Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - CONMED Corp | exhibit3212016.htm |
| EX-31.2 - EXHIBIT 31.2 - CONMED Corp | exhibit3122016.htm |
| EX-31.1 - EXHIBIT 31.1 - CONMED Corp | exhibit3112016.htm |
| EX-23 - EXHIBIT 23 - CONMED Corp | exhibit232016.htm |
| EX-21 - EXHIBIT 21 - CONMED Corp | exhibit212016.htm |
United States
Securities and Exchange Commission
Washington, D.C.
20549
Form 10-K
Annual Report Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2016 | Commission file number 0-16093 |
CONMED CORPORATION
(Exact name of registrant as specified in its charter)
New York | 16-0977505 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
525 French Road, Utica, New York | 13502 |
(Address of principal executive offices) | (Zip Code) |
(315) 797-8375
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $.01 par value per share
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer (as defined in Rule 405 of the Securities Act).
Yes ý No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§232.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act (Check one).
Large accelerated filer ý Accelerated filer o Non-accelerated filer o Smaller reporting company o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
As of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the shares of voting common stock held by non-affiliates of the registrant was approximately $1,327,544,000 based upon the closing price of the Company’s common stock on the NASDAQ Stock Market.
The number of shares of the registrant's $0.01 par value common stock outstanding as of February 21, 2017 was 27,836,532.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the Definitive Proxy Statement and any other informational filings for the 2017 Annual Meeting of Shareholders are incorporated by reference into Part III of this report.
CONMED CORPORATION
ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED DECEMBER 31, 2016
TABLE OF CONTENTS
Part I | ||
Page | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Part II | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Part III | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Part IV | ||
Item 15. | ||
Item 16. | ||
1
CONMED CORPORATION
Item 1. Business
Forward Looking Statements
This Annual Report on Form 10-K for the Fiscal Year Ended December 31, 2016 (“Form 10-K”) contains certain forward-looking statements (as such term is defined in the Private Securities Litigation Reform Act of 1995) and information relating to CONMED Corporation (“CONMED”, the “Company”, “we” or “us” — references to “CONMED”, the “Company”, “we” or “us” shall be deemed to include our direct and indirect subsidiaries unless the context otherwise requires) which are based on the beliefs of our management, as well as assumptions made by and information currently available to our management.
When used in this Form 10-K, the words “estimate”, “project”, “believe”, “anticipate”, “intend”, “expect” and similar expressions are intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors, including those identified under the caption “Item 1A-Risk Factors” and elsewhere in this Form 10-K which may cause our actual results, performance or achievements, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the following:
• | general economic and business conditions; |
• | changes in foreign exchange and interest rates; |
• | cyclical customer purchasing patterns due to budgetary and other constraints; |
• | changes in customer preferences; |
• | competition; |
• | changes in technology; |
• | the introduction and acceptance of new products; |
• | the ability to evaluate, finance and integrate acquired businesses, products and companies; |
• | changes in business strategy; |
• | the availability and cost of materials; |
• | the possibility that United States or foreign regulatory and/or administrative agencies may initiate enforcement actions against us or our distributors; |
• | future levels of indebtedness and capital spending; |
• | quality of our management and business abilities and the judgment of our personnel; |
• | the availability, terms and deployment of capital; |
• | the risk of litigation, especially patent litigation as well as the cost associated with patent and other litigation; |
• | the risk of a lack of allograft tissues due to reduced donations of such tissues or due to tissues not meeting the appropriate high standards for screening and/or processing of such tissues; |
• | compliance with and changes in regulatory requirements; and |
• | various other factors referenced in this Form 10-K. |
See “Item 7-Management’s Discussion and Analysis of Financial Condition and Results of Operations”, “Item 1-Business” and “Item 1A-Risk Factors” for a further discussion of these factors. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date of this Form 10-K or to reflect the occurrence of unanticipated events.
General
CONMED Corporation was incorporated under the laws of the State of New York in 1970. CONMED is a medical technology company that provides surgical devices and equipment for minimally invasive procedures. The Company’s products are used by surgeons and physicians in a variety of specialties including orthopedics, general surgery, gynecology, neurosurgery and gastroenterology. Headquartered in Utica, New York, the Company’s 3,300 employees distribute its products worldwide from several manufacturing locations.
We have historically used strategic business acquisitions, internal product development activities and exclusive distribution relationships to diversify our product offerings, increase our market share in certain product lines, realize economies of scale and take advantage of growth opportunities in the healthcare field.
2
We are committed to offering products with the highest standards of quality, technological excellence and customer service. Substantially all of our facilities have attained certification under the ISO international quality standards and other domestic and international quality accreditations.
Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports are accessible free of charge through the Investor Relations section of our website (http://www.conmed.com) as soon as practicable after such materials have been electronically filed with, or furnished to, the United States Securities and Exchange Commission (the "SEC"). Our SEC filings are also available for reading and copying at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (http:/www.sec.gov) containing reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
Business Strategy
Our principal objectives are to improve the quality of surgical outcomes and patient care through the development of innovative medical devices, the refinement of existing products and the development of new technologies which reduce risk, trauma, cost and procedure time. We believe that by meeting these objectives we will enhance our ability to anticipate and adapt to customer needs and market opportunities and provide shareholders with superior investment returns. We intend to achieve future growth in revenues and earnings through the following initiatives:
• | Introduction of New Products and Product Enhancements. We continually pursue organic growth through the development of new products and enhancements to existing products. We seek to develop new technologies which improve the durability, performance and usability of existing products. In addition to our internal research and development efforts, we receive new ideas for products and technologies, particularly in procedure-specific areas, from surgeons, inventors and other healthcare professionals. |
• | Pursue Strategic Acquisitions. We pursue strategic acquisitions, distribution and similar arrangements in existing and new growth markets to achieve increased operating efficiencies, geographic diversification and market penetration. Targeted companies have historically included those with proven technologies and established brand names which provide potential sales, marketing and manufacturing synergies. This includes the January 4, 2016 acquisition of SurgiQuest, Inc. ("SurgiQuest") as further described in Item 7 - Management's Discussion and Analysis of Financial Condition and Results of Operations and Note 2 to the consolidated financial statements. |
• | Realize Manufacturing and Operating Efficiencies. We continually review our production systems for opportunities to reduce operating costs, consolidate product lines or identical process flows, reduce inventory requirements and optimize existing processes. Our vertically integrated manufacturing facilities allow for further opportunities to reduce overhead and increase operating efficiencies and capacity utilization. |
• | Geographic Diversification. We believe that significant growth opportunities exist for our surgical products outside the United States. Principal international markets for our products include Europe, Latin America, Canada and Asia/Pacific Rim. Critical elements of our future sales growth in these markets include leveraging our existing relationships with international surgeons, hospitals, third-party payers and foreign distributors (including sub-distributors and sales agents), maintaining an appropriate presence in emerging market countries and continually evaluating our routes-to-market. |
• | Active Participation in the Medical Community. We believe that excellent working relationships with physicians and others in the medical industry enable us to gain an understanding of new therapeutic and diagnostic alternatives, trends and emerging opportunities. Active participation allows us to quickly respond to the changing needs of physicians and patients. In addition, we are an active sponsor of medical education both in the United States and internationally, offering training on new and innovative surgical techniques as well as other medical education materials for use with our products. |
Products
The following table sets forth the percentage of net sales for each of our product lines during each of the three years ended December 31:
3
Year Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Orthopedic surgery | 48 | % | 54 | % | 54 | % | |||||
General surgery | 45 | 38 | 38 | ||||||||
Surgical visualization | 7 | 8 | 8 | ||||||||
Consolidated net sales | 100 | % | 100 | % | 100 | % | |||||
Net sales (in thousands) | $ | 763,520 | $ | 719,168 | $ | 740,055 | |||||
The increase in the percentage of net sales to General Surgery in 2016 is driven by the acquisition of SurgiQuest, Inc. on January 4, 2016 as further described in Note 2 to the consolidated financial statements.
Orthopedic Surgery
Our orthopedic surgery product offering includes sports medicine, powered surgical instruments, and sports biologics and tissue. These products are marketed under a number of brands, including Hall®, CONMED Linvatec®, Concept® and Shutt®.
We offer a comprehensive range of devices and products to repair injuries which have occurred in the articulating joint areas of the body. Many of these injuries are the result of sports related events or similar traumas. Our sports medicine products include powered resection instruments, arthroscopes, reconstructive systems, tissue repair sets, metal and bioabsorbable implants as well as related disposable products and fluid management systems. It is our standard practice to place some of these products, such as shaver consoles and pumps, with certain customers at no charge in exchange for commitments to purchase disposable products over certain time periods. This capital equipment is loaned and subject to return if certain minimum single-use purchases are not met. Single-use products include products such as shaver blades, burs and pump tubing. In sports medicine, we compete with Smith & Nephew, plc; Arthrex, Inc.; Stryker Corporation; Johnson & Johnson: DePuy Mitek, Inc. and Zimmer Biomet, Inc.
Our powered instruments offering is sold principally under the Hall® Surgical brand name, for use in large and small bone orthopedic, arthroscopic, oral/maxillofacial, podiatric, plastic, ENT, neurological, spinal and cardiothoracic surgeries. Our newest product is the Hall 50™ Powered Instrument System, specifically designed to meet the requirements of most orthopedic applications. The modularity and versatility of the Hall 50™ Powered Instrument System allows a facility to purchase a single power system to perform total joint arthroplasty, trauma, arthroscopy and some small bone procedures. In powered instruments, our competition includes Stryker Corporation; Medtronic plc, (Midas Rex and Xomed divisions); Johnson & Johnson: DePuy Synthes, Inc.; MicroAire Surgical Instruments, LLC and Zimmer Holdings, Inc.
As more fully described in Note 5 to the consolidated financial statements, on January 3, 2012, the Company entered into the Sports Medicine Joint Development and Distribution Agreement (the "JDDA") with Musculoskeletal Transplant Foundation (“MTF”) to obtain MTF's worldwide promotion rights with respect to allograft tissues within the field of sports medicine and related products. Under the terms of this agreement, we are now the exclusive worldwide promoter of these allograft tissues, which includes the reconstruction and/or replacement of tendon, ligament, cartilage or menisci, along with the correction of deformities within the extremities.
General Surgery
Our general surgery product line offers a large range of products in the areas of advanced surgical, endoscopic technologies, and critical care.
Our advanced surgical product offering includes an extensive line of electrosurgical generators, handpieces, smoke management systems and accessories. Our endomechanical instrumentation products offer a full line of instruments including trocars, suction irrigation devices, graspers, scissors and dissectors used in minimally invasive surgery. We offer a unique and premium uterine manipulator called VCARE® for use in increasing the efficiency of laparoscopic hysterectomies and other gynecologic laparoscopic procedures. Our competition includes Medtronic plc (formerly Covidien); Johnson & Johnson: Ethicon Endo-Surgery, Inc.; ERBE Elektromedizin GmbH; Megadyne and Applied Medical Resources Corporation.
On January 4, 2016, we acquired SurgiQuest for $265 million in cash (on a cash-free, debt-free basis). SurgiQuest developed, manufactured and marketed the AirSeal® System, the first integrated access management technology for use in laparoscopic and robotic procedures. This proprietary and differentiated access system is complementary to our general surgery offering.
4
Our endoscopic technologies offering includes a comprehensive line of minimally invasive diagnostic and therapeutic products used in conjunction with procedures which require flexible endoscopy. This offering includes mucosal management devices, forceps, scope management accessories, bronchoscopy devices, dilatation, stricture management devices, hemostasis, biliary devices and polypectomy. Our competition includes Boston Scientific Corporation - Endoscopy; Cook Medical, Inc.; Merit Medical Endotek; Olympus, Inc.; STERIS Corporation - U.S. Endoscopy and Cantel Medical- Medivators, Inc.
Our critical care offering includes a line of vital signs, cardiac monitoring and patient care products including ECG electrodes & accessories and cardiac defibrillation & pacing pads. We also offer a complete line of suction instruments and tubing that are used throughout all areas of the hospital as well as in Ambulatory Surgery Centers and the emergency medical market. Finally, we offer a physician's office electrosurgical product mainly used by dermatologists. This offering's main competition includes Medtronic plc (formerly Covidien) and 3M Company.
Surgical Visualization
Our surgical visualization product line offers imaging systems for use in minimally invasive orthopedic and general surgery procedures including 2DHD and 3DHD vision technologies. Competition includes Smith & Nephew, plc; Arthrex, Inc.; Stryker Corporation; Olympus, Inc.; Richard Wolf and Karl Storz GmbH.
International
Expanding our international presence is an important component of our long-term growth plan. Our products are sold in over 100 foreign countries. International sales efforts are coordinated through local country dealers (including sub-distributors or sales agents) or through direct in-country sales. We distribute our products through sales subsidiaries and branches with offices located in Australia, Austria, Belgium, Canada, China, Denmark, Finland, France, Germany, Italy, Korea, the Netherlands, Poland, Spain, Sweden and the United Kingdom. In these countries, our sales are denominated in the local currency and amounted to approximately 30% of our total net sales in 2016. In the remaining countries where our products are sold through independent distributors, sales are denominated in United States dollars.
Competition
We compete in orthopedic, surgical visualization and general surgery medical device markets across the world. Our competitors range from large manufacturers with multiple business units to smaller manufacturers with limited product offerings. We believe we have appropriate product offerings and adequate market share to compete effectively in these markets. The global markets are constantly changing due to technological advances. We seek to closely align our research and development with our key business objectives, namely developing and improving products and processes, applying innovative technology to the manufacture of products for new global markets and reducing the cost of producing core products.
The breadth of our product lines in our key product areas enables us to meet a wide range of customer requirements and preferences. This has enhanced our ability to market our products to surgeons, hospitals, surgery centers, group purchasing organizations ("GPOs"), integrated delivery networks ("IDNs") and other customers, particularly as institutions seek to reduce costs and minimize the number of suppliers.
Marketing
A significant portion of our products are distributed domestically directly to more than 6,000 hospitals, surgery centers and other healthcare institutions as well as through medical specialty distributors. We are not dependent on any single customer and no single customer accounted for more than 10% of our net sales in 2016, 2015 and 2014.
A significant portion of our U.S. sales are to customers affiliated with GPOs, IDNs and other large national or regional accounts, as well as to the Veterans Administration and other hospitals operated by the Federal government. For hospital inventory management purposes, some of our customers prefer to purchase our products through independent third-party medical product distributors.
Our employee sales representatives are specially trained in our various product offerings. Each employee sales representative is assigned a defined geographic area and compensated on a commission basis or through a combination of salary and commission. The sales force is supervised and supported by either area directors or district managers. In certain geographies, sales agent groups are used in the United States to sell our orthopedic products. These sales agent groups are paid a commission for sales made to customers while home office sales and marketing management provide the overall direction for marketing and
5
positioning of our products. Our sales professionals provide surgeons and medical personnel with information relating to the technical features and benefits of our products.
Our health systems organization is responsible for interacting with large regional and national accounts (e.g. GPOs, IDNs, etc.). We have contracts with many such organizations and believe that the loss of any individual group purchasing contract will not materially impact our business. In addition, all of our sales professionals are required to work closely with distributors where applicable and maintain close relationships with end-users.
We sell to a diversified base of customers around the world and, therefore, believe there is no material concentration of credit risk.
Manufacturing
Raw material costs constitute a substantial portion of our cost of production. Substantially all of our raw materials and select components used in the manufacturing process are procured from external suppliers. We work closely with multiple suppliers to ensure continuity of supply while maintaining high quality and reliability. As a consequence of supply chain best practices, new product development and acquisitions, we often form strategic partnerships with key suppliers. As a consequence of these supplier partnerships, components and raw materials may be sole sourced. Due to the strength of these suppliers and the variety of products we provide, we do not believe the risk of supplier interruption poses an overall material adverse effect on our financial and operational performance. We schedule production and maintain adequate levels of safety stock based on a number of factors, including experience, knowledge of customer ordering patterns, demand, manufacturing lead times and optimal quantities required to maintain the highest possible service levels. Customer orders are generally processed for immediate shipment and backlog of firm orders is therefore not considered material to an understanding of our business.
Research and Development
New and improved products play a critical role in our continued sales growth. Internal research and development efforts focus on the development of new products and product technological and design improvements aimed at complementing and expanding existing product lines. We continually seek to leverage new technologies which improve the durability, performance and usability of existing products. In addition, we maintain close working relationships with surgeons, inventors and operating room personnel who often make new product and technology disclosures, principally in procedure-specific areas. In certain cases, we seek to obtain rights to these ideas through negotiated agreements. Such agreements typically compensate the originator through payments based upon a percentage of licensed product net sales. Annual royalty expense approximated $2.3 million, $2.3 million and $2.6 million in 2016, 2015 and 2014, respectively.
Amounts expended for Company research and development were approximately $32.3 million, $27.4 million and $27.8 million during 2016, 2015 and 2014, respectively. In 2016, the Company increased its efforts on new product development and innovation.
Intellectual Property
Patents and other proprietary rights, in general, are important to our business. We have rights to intellectual property, including United States patents and foreign equivalent patents which cover a wide range of our products. We own a majority of these patents and have exclusive and non-exclusive licensing rights to the remainder. In addition, certain of these patents have currently been licensed to third parties on a non-exclusive basis. We believe that the development of new products and technological and design improvements to existing products will continue to be of primary importance in maintaining our competitive position.
Government Regulation and Quality Systems
The development, manufacture, sale and distribution of our products are subject to regulation by numerous agencies and legislative bodies, including the U.S. Food and Drug Administration ("FDA") and comparable foreign counterparts. In the United States, these regulations were enacted under the Medical Device Amendments of 1976 to the Federal Food, Drug and Cosmetic Act and its subsequent amendments, and the regulations issued or proposed thereunder.
The FDA’s Quality System Regulations set forth requirements for our product design and manufacturing processes, require the maintenance of certain records, provide for on-site inspection of our facilities and continuing review by the FDA. Many of our products are also subject to industry-defined standards. Authorization to commercially market our products in the U.S. is granted by the FDA under a procedure referred to as a 510(k) pre-market notification. This process requires us to notify the FDA
6
of the new product and obtain FDA clearance before marketing the device. We believe that our products and processes presently meet applicable standards in all material respects.
Medical device regulations continue to evolve world-wide. Products marketed in the European Union and other countries require preparation of technical files and design dossiers which demonstrate compliance with applicable international regulations. As government regulations continue to change, there is a risk that the distribution of some of our products may be interrupted or discontinued if they do not meet the country specific requirements.
We market our products in numerous foreign countries and therefore are subject to regulations affecting, among other things, product standards, sterilization, packaging requirements, labeling requirements, import laws and onsite inspection by independent bodies with the authority to issue or not issue certifications we may require to be able to sell products in certain countries. Many of the regulations applicable to our devices and products in these countries are similar to those of the FDA. The member countries of the European Union have adopted the European Medical Device Directives, which create a single set of medical device regulations for all member countries. These regulations require companies that wish to manufacture and distribute medical devices in the European Union to maintain quality system certifications through European Union recognized Notified Bodies. These Notified Bodies authorize the use of the CE Mark allowing free movement of our products throughout the member countries. Requirements pertaining to our products vary widely from country to country, ranging from simple product registrations to detailed submissions such as those required by the FDA. We believe that our products and quality procedures currently meet applicable standards for the countries in which they are marketed.
As noted above, our facilities are subject to periodic inspection by the United States Food and Drug Administration (“FDA”) and foreign regulatory agencies or notified bodies for, among other things, conformance to Quality System Regulation and Current Good Manufacturing Practice (“CGMP”) requirements and foreign or international standards. Refer to Note 11 to the consolidated financial statements for further discussion.
Employees
As of December 31, 2016, we had approximately 3,300 full-time employees, including approximately 2,200 in operations, 160 in research and development and the remaining in sales, marketing and related administrative support. We believe that we have good relations with our employees and have never experienced a strike or similar work stoppage. None of our domestic employees are represented by a labor union.
Item 1A. Risk Factors
An investment in our securities, including our common stock, involves a high degree of risk. Investors should carefully consider the specific factors set forth below as well as the other information included or incorporated by reference in this Form 10-K. See “Forward Looking Statements”.
Our financial performance is dependent on conditions in the healthcare industry and the broader economy.
The results of our business are directly tied to the economic conditions in the healthcare industry and the broader economy as a whole. We will continue to monitor and manage the impact of the overall economic environment on the Company, including proposals for broad reform of the existing United States corporate tax system, including provisions impacting companies that import goods from Mexico or export goods from the United States. These proposals are currently under evaluation by various legislative and administrative bodies. We cannot predict the overall impact that such proposals may have on our business model, financial condition or results of operations.
In addition, approximately 21% of our revenues are derived from the sale of capital products. The sales of such products are negatively impacted if hospitals and other healthcare providers are unable to secure the financing necessary to purchase these products or otherwise defer purchases.
Our significant international operations subject us to foreign currency fluctuations and other risks associated with operating in countries outside the Untied States.
A significant portion of our revenues are derived from international sales. Approximately 48% of our total 2016 consolidated net sales were to customers outside the United States. We have sales subsidiaries in a significant number of countries in Europe as well as Australia, Canada, China and Korea. In those countries in which we have a direct presence, our sales are denominated in the local currency and those sales denominated in local currency amounted to approximately 30% of our total net sales in 2016. The remaining 18% of sales to customers outside the United States was on an export basis and transacted in United States dollars.
7
Because a significant portion of our operations consist of sales activities in jurisdictions outside the United States, our financial results may be affected by factors such as changes in foreign currency exchange rates or weak economic conditions in the markets in which we distribute products. While we have implemented a hedging strategy involving foreign currency forward contracts for 2016, our revenues and earnings are only partially protected from foreign currency translation if the United States dollar strengthens as compared with currencies such as the Euro. Further, as of the date of this Form 10-K, we have not entered into any foreign currency forward contracts beyond 2018. Our international presence exposes us to certain other inherent risks, including:
• | imposition of limitations on conversions of foreign currencies into dollars or remittance of dividends and other payments by international subsidiaries; |
• | imposition or increase of withholding and other taxes on remittances and other payments by international subsidiaries; |
• | trade barriers; |
• | political risks, including political instability; |
• | reliance on third parties to distribute our products; |
• | hyperinflation in certain countries outside the United States; and |
• | imposition or increase of investment and other restrictions by foreign governments. |
We cannot assure you that such risks will not have a material adverse effect on our business and results of operations.
Our financial performance is subject to the risks inherent in our acquisition strategy, including the effects of increased borrowing and integration of newly acquired businesses or product lines.
A key element of our business strategy has been to expand through acquisitions and we may seek to pursue additional acquisitions in the future. Our success is dependent in part upon our ability to integrate acquired companies or product lines into our existing operations. We may not have sufficient management and other resources to accomplish the integration of our past and future acquisitions and implementing our acquisition strategy may strain our relationship with customers, suppliers, distributors, personnel or others. There can be no assurance that we will be able to identify and make acquisitions on acceptable terms or that we will be able to obtain financing for such acquisitions on acceptable terms. In addition, while we are generally entitled to customary indemnification from sellers of businesses for any difficulties that may have arisen prior to our acquisition of each business, acquisitions may involve exposure to unknown liabilities and the amount and time for claiming under these indemnification provisions is often limited. As a result, our financial performance is now, and will continue to be, subject to various risks associated with the acquisition of businesses, including the financial effects associated with any increased borrowing required to fund such acquisitions or with the integration of such businesses. We incurred substantial additional debt in connection with the SurgiQuest acquisition, and we cannot ensure that we will be able to successfully advance SurgiQuest’s product lines or that risks related to the SurgiQuest acquisition will not negatively impact our financial performance.
Our financial performance may be adversely impacted by healthcare reform legislation.
Provisions of healthcare legislation, including provisions of the Patient Protection and Affordable Care Act ("ACA"), could meaningfully change the way health care is developed and delivered and may adversely affect our business and results of operations. For example, the ACA includes provisions aimed at improving quality and decreasing costs of Medicare, governing comparative effectiveness research, and implementing an independent payment advisory board and pilot programs to evaluate alternative payment methodologies. That legislation also included a 2.3% excise tax imposed upon sales within the U.S. of certain medical device products, which has been delayed until 2018. We also face uncertainties that might result in the modification or repeal of any provisions of the ACA, including as a result of current and future executive orders and legislative actions. The uncertainty associated with modifications or a repeal could generally cause healthcare markets to be unstable and we could be subject to some interruptions, the magnitude of which are impossible to determine, as healthcare providers, both facilities and medical professionals, who have benefited from the ACA determine the paths forward.
