Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT 31.1 - Aevi Genomic Medicine, Inc. | v428554_ex31-1.htm |
| EX-23.1 - EXHIBIT 23.1 - Aevi Genomic Medicine, Inc. | v428554_ex23-1.htm |
| EX-31.2 - EXHIBIT 31.2 - Aevi Genomic Medicine, Inc. | v428554_ex31-2.htm |
| EX-32.1 - EXHIBIT 32.1 - Aevi Genomic Medicine, Inc. | v428554_ex32-1.htm |
| EX-21.1 - EXHIBIT 21.1 - Aevi Genomic Medicine, Inc. | v428554_ex21-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-35112
Medgenics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 98-0217544 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
435 Devon Park Drive, Building 700 Wayne, Pennsylvania |
19087 |
| (Address of Principal Executive Offices) | (Zip Code) |
(610) 254-4201
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of exchange on which registered |
| Common stock, par value $0.0001 per share | NYSE MKT |
| Redeemable common stock purchase warrants | NYSE MKT |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
| Large accelerated filer | ¨ | Accelerated filer | x |
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of common stock held by non-affiliates of the registrant, computed by reference to the closing price of the registrant’s common stock on the NYSE MKT on June 30, 2015, as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $133.7 million.
As of February 19, 2016, the registrant had 32,921,179 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement to be issued in conjunction with the registrant’s annual meeting of stockholders to be held in 2016 are incorporated by reference in Part III of this Annual Report on Form 10-K. The proxy statement will be filed by the registrant with the Securities and Exchange Commission not later than 120 days after the end of the registrant’s fiscal year ended December 31, 2015.
MEDGENICS, INC.
TABLE OF CONTENTS
FORM 10-K
Cautionary Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, including statements regarding the progress and timing of clinical trials, the safety and efficacy of our product candidates, the goals of our development activities, estimates of the potential markets for our product candidates, estimates of the capacity of manufacturing and other facilities to support our products, our expected future revenues, operations and expenditures and projected cash needs. These statements relate to future events of our financial performance and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements. Those risks and uncertainties include, among others:
| · | our ability to obtain additional funding to develop our product candidates; |
| · | the need to obtain regulatory approval of our product candidates; |
| · | the success of our clinical trials through all phases of clinical development; |
| · | any delays in regulatory review and approval of product candidates in clinical development; |
| · | our ability to commercialize our product candidates; |
| · | market acceptance of our product candidates; |
| · | competition from existing products or new products that may emerge; |
| · | regulatory difficulties relating to products that have already received regulatory approval; |
| · | potential product liability claims; |
| · | our dependency on third-party manufacturers to supply or manufacture our products; |
| · | our ability to establish or maintain collaborations, licensing or other arrangements; |
| · | our ability and third parties’ abilities to protect intellectual property rights; |
| · | compliance with obligations under intellectual property licenses with third parties; |
| · | our ability to adequately support future growth; and |
| · | our ability to attract and retain key personnel to manage our business effectively. |
Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “can,” “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “continues,” “anticipates,” “intends,” “seeks,” “targets,” “believes,” “estimates,” “projects,” “predicts,” “potential,” or the negative of those terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including, but not limited to, those discussed in the section titled “Risk Factors” included in Part I, Item 1A of this Annual Report on Form 10-K. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Any forward-looking statement speaks only as of the date of this report and, except as required by law, we undertake no obligation to update or review publicly any forward-looking statements, whether as a result of new information, future events or otherwise. We qualify all of our forward-looking statements by these cautionary statements.
Unless the context otherwise requires, all references in this Annual Report on Form 10-K to the “Company”, “Medgenics”, “we,” “us” and “our” refer to Medgenics, Inc., a Delaware corporation organized on January 27, 2000, and its wholly-owned subsidiaries, Medgenics Medical (Israel) Ltd. and neuroFix, LLC. We use TARGTTM, TARGTEPOTM, DermaVacTM, GeneRideTM and the Medgenics logo as trademarks in the United States and elsewhere. All other trademarks or trade names referred to in this document are the property of their respective owners.
Overview
We are a clinical stage biopharmaceutical company with an emphasis on Genomic Medicine.
The National Human Genome Research Institute of the National Institute of Health defines “Genomic Medicine” as “an emerging medical discipline that involves using genomic information about an individual as part of their clinical care (e.g., for diagnostic or therapeutic decision-making) and the health outcomes and policy implications of that clinical use."
Genomic medicine is currently making an impact in the fields of oncology, pharmacology, rare and undiagnosed diseases, and infectious disease.
Medgenics has partnered with the Center for Applied Genomics (CAG) at The Children’s Hospital of Philadelphia (CHOP) to implement a genomics-medicine driven approach to drug development. CAG’s assets include a fully automated biorepository containing specimens from more than 60,000 pediatric patients, and 150,000 relatives. The sample is highly enriched for rare and orphan diseases and the large majority of patients have been genotyped. Their phenotypes are recorded in a modern electronic health record. The patients have been consented for anonymized use of their data for research and follow up contact if needed.
CAG continues to discover important and novel genetic biomarkers by both genome-wide association studies and exome sequencing and analysis of affected individuals and their family members. Such markers not only identify patients with the disease but frequently point to the cause of the disease and suggest targets and feasible intervention strategies that include protein or peptide therapy, monoclonal antibodies, drugs or gene therapy. By working initially in a pediatric population, confounding environmental factors seen in older patients are minimized.
Medgenics therapeutic strategy is to work closely with our collaborators
at CAG to identify populations of need with well-characterized, novel, genetically-defined targets. The company then
designates an actionable approach based upon the target, the biology and human pathophysiology, and the clinical
and regulatory pathways. The collaboration affords Medgenics unique and proprietary insight into these diseases
and allows the Company to better select therapeutic approaches. This, in turn, allows Medgenics to
rapidly identify appropriate approaches and molecules that have been in the clinic but not advanced due to lack of efficacy in
a different patient population or for strategic reasons. We are patient rather than technology focused and thus willing and
able to explore many therapeutic modalities incluindg small molecules, peptides, biologics as well as Medgenics’s proprietary
TARGT gene therapy technology as appropriate.
Hundreds of molecules and biologics are currently listed in proprietary databases. Many of these have remaining composition of matter patent life and many would be eligible for regulatory exclusivity based on first registration (up to 12 years for biologics), as well as orphan drug and additional pediatric exclusivity. When such molecules are available, the time to file an IND/IMPD and initiate clinical trials, cost and risk may be substantially reduced. In some cases it may be possible to advance from discovery to the clinic in less than 2 years, and to achieve proof of concept in as little as 3 years.
The availability of robust genetic biomarker(s) allows trial designs to focus on highly enriched patient populations that are more likely to respond to targeted therapies. This can allow smaller, more focused and less expensive trials. Likewise, highly targeted drugs, that are less likely to exhibit off target effects, can be used when available. This further enhances the likelihood of clinical and regulatory success and potentially requires smaller, easier-to-enroll clinical trials. Furthermore, such highly targeted therapies in specifically targeted diseases should allow the creation of higher value medicines that can address critical needs in patients suffering from rare and orphan diseases. The solid genetic foundation underlying these disease targets along with highly targeted therapies may also allow rapid label expansion into adjacent populations.
| 1 |
Medgenics initial program arising out of our Genomics Medicine strategy is the development candidate NFC-1 Through our acquisition of neuroFix, LLC, or neuroFix, we acquired the rights to develop, NFC-1, as well as the rights to certain data derived from a clinical trial and other studies of NFC-1. NFC-1 is a first-in-class, non-stimulant metabotropic glutamate receptor (mGluR) neuromodulator that is Phase 2/3 ready for the treatment of mGluR network mutation positive Attention Deficit Hyperactivity Disorder (ADHD), as well as neuropsychiatric symptoms resulting from a related rare genetic disorder, 22q11.2 Deletion Syndrome (22q11.2 DS). We intend to develop NFC-1 for the treatment of mGluR network mutation positive ADHD (mGluR+ ADHD) and certain other neurological and neuropsychological indications. A Phase 1b clinical trial of NFC-1 in adolescents with ADHD and disruptions in the mGluR gene network was recently completed showing the safety of NFC-1 as well as signaling potential efficacy in the adolescents treated.
We are also actively developing our TARGT gene therapy platform as described in more detail below.
Acquisition of neuroFix
On September 9, 2015, the Company entered into an Equity Interest Purchase Agreement with neuroFix therapeutics, Inc. (referred to as Legacy Corp), neuroFix, CHOP, Philip Harper and Hakon Hakonarson, pursuant to which we acquired all of the outstanding equity of neuroFix LLC in consideration for our payment of the following:
| · | an upfront payment of $2.0 million in cash paid upon the consummation of the acquisition, which was paid on September 9, 2015; |
| · | a payment of $6.0 million, payable as $1.2 million in cash to CHOP, $1.6 million in cash to Legacy Corp and $3.2 million in
shares of our common stock to Legacy Corp, which cash payments were made upon the completion of our registered public offering
in October 2015, at which time 459,770 shares, priced at $6.96 per share, were also issued to Legacy Corp; |
| · | additional payments of up to $450 million upon the achievement of certain developmental, regulatory and sales milestones related
to an oral formulation of NFC-1 (the “Product”) and any new chemical entity developed by us covering the same indication
as the Product (an “NCE”); and |
| · | earnout payments equal to a percentage of certain product sales by us using tiered rates ranging from the mid- to high single digits depending on the Product or NCE. |
In addition, if the Product is approved by the FDA for additional indications beyond the initial indication, additional payments of $25 million for each such additional indication will be required to be paid by the Company.
Immediately prior to and in connection with the acquisition, neuroFix entered into a license agreement with CHOP, the terms of which are described below under “Licenses.”
TARGT
TARGT therapy (Transduced Autologous Restorative Gene Therapy) is a protein therapy that uses the patient’s own skin to provide sustained production and delivery of therapeutic protein. The process starts by taking thin, small samples of dermis tissue (about 1.5-2.5 mm in diameter and approximately 30 mm in length) named a Micro-Organ (MO), by a rapid subcutaneous removal protocol, utilizing the DermaVac, a proprietary harvesting device developed by Medgenics. The MOs preserve the vital cell-to-cell relationships found in-situ in skin tissue. A viral vector, which carries the gene coding for the therapeutic protein, is used outside the body (ex-vivo) to introduce the gene into the nuclei of cells in the MO, which then secrete the therapeutic protein, thereby converting the MO into a “TARGT”. After transduction is complete, the TARGT is washed extensively to remove remaining viral vectors. The process of converting the MO into functional TARGTs takes about 7-10 days of ex-vivo treatment. Currently, TARGT manufacturing is done manually in an open system at the GMP environment, however, in the future it is desired to use a closed-system for TARGT processing and that the whole process will be done in a semi-automated fashion. Post processing, the TARGTs are implanted back into the patient in the desired location, either under the patient’s skin into the subcutaneous fat or into the Central Nervous System (“CNS”) utilizing the proprietary implantation devices developed by Medgenics. After implantation, the TARGTs integrate into the surrounding tissue and should provide sustained protein secretion within the therapeutic range for several months. Non-transduced MOs have the potential to be stored under cryo-storage conditions for later thawing and reuse for future implantations.
| 2 |
Advantages of the TARGT platform
The TARGT system offers unique advantages to current injectable protein therapy and conventional gene therapy:
Advantages over recombinant protein injection
| i) | No need for expensive protein manufacturing facility - the patient’s tissue is the bioreactor. |
| ii) | Low cost of production – relatively small amounts of vector needed. |
| iii) | Naturally, continuously produced protein with post translation modifications. |
| iv) | TARGT is a “platform” technology. Allows for low cost, fast preclinical proof of concept and a significantly less expensive pre-clinical development process into first in man. |
| v) | Delivery of protein based therapeutics into highly challenging locations with great unmet need such as the CNS. |
Advantages over traditional gene therapy
| i) | Ex-vivo processing, which allows better removal of excess viral vector particles (overcoming immune and innate immune response and safety). |
| ii) | Better dosing control and dosing flexibility. |
| iii) | TARGT removal allows dose reduction or treatment discontinuation. |
| iv) | Flexibility of viral vectors, allowing large payloads. |
First-in-line Applications:
TARGTEPO:
TARGTEPO is an endogenous Erythropoietin (EPO) secretion TARGT for treatment of End-Stage Renal Disease (ESRD) and other indications requiring EPO treatment such as β-thalassemia. Erythropoietin (EPO) is a protein that is naturally secreted by the kidneys and functions to stimulate the bone marrow to produce red blood cells. End-Stage Renal Disease (ESRD) is a condition where the kidneys have completely stopped functioning. ESRD patients must receive either dialysis or a kidney transplant in order to survive, and the disease is extremely expensive and complicated to treat (cost in the US according to the National Kidney Foundation was $48.2 billion in 2011).
In June 2014, the first patient was enrolled in our Phase 1/2 clinical trial of MDGN-201 (TARGTEPO). The aim of the ongoing trial is to validate the potential of our TARGT platform using a second-generation expression cassette of the HDAd viral vector, which was developed to enhance durability of the proposed therapeutic effect. The ongoing study is evaluating the potential of the updated platform to offer sustained production and delivery of endogenous erythropoietin (eEPO) to treat anemia in dialysis patients with ESRD. This open-label trial was expected to enroll up to 18 patients with ESRD who require recombinant humanized erythropoietin (rHuEPO) treatment for anemia. The trial endpoints include plasma eEPO levels, blood counts and safety assessment.
We have now completed enrollment in the low-dose and mid-dose cohorts of the Phase 1/2 clinical trial of TARGTEPO. All 10 patients who received TARGTEPO microorgans have shown positive response to therapy at approximately 100 times lower Cmax than rHuEPO (e.g., EPREX). Seven of Ten patients maintained hemoglobin levels within their targeted range due to red blood cell production stimulated by eEPO for at least five months following implantation without receiving any injections of rHuEPO or blood transfusions, and one patient continues to remain stable without receiving any injections of rHuEPO or blood transfusions for 19 months thus far since implantation. The treatment was well-tolerated in all patients and there have been no treatment-related serious adverse events (SAEs). One patient in the mid dose cohort was not able to maintain his hemoglobin levels within the targeted range and required injections of rHuEPO soon after implantation and exited the study. One of the mid dose patients had hemoglobin levels above 13 g/dl (which is high according to the guidelines for EPO treatment in ESRD patients) and was treated with repeated phlebotomy. Each phlebotomy resulted in a transient decrease in hemoglobin but not in eEPO. In accordance with the protocol, the investigator excised one of the implanted TARGTs. Following excision, the patient experienced a decrease both in EPO and hemoglobin, and hemoglobin stabilized to the acceptable range within the guidelines.
| 3 |
Due to the success of the 2 first cohorts, and the required excision at one of the mid dose patients, we have decided not to continue to the high dose cohort. We believe that future trials should tailor the TARGT dose according to the patient's previous response to rHuEPO to limit the need for potential excision due to overshooting of the Hb.
We also enrolled two patients in our first trial studying TARGTEPO in patients with ESRD who are undergoing peritoneal dialysis. The first patient required injection of rHuEPO close to five months post-implantation and exited the study. This patient had been implanted with one TARGT and although it was still secreting it was not sufficient to maintain the patient’s hemoglobin levels within the desired range. The second patient was stable for 6 months both in Hb levels as well as eEPO secretion. After 6 months the patient received kidney transplantation and hence exited the study and continues the safety follow up as per protocol. In the second quarter of 2015, we received clearance to proceed from the U.S. Food and Drug Administration, or the FDA, for our investigational new drug application, and we have started a Phase 2 clinical trial in the United States studying TARGTEPO in ESRD patients who are undergoing peritoneal dialysis. The first patient in this study was enrolled at the end of the second quarter of 2015, and represents the first patient that we are studying in a clinical trial in the United States. This patient required rHuEPO injection 2 months post-implantation and hence exited the study. This patient was challenging in controlling his Hb when was on rHuEPO injection prior to enrollment in the study as well as when exited the study and resumed rHuEPo injections. Currently the patient has reached the desired Hb level per guidelines and continues safety follow up as scheduled per protocol.
Orphan Diseases: Medgenics has undertaken a significant effort to identify several orphan and specialty niche markets that can leverage the TARGT’s ability to produce continuous long lasting quantities of therapeutic proteins. While the identification effort is on-going, we are beginning pre-clinical and clinical work in specific orphan markets including:
| 1) | Pre-clinical assessment of the effect of TARGTEPO in β-thalassemia intermedia. Thalassemia patients require blood transfusions, which results in deleterious effects of iron overload. It has been suggested that treatment with EPO (low dose) together with proper iron management will allow adequate maintenance of patients’ Hemoglobin while reducing or eliminating the need for blood transfusions. Following proof of concept in thalassemia mice, we intend to plan a signal finding study in thalassemia intermedia patients in Israel and/or Europe. |
| 2) | A major challenge in treating CNS conditions is the minimal penetration of protein-based drugs into the CNS. We have identified 2 groups of diseases that have significant unmet need, which can benefit from direct administration of protein based drugs into the CNS. One group of indications is Lysosomal Storage Diseases (“LSDs”), which require enzyme replacement into the CNS. The second is primary or metastatic brain tumors that may benefit from direct administration of anti-cancer antibodies into the CNS. We have shown that TARGT can secret Iduronate 2-sulfatase (IDS), the enzyme deficient in the LSD Hunter syndrome, and we will test its efficacy when such TARGT is administered directly into the CNS. We have also shown that TARGT can secrete active antibodies and we will test the efficacy of specific anti-cancer antibodies when TARGT is administered directly into the brain in the presence of brain tumors. |
Next Generation TARGT and GeneRide technology
Medgenics is currently developing the next generation of the TARGT platform which combines the benefits of both TARGT and standard cell therapy. This new platform will have several advantages over the current TARGT platform as well as cell therapy methods: i) ex-vivo transduction (which helps with residual viral wash), ii) the ability to control the implanted dose (amount of cells), iii) the potential to use higher dose than what TARGT allows, much higher number of cells with much smaller dimensions, iv) the ability to stop the treatment with excision or ablation, v) very simple harvest procedure for the patient, vi) the ability for retreatment without additional harvesting and with minimal processing time, and vii) potential for improved secretion profile while using integrated expression cassette (not episomal). For cassette integration, gene targeting by homologous recombination will be used.
| 4 |
Gene targeting by homologous recombination allows the site specific integration of a gene of choice. In recent years, sequence specific DNA nucleases have been engineered to increase the rate of gene targeting by as much as four orders of magnitude. As a consequence, gene targeting has become a preferred mode of genome engineering in diverse applications ranging from gene therapy to the design of genetically modified organisms. Compared to the common use of viral vectors, gene targeting allows controlled gene expression and reduces the risk of off-target effects such as oncogene activation. However, the risk is not diminished because, much like in viral vectors, the transgene cassette in state of the art gene targeting applications still includes a promoter. The inclusion of a promoter in the targeting vector has so far been considered a necessity although it confers many difficulties. In addition to the risk of off-target oncogene activation, the exogenous promoter can drive the expression of the transgene from the non-integrated episome as well as from off-target integrations. The promoter is often of considerable length which makes vectorization challenging. Finally, the control over the expression of the integrated gene is non-natural. In particular, the transgene may be expressed in the wrong cells in the wrong time and at the wrong amount.
Medgenics has licensed a new technology from Stanford University developed at Prof. Mark Kay's lab to perform gene targeting without a promoter, thus eliminating the above risks. The method combines the use of site-specific nucleases with a set of additional molecular tools to accomplish the integration and expression of the gene of choice under the control of a desired endogenous promoter at the native locus. Because the targeting vector bears no promoter, the chance of off-target oncogene activation is greatly reduced, episomal expression is diminished and transgene expression from off-target integration is minimized.
Most importantly, expression of the transgene is co-regulated with that the endogenous gene. Thus, the transgene will be expressed only in the desired cell type under the right conditions. This novel paradigm allows for the first time the expression of the transgene from an endogenous promoter while preserving the sequence, regulation and activity of the endogenous gene. Promoter-less gene targeting embodies the dream of genome engineering and gene therapy: achieving a desired outcome while minimizing off target effects. Additionally Dr. Mark Kay’s lab has also recently developed a method for site-specific gene targeting that does not require nucleases, providing a superior profile with respect to avoidance of toxicity and safety.
Medgenics has licensed these technologies from Stanford University, with exclusive rights to use them in the existing and future TARGT system. Additional non-exclusive rights have also been granted. We intend to adapt these technologies to the next generation TARGT system with a goal of enhancing our platform by a) greatly increasing the durability of expression as compared with the existing episomal expression system in TARGT which will be preferable for certain clinical indications, b) enhancing safety as compared with existing integrating gene therapy technologies.
Business Strategy
We are focused on finding treatments for rare and orphan diseases, with a particular focus on pediatrics, following a genomics medicine strategy. We have exclusive access to one of the world’s largest biobank of rare and orphan disease genetic information. Working closely with our partner, the Children’s Hospital of Philadelphia, the Company seeks to identify populations of patients who have a genetic commonality and who either do not benefit from currently marketed treatments, or for whom there is no approved therapy. The Company then undertakes a “rational search” approach to identify potential therapeutics that, based on their mechanism of action, are expected to have a benefit in this idenfitied population. The therapeutics could be of any form (e.g., small molecule, biologic, gene therapy using the Company’s TARGT platform) and they could be assets that have not successfully advanced through phase 3 clinical trials (reached the market( reached the market feels too broad) for a number of reasons (would also highlight that this is not for SAFETY reasons). For example, the asset could have advanced through clinical trials but failed to establish efficacy, not because of the compound, but because it was tested in a group of heterogenous patients who were not stratified by genetic commonality. The Company could therefore acquire these assets at a relatively low cost, and could advance their development relatively quickly because the asset would have already generated pre-clinical data and human safety data. In particular, if the asset is being developed for a rare and orphan disease, the asset could potentially progress to registration without a full series of Phase 1 through Phase 3 trials. The Company’s first such asset coming from this strategy, NFC-1, was acquired through its acquisition of neuroFix in September 2015. NFC-1 has a substantial pre-clinical and human safety package that should enable the Company to move NFC-1 into a Phase 1b trial for 22q deletion syndrome and Phase 2 for mGluR-positive ADHD this year.
| 5 |
Intellectual Property
Our goals are to obtain, maintain, and enforce patent and trademark protection for our products, processes, methods, and other proprietary technologies of the TARGT platform, and to preserve our trade secrets both in the United States and elsewhere in the world. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our TARGT platform through a combination of contractual arrangements, trade secrets, patents, and trademarks both in the United States and abroad.
Our ability to compete depends on our ability to maintain and enforce our intellectual property rights and operating without infringing the intellectual property of others and our ability to enforce our licenses. Our business could be materially harmed, and we could be subject to liabilities, because of lawsuits brought by others against our licensors and licensees with whom we have a strategic alliance. We will be able to protect our technology from unauthorized use by third parties only to the extent it is covered by valid and enforceable patents or is effectively maintained as trade secrets. Patents and other proprietary rights are an essential and material element of our business.
Our existing owned patent and patent application portfolio directed to TARGT platform, including TARGTEPO, currently contains 63 issued patents, and 37 pending and allowed patent applications. Applications for patents and other intellectual property rights capable of being registered have been, and will be, filed in certain key jurisdictions. As we identify additional orphan and rare disease targets, we will seek protection for the related intellectual property rights in the United States and other relevant jurisdictions.
Our licensed and owned patent portfolio has issued or pending claims covering the key elements of the TARGT platform, including, but not limited to, genetically modified dermal microorgans and methods of making same, methods for using genetically modified dermal microorgans, devices and methods for harvesting and implanting genetically modified dermal microorgans, and methods and uses for using these microorgans in the treatment of diseases and disorders or in the alleviation of symptoms of a disease or disorder.
There can be no assurance that the pending applications will result in patents ultimately being issued.
We also depend upon the skills, knowledge and experience of our scientific and technical personnel, as well as that of our advisors, consultants and other contractors, none of which is patentable. To help protect our proprietary knowledge and experience that is not patentable, and for inventions for which patents may be difficult to enforce, we rely on trade secret protection and confidentiality agreements with our employees, consultants, vendors, collaborators, advisors, customers and other third parties to protect our interests. To this end, we require all employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. We also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials. We intend to continue to take all appropriate steps to protect our intellectual property, including maintaining an active program for patent protection for novel elements in the development of our products and technology.
Licenses
neuroFix License
Immediately prior to and in connection with our acquisition of neuroFix in September 2015, neuroFix entered into a license agreement with CHOP, pursuant to which CHOP licensed to neuroFix certain technology owned and controlled by CHOP related to ADHD and certain other neurological and neuropsychological indications. Pursuant to this license agreement, CHOP licensed to neuroFix (coupled with a right to sublicense) certain patent rights and compound know-how on an exclusive, worldwide, royalty-bearing right and license basis, and certain CHOP know-how (other than compound know-how) on a non-exclusive, worldwide, royalty-bearing right and license basis. CHOP also granted to neuroFix an exclusive option during the term of the license agreement to negotiate an exclusive license to certain future CHOP intellectual property.
| 6 |
Pursuant to this license agreement, CHOP retained rights to the licensed patent rights and know-how to conduct teaching, educational, research and patient care activities itself and to conduct collaborations with certain not-for-profit, governmental, educational or non-commercial third parties and for purposes outside of the field of the license. Under the license agreement, neuroFix granted to CHOP a non-exclusive, worldwide, fully paid-up, royalty-free license under all intellectual property rights controlled by neuroFix to make and use certain products for education and non-commercial research purposes.
In addition to neuroFix having issued equity to CHOP in partial consideration for the rights granted under the license agreement (which equity was issued immediately prior to the acquisition), CHOP is eligible for certain milestone and royalty payments under the license agreement as further described below:
| · | up to $1.5 million in regulatory and sales milestone payments in connection with each FDA-approved indication obtained by neuroFix
utilizing intellectual property licensed under the license agreement; |
| · | royalty payments equal to a percentage of certain product sales by neuroFix using a fluctuating rate in the low single digits
(adjusted downward to the extent third party royalty payments exceed a certain percentage in a given calendar quarter); |
| · | annual maintenance fees of equal to or less than $100,000 depending on the year; and |
| · | a certain percentage (ranging from mid-single digits to the mid-teens depending on if other rights of neuroFix are also licensed to the sublicensee at the same time) of all sublicensee income (except any amounts attributable to sublicensed sales by a certain party in Japan). |
The license agreement will terminate, with respect to each product and each territory covered by the license agreement, upon the later of (i) the expiration of certain CHOP patent rights and (ii) January 1, 2025, at which time the license rights granted to neuroFix become perpetual, irrevocable, fully paid-up and royalty-free. The license agreement could also be subject to termination by CHOP if neuroFix is not achieving certain specified development plans and diligence events and is not undertaking commercially reasonable efforts to achieve such events.
CHOP License
In November 2014, we entered into a licensing and research collaboration agreement (the “Research Agreement” and the “License Agreement”) with CHOP. Under the terms of the License and Research Agreement, CHOP granted us (i) an exclusive, sublicensable license to use certain patent rights covering potential diagnostic and therapeutic targets, (ii) an exclusive, non-sublicensable license to use certain biospecimen and phenotypic data collected from patients with rare and orphan diseases and their family members, (iii) a non-exclusive, sublicensable license to use certain know-how related to such patent rights, biospecimen and phenotypic data, (iv) a non-exclusive and non-sublicensable license to use certain biospecimen and phenotypic data collected from patients with non-rare and orphan diseases, and (v) an exclusive option to negotiate licenses to commercialize certain inventions that may be created in the future that target rare and orphan diseases. In consideration of the licenses and option granted under the Licensing and Research Agreement, we agreed to pay to CHOP a license issuance fee of $500,000, certain maintenance fees, certain milestone payments, low single-digit royalties on net sales of all licensed products and a percentage of amounts received from sublicensing activities.
