Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Kindred Biosciences, Inc. | a8-k322016.htm |
Exhibit 99.1
Kindred Biosciences Announces Fourth Quarter and Year-End 2015 Financial Results
San Francisco, CA (March 2, 2016) Kindred Biosciences, Inc. (NASDAQ: KIN), a biopharmaceutical company focused on saving and improving the lives of pets, today announced financial results for the fourth quarter and full year ended December 31, 2015.
“We are very pleased with the progress on Zimeta™, our lead product candidate, and look forward to our first product approval and launch,” stated Richard Chin, M.D., President and CEO of KindredBio. “We are also pleased to report that the strong pipeline of additional products, including KIND-010 for management of weight in cats and feline epo for anemia in cats, are also advancing rapidly. With the rich pipeline and a strong cash position, we are well-positioned for success.”
Development and Corporate Milestones
• | Positive topline results were reported in 2015 for Zimeta (dipyrone injection), for the treatment of pyrexia (fever) in horses, in a multicenter, randomized, blinded, placebo-controlled pivotal study that enrolled 138 horses. The Company has submitted the Chemical, Manufacturing and Controls (CMC) and Effectiveness technical sections of the New Animal Drug Application (NADA) to the Food and Drug Administration (FDA) and successfully completed the Target Animal Safety Study (TASS). All other technical sections of the NADA for Zimeta are planned for submission by the end of the first quarter of 2016 and the Company anticipates the approval of Zimeta by the end of 2016 or early 2017. Zimeta was previously known as KIND-012. |
• | Pivotal study is underway for KIND-010 for the management of weight loss in cats, following the successful completion of pilot studies. Enrollment in the pivotal study is over halfway complete and topline results are expected mid-year 2016. The Company is currently conducting the TASS and preparing the CMC technical section of the NADA for submission. |
• | Pilot laboratory study has been completed for KIND-510 (feline erythropoietin), for the control of non-regenerative anemia in cats. The pilot field efficacy study has been initiated. The Company is completing a GMP manufacturing plant and anticipates proceeding to GMP manufacturing in 2016. |
• | Formulation for KIND-014, a development candidate for equine gastric ulcer syndrome in horses, is being optimized, following the completion of dose ranging and palatability studies. The Company plans to initiate a pilot field efficacy study in the second quarter of 2016. |
• | Formulation for KIND-015, a development candidate for metabolic syndrome in horses, is being optimized. The Company plans to initiate a pilot field efficacy study in the second half of 2016. |
• | Other biologics: Antibodies against cytokines and immune checkpoint are progressing on track, and initial pilot studies for some of the antibodies are anticipated in 2016. |
• | KIND-Bodies, a novel biologics scaffold that has certain advantages over antibodies, including bispecific binding, has been invented and is being further developed. |
Fourth Quarter and Full Year 2015 Financial Results
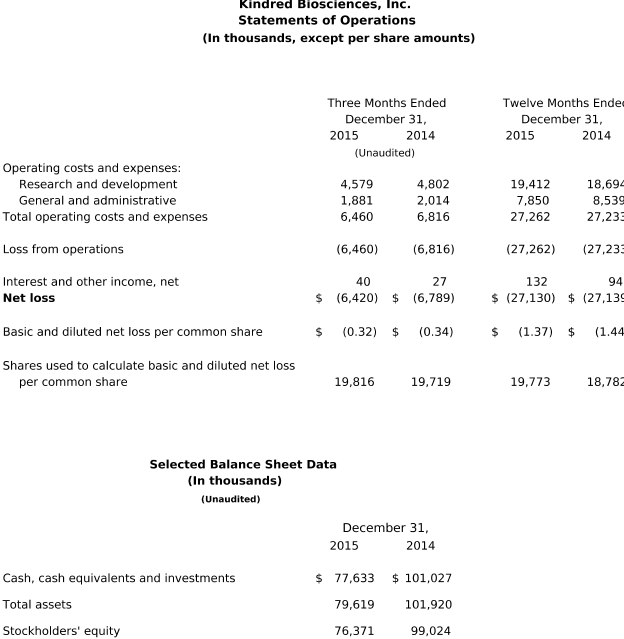
For the quarter ended December 31, 2015, KindredBio reported a net loss of $6.4 million, or $0.32 per share, compared to a net loss of $6.8 million, or $0.34 per share, for the same period in 2014. Research
Exhibit 99.1
and development expenses for the fourth quarter of 2015 totaled $4.6 million, compared to $4.8 million for the same period in 2014. General and administrative expenses for the fourth quarter of 2015 were $1.9 million, compared to $2.0 million for the same period in 2014.
For the year ended December 31, 2015, KindredBio reported a net loss of $27.1 million, or $1.37 per share, versus a net loss of $27.1 million, or $1.44 per share, for the same period in 2014.
