Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Corindus Vascular Robotics, Inc. | cvrs-8k_030817.htm |
Corindus Vascular Robotics, Inc. 8-K
Exhibit 99.1
1 Precision Vascular Robotics Corindus Vascular Robotics (CVRS) March 2017

Forward Looking Statements THIS PRESENTATION CONTAINS “FORWARD - LOOKING STATEMENTS” (AS SUCH TERM IS DEFINED IN SECTION 27 A OF THE SECURITIES ACT OF 1933 , AS AMENDED, AND SECTION 21 E OF THE SECURITIES EXCHANGE ACT OF 1934 , AS AMENDED), AND INFORMATION RELATING TO THE COMPANY, THAT ARE BASED ON THE CURRENT BELIEFS OF, AND ASSUMPTIONS MADE BY OUR MANAGEMENT AND THE INFORMATION CURRENTLY AVAILABLE TO OUR MANAGEMENT . FORWARD - LOOKING STATEMENTS RELATE TO EXPECTATIONS CONCERNING MATTERS THAT ARE NOT HISTORICAL FACTS . WORDS SUCH AS "ANTICIPATE," "BELIEVE," "ESTIMATE," "EXPECT," "INTEND," "PLAN," "PREDICT," "OPINION," "WILL" AND SIMILAR EXPRESSIONS AND THEIR VARIANTS, ARE INTENDED TO IDENTIFY FORWARD - LOOKING STATEMENTS . THESE FORWARD - LOOKING STATEMENTS INCLUDE, BUT ARE NOT LIMITED TO STATEMENTS RELATED TO OUR EXPECTED BUSINESS, PRODUCTS, ADOPTION OF ROBOTIC MEDICAL PROCEDURES, RESULTS OF OPERATIONS, FUTURE FINANCIAL CONDITION, ABILITY TO INCREASE OUR REVENUES, AND SIMILAR MATTERS . THESE FORWARD - LOOKING STATEMENTS SHOULD BE CONSIDERED IN LIGHT OF VARIOUS IMPORTANT FACTORS, INCLUDING, WITHOUT LIMITATION, THE RATE OF ADOPTION OF OUR CORPATH SYSTEM AND THE RATE OF USE OF OUR CASSETTES ; RISKS ASSOCIATED WITH MARKET ACCEPTANCE, INCLUDING PRICING AND REIMBURSEMENT ; OUR ABILITY TO ENFORCE OUR INTELLECTUAL PROPERTY RIGHTS ; OUR NEED FOR ADDITIONAL FUNDS TO SUPPORT OUR OPERATIONS ; OUR ABILITY TO MANAGE EXPENSES AND CASH FLOW ; FACTORS RELATING TO ENGINEERING, REGULATORY, MANUFACTURING, SALES AND CUSTOMER SERVICE CHALLENGES ; POTENTIAL SAFETY AND REGULATORY ISSUES THAT COULD SLOW OR SUSPEND OUR SALES ; THE EFFECT OF CREDIT, FINANCIAL AND ECONOMIC CONDITIONS ON CAPITAL SPENDING BY OUR POTENTIAL CUSTOMERS ; THE IMPACT OF GLOBAL AND REGIONAL ECONOMIC AND CREDIT MARKET CONDITIONS ON HEALTH CARE SPENDING ; HEALTH CARE REFORM LEGISLATION IN THE UNITED STATES AND ITS IMPACT ON HOSPITAL SPENDING, REIMBURSEMENT AND FEES WHICH WILL BE LEVIED ON CERTAIN MEDICAL DEVICE REVENUES, DECREASES IN HOSPITAL ADMISSIONS AND ACTIONS BY PAYERS TO LIMIT OR MANAGE SURGICAL PROCEDURES TIMING AND SUCCESS OF PRODUCT DEVELOPMENT AND MARKET ACCEPTANCE OF DEVELOPED PRODUCTS, PROCEDURE COUNTS ; REGULATORY APPROVALS, CLEARANCES AND RESTRICTIONS ; GUIDELINES AND RECOMMENDATIONS IN THE HEALTH CARE AND PATIENT COMMUNITIES, INTELLECTUAL PROPERTY POSITIONS AND LITIGATION, COMPETITION IN THE MEDICAL DEVICE INDUSTRY AND IN THE SPECIFIC MARKETS OF SURGERY IN WHICH WE OPERATE, THE INABILITY TO MEET DEMAND FOR PRODUCTS, THE RESULTS OF LEGAL PROCEEDINGS TO WHICH WE ARE OR MAY BECOME A PARTY, PRODUCT LIABILITY AND OTHER LITIGATION CLAIMS, ADVERSE PUBLICITY REGARDING OUR COMPANY AND SAFETY OF OUR PRODUCTS AND THE ADEQUACY OF TRAINING ; OUR ABILITY TO EXPAND IN FOREIGN MARKETS ; AND OTHER RISK FACTORS . READERS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD - LOOKING STATEMENTS, WHICH ARE BASED ON CURRENT EXPECTATION AND ARE SUBJECT TO RISKS, UNCERTAINTIES ; AND ASSUMPTIONS THAT ARE DIFFICULT TO PREDICT, INCLUDING THOSE RISK FACTORS DESCRIBED IN THE COMPANY’S ANNUAL REPORT ON FORM 10 - K FOR THE FISCAL YEAR ENDED ON DECEMBER 31 , 2015 . OUR ACTUAL RESULTS MAY DIFFER MATERIALLY AND ADVERSELY FROM THOSE EXPRESSED IN ANY FORWARD - LOOKING STATEMENTS . WE UNDERTAKE NO OBLIGATION TO PUBLICLY UPDATE OR RELEASE ANY REVISIONS TO THESE FORWARD - LOOKING STATEMENTS EXCEPT AS REQUIRED BY LAW . 2

