Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zyla Life Sciences | a17-1510_18k.htm |
Exhibit 99.1
Specialty Pharmaceutical Company Focused on Pain Egalet Corporate Presentation NASDAQ: EGLT 1

Forward-Looking Statements 2 Statements included in this presentation that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties, risks and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “could,” “plans,” “future,” “expects,” “goal,” “intends,” “assess,” “continue to,” “potential,” “anticipates,” “believes,” “estimates,” or “predicts,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i) our expectations regarding when our product candidates could be launched or on the market, (ii) statements regarding the potential market size for our products, (iii) the timing or likelihood of regulatory filings and approvals for our product candidates; (iv) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (v) the impact of our existing commercial presence on the launch of our new products; (vi) the impact of the addition of our new products and product candidates on our market presence; (vii) statements regarding the expansion of new prescribers and prescriptions for our products; (viii) the timing of the expansion of dosage levels for our products; and (ix) our expectations regarding our finances, our funding sources, our use of funds and potential payments under our notes and our royalty rights agreements. In addition, we, through our senior management, from time to time make forward-looking public statements concerning our expected future operations and performance and other developments. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: the success of integrating recent acquisitions; our ability to obtain regulatory approval of our product candidates; our ability to successfully commercialize SPRIX® and OXAYDO®; unexpected safety or efficacy data; competitive factors; changes in the regulatory environment for our products; general market conditions; our need for future capital; and other risk factors described in Egalet's filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this presentation whether as a result of new information or future events, except as may be required by law. Please refer to oxaydo.com and sprix.com for full prescribing information and boxed warnings.

