Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pulmatrix, Inc. | d251454d8k.htm |

September 2016 NASDAQ: PULM Engineering the Future for Inhaled Therapeutics Exhibit 99.1

Disclaimers & Forward Looking Statements This presentation contains forward-looking statements. Forward-looking statements may be identified by the use of forward-looking terms such as “anticipates,” “assumes,” “believes,” “can,” “could,” “estimates,” “expects,” “forecasts,” “guides,” “intends,” “is confident that,” “may,” “plans,” “seeks,” “projects,” “targets,” and “would” or the negative of such terms or other variations on such terms or comparable terminology. These statements are not guarantees of future performance, are based on current expectations of future events and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements, including risks that we will not have sufficient working capital, that we will have delays in obtaining, or we will be unable to obtain, FDA or other regulatory approvals for our products, unable to establish collaborations, or that our products will not be commercially viable, among other risks. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s annual report on Form 10-K filed by the Company with the Securities and Exchange Commission (SEC) on March 10, 2016. Investors and security holders are urged to read this documents free of charge on the SEC’s website at http://www.sec.gov. Forward-looking statements contained in this presentation are made as of this date, and the Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise.
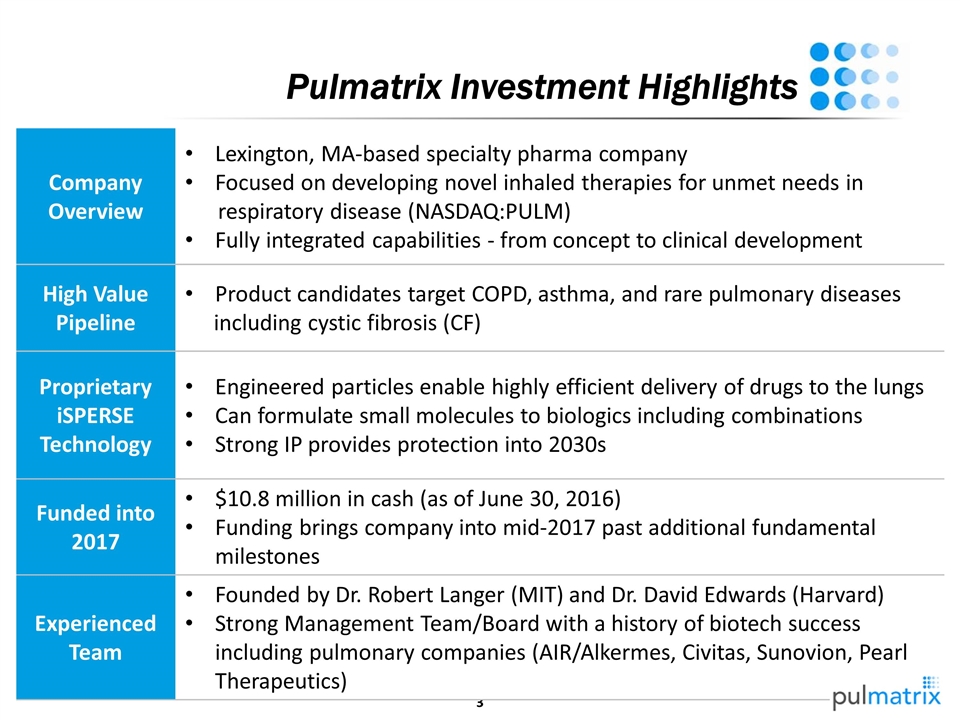
Pulmatrix Investment Highlights Company Overview Lexington, MA-based specialty pharma company Focused on developing novel inhaled therapies for unmet needs in respiratory disease (NASDAQ:PULM) Fully integrated capabilities - from concept to clinical development High Value Pipeline Product candidates target COPD, asthma, and rare pulmonary diseases including cystic fibrosis (CF) Proprietary iSPERSE Technology Engineered particles enable highly efficient delivery of drugs to the lungs Can formulate small molecules to biologics including combinations Strong IP provides protection into 2030s Funded into 2017 $10.8 million in cash (as of June 30, 2016) Funding brings company into mid-2017 past additional fundamental milestones Experienced Team Founded by Dr. Robert Langer (MIT) and Dr. David Edwards (Harvard) Strong Management Team/Board with a history of biotech success including pulmonary companies (AIR/Alkermes, Civitas, Sunovion, Pearl Therapeutics)
