Attached files
| file | filename |
|---|---|
| 8-K - 8-K JP MORGAN PRESENTATION - HALOZYME THERAPEUTICS, INC. | a8-kjpmorganpresentation.htm |

35th Annual J.P. Morgan Healthcare Conference
Building a Premier Oncology Biotech:
Two Pillar Strategy for Growth
Dr. Helen Torley
President & CEO
January 9, 2017

Forward-Looking Statements
All of the statements in this presentation that are not statements of historical
facts constitute forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995. Examples of such statements include
possible activity, benefits and attributes of PEGPH20, future product
development and regulatory events and goals, anticipated clinical trial
results and strategies, product collaborations, our business intentions and
financial estimates and results, including projected revenue amounts. These
statements are based upon management’s current plans and expectations
and are subject to a number of risks and uncertainties which could cause
actual results to differ materially from such statements. A discussion of the risks
and uncertainties that can affect these statements is set forth in the
Company’s annual and quarterly reports filed from time to time with the
Securities and Exchange Commission under the heading “Risk Factors.” The
Company disclaims any intention or obligation to revise or update any
forward-looking statements, whether as a result of new information, future
events, or otherwise.
1

Two-Pillar Strategy for Growth
Oncology Pipeline ENHANZE™ Platform
6 Global Licensing &
Collaboration Agreements
3 launched products generating
growing revenues from single-
digit royalties
1 program with FDA June action
date; 2 programs in active
clinical development
$700M in potential cumulative
milestone payments1
PEGPH202: phase 3 asset with HA-
High population of ~75,0003 in
solid tumors studied
Phase 2 Study 202 topline data
readout
Emerging applications for
PEGPH20 in immuno-oncology
2 novel preclinical assets focused
on the tumor microenvironment
2
1 Assumes all developmental and commercial milestones achieved and paid to Halozyme for Herceptin SC, Mabthera SC, HYQVIA, Daratumumab SC
and 4 non-disclosed targets.
2 PEGPH20 is an investigational drug; safety and efficacy profiles have not been established, nor is it available for commercial distribution.
3 Estimated addressable patients in U.S., EU5 based on annual Incidence of 1L Metastatic Pancreatic Cancer, Advanced Non-Small Cell Lung Cancer, 2L
Metastatic Gastric Cancer, 2L Stage IV Breast Cancer (HER2-), SEER 18 2006-2012, Globocan 2012, Medscape; and Halozyme estimates for HA-HIGH %.

Significant Milestones Achieved in 2016
3
✓
ENHANZE Platform
Royalties grew 65% versus
2015
Rituximab SC BLA filed in
the U.S.
Darzalex SC progressing
toward Phase 3 study
Roche initiates study with
Perjeta
✓
✓
✓
Oncology Pipeline
Dosed first patient, initiated
~85% of sites in HALO-301 Study
Initiated dose expansion in
Keytruda trial
Initiated HALO-Eisai Study in
Breast Cancer Patients
Signed Roche I-O/PEGPH20
clinical collaboration
Expanded pipeline with novel
preclinical assets
✓
✓
✓
✓
✓

PEGPH20 Goal: Improve Targeting of
Co-Administered Cancer Therapies

PEGPH20 Targets Hyaluronan (HA) in the Tumor
Microenvironment
PEGPH20
In HA-High Tumor Animal Models, Removal of HA by PEGPH20 Demonstrated to:
Decrease
intratumoral
pressure
Decompress
vasculature
Increase
perfusion
Increase
access for
therapeutics
Increase
access for
immune cells
5

Tumor HA Overexpression Associated with
Shorter Survival In Pancreas Cancer
6
Retrospective Evaluation of Pancreatic
Cancer Survival in ~50 Patients
HA-Low Median Survival: 24.3 months
H.R. 2.6
p=0.037
HA-High Median Survival: 9.3 months
Whatcott et al: Clin Cancer Res 2015, 21:3561-3568. HA staining by HABP. Scoring algorithm assessed
percent staining and intensity.

