Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - HTG MOLECULAR DIAGNOSTICS, INC | htgm-ex312_8.htm |
| EX-32.1 - EX-32.1 - HTG MOLECULAR DIAGNOSTICS, INC | htgm-ex321_6.htm |
| EX-23.1 - EX-23.1 - HTG MOLECULAR DIAGNOSTICS, INC | htgm-ex231_966.htm |
| EX-32.2 - EX-32.2 - HTG MOLECULAR DIAGNOSTICS, INC | htgm-ex322_7.htm |
| EX-31.1 - EX-31.1 - HTG MOLECULAR DIAGNOSTICS, INC | htgm-ex311_9.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
OR
|
¨ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 001-37369
HTG Molecular Diagnostics, Inc.
(Exact name of Registrant as specified in its Charter)
|
Delaware |
|
86-0912294 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer |
|
3430 E. Global Loop Tucson, AZ |
|
85706 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (877) 289-2615
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Name of each exchange on which registered |
|
Common Stock, par value $0.001 per share |
|
The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ¨ NO x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ¨ NO x
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES x NO ¨
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). YES x NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definition of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
|
¨ |
|
Accelerated filer |
|
¨ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
¨ (Do not check if a small reporting company) |
|
Small reporting company |
|
x |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES o NO x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on The NASDAQ Global Market on June 30, 2015, was $45,920,851.
The number of shares of Registrant’s Common Stock outstanding as of March 18, 2016 was 6,977,421.
|
|
|
Page |
|
PART I |
|
|
|
Item 1. |
1 |
|
|
Item 1A. |
39 |
|
|
Item 1B. |
65 |
|
|
Item 2. |
66 |
|
|
Item 3. |
66 |
|
|
Item 4. |
66 |
|
|
|
|
|
|
PART II |
|
|
|
Item 5. |
67 |
|
|
Item 6. |
68 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
69 |
|
Item 7A. |
80 |
|
|
Item 8. |
80 |
|
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
116 |
|
Item 9A. |
116 |
|
|
Item 9B. |
117 |
|
|
|
|
|
|
PART III |
|
|
|
Item 10. |
118 |
|
|
Item 11. |
126 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
139 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
141 |
|
Item 14. |
145 |
|
|
|
|
|
|
PART IV |
|
|
|
Item 15. |
145 |
i
Unless the context requires otherwise, references to “HTG,” “HTG Molecular Diagnostics,” “we,” “us” and “our” refer to HTG Molecular Diagnostics, Inc.
Forward-Looking Statements
This Annual Report on Form 10-K, including the sections entitled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” may contain “forward-looking statements.” We may, in some cases, use words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes, to identify these forward-looking statements. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. Forward-looking statements in this Annual Report include, but are not limited to, statements about:
|
|
· |
our ability to successfully commercialize our HTG Edge or HTG EdgeSeq systems and related applications and assays; |
|
|
· |
our ability to generate sufficient revenue or raise additional capital to meet our working capital needs; |
|
|
· |
our ability to secure regulatory clearance or approval, domestically and internationally, for the clinical use of our products; |
|
|
· |
our ability to develop new technologies beyond mRNA and miRNA to include DNA fusions and mutations or other technologies to expand our product offerings; |
|
|
· |
the implementation of our business model and strategic plans for our business; |
|
|
· |
the regulatory regime for our products, domestically and internationally; |
|
|
· |
our strategic relationships, including with manufacturers of next generation sequencing products used with our product offerings, pharmaceutical companies, manufacturers and distributors of our products, and third parties who conduct our clinical studies; |
|
|
· |
our intellectual property position; |
|
|
· |
use of available operating cash; |
|
|
· |
our ability to comply with the restrictions of our debt facility and meet our debt obligations; |
|
|
· |
our expectations regarding the market size and growth potential for our life sciences and diagnostic businesses; |
|
|
· |
any estimates regarding expenses, future revenues, capital requirements, and stock performance; |
|
|
· |
our expected use of proceeds from our initial public offering; and |
|
|
· |
our ability to sustain and manage growth, including our ability to develop new products and enter new markets. |
These forward-looking statements reflect our management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the filing date of this Annual Report and are subject to risks and uncertainties. We discuss many of these risks in greater detail under “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
Overview
We are a commercial stage company that develops and markets products based on a novel technology platform to facilitate the routine use of complex molecular profiling. Our HTG Edge and HTG EdgeSeq platforms automate sample processing and can quickly, robustly and simultaneously profile hundreds or thousands of molecular targets from samples a fraction of the size required by most current technologies. Our objective is to establish the HTG Edge and HTG EdgeSeq platforms as the standards in molecular profiling and make this capability accessible to all molecular labs from research to the clinic. We believe that our target customers
1
desire high quality molecular profiling information in a multiplexed panel format from increasingly smaller and less invasive samples, with the ability to collect such information locally to minimize turnaround time and cost. The HTG Edge and HTG EdgeSeq platforms were designed to meet these needs and empower pathologists, translational researchers and, in the future, clinical molecular labs to directly control the molecular profiling of patient samples. Our platform’s capabilities are also enabling biopharmaceutical companies to better leverage the power of molecular profiling in their preclinical and clinical research and biomarker development applications.
Molecular profiling is the analysis of multiple DNA, RNA and/or protein targets in biological samples, such as tissue, cells, blood or other biofluids, to identify expression patterns or genomic changes. Molecular profiling is enabling a paradigm shift from the traditional approach of looking at one molecule at a time to the simultaneous analysis of hundreds or thousands of molecules. There are numerous applications of molecular profiling, such as whole genome sequencing for the discovery of novel genetic variants and the assessment of patient samples to identify biomarkers or molecular markers of disease that can aid in diagnosis, gauge patient prognosis or predict response to an available therapy. Significant discoveries of new molecular targets, such as in the field of immuno-oncology, are creating substantial growth in targeted tumor profiling for molecular diagnostic testing, biomarker development and translational research in oncology and other diseases. Based on published industry reports, the cancer profiling market is estimated to be $17.8 billion today and is expected to grow to $35.0 billion by 2018.
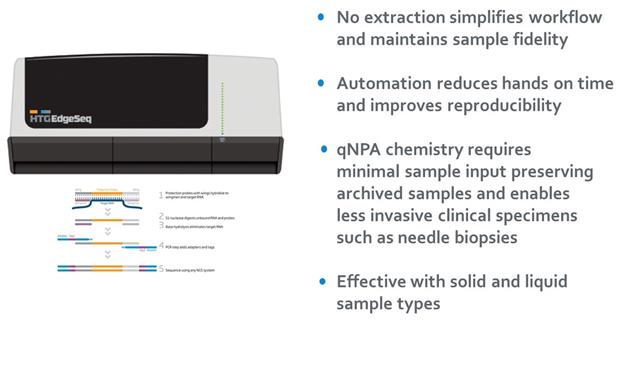
Our HTG Edge and HTG EdgeSeq platforms consist of instrumentation, consumables and software analytics that automate the molecular profiling of genes and gene activity using our proprietary nuclease protection chemistry to deliver extraction-free, multiplexed results on a wide variety of biological samples. We believe that our platforms provide significant workflow and performance advantages in molecular profiling applications including tumor profiling, biomarker development and, prospectively, molecular diagnostic testing. In 2014 we launched our HTG EdgeSeq chemistry that, together with our HTG Edge or HTG EdgeSeq instrumentation and software, automates and adapts our nuclease protection chemistry to enable analysis using next generation sequencing, or NGS, instrumentation. The HTG EdgeSeq system utilizes substantially the same sample preparation reagents as our original chemistry, but allows for read out on an NGS instrument. We believe that the HTG EdgeSeq chemistry is disruptive as it substantially simplifies current sample and library preparation methods, greatly reduces the complexity of data analytics, and provides customers additional value by expanding the utilization of their NGS instruments. By combining the power of the HTG EdgeSeq chemistry with the capabilities of NGS, we are able to analyze a wide variety of genomic profiles and sample types with high sensitivity and broad dynamic range. These technologies offer the potential to develop additional profiling panels and grow our market opportunities, and we expect to include RNA gene fusions and rearrangements, DNA mutations, detection of copy number variations, or CNV’s, and analysis of cell-free circulating DNA from liquid biopsies to our HTG EdgeSeq product portfolio.
2
The following features of our platforms are designed to enable the rapid delivery of a comprehensive molecular profile from extremely small samples:
Optimization of Sample Preparation
Improved Detection and Quantitation
3
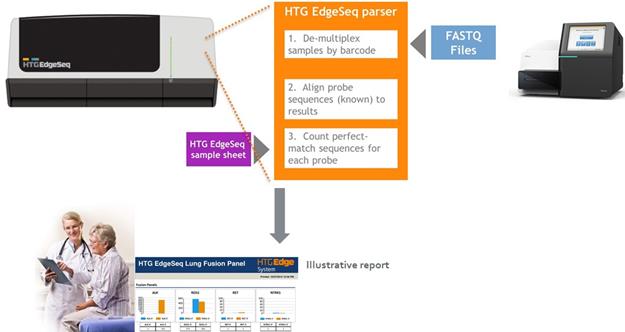
An area of dissatisfaction for customers performing NGS tests is the difficulty of analyzing the data. HTG’s platforms are designed to greatly reduce the complexity of the data analysis tasks via the HTG Parser software. The FASTQ files are downloaded from the sequencer into the HTG Parser software where standard reports are available for our research customers and, in the future, custom clinical reports will be generated for physicians and molecular lab management.
Our innovative platforms and menu of molecular profiling panels are being utilized by a wide range of customers including biopharmaceutical companies, academic institutions and molecular labs to simultaneously analyze a comprehensive set of molecular information from valuable clinical samples and substantially improve their workflow efficiency. We currently market several proprietary molecular profiling panels that address the needs of customers in high impact areas of translational research and biopharmaceutical biomarker and companion diagnostics.
Our platforms’ proprietary chemistry allows for extraction-free analysis of solid and liquid tumors including difficult clinical samples such as formalin-fixed paraffin embedded, or FFPE, tissue with as little as a single five micron section of tissue, a fraction of the amount of sample required by other technologies. The ability to provide robust data from minute samples is critically important in areas such as cancer where biopsies are becoming less invasive and smaller while the number of tests competing for the sample is growing. Our platform was designed to fit seamlessly into current surgical pathology workflows, minimize technician labor and set a new standard for ease of use.
We have a focused development pipeline of new profiling products that includes planned panels for translational research, drug development, and molecular diagnostics. Our product strategy is to build complete profiling panels of established and emerging molecular targets for broader and disease-specific approaches. For our molecular diagnostic customers where the reimbursement path is critical we expect that our planned panels will conform to approved reimbursement codes for genomic sequence procedures. We believe this will facilitate clinical customer adoption and avoid the high costs and time required to prove medical utility and seek unique reimbursement codes.
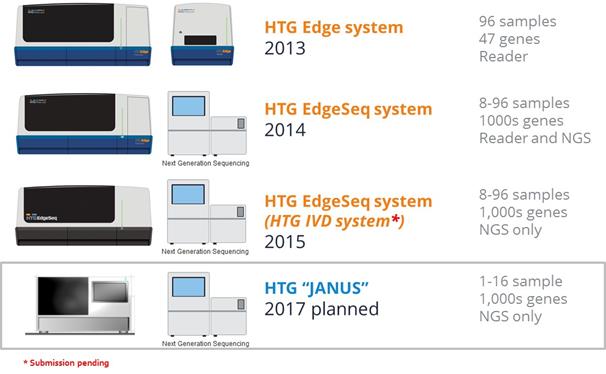
Our HTG Edge system is capable of running both our original, plate-based chemistry, which quantifies RNA using our plate reader included with the system, and our HTG EdgeSeq chemistry, which is detected using NGS instrumentation provided by the end user. We also have the flexibility to provide the HTG Edge instrument without our plate reader or, since December 2015, our dedicated HTG EdgeSeq system for those customers whose focus is NGS readout of our HTG EdgeSeq chemistry. We plan to launch a new version of our HTG EdgeSeq system in 2017 that will target the lower sample throughput clinical lab market. This program, which we refer to as “Project JANUS”, is expected to increase our addressable market by enabling efficient molecular profiling of smaller quantity batches of samples.
4
We continue to expand and evolve our assay and panel offerings, primarily focusing on our HTG EdgeSeq platform. During 2015, we launched several new HTG EdgeSeq research-use-only product offerings, including our HTG EdgeSeq Oncology Biomarker Panel, HTG EdgeSeq Immuno-Oncology Assay, HTG EdgeSeq Lymphoma Panel and HTG EdgeSeq Diffuse Large B-Cell Lymphoma Cell of Origin Assay.
We have incurred significant losses since our inception, and we have never been profitable. We incurred net losses of $21.4 million and $14.0 million for the years ended December 31, 2015 and 2014, respectively. As of December 31, 2015, we had an accumulated deficit of $89.6 million and we had available cash and cash equivalents totaling approximately $3.3 million and investments in short-term corporate and government debt securities totaling $28.2 million.
Our Strategy
Our objective is to establish the HTG Edge and HTG EdgeSeq platforms as the standards in molecular profiling, and to make their benefits accessible to all molecular labs from research to the clinic. The key components of our strategy are:
|
|
· |
Grow our installed base and promote our consumables-based business model. We plan to promote global adoption of our HTG Edge and HTG EdgeSeq platforms, including our growing menu of consumables, in molecular labs, biopharmaceutical companies and major translational research centers by demonstrating the key differentiating aspects of our platforms, such as small sample utilization, multiplexing and multi-parameter testing. These advantages result in cost, time and ease of use benefits for our global customers. We plan to expand our direct sales and support team in the United States and Europe, continue to transition from a contract sales team to our own direct sales and support team in Europe and expand our global distributor network. |
|
|
· |
Establish our systems’ workflow as the best solution for clinical sequencing. We intend to continue to establish our technology as the best sample and library preparation method for clinical applications of next generation sequencers. We believe our differentiated HTG EdgeSeq chemistry will accelerate adoption of our platforms by leveraging the large existing and growing installed base of next generation sequencers. We are engaged with industry and corporate partners to position our HTG EdgeSeq products as the benchmark for workflow in targeted sequencing applications. |
|
|
· |
Develop new molecular diagnostic panels with high medical utility. The HTG Edge and HTG EdgeSeq systems were developed with features that we believe will enhance the capabilities of local molecular labs to routinely test large panels of proven-utility RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression, all from extremely small samples, such as a single five micron section of tissue. We plan to add disease-specific panels such as lung cancer and broader profiling panels that allow our customers to use existing reimbursement codes. |
|
|
· |
Increase and strengthen companion diagnostics collaborations with biopharmaceutical companies. We believe collaborations with biopharmaceutical companies with late-stage drug development programs will lead to us generating companion diagnostic consumables revenue. We are currently working with 30 active development programs across 14 leading biopharmaceutical companies who are incorporating companion diagnostics in their drug development programs, where they are purchasing our HTG Edge and HTG EdgeSeq consumable panels, including immuno-oncology, oncology biomarker and diffuse large B-cell lymphomas, or DLBCL, panels. We plan to continue to expand our number of collaborations. In addition, we intend to opportunistically perform sample processing services for our collaboration customers, as we believe this will further strengthen our position as the technology of choice for certain of these companion diagnostic programs. |
|
|
· |
Expand the addressable market of HTG technology through new applications. We have demonstrated feasibility to add new applications in RNA-based gene fusions, expressed DNA mutations, CNVs and protein immunoassays. These new applications will allow us to develop multiple panels for use in translational, research, companion diagnostics and molecular diagnostics. We believe these applications and panels can be developed efficiently with reasonable capital investment. |
Our Market
Development of Molecular Profiling
Molecular profiling is the analysis of multiple DNA, RNA and protein targets in biological samples, such as tissue, cells, blood and other biofluids, to identify expression patterns or genomic changes. New molecular approaches are making it possible to perform these characterizations in unprecedented ways, resulting in a shift from the traditional approach of looking at one target at a time to the simultaneous analysis of hundreds or thousands of targets. There are numerous applications of molecular profiling such as whole genome sequencing for the discovery of novel genetic variants or the assessment of patient samples to identify biomarkers or molecular markers of disease that can aid in diagnosis, gauge patient prognosis or predict response to an available therapy. The
5
fundamental shift towards personalized medicine, or the use of an individual’s molecular profile to guide treatment, has led to significant growth in molecular profiling technologies and applications. We estimate that the global molecular profiling market is approximately $27.0 billion today. Based on published industry reports, cancer profiling makes up the largest segment of this market at an estimated $17.8 billion, the substantial majority of which we believe currently consists of research-use-only products, and is expected to grow to $35.0 billion by 2018. Research-use-only products are used in a wide variety of applications, including large-scale clinical trials of targeted therapies that are under development as well as basic medical research to understand and characterize tumors and tumor biology. We also estimate that genomic research makes up approximately $7.7 billion of the global molecular profiling market. Currently we are limited to marketing our HTG Edge and HTG EdgeSeq systems and proprietary profiling panels for research use only, which means that we cannot make any diagnostic or clinical claims. We intend to seek regulatory clearances and/or approvals in the United States and other jurisdictions to market certain panels for diagnostic purposes.
For decades, the treatment of disease was dominated by one-size-fits-all drug regimens. Over the last 10 years numerous molecular markers and profiling techniques have transitioned from discovery and research to inclusion in clinical guidelines. Today, there are many drugs in clinical development with a companion biomarker strategy, including many oncology drugs. Among the first of these personalized treatments were hormone therapies for breast cancer patients whose tumors expressed the estrogen receptor protein. This was followed by the use of trastuzumab for treating patients with HER2-positive breast cancers. The evolution of molecular profiling has also taken shape over the last decade in non-small cell lung cancer (NSCLC). NSCLC patients were first molecularly profiled for EGFR mutations to select patients most likely to respond to the drug, erlotinib, which was followed by ALK rearrangement testing for potential response to crizotinib. Now, additional mutations and rearrangements such as ROS1, RET, HER2, BRAF and MET, have been integrated into the NCCN guidelines for the treatment of lung cancer.
The NCCN Guidelines acknowledge that broad molecular profiling is key in improving the care of lung cancer patients. Similar trends are unfolding in other diseases such as thyroid cancer, colon cancer, and melanoma. These trends are fueling rapid growth of new molecular profiling offerings, technologies and industry business models to support the demand. The fastest growing segment of the estimated $10.6 billion molecular diagnostics market, a subset of the estimated $27.0 billion global molecular profiling market, is clinical testing in oncology. As oncologists rapidly integrate this new level of molecular profiling information into patient management strategies, a growing number of highly specialized central laboratories now offer tests that address specific clinical questions. The trend towards highly multiplexed profiling is not limited to oncology; other examples of expanding demand include prenatal, neonatal, inherited disease, and organ transplant and rejection profiling.
In addition, biopharmaceutical companies utilize molecular profiling in numerous applications. Preclinical applications include screening of compound libraries for target identification and preclinical testing for safety and fit for human use. For example, the FDA and European Medicines Agency require drug metabolism and pharmacokinetic, or DMPK, profiling studies to be performed as part of the drug development process. Metabolic gene expression response to the drug in human liver cells is assessed for each candidate drug, and it is common for biopharmaceutical companies to test the expression of three to 10 genes per drug candidate. Among the various DMPK testing technologies, such as RT-qPCR, branched DNA and microarrays, the most common is RT-qPCR. In DMPK studies, liver cells from several different human donors are challenged with the drug at various concentrations to predict how the drug will be metabolized. A large number of drug candidates are screened at this phase of development, creating significant demand for DMPK molecular profiling.
More recently, molecular profiling has moved into clinical applications. These include molecular profiling to develop clinical biomarker strategies, patient stratification for clinical trials, and companion diagnostics. The primary objective of these efforts is to improve response rates by understanding, at the molecular level, why a drug does or does not work in a patient population. Response rates for oncology therapeutics, at 25%, are among the lowest of all disease states. Combined with third-party payor pressures to lower patient treatment costs, most biopharmaceutical companies develop biomarker strategies to increase the rate of patient response to new drugs in development. Typically, they will look at a variety of biological profiling markers that include RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression. Currently, it is estimated that there are over 2,000 clinical trials underway in oncology, and the vast majority of drug developers believe personalized and targeted therapy development is important to the future success of drug development.
When a molecular biomarker panel is used for selection of patients in a Phase 3 clinical trial to demonstrate safety and efficacy of a new drug, the drug and biomarker test are often submitted to the applicable regulatory agency for approval together. Upon FDA approval of the molecular biomarker panel, or companion diagnostic, the patient must be tested with the companion diagnostic prior to treatment with the drug. Companion diagnostic tests have a clear clinical utility which generally supports favorable reimbursement. We believe there are over 900 active oncology drug development programs, most of which have molecular biomarker strategies, creating a significant opportunity for molecular diagnostic companies with the right molecular profiling solutions. We estimate this molecular profiling market for companion diagnostics to be $2.5 billion and growing to $5.6 billion by 2019.
6
Complexities and Challenges of Molecular Profiling Today
Currently, molecular profiling is conducted using a variety of profiling techniques across multiple laboratory departments, and, in many situations, sent to distant labs. These techniques include immunohistochemistry, or IHC, fluorescent in situ hybridization, or FISH, polymerase chain reaction, or PCR, gene expression arrays, or GEA, and NGS. This distributed profiling approach has accelerated the use of molecular profiling and increased the need to make the process more accessible and routine. However, molecular profiling is also highly specialized because current technologies are complex, require multiple capital-intensive workflows, and are not economically scalable to the case volume of the local laboratory. The fragmentation of methods, sample logistics, and information flows has created significant challenges for labs, physicians, and patients as discussed below.
Insufficient Sample Availability
The proliferation of new molecular profiles and technologies has led to the need for more biopsy material. However, the trend is toward less invasive procedures that produce smaller biopsies and as a result, in many situations there simply is not enough collected sample to meet all the profiling requirements. For example, the standard method of collecting tumor samples for testing in oncology is via a surgical procedure where the tumor is resected or biopsied and then stabilized, or fixed, in a formaldehyde-based fixative known as formalin. From 24 to 72 hours after the tissue is harvested, it is permanently stabilized in a hard block of paraffin where it can be stored at room temperature for decades. This preservation technique was developed over 100 years ago, well in advance of the discovery of nucleic acids such as DNA and RNA. While techniques to recover nucleic acids from these FFPE tissue samples have been developed, formalin fixation presents several technical challenges in analyzing DNA and RNA sequences. Because of the convenience of this preservation method, though, FFPE samples are the starting material for almost all tumor-profiling testing in oncology today. Very thin slices of tissue from these FFPE blocks, typically five microns in thickness, are affixed to glass slides for testing.
Most commonly, a histological stain called an H&E, for the combination of hematoxylin and eosin that comprise it, is used to differentially mark cellular structures, making it simple for a pathologist to examine the patterns of the normal and diseased tissue under a microscope. IHC stains are also performed to aid diagnosis, prognosis or help guide therapy selection, with each IHC stain consuming two slides, one for the stain, and the other for a negative control. Historically, five to ten slides in total were consumed in the complete diagnostic workup for each tumor. With the advent of new molecular techniques there are additional demands for tissue, and the molecular tests on the market today require much more than a single slide. In many of these situations there simply is not enough collected tissue to meet all the profiling requirements. The growing number of specialized tumor-profiling tests, and their appetite for FFPE tissue or other sample material, is in direct conflict with the trend towards smaller, less-invasive testing approaches.
Slow Turnaround Times
In many cases, turnaround times for comprehensive profiling are several weeks due to the logistical time to route samples to various laboratory departments and to distant specialized labs. A number of technologies for sample characterization have been introduced that determine the status of various molecular characteristics for a sample. These include RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression. For example, a single tumor specimen from a cancer patient is often profiled for multiple molecular characteristics where each characteristic is measured using a different platform. These platforms are utilized in separate laboratory departments or institutions with different technicians and clinicians, requiring that the sample and data be split into multiple workflows.
The time it takes to deliver the final report so that informed treatment decisions may be made is dependent on the turnaround time of the slowest test. A single laboratory with well-choreographed routing of tissues and information may be able to complete profiling within a week, but if part of the sample needs to be sent for additional profiling at a specialty lab, the total turnaround time may be lengthened by one or two weeks due to shipping, accessioning by the receiving laboratory, and integration of the testing results into the final report.
7
Implications of Workflow Inefficiencies on Data Quality and Integration
In addition to the challenges of splitting a single sample into multiple testing workflows, the individual workflow for molecular profiling of biological samples, including FFPE tissues, is complicated. Many of the steps from sample to result require manual intervention by a molecular technician. While these technicians are trained to standard operating procedures and proficiency tested, the levels of proficiency and precision vary among technicians. Variability introduced by technicians performing manual steps can translate to variability of results, with a test sample frequently at risk of experiencing losses of fidelity through the series of separation and transformation steps.
Sample to RNA Seq profiling of FFPE tissues
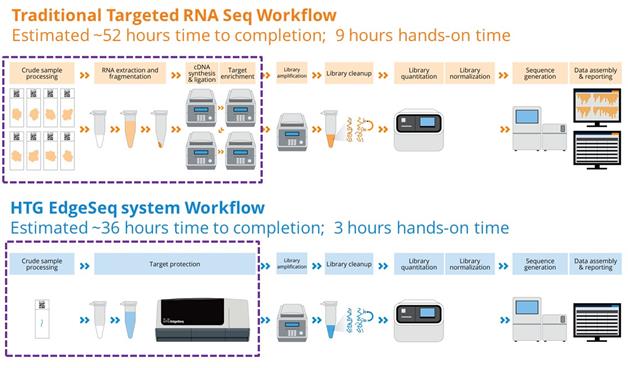
Further, the increase in number of technologies for sample characterization and fragmentation of the testing workflows can also create challenges in putting all of the results together in a timely, complete profiling report. This level of data integration is critical for the treating physicians to assure they have the complete molecular assessment prior to the patient consult. Without a complete molecular assessment, there is limited ability to discuss the diagnosis, prognosis and treatment options. Overall, we believe the critical relationship between the local pathologist and treating physician has been fractured as many tests results are now sent directly to the treating physician from these specialized and centralized CLIA labs. We believe the pathologist is critical to aggregating the diagnostic, prognostic and predictive information in a single patient molecular profile that can be utilized by the treating physician for therapeutic decisions.
Case Study of the Current Limitations in Molecular Profiling: Immuno-Oncology
A rapidly developing area of oncology drug development is the broad grouping of approaches referred to as immuno-oncology therapies. These immuno-oncology therapeutics include checkpoint inhibitors such as Keytruda (pembrolizumab) for melanoma and NSCLC, cancer vaccines such as chimeric antigen receptors in development by various companies, tyrosine kinase inhibitors such as Ibruvica (ibrutinib) for B-cell malignancies, and immunomodulatory therapies such as interferon-alpha.
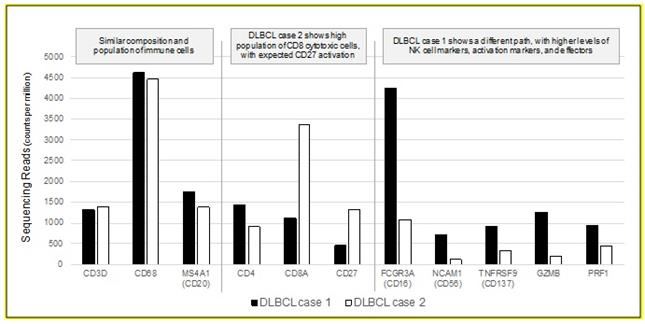
Recently, single-marker IHC tests have been approved as companion or complimentary diagnostics for anti-PD-1 checkpoint-inhibiting therapies. A proliferation of checkpoint inhibitors targeting additional ligands and receptors for CTLA4, OX40, LAG3, Tim-3 and others, coupled with combination approaches, is challenging the one-marker / one-drug diagnostic paradigm that previously advanced IHC-based companion diagnostics (e.g. HER2, ALK, c-KIT, etc). Understanding the complex biological framework of the tumor microenvironment is becoming increasingly important in interpreting the host immune response to tumors and developing the best single or combination therapy approach. The need for multiplexed gene expression assessment is illustrated in the graphic below where two histologically similar DLBCL tumors elicit very different host immune responses. Both cases have similar tumor
8
composition and populations of tumor infiltrating lymphocytes. In case 1, however, the immune system is responding primarily with natural killer lymphocytes whereas in case 2 the immune system has deployed CD8 cytotoxic lymphocytes. Based on this information and other clinical parameters, very different immunotherapeutic strategies may be deployed to combat the tumor. We believe that current molecular profiling techniques, like IHC or FISH or RT-qPCR, are ill-suited for comprehensive assessment of the tumor microenvironment.
Case Study of the Current Limitations in Molecular Profiling: NSCLC
The complexities and potential inefficiencies present in current molecular profiling techniques can be seen in non-small cell lung cancer, or NSCLC. Several clinical parameters are typically assessed using multiple testing methodologies. Personalized therapy and entry into clinical trials for NSCLC are heavily dependent on accurate histological classification, most often performed by IHC. Tumor samples are also typically tested for gene mutations, and gene rearrangements, with each of these tests performed on a different platform and separate workflow. About 70% of initial lung cancer diagnoses now have to be made based on small biopsies or cytological specimens. It is common for each of these platforms to be in separate laboratory departments with different technicians and clinicians, requiring that the already small sample be split into multiple workflows. The results from the individual testing workflows are typically aggregated into a single report, signed off by the pathologist and transmitted to the oncologist. As outlined in the diagram below, the multiple steps involved in the process may substantially slow turnaround times and result in an incomplete molecular profile not suitable to inform clinical decision making.
Example of Typical NSCLC Testing Workflow
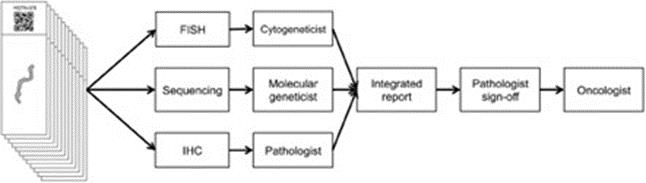
9
We have developed a novel technology platform that allows for precise, efficient molecular profiling of samples for clinical and research purposes. Our proprietary HTG Edge and HTG EdgeSeq platforms have the flexibility to work with many different biological sample types, are able to generate robust results from very small samples, and employ simple, proprietary chemistries that obviate the need for many of the steps associated with traditional molecular profiling techniques. Our platforms and chemistries enable the simultaneous detection and quantitation of hundreds or thousands of molecular targets and are capable, now or in the future, of profiling multiple parameters such as RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression in a single testing workflow that can use NGS detection for quantitative measurement.
Our HTG Edge and HTG EdgeSeq platforms, comprising instrumentation, software analytics and proprietary consumables designed exclusively for use with each respective platform. We manufacture the consumables utilized with our platforms specifically for our platforms and these consumables cannot be obtained from other sources. At the core of our solution is our proprietary chemistry called quantitative nuclease protection, or qNPA. Nuclease protection is an extremely efficient method for analyzing DNA and RNA as it eliminates the need for DNA or RNA extraction or reverse transcription. We have enhanced this method by combining it with our patented HTG Edge and HTG EdgeSeq chemistries. We designed and developed our HTG Edge and HTG EdgeSeq automation platforms to optimize the capabilities of our chemistries, provide fast turnaround time and enable ease of use to molecular labs. Our chemistries and automation platforms are highly adaptable, so when molecular profiling needs change or emerge, we expect to be able to efficiently add new applications to address these needs.
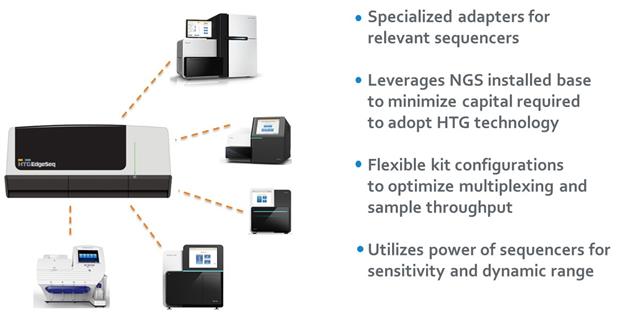
As the following diagram shows, the HTG Edge processor, software and consumables now support two methods for quantifying results, the HTG Edge reader for lower multiplexed panels and the recently launched HTG EdgeSeq chemistry for quantitation of high-plexed panels for utilizing NGS. In addition, we now offer an HTG EdgeSeq system that is dedicated to performing our HTG EdgeSeq chemistry with NSG detection.
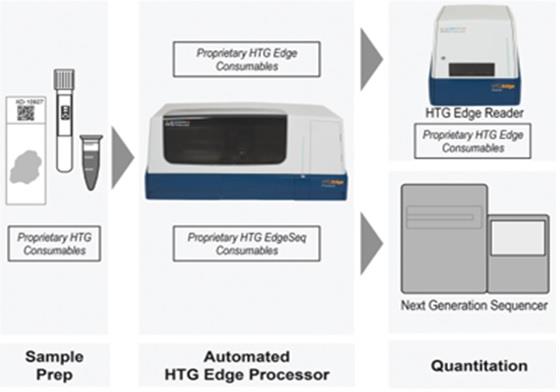
The HTG Edge and HTG EdgeSeq platforms provide data in a simple easy to use format. The entire workflow for both platforms from sample preparation to a molecular profiling report can be accomplished in 24-36 hours.
10
We believe the majority of customers in our target markets would prefer to maintain control of their samples and perform the profiling internally but are challenged by limitations in available technologies. We believe we are well positioned to democratize molecular profiling with the following key benefits:
|
|
· |
Optimize sample utilization. The HTG Edge and HTG EdgeSeq systems can analyze at least 2,500 genes from extremely small sample volumes such as a single five micron section of tissue or 12.5 microliters of plasma or serum. Our technology allows customers to do more with less, which meets the needs of clinical laboratories where today there is often not enough patient sample to do all the testing available. We believe providing customers the ability to work with extremely small sample volumes will be a significant driver of adoption of our platform. |
|
|
· |
Compatibility with multiple sample types. Our HTG Edge and HTG EdgeSeq platforms allow customers to profile and unlock genomic information from a wide variety of biological samples such as FFPE tissue, cells, blood serum and plasma. We have successfully demonstrated the ability to profile all of these and other sample types, and believe we ultimately can profile most clinically relevant sample types, including cell-free circulating nucleic acids from tumors, a rapidly developing area of investigation which is referred to as a liquid biopsy. We believe that the capabilities of our platform will allow us to efficiently expand applications, regardless of sample type. |
|
|
· |
Flexible and adaptable chemistry allows for use on multiple platforms. We believe our proprietary chemistry provides the ability to measure multiple molecular targets in all the necessary applications, including RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression and offers the ability to quantify on the HTG Edge Reader as well as a variety of NGS platforms. This flexibility provides customers the ability to optimize their use of HTG Edge and/or HTG EdgeSeq technologies based on their specific throughput, workflow and application needs. Our proprietary chemistry is simple, with fewer steps than competing technologies. For example, compared to RT-qPCR, our chemistry does not require extraction or cDNA synthesis. Compared to RNA sequencing, our chemistry does not require extraction, cDNA synthesis, shearing, rRNA depletion, ligation, adenylation, or size selection. We believe that the elimination of these steps helps prevent biases associated with these steps, sample degradation and increased opportunities for technician error. |
|
|
· |
Robust data. Molecular profiling produces large amounts of information that is used, among other things, to make important decisions, such as identifying potential drug targets or selecting a patient for a therapeutic treatment. This information is valuable only to the extent it accurately represents the true biology of the test sample and the same answer can be produced under many different conditions. Our HTG Edge and HTG EdgeSeq chemistries are highly specific and sensitive, meaning they can detect the right target even when very little is present in the sample. Our platform produces consistent results on a replicate-to-replicate, day-to-day and instrument-to-instrument basis. |
|
|
· |
Automation provides superior workflow and ease of use. Our HTG Edge and HTG EdgeSeq technologies are designed with fewer workflow steps in part due to the elimination of the need for complex biochemical processes such as extraction, cDNA synthesis, labeling, selection, depletion and shearing. This enables customers to limit hands-on time and the need for specialized skills, resulting in turnaround times of approximately 24-36 hours. Additionally, our HTG EdgeSeq application now further integrates sample preparation for targeted sequencing and greatly simplifies the bioinformatics back-end, so customers looking to leverage their NGS instrument can seamlessly add this capability to their current workflows. |
|
|
· |
Simplified bioinformatics. Our HTG Edge and HTG EdgeSeq software provides data in a simple and easy to use format through a simple graphical user interface, or GUI, that is flexible enough for researchers yet structured enough for clinical laboratories. The software is modular so that new applications can be downloaded without any changes to hardware. For applications that pair our HTG Edge processor and reader results are accessible in multiple formats through the GUI on the host computer. For HTG EdgeSeq applications, the HTG Edge and HTG EdgeSeq parser software processes the data from the NGS platform. We believe the simplicity of our bioinformatics solution will help drive the adoption of our platform. |
Current Commercial Panels Offered on the HTG Edge and HTG EdgeSeq Platforms
We currently market proprietary molecular profiling panels targeting late stage drug development programs with potential breakthrough therapies, such as immuno-oncology. We market these panels to biopharmaceutical companies, with which we collaborate in biomarker development programs. We believe these programs could facilitate our commercialization of companion diagnostic tests. In addition, our panels are used in pre-clinical and clinical research areas. Our currently marketed panels are:
11
|
|
responsive and non-responsive groups. The HTG EdgeSeq Immuno-Oncology Assay measures the expression of 549 of these immuno-oncology-associated genes. |
|
|
· |
HTG EdgeSeq Diffuse Large B-Cell Lymphoma Cell of Origin Assay. DLBCL tumors are frequently classified into either the activated B-cell like, or ABC, or germinal center B-cell like, or GCB, sub-types by measuring the molecular profile of the tumor. These two subtypes display different clinical pathologies, as patients with the GCB subtype of DLBCL tend to respond differently than those of the ABC sub-type. With many of the large number of new DLBCL-targeting drugs appearing to have greater efficacy in one of the sub-types, a need for a reliable, FFPE-based cell of origin classification assay has emerged. The HTG EdgeSeq Diffuse Large B-Cell Lymphoma Cell of Origin Assay is our most frequently purchased panel and is being utilized in numerous late-stage drug programs. |
|
|
· |
HTG EdgeSeq Oncology Biomarker Panel. We recently completed development and are now marketing our HTG EdgeSeq Oncology Biomarker Panel. This RNA expression panel measures the expression of up to 2,560 genes implicated in cancer for profiling tumor tissues, analyzing cancer pathways and identifying new biomarkers across both solid tumors and hematolymphoid neoplasms (or Heme). We worked with leading key opinion leaders to identify these genes, which we believe are a comprehensive list of genes targeting known signaling pathways and receptor gene families implicated in cancer such as the EGFR, HER2, HER3, HER4, PD-1 and FGFR genes. When paired with our existing miRNA Whole-transcriptome assay, we provide customers with a comprehensive solution for profiling their large sample archives for novel expression signatures. |
|
|
· |
HTG EdgeSeq Lymphoma Panel. Measures the expression of 93 genes frequently assessed in lymphomas, including 22 common non-Hodgkins B-Cell lymphoma markers from just 1.5mm2 of a 5 micron thick FFPE section. This is designed to enable detailed molecular profiles of lymphomas using a single FFPE slide. |
|
|
· |
HTG EdgeSeq microRNA Whole-transcriptome Assay. Human microRNAs are short non-coding strands of RNA that are used by the cell for gene regulation. The HTG EdgeSeq microRNA Whole-transcriptome assay enables the simultaneous profiling of 2,255 microRNAs, allowing new, potentially clinically relevant miRNA profiles to be discovered. This is our first marketed product on the NGS-integrated HTG EdgeSeq chemistry. Our ability to profile small FFPE samples is a significant differentiator in the rapidly growing microRNA market. |
|
|
· |
HTG Edge DMPK Assays. Biopharmaceutical companies often perform DMPK profiling studies as part of the drug development process to adhere to regulatory guidance. A set of genes known as the cytochrome P450 family is known to predict both the toxicity and stability of drugs in the human body. The HTG Edge DMPK Core CYP assay enables DMPK scientists to measure six cytochrome P450 genes recommended for in vitro pharmacokinetic studies. We are also marketing an expanded 45-gene panel that measures the expression of additional transporter genes to allow for in-depth profiling of the drug candidate. The DMPK area is a high-volume profiling market segment and creates synergies with down-stream clinical drug development programs. |
We utilize several alternative arrangements to sell our HTG Edge and HTG EdgeSeq systems and profiling panels. Our HTG Edge and HTG EdgeSeq systems can be purchased directly by our customers, who also then purchase profiling panels and other consumables from us on an as-needed basis. In some instances we provide our equipment free of charge on a limited basis to facilitate customer evaluation. We also install systems for our customers at no cost, in exchange for an agreement to purchase profiling panels and other consumables from us at a stated price over the term of the agreement. As of December 31, 2015, we had an installed base of 49 HTG Edge and HTG EdgeSeq instruments (consisting of 22 systems sold, five covered under reagent rental agreements, 19 evaluation units and three systems with key opinion leaders).
12
Product and Technology Pipeline Plan
Our objective is to establish the HTG EdgeSeq platform as a standard in molecular profiling, making this capability broadly accessible. We are leveraging our flexible and adaptable platform to develop comprehensive molecular profiling panels across an increasing set of molecular applications. We believe it is important to include applications that cover a broad set of genomic variation as well as expression-based clinical biomarkers in order to continually increase the value of the HTG Edge platform and provide a more complete profiling solution for our customers. The diagram below depicts our planned expansion of systems, applications and profiling panels which are being adding in a phased approach.
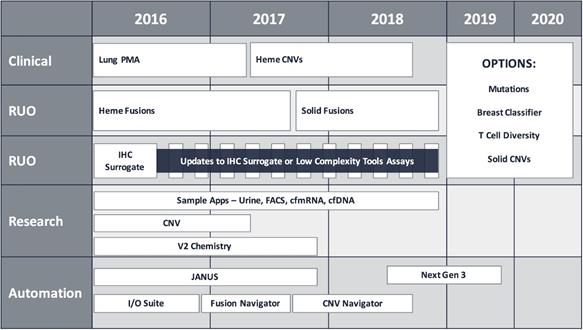
Opportunities for Comprehensive Molecular Profiling in Diagnostics
We are planning to develop a portfolio of molecular diagnostic products using our proprietary technology to provide a single, efficient testing platform for sample profiling that integrates seamlessly into a customer’s NGS testing workflow.
We are initially focused on areas where the diagnostic testing paradigm has significant inefficiencies. For example, many leading medical institutions are shifting from a single-drug / single-test approach to a broader tumor profiling strategy for their oncology practices. Rather than testing for single mutational analyses, such as KRAS for colorectal cancer, these institutions use their NGS platforms to assess the mutational status of 50 or more genes. In addition, the information a physician needs to make clinical decisions often comes from tests conducted using various testing modalities such as IHC, FISH and NGS. These complexities lead to a growing and unmet demand in clinical diagnostics for more comprehensive molecular profiles that can include RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression.
We are developing solutions to address the detection and measurement of gene rearrangements such as gene fusions and insertions. A fusion gene is a hybrid gene formed from two previously separate genes. A well-established example is the EML4-ALK fusion gene found in a subset of malignant lung cancers. Our research team previously demonstrated feasibility to simultaneously detect and measure key gene fusions in lung cancer such as ALK, ROS1, RET and NTRK1 from multiple small sample types including FFPE, cell lines and purified RNA. An insertion is the addition of one or more nucleotides into a genomic sequence; a small percentage of NSCLC exhibit HER2 exon 20 insertions.
We intend to develop our ALKPlus assay as our initial clinical diagnostic test. Our ALKPlus assay is a comprehensive fusion panel that includes rearrangements with the following genes: ALK, ROS1, RET and NTRK1 as well as insertions in HER2. This test panel is in formal development with plans to submit a modular PMA to the FDA and to seek CE/IVD marketing authorization for this panel in Europe in 2016. We have completed the initial pre-submission meeting with the FDA and expect to commence clinical trials in the second quarter of 2016. We intend to seek pre-market approval as a companion diagnostic for crizotinib therapy for ALK positive NSCLC cases. The ROS1, RET, NTRK1 and HER2 genes are expected to have RUO status, but we believe they could also be labeled as investigational use only (IUO) for certain late-stage drug development trials.
13
We are also developing enhancements to our chemistry, which we refer to as V2, that will enable a single testing workflow that provides, in parallel, reactions from a single unstained slide, histological classification of the tumor and DNA mutation status of key genes previously shown to be associated with NSCLC, such as EGFR, KRAS, BRAF and HER2, in combination with the ALKPlus panel. We refer to this as the HTG total lung solution. We intend to extend this capability to all solid tumor sample types.
We will need to develop, refine and implement new panel protocols and make changes or adjustments to our chemistry and software in order to optimize these planned applications and panels for use with our HTG EdgeSeq system. This will require substantial effort from our research scientists and the use of various laboratory equipment, supplies and materials, which combined represent the most significant costs that we expect to incur in connection with the development of these applications and profiling panels.
HTG is uniquely positioned to replace FISH testing for Heme
Another area we believe the HTG EdgeSeq platform can provide significant value to clinicians is in the diagnosis of hematolymphoid neoplasms such as leukemias, lymphomas, myelomas, and myelodysplastic syndromes. This is a large market opportunity with over 170,000 new cases reported in 2014. Across the spectrum of these diseases there is a large number of gene rearrangements and CNVs, typically performed by FISH, which physicians evaluate in a patient’s sample to make clinical decisions. The diagnoses of leukemias and lymphomas are complex and initial FISH panels are often followed with additional FISH panels, resulting in a possible delay of diagnosis and treatment.
We are in early stage development of highly multiplexed, NGS-based panels to assess most known gene rearrangements and CNVs in hematolymphoid neoplasms in a single NGS workflow. We believe improved turnaround time coupled with changing dynamics of reimbursement are causing laboratories to consider molecular options to replace FISH-based testing. The HTG EdgeSeq Heme Fusion and HTG EdgeSeq CNV / Karyotyping product concepts, with expected sample-to-answer time of 36 hours, should meet the turnaround time requirement while providing the comprehensive tumor profiling information for accurate diagnosis of hematolymphoid neoplasms.
The HTG EdgeSeq system can augment or, in some cases, replace IHC testing
IHC tests are integral to the diagnostic workup for most tumor types, providing qualitative protein expression data within the morphological context of the tissue tested. While there are over 400 different biomarkers tested by IHC in clinical practice today, we believe it is rare to test more than ten; this is due in part to tissue availability, especially for minimally invasive core needle biopsies, and limitations on the number of tests reimbursed per case. The HTG EdgeSeq IHC surrogate product concept is intended to enable the assessment of the RNA analogues for all clinical IHC biomarkers in a single NGS-based test. We believe this single-test concept will provide pathologists a standard by which to measure all tumors, augmenting or in some cases replacing their IHC testing with a quantitative assessment of the tumor biology.
Serving the decentralized markets with Project JANUS
In addition to continued menu expansion for clinical diagnostics we are now in development of our next generation instrument targeting the lower throughput clinical market segment. We believe this segment will potentially increase our addressable market for instrument sales from 100’s of labs to 1000’s of labs. The solid tumor testing market is served by a mix of centralized reference laboratories, comprehensive cancer centers, and decentralized regional hospital laboratories. Volumes of particular case types and downstream testing needs for solid tumors are somewhat unpredictable; we believe Project JANUS is well suited for managing low, medium, and surge testing volumes. Testing panels for Project JANUS include mutations, fusions, CNVs, expression classifiers, and a molecular surrogate for IHC. Our approach is to serve centralized markets with the HTG EdgeSeq system and expand to the decentralized market with Project JANUS. We believe our current HTG EdgeSeq instrument has the throughput capabilities that match the needs of the centralized market. Project JANUS is our development effort to expand our addressable market to the lower sample throughput labs and further decentralize the market.
14
Project JANUS began with the selection of our engineering partner. Project JANUS demonstrates the continuing innovation of HTG’s instrumentation platform and workflow benefits.
15
HTG Chemistries
Our core chemistry, known as qNPA, is a biochemical process capable of measuring messenger RNA, or mRNA, and microRNA gene expression levels from very small amounts of difficult to handle samples without the need for conducting RNA extraction, cDNA synthesis, RNA amplification or RNA-labeling steps. Our proprietary chemistry is well suited to measure RNA due to its simple, yet robust biochemistry designed to handle highly multiplexed assays. Two primary elements of our chemistry process are DNA to RNA hybridization and S1 nuclease digestion. Both of these elements have been widely researched for decades and are well understood.
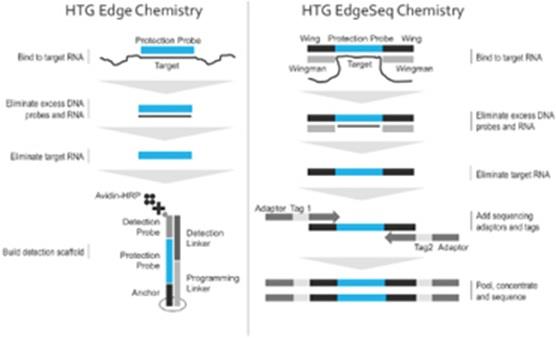
HTG Edge Chemistry
DNA nuclease protection probes, 50 bases in length, are added to the sample and hybridized to targeted RNAs, which can be both soluble and cross-linked in the biological matrix. S1 nuclease is added to remove excess, unhybridized DNA probes and RNA; the only remaining DNA protection probes are those hybridized to targeted RNA. This produces a virtual 1:1 ratio of DNA protection probes to the RNA initially targeted in the sample. Alkaline hydrolysis of the RNA releases the protection probes from the DNA:RNA duplexes. The released DNA protection probes are ready for quantitation. One of 47 unique anchor spots arrayed on the bottom of each well of a multi-well plate captures a programming linker that in turn captures a DNA protection probe. A detection scaffold consisting of a detection linker and a biotinylated detection probe is captured when a protection probe is present. After treatment with an avidin-HRP enzyme and a chemiluminescent substrate, the well is imaged and quantitated in the HTG Edge Reader. The HTG Edge system host software reports data from the reader.
HTG EdgeSeq Chemistry
DNA nuclease protection probes, which include a target-specific region flanked by universal wing sequences are hybridized to targeted RNAs, which can be both soluble and cross-linked in the biological matrix. Universal DNA wingmen probes are hybridized to the wings to prevent S1 nuclease digestion. S1 nuclease is added to remove excess, unhybridized DNA probes and RNA; the only remaining DNA protection probes are those hybridized to targeted RNA and wingmen probes. This produces a virtual 1:1 ratio of DNA detection probes to the RNA initially targeted in the sample. Alkaline hydrolysis of the RNA releases the protection probes from the DNA and RNA duplexes. The released DNA protection probes are ready for quantitation. DNA protection probes are labeled with sequencing adaptors and tags in a thermocycler. The labeled DNA protection probes are concentrated, pooled, and ready for sequencing using standard NGS protocols. Data from the NGS instrument is processed and reported by the HTG EdgeSeq Parser software.
16
Key Advantages of HTG Chemistries
|
|
· |
Multiplexing hundreds or thousands of targets. Measuring multiple genes in a single reaction can be challenging with competitive technologies due to the complex interactions of the assay sub-components. Our proprietary HTG Edge chemistry allows for 47 independent genes to be profiled in a sample. While we are currently marketing a panel with our HTG EdgeSeq chemistry that can profile up to 2,500 genes in a sample, we believe we can develop applications using our chemistry for multiplexing more than 2,500 genes if there is a customer need. The high level of gene multiplexing allows for significantly lower amounts of tissue to be used per sample than in competitive low-plex profiling technologies. |
|
|
· |
No RNA extraction. Competitive technologies for assessing RNA require RNA that is isolated and purified from other components found in the sample. These time-consuming and bias-inducing steps lead to some target loss. In FFPE tissues, a fraction of the RNA is lost in the purification process because it cannot be separated from insoluble tissue components and the fixation and embedding process or long storage times for FFPE tissue may damage the RNA and break it into smaller, more difficult to analyze fragments. This makes working with small FFPE tissues extremely challenging and can result in testing failures and loss of precious samples due to insufficient RNA recovery. These biases introduced by RNA extraction cannot be overcome and may be magnified throughout the subsequent analysis. Our proprietary chemistry does not require RNA extraction for most sample types, improves utilization of precious samples, improving workflow and reducing costs by eliminating a step known to cause bias in the data. |
|
|
· |
No cDNA synthesis. Many competitive technologies, most prominently qRT-PCR and RNA sequencing, require conversion of RNA into DNA for analysis. This process requires a viral enzyme to move along the extracted RNA to create a DNA copy of the molecule. When damaged and fragmented RNA is used, these small RNA strands become increasingly difficult to convert into DNA in an accurate and reproducible manner. Our proprietary chemistry does not require conversion of the RNA to DNA, removing a major source of bias experienced with competitive technologies. |
|
|
· |
Short protection probes. Many samples contain RNA degraded by various combinations of heat, age, poor processing, and fixation. In these samples, the RNA is damaged and fragmented into smaller strands. Utilizing short protection probes of 50 bases or less, our proprietary chemistry is more efficient than competitive technologies that require longer strands of RNA for quantitation. |
|
|
· |
Simplicity. Our proprietary chemistry is simple, with fewer steps than competing technologies. Compared to RT-qPCR, our chemistry does not require extraction or cDNA synthesis. Compared to RNA sequencing, our chemistry does not require extraction, cDNA synthesis, shearing, rRNA depletion, ligation, adenylation, or size selection. We believe that the accumulation of these steps required by other technologies results in amplification of biases, sample degradation and increased opportunities for technician error. |
HTG Edge and HTG EdgeSeq Platforms
Our HTG Edge and HTG EdgeSeq platforms were developed and are manufactured under ISO 13485:2003 guidelines using our proprietary chemistry to simplify multiplexed nucleic acid testing in research and clinical laboratories. The entire HTG Edge or HTG EdgeSeq workflow from sample preparation to a molecular profiling report can be accomplished in 24-36 hours for 96 samples. With the speed, flexibility, sensitivity, and accuracy of our HTG Edge and HTG EdgeSeq platforms, combined with the system’s ability to work effectively with small sample volumes, researchers can profile hundreds or thousands of different genes per sample.

The HTG Edge platform consists of processor and reader instruments, a host computer, and software. The HTG Edge processor is a fully automated instrument that prepares biological samples for quantitation using proprietary, electronically barcoded, single-use consumables. Both the processor and the reader have barcode scanners units to process the two-dimensional, or 2D, barcodes printed on the consumables loaded in each instrument. The barcoded consumables are single-use in order to reduce operator errors and provide chain of custody traceability for the samples. The robotic systems within each instrument are engineered for reliable performance and low maintenance. The walking path of each robot is programmed to minimize any chance of contamination of the reagents or samples.
17
Quantitation of results from the HTG Edge platform may be performed on either the proprietary HTG Edge Reader or an NGS platform. If the reader is utilized for quantitation, proprietary consumables are used in a chemiluminescent detection reaction, and the reader captures and processes these images in less than an hour to produce a quantitative result. The software reports the result on the host computer. One host computer supports up to five processors and one reader. This allows laboratories to easily expand their capacity by adding processors without purchasing an additional reader instrument.
HTG EdgeSeq applications combine either the HTG Edge processor or a dedicated HTG EdgeSeq processor with an NGS platform to enable the quantitative analysis of hundreds or thousands of targeted RNAs in a single assay. The sample is prepared for quantitation on the HTG Edge or HTG EdgeSeq processor, then labeled with molecular sequencing adaptors and tags. The labeled samples are concentrated, pooled, and sequenced on an NGS platform using standard protocols. Data from the NGS instrument are processed and reported by the HTG EdgeSeq Parser software. The HTG EdgeSeq applications currently process samples in sample volumes ranging from eight to 96.
HTG Edge and HTG EdgeSeq Platform Workflow
Our HTG Edge and HTG EdgeSeq platforms deliver complex molecular profiling information in a simple four step workflow. A technician spends minimal time preparing samples which are easily loaded into the applicable processor. Once the sample is loaded, the processor performs our proprietary chemistry protocol with no technician intervention, which is commonly referred to as walk away automation. Once the sample is processed, a technician prepares the sample for quantitation on either the HTG Edge reader, for HTG Edge systems, or an existing NGS instrument. This flexibility in quantifying molecular information provides customers the ability to optimize their use of the HTG Edge or HTG EdgeSeq platform based on their specific throughput, workflow and application requirements. Having both quantitation options allows HTG to offer a broader set of molecular profiling panels and leverage the growing NGS installed base. In comparison to RNA sequencing, there are many fewer steps required for molecular profiling using HTG EdgeSeq applications as shown in the diagrams below.
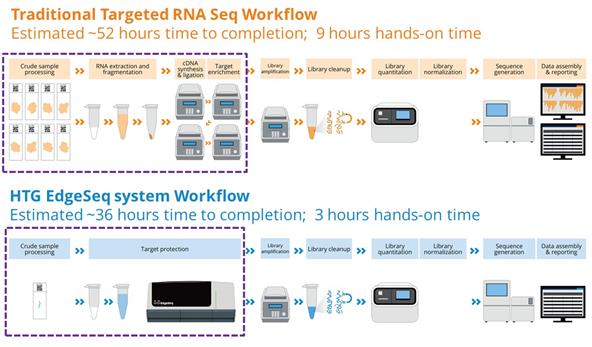
HTG Edge and HTG EdgeSeq Platform Performance
The HTG Edge and/or HTG EdgeSeq platforms enable our customers to quantitatively profile from one to thousands of genes in a single reaction using very small samples. Our chemistry works with many biological sample types including FFPE, frozen tissue, plasma, serum, whole blood preserved in PAXgene, and cultured cells. We plan to enable our customers to use a single profiling workflow for the analysis of multiple parameters such as RNA expression, RNA fusions and rearrangements, DNA mutations and protein expression. As part of our development process for new products we perform several analytical studies to demonstrate performance of the applicable platform, and we believe that our approach to multi-parameter molecular profiling is highly repeatable and reproducible.
18
Equivalent quantitative expression results from large as well as very small samples
HTG EdgeSeq assays are validated with sample inputs ranging from as much as 25 mm2 down to 1.5 mm2 tumor surface area from a single 5 µm section, enabling comprehensive molecular profiling on a large surgical resection or very small needle core biopsy. Needle core biopsies are often the only tissue available for profiling. A representation of the difference in the amount of tissue available in a surgical resection versus a core needle biopsy is shown in the graphic below. In an internal study of resections versus core needle biopsies from the same non-small cell lung cancers, Pearson correlations (log2 transformed counts) greater than 0.96 were observed.
