Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Aevi Genomic Medicine, Inc. | v441725_ex99-1.htm |
| 8-K - 8-K - Aevi Genomic Medicine, Inc. | v441725_8k.htm |
Exhibit 99.2

Medgenics / Kyowa Hakko Kirin Collaboration for Severe Pediatric Onset Inflammatory Bowel Disease June 6, 2016

Forward Looking Statement This presentation includes certain estimates and other forward - looking statements within the meaning of Section 21 E of the Securities Exchange Act of 1934 , as amended, including statements with respect to anticipated operating and financial performance, clinical results, potential partnerships, licensing opportunities and other statements of expectation . Words such as “ expects, ” “ anticipates, ” “ intends, ” “ plans, ” “ believes, ” “ assumes, ” “ seeks, ” “ estimates, ” “ should ” and variations of these words and similar expressions, are intended to identify these forward - looking statements . While we believe these statements are accurate, forward - looking statements are inherently uncertain and we cannot assure you that these expectations will occur and our actual results may be significantly different . These statements by the Company and its management are based on estimates, projections, beliefs and assumptions of management and are not guarantees of future performance . Important factors that could cause actual results to differ from those in the forward - looking statements include the factors described in the Company ’ s filings with the U . S . Securities and Exchange Commission . The Company disclaims any obligation to update or revise any forward - looking statement based on the occurrence of future events, the receipt of new information, or otherwise . 2
Agenda • Corporate Vision and Strategy • Executive summary of KHK Collaboration • Anti - LIGHT 1 Opportunity • Discovery of DcR3 role in IBD • Severe Pediatric Onset IBD • Rationale for anti - LIGHT • Development Strategy • Deal terms • Summary 1 LIGHT – also known as “ligand for herpesvirus entry mediator” is a member of the TNF ligand superfamily 3

Medgenics Vision 4 Driven by our commitment to patients we strive to create the premier genomic medicine company by : • Developing first - to - market or best - in - class therapies that are transformative to patients suffering from life altering genetic diseases • Having unparalleled understanding of the underlying science of rare diseases from pheno type to gen otype • Pushing the boundaries of medicine and technology to develop new and better therapies • Working with patient s, families and advocacy groups to increase awareness and commitment to research and improve access to therapies
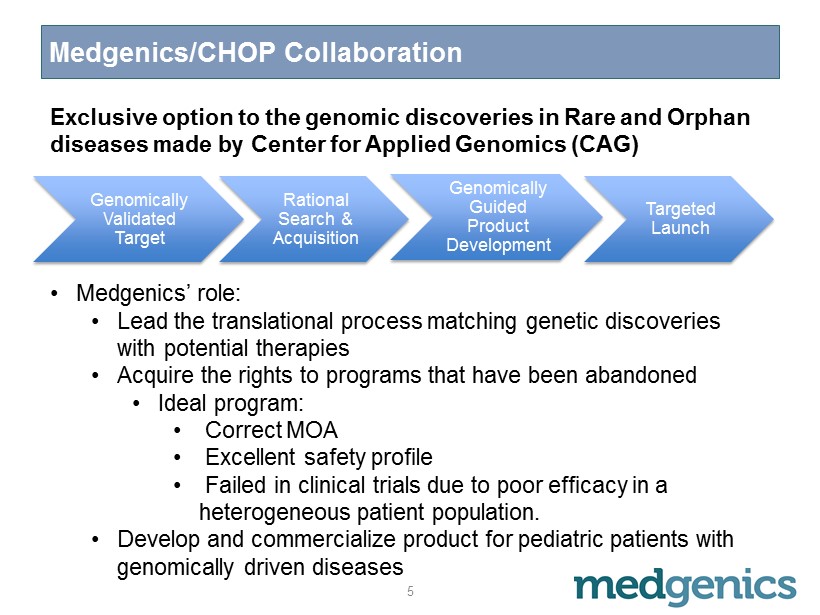
Medgenics/CHOP Collaboration 5 • Medgenics’ role: • Lead the translational process matching genetic discoveries with potential therapies • Acquire the rights to programs that have been abandoned • Ideal program: • C orrect MOA • E xcellent safety profile • F ailed in clinical trials due to poor efficacy in a heterogeneous patient population. • Develop and commercialize product for pediatric patients with genomically driven diseases Genomically Validated Target Rational Search & Acquisition Genomically Guided Product Development Targeted Launch Exclusive option to the genomic discoveries in Rare and Orphan diseases made by Center for Applied Genomics (CAG)