Research and development expenses for the year ended December 31, 2015 were $19.4 million compared to $18.7 million in 2014. Stock-based compensation expense related to research and development was $1.9 million, versus $1.5 million in 2014. The $0.7 million year-over-year increase in research and development expenses was primarily due to higher payroll and related expenses as a result of increased headcount, additional expense associated with advancing biologics programs and higher stock-based compensation expense. The increase in expense was offset, in part, by lower field trial and material costs as the development of CereKin and AtoKin were discontinued and enrollment for the SentiKind program was completed in the second quarter of 2015. Consulting expenses were also lower.
General and administrative expenses for the year ended December 31, 2015 were $7.9 million compared to $8.5 million in 2014. General and administrative stock-based compensation expense was $2.3 million in 2015, versus $3.0 million in 2014. The $0.6 million decrease in general and administrative expenses was related to lower stock-based compensation expense, consulting and professional fees, marketing and corporate expenses. The decrease was offset in part by higher payroll and related expenses due to increased headcount as the Company continues to build its corporate infrastructure and other general business expenses.
At December 31, 2015, KindredBio had $77.6 million in cash, cash equivalents and investments. Net cash used in operating activities for 2015 was approximately $22.8 million. The Company also invested approximately $1.0 million in capital expenditures for the build-out of its GMP biologics manufacturing facility.
With respect to spending in 2016, the Company plans to focus on its core pipeline and programs. Accordingly, for 2016 the Company expects to spend between $24 million and $26 million, excluding the impact of stock-based compensation expense and the impact of acquisitions, if any. The Company’s anticipated expenditures for 2016 include the development of the necessary regulatory and quality processes as KindredBio nears the filing of registration for its second lead product candidate, KIND-010, and preparing for the commercial launch of Zimeta. Additionally, the Company is addressing the necessary manufacturing requirements for possible commercialization in the following years.
Webcast and Conference Call
KindredBio will host a conference call and webcast today at 4:30 p.m. Eastern Time / 1:30 p.m. Pacific Time. Interested parties may access the call by dialing toll-free (855) 433-0927 from the US, or (484) 756-4262 internationally, and using conference ID 56547791. The call will also be webcast live at http://edge.media-server.com/m/p/nyh2g4yz. A replay will also be available at that link for 30 days.
About Kindred Biosciences
Kindred Biosciences is a development-stage biopharmaceutical company focused on saving and improving the lives of pets. Its mission is to bring to pets the same kinds of safe and effective medicines that human family members enjoy. The Company’s strategy is to identify compounds and targets that have already demonstrated safety and efficacy in humans and to develop therapeutics based on these
Exhibit 99.1
validated compounds and targets for dogs, cats and horses. The Company has a deep pipeline of novel drugs and biologics in development across many therapeutic classes.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, but not limited to, statements regarding our expectations about the trials, regulatory approval, manufacturing, distribution and commercialization of our current and future product candidates, and statements regarding our anticipated revenues, expenses, margins, profits and use of cash.
These forward-looking statements are based on our current expectations. These statements are not promises or guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results to be materially different from any future results expressed or implied by the forward-looking statements. These risks include, but are not limited to, the following: our limited operating history and expectations of losses for the foreseeable future; the absence of revenue from our product candidates for the foreseeable future; our potential inability to obtain any necessary additional financing; our substantial dependence on the success of our lead product candidates, which may not be successfully commercialized even if they are approved for marketing; the effect of competition; our potential inability to obtain regulatory approval for our existing or future product candidates; our dependence on third parties to conduct some of our development activities; our dependence upon third-party manufacturers for supplies of our product candidates; uncertainties regarding the outcomes of trials regarding our product candidates; our potential failure to attract and retain senior management and key scientific personnel; uncertainty about our ability to develop a satisfactory sales organization; our significant costs of operating as a public company; our potential inability to obtain patent protection and other intellectual property protection for our product candidates; potential claims by third parties alleging our infringement of their patents and other intellectual property rights; our potential failure to comply with regulatory requirements, which are subject to change on an ongoing basis; the potential volatility of our stock price; and the significant control over our business by our principal stockholders and management.
For a further description of these risks and other risks that we face, please see the risk factors described in our filings with the U.S. Securities and Exchange Commission (the SEC), including the risk factors discussed under the caption "Risk Factors" in our Annual Report on Form 10-K and any subsequent updates that may be contained in our Quarterly Reports on Form 10-Q filed with the SEC. As a result of the risks described above and in our filings with the SEC, actual results may differ materially from those indicated by the forward-looking statements made in this press release. Forward-looking statements contained in this press release speak only as of the date of this press release and we undertake no obligation to update or revise these statements, except as may be required by law.
Contact
Russell Radefeld
KindredBio
russell.radefeld@kindredbio.com
(650) 701-7904
Exhibit 99.1