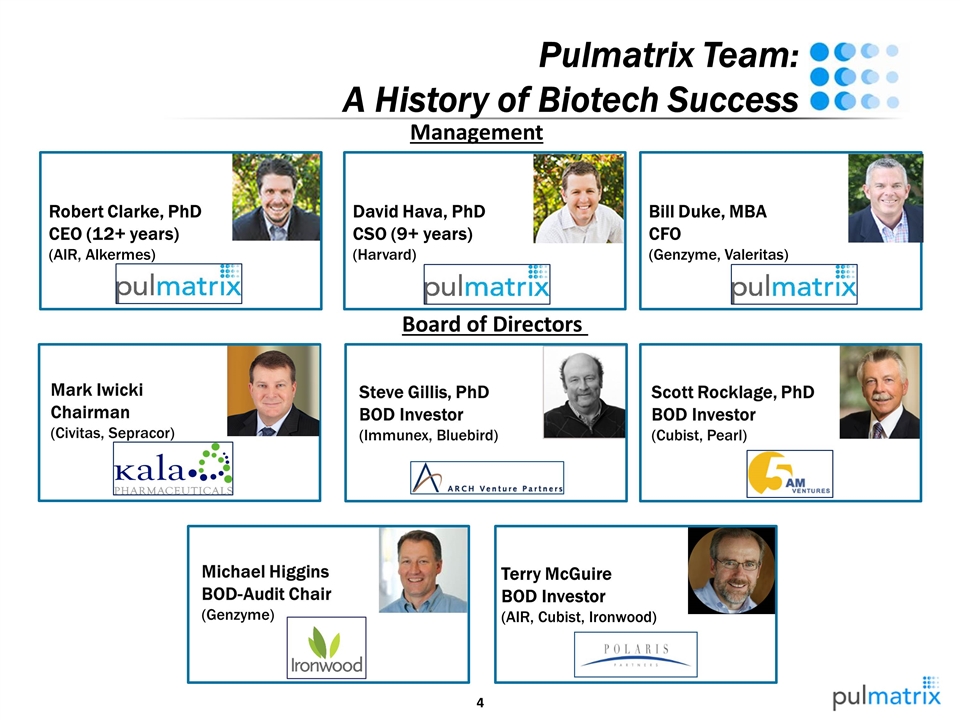
Pulmatrix Team: A History of Biotech Success Terry McGuire BOD Investor (AIR, Cubist, Ironwood) Robert Clarke, PhD CEO (12+ years) (AIR, Alkermes) Steve Gillis, PhD BOD Investor (Immunex, Bluebird) Michael Higgins BOD-Audit Chair (Genzyme) Scott Rocklage, PhD BOD Investor (Cubist, Pearl) Board of Directors Management David Hava, PhD CSO (9+ years) (Harvard) Bill Duke, MBA CFO (Genzyme, Valeritas) Mark Iwicki Chairman (Civitas, Sepracor)
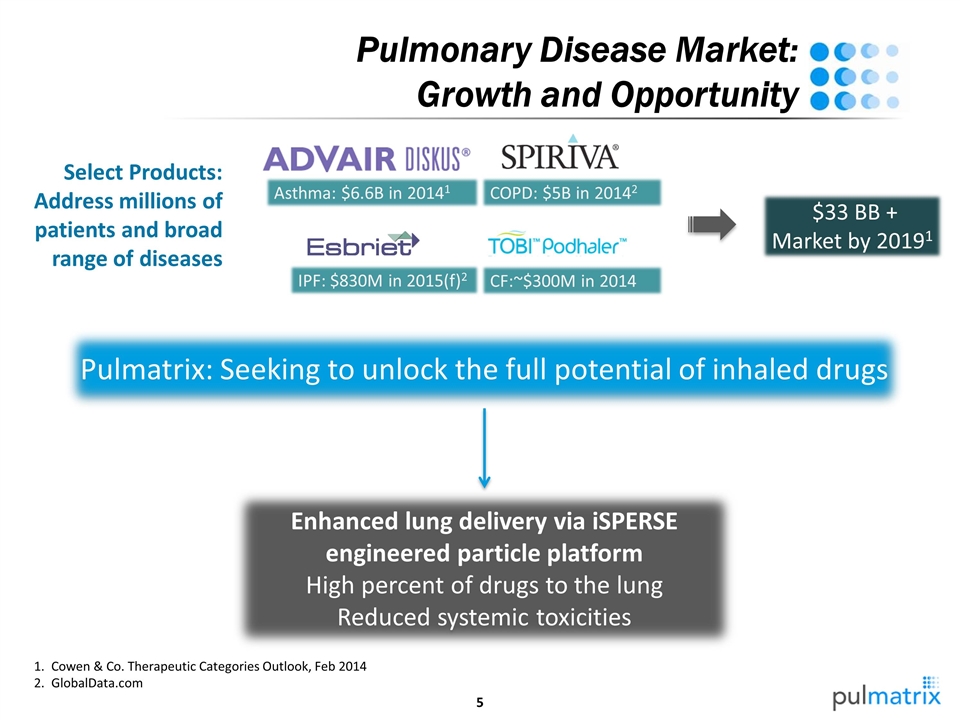
Enhanced lung delivery via iSPERSE engineered particle platform High percent of drugs to the lung Reduced systemic toxicities Pulmatrix: Seeking to unlock the full potential of inhaled drugs $33 BB + Market by 20191 Select Products: Address millions of patients and broad range of diseases Pulmonary Disease Market: Growth and Opportunity Asthma: $6.6B in 20141 COPD: $5B in 20142 IPF: $830M in 2015(f)2 CF:~$300M in 2014 Cowen & Co. Therapeutic Categories Outlook, Feb 2014 GlobalData.com
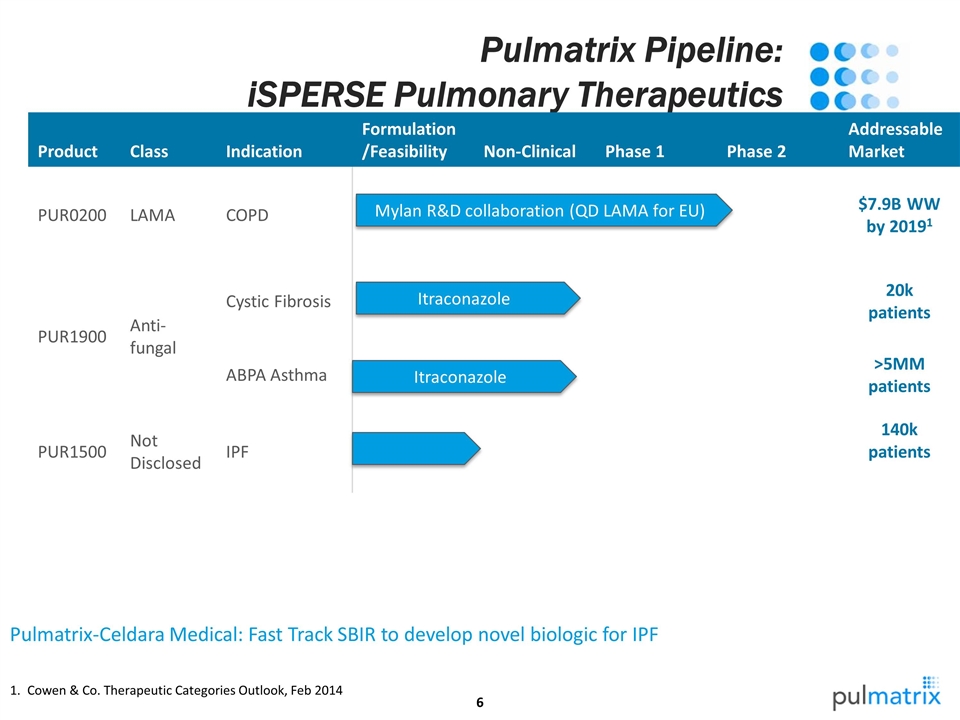
Pulmatrix Pipeline: iSPERSE Pulmonary Therapeutics Product Class Indication Formulation/Feasibility Non-Clinical Phase 1 Phase 2 Addressable Market PUR0200 LAMA COPD $7.9B WW by 20191 PUR1900 Anti-fungal Cystic Fibrosis 20k patients ABPA Asthma >5MM patients PUR1500 Not Disclosed IPF 140k patients Pulmatrix-Celdara Medical: Fast Track SBIR to develop novel biologic for IPF Mylan R&D collaboration (QD LAMA for EU) Itraconazole Cowen & Co. Therapeutic Categories Outlook, Feb 2014 Itraconazole
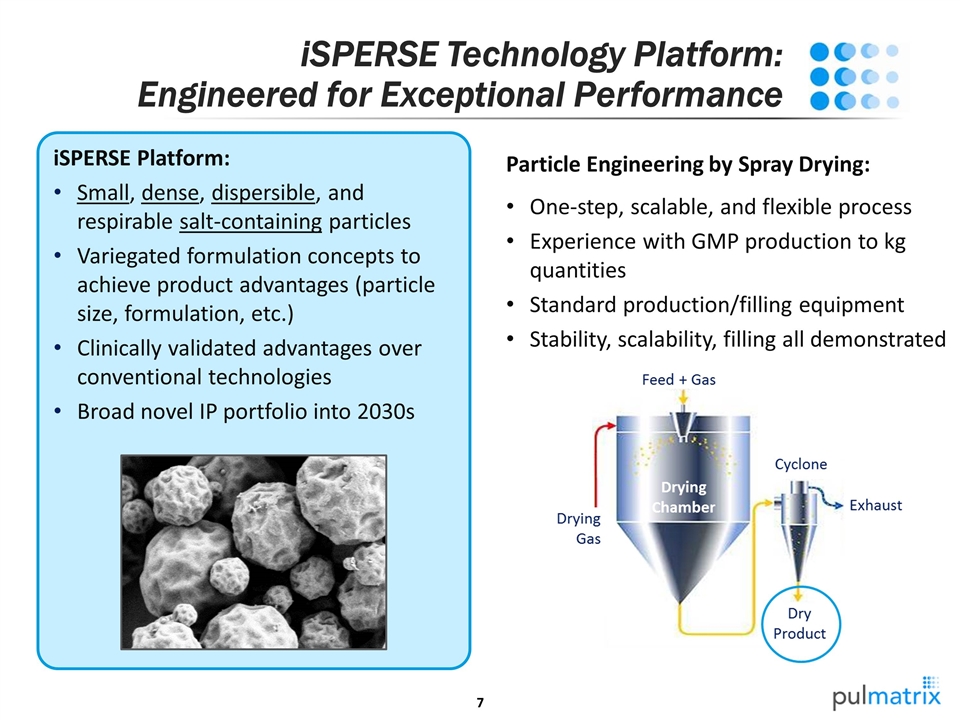
iSPERSE Platform: Small, dense, dispersible, and respirable salt-containing particles Variegated formulation concepts to achieve product advantages (particle size, formulation, etc.) Clinically validated advantages over conventional technologies Broad novel IP portfolio into 2030s Particle Engineering by Spray Drying: One-step, scalable, and flexible process Experience with GMP production to kg quantities Standard production/filling equipment Stability, scalability, filling all demonstrated iSPERSE Technology Platform: Engineered for Exceptional Performance
