Attached files
| file | filename |
|---|---|
| EX-10.13 - EX-10.13 - MEDIVATION, INC. | mdvn-ex1013_539.htm |
| EX-10.12 - EX-10.12 - MEDIVATION, INC. | mdvn-ex1012_538.htm |
| EX-10.23 - EX-10.23 - MEDIVATION, INC. | mdvn-ex1023_598.htm |
| EX-10.14 - EX-10.14 - MEDIVATION, INC. | mdvn-ex1014_540.htm |
| EX-10.8 - EX-10.8 - MEDIVATION, INC. | mdvn-ex108_666.htm |
| EX-10.33 - EX-10.33 - MEDIVATION, INC. | mdvn-ex1033_543.htm |
| EX-10.16 - EX-10.16 - MEDIVATION, INC. | mdvn-ex1016_542.htm |
| EX-10.15 - EX-10.15 - MEDIVATION, INC. | mdvn-ex1015_541.htm |
| EX-10.9 - EX-10.9 - MEDIVATION, INC. | mdvn-ex109_537.htm |
| EX-31.1 - EX-31.1 - MEDIVATION, INC. | mdvn-ex311_9.htm |
| EX-23.1 - EX-23.1 - MEDIVATION, INC. | mdvn-ex231_10.htm |
| EX-31.2 - EX-31.2 - MEDIVATION, INC. | mdvn-ex312_12.htm |
| EX-21.1 - EX-21.1 - MEDIVATION, INC. | mdvn-ex211_11.htm |
| EX-10.50 - EX-10.50 - MXCOURT - MEDIVATION, INC. | mdvn-ex1050_597.htm |
| EX-10.51 - EX-10.51 - PSU - MEDIVATION, INC. | mdvn-ex1051_599.htm |
| EX-32.1 - EX-32.1 - MEDIVATION, INC. | mdvn-ex321_8.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2015
OR
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition period from to .
Commission file number: 001-32836
MEDIVATION, INC.
(Exact name of Registrant as specified in its charter)
|
Delaware |
|
13-3863260 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
525 Market Street, 36th Floor
San Francisco, California 94105
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (415) 543-3470
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.01 per share |
|
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES o NO x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES o NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. YES x NO o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES x NO o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
Accelerated filer |
|
o |
|
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
o (Do not check if a smaller reporting company) |
Smaller reporting company |
|
o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act.). YES o NO x
The aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $7.7 billion as of June 30, 2015, based upon the closing sale price on The NASDAQ Global Market reported on June 30, 2015. Excludes an aggregate of 24,807,840 shares of the registrant’s common stock (on a post-split basis) held by officers, directors and affiliated stockholders. For purposes of determining whether a stockholder was an affiliate of the registrant at June 30, 2015, the registrant assumed that a stockholder was an affiliate of the registrant at June 30, 2015 if such stockholder (i) beneficially owned 10% or more of the registrant’s common stock, as determined based on public filings, and/or (ii) was an executive officer or director or was affiliated with an executive officer or director of the registrant at June 30, 2015. Exclusion of such shares should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.
There were 164,233,527 shares of Registrant’s Common Stock, par value $0.01 per share, outstanding as of February 16, 2016.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for the 2016 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year covered by this Form 10-K are incorporated by reference in Part III, Items 10-14 of this Form 10-K.
