executive officers”) waived all rights to receive any compensation amounts provided for in the previous employment agreements noted above.
F-37
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
17. Commitments and Contingencies – (continued)
The 2013 agreements, among other provisions, provided for a signing bonus of $1,000 on the acceptance and signing of such agreements. Upon the signing of the 2013 agreements, the Company recorded a deferred compensation obligation as an undiscounted noncurrent liability, in the amount of an aggregate $2,000 for the amount of the signing bonuses, since the payment of such obligation was not reliably determinable. The amount was classified as a noncurrent liability as an event that makes the payment due and payable had not occurred.
In June 2016, the Company reversed the deferred compensation obligation of $2,000 to additional paid in capital which is in the period the terms of 2016 agreements were accepted and replaced the previous employment agreements. As such, the executive officers, whom are also principal stockholders, have forgiven the compensation that was previously earned and due under the 2013 agreements.
The 2016 agreements, among other things, provide for an annual base salary which will be reviewed and may be adjusted periodically and, upon signing of the 2016 agreements, a signing bonus was payable within one business day after the signing the 2016 agreements. In addition, the 2016 agreements provide for an annual incentive bonus with a minimum target value equal to a certain stated percentage of annual base salary; however, any incentive bonus is determined at the discretion of the Board of Directors.
The 2016 agreements also provide for the grant of the award of restricted stock units (RSU) representing the right to receive 220,000 shares of the Company’s common stock. The RSU award will vest and be settled over a three-year period, with one- third of the units vesting on the twelve-month anniversary of the date of grant, and the remaining units vesting in equal quarterly tranches over the following twenty-four months of continuous service.
Upon the sale of the Company, the 2016 agreements provides for a bonus of (a) 1% of the amount of the net sales price of the Company that is $100,000 or less, plus (ii) an additional 0.5% of the amount of the net sales price of the Company that is more than $100,000, payable in cash or other proceeds payable to other stockholders in such Company. Under the terms of her agreement, the executives shall be entitled to this change of control bonus if the change of control transaction occurs within 12 months following the termination of the executive’s employment by the Company without cause (as such term is defined in the 2016 agreement excluding death or disability) or within 12 months following the executive’s resignation for good reason (as such term is defined in the 2016 agreement), provided that the executive remains in compliance with the confidentiality and other ongoing post-termination obligations under the 2016 agreement.
In the event of terminated without cause or resignation for good reason (as such terms are defined in the 2016 agreements) or upon death, all base salary and benefits for a period of twelve months following the effective date of such termination will be payable. Also, any earned but unpaid annual bonus, and all outstanding equity awards will accelerate immediately upon the date of termination.
F-38
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
17. Commitments and Contingencies – (continued)
Resolution of Dispute
In recognition of his continued support and cooperation, and to resolve a dispute regarding whether his options appropriately expired in the first quarter of 2016, in July 2016, the Company’s Board of Directors agreed to issue to its former chief executive officer 120,000 shares of the Company’s common stock. The expense of $300 associated with this full and final settlement was recorded at December 31, 2016.
Lease Commitments
The Company is obligated under capital leases for certain research and computer equipment that expires in October 2017 through May 2020. At December 31, 2015, the gross amount of office and computer equipment, and research equipment and the related accumulated amortization recorded under the capital leases was $69 and $21, respectively.
In February 2012, the Company entered into an operating lease for its corporate headquarters. The lease was due to expire in January 2015. In December 2013, the Company amended its lease to expand into the remaining area in the building and extended the term to December 31, 2019. In February 2014, the Company further amended the lease to expand into an adjacent building. The lease requires escalating rental payments over the lease term. Minimum rental payments under the operating lease are recognized on a straight-line basis over the term of the lease and accordingly, the Company records the difference between the cash rent payments and the recognition of rent expense as a deferred rent liability. The lease is guaranteed by the President of the Company.
On November 16, 2016, we leased an additional 25,000 square feet of research and development space, also in Flagstaff, This lease expires on November 15, 2018. The lease requires fixed rental payments over the lease term. Minimum rental payments under the operating lease are recognized on a straight-line basis over the term of the lease and accordingly, the Company records the difference between the cash rent payments and the recognition of rent expense as a deferred rent liability.
