Attached files
| file | filename |
|---|---|
| EX-32.2 - CERTIFICATION - Spotlight Innovation Inc. | stlt_ex322.htm |
| EX-32.1 - CERTIFICATION - Spotlight Innovation Inc. | stlt_ex321.htm |
| EX-31.2 - CERTIFICATION - Spotlight Innovation Inc. | stlt_ex312.htm |
| EX-31.1 - CERTIFICATION - Spotlight Innovation Inc. | stlt_ex311.htm |
| EX-10.29 - CONVERTIBLE NOTE - Spotlight Innovation Inc. | stlt_ex1029.htm |
U.S. SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
Mark One
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2015
| ¨ | ¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File No. 333-141060
Spotlight Innovation Inc. |
(Name of small business issuer in its charter) |
Nevada | 98-0518266 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
6750 Westown Parkway, Suite 200-226 West Des Moines, IA 50266 |
(Address of principal executive offices) |
(515) 274-9087 |
(Issuer's telephone number) |
Securities registered pursuant to Section 12(g) of the Act:
COMMON STOCK, $0.001
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act. ¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ¨ Yes x No
Indicate by check mark whether the registrant has (i) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (ii) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained in this form, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated file, or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filed | ¨ | Accelerated filer | ¨ |
Non-accelerated filer | ¨ | Smaller reporting company | x |
Indicate by checkmark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the average bid and asked price of the common equity, as of the last business of the registrant's most recently completed second fiscal quarter was $5,549,941.
Indicate the number of shares outstanding of each of the registrant's classes of common stock, as of the latest practicable date: As of August 25, 2016, there were 15,856,974 shares of the Company's common stock outstanding.
SPOTLIGHT INNOVATION INC.
FORM 10-K
| 2 |
This Annual Report on Form 10-K contains forward-looking statements, within the meaning of the Securities Exchange Act of 1934 and the Securities Act of 1933, that involve risks and uncertainties. Forward-looking statements convey our current expectations or forecasts of future events. All statements contained in this Annual Report other than statements of historical fact are forward-looking statements. Forward-looking statements include statements regarding our future financial position, business strategy, budgets, projected costs, plans and objectives of management for future operations. The words "may," "continue," "estimate," "intend," "plan," "will," "believe," "project," "expect," "seek," "anticipate," "should," "could," "would," "potential," or the negative of those terms and similar expressions may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. You should not place undue reliance on these forward-looking statements, which speak only as of the date of this report. All of these forward-looking statements are based on information available to us at this time, and we assume no obligation to update any of these statements. Actual results could differ from those projected in these forward-looking statements as a result of many factors, including those identified in "Business," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and elsewhere. We urge you to review and consider the various disclosures made by us in this report, and those detailed from time to time in our filings with the Securities and Exchange Commission, that attempt to advise you of the risks and factors that may affect our future results.
Overview
We are a bioscience company focused on acquiring the rights, via acquisition, license or otherwise, to innovative and proprietary technologies. We place a premium on identifying and targeting technology candidates including: cancer drugs and treatment therapies for leading disease states; drug and related treatment therapies for leading infectious diseases; and other specialty innovative and proprietary technology candidates. We provide value-added development capability and funding in order to achieve rapid approvals for investigational new drugs to commence human clinical trials. We seek to commercialize, for each technology candidate, one or more indications via: sale, out-license and or strategic relationships/marketing agreements while continuing to retain, where practical, rights/"field of use" to other indications for future commercialization. Our capabilities are intended to provide our candidates: development guidance; regulatory expertise; communications and public relations; and networking with global and U.S. pharmaceutical and biopharmaceutical companies, contract research organizations, and various other funding sources.
Going forward, we will expand our focus on diseases that are either rare (so called "orphan diseases") or new or common but underserved (e.g. feline chronic kidney disease). We believe that indications such as these offer an accelerated path to market, support form nonprofit advocacy groups, scarcity of competition, and pricing leverage (Medicare and third-party insurers). High prices for these drugs are justified by the sponsor's willingness to treat small, underserved patient populations).
We combine innovative thinking with the proven ability to:
· | Identify categories of IP that have the attributes of future growth, scalability and profit potential. | |
|
|
|
· | Provide accurate, defensible and actionable business analysis. | |
|
|
|
· | Identify existing healthcare IP companies with significant growth potential. | |
|
|
|
· | Time entry into each category based on technological and cultural readiness. | |
|
|
|
· | Identify and overcome barriers to successful commercialization and adoption. | |
|
|
|
· | Implement repeatable processes that bring speed, accuracy and efficiency to commercialization. |
As of December 31, 2015, we have three subsidiaries: Celtic Biotech Iowa, Inc., CDT Veterinary Therapeutics, Inc., and Memcine Pharmaceuticals, Inc. A description of each subsidiary and it technologies are set forth below.
| 3 |
Intellectual Property
Our goal is to protect the proprietary technologies that we believe are key to our strategy. We seek to maintain patent protection to cover our product candidates, their methods of use, related technology and other inventions that we feel important to our business. We also rely on trade secrets and monitoring of our proprietary information to protect the aspects of our business that are necessarily appropriate for patent protection. A third party may hold intellectual property, including patent rights, which are important or necessary to the development of our technologies. It may be necessary for us to use the patented or proprietary technology of third parties to commercialize our products, in which case we would be required to obtain a license from these third parties on commercially reasonable terms, or our business could be harmed, possibly materially. If we were not able to obtain a license, or were not able to obtain a license on commercially reasonable terms, our business could be harmed, possibly materially. The scope of coverage claimed in a patent application can be significantly reduced and or modified before and after the patent is issued. Consequently, we do not know whether any of our technology candidates will be protectable or remain protected by enforceable patents. We cannot predict whether the patent applications we are currently pursuing will issue as patents or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties.
Research and Development
Our business is dependent on conducting research and development. We have spent and continue to spend significant time and capital on conducting research and development. Our research and development expenses were $274,894 and $8,944 for 2015 and 2014, respectively. We anticipate that our research and development expenses will continue to be substantial and to significantly increase as we continue the development of our existing technologies.
Celtic Biotech Iowa, Inc.
On June 4, 2014, Celtic Biotech Iowa, Inc. (hereinafter "Celtic Iowa," a subsidiary of the Company) acquired Celtic Biotech Limited (hereinafter "Celtic Limited"). Celtic Limited was founded in 2003 in Dublin, Ireland and is developing novel and highly specialized treatment therapies for the treatment of solid cancers and pain in humans derived from snake venom. These compounds possess specific anti-cancer and analgesic properties which apply to a broad spectrum of treatments for solid cancerous tumors and pain management. These compounds possess specific anti-cancer and analgesic cancer indications (solid cancerous tumors) and pain management. Celtic Iowa's main focus has been the development of two specific snake venom toxins: crotoxin, as a pain and cancer therapeutic, and cardiotoxin, for cancer and chronic kidney disease. Derived from naturally specialized receptor-binding proteins, these products have the potential to reduce treatment costs, and increase survivability.
In March 2015, Celtic Iowa licensed worldwide (excluding China) rights to develop and market Cardiotoxin for the treatment of chronic kidney disease. The patent-pending invention, licensed from Zheng-Hong Qin of Soochow University, China, is titled "Use of Cardiotoxin for the Treatment of Chronic Kidney Disease." Per terms of the licensing agreement, Celtic Iowa will pay a seven percent (7%) royalty on any eventual sales of a product developed with the licensed technology. The license expires on a country-by-country basis upon the expiration date of the last valid patent claim covering the manufacture, use, importation, or sale of each product in such country, unless earlier terminated.
In March 2015, Celtic Iowa entered into an exclusive license agreement in American territories for a patent held by Instituto Butantan, São Paulo, Brazil, related to the use of Crotamine as an imaging agent for cancer and potentially for gene therapy. Pursuant to this license Celtic Iowa agreed to pay Instituto Butantan, (located in São Paulo, Brazil) $30,000 and a 10% royalty on gross sales of Crotamine. This license expires upon the expiration of the patent.
In March 2015, EPISORB, a topical gel for use as a transdermal drug delivery agent with analgesic and scar reduction creams, developed by Celtic Iowa, was registered with the U.S. Food and Drug Administration and was incorporated into the Pharmacy Benefit Managers database referenced by health insurance companies when reimbursing drugs. The product makes use of the attributes of cardiotoxin as a cell penetrating peptide and is intended for distribution through the compounding pharmacy industry.
| 4 |
In June 2015, Celtic Iowa entered into a Research Agreement with Emory University to conduct a research study titled "Development and Preclinical Validation of a Novel Fluorine-18 Labelled Crotamine Radiotracer for the Noninvasive Imaging of Lung Cancer" ("Study") in accordance with the Study protocol. The preclinical study will be designed and conducted by an Emory University research team with the specific aim of demonstrating that the cell penetrating peptide Crotamine can be made into a suitable radiopharmaceutical for imaging lung cancer tumors with positron emission tomography in a clinical setting.
In July 2015, Celtic Iowa entered into a Clinical Study Management Agreement (the "Agreement") with ImmunoClin Ltd. ("ImmunoClin") whereby ImmunoClin is to manage, on behalf of Celtic Iowa, the second part of the study Open Label Phase I Clinical Trial of Crotoxin in Patients with Advanced Cancer using an Intravenous Route of Administration". Pursuant to the Agreement the timing of the Study is expected to be 18 months, and Celtic Iowa has agreed to pay ImmunoClin an aggregate of Euro 525,330 ($576,865 USD based on current conversion rates) payable in installments (as defined in the Agreement) during the course of the Study.
In August 2015, Celtic Iowa started preparations to conduct Part 2 of the Phase I clinical study, Crotoxin in Patients with Advanced Cancer using an Intravenous Route of Administration. Crotoxin is a neurotoxin derived from the venom of the South American rattlesnake Crotalus durissus. It is expected that Part 2 of the Phase I clinical study will commence in the third quarter of 2016.
The table below shows the drug candidates being developed by Celtic Limited:
DRUG CANDIDATE | INDICATIONS | MECHANISM OF ACTION | ||
Crotoxin | Solid cancers (melanoma, breast cancer and non-small cell lung cancer) | Attacks cell membrane and triggers apoptosis (cell death). The compound also provides analgesic effects. Operates as a single product therapeutic. | ||
|
|
|
|
|
Cardiotoxin | Chronic Kidney Disease | Kidney damage resulting from chronic inflammation is "blocked" by the anti-inflammatory properties of Cardiotoxin. | ||
|
|
|
|
|
VRCTC310 (Combination of Crotoxin and Cardiotoxin) | Hematological (blood-based) cancers and pancreatic cancer | A combination product formulated to address "difficult to treat cancers" that are non-responsive to Crotoxin alone. | ||
|
|
|
|
|
Crotamine* | Cancer diagnostics (imaging, location, staging/metastasis, | When administered, Crotamine naturally seeks out and enters malignant cells, signaling the location, size and status of the tumor. |
_________________
| * | Being developed in cooperation with Emory University. |
CDT Veterinary Therapeutics, Inc.
CDT Veterinary Therapeutics, Inc. ("CDT"), was formed by the Company in November 2015 to create reformulated variants of Celtic's compounds, modified to meet the needs of the veterinary market. The specific properties of these compounds also apply to solid cancerous tumors and pain management for animals. We are researching, evaluating and developing strategic treatments for therapeutic chronic kidney disease in felines.
CDT's overall strategy is to develop products, through approval to market, and then out-license or enter into distribution arrangements with one or more companies. Current products in the veterinary market are focused on antibiotics, anti-parasitics, and vaccines. We anticipate CDT will introduce a series of therapeutic products. We anticipate commencing operations in 2016.
| 5 |
The table below shows the drug candidates being researched by CDT:
DRUG CANDIDATE | INDICATIONS | MECHANISM OF ACTION | ||
Cobramine (a less purified version of Cardiotoxin) | Feline Chronic Kidney Disease (Chronic Kidney Disease is largely non-prevalent in dogs) | Kidney damage resulting from chronic inflammation is "blocked" by the anti-inflammatory properties of Cobramine. |
Memcine Pharmaceuticals, Inc.
The Company acquired approximately eighty two percent (82%) of Memcine Pharmaceuticals, Inc. ("Memcine") in June 2015. The Company agreed to provide Memcine with up to $3,000,000 to fund the operations of Memcine via investment, grants or other means over the course of operations, upon the achievement of certain milestones, as determined by the board of directors of Memcine. Memcine, founded in 2010, holds the exclusive worldwide rights to Immunoplex,™, a vaccine platform technology developed at the University of Iowa, with universal application to numerous antigens developed to improve vaccine efficacy by using more efficient targeting and delivery.
Immunoplex™
Immunoplex™ is an immune complex consisting of an antigen which is "bound" by one or more antibodies. This technology utilizes a "universal" antibody, in conjunction with a proprietary epitope "adapter," that binds to targeted antigens to facilitate the use of asingle universal antibody to form an immune complex with any antigen, to be used as a vaccine for truly personalized cancer treatments and for prevention of specific infectious diseases. Applications for the technology are solid cancerous tumors (a broad range of cancer indications) and a virtually unlimited number of viral and bacterial infections. We believe Immunoplex™ is the first truly personalized tumor cancer treatment for any cancer indication, derived from a patient's own tumor, for which a biopsy or lumpectomy can be performed, or where ~0.1mg of cancer cells can be culled. Preliminary test data indicates that Immunoplex™ may be delivered intranasally (needle-free). We believe that this technology will provide improved safety, compliance, and vaccine efficacy, particularly for individuals with compromised immune systems.
We anticipate that this disruptive technologywillallows pharmaceutical companies to overcome the current obstacles pertaining to immune complex vaccine production:
| · | Antigen-specific antibody identification | |
|
| |
| · | Antigen-specific antibody production | |
|
| |
| · | Antigen-specific antibody testing |
| 6 |
The chart below indicates the therapy indications.
INDICATIONS | MECHANISM OF ACTION | |
CANCER (PERSONALIZED MEDICINE) |
| |
Breast cancer Melanoma Pancreatic cancer Non-small cell lung cancer | Immune complexes are recognized by Antigen Presenting Cells (APCs) through antibody-specific receptors called Fc Receptors. The immune complexes stimulate APCs that then subsume the immune complexes. The APCs use the subsumed material to educate the adaptive arm of the immune response (T and B cells) to attack the disease-related antigens. |
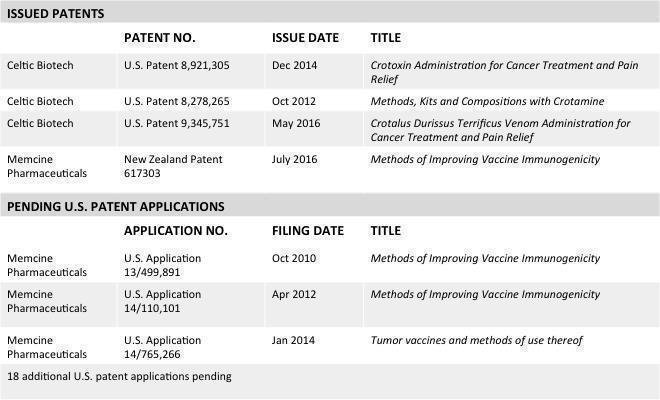
The tables below reflect the Intellectual Property of the Company:
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, and medical technology companies. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
| 7 |
There are a large number of companies developing or marketing pain therapies for the indications that we are pursuing. Many of our competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Small or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. We also compete with these companies in recruiting and retaining qualified scientific personnel and establishing clinical trial sites and patient registration for clinical trials.
FDA Approval Process
(a) Investigational New Drug Application ("IND") - At IND, the FDA decides whether it is reasonably safe for the company to move forward with testing the drug in humans after evaluating the results of preclinical testing in laboratory animals and what they propose to do for human testing.
(b) Clinical Trials- Drug studies in humans can begin only after an IND is reviewed by the FDA and a local institutional review board. At the commencement of clinical trials, they approve the clinical trial protocols, which describe the type of people who may participate in the clinical trial, the schedule of tests and procedures, the medications and dosages to be studied, the length of the study, the study's objectives, and other details.
(c) Phase 1- Phase 1 studies are usually conducted in healthy volunteers. The goal here is to determine what the drug's most frequent side effects are and, often, how the drug is metabolized and excreted. Phase 1 focuses on safety.
(d) Phase 2- Phase 2 studies begin if Phase 1 studies do not reveal unacceptable toxicity. Phase 2 focuses on effectiveness. This phase aims to obtain preliminary data on whether the drug works in people who have a certain disease or condition. For controlled trials, patients receiving the drug are compared with similar patients receiving a different treatment--usually an inactive substance (placebo).
(e) Phase 3- Phase 3 studies begin if evidence of effectiveness is shown in Phase 2. These studies gather more information about safety and effectiveness, studying different populations in a larger group and different dosages and using the drug in combination with other drugs.
(f) New Drug Application ("NDA") - The NDA is the formal step a drug sponsor takes to ask that the FDA consider approving a new drug for marketing in the United States. An NDA includes all animal and human data and analyses of the data, as well as information about how the drug behaves in the body and how it is manufactured.
Employees
We employ one person on a full-time basis (Cristopher Grunewald, our President/Chief Executive Officer) and one on a part-time basis (William Pim our Chief Financial Officer). These individuals are primarily responsible for all of our day-to-day operations. Other services are provided by outsourcing and consultant and special purpose contracts.
Investing in our common stock involves a high degree of risk. Before making an investment decision you should carefully consider the risks described below with all of the other information we include in this report and the additional information we include in the other reports we file with the Securities and Exchange Commission (the "SEC" or the "Commission"). These risks may result in material harm to our business and our financial condition and results of operations. In this event, the market price of our common stock may decline and you can lose part or all of your investment.
| 8 |
Risks Related to Our Business and Industry
We are dependent on the commercial success of our technologies which may never be successfully commercialized.
All of our product candidates are in early stages of development and subject to the risks of failure inherent in developing drug products. Accordingly, we do not have the ability to generate product revenues in the near term. Successful commercialization will depend on whether we can adequately protect against and effectively respond to any claims by holders of patents and other intellectual property rights that our products infringe their rights, whether any unanticipated adverse effects or unfavorable publicity develops in respect of our products, as well as the emergence of new or existing products as competition, which may be proven to be more clinically effective and cost-effective. If we are unable to successfully complete these tasks, we may not be able to commercialize our products at all, in which case we may be unable to generate sufficient revenues to sustain and grow our business.
We are dependent on the success of our product candidates, which may never receive regulatory approval or be successfully commercialized.
To date, we have expended significant time, resources, and effort on the development of our product candidates. All of our product candidates are in early stages of development and subject to the risks of failure inherent in developing drug products.
The ability to successfully commercialize any of our products candidates will depend on, among other things, the ability to:
| · | receive marketing approvals from the FDA and similar foreign regulatory authorities; | |
|
| |
| · | produce, through a validated process, sufficiently large quantities of our product candidates to permit successful commercialization; | |
|
| |
| · | establish commercial manufacturing arrangements with third-party manufacturers; | |
|
| |
| · | build and maintain strong sales, distribution and marketing capabilities sufficient to launch commercial sales of our product candidates; | |
|
| |
| · | establish collaborations with third parties for the commercialization of our product candidates in countries outside the United States, and such collaborators' ability to obtain regulatory and reimbursement approvals in such countries; | |
|
| |
| · | secure acceptance of our product candidates from physicians, health care payors, patients and the medical community; | |
|
| |
| · | successfully complete our clinical trials; and | |
|
| |
| · | manage our spending as costs and expenses increase due to commercialization and clinical trials. |
There are no guarantees that we will be successful in completing these tasks. If we are unable to successfully complete these tasks, we may not be able to commercialize any of our product candidates in a timely manner, or at all, in which case we may be unable to generate sufficient revenues to sustain and grow our business. In addition, if we experience unanticipated delays or problems, development costs could substantially increase and our business, financial condition and results of operations will be adversely affected.
We have limited sales and marketing experience and resources, and we may not be able to effectively market and sell our products or product candidates, if approved, in the United States.
We have limited sales and marketing experience. Further, we could face a number of additional risks in establishing internal sales and marketing capabilities, including:
| · | we may not be able to attract talented and qualified personnel to build an effective marketing or sales force capability; | |
|
| |
| · | the cost of establishing a marketing and sales force capability may not be justifiable in light of the potential revenues generated by any of our products if they were to receive final approval by the FDA; and | |
|
| |
| · | our direct sales and marketing efforts may not be successful. |
If we are unable to establish adequate sales and marketing capabilities or are unable to do so in a timely manner, we may not be able to generate product revenues and may never become profitable.
| 9 |
The commercial success of our products and product candidates, if approved, depends upon attaining market acceptance by physicians, patients, third-party payors and the medical community.
Physicians may not prescribe any of our product candidates if approved by the FDA, in which case we would not generate the revenues we anticipate. Market acceptance of any of our products or product candidates by physicians, patients, third-party payors and the medical community depends on, among other things:
| · | our ability to provide acceptable evidence of safety and efficacy; | |
|
| |
| · | acceptance by physicians and patients of each product or product candidate as a safe and effective treatment; | |
|
| |
| · | perceived advantages of our products or product candidates over alternative treatments; | |
|
| |
| · | relative convenience and ease of administration of our products or product candidates compared to existing treatments; | |
|
| |
| · | any labeling restrictions placed upon each product or product candidate in connection with its approval; | |
|
| |
| · | the prevalence and severity of the adverse side effects of each of our products or product candidates; | |
|
| |
| · | the clinical indications for which each of our products or product candidates are approved, including any potential additional restrictions placed upon each product or product candidate in connection with its approval; | |
|
| |
| · | prevalence of the disease or condition for which each product or product candidate is approved; | |
|
| |
| · | the cost of treatment in relation to alternative treatments, including generic products; | |
|
| |
| · | the extent to which each product or product candidate is approved for inclusion on formularies of hospitals and managed care organizations; | |
|
| |
| · | any negative publicity related to our or our competitors' products or product candidates, including as a result of any related adverse side effects; | |
|
| |
| · | the effectiveness of our or any current or future collaborators' sales, marketing and distribution strategies; | |
|
| |
| · | pricing and cost effectiveness; and | |
|
| |
| · | the availability of adequate reimbursement by third parties. |
If our product candidates do not achieve an adequate level of acceptance by physicians, third-party payors and patients, we may not generate sufficient revenues from these products or product candidates to become or remain profitable on a timely basis, if at all.
Final marketing approval of any of our product candidates by the FDA or other regulatory authorities may be delayed, limited, or denied, any of which would adversely affect our ability to generate operating revenues.
Our business depends on the successful development and commercialization of our products and product candidates. We are not permitted to market any of our product candidates in the United States until we receive approval of a new drug application, or NDA, from the FDA, or in any foreign jurisdiction until we receive the requisite approvals from such jurisdiction. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. We cannot predict whether or when we will obtain regulatory approval to commercialize our product candidates and we cannot, therefore, predict the timing of any future revenues from these product candidates, if any.
| 10 |
The FDA requires submission of information needed to support any changes to a previously approved drug, such as published data or new studies conducted by the applicant or clinical trials demonstrating safety and effectiveness. The FDA could refuse to file or approve our NDA submissions, request additional information before accepting our submissions for filing or require additional information to sufficiently demonstrate safety and effectiveness. The FDA has substantial discretion in the drug approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. For example, the FDA:
| · | could determine that we cannot rely on Section 505(b)(2) for any of our product candidates; | |
|
| |
| · | could determine that the information provided by us was inadequate, contained clinical deficiencies or otherwise failed to demonstrate the safety and effectiveness of any of our product candidates for any indication; | |
|
| |
| · | may not find the data from bioequivalence studies and/or clinical trials sufficient to support the submission of an NDA or to obtain marketing approval in the United States, including any findings that the clinical and other benefits of our product candidates outweigh their safety risks; | |
|
| |
| · | may disagree with our trial design or our interpretation of data from preclinical studies, bioequivalence studies and/or clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our trials; | |
|
| |
| · | may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for the supply of the active pharmaceutical ingredient, or API, used in our product candidates; | |
|
| |
| · | may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for the manufacturing of our product candidates; | |
|
| |
| · | may approve our product candidates for fewer or more limited indications than we request, or may grant approval contingent on the performance of costly post-approval clinical trials; | |
|
| |
| · | may change its approval policies or adopt new regulations; or | |
|
| |
| · | may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product candidates. |
Notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), over the last few years, some pharmaceutical companies and others have objected to the FDA's interpretation of Section 505(b)(2). If the FDA changes its interpretation of Section 505(b)(2), or if the FDA's interpretation is successfully challenged in court, this could delay or even prevent the FDA from approving any Section 505(b)(2) application that we submit. Any failure to obtain regulatory approval of our product candidates would significantly limit our ability to generate revenues, and any failure to obtain such approval for all of the indications and labeling claims we deem desirable could reduce our potential revenues.
Our trials may fail to demonstrate acceptable levels of safety, efficacy or any other requirements of our product candidates, which could prevent or significantly delay regulatory approval.
We may be unable to sufficiently demonstrate the safety and efficacy of our product candidates to obtain regulatory approval. We must demonstrate with substantial evidence gathered in well-controlled studies, and to the satisfaction of the FDA with respect to approval in the United States (and to the satisfaction of similar regulatory authorities in other jurisdictions with respect to approval in those jurisdictions), that each product candidate is safe and effective for use in the target indication. The FDA may require us to conduct or perform additional studies or trials to adequately demonstrate safety and efficacy, which could prevent or significantly delay our receipt of regulatory approval and, ultimately, the commercialization of that product candidate.
