Attached files
| file | filename |
|---|---|
| EX-10.15 - EX-10.15 - Zyla Life Sciences | eglt-20171231ex1015a53ab.htm |
| EX-32.2 - EX-32.2 - Zyla Life Sciences | eglt-20171231ex322699f44.htm |
| EX-32.1 - EX-32.1 - Zyla Life Sciences | eglt-20171231ex321f7df30.htm |
| EX-31.2 - EX-31.2 - Zyla Life Sciences | eglt-20171231ex3129b93e5.htm |
| EX-31.1 - EX-31.1 - Zyla Life Sciences | eglt-20171231ex31115d500.htm |
| EX-23.1 - EX-23.1 - Zyla Life Sciences | eglt-20171231ex231669c52.htm |
| EX-21.1 - EX-21.1 - Zyla Life Sciences | eglt-20171231ex211fbfc3c.htm |
| EX-10.34 - EX-10.34 - Zyla Life Sciences | eglt-20171231ex103478508.htm |
| EX-10.25 - EX-10.25 - Zyla Life Sciences | eglt-20171231ex10255e159.htm |
| EX-10.24 - EX-10.24 - Zyla Life Sciences | eglt-20171231ex1024adf12.htm |
| EX-10.14 - EX-10.14 - Zyla Life Sciences | eglt-20171231ex1014406df.htm |
| EX-10.13 - EX-10.13 - Zyla Life Sciences | eglt-20171231ex10137b7d3.htm |
| EX-3.1 - EX-3.1 - Zyla Life Sciences | eglt-20171231ex31bfd9d68.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10‑K
|
(Mark one) |
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2017 |
|
|
Or |
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
|
Commission file number 001‑36295
Egalet Corporation
(Exact name of registrant as specified in its charter)
|
Delaware |
46‑3575334 |
|
600 Lee Road |
19087 |
(610) 833‑4200
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Name of each exchange on which registered |
|
Common Stock, par value $0.001 per share |
|
NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well‑known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S‑T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S‑K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10‑K or any amendment to this Form 10‑K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b‑2 of the Exchange Act.
|
Large accelerated filer ☐ |
|
Accelerated filer ☒ |
|
|
|
|
|
Non-accelerated filer ☐ (Do not check if a smaller reporting company) |
|
Smaller reporting company ☐ |
|
Emerging growth company ☒ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule12b‑2 of the Act). Yes ☐ No ☒
As of June 30, 2017 (the last business day of the registrant’s most recently completed second fiscal quarter), the aggregate market value of the registrant’s voting stock held by non‑affiliates was approximately $55.3 million based on the last reported sale price of the registrant’s Common Stock on June 30, 2017.
There were 46,987,462 shares of Common Stock outstanding as of March 16, 2018.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of our Proxy Statement for the 2017 Annual Meeting of Stockholders, to be filed within 120 days of December 31, 2017, are incorporated by reference in Part III. Such Proxy Statement, except for the parts therein which have been specifically incorporated by reference, shall not be deemed “filed” for the purposes of this Annual Report on Form 10‑K.
EGALET CORPORATION
On November 26, 2013, Egalet Corporation (the “Company”) acquired all of the outstanding shares of Egalet Limited (“Egalet UK”). As a result, Egalet UK became a wholly‑owned subsidiary of the Company, and the former shareholders of Egalet UK received shares of the Company (the “Share Exchange”). Unless the context indicates otherwise, as used in this Annual Report on Form 10‑K, the terms “Egalet,” “we,” “us,” “our,” “our company” and “our business” refers to the Company for all periods subsequent to the Share Exchange, and to Egalet UK for all periods prior to the Share Exchange. The Egalet logo is our trademark and Egalet is our registered trademark. All other trade names, trademarks and service marks appearing in this Annual Report on Form 10‑K are the property of their respective owners. We have assumed that the reader understands that all such terms are source‑indicating. Accordingly, such terms, when first mentioned in this Annual Report on Form 10‑K, appear with the trade name, trademark or service mark notice and then throughout the remainder of this Annual Report on Form 10‑K without the trade name, trademark or service mark notices for convenience only and should not be construed as being used in a descriptive or generic sense. Unless otherwise indicated, all statistical information provided about our business in this report is as of December 31, 2017.
SPECIAL NOTE REGARDING FORWARD‑LOOKING STATEMENTS AND INDUSTRY DATA
This Annual Report on Form 10‑K (this “Annual Report”) includes forward‑looking statements. We may, in some cases, use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately,” “goal,” “intent,” “target,” or other words that convey uncertainty of future events or outcomes to identify these forward‑looking statements. Forward‑looking statements appear in a number of places throughout this Annual Report and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our plans to grow our business, our business strategy, the commercial success of our products and, if approved, our product candidates, our plans with regard to the commercialization of our products, including through partnerships, our plans to reformulate SPRIX, our ability to execute on our sales and marketing strategy, our ongoing and planned preclinical development and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our products and product candidates, our intellectual property position, the degree of clinical utility of our products, particularly in specific patient populations, current and future government regulations and the impact of such regulations, expectations regarding clinical trial data, including the inherent risks in conducting clinical trials for our products, our business development plans, our results of operations, the date through which our existing cash will be sufficient to fund our projected operating requirements, cash needs and ability to obtain additional funding, financial condition, liquidity, prospects, growth and strategies, foreign exchange rates, the industry in which we operate and the competition and trends that may affect the industry or us.
By their nature, forward‑looking statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on economic or other circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward‑looking statement contained in this Annual Report, we caution you that forward‑looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward‑looking statements contained in this Annual Report. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward‑looking statements contained in this Annual Report, they may not be predictive of results or developments in future periods.
Actual results could differ materially from our forward‑looking statements due to a number of factors, including risks related to:
|
· |
our ability to continue as a going concern; |
|
· |
our ability to maintain our listing on the Nasdaq Global Market; |
|
· |
our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
|
· |
our current and future indebtedness; |
1
|
· |
our ability to obtain additional financing or to refinance our existing indebtedness; |
|
· |
the level of commercial success of our products; |
|
· |
our ability to execute on our sales and marketing strategy, including developing relationships with customers, physicians, payors and other constituencies; |
|
· |
the continued development of our commercialization capabilities, including sales, marketing and market access capabilities; |
|
· |
the rate and degree of market acceptance of any of our products and product candidates; |
|
· |
the success of competing products that are or become available; |
|
· |
the entry of any generic products for SPRIX or any delay in or inability to reformulate SPRIX; |
|
· |
recently enacted and future legislation regarding the healthcare system; |
|
· |
the difficulties in obtaining and maintaining regulatory approval of our products and product candidates, and any related restrictions, limitations and/or warnings in the product label under any approval we may obtain; |
|
· |
the accuracy of our estimates of the size and characteristics of the potential markets for our products and our ability to serve those markets; |
|
· |
the performance of third parties, including contract research organizations, manufacturers and collaborators; |
|
· |
the success and timing of our preclinical studies and clinical trials; |
|
· |
obtaining and maintaining intellectual property protection for our products and product candidates and our proprietary technology; |
|
· |
litigation related to opioids and public or legislative pressure on the opioid industry; |
|
· |
the outcome of any litigation in which we are or may be involved; |
|
· |
our ability to operate our business without infringing the intellectual property rights of others; and |
|
· our ability to integrate and grow any businesses or products that we may acquire. |
You should also read carefully the factors described in the “Risk Factors” section of this Annual Report and elsewhere to better understand the risks and uncertainties inherent in our business and underlying any forward‑looking statements. As a result of these factors, we cannot assure you that the forward‑looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward‑looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward‑looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all.
2
Any forward‑looking statements that we make in this Annual Report speak only as of the date of such statement, and, except as required by applicable law, we undertake no obligation to update such statements to reflect events or circumstances after the date of this Annual Report or to reflect the occurrence of unanticipated events. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless expressed as such, and should only be viewed as historical data.
We obtained the industry, market and competitive position data in this Annual Report from our own internal estimates and research as well as from industry and general publications and research surveys and studies conducted by third parties. Any information in this Annual Report provided by IMS Health Incorporated (“IMS”) is an estimate derived from the use of information under license from the following IMS Health information services: IMS National Sales Perspectives and NPA Audits, in each case, for the period 2007‑2015. IMS expressly reserves all rights, including rights of copying, distribution and republication.
3
Overview
We are a fully integrated specialty pharmaceutical company developing, manufacturing and commercializing innovative treatments for pain and other conditions. Given the need for acute and chronic pain products and the issue of prescription abuse, we are focused on bringing non-narcotic and abuse-deterrent opioid formulations to healthcare providers. We are currently marketing SPRIX® (ketorolac tromethamine) Nasal Spray (“SPRIX Nasal Spray”), OXAYDO® (oxycodone HCI, USP) tablets for oral use only—CII (“OXAYDO”) and ARYMO® ER (morphine sulfate) extended-release (“ER”) tablets (“ARYMO ER”), an ER morphine product formulated with abuse-deterrent (“AD”) properties. In addition to ARYMO ER, an abuse-deterrent formulation (“ADF”), we have a developed a pipeline of clinical-stage product candidates using our proprietary Guardian™ Technology, a polymer matrix tablet technology that utilizes a novel application of the well-established manufacturing process of injection molding. We are focusing our resources on our commercial programs and may seek partners for our pipeline products. We plan to continue to grow our business through the revenue growth of our three approved products, business development and leveraging our proprietary Guardian Technology.
Strategy
Our goal is to be a leading specialty pharmaceutical company focused on developing, manufacturing and commercializing innovative treatments for pain and other conditions. Key elements of our strategy include:
|
· |
Drive revenue growth of SPRIX Nasal Spray, OXAYDO and ARYMO ER. Upon the approval of ARYMO ER in January 2017 and launch in April 2017, we expanded the sales force to 82 representatives. With this dedicated Egalet salesforce, we are targeting approximately 10,500 pain medicine physicians, primary care physicians, nurse practitioners, orthopedic surgeons and neurologists in the United States with the intent to build awareness and increase adoption of SPRIX, OXAYDO and ARYMO ER. |
|
· |
Expand SPRIX Nasal Spray, OXAYDO and ARYMO ER Commercial Opportunity through Partnerships. To augment our commercial efforts for all three of our products, we continue to look for partnerships that expand our reach beyond the efforts of our Egalet salesforce to access additional healthcare providers. In 2017, we signed an agreement with Ascend Therapeutics to promote SPRIX Nasal Spray to approximately 11,000 women’s healthcare providers. In early 2018, we announced an agreement with OraPharma to promote SPRIX Nasal Spray along with their own portfolio products to its target dentists, dental specialist, and oral surgeons. OraPharma began promotion of SPRIX Nasal Spray in the first quarter of 2018. We continue to seek additional partnerships for our three approved products to expand our commercial reach to specialists we do not target with our own salesforce. |
|
· |
Reformulate SPRIX Nasal Spray. We are working on a new formulation that could improve SPRIX Nasal Spray, expand the patent life of the product and potentially add new intellectual property. We plan to share the plans for the path to SPRIX Nasal Spray reformulation in mid-2018. |
3
Background on Pain and Prescription Opioid Abuse
With approximately 100 million Americans suffering from chronic pain according to the Institute of Medicine—more than those affected by heart disease, cancer, and diabetes combined—there is a substantial need for effective pain treatments. The millions suffering from acute or chronic pain every year greatly impacts the U.S. with increasing costs associated with health care, rehabilitation and lost worker productivity. According to the Institute of Medicine, pain is a significant public health problem that costs society between $560.0 and $635.0 billion annually. Nonsteroidal anti-inflammatory drugs (“NSAIDs”) are among the most widely used medications in the world due to their demonstrated efficacy in reducing pain and inflammation. In addition, according to IMS prescription data from 2017, opioids are the most widely prescribed products for pain, with prescriptions exceeding 150 million in 2017.
Drug abuse is a significant issue, with an estimated 29.0 million people suffering from drug use disorders worldwide according to the United Nations Office on Drugs and Crime, World Drug Report 2016. According to the 2016 National Survey on Drug Use and Health (NSDUH), an estimated 11.8 million Americans age 12 and older misused prescription pain relievers in the past year. The Centers for Disease Control and Prevention (“CDC”) have stated that opioids accounted for approximately 66 percent of fatal drug overdoses. Importantly, based on a 2016 national survey of people 12 and older, an estimated 53 percent of respondents who reported misusing prescription pain relievers in the past year obtained their last misused prescription pain reliever from a friend or relative. According to the CDC, the cost of prescription opioid overdose, abuse and dependence was estimated to be $78.5 billion in 2013.
Prescription medications, particularly opioids (both ER and immediate-release (“IR”) forms), are prone to being misused or abused through physical and chemical manipulation to increase the speed of the drug release into the bloodstream, accelerating and intensifying its effects. A study of prescription opioid abusers in a drug rehabilitation program published in the Journal of Pain & Palliative Care Pharmacotherapy found that 80 percent tampered with opioid tablets to accelerate drug release by chewing or administering the drug intranasally or intravenously. Common methods of manipulating medications in pill or tablet form include crushing to swallow, snort or smoke, and dissolving to inject.
In reaction to this widespread prescription opioid misuse and abuse, the U.S. government and the FDA have designated this issue as a high priority. In February 2016, the FDA announced an action plan to combat the growing problem of prescription abuse, highlighting the development of ADFs as a part of the solution. In addition, according to the CDC, the first line of therapy should be non-opioid treatments, such as NSAIDs.
Our Products
SPRIX Nasal Spray
SPRIX Nasal Spray for short‑term (up to five days) management of moderate to moderately severe pain that requires analgesia at the opioid level
SPRIX® (ketorolac tromethamine) Nasal Spray is an NSAID indicated in adult patients for the short‑term (up to five days) management of moderate to moderately severe pain that requires analgesia at the opioid level. Formulated as a nasal spray, SPRIX Nasal Spray is rapidly absorbed through the nasal mucosa, achieving peak blood levels as fast as an intramuscular injection of ketorolac. SPRIX Nasal Spray has been studied in patients with moderate to moderately severe pain. SPRIX Nasal Spray has demonstrated a 26 to 34 percent reduction in morphine use by patients over a 48‑hour period in a post‑operative setting as compared with placebo. We acquired SPRIX Nasal Spray and certain related assets from Luitpold Pharmaceuticals, Inc. (“Luitpold”) in January 2015 for $7.0 million.
Under our purchase agreement with Luitpold pursuant to which we acquired certain assets and liabilities associated with SPRIX Nasal Spray, we were assigned an exclusive license with Recordati Ireland Ltd. (“Recordati”) for intranasal formulations of ketorolac tromethamine (the “Licensed Product”), the active ingredient in SPRIX Nasal Spray. We are required to pay a fixed, single-digit royalty to Recordati on net sales of the Licensed Product. The exclusive term of the license agreement expires, on a country-by-country basis, on the later of the final expiration of any patent right in such country that contains a valid claim covering the Licensed Product, or ten years from the date of the first
4
commercial sale of the Licensed Product in such country, and thereafter the Company will retain a non-exclusive, perpetual license in such country. In addition, during the exclusivity period with respect to the United States, Canada and Latin America, the royalty payable to Recordati is decreased if no patent containing a valid claim is in force in the country at the time of sale. SPRIX Nasal Spray is currently sold in the United States and is covered by a patent that expires in December 2018 and the first commercial sale of SPRIX Nasal Spray in the United States occurred in May 2011.
Commercial Strategy
Given the issue of prescription opioid abuse and emphasis on prescribing non-narcotic treatments for pain, we believe that SPRIX Nasal Spray, a non-narcotic product that provides opioid-level pain relief, is an important treatment option for adult patients with moderate to moderately severe short-term pain. We began promotion of SPRIX Nasal Spray in 2015 and since the end of the first quarter of 2017, our salesforce has been promoting SPRIX Nasal Spray to approximately 4,500 healthcare providers. To broaden our reach to additional healthcare providers, in the third quarter we partnered with Ascend Therapeutics to promote SPRIX Nasal Spray. The Ascend salesforce began promoting SPRIX Nasal Spray to approximately 11,000 women’s healthcare providers in the same quarter. In January of 2018, we signed a co-promotion agreement with OraPharma, a division of Valeant Pharmaceuticals, to promote SPRIX Nasal Spray to dentists, dental specialists and oral surgeons in the United States through its approximately 141-person sales force. Prior to this agreement, we terminated our co-promotion agreement with Septodont, a company that previously promoted SPRIX Nasal Spray to dentists. In addition, Teva has exclusive marketing and commercialization rights to SPRIX Nasal Spray in Israel, Gaza and the West Bank once the product is registered. We are continuing to evaluate other partnership opportunities to bring SPRIX Nasal Spray to other potential specialties that treat patients with short-term pain.
OXAYDO
OXAYDO for the management of acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
OXAYDO, an approved IR oxycodone product designed using an aversion technology to discourage abuse via snorting, is indicated for the management of acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. OXAYDO was approved in 2011 prior to the U.S. FDA Guidance for Industry, Abuse-Deterrent Opioids – Evaluation and Labeling (“FDA AD Guidance”) on abuse-deterrent opioids. The label includes results from a Category 3 AD study that evaluated drug liking after snorting crushed OXAYDO compared to crushed Roxicodone (oxycodone hydrochloride tablets USP).
To expand the commercial opportunity for OXAYDO, we filed a supplemental new drug application (“sNDA”) with abuse-deterrent data and a prior approval supplement (“PAS”) with data on new dosage strengths. To contemporize the label in line with current FDA abuse-deterrent standards, we filed with the FDA a sNDA in December 2016 based on Category 1 AD data that demonstrated that OXAYDO resists syringeability, which could potentially deter abuse through the intravenous route. OXAYDO is currently available in 5 mg and 7.5 mg dosage strengths. A pharmacokinetic (“PK”) study that demonstrated bioequivalence (“BE”) of OXAYDO 15 mg to Roxicodone 15 mg served as the basis for a PAS that was submitted to the FDA in February 2017 to request approval of additional dosage strengths of 10 mg and 15 mg. We received a complete response letter (“CRL”) from the FDA in June of 2017. The FDA requested more information regarding the effect of food on OXAYDO 15 mg and the intranasal AD properties of OXAYDO 10 and 15 mg. In addition, based on discussions with the FDA regarding the sNDA, we believe a contemporary intranasal human abuse potential (“HAP”) study would be needed to complete the sNDA. Given that the issues involved in the sNDA and PAS are intertwined, we are evaluating our options and the costs associated to proceed on the AD label and/or the additional dosage strengths.
We licensed OXAYDO from Acura Pharmaceuticals (“Acura”) in January 2015 for a $5.0 million upfront payment and a $2.5 million milestone payment upon commercial launch. In addition, Acura is entitled to a one-time $12.5 million milestone payment if OXAYDO net sales reach $150.0 million in a calendar year and a tiered royalty percentage based on sales thresholds. Based on our current net sales, the royalty percentage payable to Acura is in the mid-single digits; however, the percentage may increase in future years in the event we achieve the higher sales
5
thresholds set forth in the agreement. Our royalty payment obligations commenced on the first commercial sale of OXAYDO and expire, on a country-by-country basis, upon the expiration of the last to expire valid patent claim covering OXAYDO in such country (or if there are no patent claims in such country, then upon the expiration of the last valid claim in the U.S.). Royalties will be reduced upon the entry of generic equivalents, as well for payments we are required to make to acquire intellectual property rights to commercialize OXAYDO, with an aggregate minimum floor. The term of the Acura license agreement expires, in its entirety, upon the final expiration of any such patent claim in any country. OXAYDO is currently sold in the United States and is covered by six U.S. patents that expire between 2023 and 2025. Patents covering OXAYDO in foreign jurisdictions expire in 2024. Either we or Acura may terminate the license agreement for certain customary reasons, including cause, insolvency or patent challenge. We may terminate the license agreement upon 90 days prior written notice.
Commercial Strategy
Given the increasing number of IR opioid prescriptions and the high incidence of abuse of IR opioids, we believe that OXAYDO has the potential to be an important treatment option. According to four abuse and diversion surveillance programs, IR opioid abuse was up to 4.6-fold higher than ER abuse in 2016. With over 50 million prescriptions of IR oxycodone written in 2017 according to IMS, we believe that there is a substantial need for an IR oxycodone product like OXAYDO that is designed to discourage abuse. Since our launch of OXAYDO in 2015, our sales force has been educating our target pain medicine prescribers in the United States on the attributes of OXAYDO. Starting in March 2017, the sales representatives focused on approximately 6,000 healthcare providers—individuals likely to prescribe both OXAYDO and ARYMO ER.
ARYMO ER
ARYMO ER for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
ARYMO ER, an ER, AD morphine sulfate product, is the first approved product developed using our proprietary Guardian Technology, creating tablets that are difficult to manipulate for misuse and abuse. Results from in vitro testing demonstrated that ARYMO ER tablets, in comparison to non-AD morphine sulfate extended-release tablets, have increased resistance to cutting, crushing, grinding or breaking using a variety of tools. Due to its physical and chemical properties, ARYMO ER is expected to make abuse by injection difficult. ARYMO ER has been approved in three dosage strengths: 15 mg, 30 mg and 60 mg.
Commercial Strategy
Morphine is the most commonly prescribed ER opioid, with nearly 6.5 million prescriptions written in 2017 according to IMS. With more than 98 percent of these prescriptions written for non-abuse-deterrent forms of morphine, we believe that there is a market opportunity for an ADF morphine like ARYMO ER. While the FDA has been supportive of abuse-deterrent formulations, the payors, both commercial and government, have been resistant to placing abuse-deterrent formulations on their formularies or have created barriers to prescribing. At the end of March, we began educating our target healthcare providers and payors about the benefits and risks of ARYMO ER.
In December of 2017, the FDA granted tentative approval for an expanded label for ARYMO ER. The approval was issued for a supplement submitted earlier in 2017 to update the ARYMO ER prescribing information with data from a Category 2/3 intranasal human abuse potential (“HAP”) study and an intranasal AD claim. This data was previously excluded from the label when the original new drug application (“NDA”) was approval due to exclusivity granted to another company. The final approval is expected to be granted when the exclusivity period expires on October 2, 2018.
6
Product Candidates
While we do have a pipeline of product candidates, our current focus is on our commercial products. Our primary effort with regard to our product candidates is to identify potential partners to advance them.
Guardian Technology
Overview
Our proprietary Guardian Technology is a polymer matrix tablet technology that utilizes a novel application of the well-established manufacturing process of injection molding, which results in tablets that are hard and difficult to manipulate for misuse and abuse. While our Guardian Technology creates a tablet that is extremely hard and has AD features, the construct of the tablet allows for controlled release of the active pharmaceutical ingredient (“API”) in the gastrointestinal (‘GI”) tract. This approach offers the ability to design tablets with controlled-release profiles as well as physical and chemical properties that have been demonstrated to resist both common and rigorous methods of manipulation. Tablets manufactured with Guardian Technology have been shown to have increased resistance to physical methods of manipulation, such as cutting, crushing, grinding or breaking using a variety of mechanical and electrical tools. The tablets are also resistant to chemical manipulation and turn into a viscous hydrogel on contact with liquid, making syringeability difficult.
Our first approved proprietary product developed using Guardian Technology, ARYMO ER, has a specialized matrix created through our proprietary manufacturing process which controls the release of the API into the blood stream. The matrix, which contains the API as well as inactive agents known as excipients, erodes over time in the GI tract, releasing the API.
In addition to ARYMO ER, we have a pipeline of products developed using our Guardian Technology. Egalet-002, an ER, AD oxycodone formulation, employs a similar matrix system to that used in ARYMO ER, however the Egalet-002 tablet is surrounded by a water-impermeable, non-eroding, hard shell containing polylactic acid ("PLA") that creates a cylinder, with the API-containing matrix exposed at both ends.
Our Guardian Technology employs a proven, reproducible and scalable manufacturing process. While other pharmaceutical companies typically manufacture their ADF products using conventional compression methods, our injection molding technology involves the simultaneous use of both pressure and heat to form tablets using a customized mold. This injection molding technology used to create our matrix and shell is also used in the manufacture of medical devices, including implants and diagnostics. We believe that we are the first company to combine standard pharmaceutical production with injection molding to produce an orally delivered, FDA-approved pharmaceutical product. Based on our proprietary systems and know-how, Guardian Technology may have potential in oral drug delivery for a variety of indications to support internal product development as well as those seeking access via third-party licensing.
Egalet‑002 for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate
Overview
Egalet‑002, an AD, ER, oral oxycodone formulation, has successfully completed a safety and efficacy Phase 3 program. The product is in development for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. Using our Guardian Technology, we developed Egalet‑002 to address common methods of abuse and misuse, including crushing to swallow, snort or smoke, and dissolving to inject. Egalet-002 was specifically designed to address abuse by crushing and snorting, which is the most common method of manipulating oxycodone‑based products for abuse, according to a 2011 article in the Harm Reduction Journal. To focus our efforts on our commercial products, we are not currently investing in Egalet-002. We are looking to potentially partner the program.
7
Clinical Development
On July 17, 2013, we submitted an investigational new drug (“IND”) application for Egalet‑002 to the FDA. The Egalet-002 development program was designed to use the FDA’s Section 505(b)(2) approval pathway using OxyContin as the RLD. We have conducted Phase 1 PK trials of Egalet‑002, a battery of Category 1 AD studies comparing Egalet-002 to OxyContin, a clinical alcohol interaction study and a pilot oral abuse potential study with development product. In December 2016, we announced positive results for the primary endpoint of maximum drug liking from our Category 3 intranasal HAP study comparing Egalet-002 to IR oxycodone. In 2017 we announced positive results from a safety and efficacy Phase 3 study and from an open-label, long-term safety Phase 3 study. The FDA has granted Fast Track status with respect to Egalet‑002. We have determined to delay indefinitely our previously-announced anticipated 2019 filing date for the Egalet-002 New Drug Application as we seek a partner for Egalet-002.
Completed Clinical Trials
We have performed three Phase 1 clinical trials of Egalet‑002 as part of the formulation development and optimization program. The results of these studies demonstrated that Egalet-002 exhibited different PK characteristics relative to OxyContin. In particular, the plasma concentration of oxycodone after administration of Egalet‑002 had a narrower peak‑to‑trough range than OxyContin, while maintaining a similar range of total concentration, as measured by the area under the curve (“AUC”). These results are represented in the table below as follows: Cmax or peak plasma concentration; Cmin or trough plasma concentration; and the total exposure measured as AUC. These results demonstrate that the PK profile of Egalet‑002 shows less fluctuation in plasma oxycodone concentration.
|
|
|
|
|
|
|
|
|
|
|
|
|
Percent |
|
|
Steady State |
|
Egalet‑002 |
|
OxyContin |
|
improvement |
|
||||||
|
Cmin (ng/mL) |
|
|
|
|
22 |
|
|
|
|
18 |
|
20 |
% |
|
Cmax (ng/mL) |
|
|
|
|
48 |
|
|
|
|
59 |
|
23 |
% |
|
AUC (ng/hr/mL) |
|
|
|
|
1008 |
|
|
|
|
942 |
|
N/A |
|
|
[range] |
|
[ |
687 |
‑ |
1519 |
] |
[ |
620 |
‑ |
1782 |
] |
|
|
Results from the Phase 1 clinical program also demonstrated dose proportionality of Egalet‑002 across the dosage range of 10, 20, 40 and 80 mg. In addition, one of the PK studies included a fed arm with the highest dose, 80 mg, to assess the food effect of Egalet‑002. A food effect was observed which was consistent with the magnitude of food effect observed in previous OxyContin studies.
In November 2017, we announced top-line results from a Phase 3 safety study evaluating the safety of Egalet-002. Egalet-002 was generally well-tolerated and the incidence of adverse events reported was generally consistent with outcomes expected following treatment with an ER oxycodone formulation.
Also in November 2017, we announced positive top-line results from a Phase 3 study evaluating the safety and efficacy of Egalet-002 in moderate-to-severe chronic low back pain. This second of two Phase 3 studies was a multicenter, double-blind, enriched enrollment, randomized withdrawal, efficacy and safety study of Egalet-002 versus placebo in opioid-experienced and opioid-naïve patients with moderate-to-severe chronic low back pain. The study met its primary endpoint, which showed a statistically significant difference in average pain intensity from baseline (at randomization) to week 16 between the Egalet-002 and placebo treatment groups (p<0.0001). No new safety concerns were identified in this study.
Completed Abuse‑Deterrent Studies
In accordance with the FDA AD Guidance, we commissioned a third party to conduct Category 1 AD studies of Egalet‑002 to evaluate the physical and chemical properties of Egalet‑002 compared to the ADF of OxyContin. These experiments included the full battery of Phase 1 physical manipulations and Phase 2 chemical extractions as referenced in the FDA AD Guidance to fully interrogate the AD properties of Egalet‑002 compared to OxyContin. In one study,
8
five Egalet‑002 tablets and five OxyContin tablets were milled in a coffee grinder, a household tool commonly used to defeat these tablets for recreational use and abuse, for successive rounds of 20 seconds and then placed on a sieve stack with progressively smaller filters to measure the particle size of the ground up tablets. For Egalet‑002, this can either be done with the outer shell intact or with additional effort to try to remove the shell. The result of this Category 1 study with regard to particle size reduction showed that, for Egalet‑002, 12.5 percent of particles were less than 500 microns (suitable for snorting) compared to 74.2 percent of the particles for OxyContin.
In December 2016, a randomized, double‑blind, double‑dummy, active and placebo‑controlled, crossover study comparing the abuse potential of manipulated Egalet‑002 versus manipulated IR oxycodone and manipulated OxyContin following intranasal administration in nondependent recreational opioid users was completed. For the primary comparison, the difference in maximum “Drug Liking” (Emax) between manipulated Egalet-002 (79.5 with shell on) was lower compared to crushed IR oxycodone (86.6) at a statistically significant level p = 0.0004. The difference was also statistically significant (p <0.0001) for the comparison between manipulated Egalet-002 (77.4 with shell off) and crushed IR oxycodone (86.6). For the secondary outcome of “Take Drug Again” (measured on a bipolar 100-point scale), the results were 69.9 for manipulated Egalet-002 (with shell on), 72.4 for manipulated Egalet-002 (with shell off), and 75.5 for crushed IR oxycodone which, in each case, was not a statistically significant difference. In the study’s exploratory analysis with OxyContin, “Drug Liking” Emax was similar in all treatment arms, with results of 79.5 for manipulated Egalet-002 (with shell on), 77.4 for manipulated Egalet-002 (with shell off), and 76.2 for manipulated OxyContin. “Ease of Snorting” and “Pleasantness of Snorting” were also assessed using a unipolar 100-point scale where 100 represented 'very easy'/'very pleasant' and 0 represented 'very hard'/'very unpleasant.' Ease of Snorting was harder for manipulated Egalet-002 (42.0 with shell on) and manipulated Egalet-002 (49.5 with shell off) compared to manipulated OxyContin (65.1) at statistically significant levels; (p < 0.0001 and p = 0.0050, respectively) and “Pleasantness of Snorting” was lower for manipulated Egalet-002 (40.7 with shell on) and manipulated Egalet-002 (with 44.7 shell off) compared to manipulated OxyContin (55.3) at statistically significant levels as well (p = 0.0003 and p = 0.0081, respectively).
Additional Product Candidates
We have two other product candidates that were developed using our Guardian Technology. Egalet-003, an ADF stimulant product candidate, and Egalet-004, an ADF, ER hydrocodone‑based product candidate for which an initial Phase 1 bioavailability study has been conducted. Like Egalet-002, we are not currently investing in these product candidates and are exploring the possibility of their further development with a partner.
Our proprietary Guardian Technology platform has the potential to be more broadly used with additional types of pharmaceutical products. We believe that the flexibility of our drug delivery systems can be applied to the administration of other classes of APIs, including combination products, where abuse deterrence or a specific release profile is desired. We have developed prototypes, conducted feasibility studies and are exploring additional applications of our Guardian Technology, both on our own and in collaboration with other pharmaceutical companies.
Manufacturing
Overview
Our approved products are manufactured at contract manufacturing facilities in the United States. We have agreements with UPM Pharmaceuticals to manufacture OXAYDO and Halo Pharmaceuticals, Inc. (“Halo”) to produce ARYMO ER. Jubilant Hollister Stier (“JHS”) manufactures SPRIX Nasal Spray for us and we purchase our required quantities of SPRIX Nasal Spray through purchase orders with JHS.
In February 2017, we entered into a manufacturing services agreement with Halo related to the manufacture and supply of ARYMO ER. The agreement has an initial term of five years, and under its terms we are obligated to purchase all requirements for ARYMO ER from Halo through 2019 and seventy-five percent of our requirements for the remainder of the term, subject to certain limited exceptions. We would also use Halo to produce Egalet-002.
9
Our Guardian Technology product ARYMO ER and product candidates are manufactured using our proprietary injection molding process in which the product is molded using pressure and heat. This process is reproducible, scalable and cost‑efficient, and is commonly used in the manufacture of medical devices, including implants and diagnostics. We believe that we are the first company to combine standard pharmaceutical production with plastic injection molding to produce an orally delivered, FDA-approved pharmaceutical product.
Drug Substances
The API used in SPRIX Nasal Spray is ketorolac tromethamine, in OXAYDO is oxycodone hydrochloride, in ARYMO ER is morphine sulfate, and in Egalet‑002 is oxycodone hydrochloride. We currently procure these APIs on a purchase order basis, some of which are pursuant to agreement with one of our suppliers. We acquire ketorolac from a European-based manufacturer, while we secure the opioid APIs from a U.S‑based manufacturer.
Both morphine sulfate and oxycodone hydrochloride are classified as narcotic controlled substances under U.S. federal law. OXAYDO and ARYMO ER are classified as Schedule II controlled substances by the U.S. Drug Enforcement Administration (“DEA”), meaning that these substances have the highest potential for abuse and dependence among drugs that are recognized as having an accepted medical use. We expect that Egalet‑002 also will be classified as a Schedule II controlled substance. Consequently, the manufacturing, shipping, dispensing and storing of our products and product candidates are and will be subject to a high degree of regulation, as described in more detail under the caption “Governmental Regulation—DEA Regulation.”
Intellectual Property
We regard the protection of patents, designs, trademarks and other proprietary rights that we own as critical to our success and competitive position. As of February 28, 2018, we owned 21 issued patents within the United States, and an additional 48 issued foreign patents covering our products, product candidates or technology platform. The term of our overall domestic and foreign patent portfolio related to ARYMO ER, Egalet-002 and our Guardian Technology platform, excluding possible patent extensions, extends to various dates between 2022 and 2033, if pending patent applications in each of our patent families issue as patents.
Two of our U.S. patents relate to ARYMO ER. Both of the ARYMO ER patents relate to composition of matter and method of use. On February 23, 2018, we received notice from Teva Pharmaceuticals USA, Inc. (“Teva”) that Teva has submitted an Abbreviated New Drug Application (“ANDA”) with the FDA seeking regulatory approval to market a generic version of ARYMO ER. The notice from Teva included a “Paragraph IV certification” with respect to the two ARYMO ER patents alleging that these patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of Teva’s proposed generic product. We are currently reviewing the details of Teva’s notice and Paragraph IV certification. Under the Hatch-Waxman Act, we have 45 days from receipt of the notice to determine if it will file a patent infringement suit. If the Company brings such a suit, a stay of approval will be imposed by the FDA on Teva’s ANDA for the earlier of 30 months or a decision in the infringement case that each of the patents is invalid or not infringed. The Company intends to vigorously enforce its intellectual property rights, but cannot predict the outcome of this matter.
Eleven of our U.S. and 28 of our foreign patents relate to Egalet-002 and eight of our U.S. and 20 of our foreign patents relate to our Guardian Technology.
We license seven U.S. patents and five foreign patents from Acura Pharmaceuticals, all of which cover Acura’s Aversion Technology and relate to the composition of matter. Six of the seven U.S. patents are Orange Book listed patents that cover OXAYDO. These patents expire between 2023 and 2025. In addition, we license one U.S. and five foreign patent applications relating to OXAYDO and Acura’s Aversion Technology, all of which relate to the composition of matter.
In addition, we acquired two U.S. patents that expire in 2029 and two pending U.S. patent applications, directed to processes of manufacture, devices, and compositions, related to SPRIX Nasal Spray. We also have an exclusive license to a U.S. patent listed in the Orange Book covering SPRIX Nasal Spray from Recordati that expires in December
10
2018. We are aware that there is the possibility of generic entrants and if those generic products were to come to market, there could be a material impact on our revenues. We are exploring ways to extend the patent life of SPRIX to address this issue.
As of February 28, 2018, we owned four pending patent applications under active prosecution in the United States, and an additional six pending foreign patent applications covering our products, product candidates and technology platform. We have one pending patent application in the United States and two pending foreign patent applications relating to ARYMO ER. In addition, two U.S. and one foreign patent applications relate to Egalet-002 and one U.S. and three foreign patent applications relate to our Guardian Technology. The types of protection that may be afforded by any patents that may issue from these applications include, but are not limited to, composition of matter, process of manufacturing or method of use. Our issued patents provide protection in the United States, Canada and Europe. We have applied for patents in the United States, Canada and Europe.
There currently are no material contested proceedings and/or third-party claims against our patents. See the discussion above for information on Teva’s “Paragraph IV certification” regarding our ARYMO ER Orange Book listed patents.
Research and Development
Historically, we have devoted a significant amount of resources to develop our product candidates. For the years ended December 31, 2015, 2016 and 2017, we recorded approximately $27.1 million, $33.8 million and $14.7 million, respectively, in research and development expenses. With our portfolio of commercial products, we have now shifted our focus to our commercial efforts and minimized expenditures for research and development.
Competition
Our industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition and potential competition from several sources, including pharmaceutical and biotechnology companies, generic drug companies, drug delivery companies and academic and research institutions. We believe the key competitive factors that will affect the development and commercial success of our products and product candidates include their degree of abuse deterrence, onset of action, bioavailability, therapeutic efficacy, and convenience of dosing and distribution, as well as their safety, cost and tolerability profiles and our sales and marketing capabilities. Many of our larger potential competitors have substantially greater financial, technical and human resources than we do, as well as more experience in the development of product candidates and the commercialization of products. Consequently, our competitors may develop ADF products for the treatment of moderate to severe pain or for other indications we may pursue in the future, and such competitors’ products may be more effective, better tolerated and less costly than our product candidates. Our competitors may also be more successful in manufacturing and marketing their products than we are. We will also face competition in recruiting and retaining qualified personnel.
SPRIX Nasal Spray
SPRIX Nasal Spray competes in the short‑term analgesic market which is defined as patients needing therapy for five days or less. There is a high degree of generic competition in this market; however, branded drugs continue to play a key role for patients. There are numerous categories of products in this space and various delivery methods of these analgesics including pills, gels, sprays and injectables. Product categories include NSAIDS such as ibuprofen, diclofenac, celecoxib and ketorolac and IR opioids such as oxycodone, hydrocodone and tapentadol.
OXAYDO
OXAYDO competes with other marketed branded and generic pain therapeutics. Opioid therapeutics generally fall into two classes: codeines, which include oxycodones and hydrocodones, and morphines. OXAYDO is an IR oxycodone and competes with therapeutics within both the codeine and morphine classes. These therapeutics include both Schedule II and Schedule III controlled substance products being marketed by companies such as Endo Pharmaceuticals Holdings Inc., Mallinckrodt, Pfizer, Purdue, Teva and Actavis, Inc.
11
OXAYDO will compete with ROXYBOND, an ADF, IR oxycodone developed by Inspirion and to be marketed by Daiichi Sankyo (“Daiichi”) when it is launched. OXAYDO also will compete with a considerable number of opioid product candidates under development, including abuse deterrent and tamper resistant formulations of currently available opioids, novel opioids and alternative delivery forms of various opioids under development at other pharmaceutical companies, including single‑entity ER hydrocodone product candidates, which include abuse deterrent formulations, being developed by Pfizer, Purdue and Teva. OXAYDO may also face competition from non‑opioid product candidates including new chemical entities, as well as alternative delivery forms of NSAIDs. These new opioid and non‑opioid product candidates are being developed by companies such as Acura, Collegium Pharmaceutical, Inc., Eli Lilly and Company, Elite Pharmaceuticals, Inc., Hospira, Inc., Inspirion Delivery Technologies, LLC, Intellipharmaceutics International Inc., Nektar Therapeutics, Pfizer and QRxPharma Ltd.
ARYMO ER
ARYMO ER competes with branded abuse-deterrent products as well as generic non-abuse-deterrent, long‑acting opioid products labeled for the treatment of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. These products include Pfizer’s Avinza and Embeda, Purdue’s MS Contin, Hysingla and OxyContin, Inspirion’s MorphaBond marketed by Daiichi, Collegium’s Xtampza, Pernix’s Zohydro and generic morphine products produced by Actavis, Mallinckrodt, Rhodes Pharmaceuticals and Mylan.
We believe there are ADF morphine products in clinical development by Purdue and Elite. In addition, any company that has developed an AD technology could initiate an ADF morphine program at any time.
Government Regulations
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug and Cosmetic Act (“FFDCA”) and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post‑approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution. The FDA approval process can be time consuming and cost intensive and companies may, and often do, re-evaluate the path of a particular product or product candidate at different points in the approval and post-approval process, even deciding, in some cases, to discontinue development of a product candidate or take a product off the market.
Pharmaceutical product development in the U.S. for a new product or related to changes to an approved product typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well‑controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre‑market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation, and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
12
A 30‑day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans, unless the FDA authorizes, in writing, that the clinical investigations in the IND may begin sooner than 30 days after submission. If the FDA has neither commented on nor questioned the IND within this 30‑day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with Good Clinical Practices (“GCP”), an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board (“IRB”) for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance, and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit‑risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases, the FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in some instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible. Another scenario which allows for a single Phase 3 efficacy/safety study is via the 505(b)(2) pathway when the investigational product is not bioequivalent to the RLD but can still utilize other safety data from the reference agent for a submission.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical, and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture, and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, currently $2,421,495 for FDA’s fiscal year 2018 (October 1, 2017 through September 30, 2018), and the manufacturer and/or sponsor under an approved NDA are also subject to annual product and establishment user fees, currently $97,750 per product and $512,200 per establishment in FDA’s fiscal year 2018. These fees are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in‑depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for standard review drug products are reviewed within 10-12 months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to drugs that the FDA determines offer a significant improvement in the safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition. The review process for both standard and priority review may be extended by FDA for three additional months to consider major amendments to pending NDAs.
13
The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation, and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but takes into consideration the recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practices (“cGMP”) is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a Risk Evaluation and Mitigation Strategy (“REMS”) to help ensure that the benefits of the drug outweigh the potential risks. Moreover, product approval may require substantial post‑approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Post‑Approval Requirements
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post‑marketing testing, known as Phase 4 testing, REMS, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality‑control, drug manufacture, packaging, and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality‑control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered. In addition, other regulatory action, including, among other things, warning letters, the seizure of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations, civil penalties, and criminal prosecution may be pursued.
As part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. The Prescription Drug Marketing Act (“PDMA”), imposes certain recordkeeping and reporting requirements and other limitations on the distribution of drug samples to physicians. The PDMA also requires that state licensing of distributors who distribute prescription drugs meet certain federal guidelines that include minimum standards for storage, handling and record keeping. In addition, the PDMA and a growing majority of states also impose certain drug pedigree requirements on the sale and distribution of prescription drugs. The PDMA sets forth civil and criminal penalties for violations. In 2010, a statutory provision was enacted that required manufacturers and authorized distributors of record to report on an annual basis certain information about prescription drug samples they distributed, and FDA began to enforce the requirement beginning on April 1, 2015.
14
The Hatch‑Waxman Amendments
Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, preclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding the patented method‑of‑use rather than certify to a listed method‑of‑use patent. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earliest of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant. As described above, on February 23, 2018, we received notice from Teva that Teva has submitted an ANDA to the FDA seeking regulatory approval to market a generic version of ARYMO ER. The notice from Teva included a “Paragraph IV certification” with respect to the two ARYMO ER patents alleging that these patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of Teva’s proposed generic product. We are currently reviewing the details of Teva’s notice and Paragraph IV certification and we intend to vigorously enforce our intellectual property rights, but we cannot predict the outcome of this matter.
The ANDA application also will not be approved until any applicable non‑patent exclusivity listed in the Orange Book for the referenced product has expired.
Exclusivity
Upon NDA approval of a new chemical entity (“NCE”), which is a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which FDA cannot receive any ANDA seeking approval of a generic version of that drug or any Section 505(b)(2) NDA, discussed in more detail below, that relies on the FDA’s findings regarding that drug. A drug may obtain a three‑year period of exclusivity for a change to the drug, such as the addition of a new indication to the labeling or a new formulation, during which the FDA cannot approve an ANDA or any Section 505(b)(2) NDA, if the supplement includes reports of new clinical studies (other than bioavailability studies) essential to the approval of the supplement.
15
An ANDA may be submitted one year before NCE exclusivity expires if a Paragraph IV certification is filed. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period.
Section 505(b)(2) NDAs
Most drug products obtain FDA marketing approval pursuant to an NDA or an ANDA. A third alternative is a special type of NDA, commonly referred to as a Section 505(b)(2) NDA, which enables the applicant to rely, in part, on the FDA’s findings of safety and effectiveness in the approval of a similar product or published literature in support of its application.
Section 505(b)(2) NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by, or for, the applicant and for which the applicant has not obtained a right of reference. If the Section 505(b)(2) applicant can establish that reliance on the FDA’s previous findings of safety and effectiveness is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all, or some, of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
To the extent that the Section 505(b)(2) applicant is relying on the FDA’s findings of safety and effectiveness for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. Thus approval of a Section 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non‑patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earliest of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant. As with traditional NDAs, a Section 505(b)(2) NDA may be eligible for three‑year marketing exclusivity, assuming the NDA includes reports of new clinical studies (other than bioavailability studies) essential to the approval of the NDA.
REMS
The FDA has the authority to require a REMS to ensure the safe use of the drug. In determining whether a REMS is necessary, the FDA must consider the size of the population likely to use the drug, the seriousness of the disease or condition to be treated, the expected benefit of the drug, the duration of treatment, the seriousness of known or potential adverse events, and whether the drug is a new molecular entity. If the FDA determines a REMS is necessary, the drug sponsor must agree to the REMS plan at the time of approval. A REMS may be required to include various elements, such as a medication guide or patient package insert, a communication plan to educate healthcare providers of the drug’s risks, limitations on who may prescribe or dispense the drug, or other measures that the FDA deems necessary to assure the safe use of the drug. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. In addition, the REMS must include a timetable to periodically assess the strategy. The FDA may also impose a REMS requirement on a drug already on the market if the FDA determines, based on new safety information, that a REMS is necessary to ensure that the drug’s benefits outweigh its risks. The requirement for a REMS can materially affect the potential market and profitability of a drug.
In July 2012, the FDA approved a standardized REMS for all extended-release and long acting (LA) opioid drug products to ensure their safe use. ER formulations of morphine, oxycodone, and hydrocodone, among other opioids, are required to have a REMS. This includes ARYMO ER. In September 2017, the FDA issued letters to manufacturers of IR opioid drug products announcing the agency’s intention to require a REMS for IR formulations as well.
16
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA‑regulated products, including drugs, are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved. Competitors may use this publicly‑available information to gain knowledge regarding the progress of development programs.
DEA Regulation
Our products OXAYDO and ARYMO ER are, and our product candidate, Egalet‑002, if approved, will be regulated as “controlled substances” as defined in the Controlled Substances Act of 1970 (“CSA”), which establishes registration, security, recordkeeping, reporting, storage, distribution, importation, exportation and other requirements administered by the DEA. The DEA regulates the handling of controlled substances through a closed chain of distribution. This control extends to the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce.
The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use, and may not be marketed or sold in the United States. A pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. Schedule II drugs are those that meet the following characteristics:
|
· |
high potential for abuse; |
|
· |
currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions; and |
|
· |
abuse may lead to severe psychological or physical dependence. |
OXAYDO, an IR oxycodone product designed to discourage abuse via snorting and ARYMO ER, an AD, ER morphine product, are each listed by the DEA as a Schedule II controlled substance under the CSA and we expect that Egalet‑002, an AD, ER oxycodone product candidate, if approved, will be as well. Consequently, the manufacturing, shipping, storing, selling and using of the products is subject to a high degree of regulation. Schedule II drugs are subject to the strictest requirements for registration, security, recordkeeping and reporting. Also, distribution and dispensing of these drugs are highly regulated. For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist and may not be refilled without a new prescription.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized.
In addition, a DEA quota system controls and limits the availability and production of controlled substances in Schedule I or II. Distributions of any Schedule I or II controlled substance must also be accompanied by special order forms, with copies provided to the DEA. Any of our products regulated as Schedule II controlled substances will be subject to the DEA’s production and procurement quota scheme. The DEA establishes annually an aggregate quota for how much morphine and oxycodone may be produced in total in the United States based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. The limited aggregate number of opioids that the DEA allows to be produced in the United States each year is allocated among individual companies, who must submit applications annually to the DEA for individual production and procurement quotas. We and our license partners and contract manufacturers must receive an annual quota from the DEA in order to produce or procure any Schedule I or Schedule II substance, including morphine sulfate and oxycodone hydrochloride for use in manufacturing ARYMO ER
17
and Egalet‑002 and OXAYDO respectively. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether to make such adjustments. Our, or our contract manufacturers’, quota of an active ingredient may not be sufficient to meet commercial demand or complete clinical trials. Any delay, limitation or refusal by the DEA in establishing our, or our contract manufacturers’, quota for controlled substances could delay or stop our clinical trials or product launches, which could have a material adverse effect on our business, financial position and results of operations.
To enforce these requirements, the DEA conducts periodic inspections of registered establishments that handle controlled substances. Failure to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in administrative, civil or criminal enforcement action that could have a material adverse effect on our business, results of operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate administrative proceedings to revoke those registrations. In certain circumstances, violations could result in criminal proceedings.
Individual states also independently regulate controlled substances. We and our license partners and our contract manufacturers will be subject to state regulation on distribution of these products.
International Regulation
In addition to regulations in the United States, we are subject to a variety of foreign regulations regarding safety and efficacy and governing, among other things, clinical trials and commercial sales and distribution of our products. Whether we obtain FDA approval for a product, we must obtain the necessary approvals by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and can involve additional product testing and additional review periods, and the time may be longer or shorter than that required to obtain FDA approval and, if applicable, DEA classification. The requirements governing, among other things, the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
Many foreign countries are also signatories to the internal drug control treaties and have implemented regulations of controlled substances like those in the United States. Our products will be subject to such regulation which may impose certain regulatory and reporting requirements and restrict sales of these products in those countries.
Under European Union regulatory systems, marketing authorizations may be submitted either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may apply to the remaining member states. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval.