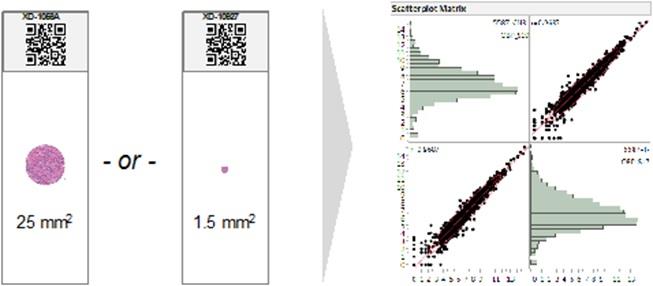
19
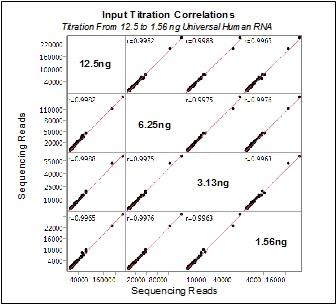
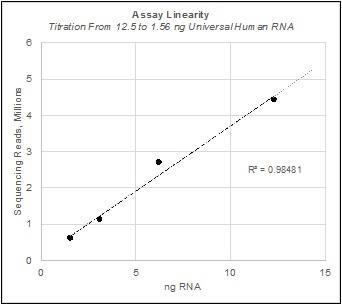
We believe obtaining molecular profiles from very small samples is of critical importance to researchers. We have demonstrated reproducible, quantitative results from a fraction of the more than 100 ng of input RNA typically required for competitive technologies. In the study shown below, the HTG EdgeSeq Oncology Biomarker Panel was used to measure mRNA expression levels in a dilution series from 12.5 ng to 1.56 ng of Universal Human RNA. In the figure titled Input Titration Correlations below, correlations between each dilution were measured by Pearson’s r and are displayed in each comparison field. A Pearson correlation between two perfectly identical molecular profiles will produce an r-value of 1.0. The r-values obtained in the study shown below, of greater than 0.99, indicate nearly identical molecular profiles were obtained. In the figure titled Assay Linearity below, the raw number of sequencing reads for each of the samples in the dilution series are shown, with the statistical measure of how close the data are to the fitted regression line, R-squared, of 0.98481. These plots demonstrate the capability of the HTG EdgeSeq applications on the HTG Edge or HTG EdgeSeq platform to deliver equivalent molecular profiles over a range of sample inputs.
|
|
|
|
20
Leveraging the dynamic range and sensitivity of NGS
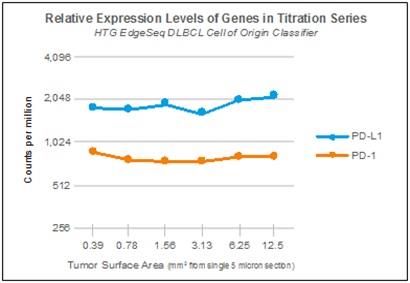
The end product of the HTG EdgeSeq chemistry process is a library of genomic fragments which, once sequenced, are compared to a reference genome consisting of one sequence per probe in the assay. NGS platforms individually sequence each genomic fragment that binds to its detection media, obtaining highly specific information about each of one of the genomic fragments analyzed in a single sequencing run. Each fragment sequence is stored as a single read. With the potential capacity of a single sequencing run numbering in the millions of reads, NGS assays are highly sensitive, detecting low abundance or rare sequences, while having a wide dynamic range, accurately counting the relative number of abundant sequences. In the development and validation of gene expression classifiers, we believe it is important that the assay system reliably measure differential gene expression for both high and low abundance genes. As an example of how the HTG EdgeSeq assays leverage the sensitivity of NGS, the graphic below shows the relative expression of the low-abundance immunotherapy targeted genes PD-1 and PD-L1 across a wide range of sample inputs using the HTG EdgeSeq Diffuse Large B-Cell Lymphoma Cell of Origin Assay.
High fidelity between processing conditions
One of the greatest challenges faced in molecular profiling of formalin fixed samples is the poor fidelity routinely seen when comparing the results across various processing conditions. One example in particular is the fidelity seen between formalin fixed samples such as FFPE to non-fixed tissue. It has been repeatedly demonstrated that the fixation process can lead to different molecular profiles in otherwise identical samples. We believe researchers often obtain sub-optimal molecular profiling results solely due to the formalin fixation process. We further believe that our chemistry overcomes the losses in fidelity between non-fixed and FFPE tissues, enabling users to obtain an accurate assessment of tumor biology from FFPE tissues.
21
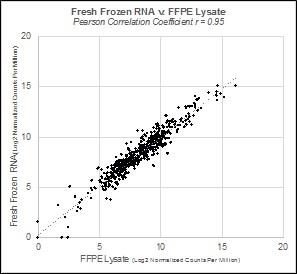
To demonstrate fidelity between non-fixed and FFPE tissues that may be achieved using the HTG EdgeSeq system, a resection from a breast cancer tumor was prospectively harvested and split, with half of the specimen immediately frozen and the other formalin fixed and paraffin embedded (FFPE). RNA from the non-fixed, fresh frozen specimen was extracted and added to the HTG lysis buffer (we recommend extracting the RNA from fresh tissue samples). The matched FFPE tissue was directly lysed in the HTG lysis buffer without extraction. Both specimens were then profiled using the HTG EdgeSeq Immuno-Oncology Assay. The scatterplot below shows the correlation between the non-fixed, fresh frozen tissue and the FFPE tissue, with a Pearson correlation coefficient of r = 0.95, indicating that the fixation process did not materially alter the molecular profile of the sample when our proprietary chemistry was used. We believe our platform enables researchers and clinicians to access their large collections of archived FFPE tissues to deliver reproducible, biologically relevant molecular profiles.
Compatibility with multiple sample types
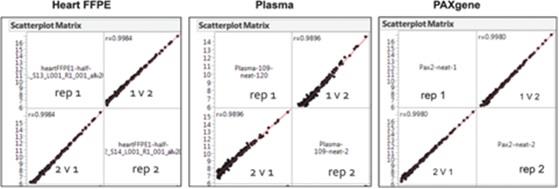
Compatibility of our proprietary chemistry with ovarian FFPE, extracted RNA, and fresh or fixed cultured cells is shown above. Below are technical replicates of heart FFPE, plasma and whole blood preserved in PAXgene profile using the HTG EdgeSeq microRNA whole transcriptome panel. We believe our proprietary chemistry is robust across many biological sample types.
Detection of gene fusions
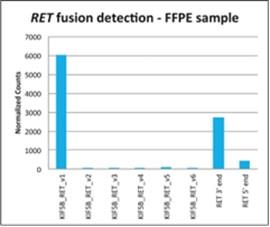
HTG EdgeSeq chemistry has demonstrated the ability to accurately detect gene rearrangements in patient-derived FFPE tissues. The example below demonstrates detection in a patient sample with a characterized gene fusion involving the KIF5B and RETS genes. Using multiple DNA protection probes, the HTG EdgeSeq platform can detect gene rearrangements and fusions. Rearrangements and fusions are detected in two ways. First is the direct measurement and detection of the unique RNA sequence created at the gene fusion site. This is indicated by the strong signal obtained from the KIF5B-RET v1 probe. The second approach involves calculating the ratio of signals obtained from probes targeting both the 5’ and 3’ end of the RET gene. Samples which do not contain a RET gene fusion will have similar RET 5’ and RET 3’ expression. In the sample above, however, which contains a RET
22
gene fusion, we see significantly higher signal from the RET 3’ probe implying the two parts of the RET gene are no longer connected, indicating a gene rearrangement has occurred.
A cell line with a known ALK fusion and normal ROS1 was tested using the same 5’ and 3’ probe ratio methodology described for the RET fusion above. As expected with a fused ALK gene, the expression of the 3’ portion of the transcript is much higher than the 5’ portion, indicating a fusion event has occurred. An example of a known non-fusion gene, ROS1, is also shown in the example where the 5’ and 3’ ends of the ROS1 are expressed at a 1:1 ratio, indicating this gene is intact.
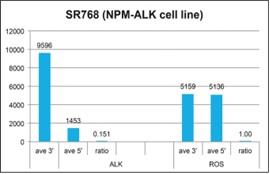
23
Discriminating between closely related sequences
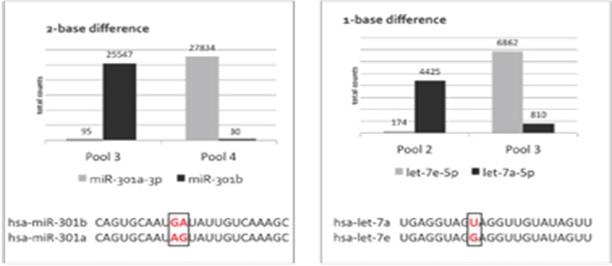
The specificity of our proprietary chemistry is demonstrated in the data chart below. Closely related synthetic miRNA pools supplied by Association of Biomolecular Resource Facilities were split between pools. All possible probes were used to profile individual pools. From 3-11% of NPP probes bound to off-target sequences with a 1-base difference (Figure 1A) while only 0.1-0.3% of NPP probes bound to off-target sequences with a 2-base difference (Figure 1B). In both examples, the on-target hybridization signal is at least 8X greater than off-target hybridization. Data was generated using the Illumina MiSeq v2 1x50 reagent kit. We believe that our proprietary chemistry is highly specific and will enable our customers to discriminate between closely related sequences.
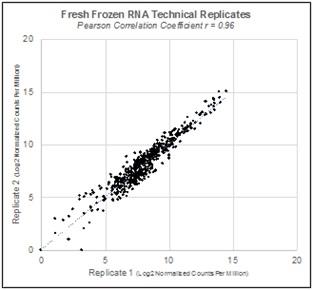
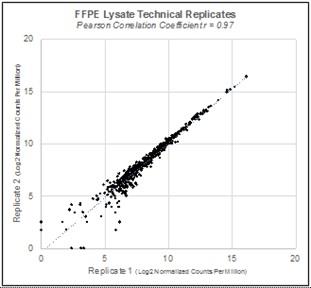
Repeatability
An important consideration in adoption of a molecular profiling platform is the degree to which the same molecular profile can be obtained from a sample tested on multiple replicates and multiple occasions. Obtaining highly similar profiles indicates that the assay is stable, and that the data generated is reliable and repeatable. The XY scatterplots below shows the correlation between the same sample on technical replicates using the HTG EdgeSeq Immuno-Oncology Assay. Extremely similar results were obtained, as demonstrated by the Pearson correlation coefficient r-values of 0.96 and 0.97 for fresh frozen and FFPE samples, respectively. We believe the HTG Edge and HTG EdgeSeq platforms and HTG EdgeSeq chemistry enables our customers to produce stable and repeatable molecular profiles. As demonstrated by the measurement of 549 mRNAs in the sample, HTG EdgeSeq chemistry has the ability to reliably detect large numbers of genes in a complex background.
|
|
|
|
24
Reproducibility
Another important consideration in adoption of a molecular profiling platform is the degree to which the same molecular profile can be obtained from a single sample tested multiple times over on the same and different instruments. Obtaining highly similar profiles indicates that the chemistry and instrument platform is stable, and that the data generated is reliable and reproducible. The XY scatterplots below shows the correlation between the same sample profiles on three separate instruments across three days using the HTG EdgeSeq Oncology Biomarker Panel. The scale of the vertical and horizontal axes represent log 2 transformed, filtered probe counts. Extremely similar results were obtained, as demonstrated by the Pearson r-values above 0.96 for all comparisons. We believe the HTG Edge platform and chemistry enables our customers to produce stable and reproducible molecular profiles.
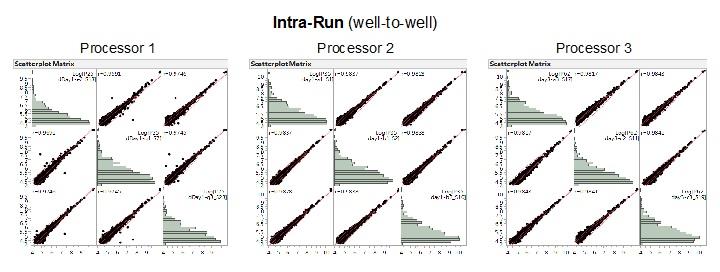
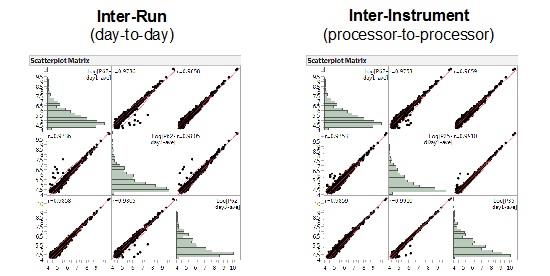
Research and Development
We have committed, and expect to commit, significant resources to developing new technologies and products, improving product performance and reliability and reducing costs. We have assembled an experienced research and development team with the scientific, engineering, software and process talent that we believe is required to successfully grow our business. As of December 31, 2015, our research and development team consisted of 22 employees across the disciplines of research and development scientist, platform development and bioinformatics.
We are currently focused on expansion of our profiling applications and test panel menu and on the development of our next generation Project JANUS instrument program. We incurred research and development expenses of $4.6 million and $3.1 million for
25
the years ended December 31, 2015 and 2014, respectively. As of December 31, 2014, we had a Small Business Innovation Research grant from the National Human Genome Research Institute within the National Institute of Health which was completed in June 2015.
We continuously seek to innovate and improve our technology to add valuable features for our customers and expand addressable market segments. Our technology advancement efforts are targeted at:
|
|
· |
Expansion of new sequencing applications to detect and measure RNA based rearrangements, DNA mutations, protein immunoassays, CVFs and circulating cell free DNA |
|
|
· |
Development of IVD products |
|
|
· |
Continued improvements to our workflow automation instrumentation, including the development of Project JANUS, a low sample volume version of the HTG EdgeSeq system. The low volume version of HTG EdgeSeq system will target sample throughput in the one to eight sample range, and is intended to be applicable to a broader set of molecular labs. This low volume version is targeted for launch no earlier than 2017. |
Our new products are developed under our ISO 13485 certified quality system and we expect to submit our first assay to FDA by the end of 2016. Our HTG Edge system is currently CE marked and we also expect to submit for CE/IVD status for the HTG EdgeSeq system by the end of 2016. We are dedicated to ongoing innovation to the HTG Edge platform technology and expanding our pipeline of product candidates. Our goal is for HTG Edge and HTG EdgeSeq systems to become the standards for rapid profiling of samples and critical clinical specimens such as tissue tumor biopsies.
Sales and Marketing
We distribute our instruments and consumables via direct sales in the U.S. and Europe and also through distributors or sales agents in parts of Europe and other countries. As of December 31, 2015, our U.S. sales and marketing organization consisted of 35 employees including 16 in direct sales or sales management, 12 in sales support and six in marketing. In addition to our direct sales team in the U.S., as of December 31, 2015, we have one direct sales employee in Europe, a contracted sales team in Europe through an agreement with Novoptim and an exclusive distribution agreement for India, Pakistan, Sri-Lanka, Bangladesh and Nepal with Innovative Life Discoveries Pvt. Ltd.
Our sales and marketing efforts are targeted at biopharmaceutical companies, clinical research centers and clinical diagnostic labs focused on sample profiling for translational research, biomarker/companion assay development and lab-developed diagnostic testing. We intend to promote adoption of our HTG Edge and HTG EdgeSeq systems, sample profiling panels and future molecular diagnostic assays, upon marketing clearance or approval by FDA, by expanding our existing U.S. sales force, building a greater direct sales presence in Europe, expanding international distribution, and continuing to collaborate with key opinion leaders to validate our platform and influence utilization of our products.
The top two customers accounted for 38% and 7% of our revenue for the year ended December 31, 2015, compared with 12% and 7% for the year ended December 31, 2014. We derived 8% and 31% of our total revenue from grants and contracts primarily from one organization during the years ended December 31, 2015 and 2014, respectively.
Manufacturing and Suppliers
We utilize third-party contract manufacturers to produce components for our HTG Edge and HTG EdgeSeq instruments and raw materials for our consumables. We complete HTG Edge and HTG EdgeSeq instruments and reagent kits at our Tucson, Arizona facility, which has been certified to ISO 13485:2003 standards. We completed a capital buildout in the first quarter of 2016 which created 5,000 square feet of dedicated manufacturing and operations space. Features include a walk-in freezer for storage of material at -20C, seven individual controlled lab areas providing climate, particulate and atmospheric pressure control that minimizes contamination of product and test processes and dedicated space for cellular manufacturing of our instruments. We believe that our existing manufacturing capacity is sufficient to meet our needs at least for the next several years.
Instruments
We assemble our HTG Edge and HTG EdgeSeq instruments at our Tucson, Arizona facility. We have qualified an alternative third-party manufacturer and we believe additional alternatives would be available if necessary. Instrument component vendors are qualified and reviewed regularly to ensure that manufacturing standards are met and maintained. We award contracts for estimated annual quantities of components and, considering the replenishment lead times of our vendors, take delivery of batches covering approximately one month of demand at a time.
26
We assemble our HTG Edge and HTG EdgeSeq reagents and consumables at our Tucson, Arizona facility. Reagent vendors are selected using precise standards, and are reviewed regularly for compliance with our specific quality requirements. We purchase reagent stock in quantities that often exceed projected annual demand. We complete batches of finished goods approximating quarterly demand and supervise inventory on a Minimum/Maximum basis to ensure that we are replenishing our finished goods and raw material ahead of demand.
Competition
We have categorized known competition into:
|
|
· |
Other molecular platform offerings such as PCR-based technologies, microarrays and next generation sequencers from companies such as Advanced Cell Diagnostics, ArcherDx, Inc., BioRad Laboratories, Roche Diagnostics, a division of the Roche Group of companies , Qiagen N.V., Illumina, Inc., Affymetrix, Inc., NanoString Technologies, Inc., Abbott Laboratories, Exiqon A/S, Fluidigm Corporation, Luminex Corporation and Thermo Fisher Scientific, Inc. |
|
|
· |
Centralized CLIA labs offering molecular profiling and gene expression tests as laboratory-developed tests, or LDTs, such as Foundation Medicine, Inc., Trovagene, Inc., Guardant Health, Inc. and Genomic Health, Inc. |
|
|
· |
NGS target enrichment technologies from companies such as Agilent Technologies, Inc. and RainDance Technologies, Inc. |
|
|
· |
Decentralized CLIA labs developing LDTs locally such as major cancer centers |
We believe that the principal competitive factors in all of our target markets include:
|
|
· |
cost of capital equipment; |
|
|
· |
cost of consumables and supplies; |
|
|
· |
reputation among customers; |
|
|
· |
innovation in product offerings; |
|
|
· |
flexibility and ease-of-use; |
|
|
· |
accuracy and reproducibility of results; and |
|
|
· |
compatibility with existing laboratory processes, tools and methods. |
We believe that additional competitive factors specific to the diagnostics market include:
|
|
· |
breadth of clinical decisions that can be influenced by information generated by tests; |
|
|
· |
volume, quality, and strength of clinical and analytical validation data; |
|
|
· |
availability of coverage and adequate reimbursement for testing services; and |
|
|
· |
economic benefit accrued to customers based on testing services enabled by product |
We believe the automation level of the HTG Edge and HTG EdgeSeq systems coupled with fast turnaround time, high multiplexing capability, lysis only / no extraction protocol and low sample requirement gives us numerous competitive advantages in our target markets.
While we believe that we compete favorably based on the factors described above, many of our competitors are either publicly traded, or are divisions of publicly traded companies, and enjoy several competitive advantages over us, including:
|
|
· |
Greater name and brand recognition, financial and human resources; |
|
|
· |
Broader product lines; |
|
|
· |
Larger sales forces and more established distributor networks; |
|
|
· |
Substantial intellectual property portfolios; |
|
|
· |
Larger and more established customer bases and relationships; and |
|
|
· |
Better established, larger scale and lower cost manufacturing capabilities. |
27
Our success depends in part on our ability to develop and maintain intellectual property rights relating to key aspects of the technology employed in our HTG Edge and HTG EdgeSeq platforms and chemistries, maintain any strategic licenses to use intellectual property owned by third parties, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and other proprietary rights of third parties. We rely upon certain patents, registered and common law trademarks, trade secrets, know-how, invention and patent assignment agreements and continuing technological innovation to develop and maintain our competitive position. We intend to aggressively protect, defend and extend the intellectual property rights in our technology.
Patents and Patent Applications
Our patent portfolio includes 13 patent families that, collectively, consist of eight issued U.S. patents, 28 granted foreign patents (variously in Australia, Canada, China, Japan, France, Germany, Italy, Spain, and United Kingdom), and 33 patent applications pending in the U.S. and foreign jurisdictions, including two patent applications now allowed in Europe and one patent application now allowed in Canada. This portfolio is directed to our nuclease-protection-based technologies as well as to lung cancer and melanoma biomarker panels and a diffuse large B-cell lymphoma, or DLBCL, cell-of-origin classifier discovered using our nuclease-protection-based technology, which will help us maintain an exclusive position in key areas of our business, including targeted nuclease-protection based sequencing, and, if we should so elect, may provide opportunities for out-licensing melanoma-DLBCL- or lung-cancer-related content. There are five pending or allowed applications and three granted patents, including one U.S. patent, directed to our novel HTG EdgeSeq methods, which patents will expire in April 2032. Worldwide, we have 26 patents in the two patent families directed to our HTG Edge plate-based methods; these patents will begin to expire at the end of December 2017 with the last ones expiring in June 2022.
Agreements with Third Parties
Asset Purchase Agreement with NuvoGen Research, LLC
We acquired some of our intellectual property, including one of our patent families, entitled “High Throughput Assay System” or similar, or Acquired Technology, from Neogen, LLC, now known as NuvoGen Research, LLC, or NuvoGen, pursuant to an Asset Purchase Agreement dated January 9, 2001, as amended in November 2003, September 2004, November 2012 and February 2014. The Acquired Technology generally relates to our array-based nuclease protection assays. Pursuant to the terms of the agreement, in exchange for the Acquired Technology, we initially paid NuvoGen 5,587 shares of our common stock, fixed payments of $740,000 over the first two years of the agreement and agreed to pay NuvoGen 6% our yearly revenue, which would be applied to any fixed payments, until the total aggregate cash compensation paid to NuvoGen under the agreement equals $15,000,000. Pursuant to the latest amendment to the agreement, through 2017, we are only required to pay a yearly fixed fee, in quarterly installments, to NuvoGen in the range of $543,750 to $800,000, and may defer any accrued revenue-based payments. Beginning in 2018, we are obligated to pay the greater of $400,000 or 6% of sales, plus amounts, if any, deferred in the 2016 and 2017 periods by which 6% of revenue exceeds the applicable fixed fee plus 5% interest on such deferred amounts until the obligation is repaid in full. We paid our fixed fees for 2015 and the first quarter of 2016 in advance, and our next payment is due in April 2016.
In a transaction related to the foregoing acquisition, NuvoGen also received a non-exclusive, royalty free license under the Acquired Technology pursuant to an agreement dated September 15, 2004. The license is limited to NuvoGen’s own internal service-oriented efforts and activities to accelerate the development of targeted drugs and other pharmaceutical compounds and agents as part of NuvoGen’s grant funded or other basic research and development of drugs intended for the treatment of cancer. Pursuant to the agreement, in the event that NuvoGen produces revenue through the sale of cancer drugs developed through use of the license for entities other than for-profit and other commercial drug-research and development service ventures, NuvoGen will pay us a royalty in the mid-single digit range percentage of such revenue for the quarter. This agreement may be terminated by either party in case of a breach by the other party, and we may terminate the agreement by giving written notice if NuvoGen files for bankruptcy or performs other similar actions.
License Agreement with Merck
In connection with the investment by Merck Capital Ventures LLC in the Company, we granted to Merck Sharpe & Dohme, or Merck, pursuant to an agreement dated February 15, 2011, a non-exclusive, worldwide, royalty-free, non-sublicenseable (except to Affiliates of Merck) license to certain of our quantitative nuclease protection, or qNPA, patent rights solely for Merck’s internal research and development purposes. We may terminate the agreement upon a breach of any material provision of the agreement by Merck, if Merck has not cured such breach within 60 days after receiving written notice from us.
28
Loan and Security Agreement with Oxford Finance, LLC and Silicon Valley Bank
On August 22, 2014, we entered into a Loan and Security Agreement, or the loan agreement, with Oxford Finance LLC, or Oxford, as collateral agent, or in such capacity, Agent, and a lender and Silicon Valley Bank, as a lender, or SVB, and together with Oxford, the Lenders, providing for term loans in an aggregate principal amount of $16.0 million.
Borrowings under the Loan Agreement may consist of up to two separate term loans. We borrowed the initial term loan in the principal amount of $11.0 million, or the Term A Loan, on August 22, 2014. The Term A Loan bears interest at a fixed rate of 8.50% per annum. On or prior to June 30, 2015, the Loan Agreement allowed us to borrow one additional term loan, or the Term B Loan, and together with the Term A Loan, the Term Loans, for up to $5.0 million, subject to the satisfaction of certain borrowing conditions. We received the proceeds, net of a $320,000 original issue discount, with a requirement to pay a final payment of 3.75% of the total amount borrowed.
In August 2015, the Company and its lenders amended the Loan Agreement to extend the availability of the Term B Loan until March 31, 2016. Pursuant to the August 2015 amendment to the Loan Agreement, we will have monthly interest-only payment obligations until April 1, 2016. Following the interest-only payment period, equal monthly payments consisting of principal and interest amortized over the remaining term of the loan through the September 1, 2018 maturity date will be due. The amendment also increased the final fee payment percentage to 4.75%. In February 2016, the Company notified its lenders, pursuant to the requirements of the amendment to the Loan Agreement, of its intention to draw the remaining $5.0 million available under the Term B Loan on or before March 31, 2016. This additional principal will be repaid evenly over the course of 30 months beginning April 1, 2016.
We may prepay all, but not less than all, of the loaned amount plus accrued and unpaid interest thereon through the prepayment date with 15 days’ advance notice to Oxford. In accordance with the August 2015 amendment, we will be obligated to pay a prepayment fee equal to (i) 3% of the principal amount prepaid if the loan is prepaid on or before the first anniversary of the August 2015 amendment, (ii) 2% of the principal amount repaid if the loan is prepaid after the first anniversary but on or prior to the second anniversary of the August 2015 amendment and (iii) 1% of the principal amount repaid if the loan is repaid after the second anniversary of the August 2015 amendment and prior to maturity. We are not entitled to reborrow any amounts of principal once such principal has been repaid.
While any amounts are outstanding under the credit facility, we are subject to a number of affirmative and restrictive covenants, including covenants regarding delivery of financial statements, maintenance of inventory, payment of taxes, maintenance of insurance, dispositions of property, business combinations or acquisitions, incurrence of additional indebtedness and transactions with affiliates, among other customary covenants. We are also restricted from paying cash dividends or making other distributions or payments on our capital stock except for repurchases of stock pursuant to employee stock purchase plans, employee restricted stock agreements, stockholder rights plans, director or consultant stock option plans, or similar plans, provided such repurchases do not exceed $100,000 in the aggregate per fiscal year.
We granted the Agent a security interest in our personal property to secure our obligations under the loan agreement. The security interest does not extend to patents, trademarks and other intellectual property rights (except for rights to payment related to the sale, licensing or disposition of such intellectual property rights) or certain other specified property.
Upon the occurrence of certain events, including but not limited to the failure by us to satisfy our payment obligations under the loan agreement, the breach of certain of our other covenants under the loan agreement, and the occurrence of a material adverse change, the Agent will have the right, among other remedies, to declare all principal and interest immediately due and payable, and will have the right to receive the final payment fee and, if the payment of principal and interest is due prior to maturity, a prepayment fee. The Agent also will have the right, among other remedies, to foreclose upon and/or sell or otherwise liquidate our personal property upon the occurrence of certain events.
As further consideration for access to the loan, we granted each of the Lenders warrants to purchase 1,256,281 shares of the Company’s Series E Preferred Stock at an exercise price of $0.2189 per share, or the Warrants. The Warrants are exercisable until August 22, 2024 and were automatically converted to warrants for common stock upon completion of our IPO in May 2015.
Development and Component Supply Agreement with Illumina, Inc.
In October 2014, we entered into a development and component supply agreement with Illumina for the development and worldwide commercialization by us of up to two complete diagnostic gene expression profiling tests for use with Illumina’s diagnostic instruments, using components supplied by Illumina. We refer to these diagnostic gene expression profiling tests as IVD test kits. The IVD test kits may be used in two discrete testing fields chosen by us, one or both of which may relate to oncology for breast, lung, lymphoma or melanoma tumors, and up to one of which may relate to transplant, chronic obstructive pulmonary disease, or
29
immunology/autoimmunity. We provided notice to Illumina of our first testing selection during the quarter ended March 31, 2015. We must provide notice to Illumina of our second selected field, if any, by October 2016.
Following our selection of the testing field for each IVD test kit, we and Illumina have agreed to negotiate a development plan for the development and regulatory approval of the applicable IVD test kit, under which development and regulatory support will be provided by Illumina. Upon mutual agreement of the first development plan in the fourth quarter of 2015, we paid Illumina a $100,000 fee. We are also required to pay Illumina up to $1.0 million in the aggregate upon achievement of specified regulatory milestones relating to the IVD test kits. In addition, we have agreed to pay Illumina a single digit percentage royalty on net sales of any IVD test kits that we commercialize pursuant to the agreement.
Pursuant to the agreement, we have agreed to obtain our requirements for certain components to be used in the development and/or commercialization of IVD test kits from Illumina. We and Illumina have also agreed to negotiate in good faith to enter into a supplemental supply agreement for the continued purchase and supply of the foregoing components.
Absent earlier termination, the agreement will expire in October 2019 or on the date which the last to expire development plan under the agreement is completed, whichever is earlier. We may terminate the agreement at any time upon 90 days’ written notice and may terminate any development plan under the agreement upon 30 days’ prior written notice. Illumina may terminate the agreement upon 30 days’ prior written notice if we undergo certain changes of control or immediately if we fail to select a testing field for an IVD test kit within 24 months following the date of the agreement. Either party may terminate the agreement upon the other party’s material breach of the agreement that remains uncured for 30 days, or upon the other party’s bankruptcy.
Authorization, Supply and Regulatory Authorization Agreement with Life Technologies Corporation
In March 2016, we entered into an Authorization, Supply and Regulation Authorization Agreement with Life Technologies Corporation (“LTC”), a wholly owned subsidiary of Thermo Fisher Scientific, Inc., for the development and worldwide commercialization by us of up to five RNA-based next generation sequencing panels (“HTG Assays”) for use with LTC’s sequencing instruments and components supplied to end‑users by LTC.
Pursuant to the agreement, we have agreed to obtain our requirements for certain components to be used in the development of HTG Assays from LTC. LTC has agreed to provide support in our efforts to obtain regulatory approval of the HTG Assays. We are required to pay LTC a milestone payment in the mid-six figure dollar range upon certain regulatory achievements for each HTG Assay. In addition, we have agreed to pay LTC a single digit percentage royalty on net sales of HTG Assays that we commercialize pursuant to the agreement.
Absent early termination, the initial term of the agreement will expire in March 2021 and thereafter will automatically renew for additional two year periods for as long as we continue to develop or sell HTG Assays, provided that neither party provides written notice of its election to terminate the agreement at least 60 days prior to expiration of the then-current term. Either party may terminate the agreement (a) upon the other party’s material breach that remains uncured for 30 days, (b) upon the other party’s bankruptcy or (c) upon written notice in the event the party providing notice reasonably determines that continued performance under the agreement may violate any regulatory law, or any other applicable law or regulation or U.S. Food and Drug Administration guidance.
Trade Secrets
We also rely on trade secrets, including unpatented know-how, technology and other proprietary information to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into nondisclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into invention or patent assignment agreements with our employees and consultants that obligate them to assign to us any inventions developed in the course of their work for us. We cannot provide any assurance, however, that we have entered into such agreements with all relevant parties, or that these parties will abide by the terms of these agreements. Despite measures taken to protect our intellectual property, unauthorized parties might copy or commercially exploit aspects of our technology or obtain and use information that we regard as proprietary.
For additional information relating to the risks associated with our intellectual property position see “Risk Factors – Risks Related to our Intellectual Property.”
30
Third-Party Coverage and Reimbursement
Clinical laboratories will acquire our instrumentation through a capital purchase, capital lease or reagent purchasing agreement. These laboratories will offer their customers a menu of testing services using our IVD test kits or their LDTs, which they may develop using components they purchase from us, or a combination of both. Our customers will generate revenue for these testing services by collecting payments from third-party payors, including public and private payors, as well as patient co-payments.
United States
In the United States, our customers will utilize the existing reimbursement framework for testing services:
Tier 1 and Tier 2 Molecular Pathology Procedures
Prior to January 1, 2013, molecular pathology procedures were reimbursed using the 83890-83912 code series, commonly known as “stacking codes”, which were based on the various technical steps performed to produce a test result. The American Medical Association (AMA) created the Tier 1 codes (81200-81383) and Tier 2 codes (81400-81408) to specifically identify the test being performed and as a replacement to stacking codes. The stacking codes were deleted from the clinical laboratory fee schedule used by the Centers for Medicare and Medicaid Services for payment determination in 2013. Private payors, for the most part, have transitioned away from the stacking codes to the Tier 1 and CMS Tier 2 codes. Single analyte tests for mutations and gene rearrangements are described by these Tier 1 and Tier 2 codes; specific examples include 81235 for epidermal growth factor receptor, or EGFR, mutation analysis and 81401 for EML4/ALK rearrangement analysis. Single analyte tests not specifically called out in Tier 1 or Tier 2 codes can be submitted for reimbursement consideration using the miscellaneous code 81479.
Genomic Sequencing Procedures.
The AMA created codes for Genomic Sequencing Procedures (GSPs) and other Molecular Multianalyte Assays as part of its calendar year 2015 update to the clinical laboratory fee schedule. After our submitted request, AMA has modified the GSP codes to clarify the definition to include DNA and RNA. These new codes will be used by our customers to seek reimbursement for multi-analyte test offerings:
|
|
· |
Gene expression classifiers for hematolymphoid neoplasms, such as the determination of activated B-cell like (ABC) or germinal center B-cell like (GCB) subtypes of diffuse large B-cell lymphomas |
|
|
· |
Gene rearrangements in solid tumors and hematolymphoid neoplasms |
|
|
· |
Copy number variations in solid tumors and hematolymphoid neoplasms |
|
|
· |
Mutations in solid tumors and hematolymphoid neoplasms |
Our customers will seek payment for Tier 1 and Tier 2 Molecular Pathology Procedures and GSPs from public and private payors. Claims for Medicare coverage are processed by private Medicare Administrative Contractors, or MACs, such as Novitas and Cahaba, and coverage for specific test codes are specified in Local Coverage Determinations, or LCDs, issued by individual MACs or National Coverage Determinations, or NCDs, which apply to all MACs. Private payors issue their own coverage determinations that are largely reflective of the CMS LCDs and NCDs. HTG closely monitors trends in coverage through interactions with customers, industry associations such as the College of American Pathologists (CAP) and the Association for Molecular Pathology, or AMP, and industry consultants; these trends are key considerations in our product development plans.
Our customers will use our products to offer testing services that provide an analysis of 5-50 genes as well as 51 or more genes. In October 2014, CMS released its preliminary determinations regarding the method for determining 2015 payment rates for new codes under the clinical laboratory fee schedule. CMS recommended that payment rates for GSPs be determined through a process known as gapfill rather than by crosswalking to allow CMS and its contractors to gather information about the manner in which the tests are performed and the resources necessary to provide them, so that ultimately CMS can set an appropriate payment rate for these tests. In the gapfill process, the local MACs determine the appropriate fee schedule amounts in the first year, and CMS calculates a national payment rate based on the median of these local fee schedule amounts in the second year. On September 27, 2015, gapfill pricing for CPTs 81445 and 81450 for the assessment of 5-50 genes in solid and liquid tumors, respectively, were set at $597 and $647 for 2015. CPT 81455 for the assessment of 51 or more genes in solid and liquid tumors has not yet been priced.
We believe that establishment of the aforementioned reimbursement codes specific to genomic sequencing procedures such as our clinical diagnostic tests currently in development is an important factor in expanding access to our products. In addition, coverage and reimbursement of our clinical diagnostic tests by government and private payors is essential to our commercial success. Accordingly, our strategy includes efforts to encourage third-party payors to establish coverage, coding and payment that will
31
facilitate access to our tests as we seek FDA approval for these tests and expand our commercialization efforts in the United States. Our success in these efforts depends in part on the extent to which governmental authorities, private health insurers and other third-party payors provide coverage for and establish adequate reimbursement levels for tests using our technology. Failure by our customers who use our tests to obtain sufficient coverage and reimbursement from healthcare payors or adverse changes in government and private third-party payors’ policies would have a material adverse effect on our business, financial condition, results of operations and future growth prospects.
In addition to uncertainties surrounding coverage policies, there are periodic changes to reimbursement. Third-party payors regularly update reimbursement amounts and also from time to time revise the methodologies used to determine reimbursement amounts. This includes annual updates to payments made under the Clinical Laboratory Fee Schedule, or CLFS, with amounts assigned to the procedure billing codes used to report the specific laboratory services. The CLFS sets the maximum amount payable under Medicare for each billing code. Payment under the CLFS has been limited from year-to-year by Congressional action such as imposition of national limitation amounts and freezes on the otherwise applicable annual consumer price index updates. In February 2012, the Middle Class Tax Relief and Job Creation Act of 2012 was signed into law which, in part, reduced the potential future cost-based increases to the CLFS by 2%. The payment amounts under the CLFS are important not only for Medicare reimbursement, but also because although there is no uniform policy of coverage and reimbursement amongst third-party payors, other third-party payors are often guided by the Medicare CLFS in establishing reimbursement rates. For example, state Medicaid programs are prohibited from paying more than the Medicare fee schedule limit for clinical laboratory services furnished to Medicaid recipients. As a result, in light of the anticipated reduction in the CLFS payment amounts, certain third party payors may also reduce reimbursement amounts.
Beginning January 1, 2016, there will also be major changes to the payment formula under the CLFS. Under the Protecting Access to Medicare Act of 2014, which was signed to law in April 2014, clinical laboratories must report laboratory test payment data for each Medicare-covered clinical diagnostic lab test that it furnishes during a time period to be defined by future regulations. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Beginning in 2017, the Medicare payment rate for each clinical diagnostic lab test will be equal to the weighted median amount for the test from the most recent data collection period. The payment rate will apply to laboratory tests furnished by a hospital laboratory if the test is separately paid under the hospital outpatient prospective payment system.
Outside the United States
Our target markets outside of the United States are Europe and Asia/Pacific.
In Europe, coverage for molecular diagnostic testing is varied. Countries with statutory health insurance (e.g., Germany, France, The Netherlands) tend to be more progressive in technology adoption with favorable reimbursement for molecular diagnostic testing. In countries such as the United Kingdom with tax-based insurance, adoption and reimbursement for molecular diagnostic testing is not uniform and is influenced by local budgets.
In Asia/Pacific, our commercial strategy is to opportunistically partner with distributors in countries with sufficient demand for our products to justify the investment by both parties. Currently we have a distribution agreement with iLife Discoveries in India, where the vast majority of molecular diagnostic testing is paid for out-of-pocket by the patient.
Our diagnostic product menu plans for Europe and Asia/Pacific consist primarily of NGS-based test kits that will satisfy existing or emerging testing demand, and we will be competing on the basis of testing efficiency, quality and price.
Government Regulation – Medical Device Regulations
United States
Our products and operations are subject to extensive and rigorous regulation by the United States Food and Drug Administration and other federal, state, local and foreign authorities. Currently we are limited to marketing our products for research use only, which means that we cannot make any diagnostic or clinical claims. However, we intend to seek regulatory clearances or approvals in the United States and other jurisdictions to market certain assays for diagnostic purposes. The clinical diagnostics under development by HTG are classified as “medical devices” under the United States Food, Drug and Cosmetic Act, or the FDCA. The FDA regulates, among other things, the research, development, testing, manufacturing, approval, labeling, storage, recordkeeping, advertising, promotion and marketing, distribution, post approval monitoring and reporting and import and export of medical devices in the United States to assure the safety and effectiveness of such products for their intended use.
32
Unless an exemption applies, each new or significantly modified medical device we seek to commercially distribute in the United States will require either a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, or approval from the FDA of a PMA application. Both the 510(k) clearance and PMA processes can be expensive, and lengthy, and require payment of significant user fees, unless an exemption is available.
Device Classification
Under the FDCA, medical devices are classified into one of three classes – Class I, Class II or Class III – depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurances with respect to safety and effectiveness.
Class I devices are those for which safety and effectiveness can be reasonably assured by adherence to a set of regulations, referred to as General Controls, which require compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. Some Class I devices, also called Class I reserved devices, also require premarket clearance by the FDA through the 510(k) premarket notification process described below. Most Class I products are exempt from the premarket notification requirements.
Class II devices are those that are subject to the General Controls, as well as Special Controls, which can include performance standards, guidelines and postmarket surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process. Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent,” as defined in the statute, to either:
|
|
· |
a device that was legally marketed prior to May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted, or |
|
|
· |
another commercially available, similar device that was cleared through the 510(k) process. |
To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence.
After a 510(k) notice is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) notification. If it is accepted for filing, the FDA begins a substantive review. By statute, the FDA is required to complete its review of a 510(k) notification within 90 days of receiving the 510(k) notification. As a practical matter, clearance often takes longer, and clearance is never assured. Although many 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including clinical data, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA application is obtained. If the FDA requires us to seek 510(k) clearance or approval of a PMA application for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. In addition, in these circumstances, we may be subject to significant regulatory fines or penalties for failure to submit the requisite PMA application(s). In addition, the FDA is currently evaluating the 510(k) process and may make substantial changes to industry requirements.
The PMA Approval Process
If the FDA determines that the device is not “substantially equivalent” to a predicate device, or if the device is classified into Class III, the device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA approval process, or seek reclassification of the device through the de novo process. A manufacturer can also submit a petition for direct de novo review if
33
the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk.
Class III devices include devices deemed by the FDA to pose the greatest risk such as life-supporting or life-sustaining devices, or implantable devices, in addition to those deemed not substantially equivalent following the 510(k) process. The safety and effectiveness of Class III devices cannot be reasonably assured solely by the General Controls and Special Controls described above. Therefore, these devices are subject to the PMA application process, which is generally more costly and time consuming than the 510(k) process. Through the PMA application process, the applicant must submit data and information demonstrating reasonable assurance of the safety and effectiveness of the device for its intended use to the FDA’s satisfaction. Accordingly, a PMA application typically includes, but is not limited to, extensive technical information regarding device design and development, pre-clinical and clinical trial data, manufacturing information, labeling and financial disclosure information for the clinical investigators in device studies. The PMA application must provide valid scientific evidence that demonstrates to the FDA’s satisfaction reasonable assurance of the safety and effectiveness of the device for its intended use.
In the United States, absent certain limited exceptions, human clinical trials intended to support medical device clearance or approval require an IDE application. Some types of studies deemed to present “non-significant risk” are deemed to have an approved IDE once certain requirements are addressed and IRB approval is obtained. If the device presents a “significant risk” to human health, as defined by the FDA, the sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of subjects. Generally, clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the study protocol and informed consent are approved by appropriate institutional review boards at the clinical trial sites. There can be no assurance that submission of an IDE will result in the ability to commence clinical trials, and although the FDA’s approval of an IDE allows clinical testing to go forward for a specified number of subjects, it does not bind the FDA to accept the results of the trial as sufficient to prove the product’s safety and efficacy, even if the trial meets its intended success criteria.
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA, by statute and by regulation, has 180 days to review a filed PMA application, although the review of an application more often occurs over a significantly longer period of time. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA. The FDA considers a PMA or PMA supplement to have been voluntarily withdrawn if an applicant fails to respond to an FDA request for information (e.g. major deficiency letter) within a total of 360 days. Before approving or denying a PMA, an FDA advisory committee may review the PMA at a public meeting and provide the FDA with the committee’s recommendation on whether the FDA should approve the submission, approve it with specific conditions, or not approve it. Prior to approval of a PMA, the FDA may conduct a bioresearch monitoring inspection of the clinical trial data and clinical trial sites, and a QSR inspection of the manufacturing facility and processes. Overall, the FDA review of a PMA application generally takes between one and three years, but may take significantly longer. The FDA can delay, limit or deny approval of a PMA application for many reasons, including:
|
|
· |
the device may not be shown safe or effective to the FDA’s satisfaction; |
|
|
· |
the data from pre-clinical studies and clinical trials may be insufficient to support approval; |
|
|
· |
the manufacturing process or facilities may not meet applicable requirements; and |
|
|
· |
changes in FDA approval policies or adoption of new regulations may require additional data. |
If the FDA evaluation of a PMA is favorable, the FDA will issue either an approval letter, or an approvable letter, which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval letter authorizing commercial marketing of the device, subject to the conditions of approval and the limitations established in the approval letter. If the FDA’s evaluation of a PMA application or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. The FDA also may determine that additional tests or clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and data is submitted in an amendment to the PMA. The PMA process can be expensive, uncertain and lengthy and a number of devices for which FDA approval has been sought by other companies have never been approved by the FDA for marketing.
New PMA applications or PMA supplements may be required for modification to the manufacturing process, labeling, device specifications, materials or design of a device that has been approved through the PMA process. PMA supplements often require
34
submission of the same type of information as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the approved PMA application and may or may not require as extensive technical or clinical data or the convening of an advisory panel, depending on the nature of the proposed change.
In approving a PMA application, the FDA may also require some form of postmarket studies or postmarket surveillance, whereby the applicant follows certain patient groups for a number of years and makes periodic reports to the FDA on the clinical status of those patients when necessary to protect the public health or to provide additional safety and effectiveness data for the device. FDA may also require postmarket surveillance for certain devices cleared under a 510(k) notification, such as implants or life-supporting or life-sustaining devices used outside a device user facility. The FDA may also approve a PMA application with other post-approval conditions intended to ensure the safety and effectiveness of the device, such as, among other things, restrictions on labeling, promotion, sale, distribution and use.
Post-Approval Requirements
After the FDA permits a device to enter commercial distribution, numerous regulatory requirements apply. These include, but are not limited to:
|
|
· |
the registration and listing regulation, which requires manufacturers to register all manufacturing facilities and list all medical devices placed into commercial distribution; |
|
|
· |
the QSR, which requires manufacturers, including third party manufacturers, to follow elaborate design, testing, production, control, supplier/contractor selection, complaint handling, documentation and other quality assurance procedures during the manufacturing process; |
|
|
· |
labeling regulations and unique device identification requirements; |
|
|
· |
advertising and promotion requirements; |
|
|
· |
restrictions on sale, distribution or use of a device; |
|
|
· |
PMA annual reporting requirements; |
|
|
· |
the FDA’s general prohibition against promoting products for unapproved or “off-label” uses; |
|
|
· |
the Medical Device Reporting, or MDR, regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to reoccur; |
|
|
· |
medical device correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
|
|
· |
recall requirements, including a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death; |
|
|
· |
an order of repair, replacement or refund; |
|
|
· |
device tracking requirements; and |
|
|
· |
postapproval study and postmarket surveillance requirements. |
Our facilities, records and manufacturing processes are subject to periodic unscheduled inspections by the FDA. Failure to comply with the applicable United States medical device regulatory requirements could result in, among other things, warning letters, untitled letters, fines, injunctions, consent decrees, civil penalties, unanticipated expenditures, repairs, replacements, refunds, recalls or seizures of products, operating restrictions, total or partial suspension of production, the FDA’s refusal to issue certificates to foreign governments needed to export products for sale in other countries, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product clearances or approvals and criminal prosecution.
Research Use Only
A research use only, or RUO, product is one that is not intended for use in a clinical investigation or for clinical diagnostic use outside an investigation and must be labeled “For Research Use Only. Not for use in diagnostic procedures.” Products that are intended for research use only and are properly labeled as RUO are exempt from compliance with the FDA requirements discussed above, including the approval or clearance and QSR requirements. A product labeled RUO but intended to be used diagnostically may
35
be viewed by the FDA as adulterated and misbranded under the FDC Act and is subject to FDA enforcement activities. The FDA may consider the totality of the circumstances surrounding distribution and use of an RUO product, including how the product is marketed, when determining its intended use.
European Union
The European Union (EU) also has adopted requirements that affect our products. These requirements include establishing standards that address creating a certified quality system as well as a number of directives that address specific product areas. The most significant of these directives is the In Vitro Diagnostic Medical Device Directive (“IVDD”), which includes:
|
|
· |
Essential Requirements. The IVDD specifies “essential requirements” that all medical devices must meet. The requirements are similar to those adopted by the FDA relating to quality systems and product labeling. |
|
|
· |
Conformity Assessment. Unlike United States regulations, which require virtually all devices to undergo some level of premarket review by the FDA, the IVDD currently allows manufacturers to bring many devices to market using a process in which the manufacturer certifies that the device conforms to the essential requirements for that device. A small number of products must go through a more formal pre-market review process. Devices that comply with the requirements of a relevant directive will be entitled to bear the CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be marketed throughout the EU and European Economic Area. |
|
|
· |
Vigilance. The IVDD also specifies requirements for post market reporting similar to those adopted by the FDA. |
Other International
A number of other countries, including Australia, Canada, China and Japan, have adopted or are in the process of adopting standards for medical devices sold in those countries. Many of these standards are loosely patterned after those adopted by the European Union, but with elements unique to each country. Although there is a trend towards harmonization of quality system standards, regulations in each country may vary substantially, which can affect timelines of introduction. We routinely monitor these developments and address compliance with the various country requirements as new standards are adopted.
Government Regulation – Fraud and Abuse and Other Healthcare Regulation
We may be subject to various federal and state healthcare laws, including, but not limited to, anti-kickback laws. Penalties for violations of these healthcare laws include, but are not limited to, criminal, civil and/or administrative penalties, damages, fines, disgorgement, individual imprisonment, possible exclusion from Medicare, Medicaid and other federal healthcare programs, and the curtailment or restructuring of operations.
Anti-Kickback Statute
The federal Anti-Kickback Statute prohibits persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service, or for the purchasing, leasing, ordering, or arranging for or recommending, any good, facility, service or item for which payment may be made in whole or in part under federal healthcare programs, such as the Medicare and Medicaid programs. The federal Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. The term “remuneration” expressly includes kickbacks, bribes, or rebates and also has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payments, ownership interests and providing anything at less than its fair market value.
There are a number of statutory exceptions and regulatory safe harbors protecting certain business arrangements from prosecution under the federal Anti-Kickback Statute. These statutory exceptions and safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they may not be prosecuted under the federal Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more applicable statutory exceptions or safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy all requirements of an applicable safe harbor may result in increased scrutiny by government enforcement authorities and will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Additionally, the intent standard under the federal Anti-Kickback Statute was amended under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the ACA, to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to
36
have committed a violation. The ACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act which is discussed below.
Federal Civil False Claims Act
The federal civil False Claims Act prohibits, among other things, persons or entities from knowingly presenting or causing to be presented a false or fraudulent claim to, or the knowing use of false statements to obtain payment from or approval by, the federal government. Suits filed under the federal civil False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government. These individuals, sometimes known as “relators” or, more commonly, as “whistleblowers”, may share in any amounts paid by the entity to the government in fines or settlement. The number of filings of qui tam actions has increased significantly in recent years, causing more healthcare companies to have to defend a case brought under the federal civil False Claim Act. If an entity is determined to have violated the federal civil False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. Many comparable state laws are broader in scope and apply to all payors, and therefore, are not limited to only those claims submitted to the federal government.
Federal Physician Self-Referral Prohibition
We are also subject to the federal physician self-referral prohibitions, commonly known as the Stark Law, which prohibits, among other things, physicians who have a financial relationship, including an investment, ownership or compensation relationship with an entity, from referring Medicare and Medicaid patients to the entity for designated health services, which include clinical laboratory services, unless an exception applies. Similarly, entities may not bill Medicare, Medicaid or any other party for services furnished pursuant to a prohibited referral. Many states have their own self-referral laws as well, which in some cases apply to all third-party payors, not just Medicare and Medicaid.
Federal Civil Monetary Penalties Statute
The federal Civil Monetary Penalties Statute, among other things, imposes fines against any person or entity who is determined to have presented, or caused to be presented, claims to a federal healthcare program that the person knows, or should know, is for an item or service that was not provided as claimed or is false or fraudulent.
Health Insurance Portability and Accountability Act of 1996
The federal Health Insurance Portability and Accountability Act, or HIPAA, created several additional federal crimes, including healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
In addition, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations established uniform standards for certain covered entities, which are certain healthcare providers, health plans and healthcare clearinghouses, as well as their business associates, governing the conduct of specified electronic healthcare transactions and protecting the security and privacy of protected health information. Among other things, HITECH also created four new tiers of civil monetary penalties and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
The Federal Physician Payments Sunshine Act
The federal Physician Payment Sunshine Act requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with certain exceptions, to report annually to CMS, information related to “payments or other transfers of value” provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by physicians, as defined above, and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1.0 million per year for “knowing failures.”
37
Many states have also adopted laws similar to each of the above federal laws, such as anti-kickback and false claims laws, which may be broader in scope and apply to items or services reimbursed by any third-party payor, including commercial insurers, as well as laws that restrict our marketing activities with health care professionals and entities, and require us to track and report payments and other transfers of value, including consulting fees, provided to healthcare professionals and entities. Some states mandate implementation of compliance programs to ensure compliance with these laws. We also are subject to foreign fraud and abuse laws, which vary by country. We may be subject to certain state and foreign laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Healthcare Reform
In March 2010, President Obama enacted the ACA, which is substantially changing healthcare financing and delivery by both governmental and private insurers, and is significantly impacting the medical device industry. The ACA’s provisions of importance to our business include, but are not limited to, a deductible 2.3% excise tax on any entity that manufactures or imports medical devices offered for sale in the U.S., with limited exceptions, which became effective January 1, 2013. However, the Consolidated Appropriations Act of 2016, signed into law in December 2015, includes a two year moratorium on the medical device excise tax that applies between January 1, 2016, and December 31, 2017. Absent further legislative action, the tax will be automatically reinstated for medical device sales beginning January 1, 2018. Since its enactment in 2010, there have been judicial and Congressional challenges to certain aspects of the ACA, and we expect additional challenges and amendments in the future.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013, and, following passage of the Bipartisan Budget Act of 2015, will stay in effect through 2025 unless Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
The full impact of the ACA, as well as other laws and reform measures that may be proposed and adopted in the future, remains uncertain, but may continue the downward pressure on medical device pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs, which could have a material adverse effect on our business operations.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Employees
As of December 31, 2015, we had 87 employees, of which 10 are employed in administration, 15 in manufacturing and operations, 22 in research and development, five in regulatory and quality affairs, and 35 in sales and marketing. We had 1 employee in Europe as of December 31, 2015, all others were in the United States. We believe that our success will depend, in part, on our ability to attract and retain qualified personnel. We have never experienced a work stoppage due to labor difficulties and believe that our relations with our employees are good. None of our U.S. employees are represented by labor unions. Collective bargaining is established by law in France and we and our European employee, who resides in France, have agreed to the terms of a National Collective Agreement applicable to our industry.
Corporate Information
We were originally incorporated in Arizona in October 1997 as “High Throughput Genomics, Inc.” In December 2000, we reincorporated in Delaware as “HTG, Inc.” and in March 2011 we changed our name to “HTG Molecular Diagnostics, Inc.” Our
38
principal executive offices are located at 3430 E. Global Loop, Tucson, AZ 85706, and our telephone number is (877) 289-2615. Our corporate website address is www.htgmolecular.com. Information contained on or accessible through our website is not a part of this report, and the inclusion of our website address in this report is an inactive textual reference only.
This report contains references to our trademarks, including HTG Edge and HTG EdgeSeq, and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this report, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our initial public offering in May 2015, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeded $700.0 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. We refer to the Jumpstart Our Business Startups Act of 2012 in this Annual Report on Form 10-K as the “JOBS Act,” and references to “emerging growth company” have the meaning associated with it in the JOBS Act.
An investment in shares of our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this report, and in our other public filings. The occurrence of any of the following risks could harm our business, financial condition, results of operations and/or growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. You should consider all of the risk factors described when evaluating our business.
Risks Related to our Business and Strategy
We have incurred losses since our inception and expect to incur losses for the foreseeable future. We cannot be certain that we will achieve or sustain profitability.
We have incurred losses since our inception and expect to incur losses in the future. We incurred net losses of $21.4 million and $14.0 million during the years ended December 31, 2015 and 2014, respectively. As of December 31, 2015, we had an accumulated deficit of $89.6 million. We expect that our losses will continue for the foreseeable future as we will be required to invest significant additional funds toward expansion of our product development and manufacturing facilities, development of our next generation instrument platforms, including our low volume HTG EdgeSeq system (Project JANUS), development of our new HTG EdgeSeq assay panels, including our initial IVD assay, and the commercialization of our HTG Edge and HTG EdgeSeq systems and our proprietary consumables. We also expect that our selling, general and administrative expenses will continue to increase due to the additional costs associated with market development activities, expanding our staff to sell and support our products, and the increased administrative costs associated with being a public company. Our ability to achieve or, if achieved, sustain profitability is based on numerous factors, many of which are beyond our control, including the market acceptance of our products, competitive product development and our market penetration and margins. We may never be able to generate sufficient revenue to achieve or, if achieved, sustain profitability.
We will need to raise additional capital to fund our operations. If we are unsuccessful in attracting new capital, we may not be able to continue operations or may be forced to sell assets to do so. Alternatively, capital may not be available to us on favorable terms, or at all. If available, financing terms may lead to significant dilution to our stockholders’ equity.
We are not profitable and have had negative cash flow from operations. To date, to fund our operations and develop and commercialize our products, we have relied primarily on equity and debt financings and revenue generated from the sale of our HTG Edge and HTG EdgeSeq systems, the sale of our proprietary consumables, and related services. We currently anticipate that our cash, cash equivalents and short-term investments in available-for-sale securities, together with funds that may be borrowed under our growth term loan, will be sufficient to enable us to fund our operations for at least the next 12 months. We will likely need to obtain additional funds to finance our operations beyond that point, as we currently project the need for additional funding around the midpoint of the second quarter 2017. In addition, our estimates of the amount of cash necessary to fund our operations and development and commercialization activities may prove to be wrong, and we could spend our available financial resources much faster than we currently expect. Additional capital may not be available, at such times or in amounts as needed by us. Even if capital is available, it might be available only on unfavorable terms. Any additional equity or convertible debt financing into which we enter could be dilutive to our
39
existing stockholders. Any future debt financing into which we enter may impose covenants upon us that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. If access to sufficient capital is not available as and when needed, our business will be materially impaired and we may be required to cease operations, curtail one or more product development or commercialization programs, or we may be required to significantly reduce expenses, sell assets, seek a merger or joint venture partner, file for protection from creditors or liquidate all our assets. Any of these factors could harm our operating results.
Payments under the instruments governing our indebtedness may reduce our working capital. In addition, a default under our term loan agreement could cause a material adverse effect on our financial position.
In August 2014, we obtained a $16.0 million term loan from Oxford Finance LLC and Silicon Valley Bank, which we collectively refer to as the lenders. Under the terms of the loan agreement, the lenders initially provided us with a term loan of $11.0 million, with an additional $5.0 million available to us until March 31, 2016, subject to the non-occurrence of a prior event of default. We have given notice to the lenders as of the date of this report of our intent to draw on this additional $5.0 million funding in March 2016. The loan, which is secured by a lien covering substantially all of our assets, excluding patents, trademarks and other intellectual property rights (except for rights to payment related to the sale, licensing or disposition of such intellectual property rights) and certain other specified property, requires us to pay interest-only payments through March 31, 2016, and principal and interest payments thereafter through September 2018. Payments under the loan could result in a significant reduction of our working capital.
Pursuant to the terms of the asset purchase agreement, as amended, with NuvoGen Research, LLC, or NuvoGen, pursuant to which we acquired certain intellectual property, we agreed to pay NuvoGen the greater of $400,000 or 6% of our yearly revenue until the total aggregate cash compensation paid to NuvoGen under the agreement equals $15.0 million. To date, we have paid NuvoGen approximately $5.8 million. We paid our fixed fees for 2015 and the first quarter of 2016 in advance, and our next payment is due in April 2016. For the remainder of 2016 and 2017, we are required to pay a yearly fixed fee, in quarterly installments, to NuvoGen in the range of $543,750 to $800,000, and may defer the accrued revenue-based payments. Beginning in 2018, we are again obligated to pay the greater of $400,000 or 6% of our annual revenue plus amounts, if any, deferred in the 2016 and 2017 periods by which 6% of revenue exceeds the applicable fixed fee plus 5% interest on any such deferred amounts until the obligation is repaid in full. Payments to NuvoGen could result in a significant reduction in our working capital.
