Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - SeaSpine Holdings Corp | spne-20151231ex231.htm |
| EX-21.1 - EXHIBIT 21.1 - SeaSpine Holdings Corp | spne-20151231ex211listofsu.htm |
| EX-10.2 - EXHIBIT 10.2 - SeaSpine Holdings Corp | spne-20151231ex102creditag.htm |
| EX-32.1 - EXHIBIT 32.1 - SeaSpine Holdings Corp | spne-20151231ex321.htm |
| EX-32.2 - EXHIBIT 32.2 - SeaSpine Holdings Corp | spne-20151231ex322.htm |
| EX-31.2 - EXHIBIT 31.2 - SeaSpine Holdings Corp | spne-20151231ex312.htm |
| EX-31.1 - EXHIBIT 31.1 - SeaSpine Holdings Corp | spne-20151231ex311.htm |
| EX-10.1 - EXHIBIT 10.1 - SeaSpine Holdings Corp | spne-20151231ex101compensa.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
or
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
COMMISSION FILE NO. 001-36905
SeaSpine Holdings Corporation
(EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
DELAWARE | 47-3251758 | |
(STATE OR OTHER JURISDICTION OF INCORPORATION OR ORGANIZATION) | (I.R.S. EMPLOYER IDENTIFICATION NO.) | |
5770 Armada Drive, Carlsbad, California | 92008 | |
(ADDRESS OF PRINCIPAL EXECUTIVE OFFICES) | (ZIP CODE) | |
REGISTRANT’S TELEPHONE NUMBER, INCLUDING AREA CODE: (760) 727-8399
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
Title of Each Class | Name of Exchange on Which Registered | |
Common Stock, Par Value $.01 Per Share | The Nasdaq Stock Market LLC | |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act. Yes o
No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
1
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | o | Accelerated filer | o |
Non-accelerated filer | x (Do not check if a smaller reporting company) | Smaller reporting company | o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
As of July 2, 2015, the aggregate market value of the registrant’s common stock held by non-affiliates was approximately $9,069,768 based upon the closing sales price of the registrant’s common stock on The Nasdaq Global Market on such date. The number of shares of the registrant’s Common Stock, $0.01 par value, outstanding as of February 29, 2016 was 11,101,777.
DOCUMENTS INCORPORATED BY REFERENCE:
Certain portions of the registrant’s definitive proxy statement relating to its scheduled June 7, 2016 Annual Meeting of Stockholders are incorporated by reference in Part III of this report.
2
SEASPINE HOLDINGS CORPORATION
INDEX
Page Number | |
3
PART I
ITEM 1. BUSINESS
Overview
SeaSpine Holdings Corporation (“SeaSpine” or “Company”) is a global medical technology company focused on the design, development and commercialization of surgical solutions for the treatment of patients suffering from spinal disorders. We have a comprehensive portfolio of orthobiologics and spinal fusion hardware solutions to meet the varying combinations of products that neurosurgeons and orthopedic spine surgeons need to perform fusion procedures in the lumbar, thoracic and cervical spine. Our orthobiologics products consist of a broad range of advanced and traditional bone graft substitutes that are designed to improve bone fusion rates following a wide range of orthopedic surgeries, including spine, hip, and extremities procedures. We manufacture most of our orthobiologics products at our Irvine, California manufacturing facility. Our spinal fusion hardware portfolio consists of an extensive line of products to facilitate spinal fusion in minimally invasive surgery (“MIS”), complex spine, deformity and degenerative procedures. We believe this broad combined portfolio of orthobiologics and spinal fusion hardware products is essential to meet the “complete solution” requirements of neurosurgeons and orthopedic spine surgeons.
Mission
We believe spine surgery patients should benefit from the most advanced scientific knowledge and technology available. The SeaSpine team is dedicated to improving the lives of spine patients, collaborating with surgeons and developing advanced and effective spinal surgery technologies. Our team collaborates closely with leading spine surgeons, global distributors and other partners on the cutting edge of spinal solutions. From research and development, to product design and engineering, to commercialization and distribution - SeaSpine’s product portfolio is dedicated to assist in restoring mobility and quality of life to patients.
Our Products
Our comprehensive offering of orthobiologics and spinal fusion hardware products has evolved to meet the surgical needs for our customers. Bone grafts and bone graft substitutes are frequently used to promote the bone healing process in orthopedic surgical procedures where a bone void or defect has been created. Our hardware products such as metal plates, rods and screws, and metal, polyetheretherketone (“PEEK”), PEEK NanoMetalene coated or machined allograft bone spacers, are used to restore and stabilize the bone structure, and our orthobiologics products can be used to support the fusion of bone. Most often autograft, the patient’s own bone, is not adequate for the complete bone healing process, so bone graft substitutes are used to either replace or supplement and extend the autograft. Bone healing requires three components-osteogenic cells which build new bone, an osteoinductive signal, which stimulates the cells to build bone, and an osteoconductive scaffold, or conductive matrix, over which the cells can migrate. Our broad orthobiologics portfolio employs these principles to provide osteoinductive and/or osteoconductive properties to support the patient’s own cells in the formation of new bone. We believe our expertise in both orthobiologic sciences and spinal fusion hardware product development allows us to offer our surgeon customers a differentiated portfolio and a “complete solution” to meet their fusion requirements.
Our orthobiologics products include a variety of bone graft substitutes including demineralized bone matrices (“DBM”) and collagen ceramic matrices that have a balance of osteoinductive and osteoconductive properties. Demineralized bone matrices consist of human cadaver (allograft) bone that has been processed to remove the mineral content but preserve the protein content and osteoinductive properties of the bone. Our most advanced bone graft substitute solution, marketed as Accell Evo3® and OsteoSurge® 300, is our third-generation demineralized bone matrix product. Utilizing our proprietary Accell technology, this optimized formulation also incorporates a standard particulate DBM in a unique biocompatible carrier designed to provide better handling and containment characteristics as compared to competitive demineralized bone matrix products. Accell Bone Matrix is an open structured, dispersed form of DBM, which provides accessibility to bone proteins without the need to be broken down after implantation in the patient. Standard particulate DBM is dense and requires more time to break down. Until these dense particles break down, access to natural bone proteins is limited. As a result, the combination of these two forms of DBM creates a favorable environment for the formation of bone over time. In addition, the carrier allows Accell Evo3 to meet the needs of challenging surgical applications where robust handling is essential. The material is considered to be a reverse-phase carrier, which means at room temperature, it is less viscous and thereby more moldable, while at body temperature after implantation, it becomes more viscous, thereby resisting irrigation and minimizing graft migration. We also offer first- and second-generation demineralized bone matrix products which also include some of the technologies in our most advanced formulation. Additional demineralized bone matrix product configurations include products designed specifically for use in spine fusion procedures. Our collagen ceramic matrix product, marketed as Isotis MozaikTM and OsteoStrux, is an engineered collagen framework with ceramic components that together provide a scaffold for bone cell migration. The ceramic components provide mineral content to foster bone formation
4
during the healing process. This product is offered in strip, putty and moldable morsel configurations to meet the varying needs and preferences of our surgeon customers. We also offer allograft cancellous bone in sponge, chips and crushed preparations, as well as synthetic beta-tricalcium phosphate synthetic bone void fillers.
Spinal fusion utilizes the body’s own bone growth processes to fuse two or more spinal vertebrae. Spinal fusion consists of a variety of different approaches or techniques and is used to treat a range of spinal conditions. Our spinal fusion hardware portfolio includes a broad offering of products to facilitate spinal fusion in MIS, complex spine, deformity and degenerative procedures. We offer MIS products consisting of multiblade adjustable retractors, tube retractors and mini-open and percutaneous solutions. We also offer rods, screws and instrumentation for posterior lumbar fusion and a broad range of PEEK and titanium coated PEEK anterior, posterior and lateral approach interbody devices, including an expandable interbody system intended for one or two adjacent levels.
Our complex spine and deformity products are used to treat multilevel conditions, including traumatic injury, tumors and abnormal curvatures of the spine. These product offerings include our Vu Mesh™ system which features a system of cages, spacers and endplates in a modular design that provide surgeons with intraoperative flexibility for their most challenging cases such as the surgical removal of a vertebral body. Our Malibu™ pedicle screw system is used in complex spine cases where its specialty screws can be leveraged to extend and capture the rod, and its specialty trauma screws can be used to help realign the vertebrae before fusing. Our Daytona® Deformity System addresses complex deformity cases by utilizing extended tab uniplanar and polyaxial screws with multiple rod options and intuitive instrumentation to create a versatile system adaptable to surgeon preference.
Our extensive line of products for degenerative cases includes devices for cervical and thoracolumbar procedures, and primarily consists of screw and plating systems and interbody devices that are typically used in open procedures. Degenerative disease refers to a variety of conditions including the degeneration of one or more of the cartilaginous discs located between the vertebral bones of the spine. Our Hollywood™, Ventura™ and Cambria NanoMetalene® Interbody Devices offer the combined benefits of a PEEK device with an innovative titanium coating. These devices are composed of PEEK-OPTIMA®, an engineered thermoplastic polymer, which has undergone a proprietary process that creates a titanium coating around the entire implant. We believe that this coating process has significant advantages over other existing material processes as it allows for the surface benefits of titanium, which is believed by scientists to encourage bone growth and cell migration, without compromising the mechanical and imaging benefits of PEEK-OPTIMA. The ultrathin NanoMetalene coating does not impair postoperative imaging, allowing surgeons to view the operative area and determine the extent of fusion of the vertebral bodies. Our cervical portfolio consists of a complete line of anterior cervical screw and plating systems, a full range of anterior cervical interbody devices and posterior cervical rod, screw and hook systems.
We currently market and sell our products in the United States and in over 30 countries worldwide. Our U.S. sales organization consists of regional business managers who oversee direct orthobiologics sales specialists and a broad network of independent orthobiologics and spine sales agents that receive commissions from us for sales that they generate. Our international sales organization is composed of a sales management team that oversees a network of independent orthobiologics and spine stocking distributors that purchase our products directly from us and independently sell them. International sales represented approximately 10%, 10% and 12% of our total revenue for the year ended December 31, 2015, 2014 and 2013, respectively.
Our History
SeaSpine was incorporated in Delaware on February 12, 2015 in connection with the spin-off of Integra LifeSciences Holdings Corporation’s (Integra) spinal fusion hardware and orthobiologics business from Integra’s diversified medical technology business on July 1, 2015. Our corporate offices are located at 5770 Armada Drive, Carlsbad, California.
We operate three facilities: our headquarters in Carlsbad, California, from which our orthobiologics and spinal fusion hardware products are designed, developed, and marketed; our Vista, California site from which our spinal fusion hardware products are procured, inspected, kitted and distributed; and our orthobiologics manufacturing facility in Irvine, California from which virtually all of our orthobiologics products are designed, developed, manufactured and distributed. We distribute our orthobiologics and spinal fusion hardware products in certain international markets through third-party logistics provider facilities in Belgium and the Netherlands.
Industry Overview
The bone graft substitutes market consists of surgical procedures in which a bone graft substitute made from donated human bone tissue or a synthetic material is implanted in the patient to augment or stimulate bone growth to aid healing. According to iData, this market was estimated at $0.9 billion in 2014 in the United States and will grow at a compound annual growth rate of 3.4% through 2021. According to the same source, spinal fusion procedures are where bone graft substitutes are most commonly
5
used, representing approximately 45% of the market, and are expected to grow faster than the other major uses with a compound annual growth rate of 4.2% through 2021. Demineralized bone matrix grafts are the largest component of the bone graft substitutes market representing approximately 43% of the overall bone graft substitute market or approximately $0.4 billion in 2014. iData estimates our share of the bone graft substitutes and demineralized bone matrices markets in the United States in 2014 to be 8.6% and 12.3%, respectively, which place us as fourth and third in market position in these markets, respectively. Orthopedic stem cell and cell therapy as well as orthopedic growth factor represent additional segments in the orthobiologics market. Products in these segments are typically used in the same surgical procedures as traditional bone graft substitutes and iData estimates these segments, collectively, at approximately $0.7 billion in 2014.
According to iData, the spinal fusion procedure market consists of products for cervical fixation, thoracolumbar fixation and interbody devices. The market for these products was $4.4 billion in 2014 in the United States and is expected to grow at a compound annual growth rate of 0.7% through 2021. The fastest growing sub segment of this market, according to iData, is the $1.6 billion interbody device market, which will grow at a compound annual growth rate of 2.9% through 2021.
Spine Anatomy
The spine is a column of bone and cartilage that consists of 33 interlocking bones, called vertebrae, which stack upon each other at a slight angle to form the spine’s S-shaped curve. With the exception of the bottom nine vertebrae, the vertebrae are separated by thin regions of cartilage known as intervertebral discs, which act as shock absorbers that facilitate motion and absorb stress during movement. The spine protects the spinal cord and acts as the core of the human skeleton, extending from the pelvis to the base of skull. Soft tissues, including ligaments, tendons and muscles are attached to the vertebrae and provide stability to the vertebral segment. The spine encloses and protects the spinal cord which carries nerves that exit through openings between the vertebrae and deliver sensation and control to the body.
Lateral View of Spine
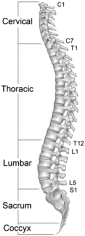
The spine consists of five regions, of which the cervical, thoracic and lumbar are the three primary regions. The cervical region consists of the seven vertebrae extending from the base of the skull to the shoulders. The thoracic, or central, region of the spine consists of the next twelve vertebrae in the middle of the back. Each vertebra in the thoracic region is connected to two ribs that protect the body’s vital organs. Below the thoracic region, the lumbar region consists of five vertebrae in the lower back and is the primary load-bearing region of the spine. The thoracic and lumbar regions are commonly referred to as thoracolumbar and many of the products and procedures to treat these regions are similar. The final two regions of the spine, the sacrum and coccyx, consist of nine naturally fused vertebrae connected to the hip bones to provide support for the spine.
In spinal fusion procedures, two or more of the vertebrae in the spine are fused together to eliminate instability as a result of deformity, degeneration or trauma affecting the vertebrae and intervertebral discs. During the surgical procedure, hardware products are used to stabilize the spine and the surgeon will often remove the damaged intervertebral disc and place a bone graft substitute product in its place to allow new bone to grow and bridge the affected vertebrae together. In addition to the bone graft substitute, the surgeon may replace the disc which was removed with an interbody device (“IBD”). An IBD may be made out of machined bone or PEEK polymer and is designed to maintain spine alignment and appropriate spacing while allowing bone to grow between the vertebrae to achieve bone fusion. Procedures that include the implantation of IBDs are often referred to by the surgical approach used to place the IBD in the disc space. A Posterior Lateral Interbody Fusion (“PLIF”) uses a direct posterior approach from the patient’s back, a Transforaminal Lumbar Interbody Fusion (“TLIF”) uses an angled approach from either the left or right side of the back, and an Anterior Lumbar Interbody Fusion (“ALIF”) uses a direct anterior approach from the patient’s front (stomach) area.
6
Our Competitive Strengths
We provide a broad portfolio of advanced and traditional orthobiologics and spinal fusion hardware solutions to assist our surgeon customers in treating patients suffering from spinal and other orthopedic disorders. Our executive management team has extensive experience in the spine and medical technology industries. We believe that our focused and experienced management team, combined with the following competitive strengths will enable us to grow our revenue and increase our presence in the markets that we serve.
• | An extensive, scaled and differentiated offering of orthobiologics products. We offer a broad range of orthobiologics products consisting of advanced and traditional bone graft substitutes that enables us to fulfill a greater portion of the orthobiologics needs of neurosurgeons and orthopedic spine surgeons than our competitors who focus primarily on offering spinal fusion hardware products. Despite our relatively small size, we are a significant participant in the U.S. market for these products, with an estimated 8.6% share in 2014 of the U.S. bone graft substitutes market, representing the fourth-largest position, according to iData. We believe that our orthobiologics portfolio offers differentiated products. For example, our third-generation demineralized bone matrix is formulated using our proprietary Accell technology and is designed to provide both immediate and sustained availability of the natural array of osteoinductive bone proteins It also provides flexibility in handling as a result of its carrier which is more moldable at room temperature and more viscous at body temperature after implantation, resisting irrigation and minimizing graft migration. Demand for this product and our other demineralized bone products has garnered us a 12.3% market share in demineralized bone matrix products in the United States in 2014, which is the third-largest position in the U.S. market, according to iData. |
• | A range of innovative, titanium coated PEEK interbody devices. Our NanoMetalene technology is an ultra-thin layer of commercially pure titanium molecularly bonded to a PEEK implant that is applied in a proprietary high-energy, low-temperature surface process and encompasses the entire implant, including the center graft aperture. We believe that our NanoMetalene technology offers advantages over existing materials as it allows for the surface benefits of titanium, which is believed by scientists to encourage bone growth and cell migration, while maintaining the mechanical and imaging benefits of PEEK, which has a modulus of elasticity similar to bone and a radiolucency for postoperative imaging, allowing surgeons to view the operative area and determine the extent of fusion of the vertebral bodies. We currently offer the NanoMetalene technology in our Ventura and Hollywood Nanometalene interbody devices for lumbar fusion and our Cambria NanoMetalene interbody device for cervical fusion. We have received U.S. Food and Drug Administration (FDA) 510(k) clearance for a number of other NanoMetalene coated PEEK interbody devices our current portfolio and we expect to launch these additional products in 2016 and beyond. |
• | A synergistic channel strategy for orthobiologics products. We maintain a dual branding strategy that allows us to market orthobiologics into territories in which we do not maintain independent spine sales agents who currently sell our hardware products. We achieve this result by marketing these products under an alternative brand through independent orthobiologics sales agents, many of whom carry competitive spinal fusion hardware products, or products for other orthopedic procedures, such as those used in large joint reconstruction. For example, we market our third-generation demineralized bone matrix product as both Accell Evo3 and OsteoSurge300 to allow differentiation between independent sales agents who sell our spinal fusion hardware, and those that sell our orthobiologics products alongside other orthopedic hardware. We believe this dual branding strategy allows us to penetrate a greater number of customer accounts than we would otherwise serve if we marketed a single line of orthobiologics brands. |
• | Our own orthobiologics design, development and manufacturing operations. While many of our spine competitors source their orthobiologics products from original equipment manufacturers to supplement their spinal fusion hardware portfolio, we design, develop and manufacture the vast majority of our orthobiologics products at our facility in Irvine, California. By controlling our own manufacturing processes, we believe we should be able to control the cost of our products more tightly and provide operational leverage with volume increases. |
Our Strategy
Our goal is to continue to scale our business in order to enhance our market position in orthobiologics and become a leader in the spinal fusion hardware market. To achieve our goals, we are investing in the following strategies:
• | Research and development to bring new products and techniques to market. We have recently increased, and intend to continue to increase our annual research and development spending as a percentage of revenue in order to drive higher revenue growth through new product sales. We plan to invest significant resources to expand our product portfolio and develop next-generation products for our existing core product lines. In order to achieve this goal, we intend to collaborate with our surgeon customers to innovate, design and develop new orthobiologics and spinal fusion hardware products. We plan to make further investments in our infrastructure by hiring additional dedicated orthobiologics engineers and |
7
scientists with expertise in material sciences and biology and hardware engineers with expertise in product design and development. By promoting a corporate culture of accountability, innovation and responsiveness to our customer needs, we plan to expedite our product launch process and bring a greater number of new products to market in the next few years than we have in recent years.
• | Commercial infrastructure to further penetrate the U.S. orthobiologics and spinal fusion hardware markets and increase our focus in international markets where we currently have a presence. We have recently increased, and intend to continue to increase the size and geographic breadth of our sales management team and network of independent sales agents in the United States. To support these efforts, we aim to develop comprehensive marketing support and physician training programs to communicate the strengths of our product platforms. We plan to expand the current schedule of hands-on cadaveric laboratory training opportunities for physicians and sales agents at our Carlsbad, California facility. In addition, we plan to increase our presence within teaching institutions that provide spinal surgery fellowship programs to educate new surgeons on the use of our products. These programs will aid surgeons in becoming comfortable with our spinal fusion hardware products and techniques. Internationally, we intend to focus our sales and marketing efforts on expanding our presence in those markets where we currently have relationships with stocking distributors. |
• | Clinical affairs programs to generate data on product efficacy. We plan to invest in additional clinical development programs to generate peer-reviewed clinical data that we believe will validate the efficacy of select orthobiologics and spinal fusion hardware solutions over competing technologies. Specifically, we believe that our third-generation demineralized bone matrix technology has benefits over other commercially available advanced bone graft substitutes in the stimulation of bone formation and bone fusion. Additionally, we plan to initiate studies to generate data on the unique surface characteristics of titanium and the mechanical properties and radiolucency of PEEK-OPTIMA as are incorporated together in a single device using our NanoMetalene technology. We believe this technology has significant advantages over existing implant materials. |
• | Opportunities to enhance our product offering through strategic alliances and acquisitions. We currently market several products under distribution agreements and licenses with third-party companies. We intend to continue to pursue alliances that will provide us with technologies to strengthen our market position. Our current business is the result of the acquisition of several companies, and we plan to continue to evaluate product alliances and acquisition opportunities as they arise to help grow our business. |
Our Products
We offer a broad portfolio of orthobiologics and spinal fusion hardware products for the treatment of patients suffering from spinal and other orthopedic disorders. The tables below group our core products into key categories and provide a summary of each technology’s features.
Orthobiologics
Our orthobiologics portfolio is used in orthopedic and dental procedures, and consists of a broad range of traditional and advanced bone graft substitutes intended to address key elements of bone regeneration, which are osteoinduction, osteoconduction and osteogenesis. Osteoinduction refers to the ability of an implant to stimulate bone forming cells based primarily on soluble growth factor signals. Osteoconduction refers to the ability of an implant to promote bone formation based primarily on a physical matrix or scaffold, when placed adjacent to viable bone tissue. Osteogenesis refers to the ability to promote new bone formation based primarily on the cells contained within the bone graft. Bone graft substitutes composed of natural biologic proteins and synthetic materials are designed to reduce the amount of autologous bone grafts needed for spinal fusion procedures. Bone graft substitutes, depending on their design, can be used entirely in place of the patient’s own bone tissue, referred to as an autograft, or by extending the volume of bone graft material from the patient by combining it with the bone graft substitute. Our products include demineralized bone matrices, collagen ceramic matrices, demineralized cancellous allograft bone and synthetic bone void fillers. We offer these products in the form of putties, pastes and strips for a range of surgical applications.
Demineralized Bone Matrix Technology
Demineralized bone matrix formulations are designed to provide proteins and other growth factors at varying stages of the bone healing process. Developed in the early 1990s, our first-generation demineralized bone matrix formulations combined particulate-demineralized bone matrix with an inert carrier engineered for easy graft handling and graft containment. The inert carrier is a highly biocompatible synthetic polymer, known as a reverse-phase medium, and has a unique property which allows the product to remain moldable at room temperature, but becomes more viscous at body temperature once implanted. In 2002, we developed a proprietary process to transform particulate-based demineralized bone matrix into a dispersed form in order to enhance the performance of the graft material. The result of this process was our second-generation demineralized bone matrix, which we refer to as Accell Bone Matrix. Accell Bone Matrix is an open structured, dispersed form of DBM, which provides accessibility
8
to bone proteins without the need to be broken down following implantation in the surgical site. Standard particulate DBM is dense and requires more time to break down. Until these dense particles break down, access to natural bone proteins is limited. Our third-generation and most advanced demineralized bone matrix solution, marketed as Accell Evo3 and OsteoSurge 300, provides an optimized formulation of Accell Bone Matrix, particulate-based demineralized bone matrix, and our reverse-phase medium carrier. Our third-generation products have an advanced handling property for bone grafting procedures and contain three times the amount of the Accell Bone Matrix compared to our second-generation technology.
Accell Technology
Our proprietary Accell technology combines our patented highly dispersed Accell Bone Matrix with a standard particulate-based demineralized bone matrix. Using a process of demineralization during manufacturing, mineral is carefully removed from the underlying organic structure, leaving behind a framework of densely packed type-1 collagen and the natural array of osteoinductive bone proteins, including bone morphogenetic proteins (“BMPs”), such as BMP-2, BMP-7 and BMP-4, and Transforming Growth Factor Beta 1. While the demineralization process allows access to the osteoinductive bone proteins, this standard particulate-form of demineralized bone matrix structure requires the body to break down the dense collagen structure in order to gain access to osteoinductive bone proteins. By contrast, during the Accell Bone Matrix production process, normal particulate-based demineralized bone matrix is converted into Accell Bone Matrix by carefully disrupting and dispersing the dense particles. This process yields a matrix with increased surface area providing for more rapid availability of the natural array of osteoinductive bone proteins. We believe that providing both the early-stage and late-stage accessibility of osteoinductive bone proteins provided by a composite of Accell Bone Matrix and the particulate-based demineralized matrix makes our product unique compared to competitive demineralized bone matrix products. The Pure Strip is a pre-shaped demineralized bone implant with an open matrix allowing bone ingrowth and providing exposure to a range of growth factors and BMP's. When hydrated, the implants can be contoured to the defect site.
Collagen Ceramic Matrix Technologies
Our collagen ceramic matrix technology leverages our long history of experience in regenerative technology and collagen engineering. Our leading products in this category are currently marketed as IsoTis Mozaik and OsteoStrux and are specifically engineered to provide a porous scaffold architecture and osteoconductivity. These products also support osteogenesis, as they are indicated for use with bone marrow aspirate, which contains osteogenic cells. They are composed of highly purified beta-tricalcium phosphate granules, which provide mineral content to foster bone formation during the healing process in a framework of type-1 collagen that provides a scaffold for bone cell migration. These products are engineered with a resorption profile consistent with the rate of natural bone formation.
Other Bone Graft Substitutes
Our other bone graft substitutes products consist of allograft cancellous bone scaffolds and synthetic bone void fillers.
Spinal Fusion Hardware
Our spinal fusion hardware portfolio consists of an extensive line of products for spinal fusion in MIS, complex spine, deformity and degenerative procedures throughout the lumbar, thoracic and cervical regions of the spine.
Minimally Invasive Surgery
Our MIS products enable a surgeon to perform a procedure less invasively than traditional open surgery, which may result in reduced postoperative pain, faster rates of healing and fewer procedure complications by minimizing incision size and tissue dissection. Our surgeon customers utilize our iPassage™ MIS Retractors and NewPort™ Tube Retractors to perform MIS fusions and decompression procedures, a surgical technique used to alleviate pain caused from compression on the spinal cord or the nerves that emanate from it. During the procedure, the surgeon makes a small incision and inserts the retractor through the skin and soft tissues down to the spinal column, creating a tunnel to the spine. The retractor is kept in place to hold the muscles open throughout the procedure. Through this tunnel, the surgeon accesses the spine, using small instruments inserts any implants necessary for fusion, such as the screws and rods of our Coral® MIS and NewPort MIS solutions. The Coral MIS product offers a mini-open muscle splitting rod delivery option for surgeons new to MIS procedures. The NewPort MIS product features extended tabs for a small incision profile and two rod delivery options for both mini-open and percutaneous approaches. Our MIS portfolio also includes interbody devices and screw systems that facilitate access to the treatment area while providing minimal anatomical disruption. These include our expandable interbody device, which is designed to minimize the amount of implant insertion force needed and an endoscopy system, which includes a complete set of decompression instruments.
Complex Spine and Deformity
9
Our spinal fusion hardware products are used in complex spine and deformity procedures involving multiple spine segments, challenging anatomy, tumors, traumatic injury and revision of previous fusion surgeries. Our complex fusion hardware portfolio allows surgeons to combine various product lines and approaches, offering several treatment options for the most difficult cases. We define deformity as any variation in the natural curvature of the spine, the most common of which is scoliosis, an abnormal lateral curvature of the spine. Our deformity platform consists of several technologies to address the needs of our deformity surgeons and the various derotation techniques that they use to correct the curvature of the spine. For example, our Daytona Deformity System addresses complex deformity cases by utilizing extended tab uniplanar and polyaxial screws with multiple rod options and intuitive instrumentation to create a versatile system adaptable to surgeon preference. Our systems are provided in multiple configurations and materials to address patient requirements, including stainless steel, titanium alloy and cobalt chrome alloy rod options, as well as 5.5 millimeter and 6.35 millimeter rod diameters. The ability to offer products with varying rod diameter and materials provides the surgeon different rod stiffness to treat individual patients. We offer both implant- and instrument-based reduction capabilities with our extended tab and locking cap products as well as our uniplanar and D-planar screws and rapid sequential reduction towers.
Degenerative
Our degenerative products include systems that are typically used in open procedures. Open procedures are still the most common surgical approach and involve a midline incision followed by retraction of the skin and soft tissues. We offer an extensive portfolio of degenerative products that are designed for use in both thoracolumbar and cervical spine cases.
Our Hollywood and Ventura NanoMetalene Interbody Device for TLIF procedures fuse the anterior column of the spine through a posterior approach that starts off to one side of the patient’s back and our Cambria NanoMetalene Interbody Device fuses the cervical spine through an anterior approach. These devices are composed of PEEK-OPTIMA® polymer, which has undergone a proprietary process that creates a titanium coating around the entire implant. We believe that this coating process has significant advantages over existing materials as it allows for the surface benefits of titanium, which is believed by scientists to encourage bone growth and cell migration, without compromising the mechanical and imaging benefits of PEEK-OPTIMA. In addition, the ultrathin NanoMetalene coating does not impair postoperative imaging, allowing surgeons to view the operative area and determine the extent of fusion of the vertebral bodies. We will continue to introduce new products for thoracolumbar and cervical applications that incorporate this unique NanoMetalene coating technology.
Thoracolumbar
We offer a comprehensive portfolio of products for the thoracic and lumbar regions of the spine, consisting of rods, screws and instrumentation for posterior lumbar fusion and a broad range of anterior, posterior and lateral interbody devices (“IBDs”), including stand-alone, zero-profile and low-profile systems and traditional PEEK-OPTIMA and innovative NanoMetalene-coated devices. Our Malibu and Coral screw and plating systems are our core products used for treating degenerative thoracolumbar spine cases. Both the Malibu and Coral screw and plating systems offer a full range of screw sizes, rod materials and lengths and unique locking caps, which minimize cross-threading and fully capture the rod.
Cervical
We offer a range of devices to treat disorders in the cervical region of the spine. Our degenerative cervical portfolio includes a full range of interbody devices including stand-alone, zero-profile systems, integrated plate interbody devices and traditional PEEK-OPTIMA and innovative NanoMetalene-coated interbody systems. In addition, we offer a variety of screw and plating systems.
Product Pipeline
We are committed to supplementing our portfolio of orthobiologics and spinal fusion hardware products through continuous innovation and bringing next-generation products to the market. We have more than ten products currently in our development pipeline, with a focus on MIS, complex spine, deformity and degenerative procedures, including advanced coating technology, as well as extensions of our orthobiologics products offering to further differentiate this portfolio from those of our competitors.
We are in the process of launching our MIS Facet Screw, a comprehensive system with a variable washer for more bone contact and a locking screwdriver to enhance stability as well as our MIS Spinous Process Clamp, a low profile, small footprint plating system. We are also launching our Smart TLIF device, a sterile packed TLIF cage prefilled with a synthetic graft material (outside the United States only).
10
Over the next 24 months, we plan to continue to build our portfolio and expect to launch a greater number of new products than we did in the past 24 months. Some of the products in our development pipeline include a next generation pedicle screw platform, a consolidated cervical stand-alone system, and our NewPort complex MIS extension. We also plan to add our NanoMetalene coating technology to more of our commercialized PEEK-OPTIMA IBD products including our ALIF and lateral systems as well as hyperlordotic cages, highly angled IBDs, which are used to ensure appropriate spine curvature.