Robotics Market 3 Robotics market expected to double in 4 years to $135B Source: International Data Corporation Auto - Driving Cars Robotics in Manufacturing 2015 2019 $135 $71 Robotics Market (Billions) Robotics in Healthcare Robots in Hazardous Areas

Global Medical Robotics Market 4 Healthcare s ector expected to grow fastest >30 companies developing robotic technologies 1 st Surgical Robot for general laparoscopic procedures Surgical robot with enhanced information for decision making Orthopedics – Spine surgery Orthopedics 1 st robotic system for PCI +

1 Market opportunity assessment based on market research reports and Corindus estimate 2 Peripheral Vascular includes lower limb, carotid, renal, iliac and AAA (abdominal aortic aneurysm) procedures 3 Millennium Research Group Interventional Market Opportunity Large & growing worldwide market • $4.5B FY2018 market estimate 1 • Non - PCI procedure types: Peripheral Vascular 2 , Neurointerventional and Structural Heart • 2018 estimated PCI procedure volume 3 : − 933,000 in the US − 1,800,000 OUS • 2018 estimated non - PCI procedure volume 3 : − 1,200,000 in the US − 1,800,000 OUS $0.88B PCI $1.14B PCI Market Opportunity OUS US $4.5B 1 $1.33B NON - PCI $1.19B NON - PCI 5
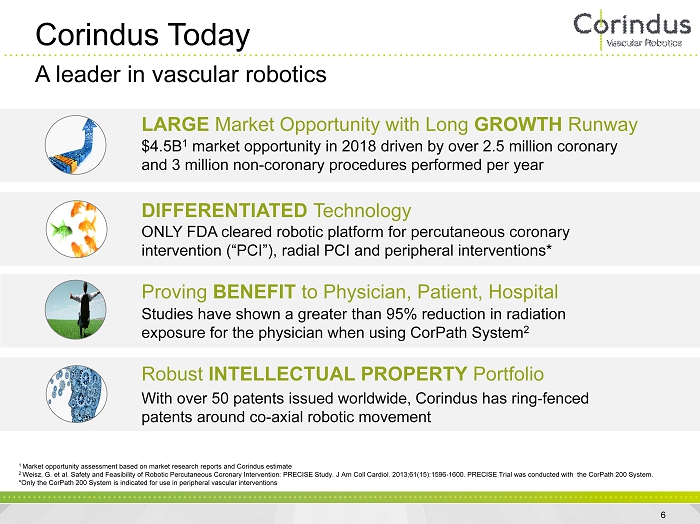
Corindus Today A leader in vascular robotics LARGE Market Opportunity with Long GROWTH Runway $4.5B 1 market opportunity in 2018 driven by over 2.5 million coronary and 3 million non - coronary procedures performed per year DIFFERENTIATED Technology ONLY FDA cleared robotic platform for percutaneous coronary intervention (“PCI”), radial PCI and peripheral interventions* Proving BENEFIT to Physician, Patient, Hospital Studies have shown a greater than 95% reduction in radiation exposure for the physician when using CorPath System 2 Robust INTELLECTUAL PROPERTY Portfolio With over 50 patents issued worldwide, Corindus has ring - fenced patents around co - axial robotic movement 1 Market opportunity assessment based on market research reports and Corindus estimate 2 Weisz , G. et al. Safety and Feasibility of Robotic Percutaneous Coronary Intervention: PRECISE Study. J Am Coll Cardiol. 2013;61(1 5): 1596 - 1600. PRECISE Trial was conducted with the CorPath 200 System. *Only the CorPath 200 System is indicated for use in peripheral vascular interventions 6