Benefits of Genomic Guided Drug Development Higher Value Medicines Improved response rates Smaller, faster, clinical trials Increased probability of regulatory success Targeted launch Potential label expansion in adjacent genetic diseases G enomic biomarkers improve overall program outcomes 6
Executive Summary • First in Class Biologic from Kyowa Hakko Kirin (KHK) • Option to Phase 2 Ready Antibody – anti - LIGHT monoclonal antibody • Significant prior investment and advanced through Phase 1 • Strong Intellectual Property and Regulatory Exclusivity • Robust patent portfolio • 1 2 years of regulatory exclusivity in USA • High Value Commercial Opportunity • Potential for additional orphan indications • Initial Disease Target: Severe Pediatric Onset Inflammatory Bowel Disease (IBD) • Ultra Orphan Population • High Unmet Need • Strong value proposition for patients, caregivers and payers 7

Executive Summary (Continued) • Rapid and Capital Efficient Global Development Pathway • Minimal investment to Proof of Concept • H1 2017 • Single Pivotal Trial for Registration • World Class Partners • The Children’s Hospital of Philadelphia • Center for Applied Genomics – largest pediatric biobank • Center for Pediatric Inflammatory Bowel Disease – world’s largest • Kyowa Hakko Kirin • Premier Antibody Discovery and Development 8

KHK: A Leading Biopharmaceutical Company in Japan • Established 1949 in Japan • ¥ 364B (~$3.0B) in consolidated net sales (2015) • Antibody - related cutting - edge technologies to discover and develop innovative new drugs aiming to become a global specialty pharmaceutical company • Major monoclonal antibodies discovered include: • Mogamulizumab : Anti - CCR4 • Collaborations with AstraZeneca, Pfizer, Ono and BMS in immuno - oncology combination therapy • Benralizumab : Anti - IL - 5Rα • Granted development and commercialization rights to AstraZeneca in certain countries including the US and Europe • KRN23 : Anti - FGF23 • Collaboration with Ultragenyx in EU and North, Central and South America • ASKP1240 : Anti - CD40 • Collaboration with Astellas 9

CAG/CHOP Discovery: Genetic Link to Pediatric Onset IBD • Established importance of the DcR3 gene in pediatric IBD • Loss of function of DcR3 correlates with severity • Prevalence between 10 - 15% Nature Genetics VOLUME 40 | NUMBER 10 | OCTOBER 2008 10
Severe Pediatric Onset IBD – Onset Prior to age 18 1 Ruel , J. et al. (2013) IBD across the age spectrum — is it the same disease? Nat . Rev. Gastroenterol . Hepatol . doi:10.1038/nrgastro.2013.240 • Distinct condition with d ominant g enetic component 1 • Greater burden of illness • More aggressive disease progression • Less responsive to typical 1 st line treatments 11
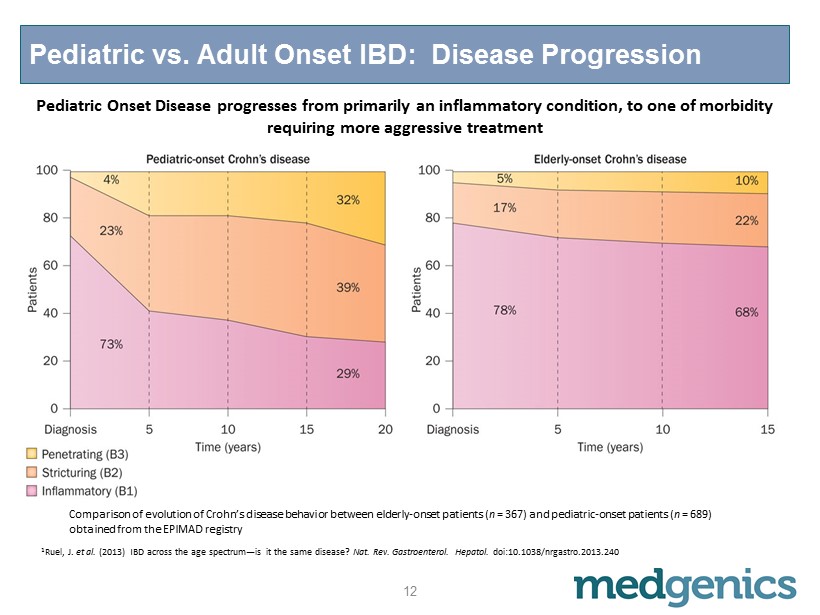
Pediatric vs. Adult Onset IBD: Disease Progression Comparison of evolution of Crohn’s disease behavior between elderly - onset patients ( n = 367) and pediatric - onset patients ( n = 689) obtained from the EPIMAD registry 1 Ruel , J. et al. (2013) IBD across the age spectrum — is it the same disease? Nat . Rev. Gastroenterol . Hepatol . doi:10.1038/nrgastro.2013.240 Pediatric Onset Disease progresses from primarily an inflammatory condition, to one of morbidity requiring more aggressive treatment 12