The License Agreement terminates upon the expiration date of the last-to-expire royalty term under the License Agreement, however (i) CHOP may terminate the License Agreement upon an uncured default by us of the License Agreement or the failure by us to meet certain development and/or commercialization milestones under the License Agreement or if we become insolvent or enter into bankruptcy proceedings, and (ii) we may terminate the License Agreement at any time with six months’ prior written notice to CHOP.
| 7 |
Trademarks
Certain names utilized for our products and tools are trademarked, and certain names utilized for our products and tools are the subject of trademark registrations and applications in certain jurisdictions. The final choice of names for products and tools has not yet been made and will be subject to marketing considerations and other factors.
The Company has filed applications in the United States for registering TARGTTM and TARGTEPOTM.
There can be no assurance that a third party will not oppose any registration, that the respective Trademark Offices will issue a registration certificate or that we will otherwise be successful in perfecting trademark rights for the marks in the United States or in foreign countries, the results of any of which would likely have a material adverse effect on our company.
Government Regulation
General
The production, distribution, and marketing of products employing our technology, and our development activities, are subject to extensive governmental regulation in the United States and in other countries. In the United States, our products are subject to the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations of the FDA, as well as to other federal, state, and local statutes and regulations. These laws, and similar laws outside the United States, govern the clinical and preclinical testing, manufacture, safety, effectiveness, approval, labeling, distribution, sale, import, export, storage, record-keeping, reporting, advertising, and promotion of our products. Product development and approval within this regulatory framework, if successful, will take many years and involve the expenditure of substantial resources. Violations of regulatory requirements at any stage may result in various adverse consequences, including the FDA’s and other health authorities’ delay in approving or refusal to approve a product. Violations of regulatory requirements also may result in enforcement actions.
The following paragraphs provide further information on certain legal and regulatory issues with a particular potential to affect our operations or future marketing of products employing our technology.
U.S. drug development
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
Our drug product candidates, including NFC-1, must be approved by the FDA through the New Drug Application (“NDA”) process, and future product candidates, including TARGT, may be subject to the Biologic License Application process (“BLA”), before they may be legally marketed in the United States. The NDA and BLA processes are collectively referred to herein as NDAs. The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| · | Completion of extensive non-clinical, sometimes referred to as non-clinical laboratory tests, non-clinical animal studies and formulation studies in accordance with applicable regulations, including the FDA’s current Good Laboratory Practice, or GLP, regulations; |
| · | Submission to the FDA of an IND application, which must become effective before human clinical trials may begin; |
| 8 |
| · | Approval by an independent institutional review board, or IRB, or ethics committee at each clinical trial site before each trial may be initiated; |
| · | Performance of adequate and well-controlled human clinical trials in accordance with applicable IND and other clinical trial-related regulations, sometimes referred to as good clinical practices, or GCPs, to establish the safety and efficacy of the proposed drug for each proposed indication; |
| · | Submission to the FDA of an NDA, for a new drug; |
| · | A determination by the FDA within 60 days of its receipt of an NDA to file the NDA for review; |
| · | Satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the drug is produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
| · | Potential FDA audit of the non-clinical and/or clinical trial sites that generated the data in support of the NDA; and |
| · | FDA review and approval of the NDA, including consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the drug in the United States. |
The non-clinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. Non-clinical tests include laboratory evaluation of product chemistry, formulation, stability and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product.
The data required to support an NDA is generated in two distinct development stages: non-clinical and clinical. For new chemical entities, the non-clinical development stage generally involves synthesizing the active component, developing the formulation and determining the manufacturing process, as well as carrying out non-human toxicology, pharmacology and drug metabolism studies in the laboratory, which support subsequent clinical testing. The conduct of the non-clinical tests must comply with federal regulations, including GLPs. The sponsor must submit the results of the non-clinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. An IND is a request for authorization from the FDA to administer an investigational drug product to humans. Some non-clinical testing may continue even after the IND is submitted, but an IND must become effective before human clinical trials may begin. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human trials. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials, including subjects will be exposed to unreasonable health risks, and places the IND on clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a drug candidate at any time before or during clinical trials due to safety concerns or non-compliance. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin, or that, once begun, issues will not arise that could cause the trial to be suspended or terminated.
The clinical stage of development involves the administration of the drug candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with Good Clinical Practices (GCP’s), which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety and assess efficacy. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries.
| 9 |
A sponsor who wishes to conduct a clinical trial outside the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of an NDA so long as the clinical trial is conducted in compliance with an international guideline for the ethical conduct of clinical research known as the Declaration of Helsinki and/or the laws and regulations of the country or countries in which the clinical trial is performed, whichever provides the greater protection to the participants in the clinical trial.
Clinical trials
Clinical trials are generally conducted in three sequential phases that may overlap, known as Phase 1, Phase 2 and Phase 3 clinical trials.
| · | Phase 1 clinical trials generally involve a small number of healthy volunteers who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the drug. |
| · | Phase 2 clinical trials typically involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, as well as identification of possible adverse effects and safety risks and preliminary evaluation of efficacy. |
| · | Phase 3 clinical trials generally involve large numbers of patients at multiple sites (from several hundred to several thousand subjects) and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use, and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. Phase 3 clinical trials may include comparisons with placebo and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing. |
Post-approval trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse events, finding from other studies, or any finding from animal or in vitro testing that suggests a significant risk for human subjects. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA, the IRB, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access to certain data from the trial. Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, we must develop methods for testing the identity, strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
| 10 |
NDA and FDA review process
The results of non-clinical studies and of the clinical trials, together with other detailed information, including extensive manufacturing information and information on the composition of the drug and proposed labeling, are submitted to the FDA in the form of an NDA requesting approval to market the drug for one or more specified indications. The FDA reviews an NDA to determine, among other things, whether a drug is safe and effective for its intended use and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. FDA approval of an NDA must be obtained before a drug may be offered for sale in the United States.
In addition, under the Pediatric Research Equity Act, or PREA, an NDA or supplement to an NDA must contain data to assess the safety and efficacy of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of pediatric data or full or partial waivers.
Under the Prescription Drug User Fee Act, or PDUFA, as amended, each NDA must be accompanied by a user fee. The FDA adjusts the PDUFA user fees on an annual basis. According to the FDA’s fee schedule for fiscal year 2016, the user fee for an application requiring clinical data, such as an NDAs, is $2.4 million. Additionally, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information rather than accepting an NDA for filing. The FDA must make a decision on accepting an NDA for filing within 60 days of receipt. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under PDUFA, the FDA has 10 months from the filing date in which to complete its initial review of a standard NDA and respond to the applicant, and six months from the filing date for a priority NDA. The FDA does not always meet its PDUFA goal dates for standard and priority NDAs, and the review process is often significantly extended by FDA requests for additional information or clarification.
After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. Before approving an NDA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether they comply with cGMPs. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. In addition, before approving an NDA, the FDA may also audit data from clinical trials to ensure compliance with GCP requirements. Additionally, the FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. The FDA will likely re-analyze the clinical trial data, which could result in extensive discussions between the FDA and the applicant during the review process. The review and evaluation of an NDA by the FDA is extensive and time consuming and may take longer than originally planned to complete, and we may not receive a timely approval, if at all.
After the FDA evaluates an NDA, it may issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. A Complete Response Letter usually describes all of the specific deficiencies in the NDA identified by the FDA. The Complete Response Letter may require additional clinical data and/or an additional pivotal Phase 3 clinical trial(s), and/or other significant and time-consuming requirements related to clinical trials, non-clinical studies or manufacturing. If a Complete Response Letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data.
| 11 |
There is no assurance that the FDA will ultimately approve a drug product for marketing in the United States and we may encounter significant difficulties or costs during the review process. If a product receives marketing approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling or may condition the approval of the NDA on other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-marketing testing or clinical trials and surveillance to monitor the effects of approved products. For example, the FDA may require Phase 4 testing which involves clinical trials designed to further assess a drug’s safety and efficacy and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. The FDA may also place other conditions on approvals including the requirement for a risk evaluation and mitigation strategy, or REMS, to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Product approvals may be withdrawn for non-compliance with regulatory requirements or if problems occur following initial marketing.
FDA Accelerated Review Programs
There are regulatory mechanisms that might potentially speed up the development and approval process for certain kinds of products. These mechanisms are Fast Track, Accelerated Approval, Priority Review, and Breakthrough status.
| · | Fast Track is a process designed to facilitate the development, and expedite the review of biological products to treat serious diseases and fill an unmet medical need by providing (1) more frequent meetings with the FDA to discuss product development, (2) more frequent written correspondence from the FDA about such things as the design of the proposed clinical trials, (3) eligibility for Accelerated Approval, and (4) a “ rolling review” process, which allows a company to submit sections of its application for review by the FDA, rather than waiting until every section of the application is completed before the entire application can be submitted for review. |
| · | Accelerated Approval allows earlier approval of biological products to treat serious diseases, and that fill an unmet medical need based on a surrogate endpoint, which can potentially reduce the time needed to conduct trials. Where the FDA approves a product on the basis of a surrogate marker, it requires the sponsor to perform post-approval studies as a condition of approval, and may withdraw approval if post-approval studies do not confirm the intended clinical benefit or safety of the product. Special rules would also apply to the submission to the FDA of advertising and promotional materials prior to use. |
| · | Priority Review designation is given to biological products that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A Priority Review means that the time it takes the FDA to review an application is reduced. The goal for completing a Priority Review is six months. Priority Review status can apply both to products that are used to treat serious diseases and to products for less serious illnesses. |
| · | Breakthrough Therapy designation is given to biological products that offer, alone or in combination, a treatment to a serious or life-threatening disorder, and preliminary clinical evidence indicates the drug may demonstrate substantial benefits over existing therapies on statistically significant clinical endpoints. Upon granting the breakthrough therapies designation, the FDA will expedite the development and review of the drug. |
| 12 |
We cannot know for sure whether the FDA would allow us to take advantage of any of these mechanisms in developing our products.
Each NDA submitted for FDA approval is usually reviewed for administrative completeness and reviewability within 45 to 60 days following submission of the application. If deemed complete, the FDA will “file” the NDA, and perform its substantive review of the application. The FDA can refuse to file a NDA that it deems incomplete or not properly reviewable. An applicant can then either request that the NDA be filed over the FDA’s protest, amend the application to address the deficiencies the FDA has alleged and resubmit it, or not pursue the application.
The FDA’s current performance goals for reviewing of NDAs are six months from submission for NDAs that the FDA designates as priority applications and 10 months from submission for standard applications. However, the FDA is not legally required to complete its review within these periods, and these performance goals may change over time. Moreover, the outcome of the review, even if generally favorable, can often be a “complete response” letter that describes additional work that must be done before the application can be approved. This work can sometimes be substantial. Also, even if the FDA approves a product, it may limit the approved therapeutic uses for the product through indications and usage statements it allows to be approved in the product labeling, require that warning statements be included in the product labeling, require that additional studies be conducted following approval as a condition of the approval, impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk evaluation and mitigation strategy (REMS), or otherwise limit the scope of any approval. Also, before any approval, facilities that manufacture the product must generally pass an FDA inspection.
Overall research, development, and approval times depend on a number of factors, including the period of review at the FDA, the number of questions posed by the FDA during review, how long it takes to respond to the FDA’s questions, the severity or life threatening nature of the disease in question, the availability of alternative treatments, the ability to take advantage of mechanisms that might facilitate development and FDA review of a product, the availability of clinical investigators and eligible patients, the rate of enrollment of patients in clinical trials, and the risks and benefits demonstrated in the clinical trials.
There are additional issues regarding our products that might be important to its research, development, and approval. Manufacturing issues regarding biological products can be particularly complex. Also the TARGT platform presents a somewhat different situation than those the FDA often deals with (i.e. a situation in which a biological therapeutic is manufactured at one or a few sites). Also, because the product will probably be considered a combination product with a device product component, there are device-related manufacturing and other compliance issues (e.g. cGMPs and adverse event reporting) that might be implicated by the product. These issues may increase the complexity of circumstances we will face with the FDA regarding approval of products using the TARGT platform.
Post-Approval Requirements
Any products for which we receive FDA approvals will be subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion, and other types of information on products that are placed on the market. Products may be promoted only for the approved indications and in accordance with the provisions of the approved label. Furthermore, product manufacturers must continue to comply with current Good Manufacturing Practices (cGMP) requirements, which are extensive and require considerable time, resources, and ongoing investment to ensure compliance. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented, and other types of changes to the approved product – such as changes in materials or adding indications or labeling claims – are also subject to further FDA review and approval.
Manufacturers and other entities involved in the manufacturing and distribution of an approved biological or medical device product are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. The cGMP requirements apply to all stages of the manufacturing process, including the production, processing, sterilization, packaging, labeling, storage, and shipment of the product. Manufacturers must establish validated systems to ensure that products meet specifications and regulatory standards, and test each product batch or lot prior to its release. On January 22, 2013, the FDA published final regulations for cGMPs for combination products. The final cGMP requirements for combination products are based upon the premise that constituent parts of a combination product retain their regulatory status after they are combined. In other words, combination products comprised of a biological product and a medical device are required to comply with both cGMPs for biological products and cGMPs for devices.
| 13 |
Manufacturers of biological products must also report to the FDA any deviations from cGMP that may affect the safety, purity, or potency of a distributed product, or any unexpected or unforeseeable event that may affect the safety, purity, or potency of a distributed product. The regulations also require investigation and correction of any deviations from cGMP and impose documentation requirements.
We might rely on third parties for the production of our products. FDA and state inspections may identify compliance issues at the facilities of contract manufacturers that may disrupt production or distribution or may require substantial resources to correct.
The FDA may withdraw a product approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Furthermore, the failure to maintain compliance with regulatory requirements may result in administrative or judicial actions, such as fines, warning letters, holds on clinical studies, product recalls or seizures, product detention or refusal to permit the import or export of products, refusal to approve pending applications or supplements, restrictions on marketing or manufacturing, injunctions or civil or criminal penalties.
In addition, from time to time, new legislation is enacted that can significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and policies are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative or FDA regulation or policy changes will be enacted or implemented and what the impact of such changes, if any, may be.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan product designation must be requested before submitting an NDA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication than that for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity.
| 14 |
To qualify for orphan designation in the European Union (EU), a medicine must meet a number of criteria:
| · | it must be intended for the treatment, prevention or diagnosis of a disease that is life-threatening or chronically debilitating; |
| · | the prevalence of the condition in the EU must not be more than five in 10,000 people or it must be unlikely that marketing of the medicine would generate sufficient returns to justify the investment needed for its development; and |
| · | no satisfactory method of diagnosis, prevention or treatment of the condition concerned can be authorized, or, if such a method exists, the medicine must be of significant benefit to those affected by the condition. |
Applications for orphan designation are examined by the EMA’s Committee for Orphan Medicinal Products (COMP), using the network of experts that the Committee has built up. The evaluation process takes a maximum of 90 days from validation. A drug that is approved as an orphan drug in by the EMA receives a special ten-year period of market exclusivity after marketing approval. If a sponsor receives orphan drug exclusivity upon approval, there can be no assurance that the exclusivity will prevent another entity or a similar product from receiving approval for the same or other uses.
Pediatric trials
The Food and Drug Administration Safety and Innovation Act, or FDASIA, which was signed into law on July 9, 2012, amended the FDCA to require that a sponsor who is planning to submit a marketing application for a drug that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within sixty days of an end-of-Phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from non-clinical studies, early phase clinical trials, and/or other clinical development programs.
Post-marketing requirements
Following approval of a new product, a pharmaceutical company and the approved product are subject to continuing regulation by the FDA, including, among other things, monitoring and recordkeeping activities, reporting to the applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling (known as “off-label use”), limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the Internet. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses. Prescription drug promotional materials must be submitted to the FDA in conjunction with their first use. Further, if there are any modifications to the drug, including changes in indications, labeling, or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require the applicant to develop additional data or conduct additional non-clinical studies and clinical trials. As with new NDAs, the review process is often significantly extended by FDA requests for additional information or clarification. Any distribution of prescription drug products and pharmaceutical samples must comply with the U.S. Prescription Drug Marketing Act, or the PDMA, a part of the FDCA.
| 15 |
In the United States, once a product is approved, its manufacture is subject to comprehensive and continuing regulation by the FDA. The FDA regulations require that products be manufactured in specific approved facilities and in accordance with cGMP. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products in accordance with cGMP regulations. NDA holders using contract manufacturers, laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified suppliers to these firms. These manufacturers must comply with cGMP regulations that require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. The discovery of violative conditions, including failure to conform to cGMP, could result in enforcement actions that interrupt the operation of any such facilities or the ability to distribute products manufactured, processed or tested by them. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved NDA, including, among other things, recall or withdrawal of the product from the market.
Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
Potential Competition with “Biosimilar” Products
The Biologics Price Competition and Innovation Act (BPCIA) was enacted as part of the Patient Protection and Affordable Care Act of 2010 (ACA), Pub. L. No. 111-148 (2010). The BPCIA authorizes the FDA to approve “abbreviated” BLAs for products whose sponsors demonstrate they are “biosimilar” to reference products previously approved under BLAs. The FDA may also separately determine whether “biosimilar” products are “interchangeable” with their reference products. However, the FDA may not approve an “abbreviated” BLA for a biosimilar product until at least twelve years after the date on which the BLA for the reference product was approved. FDA approval could be further delayed if the reference products are subject to unexpired and otherwise valid patents.
Prior to the enactment of the BPCIA, information in approved BLAs could not be relied upon by other manufacturers to establish the safety and efficacy of their products for which they were seeking FDA approval. (In contrast, since at least 1984, pharmaceutical manufacturers have been able to submit Abbreviated New Drug Applications for “generic drugs” that are materially identical to reference drugs approved under New Drug Applications.) Accordingly, if the Company’s products are approved under a BLA, other manufacturers potentially could develop and seek FDA approval of “biosimilar” products at some point in the future.
In Vitro Companion Diagnostics
FDA defines an IVD companion diagnostic device is an in vitro
diagnostic device that provides information that is essential for the safe and effective use of a corresponding therapeutic product.
The use of an IVD companion diagnostic device with a therapeutic product is stipulated in the instructions for use in the labeling
of both the diagnostic device and the corresponding therapeutic product, including the label. Such tests include genetic diagnostic
tests. Approval of such of treatment with the therapeutic product may be dependent on the approval of an IVD to
| · | Monitor response to treatment with the therapeutic product for the purpose of adjusting treatment (e.g., schedule, dose, discontinuation) to achieve improved safety or effectiveness |
| · | Identify patients in the population for whom the therapeutic product has been adequately studied, and found safe and effective, i.e., there is insufficient information about the safety and effectiveness of the therapeutic product in any other population |
| 16 |
Applications for an IVD companion diagnostic device and its corresponding therapeutic product will be reviewed and approved according to applicable regulatory requirements. The IVD companion diagnostic device application will be reviewed and approved or cleared under the device authorities of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and relevant medical device regulations; the therapeutic product application will be reviewed and approved under section 505 of the FD&C Act (i.e., drug products) or section 351 of the Public Health Service Act (i.e., biological products) and relevant drug and biological product regulations. FDA intends to review each IVD companion diagnostic device submission within the context of, or in conjunction with, its corresponding therapeutic product, and FDA review of the IVD companion diagnostic device and the therapeutic product will be carried out collaboratively among relevant FDA offices.
Ideally, a therapeutic product and its corresponding IVD companion diagnostic device should be developed contemporaneously, with the clinical performance and clinical significance of the IVD companion diagnostic device established using data from the clinical development program of the corresponding therapeutic product. Many of our current and future product development candidates, including NFC-1, will depend upon co-development of accurate genetic and other IVD. This adds additional cost and complexity to our development programs. The availability of IVD companion diagnostics can allow more efficient development programs and more appropriate use of products in the marketplace with more predictable outcomes for patients and higher value medicines.
Ultimately FDA approval of the IVD will be required to allow approval of many of our products. However, technical difficulties or other issues could delay or disrupt the development of our products.
U.S. Fraud and Abuse Laws
Anti-Kickback Statute and HIPAA Criminal Laws
We are subject to various federal and state laws pertaining to health care “fraud and abuse.” The federal Anti-Kickback Statute makes it illegal for any person, including a pharmaceutical, biologic, or medical device company (or a party acting on its behalf), to knowingly and willfully solicit, offer, receive or pay any remuneration, directly or indirectly, in exchange for, or to induce, the referral of business, including the purchase, order or prescription of a particular item or service, or arranging for the purchase, ordering, or prescription of a particular item or service for which payment may be made under federal healthcare programs such as Medicare and Medicaid. In 1996, under the Health Insurance Portability and Accountability Act (HIPAA), Pub. L. No. 104-191 (1996), the Anti-Kickback Statute was expanded to be made applicable to most federal and state-funded health care programs. The definition of “remuneration” has been broadly interpreted to include any item or service of value, including but not limited to gifts, discounts, the furnishing of free supplies or equipment, commercially unreasonable credit arrangements, cash payments, waivers of payments or providing anything at less than its fair market value. Several courts have interpreted the Anti-Kickback Statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of business reimbursable by a federal healthcare program, the statute has been violated. The ACA further amended the federal Anti-Kickback Statute to clarify that “a person need not have actual knowledge of this section or specific intent to commit a violation of this section.” Therefore, all courts are likely to use the “one purpose” test for evaluating intent. Penalties for violations include criminal penalties, civil sanctions and administrative actions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federally-funded healthcare programs. In addition, some kickback allegations have been held to violate the federal False Claims Act, which is discussed in more detail below.
The federal Anti-Kickback Statute is broad and prohibits many arrangements and practices that may be lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous and beneficial arrangements, Congress created several exceptions in the Social Security Act and has authorized the U.S. Department of Health and Human Services (HHS) to publish regulatory “safe harbors” that exempt certain practices from enforcement action under the Anti-Kickback Statute prohibitions. For example, there are safe harbors available for certain discounts to purchasers, personal services arrangements and various other types of arrangements. However, safe harbor protection is only available for transactions that satisfy all of the narrowly defined safe harbor provisions applicable to the particular remunerative relationship. We seek to comply with such safe harbors whenever possible. Conduct and business arrangements that do not strictly comply with all the provisions of an applicable safe harbor, while not necessarily illegal, face an increased risk of scrutiny by government enforcement authorities and an ongoing risk of prosecution.
| 17 |
In addition, many states have adopted laws similar to the federal Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare services reimbursed by any third-party payer, not only the Medicare and Medicaid programs or other governmental payers. At least one state, California, also has adopted a law requiring pharmaceutical companies to implement compliance programs to prevent and deter conduct that may violate fraud and abuse laws that comply with the voluntary industry guidelines and the HHS Office of Inspector General (OIG) compliance guidance. While we believe we have structured our business arrangements to comply with these laws, it is possible that the government could find that such arrangements violate these laws, which could have a material adverse effect on our business, results of operations and financial condition.
HIPAA created two new federal crimes: health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including private payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal and state health care programs such as Medicare and Medicaid. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment. Additionally, HIPAA granted expanded enforcement authority to HHS and the U.S. Department of Justice (DOJ) and provided enhanced resources to support the activities and responsibilities of the OIG and DOJ by authorizing large increases in funding for investigating fraud and abuse violations relating to health care delivery and payment.
False Claims Laws
Pursuant to various federal and state false claims laws, the submission of false or fraudulent claims for payment may lead to civil money penalties, criminal fines and imprisonment, and/or exclusion from participation in Medicare, Medicaid and other federally funded health care programs. These false claims statutes include the federal False Claims Act, 12 Stat. 696 (1863), which was significantly expanded in both the Fraud Enforcement and Recovery Act of 2009, Pub. L. No. 111-21 (2009), and in the ACA. In addition, a number of states have enacted similar laws prohibiting the submission of false or fraudulent claims to a state government.
The federal False Claims Act allows the federal government or private individuals to bring suit alleging that an entity or person knowingly submitted (or caused another person or entity to submit or conspired to submit) a false or fraudulent claim for payment to the federal government or knowingly used (or caused to be used) a false record or statement to obtain payment from the federal government. The federal False Claims Act may also be violated if a person files a false statement in order to reduce, avoid, or conceal an obligation to pay money to the federal government, or engages in conduct that may violate the federal Anti-Kickback Statute. The ACA expressly states that claims arising out of violations of the federal Anti-Kickback Statute are false claims for purposes of the federal False Claims Act. Several pharmaceutical and medical device companies have settled claims based on the federal False Claims Act for conduct involving, among other examples, providing free product to purchasers with the expectation that federally-funded health programs would be billed for the product, or instances in which a manufacturer has marketed its product for unapproved and non-reimbursable purposes or encouraged providers to bill government health care programs for unapproved uses of the product. In addition to False Claims Act cases brought directly by the federal government, individuals who file a lawsuit in the name of the federal government may be able to share in amounts recovered by the government in connection with such suits. Such suits, known as qui tam actions, have increased significantly in recent years and have increased the risk that a health care company will have to defend a false claims action, enter into settlements that may include corporate integrity agreements requiring disclosures to the federal government, pay fines or be excluded from the Medicare and/or Medicaid programs as a result of an investigation arising out of such an action. We are not aware of any false claims actions pending against us. However, no assurance can be given that such actions may not be filed against us in the future, or that any non-compliance with such laws would not have a material adverse effect on our business, results of operations and financial condition.
The foregoing description of laws and regulations affecting health care companies is not meant to be an all-inclusive discussion of aspects of federal and state fraud and abuse laws that may affect our business, results of operations and financial condition. Health care companies operate in a complicated regulatory environment. These or other statutory or regulatory initiatives may affect our revenues or operations. No assurance can be given that our practices, if reviewed, would be found to be in compliance with applicable fraud and abuse laws (including false claims laws and anti-kickback prohibitions), as such laws ultimately may be interpreted, or that any non-compliance with such laws or government investigations of alleged non-compliance with such laws would not have a material adverse effect on our business, results of operations and financial condition.
| 18 |
Other U.S. Regulatory Requirements
In the United States, the research, manufacturing, distribution, sale, and promotion of drug and biological products are subject to regulation by various federal, state, and local authorities in addition to the FDA, including the CMS (formerly the Health Care Financing Administration), other divisions of HHS (e.g., OIG), DOJ and individual United States Attorney offices within DOJ, and state and local governments. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, Pub. L. No. 101–508 (1990), and the Veterans Health Care Act of 1992, Pub. L. No. 102-585 (1992), each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection, unfair competition, and other laws. In addition, we may be subject to federal and state laws requiring the disclosure of financial arrangements with health care professionals.
Moreover, we may become subject to additional federal, state, and local laws, regulations, and policies relating to safe working conditions, laboratory practices, the experimental use of animals, and/or the use, storage, handling, transportation, and disposal of human tissue, waste, and hazardous substances, including radioactive and toxic materials and infectious disease agents used in conjunction with our research work.
Foreign Regulatory Requirements
We may be subject to widely varying foreign regulations, which may be quite different from those of the FDA, governing clinical trials, manufacture, product registration and approval, and pharmaceutical sales.
Whether or not FDA approval has been obtained, we must obtain a separate approval for a product by the comparable regulatory authorities of foreign countries prior to the commencement of product marketing in these countries. In certain countries, regulatory authorities also establish pricing and reimbursement criteria. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval.
In order to conduct clinical testing on humans in Israel, special authorization must first be obtained from the ethics committee and general manager of the institution in which the clinical studies are scheduled to be conducted, as required under the Guidelines for Clinical Trials in Human Subjects implemented pursuant to the Israeli Public Health Regulations (Clinical Trials in Human Subjects), as amended from time to time, and other applicable legislation. The institutional ethics committee must, among other things, evaluate the anticipated benefits that are likely to be derived from the project to determine if it justifies the risks and inconvenience to be inflicted on the human subjects, and the committee must ensure that adequate protection exists for the rights and safety of the participants as well as the accuracy of the information gathered in the course of the clinical testing. Since we intend to perform a portion of the clinical studies on certain of our therapeutic candidates in Israel, we will be required to obtain authorization from the ethics committee and general manager of each institution in which we intend to conduct our clinical trials, and from the Israeli Ministry of Health.
In addition, pharmaceutical products may not be imported into, or manufactured or marketed in, the State of Israel absent drug registration or the appropriate license/approval to import/manufacture for clinical trials use.