In addition, the results from the trials that we have completed for our product candidates may not be replicated in future trials, or we may be unable to demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals for our product candidates. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced development, even after promising results in earlier trials. If our product candidates are not shown to be safe and effective, our clinical development programs could be delayed or might be terminated.
Our product candidates may cause undesirable side effects or have other properties that delay or prevent their regulatory approval or limit their commercial potential.
Undesirable side effects caused by any of our product candidates could cause us or regulatory authorities to interrupt, delay or halt development and could result in the denial of regulatory approval by the FDA or other regulatory authorities, and potential products liability claims. Any undesirable side effects that are caused by any of our product candidates could have a material adverse effect upon that product candidate's development program and our business as a whole.
| 11 |
In addition, if any of our product candidates receive marketing approval, and we or others later identify undesirable side effects caused by the product candidate, a number of potentially significant negative consequences could result, including:
| · | regulatory authorities may withdraw approvals of the product candidate or otherwise require us to take the approved product off the market; | |
|
| |
| · | regulatory authorities may require additional warnings, or a narrowing of the indication, on the product label; | |
|
| |
| · | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; | |
|
| |
| · | we may be required to modify the product in some way; | |
|
| |
| · | the FDA may require us to conduct additional clinical trials or costly post-marketing testing and surveillance to monitor the safety or efficacy of the product; | |
|
| |
| · | sales of approved products may decrease significantly; | |
|
| |
| · | we could be sued and held liable for harm caused to patients; and | |
|
| |
| · | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining the commercial success of our products and product candidates and could substantially increase commercialization costs.
Delays or failures in the completion of testing of our product candidates would increase our costs and delay or limit our ability to generate revenues.
Delays or failures in the completion of clinical trials for our product candidates could significantly raise our product development costs. We do not know whether current or planned trials will be completed on schedule, if at all. The commencement and completion of clinical development can be delayed or halted for a number of reasons, including:
| · | difficulties obtaining regulatory approval to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial; | |
|
| |
| · | delays in reaching or failure to reach agreement on acceptable terms with prospective clinical research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; | |
|
| |
| · | insufficient or inadequate supply or quantity of a product candidate for use in trials; | |
|
| |
| · | difficulties obtaining institutional review board or ethics committee approval to conduct a trial at a prospective site; | |
|
| |
| · | challenges recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including competition from other programs for the treatment of similar conditions; | |
|
| |
| · | severe or unexpected drug-related side effects experienced by patients in a clinical trial; | |
|
| |
| · | difficulty retaining patients who have initiated a clinical trial but may be prone to withdraw due to side effects from the therapy, lack of efficacy or personal issues; and | |
|
| |
| · | clinical holds imposed by the FDA. |
| 12 |
Clinical trials may also be delayed as a result of ambiguous or negative interim results. In addition, clinical trials may be suspended or terminated by us, an institutional review board or ethics committee overseeing the clinical trial at a trial site (with respect to that site), the FDA or other regulatory authorities due to a number of factors, including:
| · | failure to conduct the clinical trial in accordance with regulatory requirements or the trial protocols; | |
|
| |
| · | observations during inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities that ultimately result in the imposition of a clinical hold; | |
|
| |
| · | unforeseen safety issues; or | |
|
| |
| · | lack of adequate funding to continue the trial. |
In addition, failure to conduct the clinical trial in accordance with regulatory requirements or the trial protocols may also result in the inability to use the data to support product approval. Changes in regulatory requirements and guidance may occur, and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to institutional review boards or ethics committees for reexamination, which may impact the costs, timing or successful completion of a clinical trial. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates. If we experience delays in completion of, or if we terminate any of our clinical trials, our ability to obtain regulatory approval for our product candidates may be materially harmed, and our commercial prospects and ability to generate product revenues will be diminished.
We expect intense competition and, if our competitors develop or market alternatives for treatments of our target indications, our commercial opportunities will be reduced or eliminated.
The pharmaceutical industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary therapeutics. We face competition from a number of sources, some of which may target the same indications as our products and product candidates, including large pharmaceutical companies, smaller pharmaceutical companies, biotechnology companies, academic institutions, government agencies and private and public research institutions. The availability of competing products will limit the demand and the price we are able to charge for any of our products or product candidates that are commercialized unless we are able to differentiate them. We anticipate that we will face intense competition when/if our product candidates are approved by regulatory authorities and we begin the commercialization process for our products.
In addition to already marketed competing products, we believe certain companies are developing other products which could compete with our product candidates should they be approved by regulatory authorities. Further, new developments, including the development of other drug technologies, may render our product candidates obsolete or noncompetitive. As a result, our product candidates may become obsolete before we recover expenses incurred in connection with their development or realize revenues from any commercialized product.
Further, many competitors have substantially greater:
| · | capital resources; | |
|
| |
| · | research and development resources and experience, including personnel and technology; | |
|
| |
| · | drug development, clinical trial and regulatory resources and experience; | |
|
| |
| · | sales and marketing resources and experience; | |
|
| |
| · | manufacturing and distribution resources and experience; | |
|
| |
| · | name recognition; and | |
|
| |
| · | resources, experience and expertise in prosecution and enforcement of intellectual property rights. |
As a result of these factors, our competitors may obtain regulatory approval of their products more rapidly than we are able to or may obtain patent protection or other intellectual property rights that limit or block us from developing or commercializing our product candidates. Our competitors may also develop drugs that are more effective, more useful, better tolerated, subject to fewer or less severe side effects, more widely prescribed or accepted or less costly than ours and may also be more successful than us in manufacturing and marketing their products. If we are unable to compete effectively with the products of our competitors or if such competitors are successful in developing products that compete with any of our product candidates that are approved, our business, results of operations, financial condition and prospects may be materially adversely affected. Mergers and acquisitions in the pharmaceutical industry may result in even more resources being concentrated at competitors. Competition may increase further as a result of advances made in the commercial applicability of technologies and greater availability of capital for investment.
| 13 |
Our products and our product candidates, if they receive regulatory approval, may be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements.
Even if U.S. regulatory approval is obtained, the FDA may still impose significant restrictions on a product's indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies. Our product candidates would also be, and our approved product and our collaborators' approved products are, subject to ongoing FDA requirements governing the labeling, packaging, storage, advertising, promotion, recordkeeping and submission of safety and other post-market information. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current Good Manufacturing Practices, or cGMP regulations. If we, our collaborators or a regulatory authority discovers previously unknown problems with a product, such as side effects of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory authority may impose restrictions on that product or the manufacturer, including requiring withdrawal of the product from the market or suspension of manufacturing. If we or our collaborators, or our or our collaborators' approved products or product candidates, or the manufacturing facilities for our or our collaborators' approved products or product candidates fail to comply with applicable regulatory requirements, a regulatory authority may:
| · | issue warning letters or untitled letters; | |
|
| |
| · | impose civil or criminal penalties; | |
|
| |
| · | suspend regulatory approval; | |
|
| |
| · | suspend any ongoing bioequivalence and/or clinical trials; | |
|
| |
| · | refuse to approve pending applications or supplements to applications filed by us; | |
|
| |
| · | impose restrictions on operations, including costly new manufacturing requirements, or suspension of production; or | |
|
| |
| · | seize or detain products or require us to initiate a product recall. |
In addition, our product labeling, advertising and promotion of our product candidates upon FDA approval, will be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product's approved labeling. Physicians may nevertheless prescribe our products and, upon receiving FDA approval, our product candidates to their patients in a manner that is inconsistent with the approved label. The FDA and other authorities actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant sanctions. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. If we are found to have promoted off-label uses, we may be enjoined from such off-label promotion and become subject to significant liability, which would have an adverse effect on our reputation, business and revenues, if any.
If we fail to produce our products and product candidates in the volumes that we require on a timely basis, or fail to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the development and commercialization of our products and product candidates.
We do not currently own or operate manufacturing facilities for the production of any of our product candidates beyond Phase II clinical trials, nor do we have plans to develop our own manufacturing operations for Phase III clinical materials or commercial products in the foreseeable future. We will currently depend on third-party contract manufacturers for the supply of the APIs for our product candidates, including drug substance for our preclinical research and clinical trials. Any future curtailment in the availability of raw materials could result in production or other delays with consequent adverse effects on us. In addition, because regulatory authorities must generally approve raw material sources for pharmaceutical products, changes in raw material suppliers may result in production delays or higher raw material costs.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Pharmaceutical companies often encounter difficulties in manufacturing, particularly in scaling up production of their products. These problems include manufacturing difficulties relating to production costs and yields, quality control, including stability of the product and quality assurance testing, shortages of qualified personnel, as well as compliance with federal, state and foreign regulations. If we are unable to demonstrate stability in accordance with commercial requirements, or if our manufacturers were to encounter difficulties or otherwise fail to comply with their obligations to us, our ability to obtain FDA approval and market our products and product candidates would be jeopardized. In addition, any delay or interruption in the supply of clinical trial supplies could delay or prohibit the completion of our bioequivalence and/or clinical trials, increase the costs associated with conducting our bioequivalence and/or clinical trials and, depending upon the period of delay, require us to commence new trials at significant additional expense or to terminate a trial.
| 14 |
Manufacturers of pharmaceutical products need to comply with cGMP requirements enforced by the FDA through their facilities inspection programs. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. Manufacturers of our products and product candidates may be unable to comply with these cGMP requirements and with other FDA and foreign regulatory requirements. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any of our products or product candidates is compromised due to failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for such product candidate or successfully commercialize such products or product candidates, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay in clinical developments, regulatory submissions, approvals or commercialization of our products or product candidates, entail higher costs or result in our being unable to effectively commercialize our product candidates.
We intend to rely on third-party collaborators to market and commercialize our product candidates, who may fail to effectively commercialize our product candidates.
We currently plan to utilize strategic partners or contract sales forces, where appropriate, to assist in the commercialization of our product candidates, if approved. We currently possess limited resources and may not be successful in establishing collaborations or co-promotion arrangements on acceptable terms, if at all. We also face competition in our search for collaborators and co-promoters. By entering into strategic collaborations or similar arrangements, we will rely on third parties for financial resources and for development, commercialization, sales and marketing and regulatory expertise. Our collaborators may fail to develop or effectively commercialize our product candidates because they cannot obtain the necessary regulatory approvals, they lack adequate financial or other resources or they decide to focus on other initiatives. Any failure of our third-party collaborators to successfully market and commercialize our product candidates outside of the United States would diminish our revenues and harm our results of operations.
Limitations on our patent rights relating to our product candidates may limit our ability to prevent third parties from competing against us.
Our success will depend on our ability to obtain and maintain patent protection for our proprietary technologies and our product candidates, preserve our trade secrets, prevent third parties from infringing upon our proprietary rights and operate without infringing upon the proprietary rights of others. To that end, we seek patent protection in the United States and internationally for our product candidates. Our policy is to actively seek to protect our proprietary position by, among other things, filing patent applications in the United States and abroad (including Europe, Canada and certain other countries when appropriate) relating to proprietary technologies that are important to the development of our business.
The strength of patents in the pharmaceutical industry involves complex legal and scientific questions and can be uncertain. Patent applications in the United States and most other countries are confidential for a period of time until they are published, and publication of discoveries in scientific or patent literature typically lags actual discoveries by several months or more. As a result, we cannot be certain that we were the first to conceive inventions covered by our patents and pending patent applications or that we were the first to file patent applications for such inventions. In addition, we cannot be certain that our patent applications will be granted, that any issued patents will adequately protect our intellectual property or that such patents will not be challenged, narrowed, invalidated or circumvented.
We also rely upon unpatented trade secrets, unpatented know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our employees and our collaborators and consultants. We also have agreements with our employees and selected consultants that obligate them to assign their inventions to us. It is possible that technology relevant to our business will be independently developed by a person that is not a party to such an agreement. Furthermore, if the employees and consultants that are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could otherwise become known or be independently discovered by our competitors. Any failure to adequately prevent disclosure of our trade secrets and other proprietary information could have a material adverse impact on our business.
In addition, the laws of certain foreign countries do not protect proprietary rights to the same extent or in the same manner as the United States, and therefore, we may encounter problems in protecting and defending our intellectual property in certain foreign jurisdictions.
| 15 |
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in that litigation would have a material adverse effect on our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell their approved products and our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we and our collaborators are developing product candidates. As the pharmaceutical industry expands and more patents are issued, the risk increases that our collaborators' approved products and our product candidates may give rise to claims of infringement of the patent rights of others. There may be issued patents of third parties of which we are currently unaware, that may be infringed by our product candidates, which could prevent us from being able to commercialize any of our product candidates. Because patent applications can take many years to issue, there may be currently pending applications which may later result in issued patents that our collaborators' approved products or our product candidates may infringe.
We may be exposed to, or threatened with, future litigation by third parties alleging that our collaborators' approved products or our products or product candidates infringe their intellectual property rights. If one of our collaborators' approved products or our products or product candidates is found to infringe the intellectual property rights of a third party, we or our collaborators could be enjoined by a court and required to pay damages and could be unable to commercialize the applicable approved products and product candidates unless we obtain a license to the patent. A license may not be available to us on acceptable terms, if at all. In addition, during litigation, the patent holder could obtain a preliminary injunction or other equitable relief which could prohibit us from making, using or selling our approved products, pending a trial on the merits, which may not occur for several years.
There is a substantial amount of litigation involving patent and other intellectual property rights in the pharmaceutical industry generally. If a third party claims that we or our collaborators infringe its intellectual property rights, we may face a number of issues, including, but not limited to:
| · | infringement and other intellectual property claims which, regardless of merit, may be expensive and time-consuming to litigate and may divert our management's attention from our core business; | |
|
| |
| · | substantial damages for infringement, which we may have to pay if a court decides that the product at issue infringes on or violates the third party's rights, and, if the court finds that the infringement was willful, we could be ordered to pay treble damages and the patent owner's attorneys' fees; | |
|
| |
| · | a court prohibiting us from selling Oxtellar XR, Trokendi XR, or any product candidate approved in the future, if any, unless the third party licenses its rights to us, which it is not required to do; | |
|
| |
| · | if a license is available from a third party, we may have to pay substantial royalties, fees or grant cross-licenses to our intellectual property rights; and | |
|
| |
| · | redesigning any of our product candidates so they do not infringe, which may not be possible or may require substantial monetary expenditures and time. |
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. In any infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent application at risk of not issuing.
| 16 |
Interference proceedings brought by the U.S. Patent and Trademark Office, or USPTO, may be necessary to determine the priority of inventions with respect to our patents and patent applications or those of our collaborators. An unfavorable outcome could require us to cease using the technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if a prevailing party does not offer us a license on terms that are acceptable to us. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distraction of our management and other employees. We may not be able to prevent, alone or with our collaborators, misappropriation of our proprietary rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceeding or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. There can be no assurance that our product candidate will not be subject to same risks.
We rely and will continue to rely on outsourcing arrangements for certain of our activities, including clinical research of our product candidates and manufacturing of our compounds and product candidates beyond Phase II clinical trials.
We rely on outsourcing arrangements for some of our activities, including manufacturing, preclinical and clinical research, data collection and analysis. We may have limited control over these third parties and we cannot guarantee that they will perform their obligations in an effective and timely manner. Our reliance on third parties, including third-party CROs and CMOs entails risks including, but not limited to:
| · | non-compliance by third parties with regulatory and quality control standards; | |
|
| |
| · | sanctions imposed by regulatory authorities if compounds supplied or manufactured by a third party supplier or manufacturer fail to comply with applicable regulatory standards; | |
|
| |
| · | the possible breach of the agreements by the CROs or CMOs because of factors beyond our control or the insolvency of any of these third parties or other financial difficulties, labor unrest, natural disasters or other factors adversely affecting their ability to conduct their business; and | |
|
| |
| · | termination or non-renewal of an agreement by the third parties, at a time that is costly or inconvenient for us, because of our breach of the manufacturing agreement or based on their own business priorities. |
We do not own or operate manufacturing facilities for the production of any of our product candidates, nor do we have plans to develop our own manufacturing operations for clinical materials or commercial products in the foreseeable future. We currently depend on third-party CMOs for all of our required raw materials and drug substance for our preclinical research and clinical trials. If any of these vendors are unable to perform its obligations to us, including due to violations of the FDA's requirements, our ability to meet regulatory requirements or projected timelines and necessary quality standards for successful manufacture of the various required lots of material for our development and commercialization efforts would be adversely affected. Further, if we were required to change vendors, it could result in delays in our regulatory approval efforts and significantly increase our costs. Accordingly, the loss of any of our current or future third-party manufacturers or suppliers could have a material adverse effect on our business, results of operations, financial condition and prospects.
We do not have contractual relationships for the manufacture of commercial supplies of all of our product candidates. The number of third-party manufacturers with the expertise, required regulatory approvals and facilities to manufacture drug substance and final drug product on a commercial scale is limited. Therefore, we may not be able to enter into such arrangements with third-party manufacturers in a timely manner, on acceptable terms or at all. Failure to secure such contractual arrangements would harm the commercial prospects for our product candidates, our costs could increase and our ability to generate revenues could be delayed.
| 17 |
Even if our product candidates receive regulatory approval in the United States, we or our collaborators may never receive approval to commercialize our product candidates outside of the United States.
In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other jurisdictions regarding safety and efficacy. Approval procedures vary among jurisdictions and can involve product testing and administrative review periods different from, and greater than those in the United States. The time required to obtain approval in other jurisdictions might differ from that required to obtain FDA approval. The regulatory approval process in other jurisdictions may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. For example, legislation analogous to Section 505(b)(2) of the FDCA in the United States, which relates to the ability of an NDA applicant to use published data not developed by such applicant, may not exist in other countries. In territories where data is not freely available, we may not have the ability to commercialize our products without negotiating rights from third parties to refer to their clinical data in our regulatory applications, which could require the expenditure of significant additional funds.
In addition, regulatory approval in one jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory processes in others. Failure to obtain regulatory approvals in other jurisdictions or any delay or setback in obtaining such approvals could have the same adverse effects detailed above regarding FDA approval in the United States. As described above, such effects include the risks that any of our product candidates may not be approved for all indications requested, which could limit the uses of our product candidates and have an adverse effect on their commercial potential or require costly post-marketing studies.
Guidelines and recommendations published by various organizations can reduce the use of our products and product candidates.
Government agencies promulgate regulations and guidelines directly applicable to us and to our products and product candidates. In addition, professional societies, practice management groups, private health and science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to the health care and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. Recommendations or guidelines suggesting the reduced use of our products or product candidates or the use of competitive or alternative products that are followed by patients and health care providers could result in decreased use of our products or product candidates.
We are subject to uncertainty relating to payment or reimbursement policies which, if not favorable for our products or product candidates, could hinder or prevent our commercial success.
Our ability or our collaborators' ability to successfully commercialize our product candidates, will depend in part on the coverage and reimbursement levels set by governmental authorities, private health insurers, managed care organizations and other third-party payors. As a threshold for coverage and reimbursement, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors also are increasingly challenging the effectiveness of and prices charged for medical products and services. Government authorities and these third-party payors have attempted to control costs, in some instances, by limiting coverage and the amount of reimbursement for particular medications or encouraging the use of lower-cost generic AEDs. We cannot be sure that reimbursement will be available for any of the products that we develop and, if reimbursement is available, the level of reimbursement. Reduced or partial payment or reimbursement coverage could make our product candidates, less attractive to patients and prescribing physicians. We also may be required to sell our products or product candidates at a discount, which would adversely affect our ability to realize an appropriate return on our investment in our products or product candidates or compete on price.
We expect that private insurers and managed care organizations will consider the efficacy, cost effectiveness and safety of our product candidates, in determining whether to approve reimbursement for such products or product candidates and at what level. Because each third-party payor individually approves payment or reimbursement, obtaining these approvals can be a time consuming and expensive process that could require us to provide scientific or clinical support for the use of each of our products or product candidates separately to each third-party payor. In some cases, it could take several months or years before a particular private insurer or managed care organization reviews a particular product, and we may ultimately be unsuccessful in obtaining coverage. Our competitors generally have larger organizations, as well as existing business relationships with third-party payors relating to their products. Our business would be materially adversely affected if we do not receive approval for reimbursement of our products or product candidates from private insurers on a timely or satisfactory basis. Our products and product candidates, may not be considered cost-effective, and coverage and reimbursement may not be available or sufficient to allow us to sell our products or product candidates on a profitable basis. Our business would also be adversely affected if private insurers, managed care organizations, the Medicare program or other reimbursing bodies or payors limit the indications for which our products or product candidates will be reimbursed to a smaller set than we believe they are effective in treating.
| 18 |
In some foreign countries, particularly Canada and the countries of Europe, the pricing of prescription pharmaceuticals is subject to strict governmental control. In these countries, pricing negotiations with governmental authorities can take six to 12 months or longer after the receipt of regulatory approval and product launch. To obtain favorable reimbursement for the indications sought or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our products or product candidates to other available therapies. If reimbursement for our products or product candidates is unavailable in any country in which reimbursement is sought, limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be materially harmed.
In addition, many managed care organizations negotiate the price of products and develop formularies which establish pricing and reimbursement levels. Exclusion of a product from a formulary can lead to its sharply reduced usage in the managed care organization's patient population. If our products or product candidates are not included within an adequate number of formularies or adequate payment or reimbursement levels are not provided, or if those policies increasingly favor generic products, our market share and gross margins could be negatively affected, which would have a material adverse effect on our overall business and financial condition.
We expect to experience pricing pressures due to the potential healthcare reforms discussed elsewhere in this prospectus, as well as the trend toward programs aimed at reducing health care costs, the increasing influence of health maintenance organizations and additional legislative proposals.
We face potential product liability exposure, and, if successful claims are brought against us, we may incur substantial liabilities.
The use of our product candidates in clinical trials and the sale of any of our products will expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers or others selling or otherwise coming into contact with our products and product candidates. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, product liability claims may result in:
| · | decreased demand for any product or product candidate that has received approval and is being commercialized; | |
|
| |
| · | impairment of our business reputation and exposure to adverse publicity; | |
|
| |
| · | withdrawal of bioequivalence and/or clinical trial participants; | |
|
| |
| · | initiation of investigations by regulators; | |
|
| |
| · | costs of related litigation; | |
|
| |
| · | distraction of management's attention from our primary business; | |
|
| |
| · | substantial monetary awards to patients or other claimants; | |
|
| |
| · | loss of revenues; and | |
|
| |
| · | the inability to commercialize any of our product candidates for which we obtain marketing approval. |
Insurance coverage is becoming increasingly expensive, and we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses. We intend to expand our insurance coverage to include the sale of commercial products prior to the commercialization of our products. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
| 19 |
Our failure to successfully develop and market product candidates would impair our ability to grow.
As part of our growth strategy, we intend to develop and market product candidates. We are pursuing various therapeutic opportunities through our pipeline. We may spend several years completing our development of any particular current or future internal product candidate, and failure can occur at any stage. The product candidates to which we allocate our resources may not end up being successful. In addition, because our internal research capabilities are limited, we may be dependent upon pharmaceutical companies, academic scientists and other researchers to sell or license products or technology to us. The success of this strategy depends partly upon our ability to identify, select, discover and acquire promising pharmaceutical product candidates and products.
The process of proposing, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license or acquisition of product candidates and approved products. We have limited resources to identify and execute the acquisition or in-licensing of third-party products, businesses and technologies and integrate them into our current infrastructure. Moreover, we may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. We may not be able to acquire the rights to additional product candidates on terms that we find acceptable, or at all.
In addition, future acquisitions may entail numerous operational and financial risks, including:
| · | exposure to unknown liabilities; | |
|
| |
| · | disruption of our business and diversion of our management's time and attention to develop acquired products or technologies; | |
|
| |
| · | incurrence of substantial debt, dilutive issuances of securities or depletion of cash to pay for acquisitions; | |
|
| |
| · | higher than expected acquisition and integration costs; | |
|
| |
| · | difficulty in combining the operations and personnel of any acquired businesses with our operations and personnel; | |
|
| |
| · | increased amortization expenses; | |
|
| |
| · | impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and | |
|
| |
| · | inability to motivate key employees of any acquired businesses. |
Further, any product candidate that we acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities.
Healthcare reform measures could hinder or prevent our product candidates' commercial success.
The U.S. government and other governments have shown significant interest in pursuing healthcare reform. Government-adopted reform measures could adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third-party payors. The continuing efforts of the U.S. and foreign governments, insurance companies, managed care organizations and other payors of health care services to contain or reduce healthcare costs may adversely affect our ability to set prices for any approved product candidate which we believe are fair, and our ability to generate revenues and achieve and maintain profitability.
| 20 |
In both the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals and initiatives to change the health care system in ways that could affect our ability to sell any approved product profitably. Some of these proposed and implemented reforms could result in reduced reimbursement rates for our products, which would adversely affect our business strategy, operations and financial results. For example, in March 2010, President Obama signed into law a legislative overhaul of the U.S. healthcare system, known as the Patient Protection and Affordable Care Act of 2010, as amended by the Healthcare and Education Affordability Reconciliation Act of 2010. This law, which we refer to as the PPACA, may have far reaching consequences for biopharmaceutical companies like us. As a result of this new legislation, substantial changes could be made to the current system for paying for healthcare in the United States, including changes made in order to extend medical benefits to those who currently lack insurance coverage. Extending coverage to a large population could substantially change the structure of the health insurance system and the methodology for reimbursing medical services and drugs. These structural changes could entail modifications to the existing system of private payors and government programs, such as Medicare and Medicaid, creation of a government-sponsored healthcare insurance source, or some combination of both, as well as other changes. Restructuring the coverage of medical care in the United States could impact the reimbursement for prescribed drugs, including our products and product candidates. If reimbursement for our approved products is substantially less than we expect in the future, or rebate obligations associated with them are substantially increased, our business could be materially and adversely impacted.
