Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Recro Pharma, Inc. | d199979dex991.htm |
| 8-K - FORM 8-K - Recro Pharma, Inc. | d199979d8k.htm |
Study REC-15-016 A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Evaluation of the Efficacy and Safety of IV Meloxicam (N1539) Following Bunionectomy Exhibit 99.2
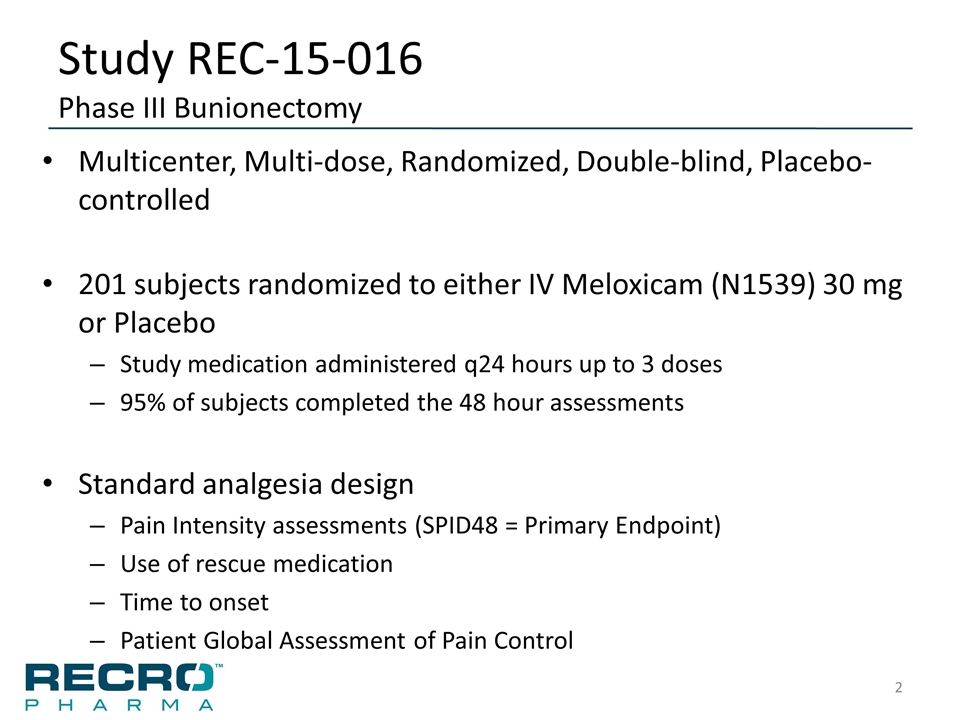
Study REC-15-016 Phase III Bunionectomy Multicenter, Multi-dose, Randomized, Double-blind, Placebo-controlled 201 subjects randomized to either IV Meloxicam (N1539) 30 mg or Placebo Study medication administered q24 hours up to 3 doses 95% of subjects completed the 48 hour assessments Standard analgesia design Pain Intensity assessments (SPID48 = Primary Endpoint) Use of rescue medication Time to onset Patient Global Assessment of Pain Control
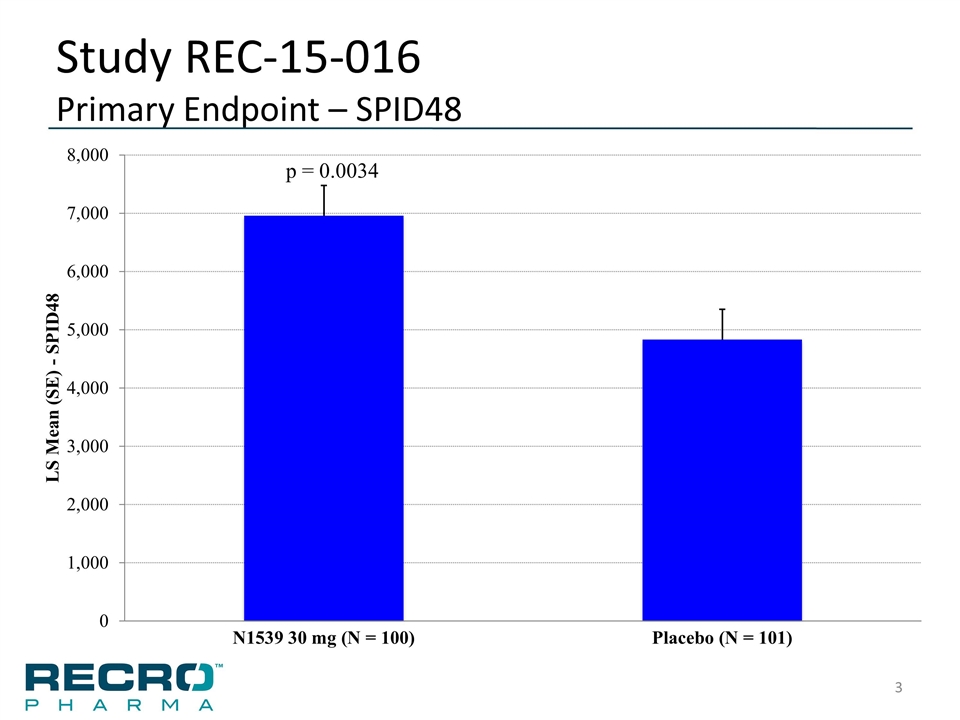