Our term loan agreement requires us, and any debt arrangements we may enter into in the future may require us, to comply with various covenants that limit our ability to, among other things:
|
|
· |
dispose of assets; |
|
|
· |
complete mergers or acquisitions; |
|
|
· |
incur indebtedness; |
|
|
· |
encumber assets; |
|
|
· |
pay dividends or make other distributions to holders of our capital stock; |
|
|
· |
make specified investments; |
|
|
· |
change certain key management personnel; and |
|
|
· |
engage in transactions with our affiliates. |
These restrictions could inhibit our ability to pursue our business strategies. If we default under our obligations under the loan agreement, the lenders could proceed against the collateral granted to them to secure our indebtedness or declare all obligation under the loan agreement to be due and payable. In certain circumstances, procedures by the lenders could result in a loss by us of all of our equipment and inventory, which are included in the collateral granted to the lenders. If any indebtedness under the loan agreement were to be accelerated, there can be no assurance that our assets would be sufficient to repay in full that indebtedness. In addition, upon any distribution of assets pursuant to any liquidation, insolvency, dissolution, reorganization or similar proceeding, the holders of secured indebtedness will be entitled to receive payment in full from the proceeds of the collateral securing our secured indebtedness before the holders of other indebtedness or our common stock will be entitled to receive any distribution with respect thereto.
40
We may incur additional indebtedness in the future. The debt instruments governing such indebtedness may contain provisions that are as, or more, restrictive than the provisions governing our existing indebtedness under the loan agreement. If we are unable to repay, refinance or restructure our indebtedness when payment is due, the lenders could proceed against the collateral granted to them to secure such indebtedness or force us into bankruptcy or liquidation.
Our HTG EdgeSeq product portfolio requires the use of next generation sequencing (“NGS”) instrumentation and reagents and could be adversely affected by actions of third party NGS product manufacturers over whom we have no control.
A key element of our strategy is to establish our HTG EdgeSeq system as the best sample and library preparation method for clinical applications of next generation sequencers. We depend at least in part on the availability of NGS instrumentation and reagents, and the ability of our HTG EdgeSeq products to operate seamlessly with NGS instrumentation. Any significant interruption or delay in the ability of our HTG EdgeSeq solution or related panels to operate on NGS instrumentation could reduce demand for our products and result in a loss of customers.
A key element of our strategy is to establish our HTG EdgeSeq system as the best front-end platform for clinical sequencing. Our reputation, and our ability to continue to establish or develop our technology for clinical applications of next generation sequencers, are dependent upon the availability of NGS instrumentation and the reliable performance of our products with NGS instrumentation. We are not able to control the providers of NGS instrumentation, which increases our vulnerability to interoperability problems with the products that they provide. For example, providers of NGS instruments may discontinue existing products, or introduce new NGS instrumentation products with little or no notice to us. This may cause some of our products not to be operable with one or more NGS instruments or may adversely affect regulatory approvals of our future IVD HTG EdgeSeq products, potentially for extended periods of time. Any interruption in the ability of our products to operate on NGS instruments could harm our reputation or decrease market acceptance of our products, and our business, financial condition and operating results may be materially and adversely affected. We also could experience additional expense in developing new products or changes to existing products to meet developments in NGS instrumentation, and our business, financial condition and operating results may be materially and adversely affected.
Current medical device regulation in the U.S. and other jurisdictions requires manufacturers of IVD molecular profiling tests that use NGS detection, referred to as NGS IVD tests, to include in regulatory submissions, technical information about the NGS products that are required for performance of, but are not supplied with, the NGS IVD test. These regulatory agencies also require that the NGS instrumentation have “locked” software for the detection of the NGS IVD test results. Thus, to obtain regulatory approval for NGS IVD tests, manufacturers like us, currently must have arrangements with NGS product manufacturers to gain access to technical information and NGS instrument software. We currently have agreements with the two most significant product manufacturers that we believe grant us the rights that we need from those manufacturers to develop, manufacture and sell up to seven future HTG EdgeSeq NGS IVD tests in specified fields, subject to, among other things, the manufacturers’ rights to terminate such agreements and discontinue products or implement product design changes that could adversely affect our HTG EdgeSeq NGS IVD tests. In addition, if regulatory agencies do not change their requirements for NGS IVD test approval or clearance and the NGS instrument manufacturers close their systems to third party NGS IVD test development (in general or with specific NGS IVD test manufacturers) and we are not able to maintain or enforce our agreements with such manufacturers, we may not be able to meet our commercial goals and our business, financial condition and operating results may be materially and adversely affected.
The development of future products is dependent on new methods and/or technologies that we may not be successful in developing.
We are planning to expand our product offerings in the fields of detecting RNA fusions and other gene rearrangements and DNA mutations, such as expressed DNA mutations, and copy number variations, or CNVs. We believe we have successfully demonstrated proof of concept that our technology is able to detect these fusions and expressed DNA mutations, but to date our work in this area has only been on a very small scale or may not have the specificity required for certain applications. We cannot guarantee that we will be able to successfully develop these applications on a commercial scale. If we are unsuccessful at developing additional applications involving RNA fusions, DNA mutations or CNVs, we may be limited in the breadth of additional products we can offer in the future, which could impact our future revenues and profits.
We are currently developing a new version of our HTG EdgeSeq system, which we expect to launch in 2017, that will target the lower-volume throughput lab market. This program, which we refer to as “Project JANUS” is expected to increase our addressable market by enabling efficient molecular profiling of smaller quantity batches of samples. This program involves the development of new chemistry that is not currently compatible with our existing HTG Edge or HTG EdgeSeq platforms. If we are unable to successfully develop this new version of our HTG EdgeSeq system and on the timeframe we anticipate, our addressable market will be limited, which could harm our business, results of operations and financial condition.
41
We may not be able to develop new products or enhance the capabilities of our systems to keep pace with rapidly changing technology and customer requirements, which could have a material adverse effect on our business and operating results.
Our success depends on our ability to develop new products and applications for our technology in existing and new markets, while improving the performance and cost-effectiveness of our systems. New technologies, techniques or products could emerge that might offer better combinations of price and performance than our current or future products and systems. Existing or future markets for our products, including gene expression analysis, liquid-based specimen analysis (e.g., plasma, blood and urine), single-cell analysis and copy number variation, as well as potential markets for our diagnostic product candidates, are characterized by rapid technological change and innovation. It is critical to our success that we anticipate changes in technology and customer requirements and successfully introduce new, enhanced and competitive technologies to meet our customers’ and prospective customers’ needs on a timely and cost-effective basis. At the same time, however, we must carefully manage the introduction of new products. If customers believe that such products will offer enhanced features or be sold for a more attractive price, they may delay purchases until such products are available. We may also have excess or obsolete inventory of older products as we transition to new products and our experience in managing product transitions is very limited. If we do not successfully innovate and introduce new technology into our product lines or effectively manage the transitions to new product offerings, our revenues and results of operations will be adversely impacted.
Competitors may respond more quickly and effectively than we do to new or changing opportunities, technologies, standards or customer requirements. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and as new companies enter the market with new technologies.
If we do not successfully manage the development and launch of new products, our financial results could be adversely affected.
We face risks associated with launching new products and with undertaking to comply with regulatory requirements for certain of our products. If we encounter development or manufacturing challenges or discover errors during our product development cycle, the product launch date(s) may be delayed. The expenses or losses associated with unsuccessful product development or launch activities or lack of market acceptance of our new products could adversely affect our business or financial condition.
If we do not achieve, sustain or successfully manage our anticipated growth, our business and growth prospects will be harmed.
Our current personnel, systems and facilities may not be adequate to support our business plan and future growth. Our need to effectively manage our operations, growth and various projects requires that we, among other things:
|
|
· |
continue to improve our operational, financial, management and regulatory compliance controls and reporting systems and procedures; |
|
|
· |
attract and retain sufficient numbers of talented employees; |
|
|
· |
manage our commercialization activities effectively and in a cost-effective manner; |
|
|
· |
manage our relationship with third parties related to the commercialization of our products; and |
|
|
· |
manage our development efforts effectively while carrying out our contractual obligations to contractors and other third parties. |
Moreover, growth will place significant strains on our management and our operational and financial systems and processes. For example, expanded market penetration of our HTG Edge and HTG EdgeSeq systems and related proprietary panels, and future development and approval of diagnostic products, are key elements of our growth strategy that will require us to hire and retain additional sales and marketing, regulatory, manufacturing and quality assurance personnel. If we do not successfully forecast the timing and cost of the development of new panels and diagnostic products, the regulatory clearance or approval for product marketing of any future diagnostic products or the demand and commercialization costs of such products, or manage our anticipated expenses accordingly, our operating results will be harmed.
Our future success is dependent upon our ability to expand our customer base and introduce new applications.
Our current customer base is primarily composed of biopharmaceutical companies, academic institutions and molecular labs that perform analyses using our HTG Edge or HTG EdgeSeq systems and consumables for research use only, which means that they may not be used for clinical diagnostic purposes. Our success will depend, in part, upon our ability to increase our market penetration among these customers and to expand our market by developing and marketing new clinical diagnostic tests and research-use-only applications, and to introduce diagnostic products into clinical laboratories after obtaining the requisite regulatory clearances or approvals. We may not be able to successfully complete development of or commercialize any of our planned future tests and
42
applications. To achieve these goals, we will need to conduct substantial research and development, conduct clinical validation studies, expend significant funds, expand and scale-up our research and development and manufacturing processes and facilities, expand and train our sales force; and seek and obtain regulatory clearance or approvals of our new tests and applications, as required by applicable regulations. Additionally, we must demonstrate to laboratory directors, physicians and third-party payors that any future diagnostic products are effective in obtaining clinically relevant information that can inform treatment decisions, and that our HTG Edge and HTG EdgeSeq systems and related panels can enable an equivalent or superior approach than other available technology. Furthermore, we expect that increasing the installed base of our HTG Edge and HTG EdgeSeq systems will increase demand for our relatively high margin panels. If we are not able to successfully increase our installed base of the HTG Edge and HTG EdgeSeq systems, sales of our panels and our margins may not meet expectations. Attracting new customers and introducing new panels requires substantial time and expense. Any failure to expand our existing customer base, or launch new panels or diagnostic products, would adversely affect our ability to improve our operating results.
Our financial results may vary significantly from quarter to quarter or may fall below the expectations of investors or securities analysts, each of which may adversely affect our stock price.
Investors should consider our business and prospects in light of the risks and difficulties we expect to encounter in the new, uncertain and rapidly evolving markets in which we compete. Because these markets are new and evolving, predicting their future growth and size is difficult. We expect that our visibility into future sales of our products, including volumes, prices and product mix between instruments and panels, will continue to be limited and could result in unexpected fluctuations in our quarterly and annual operating results.
Numerous other factors, many of which are outside our control, may cause or contribute to significant fluctuations in our quarterly and annual operating results. For example, two customers accounted for 45% of our revenue for the year ended December 31, 2015. If orders from these customers were reduced or discontinued, our revenue in future periods may materially decrease. Fluctuations in our operating results may make financial planning and forecasting difficult. In addition, these fluctuations may result in unanticipated decreases in our available cash, which could negatively affect our business and prospects. Factors that may contribute to fluctuations in our operating results include many of the risks described in this section. In addition, one or more of such factors may cause our revenue or operating expenses in one period to be disproportionately higher or lower relative to the others. Our products involve a significant capital commitment from our customers and accordingly involve a lengthy sales cycle. We may expend significant effort in attempting to make a particular sale, which may be deferred by the customer or never occur. Accordingly, comparing our operating results on a period-to-period basis may not be meaningful, and investors should not rely on our past results as an indication of our future performance. If such fluctuations occur or if our operating results deviate from our expectations or the expectations of investors or securities analysts, our stock price may be adversely affected.
Our sales cycle is lengthy and variable, which makes it difficult for us to forecast revenue and other operating results.
Our sales process involves numerous interactions with multiple individuals within any given organization, and often includes in-depth analysis by potential customers of our products (where in some instances we will provide a demonstration unit for their use and evaluation), performance of proof-of-principle studies, preparation of extensive documentation and a lengthy review process. As a result of these factors, the capital investment required in purchasing our instruments, and the budget cycles of our customers, the time from initial contact with a customer to our receipt of a purchase order can vary significantly and be up to 12 months or longer. Given the length and uncertainty of our sales cycle, we have in the past experienced, and likely will in the future experience, fluctuations in our instrument sales on a period-to-period basis. In addition, any failure to meet customer expectations could result in customers choosing to retain their existing systems or to purchase systems other than ours.
If the utility of our HTG Edge and HTG EdgeSeq systems, proprietary profiling panels and solutions in development is not supported by studies published in peer-reviewed medical publications, the rate of adoption of our current and future solutions and the rate of reimbursement of our future products by third-party payors may be negatively affected.
We anticipate that we will need to maintain a continuing presence in peer-reviewed publications to promote adoption of our solutions by biopharmaceutical companies, academic institutions and molecular labs and to promote favorable coverage and reimbursement decisions. We believe that peer-reviewed journal articles that provide evidence of the utility of our current and future solutions or the technology underlying the HTG Edge and HTG EdgeSeq platforms and future solutions are important to our commercial success. It is critical to the success of our sales efforts that we educate a sufficient number of clinicians and administrators about our HTG Edge and HTG EdgeSeq systems, our current panels and our future solutions, and demonstrate the research and clinical benefits of these solutions. Our customers may not adopt our current and future solutions, and third-party payors may not cover or adequately reimburse our future products, unless they determine, based on published peer-reviewed journal articles and the experience of other researchers and clinicians, that our system and related applications provide accurate, reliable, useful and cost-effective information. Peer-reviewed publications regarding our platforms and solutions may be limited by many factors, including
43
delays in the completion of, poor design of, or lack of compelling data from studies that would be the subject of the article. If our current and future solutions or the technology underlying our HTG Edge and HTG EdgeSeq platforms, our current tests and assays or our future solutions do not receive sufficient favorable exposure in peer-reviewed publications, the rate of research and clinician adoption and positive coverage and reimbursement decisions could be negatively affected.
We provide our HTG Edge and HTG EdgeSeq systems and profiling panels free of charge or through other arrangements to customers or key opinion leaders through evaluation agreements or reagent rental programs, and these programs may not be successful in generating recurring revenue from sales of our systems and proprietary panels.
We sell our HTG Edge and HTG EdgeSeq systems and profiling panels under different arrangements in order to expand our installed base and facilitate the adoption of our platform.
In some instances we provide equipment free of charge under evaluation agreements for a limited period of time to permit the user to evaluate the system for their purposes in anticipation of a decision to purchase the system. We retain title to the equipment under such arrangements unless a decision to purchase is made, and in most cases, require evaluation customers to purchase a minimum quantity of consumables during the evaluation period.
When we place a system under a reagent rental agreement, we install equipment in the customer’s facility without a fee and the customer agrees to purchase consumable products at a stated priced over the term of the agreement. While some of these agreements did not historically contain a minimum purchase requirement, we have included a minimum purchase requirement in all reagent rental agreements in 2015, and will continue to do so in the future. We retain title to the equipment and such title is transferred to the customer at no additional charge at the conclusion of the initial arrangement. The cost of the instrument under the agreement is expected to be recovered in the fees charged for consumables, to the extent sold, over the term of the agreement.
Other arrangements might include a collaboration agreement whereby an academic or a commercial collaborator agrees to provide samples free of charge in exchange for the use of a HTG Edge and/or HTG EdgeSeq system at no cost in furtherance of a research or clinical project.
Any of the foregoing arrangements could result in lost revenues and profit and potentially harm our long term goal of achieving profitable operations. In addition, despite the fact we require customers who receive systems we continue to own to carry insurance sufficient to protect us against any equipment losses, we cannot guarantee that they will maintain such coverage, which may expose us to a loss of the value of the equipment in the event of any loss or damage.
There are instances where we provide our systems to key opinion leaders free of charge, to gather data and publish the results of their research to assist our marketing efforts. We have no control over some of the work being performed by these key opinion leaders, or whether the results will be satisfactory. It is possible that the key opinion leader may generate data that is unsatisfactory and could potentially harm our marketing efforts. In addition, customers may from time to time create negative publicity about their experience with our systems, which could harm our reputation and negatively affect market perception and adoption of our platform.
Placing our HTG Edge and HTG EdgeSeq systems under evaluation agreements, under reagent rental agreements or with our key opinion leaders without receiving payment for the instruments could require substantial additional working capital to provide additional units for sale to our customers.
A significant amount of our inventory consists of instruments held by prospective customers who are evaluating our products and may not be converted to revenue on the timeframe that we anticipate or at all.
As of December 31, 2015, approximately $630,000 of our inventory consisted of HTG Edge or HTG EdgeSeq instruments held by customers who are evaluating and testing our products, including our HTG EdgeSeq products. If a material number of these prospective customers do not adopt our products within the time periods that we estimate, or at all, then we will not be able to convert the inventory held by these customers into revenues. If we are unable to sell this inventory to other customers or if it becomes obsolete as we introduce and expand the customer base for our dedicated HTG EdgeSeq system, which became available in the third quarter of 2015, we may be required to write off a significant portion of this inventory.
Our strategy of developing companion diagnostic products may require large investments in working capital and may not generate any revenues.
A key component of our strategy is the development of companion diagnostic products designed to determine the appropriate patient population for administration of a particular medication, to more successfully treat a variety of illnesses. Successfully developing a companion diagnostic product depends both on regulatory approval for administration of the therapeutic, as well as
44
regulatory approval of the diagnostic product. We may be successful in developing products that would be useful as companion diagnostic products, and potentially receive regulatory approval for such products, however the biopharmaceutical companies that develop the corresponding therapeutics may ultimately be unsuccessful in obtaining regulatory approval for any such therapeutic, or, even if successful, select a competing technology to use in their regulatory submission instead of ours. The development of companion diagnostic products requires a significant investment of working capital which may not result in any future income. This could require us to raise additional funds which could dilute our current investors, or could impact our ability to continue our operations in the future.
Our current business depends on levels of research and development spending by academic and governmental research institutions and biopharmaceutical companies, a reduction in which could limit demand for our products and adversely affect our business and operating results.
Our revenue will be derived initially from sales of our HTG Edge and HTG EdgeSeq systems, proprietary panels, and the development of custom panels for biopharmaceutical companies, academic institutions and molecular labs worldwide for research applications. The demand for our products will depend in part upon the research and development budgets of these customers, which are impacted by factors beyond our control, such as:
|
|
· |
changes in government programs that provide funding to research institutions and companies; |
|
|
· |
macroeconomic conditions and the political climate; |
|
|
· |
changes in the regulatory environment; |
|
|
· |
differences in budgetary cycles; |
|
|
· |
market-driven pressures to consolidate operations and reduce costs; and |
|
|
· |
market acceptance of relatively new technologies, such as ours. |
We believe that any uncertainty regarding the availability of research funding may adversely affect our operating results and may adversely affect sales to customers or potential customers that rely on government funding. In addition, academic, governmental and other research institutions that fund research and development activities may be subject to stringent budgetary constraints that could result in spending reductions, reduced allocations or budget cutbacks, which could jeopardize the ability of these customers to purchase our products. Our operating results may fluctuate substantially due to reductions and delays in research and development expenditures by these customers. Any decrease in our customers’ budgets or expenditures, or in the size, scope or frequency of capital or operating expenditures, could materially and adversely affect our business, operating results and financial condition.
We have limited experience in marketing and selling our products, and if we are unable to successfully commercialize our products, our business may be adversely affected.
We have limited experience marketing and selling our products. Our HTG Edge system was introduced for sale in the life sciences research market in the third quarter of 2013. Our HTG EdgeSeq chemistry was introduced for sale in the life sciences research market in the third quarter of 2014. Our dedicated HTG EdgeSeq system was introduced for sale in the life sciences research market in the fourth quarter of 2015. We currently market our products through our own sales force in the United States and Europe, through a third party contract sales team in Europe and a distributor in parts of Asia. In the future, we intend to expand our sales and support team in the United States, continue to build a direct sales and support team in Europe and establish additional distributor and/or third party contract sales team relationships in other parts of the world. However, we may not be able to market and sell our products effectively. Our sales of life science research products and potential future diagnostic products will depend in large part on our ability to successfully increase the scope of our marketing efforts and establish and maintain a sales force commensurate with our then applicable markets. Because we have limited experience in marketing and selling our products in the life science research market and no experience in marketing and selling our products in the diagnostic market, our ability to forecast demand, the infrastructure required to support such demand and the sales cycle to customers is unproven. If we do not build an efficient and effective sales force and distributor relationships targeting these markets, our business and operating results will be adversely affected.
If we do not obtain regulatory clearance or approval to market our products for diagnostic purposes, we will be limited to marketing our products for research use only. In addition, if regulatory limitations are placed on our diagnostic products our business and growth will be harmed.
Currently we are limited to marketing our HTG Edge and HTG EdgeSeq systems and proprietary profiling panels for research use only, which means that we cannot make any diagnostic or clinical claims. We intend to seek regulatory clearances or approvals in the United States and other jurisdictions to market certain panels for diagnostic purposes; however, we may not be successful in doing
45
so. The FDA regulates diagnostic kits sold and distributed through interstate commerce in the United States as medical devices. Unless an exemption applies, generally, before a new medical device may be sold or distributed in the United States, or may be marketed for a new use in the United States, the medical device must receive either FDA clearance of a 510(k) pre-market notification or pre-market approval. As a result, before we can market or distribute our profiling panels, including our mRNA and miRNA assays, in the United States as in vitro diagnostics, or IVD, kits for use by clinical testing laboratories, we must first obtain pre-market clearance or pre-market approval from the FDA. We have not yet applied for clearance or approval from the FDA for any of our solutions, and need to complete additional clinical validations before we submit an application, which can be a lengthy process. We are working collaboratively with multiple biopharmaceutical companies to clinically validate our HTG EdgeSeq Diffuse Large B- Cell Lymphoma Cell of Origin Assay which, in addition to classifying DLBCL as either activated B-cell (ABC) or germinal-center B-cell (GCB) subtype, includes additional drug-linked gene targets such as PD-1, PD-L1 and CD19. We are also developing an HTG EdgeSeq panel to detect certain gene fusions in lung cancer. With respect to the DLBCL assay, we plan to submit for European CE/IVD clearance in 2016 and U.S. regulatory clearances at some future time if and when our work with the biopharmaceutical companies has a positive outcome. With respect to the gene fusion panel for lung cancer, we plan to submit for U.S. and European regulatory approvals in mid-2016. We cannot provide any assurances that we will meet the regulatory clearance or approval timelines for either product. Further, even if we complete the requisite clinical validations and submit an application, we may not receive FDA clearance or approval for the commercial use of our tests on a timely basis, or at all. If we are unable to obtain regulatory clearance or approval, or if clinical diagnostic laboratories do not accept our cleared or approved tests, our ability to grow our business could be compromised.
Similarly, foreign countries have either implemented or are in the process of implementing increased regulatory controls that require that we submit applications for review and approval by foreign regulatory bodies. Once we do apply, we may not receive approval for the commercial use of our tests on a timely basis, or at all. If we are unable to achieve appropriate ex-U.S. approvals, or if clinical diagnostic laboratories outside the United States do not accept our tests, our ability to grow our business outside of the United States could be compromised.
If our HTG Edge or HTG EdgeSeq systems and proprietary profiling panels fail to achieve and sustain sufficient market acceptance, we will not generate expected revenue, and our prospects may be harmed.
We are currently focused on selling our HTG Edge and HTG EdgeSeq systems and profiling panels within the life sciences research market. We plan to develop panels for many different disease states including companion diagnostics to determine the proper course of medication for those diseases. We may experience reluctance, or refusal, on the part of physicians to order, and third-party payors to cover and provide adequate reimbursement for, our panels if the results of our research and clinical studies, and our sales and marketing activities relating to communication of these results, do not convey to physicians, third-party payors and patients that the HTG Edge and HTG EdgeSeq systems and related profiling panels provide equivalent or better diagnostic information than other available technologies and methodologies. We believe our panels represent a new methodology in diagnosing disease states, and we may have to overcome resistance among physicians to adopting it for the marketing of our products to be successful. Even if we are able to obtain regulatory approval from the FDA, the use of our panels may not become the standard diagnostic tool for those diseases on which we plan to focus our efforts. A portion of our strategy is to develop diagnostic tools in conjunction with biopharmaceutical companies to help assess the proper course of treatment for specific diseases. Even if we are successful in developing those diagnostic tools and receive regulatory approval, we still may not be successful in marketing those diagnostic tests. Furthermore, our biopharmaceutical partners may choose alternative diagnostic tests to market with their products instead of ours which could limit our diagnostic test sales and revenues.
As part of our current business model, we intend to seek to enter into strategic collaborations and licensing arrangements with third parties to develop diagnostic tests.
We have relied, and expect to continue to rely, on strategic collaborations and licensing agreements with third parties for discoveries or technologies based on which we develop profiling panels. We have entered into agreements with third parties to facilitate or enable our development of assays, and ultimately diagnostic tests, to aid in the diagnosis of breast-related disorders, lung cancer, melanoma and other diseases. We intend to enter into additional similar agreements with life sciences companies and other researchers for future diagnostic products. However, we cannot guarantee that we will enter into any additional agreements. In particular, our life sciences research customers are not obligated to collaborate with us or license technology to us, and they may choose to develop diagnostic products themselves or collaborate with our competitors. Establishing collaborations and licensing arrangements is difficult and time-consuming. Discussions may not lead to collaborations or licenses on favorable terms, or at all. Potential collaborators or licensors may elect not to work with us based upon their assessment of our financial, regulatory or intellectual property position. To the extent we enter into new collaboration or licensing agreements, they may never result in the successful development or commercialization of future tests or other products for a variety of reasons, including because our collaborators may not succeed in performing their obligations or may choose not to cooperate with us. We cannot control the amount and timing of our collaborators’ resources that will be devoted to performing their responsibilities under our agreements with them.
46
Moreover, to the extent we agree to work exclusively with a party in a given area, our opportunities to collaborate with others would be limited. Even if we establish new relationships, they may never result in the successful development or commercialization of future tests or other products. Disputes with our collaborators could also impair our reputation or result in development delays, decreased revenues and litigation expenses.
Our research and development efforts will be hindered if we are not able to contract with third parties for access to archival patient samples.
Our future development of products for clinical indications will require access to archival patient samples for which data relevant to the clinical indication of interest is known. Under standard clinical practice, tissue biopsies removed from patients are preserved and stored in formalin-fixed paraffin embedded, or FFPE, format. We rely on our ability to secure access to these archived patient samples, including FFPE tissue, plasma, serum, whole blood preserved in PAX gene, or various cytology preparations, together with the information pertaining to the clinical outcomes of the patients from which the samples were taken. Owners or custodians of relevant samples may be difficult to identify and/or identified samples may be of poor quality or limited in number or amount. Additionally, others compete with us for access to these samples for both research and commercial purposes. Even when an appropriate cohort of samples is identified, the process of negotiating access to these samples can be lengthy because it typically involves numerous parties and approval levels to resolve complex issues such as usage rights, institutional review board approval, privacy rights, publication rights, and intellectual property ownership. If we are not able to negotiate access to archived patient samples on a timely basis, or at all, or if our competitors or others secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed.
The life sciences research and diagnostic markets are highly competitive. We face competition from enhanced or alternative technologies and products, which could render our products and/or technologies obsolete. If we fail to compete effectively, our business and operating results will suffer.
We face significant competition in the life sciences research and diagnostics markets. We currently compete with both established and early-stage life sciences research companies that design, manufacture and market instruments and consumables for gene expression analysis, liquid-based specimen analysis (e.g., plasma, blood and urine), single-cell analysis, polymerase chain reaction, or PCR, digital PCR, other nucleic acid detection and additional applications. These companies use well-established laboratory techniques such as microarrays or quantitative PCR, or qPCR, as well as newer technologies such as next generation sequencing. We believe our principal competitors in the life sciences research market are Advanced Cell Diagnostics, Agilent Technologies, Inc., ArcherDx, Inc., BioRad Laboratories, Exiqon A/S, Fluidigm Corporation, Foundation Medicine, Inc. Illumina, Inc., Abbott Molecular, Luminex Corporation, Affymetrix, Inc., NanoString Technologies, Inc., Qiagen N.V., Roche Diagnostics, a division of the Roche Group of companies, and Thermo Fisher Scientific, Inc.. In addition, there are a number of other market entrants in the process of developing novel technologies for the life sciences market, including companies such as RainDance Technologies, Inc. and Wafergen Bio-Systems, Inc. One or more of our competitors could develop a product that is superior to a product we offer or intend to offer or our technology and products may be rendered obsolete or uneconomical by advances in existing technologies.
Within the diagnostic market, there are competitors that manufacture systems for sales to hospitals and laboratories and other competitors that offer tests conducted through Clinical Laboratory Improvement Amendments, or CLIA, laboratories. We will also compete with commercial diagnostics companies. Most of our current competitors are either publicly traded, or are divisions of publicly traded companies, and enjoy a number of competitive advantages over us, including:
|
|
· |
greater name and brand recognition, financial and human resources; |
|
|
· |
broader product lines; |
|
|
· |
larger sales forces and more established distributor networks; |
|
|
· |
substantial intellectual property portfolios; |
|
|
· |
larger and more established customer bases and relationships; and |
|
|
· |
better established, larger scale, and lower cost manufacturing capabilities. |
We believe that the principal competitive factors in all of our target markets include:
|
|
· |
cost of capital equipment; |
|
|
· |
cost of consumables and supplies; |
|
|
· |
reputation among customers; |
47
|
|
· |
flexibility and ease-of-use; |
|
|
· |
accuracy and reproducibility of results; and |
|
|
· |
compatibility with existing laboratory processes, tools and methods. |
We believe that additional competitive factors specific to the diagnostics market include:
|
|
· |
breadth of clinical decisions that can be influenced by information generated by tests; |
|
|
· |
volume, quality, and strength of clinical and analytical validation data; |
|
|
· |
availability of coverage and adequate reimbursement for testing services; and |
|
|
· |
economic benefit accrued to customers based on testing services enabled by products. |
Our products may not compete favorably and we may not be successful in the face of increasing competition from new products and technologies introduced by our existing competitors or new companies entering our markets. In addition, our competitors may have or may develop products or technologies that currently or in the future will enable them to produce competitive products with greater capabilities or at lower costs than ours. Any failure to compete effectively could materially and adversely affect our business, financial condition and operating results.
We are dependent on third-party suppliers for our systems and some of the components and materials used in our products, and the loss of any of these suppliers could harm our business.
We currently rely on two suppliers to supply certain components used in the processor and reader for our HTG Edge and HTG EdgeSeq systems. Our contracts with certain of these parties do not commit them to carry inventory or make available any particular quantities, and they may give other customers’ needs higher priority than ours and we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms. We also rely on third-party suppliers for various components we use to manufacture our consumable products. We periodically forecast our needs for such components and enter into standard purchase orders with them. If we were to lose such suppliers, we may not be able to identify or enter into agreements with alternative suppliers on a timely basis on acceptable terms, or at all. If we should encounter delays or difficulties in securing the quality and quantity of materials we require for our products, our supply chain would be interrupted which would adversely affect our sales. A loss of any of these suppliers could significantly delay the delivery of HTG Edge or HTG EdgeSeq systems, which in turn would materially affect our ability to generate revenue. If any of these events occur, our business and operating results could be materially harmed.
We may encounter manufacturing difficulties that could impede or delay production of our HTG Edge and HTG EdgeSeq systems.
We recently began manufacturing our HTG Edge and HTG EdgeSeq systems internally. We have limited experience with manufacturing these systems and our internal manufacturing operations may encounter difficulties involving, among other things, scale-up of manufacturing processes, production efficiency and output, regulatory compliance, quality control and quality assurance, and shortages of qualified personnel. Any failure in our planned internal manufacturing operations could cause us to be unable to meet demand for these systems, delay the delivery of these systems to customers, and harm our business relationships and reputation.
If we encounter difficulties in our planned internal manufacturing operations, we may need to engage a third-party supplier, provided we cannot be sure we will be able to do in a timely manner, or at all, or on favorable terms.
Any of these factors could cause us to delay or suspend production of our HTG Edge and HTG EdgeSeq systems, entail unplanned additional costs and materially harm our business, results of operations and financial condition.
If our Tucson facilities become unavailable or inoperable, the manufacturing of our consumables or process sales orders will be interrupted and our business could be materially harmed.
We manufacture our consumable products and our HTG Edge and HTG EdgeSeq systems in our Tucson, Arizona facilities. In addition, our Tucson facilities are the center for order processing, receipt of critical components of our HTG Edge and HTG EdgeSeq instruments manufactured by third-party contract manufacturers and shipping products to customers. We do not have redundant facilities. Damage or the inability to utilize our Tucson facilities and the equipment we use to perform research and development and manufacture our consumable products could be costly, and we would require substantial lead-time to repair or replace this facility and equipment.
48
Tucson is situated in the desert, subject to flooding, power spikes and power outages. The facility may be harmed or rendered inoperable by natural or man-made disasters, including floods, earthquakes and power outages, which may render it difficult or impossible for us to produce our tests for some period of time. The inability to manufacture consumables or to ship products to customers for even a short period of time may result in the loss of customers or harm our reputation, and we may be unable to regain those customers in the future. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
We expect to generate a portion of our revenue internationally and are subject to various risks relating to our international activities which could adversely affect our operating results.
During the years ended December 31, 2015 and 2014, approximately 13% and 14%, respectively, of our revenue was generated from sales to customers located outside of the United States. We expect that a percentage of our future revenue will continue to come from international sources, and we expect to expand our overseas operations and develop opportunities in additional areas. Engaging in international business involves a number of difficulties and risks, including:
|
|
· |
required compliance with existing and changing foreign regulatory requirements and laws; |
|
|
· |
required compliance with anti-bribery laws, such as the U.S. Foreign Corrupt Practices Act and U.K. Bribery Act, data privacy requirements, labor laws and anti-competition regulations; |
|
|
· |
export and import restrictions; |
|
|
· |
various reimbursement, pricing and insurance regimes; |
|
|
· |
laws and business practices favoring local companies; |
|
|
· |
longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
|
|
· |
political and economic instability; |
|
|
· |
potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements and other trade barriers, including transfer pricing, value added and other tax systems, double taxation and restrictions and/or taxation on repatriation of earnings; |
|
|
· |
tariffs, customs charges, bureaucratic requirements and other trade barriers; |
|
|
· |
difficulties and costs of staffing and managing foreign operations, including difficulties and costs associated with foreign employment laws; |
|
|
· |
increased financial accounting and reporting burdens and complexities; and |
|
|
· |
difficulties protecting, procuring, or enforcing intellectual property rights, including from reduced or varied protection for intellectual property rights in some countries. |
As we expand internationally our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. Historically, most of our revenue has been denominated in U.S. dollars, although we have sold our products and services in local currency outside of the United States, principally the Euro. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States. As our operations in countries outside of the United States grows, our results of operations and cash flows will be subject to fluctuations due to changes in foreign currency exchange rates, which could negatively impact our results of operations in the future. For example, if the value of the U.S. dollar increases relative to foreign currencies, in the absence of a corresponding change in local currency prices, our revenue could be adversely affected as we convert revenue from local currencies to U.S. dollars.
If we dedicate significant resources to our international operations and are unable to manage these risks effectively, our business, operating results and prospects will suffer. Moreover, we cannot be certain that the investment and additional resources required in establishing operations in other countries will produce desired levels of revenue or profitability.
In addition, any failure to comply with applicable legal and regulatory obligations could negatively impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments and restrictions on certain business activities.
49
Our reliance on distributors and sales partners for sales of our products outside of the United States could limit or prevent us from selling them in foreign markets and may impact our revenue.
We have established an exclusive distribution agreement for our HTG Edge platform and related profiling panels in the research-use-only market within parts of Asia. In addition, we have an agreement with a contract sales team in Europe, which we are in the process of transitioning to our own direct sales and support team. We intend to continue to grow our business internationally, and to do so, in addition to expanding our own direct sales and support team, we plan to attract additional distributors and sales partners to maximize the commercial opportunity for our products. We cannot guarantee that we will be successful in attracting desirable distribution and sales partners or that we will be able to enter into such arrangements on favorable terms. Distributors and sales partners may not commit the necessary resources to market and sell our products to the level of our expectations or may favor marketing the products of our competitors. If current or future distributors or sales partners do not perform adequately, or we are unable to enter into effective arrangements with distributors or sales partners in particular geographic areas, we may not realize long-term international revenue growth.
Limitations in the use of our products could harm our reputation or decrease market acceptance of our products; undetected errors or defects in our products could harm our reputation, decrease market acceptance of our products or expose us to product liability claims.
Our products are subject to the limitations set forth in the product labeling, which may not satisfy the needs of all customers. For example, in the past we have introduced new panels that initially were intended to be used with specific types of tissue samples. Because our customers desire that our panels be broadly applicable to many biological sample types, these initial limitations could harm our reputation or decrease market acceptance of our products. If that occurs, we may incur significant costs, the attention of our key personnel could be diverted, or other significant customer relations problems may arise, which could harm our business and operating results.
Similarly, our products may contain undetected errors or defects when first introduced or as new versions are released. Since our current customers use our products for research and may, if cleared or approved, in the future use them for diagnostic applications, disruptions or other performance problems with our products may damage our customers’ businesses and could harm our reputation. If that occurs, we may incur significant costs, the attention of our key personnel could be diverted, or other significant customer relations problems may arise. We may also be subject to warranty and liability claims for damages related to errors or defects in our products. A material liability claim or other occurrence that harms our reputation or decreases market acceptance of our products could harm our business and operating results.
The sale and use of products or services based on our technologies, or activities related to our research and clinical studies, could lead to the filing of product liability claims if someone were to allege that one of our products contained a design or manufacturing defect which resulted in the failure to adequately perform the analysis for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure investors that our product liability insurance could adequately protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
The enactment of legislation implementing changes in the U.S. taxation of international business activities or the adoption of other tax reform policies could materially impact our future financial position and results of operations.
Recent changes to U.S. tax laws, including limitations on the ability of taxpayers to claim and utilize foreign tax credits and the deferral of certain tax deductions until earnings outside of the United States are repatriated to the United States, as well as changes to U.S. tax laws that may be enacted in the future, could impact the tax treatment of future foreign earnings. Should the scale of our international business activities expand, any such changes in the U.S. taxation of such activities could increase our worldwide effective tax rate and harm our future financial position and results of operations.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2015, we had federal net operating loss carryforwards, or NOLs, to offset future taxable income of approximately $78.5 million, which will begin to expire in 2021 if not utilized. A lack of future taxable income would adversely affect our ability to utilize these NOLs. In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” (generally defined as a greater than 50% change, by value, in its equity ownership over a three year period) is subject to limitations on its ability to utilize its NOLs to offset future taxable income. We believe we may have already experienced an ownership change and may in the future experience one or more additional ownership changes, and as a result, our ability to use pre-ownership change NOLs and other pre-ownership change tax attributes to offset post-ownership change
50
income may be limited. Such limitations may cause a portion of our NOL and credit carryforwards to expire. In addition, future changes in our stock ownership, including as a result of our initial public offering or future offerings, as well as other changes that may be outside of our control, could result in ownership changes under Section 382 of the Code. Our NOLs may also be impaired under similar provisions of state law. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future benefits of those assets.
Acquisitions or joint ventures could disrupt our business, cause dilution to our stockholders and otherwise harm our business.
We may acquire other businesses, products or technologies as well as pursue strategic alliances, joint ventures, technology licenses or investments in complementary businesses. We have limited experience with respect to business, product or technology acquisitions or the formation of collaborations, strategic alliances and joint ventures or investing in complementary businesses. Any of these transactions could be material to our financial condition and operating results and expose us to many risks, including:
|
|
· |
disruption in our relationships with customers, distributors or suppliers as a result of such a transaction; |
|
|
· |
unanticipated liabilities related to acquired companies; |
|
|
· |
difficulties integrating acquired personnel, technologies and operations into our existing business; |
|
|
· |
diversion of management time and focus from operating our business to acquisition integration challenges; |
|
|
· |
increases in our expenses and reductions in our cash available for operations and other uses; and |
|
|
· |
possible write-offs or impairment charges relating to acquired businesses. |
Foreign acquisitions involve unique risks in addition to those mentioned above, including those related to integration of operations across different cultures and languages, currency risks and the particular economic, political and regulatory risks associated with specific countries. Also, the anticipated benefit of any acquisition may not materialize. Future acquisitions or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities or amortization expenses or write-offs of goodwill, any of which could harm our financial condition. We cannot predict the number, timing or size of future joint ventures or acquisitions, or the effect that any such transactions might have on our operating results.
If any members of our management team were to leave us or we are unable to recruit, train and retain key personnel, we may not achieve our goals.
Our future success depends on our ability to recruit, train, retain and motivate key personnel, including our senior management, research and development, manufacturing and sales and marketing personnel. If we were to lose one or more of our key employees, we may experience difficulties in competing effectively, developing our technologies and implementing our business strategies. Competition for qualified personnel is intense, and we may not be able to attract talent. Our growth depends, in part, on attracting, retaining and motivating highly trained sales personnel with the necessary scientific background and ability to understand our systems at a technical level to effectively identify and sell to potential new customers. In particular, the commercialization of our HTG Edge and HTG EdgeSeq systems and related panels requires us to continue to establish and maintain a sales and support team to optimize the market for research tools, then to fully optimize a broad array of diagnostic market opportunities if we receive approval for any future diagnostic products. We do not maintain fixed term employment contracts or, except for our Chief Executive Officer, key man life insurance with any of our employees. Because of the complex and technical nature of our products and the dynamic market in which we compete, any failure to retain our management team or to attract, train, retain and motivate other qualified personnel could materially harm our operating results and growth prospects.
Our operating results may be harmed if we are required to collect sales, services or other related taxes for our products and services in jurisdictions where we have not historically done so.
We do not believe that we are required to collect sales, use, services or other similar taxes from our customers in certain jurisdictions. However, one or more countries or states may seek to impose sales, use, services, or other tax collection obligations on us, including for past sales. A successful assertion by one or more jurisdictions that we should collect sales or other taxes on the sale of our products and services could result in substantial tax liabilities for past sales and decrease our ability to compete for future sales. Each country and each state has different rules and regulations governing sales and use taxes and these rules and regulations are subject to varying interpretations that may change over time. We review these rules and regulations periodically and, when we believe sales and use taxes apply in a particular jurisdiction, voluntarily engage tax authorities in order to determine how to comply with their rules and regulations. We cannot assure you that we will not be subject to sales and use taxes or related penalties for past sales in jurisdictions where we presently believe sales and use taxes are not due.
51
Providers of goods or services are typically held responsible by taxing authorities for the collection and payment of any applicable sales and similar taxes. If one or more taxing authorities determines that taxes should have, but have not, been paid with respect to our products and services, we may be liable for past taxes in addition to being required to collect sales or similar taxes in respect of our products and services going forward. Liability for past taxes may also include substantial interest and penalty charges. Our client contracts provide that our clients must pay all applicable sales and similar taxes. Nevertheless, clients may be reluctant to pay back taxes and may refuse responsibility for interest or penalties associated with those taxes or we may determine that it would not be feasible to seek reimbursement. If we are required to collect and pay back taxes and the associated interest and penalties and if our customers do not reimburse us for all or a portion of these amounts, we will have incurred unplanned expenses that may be substantial. Moreover, imposition of such taxes on our products and services going forward will effectively increase the cost of such products and services to our clients.
Many states are also pursuing legislative expansion of the scope of goods and services that are subject to sales and similar taxes as well as the circumstances in which a vendor of goods and services must collect such taxes. Furthermore, legislative proposals have been introduced in Congress that would provide states with additional authority to impose such taxes. Accordingly, it is possible that either federal or state legislative changes may require us to collect additional sales and similar taxes from our clients in the future.
Our insurance policies are expensive and protect us only from some business risks, which will leave us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, foreign liability, employee benefits liability, property, automobile, umbrella, workers’ compensation, crime (including cybercrime), fiduciary, products liability and directors’ and officers’ insurance. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our cash position and results of operations.
Performance issues, service interruptions or price increases by our shipping carriers could adversely affect our business and harm our reputation and ability to provide our services on a timely basis.
Expedited, reliable shipping is essential to our operations. We rely heavily on providers of transport services for reliable and secure point-to-point transport of our HTG Edge and HTG EdgeSeq systems and consumables to our customers and, as applicable, customers’ samples to our laboratory, and for enhanced tracking of these shipments. Should a carrier encounter delivery performance issues such as loss, damage or destruction of any instrumentation, consumables or samples, it would be costly to replace such instrumentation or consumables in a timely manner and may be difficult to replace customers’ samples lost or damaged in shipping, and such occurrences may damage our reputation and lead to decreased demand for our products and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters or other service interruptions affecting delivery services we use would adversely affect our ability to process orders for our products or receive recipient samples on a timely basis.
We face risks related to handling of hazardous materials and other regulations governing environmental safety.
Our operations are subject to complex and stringent environmental, health, safety and other governmental laws and regulations that both public officials and private individuals may seek to enforce. Our activities that are subject to these regulations include, among other things, our use of hazardous materials and the generation, transportation and storage of waste. We could discover that we or an acquired business is not in material compliance with these regulations. Existing laws and regulations may also be revised or reinterpreted, or new laws and regulations may become applicable to us, whether retroactively or prospectively, that may have a negative effect on our business and results of operations. It is also impossible to eliminate completely the risk of accidental environmental contamination or injury to individuals. In such an event, we could be liable for any damages that result, and any liability could exceed our resources or any applicable insurance coverage we may have, which events could adversely affect our business.
Cyber security risks and the failure to maintain the confidentiality, integrity, and availability of our computer hardware, software, and internet applications and related tools and functions could result in damage to our reputation and/or subject us to costs, fines or lawsuits.
Our business requires manipulating, analyzing and storing large amounts of data. In addition, we rely on an enterprise software system to operate and manage our business. We also maintain personally identifiable information about our employees. Our business therefore depends on the continuous, effective, reliable and secure operation of our computer hardware, software, networks, Internet servers and related infrastructure. To the extent that our hardware and software malfunction or access to our data by internal personnel is interrupted, our business could suffer. The integrity and protection of our employee and company data is critical to our business and
52
employees have a high expectation that we will adequately protect their personal information. The regulatory environment governing information, security and privacy laws is increasingly demanding and continues to evolve. Maintaining compliance with applicable security and privacy regulations may increase our operating costs. Although our computer and communications software is protected through physical and software safeguards, it is still vulnerable to fire, storm, flood, power loss, earthquakes, telecommunications failures, physical or software break-ins, software viruses and similar events. These events could lead to the unauthorized access, disclosure and use of non-public information. The techniques used by criminal elements to attack computer systems are sophisticated, change frequently and may originate from less regulated and remote areas of the world. As a result, we may not be able to address these techniques proactively or implement adequate preventative measures. If our computer systems are compromised, we could be subject to fines, damages, litigation and enforcement actions, and we could lose trade secrets, the occurrence of which could harm our business. In addition, any sustained disruption in internet access provided by other companies could harm our business.
Risks Related to Government Regulation and Diagnostic Product Reimbursement
Our “research-use-only” products for the life sciences market could become subject to regulation as medical devices by the FDA or other regulatory agencies in the future which could increase our costs and delay our commercialization efforts, thereby materially and adversely affecting our life sciences business and results of operations.
In the United States, our products are currently labeled and sold for research use only, or RUO, and not for the diagnosis or treatment of disease, and are sold to a variety of parties, including biopharmaceutical companies, academic institutions and molecular labs. Because such products are not intended for use in clinical practice in diagnostics, and the products cannot include clinical or diagnostic claims, they are exempt from many regulatory requirements otherwise applicable to medical devices. In particular, while the FDA regulations require that RUO products be labeled, “For Research Use Only. Not for use in diagnostic procedures,” the regulations do not subject such products to the FDA’s pre- and post-market controls for medical devices.
A significant change in the laws governing RUO products or how they are enforced may require us to change our business model in order to maintain compliance. For instance, in November 2013 the FDA issued a Guidance document entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, or the RUO Guidance, which highlights the FDA’s interpretation that distribution of RUO products with any labeling, advertising or promotion that suggests that clinical laboratories can validate the test through their own procedures and subsequently offer it for clinical diagnostic use as a laboratory developed test is in conflict with RUO status. The RUO Guidance further articulates the FDA’s position that any assistance offered in performing clinical validation or verification, or similar specialized technical support, to clinical laboratories, is in conflict with RUO status. If we engage in any activities that the FDA deems to be in conflict with the RUO status held by the products that we sell, we may be subject to immediate, severe and broad FDA enforcement action that would adversely affect our ability to continue operations. Accordingly, if the FDA finds that we are distributing our RUO products in a manner that is inconsistent with its regulations or guidance, we may be forced to stop distribution of our RUO tests until we are in compliance, which would reduce our revenues, increase our costs and adversely affect our business, prospects, results of operations and financial condition. In addition, the FDA’s proposed implementation for a new framework for the regulation of Laboratory Developed Tests, or LDTs, may negatively impact the LDT market and thereby reduce demand for RUO products.
In the event that the FDA requires marketing authorization of our RUO products in the future, there can be no assurance that the FDA will ultimately grant any clearance or approval requested by us in a timely manner, or at all.
Approval and/or clearance by the FDA and foreign regulatory authorities for any diagnostic tests will take significant time and require significant research, development and clinical study expenditures and ultimately may not succeed.
Before we begin to label and market our products for use as clinical diagnostics in the United States, including as companion diagnostics, unless an exemption applies, we will be required to obtain either 510(k) clearance or pre-market approval, or PMA, from the FDA. In addition, we may be required to seek FDA clearance for any changes or modifications to our products that could significantly affect their safety or effectiveness, or would constitute a change in intended use. The 510(k) clearance processes can be expensive, time-consuming and uncertain. In addition to the time required to conduct clinical trials, if necessary, it generally takes from four to twelve months from submission of an application to obtain 510(k) clearance; however, it may take longer and 510(k) clearance may never be obtained. Even if the FDA accepts a 510(k) submission for filing, the FDA may request additional information or clinical studies during its review. Our ability to obtain additional regulatory clearances for new products and indications may be significantly delayed or may never be obtained. In addition, we may be required to obtain PMAs for new products or product modifications. The requirements of the more rigorous PMA process could delay product introductions and increase the costs associated with FDA compliance. As with all in vitro diagnostic products, the FDA reserves the right to redefine the regulatory path at the time of submission or during the review process, and could require a more burdensome approach. Even if we were to obtain regulatory approval or clearance, it may not be for the uses we believe are important or commercially attractive, in which case we would not be permitted to market our product for those uses.
53
A 510(k) clearance or PMA approval for any future medical device product would likely place substantial restrictions on how the device is marketed or sold, and we will be required to continue to comply with extensive regulatory requirements, including, but not limited to, quality system regulations, or QSR, registering manufacturing facilities, listing the products with the FDA, and complying with labeling, marketing, complaint handling, adverse event and medical device reporting requirements and corrections and removals. We cannot assure you that we will successfully maintain the clearances or approvals we may receive in the future. In addition, any clearances or approvals we obtain may be revoked if any issues arises that bring into question our products’ safety or effectiveness. Any failure to maintain compliance with FDA regulatory requirements could harm our business, financial condition and results of operations.
Sales of our diagnostic products outside the United States will be subject to foreign regulatory requirements governing clinical studies, vigilance reporting, marketing approval, manufacturing, product licensing, pricing and reimbursement. These regulatory requirements vary greatly from country to country. As a result, the time required to obtain approvals outside the United States may differ from that required to obtain FDA approval and we may not be able to obtain foreign regulatory approvals on a timely basis or at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA and foreign regulatory authorities could require additional testing. In addition, the FDA regulates exports of medical devices. Failure to comply with these regulatory requirements or obtain required approvals could impair our ability to commercialize our diagnostic products outside of the United States.
We expect to rely on third parties to conduct any future studies of our diagnostic products that may be required by the FDA or other regulatory authorities, and those third parties may not perform satisfactorily.
We do not have the ability to independently conduct the clinical studies or other studies that may be required to obtain FDA and other regulatory clearance or approval for our diagnostic products, including the HTG Edge system, the HTG EdgeSeq system and related proprietary panels. Accordingly, we expect to rely on third parties, such as medical institutions and clinical investigators, and providers of NGS instrumentation, to conduct such studies and/or to provide information necessary for our submissions to regulatory authorities. Our reliance on these third parties for clinical development activities or information will reduce our control over these activities. These third-parties may not complete activities on schedule or conduct studies in accordance with regulatory requirements or our study design. Similarly, providers of NGS instrumentation may not place the same importance on our regulatory submissions as we do. Our reliance on third parties that we do not control will not relieve us of any applicable requirement to prepare, and ensure compliance with, the various procedures requires under good clinical practices, or the submission of all information required in connection with requested regulatory approvals. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our studies may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our diagnostic products.
Even if we are able to obtain regulatory approval or clearance for our diagnostic products, we will continue to be subject to ongoing and extensive regulatory requirements, and our failure to comply with these requirements could substantially harm our business.
If we receive regulatory approval or clearance for our diagnostic products, we will be subject to ongoing FDA obligations and continued regulatory oversight and review, such as compliance with QSRs, inspections by the FDA, continued adverse event and malfunction reporting, corrections and removals reporting, registration and listing, and promotional restrictions, and we may also be subject to additional FDA post-marketing obligations. If we are not able to maintain regulatory compliance, we may not be permitted to market our diagnostic products and/or may be subject to fines, injunctions, and civil penalties; recall or seizure of products; operating restrictions; and criminal prosecution. In addition, we may be subject to similar regulatory compliance actions of foreign jurisdictions.
If Medicare and other third-party payors in the United States and foreign countries do not approve coverage and adequate reimbursement for our future clinical diagnostic tests enabled by our technology, the commercial success of our diagnostic products would be compromised.
We plan to develop, obtain regulatory approval for and sell clinical diagnostics products for a number of different indications. Successful commercialization of our clinical diagnostic products depends, in large part, on the availability of coverage and adequate reimbursement for testing services using our diagnostic products from third-party payors, including government insurance plans, managed care organizations and private insurance plans. There is significant uncertainty surrounding third-party coverage and reimbursement for the use of tests that incorporate new technology, such as the HTG Edge and HTG EdgeSeq systems and related applications and assays. Reimbursement rates have the potential to fluctuate depending on the region in which the testing is provided,
54
the type of facility or treatment center at which the testing is done, and the third-party payor responsible for payment. If we are unable to obtain positive coverage decisions from third-party payors approving reimbursement for our tests at adequate levels, the commercial success of our products would be compromised and our revenue would be significantly limited. Even if we do obtain favorable reimbursement for our tests, third-party payors may withdraw their coverage policies, review and adjust the rate of reimbursement, require co-payments from patients or stop paying for our tests, which would reduce revenue for testing services based on our technology and demand for our diagnostic products.
The American Medical Association Current Procedural Terminology, or CPT, Editorial Panel recently created new CPT codes that could be used by our customers to report testing for certain large-scale multianalyte genomic sequencing procedures (GSPs), including our diagnostic products, if approved. Effective January 1, 2015, these codes allow for uniform reporting of broad genomic testing panels using technology similar to ours. While these codes will standardize reporting for these tests, coverage and payment rates for GSPs remain uncertain and we cannot guarantee that coverage and/or reimbursement for these tests will be provided in the amounts we expect, or at all. Initially, industry associations recommended that payment rates for GSPs be cross-walked to existing codes on the clinical laboratory fee schedule. On October 27, 2014, CMS issued preliminary determinations for 29 new molecular pathology codes, including the GSPs, of gapfill rather than crosswalking as recommended by the Association for Molecular Pathology. This means that local private Medicare Administrative Contractors, or MACs, such as Palmetto, Novidian, Novitas and Cahaba, were instructed to determine the appropriate fee schedule amounts in the first year, and CMS will calculate a national payment rate based on the median of those local fee schedule amounts in the second year. This process may make it more difficult for our customers to obtain coverage and adequate reimbursement for testing services using our diagnostic products. We cannot assure that CMS and other third-party payors will establish reimbursement rates sufficient to cover the costs incurred by our customers in using our clinical diagnostic products, if approved. On September 25, 2015, CMS released final 2015 pricing for 10 of these codes, and did not issue any pricing on the remaining 19. CPTs 81445 and 81450 for the assessment of 5-50 genes in solid and liquid tumors, respectively, and final gapfill pricing of $597 and $647, respectively, was set for 2015. CPT 81455 for the assessment of 51 or more genes in solid and liquid tumors has not yet been priced.
Even if we are able to establish coverage and reimbursement codes for our clinical diagnostic products in development, we will continue to be subject to significant pricing pressure, which could harm our business, results of operations, financial condition and prospects.
Third-party payors, including managed care organizations as well as government payors such as Medicare and Medicaid, have increased their efforts to control the cost, utilization and delivery of healthcare services, which may include decreased coverage or reduced reimbursement. From time to time, Congress has considered and implemented changes to the Medicare fee schedules in conjunction with budgetary legislation, and pricing and payment terms, including the possible requirement of a patient co-payment for Medicare beneficiaries for laboratory tests covered by Medicare, and are subject to change at any time. Reductions in the reimbursement rate of third-party payors have occurred and may occur in the future. Reductions in the prices at which testing services based on our technology are reimbursed in the future could result in pricing pressures and have a negative impact on our revenue. In many countries outside of the United States, various coverage, pricing and reimbursement approvals are required. We expect that it will take several years to establish broad coverage and reimbursement for testing services based on our products with payors in countries outside of the United States, and our efforts may not be successful.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws and other federal and state healthcare laws applicable to our business and marketing practices. If we are unable to comply, or have not complied, with such laws, we could face substantial penalties.
Our operations may be, and may continue to be, directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal and state anti-kickback statutes, false claims statutes, civil monetary penalties laws, patient data privacy and security laws, physician transparency laws and marketing compliance laws. These laws may impact, among other things, our proposed sales and marketing and education programs. Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Promotional activities for FDA-regulated products have been the subject of significant enforcement actions brought under healthcare reimbursement laws, fraud and abuse laws, and consumer protection statutes, among other theories. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims.
The laws that may affect our ability to operate include, but are not limited to:
55
|
|
Federal healthcare program, such as the Medicare and Medicaid programs; a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
|
|
· |
the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits, among other things, physicians who have a financial relationship, including an investment, ownership or compensation relationship with an entity, from referring Medicare and Medicaid patients to that entity for designated health services, which include clinical laboratory services, unless an exception applies. Similarly, entities may not bill Medicare, Medicaid or any other party for services furnished pursuant to a prohibited referral. Unlike the federal Anti-Kickback Statute, the Stark Law is a strict liability statute, meaning that all of the requirements of a Stark Law exception must be met in order to be compliant with the law; |
|
|
· |
Federal civil and criminal false claims laws, including the federal civil False Claims Act, and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other governmental third-party payors that are false or fraudulent, knowingly making a false statement material to an obligation to pay or transmit money to the Federal Government or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay money to the Federal Government, which may apply to entities that provide coding and billing advice to customers; the Federal Government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act; |
|
|
· |
the federal Health Insurance Portability and Accountability Act of 1996, as amended, or HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing, or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
|
|
· |
HIPAA, as amended by HITECH, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans, and healthcare clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information; |
|
|
· |
the federal Physician Payments Sunshine Act, which require certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to payments or other transfers of value made to physicians and teaching hospitals, as well as applicable manufacturers and group purchasing organizations to report annually to CMS certain ownership and investment interests held by physicians and their immediate family members; and |
|
|
· |
state law equivalents of each of the above federal laws, such as anti-kickback, self-referral, and false claims laws which may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements as well as submitting claims involving healthcare items or services reimbursed by any third-party payor, including commercial insurers; state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the Federal Government that otherwise restricts payments that may be made to healthcare providers; state laws that require device manufacturers to file reports with states regarding marketing information, such as the tracking and reporting of gifts, compensations and other remuneration and items of value provided to healthcare professionals and entities (compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships, which could potentially have a negative effect on our business and/or increase enforcement scrutiny of our activities); and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, with differing effects. |
In addition, the approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including our relationships with physicians and other health care providers, and our evaluation, reagent rental and collaboration arrangements with customers, and sales and marketing efforts could be subject to challenge under one or more of such laws.