Study 202 Design Overview
7
• Phase 2, randomized, multicenter study
• Patients with stage IV (metastatic), previously untreated PDA
• Primary & Secondary PFS Endpoint:
– 80% power at 2-sided alpha level of 0.1
CR, complete response; DCR, disease control rate; DoR, duration of response; HA, hyaluronan; KPS,
Karnofsky performance status; ORR, objective response rate; OS, overall survival; PDA, pancreatic
ductal adenocarcinoma; PFS, progression-free survival; PR, partial response; SD, stable disease.

Study 202 Study Timeline
8
2013 2014 2015 2016
Mar April Aug Feb Dec
Enrollment Clinical
Hold
Protocol
Amendment
Hold Lifted
Enrollment Follow up Data Readout
Stage 1: n=146
• Training Set for CDx
• Develop Ventana HA scoring algorithm
and cut-point
• ~40% HA High patients treated on PAG
arm discontinued PEGPH20 at clinical
hold
• Presented at ASCO 2016
Stage 2: n=133
• Validation Set for CDx
• Prospectively validate Ventana HA
scoring algorithm and cut-point used in
Phase 3 study
• To be presented at Scientific Forum 2017
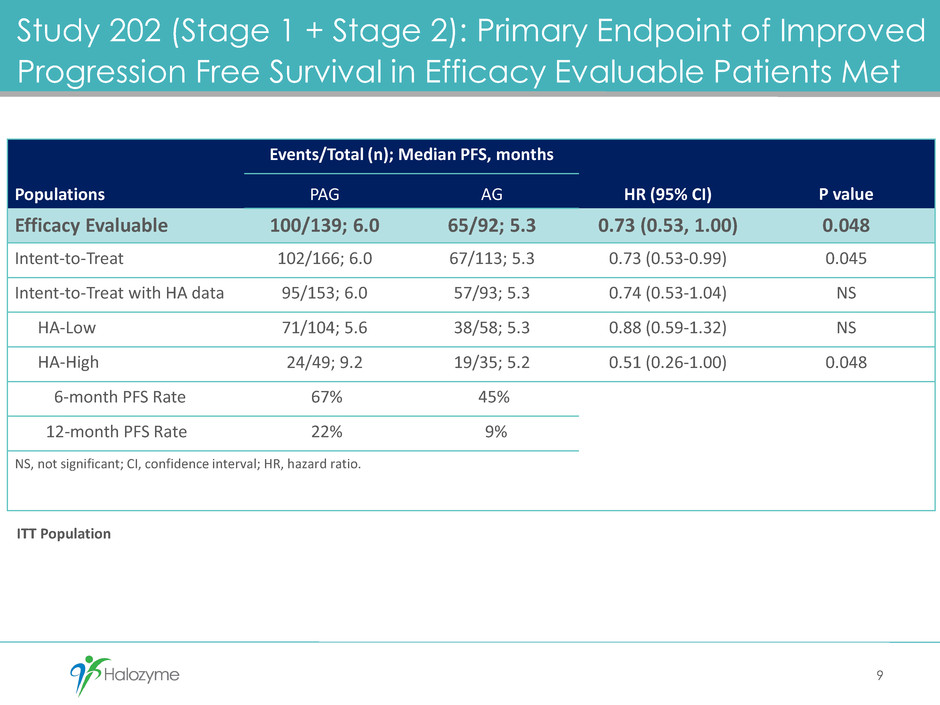
Study 202 (Stage 1 + Stage 2): Primary Endpoint of Improved
Progression Free Survival in Efficacy Evaluable Patients Met
Populations
Events/Total (n); Median PFS, months
HR (95% CI) P value PAG AG
Efficacy Evaluable 100/139; 6.0 65/92; 5.3 0.73 (0.53, 1.00) 0.048
Intent-to-Treat 102/166; 6.0 67/113; 5.3 0.73 (0.53-0.99) 0.045
Intent-to-Treat with HA data 95/153; 6.0 57/93; 5.3 0.74 (0.53-1.04) NS
HA-Low 71/104; 5.6 38/58; 5.3 0.88 (0.59-1.32) NS
HA-High 24/49; 9.2 19/35; 5.2 0.51 (0.26-1.00) 0.048
6-month PFS Rate 67% 45%
12-month PFS Rate 22% 9%
NS, not significant; CI, confidence interval; HR, hazard ratio.
9
ITT Population