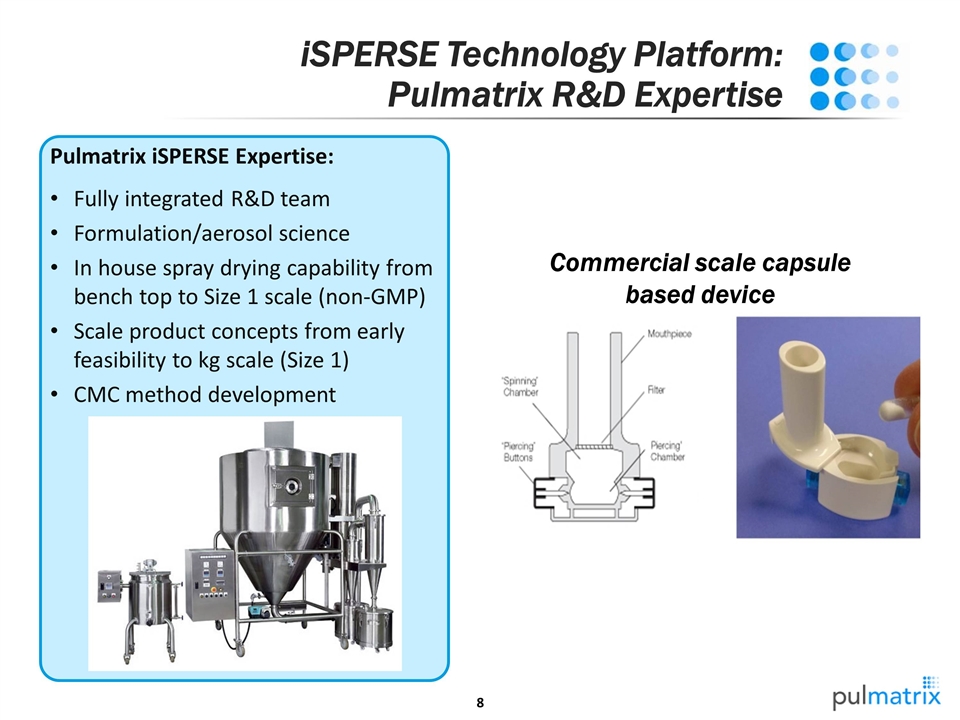
iSPERSE Technology Platform: Pulmatrix R&D Expertise Pulmatrix iSPERSE Expertise: Fully integrated R&D team Formulation/aerosol science In house spray drying capability from bench top to Size 1 scale (non-GMP) Scale product concepts from early feasibility to kg scale (Size 1) CMC method development Commercial scale capsule based device
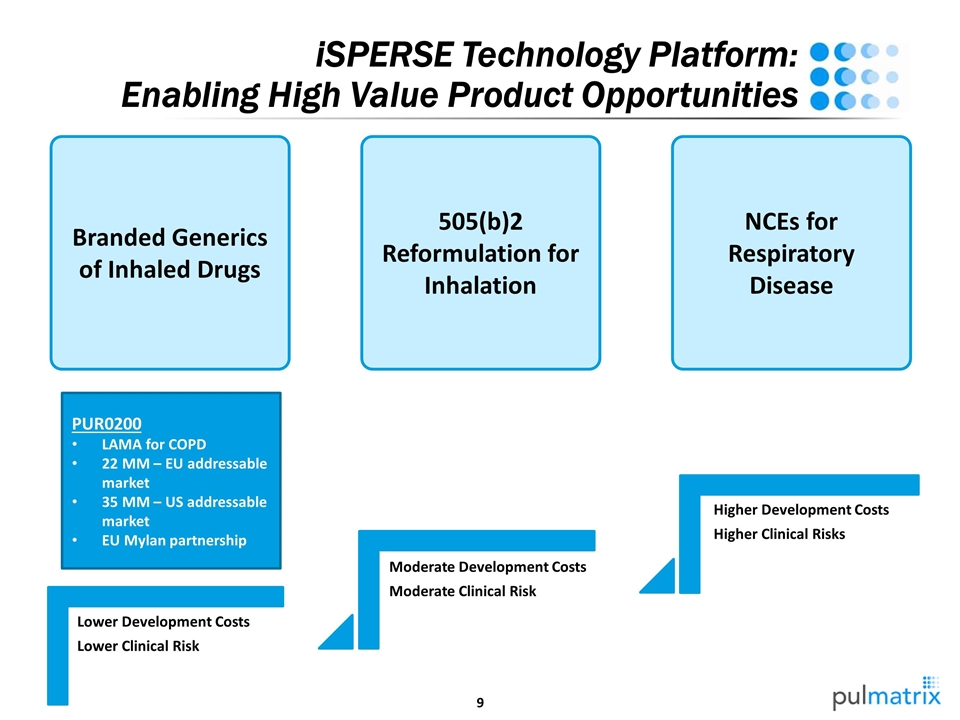
iSPERSE Technology Platform: Enabling High Value Product Opportunities Branded Generics of Inhaled Drugs 505(b)2 Reformulation for Inhalation NCEs for Respiratory Disease PUR0200 LAMA for COPD 22 MM – EU addressable market 35 MM – US addressable market EU Mylan partnership Lower Development Costs Lower Clinical Risk Moderate Development Costs Moderate Clinical Risk Higher Development Costs Higher Clinical Risks

PUR0200: Bronchodilator for COPD Product Overview Branded generic iSPERSE version of a once daily Long Acting Muscarinic Antagonist (LAMA) bronchodilator Marketed reference product generates annual worldwide sales $5B ($2B EU) Multiple clinical studies completed in both healthy volunteers and COPD patients Product Development Strategy Focused on capital efficient development pathway Initially pursuing European PK Bioequivalence Regulatory pathway Existing data sets will be used to enable U.S. development pathway - 505(b)2 Established R&D collaboration with Mylan N.V. (NASDAQ, TASE: MYL) in March, 2015 for ex-US territories US development & commercialization rights are not held by Mylan
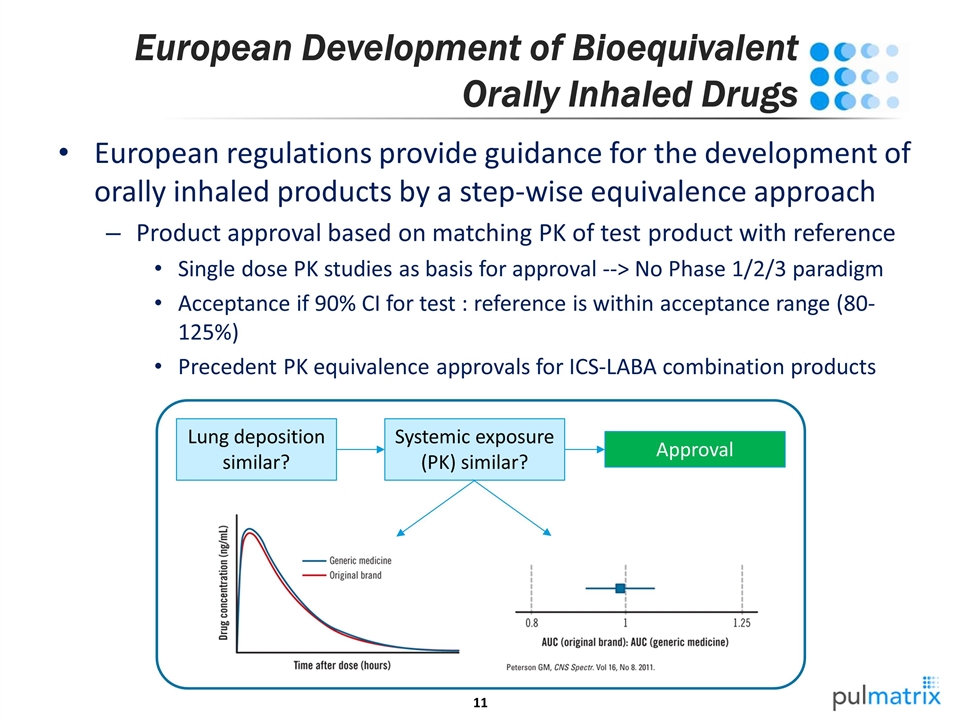
European Development of Bioequivalent Orally Inhaled Drugs European regulations provide guidance for the development of orally inhaled products by a step-wise equivalence approach Product approval based on matching PK of test product with reference Single dose PK studies as basis for approval --> No Phase 1/2/3 paradigm Acceptance if 90% CI for test : reference is within acceptance range (80-125%) Precedent PK equivalence approvals for ICS-LABA combination products Lung deposition similar? Approval Systemic exposure (PK) similar?