As a manufacturer of medical devices that interacts with physicians and health care providers domestically and internationally, we face risks under domestic and foreign regulations, including the Foreign Corrupt Practices Act.
Manufacturers of medical devices have been the subject of various investigations or enforcement actions relating to interactions with health care providers domestically or internationally. The interactions with domestic health care providers are subject to regulations, known as the Anti-Kickback Statute, the Stark Act and the False Claims Act, that generally govern incentives for health care providers, or methods of reimbursement funded in whole or in part by the government. Similarly, the Foreign Corrupt Practices Act (“FCPA”) prohibits certain conduct by manufacturers, generally described as bribery, with respect to interactions, either directly through foreign subsidiaries or indirectly through distributors, with health care providers who may be considered government officials because they are affiliated with public hospitals. The FCPA also imposes obligations on manufacturers listed
8
on U.S. stock exchanges to maintain accurate books and records, and maintain internal accounting controls sufficient to provide assurance that transactions are accurately recorded, lawful and in accordance with management’s authorization. The FCPA can pose unique challenges for manufacturers who operate in foreign cultures where conduct prohibited by the FCPA may not be viewed as illegal in local jurisdictions, and because, in some cases, a United States manufacturer may face risks under the FCPA based on the conduct of third parties over whom the manufacturer may not have complete control.
In this regard, from time to time, the Company may receive an information request or subpoena from a government agency, such as the Securities and Exchange Commission, Department of Justice, Equal Employment Opportunity Commission, the Occupational Safety and Health Administration, the Department of Labor, the Treasury Department or other federal and state agencies or foreign governments or government agencies. Alternatively, employees or private parties may provide us with reports of alleged misconduct. These information requests or subpoenas may or may not be routine inquiries, or may begin as informal or routine inquiries and over time develop into investigations or enforcement actions of various types under the FCPA or otherwise. Similarly, the employee and third party reports may prompt us to conduct internal investigations into the alleged misconduct. No inquiry that the Company currently faces or has faced to date, and no report of misconduct that the Company has received to date, has had a material adverse effect on our financial condition, results of operations or cash flows. There can be no assurance, however, that any pending inquiries will become investigations or enforcement actions, or the costs associated with responding to such inquiries, investigations, enforcement actions or investigations relating to reports of misconduct will not have a material adverse effect on our financial condition, results of operations or cash flows.
Failure to comply with regulatory requirements may result in recalls, fines or materially adverse implications.
Substantially all of our products are classified as class II medical devices subject to regulation by numerous agencies and legislative bodies, including the FDA and comparable international counterparts. As a manufacturer of medical devices, our manufacturing processes and facilities are subject to on-site inspection and continuing review by the FDA for compliance with the Quality System Regulations. We may have future inspections at our sites and there can be no assurance that the costs of responding to such inspections will not be material. Refer to Note 11 to the consolidated financial statements for further discussion.
Manufacturing and sales of our products outside the United States are also subject to international regulatory requirements which vary from country to country. Moreover, we are generally required to obtain regulatory clearance or approval prior to marketing a new product. The time required to obtain approvals from foreign countries may be longer or shorter than that required for FDA clearance, and requirements for such approvals may differ from FDA requirements. Failure to comply with applicable domestic and/or foreign regulatory requirements may result in:
• | fines or other enforcement actions; |
• | recall or seizure of products; |
• | total or partial suspension of production; |
• | loss of certification; |
• | withdrawal of existing product approvals or clearances; |
• | refusal to approve or clear new applications or notices; |
• | increased quality control costs; or |
• | criminal prosecution. |
Failure to comply with Quality System Regulations and applicable international regulations could result in a material adverse effect on our business, financial condition or results of operations.
If we are not able to manufacture products in compliance with regulatory standards, we may decide to cease manufacturing of those products and may be subject to product recall.
In addition to the Quality System Regulations, many of our products are also subject to industry-defined standards. We may not be able to comply with these regulations and standards due to deficiencies in component parts or our manufacturing processes. If we are not able to comply with the Quality System Regulations or industry-defined standards, we may not be able to fill customer orders and we may decide to cease production of non-compliant products. Failure to produce products could affect our profit margins and could lead to loss of customers.
Our products are subject to product recall and we have conducted product recalls in the past. Although no recall has had a material adverse effect on our business or financial condition, we cannot assure you that regulatory issues will not have a material adverse effect on our business, financial condition or results of operations in the future or that product recalls will not harm our reputation and our customer relationships.
9
The highly competitive market for our products may create adverse pricing pressures.
The market for our products is highly competitive and our customers have numerous alternatives of supply. Many of our competitors offer a range of products in areas other than those in which we compete, which may make such competitors more attractive to surgeons, hospitals, group purchasing organizations and others. In addition, many of our competitors are large, technically competent firms with substantial assets. Competitive pricing pressures or the introduction of new products by our competitors could have an adverse effect on our revenues. See “Products” in Item 1 - Business for a further discussion of these competitive forces.
Factors which may influence our customers’ choice of competitor products include:
•changes in surgeon preferences;
•increases or decreases in healthcare spending related to medical devices;
•our inability to supply products to them as a result of product recall, market withdrawal or back-order;
•the introduction by competitors of new products or new features to existing products;
•the introduction by competitors of alternative surgical technology; and
•advances in surgical procedures, discoveries or developments in the healthcare industry.
We use a variety of raw materials in our businesses, and significant shortages or price increases could increase our operating costs and adversely impact the competitive positions of our products.
Our reliance on certain suppliers and commodity markets to secure raw materials used in our products exposes us to volatility in the prices and availability of raw materials. In some instances, we participate in commodity markets that may be subject to allocations by suppliers. A disruption in deliveries from our suppliers, price increases or decreased availability of raw materials or commodities could have an adverse effect on our ability to meet our commitments to customers or increase our operating costs. We believe that our supply management practices are based on an appropriate balancing of the foreseeable risks and the costs of alternative practices. Nonetheless, price increases or the unavailability of some raw materials may have an adverse effect on our results of operations or financial condition.
Cost reduction efforts in the healthcare industry could put pressures on our prices and margins.
In recent years, the healthcare industry has undergone significant change driven by various efforts to reduce costs. Such efforts include national healthcare reform, trends towards managed care, cuts in Medicare, consolidation of healthcare distribution companies and collective purchasing arrangements by GPOs and IDNs. Demand and prices for our products may be adversely affected by such trends.
We may not be able to keep pace with technological change or to successfully develop new products with wide market acceptance, which could cause us to lose business to competitors.
The market for our products is characterized by rapidly changing technology. Our future financial performance will depend in part on our ability to develop and manufacture new products on a cost-effective basis, to introduce them to the market on a timely basis and to have them accepted by surgeons.
We may not be able to keep pace with technology or to develop viable new products. In addition, many of our competitors are substantially larger with greater financial resources which may allow them to more rapidly develop new products. Factors which may result in delays of new product introductions or cancellation of our plans to manufacture and market new products include:
• | capital constraints; |
• | research and development delays; |
• | delays in securing regulatory approvals; and |
• | changes in the competitive landscape, including the emergence of alternative products or solutions which reduce or eliminate the markets for pending products. |
Our new products may fail to achieve expected levels of market acceptance.
New product introductions may fail to achieve market acceptance. The degree of market acceptance for any of our products will depend upon a number of factors, including:
• | our ability to develop and introduce new products and product enhancements in the time frames we currently estimate; |
10
• | our ability to successfully implement new technologies; |
• | the market’s readiness to accept new products; |
• | having adequate financial and technological resources for future product development and promotion; |
• | the efficacy of our products; and |
• | the prices of our products compared to the prices of our competitors’ products. |
If our new products do not achieve market acceptance, we may be unable to recover our investments and may lose business to competitors.
In addition, some of the companies with which we now compete, or may compete in the future, have or may have more extensive research, marketing and manufacturing capabilities and significantly greater technical and personnel resources than we do, and may be better positioned to continue to improve their technology in order to compete in an evolving industry. See “Products” in Item 1 - Business for a further discussion of these competitive forces.
Our senior credit agreement contains covenants which may limit our flexibility or prevent us from taking actions.
Our senior credit agreement contains, and future credit facilities are expected to contain, certain restrictive covenants which will affect, and in many respects significantly limit or prohibit, among other things, our ability to:
•incur indebtedness;
•make investments;
•engage in transactions with affiliates;
•pay dividends or make other distributions on, or redeem or repurchase, capital stock;
•sell assets; and
•pursue acquisitions.
These covenants, unless waived, may prevent us from pursuing acquisitions, significantly limit our operating and financial flexibility and limit our ability to respond to changes in our business or competitive activities. Our ability to comply with such provisions may be affected by events beyond our control. In the event of any default under our credit agreement, the credit agreement lenders may elect to declare all amounts borrowed under our credit agreement, together with accrued interest, to be due and payable. If we were unable to repay such borrowings, the credit agreement lenders could proceed against collateral securing the credit agreement which consists of substantially all of our property and assets. Our credit agreement also contains a material adverse effect clause which may limit our ability to access additional funding under our credit agreement should a material adverse change in our business occur.
Our leverage and debt service requirements may require us to adopt alternative business strategies.
As of December 31, 2016, we had $499.1 million of debt outstanding, representing 45% of total capitalization. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” and Note 6 to our consolidated financial statements.
The degree to which we are leveraged could have important consequences to investors, including but not limited to the following:
• | a portion of our cash flow from operations must be dedicated to debt service and will not be available for operations, capital expenditures, acquisitions, dividends and other purposes; |
• | our ability to obtain additional financing in the future for working capital, capital expenditures, acquisitions or general corporate purposes may be limited or impaired or may be at higher interest rates; |
• | we may be at a competitive disadvantage when compared to competitors that are less leveraged; |
• | we may be hindered in our ability to adjust rapidly to market conditions; |
• | our degree of leverage could make us more vulnerable in the event of a downturn in general economic conditions or other adverse circumstances applicable to us; and |
• | our interest expense could increase if interest rates in general increase because a portion of our borrowings, including our borrowings under our credit agreement, are and will continue to be at variable rates of interest. |
We may not be able to generate sufficient cash to service our indebtedness, which could require us to reduce our expenditures, sell assets, restructure our indebtedness or seek additional equity capital.
Our ability to satisfy our obligations will depend upon our future operating performance, which will be affected by prevailing economic conditions and financial, business and other factors, many of which are beyond our control. We may not have sufficient
11
cash flow available to enable us to meet our obligations. If we are unable to service our indebtedness, we will be forced to adopt an alternative strategy that may include actions such as foregoing acquisitions, reducing or delaying capital expenditures, selling assets, restructuring or refinancing our indebtedness or seeking additional equity capital. We cannot assure you that any of these strategies could be implemented on terms acceptable to us, if at all. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources” for a discussion of our indebtedness and its implications.
We rely on a third party to obtain, process and distribute sports medicine allograft tissue. If such tissue cannot be obtained, is not accepted by the market or is not accepted under numerous government regulations, our results of operations could be negatively impacted.
A portion of our orthopedic revenues relate to our share of the service fees from the MTF allograft tissues for which we have exclusive promotion rights, as further described in our revenue recognition policy in Note 1 to the consolidated financial statements. Our primary costs related to these revenues come from our commission expense and certain marketing costs. Our ability to increase the service fees may be constrained by certain factors which are outside of our control, such as the limited supply of donors and donated tissue that meets the quality standards of MTF. Similarly, under the terms of the Joint Development and Distribution Agreement (“JDDA”), MTF remains responsible for tissue procurement and processing, shipment of tissues and invoicing of service fees to customers. To the extent MTF’s performance does not meet customer expectations or otherwise fails, CONMED may be unable to increase the allograft service fees or to find a suitable replacement for MTF on terms that are acceptable.
The FDA and several states have statutory authority to regulate allograft processing and allograft-based materials. The FDA could identify deficiencies in future inspections of MTF or MTF's suppliers or promulgate future regulatory rulings that could disrupt our business, reducing profitability.
If the Company or our business partners are unable to adequately protect our information assets from cyber-based attacks or other security incidents, our operations could be disrupted.
We are increasingly dependent on information technology, including the internet, for the storage, processing, and transmission of our electronic, business-related, information assets. We leverage our internal information technology infrastructures, and those of our business partners, to enable, sustain, and support our global business interests. In the event that the Company or our business partners are unable to prevent, detect, and remediate cyber-based attacks or other security incidents in a timely manner, our operations could be disrupted or we may incur financial or reputational losses arising from the theft, alteration, misuse, unauthorized disclosure, or destruction of our information assets.
If we infringe third parties’ patents, or if we lose our patents or they are held to be invalid, we could become subject to liability and our competitive position could be harmed.
Much of the technology used in the markets in which we compete is covered by patents. We have numerous U.S. patents and corresponding international patents on products expiring at various dates from 2017 through 2038 and have additional patent applications pending. See Item 1 Business “Research and Development” and “Intellectual Property” for a further description of our patents. The loss of our patents could reduce the value of the related products and any related competitive advantage. Competitors may also be able to design around our patents and to compete effectively with our products. In addition, the cost of enforcing our patents against third parties and defending our products against patent infringement actions by others could be substantial. We cannot assure you that:
• | pending patent applications will result in issued patents; |
• | patents issued to or licensed by us will not be challenged by competitors; |
• | our patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage; or |
• | we will be successful in defending against pending or future patent infringement claims asserted against our products. |
Ordering patterns of our customers may change resulting in reductions in sales.
Our hospital and surgery center customers purchase our products in quantities sufficient to meet their anticipated demand. Likewise, our healthcare distributor customers purchase our products for ultimate resale to healthcare providers in quantities sufficient to meet the anticipated requirements of the distributors’ customers. Should inventories of our products owned by our hospital, surgery center and distributor customers grow to levels higher than their requirements, our customers may reduce the ordering of products from us. This could result in reduced sales during a financial accounting period.
12
We can be sued for producing defective products and our insurance coverage may be insufficient to cover the nature and amount of any product liability claims.
The nature of our products as medical devices and today’s litigious environment should be regarded as potential risks which could significantly and adversely affect our financial condition and results of operations. The insurance we maintain to protect against claims associated with the use of our products has deductibles and may not adequately cover the amount or nature of any claim asserted against us. We are also exposed to the risk that our insurers may become insolvent or that premiums may increase substantially. See “Item 3 - Legal Proceedings” for a further discussion of the risk of product liability actions and our insurance coverage.
Damage to our physical properties as a result of windstorm, earthquake, fire or other natural or man-made disaster may cause a financial loss and a loss of customers.
Although we maintain insurance coverage for physical damage to our property and the resultant losses that could occur during a business interruption, we are required to pay deductibles and our insurance coverage is limited to certain caps. For example, our deductible for windstorm damage to our Florida property amounts to 2% of any loss.
Further, while insurance reimburses us for our lost gross earnings during a business interruption, if we are unable to supply our customers with our products for an extended period of time, there can be no assurance that we will regain the customers’ business once the product supply is returned to normal.
Item 1B. Unresolved Staff Comments
None.
13
Item 2. Properties
Facilities
The following table sets forth certain information with respect to our principal operating facilities. We believe that our facilities are generally well maintained, are suitable to support our business and adequate for present and anticipated needs.
Location | Square Feet | Own or Lease | Lease Expiration | ||||
Utica, NY | 500,000 | Own | — | ||||
Largo, FL | 278,000 | Own | — | ||||
Chihuahua, Mexico | 207,720 | Lease | September 2019 | ||||
Lithia Springs, GA | 188,400 | Lease | December 2019 | ||||
Brussels, Belgium | 45,531 | Lease | June 2024 | ||||
Milford, CT | 40,542 | Lease | November 2020 | ||||
Mississauga, Canada | 22,378 | Lease | December 2018 | ||||
Westborough, MA | 19,515 | Lease | June 2020 | ||||
Frenchs Forest, Australia | 16,912 | Lease | July 2020 | ||||
Seoul, Korea | 15,585 | Lease | January 2020 | ||||
Anaheim, CA | 14,037 | Lease | August 2018 | ||||
Frankfurt, Germany | 13,606 | Lease | March 2023 | ||||
Milan, Italy | 13,024 | Lease | March 2023 | ||||
Barcelona, Spain | 12,820 | Lease | December 2023 | ||||
Swindon, Wiltshire, UK | 8,562 | Lease | December 2020 | ||||
Greenwood Village, CO | 8,541 | Lease | January 2020 | ||||
Askim, Sweden | 8,353 | Lease | May 2019 | ||||
Lyon, France | 7,438 | Lease | December 2026 | ||||
Beijing, China | 6,799 | Lease | June 2017 | ||||
Copenhagen, Denmark | 5,899 | Lease | October 2018 | ||||
New York, NY | 3,473 | Lease | September 2022 | ||||
Beijing, China | 3,456 | Lease | September 2019 | ||||
Warsaw, Poland | 3,222 | Lease | February 2018 | ||||
Espoo, Finland | 3,078 | Lease | Open Ended | ||||
Shanghai, China | 2,269 | Lease | February 2018 | ||||
Innsbruck, Austria | 1,820 | Lease | June 2020 | ||||
Our principal manufacturing facilities are located in Utica, NY, Largo, FL, Anaheim, CA and Chihuahua, Mexico. Lithia Springs, GA and Brussels, Belgium are our principal distribution centers. The remaining facilities are sales and administrative offices with certain offices also including smaller distribution centers.
Item 3. Legal Proceedings
We are involved in various proceedings, legal actions and claims arising in the normal course of business, including proceedings related to product, labor and intellectual property and other matters that are more fully described in Note 11 to the consolidated financial statements. We are not a party to any pending legal proceedings other than ordinary routine litigation incidental to our business.
Item 4. Mine Safety Disclosures
Not applicable.
14
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock, par value $.01 per share, is traded on the NASDAQ Stock Market under the symbol “CNMD”. At January 31, 2017, there were 597 registered holders of our common stock and approximately 5,353 accounts held in “street name”.
The following table sets forth quarterly high and low closing sales prices for the years ended December 31, 2016 and 2015, as reported by the NASDAQ Stock Market.
2016 | |||||||
Period | High | Low | |||||
First Quarter | $ | 42.61 | $ | 36.16 | |||
Second Quarter | 47.73 | 38.97 | |||||
Third Quarter | 50.00 | 38.48 | |||||
Fourth Quarter | 46.45 | 37.75 | |||||
2015 | |||||||
Period | High | Low | |||||
First Quarter | $ | 51.88 | $ | 44.90 | |||
Second Quarter | 59.11 | 48.29 | |||||
Third Quarter | 60.19 | 47.09 | |||||
Fourth Quarter | 49.99 | 38.34 | |||||
Our Board of Directors has authorized a share repurchase program; see Note 8 to the consolidated financial statements.
On February 29, 2012, the Board of Directors adopted a cash dividend policy and declared an initial quarterly dividend of $0.15 per share. On October 28, 2013, the Board of Directors increased the quarterly dividend to $0.20 per share. The fourth quarter dividend for 2016 was paid on January 5, 2017 to shareholders of record as of December 15, 2016. The total dividend payable at December 31, 2016 was $5.6 million and is included in other current liabilities in the consolidated balance sheet. Future decisions as to the payment of dividends will be at the discretion of the Board of Directors, subject to conditions then existing, including our financial requirements and condition and the limitation and payment of cash dividends contained in debt agreements.
Refer to Item 12 for information relating to compensation plans under which equity securities of CONMED Corporation are authorized for issuance.
15
Performance Graph
The performance graph below compares the yearly percentage change in the Company’s Common Stock with the cumulative total return of the NASDAQ Composite Index and the cumulative total return of the Standard & Poor’s Health Care Equipment Index. In each case, the cumulative total return assumes reinvestment of dividends into the same class of equity securities at the frequency with which dividends are paid on such securities during the applicable fiscal year.
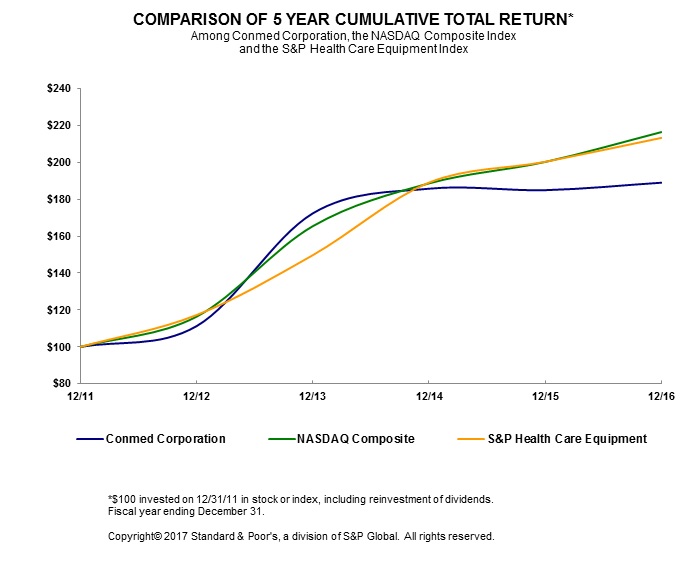
16
Item 6. Selected Financial Data
The following table sets forth selected historical financial data for the years ended December 31, 2016, 2015, 2014, 2013 and 2012. The financial data set forth below should be read in conjunction with the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 7 of this Form 10-K and the Consolidated Financial Statements of the Company and the notes thereto.
FIVE YEAR SUMMARY OF SELECTED FINANCIAL DATA
Years Ended December 31, | |||||||||||||||||||
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
(In thousands, except per share data) | |||||||||||||||||||
Statements of Operations Data (1): | |||||||||||||||||||
Net sales | $ | 763,520 | $ | 719,168 | $ | 740,055 | $ | 762,704 | $ | 767,140 | |||||||||
Cost of sales (2) | 355,190 | 337,466 | 335,998 | 350,287 | 361,297 | ||||||||||||||
Gross profit | 408,330 | 381,702 | 404,057 | 412,417 | 405,843 | ||||||||||||||
Selling and administrative expense (3) | 338,400 | 303,091 | 323,492 | 330,078 | 312,419 | ||||||||||||||
Research and development expense | 32,254 | 27,436 | 27,779 | 25,831 | 28,214 | ||||||||||||||
Income from operations | 37,676 | 51,175 | 52,786 | 56,508 | 65,210 | ||||||||||||||
Other expense (4) | 2,942 | — | — | 263 | — | ||||||||||||||
Interest expense | 15,359 | 6,031 | 6,111 | 5,613 | 5,730 | ||||||||||||||
Income before income taxes | 19,375 | 45,144 | 46,675 | 50,632 | 59,480 | ||||||||||||||
Provision for income taxes | 4,711 | 14,646 | 14,483 | 14,693 | 18,999 | ||||||||||||||
Net income | $ | 14,664 | $ | 30,498 | $ | 32,192 | $ | 35,939 | $ | 40,481 | |||||||||
Per Share Data: | |||||||||||||||||||
Basic earnings per share | $ | 0.53 | $ | 1.10 | $ | 1.17 | $ | 1.30 | $ | 1.43 | |||||||||
Diluted earnings per share | $ | 0.52 | $ | 1.09 | $ | 1.16 | $ | 1.28 | $ | 1.41 | |||||||||
Dividends per share of common stock | $ | 0.80 | $ | 0.80 | $ | 0.80 | $ | 0.65 | $ | 0.60 | |||||||||
Weighted Average Number of Common Shares In Calculating: | |||||||||||||||||||
Basic earnings per share | 27,804 | 27,653 | 27,401 | 27,722 | 28,301 | ||||||||||||||
Diluted earnings per share | 27,964 | 27,858 | 27,769 | 28,114 | 28,653 | ||||||||||||||
Other Financial Data: | |||||||||||||||||||
Depreciation and amortization | $ | 55,309 | $ | 43,879 | $ | 45,734 | $ | 47,867 | $ | 46,616 | |||||||||
Capital expenditures | 14,753 | 15,009 | 15,411 | 18,445 | 21,532 | ||||||||||||||
Balance Sheet Data (at period end): | |||||||||||||||||||
Cash and cash equivalents | $ | 27,428 | $ | 72,504 | $ | 66,332 | $ | 54,443 | $ | 23,720 | |||||||||
Total assets(5) | 1,328,983 | 1,101,700 | 1,086,703 | 1,079,881 | 1,068,620 | ||||||||||||||
Long-term obligations(5) | 634,455 | 396,909 | 389,449 | 362,336 | 336,408 | ||||||||||||||
Total shareholders’ equity | 580,576 | 585,073 | 581,298 | 606,319 | 606,998 | ||||||||||||||
(1) | Results of operations of acquired businesses have been recorded in the financial statements since the date of acquisition. Refer to Note 2 to the consolidated financial statements. |
(2) | In 2016, 2015, 2014, 2013 and 2012, we incurred charges related to the restructuring of certain of our manufacturing operations of $3.1 million, $8.0 million, $5.6 million, $6.5 million and $7.1 million, respectively; in 2016 and 2013 we |
17
incurred charges of $4.5 million and $2.1 million, respectively, related to the termination of a product offering. See additional discussion in Note 12 to the consolidated financial statements.
