Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - ULTRATECH INC | utekex32110-k2016.htm |
| EX-31.2 - EXHIBIT 31.2 - ULTRATECH INC | utekex31210k2016.htm |
| EX-31.1 - EXHIBIT 31.1 - ULTRATECH INC | utekex31110-k2016.htm |
| EX-23 - EXHIBIT 23 - ULTRATECH INC | exhibit232016.htm |
| EX-21 - EXHIBIT 21 - ULTRATECH INC | exhibit212016.htm |
| EX-10.35 - EXHIBIT 10.35 - ULTRATECH INC | exhibit1035.htm |
| EX-10.34 - EXHIBIT 10.34 - ULTRATECH INC | exhibit1034.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
————————————————————
FORM 10-K
(Mark one)
ý | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the Fiscal Year Ended December 31, 2016
Or
¨ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File Number: 0-22248
————————————————————
ULTRATECH, INC.
(Exact name of registrant as specified in its charter)
Delaware | 94-3169580 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
3050 Zanker Road San Jose, California | 95134 |
(Address of principal executive offices) | (Zip Code) |
(408) 321-8835
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered |
Common Stock, $0.001 Par Value Per Share | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No ý
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No ý
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ | Accelerated filer ý | |
Non-accelerated filer ¨ | (Do not check if a smaller reporting company) | Smaller reporting company ¨ |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No ý
The aggregate market value of voting stock held by non-affiliates of the Registrant, as of July 1, 2016, was approximately $195,162,101 (based upon the closing price for shares of the Registrant’s common stock as reported by the NASDAQ Global Market on that date, the last trading date of the Registrant’s most recently completed second quarter). Shares of common stock held by each officer, director and holder of 5% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of January 27, 2017, the Registrant had 26,950,338 shares of common stock outstanding.
————————————————————
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for the 2017 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
Ultratech, Inc.
Index
2
PART I
ITEM 1.
BUSINESS
This Annual Report on Form 10-K contains, in addition to historical information, certain forward-looking statements that involve significant risks and uncertainties, which are difficult to predict, and are not guarantees of future performance. Such statements can generally be identified by words such as “anticipates,” “expects,” “remains,” “thinks,” “intends,” “may,” “will,” “could,” “believes,” “poised,” “estimates,” “continue,” and similar expressions. Our actual results could differ materially from the information set forth in any such forward-looking statements. Factors that could cause or contribute to such differences include those discussed below, as well as those discussed under “Item 1A. Risk Factors” and elsewhere in this Annual Report on Form 10-K.
The Company
Ultratech, Inc. (referred to herein as “Ultratech,” the “Company,” “we,” “our” or “us”) develops, manufactures and markets photolithography, laser thermal processing, and inspection equipment designed to reduce the cost of ownership for manufacturers of semiconductor devices, including advanced packaging processes and various nanotechnology components such as laser diodes, high-brightness light emitting diodes (“HBLEDs”) and Micro-Electro-Mechanical Systems (“MEMS”) as well as atomic layer deposition systems (“ALD”) for customers located throughout the world. Ultratech was incorporated in the state of Delaware in 1992.
Recent Developments
On February 2, 2017, the Company, Veeco Instruments Inc., a Delaware corporation (“Veeco”) and Ulysses Acquisition Subsidiary Corp., a Delaware corporation and a wholly owned subsidiary of Veeco (“Merger Subsidiary”), entered into an Agreement and Plan of Merger (the “Merger Agreement”), pursuant to which, among other things, Merger Subsidiary will be merged with and into the Company (the “Merger”), with the Company surviving the Merger as a wholly owned subsidiary of Veeco, subject to the terms and conditions of the Merger Agreement. The boards of directors of both Veeco and the Company have approved the transaction. The closing of the Merger is subject to the adoption of the Merger Agreement by the affirmative vote of the holders of at least a majority of the outstanding shares of our common stock and to various other customary conditions, including the expiration or termination of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, (the “HSR Act”) (with respect to which the U.S. Federal Trade Commission granted early termination on February 17, 2017), the absence of any temporary restraining order, preliminary or permanent injunction or other judgment issued by any court of competent jurisdiction enjoining or otherwise prohibiting the consummation of the Merger and the SEC having declared effective a registration statement on Form S-4 with respect to the shares of Veeco common stock issuable in connection with the Merger. In addition, it is a condition to closing of the Merger that the Company and its subsidiaries, on a consolidated basis, have at least $180 million of cash on hand held in the United States. Pursuant to the Merger Agreement, and subject to the terms and conditions contained therein, upon the closing of the Merger, each share of our common stock outstanding immediately prior to the closing of the Merger (other than shares of our common stock owned by us as treasury shares or owned by Veeco, any of its subsidiaries, any of our subsidiaries or holders of our common stock, if any, who properly exercise their appraisal rights under the General Corporation Law of the State of Delaware) will be converted into the right to receive (i) $21.75 in cash without interest and (ii) 0.2675 of a share of Veeco common stock.
On February 17, 2017, we received notice from the U.S. Federal Trade Commission that it had granted early termination, effective immediately, of the applicable waiting period under the HSR Act, for the Merger. The early termination of the waiting period under the HSR Act satisfies one of the conditions to the closing of the Merger, which remains subject to other closing conditions, including the adoption of the Merger Agreement by requisite vote of our stockholders.
Our Business
Lithography and ALD
We supply step-and-repeat photolithography systems based on one-to-one (“1X”) imaging technology to customers located throughout North America, Europe and Asia. We believe that our 1X steppers utilizing the Wynne Dyson optical design offer cost and performance advantages, as compared with competitors’ contact aligners or reduction steppers, to semiconductor
3
device manufacturers for applications involving line geometries of 0.75 microns or greater (“non-critical feature sizes”) and to nanotechnology manufacturers.
Advanced packaging manufacturing processes such as flip chip, wafer level chip scale packaging (“WLCSP”) and 3D packaging techniques, require several lithography steps in the device fabrication process. We believe that the use of flip chip technology will continue to gain traction as customers migrate towards leading edge technology nodes. It is expected that wafer level packaging technologies will continue to play an important role for continued miniaturization of electronic products such as smartphones. As customers transition to sub-28 nanometers (“nm”) manufacturing technology, traditional front end scaling is becoming increasingly complex. As such, semiconductor manufacturing companies are now also focusing on various packaging technologies such as Fan Out Wafer Package (“FO-WLP”) and Through Silicon Via (“TSV”) to play an important role in delivering improved system level performance in the future. The manufacturing approach utilizing FO-WLP or TSV technology alleviates interconnect delay considerations by reducing global interconnect wiring length. These advanced packaging techniques deliver superior bandwidth performance, power management improvements and address some device latency issues. In addition, several of our customers are also evaluating other packaging approaches such as silicon interposer technology solutions for delivering improved bandwidth and system level performance.
Lithography is one of the critical process steps that affect the final device performance and associated yield for advanced packaging technology. The use of 1X stepper technology provides superior operational flexibility for thick resists utilized in the advanced packaging market. Furthermore, the use of our proprietary alignment systems enables easy insertion of our products for any back end of line application. Lastly, we have also developed a large number of application specific features which deliver technology leadership and superior economic value for our packaging customers. Our steppers are used to manufacture high volume, low cost semiconductor devices used in a variety of applications such as communications, personal computing, automotive control systems, power systems and consumer electronics. We believe that our steppers offer advantages over certain competitive reduction lithography tools with respect to field size, throughput, specialized substrate handling and cost of ownership savings.
In December 2012, we purchased certain assets of Cambridge NanoTech, Inc. (“Cambridge”) which was a technological leader in the ALD market. We will continue to pursue selling opportunities in the ALD market focusing our efforts on universities and research institutes. Additionally, we may sell these products for production applications.
Laser Anneal Technology
Device scaling has been the predominant means pursued by the semiconductor industry to achieve the gains in productivity and performance quantified by Moore’s Law. In the past several years, scaled device performance has been compromised because traditional transistor materials, such as silicon, silicon dioxide, and polysilicon, have been pushed to their fundamental materials limits. Continued scaling thus requires the introduction of new materials. For example, the traditional gate dielectric has been silicon dioxide, and as devices are scaled below 45 nm, high-k material such as hafnium oxide must be considered because silicon dioxide begins to lose its effectiveness. These new materials impose added challenges to the methods used to dope and activate silicon to produce very shallow, highly activated junctions (the main challenges regarding short channel effects include achieving maximum activation and minimal diffusion while maintaining abrupt junctions).
By leveraging our core competencies in optics engineering and system integration and our extensive knowledge of laser thermal processing, we introduced our laser spike annealing system to enable thermal annealing solutions at the 65 nm technology node and below. This advanced annealing technology provides solutions to the difficult challenge of fabricating ultra-shallow junctions and highly activated source/drain contacts. Laser thermal processing offers the flexibility to operate at near-instantaneous timeframes (microseconds to milliseconds) at temperatures below the melting point of silicon (1412° C). At these temperatures and anneal times, high activation is achieved with negligible diffusion. In addition, our proprietary hardware design minimizes the pattern density effect, reducing absorptivity variations resulting in a uniform temperature across the wafer.
Inspection
In 2012, we introduced a new in-line wafer inspection system, based on patented coherent gradient sensing technology (“CGS”) named Superfast. The CGS technology was developed at the California Institute of Technology during the 1990’s, and we acquired the rights to develop the CGS technology when we acquired certain assets of a semiconductor inspection startup, Oraxion Inc., in July 2006. We developed a next-generation system in 2007 which was primarily used internally. Our latest model introduced in 2015, Superfast 4G+, represents the fourth generation of this technology, which is focused on the inspection of a number of critical semiconductor process steps.
4
Our products and markets are more fully described below.
General Background
The fabrication of devices such as integrated circuits (“semiconductors” or “ICs”) requires a large number of complex processing steps, including deposition, photolithography and etching.
Photolithography is one of the most critical and expensive steps in IC device manufacturing. Photolithography exposure equipment is used to create device features by patterning a light-sensitive polymer coating on the wafer surface using a photomask containing the master image of a particular device layer. Typically, each exposure results in the patterning of a different deposited layer, and therefore requires a different pattern on the device. Each new device layer must be properly aligned to previously defined layers before imaging takes place, so that structures formed on the wafers are correctly placed, one on top of the other, in order to ensure a functioning device.
Since the introduction of the earliest commercial photolithography tools for IC manufacturing in the early 1960s, a number of tools have been introduced to enable manufacturers to produce ever more complex devices that incorporate progressively finer line widths. In the early 1970s, photolithography tools included contact printers and proximity aligners, which required the photomask to physically contact or nearly contact the wafer in order to transfer the entire pattern during a single exposure. By the mid-1970s, there were also projection scanners, which transferred the device image through reflective optics having a very narrow annular field that spanned the width of the wafer. Exposure was achieved by scanning the entire photomask and wafer in a single, continuous motion across the annular field. Scanners were followed by steppers, which expose a rectangular area or field on the wafer containing one or more chip patterns in a single exposure, then move or “step” the wafer to an adjacent site to repeat the exposure. This stepping process is repeated as often as necessary until the entire wafer has been exposed. By imaging a small area, steppers are able to achieve finer resolution, improved image size control and better alignment between the multiple device layers resulting in higher yield and higher performance devices than was possible with earlier tools.
The two principal types of steppers currently in use by the semiconductor industry are reduction steppers, which are the most widely-used steppers, and 1X steppers. Reduction steppers, which typically have reduction ratios of four- or five-to-one, employ photomask patterns that are four or five times larger than the device pattern that is to be exposed on the wafer surface. In addition, there is a fourth generation of lithography tools, known as step-and-scan systems that typically address device sizes of 0.35 micron and below. In contrast to steppers, which require lenses that cover the entire field, step-and-scan optical systems have an instantaneous field just large enough to span the width of a field and employ scanning to stretch coverage over the entire field. Each scan is followed by re-registration of the wafer with respect to the mask, i.e. “stepping”, to create multiple fields covering the entire wafer. The smaller instantaneous field size of step-and-scan system projection optical systems allows them to resolve finer geometries and scanning allows them to cover larger fields.
The principal advantage of reduction steppers and step-and-scan systems is that they may be used in manufacturing steps requiring critical feature sizes and are, therefore, necessary for manufacturing advanced ICs. 1X steppers, on the other hand, employ photomask patterns that are the same scale as the device pattern that is exposed on the wafer surface. The optical projection system employed in our 1X steppers is based on a Wynne Dyson design, which uses both a reflective mirror and refractive lens elements. This design approach leads to a very simple and versatile optical system that is less expensive than those employed in reduction steppers. Because our 1X optical design covers a much broader spectral range than reduction steppers, it delivers a greater proportion of the exposure energy from the lamp to the wafer surface. Depending on the size of the lamp used and the exposure energy required for an application, this can result in appreciably higher throughput. Resolution considerations currently limit 1X steppers to manufacturing steps involving less-critical, larger feature sizes. Accordingly, we believe that sales of these systems are highly dependent upon capacity expansions by our current 1X customers, or by customers making the transition to chips containing “bump” connections, that facilitate the use of higher data rates and a higher number of connections.
In the past, manufacturers of ICs and similar devices purchased capital equipment based principally on performance specifications. In view of the significant capital expenditures required to construct, equip and maintain advanced fabrication facilities, relatively short product cycles and manufacturers’ increasing concern for overall fabrication costs, we believe that focus has shifted to the total cost of ownership. Cost of ownership includes the costs associated with the acquisition of equipment, as well as components based on throughput, yield, up-time, service, labor overhead, maintenance, and various other costs associated with owning and using the equipment. As a result, in many cases, the most technologically advanced system will not necessarily be the manufacturing system that delivers the best cost of ownership.
5
In addition to enhancing our current lithography solutions, we continue to develop new tools to serve markets such as advanced annealing. The LSA family of tools is aimed at volume production of advanced semiconductors. These products, based on the same platform and stage technology as our advanced lithography tools, employ a 3500 Watt carbon dioxide laser to activate ultra-shallow, transistor junctions. Annealing times are reduced from several seconds, typical for the current generation of Rapid Thermal Processing (“RTP”) equipment, to a millisecond or less. This results in more abrupt junctions with higher dopant activation levels and leads to transistors with higher drive currents and lower leakage. While this technology is expected to be useful for multiple IC generations, we anticipate that eventually this technology will be superseded by a laser thermal processing technology that will exceed the melting point of silicon (1412°C) and reduce the processing time below one microsecond, thereby achieving even higher performance characteristics with almost “zero” thermal budget. We continue to develop our new LXA laser melt technology targeted to deliver this capability. Melt annealing has the potential to be used for multiple generations across the front end of line (“FEOL”) and middle of line (“MOL”). We believe these new laser thermal processing technologies remove several critical barriers to future device scaling and will help to extend Moore’s Law well into the future.
Thermal annealing is used by the semiconductor industry for a variety of process steps, including activation of implanted impurities, dopant activation, dielectric film formation, formation of silicides and stabilization of copper grain structures. Our LSA systems compete with annealing tools currently in use by manufacturers of semiconductor devices, including both furnaces and RTP systems. It is widely recognized that there is a need for tools that anneal at higher temperatures for shorter periods of time. We believe our current laser annealing tools are already providing this capability in a production environment for dopant activation. Our customers are also working to develop other applications for our laser annealing tools such as high-k dieletric anneal and silicide formation.
As the next-generation device nodes are developed, the inspection requirements are rapidly evolving. Tighter device specifications, new materials and new device geometries necessitate new ways to identify and eliminate the sources of defects that compromise device performance and reduce yield. The cost of these defects is also increasing; not only do individual wafers have more value, but more importantly, rapid resolution of defects and process issues can dramatically reduce time-to-market by enabling the ramp to high volume production sooner. Traditional inspection techniques can often be difficult to extend without significant increase in cost of ownership or decrease in throughput. New inspection techniques that are accurate, precise, rapid and provide large amounts of data are essential for providing device manufacturers the insight into efficiently resolving manufacturing issues during development and high-volume production alike.
Products
We currently offer two different series of 1X lithography systems for use in the semiconductor fabrication process: the 1000 series, which addresses the markets for HBLED, semiconductor fabrication and nanotechnology applications; and the AP series, which is designed to meet the requirements of the advanced packaging market. These steppers currently offer minimum feature size capabilities ranging from 2.0 microns to 0.75 microns.
The 1000 series systems are small field systems available with gh-line, i-line and broadband ghi-line illumination options. We offer the Sapphire 100C and Sapphire 100E for HBLED applications and the Star 100 for semiconductor and nanotechnology applications. These 1000 series platform systems are typically used in the manufacture of HBLEDs, power devices, ASICs, analog devices and compound semiconductors as well as a number of nanotechnology applications.
Nanotechnology manufacturing combines electronics with mechanics in small devices. We have defined a nanotechnology device as a device that has at least one dimension in the XYZ direction less than 0.1 microns. Examples include accelerometers used to activate air bags in automobiles and membrane pressure sensors used in industrial control systems. These micro-machined devices are manufactured on silicon substrates using photolithography techniques similar to those used for manufacturing semiconductors.
Our Sapphire 100C and Sapphire 100E systems are configurable with customized options designed specifically for high volume HBLED manufacturing applications. The Sapphire 100C and Sapphire 100E are also based on the 1000 Series platform, with additional features developed specifically for HBLED lithography applications. HBLED manufacturing requires special substrate handling capabilities for the small diameter sapphire and silicon carbide substrates used to manufacture the LED devices for display backlighting and general lighting applications. For HBLED applications, we believe our Sapphire 100C and our Sapphire 100E steppers offer depth of focus, productivity and yield improvement advantages over competitive product offerings.
For the advanced packaging market, we offer our AP series built on the Unity Platform®. These advanced packaging systems were developed for high volume flip-chip, wafer-level and 3-D packaging applications. They provide broadband or
6
selective exposure (gh, i, or ghi-line), and are used in conjunction with downstream processes to produce a pattern of bumps, or metal connections, on the bond pads of the die for flip chip devices. Using flip chip interconnect may result in reduced signal inductance, reduced power/ground inductance, die shrink advantages and reduced package footprint.
The AP series, consisting of the AP300W, AP300 and AP200, is built on our Unity Platform and features a customer-configurable design that supports flexible manufacturing requirements as well as tool extendibility for multiple device generations. Designed specifically for advanced packaging applications, the AP systems integrate the processing advantages associated with our advanced packaging lithography equipment with the productivity benefits of our Unity Platform. We believe that this family of lithography systems support a lower cost-of-ownership strategy due to significant throughput enhancements, higher reliability, and superior alignment and illumination systems.
We offer a family of advanced laser-based thermal annealing tools built on our Unity Platform. In addition to our flagship model, the LSA101, which enables junction activation and other advanced front-end annealing processes for the 45nm node and below, we have introduced a novel dual beam technology extending the system's capabilities to lower processing temperatures (LSA101LP). The addition of the LP dual beam is targeted to enable MOL applications such as silicide formation. The LSA201 is our latest LSA product offering, which enables full-wafer ambient control processing targeted for sub-20nm nodes. The LSA201's simple and robust design includes our patented micro-chamber design for achieving ambient control in a scanning system. The LSA201 platform can also be configured with the dual beam laser mentioned above-named the LSA201LP. As devices scale below 20nm, we believe the ambient control capability enables new applications such as high-k anneal and film property modification.
In 2012, we introduced our new in-line wafer inspection system, the Superfast 3G, which provides the flexibility to address a wide range of applications including improved overlay control and enhanced yield. In 2015, we released our next generation version, the Superfast 4G+. At advanced technology nodes, variations in stress and distortion on a wafer can have a significant impact on device performance and yield. Based on CGS technology, we believe that our Superfast system provides the industry’s highest wafer resolution for the targeted applications and measures over 3 million points of data per 300mm wafer. The Superfast system is unique in that it collects data from the whole wafer simultaneously, providing significantly higher data density without the need for targets or pads. The system’s high data density enables a wide range of applications from one measurement, as within-die, die-to-die and wafer-to-wafer process variations can be characterized quickly and comprehensively. Utilizing the Superfast system provides other key advantages as well. First, data is obtained within each die at the location of the devices (not the location of a test target) and within-die variations can be mapped via approximately 10,000 data points. Second, data acquisition is inherently faster using rapid image capture of the whole wafer with a camera. Third, data is obtained to within 2mm of the wafer edge, providing a means to characterize issues with edge-die, which dominate yield loss at leading edge nodes.
In addition to selling new systems, we sell upgrades to systems in our installed base.
Research, Development and Engineering
The semiconductor and nanotechnology industries are subject to rapid technological change and new product introductions and enhancements. We believe that continued and timely development and introduction of new and enhanced systems to serve these markets is essential for us to maintain our competitive position. We have made and continue to make substantial investments in the research and development of our core optical technology, which we believe is critical to our future financial results. We intend to continue to develop our technology and to develop innovative lower cost of ownership products and product features to meet customer demands. Current engineering projects include continued research and development and process insertion for our laser thermal processing technologies, continued development of our 1X stepper products ongoing evolution of our inspection technologies, as well as other new products. Other research and development efforts are currently focused on: performance enhancement and development of new features for existing systems, both for inclusion as a standard component in our systems and to meet special customer order requirements; increased productivity; reliability improvements; and manufacturing cost reductions. These research and development efforts are principally undertaken, by our research, development and engineering organizations and costs are generally expensed as incurred. Other operating groups within Ultratech support our research, development and engineering efforts, and the associated costs are expensed as incurred.
We work with many customers to jointly develop technology required to manufacture advanced devices or to lower the customer’s cost of ownership. We also have a worldwide engineering support organization including reticle engineering, photo processing capability and applications support.
7
We have historically devoted a significant portion of our financial resources to research and development programs and expect to continue to allocate significant resources to these efforts in the future. As of December 31, 2016, we had approximately 80 full-time employees engaged in research, development and engineering. For 2016, 2015 and 2014, total research, development and engineering expenses were approximately $35.2 million, $32.9 million and $33.6 million, respectively, and represented 18%, 22% and 22% of our net sales, respectively.
Sales and Service
We market and sell our products in North America, Europe and Asia principally through our direct sales organization. We also have service personnel based throughout the United States, Europe, Japan and the rest of Asia. We believe that as semiconductor and nanotechnology device manufacturers produce increasingly complex devices, they will require an increased level of support. Global support capability as well as product reliability, performance, yield, cost, uptime and mean time between failures are increasingly important factors by which customers evaluate potential suppliers of photolithography equipment. We believe that the strength of our worldwide service and support organization is an important factor in our ability to sell our systems, maintain customer loyalty and reduce the maintenance costs of our systems. In addition, we believe that working with our suppliers and customers is necessary to ensure that our systems are cost effective, technically advanced and designed to satisfy customer requirements.
We support our customers with field service, applications, technical support service engineers and training programs. We provide our customers with comprehensive support and service before, during and after delivery of our systems. To support the sales process and to enhance customer relationships, we work closely with prospective customers to develop hardware, applications, test specifications and benchmarks, and often design customized applications to enable prospective customers to evaluate our equipment for their specific needs. Prior to shipment, our support personnel typically assist the customer on-site preparation and inspection, and provide customers with training at our facilities or at the customer’s location. We currently offer our customers various courses of instruction on our systems, including instructions in system hardware and related applications tools for optimizing our systems to fit a customer’s particular needs. Our customer training program also includes instructions in the maintenance of our systems. Our field support personnel work with the customer to install the system and demonstrate system readiness. Technical support is also available via telephone 24 hours a day, seven days a week at our San Jose, California and Singapore locations and through our on-site personnel.
In general, we warrant our new systems against defects in design, materials and workmanship for one year. We offer our customers additional support after the warranty period for a fee in the form of service contracts for specified time periods. Service contracts include various options such as priority response, planned preventive maintenance, scheduled one-on-one training, daily on-site support, and monthly system and performance analysis.
Manufacturing
Until the third quarter of 2010, we performed all of our manufacturing activities (final assembly, system testing and certain subassembly) in clean room environments totaling approximately 25,000 square feet located in San Jose, California. Performing manufacturing operations in California exposes us to a higher risk of natural disasters, including earthquakes. In addition, California has previously experienced power shortages which have interrupted our operations. Such shortages could occur in the future and could again interrupt our operations resulting in product shipment delays, increased costs and other problems, any of which could have a material adverse effect on our business, customer relationships and results of operations.
Beginning in the fourth quarter of 2010, we started a manufacturing operation in Singapore for the production of our lithography and inspection products. This facility consists of approximately 9,000 square feet of additional clean-room production space for the manufacturing of our advanced packaging Unity AP products, HBLED, Sapphire and Superfast inspection product platforms.
Our manufacturing activities consist of assembling and testing components and subassemblies, which are then integrated into finished systems. We rely on a limited number of outside suppliers and subcontractors to manufacture certain components and subassemblies. We order one of the most critical components of our technology, the glass for our 1X lenses, from external suppliers. We design the 1X lenses and provide the lens specifications and the glass to external suppliers, who then machine the lens elements. We then assemble and test the optical 1X lenses. We have recorded the critical parameters of each of our optical lenses sold since 1988, and we believe that such information enables us to supply lenses to our customers that match the characteristics of our customers’ existing lenses.
We procure some of our other critical systems’ components, subassemblies and services from single outside suppliers or from a limited group of outside suppliers in order to ensure overall quality and timeliness of delivery. Many of these
8
components and subassemblies have significant production lead times. To date, we have been able to obtain adequate services and supplies of components and subassemblies for our systems in a timely manner. We are actively engaged with a number of our Asia-based suppliers to provide high precision parts and major opto-mechanical and electro-mechanical sub-assemblies and modules for our lithography products both in Singapore and San Jose. However, disruption or termination of certain of these sources could result in a significant adverse impact on our ability to manufacture our systems. This, in turn, would have a material adverse effect on our business, financial condition and results of operations. Our reliance on a sole or a limited group of suppliers and our reliance on subcontractors exposes us to several risks, including a potential inability to obtain an adequate supply of required components due to the suppliers’ failure or inability to provide such components in a timely manner, or at all, to our standards of quality, and reduced control over pricing and timely delivery of components. Manufacture of certain of these components and subassemblies is an extremely complex process, and long lead-times are required. Any inability to obtain adequate deliveries or any other circumstance that would require us to seek alternative sources of supply or to manufacture such components internally could delay our ability to ship our products, which could damage relationships with current and prospective customers and have a material adverse effect on our business, financial condition and results of operations.
We maintain a company-wide quality and environmental program. Our San Jose operations achieved ISO 9001:1994 certification in 1996 and ISO 14001:1996 certification in March 2001. Our San Jose ISO 9001 certification was upgraded to the ISO 9001:2000 standard in January 2002, and to the ISO 9001:2008 standard in June 2010. Our San Jose ISO 14001 certification was upgraded to the ISO 14001:2004 standard in June 2006. Our ISO 9001 and 14001 certifications were expanded to our Singapore operation in August 2011. All certifications have been maintained uninterrupted through the date of this report.
Competition
The capital equipment industry in which we operate is intensely competitive. A substantial investment is required to install and integrate capital equipment into a semiconductor, semiconductor packaging or nanotechnology device production line. We believe that once a device manufacturer or packaging subcontractor has selected a particular supplier’s capital equipment, the manufacturer generally relies upon that equipment for the specific production line application and, to the extent possible, subsequent generations of similar products. Accordingly, it is difficult to achieve significant sales to a particular customer once another supplier’s capital equipment has been selected as they are relying on reduced cost of ownership solutions and a decision by a customer to change solutions could have a material and adverse effect on our business and operating results.
Advanced Packaging and HBLED Lithography
We experience competition in the advanced packaging lithography market from various reduction steppers and proximity and projection aligner companies such as Canon Incorporated, Nikon Corporation (“Nikon”), Suss Microtec AG (“Suss Microtec”), Rudolph Technologies, Inc. (“Rudolph”), Shanghai Micro Electronics Equipment Co., Ltd (“SMEE”), ORC Manufacturing Co., Ltd., Ushio and from third party re-sale of used projection systems. We expect our competitors to continue to improve the performance of their current products and to introduce new products with improved price and performance characteristics. This could cause a decline in sales or loss of market acceptance of our steppers in our served markets, and thereby materially adversely affect our business, financial condition and results of operations. Enhancements to, or future generations of, competing products may be developed that offer superior cost of ownership and technical performance features. We believe that to be competitive, we will require significant financial resources to continue to invest in new product development, to invest in new features and enhancements to existing products, to introduce new generation stepper systems in our served markets on a timely basis, and to maintain customer service and support centers worldwide. In marketing our products, we may also face competition from suppliers employing other technologies. In addition, increased competitive pressure has led to intensified price-based competition in certain of our markets, resulting in lower prices and margins. Should these competitive trends continue, our business, financial condition and operating results may be materially adversely affected.
Our primary competition in the HBLED lithography market comes from contact, proximity and projection aligners offered by companies such as Suss Microtec, Ushio and EV Group, by stepper companies such as SMEE, as well as by third party sales of used reduction steppers including Nikon and ASML. Although contact, proximity aligners, and used reduction steppers generally have lower purchase prices than new 1X steppers, 1X steppers offer lower operating costs and favorable total cost of ownership in most applications. We believe that most device and HBLED manufacturers choose 1X steppers for the yield improvement and lower modification costs offered by the use of non-contact projection lithography.
Laser Thermal Processing
9
With respect to our laser annealing technologies, marketed under the LSA product name, our primary competition comes from companies such as SCREEN Semiconductor Solutions Co., Ltd., Applied Materials, Inc. (“Applied Materials”) and Mattson Technology. Many of these companies also offer products utilizing RTP, which is the dominant manufacturing technology for devices above 65nm. The dopant diffusion in the lateral dimension resulting from the time scales associated with RTP limits the scaling of transistors to advanced technology nodes. Shorter annealing times result in shallower and more abrupt junctions and faster transistors. We believe that RTP manufacturers recognize the need to reduce thermal budgets and are working toward this goal. Several companies offer annealing tools that incorporate flash lamp anneal (“FLA”) technology, a potential advanced annealing solution, in order to reduce annealing times and increase anneal temperatures. This technique, which employs flash lamps, has shown improvements over RTP in junction depth and sheet resistance, but we believe FLA suffers from pattern-related non-uniformities and could require additional, costly processes to equalize the reflectivity of different areas within the chip or wafer. Our proprietary laser thermal processing solution has been specifically developed to provide junction annealing on near-instantaneous timescales, while achieving high activation levels, and doing so very uniformly across the chip and within the wafer due to our unique long wavelength technology. LSA, our first implementation of laser thermal processing, activates dopants in the microsecond-to-millisecond time frame without melting. Our research indicates that, at temperatures just below the melting point of silicon time durations in the microsecond to millisecond range are required to achieve full activation, with minimal dopant diffusion.
In July 2000, we licensed certain rights to our then existing laser thermal processing technology, with reservations, to a competing manufacturer of semiconductor equipment. This company and others currently offer laser annealing tools to the semiconductor industry that compete with our offerings.