Recent Milestones 7 CAPITAL RAISE $45 MILLION ▪ Expected to close March 15, 2017 ▪ New investors included BSX, BioStar Ventures, Consonance Capital, and Hudson Executive Capital ▪ Royal Philips and HealthCor Partners Management also participated STRATEGIC AGREEMENT Expansion to Japan ▪ Japan Medicalnext became the exclusive distributor of Corindus products in Japan* ▪ Initial order for 12 CorPath GRX Systems with $2 million advance toward the purchase price GRX LAUNCH 1 st Commercial Cases ▪ First procedures using GRX System ▪ Performed at NewYork - Presbyterian, UC San Diego Health, and University of Virginia Health System Building Robotic Programs ▪ Multi - system orders at Baylor Health and the VA MULTI - SYSTEM ORDERS *PMDA approval pending

New Leadership & Strengthening Team Building operational & clinical excellence Mark Toland President & CEO 20 Year Medical Device Veteran J. Aaron Grantham, MD Chief Medical Officer Practicing Interventional Cardiologist Marty Leon, MD Executive Advisor Interventional Cardiology Thought Leader Campbell Rogers, MD Board of Directors CMO of Heartflow 8 Bill Lombardi, MD Steering Committee University of Washington David Kandzari , MD Steering Committee Piedmont Heart Sunil Rao, MD Steering Committee Duke University Ryan Madder, MD Steering Committee Spectrum Health Louis Cannon, MD Board of Directors Managing Director, BioStar Ventures
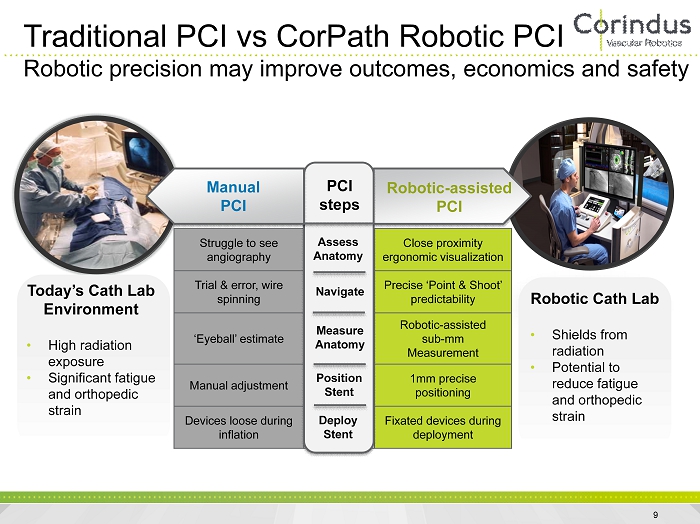
Struggle to see angiography Assess Anatomy Close proximity ergonomic visualization Trial & error, wire spinning Navigate Precise ‘Point & Shoot’ predictability ‘Eyeball’ estimate Measure Anatomy Robotic - assisted sub - mm Measurement Manual adjustment Position Stent 1mm precise positioning Devices loose during inflation Deploy Stent Fixated devices during deployment Manual PCI PCI steps Robotic - assisted PCI Assess Anatomy Navigate Measure Anatomy Position Stent Deploy Stent Traditional PCI vs CorPath Robotic PCI Robotic precision may improve outcomes, economics and safety Today’s Cath Lab Environment • High radiation exposure • Significant fatigue and orthopedic strain Robotic Cath Lab • Shields from radiation • Potential to reduce fatigue and orthopedic strain 9