(3) | Acquisition, restructuring and other expense included in selling and administrative costs are the following: |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
Restructuring costs | $ | 6,873 | $ | 13,655 | $ | 3,354 | $ | 8,750 | $ | 6,497 | |||||||||
Business and asset acquisition costs | 20,599 | 2,543 | 722 | — | 1,898 | ||||||||||||||
Gain on sale of facility | (1,890 | ) | — | — | — | — | |||||||||||||
Management restructuring costs | — | — | 12,546 | — | — | ||||||||||||||
Shareholder activism costs | — | — | 3,966 | — | — | ||||||||||||||
Patent dispute and other matters | — | — | 3,374 | 3,206 | 1,555 | ||||||||||||||
Pension settlement expense | — | — | — | 1,443 | — | ||||||||||||||
Acquisition, restructuring and other expense included in selling and administrative expense | $ | 25,582 | $ | 16,198 | $ | 23,962 | $ | 13,399 | $ | 9,950 | |||||||||
See additional discussion in Notes 2 and 12 to the consolidated financial statements.
(4) | During 2016, we incurred a $2.7 million charge related to commitment fees paid to certain of our lenders, which provided a financing commitment for the SurgiQuest acquisition and recorded a loss on the early extinguishment of debt of $0.3 million in conjunction with the fifth amended and restated senior credit agreement as further described in Note 6 to the consolidated financial statements. In 2013, we recorded a $0.3 million charge related to a loss on the early extinguishment of debt. |
(5) | In November 2015, the FASB issued ASU No. 2015-17 "Income Taxes (ASC 740): Balance Sheet Classification of Deferred Taxes". This ASU requires all deferred income tax assets and liabilities be presented as non-current in classified balance sheets. We adopted this guidance as of January 1, 2016 and applied retrospectively. |
18
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with Selected Financial Data (Item 6), and our Consolidated Financial Statements and related notes contained elsewhere in this report.
Overview of CONMED Corporation
CONMED Corporation (“CONMED”, the “Company”, “we” or “us”) is a medical technology company that provides surgical devices and equipment for minimally invasive procedures. The Company’s products are used by surgeons and physicians in a variety of specialties including orthopedics, general surgery, gynecology, neurosurgery and gastroenterology.
Our product lines consist of orthopedic surgery, general surgery and surgical visualization. Orthopedic surgery consists of sports medicine instrumentation and small bone, large bone and specialty powered surgical instruments and service fees related to the promotion and marketing of sports medicine allograft tissue. General surgery consists of a complete line of endo-mechanical instrumentation for minimally invasive laparoscopic and gastrointestinal procedures, a line of cardiac monitoring products as well as electrosurgical generators and related instruments. Surgical visualization consists of imaging systems for use in minimally invasive orthopedic and general surgery procedures including 2DHD and 3DHD vision technologies. These product lines as a percentage of consolidated net sales are as follows:
2016 | 2015 | 2014 | ||||||
Orthopedic surgery | 48 | % | 54 | % | 54 | % | ||
General surgery | 45 | 38 | 38 | |||||
Surgical visualization | 7 | 8 | 8 | |||||
Consolidated net sales | 100 | % | 100 | % | 100 | % | ||
A significant amount of our products are used in surgical procedures with approximately 79% of our revenues derived from the sale of disposable products. Our capital equipment offerings also facilitate the ongoing sale of related disposable products and accessories, thus providing us with a recurring revenue stream. We manufacture substantially all of our products in facilities located in the United States and Mexico. We market our products both domestically and internationally directly to customers and through distributors. International sales approximated 48%, 50% and 51% in 2016, 2015 and 2014, respectively.
Business Environment
On January 4, 2016, we acquired SurgiQuest, Inc. ("SurgiQuest") for $265 million in cash (on a cash-free, debt-free basis). SurgiQuest develops, manufactures and markets the AirSeal® System, the first integrated access management technology for use in laparoscopic and robotic procedures. This proprietary and differentiated access system is complementary to our current general surgery offering. Refer to Note 2 to the consolidated financial statements for further details on this acquisition.
We plan to continue to restructure both operations and administrative functions as necessary throughout the organization. We have successfully executed our restructuring plans over the past few years, however, we cannot be certain future activities will be completed in the estimated time period or that planned cost savings will be achieved.
Our facilities are subject to periodic inspection by the United States Food and Drug Administration (“FDA”) and foreign regulatory agencies or notified bodies for, among other things, conformance to Quality System Regulation and Current Good Manufacturing Practice (“CGMP”) requirements and foreign or international standards. As discussed in Note 11 to the consolidated financial statements, on August 1, 2016, we were notified by the FDA that our then outstanding warning letter was closed.
Critical Accounting Policies
Preparation of our financial statements requires us to make estimates and assumptions which affect the reported amounts of assets, liabilities, revenues and expenses. Note 1 to the consolidated financial statements describes the significant accounting policies used in preparation of the consolidated financial statements. The most significant areas involving management judgments and estimates are described below and are considered by management to be critical to understanding the financial condition and results of operations of CONMED Corporation.
Revenue Recognition
19
Revenue is recognized when title has been transferred to the customer which is at the time of shipment. The following policies apply to our major categories of revenue transactions:
• | Sales to customers are evidenced by firm purchase orders. Title and the risks and rewards of ownership are transferred to the customer when product is shipped under our stated shipping terms. Payment by the customer is due under fixed payment terms and collectability is reasonably assured. |
• | We place certain of our capital equipment with customers on a loaned basis in return for commitments to purchase related single-use products over time periods generally ranging from one to three years. In these circumstances, no revenue is recognized upon capital equipment shipment as the equipment is loaned and subject to return if certain minimum single-use purchases are not met. Revenue is recognized upon the sale and shipment of the related single-use products. The cost of the equipment is amortized over its estimated useful life. |
• | We recognize revenues related to the promotion and marketing of sports medicine allograft tissue in accordance with the contractual terms of our agreement with Musculoskeletal Transplant Foundation (“MTF”) on a net basis as our role is limited to that of an agent earning a commission or fee. MTF records revenue when the tissue is shipped to the customer. Our services are completed at this time and net revenues for the “Service Fee” for our promotional and marketing efforts are then recognized based on a percentage of the net amounts billed by MTF to its customers. The timing of revenue recognition is determined through review of the net billings made by MTF each month. Our net commission Service Fee is based on the contractual terms of our agreement and is currently 50%. This percentage can vary over the term of the agreement but is contractually determinable. Our Service Fee revenues are recorded net of amortization of the acquired assets, which are being amortized over the expected useful life of 25 years. |
• | Product returns are only accepted at the discretion of the Company and in accordance with our “Returned Goods Policy”. Historically, the level of product returns has not been significant. We accrue for sales returns, rebates and allowances based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions. |
• | Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are provided for capital equipment sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data. |
• | Amounts billed to customers related to shipping and handling have been included in net sales. Shipping and handling costs included in selling and administrative expense were $13.4 million, $12.6 million and $13.6 million for 2016, 2015 and 2014, respectively. |
• | We sell to a diversified base of customers around the world and, therefore, believe there is no material concentration of credit risk. |
• | We assess the risk of loss on accounts receivable and adjust the allowance for doubtful accounts based on this risk assessment. Historically, losses on accounts receivable have not been material. Management believes that the allowance for doubtful accounts of $2.0 million at December 31, 2016 is adequate to provide for probable losses resulting from accounts receivable. |
Inventory Valuation
We write-off excess and obsolete inventory resulting from the inability to sell our products at prices in excess of current carrying costs. The markets in which we operate are highly competitive, with new products and surgical procedures introduced on an on-going basis. Such marketplace changes may result in our products becoming obsolete. We make estimates regarding the future recoverability of the costs of our products and record a provision for excess and obsolete inventories based on historical experience and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write-downs may be required.
Goodwill and Intangible Assets
We have a history of growth through acquisitions. Assets and liabilities of acquired businesses are recorded at their estimated fair values as of the date of acquisition. Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses. Customer and distributor relationships, trademarks, tradenames, developed technology, patents
20
and other intangible assets primarily represent allocations of purchase price to identifiable intangible assets of acquired businesses. Promotional, marketing and distribution rights represent intangible assets created under our Sports Medicine Joint Development and Distribution Agreement (the "JDDA") with Musculoskeletal Transplant Foundation (“MTF”). We have goodwill of $397.7 million and other intangible assets of $419.5 million as of December 31, 2016.
Goodwill and intangible assets deemed to have indefinite lives are not amortized, but are subject to at least annual impairment testing. It is our policy to perform our annual impairment testing in the fourth quarter. The identification and measurement of goodwill impairment involves the estimation of the fair value of our business. Estimates of fair value are based on the best information available as of the date of the assessment. During 2016, we completed our goodwill impairment testing with data as of October 1, 2016. We performed a Step 1 impairment test utilizing the market capitalization approach to determine whether the fair value of a reporting unit is less than its carrying amount. Based upon our assessment, we believe the fair value continues to exceed carrying value.
Intangible assets with a finite life are amortized over the estimated useful life of the asset and are evaluated each reporting period to determine whether events and circumstances warrant a revision to the remaining period of amortization. Intangible assets subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that its carrying amount may not be recoverable. The carrying amount of an intangible asset subject to amortization is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use of the asset. An impairment loss is recognized by reducing the carrying amount of the intangible asset to its current fair value.
Customer relationship assets arose as a result of the 1997 acquisition of Linvatec Corporation. The acquisition date valuation indicated an annual attrition rate of 2.6%. Assuming an exponential attrition pattern, this equated to an average remaining useful life of approximately 38 years for the Linvatec customer relationship assets. During 2016, we acquired SurgiQuest, Inc. and recorded customer and distributor relationships with an average useful life of 22 years. Customer and distributor relationship intangible assets arising as a result of business acquisitions other than Linvatec are being amortized over a weighted average life of 21 years. The weighted average life for customer and distributor relationship assets in aggregate is 29 years.
We evaluate the remaining useful life of our customer and distributor relationship intangible assets each reporting period in order to determine whether events and circumstances warrant a revision to the remaining period of amortization. In order to further evaluate the remaining useful life of our customer and distributor relationship intangible assets, we perform an analysis and assessment of actual customer attrition and activity as events and circumstances warrant.
We test our customer and distributor relationship assets for recoverability whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. Factors specific to our customer and distributor relationship assets which might lead to an impairment charge include a significant increase in the annual customer attrition rate or otherwise significant loss of customers, significant decreases in sales or current-period operating or cash flow losses or a projection or forecast of losses. We do not believe that there have been events or changes in circumstances which would indicate the carrying amount of our customer relationship assets might not be recoverable.
Our developed technology asset arose as a result of the SurgiQuest, Inc. acquisition. This asset is amortized over a weighted average useful life of 17 years. We test for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable.
Trademarks and tradenames intangible assets are not amortized. The Company assesses the impairment of indefinite-lived intangibles annually as of October 1, 2016 and whenever an event or circumstances change that would indicate that the carrying amount may be impaired. We performed a qualitative assessment, and based upon our assessment, we believe the fair value continues to exceed carrying value.
For all other indefinite-lived intangible assets, we perform a qualitative impairment test. Based upon this assessment, we have determined that it is unlikely that our indefinite-lived intangible assets are impaired.
See Note 5 to the consolidated financial statements for further discussion of goodwill and other intangible assets.
Pension Plan
We sponsor a defined benefit pension plan (the “pension plan”) that was frozen in 2009. It covered substantially all our United States based employees at the time it was frozen. Major assumptions used in accounting for the plan include the discount rate, expected return on plan assets, rate of increase in employee compensation levels and expected mortality. Assumptions are determined based on Company data and appropriate market indicators, and are evaluated annually as of the plan’s measurement
21
date. A change in any of these assumptions would have an effect on net periodic pension costs reported in the consolidated financial statements.
The weighted-average discount rate used to measure pension liabilities at December 31, 2016 and the estimated 2017 pension expense is set by reference to the Mercer Above Mean Yield Curve. We changed to this curve at the end of 2015 as we believe it provides a better representation of the yields on bonds if we were to settle the liabilities than the prior use of the Citigroup Pension Liability Index. The 2015 pension expense was set by reference to the Citigroup Pension Liability Index. When setting the discount rate, we consider the individual characteristics of the plan, such as projected cash flow patterns. The effective rates used in determining the December 31, 2016 and 2015 pension liabilities were 4.28% and 4.54%, respectively. Effective rates of 4.54% and 3.81% were used for determining the pension liabilities that are the basis for the 2016 and 2015 pension expense, respectively. As further discussed in Note 10 to the consolidated financial statements, for 2016 we changed the method used to estimate the interest cost component of the pension expense to the spot rate approach resulting in an effective rate of interest equal to 3.77% for 2016. The rate used in determining 2017 estimated pension expense is 4.28% for the benefit obligation and 3.49% for the effective interest rate on the benefit obligation.
We have used an expected rate of return on pension plan assets of 8.0% for purposes of determining the net periodic pension benefit cost. In determining the expected return on pension plan assets, we consider the relative weighting of plan assets, the historical performance of total plan assets and individual asset classes and economic and other indicators of future performance. In addition, we consult with financial and investment management professionals in developing appropriate targeted rates of return.
Pension expense in 2017 is expected to be $1.2 million. Pension expense was $0.9 million in 2016. In addition, we do not expect to make any contributions to the pension plan for the 2017 plan year.
In performing a sensitivity analysis on our pension plan expense, we do not believe a 0.25% increase or decrease in discount rate or investment return would have a material impact on our pension expense.
See Note 10 to the consolidated financial statements for further discussion.
Stock-based Compensation
All share-based payments to employees, including stock options, grants of restricted stock units, performance share units and stock appreciation rights are recognized in the financial statements based at their fair values. Compensation expense is generally recognized using a straight-line method over the vesting period. Compensation expense for performance share units is recognized using the graded vesting method.
Income Taxes
The recorded future tax benefit arising from deductible temporary differences and tax carryforwards is approximately $72.4 million at December 31, 2016. Management believes that earnings during the periods when the temporary differences become deductible will be sufficient to realize the related future income tax benefits.
The Company is subject to taxation in the United States and various states and foreign jurisdictions. Taxing authority examinations can involve complex issues and may require an extended period of time to resolve. Our Federal income tax returns have been examined by the Internal Revenue Service (“IRS”) for calendar years ending through 2013. Tax years subsequent to 2013 are subject to future examination.
Consolidated Results of Operations
The following table presents, as a percentage of net sales, certain categories included in our consolidated statements of comprehensive income for the periods indicated:
22
Year Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Net sales | 100.0 | % | 100.0 | % | 100.0 | % | ||
Cost of sales | 46.5 | 46.9 | 45.4 | |||||
Gross profit | 53.5 | 53.1 | 54.6 | |||||
Selling and administrative expense | 44.3 | 42.1 | 43.7 | |||||
Research and development expense | 4.2 | 3.8 | 3.8 | |||||
Income from operations | 5.0 | 7.1 | 7.1 | |||||
Other expense | 0.4 | — | — | |||||
Interest expense | 2.0 | 0.8 | 0.8 | |||||
Income before income taxes | 2.6 | 6.3 | 6.3 | |||||
Provision for income taxes | 0.6 | 2.0 | 2.0 | |||||
Net income | 2.0 | % | 4.3 | % | 4.3 | % | ||
Net Sales
Net sales increased 6.2% to $763.5 million in 2016 after a decrease in sales of 2.8% in 2015 to $719.2 million from $740.1 million in 2014. The increase in 2016 is mainly due to the SurgiQuest acquisition. Excluding SurgiQuest, sales decreased 3.3% in 2016. In local currency, excluding the effects of the hedging program, sales increased 8.6% in 2016. Sales of capital equipment increased 3.8% to $157.7 million in 2016, while sales of single-use products increased 6.8% to $605.8 million in 2016. In local currency, excluding the effects of the hedging program, sales of capital equipment increased 6.1% in 2016 and single-use products increased 9.3% in 2016. The decrease in 2015 sales compared to the same period a year ago occurred across all product lines. In local currency, excluding the effects of the hedging program, sales increased 0.3% in 2015. Sales of capital equipment increased 3.8% to $151.9 million in 2015, while sales of single-use products decreased 4.5% to $567.3 million in 2015. In local currency, excluding the effects of the hedging program, sales of capital equipment increased 7.1% in 2015 while single-use products decreased 1.3% in 2015.
• | Orthopedic surgery sales decreased 4.7% in 2016 to $370.5 million after a decrease of 3.4% in 2015 to $389.0 million from $402.8 million in 2014. In 2016, the decrease was mainly due to the unfavorable impact of foreign exchange, lower sales in our capital products and resection product offering offset by increases in our procedure specific product offering. In 2015, the decrease was mainly due to lower sales in our procedure specific and resection product offerings as well as our powered instrument burs and blades offset by increases in our powered instrument handpieces. In local currency, excluding the effects of the hedging program, sales decreased 1.7% in 2016 after an increase of 0.7% in 2015. |
• | General surgery sales increased 24.5% in 2016 to $341.4 million after a decrease of 1.8% in 2015 to $274.2 million from $279.4 million in 2014. The increase in 2016 is mainly due to the SurgiQuest acquisition. Excluding SurgiQuest, general surgery sales decreased 0.4% due primarily to lower sales in our advanced surgical capital equipment products offset by higher sales in our endoscopic technologies product offering. In local currency, excluding the effects of the hedging program, sales increased 26.0% in 2016 after a 0.1% decrease in 2015. |
• | Surgical visualization sales decreased 7.8% in 2016 to $51.6 million after a decrease of 3.3% to $56.0 million in 2015 from $57.9 million in 2014. In 2016, the decrease is due to lower video system sales. The decrease in 2015 resulted from the discontinuation of an OEM video product line during 2015 offset by the increase in video system sales of our new IM8000 2DHD camera system. In local currency, excluding the effects of the hedging program, sales decreased 5.9% in 2016 and 0.1% in 2015. |
Cost of Sales
Cost of sales was $355.2 million in 2016, $337.5 million in 2015 and $336.0 million in 2014. Gross profit margins were 53.5% in 2016, 53.1% in 2015 and 54.6% in 2014. The increase in gross profit margins of 0.4 percentage points in 2016 was mainly a result of the impact of favorable production variances (1.2 percentage points) and product mix (0.3 percentage points), offset by unfavorable foreign currency exchange rates on sales (1.1 percentage points). The decrease of 1.5 percentage points in 2015 is a result of the impact of unfavorable foreign currency exchange rates on sales (1.5 percentage points) and higher costs associated with the operational restructuring (0.4 percentage points) offset by favorable production variances (0.3 percentage points) and product mix (0.1 percentage points).
23
Selling and Administrative Expense
Selling and administrative expense was $338.4 million in 2016, $303.1 million in 2015 and $323.5 million in 2014. Selling and administrative expense as a percentage of net sales were 44.3% in 2016, 42.1% in 2015 and 43.7% in 2014. The factors affecting the $35.3 million increase in 2016 compared to 2015 included $20.6 million in 2016 in investment banking fees, consulting fees and legal fees associated with the acquisition as well as the Lexion case as further described in Note 11 to the consolidated financial statements, costs associated with expensing of unvested options acquired and integration related costs associated with the acquisition of SurgiQuest as further described in Notes 2 and 12 to the consolidated financial statements and incremental on-going sales and marketing expenses primarily to support the AirSeal® products. These increases were offset by a $6.8 million decrease in severance and other related costs in 2016 from the restructuring of certain of our sales, marketing and administrative functions and a $1.9 million gain on the sale of our Centennial, Colorado facility.
The factors affecting the $20.4 million decrease in 2015 compared to 2014 included (1) $12.5 million in executive management restructuring costs in 2014 (2) $4.0 million in shareholder activism related charges in 2014 and (3) $3.4 million in legal fees associated with a patent infringement claim that we settled in the first quarter of 2014 as well as costs associated with a legal matter in which we prevailed at trial in the second quarter of 2014 as further described in Note 12 to the consolidated financial statements (4) lower medical device tax and (5) lower benefit costs offset by higher restructuring costs as also further described in Note 12 to the consolidated financial statements.
Research and Development Expense
Research and development expense was $32.3 million, $27.4 million and $27.8 million in 2016, 2015 and 2014, respectively. As a percentage of net sales, research and development expense increased to 4.2% in 2016, compared to 3.8% in 2015 and 2014. The increase of 0.4 percentage points in 2016 is due to higher project and registration related costs as the Company increased its efforts on new product development and innovation.
Other Expense
Other expense in 2016 related to costs associated with our fifth amended and restated senior credit agreement entered into on January 4, 2016 as further described in Note 6 to the consolidated financial statements. These costs include a $2.7 million charge related to commitment fees paid to certain of our lenders, which provided a financing commitment for the SurgiQuest acquisition and a loss on the early extinguishment of debt of $0.3 million.
Interest Expense
Interest expense was $15.4 million in 2016 compared to $6.0 million in 2015 and $6.1 million in 2014. Interest expense increased in 2016 compared to 2015 due to the additional borrowings and higher interest rates under the fifth amended and restated senior credit agreement as further described in Note 6 to the consolidated financial statements. Interest expense remained flat in 2015 compared to 2014 as higher weighted average borrowings were offset by lower interest rates. The weighted average interest rates on our borrowings were 2.93% in 2016 increasing from 2.23% in 2015 and 2.40% in 2014.
Provision for Income Taxes
A provision for income taxes was recorded at an effective rate of 24.3%, 32.4% and 31.0% in 2016, 2015 and 2014, respectively, as compared to the Federal statutory rate of 35.0%. The effective tax rate in 2016 is lower than that recorded in 2015 due to a higher proportion of earnings in foreign jurisdictions where the tax rates are lower than the statutory federal rate and benefits recorded in 2016 in connection with the prior year tax return finalization process. These benefits were offset by tax expense related to nondeductible SurgiQuest acquisition costs recorded in 2016. The effective tax rate in 2015 is higher than that recorded in 2014 due to the domestic impact, net of foreign tax credits, associated with the repatriation of foreign earnings to the United States, which increased tax expense by $1.1 million in the fourth quarter of 2015. Additionally, the 2015 rate increased compared to 2014 as a result of lower foreign tax benefits resulting from the change in the governmental rate upon which European permanent deductions are calculated and due to benefits recorded in 2014 related to settlements with taxing authorities. These items are offset by decreases resulting from domestic manufacturing benefits and lower state tax expense as a result of a New York State legislative change recorded in 2014. A reconciliation of the United States statutory income tax rate to our effective tax rate is included in Note 7 to the consolidated financial statements.
Non-GAAP Financial Measures
24
Net sales “on a constant currency basis” is a non-GAAP measure. The company analyzes net sales on a constant currency basis to better measure the comparability of results between periods. To measure percentage sales growth in constant currency, the Company removes the impact of changes in foreign currency exchange rates that affect the comparability and trend of net sales.
Because non-GAAP financial measures are not standardized, it may not be possible to compare this financial measure with other companies' non-GAAP financial measures having the same or similar names. This adjusted financial measure should not be considered in isolation or as a substitute for reported net sales growth, the most directly comparable GAAP financial measure. This non-GAAP financial measure is an additional way of viewing net sales that, when viewed with our GAAP results, provides a more complete understanding of our business. The Company strongly encourages investors and shareholders to review our financial statements and publicly-filed reports in their entirety and not to rely on any single financial measure.
Liquidity and Capital Resources
Our liquidity needs arise primarily from capital investments, working capital requirements and payments on indebtedness under the fifth amended and restated senior credit agreement, described below. We have historically met these liquidity requirements with funds generated from operations and borrowings under our revolving credit facility. In addition, we have historically used term borrowings, including borrowings under the fifth amended and restated senior credit agreement and borrowings under separate loan facilities, in the case of real property purchases, to finance our acquisitions. We also have the ability to raise funds through the sale of stock or we may issue debt through a private placement or public offering. Management believes that cash flow from operations, including cash and cash equivalents on hand and available borrowing capacity under our fifth amended and restated senior credit agreement, will be adequate to meet our anticipated operating working capital requirements, debt service, funding of capital expenditures and common stock repurchases in the foreseeable future.