Pediatric vs. Adult Onset IBD: Burden of Illness 1 Ruel , J. et al. (2013) IBD across the age spectrum — is it the same disease? Nat . Rev. Gastroenterol . Hepatol . doi:10.1038/nrgastro.2013.240 • Pediatric Onset Patients suffer from additional Risks/Complications, including 1 : • Malnutrition • Permanent Growth Retardation • Developmental Setbacks • Socialization Issues • Psychological Challenges • Increased Mortality • These additional morbidities drive a more aggressive approach to treating Pediatric Onset IBD 13

Treatment Objectives : • Induce remission with anti - TNFα immediately so patients can develop normally • Avoid steroids and IMs • Manage utilization of anti - TNF alphas to extend time before resistance develops Severe Pediatric Onset IBD: “Top Down” Treatment Paradigm Opportunity: • 30% of patients do not respond to anti - TNFα & • Up to 50% of patients who initially respond, resistance will develop within 3 years 1 • Creates significant opportunity for new Therapies/MOAs, that can treat anti - TNF alpha failures IBD Severity Moderate Mild Severe Step - up approach “Top - down” approach 1 Altwegg & Vincent (2014) TNF Blocking Therapies and Immunomonitoring in Patients with Inflammatory Bowel Disease. Mediators of Inflammation. doi:10.1155/2014/172821. 14
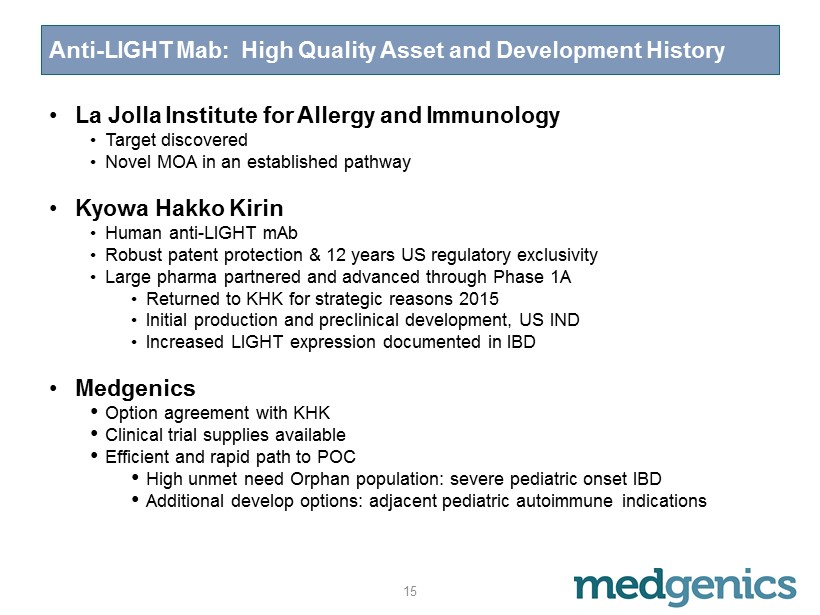
Anti - LIGHT Mab: High Quality Asset and Development History • La Jolla Institute for Allergy and Immunology • Target discovered • Novel MOA in an established pathway • Kyowa Hakko Kirin • Human anti - LIGHT m A b • Robust patent protection & 12 years US regulatory exclusivity • Large pharma partnered and advanced through Phase 1A • Returned to KHK for strategic reasons 2015 • Initial production and preclinical development, US IND • Increased LIGHT expression documented in IBD • Medgenics • Option agreement with KHK • C linical trial supplies available • Efficient and rapid path to POC • H igh unmet need Orphan population: severe pediatric onset IBD • Additional develop options: adjacent pediatric autoimmune indications 15