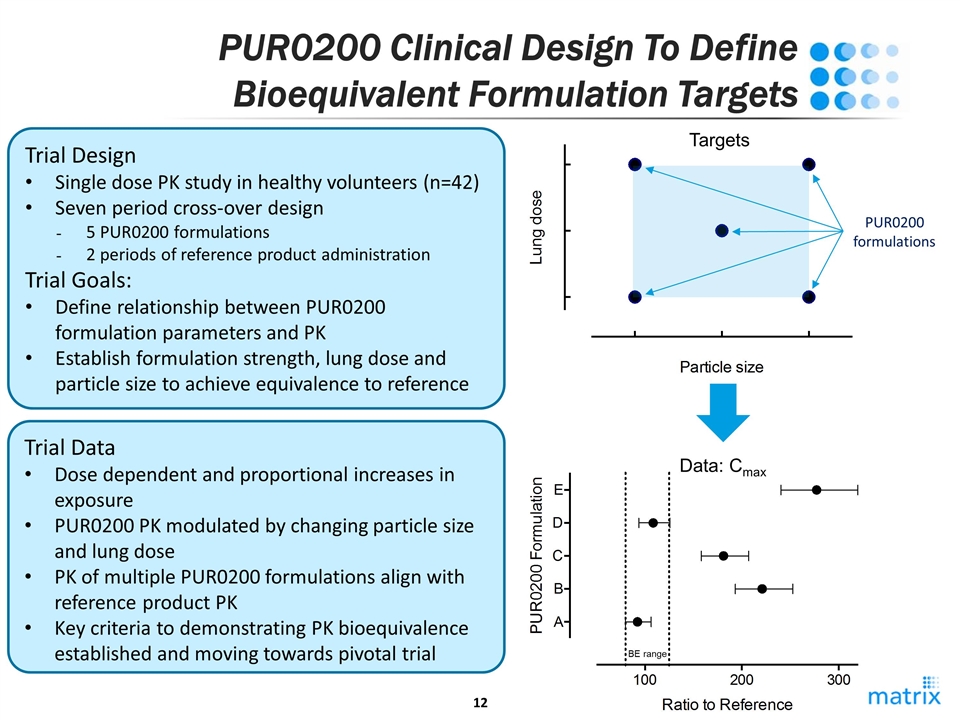
PUR0200 Clinical Design To Define Bioequivalent Formulation Targets PUR0200 formulations Trial Design Single dose PK study in healthy volunteers (n=42) Seven period cross-over design 5 PUR0200 formulations 2 periods of reference product administration Trial Goals: Define relationship between PUR0200 formulation parameters and PK Establish formulation strength, lung dose and particle size to achieve equivalence to reference Trial Data Dose dependent and proportional increases in exposure PUR0200 PK modulated by changing particle size and lung dose PK of multiple PUR0200 formulations align with reference product PK Key criteria to demonstrating PK bioequivalence established and moving towards pivotal trial Targets BE range Data: Cmax
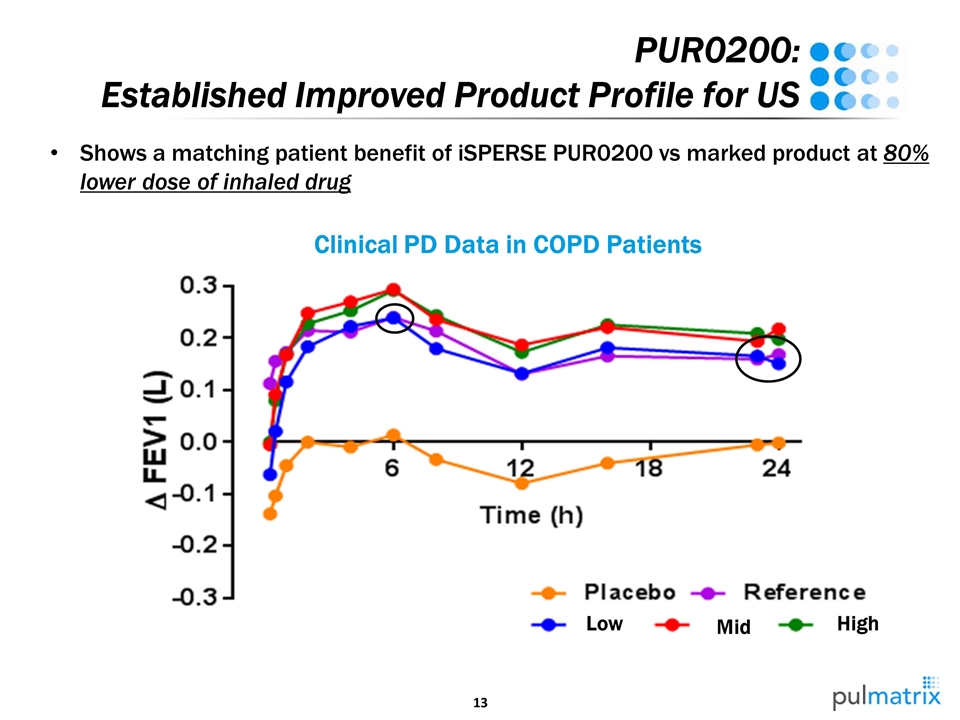
PUR0200: Established Improved Product Profile for US Low Mid High Shows a matching patient benefit of iSPERSE PUR0200 vs marked product at 80% lower dose of inhaled drug Clinical PD Data in COPD Patients
iSPERSE Technology Platform: Enabling High Value Product Opportunities Branded Generics of Inhaled Drugs 505(b)2 Reformulation for Inhalation NCEs for Respiratory Disease PUR1900 Anti-fungal 5.0 MM - addressable market Lower Development Costs Lower Clinical Risk Moderate Development Costs Moderate Clinical Risk Higher Development Costs Higher Clinical Risks
PUR1900: Targeting Fungal Infections Product Overview Inhaled formulation of Itraconazole to treat pulmonary fungal infections Superior PK profile when delivered by inhalation API is commonly used to treat Aspergillus infection Enabled by iSPERSE dry powder delivery technology PUR1900 offers possible advantages over current formulations Improves safety and tolerability profile High drug concentration at the site of infection Potential for better efficacy Product Development