Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PHASERX, INC. | v461389_8k.htm |
Exhibit 99.1

Unlocking the value of mRNA ® CORPORATE PRESENTATION March 8, 2017 NASDAQ: PZRX

.. FORWARD - LOOKING STATEMENTS This presentation contains forward - looking statements which are based on current expectations, estimates and projections. Statements that are not historical facts are forward - looking statements and typically are identified by words like “may”, “believe”, “anticipate”, “could”, “should”, “estimate”, “expect”, “intend”, “plan”, “project”, “will”, “forecast”, “budget”, “pro forma”, and similar terms. These statements are not guarantees of future performance, events or results and involve potential risks and uncertainties. Although we believe that such statements are based on reasonable assumptions, these forward - looking statements are subject to numerous factors, risks and uncertainties that could cause actual outcomes and results to be materially different from those projected or assumed in our forward - looking statements. We caution you that any forward - looking statement reflects only our belief at the time the statement is made. Accordingly, the Company’s actual results may differ from our current expectations, estimates and projections. We undertake no obligation to update any forward - looking statements, whether as a result of new information, future events or otherwise. 2

.. INVESTMENT SUMMARY 3 Strategy Team Pipeline Partnering • Broad nucleic acid experience with proven track record • Three urea cycle disorder (UCD) programs in development • Preclinical reduction in blood ammonia shown in two programs (FDA approvable endpoint for the UCDs) • Significant partnering interest in product pipeline • Hybrid mRNA Technology can be out - licensed to other firms to deliver mRNA therapeutics inside cell Opportunity • i - ERT product potential is enabled by PhaseRx’s proprietary Hybrid mRNA Technology™ • Lead development product, PRX - OTC, expected to generate Phase 2 clinical POC in 2018 with an approvable endpoint • To develop a portfolio of intracellular enzyme replacement therapy (i - ERT) products - analogous to current $4B worldwide ERT market

.. MARKET OPPORTUNITY 4 i - ERT market opportunity for UCDs is completely untapped… DISEASE INCIDENCE DRUG COMPANY 2015 SALES OTC Deficiency 1/56,500 PRX - OTC PhaseRx Pre - commercial ASL Deficiency 1/218,750 PRX - ASL PhaseRx Pre - commercial ASS1 Deficiency 1/250,000 PRX - ASS1 PhaseRx Pre - commercial DISEASE INCIDENCE DRUG COMPANY 2015 SALES Gaucher’s 1/60,000 Cerezyme , Vpriv Sanofi, Shire $1.2B w/w Pompe 1/40,000 - 1/100,000 Myozyme Sanofi $728M w/w Fabry 1/50,000 - 1/117,000 Fabrazyme , Replagal Sanofi, Shire $1.1B w/w MPS VI 1/250,000 - 1/600,000 Naglazyme Biomarin $303M w/w … and analogous to the growing $4B conventional ERT market:

.. PHASERX HYBRID MRNA TECHNOLOGY™ PLATFORM 5 Advantages of Hybrid mRNA Technology: • Targets synthesis to hepatocytes • Improved tolerability • High levels of expression and activity • Enables repeat dosing regimens Polymer Nanoparticle Inert Lipid Nanoparticle GalNAc - Targeted Polymer Provides Delivery to Cytoplasm Inert LNP Provides mRNA Protection and Delivery to Liver Both targeted to liver where they meet in endosome resulting in mRNA delivery into the interior of the cell +

.. DEVELOPMENT PIPELINE 6 PROGRAM LEAD OPTIMIZATION PRECLINICAL IND - ENABLING PHASE 2a/2b PHASE 3 PRX - OTC Ornithine Transcarbamylase Deficiency PRX - ASL Argininosuccinate Lyase Deficiency PRX - ASS1 Argininosuccinate Synthase 1 Deficiency First IND planned for 4Q 2017 - Clinical POC expected in 1H 2018 Phase 2a Data Expected 1H 2018

.. UCDS: PROGRESSIVE AND PERMANENT BRAIN DAMAGE 7 • UCDs: a family of metabolic disorders with devastating patient consequences • Each results from an inherited single - gene deficiency • Hyperammonemia causes cumulative and permanent brain damage, coma and death • Liver transplant is the only current remedy • The only drug treatment, ammonia scavengers, are palliative at best • Approvable endpoint - lowering blood ammonia levels Urea Cycle Disorders (UCDs) Goal is to deliver mRNA encoding the missing protein, thereby correcting the disease