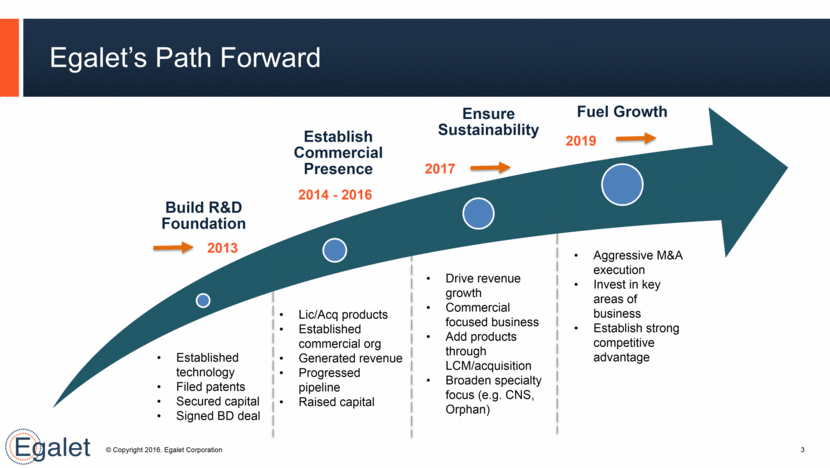
Egalet’s Path Forward 3 Established technology Filed patents Secured capital Signed BD deal Lic/Acq products Established commercial org Generated revenue Progressed pipeline Raised capital Drive revenue growth Commercial focused business Add products through LCM/acquisition Broaden specialty focus (e.g. CNS, Orphan) Aggressive M&A execution Invest in key areas of business Establish strong competitive advantage 2013 2014 - 2016 2017 2019 Build R&D Foundation Establish Commercial Presence Ensure Sustainability Fuel Growth
Focusing on Large Markets & Addressing FDA Priority 4 Rapidly growing specialty pharmaceutical company Products and pipeline target large markets and designed to address top FDA priority Ability to use business development to bring in complementary innovative products Organic growth leveraging proprietary Guardian™ Technology Strong cash position 4
Commercial Strategy 5

Commercial Experience Prior to ARYMO™ ER Launch 6 EGALET-001 EGALET-002 EGALET-002 Acquired and launched two products SPRIX Nasal Spray & OXAYDO (January 2015) Built sales, marketing, distribution, commercial operations capabilities and teams 71 Specialty sales representatives actively calling on and building relationships with targeted IR/ER opioid prescribers Acquired knowledge and experience in a highly dynamic category

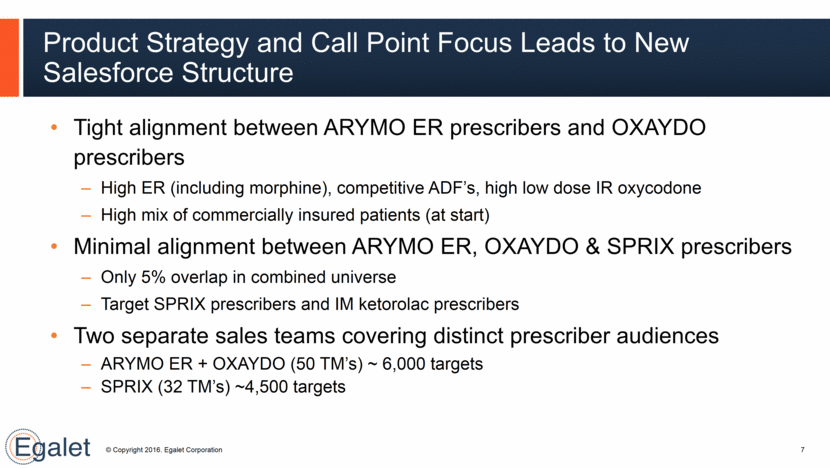
Tight alignment between ARYMO ER prescribers and OXAYDO prescribers High ER (including morphine), competitive ADF’s, high low dose IR oxycodone High mix of commercially insured patients (at start) Minimal alignment between ARYMO ER, OXAYDO & SPRIX prescribers Only 5% overlap in combined universe Target SPRIX prescribers and IM ketorolac prescribers Two separate sales teams covering distinct prescriber audiences ARYMO ER + OXAYDO (50 TM’s) ~ 6,000 targets SPRIX (32 TM’s) ~4,500 targets Product Strategy and Call Point Focus Leads to New Salesforce Structure 7