In September 2007, the Food and Drug Administration Amendments Act of 2007 was enacted, giving the FDA enhanced post-marketing authority, including the authority to require post-marketing studies and clinical trials, labeling changes based on new safety information, and compliance with risk evaluations and mitigation strategies approved by the FDA. In July 2012, the Food and Drug Administration Safety and Innovation Act was enacted, expanding drug supply chain requirements and strengthening FDA's response to drug shortages, among other things. The FDA's exercise of this authority could result in delays or increased costs during product development, clinical trials and regulatory review, increased costs to assure compliance with post-approval regulatory requirements, and potential restrictions on the sale and/or distribution of any approved product candidates.
Future federal and state proposals and health care reforms could limit the prices that can be charged for the product candidates that we develop and may further limit our commercial opportunity. Our results of operations could be materially adversely affected by the PPACA by reducing the amounts that private insurers will pay and by other health care reforms that may be enacted or adopted in the future.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We will need to manage our anticipated growth and increased operational activity. Our personnel, systems and facilities currently in place will not be adequate to support this future growth. Our future financial performance and our ability to compete effectively will depend, in part, on our ability to effectively manage any future growth. Our need to effectively execute our growth strategy requires that we:
| · | manage our regulatory approvals and clinical trials effectively; | |
|
| |
| · | manage our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors, collaborators and other third parties; | |
|
| |
| · | develop internal sales and marketing capabilities; | |
|
| |
| · | commercialize our product candidates; | |
|
| |
| · | improve our operational, financial and management controls, reporting systems and procedures; and | |
|
| |
| · | attract and motivate sufficient numbers of talented employees. |
This future growth could place a strain on our administrative and operational infrastructure and may require our management to divert a disproportionate amount of its attention away from our day-to-day activities. We may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. We may not be able to make improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls. If our management is unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate or increase our revenues could be reduced and we may not be able to implement our business strategy.
| 21 |
We may not be able to manage our business effectively if we are unable to attract and motivate key members or if we lose key members of our current management team.
We may not be able to attract or motivate qualified management and scientific and clinical personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses. Our industry has experienced a high rate of turnover of management personnel in recent years. If we are not able to attract and motivate necessary personnel to accomplish our business objectives, we may experience constraints that will significantly impede the achievement of our objectives.
We are highly dependent on the development, regulatory, commercial and financial expertise of our management. We do not have any employment agreements with any member of our management team except Mr. Grunewald. If we lose key members of our management team, we may not be able to find suitable replacements in a timely fashion, if at all. We cannot be certain that future management transitions will not disrupt our operations and generate concern among employees and those with whom we do business.
We also have scientific and clinical advisors who assist us in formulating our product development and clinical strategies. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us, or may have arrangements with other companies to assist in the development of products that may compete with ours.
If we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients' rights are and will be applicable to our business. We could be subject to healthcare fraud and abuse and patient privacy regulation by both the federal government and the states in which we conduct our business. The regulations include:
| · | the federal healthcare program anti-kickback law, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; | |
|
| |
| · | federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us which provide coding and billing advice to customers; | |
|
| |
| · | the federal Health Insurance Portability and Accountability Act of 1996, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; | |
|
| |
| · | the federal transparency requirements under the PPACA requires manufacturers of drugs, devices, biologics, and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; | |
|
| |
| · | the FDCA, which among other things, strictly regulates drug product marketing, prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; and | |
|
| |
| · | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations could be costly. If our operations are found to be in violation of any of the laws described above or any governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
| 22 |
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ individuals who were previously employed at other pharmaceutical companies, including our competitors or potential competitors and, as such, we may be subject to claims that we or these employees have used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such an event could cause interruption of our operations. For example, the loss of data from completed or ongoing bioequivalence and/or clinical trials for our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
We will need to obtain FDA approval of any proposed product names, and any failure or delay associated with such approval may adversely impact our business.
Any name we intend to use for our product candidates will require approval from the FDA regardless of whether we have secured a formal trademark registration from the USPTO. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. The FDA may object to any product name we submit if it believes the name inappropriately implies medical claims. We have in the past been required to change a proposed product name. If the FDA objects to any of our proposed product names, we may be required to adopt an alternative name for our product candidates. If we adopt an alternative name, we would lose the benefit of our existing trademark applications for such product candidate, and may be required to expend significant additional resources in an effort to identify a suitable product name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. We may be unable to build a successful brand identity for a new trademark in a timely manner or at all, which would limit our ability to commercialize our product candidates.
| 23 |
Risks Related to Our Finances and Capital Requirements
We have incurred significant operating losses since our inception and anticipate that we will incur continued losses for the foreseeable future.
In recent years, we have focused primarily on developing our current product candidates, with the goal of commercializing these products and supporting regulatory approval for these product candidates. We have financed our operations primarily through private placements of convertible securities, our collaboration and license arrangements, and the monetization of certain future royalty streams under our existing licenses. We have incurred significant operating losses since our inception. We incurred net losses of approximately $8.0 million and $3.6 million, in the years ended December 31, 2015 and 2014, respectively. As of December 31, 2015, we had an accumulated deficit of approximately $18.6 million. Substantially all of our operating losses resulted from costs incurred in connection with our development programs and from selling, general and administrative and interest costs associated with our operations. We expect our research and development costs to continue to be substantial and to increase with respect to our product candidates as we advance those product candidates through preclinical studies, clinical trials, manufacturing scale-up and other pre-approval activities. We expect to incur significant and increasing marketing and selling costs prior to and during the commercial launch of our current products. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or when, or if, we will become profitable.
Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders' equity and working capital. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. In this regard, the report of our independent registered public accounting firm with respect to our consolidated financial statements as of and for the period ended December 31, 2015 contains an explanatory paragraph stating that there is substantial doubt about our ability to continue as a going concern. In addition, we will need to obtain additional funds to develop and commercialize our other product candidates. The inclusion of a going concern statement by our auditors, our lack of cash resources and our potential inability to continue as a going concern may materially adversely affect our share price and our ability to raise new capital or to enter into critical contractual relations with third parties.
We will need additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
Developing product candidates, conducting clinical trials, establishing manufacturing relationships and marketing drugs are expensive and uncertain processes. We will need to obtain additional capital through equity offerings, debt financing, payments under new or existing licensing and research and development collaboration agreements, or any combination thereof, in order to become cash flow positive and to develop and commercialize additional product candidates. If sufficient funds on acceptable terms are not available when needed, we could be required to significantly reduce operating expenses and delay, reduce the scope of, or eliminate one or more of our development programs, which may have a material adverse effect on our business, results of operations and financial condition.
In addition, unforeseen circumstances may arise, or our strategic imperatives could change, causing us to consume capital significantly faster than we currently anticipate, requiring us to seek to raise additional funds sooner than expected. We have no committed external sources of funds.
The amount and timing of our future funding requirements will depend on many factors, including, but not limited to:
| · | the rate of progress and cost of our trials and other product development programs for our product candidates; | |
|
| |
| · | the costs and timing of in-licensing additional product candidates or acquiring other complementary companies; | |
|
| |
| · | the timing of any regulatory approvals of our product candidates; | |
|
| |
| · | our ability to successfully launch our products and to continue to increase the level of sales in the marketplace; | |
|
| |
| · | the actions of our competitors and their success in selling competitive product offerings; | |
|
| |
| · | the costs of establishing sales, marketing, manufacturing and distribution capabilities for our products; and | |
|
| |
| · | the status, terms and timing of any collaborative, licensing, co-promotion or other arrangements. |
| 24 |
Additional financing may not be available when we need it or may not be available on terms that are favorable to us. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. If adequate funds are not available to us on a timely basis, or at all, we may be required to delay, reduce the scope of or eliminate one or more of our development programs or our commercialization efforts.
We have never generated any revenues from our own sales of our products, and we may never achieve or maintain profitability.
Our ability to become profitable depends upon our ability to generate revenues from sales of our products and our product candidates. To date, we have not generated any revenues from our sales of our product candidates and have incurred significant operating losses. Our ability to generate product revenues is dependent on our ability to receive regulatory approval of our product candidates, and to successfully commercialize these products. Our ability to successfully commercialize our products depends on, among other things:
| · | our successful completion of clinical trials for our product candidates; and | |
|
| |
| · | our obtaining regulatory approvals for our product candidates. |
After our product candidates are approved for commercial sale, we anticipate incurring significant costs associated with commercialization. It is possible that we will never have sufficient product sales revenues to achieve profitability.
Our operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly and annual fluctuations. Prior to commercializing any of our product candidates, we expect that any revenues we generate will fluctuate from quarter to quarter and year to year as a result of the timing and amount of development milestones and royalty revenues received under our collaboration license agreements, as our revenues from these arrangements are principally based on the achievement of clinical and commercial milestones outside of our control.
Once we commercialize one or more of our products, our net loss and other operating results will be affected by numerous factors, including:
| · | variations in the level of expenses related to our development programs; | |
|
| |
| · | the success of our bioequivalence and clinical trials through all phases of clinical development; | |
|
| |
| · | any delays in regulatory review and approval of product candidates in clinical development; | |
|
| |
| · | potential side effects of our future products that could delay or prevent commercialization or cause an approved drug to be taken off the market; | |
|
| |
| · | any intellectual property infringement lawsuit in which we may become involved; | |
|
| |
| · | our ability to establish an effective sales and marketing infrastructure; | |
|
| |
| · | our dependency on third-party manufacturers to supply or manufacture our product candidates; | |
|
| |
| · | competition from existing products or new products that may emerge; | |
|
| |
| · | regulatory developments affecting our products and product candidates; | |
|
| |
| · | our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements; | |
|
| |
| · | the achievement and timing of milestone payments under our existing collaboration and license agreements; and | |
|
| |
| · | the level of market acceptance for any approved product candidates and underlying demand for that product and wholesalers' buying patterns. |
Due to the various factors mentioned above, and others, the results of any prior quarterly period should not be relied upon as an indication of our future operating performance. If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
| 25 |
We have limited experience operating as a public company and complying with public company obligations. Complying with these requirements has increased our costs and requires additional management resources, and we still may fail to meet all of these obligations.
We face increased legal, accounting, administrative and other costs and expenses as a public company. Compliance with the Sarbanes-Oxley Act of 2002, the Dodd-Frank Act of 2010, as well as rules of the Securities and Exchange Commission and Nasdaq, for example, has resulted in significant cost to us as well as ongoing increases in our legal, audit and financial compliance costs, particularly after we are no longer an "emerging growth company." The Securities Exchange Act of 1934, as amended, or the Exchange Act, requires, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition. Our board of directors, management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations make it more difficult and more expensive for us to obtain director and officer liability insurance, and require us to incur substantial costs to maintain the same or similar coverage.
As a public company, we are subject to Section 404 of the Sarbanes-Oxley Act relating to internal controls over financial reporting and we expect to incur significant expense and devote substantial management effort toward ensuring compliance with Section 404. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Implementing any appropriate changes to our internal controls may require specific compliance training for our directors, officers and employees, entail substantial costs to modify our existing accounting systems, and take a significant period of time to complete. Such changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate consolidated financial statements or other reports on a timely basis, could increase our operating costs and could materially impair our ability to operate our business. We cannot assure you that our internal controls over financial reporting will prove to be effective.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm after we no longer qualify as an "emerging growth company," may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
We are required to disclose changes made in our internal control procedures on a quarterly basis and our management is required to assess the effectiveness of these controls annually. However, for as long as we are an "emerging growth company" under the recently enacted JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404. We could be an "emerging growth company" for up to five years. See "Summary — Implications of being an Emerging Growth Company." An independent assessment of the effectiveness of our internal controls could detect problems that our management's assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
| 26 |
Our ability to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments is limited by provisions of the Internal Revenue Code, and may be subject to further limitation as a result of the transactions contemplated by this offering.
Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, contain rules that limit the ability of a company that undergoes an ownership change, which is generally any change in ownership of more than 50% of its stock over a three-year period, to utilize its net operating loss and tax credit carryforwards and certain built-in losses recognized in years after the ownership change. These rules generally operate by focusing on ownership changes involving stockholders owning directly or indirectly 5% or more of the stock of a company and any change in ownership arising from a new issuance of stock by the company. Generally, if an ownership change occurs, the yearly taxable income limitation on the use of net operating loss and tax credit carryforwards and certain built-in losses is equal to the product of the applicable long term tax exempt rate and the value of the company's stock immediately before the ownership change. We may be unable to offset our taxable income with losses, or our tax liability with credits, before such losses and credits expire and therefore would incur larger federal income tax liability.
In addition, it is possible that the transactions described in this offering, either on a standalone basis or when combined with future transactions, including issuances of new shares of our common stock, will cause us to undergo one or more additional ownership changes. In that event, we generally would not be able to use our pre-change loss or credit carryovers or certain built-in losses prior to such ownership change to offset future taxable income in excess of the annual limitations imposed by Sections 382 and 383 and those attributes already subject to limitations as a result of our prior ownership changes may be subject to more stringent limitations. As of December 31, 2015, we had approximately $16 million of federal net operating loss carryforwards. These federal and state net operating loss and federal and state tax credit carryforwards will begin to expire at various dates beginning in 2025, if not utilized. Our ability to utilize the aforementioned carryforwards and tax credits may be limited. As a result, we may not be able to take full advantage of these carryforwards or tax credits for federal and state tax purposes.
Risks Related to Our Indebtedness
Our level of indebtedness and debt service obligations could adversely affect our financial condition, and may make it more difficult for us to fund our operations.
In 2014, we entered into two lines of credit with the Denver Savings Bank for an aggregate amount of $1,000,000. Through December 31, 2015, we drew down $888,300 of the two lines of credit with the Denver Savings Bank. This debt financing may create additional financial risk for us, particularly if our business or prevailing financial market conditions are not conducive to paying off or refinancing our outstanding debt obligations at maturity. This indebtedness could also have important negative consequences, including:
| · | we will need to repay our debt under the two lines of credit with the Denver Savings Bank by making periodic payments of interest at the rate of 4.25%, including a final payment of $888,300 due in 2017, which will reduce the amount of money available to finance our operations, our research and development efforts and other general corporate activities; | |
|
| |
| · | we may have difficulty obtaining financing in the future for working capital, capital expenditures, acquisitions or other purposes; and | |
|
| |
| · | our failure to comply with the restrictive covenants in our loan and security agreement could result in an event of default that, if not cured or waived, would accelerate our obligation to repay this indebtedness, and the lenders could seek to enforce their security interests in the assets securing such indebtedness. |
To the extent additional debt is added to our current debt levels, the risks described above would increase.
We may not have cash available to us in an amount sufficient to enable us to make interest or principal payments on our indebtedness when due.
Since our inception, we have generated no revenue from product sales and have incurred significant operating losses. As of December 31, 2015, we had an accumulated deficit of $17.8 million. We expect to continue to incur net losses and have negative cash flow from operating activities for the foreseeable future as we continue to develop and seek marketing approval for our product candidates. As a result, we may not have sufficient funds, or may be unable to arrange for additional financing, to pay the amounts due on our outstanding indebtedness under lines of credit. Further, funds from external sources may not be available on economically acceptable terms, if at all. For example, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our product candidates or technologies, or to grant licenses on terms that are not favorable to us. If adequate funds are not available when and if needed, our ability to make interest or principal payments on our debt obligations would be significantly limited, and we may be required to delay, significantly curtail or eliminate one or more of our programs.
| 27 |
Failure to satisfy our current and future debt obligations under our secured credit facility could result in an event of default and, as a result, our lenders could accelerate all of the amounts due. In the event of an acceleration of amounts due under our secured credit facility as a result of an event of default, we may not have sufficient funds or may be unable to arrange for additional financing to repay our indebtedness. In addition, our lenders could seek to enforce their security interests in the collateral securing such indebtedness.
Risks Related to Securities Markets and Investment in Our Stock
We have never paid dividends on our capital stock, and because we do not anticipate paying any cash dividends in the foreseeable future, capital appreciation, if any, of our common stock will be your sole source of gain on an investment in our common stock.
We have paid no cash dividends on any of our classes of capital stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We currently have very limited research coverage by securities and industry analysts. If securities or industry analysts presently covering our business do not continue such coverage or if additional securities or industry analysts do not commence coverage of our Company, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
The concentration of our capital stock ownership with our directors and their affiliated entities and our executive officers will limit your ability to influence certain corporate matters.
Our directors and their affiliated entities, and our executive officers will beneficially own, in the aggregate, approximately 55.4% of our outstanding common stock. As a result, these stockholders are collectively able to significantly influence or control all matters requiring approval of our stockholders, including the election of directors and approval of significant corporate transactions such as mergers, consolidations or the sale of all or substantially all of our assets. The concentration of ownership may delay, prevent or deter a change in control of our Company even when such a change may be in the best interests of some stockholders, impede a merger, consolidation, takeover or other business combination involving us, or could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our Company or our assets and might adversely affect the prevailing market price of our common stock.
| 28 |
The price of our common stock may fluctuate substantially.
The market price for our common stock is likely to be volatile, in part because our common stock has been previously traded publicly for only a short time. In addition, the market price of our common stock may fluctuate significantly in response to a number of factors, including:
| · | the commercial performance of any of our product candidates that receive marketing approval; | |
|
| |
| · | plans for, progress in and results from clinical trials of our product candidates generally; | |
|
| |
| · | FDA or international regulatory actions, including actions on regulatory applications for any of our product candidates; | |
|
| |
| · | announcements of new products, services or technologies, commercial relationships, acquisitions or other events by us or our competitors; | |
|
| |
| · | market conditions in the pharmaceutical and biotechnology sectors; | |
|
| |
| · | fluctuations in stock market prices and trading volumes of similar companies; | |
|
| |
| · | variations in our quarterly operating results; | |
|
| |
| · | changes in accounting principles; | |
|
| |
| · | litigation or public concern about the safety of our potential products; | |
|
| |
| · | actual and anticipated fluctuations in our quarterly operating results; | |
|
| |
| · | deviations in our operating results from the estimates of securities analysts; | |
|
| |
| · | additions or departures of key personnel; | |
|
| |
| · | sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders; | |
|
| |
| · | any third-party coverage and reimbursement policies for our product candidates, and | |
|
| |
| · | discussion of us or our stock price in the financial or scientific press or in online investor communities. |
The realization of any of the risks described in these "Risk Factors" could have a dramatic and material adverse impact on the market price of our common stock. In addition, class action litigation has often been instituted against companies whose securities have experienced periods of volatility. Any such litigation brought against us could result in substantial costs and a diversion of management attention, which could hurt our business, operating results and financial condition.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
As a "smaller reporting company" as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
Our principal office space is located at 6750 Westown Parkway, Suite 200-226, West Des Moines, Iowa 50266.
Management is not aware of any legal proceedings contemplated by any governmental authority or any other party involving us or our properties. As of the date of this Annual Report, no director, officer or affiliate is (i) a party adverse to us in any legal proceeding, or (ii) has an adverse interest to us in any legal proceedings. Management is not aware of any other legal proceedings pending or that have been threatened against us or our properties.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
| 29 |
MARKET FOR COMMON EQUITY
Shares of our common stock commenced trading on the OTC Bulletin Board under the symbol "AEXP" in December 2008, and in December 2013 our symbol changed to "STLT." Shares of our common stock are currently trading on the OTCQB. The market for our common stock is limited, and can be volatile. The following table sets forth the high and low bid prices relating to our common stock on a quarterly basis for the periods indicated as quoted by the OTCQB market. These quotations reflect inter-dealer prices without retail mark-up, mark-down, or commissions, and may not reflect actual transactions.
Quarter Ended |
| High Bid |
|
| Low Bid |
| ||
|
|
|
|
|
|
| ||
December 31, 2015 |
| $ | .94 |
|
| $ | .94 |
|
September 30,2015 |
| $ | 1.00 |
|
| $ | 1.00 |
|
June 30, 2015 |
| $ | 1.05 |
|
| $ | 1.05 |
|
March 31, 2015 |
| $ | 1.25 |
|
| $ | 1.25 |
|
December 31, 2014 |
| $ | 1.85 |
|
| $ | 1.50 |
|
September 30, 2014 |
| $ | 2.06 |
|
| $ | .80 |
|
June 30, 2014 |
| $ | 1.22 |
|
| $ | .75 |
|
March 31, 2014 |
| $ | 2.00 |
|
| $ | .13 |
|
As of August 25, 2016, we had 94 shareholders of record, which does not include shareholders whose shares are held in street or nominee names.
DIVIDEND POLICY
No dividends have ever been declared by the Board of Directors on our common stock. Our losses do not currently indicate the ability to pay any cash dividends, and we do not have the intention of paying cash dividends on our common stock in the foreseeable future.
Recent Sales of Unregistered Securities
During the year ended December 31, 2015 the Company issued the following securities:
During 2015, the Company issued a consultant an aggregate of 525,000 shares of common stock and warrants to purchase an aggregate of 1,000,000 shares of common stock at a price of $1.29 per share, which expire December 19, 2017. The Company agreed to issue this consultant, provided the agreement with the consultant remains in effect, an additional 500,000 shares of common stock on each of the first anniversary (June 1, 2016) and second anniversary (June 1, 2017) of the agreement, as amended, with the consultant.
In January 2015, the Company issued 102,000 shares of common stock in exchange for cancellation of a promissory note dated September 27, 2013 in the principal amount of $40,000.
In January 2015, the Company issued 30,000 shares of common stock to an individual in exchange for providing a guarantee to the Company for a line of credit with the Denver Savings Bank.
In February 2015, the Company issued 156,139 shares of common stock to a consultant for advisory services.
| 30 |
In March 2015, the Company issued a convertible promissory note in the principal amount of $2,500,000 (the "March 2015 Note"). The March 2015 Note has a maturity date of March 27, 2020, and bears interest at the rate of Prime plus five percent (5%) during the first 36 months, increasing to an interest of prime plus six percent (6%) for the remaining 24 months. The March 2015 Note is convertible into shares of common stock of the Company at a price of $1.50 per share. The Company also issued warrants to purchase an aggregate of 250,000 shares of common stock of the Company. The warrants have a five year term and are exercisable into shares of common stock of the Company at the greater of $1.50 or 50% of the average closing bid price of the Company's common stock during the 20 days immediately following exercise thereof. In December 2015, the March 2015 Note and warrants to purchase 250,000 shares of common stock were terminated. In December 2015, the Company issued a convertible promissory note to in the principal amount of $2,500,000 (the "December 2015 Note"). Interest accrues on the principal amount of the December 2015 Note at the rate of eight percent (8%) per annum. The Company has the right to prepay all or a portion of the outstanding principal sum of the December 2015 Note as follows: (a) on or before December 31, 2016 only with the written consent of the holder, such consent not to be unreasonably withheld, for such purposes including but not limited to a NASDAQ listing of the securities of the Company or as a condition to an equity financing by the Company; and (b) at any time after December 31, 2016 upon thirty days written notice by the Company to the holder. The holder of the December 2015 Note has the right to convert all or a portion of the principal sum of the December 2015 Note into shares of common stock of the Company in denominations of not less than $250,000 as follows: (i) at any time up to December 31, 2016, at a discount of twenty percent (20%) of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $0.90 per share; (ii) at any time after December 31, 2016 and up to the close of business on December 31, 2017, at a discount of fifteen percent (15%) of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $1.35 per share; or (iii) at any time after December 31, 2017 and up to the close of business on March 27, 2020, at a discount of five percent (5%) of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $1.85 per share. The warrant to purchase 300,000 shares of Common Stock of the Company dated March 27, 2015 held by the holder of the December 2015 Note was terminated and replaced with (i) a warrant to purchase 300,000 shares of common stock at a price of $1.00 per share for a three year term, and (ii) a warrant to purchase an additional 200,000 shares of common stock of the Company at a price of $1.25 per share, for a three year term.
In December 2015, the Company issued William Pim, Chief Financial Officer of the Company, 100,000 shares of common stock pursuant to his employment agreement with the Company.
In December 2015, the Company raised $300,000 through the issuance of interesting bearing notes to seven qualified investors. The notes accrue interest at prime plus 6% payable quarterly based upon the prime rate at the end of the quarter. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each holder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years. A Qualified Financing means the issuance of equity securities to one or more investor in the amount of $2,000,000 or more of gross cash proceeds, of any conversion of outstanding securities.
