Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Aevi Genomic Medicine, Inc. | v462189_ex99-1.htm |
| 8-K - FORM 8-K - Aevi Genomic Medicine, Inc. | v462189_8-k.htm |
Exhibit 99.2

© 2017, Aevi Genomic Medicine AEVI - 001: SAGA Trial in mGluR + ADHD Topline Results March 20, 2017

© 2017, Aevi Genomic Medicine 2 Forward - Looking Statement This presentation includes certain estimates and other forward - looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, including statements with respect to anticipated operating and financial performance, clinical results, potential partnerships, licensing opportunities and other statements of expectation. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “assumes,” “seeks,” “estimates,” “should” and variations of these words and similar expressions, are intended to identify these forward - looking statements. While we believe these statements are accurate, forward - looking statements are inherently uncertain and we cannot assure you that these expectations will occur and our actual results may be significantly different. These statements by the Company and its management are based on estimates, projections, beliefs and assumptions of management and are not guarantees of future performance. Important factors that could cause actual results to differ from those in the forward - looking statements include the factors described in the Company’s filings with the U.S. Securities and Exchange Commission. The Company disclaims any obligation to update or revise any forward - looking statement based on the occurrence of future events, the receipt of new information, or otherwise.
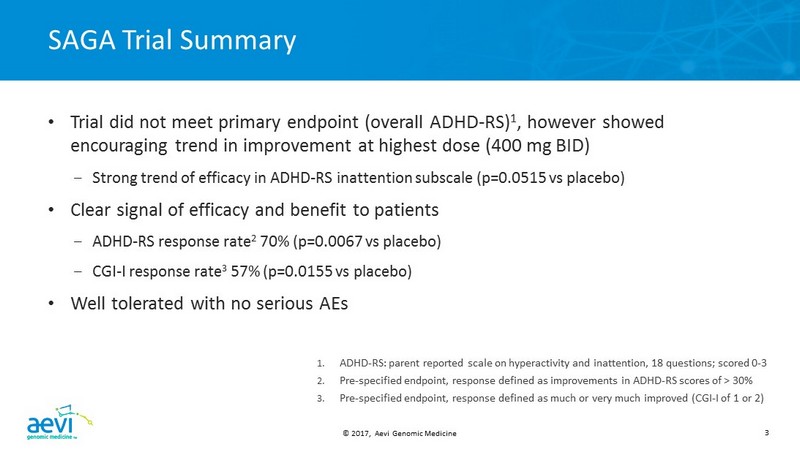
© 2017, Aevi Genomic Medicine 3 SAGA Trial Summary • Trial did not meet primary endpoint (overall ADHD - RS) 1 , however showed encouraging trend in improvement at highest dose (400 mg BID) – Strong trend of efficacy in ADHD - RS inattention subscale (p=0.0515 vs placebo) • Clear signal of efficacy and benefit to patients – ADHD - RS response rate 2 70% (p=0.0067 vs placebo) – CGI - I response rate 3 57% ( p=0.0155 vs placebo ) • Well tolerated with no serious AEs 1. ADHD - RS : parent reported scale on hyperactivity and inattention, 18 questions; scored 0 - 3 2. Pre - specified endpoint, response defined as improvements in ADHD - RS scores of > 30% 3. Pre - specified endpoint, response defined as much or very much improved (CGI - I of 1 or 2 )