Rent expense was $234 and $205 and $175 for the year ended December 31, 2016, 2015 and 2014, respectively. The future minimum lease payments under non-cancellable operating lease and future minimum capital lease payments as of December 31, 2016 are follows:
| Capital Leases |
Operating Lease |
|||||||
| Years Ending December 31, | ||||||||
| 2017 | 26 | 254 | ||||||
| 2018 | 18 | 258 | ||||||
| 2019 | 10 | 221 | ||||||
| 2020 | 3 | - | ||||||
| Total minimum lease payments | $ | 57 | $ | 733 | ||||
F-39
SENESTECH, INC.
NOTES TO THE FINANCIAL STATEMENTS
(In thousands, except share and per share data)
17. Commitments and Contingencies – (continued)
| Capital Leases | ||||
| Less: amounts representing interest (6.39%, ranging from 10.48% to 11.56%) | $ | 7 | ||
| Present value of minimum lease payments | 50 | |||
| Less: current installments under capital lease obligations | 22 | |||
| Total long-term portion | $ | 28 | ||
18. Subsequent Events
Neogen Settlement Agreement
As previously disclosed in the Company’s Current Report on Form 8-K dated and filed January 23, 2017, on January 23, 2017, the Company entered into an agreement (the “Settlement Agreement”) with Neogen Corporation (“Neogen”). Pursuant to the Settlement Agreement, the parties agreed to (a) terminate the existing Exclusive License Agreement between us and Neogen dated May 15, 2014 (the “License Agreement”), with neither Neogen or the Company having any further obligations thereunder (other than certain confidentiality obligations); (b) dismiss with prejudice the court action filed by Neogen in the District Court for the District of Arizona on January 19, 2017 (the “Court Action”), as further described below; and (c) mutually release any and all existing or future claims between the parties and their affiliates related to or arising from the License Agreement or the Court Action. Prior to the notice of filing received by SenesTech, the Company was unaware that any action was contemplated or actioned and was proceeding with all elements of the agreement in good faith. All communications prior to the complaint indicated that Neogen also was proceeding in good faith to execute on the agreement.
Under the terms of the agreement, the Company agreed to make a one-time payment in the amount of $1,000 in settlement of all claims and termination of all existing contracts between the parties. This payment was made in January, 2017. Both Neogen and the Company further agreed to drop any and all legal complaints, claims or threat of litigation for failure to perform under the previous contractual relationship.
Although notice of the legal action by Neogen and the subsequent agreement to terminate existing agreements with Neogen, occurred AFTER December 31, 2016, as per the provisions of FAS 5 Loss Contingency, included in the financial statements of the Company at December 31, 2016 is a $1,000 charge to general and administrative expenses and a corresponding accrual of contract cancellation settlement agreement related to this agreement.
In January, 2017, the Company issued 3,750 shares of common stock to a consultant for services.
In March, 2017, the Board of Directors granted 40,000 restricted stock units to a Vice President of the Company. These options vested immediately and the Company will recognize compensation expense in March of 2017 for the value of the grant.
The Company has evaluated subsequent events from the balance sheet date through March 31, 2017, the date at which the financial statements were issued, and determined that there were no other items that require adjustment to or disclosure in the financial statements.
F-40
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
| Item 9A. | Controls and Procedures. |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that the information required to be disclosed in the reports that we file or submit under the Securities Exchange Act of 1934 (“Exchange Act”) is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In connection with the preparation of this Annual Report on Form 10-K, our management carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, as of December 31, 2016, of the effectiveness of the design and operation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) and 15d-15(e) under the Exchange Act. Based upon this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2016.
This annual report does not include a report of management's assessment regarding internal control over financial reporting or an attestation report of the company's registered public accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
Changes in Internal Control over Financial Reporting
We previously identified material weaknesses in our internal control over financial reporting for the year ended December 31, 2015. See “Risk Factors — We have not fully assessed our internal control over financial reporting. We have previously identified and may in the future identify material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock.” While we have not yet assessed our internal control over financial reporting, we are in the process of implementing measures designed to improve our internal control over financial reporting, including how to remediate the control deficiencies that led to our previously identified material weaknesses, including:
| · | the appointment of a Corporate Controller in May 2016; |
| · | the establishment of formalized accounting policies and procedures and internal controls; and |
| · | the implementation of manual and automated controls to support our overall control environment and the segregation of duties and procedures. |
| Item 9B. | Other Information. |
None.
| 43 |
| Item 10. | Directors, Executive Officers and Corporate Governance. |
Certain information required by this Item regarding our directors and executive officers is set forth in Part I of this report under Item 1, “Business—Directors and Executive Officers of the Registrant” and will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the captions “Director Nominees,” “Continuing Directors” and “Executive Officers” and is incorporated herein by this reference.