Inspection
Competition for the Superfast inspection systems comes from a wide range of companies, including large companies such as KLA-Tencor, as well as numerous smaller companies developing and manufacturing inspection equipment such as Nanometrics, Rudolph, Frontier Semiconductor (FSM) and Nova Measuring Instruments. Similarly, there is an extremely wide range of technologies and approaches used by the competitors. Each individual technology will have advantages for certain specific inspection applications. In general, these competitors offer systems that inspect and measure at one location on the wafer, or one data point, at a time. This can limit overall data density to only a few hundred data points per wafer, as well as limit throughput. In addition, these competitive systems often require dedicated targets, pads or other special features in order to make a measurement, making them less flexible in production environments.
Intellectual Property Rights
Although we attempt to protect our intellectual property rights through patents, copyrights, trade secrets and other measures, we believe that our success will depend more upon the innovation, technological expertise and marketing abilities of our employees. Nevertheless, we have a policy of seeking patents when appropriate on inventions resulting from our ongoing research and development and manufacturing activities. Our intellectual property portfolio contains 1,248 patents and patent applications. We have patent expiration dates ranging from April 2017 to June 2035. In addition, we also have various registered trademarks and copyright registrations covering mainly applications used in the operation of our systems. We also rely upon trade secret protection for our confidential and proprietary information. We may not be able to protect our technology adequately and competitors may be able to develop similar technology independently. Our pending patent applications may not be issued or U.S. or foreign intellectual property laws may not protect our intellectual property rights. In addition, litigation may be necessary to enforce our patents, copyrights or other intellectual property rights, to protect our trade secrets, to determine the validity and scope of the proprietary rights of others or to defend against claims of infringement. Such litigation has resulted in, and in the future could result in, substantial costs and diversion of resources and could have a material adverse effect on our business, financial condition and results of operations, regardless of the outcome of the litigation. Patents issued to us may be challenged, invalidated or circumvented and the rights granted thereunder may not provide competitive advantages to us. Furthermore, others may independently develop similar technology or products, or, if patents are issued to us, design around the patents issued to us. Invalidation of our patents related to those technologies, or the expiration of patents covering our key technologies, could allow our competitors to more effectively compete against us, which could result in less revenue for us.
Environmental Regulations
We are subject to a variety of governmental regulations relating to the use, storage, discharge, handling, emission, generation, manufacture and disposal of toxic or other hazardous substances. We believe that we are currently in compliance in all material respects with such regulations and that we have obtained all necessary environmental permits to conduct our business. Nevertheless, the failure to comply with current or future regulations could result in substantial fines being imposed
10
on us, suspension of production, and alteration of the manufacturing process or cessation of operations. In order to comply with such regulations, we may be required to acquire expensive remediation equipment or to incur substantial expenses. Any failure by us to control the use, disposal or storage of, or adequately restrict the discharge of, hazardous or toxic substances could subject us to significant liabilities.
Customers, Applications and Markets
We sell our systems to semiconductor, advanced packaging, HBLED, MEMS and various other nanotechnology manufacturers located throughout North America, Europe and Asia. We sell our ALD systems to universities and research institutes throughout the world. Semiconductor manufacturers have purchased the 1000 Series steppers, the AP series of steppers, the NanoTech steppers, the LSA systems or the Superfast systems for the fabrication and/or packaging of microprocessors, applications processors, microcontrollers, DRAMs, ASICs and a host of other devices. Such systems could be used in mix-and-match applications with other production tools, such as for replacements of contact proximity printers for flip chip applications, and for high volume capacity production.
On a market application basis, sales to the semiconductor industry, primarily for advanced packaging and laser thermal processing applications, accounted for approximately 93% of systems revenue for the year ended December 31, 2016, as compared to 84% and 81% for the years ended December 31, 2015 and 2014, respectively. During the years ended December 31, 2016, 2015 and 2014, approximately 7%, 16% and 19%, respectively, of our systems revenue was derived from sales to nanotechnology manufacturers, including LED and MEMS manufacturers. Our future results of operations and financial position would be materially adversely impacted by a downturn in any of these industries, or by loss of market share in any of these industries.
International sales accounted for approximately 87%, 81% and 68% of total net sales for the years ended December 31, 2016, 2015 and 2014, respectively, with Asia representing 79%, 70% and 56% of total net sales for those same years and Europe representing 8%, 11% and 12% of total net sales for those same years, respectively.
Sales of our systems depend, in significant part, upon the decision of a prospective customer to increase manufacturing capacity or to restructure current manufacturing facilities, either of which typically involves a significant commitment of capital. Many of our customers in the past have cancelled or postponed the development of new manufacturing facilities and have substantially reduced their capital equipment budgets. In view of the significant investment involved in a system purchase, we have experienced and may continue to experience delays following initial qualification of our systems as a result of delays in a customer’s approval process. Additionally, we are presently receiving orders for some systems that have lengthy delivery schedules, which may be due to longer production lead times or a result of customers’ capacity scheduling requirements. For these and other reasons, our systems typically have a lengthy sales cycle during which we may expend substantial funds and management effort in securing a sale. Lengthy sales cycles subject us to a number of significant risks, including inventory obsolescence and fluctuations in operating results, over which we have little or no control. In order to maintain or exceed our present level of net sales, we are dependent upon obtaining orders for systems that will ship and be accepted in the current period. We may not be able to obtain those orders.
Backlog
We schedule production of our systems based upon order backlog, informal customer commitments and general economic forecasts for our targeted markets. We include in our backlog all accepted customer orders for our systems with assigned shipment dates within one year, as well as all orders for service, spare parts and upgrades, in each case, that management believes to be firm. However, all orders are subject to cancellation or rescheduling by the customer with limited or no penalties. Because of changes in system delivery schedules, cancellations of orders and potential delays in system shipments, our backlog at any particular date may not necessarily be representative of actual sales for any succeeding period. As of December 31, 2016, our backlog was approximately $77.2 million, including $1.9 million of products shipped but not yet installed. As of December 31, 2015, our backlog was approximately $67.6 million, including $1.3 million of products shipped but not yet installed. Cancellation, deferrals or rescheduling of orders by these customers would have a material adverse impact on our future results of operations.
Employees
At December 31, 2016, we had approximately 312 full-time employees, including 80 engaged in research, development and engineering, 31 in sales and marketing, 96 in customer service and support, 61 in manufacturing and 44 in general administration and finance. We believe our future success depends, in large part, on our ability to attract and retain highly skilled employees. None of our employees are covered by a collective bargaining agreement. We have, however, entered
11
into employment agreements with our Chief Executive Officer and Chief Financial Officer. We consider our relationships with our employees to be good.
Information Available at the Securities and Exchange Commission
Copies of any materials that we have filed with the Securities and Exchange Commission (“SEC”) can be viewed at the SEC’s Public Reference Room at 100 F Street NE, Washington, DC 20549. Information regarding the operations of the Public Reference Room can be obtained from the SEC by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains a website that contains reports, proxy and other information that we have filed with the SEC. The SEC website can be found at http://www.sec.gov.
Information Available on Our Website
Our website is located at http://www.ultratech.com. We make available, free of charge, through our website, our annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K (and amendments to those reports), as soon as reasonably practicable after such reports are filed electronically with the SEC.
ITEM IA. | RISK FACTORS |
In addition to risks described in the foregoing discussions under “Business,” including but not limited to those under “Products,” “Research, Development and Engineering,” “Sales and Service,” “Manufacturing,” “Competition,” “Intellectual Property Rights,” “Environmental Regulations,” “Customers, Applications and Markets,” “Backlog,” and “Employees,” the following risks apply to our business and us:
The semiconductor industry historically has been highly cyclical and has experienced periods of oversupply and technology changes, which have in turn affected the market for semiconductor equipment such as ours and which can adversely affect our results of operations during such periods.
Our business depends in significant part upon capital expenditures by manufacturers of semiconductors, advanced packaging semiconductors and nanotechnology components which in turn depend upon the current and anticipated market demand for such devices and products utilizing such devices. The semiconductor industry historically has been highly cyclical and has experienced recurring periods of oversupply and technology changes. This has, from time to time, resulted in significantly reduced demand for capital equipment and pricing pressure, including on the systems manufactured and marketed by us, and uncertainty among our customers. We believe that markets for new generations of semiconductors, semiconductor packaging and inspection will also be subject to similar fluctuations. Our business and operating results would be materially adversely affected by downturns or slowdowns in the semiconductor packaging market or by loss of market share. Accordingly, we may not be able to achieve or maintain our current or prior level of sales. We attempt to mitigate the risk of cyclicality by participating in multiple markets including semiconductor, semiconductor packaging, semiconductor inspection and nanotechnology sectors as well as diversifying into new markets. We believe that diversifying into new markets such as laser-based annealing for implant activation, lithography steppers for 3-D packaging, HBLED, ALD and inspection can help mitigate the effects of this cyclicality in our industry. Despite such efforts, when one or more of such markets fails to become viable as we anticipate or experience an oversupply or downturn, our net sales and operating results are materially adversely affected. For example, we have experienced less revenue from laser processing as a result of the semiconductor logic foundries’ inability to effectively increase yields in 3-D FinFET devices to a level required to invest in their production ramp and unpredictability in other markets that we serve. Customers who manufacture both logic and memory devices are expected to focus their capital spending on the memory sector at this time. We believe that as a result of cyclicality and of other factors affecting our foundry customers, such as shifting customer demand, lower capacity utilization rates and changing product designs, we have and we will continue to experience reduced sales, the push out of orders to us and uncertainty in our business.
Our quarterly revenues and operating results are difficult to predict.
Our revenues and operating results may fluctuate significantly from quarter to quarter due to a number of factors, not all of which are in our control. We manage our expense levels based in part on our expectations of future revenues, and a certain amount of those expenses are relatively fixed. As a result, a change in the timing of recognition of revenue or a change in margins or product mix can have a significant impact on our operating results in any particular quarter. Forecast uncertainty also makes it difficult for us to manage inventory levels. Factors that may cause our results of operations to fluctuate include, but are not limited to:
• | market conditions in the electronics and semiconductor industries; |
12
• | failure of suppliers to perform in a manner consistent with our expectations; |
• | customer cancellations or push-outs or delays in orders, shipments, installations and/or system acceptances; |
• | competitive factors, including customers selecting competitors’ products, the introduction of new products by our competitors or any failure of our products to gain or maintain market acceptance; |
• | changes in selling prices and product mix; |
• | manufacturing difficulties or delays; and |
• | product performance issues. |
We are dependent on our key personnel, especially Mr. Zafiropoulo, our Chief Executive Officer, and our business and results of operations would be adversely affected if we were to lose our key employees.
Our future operating results depend, in significant part, upon the continued contributions of key personnel, many of whom would be difficult to replace. We have entered into employment agreements only with our Chief Executive Officer and Chief Financial Officer, and our employees are employed “at will.” The agreements with our Chief Executive Officer and Chief Financial Officer contain vesting acceleration and severance payment provisions that could result in significant costs or charges to us should the employee be terminated without cause, die or become disabled. We do not maintain any life insurance on any of our key employees. The loss of key personnel could have a material adverse effect on our business, financial condition and results of operations. In addition, our future operating results depend in significant part upon our ability to attract and retain other qualified management, manufacturing, technical, sales and support personnel for our operations. There are only a limited number of persons with the requisite skills to serve in these positions and it may become increasingly difficult for us to hire such personnel over time. At times, competition for such personnel has been intense, particularly in the San Francisco Bay Area where we maintain our headquarters and principal operations, and we may not be successful in attracting or retaining such personnel. The failure to attract or retain such persons would have a material adverse effect on our business, financial condition and results of operations.
There are risks and uncertainties associated with the Merger with Veeco.
The Merger, whether or not consummated, may result in a loss of our key personnel and may disrupt our sales and marketing or other key business activities, including our relationships with customers, suppliers and other third parties, which may have an adverse impact on our financial performance. Our business relationships may be subject to disruption due to uncertainty associated with the Merger, which could have an adverse effect on our results of operations, cash flows and financial condition and, following the completion of the Merger, those of the combined company. Additionally, we have incurred and will continue to incur substantial financial advisory, legal, and other professional fees and expenses in connection with the Merger, which we must pay regardless of whether the Merger is completed. These payments will negatively impact our results of operations, cash flows and financial condition.
Parties with which we do business may be uncertain as to the effects on them from the Merger and the related transactions, including their current or future business relationships with us or the combined company. These relationships may be subject to disruption, as customers, suppliers and other persons with whom we have business relationships may delay or defer certain business decisions or might decide to terminate, change or renegotiate their relationships with us, or consider entering into business relationships with parties other than us or the combined company. These disruptions could have an adverse effect on our results of operations, cash flows and financial position or those of the combined company following the completion of the Merger. The risk, and adverse effect, of any disruption could be exacerbated by a delay in completion of the Merger or termination of the Merger Agreement.
The closing of the Merger is subject to customary closing conditions, including the adoption and approval of the Merger Agreement by an affirmative vote of the holders of at least a majority of the outstanding shares of our common stock, the expiration or termination of the applicable waiting period under the HSR Act, (with respect to which the U.S. Federal Trade Commission granted early termination on February 17, 2017); the absence of any temporary restraining order, preliminary or permanent injunction or other judgment issued by any court of competent jurisdiction enjoining or otherwise prohibiting the consummation of the Merger. In addition, it is a condition to closing of the Merger that the Company and its subsidiaries, on a consolidated basis, have at least $180 million of cash on hand held in the United States. These conditions are described in more detail in the Merger Agreement, which has been filed as Exhibit 2.1 to our Current Report on Form 8-K filed with the SEC on February 3, 2017. There is no assurance that all of the conditions set forth in the Merger Agreement will be satisfied or waived to the extent permitted by applicable law or that the Merger will occur when or as expected. If the Merger is not completed, the
13
share price of our common stock could decline, for reasons including the loss of the premium over the pre-announcement market price of our common stock that was to be paid upon consummation of the Merger.
Under certain circumstances set forth in the Merger Agreement, upon termination of the Merger Agreement by us or Veeco, we may be required to pay to Veeco a termination fee of $26.5 million, which could have a material adverse effect on our results of operations, cash flows and financial condition.
Some of our current and potential competitors have significantly greater resources than we do, and increased competition could adversely impact sales of our products and our gross margins.
We are a comparatively small company in the highly competitive semiconductor equipment industry where we face competition from a number of companies, many of which are larger and have greater financial, engineering, manufacturing, research and development, marketing and customer support resources than we do. As a result, our competitors may be able to devote greater resources to the development, promotion and sale of new products that are competitive with our products, which could negatively impact sales of our systems. Moreover, there has been merger and acquisition activity among our competitors and potential competitors. These transactions may result in our competitors accumulating an increased amount of resources allowing them to bring to market a portfolio of products and achieve a competitive “product grouping strategy” advantage over us. As a result, there is no assurance that we will continue to achieve market share at a desirable gross margin.
We operate in a highly competitive industry in which customers are required to invest substantial resources in each product, which makes it difficult to achieve significant sales to a particular customer once another vendor’s equipment has been purchased by that customer.
The capital equipment industry in which we operate is intensely competitive. A substantial investment is required to install and integrate capital equipment into a semiconductor, semiconductor inspection, semiconductor packaging or nanotechnology device production line. Advanced technologies have also tended to become more capital intensive.
We believe that once a device manufacturer or packaging subcontractor has selected a particular supplier’s capital equipment, the manufacturer generally relies upon that equipment for the specific production line application and, to the extent possible, subsequent generations of similar products. Accordingly, it is difficult to achieve significant sales to a particular customer once another supplier’s capital equipment has been selected.
We experience competition in advanced packaging from various proximity aligner and stepper companies such as Canon Incorporated, Nikon Corporation, ORC Manufacturing Co., Ltd., Rudolph, SMEE, Suss Microtec, Ushio as well as from the secondary market from used projection and reduction stepper systems. In nanotechnology, we experience competition from contact, proximity and projection aligner companies, such as Nikon Engineering, SMEE, Suss Microtec and Ushio as well as other third party stepper suppliers. We expect our competitors in the lithography arena to continue to improve the performance of their current products and to introduce new products with improved price and performance characteristics. This could cause a decline in sales or loss of market acceptance of our steppers in our served markets, and thereby materially adversely affect our business, financial condition and results of operations. Enhancements to, or future generations of, competing products may be developed that offer superior cost of ownership and technical performance features.
With respect to our laser annealing technologies, marketed under the LSA101 and LSA201 product names, our primary millisecond anneal competition comes from companies such as SCREEN Manufacturing Co., Ltd., Applied Materials and Mattson Technology, Inc. Some of these companies offer products utilizing RTP, which is the current prevailing manufacturing technology. RTP does not prevent semiconductor device manufacturers from scaling the lateral dimensions of their transistors to obtain improved performance, but diffusion resulting from the time scales associated with RTP limits the vertical dimension of the junctions. Faster annealing times result in shallower and more abrupt junctions and faster transistors. We believe that RTP manufacturers and semiconductor companies recognize the need to reduce thermal cycle times and are working toward this goal. In July 2000, we licensed certain rights to our then existing laser thermal processing technology, with reservations, to a competing manufacturer of semiconductor equipment. We presently anticipate that this company and others intend to offer laser annealing tools to the semiconductor industry that will compete with our offerings.
Another potential advanced annealing solution utilizes flash lamp annealing technology, or “FLA.” Several companies have published papers on annealing tools that incorporate flash lamp technology in order to reduce annealing times and increase annealing temperatures. Developers of FLA technology claim to have overcome annealing difficulties at the advanced nodes. This technique, which employs flash lamps, has shown improvements over RTP in junction depth and sheet resistance, but we believe FLA suffers from pattern-related non-uniformities and could require additional, costly processes to equalize the reflectivity of different areas within the chip or wafer. Our proprietary laser thermal processing solution has been specifically
14
developed to provide junction annealing on near-instantaneous time-scales, while achieving high activation levels. Laser thermal annealing, our first implementation of laser thermal processing, activates dopants in the microsecond-to-millisecond time frame without melting. Our research indicates that, at temperatures just below the melting point of silicon, time durations in the microsecond to millisecond range, are required to achieve full activation, and minimal dopant diffusion.
Additionally, competition to our laser thermal processing products may come from other laser annealing tools, including those presently being used by the flat panel display industry to re-crystallize silicon. Manufacturers of these tools may try to extend the use of their technologies to semiconductor device applications.
In the market for inspection systems, competition for inspection systems comes from a wide range of companies, including large companies such as KLA-Tencor, Nanometrics, Rudolph, and numerous smaller companies developing and manufacturing inspection equipment.
We believe that in order to be competitive, we will need to continue to invest significant financial resources in new product development, new features and enhancements to existing products, the introduction of new stepper systems in our served markets on a timely basis, and maintaining customer service and support centers worldwide. In marketing our products, we may also face competition from vendors employing other technologies. In addition, increased competitive pressure has led to intensified price-based competition in certain of our markets, resulting in lower prices and margins. Should these competitive trends continue, our business, financial condition and operating results may be materially adversely affected.
Our sales cycle is typically lengthy and involves a significant commitment of capital by our customers, which has subjected us, and is likely to continue to subject us, to delays in system acceptances of our products and other risks, any of which could adversely impact our results of operations by, among other things, delaying recognition of revenue with respect to those orders and resulting in increased installation, qualification and similar costs.
Sales of our systems depend, in significant part, upon the decision of a prospective customer to increase manufacturing capacity, replace older equipment or to restructure current manufacturing facilities, any of which typically involves a significant commitment of capital. Many of our customers in the past have canceled or postponed the development of new manufacturing facilities and have substantially reduced their capital equipment budgets. Many of our largest customers currently face uncertainty in the markets they serve, resulting in delays in placing new orders, the push out of previous orders to us and uncertainty in our business.
In view of the significant investment involved in a system purchase, we have experienced and may continue to experience unexpected delays following initial qualification of our systems as a result of delays in a customer’s approval process. Additionally, we are presently receiving orders for systems that have lengthy delivery schedules, which may be due to longer production lead times or a result of customers’ capacity scheduling requirements. For these and other reasons, our systems typically have a lengthy sales cycle during which we may expend substantial funds and management effort in securing a sale. Lengthy sales cycles subject us to a number of significant risks, including inventory obsolescence and fluctuations in operating results, over which we have little or no control. In order to maintain or exceed our present level of net sales, we are dependent upon obtaining orders for systems that will ship and be accepted in the current period. We may not be able to obtain those orders. Other important factors that could cause demand for our products to fluctuate include:
• | competitive pressures, including pricing pressures, from companies that have competing products; |
• | our customers’ or prospective customers’ perception of one or more of our board members; |
• | changes in customer product needs or product preferences; and |
• | strategic actions taken by our competitors. |
Our stock price has experienced significant volatility in the past and we expect this to continue in the future as a result of many factors, some of which could be unrelated to our operating performance, and such volatility can have a major impact on the number of shares subject to outstanding stock options and restricted stock units that are included in calculating our earnings per share.
We believe that factors such as announcements of developments related to our business, fluctuations in our operating results, a shortfall in revenue or earnings, changes in our or analysts’ expectations, general conditions in the semiconductor and nanotechnology industries or the worldwide or regional economies, sales of our securities into the marketplace, our share repurchase program, an outbreak or escalation of hostilities, announcements of technological innovations or new products or enhancements by us or our competitors, developments in patents or other intellectual property rights and developments in our relationships with our customers and suppliers could cause the price of our common stock to fluctuate, perhaps substantially.
15
The market price of our Common Stock has fluctuated significantly in the past and we expect it to continue to experience significant fluctuations in the future, including fluctuations that may be unrelated to our performance.
As of December 31, 2016, we had outstanding options to purchase and outstanding restricted stock units for a total of 3.0 million shares of our common stock. Among other determinants, the market price of our stock has a major impact on the number of shares subject to outstanding stock options and restricted stock units that are included in the weighted-average shares used in determining our net income per share. During periods of extreme volatility, the impact of higher stock prices can have a materially dilutive effect on our net income per share. Additionally, shares subject to outstanding options and restricted stock units are excluded from the calculation of net income per share when we have a net loss or when the exercise price and the average unrecognized compensation cost of the stock option or restricted stock unit is greater than the average market price of our common stock, as the impact of the stock options or restricted stock units would be anti-dilutive.
Our previously announced share repurchase program could affect the price of our common stock and increase volatility and may be suspended or terminated at any time, which may result in a decrease in the trading price of our common stock.
Repurchases pursuant to our share repurchase program could affect our stock price and increase its volatility. The existence of a share repurchase program could also cause our stock price to be higher than it would be in the absence of such a program and could potentially reduce the market liquidity for our stock. There can be no assurance that any share repurchases will enhance stockholder value because the market price of our common stock may decline below the levels at which we repurchased shares of common stock. Although our share repurchase program is intended to enhance long-term stockholder value, short-term stock price fluctuations could reduce the program’s effectiveness. Furthermore, the program does not obligate the Company to repurchase any dollar amount or number of shares of common stock, and may be suspended or discontinued at any time and any suspension or discontinuation could cause the market price of our stock to decline.
We sell our products primarily to a limited number of customers and to customers in a limited number of industries, which subjects us to increased risks related to the business performance of our customers, and therefore their need for our products, and the business cycles of the markets into which we sell.
Historically, we have sold a substantial portion of our systems to a limited number of customers. For the year ended December 31, 2016, Taiwan Semiconductor Manufacturing Co. Ltd. (“TSMC”), United Microelectronics Corporation, Powertech Technology, Inc. and Semiconductor Manufacturing North China (Beijing) Corporation (“SMNC”) accounted for 30%, 13%, 13% and 11% of our total systems sales, respectively. For the year ended, December 31, 2015, Siliconware Precision Industries Co. Ltd. (“SPIL”), TSMC and Intel Corporation (“Intel”) accounted for 21%, 13% and 10% of our system sales, respectively. We expect that sales to a relatively few customers will continue to account for a high percentage of our net sales in the foreseeable future and believe that our financial results depend in significant part upon the success of these major customers and our ability to meet their future capital equipment needs. Although the composition of the group comprising our largest customers may vary from period to period, the loss of a significant customer or any reduction or delay in orders by a significant customer, including reductions or delays due to market, economic or competitive conditions in the semiconductor, semiconductor packaging, semiconductor inspection or nanotechnology industries or in the industries that manufacture products utilizing integrated circuits, thin film heads or other nanotechnology components, would likely have a material adverse effect on our business, financial condition and results of operations. Our ability to maintain or increase our sales in the future depends, in part, on our ability to obtain orders from new customers as well as the financial condition and success of our existing customers, the semiconductor and nanotechnology industries and the economy in general.
In addition to the business risks associated with dependence on a few major customers, these significant customer concentrations have in the past resulted in significant concentrations of accounts receivable. These significant and concentrated receivables expose us to additional risks, including the risk of default by one or more customers representing a significant portion of our total receivables. If we were required to take additional accounts receivable reserves, our business, financial condition and results of operations would be materially adversely affected.
On a market application basis, sales to the semiconductor industry, primarily for advanced packaging applications and laser thermal processing applications, accounted for 93% of our systems revenue for the year ended December 31, 2016, as compared to 84% for the year ended December 31 2015. We sold $11.6 million of systems to nanotechnology manufacturers, including micro systems, thin film head, ALD and optical device manufacturers for the year ended December 31, 2016, as compared to $18.2 million in sales for the year ended December 31, 2015. Systems revenue from the nanotechnology sector accounted for 7% of systems revenue for the year ended December 31, 2016, as compared to 16% revenue for the year ended December 31, 2015. Our future operating results and financial condition would be materially adversely impacted by a downturn in any of these industries, or by loss of market share in any of these industries. A growing percentage of our backlog
16
of system orders is comprised of laser thermal processing tools. As our laser thermal processing tools are used for the continuation of reduced device geometries and customers seldom provide us with their future technical requirements, these tools may not meet all customers’ requirements upon initial delivery and installation at the customer’s facility. As a result, system acceptance of the tool could be delayed while we perform testing and attempt to meet their requirements, or the order could be cancelled if we are unable to meet those requirements. Should significant demand not materialize, due to technical, production, market or other factors, our business, financial position and results of operations would be materially adversely impacted.
We rely on a limited number of outside suppliers and subcontractors to manufacture certain components and subassemblies, and on single or a limited group of outside suppliers for certain materials for our products, which could result in a potential inability to obtain an adequate supply of required components due to quality problems, the suppliers’ failure or inability to provide such components in a timely manner, or at all, and reduced control over pricing and timely delivery of components and materials, any of which could adversely affect our results of operations.
Our manufacturing activities consist of assembling and testing components and subassemblies, which are then integrated into finished systems. We rely on a limited number of outside suppliers and subcontractors to manufacture certain components and subassemblies. We order one of the most critical components of our technology, the glass for our 1X lenses, from external suppliers. We design the 1X lenses and provide the lens specifications and the glass to other suppliers, who then grind and polish the lens elements. We then assemble and test the optical 1X lenses.
We procure some of our other critical systems’ components, subassemblies and services from single outside suppliers or a limited group of outside suppliers in order to ensure overall quality and timeliness of delivery. Many of these components and subassemblies have significant production lead times. To date, we have been able to obtain adequate services and supplies of components and subassemblies for our systems in a timely manner. However, disruption or termination of certain of these sources could have a significant adverse impact on our ability to manufacture our systems. In addition, our failure to timely use components in our manufacturing processes due to delays or cancellation of orders or quality problems requiring us to return components may lead to write downs of inventory. A disruption in supply or inventory window would, in turn, cause us to miss or delay shipments and would increase costs causing a material adverse effect on our business, financial condition and results of operations. Our reliance on a sole supplier or a limited group of suppliers and our reliance on subcontractors involve several risks, including a potential inability to obtain an adequate supply of required components due to the suppliers’ failure or inability to provide such components in a timely manner, to our quality standards, or at all, and reduced control over pricing and timely delivery of components. Manufacture of certain of these components and subassemblies is an extremely complex process, and long lead-times are required. Any inability to obtain adequate deliveries or any other circumstance that would require us to seek alternative sources of supply or to manufacture such components internally could delay our ability to ship our products, which could damage relationships with current and prospective customers and have a material adverse effect on our business, financial condition and results of operations.
Our industry is subject to rapid technological change and product innovation, which could result in our technologies and products being replaced by those of our competitors, which would adversely affect our business and results of operations.
The semiconductor and nanotechnology manufacturing industries are subject to rapid technological change, evolving industry standards and new product introductions and enhancements. Our ability to be competitive in these and other markets will depend, in part, upon our ability to develop new and enhanced systems and related applications, and to introduce these systems and related applications at competitive prices and on a timely and cost-effective basis to enable customers to integrate them into their operations either prior to or as they begin volume product manufacturing. We will also be required to enhance the performance of our existing systems and related applications. Our success in developing new and enhanced systems and related applications depends upon a variety of factors, including product selection, timely and efficient completion of product design, timely and efficient implementation of manufacturing and assembly processes, product performance in the field and effective sales and marketing. Product failures may result in customer dissatisfaction, high service and support costs, rework and increased inventory. Because new product development commitments must be made well in advance of sales, new product decisions must anticipate both future customer requirements and the technology that will be available to meet those requirements. We may not be successful in selecting, developing, manufacturing or marketing new products and related applications or enhancing our existing products and related applications. Any such failure would materially adversely affect our business, financial condition and results of operations. Further, we have in the past and may in the future make substantial investments in new products before we know whether they are technically feasible or commercially viable, and as a result may incur significant product development expenses that do not result in new products or revenues.
17
Because of the large number of components in our systems, significant delays can occur between a system’s introduction and our commencement of volume production of such systems. We have experienced delays from time to time in the introduction of, and technical and manufacturing difficulties with, certain of our systems and enhancements and related application tools features and options, and may experience delays and technical and manufacturing difficulties in future introductions or volume production of new systems or enhancements and related application tools features and options.
We may encounter additional technical, manufacturing or other difficulties that could further delay future introductions or volume production of systems or enhancements. Our inability to complete the development or meet the technical specifications of any of our systems or enhancements and related applications, or our inability to manufacture and ship these systems or enhancements and related tools in volume and in time to meet the requirements for manufacturing the future generation of semiconductor or nanotechnology devices would materially adversely affect our business, financial condition and results of operations. In addition, we may incur substantial unanticipated costs to ensure the functionality and reliability of our products early in the products’ life cycles. If new products have reliability or quality problems, reduced orders or higher manufacturing costs, delays in system acceptance, revenue recognition and collecting accounts receivable and additional service and warranty expenses may result. Any of such events may materially adversely affect our business, financial condition and results of operations.
We currently spend, and expect to continue to spend, significant resources to develop, introduce and commercialize our offered products and future generations of, and enhancements to these products. We may not be successful in the timely introduction of these products which may cause sales of these products to decrease or not increase as expected.