Research and Development
We have an established research and development organization dedicated to advancing our portfolio of orthobiologics and spinal fusion hardware products. Our clinical and regulatory personnel work in parallel with our product engineering personnel to facilitate regulatory clearances of our orthobiologics and spinal fusion hardware products. These teams work in close collaboration with our surgeon customers to design technologies that will aid us in increasing our competitive advantage in the United States and international markets. We have recently invested in, and intend to continue to invest in, additional resources to increase our product development efforts by expanding the size of our current orthobiologics and spinal fusion hardware product development and clinical affairs teams and by integrating both teams together in our new Carlsbad facility to better collaborate to serve the design needs of our surgeon customers and develop market ready next-generation products.
We plan to create new, innovative orthobiologics technologies that will continue to reduce the amount of autologous bone graft needed for spinal fusion procedures by extending the volume of harvested material or replacing the need for such harvesting altogether. Therefore, we are dedicated to developing technologies that have the appropriate balance of osteoinductive, osteoconductive and osteogenic properties. Our orthobiologics research and development team has extensive experience in biomaterial sciences and bringing next generation technologies to market. In addition, we collaborate with surgeons and key opinion leaders to evaluate and design new products to ensure greater acceptance of our products.
We are also committed to developing new spinal fusion hardware products that leverage our innovative NanoMetalene technology and provide next generation solutions for our existing products or extend the range of solutions that we provide. One of our primary focuses in developing new spinal fusion hardware products is to further build out our complex spine and deformity procedures platform. One particular area of effort is developing products for pediatric populations including indications in small stature pediatric deformity as well as technologies that support growth. Our organization is also committed to providing products, such as hyperlordotic cages and additional expandable technology solutions, to achieve appropriate curvature of the spine and that can improve sagittal balance, correcting the patient’s spinal alignment so that their head and shoulders are above their hips so that the patient does not lean forward. We also plan to continue to develop next generation technologies that meet global demand, particularly with respect to cost and delivery methods in a manner which supports a scalable commercial model.
Our product development efforts employ an integrated team approach that involves collaboration between surgeons, our highly skilled engineers, our machinists, as well as our regulatory personnel. Our product development team, in consultation with designing surgeons, formulates a design for the product and then our machinists build prototypes for testing in our prototyping development and testing operation at our Vista, California facility. We utilize a broad scope of technologies to allow us to meet the complex engineering related to customer requirements. As part of the development process, spine surgeons test the implantation of the products in our in-house cadaveric laboratory to ensure that all new products meet the needs of both surgeon and patient. Our team refines or redesigns the prototype as necessary based on the results of the product testing, allowing us to perform rapid iterations of the design-prototype-test development cycle. We believe that these product development efforts allow us to provide solutions that respond to the needs of neurosurgeons and orthopedic spine surgeons and their patients.
In 2016, we began the process of moving our cadaveric training laboratory and our prototyping development and testing operation from our Vista, California facility and our orthobiologics product development laboratory from our Irvine, California facility into our new Carlsbad facility. We believe that this investment will provide for better collaboration between our orthobiologics and spinal fusion hardware product development teams and further our mission to provide surgeons with the most advanced and effective spinal surgery technologies to improve the quality of patients’ lives. We expect to complete these moves into our Carlsbad facility in the second quarter of 2016.
Global Spine Community Involvement
As a key part of our strategy we continuously educate and collaborate with surgeons globally to develop and market our technologies, as well as maintain active involvement in the global spine surgeon community. We believe surgeon education on the most effective use of our products is critical to our ability to help our customers realize the value potential of our products. We provide remote and on-site cadaver training throughout the year for surgeons. Our Vista, California facility has a cadaveric laboratory which enables us to conduct hands-on training to communicate the safe and most effective use of our products.
11
In addition to surgeon education, we solicit feedback from surgeons throughout the product development process and during post market evaluation. We also work with healthcare professionals in the area of clinical research in order to support the necessary requirements for product clearances and registrations. Surgeons also actively support the training of sales agents and other salesforce personnel on end-user functionality of our products.
Sales and Distribution
We currently market and sell our products in the United States and in over 30 countries worldwide. Our U.S. sales organization consists of regional business managers who oversee direct orthobiologics sales specialists and a broad network of independent orthobiologics and spine sales agents. Our international sales organization is composed of a sales management team that oversees a network of independent orthobiologics and spine stocking distributors.
In the United States, we typically consign our orthobiologics products and consign or loan our spinal fusion hardware sets to hospitals and independent sales agents, who in turn deliver them to the hospital for a single surgical procedure or leave them with hospitals that are high volume users for use in multiple procedures. The spinal fusion hardware sets typically contain the instruments, including disposables, and spinal implants required to complete a surgery. Our sales are generated by building and maintaining relationships with the neurosurgeons and orthopedic spine surgeons who use our products in surgeries or from the hospitals that order our products directly. In international markets, we predominantly sell complete instrument and implant sets to our independent spine stocking distributors, who consign or loan these sets to surgeons. Our international sales organization is composed of a sales management team that oversees a network of independent orthobiologics and spine stocking distributors in over 30 countries that purchase our products directly from us and independently sell them. We maintain sales and marketing personnel in Switzerland and France to manage and support our stocking distributors in Europe and use third-party distribution facilities in Belgium and The Netherlands to support international distribution efforts.
We recently increased the size of our spinal fusion hardware sales management team and independent sales agents in 2015 and we anticipate adding additional independent sales agents in the United States in 2016. We also plan to invest in additional instrument sets and marketing and education efforts to support this expansion. Internationally, we intend to focus our sales and marketing efforts on expanding our presence in those markets where we currently have relationships with stocking distributors. We believe the expansion of our U.S. sales efforts will provide us with significant opportunity for future growth as we continue to penetrate existing and new markets.
Suppliers and Raw Materials
In general, raw materials essential to our businesses are readily available from multiple sources. For reasons of quality assurance, availability or cost effectiveness, certain components and raw materials are available only from sole suppliers. Our relationships with such suppliers that could not be replaced without a material expense or delay are governed by written contracts which are generally supply agreements. These agreements set forth the process by which we order components or raw materials, as applicable, from such suppliers (which process is either on a purchase order basis or based on quarterly or annual forecasts and in some cases require us to purchase minimum amounts) and the related fees for purchasing such components or raw materials. These agreements have terms from one to five years, but in most instances are terminable by us (and in limited instances the other party) for convenience, subject to a specified notice period, and are also terminable upon mutual agreement by the parties, by either party upon material breach by the other and by either party in the event the other party enters bankruptcy. These agreements also outline the rights of each party with respect to quality assurance, inspection and compliance with applicable law and contain what we believe to be customary indemnification provisions for commercial agreements. Each of these agreements is entered into in the ordinary course of our business, immaterial in amount and significance and not a contract upon which our business is substantially dependent. In addition, our policy is to maintain sufficient inventory of components and raw materials so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time.
Most of our biomaterial products contain material derived from human or bovine tissue. We take great care to provide products that are safe and free of agents that can cause disease. Only donated tissue from FDA-registered and AATB accredited tissue banks is qualified to source for our raw materials. The donors are rigorously screened, tested and processed by the tissue banks in accordance with FDA and AATB requirements. Additionally, each donor must pass all of the FDA-specified bacterial and viral testing before the raw material is distributed to us for further processing. We receive with each donor lot a certification of the safety of the raw material from the tissue bank’s medical director. As an added assurance of safety, each lot of bone is released into the manufacturing process only after our staff of quality assurance microbiologists screen the incoming bone and serology test records. During our manufacturing process, the bone particles are subjected to our proprietary process and terminally sterilized. We have demonstrated through our testing that this type of rigorous processing supports the safety and effectiveness of our demineralized bone material products.
12
The collagen used in our collagen ceramic matrix products is derived only from the deep flexor tendon of cattle less than 24 months old from the United States or New Zealand. The World Health Organization classifies different types of cattle tissue for relative risk of BSE transmission. Deep flexor tendon is in the lowest-risk category for BSE transmission (the same category as milk, for example) and is therefore considered to have a negligible risk of containing the agent that causes BSE (an improperly folded protein known as a prion).
Intellectual Property
We seek patent and trademark protection for our key technology, products and product improvements, both in the United States and in selected foreign countries. When determined appropriate, we plan to continue to enforce and defend our patent and trademark rights. In general, however, we do not rely solely on our patent and trademark estate to provide us with any significant competitive advantages as it relates to our existing product lines. We also rely upon trade secrets and continuing technological innovations to develop and maintain our competitive position. In an effort to protect our trade secrets, we have a policy of requiring our employees, consultants and advisors to execute proprietary information and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements also provide that all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential, except in specified circumstances.
IsoTis OrthoBiologics, Inc., one of our subsidiaries, owns a group of patents (6 U.S. patents and 9 foreign patents) related to reverse phase medium and the Accell process and materials. This patent group protects the Accell family of demineralized bone matrix products. The patents in this group will expire over a period of time from 2017 to 2023.
SeaSpine has licensed three U.S. patents related to certain of our pedicle screw systems from Dr. Thomas T. Haider. The license agreement, as amended, will expire when the last-to-expire licensed patent expires unless terminated earlier. The licensed patents will all expire in December, 2016. The license agreement may be terminated by either party upon any material breach by the other party, with such termination effective sixty days after giving written notice to the breaching party unless cured by the breaching party within such sixty day period. The products covered under these license agreements constitute approximately 10% of SeaSpine’s total revenue.
Our material registered and unregistered trademarks include: Accell®, Evo3®, Accell Evo3®, Accell Evo3®C, DynaGraft® II , IsoTis®, IsoTis OrthoBiologics®, OrthoBlast® II , AtollTM, CapistranoTM, Coral®, Daytona®, HollywoodTM, MalibuTM, NanoMetalene®, NewPortTM, Vu aPODTM/Vu aPODTM Prime, OsteoSurge® 100 (or 300), SeaSpine®, SierraTM and SonomaTM.
Competition
We participate in the highly competitive global orthobiologics and spine markets. We face significant competition in both of these markets from the spine divisions of large multinational medical device companies as well as smaller, emerging spine players focused on product innovation. These competitors are focused on bringing new technologies to market and acquiring technologies and technology licenses that directly compete with our products or have potential product advantages that could render our products obsolete or noncompetitive.
Our primary competitors in the combined orthobiologics and spinal fusion hardware markets include Alphatec, XTANT Medical, Baxter, Biomet, DePuy Synthes Spine (a Johnson & Johnson company) Globus Medical, Medtronic, NuVasive, K2M, LDR, Orthofix, RTI Surgical, Stryker and Zimmer and several smaller, biologically focused companies.
We anticipate that our currently marketed products and any future products will be subject to intense competition. Many of our current competitors have significantly greater financial, manufacturing and marketing resources than we do, which could make the ability to scale our business challenging. As a result, these competitors have more tenured relationships with distribution channels and we anticipate they will continue to dedicate significant resources to marketing and distributing their products. Our ability to compete will depend on our ability to launch innovative new products, garner strong relationships with surgeons, partner with key opinion leaders and demonstrate superior clinical outcomes. Because of the size of the spine market, we expect that companies will continue to dedicate significant resources to developing and commercializing competing products.
Regulation
We are a manufacturer and marketer of medical devices and a tissue bank, and therefore are subject to extensive regulation by the FDA and the Center for Medicare Services of the U.S. Department of Health and Human Services and other federal governmental agencies and, in some jurisdictions, by state and foreign governmental authorities. These regulations govern the introduction of new medical devices, the observance of certain standards with respect to the design, manufacture, testing, labeling (such as issuing a final rule in 2013 for a UDI for virtually all medical devices), promotion and sales of the devices, the maintenance
13
of certain records, the ability to track devices, the reporting of potential product defects, the import and export of devices, and other matters.
The regulatory process of obtaining product approvals and clearances can be onerous and costly. The FDA requires, as a condition to marketing a medical device in the United States that we secure a Premarket Notification clearance pursuant to Section 510(k) of the FDCA or an approved PMA application (or PMA supplement). Obtaining these approvals and clearances can take up to several years and may involve preclinical studies and clinical trials. The FDA may also require a post-approval clinical trial as a condition of approval.
To perform clinical trials for significant risk devices in the United States on an unapproved product, we are required to obtain an Investigational Device Exemption from the FDA. The FDA may also require a filing for FDA approval prior to marketing products that are modifications of existing products or new indications for existing products. Moreover, after clearance/approval is given, if the product is shown to be hazardous or defective, the FDA and foreign regulatory agencies have the power to withdraw the clearance or require us to change the device, its manufacturing process or its labeling, to supply additional proof of its safety and effectiveness or to recall, repair, replace or refund the cost of the medical device.
The FDA Safety and Innovation Act of 2012 (the “FDASIA”), which includes the Medical Device User Fee Amendments of 2012, as well as other medical device provisions, went into effect October 1, 2012. This includes performance goals and user fees paid to the FDA by medical device companies when they register and list with the FDA and when they submit an application to market a device in the United States. The FDASIA also imposes some additional requirements regarding FDA Establishment Registration and Listing of Medical Devices. All U.S. and foreign manufacturers must have a FDA Establishment Registration and complete Medical Device listings for sales in the United States.
SeaSpine manufactures medical devices derived from human tissue (demineralized bone tissue). The FDA has specific regulations governing HCT/Ps. An HCT/P is a product containing, or consisting of, human cells or tissue intended for transplantation into a human patient. Examples include bone, ligament, skin and cornea. Some HCT/Ps fall within the definition of a biological product, medical device or drug regulated under the FDCA. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to HCT/Ps and, in addition, with requirements applicable to biologics, devices or drugs, including premarket clearance or approval from the FDA.
Section 361 of the Public Health Service Act, authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as 361 HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, Good Tissue Practice when processing, storing, labeling, and distributing HCT/Ps, including required labeling information, stringent record keeping, and adverse event reporting.
The AATB has issued operating standards for tissue banking. Accreditation is voluntary, but compliance with these standards is a requirement in order to become an AATB-accredited tissue establishment. In addition, some states have their own tissue banking regulations. We are licensed or have permits for tissue banking in California, Florida, New York, Maryland, and other states that require specific licensing or registration.
National Organ Transplant Act. Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the NOTA, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks for their expenses associated with the recovery, storage and transportation of donated human tissue that they provide to us for processing. We include in our pricing structure amounts paid to tissue banks to reimburse them for their expenses associated with the recovery and transportation of the tissue, in addition to certain costs associated with processing, preservation, quality control and storage of the tissue, marketing and medical education expenses, and costs associated with development of tissue processing technologies. NOTA payment allowances may be interpreted to limit the amount of costs and expenses that we may recover in our pricing for our products, thereby reducing our future revenue and profitability.
Postmarket Requirements. After a device is cleared or approved for commercial distribution, numerous regulatory requirements apply. These include, but are not limited to, the FDA’s Quality System Regulations which cover the procedures and documentation of the design, testing, production processes, controls, quality assurance, labeling, packaging, storage and shipping of medical devices; the FDA’s general prohibition against promoting products for off-label uses; the Federal Medical Device Reporting regulation, which requires that manufacturers provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or that a malfunction occurred which would be likely to cause or contribute to a death or serious injury upon recurrence; and the Reports of Corrections and Removals regulation,
14
which requires manufacturers to report recalls and field corrective actions to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FDCA.
We are also required to register with the FDA as a medical device manufacturer. As such, our manufacturing sites are subject to periodic inspection by the FDA for compliance with the FDA’s Quality System Regulations. These regulations require that we manufacture our products and maintain our documents in a prescribed manner with respect to design, manufacturing, testing and control activities. Further, we are required to comply with various FDA requirements and other legal requirements for labeling and promotion. If the FDA believes that a company is not in compliance with applicable regulations, it may issue a warning letter, institute proceedings to detain or seize products, issue a recall order, impose operating restrictions, enjoin future violations and assess civil penalties against that company, its officers or its employees and may recommend criminal prosecution to the U.S. Department of Justice (DOJ).
Medical device regulations also are in effect in many of the countries in which we do business outside the United States. These laws range from comprehensive medical device approval and Quality System requirements for some or all of our medical device products to simpler requests for product data or certifications. The number and scope of these requirements are increasing. Under the EU Medical Devices Directive, medical devices must meet the Medical Devices Directive requirements and receive CE Mark Certification prior to marketing in the EU. CE Mark Certification requires a comprehensive Quality System program, comprehensive technical documentation and data on the product, which are then reviewed by a Notified Body. A Notified Body is an organization designated by the national governments of the EU member states to make independent judgments about whether a product complies with the requirements established by each CE marking directive. The Medical Devices Directive, ISO 9000 series and ISO 13485 are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. Other countries are also instituting regulations regarding medical devices. Compliance with these regulations requires extensive documentation and clinical reports for all of our products, revisions to labeling, and other requirements such as facility inspections to comply with the registration requirements. A recognized Notified Body audits our facilities annually to verify our compliance with these standards.
In the EU, our products that contain human-derived tissue, including demineralized bone material, are not medical devices as defined in the Medical Devices Directive (93/42/EC). They are also not medicinal products as defined in Directive 2001/83/EC. Today, regulations, if applicable, are different from one EU member state to the next. Because of the absence of a harmonized regulatory framework and the proposed regulation for advanced therapy medicinal products in the EU, the approval process for human-derived cell or tissue-based medical products may be extensive, lengthy, expensive, and unpredictable.
Certain countries, as well as the EU, have issued regulations that govern products that contain materials derived from animal sources. Regulatory authorities are particularly concerned with materials infected with the agent that causes BSE. These regulations affect our biomaterial products for the spine, which contain material derived from bovine tissue. Although we take great care to provide that our products are safe and free of agents that can cause disease, products that contain materials derived from animals, including our products, may become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for prion transmission. Significant new regulations, a ban of our products, or a movement away from bovine-derived products because of an outbreak of BSE could have a material and adverse effect on our current business or our ability to expand our business. See “Risk Factors-Risks Relating to Our Regulatory Environment-Certain of our products contain materials derived from animal sources and may become subject to additional regulation.”
We are subject to laws and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws that regulate the means by which companies in the health care industry may market their products to hospitals and health care professionals and may compete by discounting the prices of their products. The delivery of our products is subject to regulation regarding reimbursement, and federal healthcare laws apply when a customer submits a claim for a product that is reimbursed under a federally funded healthcare program. These rules require that we exercise care in structuring our sales and marketing practices and customer discount arrangements. See “Risk Factors-Risks Relating to Our Regulatory Environment-Oversight of the medical device industry might affect the manner in which we may sell medical devices and compete in the marketplace.”
Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, import-export, laws regarding transactions in foreign countries, the FCPA and local anti-bribery and other laws regarding interactions with healthcare professionals. Among other things, these laws restrict, and in some cases prohibit, United States companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices in foreign countries.
Our research, development and manufacturing processes involve the controlled use of certain hazardous materials. We are subject to country-specific, federal, state and local laws and regulations governing the use, manufacture, storage, handling and
15
disposal of these materials and certain waste products. We believe that our environmental, health and safety (“EHS”) procedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations. However, risk of accidental releases or injury from these materials is possible. These risks are managed to minimize or eliminate associated business impacts. In the event of this type of accident, we could be held liable for damages that may result, and any liability could exceed our resources. We could be subject to a regulatory shutdown of a facility that could prevent the distribution and sale of products manufactured there for a significant period of time and we could suffer a casualty loss that could require a shutdown of the facility in order to repair it, any of which could have a material and adverse effect on our business. Although we continuously strive to maintain full compliance with respect to all applicable global EHS laws and regulations, we could incur substantial costs to fully comply with future laws and regulations, and our operations, business or assets may be impacted.
In addition to the above regulations, we are and may be subject to regulation under country-specific federal and state laws, including, but not limited to, requirements regarding record keeping, and the maintenance of personal information, including personal health information. As a public company, we are subject to the securities laws and regulations, including the Sarbanes-Oxley Act. We also are subject to other present, and could be subject to possible future, local, state, federal and foreign regulations.
Reimbursement Overview
Healthcare providers that purchase medical devices generally rely on third-party payors, including the Medicare and Medicaid programs and private payors, such as indemnity insurers, employer group health insurance programs and managed care plans, to reimburse all or part of the cost of the products. As a result, demand for our products is and will continue to be dependent in part on the coverage and reimbursement policies of these payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. Reimbursement from Medicare, Medicaid and other third-party payors may be subject to periodic adjustments as a result of legislative, regulatory and policy changes as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products, as a result of these changes may affect our customers’ revenue and ability to purchase our products. Any changes in the healthcare regulatory, payment or enforcement landscape relative to our customers’ healthcare services has the potential to significantly affect our operations and revenue.
Employees
As of February 29, 2016 we had approximately 300 employees, 33 of whom were engaged in research and development, 108 in manufacturing, 109 in sales and marketing and 50 in general and administrative activities.
Available Information
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”). In accordance with the Exchange Act, we file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission. We make these reports available free of charge on our website at www.seaspine.com under the investor relations page as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Commission. All such reports were made available in this fashion during 2015.
The public can also obtain any documents that we file with the Commission at www.sec.gov. The public may read and copy any materials that we file with the Commission at the Commission’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the Commission at 1-800-SEC-0330.
ITEM 1A. RISK FACTORS
You should carefully consider the risks described below, together with all of the other information included in this Form 10-K, in evaluating the Company and our common stock. If any of the risks described below actually occurs, our business, financial results, financial condition and stock price could be materially and adversely affected.
Risks Relating to our Business
16
We expect to incur losses for the foreseeable future and cannot assure you that we will be able to generate sufficient sales to achieve or sustain profitability.
We expect to incur losses for the foreseeable future as we dedicate significant resources to our marketing and product development strategy, as well as incur increased general and administrative expenses due to operating as an independent public company following the spin-off. We intend to increase our operating expenses substantially relative to prior periods as we: (i) develop new and next generation products and product line extensions (all of which we refer to as “new products”); (ii) develop new medical techniques designed to enhance the utility of our products; (iii) collect clinical data and conduct clinical studies to differentiate our products from those of our competitors and to demonstrate the value of our products to current and prospective customers and payors; (iv) add independent sales agents and stocking distributors to increase our geographic sales coverage and penetration; (v) increase product inventory to raise the likelihood of success of new product launches; and (vi) expand our marketing campaigns and surgeon education and training programs. We cannot assure you that we will ever generate sufficient revenues from our operations to achieve profitability and, even if we achieve profitability, we cannot assure you that we will remain profitable over time. Our failure to achieve or maintain profitability could negatively affect the value of our securities and our ability to attract and retain personnel, raise capital, execute our business strategy or continue operations.
The industry and market segments in which we operate are highly competitive and, unless we successfully demonstrate to our neurosurgeon and orthopedic spine surgeon customers the merits of our products compared to those of our competitors, we may be unable to compete successfully.
There is intense competition among medical device companies that serve the spinal surgery market. We compete with established medical technology companies, as well as earlier-stage companies that often have differentiated technology and potentially superior solutions for the challenges facing our neurosurgeon and orthopedic spine surgeon customers and their patients. Many of our competitors may have access to greater financial, technical, research and development, marketing, manufacturing, sales, distribution, administrative, consulting and other resources than we do. Our competitors may be more effective at developing products, at differentiating their products from our and other competitor products and at designing, executing, analyzing the results of and publishing data from clinical studies, and they may have more established distribution networks, entrenched relationships with surgeons or greater experience in launching, marketing and selling products than we do. Our primary competitors include Alphatec, Bacterin, Baxter, Biomet, DePuy Synthes Spine (a Johnson & Johnson company), Globus Medical, Medtronic, NuVasive, K2M, LDR, Orthofix, RTI Surgical, Stryker and Zimmer, as well as several smaller, biologically-focused companies.
Our competitive position depends on our ability to achieve market acceptance for our current and future products. Market acceptance for any of our products requires, among other things, that we timely secure regulatory approval; demonstrate the value of our products, both to our surgeon customers and payors, which may require that we collect clinical data and/or conduct clinical studies; effectively educate and train surgeons and their staff on the proper use of our products; obtain and maintain coverage and adequate reimbursement for our products, both within and outside the U.S., including under Medicare and Medicaid and from private payors; attract and retain a network of independent sales agents and stocking distributors focused on neurosurgeons and orthopedic spine surgeons; develop and execute an effective marketing strategy; protect the proprietary positions of our products, including through patent protection; and consistently produce quality products in sufficient quantities to meet demand. There are significant risks associated with each of these activities and other activities required to achieve market acceptance of both our current and future products, some of which are more fully described elsewhere in this “Risk Factors” section.
Neurosurgeons and orthopedic spine surgeons play a significant role in determining the course of treatment and, ultimately, the product used to treat a patient. As a result, our success depends, in large part, on demonstrating to these surgeons the value of our products in the treatment of their patients. To do so requires that we, along with our independent sales agents and stocking distributors, demonstrate the merits of our products and underlying technology compared to those of our competitors. Surgeons who do not use our products may be hesitant to do so for the following or other reasons:
• | lack of experience with our products, techniques or technologies; |
• | existing relationships with those who sell competitive products; |
• | the time required for surgeon and medical staff education and training on new products, techniques and equipment; |
• | lack or perceived lack of clinical evidence supporting patient benefit relative to competing products; |
• | our products not being included on hospital formularies or integrated delivery network or group purchasing organization preferred vendor lists; |
• | less attractive coverage and/or reimbursement within healthcare payment systems for our products and procedures compared to other products and procedures; |
• | other costs associated with the introduction of new products and the equipment necessary to use new products; and |
17
• | perceived risk of liability that could be associated with the use of new products and techniques. |
If we are not successful in convincing surgeons of the merits of our products, or educating and training them and their staff on the proper use of our products, they may not use our products and, as a result, we may be unable to maintain or grow our sales or achieve or sustain profitability.
Changes in third-party payment systems and in the healthcare industry may require us to decrease the selling price for our products, may reduce the size of the market for our products, or may eliminate a market, any of which could have a material and adverse effect on our financial performance.
Our operations may be substantially affected by fundamental changes in the political, economic and regulatory landscape of the healthcare industry. Government and private sector initiatives to limit the growth of healthcare costs are continuing in the U.S., and in many other countries where we do business, causing the marketplace to put increased emphasis on the delivery of more cost-effective treatments. These initiatives include price regulation, competitive pricing, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements.
Maintaining and growing sales of our products depends on the availability of adequate coverage and reimbursement from third-party payors, both within and outside the U.S., including government programs such as Medicare and Medicaid, private insurance plans and managed care organizations. Hospitals and other healthcare providers that purchase our products generally rely on third-party payors to cover all or part of the costs associated with the procedures performed with our products, including the cost to purchase our product. Both the patients’ and our customers’ access to adequate coverage and reimbursement for the procedures performed with our products by government and private insurance plans is central to the acceptance of our current and future products. We may be unable to sell our products on a profitable basis, or at all, if third-party payors deny coverage or reduce their levels of payment. In addition, if our cost of production increases at a greater rate than increases in reimbursement levels for our products, our profitability may be adversely affected.
The healthcare industry, both within and outside the U.S., has experienced a trend toward cost containment as government and private insurers seek to control rising healthcare costs by imposing lower payment and negotiating reduced contract rates with service providers. Third-party payors continually review their coverage and reimbursement policies for procedures involving the use of our products and can, without notice, eliminate or reduce coverage or reimbursement for our products. For example, a major national third-party insurer in the U.S. recently reduced coverage (from all or most cases to limited indications) for biomechanical devices (e.g., spine cages) used in cervical fusion procedures, stating that the devices had not been shown to be more effective than bone graft. In addition, certain insurers have limited coverage for vertebral fusions in the lumbar spine and other insurers may adopt similar coverage decisions in the future. Patients covered by these insurers may be unwilling or unable to afford lumbar fusion surgeries to treat their conditions, which could materially harm or limit our ability to sell our products designed for such surgeries. Further, third-party payors of hospital services and hospital outpatient services annually revise their payment methodologies, which could result in stricter standards for or the elimination of reimbursement of hospital charges for certain medical procedures.
To the extent we sell our products internationally, market acceptance may depend, in part, upon the availability of coverage and reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country. As in the U.S., our products may not obtain coverage and reimbursement approvals in a timely manner, if at all, in a particular foreign market. In addition, even if we are able to obtain country-specific coverage and reimbursement approvals, we could incur considerable expense to do so. Our failure to obtain such coverage and approvals would negatively affect market acceptance of our products in the international markets in which such failure occurs and the expenses incurred in connection with obtaining such coverage and approvals could outweigh the benefits of obtaining them.
If the trend by governmental agencies and other third-party payors to reduce coverage of and/or reimbursement for procedures using our products continues, our business, results of operations and financial condition could be materially and adversely affected. Further, we cannot be certain that, under current and future payment systems, the cost of our products will be adequately incorporated into the overall cost of the procedure and, accordingly, we cannot be certain that the procedures performed with our products will be reimbursed at a cost-effective level, or at all.
Industry trends have resulted in increased downward pricing pressure on medical services and products, which may affect our ability to sell our products at prices necessary to support our current business strategy.
The trend toward healthcare cost containment and the growth of managed care organizations is placing increased emphasis on the delivery of more cost-effective medical therapies. For example:
18
• | There has been consolidation among healthcare facilities and purchasers of medical devices, particularly in the U.S. One of the results of such consolidation is that group purchasing organizations, integrated delivery networks and large single accounts use their market power to consolidate purchasing decisions, which in turn intensifies competition to provide products and services to healthcare providers and other industry participants, resulting in greater pricing pressures and the exclusion of certain suppliers from important market segments. For example, some group purchasing organizations negotiate pricing for its member hospitals and require us to discount, or limit our ability to raise, prices for certain of our products. |
• | Surgeons increasingly have moved from independent, out-patient practice settings toward employment by hospitals and other healthcare entities, which align surgeons’ product choices with their employers’ price sensitivities and adds to pricing pressures. Hospitals have introduced and may continue to introduce new pricing structures into their contracts to contain healthcare costs, including fixed price formulas and capitated and construct pricing. |
• | Certain hospitals provide financial incentives to doctors for reducing hospital costs (known as gainsharing), rewarding physician efficiency (known as physician profiling) and encouraging partnerships with healthcare service and goods providers to reduce prices. |
• | Existing and proposed laws, regulations and industry policies, in both domestic and international markets, regulate or seek to increase regulation of sales and marketing practices and the pricing and profitability of companies in the healthcare industry. |
More broadly, other provisions of the Affordable Care Act could meaningfully change the way healthcare is developed and delivered in the U.S., and may adversely affect our business and results of operations. For example, the Affordable Care Act encourages hospitals and physicians to work collaboratively through shared savings programs, such as accountable care organizations, as well as other bundled payment initiatives, which may ultimately result in the reduction of medical device purchases and the consolidation of medical device suppliers used by hospitals. There are many programs and requirements for which the details have not yet been fully established or consequences not fully understood, and it is unclear what the full impact of the legislation will be. We cannot predict accurately what healthcare programs and regulations will ultimately be implemented at the U.S. federal or state level, or the effect of any future legislation or regulation in the U.S. or elsewhere. However, any changes that lower reimbursements for our products or reduce medical procedure volumes could have a material and adverse effect on our business, financial condition and results of operations.
Further, the proliferation of medical device distributors that are owned, directly or indirectly, by physicians (commonly referred to as physician-owned distributorships, or PODs) could result in increased pricing pressure on our products or harm our ability to sell our products to physicians who own or are affiliated with these distributors. These physicians derive a proportion of their revenue from selling or arranging for the sale of medical devices for use in procedures they perform on their own patients at hospitals that agree to purchase from or through the POD, or that otherwise furnish ordering physicians with income that is based, directly or indirectly, on those orders of medical devices. The number of PODs in the spine industry may continue to grow as economic pressures increase throughout the industry and as hospitals, insurers and physicians search for ways to reduce costs and, in the case of the physicians, search for ways to increase their incomes. PODs and the physicians who own, or partially own, them have significant market knowledge and access to the surgeons who use our products and the hospitals that purchase our products. Growth in the number of PODs may reduce our ability to compete effectively for business from surgeons who own them, which could have a material and adverse effect on our business, results of operations and financial condition.