Conversion features of the Notes are as follows:
| · | Upon the occurrence of a "Qualified Financing" (as defined below) within six months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 35% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | Upon the occurrence of a Qualified Financing within six and twelve months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 75% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | The Holder has the further right to convert the full principal amount of the note prior to a Qualified Financing and after the three-month anniversary of the issuance of the note, at a price equal to the lower of $0.75 or the average price of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion. | |
|
| |
| · | The Company has recorded a Derivative Liability in the amount of $278,482 related to the $300,000 received in December 2015. The liability was recorded due to the complexity of the conversion features, noted above, and the resulting obligations that may result from any conversion of the issuance. |
| 31 |
In December 2015, pursuant to a series of termination agreements, the Company issued 500,000 shares of common stock to The Greig Companies, Inc. which are to be held by the Company and disbursed as the debt service reserve is repaid to the Company according to the terms of the Termination Agreements. As of December 31, 2015, no payments have been received from The Greig Companies, Inc. related to the debt service reserve pursuant to the terms of the Termination Agreement. The Company also issued K4 Enterprises, LLC a convertible promissory note in the principal amount of $2,500,000 (the "Note"). Interest accrues on the principal amount of the Note at the rate of eight percent (8%) per annum. The Company has the right to prepay all or a portion of the outstanding principal sum of the Note as follows: (a) on or before December 31, 2016 only with the written consent of the holder, such consent not to be unreasonably withheld, for such purposes including but not limited to a NASDAQ listing of the securities of the Company or as a condition to an equity financing by the Company; and (b) at any time after December 31, 2016 upon thirty days written notice by the Company to the holder. The holder of the Note has the right to convert all or a portion of the principal sum of the Note into shares of common stock of the Company in denominations of not less than $250,000 as follows: (i) at any time up to December 31, 2016, at a discount of twenty percent (20%) of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $0.90 per share; (ii) at any time after December 31, 2016 and up to the close of business on December 31, 2017, at a discount of fifteen percent (15%) of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $1.35 per share; or (iii) at any time after December 31, 2017 and up to the close of business on March 27, 2020, at a discount of five percent (5%) of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $1.85 per share. The warrant to purchase 300,000 shares of Common Stock of the Company dated March 27, 2015 held by the holder of the Note was terminated and replaced with (i) a warrant to purchase 300,000 shares of common stock at a price of $1.00 per share for a three year term, and (ii) a warrant to purchase an additional 200,000 shares of common stock of the Company at a price of $1.25 per share, for a three year term.
ASC 470-40 requires that a gain or loss be recognized in the early extinguishment of debt in the period the debt is extinguished. As part of the termination of the March 2015 Note, and issuance of the December 2015 Note, the Company recorded a Loss of Extinguishment of debt in the amount of $1,676,731.
During the year ended December 31, 2015, the Company issued an aggregate of 1,532,408 shares of common stock and 795,000 warrants to purchase an aggregate of 696,000 shares of common stock. Of the warrants granted, 495,000 warrants have an exercise price of $1.25 per share, and the remaining 300,000 warrants have an exercise price of $1.00 per share. 595,000 of the warrants expire three years from the date of issuance, and 200,000 warrants expire two years from the date of issuance.
The sale and issuance of the securities described above were exempt from registration under the Securities Act of 1933, as amended, by virtue of section 4(2) and Rule 506 promulgated under Regulation D.
ITEM 6. SELECTED FINANCIAL DATA.
As a "smaller reporting company" as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
| 32 |
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion should be read in conjunction with our audited financial statements and the related notes that appear elsewhere in this Annual Report. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to those discussed below and elsewhere in this Annual Report, particularly in the section entitled "Risk Factors." Our audited financial statements are stated in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles.
Overview
We are a bioscience company focused on acquiring the rights, via acquisition, license or otherwise, to innovative and proprietary technologies. We place a premium on identifying and targeting technology candidates including: cancer drugs and treatment therapies for leading disease states; drug and related treatment therapies for leading infectious diseases; and other specialty innovative and proprietary technology candidates. We provide value-added development capability and funding in order to achieve rapid approvals for investigational new drugs to commence human clinical trials. We seek to commercialize, for each technology candidate, one or more indications via: sale, out-license and or strategic relationships/marketing agreements while continuing to retain, where practical, rights, "field of use" to other indications for future commercialization. Our capabilities are intended to provide our candidates: development guidance; regulatory expertise; communications and public relations; and networking with global and U.S. pharmaceutical and biopharmaceutical companies, contract research organizations, and various other funding sources.
Going forward, we will expand our focus on diseases that are either rare (so called "orphan diseases") or new or common but underserved (e.g. feline chronic kidney disease). We believe that indications such as these offer an accelerated path to market, support form nonprofit advocacy groups, scarcity of competition, and pricing leverage (Medicare and third-party insurers). High prices for these drugs are justified by the sponsor's willingness to treat small, underserved patient populations).
Plan of Operation
Through December 31, 2015, management successfully completed two acquisitions, Celtic Biotech Iowa, Inc. and Memcine Pharmaceuticals, Inc. and created CDT Veterinary Therapeutics, Inc.
In 2016 the Company created an additional subsidiary Caretta Therepeutics, Inc.
Generally, we have financed operations to date through the proceeds of the private placement of equity and debt instruments. Management anticipates additional increases in operating expenses and capital expenditures relating to retention of additional personnel, and advancement of our technologies. We anticipate that we will finance these expenses with further issuances of equity securities and debt issuances. The Company sought to raise $850,000 in December 2015. $300,000 of those proceeds were received in 2015 and $450,000 was received in January 2016. The Company hopes to complete a financing of at least $2,000,000 in 2016. Additional issuances of equity or convertible debt securities could result in dilution to our current shareholders. Further, such securities may have rights, preferences or privileges senior to our common stock. Additional financing may not be available upon acceptable terms, or at all.
The Company has recorded a Derivative Liability in the amount of $278,482 related to the $300,000 received in December 2015. The liability was recorded due to the complexity of the conversion features, noted above, and the resulting obligations that may result from any conversion of the issuance.
| 33 |
Recent Events
In August 2016, we formed a fourth subsidiary, Caretta Therapeutics, Inc., to develop and commercialize products derived from cobra and rattlesnake venom and formulated to relieve chronic pain. The products are intended to be available in both prescription strength and Over-the-Counter formulations.
On August 19, 2016, the Company entered into a Sponsored Research Agreement (the "SRA") with the Florida State University Research Foundation ("FSURF") starting September 1, 2016, to perform certain research, over a two-year period, related to the discovery, synthetic modification, and preclinical validation of drug-like compounds intended to treat patients with Zika virus infection to be directed by Dr. Hengli Tang. The SRA provides for payments by the Company to the FSURF of $147,000 upon each of the commencement of the research and six months thereafter. The Company is also responsible for additional contributions toward the direct and indirect costs of the research as per the terms of the SRA.
Results of Operations
Fiscal Year Ended December 31, 2015 Compared to Fiscal Year Ended December 31, 2014.
Our net loss for fiscal year ended December 31, 2015 was $8,087,240, compared to a net loss of $3,625,527 for the fiscal year ended December 31, 2014, an increase of $3,601,979. During fiscal years ended December 31, 2015 and 2014, we did not generate any revenue. While operating expenses increased by $3,633,298 in 2015 over 2014, interest expense decreased $681,570 resulting from the termination of the debt agreements at December 31, 2015, and the write off of the accrued interest for those instruments. Additionally, the Company experienced the loss on the extinguishment of debt in excess of 2014 of $1,530,737 due to the extinguishment of related party debt.
General and Administrative expenses increased due to $2.0 million in share-based compensation, as well as a $212,541 write-down of an intangible asset in accordance with ASC 360-10 and an increase in research and development in the amount of $265,950.
Operating Activities
The Company has not generated revenues during the years ended December 31, 2014 or December 31, 2015. As a result, our operating activities have, to date, resulted in net cash used. For the fiscal year ended December 31, 2015, net cash used in operating activities was $1,904,348 compared to $810,660 for fiscal year ended December 31, 2014. Net cash used in operating activities during the year ended December 31, 2015 consisted primarily of our net loss of $8,087,240 which was offset by noncash amounts of $605,385 in amortization of the debt discount, $1,748,441 in share-based compensation, $3,004 in depreciation and amortization, $1,650,822 in loss on the extinguishment of debt, and $212,541 in impairment charges of long-lived asset. The increase in the use of cash for operating activities from the year ended December 31, 2015 compared to the year ended December 31, 2014 was related to obtaining financing and to maintain development and operations.
Investing Activities
For the fiscal year ended December 31, 2015, net cash flows used in investing activities was $11,101 compared to net cash flows provided by investing activities $18,405 during the year ended December 31, 2014. The change is the result of the purchase of equipment and the acquisition of Memcine in 2015, while in 2014, due to the acquisition of Celtic, the Company benefited from cash on the Celtic Balance Sheet as well as making a deposit $12,000. The Company also made payments of $4,500 on the restricted cash.
Financing Activities
We have financed our operations primarily from debt or the issuance of equity instruments. For the fiscal year ended December 31, 2015, net cash flows provided from financing activities was $2,206,300 compared to $782,221 during the fiscal year ended December 31, 2014. Cash flows from financing activities for the fiscal year ended December 31, 2015 consisted primarily of $1,920,000 in proceeds from the issuance of convertible debentures, $99,800 in proceeds from draw on line of credit, and $190,000 in proceeds from the sale of the Company's common stock and warrants. In addition these cash flows were offset $3,500 of principle payments on related party debt.
| 34 |
Liquidity and Capital Resources
We are an early stage company and have not generated any revenue to date. We have incurred recurring losses to date. Our financial statements have been prepared assuming that we will continue as a going concern and, accordingly, do not include adjustments relating to the recoverability and realization of assets and classification of liabilities that might be necessary should we be unable to continue in operation.
We expect we will require additional capital to meet our long term operating requirements. We expect to raise additional capital through, among other things, the sale of equity or debt securities, although there is no guarantee we will be able to raise such funds.
Below is a description of the material financing activities of the Company:
In June 2014, the Company entered into a series of agreements (including (i) Unit Subscription Agreements (USA Control No. 849207105 STLT No's: 01-10), (ii) Confidential Memorandum of Terms (MOT Control No. 849207105 STLT), (iii) Account Management Agreements (AMA Control No: AMA 849207105 STLT), and (iv) Escrow & Compliance Attorney Agreement, collectively referred to as the "Subscription Agreements") with nine investors, Copperbottom Investments, Ltd., Britannia Securities International, Ltd., Agri-Technologies International, Ltd., On Time Investments, ltd., RnD Company, Ltd., Rooftop Holdings, Ltd., Sequence Investments Ltd., Anybright Investments, Ltd., and Orange Investments, Ltd. (collectively the "Investors"). These agreements required the purchase by the Investors of shares of Series A Preferred Stock and Warrants of the Company based on certain formulas contained in the Subscription Agreements. These agreements were terminated as described below under the heading "Termination and Release Agreements."
In March 2015, the Company issued a Convertible Promissory Note in the principal amount of $2,500,000. In December 2015 terminated this Convertible Promissory Note, and issued a convertible note in the principal amount of $2,500,000 as described below under the heading "Termination and Release Agreements."
In December 2015, the Company sought to raise up to $850,000 through the issuance of interesting bearing notes. The notes accrue interest at prime plus 6% payable quarterly based upon the prime rate at the end of the quarter. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each holder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
A Qualified Financing means the issuance of equity securities to one or more investor in the amount of $2,000,000 or more of gross cash proceeds, of any conversion of outstanding securities.
Conversion features of the Notes are as follows:
| · | Upon the occurrence of a Qualified Financing within six months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 35% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | Upon the occurrence of a Qualified Financing within six and twelve months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 75% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | The Holder has the further right to convert the full principal amount of the note prior to a Qualified Financing and after the three-month anniversary of the issuance of the note, at a price equal to the lower of $0.75 or the average price of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion. |
At December 31, 2015 the Company had received $300,000 of the Financing, $450,000 of the funds were received in January, 2016.
| 35 |
Letters of Credit
On April 4, 2014, the Company entered into a letter of credit (the "April Letter of Credit") with Denver Savings Bank in the principal amount of $752,325. The April Letter of Credit provides that the Company can borrow up to the aforementioned principal amount from the Denver Savings Bank until April 1, 2017. Interest accrues at the rate of 4.25% per year. Through December 31, 2015, the Company has drawn down on the full principal amount of the April Letter of Credit. The loan is repayable on demand, but if no demand is made, then quarterly payments of accrued interest calculated on the amount outstanding is due and payable. As security for the April Letter of Credit a third party (the "April Cosigner") cosigned the April Letter of Credit, and pledged certain collateral. In exchange for this pledge the Company issued to the April Cosigner, 150,000 shares of common stock of the Company, and agreed to issue 30,000 shares of its common stock upon each one year anniversary of the April Letter of Credit, provided that the April Letter of Credit remains in effect. The shares of common stock had a fair value of $183,000 based on the market price on the date of grant and have been recorded as deferred financing costs.
On July 29, 2014 the Company entered into a letter of credit (the "July Letter of Credit") with Denver Savings Bank in the principal amount of $250,975. The July Letter of Credit provides that the Company can borrow up to the aforementioned principal amount from the Bank until April 1, 2017. Interest accrues at the rate of 4.25% per year. To date, the Company has drawn down on $135,975 of the July Letter of Credit. The loan is repayable on demand, but if no demand is made, then quarterly payments of accrued interest calculated on the amount outstanding is due and payable. As security for the July Letter of Credit a third party (the "July Cosigner") cosigned the July Letter of Credit, and pledged certain collateral. In exchange for this pledge, the Company issued to the July Cosigner 42,300 shares of Common Stock of the Company.
In 2014, the Company entered into a series of Convertible Promissory Notes with Warrants with Lextacan Development, Ltd., Ocana Limited, and Tosca Limited in the aggregate principal amount of approximately $865,000. This series of Convertible Promissory Notes with Warrants was issued in connection with the April Line of Credit and the July Line of Credit. In December 2015 this series of Convertible Promissory Notes with Warrants was terminated as described below under the heading "Termination and Release Agreements."
While there can be no guarantees, we intend to secure additional financings through the offerings of our common stock or debt financing. Management also intends to seek out institutional capital in the next 12 months. We will also continue to work with our existing debt holders to extend or reduce principal amount including pay-off agreements.
Termination and Release Agreements
On December 31, 2015, the Company closed a series of Termination and Release Agreements (the "Termination Agreements") which provided for the termination of certain agreements and instruments. The following is a brief summary of the transactions set forth in the Termination Agreements, and does not purport to be complete and is qualified in its entirety by reference to the full text of the Termination Agreements:
- The Company and The Greig Companies, Inc. terminated the Convertible Promissory Note with The Greig Companies, Inc. in the principal amount of $2,500,000 dated March 27, 2015. Subsequent to the termination of the foregoing Convertible Promissory Note, the Company issued a new Note to K4, LLC. $100,000 of the $600,000 debt service reserve held by The Greig Companies, Inc. shall be returned to the Company within five businessdays of the execution by all parties of the Termination Agreement, and the remaining $500,000 shall be repaid to the Company in equal quarterly payments of $42,404 over three years which includes interest accrued at the rate of 3.25% per annum. Pursuant to this termination agreement, the Company was to issue to The Greig Companies, Inc. up to 440,000 shares of Common Stock, pro rata, based on the repayment of the aforesaid amount. To date The Greig Companies, Inc. has not made any of the foregoing payments and is in default. As such, the Company has not issued the foregoing shares of Common Stock. The Company is contemplating legal action to enforce its rights under the Termination Agreements and collect the foregoing sums due thereunder.
- The Company and its counterparties terminated a series of Convertible Promissory Notes with Warrants issued in 2014 with Lextacan Development, Ltd., Ocana Limited, and Tosca Limited in the aggregate principal amount of approximately $865,000. This series of Convertible Promissory Notes with Warrants was issued in connection with the April Line of Credit and the July Line of Credit.
| 36 |
- The Company and its counterparties terminated a series of agreements (including (i) Unit Subscription Agreements (USA Control No. 849207105 STLT No's: 01-10), (ii) Confidential Memorandum of Terms (MOT Control No. 849207105 STLT), (iii) Account Management Agreements (AMA Control No: AMA 849207105 STLT), and (iv) Escrow & Compliance Attorney Agreement, collectively referred to as the "Subscription Agreements") with nine investors, Copperbottom Investments, Ltd., Britannia Securities International, Ltd., Agri-Technologies International, Ltd., On Time Investments, ltd., RnD Company, Ltd., Rooftop Holdings, Ltd., Sequence Investments Ltd., Anybright Investments, Ltd., and Orange Investments, Ltd. (collectively the "Investors"). These agreements required the purchase by the Investors of shares of Series A Preferred Stock and Warrants of the Company based on certain formulas contained in the Subscription Agreements. Pursuant to this Termination Agreement the Investors have no further obligations to provide funding to the Company, and the Company has no obligation to accept such funds. All of the shares of Series A Preferred Stock, Series C Preferred Stock, and Warrants held in trust pursuant to the Subscription Agreements are terminated, cancelled and returned to the treasury of the Company.
The following table shows the promissory notes entered into by the Company:
|
| Date of |
| Stated Interest |
|
| Original Principal |
|
| Due | |||
Promissory Note |
| Note |
| Rate |
|
| Amount |
|
| Date | |||
#1 |
| 05/29/09 |
|
| 10 | % |
| $ | 30,000 |
|
| On Demand | |
#2 |
| 06/05/09 |
|
| 10 | % |
| $ | 5,911 |
|
| On Demand | |
#3 |
| 08/16/09 |
|
| 10 | % |
| $ | 50,000 |
|
| On Demand | |
#4 |
| 09/27/10 |
|
| 10 | % |
| $ | 60,000 |
|
| On Demand | |
#5 |
| 06/02/10 |
|
| 5 | % |
| $ | 50,000 |
|
| On Demand | |
#6 |
| 02/04/11 |
|
| 5 | % |
| $ | 30,000 |
|
| On Demand | |
#7 |
| 05/04/11 |
|
| 5 | % |
| $ | 35,000 |
|
| On Demand | |
#8 |
| 08/11/11 |
|
| 10 | % |
| $ | 20,000 |
|
| On Demand | |
#9 |
| 12/05/11 |
|
| 10 | % |
| $ | 20,000 |
|
| On Demand | |
#10 |
| 04/28/12 |
|
| 10 | % |
| $ | 30,000 |
|
| On Demand | |
The Company also assumed a liability for previous advances made by our former CEO in the amount of $23,433. These advances are due on demand and do not bear interest. The Company made payments of $3,500 toward this assumed liability bringing the balance to $19,933 at December 31, 2015.
The Company also assumed $92,923 in accrued interest related to these notes. The Company recorded $28,541 and $28,720 in interest expense for the years ended December 31, 2015 and 2014 on the above promissory notes.
As of December 31, 2015, the Company has not received any demand notice from the lenders noted above for payment of principal or interest on these promissory notes payable.
As of the date of this Annual Report, we are negotiating a settlement with all creditors regarding payment provisions, there is no guarantee that we will be able to arrive at a mutually agreeable settlement or that these creditors would not demand repayment.
Danley Note
On September 15, 2012, the Company entered into a loan agreement (the "Loan") with The Danley Group ("Lender") to borrow $50,000 at an annual compounding rate of 2.7%, due March 15, 2013. In addition, the Company agreed to pay a $50,000 premium at maturity. The Company has recorded the additional premium as a debt discount and increased the face amount of the note to $100,000. The debt discount is being amortized over the life of the Loan. During the years ended December 31, 2013 and 2012, the Company amortized the debt discount and recorded $20,833 and $29,187, respectively, as additional interest expense. For the period from inception through December 31, 2013, the Company recognized and amortized $50,000 of the debt discount as interest expense. On May 8, 2013, the Loan's maturity date was extended to September 15, 2013, and Cris Grunewald, our President, signed a personal guarantee of obligations to repay a total amount of $100,000 plus accrued interest. On October 4, 2013, the Company issued 262,000 shares of common stock and 800,000 warrants to purchase one share of common stock per warrant for a further extension of the Loan's maturity date to December 31, 2013. The warrants have an exercise price of 60% of the 20 day average market price prior to the date of exercise. The Company recorded the $707,803 in fair value of the shares and warrants as interest expense. The Loan matured on December 31, 2013. As a result of the default the Company accrued interest at 10% per annum. On April 8, 2014, the Company paid $119,590 in full settlement of the Loan. No gain or loss was recorded.
| 37 |
Kopriva Note
On October 4, 2013, the Company entered into a convertible note (the "Note") dated October 4, 2013 in the amount of $40,000 from an investor. The term of this Note is fifteen months from October 4, 2013. During the term of this Note, interest shall accrue on the unpaid principal balance at a fixed rate equal to 10% per annum, compounded annually. Should the Company default on the Note, the outstanding balance of this Note shall bear interest at the default rate of 20% per annum, compounded annually. In addition to the interest accrued the holder received warrants to purchase up to 100,000 shares of common stock. The conversion feature of the Note and the exercise price of the warrants is the greater of (i) a discount of 40% to the 20 day average closing market price prior to the day that the warrant is executed or (ii) $0.20 per share. The warrants have a term of thirty-six (36) months from the date of repayment or conversion of the Note.
On December 19, 2014, the Company entered an agreement to settle the note and its accrued interest in exchange for 102,000 shares of common stock.
December 2015 Convertible Notes
In March 2015 the Company issued a Convertible Promissory Note in the principal amount of $2,500,000. In December 2015, the Company terminated this Convertible Promissory Note, and issued a convertible note in the principal amount of $2,500,000 as described above under the heading "Termination and Release Agreements."
ASC 470-40 requires that a gain or loss be recognized in the early extinguishment of debt in the period the debt is extinguished. As part of the termination of the March 2015 Note, and issuance of the December 2015 Note, the Company recorded a Loss of Extinguishment of debt in the amount of $1,676,731.
In December 2015, the Company sought to raise up to $850,000 through the issuance of interesting bearing notes to seven qualified investors. The notes accrue interest at prime plus 6% payable quarterly based upon the prime rate at the end of the quarter. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each holder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
A Qualified Financing means the issuance of equity securities to one or more investor in the amount of $2,000,000 or more of gross cash proceeds, of any conversion of outstanding securities.
Conversion features of the Notes are as follows:
| · | Upon the occurrence of a "Qualified Financing" (as defined below) within six months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 35% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | Upon the occurrence of a Qualified Financing within six and twelve months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 75% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | The Holder has the further right to convert the full principal amount of the note prior to a Qualified Financing and after the three-month anniversary of the issuance of the note, at a price equal to the lower of $0.75 or the average price of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion. | |
|
| |
| · | At December 31, 2015, the Company had received $300,000 of the Financing. The remainder of the funds, in the amount of $450,000, were received in January 2016. The Company has recorded a Derivative Liability in the amount of $278,482 related to the $300,000 received in December 2015. The liability was recorded due to the complexity of the conversion features, noted above, and the resulting obligations that may result from any conversion of the issuance. |
| 38 |
Purchase of Significant Equipment
We have not previously, nor do we intend to purchase any significant equipment during the next twelve months.
Off-Balance Sheet Arrangements
As of the date of this Annual Report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Going Concern
The independent auditors' report accompanying our December 31, 2015 and December 31, 2014 consolidated financial statements contains an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern. The financial statements have been prepared "assuming that we will continue as a going concern," which contemplates that we will realize our assets and satisfy our liabilities and commitments in the ordinary course of business. We have suffered recurring losses from operations, have a working capital deficit and are currently in default of the payment terms of certain note agreements. These factors raise substantial doubt about our ability to continue as a going concern.
Critical Accounting Policies
The following describes the critical accounting policies used in reporting our financial condition and results of operations. In some cases, accounting standards allow more than one alternative accounting method for reporting. In those cases, our reported results of operations would be different should we employ an alternative accounting method.
The significant accounting policies and bases of presentation for our consolidated financial statements are described in Note 2 "Summary of Significant Accounting Policies." The preparation of our financial statements in accordance with U.S. generally accepted accounting principles (GAAP) requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and the disclosure of contingent assets and liabilities. Actual results could differ from those estimates.
We believe the following accounting policies and estimates to be critical:
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
In-Process Research and Development
On June 4, 2014 the Company acquired 95% of Celtic Biotech Ltd in a stock exchange agreement. Through the agreement, the Company acquired In-process research and development ("IPR&D").
| 39 |
In-process research and development ("IPR&D") represents the estimated fair value assigned to research and development projects acquired in a purchased business combination that have not been completed at the date of acquisition and which have no alternative future use. IPR&D assets acquired in a business combination are capitalized as indefinite-lived intangible assets. These assets remain indefinite-lived until the completion or abandonment of the associated research and development efforts. During the periods prior to completion or abandonment, those acquired indefinite-lived assets are not amortized but are tested for impairment annually, or more frequently, if events or changes in circumstances indicate that the asset might be impaired. During periods after completion, those acquired indefinite-lived assets are amortized based on their useful life. During the year ended December 31, 2014, the Company acquired IPR&D assets in its acquisition of CBL. The fair value of the assets acquired was originally established at $6,977,347. These assets are still subject to research and development completion and accordingly, no amortization has been recorded.
Stock-Based Compensation
The Company measures the cost of employee services received in exchange for stock and stock options based on the grant date fair value of the awards under FASB ASC Topic 718, Compensation – Stock Compensation ("ASC 718"). The Company determines the fair value of stock option grants using the Black-Scholes option pricing model. The Company determines the fair value of shares of nonvested stock (also commonly referred to as restricted stock) based on the last quoted price of our stock on the date of the share grant. The fair value determined represents the cost for the award and is recognized over the vesting period during which an employee is required to provide service in exchange for the award. As share-based compensation expense is recognized based on awards ultimately expected to vest, the Company reduces the expense for estimated forfeitures based on historical forfeiture rates. Previously recognized compensation costs may be adjusted to reflect the actual forfeiture rate for the entire award at the end of the vesting period. Excess tax benefits, as defined in ASC 718, if any, are recognized as an addition to paid-in capital.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
As a "smaller reporting company" as defined by Item 10 of Regulation S-K, the Company is not required to provide this information.
| 40 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| 41 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Spotlight Innovation Inc.