Currently, we are devoting significant resources to the development, introduction and commercialization of our laser thermal processing systems, and other products, and future generations of, and enhancements to, those products. We intend to continue to develop these products and technologies, and will continue to incur significant operating expenses in the areas of research, development and engineering, manufacturing and general and administrative costs in order to develop, produce and support these new products and enhancements. There can be no assurance that our expenditures to develop these products will result in improved sales or that markets for these products will become viable. Additionally, gross profit margins and inventory levels may be further adversely impacted in the future by costs associated with the initial production of new products. Introduction of new products generally involves higher installation costs and product performance uncertainties that could delay system acceptance of our systems, resulting in a delay in recognizing revenue associated with those systems and a reduction in gross margins. These costs include, but are not limited to, additional manufacturing overhead, additional inventory write-downs, costs of demonstration systems and facilities and costs associated with the establishment of additional after-sales support organizations. Additionally, operating expenses may increase, relative to sales, as a result of adding additional marketing and administrative personnel, among other costs, to support our new products. If we are unable to achieve significantly increased net sales or if our sales fall below expectations, our operating results could be materially adversely affected.
Our ability to commercialize our laser thermal processing technologies depends on our ability to demonstrate a manufacturing-worthy tool. We do not presently have in-house capability to fabricate devices. As a result, we must rely on partnering with semiconductor companies to develop the anneal process. The development of new process technologies is largely dependent upon our ability to interest potential customers in working on developing a process and providing timely and detailed feedback for system development. Our ability to deliver timely solutions is also limited by our customers understanding and communication of the future anneal requirements.
Changes in financial accounting standards or policies in the past have affected, and in the future may affect, our reported results of operations.
We prepare our financial statements in conformity with U.S. GAAP. These principles are subject to interpretation by the FASB, the American Institute of Certified Public Accountants (AICPA), the SEC and various bodies formed to interpret and create appropriate accounting policies. A change in those policies can have a significant effect on our reported results and may affect our reporting of transactions which are completed before a change is announced.
Accounting policies affecting many other aspects of our business, including rules relating to revenue recognition, off-balance sheet transactions, employee stock options and other equity awards, restructuring, asset disposals and asset retirement obligations, derivative and other financial instruments are regularly under review and subject to revision. Changes to those rules or the questioning of how we interpret or implement those rules may have a material adverse effect on our reported financial results or on the way we conduct business. In addition, our preparation of financial statements in accordance with U.S. GAAP requires that we make estimates and assumptions that affect the recorded amounts of assets and liabilities, disclosure of those assets and liabilities at the date of the financial statements and the recorded amounts of expenses during the reporting
18
period. A change in the facts and circumstances surrounding those estimates could result in a change to our estimates and could impact our future operating results.
Our results of operations and business could be adversely affected by natural disasters, public health issues, political instability, wars, and other military action, as well as terrorist attacks and threats and government responses thereto, especially if any such actions were directed at us or our facilities or customers.
Public health issues, political instability, natural disasters, terrorist attacks in the United States and elsewhere, and government responses thereto, may disrupt our operations or those of our customers and suppliers and may affect the availability of materials needed to manufacture our products or the means to transport those materials to manufacturing facilities and finished products to customers. In addition, such events could disrupt the semiconductor market, resulting in the cancellation or delay of product orders. Significant public health issues could cause damage or disruption to international commerce by creating economic and political uncertainties that may have a significant negative impact on the global economy, us and our customers or suppliers. Should such incidents increase or other public health issues arise, we could be negatively impacted by the need for more stringent employee travel restrictions, additional limitations in the availability of freight services, governmental actions limiting the movement of products between various regions and disruptions in the operations of our customers or suppliers. Similarly, political instability could affect the ability of our suppliers to provide the materials needed in our operations or the cost of acquiring such materials. Any public health issues, political instability, natural disasters, terrorist attacks, or the ongoing war on terrorism or other wars could increase volatility in the United States and world financial markets which may depress the price of our common stock and may limit the capital resources available to us or our customers or suppliers, which could result in decreased orders from customers, less favorable financing terms from suppliers, and scarcity or increased costs of materials and components of our products. Additionally, if any of these events were to directly affect or be specifically directed at us, or occur in a country where we or our suppliers or our customers operate, our ability to conduct our business could be significantly disrupted. Any of these occurrences could have a significant impact on our operating results, revenues and costs and may result in increased volatility of the market price of our common stock.
We currently perform a significant amount of our manufacturing activities in cleanroom environments in San Jose, California, an area known for seismic activity. Performing manufacturing operations in California exposes us to a higher risk of natural disasters, including earthquakes. In addition, in the past California has experienced power shortages, which have interrupted our operations. Such shortages could occur in the future. Further, our suppliers and/or customers may operate in areas subject to natural disasters, the occurrence of which could affect their ability to continue to do business with us as expected or at all. An earthquake, other natural disaster, power shortage or other similar events could interrupt or otherwise limit our operations or those of our suppliers or customers resulting in product shipment delays, supply problems, cancellations or deferrals of product orders, increased costs and other problems, any of which could have a material adverse effect on our business, customer relationships and results of operations.
We continue to expand our manufacturing and service operations in Singapore and customer support operations in other parts of the world, which will continue to result in exposure to risks inherent in doing business outside the United States, any of which risks could harm our business, financial condition and operating results.
Foreign operations subject us to risks related to the political, economic, legal and other conditions of foreign jurisdictions. These risks include risks related to:
• | foreign exchange rate fluctuations; |
• | the need to comply with foreign government laws and regulations, including the imposition of regulatory requirements, tariffs, and import and export restrictions; |
• | general geopolitical risks such as political and economic instability and changes in diplomatic and trade relationships; |
• | the need for effective management of dispersed operations far from our headquarters in California; |
• | the potential for strain on management resources; |
• | difficulty in hiring and retaining local personnel for the successful operation of our business in each location; |
• | the need to effectively manage personnel in different languages and under different cultural and legal expectations and requirements in certain locations; |
• | meeting growth objectives and employment projections; |
• | potentially less protection of intellectual property under the laws of foreign jurisdictions in certain locations; and |
• | public safety or health concerns or natural disasters in foreign countries. |
19
These risks could, among other things, result in product shipment delays, increased costs, unexpected shutdowns or other business disruptions, or loss of benefits expected to be achieved by conducting operations in affected jurisdictions. Any of the above risks, should they occur, could have a material adverse effect on our business, financial condition and results of operations.
A substantial portion of our sales are outside of the United States, which subjects us to risks related to customer service, installation, foreign economic and political stability, uncertain regulatory and tax rules, and foreign exchange rate fluctuations, all of which make it more difficult to operate our business.
International sales accounted for approximately 87% of total net sales for the year ended December 31, 2016, as compared to 81% for the year ended December 31, 2015. We anticipate that international sales will continue to account for a significant portion of total net sales. Although we generally transact our international sales in U.S. dollars, international sales expose us to a number of additional risk factors, including fluctuations in the value of local currencies relative to the U.S. dollar, which, in turn, impact the relative cost of ownership of our products and may further impact the purchasing ability of our international customers. We have direct sales operations in Japan and orders are often denominated in Japanese yen. This may subject us to a higher degree of risk from currency fluctuations. We attempt to mitigate this exposure through foreign currency hedging. We are also subject to the risks associated with the imposition of legislation and regulations relating to the import or export of semiconductors and nanotechnology products. We cannot predict whether the United States or any other country will implement changes to quotas, duties, taxes or other charges or restrictions upon the importation or exportation of our products. These factors, or the adoption of restrictive policies, may have a material adverse effect on our business, financial condition and results of operations. Additionally, by having a significant portion of our sales occur internationally, we are continually exposed to political and economic instability; difficulties in accounts receivable collections; reduced protection of intellectual property; natural disasters; difficulties in staffing and managing foreign subsidiary and branch operations; and potentially adverse tax consequences.
To better align with the increasingly international nature of our business, we transitioned certain manufacturing processes to Singapore, thereby bringing these activities closer to our Asia-based customers. This movement is somewhat recent and our experience in international manufacturing operations is not as extensive as in the U.S. If we are unable to continue to successfully operate the site to efficiently manufacture systems, our business, financial condition and results of operations could be materially adversely impacted.
If we acquire companies, products, or technologies, we may face risks associated with those acquisitions.
We may not realize the anticipated benefits of any acquisition or investment. Acquisitions involve numerous risks, including difficulties in the assimilation of the operations, technologies, personnel and products of the acquired companies; the diversion of management’s attention from other business concerns; risks of entering markets in which we have limited or no direct experience; and the potential loss of key employees of the acquired company. In the event we acquire product lines, technologies or businesses which do not complement our business, or which otherwise do not enhance our sales or operating results, we may incur substantial write-offs and higher recurring operating costs, which could have a material adverse effect on our business, financial condition and results of operations. We may, in the future, pursue additional acquisitions of complementary product lines, technologies or businesses. Future acquisitions may require use of substantial amounts of our cash, cash equivalents and short-term investments, result in potentially dilutive issuances of equity securities, the incurrence of debt and contingent liabilities and amortization expenses and impairment charges related to goodwill and other intangible assets, which could materially adversely affect our financial condition and results of operations. In the event that any such acquisition does occur, there can be no assurance as to the effect thereof on our business or operating results.
Changes in our effective tax rate may harm our results of operations.
A number of factors may negatively impact our future effective tax rates including, but not limited to:
• | the jurisdictions in which profits are determined to be earned and taxed; |
• | changes in the valuation of our deferred tax assets and liabilities; |
• | increases in expenses not deductible for tax purposes; |
• | changes in available tax credits; |
• | changes in stock-based compensation; and |
• | changes in tax laws or the interpretation of such tax laws and changes in generally accepted accounting principles in the United States or other countries in which we operate. |
20
We are eligible for tax incentives that provide that certain income earned in Singapore would be subject to a tax holiday and/or reduced tax rates for a limited period of time under the laws of Singapore. Our ability to realize benefits from these initiatives could be materially affected if, among other things, applicable requirements are not met, the incentives are substantially modified, or if we incur losses for which we cannot take a deduction.
We may not be successful in protecting our intellectual property rights or we could be found to have infringed the intellectual property rights of others, either of which could weaken our competitive position and adversely affect our results of operations.
Although we attempt to protect our intellectual property rights through patents, copyrights, trade secrets and other measures, we believe that our success will depend more upon the innovation, technological expertise and marketing abilities of our employees. Nevertheless, we have a policy of seeking patents when appropriate on inventions resulting from our ongoing research and development and manufacturing activities. Our intellectual property portfolio has 1,248 patents and patent applications with expiration dates ranging from April 2017 to June 2035. In addition, we have various registered trademarks and copyright registrations covering mainly applications used in the operation of our systems. We also rely upon trade secret protection for our confidential and proprietary information. We may not be able to protect our technology adequately and competitors may be able to develop similar technology independently. Our pending patent applications may not be issued or U.S. or foreign intellectual property laws may not protect our intellectual property rights. In addition, litigation may be necessary to enforce our patents, copyrights or other intellectual property rights, to protect our trade secrets, to determine the validity and scope of the proprietary rights of others or to defend against claims of infringement. Such litigation has resulted in, and in the future could result in, substantial costs and diversion of resources and could have a material adverse effect on our business, financial condition and results of operations, regardless of the outcome of the litigation. Patents issued to us may be challenged, invalidated or circumvented and the rights granted thereunder may not provide competitive advantages to us. Furthermore, others may independently develop similar technology or products, or, if patents are issued to us, design around the patents issued to us. Invalidation of our patents related to those technologies, or the expiration of patents covering our key technologies, could allow our competitors to more effectively compete against us, which could result in less revenue for us.
We have from time to time been notified of claims that we may be infringing intellectual property rights possessed by third parties. We believe that the outcome of these matters will not be material to our business, results of operations or financial condition. Infringement claims by third parties or claims for indemnification resulting from infringement claims may be asserted in the future and such assertions could materially adversely affect our business, financial condition and results of operations, regardless of the outcome of any litigation. With respect to any such future claims, we may seek to obtain a license under the third party’s intellectual property rights. However, a license may not be available on reasonable terms or at all. We could decide, in the alternative, to resort to litigation to challenge such claims. Such challenges could be expensive and time consuming and could materially adversely affect our business, financial condition and results of operations, regardless of the outcome of any litigation.
We use hazardous substances in the operation of our business, and any failure on our part to comply with applicable regulations or to appropriately control the use, disposal or storage of such substances could subject us to significant liabilities.
We are subject to a variety of governmental regulations relating to environment protection and workplace safety, including the use, storage, discharge, handling, emission, generation, manufacture and disposal of toxic or other hazardous substances. The failure to comply with current or future regulations could result in substantial fines being imposed on us, suspension of production, alteration of the manufacturing process or cessation of operations. Such regulations could require us to acquire expensive remediation equipment or to incur substantial expenses to comply with environmental regulations. Any failure by us to comply with these regulations, including any failure to control the use, disposal or storage of, or adequately restrict the discharge of, hazardous or toxic substances, could subject us to significant liabilities.
Our investment portfolio may suffer losses from changes in market interest rates and changes in market conditions, which could materially and adversely affect our financial condition, results of operations or liquidity.
As of December 31, 2016, we had $267.6 million in cash, cash equivalents and short-term investments. We maintain an investment portfolio of cash equivalents and short-term investments in commercial paper and U.S. government-backed securities. These investments are subject to general credit, liquidity, and market and interest rate risks. Substantially all of these securities are subject to interest rate and credit risk and will decline in value if interest rates increase or one or more of the issuers’ credit ratings is reduced. As a result of any of the foregoing, we may experience a reduction in value or loss of liquidity of our investments, which may have a negative adverse effect on our results of operations, liquidity and financial condition. We follow an established investment policy and set of guidelines to monitor, manage and limit our exposure to interest rate and
21
credit risk. The policy sets forth credit quality standards and limits our exposure to any one issuer, as well as our maximum exposure to various asset classes.
Our restructuring activities may not achieve the results we expect and could change, which could materially and adversely affect our results of operations and financial condition.
In 2015, we implemented restructuring activities to reduce expenses, which included a reduction in our workforce. There can be no assurance that our restructuring activities will produce the cost savings we anticipate in the expected timeframe or that the cumulative restructuring charge will not have to increase in order to achieve our cost savings targets. Any delay or failure to achieve the expected cost savings and any increase in our anticipated cumulative restructuring charge would likely cause our future earnings to be lower than anticipated.
Our equity incentive plans, certain provisions of our Certificate of Incorporation and Bylaws, and certain aspects of Delaware law may discourage third parties from pursuing a change of control transaction with us.
Certain provisions of our Certificate of Incorporation, equity incentive plans, licensing agreements, Bylaws and Delaware law may discourage certain transactions involving a change in control of our company. In addition to the foregoing, the shareholdings of our officers, directors and persons or entities that may be deemed affiliates and the ability of the Board of Directors to issue “blank check” preferred stock without further stockholder approval could have the effect of delaying, deferring or inhibiting us from experiencing a change in control and may adversely affect the voting and other rights of holders of our common stock.
We are subject to laws, regulations and similar requirements, changes to which may adversely affect our business and operations.
We are subject to laws, regulations and similar requirements that affect our business and operations, including, but not limited to, the areas of commerce, import and export control, intellectual property, income and other taxes, anti-trust, anti-corruption, labor, environmental, health and safety. Our compliance in these areas may be costly, especially in areas where there are inconsistencies between the various jurisdictions in which we operate. While we have implemented policies and procedures to comply with various laws and regulations, there can be no assurance that our employees, contractors, suppliers or agents will not violate such laws and regulations or our policies. Any such violation or alleged violation could materially and adversely affect our business. Any changes or potential changes to laws, regulations or similar requirements, or our ability to respond to these changes, may significantly increase our costs to maintain compliance or result in our decision to limit our business, products or jurisdictions in which we operate, any of which could materially and adversely affect our business and operations.
The Dodd-Frank Wall Street Reform and Consumer Protection Act includes provisions regarding certain minerals and metals, known as conflict minerals, mined from the Democratic Republic of Congo and adjoining countries. These provisions require companies to undertake due diligence procedures and reports on the use of conflict minerals in its products, including products manufactured by third parties. Compliance with these provisions will cause us to incur costs to certify that our supply chain is conflict free and we may face difficulties in complying with the law if our suppliers are unwilling or unable to verify the source of their materials. Our ability to source parts containing these metals and minerals may be adversely impacted. In addition, our customers may require that we provide them with a certification and our inability to do so may disqualify us as a supplier.
We hold cash and cash equivalents at various foreign subsidiaries that may not be readily available to meet domestic cash requirements.
Our various foreign subsidiaries hold cash and cash equivalents and as we intend to reinvest certain foreign earnings indefinitely, these balances held outside the United States may not be readily available to meet our domestic cash requirements. We require a substantial amount of cash in the United States for operating requirements, purchases of property and equipment, and potentially for future acquisitions. If we are unable to meet our domestic cash requirements using domestic cash flows from operations, or domestic cash and cash equivalents, it may be necessary for us to consider repatriation of earnings that we have designated as permanently reinvested. We may also be required to repatriate cash held outside the United States to meet the closing condition of having not less than $180 million of cash on hand in the United States at the time of the closing of the Merger. The repatriation of earnings may require us to record additional income tax expense and remit additional taxes, which could have a material adverse effect on our results of operations, cash flows and financial condition.
22
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
We have no unresolved staff comments.
ITEM 2. | PROPERTIES |
We maintain our headquarters and manufacturing operations in San Jose, California in a leased facility, totaling approximately 100,000 square feet, which contains general administration and finance, marketing and sales, customer service and support, manufacturing and research, development and engineering. The lease for this facility expires in January 2021. We also rent sales and support offices in the United States and outside the U.S. in Taiwan, the Philippines, Japan, Korea, Thailand, Germany, and China, with varying terms and expiration dates. During the years ended December 31, 2016 and 2015, we either extended our leases in several of these sales facilities or negotiated new terms.
On June 28, 2016, we renegotiated the August 15, 2012 lease for our Singapore operations and entered into a new lease that will expire in June 2023. The leased facility is approximately 23,000 square feet.
In March 2013, we entered into an operating lease agreement for facilities in Waltham, Massachusetts, totaling approximately 19,000 square feet, for sales, research and development activities. The lease for this facility expires in February 2018.
In July 2015, we entered into a lease amendment for our facilities in San Jose, California. The lease amendment is to extend the building lease for five years and will expire in January 2021.
We believe that our existing facilities will be adequate to meet our currently anticipated requirements and that suitable additional or substitute space will be available as needed.
ITEM 3. LEGAL PROCEEDINGS
We are subject to claims and litigation arising in the ordinary course of business. We do not believe any liability from any reasonably foreseeable disposition of such claims and litigation, individually or in the aggregate, would have a material adverse effect on our consolidated financial statements.
ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
23
PART II
ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is traded on the NASDAQ Global Market under the symbol UTEK. The following table sets forth, for the periods indicated, the range of high and low reported sale prices of our common stock.
Fiscal 2016—Fiscal Quarter Ended | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | ||||||||||||
Market Price: | High | $ | 22.32 | $ | 23.49 | $ | 25.91 | $ | 24.11 | |||||||
Low | $ | 17.55 | $ | 19.91 | $ | 22.49 | $ | 20.88 | ||||||||
Fiscal 2015—Fiscal Quarter Ended | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | ||||||||||||
Market Price: | High | $ | 18.99 | $ | 20.89 | $ | 18.50 | $ | 20.30 | |||||||
Low | $ | 15.94 | $ | 17.26 | $ | 15.27 | $ | 14.44 | ||||||||
Our fiscal quarters in 2016 ended on April 2, 2016, July 2, 2016, October 1, 2016 and December 31, 2016, respectively. Our fiscal quarters in 2015 ended on April 4, 2015, July 4, 2015, October 3, 2015 and December 31, 2015, respectively.
As of January 27, 2017, we had approximately 170 stockholders of record.
We have not paid cash dividends on our common stock since inception, and our Board of Directors presently plans to reinvest our earnings in our business. Accordingly, it is anticipated that no cash dividends will be paid to holders of common stock in the foreseeable future.
Issuer Purchases of Equity Securities
On October 27, 2014, our Board of Directors authorized the repurchase of up to $100 million of our common stock. The share repurchase plan commenced on October 28, 2014 and will expire on October 28, 2017. Any repurchases may be made through open market purchases, block trades, privately negotiated transactions and/or derivative transactions, subject to market conditions and managements’ ongoing determination that it is the best use of available cash on hand to fund any repurchases. The repurchase authorization does not obligate us to acquire any common stock.
During the year ended December 31, 2016, we did not repurchase any common stock under the stock repurchase plan. Common stock repurchase activity under our publicly announced stock repurchase plan during the years ended December 31,
24
2015 and 2014 was as follows:
Period | Total Number of Shares Purchased | Average Price Paid Per Share | Total Number of Shares Purchased Under Publicly Announced Plan or Programs | Approximate Dollar Value of Shares That May Yet Be Purchased | ||||||||||
October 27 - 31, 2014 | — | $ | — | — | $ | 100,000,000 | ||||||||
November 1 - 30, 2014 | 779,800 | $ | 19.23 | 779,800 | $ | 85,005,000 | ||||||||
December 1 - 31, 2014 | 220,200 | $ | 19.41 | 220,200 | $ | 80,730,000 | ||||||||
May 1 - May 31, 2015 | 162,200 | $ | 20.45 | 162,200 | $ | 77,413,000 | ||||||||
June 1 - July 4, 2015 | 87,800 | $ | 20.05 | 87,800 | $ | 75,652,000 | ||||||||
July 5 - July 31, 2015 | 42,200 | $ | 15.75 | 42,200 | $ | 74,987,000 | ||||||||
August 1- August 31, 2015 | 702,700 | $ | 16.20 | 702,700 | $ | 63,607,000 | ||||||||
September 1 - October 3, 2015 | 255,100 | $ | 16.91 | 255,100 | $ | 59,292,000 | ||||||||
October 4 - October 31, 2015 | — | $ | — | — | $ | 59,292,000 | ||||||||
November 1- November 30, 2015 | — | $ | — | — | $ | 59,292,000 | ||||||||
December 1- December 31, 2015 | — | $ | — | — | $ | 59,292,000 | ||||||||
Total | 2,250,000 | $ | 18.09 | 2,250,000 | ||||||||||
25
Stock Performance Graph
The graph depicted below reflects a comparison of the cumulative total return (i.e., change in stock price plus reinvestment of dividends) of our common stock assuming $100 invested as of December 31, 2011 with the cumulative total returns of the NASDAQ Composite Index and the Philadelphia Semiconductor Index.
Comparison of Cumulative Total Returns (1)(2)(3)
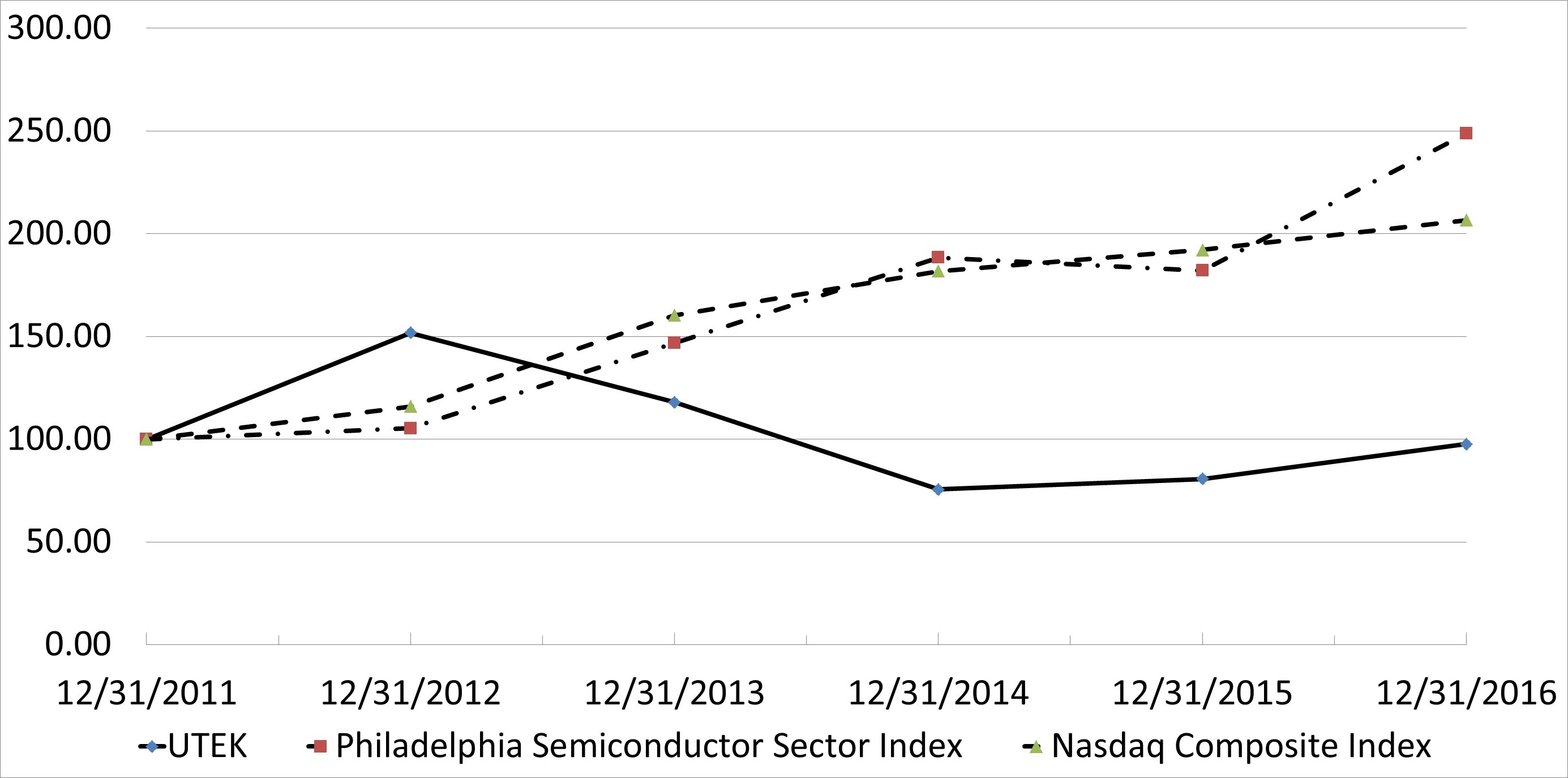
___________________
(1) | The graph covers the period from December 31, 2011 to December 31, 2016. |
(2) | No cash dividends have been declared on our common stock. |
(3) | Stockholder returns over the indicated period should not be considered indicative of future stockholder returns. |
Notwithstanding anything to the contrary set forth in any of our previous filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, which might incorporate our future filings under those statutes, the preceding Stock Performance Graph will not be incorporated by reference into any of those prior filings, nor will such report or graph be incorporated by reference into any of our future filings under those statutes.
26
ITEM 6. | SELECTED FINANCIAL DATA |
In thousands, except per share data and percentage information | 2016 (e) | 2015 (d) | 2014 (c) | 2013 (b) | 2012(a) | ||||||||||||||
Operations: | |||||||||||||||||||
Net sales | $ | 194,051 | $ | 149,176 | $ | 150,540 | $ | 157,272 | $ | 234,825 | |||||||||
Gross profit | $ | 88,735 | $ | 65,432 | $ | 63,273 | $ | 67,382 | $ | 131,810 | |||||||||
Gross profit as a percentage of net sales | 46 | % | 44 | % | 42 | % | 43 | % | 56 | % | |||||||||
Operating income (loss) | $ | 8,122 | $ | (15,040 | ) | $ | (19,063 | ) | $ | (15,058 | ) | $ | 56,398 | ||||||
Income (loss) before income taxes | $ | 8,692 | $ | (14,678 | ) | $ | (18,867 | ) | $ | (14,916 | ) | $ | 56,969 | ||||||
Pre-tax income (loss) as a percentage of net sales | 4.5 | % | (9.8 | )% | (12.5 | )% | (9.5 | )% | 24.3 | % | |||||||||
Provision (benefit) for income taxes | $ | (2,545 | ) | $ | 450 | $ | 244 | $ | (1,147 | ) | $ | 9,782 | |||||||
Net income (loss) | $ | 11,237 | $ | (15,128 | ) | $ | (19,111 | ) | $ | (13,769 | ) | $ | 47,187 | ||||||
Net income (loss) per share—basic | $ | 0.42 | $ | (0.55 | ) | $ | (0.67 | ) | $ | (0.49 | ) | $ | 1.76 | ||||||
Number of shares used in per share computation—basic | 27,012 | 27,429 | 28,437 | 28,106 | 26,881 | ||||||||||||||
Net income (loss) per share—diluted | $ | 0.41 | $ | (0.55 | ) | $ | (0.67 | ) | $ | (0.49 | ) | $ | 1.70 | ||||||
Number of shares used in per share computation—diluted | 27,333 | 27,429 | 28,437 | 28,106 | 27,705 | ||||||||||||||
Balance sheet: | |||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 267,593 | $ | 251,901 | $ | 269,730 | $ | 297,035 | $ | 302,508 | |||||||||
Working capital | $ | 342,357 | $ | 315,424 | $ | 334,434 | $ | 349,055 | $ | 349,506 | |||||||||
Total assets | $ | 415,572 | $ | 389,196 | $ | 417,518 | $ | 434,164 | $ | 436,986 | |||||||||
Long-term obligations | $ | 12,456 | $ | 13,474 | $ | 15,252 | $ | 11,923 | $ | 11,235 | |||||||||
Stockholders’ equity | $ | 365,198 | $ | 341,877 | $ | 365,120 | $ | 386,538 | $ | 375,186 | |||||||||
__________________
(a) | Operating income in 2012 includes $12.5 million of stock-based compensation expenses. |
(b) | Operating loss in 2013 includes $15.4 million of stock-based compensation expenses. |
(c) | Operating loss in 2014 includes $16.9 million of stock-based compensation expenses. |
(d) | Operating loss in 2015 includes $15.3 million of stock-based compensation expenses and $0.8 million of restructuring expenses. |
(e) | Operating income in 2016 includes $12.2 million of stock-based compensation expenses. |
27
Quarterly Data
Unaudited, in thousands, except per share data | 1st | 2nd | 3rd | 4th | |||||||||||
2016 | |||||||||||||||
Net sales | $ | 45,210 | $ | 48,924 | $ | 48,611 | $ | 51,306 | |||||||
Gross profit | $ | 18,167 | $ | 22,916 | $ | 23,433 | $ | 24,219 | |||||||
Operating income (loss) | $ | (1,382 | ) | $ | 2,597 | $ | 2,715 | $ | 4,192 | ||||||
Net income (loss) | $ | (1,154 | ) | $ | 2,610 | $ | 5,457 | $ | 4,323 | ||||||
Net income (loss) per share—basic | $ | (0.04 | ) | $ | 0.10 | $ | 0.20 | $ | 0.16 | ||||||
Number of shares used in per share computation—basic | 26,798 | 26,959 | 27,108 | 27,136 | |||||||||||
Net income (loss) per share—diluted | $ | (0.04 | ) | $ | 0.10 | $ | 0.20 | $ | 0.16 | ||||||
Number of shares used in per share computation—diluted | 26,798 | 27,193 | 27,380 | 27,403 | |||||||||||
Unaudited, in thousands, except per share data | 1st | 2nd | 3rd | 4th | |||||||||||
2015 | |||||||||||||||
Net sales | $ | 41,886 | $ | 45,921 | $ | 33,115 | $ | 28,254 | |||||||
Gross profit | $ | 19,032 | $ | 21,471 | $ | 14,061 | $ | 10,868 | |||||||
Operating income (loss) | $ | (1,435 | ) | $ | 1,463 | $ | (6,337 | ) | $ | (8,731 | ) | ||||
Net income (loss) | $ | (1,687 | ) | $ | 1,539 | $ | (6,099 | ) | $ | (8,881 | ) | ||||
Net income (loss) per share—basic | $ | (0.06 | ) | $ | 0.06 | $ | (0.22 | ) | $ | (0.33 | ) | ||||
Number of shares used in per share computation—basic | 27,733 | 27,780 | 27,248 | 26,793 | |||||||||||
Net income (loss) per share—diluted | $ | (0.06 | ) | $ | 0.06 | $ | (0.22 | ) | $ | (0.33 | ) | ||||
Number of shares used in per share computation—diluted | 27,733 | 27,980 | 27,248 | 26,793 | |||||||||||
28
ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Certain of the statements contained herein, which are not historical facts and which can generally be identified by words such as “anticipates,” “expects,” “thinks,” “remains,” “intends,” “will,” “could,” “believes,” “poised,” “estimates,” “continues,” and similar expressions, are forward-looking statements under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve risks and uncertainties, such as risks related to timing, delays, deferrals and cancellations of orders by customers, including as a result of semiconductor manufacturing capacity as well as our customers’ financial condition and demand for semiconductors; demand for consumer devices; industry growth within our served markets; continued delivery of financial performance and value; cyclicality in the semiconductor and nanotechnology industries; our dependence on new product introductions and market acceptance of new products and enhanced versions of our existing products; reliability and technical acceptance of our products; lengthy sales cycles, including the timing of system installations and acceptances; quarterly revenue fluctuations; lengthy and costly development cycles for laser-processing and lithography technologies and applications; integration, development and associated expenses of the laser processing operation; general economic and financial market conditions including impact on capital spending, as well as difficulty in predicting changes in such conditions; rapid technological change and the importance of timely product introductions; customer concentration; pricing pressures and product discounts; high degree of industry competition including competition from companies that are significantly larger than us; intellectual property matters; changes in pricing by us, our competitors or suppliers; international sales and operations; timing of new product announcements and releases by us or our competitors; improvements, including in cost and technical features, of competitors’ products; ability to volume produce systems and meet customer requirements; sole or limited sources of supply; effect of capital market fluctuations on our investment portfolio; ability and resulting costs to attract or retain key personnel; dilutive effect of employee stock option grants on net income per share, which is largely dependent upon our achieving and maintaining profitability and the market price of our stock; mix of products sold; outcome of litigation; manufacturing variances and production levels; timing and degree of success of technologies licensed to outside parties; product concentration and lack of product revenue diversification; inventory obsolescence; quality of product sourced from suppliers; asset impairment; changes to financial accounting standards; effects of certain anti-takeover provisions; future acquisitions; volatility of stock price; foreign government regulations and restrictions; business interruptions due to natural disasters or utility failures; environmental regulations; and any adverse effects of terrorist attacks in the United States or elsewhere, or government responses thereto, or military actions, on the economy, in general, or on our business in particular. Due to these and additional factors, the statements, historical results and percentage relationships set forth below are not necessarily indicative of the results of operations for any future period. These forward-looking statements are based on management’s current beliefs and expectations, some or all of which may prove to be inaccurate, and which may change. We undertake no obligation to revise or update any forward-looking statements to reflect any event or circumstance that may arise after the date of this report.
