Attached files
| file | filename |
|---|---|
| EX-99.02 - EXHIBIT 99.02 - Tonix Pharmaceuticals Holding Corp. | v442051_ex99-02.htm |
| EX-99.01 - EXHIBIT 99.01 - Tonix Pharmaceuticals Holding Corp. | v442051_ex99-01.pdf |
| 8-K - FORM 8-K - Tonix Pharmaceuticals Holding Corp. | v442051_8k.htm |
Exhibit 99.01
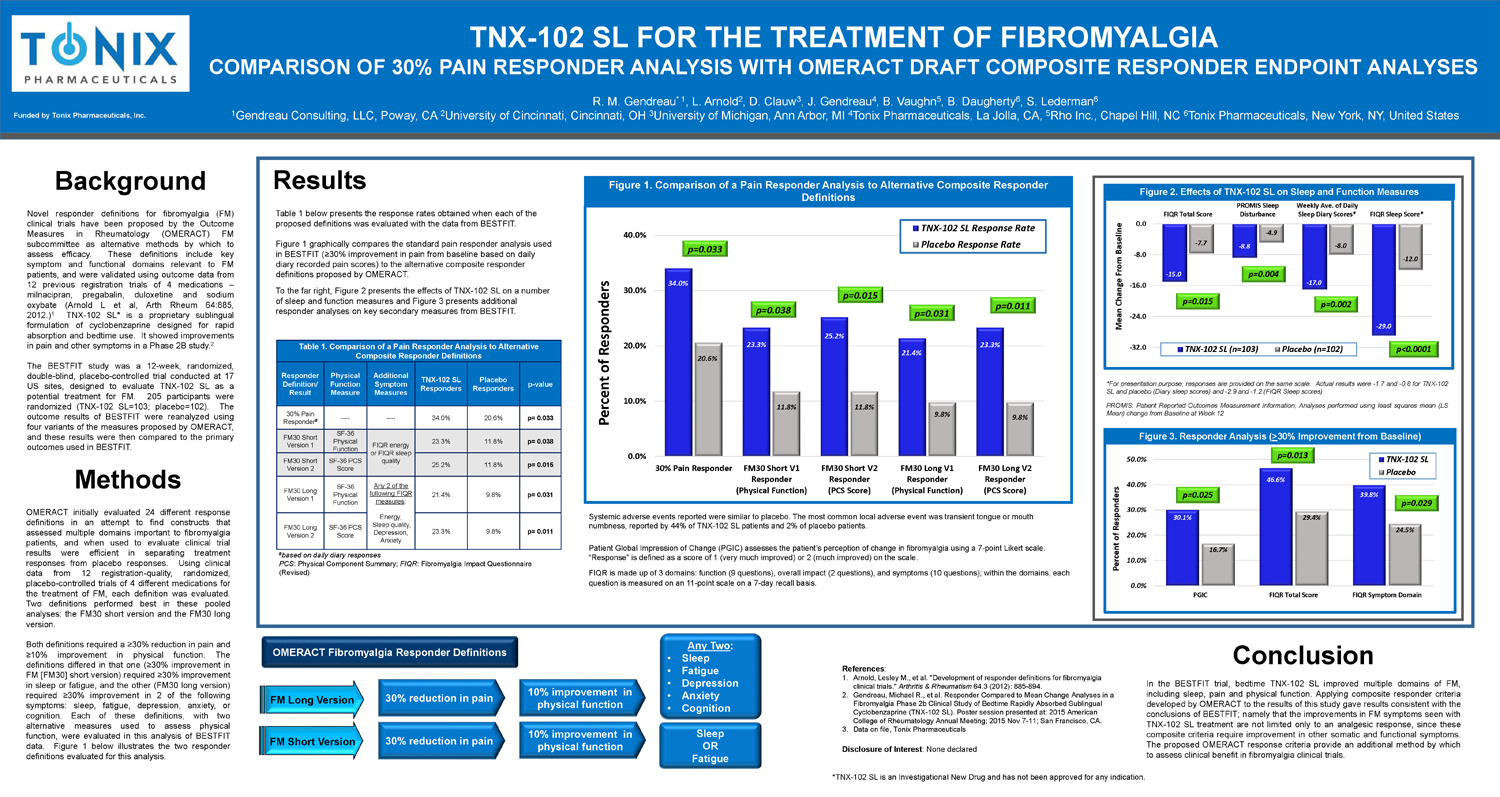
Table 1. Comparison of a Pain Responder Analysis to Alternative Composite Responder Definitions Responder Definition/ Result Physical Function Measure Additional Symptom Measures TNX - 102 SL Responders Placebo Responders p - value 30% Pain Responder # ---- ---- 34.0% 20.6% p= 0.033 FM30 Short Version 1 SF - 36 Physical Function FIQR energy or FIQR sleep quality 23.3% 11.8% p= 0.038 FM30 Short Version 2 SF - 36 PCS Score 25.2% 11.8% p= 0.015 FM30 Long Version 1 SF - 36 Physical Function Any 2 of the following FIQR measures : Energy, Sleep quality, Depression, Anxiety 21.4% 9.8% p= 0.031 FM30 Long Version 2 SF - 36 P CS Score 23.3% 9.8% p= 0.011 # based on daily diary responses PCS : Physical Component Summary; FIQR : Fibromyalgia Impact Questionnaire (Revised) Background Novel responder definitions for fibromyalgia (FM) clinical trials have been proposed by the Outcome Measures in Rheumatology (OMERACT) FM subcommittee as alternative methods by which to assess efficacy . These definitions include key symptom and functional domains relevant to FM patients, and were validated using outcome data from 12 previous registration trials of 4 medications – milnacipran , pregabalin , duloxetine and sodium oxybate (Arnold L et al, Arth Rheum 64 : 885 , 2012 . ) 1 TNX - 102 SL* is a proprietary sublingual formulation of cyclobenzaprine designed for rapid absorption and bedtime use . It showed improvements in pain and other symptoms in a Phase 2 B study . 2 The BESTFIT study was a 12 - week, randomized, double - blind, placebo - controlled trial conducted at 17 US sites, designed to evaluate TNX - 102 SL as a potential treatment for FM . 205 participants were randomized (TNX - 102 SL= 103 ; placebo= 102 ) . The outcome results of BESTFIT were reanalyzed using four variants of the measures proposed by OMERACT, and these results were then compared to the primary outcomes used in BESTFIT . R. M. Gendreau * 1 , L. Arnold 2 , D. Clauw 3 , J. Gendreau 4 , B. Vaughn 5 , B. Daugherty 6 , S. Lederman 6 1 Gendreau Consulting, LLC, Poway, CA 2 University of Cincinnati, Cincinnati, OH 3 University of Michigan, Ann Arbor, MI 4 Tonix Pharmaceuticals, La Jolla, CA, 5 Rho Inc., Chapel Hill, NC 6 Tonix Pharmaceuticals, New York, NY, United States TNX - 102 SL FOR THE TREATMENT OF FIBROMYALGIA COMPARISON OF 30% PAIN RESPONDER ANALYSIS WITH OMERACT DRAFT COMPOSITE