In addition to regulations in Europe and the United States, we will be subject to a variety of other foreign regulations governing, among other things, the conduct of clinical trials, pricing and reimbursement and commercial distribution of our products. If we fail to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Other Healthcare Laws and Compliance Requirements
In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (“HHS”) (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General and
18
other state and local government agencies. For example, pharmaceutical manufacturers’ activities (including sales and marketing activities, as well as scientific/educational grant programs, among other activities) are governed by fraud and abuse laws such as the federal Anti‑Kickback Statute, the federal False Claims Act, as amended, and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. These activities are also potentially subject to federal and state consumer protection and unfair competition laws.
The federal Anti‑Kickback Statute prohibits any person, including a prescription drug manufacturer, or a party acting on its behalf, from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce or reward either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers, on one hand, and prescribers, purchasers, and formulary managers, on the other. The term “remuneration” is not defined in the federal Anti‑Kickback Statute and has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payments, ownership interests and providing anything at less than its fair market value. Although there are several statutory exemptions and regulatory safe harbors protecting certain business arrangements from prosecution, the exemptions and safe harbors are drawn narrowly and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not meet all the criteria for safe harbor protection from federal Anti‑Kickback Statute liability in all cases. The reach of the federal Anti‑Kickback Statute was broadened by the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the “Affordable Care Act”), which, among other things, amended the intent requirement of the federal Anti‑Kickback Statute, such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti‑Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (discussed below) or the civil monetary penalties statute, which imposes fines against any person who is determined to have presented or caused to be presented claims to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Additionally, many states have adopted laws like the federal Anti‑Kickback Statute, and some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any third‑party payor, not only the Medicare and Medicaid programs in at least some cases, and do not contain safe harbors.
The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The “qui tam” provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has violated the False Claims Act, and to share in any monetary recovery. In recent years, the number of suits brought by private individuals has increased dramatically. In addition, various states have enacted false claims laws analogous to the False Claims Act. Many of these state laws apply where a claim is submitted to any third‑party payor and not merely a federal healthcare program. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The False Claims Act has been used to assert liability based on inadequate care, kickbacks and other improper referrals, improperly reported government pricing metrics such as Best Price or Average Manufacturer Price, improper use of Medicare numbers when detailing the provider of services, improper promotion of off‑label uses not expressly approved by FDA in a drug’s label, and allegations as to misrepresentations with respect to the services rendered. Our activities relating to the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products, and the sale and marketing of our products and our service arrangements or data purchases, among other activities, may be subject to scrutiny under these laws. We are unable to predict whether we would be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the cost of defending such claims, as well as any sanctions imposed, could adversely affect our financial performance. Also, the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) created several federal crimes, including healthcare fraud and false statements
19
relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third‑party payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement about the delivery of or payment for healthcare benefits, items or services.
In addition, our marketing activities may be limited by data privacy and security regulation by both the federal government and the states in which we conduct our business. For example, HIPAA and its implementing regulations established standards for “covered entities,” which are certain healthcare providers, health plans and healthcare clearinghouses, regarding the security and privacy of protected health information. While we are not a covered entity under HIPAA, many of our customers are, and this limits the information they can share with us. The American Recovery and Reinvestment Act of 2009 included expansion of HIPAA’s privacy and security standards, called the Health Information Technology for Economic and Clinical Health Act (“HITECH”), which became effective February 17, 2010. Among other things, HITECH makes HIPAA’s security standards (and certain privacy standards) directly applicable to “business associates,” which are entities that perform certain services on behalf of covered entities involving the exchange of protected health information. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. While we do not currently perform any services that would render us a business associate under HIPAA/HITECH, it is possible that we may provide such services in the future and would be subject to the applicable provisions of HIPAA/HITECH. Finally, we may be subject to state privacy and security laws, regulations and other authorities, which may limit our ability to use and disclose identifiable information, and may impose requirements related to safeguarding such information, as well as reporting on breaches.
Additionally, requirements under the federal Open Payments program, created under Section 6002 of the Affordable Care Act and its implementing regulations, require that manufacturers of drugs for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) report annually to HHS information related to “payments or other transfers of value” provided to U.S. physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals. The Open Payments program also requires that manufacturers and applicable group purchasing organizations report annually to HHS ownership and investment interests held in them by physicians (as defined above) and their immediate family members. Manufacturers’ reports are filed annually with the Centers for Medicare & Medicaid Services (“CMS”) by each March 31, covering the previous calendar year. CMS posts disclosed information on a publicly available website. There are also an increasing number of state laws that restrict or prohibit pharmaceutical manufacturers’ interactions with health care providers licensed in the respective states, and that require pharmaceutical manufacturers to, among other things, establish comprehensive compliance programs, adopt marketing codes of conduct, file periodic reports with state authorities regarding sales, marketing, pricing, and other activities, and register/license their sales representatives. A number of state laws require manufacturers to file reports regarding payments and items of value provided to health care providers (similar to the federal Open Payments program). Many of these laws contain ambiguities as to what is required to comply with the laws. These laws may affect our sales, marketing and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state and federal authorities.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties, damages, fines, imprisonment, exclusion from participation in government healthcare programs, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre‑ marketing product approvals, private qui tam actions brought by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government contracts and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. With respect to any of our products sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable privacy laws and post‑marketing requirements, including safety surveillance, anti‑fraud and abuse
20
laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
Impact of Public Pressure on Drug Pricing, Healthcare Reform and Legislation Impacting Payor Coverage
In the United States, federal and state authorities, as well as third-party payors, are increasingly attempting to limit or regulate the price of medical products and services. There is increased scrutiny of prescription drug pricing practices by federal and state lawmakers and enforcement authorities. In addition, there is an emphasis on managed healthcare in the United States, which will put additional pressure on pharmaceutical drug pricing, reimbursement and usage, which may adversely affect our future product sales and results of operations. These pressures can arise from rules and practices of managed care groups, laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general.
The Trump Administration’s budget proposal for fiscal year 2019 contains drug price control measures that could be enacted during the 2019 budget process or in other future legislation. While any proposed measure would require Congress to pass legislation to become effective, these provisions reinforce the Trump Administration’s focus on controlling drug prices. In addition, there have been several recent state and federal lawmaker inquiries and proposed legislation as was the case in California designed to, among other things, bring more transparency to drug pricing by requiring drug companies to notify insurers and government regulators of price increases and provide an explanation of the reasons for the increase. There have also been actions to review the relationship between drug pricing and manufacturer patient assistance programs and to reform government program reimbursement methodologies for drugs.
The U.S. pharmaceutical industry has already been significantly affected by major legislative initiatives, including, for example, the Affordable Care Act. The Affordable Care Act, among other things, imposes a significant annual fee on companies that manufacture or import branded prescription drug medicines. It also contains substantial provisions intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, and impose additional health policy reforms, any or all of which may affect our business. Since its enactment, there have been judicial and Congressional challenges to numerous provisions of the Affordable Care Act. Further, since 2017, both the U.S. Congress and Trump Administration have enacted legislative and regulatory changes to repeal or dismantle certain aspects of the Affordable Care Act. We continue to evaluate the effect that the Affordable Care Act and additional actions by Congress and the Trump Administration to possibly repeal and replace it has on our business.
In the future, there will likely continue to be additional proposals relating to the reform of the U.S. healthcare system, some of which could further limit coverage and reimbursement of pharmaceutical drugs. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amends the Affordable Care Act, effective January 1, 2019, to close the coverage gap in most Medicare drug plans (also known as the Medicare “Donut Hole”), and increases in 2019 the percentage that a drug manufacturer must discount the cost of prescription drugs from 50 percent under current law to 70 percent. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Third‑Party Payor Coverage and Reimbursement
The commercial success of our products and product candidates, if and when approved, depends and will depend, in part, upon the availability of coverage and adequate reimbursement from third‑party payors at the federal, state and private levels. Third‑party payors include governmental programs such as Medicare or Medicaid as well as commercial healthcare plans and pharmacy benefits managers. These third‑party payors may deny coverage or reimbursement for a product or therapy in whole or in part if they determine that the product or therapy was not medically appropriate or necessary. Also, third‑party payors will continue to control costs by limiting coverage through the use of formularies and other cost‑containment mechanisms and the amount of reimbursement for particular procedures or drug treatments.
The cost of pharmaceuticals and devices continues to generate substantial governmental and third‑party payor interest. We expect that the pharmaceutical industry will experience pricing pressures due to the trend toward managed
21
healthcare, the increasing influence of managed care organizations and additional legislative proposals. Our results of operations and business could be adversely affected by current and future third‑party payor policies as well as healthcare legislative reforms.
Some third‑party payors also require pre‑approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. While we cannot predict whether any proposed cost‑containment measures will be adopted or otherwise implemented in the future, these requirements or any announcement or adoption of such proposals could have a material adverse effect on our ability to obtain adequate prices for our product candidates and to operate profitably.
In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. There can be no assurance that our products will be considered medically reasonable and necessary for a specific indication, that our products will be considered cost‑effective by third‑party payors, that an adequate level of reimbursement will be available or that the third‑party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for influencing any act or decision of the foreign entity to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the companies to maintain books and records that accurately and fairly reflect all transactions of the companies, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Other Regulatory Requirements and Challenges to Regulatory Actions
We are also subject to various laws and regulations regarding laboratory practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances in connection with our research and other environmental and safety regulations. In each of these areas, as above, the FDA has broad regulatory and enforcement powers, including, among other things, the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals, any one or more of which could have a material adverse effect on us. Companies may petition governmental agencies, including the FDA, to discuss or take action with regard to regulatory decisions made relating to a product, product candidate or the company itself.
Employees
As of February 28, 2018, we have 131 employees, of which 127 are employed in the United States and four are employed in Denmark. Of our employees, four are in research and development, 96 are in sales and marketing and 31 are in administration. Per the Danish Salaried Act, Danish employees have the right to be represented by a labor union. We consider our employee relations to be good.
Available Information
We file electronically with the Securities and Exchange Commission (the “SEC”) annual reports on Form 10‑K, quarterly reports on Form 10‑Q, current reports on Form 8‑K and amendments to those reports filed or furnished pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. The public may read and copy any materials we have filed with or furnished to the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1‑800‑SEC‑0330. The SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Copies of our annual report on Form 10‑K, quarterly reports on Form 10‑Q, current reports on Form 8‑K, ownership reports for insiders and any amendments to these reports filed with or furnished to the SEC are available free of charge through our internet website
22
(www.egalet.com) as soon as reasonably practicable after filing with the SEC. We use the Investor Relations section of our website as a means of disclosing material non‑public information and for complying with our disclosure obligations under Regulation FD. Accordingly, investors should monitor the Investor Relations section of our website, in addition to following press releases, SEC filings and public conference calls and webcasts.
In addition, we make available free of charge on our internet website:
|
· |
our Code of Conduct; |
|
· |
the charter of our Nominating and Corporate Governance Committee; |
|
· |
the charter of our Compensation Committee; and |
|
· |
the charter of our Audit Committee. |
Investing in our securities involves a high degree of risk. You should carefully consider the risks described below, together with the other information contained in this Annual Report, including our financial statements and the related notes appearing at the end of this Annual Report, before making any investment decision regarding our securities. We cannot assure you that any of the events discussed in the risk factors below will not occur. These risks could have a material and adverse impact on our business, results of operations, financial condition and cash flows, and our future prospects would likely be materially and adversely affected. As a result, the trading price of our securities could decline and you may lose part or all of your investment.
Risks Related to Our Financial Position and Capital Needs
We have incurred significant losses since our inception and have a history of net losses and negative cash flow from operations.
We are a pharmaceutical company at an early stage of commercialization with a limited operating and commercialization history. To date, we have focused on developing ARYMO ER, our product that was approved in January 2017, and our product candidate, Egalet-002, as well as commercializing SPRIX Nasal Spray, OXAYDO and ARYMO ER. Investment in pharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidate will fail to gain regulatory approval or become commercially viable. Similarly, investment in the commercialization of a product may be slow to achieve results. As a result, there is little historical basis upon which to assess how we will respond to competitive or economic challenges or other challenges to our business. Our business and prospects must be considered in light of the risks and uncertainties frequently encountered by pharmaceutical companies in the early stages of commercialization in a difficult and changing environment.
We have generated substantial net losses and negative cash flow from operations since our inception, and we continue to incur significant commercialization and other expenses related to our ongoing operations for our products. For the years ended December 31, 2017, 2016 and 2015, we reported a net loss of approximately $69.4 million, $90.6 million and $57.9 million, respectively.
We expect to incur losses and negative cash flow for the foreseeable future. Our ability to generate sufficient revenues from our products, any products that we may in-license or acquire, and, if approved, Egalet‑002 and any other product candidates that we may develop and partner, will depend on numerous factors described in the following risk factors and elsewhere in this Annual Report. We expect that our gross margin may fluctuate from period to period as a result of changes in product mix sold, potentially by the introduction of new products by us or our competitors, manufacturing efficiencies related to our products and a variety of other factors. Even if we achieve profitability in the
23
future, we may not be able to sustain profitability in subsequent periods. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
We currently generate limited revenue from the sale of products and may never become profitable.
To date, we have generated $47.2 million in total revenue from SPRIX Nasal Spray, OXAYDO and ARYMO ER, and have generated $22.6 million in total revenue since our inception from feasibility and collaboration agreements. Under our collaboration agreement with Shionogi, we received a $10.0 million upfront payment in December 2013 and an additional $10.0 million milestone payment in April 2015, but as this agreement was terminated in December 2015, we will not generate any additional revenue under this agreement.
Our ability to generate additional revenue and become profitable depends upon our ability to, among other things, expand the marketing of SPRIX Nasal Spray, OXAYDO and ARYMO ER and any other products that we may develop, in-license or acquire in the future. Further, even if we are able to partner our product candidates and we decide to seek and are able to successfully achieve regulatory approval for them, we do not know when any of these products will generate revenue for us, if at all. Our ability to generate revenue from our products or any partnered product candidates also depends on a number of additional factors, including our ability to:
|
· |
successfully satisfy any FDA post-marketing requirements for OXAYDO and ARYMO ER, including studies and clinical trials that have been required for other immediate release and extended release/long acting opioid analgesics and individual studies and clinical trials of ARYMO ER; |
|
· |
successfully complete any necessary clinical studies and human abuse liability studies; |
|
· |
Successfully maintain all regulatory filings and labels for our products; |
|
· |
complete and submit NDAs to the FDA and obtain regulatory approval for indications for which there is a commercial market; |
|
· |
appropriately address generic entry into the markets for our products; |
|
· |
set a commercially viable price for our products; |
|
· |
obtain and maintain coverage and adequate reimbursement from third‑party payors, including government payors; |
|
· |
address limitations in our marketing ability as a result of the claims that we are permitted to include in the label for our products, including claims regarding abuse deterrence; |
|
· |
further penetrate the market for existing products and ultimately increase sales for our products relative to our competition; |
|
· |
find suitable partners to help us market, sell and distribute our products, including in other markets; |
|
· |
maintain our intellectual property rights and defend our intellectual property rights from any challenges; |
|
· |
obtain commercial quantities of our products at acceptable cost levels; |
|
· |
continue to develop and/or reconfigure a commercial organization capable of sales, marketing and distribution for the products we intend to sell ourselves in the markets in which we have retained commercialization rights; |
|
· |
obtain FDA approval for additional dosage strengths and new abuse-deterrent claims for OXAYDO; |
24
|
· |
find suitable partners to help us to develop and seek regulatory approval for our product candidates; and |
|
· |
complete and submit applications to, and obtain regulatory approval from, foreign regulatory authorities. |
In addition, our commercial infrastructure results in significant expenses. To manage operations effectively, we will need to continue to improve our operational and management controls, reporting and information technology systems and financial internal control procedures. If we are unable to manage our operations effectively, it may be difficult for us to execute our business strategy and our operating results and business could suffer. Any failure by us to manage our operations effectively could have an adverse effect on our ability to achieve our goals.
Further, because of the numerous risks and uncertainties associated with product development and clinical studies required to achieve additional claims or dosage strengths for our existing products, including that our product candidates may not advance through development or our products or product candidates may not achieve the endpoints of applicable clinical trials, we are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Even if we are able to complete the process described above, we anticipate incurring significant costs associated with commercializing these products.
Even if we are able to generate meaningful revenues from the sale of our products, we may not become profitable and may need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels, need to license or abandon one or more of our products and/or be forced to reduce our operations.
Our current significant indebtedness and any future debt obligations expose us to risks that could adversely affect our business, operating results and financial condition and may result in further dilution to our shareholders.
In April 2015, we completed an offering of $61.0 million aggregate principal amount of our 5.50% convertible senior notes due 2020, or the 5.50% Notes. Interest on the 5.50% Notes is payable semi-annually in arrears on April 1 and October 1 of each year, commencing in October 2015, and the 5.50% Notes mature on April 1, 2020. In addition, in August 2016, we completed the initial closing of our offering of up to $80.0 million aggregate principal amount of our 13% senior secured notes, or our Senior Secured Notes. We issued $40.0 million aggregate principal amount of our Senior Secured Notes at the initial closing, and an additional $40.0 million aggregate principal amount of our Senior Secured Notes in January 2017. Interest on our Senior Secured Notes accrues at a rate of 13% per annum and is payable semi-annually in arrears on March 20th and September 20th of each year. On each such payment date, we will also pay an installment of principal on the Senior Secured Notes in an amount equal to 15% (or 17% if certain sales targets are not met) of the aggregate net sales of OXAYDO, SPRIX Nasal Spray, ARYMO ER and Egalet-002 for the two-consecutive fiscal quarterly period most recently ended, less the amount of interest paid on the Senior Secured Notes on that payment date. The Senior Secured Notes mature on September 20, 2033. On December 20, 2017, we entered into exchange agreements with certain holders of our outstanding 5.50% Notes. Holders of, in the aggregate, approximately $36.4 million of outstanding principal amount of the 5.50% Notes agreed to exchange those notes for, in the aggregate, (i) approximately $23.9 million of 6.50% convertible senior notes due 2024 (‘‘6.50% Notes’’), (ii) a warrant exercisable for 3.5 million shares of our Common Stock and (iii) payments, in cash, of all accrued but unpaid interest as of the closing on the 5.50% Notes exchanged in the transaction (the “Exchange”). Interest is payable semiannually in arrears on January 1 and July 1 of each year commencing on July 1, 2018 and the 6.50% notes mature December 31, 2024. As of December 31, 2017, our total consolidated indebtedness was approximately $128.5 million
Our ability to make payments on the 5.50% Notes, the 6.50% Notes and the Senior Secured Notes depends on our ability to generate cash in the future. We expect to experience negative cash flow for the foreseeable future as we fund our operations and capital expenditures. There can be no assurance that we will be in a position to repay this indebtedness when due or obtain extensions of the maturity dates. We anticipate that we will need to secure additional funding in order for us to be able to satisfy our obligations when due. For example, on February 28, 2018 the closing price of our common stock was $0.75, which is significantly less than the mandatory conversion price under the indenture governing the 5.50% Notes. If the remaining outstanding 5.50% Notes were to mature while our stock price remains “underwater,” we would be required to repay the principal amount in cash. We cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all.
25
When we issued the 6.50% Notes in the Exchange, we were required to reserve for issuance 20,593,103 shares of our common stock, which, if issued, would dilute the stock ownership of our current equity holders. If any additional funding involves the sale of equity securities or convertible securities, it would result in the issuance of additional shares of our capital stock, which would result in dilution to our stockholders. In addition, the indebtedness under the Senior Secured Notes is secured by substantially all of our assets, including our intellectual property. As a result, a default under the Senior Secured Notes indenture could result in the loss of some or all of our assets and intellectual property, which would have a material adverse effect on our business and results of operations. Further, the indentures governing the 5.50% Notes, the 6.50% Notes and the Senior Secured Notes also contain certain customary covenants that limit or restrict our ability to, among other things, incur additional indebtedness or repay existing indebtedness, grant any security interests, pay cash dividends, repurchase our common stock, make loans, or enter into certain transactions without prior consent. If we are delisted from the Nasdaq Global Market, we could be required to repay the holders of our 5.50% Notes in cash and if we are not able to obtain or maintain listing on the Nasdaq Capital Market, we could be required to repay the holders of our 6.50% Notes in cash. The terms of the agreements governing any of our future indebtedness may have similar or additional limitations and restrictions.
This level of debt could have important consequences to you as an investor in our securities. For example, it could:
|
· |
limit our flexibility in planning for and executing the further development, clinical testing, approval and marketing of our products or product candidates, if successfully partnered; |
|
· |
place us at a competitive disadvantage compared to any of our competitors that are less leveraged than we are; |
|
· |
reduce the amount of funds available to fund working capital, capital expenditures and other general corporate purposes; |
|
· |
increase our vulnerability to both general and industry‑specific adverse economic conditions; |
|
· |
limit our ability to engage in acquisitions or other business development activities; and |
|
· |
limit our ability to obtain additional funds. |
In addition, as the magnitude of our principal and interest payments on the Senior Secured Notes will be proportionate to the revenues generated by our products, the nature of the Senior Secured Notes will reduce the amount of cash flow from net product sales that is available for other corporate purposes.
See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources” for a more detailed discussion of the 5.50% Notes, the 6.50% Notes and the Senior Secured Notes.
Servicing our debt requires a significant amount of cash, and we may not have sufficient cash flow from our business to pay our substantial debt.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the remaining 5.50% Notes, the 6.50% Notes and the Senior Secured Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations. Further, the indentures governing our 5.50% Notes, 6.50% Notes and Senior Secured
26
Notes each contain cross-default provisions which could be triggered in the event of a default under any one indenture, which would adversely impact our cash flow and financial condition.
In addition, certain provisions in our outstanding notes could require accelerated payment of principal and interest. For example, our 5.50% Notes, of which $24.6 million remain outstanding after the Exchange, provide that the delisting of our Common Stock from the Nasdaq Global Market would constitute a “fundamental change” under the 5.50% Notes, which would entitle each holder, at the holder’s option, to require us to repurchase for cash all or any portion of such holder’s 5.50% Notes at a repurchase price equal to 100% of the principal amount thereof, plus accrued and unpaid interest thereon. On November 24, 2017, we received a notice from The Nasdaq Stock Market ("Nasdaq") that we were not in compliance with Nasdaq's Listing Rule 5450(b)(2)(A) (the "Rule"), as the minimum market value of our Common Stock had been below $50 million for 30 consecutive business days. Further, on March 8, 2018, we received a notice from Nasdaq that since the minimum closing bid price for our Common Stock had been below $1.00 for 30 consecutive business days, we did not meet the minimum closing bid price requirement in the Rule.
Further, if we were to be delisted from the Nasdaq Global Market and are unable to qualify for or maintain listing on the Nasdaq Capital Market because our minimum market value of our Common Stock is below $35 million or our minimum closing bid price is below $1.00, in each case for 30 consecutive business days (or otherwise), a complete delisting from Nasdaq would qualify as a “fundamental change” under our 6.50% Notes, which would entitle the holder, at the holder’s option, to require us to repurchase for cash all or any portion of such holder’s 6.50% Notes at a repurchase price equal to 100% of the principal amount thereof. See “Risks Related to Ownership of Our Securities -- We may fail to qualify for continued listing on The NASDAQ Global Market which could make it more difficult for our stockholders to sell their shares”
If our common stock is ultimately delisted from the Nasdaq Global Market and we are required to pay the outstanding principal and interest on the 5.50% Notes at that time, or if our Common Stock is ultimately delisted from Nasdaq entirely, and we are required to, in addition, pay the outstanding principal and interest on the 6.50% Notes at that time, our financial condition would be adversely affected. In addition, the indenture governing our Senior Secured Notes limits our ability, and in some cases prohibits us, from making unscheduled payments on subordinated indebtedness, including the 5.50% Notes and the 6.50% Notes. To the extent that we are unable to make such a payment in accordance with the Senior Secured Notes Indenture, it could result in a default under that document and/or other adverse consequences.
If we are unable to refinance our remaining 5.50% Notes or if we are delisted from Nasdaq, our cash flow and financial condition would be adversely affected.
Although we recently completed the Exchange and were able to refinance $36.4 million of the previously outstanding $61 million principal amount of our 5.50% Notes, $24.6 million of our 5.50% Notes remain outstanding and if not refinanced, will mature on April 1, 2020. In addition, as noted above, if we are unable to regain compliance with the Rule, we could be delisted from the Nasdaq Global Market and such delisting would enable the holders of the remaining $24.6 million principal amount of 5.50% Notes to require us to repurchase for cash all or any portion of such holder’s 5.50% Notes at a repurchase price equal to 100% of the principal amount thereof, plus accrued and unpaid interest thereon. Such a large cash outlay would deplete our cash reserves and negatively impact our ability to fund our operations, including as a result of limitations contained in the indenture governing the Senior Secured Notes. If we are unable to refinance the 5.50% Notes, we could be forced to pay off the 5.50% Notes prior to their maturity, which would adversely impact our cash flow and financial condition. Further, if we are delisted from the Nasdaq Global Market and are not eligible for listing on the Nasdaq Capital Market because we are unable to satisfy the market value of listed securities standard and/or the equity standard, the “fundamental change” provision under the 6.50% Notes would be triggered and the holders of our 6.50% Notes would have the right to require us to repurchase for cash prior to maturity all or a portion of such holder’s 6.50% Notes at a purchase price equal to 100% of the principal amount thereof. This would also adversely impact our cash flow and financial condition and, in certain circumstances, could result in a default under our Senior Secured Notes, which would have a material adverse effect on us and could result in our seeking bankruptcy protection.
27
The report of our independent registered public accounting firm contains explanatory language that substantial doubt exists about our ability to continue as a going concern.
The report of our independent registered public accounting firm on our financial statements for the year ended December 31, 2017 contains explanatory language that substantial doubt exists about our ability to continue as a going concern. As noted, our outstanding convertible notes contain redemption features in the event we are not able to maintain our Nasdaq listing. We continue to seek other sources of capital and alternatives to maintain our listing on the Nasdaq, but if we are unable to obtain sufficient financing to support and complete these activities, then we would, in all likelihood, experience severe liquidity problems and may have to curtail our operations. In addition, volatility in the capital markets may be a significant obstacle to raising the required funds. If we curtail our operations, we may be placed into bankruptcy or undergo liquidation, the result of which will adversely affect the value of our common shares.
The consolidated financial statements included in this Annual Report on Form 10-K do not include any adjustments that might be necessary should we be unable to continue as a going concern. If the going concern basis were not appropriate for these financial statements, adjustments would be necessary in the carrying value of assets and liabilities, the reported expenses and the balance sheet classifications used.
Based on our current operating plan, if we are able to gain compliance with the continued listing requirements of the Nasdaq and avoid a delisting and a put right under our 5.50% Notes and 6.50% Notes, we estimate that our existing cash, cash equivalents and short-term investments as of December 31, 2017 will enable us to fund our cash requirements into 2020.
As of December 31, 2017, we had cash, cash equivalents and short-term investments of $91.0 million. We estimate that this amount will be sufficient to fund our cash requirements into 2020, assuming, among other assumptions, that our stock remains listed on Nasdaq. If we do not maintain compliance with Nasdaq listing requirements, there may be doubt that we can continue as a going concern, or we may be forced to sell our company or our assets, cease or wind down operations, seek protection under the provisions of the U.S. Bankruptcy Code, or otherwise liquidate and dissolve our company.
Our forecast of the period through which our financial resources will be adequate to support our operations is a forward-looking statement and involves assumptions, risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in this Form 10-K. We have based this estimate on assumptions that may prove to be incorrect, and we could use our available capital resources sooner than we currently expect or we could determine earlier that a sale of our company or our assets, petition for relief under the provisions of the U.S. Bankruptcy Code, or other liquidation or dissolution of our company is warranted.
If we require additional capital to fund our operations and we fail to obtain necessary financing, we may be unable to successfully market and promote our products, acquire new products or enhance the profiles of our products.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to commercialize our products, as well as to enhance the profiles of our products
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our products or one or more of our other research and development initiatives or delay our ability to acquire or license new products or product candidates. We also could fail to complete our post-marketing requirements, fail to maintain our regulatory approvals or our intellectual property or be required to further curtail our operations, including by discontinuing the sale of one or more of our products.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward‑looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in this “Risk Factors” section. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we
28
currently expect. Our future funding requirements, both near and long‑term, will depend on many factors, including, but not limited to:
|
· |
increasing sales of our products; |
|
· |
complying with and completing FDA post-marketing requirements on OXAYDO and ARYMO ER and PREA commitment for SPRIX Nasal Spray; |
|
· |
the initiation, progress, timing, costs and results of clinical trials for our products and any future products we may in‑license or acquire; |
|
· |
any generic entry into one or more of the markets for our products; |
|
· |
the ability to obtain abuse‑deterrent claims in the labels for our products; |
|
· |
the number and characteristics of products, any partnered product candidates, and any products that we in‑license or acquire; |
|
· |
the outcome, timing and cost of regulatory approvals by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect; |
|
· |
our ability to maintain regulatory approvals by the FDA and comparable foreign regulatory authorities once obtained; |
|
· |
our ability to obtain API through the allocation process handled by the DEA; |
|
· |
the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
· |
the effect of competing technological and market developments, including pressure on the opioid market and the expected decline in the prescribing of opioids; |
|
· |
the cost and timing of completion of commercial‑scale outsourced manufacturing activities for any products we develop, in-license or acquire; |
|
· |
the timing and amount of revenue from sales of our products; |
|
· |
our ability to achieve milestones under any license or collaboration agreement that we have entered or may enter into in the future; |
|
· |
the size and cost of our commercial infrastructure; |
|
· |
our ability to maintain coverage and adequate reimbursements from third party payors and to gain inclusion on applicable formularies; |
|
· |
our ability to continue to be listed on the Nasdaq Global Market; |
|
· |
costs and timing of completion of any outsourced commercial manufacturing supply arrangements that we may establish; and |
|
· |
costs associated with any third-party litigation. |
29
Despite our current debt levels, we may still incur additional debt or take other actions which would intensify the risks discussed above.
Despite our current consolidated debt levels, we and our subsidiaries may be able to incur certain additional debt in the future, subject to the restrictions contained in our debt instruments, some of which may be secured debt. In certain situations, the terms of the indentures governing the 5.50% Notes, 6.50% Notes and Senior Secured Notes permit us to incur additional debt, secure existing or future debt, recapitalize our debt or take a number of other actions that could have the effect of diminishing our ability to make payments on our existing debt when due. The indenture governing our Senior Secured Notes restricts our ability to incur certain additional indebtedness, including certain secured indebtedness, subject to certain exceptions, but if the Senior Secured Notes mature or are repaid, we may not be subject to such restrictions under the terms of any subsequent indebtedness.
Our recently-issued warrants to purchase our Common Stock contain anti-dilution and other provisions which may affect our ability to secure future equity financing or reduce the proceeds of any equity financing that would otherwise be available to us.
In our July 2017 equity offering, we sold our Common Stock and warrants to purchase our Common Stock. The warrants contain anti-dilution provisions, which provide that any new issuances of Common Stock or securities convertible into Common Stock at a price, exercise price or conversion price below those of the warrants will result in the exercise price of the warrants being reduced based upon the number of securities issues in such issuance and the number of shares of our Common Stock then outstanding. For example, we were required to reduce the conversion price of our outstanding warrants twice (from an initial conversion price of $2.70 to a final conversion price of $2.14) due to sales or later issuances of our Common Stock (or securities convertible into our Common Stock) at lower prices. In addition, the warrants prohibit the Company from entering into “Variable Rate Transactions” (as defined in the warrant) which are defined generally to include issuances of securities exercisable or convertible into the Company’s common stock at an exercise or conversion price that is not determinable at the time of such original issuance.
These anti-dilution and other provisions may affect our ability to secure future equity financing or reduce the proceeds of any equity financings that would be otherwise available to us.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies, products or product candidates.
We may seek additional capital through a combination of private and public equity offerings, debt financings, receivables or royalty financings, strategic partnerships and alliances and licensing arrangements. We do not currently have any committed external sources of funds. Moreover, our existing debt places significant limitations on our ability to incur additional indebtedness. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of existing stockholders. Debt, receivables and royalty financings may be coupled with an equity component, such as warrants to purchase stock, which could also result in dilution of our existing stockholders’ ownership. For example, our 5.50% Notes and 6.50% Notes are, subject to certain conditions, convertible into shares of our common stock at the option of the debt holder. The incurrence of additional indebtedness would result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens being placed on our assets and intellectual property. If we were to default on such indebtedness, we could lose such assets and intellectual property. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts, otherwise significantly curtail operations or grant rights to develop and market our technologies that we would otherwise prefer to develop and market ourselves. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us.
30
Risks Related to the Commercialization of Our Products
Our products are and may become subject to unfavorable pricing regulations, third‑party reimbursement practices or healthcare reform initiatives, which could harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. Current and future legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. For example, recent events have resulted in increased public and governmental scrutiny of the cost of drugs, especially in connection with price increases following companies’ acquisitions of the rights to certain drug products. In particular, the U.S. Senate Health, Education, Labor and Pensions Committee recently held hearings aimed at understanding drug pricing issues, which involved testimony from drug, pharmacy, and distribution groups. While no decisions resulted from the hearings, the hearings demonstrate the continued focus of the U.S. Congress on pricing issues. Our revenue and future profitability could be negatively affected if these inquiries were to result in legislative or regulatory proposals that limit our ability to increase the prices of our products. Further, legislation has been introduced in the U.S. Congress and several state legislatures that allows price controls in various circumstances, requires enhanced transparency in how pricing is established, caps or penalizes price inflation beyond certain parameters and ties pricing to federal supply schedules, among other initiatives. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we or our collaborator might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, which could negatively impact the revenues we are able to generate from the sale of the product in that particular country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more of our products.
Our ability to commercialize our products, including any products we may in-license or acquire, successfully will also depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers, pharmacy benefit managers and other organizations. Government authorities and third‑party payors, such as private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. A primary trend in the United States healthcare industry and elsewhere is cost containment. Government authorities and other third‑party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third‑party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. For example, in late 2017, we were notified by CVS Caremark, a pharmacy benefits manager, that SPRIX Nasal Spray would no longer be on its formulary for a portion of its commercial covered lives beginning January 1, 2018, which will have an adverse effect on our revenues. We cannot be sure that coverage and reimbursement will be available for our products, or any product that we commercialize, or that we will obtain such coverage and reimbursement in a timely fashion. For example, because the majority of patients taking morphine are government insured, and gaining government coverage is typically a lengthy process, we could face commercial challenges in gaining coverage for ARYMO ER. Assuming we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate or may require co‑payments that patients find unacceptably high. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Coverage and reimbursement may impact the demand for, or the price of, any of our products. If coverage and reimbursement are not available or reimbursement is available only to limited levels, we may not be able to successfully commercialize our products. In addition, when we seek coverage for our products, we may be requested to submit bids that include all three of our products for consideration at the same time, which could result in demand for and agreement to higher rebates on one or more of our products than would occur if each were bid in isolation.
There may be significant delays in obtaining coverage and reimbursement for newly-approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may only be
31
temporary. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private third-party payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Private third‑party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable reimbursement rates from both government‑funded and private payors for our products could hamper our ability to generate widespread prescription demand and would have a material adverse effect on our operating results, our ability to raise capital and our overall financial condition.
If physicians and patients do not accept and use our products, we will not achieve sufficient product revenues and our business will suffer.
If our products do not achieve coverage by third‑party payors and/or broad market acceptance by physicians and patients, the commercial success of those products and revenues that we generate from those products will be limited. Acceptance and use of our products will depend on a number of factors including:
|
· |
the timing of market introduction of competitive products; |
|
· |
the ability to obtain abuse-deterrent claims in the labels for our products at all, and broad enough abuse-deterrent claims that encompass common forms of abuse to demonstrate a substantial benefit to health care providers and patients; |
|
· |
any exclusivity rights a competitor’s products may have; |
|
· |
the results of any required Phase 4 studies following any product approval to support the continued use of any abuse-deterrent claims; |
|
· |
approved indications, warnings and precautions language that may be less desirable than anticipated; |
|
· |
perceptions by members of the healthcare community, including physicians, about the safety and efficacy of our products and, in particular, the efficacy of our abuse‑deterrent technology in reducing potential risks of unintended use; |
|
· |
published studies demonstrating the cost effectiveness of our products relative to competing products; |
|
· |
the potential and perceived advantages of our products and product candidates over alternative treatments; |
|
· |
availability of coverage and adequate reimbursement for our products from government and third-party payors; |
|
· |
the steps that prescribers and dispensers must take, since our products are controlled substances, as well as the perceived risks based upon their controlled substance status; |
|
· |
any negative publicity related to our or our competitors’ products that include the same active ingredient as our products; |
|
· |
the prevalence and severity of adverse side effects, including limitations or warnings contained in a product’s FDA approved product labeling; |
|
· |
legislative, regulatory or administrative enforcement actions against opioid manufacturers; |
|
· |
any quality issue that may arise in the manufacturing or distribution of our products; and |
32
|
· |
effectiveness of marketing and distribution efforts by us and other licensees and distributors. |
Because we expect to rely on sales generated by our products to achieve profitability in the future, the failure of our products to achieve market acceptance would harm our business prospects.
Our future prospects are dependent on the success of our products, and we may not be able to successfully commercialize these products. Failure to do so would adversely impact our financial condition and prospects.
A substantial portion of our resources are focused on the commercialization of our products, OXAYDO, SPRIX Nasal Spray and ARYMO ER. Our ability to generate significant product revenues and to achieve commercial success in the near‑term will initially depend in large part on our ability to successfully commercialize these products in the United States. If we fail to successfully commercialize our current and future products, we may be unable to generate sufficient revenues to sustain and grow our business, and our business, financial condition and results of operations will be adversely affected.
Our limited history of commercial operations makes evaluating our business and future prospects difficult, and may increase the risk of any investment in our shares.
Following our acquisition and license in January 2015 of SPRIX Nasal Spray and OXAYDO, respectively, and the approval by the FDA of ARYMO ER in January 2017, we have three products approved in the United States. However, we have a limited history of marketing these products. We began the commercial activities for OXYADO in the United States in the third quarter of 2015 and for ARYMO ER in the first quarter of 2017. SPRIX Nasal Spray has remained commercially available in the United States following our acquisition of the product on January 8, 2015. To date, sales of our marketed products, while growing, have not been significant, particularly as compared to the costs associated with the commercial infrastructure we have created and the commercialization efforts we have undertaken. We face considerable risks and difficulties as a company with limited commercial operating history. If we do not successfully address these risks, our business, prospects, operating results and financial condition will be materially and adversely harmed. Our limited commercial operating history, including our limited history commercializing SPRIX Nasal Spray, OXAYDO and ARYMO ER, makes it particularly difficult for us to predict our future operating results and appropriately budget for our expenses. In the event that actual results differ from our estimates or we adjust our estimates in future periods, our operating results and financial position could be materially affected.
We are a small company with limited sales, marketing and market access capabilities and, if we are unable to effectively utilize our sales, marketing and market access resources or enter into strategic alliances with collaborators, we may not be successful in commercializing our products or any products that we may in-license or acquire.
We have limited sales, marketing, market access and distribution capabilities compared to some of our competitors. We cannot guarantee that we will be successful in marketing our products or any products that we may in license or acquire in the United States. Factors that may inhibit our efforts to commercialize our product candidates in the United States include:
|
· |
our inability to recruit and retain adequate numbers of effective sales, marketing and market access personnel; |
|
· |
the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe our products over competitive products; and |
|
· |
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
|
· |
our inability to secure formulary coverage that provides broad product access. |
33
If we are not successful in effectively deploying our limited sales, marketing and market access capabilities or if we do not successfully enter into appropriate collaboration arrangements, we will have difficulty commercializing our products or any products that we may in-license or acquire. Outside the United States, where we intend to commercialize our products by entering into agreements with third‑party collaborators, we may have limited or no control over the sales, marketing and distribution activities of these third parties, in which case our future revenues would depend heavily on the success of the efforts of these third parties.
If we are unable to recruit, retain and effectively train qualified sales personnel, our performance could suffer.
While we compete with other pharmaceutical and biotechnology companies, many of those companies are larger or have more resources, to recruit, hire, train and retain qualified sales personnel. If we are not successful in continuing to recruit and retain sales personnel, we may not be successful in commercializing our products or any products that we may in-license or acquire. Further, we will need to provide our salesforce with the quality training, support, guidance and oversight, including with respect to compliance with applicable law, in order for them to be credible and effective. If we fail to perform these commercial functions, our products may not achieve their maximum commercial potential or any significant level of success at all, which could have a material adverse effect on our financial condition, share price and operations. The deterioration or loss of our salesforce would materially and adversely impact our ability to generate sales revenue, which would hurt our results of operations.
If we fail to obtain the necessary regulatory approvals, or if such approvals are limited, we will not be able to fully commercialize our products and our financial performance could suffer.
We have and will submit supplemental applications to the FDA for our products. The FDA may not approve supplemental applications we make to add dosage strengths for our products or to strengthen the labels for our products with additional labeling claims, including claims regarding abuse deterrence, which we believe are necessary or desirable for the successful commercialization of our products and product candidates. The FDA could also decide that any approval would require us to perform additional clinical studies, which could be costly. For example, in June 2017, we received a complete response letter from the FDA regarding the prior approval supplement of OXAYDO in 10 mg and 15 mg dosage strengths in which the FDA requested more information regarding the effect of food on OXAYDO 15 mg and the intranasal abuse-deterrent properties of OXAYDO 10 mg and 15 mg. In addition, we filed an sNDA with the FDA in December 2016 based on Category 1 AD data that demonstrated that OXAYDO resists syringeability, which could potentially deter abuse through the intravenous route. Based on discussions with the FDA regarding the sNDA, we believe a contemporary intranasal HAP study would be needed to complete the sNDA. Given that the issues involved in both the sNDA and prior approval supplement are intertwined, we are evaluating our options and the costs associated to proceed on the AD label and/or the additional dosage strengths.
Further, later discovery of previously unknown problems or adverse events could result in additional regulatory restrictions, including withdrawal of products if the benefits of such products do not outweigh the risks. The FDA may also require us to perform lengthy Phase 4 post‑approval clinical efficacy or safety trials. These trials could be very expensive. The FDA may also require us to amend our labels based on outcomes of on-going Phase 4 commitments for OXAYDO and ARYMO ER. Addressing these regulatory issues, if any, may impact the commercial availability of these products, which could have an adverse effect on our financial performance.
Our ability to market and promote our products in the United States by describing their abuse‑deterrent features will be determined by the FDA‑approved label for them.
The commercial success of certain of our products will depend upon our ability to obtain FDA approved labeling describing their abuse‑deterrent features or benefits or to strengthen our FDA approved labeling. Our failure to achieve FDA approval of product labeling containing such information, or containing such information for all potential routes of abuse, will prevent or substantially limit our advertising and promotion of the abuse‑deterrent features of our products to differentiate them from other opioid products containing the same active ingredients. This may make certain of our products less competitive in the market.
34
The FDA has publicly stated that explicit claims that a product is expected to result in a meaningful reduction of abuse must be supported by randomized, double‑blind, controlled clinical studies of the abuse potential of the drug and that explicit claims that a product has demonstrated reduced abuse in the community will be required to be supported by post‑marketing data, including formal post‑marketing studies evaluating the effect of abuse‑deterrent formulations. In addition, recent FDA advisory committee meetings have highlighted the FDA’s desire that newly approved abuse-deterrent products be supported by studies with regard to all routes of abuse. If the FDA does not approve labeling containing such information, we will not be able to promote such products based on some or all of their abuse‑deterrent features, may not be able to differentiate such products from other opioid products containing the same active ingredients. For example, in January 2017, the FDA approved abuse-deterrent labeling for ARYMO ER for the intravenous route of abuse, but did not approve the inclusion of abuse-deterrent claims for the oral and intranasal routes of abuse. The intranasal abuse-deterrent claim was blocked by another company’s exclusivity.
In addition, recent public comments from FDA and members of Congress have highlighted the importance of addressing the opioid abuse epidemic. Given the changing legislative and regulatory environment, it is difficult to predict how existing laws and regulations may affect the future approval and continued marketing of opioids, including those that fulfill current abuse-deterrent FDA guidance. For example, the FDA granted tentative approval for a supplement we submitted in early 2017 to update the ARYMO ER prescribing information with data from a Category 2/3 intranasal HAP study and an intranasal abuse-deterrent claim, which was excluded from the label at the time of the original new drug application (NDA) approval due to exclusivity granted to another company. Although we expect to receive final approval when the exclusivity period expires on October 2, 2018, given the dynamic political environment related to opioids, the FDA could change its position once the exclusivity period has passed. Further, based on discussions with the FDA regarding a sNDA we filed for OXAYDO to support an abuse-deterrent label claim for the intravenous route of abuse, we believe a contemporary intranasal HAP study would be needed to complete the sNDA. If we are unable to obtain an abuse-deterrent claim for OXAYDO, the commercial opportunity for OXAYDO could be adversely impacted.
Because the FDA closely regulates promotional materials and other promotional activities, even if the FDA initially approves product labeling that includes a description of the abuse‑deterrent characteristics of our product, the FDA may object to our marketing claims and product advertising campaigns. This could lead to the issuance of warning letters or untitled letters, suspension or withdrawal of our products from the market, recalls, fines, disgorgement of money, operating restrictions, injunctions or criminal prosecution. Any of these consequences would harm the commercial success of our products.
We may not be able to obtain three-year FDA regulatory exclusivity for certain aspects of our products and if partnered and approved, our product candidates.
Under certain circumstances, the FDA provides periods of regulatory exclusivity following its approval of an NDA, which provide the holder of an approved NDA limited protection from new competition in the marketplace for the innovation represented by its approved drug. Three-year exclusivity is available to the holder of an NDA, including a 505(b)(2) NDA, for a particular condition of approval, or change to a marketed product, such as a new formulation or new labeling information for a previously approved product, if one or more new clinical trials, other than bioavailability or bioequivalence trials, were essential to the approval of the application and were conducted or sponsored by the applicant.
There is a risk that the FDA may take the view that the studies that we are conducting are not clinical trials, other than bioavailability and bioequivalence studies, that are essential to approval and therefore do not support three-year exclusivity. Further, the FDA may decide that any exclusivity is limited (such as to a particular formulation) and does not block approval of subsequent applications for competing products that differ in certain respects from our product. Finally, to the extent that the basis for exclusivity is not clear, the FDA may determine to defer a decision until it receives an application which necessitates a decision.
If we do obtain three years of exclusivity, such exclusivity will not block all potential competitors from the market. Competitors may be able to obtain approval for similar products with different forms of abuse-deterrent mechanisms or may be able to obtain approval for similar products without an abuse-deterrent mechanism.
35
We face intense competition, including from generic products. If our competitors market or develop generic versions of our products or alternative treatments that are marketed more effectively than our products or are demonstrated to be safer or more effective than our products, our commercial opportunities will be reduced or eliminated.
Our products compete against numerous branded and generic products already being marketed and potentially those that are or will be in development. Many of these competitive products are offered in the United States by large, well‑capitalized companies.
If the FDA or other applicable regulatory authorities approve generic products that compete with any of our products, including generic products with abuse-deterrent claims, it could reduce our sales of those product. Once an NDA, including a Section 505(b)(2) application, is approved, the product covered thereby becomes a “listed drug” which can, in turn, be cited by potential competitors in support of approval of an abbreviated new drug application (“ANDA”). The FFDCA, FDA regulations and other applicable regulations and policies provide incentives to manufacturers to create modified, non‑infringing versions of a drug to facilitate the approval of an ANDA or other application for generic substitutes. Depending on the product, these manufacturers might only be required to conduct a relatively inexpensive study to show that their product has the same active ingredient(s), dosage form, strength, route of administration, and conditions of use, or labeling, as our product candidate and that the generic product is absorbed in the body at the same rate and to the same extent as, or is bioequivalent to, our products. Generic equivalents may be significantly less costly than ours to bring to market and companies that produce generic equivalents are often able to offer their products at lower prices. Thus, after the introduction of a generic competitor, a significant percentage of the sales of any branded product are typically lost to the generic product. Accordingly, competition from generic equivalents to our products would substantially limit our ability to generate revenues and therefore to obtain a return on the investments we have made in our products and product candidates. For example, the patent for SPRIX Nasal Spray expires in December 2018; however, we are a party to agreements that permit the potential launch of generics in March 2018. We cannot be certain what impact generic products would have on our revenues from SPRIX Nasal Spray, or our operating results generally. In addition, on February 23, 2018, we received notice from Teva that Teva has submitted an ANDA with the FDA seeking regulatory approval to market a generic version of ARYMO ER. The notice from Teva included a “Paragraph IV certification” with respect to two of our Orange Book-listed patents, alleging that the patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of Teva’s proposed generic product. Under the Hatch-Waxman Act, we have 45 days from receipt of the notice to determine if we will file a patent infringement suit. If we do bring such a suit, a stay of approval will be imposed by the FDA on Teva’s ANDA for the earlier of 30 months or a decision in the infringement case that each of the patents is invalid or not infringed. We intend to vigorously enforce our intellectual property rights, but cannot predict the outcome of this matter.
Our competitors may also develop products that are more effective, more abuse-deterrent, better tolerated, subject to fewer or less severe side effects, more useful, more widely‑prescribed or accepted, or less costly than ours. For each product we commercialize, sales and marketing efficiency are likely to be significant competitive factors. While we have our own internal salesforce, which markets our products in the United States, there can be no assurance that we can maintain these capabilities in a manner that will be cost efficient and competitive with the sales and marketing efforts of our competitors, especially since some or all of those competitors could expend greater economic resources than we do and/or employ third‑party sales and marketing channels.
Our products may be associated with undesirable adverse reactions or result in significant negative consequences.
Undesirable adverse reactions associated with our products could cause us, our institutional review boards, clinical trial sites or regulatory authorities to interrupt, delay or halt clinical trials and could result in a restrictive product label or the delay, denial or withdrawal of regulatory approval by the FDA or foreign regulatory authorities.
36
If we or others identify undesirable adverse events associated with any of our products a number of potentially significant negative consequences could result, including:
|
· |
we may have to significantly alter our promotional campaigns or activities or we may be forced to suspend marketing of the product entirely; |
|
· |
regulatory authorities may withdraw their approvals of the product or impose restrictions on its distribution; |
|
· |
regulatory authorities may require additional warnings or contradictions in the product label that could diminish the usage or otherwise limit the commercial success of the product; |
|
· |
we may be required to conduct additional post-marketing studies; |
|
· |
we could be sued and held liable for harm caused to patients; and |
|
· |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our products and our business, financial condition and results of operations may be adversely affected.
Recently enacted and future legislation may increase the difficulty and cost for us to commercialize our product candidates, may reduce the prices we are able to obtain for our products and our product candidates and hinder or prevent the commercial success.