© 2017, Aevi Genomic Medicine 4 A Novel Paradigm of Diagnosis and Treatment in Neuropsychiatric Diseases • Novel genomic discovery identifies disruptive mutations in the mGluR network in a significant subpopulation (20 - 25%) of ADHD patients – Role of glutamate in ADHD is supported by recent genetic 1 and neuroimaging 2,3 findings – Highly predictive biomarker facilitates precision medicine approach • mGluR network mutations also found in other neurodevelopmental disorders – 22q deletion syndrome – Autism spectrum disorder – Pediatric anxiety disorder • AEVI - 001 identified as a promising targeted therapeutic for mGluR + patients 1. Elia et al. (2012) Nat Genet , 44(1): 78 - 84 2. Gallo and Posner (2016) Lancet Psychiatry, 3(6):555 - 67 . 3. Spencer et al. (2014) J Clin Psychiatry ;75(11):1226 - 412
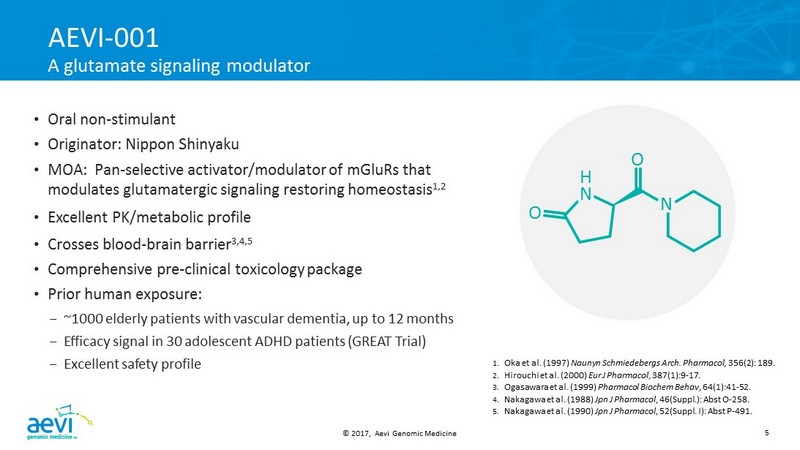
© 2017, Aevi Genomic Medicine 5 AEVI - 001 A glutamate signaling modulator • Oral non - stimulant • Originator : Nippon Shinyaku • MOA : Pan - selective activator/modulator of mGluRs that modulates glutamatergic signaling restoring homeostasis 1,2 • Excellent PK/metabolic profile • Crosses blood - brain barrier 3,4,5 • Comprehensive pre - clinical toxicology package • Prior human exposure: – ~1000 elderly patients with vascular dementia, up to 12 months – Efficacy signal in 30 adolescent ADHD patients (GREAT Trial) – Excellent safety profile 1. Oka et al. (1997) Naunyn Schmiedebergs Arch. Pharmacol , 356(2): 189. 2. Hirouchi et al. (2000) Eur J Pharmacol , 387(1):9 - 17. 3. Ogasawara et al. (1999) Pharmacol Biochem Behav , 64(1):41 - 52. 4. Nakagawa et al. (1988) Jpn J Pharmacol , 46(Suppl.): Abst O - 258. 5. Nakagawa et al. (1990) Jpn J Pharmacol , 52(Suppl. I): Abst P - 491 . O H N O N
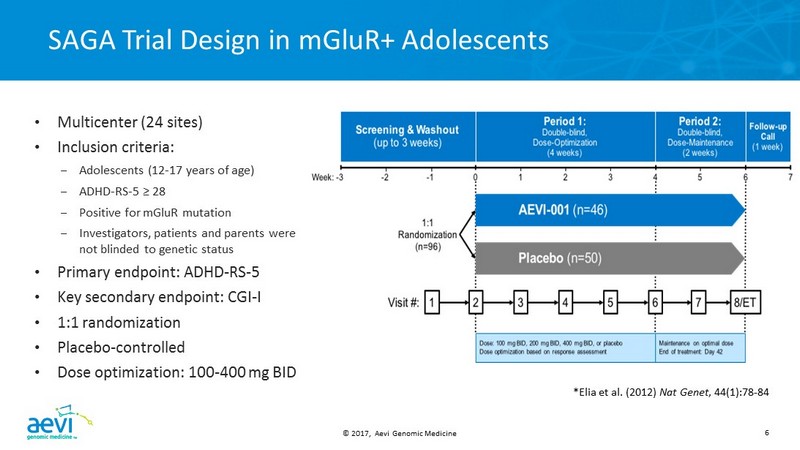