Reimbursement and Pricing Controls
Third-party payers (Medicare, Medicaid, private health insurance companies and other organizations) may affect the pricing or relative attractiveness of our product candidates by regulating the level of reimbursement provided to the physicians and clinic utilizing our product candidates or by refusing reimbursement. If reimbursement under these programs, or if the amount of time to secure reimbursement is too long, our ability to market our technology and product candidates may be adversely and materially affected. In international markets, reimbursement by private third-party medical insurance providers, including government insurers and independent providers, varies from country to country. In certain countries, our ability to achieve significant market penetration may depend upon the availability of third-party government reimbursement.
| 19 |
In many of the markets where we or our collaborative partners would commercialize a product following regulatory approval, the prices of pharmaceutical products are subject, by law, to direct price controls and to drug reimbursement programs with varying price control mechanisms. Public and private health care payers control costs and influence drug pricing through a variety of mechanisms, including the setting of reimbursement amounts for drugs and biological products covered by Medicare Part B based on their Average Sales Prices calculated by manufacturers in accordance with the Medicare Prescription Drug, Improvement, and Modernization Act of 2010, Pub. L. No. 108-173 (2003), as amended, through negotiating discounts with the manufacturers, and through the use of tiered formularies and other mechanisms that provide preferential access to certain drugs over others within a therapeutic class. Drug manufacturers also may be subject to drug rebate agreements with public or private health care payers in exchange for the manufacturers’ products being included on plan formularies.
Payers also set other criteria to govern the uses of a drug that will be deemed medically appropriate and therefore reimbursed or otherwise covered. If a payer concludes that a drug is experimental or investigational, in many cases it will deny coverage on that basis alone. Further, many public and private health care payers limit reimbursement and coverage to the uses of a drug that are either approved by the FDA or that are supported by other appropriate evidence (for example, published medical literature) and appear in a recognized drug compendium. Drug compendia are publications that summarize the available medical evidence for particular drug products and identify which uses of a drug are supported or not supported by the available evidence, whether or not such uses have been approved by the FDA. For example, in the case of Medicare coverage for physician-administered oncology drugs, the Omnibus Budget Reconciliation Act of 1993, Pub. L. No. 103-66 (1993), with certain exceptions, prohibits Medicare carriers from refusing to cover unapproved uses of an FDA-approved drug if the unapproved use is supported by one or more citations in the American Hospital Formulary Service Drug Information the American Medical Association Drug Evaluations, or the United States Pharmacopoeia Drug Information. Another commonly cited compendium, for example under Medicaid, is the DRUGDEX Information System.
Employees
We currently employ 40 full-time and two part-time employees. None of our employees is represented by a labor union and we have not experienced any strikes or work stoppages. While none of our employees is party to any collective bargaining agreements, certain provisions of the collective bargaining agreements between the Histadrut (General Federation of Labor in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Associations) are applicable to our employees in Israel by order the Israel Ministry of Labor. Such orders are part of the employment related laws and regulations which apply to our employees in Israel and set certain mandatory terms of employment. Such mandatory terms of employment primarily concern the length of the workday, minimum daily wages, pension plan benefits for all employees, insurance for work-related accidents, procedures for dismissal of employees, severance pay and other conditions of employment. We generally provide our employees with benefits and working conditions beyond the required minimums. We believe our relations with our employees are good.
Additional Information
Our principal executive offices are located at 435 Devon Park Drive, Building 700, Wayne, Pennsylvania 19087. We conduct our research and development activities primarily from our Israeli location in Misgav Business Park, Misgav. Our telephone numbers are (610) 254-4201 in the United States and +972-4-902-8900 in Israel.
Our website address is www.medgenics.com. The information on or accessible through our website is not part of this Annual Report on Form 10-K. Copies of our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to such reports are available without charge on our website or upon request to us. In addition, our Code of Business Conduct and Ethics, Audit Committee Charter, Compensation Committee Charter and Nominating and Corporate Governance Committee Charter are all available without charge on our website or upon request to us. All such requests should be sent to Medgenics, Inc., Corporate Secretary, 435 Devon Park Drive, Building 700, Wayne, Pennsylvania 19087, or by email request from our website at www.medgenics.com. Amendments to, or waivers from, our Code of Business Conduct and Ethics that apply to our executive officers will be posted to our website. We also post or otherwise make available on our website from time to time other information that may be of interest to our investors.
| 20 |
Business-Related Risks
We are a clinical stage medical technology company and have a history of significant and continued operating losses and a substantial accumulated earnings deficit and we may continue to incur significant losses.
We are a clinical stage medical technology company and since our inception have been focused on research and development and have not generated any substantial revenues. We have incurred net losses of approximately $37.99 million, $18.43 million and $17.13 million for the years ended December 31, 2015, 2014 and 2013, respectively. As of December 31, 2015, we had stockholders’ equity of approximately $50.35 million. We expect to incur additional operating losses, as well as negative cash flow from operations, for the foreseeable future, as we continue to expand our research and development and commence commercialization of our potential product candidates. Our ability to generate revenues from sales of our potential products will depend on:
| · | successful completion of necessary clinical trials which have not advanced beyond Phase 2 stage; |
| · | regulatory approval; |
| · | commercialization (through partnership or licensing deals or through internal development) and market acceptance of new technologies and product candidates under development; |
| · | medical community awareness; and |
| · | changes in regulation or regulatory policy. |
We believe that initial commercialization of any of our product candidates by us or any future strategic partners is not likely before 2018 and could easily take four years or more.
We will need substantial additional capital for the continued development of our product candidates and for our long-term operations.
As of December 31, 2015, our cash and cash equivalents were approximately $53.06 million. We believe our existing cash and cash equivalents should be sufficient to meet our operating and capital requirements at least through the end of the third quarter of 2017. However, changes in our business, whether or not initiated by us, may affect the rate at which we deplete our cash and cash equivalents. Our present and future capital requirements depend on many factors, including:
| · | the level of patient recruitment in our clinical trials, including the two planned trials for NFC-1 in the United States, and the results of the clinical trials; |
| · | the level of research and development investment required to develop our product candidates, and to maintain and improve the TARGT platform; |
| · | changes in product development plans needed to address any difficulties that may arise in manufacturing, pre-clinical activities, clinical trials or commercialization; |
| · | our ability and willingness to enter into new agreements with strategic partners, and the terms of these agreements; |
| · | our success rate in pre-clinical and clinical efforts; |
| 21 |
| · | costs of recruiting and retaining qualified personnel; |
| · | time and costs involved in obtaining regulatory approvals; and |
| · | costs of filing, prosecuting, defending, and enforcing patent claims and other intellectual property rights. |
We will require significant amounts of additional capital in the future, and such capital may not be available when we need it on terms that we find favorable, if at all. We may seek to raise these funds through public or private equity offerings, debt financings, credit facilities, or partnering or other corporate collaborations and licensing arrangements. If adequate funds are not available or are not available on acceptable terms, our ability to fund our operations, take advantage of opportunities, develop products and technologies, and otherwise respond to competitive pressures could be significantly delayed or limited, and we may need to downsize or halt our operations.
We are still in the process of clinical trials and do not have a commercialized product and may never be able to commercialize our product candidates.
We have completed a human clinical trial with respect to our TARGTEPO microorgans in pre-dialysis patients and are conducting a trial in dialysis patients in Israel with a second generation expression cassette of the HDAd viral vector. Prior to our acquisition of neuroFix, human clinical trials have also been completed by others with respect to NFC-1 in vascular dementia patients not enriched for metabotopic glutamate receptor (mGluR) neuromodulator and in mGluR mutation positive adolescents with ADHD symptoms. Only a small number of research and development programs ultimately result in commercially successful drugs and drug delivery systems. Potential products that appear to be promising at early stages of development may not reach the market for a number of reasons, including:
| · | failure to obtain approvals for clinical trials; |
| · | lack of familiarity of health care providers and patients; |
| · | low market acceptance as a result of lower demonstrated clinical safety or efficacy compared to other products or other potential disadvantages relative to alternative treatment methods; |
| · | inability to obtain favorable coverage determinations from health plans and third-party payers; |
| · | insufficient or unfavorable levels of reimbursement from government or third-party payers; |
| · | infringement on proprietary rights of others for which we (or our licensees, if any) have not received licenses; |
| · | incompatibility with other therapeutic products; |
| · | potential advantages of alternative treatment methods; |
| · | ineffective marketing and distribution support; |
| · | lack of cost-effectiveness; or |
| · | timing of market introduction of competitive products. |
If any of these potential problems occur, we may never successfully commercialize our product candidates, including NFC-1, or products based on our TARGT platform. If we are unable to develop commercially viable products, our business, results of operations and financial condition will be materially and adversely affected.
| 22 |
Our product candidates are still being developed and have not been tested on a large patient population, and, therefore, we do not know all of the possible adverse events and may not be able to commercialize our product candidates as planned.
Our product candidates have not been tested on a large number of patients, and are still in an early stage of development. While we have attained positive results in our early stages of development and early clinical trials, our product candidates are not yet fully developed or proven, and disappointing results and problems could delay or prevent the completion of our development programs and commercialization of our product candidates.
Our previous safety tests and results obtained in previous clinical trials of our product candidates may not be representative of either a larger multi-centric test or the commercial version of the technology in the general population. Specifically, the Phase 1b clinical trial for NFC-1 completed prior to our acquisition of neuroFix was conducted on a single-blinded basis and may have been subject to bias and such results may not be replicated in a double-blinded clinical trial. In addition, the full impact of our product candidates, and their many possible variations, on the body is, as yet, unknown. For instance, although no serious adverse events (SAEs) attributed to the TARGT platform were found to date in our TARGTEPO clinical trials, the possibility cannot be ruled out that treatment-related SAEs might be borne out by further trials, and if so, this could have serious implications on the viability of the technology and our business.
Treatment-related adverse events or complications in clinical trials, or post-approval, could result in limitations on the use of our product candidates and may also result in financial claims and losses against us, damage our reputation, and increase our expenses and reduce our assets. In addition, our product candidates may not gain commercial acceptance or ever be commercialized.
We are currently completely dependent upon the successful development of our lead product candidate, NFC-1, and the TARGT platform. If we fail to successfully complete their development and commercialization or enter into licensing or partnership agreements, we will not generate operating revenues.
All of our efforts are currently focused on the development of NFC-1 and our TARGT platform. There is no guarantee that we will succeed in developing NFC-1 or products based on our TARGT platform. If we or any partner(s) or collaborator(s) that we may enter into a relationship with are unable to consummate the production of NFC-1 or TARGT microorgans to provide the sustained protein or peptide therapy to treat various chronic diseases in a safe, stable, commercial end-product form, we will be unable to generate any revenues. There is no certainty as to our success, whether within a given time frame or at all. Any delays in our schedule for clinical trials, regulatory approvals or other stages in the development of our technology are likely to cause us additional expense, and may even prevent the successful finalization of any or all of our product candidates. Delays in the timing for development of our technology may also have a material adverse effect on our business, financial condition and results of operations due to the possible absence of financing sources for our operations during such additional periods of time. Although we may pursue other technologies (either developed in-house or acquired), there is no assurance that any other technology will be successfully identified or exploited.
Clinical trials involve lengthy and expensive processes with uncertain outcomes, and results of earlier studies and trials may not be predictive of future trial results.
We cannot predict whether we will encounter problems with any of our completed, ongoing or planned clinical trials, which would cause us or regulatory authorities to delay or suspend clinical trials, or delay the analysis of data from completed or ongoing clinical trials. We estimate that clinical trials involving NFC-1 and various applications of our TARGT platform will continue for several years; however, such trials may also take significantly longer to complete and may cost more money than we expect. Failure can occur at any stage of testing, and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of the current, or a future, more advanced, version of our product candidates, including but not limited to:
| · | delays in obtaining regulatory approvals to commence a clinical trial; |
| 23 |
| · | failure or inability to recruit qualified investigators; |
| · | slower than anticipated patient recruitment and enrollment; |
| · | negative or inconclusive results from clinical trials; |
| · | unforeseen safety issues; |
| · | an inability to monitor patients adequately during or after treatment; and |
| · | problems with investigator or patient compliance with the trial protocols. |
A number of companies in the medical device, biotechnology, and biopharmaceutical industries including those with greater resources and experience than us have suffered significant setbacks in advanced clinical trials, even after seeing promising results in earlier clinical trials. Despite the successful results reported in early clinical trials regarding NFC-1 and our TARGTEPO microorgans, we do not know whether any clinical trials we or any future clinical partners may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market NFC-1, TARGTEPO microorgans or any other product based on our TARGT platform. If later-stage clinical trials involving NFC-1 or our TARGT platform do not produce favorable results, we may be required to perform additional clinical trials or our ability to obtain regulatory approval may be adversely impacted, either of which may have an adverse material effect on our business, financial condition and the results of our operations.
Potential difficulty with, and delays in, recruiting additional patients for human clinical trials may adversely affect the timing of our clinical trials and our working capital requirements.
Our research and development is highly dependent on timely recruitment of the requisite number and type of patients for our clinical trials. We have previously found it very difficult to recruit such patients and the increased volume and ethnic backgrounds required for future testing may render such testing even more difficult. Such larger studies will likely be based on the use of multicenter, multinational design, which can prove difficult to manage and could result in delays in patient recruitment. In addition, as we pursue development of our product candidates in orphan and rare disease applications, we may find it difficult to find sufficient treatment-naïve patients needed for initial trials, especially within commercially-reasonable geographical regions. Delays in the recruitment of such patients could delay our trials and negatively impact our working capital requirements.
We may not successfully establish and maintain relationships with third-party service providers and collaborators, which could adversely affect our ability to develop our product candidates.
Our ability to commercialize our product candidates is dependent on our ability to reach strategic licensing and other development agreements with appropriate partners, including pharmaceutical companies and biotech firms. If we are unable to successfully negotiate such agreements, we may not be able to continue to develop our product candidates, including NFC-1 and TARGTEPO, without raising significant additional capital for commercialization.
Our core business strategy is to develop our product candidates for use in specific indications and disease markets that we would internally develop and launch. However, we do plan to explore collaborative relationships or strategic partnerships and/or license our product candidates. We may not be able to identify such collaborators and partners on a timely basis and we may not be able to enter into relationships with any future collaborator(s) or partner(s) on terms that are commercially beneficial to us or at all. In addition, such relationships and partnerships may not come to fruition or may not be successful. Our agreements with these third parties may also contain provisions that restrict our ability to develop and test our product candidates or that give third parties rights to control aspects of our product development and clinical programs.
The third-party contractors may not assign as great of a priority to our clinical development programs or pursue them as diligently as we would if we were undertaking such programs directly and, accordingly, may not complete activities on schedule, or may not conduct the studies or our clinical trials in accordance with regulatory requirements or with our trial design. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, or if their performance is substandard, we may be required to replace them.
In addition, conflicts may arise with our collaborators (e.g. those concerning the interpretation of clinical data), the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration. If any conflicts arise with our existing or future collaborators, they may act in their self-interest, which may be adverse to our best interests. The third-party contractors may also have relationships with other commercial entities, some of whom may compete with us. If the third-party contractors work with our competitors, our competitive position may be harmed.
| 24 |
In addition, although we attempt to audit and control the quality of third-party data, we cannot guarantee the authenticity or accuracy of such data, nor can we be certain that such data has not been fraudulently generated. The failure of third parties to carry out their obligations towards us would materially adversely affect our ability to develop and market product candidates.
Certain difficulties with respect to the development and use of our TARGT platform may adversely affect the timing of our clinical trials, the further development of our technology, our working capital requirements and the likelihood of commercialization of that technology.
Vectors intended for use in clinical trials must be produced by other vector suppliers who manufacture according to strict requirements of Good Manufacturing Practice (GMP). We have worked with one such GMP vector manufacturer who has supplied the GMP vectors used in our TARGTEPO human clinical trials and we intend to continue to order new GMP vectors when needed from such supplier as well as new suppliers as appropriate. There is a possibility that the existing supplier would discontinue its business or that other problems could occur with the timely production and delivery of GMP vectors. If this were to occur, we would need to establish GMP vector production at one or more alternative GMP vector manufacturers. Delays in obtaining the vectors could delay any new TARGT trials. Without the necessary vectors, we would be unable to continue the research and development of our TARGT technology, which would negatively impact our working capital requirements.
The successful adoption of the TARGT platform also relies on our ability to bring about practical, reliable and cost-effective production of TARGT microorgans on a commercial scale and its use in patients in widespread locations. This requires the design, development and commercial scale-up of TARGT manufacturing capability, intended for implementation in regional TARGT processing centers, together with appropriate logistical capabilities to enable local treatment of patients in their communities, in a cost effective and reliable manner. TARGT microorgan processing is intended to be effected using semi-automated processing stations employing sealed cassettes and other single use items for each patient. Although we have experienced initial positive results in processing MOs in individual closed processing chambers that were shipped from Israel at our contract manufacturing organization (CMO) in a GMP-certified facility in California, we or our CMO may not necessarily be able to replicate those results or be able to accommodate greater amounts. Treatment of patients in various locations is dependent upon reliable acquisition of MOs and implantation or ablation of TARGT microorgans by trained local physicians, using appropriate proprietary and nonproprietary devices and products, and upon the transport of microorgans and TARGT microorgans between the TARGT processing centers and local treatment clinics via reliable and cost effective logistical arrangements. It may also be important that the processing center not require highly skilled operators, specialist laboratories or clean rooms. The inability to adequately scale and rollout such technology could damage the cost-effectiveness and therefore one of the anticipated competitive advantages of the TARGT platform.
While our new strategic focus is on rare and orphan diseases, therapeutic proteins and peptides for these diseases have never been produced by the TARGT, and we may not be successful in creating a TARGT microorgan that produces proteins and peptides for the treatment of rare and orphan diseases.
While the TARGT microorgan’s attributes of producing low levels of autologous proteins for an extended period of time would appear to be highly amenable to treating rare and orphan diseases, we have not yet created a TARGT microorgan that addresses a rare or orphan disease and may never overcome the technical hurdles. In addition, we may target rare and orphan diseases that require peptides or proteins with post-translational modifications for efficacy, and we have not yet produced peptides or proteins with post-translational modifications in the TARGT. The production of peptides and/or proteins with post-translational modifications could require us to develop new techniques of protein production from the TARGT microorgan which may delay research and development timelines or may be simply too great to overcome.
| 25 |
We have no marketing experience, sales force or distribution capabilities. If our product candidates are approved, and we are unable to recruit key personnel to perform these functions, we may not be able to successfully commercialize the products.
Although we do not currently have any marketable products, our ability to produce revenues ultimately depends on our ability to sell our product candidates if and when they are approved by the FDA and other regulatory authorities. We currently do not have a marketing and sales staff or distribution capabilities. Developing a marketing and sales force is also time-consuming and could delay the launch of new products or expansion of existing product sales. In addition, we will compete with many companies that currently have extensive and well-funded marketing and sales operations. If we fail to establish successful marketing and sales capabilities or fail to enter into successful marketing arrangements with third parties, our ability to generate revenues will suffer.
Furthermore, even if we enter into marketing and distributing arrangements with third parties, these third parties may not be successful or effective in selling and marketing our product candidates. If we fail to create successful and effective marketing and distribution channels, our ability to generate revenue and achieve our anticipated growth could be adversely affected. If these distributors experience financial or other difficulties, sales of our products could be reduced, and our business, financial condition and results of operations could be harmed.
We are subject to intense government regulation and we may not be able to successfully complete the necessary clinical trials.
Approval for clinical trials depends, among other things, on data obtained from our pre-clinical and clinical activities, including completion of pre-clinical animal and in vitro studies in a timely manner. These pre-clinical and clinical activities must meet stringent quality assurance and compliance requirements. Data obtained from such activities are susceptible to varying interpretations, which could delay, limit or prevent regulatory approvals.
We currently have limited experience in and resources for conducting the large-scale clinical trials which may hamper our ability to obtain or comply with regulatory approval. The failure to comply with applicable regulatory requirements may result in criminal prosecution, civil penalties, product recalls, withdrawal of product approval, mandatory restrictions and other actions, which could impair our ability to conduct business.
Use of third parties to manufacture our product candidates may increase the risk that we will not have sufficient quantities of our product candidates or such quantities at an acceptable cost, and clinical development and commercialization of our product candidates could be delayed, prevented or impaired.
We do not own or operate manufacturing facilities for production of our product candidates. We lack the resources and the capabilities to manufacture any of our product candidates on a clinical or commercial scale. We currently outsource the manufacturing and packaging of our pre-clinical and clinical product candidates to third parties. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up initial production. These problems include difficulties with production costs and yields and quality control, including stability of the product candidate. The occurrence of any of these problems could significantly delay our clinical trials or the commercial availability of our products.
We do not currently have any agreements with third party manufacturers for the long-term commercial supply of any of our product candidates. We may be unable to enter into agreements for commercial supply with third party manufacturers, or may be unable to do so on acceptable terms. Even if we enter into these agreements, the manufacturers of each product candidate will be single source suppliers to us for a significant period of time.
| 26 |
Reliance on third party manufacturers entails risks, to which we would not be subject if we manufactured product candidates or products ourselves, including:
| · | reliance on the third party for regulatory compliance and quality assurance; |
| · | limitations on supply availability resulting from capacity and scheduling constraints of the third parties; |
| · | impact on our reputation in the marketplace if manufacturers of our products, once commercialized, fail to meet the demands of our customers; |
| · | the possible breach of the manufacturing agreement by the third party because of factors beyond our control; and |
| · | the possible termination or non-renewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us. |
The failure of any of our contract manufacturers to maintain high manufacturing standards could result in injury or death of clinical trial participants or patients using products. Such failure could also result in product liability claims, product recalls, product seizures or withdrawals, delays or failures in testing or delivery, cost overruns or other problems that could seriously harm our business or profitability.
Our contract manufacturers are required to adhere to FDA regulations setting forth GMP. These regulations cover all aspects of the manufacturing, testing, quality control and recordkeeping relating to our product candidates and any products that we may commercialize. Our manufacturers may not be able to comply with GMP regulations or similar regulatory requirements outside the United States. Our failure or the failure of our third party manufacturers, to comply with applicable regulations could significantly and adversely affect regulatory approval and supplies of our product candidates.
Our product candidates and any products that we may develop or acquire may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under GMP regulations and that are both capable of manufacturing for us and willing to do so. If the third parties that we engage to manufacture products for our pre-clinical tests and clinical trials should cease to continue to do so for any reason, we likely would experience delays in advancing these trials while we identify and qualify replacement suppliers and we may be unable to obtain replacement supplies on terms that are favorable to us. Later relocation to another manufacturer will also require notification, review and other regulatory approvals from the FDA and other regulators and will subject our production to further cost and instability in the availability of our product candidates. In addition, if we are not able to obtain adequate supplies of our product candidates or the drug substances used to manufacture them, it will be more difficult for us to develop our product candidates and compete effectively.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to develop product candidates and commercialize any products that obtain regulatory approval on a timely and competitive basis.
The FDA and other health authorities will regulate our product candidates and we may never receive regulatory approval to market and sell our product candidates.
Our product candidates will require regulatory approvals prior to sale. In particular, our product candidates are subject to stringent approval processes, prior to commercial marketing, by the FDA and by comparable agencies in all countries where we operate and desire to introduce our product candidates, whether sold via a strategic partner or directly by us. These requirements range from efficacy and safety assessments in Phase 3 clinical trials to long-term follow-up assessments on treated patients in clinical trials for product approval for sale. The process of obtaining FDA and corresponding foreign approvals is costly and time-consuming, and we cannot assure that such approvals will be granted. Also, the regulations we are subject to change frequently and such changes could cause delays in the development of our product candidates.
| 27 |
It typically takes a company several years or longer to satisfy the substantial requirements imposed by the FDA and comparable agencies in other countries for the introduction of therapeutic pharmaceutical and biological products. Pharmaceutical or biological products must be registered in accordance with applicable law before they can be manufactured, marketed and distributed. This registration must include medical data proving the product’s safety, efficacy and clinical testing. Also included in product registration should be references to medical publications and information about the production methods and quality control.
To obtain regulatory approvals in the United States, we or a collaborator must ultimately demonstrate to the satisfaction of the FDA that our product candidates are sufficiently safe and effective for their proposed administration to humans. Many factors, both known and unknown, can adversely impact clinical trials and the ability to evaluate a product candidate’s safety and efficacy, including:
| · | the FDA or other health regulatory authorities or instructional review boards decision(s) not to approve a clinical trial protocol or place a clinical trial on hold; |
| · | suitable patients not enrolling in a clinical trial in sufficient numbers or at the expected rate, for reasons such as the size of the prospective patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the perceptions of investigators and patients regarding safety, and the availability of other treatment options; |
| · | clinical trial data being adversely affected by trial conduct or patient withdrawal prior to completion of the trial; |
| · | competition with ongoing clinical trials and scheduling conflicts with participating clinicians; |
| · | patients experience adverse events, including treatment-related adverse events of our drug candidates, for a variety of reasons that may or may not be related to our product candidates, including the advanced stage of their disease and other medical problems; |
| · | patients in the placebo or untreated control group exhibiting greater than expected improvements or fewer than expected adverse events; |
| · | third-party clinical investigators not performing the clinical trials on the anticipated schedule or consistently with the clinical trial protocol and good clinical practices, or other third-party organizations not performing data collection and analysis in a timely or accurate manner; |
| · | service providers, collaborators or co-sponsors not adequately performing their obligations in relation to the clinical trial or cause the trial to be delayed or terminated; |
| · | being unable to obtain a sufficient supply of manufactured clinical trial materials; |
| · | regulatory inspections of manufacturing facilities requiring us or a co-sponsor to undertake corrective action or suspend the clinical trials; |
| · | interim results of the clinical trial being inconclusive or negative; |
| · | clinical trial, although approved and completed, generating data that are not considered by the FDA or others to be sufficient to demonstrate safety and efficacy; |
| · | clinical trials, although approved and completed outside the United States, not considered by the FDA or others outside the jurisdiction hosting such clinical trials to be sufficient to demonstrate safety and efficacy; and |
| · | changes in governmental regulations or administrative actions affecting the conduct of the clinical trial or the interpretation of its results. |
| 28 |
There can be no assurance that our clinical trials will in fact demonstrate, to the satisfaction of the FDA and others, that our product candidates are sufficiently safe or effective. The FDA or we may also restrict or suspend our clinical trials at any time if either believes that we are exposing the subjects participating in the trials to unacceptable health risks.
Delays in obtaining such clearances and/or changes in existing requirements could have a material adverse effect on our company by making it difficult to advance product candidates or by reducing or eliminating their potential or perceived value and, therefore, our ability to conduct our business as currently planned could materially suffer. Failure to obtain required regulatory approvals could require us to delay, curtail or cease our operations. Even if we invest the necessary time, money and resources required to advance through the FDA approval process, there is no guarantee that we will receive FDA approval of our product candidates.
Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
| · | warning letters, fines, injunctions, consent decrees and civil penalties; |
| · | repairs, replacements, refunds, recalls, or seizures of our products; |
| · | operating restrictions, partial suspension, or total shutdown of production; |
| · | refusing our requests for regulatory clearance or premarket approval of new products, new intended uses, or modifications to existing products; |
| · | withdrawing regulatory clearance or premarket approvals that have already been granted; and |
| · | criminal prosecution. |
If any of these events were to occur, it could adversely affect our business, financial condition and results of operations.
Even if we obtain regulatory approvals, our products will be subject to ongoing regulatory review and if we fail to comply with continuing regulations, we could lose those approvals and our business, financial condition and results of operations would be seriously harmed.