Strategy PUR1900 has potential to be used in multiple disease areas Initial development focused on treatment of Cystic Fibrosis (CF) FDA orphan drug designation granted for PUR1900 in CF August 2016 Severe asthma ABPA fungal infections treatment is a parallel target Focused on capital efficient 505(b)2 development pathway
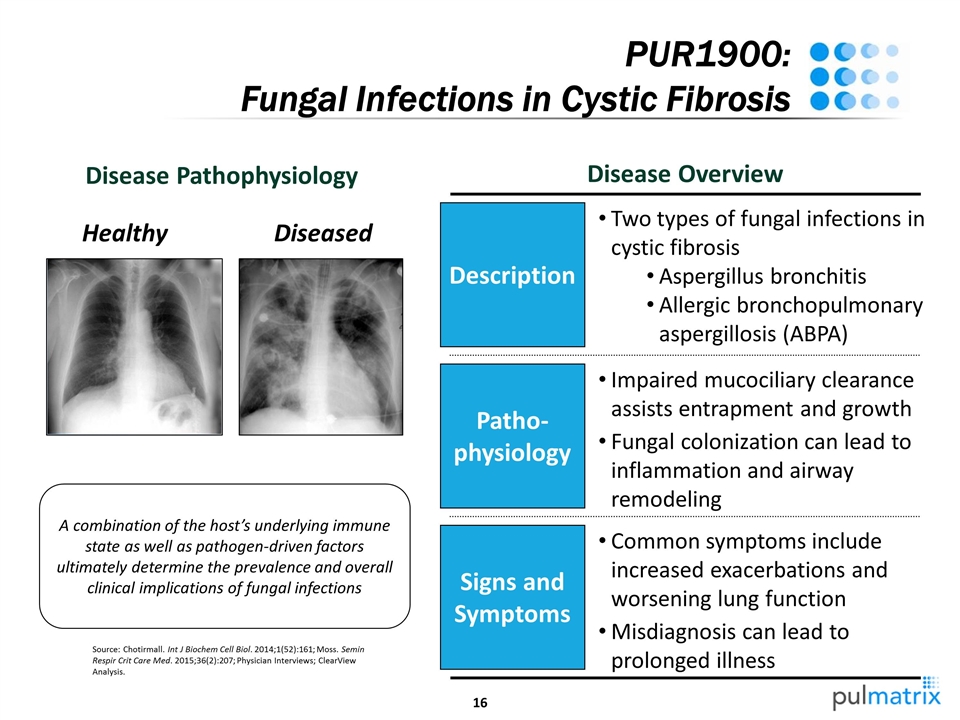
Source: Chotirmall. Int J Biochem Cell Biol. 2014;1(52):161; Moss. Semin Respir Crit Care Med. 2015;36(2):207; Physician Interviews; ClearView Analysis. PUR1900: Fungal Infections in Cystic Fibrosis A combination of the host’s underlying immune state as well as pathogen-driven factors ultimately determine the prevalence and overall clinical implications of fungal infections Disease Pathophysiology Disease Overview Healthy Diseased Description Patho-physiology Signs and Symptoms Impaired mucociliary clearance assists entrapment and growth Fungal colonization can lead to inflammation and airway remodeling Common symptoms include increased exacerbations and worsening lung function Misdiagnosis can lead to prolonged illness Two types of fungal infections in cystic fibrosis Aspergillus bronchitis Allergic bronchopulmonary aspergillosis (ABPA)
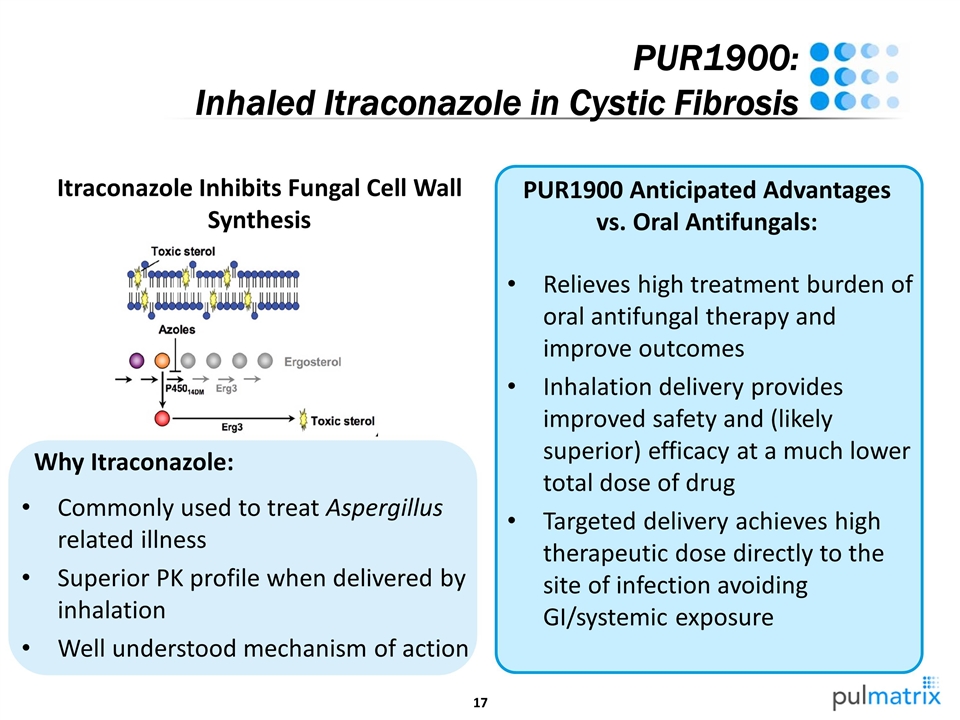
Relieves high treatment burden of oral antifungal therapy and improve outcomes Inhalation delivery provides improved safety and (likely superior) efficacy at a much lower total dose of drug Targeted delivery achieves high therapeutic dose directly to the site of infection avoiding GI/systemic exposure PUR1900: Inhaled Itraconazole in Cystic Fibrosis Itraconazole Inhibits Fungal Cell Wall Synthesis Commonly used to treat Aspergillus related illness Superior PK profile when delivered by inhalation Well understood mechanism of action Why Itraconazole: PUR1900 Anticipated Advantages vs. Oral Antifungals:
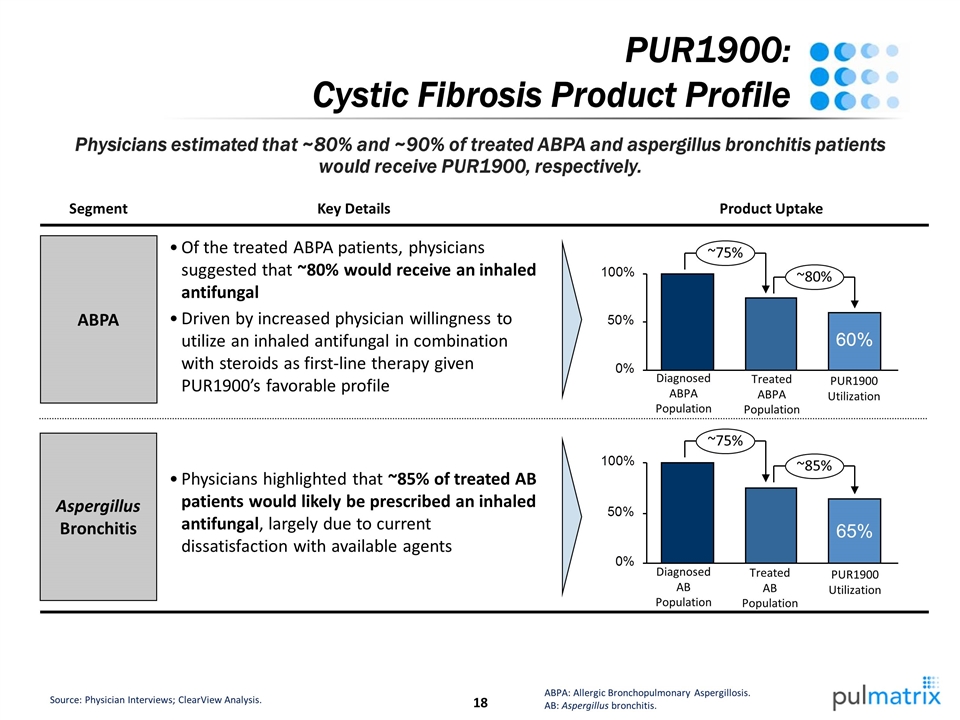
PUR1900: Cystic Fibrosis Product Profile Physicians highlighted that ~85% of treated AB patients would likely be prescribed an inhaled antifungal, largely due to current dissatisfaction with available agents Source: Physician Interviews; ClearView Analysis. ABPA: Allergic Bronchopulmonary Aspergillosis. AB: Aspergillus bronchitis. Physicians estimated that ~80% and ~90% of treated ABPA and aspergillus bronchitis patients would receive PUR1900, respectively. ~75% ~80% Of the treated ABPA patients, physicians suggested that ~80% would receive an inhaled antifungal Driven by increased physician willingness to utilize an inhaled antifungal in combination with steroids as first-line therapy given PUR1900’s favorable profile Key Details ABPA Aspergillus Bronchitis Segment Product Uptake Treated ABPA Population Diagnosed ABPA Population PUR1900 Utilization ~75% ~85% 65% Treated AB Population Diagnosed AB Population PUR1900 Utilization
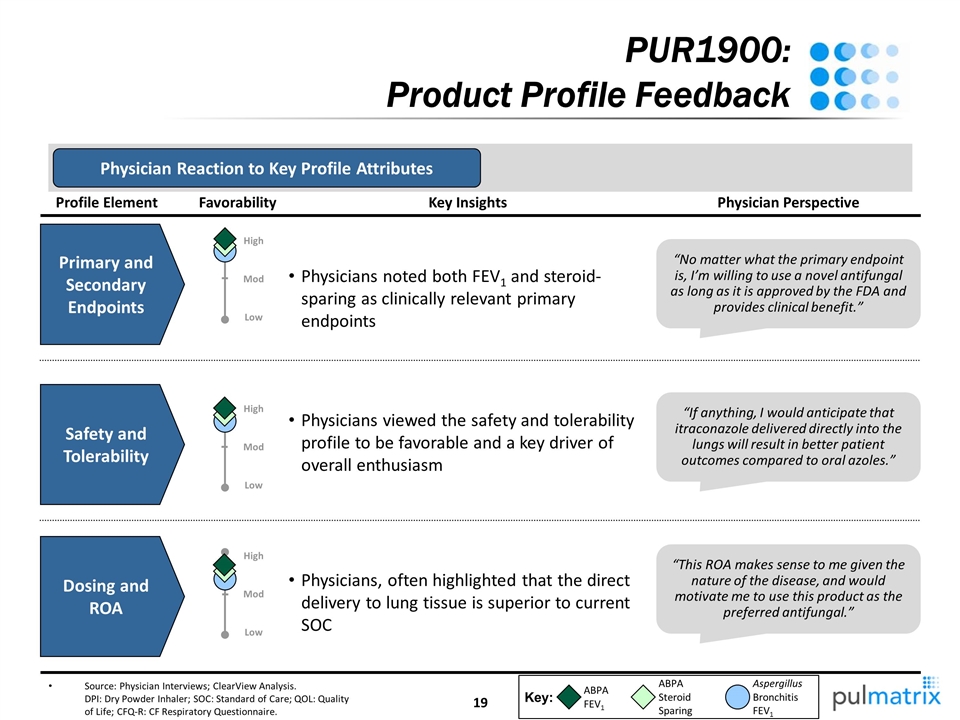
PUR1900: Product Profile Feedback Source: Physician Interviews; ClearView Analysis. DPI: Dry Powder Inhaler; SOC: Standard of Care; QOL: Quality of Life; CFQ-R: CF Respiratory Questionnaire. Profile Element Safety and Tolerability Dosing and ROA “If anything, I would anticipate that itraconazole delivered directly into the lungs will result in better patient outcomes compared to oral azoles.” “This ROA makes sense to me given the nature of the disease, and would motivate me to use this product as the preferred antifungal.” Physician Perspective Physicians viewed the safety and tolerability profile to be favorable and a key driver of overall enthusiasm Physicians, often highlighted that the direct delivery to lung tissue is superior to current SOC Key Insights Favorability Physician Reaction to Key Profile Attributes Key: ABPA FEV1 ABPA Steroid Sparing Aspergillus Bronchitis FEV1 Primary and Secondary Endpoints “No matter what the primary endpoint is, I’m willing to use a novel antifungal as long as it is approved by the FDA and provides clinical benefit.” Physicians noted both FEV1 and steroid-sparing as clinically relevant primary endpoints Low High Mod Low High Mod High Low Mod
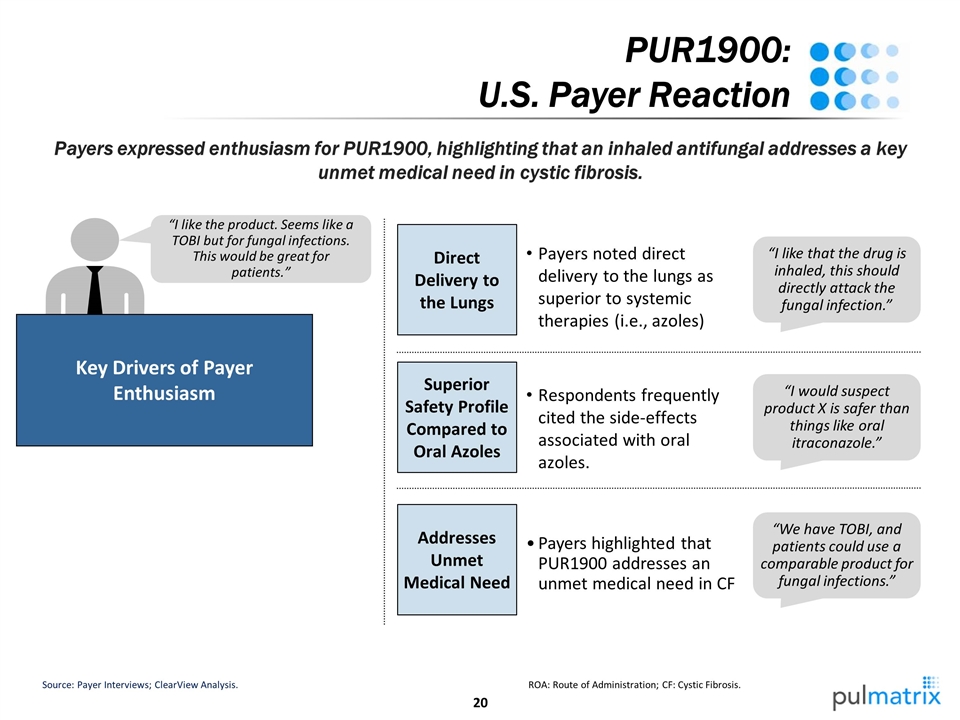
PUR1900: U.S. Payer Reaction Payers expressed enthusiasm for PUR1900, highlighting that an inhaled antifungal addresses a key unmet medical need in cystic fibrosis. Source: Payer Interviews; ClearView Analysis. ROA: Route of Administration; CF: Cystic Fibrosis. “I like the product. Seems like a TOBI but for fungal infections. This would be great for patients.” Key Drivers of Payer Enthusiasm Direct Delivery to the Lungs “I like that the drug is inhaled, this should directly attack the fungal infection.” Payers noted direct delivery to the lungs as superior to systemic therapies (i.e., azoles) “I would suspect product X is safer than things like oral itraconazole.” Superior Safety Profile Compared to Oral Azoles Respondents frequently cited the side-effects associated with oral azoles. “We have TOBI, and patients could use a comparable product for fungal infections.” Addresses Unmet Medical Need Payers highlighted that PUR1900 addresses an unmet medical need in CF
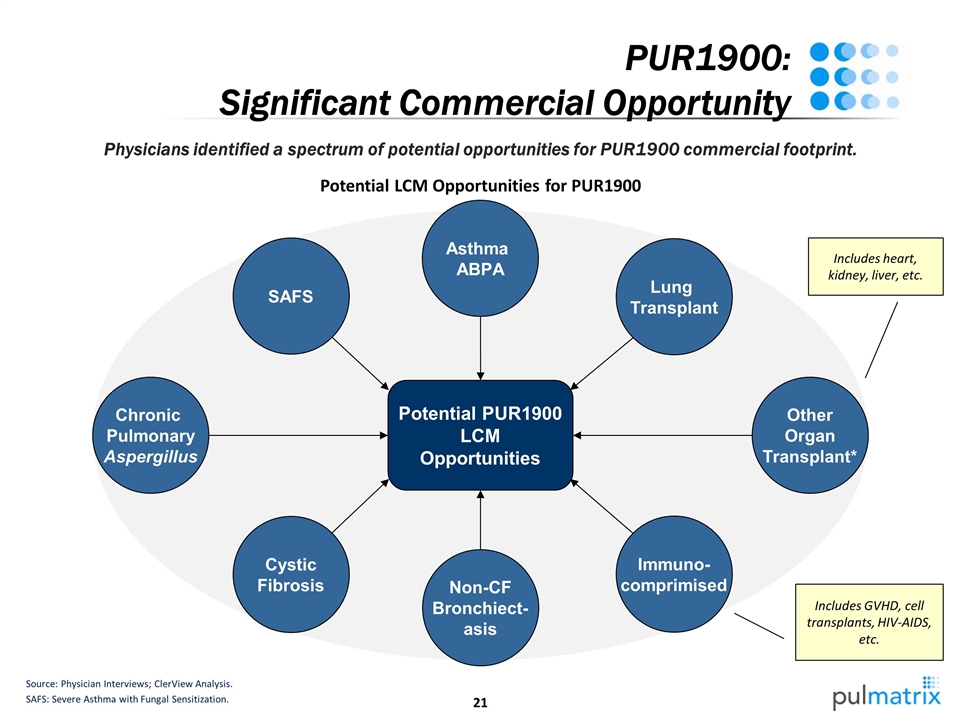
PUR1900: Significant Commercial Opportunity Source: Physician Interviews; ClerView Analysis. SAFS: Severe Asthma with Fungal Sensitization. Potential LCM Opportunities for PUR1900 Physicians identified a spectrum of potential opportunities for PUR1900 commercial footprint. Potential PUR1900 LCM Opportunities Chronic Pulmonary Aspergillus SAFS Other Organ Transplant* Asthma ABPA Lung Transplant Immuno- comprimised Cystic Fibrosis Non-CF Bronchiect- asis Includes heart, kidney, liver, etc. Includes GVHD, cell transplants, HIV-AIDS, etc.