56
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, individual imprisonment, disgorgement, exclusion from participation in Federal healthcare programs, such as Medicare and Medicaid, contractual damages, reputational harm, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk of fraud or other misconduct by our employees, independent contractors, principal investigators, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless or negligent failures to, among other things: (i) comply with the regulations of the FDA, the Centers for Medicare and Medicaid Services, or CMS, the Department of Health and Human Services Office of Inspector General, or OIG, and other similar foreign regulatory bodies; (ii) provide true, complete and accurate information to the FDA and other similar regulatory bodies; (iii) comply with manufacturing standards we have established; (iv) comply with healthcare fraud and abuse laws and regulations in the United States and similar foreign fraudulent misconduct laws; or (v) report financial information or data accurately, or disclose unauthorized activities to us. These laws may impact, among other things, our activities with collaborators and key opinion leaders, as well as our sales, marketing and education programs. In particular, the promotion, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation. We currently have a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and our code of conduct and the other precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations. Any of these actions or investigations could result in substantial costs to us, including legal fees, and divert the attention of management from operating our business.
Healthcare policy changes, including recently enacted legislation reforming the United States healthcare system, may have a material adverse effect on our financial condition and results of operations.
On April 1, 2014, the Protecting Access to Medicare Act of 2014, or PAMA, was signed into law, which, among other things, significantly alters the current payment methodology under the Medicare Clinical Laboratory Fee Schedule, or CLFS. Under the new law, starting January 1, 2016 and every three years thereafter (or annually in the case of advanced diagnostic lab tests), clinical laboratories must report laboratory test payment data for each Medicare-covered clinical diagnostic lab test that it furnishes during a time period to be defined by future regulations. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Beginning in 2017, the Medicare payment rate for each clinical diagnostic lab test will be equal to the weighted median amount for the test from the most recent data collection period. The payment rate will apply to laboratory tests furnished by a hospital laboratory if the test is separately paid under the hospital outpatient prospective payment system. It is too early to predict the impact on reimbursement for our products in development.
Also under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. For an existing test that is cleared or approved by the FDA and for which Medicare payment is made as of April 1, 2014, CMS is required to assign a unique billing code if one has not already been assigned by the agency. In addition to assigning the code, CMS was required to publicly report payment for the tests no later than January 1, 2016. We cannot determine at this time the full impact of the new law on our business, financial condition and results of operations.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the ACA or the Affordable Care Act, enacted in March 2010, makes changes that are expected to significantly impact the pharmaceutical and medical device industries and clinical laboratories. For example, the ACA includes a reduction in the annual update factor used to adjust payments under the CLFS for inflation. This update factor reflects the consumer price index for all urban consumers, or CPI-U, and the ACA reduced the CPI-U by 1.75% for the years 2011 through 2015. The ACA also imposes a
57
multifactor productivity adjustment in addition to the CPI-U, which may further reduce payment rates. These or any future proposed or mandated reductions in payments may apply to some or all of the clinical laboratory tests that our diagnostics customers use our technology to deliver to Medicare beneficiaries, and may reduce demand for our diagnostic products.
Other significant measures contained in the ACA include, for example, coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. Further, the ACA includes a deductible 2.3% excise tax on any entity that manufactures or imports medical devices offered for sale in the U.S., with limited exceptions, which became effective January 1, 2013. However, the Consolidated Appropriations Act of 2016, signed into law in December 2015, includes a two year moratorium on the medical device excise tax that applies between January 1, 2016 and December 31, 2017. Absent further legislative action, the tax will be automatically reinstated for medical device sales beginning January 1, 2018. The ACA also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013, and, following passage of the Bipartisan Budget Act of 2015, will stay in effect through 2025 unless Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Various healthcare reform proposals have also emerged from federal and state governments. Changes in healthcare law or policy, such as the creation of broad test utilization limits for diagnostic products in general or requirements that Medicare patients pay for portions of clinical laboratory tests or services received, could substantially impact the sales of our tests, increase costs and divert management’s attention from our business. Such co-payments by Medicare beneficiaries for laboratory services were discussed as possible cost savings for the Medicare program as part of the debt ceiling budget discussions in mid-2011 and may be enacted in the future. In addition, sales of our tests outside of the United States will subject us to foreign regulatory requirements, which may also change over time.
We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us. The taxes imposed by the new federal legislation and the expansion in government’s effect on the United States healthcare industry may result in decreased profits to us, lower reimbursements by payors for our products or reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations. The full impact of the ACA, as well as other laws and reform measures that may be proposed and adopted in the future, remains uncertain, but may continue the downward pressure on medical device pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs, which could have a material adverse effect on our business operations. There have been judicial and congressional challenges to certain aspects of the ACA, and we expect additional challenges and amendments in the future.
Risks Related to Intellectual Property
If we are unable to protect our intellectual property effectively, our business would be harmed.
We rely on patent protection as well as trademark, copyright, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Our U.S. and foreign patent and patent application portfolio relates to our nuclease-protection-based technologies as well as to lung cancer and melanoma and diffuse large B-cell lymphoma biomarker panels discovered using our nuclease-protection-based technology. We have exclusive or non-exclusive licenses to multiple U.S. and foreign patents and patent applications covering technologies which we intend to utilize in developing diagnostic tests for use on our HTG Edge and HTG EdgeSeq systems. Those licensed patents and patent applications cover technologies related to the diagnosis of breast cancer and melanoma.
If we fail to protect our intellectual property, third parties may be able to compete more effectively against us and we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property.
58
We cannot assure investors that any of our currently pending or future patent applications will result in issued patents, and we cannot predict how long it will take for such patents to be issued. Further, we cannot assure investors that other parties will not challenge any patents issued to us or that courts or regulatory agencies will hold our patents to be valid or enforceable. We cannot guarantee investors that we will be successful in defending challenges made against our patents. Any successful third-party challenge to our patents could result in the unenforceability or invalidity of such patents.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. Furthermore, in the biotechnology field, courts frequently render opinions that may adversely affect the patentability of certain inventions or discoveries, including opinions that may adversely affect the patentability of methods for analyzing or comparing nucleic acids molecules, such as RNA or DNA.
The patent positions of companies engaged in development and commercialization of molecular diagnostic tests are particularly uncertain. Various courts, including the U.S. Supreme Court, have recently rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to molecular diagnostics. Specifically, these decisions stand for the proposition that patent claims that recite laws of nature (for example, the relationships between gene expression levels and the likelihood of risk of recurrence of cancer) are not themselves patentable unless those patent claims have sufficient additional features that provide practical assurance that the processes are genuine inventive applications of those laws rather than patent drafting efforts designed to monopolize the law of nature itself. What constitutes a “sufficient” additional feature is uncertain. Accordingly, this evolving case law in the United States may adversely impact our ability to obtain new patents and may facilitate third-party challenges to our existing owned and licensed patents.
The laws of some non-U.S. countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
|
|
· |
We might not have been the first to make the inventions covered by each of our patents and pending patent applications. |
|
|
· |
We might not have been the first to file patent applications for these inventions. |
|
|
· |
Others may independently develop similar or alternative products and technologies or duplicate any of our products and technologies. |
|
|
· |
It is possible that none of our pending patent applications will result in issued patents, and even if they issue as patents, they may not provide a basis for commercially viable products, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties. |
|
|
· |
We may not develop additional proprietary products and technologies that are patentable. |
|
|
· |
The patents of others may have an adverse effect on our business. |
|
|
· |
We apply for patents covering our products and technologies and uses thereof, as we deem appropriate. However, we may fail to apply for patents on important products and technologies in a timely fashion or at all. |
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
In addition, competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or
59
develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We have not yet registered certain of our trademarks, including “HTG Edge,” “HTG EdgeSeq,” and “qPNA” in all of our potential markets. If we apply to register these trademarks, our applications may not be allowed for registration, and our registered trademarks may not be maintained or enforced. In addition, opposition or cancellation proceedings may be filed against our trademark applications and registrations, and our trademarks may not survive such proceedings. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would.
To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct competition. If our intellectual property does not provide adequate protection against our competitors’ products, our competitive position could be adversely affected, as could our business. Both the patent application process and the process of managing patent disputes can be time consuming and expensive.
We may need to depend on certain technologies that are licensed to us. We would not control these technologies and any loss of our rights to them could prevent us from selling some of our products.
We have entered into several license agreements with third parties for certain licensed technologies that are, or may become relevant to the products we market, or plan to market. In addition, we may in the future elect to license third party intellectual property to further our business objectives and/or as needed for freedom to operate for our products. We do not and will not own the patents, patent applications or other intellectual property rights that are a subject of these licenses. Our rights to use these technologies and employ the inventions claimed in the licensed patents, patent applications and other intellectual property rights are or will be subject to the continuation of and compliance with the terms of those licenses.
We might not be able to obtain licenses to technology or other intellectual property rights that we require. Even if such licenses are obtainable, they may not be available at a reasonable cost or multiple licenses may be needed for the same product (e.g., stacked royalties). We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Further, we could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products.
In some cases, we do not or may not control the prosecution, maintenance, or filing of the patents or patent applications to which we hold licenses, or the enforcement of these patents against third parties. As a result, we cannot be certain that drafting or prosecution of the licensed patents and patent applications by the licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights.
Certain of the U.S. patent rights we own, have licensed or may license relate to technology that was developed with U.S. government grants, in which case the U.S. government has certain rights in those inventions, including, among others, march-in license rights. In addition, federal regulations impose certain domestic manufacturing requirements with respect to any products within the scope of those U.S. patent claims.
We may be involved in lawsuits to protect or enforce our patent or other proprietary rights, to determine the scope, coverage and validity of others’ patent or other proprietary rights, or to defend against third-party claims of intellectual property infringement, any of which could be time-intensive and costly and may adversely impact our business or stock price.
We may from time to time receive notices of claims of infringement and misappropriation or misuse of other parties’ proprietary rights, including with respect to third-party trade secrets, infringement by us of third-party patents and trademarks or other rights, or challenges to the validity or enforceability of our patents, trademarks or other rights. Some of these claims may lead to litigation. We cannot assure investors that such actions will not be asserted or prosecuted against us or that we will prevail in any or all such actions.
Litigation may be necessary for us to enforce our patent and other proprietary rights or to determine the scope, coverage and validity of the proprietary rights of others. The outcome of any litigation or other proceeding is inherently uncertain and might not be favorable to us. In addition, any litigation that may be necessary in the future could result in substantial costs, even if we were to prevail, and diversion of resources and could have a material adverse effect on our business, operating results or financial condition.
As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us as a means of slowing our entry into such markets or as a means to extract substantial license and royalty payments from us. Our competitors and others may now and in the future have significantly larger and more mature patent portfolios than we currently have. In addition, future litigation may involve patent holding companies or other adverse patent owners
60
who have no relevant product revenue and against whom our own patents may provide little or no deterrence or protection. Therefore, our commercial success may depend in part on our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in our existing and targeted markets and competitors may assert that our products infringe their intellectual property rights as part of a business strategy to impede our successful entry into those markets. We have not conducted comprehensive freedom-to-operate searches to determine whether the commercialization of our products or other business activities would infringe patents issued to third parties. Third parties may assert that we are employing their proprietary technology without authorization. In addition, our competitors and others may have patents or may in the future obtain patents and claim that use of our products infringes these patents. We could incur substantial costs and divert the attention of our management and technical personnel in defending against any of these claims. Parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement against us, we may be required to pay damages and obtain one or more licenses from third parties, or be prohibited from selling certain products. We may not be able to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products to avoid infringing third-party patents or proprietary rights. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products could materially affect our ability to grow and gain market acceptance for our products.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
In addition, our agreements with some of our suppliers, distributors, customers and other entities with whom we do business require us to defend or indemnify these parties to the extent they become involved in infringement claims against us, including the claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify any of these third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, operating results, or financial condition.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our employees’ former employers.
Many of our employees were previously employed at other medical diagnostic companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. A loss of key research personnel work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Our products contain third-party open source software components, and failure to comply with the terms of the underlying open source software licenses could restrict our ability to sell our products.
Our products contain software tools licensed by third-party authors under “open source” licenses. Use and distribution of open source software may entail greater risks than use of third-party commercial software, as open source licensors generally do not provide warranties or other contractual protections regarding infringement claims or the quality of the code. Some open source licenses contain requirements that we make available source code for modifications or derivative works we create based upon the type of open source software we use. If we combine our proprietary software with open source software in a certain manner, we could, under certain open source licenses, be required to release the source code of our proprietary software to the public. This would allow our competitors to create similar products with less development effort and time and ultimately could result in a loss of product sales.
Although we monitor our use of open source software to avoid subjecting our products to conditions we do not intend, the terms of many open source licenses have not been interpreted by U.S. courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to commercialize our products. Moreover, we cannot assure investors that our processes for controlling our use of open source software in our products will be effective. If we are held to have breached the terms of an open source software license, we could be required to seek licenses from third parties to continue
61
offering our products on terms that are not economically feasible, to re-engineer our products, to discontinue the sale of our products if re-engineering could not be accomplished on a timely basis, or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, operating results, and financial condition.
We use third-party software that may be difficult to replace or cause errors or failures of our products that could lead to lost customers or harm to our reputation.
We use software licensed from third parties in our products. In the future, this software may not be available to us on commercially reasonable terms, or at all. Any loss of the right to use any of this software could result in delays in the production of our products until equivalent technology is either developed by us, or, if available, is identified, obtained and integrated, which could harm our business. In addition, any errors or defects in third-party software, or other third-party software failures could result in errors, defects or cause our products to fail, which could harm our business and be costly to correct. Many of these providers attempt to impose limitations on their liability for such errors, defects or failures, and if enforceable, we may have additional liability to our customers or third-party providers that could harm our reputation and increase our operating costs.
We will need to maintain our relationships with third-party software providers and to obtain software from such providers that do not contain any errors or defects. Any failure to do so could adversely impact our ability to deliver reliable products to our customers and could harm our results of operations.
Risks Related to Being a Public Company
If we fail to maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of The NASDAQ Global Market. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with accounting principles generally accepted in the United States, or US GAAP. Commencing with our fiscal year ending December 31, 2016, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting in our Form 10-K for that year, as required by Section 404 of the Sarbanes-Oxley Act. This will require that we incur substantial additional professional fees and internal costs to expand our accounting and finance functions and that we expend significant management efforts. We may experience difficulty in meeting these reporting requirements in a timely manner.
We continue to review, document and test our internal control over financial reporting, but we are not currently in compliance with, and we cannot be certain when we will be able to implement the requirements of, Section 404 of the Sarbanes-Oxley Act. We also continue to take steps to remediate identified deficiencies in our internal control over financial reporting. For example, in September 2014, it was determined that we did not have adequate controls in place to properly account for our obligation to NuvoGen in connection with our purchase of intellectual property under an asset purchase agreement, which resulted in a restatement of previously issued financial statements. This deficiency in our internal controls was deemed to be a material weakness. A material weakness is a significant deficiency, or a combination of significant deficiencies, in internal control over financial reporting such that it is reasonably possible that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we fail to establish and maintain proper and effective internal control over financial reporting, we may not be able to produce timely and accurate financial statements, and our ability to accurately report our financial results could be adversely affected. If that were to happen, the market price of our stock could decline and we could be subject to sanctions or investigations by NASDAQ, the Securities and Exchange Commission, or the SEC, or other regulatory authorities.
Complying with the laws and regulations affecting public companies will increase our costs and the demands on management and could harm our operating results.
As a public company, and particularly after we cease to be an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act and rules subsequently implemented by the SEC and The NASDAQ Global Market impose numerous requirements on public companies, including requiring changes in corporate governance practices. Also, the Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. Our management and other personnel will need to devote a substantial amount of time to compliance with these laws and regulations. These requirements have increased and will continue to increase our legal, accounting, and financial compliance costs and have made and will continue to make some activities more time
62
consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or to incur substantial costs to maintain the same or similar coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or our board committees or as executive officers.
As an “emerging growth company,” we expect to avail ourselves of the exemption from the requirement that our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting under Section 404. However, we may no longer avail ourselves of this exemption when we cease to be an “emerging growth company.” When our independent registered public accounting firm is required to undertake an assessment of our internal control over financial reporting, the cost of our compliance with Section 404 will correspondingly increase. Our compliance with applicable provisions of Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues as we implement additional corporate governance practices and comply with reporting requirements. Moreover, if we are not able to comply with the requirements of Section 404 applicable to us in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
Furthermore, investor perceptions of our company may suffer if deficiencies are found, and this could cause a decline in the market price of our stock. Irrespective of compliance with Section 404, any failure of our internal control over financial reporting could have a material adverse effect on our stated operating results and harm our reputation. If we are unable to implement these requirements effectively or efficiently, it could harm our operations, financial reporting, or financial results and could result in an adverse opinion on our internal controls from our independent registered public accounting firm.
We are an “emerging growth company,” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to emerging growth companies could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012, and, for as long as we continue to be an “emerging growth company,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to “emerging growth companies,” including, but not limited to, not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years following the completion of our May 5, 2015 IPO, however, we would cease to be an “emerging growth company” before the end of that five-year period as of the following December 31, if we have more than $1.0 billion in annual revenue, or if the market value of our common stock that is held by non-affiliates exceeds $700 million as of June 30 of any year, or as of the date we issue more than $1.0 billion of non-convertible debt over a three-year period. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile.
Risks Related to Our Common Stock
We expect that our stock price will fluctuate significantly.
The trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include:
|
|
· |
actual or anticipated quarterly variation in our results of operations or the results of our competitors; |
|
|
· |
announcements by us or our competitors of new products, significant contracts, commercial relationships or capital commitments; |
|
|
· |
failure to obtain or delays in obtaining product approvals or clearances from the FDA or foreign regulators; |
|
|
· |
adverse regulatory or reimbursement announcements; |
|
|
· |
issuance of new or changed securities analysts’ reports or recommendations for our stock; |
|
|
· |
developments or disputes concerning our intellectual property or other proprietary rights; |
|
|
· |
commencement of, or our involvement in, litigation; |
|
|
· |
market conditions in the life sciences and molecular diagnostics markets; |
63
|
|
· |
any future sales of our common stock or other securities; |
|
|
· |
any change to the composition of the board of directors, executive officers or key personnel; |
|
|
· |
expiration of contractual lock-up agreements with our executive officers, directors and security holders; |
|
|
· |
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
|
|
· |
general economic conditions and slow or negative growth of our markets; and |
|
|
· |
the other factors described in this section of the Annual Report captioned “Risk Factors.” |
The stock market in general, and market prices for the securities of health technology companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. In several recent situations where the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
In addition, to date our common stock has generally been sporadically and thinly traded. As a consequence, the trading of relatively small quantities of our shares may disproportionately influence the price of our common stock in either direction. The price for our common stock could decline precipitously in the event that even a moderate amount of our common stock is sold on the market without commensurate demand.
If securities or industry analysts do not publish research reports about our business, or if they issue an adverse opinion about our business, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of the analysts who cover us issues an adverse opinion about our company, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
Certain holders of our securities are entitled to rights with respect to the registration of their shares under the Securities Act of 1933, as amended, or the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by these and subsequent sales. New investors could also gain rights superior to our existing stockholders.
Pursuant to the 2014 Equity Incentive Plan, or the 2014 plan, our board of directors is authorized to grant stock options and other equity-based awards to our employees, directors and consultants. The number of shares available for future grant under the 2014 plan will automatically increase on January 1 of each year by 4% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, subject to the ability of our board of directors to take action to reduce the size of the increase in any given year. In addition, our Board of Directors has approved the granting of rights to eligible employees to purchase shares of our common stock pursuant to our 2014 Employee Stock Purchase Plan, or the ESPP, beginning January 1, 2016. The
64
number of shares of our common stock reserved for issuance under the ESPP will automatically increase on January 1 of each calendar year by the lesser of 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year and 195,000 shares, subject to the ability of our board of directors to take action to reduce the size of the increase in any given year. Currently, we plan to register the increased number of shares available for issuance under the 2014 plan and ESPP each year. Increases in the number of shares available for future grant or purchase may result in additional dilution, which could cause our stock price to decline.
Our principal stockholders and management own a significant percentage of our stock and will be able to exercise significant influence over matters subject to stockholder approval.
Our executive officers, directors and principal stockholders, together with their respective affiliates, beneficially owned a majority of our capital stock at March 24, 2016. Accordingly, our executive officers, directors and principal stockholders acting together will be able to determine the composition of the board of directors, and may be able to approve all matters requiring stockholder approval, including mergers and other business combinations, and continue to have significant influence over our operations. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material adverse effect on our stock price and may prevent attempts by our stockholders to replace or remove the board of directors or management.
We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock.
We have never declared or paid any cash dividend on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by the terms of our debt facility, and any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any return to stockholders will therefore be limited to the appreciation of their stock.
Provisions in our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders or remove our current management.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management. These provisions include:
|
|
· |
authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
|
|
· |
limiting the removal of directors by the stockholders; |
|
|
· |
creating a staggered board of directors; |
|
|
· |
prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
|
|
· |
eliminating the ability of stockholders to call a special meeting of stockholders; and |
|
|
· |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by our board of directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders. Further, other provisions of Delaware law may also discourage, delay or prevent someone from acquiring us or merging with us.
Item 1B. Unresolved Staff Comments
None.
65
Our 30,100 square feet of corporate facilities, including our administrative, laboratory and manufacturing spaces, are located in Tucson, Arizona. We occupy these facilities pursuant to two separate leases. The first lease concerns 17,500 square feet housing our administrative, manufacturing, and lab services facilities. The second lease concerns 12,600 square feet of space used for our research and development facilities. We amended these facilities leases in August 2015 to, among other things, align and extend the lease terms to end on the five year anniversary of completion of improvements to the first lease facilities. In connection with the first lease term extension the landlord agreed to perform certain capital improvements to expand and improve the existing manufacturing and administrative facilities. Base rent payable under the first lease will be approximately $27,000 per month beginning in February 2016, and base rent payable under the second lease will be approximately $16,000 per month, in each case for the remaining terms of the respective leases, which expire in January 2021. The aggregate rents payable over the renewal term of the amended first lease represent an increase of $804,000, over the prior term rental rates for a 5-year period. The amended second lease specified no rent increase over the prior term and the annual rent increases for the applicable space were eliminated.
In addition, we entered into a construction contract with a third party in the third quarter of 2015 for the expansion of and leasehold improvements in our research and development facilities, representing an expected cash outlay commitment by us of approximately $500,000 through the first quarter of 2016. Such leasehold improvements will be capitalized and amortized over the remaining life of the lease as they are completed.
Our landlord holds security deposits equal to a total of approximately $23,000. We believe that our existing facilities are adequate to meet our business requirements for the reasonably foreseeable future and that additional space will be available on commercially reasonable terms, if required.
We are not engaged in any material legal proceedings. However, in the normal course of business, we may from time to time be named as a party to legal claims, actions and complaints, including matters involving employment, intellectual property others.
Item 4. Mine Safety Disclosures.
Not applicable.
66
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is traded on The NASDAQ Global Market under the symbol “HTGM.” Trading of our common stock commenced on May 6, 2015 in connection with our initial public offering. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported on The NASDAQ Global Market.
|
Year ended December 31, 2015 |
|
High |
|
|
Low |
|
||
|
Second quarter (beginning May 6, 2015) |
|
|
19.75 |
|
|
|
10.22 |
|
|
Third quarter |
|
|
13.10 |
|
|
|
4.64 |
|
|
Fourth quarter |
|
|
9.59 |
|
|
|
4.08 |
|
On March 18, 2016, the last reported sale price of our common stock was $2.63 per share.
Holders
As of March 18, 2016, there were approximately 204 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We have never declared or paid any cash dividends on our common stock. We anticipate that we will retain all available funds and any future earnings, if any, for use in the operation of our business and do not anticipate paying cash dividends in the foreseeable future. In addition, our term loan agreement materially restricts, and future debt instruments we issue may materially restrict, our ability to pay dividends on our common stock. Payment of future cash dividends, if any, will be at the discretion of the board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, the requirements of current or then-existing debt instruments and other factors the board of directors deems relevant.
Use of Proceeds
On May 5, 2015, we commenced our initial public offering pursuant to a registration statement on Form S-1 (File 333-201313) that was declared effective by the SEC on May 5, 2015 and registered an aggregate of 4,105,500 shares of our common stock for sale to the public at a price of $14.00 per share and an aggregate offering price of approximately $57.5 million. On May 11, 2015 and May 29, 2015, we sold 3,570,000 and 90,076 shares, respectively, to the public at a price of $14.00 per share for an aggregate gross offering price of $51.3 million. Leerink Partners acted as book-running manager for the offering, and Canaccord Genuity and JMP Securities served as co-managers for the offering.
Underwriting discounts and commission for our initial public offering totaled approximately $3.6 million. We incurred additional costs of approximately $2.3 million in offering expenses, which when added to the underwriting discounts and commissions paid by us, amounts to total fees and costs of approximately $5.9 million. Thus net offering proceeds to us, after deducting underwriting discounts and commissions and offering costs, were approximately $45.4 million. No offering costs were paid directly or indirectly to any of our directors or officers (or their affiliates) or persons owning ten percent or more of any class of our equity securities.
Pending their use, the net proceeds from our IPO are being held in cash and cash equivalents or used to purchase low risk available-for-sale securities, of which $28.2 million were short-term available-for-sale securities, at fair value, and $2.6 million was long-term available-for-sale securities, at fair value, at December 31, 2015. As of December 31, 2015, we have expended approximately $14.1 million of the proceeds from our IPO on the following: (1) approximately $9.5 million for sales and marketing and general and administrative expenses; (2) approximately $2.8 million for research and development expansion efforts, including expansion of our research and development team and development of new applications and profiling panels; (3) approximately $0.5 million for payment of interest on our growth term loan; and (4) approximately $1.3 million to purchase inventory and to fund working capital and other general corporate purposes.
There has been no material change in the expected use of net proceeds from our IPO as described in our final prospectus filed with the SEC.
67
Item 6. Selected Financial Data.
The following selected financial data should be read together with our financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this filing. The selected financial data in this section are not intended to replace our financial statements and the related notes. Our historical results are not necessarily indicative of the results that may be expected in the future and results of interim periods are not necessarily indicative of the results for the entire year.
The selected statement of operations data for the years ended December 31, 2015 and 2014 and the selected balance sheet data as of December 31, 2015 and 2014 are derived from our audited financial statements appearing elsewhere in this filing.
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands, except share and per share amounts) |
|
|||||
|
Statement of Operations: |
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
4,042 |
|
|
$ |
3,329 |
|
|
Cost of revenue |
|
|
3,336 |
|
|
|
3,205 |
|
|
Gross margin |
|
|
706 |
|
|
|
124 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
14,994 |
|
|
|
9,898 |
|
|
Research and development |
|
|
4,602 |
|
|
|
3,075 |
|
|
Total operating expenses |
|
|
19,596 |
|
|
|
12,973 |
|
|
Operating loss |
|
|
(18,890 |
) |
|
|
(12,849 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Loss from stock warrant valuation |
|
|
(240 |
) |
|
|
(389 |
) |
|
Interest expense |
|
|
(1,719 |
) |
|
|
(707 |
) |
|
Interest income |
|
|
86 |
|
|
|
— |
|
|
Loss on settlement of convertible debt |
|
|
(705 |
) |
|
|
— |
|
|
Other |
|
|
81 |
|
|
|
(14 |
) |
|
Net loss before income taxes |
|
|
(21,388 |
) |
|
|
(13,958 |
) |
|
Income taxes |
|
|
10 |
|
|
|
— |
|
|
Net loss |
|
|
(21,398 |
) |
|
|
(13,958 |
) |
|
Accretion of stock issuance costs |
|
|
(35 |
) |
|
|
(103 |
) |
|
Accretion of Series E warrant discount |
|
|
(128 |
) |
|
|
(313 |
) |
|
Accretion of Series D and E redeemable convertible preferred stock dividends |
|
|
(1,166 |
) |
|
|
(3,245 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(22,726 |
) |
|
$ |
(17,619 |
) |
|
Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(5.03 |
) |
|
$ |
(175.03 |
) |
|
Shares used in computing net loss per common share, basic and diluted |
|
|
4,518,499 |
|
|
|
100,659 |
|
|
|
|
As of December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
3,294 |
|
|
$ |
3,613 |
|
|
Working capital |
|
|
28,539 |
|
|
|
2,909 |
|
|
Total assets |
|
|
39,447 |
|
|
|
8,728 |
|
|
Total long-term debt |
|
|
22,553 |
|
|
|
22,476 |
|
|
Redeemable convertible preferred stock |
|
|
— |
|
|
|
55,923 |
|
|
Accumulated deficit |
|
|
(89,566 |
) |
|
|
(68,169 |
) |
68
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis together with “Item 6. Selected Financial Data” and our financial statements and related notes included elsewhere in this Annual Report. The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those expressed or implied in any forward-looking statements as a result of various factors, including those set forth under the caption “Item 1A. Risk Factors.”
Overview
We are a commercial stage company that develops and markets products based on a novel technology platform to facilitate the routine use of complex molecular profiling. Our HTG Edge and HTG EdgeSeq platforms automate sample processing and can quickly, robustly and simultaneously profile hundreds or thousands of molecular targets from samples a fraction of the size required by current technologies. Our objective is to establish the HTG Edge and HTG EdgeSeq platforms as standards in molecular profiling and make this capability accessible to all molecular labs from research to the clinic. We believe that our target customers desire high quality molecular profiling information in a multiplexed panel format from increasingly smaller and less invasive samples, with the ability to collect such information locally to minimize turnaround time and cost.
Our HTG Edge system is capable of running both our original, plate-based chemistry, which quantifies RNA using our plate reader included with the system, and our HTG EdgeSeq chemistry, which is detected using NGS instrumentation provided by the end user. We also have the flexibility to provide the HTG Edge instrument without our plate reader or a dedicated HTG EdgeSeq system for those customers whose focus is NGS readout of our HTG EdgeSeq chemistry. We plan to launch a new version of our HTG EdgeSeq system in 2017 that will target the lower-volume throughput lab market. This program, referred to as “Project JANUS” for development, is expected to increase our addressable market by enabling efficient molecular profiling of smaller quantity batches of samples.
Our innovative platforms and menu of molecular profiling panels are being utilized by a wide range of customers including biopharmaceutical companies, academic institutions and molecular labs to simultaneously analyze a comprehensive set of molecular information from valuable clinical samples and substantially improve their workflow efficiency. We currently market several proprietary molecular profiling panels that address the needs of customers in high impact areas of translational research and biopharmaceutical biomarker and companion diagnostics. In addition, we have a focused development pipeline that includes planned panels for translational research, drug development and molecular diagnostics. Our product strategy is to build complete profiling panels of established and emerging molecular targets for broader and disease-specific approaches.
We have incurred significant losses since our inception, and we have never been profitable. We incurred net losses of $21.4 million and $14.0 million for the years ended December 31, 2015 and 2014, respectively. As of December 31, 2015, we had an accumulated deficit of $89.6 million and we had available cash and cash equivalents totaling approximately $3.3 million and investments in highly liquid corporate and government debt securities totaling $28.2 million.
Recent Developments
We continue to expand and evolve our assay and panel offerings, focusing primarily on our HTG EdgeSeq platform. Throughout the year ended December 31, 2015, we launched several new HTG EdgeSeq products for research use only, including our HTG EdgeSeq Oncology Biomarker Panel, HTG EdgeSeq Immuno-Oncology Assay, HTG EdgeSeq Lymphoma Panel and HTG EdgeSeq Diffuse Large B-Cell Lymphoma Cell of Origin Assay.
We are collaborating with The Centre of Excellence for the Prevention of Organ Failure (PROOF Centre) in Vancouver, Canada by providing technical advice on our HTG EdgeSeq system, which they are using for their development of an RNA-based liquid biopsy test from peripheral blood. The heart transplant rejection blood test is intended to provide early indication of organ rejection in heart transplant patients, allowing doctors to better monitor and treat patients post-transplant.
We completed a product development service agreement and have begun full development of our new low throughput instrument, Project JANUS. We are targeting a launch by the end of 2017. We believe that this product will expand our addressable market into the smaller clinical labs.
We hired a Vice President of European Commercial Operations and have begun implementing a direct sales and support approach in Europe.
69
Factors Affecting our Performance
We believe that our future results of operations are dependent on a number of factors discussed below. While each of these areas present significant opportunities for us, they also pose significant risks and challenges that we must successfully address. See the section entitled “Risk Factors” for further discussion of these risks.
The installed base of our HTG Edge and HTG EdgeSeq platform and proprietary panels
The growth of our business is tied to the number of HTG Edge and HTG EdgeSeq instruments we install. In addition to sales of our HTG Edge and HTG EdgeSeq systems, we place systems under reagent rental agreements, where we install instruments in the customer’s facility for no upfront charge, and the customer agrees to purchase consumable products at a stated price over the term of the agreement. In some instances we provide instruments free of charge under evaluation agreements for a limited period of time to permit the user to evaluate the system in anticipation of a decision to purchase the system. Other arrangements might include a collaboration agreement whereby an academic or commercial collaborator agrees to provide samples free of charge in exchange for the use of an HTG Edge or HTG EdgeSeq instrument at no cost in furtherance of a research or clinical project. Further, there are instances where we provide our systems to key opinion leaders free of charge, to gather data and publish the results of their research to assist in our marketing efforts. All of these efforts are intended to further our goal of increasing the installed base of our HTG Edge and HTG EdgeSeq instruments to drive instrument and consumable revenue. We refer to our installed base of HTG Edge and HTG EdgeSeq instruments as those systems that have been purchased by a customer or that have been placed with customers or key opinion leaders under reagent rental, capital purchase or evaluation agreements.
Our ability to increase instrument and consumable revenue depends on a number of factors, including (i) adoption of our HTG Edge and HTG EdgeSeq platforms by our customer base, including increasing market share for our proprietary assays for the research market; (ii) the efforts of our sales and marketing teams to demonstrate the utility of our products and technology; (iii) our ability to develop and market novel molecular profiling panels designed to meet customer needs, including unmet medical needs; (iv) our ability to demonstrate the benefits of our products to key opinion leaders so they will publish information supporting those benefits; (v) pricing and reimbursement; and (vi) our ability to expand the addressable market of our HTG Edge and HTG EdgeSeq platforms through the development of new applications. Given the length of our sales cycle, we have in the past experienced, and will likely in the future experience, fluctuations in our instrument and consumables sales on a period-to-period basis.
Our future financial performance will be driven in large part by the rate of placement of our HTG Edge and HTG EdgeSeq systems and the timely development and launch of a new version of our HTG EdgeSeq system (Project JANUS) in 2017. We plan to grow our HTG Edge and HTG EdgeSeq instrument and consumable sales through multiple commercial strategies, including expansion of our commercial efforts outside of the United States, evolving our instrument platform, assay and panel offerings, collaborating with biopharmaceutical companies on companion drug development programs and demonstrating the benefits of our products, including by expansion of our Scientific Advisory Boards with distinguished leaders in the markets we serve. As of December 31, 2015 we had an installed base of 49 HTG Edge and HTG EdgeSeq systems, and had 30 active development programs with biopharmaceutical companies. We are focused on instrument placements, consumable pull through and companion diagnostic development program progress as the primary indicators of commercial adoption and the success of our business model.
The ability to increase revenue to a level sufficient to allow us to achieve economies of scale with our cost of revenue
Our cost of revenue includes certain fixed costs related primarily to fixed headcount and facilities. Increasing our gross margin will require increases in revenues which will allow us to absorb our fixed costs of manufacturing and service. If our revenues do not increase, our gross margins could be inadequate to absorb our fixed costs of manufacturing and service.
Development of additional products
We rely on sales of HTG Edge and HTG EdgeSeq instruments and consumables to generate revenue. As part of our strategy we plan to develop new proprietary panels and applications for our HTG Edge and HTG EdgeSeq platforms. Our success in developing new products will be important in our efforts to grow our business by expanding the potential market for our products, diversifying our sources of revenue and providing recurring consumable revenue.
Timing of Research and Development Expenses
Our spending on research and development may vary substantially from period to period due to the availability and cost of clinical samples or to the timing of milestone payments under technology development agreements.
70
Financial Operations Overview and Results of Operations
Comparison of the Years Ended December 31, 2015 and 2014
|
|
|
December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
Change |
|
|||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product |
|
$ |
3,532,028 |
|
|
$ |
1,788,205 |
|
|
$ |
1,743,823 |
|
|
Service |
|
|
183,758 |
|
|
|
497,168 |
|
|
|
(313,410 |
) |
|
Other |
|
|
325,789 |
|
|
|
1,043,584 |
|
|
|
(717,795 |
) |
|
Total revenue |
|
|
4,041,575 |
|
|
|
3,328,957 |
|
|
|
712,618 |
|
|
Cost of revenue |
|
|
3,335,511 |
|
|
|
3,204,915 |
|
|
|
130,596 |
|
|
Gross margin |
|
|
706,064 |
|
|
|
124,042 |
|
|
|
582,022 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
14,994,410 |
|
|
|
9,897,697 |
|
|
|
5,096,713 |
|
|
Research and development |
|
|
4,601,718 |
|
|
|
3,075,204 |
|
|
|
1,526,514 |
|
|
Total operating expenses |
|
|
19,596,128 |
|
|
|
12,972,901 |
|
|
|
6,623,227 |
|
|
Operating loss |
|
|
(18,890,064 |
) |
|
|
(12,848,859 |
) |
|
|
(6,041,205 |
) |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from change in stock warrant valuation |
|
|
(239,683 |
) |
|
|
(388,936 |
) |
|
|
149,253 |
|
|
Interest expense |
|
|
(1,719,475 |
) |
|
|
(706,866 |
) |
|
|
(1,012,609 |
) |
|
Interest income |
|
|
85,859 |
|
|
|
— |
|
|
|
85,859 |
|
|
Loss on settlement of convertible debt |
|
|
(705,217 |
) |
|
|
— |
|
|
|
(705,217 |
) |
|
Other |
|
|
80,978 |
|
|
|
(13,747 |
) |
|
|
94,725 |
|
|
Total other expense |
|
|
(2,497,538 |
) |
|
|
(1,109,549 |
) |
|
|
(1,387,989 |
) |
|
Net loss before income taxes |
|
|
(21,387,602 |
) |
|
|
(13,958,408 |
) |
|
|
(7,429,194 |
) |
|
Income taxes |
|
|
10,189 |
|
|
|
— |
|
|
|
10,189 |
|
|
Net loss |
|
$ |
(21,397,791 |
) |
|
$ |
(13,958,408 |
) |
|
$ |
(7,439,383 |
) |
Revenue
We generate revenue from the sale of our HTG Edge and HTG EdgeSeq platforms, including our HTG Edge and HTG EdgeSeq systems, our proprietary consumables and related services, such as sample processing for pharmaceutical customers. Consumables consist primarily of our molecular profiling panels, which we also refer to as assays and test menu. Total revenue for the year ended December 31, 2015 increased by 21% to $4.0 million compared to total revenue for the year ended December 31, 2014.
Product revenue
Product revenue includes revenue from the sale of instruments and consumables. Revenue from the sale of instruments is derived from the sale of our HTG Edge and HTG EdgeSeq systems. Revenue from the sale of instruments represented 19% and 24% of our total revenue for the years ended December 31, 2015 and 2014, respectively.
In addition to the sale of our HTG Edge and HTG EdgeSeq instruments, we place systems under reagent rental agreements, whereby we install instruments in our customer’s facility for no upfront charge and the customer agrees to purchase our consumable products at a stated priced over the term of the agreement. In some instances, we provide instruments free of charge under short term evaluation agreements which allow a prospective customer to evaluate our system in anticipation of their decision to purchase the system. In almost all of our evaluation agreements, our prospective customer is required to purchase consumables during the evaluation period. We also have collaboration agreements whereby academic or other collaborators are provided the use of our instrument at no cost. We might receive free samples in exchange for the use of our instrument by a collaborator or, in the case of a key opinion leader, the collaborator might agree to develop data using our instrument and publish the results of their research to assist in our marketing efforts.
71
Revenue from the sale of consumables is derived from the sale of our research-use-only molecular profiling panels for use on our HTG Edge and HTG EdgeSeq platforms and from the sale of custom molecular profiling panels that we have developed for customers. Consumables revenue represented 68% and 30% of our total revenue for the years ended December 31, 2015 and 2014, respectively.
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Instruments |
|
$ |
789,231 |
|
|
$ |
793,505 |
|
|
Consumables |
|
|
2,742,797 |
|
|
|
994,700 |
|
|
Total product sales |
|
$ |
3,532,028 |
|
|
$ |
1,788,205 |
|
Product revenue for the year ended December 31, 2015 was $3.5 million compared to $1.8 million for the year ended December 31, 2014. Instrument revenue for the year ended December 31, 2015 was $789,000 compared to $794,000 for the year ended December 31, 2014 as one additional instrument was sold in 2014 than in 2015. Consumable revenue for the year ended December 31, 2015 was $2.7 million compared to $1.0 million for the year ended December 31, 2014. The year over year increase in consumables revenue is driven by our growing installed base, the launch of the HTG EdgeSeq chemistry in the second half of 2014 and our launch of several new HTG EdgeSeq product offerings for research only, including our HTG EdgeSeq Oncology Biomarker Panel in early 2015, followed by the HTG EdgeSeq Immuno-Oncology Assay, HTG EdgeSeq Lymphoma Panel and HTG EdgeSeq Diffuse Large B-Cell Lymphoma Cell of Origin Assay, all in the second half of 2015.
Service revenue
Service revenue consists of the design of custom panels for biopharmaceutical customers, research services and sample processing. We have strategically shifted our focus away from the service business model, primarily custom assay design services, although we continue to opportunistically pursue accretive service revenue which can be complementary to collaboration with our pharmaceutical partners who have chosen to utilize our HTG EdgeSeq system in their drug development programs. As a result, we may see periods of significant service revenue from time to time. Service revenue represented 5% and 15% of our total revenue for the years ended December 31, 2015 and 2014, respectively.
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Custom panel design |
|
$ |
101,792 |
|
|
$ |
425,168 |
|
|
Sample processing |
|
|
81,966 |
|
|
|
72,000 |
|
|
Total service revenue |
|
$ |
183,758 |
|
|
$ |
497,168 |
|
Service revenue was $184,000 for the year ended December 31, 2015 compared to $497,000 for the year ended December 31, 2014. The decrease in service revenue for the period ended December 31, 2015 compared to 2014 reflects a strategic movement away from certain service revenue, primarily custom assay design services, continuing to seek service revenue opportunities only when they are deemed to be complementary to collaborations with our pharmaceutical partners who have chosen to utilize our HTG EdgeSeq system in their drug development programs.
Other revenue
Other revenue consists grant revenues received from the National Institutes of Health, or NIH. Revenue from NIH grants represented 8% and 31% of total revenue for the years ended December 31, 2015 and 2014, respectively. In August 2013 we were awarded a grant from the NIH to develop commercial applications for our proprietary nuclease probe mediated sequencing technology. This NIH grant was completed in June 2015. We recognized $0.3 million and $1.0 million of income provided by the NIH for a Phase 2 grant in 2015 and 2014, respectively.
Cost of revenue
Cost of revenue reflects the aggregate costs incurred in manufacturing, delivering, installing and servicing instruments and the cost of manufacturing consumables. The components of our cost of revenue are materials and service costs, manufacturing costs paid to third-party manufacturers and those costs incurred internally which include direct labor costs, equipment and infrastructure expenses associated with the manufacturing and distribution of our products.
For the years ended December 31, 2015 and 2014, cost of revenue included significant fixed costs consisting primarily of manufacturing headcount, field service engineers and facilities. Due to the fixed nature of expenses associated with direct labor,
72
equipment and infrastructure, we expect cost of revenue as a percentage to decrease over time as our volume and revenue increases, absorbing those fixed costs. Additionally, cost of revenue related to grants is higher as a percentage of that specific revenue. As a result, cost of revenue as a percentage of total revenue decreased in 2015 as our grant revenue decreased. We expect to see further improvement in 2016 as grant revenue is not expected to recur.
Cost of revenue increased by $131,000, or 4% for the year ended December 31, 2015 compared to the year ended December 31, 2014. This increase was primarily due to an excess inventory reserve of approximately $210,000 recorded for deemed excess reader inventory related to our original HTG Edge system. This inventory reserve indirectly results from a faster than expected adoption of our HTG EdgeSeq products which do not utilize the reader technology. The increase in higher margin consumable sales and the decrease in lower margin grant revenue year over year also contributed to the decrease in cost of revenue as a percentage of revenue. Cost of revenue included $2.2 million in largely fixed manufacturing costs for the year ended December 31, 2015, and $1.3 million for the year ended December 31, 2014. As we grow our revenues, we do not expect these fixed costs to increase on a linear basis and expect these costs will decrease as a percentage of revenue over time.
Research and development expenses
Research and development expenses represent costs to develop new proprietary panels and evolve our HTG EdgeSeq platform. These expenses include payroll and related expenses, consulting expenses, laboratory supplies and amounts incurred under collaborative supply or development agreements. Research and development costs are expensed as incurred. We expect our research and development expenses to increase in absolute dollars in future periods as we continue to develop additional panels and evolve our systems. Further, we began the development of our next generation instrument platform, Project JANUS, in 2015. We expect research and development costs to increase in 2016 and 2017 as this project continues toward the launch of our new platform.
Research and development expenses increased by $1.5 million, or 50%, for the year ended December 31, 2015 compared to the year ended December 31, 2014. Expenses incurred for the year ended December 31, 2014 related primarily to the development of our HTG EdgeSeq systems and panels. Increases in research and development expenses in 2015 have been driven by increases in research and development headcount who support our increasing activities related to our next generation Project JANUS instrument platform and our panel development programs.
Selling, general and administrative expenses
Selling, general and administrative expenses primarily consist of personnel costs for our sales and marketing, regulatory, legal, executive management and finance and accounting functions. The expenses also include stock-based compensation, promotional expenses, consulting and facility and overhead costs. Our selling, general and administrative expenses increased in 2015 as a result of operating as a public company, including expenses related to compliance with the rules and regulations of the Securities and Exchange Commission and The NASDAQ Global Market, additional director and officer insurance costs, investor relations activities and other administrative and professional services. We expect to see increased levels of spending in 2016 as we will incur a full year of expenses as a public company. We also expect selling, general and administrative expenses to increase as we expand our commercial activities in the United States and in Europe.
Selling, general and administrative expenses increased by $5.1 million, or 51%, for the year ended December 31, 2015, compared to the year ended December 31, 2014. This increase was primarily driven by higher sales and marketing headcount costs of approximately $1.7 million, in connection with the expansion of commercial sales activities for our HTG Edge and HTG EdgeSeq systems in 2015. In addition, general and administrative costs increased by $2.5 million, primarily due to increases in payroll costs of $0.7 million, professional fees of $1.0 million, and insurance costs of $0.4 million. The increase in professional fees is related to financial audits, legal fees and consulting fees, all related to our initial public offering and subsequent costs of being a public company. Insurance costs, also increased significantly over the prior year, primarily related to increased director and officer insurance premiums.
Loss from change in stock warrant valuation
Loss from the change in stock warrant valuation for the year ended December 31, 2015 was a result of an increase in the fair value of our preferred stock warrants just prior to the exercise of Series D preferred stock warrants, the conversion to common stock warrants of our Series C-2 preferred stock warrants, convertible note warrants and growth term loan warrants at the time of our initial public offering. The increase in the fair value of our preferred stock warrants until their conversion to common stock warrants was primarily attributable to the proximity to and increased likelihood of completing an initial public offering.
73
As of December 31, 2015, we had an obligation due to NuvoGen Research, LLC, or NuvoGen, in the amount of $9.2 million under an asset purchase agreement and an obligation due to a syndicate of two lending institutions of $11.0 million under a growth term loan entered into in August 2014 and amended in August 2015. Interest expense for the year ended December 31, 2015 and 2014 related to our borrowings under our loan agreement and also included non-cash interest expense related to our obligation to NuvoGen.
Interest expense increased by $1.0 million for the year ended December 31, 2015 as compared to the year ended December 31, 2014. The increase related primarily to the growth term loan that included interest of $0.9 million and the accretion of financing costs and related premiums and discounts of $0.4 million for the year ended December 31, 2015, compared to interest of $0.4 million and accretion of financing costs and related premiums and discounts of $0.2 million for the year ended December 31, 2014. Interest expense related to the NuvoGen obligation was $0.3 million for the year ended December 31, 2015 compared to $0.2 million for the year ended December 31, 2014.
Loss on settlement of convertible debt
With completion of our initial public offering in May 2015, our remaining convertible note debt discount and deferred financing costs relating to the convertible notes totaling $0.7 million were charged to loss on settlement of convertible debt with the issuance of common stock in settlement of the convertible notes and related accrued interest.
Cash Flows for the Years Ended December 31, 2015 and 2014
The following table summarizes the primary sources and uses of cash for each of the periods presented:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(19,243,273 |
) |
|
$ |
(12,996,616 |
) |
|
Investing activities |
|
|
(32,184,889 |
) |
|
|
(857,677 |
) |
|
Financing activities |
|
|
51,108,753 |
|
|
|
15,652,396 |
|
|
(Decrease) increase in cash and cash equivalents |
|
$ |
(319,409 |
) |
|
$ |
1,798,103 |
|
Operating Activities
Net cash used in operating activities for the year ended December 31, 2015 was $19.2 million and reflected (i) the net loss of $21.4 million, (ii) net non-cash items of $3.0 million, consisting primarily of amortization and write off at the time of our initial public offering of discount on convertible notes of $0.7 million, depreciation and amortization of $0.7 million, amortization of the discount on the NuvoGen obligation of $0.3 million, amortization of the discount and final payment premium on the growth term loan of $0.3 million, provision for excess inventory of $0.2 million, share-based compensation of $0.4 million and a decrease in the warrant valuation of $0.2 million and (iii) a net cash outflow from changes in balances of operating assets and liabilities of $0.9 million. The significant items comprising the changes in balances of operating assets and liabilities were an increase of $0.7 million in inventory and an increase in prepaid and other of $0.3 million.
Net cash used in operating activities for the year ended December 31, 2014 was $13.0 million and reflected (i) the net loss of $14.0 million, (ii) net non-cash items of $1.6 million, consisting primarily of depreciation and amortization of $0.5 million, increase in bad debt reserve of $0.1 million, share-based compensation of $0.2 million and amortization of the discount on the NuvoGen obligation of $0.2 million and a decrease in the warrant valuation of $0.4 million (iii) a net cash outflow from changes in balances of operating assets and liabilities of $0.7 million. The significant items comprising the changes in balances of operating assets and liabilities were a reduction in accrued liabilities of $0.3 million and increases in accounts receivable and inventory of $0.3 million and $0.7 million, respectively.
Investing Activities
Net cash used in investing activities of $32.2 million for the year ended December 31, 2015 consisted primarily of the purchase of $38.3 million and sales, redemptions and maturities of $7.5 million of available-for-sale securities originally acquired with proceeds from our initial public offering. Prior to our initial public offering, our investing activities consisted primarily of the purchase of laboratory and office equipment.
74
Net cash used in investing activities of $0.9 million for the year ended December 31, 2014 consisted primarily of the purchase of laboratory and office equipment.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2015 of $51.1 million included $47.7 million proceeds from our IPO, partially offset by $1.0 million deferred offering costs incurred in the period, and $4.5 million in proceeds from convertible notes.
Net cash provided by financing activities for the year ended December 31, 2014 of $15.7 million included the $7.4 million net proceeds from the Series E preferred stock issuance and a $10.7 million proceeds from growth term loan initiation, partially offset by $1.2 million of payments on our NuvoGen obligation and $1.2 million of deferred offering costs incurred in the period.
Liquidity and Capital Resources
Since our inception, our operations have primarily been financed through the issuance of our common stock, redeemable convertible preferred stock, the incurrence of debt and cash received from instrument and consumables sales, services revenue and other income. Through December 31, 2015, we had received net proceeds of $52.9 million from issuances of preferred stock, including preferred stock issued on conversion of promissory notes, $45.4 million of net proceeds from the issuance of our common stock, $0.8 million in proceeds from a prior term loan, approximately $7.7 million in grants, $10.7 million from our growth term loan (net of $0.3 million original issue discount), $4.5 million from convertible note issuances and $31.3 million from product and service revenue. As of December 31, 2015, we had cash, cash equivalents and short-term available-for-sale investments totaling $31.5 million and $19.9 million of debt outstanding on our growth term loan payable, NuvoGen obligation and capital lease obligations. Amounts due on the convertible notes as well as the related accrued interest were automatically converted into 324,591 shares of our common stock in connection with the initial closing of our initial public offering in May 2015.
We have had recurring operating losses and negative cash flows from operations since inception, and we have an accumulated deficit of approximately $89.6 million as of December 31, 2015.
In February 2016, we notified our lenders, Oxford Finance LLC and Silicon Valley Bank, pursuant to requirements of the amendment to the Growth Term Loan agreement, of our intention to draw the remaining $5.0 million available under Growth Term Loan B on or before March 31, 2016. This additional principal will be repaid evenly over the course of 30 months beginning April 1, 2016.
On May 11, 2015, we completed the initial closing of our initial public offering, where we sold 3,570,000 shares of our common stock at a price of $14.00 per share, which excluded the underwriters’ exercise of their over-allotment option, available for thirty days after May 5, 2015. We received additional net proceeds of approximately $1.3 million with the sale of 90,076 additional shares of our common stock at $14.00 per share pursuant to the partial exercise by our underwriters of their over-allotment option. We raised approximately $45.4 million in net proceeds from our initial public offering, after deducting underwriting discounts and commissions of $3.6 million and offering expenses of $2.3 million. These costs have been recorded as a reduction of the proceeds received in arriving at the amount recorded in additional paid-in capital. In connection with the closing of the initial public offering, all outstanding shares of our preferred stock were converted into 2,134,192 shares of common stock.
In August 2014, we entered into an asset-secured growth capital term loan with Oxford Finance, LLC and Silicon Valley Bank. We borrowed the first tranche of the term loan in the amount of $11.0 million in August 2014. In August 2015, we and the lenders entered into an amendment to the loan and security agreement governing our growth term loan to extend the expiration of the draw period for the second tranche of $5.0 million to March 31, 2016. The loan accrues interest annually at the rate of 8.5% and matures on September 1, 2018, and pursuant to our August 2015 amendment, is payable in monthly interest-only payments through March 31, 2016. Following the interest-only payment period, equal monthly payments of principal and interest amortized over the remaining term of the loan will be due.
Funding Requirements
We believe that our cash, cash equivalents, short term investment securities and amounts available for borrowing under our growth capital term loan, will be sufficient to fund our operations and capital expenditure needs for at least the next 12 months and likely through the midpoint of the second quarter 2017. However, our estimates of the amount of cash necessary to fund our operations and development and commercialization activities may prove to be wrong, and we could spend our available financial resources faster than we currently expect.
75
We expect to need to raise additional capital to support the commercialization efforts related to our current and future products, repay debt obligations in accordance with contractual payment terms, fund our operations and further our research and development activities in the future. Future funding requirements will depend on a number of factors, including the cost and timing of establishing additional sales, marketing and distribution capabilities, the ongoing cost of research and development activities, the cost and timing of regulatory clearances and approvals, the effect of competing technology and market developments, the nature and timing of companion diagnostic development collaborations we may establish, and the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions. Additional capital may not be available, at such times or in amounts as needed by us. Even if capital is available, it might be available only on unfavorable terms. If we are unable to raise additional capital in the future when required and in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue our planned product development or the commercialization of our current and any future products, or we may be required to significantly reduce expenses, sell assets, seek a merger or joint venture partner, file for protection from creditors or liquidate our assets. If we raise additional funds through the issuance of additional debt or equity securities, it could result in increased fixed payment obligations, and/or dilution of current stockholders.
Contractual Obligations
The following table summarizes our contractual obligation as of December 31, 2015:
|
|
|
Payments due by Period |
|
|||||||||||||||||
|
|
|
Total |
|
|
Less Than 1 Year |
|
|
1 - 3 Years |
|
|
3 - 5 Years |
|
|
More Than 5 Years |
|
|||||
|
Debt obligations (1) |
|
$ |
22,247,622 |
|
|
$ |
4,452,164 |
|
|
$ |
10,696,715 |
|
|
$ |
1,200,000 |
|
|
$ |
5,898,743 |
|
|
Operating lease obligations (2) |
|
|
2,816,554 |
|
|
|
584,272 |
|
|
|
1,660,686 |
|
|
|
571,596 |
|
|
|
— |
|
|
Capital lease obligations (3) |
|
|
57,893 |
|
|
|
29,243 |
|
|
|
28,650 |
|
|
|
— |
|
|
|
— |
|
|
Total contractual obligations |
|
$ |
25,122,069 |
|
|
$ |
5,065,679 |
|
|
$ |
12,386,051 |
|
|
$ |
1,771,596 |
|
|
$ |
5,898,743 |
|
|
(1) |
Our debt obligations include amounts due to NuvoGen under an asset purchase agreement and, beginning in 2018, includes 2.5% interest on the then-remaining obligation. Through 2017, we owe minimum payments under the NuvoGen agreement. Beginning in 2018, we are obligated to pay the greater of $400,000 or 6% of sales plus amounts, if any, deferred in the 2016 and 2017 periods by which 6% of revenue exceeds the applicable fixed fee plus 5% interest on any such deferred amounts until the obligation is paid in full. Our debt obligations further include our contractual obligations pursuant to our outstanding $11.0 million term loan under our loan and security agreement with Oxford Finance LLC and Silicon Valley Bank entered into in 2014 and amended in 2015. The table above includes contractual interest payments and a final premium fee relating to this loan. Refer to footnote 8 of our audited financial statements in Item 8 for payments due under the term loan. |
|
(2) |
Our operating lease obligations consist of the leases for our laboratory and office facilities in Tucson, AZ expiring in 2021, as well as office copier and computer equipment leases. |
|
(3) |
Our capital lease obligation consists of an equipment financing arrangement with a vendor, which was entered into in December 2012. |
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update No. 2014-09, Revenue from Contracts with Customers, or ASU 2014-09, which supersedes nearly all existing revenue recognition guidance under US GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing US GAAP.
The standard is effective for annual periods beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). We are currently evaluating the impact of our pending adoption of ASU 2014-09 on our financial statements and have not yet determined the method by which we will adopt the standard.