The information required by this Item regarding compliance by our directors, executive officers and holders of ten percent of a registered class of our equity securities with Section 16(a) of the Securities Exchange Act of 1934 will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” and is incorporated herein by this reference.
The remaining information required by this Item will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the caption “Corporate Governance” and is incorporated herein by this reference.
| Item 11. | Executive Compensation. |
The information required by this Item will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the captions “Corporate Governance” and “Executive Officer Compensation” and is incorporated herein by this reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The information required by this Item regarding equity compensation plan information will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the caption “Equity Compensation Plan Information” and is incorporated herein by this reference.
The information required by this Item regarding security ownership will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the caption “Security Ownership of Principal Stockholders, Directors and Management” and is incorporated herein by this reference.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
The information required by this Item will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the captions “Corporate Governance” and “Certain Relationships and Related Transactions” and is incorporated herein by this reference.
| Item 14. | Principal Accounting Fees and Services. |
The information required by this Item with respect to principal accounting fees and services will be included in our definitive proxy statement for our 2017 annual meeting of shareholders to be filed with the SEC under the captions “Ratify Appointment of Independent Registered Public Accounting Firm” and is incorporated herein by this reference.
| 44 |
| Item 15. | Exhibits, Financial Statement Schedules. |
(a) Financial Statements and Schedules
| 1. | Financial Statements. |
The following consolidated financial statements are filed as part of this report under Item 8 of Part II, “Financial Statements and Supplementary Data.”
| A. | Balance Sheets as of December 31, 2016 and 2015. |
| B. | Statements of Operations and Comprehensive Income (Loss) for the years ended December 31, 2016 and 2015. |
| C. | Statements of Stockholders’ Equity for the years ended December 31, 2016 and 2015. |
| D. | Statements of Cash Flows for the years ended December 31, 2016 and 2015. |
| 2. | Financial Statement Schedules. |
Financial statement schedules not included herein have been omitted because they are either not required, not applicable, or the information is otherwise included herein.
| 3. | Exhibits. |
Exhibits are incorporated herein by reference or are filed with this report as indicated below (numbered in accordance with Item 601 of Regulation S-K).
(b) Exhibits
The exhibits listed in the accompanying Index to Exhibits are filed with this report or incorporated herein by reference.
(c) Financial Statement Schedules
None
| Item 16. | Form 10-K Summary. |
Not applicable.
| 45 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| SENESTECH, INC. | ||
| Date: March 31, 2017 | By: | /s/ Loretta P. Mayer, Ph.D. |
| Loretta P. Mayer | ||
| Chair of the Board, Chief Executive Officer and Chief Scientific Officer | ||
Date: March 31, 2017 |
By: | /s/ Thomas C. Chesterman |
| Thomas C. Chesterman | ||
| Chief Financial Officer and Treasurer | ||
| 46 |
POWER OF ATTORNEY
Each person whose individual signature appears below hereby authorizes and appoints Loretta P. Mayer, Ph.D. and Thomas C. Chesterman, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his true and lawful attorney-in-fact and agent to act in his name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file, any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on March 31, 2017, on behalf of the registrant and in the capacities indicated.
| Signature | Title | |
| /s/ Loretta P. Mayer, Ph.D. | ||
| Loretta P. Mayer, Ph.D. | Chair of the Board, Chief Executive Officer and Chief Scientific Officer | |
| (Principal Executive Officer) | ||
| /s/ Thomas C. Chesterman | ||
| Thomas C. Chesterman | Chief Financial Officer and Treasurer | |
| (Principal Financial and Accounting Officer) | ||
| /S/ Cheryl A. Dyer, Ph.D. | President, Chief Research Officer and Director | |
| Cheryl A. Dyer, Ph.D. | ||
| /S/ Grover Wickersham | Vice-Chair of the Board | |
| Grover Wickersham | ||
| /S/ Marc Dumont | Director | |
| Marc Dumont | ||
| /S/ Bob Ramsey | Director | |
| Bob Ramsey | ||
| /S/ Matthew K. Szot | Director | |
| Matthew K. Szot |
| /S/ Julia Williams, M.D. | ||
| Julia Williams, M.D. | Director |
| 47 |
SENESTECH, INC.