Before we can market and sell products in a particular jurisdiction, we need to obtain necessary regulatory approvals (from the FDA in the United States and from similar foreign regulatory agencies in other jurisdictions) and in some jurisdictions, reimbursement authorization. There are no guarantees that we or our commercialization partners will obtain any additional regulatory approvals for our products. Even if we or our commercialization partners obtain or maintain all of the necessary regulatory approvals, we may never generate significant revenues from any commercial sales of our products.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of any product candidates we are able to partner, restrict or regulate post‑approval activities or affect our ability to profitably sell our products.
While there have been repeated calls and attempts to repeal the Affordable Care Act, the Affordable Care Act, among other things, imposes a significant annual fee on companies that manufacture or import branded prescription drug products. It also contains substantial new provisions intended to, among other things, broaden access to health insurance, reduce or constrain the growth of health care spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, modify the definition of “average manufacturer price” for Medicaid reporting purposes thus affecting manufacturers’ Medicaid drug rebates payable to states and impose additional health policy reforms, any of which could negatively impact our business. A significant number of provisions are not yet, or have only recently become, effective, but the Affordable Care Act is likely to continue the downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Other legislative changes have also been proposed and adopted since the Affordable Care Act was enacted. For example, the Budget Control Act of 2011 resulted in aggregate reductions in Medicare payments to providers of up to 2% per fiscal year, starting in 2013, and, due to the Bipartisan Budget Act of 2015, will remain in effect through 2025 unless additional Congressional action is taken, and the American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several types of providers and increased the statute of limitations period for the
37
government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could impose additional financial pressure on our customers, which could in turn diminish demand for our products or result in pricing pressure on us. In addition, drug pricing by pharmaceutical companies has recently come under close scrutiny. The current administration has indicated that reducing the price of prescription drugs will be a priority of the administration. If healthcare policies or reforms intended to curb healthcare costs are adopted, or if we experience negative publicity with respect to pricing of our products or the pricing of pharmaceutical drugs generally, the prices that we charge for our products may be limited, our commercial opportunity may be limited and/or our revenues from sales of our products may be negatively impacted.
We expect that the Affordable Care Act, as well as other healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and additional downward pressure on the price that we receive for our products, and could seriously harm our future revenues. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from third-party payors. The implementation of cost containment measures or other healthcare reforms may compromise our ability to generate revenue, attain profitability or commercialize our products. Any new laws or regulations that have the effect of imposing additional costs or regulatory burden on pharmaceutical manufacturers, or otherwise negatively affect the industry, would adversely affect our ability to successfully commercialize our products.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent approval of any of our applications, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
The 21st Century Cures Act, which the U.S. House of Representatives passed in July 2015 and former President Obama signed into law in December 2016, provides a wide range of reforms, such as broadening the types of data required to support drug approval, extending protections from generic competition, accelerating approval of breakthrough therapies, expanding the orphan drug product program, and clarifying how manufacturers communicate about their products.
In addition, state pharmacy laws may permit pharmacists to substitute generic products for branded products if the products are therapeutic equivalents, or may permit pharmacists and pharmacy benefit managers to seek prescriber authorization to substitute generics in place of our product candidates, which could significantly diminish demand for them and significantly impact our ability to successfully commercialize our product candidates and generate revenues.
Further, various federal and state legislative and regulatory initiatives that seek to curtail patient supply of opioids, limit access to products that exceed specific morphine equivalent daily dose thresholds, or curtail or prohibit manufacturers from offering any discounts to enrollees to reduce their out-of-pocket expenses may have a negative impact on us. California recently enacted a law restricting manufacturers from paying co-pays for branded products if an AB-rated generic equivalent is available. While this law does not currently impact our products, California’s law could spur other states to adopt similar legislation, even legislation that is more restrictive. Any such law could adversely impact the utilization of our products and harm our commercial prospects. Although not successful so far, certain states have proposed legislation that would establish a tax on opioids to be paid by the manufacturer could negatively impact our financial condition.
Social issues around the abuse of opioids, including law enforcement concerns over diversion of opioid and regulatory efforts to combat abuse, could decrease the potential market for ARYMO ER and OXAYDO.
Media stories regarding prescription drug abuse and the diversion of opioids and other controlled substances are commonplace. Law enforcement and regulatory agencies may apply policies that seek to limit the availability of opioids, or remove opioids from the market entirely. Such efforts may inhibit our ability to commercialize ARYMO ER or OXAYDO. Aggressive enforcement and unfavorable publicity regarding, for example, the use or misuse of opioid drugs, the limitations or unintended consequences of abuse‑resistant formulations, public inquiries and investigations into
38
prescription drug abuse, litigation or regulatory activity relating to sales, marketing (including providing meals to doctors), distribution (including with respect to high prescribers of opioids), or storage of our drug products could harm our reputation. In addition, payments to doctors to participate in speaker programs or payments to industry groups could reflect negatively on us. Such negative publicity could reduce the potential size of the market for ARYMO ER and OXAYDO and decrease the revenues and royalties we are able to generate from their sale. Similarly, to the extent opioid abuse becomes less prevalent or a less urgent public health issue, regulators and third-party payors may not be willing to pay a premium for abuse‑deterrent formulations of opioids.
In addition, efforts by the FDA and other regulatory bodies to combat abuse of opioids may negatively impact the market for our products. For example, in February 2016, as part of a broader initiative led by HHS to address opioid-related overdose, death and dependence, the FDA released an action plan to address the opioid abuse epidemic and reassess the FDA’s approach to opioid medications. The plan identifies the FDA’s focus on implementing policies to reverse the opioid abuse epidemic, while maintaining access to effective treatments. The actions set forth in the FDA’s plan include strengthening post marketing study requirements to evaluate the benefit of long-term opioid use, changing the REMS requirements to provide additional funding for physician education courses, releasing a draft guidance setting forth approval standards for generic abuse-deterrent opioid formulations, and seeking input from the FDA’s Scientific Board to broaden the understanding of the public risks of opioid abuse. The FDA’s plan is part of a broader initiative led by the HHS to address opioid-related overdose, death and dependence. The HHS initiative’s focus is on improving physician’s use of opioids through education and resources to address opioid over-prescribing, increasing use and development of improved delivery systems for naloxone, which can reverse overdose from both prescription opioids and heroin, to reduce overdose-related deaths, and expanding the use of Medication-Assisted Treatment, which couples counseling and behavioral therapies with medication to address substance abuse. Also, as part of this initiative, the CDC has launched a state grant program to offer state health departments resources to assist with abuse prevention efforts, including efforts to track opioid prescribing through state-run electronic databases. In March 2016, as part of the HHS initiative, the CDC released a Guideline for Prescribing Opioids for Chronic Pain. The guideline is intended to assist primary care providers treating adults for chronic pain in outpatient settings. The guideline provides recommendations to improve communications between doctors and patients about the risks and benefits of opioid therapy for chronic pain, improve the safety and effectiveness of pain treatment, and reduce the risks associated with long-term opioid therapy. The guideline states that no treatment recommendations about the use of abuse-deterrent opioids can be made at this time. Many of these changes could require us to expend additional resources on commercializing ARYMO ER and OXAYDO to meet additional requirements. Advancements in the development and approval of generic abuse-deterrent opioids could also compete with and potentially impact physician use of our products and product candidates and cause our products to be less commercially successful.
The FDA continues to evaluate extended release and abuse-deterrent opioids in the post-market setting. In March 2017, the FDA’s Advisory Committee met to discuss a competitor company’s abuse-deterrent opioid. A majority of the Advisory Committee voted that the benefits do not outweigh the risks of that competitor company’s product. Upon the FDA’s subsequent request in June 2017, the drug was removed from the market. Also, in July 2017, the FDA held a public workshop to discuss available data and methods to assess the impact of opioid formulations with abuse-deterrent properties on misuse, abuse, addiction, overdose, and death in the post-market context. We expect that the FDA will continue to scrutinize the impact of abuse-deterrent opioids and in the future could impose further restrictions to products currently on the market, which may include changing labeling, imposing additional prescribing restrictions, or seeking a product’s removal from the market, which would have an adverse effect on our financial performance.
Many states and municipalities, a Native American Tribe and individual consumers have brought lawsuits against manufacturers, pharmacies and distributors of opioids, seeking damages for the costs associated with drug abuse and dependency. We may be brought into actions in the future, which could divert our attention and resources and have an adverse impact on our operations and financial condition.
Several state attorneys general, including Missouri, Ohio, New Hampshire and others, have sued opioid manufacturers, distributors and pharmacies alleging that such parties made false and misleading statements in the promotion of opioids or fueled opioid addition by selling large quantities of opioids in certain areas, resulting in high incidences of opioid overdoses and deaths. The plaintiffs in these cases are seeking to recover costs associated with drug dependency, overdose and death resulting from opioid use. These cases involve our larger competitors and largely relate
39
to time periods prior to the time that we first began commercializing OXAYDO, our abuse discouraging immediate release oxycodone, in 2015. However, we could be brought into actions in the future if potential plaintiffs view our promotion of opioids as fueling the social problems with opioids.
The U.S. Congress has also been investigating opioid manufacturers. In March 2017, the U.S. Senate began investigating the role that manufacturers may have played in the opioid addiction problem in the U.S. The Senate requested internal documents from five of our large competitors relating to the marketing tactics for opioids and what, if anything, those manufacturers knew about the dangers of those drugs, and indicated that the probe could be expanded to include other manufacturers.
Litigation involving governmental entities or class actions and governmental investigations are expensive and time consuming. If we were to be sued or investigated over our commercialization of opioids, such an action could divert our attention and resources and have an adverse impact on our operations and financial condition.
Guidelines and recommendations published by various organizations can reduce the use of our products.
Government agencies promulgate regulations and guidelines directly applicable to us and to our products. Third party payors, professional societies, practice management groups, private health and science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to the healthcare and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. For example, some governmental and large third-party payors have begun to institute limits on the number of days’ worth of opioid medication a patient can receive for the patient’s first opioid prescription. Recommendations or guidelines suggesting the reduced use of our products or the use of competitive or alternative products as the standard of care to be followed by patients and healthcare providers could result in decreased use of our products.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
We face an inherent risk of product liability as a result of the commercial sales of our products and the clinical testing of our product candidates. For example, we may be sued if any of our products or product candidates allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
|
· |
injury to our reputation; |
|
· |
decreased demand for our products; |
|
· |
withdrawal of clinical trial participants; |
|
· |
initiation of investigations by regulators; |
|
· |
costs to defend the related litigation; |
|
· |
a diversion of management’s time and our resources; |
|
· |
substantial monetary awards to trial participants or patients; |
40
|
· |
product recalls, withdrawals or labeling, marketing or promotional restrictions; |
|
· |
loss of revenue; |
|
· |
exhaustion of any available insurance and our capital resources; |
|
· |
the inability to commercialize our products; and |
|
· |
a decline in our share price. |
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We currently carry product liability insurance covering our clinical studies and commercial product sales in the amount of approximately $10 million in the aggregate. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
We intend to market our products outside of the United States, and we will be subject to the risks of doing business outside of the United States.
Because we intend to market products outside of the United States, our business is subject to risks associated with doing business outside of the United States. Accordingly, our business and financial results in the future could be adversely affected due to a variety of factors, including:
|
· |
failure to develop an international sales, marketing and distribution system for our products; |
|
· |
changes in a specific country’s or region’s political and cultural climate or economic condition; |
|
· |
unexpected changes in foreign laws and regulatory requirements; |
|
· |
difficulty of effective enforcement of contractual provisions in local jurisdictions; |
|
· |
inadequate intellectual property protection in foreign countries; |
|
· |
trade‑protection measures, import or export licensing requirements such as Export Administration Regulations promulgated by the United States Department of Commerce and fines, penalties or suspension or revocation of export privileges; |
|
· |
the effects of applicable foreign tax structures and potentially adverse tax consequences; and |
|
· |
significant adverse changes in foreign currency exchange rates. |
Risks Related to Our Business and Strategy
We face substantial competition, which may result in others commercializing products more successfully than we do.
We face and will continue to face competition from other companies in the pharmaceutical, medical devices and drug delivery industries. Our products compete a with currently marketed oral opioids, transdermal opioids, local anesthetic patches, stimulants and implantable and external infusion pumps that can be used for infusion of opioids and local anesthetics. Products of these types are marketed or in development by Purdue Pharma, Pfizer, Durect, Endo, Mallinckrodt, Zogenix, Elite Pharmaceuticals, Pain Therapeutics, Nektar, Collegium Pharmaceuticals, Inspirion, Teva,
41
Pernix, Daichii, Intellipharmaceutics, Acura Pharmaceuticals and others. Some of these companies and many others are applying significant resources and expertise to the challenges of drug delivery, and several are focusing or may focus on drug delivery to the intended site of action. Some of these current and potential future competitors may be addressing the same therapeutic areas or indications as we are. Many of our competitors have substantially more marketing, manufacturing, financial, technical, human and managerial, and research and development resources than we do, and have more institutional experience than we do.
Competitors have developed or are in the process of developing technologies that are, or in the future may be, the basis for competitive products. Some of these products may have an entirely different approach or means of accomplishing similar therapeutic effects than our products. Our competitors may develop products that are safer, more effective or less costly than our products and, therefore, present a serious competitive threat to our product offerings.
The widespread acceptance of currently available therapies with which our products compete may limit market acceptance of our products. Oral medication, transdermal drug delivery systems, such as drug patches, injectable products and implantable drug delivery devices are currently available treatments for chronic and post‑operative pain, are widely accepted in the medical community and have a long history of use. These treatments will compete with our products and the established use of these competitive products may limit the potential for our products to receive widespread acceptance.
The use of legal and regulatory strategies by competitors with innovator products, including the filing of citizen petitions, may increase our costs associated with the marketing of our products, significantly reduce the profit potential of our products, or, if successfully partnered, delay or prevent the introduction or approval of our product candidates.
Companies with innovator drugs often pursue strategies that may serve to prevent or delay competition from alternatives to their innovator products. These strategies include, but are not limited to:
|
· |
filing “citizen petitions” with the FDA that may delay competition by causing delays of our product approvals; |
|
· |
seeking to establish regulatory and legal obstacles that would make it more difficult to demonstrate a product’s bioequivalence or “sameness” to the related innovator product; |
|
· |
filing suits for patent infringement that automatically delay FDA approval of Section 505(b)(2) products; |
|
· |
obtaining extensions of market exclusivity by conducting clinical trials of innovator drugs in pediatric populations or by other methods; |
|
· |
persuading the FDA to withdraw the approval of innovator drugs for which the patents are about to expire, thus allowing the innovator company to develop and launch new patented products serving as substitutes for the withdrawn products; |
|
· |
seeking to obtain new patents on drugs for which patent protection is about to expire; and |
|
· |
initiating legislative and administrative efforts in various states to limit the substitution of innovator products by pharmacies. |
These strategies could delay, reduce or eliminate our entry into the market and our ability to generate revenues associated with our product candidates.
42
Our future success depends on our ability to retain our key personnel.
We are highly dependent upon the services of our key personnel, including our chief executive officer, Robert Radie, our chief financial officer, Stan Musial, our chief operating officer, Mark Strobeck, and our chief commercial officer, Patrick Shea. Although we have entered into employment agreements with each of them, these agreements are at‑will and do not prevent them from terminating their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of Mr. Radie, Mr. Musial, Dr. Strobeck, or Mr. Shea could impede the achievement of our corporate objectives.
We may experience issues retaining our officers, key employees and highly skilled personnel due to the fact that the exercise prices of most of our outstanding employee stock options exceed the current market price of our Common Stock.
Our performance substantially depends on the performance of our officers and other key employees. We also rely on our ability to retain and motivate qualified personnel, especially our management and highly skilled employees. Our ability to retain employees in our highly competitive industry is substantially dependent upon the attractiveness of the equity that we can offer to them. Because the exercise prices of our outstanding employee stock options exceed the market price of our Common Stock, in some cases significantly, the retention value of our outstanding stock options is currently limited. The loss of the services of any of our officers or key employees could cause us to incur increased operating expenses and divert senior management resources in searching for replacements. The loss of their services also could harm our reputations if our customers were to become concerned about our future operations. Our future success also depends on our continuing ability to identify, hire, train and retain other highly skilled personnel. Competition for these personnel is intense, especially in the pharmaceutical industry, and we may experience difficulty in hiring and retaining sufficient numbers of highly skilled employees if the market price of our Common Stock does not improve and/or the exercise prices of our outstanding employee stock options continue to exceed the market price of our Common Stock.
We may engage in future acquisitions or business development activities that could disrupt our business, cause dilution to our stockholders or cause us to recognize accounting charges in our financial statements.
We may, in the future, make acquisitions of, or investments in, companies or products that we believe have products or capabilities that are a strategic or commercial fit with our products and business or otherwise offer opportunities for our company, including in-licensing technologies. In connection with these acquisitions or investments, we may:
|
· |
pay too much for the product or business; |
|
· |
issue stock that would dilute our stockholders’ percentage of ownership; |
|
· |
incur debt and assume liabilities; and |
|
· |
incur amortization expenses related to intangible assets or incur large and immediate write‑offs. |
We also may be unable to find suitable acquisition candidates and we may not be able to complete acquisitions on favorable terms, if at all. In addition, we currently have limited capital resources and a significant amount of outstanding debt, the governing documents of which restrict our ability to make certain capital expenditures, each of which could limit our ability to engage in otherwise attractive acquisition or in-license transactions. We may also issue shares of our common stock in such a transaction, which would result in dilution to our stockholders.
43
If we do complete an acquisition, we cannot assure you that it will ultimately strengthen our competitive position or that it will not be viewed negatively by customers, financial markets or investors. Further, future acquisitions could also pose numerous additional risks to our operations, including:
|
· |
problems integrating the purchased business, products or technologies; |
|
· |
increases to our expenses; |
|
· |
the failure to have discovered undisclosed liabilities of the acquired asset or company; |
|
· |
diversion of management’s attention from their day‑to‑day responsibilities; |
|
· |
entrance into markets in which we have limited or no prior experience; and |
|
· |
potential loss of key employees, particularly those of the acquired entity. |
We may not be able to successfully complete one or more acquisitions or effectively integrate the operations, products or personnel gained through any such acquisition.
If we are unable to protect our information systems against service interruption, misappropriation of data or other failures, accidents or breaches of security, our operations could be disrupted, our reputation may be damaged, and our business and operations would suffer.
Despite the implementation of security measures, our internal computer systems, and those of our CROs and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs, our commercial activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our commercialization programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability.
Further, our reliance on information systems and other technology also gives rise to cybersecurity risks, including security breach, espionage, system disruption, theft and inadvertent release of information. We regularly make investments to upgrade, enhance or replace these systems, as well as leverage new technologies to support our growth strategies. Any delays or difficulties in transitioning to new systems or integrating them with current systems or the failure to implement our initiatives in an orderly and timely fashion could result in additional investment of time and resources, which could impair our ability to improve existing operations and support future growth, and ultimately have a material adverse effect on our business.
Changes in tax laws and regulations or in our operations may impact our effective tax rate and may adversely affect our business, financial condition and operating results.
Changes in tax laws in any of the jurisdictions in which we operate, or adverse outcomes from tax audits that we may be subject to in any of the jurisdictions in which we operate, could result in an unfavorable change in our effective tax rate, which could adversely affect our business, financial condition and operating results.
The Tax Cuts and Jobs Act (the “Tax Act”) was enacted on December 22, 2017. There are various provisions within the Tax Act that could impact our future tax position. The U.S. corporate tax rate was reduced to 21%, the Alternative Minimum Tax was repealed, and Net Operating Losses (“NOLs”) generated beginning in 2018 may be carried forward indefinitely but, limited to 80% of taxable income for utilization. However, interest deductions could be
44
limited and certain performance-based compensation deductions could be limited. In addition, we will continue to evaluate the potential impacts of the US taxation of its Controlled Foreign Corporation.
We continue to assess the impact of various U.S. federal or state legislative proposals that could result in a material increase to our U.S federal or state taxes. We cannot predict whether any specific legislation will be enacted or the terms of any such legislation. However, if such proposals were to be enacted, or if modifications were to be made to certain existing regulations, the consequences could have a material adverse impact on us, including increasing our tax burden, increasing the cost of tax compliance or otherwise adversely affecting our financial position, results of operations, cash flows and liquidity.
Fluctuations in the value of foreign currencies could negatively impact our results of operations and increase our costs.
Some payments to our employees, suppliers and contract manufacturers are denominated in foreign currencies. Our reporting currency is the U.S. dollar. Accordingly, we are exposed to foreign exchange risk, and our reported results of operations may be negatively impacted by fluctuations in the exchange rate between the U.S. dollar and the foreign currency. A significant appreciation in the foreign currency relative to the U.S. dollar would result in higher reported expenses and would cause our net losses to increase. Likewise, to the extent that we generate any revenues denominated in foreign currencies, or become required to make payments in other foreign currencies, fluctuations in the exchange rate between the U.S. dollar and those foreign currencies could also negatively impact our reported results of operations. We have not entered into any hedging contracts to mitigate the effect of changes in foreign currency exchange rates.
Our business operations may subject us to numerous commercial disputes, claims and/or lawsuits.
Operating in the pharmaceutical industry, particularly the commercialization of pharmaceutical products, involves numerous commercial relationships, complex contractual arrangements, uncertain intellectual property rights, potential product liability and other aspects that create heightened risks of disputes, claims and lawsuits. In particular, we may face claims related to the safety of our products, intellectual property matters, employment matters, tax matters, commercial disputes, competition, sales and marketing practices, environmental matters, personal injury, insurance coverage and acquisition or divestiture‑related matters. Further, pharmaceutical companies have used the Lanham Act, a private right of action that enables a party to sue a competitor for a false or misleading description or representation of fact that misrepresents the nature, characteristics, qualities, or geographic origin of the competitor’s goods, services or commercial activities. Any commercial dispute, claim or lawsuit may divert our management’s attention away from our business, we may incur significant expenses in addressing or defending any commercial dispute, claim or lawsuit, and we may be required to pay damage awards or settlements or become subject to equitable remedies that could adversely affect our operations and financial results.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Our foreign NOLs generated by Egalet UK’s operations may be carried forward indefinitely but may become subject to an annual limitation. Upon potential examination by the statutory or governing authority, it may be determined that we experienced a greater than 50% change in share capital, which would limit the availability and use of existing foreign NOLs to offset our taxable income, if any, in the future.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three‑year period, the corporation’s ability to use its pre‑change NOLs and other pre‑change tax attributes (such as research tax credits) to offset its post‑change income may be limited. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership some of which are outside our control. As a result, if we earn net taxable income, our ability to use our pre‑change NOL carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
45
Risks Related to Our Compliance with Governmental Regulations
Our products are subject to ongoing regulatory requirements, and we may face regulatory enforcement action if we do not comply with the requirements.
Manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP and other regulations. If we or a regulatory agency discover problems with a product which were previously unknown, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, our products or the manufacturing facilities for our products fail to comply with applicable regulatory requirements, a regulatory agency may:
|
· |
issue warning letters or untitled letters; |
|
· |
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; |
|
· |
require us to enter into a consent decree, which can include the imposition of various fines, reimbursements for inspection costs and penalties for noncompliance, and require due dates for specific actions; |
|
· |
seek an injunction, impose civil penalties or monetary fines or pursue criminal prosecution, require disgorgement, consider exclusion from participation in Medicare, Medicaid and other federal healthcare programs and require curtailment or restructuring of our operations; |
|
· |
suspend or withdraw regulatory approval; |
|
· |
suspend any ongoing clinical trials; |
|
· |
refuse to approve pending applications or supplements to applications filed by us; |
|
· |
deny or reduce quota allotments for the raw material for commercial production of our controlled substance products; |
|
· |
suspend or impose restrictions on operations, including costly new manufacturing requirements; or |
|
· |
seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall; or |
|
· |
refuse to allow us to enter into government contracts. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our products and generate revenue.
In addition, the FDA may impose significant restrictions on the approved indicated uses for which the product may be marketed or on the conditions of approval. For example, a product’s approval may contain requirements for potentially costly post‑approval studies and surveillance, including Phase 4 clinical trials, to monitor the safety and efficacy of the product. We currently have Phase 4 study requirements for OXAYDO and ARYMO ER. We will collect data on ARYMO ER for one year post marketing, and, depending on the results of the study, additional post-marketing investigations may be required to evaluate the effect of ARYMO ER. We are also subject to ongoing FDA obligations and continued regulatory review with respect to the manufacturing, processing, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for our product. These requirements include submissions of safety and other post‑marketing information and reports, registration, as well as continued compliance
46
with cGMPs and with GCPs and good laboratory practices, which are regulations and guidelines enforced by the FDA for all of our products in clinical and pre‑clinical development, and for any clinical trials that we conduct post‑approval. To the extent that a product is approved for sale in other countries, we may be subject to similar restrictions and requirements imposed by laws and government regulators in those countries. In addition, our product labeling, advertising and promotion are subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about prescription drug products. In particular, a drug product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling, although the FDA does not regulate the prescribing practices of physicians. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off‑label uses, and a company that is found to have improperly promoted off‑label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
Our current and future relationships with healthcare professionals, principal investigators, consultants, customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti‑kickback, fraud and abuse, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens, and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors in the United States and elsewhere play a primary role in the recommendation and prescription of any commercial products. Our current and future arrangements with healthcare professionals, principal investigators, consultants, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any product candidates for which we may obtain marketing approval. In addition, we may be subject to physician payment transparency laws and patient privacy and security regulation by the federal government and by the U.S. states and foreign jurisdictions in which we conduct our business. Restrictions under applicable federal, state and foreign healthcare laws and regulations may affect our ability to operate, including:
|
· |
the Federal anti‑kickback statute, which prohibits, among other things, knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
|
· |
the federal civil and criminal laws and civil monetary penalty laws, including the False Claims Act, which impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government, including erroneous pricing information on which mandatory rebates, discounts and reimbursement amounts are based, or in the case of the civil False Claims Act, for violations of the Anti-Kickback Statute in connection with a claim for payment or for conduct constituting reckless disregard for the truth; |
|
· |
the Foreign Corrupt Practices Act (“FCPA”), which prohibits U.S. firms and individuals from paying bribes to foreign officials in furtherance of a business deal and against the foreign official's duties and specifies required accounting transparency guidelines; |
|
· |
state and foreign anti‑kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non‑governmental payors, including private insurers; |
|
· |
HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
47
|
· |
HIPAA, as amended by HITECH and its implementing regulations, which also imposes obligations on certain covered entity healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
|
· |
laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; |
|
· |
federal laws requiring certain drug manufacturers to regularly report information related to payments and other transfers of value made to physicians and other healthcare providers, as well as ownership or investment interests held by physicians and their immediate family members, including under the federal Open Payments program, as well as other state and foreign laws regulating marketing activities; and |
|
· |
federal government price reporting laws, which require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on our products and may subject us to potentially significant discounts on our products, increased infrastructure costs, potential liability for the failure to report such prices in an accurate and timely manner and potentially limit our ability to offer certain marketplace discounts; and |
|
· |
state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, that person or entity may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, and it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, disgorgement exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations.
It is uncertain as to whether, and how, the Affordable Care Act and its related rules and regulations may be affected by the Congress and Trump Administration, given the repeated calls and attempts to amend the Affordable Care Act.
Failure to comply with ongoing governmental regulations for marketing our products could inhibit our ability to generate revenues from their sale and could also expose us to claims or other sanctions.
Advertising and promotion of our products is heavily scrutinized by the FDA, the U.S. Department of Justice, the HHS Office of the Inspector General, state attorneys general, members of Congress and the public. Violations, including unintended promotion of our products for unapproved or off‑label uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA. In addition, advertising and promotion of any product candidate that obtains approval outside of the United States will be heavily scrutinized by comparable foreign regulatory authorities.
48
In the United States, engaging in impermissible promotion of our products for off‑label uses can also subject us to false claims litigation under federal and state statutes, which can lead to civil and criminal penalties and fines and agreements that materially restrict the manner in which we promote or distribute our drug products. These false claims statutes include the federal False Claims Act, which allows any individual to bring a lawsuit against a pharmaceutical company on behalf of the federal government alleging submission of false or fraudulent claims, or causing to present such false or fraudulent claims, for payment by a federal program such as Medicare or Medicaid. If the government prevails in the lawsuit, the individual will share in any fines or settlement funds. Since 2004, these False Claims Act lawsuits against pharmaceutical companies have increased significantly in volume and breadth, leading to several substantial civil and criminal settlements based on certain sales practices promoting off‑label drug uses. This growth in litigation has increased the risk that a pharmaceutical company will have to defend a false claim action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from the Medicare, Medicaid and other federal and state healthcare programs. If we do not lawfully promote our products, even if unlawful promotion is inadvertent, we may become subject to such litigation and, if we are not successful in defending against such actions, those actions could compromise our ability to become profitable.
In addition, later discovery of previously unknown problems with a product, manufacturer or facility, or our failure to update regulatory files, may result in restrictions, including withdrawal of the product from the market. Any of the following or other similar events, if they were to occur, could delay or preclude us from further developing, marketing or realizing the full commercial potential of our products:
|
· |
failure to obtain or maintain requisite governmental approvals; |
|
· |
failure to obtain or maintain approvals of labeling with abuse‑deterrent claims; or |
|
· |
FDA required product withdrawals or warnings arising from identification of serious and unanticipated adverse side effects in our product candidates. |
ARYMO ER and OXAYDO are subject to mandatory REMS programs, which could increase the cost, burden and liability associated with the commercialization of ARYMO ER and OXAYDO.
The FDA has indicated that some opioid drugs formulated with the active ingredients fentanyl, hydromorphone, methadone, morphine, oxycodone, oxymorphone, and others will be required to have a REMS to ensure that the benefits of the drugs continue to outweigh the risks. The FDA has approved a REMS for ER and long‑acting (“LA”) and will approve a new REMS that covers both ER and IR opioids as part of a federal initiative to address prescription drug abuse and misuse. Like the ER/LA REMS, the new REMS will introduce new safety measures designed to reduce risks and improve the safe use of ER and IR opioids, while ensuring access to needed medications for patients in pain. The current ER/LA opioid REMS affects more than 20 companies that manufacture these opioid analgesics. Like the current REMS, under the new combined REMS, companies will be required to make education programs available to prescribers based on a revised FDA Blueprint. It is expected that companies will satisfy this obligation by providing educational grants to continuing education providers, who will develop and deliver the training. The REMS will also require companies to make available FDA‑approved patient education materials on the safe use of these drugs. The companies must perform periodic assessments of the implementation of the REMS and the success of the program in meeting its goals. The FDA will review these assessments and may require additional elements to achieve the goals of the program.
ARYMO ER and OXAYDO are subject to the REMS requirement. There may be increased cost, administrative burden and potential liability associated with the marketing and sale of products subject to the REMS requirement, which could reduce or remove the commercial benefits to us from the sale of these products and product candidates.
Certain of our products contain controlled substances, the manufacture, use, sale, importation, exportation and distribution of which are subject to regulation by state, federal and foreign law enforcement and other regulatory agencies.
OXAYDO and ARYMO ER and our product candidates contain, controlled substances which are subject to state, federal and foreign laws and regulations regarding their manufacture, use, sale, importation, exportation and
49
distribution. OXAYDO and ARYMO ER contain active ingredients that are classified as controlled substances under the CSA and regulations of the DEA. A number of states also independently regulate these drugs as controlled substances. Chemical compounds are classified by the DEA as Schedule I, II, III, IV or V substances, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. For our products containing controlled substances, we and our suppliers, manufacturers, contractors, customers and distributors are required to obtain and maintain applicable registrations from state, federal and foreign law enforcement and regulatory agencies and comply with state, federal and foreign laws and regulations regarding the manufacture, use, sale, importation, exportation and distribution of controlled substances. For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist and may not be refilled without a new prescription. Furthermore, the amount of Schedule II substances that can be obtained for clinical trials and commercial distribution is limited by the CSA and DEA regulations. We may not be able to obtain sufficient quantities of these controlled substances to meet the commercial demand of our products or to complete any additional clinical trials.
In addition, controlled substances are also subject to regulations governing manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal. These regulations increase the personnel needs and the expense associated with development and commercialization of product candidates that include controlled substances. Failure to obtain and maintain required registrations or to comply with any applicable regulations could delay or preclude us from developing and commercializing our product candidates that contain controlled substances and subject us to enforcement action. Because of their restrictive nature, these regulations could limit commercialization of our products and product candidates containing controlled substances.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur significant costs.
In connection with our research and development activities and our manufacture of materials and product candidates, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Our research and development involves the use, generation and disposal of hazardous materials, including chemicals, solvents, agents and biohazardous materials. Although we believe that our safety procedures for storing, handling and disposing of such materials comply with the standards prescribed by state and federal regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. We currently contract with third parties to dispose of these substances that we generate, and we rely on these third parties to properly dispose of these substances in compliance with applicable laws and regulations. If these third parties do not properly dispose of these substances in compliance with applicable laws and regulations, we may be subject to legal action by governmental agencies or private parties for improper disposal of these substances. The costs of defending such actions and the potential liability resulting from such actions are often very large. In the event we are subject to such legal action or we otherwise fail to comply with applicable laws and regulations governing the use, generation and disposal of hazardous materials and chemicals, we could be held liable for any damages that result, and any such liability could exceed our resources.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
50
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
The Sarbanes‑Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. We are required, under Section 404 of the Sarbanes‑Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment is required to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes‑Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However, for as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirement.
Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by Nasdaq, the Securities and Exchange Commission (“SEC”) or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision‑making can be faulty, and that breakdowns can occur because of simple error or mistake. In addition, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
51
Our employees, principal investigators, CROs, CMOs and other third-party manufacturers, independent contractors, consultants, collaborators or vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, CROs, CMOs and other third-party manufacturers, independent contractors, consultants, collaborators or vendors. Misconduct by any of these parties could include intentional reckless and/or negligent conduct or failures to:
|
· |
comply with FDA, DEA or similar regulations or similar regulations of comparable foreign regulatory authorities; |
|
· |
provide accurate information to the FDA or comparable foreign regulatory authorities; |
|
· |
comply with manufacturing standards we have established; |
|
· |
comply with federal and state healthcare laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities; |
|
· |
report financial information or data accurately; or |
|
· |
disclose unauthorized activities to us. |
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self‑dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by these parties could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Conduct, but it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
We are an “emerging growth company” and we intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes‑Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which could be for up to five years from our initial public offering. See “Summary—Implications of Being an Emerging Growth Company.”
If investors find our common stock less attractive as a result of our reduced reporting requirements, there may be a less active trading market for our common stock and our stock price may be more volatile. We may also be unable to raise additional capital as and when we need it.
52
We may incur increased compliance costs and our management will be required to devote substantial time to new compliance initiatives once we are no longer an “emerging growth company.”
We expect to incur significant expense and to devote substantial management effort toward ensuring compliance with Section 404 of the Sarbanes‑Oxley Act of 2002 once we lose our status as an “emerging growth company.” Compliance with the Sarbanes‑Oxley Act of 2002, the Dodd‑Frank Act of 2010, as well as rules of the Securities and Exchange Commission and Nasdaq, for example, will result in ongoing increases in our legal, accounting, administrative and other compliance costs after we are no longer an “emerging growth company.” The Exchange Act, requires, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition. Our board of directors, management and other personnel need to devote a substantial amount of time to these compliance initiatives.
We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Implementing any appropriate changes to our internal controls may require specific compliance training for our directors, officers and employees, entail substantial costs to modify our existing accounting systems, and take a significant period of time to complete. Such changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate consolidated financial statements or other reports on a timely basis, could increase our operating costs and could materially impair our ability to operate our business. It is also uncertain what the impact of the new Congress and administration will have on such regulation in light of the President’s campaign promises and executive directives to roll back aspects of, among other things, the Dodd-Frank Act.
Risks Related to Our Dependence on Third Parties
Due to the fact that we currently rely on sole suppliers to manufacture the active pharmaceutical ingredients of our products, and a sole supplier for each of our products, any production problems with our suppliers could adversely affect us.
We have relied upon supply agreements with third parties for the manufacture and supply of the bulk active pharmaceutical ingredients used in our product candidates for purposes of preclinical testing and clinical trials. We presently depend upon a sole manufacturer of our supply of APIs for each of our products. We also rely on a sole supplier for each of our products. Although we have identified alternate sources for these supplies, it would be time‑consuming and costly to qualify these sources. If our suppliers were to terminate our arrangements or fail to meet our supply needs, we could face disruptions in the distribution and sale of our products. We currently do not have secondary sources for our products.
If third‑party manufacturers of our products fail to devote sufficient time and resources to our concerns, or if their performance is substandard, we may be unable to continue to commercialize our products, and our costs may be higher than expected and could harm our business.
We have no manufacturing facilities and have limited experience in drug development and commercial manufacturing. We lack the resources and expertise to formulate, manufacture or test the technical performance of our product candidates. We currently rely on a limited number of experienced personnel and single contract manufacturers (“CMO”) to manufacture ARYMO ER, SPRIX Nasal Spray and OXAYDO. We purchase our required quantities of SPRIX Nasal Spray through purchase orders and while we have an understanding with the manufacturer of SPRIX Nasal Spray regarding an exit strategy if our relationship with that manufacturer were to be terminated, we cannot assure you that a termination of this relationship would not result in a supply disruption. Our reliance on a limited number of vendors and manufacturers exposes us to the following risks, any of which could interrupt commercialization of our products, delay our clinical trials, result in higher costs, or deprive us of potential product revenues:
|
· |
CMOs, their sub‑contractors or other third parties we rely on, may encounter difficulties in achieving the volume of production needed to satisfy clinical needs or commercial demand, may experience technical issues that impact quality or compliance with applicable and strictly enforced regulations governing the |
53
manufacture of pharmaceutical products, and may experience shortages of qualified personnel to adequately staff production operations. |
|
· |
Our CMOs could default on or, under certain circumstances, terminate their agreements or purchase orders with us to provide clinical supplies or meet our requirements for commercialization of our products. |
|
· |
For certain of our products, the use of alternate CMOs may be difficult because the number of potential CMOs that have the necessary governmental licenses to produce narcotic products is limited. In addition, the FDA and the DEA must approve any alternative manufacturer of our products before we may use the alternative manufacturer to produce our products. |
|
· |
It may be difficult or impossible for us to find a replacement CMO on acceptable terms quickly, or at all. Our CMOs and vendors may not perform as agreed or may not remain in the contract manufacturing business for the time required to successfully produce, store and distribute our products. |
The FDA and other regulatory authorities require that our products be manufactured according to cGMP and similar foreign standards. Any failure by our CMOs to comply with cGMP, including any failure to deliver sufficient quantities of products in a timely manner could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for products, or take other regulatory or legal action, including recall or seizure, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, detention or product, refusal to permit the import or export of products, injunction, imposing civil penalties, or pursuing criminal prosecution.
Our utilization of CMOs could also result in our lack of visibility throughout our supply chain, which could result in shortages in the supply of our products or, conversely, the build-up of more inventory than we require. Failures or difficulties faced at any level of our supply chain could materially adversely affect our business and delay or impede the development and commercialization of any of our products or product candidates and could have a material adverse effect on our business, results of operations, financial condition and prospects.
We rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for any supplemental drug applications relating to changes to the labels of or new dosage strengths for our products or receive regulatory approval for any product candidate we are able to partner.
We have relied upon and plan to continue to rely upon third‑party contract research organizations (“CROs”) to monitor and manage data for our ongoing preclinical and clinical programs. We rely on these parties for execution of our preclinical studies and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We also rely on third parties to assist in conducting our preclinical studies in accordance with Good Laboratory Practices (“GLP”) and the Animal Welfare Act requirements. We and our CROs are required to comply with federal regulations and current GCP which are international standards meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, advisors and monitors, enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area (“EEA”) and comparable foreign regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCP through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCP, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements. In addition, our clinical trials must be conducted with product produced under cGMP requirements. Failure to comply with these regulations may require us to repeat preclinical studies and clinical trials, which would delay the regulatory approval process.
54
Our CROs are not our employees, and except for remedies available to us under our agreements with them, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for any supplemental drug applications relating to changes to the labels of or new dosage strengths for our products or receive regulatory approval for or successfully commercialize any of our product candidates that we successfully partner. As a result, the commercial prospects for our products or partnered product candidates, as the case may be, would be harmed, our costs could increase and our ability to generate additional revenues could be delayed.
Because we have relied on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risks that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third‑party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available to identify and monitor our third‑party providers. To the extent we are unable to identify and successfully manage the performance of third‑party service providers in the future, our ability to advance any clinical trials will be compromised. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future.
If we lose our relationships with CROs, our clinical trial efforts could be delayed.
We rely on third‑party vendors and CROs for preclinical studies and clinical trials related to expanding the labels for our existing products or, if successfully partnered, developing our product candidates. Switching or adding additional CROs involves additional cost and requires management time and focus. Our CROs have the right to terminate their agreements with us in the event of an uncured material breach. In addition, some of our CROs have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the subjects participating in our clinical trials warrants such termination, if we make a general assignment for the benefit of our creditors or if we are liquidated. Identifying, qualifying and managing performance of third‑party service providers can be difficult and time‑consuming. In addition, there is a natural transition period when a new CRO commences work and the new CRO may not provide the same type or level of services as the original provider. If any of our relationships with our third‑party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms.
We may seek collaborations with third parties to market and commercialize our products, including outside of the United States, who may fail to effectively and compliantly market our products and suffer reputational harm.
We have and may continue to rely on third-party collaborators to assist us with marketing our products, including outside of the United States. For example, we have co-promotion arrangements in place in the United States with Ascend Therapeutics to promote SPRIX Nasal Spray to its target women's healthcare practitioners and with OraPharma to promote SPRIX Nasal Spray to dentists, dental specialists and oral surgeons. We currently possess limited resources and may not be successful in establishing additional collaborations or co‑promotion arrangements on acceptable terms, if at all. We also face competition in our search for collaborators and co‑promoters. By entering into strategic collaborations or similar arrangements, we will rely on third parties for financial resources and for commercialization, sales and marketing and regulatory expertise. Our collaborators may fail to market our products in a legally compliant manner, which could subject us to regulatory risk and reputational harm. Any failure of our third‑party collaborators to successfully market and commercialize our products and product candidates in a legally-compliant manner both in and outside of the United States would diminish our revenues and could harm our reputation.
55
Risks Related to Our Intellectual Property
If we are unable to obtain or maintain intellectual property rights for our technology and products, we may lose valuable assets or experience reduced market share.
We depend on our ability to protect our proprietary technology. We rely on patent and trademark laws, trade secrets and know‑how, and confidentiality, licensing and other agreements with employees and third parties, all of which offer only limited protection. Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products, including product candidates.
The steps we have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the United States. The rights already granted under any of our currently issued patents and those that may be granted from pending patent applications may not provide us with the proprietary protection or competitive advantages we are seeking. Further, given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products identical, similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights, our patent applications may not issue into patents, and any issued patents may not provide protection against competitive technologies, may be held invalid or unenforceable if challenged or may be interpreted in a manner that does not adequately protect our technology or products. Even if our patent applications issue into patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us, or otherwise provide us with any competitive advantage. The examination process may require us to narrow the claims in our patent applications, which may limit the scope of patent protection that may be obtained. Our competitors may design around or otherwise circumvent patents issued to us or licensed by us.
The patent prosecution process is expensive and time‑consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. The United States Patent and Trademark Office (“USPTO”) and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In addition, periodic maintenance fees on issued patents are required to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patents. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents.
It is also possible that we will fail to identify patentable aspects of inventions made in the course of our development and commercialization activities before it is too late to obtain patent protection on them. Further, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions typically are not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain.
Recent patent reform legislation could increase the uncertainties and costs associated with the prosecution of our patent applications and the enforcement or defense of our issued patents. The Leahy‑Smith America Invents Act (“Leahy‑Smith Act”) which was signed into law on September 16, 2011, made significant changes to U.S. patent law, including provisions that affect the way patent applications are prosecuted and litigated. Many of the substantive changes
56
to patent law associated with the Leahy‑Smith Act and, in particular, the “first to file” provisions described below, only became effective on March 16, 2013. The Leahy‑Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Pursuant to the Leahy‑Smith Act, the United States transitioned to a “first to file” system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. The Leahy‑Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post‑grant proceedings, including post‑grant review, inter partes review, and derivation proceedings. An adverse determination based on any such submission or proceeding before the USPTO or opposition before a foreign patent agency could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position with respect to third parties.
Because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, issued patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents, or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products.
If third parties claim that our technology or products infringe upon their intellectual property, this could result in costly litigation and potentially limit our ability to commercialize our products.
There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the pharmaceutical industry. We may, from time to time, be notified of claims that we are infringing upon patents, trademarks, copyrights, or other intellectual property rights owned by third parties, and we cannot provide assurances that other companies will not, in the future, pursue such infringement claims against us or any third-party proprietary technologies we have licensed.
Our commercial success depends in part upon our ability to develop product candidates and commercialize future products without infringing the intellectual property rights of others. Our products and current or future product candidates, or any uses of them, may now or in the future infringe third-party patents or other intellectual property rights. This is due in part to the considerable uncertainty within the pharmaceutical industry about the validity, scope and enforceability of many issued patents in the United States and elsewhere in the world and, to date, there is no consistency regarding the breadth of claims allowed in pharmaceutical patents. We cannot currently determine the ultimate scope and validity of patents which may be granted to third parties in the future or which patents might be asserted to be infringed by the manufacture, use and sale of our products. In part as a result of this uncertainty, there has been, and we expect that there may continue to be, significant litigation in the pharmaceutical industry regarding patents and other intellectual property rights.
Third parties may assert infringement claims against us, or other parties we have agreed to indemnify, based on existing patents or patents that may be granted in the future. We are aware of third-party patents and patent applications related to morphine or oxycodone drugs and formulations, including those listed in the FDA’s Orange Book for morphine or oxycodone products. Since patent applications are published after a certain period of time after filing, and because applications can take several years to issue, there may be currently pending third-party patent applications that are unknown to us, which may later result in issued patents. Because of the inevitable uncertainty in intellectual property litigation, any litigation could result in an adverse decision, even if the case against us was weak or flawed.
If we are found to infringe a third party’s intellectual property rights, or if a third party that we were licensing technologies from was found to infringe upon a patent or other intellectual property rights of another third party, we could be required to obtain a license from such third party to continue developing and commercializing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same
57
technologies licensed to us. In addition, in any such proceeding or litigation, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our technology or product candidates, or reengineer or rebrand our product candidates, if feasible, or force us to cease some of our business operations.
In connection with any NDA that we file under Section 505(b)(2) of the FFDCA for any of our product candidates that we successfully partner, we will also be required to notify the patent holder that we have certified to the FDA that any patents listed for the reference label drug in the FDA’s Orange Book publication are invalid, unenforceable or will not be infringed by the manufacture, use or sale of our drug. If the patent holder files a patent infringement lawsuit against us within 45 days of its receipt of notice of our certification, the FDA is automatically prevented from approving our Section 505(b)(2) NDA until the earliest of 30 months, expiration of the patent, settlement of the lawsuit or a court decision in the infringement case that is favorable to us. Accordingly, we may invest significant time and expense in the development of our product candidates only to be subject to significant delay and patent litigation before our product candidates may be commercialized. There is always a risk that someone may bring an infringement claim against us. Even if we are found not to infringe, or a plaintiff’s patent claims are found invalid or unenforceable, defending any such infringement claim would be expensive and time-consuming, and would delay launch of Egalet-002 and our other product candidates and distract management from their normal responsibilities.
Competitors may sue us as a way of delaying the introduction of our products. Any litigation, including any interference or derivation proceedings to determine priority of inventions, oppositions or other post-grant review proceedings to patents in the United States or in countries outside the United States, or litigation against our partners may be costly and time consuming and could harm our business. We expect that litigation may be necessary in some instances to determine the validity and scope of our proprietary rights. Litigation may be necessary in other instances to determine the validity, scope or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. Ultimately, the outcome of such litigation could compromise the validity and scope of our patent or other proprietary rights or hinder our ability to manufacture and market our products.
We have been, and in the future may be, forced to litigate to enforce or defend our intellectual property, and/or the intellectual property rights of our licensors, which could be expensive, time consuming and unsuccessful, and result in the loss of valuable assets.
We have been, and may in the future be, forced to litigate to enforce or defend our intellectual property rights against infringement and unauthorized use by competitors, and to protect our trade secrets. In so doing, we may place our intellectual property at risk of being invalidated, unenforceable, or limited or narrowed in scope. For example, on February 23, 2018, we received notice from Teva that Teva has submitted an ANDA with the FDA seeking regulatory approval to market a generic version of ARYMO ER. The notice from Teva included a “Paragraph IV certification” with respect to two of our Orange Book-listed patents, alleging that the patents are invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of Teva’s proposed generic product. Under the Hatch-Waxman Act, we have 45 days from receipt of the notice to determine if we will file a patent infringement suit. If we do bring such a suit, a stay of approval will be imposed by the FDA on Teva’s ANDA for the earlier of 30 months or a decision in the infringement case that each of the patents is invalid or not infringed. We intend to vigorously enforce our intellectual property rights, but cannot predict the outcome of this matter.
Further, an adverse result in any litigation or defense proceedings may place pending applications at risk of non‑issuance. In addition, if any licensor fails to enforce or defend their intellectual property rights, this may adversely affect our ability to develop and commercialize our product candidates and prevent competitors from making, using, and selling competing products. Any such litigation, even if resolved in our favor, could cause us to incur significant expenses, and distract our technical or management personnel from their normal responsibilities. Any such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct the litigation or proceedings. Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend their intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property. Litigation could result in substantial costs and diversion of management resources, which could harm our business and
58
financial results. Further, protecting against the unauthorized use of our patented technology, trademarks and other intellectual property rights is expensive, difficult and, may in some cases not be possible. In some cases, it may be difficult or impossible to detect third party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our common stock.
If we breach any of the agreements under which we license rights to products or technology from others, we could lose license rights that are material to our business or be subject to claims by our licensors.
We license rights to OXAYDO from Acura and to SPRIX Nasal Spray from Recordati, and we may enter into additional licenses in the future for products and technology that may be important to our business. Under our agreement with Acura we are subject to, and under future license agreements we may be subject to, a range of commercialization and development, sublicensing, royalty, patent prosecution and maintenance, insurance and other obligations. Under our agreement with Recordati, which was assigned to us as part of our acquisition of SPRIX Nasal Spray from Luitpold, we are obligated to use best commercial efforts to market and sell SPRIX Nasal Spray and we pay a royalty to Recordati in connection with the SPRIX Nasal Spray license. Any failure by us to comply with any of these obligations or any other breach by us of our license agreements could give the licensor the right to terminate the license in whole, terminate the exclusive nature of the license or bring a claim against us for damages. Any such termination or claim, particularly relating to our agreement with respect to OXAYDO, could have a material adverse effect on our financial condition, results of operations, liquidity or business. Even if we contest any such termination or claim and are ultimately successful, such dispute could lead to delays in the development or commercialization of products and result in time‑consuming and expensive litigation or arbitration. In addition, on termination we may be required to license to the licensor any related intellectual property that we developed.