RESPONDER ENDPOINT ANALYSES Funded by Tonix Pharmaceuticals, Inc. 34% 9.8% Conclusion In the BESTFIT trial, bedtime TNX - 102 SL improved multiple domains of FM, including sleep, pain and physical function . Applying composite responder criteria developed by OMERACT to the results of this study gave results consistent with the conclusions of BESTFIT ; namely that the improvements in FM symptoms seen with TNX - 102 SL treatment are not limited only to an analgesic response, since these composite criteria require improvement in other somatic and functional symptoms . The proposed OMERACT response criteria provide an additional method by which to assess clinical benefit in fibromyalgia clinical trials . References : 1. Arnold, Lesley M., et al. "Development of responder definitions for fibromyalgia clinical trials." Arthritis & Rheumatism 64.3 (2012): 885 - 894. 2. Gendreau, Michael R., et al. Responder Compared to Mean Change Analyses in a Fibromyalgia Phase 2b Clinical Study of Bedtime Rapidly Absorbed Sublingual Cyclobenzaprine (TNX - 102 SL). Poster session presented at: 2015 American College of Rheumatology Annual Meeting; 2015 Nov 7 - 11; San Francisco, CA. 3. Data on file, Tonix Pharmaceuticals Disclosure of Interest : None declared *TNX - 102 SL is an Investigational New Drug and has not been approved for any indication . Table 1 below presents the response rates obtained when each of the proposed definitions was evaluated with the data from BESTFIT. Figure 1 graphically compares the standard pain responder analysis used in BESTFIT (≥30% improvement in pain from baseline based on daily diary recorded pain scores) to the alternative composite responder definitions proposed by OMERACT. To the far right, Figure 2 presents the effects of TNX - 102 SL on a number of sleep and function measures and Figure 3 presents additional responder analyses on key secondary measures from BESTFIT. Results Methods OMERACT initially evaluated 24 different response definitions in an attempt to find constructs that assessed multiple domains important to fibromyalgia patients, and when used to evaluate clinical trial results were efficient in separating treatment responses from placebo responses . Using clinical data from 12 registration - quality, randomized, placebo - controlled trials of 4 different medications for the treatment of FM, each definition was evaluated . Two definitions performed best in these pooled analyses : the FM 30 short version and the FM 30 long version . Both definitions required a ≥ 30 % reduction in pain and ≥ 10 % improvement in physical function . The definitions differed in that one (≥ 30 % improvement in FM [FM 30 ] short version) required ≥ 30 % improvement in sleep or fatigue, and the other (FM 30 long version) required ≥ 30 % improvement in 2 of the following symptoms : sleep, fatigue, depression, anxiety, or cognition . Each of these definitions, with two alternative measures used to assess physical function, were evaluated in this analysis of BESTFIT data . Figure 1 below illustrates the two responder definitions evaluated for this analysis . 