Even if our product candidates receive initial regulatory approval or clearance for specific therapeutic applications, we will still be subject to ongoing reporting obligations, and such product and the related manufacturing operations will be subject to continuing regulatory review, including FDA inspections. This ongoing review may result in the withdrawal of our product from the market, the interruption of manufacturing operations and/or the imposition of labeling and/or marketing limitations related to specific applications of our product. Since many more patients will be exposed to our product candidates following their marketing approval, serious but infrequent adverse events that were not observed in clinical trials may be observed during the commercial marketing of such product. In addition, the manufacturer(s) and the manufacturing facilities that we will use to produce our product candidates will be subject to periodic review and inspection by the FDA and other similar foreign regulators. Late discovery of previously unknown problems with any product, manufacturer or manufacturing process, or failure to comply with regulatory requirements, may result in actions, such as:
| · | restrictions on such product, manufacturer or manufacturing process; |
| · | warning letters from the FDA or other regulatory authorities; |
| · | withdrawal of the product from the market; |
| · | suspension or withdrawal of regulatory approvals; |
| 29 |
| · | refusal by such regulator to approve pending applications or supplements to approved applications that we or our licensees (if any) submit; |
| · | voluntary or mandatory recall; |
| · | fines; |
| · | refusal to permit the import or export of our product; |
| · | product seizures or detentions; |
| · | injunctions or the imposition of civil or criminal penalties; and |
| · | adverse publicity. |
In addition, from time to time, legislation is drafted and introduced in the United States that could significantly change the statutory provisions governing any regulatory clearance or approval that we receive from the U.S. regulatory authorities. FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our product. We cannot predict what these changes will be, how or when they will occur or what effect they will have on the regulation of our product. If we, or our licensees, suppliers, collaborative research partners or clinical investigators are slow to adapt, or are unable to adapt, to changes in existing regulatory requirements or the adoption of new regulatory requirements or policies, we may lose marketing approval for any of the therapeutic applications of our product (to the extent that such applications are initially approved), resulting in decreased or lost revenue from milestones, product rental or usage fees, or royalties.
Even if approved by the necessary regulatory authorities, our product candidates may not gain market acceptance.
The development of a market for new technology or product is affected by numerous factors, many of which are beyond our control. There can be no assurance the any of our product candidates will gain acceptance within the markets at which they are targeted. Further, the internal structure for medical service provision varies considerably from territory to territory throughout the world and may be, in some cases, subject to public sector procurement processes, which could delay penetration of this market by our product candidates. If the market does not accept our product candidates, when and if we are able to commercialize them, then we may never become profitable. Factors that could delay, inhibit or prevent market acceptance of our product candidates may include:
| · | timing and receipt of marketing approvals; |
| · | safety and efficacy of the products; |
| · | patient reluctance to undergo the harvest and TARGT implantation procedure or physician reluctance or inability to perform the harvest and TARGT implantation procedure; |
| · | emergence of equivalent or superior products; |
| · | cost-effectiveness of products; |
| · | findings by health plans or third-party payers that the product candidates are not reasonable and necessary, or are subject to additional prerequisites for coverage; |
| · | decisions by health plans not to cover our products if they conclude that they are experimental or investigational; and |
| · | ineffective marketing. |
| 30 |
Our success is first and foremost reliant upon there being a demand for our technology or product by patients, payers, and in the case of TARGTEPO, potential strategic partners. We and potential partners will need to establish and manage reliable and cost effective production capabilities on a large scale. There is risk that such facilities may not be successfully established, may not meet their performance requirements or cost targets, or in other ways fail to deliver the requisite level of reliable and cost-effective TARGT microorgans for clinical use. In addition, sales will rely upon demand for our products, which in turn is dependent upon patient and doctor and other medical practitioner perceptions as to safety, reliability and efficacy of our product candidates. Although our product candidates will be subject to extensive testing, there can be no assurance that consumers will ultimately accept them relating to safety or efficacy.
Our efforts to comply with federal and state fraud and abuse laws could be costly, and, if we are unable to fully comply with such laws, we could face substantial penalties.
We are subject to extensive federal and state healthcare fraud and abuse laws and regulations, including, but not limited to, the following:
| · | federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs, such as Medicare and Medicaid; |
| · | federal False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| · | federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which creates federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program and which also imposes certain obligations on entities with respect to the privacy, security and transmission of individually identifiable health information; |
| · | federal False Statements Statute, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| · | federal Foreign Corrupt Practices Act (FCPA), which prohibits, among other things, making payments to foreign officials of any country outside of the United States for the purpose of obtaining or retaining business; and |
| · | state laws analogous to each of the above federal laws, such as state anti-kickback and false claims laws (some of which may apply to healthcare items or services reimbursed by any third-party payer, including commercial insurers), as well as certain state laws that require pharmaceutical and medical device companies to comply with industry voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government. |
If our past or present operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from third-party payer programs such as Medicare and Medicaid and/or the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we may do business are found to be non-compliant with applicable laws, they may be subject to criminal, civil or administrative sanctions including exclusions from government-funded health care programs, which could also negatively impact our operations. Our ongoing efforts to comply with these laws may be costly, and our failure to comply with these laws could have a material adverse effect on our business, financial condition and results of operations. The risk of our being found in violation of these laws is increased by the fact that many of them have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
| 31 |
If any of our key employees discontinue his or her services with us, our efforts to develop our business may be delayed.
Our success will depend on the retention of our directors and other current and future members of our management and technical team, including Michael F. Cola, our President and Chief Executive Officer, Brian D. Piper, our Chief Financial Officer, and Garry A. Neil, our Chief Scientific Officer, and on our ability to continue to attract and retain highly skilled and qualified personnel. There can be no assurance that we will retain the services of any of our directors, officers or employees, or attract or retain additional senior managers or skilled employees. Furthermore, we do not carry key man insurance with respect to any of such individuals.
Our lead product candidates, including NFC-1, and the TARGT platform, are still in development and are dependent on further development and testing to reach commercial production. We currently employ a small number of key personnel including top managers, scientists, engineers and clinical experts who are important to developing NFC-1 and the TARGT platform and have a high level of accumulated knowledge which would be lost if they left our company. If these employees leave our company or otherwise are unable to provide services, there could be significant implications on the timing and cost of future development of the technology. Because competition for qualified personnel in our industry is intense, we may be unable to timely find suitable replacements with the necessary scientific expertise. We cannot assure you that our efforts to attract or retain such personnel will be successful.
If we are not able to obtain and maintain adequate patent protection for our product candidates, we may be unable to prevent our competitors from using our technology.
Our ability to commercialize the TARGT platform, NFC-1 or our other product candidates, will depend, in part, on our ability, both in the United States and in other countries, to obtain patents, enforce those patents, preserve trade secrets and operate without infringing the proprietary rights of third parties. Our existing owned patent and patent application portfolio directed to TARGT platform, including TARGTEPO, currently contains 63 issued patents, and 37 pending and allowed patent applications. We have also licensed certain intellectual property in connection with NFC-1. Applications for patents and other intellectual property rights capable of being registered have been, and will be, filed in certain key jurisdictions. We may not successfully obtain patents in the countries in which patent applications have been or will be filed, and we may not develop other patentable products or processes. In addition, any future patents may not prevent other persons or companies from developing similar or medically equivalent products and other persons or companies may be issued patents that may prevent the sale of our products or that will require us to license or pay significant fees or royalties. Furthermore, our own issued and in-licensed patents may not be valid or enforceable, or be able to provide our company with meaningful protection. Patent litigation is costly and time-consuming and there can be no assurance that we will have, or will be able to devote, sufficient resources to pursue such litigation. In addition, potentially unfavorable outcomes in such proceedings could limit our intellectual property rights and activities and have an adverse effect on our business.
We cannot be certain that any of our patent applications, or those of our licensors, will result in issued patents. In addition, because the patent positions of biopharmaceutical companies are highly uncertain and involve complex legal and factual questions, the patents we own and license, or any further patents we may own or license, may not prevent other companies from developing similar or therapeutically equivalent products. Patents also will not protect our product candidates if competitors devise ways of making or using these product candidates without legally infringing our patents. In recent years, several companies have been extremely aggressive in challenging patents covering pharmaceutical products, and the challenges have often been successful. We cannot be assured that our patents will not be challenged by third parties or that we will be successful in any defense we undertake. Failure to successfully defend a patent challenge could materially and adversely affect our business.
In addition, changes in either patent laws or in interpretations of patent laws in the United States and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent defense and enforcement.
| 32 |
We also rely on trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. We seek to protect our proprietary information by requiring our employees, consultants, contractors, outside scientific collaborators and other advisors to execute non-disclosure and confidentiality agreements and our employees to execute assignment of invention agreements to us on commencement of their employment. Agreements with our employees aim to prevent employees from bringing any proprietary rights of third parties to us. We also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials. However, if our employees, consultants, contractors, outside scientific collaborators or other advisors breach their confidentiality or other obligations to us, we may not be able to successfully or effectively prevent such breach and we could be adversely impacted if the protection of our trade secrets or other intellectual property is compromised.
Even if our product candidates and the methods for treating patients for prescribed indications using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Our and our licensors’ ability to obtain patents can be highly uncertain and involve complex and in some cases unsettled legal issues and factual questions.
Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries provide different degrees of protection against the use of a patented invention by others. Therefore, if the issuance to us or our licensors, in a given country, of a patent covering an invention is not followed by the issuance, in other countries, of patents covering the same invention, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited.
Unauthorized parties may try to copy aspects of our product candidates and technologies or obtain and use information we consider proprietary. Policing the unauthorized use of our proprietary rights is difficult. We cannot guarantee that no harm or threat will be made to our or our collaborators’ intellectual property. In addition, changes in, or different interpretations of, patent laws in the United States and other countries may also adversely affect the scope of our patent protection and our competitive situation. Further, we may not have sufficient rights under our license agreements with collaborators to enforce the intellectual property licensed to us against third-party infringers.
These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be non-exclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. All of the issues described above could also impact our collaborators, which would also impact the success of the collaboration and therefore us.
Under certain of our in-licensed patents, our relevant counterpart is responsible for maintaining, controlling or enforcing the licensed intellectual property portfolio. Thus, we cannot ensure that the license rights will be adequately maintained, controlled or enforced by our relevant counterparts.
There is certain subject matter that is patent eligible in the United States but not generally patent eligible outside of the United States and vice versa. Differences in what constitutes patent eligible subject matter in various countries may limit the protection we can obtain in the United States and outside of the United States.
As we develop our product candidates, we may need to obtain licenses to protect our rights to make and use our technology. There is no assurance that we will obtain licenses for such technology or would be able to obtain licenses to any third party intellectual property on commercially reasonable terms.
| 33 |
Third parties may bring patent infringement or other intellectual property claims against us, which would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product candidate that is the subject of the suit. Additionally, if it is determined that our product candidates infringe third-party patents or other intellectual property rights, there can be no assurance that we can successfully develop non-infringing alternatives on a timely basis, or license non-infringing alternatives, if any exist, on commercially reasonable terms. A significant intellectual property impediment to our ability to develop and commercialize our product candidates could materially adversely affect our business prospects.
Even if patents are issued to us or our licensors covering embodiments of our product candidates, devices, or methods of using them, those patents can be challenged by our competitors or other third parties who can argue such patents are invalid or unenforceable, dispute the ownership of the patents, or that the claims of the issued patents should be limited or narrowly construed, which may place our company in a position without meaningful patent rights. Patents also will not protect our product candidates if competitors devise ways of making or using these product candidates without legally infringing our patent claims.
We cannot assure you that third parties cannot and will not design around our patents and develop similar products or that we will be successful in enforcing our patents on such design around products. In particular, the biosimilars pathway created under the Biologics Price Competition and Innovation Act (BPCIA) may allow for another manufacturer to develop a non-patent infringing product using data from our own clinical trials. Prior to the enactment of BPCIA, information in approved Biologic License Applications (BLAs) could not be relied upon by other manufacturers to establish the safety and efficacy of their products for which they were seeking FDA approval. Accordingly, if the TARGT platform were approved under a BLA, other manufacturers potentially could develop and seek FDA approval of “biosimilar” products at some point in the future.
The sale of any product may be reliant on licenses from third parties, and any loss of these rights would adversely affect our business.
As indicated above, we currently have licenses to certain third party intellectual property, and therefore we do not own all of the intellectual property upon which NFC-1 or a TARGT-based product may be made, used, or sold. If we fail to perform any of our obligations under these licenses, these licenses could be terminated and/or the licensed intellectual property could revert to the licensor, which may impair our ability to use or further develop our product candidates and have an adverse effect on our business.
Our business is dependent on proprietary rights that may be difficult to protect and such dependence could affect our ability to effectively compete.
In addition to our patents, we also rely on trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position especially where we do not believe that patent protection is appropriate or obtainable. However, others, including our competitors, may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. We take precautionary measures to protect our proprietary rights and information, including the use of confidentiality agreements with employees and consultants, and those with whom we have academic and commercial relationships. However, we may not have such agreements in place with all such parties and, in spite of the measures, there can still be no guarantee that agreements will not be violated or that there will be an adequate remedy available for a violation of an agreement. Any of these events could prevent us from developing or commercializing our product candidates. Trade secrets are by nature difficult to protect. Our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time-consuming, and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and/or know-how. Failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
In addition, third parties may have trademarks or pending applications on our contemplated marks, similar marks, or in confusingly similar fields of use (or may be using our contemplated marks or similar marks). We may have to change our use of certain marks which could have an adverse impact on our business and may require us to spend additional funds to develop new marks. We anticipate that we will spend both time and management resources to develop and file trademark applications in the future.
| 34 |
We are subject to intense competition from companies with greater resources and more mature products, which may result in our competitors developing or commercializing products before or more successfully than us.
While we believe our product candidates and TARGT platform have significant advantages, there are a number of well-established and substantial companies engaged in the development, production, marketing, sale and distribution of products that are potentially competitive with our product candidates or the TARGT platform in general. Many of these companies are more experienced than our company is and represent significant competition. It is also possible that other parties have in development products substantially similar to or with properties that are more efficacious, less invasive and more cost effectively delivered than our product candidates or the TARGT platform in general. The success of our competitors in developing, bringing to market, distributing and selling their products could negatively affect our result of operations and/or general acceptance of our product candidates.
We face risks related to the general economic conditions that may adversely affect our business.
In general, our operating results can be significantly and adversely affected by negative economic conditions, high labor, material and commodity costs, and unforeseen changes in demand for our potential products. These conditions have resulted and could continue to result in slower adoption of new technologies and cost containment efforts by governments and other payers for healthcare research and development, products and services.
The grants we received from the Israeli Office of the Chief Scientist place certain restrictions and royalty payment obligations on us; establishment of new Israeli national authority for technological innovation.
Through our wholly owned Israeli subsidiary, we have received an aggregate of $13.57 million in grants from the Israeli Office of the Chief Scientist (OCS). The grant agreements require repayment of the grants provided to us plus interest through the payment of royalties out of income generated by our Israeli subsidiary. Pursuant to the Israeli Encouragement of Industrial Research and Development Law, certain limitations will apply to the change of control of the grant recipient and/or Medgenics, Inc. and the financing, mortgaging, production, exportation and transfer or sale of its technology and intellectual property to third parties, which will require the OCS’s prior consent and, in case such a third party is outside of Israel, extended royalties and/or other fees. This could have a material adverse effect on and significant cash flow consequences to our company if, and when, any technologies, intellectual property or manufacturing rights are exported or transferred to third parties outside Israel. If the OCS does not wish to give its consent in any required situation or transaction, we would need to negotiate a resolution with the OCS. In any event, such a transaction, assuming the OCS approved it, would involve monetary payments, such as royalties or fees, of not less than the applicable funding received from the OCS and, in aggregate, not to exceed (depending on the proposed transaction) three or six times the applicable funding received from the OCS plus interest. Legislation has been introduced to permit licensing of technology developed with support of OCS funding outside of Israel upon OCS approval and the payment of certain fees, though it has not yet been adopted. There can be no assurance that such legislation will be adopted in the future.
We note that a National Authority for Technological Innovation (NATI), an Israeli statutory corporation, was established on January 1, 2016 with the aim, among others, of replacing many of the functions of the OCS. Going forward, NATI will be in charge of implementing Israeli government policy on technological innovation through two channels: (i) a council, comprised of government and industry representatives (Council), and (ii) through the authority itself, which will implement the means of support and encouragement to R&D. Importantly for us, all OCS programs that were in effect on January 1, 2016, will continue as programs of NATI. In addition, until the Council decides otherwise, all the rules that applied to the OCS programs continue to apply to NATI programs, including the rules regarding transfer of technology and royalty payments.
| 35 |
Health care policy changes, including U.S. health care reform legislation signed in 2010, may have a material adverse effect on us.
Health care reform is often a subject of attention in governments that are trying to control health care expenditures. Health care reform proposals have been the subject of much debate in the U.S. Congress and some state legislatures, as well as in other countries. There is no assurance that legislation or underlying rules and guidelines resulting in adverse effects on our company or our product candidates will not be adopted in a country in which we intend to operate and/or upon the distribution of our product candidates in the United States.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act (ACA) and the Health Care and Education Reconciliation Act of 2010. The legislation imposes significant new taxes on medical device makers in the form of a 2.3% excise tax on all U.S. medical device sales that began January 1, 2013. Under the law, the total cost to the medical device industry from the tax is expected to be approximately $29 billion over ten years. This significant increase in the tax burden on our industry could have a material, negative impact on our results of operations and our cash flows, especially if any of our product candidates were determined to be a medical device. Other elements of this legislation, such as comparative effectiveness research, an independent payment advisory board, payment system reforms, including shared savings pilots, and other provisions, could meaningfully change the way health care is developed and delivered, and may materially impact numerous aspects of our business.
Reimbursement policies of third-party payers may negatively affect the acceptance of our product candidates by subjecting the product candidates to sales and pharmaceutical pricing controls.
Third-party payers (Medicare, Medicaid, private health insurance companies and other organizations) may affect the pricing or relative attractiveness of our product candidates by regulating the level of reimbursement provided to the physicians and clinics utilizing our product candidates or by refusing reimbursement. If reimbursement under these programs, or if the amount of time to secure reimbursement is too long, our ability to market our technology and product candidates may be adversely and materially affected. In international markets, reimbursement by private third-party medical insurance providers, including government insurers and independent providers, varies from country to country. In certain countries, our ability to achieve significant market penetration may depend upon the availability of third-party government reimbursement. Pharmaceutical pricing is also subject to regulation in Israel as well as other countries within which we may wish to distribute our product candidates.
The ACA reduces Medicare and Medicaid payments to hospitals, clinical laboratories and pharmaceutical companies, and could otherwise reduce the volume of medical procedures. Further, the Budget Control Act enacted in August 2011 committed the U.S. federal government to significantly reduce the federal deficit over ten years. In addition to placing caps on discretionary spending through 2021, the Budget Control Act also established a budget sequestration that calls for automatic spending cuts over a nine-year period. Across-the-board spending cuts went into effect on March 1, 2013, and Medicare spending cuts that reduce Part A and Part B payments by 2% went into effect on April 1, 2013. Further, the Bipartisan Budget Act of 2013, passed in December 2013, extends the sequestration automatic Medicare spending cuts to 2023 from 2021. Although we cannot predict the full effect on our business of the implementation of existing legislation such as the ACA and the Budget Control Act, or the enactment of additional legislation, we believe that legislation or regulation that reduces reimbursement for our products could adversely affect how much or under what circumstances health care providers will prescribe or administer our products. This could materially and adversely impact our business by reducing our ability to generate revenue, raise capital, obtain additional collaborators and market our products. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact product sales.
We may experience product liability claims, which could adversely affect our business and financial condition.
We may become subject to product liability claims. We have not experienced any product liability claims to date; however, the production at commercial scale, distribution, sale and support of our product candidates may entail the risk of such claims, which is likely to be substantial in light of the use of our product candidates in the treatment of medical conditions. We carry product liability insurance coverage in connection with the clinical trials of our product candidates. Our insurance provides $5.00 million in coverage, subject to a $5,000 deductible. Our insurance must be renewed annually at a current cost of $22,000 per year to cover current and planned trials in Israel. If we are unable to obtain a renewal or if we suffer a successful product liability claim in excess of our insurance coverage, such claim could result in significant monetary liability and could have a material adverse impact on our business, operations, financial position and/or reputation.
| 36 |
Failure to maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our business and operating results. In addition, current and potential stockholders could lose confidence in our financial reporting, which could have a material adverse effect on the price of our common stock.
Effective internal controls are necessary for us to provide reliable financial reports and effectively prevent fraud. If we cannot provide reliable financial reports or prevent fraud, our results of operation could be harmed.
Section 404 of the Sarbanes-Oxley Act of 2002 requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent registered public accounting firm addressing these assessments. We continuously monitor our existing internal controls over financial reporting systems to confirm that they are compliant with Section 404, and we may identify deficiencies that we may not be able to remediate in time to meet the deadlines imposed by the Sarbanes-Oxley Act. This process may divert internal resources and will take a significant amount of time and effort to complete.
If at any time it is determined that we are not in compliance with Section 404, we may be required to implement new internal control procedures and reevaluate our financial reporting. We may experience higher than anticipated operating expenses as well as increased independent auditor fees during the implementation of these changes and thereafter. Further, we may need to hire additional qualified personnel. If we fail to maintain the adequacy of our internal controls, as such standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act, which could result in our being unable to obtain an unqualified report on internal controls from our independent auditors. Failure to maintain an effective internal control environment could also cause investors to lose confidence in our reported financial information, which could have a material adverse effect on the price of our common stock.
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses, divert management’s attention from operating our business which could have a material adverse effect on our business.
There have been changing laws, regulations and standards relating to corporate governance and public disclosure, as well as new regulations promulgated by the SEC and rules promulgated by the national securities exchanges, including the NYSE MKT. These new or changed laws, regulations and standards are subject to varying interpretations in many cases due to their lack of specificity, and as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. As a result, our efforts to comply with evolving laws, regulations and standards are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. Our board members, principal executive officer and principal financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified board members and executive officers, which could have a material adverse effect on our business. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies, we may incur additional expenses to comply with standards set by regulatory authorities or governing bodies which would have a material adverse effect on our business, financial condition and results of operations.
Security breaches and other disruptions to our information technology infrastructure could interfere with our operations or clinical trials, compromise information belonging to us and our suppliers and expose us to liability, which could adversely impact our business and reputation.
In the ordinary course of business, we rely on information technology networks and systems, some of which are managed by third parties, to process, transmit and store electronic information, and to manage or support a variety of business processes and activities, including the conduct of our clinical trials. Additionally, we collect and store sensitive data, including proprietary business information. Despite security measures and business continuity plans, our information technology networks and infrastructure may be vulnerable to damage, disruptions or shutdowns due to attack by hackers or breaches, employee error or malfeasance, power outages, computer viruses, telecommunication or utility failures, systems failures, natural disasters or other catastrophic events. Any such event could result in legal claims or proceedings, liability or penalties under privacy laws, disruption in operations and damage to our reputation, which could adversely affect our business.
| 37 |
Risk Related to our Securities
Our securities are thinly traded, resulting in relative illiquidity and price volatility, and there may not ever be an active market for our securities.
Although our common stock and a class of our warrants have been traded on the NYSE MKT since April 2011, the volumes and trading in our securities have been extremely sporadic. As a result, the ability of holders to purchase or sell our securities is limited, with low-volume trading creating wide shifts in price. For our securities to continue to be listed on the NYSE MKT, we must meet the current listing requirements of that exchange. If we were unable to meet these requirements, our securities could be delisted from the NYSE MKT. Any such delisting of our securities could have an adverse effect on the market price of, and the efficiency of the trading market for, our securities, not only in terms of the number of shares that can be bought and sold at a given price, but also through delays in the timing of transactions and less coverage of us by securities analysts, if any. Also, if in the future we were to determine that we need to seek additional equity capital, it could have an adverse effect on our ability to raise capital in the public or private equity markets.
Further, the share prices of public companies, particularly those operating in high growth sectors, are often subject to significant fluctuations. The market price of our common stock on the NYSE MKT has been volatile, ranging from $3.09 per share to $10.25 per share during the 52-week trading period ending February 19, 2016. We expect that the market price of our common stock will continue to fluctuate significantly due to factors including, but not limited to, the following:
| · | actual or anticipated variations in our operating results; |
| · | announcements of developments by us or our competitors; |
| · | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| · | adoption of new accounting standards affecting our industry; |
| · | additions or departures of key personnel; |
| · | introduction of new products by us or our competitors; |
| · | changes in market valuations of companies in our industry; |
| · | general market or economic conditions; |
| · | future issuances of our common stock or other securities; and |
| · | other events or factors, including those beyond our control. |
| 38 |
Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on its market price.
The trading market for our securities could depend in part on the research and reports that securities analysts publish about our business and us. We do not have any control over these analysts. There is no guarantee that securities analysts will cover our securities. If securities analysts do not cover our securities, the lack of research coverage may adversely affect their market prices. If we are covered by securities analysts, and our securities are the subject of an unfavorable report, the prices for our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, we could lose visibility in the financial markets, which could cause our stock price and/or trading volume to decline.
The exercise of options and warrants and other issuances of shares of common stock or securities convertible into or exercisable for shares of common stock will dilute the ownership interests of our current stockholders and may adversely affect the future market price of our common stock.
Sales of our common stock in the public market, either by us or by our current stockholders, or the perception that these sales could occur, could cause a decline in the market price of our securities. Nearly all of the shares of our common stock held by those of our current stockholders who are not affiliates may be immediately eligible for resale in the open market either in compliance with an exemption under Rule 144 promulgated under the Securities Act of 1933, as amended, or the Securities Act, or pursuant to an effective resale registration statement that we have previously filed with the SEC. Such sales, along with any other market transactions, could adversely affect the market price of our common stock.
In addition, as of December 31, 2015, there were outstanding options to purchase an aggregate of 9,309,694 shares of our common stock at exercise prices ranging from $2.66 per share to $10.80 per share, of which options to purchase 5,339,723 shares were exercisable as of such date. As of December 31, 2015, there were warrants outstanding to purchase 8,612,359 shares of our common stock, at exercise prices ranging from $2.49 per share to $11.16 per share, with a weighted average exercise price of $6.36 per share, all of which were exercisable as of December 31, 2015. The exercise of options and warrants at prices below the market price of our common stock could adversely affect the price of shares of our common stock. Additional dilution may result from the issuance of shares of our common stock in connection with collaborations or manufacturing arrangements or in connection with other financing efforts.
Any issuance of our common stock that is not made solely to then-existing stockholders proportionate to their interests, such as in the case of a stock dividend or stock split, will result in dilution to each stockholder by reducing his, her or its percentage ownership of the total outstanding shares. Moreover, if we issue options or warrants to purchase our common stock in the future and those options or warrants are exercised, stockholders may experience further dilution. In 2015, we issued 2,032,837 options resulting in significant dilution to our existing stockholders. Delaware law and our corporate governance documents do not prohibit the number of options that we may issue in the future, except to the extent we are limited by the number of our authorized shares of common stock which is currently 100,000,000 shares. Holders of shares of our common stock have no preemptive rights that entitle them to purchase their pro rata share of any offering of shares of any class or series.
Our principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
As of December 31, 2015, our officers and directors together controlled approximately 22% of our outstanding common stock on a fully diluted basis. In addition, as of December 31, 2015, our five largest stockholders other than management and the directors controlled approximately 18% of our outstanding common stock on a fully diluted basis. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock, and therefore may not be in the best interest of our other stockholders.
We have never declared or paid dividends on our capital stock and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid dividends on our capital stock and we do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain future earnings, if any, to fund the development and growth of our business. Any future determination to pay dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, operating results, capital requirements, applicable contractual restrictions and other such factors as our Board of Directors may deem relevant.
| 39 |
Provisions of Delaware law may delay or prevent efforts to acquire a controlling interest in us, even if such acquisition were in the best interests of our stockholders.
We are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law, which regulates corporate acquisitions. These provisions could discourage potential acquisition proposals and could delay or prevent a change in control transaction. They could also have the effect of discouraging others from making tender offers for our common stock. These provisions may also prevent changes in our management.
There may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
We will require additional capital in the future to continue and expand our research and development and other operations. We are not restricted from issuing additional shares of our common stock, including any securities that are convertible into or exchangeable for, or that represent the right to receive, our common stock. The market price of our common stock could decline as a result of sales of shares of our common stock or sales of such other securities or the perception that such sales could occur.
Israel-Related Risks
Our research and development activities occur primarily in Israel, and our company and our business could be adversely affected by the economic, political and military conditions in that region.