.. OTC DEFICIENCY – DIAGNOSIS AND PROGRESSION 8 • Ornithine transcarbamylase deficiency (OTCD) - 1/56,500 incidence, no ethnicity or geography spared • Awareness, diagnosis and survival rates expected to improve if corrective therapy available • Presents as a calamity at birth with vomiting, followed by seizure, coma and death if not treated • Most patients present by age 12 with toxic ammonia crises • Patients are at constant risk of ammonia toxicity, which causes cumulative and permanent neurologic impairment 0 10 20 30 40 50 60 0-30d 31d-2y 2-12 y >12 y Percent of patients Age at First OCTD Diagnosis male female Source: Summar et al., 2008 Acta Paediatr. 97:1420 - 25 Enns et al., 2007 NEJM 356: 2282 - 92

.. OTC DEFICIENCY – CURRENT THERAPY FAILS PATIENTS • Current disease management requires ammonia scavengers and adherence to protein restricted, unpalatable diet – Hyperammonemic crises still occur • Even under the controlled conditions of a clinical trial, ammonia scavengers don’t do the job: • 25% of pediatric subjects on Ravicti had hyperammonemic crises during the 12 month pivotal study 1 • Two thirds of adult patients on Ravicti with high ammonia levels at the start of the P2 trial still had high ammonia levels at the end 2 40 LARGE TABLETS per day, bad odor Oral liquid 3x per day IV scavenger given in ICU/PICU 9 1 Berry et al 2014 Mol Genet Metab. 112(1): 17 - 24 2 Lee et al 2010 Mol Genet Metab. 100(3):221 - 8
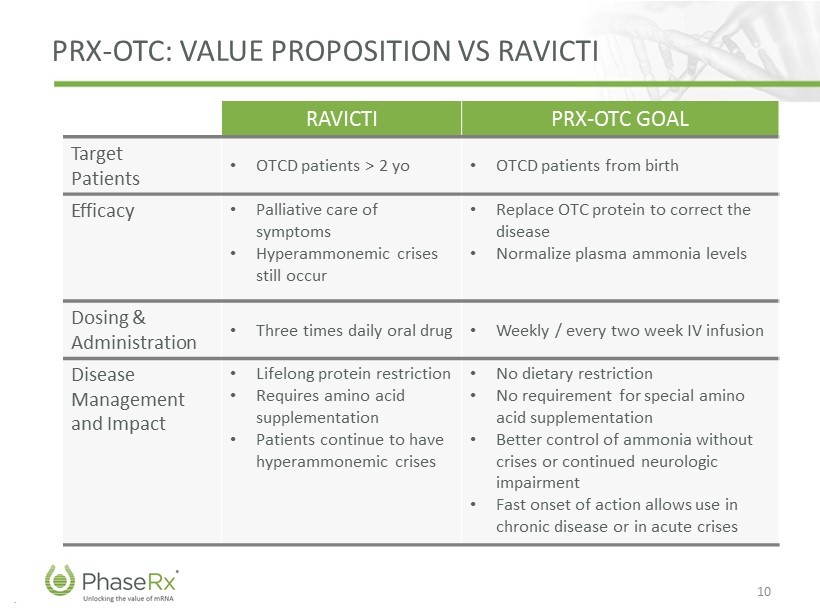
.. PRX - OTC: VALUE PROPOSITION VS RAVICTI 10 RAVICTI PRX - OTC GOAL Target Patients • OTCD patients > 2 yo • OTCD patients from birth Efficacy • Palliative care of symptoms • Hyperammonemic crises still occur • Replace OTC protein to correct the disease • Normalize plasma ammonia levels Dosing & Administration • Three times daily oral drug • Weekly / every two week IV infusion Disease Management and Impact • Lifelong protein restriction • Requires amino acid supplementation • Patients continue to have hyperammonemic crises • No dietary restriction • No requirement for special amino acid supplementation • Better control of ammonia without crises or continued neurologic impairment • Fast onset of action allows use in chronic disease or in acute crises