West Des Moines, Iowa
We have audited the accompanying consolidated balance sheets of Spotlight Innovation Inc. as of December 31, 2015 and 2014, and the related consolidated statements of operations, changes in equity, and cash flows for the years then ended. These consolidated financial statements are the responsibility of Spotlight Innovation Inc.'s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Spotlight Innovation Inc. as of December 31, 2015 and 2014, and the results of its operations and its cash flows for each of the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that Spotlight Innovation Inc. will continue as a going concern. As discussed in Note 3 to the consolidated financial statements, Spotlight Innovation Inc. has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ GBH CPAs, PC
GBH CPAs, PC
www.gbhcpas.com
Houston, Texas
August 30, 2016
| F-1 |
SPOTLIGHT INNOVATION INC.
AS OF DECEMBER 31, 2015 AND 2014
|
| December 31, 2015 |
|
| December 31, 2014 |
| ||
ASSETS |
|
|
|
|
|
| ||
|
|
|
|
|
|
| ||
Current assets: |
|
|
|
|
|
| ||
Cash |
| $ | 299,919 |
|
| $ | 9,068 |
|
Restricted cash |
|
| - |
|
|
| 122,250 |
|
Prepaid expenses |
|
| 17,500 |
|
|
| - |
|
Total current assets |
|
| 317,419 |
|
|
| 131,318 |
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
| 31,714 |
|
|
| 8,475 |
|
In-process research and development |
|
| 6,977,347 |
|
|
| 6,977,347 |
|
Total assets |
| $ | 7,326,480 |
|
| $ | 7,117,140 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 140,803 |
|
| $ | 330,099 |
|
Accrued liabilities |
|
| 527,287 |
|
|
| 457,440 |
|
Accounts payable – related parties |
|
| 3,560 |
|
|
| 348,955 |
|
Stock payable |
|
| 26,250 |
|
|
| 269,705 |
|
Warrants payable |
|
| - |
|
|
| 692,317 |
|
Notes payable |
|
| 385,373 |
|
|
| 356,131 |
|
Line of credit, net of debt discount of $19,717 and $1,611, respectively |
|
| 868,583 |
|
|
| 750,714 |
|
Short-term debt – related party |
|
| 168,949 |
|
|
| 183,000 |
|
Convertible debentures, net of debt discounts of $555,753 and $82,003, respectively |
|
| 2,244,247 |
|
|
| 670,322 |
|
Deferred liabilities |
|
| 220,465 |
|
|
| - |
|
Derivative liabilities |
|
| 278,482 |
|
|
| - |
|
Total current liabilities |
|
| 4,863,999 |
|
|
| 4,058,683 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity: |
|
|
|
|
|
|
|
|
Series A convertible preferred stock, $0.001 par value, 3,000,000 shares authorized 0 shares issued and outstanding |
|
| - |
|
|
| - |
|
Series C preferred stock, $0.001 par value, 0 shares authorized, 500,000 shares issued and outstanding |
|
| - |
|
|
| - |
|
Preferred stock, $0.001 par value, 1,500,000 shares authorized, 0 shares issued and outstanding |
|
| - |
|
|
| - |
|
Common stock, $0.001 par value, 4,000,000,000 shares authorized, 14,627,026 and 13,094,618 shares issued and outstanding, respectively |
|
| 14,627 |
|
|
| 13,095 |
|
Additional paid-in capital |
|
| 18,760,400 |
|
|
| 11,277,032 |
|
Accumulated deficit |
|
| (18,643,652 | ) |
|
| (10,601,946 | ) |
Total equity attributable to Spotlight Innovation, Inc. |
|
| 131,375 |
|
|
| 688,181 |
|
Non-controlling interest |
|
| 2,331,106 |
|
|
| 2,370,276 |
|
Total equity |
|
| 2,462,481 |
|
|
| 3,058,457 |
|
Total liabilities and equity |
| $ | 7,326,480 |
|
| $ | 7,117,140 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-2 |
SPOTLIGHT INNOVATION INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014
|
| Year Ended |
|
| Year Ended |
| ||
|
| December 31, 2015 |
|
| December 31, 2014 |
| ||
|
|
|
|
|
|
| ||
Revenue |
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
General and administrative |
|
| 4,629,495 |
|
|
| 1,477,167 |
|
Depreciation expense |
|
| 3,004 |
|
|
| 525 |
|
Research and development expenses |
|
| 274,894 |
|
|
| 8,944 |
|
Impairment of intangible assets |
|
| 212,541 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
| 5,119,934 |
|
|
| 1,486,636 |
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
| (5,119,934 | ) |
|
| (1,486,636 | ) |
|
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
Loss on debt extinguishment |
|
| (1,650,822 | ) |
|
| (120,085 | ) |
Gain on foreign currency transactions |
|
| 63,032 |
|
|
| 42,051 |
|
Interest expense |
|
| (1,379,516 | ) |
|
| (2,060,587 | ) |
Total other income (expense) |
|
| (2,967,306 | ) |
|
| (2,138,621 | ) |
|
|
|
|
|
|
|
|
|
Net loss |
|
| (8,087,240 | ) |
|
| (3,625,257 | ) |
Net loss attributable to non-controlling interest |
|
| (45,534 | ) |
|
| (1,802 | ) |
Net loss attributable to Spotlight Innovation Inc. |
| $ | (8,041,706 | ) |
| $ | (3,623,455 | ) |
|
|
|
|
|
|
|
|
|
Net loss per common share – basic and diluted |
| $ | (0.58 | ) |
| $ | (0.28 | ) |
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding – basic and diluted |
|
| 13,973,304 |
|
|
| 13,006,741 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
SPOTLIGHT INNOVATION INC.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014
|
| Common Stock |
|
| Additional Paid-In |
|
| Accumulated |
|
| Non-controlling |
|
|
|
| |||||||||
|
| Shares |
|
| Par |
|
| Capital |
|
| Deficit |
|
| Interest |
|
| Totals |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Balance at December 31, 2013 |
|
| 7,745,458 |
|
| $ | 7,745 |
|
| $ | 1,431,902 |
|
| $ | (6,978,491 | ) |
| $ | - |
|
| $ | (5,538,844 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for cash, net of costs |
|
| 380,007 |
|
|
| 380 |
|
|
| 153,876 |
|
|
| - |
|
|
| - |
|
|
| 154,256 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for debt conversion |
|
| 1,105,970 |
|
|
| 1,106 |
|
|
| 117,007 |
|
|
| - |
|
|
| - |
|
|
| 118,113 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for loan guarantee |
|
| 192,300 |
|
|
| 192 |
|
|
| 269,523 |
|
|
| - |
|
|
| - |
|
|
| 269,715 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued to extend maturity date of debt |
|
| 100,000 |
|
|
| 100 |
|
|
| 79,900 |
|
|
| - |
|
|
| - |
|
|
| 80,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature on convertible notes |
|
| - |
|
|
| - |
|
|
| 186,869 |
|
|
| - |
|
|
| - |
|
|
| 186,869 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of preferred stock in subsidiary for acquisition |
|
| - |
|
|
| - |
|
|
| 4,170,394 |
|
|
| - |
|
|
| 2,372,078 |
|
|
| 7,100,268 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for services |
|
| 74,510 |
|
|
| 75 |
|
|
| 142,867 |
|
|
| - |
|
|
| - |
|
|
| 142,942 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for stock payable |
|
| 3,496,373 |
|
|
| 3,497 |
|
|
| 4,724,694 |
|
|
| - |
|
|
| - |
|
|
| 4,728,191 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (3,623,455 | ) |
|
| (1,802 | ) |
|
| (3,625,257 | ) |
Balances at December 31, 2014 |
|
| 13,094,618 |
|
| $ | 13,095 |
|
| $ | 11,277,032 |
|
| $ | (10,601,946 | ) |
| $ | 2,370,276 |
|
|
| 3,058,457 |
|
Common shares and warrants issued for cash |
|
| 253,335 |
|
|
| 253 |
|
|
| 189,747 |
|
|
| - |
|
|
| - |
|
|
| 190,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for stock payable |
|
| 190,334 |
|
|
| 190 |
|
|
| 269,515 |
|
|
| - |
|
|
| - |
|
|
| 269,705 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for services |
|
| 1,058,739 |
|
|
| 1059 |
|
|
| 947,320 |
|
|
| - |
|
|
| - |
|
|
| 948,379 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common shares issued for loan guarantee |
|
| 30,000 |
|
|
| 30 |
|
|
| 37,470 |
|
|
| - |
|
|
| - |
|
|
| 37,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of stock options |
|
| - |
|
|
| - |
|
|
| 773,812 |
|
|
| - |
|
|
| - |
|
|
| 773,812 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature on convertible notes |
|
| - |
|
|
| - |
|
|
| 1,124,731 |
|
|
| - |
|
|
| - |
|
|
| 1,124,731 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for warrants payable |
|
| - |
|
|
| - |
|
|
| 692,317 |
|
|
| - |
|
|
| - |
|
|
| 692,317 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued with convertible debt |
|
| - |
|
|
| - |
|
|
| 907,540 |
|
|
| - |
|
|
| - |
|
|
| 907,540 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for services |
|
| - |
|
|
| - |
|
|
| 1,290,308 |
|
|
| - |
|
|
| - |
|
|
| 1,290,308 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Extinguishment of related party debt as contributed capital |
|
| - |
|
|
| - |
|
|
| 1,250,608 |
|
|
| - |
|
|
| - |
|
|
| 1,250,608 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of subsidiary - Memcine |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 6,364 |
|
|
| 6,364 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (8,041,706 | ) |
|
| (45,534 | ) |
|
| (8,087,240 | ) |
Balance at December 31, 2015 |
|
| 14,627,026 |
|
| $ | 14,627 |
|
| $ | 18,760,400 |
|
| $ | (18,643,652 | ) |
| $ | 2,331,106 |
|
|
| 2,462,481 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
SPOTLIGHT INNOVATION, INC
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014
|
| Year Ended |
|
| Year Ended |
| ||
|
| December 31, 2015 |
|
| December 31, 2014 |
| ||
CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
|
|
|
| ||
Net loss |
| $ | (8,087,240 | ) |
| $ | (3,625,257 | ) |
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
|
|
|
Share-based compensation |
|
| 1,748,441 |
|
|
| 406,953 |
|
Depreciation and amortization |
|
| 3,004 |
|
|
| 525 |
|
Impairment of intangible assets |
|
| 212,541 |
|
|
| - |
|
Bad debt expense |
|
| 122,250 |
|
|
| - |
|
Amortization of debt discount |
|
| 605,385 |
|
|
| 703,035 |
|
Amortization of deferred financing costs |
|
| - |
|
|
| 1,019,715 |
|
Interest expense from common shares and warrants issued for modification of convertible debt |
|
| 37,500 |
|
|
| 80,000 |
|
Loss on debt extinguishment |
|
| 1,650,822 |
|
|
| 120,085 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Prepaid expense |
|
| (17,500 | ) |
|
| - |
|
Accounts receivable – related party |
|
| - |
|
|
| 1,349 |
|
Accounts payable |
|
| 1,101,012 |
|
|
| 125,165 |
|
Accounts payable – related parties |
|
| (345,395 | ) |
|
| 348,955 |
|
Accrued liabilities |
|
| 1,092,116 |
|
|
| 50,866 |
|
Debt guarantee |
|
| 34,529 |
|
|
| - |
|
Foreign currency translation adjustments |
|
| (61,813 | ) |
|
| (42,051 | ) |
Net cash used in operating activities |
|
| (1,904,348 | ) |
|
| (810,660 | ) |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES |
|
|
|
|
|
|
|
|
Acquisition of Memcine, net cash received of $27,071 |
|
| (2,988 | ) |
|
| - |
|
Acquisition of Celtic Biotech |
|
| - |
|
|
| 1,655 |
|
Deposit for Celtic Iowa, Inc. |
|
| - |
|
|
| 12,250 |
|
Payment for restricted cash |
|
| - |
|
|
| 4,500 |
|
Purchases of property and equipment |
|
| (8,113 | ) |
|
| - |
|
Net cash provided by (used in) investing activities |
|
| (11,101 | ) |
|
| 18,405 |
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
Principle payments on convertible debentures |
|
| (3,500 | ) |
|
| (100,000 | ) |
Proceeds from convertible debentures, net |
|
| 1,920,000 |
|
|
| 572,221 |
|
Proceeds from line of credit, net |
|
| 99,800 |
|
|
| - |
|
Proceeds from borrowings on related-party debt |
|
| - |
|
|
| 2,500 |
|
Principal payments on related-party debt |
|
| - |
|
|
| (2,500 | ) |
Proceeds from sale of common shares and warrants |
|
| 190,000 |
|
|
| 310,000 |
|
Net cash provided by financing activities |
|
| 2,206,300 |
|
|
| 782,221 |
|
|
|
|
|
|
|
|
|
|
Increase (decrease) in cash |
|
| 290,851 |
|
|
| (10,034 | ) |
Cash, beginning of the year |
|
| 9,068 |
|
|
| 19,102 |
|
Cash, end of the year |
| $ | 299,919 |
|
| $ | 9,068 |
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flows information: |
|
|
|
|
|
|
|
|
Income taxes paid |
| $ | - |
|
| $ | - |
|
Interest paid |
| $ | 36,641 |
|
| $ | 46,590 |
|
|
|
|
|
|
|
|
|
|
Non-cash investing and financing transactions: |
|
|
|
|
|
|
|
|
Common shares issued for convertible debentures |
| $ | - |
|
| $ | 118,113 |
|
Debt discount for fair value of warrants issued with convertible debenture |
| $ | 907,540 |
|
| $ | 385,351 |
|
Common shares issued for loan guarantees |
| $ | - |
|
| $ | 269,715 |
|
Beneficial conversion feature on convertible debentures |
| $ | 1,124,731 |
|
| $ | 186,869 |
|
Debt assumed for deferred financing costs |
| $ | - |
|
| $ | 750,000 |
|
Common shares issued for stock payable |
| $ | 269,705 |
|
| $ | 4,728,191 |
|
Stock payable for convertible debt and accrued interest |
| $ | - |
|
| $ | 44,918 |
|
Settlement of warrants payable |
| $ | 692,317 |
|
| $ | - |
|
Fair values of warrants issued for service |
| $ | 1,290,308 |
|
| $ | - |
|
Debt discount for fair value of derivative liability |
| $ | 278,482 |
|
| $ | - |
|
Extinguishment of related party contributed capital |
| $ | 1,250,608 |
|
| $ | - |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
SPOTLIGHT INNOVATION INC.
NOTE 1. DESCRIPTION OF BUSINESS AND BASIS OF PRESENTATION
Spotlight Innovation Inc. (the "Company") was organized under the laws of the state of Iowa on March 23, 2012 ("inception") under the name Spotlight Innovation, LLC. The Company was founded to identify, validate and finance healthcare-focused companies founded for the purpose of commercializing intellectual property throughout the world. The Company provides strategic partners the opportunity to participate in the financing of a preferred search for, acquisition of, and/or funding of companies holding licenses for the commercialization of intellectual property developed by academic institutions. The principals of the Company have been involved in all stages of the commercialization of healthcare intellectual property over the last ten years. On December 16, 2013, Spotlight Innovation LLC was merged into Spotlight Innovation Inc. (formerly known as American Exploration Corporation) through a reverse merger transaction. The consolidated financial statements reflect operating results of the combined entities.
On June 4, 2014, the Company, through its subsidiary, Celtic Biotech Iowa, Inc. ("Celtic Iowa"), entered into a share exchange agreement with Celtic Biotech, Ltd., an Irish Limited Company ("CBL"), to provide for continued development and eventual marketing of the intellectual property of CBL. The primary intellectual property of CBL is an invention that relates to the compositions and methods combining snake venom toxin with chemotherapeutic agent(s) for cancer therapy.
On June 2, 2015, the Company concluded its purchase of 82.25% ownership interest in Memcine Pharmaceuticals, Inc., a Delaware corporation, ("Memcine") to provide for continued development of its Immunoplex™ vaccine platform technology, which is designed to use the body's own naturally occurring targeting system to deliver vaccine components to immune cells and stimulate a robust response. Memcine Pharmaceuticals has a licensing agreement for its technologies with the University of Iowa.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include those regarding the valuation of the assets acquired and liabilities assumed in the acquisition of Memcine and share-based compensation.
Principles of Consolidation
The consolidated financial statements include the Company's accounts, including those of the Company's subsidiaries Celtic Iowa and Memcine. During the year ended December 31, 2014, Celtic Iowa acquired 95% of the outstanding shares of CBL. During the year ended December 31, 2015, the Company acquired an 82.25% interest in Memcine. Accordingly, the Company has consolidated CBL, Celtic Biotech, Inc. and Memcine. All significant intercompany accounts and transactions have been eliminated.
Non-Controlling Interest
The Company is required to report its non-controlling interest in CBL and Memcine as a separate component of shareholders' equity. The Company is also required to present the consolidated net income and the portion of the consolidated net income allocable to the non-controlling interest and to the shareholders of the Company separately in its consolidated statements of operations. Losses applicable to the non-controlling interest are allocated to the non-controlling interest even when those losses are in excess of the non-controlling interest's investment basis.
| F-6 |
Loss per Common Share
Basic net income (loss) per common share is computed by dividing the net income (loss) attributable to common shareholders by the weighted-average number of common shares outstanding during the period. Diluted net income (loss) per share is computed by dividing the net income (loss) attributable to common shareholders by the weighted-average number of common and common equivalent shares outstanding during the period. Common share equivalents included in the diluted computation represent shares issuable upon assumed exercise of stock options and warrants or the assumed conversion of convertible debt instruments, using the treasury stock and "if converted" method. For periods in which net losses are incurred, weighted average shares outstanding is the same for basic and diluted loss per share calculations, as the inclusion of common share equivalents would have an anti-dilutive effect.
For the years ended December 31, 2015 and 2014, the dilutive effect of 655,200 and 5,200 options, and 1,385,000 and 1,276,671 warrants, 2,053,132 and 1,504,650 common shares issuable for conversion of convertible debt, respectively, were excluded from the diluted earnings per share calculation because their effect would have been anti-dilutive.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturity of three months or less when purchased to be cash equivalents. The Company maintains its cash in institutions insured by the Federal Deposit Insurance Corporation ("FDIC"). The Company had $299,919 and $9,068 cash equivalents at December 31, 2015 and 2014, respectively.
Financial instruments which potentially subject the Company to concentrations of credit risk include cash deposits placed with financial institutions. The Company maintains its cash in bank accounts which, at times, may exceed federally insured limits as guaranteed by the FDIC). As of December 31, 2015, the Company had no cash balances that were uninsured.
The Company had maintained cash in escrow which was restricted from immediate and general use by the Company. In December 2015, as part of the Termination and Release Agreements discussed in Note 6, the Company charged the $119,625 balance at December 31, 2015 to bad debt as the Company believes the amount to be uncollectable.
Foreign exchange and currency translation
For the years ended December 31, 2015 and 2014, the Company maintained cash accounts in U.S. dollars as well as European Union euros, and incurred certain expenses denominated in U.S. dollars and European Union euros. The Company's functional and reporting currency is the U.S. dollar. Transactions denominated in foreign currencies are translated into U.S. dollars at exchange rates in effect on the date of the transactions. Assets and liabilities are translated using exchange rates at the end of each period. Exchange gains or losses on transactions are included in earnings. Adjustments resulting from the translation process are reported in a separate component of other comprehensive income and are not included in the determination of the results of operations. For all periods presented, any exchange gains or losses or translation adjustments resulting from foreign currency transactions are included in the statements of operations as other income (expense).
In-Process Research and Development
In-process research and development ("IPR&D") represents the estimated fair value assigned to research and development projects acquired in a purchased business combination that have not been completed at the date of acquisition and which have no alternative future use. IPR&D assets acquired in a business combination are capitalized as indefinite-lived intangible assets. These assets remain indefinite-lived until the completion or abandonment of the associated research and development efforts. During the periods prior to completion or abandonment, those acquired indefinite-lived assets are not amortized but are tested for impairment annually, or more frequently, if events or changes in circumstances indicate that the asset might be impaired. During periods after completion, those acquired indefinite-lived assets are amortized based on their useful life. During the year ended December 31, 2014, the Company acquired IPR&D assets in its acquisition of CBL. The fair value of the assets acquired was $6,977,347. These assets are still subject to research and development completion and accordingly, no amortization has been recorded.
| F-7 |
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation and amortization. Maintenance and repairs are charged to expense as incurred. Renewals and betterments which extend the life or improve existing equipment are capitalized. Upon disposition or retirement of equipment, the cost and related accumulated depreciation are removed and any resulting gain or loss is reflected in operations. Depreciation is provided using the straight-line method over the estimated useful lives of the assets, which is 3-10 years.
Impairment of Long-Lived Assets and Intangibles
The Company performs impairment tests on its long-lived assets when circumstances indicate that their carrying amounts may not be recoverable. If required, recoverability is tested by comparing the estimated future undiscounted cash flows of the asset or asset group to its carrying value. If the carrying value is not recoverable, the asset or asset group is written down to fair value. For the years ended December 31, 2015, the Company recorded $212,541 in impairment to the Company's intangible assets related to the Memcine acquisition.
Deferred Financing Costs
We have incurred debt origination costs in connection with the issuance of short-term convertible debt. These costs are capitalized as deferred financing costs and amortized using the straight-line method over the term of the related convertible debt.
Stock-Based Compensation
The Company measures the cost of employee services received in exchange for stock and stock options based on the grant date fair value of the awards. The Company determines the fair value of stock option grants using the Black-Scholes option pricing model. The Company determines the fair value of shares of non-vested stock (also commonly referred to as restricted stock) based on the last quoted price of our stock on the date of the share grant. The fair value determined represents the cost for the award and is recognized over the vesting period during which an employee is required to provide service in exchange for the award. As share-based compensation expense is recognized based on awards ultimately expected to vest, the Company reduces the expense for estimated forfeitures based on historical forfeiture rates, if historical forfeiture rates are available. Previously recognized compensation costs may be adjusted to reflect the actual forfeiture rate for the entire award at the end of the vesting period. Excess tax benefits, if any, are recognized as an addition to paid-in capital.
Income Taxes
The Company utilizes the asset and liability method in accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for operating loss and tax credit carry-forwards and for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is recorded to reduce the carrying amounts of deferred tax assets unless it is more likely than not that the value of such assets will be realized.
Fair Value of Financial Instruments
The Company follows FASB ASC 820, Fair Value Measurement ("ASC 820"), which clarifies fair value as an exit price, establishes a hierarchal disclosure framework for measuring fair value, and requires extended disclosures about fair value measurements. The provisions of ASC 820 apply to all financial assets and liabilities measured at fair value.
| F-8 |
As defined in ASC 820, fair value, clarified as an exit price, represents the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a result, fair value is a market-based approach that should be determined based on assumptions that market participants would use in pricing an asset or a liability.
As a basis for considering these assumptions, ASC 820 defines a three-tier value hierarchy that prioritizes the inputs used in the valuation methodologies in measuring fair value.
Level 1 – Quoted prices in active markets for identical assets or liabilities. | |
|
|
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | |
|
|
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The Company's IPR&D assets were valued on a discounted cash flow model using the income approach. The inputs to the model were within level 3 of the fair value hierarchy.
Subsequent Events
The Company evaluated subsequent events through the date when financial statements are issued for disclosure consideration.
Recent Accounting Pronouncements
There were various accounting standards and interpretations issued recently, none of which are expected to have a material effect on the Company's operations, financial position or cash flows.
NOTE 3. GOING CONCERN
The Company is an early stage company and as such has not generated revenues from operations and there is no assurance of any future revenues. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. As of December 31, 2015, the Company has accumulated deficit of $18,643,652 and has a working capital deficit of $4,682,471. These factors raise substantial doubt as to the Company's ability to continue as a going concern.
The ability of the Company to continue as a going concern is dependent upon the Company's successful efforts to raise sufficient capital and then attain profitable operations. Management is investigating all options to raise enough funds to meet the Company's working capital requirements through either the sale of the Company's common stock or other financings. There can be no assurances, however, that management will be able to obtain sufficient additional funds when needed, or that such funds, if available, will be obtained on terms satisfactory to the Company.
| F-9 |
NOTE 4. BUSINESS COMBINATIONS
Acquisition of CBL
The Company, through its subsidiary Celtic Iowa, entered into a share exchange agreement with CBL. Celtic Iowa issued to CBL 474,419 shares of Celtic lowa Preferred Stock, with a fair value of $0.75 per share. In exchange, the Company received shares equal to 95% of the total outstanding shares of CBL.
Fair value at June 4, 2014 |
|
|
| |
Cash |
| $ | 1,656 |
|
Accounts receivable – related party |
|
| 1,349 |
|
Property, plant and equipment |
|
| 9,000 |
|
IPR&D |
|
| 6,977,347 |
|
Total assets |
|
| 6,989,352 |
|
|
|
|
|
|
Accounts payable and accrued liabilities |
|
| (242,693 | ) |
Short-term debt – related party |
|
| (204,186 | ) |
Total liabilities |
|
| (446,879 | ) |
Purchase price |
| $ | 6,542,473 |
|
The Company recorded the 5% non-controlling interest in CBL at a fair value of $327,124. The preferred shares issued are convertible into common shares of Celtic Iowa on a one for one basis and are redeemable at the Company's option in five years at the stated par value of $5 per share. The preferred shares have preferential rights to the net assets of Celtic Iowa in the case of liquidation up to the par value of the stock. While the Company maintains voting control through the common stock of Celtic Iowa and controls management decisions of Celtic Iowa, the Company's rights to the net assets are subordinated to the preferred stock up to a net asset value of $2,372,095. As a result, the Company has recorded non-controlling interest in Celtic Iowa of $2,372,078, which is the value attributed to the Preferred stock issued by Celtic Iowa. The Preferred Stock of Celtic Iowa is eliminated in consolidation.