Our principal research and development activities are based in Israel, which may be adversely affected by acts of terrorism, major hostilities, adverse legislation or litigation. If major hostilities should occur in the Middle East, including as a result of acts of terrorism in the United States or elsewhere, any such effects may not be covered by insurance. Our commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East, such as damages to our facilities and the resulting disruption to our ability to continue our product development. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot be certain that this government coverage will be maintained or will be adequate in the event we submit a claim. Any losses or damages incurred by us could have a material adverse effect on our business, financial condition and results of operations.
We are directly affected by economic, political and military conditions in that country. Our Israeli facilities are located in Misgav in northern Israel. In December 2008, in November 2012 and again in the summer of 2014, armed conflicts occurred between Israel and Hamas, an Islamist terrorist group in the Gaza Strip, including targeting civilian targets in southern Israel and the western Negev region, as well as Tel Aviv and Jerusalem. There can be no assurance that Hamas will not obtain and use longer-range missiles capable of reaching our facilities, which could result in a significant disruption of the Israel-based portion of our business.
Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions and could harm our business, financial condition and results of operations and may make it more difficult for us to raise necessary capital. For example, any major escalation in hostilities in the region could result in a portion of our employees, including officers, key personnel and consultants, being called up to perform military duty for an extended period of time. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in the agreements.
In addition to the foregoing, since the end of 2010, numerous acts of protest and civil unrest have taken place in several countries in the Middle East and North Africa, many of which involved significant violence. It cannot be predicted how the civil unrest in Egypt and the civil war in Syria or in other countries in the region will affect the political and security situation in the Middle East and on Israel’s position within the region.
| 40 |
Our operations may be disrupted by the obligations of our personnel to perform military service which could have a material adverse effect of our business.
Many of our male employees in Israel are obligated to perform up to one month (in some cases more) of annual military reserve duty until they reach the age of 45 and, in the event of a military conflict, could be called to active duty. Our operations could be disrupted by the absence of a significant number of our employees related to military service or the absence for extended periods of military service of one or more of our key employees. A disruption could have a material adverse effect on our business.
Under current U.S. and Israeli law, we may not be able to enforce employees’ covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
We have entered into non-competition agreements with our key employees. These agreements prohibit our key employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. Under applicable U.S. and Israeli law, we may be unable to enforce these agreements. If we cannot enforce our non-competition agreements with our employees, then we may be unable to prevent our competitors from benefiting from the expertise of our former employees, which could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
We may experience foreign currency exchange risks, which may increase the dollar costs of our operations in Israel.
A substantial portion of our expenses, including those related to our research and development, personnel and facilities-related expenses is incurred in New Israeli Shekels (NIS). Inflation in Israel will have the effect of increasing the dollar cost of our operations in Israel, unless it is offset on a timely basis by a devaluation of the NIS relative to the U.S. dollar. This may give rise to an exchange rate risk against NIS. We do not currently engage in hedging or use any other financial instruments or arrangements to manage this risk.
ITEM 1B - Unresolved Staff Comments.
None.
Our clinical operations are currently primarily conducted in Israel, in leased space of 7,050 sq. ft. located at Turag House, Misgav Business Center (Teradion), D.N. Misgav, Israel. Our principal executive offices are located at 435 Devon Park Drive, Building 700, Wayne, Pennsylvania 19087. We believe that these facilities are adequate to meet our current needs. We believe that if additional or alternative space is needed in the future, such space will be available on commercially reasonable terms as necessary.
We are not currently a party, as plaintiff or defendant, to any legal proceedings which, individually or in the aggregate, are expected by us to have a material effect on our business, financial condition or results of operation if determined adversely to us.
ITEM 4 -Mine Safety Disclosures.
Not applicable.
| 41 |
ITEM 5 - Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock and the series of warrants issued in connection with our U.S. initial public offering in April 2011 are traded, separately, on the NYSE MKT under the symbols “MDGN” and “MDGN.WS,” respectively. No other series of our warrants is listed on any exchange.
The following table sets forth, for the periods indicated, the high and low sale prices for our common stock as reported by the NYSE MKT:
| 2014 | High | Low | ||||||
| First Quarter (January 1, 2014 to March 31, 2014) | $ | 9.00 | $ | 5.88 | ||||
| Second Quarter (April 1, 2014 to June 30, 2014) | 8.33 | 6.08 | ||||||
| Third Quarter (July 1, 2014 to September 30, 2014) | 7.98 | 4.97 | ||||||
| Fourth Quarter (October 1, 2014 to December 31, 2014) | 6.36 | 3.68 | ||||||
| 2015 | High | Low | ||||||
| First Quarter (January 1, 2015 to March 31, 2015) | $ | 8.50 | $ | 5.00 | ||||
| Second Quarter (April 1, 2015 to June 30, 2015) | 9.63 | 5.87 | ||||||
| Third Quarter (July 1, 2015 to September 30, 2015) | 10.25 | 5.90 | ||||||
| Fourth Quarter (October 1, 2015 to December 31, 2015) | 7.72 | 5.62 | ||||||
Holders of Record
As of February 19, 2016, there were 270 holders of record of our common stock. We believe there are a substantially greater number of beneficial holders.
Stock Performance Graph
The following graph compares the cumulative total stockholder return data for our common stock from April 8, 2011 (the date on which our common stock commenced trading on the NYSE MKT) through December 31, 2015 to the cumulative return over such time period of (i) the NYSE MKT Composite Index and (ii) the RDG MicroCap Biotechnology Index. The graph assumes an investment of $100 on April 8, 2011 in (i) our common stock, (ii) the securities comprising the NYSE MKT Composite Index and (iii) the securities comprising the RDG MicroCap Biotechnology Index, including dividend reinvestment, if any. The comparisons in the graph are required by the SEC and are not intended to forecast or be indicative of possible future performance of our common stock.
The material in this section is not “soliciting material,” is not deemed “filed” with the SEC and shall not be incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent we specifically incorporate this section by reference.
| 42 |
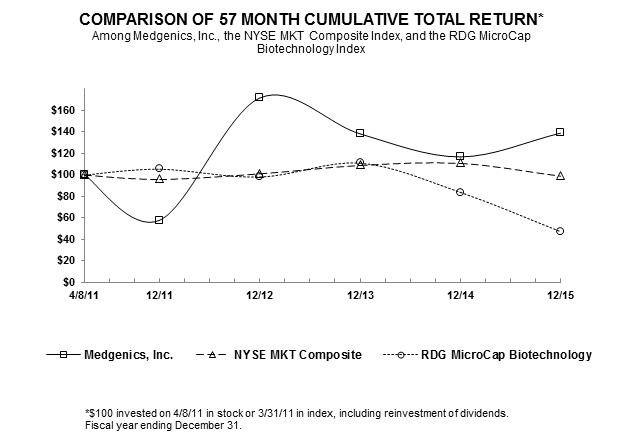
Dividends
We have never declared dividends on our equity securities, and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our Board of Directors and will depend upon such factors as earnings levels, capital requirements, our overall financial condition and any other factors deemed relevant by our Board of Directors.
Recent Sales of Unregistered Securities
In October 2015, we issued 459,770 shares of common stock to neuroFix therapeutics, Inc., priced at $6.96 per share, as partial consideration for our acquisition of neuroFix (see “Acquisition of neuroFix” in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this report for additional information regarding our acquisition of neuroFix). The issuance of these shares was exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act. There were no underwriters employed in connection with this transaction.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
| 43 |
ITEM 6 - Selected Financial Data.
The selected data presented below under the captions “Statement of Operations Data,” “Statement of Cash Flows Data” and “Balance Sheet Data” for, and as of the end of, each of the fiscal years in the five-year period ended December 31, 2015, are derived from, and should be read in conjunction with, our audited consolidated financial statements.
The information contained in this table should also be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes thereto included elsewhere in this report (in thousands of dollars except share and per share data):
| Year Ended December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development expenses, net | $ | 15,444 | $ | 8,253 | $ | 7,297 | $ | 5,431 | $ | 5,052 | ||||||||||
| Non-recurring research and development expenses resulting from acquisition | 8,170 | |||||||||||||||||||
| General and administrative expenses | 12,954 | 10,686 | 10,521 | 7,197 | 4,924 | |||||||||||||||
| Operating loss | 36,568 | (18,939 | ) | (17,818 | ) | (12,628 | ) | (9,976 | ) | |||||||||||
| Financial expenses | (1,408 | ) | (68 | ) | (20 | ) | (2,429 | ) | (214 | ) | ||||||||||
| Financial income | 1 | 586 | 726 | 5 | 2,097 | |||||||||||||||
| Loss before taxes on income | (37,975 | ) | (18,421 | ) | (17,112 | ) | (15,052 | ) | (8,093 | ) | ||||||||||
| Taxes on income | 17 | 12 | 17 | 19 | 3 | |||||||||||||||
| Loss | (37,992 | ) | (18,433 | ) | (17,129 | ) | (15,071 | ) | (8,096 | ) | ||||||||||
| Basic loss per share | $ | (1.42 | ) | $ | (0.96 | ) | $ | (0.97 | ) | $ | (1.37 | ) | $ | (0.96 | ) | |||||
| Diluted loss per share | $ | (1.45 | ) | $ | (1.00 | ) | $ | (1.06 | ) | $ | (1.37 | ) | $ | (0.96 | ) | |||||
| Weighted average number of shares used in computing basic loss per share | 26,783,623 | 19,246,611 | 17,629,436 | 11,023,881 | 8,447,908 | |||||||||||||||
| Weighted average number of shares used in computing diluted loss per share | 26,846,270 | 19,294,259 | 17,683,510 | 11,023,881 | 8,447,908 | |||||||||||||||
| Statement of Cash Flows Data: | ||||||||||||||||||||
| Net cash used in operating activities | $ | (24,347 | ) | $ | (12,195 | ) | $ | (12,732 | ) | $ | (8,619 | ) | $ | (8,027 | ) | |||||
| Net cash used in investing activities | (187 | ) | (363 | ) | (183 | ) | (63 | ) | (289 | ) | ||||||||||
| Net cash provided by financing activities | 44,310 | 23,456 | 28,874 | 10,118 | 10,452 | |||||||||||||||
| Increase in cash and cash equivalents | 19,776 | 10,898 | 15,959 | 1,436 | 2,136 | |||||||||||||||
| Balance Sheet Data: | ||||||||||||||||||||
| Cash and cash equivalents | $ | 53,064 | $ | 33,288 | $ | 22,390 | $ | 6,431 | $ | 4,995 | ||||||||||
| Current assets | 53,789 | 33,603 | 22,592 | 6,970 | 6,117 | |||||||||||||||
| Long-term assets | 469 | 677 | 495 | 737 | 745 | |||||||||||||||
| Total assets | 54,258 | 34,280 | 23,087 | 7,707 | 6,862 | |||||||||||||||
| Current liabilities | 3,908 | 3,638 | 3,014 | 2,350 | 2,059 | |||||||||||||||
| Long-term liabilities | - | 980 | 1,650 | 3,423 | 1,806 | |||||||||||||||
| Stockholders’ equity | 50,350 | 29,662 | 18,423 | 1,934 | 2,997 | |||||||||||||||
| 44 |
ITEM 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K.
Overview
We are a clinical stage biopharmaceutical company with an emphasis on Genomic Medicine.
The National Human Genome Research Institute of the National Institute of Health defines “Genomic Medicine” as “an emerging medical discipline that involves using genomic information about an individual as part of their clinical care (e.g., for diagnostic or therapeutic decision-making) and the health outcomes and policy implications of that clinical use."
Genomic medicine is currently making an impact in the fields of oncology, pharmacology, rare and undiagnosed diseases, and infectious disease.
Medgenics has partnered with the Center for Applied Genomics (CAG) at The Children’s Hospital of Philadelphia (CHOP) to implement a genomics-medicine driven approach to drug development. CAG’s assets include a fully automated biorepository containing specimens from more than 60,000 pediatric patients, and 150,000 of their relatives. The sample is highly enriched for rare and orphan diseases and the large majority of patients have been genotyped. Their phenotypes are recorded in a modern electronic health record. The patients have been consented for anonymized use of their data for research and follow up contact if needed.
CAG continues to discover important and novel genetic biomarkers by both genome-wide association studies and exome sequencing and analysis of affected individuals and their family members. Such markers not only identify patients with the disease but frequently point to the cause of the disease and suggest targets and feasible intervention strategies that include protein or peptide therapy, monoclonal antibodies, drugs or gene therapy. By working initially in a pediatric population, confounding environmental factors seen in older patients are minimized.
Our therapeutic strategy is to work closely with our collaborators at CAG to identify populations of need with well-characterized, novel, genetically-defined targets. We then designate an actionable therapeutic development approach based upon the target, the biology and human pathophysiology, and the clinical and regulatory pathways. The collaboration affords us with unique and proprietary insight into these diseases and allows us to better select therapeutic approaches including the appropriateness of our TARGT gene therapy technology. This, in turn, allows Medgenics to rapidly identify appropriate potential therapeutics that have already been tested in patients but were not advanced due to either lack of efficacy in a different patient population or for strategic reasons. We believe there are hundreds of such potential therapeutics currently listed in proprietary databases. Many of these have remaining composition of matter patent life and many would be eligible for regulatory exclusivity based on first registration (up to 12 years for biologics), as well as orphan drug and additional pediatric exclusivity. Because these potential therapeutics have already been tested in patients and have the requisite regulatory safety data generated, the time to file an IND/IMPD and initiate additional clinical trials, should substantially reduce the cost and development time.
In addition, the availability of robust genetic biomarker(s) allows trial designs to focus on highly enriched patient populations that are more likely to respond to targeted therapies. This can allow smaller, more focused and less expensive trials. Likewise, highly targeted drugs, that are less likely to exhibit off target effects, can be used when available. This further enhances the likelihood of clinical and regulatory success and potentially requires smaller, easier-to-enroll clinical trials. In some cases it may be possible to advance from discovery to the clinic in less than two years, and to achieve proof of concept in as little as three years Furthermore, such highly targeted therapies in specifically targeted diseases should allow the creation of higher value medicines that can address critical needs in patients suffering from rare and orphan diseases. The solid genetic foundation underlying these disease targets along with highly targeted therapies may also allow rapid label expansion into adjacent populations.
Our initial program arising out of our Genomics Medicine strategy is the development candidate NFC-1. Through our acquisition of neuroFix, LLC, or neuroFix, we acquired the rights to develop, NFC-1, as well as the rights to certain data derived from a clinical trial and other studies of NFC-1. NFC-1 is a first-in-class, non-stimulant metabotropic glutamate receptor (mGluR) neuromodulator that is Phase 2/3 ready for the treatment of mGluR network mutation positive Attention Deficit Hyperactivity Disorder (ADHD), as well as neuropsychiatric symptoms resulting from a related rare genetic disorder, 22q11.2 Deletion Syndrome (22q11.2 DS). We intend to develop NFC-1 for the treatment of mGluR network mutation positive ADHD (mGluR+ ADHD) and certain other neurological and neuropsychological indications. A Phase 1b clinical trial of NFC-1 in adolescents with ADHD and disruptions in the mGluR gene network was recently completed showing the safety of NFC-1 as well as signaling potential efficacy in the adolescents treated.
| 45 |
In September 2015, we completed the acquisition of neuroFix, the developer of NFC-1. We acquired all of the outstanding equity of neuroFix as further described below under “Acquisition of neuroFix.”
In November 2014, we entered into a licensing and research collaboration with CHOP (the “Research Agreement” and the “License Agreement”). Under this Research Agreement, we agreed to sponsor research at CHOP with respect to the recruitment and genetic analysis of patents with rare Mendelian diseases to accelerate discovery of diagnostic and therapeutic targets. As consideration for the research program, we were obligated to pay CHOP $4,475,765 on a specified timeline during the initial year of the Research Agreement. In exchange for our sponsorship of the research program, CHOP has granted us options over certain intellectual property created in the course of the research. The initial term of the Research Agreement was one year. In December 2015, we entered into an amendment of the Research Agreement with CHOP to extend the term for a second year (through November 10, 2016). Pursuant to the terms of the amendment, we are obligated to pay CHOP $4,475,765 in 2016 (which is equal to the funding that we paid to CHOP pursuant to the Research Agreement in 2015) and $1,856,023 in the first quarter of 2017, for a total of $6,331,788. We have the unilateral right to extend the term of the Research Agreement for an additional year beyond the extended term and to provide additional funding for such an extension. The terms of the License Agreement are described below under “Off-Balance Sheet Arrangements.”
We have generated significant losses to date, and we expect to continue to generate losses as we progress towards the commercialization of our product candidates. We have incurred net losses of approximately $37.99 million, $18.43 million and $17.13 million for the years ended December 31, 2015, 2014 and 2013, respectively. As of December 31, 2015, we had cash reserves of $53.06 million, which we believe will provide funding for the Company at least through the end of the third quarter of 2017. We are unable to predict the extent of any future losses or when we will become profitable, if at all.
Acquisition of neuroFix
On September 9, 2015, we entered into an Equity Interest Purchase Agreement with neuroFix therapeutics, Inc. (referred to as Legacy Corp), neuroFix, CHOP, Philip Harper and Hakon Hakonarson, pursuant to which we acquired all of the outstanding equity of neuroFix in consideration for our payment of the following:
| · | an upfront payment of $2.0 million in cash paid upon the consummation of the acquisition,; |
| · | a payment of $6.0 million, payable as $1.2 million in cash to CHOP, $1.6 million in cash to Legacy Corp and $3.2 million in shares of our common stock to Legacy Corp, which cash payments were made upon the completion of our registered public offering in October 2015, at which time 459,770 shares, priced at $6.96 per share, were also issued to Legacy Corp; |
| · | additional payments of up to $450 million upon the achievement of certain developmental, regulatory and sales milestones related to an oral formulation of NFC-1 (the “Product”) and any new chemical entity developed by us covering the same indication as the Product (an “NCE”); and |
| · | earnout payments equal to a percentage of certain product sales by us using tiered rates ranging from the mid- to high single digits depending on the Product or NCE. |
In addition, if the Product is approved by the FDA for additional indications beyond the initial indication, additional payments of $25 million for each such additional indication will be required to be paid by us.
Immediately prior to and in connection with the acquisition, neuroFix entered into a license agreement with CHOP, the terms of which are described below under “Off-Balance Sheet Arrangements.”
| 46 |
Recent Equity Offerings
On October 6, 2015, we completed a registered public offering of 7,078,250 shares of common stock, including 923,250 shares sold pursuant to the full exercise of the underwriters’ over-allotment option, at a price to the public of $6.50 per share. The net proceeds from this offering to us were approximately $42.88 million, after deducting underwriting discounts and commissions and offering expenses payable by us. On October 8, 2015, we issued 459,770 shares to Legacy Corp as partial consideration for the neuroFix acquisition, as described above, in a transaction exempt from registration under the Securities Act. In December 2015, we filed a resale registration statement relating to the possible resale of these shares by Legacy Corp from time to time.
On December 1, 2014, we completed a registered public offering of 5,893,750 shares of common stock, including 768,750 shares sold pursuant to the full exercise of the underwriters’ over-allotment option, at a price to the public of $4.10 per share. The net proceeds from this offering to us were approximately $22.21 million, after deducting underwriting discounts and commissions and offering expenses payable by us.
On February 13, 2013, we completed a registered public offering of 5,600,000 shares of common stock and 5,600,000 Series 2013-A warrants to purchase up to an aggregate of 2,800,000 shares of common stock. The shares of common stock and Series 2013-A warrants were sold together as a fixed combination, each consisting of one share of common stock and one Series 2013-A warrant to purchase 0.50 of a share of common stock, at a public offering price of $5.25 per combination, less the underwriting discounts and commissions payable by us, for net proceeds of approximately $26.55 million. To cover over-allotments made in connection with the offering, the underwriters purchased, at the same price, an additional 470,000 shares of common stock and an additional 840,000 Series 2013-A warrants to purchase up to 420,000 shares of common stock, for additional net proceeds of approximately $2.27 million.
The Series 2013-A warrants issued in this offering were immediately exercisable and will expire on February 13, 2018. The initial exercise price of the Series 2013-A warrants is $6.78 per whole share of common stock. The exercise price and number of shares of common stock issuable upon exercise of the Series 2013-A warrants will be subject to adjustment in the event of any stock split, reverse stock split, stock dividend, recapitalization, reorganization or similar transaction, among other events as described in the Series 2013-A warrants. However, the Series 2013-A warrants will not be adjusted for any issuances of common stock or securities convertible into or exercisable for common stock at a price below the then current exercise price of the Series 2013-A warrants. In the event of a sale of our company, each holder of Series 2013-A warrants will have the right, exercisable at its option, to require us to purchase such holder’s Series 2013-A warrants at a price determined using a Black-Scholes option pricing model under certain circumstances as described in the Series 2013-A warrants.
Financial Operations Overview
Research and Development Expense
Research and development expense consists of: (i) internal costs associated with our development activities; (ii) payments we make to third party contract research organizations, contract manufacturers, clinical trial sites and consultants; (iii) technology and intellectual property license costs; (iv) manufacturing development costs; (v) personnel related expenses, including salaries, and other related costs, including stock-based compensation expense, for the personnel involved in product development; (vi) activities related to regulatory filings and the advancement of our product candidates through preclinical studies and clinical trials; and (vii) facilities and other allocated expenses, which include direct and allocated expenses for rent, facility maintenance, as well as laboratory and other supplies. All research and development costs are expensed as incurred.
Conducting a significant amount of development is central to our business model. Product candidates in later-stage clinical development generally have higher development costs than those in earlier stages of development, primarily due to the significantly increased size and duration of the clinical trials. We plan to increase our research and development expenses for the foreseeable future in order to complete the proof of concept of our TARGTEPO microorgans with a second generation expression cassette of the HDAd viral vector and implantation protocol, and our earlier-stage research and development projects including in targeted rare and orphan disease indications.
| 47 |
The process of conducting pre-clinical studies and clinical trials necessary to obtain regulatory approval is costly and time consuming. The probability of success for each product candidate and clinical trial may be affected by a variety of factors, including, among others, the quality of the product candidate’s early clinical data, investment in the program, competition, manufacturing capabilities and commercial viability. As a result of these uncertainties, together with the uncertainty associated with clinical trial enrollments and the risks inherent in the development process, we are unable to determine the duration and completion costs of current or future clinical stages of our product candidates or when, or to what extent, we will generate revenues from the commercialization and sale of any of our product candidates. Development timelines, probability of success and development costs vary widely. We are concurrently focusing on proceeding with the approved TARGTEPO trial to obtain proof of concept with the second generation expression cassette of the HDAd viral vector and new implantation protocol and pursuing pre-clinical research and development in targeted orphan and rare disease.
Research and development expenses are shown net of participation by third parties.
General and Administrative Expense
General and administrative expense consists primarily of salaries and other related costs, including stock-based compensation expense, for persons serving as our directors and in our executive, finance and accounting functions. Other general and administrative expense includes facility-related costs not otherwise included in research and development expense, costs associated with industry and trade shows, and professional fees for legal services and accounting services. We expect that our general and administrative expenses will increase as we add personnel.
Financial Income and Expense
Financial expense consists primarily of warrant valuations and foreign currency exchange differences.
Financial income consists primarily of warrant valuations.
Results of Operations for the Years Ended December 31, 2015 and 2014
Research and Development Expenses
Gross research and development expenses for the year ended December 31, 2015 were $18.36 million, increasing from $10.49 million in 2014 primarily due to increased sub-contractor and consulting costs to advance our clinical activities related to the NFC-1 program and the CHOP collaboration, and increased non-cash stock-based compensation expenses related to options granted to research and development personnel. Research and development expenses, net for the year ended December 31, 2015 were $15.44 million, increasing from $8.25 million in 2014. The increase in the research and development expenses, net was mainly due to the increase in gross research and development expenses as detailed above, offset in part by participation by the OCS of $2.91 million in 2015 compared with $2.24 million in 2014. Non-recurring research and development costs of $8.17 million for the year ended December 31, 2015 pertain to the neuroFix acquisition described above, including $3.20 million in non-cash consideration and $0.17 million in reimbursed R&D expenses.
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2015 were $12.95 million, increasing from $10.69 million in 2014 primarily due to increased non-cash stock-based compensation expenses related to options granted to directors and general and administrative personnel and consultants of $2.01 million.
Financial Income and Expenses
Financial expenses for year ended December 31, 2015 were $1.41 million, increasing from $0.07 million in 2014. This increase of $1.34 million was mainly due to the non-cash change in valuation of the warrant liability.
| 48 |
Financial income for the year ended December 31, 2015 was de minimis, decreasing from $0.59 for the same period in 2014 mainly due to the change in non-cash valuation of the warrant liability.
We estimated the fair value of these warrants at the respective balance sheet dates using the Binomial option pricing model. We used a number of assumptions to estimate the fair value, including the remaining contractual terms of the warrants, risk-free interest rates, expected dividend yield and expected volatility of the price of the underlying common stock. All such warrants have been exercised as of December 31, 2015 thus eliminating the need for such valuations going forward.
Results of Operations for the Years Ended December 31, 2014 and 2013
Research and Development Expenses
Gross research and development expenses for the year ended December 31, 2014 were $10.49 million, increasing from $8.87 million in 2013 primarily due to increased sub-contractor and consulting costs to advance our clinical activities related to the NFC-1 program and the CHOP collaboration, and an increase in stock-based compensation expense.
Research and development expenses, net for the year ended December 31, 2014 were $8.25 million, increasing from $7.30 million in 2013. The increase in the research and development expenses, net was mainly due to the increase in gross research and development expenses as detailed above, offset in part by participation by the OCS of $2.24 million in 2014 compared with $1.57 million in 2013.
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2014 were $10.69 million, increasing from $10.52 million in 2013 primarily due to increased personnel and an increase in stock-based compensation expense offset by a decrease in professional fees.
Financial Income and Expenses
Financial expenses for year ended December 31, 2014 were $0.07 million, increasing from $0.02 million in 2013. This increase of $0.05 million was mainly due to foreign currency exchange differences.
Financial income for the year ended December 31, 2014 was $0.59 million, decreasing from $0.73 for the same period in 2013 mainly due to the non-cash change in valuation of the warrant liability.
Liquidity and Capital Resources
Sources of Liquidity
We have financed our operations primarily through a combination of equity, debt issues and grants from the OCS and other third parties.
We recorded $2.91 million, $2.24 million and $1.57 million in the years ended December 31, 2015, 2014 and 2013, respectively, from the OCS in development grants.
In the year ended December 31, 2015, options and warrants were exercised in consideration of $1.43 million and 480,122 shares of common stock were issued upon such exercises. In the year ended December 31, 2014, options and warrants were exercised in consideration of $1.24 million and 404,018 shares of common stock were issued upon such exercises. In the year ended December 31, 2013, options and warrants were exercised in consideration of $0.01 million and 19,499 shares of common stock were issued upon such exercises.
On October 6, 2015, we completed a registered public offering of 7,078,250 shares of common stock, including 923,250 shares sold pursuant to the full exercise of the underwriters’ over-allotment option, at a price to the public of $6.50 per share. The net proceeds from this offering to us were approximately $42.88 million, after deducting underwriting discounts and commissions and offering expenses payable by us.
| 49 |
On December 1, 2014, we completed a registered public offering of 5,893,750 shares of common stock, including 768,750 shares sold pursuant to the full exercise of the underwriters’ over-allotment option, at a price to the public of $4.10 per share. The net proceeds from this offering to us were approximately $22.21 million, after deducting underwriting discounts and commissions and offering expenses payable by us.
In February and March 2013, we completed a registered public offering of 6,440,000 shares of common stock and 6,440,000 Series 2013-A warrants to purchase up to an aggregate of 3,220,000 shares of common stock, including the exercise of the underwriters’ over-allotment of 840,000 shares of common stock and 840,000 Series 2013-A warrants to purchase up to 420,000 shares of common stock. The shares of common stock and Series 2013-A warrants were sold together as a fixed combination, each consisting of one share of common stock and one Series 2013-A warrant to purchase 0.50 of a share of common stock, at a public offering price of $5.25 per combination, less the underwriting discounts and commissions payable by us, for net proceeds of approximately $28.82 million.
Cash Flows
We had cash and cash equivalents of $53.06 million at December 31, 2015 and $33.29 million at December 31, 2014. The increase in our cash balance during 2015 was primarily the result of our registered public offering of common stock during the period of $42.88 million offset by the cash used in operations of $24.35 million.