Rationale for Anti - LIGHT Approach • DcR3 is strongly linked to Severe Pediatric Onset IBD 1 • Initial strategy to augment DcR3 – Very short half life – T oxicity reported w/ previous DcR3 analog • “Rational Search” based on biological pathway – LIGHT overexpressed in IBD – DcR3 LOF increases LIGHT 2 1 Hakonarson, et al 2008. Loci on 20q13 and 21q22 are associated with pediatric - onset inflammatory bowel disease. Nature Genetics 40 (10): 1211 - 1215 2 Mauri DN, et al 1998. LIGHT , a new member of the TNF superf amily , and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8 (1): 21 – 30. Decoy Receptor 3 DcR3 LIGHT Immune cell HVEM LT b R Our Approach: Therapeutic antibody which mimics DcR3 regulation by binding LIGHT 16
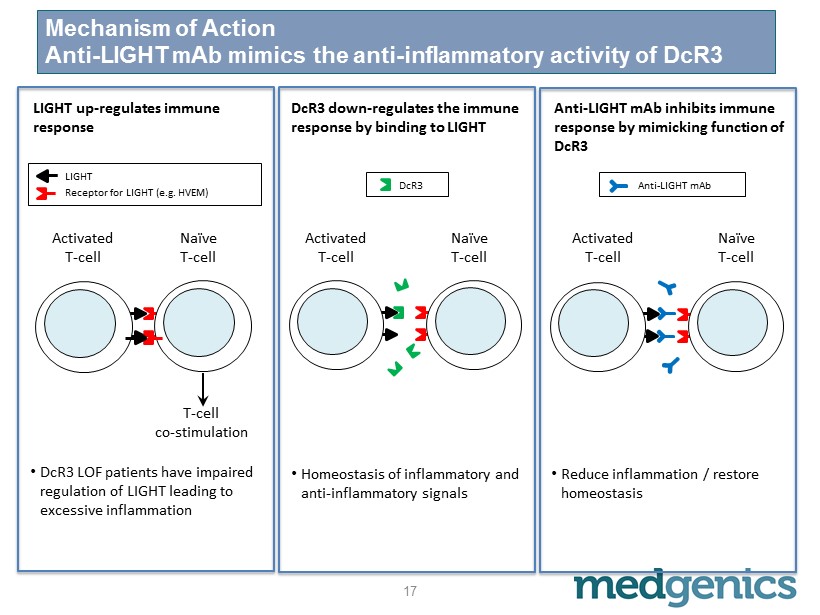
Mechanism of Action Anti - LIGHT m Ab mimics the anti - inflammatory activity of DcR3 Activated T - cell Naïve T - cell Activated T - cell Naïve T - cell Activated T - cell Naïve T - cell T - cell co - stimulation LIGHT up - regulates immune response DcR3 down - regulates the immune response by binding to LIGHT Anti - LIGHT m A b inhibits immune response by mimicking function of DcR3 • Homeostasis of inflammatory and anti - inflammatory signals • Reduce inflammation / restore homeostasis LIGHT Receptor for LIGHT (e.g. HVEM) DcR3 Anti - LIGHT m Ab • DcR3 LOF patients have impaired regulation of LIGHT leading to excessive inflammation 17

Human Biomarker Study Supports Role of LIGHT in IBD Single center non - interventional biomarker study Data on File 0 0.5 1 1.5 2 2.5 3 Normal (n=26) Ulcerative Colitis (n=27) Crohn's Disease (n=28) LIGHT mRNA Fold Increase vs Normal 18

Development Strategy • Focus on patients with Severe P ediatric O nset IBD with DcR3 mutations who have failed anti - TNFα m Ab therapy • Rapid POC • Clinical Trial Material available • Large cohort of patients identified at CHOP • Development program could be expanded to include: • All Pediatric IBD anti - TNFα failures • Additional pediatric autoimmune diseases where DcR3 is implicated (e.g., juvenile idiopathic arthritis, psoriasis, etc.) • Orphan Population and High Unmet Need Could Result in Fast Track 19