CBL has had limited activity during the years ended December 31, 2015 and 2014. Accordingly, the Company has not presented Pro Forma information for the acquisition as the impact to the Company's operations was deemed immaterial.
Memcine
On June 2, 2015, the Company acquired 82.25% of the ownership in Memcine for $30,000.
The following table summarizes the allocation of the purchase price to the net assets acquired:
Fair value at June 2, 2015 |
|
|
| |
Cash |
| $ | 27,071 |
|
Property, plant and equipment |
|
| 18,071 |
|
IPR&D |
|
| 212,541 |
|
Total assets |
|
| 257,683 |
|
|
|
|
|
|
Accounts payable and accrued liabilities |
|
| (854 | ) |
Deferred liabilities |
|
| (220,465 | ) |
Total liabilities |
|
| (221,319 | ) |
Net assets acquired |
| $ | 36,364 |
|
The Company recorded the 17.75% non-controlling interest in Memcine at a fair value of $6,364.
Memcine had immaterial activity during year ended December 31, 2015. Accordingly, the Company has not presented pro forma information for the acquisition as the impact to the Company's operations was deemed immaterial.
The Company recorded an impairment of $212,541 as of December 31, 2015 to impair the intangible assets acquired in the acquisition of Memcine.
| F-10 |
NOTE 5. NOTES PAYABLE
On December 10, 2013, the Company assumed the liabilities of American Exploration which included the following notes payable to unrelated third parties:
|
| Date |
| Stated |
|
| Original |
|
|
| |||
|
| of |
| Interest |
|
| Principal |
|
| Due | |||
Promissory Note |
| Note |
| Rate |
|
| Amount |
|
| Date | |||
#1 |
| 05/29/09 |
|
| 10 | % |
| $ | 30,000 |
|
| On Demand | |
#2 |
| 06/05/09 |
|
| 10 | % |
| $ | 5,911 |
|
| On Demand | |
#3 |
| 08/16/09 |
|
| 10 | % |
| $ | 50,000 |
|
| On Demand | |
#4 |
| 09/27/10 |
|
| 10 | % |
| $ | 60,000 |
|
| On Demand | |
#5 |
| 06/02/10 |
|
| 5 | % |
| $ | 50,000 |
|
| On Demand | |
#6 |
| 02/04/11 |
|
| 5 | % |
| $ | 30,000 |
|
| On Demand | |
#7 |
| 05/04/11 |
|
| 5 | % |
| $ | 35,000 |
|
| On Demand | |
#8 |
| 08/11/11 |
|
| 10 | % |
| $ | 20,000 |
|
| On Demand | |
#9 |
| 12/05/11 |
|
| 10 | % |
| $ | 20,000 |
|
| On Demand | |
#10 |
| 04/28/12 |
|
| 10 | % |
| $ | 30,000 |
|
| On Demand | |
Total |
|
|
|
|
|
|
| $ | 330,911 |
|
|
| |
The Company also assumed $92,923 in accrued interest related to these notes. The Company recorded $28,541 and $28,720 in interest expense for the years ended December 31, 2015 and 2014, respectively, on the above notes payable.
As of December 31, 2015, and through the date of these financial statements, the Company has not received any demand notice from the lenders noted above for payment of principal or interest on these notes payable.
The Company also assumed a liability for previous advances made by American Exploration's former CEO in the amount of $23,433. These advances are due on demand and do not bear interest. During the year ended December 31, 2015 the Company made payments on the advances in the amount of $3,500 reducing the amount to $19,933 at December 31, 2015.
NOTE 6. CONVERTIBLE DEBENTURES
Kopriva Note
On September 27, 2013, the Company entered into a convertible note in the amount of $40,000 from an investor. The term of this note is fifteen months from commencement. During the term of this note, interest shall accrue on the unpaid principal balance at a fixed rate equal to 10% per annum, compounded annually. Should the Company default on the note, the outstanding balance of this note shall bear interest at the default rate of 20% per annum, compounded annually. In addition to the interest accrued the holder received warrants to purchase up to 100,000 shares of common stock. The conversion feature of the note and the exercise price of the warrants is the greater of (i) a discount of 40% to the 20 day average closing market price prior to the day that the warrant is executed or (ii) $0.20 per share. The warrants will have a term of thirty-six (36) months from the date of repayment or conversion of the note. The relative fair value of the warrants issued on the date of grant was $25,136 and was recorded as a debt discount on the note.
| F-11 |
In connection with the note, the convertible debenture was also analyzed for a beneficial conversion feature at which time it was concluded that a beneficial conversion feature existed. The Company recorded a debt discount of $14,864 for the fair value of the beneficial conversion feature. The Company is amortizing the combined debt discounts from the warrants and beneficial conversion feature over the term of the note.
On December 19, 2014, the Company entered an agreement to extinguish the note and its accrued interest in exchange for 102,000 shares of common stock. This agreement modified the terms of the conversion which resulted in the holder receiving more shares. As a result of the modification of the conversion feature, the Company determined that the change in the fair value of the conversion feature was greater than 10% and accordingly, recorded $120,085 as a loss on debt extinguishment which was the difference in fair value of the new conversion feature and the carrying value of the note and accrued interest on the date of modification. The Company recorded the issuance of the common stock on conversion at its fair value of $165,003 based on the market price on the date of grant. As of December 31, 2014, these shares were not issued and the fair value of the common stock was recorded as a stock payable. In January 2015, the Company issued the 102,000 share of common stock in settlement of stock payable at the fair value of $165,003.
Convertible Debt Associated with Letters of Credit
On April 4, 2014, the Company entered into a line of credit (the "Line of Credit") with Denver Savings Bank in the principal amount of $752,325. The Line of Credit provides that the Company can indirectly borrow up to the aforementioned principal amount from the Bank until April 1, 2017. Interest accrues at the rate of 4.25% per year. The loan is repayable on demand, but if no demand is made, then quarterly payments of accrued interest calculated on the amount of credit outstanding. As security for the Line of Credit a third party (the "Cosigner") cosigned the Line of Credit, and pledged certain collateral. In exchange for this pledge the Company issued the Cosigner 150,000 shares of common stock of the Company, and agreed to issue 30,000 shares of its common stock upon each one year anniversary of the Line of Credit, provided that the Line of Credit remains in effect. The shares of common stock had a fair value of $183,000 based on the market price on the date of grant and have been recorded as interest expense.
On April 4, 2014, the Cosigner and three third parties entered into a Security and Loan Agreement (the "SLA"). The SLA ensures that the Cosigner will be fully remunerated should the Company default on the Line of Credit. The SLA provides a guaranty to the Cosigner from the three third parties, which have pledged to repay any outstanding amounts on the Line of Credit should the Company default on the Line of Credit.
The Company may request draw downs on the Line of Credit at any time. Once a request is made, the Cosigner withdraws the funds and issues them to the third parties. The third parties will then loan the Company the funds from the Line of Credit through convertible promissory notes (the "Convertible Notes"). As the Company is both obligated to the Bank, as primary obligor, and to the third parties through the Convertible Notes, the Company has recorded liabilities on both obligations. The amounts attributable to the Line of Credit directly are recorded as non-cash interest expense. As of December 31, 2015, the Company had borrowed $752,325 through the Line of Credit, and issued Convertible Notes with the third parties in the amount of $752,325. The Convertible Notes accrue interest at 20.5% per annum and mature in six months from the dates of issuance. The proceeds from the Convertible Notes are reduced for original issuance discount fees. The Company received $572,220 in net proceeds from the Convertible Notes. These fees were recorded as a debt discount on the Convertible Notes. The Convertible Notes also contain a conversion feature which allows the Company to convert the Convertible Notes into shares of the Company's common stock. The conversion price is the lower of 50% of the prior 20 days average market price on the date of conversion, or $0.50 per share. However, in no event will the conversion price be lower than $0.25 per shares. The Company analyzed the Convertible Notes for a beneficial conversion feature. As a result of the in-the-money conversion price, the Company determined that a beneficial conversion feature did exist and recorded a debt discount of $186,869.
The Convertible Notes also contained attached warrants that allow the holders to purchase one share of common stock of the Company for each warrant exercised. The Company was obligated to issue 752,325 warrants with an exercise price of i) lower of 50% of the prior 20 days average market price on the date of conversion, or ii) $0.50 per share. However, in no event will the exercise price be lower than $0.25 per share. The warrants have a term of three years. The Company calculated the relative fair value of the warrants using the Black-Scholes model at $385,351, which was recorded as a debt discount to the Convertible Notes. As of December 31, 2014, the Company has not issued the warrants and has recorded the relative fair value as a warrant payable. The Company issued the warrants in 2015 as settlement of warrants payable.
| F-12 |
The Company is amortizing the combined debt discounts from the original issue discounts, warrants and beneficial conversion feature over the term of the Convertible Notes. The Company recorded interest expense of $82,003 related to the amortization of the debt discounts for the year ended December 31, 2015. The Company also recorded $150,846 in interest expense for the year ended December 31, 2015.
Additional Convertible Notes Associated with Additional Line of Credit
On July 29, 2014 the Company entered into an agreement with a third party individual (the "Individual") to guarantee an additional line of credit (the "Additional Line of Credit") in the amount of $250,000 with Denver Savings Bank. In exchange for the guarantee, the Company issued 42,300 shares of its common stock to the Individual. The shares of common stock had a fair value of $33,840 based on the market price on the date of grant and have been recorded as deferred financing costs. The Company has not made any borrowings on this Additional Line of Credit.
The Individual and three third parties entered into a Security and Loan Agreement (the "SLA"). The SLA ensures that the Individual will be fully remunerated should the Company default on the Line of Credit. The SLA provides a guaranty to the Individual from the three third parties, which have pledged to repay any outstanding amounts on the Line of Credit should the Company default on the Additional Line of Credit.
As of December 31, 2015, $135,000 has been drawn on the Additional Line of Credit. Once a request is made, the Individual withdraws the funds and issues them to the third parties. The third parties will then loan the Company funds, net guarantee fees from the Line of Credit through convertible promissory notes (the "Convertible Notes"). The third party guarantors require the Company to repay all funds drawn under the Additional Line of Credit to the bank in addition to the obligation to pay the guarantors for the principal amount of the convertible notes, as a guarantee fee. As the Company is both obligated to the Bank, as a cosignor to the Additional Line of Credit and to the third parties through the Convertible Notes, the Company records liabilities on both obligations.
The Company enters into the Convertible Notes with the third parties in the amount borrowed. The Convertible Notes accrue interest at 20.5% per annum and mature in six months from the dates of issuance. The Convertible Notes contain a conversion feature which allows the Company to convert the Convertible Notes into shares of the Company's common stock. The conversion price is the lower of 50% of the prior 20 days average market price on the date of conversion, or $0.50 per share. However, in no event will the conversion price be lower than $0.25 per shares. The Company analyzed the Convertible Notes for a beneficial conversion feature. As a result of the in-the-money conversion price, the Company determined that a beneficial conversion feature did exist and recorded a debt discount of $67,015.
The Convertible Notes also contained attached warrants that allow the holders to purchase one share of common stock of the Company for each warrant exercised. The warrants have an exercise price of i) lower of 50% of the prior 20 days average market price on the date of conversion, or ii) $0.50 per share. However, in no event will the exercise price be lower than $0.25 per share. The warrants have a term of three years. The Company calculated the relative fair value of the warrants using the Black-Scholes model at $67,985, which was recorded as a debt discount to the Convertible Notes.
The Company is amortizing the combined debt discounts from the original issue discounts, warrants and beneficial conversion feature over the term of the Convertible Notes and Additional Convertible Notes. The Company recorded interest expense of $217,003 related to the amortization of the debt discounts for year ended December 31, 2015. The Company also recorded $176,252 in interest expense for year ended December 31, 2015.
On March 27, 2015, the Company entered into a convertible note (the "Greig Note") in the amount of $2,500,000 with a related party investor and received proceeds of $1,620,000, net of debt service, origination fee and legal fees. The term of the Greig Note is 60 months from commencement. During the term of the Greig Note, interest shall accrue on the unpaid principal balance at a rate equal to the prime rate plus five percentage points per annum, compounded annually, for the first 36 months; and prime rate plus six percentage points in the last 24 months. Should the Company default on the Greig Note, the outstanding balance of the Greig Note shall bear interest at the default rate of two additional percentage points per annum, compounded annually. The Company issued warrants to purchase 250,000 and 300,000 shares of the Company's common stock to K4, LLC and The Grieg Companies, respectively, as consideration for entering into the convertible note. The warrants have an exercise price equal to 50% of the 20 day average closing market price of the Company's common stock prior to the date of exercise, provided that the exercise price shall not be below $1.50 per share. The Greig Note also contains a conversion feature that allows the investor to convert the unpaid principal and accrued interest into shares of the Company's common stock at a price of $1.50 per share. The warrants will have a term of 36 months from the date of repayment or conversion of the Greig Note. The relative fair value of the warrants issued on the date of grant was $652,504 and was recorded as a debt discount on the Greig Note. The Company analyzed the Greig Note for a beneficial conversion feature. As a result of the in-the-money conversion price, the Company determined that a beneficial conversion feature did exist and recorded a debt discount of $967,496.
| F-13 |
The Company is amortizing the combined debt discounts from the original issue discounts, warrants and beneficial conversion feature over the term of the Convertible Notes. The Company accrued interest expense of $175,685 and recorded amortization of the debt discount as interest expense for $370,313 prior to the termination agreement in December 2015.
On December 31, 2015, the Company closed a series of Termination and Release Agreements (the "Termination Agreements") which provided for the termination of certain agreements and instruments. The following is a brief summary of the transactions set forth in the Termination Agreements, and does not purport to be complete and is qualified in its entirety by reference to the full text of the Termination Agreements:ASC 470-40 requires that a gain or loss be recognized in the early extinguishment of debt in the period the debt is extinguished. As part of the termination of the March 2015 Note, and issuance of the December 2015 Note, the Company recorded a Loss of Extinguishment of debt in the amount of $1,250,608.
| - | The Company and The Greig Companies terminated the Convertible Promissory Note in the principal amount of $2,500,000 dated March 27, 2015. $100,000 of the $600,000 debt service reserve held by The Greig Companies, Inc. shall be returned to the Company within five business days of the execution by all parties of the Termination Agreement, and the remaining $500,000 shall be repaid to the Company in equal quarterly payments of $42,404 over three years which includes interest accrued at the rate of 3.25% per annum. The Company issued 500,000 shares of common stock to The Greig Companies, Inc. which are to be held by the Company and disbursed as the debt reserve is repaid. As of December 31, 2015, no payments have been received from The Greig Companies and no shares have been issued. |
|
|
|
| - | Pursuant to the Termination Agreements, the Convertible Promissory Note was transferred to K4, LLC through the issuance of a new convertible promissory note in the principal amount of $2,500,000. Interest accrues on the principal amount of the note at the rate of eight percent (8%) per annum. The Company has the right to prepay all or a portion of the outstanding principal sum of the December 2015 Note as follows: (a) on or before December 31, 2016 only with the written consent of the holder, such consent not to be unreasonably withheld, for such purposes including but not limited to a NASDAQ listing of the securities of the Company or as a condition to an equity financing by the Company; and (b) at any time after December 31, 2016 upon thirty days written notice by the Company to the holder. The holder of the December 2015 Note has the right to convert all or a portion of the principal sum of the December 2015 note into shares of common stock of the Company in denominations of not less than $250,000 as follows: (i) at any time up to December 31, 2016, at a discount of 20% of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $0.90 per share; (ii) at any time after December 31, 2016 and up to the close of business on December 31, 2017, at a discount of 15% of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $1.35 per share; or (iii) at any time after December 31, 2017 and up to the close of business on March 27, 2020, at a discount of 5% of the average price per share of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion, but not less than a price of $1.85 per share. The warrant to purchase 300,000 shares of Common Stock of the Company dated March 27, 2015 held by the holder of the December 2015 Note was terminated and replaced with (i) a warrant to purchase 300,000 shares of common stock at a price of $1.00 per share for a three year term, and (ii) a warrant to purchase an additional 200,000 shares of common stock of the Company at a price of $1.25 per share, for a three year term. The issuance of the new Note resulted in a debt extinguishment in the amount of $1,676,731. |
| - | The Company and its counterparties terminated a series of Convertible Promissory Notes with Warrants with Lextacan Development, Ltd., Ocana Limited, and Tosca Limited in the aggregate principal amount of $865,000. This series of Convertible Promissory Notes with Warrants was issued in connection with the Company's existing two Lines of Credit totaling $1,003,300 with the Denver Savings Bank, of which the Company has drawn down $865,000, which remains due and payable by the Company to the Denver Savings Bank pursuant thereto. The termination resulted in $1,250,608 of extinguishment of related party debt as contributed capital. |
|
|
|
| - | The Company and its counterparties terminated a series of agreements (including (i) Unit Subscription Agreements (USA Control No. 849207105 STLT No's: 01-10), (ii) Confidential Memorandum of Terms (MOT Control No. 849207105 STLT), (iii) Account Management Agreements (AMA Control No: AMA 849207105 STLT), and (iv) Escrow & Compliance Attorney Agreement, collectively referred to as the "Subscription Agreements") with nine investors, Copperbottom Investments, Ltd., Britannia Securities International, Ltd., Agri-Technologies International, Ltd., On Time Investments, ltd., RnD Company, Ltd., Rooftop Holdings, Ltd., Sequence Investments Ltd., Anybright Investments, Ltd., and Orange Investments, Ltd. (collectively the "Investors"). These agreements required the purchase by the Investors of shares of Series A Preferred Stock and Warrants of the Company based on certain formulas contained in the Subscription Agreements. Pursuant to this Termination Agreement the Investors have no further obligations to provide funding to the Company, and the Company has no obligation to accept such funds. All of the shares of Series A Preferred Stock, Series C Preferred Stock, and Warrants held in trust pursuant to the Subscription Agreements are terminated, cancelled and returned to the treasury of the Company. |
| F-14 |
The aggregate maturities of long-term debt as of December 31, 2015 are as follows:
|
| Demand Notes |
|
| Long-Term Debt |
|
| Line of Credit |
| |||
2016 |
| $ | 385,373 |
|
| $ | - |
|
| $ | - |
|
2017 |
|
| - |
|
|
| 175,000 |
|
|
| 888,300 |
|
2018 |
|
| - |
|
|
| - |
|
|
| - |
|
2019 |
|
| - |
|
|
| - |
|
|
| - |
|
2020 |
|
| - |
|
|
| 2,500,000 |
|
|
| - |
|
Thereafter |
|
| - |
|
|
| 125,000 |
|
|
| - |
|
Total Principal |
| $ | 385,373 |
|
| $ | 2,800,000 |
|
| $ | 888,300 |
|
NOTE 7. DEFERRED LIABILITIES
In September 2012, Memcine received financial assistance from Iowa Economic Development Authority ("IEDA"). Memcine entered into an agreement with IEDA, whereby IEDA would provide $150,000 in cash proceeds to Memcine in exchange for a 2% royalty on future gross revenues of Memcine to be paid to IEDA as repayment for the financial assistance. Total royalties to be paid will be $225,000 and the payment terms are on a semi-annual basis. To date, Memcine has not produced any revenues and has not been obligated to make repayment of the financial assistance. The contract also includes an acceleration clause which would require the Company to make a lump sum repayment of the financial assistance plus accrued interest at 6% per annum beginning from the date the proceeds were disbursed to Memcine should any of the following occur:
| a) | Memcine issues an initial public offering |
|
|
| b) | Memcine relocates outside of the state of Iowa |
|
|
| c) | Memcine sells 51% or more of the company's assets or the company |
As a condition to the acquisition by the Company, Memcine applied and was granted a waiver of the acceleration clause. Accordingly, the future repayment obligation is recorded as a long-term deferred liability. As of December 31, 2015, the outstanding principal was $220,465.
NOTE 8. DERIVATIVE LIABILITIES
During the year ended December 31, 2015, the Company issued convertible notes to various investors in the principal amount of $300,000. The convertible notes accrues interest on the principal amount at a rate of Citibank Prime Rate plus six percent. The convertible notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue warrants to each noteholder equal to 33.3% of the shares of common stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
A Qualified Financing means the issuance of equity securities to one or more investor in the amount of $2,000,000 or more of gross cash proceeds, of any conversion of outstanding securities.
| F-15 |
Conversion features of the Notes are as follows:
| · | Upon the occurrence of a Qualified Financing within six months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 35% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | Upon the occurrence of a Qualified Financing within six and twelve months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 75% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | The Holder has the further right to convert the full principal amount of the note prior to a Qualified Financing and after the three-month anniversary of the issuance of the note, at a price equal to the lower of $0.75 or the average price of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion. |
Due to the structure of the agreements and some of the terms contained therein, the Company determined that the issuance of these convertible notes resulted in derivative liability. The Company utilized a Black-Scholes option-pricing model and recorded a derivative liability in the amount of $278,482 related to these convertible notes for the year ended December 31, 2015.
NOTE 9. LEASES
The Company has one lease agreement. Our subsidiary, Memcine Pharmaceuticals, Inc., entered into an office lease agreement with the University of Iowa. The lease is for the period of September 1, 2015 through August 31, 2016 at a rate of $720 per month. The lease may be renewed for successive one-year period, with the lease rate to be agreed to 45 days prior to renewal.
Lease Schedule
Year |
| Lease Obligation |
| |
2016 |
| $ | 5,760 |
|
Total |
| $ | 5,760 |
|
NOTE 10. INCOME TAXES
At December 31, 2015 and 2014, the Company's deferred tax assets consisted primarily of net operating loss carry forwards acquired from American Exploration in the merger. For the years ended December 31, 2015 and 2014, the material reconciling items between the tax benefit computed at the statutory rate and the actual benefit recognized in the financial statements consisted of expenses related to share-based compensation and the change in the valuation allowance during the applicable period. At December 31, 2015 and 2014, the Company has recorded a 100% valuation allowance as management believes it is likely that any deferred tax assets will not be realized.
As of December 31, 2015, the Company had a net operating loss carry forward of approximately $16 million, which will expire between years 2028 and 2035. Due to the change in ownership provisions of the Tax Reform Act of 1986, our net operating loss carry forwards are expected to be subject to significant annual limitations for the change in ownership that resulted in the merger with American Exploration.
| F-16 |
NOTE 11. EQUITY
The Company has authorized the issuance of 3,000,000 shares of Series A preferred stock, 500,000 shares of Series C preferred stock, 1,500,000 shares of preferred stock and 4,000,000,000 shares of common stock.
COMMON STOCK
2015 Issuances
Stock Subscriptions
During the year ended December 31, 2015, the Company issued several subscription units that consisted of common stock and warrants. The Company received subscriptions for 253,335 shares of common stock and 95,000 warrants to purchase one share of common stock for each warrant, for net cash proceeds of $190,000. The warrants have an exercise price of $1.25 per share and expire 3 years of the date of issuance. The relative fair value of the warrants using the Black-Scholes model was $78,777.
Other Stock Issuances
On January 5, 2015, the Company issued 33,334 shares of common stock for a subscription received in December 2014. The subscription consisted of 33,334 shares of common stock and 12,500 warrants to purchase one share of common stock for each warrant, for net cash proceeds of $25,000. The warrants have an exercise price of $1.25 per share and expire three years after the date of issuance. The relative fair value of the common stock was $13,702 and was recorded as a stock payable as of December 31, 2014. Warrants were issued during the year ended December 31, 2015.
On January 9, 2015, the Company issued 102,000 shares to Jennifer Kopriva in settlement of stock payable owed as of December 31, 2014 for services. The shares had a fair value of $165,003 based on the market price on the date of grant.
On January 9, 2015, the Company issued 55,000 shares to Rachel Pettit in settlement of stock payable owed as of December 31, 2014. The shares had a fair value of $91,000 based on the market price on the date of grant.
On February 3, 2015, the Company issued 25,000 shares to Daniel Pettit for services. The shares were issued at $1.65 per share and recorded as share-based compensation of $41,250.
On February 28, 2015, the Company entered into a stock for services agreement with Michael Reysack, the Company's Investor Relations Officer, to issue 156,139 shares of common stock for services. The fair value of these shares was $257,629 based on the market price on the date of grant.
On April 28, 2015, the Company issued 30,000 shares to Jared DeVries for interest on debt. The shares were issued at $1.25 per share and recorded as interest expense of $37,500.
On April 28, 2015, the Company issued 2,600 shares to Rachael Pettit for services. The shares were issued at $1.25 per share and recorded as share-based compensation of $3,250.
| F-17 |
On June 6, 2015, the Company issued 25,000 shares to Daniel Pettit for services. The shares were issued at $1.25 per share and recorded as share-based compensation of $31,250.
On July 10, 2015, the Company issued 500,000 to Sundrop Consulting in accordance with a consulting agreement. The shares were issued at $1.05 per share and recorded as share-based compensation of 525,000.