Study REC-15-016 Primary Endpoint – SPID48 p = 0.0034
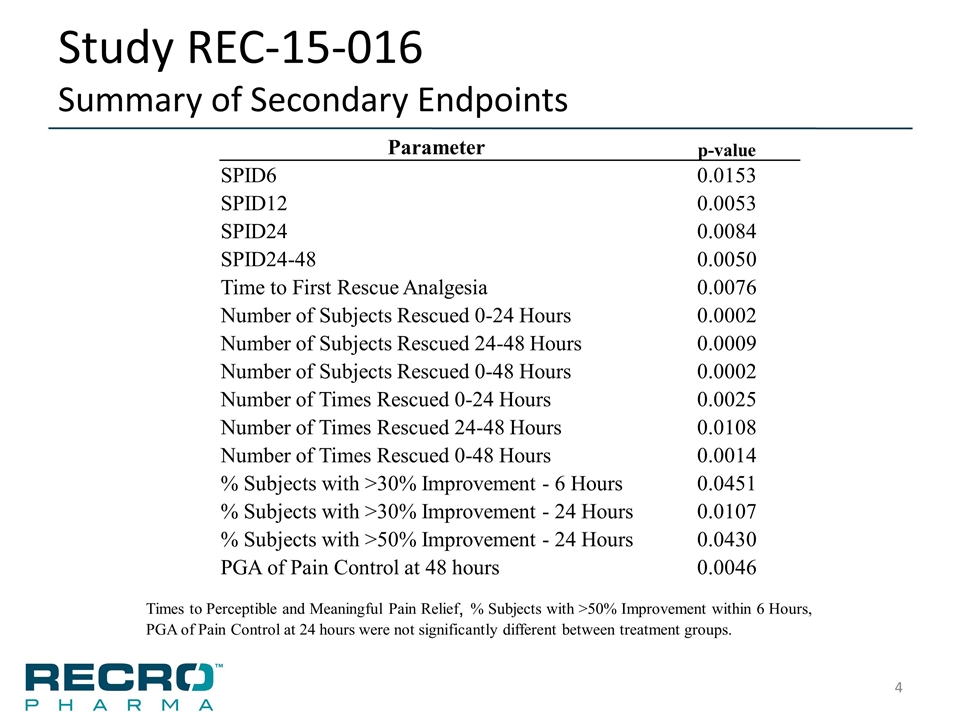
Study REC-15-016 Summary of Secondary Endpoints Parameter p-value SPID6 0.0153 SPID12 0.0053 SPID24 0.0084 SPID24-48 0.0050 Time to First Rescue Analgesia 0.0076 Number of Subjects Rescued 0-24 Hours 0.0002 Number of Subjects Rescued 24-48 Hours 0.0009 Number of Subjects Rescued 0-48 Hours 0.0002 Number of Times Rescued 0-24 Hours 0.0025 Number of Times Rescued 24-48 Hours 0.0108 Number of Times Rescued 0-48 Hours 0.0014 % Subjects with >30% Improvement - 6 Hours 0.0451 % Subjects with >30% Improvement - 24 Hours 0.0107 % Subjects with >50% Improvement - 24 Hours 0.0430 PGA of Pain Control at 48 hours 0.0046 Times to Perceptible and Meaningful Pain Relief, % Subjects with >50% Improvement within 6 Hours, PGA of Pain Control at 24 hours were not significantly different between treatment groups.