Primary Endpoint of Reduction in Incidence of
Thromboembolic (TE) Events Achieved in Stage 2
10
Treated Population
Enoxaparin
Prophylaxis Dose
TE Rate
PAG AG
Stage 1*
(Dec 2016)
N/A
43%
(32/74)
25%
(15/61)
HAhigh: 42%
(10/24)
HAhigh: 24%
(5/21)
HAlow: 46%
(21/46)
HAlow: 28%
(9/32)
Stage 2**
(Dec 2016)
Started with
40 mg/day
28%
(5/18)
29%
(2/7)
Started on
1 mg/kg/day
10%
(7/68)
6%
(2/32)
*Stage 1 data reflects additional biopsies collected: 2 HA-High (2 PAG); and 3 HA-Low (2 PAG; 1 AG)
**TE rates for all stage 2 patients are 14% (12/86) in PAG arm and 10% (4/39) in AG arm
Protocol amendment in 2014 excluded patients at high risk of TE events and
added Low Molecular Weight Heparin prophylaxis in both treatment arms

Study 202 (Stage 1 + Stage 2) Secondary Endpoint PFS
and Exploratory Endpoint OS in HA-High Patients
11
AG
8.5 months
AG
5.2 months
PAG
9.2 months
PAG
11.5 months
HR: 0.51 (0.26, 1.00);
P value: 0.048
HR
0.96 (0.57, 1.61)
Progression Free Survival Overall Survival
ITT Population
77% improvement in median PFS (secondary endpoint)

12
Treated Population
Remaining on treatment:
PAG (n=2); AG (n=1)
PAG
8.6 months
HR:
0.63 (0.21, 1.93)
AG
4.5 months
Stage 2 Secondary Endpoint PFS in HA-High
Patients
91% improvement in median PFS (secondary endpoint)

13
Treated Population
Remaining on treatment and/or follow up:
PAG (n=6); AG (n=1)
HR
0.52 (0.22, 1.23)
PAG
11.7 months
AG
7.8 months
Stage 2 Exploratory Endpoint OS in HA-High
Patients
50% improvement in median OS (exploratory endpoint)
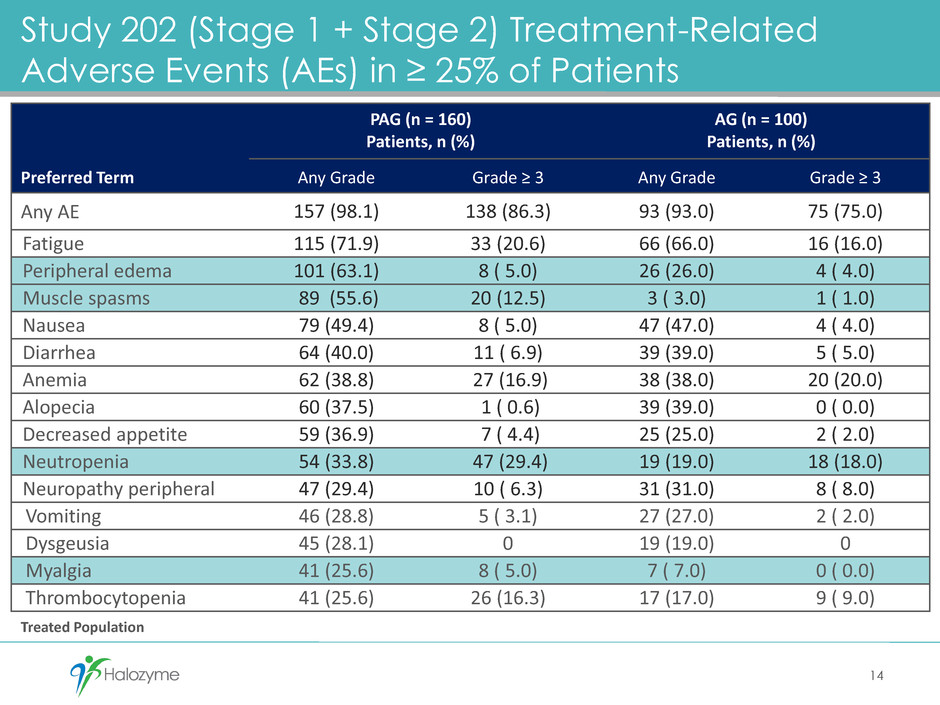
Study 202 (Stage 1 + Stage 2) Treatment-Related
Adverse Events (AEs) in ≥ 25% of Patients
14
Preferred Term
PAG (n = 160)
Patients, n (%)
AG (n = 100)
Patients, n (%)
Any Grade Grade ≥ 3 Any Grade Grade ≥ 3
Any AE 157 (98.1) 138 (86.3) 93 (93.0) 75 (75.0)
Fatigue 115 (71.9) 33 (20.6) 66 (66.0) 16 (16.0)
Peripheral edema 101 (63.1) 8 ( 5.0) 26 (26.0) 4 ( 4.0)
Muscle spasms 89 (55.6) 20 (12.5) 3 ( 3.0) 1 ( 1.0)
Nausea 79 (49.4) 8 ( 5.0) 47 (47.0) 4 ( 4.0)
Diarrhea 64 (40.0) 11 ( 6.9) 39 (39.0) 5 ( 5.0)
Anemia 62 (38.8) 27 (16.9) 38 (38.0) 20 (20.0)
Alopecia 60 (37.5) 1 ( 0.6) 39 (39.0) 0 ( 0.0)
Decreased appetite 59 (36.9) 7 ( 4.4) 25 (25.0) 2 ( 2.0)
Neutropenia 54 (33.8) 47 (29.4) 19 (19.0) 18 (18.0)
Neuropathy peripheral 47 (29.4) 10 ( 6.3) 31 (31.0) 8 ( 8.0)
Vomiting 46 (28.8) 5 ( 3.1) 27 (27.0) 2 ( 2.0)
Dysgeusia 45 (28.1) 0 19 (19.0) 0
Myalgia 41 (25.6) 8 ( 5.0) 7 ( 7.0) 0 ( 0.0)
Thrombocytopenia 41 (25.6) 26 (16.3) 17 (17.0) 9 ( 9.0)
Treated Population