ARYMO™ ER 8

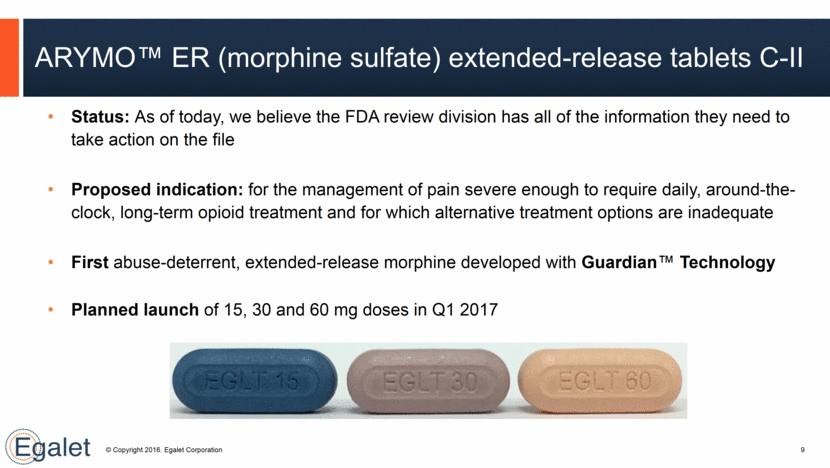
ARYMO™ ER (morphine sulfate) extended-release tablets C-II Status: As of today, we believe the FDA review division has all of the information they need to take action on the file Proposed indication: for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate First abuse-deterrent, extended-release morphine developed with Guardian™ Technology Planned launch of 15, 30 and 60 mg doses in Q1 2017 9
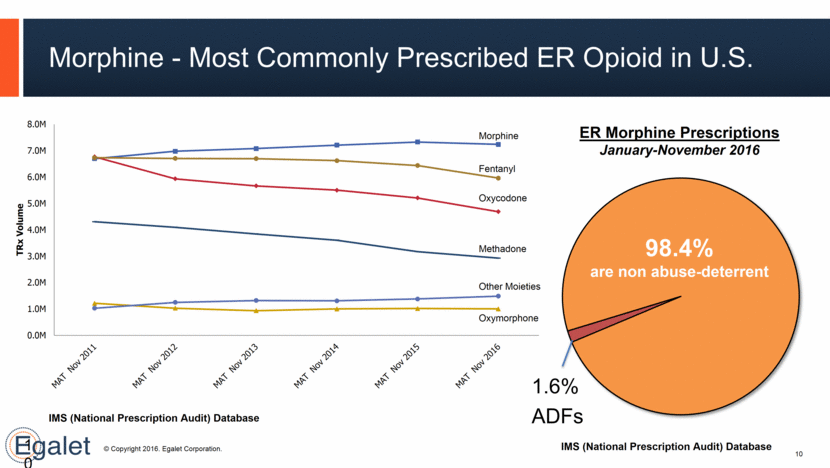
10 10 Morphine - Most Commonly Prescribed ER Opioid in U.S. 98.4% are non abuse-deterrent 1.6% ADFs ER Morphine Prescriptions January-November 2016 IMS (National Prescription Audit) Database IMS (National Prescription Audit) Database Other Moieties 0.0M 1.0M 2.0M 3.0M 4.0M 5.0M 6.0M 7.0M 8.0M TRx Volume Morphine Fentanyl Oxycodone Methadone Oxymorphone
Clinical Data Support Approval of ARYMO™ ER ARYMO™ ER bioequivalent to MS Contin at all intended dosage strengths No clinically significant food effect No evidence of alcohol dose dumping Strong Category 1 study No household tools able to grind ARYMO ER into small particles Very difficult to get into a syringe Positive Category 3 clinical human abuse potential (HAP) studies published in medical literature 11
ARYMO™ ER Commercial Launch Planned for Q1 2017 Finalized size, structure and call plan for a focused specialty sales force Wholesaler/retail pharmacy stocking and pull through planning Payer strategy ensuring adequate product access/availability across commercial, Medicare Part D and Medicaid segments developing Value proposition, product positioning and message platform being finalized Personal and non-personal support programs and tactics to accelerate brand awareness being developed 12

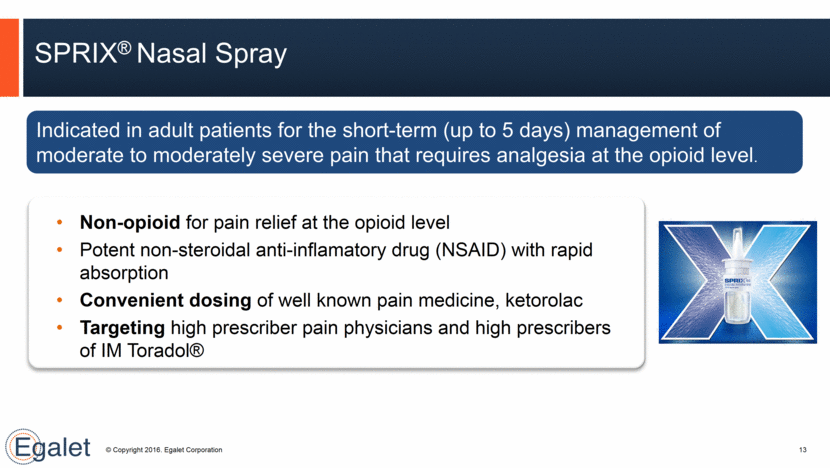