30% reduction in pain Sleep OR Fatigue Any Two : • Sleep • Fatigue • Depression • Anxiety • Cognition OMERACT Fibromyalgia Responder Definitions 34.0% 23.3% 25.2% 21.4% 23.3% 20.6% 11.8% 11.8% 9.8% 9.8% 0.0% 10.0% 20.0% 30.0% 40.0% 30% Pain Responder FM30 Short V1 Responder (Physical Function) FM30 Short V2 Responder (PCS Score) FM30 Long V1 Responder (Physical Function) FM30 Long V2 Responder (PCS Score) Percent of Responders TNX-102 SL Response Rate Placebo Response Rate p=0.033 - 15.0 - 8.8 - 17.0 - 29.0 - 7.7 - 4.9 - 8.0 - 12.0 -32.0 -24.0 -16.0 -8.0 0.0 FIQR Total Score PROMIS Sleep Disturbance Weekly Ave. of Daily Sleep Diary Scores* FIQR Sleep Score* Mean Change From Baseline TNX-102 SL (n=103) Placebo (n=102) Figure 1. Comparison of a Pain Responder Analysis to Alternative Composite Responder Definitions Figure 2. Effects of TNX - 102 SL on Sleep and Function Measures 30.1% 46.6% 39.8% 16.7% 29.4% 24.5% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% PGIC FIQR Total Score FIQR Symptom Domain Percent of Responders TNX-102 SL Placebo Systemic adverse events reported were similar to placebo. The most common local adverse event was transient tongue or mouth numbness, reported by 44% of TNX - 102 SL patients and 2% of placebo patients. Patient Global Impression of Change (PGIC) assesses the patient’s perception of change in fibromyalgia using a 7 - point Likert sc ale. “Response” is defined as a score of 1 (very much improved) or 2 (much improved) on the scale. FIQR is made up of 3 domains: function (9 questions), overall impact (2 questions), and symptoms (10 questions); within the d oma ins, each question is measured on an 11 - point scale on a 7 - day recall basis . Figure 3. Responder Analysis ( > 30% Improvement from Baseline) *For presentation purpose, responses are provided on the same scale . Actual results were - 1 . 7 and - 0 . 8 for TNX - 102 SL and placebo (Diary sleep scores) and - 2 . 9 and - 1 . 2 (FIQR Sleep scores) . PROMIS : Patient Reported Outcomes Measurement Information ; Analyses performed using least squares mean (LS Mean) change from Baseline at Week 12 FM Long Version 30% reduction in pain 10% improvement in physical function 10% improvement in physical function p=0.015 p=0.004 p=0.002 p<0.0001 p=0.025 p=0.013 p=0.029 p=0.038 p=0.015 p=0.031 p=0.011 FM Short Version