Study 202 Met Key Study Objectives
15
1. Randomized Phase 2 Study 202 met multiple key study objectives
– Primary Endpoint (PFS) achieved
– Secondary Endpoint (PFS in HA High patients) achieved
2. Stage 2 of Study 202 met primary safety endpoint & shows consistent
improvement across PFS and OS in HA High patients
– Primary Safety Endpoint achieved: Decreased TE events with protocol modifications
& LMWH prophylaxis
– Secondary Endpoint: 91% improvement in median PFS
– Exploratory Endpoint: 50% improvement in median OS
– HA algorithm and cut-point of ≥ 50% validated
3. Randomized Phase 2 Study 202 results continue to support the Phase 3 HALO
301 trial
– Same patient population as Stage 2 with LMWH prophylaxis
– Same CDx cutoff as Phase 2 study
– Two primary endpoints: PFS (Phase 2 statistically significant); and OS (Stage 2 signal)

HALO-301|Pancreatic: Phase 3 Trial Ongoing
• Randomized (2:1 PAG:AG), double-blind, placebo-controlled, global
• Interim analysis when target number of PFS events reached
• PFS powered with a hazard ratio of 0.59 (to detect a 41% risk reduction
for progression)
• First patient dosed in March 2016, study will include approximately 200
sites in 20 countries
PEGPH20 + ABRAXANE® +
gemcitabine (PAG)
ABRAXANE® + gemcitabine
(AG) + placebo
Metastatic
PDA
High-HA
patients
N=420
Primary Endpoints:
Progression-Free
Survival (PFS)
Overall Survival (OS)
16

Exploring the Pan-Tumor Potential of PEGPH20
PEGPH20 Study
Preclinical Phase 1 Phase 2 Phase 3 In Combination
With Tumor
Gemcitabine and
nab-Paclitaxel
(Abraxane®)
Pancreatic
Cancer
Pembrolizumab
(Keytruda®)
Gastric Cancer,
NSCLC
Eribulin
(Halaven®)
Breast Cancer
Atezolizumab
(Tecentriq®)
Gall Bladder,
Cholangio
Atezolizumab
(Tecentriq®)
Roche Sponsored
and Conducted
Pancreatic,
Gastric,
+4 additional
EISAI
Participating in Precision Promise Clinical Initiative (Pancreatic Action Network);
Investigator Sponsored Trials in Pancreatic Cancer: SWOG, UCSF, MSKCC
ROCHE
Planned 2H 2017 start
Planned 2H 2017 start
Dose Expansion Phase
Dose Finding Phase
17