We had total cash on hand at December 31, 2016 of $27.4 million, of which approximately $25.7 million was held by our foreign subsidiaries outside the United States with unremitted earnings. We have not repatriated, nor do we anticipate the need to repatriate, permanently reinvested earnings to the U.S. to satisfy domestic liquidity needs arising in the ordinary course of business or associated with our domestic debt service requirements. During the fourth quarter of 2015, we redeployed cash from certain non-U.S. subsidiaries for U.S. debt reduction of $33.0 million which included $9.3 million of 2015 foreign earnings not previously permanently reinvested and a $23.7 million return of accumulated foreign basis. We recorded a tax charge of $1.1 million and increased foreign borrowings under our revolving credit facility by $33.0 million related to this cash redeployment. It is our intention to permanently reinvest the remaining amount of unremitted earnings. If we were to repatriate these funds, we would be required to accrue and pay taxes on such amounts.
Operating Cash Flows
Our net working capital position was $216.6 million at December 31, 2016. Net cash provided by operating activities was $38.2 million in 2016, $48.1 million in 2015 and $65.2 million in 2014 generated on net income of $14.7 million in 2016, $30.5 million in 2015 and $32.2 million in 2014.
The decrease in cash provided by operating activities from 2016 to 2015 is mainly related to lower net income due to costs associated with the SurgiQuest acquisition and related financing costs, as discussed above. In addition, other significant changes in working capital, principally related to the SurgiQuest acquisition, which impacted cash flow in 2016 included the following:
• | A decrease in cash flows from accounts receivable reflects a $13 million increase in sales in the fourth quarter of 2016 compared to the same period a year ago offset by collections on accounts receivable acquired as a result of the SurgiQuest acquisition; |
• | A decrease in cash flows from other assets caused primarily by an increase in field inventories to support the SurgiQuest acquisition integration and anticipated sales growth; and |
• | A decrease in cash flows from other liabilities as we had a higher level of accrued expenses at the beginning of the year due to the SurgiQuest acquisition that was later paid during 2016 as well as payments related to our restructuring. |
The decrease in cash provided by operating activities in 2015 compared to 2014 is mainly related to inventory being higher as of December 31, 2015 compared to the year ended December 31, 2014 as we built additional inventory due to consolidating the Centennial, Colorado manufacturing facility into other manufacturing facilities, lower than anticipated video sales in the fourth
25
quarter and higher levels of field inventory (included in other assets) to support our salesforce. In addition, we had higher payments related to our operational and administrative restructuring during 2015 compared to 2014.
Investing Cash Flows
Net cash used in investing activities increased to $266.0 million in 2016 compared to $24.4 million in 2015 primarily due to the $256.5 million payment for the SurgiQuest acquisition compared to $9.4 million in payments related to acquiring businesses, assets and a distributor in 2015. The increase was offset by $5.2 million in proceeds from the sale of our Centennial, Colorado facility during 2016.
Net cash used in investing activities increased to $24.4 million in 2015 compared to $20.7 million in 2014 primarily due to payments related to acquiring businesses, assets and a distributor of $9.4 million in 2015 compared to payments of $5.0 million for the purchase of a business in 2014.
Capital expenditures were $14.8 million, $15.0 million and $15.4 million in 2016, 2015 and 2014, respectively. Capital expenditures are expected to be in the $15.0 million to $20.0 million range for 2017.
Financing Cash Flows
Financing activities in 2016 provided cash of $184.2 million compared to a use of cash of $9.8 million in 2015 and $26.4 million in 2014. Below is a summary of the significant financing activities:
• | During 2016, we had borrowings of $175.0 million on our term loan of which $8.8 million was repaid in accordance with the agreement, as further described below. During 2016, we had net borrowings on our revolving line of credit of $62.7 million compared to $30.7 million in 2015 and $27.0 million in 2014. |
• | Dividend payments remained consistent at $22.2 million, $22.1 million and $22.0 million in 2016, 2015 and 2014, respectively. |
• | In 2016, 2015 and 2014, we paid $16.7 million associated with the distribution and development agreement with Musculoskeletal Transplant Foundation. These payments are now complete. |
• | Debt issuance costs were $5.6 million and $1.5 million in 2016 and 2015, respectively, in conjunction with our fifth and fourth amended and restated credit agreements, respectively. |
• | Finally, during 2014, we repurchased common stock totaling $16.9 million. |
On January 4, 2016, we entered into a fifth amended and restated senior credit agreement consisting of: (a) a $175.0 million term loan facility and (b) a $525.0 million revolving credit facility both expiring on January 4, 2021. The term loan is payable in quarterly installments increasing over the term of the facility. Proceeds from the term loan facility and borrowings under the revolving credit facility were used to repay the then existing senior credit agreement and to finance the acquisition of SurgiQuest. Initial interest rates are at LIBOR plus a base rate or a Eurocurrency rate plus an applicable margin (2.77% at December 31, 2016). The applicable margin for base rate loans is 1.00% and for Eurocurrency rate loans is 2.00%.
There were $166.3 million in borrowings outstanding on the term loan as of December 31, 2016. There were $329.0 million in borrowings outstanding under the revolving credit facility as of December 31, 2016. Our available borrowings on the revolving credit facility at December 31, 2016 were $191.2 million with approximately $4.8 million of the facility set aside for outstanding letters of credit.
The fifth amended and restated senior credit agreement is collateralized by substantially all of our personal property and assets. The fifth amended and restated senior credit agreement contains covenants and restrictions which, among other things, require the maintenance of certain financial ratios and restrict dividend payments and the incurrence of certain indebtedness and other activities, including acquisitions and dispositions. We were in full compliance with these covenants and restrictions as of December 31, 2016. We are also required, under certain circumstances, to make mandatory prepayments from net cash proceeds from any issuance of equity and asset sales.
We have a mortgage note outstanding in connection with the Largo, Florida property and facilities bearing interest at 8.25% per annum with semiannual payments of principal and interest through June 2019. The principal balance outstanding on the mortgage note aggregated $3.9 million at December 31, 2016. The mortgage note is collateralized by the Largo, Florida
26
property and facilities.
Our Board of Directors has authorized a $200.0 million share repurchase program. Through December 31, 2016, we have repurchased a total of 6.1 million shares of common stock aggregating $162.6 million under this authorization and have $37.4 million remaining available for share repurchases. The repurchase program calls for shares to be purchased in the open market or in private transactions from time to time. We may suspend or discontinue the share repurchase program at any time. We did not purchase any shares of common stock under the share repurchase program during 2016. We have financed the repurchases and may finance additional repurchases through operating cash flow and from available borrowings under our revolving credit facility.
Management believes that cash flow from operations, including cash and cash equivalents on hand and available borrowing capacity under our amended and restated senior credit agreement, will be adequate to meet our anticipated operating working capital requirements, debt service, funding of capital expenditures and common stock repurchases in the foreseeable future. See “Item 1. Business – Forward Looking Statements.”
Restructuring
During 2016, 2015 and 2014, we continued our operational restructuring plan. The consolidation of our Centennial, Colorado manufacturing operations into other existing CONMED manufacturing facilities is complete. During 2014, we completed the consolidation of our Finland operations into our Largo, Florida and Utica, New York manufacturing facilities and the consolidation of our Westborough, Massachusetts manufacturing operations into our Largo, Florida and Chihuahua, Mexico facilities. We incurred $3.1 million, $8.0 million, and $5.6 million in costs associated with operational restructuring during the years ending December 31, 2016, 2015 and 2014, respectively. These costs were charged to cost of goods sold and include severance and other charges associated with the consolidation of our Finland, Westborough, Massachusetts and Centennial, Colorado operations.
During 2016, the Company discontinued our Altrus product offering as part of our ongoing restructuring and incurred $4.5 million in non-cash charges primarily related to inventory and fixed assets which were included in cost of sales.
During 2016, 2015 and 2014, we restructured certain sales, marketing and administrative functions and incurred severance and other related costs in the amount of $6.9 million, $13.7 million, and $3.4 million. These costs were charged to selling and administrative expense.
During 2016, we sold our Centennial, Colorado facility. We received net cash proceeds of $5.2 million and recorded a gain of $1.9 million in selling and administrative expense.
During 2014, we incurred $12.5 million in costs associated with restructuring of executive management. These costs include severance payments, accelerated vesting of stock-based compensation awards, accrual of the present value of deferred compensation and other benefits to our then Chief Executive Officer as defined in his termination agreement; accelerated vesting of stock-based compensation awards to certain members of executive management, consulting fees and other benefits earned as further described in our Form 8-K filing on July 23, 2014.
We have recorded an accrual in current and other long-term liabilities of $2.6 million at December 31, 2016 mainly related to severance associated with these restructurings.
During recent years we had a number of initiatives to consolidate manufacturing facilities and restructure our sales and administrative functions. Although much of this is complete, we will continue to review our operations and sales and administrative functions to reduce costs and headcount, as necessary. Such cost reductions will result in additional charges, including employee termination costs and other exit costs that will be charged to cost of sales and selling and administrative expense, as applicable.
During the year ended December 31, 2016, we had approximately $4.0 million in net savings in cost of sales from the Centennial consolidation principally as a result of lower employee costs.
Refer to Note 12 to the consolidated financial statements for further discussions regarding restructuring.
Contractual Obligations
27
The following table summarizes our contractual obligations for the next five years and thereafter (amounts in thousands) as of December 31, 2016. Purchase obligations represent purchase orders for goods and services placed in the ordinary course of business. There were no capital lease obligations as of December 31, 2016.
Payments Due by Period | |||||||||||||||||||
Total | Less than 1 Year | 1-3 Years | 3-5 Years | More than 5 Years | |||||||||||||||
Long-term debt | $ | 499,112 | $ | 10,202 | $ | 33,035 | $ | 455,875 | $ | — | |||||||||
Purchase obligations | 40,357 | 39,536 | 821 | — | — | ||||||||||||||
Operating lease obligations | 23,784 | 6,170 | 11,579 | 3,530 | 2,505 | ||||||||||||||
Total contractual obligations | $ | 563,253 | $ | 55,908 | $ | 45,435 | $ | 459,405 | $ | 2,505 | |||||||||
In addition to the above contractual obligations, we are required to make periodic interest payments on our long-term debt obligations (see additional discussion under Item 7A. “Quantitative and Qualitative Disclosures About Market Risk—Interest Rate Risk” and Note 6 to the consolidated financial statements). The above table also does not include unrecognized tax benefits of approximately $0.6 million, the timing and certainty of recognition for which is not known (See Note 7 to the consolidated financial statements).
Stock-based Compensation
We have reserved shares of common stock for issuance to employees and directors under three shareholder-approved share-based compensation plans (the "Plans"). The Plans provide for grants of stock options, stock appreciation rights (“SARs”), dividend equivalent rights, restricted stock, restricted stock units (“RSUs”), performance share units (“PSUs”) and other equity-based and equity-related awards. The exercise price on all outstanding stock options and SARs is equal to the quoted fair market value of the stock at the date of grant. RSUs and PSUs are valued at the market value of the underlying stock on the date of grant. Stock options, SARs, RSUs and PSUs are non-transferable other than on death and generally become exercisable over a five year period from date of grant. Stock options and SARs expire ten years from date of grant. SARs are only settled in shares of the Company’s stock (See Note 8 to the consolidated financial statements). Total pre-tax stock-based compensation expense recognized in the consolidated statements of comprehensive income was $8.4 million, $7.5 million and $9.3 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Other Matters
As of September 8, 2016, our credit facility was amended to allow us to seek to sell products to certain customers in Iran in compliance with applicable laws and regulations and subject to certain terms and conditions, including pre-approval by us and our lenders of the identity of any distributor and prior review of each of the end-customers. On September 13, 2016, we entered into a distribution agreement with a third-party distributor in Iran. We sold to this customer during 2016 and expect and intend that there will be sales prospectively. We intend to limit sales into Iran to products that qualify as “medical supplies” within the meaning of the general license provided by the Iranian Transactions and Sanctions Regulations set forth in the regulations promulgated by the Office of Foreign Assets Control (“OFAC”) of the United States Department of the Treasury set forth at 31 C.F.R. § 560.530. We have implemented certain controls and processes designed to ensure that the ultimate end-users for the products are those permitted under the OFAC general license, and that the sales and transactions with the Iranian distributor otherwise comply with the requirements of the OFAC regulations. The expected revenues and net profits associated with sales to the Iranian distributor are not expected to be material to our results of operations.
We do not believe that our activities to date, and do not expect that our activities in the future, will be subject to required disclosure under Section 13(r) of the Securities Exchange Act of 1934 (the “Exchange Act”), which, among other things, requires disclosure of transactions and activities knowingly entered into with the Government of Iran that do not benefit from an OFAC license and with certain designated parties. If, however, any activities in future periods are within the scope of the transactions and activities captured by Section 13(r) of the Exchange Act, we will make the required disclosures and notices.
New Accounting Pronouncements
See Note 15 to the consolidated financial statements for a discussion of new accounting pronouncements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
28
Market risk is the potential loss arising from adverse changes in market rates and prices such as commodity prices, foreign currency exchange rates and interest rates. In the normal course of business, we are exposed to various market risks, including changes in foreign currency exchange rates and interest rates. We manage our exposure to these and other market risks through regular operating and financing activities and as necessary through the use of derivative financial instruments.
Foreign currency risk
Approximately 48% of our total 2016 consolidated net sales were to customers outside the United States. We have sales subsidiaries in a significant number of countries in Europe as well as Australia, Canada, China and Korea. In those countries in which we have a direct presence, our sales are denominated in the local currency amounting to approximately 30% of our total net sales in 2016. The remaining 18% of sales to customers outside the United States was on an export basis and transacted in United States dollars.
Because a significant portion of our operations consist of sales activities in foreign jurisdictions, our financial results may be affected by factors such as changes in foreign currency exchange rates or weak economic conditions in the markets in which we distribute products. During 2016, foreign currency exchange rates, including the effects of the hedging program, caused sales to decrease by approximately $17 million and income before income taxes to decrease by approximately $12 million, compared to sales and income before income taxes in 2015.
We hedge forecasted intercompany sales denominated in foreign currencies through the use of forward contracts. We account for these forward contracts as cash flow hedges. To the extent these forward contracts meet hedge accounting criteria, changes in their fair value are not included in current earnings but are included in accumulated other comprehensive loss. These changes in fair value will be recognized into earnings as a component of sales or cost of sales when the forecasted transaction occurs. The notional contract amounts for forward contracts outstanding at December 31, 2016 which have been accounted for as cash flow hedges totaled $108.1 million. Net realized gains recognized for forward contracts accounted for as cash flow hedges approximated $1.2 million, $10.4 million and $0.6 million for the years ended December 31, 2016, 2015 and 2014, respectively. Net unrealized gains on forward contracts outstanding which have been accounted for as cash flow hedges and which have been included in other comprehensive income totaled $1.5 million at December 31, 2016. It is expected these unrealized gains will be recognized in the consolidated statement of comprehensive income in 2017 and 2018.
We also enter into forward contracts to exchange foreign currencies for United States dollars in order to hedge our currency transaction exposures on intercompany receivables denominated in foreign currencies. These forward contracts settle each month at month-end, at which time we enter into new forward contracts. We have not designated these forward contracts as hedges and have not applied hedge accounting to them. The notional contract amounts for forward contracts outstanding at December 31, 2016 which have not been designated as hedges totaled $18.4 million. Net realized gains (losses) recognized in connection with those forward contracts not accounted for as hedges approximated $0.0 million, $0.4 million and -$0.2 million for the years ended December 31, 2016, 2015 and 2014, respectively, offsetting losses on our intercompany receivables of -$0.1 million, -$0.8 million and -$0.5 million for the years ended December 31, 2016, 2015 and 2014, respectively. These gains and losses have been recorded in selling and administrative expense in the consolidated statements of comprehensive income.
We record these forward foreign exchange contracts at fair value; the net fair value for forward foreign exchange contracts outstanding at December 31, 2016 was $2.4 million and is included in prepaids and other current assets in the consolidated balance sheet.
Refer to Note 14 in the consolidated financial statements for further discussion.
Interest rate risk
At December 31, 2016, we had approximately $495.3 million of variable rate long-term debt outstanding under our senior credit agreement. Assuming no repayments, if market interest rates for similar borrowings averaged 1.0% more in 2017 than they did in 2016, interest expense would increase, and income before income taxes would decrease by $5.0 million. Comparatively, if market interest rates for similar borrowings average 1.0% less in 2017 than they did in 2016, our interest expense would decrease, and income before income taxes would increase by $5.0 million.
Item 8. Financial Statements and Supplementary Data
Our 2016 Financial Statements are included in this Form 10-K beginning on page 39 and incorporated by reference herein.
29
Item 9. Changes In and Disagreements with Accountants on Accounting and Financial Disclosures
There were no changes in or disagreement with accountants on accounting and financial disclosure.
Item 9A. Controls and Procedures
As of the end of the period covered by this report, an evaluation was carried out by CONMED Corporation’s management, with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that these disclosure controls and procedures were effective as of the end of the period covered by this report. In addition, no change in our internal control over financial reporting (as defined in Rule 13a-15 under the Securities Exchange Act of 1934) occurred during the fourth quarter of the year ended December 31, 2016 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting and the Report of Independent Registered Public Accounting Firm thereon are set forth in Part IV, Item 15 of the Annual Report on Form 10-K.
Item 9B. Other Information
Not applicable.
30
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item is incorporated herein by reference to the sections captioned “Proposal One: Election of Directors”, “Directors, Executive Officers, Other Company Officers and Nominees for the Board of Directors”, “Section 16(a) Beneficial Ownership Reporting Compliance”, “Ethics Disclosure” and "Meetings of Board of Directors and Committees, Leadership Structure and Risk Oversight” in CONMED Corporation’s definitive Proxy Statement or other informational filing to be filed with the Securities and Exchange Commission on or about April 13, 2017.
Item 11. Executive Compensation
The information required by this item is incorporated herein by reference to the sections captioned “Compensation Discussion and Analysis”, “Compensation Committee Report on Executive Compensation”, “Summary Compensation Table”, “Grants of Plan-Based Awards”, “Outstanding Equity Awards at Fiscal Year-End”, “Option Exercises and Stock Vested”, “Pension Benefits”, “Non-Qualified Deferred Compensation”, “Potential Payments on Termination or Change-in-Control”, “Director Compensation” and “Board of Directors Interlocks and Insider Participation; Certain Relationships and Related Transactions” in CONMED Corporation’s definitive Proxy Statement or other informational filing to be filed with the Securities and Exchange Commission on or about April 13, 2017.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is incorporated herein by reference to the section captioned “Security Ownership of Certain Beneficial Owners and Management” in CONMED Corporation’s definitive Proxy Statement or other informational filing to be filed with the Securities and Exchange Commission on or about April 13, 2017.
Information relating to compensation plans under which equity securities of CONMED Corporation are authorized for issuance is set forth below:
Equity Compensation Plan Information | ||||||||||
Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||
Equity compensation plans approved by security holders | 1,516,376 | $ | 42.16 | 1,514,719 | ||||||
Equity compensation plans not approved by security holders | — | — | — | |||||||
Total | 1,516,376 | $ | 42.16 | 1,514,719 | ||||||
The number of securities included in column (a) above consists of outstanding stock options, share appreciation rights (“SARs”) and performance share units, however the weighted-average exercise price in column (b) is for stock options and SARs only.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated herein by reference to the section captioned “Directors, Executive Officers and Nominees for the Board of Directors” and “Board of Directors Interlocks and Insider Participation; Certain Relationships and Related Transactions” in CONMED Corporation’s definitive Proxy Statement or other informational filing to be filed with the Securities and Exchange Commission on or about April 13, 2017.
31
Item 14. Principal Accounting Fees and Services
The information required by this item is incorporated herein by reference to the section captioned “Principal Accounting Fees and Services” in CONMED Corporation’s definitive Proxy Statement or other informational filing to be filed with the Securities and Exchange Commission on or about April 13, 2017.
32
PART IV
Item 15. Exhibits, Financial Statement Schedules
Index to Financial Statements | ||
(a)(1) | List of Financial Statements | Page in Form 10-K |
Management’s Report on Internal Control Over Financial Reporting | 39 | |
Report of Independent Registered Public Accounting Firm | 40 | |
Consolidated Balance Sheets at December 31, 2016 and 2015 | 41 | |
Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2016, 2015 and 2014 | 42 | |
Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2016, 2015 and 2014 | 43 | |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2016, 2015 and 2014 | 45 | |
Notes to Consolidated Financial Statements | 47 | |
(2) | List of Financial Statement Schedules | |
Valuation and Qualifying Accounts (Schedule II) | 75 | |
All other schedules have been omitted because they are not applicable, or the required information is shown in the financial statements or notes thereto. | ||
(3) | List of Exhibits | |
The exhibits listed on the accompanying Exhibit Index on page 36 below are filed as part of this Form 10-K. | ||
33
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on the date indicated below.