We may be subject to claims by third parties of ownership of what we regard as our own intellectual property or obligations to make compensatory payments to employees.
While it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing or obtaining such an agreement with each party who, in fact, develops intellectual property that we regard as our own. In addition, they may breach the assignment agreements or such agreements may not be self‑executing, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
In accordance with the provisions of the Danish Act on inventions of employees, we may be required to make a compensatory payment to an employee in return for the assignment to us of his or her rights to an invention made within the course of his or her employment. Any such payment would reduce the cash available to fund our operations.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
We rely on trade secrets, to protect our proprietary know‑how, technology and other proprietary information, where we do not believe patent protection is appropriate or obtainable, to maintain our competitive position. However, trade secrets are difficult to protect. We rely, in part, on non‑disclosure and confidentiality agreements that we enter into with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade
59
secret is difficult, expensive and time‑consuming, and the outcome is unpredictable. In addition, some courts both within and outside the United States may be less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such competitor, or those to whom they communicate them, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed or independently developed, our competitive position would be harmed.
We may not be able to protect our intellectual property rights throughout the world.
We rely upon a combination of patents, trade secret protection (i.e., know-how), and confidentiality agreements to protect the intellectual property of our products, including our product candidates. The strength of patents in the pharmaceutical field involves complex legal and scientific questions and can be uncertain. Where appropriate, we seek patent protection for certain aspects of our products and technology. Filing, prosecuting and defending patents on all of our products throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop and sell their own products and, further, may export otherwise infringing products to territories where we have patent protection but enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued patents or our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
The patent position of pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States, and these foreign laws may also be subject to change.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or the marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees, including our senior management, were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. These employees typically executed proprietary rights, non‑disclosure and non‑competition agreements in connection with their previous employment. Although we try to ensure that our employees do not use the proprietary information or know‑how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. We are not aware of any threatened or pending claims related to these matters, but in the future litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
We may need to license intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights, which is important or necessary to the development of our product candidates. It may be necessary for us to use the patented or proprietary technology of a third party to commercialize our own technology or products candidates, in which case we would be required to obtain a license from such third party. A license to such intellectual property may not be available or may not be available on commercially reasonable terms.
60
Risks Related to our Product Candidates
If we do not find suitable partners to assist us with the development and regulatory submissions for our product candidates, we may not be able to further develop or seek regulatory approval for Egalet-002 and our other product candidates and we may not realize any return on our investment in those assets.
Although we have completed our phase 3 studies for Egalet-002, we have determined to delay indefinitely our previously-announced anticipated 2019 filing date for the Egalet-002 NDA, unless we find a partner to share the cost. We have also ceased, for the time being, the research and development of Egalet-003, an AD stimulant, and Egalet-004, an AD, ER hydrocodone, which were developed using our Guardian Technology. We are seeking partners for those product candidates as well. If we are unable to find suitable partners, we not be able to seek regulatory approval for Egalet-002 or perform additional necessary preclinical or clinical studies for our other product candidates. As a result, we may not realize any return on the investments we have made in our product candidates or our Guardian Technology.
In addition to the level of commercial success of our products, our future growth is also dependent in part on our ability to successfully develop a pipeline of product candidates, including through potential acquisitions, and we cannot give any assurance that we will be able to find a partner for any of our product candidates, that such product candidates will receive regulatory approval or that any approved product candidates will be successfully commercialized.
Our long‑term growth will be limited unless we can successfully develop and expand our pipeline of additional product candidates, including through potential acquisitions. To date, we have only generated aggregate sales of our products of approximately $46.8 million and an aggregate of $22.6 million in revenues from various collaborative and research and development arrangements, including aggregate payments of $20.0 million under our prior collaboration and licensing agreement with Shionogi that was terminated in December 2015. To expand our growth in the long term, we must successfully research, develop, obtain regulatory approval for, manufacture, introduce, market and distribute our product candidates under development. For Egalet‑002, and each additional product candidate that we hope to commercialize, we or a collaborator must successfully meet a number of critical developmental milestones, including:
|
· |
selecting and developing a drug delivery platform technology to deliver the proper dose of drug over the desired period of time; |
|
· |
determining the appropriate drug dosage; |
|
· |
developing drug dosages that will be tolerated, safe and effective; |
|
· |
demonstrating the drug formulation will be stable for commercially reasonable time periods; |
|
· |
demonstrating through clinical trials that the drug is safe and effective in patients for the intended indication; |
|
· |
demonstrating an effective abuse-deterrent profile based on the target product profile; and |
|
· |
completing the manufacturing development and scale‑up to permit manufacture of our product candidates in commercial quantities and at acceptable prices. |
The time necessary to achieve these developmental milestones for any individual product candidate is long and uncertain, and we or a partner may not successfully complete these milestones for any of our product candidates in development. We may not be able to finalize the design or formulation of any product candidate. In addition, we may select components, solvents, excipients or other ingredients to include in our product candidates that have not been previously approved for use in pharmaceutical products, which may require us or our partner to perform additional studies and may delay clinical testing and regulatory approval of our product candidates. Even after we complete the
61
design of a product candidate, the product candidate must still be shown to be bioequivalent to an approved drug or safe and effective in required preclinical studies and clinical trials before approval for commercialization.
We have suspended our development of our product candidates and are looking for a partner for those product candidates with which we may explore possible design or formulation changes to address bioequivalence, safety, efficacy, manufacturing efficiency and performance issues. We or a partner may not be able to complete development of product candidates that will be safe and effective and that will have a commercially reasonable treatment and storage period. If we or a partner are unable to complete development of Egalet‑002 or any of our other product candidates, we will not be able to earn revenue from them.
To the extent we elect to enter into additional licensing or collaboration agreements to further develop our product candidates, our dependence on such relationships may reduce our revenues from our products or could lengthen the time for us to generate cash flows from the sale of any of our product candidates.
Our commercialization strategy for our product candidates in clinical and preclinical development will depend on our ability to enter into agreements with partners to obtain assistance and funding for the development and potential commercialization of these product candidates. Supporting diligence activities conducted by potential collaborators and negotiating the financial and other terms of a collaboration agreement can be long and complex processes with uncertain results. Even if we are successful in entering into additional collaboration agreements, collaborations may involve greater uncertainty for us, as we have less control over certain aspects of our collaborative programs than we do over our proprietary development and commercialization programs. We may determine that continuing a collaboration under the terms provided is not in our best interest, and we may terminate the collaboration. Our collaborators could delay or terminate their agreements, and our products subject to collaborative arrangements may never be successfully commercialized. Collaborations involving our product candidates pose the following risks to us:
|
· |
Collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations. |
|
· |
Collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding or external factors such as an acquisition that diverts resources or creates competing priorities. |
|
· |
Collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing. |
|
· |
Collaborators may conduct clinical trials inappropriately, or may obtain unfavorable results in their clinical trials, which may have an adverse effect on the development of our own programs. |
|
· |
Collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours. |
|
· |
A collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such products. |
|
· |
Collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation. |
62
|
· |
Disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management attention and resources. |
|
· |
Collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
|
· |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished or terminated. |
Further, our future collaborators may develop alternative products or pursue alternative technologies either on their own or in collaboration with others, including our competitors, and the priorities or focus of our collaborators may shift such that our programs receive less attention or resources than we would like, or they may be terminated altogether. Any such actions by our collaborators would compromise our ability to earn revenues. In addition, we could have disputes with our future collaborators, such as the interpretation of terms in our agreements. Any such disagreements could lead to delays in the development or commercialization of any potential products or could result in time‑consuming and expensive litigation or arbitration, which may not be resolved in our favor.
Even with respect to certain other programs that we intend to commercialize ourselves, we may enter into agreements with collaborators to share in the burden of conducting clinical trials, manufacturing and marketing our product candidates or products. In addition, our ability to apply our proprietary technologies to develop proprietary compounds will depend on our ability to establish and maintain licensing arrangements or other collaborative arrangements with the holders of proprietary rights to such compounds. We may not be able to establish such arrangements on favorable terms or at all, and our future collaborative arrangements may not be successful.
Our competitors may discover, develop or commercialize products before, and more successfully than, our product candidates.
Many of our competitors have substantially more marketing, manufacturing, financial, technical, human and managerial, and research and development resources than we do, and have more institutional experience than we do. As a result of these factors, our competitors may obtain regulatory approval of their products more rapidly than we are able to or may obtain patent protection or other intellectual property rights that allow them to develop and commercialize their products before us and limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and less costly than ours, and they may also be more successful than us in manufacturing and marketing their products.
Furthermore, if the FDA approves a competitor’s 505(b)(2) application for a drug candidate before our application for a similar drug candidate, and grants the competitor a period of exclusivity, the FDA may take the position that it cannot approve our NDA for a similar drug candidate or that our label cannot reflect certain claims because they are barred by exclusivity. For example, while the FDA approved label for ARYMO ER contains an abuse-deterrent claim for the intravenous route of abuse, the FDA did not approve the inclusion of abuse-deterrent claims for the oral and intranasal routes of abuse. The intranasal abuse-deterrent claim was blocked by another company’s exclusivity, which expires in October 2018. We also believe that several competitors are developing extended‑release oxycodone products, and if the FDA approves a competitor’s 505(b)(2) application for an extended‑release oxycodone product and grants exclusivity before our NDA for Egalet‑002 is filed and approved, we could be subject to a delay that would dramatically reduce our expected market potential for Egalet‑002. In addition, even if our 505(b)(2) application for Egalet‑002 is approved first, we may still be subject to competition from other oxycodone products, including products or other approved 505(b)(2) NDAs for different conditions of use that would not be restricted by any grant of exclusivity to us.
63
Risks Related to the Clinical Development and Regulatory Approval of Our Products and Product Candidates
The regulatory approval processes of the FDA and comparable foreign regulatory authorities can be lengthy, time‑consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates or for supplemental applications we may file for our products, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable, but typically takes many years following the commencement of preclinical studies and clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval varies among jurisdictions and may change during the course of a product candidate’s clinical development. It is possible that none of our existing product candidates or any future product candidates we may in‑license, acquire or develop will ever obtain regulatory approval. It is also possible that we may re-evaluate the path of a particular product or product candidate at different points in the approval and post-approval process, even deciding, in some cases, to discontinue development of a product candidate or take a product off the market.
Our product candidates could fail to receive regulatory approval from the FDA or a comparable foreign regulatory authority for many reasons, including:
|
· |
disagreement with or disapproval of the design or implementation of our clinical trials; |
|
· |
failure to demonstrate that a product candidate is safe and effective for its proposed indication; |
|
· |
failure to sufficiently deter abuse; |
|
· |
failure of clinical trials to meet the level of statistical significance required for approval; |
|
· |
failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
|
· |
a negative interpretation of the data from our preclinical studies or clinical trials; |
|
· |
deficiencies in the manufacturing processes or failure of third‑party manufacturing facilities with whom we contract for clinical and commercial supplies to pass inspection; or |
|
· |
insufficient data collected from clinical trials of our product candidates or changes in the approval policies or regulations that render our preclinical and clinical data insufficient to support the submission and filing of an NDA or to obtain regulatory approval. |
Any SNDA for our products could also fail to receive regulatory approval from the FDA or a comparable foreign regulatory authority if there is a disagreement with or disapproval of the design or implementation of our clinical trials; if there is a failure to demonstrate that the product sufficiently deters a particular route of abuse, if any clinical trials fail to meet the level of statistical significance required for approval; or if there are changes in the approval policies or regulations that render our clinical data insufficient to support the submission and filing of a sNDA or to obtain regulatory approval, among others.
The FDA or a comparable foreign regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or cause us to abandon the development program. Even if we obtain regulatory approval, our product candidates may be approved for fewer or more limited indications than we request, such approval may be contingent on the performance of costly post‑marketing clinical trials, or we may not be allowed to include the labeling claims necessary or desirable for the successful commercialization of such product candidate. Any FDA determination that our NDA or sNDA submission is incomplete or insufficient for filing, results in FDA refusing to file the NDA or sNDA. A refusal to file by
64
the FDA requires us to expend additional time and resources to revise and resubmit our NDA or sNDA. There is no guarantee that any revised or resubmitted NDA or sNDA filing we make will be accepted by the FDA.
In addition, under the Pediatric Research Equity Act, or PREA, a NDA or sNDA must contain data to assess the safety and effectiveness of the product candidate for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product candidate is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. We filed a sNDA for SPRIX Nasal Spray in December 2015, based on pediatric data initially generated and submitted by former sponsors. We received a refusal to file notice from the FDA on February 25, 2016. The FDA indicated that the filing review represents a preliminary review of the application and is not indicative of deficiencies that would be identified if FDA performed a complete review.
In addition, if our product candidate produces undesirable side effects or safety issues, the FDA may require the establishment of a REMS, or a comparable foreign regulatory authority may require the establishment of a similar strategy, that may, for instance, restrict distribution of our products and impose burdensome implementation requirements on us. For example, certain of our products are and we expect that Egalet‑002, if approved, will be subject to REMS or other post-marketing requirements, such as lengthy and costly post-marketing studies. Any of the foregoing scenarios could materially harm the commercial prospects for our products and product candidates.
To market and sell our products outside of the United States, we must obtain separate marketing approvals and comply with numerous and various regulatory requirements and regimes, which can involve additional testing, may take substantially longer than the FDA approval process, and still generally include all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. FDA approval does not ensure approval by regulatory authorities in other countries or jurisdictions, approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA, and we may not obtain any regulatory approvals on a timely basis, if at all. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of any of our product candidates by regulatory authorities in the European Union, China or another country, the commercial prospects of that product candidate may be significantly diminished, and our business prospects could decline.
If we fail to obtain the necessary regulatory approvals for any of our product candidates that we are able to partner, or if such approvals are limited, we will not be able to fully commercialize our product candidates.
Even if we comply with all FDA pre‑approval regulatory requirements, the FDA may not determine that some or all of our product candidates are safe and effective, and we may never obtain regulatory approval for some or all of our product candidates. If we fail to obtain regulatory approval for some or all of our product candidates, we will have fewer commercial products, and correspondingly lower product revenues. Even if our product candidates receive regulatory approval, such approval may involve limitations on the indications and conditions of use or marketing claims for our products. For example, the FDA approved label for ARYMO ER contains abuse-deterrent claims for the intravenous route of abuse, but the FDA did not approve the inclusion of abuse-deterrent claims for the oral and intranasal routes of abuse. The intranasal abuse-deterrent claim was blocked by another company’s exclusivity.
Further, while the FDA has issued guidance on requirements for abuse-deterrent labeling, including the studies that must be performed, and although we intend to conduct such studies if we are able to find suitable partners for our product candidates, there can be no assurance that our product candidates that are approved will receive FDA‑approved labeling that describes any or all of the abuse‑deterrent features of such product candidates.
Any product candidates that we are able to partner may also have other properties that could delay or prevent their regulatory approval or limit the commercial profile of their approved product label. These properties may come to light in the form of adverse events experienced during clinical trials.
We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes may be on the marketing approval of
65
any product candidates we are able to partner. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval of any NDAs we file, as well as subject us to more stringent product labeling and post-marketing testing and other requirements. Even if we are able to partner some of our product candidates and obtain regulatory approval for them, they may be subject to mandatory REMs programs, which could increase the cost, burden and liability associated with their commercialization.
In jurisdictions outside the United States, we must receive marketing authorizations from the appropriate regulatory authorities before commercializing our product candidates. Regulatory approval processes outside the United States generally include requirements and risks similar to, and in many cases in excess of, the risks associated with FDA approval.
If we are unable to design, conduct and complete clinical trials successfully, our product candidates will not be able to receive regulatory approval.
In order to obtain FDA approval for any of our product candidates, we must submit to the FDA an NDA with substantial evidence that demonstrates that the product candidate is both safe and effective in humans for its intended use. This demonstration requires significant research, preclinical studies and clinical trials.
Clinical trials are time‑consuming, very expensive and difficult to design and implement, in part because they are subject to rigorous requirements. We could encounter problems that cause abandonment or repetition of clinical trials. If patients participating in clinical trials suffer drug‑related adverse reactions during the course of such clinical trials, or if we or the FDA believe that participating patients are being exposed to unacceptable health risks, such clinical trials will have to be suspended or terminated. Suspensions, termination or the need to repeat a clinical trial can occur at any stage.
We may be unable to establish bioequivalence for our product candidates at a statistically significant level, which would require us to design and complete additional clinical trials to establish the safety and efficacy of our product candidates.
The clinical trial success of each of our product candidates designed to reduce potential risks of unintended use and abuse depends on reaching statistically significant changes in patients’ symptoms based on clinician‑rated scales. There is a lack of consensus regarding standardized processes for assessing clinical outcomes based on clinician‑rated scales. Accordingly, the scores from our clinical trials may not be reliable, useful or acceptable to the FDA or other regulatory agencies.
Changes in standards related to clinical trial design could affect our ability to design and conduct clinical trials as planned. For example, we have conducted or will conduct clinical trials comparing our product candidates to both placebo and other approved drugs, but regulatory authorities may not allow us to compare our product candidates to a placebo in a particular clinical indication where products are available. In that case, both the cost and the amount of time required to conduct a clinical trial could increase. The FDA may disagree with our trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical trials. The FDA may also approve a product candidate for fewer or more limited indications than we request, or may grant approval contingent on the performance of costly post-approval clinical trials. In addition, the FDA may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product candidates. Approval may be contingent on a post-marketing REMS, which could limit the labeling, distribution or promotion of a drug product.
Any of these delays or additional requirements could cause our product candidates to not be approved, or if approved, significantly impact the timing and commercialization of our product candidates and significantly increase our overall costs of drug development.
66
If we are unable to conduct and complete clinical trials on schedule, or if there is a delay in the approval process, the cost of seeking necessary regulatory approvals will be significantly increased.
The clinical trial process consumes a significant amount of time. The length of clinical trials will depend upon, among other factors, the number of patients required to be enrolled in such studies and the rate of trial site and patient enrollment. We may fail to obtain adequate levels of patient enrollment in our clinical trials. Delays in planned patient enrollment may result in increased costs, delays or termination of clinical trials. In addition, even if we enroll the number of patients we expect in the time frame we expect, such clinical trials may not provide the data necessary to support regulatory approval for the product candidates for which they were conducted. In addition, we may fail to effectively oversee and monitor these clinical trials, which would result in increased costs or delays of our clinical trials. Even if these clinical trials are completed, we may fail to complete and submit an NDA or sNDA as scheduled.
Even if clinical trials are completed as planned, their results may not support expectations or intended marketing claims. The clinical trials process may fail to demonstrate that our product candidates are safe and effective for indicated uses or could fail to support a labeling claim. Such failure may cause us to abandon a product candidate, could delay development of other product candidates or could cause us to abandon a labeling claim that we are seeking, or the FDA could require additional studies, in which case we would have to expend additional time and resources which would likely delay the date of potentially receiving regulatory approval. The approval process may also be delayed by changes in government regulation, future legislation or administrative action or changes in FDA policy that occur prior to or during our regulatory review. Delays in obtaining regulatory approvals would:
|
· |
delay commercialization of, and product revenues from, our product candidates; and |
|
· |
diminish the competitive advantages that we may have otherwise enjoyed, which would have an adverse effect on our operating results and financial condition. |
Because the results of preclinical studies and early‑stage clinical trials are not necessarily predictive of future results, any product candidate we advance into additional clinical trials may not continue to have favorable results or receive regulatory approval.
Success in preclinical studies and early clinical trials does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of an investigational drug. Many companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience, have suffered significant setbacks in clinical trials, even after reporting promising results in earlier clinical trials. We do not know whether the clinical trials we may conduct will demonstrate adequate efficacy and safety or otherwise provide adequate information to result in regulatory approval to market any of our product candidates in any particular jurisdiction. If later‑stage clinical trials do not produce favorable results, our ability to achieve regulatory approval for any of our product candidates may be compromised.
If the FDA does not conclude that our product candidates are sufficiently bioequivalent, or have comparable bioavailability, to approved drugs, or if the FDA does not allow us to pursue the Section 505(b)(2) approval pathway as anticipated, the approval pathway for those product candidates will likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated, and the FDA may not approve those product candidates.
A key element of our strategy with regard to any product candidate that we are able to partner is to seek FDA approval for our product candidates through the Section 505(b)(2) regulatory pathway. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Such reliance is typically predicated on a showing of bioequivalence or comparable bioavailability to an approved drug.
If the FDA does not allow us to pursue the Section 505(b)(2) approval pathway for our product candidates, or if we cannot demonstrate bioequivalence or comparable bioavailability of our product candidates to products at a statistically significant level, we may need to conduct additional clinical trials, provide additional data and information,
67
and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for these product candidates, and complications and risks associated with these product candidates, would likely substantially increase. Moreover, our inability to pursue the Section 505(b)(2) approval pathway could result in new competitive products reaching the market more quickly than our product candidates, which could hurt our competitive position and our business prospects. Even if we are allowed to pursue the Section 505(b)(2) approval pathway, we cannot assure you that our product candidates will receive the requisite approvals for commercialization on a timely basis, if at all.
In addition, notwithstanding the approval of a number of products by the FDA under Section 505(b)(2) over the last few years, pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA’s interpretation of Section 505(b)(2) is successfully challenged, the FDA may change its policies and practices with respect to Section 505(b)(2) regulatory approvals, which could delay or even prevent the FDA from approving any NDA that we submit under Section 505(b)(2).
Even if our product candidates are approved under Section 505(b)(2), the approval may be subject to limitations on the indicated uses for which the products may be marketed or to other conditions of approval, or may contain requirements for costly post‑marketing testing and surveillance to monitor the safety or efficacy of the products.
Our decision to seek approval of our product candidates under Section 505(b)(2) may increase the risk that patent infringement suits are filed against us, which would delay the FDA’s approval of such product candidates.
In connection with any NDA that we submit under Section 505(b)(2), we will also be required to notify the patent holder that we have certified to the FDA that any patents listed for the approved drug, also known as a reference listed drug, in the FDA’s Orange Book publication are invalid, unenforceable or will not be infringed by the manufacture, use or sale of our drug. If the patent holder files a patent infringement lawsuit against us within 45 days of its receipt of notice of our certification, the FDA is automatically prevented from approving our Section 505(b)(2) NDA until the earliest of 30 months, expiration of the patent, settlement of the lawsuit or a court decision in the infringement case that is favorable to us. Accordingly, we may invest significant time and expense in the development of our product candidates only to be subject to significant delay and patent litigation before our product candidates may be commercialized. With regard to Egalet‑002, we are aware of litigation involving the sponsor for the RLD for oxycodone and a number of generic manufacturers related to patents listed in the Orange Book that expire on various dates between 2017 and 2025. There is a risk that the sponsor for the RLD may bring an infringement claims against us. Even if we are found not to infringe, or a plaintiff’s patent claims are found invalid or unenforceable, defending any such infringement claim would be expensive and time‑consuming, and would delay launch of Egalet‑002 and distract management from their normal responsibilities.
Conducting clinical trials for our products and product candidates and any commercial sales of our products or future sales of a product candidate may expose us to expensive product liability claims, and we may not be able to maintain product liability insurance on reasonable terms or at all.
The commercial use of our products and clinical use of our products and product candidates expose us to the risk of product liability claims. This risk exists even if a product is approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA, such as the case with our products, or an applicable foreign regulatory authority.
We currently carry clinical trial and product liability insurance with coverage up to approximately $10 million. We may face product liability claims for our products and product candidates, regardless of FDA approval for commercial manufacturing and sale. Product liability claims may be brought against us by consumers, pharmaceutical companies, subjects enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling our products. If we cannot successfully defend ourselves against claims that our products or product candidates caused injuries, we could incur substantial liabilities. We may not be able to obtain insurance coverage at a reasonable cost or in
68
an amount adequate to satisfy any liability that may arise. Regardless of merit or eventual outcome, liability claims may result in:
|
· |
decreased demand for our products; |
|
· |
termination of clinical trial sites or entire trial programs; |
|
· |
injury to our reputation and significant negative media attention; |
|
· |
withdrawal of clinical trial participants; |
|
· |
significant costs to defend the related litigation; |
|
· |
substantial monetary awards to trial subjects or patients; |
|
· |
loss of revenue; |
|
· |
diversion of management and scientific resources from our business operations; |
|
· |
product recall or withdrawal from the market; |
|
· |
the inability to commercialize any products that we may develop; and |
|
· |
an increase in product liability insurance premiums or an inability to maintain product liability insurance coverage. |
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of our product candidates. Any agreements we may enter into in the future with collaborators in connection with the development or commercialization of our product candidates may entitle us to indemnification against product liability losses, but such indemnification may not be available or adequate should any claim arise.
Risks Related to Ownership of Our Securities
We may fail to qualify for continued listing on Nasdaq, which could make it more difficult for our stockholders to sell their shares.
Our common stock, par value $0.001 per share (the “Common Stock”) is listed on The Nasdaq Global Market and therefore we are required to satisfy the continued listing requirements of Nasdaq for inclusion in the Global Market to maintain such listing, including, among other things, the maintenance of a minimum closing bid price of $1.00 per share and either (i) a market value of listed securities of at least $50 million, (ii) stockholders’ equity of at least $10 million or (iii) total assets and total revenue in the most recent fiscal year (or two of the three most recent fiscal years) of at least $50 million. On November 24, 2017, we received a notice from Nasdaq that we were not in compliance with Nasdaq’s Listing Rule 5450(b)(2)(A), as the minimum market value of our Common Stock was below $50 million for 30 consecutive business days. The notification of noncompliance had no immediate effect on the listing or trading of our Common Stock on the Global Market and we have 180 days, or until May 23, 2018, to achieve compliance with the minimum market value requirement. To regain compliance, the minimum market value of our Common Stock must meet or exceed $50 million for a minimum of ten consecutive business days during the 180-day grace period. Our failure to regain compliance during this period could result in delisting. In addition, on March 8, 2018, we received a notice from Nasdaq that were not in compliance with Nasdaq’s Listing Rule 5450(a), as the minimum closing bid price for our Common Stock had been below $1.00 for 30 consecutive business days. We have 180 days, or until September 4, 2018, to achieve compliance with the minimum closing bid price requirement. To regain compliance, the closing bid price of
69
our common stock must meet or exceed $1.00 for a minimum of ten consecutive business days during this 180-day grace period.
In the event that we do not regain compliance with the Nasdaq’s Listing Rules prior to the expiration of the grace period, or fail to meeting any of Nasdaq’s other continued listing requirements, we expect to receive written notification that our Common Stock is subject to delisting. If we receive such a delisting notification, we may either apply for listing on The Nasdaq Capital Market, provided we meet the continued listing requirements of that market, or appeal the decision to a Nasdaq Hearings Panel. In the event of an appeal, our Common Stock would remain listed on The Nasdaq Global Market pending a decision by the panel following the hearing. There can be no assurance that we will be able to regain compliance with the continued listing requirements. If our Common Stock is delisted by Nasdaq, we could face significant material adverse consequences, including:
|
· |
holders of our 5.50% Notes could require us to repurchase the notes, including both principal and accrued interest, for cash if we are delisted from the Nasdaq Global Market and holders of our 6.50% Notes could require us to repurchase the notes for cash if we are delisted from Nasdaq entirely; |
|
· |
a limited availability of market quotations for our Common Stock; |
|
· |
reduced liquidity with respect to our Common Stock; |
|
· |
a determination that our shares are “penny stock,” which will require brokers trading in our shares to adhere to more stringent shares, and which may limit demand for our Common Stock among certain investors; |
|
· |
a limited amount of news and analyst coverage for our company; and |
|
· |
a decreased ability to issue additional securities or obtain additional financing in the future. |
We are party to ongoing stockholder litigation, and in the future could be party to additional stockholder litigation, which is expensive and could harm our business, financial condition and operating results and could divert management attention.
The market price of our common stock has been and may continue to be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We have been and may be the target of this type of litigation in the future as a result of changes in our stock price, past transactions or other matters. Securities litigation against us, whether or not resolved in our favor, could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business or results of operations. For example, and as further described in Item 3, “Legal Proceedings,” and Note 13, “Commitments and Contingencies” to the financial statements accompanying this Annual Report on Form 10-K, we are currently defending two putative securities class actions that were filed in the U.S. District Court for the Eastern District of Pennsylvania on January 27, 2017 and February 10, 2017, respectively. On May 1, 2017, the Court entered an order consolidating the two cases before it and appointing a lead plaintiff. On July 3, 2017, the plaintiffs filed their consolidated amended complaint, which asserts claims for purported violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. The plaintiffs brought their claims individually and on behalf of a putative class of all persons who purchased or otherwise acquired shares of the Company between November 4, 2015 and January 9, 2017 inclusive. The consolidated amended complaint bases its claims on allegedly false and/or misleading statements and/or failures to disclose information about the likelihood that ARYMO ER would be approved for intranasal abuse-deterrent labeling. The defendants moved to dismiss the consolidated amended complaint and after oral argument on the motion to dismiss on February 20, 2018, the Court entered an order pursuant to which the plaintiffs filed a motion for leave to file a second amended complaint on March 6, 2018. The defendants response is due on March 20, 2018. We cannot determine the likelihood of, nor can we reasonably estimate the range of, any potential loss, if any, from these lawsuits.
We may not be able to fully redeem the holders of our warrants if we experience a Fundamental Transaction.
The holders of our warrants may require us to redeem the warrants for cash if we experience certain “Fundamental Transaction” (as defined in the warrants), which generally includes any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our
70
outstanding common stock. There can be no assurance that in the event of a Fundamental Transaction we will be able to sufficiently compensate the holders of the warrants in accordance with the terms thereof. In addition, the provisions in the warrant related to Fundamental Transaction may delay a potential change of control even if such change may be beneficial to some or all our stockholders. These restrictions may also adversely affect the market price of shares of our common stock.
Future issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans or the exercise or our outstanding warrants, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of common stock or common stock‑related securities, together with the exercise of outstanding options and any additional shares issued in connection with acquisitions, if any, may result in material dilution to our investors. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our common stock.
Pursuant to our 2013 Stock‑Based Incentive Compensation Plan, as amended, or the 2013 Stock Plan, our compensation committee is authorized to grant equity‑based incentive awards to our directors, executive officers and other employees and service providers, including officers, employees and service providers of our subsidiaries and affiliates. The number of shares of our common stock we have reserved for issuance under our 2013 Stock Plan is currently 6,280,000, and future option grants and issuances of common stock under our 2013 Stock Plan may adversely affect the market price of our common stock. In addition, in June 2016 our stockholders approved our Employee Stock Purchase Plan, or ESPP, and the reservation of 750,000 shares for issuance thereunder, and in December 2016, our Board adopted our 2017 Inducement Plan (the “Inducement Plan”) and reserved 300,000 shares for issuance under the Inducement Plan for grants to new hires as an inducement for them to join the Company in accordance with Nasdaq Listing Rule 5635(c)(4).
In our July 2017 equity offering, we sold our Common Stock and warrants to purchase our Common Stock. The warrants also contain anti-dilution provisions, which provide that any new issuances of Common Stock or securities convertible into Common Stock at a price, exercise price or conversion price below those of the warrants will result in the exercise price of the warrants being reduced based upon the number of securities issues in such issuance and the number of shares of our Common Stock then outstanding, which could cause further dilution.
Further, our 6.50% Notes are convertible into shares of our Common Stock at any time, and our 5.50% Notes are convertible into shares of our Common Stock under certain specified circumstances, each of which could result in additional issuances of shares and cause further dilution.
Our share price has been and may continue to be volatile, which could subject us to securities class action litigation and result in substantial losses to our stockholders.
The trading price of our common stock is highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this Annual Report, these factors include:
|
· |
the success of competitive products or technologies; |
|
· |
regulatory actions with respect to our products or our competitors’ products, including regulatory decisions regarding the content of the labels for our products or our competitors’ products; |
|
· |
actual or anticipated changes in our growth rate relative to our competitors; |
|
· |
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
71
|
· |
announcements related to third-party payor arrangements; |
|
· |
actions taken by third-party payors to reduce the utilization of opioids; |
|
· |
results of clinical trials of our product candidates or those of our competitors; |
|
· |
regulatory or legal developments in the United States and other countries; |
|
· |
developments or disputes concerning patent applications, issued patents or other proprietary rights; |
|
· |
the recruitment or departure of key personnel; |
|
· |
the level of expenses related to any of our product candidates or clinical development programs; |
|
· |
the results of our efforts to in‑license or acquire additional product candidates or products; |
|
· |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
|
· |
variations in our financial results or those of companies that are perceived to be similar to us; |
|
· |
fluctuations in the valuation of companies perceived by investors to be comparable to us; |
|
· |
share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
|
· |
announcement or expectation of additional financing efforts; |
|
· |
sales of our common stock by us, our insiders or our other stockholders; |
|
· |
changes in the structure of healthcare payment systems; |
|
· |
the receipt of a going concern opinion; |
|
· |
market conditions in the pharmaceutical and biotechnology sectors; and |
|
· |
general economic, industry and market conditions. |
If any of these risks occurs, our stock price may fall and we may be exposed to class action lawsuits that, even if unsuccessful, could be costly to defend and distracting to management. For example, as discussed above and as further described in Item 3, “Legal Proceedings,” and Note 13, “Commitments and Contingencies” to the financial statements accompanying this Annual Report on Form 10-K, we are currently defending two putative securities class actions that were filed in the U.S. District Court for the Eastern District of Pennsylvania on January 27, 2017 and February 10, 2017, respectively, that were consolidated on May 1, 2017. We cannot determine the likelihood of, nor can we reasonably estimate the range of, any potential loss, if any, from these lawsuits.
In addition, the stock market in general, and pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks, including those described in these “Risk Factors,” could have a dramatic and material adverse impact on the market price of our common stock.
72
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will continue to cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our share price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Actual or potential sales of our common stock by our directors or employees, including our executive officers, pursuant to pre-arranged stock trading plans could cause our stock price to fall or prevent it from increasing for numerous reasons, and actual or potential sales by such persons could be viewed negatively by investors.
In accordance with the guidelines specified under Rule 10b5-1 of the Exchange Act and our policies regarding stock transactions, our directors and employees, including our executive officers, have adopted and may in the future adopt stock trading plans pursuant to which they may sell shares of our common stock from time to time in the future. Generally, sales under such plans by our executive officers and directors require public filings. Actual or potential sales of our common stock by such persons could cause the market price of our common stock to fall or prevent it from increasing for numerous reasons. For example, actual or potential sales by such persons could be viewed negatively by investors.
Some provisions of our charter documents and Delaware law have anti‑takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws, as well as provisions of Delaware law, could make it more difficult for a third‑party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These provisions include:
|
· |
authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
|
· |
prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of stockholders to elect director candidates; |
|
· |
prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
|
· |
eliminating the ability of stockholders to call a special meeting of stockholders; |
|
· |
establishing a staggered board of directors; and |
|
· |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management.
Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may also discourage, delay or prevent a third party from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Delaware law, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held
73
the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our amended and restated certificate of incorporation or amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our amended and restated certificate of incorporation provides that, with certain limited exceptions, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for any stockholder (including any beneficial owner) to bring (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of fiduciary duty owed by any director or officer of the Company owed to us or our stockholders, (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law or our certificate of incorporation or our bylaws, or (iv) any action asserting a claim against the Company governed by the internal affairs doctrine, in each case subject to the Court of Chancery having personal jurisdiction of the indispensable parties named as defendants. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have received notice of and consented to the foregoing provisions. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, if a court were to find this choice of forum provision inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition or results of operations.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
Facilities
Our corporate headquarters are located in Wayne, Pennsylvania, where we lease 19,797 square feet of office space under a lease agreement that expires in February 2022 unless terminated earlier. We also maintain a research laboratory, pilot manufacturing and administrative facility in Vaerlose, Denmark, where we lease 12,895 square feet of space under a lease agreement that automatically renews every 12 months (currently through August 2018, after which we plan to vacate or renegotiate the lease).
We believe that our existing facilities are adequate for our current needs.
74
On January 27, 2017 and February 10, 2017, respectively, two putative securities class actions were filed in the U.S. District Court for the Eastern District of Pennsylvania that named as defendants Egalet Corporation and current officers Robert S. Radie, Stanley J. Musial, and Jeffrey M. Dayno. These two complaints, captioned Mineff v. Egalet Corp. et al., No. 2:17-cv-00390-MMB and Klein v. Egalet Corp. et al., No. 2:17-cv-00617-MMB, assert securities fraud claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 (the “Exchange Act”) on behalf of putative classes of persons who purchased or otherwise acquired Egalet Corporation securities between December 15, 2015 and January 9, 2017. On May 1, 2017, the Court entered an order consolidating the two cases before it, appointing the Egalet Investor Group (consisting of Joseph Spizzirri, Abdul Rahiman and Kyle Kobold) as lead plaintiff and approving their selection of lead and liaison counsel. On July 3, 2017, the plaintiffs filed their consolidated amended complaint, which named the same defendants and also asserts claims for purported violations of Sections 10(b) and 20(a) of the Exchange Act. Plaintiffs bring their claims individually and on behalf of a putative class of all persons who purchased or otherwise acquired shares of the Company between November 4, 2015 and January 9, 2017 inclusive. The consolidated amended complaint bases its claims on allegedly false and/or misleading statements and/or failures to disclose information about the likelihood that ARYMO ER would be approved for intranasal abuse-deterrent labeling. The defendants moved to dismiss the Consolidated Amended Complaint on September 1, 2017, the plaintiffs filed their opposition on October 31, 2017, and the defendants filed their reply on December 8, 2017. The Court heard oral arguments on the motion to dismiss on February 20, 2018, and entered an order pursuant to which the plaintiffs filed a motion for leave to file a second amended complaint on March 6, 2018, and the defendants response is due on March 20, 2018. The Company disputes the allegations in the lawsuit and intends to defend these actions vigorously. The Company cannot determine the likelihood of, nor can it reasonably estimate the range of, any potential loss, if any, from these lawsuits.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
75
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our Common Stock trades on the NASDAQ Global Market under the symbol “EGLT.” The following table sets forth the high and low sales price of our Common Stock, as reported by the Nasdaq Global Market for the periods indicated:
|
|
Year Ended December 31, 2017 |
||||||
|
|
|
High |
|
Low |
|
||
|
Year Ended December 31, 2017 |
|
|
|
|
|
|
|
|
Fourth Quarter |
|
$ |
1.32 |
|
$ |
0.83 |
|
|
Third Quarter |
|
|
2.54 |
|
|
1.02 |
|
|
Second Quarter |
|
|
4.88 |
|
|
2.14 |
|
|
First Quarter |
|
|
8.38 |
|
|
4.45 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2016 |
||||||
|
|
|
High |
|
Low |
|
||
|
Year Ended December 31, 2016 |
|
|
|
|
|
|
|
|
Fourth Quarter |
|
$ |
9.40 |
|
$ |
4.50 |
|
|
Third Quarter |
|
|
9.15 |
|
|
4.83 |
|
|
Second Quarter |
|
|
7.50 |
|
|
4.34 |
|
|
First Quarter |
|
|
11.81 |
|
|
5.60 |
|
Stockholders
As of February 22, 2017, there were 8 record holders for shares of our Common Stock.
Securities Authorized for Issuance Under Equity Compensation Plans
Information regarding securities authorized for issuance under our equity compensation plans is contained in Part III, Item 12 of this Annual Report.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our Common Stock for the foreseeable future.
Performance Graph
The performance graph below compares the cumulative total stockholder return on our Common Stock beginning on February 6, 2014, the date our stock began trading on the NASDAQ Global Market, and for each subsequent quarter period end through and including December 31, 2017, with the cumulative return of the NASDAQ Composite Index and NASDAQ Biotechnology Index.
76
The performance graph comparison assumes $100 was invested in our Common Stock and in each of the other indices described above on February 6, 2014. The stock performance shown on the graph below is not necessarily indicative of future price performance.
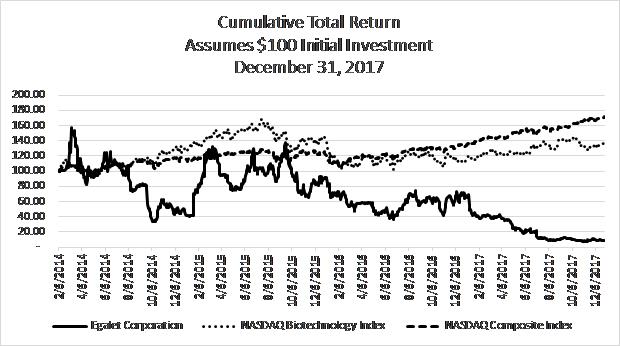
The performance graph above is being furnished solely to accompany this Annual Report on Form 10‑K pursuant to Item 201(e) of Regulation S‑K, is not being filed for purposes of Section 18 of the Exchange Act, shall not be deemed to be “soliciting material” or subject to Rule 14A of the Exchange Act and is not to be incorporated by reference into any filing of the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing, except to the extent that we specifically incorporate this information by reference.
Issuer Purchases of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Annual Report.
Recent Sales of Unregistered Securities
As further described in our Current Report on Form 8-K filed with the SEC on December 28, 2017, in December 2017 we entered into exchange agreements (the “Exchange Agreements”) with certain holders (the “Holders”) of the Company’s 5.50% convertible senior notes due 2020 (the “5.50% Notes”) pursuant to which the Holders agreed to exchange, in the aggregate, approximately $36.4 million of outstanding principal amount of the 5.50% Notes for, in the aggregate, (i) approximately $23.9 million of the Company’s new 6.50% convertible senior notes due 2024 (the “New Notes”), (ii) a warrant exercisable for 3.5 million shares of the Company’s common stock and (iii) payments, in cash, of all accrued but unpaid interest as of the closing on the 5.50% Notes exchanged in the transaction (the “Exchange”). The 6.50% Notes are convertible into shares of our common stock on the terms set forth in the indenture governing the 6.50% Notes. Refer to Note 9- Long term debt for additional information.
77
ITEM 6. SELECTED FINANCIAL DATA
The selected financial data set forth below is derived from our audited consolidated financial statements and may not be indicative of future operating results. The following selected consolidated financial data should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and the notes thereto included elsewhere in this report. The selected financial data in this section are not intended to replace our Consolidated Financial Statements and the related notes. Our historical results are not necessarily indicative of our future results.
Prior to the Share Exchange, we had nominal assets and no operations. We have derived the following consolidated historical statement of operations data for the years ended December 31, 2015, 2016 and 2017 and balance sheet data as of December 31, 2016 and 2017 from our audited financial statements included elsewhere in this report. The consolidated historical statement of operations data for the year ended December 31, 2013 and 2014 and the balance sheet data as of December 31, 2013, 2014 and 2015 are derived from our audited financial statements, which are not included herein. Our historical results are not necessarily indicative of the results that may be expected in the future for any full year or any other interim period.
|
(in thousands) |
|
Year Ended |
|||||||||||||
|
|
|
December 31, |
|||||||||||||
|
|
|
2013 |
|
2014 |
|
2015 |
|
2016 |
|
2017 |
|||||
|
Consolidated Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
— |
|
$ |
1,920 |
|
$ |
22,830 |
|
$ |
16,964 |
|
$ |
26,136 |
|
Cost and Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of product rights) |
|
|
— |
|
|
— |
|
|
3,271 |
|
|
3,660 |
|
|
5,153 |
|
Amortization of product rights |
|
|
— |
|
|
— |
|
|
1,958 |
|
|
2,006 |
|
|
2,082 |
|
General and administrative |
|
|
5,095 |
|
|
15,715 |
|
|
26,474 |
|
|
30,670 |
|
|
34,065 |
|
Sales and marketing |
|
|
— |
|
|
946 |
|
|
16,289 |
|
|
27,892 |
|
|
35,532 |
|
Research and development |
|
|
6,280 |
|
|
22,395 |
|
|
27,054 |
|
|
33,759 |
|
|
14,744 |
|
Restructuring charges |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
2,760 |
|
Total costs and expenses |
|
|
11,375 |
|
|
39,056 |
|
|
75,046 |
|
|
97,987 |
|
|
94,336 |
|
Loss from operations |
|
|
(11,375) |
|
|
(37,136) |
|
|
(52,216) |
|
|
(81,023) |
|
|
(68,200) |
|
Change in fair value of derivative liability |
|
|
— |
|
|
— |
|
|
(260) |
|
|
(644) |
|
|
(2,546) |
|
Interest expense, net |
|
|
8,842 |
|
|
7,079 |
|
|
7,477 |
|
|
12,109 |
|
|
17,666 |
|
Other gain |
|
|
(222) |
|
|
(1,045) |
|
|
(864) |
|
|
(797) |
|
|
(750) |
|
Gain on extinguishment of debt |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(13,221) |
|
Loss (gain) on foreign currency exchange |
|
|
190 |
|
|
(3) |
|
|
82 |
|
|
2 |
|
|
10 |
|
|
|
|
8,810 |
|
|
6,031 |
|
|
6,435 |
|
|
10,670 |
|
|
1,159 |
|
Loss before provision (benefit) for income taxes |
|
|
(20,185) |
|
|
(43,167) |
|
|
(58,651) |
|
|
(91,693) |
|
|
(69,359) |
|
Provision (benefit) for income taxes |
|
|
22 |
|
|
47 |
|
|
(718) |
|
|
(1,061) |
|
|
— |
|
Net loss |
|
$ |
(20,207) |
|
$ |
(43,214) |
|
$ |
(57,933) |
|
$ |
(90,632) |
|
$ |
(69,359) |
78
|
(in thousands) |
|
As of |
|
As of |
|
As of |
|
As of |
|
As of |
|
|||||
|
|
|
December 31, 2013 |
|
December 31, 2014 |
|
December 31, 2015 |
|
December 31, 2016 |
|
December 31, 2017 |
|
|||||
|
Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and marketable securities, available for sale |
|
$ |
15,700 |
|
$ |
52,738 |
|
$ |
145,707 |
|
$ |
86,826 |
|
$ |
91,043 |
|
|
Total assets |
|
|
20,363 |
|
|
60,570 |
|
|
172,416 |
|
|
114,858 |
|
|
119,858 |
|
|
Long term liabilities |
|
|
9,614 |
|
|
8,880 |
|
|
54,530 |
|
|
84,637 |
|
|
116,266 |
|
|
Total liabilities |
|
|
30,236 |
|
|
16,309 |
|
|
83,194 |
|
|
109,532 |
|
|
159,233 |
|
|
Convertible preferred stock |
|
|
14,957 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Accumulated deficit |
|
|
(33,399) |
|
|
(76,613) |
|
|
(134,546) |
|
|
(225,178) |
|
|
(295,300) |
|
|
Total stockholders’ (deficit) equity |
|
|
(24,830) |
|
|
44,261 |
|
|
89,222 |
|
|
5,326 |
|
|
(39,375) |
|
79
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our historical consolidated financial statements and the related notes thereto appearing in this Annual Report. In addition to historical information, some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business and related financing, includes forward‑looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report, our actual results could differ materially from the results described in or implied by the forward‑looking statements contained in the following discussion and analysis.
Overview
We are a fully integrated specialty pharmaceutical company developing, manufacturing and commercializing innovative treatments for pain and other conditions. Given the need for acute and chronic pain products and the issue of prescription abuse, we are focused on bringing non-narcotic and abuse-deterrent opioid formulations to patients and healthcare providers. We are currently marketing SPRIX® (ketorolac tromethamine) Nasal Spray (“SPRIX Nasal Spray”), OXAYDO® (oxycodone HCI, USP) tablets for oral use only—CII (“OXAYDO”) and ARYMO® ER (morphine sulfate) extended-release (“ER”) tablets (“ARYMO ER”), an ER morphine product formulated with abuse-deterrent (“AD”) properties. We have a pipeline of clinical-stage, product candidates developed using our proprietary Guardian™ Technology, for which we are seeking partners. Our lead product candidate, Egalet-002, an abuse-deterrent, extended-release, oral oxycodone formulation, has completed Phase 3 safety and efficacy studies. We plan to continue to grow our business through the revenues of our three approved products, business development and leveraging our proprietary Guardian Technology.
With approximately 100 million Americans suffering from chronic pain according to the Institute of Medicine and an estimated 29.0 million people suffering from drug use disorders worldwide according to the United Nations Office on Drugs and Crime World Drug Report 2016, there is a need for pain treatments and there is also a need to decrease the abuse of pain medications. In reaction to this widespread prescription opioid misuse and abuse, the U.S. government and the FDA have designated this issue as a high priority. In February 2016, the FDA announced an action plan to combat the growing problem of prescription opioid abuse, importantly highlighting the development of AD formulations as a part of the solution. In addition, the Centers for Disease Control and Prevention (“CDC”) has stated that the first line of therapy should be non-opioid treatments, such as non-steroidal anti-inflammatory drugs (“NSAIDs”). We are committed to developing, manufacturing and marketing non-narcotic treatment options such as NSAIDs and abuse-deterrent formulation opioids to provide treatment options that are less likely to be abused or deter abuse.
Our goal is to be a leading specialty pharmaceutical company focused on developing and commercializing innovative treatments for pain and other conditions. With the launch of ARYMO ER at the end of March 2017, we expanded our salesforce and now have 82 territory managers promoting SPRIX Nasal Spray, OXAYDO and ARYMO ER to approximately 10,500 healthcare providers. In addition, we have an account management team calling on commercial and government payors and a trade team focused on channel partners.
To broaden our reach, in the third quarter, we partnered with Ascend Therapeutics to promote SPRIX to approximately 11,000 women’s healthcare providers, which it began in the same quarter. In January 2018, we entered into a co-promotion arrangement with OraPharma, a division of Valeant Pharmaceuticals, to promote SPRIX Nasal Spray in addition along with OraPharma’s portfolio products with its 141-person salesforce exclusively to dentists, dental specialists and oral surgeons in the United States. Prior to this agreement, we terminated our partnership with Septodont, a company that previously promoted SPRIX to dentists. In addition, Teva has exclusive marketing and commercialization rights to SPRIX Nasal Spray in Israel, Gaza and the West Bank once the product is registered. We are continuing to evaluate other partnership opportunities to bring SPRIX Nasal Spray to other potential specialties that treat patients with short-term pain.