SPRIX® Nasal Spray 13 Non-opioid for pain relief at the opioid level Potent non-steroidal anti-inflamatory drug (NSAID) with rapid absorption Convenient dosing of well known pain medicine, ketorolac Targeting high prescriber pain physicians and high prescribers of IM Toradol® Indicated in adult patients for the short-term (up to 5 days) management of moderate to moderately severe pain that requires analgesia at the opioid level.
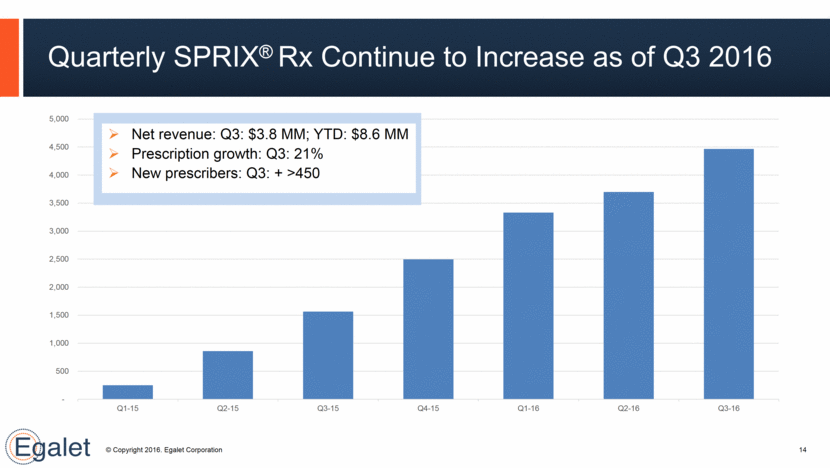
14 Quarterly SPRIX® Rx Continue to Increase as of Q3 2016 Net revenue: Q3: $3.8 MM; YTD: $8.6 MM Prescription growth: Q3: 21% New prescribers: Q3: + >450
OXAYDO®, Only IR Oxycodone Designed to Discourage Nasal Abuse 15 Immediate-release oxycodone with aversion technology Designed to discourage abuse via most common non-oral route of abuse for oxycodone—snorting Approved in 5 mg and 7.5 mg doses Demonstrated BE at 15 mg Abuse-deterrent Category 1 data showed resists syringeability and sNDA under review at FDA For the management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate. Goal: 10 and 15 mg dosage strengths in 2H 2017
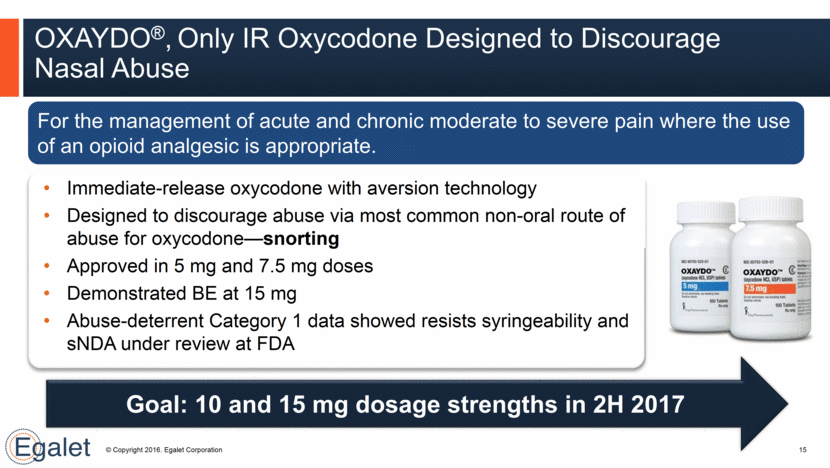
Weekly Rx for OXAYDO® Continues to Increase Net revenue: Q3: $958K; YTD: $2.1 MM Prescription growth: Q3: 10% New prescribers: Q3: + >395 16
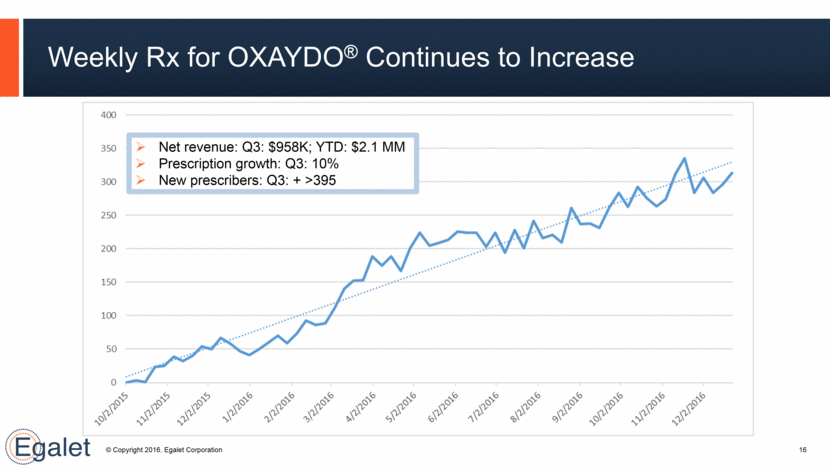
Robust Pipeline 17 Approved Late-Stage Early-Stage SPRIX® (ketorolac tromethamine) Nasal Spray Short-term pain ARYMO™ ER, AD morphine Chronic pain Egalet-002, AD, ER oxycodone Chronic pain Egalet-004, AD ER Hydrocodone Chronic pain OXAYDO® (oxycodone HCl, USP) Tablets for oral use only – CII Acute and chronic pain Egalet-003, AD Stimulant Ph 1 Ph 2 Pivotal NDA Filed Marketed Guardian Technology Commercial Products SPRIX® Nasal Spray for outside US Short-term pain Preclinical Partnered Products
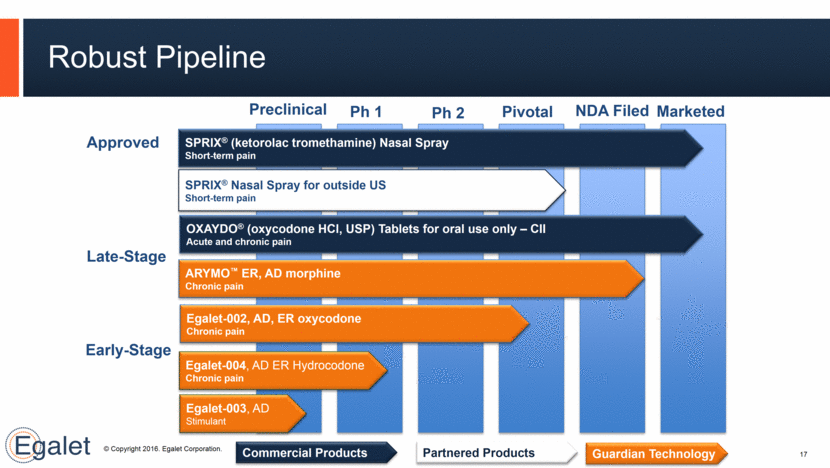
Guardian™ Technology Confers Physical and Chemical Barriers to Abuse 18 Polyethylene oxide (PEO) + Chemical (polymer matrix) Injection molding machine Physical (hardness) Well-characterized process, differentiated by heat & pressure Viscous hydrogel on contact with liquid Extended-release profile with physical and chemical abuse-deterrent properties