CONMED CORPORATION |
By: /s/ Curt R. Hartman |
Curt R. Hartman |
(President and Chief |
Executive Officer) |
Date: |
February 27, 2017 |
34
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ MARK E. TRYNISKI | Chairman of the Board | |||
Mark E. Tryniski | of Directors | February 27, 2017 | ||
/s/ CURT R. HARTMAN | President, Chief Executive | |||
Curt R. Hartman | Officer and Director | February 27, 2017 | ||
/s/ LUKE A. POMILIO | Executive Vice President-Finance | |||
Luke A. Pomilio | and Chief Financial Officer (Principal Financial Officer) | February 27, 2017 | ||
/s/ TERENCE M. BERGE | Vice President- | |||
Terence M. Berge | Corporate Controller | February 27, 2017 | ||
/s/ DAVID BRONSON | ||||
David Bronson | Director | February 27, 2017 | ||
/s/ BRIAN CONCANNON | ||||
Brian Concannon | Director | February 27, 2017 | ||
/s/ CHARLES M. FARKAS | ||||
Charles M. Farkas | Director | February 27, 2017 | ||
/s/ MARTHA GOLDBERG ARONSON | ||||
Martha Goldberg Aronson | Director | February 27, 2017 | ||
/s/ JO ANN GOLDEN | ||||
Jo Ann Golden | Director | February 27, 2017 | ||
/s/ DIRK M. KUYPER | ||||
Dirk M. Kuyper | Director | February 27, 2017 | ||
/s/ JEROME J. LANDE | ||||
Jerome J. Lande | Director | February 27, 2017 | ||
/s/ JOHN WORKMAN | ||||
John Workman | Director | February 27, 2017 | ||
35
Exhibit Index
Exhibit No. | Description | |
3.1 | - | Amended and Restated By-Laws, as adopted by the Board of Directors on April 29, 2011 (Incorporated by reference to the Company’s Current Report on Form 10-Q filed with the Securities and Exchange Commission on May 2, 2011). |
3.2 | - | 1999 Amendment to Certificate of Incorporation and Restated Certificate of Incorporation of CONMED Corporation (Incorporated by reference to Exhibit 3.2 of the Company’s Annual Report on Form 10-K for the year ended December 31, 1999). |
4.1 | - | See Exhibit 3.1. |
4.2 | - | See Exhibit 3.2. |
4.3 | - | Guarantee and Collateral Agreement, dated August 28, 2002, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank (Incorporated by reference to Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2002). |
4.4 | - | First Amendment to Guarantee and Collateral Agreement, dated June 30, 2003, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003). |
4.5 | - | Second Amendment to Guarantee and Collateral Agreement, dated April 13, 2006, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 19, 2006). |
4.6 | - | Third Amendment to Guarantee and Collateral Agreement, dated as of January 17, 2013, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank (Incorporated by reference to Exhibit 4.6 of the Company's Annual Report on Form 10-K for the year ended December 31, 2012). |
4.7 | - | Fourth Amendment to Guarantee and Collateral Agreement, dated as of January 4, 2016, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank (Incorporated by reference to Exhibit 10.2 of the Company's Current Report on Form 8-K filed with the Securities and Exchange Commission on January 4, 2016). |
10.1+ | - | Employment Agreement between the Company and Curt R. Hartman, dated November 9, 2014 (Incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2014). |
10.2+ | - | Amended and Restated Employment Agreement, dated October 30, 2009, by and between CONMED Corporation and Joseph J. Corasanti, Esq. (Incorporated by reference to the Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2009). |
10.3 | - | Amended and Restated 1999 Long Term Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on November 3, 2009). |
36
10.4 | - | 2002 Employee Stock Purchase Plan (Incorporated by reference to the Company’s Definitive Proxy Statement for the 2002 Annual Meeting filed with the Securities and Exchange Commission on April 17, 2002). |
10.5 | - | Amendment to CONMED Corporation 2002 Employee Stock Purchase Plan (Incorporated by reference to Exhibit 10.11 of the Company’s Annual Report on Form 10-K for the year ended December 31, 2005). |
10.6 | - | 2006 Stock Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on August 8, 2006). |
10.7 | - | Amended and Restated 2007 Non-Employee Director Equity Compensation Plan of CONMED Corporation (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on August 3, 2010). |
10.8 | - | Amended and Restated Long Term Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on July 27, 2012). |
10.9 | - | Amended and Restated 2015 Long-Term Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company's Registration Statement on Form S-8 on October 23, 2015). |
10.10 | - | Amended and Restated 2016 Non-Employee Director Equity Compensation Plan (Incorporated by reference to Exhibit 4.3 of the Company's Registration Statement on Form S-8 on October 28, 2016). |
10.11 | - | Fifth Amended and Restated Credit Agreement, dated January 4, 2016, among CONMED Corporation, JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 4, 2016). |
10.12 | - | First Amendment to the Fifth Amended and Restated Credit Agreement, dated January 4, 2016, among CONMED Corporation, JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on October 28, 2016). |
10.13 | - | Sports Medicine Joint Development and Distribution Agreement by and between Musculoskeletal Transplant Foundation, Inc. and CONMED Corporation dated as of January 3, 2012 (Incorporated by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K dated January 3, 2012). |
10.14+ | - | Employment Agreement between the Company and Patrick Beyer, dated December 9, 2014 (Incorporated by reference to Exhibit 10.21 of the Company's Annual Report on Form 10-K for the year ended December 31, 2014). |
10.15+ | - | Separation Agreement, by and between CONMED Corporation and Joseph J. Corasanti, dated July 22, 2014. (Incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on July 23, 2014). |
10.16+ | - | Retirement Agreement, by and between CONMED Corporation and Robert D. Shallish, Jr., dated December 9, 2014. (Incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 9, 2014). |
10.17 | - | Agreement and Plan of Merger, dated November 15, 2015, by and among CONMED Corporation, Nemo Acquisition Sub, Inc., SurgiQuest, Inc. and Shareholder Representative Services LLC (Incorporated by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K filed with the Securities and Exchange Commission on November 16, 2015). |
37
14 | - | Code of Ethics. The CONMED code of ethics may be accessed via the Company’s website at http://www.conmed.com/conmed_investor_template.php |
21* | - | Subsidiaries of the Registrant. |
23* | - | Consent of Independent Registered Public Accounting Firm. |
31.1* | - | Certification of Curt R. Hartman pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2* | - | Certification of Luke A. Pomilio. pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1* | - | Certifications of Curt R. Hartman and Luke A. Pomilio pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
101* | - | The following materials from CONMED Corporation's Annual Report on Form 10-K for the year ended December 31, 2016 formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Comprehensive Income for the three years ended December 31, 2016, (ii) Consolidated Balance Sheets at December 31, 2016 and 2015, (iii) Consolidated Statements of Shareholders' Equity for the three years ended December 31, 2016 (iv) Consolidated Statements of Cash Flows for the three years ended December 31, 2016, (v) Notes to the Consolidated Financial Statements for the year ended December 31, 2016 and (vi) Schedule II - Valuation and Qualifying Accounts. In accordance with Rule 406T of Regulation S-T, the XBRL related information in Exhibit 101 to this Annual Report on Form 10-K shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and shall not be part of any registration statement or other document filed under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing. |
* | Filed herewith | |
+ | Management contract or compensatory plan or arrangement | |
38
MANAGEMENT’S REPORT ON INTERNAL CONTROL
OVER FINANCIAL REPORTING
The management of CONMED Corporation is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and dispositions of assets; provide reasonable assurances that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures are being made only in accordance with authorizations of management and the directors of the Company; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Management assessed the effectiveness of CONMED’s internal control over financial reporting as of December 31, 2016. In making its assessment, management utilized the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in “Internal Control-Integrated Framework”, released in 2013. Management has concluded that based on its assessment, CONMED’s internal control over financial reporting was effective as of December 31, 2016. The effectiveness of the Company’s internal control over financial reporting as of December 31, 2016 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
/s/ Curt R. Hartman
Curt R. Hartman
President and
Chief Executive Officer
/s/ Luke A. Pomilio
Luke A. Pomilio
Executive Vice President-Finance and
Chief Financial Officer
39
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of CONMED Corporation
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of comprehensive income, of shareholders’ equity and of cash flows present fairly, in all material respects, the financial position of CONMED Corporation and its subsidiaries at December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 15 to the consolidated financial statements, the Company changed the manner in which it accounts for the classification of deferred taxes in 2016.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Rochester, New York
February 27, 2017
40
CONMED CORPORATION
CONSOLIDATED BALANCE SHEETS
December 31, 2016 and 2015
(In thousands except share and per share amounts)
2016 | 2015 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 27,428 | $ | 72,504 | |||
Accounts receivable, less allowance for doubtful | |||||||
accounts of $2,031 in 2016 and $1,336 in 2015 | 148,244 | 133,863 | |||||
Inventories | 135,869 | 133,361 | |||||
Prepaid expenses and other current assets | 18,971 | 20,076 | |||||
Total current assets | 330,512 | 359,804 | |||||
Property, plant and equipment, net | 122,029 | 125,452 | |||||
Deferred income taxes | 3,712 | 4,238 | |||||
Goodwill | 397,664 | 260,651 | |||||
Other intangible assets, net | 419,549 | 308,171 | |||||
Other assets | 55,517 | 43,384 | |||||
Total assets | $ | 1,328,983 | $ | 1,101,700 | |||
LIABILITIES AND SHAREHOLDERS' EQUITY | |||||||
Current liabilities: | |||||||
Current portion of long-term debt | $ | 10,202 | $ | 1,339 | |||
Accounts payable | 41,647 | 34,720 | |||||
Accrued compensation and benefits | 32,036 | 31,823 | |||||
Other current liabilities | 30,067 | 51,836 | |||||
Total current liabilities | 113,952 | 119,718 | |||||
Long-term debt | 488,288 | 269,471 | |||||
Deferred income taxes | 119,143 | 103,379 | |||||
Other long-term liabilities | 27,024 | 24,059 | |||||
Total liabilities | 748,407 | 516,627 | |||||
Commitments and contingencies (Note 11) | |||||||
Shareholders' equity: | |||||||
Preferred stock, par value $.01 per share; authorized | |||||||
500,000 shares, none issued or outstanding | — | — | |||||
Common stock, par value $.01 per share; 100,000,000 | |||||||
authorized; 31,299,194 issued in 2016 and 2015, respectively | 313 | 313 | |||||
Paid-in capital | 329,276 | 324,915 | |||||
Retained earnings | 406,932 | 414,506 | |||||
Accumulated other comprehensive loss | (58,526 | ) | (53,894 | ) | |||
Less: Treasury stock, at cost; | |||||||
3,471,121 and 3,590,409 shares in | |||||||
2016 and 2015, respectively | (97,419 | ) | (100,767 | ) | |||
Total shareholders' equity | 580,576 | 585,073 | |||||
Total liabilities and shareholders' equity | $ | 1,328,983 | $ | 1,101,700 | |||
The accompanying notes are an integral part of the consolidated financial statements.
41
CONMED CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
Years Ended December 31, 2016, 2015 and 2014
(In thousands except per share amounts)
2016 | 2015 | 2014 | |||||||||
Net sales | $ | 763,520 | $ | 719,168 | $ | 740,055 | |||||
Cost of sales | 355,190 | 337,466 | 335,998 | ||||||||
Gross profit | 408,330 | 381,702 | 404,057 | ||||||||
Selling and administrative expense | 338,400 | 303,091 | 323,492 | ||||||||
Research and development expense | 32,254 | 27,436 | 27,779 | ||||||||
Operating expenses | 370,654 | 330,527 | 351,271 | ||||||||
Income from operations | 37,676 | 51,175 | 52,786 | ||||||||
Other expense | 2,942 | — | — | ||||||||
Interest expense | 15,359 | 6,031 | 6,111 | ||||||||
Income before income taxes | 19,375 | 45,144 | 46,675 | ||||||||
Provision for income taxes | 4,711 | 14,646 | 14,483 | ||||||||
Net income | $ | 14,664 | $ | 30,498 | $ | 32,192 | |||||
Per share data: | |||||||||||
Basic | $ | 0.53 | $ | 1.10 | $ | 1.17 | |||||
Diluted | $ | 0.52 | $ | 1.09 | $ | 1.16 | |||||
Dividends per share of common stock | $ | 0.80 | $ | 0.80 | $ | 0.80 | |||||
Other comprehensive income (loss), before tax: | |||||||||||
Foreign currency translation adjustments | $ | (4,501 | ) | $ | (16,775 | ) | $ | (15,069 | ) | ||
Pension liability | (755 | ) | 7,578 | (18,781 | ) | ||||||
Cash flow hedging gain (loss) | 547 | (3,291 | ) | 7,393 | |||||||
Other comprehensive income, before tax | 9,955 | 18,010 | 5,735 | ||||||||
Provision (benefit) for income taxes related to items of other comprehensive income | (77 | ) | 1,584 | (4,207 | ) | ||||||
Comprehensive income | $ | 10,032 | $ | 16,426 | $ | 9,942 | |||||
The accompanying notes are an integral part of the consolidated financial statements.
42
CONMED CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
Years Ended December 31, 2016, 2015 and 2014
(In thousands)
Common Stock | Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Loss | Treasury Stock | Shareholders’ Equity | |||||||||||||||||||||
Shares | Amount | |||||||||||||||||||||||||
Balance at December 31, 2013 | 31,299 | $ | 313 | $ | 326,436 | $ | 395,889 | $ | (17,572 | ) | $ | (98,747 | ) | $ | 606,319 | |||||||||||
Common stock issued | ||||||||||||||||||||||||||
under employee plans | (16,658 | ) | 10,519 | (6,139 | ) | |||||||||||||||||||||
Repurchase of treasury stock | (16,862 | ) | (16,862 | ) | ||||||||||||||||||||||
Tax benefit arising from | ||||||||||||||||||||||||||
common stock issued under | ||||||||||||||||||||||||||
employee plans | 644 | 644 | ||||||||||||||||||||||||
Stock-based compensation | 9,330 | 9,330 | ||||||||||||||||||||||||
Dividends on common stock | (21,936 | ) | (21,936 | ) | ||||||||||||||||||||||
Comprehensive income (loss): | ||||||||||||||||||||||||||
Foreign currency translation adjustments | (15,069 | ) | ||||||||||||||||||||||||
Pension liability (net of income tax benefit of $6,939) | (11,842 | ) | ||||||||||||||||||||||||
Cash flow hedging gain (net of income tax expense of $2,732) | 4,661 | |||||||||||||||||||||||||
Net income | 32,192 | |||||||||||||||||||||||||
Total comprehensive income | 9,942 | |||||||||||||||||||||||||
Balance at December 31, 2014 | 31,299 | $ | 313 | $ | 319,752 | $ | 406,145 | $ | (39,822 | ) | $ | (105,090 | ) | $ | 581,298 | |||||||||||
Common stock issued | ||||||||||||||||||||||||||
under employee plans | (6,297 | ) | 4,323 | (1,974 | ) | |||||||||||||||||||||
Tax benefit arising from | ||||||||||||||||||||||||||
common stock issued under | ||||||||||||||||||||||||||
employee plans | 3,961 | 3,961 | ||||||||||||||||||||||||
Stock-based compensation | 7,499 | 7,499 | ||||||||||||||||||||||||
Dividends on common stock | (22,137 | ) | (22,137 | ) | ||||||||||||||||||||||
Comprehensive income (loss): | ||||||||||||||||||||||||||
Foreign currency translation adjustments | (16,775 | ) | ||||||||||||||||||||||||
Pension liability (net of income tax expense $2,800) | 4,778 | |||||||||||||||||||||||||
Cash flow hedging loss (net of income tax benefit of $1,216) | (2,075 | ) | ||||||||||||||||||||||||
43
Common Stock | Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Loss | Treasury Stock | Shareholders’ Equity | |||||||||||||||||||||
Shares | Amount | |||||||||||||||||||||||||
Net income | 30,498 | |||||||||||||||||||||||||
Total comprehensive income | 16,426 | |||||||||||||||||||||||||
Balance at December 31, 2015 | 31,299 | $ | 313 | $ | 324,915 | $ | 414,506 | $ | (53,894 | ) | $ | (100,767 | ) | $ | 585,073 | |||||||||||
Common stock issued | ||||||||||||||||||||||||||
under employee plans | (4,217 | ) | 3,348 | (869 | ) | |||||||||||||||||||||
Tax benefit arising from | ||||||||||||||||||||||||||
common stock issued | ||||||||||||||||||||||||||
under employee plans | 203 | 203 | ||||||||||||||||||||||||
Stock-based compensation | 8,375 | 8,375 | ||||||||||||||||||||||||
Dividends on common stock | (22,238 | ) | (22,238 | ) | ||||||||||||||||||||||
Comprehensive income (loss): | ||||||||||||||||||||||||||
Foreign currency translation adjustments | (4,501 | ) | ||||||||||||||||||||||||
Pension liability (net of income tax benefit of $279) | (476 | ) | ||||||||||||||||||||||||
Cash flow hedging gain (net of income tax expense of $202) | 345 | |||||||||||||||||||||||||
Net income | 14,664 | |||||||||||||||||||||||||
Total comprehensive income | 10,032 | |||||||||||||||||||||||||
Balance at December 31, 2016 | 31,299 | $ | 313 | $ | 329,276 | $ | 406,932 | $ | (58,526 | ) | $ | (97,419 | ) | $ | 580,576 | |||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
44
CONMED CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years Ended December 31, 2016, 2015 and 2014
(In thousands)
2016 | 2015 | 2014 | |||||||||
Cash flows from operating activities: | |||||||||||
Net income | $ | 14,664 | $ | 30,498 | $ | 32,192 | |||||
Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
Depreciation | 20,479 | 18,704 | 19,792 | ||||||||
Amortization | 34,830 | 25,175 | 25,942 | ||||||||
Stock-based compensation | 8,375 | 7,499 | 9,330 | ||||||||
Deferred income taxes | (2,871 | ) | 2,251 | (284 | ) | ||||||
Gain on sale of facility | (1,890 | ) | — | — | |||||||
Income tax benefit of stock option exercises | 203 | 3,961 | 644 | ||||||||
Excess tax benefit from stock option exercises | (483 | ) | (4,081 | ) | (922 | ) | |||||
Loss on early extinguishment of debt | 254 | — | — | ||||||||
Increase (decrease) in cash flows from changes in assets and | |||||||||||
liabilities, net of acquired assets: | |||||||||||
Accounts receivable | (6,380 | ) | (9,643 | ) | 5,255 | ||||||
Inventories | 3,103 | (18,581 | ) | (10,449 | ) | ||||||
Accounts payable | 2,094 | 11,508 | (3,449 | ) | |||||||
Income taxes | (200 | ) | (1,357 | ) | 5,291 | ||||||
Accrued compensation and benefits | (2,598 | ) | (3,964 | ) | 3,572 | ||||||
Other assets | (23,234 | ) | (12,005 | ) | (11,037 | ) | |||||
Other liabilities | (8,124 | ) | (1,897 | ) | (10,701 | ) | |||||
23,558 | 17,570 | 32,984 | |||||||||
Net cash provided by operating activities | 38,222 | 48,068 | 65,176 | ||||||||
Cash flows from investing activities: | |||||||||||
Payments related to business and asset acquisitions, net of cash acquired | (256,450 | ) | (9,353 | ) | (5,265 | ) | |||||
Proceeds from sale of a facility | 5,178 | — | — | ||||||||
Purchases of property, plant and equipment | (14,753 | ) | (15,009 | ) | (15,411 | ) | |||||
Net cash used in investing activities | (266,025 | ) | (24,362 | ) | (20,676 | ) | |||||
Cash flows from financing activities: | |||||||||||
Repurchase of common stock | — | — | (16,862 | ) | |||||||
Excess tax benefit from stock option exercises | 483 | 4,081 | 922 | ||||||||
Payments on term loan | (8,750 | ) | — | — | |||||||
Proceeds from term loan | 175,000 | — | — | ||||||||
Payments on revolving line of credit | (162,347 | ) | (112,000 | ) | (86,000 | ) | |||||
Proceeds from revolving line of credit | 225,000 | 142,680 | 113,000 | ||||||||
Payments related to distribution agreement | (16,667 | ) | (16,667 | ) | (16,667 | ) | |||||
Payments on mortgage notes | (1,339 | ) | (1,234 | ) | (1,140 | ) | |||||
Payments related to debt issuance costs | (5,556 | ) | (1,485 | ) | — | ||||||
Dividends paid on common stock | (22,213 | ) | (22,105 | ) | (21,959 | ) | |||||
Other, net | 591 | (3,043 | ) | 2,316 | |||||||
Net cash provided by (used in) financing activities | 184,202 | (9,773 | ) | (26,390 | ) | ||||||
Effect of exchange rate changes | |||||||||||
on cash and cash equivalents | (1,475 | ) | (7,761 | ) | (6,221 | ) | |||||
Net increase (decrease) in cash and cash equivalents | (45,076 | ) | 6,172 | 11,889 | |||||||
45
2016 | 2015 | 2014 | |||||||||
Cash and cash equivalents at beginning of year | 72,504 | 66,332 | 54,443 | ||||||||
Cash and cash equivalents at end of year | $ | 27,428 | $ | 72,504 | $ | 66,332 | |||||
Non-cash investing activities: | |||||||||||
Contractual obligations for acquisition of a business | $ | — | $ | 440 | $ | 10,137 | |||||
Non-cash financing activities: | |||||||||||
Dividends payable | $ | 5,566 | $ | 5,542 | $ | 5,510 | |||||
Supplemental disclosures of cash flow information: | |||||||||||
Cash paid during the year for: | |||||||||||
Interest | $ | 13,758 | $ | 5,434 | $ | 5,532 | |||||
Income taxes | 9,588 | 10,261 | 10,206 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
46
CONMED CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands except per share amounts)
Note 1 — Operations and Significant Accounting Policies
Organization and operations
CONMED Corporation (“CONMED”, the “Company”, “we” or “us”) is a medical technology company that provides surgical devices and equipment for minimally invasive procedures. The Company’s products are used by surgeons and physicians in a variety of specialties including orthopedics, general surgery, gynecology, neurosurgery and gastroenterology.
Principles of consolidation
The consolidated financial statements include the accounts of CONMED Corporation and its controlled subsidiaries. All significant intercompany accounts and transactions have been eliminated.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and judgments which affect the reported amounts of assets, liabilities, related disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Estimates are used in accounting for, among other things, allowances for doubtful accounts, rebates and sales allowances, inventory allowances, purchased in-process research and development, pension benefits, goodwill and intangible assets, contingent consideration, contingencies and other accruals. We base our estimates on historical experience and on various other assumptions which are believed to be reasonable under the circumstances. Due to the inherent uncertainty involved in making estimates, actual results reported in future periods may differ from those estimates. Estimates and assumptions are reviewed periodically, and the effect of revisions is reflected in the consolidated financial statements in the period they are determined to be necessary.
Cash and cash equivalents
We consider all highly liquid investments with an original maturity of three months or less to be cash equivalents.
Inventories
Inventories are valued at the lower of cost or market. Cost is determined on the FIFO (first-in, first-out) method of accounting.
We write-off excess and obsolete inventory resulting from the inability to sell our products at prices in excess of current carrying costs. We make estimates regarding the future recoverability of the costs of our products and record a provision for excess and obsolete inventories based on historical experience and expected future trends.
Property, plant and equipment
Property, plant and equipment are stated at cost and depreciated using the straight-line method over the following estimated useful lives:
Building and improvements | 12 to 40 years | |
Leasehold improvements | Shorter of life of asset or life of lease | |
Machinery and equipment | 2 to 15 years | |
Goodwill and other intangible assets
We have a history of growth through acquisitions. Assets and liabilities of acquired businesses are recorded at their estimated fair values as of the date of acquisition. Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses. Customer and distributor relationships, trademarks, tradenames, developed technology, patents
47
and other intangible assets primarily represent allocations of purchase price to identifiable intangible assets of acquired businesses. Promotional, marketing and distribution rights represent intangible assets created under our Sports Medicine Joint Development and Distribution Agreement (the "JDDA") with Musculoskeletal Transplant Foundation (“MTF”). We have goodwill of $397.7 million and other intangible assets of $419.5 million as of December 31, 2016.
Goodwill and intangible assets deemed to have indefinite lives are not amortized, but are subject to at least annual impairment testing. It is our policy to perform our annual impairment testing in the fourth quarter. The identification and measurement of goodwill impairment involves the estimation of the fair value of our business. Estimates of fair value are based on the best information available as of the date of the assessment. During 2016, we completed our goodwill impairment testing with data as of October 1, 2016. We performed a Step 1 impairment test utilizing the market capitalization approach to determine whether the fair value of a reporting unit is less than its carrying amount. Based upon our assessment, we believe the fair value continues to exceed carrying value.
Intangible assets with a finite life are amortized over the estimated useful life of the asset and are evaluated each reporting period to determine whether events and circumstances warrant a revision to the remaining period of amortization. Intangible assets subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that its carrying amount may not be recoverable. The carrying amount of an intangible asset subject to amortization is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use of the asset. An impairment loss is recognized by reducing the carrying amount of the intangible asset to its current fair value.
Customer relationship assets arose as a result of the 1997 acquisition of Linvatec Corporation. The acquisition date valuation indicated an annual attrition rate of 2.6%. Assuming an exponential attrition pattern, this equated to an average remaining useful life of approximately 38 years for the Linvatec customer relationship assets. During 2016, we acquired SurgiQuest, Inc. and recorded customer and distributor relationships with an average useful life of 22 years. Customer and distributor relationship intangible assets arising as a result of business acquisitions other than Linvatec are being amortized over a weighted average life of 21 years. The weighted average life for customer and distributor relationship assets in aggregate is 29 years.
We evaluate the remaining useful life of our customer and distributor relationship intangible assets each reporting period in order to determine whether events and circumstances warrant a revision to the remaining period of amortization. In order to further evaluate the remaining useful life of our customer and distributor relationship intangible assets, we perform an analysis and assessment of actual customer attrition and activity as events and circumstances warrant.
We test our customer and distributor relationship assets for recoverability whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. Factors specific to our customer and distributor relationship assets which might lead to an impairment charge include a significant increase in the annual customer attrition rate or otherwise significant loss of customers, significant decreases in sales or current-period operating or cash flow losses or a projection or forecast of losses. We do not believe that there have been events or changes in circumstances which would indicate the carrying amount of our customer relationship assets might not be recoverable.
Our developed technology asset arose as a result of the SurgiQuest, Inc. acquisition. This asset is amortized over a weighted average useful life of 17 years. We test for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable.
Trademarks and tradenames intangible assets are not amortized. The Company assesses the impairment of indefinite-lived intangibles annually as of October 1, 2016 and whenever an event or circumstances change that would indicate that the carrying amount may be impaired. We performed a qualitative assessment, and based upon our assessment, we believe the fair value continues to exceed carrying value.
For all other indefinite-lived intangible assets, we perform a qualitative impairment test. Based upon this assessment, we have determined that it is unlikely that our indefinite-lived intangible assets are impaired.
Other long-lived assets
We review other long-lived assets consisting of intangible assets subject to amortization, property, plant and equipment and field inventory for impairment whenever events or circumstances indicate that such carrying amounts may not be recoverable. If the sum of the expected future undiscounted cash flows is less than the carrying amount of the asset, an impairment loss is recognized by reducing the recorded value to its current fair value.
48
The Company maintains field inventory consisting of capital equipment for customer demonstration and evaluation purposes. Field inventory is generally not sold to customers but rather continues to be used over its useful life for demonstration, evaluation and loaner purposes. An annual wear and tear provision has been recorded on field inventory.
In the fourth quarter of 2016 and as a result of the SurgiQuest acquisition, the Company determined it needed to expand its field inventory and reevaluated its prior accounting classification as inventory. As a result, the equipment is classified as a long term asset and amortized over its useful life. The net book value of such equipment at December 31, 2016 and 2015 is $44.8 million and $33.5 million, respectively; the prior year balance is presented in other long term assets.
Translation of foreign currency financial statements
Assets and liabilities of foreign subsidiaries have been translated into United States dollars at the applicable rates of exchange in effect at the end of the period reported. Revenues and expenses have been translated at the applicable weighted average rates of exchange in effect during the period reported. Translation adjustments are reflected in accumulated other comprehensive loss. Transaction gains and losses are included in net income.