.. HUMAN OTC LEVELS IN CD1 MICE • Study Design: – CD1 mice were treated with a single dose of OTC mRNA – Livers collected at endpoints were examined by western analysis and for OTC enzyme activity • Results: – High levels of protein expression by Western blot analysis – Duration of OTC expression is out to day >10 following single dose – 200% wild - type OTC enzyme activity 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Buffer Day 2 Day 4 OTC Ab HSP90 Day 8 Day 10 11 OTC Protein Western Blot Analysis 50 100 150 200 250 Buffer OTC mRNA % of Wild - Type OTC Enzyme Activity at Day 10

.. 0 50 100 150 200 250 300 350 Buffer OTC mRNA Normal Mice Hyperammonemia Induced spf-ash Mice Plasma Ammonia (µM) Day 14 Day 21 PRX - OTC EFFICACY: REDUCTION IN TOXIC AMMONIA 12 • Hyperammonemia can be induced in OTC - spf ash mice • Hyperammonemia is the hallmark of human OTCD and causes CNS damage and death in OTCD patients • Reduction in ammonia levels is an approvable endpoint that served as the basis for Ravicti approval by FDA • Treatment with PRX - OTC shows normalization of blood ammonia levels in hyperammonemic mice *** p < 0.001

.. 0 5 10 15 20 25 30 35 0 20 40 60 80 100 Buffer OTC mRNA Days Post Induction of Hyperammonemia % S u r v i v a l Control mRNA PRX - OTC EFFICACY: SURVIVAL • Treatment with PRX - OTC results in complete survival of hyperammonemia induced OTC - spf ash mice • All mice succumbed to hyperammonemia in both negative control groups 13 **** p < 0.0001

.. NON - HUMAN PRIMATE SAFETY STUDY • Study Design – Single - dose study in cynomolgus monkeys (N=3/group) – Hybrid mRNA Technology formulated with human EPO ( hEPO ) mRNA – Endpoints: safety and EPO expression • Results – Dose responsive increase in blood EPO levels from 0.1 to 1 mg/kg mRNA – No noteworthy changes in serum chemistry, hematology, or cytokines • Conclusion – Hybrid mRNA Technology showed very good expression and tolerability in this large animal study 14 0 50 100 150 200 250 300 350 Buffer 0.1 mg/kg 0.3 mg/kg 1 mg/kg ng/mL hEPO Protein Levels 2 h 6 h 12h 24 h 48 h 72 h * * * No 12 h data 0 50 100 150 200 Buffer 0.1 mg/kg 0.3 mg/kg 1 mg/kg U/L ALT Levels Pre-dose 1 day 2 days 7 days hEPO mRNA Dose 0 200 400 600 800 1000 Buffer 0.1 mg/kg 0.3 mg/kg 1 mg/kg (pg/mL) Cytokines 6 h Post Dose IP-10 IL-6 TNF - α IFN - γ IL-12

.. PRX - OTC: DEVELOPMENT PLAN • Working with CMOs to scale up and manufacture PRX - OTC • Dose range finding studies – Planned for Q2 2017 • IND enabling GLP toxicology – Planned for H2 2017 • IND filing – Planned submission Q4 2017 • Clinical trial expected to begin patient enrollment in H1 2018 – Single - dose escalating trial • Safety; measurement of blood ammonia and urea – Multiple - dose trial expected • Safety; measurement of blood ammonia and urea 15