76
In August 2014, the FASB issued Accounting Standards Update No. 2014-15, Going Concern, or ASU 2014-15. ASU 2014-15 provides US GAAP guidance on management’s responsibility in evaluating whether there is substantial doubt about a company’s ability to continue as a going concern and about related footnote disclosures. For each reporting period, management will be required to evaluate whether there are conditions or events that raise substantial doubt about a company’s ability to continue as a going concern within one year from the date the financial statements are issued. The standard will be effective for annual periods ending after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early application is permitted for annual or interim reporting periods for which the financial statements have not previously been issued. We are currently evaluating the impact of our pending adoption of ASU 2014-15 on our financial statements and anticipate adoption in the first quarter 2016. Based upon current projections, without receipt of a firm commitment for an additional capital infusion in the first quarter, management believes that adoption of this standard in the first quarter may result the need for additional liquidity disclosures regarding the entity’s ability to continue as a going concern to be included in our Quarterly Report on Form 10-Q for the quarter ending March 31, 2016.
In April 2015, the FASB issued an accounting standards update entitled “ASU 2015-03, Interest – Imputation of Interest: Simplifying the presentation of Debt Issuance Costs”. The standard requires entities to present debt issuance costs on the balance sheet as a direct deduction from the related debt liability rather than as an asset, and the amortization is reported as interest expense. The result of application of this guidance would be to reduce the deferred financing costs balance, with a corresponding reduction to the long term liabilities to which the debt issuance costs relate in the balance sheets. The standard does not affect recognition and measurement of debt issuance costs. We expect to adopt ASU 2015-03 on January 1, 2016.
In April 2015, the FASB issued ASU No. 2015-05, Intangibles - Goodwill and Other - Internal-Use Software (Subtopic 350-40): Customer's Accounting for Fees Paid in a Cloud Computing Arrangement. Under this standard, if a cloud computing arrangement includes a software license, the software license element of the arrangement should be accounted for consistent with the acquisition of other software licenses. If a cloud computing arrangement does not include a software license, the arrangement should be accounted for as a service contract. We expect to adopt ASU 2015-05 on January 1, 2016. The adoption of this standard is not expected to have a significant impact on our financial position or results of operations.
In July 2015, the FASB issued ASU 2015-11, Inventory: Simplifying the Measurement of Inventory (“ASU 2015-11”). The standard requires inventory within the scope of the ASU to be measured using the lower of cost and net realizable value. The changes apply to all types of inventory, except those measured using LIFO or retail inventory method, and are intended to more clearly articulate the requirements for the measurement and disclosure of inventory and to simplify the accounting for inventory by eliminating the notions of replacement cost and net realizable value less a normal profit margin. The standard will be effective for fiscal years and interim periods within those fiscal years beginning after December 15, 2016. We do not believe the adoption of this standard will have a significant impact on our financial statements.
In November 2015, the FASB issued an accounting standards update entitled “ASU No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes.” The standard requires that all deferred tax assets and liabilities, along with any related valuation allowance, be classified as noncurrent on the balance sheet. The guidance is effective for annual periods, and the interim periods within those annual periods, beginning after December 15, 2016. Early adoption is permitted. We early adopted ASU No. 2015-17 on a prospective basis for the year ended December 31, 2015 and adoption did not have a material impact on our financial statements.
In February 2016, the FASB issued Accounting Standards Update No 2016-02, Leases. Under the new guidance, lessees will be required to recognize for all leases (with the exception of short-term leases) a lease liability, which is a lessee’s obligation to make lease payments arising from a lease, measured on a discounted basis and a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term.
The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods for those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. We are currently evaluating the effect that this guidance will have on our financial statements.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operation is based on our financial statements, which have been prepared in accordance with US GAAP. The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Items subject to estimates based on judgments include, but are not limited to: revenue recognition, stock-based compensation expense, fair value measurements, the resolution of uncertain tax position, income tax valuation allowances,
77
recovery of long-lived assets and provisions for doubtful accounts, inventory obsolescence and inventory valuation. Actual results could differ from these estimates and such differences could affect the results of operations in future periods.
Revenue Recognition
Product revenue
We recognize revenue from the sale of instruments, consumables and related services when the following four basic criteria are met: (1) a contract has been entered into with a customer or persuasive evidence of an arrangement exists, (2) delivery has occurred or services rendered, (3) the fee is fixed and determinable, and (4) collectability is reasonably assured.
Sale of instruments and consumables
Instrument product revenue is generally recognized upon installation and calibration of the instrument by our field service engineers, unless the customer has specified any other acceptance criteria. The sale of instruments and related installation and calibration are considered to be one unit of accounting, as instruments are required to be professionally installed and calibrated before use. Installation generally occurs within a month of shipment.
Consumables are considered to be separate units of accounting as they are sold separately. Consumables revenue is recognized upon transfer of ownership, which is generally upon shipment. Our standard terms and conditions provide that no right of return exists for instruments or consumables unless replacement is necessary due to delivery of defective or damaged products.
When a contract involves multiple elements, the items included in the arrangement, referred to as deliverables, are evaluated to determine whether they represent separate units of accounting. We perform this evaluation at the inception of an arrangement and as each item is delivered in the arrangement. Generally, we account for a deliverable (or a group of deliverables) separately if the delivered item has stand-alone value to the customer and delivery or performance of the undelivered item or service is probable and substantially in our control. When multiple elements can be separated into separate units of accounting, arrangement consideration is allocated at the inception of the arrangement, based on each deliverables’ relative selling price. All revenue from contracts determined not to have separate units of accounting is recognized based on consideration of the most substantive delivery factor of all the elements in the contract.
We provide instruments to customers under reagent rental agreements. Under these agreements, we install instruments in the customer’s facility without a fee and the customer agrees to purchase consumable products at a stated price over the term of the agreement. While some of these agreements did not historically contain a minimum purchase requirement, we have included a minimum purchase requirement in all reagent rental agreements entered into in 2015, and will continue to do so on future agreements. Terms range from several months to multiple years and may automatically renew in several month or multiple year increments unless either party notifies the other in advance that the agreement will not renew. This represents a multiple element arrangement and because all consideration under the reagent rental agreement is contingent on the sale of consumables, no consideration is allocated to the instrument and no revenue is recognized upon installation of the instrument. The cost of the instrument under the agreement is expected to be recovered in the fees charged for consumables, to the extent sold, over the term of the agreement. Revenue is recognized as consumables are shipped.
We retain title to the instrument in reagent rental agreements and such title is transferred to the customer at no additional charge at the conclusion of the initial arrangement. Because the pattern of revenue from the arrangement cannot be reasonably estimated, the cost of the instrument is amortized on a straight-line basis over the term of the arrangement, unless there is no minimum consumable product purchase in which case the instrument would be expensed as cost of revenue. Cost to maintain the equipment while title remains with us is charged to cost of sales as incurred.
Service revenue
For contracts related to custom panel design services and sample processing, we utilize a proportional performance revenue recognition model, under which revenue is recognized as performance occurs based on the relative outputs of the performance that have occurred up to that point in time under the respective agreement. We include all applicable costs incurred related to custom panel design services, including research and development costs and general and administrative expenses, in cost of revenue.
Anticipated losses, if any, on contracts are charged to earnings as soon as they are identified. Anticipated losses cover all costs allocable to contracts. Revenue arising from claims or change orders is recorded either as income or as an offset against a potential loss only when the amount of the claim can be estimated and its realization is probable.
78
Other revenue includes grant revenue. Grant revenue is earned when expenditures relating to the projects under these awards are incurred.
We establish the fair value of all of our financial assets and liabilities that are recognized and disclosed at fair value in the financial statements using the price that would be received to sell an asset or paid to transfer a financial liability in an orderly transaction between market participants at the measurement date. A fair value hierarchy is used to measure fair value. The three levels of the fair value hierarchy are as follows:
Level 1 – Quoted prices in active markets for identical assets and liabilities.
Level 2 – Pricing inputs are based on quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets.
Level 3 – Valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable and include situations where there is little, if any, market activity for the investment.
Our portfolio of securities comprises U.S. Treasuries, U.S. government sponsored agency obligations and high credit quality corporate debt securities classified as available-for-sale securities.
As discussed in Note 4, Fair Value, in Part II, Item 8 of this Form 10-K, a majority of our security holdings have been classified as Level 2. These securities have been initially valued at the transaction price and subsequently valued utilizing a third party service provider who assesses the fair value using inputs other than quoted prices that are observable either directly or indirectly, such as yield curve, volatility factors, credit spreads, default rates, loss severity, current market and contractual prices for the underlying instrument or debt, broker and dealer quotes, as well as other relevant economic measures. We perform certain procedures to corroborate the fair value of these holdings, and in the process, we apply judgment and estimates that if changed, could significantly affect our statement of financial position.
Inventory Valuation
Inventory consists of raw materials and finished goods which are stated at the lower of cost (first-in, first-out) or market. We reserve or write down inventory for estimated obsolescence, inventory in excess of reasonably expected near term sales or unmarketable inventory, in an amount equal to the difference between the cost of inventory and the estimated market value, based upon assumption about future demand and market conditions. If actual market conditions are less favorable than those projected, additional inventory adjustments may be required. Inventory impairment charges establish a new cost basis for inventory and charges are not reversed subsequently to income, even if circumstances later suggest that increased carrying amounts are recoverable.
Stock-Based Compensation
We recognize compensation costs related to share-based payments to employees, including grants of stock options and restricted stock units, based on the estimated fair value of the awards on the date of grant, net of estimated forfeitures. We estimate the grant date fair value, and the resulting stock-based compensation expense, using the Black-Scholes option-pricing model. The grant date fair value of stock-based awards is expensed on a straight-line basis over the vesting period of the respective award. We account for stock-based compensation arrangements with non-employees using a fair value approach. The fair value of these options is measured using the Black-Scholes option-pricing model reflecting the same assumptions as applied to employee options in each of the reported periods, other than the expected life, which is assumed to be the remaining contractual life of the option. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions, which determine the fair value of stock-based awards, including estimating the value per share of our stock, risk-free interest rate, expected term and volatility. The assumptions used in calculating the fair value of stock-based awards represent our best estimates based on management judgment and subjective future expectations. These estimates involve inherent uncertainties. If we had made different assumptions, our stock-based compensation expense, net loss and net loss per share of common stock could have been significantly different.
79
We account for income taxes under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts and tax base of assets and liabilities using enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is established against net deferred tax assets for the uncertainty it presents of our ability to use the net deferred tax assets, in this case the net operating tax loss carryforwards and research and development tax credits in the future. In assessing the realizability of net deferred tax assets we have assessed the likelihood that net deferred tax assets will be recovered from future taxable income, and to the extent that recovery is not likely or there is insufficient operating history, a valuation allowance is established. We record the valuation allowance in the period we determine that it is “more likely than not” that net deferred tax assets will not be realized. For the years ended December 31, 2015 and 2014, we have provided a full valuation allowance for all net deferred tax assets due to their current realization being considered remote in the near term. Uncertain tax positions taken or expected to be taken in a tax return are accounted for using the more-likely-than-not threshold for financial statement recognition and measurement.
Emerging Growth Company Status
We are an emerging growth company as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks in the ordinary course of our business. These risks primarily relate to interest rates. We had cash and cash equivalents of $3.3 million at December 31, 2015, which consist of bank deposits and money market funds. Such interest-bearing instruments carry a degree of risk; however, we have not been exposed to, nor do we anticipate being exposed to, material risks due to changes in interest rates. Our debt is at fixed interest rates. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our financial statements. Our investments in available-for-sale securities are at some risk for losses from possible volatility in the market. However, our investments are in a low risk portfolio comprising U.S. Treasuries, U.S. government sponsored agency obligation and high credit quality corporate debt securities which have an average credit rating of AA. We do not anticipate exposure to significant market risk based upon our conservative investment policy.
Item 8. Financial Statements and Supplementary Data.
80
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of HTG Molecular Diagnostics, Inc.
Tucson, Arizona
We have audited the accompanying balance sheets of HTG Molecular Diagnostics, Inc. (the “Company”) as of December 31, 2015 and 2014 and the related statements of operations, comprehensive loss, redeemable convertible preferred stock and stockholders’ equity (deficit), and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2015 and 2014, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO USA, LLP
Phoenix, Arizona
March 24, 2016
F-2
HTG Molecular Diagnostics, Inc.
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
3,293,983 |
|
|
$ |
3,613,392 |
|
|
Short-term investments available-for-sale, at fair value |
|
|
28,201,507 |
|
|
|
— |
|
|
Accounts receivable, net |
|
|
716,246 |
|
|
|
801,125 |
|
|
Inventory, net of allowance of $284,319 and $54,269 at December 31, 2015 and 2014 |
|
|
2,201,301 |
|
|
|
1,685,814 |
|
|
Prepaid insurance |
|
|
234,777 |
|
|
|
20,905 |
|
|
Prepaid expenses and other |
|
|
210,440 |
|
|
|
91,130 |
|
|
Total current assets |
|
|
34,858,254 |
|
|
|
6,212,366 |
|
|
|
|
|
|
|
|
|
|
|
|
Long-term investments available-for-sale, at fair value |
|
|
2,603,901 |
|
|
|
— |
|
|
Deferred financing and offering costs |
|
|
52,377 |
|
|
|
1,369,281 |
|
|
Property and equipment, net |
|
|
1,932,213 |
|
|
|
1,146,599 |
|
|
Total assets |
|
$ |
39,446,745 |
|
|
$ |
8,728,246 |
|
The accompanying notes are an integral part of these financial statements.
F-3
HTG Molecular Diagnostics, Inc.
Balance Sheets (Continued)
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Liabilities and stockholders’ equity (deficit) |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
724,805 |
|
|
$ |
948,429 |
|
|
Accrued liabilities |
|
|
1,944,511 |
|
|
|
1,499,750 |
|
|
Deferred revenue |
|
|
47,476 |
|
|
|
41,248 |
|
|
NuvoGen obligation |
|
|
543,750 |
|
|
|
— |
|
|
Term loan |
|
|
3,059,068 |
|
|
|
813,715 |
|
|
Total current liabilities |
|
|
6,319,610 |
|
|
|
3,303,142 |
|
|
Redeemable convertible preferred stock warrant liability |
|
|
— |
|
|
|
730,543 |
|
|
Term loan payable - non-current, net of discount |
|
|
7,789,963 |
|
|
|
9,705,655 |
|
|
NuvoGen obligation - non-current, net of discount |
|
|
8,415,122 |
|
|
|
8,677,859 |
|
|
Other |
|
|
28,652 |
|
|
|
58,380 |
|
|
Total liabilities |
|
|
22,553,347 |
|
|
|
22,475,579 |
|
|
Redeemable convertible preferred stock: |
|
|
|
|
|
|
|
|
|
Series A, $0.001 par value; $0 and $1,406,369 liquidation value at December 31, 2015 and 2014, respectively; 0 and 1,292,084 shares authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at December 31, 2015 and 1,292,084 shares both issued and outstanding at December 31, 2014 |
|
|
— |
|
|
|
1,402,687 |
|
|
Series B, $0.001 par value; $0 and $2,104,811 liquidation value at December 31, 2015 and 2014, respectively; 0 and 11,919,624 shares authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at December 31, 2015 and 6,789,712 shares both issued and outstanding at December 31, 2014 |
|
|
— |
|
|
|
2,099,310 |
|
|
Series C-1, $0.001 par value; $0 and $4,581,944 liquidation value at December 31, 2015 and 2014, respectively; 0 and 17,530,800 shares authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at December 31, 2015 and 13,242,612 shares both issued and outstanding at December 31, 2014 |
|
|
— |
|
|
|
4,567,525 |
|
|
Series C-2, $0.001 par value; $0 and $2,244,343 liquidation value at December 31, 2015 and 2014, respectively; 0 and 19,262,522 shares authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at December 31, 2015 and 9,948,331 shares both issued and outstanding at December 31, 2014 |
|
|
— |
|
|
|
2,223,506 |
|
|
Series D, $0.001 par value; $0 and $68,001,923 liquidation value at December 31, 2015 and 2014, respectively; 0 and 237,031,908 shares authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at December 31, 2015 and 139,529,173 shares both issued and outstanding at December 31, 2014 |
|
|
— |
|
|
|
37,174,666 |
|
|
Series E, $0.001 par value; $0 and $20,839,001 liquidation value at December 31, 2015 and 2014, respectively; 0 and 185,046,445 shares authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at December 31, 2015, 45,938,041 shares both issued and outstanding at December 31, 2014 |
|
|
— |
|
|
|
8,454,899 |
|
|
Total redeemable convertible preferred stock |
|
|
— |
|
|
|
55,922,593 |
|
|
Commitments and Contingencies |
|
|
|
|
|
|
|
|
|
Stockholders’ equity (deficit): |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 10,000,000 and 0 authorized at December 31, 2015 and 2014, respectively; no shares issued and outstanding at either date. |
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par value; 200,000,000 and 600,000,000 shares authorized at December 31, 2015 and 2014, respectively; 6,845,638 and 6,844,242 shares issued and outstanding, respectively, at December 31, 2015, 334,003 and 332,607 shares issued and outstanding, respectively, at December 31, 2014 |
|
|
6,844 |
|
|
|
333 |
|
|
Additional paid-in-capital (distributions in excess of capital) |
|
|
106,569,405 |
|
|
|
(1,426,556 |
) |
|
Treasury stock – 1,396 shares, at cost |
|
|
(75,000 |
) |
|
|
(75,000 |
) |
|
Accumulated other comprehensive loss |
|
|
(41,357 |
) |
|
|
— |
|
|
Accumulated deficit |
|
|
(89,566,494 |
) |
|
|
(68,168,703 |
) |
|
Total stockholders’ equity (deficit) |
|
|
16,893,398 |
|
|
|
(69,669,926 |
) |
|
Total liabilities and stockholders' equity (deficit) |
|
$ |
39,446,745 |
|
|
$ |
8,728,246 |
|
The accompanying notes are an integral part of these financial statements.
F-4
HTG Molecular Diagnostics, Inc.
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Revenue: |
|
|
|
|
|
|
|
|
|
Product |
|
$ |
3,532,028 |
|
|
$ |
1,788,205 |
|
|
Service |
|
|
183,758 |
|
|
|
497,168 |
|
|
Other |
|
|
325,789 |
|
|
|
1,043,584 |
|
|
Total revenue |
|
|
4,041,575 |
|
|
|
3,328,957 |
|
|
Cost of revenue |
|
|
3,335,511 |
|
|
|
3,204,915 |
|
|
Gross margin |
|
|
706,064 |
|
|
|
124,042 |
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
14,994,410 |
|
|
|
9,897,697 |
|
|
Research and development |
|
|
4,601,718 |
|
|
|
3,075,204 |
|
|
Total operating expenses |
|
|
19,596,128 |
|
|
|
12,972,901 |
|
|
Operating loss |
|
|
(18,890,064 |
) |
|
|
(12,848,859 |
) |
|
|
|
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Loss from change in stock warrant valuation |
|
|
(239,683 |
) |
|
|
(388,936 |
) |
|
Interest expense |
|
|
(1,719,475 |
) |
|
|
(706,866 |
) |
|
Interest income |
|
|
85,859 |
|
|
|
— |
|
|
Loss on settlement of convertible debt |
|
|
(705,217 |
) |
|
|
— |
|
|
Other |
|
|
80,978 |
|
|
|
(13,747 |
) |
|
Total other expense |
|
|
(2,497,538 |
) |
|
|
(1,109,549 |
) |
|
Net loss before income taxes |
|
|
(21,387,602 |
) |
|
|
(13,958,408 |
) |
|
Income taxes |
|
|
10,189 |
|
|
|
— |
|
|
Net loss |
|
|
(21,397,791 |
) |
|
|
(13,958,408 |
) |
|
Accretion of stock issuance costs |
|
|
(35,046 |
) |
|
|
(102,677 |
) |
|
Accretion of Series E warrant discount |
|
|
(127,616 |
) |
|
|
(313,152 |
) |
|
Accretion of Series D and E redeemable convertible preferred stock dividends |
|
|
(1,165,932 |
) |
|
|
(3,244,572 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(22,726,385 |
) |
|
$ |
(17,618,809 |
) |
|
Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(5.03 |
) |
|
$ |
(175.03 |
) |
|
Shares used in computing net loss per share attributable to common stockholders, basic and diluted |
|
|
4,518,499 |
|
|
|
100,659 |
|
The accompanying notes are an integral part of these financial statements.
F-5
HTG Molecular Diagnostics, Inc.
Statements of Comprehensive Loss
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Net loss |
|
$ |
(21,397,791 |
) |
|
$ |
(13,958,408 |
) |
|
Other comprehensive loss, net of tax effect: |
|
|
|
|
|
|
|
|
|
Unrealized loss on short and long-term investments |
|
|
(41,357 |
) |
|
|
— |
|
|
Comprehensive loss |
|
$ |
(21,439,148 |
) |
|
$ |
(13,958,408 |
) |
|
Accretion of stock issuance costs |
|
|
(35,046 |
) |
|
|
(102,677 |
) |
|
Accretion of Series E warrant discount |
|
|
(127,616 |
) |
|
|
(313,152 |
) |
|
Accretion of Series D and E redeemable convertible preferred stock dividends |
|
|
(1,165,932 |
) |
|
|
(3,244,572 |
) |
|
Comprehensive loss attributable to common stockholders |
|
$ |
(22,767,742 |
) |
|
$ |
(17,618,809 |
) |
The accompanying notes are an integral part of these financial statements.
F-6
HTG Molecular Diagnostics, Inc.
Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
|
|
|
Redeemable Convertible Preferred Stock |
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|
|
Series A |
|
|
Series B |
|
|
Series C-1 |
|
|
Series C-2 |
|
|
Series D |
|
|
Series E |
|
||||||||||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
||||||||||||
|
Balance at January 1, 2014 |
|
|
1,292,084 |
|
|
$ |
1,401,677 |
|
|
|
11,919,624 |
|
|
$ |
3,795,690 |
|
|
|
16,240,450 |
|
|
$ |
5,596,660 |
|
|
|
19,104,610 |
|
|
$ |
4,270,835 |
|
|
|
143,737,467 |
|
|
$ |
35,803,437 |
|
|
|
— |
|
|
$ |
— |
|
|
Issuance of preferred stock Series E, net of issuance cost of $170,546 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
34,453,538 |
|
|
|
5,494,766 |
|
|
Exercise of Series D warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
25,125 |
|
|
|
4,271 |
|
|
|
|
|
|
|
|
|
|
Exercise of Series E warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11,484,503 |
|
|
|
1,889,201 |
|
|
Exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Accretion of redeemable convertible preferred stock issuance costs |
|
|
— |
|
|
|
1,010 |
|
|
|
— |
|
|
|
2,647 |
|
|
|
— |
|
|
|
4,853 |
|
|
|
— |
|
|
|
6,665 |
|
|
|
— |
|
|
|
57,049 |
|
|
|
— |
|
|
|
30,453 |
|
|
Accretion of Series E warrant discount |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
313,152 |
|
|
Adjustment to Series B book value |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(112,903 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Accretion of Series D and E redeemable convertible preferred stock dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,517,245 |
|
|
|
— |
|
|
|
727,327 |
|
|
Optional conversion of preferred stock to common |
|
|
— |
|
|
|
— |
|
|
|
(5,129,912 |
) |
|
|
(1,586,124 |
) |
|
|
(2,997,838 |
) |
|
|
(1,033,988 |
) |
|
|
(9,156,279 |
) |
|
|
(2,053,994 |
) |
|
|
(4,233,419 |
) |
|
|
(1,207,336 |
) |
|
|
— |
|
|
|
— |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Balance at December 31, 2014 |
|
|
1,292,084 |
|
|
$ |
1,402,687 |
|
|
|
6,789,712 |
|
|
$ |
2,099,310 |
|
|
|
13,242,612 |
|
|
$ |
4,567,525 |
|
|
|
9,948,331 |
|
|
$ |
2,223,506 |
|
|
|
139,529,173 |
|
|
$ |
37,174,666 |
|
|
|
45,938,041 |
|
|
$ |
8,454,899 |
|
|
Exercise of Series D and Series E warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
723,505 |
|
|
|
93,550 |
|
|
|
51,681 |
|
|
|
11,312 |
|
|
Exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Accretion of redeemable convertible preferred stock issuance costs |
|
|
— |
|
|
|
320 |
|
|
|
— |
|
|
|
839 |
|
|
|
— |
|
|
|
1,538 |
|
|
|
— |
|
|
|
2,113 |
|
|
|
— |
|
|
|
18,109 |
|
|
|
— |
|
|
|
12,127 |
|
|
Accretion of Series E warrant discount |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
127,616 |
|
|
Accretion of Series D and E redeemable convertible preferred stock dividend |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
876,955 |
|
|
|
— |
|
|
|
288,977 |
|
|
Conversion of outstanding principal and accrued interest on convertible notes to common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Conversion of warrants from warrants for preferred stock to warrants for common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Conversion of preferred stock into common stock at initial public offering |
|
|
(1,292,084 |
) |
|
|
(1,403,007 |
) |
|
|
(6,789,712 |
) |
|
|
(2,100,149 |
) |
|
|
(13,242,612 |
) |
|
|
(4,569,063 |
) |
|
|
(9,948,331 |
) |
|
|
(2,225,619 |
) |
|
|
(140,252,678 |
) |
|
|
(38,163,280 |
) |
|
|
(45,989,722 |
) |
|
|
(8,894,931 |
) |
|
Issuance of common stock from initial public offering net of issuance costs of $5.9 million |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Balance at December 31, 2015 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
The accompanying notes are an integral part of these financial statements.
F-7
HTG Molecular Diagnostics, Inc.
Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit) (Continued)
|
|
|
Stockholders’ Equity (Deficit) |
|
|||||||||||||||||||||||||
|
|
|
|
|
|
Additional Paid-In-Capital |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|||||||
|
|
|
Common Stock |
|
|
(Distributions in |
|
|
Treasury |
|
|
Accumulated Other |
|
|
Accumulated |
|
|
Stockholders' |
|
||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Excess of Capital) |
|
|
Stock |
|
|
Comprehensive Loss |
|
|
Deficit |
|
|
Equity (Deficit) |
|
|||||||
|
Balance at January 1, 2014 |
|
|
96,349 |
|
|
$ |
97 |
|
|
$ |
(3,966,712 |
) |
|
$ |
(75,000 |
) |
|
$ |
— |
|
|
$ |
(54,210,295 |
) |
|
$ |
(58,251,910 |
) |
|
Issuance of preferred stock Series E, net of issuance cost of $170,546 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Exercise of Series D warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Exercise of Series E warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Exercise of stock options |
|
|
9,909 |
|
|
|
10 |
|
|
|
21,472 |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
21,482 |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
184,966 |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
184,966 |
|
|
Accretion of redeemable convertible preferred stock issuance costs |
|
|
— |
|
|
|
— |
|
|
|
(102,677 |
) |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
(102,677 |
) |
|
Accretion of Series E warrant discount |
|
|
— |
|
|
|
— |
|
|
|
(313,152 |
) |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
(313,152 |
) |
|
Adjustment to Series B book value |
|
|
— |
|
|
|
— |
|
|
|
112,903 |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
112,903 |
|
|
Accretion of Series D and E redeemable convertible preferred stock dividends |
|
|
— |
|
|
|
— |
|
|
|
(3,244,572 |
) |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
(3,244,572 |
) |
|
Optional conversion of preferred stock to common |
|
|
226,349 |
|
|
|
226 |
|
|
|
5,881,216 |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
5,881,442 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
(13,958,408 |
) |
|
|
(13,958,408 |
) |
|
Balance at December 31, 2014 |
|
|
332,607 |
|
|
$ |
333 |
|
|
$ |
(1,426,556 |
) |
|
$ |
(75,000 |
) |
|
$ |
— |
|
|
$ |
(68,168,703 |
) |
|
$ |
(69,669,926 |
) |
|
Exercise of Series D and Series E warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Exercise of stock options |
|
|
13,960 |
|
|
|
14 |
|
|
|
34,867 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
34,881 |
|
|
Stock issued under stock purchase plan |
|
|
4,184 |
|
|
|
4 |
|
|
|
19,996 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
20,000 |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
405,426 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
405,426 |
|
|
Accretion of redeemable convertible preferred stock issuance costs |
|
|
— |
|
|
|
— |
|
|
|
(35,046 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(35,046 |
) |
|
Accretion of Series E warrant discount |
|
|
— |
|
|
|
— |
|
|
|
(127,616 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(127,616 |
) |
|
Accretion of Series D and E redeemable convertible preferred stock dividends |
|
|
— |
|
|
|
— |
|
|
|
(1,165,932 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,165,932 |
) |
|
Settlement of outstanding principal and accrued interest on convertible notes by issuance of common stock |
|
|
324,591 |
|
|
|
324 |
|
|
|
4,544,060 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,544,384 |
|
|
Conversion of warrants from warrants for preferred stock to warrants for common stock |
|
|
— |
|
|
|
— |
|
|
|
1,616,140 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,616,140 |
|
|
Conversion of preferred stock into common stock at initial public offering |
|
|
2,508,824 |
|
|
|
2,509 |
|
|
|
57,353,540 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
57,356,049 |
|
|
Issuance of common stock from initial public offering net of issuance costs of $5.9 million |
|
|
3,660,076 |
|
|
|
3,660 |
|
|
|
45,350,526 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
45,354,186 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(21,397,791 |
) |
|
|
(21,397,791 |
) |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(41,357 |
) |
|
|
— |
|
|
|
(41,357 |
) |
|
Balance at December 31, 2015 |
|
|
6,844,242 |
|
|
$ |
6,844 |
|
|
$ |
106,569,405 |
|
|
$ |
(75,000 |
) |
|
$ |
(41,357 |
) |
|
$ |
(89,566,494 |
) |
|
$ |
16,893,398 |
|
The accompanying notes are an integral part of these financial statements.
F-8
HTG Molecular Diagnostics, Inc.
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(21,397,791 |
) |
|
$ |
(13,958,408 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
662,562 |
|
|
|
496,595 |
|
|
Accretion of discount on NuvoGen obligation |
|
|
281,013 |
|
|
|
187,017 |
|
|
Bad debt expense, net of recoveries |
|
|
44,854 |
|
|
|
115,068 |
|
|
Provision for excess inventory |
|
|
230,754 |
|
|
|
54,269 |
|
|
Amortization of deferred financing costs |
|
|
44,666 |
|
|
|
13,841 |
|
|
Amortization of discount on term loan |
|
|
170,954 |
|
|
|
84,676 |
|
|
Amortization of final payment premium on term loan |
|
|
158,707 |
|
|
|
56,201 |
|
|
Loss on settlement of convertible notes |
|
|
705,217 |
|
|
|
— |
|
|
Amortization of discount on convertible notes |
|
|
90,222 |
|
|
|
— |
|
|
Stock-based compensation expense |
|
|
405,426 |
|
|
|
184,966 |
|
|
Change in redeemable convertible preferred stock warrant liability |
|
|
239,683 |
|
|
|
388,936 |
|
|
Loss on disposal of assets |
|
|
12,125 |
|
|
|
35,960 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
40,025 |
|
|
|
(312,340 |
) |
|
Inventory |
|
|
(746,241 |
) |
|
|
(746,982 |
) |
|
Prepaid expenses and other |
|
|
(334,536 |
) |
|
|
52,092 |
|
|
Accounts payable |
|
|
(312,275 |
) |
|
|
198,193 |
|
|
Accrued liabilities |
|
|
455,620 |
|
|
|
278,291 |
|
|
Deferred revenue |
|
|
6,228 |
|
|
|
(95,442 |
) |
|
Other long term liabilities |
|
|
(486 |
) |
|
|
(29,549 |
) |
|
Net cash used in operating activities |
|
|
(19,243,273 |
) |
|
|
(12,996,616 |
) |
|
Investing activities |
|
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(1,338,124 |
) |
|
|
(857,677 |
) |
|
Sales, redemptions and maturities of available-for-sale securities |
|
|
7,500,000 |
|
|
|
— |
|
|
Purchase of available-for-sale securities |
|
|
(38,346,765 |
) |
|
|
— |
|
|
Net cash used in investing activities |
|
|
(32,184,889 |
) |
|
|
(857,677 |
) |
|
Financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
34,881 |
|
|
|
21,482 |
|
|
Proceeds from stock issued under stock purchase plan |
|
|
20,000 |
|
|
|
— |
|
|
Draws on line of credit |
|
|
— |
|
|
|
750,000 |
|
|
Payments on line of credit |
|
|
— |
|
|
|
(750,000 |
) |
|
Payments on NuvoGen obligation |
|
|
— |
|
|
|
(1,206,250 |
) |
|
Proceeds from exercise of Series D and E warrants |
|
|
8,948 |
|
|
|
251 |
|
|
Deferred offering costs |
|
|
(1,002,930 |
) |
|
|
(1,205,122 |
) |
|
Deferred financing costs |
|
|
(75,523 |
) |
|
|
— |
|
|
Payments on equipment lease |
|
|
(29,242 |
) |
|
|
(21,932 |
) |
|
Proceeds from term loan |
|
|
— |
|
|
|
10,680,000 |
|
|
Proceeds from sale of Series E preferred stock, net of issuance costs |
|
|
— |
|
|
|
7,383,967 |
|
|
Proceeds from issuance of convertible note warrants |
|
|
1,354 |
|
|
|
— |
|
|
Settlement of fractional common shares |
|
|
(2,925 |
) |
|
|
— |
|
|
Proceeds from convertible notes |
|
|
4,500,000 |
|
|
|
— |
|
|
Proceeds from initial public offering |
|
|
47,654,190 |
|
|
|
— |
|
|
Net cash provided by financing activities |
|
|
51,108,753 |
|
|
|
15,652,396 |
|
|
(Decrease) increase in cash and cash equivalents |
|
|
(319,409 |
) |
|
|
1,798,103 |
|
|
Cash and cash equivalents at beginning of year |
|
|
3,613,392 |
|
|
|
1,815,289 |
|
|
Cash and cash equivalents at end of year |
|
$ |
3,293,983 |
|
|
$ |
3,613,392 |
|
|
Noncash investing and financing activities |
|
|
|
|
|
|
|
|
|
Accretion of preferred stock issuance costs |
|
$ |
35,046 |
|
|
$ |
102,677 |
|
|
Net exercise of Series D and Series E warrants |
|
|
(95,914 |
) |
|
|
(4,020 |
) |
|
Accretion of Series E warrant discount |
|
|
127,616 |
|
|
|
313,152 |
|
|
Adjustment to Series B book value |
|
|
— |
|
|
|
(112,903 |
) |
|
Accretion of Series D and E redeemable convertible preferred stock dividends |
|
|
1,165,932 |
|
|
|
3,244,572 |
|
|
Deferred offering costs reclassified to distributions in excess of capital |
|
|
2,297,079 |
|
|
|
— |
|
|
Accrual of deferred offering and finance costs |
|
|
— |
|
|
|
178,000 |
|
|
Allocation of Series E warrant convertible notes debt discount |
|
|
741,828 |
|
|
|
— |
|
|
Allocation of Series E warrant debt discount |
|
|
— |
|
|
|
301,507 |
|
|
Conversion of convertible notes and related accrued interest to common stock |
|
|
4,544,384 |
|
|
|
— |
|
|
Fixed asset purchases payable at period end |
|
|
122,176 |
|
|
|
— |
|
|
Conversion of convertible preferred stock to common stock |
|
|
(57,356,049 |
) |
|
|
(5,881,442 |
) |
|
Reclassification of convertible preferred stock liability warrants to equity warrants |
|
|
(1,616,140 |
) |
|
|
— |
|
|
Supplemental cash flow information |
|
|
|
|
|
|
|
|
|
Cash paid for interest |
|
$ |
935,004 |
|
|
$ |
368,331 |
|
The accompanying notes are an integral part of these financial statements.
F-9
HTG Molecular Diagnostics, Inc.
Note 1. Description of Business
HTG Molecular Diagnostics, Inc. (the “Company”) is a commercial stage company that develops and markets a novel technology platform to facilitate the routine use of complex molecular profiling. The Company’s HTG Edge and HTG EdgeSeq platforms, consisting of instrumentation, consumables and software analytics, are used in sample profiling applications including tumor profiling, molecular diagnostic testing and biomarker development. The Company’s HTG Edge and HTG EdgeSeq platforms automate the molecular profiling of genes and gene activity using its proprietary nuclease protection chemistry on a wide variety of biological samples. The Company derives revenue from the sale of instruments, consumables and related services.
The Company operates in one segment and its customers are primarily located in the United States. For the year ended December 31, 2015, approximately 13% of the Company’s revenue was generated from sales to customers located outside of the United States, compared to 14% for the year ended December 31, 2014.
Reverse Stock Split
On April 27, 2015, the Company effected a one-for-107.39 reverse stock split of its outstanding common stock. All applicable common share and per common share information has been retroactively adjusted to reflect the effect of this reverse stock split. The reverse stock split did not change the number of shares of convertible preferred stock outstanding, but did affect the conversion ratios associated with the convertible preferred stock.
Initial Public Offering
On May 11, 2015, the Company successfully completed its initial public offering (“IPO”), in which the Company sold 3,570,000 shares of common stock at $14.00 per share for total gross proceeds of approximately $50 million. An additional 90,076 shares of common stock were subsequently sold pursuant to the partial exercise by the underwriters of their over-allotment option resulting in additional gross proceeds of approximately $1.3 million. After underwriters’ fees and commissions and other expenses of the offering, the Company’s aggregate net proceeds were approximately $45.4 million. All outstanding shares of the Company’s redeemable convertible preferred stock converted into shares of common stock in connection with the IPO. Following the IPO, there were no shares of preferred stock outstanding.
Note 2. Summary of Significant Accounting Policies
Accounting Principles
The financial statements and accompanying notes were prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
Liquidity
The Company expects that its cash and cash equivalents, together with interest thereon, will be sufficient to fund its operations for at least the next 12 months and likely through the midpoint of the second quarter 2017. However, the Company has had recurring operating losses and negative cash flows from operations since its inception. As such, the Company expects that it will need to raise additional equity or debt capital at some point in the future until its revenue reaches a level sufficient to provide for self-sustaining cash flows. There can be no assurance that additional equity or debt financing will be available on acceptable terms, or at all, or that the Company’s revenue will reach a level sufficient to provide for self-sustaining cash flows.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. The Company’s significant estimates include revenue recognition, stock-based compensation expense, the value of the warrant liability, the resolution of uncertain tax positions, income tax valuation allowances, recovery of long-lived assets and provisions for doubtful accounts, inventory obsolescence and inventory valuation. Actual results could materially differ from those estimates.
F-10
The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash and cash equivalents. Cash and cash equivalents consist of cash on deposit with financial institutions, money market instruments and high credit quality corporate debt securities purchased with a term of three months or less.
Accounts Receivable, net
Accounts receivable represent valid claims against debtors and have been reported net of an allowance for doubtful accounts of $0 and $85,068 at December 31, 2015 and 2014, respectively. The Company reviews accounts receivable to identify where collectability may not be probable based on the specific identification method. Bad debt expense, net of recoveries, was $44,854 and $115,068 for the years ended December 31, 2015 and 2014, respectively.
Investments
The Company classifies its securities as available-for-sale, which are reported at estimated fair value with unrealized gains and losses included in accumulated other comprehensive loss, net of tax. Realized gains, realized losses and declines in value of securities judged to be other-than-temporary, are included in other income (expense) within the statements of operations. The cost of investments for purposes of computing realized and unrealized gains and losses is based on the specific identification method. Interest and dividends earned on all securities are included in other income (expense) within the statements of operations. Investments in securities with maturities of less than one year, or where management’s intent is to use the investments to fund current operations, or to make them available for current operations, are classified as short-term investments.
If the estimated fair value of a security is below its carrying value, the Company evaluates whether it is more likely than not that it will sell the security before its anticipated recovery in market value and whether evidence indicating that the cost of the investment is recoverable within a reasonable period of time outweighs evidence to the contrary. The Company also evaluates whether or not it intends to sell the investment. If the impairment is considered to be other-than-temporary, the security is written down to its estimated fair value. In addition, the Company considers whether credit losses exist for any securities. A credit loss exists if the present value of cash flows expected to be collected is less than the amortized cost basis of the security. Other-than-temporary declines in estimated fair value and credit losses are charged against other income (expense).
Fair Value of Financial Instruments
The carrying value of financial instruments classified as current assets and current liabilities approximate fair value due to their liquidity and short-term nature. Investments that are classified as available-for-sale are recorded at fair value, which was determined using quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. The carrying amounts of the Company’s asset-secured growth capital term loan (the “Growth Term Loan”) and convertible notes were estimated using Level 3 inputs and approximate fair value since the interest rate approximates the market rate for debt securities with similar terms and risk characteristics.
Fair value measurements are based on the premise that fair value represents an exit price representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the following three-tier fair value hierarchy has been used in determining the inputs used in measuring fair value:
|
Level 1 |
|
– |
|
Quoted prices in active markets for identical assets or liabilities on the reporting date. |
|
|
|
|
|
|
|
Level 2 |
|
– |
|
Pricing inputs are based on quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant assumptions are observable in the market or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
|
|
|
|
|
|
|
Level 3 |
|
– |
|
Pricing inputs are generally unobservable and include situations where there is little, if any, market activity for the investment. The inputs into the determination of fair value require management’s judgment or estimation of assumptions that market participants would use in pricing the assets or liabilities. The fair values are therefore determined using factors that involve considerable judgment and interpretations, including but not limited to private and public comparables, third-party appraisals, discounted cash flow models, and fund manager estimates. |
F-11
Inventory, consisting of raw materials and finished goods, is stated at the lower of cost (first-in, first-out) or market. The Company reserves or writes down its inventory for estimated obsolescence, inventory in excess of reasonably expected near term sales or unmarketable inventory, in an amount equal to the difference between the cost of inventory and the estimated market value, based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by the Company, additional inventory write-downs may be required. Inventory impairment charges establish a new cost basis for inventory and charges are not reversed subsequently to income, even if circumstances later suggest that increased carrying amounts are recoverable.
For the year ended December 31, 2015, the Company recorded an increase in the inventory reserve of $230,050, to adjust for estimated shrinkage and excess inventory. For the year ended December 31, 2014 the Company wrote off inventory previously reserved of $536,119, partially offset by the recording of an increase of $54,269 for estimated obsolescence, resulting in a decrease in the inventory reserve of $481,850. For the years ended December 31, 2015 and 2014, $230,754 and $54,269 have been included in cost of revenue, respectively.
In the first quarter of 2015, the Company determined that the average period of time customers use to evaluate the Company’s equipment generally ranges from 90 to 180 days, and in certain circumstances the evaluation period may need to be extended beyond that period. HTG Edge or HTG EdgeSeq instruments at customer locations under evaluation agreements are included in finished goods inventory. Equipment that is under evaluation for purchase remains in inventory as the Company maintains title to the equipment throughout the evaluation period. If the customer has not completed the purchase of the instrument by the end of the initial evaluation period, the Company will determine whether to extend the evaluation period or have the equipment returned to the Company. However, in no case will the evaluation period exceed one year. If the customer has not purchased the equipment or entered into a reagent rental agreement with the Company after a one-year evaluation period, the cost of the equipment is written off to cost of revenue if the customer is allowed to continue use of the equipment.
Prior to January 1, 2015, the Company recorded equipment under evaluation more than 90 days in property and equipment. The Company accordingly has reclassified from property and equipment to inventory field equipment with a net book value of $210,606 at December 31, 2014 under evaluation at that time for longer than 90 days and less than one year.
Property and Equipment, net
Property and equipment are stated at historical cost and depreciated over their useful lives, which range from three to five years, using the straight-line method. Leasehold improvements are amortized using the straight-line method over the lesser of the remaining lease term or the estimated useful life. Field equipment is amortized using the straight-line method over the lesser of the period of the related reagent rental agreement or the estimated useful life. Depreciation and leasehold improvement amortization expense was $662,562 for the year ended December 31, 2015, and $481,012 for the year ended December 31, 2014.
Costs incurred in the development and installation of software for internal use are expensed or capitalized, depending on whether they are incurred in the preliminary project stage (expensed), application development stage (capitalized), or post-implementation stage (expensed). Amounts capitalized following project completion are amortized on a straight-line basis over the useful life of the developed asset, which is generally three years. There was no amortization expense for capitalized software costs for the year ended December 31, 2015. Amortization expense for capitalized software costs was $15,583 for the year ended December 31, 2014.
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flow, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Although the Company has accumulated losses since inception, the Company believes the future cash flows will be sufficient to exceed the carrying value of the Company’s long-lived assets. There were no impairments of long-lived assets during the years ended December 31, 2015 or 2014.
Stock Issuance Costs
Certain costs incurred in connection with the issuance of the Company’s redeemable convertible preferred stock (the “Convertible Preferred Stock”) were being deferred and accreted. Stock issuance costs have historically been accreted to distributions in excess of capital using the effective interest method. Accretion was $35,046 for the year ended December 31, 2015, and $102,677 for the year ended December 31, 2014. Upon automatic conversion of the Convertible Preferred Stock to common stock in connection with the closing of the Company’s IPO in May 2015, issuance costs were no longer accreted.
F-12
Deferred Financing Costs and Debt Discounts
Certain costs incurred in connection with the Growth Term Loan have been deferred and are being amortized. Debt issuance costs and debt discount are amortized over the term of the Growth Term Loan using the effective interest method. In addition, prior to the IPO, costs incurred in connection with the issuance of notes under the Company’s two note and warrant purchase agreements dated December 30, 2014 (the “Note Agreements”) in February and March 2015 were capitalized and amortized over the term of the Note Agreements using the straight-line accretion method, which approximated the effective interest method in this instance. The Company has recorded approximately $52,377 and $75,131 of deferred financing costs in the accompanying balance sheets as of December 31, 2015 and 2014, respectively. Deferred financing cost amortization expense for the years ended December 31, 2015 and 2014 was $44,666 and $13,841, respectively. Amortization of growth term loan discount was $170,954 and $84,676 for the years ended December 31, 2015 and 2014. Amortization of the Note Agreement discount was $90,222 and $0 for the years ended December 31, 2015 and 2014. Growth term loan discount and amortization of the Note Agreement discount are included in interest expense in the accompanying statements of operations. The Company compared the value of the common stock issued to settle the debt with the carrying amount of the debt at the IPO closing date of May 2015, net of unamortized discount. The Company recorded a loss on settlement of convertible debt of $705,217, comprising $651,606 to write off unamortized discount and $53,611 to write off unamortized deferred financing costs relating to the convertible notes which were settled with common stock at the IPO, in the accompanying statements of operations for the year ended December 31, 2015.
Deferred Offering Costs
Deferred offering costs represent legal, accounting and other direct costs related to the IPO, which was completed in May 2015. In accounting for the IPO in May 2015, direct offering costs of approximately $2.3 million were reclassified to additional paid-in capital and are shown, along with underwriters’ fees paid, net against IPO proceeds received. The Company recorded $0 and $1.3 million of deferred offering costs as a non-current asset in the accompanying balance sheets as of December 31, 2015 and 2014, respectively.
Deferred Revenue
Deferred revenue represents cash receipts for products or services to be provided in future periods. When products are delivered or services are rendered, deferred revenue is then recognized as earned.
Revenue Recognition
The Company recognizes revenue from the sale of instruments, consumables and related services when the following four basic criteria are met: (1) a contract has been entered into with a customer or persuasive evidence of an arrangement exists, (2) delivery has occurred or services rendered, (3) the fee is fixed and determinable, and (4) collectability is reasonably assured.
Sale of instruments and consumables
The Company had product revenue consisting of revenue from the sale of instruments and consumables for the years ended December 31, 2015 and 2014 as follows:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Instruments |
|
$ |
789,231 |
|
|
$ |
793,505 |
|
|
Consumables |
|
|
2,742,797 |
|
|
|
994,700 |
|
|
Total product sales |
|
$ |
3,532,028 |
|
|
$ |
1,788,205 |
|
Instrument product revenue is generally recognized upon installation and calibration of the instrument by field service engineers, unless the customer has specified any other acceptance criteria. The sale of instruments and related installation and calibration are considered to be one unit of accounting, as instruments are required to be professionally installed and calibrated before use. Installation generally occurs within a month of shipment.
Consumables are considered to be separate units of accounting as they are sold separately. Consumables revenue is recognized upon transfer of ownership, which is generally upon shipment. The Company’s standard terms and conditions provide that no right of return exists for instruments or consumables, unless replacement is necessary due to delivery of defective or damaged products. Shipping and handling fees charged to the Company’s customers for instruments and consumables shipped are included in the statements of operations as part of product revenue. Shipping and handling costs for sold products shipped to the Company’s customers are included on the statements of operations as part of cost of revenue.
F-13
When a contract involves multiple elements, the items included in the arrangement, referred to as deliverables, are evaluated to determine whether they represent separate units of accounting. The Company performs this evaluation at the inception of an arrangement and as each item is delivered in the arrangement. Generally, the Company accounts for a deliverable (or a group of deliverables) separately if the delivered item has stand-alone value to the customer and delivery or performance of the undelivered item or service is probable and substantially in the Company’s control. When multiple elements can be separated into separate units of accounting, arrangement consideration is allocated at the inception of the arrangement, based on each deliverables’ relative selling price. All revenue from contracts determined not to have separate units of accounting is recognized based on consideration of the most substantive delivery factor of all the elements in the contract.
The Company provides instruments to certain customers under reagent rental agreements. Under these agreements, the Company installs instruments in the customer’s facility without a fee and the customer agrees to purchase consumable products at a stated price over the term of the agreement; in some instances the agreements do not contain a minimum purchase requirement. Terms range from several months to multiple years and may automatically renew in several month or multiple year increments unless either party notifies the other in advance that the agreement will not renew. This represents a multiple element arrangement and because all consideration under the reagent rental agreement is contingent on the sale of consumables, no consideration has been allocated to the instrument and no revenue has been recognized upon installation of the instrument. The Company expects to recover the cost of the instrument under the agreement through the fees charged for consumables, to the extent sold, over the term of the agreement. Revenue is recognized as consumables are shipped.
In reagent rental agreements, the Company retains title to the instrument and title is transferred to the customer at no additional charge at the conclusion of the initial arrangement. Because the pattern of revenue from the arrangement cannot be reasonably estimated, the cost of the instrument is amortized on a straight-line basis over the term of the arrangement, unless there is no minimum consumable product purchase in which case the instrument would be expensed as cost of revenue. Cost to maintain the instrument while title remains with the Company is charged to cost of revenue as incurred.
The Company offers customers the opportunity to purchase separately-priced extended warranty contracts to provide for service upon conclusion of the standard one-year warranty period. The revenue from these contracts is recorded as a component of deferred revenue in the accompanying balance sheets at the inception of the contract and is recognized as revenue over the contract service period.
Service Revenue
For contracts related to custom panel design services and sample processing, the Company utilizes a proportional performance revenue recognition model, under which revenue is recognized as performance occurs based on the relative outputs of the performance that have occurred up to that point in time under the respective agreement. The Company includes all applicable costs incurred related to custom panel design services, including research and development costs and general and administrative expenses, in cost of revenue.
Anticipated losses, if any, on contracts are charged to earnings as soon as they are identified. Anticipated losses cover all costs allocable to contracts. Revenue arising from claims or change orders is recorded either as income or as an offset against a potential loss only when the amount of the claim can be estimated and its realization is probable.
Other Revenue
Other revenue includes license and grant revenue. Grant revenue is earned when expenditures relating to the projects under these awards are incurred.
Product Warranty
The Company generally provides a one-year warranty on its HTG Edge and HTG EdgeSeq systems covering the performance of system hardware and software in conformance with customer specifications under normal use and protecting against defects in materials and workmanship. The Company may, at its option, replace, repair or exchange products covered under valid warranty claims. A provision for estimated warranty costs is recognized at the time of sale, through cost of revenue, based upon recent historical experience and other relevant information as it becomes available. The Company continuously assesses the adequacy of its product warranty accrual by reviewing actual claims and makes adjustments to the provision as needed. Due to a lack of historical data and a low rate of warranty claims, the Company had not recorded any liability for product warranty prior to the current period. Note 14 “Commitments and Contingencies” contains additional information relating to the Company’s product warranties.
F-14
Research and Development Expenses
Research and development expenses represent both costs incurred internally for research and development activities and costs incurred externally to fund research activities. All research and development costs are expensed as incurred. During the year ended December 31, 2015, the Company entered into a Development and Professional Services agreement with Invetech PTY Ltd. (Note 9) for the development of its low volume throughput HTG EdgeSeq platform, for which $325,000 of research and development expense was recorded for the year ended December 31, 2015.
Advertising
All costs associated with advertising and promotions are expensed as incurred. The amount of advertising and promotion expense was $11,761 and $18,581 for the years ended December 31, 2015 and 2014, respectively, and is included as a component of selling, general and administrative expenses on the accompanying statement of operations.
Stock-Based Compensation
The Company recognizes expense for share-based payments to employees, including grants of stock options and restricted stock units, based on the fair value of awards on the date of grant. The fair value of each employee stock option granted pursuant to the Company’s equity incentive plans is estimated on the date of grant using the Black-Scholes option pricing model. The determination of the fair value of share-based payment awards utilizing the Black-Scholes option pricing model is affected by the fair value of the Company’s stock price and a number of assumptions, including volatility, expected term, risk-free interest rate, and dividend yield. Generally these assumptions are based on historical information and judgment is required to determine if historical trends may be indicators of future outcomes.
The Company recognizes compensation cost for share-based payment awards with service conditions that have a graded vesting schedule on a straight-line basis over the requisite service period. However, the amount of compensation cost recognized at any time generally equals the portion of grant-date fair value of the award that is vested at that date, net of estimated forfeitures. Forfeitures are revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates and an adjustment to stock-based compensation expense will be recognized at that time. Changes to assumptions used in Black-Scholes option valuation calculation and the forfeiture rate could significantly impact the compensation expense recognized by the Company. The Company considered its historical experience of pre-vesting option forfeitures as the basis to arrive at its estimated pre-vesting option forfeiture rates of 17% and 2.8% per year for the years ended December 31, 2015 and 2014, respectively. The Company reports cash flows resulting from tax deductions in excess of the compensation cost recognized from those options (excess tax benefits) as financing cash flows, if they should arise.
The Company uses fair value to account for restricted stock units (RSUs). The RSUs are valued based on the quoted market price of common stock on the date of grant and amortized ratably over the life of the award.
For share-based payments to nonemployee consultants, the fair value of the share-based consideration issued is used to measure the transaction, as the Company believes this to be a more reliable measure of fair value than the services received. The fair value of the award is measured at the fair value of the Company’s stock options on the date that the commitment for performance by the nonemployee consultant has been reached or performance is complete. Stock-based compensation costs are recognized as expense over the requisite service period, which is generally the vesting period for awards, on a straight-line basis.
Black-Scholes assumptions used to calculate the fair value of options granted during the years ended December 31, 2015 and 2014 were as follows:
|
|
|
2015 |
|
2014 |
|
|
|
Fair value of common stock |
|
$ 4.88 - 14.64 |
|
$ 2.15 - 12.89 |
|
|
|
Risk-free interest rate |
|
1.36% - 2.22% |
|
1.65% - 2.03% |
|
|
|
Expected volatility |
|
60.5% - 75.0% |
|
|
70% |
|
|
Expected term |
|
5.0 to 10 years |
|
5.4 to 6.1 years |
|
|
|
Expected dividend yield |
|
—% |
|
—% |
|
|
Black-Scholes option pricing model was developed for use in estimating the fair value of short-traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of assumptions. The volatility assumption for 2015 is based on the volatility of the Company’s stock in recent periods as well as that of publicly traded industry competitors. The volatility assumption for 2014 is based on the volatility of publicly traded industry competitors and adjusted for future expectations.
F-15
The expected term of options is based on the utilization of the simplified method, which utilizes the contract term and vesting period to derive the expected term. The Company has elected to use the simplified method because the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term due to its equity shares being publicly traded for less than one year. The risk-free interest rate assumption is based on observed interest rates appropriate for the expected terms of the stock option grant. The Company does not anticipate paying a dividend, and, therefore, no expected dividend yield was used.
Stock-based compensation cost amounted to the following:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Selling, general and administrative |
|
$ |
367,000 |
|
|
$ |
163,540 |
|
|
Research and development |
|
|
38,426 |
|
|
|
21,426 |
|
|
|
|
$ |
405,426 |
|
|
$ |
184,966 |
|
The weighted-average fair value of stock options granted was $3.61 and $2.51 for the years ended December 31, 2015 and 2014, respectively. As of December 31, 2015, total unrecognized compensation cost related to stock-based compensation awards was approximately $788,875, which is expected to be recognized over approximately 2.9 years.
Income Taxes
The Company accounts for income taxes under the asset and liability method. Under the asset and liability method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is established against net deferred tax assets for the uncertainty it presents of the Company’s ability to use the net deferred tax assets, in this case the net operating tax loss carryforwards and research and development tax credits in the future. In assessing the realizability of net deferred tax assets, the Company assesses the likelihood that net deferred tax assets will be recovered from future taxable income, and to the extent that recovery is not likely or there is insufficient operating history, a valuation allowance is established. The Company records the valuation allowance in the period the Company determines it is “more likely than not” that net deferred tax assets will not be realized. For the years ended December 31, 2015 and 2014, the Company has provided a full valuation allowance for all net deferred tax assets due to their current realization being considered remote in the near term. The Company accounts for uncertain tax position taken or expected to be taken in a tax return using the more-likely-than-not threshold for financial statement recognition and measurement. The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. No material uncertain tax positions have been identified or recorded in the financial statements as of December 31, 2015 and 2014.
In November 2015, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”). ASU No. 2015-17 requires that all deferred tax assets and liabilities, along with any related valuation allowance, be classified as noncurrent on the balance sheet. The ASU is effective for annual periods, and the interim periods within those annual periods, beginning after December 31, 2016. Early adoption is permitted. The Company early adopted ASU No. 2015-17 on a prospective basis for the year ended December 31, 2015. Adoption did not have a material impact on the Company’s financial statements as of December 31, 2015.
Comprehensive loss
Comprehensive loss includes certain changes in equity that are excluded from net loss. Specifically, unrealized gains and losses on short and long-term available-for-sale investments are included in comprehensive loss.
Concentration Risks
Financial instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and uncollateralized accounts receivable. The Company maintains the majority of its cash balances in the form of cash deposits in bank checking and money market accounts in amounts in excess of federally insured limits. Management believes, based upon the quality of the financial institution, that the credit risk with regard to these deposits is not significant.
The Company sells its instruments, consumables, sample processing services, custom panel design services and contract research services primarily to biopharmaceutical companies, academic institutions and molecular labs. The Company routinely assesses the financial strength of its customers and credit losses have been minimal to date.
F-16
The top two customers accounted for 38% and 7% of the Company’s revenue for the year ended December 31, 2015, compared with 12% and 7% for the year ended December 31, 2014. The Company derived 8% and 31% of its total revenue from grants and contracts, primarily from one organization during the years ended December 31, 2015 and 2014. The largest two customers accounted for approximately 32% and 25% of the Company’s net accounts receivable as of December 31, 2015. One customer accounted for approximately 31% of the Company’s net accounts receivable at December 31, 2014.
The Company currently relies on a single supplier to supply several of the subcomponents used in the HTG Edge and HTG EdgeSeq processors. A loss of this supplier could significantly delay the delivery of HTG Edge and HTG EdgeSeq systems, which in turn would materially affect the Company’s ability to generate revenue.
New Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”), which supersedes nearly all existing revenue recognition guidance under US GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing US GAAP.
The revised revenue standard is effective for public entities for annual periods beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). The Company is currently evaluating the impact of the pending adoption of ASU 2014-09 on its financial statements and has not yet determined the method by which it will adopt the standard.