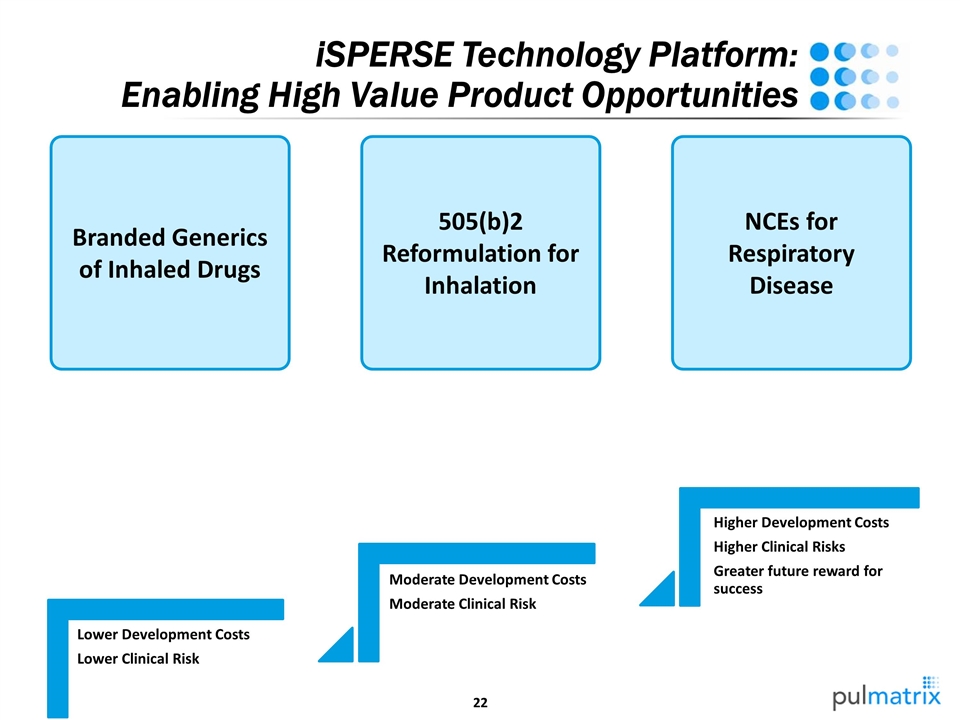
iSPERSE Technology Platform: Enabling High Value Product Opportunities Branded Generics of Inhaled Drugs 505(b)2 Reformulation for Inhalation NCEs for Respiratory Disease Lower Development Costs Lower Clinical Risk Moderate Development Costs Moderate Clinical Risk Higher Development Costs Higher Clinical Risks Greater future reward for success
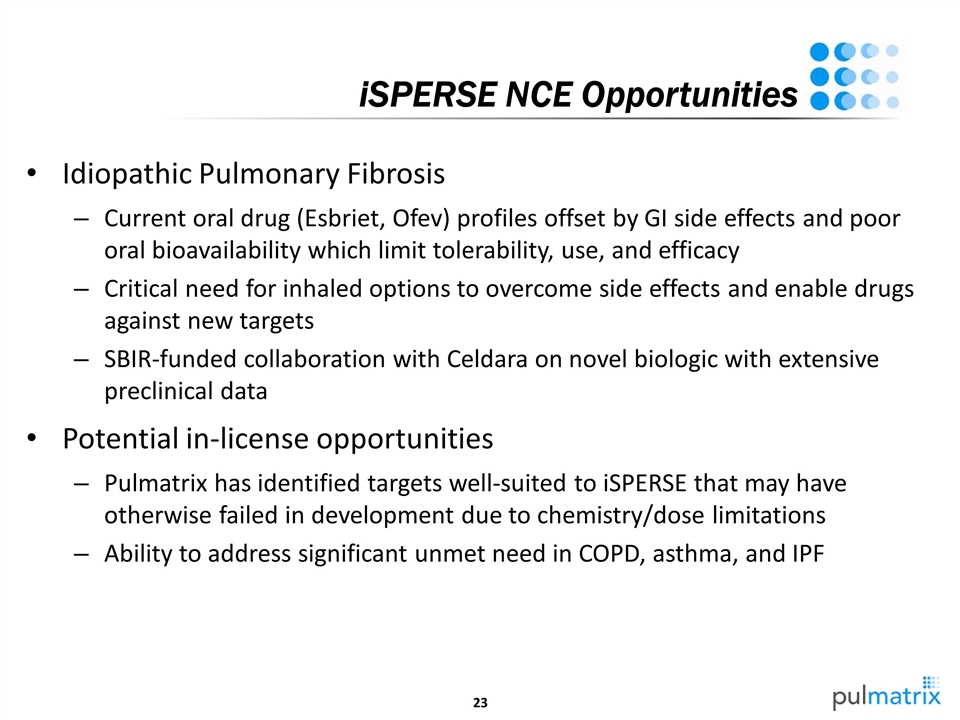
iSPERSE NCE Opportunities Idiopathic Pulmonary Fibrosis Current oral drug (Esbriet, Ofev) profiles offset by GI side effects and poor oral bioavailability which limit tolerability, use, and efficacy Critical need for inhaled options to overcome side effects and enable drugs against new targets SBIR-funded collaboration with Celdara on novel biologic with extensive preclinical data Potential in-license opportunities Pulmatrix has identified targets well-suited to iSPERSE that may have otherwise failed in development due to chemistry/dose limitations Ability to address significant unmet need in COPD, asthma, and IPF
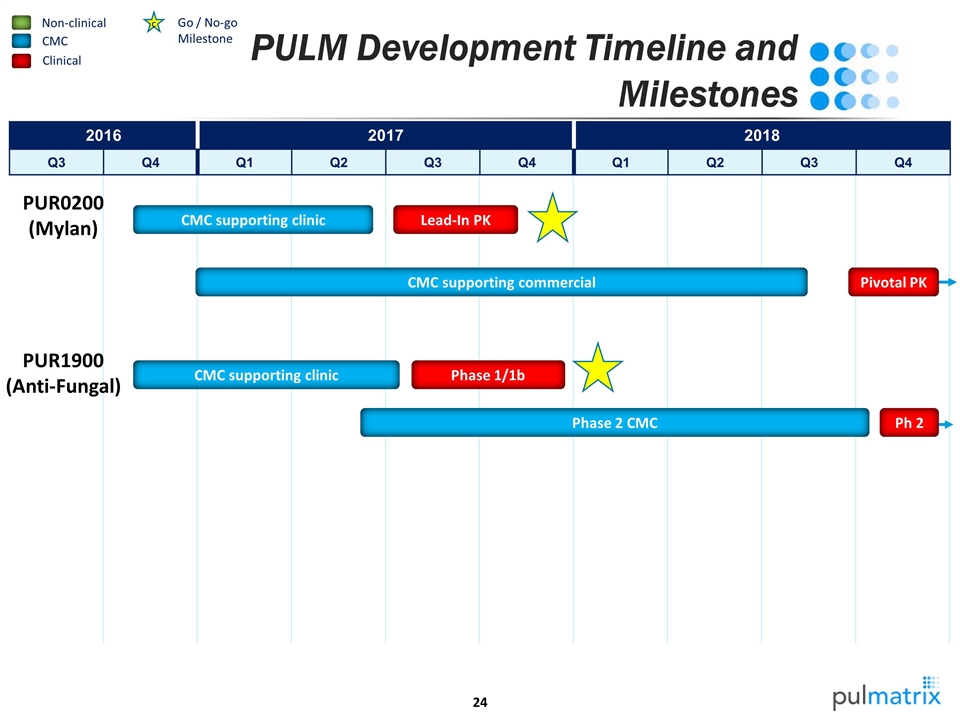
PULM Development Timeline and Milestones Non-clinical CMC 2016 2017 2018 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Clinical c Go / No-go Milestone PUR0200 (Mylan) CMC supporting clinic Lead-In PK PUR1900 (Anti-Fungal) Pivotal PK CMC supporting commercial CMC supporting clinic Phase 1/1b Ph 2 Phase 2 CMC
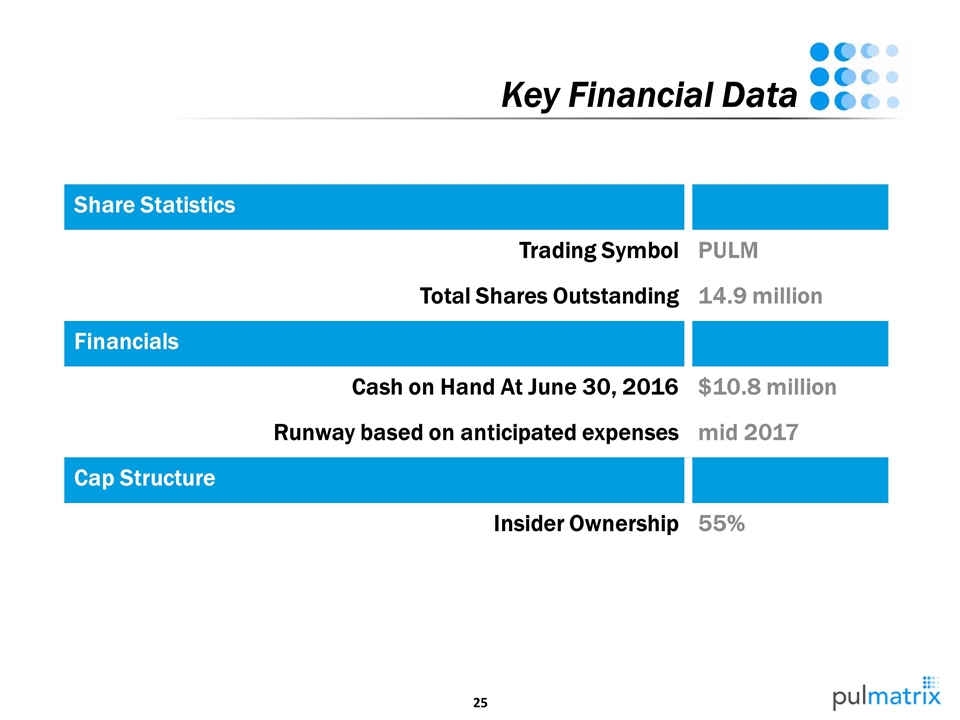
Share Statistics Trading Symbol PULM Total Shares Outstanding 14.9 million Financials Cash on Hand At June 30, 2016 $10.8 million Runway based on anticipated expenses mid 2017 Cap Structure Insider Ownership 55% Key Financial Data

September 2016 NASDAQ: PULM Engineering the Future for Inhaled Therapeutics