Rapid Pathway to Approval Phase 2/3 mGluR+ ADHD, Adolescents (N = 90) Severe Pediatric Onset IBD POC Trial Signal Finding Trial design: - Single center (CHOP ) - N = up to 12 patients - Duration 8 – 12 weeks - Ascending dose Endpoints : - Endoscopic Evaluation , Pediatric Crohn’s Disease Activity Index (PCDAI), Safety Estimated Cost: ~$2M Single Phase 3 Pivotal Registration Trial Long Term Safety Trial 20
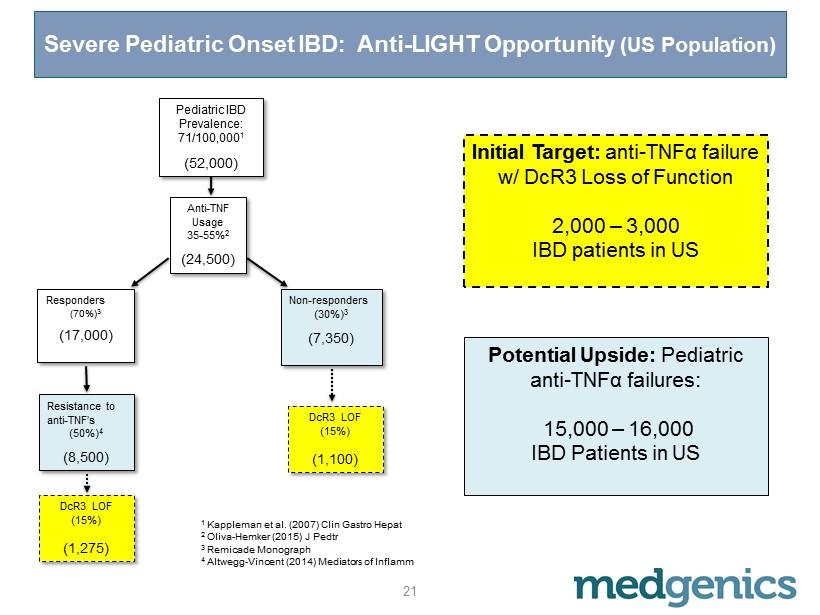
Severe Pediatric Onset IBD: Anti - LIGHT Opportunity (US Population) Potential Upside: Pediatric anti - TNFα failures: 15,000 – 16,000 IBD Patients in US 1 Kappleman et al. (2007) Clin Gastro Hepat 2 Oliva - Hemker (2015) J Pedtr 3 Remicade Monograph 4 Altwegg - Vincent (2014) Mediators of Inflamm Initial Target: anti - TNFα failure w/ DcR3 Loss of Function 2,000 – 3,000 IBD patients in US Pediatric IBD Prevalence: 71/100,000 1 (52,000) Anti - TNF Usage 35 - 55% 2 (24,500) Responders (70%) 3 (17,000) Resistance to anti - TNF’s (50%) 4 (8,500) DcR3 LOF (15%) (1,275) Non - responders (30%) 3 (7,350) DcR3 LOF (15%) (1,100) 21
Severe Pediatric Onset IBD Commercial Opportunity • With conservative p ricing e stimates and assuming a 15% prevalence rate of DcR3 LOF, the Severe Pediatric Onset opportunity in the US: • $300 - $400M • DcR3 LOF mutation who fail to respond to anti - TNFα • Significant upside potential • anti - TNFα failures • additional pediatric autoimmune diseases where DcR3 LOF is found, e.g., juvenile idiopathic arthritis, psoriasis, etc. 22

Deal Terms • MDGN to: • Requalify KHK clinical supplies • C onduct s ignal f inding s tudy (~$2M) • Exercise option to license on positive data (low single - digit millions USD) • Following option exercise, KHK elects either: • Partnership • “Co - Development/Co - Commercialization ” – Parties share sales and costs equally in US and Canada; MDGN receives double - digit royalty on ex - North Americ a net sales • License • MDGN funds development and commercializes in North America and EU, and retains approximately 75% of profits, MDGN receives double - digit royalty on ROW sales • S uccess - based development milestones payable in both scenarios 23

Summary • First - in - Class Biologic from Kyowa Hakko Kirin • Initial Development in Severe Pediatric Onset IBD • Phase 2 Ready Antibody • Strong Regulatory Exclusivity and Robust Intellectual Property Portfolio • High Value Commercial Opportunity with Potential for Indication Expansion • World Class Partners • Kyowa Hakko Kirin • The Children’s Hospital of Philadelphia • Rapid and Capital Efficient Global Development Pathway • Minimal investment to POC • Single Pivotal Trial for registration 24