80
In September 2016, we announced Category 1 abuse-deterrent data that demonstrated that OXAYDO resists syringeability which could potentially deter abuse through the intravenous route. This data was the basis for an sNDA filed in December 2016 with the FDA. Based on conversations with the FDA, we do not anticipate the FDA taking action on this file in the near-term. OXAYDO is currently available in 5 mg and 7.5 mg dosage strengths. In September 2016, a pharmacokinetic study demonstrated bioequivalence (“BE”) of OXAYDO 15 mg to Roxicodone 15 mg. This data served as the basis for a prior approval supplement that was submitted to the FDA in February 2017 to request approval of additional dosage strengths of 10 mg and 15 mg. We received a complete response letter (CRL) from the FDA in June of 2017 in which the FDA requested more information regarding the effect of food on OXAYDO 15 mg and the intranasal abuse-deterrent properties of OXAYDO 10 and 15 mg. We are evaluating our options regarding how to address the CRL.
In December of 2017, the FDA granted tentative approval for an expanded label for ARYMO ER. The approval was issued for a supplement submitted earlier in 2017 to update the ARYMO ER prescribing information with data from a Category 2/3 intranasal human abuse potential (“HAP”) study and an intranasal AD claim. This data was previously excluded from the label based on the original new drug application (“NDA”) approval due to exclusivity granted to another company. The final approval is expected to be granted when the exclusivity period expires on October 2, 2018.
Our proprietary Guardian Technology is a polymer matrix tablet technology that utilizes a novel application of the well-established manufacturing process of injection molding, which results in tablets that are hard and difficult to manipulate for misuse and abuse. Our proprietary product, ARYMO ER, is the first product approved using the Guardian Technology. In addition to ARYMO ER, we have a pipeline of products developed using our Guardian Technology. Egalet-002, our current lead product candidate, employs a similar matrix system to that used in ARYMO ER, however the Egalet-002 tablet is surrounded by a water-impermeable, non-eroding, hard shell containing polylactic acid ("PLA") that creates a cylinder, with the API-containing matrix exposed at both ends. Egalet‑002, an AD, ER, oral oxycodone formulation, completed a Phase 3 program in 2017. The product is in development for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. Using our Guardian Technology, we developed Egalet‑002 to address common methods of abuse and misuse, including crushing to swallow, snort or smoke, and dissolving to inject. Egalet-002 was specifically designed to address abuse by crushing and snorting, which is the most common method of manipulating oxycodone‑based products for abuse, according to a 2011 article in the Harm Reduction Journal. To focus on our commercial products, we are not investing any additional resources in Egalet-002 at this time and are looking to potentially partner the program.
In addition to growing through product sales, we plan to conduct business development to build on our product portfolio and leverage Guardian Technology to fuel pipeline growth. Our business development activities will be concentrated in three areas: 1) augment our product portfolio through potential in-licenses and product acquisitions; 2) enhance the opportunity for our existing products through partnerships that access healthcare providers and patients outside our commercial focus in the United States or markets outside the United States; and 3) develop partnerships to leverage our Guardian Technology by collaborating around our current product candidates or exploring new product opportunities or signing revenue generating license agreements with third parties.
Financial Operations
Our net losses were $57.9 million, $90.6 million and $69.4 million for the years ended December 31, 2015, 2016 and 2017, respectively. We recognized total revenues of $22.8 million, $17.0 million and $26.1 million for the years ended December 31, 2015, 2016 and 2017, respectively. Included in those amounts are net product sales of $4.2 million, $16.9 million and $26.1 million for the years ended December 31, 2015, 2016 and 2017, respectively. As of December 31, 2017, we had an accumulated deficit of $295.3 million. We expect to incur significant expenses and operating losses for the foreseeable future as we incur significant commercialization expenses as we continue to grow our sales, marketing and distribution infrastructure to sell our commercial products in the U.S. Additionally, we expect to continue to protect and expand our intellectual property portfolio.
Until we become profitable, if ever, we will seek to fund our operations primarily through public or private equity or debt financings or other sources. Other additional financing may not be available to us on acceptable terms, or
81
at all. Our failure to raise capital as and when needed could have a material adverse effect on our financial condition and our ability to pursue our business strategy. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
Financial Operations Overview
Revenue
We have generated $47.2 million through December 31, 2017 in net product revenue from our approved products, ARYMO ER, SPRIX Nasal Spray and OXAYDO, and have generated $22.6 million in total revenue through December 31, 2017, from feasibility and collaboration agreements. Our ability to generate additional revenue and become profitable depends upon our ability to expand the marketing of our approved products and commercialize our product candidates, or other product candidates that we may in-license or acquire in the future. To date, we have generated no revenues from Egalet‑002, our clinical stage product candidate.
Cost of Sales (excluding amortization of product rights)
Cost of sales includes the cost of inventory sold or reserved, which includes manufacturing, supply chain costs, product shipping and handling costs, and product royalties. The cost of sales associated with the deferred product revenues is recorded as deferred costs, which are included in inventory, until such time the deferred revenue is recognized.
Amortization of Product Rights
Amortization of product rights consists of the amortization expense associated with the intangible product rights related to the SPRIX Nasal Spray acquisition and OXAYDO license. These expenses are recognized on a straight-line basis over the useful life of the related intangible asset.
General & Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel in our executive, finance and other administrative areas. Other general and administrative expenses include facility costs and professional fees for legal, patent, regulatory fees, consulting and accounting services. We anticipate that our general and administrative expenses will increase in the future due to the growth of our commercialization efforts for our approved products and, if approved, our product candidates and to fund ongoing public company costs. These increases will likely include increased costs for insurance, costs related to the hiring of additional personnel and payments to outside consultants, regulatory fees, and legal and accounting fees, among other expenses.
Sales & Marketing Expenses
Sales and marketing expenses consist primarily of salaries and related costs for personnel in our sales and marketing departments, along with our third party contracted sales force. Other sales and marketing costs include professional fees for consulting and promotional materials. We anticipate that our sales and marketing expenses will continue to increase as we grow our commercial operations for our approved products and, if approved, our product candidates. These increases will likely include increased costs for hiring of additional personnel, outside consultants and marketing programs, among other expenses.
Research and Development Expenses
Research and development expenses consist primarily of costs associated with the development and clinical testing of Egalet‑002 and life cycle management opportunities for our approved products. Our research and development expenses consist of:
|
· |
employee‑related expenses, including salaries, benefits, and travel expense; |
82
|
· |
expenses incurred under agreements with Clinical Research Organizations (“CROs”) and investigative sites that conduct our clinical trials; |
|
· |
the cost of acquiring, developing and manufacturing clinical trial materials; |
|
· |
facilities and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, other supplies; and |
|
· |
costs associated with preclinical activities |
We expense research and development costs to operations as incurred. We account for non‑refundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been received, rather than when the payment is made.
We do not currently utilize a formal time allocation system to capture personnel related expenses on a project‑by‑project basis because we record expenses by functional department. However, we do allocate third party research and development expenses to our product and product candidates, as shown in the table below.
The following table summarizes our research and development expenses for the years ended December 31, 2015, 2016 and 2017:
|
(in thousands) |
|
Year Ended |
|
Year Ended |
|
Year Ended |
|
|||
|
|
|
December 31, |
|
December 31, |
|
December 31, |
|
|||
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||
|
ARYMO ER |
|
$ |
7,777 |
|
$ |
5,252 |
|
$ |
663 |
|
|
Egalet‑002 |
|
|
11,613 |
|
|
17,883 |
|
|
8,221 |
|
|
OXAYDO |
|
|
— |
|
|
4,091 |
|
|
225 |
|
|
Other clinical and preclinical development |
|
|
3,853 |
|
|
2,054 |
|
|
2,892 |
|
|
Personnel related |
|
|
3,811 |
|
|
4,479 |
|
|
2,743 |
|
|
|
|
$ |
27,054 |
|
$ |
33,759 |
|
$ |
14,744 |
|
We incurred research and development expenses of $27.1 million, $33.8 million and $14.7 million during the years ended December 31, 2015, 2016 and 2017, respectively. We anticipate that our future research and development expense will continue to decline as we are seeking partners for each of our product candidates.
It is difficult to determine with certainty the duration and completion costs of our current or future preclinical programs and clinical trials of our product candidates, or if, when or to what extent we will generate revenues from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors, including the uncertainties of future clinical and preclinical studies, uncertainties in clinical trial enrollment rate and significant and changing government regulation. In addition, the probability of success for each product candidate will depend on numerous factors, including competition, manufacturing capability and commercial viability. A change in the outcome of any of these variables with respect to the development of our product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if regulatory authorities were to require us to conduct clinical trials beyond those which we currently anticipate will be required for the completion of our clinical pipeline or if we experience significant delays in enrollment in any clinical trials, we could be required to expend significant additional financial resources and time on the completion of the clinical development.
The successful development of our product candidates is highly uncertain due to the numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
|
· |
the scope, rate of progress and expense of our research and development activities; |
83
|
· |
clinical trial results; |
|
· |
the terms and timing of regulatory approvals; and |
|
· |
the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights. |
Due to these uncertainties, we are unable to determine with certainty the duration and completion costs of our development projects or when and to what extent we will receive revenue from the commercialization and sale of our product candidates.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, debt, stock-based compensation, income taxes and accrued research and development expenses, as described in greater detail below. We base our estimates on our limited historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily-apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in more detail in the notes to our financial statements appearing at the end of this filing. However, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our financial condition and results of operations.
Revenue Recognition
We generate revenue from product sales of our approved products and collaborative research and development agreements that we have entered or may enter into from time to time.
Net Product Sales
We recognize revenue in accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 605, Revenue Recognition, when the following criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred; and risk of loss has passed; the seller’s price to the buyer is fixed or determinable and collectability is reasonably assured. We determine that persuasive evidence of an arrangement exists based on written contracts that define the terms of the arrangements. Pursuant to the contract terms, we determine when title to products and associated risk of loss has passed on to the customer. We assess whether the price is fixed or determinable based on the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. We assess collectability based primarily on the customer’s payment history and creditworthiness.
We sell SPRIX Nasal Spray in the U.S. to a single specialty pharmaceutical distributor subject to rights of return. We have limited SPRIX Nasal Spray sales history under the current distribution model and pricing and have determined that at this time we cannot reliably estimate expected returns of the product at the time of shipment. Accordingly, we defer recognition of revenue on product shipments of SPRIX Nasal Spray until the right of return no longer exists, which occurs at the earlier of the time SPRIX Nasal Spray units are dispensed through patient prescriptions or expiration of the right of return. Units dispensed are generally not subject to return, except in the rare cases where the product malfunctions or the product is damaged in transit. We calculate patient prescriptions dispensed using an analysis of third-party information. As of December 31, 2017, we had deferred revenue of $772,000 related to sales of SPRIX Nasal Spray to our specialty pharmaceutical distributor.
84
We sell ARYMO ER and OXAYDO in the U.S. to several wholesalers, all subject to rights of return. We have limited ARYMO ER and OXAYDO sales history under the current distribution model and pricing, and have determined that at this time we cannot reliably estimate expected returns of the product at the time of shipment. Accordingly, we defer recognition of revenue on product shipments of ARYMO ER and OXAYDO until the right of return no longer exists, which occurs at the earlier of the time units are dispensed through patient prescriptions or expiration of the right of return. We calculate patient prescriptions dispensed using an analysis of third-party information. As of December 31, 2017, we had deferred revenue of $1.8 million and $4.9 million related to sales of ARYMO ER and OXAYDO to the wholesalers.
The following table presents an analysis of our provision for product sales allowances and accruals for the year ended December 31, 2017:
|
(in thousands) |
|
Fees and distribution costs |
|
Co-pay assistance |
|
Rebates |
|
Total |
||||
|
Balances at December 31, 2016 |
|
$ |
— |
|
$ |
1,021 |
|
$ |
— |
|
$ |
1,021 |
|
Allowances for current period sales |
|
|
4,586 |
|
|
32,685 |
|
|
3,740 |
|
|
41,011 |
|
Credits or payments made for current period sales |
|
|
(4,088) |
|
|
(30,062) |
|
|
(3,161) |
|
|
(37,311) |
|
Balance at December 31, 2017 |
|
$ |
498 |
|
$ |
3,644 |
|
$ |
579 |
|
$ |
4,721 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total gross product sales |
|
|
|
|
|
|
|
|
|
|
$ |
67,147 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total provision for product sales allowances and accruals as a percentage of total gross sales |
|
|
|
|
|
|
|
|
|
|
|
61% |
Intangible and Long-Lived Assets
Intangible assets consist of product rights related to the product acquisition of SPRIX Nasal Spray from Luitpold, product rights associated with the Collaboration and License Agreement with Acura to commercialize OXAYDO tablets, and in‑process research and development (“IP R&D”) related to our drug delivery platform technology.
Long‑lived assets, including intangible assets and property and equipment, are assessed for impairment whenever events or changes in circumstances indicate the carrying amount of the asset may not be recoverable. Recoverability of long‑lived assets that will continue to be used in our operations is measured by comparing the carrying amount of the asset to the forecasted undiscounted future cash flows related to that asset. In the event the carrying value of the asset exceeds the undiscounted future cash flows, and the carrying value is not considered recoverable, an impairment loss is measured as the excess of the asset’s carrying value over its fair value, generally based on a discounted future cash flow method.
Events giving rise to impairment are an inherent risk in the pharmaceutical industry and are difficult to predict. Factors that we consider in deciding when to perform an impairment review include significant under‑performance of the asset in relation to expectations, significant negative industry or economic trends and significant changes or planned changes in our use of the assets. We did not record any impairment charges for the years ended December 31, 2015, 2016 and 2017.
Stock‑Based Compensation Expense
We apply the fair value recognition provisions of FASB ASC Topic 718, Compensation—Stock Compensation. Determining the amount of share-based compensation expense to be recorded requires us to develop
85
estimates of the fair value of stock options as of their grant date. We recognize share-based compensation expense ratably over the requisite service period, which in most cases is the vesting period of the award. Calculating the fair value of share-based awards requires that we make highly subjective assumptions.
We use the Black-Scholes option pricing model to value our stock option awards. Use of this valuation methodology requires that we make assumptions as to the volatility of our Common Stock, the expected term of our stock options, and the risk-free interest rate for a period that approximates the expected term of our stock options and our expected dividend yield. For the year ended December 31, 2017 we estimated our expected volatility based on our actual historical volatility by using daily closing prices over a period of the expected term of the associated award. Prior to January 1, 2017, we utilized data from a representative group of companies to estimate expected stock price volatility. We selected companies from the pharmaceutical industry with similar characteristics to us, including those in the early stage of product development and with a therapeutic focus, to comprise our representative group.
We use the simplified method as prescribed by the SEC Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment, to calculate the expected term of stock option grants to employees as we do not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term of stock options granted to employees. We utilize a dividend yield of zero based on the fact that we have never paid cash dividends and have no current intention to pay cash dividends. The risk-free interest rate used for each grant is based on the U.S. Treasury yield curve in effect at the time of grant for instruments with a similar expected life. The weighted-average assumptions used to estimate the fair value of stock options using the Black-Scholes option pricing model were as follows for the year ended December 31, 2015, 2016 and 2017:
|
|
|
2015 |
|
2016 |
|
2017 |
|||
|
Risk-free interest rate |
|
1.76 |
% |
|
1.66 |
% |
|
1.93 |
% |
|
Expected term of options (in years) |
|
6.27 |
|
|
6.19 |
|
|
6.15 |
|
|
Expected volatility |
|
72.93 |
% |
|
68.94 |
% |
|
80.57 |
% |
|
Dividend yield |
|
— |
|
|
— |
|
|
— |
|
Convertible Debt Accounting
We perform an assessment of all embedded features of a debt instrument to determine if (1) such features should be bifurcated and separately accounted for, and (2) if bifurcation requirements are met, whether such features should be classified and accounted for as equity or liability instruments. If the embedded feature meets the requirements to be bifurcated and accounted for as a liability, the fair value of the embedded feature is measured initially, included as a liability on our Consolidated Balance Sheet, and re-measured to fair value at each reporting period. Any changes in fair value are recorded in our Consolidated Statement of Operations. We monitor, on an ongoing basis, whether events or circumstances could give rise to a change in our classification of embedded features.
5.50% Notes
We determined the embedded conversion options in the 5.50% Convertible Senior Notes due 2020 (the “5.50% Notes”) are not required to be separately accounted for as derivatives. However, since the 5.50% Notes can be settled in cash or shares of our Common Stock or a combination of cash and shares of our Common Stock at our option, we are required to separate the 5.50% Notes into a liability and equity component. The carrying amount of the liability component is calculated by measuring the fair value of a similar liability that does not have an associated equity component. The carrying amount of the equity component representing the embedded conversion option is determined by deducting the fair value of the liability component from the initial proceeds. The excess of the principal amount of the liability component over its carrying amount is amortized to interest expense over the expected life of the 5.50% Notes using the effective interest method. The equity component is not re-measured as long as it continues to meet the conditions for equity classification.
86
The fair value of the liability component of the 5.50% Notes was estimated at $40.6 million at issuance in April 2015. Therefore, the difference between the $61.0 million face value of the 5.50% Notes and the $40.6 million estimated fair value of the liability component is being amortized to interest expense over the term of the 5.50% Notes through April 1, 2020 using the effective interest method.
On a quarterly basis, we perform an assessment to determine whether the 5.50% Notes have become convertible at the option of the holder, based on meeting any of the conversion criteria described above. Should the 5.50% Notes become convertible, we then assess our intent and ability to settle the 5.50% Notes in cash, shares of our Common Stock, or a combination of cash and shares of our Common Stock.
The 5.50% Notes include an interest make-whole feature whereby if a noteholder converts any of the 5.50% Notes prior to April 1, 2018, we will, in addition to the other consideration payable or deliverable in connection with such conversion, make an interest make-whole payment to the converting holder equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the 5.50% Notes to be converted had such notes remained outstanding from the conversion date through April 1, 2018, computed using a discount rate equal to 2%. We have determined that this feature is an embedded derivative and have recognized the fair value of this derivative as a liability in our Consolidated Balance Sheets, with subsequent changes to fair value recorded through earnings at each reporting period on our Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities. The fair value of this embedded derivative was determined based on a binomial tree lattice model.
The estimated fair value of the liability component at the date of issuance was determined using valuation models that are complex and subject to judgment. Significant assumptions within the valuation models included an implied credit spread, the expected volatility and dividend yield of our Common Stock and the risk-free interest rate for notes with a similar term.
6.50% Notes
We determined the embedded conversion options in the 6.50% Convertible Senior Notes due 2024 (the “6.50% Notes”) are required to be separately accounted for as derivatives as we did not have enough available authorized shares to cover the conversion obligation as of the date of issuance, December 27, 2017 or as of December 31, 2017. The value of the liability component is calculated by measuring the fair value of a similar liability that does not have an associated conversion component. The excess of the principal amount of the liability component over its carrying amount is amortized to interest expense over the expected life of the 6.50% Notes using the effective interest method.
The fair value of the liability component of the 6.50% Notes was estimated at $4.6 million at issuance in December 2017. Therefore, the difference between the $23.9 million face value of the 6.50% Notes and the $4.6 million estimated fair value of the liability component is being amortized to interest expense over the term of the 6.50% Notes through December 31, 2024 using the effective interest method.
The estimated fair value of the liability component at the date of issuance was determined using valuation models that are complex and subject to judgment. Significant assumptions within the valuation models included an implied credit spread, the expected volatility and dividend yield of our Common Stock and the risk-free interest rate for notes with a similar term.
The 6.50% Notes include an interest make-whole feature whereby if a noteholder converts any of the 6.50% Notes prior to January 1, 2021, we will, in addition to the other consideration payable or deliverable in connection with such conversion, make an interest make-whole payment to the converting holder equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the 6.50% Notes to be converted had such notes remained outstanding from the conversion date through January 1, 2021, computed using a discount rate equal to 2%. We have determined that this feature is an embedded derivative and have recognized the fair value of this derivative as a liability in our Consolidated Balance Sheets, with subsequent changes to fair value recorded through earnings at each reporting period on our Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities. The fair value of this embedded derivative was determined based on a binomial tree lattice model.
87
Warrant Liability Accounting
In July 2017, we entered into an underwriting agreement with Cantor Fitzgerald & Co. relating to an underwritten public offering (the “July 2017 Equity Offering”) of 16,666,667 shares of our Common Stock and accompanying warrants to purchase 16,666,667 shares of Common Stock, at a combined public offering price of $1.80 per share and accompanying warrant, for gross proceeds of $30.0 million. Each warrant has an exercise price of $2.70, subject to adjustment in certain circumstances. As of December 31, 2017, the warrant exercise price was adjusted to $2.14. The shares of Common Stock and warrants were issued separately. The warrants may be exercised at any time on or after the date of issuance and will expire five years from the date of issuance.
We accounted for the warrants using ASC 480 – Distinguishing Liabilities from Equity and determined that the warrants were a freestanding financial instrument that are subject to liability classification. Pursuant to the terms of the agreement, we could be required to settle the warrants in cash in the event of an acquisition of the Company, and as a result the warrants are required to be measured at fair value and reported as a current liability in our Consolidated Balance Sheet. The warrant exercise price is subject to adjustment upon the issuance of certain equity securities at a price less than the exercise price of the warrants then in effect.
Accrued Research and Development Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses, including clinical trial expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. The significant estimates in our accrued research and development expenses are related to fees paid to vendors in connection with research and development activities for which we have not yet been invoiced. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows in accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we will adjust the accrual accordingly. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. We do not anticipate the future settlement of existing accruals to differ materially from our estimates.
Income Taxes
Our income tax expense, deferred tax assets and reserves for unrecognized tax benefits reflect management’s best assessment of estimated future taxes to be paid. We are subject to income taxes in the U.S., Denmark, and the United Kingdom (“U.K.”). Significant judgments and estimates are required in determining the consolidated income tax expense, including a determination of whether and how much of a tax benefit taken by us in our tax filings or positions is more likely to be realized than not.
We believe that it is more likely than not that the benefit from some of our U.S. federal, U.S. state, Denmark, and U.K. net operating loss carryforwards will not be realized. At December 31, 2017, in recognition of this risk, we have provided a valuation allowance of approximately $61.0 million on the deferred tax assets relating to these net operating loss carryforwards and other deferred tax assets. If our assumptions change and we determine we will be able to realize these net operating losses, the tax benefits relating to any reversal of the valuation allowance on deferred tax assets at December 31, 2017 will be accounted for as a reduction of income tax expense.
We recognize tax liabilities in accordance with ASC Topic 740 – Tax Provisions and we adjust these liabilities when our judgment changes as a result of the evaluation of new information not previously available. Due to the
88
complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from our current estimate of the tax liabilities. These differences will be reflected as increases or decreases to income tax expense in the period in which they are determined.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act makes broad and complex changes to the U.S. tax code, including, but not limited to, reducing the top U.S. federal corporate tax rate to 21%; requiring companies to pay a one-time transition tax on certain un-repatriated earnings of foreign subsidiaries; generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; eliminating the corporate alternative minimum tax (“AMT”) and changing how existing AMT credits can be realized; creating the base erosion anti-abuse tax, a new minimum tax; creating a new limitation on deductible interest expense; and changing rules related to uses and limitations of net operating loss carryforwards created in tax years beginning after December 31, 2017.
The Tax Act reduces our U.S. corporate income tax rate from 34% to 21%, effective January 1, 2018. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. As a result of the reduction in the U.S. corporate income tax rate to 21% under the Tax Act, we revalued our ending net deferred tax assets and liabilities at December 31, 2017.
The Tax Act provided for a one-time transition tax on the deemed repatriation of post-1986 undistributed foreign subsidiary earnings and profits. We did not have to recognize any income tax expense related to the transition tax due to current and historical losses at its controlled foreign corporation.
The global intangible low-taxed income tax and base erosion provisions are effective for taxable years beginning after December 31, 2017. The Company does not currently expect these provisions to have a material impact on its tax rate due to losses at its controlled foreign corporation and they are currently below the gross receipts threshold for purposes of the base erosion provisions.
Due to the timing of the new tax law and the substantial changes it brings, the SEC Staff issued SAB 118, which provides registrants a measurement period to report the impact of the new U.S. tax law. During the measurement period, provisional amounts for the effects of the law are recorded to the extent a reasonable estimate can be made. To the extent that all information necessary is not available, prepared or analyzed, companies may recognize provisional estimated amounts for a period of up to one year following enactment of the Tax Act. In connection with our initial analysis of the impact of the Tax Act, we have reasonably estimated there to be no income tax expense in the year ended December 31, 2017. We will continue to monitor for future updates to guidance or interpretations issued by the Internal Revenue Service.
89
Results of Operations
Comparison of Years Ended December 31, 2016 and 2017
|
(in thousands) |
|
|
Year Ended |
|||||||
|
|
|
|
December 31, |
|||||||
|
|
|
|
2016 |
|
2017 |
|
Change |
|||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
|
$ |
16,864 |
|
$ |
26,136 |
|
$ |
9,272 |
|
Collaboration revenues |
|
|
|
100 |
|
|
— |
|
|
(100) |
|
Total revenue |
|
|
|
16,964 |
|
|
26,136 |
|
|
9,172 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost and Expenses |
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of product rights) |
|
|
|
3,660 |
|
|
5,153 |
|
|
1,493 |
|
Amortization of product rights |
|
|
|
2,006 |
|
|
2,082 |
|
|
76 |
|
General and administrative |
|
|
|
30,670 |
|
|
34,065 |
|
|
3,395 |
|
Sales and marketing |
|
|
|
27,892 |
|
|
35,532 |
|
|
7,640 |
|
Research and development |
|
|
|
33,759 |
|
|
14,744 |
|
|
(19,015) |
|
Restructuring charges |
|
|
|
— |
|
|
2,760 |
|
|
2,760 |
|
Total costs and expenses |
|
|
|
97,987 |
|
|
94,336 |
|
|
(3,651) |
|
Loss from operations |
|
|
|
(81,023) |
|
|
(68,200) |
|
|
12,823 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense: |
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability |
|
|
|
(644) |
|
|
(2,546) |
|
|
(1,902) |
|
Interest expense, net |
|
|
|
12,109 |
|
|
17,666 |
|
|
5,557 |
|
Other gain |
|
|
|
(797) |
|
|
(750) |
|
|
47 |
|
Gain on extinguishment of debt |
|
|
|
— |
|
|
(13,221) |
|
|
|
|
Loss on foreign currency exchange |
|
|
|
2 |
|
|
10 |
|
|
8 |
|
|
|
|
|
10,670 |
|
|
1,159 |
|
|
(9,511) |
|
Loss before benefit for income taxes |
|
|
|
(91,693) |
|
|
(69,359) |
|
|
22,334 |
|
Benefit for income taxes |
|
|
|
(1,061) |
|
|
— |
|
|
1,061 |
|
Net loss |
|
|
$ |
(90,632) |
|
$ |
(69,359) |
|
$ |
21,273 |
Net product sales
Net product sales increased from $16.9 million for the year ended December 31, 2016 to $26.1 million for the year ended December 31, 2017. The increase was primarily due to continued growth of SPRIX Nasal Spray and OXAYDO product sales and the commercial launch of ARYMO ER in 2017.
Collaboration revenues
Collaboration revenues were $100,000 for the year ended December 31, 2016 and consisted entirely of revenues recognized under our SPRIX Nasal Spray marketing agreement with Septodont. There were no collaboration revenues for the year ended December 31, 2017.
Cost of sales (excluding amortization of product rights)
Cost of sales (excluding amortization of product rights) was $3.7 million for the year ended December 31, 2016 related to the product sales of SPRIX Nasal Spray and OXAYDO. Cost of sales (excluding amortization of product rights) was $5.2 million for the year ended December 31, 2017.
90
Cost of sales (excluding amortization of product rights) for SPRIX Nasal Spray reflects the fair value of finished goods inventory assumed as part of the SPRIX Nasal Spray acquisition as well as the average cost of inventory subsequently produced and dispensed to patients. The cost of sales (excluding amortization of product rights) for OXAYDO reflects the average costs of inventory dispensed to patients during each period.
Amortization of product rights
Amortization of product rights of $2.0 million for the year ended December 31, 2016 was composed of $1.1 million and $903,000, related to intangible assets acquired in the OXAYDO License and SPRIX Nasal Spray acquisition, respectively. Amortization of product rights of $2.1 million for the year ended December 31, 2017 was composed of $1.1 million and $1.0 million, related to intangible assets acquired in the OXAYDO License and SPRIX Nasal Spray acquisition, respectively.
General and administrative expenses
General and administrative expenses increased by $3.4 million, or 11.1%, from $30.7 million for the year ended December 31, 2016 to $34.1 million for the year ended December 31, 2017.
This increase was attributable to increases in FDA-mandated post-marketing studies of $5.3 million in the year ended December 31, 2017 and increases in annual regulatory filing fees of $1.4 million for Oxaydo, ARYMO ER and SPRIX Nasal Spray. These increases were partially offset by a reduction in professional fees of $2.9 million, primarily due to consulting fees incurred in 2016 related to preparation for the ARYMO ER FDA Advisory Committee meeting.
Sales and marketing expenses
Sales and marketing expenses increased by $7.6 million, or 27.4%, from $27.9 million for the year ended December 31, 2016 to $35.5 million for the year ended December 31, 2017.
This increase was attributable to costs related to the commercial launch of ARYMO ER in early 2017, totaling $2.8 million. There was an increase of $5.5 million in sales force expenses due to the expansion of the sales force promoting SPRIX Nasal Spray, OXAYDO and ARYMO ER. These increases were offset by decreases in promotional expenses for OXAYDO of $1.6 million.
Research and development expenses
Research and development expenses decreased by $19.0 million, or 56.3%, from $33.8 million for the year ended December 31, 2016 to $14.7 million for the year ended December 31, 2017.
This decrease was driven primarily by decreases in our development program expenses for ARYMO ER (formerly Egalet-001), Egalet-002 and OXAYDO of $4.6 million, $9.7 million and $4.1 million, respectively. There were also decreases in compensation and benefit expense of $1.5 million due to the reduction in research and development personnel pursuant to the expense reduction plan announced in August 2017.
Restructuring Expenses
Restructuring expenses of $2.8 million for the year ended December 31, 2017 reflect costs related to the expense reduction plan announced in August 2017 to decrease the operating expenses that do not directly support the growth of our commercial business.
91
Change in fair value of derivative liability
The interest make whole provisions of the 5.50% Notes and the 6.50% Notes, along the warrant liability associated with our July 2017 Equity offering are subject to re-measurement at each balance sheet date and we recognize any change in fair value in our statements of operations and comprehensive loss as a change in fair value of the derivative liabilities. During the years ended December 31, 2016 and 2017, we recognized a change in the fair value of our derivative liabilities $644,000 and $2.5 million respectively. The change in fair value of the derivative liability is due the changes in the value of our Common Stock during the years ended December 31, 2016 and 2017.
Interest expense, net
Interest expense increased from $12.1 million for the year ended December 31, 2016 to $17.7 million for the year ended December 31, 2017. Interest expense for the year ended December 31, 2016 was primarily attributable to the interest expense on the 5.50% convertible notes and the Hercules Loan Agreement. Interest expense for the year ended December 31, 2017 was primarily attributable to the interest expense on the 5.50% convertible notes and the 13% senior secured notes.
Refer to Note 9 — Long Term Debt in the Notes to our Consolidated Financial Statements, for additional information about our long-term debt at December 31, 2017.
Other gain
Other gain of $797,000 for the year ended December 31, 2016 consisted primarily of a Danish research and development tax credit. Other gain of $750,000 for the year ended December 31, 2017 consisted primarily of Danish research and development tax credit of $880,000 offset by losses of $184,000 for the retirement of a leasehold improvement in the U.S.
Gain on Extinguishment of Debt
We recognized a gain of $13.2 million for the year ended December 31, 2017 related to the note exchange of a portion of our 5.50% convertible notes in 2017.
Refer to Note 9 — Long Term Debt in the Notes to our Consolidated Financial Statements, for additional information about our long-term debt at December 31, 2017.
Loss on foreign currency exchange
We recognized a loss on foreign currency exchange of $2,000 and $10,000, during the years ended December 31, 2016 and 2017, respectively. The change was primarily attributable to a change in the average rates of currency in which we transacted during 2016 compared to 2017.
Benefit for income taxes
We had a tax benefit of $1.1 million during the year ended December 31, 2016. We had no tax benefit during the year ended December 31, 2017. The tax benefit in the year ended December 31, 2016 relates to a state tax benefit associated with the 5.50% Notes.
92
Comparison of Years Ended December 31, 2015 and 2016
|
(in thousands) |
|
|
Year Ended |
|||||||
|
|
|
|
December 31, |
|||||||
|
|
|
|
2015 |
|
2016 |
|
Change |
|||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
|
$ |
4,184 |
|
$ |
16,864 |
|
$ |
12,680 |
|
Related party revenues |
|
|
|
18,646 |
|
|
— |
|
|
(18,646) |
|
Collaboration revenues |
|
|
|
— |
|
|
100 |
|
|
100 |
|
Total revenue |
|
|
|
22,830 |
|
|
16,964 |
|
|
(5,866) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost and Expenses |
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of product rights) |
|
|
|
3,271 |
|
|
3,660 |
|
|
389 |
|
Amortization of product rights |
|
|
|
1,958 |
|
|
2,006 |
|
|
48 |
|
General and administrative |
|
|
|
26,474 |
|
|
30,670 |
|
|
4,196 |
|
Sales and marketing |
|
|
|
16,289 |
|
|
27,892 |
|
|
11,603 |
|
Research and development |
|
|
|
27,054 |
|
|
33,759 |
|
|
6,705 |
|
Total costs and expenses |
|
|
|
75,046 |
|
|
97,987 |
|
|
22,941 |
|
Loss from operations |
|
|
|
(52,216) |
|
|
(81,023) |
|
|
(28,807) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense: |
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liability |
|
|
|
(260) |
|
|
(644) |
|
|
(384) |
|
Interest expense, net |
|
|
|
7,477 |
|
|
12,109 |
|
|
4,632 |
|
Other gain |
|
|
|
(864) |
|
|
(797) |
|
|
67 |
|
Loss on foreign currency exchange |
|
|
|
82 |
|
|
2 |
|
|
(80) |
|
|
|
|
|
6,435 |
|
|
10,670 |
|
|
4,235 |
|
Loss before benefit for income taxes |
|
|
|
(58,651) |
|
|
(91,693) |
|
|
(33,042) |
|
Benefit for income taxes |
|
|
|
(718) |
|
|
(1,061) |
|
|
(343) |
|
Net loss |
|
|
$ |
(57,933) |
|
$ |
(90,632) |
|
$ |
(32,699) |
Net product sales
Net product sales increased from $4.2 million for the year ended December 31, 2015 to $16.9 million for the year ended December 31, 2016. The increase was due to continued growth of SPRIX Nasal Spray product sales and a full year of OXAYDO product sales in 2016.
Collaboration revenues
There were no collaboration revenues for the year ended December 31, 2015. Collaboration revenues were $100,000 for the year ended December 31, 2016 and consisted entirely of revenues recognized under our SPRIX Nasal Spray marketing agreement with Septodont.
Related party revenues
Related party revenues for the year ended December 31, 2015 were $18.6 million due to the termination of the Shionogi agreement which resulted in the recognition of $16.9 million in related party revenues that otherwise would have been classified as deferred revenue as of December 31, 2015 and recognized as revenue over the life of the agreement. There were no related party revenues for the year ended December 31, 2016, as a result of the termination of the Shionogi agreement in December 2015. Refer to Note 15 – Acquisition and License and Collaboration Agreements in the Notes to our Consolidated Financial Statements for additional information about the Shionogi agreement.
93
Cost of sales (excluding amortization of product rights)
Cost of sales (excluding amortization of product rights) was $3.3 million for the year ended December 31, 2015 related to the product sales of SPRIX Nasal Spray and OXAYDO, which began in 2015. The cost of sales in 2015 included $1.6 million related to an inventory write down due to SPRIX Nasal Spray product expiration for product that was purchased from Luitpold as part of the initial acquisition of SPRIX Nasal Spray.
Cost of sales (excluding amortization of product rights) was $3.7 million for the year ended December 31, 2016. The cost of sales in 2016 included $310,000 related to an inventory write down due to OXAYDO product expiration.
Cost of sales (excluding amortization of product rights) for SPRIX Nasal Spray reflects the fair value of finished goods inventory assumed as part of the SPRIX Nasal Spray acquisition as well as the average cost of inventory subsequently produced and dispensed to patients. The cost of sales (excluding product amortization rights) for OXAYDO reflects the average costs of inventory dispensed to patients during the period.
Amortization of product rights
Amortization of product rights was $2.0 million for each of the years ended December 31, 2015 and 2016 was composed, in each case, of $1.1 million and $903,000, related to intangible assets acquired in the OXAYDO License and SPRIX Nasal Spray acquisition, respectively.
General and administrative expenses
General and administrative expenses increased by $4.2 million, or 15.8%, from $26.5 million for the year ended December 31, 2015 to $30.7 million for the year ended December 31, 2016.
This increase was attributable to increases in salary, benefits and stock-based compensation expense of $4.5 million due to an increase in headcount. There were also increased professional and administrative fees of $4.0 million due to increased medical publication expenses, depreciation expense associated with the manufacturing equipment and regulatory professional fees related to preparation for the ARYMO ER FDA Advisory Committee meeting that was held in 2016. These increases were partially offset by a $4.1 million decrease related to the regulatory filing fees for ARYMO ER and SPRIX Nasal Spray that were incurred during 2015. The regulatory fees during 2016 were composed of annual Pharmaceutical Drug User Fee Act (“PDUFA”) product and establishment fees.
Sales and marketing expenses
Sales and marketing expenses increased by $11.6 million, or 71.2%, from $16.3 million for the year ended December 31, 2015 to $27.9 million for the year ended December 31, 2016.
This increase was attributable to the continued growth of our commercial operations in the United States. There was an increase of $5.9 million in sales force expenses due to the expansion of the contracted sales force. There was a full year of sales force activities during 2016 compared to 6 months of sales force activity during 2015. As of December 31, 2016 and 2017, we had a contract sales force of 43 and 64 territory managers, respectively. There were also increases in salary and benefits of $1.2 million and increases in promotional expenses for OXAYDO of $1.0 million and launch planning expenses for ARYMO ER of $1.1 million.
In January 2017, we internalized our commercial sales force resulting in the creation of a specialty sales force of approximately 72 people. We believe the internalization of our sales force will not have a material impact on our sales and marketing expenses in 2017.
94
Research and development expenses
Research and development expenses increased by $6.7 million, or 24.8%, from $27.1 million for the year ended December 31, 2015 to $33.8 million for the year ended December 31, 2016.
This increase was driven primarily by increases in our development program expenses of $6.3 million related to Egalet-002 Phase 3 clinical efficacy and safety trials and Human Abuse Potential (“HAP”) trials, and $4.3 million related to OXAYDO bioequivalence and HAP trials, respectively. These increases were partially offset by a decrease in ARYMO ER development program expenses of $2.5 million and a decrease in other research and development activities of $1.8 million.
Change in fair value of derivative liability
The interest make whole provisions of the 5.50% Notes is subject to re-measurement at each balance sheet date and we recognize any change in fair value in our statements of operations and comprehensive loss as a change in fair value of the derivative liability. During the years ended December 31, 2015 and 2016, we had a gain of $260,000 and $644,000, respectively, as a result of the change in the fair value of our derivative liability. The change in fair value of the derivative liability is due the changes in the value of our Common Stock during the years ended December 31, 2015 and 2016.
Interest expense, net
Interest expense increased from $7.5 million for the year ended December 31, 2015 to $12.1 million for the year ended December 31, 2016. Interest expense for the year ended December 31, 2016 was primarily attributable to the interest expense on the 5.50% convertible notes and the Hercules Loan Agreement.
Interest expense for the year ended December 31, 2016 primarily consisted of interest expense on the Hercules Loan Agreement prior to its termination, the 5.50% Notes and the 13% Senior Secured Notes (the “13% Notes”). The interest expense of $12.1 million for the year ended December 31, 2017 was composed of $6.1 million of coupon interest, debt discount amortization of $5.7 million and $800,000 in debt extinguishment costs related to the repayment in full of the Hercules Loan Agreement. Interest expense, net, was partially offset by interest income of $682,000 from our marketable securities.
Refer to Note 9 — Long-Term Debt in the Notes to our Consolidated Financial Statements, for additional information about our long-term debt at December 31, 2017.
Other gain
Other gain was $864,000 and $797,000 for the years ended December 31, 2015 and 2016, respectively, and in both years consisted primarily of a Danish research and development tax credit.
Loss on foreign currency exchange
We recognized a loss on foreign currency exchange of $82,000 and $2,000, during the years ended December 31, 2015 and 2016 respectively. The change was primarily attributable to a change in the average rates of currency in which we transacted during 2015 compared to 2016.
Benefit for income taxes
We had a tax benefit of $718,000 during the year ended December 31, 2015 compared to a tax benefit of $1.1 million during the year ended December 31, 2016. The tax benefit in each of the years ended December 31, 2015 and 2016 relates to a state tax benefit associated with the 5.50% Notes.
95
Liquidity and Capital Resources
Since our inception, we have incurred net losses and generally negative cash flows from our operations. We incurred net losses of $90.6 million and $69.4 million for the years ended December 31, 2016 and 2017, respectively. Our operating activities used $72.9 million and $65.2 million of cash during the years ended December 31, 2016 and 2017, respectively. At December 31, 2017, we had an accumulated deficit of $295.3 million, a working capital surplus of $59.0 million and cash, cash equivalents and marketable securities totaling $91.0 million.
Since our initial public offering (“IPO”) we have engaged in the following financing transactions.
In January 2015, we entered into the Loan Agreement with Hercules and certain other lenders, pursuant to which we borrowed $15.0 million under a term loan. In August 2016, we repaid all outstanding obligations under the Loan Agreement, using the proceeds from the 13% Notes. Refer to Note 6 — Long-term Debt in the Notes to our Unaudited Consolidated Financial Statements for additional information.
In April and May 2015, we issued through a private placement $61.0 million in aggregate principal amount of the 5.50% Notes. Interest on the 5.50% Notes is payable semi-annually in arrears on April 1 and October 1 of each year, commencing on October 1, 2015. Refer to Note 9 — Long-term Debt for additional information.
In July 2015, we completed an underwritten public offering of 7,666,667 shares of Common Stock (including the exercise in full of the underwriters’ option to purchase additional shares) at an offering price of $11.25 per share for gross proceeds of $86.3 million. The net offering proceeds from the sale were $80.8 million, after deducting underwriting discounts and commissions of $5.2 million and offering costs of $293,000.
Through December 31, 2015, we received $4.1 million in payments from our collaborative research and development agreements along with aggregate upfront and milestone payments of $20.0 million under our collaborative research and license agreement with Shionogi Limited.
In August 2016, we issued $40.0 million in aggregate principal amount of the 13% Senior Secured Notes and issued another $40.0 million in aggregate principal amount following FDA approval of ARYMO ER in January 2017 (the “13% Notes”). Interest on the 13% Notes accrues at a rate of 13% per annum and is payable semi-annually in arrears on March 20 and September 20 of each year (each, a “Payment Date”) commencing on March 20, 2017. On each Payment Date commencing on March 20, 2018, we will also pay an installment of principal on the 13% Notes in an amount equal to 15% (or 17% if certain sales targets are not met) of the aggregate net sales of OXAYDO (oxycodone HCI, USP) tablets for oral use only – CII, SPRIX Nasal Spray, ARYMO ER and Egalet-002, if approved, for the two consecutive fiscal quarter periods most recently ended, less the amount of interest paid on the 13% Notes on such Payment Date.
In March 2017, we initiated sales of shares under our July 2015 Controlled Equity Offering Sales Agreement (“2015 Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”) and sold an aggregate of 1,540,597 shares of Common Stock through December 31, 2017, resulting in net proceeds of $4.1 million after deducting commissions of $126,000. Under the 2015 Sales Agreement, we may, at our discretion, from time to time sell shares of our Common Stock, for an aggregate offering price of up to $30.0 million (inclusive of amounts sold to date). We provided Cantor with customary indemnification rights, and Cantor is entitled to a commission at a fixed rate of 3% of the gross proceeds per share sold. Sales of the shares under the 2015 Sales Agreement have been made and, any additional sales under the 2015 Sales Agreement, will be made in transactions deemed to be “at the market offerings”, as defined in Rule 415 under the Securities Act of 1933, as amended.
In July 2017, we completed an underwritten public offering of 16,666,667 shares of our Common Stock and accompanying warrants to purchase 16,666,667 shares of our Common Stock, at a combined public offering price of $1.80 per share and accompanying warrant for gross proceeds of $30.0 million. The net offering proceeds were $28.6 million after deducting underwriting discounts and commissions of $1.4 million. Each warrant has an exercise price of $2.70, subject to adjustment in certain circumstances. As of December 31, 2017, the exercise price of the warrants was $2.14. The shares of Common Stock and warrants were issued separately.
96
In December 2017, the Company entered into exchange agreements (the “Exchange Agreements”) with certain holders (the “Holders”) of the Company’s 5.50% Notes pursuant to which the Holders agreed to exchange, in the aggregate, approximately $36.4 million of outstanding principal amount of the 5.50% Notes for, in the aggregate, (i) approximately $23.9 million of the Company’s new 6.50% Notes, (ii) a warrant exercisable for 3.5 million shares of the Company’s Common Stock and (iii) payments, in cash, of all accrued but unpaid interest as of the closing on the 5.50% Notes exchanged in the transaction (the “Exchange”). The 6.50% Notes will pay interest semiannually in arrears on January 1 and July 1 of each year commencing July 1, 2018 at a rate of 6.50% per year.
Cash Flows
Comparison of Years Ended December 31, 2016 and 2017
The following table summarizes our cash flows for the years ended December 31, 2016 and 2017:
|
(in thousands) |
|
Year Ended |
||||
|
|
|
December 31, |
||||
|
|
|
2016 |
|
2017 |
||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
Operating activities |
|
$ |
(72,867) |
|
$ |
(65,164) |
|
Investing activities |
|
|
48,865 |
|
|
(17,984) |
|
Financing activities |
|
|
21,647 |
|
|
69,353 |
|
Effect of foreign currency translation on cash |
|
|
45 |
|
|
530 |
|
Net decrease in cash |
|
$ |
(2,310) |
|
$ |
(13,265) |
Cash Flows from Operating Activities
Net cash used in operating activities was $72.9 million for the year ended December 31, 2016 and consisted primarily of a net loss of $90.6 million. This net loss was partially offset by $6.3 million in stock-based compensation expense, $5.9 million of non-cash interest and debt discount amortization, $4.1 million of noncash depreciation and amortization expense and $1.7 million in net cash inflows from changes in operating assets and liabilities. Cash inflows from changes in operating assets and liabilities were primarily due to an increase in accrued expenses of $10.5 million, primarily driven by our commercial operations, clinical studies and interest obligations, and a decrease in deposits and other assets of $1.4 million. These inflows were partially offset by a decrease in deferred revenue of $6.2 million related to the product sales of SPRIX Nasal Spray and OXAYDO.
Net cash used in operating activities was $65.2 million for the year ended December 31, 2017 and consisted primarily of a net loss of $69.4 million. This net loss included a non-cash gain on extinguishment of debt of $13.2 million, which was partially offset by $5.9 million in stock-based compensation expense, $5.1 million of non-cash interest and debt discount amortization, $5.0 million of non-cash depreciation and amortization expense, and $3.9 million in net cash inflows from changes in operating assets and liabilities. Cash inflows from changes in operating assets and liabilities were primarily due to an increase in accounts payable and accrued expenses of $5.5 million primarily driven by our commercial operations, clinical studies and manufacturing operations, and an increase in deferred revenue of $3.5 million related to the product sales of SPRIX Nasal Spray, OXAYDO and ARYMO ER. These inflows were partially offset by cash outflows due to a decrease in accounts receivable of $3.0 million and a decrease in inventory of $1.5 million.
Cash Flows from Investing Activities
Net cash provided by investing activities for the year ended December 31, 2016 was $48.9 million. Cash inflows consisted of $115.6 million provided by the sale and maturity of investments. These inflows were partially offset by cash outflows for purchases of investments of $59.5 million and purchases of property and equipment of $7.2
97
million, primarily related to the expansion of our commercial manufacturing capability at our third-party contract manufacturer, Halo Pharmaceuticals.
Net cash used in investing activities for the year ended December 31, 2017 was $18.0 million. Cash outflows for the year ended December 31, 2017 consisted of the purchase of investments for $101.3 million and an increase in restricted cash of $400,000. These outflows were partially offset by inflows from the sale and maturity of investments of $83.8 million.
Cash Flows from Financing Activities
Net cash provided by financing activities was $21.6 million for the year ended December 31, 2016 and consisted of the net proceeds from the first tranche of the 13% Notes of $37.2 million, partially offset by the $15.9 million repayment of the Hercules Loan Agreement.
Net cash provided by financing activities was $69.4 million for the year ended December 31, 2017 and consisted of $38.3 million in net proceeds from the issuance of the 13% Notes and Royalty Rights and $32.9 million in net proceeds from the issuance of Common Stock and warrants pursuant to our July 2017 underwritten public offering and our “at-the-market” offering.
Comparison of Years Ended December 31, 2015 and 2016
The following table summarizes our cash flows for the years ended December 31, 2015 and 2016:
|
(in thousands) |
|
Year Ended |
||||
|
|
|
December 31, |
||||
|
|
|
2015 |
|
2016 |
||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
Operating activities |
|
$ |
(38,906) |
|
$ |
(72,867) |
|
Investing activities |
|
|
(119,787) |
|
|
48,865 |
|
Financing activities |
|
|
152,293 |
|
|
21,647 |
|
Effect of foreign currency translation on cash |
|
|
327 |
|
|
45 |
|
Net increase (decrease) in cash |
|
$ |
(6,073) |
|
$ |
(2,310) |
Cash Flows from Operating Activities
Net cash used in operating activities was $38.9 million for the year ended December 31, 2015 and consisted primarily of a net loss of $57.9 million. These outflows were partially offset by $5.2 million in stock-based compensation expense, $4.0 million of non-cash interest and debt discount amortization, $3.0 million of noncash depreciation and amortization expense and $7.0 million in net cash inflows from changes in operating assets and liabilities. Cash inflows from changes in operating assets and liabilities were primarily due to an increase in accounts payable and accrued expenses of $6.3 million primarily driven by our commercial operations, clinical studies and manufacturing operations, and an increase due to deferred revenue related to the product sales of SPRIX Nasal Spray and OXAYDO.