In August 2014, the FASB issued ASU No. 2014-15, Going Concern (“ASU 2014-15”). ASU 2014-15 provides US GAAP guidance on management’s responsibility in evaluating whether there is substantial doubt about a company’s ability to continue as a going concern and about related footnote disclosures. For each reporting period, management will be required to evaluate whether there are conditions or events that raise substantial doubt about a company’s ability to continue as a going concern within one year from the date the financial statements are issued. The standard will be effective for annual periods ending after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early application is permitted for annual or interim reporting periods for which the financial statements have not previously been issued. The Company does not believe the adoption of this standard will have a significant impact on its financial statements and is still assessing when the standard will be adopted.
In April 2015, the FASB issued ASU No. 2015-03, Interest – Imputation of Interest: Simplifying the presentation of Debt Issuance Costs (“ASU 2015-03”). The standard requires entities to present debt issuance costs on the balance sheet as a direct deduction from the related debt liability rather than as an asset, and to report amortization as interest expense. The result of application of this guidance would be to reduce the deferred financing costs balance, with a corresponding reduction to the long term liabilities to which the debt issuance costs relate in the balance sheets. The standard does not affect recognition and measurement of debt issuance costs. The Company expects to adopt ASU 2015-03 on January 1, 2016 and does not expect this standard to have a significant impact on its financial statements.
In April 2015, the FASB issued ASU No. 2015-05, Intangibles – Goodwill and other – Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement. Under this standard, if a cloud computing arrangement includes a software license, the software license element of the arrangement should be accounted for consistent with the acquisition of other software licenses. If a cloud computing arrangement does not include a software license, the arrangement should be accounted for as a service contract. The new standard will be effective for the Company on January 1, 2016. The adoption of this standard is not expected to have a significant impact on the Company’s financial position or results of operations.
In July 2015, the FASB issued ASU 2015-11, Inventory: Simplifying the Measurement of Inventory (“ASU 2015-11”). The standard requires inventory within the scope of the ASU to be measured using the lower of cost and net realizable value. The changes apply to all types of inventory, except those measured using LIFO or retail inventory method, and are intended to more clearly articulate the requirements for the measurement and disclosure of inventory and to simplify the accounting for inventory by eliminating the notions of replacement cost and net realizable value less a normal profit margin. The standard will be effective for fiscal years and interim periods within those fiscal years beginning after December 15, 2016. The Company does not believe the adoption of this standard will have a significant impact on its financial statements.
In February 2016, the FASB issued ASU No 2016-02, Leases. Under the new guidance, lessees will be required to recognize for all leases (with the exception of short-term leases) a lease liability, which is a lessee’s obligation to make lease payments arising from a
F-17
lease, measured on a discounted basis and a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term. Leases will be classified as either financing or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods for those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The Company is currently evaluating the effect the guidance will have on its financial statements.
Note 3. Inventory
Inventory, net of allowance, consisted of the following as of the date indicated:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Raw materials |
|
$ |
1,198,605 |
|
|
$ |
1,029,927 |
|
|
Work in process |
|
|
— |
|
|
|
— |
|
|
Finished goods |
|
|
1,002,696 |
|
|
|
655,887 |
|
|
|
|
$ |
2,201,301 |
|
|
$ |
1,685,814 |
|
Note 4. Fair Value
Financial assets and liabilities measured at fair value are classified in their entirety into the fair value hierarchy, based on the lowest level input significant to the fair value measurement. The following table classifies the Company’s financial assets and liabilities measured at fair value on a recurring basis at December 31, 2015 and 2014, respectively into the fair value hierarchy:
|
|
|
Balance at December 31, 2015 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Asset included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market securities |
|
$ |
3,290,490 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,290,490 |
|
|
Available-for-sale investments at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government obligations |
|
$ |
3,298,014 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,298,014 |
|
|
U.S. government agency obligations |
|
$ |
— |
|
|
$ |
14,589,378 |
|
|
$ |
— |
|
|
$ |
14,589,378 |
|
|
Corporate debt securities |
|
$ |
— |
|
|
$ |
12,918,016 |
|
|
$ |
— |
|
|
$ |
12,918,016 |
|
|
|
|
Balance at December 31, 2014 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Asset included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market securities |
|
$ |
3,608,890 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,608,890 |
|
|
Liabilities included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Growth Term Loan warrants |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
301,508 |
|
|
$ |
301,508 |
|
|
Convertible Preferred Stock warrants |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
429,035 |
|
|
$ |
429,035 |
|
There were no other financial instruments subject to fair value measurement on a recurring basis. Transfers to and from Levels 1, 2 and 3 are recognized at the end of the reporting period. There were no transfers between levels for the years ended December 31, 2015 and 2014.
Level 1 instruments include investments in money market funds and U.S. Treasuries. These instruments are valued using quoted market prices for identical unrestricted instruments in active markets. The Company defines active markets for debt instruments based on both the average daily trading volume and the number of days with trading activity. Level 2 instruments include U.S. Government agency obligations and corporate debt securities. Valuations of Level 2 instruments can be verified to quoted prices, recent trading activity for identical or similar instruments, broker or dealer quotations or alternative pricing sources with reasonable levels of price transparency. Consideration is given to the nature of the quotations (e.g. indicative or firm) and the relationship of recent market activity to the prices provided from alternative pricing sources.
F-18
Fair values of these assets and liabilities are based on prices provided by independent market participants that are based on observable inputs using market-based valuation techniques. These valuation models and analytical tools use market pricing or similar instruments that are both objective and publicly available, including matrix pricing or reported trades, benchmark yields, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids and/or offers. The Company did not adjust any of the valuations received from these third parties with respect to any of its level 1 securities for either of the years ended December 31, 2015 or 2014.
The Company’s portfolio of available-for-sale securities comprises U.S. Treasuries, U.S. government sponsored agency obligations and high credit quality corporate debt securities classified as available-for-sale securities. Initial investment in available-for-sale securities was made during the quarter ended June 30, 2015 as a result of funding received through the Company’s IPO.
Level 3 instruments include Redeemable Convertible Preferred Stock warrant liabilities. The Company used its May 2015 IPO pricing as an input for measurement of the fair value of its Level 3 preferred stock warrant liabilities at May 11, 2015 and the Black-Scholes option pricing model, and other valuation models for measuring the fair value of its Level 3 Preferred Stock warrant liabilities at December 31, 2014. The Company’s warrant liabilities at December 31, 2014 were categorized as Level 3 because they were valued based on unobservable inputs and management judgment due to the absence of quoted market prices, inherent lack of liquidity and the long-term nature of such financial instruments. As a result of their conversion to common stock warrants on May 11, 2015 with the Company’s IPO, there were no warrant liabilities outstanding at December 31, 2015.
The December 31, 2014 fair value assessments used the Black-Sholes option pricing model using the following assumptions:
|
|
|
December 31, 2014 |
|
|
|
Fair value of Series B/C/D Stock and Series E Stock shares on grant date or measurement date |
|
$0.14 - $0.22 |
|
|
|
Exercise price |
|
$0.01-$0.346 |
|
|
|
Expected risk-free interest rate |
|
|
1.20% |
|
|
Expected volatility |
|
|
70% |
|
|
Expected term |
|
4.1 years |
|
|
|
Expected dividend yield |
|
0 - 8% |
|
|
The volatility assumption is based on the volatility of publicly traded industry competitors as adjusted for future expectations. The expected term was based on the Company’s historical experience and future expectations with regard to the exercise of the Convertible Preferred Stock warrants and the probability of conversion of the underlying Convertible Preferred Stock. The risk-free interest rate assumption is based on observed interest rates appropriate for the expected terms of the warrants. At December 31, 2014 the fair value of the Convertible Preferred Stock was determined by a valuation model that considered both income and market-based valuations of the Company’s enterprise value.
The expected dividend yield at December 31, 2014 is consistent with the dividend rate on the Convertible Preferred Stock. The assumptions used in the Black-Scholes option pricing model are inherently subjective and involve significant judgment. Any change in the fair value was recognized as a component of other income (expense) in the statements of operations.
F-19
A reconciliation of the beginning and ending liabilities measured at fair value and classified as Level 3 for the years ended December 31, 2015 and 2014 are as follows:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Beginning balance |
|
$ |
730,543 |
|
|
$ |
44,120 |
|
|
Issuance of Series E Convertible Preferred Stock Warrants |
|
|
— |
|
|
|
2,190,708 |
|
|
Exercise of Series E Convertible Preferred Stock Warrants |
|
|
(4,116 |
) |
|
|
(1,889,201 |
) |
|
Exercise of Series D Convertible Preferred Stock Warrants |
|
|
(91,798 |
) |
|
|
(4,020 |
) |
|
Reclassification of Series C-2 Convertible Preferred Stock Warrants to Common Stock Warrants |
|
|
(555 |
) |
|
|
— |
|
|
Reclassification of Growth Term Loan Warrants to Common Stock Warrants |
|
|
(229,550 |
) |
|
|
— |
|
|
Issuance of Convertible Note Warrants |
|
|
741,828 |
|
|
|
— |
|
|
Reclassification of Convertible Note Warrants to Common Stock Warrants |
|
|
(1,386,035 |
) |
|
|
— |
|
|
Change in Convertible Preferred Stock warrant valuation |
|
|
239,683 |
|
|
|
388,936 |
|
|
Ending balance |
|
$ |
— |
|
|
$ |
730,543 |
|
Note 5. Property and Equipment
Property and equipment, net consists of the following:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Office equipment |
|
$ |
257,296 |
|
|
$ |
414,424 |
|
|
Leasehold improvements |
|
|
224,061 |
|
|
|
234,602 |
|
|
Laboratory and manufacturing equipment |
|
|
2,442,191 |
|
|
|
2,003,693 |
|
|
Field equipment |
|
|
180,355 |
|
|
|
286,044 |
|
|
Software |
|
|
140,248 |
|
|
|
140,248 |
|
|
Construction in progress |
|
|
175,501 |
|
|
|
6,125 |
|
|
|
|
|
3,419,652 |
|
|
|
3,085,136 |
|
|
Less: accumulated depreciation and amortization |
|
|
(1,487,439 |
) |
|
|
(1,938,537 |
) |
|
|
|
$ |
1,932,213 |
|
|
$ |
1,146,599 |
|
During the year ended December 31, 2015, the Company retired fully-depreciated property and equipment with a gross carrying value of $1,066,246, previously contained in office equipment, leasehold improvements and laboratory and manufacturing equipment.
Note 6. Accrued Liabilities
Accrued liabilities consist of the following:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Employee compensation and benefits |
|
$ |
1,240,314 |
|
|
$ |
881,821 |
|
|
Employee compensation for future absences |
|
|
154,107 |
|
|
|
136,040 |
|
|
Interest |
|
|
77,917 |
|
|
|
77,917 |
|
|
Professional fees |
|
|
140,385 |
|
|
|
70,000 |
|
|
Offering costs |
|
|
— |
|
|
|
178,000 |
|
|
Other accrued liabilities |
|
|
331,788 |
|
|
|
155,972 |
|
|
|
|
$ |
1,944,511 |
|
|
$ |
1,499,750 |
|
F-20
Note 7. Available for Sale Securities
The following is a summary of the Company’s available-for-sale securities at December 31, 2015:
|
|
|
|
|
|
|
Gross |
|
|
Gross |
|
|
Fair Value |
|
|||
|
|
|
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
(Net Carrying |
|
||||
|
|
|
Cost |
|
|
Gains |
|
|
Losses |
|
|
Amount) |
|
||||
|
US Treasury securities and obligations of US government agencies |
|
$ |
17,914,136 |
|
|
$ |
— |
|
|
$ |
(26,744 |
) |
|
$ |
17,887,392 |
|
|
Corporate debt securities |
|
|
12,932,629 |
|
|
|
397 |
|
|
|
(15,010 |
) |
|
|
12,918,016 |
|
|
Total available-for-sale securities |
|
$ |
30,846,765 |
|
|
$ |
397 |
|
|
$ |
(41,754 |
) |
|
$ |
30,805,408 |
|
The net adjustment to unrealized holding gains (losses) on available-for-sale securities in other comprehensive income totaled ($41,357) and $0, for the years ended December 31, 2015 and 2014, respectively.
Contractual maturities of debt investment securities at December 31, 2015, are shown below. Expected maturities will differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
|
|
|
Under |
|
|
|
|
|
|
|
|
|
|
|
|
|
1 Year |
|
|
1 to 2 Years |
|
|
Total |
|
|||
|
US Treasury securities and obligations of US government agencies |
|
$ |
16,592,324 |
|
|
$ |
1,295,068 |
|
|
$ |
17,887,392 |
|
|
Corporate debt securities |
|
|
11,609,183 |
|
|
|
1,308,833 |
|
|
|
12,918,016 |
|
|
Total available-for-sale securities |
|
$ |
28,201,507 |
|
|
$ |
2,603,901 |
|
|
$ |
30,805,408 |
|
The following table shows the gross unrealized losses and fair values of the Company’s investments that have unrealized losses, aggregated by investment category and length of time that individual securities have been in a continuous unrealized loss position as of December 31, 2015:
|
|
|
Under 1 Year |
|
|
1 to 2 Years |
|
|
Total |
|
|||||||||||||||
|
|
|
|
|
|
|
Gross |
|
|
|
|
|
|
Gross |
|
|
|
|
|
|
Gross |
|
|||
|
|
|
Fair |
|
|
Unrealized |
|
|
Fair |
|
|
Unrealized |
|
|
Fair |
|
|
Unrealized |
|
||||||
|
|
|
Value |
|
|
Losses |
|
|
Value |
|
|
Losses |
|
|
Value |
|
|
Losses |
|
||||||
|
US Treasury securities and obligations of US government agencies |
|
$ |
17,887,392 |
|
|
$ |
(26,744 |
) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
17,887,392 |
|
|
$ |
(26,744 |
) |
|
Corporate debt securities |
|
|
5,822,919 |
|
|
|
(15,010 |
) |
|
|
— |
|
|
|
— |
|
|
|
5,822,919 |
|
|
|
(15,010 |
) |
|
Total available-for-sale securities with unrealized losses |
|
$ |
23,710,311 |
|
|
$ |
(41,754 |
) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
23,710,311 |
|
|
$ |
(41,754 |
) |
For debt securities, the Company determines whether it intends to sell or if it is more likely than not that it will be required to sell impaired securities. This determination considers current and forecasted liquidity requirements, regulatory and capital requirements and securities portfolio management. For all impaired debt securities for which there was no intent or expected requirement to sell, the evaluation considers all available evidence to assess whether it is likely the amortized cost value will be recovered. The Company conducts a regular assessment of its debt securities with unrealized losses to determine whether securities have other-than-temporary impairment considering, among other factors, the nature of the securities, credit rating or financial condition of the issuer, the extent and duration of the unrealized loss, expected cash flows of underlying collateral, market conditions and whether the Company intends to sell or it is more likely than not the Company will be required to sell the debt securities. The Company did not have any other-than-temporary impairment in its US Treasury securities, obligations of US government agencies or corporate debt securities for the period ended December 31, 2015.
The Company began investing in marketable securities upon receipt of proceeds from its IPO in May 2015. Prior to that date, the Company did not have any investments in marketable securities.
Note 8. Debt Obligations.
Growth Term Loan
In August 2014, the Company entered into the Growth Term Loan with a syndicate of two lending institutions. The first tranche of the Growth Term Loan (“Growth Term Loan A”) of $11.0 million was funded at the August 2014 closing. A second tranche of $5.0
F-21
million (“Growth Term Loan B”) was initially available to be drawn until July 4, 2015. In August 2015, the Company and its lenders amended the Growth Term Loan agreement to extend the availability of Growth Term Loan B until March 31, 2016. The Company received the Growth Term Loan A proceeds net of a $0.3 million original issue discount. The Company also recorded a discount for the issuance of warrants with Growth Term Loan A (See Note 11). The original issuance discount and warrant discount are being amortized, using the effective interest method, over the term of the Growth Term Loan A. Growth Term Loan discount amortization expense was $170,954 and $84,676 for the years ended December 31, 2015 and 2014, respectively, and is included in interest expense in the accompanying statements of operations. The Growth Term Loan A bears interest at the fixed rate of 8.5% and matures in September 2018. Pursuant to the August 2015 amendment to the Growth Term Loan agreement, the Company will have monthly interest-only payment obligations until April 1, 2016. Following the interest-only payment period, equal monthly payments of $408,296 consisting of principal and interest amortized over the remaining term of the loan through September 2018 will be due. The balance sheets and the principal repayments table below have been adjusted to reflect this amended payment schedule. The August 2015 amendment further changed the prepayment fee schedule for Growth Term Loan A such that the Company will be obligated to pay a prepayment fee equal to (i) 3% of the principal amount prepaid if the Growth Term Loan is prepaid on or before the first anniversary of the August 2015 amendment, (ii) 2% of the principal amount repaid if the Growth Term Loan is prepaid after the first anniversary but on or prior to the second anniversary of the August 2015 amendment and (iii) 1% of the principal amount repaid if the Growth Term Loan is repaid after the second anniversary of the August 2015 amendment and prior to maturity. The amendment also increased the final payment percentage to 4.75%. Final fee premium amortization expense was $158,707 and $56,201 for the years ended December 31, 2015 and 2014, respectively, and is included in interest expense in the accompanying statements of operations. The Growth Term Loan requires the Company to maintain compliance with specific reporting covenants and does not require financial covenants. The Growth Term Loan is secured by a lien covering substantially all of the Company’s assets, excluding patents, trademarks and other intellectual property rights (except for rights to payment related to the sale, licensing or disposition of such intellectual property rights) and certain other specified property. The Company paid $0.1 million in financing costs upon entering the Growth Term Loan. The agreement included Convertible Preferred Stock warrants to purchase 2,512,562 shares of Series E Stock (the “Series E Loan Warrants”) at a price of $0.2189 per share or at the purchase price of the next round of equity sold if no further shares of Series E Stock were sold. The warrants to purchase shares of Series E Stock expire on August 22, 2024 (See Note 11) and were automatically converted to warrants for common stock upon completion of the Company’s IPO in May 2015.
The principal repayments due under the term loan as of December 31, 2015 before consideration of the additional $5.0 million Growth Term Loan B funds expected to be drawn in March 2016, are as follows:
|
2016 |
|
$ |
3,059,068 |
|
|
2017 |
|
|
4,393,103 |
|
|
2018 |
|
|
3,547,829 |
|
|
Total Growth Term Loan payments |
|
|
11,000,000 |
|
|
Less discount |
|
|
(365,877 |
) |
|
Plus final fee premium |
|
|
214,908 |
|
|
Total Growth Term Loan, net |
|
$ |
10,849,031 |
|
Convertible Notes
On December 30, 2014, the Company entered into two, separate subordinated convertible promissory note agreements (“the Note Agreements”). The Note Agreements provided that upon a qualified equity financing, pursuant to which the Company raised either through a qualified initial public offering of common stock or through a qualifying private placement of Convertible Preferred Stock, gross offering proceeds of at least $20,000,000 from the sale of shares to new investors, the outstanding principal amount and all accrued but unpaid interest under convertible notes issued pursuant to the Note Agreements would automatically convert into shares of common stock or preferred stock, whichever was sold in the offering. The number of shares into which the convertible notes were convertible was equal to the outstanding principal and accrued interest divided by the price per share paid by investors purchasing such newly issued equity securities. The Note Agreements also provided that in the event of a sale of the Company, the holders could elect to receive two times the outstanding principal and accrued interest or the number of shares of Series E Convertible Preferred Stock as was equal to the outstanding principal and accrued interest divided by $0.2189.
Under the first Note Agreement, the Company was able to issue up to $7,339,165 of notes to existing investors at five future, individual closings of up to $1,500,000 each upon ten days’ notice to participating investors. When issued, the notes would bear annual interest at 8% and mature on March 31, 2016. In addition, pursuant to the first Note Agreement the Company issued to participating investors warrants to purchase up to 5,029,114 shares of Series E Convertible Preferred Stock at $0.2189 per share, discussed further in Note 11.
F-22
Under the second Note Agreement, the Company was able to issue up to $6,203,971 of subordinated convertible notes to existing investors at four individual closings of up to $1,703,971 each upon ten days’ notice to participating investors. When issued, the notes would bear annual interest at 8% and mature on March 31, 2016. In addition, pursuant to the second Note Agreement the Company issued to participating investors warrants to purchase up to 4,282,472 shares of Series E Convertible Preferred Stock at $0.2189 per share, discussed further in Note 11.
Under each of the Note Agreements, the failure of a principal or major participating investor to invest their committed amount at each of the closings resulted in the conversion of a percentage of the investor’s preferred shares into common shares at the applicable conversion rates in effect pursuant to the Company’s restated certificate of incorporation. Under the first Note Agreement, failure of a principal investor to purchase their committed amount resulted in the conversion of their entire holdings of Convertible Preferred Stock into common stock, at conversion rates then in effect. Under the second Note Agreement, up to 80% of the total Convertible Preferred Stock held by such investor will be converted into common stock, at conversion rates then in effect. In addition, failure to purchase the full committed amount in any closing will result in the termination of the applicable investor’s warrants, in whole or in part, and the forgiveness and extinguishment of 80% of the aggregate principal amount of the applicable investor’s outstanding notes, if any. In the event the Company sold new shares of stock in a qualified initial public offering or preferred stock in a private placement, an investor’s failure to participate in the subsequent equity financing, in an amount equal to their applicable remaining committed amount under the Note Agreements, would result in the conversion of up to 80% of the non-participating investor’s aggregate Convertible Preferred Stock holding as of the closing date of the subsequent equity financing, along with a reduction of up to 80% of the non-participating investor’s warrants.
Pursuant to the provisions of the second Note Agreement, certain preferred shares were optionally converted into common stock as of December 30, 2014.
Certain provisions of the first Note Agreement terminate (including the investors’ obligations to purchase notes thereunder) immediately prior to the earlier to occur of the closing of (i) a qualified initial public offering or (ii) a qualified private placement. Certain provisions of the second Note Agreement terminate (including the investors’ obligations to purchase notes thereunder) immediately prior to the earlier to occur of (x) the time at which a registration statement covering a public offering of the Company’s securities under the Securities Act of 1933, as amended, becomes effective or (y) the initial closing of a qualified private placement. Such provisions of the Note Agreements (including the investors’ obligations to purchase notes thereunder) terminated in connection with the completion of the Company’s IPO.
As of December 31, 2014, there were no borrowings under the Note Agreements. Draws under the first Note Agreement in February, March and April 2015 totaled $4.5 million through the Company’s IPO in May 2015. There were no draws under the second Note Agreement prior to the Company’s IPO in May 2015.
The Company recorded a $741,828 discount for the estimated fair value of warrants issued in connection with the debt (See Note 11). The warrant discount is being amortized, using the effective interest method, over the term of the Convertible Notes. Amortization expense of $90,222 for the year ended December 31, 2015, is included in interest expense in the statements of operations. There was no amortization expense for the year ended December 31, 2014.
Settlement of Convertible Debt upon IPO Closing
Upon closing of the Company’s IPO in May 2015, all outstanding principal ($4.5 million) and accrued interest under the convertible notes issued pursuant to the first Note Agreement was converted into 324,591 shares of common stock at a conversion price of $14.00 per share. The Company evaluated the terms of the Note Agreement at inception and concluded that it should be accounted for as share settled debt as it falls within the scope of ASC 480, Distinguishing Liabilities from Equity. While the issued debt Note Agreements contain multiple events that could trigger settlement at different values, the Company evaluated all of the possible outcomes and considered the qualified IPO outcome to be predominant at more than a 50% probability of occurrence. The IPO settlement triggering event settles the fixed monetary amount of the debt known at inception with a variable number of shares of common stock based on the price of the common stock at settlement and therefore meets the definition of share settled debt. The Company compared the value of the common stock issued to settle the debt with the carrying amount of the debt at the IPO closing date of May 2015, net of unamortized discount and deferred financing costs, and recognized a loss on settlement of debt of $705,217 in the accompanying statements of operations for the year ended December 31, 2015. The value of the debt adjusted to the value of the common stock paid was reclassified from debt to equity.
F-23
NuvoGen Obligation
The Company entered into an asset purchase agreement in 2001, as amended, with NuvoGen Research, LLC (“NuvoGen”) to acquire certain intellectual property from NuvoGen. The Company accounted for the transaction as an asset acquisition. However, as the intellectual property was determined to not have an alternative future use, the upfront consideration was expensed. In exchange for the intellectual property, the Company initially paid NuvoGen 5,587 shares of the Company’s common stock, fixed payments of $740,000 over the first two years of the agreement and agreed to pay NuvoGen 6% of its yearly revenue, which would be applied to any fixed payments, until the total aggregate cash compensation paid to NuvoGen under the agreement equaled $15,000,000. Certain terms of the agreement were amended in November 2003, September 2004, November 2012 and February 2014. Pursuant to the latest amendment to the agreement, through 2017, the Company is only required to pay a yearly fixed fee, in quarterly installments, to NuvoGen in the range of $543,750 to $800,000, and may defer any accrued revenue-based payments. Beginning in 2018, we are obligated to pay the greater of $400,000 or 6% of the Company’s applicable annual revenues, plus amounts, if any, deferred in the 2016 and 2017 periods by which 6% of revenue exceeds the applicable fixed fee plus 5% interest on such deferred amounts until the obligation is paid in full. The obligation is currently non-interest bearing and was, but is no longer, secured by certain patents and trademarks.
The Company recorded the obligation at the estimated present value of the future payments using a discount rate of 2.5%, the Company’s estimate of its effective borrowing rate for similar obligations. Unamortized debt discount was $283,621 and $564,634 at December 31, 2015 and 2014, respectively.
Pursuant to the closing of the Growth Term Loan in August 2014 (See Note 8), the Company agreed to accelerate certain minimum payments pursuant to the asset purchase agreement and NuvoGen agreed to terminate its security interest in the originally pledged patents and trademarks. Remaining minimum payments that were otherwise due for 2014, 2015 and the first quarter of 2016, amounting to $868,750 were paid in advance. No further payment is due under the asset purchase agreement until the second quarter of 2016. The acceleration of payments did not significantly change the minimum cash flows and therefore had no significant accounting effect.
There were no payments made to NuvoGen during the year ended December 31, 2015. NuvoGen was paid $1,206,250 during the year ended December 31, 2014. Discount accreted during the years ended December 31, 2015 and 2014 was $281,013, and $187,017, respectively.
The remaining payments due to NuvoGen at December 31, 2015, are as follows:
|
2016 |
|
$ |
543,750 |
|
|
2017 |
|
|
800,000 |
|
|
2018 |
|
|
400,000 |
|
|
2019 |
|
|
400,000 |
|
|
2020 |
|
|
400,000 |
|
|
2021 and beyond |
|
|
6,698,743 |
|
|
Total NuvoGen obligation payments |
|
|
9,242,493 |
|
|
Less discount |
|
|
(283,621 |
) |
|
Total NuvoGen obligation, net |
|
$ |
8,958,872 |
|
Illumina, Inc. Agreement
In October 2014, the Company entered into a development and component supply agreement with Illumina, Inc. for the development and worldwide commercialization by the Company of up to two complete diagnostic gene expression profiling tests for with Illumina’s diagnostic instruments, using components supplied by Illumina. The Company refers to these diagnostic gene expression profiling tests as IVD test kits. The IVD test kits may be used in two discrete testing fields chosen by the Company, one or both of which may relate to oncology for breast, lung, lymphoma or melanoma tumors, and up to one of which may relate to transplant, chronic obstructive pulmonary disease, or immunology/autoimmunity. The Company provided notice to Illumina of its first testing selection during the quarter ended March 31, 2015. The Company must provide notice to Illumina of its second selected field, if any, by October 2016.
Following the Company’s selection of the testing field for each IVD test kit, the Company and Illumina have agreed to negotiate a development plan for the development and regulatory approval of the applicable IVD test kit. Illumina has agreed to provide development and regulatory support as part of the plan. Upon mutual agreement of the first development plan in the fourth quarter of
F-24
2015, the Company paid Illumina a $100,000 fee. The Company is also required to pay Illumina up to $1.0 million in the aggregate upon achievement of specified regulatory milestones relating to the IVD test kits. In addition, the Company has agreed to pay Illumina a single digit percentage royalty on net sales of any IVD test kits that the Company commercializes pursuant to the agreement. The first development plan was finalized on October 29, 2015. Ongoing research and development costs for these programs have been expensed as incurred and $100,000 has been paid to Illumina relating to this agreement as of December 31, 2015.
The agreement will expire on the earlier of October 2019 or the date which the last to expire development plan under the agreement is completed. The Company may terminate the agreement at any time upon 90 days’ written notice and may terminate any development plan under the agreement upon 30 days’ prior written notice. Illumina may terminate the agreement upon 30 days’ prior written notice if the Company undergoes certain changes of control or immediately if the Company fails to select a testing field for an IVD test kit within 24 months following the date of the agreement. Either party may terminate the agreement upon the other party’s material breach of the agreement that remains uncured for 30 days, or upon the other party’s bankruptcy.
Invetech PTY Ltd. Agreement
In September 2015, the Company entered into a development and professional services agreement with Invetech PTY Ltd., for the conduct of research and development of a next generation automated sample library preparation instrument. This instrument is to be a low volume throughput version of the Company’s existing HTG EdgeSeq system technology and is being referred to as Project JANUS during development.
The agreement requires the execution of a development plan for each stage of the project. Upon full execution and delivery of each development plan, the Company will pay Invetech development fee installments for that development plan. The initial installments of the Stage 0 development fee were earned in the third and fourth quarters of 2015, resulting in $325,000 of research and development expense relating to this agreement being included in the statements of operations for the year ended December 31, 2015. The project will move into Stage 1.1, initial prototypes, development phase, in the first quarter 2016. This stage is anticipated to be completed in 16 to 20 weeks of its start date and includes milestone payments due to Invetech during that period of approximately $1.5 million.
The agreement will remain in effect through completion of all tasks and acceptance of all deliverables under each development plan unless terminated earlier as provided for in the agreement.
Note 10. Net Loss Per Share
Net loss attributable to common stockholders per share is computed by dividing the net loss allocable to common stockholders by the weighted-average number of shares of common stock or common stock equivalents outstanding. Outstanding stock options, warrants and Convertible Preferred Stock have not been included in the calculation of diluted net loss attributable to common stockholders per share because to do so would be anti-dilutive. Accordingly, the numerator and the denominator used in computing both basic and diluted net loss per share for each period are the same. The following table provides a reconciliation of the numerator and denominator used in computing basic and diluted net loss per share for the periods presented:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Numerator: |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(21,397,791 |
) |
|
$ |
(13,958,408 |
) |
|
Accretion of stock issuance costs |
|
|
(35,046 |
) |
|
|
(102,677 |
) |
|
Accretion of Series E warrant discount |
|
|
(127,616 |
) |
|
|
(313,152 |
) |
|
Accretion of Series D and E Redeemable Convertible Preferred Stock dividends |
|
|
(1,165,932 |
) |
|
|
(3,244,572 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(22,726,385 |
) |
|
$ |
(17,618,809 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding-basic and diluted |
|
|
4,518,499 |
|
|
|
100,659 |
|
|
Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(5.03 |
) |
|
$ |
(175.03 |
) |
F-25
The following outstanding options, warrants and Convertible Preferred Stock were excluded from the computation of diluted net loss per share for the periods presented because their effect would have been anti-dilutive:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Options to purchase common stock |
|
|
736,645 |
|
|
|
595,577 |
|
|
Restricted stock units |
|
|
27,500 |
|
|
|
— |
|
|
Convertible Preferred Stock (as converted) |
|
|
— |
|
|
|
2,126,982 |
|
|
Convertible Preferred Stock warrants (as converted) |
|
|
— |
|
|
|
50,681 |
|
|
Common stock warrant |
|
|
169,099 |
|
|
|
931 |
|
Note 11. Warrants
In connection with certain of its Convertible Preferred Stock issuances, convertible debt financings and other financing arrangements, the Company issued warrants for shares of its common stock and various issues of its Convertible Preferred Stock.
On August 22, 2014, in connection with the Company’s entry into the Growth Term Loan, the Company issued to the lenders Series E Loan Warrants exercisable for an aggregate of 2,512,562 shares of Series E Convertible Preferred Stock at a price of $0.2189 per share. The warrants provided for cashless exercise at the option of the holders, and also contained provisions for the adjustment of the number of shares issuable upon the exercise of the warrant in the event of stock splits, recapitalizations, reclassifications, consolidations or dilutive issuances. In connection with the completion of the IPO in May 2015, the Series E Loan Warrants became exercisable for an aggregate of 23,396 shares of the Company’s common stock at an exercise price of $23.51 per share. The Series E Loan Warrants expire by their terms on August 22, 2024, provided that the warrants will be automatically exercised on a cashless basis upon expiration if not previously exercised if the fair market value of a share of the Company’s common stock exceeds the per share exercise price.
The Company allocated the total proceeds of the Growth Term Loan and the Series E Loan Warrants based on the residual value method. Assumptions used to determine the initial fair value included fair value of shares of Series E Stock on grant date $0.31; Exercise price $0.2189; Expected risk-free interest rate 1.6%; Expected volatility 70.0%; Expected term 4.5 years; and Expected dividend yield 8.0%. The fair value of the Series E Warrants of $0.12 per share, or $301,507, was recorded as a discount on the Growth Term Loan to be accreted over the term of the Growth Term Loan using the effective interest method. The Company has historically accounted for the Series E Loan Warrants as liabilities as such warrants were indexed to shares that could be redeemed for cash outside the control of the Company. The preferred warrants were valued using the Black-Scholes option pricing model as of December 31, 2014. At December 31, 2014, the fair value of the Series E Loan Warrants was $301,507. Refer to Note 4 for assumptions used to value these warrants.
On December 30, 2014, in connection with the Company’s Note and Warrant Purchase Agreements (Note 8) the Company agreed to issue warrants (the “Convertible Note Warrants”) exercisable for an aggregate of 9,311,586 shares of Series E Stock at a price of $0.2189 per share, for aggregate consideration of $1,354. The Convertible Note Warrants were issued on January 15, 2015. The warrants provide for cashless exercise at the option of the holders, and also contained provisions for the adjustment of the number of shares issuable upon the exercise of the warrant in the event of stock splits, recapitalizations, reclassifications, consolidations or dilutive issuances. The Convertible Note Warrants expire by their terms on January 15, 2022. As the Convertible Note Warrants were issued in conjunction with and in order to establish a lending facility commitment, they were being accounted for as a debt discount to be amortized over the life of the Note Agreements using the effective interest method and as a warrant liability, at fair value, as they were indexed to shares that could be redeemed for cash outside the control of the Company.
The Company recorded the initial $741,828 of the Convertible Note Warrants using the Black-Scholes option pricing model and assumptions further described in Note 4, specifically using a 7 year term, a 1.6% risk free interest rate, 75% volatility, 0% dividend yield and a common stock price of $0.13 which is a pre-IPO equivalent to the Company’s $14.00 per share offering price in May 2015, which the Company considered to approximate the fair value at January 14, 2015 as the operations of the business had not changed during the intervening period.
Upon closing of the IPO, the Company’s Convertible Preferred Stock warrants expired, were exchanged for common stock warrants or were exercised by the holders, depending on the terms of the respective agreements and actions of the holders. The Company computed the fair value of the warrants immediately prior to the conversion date and the change in fair value was recorded to loss from change in stock warrant valuation within the statements of operations.
F-26
The Company’s Series C-1 Convertible Preferred Stock warrants expired upon closing of the IPO as they were not exercised prior to or contemporaneously with the closing of the IPO. As of May 11, 2015, 1,290,350 Series C-1 preferred stock warrants were forfeited and cancelled as they were not exercised prior to the IPO.
Each share of Convertible Preferred Stock outstanding immediately prior to the closing of the IPO automatically converted into shares of the Company’s common stock. Due to the conversion of the underlying Series C-2 Convertible Preferred Stock and Series E Convertible Preferred Stock into common stock, the Series C-2, Series E and Convertible Note preferred warrants became exercisable for common stock. Following conversion to common stock warrants, these warrants were determined to meet the criteria for equity classification. Upon completion of the IPO, and the conversion of the Company’s Series C-2 Convertible Preferred Stock into common stock, the Series C-2 Convertible Preferred Stock warrants converted to warrants for purchase of 1,488 shares of the Company’s common stock at an exercise price of $24.23 per share. The Convertible Note Warrants converted to warrants for the purchase of 144,772 shares of the Company’s common stock at the initial public offering price of $14.00 per share. Series E Loan Warrants converted to warrants for the purchase of 23,396 shares of the Company’s common stock at the exercise price of $23.51 per share.
During 2015, all Series D Convertible Preferred Stock warrant holders elected to exercise their warrants, contingent and effective upon closing of the IPO. Upon completion of the IPO, the Company issued 723,050 shares of its Series D Convertible Preferred Stock upon the exercise of 769,059 outstanding warrants, for which it received aggregate cash consideration of approximately $1,752 for such exercises. Certain warrant holders elected to exercise pursuant to the terms of the net exercise provision of the warrant. For purposes of such net exercise, the fair market value of the Company’s common stock was equal to the IPO price of $14.00. The Series D Convertible Preferred Stock converted into 6,729 shares of the Company’s common stock on May 11, 2015. The Series C-2 Redeemable Convertible Preferred Stock warrants expired without exercise in December 2015.
|
Security |
|
Number of Preferred and Common Warrants at December 31, 2014 |
|
|
Number of Common Warrants at December 31, 2015 |
|
|
Exercise Price/Share |
|
|
Expiration Date |
|
|||
|
Series C-1 redeemable convertible preferred stock warrants |
|
|
1,290,350 |
|
|
|
— |
|
|
$ |
37.16 |
|
|
2016-2017 |
|
|
Series C-2 redeemable convertible preferred stock warrants |
|
|
157,912 |
|
|
|
— |
|
|
|
24.23 |
|
|
2015 |
|
|
Series D redeemable convertible preferred stock warrants |
|
|
769,059 |
|
|
|
— |
|
|
|
1.07 |
|
|
2020 |
|
|
Series E redeemable convertible preferred stock warrants |
|
|
2,512,562 |
|
|
|
23,396 |
|
|
|
23.51 |
|
|
2024 |
|
|
Convertible note warrants |
|
|
— |
|
|
|
144,772 |
|
|
|
14.00 |
|
|
2022 |
|
|
Common stock warrants |
|
|
931 |
|
|
|
931 |
|
|
|
6.45 |
|
|
2019 |
|
The fair value of the Convertible Preferred Stock warrant liability was $0 and $730,543 at December 31, 2015 and 2014, respectively. During the year ended December 31, 2015 the Company recorded a loss of $239,683, on the change in fair value of the Convertible Preferred Stock warrants, as the fair value of all Convertible Preferred Stock warrants was updated prior to conversion at IPO, with the fair value change being charged to loss from change in stock warrant valuation in the statements of operations. A loss of $388,936 was recorded during the year ended December 31, 2014 on the change in fair value of the Convertible Preferred Stock warrants. The Convertible Preferred Stock warrant liability for outstanding Series C-2, Series D and Series E warrants was reclassified to additional paid-in-capital and recorded as common stock warrants upon the closing of the Company’s IPO.
Note 12. Redeemable Convertible Preferred Stock
On February 4, 2014, the Company entered into the Series E Convertible Preferred Stock and Warrant Purchase Agreement (the “Series E Agreement”) authorizing the sale and issuance of up to 99,132,024 shares of its Series E Stock for $0.2189 per share and warrants (the “Series E Warrants”) to purchase up to an aggregate of 33,044,008 shares of Series E Stock at an exercise price of $0.001 per share. Pursuant to the Series E Agreement, up to 49,566,012 shares of Series E Stock together with Series E Warrants to purchase up to an aggregate of 16,522,004 shares of Series E Stock would be offered at one or more closings of a first tranche and the remainder of which will be offered in a second tranche.
As a result of the Series E Preferred Agreement, the Company’s authorized shares were increased to 600,000,000 shares of Common Stock and 472,083,383 shares of Convertible Preferred Stock.
On February 4, 2014 the Company issued 34,099,476 shares of Series E Stock pursuant to the Series E Agreement at a price per share of $0.2189 per share. Along with the shares of Series E Stock, one Series E Warrant was issued for every three shares of Series E Stock purchased with a purchase price of $0.0001 per Series E Warrant and an exercise price of $0.001 per share of Series E Stock for a total of 11,366,486 Series E Warrants. Each Series E Stock purchaser was required to exercise the Series E Warrant in a
F-27
simultaneous transaction with the purchase of shares of Series E Stock. Together with the Series E Warrants, the issuance of shares of Series E Stock and Series E Warrants resulted in gross proceeds to the Company of $7,476,879.
On March 31, 2014, pursuant to a rights offering, the Company issued 354,062 shares of Series E Stock pursuant to the Series E Agreement at a price per share of $0.2189 per share. Along with the shares of Series E Stock, one Series E Warrant was issued for each three shares of Series E Stock purchased with a purchase price of $0.0001 per Series E Warrant and an exercise price of $0.001 per share of Series E Stock for a total of 118,017 Series E Warrants. Each Series E Stock purchaser was required to exercise the Series E Warrant in a simultaneous transaction with the purchase of shares of Series E Stock. Together with Series E Warrants, the issuance of shares of Series E Stock and Series E Warrants resulted in gross proceeds to the Company of $77,634. The Series E Agreement provided for a second tranche on or before November 30, 2014, contingent upon the achievement of certain milestones.
It was determined that, given the nominal strike price of the Series E Warrants and the fact that the Series E Preferred Warrants were required to be exercised immediately upon issuance, the per share value of the Series E Warrants would be similar to the effective per share value of the Series E Stock, or $0.1645 per share.
The second closing under the Series E Agreement, originally scheduled to occur on or before November 31, 2014, was replaced by an alternative financing in the form of subordinated convertible promissory notes as discussed in Note 8.
Each series of Convertible Preferred Stock had a par value of $0.001 per share.
The Series D Convertible Preferred Stock and Series E Convertible Preferred Stock liquidation preference was equal to two times the original issue price of the Series D Convertible Preferred Stock or Series E Convertible Preferred Stock, respectively, plus any accrued but unpaid dividends whether or not declared. The Company has historically accreted up to the redemption amount and not the liquidation value, because additional amounts due under the liquidation rights were not considered probable at initial recording or as of the Company’s IPO in May 2015 or at December 31, 2014.
The shares of each series of Convertible Preferred Stock historically had the right to redemption of their respective class of shares on a date beginning not prior to February 2019 (the “Series E Preferred Stock Redemption Date”). In order to effect each respective redemption, the holders of at least 60% of the voting power of the then outstanding shares of Series D Convertible Preferred Stock (the “Series D Stock”) or Series E Convertible Preferred Stock (the “Series E Stock”) needed to vote in favor of a redemption and provide written notice to the Company at least 60 days prior to the Series E Preferred Stock Redemption Date of such election. The holders of at least a majority in the voting power of the then outstanding shares of the Series A, B and C Convertible Preferred Stock (the “Series C Stock”) could then vote in favor of a redemption, respectively. The holders of at least 66 2/3% of the voting power of the then outstanding shares of Series A Redeemable Convertible Preferred Stock (the “Series A Stock”) could then vote in favor of a Series A Stock redemption. The Series D Stock and Series E Stock redemption value was equal to the Series D Stock and Series E Stock original issue price per share (as adjusted for any stock dividends, combinations, splits, recapitalizations and the like) plus all dividends declared but unpaid thereon together with any accruing dividends accrued but unpaid thereon, whether or not declared with respect to such shares. The Series A/B/C/D and Series E Stock redemption value was equal to the respective series original issue price per share (as adjusted for any stock dividends, combinations, splits, recapitalizations and the like) plus all accrued and unpaid dividends with respect to such shares. If the Company did not have sufficient funds or other assets legally available to redeem all shares to be redeemed at any redemption date, it was to redeem as applicable (i) pro rata to Series E Stock, until all shares were redeemed, (ii) pro rata to Series D Stock until all shares were redeemed, (iii) pro rata to Series C Stock, until all shares were redeemed, (iv) pro rata to Series A Stock and Series B Convertible Preferred Stock (the “Series B Stock”), until all shares were redeemed. If any shares to be redeemed remained outstanding, the Company was to redeem the remaining shares in accordance with the previous sentence as soon as sufficient funds were legally available. Due to the redemption feature, the preferred shares were historically recorded as mezzanine equity.
|
Preferred Shares |
|
Carrying Value at 5/11/15 |
|
|
Preferred Shares Before IPO |
|
|
Common Shares After IPO |
|
|||
|
Series A Stock |
|
$ |
1,403,007 |
|
|
|
1,292,084 |
|
|
|
38,973 |
|
|
Series B Stock |
|
|
2,100,149 |
|
|
|
6,789,712 |
|
|
|
75,835 |
|
|
Series C-1 Stock |
|
|
4,569,063 |
|
|
|
13,242,612 |
|
|
|
191,406 |
|
|
Series C-2 Stock |
|
|
2,225,619 |
|
|
|
9,948,331 |
|
|
|
93,757 |
|
|
Series D Stock |
|
|
30,370,273 |
|
|
|
140,252,678 |
|
|
|
1,305,984 |
|
|
Series E Stock |
|
|
7,879,873 |
|
|
|
45,989,722 |
|
|
|
428,237 |
|
|
Carrying value, excluding dividends |
|
$ |
48,547,984 |
|
|
|
217,515,139 |
|
|
|
2,134,192 |
|
|
Series D Stock and Series E Stock cumulative dividends |
|
|
8,808,065 |
|
|
|
— |
|
|
|
374,632 |
|
|
Total |
|
$ |
57,356,049 |
|
|
|
217,515,139 |
|
|
|
2,508,824 |
|
F-28
As of the Company’s IPO in May 2015, Series A Stock, Series B Stock, Series C-1 Stock, Series C-2 Stock, Series D Stock and Series E Stock conversion ratios were 0.030, 0.011, 0.014, 0.009, 0.009 and 0.009, respectively, after consideration of the one-for-107.39 reverse split. When converted at May 11, 2015, all of the outstanding preferred shares and cumulative accrued dividends on Series D Stock and Series E Stock were converted into 2,134,192 and 374,632 shares of Common Stock, respectively.
The Company has historically accounted for the Series E Warrants as liabilities as such warrants were indexed to shares that could be redeemed for cash outside the control of the Company. The Company allocated the total proceeds first to the Series E Warrants based on their fair value of approximately $1,890,000 and the remainder amounting to approximately $5,665,000 of the proceeds allocated to the Preferred Shares. The fair value of the Series E Warrants was estimated to approximate the fair value of the Series E Stock because of their nominal price. The amount allocated to the Series E Warrants represented a discount to the Preferred Shares and was being accreted using the effective interest method up to the redemption amount from the respective issuance date to redemption date of five years. Accretion for the years ended December 31, 2015 and 2014 was $127,616 and $313,152, respectively. Upon immediate exercise of the Series E Warrants, the amount recorded as warrant liability was reclassified to Preferred Shares, and issuance costs of $48,384 which had been allocated to the warrants were expensed. The Company was not accreting to the amount resulting from additional liquidation preference rights under the agreement because they were not considered probable at initial recording or at any point between then and the May 2015 IPO. The Company additionally analyzed the issuance of Series E Warrants with the Series E Stock, noting there was no resulting beneficial conversion feature. Following the IPO, all outstanding warrants previously exercisable for Convertible Preferred Stock became exercisable for common stock. The previously reported warrant liability associated with the convertible warrants was applied to additional paid-in-capital.
The Company’s Series D Stock and Series E Stock accrued cumulative dividends at 8% per annum on the original issue price of $0.2189, whether or not declared by the Board of Directors. In connection with the Company’s Growth Term Loan, the holders of both the Series D Stock and Series E Stock agreed to waive their rights to cash dividends. As a result, only a share dividend, based on the cumulative dividends divided by the original issue price, could be paid upon declaration by the Board of Directors or upon the automatic conversion of the Convertible Preferred Stock. Dividends were being accreted based on the number of days outstanding. At December 31, 2015 and 2014, the cumulative Series D Stock and Series E Stock dividends were $0 and $7,643,378, respectively. All shares of Series D and Series E, together with accrued dividends, automatically converted into 374,632 shares of the Company’s common stock (on as converted method) in connection with the initial closing of the IPO on May 11, 2015.
The value of the shares of Series A/B/C/D Stock and Series E Stock was recorded at the amount initially received on the date of issuance or upon conversion from debt, less any applicable discounts for warrants and issuance costs, adjusted for the accretion of discounts utilizing the effective interest method up to the redemption amount from the respective issuance date to the Series E Preferred Stock Redemption Date, and the accretion of cumulative dividends. The redemption value for Series A/B/C Stock agrees to the liquidation value presented on face of the balance sheet. The redemption value of Series D Stock and Series E Stock was $0 at December 31, 2015.
On January 30, 2015, 51,681 shares of Series E Convertible Preferred Stock were purchased by a convertible note warrant holder for cash consideration of approximately $7,196.
Note 13. Stockholders’ Equity (Deficit)
Common Stock
The Company amended its certificate of incorporation on May 11, 2015, to decrease the number of authorized shares from 600,000,000 to 200,000,000 shares. The 200,000,000 authorized shares of common stock have a par value of $0.001 per share. As of December 31, 2015, 6,845,638 shares were issued. As of December 31, 2014, 334,003 of the 600,000,000 shares were issued.
Each share of common stock is entitled to one vote. All shares of common stock rank equally as to voting and all other matters that the holders are entitled to vote on. The shares of common stock have no preemptive or conversion rights, no redemption or sinking fund provisions, no liability for further call or assessment, and are not entitled to cumulative voting rights.
Treasury Stock
Shares of common stock repurchased by the Company are recorded as treasury stock and result in an increase of stockholders’ equity (deficit) on the balance sheets. Reacquired common shares may be retired by resolution of the Board of Directors and resume the status of authorized and unissued common shares. There was no new treasury stock activity for the years ended December 31, 2015 and 2014.
F-29
Pursuant to the Company’s certificate of incorporation the Company has been authorized to issue 10,000,000 shares of preferred stock, each having a par value of $0.001. The preferred stock may be issued from time to time in one or more series with the authorization of the Company’s Board of Directors. The Board of Directors has the ability to determine voting power for each series issued, as well as designation, preferences, and relative, participating, optional or other rights and such qualifications, limitations or restrictions thereof.
Stock-based Compensation
The Company initially established the 2001 Stock Option Plan (the “2001 Plan”), which included incentive and nonqualified stock options and restricted stock to be granted to directors, officers, employees, consultants and others. The 2001 plan terminated and no further awards were granted under the 2001 Plan upon the effective date of the Company’s 2011 Equity Incentive Plan (the “2011 Plan).
In February 2014, pursuant to the Series E Agreement, the amount of shares reserved under the 2011 Plan was increased to 20% of the total outstanding shares of the Company calculated on a fully diluted basis. The shares reserved under the 2011 Plan were required to be kept at that percentage with each subsequent equity financing. No new equity awards may be granted under the 2011 Plan.
On May 11, 2015, 940,112 shares were reserved for issuance under the Company’s 2014 Equity Incentive Plan (the “2014 Plan”), including 14,006 shares from the 2011 Plan. As of December 31, 2015, there were 756,332 shares available for issuance under the 2014 Plan.
As of December 31, 2015, options to purchase 736,645 shares of common stock were outstanding, including 410,649 options that were fully vested. The remaining options vest over 2.9 years.
The Company issued 27,500 RSUs in the year ended December 31, 2015 at a weighted average grant date fair value of $5.45. The RSUs vest 100% on March 31, 2016. As of December 31, 2015, there is $62,562 of unrecognized compensation expense related to the RSUs, which will be recognized over the weighted average remaining service period of three months. There is an additional $788,875 of unrecognized compensation expense of related to unvested stock options, which the Company expects to recognize over a weighted-average period of approximately 2.9 years.
The exercise price of options and restricted stock granted is generally equal to the estimated fair value of the Company’s common stock on the date of grant, as determined by the Company’s Board of Directors. All options granted have a ten-year term. The vesting period of options and restricted stock grants is established by the Board of Directors but typically ranges between three and four years.
A summary of the Plans’ stock option activity is as follows:
|
|
|
Number of Shares |
|
|
Weighted- Average Exercise Price Per Share |
|
|
Weighted- Average Remaining Contractual Life (Years) |
|
|
Aggregate Intrinsic Value |
|
||||
|
Balance at January 1, 2014 |
|
|
349,046 |
|
|
$ |
3.14 |
|
|
7.5 |
|
|
|
|
|
|
|
Granted |
|
|
314,685 |
|
|
|
4.01 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(9,909 |
) |
|
|
2.17 |
|
|
|
|
|
|
$ |
— |
|
|
Forfeited |
|
|
(54,739 |
) |
|
|
2.15 |
|
|
|
|
|
|
|
|
|
|
Expired |
|
|
(3,506 |
) |
|
|
2.98 |
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2014 |
|
|
595,577 |
|
|
|
3.71 |
|
|
|
7.8 |
|
|
$ |
— |
|
|
Granted |
|
|
171,408 |
|
|
|
6.11 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(13,960 |
) |
|
|
2.50 |
|
|
|
|
|
|
$ |
86,070 |
|
|
Forfeited |
|
|
(16,380 |
) |
|
|
8.57 |
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2015 |
|
|
736,645 |
|
|
|
4.18 |
|
|
|
7.5 |
|
|
$ |
947,794 |
|
|
Vested and expected to vest at December 31, 2015 |
|
|
687,488 |
|
|
|
4.01 |
|
|
|
7.3 |
|
|
$ |
723,894 |
|
|
Exercisable at December 31, 2014 |
|
|
294,851 |
|
|
|
3.42 |
|
|
|
6.5 |
|
|
$ |
— |
|
|
Exercisable at December 31, 2015 |
|
|
410,649 |
|
|
|
3.55 |
|
|
|
6.3 |
|
|
$ |
641,115 |
|
F-30
In December 2015, the Board of Directors adopted a Stock Purchase Plan (the “Purchase Plan”) which allows directors, any individual deemed by the Board of Directors to be an officer for purposes of Section 16 of the Exchange Act, and anyone designated by the Board of Directors as eligible to participate in the Purchase Plan to purchase shares of the Company’s common stock from the Company at fair market value. The aggregate number of shares of common stock that may be issued under the Purchase Plan shall not exceed 250,000 shares of common stock, and a maximum of 7,500 shares of common stock may be purchased by any one participant on any one purchase date. The Board of Directors or an authorized committee must review and approve each individual request to purchase common stock under the Purchase Plan. Cash received from the sale of common stock by the Company to eligible participants for the year ended December 31, 2015 was $20,000 which resulted in the sale of 4,184 shares of the Company’s common stock at fair market value.
2014 Employee Stock Purchase Plan
In April 2015, the Company’s stockholders approved the 2014 Employee Stock Purchase Plan (“ESPP”), which became effective in May 2015. Initially, the ESPP authorizes the issuance of up to 110,820 shares of common stock pursuant to purchase rights granted to the Company’s employees or to employees of any of the Company’s designated affiliates. The number of shares of common stock reserved for issuance automatically increases on January 1 of each calendar year, from January 1, 2016 to January 1, 2024 by the least of (i) 1% of the total number of shares of the Company’s common stock outstanding on December 31 of the preceding calendar year, (2) 195,000 shares, or (3) a number determined by the Company’s board of directors that is less than (1) and (2). The ESPP enables participants to contribute up to 15% of such participant’s eligible compensation during a defined period (not to exceed 27 months) to purchase common stock of the Company. The purchase price of common stock under the ESPP will be the lesser of: (i) 85% of the fair market value of the Company’s common stock at the applicable purchase date. As of December 31, 2015, no shares of common stock were issued and 110,820 shares of common stock were reserved for future issuance under the ESPP.
Note 14. Commitments and Contingencies
Legal Matters
The Company’s industry is characterized by frequent claims and litigation, including claims regarding intellectual property and product liability. As a result, the Company may be subject to various legal proceedings from time to time. The results of any current or future litigation cannot be predicted with certainty, and regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors. Any current litigation is considered immaterial and counter claims have been assessed as remote.
Severance Agreements and Retention Plans
In October 2012, the Board of Directors and 60% of requisite preferred holders approved a plan to set aside no less than 15% of the proceeds of any sale of the Company to be distributed to the Company’s employees, directors or consultants as designated by the Board of Directors (the “HTG Employee Retention Plan”). The HTG Employee Retention Plan terminated pursuant to its terms upon the closing of the IPO.
The Company has entered into employment agreements or other arrangements with certain named executive officers, which provides salary continuation payments, bonuses and, in certain instances, the acceleration of the vesting of certain equity awards to individuals in the event that the individual is terminated other than for cause, as defined in the applicable agreement.
Indemnification Agreements
In the course of operating its business, the Company has entered into, and continues to enter into, separate indemnification agreements with the Company’s directors and executive officers, in addition to the indemnification provided for in the Company’s amended and restated bylaws. These agreements may require the Company to indemnify its directors and executive officers for certain expenses incurred by a director executive officer in any action or proceeding arising out of their services as one of the Company’s directors or executive officers.
Leases
The Company leases office and laboratory space under two non-cancelable operating leases in Tucson, Arizona, which were set to expire in November and December 2015, respectively. Under the terms of the two Tucson leases, the Company had one option to extend the leases for five years at the end of the initial seven year lease term. The Company amended its existing facilities leases in
F-31
August 2015 to extend the terms for five years and require the landlord to undergo capital improvements to expand and improve the existing research, development, operations and administration office facilities. The lease amendment includes an increase of $804,000 in total monthly rent over the remaining life of the lease.
In addition, the Company entered into a construction contract with a third party in the third quarter 2015 for the expansion of and leasehold improvements in its laboratory, manufacturing and office spaces, representing an expected cash outlay commitment by the Company of approximately $500,000 through the first quarter of 2016. Such leasehold improvements will be capitalized and amortized over the remaining life of the lease as they are completed.
As a result of the amendments, the Company’s annual minimum lease payments before common area maintenance charges, which will commence in 2016 and continue through 2021 are as follows:
|
2016 |
|
$ |
494,354 |
|
|
2017 |
|
|
510,125 |
|
|
2018 |
|
|
512,533 |
|
|
2019 |
|
|
514,977 |
|
|
2020 |
|
|
517,457 |
|
|
2021 and beyond |
|
|
43,139 |
|
|
|
|
$ |
2,592,585 |
|
Rent expense and common area maintenance costs for all operating leases were $443,085and $368,937 for the years ended December 31, 2015 and 2014, respectively.
Merck Non-Exclusive License Agreement
In June 2012, the Company entered into a non-exclusive license agreement with Merck Sharp & Dohme Cor. (“Merck”) whereby the Company agreed to sublicense certain intellectual property related to breast cancer biomarkers with the intent to develop, manufacture and commercialize a diagnostic test utilizing this technology. The Company agreed to pay Merck certain contingent milestone payments between $50,000 and $1,000,000 and future royalties of 3%-6% of sales derived from such products developed that utilize the licensed technology. No amounts have been accrued or paid under this agreement as the Company has not achieved any of the milestone targets or developed any products that utilize the licensed technology.
Product Warranty
The following is a summary of the Company’s general product warranty liability for the year ended December 31, 2015:
|
Warranty reserve, January 1, 2015 |
|
$ |
— |
|
|
Cost of warranty claims |
|
|
(545 |
) |
|
Warranty accrual |
|
|
20,758 |
|
|
Warranty reserve, December 31, 2015 |
|
$ |
20,213 |
|
Prior to the quarter ended September 30, 2015, no warranty reserves were recorded by the Company.
Defined Contribution Plan
Effective January 1, 2003, the Company established a defined contribution plan (the 401(k) Plan) under Internal Revenue Code section 401(k). All employees upon hire who are over the age of 21 are eligible for participation in the 401(k) Plan. The Company may make discretionary contributions to the 401(k) Plan, but has not done so during the years ended December 31, 2015 and 2014.