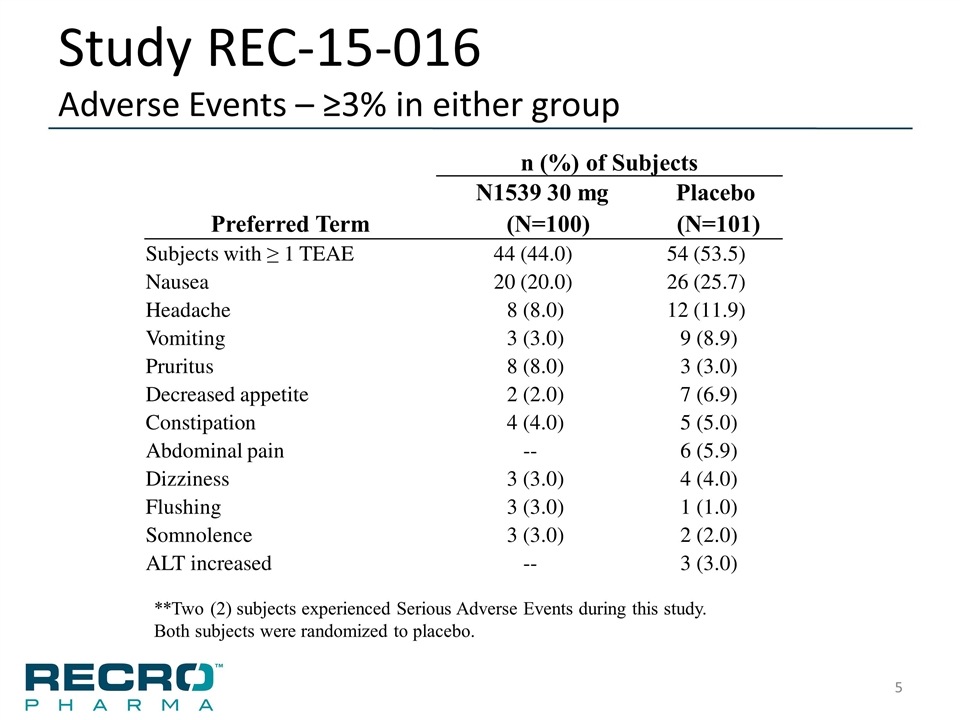
Study REC-15-016 Adverse Events – ≥3% in either group n (%) of Subjects N1539 30 mg Placebo Preferred Term (N=100) (N=101) Subjects with ≥ 1 TEAE 44 (44.0) 54 (53.5) Nausea 20 (20.0) 26 (25.7) Headache 8 (8.0) 12 (11.9) Vomiting 3 (3.0) 9 (8.9) Pruritus 8 (8.0) 3 (3.0) Decreased appetite 2 (2.0) 7 (6.9) Constipation 4 (4.0) 5 (5.0) Abdominal pain -- 6 (5.9) Dizziness 3 (3.0) 4 (4.0) Flushing 3 (3.0) 1 (1.0) Somnolence 3 (3.0) 2 (2.0) ALT increased -- 3 (3.0) **Two (2) subjects experienced Serious Adverse Events during this study. Both subjects were randomized to placebo.