OVERVIEW
Ultratech, Inc. develops, manufactures and markets photolithography, laser thermal processing, and inspection equipment designed to reduce the cost of ownership for manufacturers of semiconductor devices, including advanced packaging processes and various nanotechnology components such as laser diodes, high-brightness light emitting diodes (“HBLEDs”) and Micro-Electro-Mechanical Systems (“MEMS”) as well as atomic layer deposition systems (“ALD”) for customers located throughout the world. Ultratech was incorporated in the state of Delaware in 1992.
We supply step-and-repeat photolithography systems based on one-to-one imaging technology. Within the semiconductor industry, we target the market for advanced packaging applications. Our laser thermal processing equipment is targeted at advanced annealing applications within the semiconductor industry. Within the nanotechnology industry, our target markets include thin film head magnetic recording devices, ink jet print heads, HBLEDs and atomic layer deposition for the nanotechnology portion of our defined served market.
On February 2, 2017, we entered into the Merger Agreement with Veeco and Merger Subsidiary, pursuant to which, among other things, Merger Subsidiary will be merged with and into the Company, with the Company surviving the Merger as a wholly owned subsidiary of Veeco, subject to the terms and conditions of the Merger Agreement, as discussed in more detail under the heading “Recent Developments” on page 3 and in Note 18 to the Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K beginning on page 51.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
29
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities at the date of the consolidated financial statements. By their nature, these estimates and judgments are subject to an inherent degree of uncertainty. On an on-going basis, we evaluate our estimates, including those related to revenues, inventory valuation, warranty obligations, purchase order commitments, bad debts, deferred income taxes, restructuring liabilities, asset retirement obligations, stock based-compensation and contingencies and litigation. Management bases its estimates and judgments on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies are affected by our more significant judgments and estimates used in the preparation of our consolidated financial statements. We have reviewed these policies with our Audit Committee.
Revenue Recognition
We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the arrangement consideration is fixed or determinable, and collectability is reasonably assured. We derive revenue from four sources: system sales, spare parts sales, service contracts and license fees.
Provided all other criteria are met, we recognize revenues on system sales when system acceptance provisions have been met in accordance with the terms and conditions of the arrangement. In the event that terms of the sale provide for a lapsing system acceptance period, we recognize revenue upon the expiration of the lapsing acceptance period or system acceptance, whichever occurs first. In these instances, which are infrequent, revenue is recorded only if the product has met product specifications prior to shipment and management deems that no significant uncertainties as to product performance exist.
Our transactions frequently include the sale of systems and services under multiple element arrangements. In transactions with multiple deliverables, revenue is recognized upon the delivery of the separate elements and when system acceptance has occurred or we are otherwise released from our system acceptance obligations.
For multiple element arrangements, the total consideration for an arrangement is allocated among the separate elements in the arrangement based on a selling price hierarchy. The selling price hierarchy for a deliverable is based on (i) vendor specific objective evidence (“VSOE”); if available; (ii) third party evidence of selling price if VSOE is not available; or (iii) an estimated selling price, if neither VSOE nor third party evidence is available. If we have not established VSOE and cannot obtain third party evidence of selling price, we determine our estimate of the relative selling price by considering our production costs and historical margins of similar products or services. We believe this best represents the price at which we would transact a sale if the product or service were sold on a stand-alone basis. We regularly review the method used to determine our relative selling price and update any estimates accordingly. We limit the amount of revenue recognized for delivered elements to the amount that is not contingent on the future delivery of products or services or other future performance obligations.
We generally recognize revenue from spare parts sales upon shipment, as our products are generally sold on terms that transfer title and risk of ownership when it leaves our site. We sell service contracts for which revenue is deferred and recognized ratably over the contract period (for time-based service contracts) or as service hours are delivered (for contracts based on a purchased quantity of hours). We recognize license revenue from transactions in which our systems are re-sold by our customers to third parties, as well as from royalty arrangements.
Costs related to deferred product revenues are capitalized (deferred) and recognized at the time of revenue recognition. Deferred product revenue and costs are netted on our balance sheet, under the caption “deferred product and services income.” The gross amount of deferred revenues and deferred costs at December 31, 2016 were $4.6 million and $0.2 million, respectively, as compared to $5.3 million and $0.8 million, respectively, at December 31, 2015.
Costs incurred for shipping and handling are included in cost of sales.
Inventories and Purchase Order Commitments
30
Inventories are stated at the lower of cost or market using standard costs that generally approximate actual costs on a first-in, first-out basis. We maintain a perpetual inventory system and continuously record the quantity on-hand and standard cost for each product, including purchased components, subassemblies, and finished goods. We maintain the integrity of perpetual inventory records through periodic physical counts of quantities on hand.
The semiconductor industry is characterized by rapid technological change, changes in customer requirements and evolving industry standards. We perform a detailed assessment of inventory at each balance sheet date, which includes a review of, among other factors, demand requirements and market conditions. Based on this analysis, we may record adjustments to the value of our inventory. Although we make every effort to ensure the accuracy of our forecasts of product demand, any significant unanticipated changes in demand, product mix or technological developments would significantly impact the value of our inventory and our reported operating results. In the future, if we find that our estimates are too optimistic and we determine that our inventory needs to be reserved, we will be required to recognize such costs in our cost of sales at the time of such determination.
Warranty Obligations
We recognize the estimated cost of our product warranties at the time revenue is recognized. Our warranty obligation is affected by product failure rates, material usage rates and the efficiency by which the product failure is corrected. Should actual product failure rates, material usage rates and labor efficiencies differ from our estimates, revisions to the estimated warranty liability would be required which could result in future charges or credits to our gross profit. We believe our warranty accrual, as of December 31, 2016, will be sufficient to satisfy outstanding obligations as of that date.
Allowance for Bad Debts
We maintain an allowance for estimated losses resulting from the inability of our customers to make required payments. This reserve is established based upon historical trends, current economic conditions, delinquency status based on contractual terms and an analysis of specific exposures. If the financial conditions of our customers were to deteriorate, or even a single customer was otherwise unable to make payments, additional allowances may be required. The typical selling price of our systems is between $0.1 million and $6.0 million. Accordingly, a single customer default could have a material adverse effect on our results of operations. Our bad debt reserve for accounts receivable was $0.5 million at December 31, 2016 and 2015.
Intangible Assets
Purchased technology, patents and other intangible assets are presented at cost, net of accumulated amortization, and are amortized over their estimated useful lives using the straight-line method. We review for impairment whenever events or changes in circumstances indicate that the carrying amount of the intangible asset may not be recoverable and the carrying amount exceeds its fair value.
Stock-Based Compensation
Under the fair value recognition provisions of ASC Topic 718, Compensation—Stock Compensation (“ASC 718”), share-based compensation cost is measured at the grant date based on the value of the award and is recognized as expense over the vesting period. Determining the fair value of share-based awards at the grant date requires judgment, including estimating our stock price volatility, employee stock option exercise behaviors and employee option forfeiture rates. If actual results differ significantly from these estimates, stock-based compensation expense recognized in our results of operations could be materially affected. As stock-based compensation expense recognized in the Consolidated Statement of Operations is based on awards that ultimately are expected to vest, the amount of the expense has been reduced for estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience. If factors change and we employ different assumptions in the application of ASC 718, the compensation expense that we record in future periods may differ significantly from what we have recorded in the current period.
Deferred Income Taxes
Deferred income taxes are provided for the tax effect of temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements. ASC Topic 740, Income Taxes (“ASC 740”), provides for recognition of deferred tax assets if the realization of such deferred tax assets is more likely than not to occur. Realization of our net deferred tax assets is dependent upon the generation of sufficient taxable income in future years in appropriate tax
31
jurisdictions to obtain the benefit of the reversal of temporary differences, net operating loss carry-forwards, and tax credit carry-forwards. Each quarter we assess the likelihood that we will be able to recover our deferred tax assets. We consider available evidence, both positive and negative, including historical levels of income, expectations and risks associated with estimates of future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance. As a result of our analysis, we concluded that it is more likely than not that, as of December 31, 2016, our net deferred tax assets will not be realized, with the exception of those in Japan and Taiwan. Therefore, we continue to provide a full valuation allowance against net deferred tax assets outside of Japan and Taiwan. We continue to monitor the relative weight of positive and negative evidence of future profitability in relevant jurisdictions. However, as of December 31, 2016, we have determined that the following negative evidence outweighs the positive evidence such that it is not more likely than not that we will generate sufficient taxable income in the relevant jurisdictions to utilize our deferred tax assets and release the associated valuation allowance:
• | Movement of certain product manufacturing to Singapore, resulting in U.S. taxable losses, |
• | Cumulative three year U.S. taxable loss, |
• | Inherent earnings volatility of our industry resulting in our inability to forecast long term earnings, and |
• | Usage limitations resulting in a longer period being required to realize our deferred tax assets. |
As of December 31, 2016, we had recorded a valuation allowance of approximately $62.7 million against our net deferred tax assets except for those in Japan and Taiwan. As of December 31, 2016, we had recorded approximately $0.4 million of net foreign deferred tax assets related to our operations in Japan and Taiwan. Based on projected future pre-tax income in Japan and Taiwan, these assets were not subject to a valuation allowance as it is more likely than not that they will be realized in the future.
Impact of Recently Issued Accounting Standards
In May 2014, the Financial Accounting Standards Board (“FASB”) issued a new standard to achieve a consistent application of revenue recognition within the U.S., resulting in a single revenue model to be applied by reporting companies under U.S. GAAP. Under the new model, recognition of revenue occurs when a customer obtains control of promised goods or services in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In addition, the new standard requires that reporting companies disclose the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. On July 9, 2015, the FASB agreed to delay the effective date by one year. As currently issued and amended and in accordance with the agreed upon delay, the new standard is effective for us beginning in the first quarter of 2018. Early adoption is permitted, but not before the original effective date of the standard. The new standard is required to be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying it recognized at the date of initial application. While we continue to assess all potential impacts of the standard, we currently believe the most significant impact will likely relate to systems revenue. Under the new standard we are evaluating whether our systems revenue would be recognized on shipment. We are planning to adopt the standard on January 1, 2018. We plan to adopt the standard retrospectively with the cumulative effect of initially applying it recognized at the date of initial application ("modified retrospective" approach).
In February 2016, the FASB issued ASU 2016-02, “Leases” (Topic 842) which supersedes Topic 840, Leases. The update requires companies to generally recognize on the balance sheet operating and financing lease liabilities and corresponding right-of-use assets. The new standard is effective for us in our first quarter of fiscal year 2020 on a modified retrospective basis and earlier adoption is permitted. We are currently evaluating the impact of our pending adoption of ASU 2016-02 on our condensed consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, “Compensation - Stock Compensation” (Topic 718): Improvements to Employee Share-Based Payment Accounting. The update involves several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. It is effective for annual periods beginning after December 15, 2016. While we are continuing to assess all potential impacts of the standard, we currently believe it will not have a significant impact on our income tax provision, classification of awards or statement of cash flows.
RESULTS OF OPERATIONS
We derive a substantial portion of our total net sales from sales of a relatively small number of newly manufactured systems, which typically range in price from $0.1 million to $6.0 million. As a result of the larger sale prices, the timing and recognition of revenue from a single transaction has had and most likely will continue to have a significant impact on our net sales and operating results for any particular period.
32
Our backlog at the beginning of a period typically does not include all of the sales needed to achieve our sales objectives for that period. In addition, orders in backlog are subject to cancellation, shipment or system acceptance delays, and deferral or rescheduling by a customer with limited or no penalties. Consequently, our net sales and operating results for a period have been and will continue to be dependent upon our obtaining orders for systems to be shipped and accepted in the same period in which the order is received. Our business and financial results for a particular period could be materially adversely affected if an anticipated order for even one system is not received in time to permit shipment and system acceptance during that period. Furthermore, a substantial portion of our shipments has historically occurred near the end of each quarter. Delays in installation and system acceptance due, for example, to our inability to successfully demonstrate the agreed-upon specifications or criteria at the customer’s facility, or to the failure of the customer to permit installation of the system in the agreed upon time, may cause net sales in a particular period to fall significantly below our expectations, which may materially adversely affect our operating results for that period. This risk is especially applicable in connection with the introduction and initial sales of a new product line or as a result of industry trends impacting our foundry customers, including a shift toward semiconductors utilizing new technology transistor architecture. Additionally, the failure to receive anticipated orders or delays in shipments due, for example, to rescheduling, delays, deferrals or cancellations by customers, additional customer configuration requirements, or to unexpected manufacturing, or performance difficulties, or delays in deliveries by suppliers due to their long production lead times, or quality issues with components sourced from suppliers, have caused and may continue to cause net sales in a particular period to fall significantly below our expectations and for our operating results to vary from period to period, materially adversely affecting our operating results for that period. In particular, the long manufacturing and acceptance cycles of our advanced packaging family of wafer steppers, laser thermal processing and inspection systems and the long lead time for lenses and other materials, could cause shipments and acceptances of such products to be delayed from one quarter to the next, which could materially adversely affect our financial condition and results of operations for a particular quarter.
Additionally, the need for continued expenditures for research and development, capital equipment, ongoing training and worldwide customer service and support, among other factors, will make it difficult for us to reduce our operating expenses in a particular period if we fail to achieve our net sales goals for the period.
Sales Summary
2016 | 2015 | 2014 | ||||
International sales | 87% | 81% | 68% | |||
Domestic sales | 13% | 19% | 32% | |||
Total sales | 100% | 100% | 100% | |||
Semiconductor system sales | 93% | 84% | 81% | |||
Nanotechnology system sales | 7% | 16% | 19% | |||
Total system sales | 100% | 100% | 100% | |||
Net Sales
In millions | 2016 | 2015 | 2014 | 2016 vs 2015 | 2015 vs 2014 | ||||||||||
Sales of: | |||||||||||||||
Systems | $ | 157.2 | $ | 117.2 | $ | 114.5 | 34% | 2% | |||||||
Spare parts | 24.3 | 18.5 | 21.6 | 31% | (14)% | ||||||||||
Services | 11.9 | 13.1 | 14.0 | (9)% | (6)% | ||||||||||
Licenses | 0.7 | 0.4 | 0.4 | 75% | —% | ||||||||||
Total net sales | $ | 194.1 | $ | 149.2 | $ | 150.5 | 30% | (1)% | |||||||
2016 vs. 2015
Net sales consist of revenues from system sales (systems and system upgrades), spare parts sales, services and licensing of technologies. For the year ended December 31, 2016, system sales accounted for approximately 81% of total net sales. Sales of spare parts, services and licenses accounted for the remaining 19% of total net sales.
33
System sales increased 34% to $157.2 million primarily attributable to an increase in lithography, inspection and laser thermal processing system sales, partially offset by a decrease in system upgrades and nanotechnology sales in 2016, as compared to 2015.
On a product market application basis, system sales to the semiconductor industry were $145.6 million for the year ended December 31, 2016, an increase of 47% as compared to $98.9 million in 2015. This increase was primarily due to more system units sold. Semiconductor industry sales consist of sales to the advanced packaging market, semiconductor and semiconductor inspection market. System sales to the semiconductor industry are highly dependent on customer capacity demand.
System sales to the nanotechnology market were $11.6 million for the year ended December 31, 2016, a 36% decrease as compared with $18.2 million in 2015. The decrease in sales to the nanotechnology market was primarily due to a decrease in HBLED system sales, partially offset by an increase in ALD system sales. System sales to the nanotechnology market are highly dependent on capacity demand in the industries we serve, including HBLEDs, ALD, thin film heads, automotive MEMS, LED/laser diodes, and ink jet print heads. We therefore expect to experience significant variations in sales within this market from period to period.
Sales of spare parts for the year ended December 31, 2016 increased 31% to $24.3 million, as compared to $18.5 million in 2015. The increase in sales of spare parts was the result of an increase in spare parts demand. Services revenue decreased 9% to $11.9 million for the year ended December 31, 2016 as compared to $13.1 million in 2015. The decrease in services revenue was the result of a decrease in the number of service contracts.
Revenues from licensing activities for the year ended December 31, 2016, increased 75% to $0.7 million as compared to $0.4 million in 2015. Pursuant to our license arrangements, such transactions are subject to a license fee based on units sold. Future revenues from licensing activities, if any, will be contingent upon existing and future licensing arrangements. We may not be successful in generating licensing revenues and do not anticipate the recognition of significant levels of licensing income in the future.
For the year ended December 31, 2016, international net sales were $169.2 million, or 87% of total net sales, as compared with $120.9 million, or 81% of total net sales in 2015. For the year ended December 31, 2016 as compared to the corresponding 2015 period, sales to Greater China increased by $52.9 million and sales to Korea increased by $2.1 million, partially offset by (i) a decrease in sales to Japan of $4.0 million, (ii) a decrease in sales to the rest of the world of $1.6 million and (iii) a decrease in sales to Europe of $1.1 million. We expect sales to international customers to continue to represent a significant amount of our future revenues as our customer base in Asia continues to expand its capacity.
Our revenue derived from sales in foreign countries is not generally subject to significant exchange rate fluctuations, principally because sales contracts for our systems are generally denominated in U.S. dollars. However, we do sell systems and spare parts into Japan and this subjects us to the risk of currency exchange rate fluctuations since the orders are often denominated in Japanese yen. We attempt to mitigate this risk by entering into monthly foreign currency forward exchange contracts, as balance sheet derivatives, which includes a portion of our receivables that are denominated in Japanese yen from parts sales. For system sales, we attempt to mitigate foreign currency risk by entering into foreign currency forward contracts that generally have maturities of nine months or less, as cash flow derivatives. We had approximately $754.3 million of Japanese yen-denominated receivables at December 31, 2016. International sales expose us to a number of additional risks, including fluctuations in the value of local currencies relative to the U.S. dollar, which impact the relative cost of ownership of our products and, thus, the customer’s willingness to purchase our product. (See “Risk Factors - A substantial portion of our sales are outside of the United States, which subjects us to risks related to customer service, installation, foreign economic and political stability, uncertain regulatory and tax rules, and foreign exchange rate fluctuations, all of which make it more difficult to operate our business.”).
2015 vs. 2014
For the year ended December 31, 2015, system sales accounted for approximately 79% of total net sales. Sales of spare parts, services and licenses accounted for the remaining 21% of total net sales. System sales increased 2% to $117.2 million primarily attributable to an increase in lithography and inspection system sales, partially offset by a decrease in system upgrades, nanotechnology and laser thermal processing system sales in 2015, as compared to 2014.
On a product market application basis, system sales to the semiconductor industry were $98.9 million for the year ended December 31, 2015, an increase of 6% as compared to $92.9 million in 2014. This increase was primarily due to more system units sold.
34
System sales to the nanotechnology market were $18.2 million for the year ended December 31, 2015, a 16% decrease as compared with $21.7 million in 2014. The decrease in sales to the nanotechnology market was primarily due to a decrease in HBLED system sales, partially offset by an increase in ALD system sales.
Sales of spare parts for the year ended December 31, 2015 decreased 14% to $18.5 million, as compared to $21.6 million in 2014. The decrease in sales of spare parts was the result of a decrease in spare parts demand. Services revenue decreased 6% to $13.1 million for the year ended December 31, 2015 as compared to $14.0 million in 2014. The decrease in service revenue was the result of a decrease in the number of service contracts.
Revenues from licensing activities was unchanged for the year ended December 31, 2015, when compared with 2014.
For the year ended December 31, 2015, international net sales were $120.9 million, or 81% of total net sales, as compared with $102.9 million, or 68% of total net sales in 2014. For the year ended December 31, 2015 as compared to the corresponding 2014 period, sales to Greater China increased by $22.7 million, sales to Japan increased by $8.6 million and sales to the rest of the world increase by $10.0 million, partially offset by a decrease in sales to Korea of $21.4 million and a decrease in sales to Europe of $1.9 million.
Gross Profit
2016 vs. 2015
Gross margins were 46% and 44% for 2016 and 2015, respectively. The 2% increase in gross margin was due to an increase of 5% resulting from manufacturing efficiencies created by greater production volume and a 2% increase due to improved service utilization, partially offset by a 5% decrease in gross margin resulting from higher material cost due to product mix.
Our gross profit as a percentage of sales has been and most likely will continue to be significantly affected by a variety of factors, including: the introduction of new products, which typically have higher manufacturing costs until manufacturing efficiencies are realized and which are typically discounted more than existing products until the products gain market acceptance; the mix of products sold; the rate of capacity utilization; write-down of inventory and open purchase commitments; product discounts, pricing and competition in our targeted markets; and non-linearity of shipments during the period that can result in manufacturing inefficiencies.
2015 vs. 2014
Gross margins were 44% and 42% for 2015 and 2014, respectively. The 2% increase in gross margin was due to an improvement of 3% resulting from manufacturing efficiencies, partially offset by increases in other manufacturing costs which reduced total gross margin by 1%.
Research, Development and Engineering Expenses
In millions | 2016 | 2015 | 2014 | 2016 vs 2015 | 2015 vs 2014 | ||||||||||
Research, development and engineering expenses | $ | 35.2 | $ | 32.9 | $ | 33.6 | 7% | (2)% | |||||||
% of revenue | 18 | % | 22 | % | 22 | % | |||||||||
An inherent delay exists between the time when product development activities and expenditures occur and when resultant product revenue is ultimately realized. We expect current year research, development and engineering program investments to contribute to revenue in future years.
2016 vs. 2015
Research, development and engineering expenses in 2016 increased to $35.2 million, as compared to $32.9 million in 2015. The $2.3 million increase was primarily due to (i) a $2.2 million increase in engineering support costs and (ii) a $0.8 million increase in executive bonus plan expenses, partially offset by (a) a $0.5 million decrease in research and development expense primarily due to the restructuring activities undertaken during 2015 and (b) a $0.2 million decrease in stock-based
35
compensation expense. As a percentage of net sales, research, development and engineering expenses for the year ended December 31, 2016 decreased to 18% from 22% compared to 2015. The percentage decrease was primarily due to the increase in net sales as compared to 2015.
2015 vs. 2014
Research, development and engineering expenses in 2015 decreased to $32.9 million, as compared to $33.6 million in 2014. The $0.7 million decrease was primarily due to (i) a $1.0 million decrease in research and development expense primarily due to reduction in headcount and related expenses as well as the timing of certain research and development projects and (ii) a $0.1 million decrease in other research and development expenses, partially offset by (a) a $0.3 million increase in stock-based compensation expense. As a percentage of net sales, research, development and engineering expenses for the year ended December 31, 2015 remained flat at 22% compared to 2014.
Selling, General and Administrative Expenses
In millions | 2016 | 2015 | 2014 | 2016 vs 2015 | 2015 vs 2014 | ||||||||||
Selling, general and administrative expenses | $ | 45.4 | $ | 46.8 | $ | 48.7 | -3% | -4% | |||||||
% of revenue | 23 | % | 31 | % | 32 | % | |||||||||
2016 vs. 2015
Selling, general and administrative expenses decreased to $45.4 million in 2016, as compared to $46.8 million in 2015. The $1.4 million decrease was primarily due to (i) a $2.7 million decrease in stock-based compensation expense, (ii) a $2.1 million decrease associated with reduced headcount resulting from the 2015 restructuring and (iii) a $1.3 million decrease in service support cost, partially offset by (a) a $3.0 million increase in executive bonus plan expense, (b) a $1.0 million increase in non-recurring legal expenses related to the proxy contest, (c) a $0.5 million increase in health care expense and (d) a $0.2 million increase in other sales and marketing expense. As a percentage of net sales, selling, general and administrative expenses for the year ended December 31, 2016 were 23% compared to 31% for 2015. The decrease was due to the decrease in selling, general and administrative expenses and the increase in net sales as compared to 2015.
2015 vs. 2014
Selling, general and administrative expenses decreased to $46.8 million in 2015, as compared to $48.7 million in 2014. The $1.9 million decrease was primarily due to (i) a $1.8 million decrease in stock-based compensation expense, (ii) a $1.5 million decrease in travel expenses as a result of cost reduction measures, and (iii) a $0.9 million decrease associated with reductions in headcount, partially offset by (a) a $1.0 million increase in legal expenses, (b) a $0.7 million increase in bonus plan expenses, (c) a $0.3 increase in employee benefit costs and (d) a $0.3 million increase in certain marketing activities. As a percentage of net sales, selling, general and administrative expenses for the year ended December 31, 2015 were 31% compared to 32% for 2014. The decrease was due primarily to the decrease in selling, general and administrative expenses as compared to 2014.
Restructuring Expenses
In millions | 2016 | 2015 | 2014 | 2016 vs 2015 | 2015 vs 2014 | ||||||||||
Restructuring expenses | $ | — | $ | 0.8 | $ | — | -100% | 100% | |||||||
% of revenue | — | % | 1 | % | — | % | |||||||||
During the year ended December 31, 2016, there were no restructuring efforts. However, during the year ended December 31, 2015, we made reductions in our workforce as part of our efforts to remain competitive in the current environment. The restructuring costs totaled $0.8 million and were related to severance and benefits for headcount reductions in certain functions in the U.S. and certain foreign countries.
Interest and Other Income, Net
36
In millions | 2016 | 2015 | 2014 | ||||||||
Interest income | $ | 1.1 | $ | 0.5 | $ | 0.1 | |||||
Other income (expense), net | (0.5 | ) | (0.1 | ) | 0.1 | ||||||
Interest and other income, net | $ | 0.6 | $ | 0.4 | $ | 0.2 | |||||
Interest and other income, net, was $0.6 million for the year ended December 31, 2016, as compared with $0.4 million and $0.2 million for 2015 and 2014, respectively. We recorded $1.1 million of interest income during the year ended December 31, 2016. The $0.6 million increase was primarily due to higher returns on the short-term investments balance during the year ended December 31, 2016 as compared to 2015. For the year ended December 31, 2015 interest and other income, net was $0.4 million, as compared with $0.2 million for 2014. We recorded $0.5 million of interest income during the year ended December 31, 2015. The $0.4 million increase was primarily due to higher returns on the short-term investments balance during the year ended December 31, 2015, as compared to 2014. We presently maintain an investment portfolio with a weighted-average maturity of less than one year. Consequently, changes in short-term interest rates have a significant impact on our interest income. Future changes in short-term interest rates are expected to continue to have a significant impact on our interest income.
Other expense net of $0.5 million for the year ended December 31, 2016 was primarily the result of $0.5 million of losses from foreign currency movements. In 2015, other income, net of $0.1 million, was primarily the result of $0.2 million of losses from foreign currency movements, partially offset by $0.1 million in miscellaneous other income. In 2014, other expense, net of $0.1 million was the result of $0.5 million in miscellaneous other income, partially offset by $0.4 million of losses from foreign currency movements.