Net cash used in operating activities was $72.9 million for the year ended December 31, 2016 and consisted primarily of a net loss of $90.6 million. This net loss was partially offset by $6.3 million in stock-based compensation expense, $5.9 million of non-cash interest and debt discount amortization, $4.1 million of noncash depreciation and amortization expense and $1.7 million in net cash inflows from changes in operating assets and liabilities. Cash inflows from changes in operating assets and liabilities were primarily due to an increase in accrued expenses of $10.5 million, primarily driven by our commercial operations, clinical studies and interest obligations, and a decrease in deposits and other assets of $1.4 million. These inflows were partially offset by a decrease in deferred revenue of $6.2 million related to the product sales of SPRIX Nasal Spray and OXAYDO.
98
Cash Flows from Investing Activities
Net cash used in investing activities for the year ended December 31, 2015 was $119.8 million. Cash outflows for the year ended December 31, 2016 consisted of the purchase of investments for $110.2 million, the purchase of SPRIX Nasal Spray for $8.1 million, the in-license of OXAYDO for $7.7 million, and payments and deposits for property and equipment of $4.0 million. These outflows were partially offset by inflows from the maturity and sales of investments of $10.2 million.
Net cash provided by investing activities for the year ended December 31, 2016 was $48.9 million. Cash inflows consisted of $115.6 million provided by the sale and maturity of investments. These inflows were partially offset by cash outflows for purchases of investments of $59.5 million and purchases of property and equipment of $7.2 million, primarily related to the expansion of our commercial manufacturing capability at our third-party contract manufacturer, Halo Pharmaceuticals.
Cash Flows from Financing Activities
Net cash provided by financing activities was $152.3 million for the year ended December 31, 2015 and consisted of the net proceeds from Hercules Loan Agreement of $14.7 million, net proceeds from the issuance of the 5.50% Notes of $56.7 million and the net proceeds from the July 2015 equity offering of $80.8 million.
Net cash provided by financing activities was $21.6 million for the year ended December 31, 2016 and consisted of the net proceeds from the first tranche of the 13% Notes of $37.2 million, partially offset by the $15.9 million repayment of the Hercules Loan Agreement.
Operating and Capital Expenditure Requirements
We have not achieved profitability since our inception and we expect to continue to incur net losses for the foreseeable future. Our primary uses of capital are, and we expect will continue to be, compensation and related expenses, sales and marketing expenses, commercial infrastructure, legal and other regulatory expense, business development opportunities and general overhead costs, including interest and principal repayments on our outstanding debt. We expect our cash expenditures to decrease in the near term as a result of our corporate restructuring initiative and the reduction of our research and development activities.
Because our approved products are in the early stages of commercialization that will require significant investment and our product candidates are in various stages of clinical and preclinical development, and the outcome of these efforts is uncertain, we cannot estimate the actual amounts necessary to successfully complete the commercialization and development of our products and product candidates or whether, or when, we may achieve profitability. Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity or debt financings and collaboration arrangements. In order to meet these additional cash requirements, we may seek to sell additional equity or convertible debt securities that may result in dilution to our stockholders. The indenture governing the 13% Notes contains covenants that restrict our ability to issue additional indebtedness. Although our ability to issue additional indebtedness is significantly limited by such covenants, if we raise additional funds through the issuance of convertible debt securities, these securities could have rights senior to those of our Common Stock and could contain covenants that restrict our operations. We may also seek to raise additional financing through the issuance of debt which, if available and permitted pursuant to the documents governing the 13% Notes and our other existing indebtedness, may involve agreements that include restrictive covenants limiting our ability to take important actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration arrangements in the future, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. There can be no assurance that we will be able to obtain additional equity or debt financing on terms acceptable to us, if at all. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
99
As of December 31, 2017, we had cash, cash equivalents and short-term investments of $91 million. We estimate that this amount will be sufficient to fund our cash requirements into 2020, assuming that our stock remains listed on the Nasdaq. If we do not maintain compliance with Nasdaq listing requirements, there may be doubt that we can continue as a going concern, or we may be forced to sell our company or our assets, cease or wind down operations, seek protection under the provisions of the U.S. Bankruptcy Code, or otherwise liquidate and dissolve our company. Our future operating and capital requirements will depend on many factors, including:
|
· |
the commercial success of our approved products |
|
· |
the cost of our current commercialization activities, including marketing, sales and distribution costs, as well as, commercialization activities for any future product candidates that are approved for sale; our ability to establish collaborations or product acquisitions on favorable terms, if at all; |
|
· |
the results of our clinical trials; |
|
· |
the scope, progress, results and costs of product development of our product candidates; |
|
· |
the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending intellectual property-related claims; |
|
· |
our ability to raise additional capital, which is currently limited by the covenants and other restrictions contained in the agreements governing our existing indebtedness; and |
|
· |
our continued listing on the Nasdaq. |
Please see “Risk Factors” for additional risks associated with our substantial capital requirements.
Contractual Obligations and Commitments
The following table represents our contractual obligations and commitments as of December 31, 2017.
|
(in thousands) |
|
Payments Due By Period |
|
|||||||||||||
|
|
|
|
|
|
Less than |
|
|
|
|
|
|
|
More than |
|
||
|
|
|
Total |
|
1 year |
|
1 to 3 years |
|
3 to 5 years |
|
5 years |
|
|||||
|
Operating lease obligations(1) |
|
$ |
2,244 |
|
$ |
523 |
|
$ |
1,076 |
|
$ |
645 |
|
$ |
— |
|
|
13% Notes (2) |
|
|
151,887 |
|
|
10,400 |
|
|
24,305 |
|
|
33,311 |
|
|
83,871 |
|
|
5.50% convertible notes (3) |
|
|
28,189 |
|
|
1,408 |
|
|
26,781 |
|
|
— |
|
|
— |
|
|
6.50% convertible notes (4) |
|
|
34,774 |
|
|
794 |
|
|
3,105 |
|
|
3,105 |
|
|
27,770 |
|
|
Supply Agreements (5) |
|
|
15,300 |
|
|
3,600 |
|
|
7,200 |
|
|
4,500 |
|
|
— |
|
|
Total |
|
$ |
232,394 |
|
$ |
16,725 |
|
$ |
62,467 |
|
$ |
41,561 |
|
$ |
111,641 |
|
|
(1) |
Operating lease obligations reflect our obligation to make payments in connection with the leases for our office space. |
|
(2) |
On August 31, 2016, we completed the initial closing (the “Initial Closing”) of our offering of up to $80.0 million aggregate principal amount of our 13% Notes. We issued $40.0 million aggregate principal amount of the 13% Notes at the Initial Closing, and issued an additional $40.0 million aggregate principal amount of the 13% Notes upon FDA approval of ARYMO™ ER. Interest on the 13% Notes accrues at a rate of 13% per annum and is payable semi-annually in arrears on each Payment Date, which occurs on March 20 and September 20 of each year which commenced on March 20, 2017. On each Payment Date commencing on March 20, 2018, we will pay an installment of principal on the 13% Notes in an amount equal to 15% (or 17% if certain sales targets are not met) of the aggregate net sales of OXAYDO, SPRIX Nasal Spray, ARYMO ER and Egalet-002, if approved, for the two |
100
consecutive fiscal quarter period most recently ended, less the amount of interest paid on the 13% Notes on such Payment. |
In connection with the Initial Offering in August 2016, we entered into royalty rights agreements with each of the 13% Notes Purchasers pursuant to which we sold to such Purchasers the right to receive, in the aggregate, a payment equal to 1.5% of the aggregate net sales of OXAYDO and SPRIX Nasal Spray from the Initial Closing through December 31, 2019, inclusive (the “Royalty Rights”). Following the approval of ARYMO ER in January 2017, the Royalty Rights will continue through December 31, 2020 and we also entered into separate Royalty Rights agreements with each of the Purchasers pursuant to which we sold to such Purchasers the right to receive 1.5% of the aggregate net sales of ARYMO ER payable from the date of first sale of ARYMO ER through December 31, 2020, inclusive. The above table does not include any potential payments related to the Royalty.
|
(3) |
In April 2015, we issued, through a private placement, $60.0 million in aggregate principal amount of the 5.50% Notes. On May 6, 2015, we issued an additional $1.0 million in principal amount pursuant to the initial purchasers’ exercise of their 30-day over-allotment for aggregate gross proceeds of $61.0 million. Interest on the 5.50% Notes is payable semi-annually in arrears on April 1 and October 1 of each year, commencing October 1, 2015. |
In December 2017, we exchanged $36.4 million in face value of the 5.50% Notes for $23.9 million in face value of the 6.50% Notes described below.
|
(4) |
In December 2017, we exchanged $36.4 million in face value of the 5.50% Notes for $23.9 million in face value of the 6.50% Notes. Interest on the 6.50% Notes is payable semi-annually in arrears on January 1 and July 1 of each year, commencing July 1, 2018. |
|
(5) |
On February 28, 2017, we entered into a Drug Product Manufacturing Services Agreement (the “Manufacturing Agreement”) with Halo Pharmaceutical, Inc. (“Halo”) pursuant to which we engaged Halo to provide certain services related to the manufacture and supply of ARYMO ER tablets for our commercial use in the United States. We are obligated to purchase all of our requirements for ARYMO ER from Halo through 2019, and seventy-five percent of our requirements thereafter, subject to certain limited exceptions. We will purchase ARYMO ER pursuant to binding purchase orders at a fixed price based on dosage strength, with specified percentage rebates for annual volumes of product ordered over a specified amount. In addition, we have agreed to purchase certain minimum amounts of manufacturing and additional services per calendar quarter from Halo over the term of the Agreement (the “Quarterly Minimum”). If we fail to meet the Quarterly Minimum, we will be required to pay to Halo the resulting shortfall. |
We have employment agreements with our executive officers that require the funding of a specific level of payments if specified events occur, such as a change in control or termination without cause. However, because of the contingent nature of those payments, they are not presented in the table.
In addition, in the course of normal business operations, we have agreements with contract service providers to assist in the performance of our research and development, commercial and manufacturing activities. We can elect to discontinue the work under these agreements at any time. We could also enter into additional collaborative research, contract research, manufacturing and supplier agreements in the future, which may require upfront payments or long‑term commitments of cash.
Purchase Commitments
Other than described above with respect to the purchase of ARYMO ER, we have no material non‑cancelable purchase commitments with service providers as we have generally contracted on a cancelable purchase order basis.
Off‑Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off‑balance sheet arrangements, as defined under SEC rules.
101
JOBS Act
As an “emerging growth company” under the JOBS Act of 2012, we can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are electing not to delay our adoption of such new or revised accounting standards. As a result of this election, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non‑emerging growth companies.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate and foreign currency fluctuations.
Interest Rate Risk
We had cash, cash equivalents and marketable securities of $86.8 million and $91.0 million at December 31, 2016 and December 31, 2017, respectively, consisting primarily of funds in cash, money market accounts and corporate debt securities. The primary objective of our investment activities is to preserve principal and liquidity while maximizing income without significantly increasing risk. We do not enter into investments for trading or speculative purposes. Due to the short‑term nature of our investment portfolio, we do not believe an immediate 10% increase in interest rates would have a material effect on the fair market value of our portfolio, and accordingly we do not expect our operating results or cash flows to be materially affected by a sudden change in market interest rates.
Foreign Currency Exchange Risk
With international operations, we face exposure to adverse movements in foreign currency exchange rates. These exposures may change over time as business practices evolve. As a result of this exposure, adverse movement in foreign currency exchange rates may have a material adverse impact on our financial results. We are party to contracts which are primarily denominated in U.S. dollars and Danish Krone.
All assets and liabilities of our international subsidiary, Egalet Ltd. which maintains its financial statements in the local currency, are translated to U.S. dollars at period‑end exchange rates. Translation adjustments arising from the use of differing exchange rates are included in accumulated other comprehensive income in stockholders’ equity. Gains and losses on foreign currency transactions are included in loss (gain) on foreign currency exchange. The reported results of our foreign operations will be influenced by their translation into U.S. dollars by currency movements against the U.S. dollar.
A 10% increase in foreign currency exchange rates would have increased our 2017 net loss from $69.4 million to $71.1 million, an increase of $1.7 million.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data required by this item are listed in Item 15 – “Exhibits and Financial Statement Schedules” of this Annual Report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
102
ITEM 9A. CONTROLS AND PROCEDURES
Conclusions Regarding the Effectiveness of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the timelines specified in the SEC rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost‑benefit relationship of possible controls and procedures. Because of its inherent limitations, disclosure controls and procedures may not prevent all misstatements.
As required by SEC Rule 13a‑15(b), we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as of the end of the period covered by this report. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2017 at the reasonable assurance level.
Management’s Annual Report on Internal Control Over Financial Reporting
Internal control over financial reporting refers to the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that: (1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Management is responsible for establishing and maintaining adequate internal control over our financial reporting, as such term is defined in Rules 13a‑15(f) and 15d‑15(e) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting. Management has used the framework set forth in the report entitled “Internal Control—Integrated Framework (2013)” published by the Committee of Sponsoring Organizations of the Treadway Commission to evaluate the effectiveness of our internal control over financial reporting. Based on its evaluation, management has concluded that our internal control over financial reporting was effective as of December 31, 2017, the end of our most recent fiscal year.
Our independent registered public accounting firm has not performed an evaluation of our internal control over financial reporting during any period in accordance with the provisions of the Sarbanes‑Oxley Act. For as long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to take advantage of the exemption
103
permitting us not to comply with the requirement that our independent registered public accounting firm provide an attestation on the effectiveness of our internal control over financial reporting.
Changes in Internal Control Over Financial Reporting
During the year ended December 31, 2017, we implemented a new enterprise resource planning (“ERP”) system. As appropriate, we have modified the design and documentation of internal control processes and procedures relating to the new system and interfaces to simplify and synchronize our existing internal control over financial reporting.
With the exception of the ERP implementation described above, there were no changes in our internal control over financial reporting during the year ended December 31, 2017, which were identified in connection with management’s evaluation required by paragraph (d) of Rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
.
None.
104
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information with respect to this item will be set forth in the Proxy Statement for the 2018 Annual Meeting of Stockholders (“Proxy Statement”) or an amendment to this Annual Report on Form 10-K (“Form 10-K/A”) under the headings “Election of Directors,” “Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Code of Ethics” and “Corporate Governance” and is incorporated herein by reference. The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
ITEM 11. EXECUTIVE COMPENSATION
Information with respect to this item will be set forth in the Proxy Statement or Form 10-K/A under the headings “Executive Compensation” and “Director Compensation,” and is incorporated herein by reference. The Proxy Statement or Form 10-K/A will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information with respect to this item will be set forth in the Proxy Statement or Form 10-K/A under the headings “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation,” and is incorporated herein by reference. The Proxy Statement of Form 10-K/A will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information with respect to this item will be set forth in the Proxy Statement or Form 10-K/A under the headings “Certain Relationships and Related Party Transactions” and “Corporate Governance” and is incorporated herein by reference. The Proxy Statement of Form 10-K/A will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Information with respect to this item will be set forth in the Proxy Statement or Form 10-K/A under the heading “Ratification of the Selection of Independent Registered Public Accounting Firm,” and is incorporated herein by reference. The Proxy Statement Form 10-K/A will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
105
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
(a) |
(1) |
Financial Statements: See Index to Consolidated Financial Statements on page F‑1. |
|
|
(3) |
Exhibits: See Exhibits Index on page 101. |
106
ITEM 16. FORM 10-K SUMMARY
None.
|
Exhibit |
|
Exhibit Description |
|
2.1^ |
|
|
| 3.1 |
|
|
| 3.2 |
|
|
| 4.1 |
|
|
| 4.2 |
|
|
| 4.3 |
|
|
| 4.4 |
|
|
| 4.5 |
|
|
| 4.6 |
|
|
| 10.1 |
|
|
| 10.2 |
|
|
| 10.3 |
|
|
| 10.4 |
|
107
| 10.5 |
|
|
| 10.6 |
|
|
| 10.7 |
|
|
| 10.8 |
|
|
| 10.9 |
|
|
| 10.10 |
|
|
| 10.11 |
|
|
|
10.12* |
|
|
|
10.13* |
|
|
|
10.14* |
|
|
|
10.15* |
|
|
|
10.16* |
|
|
| 10.17 |
|
|
| 10.18 |
|
|
|
10.19+ |
|
|
|
10.20+ |
|
|
|
10.21+ |
|
|
|
10.22+ |
|
|
|
10.23+ |
|
|
|
10.24+ |
|
Employment Agreement by and between Egalet Corporation and Barbara Carlin (filed herewith). |
|
10.25+ |
|
Employment Agreement by and between Egalet Corporation and Megan Timmins (filed herewith). |
|
10.26+ |
|
108
|
10.27+ |
|
|
|
10.28+ |
|
|
|
10.29+ |
|
|
|
10.30+ |
|
|
|
10.31+ |
|
|
|
10.32+ |
|
|
|
10.33+ |
|
|
|
10.34+ |
|
Form of Restricted Stock Unit Award Agreement (filed herewith). |
|
10.35+ |
|
|
|
10.36+ |
|
|
|
10.37+ |
|
|
| 10.38 |
|
|
| 21.1 |
|
|
| 23.1 |
|
|
| 31.1 |
|
|
| 31.2 |
|
|
| 32.1 |
|
|
| 32.2 |
|
|
|
101.INS |
|
XBRL Instance Document (filed herewith) |
|
101.SCH |
|
XBRL Taxonomy Extension Schema (filed herewith) |
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase (filed herewith) |
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase (filed herewith) |
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase (filed herewith) |
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase (filed herewith) |
+Indicates management contract or compensatory plan.
*Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
^All exhibits and schedules have been omitted pursuant to Item 601(b)(2) of Regulation S‑K. The Company will furnish the omitted exhibits and schedules to the SEC upon request by the SEC.
109
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: March 16, 2018 |
Egalet Corporation |
|
|
|
By: |
/s/ Robert Radie |
|
|
|
|
|
|
|
Robert Radie |
|
|
|
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Robert Radie |
|
Director, President and Chief Executive Officer |
|
March 16, 2018 |
|
Robert Radie |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Stan Musial |
|
Chief Financial Officer (Principal Financial Officer) |
|
March 16, 2018 |
|
Stan Musial |
|
|
|
|
|
|
|
|
|
|
|
/s/ Barbara Carlin |
|
Chief Accounting Officer (Principal Accounting Officer) |
|
March 16, 2018 |
|
Barbara Carlin |
|
|
|
|
|
|
|
|
|
|
|
/s/ Timothy P. Walbert |
|
Chairman, Board of Directors |
|
March 16, 2018 |
|
Timothy P. Walbert |
|
|
|
|
|
|
|
|
|
|
|
/s/ Elaine Hochberg |
|
Director |
|
March 16, 2018 |
|
Elaine Hochberg |
|
|
|
|
|
|
|
|
|
|
|
/s/ Nicholas Nicolaides |
|
Director |
|
March 16, 2018 |
|
Nicholas Nicolaides |
|
|
|
|
|
|
|
|
|
|
|
/s/ John Osborn |
|
Director |
|
March 16, 2018 |
|
John Osborn |
|
|
|
|
|
|
|
|
|
|
|
/s/ Robert Roche |
|
Director |
|
March 16, 2018 |
|
Robert Roche |
|
|
|
|
|
|
|
|
|
|
|
/s/ Andrea Heslin Smiley |
|
Director |
|
March 16, 2018 |
|
Andrea Heslin Smiley |
|
|
|
|
|
|
|
|
|
|
|
/s/ Gregory Weaver |
|
Director |
|
March 16, 2018 |
|
Gregory Weaver |
|
|
|
|
110
|
Egalet Corporation and Subsidiaries |
|
|
|
Audited Financial Statements |
|
|
|
|
F-2 |
|
|
Consolidated balance sheets as of December 31, 2016 and 2017 |
|
F-3 |
|
Consolidated statements of operations for the years ended December 31, 2015, 2016 and 2017 |
|
F-4 |
|
Consolidated statements of comprehensive loss for the years ended December 31, 2015, 2016 and 2017 |
|
F-5 |
|
|
F-6 |
|
|
Consolidated statements of cash flows for the years ended December 31, 2015, 2016 and 2017 |
|
F-7 |
|
|
F-8 |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Egalet Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Egalet Corporation (the Company) as of December 31, 2017 and 2016, the related consolidated statements of operations, comprehensive loss, changes in stockholders’ (deficit) equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has incurred recurring operating losses and negative cash flows from operations and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2015.
Philadelphia, Pennsylvania
March 16, 2018
F-2
Egalet Corporation and Subsidiaries
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2016 |
|
December 31, 2017 |
|
||
|
|
|
|
|
|
|
|
|
|
Assets |
|||||||
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
44,355 |
|
$ |
31,090 |
|
|
Marketable securities, available for sale |
|
|
42,471 |
|
|
59,953 |
|
|
Accounts receivable |
|
|
1,108 |
|
|
4,120 |
|
|
Inventory |
|
|
1,700 |
|
|
3,225 |
|
|
Prepaid expenses and other current assets |
|
|
2,537 |
|
|
2,672 |
|
|
Other receivables |
|
|
1,001 |
|
|
893 |
|
|
Total current assets |
|
|
93,172 |
|
|
101,953 |
|
|
Intangible assets, net |
|
|
8,350 |
|
|
6,583 |
|
|
Restricted cash |
|
|
- |
|
|
400 |
|
|
Property and equipment, net |
|
|
12,709 |
|
|
9,911 |
|
|
Deposits and other assets |
|
|
627 |
|
|
1,011 |
|
|
Total assets |
|
$ |
114,858 |
|
$ |
119,858 |
|
|
Liabilities and stockholders’ equity (deficit) |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
|
2,392 |
|
|
10,160 |
|
|
Accrued expenses |
|
|
18,147 |
|
|
16,104 |
|
|
Deferred revenue |
|
|
3,975 |
|
|
7,456 |
|
|
Debt - current |
|
|
381 |
|
|
1,081 |
|
|
Warrant liability |
|
|
- |
|
|
8,166 |
|
|
Total current liabilities |
|
|
24,895 |
|
|
42,967 |
|
|
Debt - non-current portion, net |
|
|
83,711 |
|
|
98,890 |
|
|
Deferred income tax liability |
|
|
23 |
|
|
26 |
|
|
Derivative liability |
|
|
12 |
|
|
16,623 |
|
|
Other liabilities |
|
|
891 |
|
|
727 |
|
|
Total liabilities |
|
|
109,532 |
|
|
159,233 |
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 13) |
|
|
|
|
|
|
|
|
Stockholders’ equity (deficit) |
|
|
|
|
|
|
|
|
Common stock--$0.001 par value; 75,000,000 shares authorized at December 31, 2016 and December 31, 2017; 25,189,125 and 45,939,663 shares issued and outstanding at December 31, 2016 and December 31, 2017, respectively |
|
|
25 |
|
|
46 |
|
|
Additional paid-in capital |
|
|
230,379 |
|
|
254,871 |
|
|
Accumulated other comprehensive income |
|
|
100 |
|
|
1,008 |
|
|
Accumulated deficit |
|
|
(225,178) |
|
|
(295,300) |
|
|
Total stockholders' equity (deficit) |
|
|
5,326 |
|
|
(39,375) |
|
|
Total liabilities and stockholders’ equity (deficit) |
|
$ |
114,858 |
|
$ |
119,858 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Egalet Corporation and Subsidiaries
Consolidated Statements of Operations
(in thousands, except per share data)
|
|
|
Year Ended |
|
|||||||
|
|
|
December 31, |
|
|||||||
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
4,184 |
|
$ |
16,864 |
|
$ |
26,136 |
|
|
Related party revenue |
|
|
18,646 |
|
|
— |
|
|
— |
|
|
Collaboration revenues |
|
|
— |
|
|
100 |
|
|
— |
|
|
Total revenue |
|
|
22,830 |
|
|
16,964 |
|
|
26,136 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost and Expenses |
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of product rights) |
|
|
3,271 |
|
|
3,660 |
|
|
5,153 |
|
|
Amortization of product rights |
|
|
1,958 |
|
|
2,006 |
|
|
2,082 |
|
|
General and administrative |
|
|
26,474 |
|
|
30,670 |
|
|
34,065 |
|
|
Sales and marketing |
|
|
16,289 |
|
|
27,892 |
|
|
35,532 |
|
|
Research and development |
|
|
27,054 |
|
|
33,759 |
|
|
14,744 |
|
|
Restructuring charges |
|
|
— |
|
|
— |
|
|
2,760 |
|
|
Total costs and expenses |
|
|
75,046 |
|
|
97,987 |
|
|
94,336 |
|
|
Loss from operations |
|
|
(52,216) |
|
|
(81,023) |
|
|
(68,200) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense: |
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of warrant and derivative liability |
|
|
(260) |
|
|
(644) |
|
|
(2,546) |
|
|
Interest expense, net |
|
|
7,477 |
|
|
12,109 |
|
|
17,666 |
|
|
Other gain |
|
|
(864) |
|
|
(797) |
|
|
(750) |
|
|
Gain on extinguishment of debt |
|
|
— |
|
|
— |
|
|
(13,221) |
|
|
Loss on foreign currency exchange |
|
|
82 |
|
|
2 |
|
|
10 |
|
|
|
|
|
6,435 |
|
|
10,670 |
|
|
1,159 |
|
|
Loss before benefit for income taxes |
|
|
(58,651) |
|
|
(91,693) |
|
|
(69,359) |
|
|
Benefit for income taxes |
|
|
(718) |
|
|
(1,061) |
|
|
— |
|
|
Net loss |
|
$ |
(57,933) |
|
$ |
(90,632) |
|
$ |
(69,359) |
|
|
Per share information: |
|
|
|
|
|
|
|
|
|
|
|
Net loss per share of common stock, basic and diluted |
|
$ |
(2.94) |
|
$ |
(3.70) |
|
$ |
(2.05) |
|
|
Weighted-average shares outstanding, basic and diluted |
|
|
19,738,042 |
|
|
24,514,645 |
|
|
33,755,462 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Egalet Corporation and Subsidiaries
Consolidated Statements of Comprehensive Loss
(in thousands)
|
|
|
Year Ended December 31, |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||
|
Net loss |
|
$ |
(57,933) |
|
$ |
(90,632) |
|
$ |
(69,359) |
|
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
Unrealized (loss) gain on available for sale securities |
|
|
(147) |
|
|
138 |
|
|
(37) |
|
|
Foreign currency translation adjustments |
|
|
277 |
|
|
3 |
|
|
945 |
|
|
Comprehensive loss |
|
$ |
(57,803) |
|
$ |
(90,491) |
|
$ |
(68,451) |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Egalet Corporation and Subsidiaries
Consolidated Statements of Changes in Stockholders’ Equity (Deficit)
(in thousands, except per share data)
|
|
|
|
|
|||||||||||||||
|
|
|
Common Stock |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.001 |
|
Additional |
|
|
|
|
Accumulated |
|
|
|
|
|||
|
|
|
Number of |
|
Par |
|
Paid-in |
|
Accumulated |
|
Comprehensive |
|
|
|
|
||||
|
|
|
Shares |
|
Value |
|
Capital |
|
Deficit |
|
Income |
|
Total |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2014 |
|
17,283,663 |
|
|
17 |
|
|
121,028 |
|
|
(76,613) |
|
|
(171) |
|
|
44,261 |
|
|
Issuance of warrants |
|
— |
|
|
— |
|
|
329 |
|
|
— |
|
|
— |
|
|
329 |
|
|
Exercise of warrants |
|
61,644 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Issuance of restricted shares of common stock |
|
75,000 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Forfeiture of restricted shares of common stock |
|
(13,920) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Issuance of common stock, net of costs |
|
7,666,667 |
|
|
8 |
|
|
80,775 |
|
|
— |
|
|
— |
|
|
80,783 |
|
|
Issuance of convertible debt |
|
— |
|
|
— |
|
|
16,341 |
|
|
— |
|
|
— |
|
|
16,341 |
|
|
Exercise of stock options |
|
12,500 |
|
|
— |
|
|
112 |
|
|
— |
|
|
— |
|
|
112 |
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
5,199 |
|
|
— |
|
|
— |
|
|
5,199 |
|
|
Unrealized loss on available for sale securities |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(147) |
|
|
(147) |
|
|
Foreign currency translation adjustment |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
277 |
|
|
277 |
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(57,933) |
|
|
— |
|
|
(57,933) |
|
|
Balance at December 31, 2015 |
|
25,085,554 |
|
|
25 |
|
|
223,784 |
|
|
(134,546) |
|
|
(41) |
|
|
89,222 |
|
|
Issuance of restricted shares of common stock |
|
72,500 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Forfeiture of restricted shares of common stock |
|
(21,432) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Issuance of common stock, net of costs |
|
52,503 |
|
|
— |
|
|
272 |
|
|
— |
|
|
— |
|
|
272 |
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
6,323 |
|
|
— |
|
|
— |
|
|
6,323 |
|
|
Unrealized gain on available for sale securities |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
138 |
|
|
138 |
|
|
Foreign currency translation adjustment |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
3 |
|
|
3 |
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(90,632) |
|
|
— |
|
|
(90,632) |
|
|
Balance, December 31, 2016 |
|
25,189,125 |
|
|
25 |
|
|
230,379 |
|
|
(225,178) |
|
|
100 |
|
|
5,326 |
|
|
Cumulative adjustment - ASU 2016-09 |
|
— |
|
|
— |
|
|
763 |
|
|
(763) |
|
|
— |
|
|
— |
|
|
Forfeiture of restricted shares of common stock |
|
(32,771) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Issuance of common stock, net of costs |
|
18,283,309 |
|
|
18 |
|
|
23,204 |
|
|
— |
|
|
— |
|
|
23,222 |
|
|
Exchange of convertible debt and issuance of warrants |
|
2,500,000 |
|
|
3 |
|
|
(5,393) |
|
|
— |
|
|
— |
|
|
(5,390) |
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
5,918 |
|
|
— |
|
|
— |
|
|
5,918 |
|
|
Unrealized loss on available for sale securities |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(37) |
|
|
(37) |
|
|
Foreign currency translation adjustment |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
945 |
|
|
945 |
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(69,359) |
|
|
— |
|
|
(69,359) |
|
|
Balance, December 31, 2017 |
|
45,939,663 |
|
$ |
46 |
|
$ |
254,871 |
|
$ |
(295,300) |
|
$ |
1,008 |
|
$ |
(39,375) |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Egalet Corporation and Subsidiaries
Consolidated Statements of Cash Flows
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|||||||
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||
|
Operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(57,933) |
|
$ |
(90,632) |
|
$ |
(69,359) |
|
|
Adjustment to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization of intangibles |
|
|
3,014 |
|
|
4,118 |
|
|
5,062 |
|
|
Change in fair value of warrant and derivative liability |
|
|
(260) |
|
|
(644) |
|
|
(2,546) |
|
|
Stock-based compensation expense |
|
|
5,199 |
|
|
6,323 |
|
|
5,918 |
|
|
Noncash interest and amortization of debt discount |
|
|
3,957 |
|
|
5,911 |
|
|
5,070 |
|
|
Amortization of premium (discount) on marketable securities |
|
|
829 |
|
|
607 |
|
|
(24) |
|
|
Deferred income taxes |
|
|
(718) |
|
|
(1,061) |
|
|
— |
|
|
Loss (gain) on extinguishment of debt |
|
|
— |
|
|
800 |
|
|
(13,221) |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Related party receivable |
|
|
563 |
|
|
57 |
|
|
— |
|
|
Accounts receivable |
|
|
(295) |
|
|
(813) |
|
|
(3,012) |
|
|
Inventory |
|
|
1,571 |
|
|
137 |
|
|
(1,525) |
|
|
Prepaid expenses and other current assets |
|
|
(631) |
|
|
(1,243) |
|
|
(132) |
|
|
Other receivables |
|
|
(144) |
|
|
28 |
|
|
219 |
|
|
Deposits and other assets |
|
|
(2,050) |
|
|
1,481 |
|
|
(375) |
|
|
Accounts payable |
|
|
1,147 |
|
|
(2,945) |
|
|
7,597 |
|
|
Accrued expenses |
|
|
5,123 |
|
|
10,553 |
|
|
(2,132) |
|
|
Deferred revenue |
|
|
1,485 |
|
|
(6,153) |
|
|
3,475 |
|
|
Other current liabilities |
|
|
116 |
|
|
(187) |
|
|
— |
|
|
Other liabilities |
|
|
121 |
|
|
796 |
|
|
(179) |
|
|
Net cash used in operating activities |
|
|
(38,906) |
|
|
(72,867) |
|
|
(65,164) |
|
|
Investing activities: |
|
|
|
|
|
|
|
|
|
|
|
Payments for purchase of property and equipment |
|
|
(2,747) |
|
|
(7,237) |
|
|
(88) |
|
|
Increase in restricted cash |
|
|
(1,223) |
|
|
— |
|
|
(400) |
|
|
Purchases of investments |
|
|
(110,216) |
|
|
(59,545) |
|
|
(101,338) |
|
|
Sales of investments |
|
|
3,400 |
|
|
14,370 |
|
|
12,195 |
|
|
Maturity of investments |
|
|
6,799 |
|
|
101,277 |
|
|
71,647 |
|
|
Purchase of SPRIX Nasal Spray |
|
|
(8,128) |
|
|
— |
|
|
— |
|
|
License of OXAYDO |
|
|
(7,672) |
|
|
— |
|
|
— |
|
|
Net cash (used in) provided by investing activities |
|
|
(119,787) |
|
|
48,865 |
|
|
(17,984) |
|
|
Financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from issuance of common stock and warrants |
|
|
80,896 |
|
|
272 |
|
|
32,888 |
|
|
Payments on borrowings |
|
|
— |
|
|
(15,856) |
|
|
— |
|
|
Net proceeds from debt and royalty rights |
|
|
71,397 |
|
|
37,231 |
|
|
38,304 |
|
|
Exchange of convertible notes |
|
|
— |
|
|
— |
|
|
(1,532) |
|
|
Royalty payments in connection with the 13% Notes |
|
|
— |
|
|
— |
|
|
(307) |
|
|
Net cash provided by financing activities |
|
|
152,293 |
|
|
21,647 |
|
|
69,353 |
|
|
Effect of foreign currency translation on cash and cash equivalents |
|
|
327 |
|
|
45 |
|
|
530 |
|
|
Net decrease in cash and cash equivalents |
|
|
(6,073) |
|
|
(2,310) |
|
|
(13,265) |
|
|
Cash at beginning of period |
|
|
52,738 |
|
|
46,665 |
|
|
44,355 |
|
|
Cash at end of period |
|
$ |
46,665 |
|
$ |
44,355 |
|
$ |
31,090 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
Cash interest payments |
|
$ |
3,461 |
|
$ |
4,712 |
|
$ |
12,817 |
|
|
Non-cash purchases of property and equipment |
|
$ |
2,084 |
|
$ |
— |
|
$ |
— |
|
|
Non-cash financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Fair value of warrants issued in connection with debt and common stock |
|
$ |
329 |
|
$ |
— |
|
$ |
9,667 |
|
|
Reclassification to additional paid in capital related to convertible note exchange |
|
$ |
— |
|
$ |
— |
|
$ |
9,030 |
|
|
Fair value of warrants issued in connection with convertible note exchange |
|
$ |
— |
|
$ |
— |
|
$ |
3,640 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Egalet Corporation and Subsidiaries
Notes to the Consolidated Financial Statements
December 31, 2015, 2016 and 2017
1. Organization and Description of the Business
Egalet Corporation (the “Company”) is a fully integrated specialty pharmaceutical company manufacturing and commercializing innovative treatments for pain and other conditions. Given the need for acute and chronic pain products and the issue of prescription abuse, the Company is focused on bringing non-narcotic and abuse-deterrent (“AD”) formulations to patients and healthcare providers. The Company is currently marketing ARYMO® ER (morphine sulfate) extended-release (“ER”) tablets, for oral use CII (“ARYMO ER”), SPRIX® (ketorolac tromethamine) Nasal Spray (“SPRIX Nasal Spray”), and OXAYDO® (oxycodone HCI, USP) tablets for oral use only—CII (“OXAYDO”).
ARYMO ER is an ER morphine product formulated with AD properties and approved for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. ARYMO ER is the Company’s first product developed using its proprietary Guardian™ Technology. SPRIX Nasal Spray is a nonsteroidal anti-inflammatory drug indicated in adult patients for the short‑term (up to five days) management of moderate to moderately severe pain that requires analgesia at the opioid level. OXAYDO is an immediate release (“IR”) oxycodone product designed to discourage abuse via snorting, indicated for the management of acute and chronic pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
The Company also has a pipeline of products developed using Guardian Technology that it may look to partner out to other companies. The Company plans to continue to grow through the revenues of its three commercial products, business development opportunities and leveraging its proprietary Guardian Technology.
Liquidity and Substantial Doubt in Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred recurring operating losses since inception. As of December 31, 2017, the Company had an accumulated deficit of $295.3 million and will need to improve cash generated from product sales and may require additional capital to fund its commercialization strategies for SPRIX Nasal Spray, OXAYDO and ARYMO ER. The Company anticipates operating losses to continue for the foreseeable future due to, among other things, costs related to its commercial infrastructure.
As of December 31, 2017, and through the date of this report, the Company was not in compliance with Nasdaq Global Market listing requirements. The Company’s outstanding convertible notes in the amount of $48.5 million contain redemption features in the event it is not able to maintain its Nasdaq listing – refer to Note 9 – Long Term Debt for further information. These factors, in combination with others described above, raise substantial doubt about the ability of the Company to continue as a going concern within one year after the date that these financial statements are issued. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company continues to seek other sources of capital and alternatives, such as contemplating a reverse stock split, to maintain its listing on the Nasdaq. However, if the Company is unable to raise sufficient capital or maintain its Nasdaq listing to prevent the redemption of its convertible notes, the Company will not have sufficient liquidity to fund its business operations for at least the next year following the date that the financial statements are issued. Accordingly, management has concluded that substantial doubt exists with respect to the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
F-8
2. Summary of Significant Accounting Policies and Basis of Accounting
Basis of Accounting
The consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The Company’s consolidated financial statements include the accounts of Egalet Corporation and its wholly‑owned subsidiaries, Egalet Limited and Egalet US, Inc. The Company’s consolidation policy requires the consolidation of entities where a controlling financial interest is held. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period.
Significant areas that require management’s estimates include intangible assets, revenue recognition, useful lives of assets, accrued research and development expenses, the outcome of litigation, convertible debt, share-based payments, warrant and derivative liabilities and income taxes. The Company is subject to risks and uncertainties due to changes in, among other things, the healthcare environment, regulatory oversight, competition, and legislation that may cause actual results to differ from estimated results.
Segment and Geographic Information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision‑making group, in deciding how to allocate resources and in assessing performance. The Company globally manages the business within one reportable segment. Segment information is consistent with how management reviews the business, makes investing and resource allocation decisions and assesses operating performance. As of December 31, 2017, long‑lived assets based upon geographic location were located in both the United States and Europe, with a net book value of $9.4 million and $505,000 respectively. For the year ended December 31, 2015, revenue based upon geographic location was derived substantially from Europe. For the year ended December 31, 2016, revenue from product sales was derived entirely from the United States, while related party revenue was derived entirely from Europe. For the year ended December 31, 2017, revenue from product sales and collaborations was derived entirely from the United States.
Concentrations of Credit Risk and Off‑Balance Sheet Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are primarily cash and investments in marketable securities and accounts receivable. The Company maintains its cash balances in accounts with financial institutions that management believes are creditworthy. The Company invests cash that is not currently being used for operational purposes in accordance with its investment policy. The policy allows for the purchase of low-risk debt securities issued by U.S. government agencies and very highly rated corporations, subject to certain concentration limits. The Company believes its established guidelines for investment of its excess cash maintain safety and liquidity through its policies on diversification and investment maturity.
F-9
The below table represents the Company’s accounts receivable concentration by customer at December 31, 2016 and 2017:
|
|
2016 |
|
2017 |
|
||
|
Customer A |
|
39.0 |
|
|
31.0 |
% |
|
Customer B |
|
26.0 |
|
|
24.0 |
% |
|
Customer C |
|
23.0 |
|
|
38.0 |
% |
|
Customer D |
|
10.0 |
|
|
— |
% |
|
Customer E |
|
1.0 |
|
|
5.0 |
% |
|
Total |
|
99.0 |
|
|
98.0 |
% |
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Cash balances of $28.0 million and $3.1 million were maintained at financial institutions in the United States and Denmark, respectively, at December 31, 2017. Bank deposits are insured up to approximately $250,000 and $121,000 for U.S. and Danish financial institutions, respectively.
Marketable Securities, Available-for-Sale
Marketable securities consist of securities with original maturities greater than three months and are composed of securities issued by U.S. government agencies and corporate debt securities. Marketable securities have been classified as current assets in the accompanying Consolidated Balance Sheets based upon the nature of the securities and their intended use to fund operations.
Management determines the appropriate classification of securities at the time of purchase. The Company has classified its investment portfolio as available-for-sale in accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 320, Investments—Debt and Equity Securities. The Company’s available-for-sale securities are carried at fair value with unrealized gains and losses reported in other comprehensive income (loss). Realized gains and losses are determined using the specific identification method and are included in interest expense. Marketable securities are evaluated periodically for impairment. If it is determined that a decline of any investment is other than temporary, then the carrying amount of the investment is written down to fair value and the write-down is included in the Consolidated Statements of Comprehensive Loss as a loss.
Fair Value Measurements
The carrying amounts reported in the Company’s consolidated financial statements for cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximate their respective fair values because of the short-term nature of these accounts. The carrying value of the derivative liabilities are the estimated fair value of the liability as further described in Note 4 – Fair value measurements.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Fair value should be based on the assumptions that market participants would use when pricing an asset or liability and is based on a fair value hierarchy that prioritizes the information used to develop those assumptions. The fair value hierarchy gives the highest priority to quoted prices in active markets (observable inputs) and the lowest priority to the Company’s assumptions (unobservable inputs). Fair value measurements should be disclosed separately by level within the fair value hierarchy. For assets and liabilities recorded at fair value, it is the Company’s policy to maximize the use of observable inputs and minimize the use of unobservable inputs when developing fair value measurements, in accordance with established fair value hierarchy.
F-10
Financial assets that the Company measures at fair value on a recurring basis include cash equivalents and marketable securities. These financial assets are generally classified as Level 1 or 2 within the fair value hierarchy. In general, fair values determined by Level 1 inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities. Fair values determined by Level 2 inputs utilize data points that are observable, such as quoted prices (adjusted), interest rates and yield curves. Fair values determined by Level 3 inputs utilize unobservable data points for the asset or liability, and include situations where there is little, if any, market activity for the asset or liability. The fair value hierarchy level is determined by the lowest level of significant input.
The Company’s financial assets have been initially valued at the transaction price and subsequently valued at the end of each reporting period, typically utilizing third-party pricing services or other market observable data. The pricing services utilize industry standard valuation models, including both income and market-based approaches, and observable market inputs to determine value. These observable market inputs include reportable trades, benchmark yields, credit spreads, broker/dealer quotes, bids, offers, current spot rates and other industry and economic events. The Company validates the prices provided by its third-party pricing services by reviewing their pricing methods and matrices, obtaining market values from other pricing sources, analyzing pricing data in certain instances and confirming that the relevant markets are active. The Company did not adjust or override any fair value measurements provided by its pricing services as of December 31, 2016 and December 31, 2017.
Financial liabilities that the Company measures at fair value on a recurring basis include derivative liabilities consisting of the interest make whole feature of the 5.50% and 6.50% Notes, the conversion feature of the 6.50% Notes and the warrant liability associated with the July Equity offering. These financial liabilities are classified as Level 3 within the fair value hierarchy. Fair values determined by Level 3 inputs utilize unobservable data points for the asset or liability, and include situations where there is little, if any, market activity for the asset or liability. The fair value hierarchy level is determined by the lowest level of significant input.
The Company’s financial liabilities have been initially and subsequently valued at the end of each reporting period, typically utilizing third-party valuation services. The valuation services utilize industry standard valuation models, including both income and market-based approaches and observable market inputs for similar instruments to determine value, if available. These observable market inputs include reportable trades, benchmark yields, credit spreads, broker/dealer quotes, bids, offers, current spot rates and other industry and economic events. The Company validates the valuations provided by its third-party valuation services by reviewing their pricing valuation and matrices and confirming the relevant markets are active. The Company did not adjust or override any fair value measurements provided by its valuation services as of December 31, 2016 and December 31, 2017.
During the years ended December 31, 2016 and 2017, there were no transfers between Level 1, Level 2, or Level 3 financial assets or liabilities. The Company did not have any non-recurring fair value measurements on any assets or liabilities at December 31, 2016 and December 31, 2017.
Stock-Based Compensation
The Company uses the Black-Scholes valuation model in determining the fair value of equity awards. For stock options granted to employees and directors with only service-based vesting conditions, the Company measures stock-based compensation cost at the grant date based on the estimated fair value of the award and recognizes it as expense over the requisite service period on a straight-line basis. The Company records the expense of services rendered by non-employees based on the estimated fair value of the stock option as of the respective vesting date. Further, the Company expenses the fair value of non-employee stock options that contain only service-based vesting conditions over the requisite service period of the underlying stock options.
The fair value for restricted stock awards is determined based on the closing market price of the Company’s Common Stock on the grant date of the awards. The expense is recognized over the requisite service period on a straight-line basis.
F-11
Property and Equipment
Property and equipment consist primarily of laboratory and manufacturing equipment, furniture, fixtures, and other property, all of which are stated at cost, less accumulated depreciation. Property and equipment are depreciated using the straight‑line method over the estimated useful lives of the assets. Maintenance and repairs are expensed as incurred. The following estimated useful lives were used to depreciate the Company’s assets:
|
|
|
Estimated Useful Life |
|
||
|
Laboratory and manufacturing equipment |
|
3 |
- |
10 |
years |
|
Furniture, fixtures and other property |
|
3 |
- |
7 |
years |
Upon retirement or sale, the cost of the disposed asset and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is charged to income.
Intangible and Long-Lived Assets
Intangible and long-lived assets consist of in‑process research and development (“IPR&D”) and product rights. IPR&D is considered an indefinite‑lived intangible asset and is assessed for impairment annually or more frequently if impairment indicators exist. If the associated research and development effort is abandoned, the related assets would be written‑off and the Company would record a non‑cash impairment loss on its consolidated statement of operations. For those product candidates that reach commercialization, the IPR&D asset will be amortized over its estimated useful life.
Long-lived intangible assets acquired as part of the SPRIX Nasal Spray acquisition and OXAYDO license are being amortized on a straight-line basis over their estimated useful lives of 5 years and 7 years, respectively. The Company estimated the useful life of the assets by considering competition by products prescribed for the same indication, the likelihood and estimated future entry of non-generic and generic competition for the same or similar indication and other related factors. The factors that drive the estimate of the life are often uncertain and are reviewed on a periodic basis or when events occur that warrant review.
The Company assesses the recoverability of its long‑lived assets, which include property and equipment and product rights whenever significant events or changes in circumstances indicate impairment may have occurred. If indicators of impairment exist, projected future undiscounted cash flows associated with the asset are compared to its carrying amount to determine whether the asset’s value is recoverable. Any resulting impairment is recorded as a reduction in the carrying value of the related asset and a charge to operating results. For the years ended December 31, 2015, 2016 and 2017, the Company determined that there was no impairment of its intangible and other long‑lived assets.
Net Product Sales
The Company recognizes revenue in accordance with FASB ASC 605, Revenue Recognition, when the following criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred and risk of loss has passed; and the seller’s price to the buyer is fixed or determinable and collectability is reasonably assured. The Company determines that persuasive evidence of an arrangement exists based on written contracts that define the terms of the arrangements. The Company determines when title to products and associated risk of loss has passed on to the customer pursuant to contract terms. The Company assesses whether the price is fixed or determinable based on the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. The Company assesses collectability based primarily on the customer’s payment history and creditworthiness.
The Company sells SPRIX Nasal Spray in the U.S. to a single specialty pharmaceutical distributor subject to rights of return. The Company has limited SPRIX Nasal Spray sales history under the current distribution model and pricing, and the Company has determined that it cannot reliably estimate expected returns of the product at the time of shipment. Accordingly, the Company defers recognition of revenue on product shipments of SPRIX Nasal Spray until the right of return no longer exists, which occurs at the earlier of the time SPRIX Nasal Spray units are dispensed through patient prescriptions or expiration of the right of return. Units dispensed are generally not subject to return,
F-12
except in the rare cases where the product malfunctions or the product is damaged in transit. The Company calculates patient prescriptions dispensed using an analysis of third-party information.
The Company sells OXAYDO and ARYMO ER in the U.S. to several wholesalers, all subject to rights of return. The Company has limited OXAYDO and ARYMO ER sales history under the current distribution model and pricing and has determined that it cannot reliably estimate expected returns of the product at the time of shipment. Accordingly, the Company defers recognition of revenue on product shipments of OXAYDO and ARYMOE ER until the right of return no longer exists, which occurs at the earlier of the time OXAYDO and ARYMO ER units are dispensed through patient prescriptions or expiration of the right of return. Units dispensed are generally not subject to return, except in the rare cases where the product malfunctions or the product is damaged in transit. The Company calculates patient prescriptions dispensed using an analysis of third-party information.
Product Sales Allowances
The Company recognizes product sales allowances as a reduction of product sales in the same period the related revenue is recognized. Product sales allowances are based on amounts owed or to be claimed on the related sales. These estimates take into consideration the terms of the Company’s agreements with customers and third-party payors that may result in future rebates or discounts taken. In certain cases, such as patient assistance programs, the Company recognizes the cost of patient discounts as a reduction of revenue based on estimated utilization. If actual future results vary, the Company may need to adjust these estimates, which could have an effect on product revenue in the period of adjustment. The Company’s product sales allowances include:
Specialty Pharmacy Fees. The Company offers a discount to a certain specialty pharmaceutical distributor based on a contractually determined rate. The Company accrues the discount on shipment to the respective distributor and recognizes the discount as a reduction of revenue in the same period the related revenue is recognized.
Wholesaler Fees. The Company pays certain pharmaceutical wholesalers fees based on a contractually determined rate. The Company accrues the fees on shipment to the respective wholesalers and recognizes the fees as a reduction of revenue in the same period the related revenue is recognized.
Prompt Pay Discounts. The Company offers cash discounts to its customers, generally 2% of the sales price, as an incentive for prompt payment. The Company accounts for cash discounts by reducing accounts receivable by the prompt pay discount amount and recognizes the discount as a reduction of revenue in the same period the related revenue is recognized.
Patient Discount Programs. The Company offers co-pay discount programs for SPRIX Nasal Spray, ARYMO ER and OXAYDO to patients, in which patients receive a co-pay discount on their prescriptions. The Company utilizes data provided by independent third parties to determine the total amount that was redeemed and recognizes the discount as a reduction of revenue in the same period the related revenue is recognized.