Net cash used in operating activities of $24.35 million, $12.20 million and $12.73 million for the years ended December 31, 2015, 2014 and 2013, respectively, primarily reflected our net cash expenses for our operations, including our acquisition of neuroFix and amounts paid to CHOP under the License and Research Agreement.
Our cash used in investing activities relates mainly to our purchases of property and equipment.
Net cash provided by financing activities was $44.31 million, $23.46 million and $28.87 million for the years ended December 31, 2015, 2014 and 2013, respectively. Our cash flows from financing activities during the year ended December 31, 2015 were primarily the result of our registered public offering of common stock in October 2015 from which the net proceeds were approximately $42.88 million. Our cash flows from financing activities during the year ended December 31, 2014 were primarily the result of our registered public offering of common stock in December 2014 from which the net proceeds were approximately $22.21 million. Our cash flows from financing activities during the year ended December 31, 2013 were primarily the result of our registered public offering of common stock and Series 2013-A warrants in February 2013 from which the net proceeds were approximately $28.82 million.
Funding Requirements
Our future capital requirements will depend on a number of factors, including our success in targeting rare and orphan disease candidates, the timing and outcome of clinical trials and regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims and other intellectual property rights, the acquisition of licenses to new products or compounds, the status of competitive products, the availability of financing, and our success in developing markets for our product candidates.
Without taking into account any revenue we may receive as a result of licensing or other commercialization agreements, we believe that cash on hand, including the net proceeds we received from our public offering of common stock in the fourth quarter of 2015, will be sufficient to enable us to fund our operating expenses and capital expenditure requirements at least through the end of the third quarter of 2017. We have based this estimate on assumptions that may prove to be wrong and we could use our available resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
| 50 |
We do not anticipate that we will generate revenue from the sale of products for several years or more given the uncertainty of drug development; we do, however, intend to seek licensing or other commercialization agreements for existing and new TARGT applications. In the absence of additional funding or adequate funding from commercialization agreements, we expect our continuing operating losses to result in decreases in our cash balances. Absent significant corporate collaboration and licensing arrangements, we will need to finance our future cash needs through public or private equity offerings or debt financings. We do not currently have any commitments for future external funding. We may need to raise additional funds more quickly if one or more of our assumptions prove to be incorrect or if we choose to expand our product development efforts more rapidly than we presently anticipate, and we may decide to raise additional funds even before we need them if the conditions for raising capital are favorable. We may seek to encourage holders of our warrants to exercise, sell additional equity or debt securities or obtain a bank credit facility. The sale of additional equity or debt securities, if convertible, could result in dilution to our stockholders. The incurrence of indebtedness would result in increased fixed obligations and could also result in covenants that would restrict our operations.
Our plans include seeking additional investments and commercial agreements to continue our operations. However, there is no assurance that we will be successful in our efforts to raise the necessary capital and/or reach such commercial agreements to continue our planned research and development activities.
Contractual Obligations
The following table sets forth our contractual payment obligations as of December 31, 2015 for the periods indicated below:
| Contractual Obligations | Total | Less than 1 Year | 1 – 3 Years | 3 – 5 Years | More than 5 Years and Thereafter | |||||||||||||||
| Operating lease obligations | $ | 184,000 | $ | 141,000 | $ | 43,000 | $ | - | $ | - | ||||||||||
| Purchase obligations | $ | 7,484,000 | $ | 5,628,000 | $ | 1,856,000 | $ | - | $ | - | ||||||||||
| Total | $ | 7,668,000 | $ | 5,769,000 | $ | 1,899,000 | $ | - | $ | - | ||||||||||
We are a party to license and research and development agreements with universities and other third parties, as well as patent assignment agreements, under which we have obtained rights to patents, patent applications and know-how. We enter into contracts in the normal course of business with CROs for clinical trials, clinical and commercial supply manufacturing, with vendors for preclinical research studies and for other services and products for operating purposes. Our agreements generally provide for termination within 30-60 days of notice. Such agreements are cancelable contracts and not included in the table of contractual obligations and commitments. We have included as purchase obligations our commitments under agreements to the extent they are quantifiable and are not cancelable.
Critical Accounting Policies
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on our historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results and experiences may differ materially from these estimates.
While our significant account policies are more fully described in Note 2 to our financial statements included elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
| 51 |
Liability in Respect of Warrants
In the past, we issued warrants whose exercise price is subject to downward adjustment. In accordance with Accounting Standards Codification No. 815-40-15-7I, we classified these warrants as a liability at their fair value. The warrants liability were re-measured at each reporting period until exercised or expired. The increase in the fair value of the warrants during the year ended December 31, 2015 of $1.37 million, and the decrease in the fair value of the warrants during the year ended December 31, 2014 and 2013 of $0.58 million and $0.72 million respectively, are reported in the Statements of Operations as financial expense and income, respectively.
We estimated the fair value of these warrants at the respective balance sheet dates using the Binomial option pricing model. We used a number of assumptions to estimate the fair value, including the remaining contractual terms of the warrants, risk-free interest rates, expected dividend yield and expected volatility of the price of the underlying common stock. These warrants have been exercised as of December 31, 2015 thus eliminating the need for such valuations going forward.
Stock-Based Compensation
We account for stock options according to the Accounting Standards Codification No. 718 (ASC 718) “Compensation - Stock Compensation.” Under ASC 718, stock-based compensation cost is measured at grant date, based on the estimated fair value of the award, and is recognized as an expense over the employee’s requisite service period on a straight-line basis.
We account for stock options granted to non-employees on a fair value basis using an option pricing method in accordance with ASC 718. The initial non-cash charge to operations for non-employee options with vesting are revalued at the end of each reporting period based upon the change in the fair value of the options and amortized to consulting expense over the related vesting period.
For the purpose of valuing options and warrants granted to our employees, non-employees and directors and officers during the years ended December 31, 2015, 2014 and 2013, we used the Binomial options pricing model. To determine the risk-free interest rate, we utilized the U.S. Treasury yield curve in effect at the time of grant with a term consistent with the expected term of our awards. We estimated the expected life of the options granted based on anticipated exercises in the future periods assuming the success of our business model as currently forecast. The expected dividend yield reflects our current and expected future policy for dividends on our common stock. The expected stock price volatility for our stock options was calculated by examining historical volatilities for publicly traded industry peers as we do not have sufficient trading history for our common stock. We will continue to analyze the expected stock price volatility and expected term assumptions as more historical data for our common stock becomes available. We currently estimate that we will experience 8% forfeitures for those options currently outstanding.
Off-Balance Sheet Arrangements
neuroFix License
Immediately prior to and in connection with our acquisition of neuroFix in September 2015, neuroFix entered into a license agreement with CHOP, pursuant to which CHOP licensed to neuroFix certain technology owned and controlled by CHOP related to ADHD and certain other neurological and neuropsychological indications. Pursuant to this license agreement, CHOP licensed to neuroFix (coupled with a right to sublicense) certain patent rights and compound know-how on an exclusive, worldwide, royalty-bearing right and license basis, and certain CHOP know-how (other than compound know-how) on a non-exclusive, worldwide, royalty-bearing right and license basis. CHOP also granted to neuroFix an exclusive option during the term of the license agreement to negotiate an exclusive license to certain CHOP intellectual property.
Pursuant to this license agreement, CHOP retained rights to the licensed patent rights and know-how to conduct teaching, educational, research and patient care activities itself and to conduct collaborations with certain not-for-profit, governmental, educational or non-commercial third parties and for purposes outside of the field of the license. Under the license agreement, neuroFix granted to CHOP a non-exclusive, worldwide, fully paid-up, royalty-free license under all intellectual property rights controlled by neuroFix to make and use certain products for education and non-commercial research purposes.
| 52 |
In addition to neuroFix having issued equity to CHOP in partial consideration for the rights granted under the license agreement (which equity was issued immediately prior to the acquisition), CHOP is eligible for certain milestone and royalty payments under the license agreement as further described below:
| · | up to $1.5 million in regulatory and sales milestone payments in connection with each FDA-approved indication obtained by neuroFix utilizing intellectual property licensed under the license agreement; |
| · | royalty payments equal to a percentage of certain product sales by neuroFix using a fluctuating rate in the low single digits (adjusted downward to the extent third party royalty payments exceed a certain percentage in a given calendar quarter); |
| · | annual maintenance fees of equal to or less than $100,000 depending on the year; and |
| · | a certain percentage (ranging from mid-single digits to the mid-teens depending on if other rights of neuroFix are also licensed to the sublicensee at the same time) of all sublicensee income (except any amounts attributable to sublicensed sales by a certain party in Japan). |
The license agreement will terminate, with respect to each product and each territory covered by the license agreement, upon the later of (i) the expiration of certain CHOP patent rights and (ii) January 1, 2025, at which time the license rights granted to neuroFix become perpetual, irrevocable, fully paid-up and royalty-free. The license agreement could also be subject to termination by CHOP if neuroFix is not achieving certain specified development plans and diligence events and is not undertaking commercially reasonable efforts to achieve such events.
CHOP License
In November 2014, we entered into a licensing and research collaboration agreement (the “Research Agreement” and the “License Agreement”) with the Children’s Hospital of Philadelphia (“CHOP”). Under the terms of the License and Research Agreement, CHOP granted us (i) an exclusive, sublicensable license to use certain patent rights covering potential diagnostic and therapeutic targets, (ii) an exclusive, non-sublicensable license to use certain biospecimen and phenotypic data collected from patients with rare and orphan diseases and their family members, (iii) a non-exclusive, sublicensable license to use certain know-how related to such patent rights, biospecimen and phenotypic data, (iv) a non-exclusive and non-sublicensable license to use certain biospecimen and phenotypic data collected from patients with non-rare and orphan diseases, and (v) an exclusive option to negotiate licenses to commercialize certain inventions that may be created in the future that target rare and orphan diseases. In consideration of the licenses and option granted under the Licensing and Research Agreement, we agreed to pay to CHOP a license issuance fee of $500,000, certain maintenance fees, certain milestone payments, low single-digit royalties on net sales of all licensed products and a percentage of amounts received from sublicensing activities.
The License Agreement terminates upon the expiration date of the last-to-expire royalty term under the License Agreement, however (i) CHOP may terminate the License Agreement upon an uncured default by us of the License Agreement or the failure by us to meet certain development and/or commercialization milestones under the License Agreement or if we become insolvent or enter into bankruptcy proceedings, and (ii) we may terminate the License Agreement at any time with six months’ prior written notice to CHOP.
Stanford University License
In May 2014, we entered into a non-exclusive agreement with the Board of Trustees of the Leland Stanford Junior University (Stanford) to protect our right to make and use certain embodiments of our TARGT platform, including TARGTEPOTM. According to the agreement, Stanford granted us a non-exclusive license to a) a patent application and any patents that grant therefrom; and b) the right to use a biological material, in a specific field of use, for commercial development, production and marketing of certain products. In consideration, we agreed to pay Stanford the following amounts:
| · | an issue royalty of $25,000 upon signing the agreement; |
| 53 |
| · | license maintenance fees of: (i) $10,000 in May 2015 and May 2016, (ii) $20,000 in May 2017 and May 2018, and (iii) $50,000 in May 2019 and each year thereafter; |
| · | royalties at a rate of 1.5% of net sales (although certain credits may apply for license maintenance fees); and |
| · | milestone payments of: (i) $50,000 upon dosing of the first patient with a licensed product, (ii) $150,000 upon the first approval in the United States of a licensed product, and (iii) $150,000 upon the first approval in Europe or Japan of a licensed product, even if there are no patent rights in that country. |
The Stanford license began on May 30, 2014 and will end upon the expiration date of the licensed patent.
OCS Agreements
Under agreements with the OCS in Israel regarding research and development projects, our Israeli subsidiary is committed to pay royalties to the OCS at rates between 3.5% and 5% of the income resulting from this research and development, at an amount not to exceed the amount of the grants received by our subsidiary as participation in the research and development program, plus interest at LIBOR. The obligation to pay these royalties is contingent on actual income and in the absence of such income no payment is required. As of December 31, 2015, the principal amount of the aggregate contingent liability amounted to approximately $13.57 million.
Selected Quarterly Financial Data (unaudited)
| Three Months Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| 2015: | ||||||||||||||||
| Gross R&D expenses | $ | (3,901 | ) | $ | (4,458 | ) | $ | (4,568 | ) | $ | (5,429 | ) | ||||
| Net R&D expenses | $ | (3,901 | ) | $ | (3,028 | ) | $ | (4,201 | ) | $ | (4,314 | ) | ||||
| Non-recurring R&D expenses resulting from acquisition | — | — | (8,170 | ) | — | |||||||||||
| G&A expenses | $ | (3,947 | ) | $ | (3,889 | ) | $ | (2,996 | ) | $ | (2,122 | ) | ||||
| Operating loss | $ | (7,848 | ) | $ | (6,917 | ) | $ | (15,367 | ) | $ | (6,436 | ) | ||||
| Financial Expenses | $ | (1,078 | ) | $ | (8 | ) | $ | (1,192 | ) | $ | (21 | ) | ||||
| Financial Income | $ | 5 | $ | 843 | $ | 44 | $ | — | ||||||||
| Loss | $ | (8,922 | ) | $ | (6,086 | ) | $ | (16,521 | ) | $ | (6,463 | ) | ||||
| Basic loss per share | $ | (0.36 | ) | $ | (0.24 | ) | $ | (0.66 | ) | $ | (0.20 | ) | ||||
| Diluted loss per share | $ | (0.36 | ) | $ | (0.28 | ) | $ | (0.66 | ) | $ | (0.20 | ) | ||||
| Weighted average number of shares used in computing basic loss per share | 24,843,516 | 24,906,823 | 24,982,577 | 32,339,001 | ||||||||||||
| Weighted average number of shares used in computing diluted loss per share | 24,843,516 | 25,094,763 | 24,982,577 | 32,339,001 | ||||||||||||
| 2014: | ||||||||||||||||
| Gross R&D expenses | $ | (2,142 | ) | $ | (1,955 | ) | $ | (2,281 | ) | $ | (4,112 | ) | ||||
| Net R&D expenses | $ | (2,142 | ) | $ | (773 | ) | $ | (1,565 | ) | $ | (3,773 | ) | ||||
| G&A expenses | $ | (3,093 | ) | $ | (2,864 | ) | $ | (2,305 | ) | $ | (2,424 | ) | ||||
| Operating loss | $ | (5,235 | ) | $ | (3,637 | ) | $ | (3,870 | ) | $ | (6,197 | ) | ||||
| Financial Expenses | $ | (120 | ) | $ | (217 | ) | $ | (64 | ) | $ | (7 | ) | ||||
| Financial Income | $ | 3 | $ | 15 | $ | 896 | $ | 12 | ||||||||
| Loss | $ | (5,357 | ) | $ | (3,840 | ) | $ | (3,040 | ) | $ | (6,196 | ) | ||||
| Basic loss per share | $ | (0.28 | ) | $ | (0.21 | ) | $ | (0.16 | ) | $ | (0.30 | ) | ||||
| Diluted loss per share | $ | (0.28 | ) | $ | (0.21 | ) | $ | (0.21 | ) | $ | (0.30 | ) | ||||
| Weighted average number of shares used in computing basic loss per share | 18,872,001 | 18,715,541 | 18,817,557 | 20,820,818 | ||||||||||||
| Weighted average number of shares used in computing diluted loss per share | 18,872,001 | 18,715,541 | 18,938,723 | 20,890,247 | ||||||||||||
| 54 |
ITEM 7A - Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
We have no debt outstanding nor do we have any investments in debt instruments other than highly liquid short-term investments. We invest a major portion of our cash surplus in money market funds in the United States. Given the historic low levels of interest rates, we estimate that a further decline in the interest rate we are receiving will not result in a material adverse effect to our business. Accordingly, we consider our interest rate risk exposure to be insignificant at this time.
Foreign Currency Exchange Risk and Inflation
Approximately 20% of our costs, including salaries, lab materials and office expenses, are incurred in NIS. Inflation in Israel may have the effect of increasing the U.S. dollar cost of our operations in Israel. If the U.S. dollar declines in value in relation to the NIS, it will become more expensive for us to fund our operations in Israel. We do not believe that inflation has had a material effect on our results of operations for the years ended December 31, 2015, 2014 or 2013, nor do we expect that inflation will have a material impact on our results of operations for the year ending December 31, 2016.
The exchange rate of the U.S. dollar to the NIS, based on exchange rates published by the Bank of Israel, was as follows:
| Year Ended December 31, | ||||||||||||
| 2013 | 2014 | 2015 | ||||||||||
| Average rate for year | 3.6107 | 3.5779 | 3.8858 | |||||||||
| Rate at year-end | 3.471 | 3.889 | 3.902 | |||||||||
Currency fluctuations may affect us by increasing or decreasing costs. Currency fluctuations had no material effect on our results of operations for the years ended December 31, 2013, 2014 and 2015.
To date, we have not engaged in hedging transactions. In the future, we may enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rate of the U.S. dollar against the NIS. These measures, however, may not adequately protect us from material adverse effects due to the impact of inflation in Israel.
| 55 |
ITEM 8 - Financial Statements and Supplementary Data.
MEDGENICS, INC. AND ITS SUBSIDIARY
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2015
INDEX
| F-1 |
 |
Kost Forer Gabbay & Kasierer 2 Pal-Yam Blvd. Haifa 3309502, Israel |
Tel: +972-4-8654000 Fax: +972-3-5633439 ey.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
MEDGENICS, INC.
We have audited the accompanying consolidated balance sheets of Medgenics, Inc. ("the Company") and its subsidiary as of December 31, 2014 and 2015, and the related consolidated statements of operations, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company and its subsidiary as of December 31, 2014 and 2015 and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company's internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 10, 2016, expressed an unqualified opinion thereon.
| Haifa, Israel | /s/ KOST FORER GABBAY & KASIERER |
| February 26, 2016 | A Member of EY Global |
| F-2 |
 |
Kost Forer Gabbay & Kasierer 2 Pal-Yam Blvd. Haifa 3309502, Israel |
Tel: +972-4-8654000 Fax: +972-3-5633439 ey.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
MEDGENICS, INC.
We have audited Medgenics, Inc. ("the Company") and its subsidiary internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Medgenics, Inc. and its subsidiary maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Medgenics, Inc. and its subsidiary as of December 31, 2014 and 2015, and the related consolidated statements of operations, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2015 and our report dated February 10, 2016 expressed an unqualified opinion thereon.
| Haifa, Israel | /s/ KOST FORER GABBAY & KASIERER |
| February 26, 2016 | A Member of EY Global |
| F-3 |
MEDGENICS, INC. AND ITS SUBSIDIARY
U.S. dollars in thousands (except share and per share data)
| December 31, | ||||||||||||
| Note | 2014 | 2015 | ||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS: | ||||||||||||
| Cash and cash equivalents | $ | 33,288 | $ | 53,064 | ||||||||
| Prepaid expenses and other current assets | 315 | 747 | ||||||||||
| Total current assets | 33,603 | 53,811 | ||||||||||
| LONG-TERM ASSETS: | ||||||||||||
| Restricted lease deposits | 5(e) | 83 | 23 | |||||||||
| Severance pay fund | 99 | - | ||||||||||
| Property and equipment, net | 3 | 495 | 424 | |||||||||
| Total long-term assets | 677 | 447 | ||||||||||
| Total assets | $ | 34,280 | $ | 54,258 | ||||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||||||
| CURRENT LIABILITIES: | ||||||||||||
| Trade payables | $ | 1,076 | $ | 1,322 | ||||||||
| Other accounts payable and accrued expenses | 4 | 2,562 | 2,586 | |||||||||
| Total current liabilities | 3,638 | 3,908 | ||||||||||
| LONG-TERM LIABILITIES: | ||||||||||||
| Accrued severance pay | 368 | - | ||||||||||
| Liability in respect of warrants | 9 | 612 | - | |||||||||
| Total long-term liabilities | 980 | - | ||||||||||
| Total liabilities | 4,618 | 3,908 | ||||||||||
| COMMITMENTS AND CONTINGENCIES | 5 | |||||||||||
| STOCKHOLDERS' EQUITY: | 6 | |||||||||||
| Common stock - $0.0001 par value; 100,000,000 shares authorized; 32,869,217 shares issued and 32,860,717 shares outstanding at December 31,2015; 24,851,075 shares issued and 24,818,075 shares outstanding at December 31,2014 | 3 | 4 | ||||||||||
| Additional paid-in capital | 129,797 | 188,476 | ||||||||||
| Accumulated deficit | (100,138 | ) | (138,130 | ) | ||||||||
| Total stockholders' equity | 29,662 | 50,350 | ||||||||||
| Total liabilities and stockholders' equity | $ | 34,280 | $ | 54,258 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
MEDGENICS, INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
U.S. dollars in thousands (except share and per share data)
| Year ended | ||||||||||||||||
| December 31, | ||||||||||||||||
| Note | 2013 | 2014 | 2015 | |||||||||||||
| Research and development expenses | $ | 8,870 | $ | 10,490 | $ | 18,356 | ||||||||||
| Less: | ||||||||||||||||
| Participation by the Office of the Chief Scientist | 5(d) | (1,573 | ) | (2,237 | ) | (2,912 | ) | |||||||||
| Research and development expenses, net | 7,297 | 8,253 | 15,444 | |||||||||||||
| Non-recurring research and development expenses resulting from acquisition | - | - | 8,170 | |||||||||||||
| General and administrative expenses | 10,521 | 10,686 | 12,954 | |||||||||||||
| Operating loss | (17,818 | ) | (18,939 | ) | (36,568 | ) | ||||||||||
| Financial expenses | 8 | (20 | ) | (68 | ) | (1,408 | ) | |||||||||
| Financial income | 8 | 726 | 586 | 1 | ||||||||||||
| Loss before taxes on income | (17,112 | ) | (18,421 | ) | (37,975 | ) | ||||||||||
| Taxes on income | 7(e) | 17 | 12 | 17 | ||||||||||||
| Loss | $ | (17,129 | ) | $ | (18,433 | ) | $ | (37,992 | ) | |||||||
| Basic loss per share | 10 | $ | (0.97 | ) | $ | (0.96 | ) | $ | (1.42 | ) | ||||||
| Diluted loss per share | 10 | $ | (1.06 | ) | $ | (1.00 | ) | $ | (1.45 | ) | ||||||
| Weighted average number of shares of common stock used in computing basic loss per share | 17,629,436 | 19,246,611 | 26,783,623 | |||||||||||||
| Weighted average number of shares of common stock used in computing diluted loss per share | 17,683,510 | 19,294,259 | 26,846,270 | |||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
MEDGENICS, INC. AND ITS SUBSIDIARY
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
U.S. dollars in thousands (except share and per share data)
| Additional paid-in | Accumulated | Total stockholders' | ||||||||||||||||||
| Common stock | capital | deficit | equity | |||||||||||||||||
| Shares | Amount | |||||||||||||||||||
| Balance as of December 31, 2012 | 12,307,808 | 1 | 66,509 | (64,576 | ) | 1,934 | ||||||||||||||
| Issuance of common stock and warrants at $5.24 per share and $0.01 per warrant, net | 6,070,000 | 1 | 28,820 | - | 28,821 | |||||||||||||||
| Stock based compensation related to the issuance of common stock to consultants | 55,000 | (*) | 548 | - | 548 | |||||||||||||||
| Stock based compensation related to the issuance and vesting of restricted common stock to directors and an employee | 45,000 | (*) | 388 | - | 388 | |||||||||||||||
| Stock based compensation related to options and warrants granted to consultants and employees | - | - | 3,848 | - | 3,848 | |||||||||||||||
| Exercise of warrants and options | 19,499 | (*) | 13 | - | 13 | |||||||||||||||
| Loss | - | - | - | (17,129 | ) | (17,129 | ) | |||||||||||||
| Balance as of December 31, 2013 | 18,497,307 | $ | 2 | $ | 100,126 | $ | (81,705 | ) | $ | 18,423 | ||||||||||
| Issuance of common stock at $4.10 per share, net | 5,893,750 | 1 | 22,211 | - | 22,212 | |||||||||||||||
| Stock based compensation related to the issuance and vesting of restricted common stock to directors and an employee | 23,000 | (*) | 411 | - | 411 | |||||||||||||||
| Stock-based compensation related to options and warrants granted to consultants, directors and employees | - | - | 5,791 | - | 5,791 | |||||||||||||||
| Exercise of warrants and options | 404,018 | (*) | 1,258 | - | 1,258 | |||||||||||||||
| Loss | - | - | - | (18,433 | ) | (18,433 | ) | |||||||||||||
| Balance as of December 31, 2014 | 24,818,075 | $ | 3 | $ | 129,797 | $ | (100,138 | ) | $ | 29,662 | ||||||||||
| Issuance of common stock at $6.50 per share, net | 7,078,250 | 1 | 42,881 | - | 42,882 | |||||||||||||||
| Stock based consideration related to acquisition | 459,770 | (*) | 3,200 | - | 3,200 | |||||||||||||||
| Stock-based compensation related to vesting of restricted common stock to directors | 24,500 | (*) | - | - | - | |||||||||||||||
| Stock-based compensation related to options and warrants granted to consultants, directors and employees | - | - | 9,188 | - | 9,188 | |||||||||||||||
| Exercise of warrants and options | 480,122 | (*) | 3,410 | - | 3,410 | |||||||||||||||
| Loss | - | - | - | (37,992 | ) | (37,992 | ) | |||||||||||||
| Balance as of December 31, 2015 | 32,860,717 | $ | 4 | $ | 188,476 | $ | (138,130 | ) | $ | 50,350 | ||||||||||
(*) Represents an amount lower than $1.