Provision for Income Taxes
For the year ended December 31, 2016, we recorded income tax benefit of $2.5 million as compared to an income tax expense of $0.5 million and an income tax expense of $0.2 million, respectively, in 2015 and 2014. The income tax benefit recorded in 2016 resulted primarily from the reversal of previously accrued liabilities for uncertain tax positions due to the lapsing of the statute of limitations, partially offset by tax expense recorded from foreign jurisdictions. The income tax expense recorded in 2015 and 2014 resulted primarily from foreign taxes. For the year ended December 31, 2016, the actual tax benefit recorded differs from the federal tax rate of 35% primarily due to the reversal of accrued liabilities related to uncertain tax positions and the fact that the U.S. taxable loss was not realized due to a valuation allowance. The actual income tax expense recorded for the years ended 2015 and 2014 differs from the federal tax rate of 35% primarily due to current tax expense in foreign jurisdictions and the fact that in both 2015 and 2014 the U.S. taxable loss was not realized due to a valuation allowance.
We are eligible for tax incentives that provide that certain income earned in Singapore is subject to a tax holiday and/or reduced tax rates for a limited period of time under the laws of Singapore. To realize these benefits, we must meet certain requirements relating to employment and investment activities. This exemption is expected to expire within four years. In 2016 the tax benefit attributable to the tax holiday was approximately $6.0 million with a $0.22 impact on earnings per share. In 2015, the tax benefit attributable to the tax holiday was approximately $3.0 million with a $0.11 impact on diluted earnings per share. In 2014, the tax benefit attributable to the tax holiday was approximately $1 million with a $0.04 impact on diluted earnings per share. Our ability to realize benefits from these initiatives could be materially adversely affected if, among other things, applicable requirements are not met, the incentives are substantially modified, or if we incur losses for which we cannot take a deduction.
Income taxes can be affected by estimates of whether, and within which jurisdictions, future earnings will occur and how, when and if cash is repatriated to the United States, combined with other aspects of an overall income tax strategy. Additionally, taxing jurisdictions could retroactively disagree with our tax treatment of certain items, and some historical transactions have income tax effects going forward. Accounting rules require these future effects to be evaluated using current laws, rules and regulations, each of which can change at any time and in an unpredictable manner. We believe we have adequately provided for any reasonably foreseeable outcome related to these matters and we do not anticipate any material earnings impact from their ultimate resolutions.
In accordance with ASC 740, we had unrecognized benefits of $15.2 million as of December 31, 2016 due to uncertain tax positions. We continue to recognize interest and penalties as a component of income tax provision and accrued an insignificant amount for these items for the year. During the year ended December 31, 2016, the balance related to uncertain tax positions decreased by $2.2 million, net, as discussed above. If we are able to eventually recognize these uncertain tax positions, $15.2 million of the unrecognized benefit on December 31, 2016, would reduce our effective tax rate. We currently
37
have a full valuation allowance against our U.S. net deferred tax asset which would impact the timing of the effective tax rate benefit should any of these uncertain tax positions be favorably settled in the future.
Each quarter we assess the likelihood that we will be able to recover our deferred tax assets. We consider available evidence, both positive and negative, including historical levels of income, expectations and risks associated with estimates of future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance. As a result of our analysis, and as further described in the Critical Accounting Policies and Estimates section, deferred income taxes, we concluded that it is more likely than not that, as of December 31, 2016, our net deferred tax assets will not be realized, with the exception of those in Japan and Taiwan. Therefore, we continue to provide a full valuation allowance against net deferred tax assets outside of Japan and Taiwan. It is possible that sometime in the next 12 months the positive evidence will be sufficient to release a material amount of our valuation allowance; however there is no assurance that this will occur.
We are subject to federal and state tax examination for years 2000 forward and 1997 forward, respectively, by virtue of the tax attributes carrying forward from those years. We are also subject to audits in the foreign jurisdictions in which we operate for years 2003 and forward. There are no material income tax examinations currently in progress.
Outlook
The anticipated timing of orders, shipments and system acceptances usually requires that we fill a number of production slots in any given period in order to meet our sales targets. If we are unsuccessful in our efforts to secure those production orders, or if existing production orders are delayed or cancelled, our results of operations will be materially adversely impacted. Additionally, we may not exceed or even maintain our current or prior levels of net sales for any period in the future for the reasons enumerated in this report. We believe that the market acceptance and volume production of our advanced packaging systems, laser processing systems and inspection systems are of critical importance to our future financial results. At December 31, 2016, these systems represented approximately 77% of our backlog.
LIQUIDITY AND CAPITAL RESOURCES
Net cash provided by operating activities was $22.0 million for the year ended December 31, 2016, as compared with $8.4 million for the comparable period in 2015. Net cash provided by operating activities during the year ended December 31, 2016 was attributable to $31.8 million of cash generated from operations after adjustments for non-cash charges such as stock-based compensation, depreciation and amortization of intangibles and others, partially offset by $9.7 million of working capital cash used by changes in operating assets and liabilities as described below.
Working capital uses of cash of $32.4 million were due to (i) a $26.4 million increase in accounts receivables, (ii) a $4.7 million increase in prepaid expenses and other assets, (iii) a $1.2 million decrease in other liabilities and (iv) a $0.1 million decrease in deferred product and service income. Working capital sources of cash of $22.7 million were due to (a) a $14.9 million decrease in inventory, (b) a $1.9 million increase in accounts payable and (c) a $5.9 million increase in accrued expenses.
We believe that because of the relatively long manufacturing cycle of certain systems, particularly newer products, our inventories will continue to represent a significant portion of working capital. Currently, we are devoting significant resources to the development, introduction and commercialization of our laser thermal processing systems and inspection systems and to the development of our next generation 1X lithography technologies. We intend to continue to incur significant operating expenses in the areas of research, development and engineering, manufacturing, and selling, general and administrative costs in order to further develop, produce and support these new products. There can be no assurance that any expenditures to develop these products will result in improved sales or that markets for these products will become viable. Additionally, gross profit margins, inventory and capital equipment levels may be adversely impacted in the future by costs associated with the initial production of the laser thermal processing systems, inspection systems and by future generations of our 1X wafer steppers. These costs include, but are not limited to, additional manufacturing overhead, costs of demonstration systems and facilities and the establishment of additional after-sales support organizations. Additionally, there can be no assurance that operating expenses will not increase, relative to sales, as a result of adding technical, marketing and administrative personnel, among other costs, to support our new products. If we are unable to achieve significantly increased net sales or if our sales fall below expectations, our cash flow and operating results will be materially adversely affected until, among other factors, costs and expenses can be reduced. Our failure to achieve our sales targets for these new products could result in additional inventory write-offs and asset impairment charges, either of which could materially adversely impact our results of operations.
38
During the year ended December 31, 2016, net cash provided by investing activities was $6.0 million, as compared with net cash provided by investing activities of $10.0 million for the comparable period in 2015. Net cash provided by investing activities during the year ended December 31, 2016 was attributable to maturities of available-for-sale investment securities of $8.2 million, net of purchases, partially offset by capital expenditures of $2.2 million.
Net cash used in financing activities was $4.3 million during the year ended December 31, 2016, as compared with net cash used in financing activities of $23.4 million for the comparable period in 2015. Net cash used in financing activities during the year ended December 31, 2016 was primarily attributable to (i) the net payment of notes payable of $3.6 million, (ii) tax payments for the release of restricted stock units of $2.6 million, partially offset by proceeds received from the issuance of common stock under our stock-based compensation plans of $1.9 million, net.
Historically, our principal source of liquidity has been cash provided by our operations. At December 31, 2016, we had working capital of $342.4 million. At December 31, 2016, our cash, cash equivalents and short-term investments, net of related borrowings under our line of credit, was $266.1 million.
At December 31, 2016, $75.9 million of our cash, cash equivalents, and investments was held by foreign subsidiaries. Amounts held by foreign subsidiaries are generally subject to U.S. income taxation on repatriation to the United States. We currently have no plans to repatriate any foreign earnings back to the United States as we believe our cash flows provided by our U.S. operations will meet our future U.S. liquidity needs.
In December 2004, we entered into a line of credit agreement with a brokerage firm replacing a similar arrangement that we had with a different firm. Under the terms of this agreement, we may borrow funds at a cost equal to the current Federal funds rate plus 125 basis points (i.e. 1.91% as of December 31, 2016). Certain of our cash, cash equivalents and short-term investments secure outstanding borrowings under this facility. We may borrow up to 75% of our total cash, cash equivalents and investments balance in this brokerage account. Funds are advanced to us under this facility based on pre-determined advance rates on the cash and securities held by us in this brokerage account. This agreement has no set expiration date and there are no loan covenants. As of December 31, 2016 and 2015, balances of $1.5 million and $5.1 million, respectively were outstanding under this facility with a related collateral requirement of approximately $2.0 million of our cash, cash equivalents and investments.
The following summarizes our contractual obligations at December 31, 2016, and the effect such obligations are expected to have on our liquidity and cash flows in future periods:
In millions | Total | Less than 1 year | 1-3 years | 3-5 years | After 5 years | ||||||||||||||
Notes payable obligations | $ | 1.5 | $ | 1.5 | $ | — | $ | — | $ | — | |||||||||
Non-cancelable capital lease obligations | 0.5 | 0.5 | — | — | — | ||||||||||||||
Non-cancelable operating lease obligations | 14.2 | 3.5 | 6.2 | 3.7 | 0.8 | ||||||||||||||
Long-term payables | 9.8 | — | 1.8 | — | 8.0 | ||||||||||||||
Asset retirement obligations | 2.7 | — | 2.7 | — | — | ||||||||||||||
Open purchase order commitments | 16.6 | 16.6 | — | — | — | ||||||||||||||
Total contractual cash obligations | $ | 45.3 | $ | 22.1 | $ | 10.7 | $ | 3.7 | $ | 8.8 | |||||||||
The amounts shown in the table above for open purchase order commitments are primarily related to the purchase of inventories, equipment and leasehold improvements. We record charges to operations for purchase order commitments we deem in excess of normal operating requirements (see “Critical Accounting Policies and Estimates”).
The development and manufacture of new systems and enhancements are highly capital-intensive. In order to be competitive, we believe we must continue to make significant expenditures for capital equipment; sales, service, training and support capabilities; systems, procedures and controls; and expansion of operations and research and development, among many other items. We expect that cash generated from operations and our cash, cash equivalents and short-term investments will be sufficient to meet our cash requirements for at least the next twelve months. However, in the near-term, we may continue to utilize existing and future lines of credit, and other sources of financing, in order to maintain our present levels of cash, cash equivalents and short-term investments. Beyond the next twelve months, we may require additional equity or debt financing to address our working capital or capital equipment needs. In addition, we may seek to raise equity or debt capital at any time that we deem market conditions to be favorable. Additional financing, if needed, may not be available on reasonable terms, or at all.
39
We have in the past, and may in the future, pursue acquisitions of complementary product lines, technologies or businesses. Future acquisitions may require the use of substantial amounts of our cash, cash equivalents and short-term investments, the issuance of potentially dilutive equity securities, the incurrence of debt and contingent liabilities and amortization expenses and impairment charges related to goodwill and other intangible assets, which could materially adversely affect our financial condition and results of operations. In addition, acquisitions involve numerous risks, including difficulties in the assimilation of the operations, technologies, personnel and products of the acquired companies; the diversion of management’s attention from other business concerns; risks of entering markets in which we have limited or no direct experience; and the potential loss of key employees of the acquired company. In the event we acquire product lines, technologies or businesses which do not complement our business, or which otherwise do not enhance our sales or operating results, we may incur substantial write-offs and higher recurring operating costs, which could have a material adverse effect on our business, financial condition and results of operations. In the event that any such acquisition does occur, there can be no assurance as to the effect thereof on our business or operating results.
Off-Balance Sheet Transactions
Our off-balance sheet transactions consist of certain financial guarantees, both expressed and implied, related to indemnification for product liability, patent infringement and latent product defects. Other than liabilities recorded pursuant to known product defects, at December 31, 2016, we did not record a liability associated with these guarantees, as we have little or no history of costs associated with such indemnification requirements. See Note 17 to our Consolidated Financial Statements for additional information.
Foreign Currency
As part of our overall strategy to manage the level of exposure to the risk of foreign currency exchange rate fluctuations, we attempt to hedge most of our Japanese yen-denominated foreign currency exposures. We use foreign currency forward contracts to hedge the risk that outstanding Japanese yen-denominated receipts from customers, for actual or forecasted sales of equipment after receipt of customer orders, may be adversely affected by changes in foreign currency exchange rates. We use foreign currency forward exchange contracts and natural hedges to offset substantial portions of the potential gains or losses associated with our Japanese yen-denominated assets and liabilities due to exchange rate fluctuations. We enter into foreign currency forward contracts that generally have maturities of nine months or less.
40
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate Risk
Our exposure to market risk due to potential changes in interest rates, relates primarily to our investment portfolio, which consisted primarily of fixed interest rate instruments as of December 31, 2016 and 2015. We maintain an investment policy designed to ensure the safety and preservation of our invested funds by limiting market risk and the risk of default.
Certain of our cash, cash equivalents and investments serve as collateral for a line of credit we maintain with a brokerage firm. The line of credit is used for liquidity purposes, mitigating the need to liquidate investments in order to meet our current operating cash requirements.
The following table presents the hypothetical changes in fair values in the financial instruments held by us at December 31, 2016 that are sensitive to changes in interest rates. These instruments are comprised of cash equivalents and investments. These instruments are held for purposes other than trading. The modeling techniques used to measure the change in fair values arising from selected hypothetical changes in interest rates. Assumed market value changes to our portfolio reflect immediate hypothetical parallel shifts in the yield curve of plus or minus 50 basis points (“BPS”), 100 BPS, and 150 BPS:
Cash equivalents and available-for-sale investments, in thousands | Valuation of securities given an interest rate decrease of X basis points | No change in interest rate | Valuation of securities given an interest rate increase of X basis points | ||||||||||||||||||||||||
(150 BPS) | (100 BPS) | (50 BPS) | 0 BPS | 50 BPS | 100 BPS | 150 BPS | |||||||||||||||||||||
Commercial paper | $ | 23,983 | $ | 23,882 | $ | 23,782 | $ | 23,684 | $ | 23,586 | $ | 23,489 | $ | 23,394 | |||||||||||||
Money market funds | 2,266 | 2,266 | 2,266 | 2,266 | 2,266 | 2,266 | 2,266 | ||||||||||||||||||||
U.S. treasury bills and notes | 29,596 | 29,551 | 29,506 | 29,461 | 29,417 | 29,374 | 29,330 | ||||||||||||||||||||
Securities and obligations of U.S. government agencies | 129,622 | 128,975 | 128,336 | 127,704 | 127,079 | 126,461 | 125,850 | ||||||||||||||||||||
Total investments | $ | 185,467 | $ | 184,674 | $ | 183,890 | $ | 183,115 | $ | 182,348 | $ | 181,590 | $ | 180,840 | |||||||||||||
During the year ended December 31, 2016, we did not materially alter our investment objectives or criteria and believe that, although the composition of our portfolio has changed from the preceding year, the portfolio’s sensitivity to changes in interest rates is materially the same.
Credit Risk
We mitigate credit default risk by attempting to invest in high credit quality securities and by positioning our portfolio to respond appropriately to a significant reduction in a credit rating of any investment issuer or guarantor. Our portfolio includes only marketable securities with active secondary or resale markets to ensure portfolio liquidity and is diversified in accordance with our investment policy. To date, we have not experienced significant liquidity problems with our portfolio. Our single largest holding at December 31, 2016, excluding the U.S. government and its agencies, was a $2.3 million money market fund.
As of December 31, 2016, we did not have any investments in mortgage backed or auction rate securities. However, we intend to closely monitor developments in the credit markets and make appropriate changes to our investment policy as deemed necessary or advisable. Based on our ability to liquidate our investment portfolio and our expected operating cash flows, we do not anticipate any liquidity constraints as a result of the current credit environment.
Foreign Exchange Risk
The majority of our revenue, expense and capital purchasing activities are transacted in U.S. dollars. However, we do enter into these transactions in other currencies, primarily Japanese yen. To protect against reductions in value and the volatility of future cash flows caused by changes in currency exchange rates, we have established cash flow and balance sheet hedging programs.
41
We use foreign currency forward contracts to hedge the risk that outstanding Japanese yen-denominated receipts from customers for actual or forecasted sales of equipment may be adversely affected by changes in foreign currency exchange rates. Our hedging programs reduce, but do not always entirely eliminate, the impact of currency movements. See “Derivative instruments and hedging” in Note 4 of Notes to Consolidated Financial Statements for additional disclosures.
42
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The Selected Financial Data information contained in Item 6 of Part II hereof is hereby incorporated by reference into this Item 8 of Part II of this Form 10-K.
ULTRATECH, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Consolidated Financial Statements included in Item 8:
Page Number | |
43
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Ultratech, Inc.
We have audited the accompanying consolidated balance sheets of Ultratech, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016. Our audits also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Ultratech, Inc. at December 31, 2016 and 2015, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Ultratech, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP | |
San Jose, California | |
March 1, 2017 | |
44
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Ultratech, Inc.
We have audited Ultratech, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Ultratech, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Ultratech, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Ultratech, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016 of Ultratech, Inc. and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP | |
San Jose, California | |
March 1, 2017 | |
45
ULTRATECH, INC.
CONSOLIDATED BALANCE SHEETS
In thousands, except share and per share amounts | December 31, 2016 | December 31, 2015 | ||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 86,744 | $ | 63,008 | ||||
Short-term investments | 180,849 | 188,893 | ||||||
Accounts receivable, net of allowance for doubtful accounts of $498 and $498 at December 31, 2016 and 2015, respectively | 54,549 | 28,108 | ||||||
Inventories, net | 50,475 | 65,398 | ||||||
Prepaid expenses and other current assets | 7,658 | 3,862 | ||||||
Total current assets | 380,275 | 349,269 | ||||||
Property, plant, and equipment, net | 13,869 | 17,280 | ||||||
Intangible assets, net | 10,630 | 12,288 | ||||||
Other assets | 10,798 | 10,359 | ||||||
Total assets | $ | 415,572 | $ | 389,196 | ||||
Liabilities and Stockholders’ Equity | ||||||||
Current liabilities: | ||||||||
Notes payable | $ | 1,500 | $ | 5,120 | ||||
Accounts payable | 14,038 | 12,080 | ||||||
Accrued expenses | 18,028 | 12,146 | ||||||
Deferred product and services income | 4,352 | 4,499 | ||||||
Total current liabilities | 37,918 | 33,845 | ||||||
Other liabilities | 12,456 | 13,474 | ||||||
Commitments and contingencies | ||||||||
Stockholders’ equity: | ||||||||
Preferred stock, $0.001 par value: 2,000,000 shares authorized; none issued | — | — | ||||||
Common stock, $0.001 par value: 80,000,000 shares authorized; 31,033,139 and 30,606,577 shares issued at December 31, 2016 and 2015, respectively; and 26,950,338 and 26,522,276 outstanding at December 31, 2016 and 2015, respectively | 31 | 30 | ||||||
Additional paid-in capital | 378,110 | 366,629 | ||||||
Treasury stock: 4,082,801and 4,084,301 shares at December 31, 2016 and 2015, respectively | (67,163 | ) | (67,185 | ) | ||||
Accumulated other comprehensive loss, net | (619 | ) | (1,199 | ) | ||||
Retained earnings | 54,839 | 43,602 | ||||||
Total stockholders’ equity | 365,198 | 341,877 | ||||||
Total liabilities and stockholders’ equity | $ | 415,572 | $ | 389,196 | ||||
See accompanying notes to consolidated financial statements.
46
ULTRATECH, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
Years Ended December 31, | |||||||||||
In thousands, except per share amounts | 2016 | 2015 | 2014 | ||||||||
Net sales | |||||||||||
Products | $ | 181,433 | $ | 135,628 | $ | 136,169 | |||||
Services | 11,891 | 13,148 | 13,973 | ||||||||
Licenses | 727 | 400 | 398 | ||||||||
Total net sales | 194,051 | 149,176 | 150,540 | ||||||||
Cost of sales | |||||||||||
Cost of products sold | 93,122 | 71,159 | 74,872 | ||||||||
Cost of services | 12,194 | 12,585 | 12,395 | ||||||||
Gross profit | 88,735 | 65,432 | 63,273 | ||||||||
Research, development and engineering | 35,201 | 32,886 | 33,590 | ||||||||
General and administrative | 45,412 | 46,835 | 48,746 | ||||||||
Restructuring | — | 751 | — | ||||||||
Operating income (loss) | 8,122 | (15,040 | ) | (19,063 | ) | ||||||
Interest expense | (43 | ) | (80 | ) | (35 | ) | |||||
Interest and other income, net | 613 | 442 | 231 | ||||||||
Income (loss) before income taxes | 8,692 | (14,678 | ) | (18,867 | ) | ||||||
Provision (benefit) for income taxes | (2,545 | ) | 450 | 244 | |||||||
Net income (loss) | $ | 11,237 | $ | (15,128 | ) | $ | (19,111 | ) | |||
Net income (loss) per share—basic | |||||||||||
Net income (loss) per share | $ | 0.42 | $ | (0.55 | ) | $ | (0.67 | ) | |||
Number of shares used in per share computations—basic | 27,012 | 27,429 | 28,437 | ||||||||
Net income (loss) per share—diluted | |||||||||||
Net income (loss) per share | $ | 0.41 | $ | (0.55 | ) | $ | (0.67 | ) | |||
Number of shares used in per share computations—diluted | 27,333 | 27,429 | 28,437 | ||||||||
See accompanying notes to consolidated financial statements.
47
ULTRATECH, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
Years Ended December 31, | ||||||||||||
(In thousands) | 2016 | 2015 | 2014 | |||||||||
Net income (loss) | $ | 11,237 | $ | (15,128 | ) | $ | (19,111 | ) | ||||
Other comprehensive income (loss), net of tax: | ||||||||||||
Change in unrealized loss on investments | 53 | (258 | ) | (86 | ) | |||||||
Change in minimum postretirement medical obligation | 527 | 318 | (1,066 | ) | ||||||||
Change in unrealized loss on hedge contracts | — | — | (40 | ) | ||||||||
Other comprehensive income (loss) | 580 | 60 | (1,192 | ) | ||||||||
Total comprehensive income (loss) | $ | 11,817 | $ | (15,068 | ) | $ | (20,303 | ) | ||||
See accompanying notes to consolidated financial statements.
48
ULTRATECH, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years Ended December 31, | |||||||||||
In thousands | 2016 | 2015 | 2014 | ||||||||
Cash flows from operating activities: | |||||||||||
Net income (loss) | $ | 11,237 | $ | (15,128 | ) | $ | (19,111 | ) | |||
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | |||||||||||
Depreciation | 5,350 | 6,461 | 6,353 | ||||||||
Amortization of securities discount | (82 | ) | (23 | ) | (200 | ) | |||||
Amortization of intangible assets | 1,658 | 1,707 | 1,740 | ||||||||
Amortization of other | 1,088 | 885 | 827 | ||||||||
Accretion of asset retirement obligations | 158 | 147 | 136 | ||||||||
Loss on disposal of equipment | 133 | — | 9 | ||||||||
Provision for inventory reserves and purchase order commitments | — | — | 306 | ||||||||
Stock-based compensation | 12,220 | 15,262 | 16,827 | ||||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable | (26,441 | ) | 16,109 | (9,776 | ) | ||||||
Inventories | 14,922 | (13,061 | ) | (4,830 | ) | ||||||
Prepaid expenses and other current assets | (3,796 | ) | 1,913 | (316 | ) | ||||||
Other assets | (921 | ) | (616 | ) | (638 | ) | |||||
Accounts payable | 1,959 | (6 | ) | 3,172 | |||||||
Accrued expenses | 5,882 | 620 | (3,462 | ) | |||||||
Deferred product and services income | (147 | ) | (4,139 | ) | 2,064 | ||||||
Other liabilities | (1,176 | ) | (1,701 | ) | 245 | ||||||
Net cash provided by (used in) operating activities | 22,044 | 8,430 | (6,654 | ) | |||||||
Cash flows from investing activities: | |||||||||||
Capital expenditures | (2,153 | ) | (2,587 | ) | (2,823 | ) | |||||
Purchase of investments in securities | (174,115 | ) | (233,636 | ) | (230,033 | ) | |||||
Proceeds from maturities of investments | 182,294 | 246,252 | 231,889 | ||||||||
Net cash provided by (used in) investing activities | 6,026 | 10,029 | (967 | ) | |||||||
Cash flows from financing activities: | |||||||||||
Proceeds from notes payable | 8,820 | 20,480 | 20,480 | ||||||||
Repayment of notes payable | (12,440 | ) | (20,480 | ) | (20,480 | ) | |||||
Proceeds from issuance of common stock for stock option exercises | 1,857 | 332 | 4,279 | ||||||||
Tax payments for restricted stock unit releases | (2,571 | ) | (2,333 | ) | (2,950 | ) | |||||
Repurchase of common stock | — | (21,438 | ) | (19,270 | ) | ||||||
Net cash used in financing activities | (4,334 | ) | (23,439 | ) | (17,941 | ) | |||||
Net increase (decrease) in cash and cash equivalents | 23,736 | (4,980 | ) | (25,562 | ) | ||||||
Cash and cash equivalents at beginning of period | 63,008 | 67,988 | 93,550 | ||||||||
Cash and cash equivalents at end of period | $ | 86,744 | $ | 63,008 | $ | 67,988 | |||||
Supplemental disclosures of cash flow information: | |||||||||||
Cash paid during the period for income taxes | $ | 416 | $ | 325 | $ | 396 | |||||
Other non-cash changes: | |||||||||||
Systems transferred from inventory and other assets to equipment, net | $ | — | $ | 338 | $ | 302 | |||||
Capital lease of equipment | $ | — | $ | — | $ | 2,460 | |||||
See accompanying notes to consolidated financial statements.
49
ULTRATECH, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
Stockholders’ Equity | ||||||||||||||||||||||||||
Common Stock | Additional Paid-in Capital | Treasury Stock | Accumulated Other Comprehensive Income (Loss) | Retained Earnings | Total Stockholders’ Equity | |||||||||||||||||||||
In thousands, except share data | Shares | Amount | ||||||||||||||||||||||||
Balance at December 31, 2013 | 27,854,553 | 29 | 335,232 | (26,497 | ) | (67 | ) | 77,841 | 386,538 | |||||||||||||||||
Net issuance of common stock under stock option plans | 541,221 | 1 | 1,307 | 20 | 1,328 | |||||||||||||||||||||
Stock-based compensation | — | — | 16,827 | — | — | — | 16,827 | |||||||||||||||||||
Repurchase of common stock | (1,000,000 | ) | (19,270 | ) | (19,270 | ) | ||||||||||||||||||||
Other comprehensive loss | — | — | — | — | (1,192 | ) | — | (1,192 | ) | |||||||||||||||||
Net loss | — | — | — | — | — | (19,111 | ) | (19,111 | ) | |||||||||||||||||
Total comprehensive loss | (20,303 | ) | ||||||||||||||||||||||||
Balance at December 31, 2014 | 27,395,774 | 30 | 353,366 | (45,747 | ) | (1,259 | ) | 58,730 | 365,120 | |||||||||||||||||
Net issuance of common stock under stock option plans | 376,502 | — | (1,999 | ) | — | — | — | (1,999 | ) | |||||||||||||||||
Stock-based compensation | — | — | 15,262 | — | — | — | 15,262 | |||||||||||||||||||
Repurchase of common stock | (1,250,000 | ) | — | — | (21,438 | ) | — | — | (21,438 | ) | ||||||||||||||||
Other comprehensive income | — | — | — | — | 60 | — | 60 | |||||||||||||||||||
Net loss | — | — | — | — | — | (15,128 | ) | (15,128 | ) | |||||||||||||||||
Total comprehensive loss | (15,068 | ) | ||||||||||||||||||||||||
Balance at December 31, 2015 | 26,522,276 | $ | 30 | $ | 366,629 | $ | (67,185 | ) | $ | (1,199 | ) | $ | 43,602 | $ | 341,877 | |||||||||||
Net issuance of common stock under stock option plans | 428,062 | 1 | (739 | ) | 22 | — | — | (716 | ) | |||||||||||||||||
Stock-based compensation | — | — | 12,220 | — | — | — | 12,220 | |||||||||||||||||||
Other comprehensive income | — | — | — | — | 580 | — | 580 | |||||||||||||||||||
Net income | — | — | — | — | — | 11,237 | 11,237 | |||||||||||||||||||
Total comprehensive income | 11,817 | |||||||||||||||||||||||||
Balance at December 31, 2016 | 26,950,338 | $ | 31 | $ | 378,110 | $ | (67,163 | ) | $ | (619 | ) | $ | 54,839 | $ | 365,198 | |||||||||||
See accompanying notes to consolidated financial statements.
50
ULTRATECH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. COMPANY AND INDUSTRY INFORMATION
Nature of Operations
Ultratech, Inc. develops, manufactures and markets photolithography, laser thermal processing and inspection equipment for manufacturers of integrated circuits and nanotechnology components located throughout North America, Europe, Singapore, Japan, Taiwan, Korea and the rest of Asia.
We supply step-and-repeat photolithography systems based on one-to-one imaging technology. Within the integrated circuit industry, we target the market for advanced packaging applications. Within the nanotechnology industry, our target markets include laser diodes, high-brightness light emitting diodes (“HBLEDs”), Micro-Electro-Mechanical Systems (“MEMS”) and atomic layer deposition (“ALD”). Our laser thermal processing equipment is targeted at advanced annealing applications within the semiconductor industry.
Major Customers
In 2016, Taiwan Semiconductor Manufacturing Co. Ltd. (“TSMC”), United Microelectronics Corporation, Powertech Technology, Inc. and Semiconductor Manufacturing North China (Beijing) Corporation (“SMNC”) accounted for 30%, 13%, 13% and 11% of our total systems sales, respectively. In 2015, Siliconware Precision Industries Co. Ltd. (“SPIL”), TSMC and Intel Corporation (“Intel”) accounted for 21%, 13% and 10% of our total system sales, respectively. In 2014, Intel, Samsung and Global Foundries Incorporated (“GFI”) accounted for 18%, 14% and 13% of our total system sales, respectively.
At December 31, 2016, TSMC, Powertech Technology, Inc., Flash Forward, Ltd., SMNC and United Microelectronics Corporation accounted for 22%, 19%, 12%, 11% and 11% of our accounts receivable, respectively. At December 31, 2015, TSMC and Flash Forward, Ltd. accounted for 36% and 19% of our accounts receivable, respectively.
Business Segments
In evaluating our business, we give consideration to the Chief Executive Officer’s review of financial information and the organizational structure of our management. Based on this review, we concluded that, at the present time, resources are allocated and other financial decisions are made based on consolidated financial information. Accordingly, we have determined that we operate in one business segment, which is the manufacture and distribution of capital equipment to manufacturers of integrated circuits and nanotechnology components.