Foreign exchange and hedging activity
We manage our foreign currency transaction risks through the use of forward contracts to hedge forecasted cash flows associated with foreign currency transaction exposures. We account for these forward contracts as cash flow hedges. To the extent these forward contracts meet hedge accounting criteria, changes in their fair value are not included in current earnings but are included in accumulated other comprehensive loss. These changes in fair value will be reclassified into earnings as a component of sales or cost of sales when the forecasted transaction occurs.
We also enter into forward contracts to exchange foreign currencies for United States dollars in order to hedge our currency transaction exposures on intercompany receivables denominated in foreign currencies. These forward contracts settle each month at month-end, at which time we enter into new forward contracts. We have not designated these forward contracts as hedges and have not applied hedge accounting to them. We record these forward contracts at fair value with resulting gains and losses included in selling and administrative expense in the consolidated statements of comprehensive income.
Income taxes
Deferred income tax assets and liabilities are based on the difference between the financial statement and tax basis of assets and liabilities and operating loss and tax credit carryforwards as measured by the enacted tax rates that are anticipated to be in effect in the respective jurisdictions when these differences reverse. The deferred income tax provision generally represents the net change in the assets and liabilities for deferred income taxes. A valuation allowance is established when it is necessary to reduce deferred income tax assets to amounts for which realization is likely. In assessing the need for a valuation allowance, we estimate future taxable income, considering the feasibility of ongoing tax planning strategies and the realizability of tax loss carryforwards. Valuation allowances related to deferred tax assets may be impacted by changes to tax laws, changes to statutory tax rates, reversal of temporary differences and ongoing and future taxable income levels.
Deferred income taxes are not provided on the unremitted earnings of subsidiaries outside of the United States when it is expected that these earnings are permanently reinvested. Such earnings may become taxable upon a repatriation of assets from a subsidiary or the sale or liquidation of a subsidiary. Deferred income taxes are provided when the Company no longer considers subsidiary earnings to be permanently invested, such as in situations where the Company’s subsidiaries plan to make future dividend distributions.
Revenue recognition
Revenue is recognized when title has been transferred to the customer which is at the time of shipment. The following policies apply to our major categories of revenue transactions:
• | Sales to customers are evidenced by firm purchase orders. Title and the risks and rewards of ownership are transferred to the customer when product is shipped under our stated shipping terms. Payment by the customer is due under fixed payment terms and collectability is reasonably assured. |
• | We place certain of our capital equipment with customers on a loaned basis in return for commitments to purchase related single-use products over time periods generally ranging from one to three years. In these circumstances, no revenue is |
49
recognized upon capital equipment shipment as the equipment is loaned and subject to return if certain minimum single-use purchases are not met. Revenue is recognized upon the sale and shipment of the related single-use products. The cost of the equipment is amortized over its estimated useful life.
• | We recognize revenues related to the promotion and marketing of sports medicine allograft tissue in accordance with the contractual terms of our agreement with Musculoskeletal Transplant Foundation (“MTF”) on a net basis as our role is limited to that of an agent earning a commission or fee. MTF records revenue when the tissue is shipped to the customer. Our services are completed at this time and net revenues for the “Service Fee” for our promotional and marketing efforts are then recognized based on a percentage of the net amounts billed by MTF to its customers. The timing of revenue recognition is determined through review of the net billings made by MTF each month. Our net commission Service Fee is based on the contractual terms of our agreement and is currently 50%. This percentage can vary over the term of the agreement but is contractually determinable. Our Service Fee revenues are recorded net of amortization of the acquired assets, which are being amortized over the expected useful life of 25 years. |
• | Product returns are only accepted at the discretion of the Company and in accordance with our “Returned Goods Policy”. Historically, the level of product returns has not been significant. We accrue for sales returns, rebates and allowances based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions. |
• | Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are provided for capital equipment sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data. |
• | Amounts billed to customers related to shipping and handling have been included in net sales. Shipping and handling costs included in selling and administrative expense were $13.4 million, $12.6 million and $13.6 million for 2016, 2015 and 2014, respectively. |
• | We sell to a diversified base of customers around the world and, therefore, believe there is no material concentration of credit risk. |
• | We assess the risk of loss on accounts receivable and adjust the allowance for doubtful accounts based on this risk assessment. Historically, losses on accounts receivable have not been material. Management believes that the allowance for doubtful accounts of $2.0 million at December 31, 2016 is adequate to provide for probable losses resulting from accounts receivable. |
Earnings per share
Basic earnings per share (“basic EPS”) is computed by dividing net income by the weighted average number of common shares outstanding for the reporting period. Diluted earnings per share (“diluted EPS”) gives effect to all dilutive potential shares outstanding resulting from employee stock options, restricted stock units, performance share units and stock appreciation rights during the period. The following table sets forth the computation of basic and diluted earnings per share at December 31, 2016, 2015 and 2014, respectively:
2016 | 2015 | 2014 | |||||||||
Net income | $ | 14,664 | $ | 30,498 | $ | 32,192 | |||||
Basic-weighted average shares outstanding | 27,804 | 27,653 | 27,401 | ||||||||
Effect of dilutive potential securities | 160 | 205 | 368 | ||||||||
Diluted-weighted average shares outstanding | 27,964 | 27,858 | 27,769 | ||||||||
Net income (per share) | |||||||||||
Basic | $ | 0.53 | $ | 1.10 | $ | 1.17 | |||||
Diluted | 0.52 | 1.09 | 1.16 | ||||||||
50
The shares used in the calculation of diluted EPS exclude options and stock appreciation rights ("SARs") to purchase shares where the exercise price was greater than the average market price of common shares for the year and the effect of the inclusion would be anti-dilutive. Such shares aggregated approximately 1.4 million, 0.5 million and 0.0 million at December 31, 2016, 2015 and 2014, respectively.
Stock-based compensation
All share-based payments to employees, including grants of employee stock options, restricted stock units, performance share units and stock appreciation rights are recognized in the financial statements based at their fair values. Compensation expense is generally recognized using a straight-line method over the vesting period. Compensation expense for performance share units is recognized using the graded vesting method.
We issue shares under our stock based compensation plans out of treasury stock whereby treasury stock is reduced by the weighted average cost of such treasury stock. To the extent there is a difference between the cost of the treasury stock and the exercise price of shares issued under stock based compensation plans, we record gains to paid in capital; losses are recorded to paid in capital to the extent any gain was previously recorded, otherwise the loss is recorded to retained earnings.
Accumulated other comprehensive loss
Accumulated other comprehensive loss consists of the following:
51
Cash Flow Hedging Gain (Loss) | Pension Liability | Cumulative Translation Adjustments | Accumulated Other Comprehensive Loss | ||||||||||||
Balance, December 31, 2013 | $ | (1,385 | ) | $ | (18,918 | ) | $ | 2,731 | $ | (17,572 | ) | ||||
Other comprehensive income (loss) before reclassifications, net of tax | 5,061 | (10,551 | ) | (15,069 | ) | (20,559 | ) | ||||||||
Amounts reclassified from accumulated other comprehensive income (loss) before taxa | (635 | ) | (2,048 | ) | — | (2,683 | ) | ||||||||
Income tax provision (benefit) | 235 | 757 | — | 992 | |||||||||||
Net current-period other comprehensive income (loss) | 4,661 | (11,842 | ) | (15,069 | ) | (22,250 | ) | ||||||||
Balance, December 31, 2014 | $ | 3,276 | $ | (30,760 | ) | $ | (12,338 | ) | $ | (39,822 | ) | ||||
Other comprehensive income (loss) before reclassifications, net of tax | 4,482 | 2,739 | (16,775 | ) | (9,554 | ) | |||||||||
Amounts reclassified from accumulated other comprehensive income (loss) before taxa | (10,399 | ) | 3,233 | — | (7,166 | ) | |||||||||
Income tax provision (benefit) | 3,842 | (1,194 | ) | — | 2,648 | ||||||||||
Net current-period other comprehensive income (loss) | (2,075 | ) | 4,778 | (16,775 | ) | (14,072 | ) | ||||||||
Balance, December 31, 2015 | $ | 1,201 | $ | (25,982 | ) | $ | (29,113 | ) | $ | (53,894 | ) | ||||
Other comprehensive income (loss) before reclassifications, net of tax | 1,088 | (2,229 | ) | (4,501 | ) | (5,642 | ) | ||||||||
Amounts reclassified from accumulated other comprehensive income (loss) before taxa | (1,179 | ) | 2,780 | — | 1,601 | ||||||||||
Income tax provision (benefit) | 436 | (1,027 | ) | — | (591 | ) | |||||||||
Net current-period other comprehensive income (loss) | 345 | (476 | ) | (4,501 | ) | (4,632 | ) | ||||||||
Balance, December 31, 2016 | $ | 1,546 | $ | (26,458 | ) | $ | (33,614 | ) | $ | (58,526 | ) | ||||
(a) The cash flow hedging gain (loss) and pension liability accumulated other comprehensive income components are included in sales or cost of sales and as a component of net periodic pension cost, respectively. Refer to Note 14 and Note 10, respectively, for further details.
Note 2 – Business Acquisition
On January 4, 2016, we acquired all of the stock of SurgiQuest, Inc. ("SurgiQuest") for $257.7 million in cash (based on an aggregate purchase price of $265 million as adjusted pursuant to the merger agreement governing the acquisition). SurgiQuest developed, manufactured and marketed the AirSeal® System, the first integrated access management technology for use in laparoscopic and robotic procedures. This proprietary and differentiated access system is complementary to our current general surgery offering. The acquisition was funded through a combination of cash on hand and long-term borrowings.
The following table summarizes the fair values of the assets acquired and liabilities assumed as a result of the SurgiQuest acquisition.
52
Cash | $ | 1,305 | |
Accounts receivable | 10,032 | ||
Inventory | 4,267 | ||
Other current assets | 728 | ||
Current assets acquired | 16,332 | ||
Property, plant & equipment | 3,332 | ||
Goodwill | 136,687 | ||
Customer and distributor relationships | 76,420 | ||
Developed technology | 49,600 | ||
Trademarks and tradenames | 4,780 | ||
Other non-current assets | 1,553 | ||
Total assets acquired | $ | 288,704 | |
Accounts payable | $ | 5,012 | |
Other current liabilities | 6,004 | ||
Current liabilities assumed | 11,016 | ||
Deferred income taxes | 19,505 | ||
Other long-term liabilities | 454 | ||
Total liabilities assumed | 30,975 | ||
Net assets acquired | $ | 257,729 | |
The goodwill recorded as part of the acquisition primarily represents revenue synergies, as well as operating efficiencies and cost savings. Goodwill deductible for tax purposes is $11.5 million. The weighted amortization period for intangibles acquired is 20 years. Customer and distributor relationships, developed technology and trademarks and tradenames are being amortized over a weighted average life of 22, 17 and 23 years, respectively.
The unaudited pro forma information for the years ended December 31, 2016 and 2015, assuming SurgiQuest occurred as of January 1, 2015 are presented below. This information has been prepared for comparative purposes only and does not purport to be indicative of the results of operations which actually would have resulted had the SurgiQuest acquisition occurred on the dates indicated, or which may result in the future.
December 31, | |||||||
2016 | 2015 | ||||||
Net sales | $ | 763,520 | $ | 768,726 | |||
Net income | 29,153 | (9,673 | ) | ||||
These pro forma results include certain adjustments, primarily due to increases in amortization expense due to fair value adjustments of intangible assets, increases in interest expense due to additional borrowings incurred to finance the acquisition, and acquisition related costs including transaction costs such as legal, accounting, valuation and other professional services as well as integration costs such as severance and retention.
Acquisition related costs included in the determination of pro forma net income for the year ended December 31, 2015 totaled $20.6 million. Such amounts are excluded from the determination of pro forma net income for the year ended December 31, 2016.
Net sales associated with SurgiQuest of $68.4 million have been recorded in the consolidated statement of comprehensive income for the year ended December 31, 2016. It is impracticable to determine the earnings recorded in the consolidated statement of comprehensive income associated with the SurgiQuest acquisition for the year ended December 31, 2016 as these amounts are not separately measured.
Note 3 — Inventories
53
Inventories consist of the following at December 31:
2016 | 2015 | ||||||
Raw materials | $ | 42,821 | $ | 47,681 | |||
Work in process | 13,315 | 13,922 | |||||
Finished goods | 79,733 | 71,758 | |||||
$ | 135,869 | $ | 133,361 | ||||
Note 4 — Property, Plant and Equipment
Property, plant and equipment consist of the following at December 31:
2016 | 2015 | ||||||
Land | $ | 4,027 | $ | 4,027 | |||
Building and improvements | 90,780 | 90,272 | |||||
Machinery and equipment | 205,674 | 193,630 | |||||
Construction in progress | 7,229 | 5,281 | |||||
307,710 | 293,210 | ||||||
Less: Accumulated depreciation | (185,681 | ) | (167,758 | ) | |||
$ | 122,029 | $ | 125,452 | ||||
We lease various manufacturing facilities, office facilities and equipment under operating leases. Leasehold improvements related to these facilities are included in building and improvements above. Rental expense on these operating leases was approximately $6,043, $5,464 and $5,897 for the years ended December 31, 2016, 2015 and 2014, respectively. The aggregate future minimum lease commitments for operating leases at December 31, 2016 are as follows:
2017 | $ | 6,170 | |
2018 | 5,862 | ||
2019 | 5,717 | ||
2020 | 2,348 | ||
2021 | 1,182 | ||
Thereafter | 2,505 | ||
Note 5 – Goodwill and Other Intangible Assets
The changes in the net carrying amount of goodwill for the years ended December 31, are as follows:
2016 | 2015 | ||||||
Balance as of January 1, | $ | 260,651 | $ | 256,232 | |||
Goodwill resulting from business acquisitions | 136,687 | 5,369 | |||||
Reduction in goodwill resulting from a business acquisition purchase price allocation adjustment | — | (525 | ) | ||||
Foreign currency translation | 326 | (425 | ) | ||||
Balance as of December 31, | $ | 397,664 | $ | 260,651 | |||
54
Assets and liabilities of acquired businesses are recorded at their estimated fair values as of the date of acquisition. Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses. During 2016, the Company acquired SurgiQuest, Inc. (SurgiQuest) as further described in Note 2. Goodwill resulting from the acquisition amounted to $136.7 million and acquired amortizing intangible assets including customer and distributor relationships, developed technology and trademarks and tradenames amounted to $130.8 million. During 2015, the Company entered into three acquisitions totaling a cash purchase price of $6.1 million. The purchase price in a prior acquisition was allocated based on information available at the acquisition date. During the quarter ended March 31, 2015, we recorded a measurement period adjustment, which reduced goodwill by $0.5 million. The amount was not considered material and therefore prior periods have not been revised.
Total accumulated impairment losses aggregated $106,991 at December 31, 2016 and 2015, respectively.
Other intangible assets consist of the following:
December 31, 2016 | December 31, 2015 | ||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | ||||||||||||
Amortized intangible assets: | |||||||||||||||
Customer and distributor relationships | $ | 213,259 | $ | (75,164 | ) | $ | 136,871 | $ | (64,423 | ) | |||||
Promotional, marketing and distribution rights | 149,376 | (30,000 | ) | 149,376 | (24,000 | ) | |||||||||
Patents and other intangible assets | 67,509 | (40,335 | ) | 66,688 | (42,885 | ) | |||||||||
Developed technology | 49,600 | (1,240 | ) | — | — | ||||||||||
Unamortized intangible assets: | |||||||||||||||
Trademarks and tradenames | 86,544 | — | 86,544 | — | |||||||||||
$ | 566,288 | $ | (146,739 | ) | $ | 439,479 | $ | (131,308 | ) | ||||||
On January 3, 2012, the Company entered into the JDDA with MTF to obtain MTF's worldwide promotion rights with respect to allograft tissues within the field of sports medicine and related products. The initial consideration from the Company included a $63.0 million up-front payment for the rights and certain assets, with an additional $84.0 million contingently payable over a four year period depending on MTF meeting supply targets for tissue. On January 6, 2016, January 5, 2015 and January 3, 2014, we paid equal installments of $16.7 million and on January 3, 2013, we paid $34.0 million of the additional consideration.
Amortization expense related to intangible assets which are subject to amortization totaled $20.0 million, $12.6 million and $13.0 million for the years ending December 31, 2016, 2015 and 2014, respectively, and is included as a reduction of revenue (for amortization related to our promotional, marketing and distribution rights) and in selling and administrative expense (for all other intangible assets) in the consolidated statements of comprehensive income. The weighted average amortization period for intangible assets which are amortized is 25 years. Customer and distributor relationships are being amortized over a weighted average life of 29 years. Developed technology is being amortized over a weighted average life of 17 years. Promotional, marketing and distribution rights are being amortized over a weighted average life of 25 years. Patents and other intangible assets are being amortized over a weighted average life of 13 years. Included in patents and other intangible assets at December 31, 2016 is an in-process research and development asset that is not currently amortized.
The estimated amortization expense related to intangible assets at December 31, 2016 and for each of the five succeeding years is as follows:
55
Amortization included in expense | Amortization recorded as a reduction of revenue | Total | |||||||
2017 | 15,539 | 6,000 | $ | 21,539 | |||||
2018 | 15,857 | 6,000 | $ | 21,857 | |||||
2019 | 15,711 | 6,000 | $ | 21,711 | |||||
2020 | 15,732 | 6,000 | $ | 21,732 | |||||
2021 | 14,356 | 6,000 | $ | 20,356 | |||||
Note 6 — Long Term Debt
Long-term debt consists of the following at December 31:
2016 | 2015 | ||||||
Revolving line of credit | $ | 329,000 | $ | 265,609 | |||
Term loan, net of deferred debt issuance costs of $622 and $0 in 2016 and 2015, respectively | 165,628 | — | |||||
Mortgage notes | 3,862 | 5,201 | |||||
Total debt | 498,490 | 270,810 | |||||
Less: Current portion | 10,202 | 1,339 | |||||
Total long-term debt | $ | 488,288 | $ | 269,471 | |||
On January 4, 2016, we entered into a fifth amended and restated senior credit agreement consisting of: (a) a $175.0 million term loan facility and (b) a $525.0 million revolving credit facility both expiring on January 4, 2021. The term loan is payable in quarterly installments increasing over the term of the facility. Proceeds from the term loan facility and borrowings under the revolving credit facility were used to repay the then existing senior credit agreement and to finance the acquisition of SurgiQuest. Initially, the interest rates were at LIBOR plus a base rate or a Eurocurrency rate plus an applicable margin. The applicable margin for base rate loans is 1.00% and for Eurocurrency rate loans is 2.00% (2.77% at December 31, 2016). In conjunction with this agreement, we incurred charges included in other expense in the statements of comprehensive income related to commitment fees paid to certain of our lenders, which provided a financing commitment for the SurgiQuest acquisition totaling $2.7 million and recorded a loss on the early extinguishment of debt of $0.3 million.
There were $166.3 million in borrowings outstanding on the term loan as of December 31, 2016. There were $329.0 million in borrowings outstanding under the revolving credit facility as of December 31, 2016. Our available borrowings on the revolving credit facility at December 31, 2016 were $191.2 million with approximately $4.8 million of the facility set aside for outstanding letters of credit.
The fifth amended and restated senior credit agreement is collateralized by substantially all of our personal property and assets. The fifth amended and restated senior credit agreement contains covenants and restrictions which, among other things, require the maintenance of certain financial ratios and restrict dividend payments and the incurrence of certain indebtedness and other activities, including acquisitions and dispositions. We were in full compliance with these covenants and restrictions as of December 31, 2016. We are also required, under certain circumstances, to make mandatory prepayments from net cash proceeds from any issuance of equity and asset sales.
We have a mortgage note outstanding in connection with the Largo, Florida property and facilities bearing interest at 8.25% per annum with semiannual payments of principal and interest through June 2019. The principal balance outstanding on the mortgage note aggregated $3.9 million at December 31, 2016. The mortgage note is collateralized by the Largo, Florida property and facilities.
The scheduled maturities of long-term debt outstanding at December 31, 2016 are as follows:
56
2017 | $ | 10,202 | |
2018 | 14,699 | ||
2019 | 18,336 | ||
2020 | 17,500 | ||
2021 | 438,375 | ||
Thereafter | — | ||
Note 7 — Income Taxes
The provision for income taxes for the years ended December 31, 2016, 2015 and 2014 consists of the following:
2016 | 2015 | 2014 | |||||||||
Current tax expense: | |||||||||||
Federal | $ | 312 | $ | 4,208 | $ | 2,256 | |||||
State | 159 | 1,238 | 516 | ||||||||
Foreign | 7,111 | 6,949 | 11,995 | ||||||||
7,582 | 12,395 | 14,767 | |||||||||
Deferred income tax expense (benefit) | (2,871 | ) | 2,251 | (284 | ) | ||||||
Provision for income taxes | $ | 4,711 | $ | 14,646 | $ | 14,483 | |||||
A reconciliation between income taxes computed at the statutory federal rate and the provision for income taxes for the years ended December 31, 2016, 2015 and 2014 follows:
2016 | 2015 | 2014 | ||||||
Tax provision at statutory rate based on income before income taxes | 35.0 | % | 35.0 | % | 35.0 | % | ||
Foreign income taxes | (6.8 | ) | (3.6 | ) | (4.8 | ) | ||
Federal research credit | (5.6 | ) | (2.0 | ) | (2.1 | ) | ||
Settlement of taxing authority examinations | (3.5 | ) | (0.6 | ) | (3.7 | ) | ||
European permanent deduction | (3.4 | ) | (2.1 | ) | (3.8 | ) | ||
Non deductible/non-taxable items | 7.2 | 1.8 | 1.8 | |||||
State income taxes, net of federal tax benefit | 1.7 | 3.2 | 1.7 | |||||
Impact of repatriation of foreign earnings | — | 2.5 | — | |||||
New York State corporate tax reform | — | — | 5.5 | |||||
Stock-based compensation | — | — | (0.2 | ) | ||||
Other, net | (0.3 | ) | (1.8 | ) | 1.6 | |||
24.3 | % | 32.4 | % | 31.0 | % | |||
The tax effects of the significant temporary differences which comprise the deferred income tax assets and liabilities at December 31, 2016 and 2015 are as follows:
57
2016 | 2015 | ||||||
Assets: | |||||||
Inventory | $ | 3,769 | $ | 3,938 | |||
Net operating losses | 34,669 | 6,421 | |||||
Capitalized research and development | 6,257 | 5,733 | |||||
Deferred compensation | 2,544 | 2,557 | |||||
Accounts receivable | 3,186 | 2,938 | |||||
Compensation and benefits | 6,645 | 7,365 | |||||
Accrued pension | 4,530 | 3,944 | |||||
Research and development credit | 8,164 | 7,094 | |||||
Other | 2,001 | 3,245 | |||||
Foreign tax credit | 1,112 | — | |||||
Less: valuation allowances | (441 | ) | (124 | ) | |||
72,436 | 43,111 | ||||||
Liabilities: | |||||||
Goodwill and intangible assets | 168,509 | 122,623 | |||||
Depreciation | 9,099 | 11,999 | |||||
State taxes | 10,123 | 7,427 | |||||
Contingent interest | 136 | 203 | |||||
187,867 | 142,252 | ||||||
Net liability | $ | (115,431 | ) | $ | (99,141 | ) | |
Income before income taxes consists of the following U.S. and foreign income:
2016 | 2015 | 2014 | |||||||||
U.S. income | $ | (6,128 | ) | $ | 18,119 | $ | 12,374 | ||||
Foreign income | 25,503 | 27,025 | 34,301 | ||||||||
Total income | $ | 19,375 | $ | 45,144 | $ | 46,675 | |||||
As of December 31, 2016, the amount of federal net operating loss carryforward was $99.3 million and begins to expire in 2026. As of December 31, 2016, the amount of federal research credit carryforward available was $8.1 million. These credits begin to expire in 2027.
In New York State, corporate tax reform enacted in March 2014 changed the tax rate of a manufacturing company such as our Company to essentially 0%. Previously recorded New York State net deferred tax assets of $2.3 million, including $3.3 million of future tax benefits associated with state tax credits, have been written off as a non-cash charge to income tax expense.
During the fourth quarter of 2015, the Company repatriated $9.3 million of 2015 foreign earnings and recorded a tax charge of $1.1 million. The repatriated earnings represented a portion of the 2015 earnings of certain foreign subsidiaries and affiliates and thus were not previously permanently reinvested. There has been no change in our longer term international plans as our intent to indefinitely reinvest the remaining foreign earnings accumulated through the year ended December 31, 2016 has not changed.