On November 25, 2015, the Company issued 250,000 shares to the Community Foundation for Greater Des Moines as part of an agreement with Sundrop Consulting. The shares were issued in exchange for 1,000,000 warrants that were terminated. No additional expense was recorded as the fair value of the warrants exceeded the fair value of the common stock.
On December 8, 2015, the Company issued 100,000 shares of the Company's common stock to the Company's CFO for services and recorded the shares at the fair value at the date of issuance recording the transaction as share-based compensation of $90,000.
2014 Issuances
Stock Subscriptions
During the year ended December 31, 2014, the Company issued several subscription units that consisted of common stock and warrants. The Company received subscriptions to acquire 413,341 shares of common stock and 155,000 warrants to purchase one share of common stock for each warrant, for net cash proceeds of $310,000. The warrants have an exercise price of $1.25 per share and expire 3 years of the date of issuance. As of December 31, 2014, the Company had not issued 155,000 warrants and 33,334 shares of common stock attributable to a subscription in the amount of $25,000. Accordingly, the Company has recorded the relative fair value of the warrants and common stock as payables in the amounts of $142,042 and $13,702, respectively.
Other Stock Issuances
During the year ended December 31, 2014, the Company issued 1,105,970 shares of common stock in full extinguishment of a debenture with a principal balance of $110,598 and $7,515 of accrued interest. The shares were issued at 50% of the market price on the conversion date. No gain or loss was recorded on the conversion.
The Company issued 150,000 shares to an individual in return for his guarantee of the Letter of Credit at Denver Savings Bank. The shares were valued at the market price of $183,000 at the time of grant. (See Note 7.)
On July 29, 2014, the Company entered into an agreement with a third party individual to guarantee an additional Line of Credit in the amount of $250,975 with Denver Savings Bank. In exchange for the guarantee, the Company issued 42,300 shares of its common stock to the individual. The shares of common stock had a fair value of $86,715 based on the market price on the date of grant and have been recorded as deferred financing costs. The Company has not made any borrowings on this Line of Credit.
The Company issued 100,000 shares to an individual as a fee for extending the due date of his debenture. The debenture was paid on April 8, 2014 and the shares were issued on June 11, 2014. The share value was stated at the market price of $80,000 at the time of grant.
On July 30, 2014, the Company entered into an agreement with BFS Financial, Inc. ("BFS") and issued 50,000 shares of the Company's common stock. The agreement calls for BFS to assist the Company in raising capital. BFS is entitled to a 12% fee for all proceeds received by the Company from investors introduced to the Company by BFS for up to one year. The Company is also obligated to issue BFS an additional 50,000 shares of common stock upon receiving the first $100,000 in proceeds. The Company valued the shares issued at $102,500 based on the market price on the date of grant. In October 2014, the Company terminated the agreement with BFS. Accordingly, the additional 50,000 shares of common stock will not be issued.
| F-18 |
On October 30, 2014, the Company issued 18,450 shares of common stock to a third-party in settlement of the stock payable owed to the third-party for services performed. The shares had a fair value of $30,443 based on the market price on the date of grant.
On October 24, 2014, the Company issued 4,242 shares of common stock to a third-party in settlement of the stock payable owed to the third-party for services performed. The shares had a fair value of $6,999 based on the market price on the date of grant.
OPTIONS
Upon the acquisition of American Exploration, the Company adopted the 2009 Stock Option Plan (the "2009 Plan"). The 2009 Plan allows the Company to issue options to officers, directors and employees, as well as consultants, to purchase up to 7,000,000 shares of common stock.
The Company, as part of the merger, issued and exchanged 5,200 stock options to individuals who previously held stock options in American Exploration. These stock options were valued at $6,934 using the Black-Scholes model which was included in the purchase price of American Exploration.
2015 EQUITY INCENTIVE PLAN
On November 25, 2015, the Company authorized the Spotlight Innovation, Inc. 2015 Equity Incentive Plan (the "2015 Plan") to:
| · | Encourage selected employees, directors and consultants to improve operations and increase profits; | |
|
| |
| · | Encourage selected employees, directors and consultants to accept or continue employment or association with the Company it its Subsidiaries; | |
|
| |
| · | Increase the interest of selected employees, directors and consultants in the Company's welfare through participation in the growth in value of the common stock of the Company; and | |
|
| |
| · | Align the interest of the Company with selected employees, directors and consultants. |
Eligible persons who at the date of grant of an Option is an employee of the Company or of any Subsidiary of the Company is eligible to receive NQSOs or ISOs under the 2015 Plan. Every person who at the date of the grant is a consultant, or non-employee director of, the Company or any Subsidiary of the Company is eligible to receive NQSOs under the 2015 Plan.
The total number of shares which may be issued under the Options granted pursuant to the 2015 Plan shall not exceed the 3,600,000 Shares. The shares covered by the portion of any grant under the 2015 Plan which expires unexercised shall become available again for grant under the 2015 Plan.
On November 25, 2015, the Company granted incentive stock options and non-qualified stock options to acquire an aggregate of 2,600,000 shares of the Company's common stock under the Company's 2015 Plan to various officers and consultants of the Company. The options have exercise prices of $1.10 to $1.21. Each option was granted under a three-year vesting term, 25% upon grant, and 25% on each of the first, second and third anniversary of grant date. Of the 2,600,000 options granted, 150,000 were issued to an executive officer and the remaining 1,600,000 were issued to certain consultants of the Company.
| F-19 |
The fair value of each option award is estimated on the date of grant using a Black-Sholes valuation model that uses the assumptions noted in the following table.
|
| 2015 Equity Option Plan |
| |
Expected Volatility |
|
| 142.16 | % |
Expected Dividends |
| $ | 0 |
|
Expected Term in years |
|
| 3 |
|
The weighted-average grant-date fair value of options granted in 2015 is $1.04.
A summary of the stock option activity for the years ended December 31, 2015 and 2014 is presented below:
|
| Options |
|
| Weighted-Average Exercise Price |
| ||
Outstanding December 31, 2013 |
|
| 5,200 |
|
| $ | 359.04 |
|
Granted |
|
| - |
|
|
| - |
|
Exercised |
|
| - |
|
|
| - |
|
Expired |
|
| - |
|
|
| - |
|
Outstanding December 31, 2014 |
|
| 5,200 |
|
|
| 359.04 |
|
Granted |
|
| 2,600,000 |
|
|
| 1.11 |
|
Exercised |
|
| - |
|
|
| - |
|
Expired |
|
| - |
|
|
| - |
|
Outstanding December 31, 2015 |
|
| 2,605,200 |
|
| $ | 1.82 |
|
Exercisable December 31, 2015 |
|
| 655,200 |
|
| $ | 3.95 |
|
The following table provides information as of December 31, 2015 regarding shares authorized for issuance under our equity compensation plans, including individual compensation arrangements.
|
| Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) |
|
| Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights |
|
| Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding column (a)) |
| |||
Equity Compensation Plans Approved by Security Holders |
|
| 3,605,200 |
|
| $ | 3.95 |
|
|
| 5,050,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity Compensation Plans Not Approved by Security Holders |
|
| 1,364,171 | (1) |
|
| 1.40 |
|
|
| - |
|
Total |
|
| 4,969,371 |
|
| $ | 2.23 |
|
|
| 5,050,000 |
|
_____________
| (1) | Includes options to purchase 4,000,000- shares of common stock, up to 8,000 shares of common stock reserved under the 2009 Plan, and 3,600,000 shares of common stock reserved under the 2015 Plan. |
| F-20 |
Warrants
During the year ended December 31, 2015, the Company received subscriptions to acquire 253,335 shares of common stock and 95,000 warrants to purchase one share of common stock each, for net cash proceeds of $190,000. The warrants have an exercise price of $1.25 per share, and expire three years of the date of issuance. The relative fair value of the warrants based on the Black-Scholes model was $78,777.
The Company issued warrants to purchase 135,000 shares of common stock in conjunction with the Additional Convertible Notes during January 2015. The exercise price is equal to the lower of: (i) 50% of the prior 20 days average market price on the date of exercise, or ii) $0.50 per share. However, in no event will the exercise price be lower than $0.25 per share. The warrants have a term of three years. The relative fair value of the warrants based on the Black-Scholes model was $67,985. As of December 31, 2015, the Company entered into a termination agreement and terminated the Additional Convertible Notes issued January 2015, cancelling the warrants to purchase 135,000 shares of common stock.
The Company granted warrants to purchase 250,000 and 300,000 shares of common stock to related parties in conjunction with a convertible note dated March 27, 2015. The warrants have an exercise price of $1.50 per share, and expire thirty-six (36) months from the date of repayment or conversion of the convertible note. The relative fair value of the warrants based on the Black-Scholes model was $652,504. As of December 31, 2015, the Company entered into a termination agreement and terminated the convertible note dated March 27, 2015, cancelling the warrants to purchase 250,000 and 300,000 shares of common stock.
On March 30, 2015, the Company issued warrants to purchase 359,620 shares of common stock to a related party in conjunction with a settlement of an outstanding payable in the amount of $419,413. The warrants have an exercise price of $1.25 per share, and expire five years from the date of issuance. The fair value of the warrants based on the Black-Scholes model was $449,398. Accordingly, the Company recorded a loss on extinguishment of debt of $29,984. Pursuant to the December 2015 termination agreements, the warrants to purchase 359,620 share of common stock were cancelled.
On June 3, 2015, the Company issued warrants to purchase 200,000 shares of common stock to an unrelated third party in conjunction to a services agreement. The warrants were priced at $1.25 per share and expire in 1.7 years from issuance. The fair value of the warrants based upon the Black-Scholes model is $246,852.
On June 23, 2015, the Company issued warrants to purchase 1,000,000 shares of common stock to an unrelated party in conjunction to a services agreement. The warrants have an exercise price of $1.25 per share, and expire in 2.2 years from issuance. The fair value of the warrants based upon the Black-Scholes model is $1,043,457. As of December 31, 2015, these warrants were forfeited.
On December 31, 2015, the Company issued warrants to purchase 200,000 and 300,000 shares of Common Stock in conjunction with a convertible note dated December 31, 2015. The warrants have an exercise price of $1.25 and $1.00 per share, respectively, and expire thirty-six (36) months from the date of issuance. The relative fair value of the warrants based on the Black-Scholes model was $187,051 and $280,726, respectively.
The fair value of the above warrants was determined by using the Black-Scholes option-pricing model. Variables used in the model for the warrants issued include: i) discount rates ranging from 0.69% to 1.31%; ii) expected terms ranging from 2.00 to 5.00 years; iii) expected volatility ranging from 323.95% to 393.03%; iv) zero expected dividends and v) stock price of $0.94 to $1.65.
During the year ended December 31, 2015, the Company issued 692,317 warrants that were granted in the year ended December 31, 2014 and were recorded as a warrant payable. The Company recorded $692,317 in additional paid-in capital for the issuance of the warrants in satisfaction of the warrants payable.
During the year ended December 31, 2014, the Company issued subscription units that consisted of common stock and warrants. The Company received subscriptions to acquire 413,341 shares of common stock and 155,000 warrants to purchase one share of common stock each for net cash proceeds of $310,000. The warrants have an exercise price of $1.25 per share and expire three years from the date of issuance. The relative fair value of the warrants was $142,042.
As of December 31, 2014, the Company was obligated to issue 752,325 warrants to purchase one share of common stock for each warrant, in conjunction with the issuance of the Convertible Notes. The warrants have an exercise price of the lower of i) 50% of the prior 20 days average market price on the date of conversion, or ii) $0.50 per share. However, in no event with the exercise price be lower than $0.25 per share. The warrants have a term of three years. The Company calculated the relative fair value of the warrants using the Black-Scholes model at $385,351. However, as of December 31, 2014, the Company has not issued these warrants and has recorded them as a warrant payable.
On December 19, 2014, the Company agreed to issue 100,000 warrants to purchase one share of common stock for each warrant in connection with a consulting agreement. The warrants have a term of three years and an exercise price of $1.29 per share. The fair value of the warrants on the date of grant using the Black-Scholes model was $164,924. As of December 31, 2014, these warrants have not been issued and are recorded in warrants payable.
The fair value of the above 2014 warrants was determined by using the Black-Scholes option-pricing model. Variables used in the model for the warrants issued include: i) discount rates ranging from 0.79% to 1.10%; ii) expected terms of 3.0 years; iii) expected volatility ranging from 399.22% to 409.43%; iv) zero expected dividends and v) stock prices ranging from $0.75 to 2.00.
| F-21 |
A summary of the warrant activity for the years ended December 31, 2015 and 2014 is presented below:
|
| Warrants |
|
| Weighted-Average Exercise Price |
| ||
Outstanding December 31, 2013 |
|
| 1,381,671 |
|
| $ | 1.41 |
|
Granted |
|
| 1,005,000 |
|
|
| 0.72 |
|
Exercised |
|
| - |
|
|
| - |
|
Expired/forfeited |
|
| (160,000 | ) |
|
| 1.46 |
|
Outstanding at December 31, 2014 |
|
| 2,226,671 |
|
|
| 1.10 |
|
Granted |
|
| 2,839,620 |
|
|
| 1.25 |
|
Exercised |
|
|
|
|
|
| - |
|
Expired/forfeited |
|
| (3,054,620 | ) |
|
| 1.11 |
|
Outstanding December 31, 2015 |
|
| 2,011,671 |
|
| $ | 1.29 |
|
Exercisable December 31, 2015 |
|
| 2,011,671 |
|
| $ | 1.29 |
|
The weighted average remaining contractual term of the outstanding warrants and exercisable warrants as of December 31, 2015 is 1.58 years.
NOTE 12. PRIVATE PLACEMENT
On June 4, 2014, the Company entered into a series of agreements related to an equity arrangement for the sole purpose of funding acquisitions. The agreements require the Company to issue 1,051,200 Convertible Series A Preferred Stock in exchange for $41,418,000 to nine investors, through a private placement of 900 Units, under certain circumstances, consisting of 1,168 Convertible Series A Preferred Stock (convertible into common shares of the Company) and warrants to purchase 413,964,900 common shares of the Company. The warrants have exercise prices from $0.25 to $0.7035 per share and terms of four to six years. The Company can receive up to $165,681,009 if all the warrants are exercised. The Convertible Series A Preferred Stock may convert into common shares of the Company at a rate of 1 share of Series A Preferred stock for 100 shares of common stock and must be converted within three years.
Under the terms of the agreements the Investors' cash of $41,418,000 ($46,020 per Unit sold) has been deposited in a restricted account with an Intermediary, whereby an Account Management Agreement between the Investors, the Company, and the Intermediary governs the release of funds to the Company from the restricted account. The Company has placed with the Intermediary, the Securities to be released to the investors at the same time and ratio as the funds are released to the Company. The Company will record the fair value of the Securities as they are issued to the investors by the Intermediary.
On December 31, 2015, the Company entered into the Termination Agreements which provided for the termination of all of the agreements and instruments referenced above, as described in Note 6.
NOTE 13. RELATED PARTY TRANSACTIONS
As a result of the acquisition of CBL, as disclosed in Note 4, the Company assumed two short-term notes payable due to a related party. The debts are denominated in Euros, and on June 4, 2014, the date of acquisition, the carrying amount of the debts was approximately $204,186. The notes accrue compounded interest at 5% per annum and were due in November and December 2014. As of December 31, 2015, these notes were still outstanding and the carrying value of the notes was $168,949. The Company has negotiated an extension of the due date to December 31, 2015 for the note that was due in December 2014. The notes are currently in default.
On March 30, 2015, the Company entered into a consulting agreement with The Greig Companies Inc., whose principal is a significant shareholder of the Company. The consultant will provide corporate and strategic growth advisory services. The Company has agreed to pay the consultant a $10,000 monthly fee with an additional per diem reimbursement of $1,250 for days traveling. The term of the contract is three years. In August 2015, the Company terminated this agreement pursuant to the Termination Agreements described in Note 6.
| F-22 |
NOTE 14. COMMITMENTSAND CONTINGENCIES
The Company, through its subsidiary Celtic Biotech Iowa, Inc., entered into a license agreement to license technology for novel therapy for chronic kidney disease owned by Zheng-Hong Qin of Wilmington, Massachusetts. The agreement provides for a 7% royalty payment on all net sales related to the licensed therapies. As of December 31, 2015, no sales have been made.
On December 19, 2014, the Company entered into an agreement with a third party to provide investor relations consulting services. The Company agreed to issue 25,000 shares of restricted common stock at the time of the agreement and up to 150,000 additional shares based on milestones achieved by the consultant. As of December 31, 2014, the consultant had achieved one of the milestones and was due an additional 25,000 shares. The fair value of the 50,000 shares of common stock to be issued to the consultant was $91,000 based on the market price on the dates of grant which was recorded as a stock payable at December 31, 2014. During the three months ended March 31, 2015, the consultant met an additional milestone which required the Company to issue 25,000 shares of common stock. The shares were valued at $41,250 based on the market price on the date grant. All of the shares earned by the consultant were issued in January and February of 2015. On April 29, 2015, the consulting agreement was mutually terminated. As a result of the termination of the agreement, 100,000 warrants were cancelled.
On April 29, 2015, the Company entered into a three-year consulting agreement with Sundrop Consulting, Inc. ("Sundrop") and agreed to compensate Sundrop as follows: $10,669 per month; 25,000 shares of common stock upon execution of the agreement; a warrant to purchase 100,000 shares of the Company's common stock at $1.29 per share with an expiration date of December 19, 2017. In addition, the Company agreed to pay a bonus of $300,000 in cash and 500,000 shares of common stock if the Company receives $10 million in financing through the efforts of Sundrop; a bonus of $100,000 in cash and 300,000 shares of common stock if the Company achieves a listing of its securities on the New York Stock Exchange or Nasdaq; and a bonus of $750,000 in cash and 1.5 million shares of common stock if the Company receives $40 million in financing through the efforts of Sundrop.
On June 23, 2015, the Company amended the agreement with Sundrop as follows: a) the Company will compensate Sundrop $10,669 per month in bi-weekly installments of $5,334, b) bonus provision whereby the Company will issue 500,000 shares of Common Stock at the signing of the agreement with an additional 500,000 issued at the one year anniversary of the agreement and 500,000 issued at the two year anniversary of the agreement providing the agreement remains in effect, c) Sundrop is issued warrants for the right to purchase 1,000,000 shares of common stock at $1.25 per share expiring on December 19, 2017, d) an annual raise of $10,000 for every $1,000,000 in financing received by the Company during the term of the agreement.
On July 1, 2015, the Company entered into an agreement with DAK Capital, LLC ("DAK") to provide investment banking and capital advisory services in connection with the Company's efforts to raise capital and with respect to such other matters as to which the Company and DAK may agree. The agreement has a minimum term of six months and a monthly non-refundable payment of $10,000 to DAK. In the event that DAK completes a transaction for the benefit of the Company of at least $10 million during this six-month term, the agreement is automatically extended for eighteen additional months. Each transaction is subject to payment of a success fee of 8% to DAK, as well as warrants equal to the amount of the transaction, each with a term of five years.
On July 14, 2015, CBL entered into a clinical agreement with Immunoclin, Ltd., a United Kingdom corporation, to carry out the phase 1b (and potentially phase 2) trial of the drug Crotoxin. The agreement calls for Celtic Iowa to pay to Immunoclin, Ltd. 525,330 Euro (approximately $573,944) over the period of the trial, which is expected to last 18 months.
NOTE 15. SUBSEQUENT EVENTS
On January 5, 2016, the Company received $50,000 in proceeds from Shawn W. Rouse related to the subscription agreement entered into by and between Mr. Rouse and the Company. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each holder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
A Qualified Financing means the issuance of equity securities to one or more investor in the amount of $2,000,000 or more of gross cash proceeds, of any conversion of outstanding securities.
| F-23 |
Conversion features of the Notes are as follows:
| · | Upon the occurrence of a Qualified Financing within six months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 35% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | Upon the occurrence of a Qualified Financing within six and twelve months from the date of issuance, the note automatically converts, without any further action by the holder, into shares of common stock of the Company at a 75% discount to the pre-money valuation at the time of such Qualified Financing. | |
|
| |
| · | The Holder has the further right to convert the full principal amount of the note prior to a Qualified Financing and after the three-month anniversary of the issuance of the note, at a price equal to the lower of $0.75 or the average price of the common stock of the Company during the 20 consecutive trading days immediately prior to such conversion. |
On January 11, 2016, Jennifer Korpriva exercised her warrants to purchase to purchase 100,000 shares of Spotlight Common stock. The exercise price was at 60% of the average closing market price for the 20 trading days preceding the exercise. Total proceeds received from the exercise was $55,140.
On January 14, 2016, the Company received $25,000 in proceeds from Eric Turner related to the subscription agreement entered into by and between Mr. Turner and the Company. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each noteholder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
On January 15, 2016, the Company received $325,000 in proceeds from K4 Enterprises, LLC related to the subscription agreement entered into by and between K4 Enterprises and the Company. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each holder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
On February 12, 2016, the Company received $50,000 in proceeds from Craig Lang related to the subscription agreement entered into by and between Craig Lang and the Company. The notes convert into shares of common stock of the Company in certain instances as discussed below. Upon the closing of a Qualified Financing, the Company will issue to each holder of a Note warrants equal to 33.3% of the shares of Common Stock issuable thereunder, at an exercise price equal to 110% of the price offered in the Qualified Financing. Such warrants shall have a term of three years.
On August 19, 2016, the Company entered into a Sponsored Research Agreement (the "SRA") with the Florida State University Research Foundation ("FSURF") starting September 1, 2016, to perform certain research, over a two-year period, related to the discovery, synthetic modification, and preclinical validation of drug-like compounds intended to treat patients with Zika virus infection to be directed by Dr. Hengli Tang. The SRA provides for payments by the Company to the FSURF of $147,000 upon each of the commencement of the research and six months thereafter. The Company is also responsible for additional contributions toward the direct and indirect costs of the research as per the terms of the SRA.
| F-24 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
CEO/CFO Certifications
Attached to this Annual Report on Form 10-K as Exhibits 31.1 and 31.2, there are two certifications, and the Section 302 certifications, one by each of our Chief Executive Officer (CEO) and our Chief Financial Officer (CFO). This Item 9A contains information concerning the evaluation of our disclosure controls and procedures and internal control over financial reporting that is referred to in the Section 302 Certifications and this information should be read in conjunction with the Section 302 Certifications for a more complete understanding of the topics presented.
Evaluation of Disclosure Controls and Procedures
We have performed an evaluation under the supervision and with the participation of our management, including our Chief Executive Officer (CEO) and Chief Financial Officer (CFO), of the effectiveness of our disclosure controls and procedures, (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2015. Based on that evaluation, our management, including our CEO and CFO, concluded that our disclosure controls and procedures were not effective as of December 31, 2015 to provide reasonable assurance that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms and (ii) accumulated and communicated to our management, including our principal executive officer, as appropriate to allow timely decisions regarding required disclosure due to the material weaknesses described below.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our CEO and CFO, we evaluated the effectiveness of our internal control over financial reporting as of December 31, 2015, and concluded that our internal control over financial reporting was not effective at December 31, 2015. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") in Internal Control-Integrated Framework. Management's evaluation included such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment. We identified a material weakness in our internal control over financial reporting primarily attributable to limited accounting and SEC reporting expertise within the Company and the Company has insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of accounting principles generally accepted in the United States of America ("GAAP") and SEC disclosure requirements. Due to being an early stage company, we have limited financial ability to remedy this staffing deficiency at this time; however, we will add additional accounting and SEC reporting expertise in the future as funds permit. Notwithstanding this material weakness, management has concluded that our audited consolidated financial statements included in this Annual Report on Form 10-K are fairly stated in all material respects in accordance with generally accepted accounting principles for each of the periods presented herein.
| 42 |
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by our registered public accounting firm pursuant to the rules of the SEC that permit us to provide only management's report in this Annual Report on Form 10-K.
Inherent Limitations On Effectiveness Of Controls
We believe that a controls system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives, and our CEO and CFO, has concluded that these controls and procedures are not effective at the "reasonable assurance" level.
Changes In Internal Controls Over Financial Reporting
There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the quarter ended December 31, 2015 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
| 43 |
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors and Executive Officers
All of our directors hold office until the next annual general meeting of the shareholders or until their successors are elected and qualified. Our officers are appointed by our board of directors and hold office until their earlier death, retirement, resignation or removal.
Our directors and executive officers, their ages and positions held are as follows:
Name |
| Age |
| Position with the Company |
Cristopher Grunewald |
| 42 |
| President, Chief Executive Officer and Director |
William Pim |
| 62 |
| Chief Financial Officer |
John M. Krohn |
| 57 |
| Director |
Craig A. Lang |
| 64 |
| Director |
Jack Price and Steven Katz served as members of the Board of Directors of the Company from February 24, 2016, until their resignations from the Board of Directors on June 15, 2016, and June 14, 2016 respectively.
Business Experience
The following is a brief account of the education and business experience of each director, executive officer and key employee during at least the past five years, indicating each person's principal occupation during the period, and the name and principal business of the organization by which he or she was employed, and including other directorships held in reporting companies.