In addition, the largest device companies with multiple product franchises have increased their effort to leverage and contract broadly with customers across franchises by providing volume discounts and multi-year arrangements that could prevent our access to these customers or make it difficult (or impossible) to compete on price.
We may be unable to develop new products in a timely and consistent manner, and failure to do so may adversely affect the attractiveness of our overall product portfolio to our surgeon customers and negatively impact our sales and market share.
To be and remain competitive, we need to develop new products on a regular basis and respond to technological advances. Doing so is technologically challenging and involves significant risks and uncertainty. Despite substantial investments of time and resources, our research and development efforts may not result in technically feasible new products. Even if technically feasible, the anticipated time and cost of obtaining regulatory approval and/or commercializing a new product may be too great to justify continued development. Even if we determine to continue development, competitors could develop similar products that are more effective, are less expensive to manufacture, are priced more competitively or are ready for commercial introduction before our products. If we are unable to develop technically and commercially viable products on a consistent basis and before our competitors, our future prospects could be materially and adversely affected.
19
If we are unable to maintain and expand our network of independent sales agents and stocking distributors, we may not be able to maintain or grow our revenue.
Our ability to generate revenue depends on the sales and marketing efforts of independent sales agents and stocking distributors. Some of our independent sales agents account for a significant portion of our sales volume. If our independent sales agents and stocking distributors fail to adequately promote, market and sell our products, our sales could significantly decrease.
Further, we face significant challenges and risks in managing our geographically dispersed distribution network and retaining the independent sales agents and stocking distributors who make up that network, and as we launch new products and increase our marketing efforts with respect to existing products, we plan to expand the reach of our marketing and sales networks and may need to hire new independent sales agents and stocking distributors. Independent sales agents and stocking distributors require significant technical expertise in various areas-such as spinal care practices, spine injuries and disease and spinal health-and they require training and time to achieve full productivity. Because of the intense competition for their services, we may be unable to attract or retain additional qualified independent sales agents or stocking distributors or be able to enter into agreements with them on favorable or commercially reasonable terms, if at all. Our success will depend largely on our ability to continue to hire, train, retain and motivate qualified independent sales agents and stocking distributors. If we are unable to expand our sales and marketing capabilities domestically and internationally, if we fail to train new independent sales agents and stocking distributors adequately, or if we experience high turnover in our sales network, we may not be able to commercialize our products adequately, or at all, which would adversely affect our business, results of operations and financial condition.
Moreover, our independent sales agents and stocking distributors are not our employees, we have limited control over their activities and, generally, we do not enter into exclusive relationships with them. If one or more of them were to be retained by a competitor, whether or an exclusive or non-exclusive basis, they may divert business from us to our competitor, which could materially and adversely affect our sales.
Sales of, or the price at which we sell, our products may be adversely affected unless the safety and efficacy of our products, alone and relative to competitive products, is demonstrated in clinical studies.
Generally, we have obtained 510(k) clearance to manufacture, market and sell the products we market in the U.S. and the right to affix the CE mark to the products we market in the European Economic Area, or EEA. However, to date, we have not been required to generate new clinical data to support our current 510(k) clearances, CE marks, or product registrations in other countries and, accordingly, we do not have our own clinical data regarding our currently marketed products. Certain of our competitors have clinical data supporting the safety and efficacy of their products, which may place our products at a competitive disadvantage and materially and adversely impact our revenues.
In part due to the increased emphasis on the delivery of more cost-effective treatments, purchasing decisions of our customers increasingly will be based on clinical data that demonstrates the value of our products or the effectiveness of our products relative to others. Conducting clinical studies is expensive and time-consuming and outcomes are uncertain. See “Risks Relating to Our Regulatory Environment-Clinical studies are expensive and subject to extensive regulation and their results may not support our product candidate claims or may result in the discovery of adverse effects,” below. We may elect not to, or may be unable to, fund the clinical studies necessary to generate the data required for all of our products to compete effectively, in part due to the breadth our product portfolio. Currently, we do not expect to undertake such clinical studies for all of our products and will only do so where we anticipate the benefits will outweigh the costs on a risk-adjusted basis. However, even when we elect and are able to fund such clinical studies on one or more of our products, they may not be successful. Data we generate may not be consistent with our existing data and may demonstrate less favorable safety or efficacy, which could reduce demand for our products and negatively impact future sales. Neurosurgeons and orthopedic spine surgeons may be less likely to use our products if more robust, or any, clinical data supporting the safety and efficacy of competing products is available. If we are unable to or unwilling to generate clinical data supporting the safety and efficacy of our products, our business, results of operations and financial condition could be materially and adversely affected.
If any of our manufacturing, development or research facilities are damaged and/or our manufacturing processes are interrupted, we could experience supply disruptions, lost revenues and our business could be seriously harmed.
Damage to our manufacturing, development or research facilities or disruption to our business operations for any reason, including natural disaster (such as earthquake, wildfires and other fires or extreme weather), power loss, communications failure, unauthorized entry or other events, such as a flu or other health epidemic, could cause us to discontinue development and/or manufacturing of some or all of our products for an undetermined period of time. In addition, our facilities would be difficult to replace and would require substantial lead time to repair or replace. The property damage and business interruption insurance coverage on these facilities that we maintain might not cover all losses under such circumstances, and we may not be able to renew
20
or obtain such insurance in the future on acceptable terms with adequate coverage or at reasonable costs. In particular, we manufacture our orthobiologics products in one facility located in Irvine, California and any damage to that facility could adversely affect our ability to timely satisfy demand for those products. Any significant disruption to our manufacturing operations and to our ability to meet market demand likely would have an adverse impact on our sales and revenues as key stakeholders, including our independent sales agents and stocking distributors and surgeon customers, transition to what they perceive as more reliable sources of products.
We are dependent on a limited number of third-party suppliers for components and raw materials and the loss of any of these suppliers, or their inability to provide us with an adequate supply of materials that meet our quality and other requirements, could harm our business.
Outside suppliers, some of whom are sole-source suppliers, provide us with spine hardware products and raw materials and components used in the manufacture of our orthobiologics and hardware products. We strive to maintain sufficient inventory of spine hardware products, raw materials and components so that our production will not be significantly disrupted if a particular product, raw material or component is not available to us for a period of time. Although we believe there are alternative supply sources, replacing our current suppliers may be impractical or difficult in many instances. For example, we could have difficulty obtaining similar products from other suppliers that are acceptable to the U.S. Food and Drug Administration, or the FDA, or other foreign regulatory authorities. If we are unable to obtain sufficient quantities of spine hardware products, raw materials or components that meet our quality and other requirements on a timely basis for any reason, we may not be able to produce sufficient quantities of our products to meet market demand until a new or alternative supply source is identified and qualified and, as a result, we could lose customers, our reputation could be harmed and our business could suffer. In 2013, we experienced supply shortages in collagen ceramic matrix bone void fillers, which adversely affected sales of our orthobiologics products, even after the supply shortage was resolved.
Further, the FDA Safety and Innovation Act of 2012, or the FDASIA, which includes the Medical Device User Fee Amendments of 2012, as well as other medical device provisions, went into effect October 1, 2012. Under FDASIA, all U.S. and foreign manufacturers must have a FDA Establishment Registration and complete Medical Device listings for sales in the U.S. While our facilities materially comply with these requirements, we also source products from foreign contract manufacturers. It is possible that some of our foreign contract manufacturers will not comply with applicable requirements and choose not to register with the FDA. In such an event, we will need to determine if there are alternative foreign contract manufacturers who comply with the FDA Establishment Registration requirements. If such a foreign contract manufacturer is a sole supplier of one of our products, there is a risk that we may not be able to source another supplier.
Furthermore, an uncorrected defect or supplier’s variation in a component or raw material that is incompatible with our manufacturing, unknown to us, could harm our ability to manufacture products.
In addition, we rely on a small number of tissue banks accredited by the American Association of Tissue Banks for the supply of human tissue, a crucial component of our orthobiologics products that serve as bone graft substitutes. We cannot be certain that these tissue banks will be able to fulfill our requirements or that we will be able to successfully negotiate with other accredited tissue facilities on commercially reasonable terms.
We are dependent on information technology and if our information technology fails to operate adequately or fails to properly maintain the integrity of our data, our business could be materially and adversely affected.
We depend significantly on sophisticated information technology, or IT, for our infrastructure and to support business decisions. As a result of technology initiatives and changes in our system platforms due, in part, to our relatively recently becoming an independent public company, we are in the process of consolidating and integrating our systems. Our IT needs require an ongoing commitment of significant resources to maintain, protect and enhance existing systems and develop new systems to keep pace with new technology, evolving regulatory standards, the increasing need to protect patient and customer information and changing customer patterns. Currently, we do not have an IT disaster recovery plan. Any significant breakdown, intrusion, interruption, corruption or destruction of these systems could have a material and adverse effect on our business, financial condition and results of operations.
In addition, although our computer and information systems are protected through physical and software safeguards, they are still vulnerable to system malfunction, computer viruses, and cyber-attacks. These events could lead to the unauthorized access and result in the misappropriation or unauthorized disclosure of confidential information belonging to us or to our employees, partners, customers or suppliers. The techniques used by criminal elements to attack computer systems are sophisticated, change frequently and may originate from less regulated and remote areas of the world. As a result, we may not be able to address these techniques proactively or implement adequate preventative measures. If our IT systems are compromised, due to a data breach or
21
otherwise, we could be subject to fines, damages, litigation and enforcement actions and we could lose trade secrets or other confidential information, the occurrence of which could harm our business and could have a material and adverse effect on our business, financial condition and results of operations.
We expend substantial resources to comply with laws and regulations relating to public companies, and any failure to maintain compliance could subject us to regulatory scrutiny and cause investors to lose confidence in our company, which could harm our business and have a material adverse effect on our stock price.
Laws and regulations affecting public companies, including provisions of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and the Sarbanes-Oxley Act of 2002, or SOX, and the related rules and regulations adopted by the U.S. Securities and Exchange Commission, or SEC, and by the NASDAQ Global Market have resulted in, and will continue to result in, significant costs to us as we evaluate the implications of these rules and respond to their requirements. For example, compliance with Section 404 of SOX, including performing the system and process documentation and evaluation necessary to issue our annual report on the effectiveness of our internal control over financial reporting and, if applicable, obtain the required attestation report from our independent registered public accounting firm, requires us to incur substantial expense and expend significant management time. Further, we (through our former parent company) have in the past discovered, and in the future may discover, areas of internal controls that need improvement. If we identify deficiencies in our internal controls that are deemed to be material weaknesses, we could become subject to scrutiny by regulatory authorities and lose investor confidence in the accuracy and completeness of our financial reports, which could have a material adverse effect on our stock price. Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations, including the possibility of human error and circumvention by collusion or overriding of controls. Accordingly, even an effective internal control system may not prevent or detect material misstatements on a timely basis, or at all. Also, previously effective controls may become inadequate over time as a result of changes in our business or operating structure, and we may fail to take measures to evaluate the adequacy of and update these controls, as necessary, which could lead to a material misstatement.
In addition, new laws and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the coverage that is the same or similar to our current coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or board committees, and as our executive officers. We cannot predict or estimate with any reasonable accuracy the total amount or timing of the costs we may incur to comply with these laws and regulations.
Our business could suffer if we lose the services of key members of our senior management or fail to hire and retain other personnel on whom our business relies.
Our ability to execute our business strategy and compete in the highly competitive medical device industry depends, in part, on our ability to attract and retain highly qualified personnel. Companies in the medical device industry in general have experienced a high rate of personnel turnover. Loss of key employees, including any of our scientific, technical and managerial personnel, could adversely affect our ability to successfully execute our current business strategy, which could have a material and adverse effect on our business, results of operations and financial condition. Moreover, replacing key employees may be a difficult, costly and protracted process, and we may not have other personnel with the capacity to assume all of the responsibilities of a departing employee. Competition for qualified personnel, particularly for key positions, is intense among companies in our industry, particularly in the San Diego, California area, and many of the organizations against which we compete for qualified personnel have greater financial and other resources and different risk profiles than our company, which may make them more attractive employers. All of our employees, including our management personnel, may terminate their employment with us at any time without notice. If we cannot attract and retain skilled personnel, as needed, we may not achieve our financial and other goals.
Moreover, future internal growth could impose significant added responsibilities on our management, and we will need to identify, recruit, maintain, motivate and integrate additional employees to manage growth effectively. If we are unable to effectively manage such growth, our expenses may increase more than expected, we may not be able to achieve our goals, and our ability to generate and/or grow revenue could be diminished.
We may have significant product liability exposure and our insurance may not cover all potential claims.
We are exposed to product liability and other claims if our products are alleged to have caused harm. For example, the development of allograft implants and technologies for human tissue repair and treatment may entail particular risk of transmitting diseases to human recipients, and any such transmission could result in the assertion of product liability claims against us. Further, successful product liability claims made against one or more of our competitors could cause claims to be made against us or expose us to a perception that we are vulnerable to similar claims. Product liability claims are expensive to defend, divert our management’s
22
attention and, if we are not successful in defending the claim, can result in substantial damages awarded against us.
In addition, we may not be able to obtain or renew insurance on acceptable terms with adequate coverage or at reasonable cost. Even if we obtain insurance, a claim could exceed the amount of our insurance coverage or it may be excluded from coverage under the terms of the policy. If we do not have adequate insurance coverage, products liability claims could have a material and adverse effect on our ability to successfully market our products and on our financial condition and results of operations.
Even if a product liability claim is not successful or is not fully pursued, the adverse publicity surrounding any assertion that our products caused harm could have a material and adverse effect on our reputation with existing and potential customers and on our business, financial condition and results of operations.
Our strategy could involve growth through acquisitions, which would require us to incur substantial costs and potential liabilities for which we may never realize the anticipated benefits.
We may grow our business through acquisitions, a strategy which ultimately could prove unsuccessful. Any new acquisition could result in material transaction expenses, increased interest and amortization expense, increased depreciation expense, increased operating expense and possible in-process research and development charges for acquisitions that do not meet the definition of a “business,” any of which could have a material and adverse effect on our operating results.
In addition, businesses that we acquire may not have adequate financial, disclosure, regulatory, quality or other compliance controls in place at the time we acquire them, which may create uncertainty regarding the actual condition and financial results of the acquired business and our assumptions regarding synergies and future results. Following any acquisition, we must integrate the new business, which includes incorporating it into our financial, compliance, regulatory and quality systems. Failure to timely and successfully integrate acquired businesses may result in non-compliance with regulatory or other requirements and may result in unexpected costs, including as a result of inadequate cost containment and unrealized economies of scale. In addition, acquisitions involve other risks, including diversion of management resources, risks associated with entering markets in which our marketing and sales personnel may have limited experience and disruption to existing relationships with employees, suppliers, customers and sales agents, both with respect to us and the acquired company. As a result of any of the foregoing, we may not realize the expected benefit from any acquisition. If we cannot integrate acquired businesses, products or technologies, our business, financial conditions and results of operations could be materially and adversely affected.
Furthermore, as a result of acquisitions of other healthcare businesses, we may be subject to the risk of unanticipated business uncertainties, regulatory and other compliance matters or legal liabilities relating to those acquired businesses for which the sellers of the acquired businesses may not indemnify us, for which we may not be able to obtain insurance (or adequate insurance) or for which the indemnification may not be sufficient to cover the ultimate liabilities.
We are exposed to a variety of risks relating to our international sales and operations, including fluctuations in exchange rates, delays in collection of accounts receivable and local economic conditions.
As a result of our international sales and operations, we generate revenues in various foreign currencies including euros, British pounds, Swiss francs and New Zealand dollars, and in U.S. dollar-denominated transactions conducted with customers who generate revenue in currencies other than the U.S. dollar. We also incur operating expenses in euros. We cannot predict accurately the consolidated effects of exchange rate fluctuations upon our future operating results because of the variability of currency exposure in our revenues and operating expenses and the potential volatility of currency exchange rates. Although we address currency risk management through regular operating and financing activities, those actions may not prove to be fully effective.
In addition, for those foreign customers who purchase our products in U.S. dollars, currency exchange rate fluctuations between the U.S. dollar and the currencies in which those customers do business may have a negative effect on the demand for our products in foreign countries where the U.S. dollar has increased in value compared to the local currency.
Converting our earnings from international operations to U.S. dollars for use in the U.S. can also raise challenges, including problems moving funds out of the countries in which the funds were earned and difficulties in collecting accounts receivable in foreign countries where the usual accounts receivable payment cycle is longer.
Local economic conditions, legal, regulatory or political considerations, disruptions from strikes, the effectiveness of our independent stocking distributors, local competition, in-country reimbursement methodologies and changes in local medical practice could also affect our sales in foreign markets.
23
We may be subject to damages resulting from claims that we, our employees, or our independent sales agents or stocking distributors have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Many of our employees were previously employed at other medical device companies, including our competitors or potential competitors, in some cases immediately prior to joining us. In addition, many of our independent sales agents and stocking distributors sell, or in the past have sold, products of our competitors. See “If we are unable to maintain and expand our network of independent sales agents and stocking distributors, we may not be able to maintain or grow our revenue,” and “Our business could suffer if we lose the services of key members of our senior management or fail to hire and retain other personnel on whom our business relies,” above. We may be subject to claims that we, our employees or our independent sales agents or stocking distributors have intentionally, inadvertently or otherwise used or disclosed trade secrets or other proprietary information of former employers or competitors. In addition, we have been and may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Litigation is expensive, time-consuming and could divert management attention and resources away from our business. Even if we prevail, the cost of litigation could affect our profitability. If we do not prevail, in addition to any damages we might have to pay, we may lose valuable intellectual property rights or employees, independent sales agents or stocking distributors. There can be no assurance that this type of litigation or the threat thereof will not adversely affect our ability to engage and retain key employees, sales agents or stocking distributors.
We might not be able to engage in desirable strategic transactions and equity issuances because of certain restrictions relating to requirements for tax-free distributions.
Our ability to engage in significant equity transactions could be limited or restricted in order to preserve, for U.S. federal income tax purposes, the tax-free nature of the distribution and related activities associated with our separation from Integra LifeSciences Holdings Corporation, or Integra, our former parent. Even if the distribution and related activities otherwise qualify for tax-free treatment under Section 355 of the Internal Revenue Code of 1986, as amended, or the Code, they may result in corporate-level taxable gain to Integra under Section 355(e) of the Code if there is a 50% or greater change in ownership, by vote or value, of shares of our stock or Integra’s stock occurring as part of a plan or series of related transactions that includes the distribution. Any acquisitions or issuances of our stock or Integra’s stock within two years after the distribution are generally presumed to be part of such a plan, although we or Integra may be able to rebut that presumption.
Under the Tax Matters Agreement we entered into with Integra, we are prohibited from taking or failing to take any action that prevents distribution activities from being tax-free. Further, during the two-year period following the distribution, without obtaining the consent of Integra, a private letter ruling from the Internal Revenue Service or an unqualified opinion of a nationally recognized law firm, we are prohibited from taking certain specified actions that could affect the treatment of the distribution.
These restrictions may limit our ability to pursue strategic transactions or engage in new business or other transactions that may maximize the value of our business. Moreover, the Tax Matters Agreement also provides that we are responsible for any taxes imposed on Integra or any of its affiliates as a result of the failure of the distribution to qualify for favorable treatment under the Code if such failure is attributable to certain actions taken after the distribution by or in respect of us, any of our affiliates or our stockholders.
We are subject to continuing contingent liabilities of Integra.
Even after our separation from Integra, there are several significant areas where Integra’s liabilities may become our obligations. For example, under the Code and the related rules and regulations, each corporation that was a member of the Integra consolidated U.S. federal income tax reporting group during any taxable period or portion of any taxable period ending on or before the effective time of the distribution is jointly and severally liable for the U.S. federal income tax liability of the entire Integra consolidated tax reporting group for that taxable period. In addition, the Tax Matters Agreement allocates the responsibility for prior period taxes of the Integra consolidated tax reporting group between us and Integra. Pursuant to this allocation, we may be responsible for taxes that we would not have otherwise incurred, or that we would have incurred but in different amounts and/or at different times, on a standalone basis outside of the Integra consolidated group, and the amount of such taxes could be significant. If Integra is unable to pay any prior period taxes for which it is responsible, we could be required to pay the entire amount of such taxes.
We have overlapping board membership with Integra, which may lead to conflicting interests, and one of our directors continues to own a substantial amount of Integra common stock and equity awards covering Integra stock.
Several of our board members also serve as board members of Integra. Our directors who are members of Integra’s board
24
of directors have fiduciary duties to Integra’s stockholders, as well as fiduciary duties to our stockholders. In addition, several of our directors own or have rights to acquire Integra common stock (in at least one case, a substantial amount).
As a result of the foregoing, there may be the appearance of a conflict of interest and there is the potential for a conflict of interest with respect to matters involving or affecting both companies, such as when we or Integra consider acquisitions and other corporate opportunities that may be suitable for each company. In addition, potential conflicts of interest could arise in connection with the resolution of any dispute that may arise between Integra and us regarding the terms of the agreements governing our separation from Integra, the Tax Matters Agreement or under other agreements between Integra and us, including with respect to indemnification matters. From time to time, we may enter into transactions with Integra and/or its subsidiaries or other affiliates. There can be no assurance that the terms of any such transactions will be as favorable to us, Integra or any of our or their subsidiaries or affiliates as would be the case were there no overlapping board membership or ownership interest.
Risks Relating to Our Financial Results and Need for Financing
Our sales volumes and our operating results may fluctuate.
Our sales volumes and our operating results, including components of operating results, such as gross margin and cost of goods sold, may fluctuate from time to time, including over the course of a fiscal year, and such fluctuations could affect our stock price. Our operating results have fluctuated in the past and can be expected to fluctuate from time to time in the future. Some of the factors that may cause these fluctuations include:
• | economic conditions worldwide, which could affect the ability of hospitals and other customers to purchase our products and could result in a reduction in elective and non-reimbursed operative procedures; |
• | increased competition; |
• | market acceptance of our existing products, as well as products in development, and the demand for, and pricing of, our products and the products of our competitors; |
• | costs, benefits and timing of new product introductions; |
• | the timing of or failure to obtain regulatory clearances or approvals for new products; |
• | lost sales and other expenses resulting from stoppages in our or third parties’ production, including as a result of product recalls or field corrective actions; |
• | the availability and cost of components and materials, including raw materials such as human tissue; |
• | our ability to purchase or manufacture and ship our products efficiently and in sufficient quantities to meet sales demands; |
• | the timing of our research and development expenditures; |
• | expenditures for major initiatives; |
• | reimbursement, changes in reimbursement or denials in coverage for our products by third-party payors, such as Medicare, Medicaid, private and public health insurers and foreign governmental health systems; |
• | the ability of our independent sales agents and stocking distributors to achieve expected sales targets and for new agents and distributors to become familiar with our products in a timely manner; |
• | peer-reviewed publications discussing the clinical effectiveness of our products; |
• | inspections of our manufacturing facilities for compliance with Quality System Regulations (Good Manufacturing Practices), which could result in Form 483 observations, warning letters, injunctions or other adverse findings from the FDA or equivalent foreign regulatory bodies, and corrective actions, procedural changes and other actions, including product recalls, that we determine are necessary or appropriate to address the results of those inspections, any of which may affect production and our ability to supply our customers with our products; |
• | the costs to comply with new regulations from the FDA or equivalent foreign regulatory bodies, such as the requirements to establish a unique device identification system to adequately identify medical devices through their distribution and use; |
• | the increased regulatory scrutiny of certain of our products, including products we manufacture for others, which could result in their being removed from the market; |
• | fluctuations in foreign currency exchange rates; and |
• | the impact of acquisitions, including the impact of goodwill and intangible asset impairment charges, if future operating results of the acquired businesses are significantly less than the results anticipated at the time of the acquisitions. |
In addition, we may experience meaningful variability in our sales and gross profit among quarters, as well as within each quarter, as a result of a number of factors, including but not limited to (and in addition to those listed above):
25
• | the number of products sold in the quarter; |
• | the unpredictability of sales of full sets of spinal implants and instruments to our international stocking distributors; and |
• | the number of selling days in the quarter. |
Our future capital needs are uncertain and we may need to raise additional funds in the future, and such funds may not be available on acceptable terms or at all.
We believe that our cash and the borrowing capacity that we have under the credit facility that we entered into in December 2015 will be sufficient to meet our projected operating requirements over the next 12 months. That said, continued expansion of our business will be expensive, and we may seek additional capital. Our capital requirements will depend on many factors, including, but not limited to:
• | the revenue generated by sales of our products; |
• | the costs associated with expanding our sales and marketing efforts; |
• | the expenses we incur in procuring, manufacturing and selling our products; |
• | the scope, rate of progress and cost of our clinical studies; |
• | the cost of obtaining and maintaining regulatory approval or clearance of our products and products in development; |
• | the costs associated with complying with state, federal and international laws and regulations; |
• | the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights; |
• | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patent or other intellectual property rights; |
• | the cost of enforcing or defending against non-competition claims; |
• | the number and timing of acquisitions and other strategic transactions; |
• | the costs associated with increased capital expenditures, including fixed asset purchases of instrument sets which we consign to hospitals and independent sales agents to support surgeries; and |
• | anticipated and unanticipated general and administrative expenses, including insurance expenses. |
As a result of these factors, we may seek to raise additional capital to:
• | maintain, and, where necessary, increase appropriate product inventory levels; |
• | fund our operations and clinical studies; |
• | continue, and, where appropriate, increase our research and development activities; |
• | file, prosecute and defend our intellectual property rights, and defend, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
• | address the FDA or other governmental, legal or enforcement actions and remediate underlying problems and address investigations or inquiries into sales and marketing practices from governmental agencies worldwide; |
• | commercialize our new products, if any such products receive regulatory clearance or approval for sale; and |
• | acquire companies new products, technology or intellectual property. |
Such capital, which we may seek to raise through public or private equity offerings, issuing debt or existing, expanded or new credit facilities, or other sources, may not be available to us on favorable terms, or at all. In addition, our December 2015 credit agreement prohibits us from incurring indebtedness without the lender’s consent. If we issue equity securities to raise additional capital, our existing stockholders may experience dilution, and the new equity securities may have rights, preferences and privileges senior to those of our existing stockholders. See “Risks Relating to Owning Our Common Stock-Your percentage of ownership in us may be diluted in the future and issuances of substantial amounts of our common stock, or the perception that such issuances may occur, could cause the market price of our common stock to decline significantly, even if our business is performing well.,” and “Risks Relating to Owning Our Common Stock-We may issue preferred stock with terms that could dilute the voting power or reduce the value of our common stock,” below. If we raise additional capital through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our products, potential products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise capital on acceptable terms, we may not be able to develop or enhance our products, execute our business plan, take advantage of future opportunities or respond to competitive pressures, changes in our supplier relationships or unanticipated customer requirements. Any of these events could adversely affect our ability to achieve our business and financial goals or to achieve or maintain profitability, and could have a material and adverse effect on our business, results of operations and financial condition.
26
Our future financial results could be adversely affected by impairments or other charges, including as a result of the high levels of inventory we maintain, which could consume a significant amount of our resources and reduce our cash flows.
Because we maintain substantial inventory levels to meet the needs of our customers, we are subject to the risk of inventory excess, obsolescence and shelf-life expiration. Many of our spine hardware products come in sets. Each set includes a significant number of components in various sizes so that the surgeon may select the appropriate spinal implant based on the patient’s needs. In a typical surgery, not all of the implants in the set are used, and therefore certain sizes of implants placed in the set or that we purchase for replenishment inventory may become obsolete before they can be used. In addition, in order to market our products effectively, we often must provide hospitals and independent sales agents with consigned sets that typically consist of spinal implants and instruments, including products to ensure redundancy and products of different sizes. Further, the use of our orthobiologics products is limited by the sterilization expiration date, which ranges from one to five years. Therefore, these products may expire before they can be used. If a substantial portion of our inventory is deemed excess, becomes obsolete or expires, we may be required to incur impairment charges that could have a material and adverse effect on our financial results.
Further, we assess periodically impairment of our long-lived assets, including finite-lived intangible assets, whenever events or changes in circumstances indicate that the carrying value may not be recoverable. As of December 31, 2015, we had $39.6 million of net finite-lived intangible assets, consisting of technology and customer relationships. In addition, we continually assess the profitability of our product lines and, after such assessment, may discontinue certain products or product lines in the future. As a result, we may record impairment charges or accelerate amortization on certain technology-related intangible assets in the future. Impairment charges as a result of any of the foregoing could be significant and could have a material and adverse effect on our reported financial results for the period in which the charge is taken, which could have a material and adverse effect on the market price of our common stock.
Certain of our historical financial information may not be representative of the results we would have achieved as an independent public company and may not be a reliable indicator of our future results.
For periods prior to our separation from Integra, financial information included in this Form 10-K may not necessarily reflect what our financial position, results of operations or cash flows would have been had we been an independent entity during the periods presented or those that we will achieve in the future. The costs and expenses reflected in these prior periods include an allocation for certain corporate functions historically provided by Integra, including shared services and infrastructure provided by Integra to us, such as costs for IT, including the costs of a multi-year global implementation of a enterprise resource planning system, accounting and legal services, real estate and facilities, corporate advertising, insurance services and related treasury, and other corporate and infrastructure services that may be different from the comparable expenses that we would have incurred had we operated as an independent public company. Financial data presented as of and for these periods does not reflect changes in our cost structure and operations as a result of operating as an independent public company, including changes in our employee base, increased costs associated with reduced economies of scale and increased costs associated with SEC reporting and requirements. Accordingly, financial data as of and for these periods should not be assumed to be indicative of what our financial condition or results of operations actually would have been as an independent public company or to be a reliable indicator of what our financial condition or results of operations actually could be in the future.
Continuing economic instability, including challenges faced by European countries, may adversely affect the ability of hospitals and other customers to access funds or otherwise have available liquidity, which could reduce orders for our products or impede our ability to obtain new customers, particularly in European markets.
Continuing economic instability, including challenges faced by European countries, may adversely affect the ability of hospitals and other customers to access funds to enable them to fund their operating budgets. As a result, hospitals and other customers may reduce budgets or put all or part of their budgets on hold or close their operations, which could have a negative effect on our sales and could impede our ability to obtain new customers, particularly in European markets. Governmental austerity policies in Europe and other markets have reduced and could continue to reduce the amount of money available to purchase medical products, including our products. If such conditions persist, they could have a material and adverse effect on our business, financial condition and results of operations.
Risks Relating to Our Regulatory Environment
We are subject to stringent domestic and foreign medical device regulation and any adverse regulatory action may materially and adversely affect our financial condition and business operations.
Our products, development activities and manufacturing processes are subject to extensive and rigorous regulation by numerous federal and state government agencies, including the FDA and comparable foreign agencies. To varying degrees, each
27
of these agencies monitors and enforces our compliance with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our products. For example, we are required to comply with the FDA’s Quality System Regulation, which mandates that manufacturers of medical devices adhere to certain quality assurance standards pertaining to, among other things, validation of manufacturing processes, controls for purchasing product components and documentation practices.
In addition, we must engage in extensive recordkeeping and reporting. For example, the Federal Medical Device Reporting regulation requires us to provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or that a malfunction occurred that would be likely to cause or contribute to a death or serious injury upon recurrence.
Compliance with applicable regulatory requirements is subject to continual review and we must make our manufacturing facilities and records available for periodic unscheduled inspections by governmental agencies, including the FDA, state authorities and comparable agencies in other countries. If we fail to pass an FDA Quality System Regulation inspection or to comply with applicable regulatory requirements, we may receive a notice of a violation in the form of inspectional observations on Form FDA 483, a warning letter, or could otherwise be required to take corrective action and, in severe cases, we could suffer a disruption of our operations and manufacturing delays. If we fail to take adequate corrective actions, we could be subject to enforcement actions, including significant fines, suspension of approvals, seizures or recalls of products, operating restrictions and criminal prosecutions.