INDEX TO EXHIBITS
| Incorporated by Reference | ||||||||||||
| Exhibit Number |
Description | Filed or Furnished Herewith |
Form | Filing Date | Exhibit | File No. | ||||||
| 3.1 | Amended and Restated Certificate of Incorporation | S-1/A | 10/20/2016 | 3.3 | 333-213736 | |||||||
| 3.2 | Amended and Restated Bylaws | S-1 | 9/21/2016 | 3.5 | 333-213736 | |||||||
| 10.1(1) | SenesTech, Inc. 2008 – 2009 Non-Qualified Stock Option Plan and form of agreement thereunder | S-1 | 9/21/2016 | 10.1 | 333-213736 | |||||||
| 10.2(1) | SenesTech, Inc. 2015 Equity Incentive Plan and forms of agreement thereunder | S-1 | 9/21/2016 | 10.2 | 333-213736 | |||||||
| 10.3(1) | Form of Restricted Stock Unit Agreement | 8-K | 12/21/2016 | 4.1 | 001-37941 | |||||||
| 10.4(1) | Form of Indemnification Agreement | S-1 | 9/21/2016 | 10.2 | 333-213736 | |||||||
| 10.5(1) | Employment Letter Agreement by and between the Registrant and Loretta P. Mayer, Ph.D. dated June 30, 2016 | S-1 | 9/21/2016 | 10.7 | 333-213736 | |||||||
| 10.6(1) | Employment Letter Agreement by and between the Registrant and Cheryl A. Dyer, Ph.D. dated June 30, 2016 | S-1 | 9/21/2016 | 10.8 | 333-213736 | |||||||
| 10.7(1) | Employment Offer Letter by and between the Registrant and Thomas Chesterman dated November 20, 2015. | S-1 | 9/21/2016 | 10.9 | 333-213736 | |||||||
| 10.8 | Lease by and between the Registrant and Caden Court, LLC, dated as of December 20, 2011 and amendments thereto dated December 6, 2013 and February 27, 2014. | S-1 | 9/21/2016 | 10.5 | 333-213736 | |||||||
| 10.9(2) | Agency Agreement by and between the Registrant, Inmet S.A. and Bioceres, Inc. dated January 21, 2016. | S-1 | 9/21/2016 | 10.10 | 333-213736 | |||||||
| 10.10(2) | Services Agreement by and between the Registrant, Inmet S.A. and Bioceres, Inc. dated January 21, 2016. | S-1 | 9/21/2016 | 10.11 | 333-213736 | |||||||
| 10.11(2) | Marketing, Sales and Distribution Agreement by and between the Registrant and NeoVenta Solutions, Inc. dated September 26, 2015. | S-1 | 9/21/2016 | 10.13 | 333-213736 | |||||||
| 10.12 | Settlement Agreement and Release dated January 23, 2017 by and between Neogen Corporation and the Registrant. | 8-K | 1/23/2017 | 1.1 | 001-37941 | |||||||
| 21.1 | Subsidiaries of the Registrant | X | ||||||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm | X | ||||||||||
| 31.1 | Certification of Chief Executive Officer pursuant to Exchange Act Rule 13a-14(a) under the Securities and Exchange Act of 1934 | X | ||||||||||
| 31.2 | Certification of Chief Financial Officer pursuant to Exchange Act Rule 13a-14(a) under the Securities and Exchange Act of 1934 | X | ||||||||||
| 32.1 | Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 32.2 | Certification of Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| |
| (1) | Indicates a management contract or compensatory plan or arrangement. |
| (2) | Confidential treatment has previously been granted by the SEC for certain portions of the referenced exhibit. |