Note 15. Income Taxes
The Company provides for income taxes based upon management’s estimate of taxable income or loss for each respective period. The Company recognizes an asset or liability for the deferred tax consequences of temporary differences between the tax bases of assets and liabilities and their reported amounts in the financial statements. These temporary differences would result in deductible or taxable amounts in future years, when the reported amounts of the assets are recovered or liabilities are settled, respectively.
F-32
In each period since inception, the Company has recorded a valuation allowance for the full amount of its net deferred tax assets, as the realization of the net deferred tax assets is uncertain. As a result, the Company has not recorded any federal or state income tax benefit in the statements of operations; however, state income tax expense has been recorded for state minimum taxes.
The Company periodically reviews its filing positions for all open tax years in all U.S. Federal, state and international jurisdictions where the Company is or might be required to file tax returns or other required reports.
The Company applies a two-step approach to recognizing and measuring uncertain tax positions. The Company evaluates the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation process, if any. The term “more likely than not” means a likelihood of more than 50 percent. If the tax position is not more likely than not to be sustained on audit, the Company may not recognize any of the potential tax benefit associated with the position. The Company recognizes a benefit for a tax position that meets the “more likely than not” criterion at the largest amount of tax benefit that is greater than 50 percent likely of being realized upon its effective resolution. Unrecognized tax benefits involve management’s judgment regarding the likelihood of the benefit being sustained. The final resolution of uncertain tax positions could result in adjustments to recorded amounts and may affect the Company’s results of operations, financial position and cash flows. The Company has not identified any uncertain tax positions at December 31, 2015 or December 31, 2014.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest or penalties at December 31, 2015 and 2014, respectively, and has not recognized interest or penalties during the years ended December 31, 2015 and 2014, respectively, since there are no material unrecognized tax benefits. Management believes no material change to the amount of unrecognized tax benefits will occur within in the next 12 months.
The components of income tax expense are as follows:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Current: |
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
— |
|
|
$ |
— |
|
|
State |
|
|
10,189 |
|
|
|
— |
|
|
Total current income tax expense |
|
$ |
10,189 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
Deferred: |
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
— |
|
|
$ |
— |
|
|
State |
|
|
— |
|
|
|
— |
|
|
Total deferred income tax expense |
|
$ |
— |
|
|
$ |
— |
|
|
Total income tax expense |
|
$ |
10,189 |
|
|
$ |
— |
|
The Company’s actual income tax expense for the years 2015 and 2014 differ from the expected amount computed by applying the statutory federal income tax rate of 34% to loss before income taxes as follows:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Computed tax (benefit) at 34% |
|
$ |
(7,271,785 |
) |
|
$ |
(4,745,859 |
) |
|
State taxes, net of federal benefit |
|
|
(918,079 |
) |
|
|
(233,632 |
) |
|
Stock-based compensation |
|
|
102,699 |
|
|
|
59,119 |
|
|
Expiring state net operating loss ("NOL") carryforwards |
|
|
(20,086 |
) |
|
|
131,342 |
|
|
Return to provision |
|
|
(27,934 |
) |
|
|
(4,269 |
) |
|
Other |
|
|
44,679 |
|
|
|
37,969 |
|
|
Research and development tax credit - state |
|
|
(300,213 |
) |
|
|
(20,720 |
) |
|
Research and development tax credit - federal |
|
|
(342,681 |
) |
|
|
(11,224 |
) |
|
Change in valuation reserve |
|
|
8,743,589 |
|
|
|
4,787,274 |
|
|
|
|
$ |
10,189 |
|
|
$ |
— |
|
F-33
Deferred tax assets and liabilities comprise the following:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
$ |
28,795,562 |
|
|
$ |
20,977,060 |
|
|
Research and development credits |
|
|
1,435,314 |
|
|
|
796,328 |
|
|
Deferred revenue |
|
|
17,396 |
|
|
|
15,166 |
|
|
Inventory reserve |
|
|
104,182 |
|
|
|
19,953 |
|
|
Fixed assets and intangibles |
|
|
50,128 |
|
|
|
53,822 |
|
|
Change in fair value of warrant liability |
|
|
111,993 |
|
|
|
3,541 |
|
|
Accrued NuvoGen liability |
|
|
3,282,756 |
|
|
|
3,190,603 |
|
|
Capitalized research and development |
|
|
27,583 |
|
|
|
55,354 |
|
|
Other |
|
|
115,792 |
|
|
|
85,290 |
|
|
|
|
|
33,940,706 |
|
|
|
25,197,117 |
|
|
Valuation allowance |
|
|
(33,940,706 |
) |
|
|
(25,197,117 |
) |
|
Deferred tax asset, net |
|
$ |
— |
|
|
$ |
— |
|
As of December 31, 2015, the Company has estimated federal and state NOL carryforwards of approximately $78,496,747 and $52,447,023 for federal and state income tax purposes, respectively. The Company’s federal NOLs are scheduled to expire from 2021 through 2035. The Company’s state NOLs are scheduled to expire from 2016 through 2035. The Company’s federal and state tax credit carryforwards begin expiring in 2021 and 2016, respectively. The Company’s federal NOL carryforwards have the following expiration dates:
|
|
|
Year of Expiration |
|
Carryforwards |
|
|
|
Federal NOL carryforwards |
|
|
|
|
|
|
|
|
|
2021 |
|
$ |
211,806 |
|
|
|
|
2023 |
|
|
1,635,651 |
|
|
|
|
2024 |
|
|
1,217,290 |
|
|
|
|
2025 |
|
|
1,409,498 |
|
|
|
|
2026 |
|
|
1,175,594 |
|
|
|
|
2027 |
|
|
1,676,458 |
|
|
|
|
2028 |
|
|
3,037,785 |
|
|
|
|
2029 |
|
|
3,753,314 |
|
|
|
|
2030 |
|
|
623,235 |
|
|
|
|
2031 |
|
|
5,435,312 |
|
|
|
|
2032 |
|
|
10,913,787 |
|
|
|
|
2033 |
|
|
12,095,966 |
|
|
|
|
2034 |
|
|
14,190,409 |
|
|
|
|
2035 |
|
|
21,120,642 |
|
|
|
|
|
|
$ |
78,496,747 |
|
For financial reporting purposes, valuation allowances of $33,940,706 and $25,197,117 at December 31, 2015 and 2014, respectively, have been established to offset deferred tax assets relating mainly to NOLs and research and development credits. The increase in the valuation allowance of $8,743,589 for the year ended December 31, 2015 was due primarily to increased operating losses. The Company has established a valuation allowance against the entire tax asset. As a result, the Company does not recognize any tax benefit until it is in a taxpaying position and, therefore, more likely to realize the tax benefit. Past and subsequent equity offerings by the Company, and other transactions that have an impact on the Company’s ownership structure, may trigger Sections 382 and 383 provisions of the Internal Revenue Code on special limitations on net operating losses and credits following ownership change. Such limitations may limit or eliminate the potential future tax benefit to be realized by the Company from its accumulated NOLs and research and development credits.
The Company files income tax returns in the United States, Arizona, California, Texas and various other state jurisdictions, with varying statutes of limitations. As of December 31, 2015, the earliest year subject to examination is 2012 for US federal tax purposes. The earliest year subject to examination is 2011 for Arizona, California and Texas, and 2013 for the remaining state jurisdictions.
F-34
However, the Company’s net operating loss carryforwards for periods ending December 31, 2001 and thereafter remain subject to examination by the United States and certain states.
Note 16. Related-Party Transactions
In March 2014 and July 2013, the Company entered into two separate consulting agreements to assess potential markets for its products with a holder of Series D Stock and Series E Stock. The stockholder was paid $0 for the year ended December 31, 2015, and $99,800 for the year ended December 31, 2014, under those agreements. An employee of the stockholder is a member of the Board of Directors. At December 31, 2015, there were no further amounts due under the consulting agreements.
Note 17. Subsequent Events
In March 2016, the Company entered into an Authorization, Supply and Regulatory Authorization Agreement with Life Technologies Corporation (“LTC”), a wholly owned subsidiary of Thermo Fisher Scientific, Inc., for the development and worldwide commercialization by the Company of up to five RNA-based next generation sequencing panels (“HTG Assays”) for use with LTC’s sequencing instruments and components supplied to end‑users by LTC.
Pursuant to the agreement, the Company has agreed to obtain its requirements for certain components to be used in the development of HTG Assays from LTC. As such, on March 21, 2016, the Company placed a non-cancellable purchase order for certain LTC products for approximately $250,000. LTG has agreed to provide support in the Company’s efforts to obtain regulatory approval of the HTG Assays. The Company is required to pay LTC a milestone payment in the mid-six figure dollar range upon certain regulatory achievements for each HTG Assay. In addition, the Company has agreed to pay LTC a single digit percentage royalty on net sales of any HTG Assays that the Company commercializes pursuant to the agreement.
Absent early termination, the initial term of the agreement will expire in March 2021 and thereafter will automatically renew for additional two year terms for as long as the Company continues to develop or sell HTG Assays. Either party may terminate the agreement by written notice delivered to the other party at least 60 days prior to the expiration of any then-current term. Either party may terminate the agreement (a) upon the other party’s material breach that remains uncured for 30 days, (b) upon the other party’s bankruptcy or (c) upon written notice in the event the party providing notice reasonably determines that continued performance under the agreement would violate any regulatory law, or any other applicable law or regulation or U.S. Food and Drug Administration guidance.
F-35
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures.
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC is recorded, processed, summarized and reported with the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable and not absolute assurance of achieving the desired control objectives. In reaching a reasonable level of assurance, management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As of December 31, 2015, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended. Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that, as of the end of the period covered by this annual report, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting.
This annual report does not include a report management’s assessment regarding internal control over financial reporting or an attestation report of the Company’s registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Control over Financial Reporting.
There were no changes in our internal controls over financial reporting during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
We are in the very early stages of the costly and challenging process of compiling the systems and processing documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. We are not currently in compliance with, and we cannot be certain when we will be able to implement the requirements of, Section 404 of the Sarbanes-Oxley Act.
We continue to review, document and test our internal control over financial reporting. We also continue to take steps to remediate certain identified deficiencies in our internal control over financial reporting.
Commencing with our fiscal year ending December 31, 2016, we must perform system and process evaluation and testing of our internal controls over financial reporting, to allow management to report on the effectiveness of our internal controls over financial reporting in our Form 10-K for that year, as required by Section 404 of the Sarbanes-Oxley Act.
We might not be able to complete our evaluation, testing or any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are designed and operating effectively, which could result in a loss of investor confidence in the accuracy and completeness of our financial reports.
The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, any system of internal control over financial reporting, including ours, no matter
116
how well designed and operated, can only provide reasonable, not absolute assurances. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. We intend to continue to monitor and upgrade our internal controls as necessary or appropriate for our business, but cannot assure you that such improvements will be sufficient to provide us with effective internal control over financial reporting.
None.
117
Item 10. Directors, Executive Officers and Corporate Governance.
Executive Officers and Directors
The following table sets forth certain information regarding our executive officers and directors:
|
Name |
|
Age |
|
Position(s) |
|
Executive Officers |
|
|
|
|
|
Timothy B. Johnson |
|
54 |
|
President and Chief Executive Officer and Director |
|
John L. Lubniewski |
|
52 |
|
Chief Business Officer |
|
Patrick C. Roche, Ph.D. |
|
63 |
|
Senior Vice President for Research and Development |
|
Shaun D. McMeans |
|
54 |
|
Vice President of Finance & Administration and Chief Financial Officer |
|
Debra A. Gordon, Ph.D., J.D. |
|
56 |
|
Vice President and Chief Legal Counsel |
|
|
|
|
||
|
Non-Employee Directors |
|
|
|
|
|
Peter T. Bisgaard (2)(3) |
|
42 |
|
Director |
|
Harry A. George (1) |
|
67 |
|
Director |
|
Mary (Molly) Hoult (1) |
|
50 |
|
Director |
|
James T. LaFrance (3) |
|
58 |
|
Director |
|
Lee McCracken (2)(3) |
|
48 |
|
Director |
|
Lewis J. Shuster (1) |
|
60 |
|
Director |
|
(1) |
Member of the audit committee. |
|
(2) |
Member of the compensation committee. |
|
(3) |
Member of the nominating and corporate governance committee. |
Executive Officers
Timothy (TJ) B. Johnson. Mr. Johnson has served as our President and Chief Executive Officer and as a member of our board of directors since January 2008. Mr. Johnson joined us from LVC Consulting, a consulting company, where he was a partner from April 2007 to January 2008. Prior to April 2007, Mr. Johnson spent five years in leadership roles at Ventana Medical Systems, Inc. or Ventana, a medical diagnostics company, prior to its acquisition by Roche Holdings, Inc., or Roche. At Ventana, Mr. Johnson held the positions of Senior Vice President, Global Business Services, Senior Vice President, Corporate Development and Operations, and Vice President/General Manager, Operations and Lean Systems. In these roles, Mr. Johnson’s responsibilities included product technical support, worldwide marketing, corporate development, strategic planning and manufacturing. Prior to working at Ventana, Mr. Johnson had an 12 year career at Hillenbrand Industries, Inc., a global diversified industrial company, where he held several leadership roles in the corporate offices and in the Hill-Rom Division, including Vice President, Global Marketing, Vice President/General Manager, Hill-Rom AirShields, Vice President, Operations, and Vice President, Continuous Improvement and Strategic Planning. Mr. Johnson spent part of his early career at PricewaterhouseCoopers LLP, a public accounting firm. Mr. Johnson previously served on the board of directors of Kalypto Medical, Inc. and was the Industry co-chair for the Biosciences Leadership Council of Southern Arizona. He earned a B.S. in Business from Indiana University. Our board of directors believes that Mr. Johnson’s extensive executive background in general management, strategic planning and managing operations of a diagnostic company and service as our President and Chief Executive Officer qualify him to serve on our board of directors.
John L. Lubniewski. Mr. Lubniewski has served as our Chief Business Officer since April 2011. Mr. Lubniewski joined us from Ventana, a medical diagnostics company and member of the Roche Group and global headquarters of Roche Tissue Diagnostics, or RTD, where he served in leadership roles for nine years both before and after the acquisition of Ventana by Roche in March 2008. From August 2010 to April 2011, Mr. Lubniewski was Senior Vice President and Lifecycle Leader, Advanced Staining Platforms at Ventana. From January 2008 to August 2010, Mr. Lubniewski served as Senior Vice President and Lifecycle Leader, Clinical Assays at RTD, with responsibility for three lifecycle teams, technical marketing and medical marketing and global accountability for all RTD clinical assay products. Prior to the Roche acquisition of Ventana, Mr. Lubniewski served at Ventana as Senior Vice President, Advanced Staining Business Unit, Vice President Worldwide Marketing and Translational Diagnostic Business Unit, and General Manager, Research Products. In these roles, Mr. Lubniewski was responsible for a variety of assay and platform development and commercialization efforts. Prior to Ventana, Mr. Lubniewski worked for over ten years at Corning, Inc., a manufacturing company, in a variety of divisional, sector and corporate sales and marketing roles. Mr. Lubniewski earned a B.S. in Chemical Engineering from Clarkson University.
118
Shaun D. McMeans. Mr. McMeans has served as our Vice President of Finance & Administration and Chief Financial Officer since February 2012. Prior to joining us, Mr. McMeans was Vice President – Finance of Securaplane Technologies, Inc., a product supply company and division of Meggitt PLC, an aerospace, defense and energy conglomerate, from May 2011 to February 2012. Mr. McMeans was a financial consultant from February 2008 to April 2011, working both in an individual capacity and as a partner for Tatum LLC, a consulting company. Prior to February 2008, Mr. McMeans was Chief Financial Officer for The Long Companies, a full service residential and commercial real estate division of Berkshire Hathaway, Inc. Mr. McMeans also worked for over five years at LXU Healthcare, Inc., a manufacturer and distributor of specialty surgical equipment, as Controller and then Chief Financial and Operating Officer. In his early career, Mr. McMeans worked in roles of increasing responsibility, including Director of Finance, for Burnham Holdings, Inc., formerly Burnham Corporation, a manufacturer and distributor of residential and commercial hydronic heating equipment. Mr. McMeans received his B.S. in Accounting from The Pennsylvania State University.
Patrick (Pat) C. Roche, Ph.D. Dr. Roche has served as our Senior Vice President for Research and Product Development since April 2014. Dr. Roche joined us from Ventana, a medical diagnostics company and member of the Roche Group and global headquarters of RTD, where he worked for 12 years and held a number of positions of increasing responsibility, including Vice President, Head Biomarker Strategy, Translational Diagnostics, from August 2009 to April 2014, and Vice President, Assay Development and Clinical Studies, from March 2007 to July 2009. In these roles, Dr. Roche was responsible for interfacing with pharmaceutical partners in their development of targeted cancer therapeutics and facilitating the transition of biomarkers into companion diagnostics and for leading reagent product development and launching over 30 in vitro diagnostic products, including the FDA-approved c-kit and HER2 tests. Prior to working at Ventana, Dr. Roche was at the Mayo Clinic Rochester, a medical research group, where he served as Director of the Immunohistochemistry Laboratory and as Associate Professor of Laboratory Medicine and Pathology. Dr. Roche has co-authored more than 125 peer-reviewed publications and is an inventor on a number of patent filings and two issued U.S. patents. Dr. Roche received a B.S. in Biological Sciences from the University of Southern California, and a Ph.D. in Experimental Pathology from the University of Southern California, School of Medicine.
Debra (Deb) A. Gordon, Ph.D., J.D. Dr. Gordon has served as our Vice President and Chief Legal Counsel since June 2011. Prior to joining us, Dr. Gordon was General Counsel and Head, Patent Legal at Ventana, a medical diagnostics company and a member of the Roche Group and the global headquarters for RTD, from October 2008 to June 2011. Dr. Gordon was promoted to leadership of the Ventana legal function after serving at Ventana from February 2007 as a patent attorney supporting the assay development functions. For nearly ten years preceding Dr. Gordon’s in-house legal experiences, Dr. Gordon practiced as a business transactional attorney at the law firms Lewis and Roca LLP (now, Lewis Roca Rothgerber LLP) and Perkins Coie LLP, and as an intellectual property attorney at the law firm Klarquist Sparkman LLP. Prior to law school, Dr. Gordon completed post-doctoral fellowships at Brandeis University and the University of Arizona. Dr. Gordon is an author on 13 peer-reviewed scientific publications and an inventor on two patent filings, one of which has issued in the U.S. and several other jurisdictions. Dr. Gordon received a B.S. in Biology and Chemistry and a M.S. in Chemistry from Western Washington University, a Ph.D. in Physiology from the University of Arizona, College of Medicine, and a J.D. with honors from the University of Arizona, James E. Rogers College of Law.
Non-Employee Directors
Peter T. Bisgaard. Mr. Bisgaard has served on our board of directors since March 2011, and as the chairman of our board of directors since September 2013. Mr. Bisgaard is currently employed as a Partner with Novo Ventures (US) Inc., a company that provides consultancy services to Novo A/S, a Danish limited liability company that manages investments and financial assets. Mr. Bisgaard joined Novo Ventures (US) Inc. in 2009. From 2001 to 2009, Mr. Bisgaard was employed as a Partner with Novo A/S. From 1998 to 2001, Mr. Bisgaard was employed with McKinsey & Co., a management consulting firm, where he focused on strategy development, mergers, acquisitions and alliances in various industries. Mr. Bisgaard was a member of the board of directors of Nevro Corporation, Alder BioPharmaceuticals, Inc. and Otonomy, Inc. Nevro is a medical device company listed on NYSE and Alder and Otonomy are both clinical-stage biopharmaceutical companies listed on NASDAQ. Further, Mr. Bisgaard is on the board of directors of the private companies Ceterix Orthopaedics Inc. and RA Pharma, and has served on the boards of directors of numerous other private biotech companies. Mr. Bisgaard holds an MSc from the Technical University of Denmark and was awarded a post-graduate degree in Mathematical Modeling in Economics by the European Consortium for Mathematics in the Industry. Our board of directors believes that Mr. Bisgaard’s strong financial expertise, extensive industry experience, experience serving on the board of directors for several biopharmaceutical and medtech companies, and experience with venture capital investments qualify him to serve on our board of directors.
Harry A. George. Mr. George has served on our board of directors since 2002 and served as the chairman of our board of directors from December 2007 until September 2013. Mr. George co-founded Solstice Capital, a venture capital firm, in 1995 and serves as its managing general partner. Mr. George has served as a member of the board of directors of a number of private and public companies and is currently serving on the boards of directors of Calimmune, Inc., Tempronics, Inc., Medipacs, Inc., Post.Bid.Ship, Inc., AdiCyte, Inc. and Sharklet. Mr. George is an advisor to Tech Launch Arizona and a board member of the University Venture Fund, Catapult. Additionally, Mr. George is a member of the Southern Arizona Leadership Council and serves on its board of
119
directors and has also served on the boards of directors of Bio5 Institute, the Arizona-Sonora Desert Museum, the Tucson Museum of Art, and the Pima County Bond Advisory Committee. Prior to 1995, Mr. George was co-founder, Director, and Vice-President of Finance for Interleaf Inc., a software products company. Prior to his time at Interleaf, Mr. George was co-founder, Director and Vice President of Finance of Kurzweil Computer Products, Inc., a computer products company, which subsequently was purchased by Xerox Imaging Systems. Mr. George received an A.B. from Bowdoin College and, in 2012, received an Honorary Doctorate of Science from the University of Arizona. Also in 2012, the Arizona BioIndustry Association conferred upon Mr. George the John McGarrity Bioscience Leader of the Year Award. Our board of directors believes Mr. George’s detailed knowledge of our company and long tenure with us, together with his more than 40 years of experience serving as founder, operating officer, or investor with successful rapid growth technology-related companies qualify him to serve on our board of directors.
Mary (Molly) Hoult. Ms. Hoult has served on our board of directors since February 2011. Ms. Hoult has worked for Fletcher Spaght, Inc., a venture capital and consulting firm, since August 2009, where she, as a vice president, co-directs the life science consulting practice and evaluates venture capital investments for life science tools, diagnostics, and pharmaceutical sectors. From January 2006 to June 2009, Ms. Hoult was a principal in Hoult Consulting, a consulting company, and, prior to that, served in roles of increasing responsibility at a number of life science companies, including Blue Heron Biotechnology, Inc., Xcyte Therapies, Inc., ZymoGenetics, Inc. and NeoPath, Inc. Ms. Hoult earned a B.A. in Biology and Environmental Studies from Dartmouth College and an M.B.A. from the Stanford Graduate School of Business. Our board of directors believes that Ms. Hoult’s more than 20 years of biotechnology, life science and healthcare experience as a consultant and in marketing, sales and business development roles for various biotechnology and diagnostics firms qualify her to serve on our board of directors.
James (Jim) T. LaFrance. Mr. LaFrance has served on our board of directors since December 2015. Mr. LaFrance has almost thirty years of diagnostic industry experience working since January 2015 as a sales, marketing, strategy development and commercial operational management consultant for LaFrance Consulting LLC, a firm he founded. He served as interim Chief Executive Officer of Vermillion, Inc. (NASDAQ: VRML), a bioanalytics service provider, from April 2014 to December 2014, where he also has served as chairman of the board of directors since December 2013. From November 2013 to March 2014, he served as a consultant in the medical diagnostics sector for his firm, LaFrance Consulting LLC. Prior to that, he was head of digital pathology and Chief Executive Officer of Omnyx, LLC for GE Healthcare from January 2012 to October 2013. From August 2009 to December 2011, Mr. LaFrance further served as a consultant in the medical diagnostics for LaFrance Consulting LLC. For eight years earlier in his career, Mr. LaFrance held a series of commercial, strategic marketing and business development leadership roles at Ventana Medical Systems (now Roche Tissue Diagnostics), or Ventana, including general management of North American and international commercial operations. Prior to working for Ventana, Mr. LaFrance served in leadership roles in strategic marketing and business development at Bayer Diagnostics. He earned a Bachelor of Arts degree in Economics from the University of Connecticut and holds a Masters in Business Administration from the University of Notre Dame. Our board of directors believes that Mr. LaFrance’s extensive industry and executive experience, and his experience serving on the board of directors of another public company qualify him to serve on our board of directors.
Lee McCracken. Mr. McCracken has served on our board of directors since October 2015. Mr. McCracken currently is the Chief Executive Officer of Gensignia Life Sciences, Inc., a molecular diagnostics company, a position that he has held since April 2014. Prior to that, from April 2013 to March 2014, he served as a strategic and restructuring consultant in the regenerative medicine and diagnostic sectors in his firm, McCracken Consulting. From October 2012 to March 2013, Mr. McCracken served as the President and Chief Executive officer of Pathwork Diagnostics, Inc., a molecular diagnostics company. From January 2012 to September 2012, he was a strategic consultant in the molecular diagnostics sector in his firm, McCracken Consulting. Beginning in 2004 through December 2011, he was the Corporate Head of Business Development and consultant for Prometheus Laboratories, Inc., a pharmaceutical and medical diagnostics company. Earlier in his career, Mr. McCracken held a number of executive positions or roles with significant responsibility at several biotechnology and therapeutics companies, including GenStar Therapeutics Corporation, CombiChem Inc., Allergan Inc., as well as at the investment companies, 3i Capital and Union Venture. Mr. McCracken received his M.B.A from the Anderson School of Management at the University of California, Los Angeles, his Master of Computer Science (MCS) from the University of Dayton, and his B.S. in Commerce from Santa Clara University. He currently serves as a director of several private companies including Gensignia Life Sciences, Inc. Our board of directors believes Mr. McCracken’s extensive executive and industry experience and his broad knowledge of molecular diagnostics qualify him to serve on our board of directors.
Lewis (Lew) J. Shuster. Mr. Shuster has served on our board of directors since March 2014. In 2002, Mr. Shuster founded Shuster Capital, a strategic and operating advisor to and angel investor in life science companies, and has served as its chief executive officer since that time. From June 2003 to November 2007, Mr. Shuster served as chief executive officer of Kemia, Inc., a drug discovery and development company. From February 2000 to December 2001, Mr. Shuster held various operating executive positions at Invitrogen Corporation, a biotechnology company that merged with Applied Biosystems Inc. and became Life Technologies Corporation. Between 1994 and 1999, Mr. Shuster served as chief financial officer and other executive positions at Pharmacopeia, Inc., a drug discovery product and service company. Mr. Shuster joined Human Genome Sciences, Inc. as its first employee in September 1992 and served as its executive vice president, operations and finance until 1994. Since June 2011 Mr. Shuster has served
120
as a member of the board of directors of Response Biomedical Corporation. From April 2010 to March 2013, Mr. Shuster served as a director of Complete Genomics, Inc., a life science company, from September 2009 to February 2010, as a director of Sorrento Therapeutics, Inc., and, from April 2011 to March 2016, as a director of Mast Therapeutics, Inc., both biopharmaceutical companies. Mr. Shuster received a B.A. in Economics from Swarthmore College and an M.B.A. from Stanford University. Our board of directors believes that Mr. Shuster’s extensive executive background in strategic planning and managing rapid operations growth for multiple public and private life science companies qualify him to serve on our board of directors.
Board Composition
Our business and affairs are organized under the direction of our board of directors, which consists of seven members as of December 31, 2015. The primary responsibilities of our board of directors are to provide oversight, strategic guidance, counseling and direction to our management. Our board of directors meets on a regular basis and additionally as required.
Certain of our current directors were elected to serve as a member of our board of directors pursuant to the voting provisions in our amended and restated investor rights agreement, dated February 4, 2014, by and among us and certain of our stockholders, or the investor rights agreement. Pursuant to the investor rights agreement, Mr. Bisgaard and Ms. Hoult, were selected to serve on our board of directors as the representative of our preferred stockholders, with Mr. Bisgaard designated by Novo A/S and Ms. Hoult designated by Fletcher Spaght Ventures II, L.P. Mr. Johnson was selected to serve on our board of directors as the representative of our common stockholders. The voting provisions in the investor rights agreement terminated upon the closing of our IPO, and members previously elected to our board of directors pursuant to the investor rights agreement will continue to serve as directors until their successors are duly elected and qualified by holders of our common stock.
Our board of directors has determined that all of our directors other than Mr. Johnson are independent directors, as defined by Rule 5605(a)(2) of the NASDAQ Listing Rules. The NASDAQ independence definition includes a series of objective tests, including that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by NASDAQ rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
In accordance with the terms of our amended and restated certificate of incorporation, our board of directors is divided into three classes, as follows:
|
|
· |
Class I, which consists of Ms. Hoult, Mr. McCracken and Mr. LaFrance, whose terms will expire at our annual meeting of stockholders to be held in 2016; |
|
|
· |
Class II, which consists of Mr. Bisgaard and Mr. George, whose terms will expire at our annual meeting of stockholders to be held in 2017; and |
|
|
· |
Class III, which consists of Mr. Johnson and Mr. Shuster, whose terms will expire at our annual meeting of stockholders to be held in 2018. |
At each annual meeting of stockholders, the successors to directors whose terms then expire will serve until the third annual meeting following their election and until their successors are duly elected and qualified. The authorized number of directors may be changed only by resolution of our board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in our control or management. Our directors may be removed for cause by the affirmative vote of the holders of at least 66 2/3% of our voting stock.
Board Leadership Structure
Our board of directors is currently chaired by Mr. Bisgaard. As a general policy, our board of directors believes that separation of the positions of Chairman and Chief Executive Officer reinforces the independence of the board of directors from management, creates an environment that encourages objective oversight of management’s performance and enhances the effectiveness of the board of directors as a whole. As such, Mr. Johnson serves as our President and Chief Executive Officer while Mr. Bisgaard serves as our Chairman of the board of directors but is not an officer. We expect the positions of Chairman of the board of directors and Chief Executive Officer to continue to be held by two individuals in the future.
121
Role of the Board in Risk Oversight
One of the key functions of our board of directors is informed oversight of our risk management process. The board of directors does not have a standing risk management committee, but rather administers this oversight function directly through the board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. The risk oversight process includes receiving regular reports from board committees and members of senior management to enable our board to understand our risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, strategic and reputational risk. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The audit committee also monitors compliance with legal and regulatory requirements. Oversight by the audit committee includes direct communication with our external auditors. Our nominating and corporate governance committee monitors the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee.
Audit Committee
Our audit committee consists of Mr. Shuster, Mr. George and Ms. Hoult. Mr. Shuster serves as the chair of our audit committee. Our board of directors has determined that each of the members of our audit committee satisfies the NASDAQ Stock Market and SEC independence requirements. The functions of this committee include, among other things:
|
|
· |
evaluating the performance, independence and qualifications of our independent auditors and determining whether to retain our existing independent auditors or engage new independent auditors; |
|
|
· |
reviewing and approving the engagement of our independent auditors to perform audit services and any permissible non-audit services; |
|
|
· |
monitoring the rotation of partners of our independent auditors on our engagement team as required by law; |
|
|
· |
prior to engagement of any independent auditor, and at least annually thereafter, reviewing relationships that may reasonably be thought to bear on their independence, and assessing and otherwise taking the appropriate action to oversee the independence of our independent auditor; |
|
|
· |
reviewing our annual and quarterly financial statements and reports, including the disclosures contained under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and discussing the statements and reports with our independent auditors and management; |
|
|
· |
reviewing with our independent auditors and management significant issues that arise regarding accounting principles and financial statement presentation and matters concerning the scope, adequacy and effectiveness of our financial controls; |
|
|
· |
reviewing with management and our auditors any earnings announcements and other public announcements regarding material developments; |
|
|
· |
establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters and other matters; |
|
|
· |
preparing the report that the SEC requires in our annual proxy statement; |
|
|
· |
reviewing and providing oversight of any related-person transactions in accordance with our related person transaction policy and reviewing and monitoring compliance with legal and regulatory responsibilities, including our code of business conduct and ethics; |
|
|
· |
reviewing our major financial risk exposures, including the guidelines and policies to govern the process by which risk assessment and risk management is implemented; |
122
|
|
· |
reviewing and evaluating on an annual basis the performance of the audit committee, including compliance of the audit committee with its charter. |
Our board of directors has determined that each member of the audit committee meets the requirements for financial literacy under the applicable rules and regulations of the SEC and the NASDAQ Stock Market. It has also determined that Mr. Shuster qualifies as an audit committee financial expert within the meaning of SEC regulations and meets the financial sophistication requirements of the NASDAQ Listing Rules. In making this determination, our board of directors has considered Mr. Shuster’s formal education and experience in financial and executive roles. Both our independent registered public accounting firm and management periodically meet privately with our audit committee. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and the NASDAQ Stock Market.
Compensation Committee
Our compensation committee consists of Mr. Bisgaard and Mr. McCracken. Dr. George was previously on the compensation committee and served as its chair until his board resignation in October 2015, at which time Mr. McCracken was appointed by our board of directors to serve as the chair of our compensation committee. Our board of directors has determined that each of the members of our compensation committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Securities Exchange Act of 1934, as amended, or Exchange Act, is an outside director, as defined pursuant to Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or the Code, and satisfies the NASDAQ Stock Market independence requirements. The functions of this committee include, among other things:
|
|
· |
reviewing, modifying and approving (or if it deems appropriate, making recommendations to the full board of directors regarding) our overall compensation strategy and policies; |
|
|
· |
reviewing and recommending to our board of directors the compensation and other terms of employment of our executive officers; |
|
|
· |
reviewing and recommending to our board of directors the performance goals and objectives relevant to the compensation of our executive officers and assessing their performance against these goals and objectives; |
|
|
· |
reviewing and approving (or if it deems it appropriate, making recommendations to the full board of directors regarding) the equity incentive plans, compensation plans and similar programs advisable for us, as well as modifying, amending or terminating existing plans and programs; |
|
|
· |
evaluating risks associated with our compensation policies and practices and assessing whether risks arising from our compensation policies and practices for our employees are reasonably likely to have a material adverse effect on us; |
|
|
· |
reviewing and approving (or if it deems it appropriate, making recommendations to the full board of directors regarding) the type and amount of compensation to be paid or awarded to our non-employee board members; |
|
|
· |
establishing policies for allocating between long-term and currently paid out compensation, between cash and non-cash compensation and the factors used in deciding between the various forms of compensation; |
|
|
· |
establishing policies with respect to votes by our stockholders to approve executive compensation as required by Section 14A of the Exchange Act and determining our recommendations regarding the frequency of advisory votes on executive compensation; |
|
|
· |
reviewing and assessing the independence of compensation consultants, legal counsel and other advisors as required by Section 10C of the Exchange Act; |
|
|
· |
establishing elements of corporate performance for purposes of increasing or decreasing compensation; |
|
|
· |
administering our equity incentive plans; |
|
|
· |
establishing policies with respect to equity compensation arrangements; |
|
|
· |
reviewing regional and industry-wide compensation practices and trends to assess the competitiveness of our executive compensation programs and evaluating the effectiveness of our compensation policy and strategy in achieving expected benefits to us; |
|
|
· |
reviewing the adequacy of its charter on a periodic basis; |
|
|
· |
reviewing with management and approving our disclosures under the caption “Compensation Discussion and Analysis” in our periodic reports or proxy statements to be filed with the SEC, if applicable; |
123
|
|
· |
reviewing and assessing on an annual basis the performance of the compensation committee. |
The compensation committee operates under a written charter that satisfies the applicable standards of the SEC and the NASDAQ Stock Market.
Nominating and Corporate Governance Committee
Immediately following our initial public offering in May 2015, the nominating and corporate governance committee consisted of Mr. Bisgaard, Dr. Simeon George and Mr. Donald Grimm. Mr. Grimm served as the chair of our nominating and corporate governance committee until his resignation in October 2015. At that time, Mr. Bisgaard was appointed by our board of directors to be the chair of our nominating and corporate governance committee and Mr. McCracken was appointed to be a member of the committee replacing Dr. George upon his resignation from our board of directors. Our board of directors has determined that each of the members of this committee satisfies the NASDAQ Stock Market independence requirements. The functions of this committee include, among other things:
|
|
· |
identifying, reviewing and evaluating candidates to serve on our board of directors consistent with criteria approved by our board of directors; |
|
|
· |
determining the minimum qualifications for service on our board of directors; |
|
|
· |
evaluating director performance on the board and applicable committees of the board and determining whether continued service on our board is appropriate; |
|
|
· |
evaluating, nominating and recommending individuals for membership on our board of directors; |
|
|
· |
evaluating nominations by stockholders of candidates for election to our board of directors; |
|
|
· |
considering and assessing the independence of members of our board of directors; |
|
|
· |
developing a set of corporate governance policies and principles, including a code of business conduct and ethics, periodically reviewing and assessing these policies and principles and their application and recommending to our board of directors any changes to such policies and principles; |
|
|
· |
assist the chair of our board of directors or lead independent director in developing effective board of directors meeting practices and procedures; |
|
|
· |
oversee and review the processes and procedures used by us to provide information to our board of directors and its committees; |
|
|
· |
assist the members of our compensation committee, as requested, in determining the compensation paid to non-employee directors for their service on our board of directors and its committees and recommend any changes considered appropriate to our full board of directors for approval; |
|
|
· |
periodically review with our Chief Executive Officer the plans for succession to the offices of our Chief Executive Officer and other key executive officers and make recommendations to our board of directors with respect to the selection of appropriate individuals to succeed those positions; |
|
|
· |
reviewing the adequacy of its charter on an annual basis; and |
|
|
· |
annually evaluating the performance of the nominating and corporate governance committee. |
The nominating and corporate governance committee operates under a written charter, which the nominating and corporate governance committee reviews and evaluates at least annually.
The nominating and corporate governance committee will consider qualified director candidates recommended by stockholders in compliance with our procedures and subject to applicable inquiries. The nominating and corporate governance committee’s evaluation of candidates recommended by stockholders does not differ materially from its evaluation of candidates recommended from other sources. Any stockholder may recommend nominees for director by writing to Peter T. Bisgaard, Chairman of the nominating and corporate governance committee of the Board of Directors, HTG Molecular Diagnostics, Inc., 3430 E. Global Loop, Tucson, Arizona 85706, giving the name and address of the stockholder on whose behalf the submission is made, the number of Company shares that are owned beneficially by such stockholder as of the date of the submission, the full name of the proposed candidate, a description of the proposed candidate’s business experience for at least the previous five years, complete biographical information for the proposed candidate and a description of the proposed candidate’s qualifications as a director. All of these
124
communications will be reviewed by our nominating and corporate governance committee, for further review and consideration in accordance with this policy.
Compensation Committee Interlocks and Insider Participation
We have established a compensation committee which has and will make decisions relating to compensation of our executive officers. Mr. McCracken became the chairman of the compensation committee upon joining our board of directors in October 2015. Mr. Bisgaard serves on the compensation committee as did Dr. George until his resignation in October 2015. None of these individuals has ever been an executive officer or employee of ours.
None of our executive officers currently serves, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Section 16(a) Beneficial Ownership Reporting Compliance
Under Section 16(a) of the Exchange Act, directors, officers and beneficial owners of 10% or more of our common stock are required to file with the SEC on a timely basis initial reports of beneficial ownership and reports of changes regarding their beneficial ownership of our common stock. Officers, directors and 10% beneficial owners are required by SEC regulations to furnish us with copies of all Section 16(a) forms that they file.
Based solely on our review of the copies of such forms received and the written representations from certain reporting persons, we have determined that no officer, director or 10% beneficial owner known to us was delinquent with respect to their reporting obligations as set forth in Section 16(a) of the Exchange Act during the fiscal year ended December 31, 2015, with the exception of Lee R. McCracken, who received an initial stock option grant on October 27, 2015 and filed Forms 3 and Form 4 late on December 7, 2015.
Limitation on Liability and Indemnification of Directors and Officers
Our amended and restated certificate of incorporation and bylaws limits our directors’ and officers’ liability to the fullest extent permitted under Delaware corporate law. Delaware corporate law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability:
|
|
· |
for any transaction from which the director derives an improper personal benefit; |
|
|
· |
for any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
|
|
· |
under Section 174 of the Delaware General Corporation Law (unlawful payment of dividends or redemption of shares); or |
|
|
· |
for any breach of a director’s duty of loyalty to the corporation or its stockholders. |
If the Delaware General Corporation Law is amended to authorize corporate action further eliminating or limiting the personal liability of directors or officers, then the liability of our directors or officers shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
Delaware law and our amended and restated bylaws provide that we will, in certain situations, indemnify any person made or threatened to be made a party to a proceeding by reason of that person’s former or present official capacity with us against judgments, penalties, fines, settlements and reasonable expenses. Any person is also entitled, subject to certain limitations, to payment or reimbursement of reasonable expenses (including attorneys’ fees and disbursements) in advance of the final disposition of the proceeding.
In addition, we have entered, and intend to continue to enter, into separate indemnification agreements with our directors and executive officers. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or as a director or executive officer of any other company or enterprise to which the person provides services at our request.
We believe that these provisions in our amended and restated certificate of incorporation and amended bylaws and these indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain a directors’
125
and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or control persons, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Stockholder Communications with the Board of Directors
We have adopted a formal process by which stockholders may communicate with our board of directors or any individual director. Stockholders who wish to communicate with our board of directors or any individual director may do so by sending written communications addressed to our Secretary at 3430 E. Global Loop, Tucson, Arizona, 85706. These communications will be reviewed by our Secretary, who will determine whether the communication is appropriate for presentation to our board of directors or the relevant director. The purpose of this screening is to allow our board of directors to avoid having to consider irrelevant or inappropriate communications (such as advertisements, solicitations and hostile communications).
Code of Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our officers, directors and employees. The Code of Business Conduct and Ethics is available on our website at www.htgmolecular.com. If we make any substantive amendments to the Code of Business Conduct and Ethics or grant any waiver from a provision of the Code to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website.
Item 11. Executive Compensation.
Our named executive officers for the year ended December 31, 2015, which consist of our principal executive officer and our two other most highly compensated executive officers as of December 31, 2015, are as follows:
|
|
· |
Timothy B. Johnson, our President and Chief Executive Officer; |
|
|
· |
John Lubniewski, our Chief Business Officer; and |
|
|
· |
Shaun McMeans, our Vice President of Finance and Administration and Chief Financial Officer. |
Summary Compensation Table
|
Name and principal position |
|
Year |
|
Salary ($) |
|
|
Restricted Stock Unit Awards ($) (1) |
|
|
Option Awards ($) (2) |
|
|
Non-equity incentive plan compensation ($) (3) |
|
|
|
All Other Compensation ($) (4) |
|
|
Total ($) |
|
||||||
|
Timothy B. Johnson |
|
2015 |
|
|
382,952 |
|
|
|
81,750 |
|
|
|
— |
|
|
|
120,000 |
|
|
|
|
647 |
|
|
|
503,599 |
|
|
President and Chief Executive Officer |
|
2014 |
|
|
350,000 |
|
|
|
— |
|
|
|
133,518 |
|
|
|
105,000 |
|
|
|
|
836 |
|
|
|
589,354 |
|
|
John L. Lubniewski |
|
2015 |
|
|
289,604 |
|
|
|
13,625 |
|
|
|
— |
|
|
|
70,440 |
|
|
|
|
647 |
|
|
|
360,691 |
|
|
Vice President and Chief Business Officer |
|
2014 |
|
|
281,875 |
|
|
|
— |
|
|
|
70,342 |
|
|
|
68,400 |
|
|
|
|
836 |
|
|
|
421,453 |
|
|
Shaun D. McMeans |
|
2015 |
|
|
226,362 |
|
|
|
27,250 |
|
|
|
— |
|
|
|
57,600 |
|
|
|
|
532 |
|
|
|
284,494 |
|
|
Vice President, Treasurer and Chief Financial Officer |
|
2014 |
|
|
200,000 |
|
|
|
— |
|
|
|
59,762 |
|
|
|
48,000 |
|
|
|
|
709 |
|
|
|
308,471 |
|
|
(1) |
The dollar amounts in this column represent the aggregate grant date fair value of restricted stock unit awards granted in 2015 and 2014, as applicable. For further discussion of valuation assumptions, see Note 13 “Stock-based Compensation” to our financial statements included elsewhere in this Annual Report on Form 10-K. |
|
(2) |
The dollar amounts in this column represent the aggregate grant date fair value of stock option awards granted in 2015 and 2014, as applicable. These amounts have been computed in accordance with FASB ASC Topic 718, using the Black-Scholes option pricing model. Pursuant to SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. For a discussion of valuation assumptions, see Note 13 “Stock-based Compensation” to our financial statements included elsewhere in this Annual Report on Form 10-K. |
|
(3) |
Amounts shown represent annual performance-based bonuses earned for 2015 and 2014. The annual performance-based bonuses earned for 2015 were paid in the form of fully vested shares of common stock granted under the 2014 plan. For more information, see below under “—Annual Performance-Based Bonus Opportunity.” |
126
|
(4) |
Amount shown represents premiums for life, disability and accidental death and dismemberment insurance paid by us on behalf of the named executive officer. |
Annual Base Salary
The base salary of our named executive officers is generally set forth in each officer’s employment letter agreement with us and periodically reviewed and adjusted as necessary by our board of directors, based on the recommendation of the compensation committee of our board of directors. The 2015 base salaries for our named executive officers, which were effective as of January 1, 2015, were $350,000, $285,000 and $200,000, for Mr. Johnson, Mr. Lubniewski and Mr. McMeans, respectively. Mr. Johnson’s annual base salary was increased to $400,000 in May 2015. Mr. Lubniewski’s annual base salary was increased to $293,500 in June 2015. Mr. McMeans’ annual base salary was increased to $240,000 in May 2015.
Annual Performance-Based Bonus Opportunity
In addition to base salaries, our named executive officers are eligible to receive annual performance-based bonuses, which are designed to provide appropriate incentives to our executives to achieve defined annual corporate goals and to reward our executives for individual achievement towards these goals. The annual performance-based bonus each named executive officer is eligible to receive is generally based on the extent to which we achieve the corporate goals that our board of directors establishes each year. At the end of the year, our board of directors reviews our performance against each corporate goal and approves the extent to which we achieved each of our corporate goals.
Our board of directors will generally consider each named executive officer’s individual contributions towards reaching our annual corporate goals but does not typically establish specific individual goals for our named executive officers. There is no minimum bonus percentage or amount established for the named executive officers and, as a result, the bonus amounts vary from year to year based on corporate and individual performance. For 2015, Mr. Johnson was eligible to receive a target bonus of up to 50% of his base salary pursuant to the terms of his employment letter agreement described below. For 2015, Mr. Lubniewski was eligible to receive a target bonus of up to 40% of his base salary pursuant to the terms of his employment letter agreement described below. For 2015, Mr. McMeans was eligible to receive a target bonus of up to 40% of his base salary pursuant to the terms of his employment letter agreement described below.
The corporate goals established by our board of directors for 2015 were based upon market adoption, menu expansion, market development and financial goals. Specific goals included expanded placement of HTG Edge and HTG EdgeSeq instruments, the launching of new assays, product development milestone achievement and expansion of relationships through consortium agreements and external publications. The menu expansion goals were weighted at 40% towards overall corporate goal achievement, the market adoption goals were weighted 40% towards overall corporate goal achievement, the market development goals were weighted at 10% towards overall corporate goal achievement and the financial goals were weighted 10% towards overall corporate goal achievement. There was no minimum percentage of corporate goals that must be achieved in order to earn a bonus. No specific individual goals were established for any of our named executive officers for 2015.
In February 2016, our board of directors determined that the 2015 corporate goals had been achieved at an aggregate level of 60%. As a result, on March 4, 2016, our board of directors awarded the following bonuses to our named officers:
|
Executive Officer |
|
Title |
|
2015 Bonus Amount |
|
|
|
Timothy B. Johnson |
|
President and Chief Executive Officer |
|
$ |
120,000 |
|
|
John L. Lubniewski |
|
Chief Business Officer |
|
$ |
70,440 |
|
|
Shaun D. McMeans |
|
Vice President of Finance & Administration and Chief Financial Officer |
|
$ |
57,600 |
|
Also on March 4, 2016, our board of directors determined to pay the 2015 bonus awards entirely in the form of stock grants under the 2014 plan. The stock grants were issuances of fully vested shares, in an amount equivalent in value, based on our stock price as of March 4, 2016, to the bonus each named executive was awarded as reflected above. Accordingly, Mr. Johnson, Mr. Lubniewski and Mr. McMeans earned 43,795, 25,708 and 21,021 shares, respectively, a portion of which shares was sold shortly thereafter to satisfy the tax withholding requirements for such shares. The named executive officers did not have any control over, or ability to elect, whether bonuses were paid in the form of cash or stock.
127
Our equity-based incentive awards are designed to align our interests with those of our employees and consultants, including our named executive officers. Our board of directors is responsible for approving equity grants. As of December 31, 2015, our named executive officers have been granted both stock option awards and restricted stock units. Vesting of the stock option and restricted stock unit awards is tied to continuous service with us and serves as an additional retention measure. Our executives generally are awarded an initial grant upon commencement of employment. Additional grants may occur periodically in order to specifically incentivize executives with respect to achieving certain corporate goals or to reward executives for exceptional performance.
Prior to the initial public offering, we granted all equity awards pursuant to the 2011 plan and the 2001 plan. All equity awards granted since our initial public offering have been granted pursuant to the 2014 plan, the terms of which are described below under “—Equity Benefit Plans.” All options are granted with a per share exercise price equal to no less than the fair market value of a share of our common stock on the date of the grant of such award.
Generally our stock option awards vest over a four-year period subject to the holder’s continuous service to us and may be granted with an early exercise feature. Such early exercise feature allows the holder to exercise and receive unvested shares of our stock, so that the holder may have a greater opportunity for gains on the shares to be taxed at long-term capital gains rates rather than ordinary income rates. From time to time as our board of directors considers appropriate, we may grant stock options or restricted stock units that vest upon achievement of performance goals.
On May 11, 2015, our board of directors granted Mr. Johnson, Mr. Lubniewski and Mr. McMeans restricted stock unit awards for 15,000, 2,500 and 5,000 shares of our common stock, respectively, which will vest in full on March 31, 2016, subject to continued service by each of these individuals though that date. See “—Outstanding Equity Awards at Fiscal Year-End.”
Agreements with Named Executed Officers
We have entered into letter agreements with each of our named executive officers. The letter agreements generally provide for at-will employment and set forth the named executive officer’s initial base salary, eligibility for employee benefits, in some cases, and severance benefits upon a qualifying termination of employment. In addition, each of our named executive officers has executed a form of our standard confidential information and invention assignment agreement. The key terms of the letter agreements with our named executive officers are described below. Any potential payments and benefits due upon a qualifying termination of employment or a change in control are further described below under “– Potential Payments and Benefits upon Termination or Change in Control.”
Employment Letter Agreement with Mr. Johnson. We entered into an amended and restated letter agreement with Mr. Johnson in December 2014 that replaced his previous letter agreement and became effective at completion of our IPO. The agreement sets forth certain agreed upon terms and conditions of employment. Under the amended and restated letter agreement, Mr. Johnson serves as our President and Chief Executive Officer. Mr. Johnson is entitled to an annual base salary of $400,000, is eligible to receive an annual target performance bonus of up to 50% of his base salary as determined by the board of directors, and certain severance benefits, the terms of which are described below under “—Potential Payments and Benefits upon Termination or Change of Control.” Mr. Johnson’s base salary and target bonus percentage are subject to modification from time to time in the discretion of our board of directors or any authorized committee thereof.
Employment Letter Agreement Mr. Lubniewski. We entered into an amended and restated letter agreement with Mr. Lubniewski in December 2014 that replaced his previous letter agreement and became effective at completion of our IPO. The agreement sets forth certain agreed upon terms and conditions of employment. Under the amended and restated letter agreement, Mr. Lubniewski serves as our Chief Business Officer. Mr. Lubniewski was initially entitled to receive an annual base salary of $293,500, is eligible to receive an annual target performance bonus of up to 40% of his base salary as determined by the board of directors, and certain severance benefits, the terms of which are described below under “—Potential Payments and Benefits upon Termination or Change of Control.” Mr. Lubniewski’s base salary and target bonus percentage are subject to modification from time to time in the discretion of our board of directors or any authorized committee thereof. In March 2016, our board of directors approved an increase to Mr. Lubniewski’s annual base salary to $300,837.50, effective March 1, 2016.
Employment Letter Agreement with Mr. McMeans. We entered into an amended and restated letter agreement with Mr. McMeans in December 2014 that replaced his previous letter agreement and became effective at completion of our IPO. The agreement sets forth certain agreed upon terms and conditions of employment. Under the amended and restated letter agreement, Mr. McMeans serves as our Vice President of Finance & Administration and Chief Financial Officer. Mr. McMeans is entitled to an annual base salary of $240,000, is eligible to receive an annual target performance bonus of up to 40% of his base salary as determined by the board of directors, and certain severance benefits, the terms of which are described below under “—Potential Payments and Benefits upon Termination or Change of Control.” Mr. McMeans’ base salary and target bonus percentage are subject
128
to modification from time to time in the discretion of our board of directors or any authorized committee thereof. In March 2016, our board of directors approved an increase to Mr. McMeans’ annual base salary to $246,000, effective March 1, 2016.
Potential Payments and Benefits upon Termination or Change of Control
Under the terms of our named executive officers’ amended and restated letter agreements upon the executive’s termination without “cause,” or resignation for “good reason,” each as defined below, including any such termination that occurs in connection with a change of control, each of our named executive officers is eligible to receive continued base salary payments and COBRA premium payments for 12 months for Mr. Johnson and nine months for Mr. Lubniewski and Mr. McMeans.
For purposes of the amended and restated letter agreements, “cause” generally means the occurrence of any of the following events, conditions or actions with respect to the executive: (1) conviction of any felony or crime involving fraud or dishonesty; (2) participation in any material fraud, material act of dishonesty or other material act of misconduct against us; (3) willful and habitual neglect of the executive’s duties after written notice and opportunity to cure; (4) material violation of any fiduciary duty or duty of loyalty owed to us; (5) breach of any material term of any material contract with us which has a material adverse effect on us; (6) knowing violation of any material company policy which has a material adverse effect on us; or (7) knowing violation of state or federal law in connection with the performance of the executive’s job which has a material adverse effect on us.
For purposes of each of the named executive officer’s amended and restated letter agreements, “good reason” generally means the following events, conditions or actions taken by us with respect to the executive without cause and without the executive’s express written consent: (1) a material reduction in base salary; (2) a material reduction in the executive’s authority, duties or responsibilities; (3) a material reduction in the authority, duties or responsibilities of the supervisor to whom the executive is required to report; or (4) a relocation of the executive’s principal place of employment to a place that increases the executive’s one-way commute by more than 50 miles.
Each of our named executive officers holds stock options and restricted stock units under our equity incentive plans that were granted subject to our form of stock option and restricted stock unit agreements. A description of the termination and change of control provisions in such equity incentive plans and stock options and restricted stock units granted thereunder is provided below under “– Equity Benefit Plans” and the specific vesting terms of each named executive officer’s stock options and restricted stock units are described below under “– Outstanding Equity Awards at Fiscal Year-End.”
129
Outstanding Equity Awards at Fiscal Year-End
The following table presents information concerning equity awards held by our named executive officers as of December 31, 2015, granted under the 2001 plan, the 2011 plan and the 2014 plan.
|
|
|
|
|
|
Option Awards |
|
Stock Awards |
|
|||||||||||||||||||||||||||||
|
Name |
|
|
Grant Date/Vesting Commencement Date |
|
Number of Securities Underlying Unexercised Options (#) Exercisable |
|
|
Number of Securities Underlying Unexercised Options (#) Unexercisable |
|
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
|
|
Option Exercise Price ($) |
|
|
Option Expiration Date |
|
Number of Shares or Units of Stock That Have Not Vested (#) |
|
|
Market Value of Shares or Units of Stock That Have Not Vested ($) |
|
|
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
|
|
Equity incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
|
||||||||
|
Timothy B. Johnson |
|
(1) |
1/16/2008 |
|
|
25,048 |
|
|
|
— |
|
|
|
— |
|
|
|
6.44 |
|
|
1/16/2018 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
10/23/2008 |
|
|
14,407 |
|
|
|
— |
|
|
|
— |
|
|
|
6.44 |
|
|
10/23/2018 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
3/01/2009 |
|
|
4,655 |
|
|
|
— |
|
|
|
— |
|
|
|
4.30 |
|
|
3/01/2019 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
3/01/2010 |
|
|
6,983 |
|
|
|
— |
|
|
|
— |
|
|
|
4.30 |
|
|
3/01/2020 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
4/13/2010 |
|
|
675 |
|
|
|
— |
|
|
|
— |
|
|
|
4.30 |
|
|
4/13/2020 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
10/21/2010 |
|
|
581 |
|
|
|
— |
|
|
|
— |
|
|
|
4.30 |
|
|
10/21/2020 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
1/20/2011 |
|
|
675 |
|
|
|
— |
|
|
|
— |
|
|
|
4.30 |
|
|
1/20/2021 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
4/26/2011 |
|
|
29,797 |
|
|
|
— |
|
|
|
— |
|
|
|
2.15 |
|
|
4/26/2021 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
2/01/2013 |
|
|
13,740 |
|
|
|
4,588 |
|
|
|
— |
|
|
|
2.15 |
|
|
2/01/2023 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
8/06/2013 |
|
|
5,810 |
|
|
|
3,501 |
|
|
|
— |
|
|
|
2.15 |
|
|
8/06/2023 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
3/20/2014 |
|
|
21,632 |
|
|
|
21,635 |
|
|
|
— |
|
|
|
2.15 |
|
|
3/19/2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
12/29/2014 |
|
|
2,905 |
|
|
|
6,406 |
|
|
|
— |
|
|
|
12.89 |
|
|
12/28/2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(3) |
5/11/15 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
n/a |
|
|
15,000 |
|
|
$ |
65,400 |
|
|
|
— |
|
|
|
— |
|
|
John L. Lubniewski |
|
(1) |
4/26/2011 |
|
|
13,036 |
|
|
|
— |
|
|
|
— |
|
|
|
2.15 |
|
|
4/26/2021 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(1) |
3/08/2012 |
|
|
2,793 |
|
|
|
— |
|
|
|
— |
|
|
|
2.15 |
|
|
3/08/2022 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
2/01/2013 |
|
|
2,616 |
|
|
|
887 |
|
|
|
— |
|
|
|
2.15 |
|
|
2/01/2023 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
8/06/2013 |
|
|
8,140 |
|
|
|
4,896 |
|
|
|
— |
|
|
|
2.15 |
|
|
8/06/2023 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
3/20/2014 |
|
|
14,904 |
|
|
|
14,914 |
|
|
|
— |
|
|
|
2.15 |
|
|
3/20/2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
12/29/2014 |
|
|
1,160 |
|
|
|
2,564 |
|
|
|
— |
|
|
|
12.89 |
|
|
12/29/2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(3) |
5/11/15 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
n/a |
|
2,500 |
|
|
$ |
10,900 |
|
|
|
— |
|
|
|
— |
|
|
|
Shaun D. McMeans |
|
(1) |
2/13/2012 |
|
|
8,380 |
|
|
|
— |
|
|
|
— |
|
|
|
2.15 |
|
|
2/13/2022 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
2/01/2013 |
|
|
1,380 |
|
|
|
474 |
|
|
|
— |
|
|
|
2.15 |
|
|
2/01/2023 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
8/06/2013 |
|
|
8,140 |
|
|
|
4,896 |
|
|
|
— |
|
|
|
2.15 |
|
|
8/06/2023 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
3/20/2014 |
|
|
15,152 |
|
|
|
15,156 |
|
|
|
— |
|
|
|
2.15 |
|
|
3/20/2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(2) |
12/29/2014 |
|
|
725 |
|
|
|
1,602 |
|
|
|
— |
|
|
|
12.89 |
|
|
12/29/2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(3) |
5/11/15 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
n/a |
|
5,000 |
|
|
$ |
21,800 |
|
|
|
— |
|
|
|
— |
|
|
|
(1) |
Fully vested. |
|
(2) |
Options vest over four years as follows: 1/16 of the outstanding shares vest at the end of each calendar quarter over a period of approximately four years, subject to the individual’s continued service with us through each vesting date. |
|
(3) |
Restricted stock units vest 100% on March 31, 2016, subject to the individual’s continued service with us through that date. |
Equity Benefit Plans
2014 Equity Incentive Plan
Our board of directors adopted the 2014 plan in December 2014 and our stockholders approved the 2014 plan in April 2015. The 2014 plan became effective on May 5, 2015 in connection with our initial public offering.