Rebates. Managed care rebates are payments to third parties, primarily pharmacy benefit managers and other health insurance providers. The reserve for these rebates is based on a combination of actual utilization provided by the third party and an estimate of customer buying patterns and applicable contractual rebate rates to be earned over each period. The Company recognizes the discount as a reduction of revenue in the same period the related revenue is recognized.
Related Party Revenues
During 2013, the Company entered into a collaborative research and license agreement with Shionogi Limited (“Shionogi”). The agreement contained multiple deliverables including (i) licenses, (ii) research and development activities, and (iii) royalty and related commissions. Revenue was recognized when the Company satisfied its service obligations under a written contract with the Company’s customer (or collaboration partner) where the price for the services have been agreed upon and when the Company has reasonable assurance that the resulting receivable will be collected within contractually agreed upon terms. The Company had adopted the provisions of Accounting Standards
F-13
Update (“ASU”) 2009‑13, “Multiple‑Deliverable Revenue Arrangements,” which amends ASC 605‑25, and also adopted ASU 2010‑17, “Revenue Recognition—Milestone Method.” In accordance with ASU 2009‑13, the Company considers whether the deliverables under the arrangement represent separate units of accounting. In determining the units of accounting, management evaluates certain criteria, including whether the deliverables have stand‑alone value. In December 2015, the Company received notice from Shionogi that the collaboration and license agreement was terminated for convenience.
Inventories and Cost of Sales
Inventories are stated at the lower of cost or market net of reserve for excess and obsolete inventory and cost is determined using the average-cost method. At each of December 31, 2016 and 2017, inventory consisted of raw materials, work in process, finished goods and deferred cost of goods.
Cost of sales includes the cost of inventory sold or reserved, which includes manufacturing and supply chain costs, product shipping and handling costs, and product royalties. The cost of sales associated with the deferred product revenues are recorded as deferred costs, which are included in inventory, until such time the deferred revenue is recognized.
Long Term Debt
Hercules Loan Agreement
In January 2015, the Company entered into the Loan and Security Agreement, which was subsequently amended in December 2015 (as amended, the “Loan Agreement”), with Hercules Technology Growth Capital, Inc. (“Hercules”) and certain other lenders, pursuant to which the Company borrowed $15.0 million under a term loan. The term loan bore an interest rate per annum equal to the greater of either (i) 9.40% plus the prime rate as reported in The Wall Street Journal minus 3.25% or (ii) 9.40%. Under the Loan Agreement, the Company made interest only payments through July 1, 2016, with the potential for the interest only period to be extended to January 1, 2017, subject to the FDA’s acceptance of the Company’s new drug application for its product candidate ARYMO ER, the Company’s receipt of at least $5.0 million of product revenue for any consecutive three-month period prior to June 30, 2016 and there being no event of default under the Loan Agreement. The Company did not receive at least $5.0 million of product revenue in a consecutive three-month period prior to June 30, 2016, and as a result began repaying the principal balance on July 1, 2016. In connection with the Loan Agreement, the Company granted a security interest in substantially all of its assets, excluding intellectual property and certain new drug applications and related approvals, as collateral for the obligations under the Loan Agreement.
On August 31, 2016, the Company repaid all outstanding obligations under the Loan Agreement with the proceeds of the 13.0% Notes (as defined below). As a result of the repayment, the Company recorded debt extinguishment costs of $800,000 during the year ended December 31, 2016 which is classified as interest expense on the Company’s Consolidated Statement of Operations.
5.50% Notes
In April and May 2015, the Company issued through a private placement $61.0 million in aggregate principal amount of the 5.50% Notes (the “5.50% Notes”) in two separate closings. Interest on the 5.50% Notes is payable semi-annually in arrears on April 1 and October 1 of each year, commencing October 1, 2015. The 5.50% Notes are convertible at 67.2518 shares per $1,000 principal amount of the 5.50% Notes (equivalent to an initial conversion price of approximately $14.87 per share of Common Stock).
In December 2017, the Company closed exchange agreements with certain holders of the outstanding 5.50% notes for $36.4 million in principal value of the 5.50% Notes. The total face value of the outstanding 5.50% notes was reduced from $61.0 million to $24.6 million as a result of the Exchange. As part of the exchange, the Company issued $23.9 million in principal amount of new 6.50% convertible notes due December 31, 2024 (the “6.50% Notes”). See below for further information.
F-14
13.0% Notes
In August 2016, the Company completed the initial closing (the “Initial Closing”) of its offering (the “Offering”) of up to $80.0 million aggregate principal amount of its 13.0% senior secured notes (the “13.0% Notes”) and entered into an indenture (the “Indenture”) governing the Notes with the guarantors party thereto (the “Guarantors”) and U.S. Bank National Association, a national banking association, as trustee (the “Trustee”) and collateral agent (the “Collateral Agent”). The Company issued $40.0 million aggregate principal amount of the 13% Notes at the Initial Closing and issued an additional $40.0 million aggregate principal amount of the Notes on approval from the FDA of ARYMO™ ER in January 2017 (the “Second Closing”). Net proceeds from the Initial Closing and Second Closing were $37.2 million, and $38.3 million respectively, after deducting offering expenses. The Notes were sold only to qualified institutional buyers within the meaning of Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”).
6.50% Notes
In December 2017, the Company issued $23.9 million in principal amount of the 6.50% Notes. The 6.50% notes were issued to existing 5.50% Note holders in exchange for $36.4 million in face value of the 5.50% Notes. Interest on the 6.50% Notes is payable semi-annually in arrears on January 1 and July 1 of each year, commencing July 1, 2018. The 6.50% Notes are convertible at 749.6252 shares per $1,000 principal amount of the 6.50% Notes (equivalent to an initial conversion price of approximately $1.33 per share of Common Stock).
Refer to Note 9 - Long Term Debt for additional information.
Interest Make-Whole Derivative
The 5.50% Notes include an interest make-whole feature whereby if a noteholder converts any of the 5.50% Notes prior to April 1, 2018, subject to certain restrictions, they are entitled, in addition to the other consideration payable or deliverable in connection with such conversion, to an interest make-whole payment equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the notes to be converted had such notes remained outstanding from the conversion date through April 1, 2018, computed using a discount rate equal to 2%. The Company has determined that this feature is an embedded derivative and has recognized the fair value of this derivative as a liability on the Company’s Consolidated Balance Sheet, with subsequent changes to fair value recorded through earnings at each reporting period on the Company’s Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities. The fair value of this embedded derivative was determined based on a binomial tree lattice model.
The 6.50% Notes include an interest make-whole feature whereby if a noteholder converts any of the 6.50% Notes prior to January 1, 2021, subject to certain restrictions, they are entitled, in addition to the other consideration payable or deliverable in connection with such conversion, to an interest make-whole payment equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the notes to be converted had such notes remained outstanding from the conversion date through January 1, 2021, computed using a discount rate equal to 2%. The Company has determined that this feature is an embedded derivative and has recognized the fair value of this derivative as a liability on the Company’s Consolidated Balance Sheet, with subsequent changes to fair value recorded through earnings at each reporting period on the Company’s Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities. The fair value of this embedded derivative was determined based on a binomial tree lattice model.
Warrant Liability
On July 6, 2017, the Company entered into an underwriting agreement with Cantor Fitzgerald & Co. relating to an underwritten public offering (the “July 2017 Equity Offering”) of 16,666,667 shares of the Company’s Common Stock and accompanying warrants to purchase 16,666,667 shares of Common Stock, at a combined public offering price
F-15
of $1.80 per share and accompanying warrant, for gross proceeds of $30.0 million. Each warrant was issued at an exercise price of $2.70, subject to adjustment in certain circumstances. The shares of Common Stock and warrants were issued separately. The warrants may be exercised at any time on or after the date of issuance and will expire five years from the date of issuance.
The Company accounted for the warrants using ASC 480 – Distinguishing Liabilities from Equity and determined that the warrants were a freestanding financial instrument that are subject to liability classification. Pursuant to the terms of the agreement, the Company could be required to settle the warrants in cash in the event of an acquisition of the Company, and as a result the warrants are required to be measured at fair value and reported as a current liability in the Company’s Consolidated Balance sheet, with subsequent changes to fair value recorded through earnings at each reporting period on the Company’s Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities.
Common Stock Warrants
The Company issued warrants to Hercules in connection with the Loan Agreement with Hercules and certain other lenders. The Company evaluated the warrants under ASC 480 - Distinguishing Liabilities from Equity and determined the warrants are classified as equity. The fair value of the warrants on the date of grant was recorded as a debt discount. On August 3, 2015, Hercules exercised the warrant in full, electing the net issuance option. As a result, the Company issued 61,644 shares of the its Common Stock to Hercules.
Research and Development Expenses
Research and development costs are charged to expense as incurred. These costs include, but are not limited to, employee‑related expenses, including salaries, benefits and travel; expenses incurred under agreements with contract research organizations and investigative sites that conduct clinical trials and preclinical studies; the cost of acquiring, developing and manufacturing clinical trial materials; the costs of manufacturing scale-up and optimization: the costs of pre-approval product manufactured; facilities, other expenses which include direct and allocated expenses for rent and maintenance of facilities, insurance and other supplies; and costs associated with preclinical activities.
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information provided to the Company by its vendors with respect to their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the consolidated financial statements as prepaid or accrued research and development expense as the case may be.
Foreign Currency Translation
The reporting currency of the Company is the U.S. dollar. The functional currency of the Company’s non‑U.S. operations is the Danish Krone. Assets and liabilities of foreign operations are translated into U.S. dollars based on exchange rates at the end of each reporting period. Revenues and expenses are translated at average exchange rates during the reporting period. Gains and losses arising from the translation of assets and liabilities are included as a component of accumulated other comprehensive loss or income on the Company’s Consolidated Balance Sheets. Gains and losses resulting from foreign currency transactions are reflected within the Company’s Consolidated Statement of Operations. The Company has not utilized any foreign currency hedging strategies to mitigate the effect of its foreign currency exposure.
Intercompany payables and receivables are considered to be long-term in nature and any change in balance due to foreign currency fluctuation is included as a component of the Company's Consolidated Statements of Comprehensive Loss and Accumulated Other Comprehensive Loss within the Company's Consolidated Balance Sheets.
F-16
Comprehensive Loss
Comprehensive loss is defined as changes in stockholders’ equity exclusive of transactions with owners (such as capital contributions and distributions). Comprehensive loss is composed of net loss, foreign currency translation adjustments and unrealized gains or losses on marketable securities classified as available for sale.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Current tax liabilities or receivables are recognized for the amount of taxes the Company estimates are payable or refundable for the current year. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss and credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. A valuation allowance is provided when it is more likely than not that some portion or the entire deferred tax asset will not be realized. The Company recognizes the benefit of an uncertain tax position that it has taken or expects to take on its income tax return if such a position is more likely than not to be sustained. Then, the tax benefit recognized is the largest amount of benefit, determined on a cumulative probability basis, which is more likely than not to be realized upon ultimate settlement. The Company recognizes interest and penalties related to unrecognized tax benefits within the income tax expense line in the Company’s Consolidated Statement of Operations and Comprehensive Loss. Accrued interest and penalties are included within the related tax liability line in the Company’s Consolidated Balance Sheet. The Company did not have any accrued interest or penalties associated with any unrecognized tax positions at December 31, 2016 and 2017, and there were no such interest or penalties recognized during the years ended December 31, 2015, 2016 and 2017.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act makes broad and complex changes to the U.S. tax code, including, but not limited to, reducing the top U.S. federal corporate tax rate to 21 percent; requiring companies to pay a one-time transition tax on certain un-repatriated earnings of foreign subsidiaries; generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; eliminating the corporate alternative minimum tax (AMT) and changing how existing AMT credits can be realized; creating the base erosion anti-abuse tax (BEAT), a new minimum tax; creating a new limitation on deductible interest expense; and changing rules related to uses and limitations of net operating loss carryforwards created in tax years beginning after December 31, 2017.
The Tax Act reduces the Company’s U.S. corporate income tax rate from 34% to 21%, effective January 1, 2018. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. As a result of the reduction in the U.S. corporate income tax rate to 21% under the Tax Act, the Company revalued its ending net deferred tax assets and liabilities at December 31, 2017.
The Tax Act provided for a one-time transition tax on the deemed repatriation of post-1986 undistributed foreign subsidiary earnings and profits (“E&P”). The Company did not have to recognize any income tax expense related to the transition tax due to current and historical losses at its controlled foreign corporation.
The global intangible low-taxed income tax and base erosion provisions are effective for taxable years beginning after December 31, 2017. The Company does not currently expect these provisions to have a material impact on its tax rate due to losses at its controlled foreign corporation and they are currently below the gross receipts threshold for purposes of the base erosion provisions.
Due to the timing of the new tax law and the substantial changes it brings, the Staff of the Securities and Exchange Commission (the "SEC") issued Staff Accounting Bulletin No. 118 ("SAB 118"), which provides registrants a measurement period to report the impact of the new US tax law. During the measurement period, provisional amounts for the effects of the law are recorded to the extent a reasonable estimate can be made. To the extent that all information
F-17
necessary is not available, prepared or analyzed, companies may recognize provisional estimated amounts for a period of up to one year following enactment of the TCJA. In connection with our initial analysis of the impact of the Act, we have reasonably estimated there to be no income tax expense. The Company will continue to monitor for future updates to guidance or interpretations issued by the IRS.
Clinical Trial Expense Accruals
As part of the process of preparing its consolidated financial statements, the Company is required to estimate its expenses resulting from its obligations under contracts with vendors, clinical research organizations and consultants and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided under such contracts. The Company’s objective is to reflect the appropriate clinical trial expenses in its consolidated financial statements by matching those expenses with the period in which services are performed and efforts are expended. The Company accounts for these expenses according to the progress of the trial as measured by patient progression and the timing of various aspects of the trial. The Company determines accrual estimates through financial models taking into account discussion with applicable personnel and outside service providers as to the progress or state of consummation of trials, or the services completed. During the course of a clinical trial, the Company adjusts its clinical expense recognition if actual results differ from its estimates. The Company makes estimates of its accrued expenses as of each balance sheet date based on the facts and circumstances known to it at that time. The Company’s clinical trial accruals are dependent upon the timely and accurate reporting of contract research organizations and other third‑party vendors. Although the Company does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in it reporting amounts that are too high or too low for any particular period. For the years ended December 31, 2015, 2016 and 2017, there were no material adjustments to the Company’s prior period estimates of accrued expenses for clinical trials.
Basic and Diluted Net Loss Per Share of Common Stock
Basic net loss per share of Common Stock is computed by dividing net loss applicable to Common Stockholders by the weighted‑average number of common shares outstanding during the period. Diluted net loss per share of Common Stock is computed by dividing the net loss applicable to Common Stockholders by the sum of the weighted‑average number of common shares outstanding during the period plus the potential dilutive effects of Common Stock options and warrants outstanding during the period calculated in accordance with the treasury stock method, plus the potential dilutive effects of the 5.50% and 6.50% Notes using the if-converted method. Because the impact of these items is anti‑dilutive during periods of net loss, there was no difference between basic and diluted net loss per share of Common Stock for the years ended December 31, 2015, 2016 and 2017.
Customer Concentration
Customer concentration for the years ended December 31, 2016 and 2017 are as follows:
|
|
2016 |
|
2017 |
|
||
|
Customer A |
|
— |
|
|
— |
% |
|
Customer B |
|
78.7 |
|
|
76.0 |
% |
|
Customer C |
|
8.2 |
|
|
9.7 |
% |
|
Customer D |
|
5.6 |
|
|
7.2 |
% |
|
Customer E |
|
5.4 |
|
|
5.8 |
% |
|
Total |
|
97.9 |
|
|
98.7 |
% |
F-18
Recent Accounting Pronouncements
In March 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-09, Improvements to Employee Share-Based Payment Accounting, which provides for simplification of certain aspects of employee share-based payment accounting including income taxes, classification of awards as either equity or liabilities, accounting for forfeitures and classification on the statement of cash flows. ASU 2016-09 became effective for the Company in the first quarter of 2017 and was applied using a modified retrospective transition approach. Under ASU 2016-09, the Company has elected to no longer estimate forfeiture rates as part of its stock-based compensation expense and will true up forfeitures as they occur. As a result of the adoption of ASU 2016-09, the Company recorded a cumulative adjustment of $763,000, which increased its accumulated deficit as of January 1, 2017.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. ASU 2016-02 is effective for annual periods beginning after December 15, 2018, including interim periods within those annual periods, with early adoption permitted. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The Company is currently evaluating the impact that the standard will have on the financial statements, and has not yet determined what effect, if any, the impact of adoption will be.
In January 2016, the FASB issued ASU 2016-01, Financial Instruments - Overall (Subtopic 825-10), Recognition and Measurement of Financial Assets and Financial Liabilities, which addresses certain aspects of recognition, measurement, presentation, and disclosure of financial instruments. ASU 2016-01 will be effective for annual periods and interim periods within those annual periods beginning after December 15, 2017 and early adoption is not permitted. The Company is currently evaluating the impact that the standard will have on the financial statements, and has not yet determined what effect, if any, the impact of adoption will be.
In May 2014, the FASB issued new guidance related to revenue recognition, ASU 2014-09, Revenue from Contracts with Customers (“ASC 606”), which outlines a comprehensive revenue recognition model and supersedes most current revenue recognition guidance. The new guidance requires an entity to recognize revenue upon transfer of goods or services to a customer at an amount that reflects the expected consideration to be received in exchange for those goods or services. ASC 606 defines a five-step approach for recognizing revenue, which may require an entity to use more judgment and make more estimates than under the current guidance. The new guidance becomes effective in calendar year 2018 and early adoption in calendar year 2017 is permitted. Two methods of adoption are permitted: (a) full retrospective adoption, meaning the standard is applied to all periods presented; or (b) modified retrospective adoption, meaning the cumulative effect of applying the new guidance is recognized at the date of initial application as an adjustment to the opening retained earnings balance.
In March 2016, April 2016 and December 2016, the FASB issued ASU No. 2016-08, Revenue From Contracts with Customers: Principal Versus Agent Considerations, ASU No. 2016-10, Revenue From Contracts with Customers: Identifying Performance Obligations and Licensing, and ASU No. 2016-20, Technical Corrections and Improvements to Topic 606, Revenue From Contracts with Customers, respectively, which further clarify the implementation guidance on principal versus agent considerations contained in ASU No. 2014-09. In May 2016, the FASB issued ASU 2016-12 Revenue from Contracts with Customers, narrow-scope improvements and practical expedients which provides clarification on assessing the collectability criterion, presentation of sales taxes, measurement date for non-cash consideration and completed contracts at transition (collectively “ASC 606”). These standards will be effective for the Company beginning in the first quarter of 2018. Early adoption is permitted.
The Company has formed a task force that has analyzed the Company’s customer contracts and the impact the standard will have on previously reported revenues and future revenues. Under ASC 606, the Company will recognize net product sales at the time it ships its products to its customers (primarily wholesalers and specialty pharmacies), rather than the current policy of recognizing net product sales when prescriptions are dispensed to patients. As a result, the Company will recognize the majority of net product sales under such contracts earlier under ASC 606 than it would have
F-19
recognized under current guidance. The Company expects that it will record a one-time adjustment to retained earnings at the adoption of the new standard in the first quarter of 2018, which it estimates to be between $2.0 million and $2.5 million.
The Company plans to adopt the new standard effective January 1, 2018 using the modified retrospective approach.
3. Investments
Marketable securities consisted of the following as of December 31, 2017:
|
(in thousands) |
|
Cost Basis |
|
Unrealized Gains |
|
Unrealized Losses |
|
Fair Value |
||||
|
Corporate notes and bonds |
|
$ |
60,000 |
|
$ |
— |
|
$ |
(47) |
|
$ |
59,953 |
|
Total |
|
$ |
60,000 |
|
$ |
— |
|
$ |
(47) |
|
$ |
59,953 |
Marketable securities consisted of the following as of December 31, 2016:
|
(in thousands) |
|
Cost Basis |
|
Unrealized Gains |
|
Unrealized Losses |
|
Fair Value |
||||
|
Corporate notes and bonds |
|
$ |
42,481 |
|
$ |
4 |
|
$ |
(14) |
|
$ |
42,471 |
|
Total |
|
$ |
42,481 |
|
$ |
4 |
|
$ |
(14) |
|
$ |
42,471 |
At December 31, 2017, the Company held 10 marketable securities, which were in a continuous loss position for less than one year. The unrealized losses are the result of current economic and market conditions and the Company has determined that only a temporary impairment existed at December 31, 2017.
The fair value of marketable securities at December 31, 2017 with a maturity of less than one year was $60.0 million. The Company had no marketable securities with a maturity of greater than one year as of December 31, 2017.
4. Fair Value Measurements
The Company measures certain assets and liabilities at fair value in accordance with ASC 820, Fair Value Measurements and Disclosures. ASC 820 defines fair value as the price that would be received to sell an asset or paid to transfer a liability (the exit price) in an orderly transaction between market participants at the measurement date. The guidance in ASC 820 outlines a valuation framework and creates a fair value hierarchy in order to increase the consistency and comparability of fair value measurements and the related disclosures. In determining fair value, the Company maximizes the use of quoted prices and observable inputs. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from independent sources. The fair value hierarchy is broken down into three levels based on the source of inputs as follows:
|
· |
Level 1—Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities. |
|
· |
Level 2—Valuations based on observable inputs and quoted prices in active markets for similar assets and liabilities. |
|
· |
Level 3—Valuations based on inputs that are unobservable and models that are significant to the overall fair value measurement. |
The following fair value hierarchy table presents information about each major category of our financial assets and liabilities measured at fair value on a recurring basis:
F-20
|
(in thousands) |
|
Fair Value Measurements as of December 31, 2017 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
||||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents (money market funds and commercial paper) |
|
$ |
16,973 |
|
$ |
— |
|
$ |
— |
|
$ |
16,973 |
|
Marketable securities, available-for-sale |
|
|
— |
|
|
59,953 |
|
|
— |
|
|
59,953 |
|
Total assets |
|
$ |
16,973 |
|
$ |
59,953 |
|
$ |
— |
|
$ |
76,926 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest make-whole derivatives |
|
$ |
— |
|
$ |
— |
|
$ |
2,589 |
|
$ |
2,589 |
|
Conversion feature, 6.50% Notes |
|
|
— |
|
|
— |
|
|
14,034 |
|
|
14,034 |
|
Warrant liability |
|
|
— |
|
|
— |
|
|
8,166 |
|
|
8,166 |
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
24,789 |
|
$ |
24,789 |
|
(in thousands) |
|
Fair Value Measurements as of December 31, 2016 |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
||||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents (money market funds) |
|
$ |
27,635 |
|
$ |
— |
|
$ |
— |
|
$ |
27,635 |
|
Marketable securities, available-for-sale |
|
|
— |
|
|
42,471 |
|
|
— |
|
|
42,471 |
|
Total assets |
|
$ |
27,635 |
|
$ |
42,471 |
|
$ |
— |
|
$ |
70,106 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest make-whole derivatives |
|
$ |
— |
|
$ |
— |
|
$ |
12 |
|
$ |
12 |
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
12 |
|
$ |
12 |
The 5.50% Notes and 6.50% Notes include an interest make-whole feature whereby if a noteholder converts any of the 5.50% Notes prior to April 1, 2018, or any of the 6.50% Notes prior to July 1, 2021, the Company will, in addition to the other consideration payable or deliverable in connection with such conversion, make an interest make-whole payment to the converting holder equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the notes to be converted had such notes remained outstanding from the conversion date through April 1, 2018 (5.50% Notes), or July 1, 2021 (6.50% Notes), computed using a discount rate equal to 2%.
The embedded conversion options in the 6.50% Notes are required to be separately accounted for as derivatives as the Company did not have enough available authorized shares to cover the conversion obligation as of the date of issuance as of December 31, 2017. In February 2018, the Company received Shareholder approval for an additional 200,000,000 authorized shares, refer to Footnote 18 – Subsequent Event for further details.
The Company has determined that the above features are embedded derivatives and has recognized the fair value of the derivatives as liabilities in the Company’s Consolidated Balance Sheet, with subsequent changes to fair value recorded through earnings at each reporting period on the Company’s Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities.
The following tables set forth a summary of changes in the fair value of Level 3 liabilities for the year ended December 31, 2017:
F-21
|
(in thousands) |
|
|
|
|
|
|
|
|
Fair Value |
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
Change in |
|
|
December 31, |
|
|
|
|
2016 |
|
|
Additions |
|
|
2017 |
|
|
2017 |
|
Interest make-whole derivatives |
|
|
12 |
|
$ |
2,683 |
|
$ |
(106) |
|
$ |
2,589 |
|
Conversion feature, 6.50% Notes |
|
|
— |
|
|
14,973 |
|
|
(939) |
|
|
14,034 |
|
Warrant liability |
|
|
— |
|
|
9,667 |
|
|
(1,501) |
|
|
8,166 |
|
Total liabilities |
|
$ |
12 |
|
$ |
27,323 |
|
$ |
(2,546) |
|
$ |
24,789 |
The following tables set forth a summary of changes in the fair value of Level 3 liabilities for the year ended December 31, 2016:
|
(in thousands) |
|
|
|
|
|
|
|
|
Fair Value |
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
Change in |
|
|
December 31, |
|
|
|
|
2015 |
|
|
Additions |
|
|
2016 |
|
|
2016 |
|
Interest make-whole derivatives |
|
|
656 |
|
$ |
— |
|
$ |
(644) |
|
$ |
12 |
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
(644) |
|
$ |
12 |
The fair value of the Company’s warrant liability was estimated utilizing a lattice tree model both for the initial valuation and as of December 31, 2017. This fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement within the fair value hierarchy. The fair value measurement was based on several factors including:
|
· |
Various future financing scenarios |
|
· |
Volatility of the Company’s Common Stock and risk-free rate |
As of December 31, 2017, the fair value of the Company’s 5.50% Notes and 6.50% Notes and the included interest make whole features, along with the conversion feature of the 6.50% Notes were estimated utilizing the binomial lattice tree model. This fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement within the fair value hierarchy. The fair value measurement was based on several factors including:
Credit spread at the valuation date
Discount yield as of the valuation date
The fair value and carrying value of the Company’s 5.50% Notes and 6.50% Notes at December 31, 2017 were as follows:
|
(in thousands) |
|
Fair Value |
|
Carrying Value |
|
Face Value |
|||
|
|
|
|
|
|
|
|
|
|
|
|
5.50% Notes due 2020 |
|
$ |
11,699 |
|
$ |
20,428 |
|
$ |
24,650 |
|
6.50% Notes due 2024 |
|
$ |
4,643 |
|
$ |
2,969 |
|
$ |
23,888 |
The fair value of the Company’s 13.0% notes approximates its carrying value of $75.5 million as the interest rate is reflective of the interest rates on debt the Company could currently obtain with similar terms and conditions and thus represents a Level 2 measurement within the fair value hierarchy.
F-22
5. Inventory
Inventory is stated at the lower of cost or market using actual cost net of reserve for excess and obsolete inventory. The following represents the components of inventory at December 31, 2016 and 2017.
|
|
|
December 31, |
||||
|
(in thousands) |
|
2016 |
|
2017 |
||
|
Raw materials |
|
$ |
779 |
|
$ |
850 |
|
Work in process |
|
|
— |
|
|
772 |
|
Finished goods |
|
|
820 |
|
|
1,446 |
|
Deferred cost of sales |
|
|
101 |
|
|
157 |
|
Total |
|
$ |
1,700 |
|
$ |
3,225 |
During the years ended December 31, 2016 and 2017 the Company recorded a reserve for excess and obsolete inventory of $477,000 and $542,000, respectively.
6. Property and Equipment
Property and equipment and related accumulated depreciation are as follows:
|
|
|
December 31, |
|
||||
|
(in thousands) |
|
2016 |
|
2017 |
|
||
|
Laboratory and manufacturing equipment |
|
$ |
16,288 |
|
$ |
14,911 |
|
|
Furniture, fixtures and other property |
|
|
779 |
|
|
888 |
|
|
Construction in process |
|
|
— |
|
|
241 |
|
|
Less accumulated depreciation |
|
|
(4,358) |
|
|
(6,129) |
|
|
Property and equipment, net |
|
$ |
12,709 |
|
$ |
9,911 |
|
Depreciation expense was $1.1 million $2.1 million and $2.8 million for the years ended December 31, 2015, 2016 and 2017, respectively.
F-23
7. Intangible Assets
The following represents the balance of the intangible assets including accumulated amortization at December 31, 2017:
|
|
|
Gross |
|
|
|
|
Net |
|
Remaining Useful |
|
||
|
|
|
Intangible |
|
Accumulated |
|
Intangible |
|
Life |
|
|||
|
(in thousands) |
|
Assets |
|
Amortization |
|
Assets |
|
(in years) |
|
|||
|
OXAYDO product rights |
|
$ |
7,695 |
|
$ |
(3,273) |
|
$ |
4,422 |
|
4.00 |
|
|
SPRIX Nasal Spray product rights |
|
|
4,978 |
|
|
(2,964) |
|
|
2,014 |
|
2.00 |
|
|
IP R&D |
|
|
183 |
|
|
(36) |
|
|
147 |
|
4.00 |
|
|
Total |
|
$ |
12,856 |
|
$ |
(6,273) |
|
$ |
6,583 |
|
|
|
The following represents the balance of the intangible assets including accumulated amortization at December 31, 2016:
|
|
|
Gross |
|
|
|
|
Net |
|
Remaining Useful |
||
|
|
|
Intangible |
|
Accumulated |
|
Intangible |
|
Life |
|||
|
(in thousands) |
|
Assets |
|
Amortization |
|
Assets |
|
(in years) |
|||
|
OXAYDO product rights |
|
$ |
7,520 |
|
$ |
(2,124) |
|
$ |
5,396 |
|
5.00 |
|
SPRIX Nasal Spray product rights |
|
|
4,620 |
|
|
(1,827) |
|
|
2,793 |
|
3.00 |
|
IP R&D |
|
|
161 |
|
|
— |
|
|
161 |
|
Indefinite |
|
Total |
|
$ |
12,301 |
|
$ |
(3,951) |
|
$ |
8,350 |
|
|
There was no impairment to intangible assets recognized in the years ended December 31, 2016 and 2017.
Estimated amortization of the intangible assets for the five years subsequent to December 31, 2017 is as follows:
|
(in thousands) |
|
|
|
|
2018 |
|
$ |
2,136 |
|
2019 |
|
$ |
2,136 |
|
2020 |
|
$ |
1,145 |
|
2021 |
|
$ |
1,136 |
|
2022 |
|
$ |
30 |
Collaboration and License Agreement with Acura
In January 2015, the Company entered into a Collaboration and License Agreement with Acura Pharmaceuticals, Inc. (“Acura”) to commercialize OXAYDO (oxycodone hydrochloride) tablets containing Acura’s Aversion® Technology. The Company paid Acura an upfront payment of $5.0 million in January 2015 and a $2.5 million milestone payment in October 2016 as a result of the first commercial sale of OXAYDO. The Company also incurred transaction costs of $172,000 associated with the transaction. Refer to Note 15 — Acquisitions and license and collaboration agreements for additional details.
During each of the years ended December 31, 2015, 2016 and 2017, the Company recognized amortization expense of $1.1 million related to the OXAYDO product right intangible asset.
Purchase Agreement with Luitpold
In January 2015, the Company entered into a purchase agreement with Luitpold Pharmaceuticals, Inc. (“Luitpold”). Pursuant to the purchase agreement, the Company acquired specified assets and liabilities associated with
F-24
SPRIX (ketorolac tromethamine) Nasal Spray for a purchase price of $7.0 million. The Company recorded an intangible asset of $4.6 million related to this transaction. Refer to Note 15 – Acquisitions and license and collaboration agreements for additional details.
During the years ended December 31, 2015, 2016 and 2017, the Company recognized amortization expense of $903,000, $924,000 and $960,000, respectively, related to the SPRIX Nasal Spray product rights intangible asset.
In-Process Research and Development (“IP R&D”)
In connection with the acquisition of Egalet A/S in 2010, the Company recognized an IP R&D asset related to the drug delivery platform specifically designed to help deter physical abuse of pain medications. Through December 31, 2016, the IP R&D was considered an indefinite-lived intangible asset and was assessed for impairment annually or more frequently if impairment indicators exist. Following the approval of ARYMO ER in January 2017, the Company began to amortize the intangible asset over a useful life of five years. For the year ended December 31, 2017, the Company recognized amortization expense of $36,000. There was no amortization expense recognized for the years ended December 31, 2015 and 2016.
8. Accrued Expenses
Accrued expenses were as follows:
|
(in thousands) |
|
December 31, |
||||
|
|
|
2016 |
|
2017 |
||
|
Sales allowances |
|
$ |
1,192 |
|
$ |
4,721 |
|
Payroll and bonuses |
|
|
3,927 |
|
|
4,349 |
|
Interest |
|
|
2,600 |
|
|
3,270 |
|
Sales and marketing |
|
|
3,182 |
|
|
1,247 |
|
Royalties |
|
|
672 |
|
|
800 |
|
Professional services |
|
|
798 |
|
|
627 |
|
Manufacturing services |
|
|
996 |
|
|
579 |
|
Clinical research |
|
|
4,128 |
|
|
355 |
|
Other |
|
|
652 |
|
|
156 |
|
|
|
$ |
18,147 |
|
$ |
16,104 |
9. Long Term Debt
Hercules Loan and Security Agreement
In January 2015, the Company entered into the Loan Agreement with Hercules and certain other lenders, pursuant to which the Company borrowed $15.0 million under a term loan. The term loan bore an interest rate per annum equal to the greater of either (i) 9.40% plus the prime rate as reported in The Wall Street Journal minus 3.25% or (ii) 9.40%. Pursuant to the terms of the Loan Agreement, the Company made interest-only payments for 12 months beginning on February 1, 2015, and then was scheduled to repay the principal balance of the loan in 30 equal monthly payments of principal and interest through the scheduled maturity date of July 1, 2018. In connection with the Loan Agreement, the Company granted a security interest in substantially all of its assets, excluding intellectual property and certain new drug applications and related approvals, as collateral for the obligations under the Loan Agreement.
The Loan Agreement also contained representations and warranties, and indemnification in favor of Hercules. The Company was required to comply with various customary covenants, including, among others, restrictions on indebtedness, investments, distributions, transfers of assets and acquisitions. The Loan Agreement contained several events of default, including, among others, payment defaults, breaches of covenants or representations, material
F-25
impairment in the perfection of Hercules’ security interest or in the collateral and events related to bankruptcy or insolvency. Upon an event of default, Hercules could declare all outstanding obligations immediately due and payable, and Hercules could take such further actions as set forth in the Loan Agreement, including collecting or taking such other action with respect to the collateral pledged in connection with the Loan Agreement.
In connection with the Loan Agreement, the Company issued Hercules a warrant (the “Warrant”) to purchase $600,000 in shares of the Company’s Common Stock at an exercise price of $5.29 per share (or, approximately 113,421 shares of Common Stock). The Warrant was considered a standalone instrument since it could be exercised separately from the Loan Agreement. The Warrant was exercisable for a period of five years beginning on the date of issuance and had a fair value of $328,610 that is included in stockholders’ equity. The fair value of the Warrant was recorded as a debt discount and was determined through the use of a Black-Scholes calculation using the below assumptions:
|
Risk-free interest rate |
|
1.5 |
% |
|
Expected term (in years) |
|
5 |
|
|
Expected volatility |
|
71.68 |
% |
|
Dividend yield |
|
— |
|
On August 3, 2015, Hercules exercised the warrant in full, electing the net issuance option. As a result, the Company issued 61,644 shares of the Company’s Common Stock to Hercules.
On December 9, 2015, the Company entered into an amendment (the “Amendment”) to the Loan agreement which, among other things, provides that the interest-only period of the term loan would be extended for an additional six months to July 1, 2016. The Amendment also provided that the interest-only period may be further extended to January 1, 2017, subject to the FDA’s acceptance of the Company’s new drug application for its product candidate ARYMO ER, the Company’s receipt of at least $5.0 million of product revenue for any consecutive three-month period prior to June 30, 2016 and there being no default or event of default under the Loan Agreement.
On August 31, 2016, the Company repaid all outstanding obligations under the Loan Agreement with the proceeds of the 13% Notes (as defined below). As a result of the repayment, the Company recorded debt extinguishment costs of $800,000 during the year ended December 31, 2016 which is classified as interest expense on the Company’s Consolidated Statement of Operations.
5.50% Convertible Senior Notes Due 2020
On April 7, 2015, the Company completed the issuance through a private placement of $60.0 million in aggregate principal amount of the 5.50% Notes. On May 6, 2015, the Company issued an additional $1.0 million in principal amount pursuant to the initial purchasers’ exercise of their 30-day over-allotment for aggregate gross proceeds of $61.0 million. Interest on the 5.50% Notes is payable semi-annually in arrears on April 1 and October 1 of each year, commencing October 1, 2015.
The 5.50% Notes are general, unsecured and unsubordinated obligations and will rank senior in right of payment to all of the Company’s indebtedness that is expressly subordinated in right of payment to the notes. The 5.50% Notes rank equal in right of payment to the Company’s existing and future indebtedness and other liabilities that are not so subordinated. The 5.50% Notes are effectively subordinated to any of the Company’s future secured indebtedness to the extent of the value of the assets securing such indebtedness and rank structurally junior to all indebtedness and other liabilities incurred by the Company’s subsidiaries, including trade payables.
The Company may not redeem the 5.50% Notes prior to maturity. The 5.50% Notes are convertible prior to maturity, subject to certain conditions described below, into shares of the Company’s Common Stock at an initial conversion rate of 67.2518 shares per $1,000 principal amount of the 5.50% Notes (equivalent to an initial conversion price of approximately $14.87 per share of Common Stock). This conversion rate is subject to adjustment upon the occurrence of certain specified events but will not be adjusted for accrued and unpaid interest. The Company will satisfy the conversion obligation by paying or delivering, as the case may be, cash, shares of the Company’s Common Stock or a combination thereof, at the Company’s election.
F-26
Holders may convert all or any portion of their notes, in multiples of $1,000 principal amount, at their option at any time prior to the close of business on the business day immediately preceding January 1, 2020 only under the following circumstances:
on or after the date that is six months after the last date of original issuance of the notes, if the last reported sale price of the Company’s Common Stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending within the five trading days immediately preceding a conversion date is greater than or equal to the conversion price for the notes on each applicable trading day;
during the five-business day period after any five consecutive trading day period, the measurement period in which the trading price per $1,000 principal amount of notes for each trading day of the measurement period was less than 98% of the product of the last reported sale price of the Company’s Common Stock and the conversion rate on each such trading day; or
upon the occurrence of specified corporate events.
On or after January 1, 2020 until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or any portion of their notes, in multiples of $1,000 principal amount, at the option of the holder regardless of the foregoing circumstances.
The conversion rate for the 5.50% Notes is initially 67.2518 shares of Common Stock per $1,000 principal amount of notes (equivalent to an initial conversion price of approximately $14.87 per share of Common Stock), subject to adjustment.
Upon conversion, the Company will pay or deliver, as the case may be, cash, shares of Common Stock or a combination of cash and shares of the Company’s Common Stock, at the Company’s election, and an interest make-whole payment in shares of the Common Stock, if applicable. If the Company satisfies the conversion obligation solely in cash or through payment and delivery, as the case may be, of a combination of cash and shares Common Stock, the amount of cash and shares of Common Stock, if any, due upon conversion will be based on a daily conversion value calculated on a proportionate basis for each trading day in a 50-trading day observation period.
In addition, following certain corporate events that occur prior to the maturity date, the Company will increase the conversion rate for a holder who elects to convert its 5.50% Notes in connection with such a corporate event in certain circumstances. Holders will not receive any additional cash payment or additional shares representing accrued and unpaid interest, if any, upon conversion of a 5.50% Note, except in limited circumstances. Instead, interest will be deemed to be paid by the cash, shares the Company’s Common Stock or a combination of cash and shares of the Company’s Common Stock paid or delivered, as the case may be, to the holders upon conversion of a 5.50% Note.
On or after the date that is six months after the last date of original issuance of the 5.50% Notes, if the last reported sale price of the Company’s Common Stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending within the five trading days immediately preceding a conversion date is greater than or equal to the conversion price for the 5.50% Notes on each applicable trading day, the Company will, in addition to the other consideration payable or deliverable in connection with such conversion, make an interest make-whole payment to the converting holder equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the 5.50% Notes to be converted had such 5.50% Notes remained outstanding from the conversion date through April 1, 2018, computed using a discount rate equal to 2%. The Company will pay any interest make-whole payment by delivering shares of the Company’s Common Stock valued at 95% of the simple average of the daily volume weighted average price for the 10 trading days ending on and including the trading day immediately preceding the conversion date. Notwithstanding the foregoing, the number of shares the Company may deliver in connection with a conversion of the 5.50% Notes, including those delivered in connection with an interest make-whole payment, will not exceed 77.3395 shares of the Company’s Common Stock per $1,000 principal amount of 5.50% Notes, subject to adjustment. The Company will not be required to make any cash payments in lieu of any fractional shares or have any further obligation to deliver any shares of Common Stock or pay any cash in excess of the
F-27
threshold described above. In addition, if in connection with any conversion the conversion rate is adjusted, then such holder will not receive the interest make-whole payment with respect to such 5.50% Notes.
Certain provisions in the 5.50% notes could require accelerated payment of principal and interest. The 5.50% Notes provide that the delisting of our Common Stock from the Nasdaq Global Market would constitute a “fundamental change” under the 5.50% Notes, which would entitle the holder, at the holder’s option, to require the Company to repurchase for cash all or any portion of such holder’s 5.50% Notes at a repurchase price equal to 100% of the principal amount thereof, plus accrued and unpaid interest thereon. On November 24, 2017, we received a notice from The Nasdaq Stock Market ("Nasdaq") that we were not in compliance with Nasdaq's Listing Rule 5450(b)(2)(A) (the "Rule"), as the minimum market value of our Common Stock had been below $50 million for 30 consecutive business days. Further, on March 8, 2018, we received a notice from Nasdaq that since the minimum closing bid price for our Common Stock had been below $1.00 for 30 consecutive business days, we did not meet the minimum closing bid price requirement in the Rule. Refer to Note 1- Organization and Description of the Business for further details.
The Company accounts for convertible debt instruments by recording the liability and equity components of the convertible debt separately. The liability is computed based on the fair value of a similar debt instrument that does not include the conversion option. The liability component includes both the value of the embedded interest make-whole derivative and the carrying value of the 5.50% Notes. The equity component is computed based on the total debt proceeds less the fair value of the liability component. The equity component is also recorded as debt discount and amortized as interest expense over the expected term of the 5.50% Notes, using the effective interest method.
The liability component of the 5.50% Notes on the date of issuance was computed as $41.6 million, including the value of the embedded interest make-whole derivative of $0.9 million and the carrying value of the 5.50% Notes of $40.6 million. Accordingly, the equity component on the date of issuance was $19.4 million. The discount on the 5.50% Notes is being amortized to interest expense over the term of the 5.50% Notes, using the effective interest method.
The conversion criteria for the 5.50% Notes have not been met at December 31, 2017. Should the 5.50% Notes become convertible, management will determine whether the intent is to settle in cash which would result in the liability component of the 5.50% Notes being classified as a current liability and the equity component being presented as redeemable equity if the liability is considered current.
Transaction costs of $4.1 million related to the issuance of the 5.50% Notes are allocated to the liability and equity components in proportion to the allocation of the proceeds and accounted for as debt discount and equity issuance costs, respectively. Approximately $1.3 million of the transaction costs were allocated to equity and the remaining $2.8 million was recorded as debt discount.
In December 2017, the Company exchanged, with certain existing 5.50% Noteholders, $36.4 million in principal value of the 5.50% Notes for 6.50% Notes in the amount of $23.9 million. This exchange was accounted for as a debt extinguishment and the gain on debt extinguishment of $13.2 million, inclusive of the make-whole payments and write-off of deferred financing fees is reflected in the Company’s Consolidated Statements of Operations during the year ended December 31, 2017.
The following table summarizes how the issuance of the 5.50% Notes is reflected in the Company’s Consolidated Balance Sheets at December 31, 2016 and 2017:
|
|
|
December 31, 2016 |
|
December 31, 2017 |
||
|
(in thousands) |
|
|
|
|
|
|
|
Principal |
|
$ |
61,000 |
|
$ |
24,650 |
|
Unamortized debt discount |
|
|
(15,091) |
|
|
(4,222) |
|
Carrying value |
|
$ |
45,909 |
|
$ |
20,428 |
F-28
6.50% Convertible Notes due 2024
In December 2017, the Company entered into exchange agreements (the “Exchange Agreements”) with certain holders (the “Holders”) of the Company’s 5.50% Notes pursuant to which the Holders agreed to exchange, in the aggregate, approximately $36.4 million of outstanding principal amount of the 5.50% Notes for, in the aggregate, (i) approximately $23.9 million of the Company’s new 6.50% Notes, (ii) a warrant exercisable for 3.5 million shares of the Company’s Common Stock at an exercise price of $0.01 per share and (iii) payments, in cash, of all accrued but unpaid interest as of the closing on the 5.50% Notes exchanged in the transaction (the “Exchange”). At the closing of the Exchange, 2.5 million warrants were exercised. The remaining 1.0 million warrants were exercised in January 2018.
The Company consummated the Exchange in reliance upon the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended (the “Securities Act”), and the 6.50% Notes and any Common Stock issuable upon conversion of the 6.50% Notes have not been registered under the Securities Act and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act. The 6.50% Notes were issued pursuant to an indenture (the “Indenture”), dated December 27, 2017, by and among the Company, the subsidiary guarantors party thereto as of the date thereof, and The Bank of New York Mellon, as trustee (the “Trustee”).
The 6.50% Notes will pay interest semiannually in arrears on January 1 and July 1 of each year commencing July 1, 2018 at a rate of 6.50% per year, which rate is subject to adjustment in accordance with the terms of the Indenture and as described below. The 6.50% Notes are general unsecured obligations of the Company and rank equally in right of payment with all of its other existing and future senior unsecured indebtedness and senior in right of payment to all of its existing and future subordinated indebtedness. The 6.50% Notes will mature on December 31, 2024, unless earlier repurchased, redeemed or converted in accordance with the terms of the Indenture prior to such date. Subject to certain conditions, on or after January 1, 2021, the Company may redeem for cash all or a part of the 6.50% Notes. The 6.50% Notes will be convertible at any time until the close of business on the business day immediately preceding the maturity date. Upon conversion and subject to certain conditions, holders of the 6.50% Notes will receive shares of the Company’s Common Stock at an initial conversion rate of 749.6252 shares of Common Stock per $1,000 principal amount of 6.50% Notes, which is equivalent to an initial conversion price of approximately $1.33 per share, and is subject to adjustment under the terms of the Indenture. Similar to the 5.50% Notes, the 6.50% Notes will provide for an interest make-whole payment in connection with conversions that occur prior to January 1, 2021. For any Conversion Date that occurs prior to the close of business on the business day immediately preceding January 1, 2021, the Company shall, in addition to the other consideration payable or deliverable in connection with any conversion of Notes, make an interest make-whole payment in cash or in shares of Common Stock, at the Company’s election, to such converting Holder equal to the sum of the present value of the remaining scheduled payments of interest that would have been made on the Notes to be converted had such Notes remained outstanding from the Conversion Date through January 1, 2021. The present values will be computed using a discount rate equal to 2% by a U.S. nationally recognized independent investment banking firm.
If an event of default (as defined in the Indenture) occurs and is continuing (other than specified events of bankruptcy or insolvency with respect to the Company), the Trustee or the holders of at least 25% in aggregate principal amount of the outstanding 6.50% Notes may declare all the outstanding 6.50% Notes to be due and payable immediately. If an event of default relating to specified events of bankruptcy or insolvency with respect to the Company occurs, all the outstanding 6.50% Notes will immediately become due and payable without any declaration or other act on the part of the trustee or any holders of the 6.50% Notes. Notwithstanding the foregoing, the Indenture provides that, to the extent the Company elects, the sole remedy for an event of default relating to certain failures by the Company to comply with certain reporting covenants in the Indenture will, for the first 180 days after such event of default, consist exclusively of the right to receive additional interest on the 6.50% Notes.
In addition, the Indenture requires the Company to use its reasonable best efforts to (i) seek stockholder approval of an amendment to the Company’s Third Amended & Restated Certificate of Incorporation, as amended, in order to increase the amount of authorized shares available for issuance thereunder, and (ii) if and when such approval is obtained, to reserve from such amount the number of shares that may be issued in respect of the 6.50% Notes and any other securities issued in connection with the Exchange. In the Exchange Agreements, the Holders have granted a proxy
F-29
to the Company authorizing the Company to vote any shares of Common Stock held by such Holder in favor of such amendment at any applicable meeting of the Company’s stockholders. If the Company is unable to obtain stockholder approval on or prior to July 1, 2018, the interest rate of the 6.50% Notes will increase to 10.0% unless and until such stockholder approval is obtained. The Exchange Agreements also provide that, for a period of nine months, the Company will not enter into additional exchange transactions with the other holders of the 5.50% Notes the economic terms of which, taken is a whole, are more favorable to the 5.50% Note holders than the December 2017 Exchange. In February 2018, the Company received stockholder approval to increase authorized shares by 200,000,000 additional shares. Refer to Footnote 18 – Subsequent Events for further details.
Certain provisions in the 6.50% notes could require accelerated payment of principal and interest. The 6.50% Notes provide that the delisting of the Company’s Common Stock from the Nasdaq exchange would constitute a “fundamental change” under the 6.50% Notes, which would entitle the holder, at the holder’s option, to require the Company to repurchase for cash all or any portion of such holder’s 6.50% Notes at a repurchase price equal to 100% of the principal amount thereof, plus accrued and unpaid interest thereon. On November 24, 2017, the Company received a notice from The Nasdaq Stock Market ("Nasdaq") that it was not in compliance with Nasdaq Global Market’s Listing Rule 5450(b)(2)(A) (the "Rule"), as the minimum market value of our Common Stock had been below $50 million for 30 consecutive business days. Further, on March 8, 2018, the Company received a notice from Nasdaq that since the minimum closing bid price for the Company’s Common Stock had been below $1.00 for 30 consecutive business days, it did not meet the minimum closing bid price requirement in the Rule. The Company has the option to move to the Nasdaq Capital Market if it is delisted from the Nasdaq Global Market, provided it meets the minimum listing requirements for the Nasdaq Capital Market at the time of the move. The move from the Nasdaq Global Market to the Nasdaq Capital Market would not be considered a fundamental change under the 6.50% Notes. Refer to Note 1- Organization and Description of the Business for further details.