U.S. income and foreign withholding taxes have not been recognized on the excess of the amount for financial reporting over the tax basis of investments in foreign subsidiaries that are essentially permanent in duration. The amount of such temporary differences totaled $108.8 million as of December 31, 2016. It is not practicable given the complexities of the hypothetical foreign tax credit calculation to determine the tax liability on this temporary difference.
58
The Company is subject to taxation in the United States and various states and foreign jurisdictions. Taxing authority examinations can involve complex issues and may require an extended period of time to resolve. Our federal income tax returns have been examined by the Internal Revenue Service (“IRS”) for calendar years ending through 2013.
We recognize tax liabilities in accordance with the provisions for accounting for uncertainty in income taxes. Such guidance prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return.
The following table summarizes the activity related to our unrecognized tax benefits for the years ending December 31,:
2016 | 2015 | 2014 | |||||||||
Balance as of January 1, | $ | 616 | $ | 581 | $ | 1,689 | |||||
Increases for positions taken in prior periods | — | 100 | 45 | ||||||||
Increases for positions taken in current periods | 1,584 | — | — | ||||||||
Decreases in unrecorded tax positions related to settlement with the taxing authorities | (361 | ) | — | (1,073 | ) | ||||||
Decreases in unrecorded tax positions related to lapse of statute of limitations | — | (65 | ) | (80 | ) | ||||||
Balance as of December 31, | $ | 1,839 | $ | 616 | $ | 581 | |||||
If the total unrecognized tax benefits of $1.8 million at December 31, 2016 were recognized, it would reduce our annual effective tax rate. The amount of interest accrued in 2016 related to these unrecognized tax benefits was not material and is included in the provision for income taxes in the consolidated statements of comprehensive income.
Note 8 – Shareholders’ Equity
On February 29, 2012, the Board of Directors adopted a cash dividend policy and declared an initial quarterly dividend of $0.15 per share. On October 28, 2013, the Board of Directors increased the quarterly dividend to $0.20 per share. The fourth quarter dividend for 2016 was paid on January 5, 2017 to shareholders of record as of December 15, 2016. The total dividend payable was $5.6 million and $5.5 million at December 31, 2016 and 2015, respectively, and is included in other current liabilities in the consolidated balance sheet.
Our shareholders have authorized 500,000 shares of preferred stock, par value $.01 per share, which may be issued in one or more series by the Board of Directors without further action by the shareholders. As of December 31, 2016 and 2015, no preferred stock had been issued.
Our Board of Directors has authorized a $200.0 million share repurchase program. Through December 31, 2016, we have repurchased a total of 6.1 million shares of common stock aggregating $162.6 million under this authorization and have $37.4 million remaining available for share repurchases. The repurchase program calls for shares to be purchased in the open market or in private transactions from time to time. We may suspend or discontinue the share repurchase program at any time. During 2016 and 2015 we did not repurchase any shares. During 2014, we repurchased 0.4 million shares for an aggregate cost of $16.9 million.
We have reserved 8.9 million shares of common stock for issuance to employees and directors under three shareholder approved share-based compensation plans (the "Plans") of which approximately 1.5 million shares remain available for grant at December 31, 2016. The exercise price on all outstanding stock options and stock appreciation rights (“SARs”) is equal to the quoted fair market value of the stock at the date of grant. Restricted stock units (“RSUs”) and performance stock units (“PSUs”) are valued at the market value of the underlying stock on the date of grant. Stock options, SARs, RSUs and PSUs are non-transferable other than on death and generally become exercisable over a five year period from date of grant. Stock options and SARs expire ten years from date of grant. SARs are only settled in shares of the Company’s stock. The issuance of shares pursuant
59
to the exercise of stock options and SARs and vesting of RSUs and PSUs are from the Company’s treasury stock.
Total pre-tax stock-based compensation expense recognized in the consolidated statements of comprehensive income was $8.4 million, $7.5 million and $9.3 million for the years ended December 31, 2016, 2015 and 2014, respectively. These amounts are included in selling and administrative expenses, and in 2016, 2015 and 2014, $0.7 million, $1.0 million and $3.9 million, respectively, of the total relates to acceleration of awards associated with the Company's restructuring as further described in Note 12. Tax related benefits of $3.1 million, $2.7 million and $3.4 million were also recognized for the years ended December 31, 2016, 2015 and 2014, respectively. Cash received from the exercise of stock options was $0.0 million, $0.2 million and $1.8 million for the years ended December 31, 2016, 2015 and 2014, respectively, and is reflected in cash flows from financing activities in the consolidated statements of cash flows.
The Company uses the Black-Scholes option pricing model to estimate the fair value of stock options and SARs at the date of grant. Use of a valuation model requires management to make certain assumptions with respect to select model inputs. Expected volatilities are based upon historical volatility of the Company’s stock over a period equal to the expected life of each stock option and SAR grant. The risk free interest rate is based on the stock option and SAR grant date for a traded U.S. Treasury bond with a maturity date closest to the expected life. The expected annual dividend yield is based on the Company's anticipated cash dividend payouts. The expected life represents the period of time that the stock options and SARs are expected to be outstanding based on a study of historical data of option holder exercise and termination behavior.
The following table illustrates the assumptions used in estimating fair value in the years ended December 31, 2016, 2015 and 2014:
2016 | 2015 | 2014 | |||||||||
Grant date fair value of stock options and SARs | $ | 8.61 | $ | 11.37 | $ | 13.40 | |||||
Expected stock price volatility | 26.88 | % | 25.96 | % | 34.85 | % | |||||
Risk-free interest rate | 1.45 | % | 1.49 | % | 1.53 | % | |||||
Expected annual dividend yield | 2.10 | % | 1.55 | % | 1.80 | % | |||||
Expected life of options & SARs (years) | 6.0 | 5.7 | 6.4 | ||||||||
The following table illustrates the stock option and SAR activity for the year ended December 31, 2016:
Number of Shares (in 000’s) | Weighted- Average Exercise Price | |||||
Outstanding at December 31, 2015 | 920 | $ | 43.47 | |||
Granted | 1,001 | $ | 39.99 | |||
Forfeited | (93 | ) | $ | 46.78 | ||
Exercised | (75 | ) | $ | 23.55 | ||
Outstanding at December 31, 2016 | 1,753 | $ | 42.16 | |||
Exercisable at December 31, 2016 | 340 | $ | 37.96 | |||
Stock options & SARs expected to vest | 1,414 | $ | 43.17 | |||
The weighted average remaining contractual term for SARs and stock options outstanding and exercisable at December 31, 2016 was 8.0 years and 4.8 years, respectively. The aggregate intrinsic value of SARs and stock options outstanding and exercisable at December 31, 2016 was $7.5 million and $3.2 million, respectively. The aggregate intrinsic value of stock options and SARs exercised during the years ended December 31, 2016, 2015 and 2014 was $1.4 million, $2.8 million and $10.7 million, respectively.
The following table illustrates the RSU and PSU activity for the year ended December 31, 2016:
60
Number of Shares (in 000’s) | Weighted- Average Grant-Date Fair Value | |||||
Outstanding at December 31, 2015 | 353 | $ | 41.23 | |||
Granted | 83 | $ | 40.27 | |||
Vested | (110 | ) | $ | 43.16 | ||
Forfeited | (29 | ) | $ | 39.62 | ||
Outstanding at December 31, 2016 | 297 | $ | 41.01 | |||
The weighted average fair value of awards of RSUs and PSUs granted in the years ended December 31, 2016, 2015 and 2014 was $40.27, $45.75 and $43.21, respectively.
The total fair value of shares vested was $4.7 million, $6.0 million and $11.6 million for the years ended December 31, 2016, 2015 and 2014, respectively.
As of December 31, 2016, there was $18.8 million of total unrecognized compensation cost related to nonvested stock options, SARs, RSUs and PSUs granted under the Plans which is expected to be recognized over a weighted average period of 3.2 years.
We offer to our employees a shareholder-approved Employee Stock Purchase Plan (the “Employee Plan”), under which we have reserved 1.0 million shares of common stock for issuance to our employees. The Employee Plan provides employees with the opportunity to invest from 1% to 10% of their annual salary to purchase shares of CONMED common stock at a purchase price equal to 95% of the fair market value of the common stock on the exercise date. During 2016, we issued approximately 19,300 shares of common stock under the Employee Plan. No stock-based compensation expense has been recognized in the accompanying consolidated financial statements as a result of common stock issuances under the Employee Plan.
Note 9 — Business Segments and Geographic Areas
We are accounting and reporting for our business as a single operating segment entity engaged in the development, manufacturing and sale on a global basis of surgical devices and related equipment. Our chief operating decision maker (the executive management team) evaluates the various global product portfolios on a net sales basis and evaluates profitability, investment and cash flow metrics on a consolidated worldwide basis due to shared infrastructure and resources.
Our product lines consist of orthopedic surgery, general surgery and surgical visualization. Orthopedic surgery consists of sports medicine instrumentation and small bone, large bone and specialty powered surgical instruments and service fees related to the promotion and marketing of sports medicine allograft tissue. General surgery consists of a complete line of endo-mechanical instrumentation for minimally invasive laparoscopic and gastrointestinal procedures, a line of cardiac monitoring products as well as electrosurgical generators and related instruments. Surgical visualization consists of imaging systems for use in minimally invasive orthopedic and general surgery procedures including 2DHD and 3DHD vision technologies. These product lines' net sales are as follows:
2016 | 2015 | 2014 | |||||||||
Orthopedic surgery | $ | 370,472 | $ | 388,948 | $ | 402,750 | |||||
General surgery | 341,417 | 274,190 | 279,356 | ||||||||
Surgical visualization | 51,631 | 56,030 | 57,949 | ||||||||
Consolidated net sales | $ | 763,520 | $ | 719,168 | $ | 740,055 | |||||
Net sales information for geographic areas consists of the following:
61
2016 | 2015 | 2014 | |||||||||
United States | $ | 399,107 | $ | 361,452 | $ | 360,960 | |||||
Americas (excluding the United States) | 87,532 | 86,867 | 94,770 | ||||||||
Europe, Middle East & Africa | 147,985 | 145,565 | 158,040 | ||||||||
Asia Pacific | 128,896 | 125,284 | 126,285 | ||||||||
Total | $ | 763,520 | $ | 719,168 | $ | 740,055 | |||||
Sales are attributed to countries based on the location of the customer. There were no significant investments in long-lived assets located outside the United States at December 31, 2016 and 2015. No single customer represented over 10% of our consolidated net sales for the years ended December 31, 2016, 2015 and 2014.
Note 10 — Employee Benefit Plans
We sponsor an employee savings plan (“401(k) plan”) covering substantially all of our United States based employees. We also sponsor a defined benefit pension plan (the “pension plan”) that was frozen in 2009. It covered substantially all our United States based employees at the time it was frozen.
Total employer contributions to the 401(k) plan were $7.1 million, $7.6 million and $6.9 million during the years ended December 31, 2016, 2015 and 2014, respectively.
We use a December 31, measurement date for our pension plan. Gains and losses are amortized on a straight-line basis over the average remaining service period of active participants.
The following table provides a reconciliation of the projected benefit obligation, plan assets and funded status of the pension plan at December 31:
2016 | 2015 | ||||||
Accumulated Benefit Obligation | $ | 82,005 | $ | 78,437 | |||
Change in benefit obligation | |||||||
Projected benefit obligation at beginning of year | $ | 78,437 | $ | 91,107 | |||
Service cost | 452 | 240 | |||||
Interest cost | 2,878 | 3,394 | |||||
Actuarial (gain) loss | 4,844 | (11,806 | ) | ||||
Benefits paid | (1,814 | ) | (1,620 | ) | |||
Settlement | (2,792 | ) | (2,878 | ) | |||
Projected benefit obligation at end of year | $ | 82,005 | $ | 78,437 | |||
Change in plan assets | |||||||
Fair value of plan assets at beginning of year | $ | 67,168 | $ | 73,431 | |||
Actual gain (loss) on plan assets | 6,499 | (1,765 | ) | ||||
Benefits paid | (1,814 | ) | (1,620 | ) | |||
Settlement | (2,792 | ) | (2,878 | ) | |||
Fair value of plan assets at end of year | $ | 69,061 | $ | 67,168 | |||
Funded status | $ | (12,944 | ) | $ | (11,269 | ) | |
Amounts recognized in the consolidated balance sheets consist of the following at December 31,:
62
2016 | 2015 | ||||||
Other long-term liabilities | $ | (12,944 | ) | $ | (11,269 | ) | |
Accumulated other comprehensive loss | (41,960 | ) | (41,205 | ) | |||
The following actuarial assumptions were used to determine our accumulated and projected benefit obligations as of December 31,:
2016 | 2015 | ||||
Discount rate | 4.28 | % | 4.54 | % | |
Accumulated other comprehensive loss for the years ended December 31, 2016 and 2015 consists of net actuarial losses of $41,960 and $41,205, respectively, not yet recognized in net periodic pension cost (before income taxes).
Other changes in plan assets and benefit obligations recognized in other comprehensive income in 2016 are as follows:
Current year actuarial loss | $ | (3,535 | ) |
Amortization of actuarial loss | 2,780 | ||
Total recognized in other comprehensive loss | $ | (755 | ) |
The estimated portion of net actuarial loss in accumulated other comprehensive loss that is expected to be recognized as a component of net periodic pension cost in 2017 is $2.8 million.
Net periodic pension cost for the years ended December 31, consists of the following:
2016 | 2015 | 2014 | |||||||||
Service cost | $ | 452 | $ | 240 | $ | 271 | |||||
Interest cost on projected benefit obligation | 2,878 | 3,394 | 3,465 | ||||||||
Expected return on plan assets | (5,189 | ) | (5,697 | ) | (2,297 | ) | |||||
Amortization of loss | 2,780 | 3,233 | (2,048 | ) | |||||||
Net periodic pension (income) cost | $ | 921 | $ | 1,170 | $ | (609 | ) | ||||
The following actuarial assumptions were used to determine our net periodic pension benefit cost for the years ended December 31,:
2016 | 2015 | 2014 | ||||||
Discount rate on benefit obligation | 4.54 | % | 3.81 | % | 4.75 | % | ||
Effective rate for interest on benefit obligation | 3.77 | % | 3.81 | % | 4.75 | % | ||
Expected return on plan assets | 8.00 | % | 8.00 | % | 8.00 | % | ||
In 2016, we changed the method we used to estimate the interest cost component of net periodic pension cost. Historically, we estimated the interest cost component using a single weighted-average discount rate derived from the yield curve used to measure the benefit obligation at the beginning of the period. We have elected to use a full yield curve approach in the estimation of this component of benefit cost by applying the specific spot rates along the yield curve used in the determination of the benefit obligation that correlate to the relevant projected cash flows ("spot rate approach"). This change provides a more precise measurement of interest cost. This change did not affect the measurement of our total benefit obligation. We have accounted for this change as a change in estimate and therefore accounted for it prospectively in 2016.
63
In determining the expected return on pension plan assets, we consider the relative weighting of plan assets, the historical performance of total plan assets and individual asset classes and economic and other indicators of future performance. In addition, we consult with financial and investment management professionals in developing appropriate targeted rates of return.
Asset management objectives include maintaining an adequate level of diversification to reduce interest rate and market risk and providing adequate liquidity to meet immediate and future benefit payment requirements.
The allocation of pension plan assets by category is as follows at December 31,:
Percentage of Pension Plan Assets | Target Allocation | |||||||
2016 | 2015 | 2017 | ||||||
Equity securities | 86 | % | 86 | % | 75 | % | ||
Debt securities | 14 | 14 | 25 | |||||
Total | 100 | % | 100 | % | 100 | % | ||
As of December 31, 2016, the Plan held 27,562 shares of our common stock, which had a fair value of $1.2 million. We believe that our long-term asset allocation on average will approximate the targeted allocation. We regularly review our actual asset allocation and periodically rebalance the pension plan’s investments to our targeted allocation when deemed appropriate.
FASB guidance defines fair value and establishes a framework for measuring fair value and related disclosure requirements. A valuation hierarchy was established for disclosure of the inputs to the valuations used to measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable for the asset or liability, including interest rates, yield curves and credit risks, or inputs that are derived principally from, or corroborated by, observable market data through correlation. Level 3 inputs are unobservable inputs based on our own assumptions used to measure assets and liabilities at fair value. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
Following is a description of the valuation methodologies used for assets measured at fair value. There have been no changes in the methodologies used at December 31, 2016 and 2015:
Common Stock: | Common stock is valued at the closing price reported on the common stock’s respective stock exchange and is classified within level 1 of the valuation hierarchy. |
Money Market Fund: | These investments are public investment vehicles valued using $1 for the Net Asset Value (NAV). The money market fund is classified within level 2 of the valuation hierarchy. |
Mutual Funds: | These investments are public investment vehicles valued using the NAV provided by the administrator of the fund. The NAV is based on the value of the underlying assets owned by the fund, minus its liabilities, and then divided by the number of shares outstanding. The NAV is a quoted price in an active market and is classified within level 1 of the valuation hierarchy. |
Fixed Income Securities: | Valued at the closing price reported on the active market on which the individual securities are traded and are classified within level 1 of the valuation hierarchy. |
The methods described above may produce a fair value calculation that may not be indicative of net realizable value or reflective of future fair values. Furthermore, while the pension plan believes its valuation methods are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different fair value measurement at the reporting date.
The following table sets forth by level, within the fair value hierarchy, the pension plan's assets at fair value as of December 31, 2016 and December 31, 2015:
64
December 31, 2016 | Level 1 | Level 2 | Total | ||||||||
Common Stock | $ | 34,856 | $ | — | $ | 34,856 | |||||
Money Market Fund | — | 1,710 | 1,710 | ||||||||
Mutual Funds | 24,626 | — | 24,626 | ||||||||
Fixed Income Securities | 7,869 | — | 7,869 | ||||||||
$ | 67,351 | $ | 1,710 | $ | 69,061 | ||||||
December 31, 2015 | Level 1 | Level 2 | Total | ||||||||
Common Stock | $ | 34,466 | $ | — | $ | 34,466 | |||||
Money Market Fund | — | 1,302 | 1,302 | ||||||||
Mutual Funds | 23,576 | — | 23,576 | ||||||||
Fixed Income Securities | 7,824 | — | 7,824 | ||||||||
$ | 65,866 | $ | 1,302 | $ | 67,168 | ||||||
We do not expect to make any contributions to our pension plan for 2017.
The following table summarizes the benefits and settlements expected to be paid by our pension plan in each of the next five years and in aggregate for the following five years. The expected payments are estimated based on the same assumptions used to measure the Company’s projected benefit obligation at December 31, 2016 and reflect the impact of expected future employee service.
2017 | $5,033 | ||
2018 | 5,368 | ||
2019 | 5,465 | ||
2020 | 5,696 | ||
2021 | 5,545 | ||
2022-2026 | 25,194 | ||
Note 11 — Legal Matters and Contingencies
From time to time, we are subject to claims alleging product liability, patent infringement or other claims incurred in the ordinary course of business. These may involve our United States or international operations, or sales by foreign distributors. Likewise, from time to time, the Company may receive an informal information request or subpoena from a government agency such as the Securities and Exchange Commission, Department of Justice, Equal Employment Opportunity Commission, the Occupational Safety and Health Administration, the Department of Labor, the Treasury Department or other federal and state agencies or foreign governments or government agencies. These information requests or subpoenas may or may not be routine inquiries, or may begin as informal or routine inquiries and over time develop into enforcement actions of various types. Likewise, we receive reports of alleged misconduct from employees and third parties, which we investigate as appropriate.
Manufacturers of medical devices have been the subject of various enforcement actions relating to interactions with health care providers domestically or internationally whereby Companies are claimed to have provided health care providers inappropriate incentives to purchase their products. Similarly, the Foreign Corrupt Practices Act (“FCPA”) imposes obligations on manufacturers with respect to interactions with health care providers who may be considered government officials if they are affiliated with public hospitals. The FCPA also requires publicly listed manufacturers to maintain accurate books and records, and maintain internal accounting controls sufficient to provide assurance that transactions are accurately recorded, lawful and in accordance with management’s authorization. The FCPA poses unique challenges both because manufacturers operate in foreign cultures in which conduct illegal under the FCPA may not be illegal in local jurisdictions, and because, in some cases, a United States manufacturer may face risks under the FCPA based on the conduct of third parties over whom the manufacturer may not have complete control.
Manufacturers of medical products may face exposure to significant product liability claims. To date, we have not experienced any product liability claims that have been material to our financial statements or financial condition, but any such
65
claims arising in the future could have a material adverse effect on our business or results of operations. We currently maintain commercial product liability insurance of $25 million per incident and $25 million in the aggregate annually, which we believe is adequate. This coverage is on a claims-made basis. There can be no assurance that claims will not exceed insurance coverage, that the carriers will be solvent or that such insurance will be available to us in the future at a reasonable cost.
We establish reserves sufficient to cover probable losses associated with any such pending claims. We do not expect that the resolution of any pending claims, investigations or reports of alleged misconduct will have a material adverse effect on our financial condition, results of operations or cash flows. There can be no assurance, however, that future claims or investigations, or the costs associated with responding to such claims, investigations or reports of misconduct, especially claims and investigations not covered by insurance, will not have a material adverse effect on our financial condition, results of operations or cash flows.
Our operations are subject, and in the past have been subject, to a number of environmental laws and regulations governing, among other things, air emissions; wastewater discharges; the use, handling and disposal of hazardous substances and wastes; soil and groundwater remediation and employee health and safety. In some jurisdictions, environmental requirements may be expected to become more stringent in the future. In the United States, certain environmental laws can impose liability for the entire cost of site restoration upon each of the parties that may have contributed to conditions at the site regardless of fault or the lawfulness of the party’s activities. While we do not believe that the present costs of environmental compliance and remediation are material, there can be no assurance that future compliance or remedial obligations would not have a material adverse effect on our financial condition, results of operations or cash flows.
The FDA inspected our Centennial, Colorado facility in 2013 and 2014 and ultimately issued a Warning Letter on January 30, 2014. Accordingly, we took corrective action and, on August 1, 2016, we received notification from the FDA that the Warning Letter was closed. The costs of remediation relating to the January 30, 2014 warning letter were not material to our consolidated results of operations. We may have future inspections at other sites and there can be no assurance that the costs of responding to such inspections will not be material.
In September 2013, Lexion Medical ("Lexion") filed suit against SurgiQuest in federal court in the District of Minnesota alleging false advertising under the Lanham Act, as well as various state law claims, including common law trade libel and unfair competition. In March 2014, SurgiQuest’s motion to dismiss for lack of personal jurisdiction was granted and that same day, SurgiQuest filed suit against Lexion in federal court in the District of Delaware seeking, among other claims, a declaratory judgment that SurgiQuest’s actions did not violate the Lanham Act. Lexion filed an answer generally denying SurgiQuest’s claims, and asserted counterclaims that were substantially similar to the claims Lexion brought in the Minnesota action. On January 4, 2016, SurgiQuest became a subsidiary of CONMED as further described in Note 2, and we assumed the costs and liabilities related to the Lexion lawsuit subject to the terms of the merger agreement referenced in Note 2. Discovery is now largely complete, and the case is currently scheduled to go to trial on April 3, 2017. Based on its expert's reports, Lexion is seeking damages of $14.8 million for alleged lost profits and $18.7 million for costs related to alleged “corrective advertising” as well as an unspecified sum for disgorgement of SurgiQuest’s alleged profit. We believe that there is no factual and/or legal merit to Lexion’s claims against SurgiQuest, and intend to vigorously defend the claims asserted by Lexion.
In 2014, the Company acquired EndoDynamix, Inc. The agreements governing the terms of the acquisition provide that, if various conditions are met, certain contingent payments relating to the first commercial sale of the products (the milestone payment), as well as royalties based on sales (the revenue based payments), are due to the seller. We have notified the seller that there is a need to redesign the product, and that as a consequence, the first commercial sale has been delayed. Consequently, the payment of contingent milestone and revenue-based payments have been delayed. On January 18, 2017, the seller provided notice ("the Notice") seeking $12.7 million, which essentially represents the sum of the projected contingent milestone and revenue-based payments on an accelerated basis. CONMED responded to the Notice denying that there was any basis for acceleration of the payments due under the acquisition agreements. On February 22, 2017, the representative of the former shareholders of EndoDynamix filed a complaint in Delaware Chancery Court claiming breach of contract and seeking the contingent payments on an accelerated basis. We do not believe that there is a legitimate basis for seeking the acceleration of the contingent payments, and expect to defend the claims asserted by the sellers of EndoDynamix in the Delaware Court.