.. ASL DEFICIENCY: DIAGNOSIS AND PROGRESSION • ASL deficiency (ASLD) leads to argininosuccinic aciduria, an autosomal recessive inherited disease causing: – elevated blood ammonia – accumulation of argininosuccinic acid (ASA) in blood and urine – developmental delay and retardation – effects on liver, CNS, and vascular system – hypertension • Lowering of blood ammonia is believed to be an approvable endpoint for ASL deficiency 16 Urea Cycle Disorders: ASL Deficiency
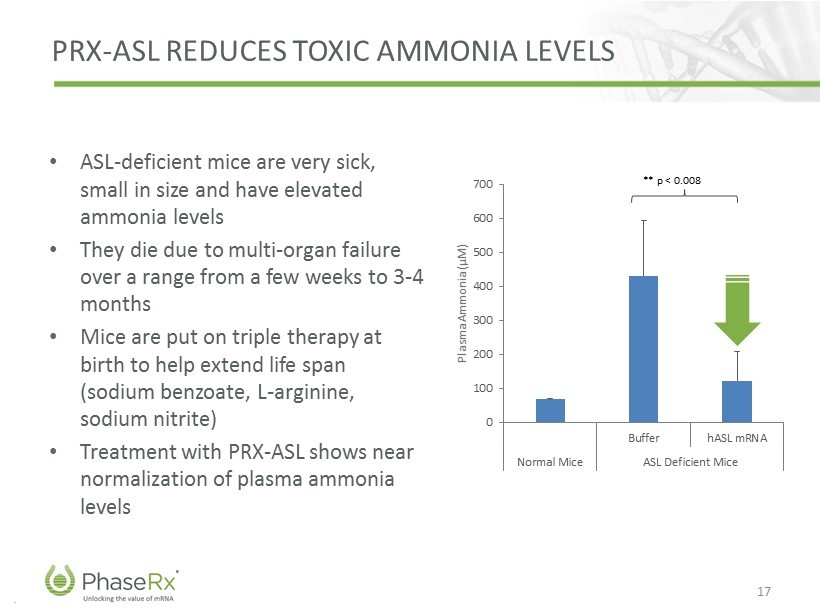
.. PRX - ASL REDUCES TOXIC AMMONIA LEVELS • ASL - deficient mice are very sick, small in size and have elevated ammonia levels • They die due to multi - organ failure over a range from a few weeks to 3 - 4 months • Mice are put on triple therapy at birth to help extend life span (sodium benzoate, L - arginine, sodium nitrite) • Treatment with PRX - ASL shows near normalization of plasma ammonia levels 17 0 100 200 300 400 500 600 700 Buffer hASL mRNA Normal Mice ASL Deficient Mice Plasma Ammonia (µM) ** p < 0.008

.. MRNA THERAPEUTIC PRODUCT GENERATOR 18 Ability to rapidly manufacture new mRNAs Known sequences of all mRNAs Proprietary PhaseRx Hybrid mRNA Technology i - ERT Products to Treat: • Organic Acidemias • Glycogen Storage Diseases • Porphyria • Hyperoxalurea • Urea Cycle Disorders • Phenylketonuria • Tyrosinemia • Wilson’s Disease In Vivo Gene Editing: Strong belief technology is enabling for in vivo gene editing

.. PARTNERING OPPORTUNITIES 19 Therapeutic Focus • Rare diseases • Patients failed by existing treatments Value to Partners • Correct physiology of devastating diseases • In vivo instead of ex vivo delivery • Specific activity in liver and avoiding off - target effects • High levels of expression and activity • Achieving the above with repeat dosing regimens PhaseRx Technology Platform In Vivo Gene Editing mRNA Therapeutics PhaseRx Development Pipeline PRX - OTC PRX - ASL PRX - ASS1

.. EXPANDING INTELLECTUAL PROPERTY • Initial IP exclusively licensed from the University of Washington and developed internally – Portfolio covers all the world's major pharmaceutical markets – Over 30 patents issued and over 35 applications pending worldwide • Diblock Polymer family – Issued in 6 territories, pending in 7 more – Provides exclusivity until at least 2029 • Polymer Nanoparticle family – Issued in 3 territories, pending in 4 more – Provides exclusivity until at least 2029 • Hybrid mRNA Technology family – International PCT patent application is pending – Should provide exclusivity until at least 2036 20

.. MANAGEMENT TEAM: BROAD NUCLEIC ACID EXPERIENCE 21 • Robert Overell, Ph.D., President and Chief Executive Officer Frazier Healthcare Ventures, Targeted Genetics, Immunex • Michael Houston, Ph.D., Chief Scientific Officer Nastech /MDRNA • Gordon Brandt, M.D., Chief Medical Officer Nastech /MDRNA • Helen Tsui, CPA , Senior Vice President of Finance and Principal Accounting Officer Dendreon • James Watson, MBA, Head of Corporate Development Alvine , Burrill Investment Banking, Incyte, Lilly • Mary Prieve, Ph.D., Vice President, Biology Nastech /MDRNA • Sean Monahan, Ph.D., Vice President, Chemistry Mirus/Roche, Schering AG