Egalet-002, AD/ER Oxycodone, in Late-Stage Development* *for the management of pain severe enough to require daily, around-the-clock, opioid treatment and for which alternative treatments are inadequate 19 Pivotal Phase 3 program ongoing Hard matrix surrounded by hard shell Resistant to particle size reduction & chemical extraction Forms viscous hydrogel on contact with liquid can’t draw up into a syringe Reduces risk of accidental misuse Reduces risk of intential abuse via snorting or IV injection Oxycodone is highest selling ER opioid; $2.5 B in sales in 2013 Completion of Phase 3 program 4Q17
Financials and Conclusion 20

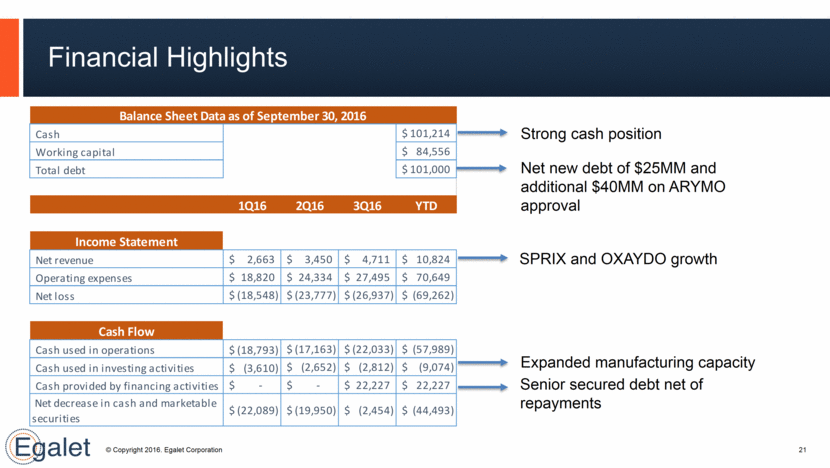
Financial Highlights 21 Strong cash position Net new debt of $25MM and additional $40MM on ARYMO approval SPRIX and OXAYDO growth Expanded manufacturing capacity Senior secured debt net of repayments Cash 101,214 $ Working capital 84,556 $ Total debt 101,000 $ 1Q16 2Q16 3Q16 YTD Net revenue 2,663 $ 3,450 $ 4,711 $ 10,824 $ Operating expenses 18,820 $ 24,334 $ 27,495 $ 70,649 $ Net loss (18,548) $ (23,777) $ (26,937) $ (69,262) $ Cash used in operations (18,793) $ (17,163) $ (22,033) $ (57,989) $ Cash used in investing activities (3,610) $ (2,652) $ (2,812) $ (9,074) $ Cash provided by financing activities - $ - $ 22,227 $ 22,227 $ (22,089) $ (19,950) $ (2,454) $ (44,493) $ Balance Sheet Data as of September 30, 2016 Net decrease in cash and marketable securities Cash Flow Income Statement
2018 30 April, 2016 06 September, 2016 31 December, 2016 30 June, 2017 30 March, 2018 29 February, 2016 30 September, 2016 14 October, 2016 31 December, 2016 30 June, 2017 30 June, 2017 30 September, 2017 36.6 wks 2016 - 2016 117.2 wks January 1, 2016 - March 31, 2018 43.4 wks January 1, 2016 - October 31, 2016 91.2 wks January 1, 2016 - September 30, 2017 Sustainable Growth with Numerous Milestones H2 2016 Egalet-002 Cat 3 IN HAP ARYMO ER Approval Q1 2017 ARYMO ER launch H2 2017 New dosage strengths of OXAYDO available Q4 2017 Complete Egalet-002 Phase 3 program 2018 submit Egalet-002 NDA 22

Thank You @EgaletCorp Egalet.com 23