CorPath Clinical Benefit 10 Bedside improvements to enhance workflow Open architecture Potential to improve patient outcomes Evidence of success in complex PCI 1mm movements for precise positioning Sub mm measurement to select the most appropriate stent Reduce stent utilization Radiation protection to the physician Potential to reduce long - term orthopedic issues Smilowitz et al, “Robotic - Enhanced PCI Compared to the Traditional Manual Approach”, J Invasive Cardiol, 2014;26(7):318 - 321 . Campbell PT. et al. The Impact of Precise Robotic Lesion Length Measurement on Stent Length Selection: Ramification for stent sa vings. Cardiovasc Revasc Med. 2015;piii:S1553 - 8389 . Weisz , G. et al. Safety and Feasibility of Robotic Percutaneous Coronary Intervention: PRECISE Study. J Am Coll Cardiol. 2013;61(1 5): 1596 - 1600. PRECISE Trial was conducted with the CorPath 200 System. Clinical trials were conducted using the CorPath 200 System
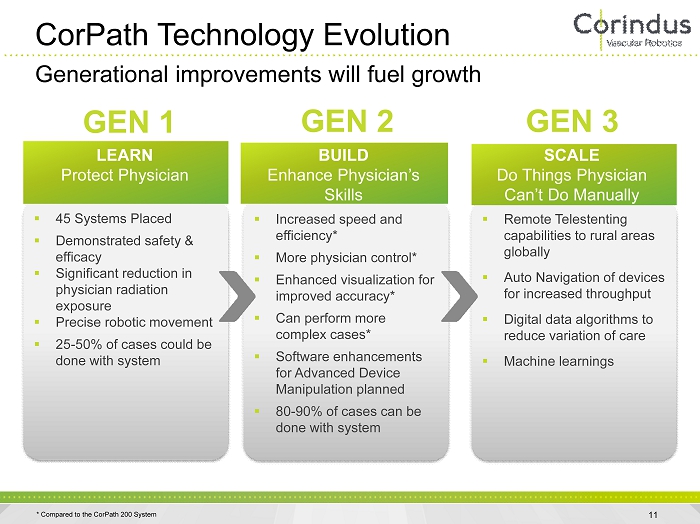
CorPath Technology Evolution 11 Generational improvements will fuel growth ▪ 45 Systems Placed ▪ Demonstrated safety & efficacy ▪ Significant reduction in physician radiation exposure ▪ Precise robotic movement ▪ 25 - 50% of cases could be done with system ▪ Increased speed and efficiency* ▪ More physician control* ▪ Enhanced visualization for improved accuracy* ▪ Can perform more complex cases* ▪ Software enhancements for Advanced Device Manipulation planned ▪ 80 - 90% of cases can be done with system ▪ Remote Telestenting capabilities to rural areas globally ▪ Auto Navigation of devices for increased throughput ▪ Digital data algorithms to reduce variation of care ▪ Machine learnings BUILD Enhance Physician’s Skills SCALE Do Things Physician Can’t Do Manually GEN 1 GEN 2 GEN 3 LEARN Protect Physician * Compared to the CorPath 200 System

The Next Generation is Here 12 CorPath GRX System * Compared to the CorPath 200 System IMPROVED WORKFLOW FOR BEDSIDE USER* • Extended reach arm • Bedside touchscreen for step - by - step instructions INCREASED PROCEDURAL CONTROL FOR PHYSICIAN* • Active Guide Catheter Management • 40’’ Power Vision Monitor