© 2017, Aevi Genomic Medicine 6 SAGA Trial Design in mGluR + Adolescents *Elia et al. (2012) Nat Genet , 44(1):78 - 84 • Multicenter (24 sites) • Inclusion criteria: – Adolescents (12 - 17 years of age) – ADHD - RS - 5 ≥ 28 – Positive for mGluR mutation – Investigators, patients and parents were not blinded to genetic status • Primary endpoint: ADHD - RS - 5 • Key secondary endpoint: CGI - I • 1:1 randomization • Placebo - controlled • Dose optimization: 100 - 400 mg BID
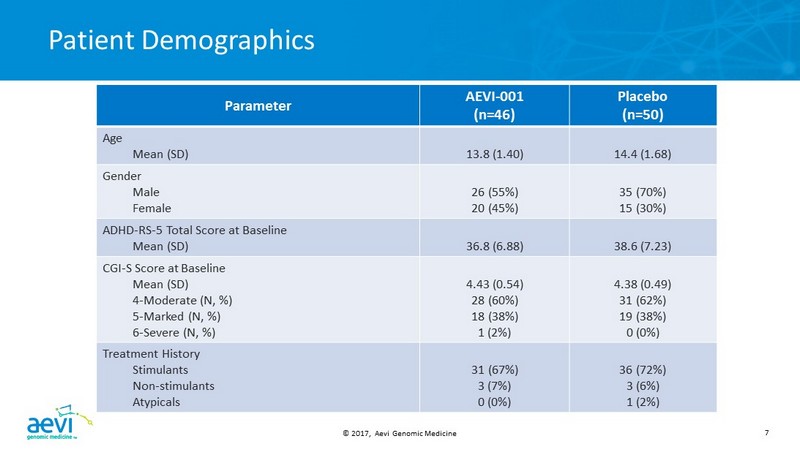
© 2017, Aevi Genomic Medicine 7 Patient Demographics Parameter AEVI - 001 (n=46) Placebo (n=50) Age Mean (SD) 13.8 (1.40) 14.4 (1.68) Gender Male Female 26 (55%) 20 (45%) 35 (70%) 15 (30%) ADHD - RS - 5 Total Score at Baseline Mean (SD) 36.8 (6.88) 38.6 (7.23) CGI - S Score at Baseline Mean (SD) 4 - Moderate (N, %) 5 - Marked (N, %) 6 - Severe (N, %) 4.43 (0.54) 28 (60%) 18 (38%) 1 (2%) 4.38 (0.49) 31 (62%) 19 (38%) 0 (0%) Treatment History Stimulants Non - stimulants Atypicals 31 (67%) 3 (7%) 0 (0%) 36 (72%) 3 (6%) 1 (2%)
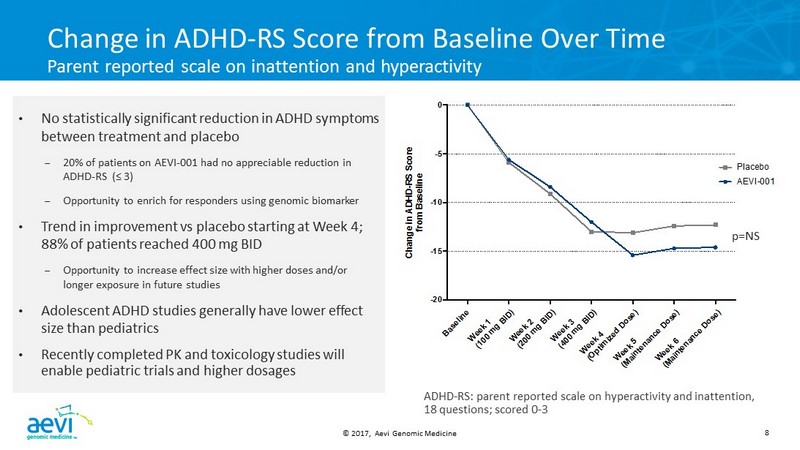
© 2017, Aevi Genomic Medicine 8 B a s e l i n e W e e k 1 ( 1 0 0 m g B I D ) W e e k 2 ( 2 0 0 m g B I D ) W e e k 3 ( 4 0 0 m g B I D ) W e e k 4 ( O p t i m i z e d D o s e ) W e e k 5 ( M a i n t e n a n c e D o s e ) W e e k 6 ( M a i n t e n a n c e D o s e ) -20 -15 -10 -5 0 C h a n g e i n A D H D - R S S c o r e f r o m B a s e l i n e AEVI-001 Placebo Change in ADHD - RS Score from Baseline Over Time Parent reported scale on inattention and hyperactivity • No statistically significant reduction in ADHD symptoms between treatment and placebo – 20 % of patients on AEVI - 001 had no appreciable reduction in ADHD - RS (≤ 3 ) – Opportunity to enrich for responders using genomic biomarker • Trend in improvement vs placebo starting at Week 4; 88 % of patients reached 400 mg BID – Opportunity to increase effect size with higher doses and/or longer exposure in future studies • Adolescent ADHD studies generally have lower effect size than pediatrics • Recently completed PK and toxicology studies will enable pediatric trials and higher dosages p =NS ADHD - RS: parent reported scale on hyperactivity and inattention, 18 questions; scored 0 - 3