The FDA has been increasing its scrutiny of the medical device industry and the government is expected to continue to scrutinize the industry closely. Moreover, allegations may be made against us or against our suppliers, including donor recovery groups or tissue banks, claiming that the acquisition or processing of biomaterials products does not comply with applicable FDA regulations or other relevant statutes and regulations. Allegations like these could cause regulators or other authorities to investigate or take other action against us or our suppliers, or could cause negative publicity for us or our industry generally. If the FDA were to investigate us, because of an allegation or otherwise, and if the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could ban such medical devices, detain or seize such medical devices, order a recall, repair, replacement or refund of such devices, require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health, restrict manufacturing and impose other operating restrictions, enjoin and restrain certain violations of applicable law pertaining to medical devices and assess civil or criminal penalties against our officers, employees or us. The FDA may also recommend prosecution to the U.S. Department of Justice. Any notice or communication from the FDA regarding a failure to comply with applicable requirements, or negative publicity or product liability claims resulting from any adverse regulatory action, could materially and adversely affect our product sales and overall business.
Further, our suppliers also are subject to a wide array of regulatory and other requirements, including quality control, quality assurance and the maintenance of records and documentation. Our suppliers may be unable to comply with these requirements and with other FDA, state and foreign regulatory requirements. We have little control over their ongoing compliance with these regulations. Their failure to comply may expose us to regulatory action and other liability, including fines and civil penalties, suspension of production, suspension or delay in new product approval or clearance, product seizure or recall, or withdrawal of product approval or clearance.
Foreign governmental regulations have become increasingly stringent and more common, and we may become subject to even more rigorous regulation by foreign governmental authorities in the future. Penalties for a company’s noncompliance with foreign governmental regulation could be severe, including revocation or suspension of a company’s business license and criminal sanctions. Any domestic or foreign governmental medical device law or regulation imposed in the future may have a material and adverse effect on our financial condition and business operations.
There is no guarantee that the FDA will grant 510(k) clearance or premarket approval, or that equivalent foreign regulatory authorities will grant the foreign equivalent, of our future products, and failure to obtain necessary clearances or approvals for our future products would adversely affect our ability to grow our business.
In general, unless an exemption applies, a medical device and modifications to the device or its indications must receive either premarket approval or premarket clearance from the FDA before it can be marketed in the U.S. While in the past we have received such clearances, we may not be successful in the future in receiving approvals and clearances in a timely manner, or at all. The process of obtaining approval or clearance from the FDA and comparable foreign regulatory agencies for new products, or for enhancements or modifications to existing products, could:
• | take a significant amount of time; |
28
• | require the expenditure of substantial resources; |
• | involve rigorous and expensive pre-clinical and clinical testing, as well as post-market surveillance; |
• | involve modifications, repairs or replacements of our products; and |
• | result in limitations on the indicated uses of our products. |
Some of our new products will require FDA 510(k) clearance or approval of a premarket approval application, or PMA, prior to being marketed. Any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, including significant design and manufacturing changes, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or, possibly, approval of a PMA. Similarly, modifications to PMA-approved products may require submission and approval of a supplement PMA. The FDA requires every manufacturer to determine whether a new 510(k) or supplement PMA is needed in the first instance, and the FDA has issued guidance on assessing modifications to 510(k)-cleared and PMA-approved devices to assist manufacturers with making these determinations. However, the FDA may review any such determination and the FDA may not agree with our determinations regarding whether new clearances or approvals are necessary. We have modified some of our 510(k)-cleared products and have determined, based on our understanding of FDA guidance, that certain changes did not require new 510(k) clearances. If the FDA disagrees with our determination and requires us to seek new 510(k) clearances, or PMA approval, for modifications to our previously cleared products, we may be required to stop marketing or distributing our products, we may need to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Significant delays in receiving clearance or approval, or the failure to receive clearance or approval for our new products would have a material and adverse effect on our ability to expand our business.
Outside the U.S., clearance or approval procedures can vary among countries and can involve additional product testing and validation and additional administrative review periods. The time required to obtain clearance or approval in other countries might differ from that required to obtain FDA clearance or approval. The regulatory process in other countries may include all of the risks to which we are exposed in the U.S., as well as other risks. Favorable regulatory action in one country does not ensure favorable regulatory action in another, but a failure or delay in obtaining regulatory clearance or approval in one country may have a negative effect on the regulatory process in others. Failure to obtain clearance or approval in other countries or any delay or setback in obtaining such clearance or approval have a material and adverse effect on our business, including that our products may not be cleared or approved for all indications requested, which could limit the uses of our products and have an adverse effect on product sales.
In the EEA, we must inform the Notified Body that carried out the conformity assessment of the medical devices we market or sell in the EEA of any planned substantial change to our quality system or any change to our devices that could affect compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive or the devices’ intended purpose. The Notified Body will then assess the change and verify whether it affects the products’ conformity with the Essential Requirements or the conditions for the use of the device. If the assessment is favorable, the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the Essential Requirements. If it is not, we may not be able to continue to market and sell the applicable product in the EEA, which could have a material and adverse effect on our business, results of operations and financial condition.
We cannot be certain that we will receive required approval or clearance from the FDA and foreign regulatory agencies for new products, including modifications to existing products, on a timely basis, or at all. The failure to receive approval or clearance for new on a timely basis would have a material and adverse effect on our financial condition and results of operations.
Certain of our products are derived from human tissue and are or could be subject to additional regulations and requirements.
Some of our orthobiologics products are derived from human bone tissue, and as a result are also subject to FDA and certain state regulations regarding human cells, tissues and cellular or tissue-based products, or HCT/Ps. An HCT/P is a product containing or consisting of human cells or tissue intended for transplantation into a human patient. Examples include bone, ligament, skin and cornea.
Some HCT/Ps also meet the definition of a biological product, medical device or drug regulated under the Federal Food, Drug and Cosmetic Act. Section 361 of the Public Health Service Act authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “361 HCT/Ps” are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, Good Tissue Practice when processing, storing, labeling and distributing HCT/Ps, including required labeling information, stringent record keeping and adverse event reporting. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to 361 HCT/Ps and, in addition, with requirements applicable to biologics, devices or drugs, including premarket clearance or approval. We have received required approvals for our products that are regulated as 361 HCT/Ps. However, there
29
have been occasions in the past, and there could be occasions in the future, when the FDA requires us to obtain a 510(k) clearance for our products that are regulated as 361 HCT/Ps. The process of obtaining a 510(k) clearance could take time and consume resources, and the failure to receive such a clearance would render us unable to market and sell such products, which could have a material and adverse effect on our business.
The American Association of Tissue Banks has issued operating standards for tissue banking. Accreditation is voluntary, but compliance with these standards is a requirement in order to become a licensed tissue bank. In addition, some states have their own tissue banking regulations. In addition, procurement of certain human organs and tissue for transplantation is subject to the National Organ Transplant Act, or NOTA, which prohibits the transfer of certain human organs, including skin and related tissue, for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks for their expenses associated with the recovery, storage and transportation of donated human tissue that they provide to us for processing. We include in our pricing structure amounts paid to tissue banks to reimburse them for their expenses associated with the recovery and transportation of the tissue, in addition to certain costs associated with processing, preservation, quality control and storage of the tissue, marketing and medical education expenses and costs associated with development of tissue processing technologies. NOTA payment allowances may be interpreted to limit the amount of costs and expenses that we can recover in our pricing for our products, thereby reducing our future revenue and profitability. If we were to be found to have violated NOTA’s prohibition on the sale or transfer of human tissue for valuable consideration, we would potentially be subject to criminal enforcement sanctions, which could materially and adversely affect our results of operations.
Because of the absence of a harmonized regulatory framework and the proposed regulation for advanced therapy medicinal products in the European Union, or EU, as well as for other countries, the approval process in the EU for human-derived cell or tissue-based medical products could be extensive, lengthy, expensive and unpredictable. Among others, some of our orthobiologics products are subject to EU member states’ regulations that govern the donation, procurement, testing, coding, traceability, processing, preservation, storage and distribution of HCT/Ps. These EU member states’ regulations include requirements for registration, listing, labeling, adverse-event reporting and inspection and enforcement. Some EU member states have their own tissue banking regulations. Non-compliance with various regulations governing our products in any EU member state could result in the banning of our products in such member state or enforcement actions being brought against us, which could have a material and adverse effect on our business, results of operations and financial condition.
Certain of our products contain materials derived from animal sources and may become subject to additional regulation.
Certain of our products contain material derived from bovine tissue. Products that contain materials derived from animal sources, including food, pharmaceuticals and medical devices, are subject to scrutiny in the media and by regulatory authorities. Regulatory authorities are concerned about the potential for the transmission of disease from animals to humans via those materials. This public scrutiny has been particularly acute in Japan and Western Europe with respect to products derived from animal sources, largely due to concern that materials infected with the agent that causes bovine spongiform encephalopathy, or BSE, otherwise known as mad cow disease, may, if ingested or implanted, cause a variant of the human Creutzfeldt-Jakob disease, an ultimately fatal disease with no known cure. Cases of BSE in cattle discovered in Canada and the U.S. have increased awareness of the issue in North America.
We take steps designed to minimize the risk that our products contain agents that can cause disease, such as obtaining our collagen from countries considered to be BSE-free. Nevertheless, products that contain materials derived from animals, including our products, could become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for the transmission of infectious or other agents. Significant new regulation, or a ban of our products, could have a material and adverse effect on our current business or our ability to expand our business.
Certain countries, such as Japan, China, Taiwan and Argentina, have already issued regulations that require our collagen products be processed from bovine tendon sourced from countries where no cases of BSE have occurred. The collagen raw material we use in our products is sourced from New Zealand. Our supplier has obtained approval from certain countries, including the U.S., the European Union, Japan, Taiwan, China and Argentina, for the use of such collagen raw material in products sold in those countries. If we cannot continue to obtain collagen raw material from a qualified source of tendon from a country that has never had a case of BSE, we will not be permitted to sell our collagen products in certain countries, which could have a material and adverse effect on our business, results of operations and financial condition.
Clinical studies are expensive and subject to extensive regulation and their results may not support our product candidate claims or may result in the discovery of adverse effects.
In the development of new products or new indications for, or modifications to, existing products, we may conduct or sponsor
30
pre-clinical testing, clinical studies or other clinical research. We are currently conducting post-market clinical studies of some of our products to gather information about their performance or optimal use. The data collected from these clinical studies may ultimately be used to support additional market clearance or approval for these products or future products. If any of our new products require premarket clinical studies, these studies are expensive, the outcomes are inherently uncertain and they are subject to extensive regulation and review by numerous governmental authorities both in the U.S. and abroad, including by the FDA and, if federal funds are involved or if an investigator or site has signed a federal assurance, are subject to further regulation by the Office for Human Research Protections and the National Institutes of Health. For example, clinical studies must be conducted in compliance with FDA regulations, local regulations, and according to principles and standards collectively referred to as “Good Clinical Practices.” Failure to comply with applicable regulations could result in regulatory and legal enforcement action, including fines, penalties, suspension of studies, and also could invalidate the data and make it unusable to support a FDA submission.
Even if any of our future premarket clinical studies are completed as planned, we cannot be certain that their results will support our product candidates and/or proposed claims or that the FDA or foreign authorities and Notified Bodies will agree with our interpretation and conclusions regarding the data they generate. Success in pre-clinical studies and early clinical studies does not ensure that later clinical studies will be successful, and we cannot be sure that the results of later studies will replicate those of earlier or prior studies. The clinical study process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product candidate and may delay development of others. Any delay or termination of our clinical studies will delay the filing of our product submissions and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patient subjects enrolled in our clinical studies of our marketed products will experience adverse side effects that are not currently part of the product candidate’s profile and, if so, these findings may result in lower market acceptance, which could have a material and adverse effect on our business, results of operations and financial condition.
If the third parties on which we rely to conduct our clinical studies and to assist us with pre-clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance, approval or a CE Certificate of Conformity for or commercialize our products.
We often must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to assist in conducting our clinical studies and other development activities. If these third parties do not successfully carry out their contractual duties, comply with applicable regulatory obligations or meet expected deadlines, or if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to clinical protocols, to applicable regulatory requirements or otherwise, our pre-clinical development activities and clinical studies may be extended, delayed, suspended or terminated. Under these circumstances we may not be able to obtain regulatory clearance/approval or a CE Certificate of Conformity for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be materially and adversely affected.
Oversight of the medical device industry might affect the manner in which we may sell medical devices and compete in the marketplace.
The U.S. Office of the Inspector General for the U.S. Department of Health and Human Services, the FDA, the U.S. Department of Justice and other regulatory agencies actively enforce regulations prohibiting the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. Physicians may prescribe our products for off-label uses, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if a regulatory agency determines that our promotional materials, training or activities constitute improper promotion of an off-label use, the regulatory agency could request that we modify our promotional materials, training or activities, or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and/or criminal penalties. Although our policy is to refrain from statements and activities that could be considered off-label promotion of our products, any regulatory agency could disagree and conclude that we have engaged in off-label promotion and, potentially, caused the submission of false claims. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. See “Risks Relating to our Business-We may have significant product liability exposure and our insurance may not cover all potential claims,” above.
There are also multiple other laws and regulations that govern the means by which companies in the healthcare industry may market their products to healthcare professionals and may compete by discounting the prices of their products, including, for example, the federal Anti-Kickback Statute, the federal False Claims Act, the federal Health Insurance Portability and Accountability Act of 1996, state law equivalents to these federal laws that are meant to protect against fraud and abuse, the Foreign Corrupt Practices Act of 1977 and analogous laws in foreign countries. Violations of these laws are punishable by criminal and civil sanctions, including, but not limited to, penalties, fines and exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid, and imprisonment. Federal and state government agencies, as well as private
31
whistleblowers, have significantly increased investigations and enforcement activity under these laws. Although we exercise care in structuring our sales and marketing practices, customer discount arrangements and interactions with healthcare professionals to comply with these laws and regulations, we cannot provide assurance that government officials will not assert that our practices are in compliance or that government regulators or courts will interpret those laws or regulations in a manner consistent with our interpretation. Even if an investigation is not successful or is not fully pursued, we may spend considerable time and resources defending ourselves and the adverse publicity surrounding any assertion that we may have engaged in violative conduct could have a material and adverse effect on our reputation with existing and potential customers and on our business, financial condition and results of operations.
Federal and state laws are also sometimes open to interpretation, and from time to time we may find ourselves at a competitive disadvantage if our interpretation differs from that of our competitors. AdvaMed (U.S.), EucoMed (Europe), MEDEC (Canada) and MTAA (Australia), some of the principal trade associations for the medical device industry, have promulgated model codes of ethics that set forth standards by which its members should (and non-member companies may) abide in the promotion of their products in various regions. We have implemented policies and procedures for compliance consistent with those promulgated by these associations, and we train our sales and marketing personnel on our policies regarding sales and marketing practices. Nevertheless, the sales and marketing practices of our industry have been the subject of increased scrutiny from federal and state government agencies, we believe this trend will continue and that it could affect our ability to retain customers and other relationships important to our business.
For example, prosecutorial scrutiny and governmental oversight, at both the state and federal levels, over some major device companies regarding the retention of healthcare professionals have limited the manner in which medical device companies may retain healthcare professionals as consultants. Various hospital organizations, medical societies and trade associations are establishing their own practices that may require detailed disclosures of relationships between healthcare professionals and medical device companies or ban or restrict certain marketing and sales practices, such as gifts and business meals. In addition, the Affordable Care Act, as well as certain state laws, require detailed disclosure of certain financial relationships, gifts and other remuneration made to certain healthcare professionals and teaching hospitals, the publicity surrounding which could have a negative impact on our relationships with our customers and ability to seek input on product design or involvement in research. As a result of laws, rules and regulations or our own or third-party policies that prohibit or restrict interactions, or the growing perception that any interaction between healthcare professionals and industry are tainted, we may be unable to engage with our healthcare professional customers in the same manner or to the same degree, or at all, as would otherwise be the case, which may adversely affect our ability to understand our customer’s needs and to incorporate into our development programs feedback that addresses these needs. If we are unable to develop and commercialize new products that address the needs of our surgeon customers and their patients, our products may not be broadly accepted in the marketplace, or at all, which would have a negative effect on our business, results of operations and financial condition.
Unfavorable media reports or other negative publicity concerning both alleged improper methods of tissue recovery from donors and disease transmission from donated tissue could limit widespread acceptance of some of our products.
Unfavorable reports of improper or illegal tissue recovery practices, both in the U.S. and internationally, as well as incidents of improperly processed tissue leading to the transmission of disease, may affect the rate of future tissue donation and market acceptance of technologies incorporating human tissue. In addition, negative publicity could cause the families of potential donors to become reluctant to agree to donate tissue to for-profit tissue processors. For example, the media has reported examples of alleged illegal harvesting of body parts from cadavers and resulting recalls conducted by certain companies selling human tissue based products affected by the alleged illegal harvesting. These reports and others could have a negative effect on our tissue regeneration business.
Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs and import-export practices, laws regarding transactions in foreign countries, the Foreign Corrupt Practices Act of 1977 and local anti-bribery and other laws regarding interactions with healthcare professionals, and product registration requirements.
Numerous laws restrict, and in some cases prohibit, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices and effecting product registrations in foreign countries. Compliance with these regulations is costly. Any failure to comply with applicable foreign legal and regulatory obligations could adversely affect us in a variety of ways, including the suspension or withdrawal of our CE Certificates of Conformity; the imposition of significant criminal, civil and administrative penalties, including imprisonment of individuals and fines; disrupting our shipping activities, including denial of export privileges and seizure of shipments; requiring us to recall or withdraw products; imposing restrictions on our business activities; and disrupting our sales activities and adversely affect our overall business.
32
New regulations related to “conflict minerals” may force us to incur additional expenses, may make our supply chain more
complex and may result in damage to our reputation with customers.
We are subject to SEC regulations that require us to determine whether our products contain certain specified minerals, referred to under the regulations as “conflict minerals,” and, if so, to perform an extensive inquiry into our supply chain, in an effort to determine whether or not such conflict minerals originate from the Democratic Republic of Congo (“DRC”) or an adjoining country. Compliance with these regulations has increased our costs, and we expect our costs may increase in the future. We have determined that certain of our products contain such specified minerals. We are continuing to conduct inquiries into our supply chain in connection with the preparation of our conflict minerals report for 2016. Compliance with these requirements has been time-consuming for management and our supply chain personnel (as well as time-consuming for our suppliers), and we expect that compliance will continue to require the expenditure of significant amounts of time and money by us and them. In addition, to the extent any of our disclosures are perceived by the market to be “negative,” it may cause customers to refuse to purchase our products. Further, if we determine to make any changes to products, processes, or sources of supply, it may result in additional costs, which may adversely affect our business, financial condition and results of operations.”
We are subject to requirements relating to hazardous materials which may impose significant compliance or other costs on us.
Our research, development and manufacturing processes involve the controlled use of certain hazardous materials. In addition, we lease facilities at which hazardous materials could have been used in the past. For these reasons, we are subject to federal, state, foreign and local laws and regulations governing the use, manufacture, storage, handling, treatment, remediation and disposal of hazardous materials and certain waste products. For example, our allograft bone tissue processing may generate waste materials that in the U.S. are classified as medical waste. Although we believe that our procedures for handling and disposing of hazardous materials comply with applicable laws as currently in effect, new laws may become effective, or existing laws may be amended in ways, that increase our cost of compliance, in some cases materially.
Furthermore, there is a risk that accidental contamination or injury has occurred in connection with one of our facilities. If such accidental contamination or injury occurred, we could be held liable for any damages that result and any related liability could exceed the limits or fall outside the coverage of our insurance and could exceed our resources. We may not be able to maintain insurance on acceptable terms or at all, which could have a material and adverse effect on our business, results of operations and financial condition.
Risks Relating to Our Intellectual Property
Our intellectual property rights may not provide meaningful commercial protection for our products, potentially enabling third parties to use our technology or very similar technology in ways that could reduce our ability to compete in the marketplace.
Our success will depend in part on our ability to obtain and maintain patent and other exclusivity with respect to our products; prevent third parties from infringing upon our proprietary rights; maintain proprietary know-how and trade secrets; operate without infringing upon the patents and proprietary rights of others; and obtain appropriate licenses to patents or proprietary rights held by third parties if infringement would otherwise occur, both in the U.S. and in foreign countries.
We own or have licensed patents that cover aspects of some of our product lines. Our patents, however, may not provide us with any significant competitive advantage. Others may challenge our patents and, as a result, our patents could be narrowed, invalidated or rendered unenforceable. Competitors may develop products similar to ours that our patents do not cover. In addition, our current and future patent applications may not result in the issuance of patents in the U.S. or foreign countries. Further, there is a substantial backlog of patent applications at the U.S. Patent and Trademark Office, and the approval or rejection of patent applications may take several years.
In an effort to protect our trade secrets, we require our employees, consultants and advisors to execute confidentiality and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements provide that, except in specified circumstances, all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential. We cannot assure you, however, that these agreements will provide meaningful protection for our trade secrets or other proprietary information in the event of the unauthorized use or disclosure of confidential information. In addition, we cannot assure you that others will not independently develop substantially equivalent proprietary information and procedures or otherwise gain access to our trade secrets, that our trade secrets will not be disclosed or that we can otherwise protect our rights to unpatented trade secrets.
If we are unable to obtain, protect and enforce patents on our technology and to protect our trade secrets, such inability could have a material and adverse effect on our business, results of operations and financial condition.
33
Our success will depend partly on our ability to operate without infringing or misappropriating the proprietary rights of others.
To protect or enforce our intellectual property rights, we may have to initiate or defend litigation against or by third parties, such as infringement suits, opposition proceedings or seeking a court declaration that we do not infringe the proprietary rights of others or that their rights are invalid or unenforceable. Litigation is expensive, time-consuming and could divert management attention and resources away from our business. Even if we prevail, the cost of litigation could affect our profitability.
If we do not prevail in any litigation, in addition to any damages we might have to pay, we may be required to stop the infringing activity (which could include requiring us to stop selling the products in question) or obtain a license for the proprietary rights involved. Any required license may be unavailable to us on acceptable terms, if at all. In addition, some licenses may be nonexclusive, in which case our competitors could access the same proprietary rights we license. If we fail to obtain a required license or are unable to design our products so as not to infringe on the proprietary rights of others, we may be unable to sell some of our products, which could have a material and adverse effect on our revenues and profitability.
Risks Relating to Owning Our Common Stock
The market price of our common stock has been and likely will continue to be volatile.
The market price of our common stock is likely to be volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this Form 10-K, these factors include:
• | actual or anticipated fluctuations in our quarterly financial condition and operating performance; |
• | introduction of new products by us or our competitors; |
• | announcements by us or our competitors of significant acquisitions or dispositions; |
• | our ability to obtain financing as needed; |
• | a shift in our investor base, including sales of our shares by existing stockholders;; |
• | any major change in our board of directors or management; |
• | threatened or actual litigation or governmental investigations; |
• | the number of shares of our common stock publicly owned and available for trading; |
• | the operating and stock price performance of similar companies; |
• | changes in earnings estimates by securities analysts or our ability to meet earnings guidance; |
• | publication of research reports about us or our industry or changes in recommendations or withdrawal of research coverage by securities analysts; |
• | changes in laws or regulations affecting our business, including tax legislation; |
• | the success or failure of our business strategy; |
• | investor perception of us and our industry; |
• | changes in accounting standards, policies, guidance, interpretations or principles; |
• | the overall performance of the equity markets; |
• | general political and economic conditions, and other external factors. |
In addition, the stock market in general, and the market for medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. This could limit or prevent investors from readily selling their shares and may otherwise negatively affect the liquidity of our common stock. Securities class action litigation has often been instituted against companies following periods of volatility in the overall market and in the market price of a company’s securities. This litigation, if instituted against us, could result in very substantial costs, divert our management’s attention and resources, and harm our business, financial condition and results of operation.
Your percentage of ownership in us may be diluted in the future and issuances of substantial amounts of our common stock, or the perception that such issuances may occur, could cause the market price of our common stock to decline significantly, even if our business is performing well.
As with any public company, your percentage ownership in us may be diluted in the future because of equity issuances for acquisitions and investments, capital-raising transactions or otherwise, including equity awards that we have granted and we expect to grant to our directors, officers and employees. For example, in connection with our separation from Integra, we granted to certain Integra directors and officers equity awards equivalent to approximately 1.9% of our then-outstanding common stock and granted to our directors stock option awards equivalent to approximately 2.6% of our then-outstanding common stock. Further, the market price of our common stock could decline as a result of the issuance, including sale, of a large number of shares of our
34
common stock, and the perception that these sales could occur may also depress the market price of our common stock. A decline in the price of our common stock might impede our ability to raise capital through the issuance of additional shares of our common stock or other equity securities.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we are taking advantage of certain exemptions and relief from various reporting requirements that are applicable to public companies that are not emerging growth companies. In particular, while we are an emerging growth company: (i) we will not be required to comply with the auditor attestation requirements of Section 404(b) of SOX; (ii) we will be exempt from any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotations or a supplement to the auditor’s report on financial statements; (iii) we will be subject to reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and (iv) we will not be required to hold nonbinding advisory votes on executive compensation or stockholder approval of any golden parachute payments not previously approved.
In addition, as an emerging growth company we are not required to comply with any new or revised accounting standard applicable to public companies until such date that a private company is required to comply with such standard. We elected not to comply with such new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies, therefore our financial statements may not be comparable to the financial statements of public companies that are not emerging growth companies.
We will remain an emerging growth company until the earliest of: (i) December 31, 2020 (the fiscal year-end following the fifth anniversary of the completion of the spin-off); (ii) the end of the fiscal year in which the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of the last business day of the second fiscal quarter of that year; (iii) the end of the fiscal year in which our annual revenues exceed $1.0 billion; and (iv) the date on which we issue more than $1.0 billion in nonconvertible debt in any three-year period.
The exact implications of the JOBS Act are still subject to interpretations and guidance by the SEC and other regulatory agencies, and we cannot assure you that we will be able to take advantage of all of the benefits of the JOBS Act. In addition, investors may find our common stock less attractive because we rely on the exemptions available to, and relief granted to, emerging growth companies by the JOBS Act. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may decline and/or become more volatile.
If, once we are no longer an emerging growth company, our independent registered public accounting firm cannot provide an unqualified attestation report on the effectiveness of our internal control over financial reporting, investor confidence and, in turn, the market price of our common stock, could decline.
We may issue preferred stock with terms that could dilute the voting power or reduce the value of our common stock.
While we have no specific plan to issue preferred stock, our amended and restated certificate of incorporation authorizes us to issue, without stockholder approval, one or more series of preferred stock having such designation, powers, privileges, preferences, including preferences over our common stock respecting dividends and distributions, terms of redemption and relative participation, optional, or other rights, if any, of the shares of each such series of preferred stock and any qualifications, limitations or restrictions thereof, as our board of directors may determine. The terms of one or more series of preferred stock could dilute the voting power or reduce the value of our common stock. For example, the repurchase or redemption rights or liquidation preferences we could assign to holders of preferred stock could affect the residual value of the common stock.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. If current or future analysts who cover us were to downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts were to stop covering us or were to stop regularly publishing reports on us, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
35
We do not anticipate paying cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We have paid no cash dividends on any of our common stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, with respect to our common stock, we do not expect to pay any cash dividends in the foreseeable future, and payment of cash dividends, if any, will depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors. Furthermore, we are subject to various laws and regulations that may restrict our ability to pay dividends and are subject to contractual restrictions on, or prohibitions against, the payment of dividends. Due to our intent to retain any future earnings rather than pay cash dividends on our common stock and applicable laws, regulations and contractual obligations that may restrict our ability to pay dividends on our common stock, the success of your investment in our common stock will likely depend entirely upon any future appreciation and our common stock may not appreciate. Investors seeking cash dividends should not invest in our common stock.
Certain provisions in our charter documents and Delaware law could discourage takeover attempts and lead to management entrenchment and, therefore, may depress the market price of our common stock.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could have the effect of delaying or preventing changes in control or changes in our management without the consent of our board of directors, including, among other things:
• | a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors; |
• | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
• | the ability of our board of directors to determine to issue shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquirer; |
• | the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of our board of directors or by the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
• | limitations on the removal of directors; |
• | a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; |
• | the requirement that a special meeting of stockholders be called only by the chairman of our board of directors, our chief executive officer, our president (in absence of a chief executive officer) or our board of directors, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; |
• | the requirement for the affirmative vote of holders of at least 66 2⁄3% of the voting power of all of the then outstanding shares of our voting stock, voting together as a single class, to amend the provisions of our amended and restated certificate of incorporation relating to the issuance of preferred stock and management of our business or our amended and restated bylaws, which may inhibit the ability of an acquirer from amending our amended and restated certificate of incorporation or amended and restated bylaws to facilitate a hostile acquisition; |
• | the ability of our board of directors, by majority vote, to amend our amended and restated bylaws, which may allow our board of directors to take additional actions to prevent a hostile acquisition and inhibit the ability of an acquirer from amending our amended and restated bylaws to facilitate a hostile acquisition; and |
• | advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us. |
We believe that these provisions protect our stockholders from coercive or harmful takeover tactics by requiring potential acquirers to negotiate with our board of directors and by providing our board of directors with adequate time to assess any acquisition proposal. However, these provisions may discourage, delay or prevent a transaction involving a change in control that is in the best interest of our stockholders. Even in the absence of a takeover attempt, the existence of these provisions may adversely affect the prevailing market price of our common stock if they are viewed as discouraging future takeover attempts.
We are also subject to certain anti-takeover provisions under the DGCL. Under the DGCL, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three
36
years or, among other things, our board of directors has approved the transaction.
Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain litigation that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us.
Our amended and restated certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for: (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers or other employees or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the DGCL or our amended and restated certificate of incorporation or amended and restated bylaws; or (iv) any action asserting a claim governed by the internal affairs doctrine. Our amended and restated certificate of incorporation further provides that any person or entity purchasing or acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions described above. These provisions may limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us.
ITEM 1B. UNRESOLVED STAFF COMMENTS
As of the filing of this Annual Report on Form 10-K, we had no unresolved comments from the staff of the Securities and Exchange Commission that were received not less than 180 days before the end of our 2015 fiscal year.
ITEM 2. PROPERTIES
Our principal executive offices are located in Carlsbad, California, effective October, 1, 2015. We design, develop and market most of our orthobiologics and spinal fusion hardware products at the 82,000 square foot Carlsbad facility. We also operate out of facilities in Vista and Irvine, California. We procure, inspect, kit and distribute spinal fusion hardware products and we currently maintain our cadaveric training laboratory and our prototyping development and testing operation in two adjacent buildings in Vista that are 22,000 and 18,000 square feet, respectively. We design, develop, manufacture and distribute virtually all of our orthobiologics products in our 70,000 square feet Irvine facility. We conduct sales, marketing, corporate, and general and administrative functions from the Carlsbad facility. Our Carlsbad, Vista and Irvine facilities are all leased, with the lease term expiring for the Carlsbad facility in 2027, for the Vista facility in 2016, and for the Irvine facility in 2023. All current operations at the Vista facility will be transitioned to the Carlsbad facility or other locations before the end of the lease term. We believe that our facilities are sufficient to meet our current needs and that renewal of this space will be available when needed on acceptable terms.