51
Enterprise-Wide Disclosures
Our products are manufactured in the United States and Singapore, and are sold worldwide. We market our products internationally through domestic and foreign-based sales and service offices. The following table presents enterprise-wide sales to external customers and long-lived assets by geographic region:
In thousands | 2016 | 2015 | 2014 | ||||||||
Net sales: | |||||||||||
United States | $ | 24,871 | $ | 28,297 | $ | 47,629 | |||||
International: | |||||||||||
Greater China | 120,690 | 67,927 | 45,227 | ||||||||
Rest of the world | 17,730 | 19,365 | 9,208 | ||||||||
Europe | 14,578 | 15,639 | 17,688 | ||||||||
Japan | 9,577 | 13,528 | 5,036 | ||||||||
Korea | 6,605 | 4,420 | 25,752 | ||||||||
Subtotal | 169,180 | 120,879 | 102,911 | ||||||||
Total | $ | 194,051 | $ | 149,176 | $ | 150,540 | |||||
Long-lived assets: | |||||||||||
United States | $ | 30,760 | $ | 34,124 | $ | 39,577 | |||||
Singapore | 3,836 | 5,049 | 5,367 | ||||||||
Rest of the world | 702 | 756 | 994 | ||||||||
Total | $ | 35,298 | $ | 39,929 | $ | 45,938 | |||||
The rest of the world is comprised of sales to customers and long-lived assets in countries that are individually insignificant.
With the exception of Japan, our operations in foreign countries are not currently subject to significant currency exchange rate fluctuations, principally because sales contracts for our systems are generally denominated in U.S. dollars. In Japan, we sell our products in both U.S. dollars and Japanese yen. However, we attempt to mitigate our currency exchange rate exposure through the use of currency forward contracts. (See “Derivative Instruments and Hedging” in Note 4.)
2. CONCENTRATIONS OF RISKS
Financial instruments that potentially subject us to concentrations of credit risk consist principally of cash equivalents, short-term investments and trade receivables. These credit risks include the potential inability of an issuer or customer to honor their obligations under the terms of the instrument or the sales agreement. We place our cash equivalents and investments with high credit-quality financial institutions. We invest our excess cash in commercial paper, readily marketable debt instruments and collateralized funds of United States and state government entities. We have established guidelines relative to credit ratings, diversification and maturities that seek to maintain principal balance and liquidity.
A majority of our trade receivables are derived from sales in various geographic areas, principally the United States, Europe, Korea, Japan, Taiwan and the rest of Asia, to large companies within the integrated circuit and nanotechnology industries. We perform ongoing credit evaluations of our customers’ financial condition and require collateral, whenever deemed necessary. As of December 31, 2016 and 2015, the recorded value of our accounts receivable approximated fair value due to the short-term nature of our accounts receivable.
Sole-source and single-source suppliers provide critical components and services for the manufacture of our products. The reliance on sole or limited groups of suppliers may subject us from time to time to quality, allocation and pricing constraints.
3. BASIS OF PRESENTATION
The accompanying consolidated financial statements include the accounts of Ultratech and our subsidiaries, all of which are wholly owned. Intercompany balances and transactions have been eliminated.
52
The U.S. dollar is the functional currency for all foreign operations. Foreign exchange gains and losses which result from the process of remeasuring foreign currency financial statements into U.S. dollars or from foreign currency exchange transactions during the period, are included in interest and other income, net. Net foreign exchange losses during the years ended December 31, 2016, 2015 and 2014 were $0.5 million, $0.2 million, and $0.4 million, respectively.
Our fiscal quarters in 2016 ended on April 2, 2016, July 2, 2016, October 1, 2016 and December 31, 2016. Our fiscal quarters in 2015 ended on April 4, 2015, July 4, 2015, October 3, 2015 and December 31, 2015.
We have evaluated subsequent events, as defined by Accounting Standard Codification (“ASC”) Topic 855, through March 1, 2017, which is the issuance date of our financial statements.
Use of Estimates
The preparation of the financial statements and related disclosures in conformity with accounting principles generally accepted in the United States requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities at the date of the consolidated financial statements. By their nature, these estimates and judgments are subject to an inherent degree of uncertainty. On an ongoing basis, management evaluates its estimates, including those related to inventory valuation and purchase order commitments, warranty obligations, asset retirement obligations, bad debts, estimated useful lives of fixed assets, intangible assets, asset impairment, income taxes, deferred income tax valuation allowance, stock-based compensation, and contingencies and litigation. Management bases its estimates on historical experience and on various other analyses and assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
4. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Cash Equivalents
Cash equivalents consist of highly liquid investments with an original maturity date at acquisition of three months or less. The carrying value of cash equivalents approximates fair value.
Investments
Management determines the appropriate classification of its investments at the time of purchase and re-evaluates the classification at each balance sheet date. At December 31, 2016 and 2015, all investments and cash equivalents in our portfolio were classified as “available-for-sale” and are stated at fair value, with the unrealized gains and losses, net of tax, reported in accumulated other comprehensive income (loss), as a separate component of stockholders’ equity. The fair value of short-term investments are estimated based on quoted prices in active markets or significant other observable inputs as of December 31, 2016 and 2015.
The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization, as well as interest, dividends, realized gains and losses and declines in value judged to be other than temporary are included in interest and other income, net. The cost of securities sold is based on the specific identification method.
Allowance for Bad Debts
We maintain an allowance for uncollectible accounts receivable based upon expected collectability. This reserve is established based upon historical trends, current economic conditions, delinquency status based on contractual terms and an analysis of specific exposures.
Inventories
Inventories are stated at the lower of cost or market using standard costs that generally approximate actual costs on a first-in, first-out basis. We maintain a perpetual inventory system and continuously record the quantity on-hand and standard cost for each product, including purchased components, subassemblies, and finished goods. We maintain the integrity of perpetual inventory records through periodic physical counts of quantities on hand. The semiconductor industry is characterized by rapid technological change, changes in customer requirements and evolving industry standards. We perform a detailed
53
assessment of inventory at each balance sheet date, which includes a review of, among other factors, demand requirements and market conditions. Based on this analysis, we may record adjustments to the value of our inventory. During the years ended December 31, 2016 and 2015 we have recorded zero in reserves and recorded no charges for excess and obsolete inventories and open purchase order commitments.
Long-lived Assets
Equipment and leasehold improvements are stated at cost, less accumulated depreciation and amortization. Equipment is depreciated on a straight-line basis over the estimated useful lives (i.e. three to nine years). Leasehold improvements are amortized on a straight-line basis over the life of the related assets or the lease term, whichever is shorter. Depreciation and amortization expense for the years ended December 31, 2016, 2015 and 2014 was $5.4 million, $6.5 million and $6.4 million, respectively.
We review long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of these assets may not be recoverable. We assess these assets for impairment based on estimated future cash flows from these assets. No asset impairment charges have been recorded during the years ended December 31, 2016, 2015 and 2014.
Intangible Assets
Purchased technology, patents and other intangible assets are presented at cost, net of accumulated amortization, and are amortized over their estimated useful lives using the straight-line method. Amortization expense for each of the years ended December 31, 2016, 2015 and 2014 was $1.7 million.
We review for impairment whenever events or changes in circumstances indicate that the carrying amount of the intangible asset may not be recoverable and the carrying amount exceeds its fair value. No intangible assets impairment charges have been recorded during the years ended December 31, 2016, 2015 and 2014.
Derivative Instruments and Hedging
The majority of our revenue, expense and capital purchasing activities are transacted in U.S. dollars. However, we also enter into these transactions in other currencies, primarily Japanese yen. Our policy is to minimize foreign currency denominated transaction and remeasurement exposures with derivative instruments, mainly forward contracts. The gains and losses on these derivatives are intended to at least partially offset the transaction and remeasurement gains and losses recognized in earnings. We do not enter into foreign exchange forward contracts for speculative purposes. Under ASC Topic 815, Derivatives and Hedging (“ASC 815”) all derivatives are recorded on the balance sheet at fair value. The gains and losses resulting from changes in fair value are accounted for depending on the use of the derivative and whether it is designated and qualifies for hedge accounting.
The fair value of derivative instruments recorded in our Consolidated Balance Sheets is as follows:
Fair Value as of | ||||||||||
(In thousands) | Balance Sheets Location | December 31, 2016 | December 31, 2015 | |||||||
Foreign exchange contracts | Other current assets | $ | 5 | $ | 43 | |||||
Other current liabilities | $ | (5 | ) | $ | (4 | ) | ||||
Total derivatives | $ | — | $ | 39 | ||||||
Our derivative financial instruments are subject to both credit and market risk. Credit risk is the risk of loss due to failure of a counter-party to perform its obligations in accordance with contractual terms. Market risk is the potential change in an investment’s value caused by fluctuations in interest and currency exchange rates, credit spreads or other variables. We monitor the credit-worthiness of the financial institutions that are counter-parties to our derivative financial instruments and do not consider the risks of counter-party nonperformance to be material. Credit and market risks, as a result of an offset by the underlying cash flow being hedged, related to derivative instruments were not considered material at December 31, 2016 and 2015.
Cash Flow Hedging
54
We designate and document as cash flow hedges foreign exchange forward contracts that are used by us to hedge the risk that forecasted revenue may be adversely affected by changes in foreign currency exchange rates. The effective portion of the contracts’ gains or losses is included in accumulated other comprehensive income (loss) (“OCI”) until the period in which the forecasted sale being hedged is recognized, at which time the amount in OCI is reclassified to earnings as a component of revenue. To the extent that any of these contracts are not considered to be effective in offsetting the change in the value of the forecasted sales being hedged, the ineffective portion of these contracts is immediately recognized in income as a component of interest and other income, net. We calculate hedge effectiveness at a minimum each fiscal quarter. We measure hedge effectiveness by comparing the cumulative change in the spot rate of the derivative with the cumulative change in the spot rate of the anticipated sales transactions. The maturity of these instruments is generally nine months or less. We record any excluded components of the hedge in interest and other income, net.
In the event the underlying forecasted transaction does not occur within the designated hedge period or it becomes probable that the forecasted transaction will not occur, the related gains and losses on the cash flow hedge are reclassified from OCI to interest and other income, net on the consolidated statement of operations.
At December 31, 2016, we had two currency forward contracts for the sale of Japanese yen of 697 million. At December 31, 2015, we had one currency forward contract for the sale of Japanese yen of 661 million. During the year ended December 31, 2016, and 2015, we reclassified into earnings an accumulated loss of $0.1 million and $0.1 million, respectively, that was recorded as a component of other comprehensive income (loss).
Balance Sheet Derivatives
We manage the foreign currency risk associated with yen-denominated assets and liabilities using foreign exchange forward contracts with maturities of less than nine months. The change in fair value of these derivatives is recognized as a component of interest and other income, net and is intended to offset the remeasurement gains and losses associated with the non-functional currency denominated assets and liabilities.
At December 31, 2016 and 2015, we had currency sell-forward contracts classified as fair value hedges for the sale of Japanese yen of $0.4 million and $3.2 million, respectively. At December 31, 2016 and 2015, we had buy-forward contracts for the purchase of Japanese yen of $0.4 million and $0.3 million, respectively. The fair value of derivatives at December 31, 2016 and 2015 was insignificant.
The following sets forth the effect of the derivative instruments on our Consolidated Statements of Operations for the years ended:
Location of Gain/(Loss) Recognized in Statement of Operations on Derivatives (In thousands) | December 31, 2016 | December 31, 2015 | ||||||
Interest and other income (expense), net | ($220 | ) | $110 | |||||
Revenue Recognition
We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the arrangement consideration is fixed or determinable, and collectability is reasonably assured. We derive revenue from four sources: system sales, spare parts sales, service contracts and license fees.
Provided all other criteria are met, we recognize revenues on system sales when system acceptance provisions have been met in accordance with the terms and conditions of the arrangement. In the event that terms of the sale provide for a lapsing system acceptance period, we recognize revenue upon the expiration of the lapsing acceptance period or system acceptance, whichever occurs first. In these instances, which are infrequent, revenue is recorded only if the product has met product specifications prior to shipment and management deems that no significant uncertainties as to product performance exist.
Our transactions frequently include the sale of systems and services under multiple element arrangements. In transactions with multiple deliverables, revenue is recognized upon the delivery of the separate elements and when system acceptance has occurred or we are otherwise released from our system acceptance obligations.
55
For multiple element arrangements, the total consideration for an arrangement is allocated among the separate elements in the arrangement based on a selling price hierarchy. The selling price hierarchy for a deliverable is based on (i) vendor specific objective evidence (“VSOE”); if available; (ii) third party evidence of selling price if VSOE is not available; or (iii) an estimated selling price, if neither VSOE nor third party evidence is available. If we have not established VSOE and cannot obtain third party evidence of selling price, we determine our estimate of the relative selling price by considering our production costs and historical margins of similar products or services. We believe this best represents the price at which we would transact a sale if the product or service were sold on a stand-alone basis. We regularly review the method used to determine our relative selling price and update any estimates accordingly. We limit the amount of revenue recognized for delivered elements to the amount that is not contingent on the future delivery of products or services or other future performance obligations.
We generally recognize revenue from spare parts sales upon shipment, as our products are generally sold on terms that transfer title and risk of ownership when it leaves our site. We sell service contracts for which revenue is deferred and recognized ratably over the contract period (for time-based service contracts) or as service hours are delivered (for contracts based on a purchased quantity of hours). We recognize license revenue from transactions in which our systems are re-sold by our customers to third parties, as well as from royalty arrangements.
Costs related to deferred product revenues are capitalized (deferred) and recognized at the time of revenue recognition. Deferred product revenue and costs are netted on our balance sheet, under the caption “deferred product and services income.” The gross amount of deferred revenues and deferred costs at December 31, 2016 was $4.6 million and $0.2 million, respectively. The gross amount of deferred revenues and deferred costs at December 31, 2015 was $5.3 million and $0.8 million, respectively.
Costs incurred for shipping and handling are included in cost of sales.
Warranty Accrual
We generally warrant our products for material and labor to repair the product for a period of 12 months for new products, or three months for refurbished products, from the date of system acceptance. Accordingly, an accrual for the estimated cost of the warranty is recorded at the time the product is shipped and the related charge is recorded in the statement of operations at the time revenue is recognized.
Research, Development and Engineering Expenses
We are actively engaged in basic technology and applied research programs designed to develop new products and product applications. In addition, substantial ongoing product and process improvement engineering and support programs relating to existing products are conducted within engineering departments and elsewhere. Research, development and engineering costs are charged to operations as incurred.
Stock-Based Compensation
Under the fair value recognition provisions of ASC Topic 718, Compensation—Stock Compensation (“ASC 718”), share-based compensation cost is measured at the grant date based on the value of the award and is recognized as expense over the vesting period. Determining the fair value of share-based awards at the grant date requires judgment, including estimating our stock price volatility, employee stock option exercise behaviors and employee option forfeiture rates. As stock-based compensation expense recognized in the Consolidated Statement of Operations is based on awards that ultimately are expected to vest, the amount of the expense has been reduced for estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience.
Deferred Income Taxes
Deferred income taxes are provided for the tax effect of temporary differences between the tax basis of assets and liabilities and their reported amounts in the financial statements. ASC Topic 740, Income Taxes (“ASC 740”) provides for recognition of deferred tax assets if the realization of such deferred tax assets is more likely than not to occur. Realization of our net deferred tax assets is dependent upon our generation of sufficient taxable income in future years in appropriate tax jurisdictions to obtain the benefit of the reversal of temporary differences, net operating loss carry-forwards, and tax credit carry-forwards. The amount of deferred tax assets considered realizable is subject to adjustment in future periods if estimates of future taxable income are changed. With the exception of certain international jurisdictions (i.e. Japan and Taiwan), we have
56
determined that at this time it is more likely than not that deferred tax assets attributable to the remaining jurisdictions will not be realized, primarily due to uncertainties related to our ability to utilize the net operating loss and tax credit carry-forwards before they expire based on the fact that it is more likely than not we will not generate sufficient taxable income in the relevant jurisdictions. Accordingly, we have established a valuation allowance for such deferred tax assets. Management continues to monitor the relative weight of positive and negative evidence of future profitability in relevant jurisdictions. See Note 14 Income Taxes for further details.
Taxes Collected from Customers
We collect taxes from our customers for sales transactions as assessed by respective governmental authorities. On our consolidated statements of operations these taxes are presented on a net basis and are excluded from revenues and expenses.
Impact of Recently Issued Accounting Standards
In May 2014, the Financial Accounting Standards Board (“FASB”) issued a new standard to achieve a consistent application of revenue recognition within the U.S., resulting in a single revenue model to be applied by reporting companies under U.S. GAAP. Under the new model, recognition of revenue occurs when a customer obtains control of promised goods or services in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In addition, the new standard requires that reporting companies disclose the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. On July 9, 2015, the FASB agreed to delay the effective date by one year. As currently issued and amended and in accordance with the agreed upon delay, the new standard is effective for us beginning in the first quarter of 2018. Early adoption is permitted, but not before the original effective date of the standard. The new standard is required to be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying it recognized at the date of initial application. While we continue to assess all potential impacts of the standard, we currently believe the most significant impact will likely relate to systems revenue. Under the new standard we are evaluating whether our systems revenue would be recognized on shipment. We are planning to adopt the standard on January 1, 2018. We plan to adopt the standard retrospectively with the cumulative effect of initially applying it recognized at the date of initial application ("modified retrospective" approach).
In February 2016, the FASB issued ASU 2016-02, “Leases” (Topic 842) which supersedes Topic 840, Leases. The update requires companies to generally recognize on the balance sheet operating and financing lease liabilities and corresponding right-of-use assets. The new standard is effective for us in our first quarter of fiscal 2020 on a modified retrospective basis and earlier adoption is permitted. We are currently evaluating the impact of our pending adoption of ASU 2016-02 on our condensed consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, “Compensation - Stock Compensation” (Topic 718): Improvements to Employee Share-Based Payment Accounting. The update involves several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. It is effective for annual periods beginning after December 15, 2016. While we are continuing to assess all potential impacts of the standard, we currently believe it will not have a significant impact on our income tax provision, classification of awards and statement of cash flows.
5. STOCK-BASED COMPENSATION
The following table shows total stock-based compensation expense recognized under ASC Topic 718, Compensation—Stock Compensation (“ASC 718”), for employees and directors and the effect on the accompanying Consolidated Statement of Operations for the years ended December 31:
In thousands | 2016 | 2015 | 2014 | ||||||||
Cost of sales | $ | 750 | $ | 833 | $ | 800 | |||||
Research, development, and engineering | 3,016 | 3,230 | 2,875 | ||||||||
Selling, general and administrative expenses | 8,454 | 11,199 | 13,152 | ||||||||
Total stock-based compensation expense | $ | 12,220 | $ | 15,262 | $ | 16,827 | |||||
Compensation cost capitalized as part of inventory was $0.5 million, $0.8 million and $0.5 million during the years ended December 31, 2016, 2015 and 2014, respectively.
57
The estimated fair value of our stock-based awards, less expected forfeitures, is amortized over the awards’ vesting period using a single grant approach on a ratable basis for awards granted after the adoption of ASC 718 and using a multiple grant approach on an accelerated basis for awards granted prior to the adoption of ASC 718.
The fair value of each option award is estimated on the date of grant using the Black-Scholes valuation model and the assumptions noted in the following table. The expected life of options is based on observed historical exercise patterns. Groups of employees that have similar historical exercise patterns have been considered separately for valuation purposes. The Black-Scholes valuation input for expected volatility used for our stock options for all years presented was based on the historical volatility of our common stock. The risk free interest rate is based on the implied yield on a U.S. Treasury zero-coupon issue with a remaining term equal to the expected term of the option. The dividend yield reflects that we have not paid any cash dividends since inception and do not intend to pay any cash dividends in the foreseeable future.
We used the following weighted-average assumptions to estimate the fair value of stock options at the date of grant using the Black-Scholes option-pricing model for the years ended December 31:
2016 | 2015 | 2014 | |||||
Expected life (in years) | N/A | 5.9 | 5.9 | ||||
Risk-free interest rate | N/A | 1.7 | % | 1.7 | % | ||
Volatility factor | N/A | 43 | % | 47 | % | ||
Dividend yield | N/A | — | — | ||||
During the year ended December 31, 2016, we did not grant any stock options. The weighted- average fair value of stock options granted during the years ended December 31, 2015 and 2014 was $7.19, and $10.40 respectively.
1993 Stock Option Plan/Stock Issuance Plan
On July 19, 2011, our stockholders approved amendments to our 1993 Stock Option/Stock Issuance Plan which increased the number of shares available for issuance pursuant to the 1993 Plan by 3.3 million shares. The amendments, which were adopted by our Board of Directors on May 31, 2011, effective as of their approval by our stockholders, increased the share reserve, altered share-counting procedures, made changes to the non-employee director automatic grant program and enabled the granting of performance-based awards under the plan. These plans provide for the grant of stock-based awards to our eligible employees, consultants and advisers and non-employee directors.
Under our 1993 Stock Option Plan/Stock Issuance Plan, as amended and restated as of May 31, 2011, officers and other key employees, non-employee Board members and consultants may receive equity incentive awards in the form of stock options to purchase shares of common stock at no less than 100% of fair value at the grant date or restricted stock or restricted stock units. Options historically have vested in equal monthly installments over a fifty-month period or one hundred-month period, with a minimum vesting period of twelve months from the grant date, and generally expire ten years from the date of grant or upon the expiration of a limited period following any earlier termination of employment. The plan was amended in January 2006 to allow the issuance of shares pursuant to restricted stock unit awards. These awards when granted generally vest in equal monthly installments over the vesting period but which defer the issuance of the vested shares until the end of the issuance period measured from the award date, subject to earlier issuance upon termination of employment under certain circumstances or a change in control. Awards under the plan may be subject to accelerated vesting under certain circumstances should a change in control occur. The plan terminates on the earlier of June 6, 2020 or the date on which all shares available for issuance under the plan have been issued. Under the plan, approximately 0.4 million, 0.4 million and 0.2 million shares were available for issuance at December 31, 2016, 2015 and 2014, respectively.
1998 Supplemental Stock Option/Stock Issuance Plan
Under our 1998 Supplemental Stock Option/Stock Issuance Plan, as amended, eligible employees (i.e. other than executive officers and employees holding the title of Vice President or General Manager) were able to receive options to purchase shares of common stock at not less than 100% of fair value on the grant date. These options generally vest in equal monthly installments over a fifty-month period, with a minimum vesting period of twelve months from grant date, and generally expire ten years from date of grant, subject to earlier termination following the optionee’s cessation of employee status. Direct stock issuances may also be made under the plan, subject to similar vesting provisions. The plan was amended in January 2008 to allow the issuance of shares pursuant to restricted stock unit awards. Since the plan terminated on October 19, 2008, there were no options available for issuance under the plan at December 31, 2016, 2015 and 2014.
58
Stock Option Activity
The following table is a summary of our stock option activity and related information for the year ended December 31, 2016:
Options | Weighted- Average Exercise Price | Weighted Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value as of December 31, 2016 (in thousands) | |||||||||
Outstanding at January 1, 2016 | 2,395,107 | $ | 24.13 | |||||||||
Granted | — | $ | — | |||||||||
Exercised | (109,045 | ) | $ | 16.65 | ||||||||
Forfeited and expired | (46,620 | ) | $ | 24.51 | ||||||||
Outstanding at December 31, 2016 | 2,239,442 | $ | 24.48 | 5.08 | $ | 5,327 | ||||||
Exercisable at December 31, 2016 | 1,585,813 | $ | 24.20 | 4.95 | $ | 4,096 | ||||||
Vested and expected to vest as of December 31, 2016, net of anticipated forfeitures | 2,174,579 | $ | 24.43 | 5.08 | $ | 5,237 | ||||||
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (the difference between our closing stock price on the last trading day of the year ended December 31, 2016 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2016. Total intrinsic value of options exercised (selling price of the exercised stock less the option strike price multiplied by the share amount) in the year ended December 31, 2016 was $0.7 million as compared to $0.1 million and $1.8 million in 2015 and 2014, respectively. Cash received from option exercises in the years ended December 31, 2016 and 2015 was $1.9 million and $0.3 million, respectively.
The following table is a summary of our option activity for the years ended December 31:
2015 | 2014 | ||||||||||||
Options | Weighted- Average Exercise Price | Options | Weighted- Average Exercise Price | ||||||||||
Outstanding at January 1 | 2,695,276 | $ | 24.09 | 2,659,884 | $ | 23.51 | |||||||
Granted | 31,500 | $ | 16.71 | 462,250 | $ | 22.99 | |||||||
Exercised | (21,730 | ) | $ | 15.29 | (274,551 | ) | $ | 15.46 | |||||
Forfeited and expired | (309,939 | ) | $ | 23.63 | (152,307 | ) | $ | 26.23 | |||||
Outstanding at December 31 | 2,395,107 | $ | 24.13 | 2,695,276 | $ | 24.09 | |||||||
The following table shows the related information for options outstanding and options exercisable as of December 31, 2016:
59
Options Outstanding | Options Exercisable | |||||||||||||||
Range of Exercise Prices | Options | Weighted- Average Remaining Contractual Life (Years) | Weighted- Average Exercise Price | Options | Weighted- Average Exercise Price | |||||||||||
$9.66 - $15.65 | 248,914 | 2.44 | $ | 12.60 | 207,434 | $ | 12.31 | |||||||||
$15.68 - $18.65 | 233,231 | 6.27 | $ | 17.71 | 151,036 | $ | 17.86 | |||||||||
$18.80 - $22.00 | 247,124 | 4.31 | $ | 20.74 | 178,284 | $ | 20.64 | |||||||||
$22.53 - $24.10 | 263,740 | 5.47 | $ | 23.16 | 181,485 | $ | 23.17 | |||||||||
$24.57 - $27.75 | 391,028 | 5.95 | $ | 26.74 | 286,208 | $ | 26.75 | |||||||||
$28.92 - $29.54 | 275,626 | 5.57 | $ | 29.10 | 199,898 | $ | 29.17 | |||||||||
$30.12 - $30.12 | 194,860 | 5.56 | $ | 30.12 | 119,128 | $ | 30.12 | |||||||||
$30.91-$30.91 | 158,751 | 4.32 | $ | 30.91 | 111,900 | $ | 30.91 | |||||||||
$31.24 - $31.24 | 211,168 | 5.19 | $ | 31.24 | 135,440 | $ | 31.24 | |||||||||
$31.91 - $31.91 | 15,000 | 5.46 | $ | 31.91 | 15,000 | $ | 31.91 | |||||||||
Outstanding at December 31 | 2,239,442 | 5.08 | $ | 24.48 | 1,585,813 | $ | 24.20 | |||||||||
As of December 31, 2016, $9.1 million of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted-average period of 2.17 years.
Restricted Stock Unit Activity
The following table is a summary of our restricted stock unit activity and related information as of December 31:
2016 | 2015 | 2014 | ||||||||||||||||||
Shares | Weighted- Average Grant Date Fair Value | Shares | Weighted-Average Grant Date Fair Value | Shares | Weighted-Average Grant Date Fair Value | |||||||||||||||
Nonvested stock at January 1 | 884,160 | $ | 22.54 | 1,328,357 | $ | 23.43 | 1,089,354 | $ | 27.75 | |||||||||||
Granted | 47,500 | $ | 24.41 | 56,000 | $ | 16.54 | 694,250 | $ | 19.26 | |||||||||||
Vested | (346,651 | ) | $ | 22.86 | (421,375 | ) | $ | 24.27 | (403,116 | ) | $ | 27.55 | ||||||||
Forfeited | (34,485 | ) | $ | 20.45 | (78,822 | ) | $ | 24.00 | (52,131 | ) | $ | 26.40 | ||||||||
Nonvested stock at December 31 | 550,524 | $ | 22.63 | 884,160 | $ | 22.54 | 1,328,357 | $ | 23.43 | |||||||||||
A total of 235,208 shares of our common stock subject to restricted stock units was vested but not yet distributed as of December 31, 2016. Stock-based compensation expense related to our restricted stock units for the year ended December 31, 2016 was $7.8 million. As of December 31, 2016, $12.3 million of total unrecognized compensation cost related to unvested restricted stock units is expected to be recognized over a weighted-average period of 2.61 years. Total fair value of vested shares in the year ended December 31, 2016 was $7.9 million compared to $10.2 million and $11.1 million in 2015 and 2014, respectively.
6. Common Stock Repurchase Program
On October 27, 2014, our Board of Directors authorized the repurchase of up to $100 million of the Company’s common stock. Any repurchases may be made through open market purchases, block trades, privately negotiated transactions and/or derivative transactions, subject to market conditions and managements’ ongoing determination that it is the best use of available cash on hand to fund any repurchases. The repurchase authorization does not obligate us to acquire any common stock.
During the year ended December 31, 2016, we did not repurchase any common stock under the stock repurchase plan.
60
7. BASIC AND DILUTED NET INCOME (LOSS) PER SHARE
The following sets forth the computation of basic and diluted net income (loss) per share:
Years Ended December 31, | |||||||||||
In thousands, except per share amounts | 2016 | 2015 | 2014 | ||||||||
Numerator: | |||||||||||
Net income (loss) | $ | 11,237 | $ | (15,128 | ) | $ | (19,111 | ) | |||
Denominator: | |||||||||||
Basic weighted-average shares outstanding | 27,012 | 27,429 | 28,437 | ||||||||
Effect of dilutive employee stock options and restricted stock units | 321 | — | — | ||||||||
Diluted weighted-average shares outstanding | 27,333 | 27,429 | 28,437 | ||||||||
Net income (loss) per share—basic | $ | 0.42 | $ | (0.55 | ) | $ | (0.67 | ) | |||
Net Income (loss) per share—diluted | $ | 0.41 | $ | (0.55 | ) | $ | (0.67 | ) | |||
The dilutive effect of outstanding options and restricted stock units is reflected in diluted net income per share, but not diluted net loss per share, by application of the treasury stock method, which includes consideration of stock-based compensation required by U.S. GAAP. As the Company reported net losses for each of the years ended December 31, 2015 and 2014, both basic and diluted net loss per share are the same.
For the year ended December 31, 2016, a total of 2.0 million shares of common stock subject to options and restricted stock units was excluded from the computation of diluted net income per share as the resulting effect would have been anti-dilutive, compared to 3.0 million shares and 2.7 million shares for the years ended December 31, 2015 and 2014, respectively. Options and restricted stock units are anti-dilutive when we have a net loss or when the exercise price of the stock option and the average unrecognized compensation cost of the stock option or restricted stock unit are greater than the average market price of our common stock.
8. FAIR VALUE MEASUREMENTS
On January 1, 2008, we adopted ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”) for financial assets and liabilities recognized at fair value on a recurring basis. On January 1, 2009, we adopted ASC 820 for all nonfinancial assets and nonfinancial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis. These nonfinancial items include assets and liabilities such as reporting units measured at fair value in a goodwill impairment test and nonfinancial assets acquired and liabilities assumed in a business combination. The adoption of this deferred portion of ASC 820 did not have any impact on our results of operations or financial position.
Fair value is defined under ASC 820 as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
• | Level 1—Quoted prices for identical assets or liabilities in active markets. |
• | Level 2—Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets. |
• | Level 3—Model derived valuations in which one or more significant inputs or significant value drivers are unobservable. |
We measure certain financial assets and liabilities at fair value on a recurring basis, including available-for-sale securities and foreign currency derivatives. There were no movements between levels one and two for any years presented.