The accompanying notes are an integral part of the consolidated financial statements.
| F-6 |
MEDGENICS, INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year ended December 31 | ||||||||||||
| 2013 | 2014 | 2015 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Loss | $ | (17,129 | ) | $ | (18,433 | ) | $ | (37,992 | ) | |||
| Adjustments to reconcile loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 177 | 225 | 240 | |||||||||
| Loss from disposal of property and equipment | 1 | - | 18 | |||||||||
| Stock based compensation related to options, warrants and restricted shares granted to employees, directors and consultants | 4,784 | 6,202 | 9,188 | |||||||||
| Stock based consideration related to acquisition | - | - | 3,200 | |||||||||
| Changes in fair value of warrants classified as a liability | (720 | ) | (585 | ) | 1,370 | |||||||
| Accrued severance pay, net | (416 | ) | (74 | ) | (269 | ) | ||||||
| Change in operating assets and liabilities: | ||||||||||||
| Accounts receivable and prepaid expenses | 360 | (113 | ) | (432 | ) | |||||||
| Trade payables | 185 | 14 | 246 | |||||||||
| Other accounts payable and accrued expenses | 29 | 610 | 24 | |||||||||
| Restricted lease deposits | (3 | ) | (41 | ) | 60 | |||||||
| Net cash used in operating activities | (12,732 | ) | (12,195 | ) | (24,347 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchase of property and equipment | (186 | ) | (364 | ) | (187 | ) | ||||||
| Proceeds from disposal of property and equipment | 3 | 1 | - | |||||||||
| Net cash used in investing activities | $ | (183 | ) | $ | (363 | ) | $ | (187 | ) | |||
The accompanying notes are an integral part of the consolidated financial statements.
| F-7 |
MEDGENICS, INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year ended | ||||||||||||
| December 31 | ||||||||||||
| 2013 | 2014 | 2015 | ||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from issuance of shares and warrants, net | $ | 28,861 | $ | 22,212 | $ | 42,882 | ||||||
| Proceeds from exercise of options and warrants | 13 | 1,244 | 1,428 | |||||||||
| Net cash provided by financing activities | 28,874 | 23,456 | 44,310 | |||||||||
| Increase in cash and cash equivalents | 15,959 | 10,898 | 19,776 | |||||||||
| Balance of cash and cash equivalents at the beginning of the period | 6,431 | 22,390 | 33,288 | |||||||||
| Balance of cash and cash equivalents at the end of the period | $ | 22,390 | $ | 33,288 | $ | 53,064 | ||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid during the period for taxes | $ | 17 | $ | 12 | $ | 19 | ||||||
| Supplemental disclosure of non-cash flow information: | ||||||||||||
| Classification of liability in respect of warrants into equity due to the exercise of warrants | $ | - | $ | 14 | $ | 1,982 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-8 |
MEDGENICS, INC. AND ITS SUBSIDIARY
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| NOTE 1:- | GENERAL |
| a. | Medgenics, Inc. (the "Company") was incorporated in January 2000 in Delaware. The Company has a wholly-owned subsidiary, Medgenics Medical Israel Ltd. (the "Subsidiary"), which was incorporated in Israel in March 2000. The Company and the Subsidiary are engaged in the research and development of products in the field of biotechnology and associated medical equipment industry. | |
| The Company's common stock is traded on the NYSE MKT. | ||
| b. | The Company has not generated any revenue from the sale of products since its inception. The Company has experienced accumulated losses since its inception of $138,130 and incurred a loss of $37,992 during the year ended December 31, 2015. The Company expects to incur losses and have negative cash flows from operating activities as it expands its portfolio and engages in further research and development activities, particularly conducting preclinical studies and clinical trials. | |
| c. | In September 2015, the Company acquired neuroFix, LLC, a Delaware limited liability company (“neuroFix”). As a result of neuroFix lacking outputs necessary to be considered a business as that term is defined in ASC 805 - Business Combination, the acquisition was determined not to be a business since no significant processes were acquired. Therefore, the transaction was treated as an asset acquisition. The Company determined that the acquired assets can only be economically used for the specific and intended purpose and have no alternative future use after taking into consideration that further research and development, regulatory marketing approval efforts will be required in order to reach technological feasibility. Accordingly, the entire initial purchase consideration of $8,170 thousand was immediately expensed to non-recurring research and development expenses. See note 5(b). |
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES |
| The consolidated financial statements are prepared in accordance with United States Generally Accepted Accounting Principles (“U.S. GAAP”), applied on a consistent basis, as follows: |
| a. | Use of estimates: | |
| The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions. The Company's management believes that the estimates and assumptions used are reasonable based upon information available at the time they are made. These estimates and assumptions can affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates. | ||
| b. | Financial statements in U.S. dollars: | |
| The majority of the Subsidiary's research and development operations are currently conducted in Israel; however, the majority of expenses are denonimated in U.S. dollars (“dollars”) and it is anticipated that the majority of the Company's revenues will be generated outside Israel and will be denominated in dollars. In addition, financing activities including equity transactions and cash investments, are made mainly in dollars. The Company's management believes that the dollar is the primary currency of the economic environment in which the Company and its Subsidiary operate. Thus, the functional currency of the Company and the Subsidiary is the dollar. | ||
| Accordingly, transactions and balances denominated in dollars are presented at their original amounts. Non-dollar transactions and balances have been re-measured to dollars, in accordance with ASC 830, "Foreign Currency Matters" of the Financial Accounting Standards Board (“FASB”). All exchange gains and losses from re-measurement of monetary balance sheet items denominated in non-dollar currencies are reflected in the Statements of Operations as financial income or expenses, as appropriate. |
| F-9 |
MEDGENICS, INC. AND ITS SUBSIDIARY
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| c. | New accounting pronouncements: |
In 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity s Ability to Continue as a Going Concern, which defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. The Company is evaluating the effect, if any, that the adoption of this guidance will have on the Company’s financial statements. |
| d. | Principles of consolidation: |
| The consolidated financial statements include the accounts of the Company and the Subsidiary. Intercompany transactions and balances have been eliminated upon consolidation. |
| e. | Cash equivalents: |
| The Company and the Subsidiary consider all highly liquid investments originally purchased with maturities of three months or less to be cash equivalents. |
| f. | Property and equipment: |
| Property and equipment are stated at cost net of accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. |
| The annual rates of depreciation are as follows: |
| % | ||
| Furniture and office equipment | 6 - 15 | |
| Computers and peripheral equipment | 33 | |
| Laboratory equipment | 15 - 33 | |
| Leasehold improvements | The shorter of term of the lease or the useful life of the asset |
| g. | Impairment of long-lived assets: |
| Long-lived assets are reviewed for impairment in accordance with ASC 360, "Property, Plant, and Equipment ” ("ASC 360"), whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of the asset to the future undiscounted cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds the fair value of the asset. For the years ended December 31, 2013, 2014 and 2015, no impairment losses have been identified. |
| F-10 |
MEDGENICS, INC. AND ITS SUBSIDIARY
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| h. | Severance pay: |
| The Subsidiary’s liability for severance pay is based upon Israeli severance pay law, and, in prior years, upon additional contractual obligations. The Subsidiary’s liability for all of its employees is fully provided by an accrual and is funded by monthly deposits with insurance policies. As part of employment agreements, the Subsidiary and, as of December 31, 2015, all of its employees agreed to the terms set forth in Section 14 of the Israeli Severance Pay Law, according to which amounts deposited in severance pay funds by the Subsidiary shall be the only severance payments released to the employee upon termination of employment, voluntarily or involuntarily. Accordingly, the financial statements do not include the severance pay fund and the severance pay accrual in connection with these employees, as the Subsidiary is legally released from the obligation to employees once the deposit amount have been paid. | |
| Severance expenses for the years ended December 31, 2013, 2014 and 2015 amounted to $186, $171 and $156, respectively. |
| i. | Income taxes: |
| The Company accounts for income taxes in accordance with ASC 740, “Income Taxes” (“ASC 740”). ASC 740 prescribes the use of the liability method whereby deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value. As of December 31, 2015, a full valuation allowance was provided by the Company. | |
| The Company also accounts for income taxes in accordance with ASC 740-10, “Accounting for Uncertainty in Income Taxes” (“ASC 740-10”). ASC 740-10 contains a two-step approach for recognizing and measuring uncertain tax positions accounted for in accordance with ASC 740-10. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. As of December 31, 2014 and 2015, no liability has been recorded as a result of ASC 740-10. |
| F-11 |
MEDGENICS, INC. AND ITS SUBSIDIARY
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| j. | Accounting for stock based compensation: |
| The Company applies ASC 718, “Compensation-Stock Compensation” (“ASC 718”) which requires the measurement and recognition of compensation expense based on estimated fair values for all share-based payment awards made to employees and directors. | |
| The Company recognized compensation expenses for awards granted based on the straight line method over the requisite service period of each of the grants, net of estimated forfeitures. |
| In 2013, 2014 and 2015, the Company estimated the fair value of stock options granted to employees and directors using the Binominal options pricing model with the following assumptions: |
| 2013 | 2014 | 2015 | ||||||||||
| Dividend yield | 0 | % | 0 | % | 0 | % | ||||||
| Expected volatility | 78-83 | % | 70.2-82.4 | % | 75.7-78.3 | % | ||||||
| Risk-free interest rate | 1.4-2.8 | % | 1.4-3 | % | 1.9-2.3 | % | ||||||
| Suboptimal exercise factor | 1.5 | 1.5 | 1.5-2.5 | |||||||||
| Contractual life (years) | 5-10 | 5-10 | 10 | |||||||||
| The Company uses historical data to estimate pre and post vesting exit rate within the valuation model; separate groups of employees that have similar historical exercise behavior are considered separately for valuation purposes. | |
| The suboptimal exercise factor represents the value of the underlying stock as a multiple of the exercise price of the option which, if achieved, results in exercise of the option. | |
| The risk-free interest rate assumption is based on observed interest rates appropriate for the term of the Company’s stock options. | |
| The Company has historically not paid dividends and has no foreseeable plans to pay dividends. | |
| The Company applies ASC 718 and ASC 505-50, “Equity-Based Payments to Non-Employees” (“ASC 505-50”), with respect to options issued to non-employees. ASC 718 requires the use of option valuation models to measure the fair value of the options. The fair value of these options was estimated at grant date and at the end of each reporting period, using the Binomial option pricing model with the following assumptions: |
| 2013 | 2014 | 2015 | ||||||||||
| Dividend yield | 0 | % | 0 | % | 0 | % | ||||||
| Expected volatility | 80-82 | % | 70-81 | % | 69-75 | % | ||||||
| Risk-free interest rate | 2.7-3.0 | % | 1.6-2.7 | % | 1.1-2.3 | |||||||
| Contractual life (years) | 8.3-9.8 | 4.8-9.6 | 3.8-9.8 | |||||||||
| F-12 |
MEDGENICS, INC. AND ITS SUBSIDIARY
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| k. | Loss per share: |
| Basic loss per share is computed based on the weighted average number of shares of common stock outstanding during each year. Diluted loss per share is computed based on the weighted average number of shares of common stock outstanding during each year, plus the dilutive effect of options, warrants and restricted shares considered to be outstanding during each year, in accordance with ASC 260, “Earnings Per Share “ (“ASC 260”). |
| l. | Research and development expenses, net: |
| All research and development expenses are charged to the Consolidated Statements of Operations as incurred. Grants from the Office of the Chief Scientist in Israel (“OCS”) related to such research and development expenses are offset against the expense at the later of when receipt is assured or the expenses are incurred. |
| m. | Grants: |
| Royalty-bearing grants from the OCS for funding approved research and development projects are recognized at the time the Subsidiary is entitled to such grants, on the basis of the costs incurred, and are presented as a deduction from research and development expenses. |
| F-13 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| n. | Concentrations of credit risks: |
| Financial instruments that potentially subject the Company and the Subsidiary to concentrations of credit risk consist principally of cash and cash equivalents. | |
| Cash and cash equivalents are invested in major banks and financial institutions in Israel and the United States. Such deposits in the United States may be in excess of insured limits and are not insured in other jurisdictions. Management believes that the financial institutions that hold the Company’s and the Subsidiary’s investments are institutions with high credit standing and accordingly, minimal credit risk exists with respect to these investments. | |
| The Company has no off-balance-sheet concentrations of credit risk such as foreign exchange contracts, option contracts or other foreign hedging arrangements. |
| o. | Fair value of financial instruments: |
| The carrying amount of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities are generally considered to be representative of their respective fair values because of the short-term nature of those instruments. The liability in respect of warrants is presented at fair value. | |
| The Company applies ASC 820, “Fair Value Measurements and disclosures” (“ASC 820”). ASC 820 clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. | |
| As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering such assumptions, ASC 820 establishes a three-tier value hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value: |
| Level 1 Inputs- | Quoted prices for identical instruments in active markets. | |
| Level 2 Inputs - | Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable. | |
| Level 3 Inputs- | Valuation derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
| The financial instruments carried at fair value on the Company’s balance sheet as of December 31, 2014 were warrants with down-round protection classified as a liability. These warrants were exercised in 2015. |
| F-14 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 3:- | PROPERTY AND EQUIPMENT, NET |
| Composition of property and equipment is as follows: |
| December 31, | ||||||||
| 2014 | 2015 | |||||||
| Cost: | ||||||||
| Furniture and office equipment | $ | 127 | $ | 109 | ||||
| Computers and peripheral equipment | 123 | 179 | ||||||
| Laboratory equipment | 670 | 709 | ||||||
| Leasehold improvements | 576 | 584 | ||||||
| Total cost | 1,496 | 1,581 | ||||||
| Total accumulated depreciation | 1,001 | 1,157 | ||||||
| Depreciated cost | $ | 495 | $ | 424 | ||||
| Depreciation expenses for the years ended December 31, 2013, 2014 and 2015 amounted to $177, $225 and $240, respectively. |
| NOTE 4:- | OTHER ACCOUNTS PAYABLE AND ACCRUED EXPENSES |
| December 31, | ||||||||
| 2014 | 2015 | |||||||
| Employees and payroll accruals | $ | 1,561 | $ | 1,534 | ||||
| Accrued expenses | 1,001 | 1,052 | ||||||
| $ | 2,562 | $ | 2,586 | |||||
| F-15 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 5:- | COMMITMENTS AND CONTINGENCIES |
| a. | In November 2014 the Company entered into a research agreement with the Children’s Hospital of Philadelphia (CHOP). Under the terms of the agreement, the Company agreed to sponsor research at CHOP with respect to the recruitment and genetic analysis of patients with rare Mendelian diseases to accelerate discovery of diagnostic and therapeutic targets. As consideration for the research program, the Company was obligated to pay CHOP $4,476 in 2015 in exchange for the Company’s sponsorship of the research program. The $4,476 was expensed over the period of the agreement ended November 2015. |
CHOP has granted the Company options over certain intellectual property created in the course of the research. The initial term of the Research Agreement is one year. The Company has the unilateral right to extend the term of the Research Agreement for an additional two-year term beyond the initial term and to provide additional funding for such an extension.
In December 2015 the Company extended the term of the Research Agreement for an additional year for which the Company is obligated to pay CHOP $4,476 in 2016, and $1,856 in the first quarter of 2017, for a total of $6,332. The $6,332 will be expensed over the period of the agreement ending in November 2016.
| b. | In September 2015, the Company entered into an Equity Interest Purchase Agreement (the “Purchase Agreement”) with neuroFix Therapeutics, Inc., a Delaware corporation (“Legacy Corp.), neuroFix, LLC, a Delaware limited liability company (“neuroFix”), The Children’s Hospital of Philadelphia, a Pennsylvania nonprofit corporation (“CHOP”), Philip Harper, an individual, and Hakon Hakonarson, an individual, pursuant to which the Company acquired all of the equity interests of neuroFix. Immediately prior to the execution of the Purchase Agreement, Legacy Corp. had contributed its assets to neuroFix. |
Under the terms of the Purchase Agreement, Legacy Corp., neuroFix, CHOP, Harper and Hakonarson agreed to consummate the neuroFix Acquisition in consideration for certain upfront, milestone and earnout payments related to certain product sales by the Company. The payments made or to be made by the Company are as follows:
| · | an upfront payment of $2,000 in cash paid upon the consummation of the neuroFix Acquisition; |
| · | a payment of $6,000 payable as $2,800 in cash and $3,200 in the Company’s common stock, upon the earlier to occur of (i) the achievement of a corporate milestone and (ii) March 31, 2016. In October 2015, the cash payment was made and 459,770 shares of common shares were issued; |
| · | additional payments of up to $450,000 upon the achievement of certain developmental, regulatory and sales milestones; and |
| · | earnout payments equal to a percentage of certain product sales by the Company using tiered rates ranging from the mid-to-high single digits. |
In addition to the foregoing, in the event a certain product is approved by the FDA for additional indications beyond the initial indication, additional payments of $25,000 for each such additional indication shall be paid by the Company to Legacy Corp. and CHOP.
In September 2015, the Company also entered into a reimbursement agreement with Legacy Corp. whereby the Company agreed to reimburse Legacy Corp. for various costs totaling $170.
| F-16 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 5:- | COMMITMENTS AND CONTINGENCIES (Cont.) |
| c. | License agreements: |
| 1. | In May 2014, the Company signed an agreement with the Board of Trustees of the Leland Stanford Junior University (“Stanford”). According to the agreement, Stanford granted the Company a non-exclusive license for a patent for commercial development, production and marketing of certain products based on its know-how. In consideration, the Company agreed to pay Stanford the following amounts: |
An issue royalty of $25 upon signing the agreement.
ii) License maintenance fees of:
1. $10 in each of May 2015 and May 2016;
2. $20 in May 2017 and May 2018; and
3. $50 in May 2019 and each year thereafter
iii) Royalties at a rate of 1.5% of net sales.
iv) Milestone payments of:
1. $50 upon dosing of the first patient with a licensed product;
2. $150 upon the first approval in the U.S. of a licensed product; and
3. $150 upon the first approval in Europe or Japan of a licensed product.
| 2. | In November 2014 the Subsidiary entered into a license agreement with the Children’s Hospital of Philadelphia (CHOP). According to the agreement CHOP granted an exclusive license to the Subsidiary to use the rare and orphan disease samples at the Center for Applied Genomics biobank for the purpose of developing and commercializing therapeutic treatments and diagnostic targets for rare and orphan diseases. |
A License Issuance Fee of $500 was paid and expensed in 2014. Beginning in 2016 for a period of five years the Company will pay to CHOP an annual license maintenance fee of $100. For the remainder of the term of the agreement the Company shall pay an annual license fee of $200. The Company will pay to CHOP certain milestone payments, ranging from $250 to $500; low single-digit royalties on net sales of all licensed products and a percentage of amounts received from sublicensing activities.
The License Agreement terminates upon the expiration date of the last-to-expire royalty term under the License Agreement. The Company may terminate the License Agreement at any time with six months’ prior written notice to CHOP, and CHOP may terminate the License Agreement upon (i) an uncured default by the Company of the License Agreement, (ii) the failure by the Company to meet certain development and/or commercialization milestones under the License Agreement, or (iii) the Company becoming insolvent or entering into bankruptcy proceedings.
| 3. | Immediately prior to and in connection with the Acquisition, neuroFix entered into a License Agreement (the “License Agreement”) with CHOP, pursuant to which CHOP would license to neuroFix certain technology owned and controlled by CHOP related to ADHD and certain other neurological and neuropsychological indications. Pursuant to the License Agreement, CHOP licenses to neuroFix (coupled with a right to sublicense) certain patent rights and compound know-how on an exclusive, worldwide, royalty-bearing right and license basis, and certain CHOP know-how (other than compound know-how) on a non-exclusive, worldwide, royalty-bearing right and license basis. CHOP also grants to neuroFix an exclusive option during the term of the License Agreement to negotiate an exclusive license to certain CHOP intellectual property. |
Pursuant to the License Agreement, CHOP retains rights to the licensed patent rights and know-how to conduct teaching, educational, research and patient care activities itself and to conduct collaborations with certain not-for-profit, governmental, educational or non-commercial third parties and for purposes outside of the field of the license. Under the License Agreement, neuroFix grants to CHOP a non-exclusive, worldwide, fully paid-up, royalty-free license under all intellectual property rights controlled by neuroFix to make and use certain products for education and non-commercial research purposes.
In addition to neuroFix having issued equity to CHOP in partial consideration for the rights granted under the License Agreement (which equity was issued immediately prior to the Acquisition described above), CHOP is eligible for certain milestone and royalty payments under the License Agreement as further described below:
| · | up to $1,500 in regulatory and sales milestone payments in connection with each FDA-approved indication obtained by neuroFix utilizing intellectual property licensed under the License Agreement; |
| · | royalty payments equal to a percentage of certain product sales by neuroFix using a fluctuating rate in the low single digits (adjusted downward to the extent third party royalty payments exceed a certain percentage in a given calendar quarter); |
| · | annual maintenance fees of equal to or less than $100 depending on the year; and |
| · | a certain percentage (ranging from mid-single digits to the mid-teens depending on if other rights of neuroFix are also licensed to the sublicensee at the same time) of all sublicensee income (except any amounts attributable to sublicensed sales by a certain party in Japan). |
The License Agreement will terminate, with respect to each product and each territory covered by the License Agreement, upon the later of (i) the expiration of the certain CHOP patent rights and (ii) January 1, 2025, at which time the license rights granted to neuroFix become perpetual, irrevocable, fully paid-up and royalty-free. The License Agreement could also be subject to termination by CHOP if neuroFix is not achieving certain specified development plans and diligence events and is not undertaking commercially reasonable efforts to achieve such events.
| F-17 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 5:- | COMMITMENTS AND CONTINGENCIES (Cont.) |
| d. | Office of the Chief Scientist (OCS): |
Under agreements with the OCS in Israel regarding research and development projects, the Subsidiary is committed to pay royalties to the OCS at rates between 3.5% and 5% of the commercial revenues resulting from this research and development, at an amount not to exceed the amount of the grants received by the Subsidiary as participation in the research and development program, plus interest at LIBOR. The obligation to pay these royalties is contingent on actual income and in the absence of such income no payment is required. As of December 31, 2015, the principal amount of the aggregate contingent liability was $13,565.
In June 2015, the Subsidiary received approval for an additional Research and Development program from the OCS at the Ministry of Economy of Israel for the period from December 2014 through December 2015. The approval, amended in December 2015, allows for a grant of up to approximately $3,300 based on research and development expenses, not funded by others, of up to $6,400. As of December 31, 2015, $2,383 was received from the OCS under this plan and $205 was recorded as a receivable. |
| e. | Lease Agreements: |
| 1. | The facilities of the Subsidiary are rented under an operating lease agreement for a period ending December 2015 and the Subsidiary has exercised its option to renew the lease for an additional period through December 2016. Future minimum lease commitment under the existing non-cancelable operating lease agreement is approximately $81 for 2016. | |
| As of December 31, 2015 the Subsidiary pledged a bank deposit which is used as a bank guarantee at an amount of $22 to secure its payments under the lease agreement. | ||
| 2. | The offices of the Company are rented under an operating lease agreement and are cancelable by either party with 60 days’ notice. Future minimum lease commitment under the existing operating lease agreement is $11. | |
| 3. | The Subsidiary leases vehicles under standard commercial operating leases. Future minimum lease commitments under various non-cancelable operating lease agreements in respect of motor vehicles are as follows: |
| Year | ||||
| 2016 | $ | 49 | ||
| 2017 | 36 | |||
| 2018 | 7 | |||
| $ | 92 | |||
| As of December 31, 2015, the Subsidiary paid three months lease installments in advance which amounted to $23. |
| F-18 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 5:- | COMMITMENTS AND CONTINGENCIES (Cont.) |
| f. | Over the past three years, four executives joined the Company. Per their employment agreements, if terminated without cause, these executives will be entitled to severance pay in the aggregate amount of $3,867. |
Subsequent to the Balance Sheet date, in February 2016, the Company announced that one of such executives will be leaving the Company and will be receiving compensation in the approximate amount of $1,000. In addition, all options granted to such executive under the Company’s stock incentive plan will become fully vested and shall remain exercisable through the 24-month anniversary of the termination date.
| NOTE 6:- | STOCKHOLDERS’ EQUITY |
| a. | Common stock: |
| The common stock confers upon the holders the right to receive notice to participate and vote in annual and special meetings of the stockholders of the Company and the right to receive dividends, if declared. |
| b. | Issuance of shares, stock options and warrants to investors: |
| 1. | In February 2013, the Company completed an underwritten public offering of 5,600,000 shares of common stock and Series 2013-A warrants to purchase up to an aggregate of 2,800,000 shares of common stock. The shares and the warrants were sold together as a fixed combination at a price to the public of $5.25 per fixed combination. Each combination consisted of one share of common stock and a warrant to purchase one-half of a share of common stock at an exercise price of $6.78 per share. These warrants will expire on February 13, 2018. In March 2013, the underwriters exercised their option and purchased 470,000 shares of common stock at $5.24 per share and 840,000 warrants (to purchase up to an aggregate of 420,000 shares) at $0.01 per warrant. Gross proceeds were $31,871 or approximately $28,821 in net proceeds after deducting underwriting discounts and commissions of $2,550 and other offering costs of approximately $500.
| |
| 2. | In December 2014, the Company completed an underwritten public offering of 5,125,000 shares of common stock. The shares were sold to the public at a price of $4.10 per share. In addition, the underwriters exercised their option and purchased 768,750 shares of common stock at $4.10 per share. Gross proceeds were $24,164 or approximately $22,212 in net proceeds after deducting underwriting discounts and commissions of $1,450 and other offering costs of approximately $507. |
| F-19 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 6:- | STOCKHOLDERS’ EQUITY (Cont.) |
| 3. | In October 2015, the Company completed an underwritten public offering of 7,078,250 shares of common stock, including 923,250 shares sold pursuant to the full exercise of an option granted to the underwriters to purchase additional shares of common stock. The shares were offered to the public at a price of $6.50 per share. Gross proceeds were $46,008 or approximately $42,882 in net proceeds after deducting underwriting discounts and commissions of $2,761 and other offering costs of approximately $365. |
| c. | Issuance of stock options warrants and restricted stock to employees and directors: |
| 1. | In 2006, the Company adopted a stock incentive plan (the “stock incentive plan”) according to which options, restricted stock and other awards related to common stock of the Company may be granted to directors, employees and consultants (non-employees) of the Company and the Subsidiary, as determined by the Company’s Board of Directors from time to time. The options outstanding are exercisable within a designated period from the date of grant and at an exercise price, each as determined by the Company’s Board of Directors. The options outstanding to employees, directors and consultants will vest over a period of two to four years from the date of grant. Any option which is cancelled or forfeited before expiration becomes available for future grants.
| |
| 2. | In March 2013, the Company’s Board of Directors approved an amendment to the stock incentive plan increasing the number of shares of common stock authorized for issuance thereunder to a total of 4,178,571 shares of common stock. | |
| 3. | In the year ended December 31, 2015, upon retirement of two directors from the Board of Directors, the Compensation Committee of the Board of Directors approved to allow their options to vest pursuant to their original terms and all their options will have a 10 year contractual life from the date of being granted.
As a result of the modification, the Company recorded incremental compensation cost of $1,427 on the modification date. The fair value was estimated using Binomial model with the following weighted-average assumptions: expected stock price volatility range of 70.5%-78.6%, risk-free interest rate of 1.6%-2.0% and expected dividend yield of 0%.
No future compensation will be recorded on these options.
In addition, the Compensation Committee of the Board of Directors approved two employees’ options to vest upon termination and all their options will have a 10 year contractual life from the date of being granted.
As a result of the modification, the Company recorded incremental compensation cost of $266 in 2015. The fair value was estimated using Binomial model with the following weighted-average assumptions: expected stock price volatility range of 70.1%-79.8%, risk-free interest rate of 1.4%-2.5% and expected dividend yield of 0%.
Future compensation to be recorded on these options is $74.