Focus: Tumors with High Unmet Need
18
1L Metastatic Pancreatic Cancer
Annual Incidence
(US and EU 5)
~65,000
Estimated % HA-HIGH 35-40%
Target HA-HIGH Population ~25,0001
Advanced Non-Small Cell Lung,
2L Metastatic Gastric,
2L Stage IV Breast (HER2-)
Annual Incidence
(US and EU 5)
~180,000
Projected % HA-HIGH 30%
Target HA-HIGH Population ~50,0001
1Annual Incidence, SEER 18 2006-2012, Globocan 2012, Medscape; Estimated HA-High %, Halozyme estimates.

ENHANZE™ Platform

ENHANZE Value Demonstration
Product
ENHANZE Value
Proposition
ENHANZE
Subcutaneous
Alternative
Number and
frequency of
injections per
month
Alternate SC:
Lifecycle
Management
2030
patent extension1
2014
EU patent expiration
for Herceptin IV2
Dosing time3,4 ~8 minutes / visit
1.5-6 hours / visit
for MabThera IV
1 European patent: EP2459167B1, U.S. patent: 9345661
2 Generics and Biosimilars Initiative, Aug. 12, 2016 (http://www.gabionline.net/Biosimilars/General/Biosimilars-of-trastuzumab)
3 Shpilberg O, et al. British J Cancer. 2013; 109(6):1556–1561
4 De Cock E, et al. Plos One. 2016; 11(6):e0157957
20

ENHANZE Portfolio Opportunity
4 Non-Disclosed Targets
26 Additional Targets Licensed
Additional Targets Available2
Potential Future
Opportunity
1 Mean analyst estimates for global revenue, Bloomberg; Analyst model estimates. ENHANZE platform royalty
revenue will depend upon indications evaluated by partners and market penetration.
2 Estimate of 150 available targets based on Informa PLC data search: monoclonal antibodies, siRNA/RNAi, select
small molecules, delivered Intramuscular, intravenous and subcutaneously.
The Top 3
• $5B+ Royalty-eligible Sales
in 20251
• Recurring mid-single digit
royalties
Partnered Pipeline
• $10B+ Sales in 20251
• Recurring mid-single
digit royalties
21

Genentech’s BLA for Rituximab SC in Multiple
Blood Cancer Indications Under FDA Review
22
Information provided during Roche analyst event at 2016 American Society of Hematology Annual
Meeting (Dec. 5, 2016).

Financial Update

2017 Financial Guidance
2016 2017 Notes
Net Revenue $145M to $150M $115M to $130M
• 2017 guidance excludes new
ENHANZE partnerships
• Robust royalty growth
projected to continue in 2017
• 2016 included ~$20M of one-
time reimbursed R&D
expenses
Operating
Expenses
$240M to $245M $240M to $250M
• Continued investment in
Phase 3 pancreas and pan-
tumor studies
Year-end Cash $180M to $190M $100M to $110M
• Royalty-backed loan
repayment starts 2017
24

Value Enhancing Milestones Throughout 2017
Goal Target Date
Study 202: Top-line Stage 2, Combined Phase 2 Data Presented
Study 202: Data Presented at Scientific Forum 2017
PEGPH20 Tumor Microenvironment Immunology Data Presented
at Scientific Forum
1H 2017
Initiation of Genentech/Halozyme Clinical Collaboration trials –
Atezolizumab + PEGPH20
2H 2017
Rituximab SC U.S. Action Date June 2017
Support ENHANZE Partners’ Progress; Sign New ENHANZE Platform
Agreement
2017
25
✓

35th Annual J.P. Morgan Healthcare Conference
Building a Premier Oncology Biotech:
Two Pillar Strategy for Growth
Dr. Helen Torley
President & CEO
January 9, 2017