The fair value of these certain financial assets and liabilities was determined at December 31, 2016 and 2015:
61
Fair Value Measurements at December 31, 2016 Using | |||||||||||||||
In thousands | Total | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||
Available-for-sale securities(1) | |||||||||||||||
Commercial paper | $ | 23,684 | $ | — | $ | 23,684 | $ | — | |||||||
Money market funds | 2,266 | 2,266 | — | — | |||||||||||
U.S. treasury bills and notes | 29,461 | 29,461 | — | — | |||||||||||
Securities and obligations of U.S. government agencies | 127,704 | — | 127,704 | — | |||||||||||
183,115 | 31,727 | 151,388 | — | ||||||||||||
Foreign currency derivatives(2) | 4 | — | 4 | — | |||||||||||
Foreign currency derivatives(3) | (4 | ) | — | (4 | ) | — | |||||||||
$ | 183,115 | $ | 31,727 | $ | 151,388 | $ | — | ||||||||
Fair Value Measurements at December 31, 2015 Using | |||||||||||||||
In thousands | Total | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||
Available-for-sale securities(1) | |||||||||||||||
Commercial paper | $ | 9,996 | $ | — | $ | 9,996 | $ | — | |||||||
Money market funds | 9,074 | 9,074 | — | — | |||||||||||
U.S. treasury bills and notes | 56,872 | 56,872 | — | — | |||||||||||
Securities and obligations of U.S. government agencies | 128,023 | — | 128,023 | — | |||||||||||
203,965 | 65,946 | 138,019 | — | ||||||||||||
Foreign currency derivatives(2) | 43 | — | 43 | — | |||||||||||
Foreign currency derivatives(3) | (4 | ) | — | (4 | ) | — | |||||||||
$ | 204,004 | $ | 65,946 | $ | 138,058 | $ | — | ||||||||
___________________
(1) | Included in cash and cash equivalents and short-term investments on our consolidated balance sheet. Cash equivalents at December 31, 2016 and 2015 were $2.3 million and $15.1 million, respectively. |
(2) | Included in current assets on our consolidated balance sheet. Consisted of forward foreign exchange contracts for the Japanese yen. See Note 4 - Derivative Instruments and Hedging. |
(3) | Included in current liabilities on our consolidated balance sheet. Consisted of forward foreign exchange contracts for the Japanese yen. See Note 4 - Derivative Instruments and Hedging. |
9. ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS)
Accumulated other comprehensive income (loss) is comprised of the following items, net of tax:
In thousands | December 31, 2016 | December 31, 2015 | |||||
Unrealized gain (loss) on: | |||||||
Available-for-sale investments | $ | 53 | $ | (377 | ) | ||
Postretirement benefit obligation | 527 | 317 | |||||
Accumulated other comprehensive income (loss) at end of period | $ | 580 | $ | (60 | ) | ||
62
The amount of gain on foreign exchange contracts reclassified to earnings was $0.1 million for the years ended December 31, 2016, and 2015. Unrealized gains and losses on investments affect the short-term investment line on our consolidated balance sheet. Amounts in other comprehensive income for changes in the minimum post-retirement obligation are recorded on our consolidated balance sheets in other liabilities or expensed to our consolidated statement of operations in selling, general, and administrative expenses.
10. INVESTMENTS
We classified all of our investments as “available-for-sale” as of December 31, 2016 and 2015. Accordingly, we state our investments at estimated fair value. Fair values are determined based on quoted market prices or pricing models using current market rates. We deem all investments to be available to meet current working capital requirements.
The following is a summary of our investments:
December 31, 2016 | December 31, 2015 | |||||||||||||||||||||||||||||||
Cash equivalents and available- for-sale investments, In thousands | Amortized Cost | Accumulated Other Comprehensive Income | Estimated Fair Value | Amortized Cost | Accumulated Other Comprehensive Income | Estimated Fair Value | ||||||||||||||||||||||||||
Gains | Losses | Gains | Losses | |||||||||||||||||||||||||||||
Commercial paper | $ | 23,744 | $ | — | $ | 60 | $ | 23,684 | $ | 9,996 | $ | — | $ | — | $ | 9,996 | ||||||||||||||||
Money market funds | 2,266 | — | — | 2,266 | 9,074 | — | — | 9,074 | ||||||||||||||||||||||||
U.S. treasury bills and notes | 29,467 | 1 | 7 | 29,461 | 56,929 | 1 | 58 | 56,872 | ||||||||||||||||||||||||
Securities and obligations of U.S. government agencies | 127,961 | 8 | 265 | 127,704 | 128,343 | 3 | 323 | 128,023 | ||||||||||||||||||||||||
Total | $ | 183,438 | $ | 9 | $ | 332 | $ | 183,115 | $ | 204,342 | $ | 4 | $ | 381 | $ | 203,965 | ||||||||||||||||
The following is a reconciliation of our investments to the balance sheet classifications at December 31, 2016 and 2015:
In thousands | 2016 | 2015 | |||||
Cash equivalents | $ | 2,266 | $ | 15,072 | |||
Short-term investments | 180,849 | 188,893 | |||||
Investments, at estimated fair value | $ | 183,115 | $ | 203,965 | |||
Gross realized gains and losses on sales of investments were insignificant in each of the years ended December 31, 2016, 2015 and 2014.
The gross amortized cost and estimated fair value of our investments at December 31, 2016, by contractual maturity, are shown below. Expected maturities will differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
In thousands | Gross Amortized Cost | Fair Value | |||||
Due in one year or less | $ | 112,164 | $ | 112,058 | |||
Due after one year through five years | 71,274 | 71,057 | |||||
Total | $ | 183,438 | $ | 183,115 | |||
63
The following table provides the breakdown of the cash equivalents and investments with unrealized losses at December 31, 2016:
In Loss Position for Less Than 12 Months | In Loss Position for More Than 12 Months | Total | ||||||||||||||||||||||
Investments, In thousands | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | ||||||||||||||||||
Commercial paper | 17,693 | 60 | 17,693 | 60 | ||||||||||||||||||||
U.S. treasury bills and notes | $ | 18,463 | $ | 7 | $ | — | $ | — | $ | 18,463 | $ | 7 | ||||||||||||
Securities and obligations of U.S. government agencies | 103,426 | 265 | — | — | 103,426 | 265 | ||||||||||||||||||
Total | $ | 139,582 | $ | 332 | $ | — | $ | — | $ | 139,582 | $ | 332 | ||||||||||||
The following table provides the breakdown of the cash equivalents and investments with unrealized losses at December 31, 2015:
In Loss Position for Less Than 12 Months | In Loss Position for More Than 12 Months | Total | ||||||||||||||||||||||
Investments, In thousands | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | ||||||||||||||||||
U.S. corporate debt securities | $ | 51,872 | $ | 58 | $ | — | $ | — | $ | 51,872 | $ | 58 | ||||||||||||
Securities and obligations of U.S. government agencies | $ | 110,345 | $ | 323 | $ | — | $ | — | $ | 110,345 | $ | 323 | ||||||||||||
Total | $ | 162,217 | $ | 381 | $ | — | $ | — | $ | 162,217 | $ | 381 | ||||||||||||
We review our investment portfolio regularly for impairment. A security is considered impaired when its fair value is less than its cost basis. If we intend to sell an impaired debt security or it is more likely than not that we will be required to sell it prior to recovery of its amortized cost basis, and other-than-temporary-impairment (“OTTI”) is deemed to have occurred. In these instances, the OTTI loss is recognized in earnings equal to the entire difference between the debt security’s amortized cost basis and its fair value at the balance sheet date.
If we do not intend to sell an impaired debt security and it is not more likely than not that we will be required to sell it prior to recovery of its amortized cost basis, we must determine whether it will recover its amortized cost basis. If we conclude it will not, a credit loss exists and the resulting OTTI is separated into:
• | The amount representing the credit loss, which is recognized in earnings, and |
• | The amount related to all other factors, which is recognized in other comprehensive income. |
As part of this assessment we will consider the various characteristics of each security, including, but not limited to the following: the length of time and the extent to which the fair value has been less than the amortized cost basis; adverse conditions specifically related to the security, an industry, or a geographic area; the payment structure of the debt security; failure of the issuer of the security to make scheduled interest or principal payments; any changes to the rating of the security by a rating agency and related outlook or status; recoveries or additional declines in fair value subsequent to the balance sheet date. The relative importance of this information varies based on the facts and circumstances surrounding each security, as well as the economic environment at the time of assessment.
We have not recorded any OTTI on our investments for the years ended December 31, 2016, 2015 and 2014.
64
11. BALANCE SHEET DETAIL
In thousands | December 31, 2016 | December 31, 2015 | |||||
Inventories, net: | |||||||
Raw materials | $ | 17,273 | $ | 24,898 | |||
Work-in-process | 15,510 | 18,284 | |||||
Finished products | 17,692 | 22,216 | |||||
Total (net of reserves) | $ | 50,475 | $ | 65,398 | |||
Property, plant, and equipment, net | |||||||
Machinery and equipment | $ | 46,581 | $ | 46,369 | |||
Leasehold improvements | 15,695 | 15,603 | |||||
Office equipment and furniture | 14,755 | 14,619 | |||||
77,031 | 76,591 | ||||||
Accumulated depreciation and amortization | (63,162 | ) | (59,311 | ) | |||
Total | $ | 13,869 | $ | 17,280 | |||
Other assets: | |||||||
Deferred compensation plan assets | 5,062 | 4,341 | |||||
Demonstration and laboratory equipment | 4,540 | 5,022 | |||||
Other | 1,196 | 996 | |||||
Total | $ | 10,798 | $ | 10,359 | |||
Accrued expenses: | |||||||
Accrued payroll-related liabilities | $ | 11,494 | $ | 6,417 | |||
Warranty accrual | 1,884 | 1,586 | |||||
Capital lease, current portion | 504 | 831 | |||||
Advanced billings | 583 | 543 | |||||
Other | 3,563 | 2,769 | |||||
Total | $ | 18,028 | $ | 12,146 | |||
Other Liabilities: | |||||||
Deferred compensation plan liabilities | $ | 6,100 | $ | 5,247 | |||
Income tax payable - long term | 26 | 2,523 | |||||
Asset retirement obligations | 2,706 | 2,540 | |||||
Postretirement benefits obligation | 1,869 | 2,231 | |||||
Capital lease - long term | — | 575 | |||||
Deferred service income - long term | 1,145 | 49 | |||||
Other | 610 | 309 | |||||
Total | $ | 12,456 | $ | 13,474 | |||
Warranty Accrual
We generally warrant our products for a period of 12 months for new products, or three months for refurbished products, from the date of system acceptance for material and labor to repair the product; accordingly, an accrual for the estimated cost of the warranty is recorded at the time the product is shipped. Extended warranty terms, if granted, result in deferral of revenue equating to our standard pricing for similar service contracts. Recognition of the related warranty cost is deferred until product revenue is recognized. Factors that affect our warranty liability include the number of installed units, historical and anticipated rates of warranty claims, and cost per claim. We periodically assess the adequacy of our recorded warranty liabilities and adjust the amounts as necessary.
Changes in our product liability are as follows:
65
In thousands | December 31, 2016 | December 31, 2015 | |||||
Balance, beginning of year | $ | 1,586 | $ | 1,501 | |||
Liabilities accrued for warranties issued | 2,529 | 1,896 | |||||
Warranty claims paid and utilized | (2,137 | ) | (2,155 | ) | |||
Changes in accrued warranty liabilities | (94 | ) | 344 | ||||
Balance, end of year | $ | 1,884 | $ | 1,586 | |||
Deferred Service Income
We sell service contracts for which revenue is deferred and recognized ratably over the contract period (for time based service contracts) or as service hours are delivered (for contracts based on a purchased quantity of hours). Changes in our deferred service revenue are as follows:
In thousands | December 31, 2016 | December 31, 2015 | |||||
Balance, beginning of year | $ | 4,011 | $ | 5,233 | |||
Service contracts billed during year | 3,891 | 3,244 | |||||
Service contract revenue recognized during year | (4,060 | ) | (4,466 | ) | |||
Balance, end of year | $ | 3,842 | $ | 4,011 | |||
Balance sheet classification | |||||||
Service contracts classified as short-term | $ | 2,697 | $ | 3,962 | |||
Service contracts classified as long-term | 1,145 | 49 | |||||
Balance, end of year | $ | 3,842 | $ | 4,011 | |||
Asset Retirement Obligations
In accordance with ASC 410, Asset Retirement and Environmental Obligations, an entity is required to recognize a liability for the fair value of a conditional asset retirement obligation (“ARO”) if the fair value of the liability can be reasonably estimated, even if conditional on a future event. The ARO liability is principally for estimable retirement obligations related to remediation costs, which we estimate will be incurred upon the expiration of certain operating leases.
The following table sets forth an analysis of the ARO activity for the years ended December 31, 2016 and 2015:
In thousands | 2016 | 2015 | |||||
Balance as of January 1 | $ | 2,540 | $ | 2,397 | |||
Accretion expense | 166 | 143 | |||||
Liabilities incurred | — | — | |||||
Balance as of December 31 | $ | 2,706 | $ | 2,540 | |||
Advanced Billings
On occasion, we require, or our customers pay, a deposit in advance of order shipment. These amounts are classified as advanced billings until the related order ships. At December 31, 2016, we have received $0.6 million in advanced billings from our customers and $0.5 million at December 31, 2015.
12. NOTES PAYABLE
In December 2004, we entered into a line of credit agreement with a brokerage firm. Under the terms of this agreement, we may borrow funds at a cost equal to the current federal funds rate plus 125 basis points (1.91% as of December 31, 2016). Certain of our cash, cash equivalents and short-term investments secure borrowings outstanding under this facility, but we are not restricted in the use of those assets. Funds are advanced to us under this facility based on pre-determined advance rates on the cash and securities held by us in this brokerage account. This agreement has no set expiration
66
date and there are no loan covenants, other than the aforementioned collateral requirement which does not legally restrict the cash and securities. At each of the years ended December 31, 2016 and 2015, $1.5 million and $5.1 million was outstanding under this facility, with a related collateral requirement of approximately of $2.0 million and $6.8 million of our cash, cash equivalents and short-term investments.
13. EMPLOYEE BENEFIT PLANS
Employee bonus plans
We currently sponsor an executive incentive bonus plan that distributes employee awards based on the achievement of predetermined targets. We recorded charges of $6.3 million, $2.1 million, and $1.5 million under this bonus plan for the years ended December 31, 2016, 2015, and 2014, respectively.
Employee Savings and Retirement Plans
We sponsor a 401(k) employee salary deferral plan that allows voluntary contributions by all full-time employees up to the Internal Revenue Service limits of their pretax earnings. We may also make matching contributions to this plan at our discretion. Employees are eligible for the matching plan if they contribute at least 2% of their compensation. Our contributions, when made, are limited to a maximum of 3% of the employee’s compensation if the company exceeds certain income levels. During the year ended December 31, 2016, 2015 and 2014, we made zero contributions to the plan.
We also sponsor an executive non-qualified deferred compensation plan (the “Plan”) that allows qualifying executives to defer current cash compensation. At December 31, 2016 Plan assets of $5.1 million, representing the cash surrender value of life insurance policies held by us, and liabilities of $6.1 million are included in our consolidated balance sheets under the captions “other assets” and “other liabilities,” respectively.
Postretirement Benefits
We have committed to providing lifetime postretirement medical and dental benefits to our Chief Executive Officer and Chief Financial Officer and their spouses, commencing after retirement. These medical and dental benefits are similar to the benefits provided to all full-time employees while employed by us, except that we are paying the entire cost of these benefits.
The following table sets forth the amounts of unrecognized prior service cost and unrecognized actuarial (gain) loss included in accumulated other comprehensive loss:
In thousands | December 31, 2016 | December 31, 2015 | |||||
Prior service cost | $ | 381 | $ | 767 | |||
Net actuarial (gain) loss | (88 | ) | 53 | ||||
Amount included in accumulated other comprehensive income | $ | 293 | $ | 820 | |||
The reconciliation of the beginning and ending balance of the accumulated postretirement benefit obligation and the fair value of plan assets is as follows:
In thousands | December 31, 2016 | December 31, 2015 | |||||
Benefit obligation at beginning of year | $ | 2,231 | $ | 2,255 | |||
Interest cost | 87 | 86 | |||||
Actuarial gain | (448 | ) | (110 | ) | |||
Benefit obligation at end of year | 1,870 | 2,231 | |||||
Funded status at end of year | $ | (1,870 | ) | $ | (2,231 | ) | |
Amounts recognized in the statement of financial position are included in noncurrent liabilities.
67
Weighted-average discount rates as of December 31, 2016 were 3.8% and 4.0% for each of the Chief Executive Officer’s plan and the Chief Financial Officer’s plan, respectively, as compared to 4.0% and 4.2%, as of December 31, 2015.
For measurement purposes, a 5% annual rate of increase in the per capita cost of covered health care benefits was assumed for the year ended December 31, 2016 and the rate was assumed to remain at 5% thereafter.
Components of net periodic benefit cost and other amounts are recognized in selling, general, and administrative expense on our Consolidated Statements of Operations are as follows:
In thousands | December 31, 2016 | December 31, 2015 | |||||
Interest cost | $ | 87 | $ | 86 | |||
Actuarial gain | $ | (194 | ) | $ | — | ||
Net periodic benefit (gain) loss | $ | (107 | ) | $ | 86 | ||
Other changes in plan benefit obligations from other comprehensive loss recognized in selling, general, and administrative expense on our Consolidated Statements of Operations are as follows:
In thousands | December 31, 2016 | December 31, 2015 | |||||
Amortization of net loss | — | 15 | |||||
Total recognized from other comprehensive loss | $ | — | $ | 15 | |||
Total recognized net periodic benefit (gain) loss and changes in plan benefit obligations | $ | (107 | ) | $ | 101 | ||
The expected benefit payments are as follows:
In thousands | |||
2020 and thereafter | $ | 1,870 | |
Total expected benefit payments | $ | 1,870 | |
Assumed health care cost trend rates have a significant effect on the amounts reported for the health care plan. A one-percentage-point change in assumed health care cost trend rates would have the following effects:
December 31, 2016 | |||||||
In thousands | 1-Percentage- Point Increase | 1-Percentage- Point Decrease | |||||
Effect on total of service and interest cost components | $ | 21.3 | $ | (21.3 | ) | ||
Effect on postretirement benefit obligation | $ | 297 | $ | (245 | ) | ||
14. INCOME TAXES
The domestic and foreign components of income (loss) before income taxes is as follows:
Years Ended December 31, | |||||||||||
In thousands | 2016 | 2015 | 2014 | ||||||||
Domestic | $ | (31,061 | ) | $ | (37,696 | ) | $ | (16,027 | ) | ||
Foreign | 39,753 | 23,018 | (2,840 | ) | |||||||
Income (loss) before income taxes | $ | 8,692 | $ | (14,678 | ) | $ | (18,867 | ) | |||
The components of the provision (benefit) for income taxes were as follows:
68
Years Ended December 31, | |||||||||||
In thousands | 2016 | 2015 | 2014 | ||||||||
Federal: | |||||||||||
Current | $ | (2,405 | ) | $ | — | $ | — | ||||
Deferred | (212 | ) | — | — | |||||||
(2,617 | ) | — | — | ||||||||
State: | |||||||||||
Current | (131 | ) | (23 | ) | (18 | ) | |||||
Deferred | — | — | — | ||||||||
(131 | ) | (23 | ) | (18 | ) | ||||||
Foreign: | |||||||||||
Current | 250 | 289 | 390 | ||||||||
Deferred | (47 | ) | 184 | (128 | ) | ||||||
203 | 473 | 262 | |||||||||
Total income tax provision (benefit) | $ | (2,545 | ) | $ | 450 | $ | 244 | ||||
The difference between the provision (benefit) for income taxes and the amount computed by applying the U.S. federal statutory rate of 35 percent to income (loss) before income taxes is explained below:
Years Ended December 31, | |||||||||||
In thousands | 2016 | 2015 | 2014 | ||||||||
Tax computed at statutory rate | $ | 3,042 | $ | (5,136 | ) | $ | (6,602 | ) | |||
Foreign taxes | (13,710 | ) | (7,583 | ) | 1,256 | ||||||
Credits | (625 | ) | (169 | ) | (282 | ) | |||||
ASU 740-10 Release | (2,502 | ) | — | — | |||||||
Change in valuation allowance | 11,250 | 13,338 | 5,872 | ||||||||
Income tax provision (benefit) | $ | (2,545 | ) | $ | 450 | $ | 244 | ||||
To better align with the international nature of our business, we transitioned certain manufacturing processes to Singapore, thereby bringing these activities closer to our Asia-based customers. We have qualified for tax incentives that provide that certain income earned in Singapore is subject to a tax holiday and/or reduced tax rates for a limited period of time under the laws of Singapore. To realize these benefits, we must meet certain requirements relating to employment and investment activities. This exemption is expected to expire within four years. In 2016, the tax benefit attributable to tax holidays was approximately $6.0 million with a $0.22 impact on diluted earnings per share. In 2015, the tax benefit attributable to tax holidays was approximately $3.0 million with a $0.11 impact on diluted earnings per share. In 2014, the tax benefit attributable to tax holidays was approximately $1 million with a $0.04 impact on diluted earnings per share. Our ability to realize benefits from these initiatives could be materially adversely affected if, among other things, applicable requirements are not met, the incentives are substantially modified, or if we incur losses for which we cannot take a deduction.
69
Significant components of deferred income tax assets and liabilities are as follows:
In thousands | 2016 | 2015 | 2014 | ||||||||
Deferred tax assets: | |||||||||||
Net operating loss carry-forwards | $ | 30,702 | $ | 21,550 | $ | 20,661 | |||||
Tax credit carry-forwards | 17,654 | 16,127 | 16,050 | ||||||||
Other non-deductible accruals and reserves | 6,878 | 5,538 | 5,730 | ||||||||
Stock-based compensation | 6,340 | 6,515 | 6,440 | ||||||||
Inventory valuation | 3,101 | 3,838 | 4,101 | ||||||||
Bad debt reserve | 182 | 184 | 181 | ||||||||
Warranty reserves | 231 | 211 | 290 | ||||||||
Deferred product and services income | 3 | 411 | 328 | ||||||||
Basis difference in assets | (69 | ) | 44 | 133 | |||||||
Total deferred tax assets | 65,022 | 54,418 | 53,914 | ||||||||
Valuation allowance | (62,739 | ) | (52,380 | ) | (51,558 | ) | |||||
Net deferred tax assets | $ | 2,283 | $ | 2,038 | $ | 2,356 | |||||
Deferred tax liabilities: | |||||||||||
Other | (1,919 | ) | (1,854 | ) | (1,695 | ) | |||||
Total deferred tax liabilities | (1,919 | ) | (1,854 | ) | (1,695 | ) | |||||
Net deferred tax assets | $ | 364 | $ | 184 | $ | 661 | |||||
We have not provided for U.S. income taxes and foreign withholding taxes on the undistributed earnings of foreign subsidiaries as of December 31, 2016 because we intend to permanently reinvest such earnings outside the United States given that we have moved certain manufacturing processes to Singapore and that our future U.S. cash flows are expected to meet our future U.S. cash needs. As of December 31, 2016, the cumulative amount of earnings for which U.S. income taxes have not been provided is approximately $92.8 million. Determination of the amount of unrecognized deferred tax liability related to these earnings is not practicable.
We currently have a full valuation allowance against our U.S. net deferred tax asset. Each quarter we assess the likelihood that we will be able to recover our deferred tax assets. As a result of our analysis, we concluded that it is more likely than not that, as of December 31, 2016, our net deferred tax assets will not be realized, with the exception of those in Japan and Taiwan. Therefore, we continue to provide a full valuation allowance against net deferred tax assets outside of Japan and Taiwan. Management continues to monitor the relative weight of positive and negative evidence of future profitability in relevant jurisdictions. However, as of December 31, 2016, we have determined that the following negative evidence outweighs the positive evidence such that it is not more likely than not we will generate sufficient taxable income in the relevant jurisdictions to utilize our deferred tax assets and release the associated valuation allowance:
• | Movement of certain product manufacturing to Singapore, resulting in U.S. taxable losses, |
• | Cumulative three years U.S. taxable loss, |
• | Inherent earnings volatility of our industry resulting in our inability to forecast long term earnings, and |
• | Usage limitations resulting in a longer period being required to realize our deferred tax assets. |
The net valuation allowance increased by $10.4 million during the year ended December 31, 2016, and increased by $0.8 million and $3.7 million during the years ended December 31, 2015 and 2014, respectively. The increase in valuation allowance in 2016 was primarily due to an increase in net operating loss carry-forwards and tax credit carry-forwards.
Approximately $4.5 million of the valuation allowance as of December 31, 2016 is attributable to pre-2006 windfall stock option deductions, the benefit of which will be credited to paid-in capital if and when realized through a reduction in income taxes payable. Beginning in 2006, we are tracking the windfall stock option deductions off balance sheet, as required by ASC 718. As of December 31, 2016, we have a previously recorded balance of $41.6 million of windfall stock option deductions that are being tracked off balance sheet. If and when realized, a tax benefit of $15.2 million associated with those deductions will be credited to additional paid-in capital. In 2016, no entry to paid-in capital was made to reflect the benefit from windfall stock option deductions, which is the excess of federal income tax liabilities expected on the tax return without windfall stock option deductions over federal income tax liabilities expected on the tax return with windfall stock option deductions, because we are in a loss position in this year. Prior to 2016, a tax benefit of $9.9 million associated with those deductions had been credited to additional paid-in capital.
70
As of December 31, 2016, we had net operating loss carry-forwards for federal and California tax purposes of $80.0 million and $26.0 million, respectively. We also had federal and California research and development tax credit carry-forwards of approximately $6.5 million and $14.5 million, respectively. The federal and state net operating loss carry-forwards will expire at various dates beginning in 2019 through 2037, if not utilized. The federal tax credit carry-forwards will expire at various dates beginning in 2021 through 2035, if not utilized. The California tax credit carry-forwards have no expiration date.
Utilization of our net operating loss and tax credit carry-forwards is subject to an annual limitation due to an ownership change, as defined by the IRS code section 382 that occurred in 2007. None of the net operating loss or tax credit carry-forwards is anticipated to expire as a result of the ownership change. Any future changes of ownership could result in the expiration of net operating losses or credits before utilization.
During the year ended December 31, 2016, our reserve for uncertain tax positions decreased by $2.2 million, primarily due to the release of $2.4 million of a federal tax reserve where the statute of limitations had lapsed. Interest and penalties related to the reserve for uncertain tax positions were insignificant in 2016 and 2015.
If we are able to eventually recognize these uncertain tax positions, $17.4 million of the unrecognized benefit on January 1, 2016 and $15.2 million of the unrecognized benefit on December 31, 2016, would reduce our effective tax rate. We currently have a full valuation allowance against our U.S. net deferred tax asset which would impact the timing of the effective tax rate benefit should any of these uncertain tax positions be favorably settled in the future.
We are subject to federal and state tax examination for years 2000 forward and 1997 forward, respectively, by virtue of the tax attributes carrying forward from those years. We are also subject to audits in the foreign jurisdictions in which we operate for years 2003 and forward. There are no income tax examinations currently in progress.
A reconciliation of the change in the uncertain income tax benefits from January 1, 2015 to December 31, 2016 is as follows:
In thousands | 2016 | 2015 | |||||
Balance at January 1 | $ | 17,402 | $ | 8,146 | |||
Tax positions related to the current year: | |||||||
Additions | 228 | 9,336 | |||||
Tax positions related to the prior years: | |||||||
Additions | 321 | — | |||||
Reductions | (218 | ) | (78 | ) | |||
Lapses in statutes of limitations | (2,502 | ) | (2 | ) | |||
Balance at December 31 | $ | 15,231 | $ | 17,402 | |||
15. COMMITMENTS AND CONTINGENCIES
Commitments
We lease our facilities and certain equipment under operating leases. During 2015, we extended our lease in San Jose, California. Certain of our leasing arrangements subject us to letter of credit requirements to provide a $2.4 million bank letter of credit as security to the landlord. In addition, certain of our leases require us to restore the facilities back to the original condition at the end of lease terms. As such, we recorded asset retirement obligations related to remediation costs as disclosed in Note 11 herein.
On June 28, 2016, we renegotiated the August 15, 2012 lease for our Singapore operations and entered into a new lease that will expire in June 2023.
In March 2013, we entered into an operating lease agreement for facilities in Waltham, Massachusetts, for sales, research and development activities. The lease for this facility expires in February 2018.
In August 2014, we entered into a thirty-six month capital lease agreement for equipment valued at $2.5 million. At the end of the lease term, we have an option to purchase the leased equipment for a bargain purchase price. Accordingly, this lease is classified as a capital lease. The amortization of these leased assets is included with depreciation expense. As of December 31, 2016, the total amortization of the leased equipment is $1.1 million.
71
In July 2015, we entered into a lease amendment for our facilities in San Jose, California. The lease amendment is to extend the building lease for five years and will expire in January 2021. We account for this lease as an operating lease; any improvements to the leased property are capitalized and classified as leasehold improvements.
The table below summarizes our capital and operating lease commitments as of December 31, 2016:
In thousands | Capital Leases | Operating Leases | |||||
For the years: | |||||||
2017 | $ | 582 | $ | 3,465 | |||
2018 | — | 3,140 | |||||
2019 | — | 3,066 | |||||
2020 | — | 3,135 | |||||
2021 | — | 1,412 | |||||
Total minimum lease payments | 582 | $ | 14,218 | ||||
Interest component | (7 | ) | |||||
Present value of total capital lease payments | $ | 575 | |||||
Rent expense was approximately $3.4 million, $2.7 million and $2.7 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Our open purchase order commitments, which primarily relate to purchases of inventories and equipment, were approximately $16.6 million as of December 31, 2016.
Legal Proceedings
We are subject to claims and litigation arising in the ordinary course of business. We do not believe any liability from any reasonably foreseeable disposition of such claims and litigation, individually or in the aggregate, would have a material adverse effect on our consolidated financial statements. At December 31, 2016, there were no significant outstanding legal matters.
16. INTANGIBLE ASSETS, NET
As of December 31, 2016, the gross carrying amount of all our intangible assets was $18.1 million and accumulated amortization was $7.5 million. The weighted average remaining life of all our intangible assets was 8.9 years as of December 31, 2016. Amortization expense for our intangibles was $1.7 million for each of the years ended December 31, 2016, 2015 and 2014.