Building Robust Robotic Programs Driving strategic growth 13 Commercial Focus on Developing New Programs and Growing Adoption Team Approach Commitment to Building a Program Ongoing Training Adequate Procedural Volume Physicians, techs, nurses and administration Buy in from all stakeholders Basic, intermediate and advanced Support multiple users

Addressing Healthcare Challenges Overdue paradigm shift into the digital age 14 Safer Work Environment Safer Work Environment Reduce Cost Expand Access To Care Improve Efficiency Enhance Quality

Future of Cardiovascular Robotics 15 The only platform for advanced capabilities Expanded compatibility, precise actuation, multiple device control, quick exchange. Advanced Device Manipulation Algorithms to guide peri - procedure lesion assessment, treatment plan, and device selection. Prescriptive Analytics Automated wire techniques scaled to full auto - navigation of wires and catheters. Robotic - Assisted Procedures Tele - treatment capabilities spanning from remote case proctoring to remote catheterization. Remote Capabilities Planned features aimed at improving workflow, expanding access, and reducing variability of care.

Technology Enablers 16 Third - party technologies that compliment robotics Advanced Imaging ▪ 3D Image Reconstruction ▪ CT Fractional Flow Reserve (FFR ) ▪ Angiographic FFR Data Integration ▪ Machine Learnings ▪ Descriptive and Prescriptive Data ▪ Algorithms Autonomous ▪ Sensors ▪ Mapping ▪ Closed Loop Movement Communication ▪ Advancement of 5G ▪ Security Solutions ▪ Latency Solutions ▪ No Geographic Boundaries

Telestenting First - in - Human Study Study showed 86.4% technical success and 95% procedural success 17 REMOTE - PCI REMOTE - PCI Study explored feasibility of remote telestenting using a robotic system Single - center prospective observational study performed at Spectrum Health, Grand Rapids, MI 20 patients treated via physician at remote cockpit leveraging telehealth technology Madder R, et al. “Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location f rom the patient: an early exploration into the feasibility of telestenting (the REMOTE - PCI study ).” Eurointervention , 2017;12:1569 - 1576. This study, conducted under local IRB, may involve off - label usage and was not sponsored by Corindus.

Vascular Robotic Clinical Roadmap Demonstrating excellence in multiple lesion types and anatomies Product Evolution Robotic system capability Exploratory Feasibility Expand Use NEURO PERIPHERAL PCI 18 STRUCTURAL CorPath 200 and CorPath GRX Systems are indicated for PCI. Only the CorPath 200 System is indicated for use in peripheral vascular interventions. CorPath Systems are not indicated for use in neuro or structural interventions. • Below the Knee • Ostial Stenting • Atherectomy • Drug Eluting Balloons • Left Main Intervention • Complex PCI & CTO • Ostial Lesion • Bioabsorbable Stents • Staff Radiation Protection • Outcomes • Remote PCI • Exploratory Work

19 Corindus Vascular Robotics Strategic Objectives Near Term Mid to Long Term Mid to Long Term x Recurring revenue streams and NG3 system launch x Expansion into additional disease states (neurovascular and structural heart) x Global expansion and remote tele - proctoring, with specific focus on China x Establish at least 25 new robotic programs x Pursue co - development opportunities, add at least one additional collaboration x Drive system utilization x Prepare Japan distributor for launch x Ramp up educational and training opportunities for physicians x Further clinical trial development

1 As of December 31, 2016 2 As of September 30, 2016 3 Outstanding principal balance 4 Per our press release filed on January 17, 2017 Financial Snapshot Key Financial Metrics Cash and Cash Equivalents 1 $9.2 million Debt 2,3 $4.7 million Basic Shares Outstanding 2 119,025,221 Guidance Year Robotic Programs Revenue 2017 Establish at least 25 new robotic programs $13 - $15 million 4 20

Corindus Vascular Robotics, Inc. is a global technology leader in robotic - assisted vascular interventions. The company's CorPath ® System is the first FDA - cleared medical device to bring robotic - assisted precision to percutaneous coronary interventions. With the CorPath System, Corindus Vascular Robotics brings robotic precision to interventional procedures to help optimize clinical outcomes and minimize the costs associated with complications of improper stent placement with manual procedures. Visit us at www.corindus.com About Corindus Vascular Robotics 21