The Company accounted for the above changes in terms of the options under the provisions of ASC 718 as modifications. A modification to the terms of an award should be treated as an exchange of the original award for a new award with total compensation cost equal to the grant-date fair value of the original award plus the incremental value measured at the same date. Under ASC 718, the calculation of the incremental value is based on the excess of the fair value of the (modified) award based on current circumstances over the fair value of the original option measured immediately before its terms are modified based on current circumstances. That is, the original (pre-modification) award will be valued based on current assumptions, without regard to the assumptions made on the grant date. | |
| 4. | In April 2014, stockholders approved an amendment to the Company’s Stock Incentive Plan, increasing the number of shares authorized to be issued under such plan by 2,000,000 shares. | |
| 5. | Subsequent to the Balance Sheet date, in February 2016, the Company granted 100,000 options to an employee. These options are exercisable at $3.64 per share, have a 10 year term and vest in three equal annual tranches. |
| F-20 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 6:- | STOCKHOLDERS’ EQUITY (Cont.) |
| 6. | A summary of the Company’s activity for shares of restricted stock granted to employees and directors is as follows: |
| Number of shares of restricted stock as of December 31, 2014 | 24,500 | |||
| Vested in 2015 | (24,500 | ) | ||
| Number of shares of restricted stock as of December 31, 2015 | - |
| 7. | A summary of the Company’s activity for options and warrants granted to employees and directors is as follows: |
| Number of options and warrants | Weighted average exercise price | Weighted average remaining contractual terms (years) | Aggregate intrinsic value | |||||||||||||
| Outstanding at December 31, 2014 | 8,211,124 | $ | 5.48 | 6.79 | $ | 5,490 | ||||||||||
| Granted | 2,012,837 | $ | 6.96 | |||||||||||||
| Exercised | (80,250 | ) | $ | 3.38 | ||||||||||||
| Forfeited | (78,175 | ) | $ | 7.50 | ||||||||||||
| Outstanding at December 31, 2015 | 10,065,536 | $ | 5.78 | 6.48 | $ | 10,349 | ||||||||||
| Vested and expected to vest, December 31, 2015 | 9,737,303 | $ | 5.77 | 6.42 | $ | 10,174 | ||||||||||
| Exercisable at December 31, 2015 | 5,884,314 | $ | 5.54 | 5.12 | $ | 8,073 | ||||||||||
| F-21 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 6:- | STOCKHOLDERS’ EQUITY (Cont.) |
| The aggregate intrinsic value represents the total intrinsic value (the difference between the Company’s common stock fair value as of December 31, 2014 and 2015, respectively, and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2014 and 2015, respectively. | |
| Calculation of aggregate intrinsic value is based on the closing share price of the Company’s common stock as reported on the NYSE MKT as of December 31, 2013 ($5.99 per share), December 31, 2014 ($5.06 per share) and December 31, 2015 ($6.02 per share), respectively. | |
| The weighted average grant date fair value of options and warrants granted to employees and directors during the years ended December 31, 2013, 2014 and 2015 was $4.73, $3.56 and $4.08, respectively. | |
| As of December 31, 2015, there was $8,892 of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted to employees and directors. That cost is expected to be recognized over a weighted-average period of 1.4 years. |
| d. | Issuance of shares, stock options and warrants to consultants: |
| 1. | In January 2013, the Company issued a total of 55,000 shares of common stock to two consultants. Total compensation, measured as the grant date fair market value of the stock, amounted to $548 and was recorded as an operating expense in the Consolidated Statement of Operations in 2013. |
| F-22 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 6:- | STOCKHOLDERS’ EQUITY (Cont.) |
| 2. | A summary of the Company’s activity for options granted under the stock incentive plan and warrants to consultants is as follows: |
| Weighted | ||||||||||||||||
| average | ||||||||||||||||
| remaining | ||||||||||||||||
| Number of | Weighted | contractual | Aggregate | |||||||||||||
| warrants and | average | terms | intrinsic | |||||||||||||
| Options | exercise price | (years) | value price | |||||||||||||
| Outstanding at December 31, 2014 | 473,826 | $ | 6.53 | 3.29 | $ | 207 | ||||||||||
| Granted | 20,000 | $ | 7.37 | |||||||||||||
| Exercised | (12,928 | ) | $ | 3.41 | ||||||||||||
| Outstanding at December 31, 2015 | 480,898 | $ | 6.65 | 2.67 | $ | 391 | ||||||||||
| Exercisable at December 31, 2015 | 439,232 | $ | 6.67 | 2.22 | $ | 378 | ||||||||||
| F-23 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 6:- | STOCKHOLDERS’ EQUITY (Cont.) |
| The aggregate intrinsic value represents the total intrinsic value (the difference between the Company’s common stock fair value as of December 31, 2014 and 2015 respectively and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2014 and 2015, respectively. | |
| Calculation of aggregate intrinsic value is based on the closing share price of the Company’s common stock as reported on the NYSE MKT as of December 31, 2013 ($5.99 per share), December 31, 2014 ($5.06 per share) and December 31, 2015 ($6.02 per share), respectively. | |
| The weighted average grant date fair value of options and warrants granted to consultants during the years ended December 31, 2013, 2014 and 2015 was $4.39, $2.91 and $7.37, respectively. | |
| As of December 31, 2015, there was $154 of total unrecognized compensation cost related to share-based compensation arrangements granted to consultants. That cost is expected to be recognized over a weighted-average period of 1.7 years. |
| e. | Compensation expenses: |
| Compensation expense related to shares, warrants and options granted to employees, directors and consultants was recorded in the Consolidated Statements of Operations in the following line items: |
| Year ended December 31, | ||||||||||||
| 2013 | 2014 | 2015 | ||||||||||
| Research and development expenses | $ | 450 | $ | 1,115 | $ | 2,087 | ||||||
| General and administrative expenses | 4,334 | 5,087 | 7,101 | |||||||||
| $ | 4,784 | $ | 6,202 | $ | 9,188 | |||||||
| F-24 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands (except share and per share data) |
| NOTE 6:- | STOCKHOLDERS’ EQUITY (Cont.) |
| f. | Summary of shares to be issued upon exercise of options and warrants: |
| A summary of shares to be issued upon exercise of all the options and warrants, segregated into ranges, as of December 31, 2015 is presented in the following table: |
| As of December 31, 2015 | ||||||||||||||||
| Shares to be | Shares to be | |||||||||||||||
| Issued upon | Issued upon | Weighted Average | ||||||||||||||
| Exercise of | Exercise of | Remaining | ||||||||||||||
| Exercise | Options and | Options and | Contractual Terms | |||||||||||||
| Price per | Warrants | Warrants | of Options and | |||||||||||||
| Options / Warrants | Share ($) | Outstanding | Exercisable | Warrants (in years) | ||||||||||||
| Options: | ||||||||||||||||
| Granted to Employees and Directors | ||||||||||||||||
| 2.66-3.14 | 196,000 | 196,000 | 6.0 | |||||||||||||
| 3.86-4.99 | 3,606,429 | 2,490,763 | 7.2 | |||||||||||||
| 5.13-7.37 | 4,008,677 | 1,481,593 | 8.1 | |||||||||||||
| 8.19-10.80 | 1,399,240 | 1,113,685 | 3.5 | |||||||||||||
| 9,210,346 | 5,282,041 | |||||||||||||||
| Granted to Consultants | 5.02-7.37 | 99,348 | 57,682 | 6.6 | ||||||||||||
| Total Shares to be Issued upon Exercise of Options | 9,309,694 | 5,339,723 | ||||||||||||||
| Warrants: | ||||||||||||||||
| Granted to Employees and Directors | 2.49 | 855,190 | 855,190 | 0.2 | ||||||||||||
| Granted to Consultants | 3.76-4.99 | 180,077 | 180,077 | 1.8 | ||||||||||||
| 9.17-11.16 | 201,473 | 201,473 | 1.5 | |||||||||||||
| 381,550 | 381,550 | |||||||||||||||
| Granted to Investors | 4.99-6.00 | 2,792,699 | 2,792,669 | 0.3 | ||||||||||||
| 6.78-8.34 | 4,582,920 | 4,582,920 | 2.0 | |||||||||||||
| 7,375,619 | 7,375,619 | |||||||||||||||
| Total Shares to be Issued upon Exercise of Warrants | 8,612,359 | 8,612,359 | ||||||||||||||
| Total Shares to be Issued upon Exercise of Options and Warrants | 17,922,053 | 13,952,082 | ||||||||||||||
| F-25 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands |
| NOTE 7:- | TAXES ON INCOME |
| a. | Tax laws applicable to the Company and the Subsidiary: |
| 1. | The Company is taxed under U.S. tax law. | |
| 2. | The Subsidiary is taxed under Israeli income tax law. | |
| Results of the Subsidiary for tax purposes are measured and reflected in nominal New Israeli Shekel (NIS.) The difference between the rate of change in nominal NIS value and the rate of change in the NIS/U.S. dollar exchange rate causes a difference between taxable income or loss and the income or loss before taxes reflected in the financial statements. In accordance with ASC 740-10, the Company has not provided deferred income taxes on this difference between the reporting currency and the tax bases of assets and liabilities. | ||
| 3. | The Law for the Encouragement of Capital Investments, 1959 (the “ECI Law”): | |
| According to the ECI Law, the Subsidiary is entitled to various tax benefits by virtue of the “beneficiary enterprise” status granted to part of its enterprises, as implied by this ECI Law. The principal benefits by virtue of the ECI Law are tax benefits and reduced tax rates. | ||
The Subsidiary has chosen the alternative track under the ECI Law. Under this track, the Subsidiary is tax exempt for ten years within the benefit period on part of its taxable income.
| ||
| The income qualifying for tax benefits under the alternative track is the taxable income of a company that has met certain conditions as determined by the ECI Law (“a beneficiary company”), and which is derived from an industrial enterprise. The ECI Law specifies the types of qualifying income that is entitled to tax benefits under the alternative track whereby income from an industrial enterprise includes, among others, revenues from the production and development of software products and revenues from industrial research and development activities performed for a foreign resident (and approved by the Head of the Administration of Industrial Research and Development). | ||
| The benefit period starts at the later of the year elected (2011) and the first year the Subsidiary earns taxable income provided that 12 years have not passed since the beginning of the year of election as allowed for companies in development area A. The Subsidiary is located in development area A. | ||
| If a dividend is distributed out of tax exempt profits, as above, the Subsidiary will become liable for tax at the rate applicable to its profits from the beneficiary enterprise in the year in which the income was earned, as if it was not under the alternative track. The Company currently does not have tax exempt profits as the period of benefits has not commenced yet. | ||
| The above benefits are conditional upon the fulfillment of the conditions stipulated by the ECI Law, regulations published thereunder and the letters of approval for the investments in the approved enterprises, as above. Non-compliance with the conditions may cancel all or part of the benefits and refund of the amount of the benefits, including interest. The Company’s management believes that the Subsidiary is meeting the aforementioned conditions. |
| F-26 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands |
| NOTE 7:- | TAXES ON INCOME (Cont.) |
| b. | Tax rates applicable to the Company and the Subsidiary: |
| 1. | The Subsidiary: |
| During the year 2013 the Israeli corporate tax rate was 25%. In 2014 and 2015 the tax rate increased to 26.5%. |
In January 2016, the Israeli government tentatively approved a decrease in the corporate tax rate from 26.5% to 25%. The effect of this change is not included in these financial statements but will be included in the financial statements when the decision becomes final in 2016.
| 2. | The Company: |
| The tax rates applicable to the Company whose place of incorporation is the U.S. are corporate (progressive) tax at the rate of up to 35%, excluding state tax, which rates depend on the state in which the Company conducts its business. |
| c. | Tax assessments: |
| The Company files income tax returns in the U.S. federal jurisdiction and state jurisdiction. The U.S. tax authorities have not conducted an examination in respect of the Company's U.S. federal income tax returns since inception. The Subsidiary has tax assessments, deemed final under the law, up to and including the year 2008. |
| d. | Carryforward losses for tax purposes: | |
| As of December 31, 2015, the Company had a U.S. federal net operating loss carryforward for income tax purposes in the amount of approximately $68,342. Net operating loss carryforward arising in taxable years beginning after January 2000 (inception date) can be carried forward and offset against taxable income for 20 years and expiring between 2020 and 2034. | ||
| Utilization of U.S. net operating losses may be subject to substantial annual limitations due to the "change in ownership" provisions of the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of net operating losses before utilization. | ||
| The Subsidiary has accumulated net operating losses for tax purposes as of December 31, 2015, in the amount of approximately $35,900, which may be carried forward and offset against taxable income and capital gain in the future for an indefinite period. | ||
| e. | Taxes on income included in the Consolidated Statements of Operations: | |
| Taxes on income derived from tax prepayments related to non-deductible expenses in Israel. |
| F-27 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands |
| NOTE 7:- | TAXES ON INCOME (Cont.) |
| f. | Deferred income taxes: |
| Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company's deferred tax assets are as follows: |
| December 31, | ||||||||
| 2014 | 2015 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforward | $ | 21,412 | $ | 30,900 | ||||
| Allowances and reserves | 529 | 2,307 | ||||||
| Total deferred tax assets before valuation allowance | 21,941 | 33,207 | ||||||
| Valuation allowance | (21,941 | ) | (33,207 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
| As of December 31, 2015, the Company and the Subsidiary have provided valuation allowances in respect of deferred tax assets resulting from tax loss carryforward and other temporary differences, since they have a history of operating losses and current uncertainty concerning their ability to realize these deferred tax assets in the future. Management currently believes that it is more likely than not that the deferred tax regarding the loss carryforward and other temporary differences will not be realized in the foreseeable future. | |
| In 2013, 2014 and 2015, the main reconciling item of the statutory tax rate of the Company and the Subsidiary (26.5% to 35% in 2014 and in 2015) to the effective tax rate (0%) is tax loss carryforwards and other deferred tax assets for which a full valuation allowance was provided. |
| F-28 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands |
| NOTE 8:- | FINANCIAL INCOME (EXPENSE) |
| Year ended December 31, | ||||||||||||
| 2013 | 2014 | 2015 | ||||||||||
| Financial expenses: | ||||||||||||
| Bank charges | $ | (19 | ) | $ | (20 | ) | $ | (20 | ) | |||
| Warrant valuation | - | - | (1,370 | ) | ||||||||
| Foreign currency remeasurement adjustments | - | (46 | ) | (17 | ) | |||||||
| Others | (1 | ) | (2 | ) | (1 | ) | ||||||
| $ | (20 | ) | $ | (68 | ) | $ | (1,408 | ) | ||||
| Financial income: | ||||||||||||
| Foreign currency remeasurement adjustments | $ | 2 | $ | - | $ | - | ||||||
| Warrant valuation | 720 | 585 | - | |||||||||
| Interest on cash equivalents, short-term bank deposits and others | 3 | 1 | 1 | |||||||||
| Others | 1 | - | - | |||||||||
| $ | 726 | $ | 586 | $ | 1 | |||||||
| F-29 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands |
| NOTE 9:- | FAIR VALUE MEASUREMENTS |
| The Company classified certain warrants with down-round protection issued to investors through the years 2006 and 2007 and warrants issued to the purchasers of convertible debentures in 2010 as a liability at their fair value according to ASC 815-40-15-7I. The liability in respect of these warrants is remeasured at each reporting period until exercised or expired. Changes in the fair value of these warrants are reported in the Consolidated Statements of Operations as financial income or expense. As this class of warrants have now all been exercised or expired, there will be no need for additional warrant revaluation work going forward. |
| The fair value of these warrants was estimated at December 31, 2013, 2014 and in June 30, 2015 using the Binomial pricing model with the following assumptions: |
| December 31, | June 30, | |||||||||||
| 2013 | 2014 | 2015(*) | ||||||||||
| Dividend yield | 0 | % | 0 | % | 0 | % | ||||||
| Expected volatility | 78.1 | % | 53.3 | % | 51.6 | % | ||||||
| Risk-free interest rate | 0.3 | % | 0.2 | % | 0.01 | % | ||||||
| Contractual life (in years) | 1.7 | 0.7 | 0.2 | |||||||||
| (*) | These warrants were fully exercised in September 2015. |
| The changes in level 3 liabilities measured at fair value on a recurring basis: |
| Fair value of liability in respect of warrants | ||||
| Balance as of December 31, 2012 | $ | 1,931 | ||
| Change in the liability in respect of warrants | (720 | ) | ||
| Balance as of December 31, 2013 | 1,211 | |||
| Classification of liability in respect of warrants into equity due to the exercise of warrants | (14 | ) | ||
| Change in the liability in respect of warrants | (585 | ) | ||
| Balance as of December 31, 2014 | 612 | |||
| Classification of liability in respect of warrants into equity due to the exercise of warrants | (1,982 | ) | ||
| Change in the liability in respect of warrants | 1,370 | |||
| Balance as of December 31, 2015 | $ | - | ||
| F-30 |
MEDGENICS, INC. AND ITS SUBSIDIARY
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| U.S. dollars in thousands |
| NOTE 10:- | LOSS PER SHARE |
| Details in the computation of diluted loss per share: |
| Year ended December 31, | ||||||||||||||||||||||||
| 2013 | 2014 | 2015 | ||||||||||||||||||||||
| Weighted average number of shares | Loss | Weighted average number of shares | Loss | Weighted average number of shares | Loss | |||||||||||||||||||
| For the computation of basic loss | 17,629,436 | $ | 17,129 | 19,246,611 | $ | 18,433 | 26,783,623 | $ | 37,992 | |||||||||||||||
| Effect of potential dilutive common shares issuable upon exercise of warrants classified as liability | 54,074 | 1,615 | (*) | 47,648 | 891 | (*) | 62,647 | 824 | (*) | |||||||||||||||
| For the computation of diluted loss | 17,683,510 | $ | 18,744 | 19,294,259 | $ | 19,324 | 26,846,270 | $ | 38,816 | |||||||||||||||
| (*) | Financial income resulted from changes in fair value of warrants classified as liability. |
| The total weighted average number of shares related to the outstanding options, warrants and restricted shares excluded from the calculations of diluted loss per share due to their anti-dilutive effect was 14,838,907, 16,426,096 and 17,626,215 for the years ended December 31, 2013, 2014 and 2015, respectively. |
* * * * * * * * * * * * * * * * * * *
| F-31 |
ITEM 9 - Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
ITEM 9A - Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
As required by Exchange Act Rule 13a-15(b), in connection with the filing of this Annual Report on Form 10-K, we carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 2015, the end of the period covered by this report.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our evaluation under the framework set forth in Internal Control – Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2015. Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, the independent registered public accounting firm that audited the financial statements included in this Annual Report on Form 10-K, has issued an attestation report on our internal control over financial reporting as of December 31, 2015, which is included herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
| 56 |
ITEM 10 - Directors, Executive Officers and Corporate Governance.
Information required by Item 10 is incorporated by reference to our definitive proxy statement for our annual stockholders’ meeting presently scheduled to be held in April 2016.
ITEM 11 - Executive Compensation.
Information required by Item 11 is incorporated by reference to our definitive proxy statement for our annual stockholders’ meeting presently scheduled to be held in April 2016.
ITEM 12 - Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information required by Item 12 is incorporated by reference to our definitive proxy statement for our annual stockholders’ meeting presently scheduled to be held in April 2016.
ITEM 13 - Certain Relationships and Related Transactions, and Director Independence.
Information required by Item 13 is incorporated by reference to our definitive proxy statement for our annual stockholders’ meeting presently scheduled to be held in April 2016.
ITEM 14 - Principal Accountant Fees and Services.
Information required by Item 14 is incorporated by reference to our definitive proxy statement for our annual stockholders’ meeting presently scheduled to be held in April 2016.
| 57 |
ITEM 15 - Exhibits and Financial Statement Schedules.
(a)(1) Financial Statements.
(a)(2) Financial Statement Schedules. No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
(a)(3) Exhibits. The list of exhibits filed with or incorporated by reference in this Annual Report on Form 10-K is set forth below.
| Exhibit No. | Description | |
| 3.1 | Amended and Restated Certificate of Incorporation (previously filed as Exhibit 3.1 to the Company’s Registration Statement on Form S-1 filed November 5, 2010 (File No. 333-170425) and incorporated herein by reference). | |
| 3.2 | Certificate of Amendment to Amended and Restated Certificate of Incorporation (previously filed as Exhibit 3.2 to the Company’s Registration Statement on Form S-1 filed November 5, 2010 (File No. 333-170425) and incorporated herein by reference). | |
| 3.3 | Certificate of Amendment to Amended and Restated Certificate of Incorporation dated as of February 14, 2011 (previously filed as Exhibit 4.3 to the Company’s Post-Effective Amendment No. 1 to Form S-1 on Form S-3 filed July 16, 2012 (File No. 333-170425) and incorporated herein by reference). | |
| 3.4 | Second Amended and Restated By-Laws (previously filed as Exhibit 3.3 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2011 (File No. 001-35112) and incorporated herein by reference). | |
| 4.1 | Specimen Common Stock Certificate (previously filed as Exhibit 4.1 to the Company’s Amendment No. 4 to Registration Statement on Form S-1 filed February 22, 2011 (File No. 333-170425) and incorporated herein by reference). | |
| 4.2 | Registration Rights Agreement, dated as of May 25, 2009, between the Company and the person named therein (previously filed as Exhibit 4.2 to the Company’s Registration Statement on Form S-1 filed November 5, 2010 (File No. 333-170425) and incorporated herein by reference). | |
| 4.3 | Registration Rights Agreement, dated as of September 15, 2010, between the Company and the persons named therein (previously filed as Exhibit 4.3 to the Company’s Registration Statement on Form S-1 filed November 5, 2010 (File No. 333-170425) and incorporated herein by reference). | |
| 4.4 | Specimen Warrant Certificate (previously filed as Exhibit 4.4 to the Company’s Amendment No. 4 to Registration Statement on Form S-1 filed February 22, 2011 (File No. 333-170425) and incorporated herein by reference). | |
| 58 |
| Exhibit No. | Description | |
| 4.5 | Form of Warrant Agreement (previously filed as Exhibit 4.5 to the Company’s Amendment No. 4 to Registration Statement on Form S-1 filed February 22, 2011 (File No. 333-170425) and incorporated herein by reference). | |
| 4.6 | Form of Warrant Certificate, dated as of June 18, 2012 (previously filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed June 19, 2012 (File No. 001-35112) and incorporated herein by reference). | |
| 4.7 | Warrant Agreement, dated as of June 18, 2012, between Medgenics, Inc. and Corporate Stock Transfer, Inc., as warrant agent (previously filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K filed June 19, 2012 (File No. 001-35112) and incorporated herein by reference). | |
| 4.8 | Common Stock Purchase Warrant, dated as of June 18, 2012, issued to Maxim Partners LLC (previously filed as Exhibit 10.4 to the Company’s Current Report on Form 8-K filed June 19, 2012 (File No. 001-35112) and incorporated herein by reference). | |
| 4.9 | Registration Rights Agreement, dated as of June 18, 2012, by and among Medgenics, Inc. and the investors party thereto (previously filed as Exhibit 10.5 to the Company’s Current Report on Form 8-K filed June 19, 2012 (File No. 001-35112) and incorporated herein by reference). | |
| 4.10 | Warrant Agreement, dated as of February 8, 2013, between Medgenics, Inc. and Corporate Stock Transfer, Inc. (previously filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed February 8, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 4.11 | Form of Series 2013-A Warrant Certificate (previously filed as Exhibit 4.2 to the Company’s Current Report on Form 8-K filed February 8, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.1† | Medgenics, Inc. Stock Incentive Plan, as amended and restated effective March 5, 2012 (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 5, 2012 (File No. 001-35112) and incorporated herein by reference). | |
| 10.2† | First Amendment of the Medgenics, Inc. Stock Incentive Plan (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed May 1, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.3† | Second Amendment of the Medgenics, Inc. Stock Incentive Plan (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 9, 2014 (File No. 001-35112) and incorporated herein by reference). | |
| 10.4† | Form of Non-Qualified Stock Option Award Agreement under the Medgenics, Inc. Stock Incentive Plan (previously filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.5† | Form of Restricted Stock Award Agreement under the Medgenics, Inc. Stock Incentive Plan (previously filed as Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.6† | Form of Non-Qualified Stock Option Award Terms (Outside of Plan) (previously filed as Exhibit 4.7 to the Company’s Registration Statement on Form S-8 filed October 15, 2013 (File No. 333-191733) and incorporated herein by reference). | |
| 59 |
| Exhibit No. | Description | |
| 10.7† | Employment Agreement, dated as of September 13, 2013, between Medgenics, Inc. and Michael Cola (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed September 16, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.8† | Executive Director Appointment Letter, dated as of September 13, 2013, between Medgenics, Inc. and Michael Cola (previously filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed September 16, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.9† | Employment Agreement, dated as of September 13, 2013, between Medgenics, Inc. and John Leaman (previously filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K filed September 16, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.10† | Employment Agreement, dated as of September 13, 2013, between Medgenics, Inc. and Garry Neil (previously filed as Exhibit 10.4 to the Company’s Current Report on Form 8-K filed September 16, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.11† | Employment Agreement, dated as of September 8, 2014, between Medgenics, Inc. and Scott Applebaum (previously filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 (File No. 001-35112) and incorporated herein by reference). | |
| 10.12† | Non-Executive Director Appointment Letter, dated as of November 14, 2007, for Eugene Andrew Bauer (previously filed as Exhibit 10.12 to the Company’s Registration Statement on Form S-1 filed November 5, 2010 (File No. 333-170425) and incorporated herein by reference). | |
| 10.13† | Non-Executive Director Appointment Letter, dated as of June 6, 2011, for Isaac Blech (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed July 5, 2011 (File No. 001-35112) and incorporated herein by reference). | |
| 10.14† | Medgenics, Inc. Non-Qualified Stock Option Award Terms between Medgenics, Inc. and Sol J. Barer (previously filed as Exhibit 4.7 to the Company’s Registration Statement on Form S-8 filed August 1, 2012 (File No. 333-182992) and incorporated herein by reference). | |
| 10.15† | Director Appointment Letter, dated as of August 6, 2012, between Medgenics, Inc. and Sol J. Barer (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed August 8, 2012 (File No. 001-35112) and incorporated herein by reference). | |
| 10.16† | Non-Executive Director Appointment Letter, dated as of March 8, 2013, between Medgenics, Inc. and Joseph J. Grano, Jr. (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed March 14, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.17† | Non-Executive Director Appointment Letter, dated as of October 16, 2013, between Medgenics, Inc. and Wilbur H. Gantz (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed October 18, 2013 (File No. 001-35112) and incorporated herein by reference). | |
| 10.18† | Non-Executive Director Appointment Letter, dated as of May 21, 2015, between Medgenics, Inc. and Barbara G. Duncan (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed June 10, 2015 (File No. 001-35112) and incorporated herein by reference). | |
| 10.19* | Sponsored Research Agreement, dated as of November 12, 2014, between Medgenics Medical Israel Ltd. and The Children’s Hospital of Philadelphia (previously filed as Exhibit 10.28 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2014 (File No. 001-35112) and incorporated herein by reference). | |
| 60 |
| Exhibit No. | Description | |
| 10.20 | Amendment #1 to Sponsored Research Agreement, dated December 18, 2015, by and between Medgenics Medical Israel Ltd. and the Children’s Hospital of Philadelphia (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed December 22, 2015 (File No. 001-35112) and incorporated herein by reference). | |
| 10.21* | License Agreement, dated as of November 12, 2014, between Medgenics Medical Israel Ltd. and The Children’s Hospital of Philadelphia (previously filed as Exhibit 10.29 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2014 (File No. 001-35112) and incorporated herein by reference). | |
| 10.22* | Equity Interest Purchase Agreement, dated as of September 9, 2015, among Medgenics, Inc., neuroFix therapeutics, inc., neuroFix, LLC, The Children’s Hospital Of Philadelphia, Philip Harper and Hakon Hakonarson (previously filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 (File No. 001-35112) and incorporated herein by reference). | |
| 10.23* | License Agreement, dated as of September 9, 2015, between neuroFix, LLC and The Children’s Hospital Of Philadelphia (previously filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 (File No. 001-35112) and incorporated herein by reference). | |
| 10.24 | Purchase Agreement dated October 1, 2015 by and among Medgenics, Inc. and Piper Jaffray & Co., as representative of the several underwriters set forth on Schedule I thereto (previously filed as Exhibit 1.1 to the Company’s Current Report on Form 8-K filed October 7, 2015 and incorporated herein by reference). | |
| 10.25† | Letter Agreement, by and between Medgenics, Inc. and Brian Piper, dated February 1, 2016 (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed February 3, 2016 (file No. 001-35112) and incorporated herein by reference). | |
|
10.26† |
Agreement and Release and Waiver, by and between Medgenics, Inc. and John Leaman, dated February 1, 2016 (previously filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed February 3, 2016 (file No. 001-35112) and incorporated herein by reference). | |
| 21.1 | Subsidiaries of the Company (filed herewith). | |
| 23.1 | Consent of Kost Forer Gabbay & Kasierer (Ernst & Young) (filed herewith). | |
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). | |
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). | |
| 32.1 | Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (furnished herewith). | |
| 101 | Interactive Data File (filed herewith). |
| † | Indicates a management contract or compensatory plan or arrangement contemplated by Item 15(a)(3) of Form 10-K. |
| * | Portions of this exhibit have been omitted pursuant to a request for confidential treatment on file with the Securities and Exchange Commission. |
| 61 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| MEDGENICS, INC. | ||
| Date: February 26, 2016 | By: | /s/ Michael F. Cola |
|
Michael F. Cola President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/ Michael F. Cola | President, Chief Executive Officer and Director | February 26, 2016 | ||
| Michael F. Cola | (Principal Executive Officer) | |||
| /s/ Brian D. Piper | Chief Financial Officer | February 26, 2016 | ||
| Brian D. Piper | (Principal Financial Officer & Principal Accounting Officer) | |||
| /s/ Sol J. Barer | Chairman of the Board of Directors | February 26, 2016 | ||
| Sol J. Barer | ||||
| /s/ Eugene A. Bauer | Director | February 26, 2016 | ||
| Eugene A. Bauer | ||||
| /s/ Isaac Blech | Director | February 26, 2016 | ||
| Isaac Blech | ||||
| /s/ Alastair Clemow | Director | February 26, 2016 | ||
| Alastair Clemow | ||||
| /s/ Barbara Duncan | Director | February 26, 2016 | ||
| Barbara Duncan | ||||
| /s/ Wilbur H. Gantz | Director | February 26, 2016 | ||
| Wilbur H. Gantz | ||||
| /s/ Joseph J. Grano, Jr. | Director | February 26, 2016 | ||
| Joseph J. Grano, Jr. |
| 62 |