At the date of Exchange, December 27, 2017, the Company did not have sufficient unissued authorized shares to cover the conversion of the outstanding 6.50% Notes and as a result was required to account for the bifurcated conversion feature as a derivative liability which results in a debt discount on the 6.50% Notes. The fair value of the derivative liability for the conversion feature at the date of Exchange was determined to be approximately $15.0 million and is classified as a liability in the Company’s Consolidated Balance Sheet, with subsequent changes to fair value recorded through earnings at each reporting period on the Company’s Consolidated Statements of Operations and Comprehensive Loss as change in fair value of derivative liabilities. As of December 31, 2017, the derivative liability for the conversion feature on the 6.50% Notes was $14.0 million.
The conversion criteria for the 6.50% Notes have not been met at December 31, 2017. Should the 6.50% Notes become convertible, management will determine whether the intent is to settle in cash which would result in the liability component of the 6.50% Notes being classified as a current liability and the equity component being presented as redeemable equity if the liability is considered current.
Transaction costs of $1.7 million were incurred related to the issuance of the 6.50% Notes are accounted for as debt discount.
The following table summarizes how the issuance of the 6.50% Notes is reflected in the Company’s Consolidated Balance Sheets at December 31, 2017:
|
|
|
December 31, 2017 |
|
|
(in thousands) |
|
|
|
|
Principal |
|
$ |
23,888 |
|
Unamortized debt discount |
|
|
(20,919) |
|
Carrying value |
|
$ |
2,969 |
13% Senior Secured Notes (the “13% Notes”)
F-30
On August 31, 2016, the Company completed the Initial Closing of its Offering of up to $80.0 million in aggregate principal amount of its 13% Notes and entered into the Indenture governing the 13% Notes with the Guarantors and U.S. Bank as the Trustee and Collateral Agent.
The Company issued $40.0 million aggregate principal amount of the 13% Notes at the Initial Closing, and issued an additional $40.0 million aggregate principal amount of the Notes on approval from the FDA of ARYMO™ ER in January 2017 (the “Second Closing”). Net proceeds from the Initial Closing and Second Closing were $37.2 million, and $38.3 million respectively, after deducting the estimated Offering expenses payable by the Company in connection with the Initial Closing and Second Closing. The Notes were sold only to qualified institutional buyers within the meaning of Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”).
The Company has used and will use the net proceeds from the 13% Notes and the Royalty Rights (as defined below) to repay all outstanding obligations to Hercules under the Loan Agreement with Hercules, to support the approval and commercialization of ARYMO ER, to support the development of Egalet-002 and for general corporate purposes.
Prior to the Second Closing, interest on the 13% Notes accrued at a rate of 13% per annum and was payable semi-annually in arrears on March 20 and September 20 of each year (each, a “Payment Date”) commencing on March 20, 2017. On each Payment Date commencing on March 20, 2018, the Company was obligated to also pay an installment of principal of the Notes pursuant to a straight-line fixed amortization schedule. Following the approval of ARYMO ER from the FDA in January 2017, in lieu of the straight-line fixed amortization schedule, on each Payment Date commencing on March 20, 2018, the Company will pay an installment of principal on the Notes in an amount equal to 15% (or 17% if certain sales targets are not met) of the aggregate net sales of OXAYDO, SPRIX Nasal Spray, ARYMO ER and Egalet-002, if approved, for the two consecutive fiscal quarterly period most recently ended, less the amount of interest payable on the 13% Notes on such Payment Date.
The Notes are senior secured obligations of the Company and will be equal in right of payment to all existing and future indebtedness of the Company (including the Company’s outstanding 5.50% convertible senior notes due 2020), will be senior in right of payment to all existing and future subordinated indebtedness of the Company, will have the benefit of a security interest in the Notes collateral and will be junior in lien priority in respect of any collateral that secures any first priority lien obligations incurred, which includes intellectual property, from time to time in accordance with the Indenture. The stated maturity date prior to the approval of ARYMO ER of the 13% Notes was March 20, 2020. Following ARYMO ER approval by the FDA in January 2017, the stated maturity date of the Notes will be September 30, 2033. Upon the occurrence of a Change of Control, subject to certain conditions, or certain Asset Sales events (each, as defined in the Indenture), holders of the Notes may require the Company to repurchase for cash all or part of their Notes at a repurchase price equal to 101.00% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest to the date of repurchase.
The Company may redeem the 13% Notes at its option, in whole or in part from time to time, prior to August 31, 2018, at a redemption price equal to 100.00% of the principal amount of the Notes being redeemed, plus accrued and unpaid interest, if any, through the redemption date, plus a make-whole premium computed using a discount rate equal to the treasury rate in respect of such redemption date plus 100 basis points. The Company may redeem the 13% Notes at its option, in whole or in part from time to time, on or after August 31, 2018 at a redemption price equal to: (i) from and including August 31, 2018 to and including August 30, 2019, 109.00% of the principal amount of the 13% Notes to be redeemed, (ii) from and including August 31, 2019 to and including August 30, 2020, 104.50% of the principal amount of the 13% Notes to be redeemed, and (iii) from and including August 31, 2020 and thereafter, 100.00% of the principal amount of the 13% Notes to be redeemed, in each case, plus accrued and unpaid interest to the redemption date. In addition, prior to August 31, 2018, the Company may redeem, at its option, up to 35% of the aggregate principal amount of the 13% Notes with the proceeds of one or more public or private equity offerings at a redemption price equal to 113.50% of the aggregate principal amount of the 13% Notes to be redeemed, plus accrued and unpaid interest to the date of redemption in accordance with the Indenture; provided that at least 65% of the aggregate principal amount of 13% Notes issued under the Indenture remains outstanding immediately after each such redemption and provided further that each such redemption occurs within 90 days of the date of closing of each such equity offering. No sinking fund is provided for the 13% Notes, which means that the Company is not required to periodically redeem or retire the 13% Notes.
F-31
The obligations of the Company under the Indenture and the Notes are unconditionally guaranteed on a secured basis by the Guarantors. Under the terms of the Indenture, the Company may designate entities within its corporate structure as unrestricted subsidiaries, which entities will therefore not be guarantors provided that certain conditions set forth in the Indenture are met.
Pursuant to the Indenture, the Company and its restricted subsidiaries must also comply with certain affirmative covenants, such as furnishing financial statements to the holders of the Notes, and negative covenants, including limitations on the following: the incurrence of debt; the issuance of preferred and/or disqualified stock; the payment of dividends, the repurchase of shares and under certain conditions making certain other restricted payments; the prepayment, redemption or repurchase of subordinated debt; the merger, amalgamation or consolidation involving the Company; engaging in certain transactions with affiliates; and the making of investments other than those permitted by the Indenture.
The Indenture governing the 13% Notes contains customary events of default with respect to the 13% Notes (including the Company’s failure to make any payment of principal or interest on the 13% Notes when due and payable), and upon certain events of default occurring and continuing, the Trustee by notice to the Company, or the holders of at least 25% in principal amount of the outstanding 13% Notes by notice to the Company and the Trustee, may (subject to the provisions of the Indenture) declare 100% of the principal of and accrued and unpaid interest, if any, on all of the 13% Notes to be due and payable. Upon such a declaration of acceleration, such principal and accrued and unpaid interest, if any, as well as the then-applicable optional redemption premium under the Indenture, will be due and payable immediately. In the case of certain events of bankruptcy, insolvency or reorganization involving the Company or a Restricted Subsidiary (as defined in the Indenture), the 13% Notes will automatically become due and payable.
In connection with the Offering on August 31, 2016, the Company entered into royalty rights agreements with each of the Purchasers pursuant to which the Company sold to such Purchasers the right to receive, in the aggregate, a payment equal to 1.5% of the aggregate net sales of OXAYDO and SPRIX Nasal Spray from the Initial Closing through December 31, 2019, inclusive (the “Royalty Rights”). Following the approval of ARYMO ER in January 2017, Royalty Rights will continue through December 31, 2020 and include royalties of ARYMO ER as described below.
The Company also entered into separate royalty rights agreements with each of the Purchasers pursuant to which the Company sold to such Purchasers the right to receive 1.5% of the aggregate net sales of ARYMO ER payable from the date of first sale of ARYMO ER through December 31, 2020, inclusive. The royalty rights agreements also include other terms and conditions customary in agreements of this type.
The Company incurred fees and legal expenses of $4.5 million in connection with the issuance of the 13% Notes, which have been recorded as a discount on the debt in the Company’s consolidated balance sheets and are amortized using the effective interest method. The Company calculated an effective interest rate of 14.6% upon origination of the 13% Notes based on its best estimate of future cash outflows.
The Royalty Rights were determined to be a freestanding element with respect to the 13% Notes and the Company is accounting for the Royalty Rights obligation relating to future royalties as a debt instrument. The Company has Royalty Rights obligations of $4.1 million as of December 31, 2017, which are classified as current and non-current debt in the consolidated balance sheet.
The accounting for the 13% Notes requires the Company to make certain estimates and assumptions about the future net sales of ARYMO ER, OXAYDO and SPRIX Nasal Spray in the U.S. The estimates of the magnitude and timing of ARYMO ER, OXAYDO and SPRIX Nasal Spray net sales are subject to significant variability due to the recent product launch and the extended time period associated with the financing transaction, and are thus subject to significant uncertainty. Therefore, these estimates and assumptions are likely to change as the Company gains experience marketing ARYMO ER, OXAYDO and SPRIX Nasal Spray, which may result in future adjustments to the portion of the debt that is classified as a current liability, the amortization of debt issuance costs and discount as well as the accretion of the interest expense. Any such adjustments could be material. The fair value of the Royalty Rights associated with
F-32
certain net product sales was estimated to be approximately $5.0 million using a probability-weighted present value analysis.
The following table summarizes how the issuance of the 13% Notes is reflected in the Company’s Consolidated Balance Sheet at December 31, 2016 and 2017:
|
|
|
December 31, 2016 |
|
December 31, 2017 |
||
|
(in thousands) |
|
|
|
|
|
|
|
Gross proceeds |
|
$ |
40,000 |
|
$ |
80,000 |
|
Unamortized debt discount |
|
|
(5,187) |
|
|
(7,572) |
|
Carrying value |
|
$ |
34,813 |
|
$ |
72,428 |
Current and non-current debt on the Company’s consolidated balance sheet at December 31, 2016 includes the carrying value of the 5.50% Notes and the 13% Notes, as well as $3.3 million, for the Royalty Rights issued in connection with the debt.
Current and non-current debt on the Company’s Consolidated Balance Sheet at December 31, 2017 includes the carrying value of the 5.50% Notes, 6.50% Notes and the 13% Notes, as well as $4.1 million for the Royalty Rights issued in connection with the debt.
The following table sets forth the Company’s net interest expense incurred for the year ended December 31, 2015, 2016 and 2017:
|
|
|
Year Ended |
|
Year Ended |
Year Ended |
|||
|
|
|
December 31, |
|
December 31, |
December 31, |
|||
|
(in thousands) |
|
2015 |
|
2016 |
2017 |
|||
|
5.50% Notes |
$ |
5,865 |
|
$ |
7,999 |
|
$ |
7,998 |
|
6.50% Notes |
|
— |
|
|
— |
|
|
50 |
|
13% Notes |
|
— |
|
|
2,713 |
|
|
10,506 |
|
Hercules Loan and Security Agreement |
|
2,005 |
|
|
2,078 |
|
|
— |
|
Amortization of premium on marketable securities |
|
832 |
|
|
601 |
|
|
(38) |
|
Interest income on investments |
|
(1,225) |
|
|
(1,282) |
|
|
(850) |
|
Total |
$ |
7,477 |
|
$ |
12,109 |
|
$ |
17,666 |
F-33
The following table sets forth the Company’s future principal payments as of December 31, 2017, and excludes payments to be made under the Royalty Rights agreements, which are in included in the carrying value of the Company’s current and non-current debt on the Company’s consolidated balance sheet at December 31, 2017.
|
(in thousands) |
|
|
|
|
2018 |
|
$ |
— |
|
2019 |
|
|
320 |
|
2020 |
|
|
27,943 |
|
2021 |
|
|
6,092 |
|
2022 |
|
|
8,563 |
|
2023 |
|
|
10,632 |
|
2024 |
|
|
35,947 |
|
2025 |
|
|
13,678 |
|
2026 |
|
|
15,514 |
|
2027 |
|
|
9,849 |
10. Stock-Based Compensation Expense
2013 Stock-Based Incentive Plan
In November 2013, the Company adopted its 2013 Stock-Based Incentive Plan (as subsequently amended and restated from time to time, the “2013 Plan”). Pursuant to the Plan, the Company’s compensation committee is authorized to grant equity-based incentive awards to its directors, executive officers and other employees and service providers, including officers, employees and service providers of its subsidiaries and affiliates. The number of shares of the Company’s Common Stock initially reserved for issuance under the 2013 Plan was 1,680,000, in the form of restricted stock and stock options. Share increases of 2,000,000 and 2,600,000 to the number of shares originally reserved for issuance under the 2013 Plan were authorized by the Company’s stockholders in June 2014 and June 2016, respectively. The amount, terms of grants and exercisability provisions are determined by the compensation committee. The term of the stock options may be up to 10 years, and stock options are exercisable in cash or as otherwise determined by the compensation committee. All stock options vest over time as stipulated in the individual award agreements. In September 2015, the compensation committee voted to amend the 2013 Plan to, among other things, allow for monthly vesting of stock options granted thereunder.
2017 Inducement Plan
In December 2016, the Company adopted its 2017 Inducement Plan (the “Inducement Plan”). Pursuant to the Plan, the Company’s compensation committee is authorized to grant equity-based incentive awards to its employees, including employees of its subsidiaries, who were not previously employees or Non-Employee Directors of the Company or any of its subsidiaries (or who have had a bona fide period of non-employment with the Company and its subsidiaries) in compliance with Rule 5635(c)(4) of the Nasdaq Global Market. The number of shares of the Company’s Common Stock initially reserved for issuance under the Plan was 300,000, in the form of Common Stock, deferred stock, restricted stock, restricted stock units and stock options. The amount, terms of grants and exercisability provisions are determined by the compensation committee of the Company’s board of directors. The term of stock options issued under the Inducement Plan may be up to 10 years, and stock options are exercisable in cash or as otherwise determined by the compensation committee of the Company’s board of directors. All stock options vest over time as stipulated in the individual award agreements.
Shares Reserved for Future Issuance
As of December 31, 2017, the Company has reserved the following shares of the Company’s Common Stock
F-34
for issuance:
|
Shares initially reserved under the 2013 Plan |
|
1,680,000 |
|
|
Shares reserved under the Inducement Plan |
|
300,000 |
|
|
Shares reserved under the Purchase Plan |
|
750,000 |
|
|
Authorized increase to the 2013 Plan |
|
4,600,000 |
|
|
Common stock options granted under the 2013 Plan |
|
(5,190,938) |
|
|
Common stock options granted under the Inducement Plan |
|
(212,500) |
|
|
Restricted stock awards granted under the 2013 Plan |
|
(1,543,660) |
|
|
Common stock issued under the Purchase Plan |
|
(98,548) |
|
|
Stock options and restricted stock awards forfeited |
|
1,303,449 |
|
|
Remaining shares available for future grant |
|
1,587,803 |
|
The estimated grant-date fair value of the Company’s share-based awards is amortized ratably over the awards’ service periods. Stock-based compensation expense recognized was as follows:
|
|
|
Year Ended December 31, |
||||
|
(in thousands) |
|
2015 |
|
2016 |
|
2017 |
|
General and administrative |
$ |
4,049 |
|
5,157 |
|
4,458 |
|
Sales and marketing |
|
218 |
|
364 |
|
524 |
|
Research and development |
|
932 |
|
802 |
|
570 |
|
Restructuring charges |
|
— |
|
— |
|
364 |
|
Total stock based compensation expense |
$ |
5,199 |
|
6,323 |
|
5,916 |
Stock Options Outstanding under the 2013 Stock-Based Incentive Plan
|
|
|
Stock Options Outstanding |
|
|||||
|
|
|
|
|
|
|
|
Weighted-average |
|
|
|
|
|
|
|
|
|
Remaining |
|
|
|
|
Number of |
|
Weighted-Average |
|
Contractual |
|
|
|
|
|
Shares |
|
Exercise Price |
|
Term (in years) |
|
|
|
Outstanding at December 31, 2016 |
|
2,952,572 |
|
$ |
8.63 |
|
|
|
|
Granted |
|
2,098,581 |
|
|
3.62 |
|
|
|
|
Exercised |
|
— |
|
|
— |
|
|
|
|
Forfeited |
|
(824,966) |
|
|
6.74 |
|
|
|
|
Cancelled |
|
(115,575) |
|
|
10.26 |
|
|
|
|
Outstanding at December 31, 2017 |
|
4,110,612 |
|
$ |
6.41 |
|
8.26 |
|
|
Vested or expected to vest at December 31, 2017 |
|
4,110,612 |
|
$ |
6.41 |
|
8.26 |
|
|
Exercisable at December 31, 2017 |
|
1,300,006 |
|
$ |
8.35 |
|
6.85 |
|
The intrinsic value of the Company’s 4,110,612 stock options outstanding as of December 31, 2017 was $1,400 based on a per share price of $1.00, the Company’s closing stock price on that date, and a weighted-average exercise price of $6.41 per share.
The intrinsic value of stock options exercised during the years ended December 31, 2015 and 2016 was $64,000 and $32,700, respectively. There were no options exercised in the year ended December 31, 2017.
The Company uses the Black-Scholes valuation model in determining the fair value of equity awards. For stock options granted to employees and directors with only service-based vesting conditions, the Company measures stock-based compensation cost at the grant date based on the estimated fair value of the award, and recognize it as expense over the requisite service period on a straight-line basis. The Company records the expense of services rendered by non-
F-35
employees based on the estimated fair value of the stock option as of the respective vesting date. Further, the Company expenses the fair value of non-employee stock options that contain only service-based vesting conditions over the requisite service period of the underlying stock options.
On June 8, 2017, the Company granted stock options for 630,000 shares of the Company’s Common Stock to nine senior executives (the “June 2017 Grant”). The contractual term of each of the grants made in the June 2017 Grant is 10 years and the exercise price is $2.38 per share. Provided that the grantee is still employed by the Company, the vesting terms of the June 2017 Grant include a combination of market and service-based conditions as follows:
|
· |
25% of the award will vest on the later of (i) the six-month anniversary of the grant and (ii) the date on which the average closing price of the Company's Common Stock on Nasdaq is at least $3.33 for 30 consecutive trading days. |
|
· |
25% of the award will vest on the later of (i) the twelve-month anniversary of the grant and (ii) the date on which the average closing price of the Company's Common Stock on Nasdaq is at least $4.05 for 30 consecutive trading days. |
|
· |
50% of the award will vest on the later of (i) the twenty-four-month anniversary of the grant and (ii) the date on which the average closing price of the Company's Common Stock on Nasdaq is at least $4.76 for 30 consecutive trading days. |
The Company used the binomial model to estimate the compensation cost for the June 2017 Grant. Key assumptions used in calculating the total estimated compensation cost of $1.3 million included (i) an estimated term of 5.6 years, (ii) expected volatility of 95.54%, (iii) expected dividends of $0.00 and (iv) a risk-free return of 1.80%. Stock-based compensation expense related to the June 2017 Grant will be recognized ratably over the requisite service period of 5.6 years and amounted to $136,000 for the year ended December 31, 2017.
The per-share weighted-average grant date fair value of the options granted to employees during the years ended December 31, 2015, 2016 and 2017 was estimated at $6.44, $4.62 and $3.62, respectively, per share on the date of grant using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
|
|
2015 |
|
2016 |
|
2017 |
|||
|
Risk-free interest rate |
|
1.76 |
% |
|
1.66 |
% |
|
1.93 |
% |
|
Expected term of options (in years) |
|
6.27 |
|
|
6.19 |
|
|
6.15 |
|
|
Expected volatility |
|
72.93 |
% |
|
68.94 |
% |
|
80.57 |
% |
|
Dividend yield |
|
— |
|
|
— |
|
|
— |
|
The weighted-average valuation assumptions were determined as follows:
|
· |
Risk-free interest rate: The Company based the risk-free interest rate on the interest rate payable on U.S. Treasury securities in effect at the time of grant for a period that is commensurate with the assumed expected option term. |
|
· |
Expected term of stock options: The Company estimated the expected life of its employee stock options using the “simplified” method, as prescribed in Staff Accounting Bulletin (“SAB”) No. 107, Share Based Payments, whereby the expected life equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. |
|
· |
Expected stock price volatility: The Company estimated the expected volatility based on its actual historical volatility of the Company’s stock price. The Company calculated the historical volatility by using daily closing prices over a period of the expected term of the associated award. A decrease in the expected volatility would have decreased the fair value of the underlying instrument. |
F-36
Prior to January 1, 2017, the Company estimated the expected volatility based on actual historical volatility of the stock price of similar companies with publicly-traded equity securities. The Company calculated the historical volatility of the selected companies by using daily closing prices over a period of the expected term of the associated award. The impact of this change had an immaterial effect on the Company’s financial results for the year ended December 31, 2017.
|
· |
Expected annual dividend yield: The Company estimated the expected dividend yield based on consideration of its historical dividend experience and future dividend expectations. The Company has not historically declared or paid dividends to stockholders. Moreover, it does not intend to pay dividends in the future, but instead expects to retain any earnings to invest in the continued growth of the business. Accordingly, the Company assumed an expected dividend yield of 0.0%. |
As of December 31, 2017, there was $8.4 million of total unrecognized compensation expense, related to unvested options granted under the Plan, which will be recognized over the weighted-average remaining period of 2.55 years.
Restricted Stock Granted under the 2013 Stock-Based Incentive Plan
A summary of the status of the Company’s restricted stock awards at December 31, 2017 and of changes in restricted stock awards outstanding under the Plan for the year ended December 31, 2017 is as follows:
|
|
|
|
|
Weighted-average |
|
|
|
|
|
Number of |
|
Grant Date Fair |
|
|
|
|
|
Shares |
|
Value per Share |
|
|
|
Unvested at December 31, 2016 |
|
543,577 |
|
$ |
10.77 |
|
|
Granted |
|
— |
|
$ |
— |
|
|
Forfeited |
|
(32,771) |
|
$ |
7.48 |
|
|
Vested restricted stock awards |
|
(485,759) |
|
$ |
11.19 |
|
|
Unvested at December 31, 2017 |
|
25,047 |
|
$ |
7.07 |
|
For stock awards that vest subject to the satisfaction of service requirements, compensation expense is measured based on the fair value of the award on the date of grant and is recognized as expense on a straight-line basis (net of estimated forfeitures) over the requisite service period. All restricted stock awards issued above vest over time as stipulated in the individual award agreements. In the event of a change in control of the Company, the unvested awards will be accelerated and fully vested immediately prior to the change in control. There are no performance based features or market conditions.
The fair value of restricted stock awards vested for the years ended December 31, 2015, 2016 and 2017, was $2.5 million, $1.0 million and $1.8 million, respectively.
As of December 31, 2017, there was $177,000 of total unrecognized stock-based compensation expense, related to restricted stock under the Plan, which will be recognized over the weighted-average remaining period of 2.75 years.
Employee Stock Purchase Plan
In January 2016, the Company established an Employee Stock Purchase Plan (the “Purchase Plan”), which was approved by the Company’s stockholders in June 2016. A total of 750,000 shares of Common Stock were originally approved for future issuance under the Purchase Plan pursuant to purchase rights granted to the Company’s employees. Under the Company’s Purchase Plan, eligible employees can purchase the Company’s Common Stock through accumulated payroll deductions at such times as are established by the administrator. The Purchase Plan is administered by the compensation committee. Under the Purchase Plan, eligible employees may purchase the Company’s Common Stock at 85% of the lower of the fair market value of a share of the Company’s Common Stock on the first day of an offering period or on the last day of the offering period. Eligible employees may contribute up to 10% of their eligible compensation. A participant may purchase a maximum of 1,500 shares of Common Stock per offering period. Under the
F-37
Purchase Plan, a participant may not accrue rights to purchase more than $25,000 worth of the Company’s Common Stock for each calendar year in which such right is outstanding.
Effective January 1, 2016, employees who elected to participate in the Purchase Plan commenced payroll withholdings that accumulate during the six month offering periods each calendar year while the Purchase Plan is effective:
|
|
|
|
January 1 through June 30, and |
|
|
|
|
July 1 through December 31 |
At the end of each offering period, shares of the Company’s Common Stock may be purchased at 85% of the lower of the fair market value of the Company’s Common Stock on the first or last day of the respective offering period. In accordance with the guidance in ASC 718-50 – Compensation – Stock Compensation, the ability to purchase shares of the Company’s Common Stock at the lower of the price on the first day of the offering period or the last day of the offering period (i.e. the purchase date) represents an option and, therefore, the Purchase Plan is a compensatory plan under this guidance. Accordingly, stock-based compensation expense is determined based on the option’s grant-date fair value and is recognized over the requisite service period of the option. The Company has recognized stock-based compensation expense of $111,000 and $107,000 for the years ended December 31, 2016 and 2017, respectively, related to the Purchase Plan.
11. Income Taxes
Income taxes have been recorded on the following losses before income taxes:
|
(in thousands) |
|
|
As of December 31, |
|
||||||||||
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||||||
|
Domestic operations |
|
$ |
(45,361) |
|
$ |
(52,545) |
|
$ |
(52,568) |
|
||||
|
Foreign operations |
|
|
(13,290) |
|
|
(39,148) |
|
|
(16,791) |
|
||||
|
Loss before provision for income taxes |
|
$ |
(58,651) |
|
$ |
(91,693) |
|
$ |
(69,359) |
|
||||
The benefit for income taxes consists of the following for 2015, 2016 and 2017:
|
|
|
As of |
|
|||||||
|
(in thousands) |
|
December 31, |
|
|||||||
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||
|
Current: |
|
|
|
|
|
|
|
|
|
|
|
U.S. federal |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
State and local |
|
|
— |
|
|
— |
|
|
— |
|
|
Foreign |
|
|
— |
|
|
— |
|
|
— |
|
|
Total current taxes |
|
|
— |
|
|
— |
|
|
— |
|
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
U.S. federal |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
State and local |
|
|
(700) |
|
|
(1,061) |
|
|
— |
|
|
Foreign |
|
|
(18) |
|
|
— |
|
|
— |
|
|
Total deferred taxes |
|
|
(718) |
|
|
(1,061) |
|
|
— |
|
|
Total income tax benefit |
|
$ |
(718) |
|
$ |
(1,061) |
|
$ |
— |
|
For the years ended December 31, 2015, 2016 and 2017, the Company had no interest or penalties accrued related to unrecognized tax benefits. Any interest and penalties relating to unrecognized tax benefits will be recorded as a
F-38
component of income tax expense. The following table indicates the changes to the Company’s unrecognized tax benefits:
|
(in thousands) |
|
For the Year Ended |
|
|||||||
|
|
|
December 31, |
|
|||||||
|
|
|
2015 |
|
2016 |
|
2017 |
|
|||
|
Beginning balance |
|
$ |
59 |
|
$ |
73 |
|
$ |
73 |
|
|
Increase related to prior tax years |
|
|
14 |
|
|
— |
|
|
— |
|
|
Increase related to current year |
|
|
— |
|
|
— |
|
|
— |
|
|
Ending balance |
|
$ |
73 |
|
$ |
73 |
|
$ |
73 |
|
Of the Company’s unrecognized tax benefits, none would affect the Company’s effective tax rate in the period recognized due to the offsetting impact of the valuation allowance recorded against the Company’s net operating losses. The Company does not expect its unrecognized tax benefit liability to change significantly over the next 12 months.
The principal components of the Company’s deferred tax assets and liabilities were as follows:
|
(in thousands) |
|
|
As of December 31, |
|
|||
|
|
|
2016 |
|
2017 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
Inventory |
|
$ |
714 |
|
$ |
96 |
|
|
Accrued expenses |
|
|
1,049 |
|
|
725 |
|
|
Deferred revenue |
|
|
1,484 |
|
|
1,843 |
|
|
Stock-based compensation expense |
|
|
1,183 |
|
|
1,072 |
|
|
Intangible assets |
|
|
1,307 |
|
|
969 |
|
|
Other |
|
|
366 |
|
|
341 |
|
|
Other debt |
|
|
1,288 |
|
|
1,149 |
|
|
Net operating losses |
|
|
51,932 |
|
|
56,943 |
|
|
Deferred tax assets |
|
|
59,323 |
|
|
63,138 |
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Fixed assets |
|
$ |
(651) |
|
$ |
(747) |
|
|
Convertible notes |
|
|
(5,039) |
|
|
(1,445) |
|
|
Deferred tax liabilities |
|
|
(5,690) |
|
|
(2,192) |
|
|
Net deferred tax assets |
|
|
53,633 |
|
|
60,946 |
|
|
Less: valuation allowance |
|
|
(53,656) |
|
|
(60,966) |
|
|
Net deferred tax liabilities after valuation allowance |
|
$ |
(23) |
|
$ |
(20) |
|
As of December 31, 2017, the Company had foreign net operating loss (“NOL”) carry forwards of $97.1 million from its operations in Denmark, which are available to reduce future foreign taxable income. The NOL carry forwards are not subject to future expiration and may be carried forward indefinitely. However, if there is a more than 50% change of stockholders by value or vote at the end of the tax year as compared to the beginning of the tax year, these existing foreign NOLs may not be available to offset certain types of future foreign income (generally, “net financial income”, which includes interest income net of interest expense, dividends, and capital gains and losses). The Company files income tax returns in the U.K., because Egalet Limited (“Egalet UK”) was incorporated in that jurisdiction; however, Egalet UK has no business operations in the U.K. and the Company has no plans to commence operations in that jurisdiction in the foreseeable future. As such, the Company has determined that it will not record U.K. NOL’s as a component of their deferred tax inventory, since there is currently no expectation that the NOLs will ever be realized. As of December 31, 2017, the Company had U.S. federal and state NOL’s of $137.9 million and $91.8 million, respectively. These domestic NOL carry forwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three‑ year period in excess of 50%. This could limit the amount of NOLs that the Company can utilize annually to offset future domestic taxable income or tax liabilities, if any. The amount of the annual limitation, if any, will be determined based on the value of the Company immediately
F-39
prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. These federal and state NOL’s will begin to expire in 2033 and through 2036.
ASC 740 – Tax Provisions requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, the Company has recorded a full valuation allowance against its net deferred tax assets at December 31, 2016 and 2017, respectively, because the Company has determined that is it more likely than not that these assets will not be fully realized. The Company experienced a net change in valuation allowance of $27.9 million and $7.3 million, which includes the impact of the Tax Reform for the years ended December 31, 2016 and 2017, respectively.
At December 31, 2017, no provision has been made for U.S. federal and state income taxes of foreign earnings due to the history of foreign losses. However, the Company expects that the future earnings, if any, of its foreign subsidiaries will be reinvested indefinitely. Upon becoming profitable, if ever, distribution of these earnings, in the form of dividends or otherwise, may result in the Company falling subject to U.S. income taxes and foreign withholding taxes. The determination of the amount of unrecognized deferred U.S. income tax expense and foreign withholding tax liabilities on these future earnings, if any, is not practicable because of the complexities with the hypothetical calculations.
The Company files income tax returns in Denmark, the U.K., the United States, and in various U.S. states. The foreign tax returns are subject to tax examinations for the tax years ended July 31, 2012 through December 31, 2017. The domestic tax returns are subject to tax examinations for the tax years ended December 31, 2014 through December 31, 2017. However, to the extent the Company utilizes in the future any tax attribute NOL carry forwards from a tax period that may otherwise be closed to examination, the Internal Revenue Service, state tax authorities, or other governing parties may still adjust the NOL upon their examination of the future period in which the attribute was utilized.
A reconciliation of income tax expense (benefit) at the statutory federal income tax rate and income taxes as reflected in the financial statements is as follows:
|
|
|
For the Year |
|
||
|
|
|
Ended |
|
||
|
(in thousands) |
|
December 31, |
|
||
|
|
|
2016 |
|
2017 |
|
|
Federal income tax at the statutory rate |
|
34.0 |
% |
34.0 |
% |
|
Permanent tax items |
|
(0.5) |
|
(0.3) |
|
|
State income tax, net of federal benefit |
|
2.2 |
|
2.6 |
|
|
Change in foreign tax rate |
|
(5.4) |
|
(3.1) |
|
|
Equity compensation shortfall |
|
|
|
(0.7) |
|
|
Tax reform rate change |
|
|
|
(25.8) |
|
|
Change in valuation allowance |
|
(29.2) |
|
(6.7) |
|
|
Effective income tax rate |
|
1.1 |
% |
— |
% |
|
The Cuts and Jobs Act (the “Tax Act”) was enacted on December 22, 2017 and was the driver of the rate change item above. |
|||||
12. Employee Benefit Plans
The Company's 401(k) Employee Savings Plan (the "401(k) Plan") is available to all U.S. employees meeting certain eligibility criteria. As the Company has elected a Safe-Harbor provision for the 401(k) Plan, participants are always fully vested in their employer contributions. The Company matches 100% of the first 3% of participating employee contributions and 50% of the next 2% of participating employee contributions. The Company contributed approximately $199,000, $308,000 and $617,000 to the 401(k) Plan in the years ended December 31, 2015, 2016 and 2017, respectively. The Company's contributions are made in cash. The Company's Common Stock is not an investment option available to participants in the 401(k) Plan.
F-40
For its employees based in Denmark, the Company subscribes to a state plan for which the expense for the financial year is equal to the contributions called by, and thus payable to, such plan. Under Denmark’s state plan, contributions paid by the Company are in full discharge of the Company’s liability and are recognized as an expense for the period. For the years ended December 31, 2015, 2016 and 2017, the Company recorded $230,000, $256,000 and $202,000, respectively, for contributions under its state plan for Denmark employees.
13. Commitments and Contingencies
Operating Leases
The Company’s corporate U.S. headquarters are located in Wayne, Pennsylvania, where it leases 19,797 square feet of office space under a lease agreement that expires in February 2022 unless terminated earlier. The Company also maintains a research laboratory, pilot manufacturing and administrative facility in Vaerlose, Denmark, where it leases 12,895 square feet of space under a lease agreement that automatically renews every 12 months (currently through August 2018 unless terminated earlier).
The following is a schedule by year of the future minimum rental payments required under non-cancelable leases as of December 31, 2017:
|
(in thousands) |
|
|
|
|
2018 |
|
|
523 |
|
2019 |
|
|
533 |
|
2020 |
|
|
543 |
|
2021 |
|
|
553 |
|
2022 |
|
|
92 |
|
Total minimum lease payments |
|
$ |
2,244 |
Rent expense was $408,000, $666,000 and $617,000 for the years ended December 31, 2015, 2016 and 2017, respectively.
Legal Proceedings
On January 27, 2017 and February 10, 2017, respectively, two putative securities class actions were filed in the U.S. District Court for the Eastern District of Pennsylvania that named as defendants Egalet and current officers Robert S. Radie, Stanley J. Musial, and Jeffrey M. Dayno. These two complaints, captioned Mineff v. Egalet Corp. et al., No. 2:17-cv-00390-MMB and Klein v. Egalet Corp. et al., No. 2:17-cv-00617-MMB, assert securities fraud claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 (the “Exchange Act”) on behalf of putative classes of persons who purchased or otherwise acquired Egalet Corporation securities between December 15, 2015 and January 9, 2017. On May 1, 2017, the Court entered an order consolidating the two cases before it, appointing the Egalet Investor Group (consisting of Joseph Spizzirri, Abdul Rahiman and Kyle Kobold) as lead plaintiff and approving their selection of lead and liaison counsel. On July 3, 2017, the plaintiffs filed their consolidated amended complaint, which named the same defendants and also asserts claims for purported violations of Sections 10(b) and 20(a) of the Exchange Act. Plaintiffs bring their claims individually and on behalf of a putative class of all persons who purchased or otherwise acquired shares of the Company between November 4, 2015 and January 9, 2017 inclusive. The consolidated amended complaint bases its claims on allegedly false and/or misleading statements and/or failures to disclose information about the likelihood that ARYMO ER would be approved for intranasal abuse-deterrent labeling. The defendants moved to dismiss the Consolidated Amended Complaint on September 1, 2017, the plaintiffs filed their opposition on October 31, 2017, and the defendants filed their reply on December 8, 2017. The Court heard oral argument on the motion to dismiss on February 20, 2018, and entered an order pursuant to which the plaintiffs filed a motion for leave to file a second amended complaint on March 6, 2018, and the defendants response is due on March 20, 2018. The Company disputes the allegations in the lawsuit and intends to defend these actions vigorously. The Company cannot determine the likelihood of, nor can it reasonably estimate the range of, any potential loss, if any, from these lawsuits.
F-41
14. Net Loss Per Share of Common Stock
The following table sets forth the computation of basic and diluted loss per share of the Company’s Common Stock for the years ended December 31, 2015, 2016 and 2017:
|
(in thousands, except share and per share data) |
|
Year Ended December 31, |
|||||||
|
|
|
2015 |
|
2016 |
|
2017 |
|||
|
Basic and diluted net loss per common share calculation: |
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(57,933) |
|
$ |
(90,632) |
|
$ |
(69,359) |
|
Weighted average common stock outstanding |
|
|
19,738,042 |
|
|
24,514,645 |
|
|
33,755,462 |
|
Net loss per share of common stock—basic and diluted |
|
$ |
(2.94) |
|
$ |
(3.70) |
|
$ |
(2.05) |
The following outstanding securities for the year ended December 31, 2015, 2016 and 2017 have been excluded from the computation of diluted weighted shares outstanding, as they would have been anti‑dilutive:
|
|
|
|
Year Ended December 31, |
||||
|
|
|
|
2015 |
|
2016 |
|
2017 |
|
Stock options outstanding |
|
|
1,755,808 |
|
2,952,572 |
|
4,110,612 |
|
Unvested restricted stock awards |
|
|
679,866 |
|
543,577 |
|
25,047 |
|
Common shares issuable upon conversion of the 5.50% Notes |
|
|
4,102,360 |
|
4,102,360 |
|
1,657,757 |
|
Common shares issuable upon conversion of the 6.50% Notes |
|
|
— |
|
— |
|
17,907,047 |
|
Common shares issuable upon exercise of warrants |
|
|
— |
|
— |
|
17,666,667 |
|
Total |
|
|
6,538,034 |
|
7,598,509 |
|
41,367,130 |
15. Acquisitions and License and Collaboration Agreements
License and collaboration agreement with Shionogi
In November 2013, the Company entered into a license and collaboration agreement with Shionogi, granting Shionogi an exclusive, royalty-bearing, worldwide license to develop, manufacture and commercialize abuse deterrent (“AD”) hydrocodone-based product candidates using certain of the Company’s core technologies. The collaboration allowed Shionogi to develop and commercialize an AD single-agent hydrocodone-based product and up to 20 different AD combination product candidates containing hydrocodone. In December 2015, the Company received notice from Shionogi that the collaboration and license agreement was terminated for convenience.
Under the terms of the agreement, the Company received an upfront payment of $10.0 million and payment of $10.0 million in April 2015 upon submission of an investigational new drug (“IND”) application by Shionogi. Prior to the termination of the agreement, the Company was eligible to receive regulatory milestone payments under the agreement as follows: (i) up to an additional $50.0 million upon successful achievement of specified regulatory milestones for the first licensed product candidate; (ii) up to $42.5 million upon successful achievement of specified regulatory milestones for a defined combination product candidate; (iii) up to $25.0 million upon successful achievement of specified regulatory milestones for a second product candidate (other than the defined combination product candidate); and (iv) up to $12.5 million upon successful achievement of specified regulatory milestones for further
F-42
product candidates. In addition, the Company was eligible to receive up to an aggregate of $185.0 million based on successful achievement of specified net sales thresholds of licensed products.
The Company determined that the deliverables under the Shionogi agreement were the exclusive, royalty‑bearing, worldwide license to its AD hydrocodone‑based product candidates using certain of the Company’s core technologies, the research and development services to be completed by the Company and the Company’s obligation to serve on a joint committee. The license did not have standalone value to Shionogi and was not separable from the research and development services, because of the uncertainty of Shionogi’s ability to develop the product candidates without the research and development services of the Company during the transfer period and over the term of the agreement.
Due to the lack of standalone value for the license and research and development services, the upfront and IND payments were recorded as deferred revenue and were recognized ratably using the straight-line method through November 2030, the expected term of the agreement. As a result of the termination of the agreement, the Company recognized all remaining deferred revenue related to the Shionogi agreement as revenue in December 2015, as the Company had no further material obligations under the agreement at that time. For the year ended December 31, 2015, the Company recognized revenue of $17.9 million related to the amortization of deferred revenue.
Additionally, during the year ended December 31, 2015, the Company recognized revenue of $699,000, related to certain development costs incurred under the Company’s collaboration agreement.
Collaboration Agreement with Septodont
In February 2016, the Company entered in a Collaboration Agreement with Septodont, Inc. (“Septodont”). Under the agreement, Septodont promotes SPRIX Nasal Spray exclusively to dentists in the United States using its focused specialty sales force. Under the terms of the agreement, The Company received an upfront licensing fee of $100,000 and can earn sales-based milestones and share in profits from net sales of SPRIX to dentists in the U.S.
Collaboration and License Agreement with Acura
In January 2015, the Company entered into a Collaboration and License Agreement with Acura to commercialize OXAYDO™ (oxycodone hydrochloride) tablets containing Acura’s Aversion® Technology. Under the terms of the Collaboration and License Agreement, Acura transferred the approved NDA for OXAYDO to the Company and the Company was granted an exclusive license under Acura’s intellectual property rights for development and commercialization of OXAYDO worldwide in all strengths.
The Company paid Acura an upfront payment of $5.0 million in January 2015 and a $2.5 million milestone in October 2015 upon the first commercial sale of OXAYDO. In addition, Acura will be entitled to a one-time $12.5 million milestone payment when OXAYDO net sales reach a specified level of $150.0 million in a calendar year.
The Company has recorded a product rights intangible asset of $7.7 million related to the arrangement, which includes $172,000 of transaction costs related to the agreement. The intangible asset is being amortized over a useful life of 7 years, which coincides with the patent protection of OXAYDO in the United States.
In addition, Acura will receive from the Company, a stepped royalty at percentage rates ranging from mid-single digits to double-digits on net sales during a calendar year based on OXAYDO net sales during such year. Based on our current net sales, the royalty percentage payable to Acura is in the mid-single digits; however, the percentage may increase in future years in the event we achieve the higher sales thresholds set forth in the agreement. The Company’s royalty payment obligations commence on the first commercial sale of OXAYDO and expire, on a country-by-country basis, upon the expiration of the last to expire valid patent claim covering OXAYDO in such country (or if there are no patent claims in such country, then upon the expiration of the last valid claim in the U.S.). Royalties will be reduced upon the entry of generic equivalents, as well for payments required to be made by the Company to acquire intellectual property rights to commercialize OXAYDO, with an aggregate minimum floor. The term of the Acura license agreement expires, in its entirety, upon the final expiration of any such patent claim in any country. OXAYDO is
F-43
currently sold in the United States and is covered by six U.S. patents that expire between 2023 and 2025. Patents covering OXAYDO in foreign jurisdictions expire in 2024. Either the Company or Acura may terminate the license agreement for certain customary reasons, including cause, insolvency or patent challenge. The Company may terminate the license agreement upon 90 days prior written notice.
Purchase Agreement with Luitpold
In January 2015, the Company entered into and consummated a purchase agreement with Luitpold. Pursuant to the purchase agreement, the Company acquired specified assets and liabilities associated with SPRIX® (ketorolac tromethamine) Nasal Spray for a purchase price of $7.0 million, of which $315,000 was deposited into an escrow account to secure Luitpold’s indemnification obligations under the purchase agreement. The Company concurrently purchased an additional $1.1 million of glassware, equipment and active pharmaceutical ingredient (“API”) from Luitpold and agreed to purchase an additional $340,000 of API after closing.
Under the purchase agreement with Luitpold pursuant to which the Company acquired certain assets and liabilities associated with SPRIX Nasal Spray, the Company was assigned an exclusive license with Recordati Ireland Ltd. (“Recordati”) for intranasal formulations of ketorolac tromethamine (the “Licensed Product”), the active ingredient in SPRIX Nasal Spray. The Company was required to pay a fixed, single-digit royalty to Recordati on net sales of the Licensed Product. The exclusive term of the license agreement expires, on a country-by-country basis, on the later of the final expiration of any patent right in such country that contains a valid claim covering the Licensed Product, or ten years from the date of the first commercial sale of the Licensed Product in such country, and thereafter the Company will retain a non-exclusive, perpetual license in such country. In addition, during the exclusivity period with respect to the United States, Canada and Latin America, the royalty payable to Recordati is decreased if no patent containing a valid claim is in force in the country at the time of sale. SPRIX Nasal Spray is currently sold in the United States and is covered by a patent that expires in December 2018 and the first commercial sale of SPRIX Nasal Spray in the United States occurred in May 2011.
The Company accounted for the arrangement as a business combination and the purchase price has been allocated to the acquisition date fair values as follows:
|
|
|
Purchase Price |
|
|
(in thousands) |
|
Allocation |
|
|
Inventory |
|
$ |
3,408 |
|
Property, plant & equipment |
|
|
100 |
|
Finite lived intangible-intellectual property |
|
|
4,620 |
|
Net assets acquired |
|
$ |
8,128 |
The Company incurred $1.1 million of SPRIX Nasal Spray acquisition-related costs, which were recorded as general and administrative expense in the Consolidated Statement of Operations in the year ended December 31, 2015.
16. Stockholders’ Equity
At the Market Offering
In July 2015, the Company entered into a Controlled Equity Offering Sales Agreement (“2015 Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), under which the Company could, at its discretion, from time to time, sell shares of its Common Stock, for an aggregate offering price of up to $30.0 million. The Company provided Cantor with customary indemnification rights, and Cantor is entitled to a commission at a fixed rate of 3% of the gross proceeds per share sold. Sales of the shares under the 2015 Sales Agreement have been and, if there are additional sales
F-44
under the 2015 Sales Agreement, will be, made in transactions deemed to be “at the market offerings”, as defined in Rule 415 under the Securities Act of 1933, as amended.
The Company initiated sales of shares under the 2015 Sales Agreement in March 2017 and sold an aggregate of 1,540,597 shares of Common Stock through December 31, 2017, resulting in net proceeds of $4.1 million after deducting commissions of $126,000. The Company has not sold any additional shares under the Sales Agreement during the period subsequent to December 31, 2017.
July 2017 Equity Offering
In July 2017, the Company entered into an underwriting agreement with Cantor Fitzgerald & Co. relating to an underwritten public offering (the “July 2017 Equity Offering”) of 16,666,667 shares of the Company’s Common Stock and accompanying warrants to purchase 16,666,667 shares of Common Stock, at a combined public offering price of $1.80 per share and accompanying warrant, for gross proceeds of $30.0 million. The net offering proceeds were $28.6 million after deducting underwriting discounts and commissions and offering-related costs of $1.4 million. Each warrant has an exercise price of $2.70, subject to adjustment in certain circumstances. The shares of Common Stock and warrants were issued separately. The warrants may be exercised at any time on or after the date of issuance and will expire five years from the date of issuance.
The Company accounted for the warrants using ASC 480 – Distinguishing Liabilities from Equity and determined that the warrants were a freestanding financial instrument that are subject to liability classification. Pursuant to the terms of the agreement, the Company could be required to settle the warrants in cash in the event of an acquisition of the Company, and as a result the warrants are required to be measured at fair value and reported as a liability in the Company’s consolidated balance sheet. The warrant exercise price is subject to adjustment upon the issuance of certain equity securities at a price less than the exercise price of the warrants then in effect.
The fair value of the warrants to purchase shares of the Company’s Common Stock in connection with the July 2017 Equity Offering was $9.7 million on the date of issuance, which was determined using a lattice model that takes into account various future financing scenarios and the impact of those scenarios on the fair value of the warrants. The fair value of the warrants of $9.7 million on the date of issuance was recorded as a liability which will be marked to its estimated fair value at each reporting period.
17. Quarterly Financial Information (unaudited)
This table summarizes the unaudited consolidated financial results of operations for the quarters ended:
|
(in thousands) |
|
March 31, |
|
June 30, |
|
September 30, |
|
December 31, |
|
||||
|
2017 Quarter Ended |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
$ |
5,427 |
|
$ |
6,255 |
|
$ |
6,651 |
|
$ |
7,803 |
|
|
Total costs and expenses |
|
|
26,097 |
|
|
27,999 |
|
|
22,485 |
|
|
17,755 |
|
|
Other loss (gain) |
|
|
4,703 |
|
|
4,735 |
|
|
3,114 |
|
|
(11,393) |
|
|
Net (loss) income |
|
|
(25,373) |
|
|
(26,479) |
|
|
(18,948) |
|
|
1,441 |
|
|
Net (loss) income per share of common stock, basic (1) |
|
|
(1.02) |
|
|
(1.04) |
|
|
(0.46) |
|
|
0.03 |
|
|
Net (loss) income per share of common stock, diluted (1) |
|
|
(1.02) |
|
|
(1.04) |
|
|
(0.46) |
|
|
0.03 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2016 Quarter Ended |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
2,663 |
|
$ |
3,450 |
|
$ |
4,711 |
|
$ |
6,140 |
|
|
Total costs and expenses |
|
|
19,702 |
|
|
25,118 |
|
|
28,409 |
|
|
24,758 |
|
|
Other expense |
|
|
1,694 |
|
|
2,346 |
|
|
3,597 |
|
|
3,033 |
|
|
Net loss |
|
|
(18,548) |
|
|
(23,777) |
|
|
(26,937) |
|
|
(21,370) |
|
|
Net loss per share of common stock, basic and diluted (1) |
|
|
(0.76) |
|
|
(0.97) |
|
|
(1.10) |
|
|
(0.87) |
|
|
(1) |
Net loss per share amounts may not agree to the per share amounts for the full year due to the use of weighted |
F-45
average shares for each period. |
18. Subsequent Events
Special Meeting of Stockholders
In February 2018, the Company held a special meeting of stockholders (the “Special Meeting”). At the Special Meeting, the Company’s stockholders approved an amendment to the Company’s Third Amended and Restated Certificate of Incorporation to increase the number of shares of authorized common stock from 75,000,000 to 275,000,000 shares (the “Charter Amendment”). As the Company had reserved sufficient shares of their common stock to satisfy the conversion provisions of the 6.50% Notes, the conversion feature is considered indexed to their stock and the fair value of the conversion feature will be reclassified from a liability into stockholders' in the first quarter of 2018.
In addition, at the Special Meeting, the Company’s stockholders approved an amendment to the Company’s Charter to effect a reverse stock split of the shares of the Company’s common stock at a ratio of not less than 1-to-2 and not greater than 1-to-20, with the exact ratio and effective time of the reverse stock split to be determined by our Board of Directors, if at all.
F-46