130
Stock Awards. The 2014 plan provides for the grant of incentive stock options, or ISOs, nonstatutory stock options, or NSOs, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance-based stock awards, and other forms of equity compensation, or collectively, stock awards, all of which may be granted to employees, including officers, non-employee directors and consultants of us and our affiliates. Additionally, the 2014 plan provides for the grant of performance cash awards. ISOs may be granted only to employees. All other awards may be granted to employees, including officers, and to non-employee directors and consultants.
Share Reserve. Initially, the aggregate number of shares of our common stock that may be issued pursuant to stock awards under the 2014 plan was the sum of (1) 925,616 shares, plus (2) the number of shares (not to exceed 608,819 shares) (i) reserved for issuance under our 2011 plan at the time the 2014 plan became effective, and (ii) any shares subject to outstanding stock options or other stock awards that were granted under our 2011 plan or 2001 plan that, on or after the effective date of the 2014 plan, are forfeited, terminate, expire or are otherwise not issued. Additionally, the number of shares of our common stock reserved for issuance under the 2014 plan automatically increases on January 1 of each year, beginning on January 1, 2016 and continuing through and including January 1, 2024, by 4% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares determined by our board of directors. The maximum number of shares of our common stock that may be issued upon the exercise of ISOs under the 2014 plan is 1,800,000 shares.
No person may be granted stock awards covering more than 200,000 shares of our common stock under the 2014 plan during any calendar year pursuant to stock options, stock appreciation rights and other stock awards whose value is determined by reference to an increase over an exercise or strike price of at least 100% of the fair market value on the date the stock award is granted. Additionally, no person may be granted in a calendar year a performance stock award covering more than 200,000 shares of our common stock or a performance cash award having a maximum value in excess of $2,000,000. Such limitations are designed to help assure that any deductions to which we would otherwise be entitled with respect to such awards will not be subject to the $1,000,000 limitation on the income tax deductibility of compensation paid to any covered executive officer imposed by Section 162(m) of the Code.
If a stock award granted under the 2014 plan expires or otherwise terminates without being exercised in full, or is settled in cash, the shares of our common stock not acquired pursuant to the stock award again will become available for subsequent issuance under the 2014 plan. In addition, the following types of shares of our common stock under the 2014 plan may become available for the grant of new stock awards under the 2014 plan: (1) shares that are forfeited to or repurchased by us prior to becoming fully vested; (2) shares withheld to satisfy income or employment withholding taxes; or (3) shares used to pay the exercise or purchase price of a stock award. Shares issued under the 2014 plan may be previously unissued shares or reacquired shares bought by us on the open market. As of December 31, 2015, option awards covering an aggregate of 193,490 shares of our common stock have been granted under the 2014 plan and were outstanding, and no shares of our common stock had been issued under the 2014 plan.
Administration. Our board of directors, or a duly authorized committee thereof, has the authority to administer the 2014 plan. Our board of directors may also delegate to one or more of our officers the authority to (1) designate employees (other than other officers) to be recipients of certain stock awards, and (2) determine the number of shares of common stock to be subject to such stock awards. Subject to the terms of the 2014 plan, our board of directors or the authorized committee, referred to herein as the plan administrator, determines recipients, dates of grant, the numbers and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability, vesting schedule and change of control provision applicable to a stock award, if any. Subject to the limitations set forth below, the plan administrator will also determine the exercise price, strike price or purchase price of awards granted and the types of consideration to be paid for the award.
The plan administrator has the authority to modify outstanding awards under the 2014 plan. Subject to the terms of the 2014 plan, the plan administrator has the authority to reduce the exercise, purchase or strike price of any outstanding stock award, cancel any outstanding stock award in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Stock Options. ISOs and NSOs are granted pursuant to stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for a stock option, within the terms and conditions of the 2014 plan, provided that the exercise price of a stock option generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2014 plan vest at the rate specified by the plan administrator.
The plan administrator determines the term of stock options granted under the 2014 plan, up to a maximum of ten years. Unless the terms of an optionholder’s stock option agreement provide otherwise, if an optionholder’s service relationship with us, or any of our affiliates, ceases for any reason other than disability, death or cause, the optionholder may generally exercise any vested options for a period of three months following the cessation of service. The option term may be extended in the event that exercise of the option following such a termination of service is prohibited by applicable securities laws or our insider trading policy. If an
131
optionholder’s service relationship with us or any of our affiliates ceases due to disability or death, or an optionholder dies within a certain period following cessation of service, the optionholder or a beneficiary may generally exercise any vested options for a period of 12 months in the event of disability and 18 months in the event of death. In the event of a termination for cause, options generally terminate immediately upon the termination of the individual for cause. In no event may an option be exercised beyond the expiration of its term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the plan administrator and may include (1) cash, check, bank draft or money order, (2) a broker-assisted cashless exercise, (3) the tender of shares of our common stock previously owned by the optionholder, (4) a net exercise of the option if it is an NSO, and (5) other legal consideration approved by the plan administrator.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An optionholder may designate a beneficiary, however, who may exercise the option following the optionholder’s death.
Tax Limitations on Incentive Stock Options. The aggregate fair market value, determined at the time of grant, of our common stock with respect to ISOs that are exercisable for the first time by an optionholder during any calendar year under all of our stock plans may not exceed $100,000. Options or portions thereof that exceed such limit will generally be treated as NSOs. No ISO may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates unless (1) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and (2) the term of the ISO does not exceed five years from the date of grant.
Restricted Stock Awards. Restricted stock awards are granted pursuant to restricted stock award agreements adopted by the plan administrator. Restricted stock awards may be granted in consideration for (1) cash, check, bank draft or money order, (2) services rendered to us or our affiliates, or (3) any other form of legal consideration. Common stock acquired under a restricted stock award may, but need not, be subject to a share repurchase option in our favor in accordance with a vesting schedule to be determined by the plan administrator. A restricted stock award may be transferred only upon such terms and conditions as set by the plan administrator. Except as otherwise provided in the applicable award agreement, restricted stock awards that have not vested may be forfeited or repurchased by us upon the participant’s cessation of continuous service for any reason.
Restricted Stock Unit Awards. Restricted stock unit awards are granted pursuant to restricted stock unit award agreements adopted by the plan administrator. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award may be settled by cash, delivery of stock, a combination of cash and stock as deemed appropriate by the plan administrator, or in any other form of consideration set forth in the restricted stock unit award agreement. Additionally, dividend equivalents may be credited in respect of shares covered by a restricted stock unit award. Except as otherwise provided in the applicable award agreement, restricted stock units that have not vested will be forfeited upon the participant’s cessation of continuous service for any reason.
Stock Appreciation Rights. Stock appreciation rights are granted pursuant to stock appreciation grant agreements adopted by the plan administrator. The plan administrator determines the strike price for a stock appreciation right, which generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Upon the exercise of a stock appreciation right, we will pay the participant an amount equal to the product of (1) the excess of the per share fair market value of our common stock on the date of exercise over the strike price, multiplied by (2) the number of shares of common stock with respect to which the stock appreciation right is exercised. A stock appreciation right granted under the 2014 plan vests at the rate specified in the stock appreciation right agreement as determined by the plan administrator.
The plan administrator determines the term of stock appreciation rights granted under the 2014 plan, up to a maximum of ten years. Unless the terms of a participant’s stock appreciation right agreement provides otherwise, if a participant’s service relationship with us or any of our affiliates ceases for any reason other than cause, disability or death, the participant may generally exercise any vested stock appreciation right for a period of three months following the cessation of service. The stock appreciation right term may be further extended in the event that exercise of the stock appreciation right following such a termination of service is prohibited by applicable securities laws. If a participant’s service relationship with us, or any of our affiliates, ceases due to disability or death, or a participant dies within a certain period following cessation of service, the participant or a beneficiary may generally exercise any vested stock appreciation right for a period of 12 months in the event of disability and 18 months in the event of death. In the event of a termination for cause, stock appreciation rights generally terminate immediately upon the occurrence of the event giving rise to the termination of the individual for cause. In no event may a stock appreciation right be exercised beyond the expiration of its term.
Performance Awards. The 2014 plan permits the grant of performance-based stock and cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid
132
to a covered executive officer imposed by Section 162(m) of the Code. To help assure that the compensation attributable to performance-based awards will so qualify, our compensation committee can structure such awards so that stock or cash will be issued or paid pursuant to such award only after the achievement of certain pre-established performance goals during a designated performance period.
The performance goals that may be selected include one or more of the following: (1) earnings (including earnings per share and net earnings); (2) earnings before interest, taxes and depreciation; (3) earnings before interest, taxes, depreciation and amortization; (4) earnings before interest, taxes, depreciation, amortization and legal settlements; (5) earnings before interest, taxes, depreciation, amortization, legal settlements and other income (expense); (6) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense) and stock-based compensation; (7) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense), stock-based compensation and changes in deferred revenue; (8) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense), stock-based compensation, other non-cash expenses and changes in deferred revenue; (9) total stockholder return; (10) return on equity or average stockholder’s equity; (11) return on assets, investment, or capital employed; (12) stock price; (13) margin (including gross margin); (14) income (before or after taxes); (15) operating income; (16) operating income after taxes; (17) pre-tax profit; (18) operating cash flow; (19) sales or revenue targets; (20) increases in revenue or product revenue; (21) expenses and cost reduction goals; (22) improvement in or attainment of working capital levels; (23) economic value added (or an equivalent metric); (24) market share; (25) cash flow; (26) cash flow per share; (27) cash balance; (28) cash burn; (29) cash collections; (30) share price performance; (31) debt reduction; (32) implementation or completion of projects or processes (including, without limitation, clinical trial initiation, clinical trial enrollment and dates, clinical trial results, regulatory filing submissions, regulatory filing acceptances, regulatory or advisory committee interactions, regulatory approvals, and product supply); (33) stockholders’ equity; (34) capital expenditures; (35) debt levels; (36) operating profit or net operating profit; (37) workforce diversity; (38) growth of net income or operating income; (39) billings; (40) bookings; (41) employee retention; (42) initiation of studies by specific dates; (43) budget management; (44) submission to, or approval by, a regulatory body (including, but not limited to the U.S. Food and Drug Administration) of an applicable filing or a product; (45) regulatory milestones; (46) progress of internal research or development programs; (47) acquisition of new customers; (48) customer retention and/or repeat order rate; (49) improvements in sample and test processing times; (50) progress of partnered programs; (51) partner satisfaction; (52) timely completion of clinical trials; (53) submission of 510(k)s or pre-market approvals and other regulatory achievements; (54) milestones related to samples received and/or tests or panels run; (55) expansion of sales in additional geographies or markets; (56) research progress, including the development of programs; (57) strategic partnerships or transactions (including in-licensing and out-licensing of intellectual property); and (58) to the extent that an award is not intended to comply with Section 162(m) of the Code, other measures of performance selected by our board of directors.
The performance goals may be based on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless specified otherwise (i) in the award agreement at the time the award is granted or (ii) in such other document setting forth the performance goals at the time the goals are established, we will appropriately make adjustments in the method of calculating the attainment of performance goals as follows: (1) to exclude restructuring and/or other nonrecurring charges; (2) to exclude exchange rate effects; (3) to exclude the effects of changes to generally accepted accounting principles; (4) to exclude the effects of any statutory adjustments to corporate tax rates; (5) to exclude the effects of any “extraordinary items” as determined under generally accepted accounting principles; (6) to exclude the dilutive effects of acquisitions or joint ventures; (7) to assume that any business divested by us achieved performance objectives at targeted levels during the balance of a performance period following such divestiture; (8) to exclude the effect of any change in the outstanding shares of our common stock by reason of any stock dividend or split, stock repurchase, reorganization, recapitalization, merger, consolidation, spin-off, combination or exchange of shares or other similar corporate change, or any distributions to common stockholders other than regular cash dividends; (9) to exclude the effects of stock-based compensation and the award of bonuses under our bonus plans; (10) to exclude costs incurred in connection with potential acquisitions or divestitures that are required to be expensed under generally accepted accounting principles; (11) to exclude the goodwill and intangible asset impairment charges that are required to be recorded under generally accepted accounting principles; (12) to exclude the effect of any other unusual, non-recurring gain or loss or other extraordinary item; and (13) to exclude the effects of the timing of acceptance for review and/or approval of submissions to the FDA or any other regulatory body. In addition, we retain the discretion to reduce or eliminate the compensation or economic benefit due upon attainment of the performance goals and to define the manner of calculating the performance criteria we select to use for such performance period. The performance goals may differ from participant to participant and from award to award.
Other Stock Awards. The plan administrator may grant other awards based in whole or in part by reference to our common stock. The plan administrator will set the number of shares under the stock award and all other terms and conditions of such awards.
Changes to Capital Structure. In the event that there is a specified type of change in our capital structure, such as a stock split or recapitalization, appropriate adjustments will be made to (1) the class and maximum number of shares reserved for issuance under the 2014 plan, (2) the class and maximum number of shares by which the share reserve may increase automatically each year, (3) the class
133
and maximum number of shares that may be issued upon the exercise of ISOs, (4) the class and maximum number of shares subject to stock awards that can be granted in a calendar year (as established under the 2014 plan pursuant to Section 162(m) of the Code) and (5) the class and number of shares and exercise price, strike price, or purchase price, if applicable, of all outstanding stock awards.
Corporate Transactions. In the event of certain specified significant corporate transactions, the plan administrator has the discretion to take any of the following actions with respect to stock awards:
|
|
· |
arrange for the assumption, continuation or substitution of a stock award by a surviving or acquiring entity or parent company; |
|
|
· |
arrange for the assignment of any reacquisition or repurchase rights held by us to the surviving or acquiring entity or parent company; |
|
|
· |
accelerate the vesting of the stock award and provide for its termination at or prior to the effective time of the corporate transaction; |
|
|
· |
arrange for the lapse of any reacquisition or repurchase right held by us; |
|
|
· |
cancel or arrange for the cancellation of the stock award in exchange for such cash consideration, if any, as our board of directors may deem appropriate; or |
|
|
· |
make a payment equal to the excess of (1) the value of the property the participant would have received upon exercise of the stock award over (2) the exercise price otherwise payable in connection with the stock award. |
Our plan administrator is not obligated to treat all stock awards, even those that are of the same type, in the same manner.
Under the 2014 plan, a corporate transaction is generally the consummation of (1) a sale or other disposition of all or substantially all of our assets, (2) a sale or other disposition of at least 90% of our outstanding securities, (3) a merger, consolidation or similar transaction following which we are not the surviving corporation, or (4) a merger, consolidation or similar transaction following which we are the surviving corporation but the shares of our common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction.
Change of Control. The plan administrator may provide, in an individual award agreement or in any other written agreement between a participant and us that the stock award will be subject to additional acceleration of vesting and exercisability in the event of a change of control. For example, certain of our employees may receive an award agreement that provides for vesting acceleration upon the individual’s termination without cause or resignation for good reason (including a material reduction in the individual’s base salary, duties, responsibilities or authority, or a material relocation of the individual’s principal place of employment with us) in connection with a change of control. Under the 2014 plan, a change of control is generally (1) the acquisition by a person or entity of more than 50% of our combined voting power other than by merger, consolidation or similar transaction; (2) a consummated merger, consolidation or similar transaction immediately after which our stockholders cease to own more than 50% of the combined voting power of the surviving entity; (3) a consummated sale, lease or exclusive license or other disposition of all or substantially of our assets; or (4) our stockholders approve a plan of our complete dissolution or liquidation or our complete dissolution or liquidation otherwise occurs.
All options granted under the 2014 plan to our named executive officers provide that vesting and exercisability of such options will be accelerated in full following a change in control if, immediately prior to or within 12 months after the effective time of such change in control, the optionholder’s continuous service terminates due to an involuntary termination without cause or due to a voluntary termination with good reason. Several terms are specifically defined in the 2014 plan for purposes of this “double-trigger” provision; in particular, (i) “good reason” is generally defined as (1) a material reduction in the optionholders’s annual base salary, except pursuant to a salary reduction program affecting substantially all of our employees that does not disproportionately affect the optionholder; (2) a material reduction in the optionholder’s authority, duties or responsibilities; (3) any failure by us to continue any material benefit plan or program in which the optionholder was participating immediately prior to the change in control, or any action by us that would adversely affect the optionholder’s participation in or reduce his/her benefits under such benefit plan or program, or deprive him/her of any fringe benefit enjoyed immediately prior to the change in control, unless, taken as a whole, we provide for optionholder participation in comparable benefit plans or programs; (4) a relocation of the optionholder’s principal place of employment more than 50 miles; or (5) a material breach by us of any provision of the 2014 plan or an option agreement under the 2014 plan or any other material agreement between the optionholder and us concerning the terms and conditions of employment or service with us; and (ii) “cause” is generally defined as the occurrence of any of the following events: (A) the optionholder’s commission of any felony or any crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof; (B) the optionholder’s attempted commission of, or participation in, a fraud or act of dishonesty against us; (C) the optionholder’s intentional, material violation of any contract or agreement between the optionholder and us or of any statutory duty
134
owed to us; (D) the optionholder’s unauthorized use or disclosure of our confidential information or trade secrets; or (E) the optionholder’s gross misconduct.
Amendment and Termination. Our board of directors has the authority to amend, suspend, or terminate the 2014 plan, provided that such action does not materially impair the existing rights of any participant without such participant’s written consent. No ISOs may be granted after the tenth anniversary of the date our board of directors adopted the 2014 plan.
2011 Equity Incentive Plan
General. Our board of directors and our stockholders approved our 2011 plan in March 2011. The 2011 plan was subsequently amended by our board of directors and our stockholders, most recently in February 2014. The 2011 plan is the successor to and continuation of our 2001 plan. As of December 31, 2015, option awards under the 2011 plan covering an aggregate of 409,139 shares of our common stock were outstanding. No additional awards will be granted under the 2011 plan and all outstanding awards granted under the 2011 plan that are repurchased, forfeited, expire or are cancelled will become available for grant under the 2014 plan in accordance with its terms. Our board of directors, or a duly authorized committee thereof, has the authority to administer the 2011 plan. Our board of directors may also delegate certain authority to one or more of our officers The plan administrator has the authority to modify outstanding awards under our 2011 plan, including the authority to reduce the exercise, purchase or strike price of any outstanding stock award, cancel any outstanding stock award in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Stock Options. ISOs and NSOs are granted pursuant to stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for a stock option, within the terms and conditions of the 2011 plan, provided that the exercise price of a stock option generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2011 plan vest at the rate specified by the plan administrator.
The plan administrator determines the term of stock options granted under the 2011 plan, up to a maximum of 10 years. Unless the terms of an optionholder’s stock option agreement provide otherwise, if an optionholder’s service relationship with us, or any of our affiliates, ceases for any reason other than disability, death or cause, the optionholder may generally exercise any vested options for a period of three months following the cessation of service. The option term may be extended in the event that exercise of the option following such a termination of service is prohibited by applicable securities laws or our insider trading policy. If an optionholder’s service relationship with us or any of our affiliates ceases due to disability or death, or an optionholder dies within a certain period following cessation of service, the optionholder or a beneficiary may generally exercise any vested options for a period of 12 months in the event of disability and 18 months in the event of death. In the event of a termination for cause, options generally terminate immediately upon the termination of the individual for cause. In no event may an option be exercised beyond the expiration of its term.
Corporate Transactions. Unless otherwise provided in a stock award agreement or other written agreement between us and a participant, in the event of certain specified significant corporate transactions, the plan administrator has the discretion to take any of the following actions with respect to stock awards:
|
|
· |
arrange for the assumption, continuation or substitution of a stock award by a surviving or acquiring entity or parent company; |
|
|
· |
arrange for the assignment of any reacquisition or repurchase rights held by us to the surviving or acquiring entity or parent company; |
|
|
· |
accelerate the vesting, in whole or in part, of the stock award and provide for its termination prior to the effective time of the corporate transaction; |
|
|
· |
arrange for the lapse of any reacquisition or repurchase right held by us; |
|
|
· |
cancel or arrange for the cancellation of the stock award in exchange for such cash consideration, if any, as our board of directors may deem appropriate; or |
|
|
· |
make a payment equal to the excess of (a) the value of the property the participant would have received upon exercise of the stock award over (b) the exercise price otherwise payable in connection with the stock award. |
Our plan administrator is not obligated to treat all stock awards, even those that are of the same type, in the same manner.
135
Under the 2011 plan, a corporate transaction is generally defined as the consummation of (1) a sale or other disposition of all or substantially all of our assets, (2) a sale or other disposition of at least 90% of our outstanding securities, (3) a merger, consolidation or similar transaction following which we are not the surviving corporation, or (4) a merger, consolidation or similar transaction following which we are the surviving corporation but the shares of our common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction.
Change of Control. The plan administrator may provide, in an individual award agreement or in any other written agreement between a participant and us that the stock award will be subject to additional acceleration of vesting and exercisability in the event of a change of control. Under the 2011 plan, a change of control is generally defined as (1) the acquisition by a person or entity of more than 50% of our combined voting power other than by merger, consolidation or similar transaction, (2) a consummated merger, consolidation or similar transaction immediately after which our stockholders cease to own more than 50% of the combined voting power of the surviving entity, (3) approval by the stockholders or our board of directors of a plan of complete dissolution or liquidation of us or our complete dissolution or liquidation occurs or (4) a consummated sale, lease or exclusive license or other disposition of all or substantially of our assets.
Certain options granted under the 2011 plan, including the options held by our named executive officers, provide that if immediately prior to a change of control the participant’s service with the Company has not terminated, the option will accelerate vesting with respect to 25% of the then-unvested portion of the option; if the option continues, the remaining 75% of the unvested option will continue to vest on the option’s original schedule prior to the change of control and will accelerate vesting in full in the event that the participant’s continuous service is terminated without cause or by the participant for good reason within the 12 months following the change of control. “Good reason” for purposes of this “double-trigger” provision is generally defined as (1) an assignment of duties or responsibilities to the participant that results in a material diminution of the participant’s function; (2) a material reduction in the participant’s annual base salary; (3) failure to continue the participant’s benefit plans or programs, any action that would adversely affect the participant’s participation in any benefit plan, reduce the participant’s benefits under any benefit plan or deprive the participant of any fringe benefit; or (4) a relocation of the participant’s business office more than 50 miles.
2001 Stock Option Plan
Our board of directors and our stockholders approved our 2001 plan, which became effective in February 2001. The 2001 plan terminated and no further awards were granted under the 2001 plan upon the effective date of the 2011 plan. As of December 31, 2015, there were outstanding stock options under our 2001 plan covering a total of 161,516 shares of our common stock.
2014 Employee Stock Purchase Plan
General. Our board of directors adopted the ESPP in December 2014 and our stockholders approved the ESPP in April 2015. The ESPP became effective on May 5, 2015 in connection with our initial public offering. The purpose of the ESPP is to retain the services of new employees and secure the services of new and existing employees while providing incentives for such individuals to exert maximum efforts toward our success and that of our affiliates. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code. As of the date hereof, no shares of our common stock have been purchased under the ESPP. Our board of directors has delegated its authority to administer the ESPP to our compensation committee.
The ESPP initially authorized the issuance of 110,820 shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. The number of shares of our common stock reserved for issuance automatically increases on January 1 of each calendar year, from January 1, 2016 through January 1, 2024 by the least of (1) 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, (2) 195,000 shares, or (3) a number determined by our board of directors that is less than (1) and (2).
Offerings and Purchases. The ESPP is implemented through a series of offerings of purchase rights to eligible employees. Under the ESPP, we may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. Generally, all regular employees, including executive officers, subject to certain restrictions, employed by us or by any of our designated affiliates, may participate in the ESPP and may contribute, normally through payroll deductions, up to 15% of their earnings for the purchase of our common stock under the ESPP. Unless otherwise determined by our board of directors, common stock will be purchased for accounts of employees participating in the ESPP at a price per share equal to the lower of (1) 85% of the fair market value of a share of our common stock on the first date of an offering or (2) 85% of the fair market value of a share of our common stock on the date of purchase.
Changes to Capital Structure. In the event that there occurs a change in our capital structure through such actions as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash,
136
large nonrecurring cash dividend, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or similar transaction, the board of directors will make appropriate adjustments to (1) the number of shares reserved under the ESPP, (2) the maximum number of shares by which the share reserve may increase automatically each year and (3) the number of shares and purchase price of all outstanding purchase rights.
Corporate Transactions. In the event of certain significant corporate transactions, including the consummation of: (1) a sale of all our assets, (2) the sale or disposition of 90% of our outstanding securities, (3) a merger or consolidation where we do not survive the transaction and (4) a merger or consolidation where we do survive the transaction but the shares of our common stock outstanding immediately prior to such transaction are converted or exchanged into other property by virtue of the transaction, any then-outstanding rights to purchase our stock under the ESPP may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase rights, then the participants’ accumulated payroll contributions will be used to purchase shares of our common stock within ten business days prior to such corporate transaction, and such purchase rights will terminate immediately.
Plan Amendments, Termination. Our board of directors has the authority to amend or terminate our ESPP, provided that except in certain circumstances any such amendment or termination may not materially impair any outstanding purchase rights without the holder’s consent. We will obtain stockholder approval of any amendment to our ESPP as required by applicable law or listing requirements.
401(k) Plan
We maintain a tax-qualified retirement plan that provides eligible employees with an opportunity to save for retirement on a tax advantaged basis. All participants’ interests in their deferrals are 100% vested when contributed. In 2015, we made no matching contributions into the 401(k) plan. Pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. The 401(k) plan is intended to qualify under Sections 401(a) and 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan, and all contributions are deductible by us when made.
Director Compensation
The following table sets forth in summary form information concerning the compensation that we paid or awarded during the year ended December 31, 2015 to each of our non-employee directors:
|
Name |
|
Fees Earned ($) |
|
|
Option Awards ($) (5) |
|
|
Total ($) |
|
|||
|
Peter Bisgaard (6) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Harry A. George |
|
|
22,885 |
|
|
|
— |
|
|
|
22,885 |
|
|
Simeon J. George, M.D. (1) (6) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Donald W. Grimm (2) |
|
|
20,692 |
|
|
|
— |
|
|
|
20,692 |
|
|
Mary (Molly) Hoult |
|
|
22,885 |
|
|
|
— |
|
|
|
22,885 |
|
|
James T. LaFrance |
|
|
2,973 |
|
|
|
13,394 |
|
|
|
16,367 |
|
|
Lee McCracken |
|
|
7,233 |
|
|
|
14,169 |
|
|
|
21,402 |
|
|
Larry Senour (3) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Lewis J. Shuster |
|
|
38,077 |
|
|
|
— |
|
|
|
38,077 |
|
|
James R. Weersing (4) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
(1) |
Dr. George resigned from our board of directors on October 27, 2015. |
|
(2) |
Mr. Grimm resigned from our board of directors on November 23, 2015. |
|
(3) |
Mr. Senour resigned from our board of directors on May 5, 2015. |
|
(4) |
Mr. Weersing’s service on our board of directors ended on April 24, 2015. |
|
(5) |
As of December 31, 2015, the aggregate number of shares outstanding under all options to purchase our common stock held by our non-employee directors were: Mr. LaFrance: 5,000; Mr. McCracken: 5,000; Mr. Shuster: 12,569 and Mr. Weersing: 7,840. None of the other directors listed in the table above held any options to purchase our common stock as of December 31, 2015. The dollar amounts in this column represent the aggregate grant date fair value of stock option awards granted in 2015. These amounts have been computed in accordance with FASB ASC Topic 718, using the Black-Scholes option pricing model. Pursuant to SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. For a discussion of valuation assumptions, see Note 13 “Stock-based Compensation” to our financial statements included elsewhere in this Annual Report. |
137
|
(6) |
Mr. Bisgaard and Dr. George waived compensation under our non-employee director compensation policy implemented upon completion of our IPO. |
We did not pay cash or equity compensation to any of our non-employee directors in 2015 prior to our IPO for service on our board of directors, except that we paid Mr. Shuster annual cash compensation of $25,000 for his service as the Chairman of the audit committee.
We have reimbursed and will continue to reimburse all of our non-employee directors for their travel, lodging and other reasonable expenses incurred in attending meetings of our board of directors and committees of our board of directors, and will pay for the travel, lodging and other reasonable expenses incurred by our employee directors to attend meetings of our board of directors and, as applicable, committees of our board of directors.
We implemented a compensation policy for our non-employee directors upon completion of our IPO which provides that each such non-employee director will receive the following compensation for service on our board of directors:
|
|
· |
an annual cash retainer of $35,000; |
|
|
· |
an additional annual cash retainer of $5,000 for service as chairman of our board of directors; |
|
|
· |
an additional annual cash retainer of $10,000, $5,000 and $2,500 for service as chairman of our audit committee, compensation committee and nominating and corporate governance committee, respectively; |
|
|
· |
an automatic annual option grant to purchase 2,500 shares of our common stock for each non-employee director serving on the board of directors on the date of each annual stockholder meeting, in each case vesting monthly in equal installments over a one-year period such that the stock option is fully vested on the first anniversary of the date of grant; and |
|
|
· |
upon first joining our board of directors an automatic initial option grant to purchase 5,000 shares of our common stock on the date of grant. One-third of the shares will vest twelve months after the date of grant and the remaining shares will vest monthly in equal installments over a two-year period thereafter such that the stock option is fully vested on the third anniversary of the date of grant. A director who, in the one year prior to his or her initial election to serve on the board of directors as a non-employee director, served as an employee of the company will not be eligible for an initial grant. |
Each of the option grants described above will vest and become exercisable subject to the director’s continuous service with us through each applicable vesting date, provided that each option will vest in full upon a change of control, as defined under the 2014 plan. The options will be granted under the 2014 plan, the terms of which are described in more detail above under “– Equity Benefit Plans – 2014 Equity Incentive Plan.”
138
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides certain information with respect to all of the Company’s equity compensation plans in effect as of December 31, 2015.
Equity Compensation Plan Information
|
Plan Category |
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) |
|
|
Weighted-average exercise price of outstanding options, warrants and rights (b) |
|
|
Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a)) (c ) |
|
|||
|
Equity compensation plans approved by security holders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
2001 Stock Option Plan |
|
|
161,516 |
|
|
$ |
4.24 |
|
|
|
— |
|
|
2011 Equity Incentive Plan |
|
|
409,139 |
|
|
|
3.49 |
|
|
|
— |
|
|
2014 Equity Incentive Plan |
|
|
193,490 |
|
|
|
6.73 |
|
|
|
756,332 |
|
|
2014 Employee Stock Purchase Plan |
|
|
— |
|
|
N/A |
|
|
|
110,820 |
|
|
|
Equity compensation plans not approved by security holders |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
|
764,145 |
|
|
|
|
|
|
|
867,152 |
|
Principal Stockholders
The following table sets forth certain information regarding the ownership of the Company’s common stock as of February 29, 2016 by: (i) each director; (ii) each of our executive officers named in the Summary Compensation Table above; (iii) all executive officers and directors of the Company as a group; and (iv) all those known by the Company to be beneficial owners of more than five percent of its common stock.
139
The table is based upon information supplied by officers, directors and principal stockholders, Schedules 13G filed with the SEC and other sources believed to be reliable by us. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, the Company believes that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 6,844,242 shares outstanding on February 29, 2016, adjusted as required by rules promulgated by the SEC. Unless otherwise indicated, the address for each person or entity listed in the table is c/o HTG Molecular Diagnostics, Inc., 3430 E. Global Loop, Tucson, Arizona 85706.
|
|
|
Common Stock Beneficially Owned |
|
|||||
|
Name and address of beneficial owner |
|
Shares |
|
|
Percentage |
|
||
|
Greater than 5% stockholders |
|
|
|
|
|
|
|
|
|
Novo A/S (1) |
|
|
915,079 |
|
|
|
13.3 |
% |
|
Turborg Havnevej 19 |
|
|
|
|
|
|
|
|
|
DK-2900 Hellerup, Denmark |
|
|
|
|
|
|
|
|
|
S.R. One Limited (2) |
|
|
727,675 |
|
|
|
10.6 |
% |
|
161 Washington Street, Suite 500 |
|
|
|
|
|
|
|
|
|
Conshohocken, PA 19428-2077 |
|
|
|
|
|
|
|
|
|
Entities affiliated with BlackRock, Inc. (3) |
|
|
711,897 |
|
|
|
10.4 |
% |
|
55 East 52nd Street |
|
|
|
|
|
|
|
|
|
New York, NY 10055 |
|
|
|
|
|
|
|
|
|
FMR LLC (4) |
|
|
673,461 |
|
|
|
9.8 |
% |
|
245 Summer Street |
|
|
|
|
|
|
|
|
|
Boston, MA 02210 |
|
|
|
|
|
|
|
|
|
Merck Capital Ventures, LLC (5) |
|
|
617,797 |
|
|
|
9.0 |
% |
|
One Merck Drive |
|
|
|
|
|
|
|
|
|
P.O. Box 1000 |
|
|
|
|
|
|
|
|
|
Whitehouse Station, NJ 08889-0100 |
|
|
|
|
|
|
|
|
|
Entities affiliated with Putnam Investments LLC (6) |
|
|
575,692 |
|
|
|
8.4 |
% |
|
One Post Office Square |
|
|
|
|
|
|
|
|
|
Boston, MA 02109 |
|
|
|
|
|
|
|
|
|
Entities affiliated with Fletcher Spaght Ventures (7) |
|
|
437,396 |
|
|
|
6.4 |
% |
|
222 Berkeley Street |
|
|
|
|
|
|
|
|
|
Boston, MA 02116 |
|
|
|
|
|
|
|
|
|
Directors and named executive officers |
|
|
|
|
|
|
|
|
|
Timothy B. Johnson (8) |
|
|
200,729 |
|
|
|
2.9 |
% |
|
John L. Lubniewski (9) |
|
|
75,974 |
|
|
|
1.1 |
% |
|
Shaun D. McMeans (10) |
|
|
60,822 |
|
|
* |
|
|
|
Peter T. Bisgaard (11) |
|
|
— |
|
|
* |
|
|
|
Harry A. George (12) |
|
|
144,366 |
|
|
|
2.1 |
% |
|
Mary (Molly) Hoult (13) |
|
|
— |
|
|
* |
|
|
|
Lewis J. Shuster (14) |
|
|
12,334 |
|
|
* |
|
|
|
Lee McCracken |
|
|
— |
|
|
* |
|
|
|
James T. LaFrance |
|
|
— |
|
|
* |
|
|
|
All current executive officers and directors as a group (11 persons) (15) |
|
|
576,295 |
|
|
|
8.1 |
% |
|
* |
Represents beneficial ownership of less than one percent. |
|
(1) |
Includes 49,786 shares issuable upon the exercise of warrants. The board of directors of Novo A/S, a Danish limited liability company, consists of Sten Scheibye, Göran Ando, Jeppe Christiansen, Steen Riisgaard and Per Wold-Olsen, who have shared investment and voting control with respect to the shares held by Novo A/S and may exercise such control only with the support of a majority of the members of the Novo A/S board of directors. No individual member of the Novo A/S board of directors is deemed to hold any beneficial ownership or reportable pecuniary interest in the shares held by Novo A/S. |
|
(2) |
Includes 43,538 shares issuable upon the exercise of warrants. Voting and/or dispositive powers with respect to shares held in the name of S.R. One Limited are exercised by S.R. One Limited through a collective vote of S.R. One Limited’s principals, on a majority vote basis. The current roster of S.R. One Limited principals may be found at http://www.srone.com/Team.aspx |
|
(3) |
This information is based on the Schedule 13G/A filed on January 8, 2016 with the SEC. |
140
|
common shares of the FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of the Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FRM LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act advised by Fidelity Management & Research Company, a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. Fidelity Management & Research Company carries out the voting of the shares under written guidelines established by Fidelity Funds’ Board of Trustees. This information is based on the Schedule 13G filed on February 12, 2016 with the SEC. |
|
(5) |
Includes 28,436 shares issuable upon the exercise of warrants. |
|
(6) |
The number of shares beneficially owned consists of (a) 545,443 shares held by Putnam Investment Management and (b) 30,249 shares held by The Putnam Advisory Company, LLC, both wholly owned subsidiaries of Putnam Investments, LLC. Putnam Investment Management is the investment adviser to the Putnam family of mutual funds and the Putnam Advisory Company, LLC is the investment adviser to Putnam’s institutional clients. Both subsidiaries have dispositive power over the shares as investment managers. In the case of shares held by the Putnam mutual funds managed by Putnam Investment Management, LLC, the mutual funds, through their boards of trustees, have voting power. Unless otherwise indicated, The Putnam Advisory Company, LLC has sole voting power over the shares held by its institutional clients. This information is based on the Schedule 13G filed on February 16, 2016 with the SEC. |
|
(7) |
Consists of (a) 263,890 shares of common stock and 13,473 shares issuable upon exercise of warrants held by Fletcher Spaght Ventures II, L.P., (b) 26,571 shares of common stock and 1,356 shares issuable upon exercise of warrants held by FSV II, L.P. and (c) 125,689 shares of common stock and 6,417 shares issuable upon exercise of warrants held by FSV II-B, L.P. |
|
(8) |
Includes 145,044 shares that Mr. Johnson has the right to acquire from us within 60 days of February 29, 2016 pursuant to the exercise of stock options and 15,000 shares issuable within 60 days of February 29, 2016 pursuant to the vesting of restricted stock units. |
|
(9) |
Includes 50,151 shares that Mr. Lubniewski has the right to acquire from us within 60 days of February 29, 2016 pursuant to the exercise of stock options and 2,500 shares issuable within 60 days of February 29, 2016 pursuant to the vesting of restricted stock units. |
|
(10) |
Includes 39,245 shares that Mr. McMeans has the right to acquire from us within 60 days of February 29, 2016 pursuant to the exercise of stock options and 5,000 shares issuable within 60 days of February 29, 2016 pursuant to the vesting of restricted stock units. |
|
(11) |
Mr. Bisgaard is employed as a partner of Novo Ventures (US), Inc., a consultant to Novo A/S, but does not have voting or investment power over the shares beneficially held by Novo A/S. |
|
(12) |
Consists of shares beneficially owned by Solstice Capital II LP. Mr. George is the managing member of Solstice Capital and has joint voting and investment power over the shares held by Solstice Capital II LP. |
|
(13) |
Ms. Hoult is a vice president of Fletcher Spaght but does not have voting or investment power over the shares held by the entities affiliated with Fletcher Spaght. |
|
(14) |
Includes 12,334 shares that Mr. Shuster has the right to acquire from us within 60 days of February 29, 2016 pursuant to the exercise of stock options. |
|
(15) |
The number of shares beneficially owned consists of (a) the shares described in Notes (8) through (14), and (b) 39,275 shares beneficially owned, 37,795 shares issuable upon exercise of options within 60 days of February 29, 2016 and 5,000 shares issuable within 60 days of February 29, 2016 pursuant to the vesting of restricted stock units held by two additional executive officers. |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
We have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related-person transactions.” For purposes of our policy only, a “related-person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants involving an amount that exceeds the lesser of $120,000 or one percent of the average of the Company’s total assets at year-end for the last two completed fiscal years.
Transactions involving compensation for services provided to us as an employee, consultant or director are not considered related-person transactions under this policy. A “related person” is any executive officer, director or a holder of more than five percent of our common stock, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, where a transaction has been identified as a related-person transaction, management must present information regarding the proposed related-person transaction to our audit committee (or, where review by our audit committee would be inappropriate, to another independent body of our board of directors) for review. The presentation must include a description of,
141
among other things, the material facts, the direct and indirect interests of the related persons, the benefits of the transaction to us and whether any alternative transactions are available. To identify related-person transactions in advance, we rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related-person transactions, our audit committee or other independent body of our board of directors takes into account the relevant available facts and circumstances including, but not limited to:
|
|
· |
the risks, costs and benefits to us; |
|
|
· |
the impact on a director’s independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated; |
|
|
· |
the terms of the transaction; |
|
|
· |
the availability of other sources for comparable services or products; and |
|
|
· |
the terms available to or from, as the case may be, unrelated third parties or to or from our employees generally. |
In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval.
The following includes a summary of transactions since January 1, 2014 to which we have been a party, in which the amount involved in the transaction exceeded the lesser of $120,000 or one percent of the average of the Company’s total assets at year-end for the last two completed fiscal years, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive Compensation” and “Director Compensation.”
Preferred Stock and Warrant Financings
From February 4, 2014 through March 31, 2014, we issued and sold to investors in two closings an aggregate of 34,453,538 shares of our Series E preferred stock at a purchase price of $0.2189 per share, for aggregate consideration of $7.5 million. We also issued to investors in our Series E preferred stock financing warrants to purchase up to an aggregate of 11,484,503 shares of our Series E preferred stock at an issue price of $0.0001 per share underlying the warrants, for aggregate consideration of $1,148. The Series E warrants were exercisable for $0.001 per share and were each exercised immediately upon issuance for an aggregate cash purchase price of $11,484.
The participants in these preferred stock financings included the following executive officers, directors and holders of more than 5% of our capital stock:
|
Participants |
|
Series E Preferred Stock (1) |
|
|
|
Executive Officers and Directors |
|
|
|
|
|
Timothy B. Johnson |
|
|
60,910 |
|
|
John L. Lubniewski |
|
|
76,138 |
|
|
Debra A. Gordon |
|
|
7,646 |
|
|
Shaun D. McMeans |
|
|
152,276 |
|
|
James R. Weersing (2) |
|
|
609,106 |
|
|
5% or Greater Stockholders |
|
|
|
|
|
Novo A/S |
|
|
15,227,653 |
|
|
S.R. One, Limited |
|
|
15,227,653 |
|
|
Merck Capital Ventures, LLC |
|
|
7,613,826 |
|
|
Entities affiliated with Fletcher Spaght Associates |
|
|
6,091,062 |
|
|
(1) |
Includes the shares of Series E preferred stock issued to the participant upon exercise of the Series E preferred stock warrants issued in the financing. |
|
(2) |
Mr. Weersing served on our board of directors until April 2015. Mr. Weersing participated in the financings through his affiliated family trust. |
142
Certain of our directors have affiliations with the investors that participated in the preferred stock financings described above, as indicated in the table below:
|
Director |
|
Principal Stockholder |
|
Peter T. Bisgaard |
|
Novo A/S |
|
Simeon J. George, M.D. |
|
S.R. One Limited |
|
Mary (Molly) Hoult |
|
Fletcher Spaght Associates |
|
Larry Senour (1) |
|
Merck Capital Ventures, LLC |
|
|
(1) |
Mr. Senour resigned from our board of directors on May 5, 2015. |
Convertible Note and Warrant Financings
In December 2014, we entered into two separate note and warrant purchase agreements, or collectively, the 2014 note purchase agreements, with certain of our existing investors, including beneficial owners of more than 5% of our capital stock and certain entities affiliated with members of our board of directors. The first note and warrant purchase agreement, or the first note purchase agreement, provided for the sale and issuance by us of up to an aggregate of $7.3 million in principal amount of convertible notes in a series of closings, each of which were to be approved by the unanimous vote or written consent of those members of our board of directors who were not an affiliate of any of the investors under such agreement. The second note and warrant purchase agreement, or the second note purchase agreement, provided for the sale and issuance by us of up to an aggregate of $6.2 million in principal amount of convertible notes in a series of closings, each of which were to be approved by (i) our board of directors, including a majority of the directors elected by the holders of our Series E preferred stock, and (ii) investors whose purchase amount for such closing equaled or exceeded 50% of the aggregate principal amount of notes to be sold at such closing. Notes issued under the 2014 note purchase agreements accrued interest at a rate of 8% per annum, compounded annually, and became due and payable on March 31, 2016, subject to their earlier conversion in the event we completed an initial public offering in which we received gross offering proceeds of at least $20.0 million from the sale of shares to investors who were not holders of our securities, or a qualified initial public offering, or a private placement of our preferred stock (whether in one single transaction or several tranches) resulting in aggregate gross proceeds of at least $20.0 million from sales of securities to investors who were not holders of our securities, or a qualified private placement of our equity securities. The number of shares into which the notes may be converted, common shares in the case of a qualified initial public offering or preferred shares in the case of a qualified private placement, was equal to the outstanding principal and accrued interest divided by the price per share paid by investors purchasing such newly issued equity securities. Certain provisions of the first note purchase agreement terminated (including the investors’ obligations to purchase notes thereunder) immediately prior to the earlier to occur of the closing of (i) a qualified initial public offering or (ii) a qualified private placement and certain provisions of the second note purchase agreement terminated (including the investors’ obligations to purchase notes thereunder) immediately prior to the earlier to occur of (x) the time at which a registration statement covering a public offering of our securities under the Securities Act of 1933, as amended, becomes effective or (y) the initial closing of a qualified private placement. Such provisions of the 2014 note purchase agreements (including the investors’ obligations to purchase notes thereunder) terminated in connection with the closing of our initial public offering in May 2015. In February and March 2015, we issued an aggregate of $3.0 million in principal amount of the 2015 notes to investors in two closings under the first note purchase agreement and expect to issue an aggregate of $1.5 million in principal amount of the 2015 notes in the Third Note Closing.
In January 2015, in connection with the 2014 note purchase agreements, we issued warrants, or the 2015 warrants, which were initially exercisable for an aggregate of 9,311,586 shares of our Series E preferred stock at an exercise price of $0.2189 per share. In connection with the closing of our initial public offering, the 2015 warrants became exercisable for an aggregate of 144,772 shares of our common stock at an exercise price of $14.00 per share.
143
The participants in these convertible note and warrant financings included the following director and holders of more than 5% of our capital stock:
|
Participants |
|
Shares of Series E Preferred Stock Underlying 2015 Warrants |
|
|
Aggregate Principal Amount of 2015 Notes (in thousands) (2) |
|
||
|
Directors |
|
|
|
|
|
|
|
|
|
James R. Weersing (1) |
|
|
51,126 |
|
|
|
— |
|
|
5% or Greater Stockholders |
|
|
|
|
|
|
|
|
|
Novo A/S |
|
|
3,184,170 |
|
|
|
1,535 |
|
|
S.R. One, Limited |
|
|
2,784,593 |
|
|
|
1,535 |
|
|
Merck Capital Ventures, LLC |
|
|
1,818,681 |
|
|
|
768 |
|
|
Entities affiliated with Fletcher Spaght Associates |
|
|
1,358,988 |
|
|
|
614 |
|
|
(1) |
Mr. Weersing served on our board of directors until April 2015. Mr. Weersing participated through his affiliated family trust. |
|
(2) |
Includes amounts purchased in note closings in February, March and April 2015. |
Investor Rights Agreement
In connection with our preferred stock financings, we entered into an Amended and Restated Investor Rights Agreement containing registration rights, information rights, voting rights and rights of first refusal, among other things, with certain holders of our convertible preferred stock and certain holders of our common stock. In connection our IPO, we entered into a further Amended and Restated Investor Rights Agreement with these holders which eliminated all of the rights and obligations provided in the first Amended and Restated Investor Rights Agreement, except for the registration rights granted under the new agreement. The holders of at least 50% of the registrable securities have the right to make up to two demands that we file a registration statement under the Securities Act covering such holders’ registrable securities then outstanding (provided that the anticipated aggregate offering price of securities requested to be sold under such registration statement is at least $7.5 million), subject to specified exceptions, conditions and limitations. If we are eligible to file a registration statement on Form S-3, the holders of at least 50% or more of the outstanding registrable securities have the right to demand that we file a registration statement on Form S-3 so long as the aggregate amount of securities to be sold under the registration statement on Form S-3 is at least $5.0 million, subject to specified exceptions, conditions and limitations. If we propose to register any securities for public sale, holders of registration rights will have the right to include their shares in the registration statement (except with respect to this offering, for which the holders have waived any and all rights to have their shares included). The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement, subject to specified conditions and limitations. Generally, we are required to bear all registration and selling expenses incurred in connection with the demand, piggyback and Form S-3 registrations described above, other than underwriting discounts and commissions. The demand, piggyback and Form S-3 registration rights discussed above will terminate in May 2017 or, as to a given holder of registrable securities, when such holder is able to sell all of its registrable securities in a single 90-day period under Rule 144 of the Securities Act.
Employment Arrangements
We currently have written employment agreements with our executive officers. For information about our employment agreements with our named executive officers, refer to “Executive Compensation – Agreements with our Named Executive Officers.”
Stock Options Granted to Executive Officers and Directors
We have granted stock options to our executive officers and directors, as more fully described in “Executive Compensation – Outstanding Equity Awards at Fiscal Year-End.”
Indemnification Agreements
We have entered into, and intend to continue to enter into, separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amended and restated bylaws. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or any other company or enterprise to which the person provides services at our request. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
144
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Director Independence
Our board of directors has determined that all of our directors other than Mr. Johnson are independent directors, as defined by Rule 5605(a)(2) of the NASDAQ Listing Rules. The NASDAQ independence definition includes a series of objective tests, including that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by NASDAQ rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management.
Item 14. Principal Accounting Fees and Services.
The following table summarizes the fees of BDO USA, LLP, our independent registered public accounting firm, for 2015 and 2014.
|
|
|
As of December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Fee Category |
|
|
|
|
|
|
|
|
|
Audit fees (1) |
|
$ |
420,471 |
|
|
$ |
288,316 |
|
|
Audit-related fees |
|
|
— |
|
|
|
— |
|
|
Tax fees |
|
|
— |
|
|
|
— |
|
|
All other fees |
|
|
— |
|
|
|
— |
|
|
Total fees |
|
$ |
420,471 |
|
|
$ |
288,316 |
|
|
(1) |
Audit fees consist of fees for professional services provided in connection with the audit of our annual financial statements, review of our quarterly financial statements and initial public offering, as well as fees for comfort letters, S-8, S-1 and Yellowbook audit services (for the year ended December 31, 2015 only) |
Pre-Approval Policies and Procedures
Pursuant to its charter, the audit committee must review and approve, in advance, the scope and plans for the audits and the audit fees and approve in advance (or, where permitted under the rules and regulations of the SEC, subsequently) all non-audit services to be performed by the independent auditor that are not otherwise prohibited by law and any associated fees. The audit committee may delegate to one or more members of the committee the authority to pre-approve audit and permissible non-audit services, as long as this pre-approval is presented to the full committee at scheduled meetings. All fees described above were pre-approved by the audit committee.
PART IV
Item 15. Exhibits, Financial Statement Schedules.
|
(a)(1) |
Financial Statements - The financial statements filed as part of this Annual Report on Form 10-K are listed on the Index to Financial Statements in Item 8. |
|
(a)(2) |
Financial Statement Schedules |
All schedules are omitted because they are not applicable or the required information is shown in the financial statements or the notes thereto.
145
The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
|
(b) |
Exhibits. |
The exhibits listed on the Exhibit Index (following the Signatures section of this report) are filed herewith or are incorporated by reference to exhibits previously filed with the SEC.
146
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
HTG Molecular Diagnostics, Inc. |
|
|
|
|
|
|
|
Date: March 24, 2016 |
|
By: |
/s/ Timothy Johnson |
|
|
|
|
Timothy Johnson |
|
|
|
|
President and Chief Executive Officer (Principal Executive Officer) |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Timothy Johnson and Shaun D. McMeans, jointly and severally, his or her attorneys-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this report, and to file the same, with exhibits thereto and other documents in connection therewith with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated:
|
Signature |
|
Title |
|
Date |
|
|
|
|
||
|
/s/ Timothy Johnson Timothy Johnson |
|
President, Chief Executive Officer and Member of the Board of Directors (Principal Executive Officer) |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ Shaun D. McMeans Shaun D. McMeans |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ Peter T. Bisgaard Peter T. Bisgaard |
|
Chairman of the Board of Directors |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ Harry A. George Harry A. George |
|
Member of the Board of Directors |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ Mary Hoult Mary Hoult |
|
Member of the Board of Directors |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ James T. LaFrance James T. LaFrance |
|
Member of the Board of Directors |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ Lee R. McCracken Lee R. McCracken |
|
Member of the Board of Directors |
|
March 24, 2016 |
|
|
|
|
||
|
/s/ Lewis J. Shuster Lewis J. Shuster |
|
Member of the Board of Directors |
|
March 24, 2016 |
147
|
Exhibit Number |
|
Description |
|
|
|
|
|
2.1 |
|
Asset Purchase Agreement dated January 9, 2001, as amended by and between the Registrant, NuvoGen, L.L.C., Stephen Felder and Richard Kris (incorporated by reference to Exhibit 2.1 to the Registrant’s registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
3.1 |
|
Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 12, 2015). |
|
|
|
|
|
3.2 |
|
Amended and Restated Bylaws of the Registrant (incorporated by reference to Exhibit 3.2 to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 12, 2015). |
|
|
|
|
|
4.1 |
|
Reference is made to Exhibits 3.1 and 3.2. |
|
|
|
|
|
4.2 |
|
Form of Common Stock Certificate of the Registrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.3 |
|
Common Stock Warrant issued by the Registrant to the University of Arizona, dated March 13, 2009 (incorporated by reference to Exhibit 4.2 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.4 |
|
Series C-2 Preferred Stock Warrant issued by the Registrant to Silicon Valley Bank, dated December 24, 2008 (incorporated by reference to Exhibit 4.3 to the Registrant’s Registration Statement on Form S-1, as amended (file No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.5 |
|
Series E Preferred Stock Warrant issued by the Registrant to Silicon Valley Bank, dated August 22, 2014 (incorporated by reference to Exhibit 4.4 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.6 |
|
Series E Preferred Stock Warrant issued by the Registrant to Oxford Finance LLC, dated August 22, 2014 (incorporated by reference to Exhibit 4.5 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.7 |
|
Form of Warrant issued by Registrant to bridge financing investors (incorporated by reference to Exhibit 4.6 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.8 |
|
Form of Warrant issued by Registrant to bridge financing investors (incorporated by reference to Exhibit 4.7 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
4.9 |
|
Amended and Restated Investor Rights Agreement by and among the Registrant and certain of its stockholders, dated May 11, 2015 (incorporated by reference to Exhibit 4.8 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.1+ |
|
Form of Indemnity Agreement by and between the Registrant and its directors and officers (incorporated by reference to Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.2+ |
|
HTG Molecular Diagnostics, Inc. 2001 Stock Option Plan and Forms of Stock Option Agreement and Stock Option Grant Notice thereunder (incorporated by reference to Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.3+ |
|
HTG Molecular Diagnostics, Inc. 2011 Equity Incentive Plan and Forms of Stock Option Agreement, Notice of Exercise and Stock Option Grant Notice thereunder (incorporated by reference to Exhibit 10.3 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.4+ |
|
HTG Molecular Diagnostics, Inc. 2014 Equity Incentive Plan and Forms of Stock Option Agreement, Notice of Exercise and Stock Option Grant Notice thereunder (incorporated by reference to Exhibit 99.3 to the Registrant’s Registration Statement on Form S-8 (File No. 333-203930), filed with the SEC on May 7, 2015). |
148
|
Exhibit Number |
|
Description |
|
|
|
|
|
10.5+ |
|
HTG Molecular Diagnostics, Inc. 2014 Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.5 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.6+ |
|
HTG Molecular Diagnostics, Inc. Non-Employee Director Compensation Policy (incorporated by reference to Exhibit 10.6 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.7+ |
|
Employment Letter Agreement dated December 24, 2014 by and between the Registrant and Timothy Johnson (incorporated by reference to Exhibit 10.7 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.8+ |
|
Employment Letter Agreement dated December 18, 2014 by and between the Registrant and John Lubniewski (incorporated by reference to Exhibit 10.8 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.9+ |
|
Employment Letter Agreement dated December 15, 2014 by and between the Registrant and Debra Gordon (incorporated by reference to Exhibit 10.9 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.10+ |
|
Employment Letter Agreement dated December 15, 2014 by and between the Registrant and Shaun McMeans (incorporated by reference to Exhibit 10.10 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.11+ |
|
Employment Letter Agreement dated December 15, 2014 by and between the Registrant and Patrick Roche (incorporated by reference to Exhibit 10.11 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.12 |
|
Standard Commercial-Industrial Multi Tenant Triple Net Lease dated July 11, 2008 by and between the Registrant and Pegasus Properties LP (incorporated by reference to Exhibit 10.12 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.13 |
|
Standard Commercial-Industrial Multi Tenant Triple Net Lease dated May 11, 2011 by and between the Registrant and Pegasus Properties LP (incorporated by reference to Exhibit 10.13 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.14* |
|
First Amendment to Lease Agreement (Suite 100 – Administration), dated August 4, 2015, by and between Pegasus Properties, L.P. and the Registrant (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on November 12, 2015). |
|
|
|
|
|
10.15* |
|
First Amendment to Lease Agreement (Suite 300 – Laboratory), dated August 4, 2015, by and between Pegasus Properties, L.P. and the Registrant (incorporated by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on November 12, 2015). |
|
|
|
|
|
10.16 |
|
Loan and Security Agreement, dated August 22, 2014, by and among the Registrant, Oxford Finance LLC and Silicon Valley Bank (incorporated by reference to Exhibit 10.15 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.17 |
|
First Amendment to Loan and Security Agreement and First Amendment to Disbursement Letter, dated August 4, 2015, by and between Registrant, Oxford Finance LLC and Silicon Valley Bank (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on August 7, 2015). |
|
|
|
|
|
10.18 |
|
Termination of Security Agreement, Release of Security Interest and Understanding Regarding Asset Purchase Agreement, dated August 22, 2014, by and between the Registrant and NuvoGen Research LLC (incorporated by reference to Exhibit 10.16 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.19* |
|
IVD Test Development and Component Supply Agreement, dated October 15, 2014, by and between the Registrant and Illumina, Inc. (incorporated by reference to Exhibit 10.17 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
149
|
Exhibit Number |
|
Description |
|
|
|
|
|
10.20 |
|
Note and Warrant Purchase Agreement, dated December 30, 2014, by and among the Registrant and the entities and persons listed therein (incorporated by reference to Exhibit 10.18 to the Registrant’s Registration Statement on Form S-1, as amended (File No. 333-201313), originally filed with the SEC on December 30, 2014). |
|
|
|
|
|
10.21 |
|
HTG Molecular Diagnostics, Inc. Stock Purchase Plan (incorporated by reference to Exhibit 99.1 to the Registrant’s Registration Statement on Form S-8 (File No. 333-208325), filed with the SEC on December 3, 2015). |
|
|
|
|
|
10.22* |
|
Authorization, Supply, and Regulatory Authorization Agreement, dated March 16, 2016, by and between HTG Molecular Diagnostics, Inc. and Life Technologies Corporation (incorporated by reference to Exhibit 99.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on March 21 ,2016). |
|
|
|
|
|
23.1 |
|
Consent of Independent Registered Public Accounting Firm. |
|
|
|
|
|
24.1 |
|
Power of Attorney. Reference is made to the signature page hereto. |
|
|
|
|
|
31.1 |
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
31.2 |
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
32.1 |
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
32.2 |
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
101.INS |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
* |
We have requested confidential treatment for certain portions of this agreement. Omitted portions have been filed separately with the SEC. |
|
+ |
Indicates management contract or compensatory plan. |
150