.. BOARD OF DIRECTORS • Steven Gillis, Ph.D., Chairman Managing Director, ARCH Venture Partners • Brian Atwood Managing Director, Versant Ventures • Michelle Griffin Former Corixa , Trubion CFO/COO • Paul Johnson, Ph.D. Cofounder and former CSO • Robert Overell, Ph.D. President and CEO • Peggy Phillips Former Immunex COO • Jack Schmidt, M.D. Former Alnylam CSO 22
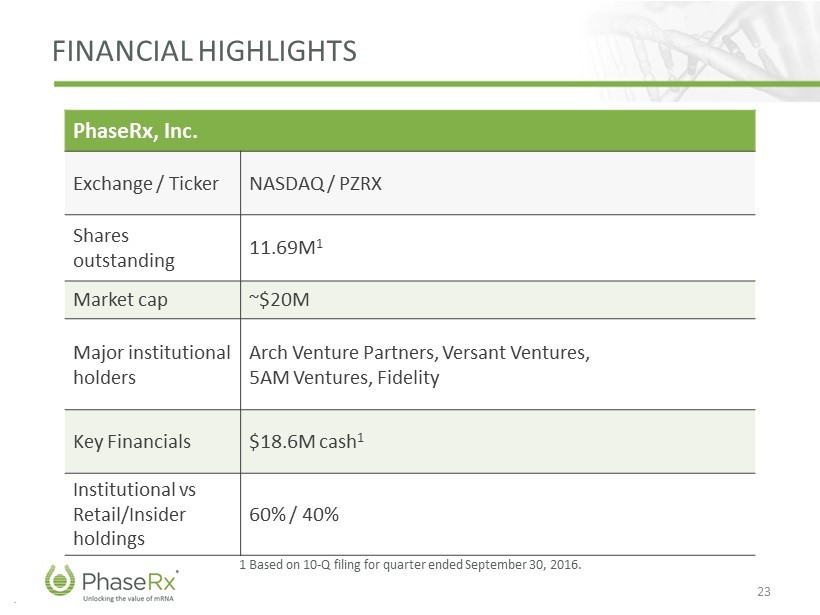
.. FINANCIAL HIGHLIGHTS 23 1 Based on 10 - Q filing for quarter ended September 30, 2016. PhaseRx , Inc. Exchange / Ticker NASDAQ / PZRX Shares outstanding 11.69 M 1 Market cap ~$20M Major institutiona l holders Arch Venture Partners, Versant Ventures, 5AM Ventures, Fidelity Key Financials $18.6M cash 1 Institutiona l vs Retail/Insider holdings 60% / 40%

.. NEAR - TERM POTENTIAL MILESTONES 24 EVENT ANTICIPATED TIMING POC in Second Disease Model 2Q 2016 – Achieved Declare Lead Development Candidate 2Q 2016 - Achieved Large Animal Tolerability Study 4Q 2016 - Achieved GMP Manufacturing 3Q 2017 File IND 4Q 2017 Phase 2a Single - dose Safety and Efficacy Data 2018 Phase 2b Repeat - dose Safety and Efficacy Data 2018 Corporate Partnership (s) Discussions Ongoing

.. INVESTMENT SUMMARY 25 Strategy Team Pipeline Partnering • Broad nucleic acid experience with proven track record • Three urea cycle disorder (UCD) programs in development • Preclinical reduction in blood ammonia shown in two programs (FDA approvable endpoint for the UCDs) • Significant partnering interest in product pipeline • Hybrid mRNA Technology can be out - licensed to other firms to deliver mRNA therapeutics inside cell Opportunity • i - ERT product potential is enabled by PhaseRx’s proprietary Hybrid mRNA Technology™ • Lead development product, PRX - OTC, expected to generate Phase 2 clinical POC in 2018 with an approvable endpoint • To develop a portfolio of intracellular enzyme replacement therapy (i - ERT) products - analogous to current $4B worldwide ERT market

Unlocking the value of mRNA ® Investor Contact: Erin S. Cox, Director of Investor Relations erin@phaserx.com