Our manufacturing facilities are registered with the FDA. Our facilities are subject to FDA inspection to ensure compliance with Quality System regulations. For further information regarding the status of FDA inspections, see the "Regulation" section in this Form 10-K.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we are subject to legal proceedings and claims in the ordinary course of business. While management presently believes that the ultimate outcome of these proceedings, individually and in the aggregate, will not materially harm our financial position, cash flows, or overall trends in results of operations, legal proceedings are subject to inherent uncertainties, and unfavorable rulings or outcomes could occur that have individually or in aggregate, a material adverse effect on our business, financial condition or operating results. The Company is not currently subject to any pending material litigation, other than ordinary routine litigation incidental to our business, as described above.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information, Holders and Dividends
37
Our common stock is listed on the NASDAQ Global Select Market (“NASDAQ”) under the symbol “SPNE.” SeaSpine’s common stock began trading on NASDAQ on a “when-issued” basis on June 17, 2015, prior to SeaSpine’s separation from Integra, and on a “regular way” basis on July 2, 2015, which was the day following the separation from Integra. There was no public market for SeaSpine common stock prior to July 2, 2015. The following table lists the high and low sales prices for our common stock for each quarter since July 2, 2015:
2015 | ||||
High | Low | |||
Fourth Quarter | 17.18 | 14.66 | ||
Third Quarter | 19.11 | 13.93 | ||
We have not paid any cash dividends on our common stock since our formation. Any future determinations to pay cash dividends on the common stock will be at the discretion of our Board of Directors and will depend upon our results of operations, cash flows, and financial condition and other factors deemed relevant by the Board of Directors. We did not repurchase any of our common stock during 2015.
The number of stockholders of record as of February 29, 2016 was approximately 394, which includes stockholders whose shares were held in nominee name.
Equity Compensation Plan Information
Information about our equity compensation plan is incorporated herein by reference to Part III, Item 12 of this Annual Report.
Sales of Unregistered Securities
There were no sales of unregistered securities during the years ended December 31, 2015.
Sale of Registered Securities
On July 1, 2015, Integra distributed, as a dividend, approximately 11.0 million shares of our common stock to Integra shareholders as part of the spin-off. Each holder of Integra common stock received one share of SeaSpine common stock for every three shares of Integra common stock held by such holder as of the record date for the dividend.
ITEM 6. | SELECTED FINANCIAL DATA |
The following table summarizes certain selected financial data derived from our audited financial statements. The information presented should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited financial statements and notes thereto appearing elsewhere in this Annual Report on Form 10-K. For periods prior to the spin-off, the consolidated statements of operations data for the years ended December 31, 2014, 2013 and 2012 were derived from the audited consolidated financial statements of the orthobiologics and spinal fusion hardware business of Integra. Subsequent to the spin-off, the Company’s financial statements are presented on a consolidated basis, as the Company became a separate publicly-traded company on July 1, 2015. As a result, the consolidated financial results and balance sheet data for certain of the periods presented below may not be directly comparable.
38
Year Ended December 31, | ||||||||||||||||
2015 | 2014 | 2013 | 2012 | |||||||||||||
(In thousands, except per share data) | ||||||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||||
Total revenue, net | $ | 133,178 | $ | 138,695 | $ | 146,586 | $ | 147,510 | ||||||||
Cost of goods sold | 61,119 | 56,714 | 55,532 | 54,856 | ||||||||||||
Gross profit | 72,059 | 81,981 | 91,054 | 92,654 | ||||||||||||
Operating expenses: | ||||||||||||||||
Selling, general and administrative | 110,551 | 88,213 | 93,009 | 94,747 | ||||||||||||
Research and development | 8,353 | 8,527 | 9,893 | 12,269 | ||||||||||||
Intangible amortization | 5,331 | 5,590 | 5,598 | 5,716 | ||||||||||||
Total operating expenses | 124,235 | 102,330 | 108,500 | 112,732 | ||||||||||||
Operating loss | (52,176 | ) | (20,349 | ) | (17,446 | ) | (20,078 | ) | ||||||||
Other expense, net | (877 | ) | (269 | ) | (4,556 | ) | (8,194 | ) | ||||||||
Loss before income taxes | (53,053 | ) | (20,618 | ) | (22,002 | ) | (28,272 | ) | ||||||||
Provision for income taxes | 2,479 | 3,927 | 3,744 | 2,152 | ||||||||||||
Net loss | $ | (55,532 | ) | $ | (24,545 | ) | $ | (25,746 | ) | $ | (30,424 | ) | ||||
Net loss per share (basic and diluted) | $ | (4.99 | ) | $ | (2.22 | ) | $ | (2.33 | ) | $ | (2.75 | ) | ||||
As of December 31, | ||||||||||||||||
2015 | 2014 | 2013 | 2012 | |||||||||||||
(In thousands) | ||||||||||||||||
Consolidated Balance Sheets Data: | ||||||||||||||||
Working capital | $ | 87,687 | $ | 28,664 | $ | 37,857 | $ | 36,871 | ||||||||
Total assets | 176,389 | 139,642 | 153,493 | 157,387 | ||||||||||||
Long term debt (1) | 328 | — | — | 126,963 | ||||||||||||
Stockholders' equity | 147,339 | 91,284 | 111,495 | (5,624 | ) | |||||||||||
(1) In December 2015, the Company entered into a three year, maximum borrowing capacity of $30.0 million credit facility with Wells Fargo Capital Finance. The 2012 long term debt was related to a related-party loan from Integra arising from a prior acquisition. See Note 4 to the Consolidated Financial Statements for detailed discussion.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The matters discussed in these forward-looking statements are subject to risk and uncertainties that could cause actual results to differ materially from those made, projected or implied in the forward-looking statements. Such risks and uncertainties may also give rise to future claims and increase exposure to contingent liabilities. Please see the “Risk Factors” section for a discussion of the uncertainties, risks and assumptions associated with these statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
You can identify these forward-looking statements by forward-looking words such as “believe,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would” and similar expressions.
These risks and uncertainties arise from (among other factors) the following:
• | general economic and business conditions, in both domestic and international markets; |
• | our expectations and estimates concerning future financial performance, financing plans and the impact of competition; |
39
• | anticipated trends in our business, including healthcare reform in the United States, increased pricing pressure from our competitors or hospitals and changes in third-party payment systems; |
• | physicians’ willingness to adopt our recently launched and planned products, customers’ continued willingness to pay for our products and third-party payors’ willingness to provide or continue coverage and appropriate reimbursement for any of our products and our ability to secure regulatory approval for products in development; |
• | existing and future regulations affecting our business, both in the United States and internationally, and enforcement of those regulations; |
• | anticipated demand for our products and our ability to produce our products in sufficient quantities to meet customer demand; |
• | our ability to manage timelines and costs related to manufacturing our products; |
• | our ability to maintain and expand our marketing and sales networks and the costs related thereto; |
• | our ability to successfully develop new products and the costs associated with designing and developing those new products; |
• | our ability to support the safety and efficacy of our products with long-term clinical data; |
• | our ability to obtain additional debt and equity financing to fund capital expenditures and working capital requirements and acquisitions; |
• | our dependence on a limited number of third-party suppliers for components and raw materials; |
• | our ability to protect our intellectual property, including unpatented trade secrets, and to operate without infringing or misappropriating the proprietary rights of others; |
• | our ability to complete acquisitions, integrate operations post-acquisition and maintain relationships with customers of acquired entities; and |
• | other risk factors described in the section entitled “Risk Factors.” |
These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in this report.
Spin-off from Integra
SeaSpine was incorporated in Delaware on February 12, 2015 in connection with the spin-off of Integra’s spinal fusion hardware and orthobiologics business from Integra’s diversified medical technology business on July 1, 2015.
For periods prior to the spin-off, our consolidated financial statements were prepared on a stand-alone basis and were derived from Integra’s consolidated financial statements and accounting records. Therefore, these financial statements reflected, in conformity with accounting principles generally accepted in the United States, the financial position, results of operations, comprehensive loss and cash flows as the orthobiologics and spinal fusion hardware business was historically operated as part of Integra. They may not be indicative of our future performance and do not necessarily reflect what our consolidated results of operations, financial condition and cash flows would have been had we operated as a separate, publicly-traded company during the periods presented, particularly because we implemented many changes in our operations and capitalization after the spin-off from Integra.
The consolidated financial statements included the attribution of certain assets and liabilities that were historically held at the Integra corporate level but which were specifically identified or attributable to us. However, cash held by Integra was not attributed to us. Integra’s debt and related interest expense also were not allocated to us for any of the periods presented since we were not the legal obligor of the debt and Integra’s borrowings were not directly attributable to us. Integra managed cash centrally and substantially all cash generated by our business through May 4, 2015, the date we implemented a separate
40
enterprise resource planning ("ERP") system for SeaSpine, was assumed to be remitted to Integra. All significant related party transactions between us and Integra were included in the consolidated financial statements and, prior to the spin-off, were considered to be effectively settled for cash at the time the transaction was recorded, with the exception of the purchases from Integra of Mozaik raw materials and finished goods for all periods presented. Prior to the spin-off, SeaSpine purchased a portion of raw materials and finished goods from Integra for the Mozaik family of products, and SeaSpine contract manufactured certain finished goods for Integra. The total net effect of the settlement of the transactions considered to be effectively settled for cash was reflected in the consolidated statements of cash flows as a financing activity and in the consolidated balance sheet as Integra net investment.
Our consolidated statements of operations included our direct expenses for cost of goods sold, research and development, sales and marketing, distribution, and administration as well as allocations of expenses arising from shared services and infrastructure provided by Integra to us, such as costs of information technology, including the costs of a multi-year global ERP implementation, accounting and legal services, real estate and facilities, corporate advertising, insurance services and related treasury, and other corporate and infrastructure services. In addition, other costs allocated to us include restructuring costs, share-based compensation expense and retirement plan expenses related to Integra’s corporate and shared services employees. These operating expenses were allocated to us using estimates that we considered to be a reasonable reflection of the utilization of services provided to or benefits received by us. We expect, however, that the actual expenses that we would have incurred had we been operating as a separate, publicly-traded company for the periods presented would have been lower, in the aggregate, as they would not include the allocation of the multi-year ERP implementation and other corporate strategic initiatives of Integra in place at the time. The allocation methods include pro-rata basis of revenue, standard cost of sales or other measures.
Integra continues to provide some of the services related to these functions to us after the spin-off on a transitional basis for a fee under a transition services agreement. In addition, costs associated with supply agreements with Integra for our Mozaik product line are expected to be at materially different terms than those that were incurred while the business was part of Integra. Also, we are incurring costs as an independent, publicly-traded company that are different from the costs historically allocated to us by Integra. We currently estimate the total operating costs will be $14.0 million to $15.0 million on an annual pre-tax basis for the year ended December 31, 2016.
Subsequent to the spin-off, our financial statements as of and for the year ended December 31, 2015 are presented on a consolidated basis, as we became a separate publicly-traded company on July 1, 2015.
We incurred $20.1 million of non-recurring transaction and spin-off related costs and transition service fees from Integra in the year ended December 31, 2015, including $5.2 million incurred on or after the July 1, 2015 spin-off. These costs include, among other things, branding, legal, accounting and other advisory fees and other costs to separate and transition from Integra.
Overview
We are a global medical technology company focused on the design, development and commercialization of surgical solutions for the treatment of patients suffering from spinal disorders. We have a comprehensive portfolio of orthobiologics and spinal fusion hardware solutions to meet the varying combinations of products that neurosurgeons and orthopedic spine surgeons need to perform fusion procedures in the lumbar, thoracic and cervical spine. We believe this broad combined portfolio of orthobiologics and spinal fusion hardware products is essential to meet the “complete solution” requirements of neurosurgeons and orthopedic spine surgeons.
We report revenue in two product categories: orthobiologics and spinal fusion hardware. Our orthobiologics products consist of a broad range of advanced and traditional bone graft substitutes that are designed to improve bone fusion rates following a wide range of orthopedic surgeries, including spine, hip, and extremities procedures. Our spinal fusion hardware portfolio consists of an extensive line of products to facilitate spinal fusion in MIS, complex spine, deformity and degenerative procedures.
Our U.S. sales organization consists of regional business managers who oversee a broad network of direct orthobiologics sales specialist and independent orthobiologics and spinal fusion hardware sales agents, to whom we consign and loan our products and pay commissions based on the sales of our products that they generate. These sales are generated by building and maintaining relationships with the neurosurgeons and orthopedic spine surgeons who use our products in surgeries or from the hospitals that order our products directly. Our international sales organization is composed of a sales management team that oversees a network of independent orthobiologics and spinal fusion hardware stocking distributors in over 30 countries that purchase products directly from us and independently sell them. For the year ended December 31, 2015, international sales accounted for approximately 10% of our revenue. Our policy is not to sell our products through or participate in physician-owned distributorships.
41
For the year ended December 31, 2015, our total revenue, net was $133.2 million and our net loss was $55.5 million. For the same period, our orthobiologics sales were $67.3 million, and our spinal fusion hardware sales were $65.9 million. We expect to continue to incur losses as we further invest in the expansion of our business, primarily in sales, marketing and research and development, and from the general and administrative expenses we expect to incur due to our operation as an independent, publicly-traded company. As of December 31, 2015, our cash balance was $33.4 million. In connection with the spin-off, Integra contributed $34.0 million in cash to us.
As of February 29, 2016, we had approximately 300 employees.
Components of Our Results of Operations
Revenue
Our net revenue is derived primarily from the sale of orthobiologics and spinal fusion hardware products across North America, Europe, Asia Pacific and Latin America. Sales are reported net of returns, group purchasing organization fees and other customer allowances.
In the United States, we generate most of our revenue by consigning our orthobiologics products and consigning or loaning our spinal fusion hardware sets to hospitals and independent sales agents, who in turn deliver them to hospitals for a single surgical procedure, after which they are returned to us, or leave them with hospitals that are high volume users for multiple procedures. The spinal fusion hardware sets typically contain the instruments, including disposables, and spinal implants required to complete a surgery. We ship replacement inventory to independent sales agents to replace the consigned inventory used in surgeries and maintain and replenish loaned sets at our facility, and return them to a hospital or independent sales agent for the next procedure. We recognize revenue on these consigned or loaned products when they have been used or implanted in a surgical procedure.
For all other transactions, including sales to international stocking distributors and private label partners, we recognize revenue when the products are shipped to the customer or stocking distributor and the transfer of title and risk of loss occurs. There is generally no customer acceptance or other condition that prevents us from recognizing revenue in accordance with the delivery terms.
We entered into certain supply agreements with Integra prior to the spin-off, pursuant to which Integra provides us with certain raw materials and we will provide each other with finished product for further sale in the operation of each other’s business. These supply agreements reflect new pricing compared to our historical related party arrangements.
Cost of Goods Sold
Cost of goods sold primarily consists of the costs of finished goods purchased directly from third parties and raw materials used in the manufacture of our products, plant and equipment overhead, labor costs, packaging costs, amortization of technology-related intangible assets and freight. The majority of our orthobiologics products are designed and manufactured internally. The cost of human tissue and fixed manufacturing overhead costs are significant drivers of the costs of goods sold and consequently our orthobiologics products, at current production volumes, generate lower gross margin than our spinal fusion hardware products. We rely on third-party suppliers to manufacture our spinal fusion hardware products, and we assemble them into surgical sets in-house. Other costs included in cost of goods sold include royalties, shipping, inspection and charges for expired, excess and obsolete inventory. We expect our cost of goods sold to continue to increase in absolute dollars due primarily to increased sales volume.
Selling, General and Administrative Expense
Our selling, general and administrative (“SG&A”) expenses consist primarily of sales commissions to independent sales agents, cost of medical education and training, payroll and other headcount related expenses, depreciation and other expenses recorded against instrument sets, stock-based compensation, the medical device excise tax, marketing expenses, supply chain and distribution expenses, and expenses for information technology, legal, human resources, insurance, finance, facilities, and management, the substantial majority of which were allocated from Integra prior to the spin-off. Subsequent to the spin-off, we are incurring these administrative expenses directly as an independent, publicly-traded company.
We expect our SG&A expenses, excluding allocations from Integra incurred prior to the spin-off, to increase as we continue to hire additional personnel to support the growth of our business, expand our product portfolio and add related sales and marketing personnel, and as a result of being an independent, publicly-traded company.
42
Research and Development Expense
Our research and development (“R&D”) expenses primarily consist of expenses related to the headcount for engineering, product development, clinical affairs and regulatory functions as well as consulting services, third-party prototyping services, outside research and clinical studies activities, and materials, production and other costs associated with development of our products. We expense R&D costs as they are incurred.
While our R&D expenses fluctuate from period to period based on the timing of specific initiatives, we expect that these costs will increase over time as we continue to design and commercialize new products and expand our product portfolio, add related personnel and conduct additional clinical activities.
Intangible Amortization
Our intangible amortization, including the amounts reported in cost of goods sold, consists of acquisition-related amortization and impairments related to product discontinuations. We expect total annual amortization expense (including amounts reported in cost of goods sold) to be approximately $7.0 million in 2016, $5.8 million in 2017, $5.5 million in 2018, $4.8 million in 2019 and $3.9 million in 2020.
Other Expense, Net
Other expense, net consists of non-operating items such as interest income and expense, and foreign exchange transaction gains and losses on related party transactions and balances.
RESULTS OF OPERATIONS
Year Ended December 31, | ||||||||||||
(In thousands, except percentages) | 2015 | 2014 | 2013 | |||||||||
Total revenue, net | $ | 133,178 | $ | 138,695 | $ | 146,586 | ||||||
Cost of goods sold | 61,119 | 56,714 | 55,532 | |||||||||
Gross profit | 72,059 | 81,981 | 91,054 | |||||||||
Gross margin | 54.1 | % | 59.1 | % | 62.1 | % | ||||||
Operating expenses: | ||||||||||||
Selling, general and administrative | 110,551 | 88,213 | 93,009 | |||||||||
Research and development | 8,353 | 8,527 | 9,893 | |||||||||
Intangible amortization | 5,331 | 5,590 | 5,598 | |||||||||
Total operating expenses | 124,235 | 102,330 | 108,500 | |||||||||
Operating loss | (52,176 | ) | (20,349 | ) | (17,446 | ) | ||||||
Other expense, net | (877 | ) | (269 | ) | (4,556 | ) | ||||||
Loss before income taxes | (53,053 | ) | (20,618 | ) | (22,002 | ) | ||||||
Provision for income taxes | 2,479 | 3,927 | 3,744 | |||||||||
Net loss | $ | (55,532 | ) | $ | (24,545 | ) | $ | (25,746 | ) | |||
Year Ended December 31, 2015 Compared to Year Ended December 31, 2014
Revenue
Total revenue, net decreased in 2015 by $5.5 million, or 4%, to $133.2 million compared to $138.7 million for the prior year.
43
Year Ended December 31, | ||||||||
2015 | 2014 | |||||||
(In millions) | ||||||||
Orthobiologics | $ | 67.3 | 67.6 | |||||
% of total revenue, net | 51 | % | 49 | % | ||||
Spinal Fusion Hardware | 65.9 | 71.1 | ||||||
% of total revenue, net | 49 | % | 51 | % | ||||
Total revenue, net | $ | 133.2 | $ | 138.7 | ||||
Orthobiologics revenues totaled $67.3 million in 2015, roughly flat compared to the prior year. Sales in the United States increased 2% in 2015 to $59.9 million primarily because of increased demand for demineralized bone matrix products, especially our third-generation products. This growth was somewhat offset by lower sales of our synthetic bone matrix products. A decrease of $1.0 million in international orthobiologics revenues compared to the prior year period further offset the increase in domestic orthobiologics revenues. Supply shortages in our third-generation demineralized bone matrix products limited growth in the orthobiologics portfolio in 2015. However, we expect that recent increases in production capacity and output have alleviated these supply issues and do not expect supply to limit revenue growth in 2016.
Spinal fusion hardware revenues totaled $65.9 million in 2015, a decrease of 7% from the prior year. The decrease was mostly due to declined sales of $5.0 million in the U.S. market. The U.S. hardware business continued to face pricing pressures and lower demand for our older product lines (in aggregate 108,000 items were sold in 2015 as compared to 136,000 in 2014), while delays in introducing some of our new products in 2015 negatively impacted revenue in the current year. However, the decline in sales of U.S. spinal fusion hardware products decelerated in the second half of 2015, with fourth quarter of 2015 sales roughly flat vs. the prior year, as sales of new and recently launched products began to accelerate. We expect sales of these new and recently launched products to continue to accelerate in 2016 and offset the anticipated continued decline in sales our older spinal fusion hardware product lines. Sales of our spinal hardware fusion products internationally totaled $5.5 million in 2015, roughly flat compared to the prior year.
The following table sets forth our total revenue, net by geography:
Year Ended December 31, | ||||||||
2015 | 2014 | |||||||
(In millions) | ||||||||
United States | $ | 120.3 | $ | 124.4 | ||||
International | 12.9 | 14.3 | ||||||
Total revenue, net | $ | 133.2 | $ | 138.7 | ||||
Cost of Goods Sold and Gross Margin
Cost of goods sold in 2015 increased $4.4 million to $61.1 million. Gross margin was 54.1% in 2015 and 59.1% for the prior year. The decrease in gross margin was mainly driven by a higher percentage of sales in 2015 being derived from orthobiologics products, which have lower gross margin than our hardware products, and by additional charges for excess and obsolete spinal fusion hardware inventory recorded in 2015, a large portion of which relates to a shift in our international strategy after the spin-off. During the third quarter of 2015, we assessed our growth strategy for international markets and determined that we will deploy and invest our limited sales and marketing resources dedicated to international markets in a more targeted manner in fewer countries. As we introduce more new products in the future, we expect to leverage those new product launches to lead our international expansion activities. As a result of this shift in international strategy, we recorded $2.6 million of excess and obsolete charges in the third quarter of 2015 against certain inventory targeted for international distribution that that we no longer expect that we will be able to sell. The higher costs were partially offset by total lower manufacturing costs in 2015 resulting from increased production volumes and more efficient production of our orthobiologics product portfolio, and a $1.0 million charge recorded in 2014 related to a discontinued product line. Cost of goods sold included $2.7 million and $2.6 million of amortization for technology-based intangible assets in 2015 and 2014, respectively. Allocations from Integra accounted for $0.5 million of expense in 2015 compared to $1.3 million for the prior year.
In December 2015, we began to manufacture the majority of our collagen ceramic matrix product supply needs in our Irvine facility. Prior to that, we purchased most of our supply of collagen ceramic matrix products from Integra either a) subsequent to
44
the spin-off under our supply agreement with Integra or b) prior to the spin-off at a calculated transfer price. We typically manufacture those products internally at a lower cost than the price that we purchased those products from Integra. Accordingly, as we begin to sell in 2016 those products we manufacture internally, we expect our gross margin to increase.
Selling, General and Administrative
SG&A expenses increased $22.3 million to $110.6 million in 2015. The increase in SG&A expenses was mainly driven by an increase of $17.1 million of nonrecurring spin-off related charges, $1.9 million of fees incurred under a transition services agreement with Integra, and an approximately $14.8 million increase in direct operating expenses after the spin-off that were previously accounted for in the allocation from Integra, including higher salary costs due to increased sales, marketing and administrative headcount, and increased costs associated with being an independent, publicly-traded company, such as higher stock-based compensation expense, higher salary costs due to the hiring of an executive management team, medical device exercise tax expenses, and increased audit, legal, insurance, and information technology-related fees. These increases were offset by a $9.0 million reduction in allocations from Integra, $0.6 million lower sales commissions resulting from the decrease in domestic sales, and $1.0 million of lower instrument set depreciation as more of our older spinal fusion hardware product lines became fully depreciated. Allocations from Integra accounted for $8.6 million of expense in 2015 compared to $17.6 million for the prior year.
Research and Development
R&D expenses decreased $0.2 million to $8.4 million, or 6% of revenue, in 2015. The decrease in R&D expenses in 2015 was primarily the result of $0.2 million lower allocations from Integra in 2015 as the spin-off was completed on July 1, 2015. Allocations from Integra totaled $0.3 million and $0.5 million in 2015 and 2014, respectively. In 2016, we plan to increase our investment in R&D to between 7%-9% of revenues as we continue to add personnel and accelerate the design and commercialization of new products to expand our product portfolio and conduct additional clinical activities.
Intangible Amortization
Intangible amortization expense, excluding $2.7 million of reported in cost of goods sold for technology-based intangible assets, decreased $0.3 million to $5.3 million in 2015, primarily due to non-compete agreements that were fully amortized during the second quarter of 2015.
Other Expense, Net
Other expense, net increased $0.6 million to $0.9 million in 2015, primarily due to foreign exchange remeasurement losses offset by the positive impact of foreign exchange rates on related party loans.
Income Taxes
Year Ended December 31, | |||||||
2015 | 2014 | ||||||
(In thousands) | |||||||
Loss before income taxes | $ | (53,053 | ) | $ | (20,618 | ) | |
Provision for income taxes | 2,479 | 3,927 | |||||
Effective tax rate | (4.7 | )% | (19.0 | )% | |||
The primary drivers of the effective tax rate in 2015 and 2014 were pretax losses incurred by the consolidated U.S. tax group that received no corresponding tax benefit and pretax income incurred by a U.S. subsidiary not included in the Company’s U.S. consolidated federal income tax return prior to September 1, 2015.
We reported income tax expense in 2015 and 2014 related to the taxable income generated by a U.S. subsidiary that was not part of the U.S. consolidated tax group through August 31, 2015. As such, despite the losses before income taxes reported in those periods, the taxable income generated by such U.S. subsidiary was not allowed to be offset against the taxable losses generated by our other U.S. subsidiaries through August 31, 2015. Effective September 1, 2015, we made an election that allows us to offset any future taxable losses generated by our U.S. subsidiaries against any future taxable income generated by our U.S. subsidiaries.
45
The income tax provision in the consolidated statements of operations for periods prior to the spin-off was calculated using the separate return method, as if we had filed a separate tax return and operated as a stand-alone business. However, because Integra historically generated taxable income in excess of our pretax losses incurred prior to the spinoff and all of our U.S. subsidiaries that incurred these pretax losses were included in Integra’s U.S. consolidated tax group, those pretax losses were more than offset by Integra’s taxable income. Therefore, there were no U.S. net operating losses available to us for future use at the date of the spin-off.
In addition, for any pretax losses incurred subsequent to the spin-off by the consolidated U.S. tax group or otherwise, we recorded no corresponding tax benefit because we have concluded that it is more-likely-than-not that we will be unable to realize the benefit from any resulting deferred tax assets. We will continue to assess our position in future periods to determine if it is appropriate to reduce a portion of our valuation allowance in the future.
Year Ended December 31, 2014 Compared to Year Ended December 31, 2013
Revenue
Total revenue, net decreased by $7.9 million, or 5.4%, to 138.7 million in 2014 compared to $146.6 million in 2013.
Year Ended December 31, | ||||||||
2014 | 2013 | |||||||
(In millions) | ||||||||
Orthobiologics | $ | 67.6 | $ | 66.7 | ||||
% of total revenue, net | 49 | % | 45 | % | ||||
Spinal Fusion Hardware | 71.1 | 79.9 | ||||||
% of total revenue, net | 51 | % | 55 | % | ||||
Total revenue, net | $ | 138.7 | $ | 146.6 | ||||
Orthobiologics revenues totaled $67.6 million in 2014, an increase of 1.4% compared to 2013. Sales in the United States increased 3% in 2014 to $58.9 million and were led by increased demand for demineralized bone matrix products, especially our third-generation products. Sales of our orthobiologics products decreased internationally in 2014 compared to 2013, primarily as a result of the discontinuation of a product line in early 2014.
Spinal fusion hardware revenues totaled $71.1 million in 2014, a decrease of 11% compared to 2013. Sales in the United States decreased 8.3% compared to the prior year. The U.S. hardware business continued to face pricing pressures and lower demand for our older product lines. International sales decreased as a result of large stocking orders from new stocking distributors in 2013 that did not recur in 2014.
The following table sets forth our total revenue, net by geography:
Year Ended December 31, | ||||||||
2014 | 2013 | |||||||
(In millions) | ||||||||
United States | $ | 124.4 | $ | 128.7 | ||||
International | 14.3 | 17.9 | ||||||
Total revenue, net | $ | 138.7 | $ | 146.6 | ||||
Cost of Goods Sold and Gross Margin
Costs of goods sold increased by $1.2 million, or 2.1%, to $56.7 million in 2014 compared to $55.5 million in 2013. Gross margin was 59.1% in 2014 and 62.1% in 2013. The decrease in gross margin from 2013 to 2014 resulted primarily from increased manufacturing costs and because our lower-margin orthobiologics products represented a greater percentage of our revenues.
Cost of goods sold in 2014 and 2013 included $0.3 million and $0.8 million, respectively, in fair value inventory purchase accounting adjustments recorded in connection with acquisitions and $2.6 million of amortization for technology-based
46
intangible assets in both 2014 and 2013. Allocations from Integra accounted for $1.3 million of expense in 2014 as compared to $1.2 million in 2013.
Selling, General and Administrative
SG&A expenses decreased by $4.8 million, or 5.2%, to $88.2 million in 2014 compared to $93.0 million in 2013, driven by lower sales commissions from fewer domestic sales, decreased instrument set depreciation and the impact of costs for structural optimization incurred in 2013 arising from the closure of our facilities in northeast Ohio. Allocations from Integra accounted for $17.6 million of expense in 2014 as compared to $17.4 million in 2013.
Research and Development
R&D expenses decreased by 13.8% to $8.5 million in 2014 compared to $9.9 million in 2013. The decrease in R&D expenses from 2013 to 2014 resulted mostly from a reduction in compensation-related costs because of planned and unplanned turnover in headcount and decreased external spending and project delays, in part because Integra decided to prioritize R&D spending in other areas of its business. Allocations from Integra accounted for $0.5 million of expense in 2014 compared to $0.4 million in 2013.
Intangible Amortization
Amortization expense, excluding $2.6 million reported in cost of goods sold for technology-based intangible assets, was $5.6 million in 2014 and 2013.
Other Expense, Net
Other expense, net was $0.3 million in 2014 compared to $4.6 million in 2013. Related-party interest expense decreased $4.6 million in 2014 primarily as a result of the capitalization of related-party loan activity in July 2013.
Income Taxes
We recorded income tax expense of $3.9 million and $3.7 million in 2014 and 2013, respectively. Our effective income tax rate was (19.0)% and (17.0)% of loss before income taxes in 2014 and 2013, respectively. We reported income tax expense in 2014 and 2013, despite the fact that we reported losses before income taxes, because our legal entity structure at that time did not permit us to offset taxable losses generated by certain U.S. subsidiaries against the taxable income generated by another of our U.S. subsidiaries. Effective September 1, 2015, we made an election that will allow us to offset any future taxable losses generated by our U.S. subsidiaries against any future taxable income generated by our U.S. subsidiaries. There is no future tax benefit for such prior year losses because we have no assurance that future taxable income will be generated to allow for the recognition of such losses.
The income tax provision in the consolidated statements of operations for periods prior to the spin-off was calculated using the separate return method, as if we had filed a separate tax return and operated as a stand-alone business. However, because Integra historically generated taxable income in excess of our pretax losses incurred prior to the spin-off and all of our U.S. subsidiaries that incurred these pretax losses were included in Integra’s U.S. consolidated tax group, those pretax losses were more than offset by Integra’s taxable income. Therefore, there were no U.S. net operating losses available to us for future use at the date of the spin-off.
In addition, for any pretax losses incurred subsequent to the spin-off by the consolidated U.S. tax group or otherwise, we recorded no corresponding tax benefit because we have concluded that it is more-likely-than-not that we will be unable to realize the value of any resulting deferred tax assets. We will continue to assess our position in future periods to determine if it is appropriate to reduce a portion of our valuation allowance in the future.
47
Business Factors Affecting the Results of Operations
Special Charges
We define special charges as expenses for which the amount or timing can vary significantly from period to period, and for which the amounts are non-cash in nature, or the amounts are not expected to recur at the same magnitude.
We believe that identification of these special charges provides important supplemental information to investors regarding financial and business trends relating to our financial condition and results of operations. Investors may find this information useful in assessing comparability of our operating performance from period to period, against the business model objectives that management has established, and against other companies in our industry. We provide this information to investors so that they can analyze our operating results in the same way that management does and use this information in their assessment of the core business and valuation of SeaSpine.