Note 12 — Acquisition, Restructuring and Other Expense
Acquisition, restructuring and other expense for the year ended December 31, consists of the following:
66
2016 | 2015 | 2014 | |||||||||
Consolidation costs | $ | 3,066 | $ | 8,016 | $ | 5,612 | |||||
Termination of a product offering | 4,546 | — | — | ||||||||
Restructuring costs included in cost of sales | $ | 7,612 | $ | 8,016 | $ | 5,612 | |||||
Restructuring costs | $ | 6,873 | $ | 13,655 | $ | 3,354 | |||||
Business and asset acquisition costs | 20,599 | 2,543 | 722 | ||||||||
Gain on sale of facility | (1,890 | ) | — | — | |||||||
Management restructuring costs | — | — | 12,546 | ||||||||
Shareholder activism costs | — | — | 3,966 | ||||||||
Patent dispute and other matters | — | — | 3,374 | ||||||||
Acquisition, restructuring and other expense included in selling and administrative expense | $ | 25,582 | $ | 16,198 | $ | 23,962 | |||||
Debt refinancing costs included in other expense | $ | 2,942 | $ | — | $ | — | |||||
During 2016, we incurred $20.6 million in costs associated with the January 4, 2016 acquisition of SurgiQuest, Inc. as further described in Note 2. These costs include investment banking fees, consulting fees, legal fees associated with the acquisition as well as the Lexion case as further described in Note 11, costs associated with expensing of unvested options acquired and integration related costs. During 2015, we incurred $2.5 million in costs associated with the acquisition of SurgiQuest and other acquisitions during the year. During 2014, we incurred $0.7 million in costs associated with the purchase of a business.
During 2014, we incurred $4.0 million in professional fees related to shareholder activism.
During 2014, we incurred $3.4 million, in legal and settlement costs. The 2014 patent infringement claim costs totaled $1.9 million, including $0.9 million in settlement costs during the first quarter of 2014. The remaining $1.5 million in 2014 legal costs were associated with a legal matter in which we prevailed at trial, and consulting fees.
During 2016, we incurred a $2.7 million charge related to commitment fees paid to certain of our lenders, which provided a financing commitment for the SurgiQuest acquisition and recorded a loss on the early extinguishment of debt of $0.3 million in conjunction with the fifth amended and restated senior credit agreement as further described in Note 6.
During 2016, 2015 and 2014, we continued our operational restructuring plan. The consolidation of our Centennial, Colorado manufacturing operations into other existing CONMED manufacturing facilities is complete. During 2014, we completed the consolidation of our Finland operations into our Largo, Florida and Utica, New York manufacturing facilities and the consolidation of our Westborough, Massachusetts manufacturing operations into our Largo, Florida and Chihuahua, Mexico facilities. We incurred $3.1 million, $8.0 million and $5.6 million in costs associated with the operational restructuring during the years ending December 31, 2016, 2015 and 2014, respectively. These costs were charged to cost of sales and include severance and other charges associated with the consolidation of operations.
During 2016, the Company discontinued our Altrus product offering as part of our ongoing restructuring and incurred $4.5 million in non-cash charges primarily related to inventory and fixed assets which were included in cost of sales during 2016.
During 2016, we sold our Centennial, Colorado facility. We received net cash proceeds of $5.2 million and recorded a gain of $1.9 million on the sale.
During 2016, 2015 and 2014, we restructured certain selling and administrative functions and incurred $6.9 million, $13.7 million and $3.4 million, respectively, in related costs consisting principally of severance charges.
During 2014, we incurred $12.5 million in costs associated with restructuring of executive management. These costs include severance payments, accelerated vesting of stock-based compensation awards, accrual of the present value of deferred compensation and other benefits to our then Chief Executive Officer as defined in his termination agreement; accelerated vesting of stock-based compensation awards to certain members of executive management, consulting fees and other benefits earned as further described in our Form 8-K filing on July 23, 2014.
67
We have recorded an accrual in current and other long term liabilities of $2.6 million at December 31, 2016 mainly related to severance costs associated with restructuring. Below is a rollforward of the costs incurred and cash expenditures associated with these activities during 2016, 2015 and 2014:
Balance as of January 1, 2014 | $ | 3,128 | ||
Expenses incurred | 21,512 | |||
Payments made | (16,386 | ) | ||
Balance as of December 31, 2014 | 8,254 | |||
Expenses incurred | 21,671 | |||
Payments made | (22,750 | ) | ||
Balance as of December 31, 2015 | 7,175 | |||
Expenses incurred | 9,939 | |||
Payments made | (14,471 | ) | ||
Balance as of December 31, 2016 | $ | 2,643 | ||
A significant portion of this accrual will be paid out in 2017.
Note 13 — Guarantees
We provide warranties on certain of our products at the time of sale and sell extended warranties. The standard warranty period for our capital and reusable equipment is generally one year and our extended warranties can vary in length. Liability under service and warranty policies is based upon a review of historical warranty and service claim experience. Adjustments are made to accruals as claim data and historical experience warrant.
Changes in the carrying amount of service and product warranties for the year ended December 31, are as follows:
2016 | 2015 | 2014 | |||||||||
Balance as of January 1, | $ | 2,509 | $ | 2,286 | $ | 2,422 | |||||
Provision for warranties | 2,967 | 3,836 | 3,492 | ||||||||
Claims made | (3,522 | ) | (3,613 | ) | (3,628 | ) | |||||
Balance as of December 31, | $ | 1,954 | $ | 2,509 | $ | 2,286 | |||||
Note 14 – Fair Value Measurement
We enter into derivative instruments for risk management purposes only. We operate internationally and, in the normal course of business, are exposed to fluctuations in interest rates, foreign exchange rates and commodity prices. These fluctuations can increase the costs of financing, investing and operating the business. We use forward contracts, a type of derivative instrument, to manage certain foreign currency exposures.
68
By nature, all financial instruments involve market and credit risks. We enter into forward contracts with major investment grade financial institutions and have policies to monitor the credit risk of those counterparties. While there can be no assurance, we do not anticipate any material non-performance by any of these counterparties.
Foreign Currency Forward Contracts. We hedge forecasted intercompany sales denominated in foreign currencies through the use of forward contracts. The notional contract amounts for forward contracts outstanding at December 31, 2016 which have been accounted for as cash flow hedges totaled $108.1 million. Net realized gains recognized for forward contracts accounted for as cash flow hedges approximated $1.2 million, $10.4 million and $0.6 million for the years ended December 31, 2016, 2015 and 2014, respectively. Net unrealized gains on forward contracts outstanding which have been accounted for as cash flow hedges and which have been included in other comprehensive income totaled $1.5 million at December 31, 2016. It is expected these unrealized gains will be recognized in the consolidated statement of comprehensive income in 2017 and 2018.
We also enter into forward contracts to exchange foreign currencies for United States dollars in order to hedge our currency transaction exposures on intercompany receivables denominated in foreign currencies. The notional contract amounts for forward contracts outstanding at December 31, 2016 which have not been designated as hedges totaled $18.4 million. Net realized gains (losses) recognized in connection with those forward contracts not accounted for as hedges approximated $0.0 million, $0.4 million and -$0.2 million for the years ended December 31, 2016, 2015 and 2014, respectively, offsetting losses on our intercompany receivables of -$0.1 million, -$0.8 million and -$0.5 million for the years ended December 31, 2016, 2015 and 2014, respectively. These gains and losses have been recorded in selling and administrative expense in the consolidated statements of comprehensive income.
We record these forward foreign exchange contracts at fair value; the following tables summarize the fair value for forward foreign exchange contracts outstanding at December 31, 2016 and 2015:
December 31, 2016 | Asset Fair Value | Liabilities Fair Value | Net Fair Value | ||||||||
Derivatives designated as hedged instruments: | |||||||||||
Foreign exchange contracts | $ | 3,962 | $ | (1,510 | ) | $ | 2,452 | ||||
Derivatives not designated as hedging instruments: | |||||||||||
Foreign exchange contracts | 48 | (54 | ) | (6 | ) | ||||||
Total derivatives | $ | 4,010 | $ | (1,564 | ) | $ | 2,446 | ||||
December 31, 2015 | Asset Fair Value | Liabilities Fair Value | Net Fair Value | ||||||||
Derivatives designated as hedged instruments: | |||||||||||
Foreign exchange contracts | $ | 2,931 | $ | (1,026 | ) | $ | 1,905 | ||||
Derivatives not designated as hedging instruments: | |||||||||||
Foreign exchange contracts | 4 | (38 | ) | (34 | ) | ||||||
Total derivatives | $ | 2,935 | $ | (1,064 | ) | $ | 1,871 | ||||
Our forward foreign exchange contracts are subject to a master netting agreement and qualify for netting in the consolidated balance sheets. Accordingly, at December 31, 2016 and December 31, 2015 we have recorded the net fair value of $2.4 million and $1.9 million, respectively, in prepaids and other current assets.
Fair Value Disclosure. FASB guidance defines fair value and establishes a framework for measuring fair value and related disclosure requirements. This guidance applies when fair value measurements are required or permitted. The guidance indicates, among other things, that a fair value measurement assumes that the transaction to sell an asset or transfer a liability occurs in the principal market for the asset or liability or, in the absence of a principal market, the most advantageous market for the asset or liability. Fair value is defined based upon an exit price model.
69
Valuation Hierarchy. A valuation hierarchy was established for disclosure of the inputs to the valuations used to measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable for the asset or liability, including interest rates, yield curves and credit risks, or inputs that are derived principally from or corroborated by observable market data through correlation. Level 3 inputs are unobservable inputs based on our own assumptions used to measure assets and liabilities at fair value. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement. There have been no significant changes in the assumptions since the acquisition.
Valuation Techniques. Assets and liabilities carried at fair value and measured on a recurring basis as of December 31, 2016 consist of forward foreign exchange contracts and contingent liabilities associated with a business acquisition. The Company values its forward foreign exchange contracts using quoted prices for similar assets. The most significant assumption is quoted currency rates. The value of the forward foreign exchange contract assets and liabilities were valued using Level 2 inputs and are listed in the table above.
Certain acquisitions involve the potential for the payment of future contingent consideration upon the achievement of certain product development milestones and revenue based payments. Contingent consideration is recorded at the estimated fair value of the contingent milestone and revenue based payments on the acquisition date. The fair value of the contingent consideration is remeasured at the estimated fair value at each reporting period with the change in fair value recognized as income or expense within selling and administrative expenses in the consolidated statements of comprehensive income. We remeasure the liability on a recurring basis using Level 3 inputs as defined under authoritative guidance for fair value measurements.
The carrying amounts reported in our balance sheets for cash and cash equivalents, accounts receivable, accounts payable and long-term debt approximate fair value.
Note 15 - New Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update ("ASU") No. 2014-09, Revenue from Contracts with Customers. This ASU is a comprehensive new revenue recognition model that requires a company to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration the company expects to receive in exchange for those goods or services. In March, April and May 2016, the FASB issued ASU 2016-08 related to principal versus agent considerations; ASU 2016-10 related to identifying performance obligations and licensing; and ASU 2016-12 clarifying the guidance on assessing collectability, presenting sales taxes, measuring noncash consideration, and certain transition matters, respectively. These additional ASUs provide supplemental adoption guidance and clarification to ASU 2014-09. The guidance in these ASUs is effective for annual reporting periods beginning after December 15, 2017 and early adoption is permitted as of January 1, 2017. The standard allows the option of either a full retrospective adoption, meaning the standard is applied to all periods presented, or a modified retrospective adoption, meaning the standard is applied only to the most current period. The Company will adopt the new standard on January 1, 2018. The Company is currently evaluating the impact of adopting this new guidance on the consolidated financial statements, however we currently anticipate applying the modified retrospective approach.
In August 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. This ASU establishes specific guidance to an organization's management on their responsibility to evaluate whether there is substantial doubt about the organization's ability to continue as a going concern. The provisions of this ASU are effective for annual periods ending after December 15, 2016, and interim periods thereafter. We implemented this guidance in 2016 and it did not impact our financial statements or disclosures.
In July 2015, the FASB issued ASU No. 2015-11, Simplifying the Measurement of Inventory. An entity should measure inventory within the scope of this Update at the lower of cost and net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. Subsequent measurement is unchanged for inventory measured using LIFO or the retail inventory method. This ASU is effective for annual periods beginning after December 15, 2016. The Company does not believe this new guidance will have a material impact on the consolidated financial statements.
In August 2015, the FASB issued ASU No. 2015-15, Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements. This ASU was issued to clarify the guidance included in ASU 2015-03 "Simplifying the Presentation of Debt Issuance Costs", which requires entities to present debt issuance costs related to a recognized debt liability as a direct deduction from the carrying amount of that debt liability. ASU 2015-03 does not address presentation or subsequent
70
measurement of debt issuance costs related to line-of-credit arrangements. Given the absence of authoritative guidance within Update 2015-03 for debt issuance costs related to line-of-credit arrangements, ASU 2015-15 was issued to clarify that the SEC staff would not object to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. This ASU is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company adopted this guidance as of January 1, 2016, continuing to account for debt issuance costs related to the line-of-credit arrangement as an asset, and it did not have a material impact on our consolidated financial statements.
In September 2015, the FASB issued ASU No. 2015-16, Simplifying the Accounting for Measurement-Period Adjustments. This ASU simplifies the accounting for changes in measurement period adjustments associated with a business combination. It requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. This ASU is effective for annual periods beginning after December 15, 2015. The Company adopted this guidance as of January 1, 2016 and it did not have a material impact on our consolidated financial statements.
In November 2015, the FASB issued ASU No. 2015-17, Income Taxes (ASC 740): Balance Sheet Classification of Deferred Taxes. This ASU requires all deferred income tax assets and liabilities be presented as non-current in classified balance sheets. This can be applied prospectively or retrospectively and we must disclose the reason for the change in accounting principle, the application applied and if applied retrospectively, include quantitative information about the effects of the change on prior periods. This standard is effective for annual and interim periods beginning after December 15, 2016. The Company retrospectively implemented this new guidance in the first quarter of 2016. The table below summarizes the adjustments made to conform prior period classification with the new guidance:
December 31, 2015 | |||||||||||
As Previously Filed | Reclass | As Adjusted | |||||||||
Current deferred income tax assets | $ | 14,150 | $ | (14,150 | ) | $ | — | ||||
Long-term deferred income tax assets | 1,332 | 2,906 | 4,238 | ||||||||
Long-term deferred income tax liabilities | (114,623 | ) | 11,244 | (103,379 | ) | ||||||
$ | (99,141 | ) | $ | — | $ | (99,141 | ) | ||||
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). This ASU requires lessees to put most leases on their balance sheets but recognize the expenses on their income statements in a manner similar to current practice. It states that a lessee would recognize a lease liability for the obligation to make lease payments and a right-to-use asset for the right to use the underlying asset for the lease term. The new standard is effective for interim and annual periods beginning after December 15, 2018 and early adoption is permitted. The Company is currently evaluating the impact of of adopting this new guidance on the consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, Improvements to Employee Share-Based Payment Accounting. This ASU requires all tax effects to run through the statement of operations, where historically tax benefits in excess of compensation cost ran through equity. It also allows employers to withhold the maximum amount of individual tax withholdings without resulting in liability accounting. Finally, the ASU allows companies to make an accounting policy election regarding the impact of forfeitures on expense related to share based awards. This new guidance is effective for periods beginning after December 15, 2016, however early adoption is permitted. The Company is currently evaluating the impact of adopting this new guidance on the consolidated financial statements.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments (A Consensus of the FASB Emerging Issues Task Force). This ASU provides amendments to specific statement of cash flows classification issues. This new guidance is effective for periods beginning after December 15, 2017, however early adoption is permitted. The Company does not believe this new guidance will have a material impact on the consolidated financial statements.
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash. The amendments in this Update require that a statement a of cash flows explain the change during the period in the total cash, cash equivalents and amounts generally described as restricted cash or restricted cash equivalents. The ASU is effective for periods
71
beginning after December 15, 2017, however early adoption is permitted. The Company is currently assessing the impact of this guidance on our consolidated financial statements.
In January 2017, the FASB issued ASU 2017-01, Clarifying the Definition of a Business. This ASU states when substantially all of the fair value of gross assets acquired is concentrated in a single asset (or a group of similar assets), the assets acquired would not represent a business. In addition, this guidance states in order to be a business, an input and a substantive process must significantly contribute to the ability to produce outputs. This new guidance is effective for periods beginning after December 15, 2017, however early adoption is permitted. The Company is currently assessing the impact of this guidance on our consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04, Intangibles - Goodwill and Other (Topic 350): Simplifying the Accounting for Goodwill Impairment. This ASU removes Step 2 of the goodwill impairment test, which requires hypothetical purchase price allocation. A goodwill impairment will now be the amount by which the reporting unit's carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. The Company is currently assessing the impact of this guidance on our consolidated financial statements.
Note 16 — Selected Quarterly Financial Data (Unaudited)
Selected quarterly financial data for 2016 and 2015 are as follows:
Three Months Ended | |||||||||||||||
March | June | September | December | ||||||||||||
2016 | |||||||||||||||
Net sales | $ | 181,201 | $ | 193,433 | $ | 184,792 | $ | 204,094 | |||||||
Gross profit | 97,740 | 102,422 | 101,209 | 106,959 | |||||||||||
Net income (loss) | (2,265 | ) | 2,884 | 7,337 | 6,708 | ||||||||||
EPS: | |||||||||||||||
Basic | $ | (.08 | ) | $ | .10 | $ | .26 | $ | .24 | ||||||
Diluted | (.08 | ) | .10 | .26 | .24 | ||||||||||
Three Months Ended | |||||||||||||||
March | June | September | December | ||||||||||||
2015 | |||||||||||||||
Net sales | $ | 177,940 | $ | 181,027 | $ | 169,184 | $ | 191,017 | |||||||
Gross profit | 92,282 | 93,498 | 93,546 | 102,376 | |||||||||||
Net income | 6,312 | 7,461 | 8,873 | 7,852 | |||||||||||
EPS: | |||||||||||||||
Basic | $ | .23 | $ | .27 | $ | .32 | $ | .28 | |||||||
Diluted | .23 | .27 | .32 | .28 | |||||||||||
Items Included In Selected Quarterly Financial Data:
2016
First Quarter
During the first quarter of 2016, we incurred $0.9 million in costs associated with the consolidation of our Centennial, Colorado manufacturing operations into other existing CONMED manufacturing facilities. These costs were charged to cost of sales and include severance and other charges associated with the consolidation – see Note 12.
During the first quarter of 2016, we incurred $9.0 million in costs associated with the January 4, 2016 acquisition of SurgiQuest, Inc. These costs include investment banking fees, consulting fees, legal fees associated with the acquisition as well as the Lexion case as further described in Note 11, costs associated with expensing of unvested options acquired and integration related costs and were charged to selling and administrative expense - see Note 2 and 12.
72
During the first quarter of 2016, we incurred a $2.7 million charge related to commitment fees paid to certain of our lenders, which provided a financing commitment for the SurgiQuest acquisition and recorded a loss on the early extinguishment of debt of $0.3 million in conjunction with the fifth amended and restated senior credit agreement. These costs were charged to other expense - see Note 6 and 12.
During the first quarter of 2016, we recorded a charge of $2.8 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
Second Quarter
During the second quarter of 2016, we incurred $0.1 million in costs associated with the consolidation of our Centennial, Colorado manufacturing operations into other existing CONMED manufacturing facilities. These costs were charged to cost of sales and include severance and other charges associated with the consolidation – see Note 12.
During the second quarter of 2016, the Company discontinued our Altrus product offering as part of our ongoing restructuring and incurred $4.5 million in non-cash charges which were included in cost of sales - see Note 12.
During the second quarter of 2016, we incurred $5.0 million in costs associated with the acquisition of SurgiQuest, Inc. which include consulting fees, legal fees associated with the acquisition as well as the Lexion case as further described in Note 11, costs associated with expensing of unvested options acquired and integration related costs and were charged to selling and administrative expense - see Note 2 and 12.
During the second quarter of 2016, we recorded a charge of $1.0 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
Third Quarter
During the third quarter of 2016, we incurred $3.3 million in costs associated with the acquisition of SurgiQuest, Inc. which include consulting fees, legal fees associated with the Lexion case as further described in Note 11, costs associated with expensing of unvested options acquired and integration related costs and were charged to selling and administrative expense - see Note 2 and 12.
During the third quarter of 2016, we sold our Centennial, Colorado facility. We received net cash proceeds of $5.2 million and recorded a gain of $1.9 million on the sale of our facility in selling and administrative expense - see Note 12.
During the third quarter of 2016, we recorded a charge of $0.4 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
Fourth Quarter
During the fourth quarter of 2016, we incurred $2.1 million in severance and other related costs associated with restructuring. These costs were charged to cost of sales - see Note 12.
During the fourth quarter of 2016, we incurred $3.2 million in costs associated with the acquisition of SurgiQuest, Inc. which include consulting fees, legal fees associated with the Lexion case as further described in Note 11, costs associated with expensing of unvested options acquired and integration related costs and were charged to selling and administrative expense - see Note 2 and Note 12.
During the fourth quarter of 2016, we recorded a charge of $2.8 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
2015
First Quarter
73
During the first quarter of 2015, we incurred $2.3 million in costs associated with the consolidation of our Centennial, Colorado manufacturing operations into other existing CONMED manufacturing facilities. These costs were charged to cost of sales and include severance and other charges associated with the consolidation – see Note 12.
During the first quarter of 2015, we recorded a charge of $6.2 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
Second Quarter
During the second quarter of 2015, we incurred $1.5 million in costs associated with the consolidation of our Centennial, Colorado manufacturing operations into other existing CONMED manufacturing facilities. These costs were charged to cost of sales and include severance and other charges associated with the consolidation – see Note 12.
During the second quarter of 2015, we recorded a charge of $2.2 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
Third Quarter
During the third quarter of 2015, we incurred $1.3 million in costs associated with the consolidation of our Centennial, Colorado manufacturing operations into our other existing CONMED manufacturing facilities. These costs were charged to cost of sales and include severance and other charges associated with the consolidation – see Note 12.
During the third quarter of 2015, we recorded a charge of $1.1 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
Fourth Quarter
During the fourth quarter of 2015, we incurred $2.8 million in costs associated with the consolidation of our Centennial, Colorado manufacturing operations into our other existing CONMED manufacturing facilities. These costs were charged to cost of sales and include severance and other charges associated with the consolidation – see Note 12.
During the fourth quarter of 2015, we recorded a charge of $4.3 million to selling and administrative expense related to the restructuring of certain selling and administrative functions which includes severance costs and other related costs - see Note 12.
During the fourth quarter of 2015, we recorded a charge of $2.1 million to selling and administrative expense associated with the purchase of SurgiQuest, Inc and other acquisitions - see Note 2 and Note 12.
74
SCHEDULE II—Valuation and Qualifying Accounts
(In thousands)
Additions | ||||||||||||||||
Balance at Beginning of Period | Charged to Costs and Expenses | |||||||||||||||
Balance at End of Period | ||||||||||||||||
Description | Deductions | |||||||||||||||
2016 | ||||||||||||||||
Allowance for bad debts | $ | 1,336 | $ | 983 | $ | (288 | ) | $ | 2,031 | |||||||
Sales returns and | ||||||||||||||||
allowance | 3,417 | 254 | (315 | ) | 3,356 | |||||||||||
Deferred tax asset | ||||||||||||||||
valuation allowance | 124 | 317 | — | 441 | ||||||||||||
2015 | ||||||||||||||||
Allowance for bad debts | $ | 1,239 | $ | 493 | $ | (396 | ) | $ | 1,336 | |||||||
Sales returns and | ||||||||||||||||
allowance | 3,081 | 521 | (185 | ) | 3,417 | |||||||||||
Deferred tax asset | ||||||||||||||||
valuation allowance | 293 | — | (169 | ) | 124 | |||||||||||
2014 | ||||||||||||||||
Allowance for bad debts | $ | 1,384 | $ | 517 | $ | (662 | ) | $ | 1,239 | |||||||
Sales returns and | ||||||||||||||||
allowance | 3,098 | 252 | (269 | ) | 3,081 | |||||||||||
Deferred tax asset | ||||||||||||||||
valuation allowance | — | 293 | — | 293 | ||||||||||||
Item 16. Form 10-K Summary
Registrants may voluntarily provide a summary of information required by Form 10-K under this Item 16. The Company has elected not to include such summary information.
75