Cristopher Grunewald
President, Chief Executive Officer, Director
Mr. Grunewald is the founder, President, Chief Executive Officer and Director of the Company since inception. Prior experience includes leading corporate development and operations projects and providing decision support for early stage companies. Prior experience also includes serving as Vice President for Real Estate Acquisitions with DC Investments and executing an extensive expansion plan into various new markets. Mr. Grunewald was a director with GOOI Global from December 13, 2013 to February 17, 2016, Military service with U.S. Marines, and training from the National Geospatial Intelligence Agency. B.S. in Liberal Science and Technology from Iowa State University.
| 44 |
William Pim
Chief Financial Officer
Mr. Pim joined the Company as Chief Financial Officer in November 2015. Mr. Pim has over three decades of financial and business development experience with expertise in auditing, financial reporting, SEC compliance, strategic planning, risk management and mergers and acquisitions. He has held senior level financial positions with: Mark Seed Company; Adrian Carriers; Iowa Renewable Energy, Inc. (where Mr. Pim was a co-founder and a Director from October 2005 to October 2010); Iowa Foundation for Medical Care; and Heartland Express. Mr. Pim, a C.P.A. received a B.S. degree in Accounting from the University of Iowa.
John M. Krohn
Director
Mr. Krohn joined the Company as a member of the Board of Directors of the Company in February 2016. Mr. Krohn is a Senior Financial Services Advisor with Principal Financial Group, a global investment management leader, offering retirement services, insurance solutions and asset management. Mr. Krohn is a registered investment advisor and holds Series 7, 63 and 65 licenses. He was inducted into the Principal Financial Group Agent Hall of Fame and is the 2014 recipient of the Lifetime Achievement Award for life insurance production. Mr. Krohn, a CPA, is a 1981 graduate of the University of Iowa with a B.S. degree in Accounting.
Craig A. Lang
Director
Mr. Lang joined the Company as a member of the Board of Directors of the Company in February 2016. Craig A. Lang is President of The Prairie Strategy Group, a policy, communication and logistics consulting company focused on the worldwide need for affordable food and energy. He is also President of Windward Iowa, an organization advocating for clean wind energy and the advancement and modernization of electric transmission lines across the United States. From 2001 to 2011, he was Chairman of the Board of FBL Financial, an insurance and annuity company, focusing on markets in the Midwest and Western states. From 2008 to 2013, Mr. Lang served as a board member of, and from 2011 to 2013, as President of, the Board of Regents of the State of Iowa, a group which governs five public educational institutions in the State. Mr. Lang received a Bachelor of Science degree from Iowa State University in 1973.
Committees of The Board Of Directors
During the year ending December 31, 2015, the Board of Directors consisted of only one member and as such we did not establish an audit committee, a compensation committee nor a nominating committee.
Family Relationships
There are no family relationships among our directors or officers.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and officers, and the persons who beneficially own more than ten percent of our common stock, to file reports of ownership and changes in ownership with the Securities and Exchange Commission. Copies of all filed reports are required to be furnished to us pursuant to Rule 16a-3 promulgated under the Exchange Act. Based solely on the reports received by us and on the representations of the reporting persons, we believe that these persons have complied with all applicable filing requirements during the fiscal year ended December 31, 2015; with the exception of John M. Krohn (Director) and Sundrop Consulting, Inc.
| 45 |
ITEM 11. EXECUTIVE COMPENSATION.
The following table sets forth the compensation paid to our Chief Executive Officer, Chief Financial Officer and those executive officers that earned in excess of $120,000during the last two fiscal years ended December 31, 2015 and 2014 (collectively, the "Named Executive Officers"):
Summary Compensation Table
Name and Principal Position |
| Year |
| Salary ($) |
|
| Bonus ($) |
|
| Stock Awards ($) |
|
| Option Awards ($) |
|
| Non-Equity Incentive Plan Compensation ($) |
|
| Non-Qualified Deferred Compensation Earnings ($) |
|
| All Other Compensation ($) |
|
| Total($) |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Cristopher Grunewald, |
| 2015 |
|
| 120,000 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
| (4 | ) |
|
| 120,000 |
| |
President, CEO (1) |
| 2014 |
|
| 120,000 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
| (4 | ) |
|
| 120,000 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
William Pim, |
| 2015 |
|
| 7,000 |
|
|
| 0 |
|
|
| 90,000 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
| 0 |
|
|
| 97,000 |
| |
CFO (2) |
| 2014 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
| 0 |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
David Hostelley, |
| 2015 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Former CFO (3) |
| 2014 |
|
| 23,500 |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 23,500 |
|
_______________
(1) | Mr. Grunewald became President and CEO of the Company on July 24, 2013. |
(2) | Mr. Pim became CFO of the Company on November 6, 2015. |
(3) | Mr. Hostelley served as CFO of the Company from June 20, 2014 through his resignation on August 31, 2015. |
(4) | Amount represents an estimated amount due Mr. Grunewald pursuant to his employment agreement dated December 16, 2013 if Mr. Grunewald's employment is terminated without cause or he resigns for good reason, each such term as defined in Mr. Grunewald's employment agreement. |
Agreements
Below are the agreements the Company is a party to with the named executive officers:
In December 2013, we entered into an agreement with Cristopher Grunewald to serve as President and CEO. The agreement was for a ten- year term and provided for an annual salary of $120,000 and the issuance of 3,200,000 shares of common stock. The salary shall increase at the rate of a minimum of five percent (5%) per annum on a cumulative basis, with the Board of Directors of the Employer having discretion to provide additional increases. The agreement also calls for the Company to pay Mr. Grunewald certain sums in the event he is terminated by the Company without cause, based upon the remainder of the original term of the Agreement.
In November 2015, we entered into an agreement with Mr. Pim to serve as Chief Financial Officer of the Company for one year. This agreement obligates us to pay Mr. Pim a salary of $3,500 per month, and to grant him 100,000 shares of Common Stock of the Company.
| 46 |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information with respect to outstanding equity awards at December 31, 2015 with respect to the named executive officers.
|
| Individual Grants | |||||||||||||||
Name |
| Year |
| Number of |
|
| Number of |
|
| Exercise |
|
| Expiration | ||||
Cristopher Grunewald |
| 11/25/2015(1) |
|
| 37,500 |
|
|
| 112,500 |
|
| $ | 1.21 |
|
| 11/25/2025 | |
_______________
| (1) | These shares vest twenty five percent (25%) upon grant, and twenty five percent (25%) on each of the first, second and third anniversary of the grant date. |
Equity Compensation Plans
We have two equity compensation plans, the Spotlight Innovation Inc. 2009 Stock Option Plan (the "2009 Plan") and the Spotlight Innovation Inc. 2015 Equity Incentive Plan (the "2015 Plan").
The following table provides information as of December 31, 2015 regarding shares authorized for issuance under our equity compensation plans, including individual compensation arrangements.
|
| Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) |
|
| Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights |
|
| Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding column (a)) |
| |||
Equity Compensation Plans Approved by Security Holders |
|
| 3,605,200 |
|
| $ | 3.95 |
|
|
| 5,050,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity Compensation Plans Not Approved by Security Holders |
|
| 1,364,171 | (1) |
|
| 1.40 |
|
|
| - |
|
Total |
|
| 4,969,371 |
|
| $ | 2.23 |
|
|
| 5,050,000 |
|
______________
| (2) | Includes warrants to purchase ---- shares of common stock, up to 8,000 shares of common stock reserved under the 2009 Plan, and 3,600,000 shares of common stock reserved under the 2015 Plan. |
| 47 |
2009 Plan
The 2009 Plan provides that, subject to the provisions of the 2009 Plan, the Board of Directors may grant to any key individuals who are our employees eligible to receive options, one or more incentive stock options to purchase the number of shares of common stock allotted by the Board of Directors (the "Incentive Stock Options"). The option price per share of common stock deliverable upon the exercise of an Incentive Stock Option shall be at least 100% of the fair market value of our common shares, and in the case of an Incentive Stock Option granted to an optionee who owns more than 10% of the total combined voting power of all classes of our stock, shall not be less than 100% of the fair market value of our common shares. The option term of each Incentive Stock Option shall be determined by the Board of Directors, which shall not commence sooner than from the date of grant and shall terminate no later than ten (10) years from the date of grant of the Incentive Stock Option, subject to possible early termination as described above.
2015 Plan
Effective November 25, 2015, our Board of Directors adopted the Spotlight Innovation Inc. 2015 Equity Incentive Plan (the "2015 Plan"). The 2015 Plan allows us to grant certain options to our directors, officers, employees, and eligible consultants. The 2015 Plan allows us to grant options to our officers, directors, and employees. In addition, we may grant options to individuals who act as our consultants. A total of 3,600,000 shares of our common stock are available for issuance under the 2015 Plan. Pursuant to the 2015 Plan employees are eligible to receive incentive stock options or non-qualified stock options, and consultants and directors are eligible to receive non-qualified stock options. The 2015 Plan has not been approved by our stockholders. On November 25, 2015, the Company granted incentive stock options and non-qualified stock options to acquire an aggregate of 2,600,000 shares of the Company's common stock under the Company's 2015 Plan to various officers and consultants of the Company. Each option was granted under a three-year vesting term, Twenty Five Percent (25%) upon grant, and Twenty Five Percent (25%) on each of the first, second and third anniversary of grant date. Of the 2,600,000 options granted, 150,000 were issued to an executive officer and the remaining 1,600,000 were issued to certain consultants of the Company.
Director Compensation
During the Company's last completed fiscal year ending December 31, 2015, directors were not compensated for serving as a director of the Company. Our employee director (Cristopher Grunewald) receives no compensation for serving as a director. Commencing February 26, 2016, each non-executive officer member of the Board of Directors will receive: (i) an annual cash retainer of $12,000, payable quarterly at the beginning of each calendar quarter, and (ii) an option, to purchase 75,000 shares of the Company's Common Stock with an exercise price equal to the closing price on the date prior to the date of the grant. Subject to continued service as a Director, these options will vest one third upon issuance, one third on the one year anniversary as a member of the Board of Directors, and one third on the second year anniversary as a member of the Board of Directors.
Risk Oversight
Our board as a whole has responsibility for risk oversight, with reviews of certain areas being conducted by the relevant board committees that report on their deliberations to the full board, as further described below. Given the small size of the board, the board feels that this structure for risk oversight is appropriate (except for those risks that require risk oversight by independent directors only).
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of August 24, 2016 by each stockholder known by us to be the beneficial owner of more than 5% of our common stock and by each of our current directors and executive officers. Each person has sole voting and investment power with respect to the shares of common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
| 48 |
Beneficial ownership is determined in accordance with the rules and regulations of the United States Securities and Exchange Commission ("SEC"). In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options held by that person that are currently exercisable or exercisable within sixty (60) days of August 24, 2016 are deemed outstanding. These shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. Except as indicated in the footnotes to this table and pursuant to applicable community property laws, we believe each stockholder named in the table has sole voting and investment power with respect to the shares set forth opposite that stockholders' name.
Name and Address of Beneficial Owner(8) |
| Amount and Nature of Beneficial Ownership |
|
| Percentage of Beneficial Ownership |
| ||
Directors and Executive Officers: |
|
|
|
|
|
| ||
Cristopher Grunewald (1) |
|
| 7,303,627 |
|
|
| 48.9 | % |
William Pim (2) |
|
| 100,000 |
|
| * |
| |
John M. Krohn (3) |
|
| 622,698 |
|
|
| 4.2 | % |
Craig A. Lang (4) |
|
| 25,000 |
|
| * |
| |
|
|
|
|
|
|
|
|
|
All executive officers and directors as a group (6 persons) |
|
| 8,101,325 |
|
|
| 55.4 | % |
| 5% or Greater Beneficial Owners: |
|
| 278,779 |
|
|
| 1.9 | % |
K4 Enterprises, LLC (7) |
|
| 500,000 |
|
|
| 3.4 | % |
______________
* | Less than one percent. |
|
|
(1) | Mr. Grunewald is the President, Chief Executive Officer, and a director of the Company. The number of shares beneficially owned consists of (a) 7,303,627 shares of common stock, and (b) 37,500 shares of common stock issuable to Mr. Grunewald upon exercise of options that vest within 60 days of -August 24, 2016. |
(2) | Mr. Pim is the Chief Financial Officer of the Company. The address for Mr. Pim is c/o Spotlight Innovation Inc.6750 Westown Parkway, Suite 200-226, West Des Moines, IA 50266. |
(3) | Mr. Krohn is a member of the Board of Directors of the Company. The address for Mr. Krohn is 124 62nd Street, West Des Moines, IA 50266 |
(4) | Mr. Lang is a member of the Board of Directors of the Company. The number of shares beneficially owned consists of 25,000 shares of common stock issuable to Mr. Lang upon exercise of options that vest within 60 days of August 24, 2016. The address for Mr. Lang is 4245 180th Street Brooklyn, Iowa 52211. |
(5) | Mr. Price is a former member of the Board of Directors of the Company. The number of shares beneficially owned consists of 25,000 shares of common stock issuable to Mr. Price upon exercise of options that vest within 60 days of August 24, 2016. The address for Mr. Price is 12942 North East 24th Street Bellevue, Washington 98005. |
(6) | Mr. Katz is a former member of the Board of Directors of the Company. The number of shares beneficially owned consists of 25,000 shares of common stock issuable to Mr. Katz upon exercise of options that vest within 60 days of August 24, 2016. The address for Mr. Katz is Briar Ridge Plaza, 440 South Main Street, Milltown, NJ 08850. |
(7) | Mr. Kemery is the controlling principal and owner of K4 Enterprises, LLC. |
(8) | Does not include options to purchase 148,571 shares of Common Stock at $35 issued in 2016 to former director of the Company (Steven Katz). |
Changes In Control
We are unaware of any contract, or other arrangement or provision, the operation of which may at a subsequent date result in a change of control of our Company.
| 49 |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Since January 1, 2015, except as described in Item 11 above, there has not been any transaction or series of transactions to which we were or are a party in which the amount involved exceeded or exceeds $120,000 and in which any director, executive officer, holder of more than 5% of any class of our voting securities or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest. We believe the transactions set forth below were executed on terms no less favorable to us than we could have obtained from unaffiliated third parties.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
On January 18, 2016, the Company notified GBH CPAs, PC ("GBH") of its dismissal as the Company's independent registered public accounting firm effective as of that date. On January 19, 2016, the Company selected WithumSmith+Brown, PC as its new independent registered public accounting firm for the fiscal year ended December 31, 2015. The decision to engage WithumSmith+Brown, PC as the Company's independent registered public accounting firm was approved by the Company's Board of Directors. On June 22, 2016, the Company notified WithumSmith+Brown, PC of its dismissal as the Company's independent registered public accounting firm. On June 22, 2016, the Company selected GBH as its independent registered public accounting firm.
Principal Accounting Fees & Services |
| 2015 |
|
| 2014 |
| ||
Audit Fees |
| $ | 99,800 |
|
| $ | 82,500 |
|
Audit Related Fees |
|
| - |
|
|
| 19,950 |
|
Tax Fees |
|
| 8,490 |
|
|
| - |
|
All Other Fees |
|
| - |
|
|
| - |
|
Total Fees |
| $ | 108,290 |
|
| $ | 102,450 |
|
Audit Fees
These amounts include fees for professional services rendered in auditing our financial statements set forth in our Forms 10-K for the years ended December 31, 2015 and 2014 year-end audit and the reviews of our quarterly financial statements set forth in our Forms 10-Q in 2015 and 2014.
Audit-Related Fees
These amounts consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the Company's consolidated financial statements and are not reported under "Audit Fees." These fees were for professional services incurred in connection with accounting consultations and consultation regarding financial accounting and reporting standards.
Tax Fees
These amounts consisted of fees for services including tax compliance and the preparation of tax returns and tax consultation services.
| 50 |
ITEM 15. EXHIBITS AND FINANCIAL SCHEDULES
(a)(1) Index to consolidated Financial Statements
The Financial Statements listed in the Index to Consolidated Financial Statements are filed as part of this Annual Report on Form 10-K. See Part II, Item 8, "Financial Statement and Supplementary Data."
(a)(2) Financial Statement Schedules
Other financial statement schedules for the years ended December 31, 2015 and 2014 have been omitted since they are either not required, not applicable, or the information is otherwise included in the consolidated financial statements or the notes to consolidated financial statements.
(a)(3) Exhibits
The Exhibits listed in the accompanying Exhibit Index are attached and incorporated herein by reference and filed as part of this report.
| 51 |
SIGNATURES
Pursuant to the requirements of Securities 13 or 15(d) of the Securities and Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| SPOTLIGHT INNOVATION INC. | |||
Dated: August 30, 2016 | By: | /s/ Cristopher Grunewald | |
Cristopher Grunewald, President/Chief Executive Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ Cristopher Grunewald | President and Chief Executive Officer and Director (Principal Executive Officer) | August 30, 2016 | ||
Cristopher Grunewald |
|
|
|
|
|
|
|
|
|
/s/ William Pim |
| Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
| August 30, 2016 |
William Pim |
|
| ||
|
|
|
|
|
/s/ John M. Krohn |
| Director |
| August 30, 2016 |
John M. Krohn |
|
| ||
|
| |||
/s/ Craig A. Lang |
| Director |
| August 30, 2016 |
Craig A. Lang |
|
| ||
|
|
|
|
|
/s/ Jack E. Price |
| Director |
| August 30, 2016 |
Jack E. Price |
|
| ||
|
|
|
|
|
| 52 |
EXHIBIT NO. | DOCUMENT | |
3.1* | Articles of Incorporation (Incorporated by reference to Exhibit 3.1 on Form SB-2 filed March 5, 2006). | |
3.2* | Bylaws. (Incorporated by reference to Exhibit 3.2 on Form SB-2 filed March 5, 2006). | |
3.3* | Articles of Merger between Minhas Energy Consultants and American Energy Corp. (Incorporated by reference to Exhibit 3.1(2) on Form 8-K filed August 8, 2008). | |
3.4* | Certificate of Change dated May 26, 2010. (Incorporated by reference to Exhibit 3.1 on Form 8-K filed May 27, 2010). | |
3.5* | Certificate of Change dated October 7, 2010. (Incorporated by reference to Exhibit 3.1.2 filed October 14, 2014). | |
3.4* | Certificate of Amendment to Articles of Incorporation dated December 9, 2013. (Incorporated by reference to Exhibit 3.1 on Form 8-K filed December 13, 2013). | |
3.5* | Articles of Merger between Spotlight Innovation, LLC and Spotlight Innovation Inc. dated December 10, 2013. (Incorporated by reference to Exhibit 3.2 on Form 8-K filed December 13, 2013). | |
3.6* | Certificate of Designation dated December 10, 2013. (Incorporated by reference to Exhibit 4.1 on Form 8-K filed December 13, 2013). | |
3.7* | Certificate of Amendment to Articles of Incorporation. (Incorporated by reference to Exhibit 3.1 on Form 8-K filed July 1, 2014). | |
3.8* | Series A Preferred Stock and Series C Preferred Stock Certificates of Designation filed with the State of Nevada August 21, 2014. (Incorporated by reference to Exhibit 3.1 on Form 8-K filed August 25, 2014). | |
10.1* | Option Agreement between American Energy Corporation and Westrock Land Corporation dated October 2008. (Incorporated by reference to Exhibit 10.1 on Form 8-K filed November 6, 2008 and Exhibit 10.1 on Amendment No. 1 to Form 8-K filed January 26, 2009). | |
10.2* | 5% Convertible Debenture dated October 13, 2009 between American Exploration Corporation and DMS Ltd. (Incorporated by reference to Exhibit 10.1 on Form 8-K filed October 19, 2009). | |
10.3* | Stock Option Plan of American Exploration Corporation dated September 14, 2009. (5) Incorporated by reference from Quarterly Report on Form 10-Q filed with the Commission on November 20, 2009 | |
10.4* | Merger Agreement and Plan of Merger dated March 22, 2010 between American Exploration Corporation and Mainland Resources Inc. (Incorporated by reference to Exhibit 2.1 on Form 8-K filed March 26, 2010). | |
10.5* | Merger Agreement dated February 15, 2013 between American Exploration Corp. and Spotlight Innovation Inc. (Incorporated by reference to Exhibit 10.1 on Form 8-K filed February 15, 2013). | |
10.6* | Employment Agreement between Spotlight Innovation Inc. and Cristopher Grunewald dated December 16, 2013. (Incorporated by reference to Exhibit 10.1 on Form 8-K filed December 20, 2013). | |
10.7* | Exclusive License Agreement between Celtic Biotech Limited and Celtic Biotech Iowa, Inc. dated March 10, 2014. (Incorporated by reference to Exhibit 10.2 on Form 8-K/A filed March 18, 2014). |
| 53 |
10.8* | Agreement between Spotlight Innovation Inc. and Jared DeVries executed April 4, 2014. (Incorporated by reference to Exhibit 10.4 Form 8-K filed April 9, 2014). | |
10.9* | Share Exchange Agreement between Celtic Biotech Limited and Celtic Biotech Iowa, Inc. dated June 4, 2014. (Incorporated by reference to Exhibit 10.3 on Form 8-K filed June 6, 2014). | |
10.10* | Unit Subscription Agreement dated as of June 9, 2014. (Incorporated by reference to Exhibit 10.4 on Form 8-K filed June 13, 2014). | |
10.11* | Account Management Agreement dated as of June 9, 2014. (Incorporated by reference to Exhibit 10.5 on Form 8-K filed June 13, 2014). | |
10.12* | Escrow & Compliance Attorney Agreement dated as of June 9, 2014. (Incorporated by reference to Exhibit 10.6 on Form 8-K filed June 13, 2014). | |
10.13* | Form of Warrant Agreement dated as of June 9, 2014. (Incorporated by reference to Exhibit 10.7 on Form 8-K filed June 13, 2014). | |
10.14* | Memorandum of Terms. (Incorporated by to Exhibit 10.8 on Form 8-K filed June 13, 2014). | |
10.15* | Agreement between Spotlight Innovation Inc. and David Hostelley dated June 20, 2014. (Incorporated by reference to Exhibit 10.9 on Form 8-K filed with the Commission June 24, 2014). | |
|
|
|
10.16* | Securities Purchase Agreement between Spotlight Innovation Inc. and Memcine Pharmaceuticals, Inc. (Incorporated by reference to Exhibit 10.1 on Form 8-K filed March 10, 2015). | |
|
|
|
10.17* | Convertible Promissory Note dated March 27, 2015. (Incorporated by reference to Exhibit 10.17 to the Form 8-K filed on April 3, 2015). | |
10.18* | Shareholders Agreement of Memcine Pharmaceuticals, Inc. (Incorporated by reference to Exhibit 10.18 to the Form 8-K filed on June 5, 2015). | |
10.19* | Agreement between Memcine Pharmaceuticals, Inc. and Dr. Ton Vanden Bush, Ph.D. dated June 1, 2015. (Incorporated by reference to Exhibit 10.19 to the Form 8-K filed on June 5, 2015). | |
10.20* | Clinical Study Management Agreement between Celtic Biotech, Inc. and Immunoclin Ltd. dated July 14, 2015. (Incorporated by reference to Exhibit 10.20 to the Form 8-K filed on July 21, 2015). | |
10.21* | Agreement between Spotlight Innovation Inc. and William Pim dated November 6, 2015. (Incorporated by reference to Exhibit 10.21 to the Form 8-K filed on November 10, 2015). | |
10.22* | Spotlight Innovation Inc, 2015 Equity Incentive Plan. (Incorporated by reference to Exhibit 10.22 to the Form 8-K filed on December 1, 2015). | |
10.23* | Termination and Release Agreement between Spotlight Innovation Inc. and The Greig Companies, Inc. (Incorporated by reference to Exhibit 10.23 to the Form 8-K filed on January 7, 2016). | |
10.24* | Termination and Release Agreement between Spotlight Innovation Inc. and Lextacan Development, Ltd., Ocana Limited, Tosca Limited. (Incorporated by reference to Exhibit 10.24 to the Form 8-K filed on January 7, 2016). | |
10.25* | Termination and Release Agreement between Spotlight Innovation Inc. and Britannia Securities International, Ltd., Agri-Technologies International, Ltd., On Time Investments, Ltd., RnD Company, Ltd., Rooftop Holdings, Ltd., Sequence Investments Ltd., Anybright Investments, Ltd., Orange Investments, Ltd, Elco Securities, Ltd., Jonathan Kramer, and Catwalk Capital, LLC. (Incorporated by reference to Exhibit 10.25 to the Form 8-K filed on January 7, 2016). |
| 54 |
10.26* | Convertible Note dated December 31, 2015 in the principal amount of $2,500,000 (Incorporated by reference to Exhibit 10.26 to the Form 8-K filed on January 7, 2016). | |
10.27* | Line of Credit with Denver Savings Bank in the Principal sum of $752,325 dated April 4, 2014 (incorporated by reference to Exhibit 10.27 to the Form 8-K filed on January 7, 2016). | |
10.28* | Line of Credit with Denver Savings Bank in the Principal sum of $250,000 dated July 29, 2014 (incorporated by reference to Exhibit 10.28 to the Form 8-K filed on January 7, 2016). | |
21** | Subsidiaries of the Registrant. | |
23.1** | Consent of GBH CPAS, PC. | |
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act. | ||
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act. | ||
101.INS ** | XBRL Instance Document. | |
101.SCH ** | XBRL Taxonomy Extension Schema Documents. | |
101.CAL ** | XBRL Taxonomy Extension Calculation Linkbase Document. | |
101.DEF ** | XBRL Taxonomy Extension Definition Linkbase Document. | |
101.LAB ** | XBRL Taxonomy Extension Label Linkbase Document. | |
101.PRE ** | XBRL Taxonomy Extension Presentation Linkbase Document. |
| * | Previously filed. |
|
|
| ** | Filed herewith. |
55 |