© 2017, Aevi Genomic Medicine 9 W e e k 1 ( 1 0 0 m g B I D ) W e e k 2 ( 2 0 0 m g B I D ) W e e k 3 ( 4 0 0 m g B I D ) W e e k 4 ( O p t i m i z e d D o s e ) W e e k 5 ( M a i n t e n a n c e D o s e ) W e e k 6 ( M a i n t e n a n c e D o s e ) 0 20 40 60 80 P e r c e n t a g e o f p a t i e n t s w h o a c h i e v e d r e d u c t i o n i n A D H D - R S > 3 0 % AEVI-001 Placebo Clinically and Statistically Significant Response Rate (ADHD - RS Reduction > 30%) • Significantly more treated patients were responders as measured by ADHD - RS* • 70% response rate in treatment arm vs 42% in placebo arm (p=0.0067) at Week 6 − Concerta achieved a response rate of 73.3% in an adolescent ADHD study 1 • Results warrant consideration of higher doses and/or longer exposure in future studies * Pre - specified endpoint, response defined as improvements in ADHD - RS scores of > 30 % p=0.0067 p=0.0203 1. Wilens et al. (2006) Arch Pediatr Adolesc Med . 160(1):82 - 90
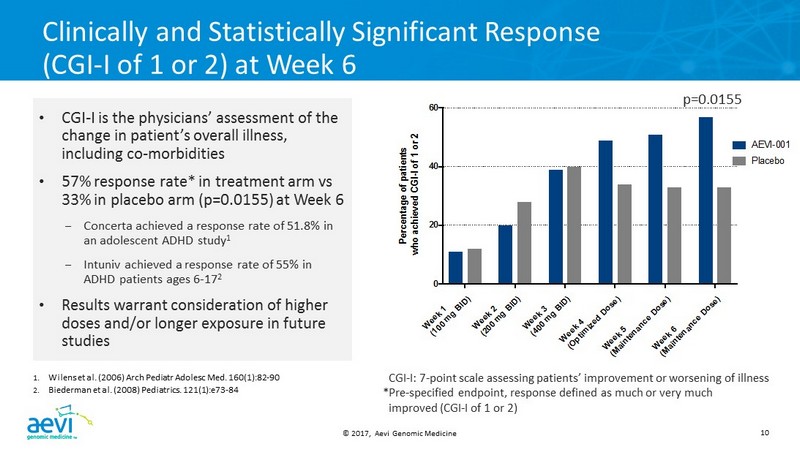
© 2017, Aevi Genomic Medicine 10 W e e k 1 ( 1 0 0 m g B I D ) W e e k 2 ( 2 0 0 m g B I D ) W e e k 3 ( 4 0 0 m g B I D ) W e e k 4 ( O p t i m i z e d D o s e ) W e e k 5 ( M a i n t e n a n c e D o s e ) W e e k 6 ( M a i n t e n a n c e D o s e ) 0 20 40 60 P e r c e n t a g e o f p a t i e n t s w h o a c h i e v e d C G I - I o f 1 o r 2 AEVI-001 Placebo Clinically and Statistically Significant Response (CGI - I of 1 or 2) at Week 6 • CGI - I is the physicians’ assessment of the change in patient’s overall illness, including co - morbidities • 57% response rate* in treatment arm vs 33% in placebo arm (p=0.0155) at Week 6 – Concerta achieved a response rate of 51.8% in an adolescent ADHD study 1 – Intuniv achieved a response rate of 55% in ADHD patients ages 6 - 17 2 • Results warrant consideration of higher doses and/or longer exposure in future studies 1. Wilens et al. (2006) Arch Pediatr Adolesc Med. 160(1):82 - 90 2. Biederman et al. (2008) Pediatrics . 121(1):e73 - 84 CGI - I : 7 - point scale assessing patients’ improvement or worsening of illness *Pre - specified endpoint, response defined as much or very much improved (CGI - I of 1 or 2) p=0.0155

© 2017, Aevi Genomic Medicine 11 AEVI - 001: Safety and Tolerability • AEVI - 001 was well - tolerated; 88% achieved highest dose of 400 mg BID • No serious adverse events • Majority of AEs judged to be mild or moderate AEVI - 001 Placebo Number of subjects with ≥1 AE 33/47 (70.2%) 28/50 (56%) AEs > 5% Weight gain Fatigue Headache Nausea 7 (14.9%) 7 (14.9%) 4 (8.5%) 3 (6.4%) 2 (4%) 3 (6%) 5 (10%) 4 (8%)

© 2017, Aevi Genomic Medicine 12 AEVI - 001: Next Steps • Enrich ADHD study population to enhance response rate and effect size: – Refine responders using genomic biomarker – Higher exposure: dose and duration – Pediatric (ages 6 - 12) ADHD study: – Higher responses, better compliance, lower placebo effect • Additional signal finding studies planned in: – Autism spectrum disorder – Pediatric anxiety disorder • FDA meeting planned mid - 2017 – Development plan including companion diagnostic • Full data to be presented at World Congress on ADHD (April 20 - 23, Vancouver, Canada)

© 2017, Aevi Genomic Medicine 13 Thank you