Loss before income taxes includes the following special charges:
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
SeaSpine spin-off related charges | $ | 17,278 | $ | 2,310 | $ | — | |||||
Transition services agreement charges | 2,809 | — | — | ||||||||
Discontinued product line charges | — | 860 | — | ||||||||
Structural optimization charges | — | — | 3,462 | ||||||||
Acquisition-related charges | — | 257 | 796 | ||||||||
Total | $ | 20,087 | $ | 3,427 | $ | 4,258 | |||||
The items reported above are reflected in the consolidated statements of operations as follows:
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
Cost of goods sold | $ | 648 | $ | 1,117 | $ | 796 | |||||
Selling, general and administrative | 19,439 | 2,310 | 3,462 | ||||||||
Total | $ | 20,087 | $ | 3,427 | $ | 4,258 | |||||
These special charges are directly related to the SeaSpine business and do not include allocations from Integra. SeaSpine spin-off related charges include legal, accounting, program management and outside consulting expenses incurred as part of the spin-off from Integra, and incremental personnel costs associated with becoming an independent, publicly-traded company. Discontinued product line charges are related to the exit of one of our product lines sold internationally. Acquisition-related charges include transaction fees and the amortization of inventory fair value adjustments related to acquisitions.
Liquidity and Capital Resources
Overview
Prior to the spin-off, Integra provided financing, cash management and other treasury services to us, and we transferred the majority of cash from operations to Integra; accordingly we generally had no significant cash. With the implementation of our own global ERP system on May 4, 2015, we began to collect against our own accounts receivable, including accounts receivable with Integra, and to directly pay some of our obligations. Effective with the spin-off, we no longer transfer any of our cash to Integra and began to directly pay all of our obligations. Cash historically transferred to and from Integra prior to the spin-off has been reflected in the consolidated statement of cash flows as Integra net investment and in the consolidated balance sheet through Integra net investment.
We believe that our cash and cash equivalents in the U.S., and the maximum of $30.0 million borrowing capacity that we have under a credit facility that we entered into in December 2015, will be sufficient to fund our operations for at least the next twelve months.
Cash and Cash Equivalents
48
We had cash and cash equivalents totaling approximately $33.4 million and $0.7 million at December 31, 2015 and December 31, 2014, respectively.
Cash Flows
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
Net cash (used in) provided by operating activities | $ | (32,566 | ) | $ | 806 | $ | (7,480 | ) | |||
Net cash used in investing activities | (11,705 | ) | (3,804 | ) | (5,550 | ) | |||||
Net cash provided by financing activities | 77,130 | 3,012 | 13,581 | ||||||||
Effect of exchange rate fluctuations on cash | (82 | ) | (8 | ) | 4 | ||||||
Net increase in cash and cash equivalents | $ | 32,777 | $ | 6 | $ | 555 | |||||
Net Cash Flows (Used in) Provided by Operating Activities
We used $32.6 million and generated operating cash flows of $0.8 million in 2015 and 2014, respectively.
Operating cash outflows for the year ended December 31, 2015 increased by $33.4 million compared to the year ended December 31, 2014. Net loss plus adjustments to reconcile net loss to net cash (used in) provided by operating activities decreased cash flows by $24.9 million, largely driven by spin-off related charges, and higher operating expenses related to being a separate, publicly traded entity after the spin-off. Among the changes in working capital, purchases of inventory used $3.2 million more cash and the increase in accounts receivable decreased operating cash flow activities by $5.0 million in 2015.
Operating cash flows for 2014 improved as compared to the same period in 2013. Cash generated by working capital changes
increased by $8.7 million. Net loss plus items included in that loss for non-cash expenses were flat compared to the prior year. The main driver of the change in working capital was a $7.5 million reduction in inventory builds in 2014 compared to 2013. In 2013, we invested a substantial amount in inventory to support new product launches in Europe and to achieve safety stock levels more appropriate for the business.
Net Cash Flows Used in Investing Activities
Net cash used in investing activities was $11.7 million for in 2015 compared to $3.8 million in 2014. The increased use of cash was primarily attributable to the implementation of a global ERP system and new hardware and software required to meet our needs after the spin-off and $4.6 million of purchases of spinal fusion hardware sets and instruments related to existing products and new product launches.
Net cash used in investing activities was $3.8 million in 2014 compared to $5.6 million in 2013. The decrease in cash used was attributable to fewer purchases of spine hardware sets and instruments in 2014.
Net Cash Flows Provided by Financing Activities
Net cash provided by financing activities was $77.1 million in 2015 compared to net cash provided by financing activities of $3.0 million in 2014. The increase in cash resulted from a higher investment from Integra and the $34 million cash contribution from Integra in connection with the spin-off.
Net cash provided by financing activities was $3.0 million in 2014 compared to net cash provided by financing activities of $13.6 million in 2013. The decrease in cash resulted from a decrease in investment from Integra because of improved cash flows in 2014 compared to 2013.
Credit Facility
On December 24, 2015, we entered into a three-year credit facility (the "Credit Facility") with Wells Fargo Capital Finance, as Administrative Agent and as a Lender. The Credit Facility provides a revolving line of credit of up to $30.0 million in borrowing capacity with a maturity date of December 24, 2018, which maturity date is subject to a one-time one-year extension at our election. In connection with the Credit Facility, we were required to become guarantors and to provide a security interest in substantially all our assets for the benefit of Agent and the Lender.
49
Our borrowings under the Credit Facility shall accrue interest at the rate then applicable to the Base Rate (as customarily defined) Loans, unless and until converted into LIBOR Rate Loans in accordance with the terms of the Credit Facility. Borrowings bear interest at a floating annual rate equal to (a) during any month for which our average excess availability (as customarily defined) is greater than $20.0 million, base rate plus (i) 1.25 percentage points for base rate loans and (ii) LIBOR rate plus 2.25 percentage points for LIBOR loans, (b) during any month for which our average excess availability is greater than $10.0 million but less than or equal to $20.0 million, (i) base rate plus 1.50 percentage points for base rate loans and (ii) LIBOR rate plus 2.50 percentage points for LIBOR loans and (c) during any month for which our average excess availability is less than or equal to $10.0 million, (i) base rate plus 1.75 percentage points for base rate loans and (ii) LIBOR rate plus 2.75 percentage points for LIBOR loans.
We will also pay an annual unused line fee in an amount equal to 0.375% times the unused Credit Facility amount. The unused line fee is due and payable on the first day of each month. At December 31, 2015, there was $0.3 million outstanding under the Credit Facility. Debt issuance costs and legal fees related to the financing totaling $0.4 million were recorded as a deferred asset and are subsequently being amortized ratably over the term of the Credit Facility.
The Credit Facility contains various customary affirmative and negative covenants agreed to by us, including prohibiting us from incurring indebtedness without the lender’s consent. The Credit Facility also includes a financial covenant that requires us to maintain a minimum fixed charge coverage ratio of 1.10 to 1.00 for the applicable measurement period if our Total Liquidity (as defined in the Credit Facility) is less than $5.0 million. We were in compliance with all such covenants at December 31, 2015.
The Credit Facility also includes customary events of default, including events of default relating to non-payment of amounts due under the Credit Facility, material inaccuracy of representations and warranties, violation of covenants, bankruptcy and insolvency, failure to comply with health care laws, violation of certain of our existing agreements, and the occurrence of a change of control. Under the Credit Facility, if an event of default occurs, lenders holding a majority of the revolving commitments will have the right to terminate the commitments and accelerate the maturity of any loans outstanding.
Off-Balance Sheet Arrangements
There were no off-balance sheet arrangements as of December 31, 2015 that have or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to our business.
Contractual Obligations and Commitments
As of December 31, 2015, we were obligated to pay the following amounts under various agreements:
Total | Less than 1 Year | 1-3 Years | 4-5 Years | More than 5 Years | |||||||||||||||
(In millions) | |||||||||||||||||||
Employment Agreements | $ | 1.6 | $ | 0.5 | $ | 1.1 | $ | — | $ | — | |||||||||
Operating Leases | 23.3 | 2.3 | 4.4 | 4.6 | 12.0 | ||||||||||||||
Purchase Obligations | 6.7 | 6.7 | — | — | — | ||||||||||||||
Credit Facility | 0.3 | — | 0.3 | — | — | ||||||||||||||
Other | 2.2 | 0.3 | 0.9 | 1.0 | — | ||||||||||||||
Total | $ | 34.1 | $ | 9.8 | $ | 6.7 | $ | 5.6 | $ | 12.0 | |||||||||
Excluded from the table is the liability for uncertain tax benefits, including interest and penalties, totaling approximately $0.3 million. This liability for uncertain tax benefits has been excluded because we cannot make a reliable estimate of the period in which such liability may be realized. "Other" includes minimum royalties and milestone payments for certain license agreements.
Critical Accounting Polices and the Use of Estimates
Our discussion and analysis of financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of
50
assets and liabilities, the disclosure of contingent liabilities, and the reported amounts of revenues and expenses. Significant estimates affecting amounts reported or disclosed in the consolidated financial statements include revenue recognition, allowances for doubtful accounts receivable and sales returns and allowances, net realizable value of inventories, amortization periods for acquired intangible assets, estimates of projected cash flows and discount rates used to value intangible assets and test them for impairment, estimates of projected cash flows and depreciation and amortization periods for long-lived assets, valuation of stock-based compensation, computation of taxes and valuation allowances recorded against deferred tax assets, and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from these estimates.
We believe that the following accounting policies, which form the basis for developing these estimates, are those that are most critical to the presentation of our consolidated financial statements and require the more difficult subjective and complex judgments:
Revenue Recognition
Our net sales are derived primarily from the sale of orthobiologics and spinal fusion hardware products globally. Sales are reported net of returns, group purchasing organization fees and other customer allowances.
Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred, title and risk of loss have passed to the customer, there is a fixed or determinable sales price and collectability of that sales price is reasonably assured.
In the United States, we generate most of our revenue by consigning our orthobiologics products and consigning or loaning our spinal fusion hardware sets to hospitals and independent sales agents, who in turn deliver them to the hospital for a single surgical procedure or leave them with hospitals that are high volume users for use in multiple procedures. The spinal fusion hardware sets typically contain the instruments, including disposables, and spinal implants required to complete a surgery. We ship replacement inventory to independent sales agents to replace the consigned inventory used in surgeries and maintain and replenish the loaned sets and return them to a hospital or independent sales agent for the next procedure. We recognize revenue on these consigned or loaned products when they have been used or implanted in a surgical procedure.
For all other transactions, including sales to international stocking distributors, we recognize revenue when the products are shipped to the customer or stocking distributor and the transfer of title and risk of loss occurs. There are generally no customer acceptance or other conditions that prevent us from recognizing revenue in accordance with the delivery terms.
Product royalties are estimated and recognized in the same period that the royalty-based products are sold by licensees. We estimate and recognize royalty revenue based upon communication with licensees, historical information and expected sales trends. Differences between actual revenues and estimated royalty revenues are adjusted in the period in which they become known, which is typically the following quarter. Historically, such adjustments have not been significant.
Allowance for Doubtful Accounts Receivable
We evaluate the collectability of accounts receivable based on a combination of factors. In circumstances where a specific customer is unable to meet its financial obligations to us, we record an allowance to reduce the net recognized receivable to the amount that we reasonably expect to collect. For all other customers, we record allowances for doubtful accounts based on the length of time the receivables are past due, the current business environment and our historical experience. If the financial condition of customers or the length of time that receivables are past due were to change, we may change the recorded amount of allowances for doubtful accounts in the future through charges or reductions to SG&A expense.
Inventories
Inventories, consisting of purchased materials, direct labor and manufacturing overhead, are stated at the lower of cost, the value determined by the first-in, first-out method, or the market methods. At each balance sheet date, we evaluate ending inventories for excess quantities, obsolescence or shelf-life expiration. Our evaluation includes an analysis of our current and future strategic plans, historical sales levels by product, projections of future demand by product, the risk of technological or competitive obsolescence for our products, general market conditions, a review of the shelf-life expiration dates for our products, and the feasibility of reworking or using excess or obsolete products or components in the production or assembly of other products that are not obsolete or for which we do not have excess quantities in inventory. To the extent that we determine there are excess or obsolete quantities or quantities with a shelf life that is too near its expiration for us to reasonably expect that we can sell those products prior to their expiration, we adjust their carrying value to estimated net realizable value. If future demand or market conditions are lower than our projections or if we are unable to rework excess or obsolete quantities into
51
other products, we may record further adjustments to the carrying value of inventory through a charge to cost of goods sold in the period the revision is made. In addition, we capitalize inventory costs associated with certain products prior to regulatory approval, based on management’s judgment of probable economic benefit. We could be required to expense previously capitalized costs related to pre-approval inventory upon a change in such judgment, due to, among other potential factors, a denial or delay of approval by necessary regulatory bodies or a decision by management to discontinue the related development program.
Property, Plant and Equipment
Property, plant and equipment is carried at cost less accumulated depreciation. Depreciation is computed using the straight-line method based on an asset’s estimated useful life. Effective July 2015, we changed the estimated useful lives for leasehold improvements at our Vista facility to less than one year to align with the lease term expiring on the May 23, 2016 for our leased offices located in Vista. This change was triggered by our execution of a sublease for the new facility located in Carlsbad in July 2015. All current operations at the Vista facility will be transitioned to the Carlsbad facility or other locations before the end of the Vista lease term. Maintenance and repairs on all property and equipment are expensed as incurred.
Valuation of Identifiable Intangible Assets
We review identifiable intangible assets with definite lives for impairment annually. We continually assess whether events or changes in circumstances represent a triggering event that would require us to complete an impairment assessment. Factors that we consider in determining whether a triggering event has occurred include a significant change in the business climate, legal factors, operating performance indicators, competition, sale or disposition of significant assets or products. Application of these impairment tests requires significant judgments, including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth for our business, the useful life over which cash flows will occur and determination of our weighted-average cost of capital.
Should a triggering event be deemed to occur, we are required to estimate the expected net cash flows to be realized over the life of the asset and/or the asset’s fair value. Fair values are determined by a discounted cash flow model. These estimates are also subject to significant management judgment including the determination of many factors such as revenue growth rates, cost growth rates, terminal value assumptions and discount rates. Changes in these estimates can have a significant impact on the determination of cash flows and fair value and could potentially result in future material impairments.
We initially record identifiable intangible assets at fair value at the time of acquisition, generally using an income or cost approach. We capitalize costs incurred to renew or extend the term of recognized intangible assets and amortize those costs over their expected useful lives.
Valuation of Stock-Based Compensation
The estimated fair value of stock-based awards exchanged for employee and non-employee director services are expensed over the requisite service period.
For purposes of calculating stock-based compensation, we estimate the fair value of stock options using a Black-Scholes option-pricing model. The determination of the fair value of stock-based payment awards utilizing the Black-Scholes model is affected by our stock price and a number of assumptions, including expected volatility, expected term, risk-free interest rate and expected dividends. Due to our limited historical data as a separate public company, the expected volatility is calculated based upon the historical volatility of comparable companies in the medical device industry whose share prices are publicly available for a sufficient period of time. The expected term of options is calculated using the simplified method as prescribed by accounting guidance for stock-based compensation. The risk-free interest rates are derived from the U.S. Treasury yield curve in effect on the date of grant for instruments with a remaining term similar to the expected term of the options. We considered that we have never paid cash dividends and do not currently intend to pay cash dividends. The fair value of restricted stock units granted is based on the market price of our common stock on the date of grant. In addition, we apply an expected forfeiture rate when amortizing stock-based compensation expense. The expected forfeiture rate of stock options is based on historical patterns of the employee turnover rate and is estimated to be 10% annually for stock-based compensation expense recorded for the year ended December 31, 2015. As individual grant awards become fully vested, stock-based compensation expense is adjusted to recognize actual forfeitures.
If factors change and we employ different assumptions, stock-based compensation expense may differ significantly from what we have recorded in the past. If there is a difference between the assumptions used in determining stock-based compensation expense and the actual factors which become known over time, specifically with respect to anticipated forfeitures, we may
52
change the input factors used in determining stock-based compensation costs for future grants. These changes, if any, may materially impact our results of operations in the period such changes are made.
Income Taxes
The income tax provision in the consolidated statements of operations for periods prior to the spin off was calculated using the separate return method, as if we filed a separate tax return and operated as a stand-alone business. Therefore, cash tax payments and items of current and deferred taxes may not be reflective of our actual tax balances included in Integra’s historical consolidated income tax return. More specifically, the presentation of substantial net operating losses, and any related valuation allowances, presented herein prior to the spin-off do not represent actual net operating losses that have been incurred by us or that are available for carryforward to a future tax year.
We reported income tax expense for the year ended December 31, 2015 related to the taxable income generated
by a U.S. subsidiary that was not part of the U.S. consolidated tax group as of August 31, 2015. As such, despite the
reported losses before income taxes in those periods, the taxable income generated by such U.S. subsidiary was not
allowed to be offset against the taxable losses generated by our other U.S. subsidiaries through August 31, 2015. Effective
September 1, 2015, we made an election that will allow us to offset any future taxable losses generated by our U.S. subsidiaries against any future taxable income generated by our U.S. subsidiaries.
Changes in the tax rates of the various jurisdictions in which we operate affect our profits. In addition, we maintain a reserve for uncertain tax benefits, changes to which could impact our effective tax rate in the period such changes are made. The effective tax rate can also be impacted by changes in valuation allowances of deferred tax assets, and tax law changes.
Our provision for income taxes may change period-to-period based on specific events, such as the settlement of income tax audits and changes in tax laws, as well as general factors, including the geographic mix of income before taxes, state and local taxes.
We recognize a tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is not material for any period presented.
We believe that we have identified all reasonably identifiable exposures and the reserve we have established for identifiable exposures is appropriate under the circumstances; however, it is possible that additional exposures exist and that exposures will be settled at amounts different than the amounts reserved. It is also possible that changes in facts and circumstances could cause us to either materially increase or reduce the carrying amount of our tax reserves.
Our deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and their basis for income tax purposes, and also the temporary differences created by the tax effects of capital loss, net operating loss and tax credit carryforwards. We record valuation allowances to reduce deferred tax assets to the amounts that are more likely than not to be realized. We could recognize no benefit from our deferred tax assets or we could recognize some or all of the future benefit depending on the amount and timing of taxable income we generate in the future.
Loss Contingencies
We are subject to claims and lawsuits in the ordinary course of our business, with respect to our products, our current or former employees, and involving commercial disputes. We accrue for loss contingencies when it is deemed probable that a loss has been incurred and that loss is estimable. The amounts accrued are based on the full amount of the estimated loss before considering insurance proceeds, if applicable, and do not include an estimate for legal fees expected to be incurred in connection with the loss contingency. We accrue legal fees expected to be incurred in connection with loss contingencies as those fees are incurred by outside counsel as a period cost. Our financial statements do not reflect any material amounts related to possible unfavorable outcomes of claims and lawsuits to which we are currently a party because we currently believe that such claims and lawsuits are not expected, individually or in the aggregate, to result in a material and adverse effect on our financial condition.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to various market risks, including changes in foreign currency exchange rates and interest rates that could adversely affect our results of operations and financial condition.
53
Foreign Currency Exchange Risk
We operate on a global basis and are exposed to the risk that changes in foreign currency exchange rates could adversely affect our financial condition, results of operations and cash flows. In 2015, 2014 and 2013, we generated revenues outside the United States in multiple foreign currencies including euros, British pounds, Swiss francs and New Zealand dollars, and in U.S. dollar-denominated transactions conducted with customers who generated revenue in currencies other than the U.S. dollar. We also incur operating expenses in euros. As a result, changes in the exchange rates of any such foreign currency vs. the U.S. dollar may affect our revenues, gross profits and net loss and may also affect the book value of our assets and the amount of stockholders’ equity. We cannot predict the consolidated effects of exchange rate fluctuations upon our future operating results because of the variability of foreign currency exposure in our revenues and operating expenses and the potential volatility of currency exchange rates.
Interest Rate Risk
Our primary exposure to market risk is interest expense and interest income sensitivity, which is affected by changes in the general level of U.S. interest rates.
Our cash and cash equivalents as of December 31, 2015 consisted of cash and bank deposit sweep. We are exposed to market risk related to fluctuations in interest rates and market prices. We currently do not hedge interest rate exposure. However, because of the short-term nature of the instruments in our portfolio, a sudden change in market interest rates would not be expected to have a material impact on our financial condition and/or results of operation.
We had outstanding borrowings under the Credit Facility of $0.3 million as of December 31, 2015. Interest expense is accrued at an annual interest rate plus a margin with a range of 1.25 and 1.75 percentage points. The annual interest rate is equal to the greatest of (a) the Federal Funds Rate plus ½%, (b) the LIBOR Rate (which rate shall be calculated based upon an interest period of 1 month and shall be determined on a daily basis), plus 1 percentage point, and (c) the rate of interest announced within Wells Fargo at its principal office in San Francisco as its “prime rate.” A hypothetical 100 basis point change in interest rates would not be expected to have a material effect on our net loss for the period or cash flow.
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Financial statements and the financial statement schedules specified by this Item, together with the report thereon of PricewaterhouseCoopers LLP, are presented following Item 15 of this report.
Information on quarterly results of operations is set forth in our financial statements under Note 12, “Selected Quarterly Information — Unaudited,” to our consolidated financial statements.
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES |
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the direction of our Chief Executive Officer and Chief Financial Officer, we evaluated our disclosure controls and procedures and concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report.
Management’s Annual Report on Internal Control Over Financial Reporting
This annual report on Form 10-K does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our independent registered public accounting firm due to a transition period established by the rules of the SEC for newly public companies.
54
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting identified in connection with the evaluation required by Rules 13a-15(d) and 15d-15(d) under the Securities and Exchange Act of 1934, as amended, that occurred during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
Part III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Information required by this item will be contained in our definitive proxy statement to be filed with the SEC in connection with our 2016 Annual Meeting of Stockholders, or the Definitive Proxy Statement, which is expected to be filed not later than 120 days after the end of our fiscal year ended December 31, 2015, under the headings “Board of Directors Information,” “Corporate Governance,” “Executive Officers,” and “Section 16(a) Beneficial Ownership Reporting Compliance,” and is
incorporated herein by reference.
Item 11. EXECUTIVE COMPENSATION.
The information required by this item will be set forth in the Definitive Proxy Statement and is incorporated in this report by
reference.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by this item will be set forth in the Definitive Proxy Statement and is incorporated in this report by
reference.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required by this item will be set forth in the Definitive Proxy Statement and is incorporated in this report by
reference.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The information required by this item will be set forth in the Definitive Proxy Statement and is incorporated in this report by reference.
PART IV
ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) Documents filed as a part of this report.
1. Financial Statements.
The following financial statements and financial statement schedules are filed as a part of this report:
55
2. Financial Statement Schedules. | |
All other schedules not listed above have been omitted, because they are not applicable or are not required, or because the required information is included in the consolidated financial statements or notes thereto.
3. Exhibits required to be filed by Item 601 of Regulation S-K. See Item 15(b) below.
(b) | Exhibits: The following exhibits are filed as a part of this report: | |||||||||
Incorporated by Reference | ||||||||||
Exhibit No. | Description | Filed Herewith | Form | File/Film No. | Date Filed | |||||
2.1 | Separation and Distribution Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of June 30, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
3.1 | Amended and Restated Certificate of Incorporation of SeaSpine Holdings Corporation. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
3.2 | Amended and Restated Bylaws of SeaSpine Holdings Corporation. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
4.1 | Form of Common Stock Certificate of SeaSpine Holdings Corporation. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.1 | Transition Services Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.2 | Tax Matters Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.3 | Employee Matters Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
56
10.4 | Microfibrillar Collagen Supply Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.5 | Collagen Ceramic Supply Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.6 | Demineralized Bone Matrix and Collagen Ceramic Products Supply Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.7 | Brian Baker Letter Agreement, dated February 25, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.8 | Form of Indemnification Agreement entered into between SeaSpine Holdings Corporation and each of its directors and executive officers. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.9 | SeaSpine Holdings Corporation 2015 Incentive Award Plan. | Form S-8 | 333-205334-1598733 | 6/29/2015 | ||||||
10.10 | Form of SeaSpine Holdings Corporation 2015 Incentive Award Plan Stock Option Agreement. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.11 | SeaSpine Holdings Corporation 2015 Employee Stock Purchase Plan. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.12 | Employment Agreement, by and between SeaSpine Holdings Corporation, SeaSpine Orthopedics Corporation and Keith Valentine, dated April 28, 2015. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.13 | John Bostjancic Letter Agreement, dated March 30, 2015. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.14 | John Winge Letter Agreement, dated January 22, 2015. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.15 | Amended and Restated Lease between Salma Jason Monica Limited Partnership and SeaSpine, Inc., dated as of May 23, 2011 for property at 2384 La Miranda, Vista, CA. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.16 | Amended and Restated Lease between Salma Jason Monica Limited Partnership and SeaSpine, Inc., dated as of May 23, 2011 for property at 2302 La Miranda, Vista, CA | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
57
10.17 | Amended and Restated Lease between Monarch RRC Properties, LLC (assignee of original landlord, New Goodyear LTD) and IsoTis Orthobiologics, Inc., dated as of February 23, 2006, for property at 2 Goodyear, Irvine, CA (the “Irvine Industrial Real Estate Lease”). | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.18 | Amendment No. 1 to Irvine Industrial Real Estate Lease, dated as of May 26, 2011. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.19 | Amendment No. 2 to Irvine Industrial Real Estate Lease, dated as of May 14, 2013. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.20 | Sublease Agreement between SeaSpine Orthopedics Corporation, and SkinMedica, Inc., dated as of July 8, 2015. | Form 8-K | 001-36905-151103433 | 9/11/2015 | ||||||
10.21 | SeaSpine Holdings Corporation Senior Leadership Retention and Severance Plan, effective January 27, 2016. | Form 8-K | 001-36905-161378936 | 2/2/2016 | ||||||
10.22 | SeaSpine Holdings Corporation 2015 Incentive Award Plan Annual Incentive Program. | Form 8-K | 001-36905-161472253 | 3/1/2016 | ||||||
10.23 | SeaSpine Holdings Corporation Non-Employee Director Compensation Program, effective October 13, 2015. | X | ||||||||
10.24 | Credit Agreement between SeaSpine Holdings Corporation, and Wells Fargo Bank, National Association, dated as of December 24, 2015. | X | ||||||||
21.1 | List of subsidiaries of SeaSpine Holdings Corporation. | X | ||||||||
23.1 | Consent of Pricewaterhouse Coopers LLP, Independent Registered Public Accounting Firm. | X | ||||||||
31.1 | Certification of Principal Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
31.2 | Certification of Principal Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
32.1 | Certification of Principal Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
58
32.2 | Certification of Principal Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
†101.INS | XBRL Instance Document | X | ||||||||
†101.SCH | XBRL Taxonomy Extension Schema Document | X | ||||||||
†101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||
†101.DEF | XBRL Definition Linkbase Document | X | ||||||||
†101.LAB | XBRL Taxonomy Extension Labels Linkbase Document | X | ||||||||
†101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||
† The financial information of SeaSpine Holdings Corporation Annual Report on Form 10-K for the year ended December 31, 2015 filed on March 16, 2016 formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Operations, (ii) the Consolidated Statements of Comprehensive Loss, (iii) the Consolidated Balance Sheets, (iv) Parenthetical Data to the Consolidated Balance Sheets, (v) the Consolidated Statements of Cash Flows, (vi) the Consolidated Statements of Equity, and (vii) Notes to Consolidated Financial Statements, is furnished electronically herewith.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
SEASPINE HOLDINGS CORPORATION | |||
Date: | March 16, 2016 | /s/ Keith C. Valentine | |
Keith C. Valentine | |||
President and Chief Executive Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated:
59
Date: | March 16, 2016 | /s/ Keith C. Valentine | ||
Keith C. Valentine | ||||
President and Chief Executive Officer | ||||
Date: | March 16, 2016 | /s/ John J. Bostjancic | ||
John J. Bostjancic | ||||
Chief Financial Officer | ||||
Date: | March 16, 2016 | /s/ Kirtley Stephenson | ||
Kirtley Stephenson | ||||
Chairman of the Board of Directors | ||||
James M. Sullivan | ||||
James M. Sullivan | ||||
Director | ||||
Date: | March 16, 2016 | /s/ Michael Fekete | ||
Michael Fekete | ||||
Director | ||||
Date: | March 16, 2016 | /s/ John B. Henneman III | ||
John B. Henneman III | ||||
Director | ||||
Date: | March 16, 2016 | /s/ Stuart M. Essig | ||
Stuart M. Essig | ||||
Director | ||||
Date: | March 16, 2016 | /s/ Cheryl Blanchard | ||
Cheryl Blanchard | ||||
Director | ||||
Date: | March 16, 2016 | /s/ Keith Bradley Ph.D. | ||
Keith Bradley Ph.D. | ||||
Director | ||||
60
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of SeaSpine Holdings Corporation:
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of SeaSpine Holdings Corporation and its subsidiaries at December 31, 2015 and December 31, 2014, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement Schedule II - Valuation and Qualifying Accounts presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
San Diego, California
March 16, 2016
F- 1
SEASPINE HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Total revenue, net | $ | 133,178 | $ | 138,695 | $ | 146,586 | |||||
Cost of goods sold | 61,119 | 56,714 | 55,532 | ||||||||
Gross profit | 72,059 | 81,981 | 91,054 | ||||||||
Operating expenses: | |||||||||||
Selling, general and administrative | 110,551 | 88,213 | 93,009 | ||||||||
Research and development | 8,353 | 8,527 | 9,893 | ||||||||
Intangible amortization | 5,331 | 5,590 | 5,598 | ||||||||
Total operating expenses | 124,235 | 102,330 | 108,500 | ||||||||
Operating loss | (52,176 | ) | (20,349 | ) | (17,446 | ) | |||||
Other expense, net | (877 | ) | (269 | ) | (4,556 | ) | |||||
Loss before income taxes | (53,053 | ) | (20,618 | ) | (22,002 | ) | |||||
Provision for income taxes | 2,479 | 3,927 | 3,744 | ||||||||
Net loss | $ | (55,532 | ) | $ | (24,545 | ) | $ | (25,746 | ) | ||
Net Loss per share, basic and diluted | $ | (4.99 | ) | $ | (2.22 | ) | $ | (2.33 | ) | ||
Weighted average shares used to compute basic and diluted net loss per share | 11,139 | 11,048 | 11,048 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F- 2
SEASPINE HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net loss | $ | (55,532 | ) | $ | (24,545 | ) | $ | (25,746 | ) | ||
Other comprehensive income (loss) | |||||||||||
Change in foreign currency translation adjustments | 498 | (961 | ) | 256 | |||||||
Comprehensive loss | $ | (55,034 | ) | $ | (25,506 | ) | $ | (25,490 | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
F- 3
SEASPINE HOLDINGS CORPORATION
CONSOLIDATED BALANCE SHEETS
(In thousands, except par value data)
December 31, 2015 | December 31, 2014 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 33,429 | $ | 652 | |||
Trade accounts receivable, net of allowances of $764 and $558 | 25,326 | 22,538 | |||||
Inventories | 51,271 | 49,862 | |||||
Prepaid expenses and other current assets | 3,696 | 1,564 | |||||
Total current assets | 113,722 | 74,616 | |||||
Property, plant and equipment, net | 21,958 | 16,360 | |||||
Intangible assets, net | 39,632 | 46,891 | |||||
Other assets | 1,077 | 1,775 | |||||
Total assets | $ | 176,389 | $ | 139,642 | |||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable, trade | $ | 13,689 | $ | 36,637 | |||
Income taxes payable | — | 608 | |||||
Accrued compensation | 4,177 | 2,408 | |||||
Accrued commissions | 4,227 | 3,892 | |||||
Accrued expenses and other current liabilities | 3,942 | 2,407 | |||||
Total current liabilities | 26,035 | 45,952 | |||||
Long-term borrowings under credit facility | 328 | — | |||||
Other liabilities | 2,687 | 2,406 | |||||
Total liabilities | 29,050 | 48,358 | |||||
Commitments and contingencies | |||||||
Stockholders' equity: | |||||||
Preferred stock, $0.01 par value; 15,000 authorized at December 31, 2015; no shares issued and outstanding at December 31, 2015 | — | — | |||||
Common stock, $0.01 par value; 60,000 authorized; 11,102 shares issued and outstanding at December 31, 2015, and no shares issued and outstanding at December 31, 2014 | 111 | — | |||||
Additional paid-in capital | 173,786 | — | |||||
Integra net investment prior to the spin-off | — | 90,391 | |||||
Accumulated other comprehensive income | 1,391 | 893 | |||||
Accumulated deficit | (27,949 | ) | — | ||||
Total stockholders' equity | 147,339 | 91,284 | |||||
Total liabilities and stockholders' equity | $ | 176,389 | $ | 139,642 | |||
The accompanying notes are an integral part of these consolidated financial statements.