The following table summarizes the balances and activity of our intangibles:
(In thousands) | At December 31, 2016 | At December 31, 2015 | ||||||||||||||||||||||||
Category | Weighted Average Years | Gross Carrying Amount | Accumulated Amortization | Net Book Value | Gross Carrying Amount | Accumulated Amortization | Net Book Value | |||||||||||||||||||
Developed technology | 6 | $ | 1,080 | $ | (432 | ) | $ | 648 | $ | 1,080 | $ | (324 | ) | $ | 756 | |||||||||||
Patents | 9 | 15,946 | (6,384 | ) | 9,562 | 15,946 | (5,000 | ) | 10,946 | |||||||||||||||||
Trade names | 6 | 209 | (82 | ) | 127 | 209 | (62 | ) | 147 | |||||||||||||||||
Customer relationships | 2 | 878 | (585 | ) | 293 | 878 | (439 | ) | 439 | |||||||||||||||||
Total | $ | 18,113 | $ | (7,483 | ) | $ | 10,630 | $ | 18,113 | $ | (5,825 | ) | $ | 12,288 | ||||||||||||
Intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Based on the intangible assets recorded as of December 31, 2016, and assuming no subsequent additions to or impairment of the underlying assets, the remaining estimated annual amortization expense is expected to be as follows:
72
Year | (In thousands) | ||
2017 | 1,602 | ||
2018 | 1,479 | ||
2019 | 1,222 | ||
2020 | 1,110 | ||
2021 | 1,049 | ||
Thereafter | 4,168 | ||
Total | $ | 10,630 | |
17. FINANCIAL GUARANTEES
Our off-balance sheet transactions consist of certain financial guarantees, both express and implied, related to indemnification for product liability, patent infringement and latent product defects. Other than liabilities recorded pursuant to known product defects, at December 31, 2016, we did not record a liability associated with these guarantees, as we have little or no history of costs associated with such indemnification requirements. Contingent liabilities associated with product liability may be mitigated by insurance coverage we maintain.
18. SUBSEQUENT EVENT
On February 2, 2017, the Company, Veeco Instruments Inc., a Delaware corporation (“Veeco”) and Ulysses Acquisition Subsidiary Corp., a Delaware corporation and a wholly owned subsidiary of Veeco (“Merger Subsidiary”), entered into an Agreement and Plan of Merger (the “Merger Agreement”), pursuant to which, among other things, Merger Subsidiary will be merged with and into the Company (the “Merger”), with the Company surviving the Merger as a wholly owned subsidiary of Veeco, subject to the terms and conditions of the Merger Agreement. The boards of directors of both Veeco and the Company have approved the transaction. The closing of the Merger is subject to the adoption of the Merger Agreement by the affirmative vote of the holders of at least a majority of the outstanding shares of our common stock and to various other customary conditions, including the expiration or termination of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, (the “HSR Act”) (with respect to which the U.S. Federal Trade Commission granted early termination on February 17, 2017), the absence of any temporary restraining order, preliminary or permanent injunction or other judgment issued by any court of competent jurisdiction enjoining or otherwise prohibiting the consummation of the Merger and the SEC having declared effective a registration statement on Form S-4 with respect to the shares of Veeco common stock issuable in connection with the Merger. In addition, it is a condition to closing of the Merger that the Company and its subsidiaries, on a consolidated basis, have at least $180 million of cash on hand held in the United States. Pursuant to the Merger Agreement, and subject to the terms and conditions contained therein, upon the closing of the Merger, each share of our common stock outstanding immediately prior to the closing of the Merger (other than shares of our common stock owned by us as treasury shares or owned by Veeco, any of its subsidiaries, any of our subsidiaries or holders of our common stock, if any, who properly exercise their appraisal rights under the General Corporation Law of the State of Delaware) will be converted into the right to receive (i) $21.75 in cash without interest and (ii) 0.2675 of a share of Veeco common stock.
On February 17, 2017, we received notice from the U.S. Federal Trade Commission that it had granted early termination, effective immediately, of the applicable waiting period under the HSR Act, for the Merger. The early termination of the waiting period under the HSR Act satisfies one of the conditions to the closing of the Merger, which remains subject to other closing conditions, including the adoption of the Merger Agreement by requisite vote of our stockholders.
.
73
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
ITEM 9A. | CONTROLS AND PROCEDURES |
Controls and Procedures
We conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as of the end of the period covered by this report (the “Evaluation Date”). Based upon the evaluation, our principal executive officer and principal financial officer concluded as of the Evaluation Date that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission (“SEC”) rules and forms.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Management is further required to apply judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control over financial reporting is designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Management, including our principal executive officer and principal financial officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2016. In making this assessment, management used the criteria set forth by the committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework (2013 framework). Based on this assessment, our management has concluded that, as of December 31, 2016, our internal control over financial reporting is effective based on those criteria. Our management has also concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
Ernst & Young, LLP, the independent registered public accounting firm who also audited our consolidated financial statements, has issued an attestation report on our internal control over financial reporting. This attestation report appears elsewhere herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
ITEM 9B. | OTHER INFORMATION |
None.
74
PART III
The information required by Part III is omitted from this Report and is incorporated herein by reference from our definitive proxy statement to be filed within 120 days after the end of our fiscal year pursuant to Regulation 14A for our 2017 Annual Meeting of Stockholders.
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
All of our directors, officers and employees must act in accordance with the Code of Business Conduct and Ethics, which has been adopted by our Board of Directors. In addition, our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions must act in accordance with the Code of Ethics for Corporate Officers and Director-level Employees, which has been adopted by our Board of Directors. Copies of these Codes are available in the Corporate Governance sub-section of the Investors section of our website at http://www.ultratech.com. To the extent required by applicable rules of the NASDAQ Stock Market LLC and the SEC, any future amendments or waivers to these Codes will be posted on our website at the location specified above.
The information concerning our directors required by this Item is incorporated by reference from the Item captioned “Election of Directors” in our Proxy Statement for the 2017 Annual Meeting of Stockholders (the “Proxy Statement”). Other information required by this Item is incorporated herein by reference from the Item captioned “Section 16(a) Beneficial Ownership Reporting Compliance” in the Proxy Statement.
Executive Officers of the Registrant
As of December 31, 2016, the executive officers of Ultratech, who are appointed by and serve at the discretion of the Board of Directors, were as follows:
Name | Age | Position with the Company | ||
Arthur W. Zafiropoulo | 77 | Chairman of the Board of Directors, Chief Executive Officer and President | ||
Bruce R. Wright | 68 | Senior Vice President, Finance, Chief Financial Officer and Secretary | ||
Tammy D. Landon | 55 | Senior Vice President, Operations | ||
Dave Ghosh | 54 | Senior Vice President, Global Sales and Services | ||
Mr. Zafiropoulo founded Ultratech in September 1992 to acquire certain assets and liabilities of the Ultratech Stepper Division (the “Predecessor”) of General Signal Technology Corporation (“General Signal”) and, since March 1993, has served as Chief Executive Officer and Chairman of the Board. Additionally, Mr. Zafiropoulo served as President of Ultratech from March 1993 to March 1996, from May 1997 until April 1999 and from April 2001 to January 2004. In October 2006, he resumed the responsibilities of President and Chief Operating Officer. Between September 1990 and March 1993, he was President of the Predecessor. From February 1989 to September 1990, Mr. Zafiropoulo was President of General Signal’s Semiconductor Equipment Group International, a semiconductor equipment company. From August 1980 to February 1989, Mr. Zafiropoulo was President and Chief Executive Officer of Drytek, Inc., a plasma dry-etch company that he founded in August 1980, and which was later sold to General Signal in 1986. From July 1987 to September 1989, Mr. Zafiropoulo was also President of Kayex, a semiconductor equipment manufacturer, which was a unit of General Signal. From July 2001 to July 2002, Mr. Zafiropoulo served as Vice Chairman of Semiconductor Equipment and Materials International (“SEMI”), an international trade association representing the semiconductor, flat panel display equipment and materials industry. From July 2002 to June 2003, Mr. Zafiropoulo served as Chairman of SEMI, and Mr. Zafiropoulo has been on the Board of Directors of SEMI since July 1995. In December 2007, Mr. Zafiropoulo was elected as Director Emeritus of SEMI. Beginning January 1, 2013, Mr. Zafiropoulo is a member of the Board of Trustees at Northeastern University.
Mr. Wright has served as Senior Vice President, Finance, Chief Financial Officer and Secretary since joining Ultratech in June 1999. From May 1997 to May 1999, Mr. Wright served as Executive Vice President, Finance and Chief Financial Officer of Spectrian Corporation, a radio frequency amplifier company. From November 1994 through May 1997, Mr. Wright was Senior Vice President of Finance and Administration, and Chief Financial Officer of Tencor Instruments until its acquisition by KLA Instruments Corporation in 1997, which formed KLA-Tencor Corporation, and from December 1991 through October 1994, Mr. Wright was Vice President and Chief Financial Officer of Tencor Instruments. Mr. Wright serves on the Board of Directors of Xcerra Corporation, a global provider of automated test equipment solutions for the testing of semiconductor integrated circuits.
75
Ms. Landon has served as Senior Vice President of Operations at Ultratech since May 2010. Since joining Ultratech in 2000, Ms. Landon served Ultratech in various capacities, including serving as Senior Director of Corporate Quality. Ms. Landon has more than 30 years of experience in manufacturing, project management and engineering positions in the semiconductor and defense industries. Ms. Landon has a bachelor’s degree in biochemistry as well as a bachelor’s degree in industrial technology from California Polytechnic State University, San Luis Obispo.
Mr. Ghosh has served as Senior Vice President of Global Sales and Service at Ultratech since January 2016. Since joining Ultratech in 1989, Mr. Ghosh served Ultratech in various capacities, including Senior Vice President of Corporate and Customer Service from January 2013 to January 2016, Vice President of Human Resources and Corporate Services from August 2009 to January 2013 and Vice President of Corporate Services from January 2004 to August 2009. Mr. Ghosh’s experience spans over 30 years in a variety of operations and service positions. Mr. Ghosh earned his bachelor’s degree in industrial technology from San Jose State University.
ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item is incorporated by reference from the Item captioned “Executive Compensation” in the Proxy Statement.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item is incorporated by reference from the Items captioned “Election of Directors,” “Ownership of Securities” and “Equity Compensation Information for Plans or Individual Arrangements with Employees and Non-Employees” in the Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item is incorporated by reference from the items captioned “Election of Directors” and “Certain Relationships and Related Transactions” in the Proxy Statement.
ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this Item is incorporated by reference from the item captioned “Fees billed to Ultratech by Ernst & Young LLP during fiscal year 2016” in the Proxy Statement.
PART IV
ITEM 15. | FINANCIAL STATEMENTS, FINANCIAL STATEMENT SCHEDULES, AND EXHIBITS |
(a) | The following documents are filed as part of this Report on Form 10-K |
(1) | Financial Statements |
The financial statements (including the notes thereto) listed in the Index to Consolidated Financial Statement Schedule (set forth in Item 8 of Part II of this Form 10-K) are filed within this Annual Report on Form 10-K.
(2) | Financial Statement Schedules |
The following consolidated financial statement schedule is included herein:
Page Number | |
Schedule II Valuation and Qualifying Accounts | |
Schedules other than those listed above have been omitted since they are either not required, are not applicable, or the required information is shown in the financial statements or related notes.
(3) | Exhibits |
Except as indicated in Exhibit 32.1, the following exhibits are filed as part of, or incorporated in reference into this Annual Report on Form 10-K:
76
Exhibit | Description |
2.1 (21) | Agreement and Plan of Merger, dated as of February 2, 2017, among Ultratech, Inc., Veeco Instruments Inc. and Ulysses Acquisition Subsidiary Corp. |
3.1(1) | Amended and Restated Certificate of Incorporation of the Registrant, filed October 6, 1993. |
3.1.1(1) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed May 17, 1995. |
3.1.2(1) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed June 17, 1998. |
3.1.3(1) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed June 20, 2003. |
3.1.4(8) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed August 31, 2009. |
3.2(2) | Amended and Restated Bylaws of Registrant. |
3.2.1 (22) | Amendment to the Amended and Restated Bylaws of the Registrant dated February 17, 2016. |
3.2.1 (21) | Amendment to the Amended and Restated Bylaws of the Registrant dated February 2, 2017 |
4.1(3) | Specimen Common Stock Certificate of Registrant. |
10.1 | 1993 Stock Option/Stock Issuance Plan (amended and restated as of May 31, 2011; further amended as of October 20, 2015). |
10.2(3) | Form of Indemnification Agreement entered into between the Registrant and certain of its officers and directors. |
10.3(4) | Form of Indemnification Agreement entered into between the Registrant and certain officers. |
10.4(3) | Standard Industrial Lease—Single Tenant, Full Net between The Equitable Life Assurance Society of the United States, as Landlord, and Registrant, as Tenant, dated August 27, 1993. |
10.4.1(4) | First Amendment to Lease between The Equitable Life Assurance Society of the United States, as Landlord, and Registrant, as Tenant, dated November 1999. |
10.5(5) | Profit Sharing Plan. |
10.6(6) | 1998 Supplemental Stock Option/ Stock Issuance Plan (amended and restated effective January 29, 2008). |
10.7(7) | Private Wealth Management Client Agreement with Morgan Stanley, dated December 16, 2004. |
10.8(9) | Amended and Restated Employment Agreement between Registrant and Mr. Arthur Zafiropoulo, Chief Executive Officer, dated as of October 14, 2008 |
10.8.1(24) | Amendment dated as of August 17, 2016 to the Amended and Restated Employment Agreement dated as of January 1, 2009 between the Registrant and Arthur W. Zafiropoulo. |
10.9(9) | Amended and Restated Employment Agreement between Registrant and Mr. Bruce Wright, Chief Financial Officer, dated as of October 14, 2008. |
10.9.1(24) | Amendment dated as of August 17, 2016 to the Amended and Restated Employment Agreement dated as of January 1, 2009 between the Registrant and Bruce R. Wright. |
77
10.10(9) | Form of Restricted Stock Unit Issuance Agreement for Executive Officers with Employment Agreements. |
10.11(16) | New Form of Restricted Stock Unit Issuance Agreement for Executive Officers with Employment Agreements. |
10.12(9) | Amended and Restated Non-Qualified Supplemental Deferred Compensation Plan. |
10.13(9) | Adoption Agreement Related to Amended and Restated Non-Qualified Supplemental Deferred Compensation Plan. |
10.14(9) | Amendment No. 1 to Amended and Restated Non-Qualified Supplemental Deferred Compensation Plan. |
10.15(9) | Special Form of Stock Option Agreement for Executive Officers with Employment Agreements. |
10.16(9) | Special Form of Stock Option Agreement for Executive Officers without Employment Agreements. |
10.17(9) | Regular Form of Stock Option Agreement. |
10.18(10) | Description of 2012 Management Incentive Compensation Plan. |
10.19(11) | New Form of Indemnification Agreement entered into between the Registrant and each of its officers and directors. |
10.20(12) | Second Amendment to Lease (3050 Zanker), entered into on October 30, 2009, by and between LaSalle Montague, Inc. and the Registrant. |
10.21(13) | Letter Amendment to Employment Agreement - Arthur W. Zafiropoulo. |
10.22(13) | Letter Amendment to Employment Agreement - Bruce R. Wright. |
10.23(14) | Singapore Lease Agreement dated February 17, 2010. |
10.24(15) | Ultratech, Inc. Long-Term Incentive Compensation Plan as Amended and Restated January 24, 2011. |
10.25(15) | Amendment No. 2 to Ultratech, Inc. Non-Qualified Supplemental Deferred Compensation Plan, as amended and restated and subsequently amended effective as of January 1, 2005. |
10.26(15) | Description of 2011 Management Incentive Compensation Plan. |
10.27(17) | Form of Restricted Stock Unit Issuance Agreement for Chief Executive Officer Grants. |
10.28(17) | Form of Restricted Stock Unit Issuance Agreement for Chief Financial Officer Grants. |
10.29(18) | Patent Assignment Agreement, between the Registrant and International Business Machines Corporation, dated June 26, 2012. |
10.30(19) | New Singapore Lease (1Kaki Bukit View, Singapore), among the Registrant, Ascendas, Inc., and Singapore SE, dated August 15, 2012. |
10.31(20) | Fourth Amendment to Lease (3050 Zanker) dated as of July 21, 2015 by and among Metropolitan Life Insurance Company, Metlife Insurance Company USA, General American Insurance Company, Tenants-in-Common, and Ultratech, Inc. |
10.32 (21) | Form of Support Agreement |
78
10.33 (23) | Lease Agreement dated June 28, 2016 between Ultratech SE Asia PTE, Ltd. and Ascendas Services Pte. Ltd. |
10.34 | Form of Change in Control Severance Agreement between the Registrant and Tammy Landon. |
10.35 | Form of Change in Control Severance Agreement between the Registrant and Dave Ghosh. |
21 | Subsidiaries of Registrant. |
23 | Consent of Independent Registered Public Accounting Firm. |
24 | Power of Attorney (contained in Signature page hereto). |
31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1* | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
* | Exhibit 32.1 is being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall such exhibit be deemed to be incorporated by reference in any registration statement or other document filed under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except as otherwise stated in such filing. |
101.INS | XBRL Instance Document |
101.SCH | XBRL Taxonomy Extension Schema Document |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
___________________
(1) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended June 28, 2003 (Commission File No. 0-22248). |
(2) | Incorporated by reference to our Current Report on Form 8-K filed on April 17, 2012 (Commission File No. 0-22248). |
(3) | Incorporated by reference to our Registration Statement on Form S-1 declared effective with the Securities and Exchange Commission on September 28, 1993. (Commission File No. 33-66522). |
(4) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2002 (Commission File No. 0-22248). |
(5) | Incorporated by reference to our 1993 Annual Report on Form 10-K (Commission File No. 0-22248). |
(6) | Incorporated by reference to our Current Report on Form 8-K filed on February 1, 2008 (Commission File No. 0-22248). |
(7) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2004 (Commission File No. 0-22248). |
(8) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended October 3, 2009 (Commission File No. 0-22248). |
(9) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2008 (Commission File No. 0-22248). |
79
(10) | Incorporated herein by reference to our Current Report on Form 8-K filed on January 27, 2012 (Commission File No. 0-22248). |
(11) | Incorporated by reference to our Current Report on Form 8-K filed on January 30, 2009 (Commission File No. 0-22248). |
(12) | Incorporated by reference to our Current Report on Form 8-K filed on November 5, 2009 (Commission File No. 0-22248). |
(13) | Incorporated by reference to our Current Report on Form 8-K filed on April 23, 2010 (Commission File No. 0-22248). |
(14) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended July 3, 2010 (Commission File No. 0-22248). |
(15) | Incorporated herein by reference to our Current Report on Form 8-K filed on January 28, 2011 (Commission File No. 0-22248). |
(16) | Incorporated herein by reference to our Annual Report on Form 10-K for the year ended December 31, 2010 (Commission File No. 0-22248). |
(17) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 |
(Commission File No. 0-22248).
(18) Incorporated by reference to our Current Report on Form 8-K filed on June 28, 2012 (Commission File No. 0-22248).
(19) Incorporated by reference to our Current Report on Form 8-K filed on August 21, 2012 (Commission File No. 0-22248).
(20) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended July 4, 2015 (Commission File No. 0-22248). |
(21) | Incorporated by reference to our Current Report on Form 8-K filed on February 3, 2017 (Commission File No. 0-22248). |
(22) | Incorporated by reference to our Current Report on Form 8-K filed on July 21, 2016 (Commission File No. 0-22248). |
(23) | Incorporated by reference to our Current Report on Form 8-K filed on June 30, 2016 (Commission File No. 0-22248). |
(24) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended October 1, 2016 filed on October 28, 2016 (Commission File No. 0-22248). |
(b) Exhibits. See list of exhibits under (a)(3) above.
(c) Financial Statement Schedules. See list of schedules under (a)(2) above.
80
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunder duly authorized.
ULTRATECH, INC. | |||
Date: | March 1, 2017 | By: | /s/ ARTHUR ZAFIROPOULO |
Arthur Zafiropoulo | |||
Chairman of the Board of Directors and Chief Executive Officer | |||
The undersigned directors and officers of Ultratech, Inc. (the “Company”), a Delaware corporation, hereby constitute and appoint Arthur W. Zafiropoulo and Bruce R. Wright, and each of them with full power to act without the other, the undersigned’s true and lawful attorney-in-fact, with full power of substitution and re-substitution, for the undersigned and in the undersigned’s name, place and stead in the undersigned’s capacity as an officer and/or director of the Company, to execute in the name and on behalf of the undersigned this Report and to file such Report, with exhibits thereto and other documents in connection therewith and any and all amendments thereto, with the Securities and Exchange Commission, granting unto said attorneys-in-fact, and each of them, full power and authority to do and perform each and every act and thing necessary or desirable to be done and to take any other action of any type whatsoever in connection with the foregoing which, in the opinion of such attorney-in-fact, may be of benefit to, in the best interest of, or legally required of, the undersigned, it being understood that the documents executed by such attorney-in-fact on behalf of the undersigned pursuant to this Power of Attorney shall be in such form and shall contain such terms and conditions as such attorney-in-fact may approve in such attorney-in-fact’s discretion.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below (and the above Powers of Attorney granted) by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ARTHUR ZAFIROPOULO | Chairman of the Board of Directors and Chief Executive Officer (Principal Executive Officer) | March 1, 2017 | ||
Arthur Zafiropoulo | ||||
/s/ BRUCE WRIGHT | Senior Vice President, Finance, Chief Financial Officer and Secretary (Principal Financial and Accounting Officer) | March 1, 2017 | ||
Bruce Wright | ||||
/s/ DENNIS RANEY | Director | March 1, 2017 | ||
Dennis Raney | ||||
/s/ RONALD BLACK | Director | March 1, 2017 | ||
Ronald Black | ||||
/s/ HENRI RICHARD | Director | March 1, 2017 | ||
Henri P Richard | ||||
/s/ PARAMESH GOPI | Director | March 1, 2017 | ||
Paramesh Gopi | ||||
/s/ BEATRIZ INFANTE | Director | March 1, 2017 | ||
Beatriz Infante | ||||
/s/ MICHAEL CHILD | Director | March 1, 2017 | ||
Michael Child | ||||
81
SCHEDULE II
ULTRATECH, INC.
VALUATION AND QUALIFYING ACCOUNTS
(in thousands)
Description | Balance at Beginning of Year | Charged (Credited) to Costs and Expenses | Balance at End of Year | ||||||||
Allowance for doubtful accounts: | |||||||||||
2014 | $ | 498 | $ | — | $ | 498 | |||||
2015 | $ | 498 | $ | — | $ | 498 | |||||
2016 | $ | 498 | $ | — | $ | 498 | |||||
82
EXHIBIT INDEX
Exhibit | Description |
2.1(21) | Agreement and Plan of Merger, dated as of February 2, 2017, among Ultratech, Inc., Veeco Instruments Inc. and Ulysses Acquisition Subsidiary Corp. |
3.1(1) | Amended and Restated Certificate of Incorporation of the Registrant, filed October 6, 1993. |
3.1.1(1) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed May 17, 1995. |
3.1.2(1) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed June 17, 1998. |
3.1.3(1) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed June 20, 2003. |
3.1.4(8) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Registrant, filed August 31, 2009. |
3.2(2) | Amended and Restated Bylaws of Registrant. |
3.2.1 (22) | Amendment to the Amended and Restated Bylaws of the Registrant dated February 17, 2016. |
3.2.1(21) | Amendment to the Amended and Restated Bylaws of the Registrant dated February 2, 2017. |
4.1(3) | Specimen Common Stock Certificate of Registrant. |
10.1 | 1993 Stock Option/Stock Issuance Plan (amended and restated as of May 31, 2011); further amended as of October 20, 2015. |
10.2(3) | Form of Indemnification Agreement entered into between the Registrant and certain of its officers and directors. |
10.3(4) | Form of Indemnification Agreement entered into between the Registrant and certain officers. |
10.4(3) | Standard Industrial Lease—Single Tenant, Full Net between The Equitable Life Assurance Society of the United States, as Landlord, and Registrant, as Tenant, dated August 27, 1993. |
10.4.1(4) | First Amendment to Lease between The Equitable Life Assurance Society of the United States, as Landlord, and Registrant, as Tenant, dated November 22, 1999. |
10.5(5) | Profit Sharing Plan. |
10.6(6) | 1998 Supplemental Stock Option/ Stock Issuance Plan (amended and restated effective January 29, 2008). |
10.7(7) | Private Wealth Management Client Agreement with Morgan Stanley, dated December 16, 2004. |
10.8(9) | Amended and Restated Employment Agreement between Registrant and Mr. Arthur Zafiropoulo, Chief Executive Officer, dated as of October 14, 2008 |
10.8.1 (24) | Amendment dated as of August 17, 2016 to the Amended and Restated Employment Agreement dated as of January 1, 2009 between the Registrant and Arthur W. Zafiropoulo. |
10.9(9) | Amended and Restated Employment Agreement between Registrant and Mr. Bruce Wright, Chief Financial Officer, dated as of October 14, 2008. |
83
10.9.1 (24) | Amendment dated as of August 17, 2016 to the Amended and Restated Employment Agreement dated as of January 1, 2009 between the Registrant and Bruce R. Wright. |
10.10(9) | Form of Restricted Stock Unit Issuance Agreement for Executive Officers with Employment Agreements. |
10.11(16) | New Form of Restricted Stock Unit Issuance Agreement for Executive Officers with Employment Agreements. |
10.12(9) | Amended and Restated Non-Qualified Supplemental Deferred Compensation Plan. |
10.13(9) | Adoption Agreement Related to Amended and Restated Non-Qualified Supplemental Deferred Compensation Plan. |
10.14(9) | Amendment No. 1 to Amended and Restated Non-Qualified Supplemental Deferred Compensation Plan. |
10.15(9) | Special Form of Stock Option Agreement for Executive Officers with Employment Agreements. |
10.16(9) | Special Form of Stock Option Agreement for Executive Officers without Employment Agreements. |
10.17(9) | Regular Form of Stock Option Agreement. |
10.18(10) | Description of 2012 Management Incentive Compensation Plan. |
10.19(11) | New Form of Indemnification Agreement entered into between the Registrant and each of its officers and directors. |
10.20(12) | Second Amendment to Lease (3050 Zanker), entered into on October 30, 2009, by and between LaSalle Montague, Inc. and the Registrant. |
10.21(13) | Letter Amendment to Employment Agreement - Arthur W. Zafiropoulo. |
10.22(13) | Letter Amendment to Employment Agreement - Bruce R. Wright. |
10.23(14) | Singapore Lease Agreement dated February 17, 2010. |
10.24(15) | Ultratech, Inc. Long-Term Incentive Compensation Plan as Amended and Restated January 24, 2011. |
10.25(15) | Amendment No. 2 to Ultratech, Inc. Non-Qualified Supplemental Deferred Compensation Plan, as amended and restated and subsequently amended effective as of January 1, 2005. |
10.26(15) | Description of 2011 Management Incentive Compensation Plan. |
10.27(17) | Form of Restricted Stock Unit Issuance Agreement for Chief Executive Officer Grants. |
10.28(17) | Form of Restricted Stock Unit Issuance Agreement for Chief Financial Officer Grants. |
10.29(18) | Patent Assignment Agreement, between the Registrant and International Business Machines Corporation, dated June 26, 2012. |
10.30(19) | New Singapore Lease (1Kaki Bukit View, Singapore), among the Registrant, Ascendas, Inc., and Singapore SE, dated August 15, 2012. |
10.31(20) | Fourth Amendment to Lease (3050 Zanker) dated as of July 21, 2015 by and among Metropolitan Life Insurance Company, Metlife Insurance Company USA, General American Insurance Company, Tenants-in-Common, and Ultratech, Inc. |
10.32(21) | Form of Support Agreement |
84
10.33 (23) | Lease Agreement dated June 28, 2016 between Ultratech SE Asia PTE, Ltd. and Ascendas Services Pte. Ltd. |
10.34 | Form of Change in Control Severance Agreement between the Registrant and Tammy Landon. |
10.35 | Form of Change in Control Severance Agreement between the Registrant and Dave Ghosh. |
21 | Subsidiaries of Registrant. |
23 | Consent of Independent Registered Public Accounting Firm. |
24 | Power of Attorney (contained in Signature page hereto). |
31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1* | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
* | Exhibit 32.1 is being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall such exhibit be deemed to be incorporated by reference in any registration statement or other document filed under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except as otherwise stated in such filing. |
101.INS | XBRL Instance Document |
101.SCH | XBRL Taxonomy Extension Schema Document |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
___________________
(1) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended June 28, 2003 (Commission File No. 0-22248). |
(2) | Incorporated by reference to our Current Report on Form 8-K filed on April 17, 2012 (Commission File No. 0-22248). |
(3) | Incorporated by reference to our Registration Statement on Form S-1 declared effective with the Securities and Exchange Commission on September 28, 1993. (Commission File No. 33-66522). |
(4) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2002 (Commission File No. 0-22248). |
(5) | Incorporated by reference to our 1993 Annual Report on Form 10-K (Commission File No. 0-22248). |
(6) | Incorporated by reference to our Current Report on Form 8-K filed on February 1, 2008 (Commission File No. 0-22248). |
(7) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2004 (Commission File No. 0-22248). |
(8) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended October 3, 2009 (Commission File No. 0-22248). |
(9) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2008 (Commission File No. 0-22248). |
85
(10) | Incorporated herein by reference to our Current Report on Form 8-K filed on January 27, 2012 (Commission File No. 0-22248). |
(11) | Incorporated by reference to our Current Report on Form 8-K filed on January 30, 2009 (Commission File No. 0-22248). |
(12) | Incorporated by reference to our Current Report on Form 8-K filed on November 5, 2009 (Commission File No. 0-22248). |
(13) | Incorporated by reference to our Current Report on Form 8-K filed on April 23, 2010 (Commission File No. 0-22248). |
(14) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended July 3, 2010 (Commission File No. 0-22248). |
(15) | Incorporated herein by reference to our Current Report on Form 8-K filed on January 28, 2011 (Commission File No. 0-22248). |
(16) | Incorporated herein by reference to our Annual Report on Form 10-K for the year ended December 31, 2010 (Commission File No. 0-22248). |
(17) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 |
(Commission File No. 0-22248)
(18) Incorporated by reference to our Current Report on Form 8-K filed on June 28, 2012 (Commission File No. 0-22248).
(19) Incorporated by reference to our Current Report on Form 8-K filed on August 21, 2012 (Commission File No. 0-22248).
(20) Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended July 4, 2015 (Commission File No. 0-22248).
(21) Incorporated by reference to our Current Report on Form 8-K filed on February 3, 2017 (Commission File No. 0-22248).
(22) | Incorporated by reference to our Current Report on Form 8-K filed on July 21, 2016 (Commission File No. 0-22248). |
(23) | Incorporated by reference to our Current Report on Form 8-K filed on June 30, 2016 (Commission File No. 0-22248). |
(24) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended October 1, 2016 filed on October 28, 2016 (Commission File No. 0-22248). |
86