F- 4
SEASPINE HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
OPERATING ACTIVITIES: | |||||||||||
Net loss | $ | (55,532 | ) | $ | (24,545 | ) | $ | (25,746 | ) | ||
Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | |||||||||||
Depreciation and amortization | 12,445 | 12,961 | 13,579 | ||||||||
Instrument replacement expense | 1,228 | 1,732 | 2,417 | ||||||||
Impairment of construction in progress | 594 | — | — | ||||||||
Provision for excess and obsolete inventories | 7,327 | 2,500 | 2,431 | ||||||||
Loss on disposal of property and equipment | — | 292 | — | ||||||||
Deferred income tax benefit | (282 | ) | (673 | ) | (697 | ) | |||||
Stock-based compensation | 3,816 | 551 | 706 | ||||||||
Amortization of inventory step-up | — | 258 | 795 | ||||||||
Allocation of non-cash charges from Integra | 563 | 1,934 | 1,415 | ||||||||
Changes in assets and liabilities | |||||||||||
Accounts receivable | (2,004 | ) | 2,997 | 2,314 | |||||||
Inventories | (8,365 | ) | (5,185 | ) | (12,633 | ) | |||||
Prepaid expenses and other current assets | (2,867 | ) | 256 | 754 | |||||||
Other non-current assets | 1,335 | 499 | 149 | ||||||||
Accounts payable | 5,818 | 5,797 | 7,944 | ||||||||
Income taxes payable | (320 | ) | 507 | 101 | |||||||
Accrued commissions | 335 | 344 | 1,282 | ||||||||
Accrued compensation, accrued expenses and other current liabilities | 3,316 | 875 | (2,826 | ) | |||||||
Other non-current liabilities | 27 | (294 | ) | 535 | |||||||
Net cash (used in) provided by operating activities | (32,566 | ) | 806 | (7,480 | ) | ||||||
INVESTING ACTIVITIES: | |||||||||||
Purchases of property and equipment | (11,555 | ) | (3,804 | ) | (5,550 | ) | |||||
Technology license milestone payment | (150 | ) | — | — | |||||||
Net cash used in investing activities | (11,705 | ) | (3,804 | ) | (5,550 | ) | |||||
FINANCING ACTIVITIES: | |||||||||||
Debt issuance costs | (80 | ) | — | — | |||||||
Integra net investment prior to the spin-off | 77,173 | 3,012 | 13,581 | ||||||||
Excess tax benefits from stock-based compensation arrangements | 37 | — | — | ||||||||
Net cash provided by financing activities | 77,130 | 3,012 | 13,581 | ||||||||
Effect of exchange rate changes on cash and cash equivalents | (82 | ) | (8 | ) | 4 | ||||||
Net change in cash and cash equivalents | 32,777 | 6 | 555 | ||||||||
Cash and cash equivalents at beginning of period | 652 | 646 | 91 | ||||||||
Cash and cash equivalents at end of period | $ | 33,429 | $ | 652 | $ | 646 | |||||
Non-cash financing activities: | |||||||||||
Settlement of related-party payable to Integra net investment | $ | 29,022 | $ | — | $ | — | |||||
Long-term borrowings under credit facility | $ | 328 | $ | — | $ | — | |||||
Non-cash investing activities: | |||||||||||
Property and equipment in liabilities | $ | 638 | $ | 300 | $ | 500 | |||||
Supplemental cash flow information: | |||||||||||
Income taxes paid | $ | 2,982 | $ | 4,200 | $ | 3,900 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F- 5
SEASPINE HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(In thousands)
Common Stock | Additional | Integra | Accumulated Other | Total | ||||||||||||||||||||||
Number of | Paid-In | Net | Comprehensive | Accumulated | Stockholders' | |||||||||||||||||||||
Shares | Amount | Capital | Investment | Income (Loss) | Deficit | Equity | ||||||||||||||||||||
Balance December 31, 2012 | — | $ | — | $ | — | $ | (7,222 | ) | $ | 1,598 | $ | — | $ | (5,624 | ) | |||||||||||
Net loss | — | — | — | (25,746 | ) | — | — | (25,746 | ) | |||||||||||||||||
Other comprehensive income | — | — | — | — | 256 | — | 256 | |||||||||||||||||||
Net transfers to Integra | — | — | — | 142,609 | — | — | 142,609 | |||||||||||||||||||
Balance December 31, 2013 | — | $ | — | $ | — | $ | 109,641 | $ | 1,854 | $ | — | $ | 111,495 | |||||||||||||
Net loss | — | — | — | (24,545 | ) | — | — | (24,545 | ) | |||||||||||||||||
Other comprehensive loss | — | $ | — | — | — | (961 | ) | — | (961 | ) | ||||||||||||||||
Net transfers to Integra | — | — | — | 5,295 | — | — | 5,295 | |||||||||||||||||||
Balance December 31, 2014 | — | $ | — | $ | — | $ | 90,391 | $ | 893 | $ | — | $ | 91,284 | |||||||||||||
Net loss | — | — | — | (27,583 | ) | — | (27,949 | ) | (55,532 | ) | ||||||||||||||||
Net transfer from Integra | — | — | — | 107,433 | — | — | 107,433 | |||||||||||||||||||
Reclassification of Integra net investment in connection with spin-off | — | — | 170,241 | (170,241 | ) | — | — | |||||||||||||||||||
Other comprehensive income | — | — | — | — | 498 | — | 498 | |||||||||||||||||||
Issuance of common stock in connection with spin-off | 11,048 | 110 | (110 | ) | — | — | — | — | ||||||||||||||||||
Restricted stock awards issued | 66 | 1 | (1 | ) | — | — | — | — | ||||||||||||||||||
Restricted stock awards forfeited | (12 | ) | — | — | — | — | — | — | ||||||||||||||||||
Stock-based compensation | — | — | 3,619 | — | — | — | 3,619 | |||||||||||||||||||
Excess tax benefits from stock-based compensation arrangements | — | — | 37 | — | — | — | 37 | |||||||||||||||||||
Balance December 31, 2015 | 11,102 | $ | 111 | $ | 173,786 | $ | — | $ | 1,391 | $ | (27,949 | ) | $ | 147,339 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F- 6
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. BUSINESS
Spin-off from Integra
As of June 30, 2015, SeaSpine Holdings Corporation ("SeaSpine," or the "Company") was a subsidiary of Integra LifeSciences Holdings Corporation (“Integra”). On July 1, 2015, Integra completed the spin-off of its orthobiologics and spinal fusion hardware business into SeaSpine, which was created to be a separate, independent, publicly-traded medical technology company focused on the design, development and commercialization of surgical solutions for the treatment of patients suffering from spinal disorders. Unless the context indicates otherwise, (i) references to "SeaSpine," the "Company," and the "Business," refer to SeaSpine Holdings Corporation and its orthobiologics and spinal fusion hardware business and (ii) references to "Integra" refer to Integra LifeSciences Holdings Corporation and its subsidiaries other than SeaSpine.
On July 1, 2015 (the "Distribution Date"), SeaSpine common stock was distributed, on a pro rata basis, to Integra’s stockholders of record as of 5:00 p.m. Eastern Time on June 19, 2015 (the "Record Date"). On the Distribution Date, each holder of Integra common stock received one share of SeaSpine common stock for every three shares of Integra common stock held by such holder as of the Record Date. The spin-off was completed pursuant to a Separation and Distribution Agreement and several other agreements with Integra or its subsidiaries related to the spin-off, including an Employee Matters Agreement, a Tax Matters Agreement, a Transition Services Agreement and several Supply Agreements, each of which is filed as an Exhibit to the Current Report on Form 8-K filed with the U.S. Securities and Exchange Commission ("SEC") on July 1, 2015 and incorporated by reference herein. These agreements govern the relationship between SeaSpine and Integra following the spin-off and provide for the allocation of various assets, liabilities, rights and obligations. These agreements also include arrangements for transition services, products and raw materials to be provided by Integra to SeaSpine and transition services and products to be provided by SeaSpine to Integra. For a discussion of each agreement, see the section entitled “Certain Relationships and Related Party Transactions" in the SeaSpine Information Statement included as Exhibit 99.1 to the Registration Statement on Form 10, as amended, filed with the SEC on June 9, 2015 (the “Information Statement”).
The SeaSpine Registration Statement on Form 10 became effective on June 9, 2015, and SeaSpine common stock began “regular-way” trading on the NASDAQ Global Market on July 2, 2015 under the symbol “SPNE.”
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Company prepared the consolidated financial statements included in this report in accordance with accounting principles generally accepted in the U.S. (“GAAP”). For periods prior to the spin-off, the Company’s consolidated financial statements were prepared on a stand-alone basis and derived from Integra's consolidated financial statements and accounting records related to its orthobiologics and spinal fusion hardware business. The Company relied on Integra for a significant portion of its operational and administrative support. The consolidated financial statements included allocations of certain Integra corporate expenses, including information technology resources and support; finance, accounting, auditing services; real estate and facility management services; human resources activities; certain procurement activities; treasury services, legal advisory services and costs for research and development. These costs were allocated to the Company on the basis of direct usage when identifiable, with the remainder allocated on a pro-rata basis of revenue, standard costs of sales, or other measures.
Integra used a centralized approach to cash management and financing of its operations and substantially all cash generated by the Company through May 4, 2015, the date the Company implemented a separate enterprise resource planning ("ERP") system for SeaSpine, was assumed to be remitted to Integra. Prior to the spin-off, cash management and financing transactions relating to the Company were accounted for through the Integra invested equity account. Accordingly, none of the Integra cash and cash equivalents at the corporate level were assigned to SeaSpine in the consolidated financial statements. Integra’s debt and related interest expense were not allocated to SeaSpine for any of the periods presented since the Company was not the legal obligor of the debt and Integra’s borrowings were not directly attributable to SeaSpine.
Subsequent to the spin-off, the Company’s financial statements are presented on a consolidated basis, as the Company became a separate publicly-traded company on July 1, 2015. The Company performs its operational and administrative support using internal resources and purchased services, some of which have been provided by Integra for a fee pursuant to a transition services agreement.
See Note 3, “Transactions with Integra,” for further information regarding the relationships the Company has with Integra.
F- 7
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Principles of Consolidation
For periods prior to the spin-off, the consolidated financial statements include certain assets and liabilities that have historically been held at the Integra level but were specifically identifiable or otherwise attributable to the Company. All significant intra-company transactions within Integra's pre-spin off orthobiologics and spinal fusion hardware business have been eliminated. All significant transactions between the Company and other businesses of Integra before the spin-off are included in these consolidated financial statements.
For periods subsequent to the spin-off, the consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities, and the reported amounts of revenues and expenses. Significant estimates affecting amounts reported or disclosed in the consolidated financial statements include allowances for doubtful accounts receivable and sales returns and allowances, net realizable value of inventories, amortization periods for acquired intangible assets, discount rates and estimated projected cash flows used to value and test impairments of long-lived assets, estimates of projected cash flows, depreciation and amortization periods for long-lived assets, computation of taxes, valuation allowances recorded against deferred tax assets, the valuation of stock-based compensation and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from these estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of 90 days or less at the date of purchase to be cash equivalents. Cash and cash equivalents include cash readily available in checking and bank deposit sweep accounts.
Fair Value of Financial Instruments
The carrying amounts of cash, cash equivalents, receivables, accounts payable and accrued expenses at December 31, 2015 and December 31, 2014, are considered to approximate fair value because of the short term nature of those items.
The Company measures certain assets and liabilities in accordance with authoritative guidance which requires fair value measurements to be classified and disclosed in one of the following three categories:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities.
Level 2: Observable prices that are based on inputs not quoted on active markets, but corroborated by market data.
Level 3: Unobservable inputs are used when little or no market data is available.
The carrying amount of debt outstanding pursuant to the Credit Facility approximates fair value as interest rates on this instrument approximate current market rates. This fair value measurement is categorized within Level 2 of the fair value hierarchy.
Trade Accounts Receivable and Allowances for Doubtful Accounts Receivable
Trade accounts receivable in the accompanying consolidated balance sheets are presented net of allowances for doubtful accounts.
The Company grants credit to customers in the normal course of business, but generally does not require collateral or any other security to support its receivables.
The Company evaluates the collectability of accounts receivable based on a combination of factors. In circumstances where a specific customer is unable to meet its financial obligations to the Company, a provision to the allowances for doubtful accounts is recorded to reduce the net recognized receivable to the amount that is reasonably expected to be collected. For all other customers, a provision to the allowances for doubtful accounts is recorded based on factors including the length of time the receivables are past due, the current business environment and the Company’s historical experience. Provisions to the allowances for doubtful accounts are recorded to selling, general and administrative expenses. Account balances are charged off against the allowance when it is probable that the receivable will not be recovered.
Inventories
F- 8
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Inventories, consisting of purchased materials, direct labor and manufacturing overhead, are stated at the lower of cost, the value determined by the first-in, first-out method, or market.
At each balance sheet date, the Company evaluates inventories for excess quantities, obsolescence or shelf life expiration. This evaluation includes analysis of our current and future strategic plans, historical sales levels by product, projections of future demand, the risk of technological or competitive obsolescence for products, general market conditions, a review of the shelf life expiration dates for products, as well as the feasibility of reworking or using excess or obsolete products or components in the production or assembly of other products that are not obsolete or for which there are not excess quantities in inventory. To the extent that management determines there are excess or obsolete inventory or quantities with a shelf life that is too near its expiration for the Company to reasonably expect that it can sell those products prior to their expiration, the Company adjusts the carrying value to estimated net realizable value.
The Company capitalizes inventory costs associated with certain products prior to regulatory approval, based on management’s judgment of probable economic benefit. The Company could be required to expense previously capitalized costs related to pre-approval inventory upon a change in such judgment, due to, among other potential factors, a denial or delay of approval by necessary regulatory bodies or a decision by management to discontinue the related development program. No such amounts were capitalized at December 31, 2015 or 2014.
Property, Plant, and Equipment
Property, plant and equipment are stated at historical cost less accumulated depreciation and any impairment charges. The
Company provides for depreciation using the straight-line method over the estimated useful lives of the assets. Leasehold
improvements are amortized over the lesser of the lease term or the useful life. The cost of major additions and
improvements is capitalized, while maintenance and repair costs that do not improve or extend the lives of the respective
assets are charged to operations as incurred. The cost of computer software obtained for internal use is accounted for in
accordance with the Accounting Standards Codification 350-40, Internal-Use Software.
The cost of purchased instruments which the Company consigns to hospitals and independent sales agents to support surgeries is initially capitalized as construction in progress. The amount is then reclassified to instrument sets and depreciation is initiated when instruments are put together in a newly built set with spinal implants, or directly expensed for the instruments that are used to replace damaged instruments in an existing set. The depreciation expense and direct expense for replacement instruments are recorded in selling, general and administrative expense.
Identifiable Intangible Assets
Identifiable intangible assets are initially recorded at fair value at the time of acquisition generally using an income or cost approach. The Company capitalizes costs incurred to renew or extend the term of recognized intangible assets and amortizes those costs over their expected useful lives.
Long-Lived Assets
Long-lived assets held and used by the Company, including property, plant and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. For purposes of evaluating the recoverability of long-lived assets to be held and used, a recoverability test is performed using projected undiscounted net cash flows applicable to the long-lived assets. If an impairment exists, the amount of such impairment is calculated based on the estimated fair value of the asset. Impairments to long-lived assets to be disposed of are recorded based upon the difference between the carrying value and the fair value of the applicable assets. There was no impairment of intangible or tangible long-lived assets in any of the periods presented.
Foreign Currency
The Company generates revenues outside the United States in multiple foreign currencies including euros, British pounds, Swiss francs and New Zealand dollars, and in U.S. dollar-denominated transactions conducted with customers who generate revenue in currencies other than the U.S. dollar. The Company also incurs operating expenses in euros. All assets and liabilities of foreign subsidiaries which have a functional currency other than the U.S. dollar are translated at the rate of exchange at year-end, while elements of the income statement are translated at the average exchange rates in effect during the year. The net effect of these translation adjustments is shown as a component of accumulated other comprehensive income (loss). These currency translation adjustments are not currently adjusted for income taxes as they relate to permanent investments in non-U.S. subsidiaries. Foreign currency transaction gains and losses are reported in other income (expense), net.
F- 9
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Income Taxes
In the Company’s consolidated financial statements prior to the spin-off, income tax expense and deferred tax balances were calculated on a separate return basis although the Company’s operations had historically been included in the tax returns filed by the respective Integra entities of which the Company’s business was a part.
Prior to the spin-off, the Company maintained an income taxes payable to/from account with Integra. The Company was deemed to settle current tax balances with the Integra tax paying entities in the respective jurisdictions. The Company’s current income tax balances were reflected as income taxes payable and settlements, which are deemed to occur in the year following incurrence, were reflected as changes in net Integra investment in the consolidated balance sheets.
We recognize tax benefits in our financial statements when our uncertain tax positions are more likely than not to be sustained upon audit. The amount we recognize is measured as the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. We recognize deferred tax assets for deductible temporary differences, operating loss carryforwards and tax credit carryforwards. Deferred tax assets are reduced by valuation allowance if it is more likely than not that some portion, or all, of the deferred tax assets will not be realized.
Revenue Recognition
Our net sales are derived primarily from the sale of orthobiologics and spinal fusion hardware products globally. Sales are reported net of returns, group purchasing organization fees and other customer allowances.
Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred title and risk of loss have passed to the customer, there is a fixed or determinable sales price and collectability of that sales price is reasonably assured.
In the United States, we generate most of our revenue by consigning our orthobiologics products and consigning or loaning our spinal fusion hardware sets to hospitals and independent sales agents, who in turn deliver them to the hospital for a single surgical procedure or leave them with hospitals that are high volume users for use in multiple procedures. The spinal fusion hardware sets typically contain the instruments, including disposables, and spinal implants required to complete a surgery. We ship replacement inventory to independent sales agents to replace the consigned inventory used in surgeries and maintain and replenish the loaned sets and return them to a hospital or independent sales agent for the next procedure. We recognize revenue on these consigned or loaned products when they have been used or implanted in a surgical procedure.
For all other transactions, including sales to international stocking distributors, we recognize revenue when the products are shipped to the customer or stocking distributor and the transfer of title and risk of loss occurs. There are generally no customer acceptance or other conditions that prevent us from recognizing revenue in accordance with the delivery terms.
Product royalties are estimated and recognized in the same period that the royalty-based products are sold by licensees. The Company estimates and recognizes royalty revenue based upon communication with licensees, historical information and expected sales trends. Differences between actual revenues and estimated royalty revenues are adjusted in the period in which they become known, which is typically the following quarter. Historically, such adjustments have not been significant.
Shipping and Handling Fees and Costs
Amounts billed to customers for shipping and handling are included in revenues. The related shipping and freight charges incurred by the Company are included in cost of goods sold. Shipping and handling costs of $1.2 million, $1.0 million, and $1.1 million were recorded in selling, general and administrative expense during the years ended December 31, 2015, 2014 and 2013, respectively.
Research and Development
Research and development costs, including salaries, depreciation, consultant and other external fees, and facility costs directly attributable to research and development activities, are expensed in the period in which they are incurred.
Stock-Based Compensation
For periods prior to the spin-off, the Company’s stock-based compensation was derived from the equity awards granted by Integra to individuals who would become the Company’s employees. Stock-based compensation expense has been allocated to the
F- 10
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Company based on the awards and terms previously granted to its employees. As those stock-based compensation plans were Integra’s plans, the amounts have been recognized in the consolidated statements of operations and the Integra net investment account on the consolidated balance sheet. For periods after the spin-off, the Company's stock-based compensation has been recognized through the consolidated statement of operations and the Company's additional paid-in capital account on the consolidated balance sheet.
The Company applies the authoritative guidance for stock-based compensation. This guidance requires companies to recognize the expense related to the fair value of their stock-based compensation awards. Stock-based compensation expense for stock option awards granted after January 1, 2006 was based on the fair value on the grant date using the Black-Scholes-Merton option pricing model. The fair value of performance awards of restricted stock granted prior to the spin-off was based on the Integra’s stock price at the grant date and the assessed probability of meeting future performance targets. The long form method was used in the determination of the windfall tax benefit in accordance with the guidance.
The stock-based compensation is initially measured at the fair value of the awards on the grant date and is then recognized on a ratable basis in the financial statements over the requisite service period of the award. Stock-based compensation expense was $4.4 million in 2015, $0.6 million in 2014, and $0.7 million in 2013.
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash, which is held at major financial institutions, and trade receivables.
The Company’s products are sold on an uncollateralized basis and on credit terms based upon a credit risk assessment of each customer. A portion of the Company’s trade receivables to customers outside the United States includes sales to foreign distributors, who then sell to government owned or supported healthcare systems. The ongoing economic conditions in certain European countries, especially Greece, Ireland, Italy, Portugal and Spain remain uncertain. Accounts receivable from customers in these countries are not a material amount of the Company’s overall receivables.
None of the Company’s customers accounted for 10% or more of the combined net sales during the years ended December 31, 2015, 2014 or 2013.
Recently Issued Accounting Standards
In May 2014, the Financial Accounting Standards Board ("FASB") issued Update No. 2014-09, Revenue from Contracts with Customers (Topic 606). The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should: 1) identify the contract(s) with a customer, 2) identify the performance obligations in the contract, 3) determine the transaction price, 4) allocate the transaction price to the performance obligations in the contract, and 5) recognize revenue when (or as) the entity satisfies a performance obligation. In July 2015, the FASB deferred for one year the effective date of the new revenue standard, but early adoption is permitted. The new standard will be effective for the Company on January 1, 2018. The Company is in the process of evaluating the impact of this standard on its financial statements.
In August 2014, the FASB issued Update No. 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The amendment requires management to evaluate, for each annual and interim reporting period, whether there are conditions and events, considered in the aggregate, that raise substantial doubt about an entity’s ability to continue as a going concern within one year after the date the financial statements are issued or are available to be issued. If substantial doubt is raised, additional disclosures around management’s plan to alleviate these doubts are required. This update will become effective for all annual periods and interim reporting periods beginning after December 15, 2016. The implementation of the amended guidance is not expected to have an impact on current disclosures in our financial statements.
In April 2015, the FASB issued Update No. 2015-03, Simplifying the Presentation of Debt Issuance Costs. The new standard will require debt issuance costs to be presented on the balance sheet as a direct reduction of the carrying value of the associated debt liability, consistent with the presentation of debt discounts. The recognition and measurement requirements will not change as a result of this guidance. The standard is effective for the annual reporting periods beginning after December 15, 2015 and requires a retrospective application. The guidance in Accounting Standards Update (ASU) 2015-03 does not address presentation or subsequent measurement of debt issuance costs related to line-of-credit arrangements. In August 2015, the FASB issued ASU 2015-15, Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements. Under the new standard, the SEC staff will not object to an entity deferring and presenting debt issuance costs as an asset and subsequently
F- 11
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. The implementation of the amended guidance is not expected to have an impact on current disclosures in our financial statements.
In July 2015, the FASB issued Update No. 2015-11, Simplifying the Measurement of Inventory. The new guidance requires an entity to measure inventory within the scope of the amendment at the lower of cost and net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The guidance is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The implementation of the amended guidance is not expected to have an impact on our financial statements.
In November 2015, the FASB issued Update No. 2015-17, Income Taxes - Balance Sheet Reclassification of Deferred Taxes (Topic 740). This ASU requires that deferred tax liabilities and assets be classified as non-current in a classified statement of financial position. The current requirement that deferred tax liabilities and assets of a tax-paying component of an entity be offset and presented as a single amount is not affected by the amendments in this update. The amendments in this update are effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted and the amendments may be applied either prospectively to all deferred tax liabilities and assets or retrospectively to all periods presented. The Company early adopted this ASU in the fourth quarter of 2015 on a prospective basis and included the current portion of deferred tax assets within the non-current portion of deferred tax assets within the consolidated balance sheet. The Company did not adjust our prior period consolidated balance sheet as a result of the adoption of this ASU.
In February 2016, the FASB issued Update No. 2016-02, Leases (ASC 842), which sets out the principles for the recognition, measurement, presentation and disclosure of leases for both parties to a contract (i.e. lessees and lessors). The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight line basis over the term of the lease, respectively. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months regardless of their classification. Leases with a term of 12 months or less will be accounted for similar to existing guidance for operating leases today. ASC 842 supersedes the previous leases standard, ASC 840 Leases. The standard is effective on January 1, 2019, with early adoption permitted. The Company is in the process of evaluating the impact of this new guidance.
Net Loss Per Share
For periods prior to the spin-off, basic and diluted net loss per share was calculated based on the approximately 11.0 million shares of SeaSpine common stock that were distributed to Integra shareholders on July 1, 2015. For periods subsequent to the spin-off, basic and diluted net loss per share was calculated using the weighted-average number of shares of common stock outstanding during the period. The weighted average number of shares used to compute diluted net loss per share excludes any assumed exercise of stock options, and any assumed issuance of common stock under restricted stock units as the effect would be antidilutive. Common stock equivalents of 2.0 million shares for the year ended December 31, 2015 were excluded from the calculation because of their antidilutive effect.
Year Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
(In thousands, except per share data) | ||||||||||||
Net loss | $ | (55,532 | ) | $ | (24,545 | ) | $ | (25,746 | ) | |||
Loss Per Share Data | ||||||||||||
Loss per share | ||||||||||||
Basic and diluted | $ | (4.99 | ) | $ | (2.22 | ) | $ | (2.33 | ) | |||
Weighted average number of shares outstanding | ||||||||||||
Basic and diluted | 11,139 | 11,048 | 11,048 | |||||||||
3. TRANSACTIONS WITH INTEGRA
Related-party Transactions
F- 12
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Prior to the spin-off, and pursuant to certain supply agreements subsequent to the spin-off, SeaSpine purchased a portion of raw materials and finished goods from Integra for the Company's Mozaik family of products, and SeaSpine contract manufactured certain finished goods for Integra. The Company's purchases of raw materials and Mozaik product finished goods from Integra for the years ended December 31, 2015, 2014 and 2013 totaled $6.2 million, $6.2 million and $7.9 million, respectively. The amount of finished goods sold by SeaSpine to Integra under its contract manufacturing arrangement was immaterial for all periods presented.
Pursuant to a transition services agreement, Integra and SeaSpine will provide certain services following the spin-off, and Integra and SeaSpine will indemnify each other against certain liabilities arising from their respective businesses. Under this agreement, Integra provides us with certain support functions, including information technology, accounting and other financial functions, regulatory affairs and quality assurance, human resources and other administrative support. In addition, SeaSpine provides limited information technology and systems support services to Integra. The Company incurred approximately $2.8 million of costs under the agreement for the year ended December 31, 2015, of which $1.5 million was outstanding at December 31, 2015. The amount of services provided by SeaSpine to Integra was immaterial for the year ended December 31, 2015. Subsequent to the spin-off, Integra also collected trade receivables from customers on behalf of the Company, of which $1.3 million was outstanding as of December 31, 2015 and recorded in Other Current Assets.
Allocated Costs
For periods prior to the spin-off, the consolidated statements of operations included direct expenses for cost of goods sold, research and development, sales and marketing, customer service, and administration as well as allocations of expenses arising from shared services and infrastructure provided by Integra to the Company, such as costs of information technology, including the costs of a multi-year global enterprise resource planning implementation, accounting and legal services, real estate and facilities management, corporate advertising, insurance and treasury services, and other corporate and infrastructure services. These allocations are included in the table below. These expenses were allocated to the Company using estimates that the Company considers to be a reasonable reflection of the utilization of services provided to or benefits received from the Company. The allocation methods include pro-rata basis of revenue, standard cost of sales or other measures.
Year Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
Cost of goods sold | $ | 488 | $ | 1,304 | $ | 1,166 | ||||||
Selling, general and administrative | 8,633 | 17,602 | 17,408 | |||||||||
Research and development | 253 | 490 | 427 | |||||||||
Total Allocated Costs | $ | 9,374 | $ | 19,396 | $ | 19,001 | ||||||
Included in the above amounts are certain non-cash allocated costs, including stock-based compensation. Such amounts were $0.6 million, $1.9 million and $1.4 million for the years ended December 31, 2015, 2014 and 2013, respectively.
All significant related party transactions between SeaSpine and Integra were included in the consolidated financial statements and, prior to the spin-off, were considered to be effectively settled for cash at the time the transaction was recorded, with the exception of the purchases from Integra of Mozaik raw materials and finished goods for all periods presented. The total net effect of the transactions considered to be effectively settled for cash was reflected in the consolidated statement of cash flows as a financing activity and in the consolidated balance sheet as Integra net investment.
The following table summarizes the components of the net increase (decrease) in Integra net investment for the years ended December 31, 2015, 2014 and 2013:
F- 13
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Year Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
Cash pooling and general financing activities (a) | $ | 68,386 | $ | (14,451 | ) | $ | (4,005 | ) | ||||
Corporate Allocations (excluding non-cash adjustments) | 8,787 | 17,463 | 17,586 | |||||||||
Total Integra net investment in financing activities within cash flow statement | 77,173 | 3,012 | 13,581 | |||||||||
Non-cash adjustments (b) | 29,806 | 2,485 | 2,122 | |||||||||
Interest on long term loan (c) | — | — | (4,617 | ) | ||||||||
Net capitalization of related-party loan | — | — | 131,580 | |||||||||
Spin-off related adjustment (d) | 161 | — | — | |||||||||
Reclassification of Integra net investment in connection with the spin-off | (170,241 | ) | — | — | ||||||||
Foreign exchange impact | 293 | (202 | ) | (57 | ) | |||||||
Net (decrease) increase in Integra investment | $ | (62,808 | ) | $ | 5,295 | $ | 142,609 | |||||
(a) | Includes financing activities for capital transfers, cash sweeps and other treasury services. |
(b) | Reflects allocation of non-cash charges from Integra, stock-based compensation and settlement of related-party payable to Integra net investment. |
(c) | Interest on long-term loan capitalized in 2013. |
(d) | During the year ended December 31, 2015, certain spin-off related adjustments were recorded in stockholders' equity, to reflect the appropriate opening balances related to SeaSpine’s legal entities at the Distribution Date. |
4. DEBT AND INTEREST
Related -Party Loans
The Company had $132.0 million in related-party loans from Integra arising from a prior acquisition. During 2013, those loans and the associated accrued interest were forgiven and capitalized as part of Integra's net investment. The company recorded $4.6 million of interest expense for the year ended December 31, 2013, which was reflected as interest expense in the Company’s consolidated financials.
Credit Agreement
On December 24, 2015, the Company entered into a three-year credit facility (the "Credit Facility") with Wells Fargo Capital Finance, as Administrative Agent and as a Lender. The Credit Facility provides an asset-backed revolving line of credit of up to $30.0 million in borrowing capacity with a maturity date of December 24, 2018, which maturity date is subject to a one-time one-year extension at the Company's election. In connection with the Credit Facility, the Company was required to become guarantors and to provide a security interest in substantially all its assets for the benefit of Agent and the Lender.
Borrowings under the Credit Facility shall accrue interest at the rate then applicable to the Base Rate (as customarily defined) Loans, unless and until converted into LIBOR Rate Loans in accordance with the terms of the Credit Facility. Borrowings bear interest at a floating annual rate equal to (a) during any month for which the Company's average excess availability (as customarily defined) is greater than $20.0 million, base rate plus (i) 1.25 percentage points for base rate loans and (ii) LIBOR rate plus 2.25 percentage points for LIBOR loans, (b) during any month for which the Company's average excess availability is greater than $10.0 million but less than or equal to $20.0 million, (i) base rate plus 1.50 percentage points for base rate loans and (ii) LIBOR rate plus 2.50 percentage points for LIBOR loans and (c) during any month for which the Company's average excess availability is less than or equal to $10.0 million, (i) base rate plus 1.75 percentage points for base rate loans and (ii) LIBOR rate plus 2.75 percentage points for LIBOR loans.
The Company will also pay an annual unused line fee in an amount equal to 0.375% times the unused Credit Facility amount. The unused line fee is due and payable on the first day of each month. At December 31, 2015, there was $0.3 million outstanding under the Credit Facility. Debt issuance costs and legal fees related to the financing totaling $0.4 million were recorded as a deferred asset and are subsequently being amortized ratably over the term of the Credit Facility.
The Credit Facility contains various customary affirmative and negative covenants agreed to by the Company, including prohibiting the Company from incurring indebtedness without the lender’s consent. The Credit Facility also includes a financial covenant, that requires the Company to maintain a minimum fixed charge coverage ratio of 1.10 to 1.00 for the applicable measurement period, if the Company's Total Liquidity (as defined in the Credit Facility) is less than $5.0 million. The Company was in compliance with all such covenants at December 31, 2015.
F- 14
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Credit Facility also includes customary events of default, including events of default relating to non-payment of amounts due under the Credit Facility, material inaccuracy of representations and warranties, violation of covenants, bankruptcy and insolvency, failure to comply with health care laws, violation of certain of the Company’s existing agreements, and the occurrence of a change of control. Under the Credit Facility, if an event of default occurs, lenders holding a majority of the revolving commitments will have the right to terminate the commitments and accelerate the maturity of any loans outstanding.
5. BALANCE SHEET DETAILS
Inventories. Inventories consisted of the following:
December 31, 2015 | December 31, 2014 | ||||||
(In thousands) | |||||||
Finished goods | $ | 29,845 | $ | 32,364 | |||
Work in process | 15,574 | 11,675 | |||||
Raw materials | 5,852 | 5,823 | |||||
$ | 51,271 | $ | 49,862 | ||||
Property, Plant and Equipment. Property, plant and equipment, net and corresponding useful lives were as follows:
December 31, 2015 | December 31, 2014 | Useful Lives | |||||||
(In thousands) | |||||||||
Leasehold improvement | $ | 4,830 | $ | 4,262 | Lease term | ||||
Machinery and production equipment | 6,404 | 5,810 | 3-20 years | ||||||
Spinal fusion hardware instrument sets | 25,080 | 22,122 | 5 years | ||||||
Information systems and hardware | 6,872 | 1,720 | 3-7 years | ||||||
Furniture and fixtures | 944 | 657 | 3-15 years | ||||||
Construction in progress | 8,375 | 8,789 | |||||||
Total | 52,505 | 43,360 | |||||||
Less accumulated depreciation and amortization | (30,547 | ) | (27,000 | ) | |||||
Property, plant and equipment, net | $ | 21,958 | $ | 16,360 | |||||
Depreciation expenses totaled $4.5 million, $4.8 million and $5.4 million for the years ended December 31, 2015, 2014, and 2013, respectively. The cost of purchased instruments used to replace damaged instruments in existing sets and recorded directly to expense totaled $1.2 million, $1.7 million and $2.4 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Identifiable Intangible Assets. The components of the Company’s identifiable intangible assets were as follows:
December 31, 2015 | |||||||||||||
Weighted Average Life | Cost | Accumulated Amortization | Net | ||||||||||
(In thousands) | |||||||||||||
Completed technology | 12 years | $ | 31,169 | $ | (19,280 | ) | $ | 11,889 | |||||
Customer relationships | 12 years | 56,830 | (29,087 | ) | 27,743 | ||||||||
Trademarks/brand names | — | 300 | (300 | ) | — | ||||||||
$ | 88,299 | $ | (48,667 | ) | $ | 39,632 | |||||||
F- 15
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
December 31, 2014 | |||||||||||||
Weighted Average Life | Cost | Accumulated Amortization | Net | ||||||||||
(In thousands) | |||||||||||||
Completed technology | 12 years | $ | 30,419 | $ | (16,582 | ) | $ | 13,837 | |||||
Customer relationships | 12 years | 56,830 | (23,963 | ) | 32,867 | ||||||||
Trademarks/brand names | — | 300 | (300 | ) | — | ||||||||
Non-Compete agreements | 4 years | 1,900 | (1,713 | ) | 187 | ||||||||
$ | 89,449 | $ | (42,558 | ) | $ | 46,891 | |||||||
Annual amortization expense (including amounts reported in cost of goods sold) is expected to be approximately, $7.0 million in 2016, $5.8 million in 2017, $5.5 million in 2018, $4.8 million in 2019, and $4.0 million in 2020. Amortization of product technology-based intangible assets totaled $2.7 million, $2.6 million and $2.6 million for the years ended December 31, 2015, 2014 and 2013, respectively, and is presented by the Company within cost of goods sold.
6. STOCK-BASED COMPENSATION
Stock-based compensation expense, all related to employees and non-employee directors, was recognized as follows:
Year Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
(In thousands) | ||||||||||||
Selling, general and administrative | $ | 3,993 | $ | 519 | $ | 619 | ||||||
Research and development | 242 | 18 | 78 | |||||||||
Cost of goods sold | 168 | 14 | 9 | |||||||||
Total stock-based compensation expense | 4,403 | 551 | 706 | |||||||||
Total estimated tax benefit related to stock-based compensation expense | 37 | 203 | 271 | |||||||||
Net effect on net income | $ | 4,366 | $ | 348 | $ | 435 | ||||||
Equity Award Plans
As of June 30, 2015, Integra had stock options, restricted stock awards, performance stock awards, contract stock awards and restricted stock units outstanding under three plans, the 2000 Equity Incentive Plan, the 2001 Equity Incentive Plan, and the 2003 Equity Incentive Plan. In connection with the spin-off, Integra equity awards granted to individuals who became employees of the Company were converted to SeaSpine equity awards. In general, each award is subject to the same terms and conditions as were in effect prior to the spin-off.
In May 2015, the Company adopted a 2015 Incentive Award Plan (the "2015 Plan"), under which the Company can grant its employees and non-employee directors incentive stock options and non-qualified stock options, restricted stock, performance stock, dividend equivalent rights, stock appreciation rights, stock payment awards and other incentive awards. The Company may issue up to 2,000,000 shares of its common stock under the 2015 Plan.
Restricted Stock Awards, Restricted Stock Units and Performance Stock Awards
Performance stock awards, restricted stock awards and restricted stock units generally have requisite service periods of three years. Performance stock awards are subject to graded vesting and the Company expenses their fair value over the requisite service period. The Company expenses the fair value of restricted stock awards and restricted stock units on an accelerated basis over the vesting period or requisite service period, whichever is shorter. Stock-based compensation expense related to restricted stock awards, restricted stock units and performance stock awards includes an estimate for forfeitures. The expected forfeiture rate of all equity based compensation is based on historical patterns of the Company’s employees and is estimated to be 10% annually for the twelve months ended December 31, 2015.
The following table summarizes awards of restricted stock awards, restricted stock units and performance stock awards to SeaSpine employees for the year ended December 31, 2015:
F- 16
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Restricted Stock and Performance Stock Awards | |||
Shares (In thousands) | Weighted Average Grant Date Fair Value Per Share | ||
Unvested, January 1, 2015 | — | $— | |
Granted | 138 | 12.81 | |
Cancellations | (13) | 4.40 | |
Released/Vested | (62) | 17.88 | |
Unvested, December 31, 2015 | 63 | $9.58 | |
The total fair value of shares vested in 2015, 2014 and 2013 was $1.1 million, $0.7 million, and $0.6 million, respectively.
The Company recognized $0.3 million, $0.6 million and $0.7 million in expense related to such awards during the years ended December 31, 2015, 2014 and 2013, respectively. As of December 31, 2015, there was approximately $0.3 million of total unrecognized compensation expense related to unvested awards. This cost is expected to be recognized over a weighted-average period of approximately one year.
Stock Options
Stock option grants to employees generally have requisite service periods of four years, and stock option grants to non-employee directors generally have a requisite service period of one year. Both are subject to graded vesting. The Company records stock-based compensation expense associated with stock options on an accelerated basis over the various vesting periods within each grant and based on their fair value at the date of grant using the Black-Scholes-Merton option pricing model. The following weighted-average assumptions were used in the calculation of fair value for options grants for the year ended December 31, 2015:
December 31, 2015 | ||
Expected dividend yield | 0 | % |
Risk-free interest rate | 1.55 | % |
Expected volatility | 38.17 | % |
Expected term (in years) | 5.1 | |
The Company considered that it has never paid cash dividends and does not currently intend to pay cash dividends. The risk-free interest rates are derived from the U.S. Treasury yield curve in effect on the date of grant for instruments with a remaining term similar to the expected term of the options. Due to the Company’s limited historical data, the expected volatility is calculated based upon the historical volatility of comparable companies in the medical device industry whose share prices are publicly available for a sufficient period of time. The expected term of options is calculated using the simplified method as prescribed by accounting guidance for stock-based compensation. In addition, the Company applies an expected forfeiture rate when amortizing stock-based compensation expense. The expected forfeiture rate of stock options is based on historical patterns of the employee turnover rate and is estimated to be 10% annually for stock-based compensation expense recorded for the year ended December 31, 2015. As individual grant awards become fully vested, stock-based compensation expense is adjusted to recognize actual forfeitures.
A summary of the options issued during the year ended December 31, 2015 and the total number of options outstanding as of that date and changes since January 1, 2015 are set forth below:
Number of Shares Outstanding (In thousands) | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (In years) | Aggregate Intrinsic Value (In thousands) | ||||||||||
Outstanding, January 1, 2015 | — | $ | — | — | $ | — | |||||||
Granted | 2,009 | 14.87 | — | — | |||||||||
Exercised | — | — | — | — | |||||||||
Forfeited | (36 | ) | 15.61 | — | — | ||||||||
Outstanding, December 31, 2015 | 1,973 | $ | 14.86 | 7.20 | $ | 4,585 | |||||||
Vested or expected to vest, December 31, 2015 | 1,800 | $ | 14.78 | 7.17 | $ | 4,314 | |||||||
Exercisable, December 31, 2015 | 472 | $ | 12.46 | 4.53 | $ | 2,230 | |||||||
F- 17
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The weighted average grant date fair value of options granted during the year ended December 31, 2015 was $5.57. The total fair value of shares vested during the year ended December 31, 2015 was $0.7 million.
The Company recognized $4.4 million in expense related to stock options for the year ended December 31, 2015. As of December 31, 2015, there was approximately $4.7 million of total unrecognized compensation expense related to unvested stock options. These costs are expected to be recognized over a weighted-average period of approximately 1.3 years. As of December 31, 2015, the Company had 388,000 shares remaining in the 2015 Plan available for grant.
Employee Stock Purchase Plan
In May 2015, the Company adopted a 2015 Employee Stock Purchase Plan (the “ESPP”), which was amended in December 2015. The ESPP enables eligible employees to purchase shares of the Company’s common stock through payroll deductions of up to 15% of eligible compensation during an offering period. Generally, each offering will be for a period of twenty-four months as determined by the Company's Board of Directors. There are four six-month purchase periods in each offering period for contributions to be made and to be converted into shares at the end of the purchase period. In no event may an employee invest more than 2,500 shares per purchase period based on the closing price on the first trading date of an offering period or more than $25,000 worth of stock during each calendar year. The purchase price is 85% of the market price of the stock at the first trading date of an offering period or any purchase date during an offering period (June 30 or December 31), whichever is less.
The ESPP authorizes the issuance of 400,000 shares of common stock pursuant to purchase rights granted to employees. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Internal Revenue Code of 1986. The first offering period commenced on January 1, 2016 and will end on December 31, 2017. As of December 31, 2015, no shares of common stock have been purchased under the ESPP.
7. LEASE
The Company entered into a sublease agreement for an office located in Carlsbad, California, which became effective September 8, 2015 upon the Company's receipt of the consent to sublease of the landlord. The term of the lease agreement is from October 1, 2015 through April 28, 2027 at an average annual cost of approximately $1.4 million. The Company leases administrative, manufacturing, research, and distribution facilities and various manufacturing, office and transportation equipment through operating lease agreements. Future minimum lease payments under these operating leases at December 31, 2015 are as follows:
Payments Due by Calendar Year | |||
(In thousands) | |||
2016 | $ | 2,287 | |
2017 | 2,152 | ||
2018 | 2,207 | ||
2019 | 2,262 | ||
2020 | 2,317 | ||
Thereafter | 12,107 | ||
Total minimum lease payments | $ | 23,332 | |
Total rental expense for the years ended December 31, 2015, 2014, and 2013 was $2.5 million, $2.1 million and $2.1 million,
respectively.
8. INCOME TAXES
The Company is subject to income taxes in the U.S. and Switzerland. Income taxes are accounted for under the asset and liability method. Deferred income tax assets and liabilities are calculated based on the difference between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases using the enacted income tax rates expected to be in effect during the years in which the temporary differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Significant judgment is required in determining whether a valuation allowance should be recorded against deferred tax assets. In assessing the need for a valuation allowance, management considers all available evidence for each jurisdiction including past operating results, estimates of future taxable
F- 18
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
income and the feasibility of ongoing tax planning strategies. In the event that the Company changes its determination as to the amount of deferred tax assets that can be realized, the Company will adjust its valuation allowance with a corresponding impact to income tax expense in the period in which such determination is made.
Prior to the Spin-off
Prior to the spin-off, the income tax provision in the consolidated statements of operations has been calculated using the separate return method, as if the Company filed a separate tax return and operated as a stand-alone business. Therefore, cash tax payments and items of current and deferred taxes may not be reflective of actual tax balances included in Integra’s historical consolidated income tax return. More specifically, the presentation of substantial net operating losses, and any related valuation allowances, presented herein do not represent actual net operating losses that have been incurred by the Company or that are available for carryforward to a future tax year.
After the Spin-off
Subsequent to the spin-off on July 1, 2015, the deferred tax balances were adjusted to reflect only those tax attributes that carryforward with the Company. The adjustment to deferred taxes was recorded through stockholders' equity. The Company also made an election to change the tax classification for its foreign entity. This election resulted in both the foreign entity and its U.S. subsidiary to be included in the consolidated federal tax group on September 1, 2015.
Income Tax Provision (Benefit)
The Company reported income tax expense despite the reported losses before income taxes, because the legal entity structure did not permit the Company to offset taxable losses generated by certain U.S. subsidiaries against the taxable income generated by its other U.S. subsidiaries.
Income/(loss) before income taxes consisted of the following:
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
United States operations | $ | (51,305 | ) | $ | (22,097 | ) | $ | (22,157 | ) | ||
Foreign operations | (1,748 | ) | 1,479 | 155 | |||||||
$ | (53,053 | ) | $ | (20,618 | ) | $ | (22,002 | ) | |||
A reconciliation of the U.S. federal statutory rate to the Company’s effective tax rate is as follows:
Year Ended December 31, | |||||
2015 | 2014 | 2013 | |||
Federal statutory rate | 35.0% | 35.0% | 35.0% | ||
Increase (decrease) in income taxes resulting from: | |||||
State income taxes, net of federal tax benefit | 0.1% | 2.3% | 2.0% | ||
Foreign operations | (0.7)% | (1.1)% | 0.5% | ||
Changes in valuation allowances | (16.7)% | (57.9)% | (56.1)% | ||
Pre-Spin losses with no tax benefit | (22.7)% | —% | —% | ||
Uncertain tax positions | —% | 0.4% | 0.4% | ||
Research and development credit | —% | 0.2% | 0.3% | ||
Return to provision | —% | 0.6% | (0.4)% | ||
Domestic manufacturing deduction | 0.5% | 2.0% | 1.6% | ||
Other | (0.2)% | (0.5)% | (0.3)% | ||
Effective tax rate | (4.7)% | (19.0)% | (17.0)% | ||
The effective tax rate for 2015 includes pre-spin net operating losses for which the Company will receive no tax benefit as such losses were utilized by Integra prior to the spin-off.
F- 19
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The provision/(benefit) for income taxes consisted of the following:
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
Current: | |||||||||||
Federal | $ | 2,655 | $ | 3,944 | $ | 3,994 | |||||
State | 106 | 252 | 294 | ||||||||
Foreign | — | 404 | 153 | ||||||||
Total current | $ | 2,761 | $ | 4,600 | $ | 4,441 | |||||
Deferred: | |||||||||||
Federal | — | (741 | ) | (744 | ) | ||||||
State | — | (60 | ) | (54 | ) | ||||||
Foreign | (282 | ) | 128 | 101 | |||||||
Total deferred | $ | (282 | ) | $ | (673 | ) | $ | (697 | ) | ||
Provision for income taxes | $ | 2,479 | $ | 3,927 | $ | 3,744 | |||||
The income tax effects of significant temporary differences that give rise to deferred tax assets and liabilities, shown before jurisdictional netting, are presented below:
Year Ended December 31, | |||||||
2015 | 2014 | ||||||
(In thousands) | |||||||
Deferred tax assets: | |||||||
Doubtful accounts | $ | 272 | $ | 88 | |||
Inventory related items | 11,170 | 8,435 | |||||
Tax credits | — | 579 | |||||
Accrued vacation | 425 | 374 | |||||
Accrued bonus | 740 | 335 | |||||
Stock compensation | 1,466 | 627 | |||||
Net operating loss carryforwards | 7,045 | 44,966 | |||||
Intangible & fixed assets | 25,354 | 28,609 | |||||
Other | 649 | 419 | |||||
Total deferred tax assets | 47,121 | 84,432 | |||||
Less valuation allowance | (46,638 | ) | (83,457 | ) | |||
Deferred tax assets after valuation allowance | $ | 483 | $ | 975 | |||
Deferred tax liabilities: | |||||||
Other | — | (60 | ) | ||||
Total deferred tax liabilities | $ | — | $ | (60 | ) | ||
Net deferred tax assets | $ | 483 | $ | 915 | |||
At December 31, 2015 we had net operating loss carryforwards of $13.3 million for federal and state income tax purposes. We also had foreign net operating loss carryforwards of $8.9 million. These tax loss carryforwards expire in various periods through 2035. The tax benefit recorded for net operating losses, net of valuation allowance, is $0.3 million which relates only to foreign net operating losses.
At December 31, 2014 we had net operating loss carryforwards of $113.1 million for federal income tax purposes, and $57.6 million for state income tax purposes. These losses have been recognized in the Integra tax returns and are not available to offset future taxable income.
A valuation allowance of $46.6 million, and $83.5 million is recorded against the Company’s gross deferred tax assets of $47.1 million, and $84.4 million recorded at December 31, 2015 and 2014, respectively.
F- 20
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The valuation allowance relates to deferred tax assets for certain items that will be deductible for income tax purposes under very limited circumstances and for which the Company believes it is not more likely than not that it will realize the associated tax benefit. However, in the event that the Company determines that it would be able to realize more or less than the recorded amount of net deferred tax assets, an adjustment to the deferred tax asset valuation allowance would be recorded in the period such a determination is made. In assessing the realizability of deferred tax assets, management considers whether it is more-likely-than-not that some portion of all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods), projected future taxable income, and tax planning strategies in making this assessment. Based upon the levels of historical taxable income, projections of future taxable income and the reversal of deferred tax liabilities over the periods in which the deferred tax assets are deductible, management believes it is more-likely-than-not that the Company will realize the benefits of these deductible differences, net of the existing valuation allowance. The amount of deferred tax asset considered realizable, however, could change in the near term if estimates which require significant judgment of future taxable income during the carryforward period are increased or decreased.
A reconciliation of the Company’s uncertain tax benefits is as follows:
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
Balance, beginning of year | $ | 113 | $ | 187 | $ | 262 | |||||
Gross increases: | |||||||||||
Prior years’ tax positions | 90 | 13 | 100 | ||||||||
Additions to tax positions in prior years due to spin-off | 185 | — | — | ||||||||
Gross decreases: | |||||||||||
Settlements | — | — | — | ||||||||
Statute of limitations lapses | (90 | ) | (87 | ) | (175 | ) | |||||
Balance, end of year | $ | 298 | $ | 113 | $ | 187 | |||||
Approximately $0.3 million of the balance at December 31, 2015 relates to uncertain tax positions that, if recognized, would affect the annual effective tax rate. Included in the balance of uncertain tax positions at December 31, 2015 is $0.2 million of prior year items that were spun off from Integra, but not originally reported in their respective years. There is $0.1 million related to tax positions for which it is reasonably possible that the total amounts could be reduced during the twelve months following December 31, 2015, as a result of expiring statutes of limitations.
The Company recognizes interest and penalties relating to uncertain tax positions in income tax expense. The amounts recorded in 2015, 2014 and 2013 were not significant.
The Company files income tax returns as prescribed by tax laws of the jurisdictions in which they operate. In the normal course of business, the Company is subject to examination by federal, state, local and foreign jurisdictions where applicable based on the statute of limitations that apply in each jurisdiction. The Company has no open tax audits with any taxing authority as of December 31, 2015.
9. COMMITMENTS AND CONTINGENCIES
In consideration for certain technology, manufacturing, distribution, and selling rights and licenses granted to the Company, the Company has agreed to pay royalties on sales of certain products sold by the Company. The royalty payments that the Company made under these agreements were included on the consolidated statements of operations as a component of cost of goods sold.
The Company is subject to various claims, lawsuits and proceedings in the ordinary course of its business with respect to its products, its current or former employees, and involving commercial disputes, some of which have been settled by the Company. In the opinion of management, such claims are either adequately covered by insurance or otherwise indemnified, or are not expected, individually or in the aggregate, to result in a material adverse effect on the Company's financial condition. However, it is possible that our results of operations, financial position and cash flows in a particular period could be materially affected by these contingencies.
F- 21
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company accrues for loss contingencies when it is deemed probable that a loss has been incurred and that loss is estimable. The amounts accrued are based on the full amount of the estimated loss before considering insurance proceeds, and do not include an estimate for legal fees expected to be incurred in connection with the loss contingency. The Company accrues legal fees expected to be incurred in connection with loss contingencies as those fees are incurred by outside counsel as a period cost. The Company does not believe there are any pending legal proceedings that would have a material impact on the Company’s financial position, liquidity or results of operations.
10. SEGMENT AND GEOGRAPHIC INFORMATION
Subsequent to the spin-off from Integra, management assessed its segment reporting based on how it internally manages and reports the results of its business to its chief operating decision maker. The Company’s management reviews financial results, manages the business and allocates resources on an aggregate basis. Therefore, financial results are reported in a single operating segment: the development, manufacture and marketing of orthobiologics and spinal fusion hardware. The Company reports revenue in two product categories: orthobiologics and spinal fusion hardware. Orthobiologics products consist of a broad range of advanced and traditional bone graft substitutes that are designed to improve bone fusion rates following surgery. The spinal fusion hardware portfolio consists of an extensive line of products for minimally invasive surgery, complex spine, deformity and degenerative procedures.
Revenue, net consisted of the following:
Year Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
(In thousands) | ||||||||||||
Orthobiologics | $ | 67,258 | $ | 67,594 | $ | 66,669 | ||||||
Spinal fusion hardware | 65,920 | 71,101 | 79,917 | |||||||||
Total Revenue, net | $ | 133,178 | $ | 138,695 | $ | 146,586 | ||||||
The Company attributes revenues to geographic areas based on the location of the customer. Total revenue by major geographic area consisted of the following:
Year Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
(In thousands) | ||||||||||||
United States | $ | 120,259 | $ | 124,365 | $ | 128,653 | ||||||
International | 12,919 | 14,330 | 17,933 | |||||||||
Total Revenue, net | $ | 133,178 | $ | 138,695 | $ | 146,586 | ||||||
11. EMPLOYEE BENEFIT PLAN
The Company has a defined contribution savings plan under section 401(k) of the IRC. The plan covers substantially all employees. The Company matches employee contributions made to the plan according to a specified formula. The Company’s matching contributions totaled approximately $0.5 million, $0.3 million and $0.1 million for the years ended 2015, 2014 and 2013, respectively.
F- 22
SEASPINE HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
12. SELECTED QUARTERLY INFORMATION - UNAUDITED
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
(In thousands, except per share data) | |||||||||||||||
Total revenue, net: | |||||||||||||||
2015 | $ | 32,314 | $ | 33,461 | $ | 32,679 | $ | 34,724 | |||||||
2014 | 34,175 | 35,766 | 33,606 | 35,148 | |||||||||||
Gross profit: | |||||||||||||||
2015 | $ | 19,713 | $ | 18,955 | $ | 15,338 | $ | 18,053 | |||||||
2014 | 21,580 | 20,566 | 19,324 | 20,511 | |||||||||||
Net loss: | |||||||||||||||
2015 | $ | (9,898 | ) | $ | (17,685 | ) | $ | (14,199 | ) | $ | (13,750 | ) | |||
2014 | (5,560 | ) | (5,429 | ) | (5,316 | ) | (8,241 | ) | |||||||
Basic/diluted net loss per common share(1): | |||||||||||||||
2015 | $ | (0.90 | ) | $ | (1.60 | ) | $ | (1.27 | ) | $ | (1.23 | ) | |||
2014 | (0.50 | ) | (0.49 | ) | (0.48 | ) | (0.75 | ) | |||||||
(1) Per common share amounts for the quarters and full years have been calculated separately. Accordingly, quarterly amounts
do not necessarily add to the annual amount because of differences in the weighted average common shares outstanding during each period principally due to the effect of the Company’s issuing or retiring shares of its common stock during the year.
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
Balance at Beginning of Period | Charged to Costs and Expenses | Charged to Other Accounts | Additions/Deductions | Balance at End of Period | |||||||||||||||
Description | |||||||||||||||||||
(In thousands) | |||||||||||||||||||
Year ended December 31, 2015: | |||||||||||||||||||
Allowance for doubtful accounts and sales returns and allowances | $ | 558 | $ | 55 | $ | — | $ | 151 | $ | 764 | |||||||||
Deferred tax asset valuation allowance | 83,457 | (36,819 | ) | — | — | 46,638 | |||||||||||||
Year ended December 31, 2014: | |||||||||||||||||||
Allowance for doubtful accounts and sales returns and allowances | $ | 1,068 | $ | (267 | ) | $ | — | $ | (238 | ) | $ | 563 | |||||||
Deferred tax asset valuation allowance | 73,461 | 10,483 | (487 | ) | — | 83,457 | |||||||||||||
Year ended December 31, 2013: | |||||||||||||||||||
Allowance for doubtful accounts and sales returns and allowances | $ | 2,384 | $ | (691 | ) | $ | — | $ | (625 | ) | $ | 1,068 | |||||||
Deferred tax asset valuation allowance | 66,497 | 6,569 | 395 | — | 73,461 | ||||||||||||||
F- 23
EXHIBIT INDEX | ||||||||||
Incorporated by Reference | ||||||||||
Exhibit No. | Description | Filed Herewith | Form | File/Film No. | Date Filed | |||||
2.1 | Separation and Distribution Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of June 30, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
3.1 | Amended and Restated Certificate of Incorporation of SeaSpine Holdings Corporation. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
3.2 | Amended and Restated Bylaws of SeaSpine Holdings Corporation. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
4.1 | Form of Common Stock Certificate of SeaSpine Holdings Corporation. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.1 | Transition Services Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.2 | Tax Matters Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.3 | Employee Matters Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.4 | Microfibrillar Collagen Supply Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.5 | Collagen Ceramic Supply Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.6 | Demineralized Bone Matrix and Collagen Ceramic Products Supply Agreement between Integra LifeSciences Holdings Corporation and SeaSpine Holdings Corporation, dated as of July 1, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.7 | Brian Baker Letter Agreement, dated February 25, 2015. | Form 8-K | 001-36905-15966132 | 7/1/2015 | ||||||
10.8 | Form of Indemnification Agreement entered into between SeaSpine Holdings Corporation and each of its directors and executive officers. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.9 | SeaSpine Holdings Corporation 2015 Incentive Award Plan. | Form S-8 | 333-205334-1598733 | 6/29/2015 | ||||||
10.10 | Form of SeaSpine Holdings Corporation 2015 Incentive Award Plan Stock Option Agreement. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.11 | SeaSpine Holdings Corporation 2015 Employee Stock Purchase Plan. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.12 | Employment Agreement, by and between SeaSpine Holdings Corporation, SeaSpine Orthopedics Corporation and Keith Valentine, dated April 28, 2015. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.13 | John Bostjancic Letter Agreement, dated March 30, 2015. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.14 | John Winge Letter Agreement, dated January 22, 2015. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.15 | Amended and Restated Lease between Salma Jason Monica Limited Partnership and SeaSpine, Inc., dated as of May 23, 2011 for property at 2384 La Miranda, Vista, CA. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.16 | Amended and Restated Lease between Salma Jason Monica Limited Partnership and SeaSpine, Inc., dated as of May 23, 2011 for property at 2302 La Miranda, Vista, CA | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.17 | Amended and Restated Lease between Monarch RRC Properties, LLC (assignee of original landlord, New Goodyear LTD) and IsoTis Orthobiologics, Inc., dated as of February 23, 2006, for property at 2 Goodyear, Irvine, CA (the “Irvine Industrial Real Estate Lease”). | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.18 | Amendment No. 1 to Irvine Industrial Real Estate Lease, dated as of May 26, 2011. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.19 | Amendment No. 2 to Irvine Industrial Real Estate Lease, dated as of May 14, 2013. | Form 10 | 001-36905-15904590 | 6/1/2015 | ||||||
10.20 | Sublease Agreement between SeaSpine Orthopedics Corporation, and SkinMedica, Inc., dated as of July 8, 2015. | Form 8-K | 001-36905-151103433 | 9/11/2015 | ||||||
10.21 | SeaSpine Holdings Corporation Senior Leadership Retention and Severance Plan, effective January 27, 2016. | Form 8-K | 001-36905-161378936 | 2/2/2016 | ||||||
10.22 | SeaSpine Holdings Corporation 2015 Incentive Award Plan Annual Incentive Program. | Form 8-K | 001-36905-161472253 | 3/1/2016 | ||||||
10.23 | SeaSpine Holdings Corporation Non-Employee Director Compensation Program, effective October 13, 2015. | X | ||||||||
10.24 | Credit Agreement between SeaSpine Holdings Corporation, and Wells Fargo Bank, National Association, dated as of December 24, 2015. | X | ||||||||
21.1 | List of subsidiaries of SeaSpine Holdings Corporation. | X | ||||||||
23.1 | Consent of Pricewaterhouse Coopers LLP, Independent Registered Public Accounting Firm. | X | ||||||||
31.1 | Certification of Principal Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
31.2 | Certification of Principal Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
32.1 | Certification of Principal Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
32.2 | Certification of Principal Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
†101.INS | XBRL Instance Document | X | ||||||||
†101.SCH | XBRL Taxonomy Extension Schema Document | X | ||||||||
†101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||
†101.DEF | XBRL Definition Linkbase Document | X | ||||||||
†101.LAB | XBRL Taxonomy Extension Labels Linkbase Document | X | ||||||||
†101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||
† The financial information of SeaSpine Holdings Corporation Annual Report on Form 10-K for the year ended December 31, 2015 filed on March 16, 2016 formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Operations, (ii) the Consolidated Statements of Comprehensive Loss, (iii) the Consolidated Balance Sheets, (iv) Parenthetical Data to the Consolidated Balance Sheets, (v) the Consolidated Statements of Cash Flows, (vi) the Consolidated Statements of Equity, and (vii) Notes to Consolidated Financial Statements, is furnished electronically herewith.