2015 ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
|
|
|
Page |
|
|
|
|
|
PART I |
|
|
|
Item 1. |
2 |
|
|
Item 1A. |
21 |
|
|
Item 1B. |
44 |
|
|
Item 2. |
45 |
|
|
Item 3. |
45 |
|
|
Item 4. |
45 |
|
|
|
|
|
|
PART II |
|
|
|
Item 5. |
46 |
|
|
Item 6. |
48 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
49 |
|
Item 7A. |
67 |
|
|
Item 8. |
68 |
|
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
68 |
|
Item 9A. |
68 |
|
|
Item 9B. |
69 |
|
|
|
|
|
|
PART III |
|
|
|
Item 10. |
70 |
|
|
Item 11. |
70 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder |
70 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
70 |
|
Item 14. |
70 |
|
|
|
|
|
|
PART IV |
|
|
|
Item 15. |
71 |
|
|
|
|
|
|
72 |
||
|
|
|
|
|
FINANCIAL STATEMENTS |
|
|
|
|
Report of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm |
73 |
|
|
74 |
|
|
|
75 |
|
|
|
76 |
|
|
|
77 |
|
|
|
78 |
|
|
|
79 |
|
i
This Annual Report on Form 10-K, or Annual Report, includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. We intend that these forward-looking statements be subject to the safe harbors created by those provisions. Forward-looking statements are generally written in the future tense and/or are preceded by words such as “may,” “should,” “could,” “expect,” “suggest,” “believe,” “estimate,” “continue,” “anticipate,” “intend,” “plan,” or similar words, or negatives of such terms or other variations on such terms of comparable terminology. These forward-looking statements include, but are not limited to, statements regarding the commercialization of XTANDI ® (enzalutamide) capsules, or XTANDI, the continuation and success of our collaboration with Astellas Pharma, Inc., or Astellas, the timing, progress and results of our clinical trials, and our future drug development activities, including those with respect to talazoparib (which is referred to as MDV3800) and pidilizumab (which is referred to as MDV9300). The forward-looking statements contained in this Annual Report involve a number of risks, uncertainties, and assumptions, many of which are outside of our control. Factors that could cause actual results to differ materially from projected results include, but are not limited to, those discussed in “Risk Factors” included elsewhere in this Annual Report. Readers are expressly advised to review and consider those Risk Factors. Although we believe that the assumptions underlying the forward-looking statements contained in this Annual Report are reasonable, any of the assumptions could be inaccurate, and therefore there can be no assurance that the results anticipated by such statements will occur. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by us or any other person that the results or conditions described in such statements or our objectives and plans will be achieved. Furthermore, past financial or operations performance is not necessarily indicative of future performance. We disclaim any intention or obligation to update, supplement, or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
1
OVERVIEW
We are a biopharmaceutical company focused on the development and commercialization of medically innovative therapies to treat serious diseases for which there are limited treatment options. We have one commercial product, XTANDI® (enzalutamide) capsules, or XTANDI, through our collaboration with Astellas Pharma, Inc., or Astellas. XTANDI has received marketing approval in the United States, Europe and numerous other countries worldwide for the treatment of patients with metastatic castration-resistant prostate cancer, or mCRPC, and in Japan for the treatment of patients with castration-resistant prostate cancer, or CRPC. We and Astellas are also conducting investigational studies of enzalutamide in prostate cancer, advanced breast cancer, and hepatocellular carcinoma. Under our collaboration agreement with Astellas, we share equally with Astellas all profits (losses) related to U.S. net sales of XTANDI. We also receive royalties ranging from the low teens to the low twenties as a percentage of ex-U.S. XTANDI net sales. The collaboration also involved certain milestone payments from Astellas to us upon the achievement of defined development, regulatory and sales events, all of which have been achieved as of December 31, 2015.
We seek to become a more fully-integrated biopharmaceutical company through the continued commercialization of XTANDI, the acquisition or in-license and development and commercialization of other product opportunities, and through the advancement of our own proprietary research and development programs. We expect that our future growth may come from both internal research efforts and third-party business development activities. In the fourth quarter of 2015, we acquired all worldwide rights to talazoparib (which we refer to as MDV3800), an orally available poly-ADP ribose polymerase, or PARP, inhibitor, from BioMarin Pharmaceutical Inc., or BioMarin. MDV3800 is currently in a Phase 3 clinical trial for the treatment of patients with germline BRCA, or gBRCA, mutated advanced breast cancer (i.e., advanced breast cancer in patients whose BRCA genes contain germline mutations). We are targeting a number of other indications in which to investigate MDV3800, including breast cancer (beyond gBRCA mutations), prostate cancer, small cell lung cancer, and ovarian cancer. In the fourth quarter of 2014, we licensed exclusive worldwide rights to pidilizumab (which we refer to as MDV9300), an antibody with immune-mediated anti-tumor effects for all potential indications from CureTech, Ltd., or CureTech. Under the license agreement, we are responsible for all development, regulatory, manufacturing, and commercialization activities for MDV9300. We initiated a Phase 2 clinical trial evaluating MDV9300 in patients with relapsed or refractory diffuse large B-cell lymphoma in the fourth quarter of 2015, which is on partial clinical hold pending our revision of certain investigator brochure, protocols and informed consent documents. We submitted the revised documents to the FDA in early February 2016 and the FDA has 30 days thereafter to notify us if the partial hold is lifted. We also plan to develop MDV9300 in other hematologic malignancies such as multiple myeloma.
In addition to the above activities, we have various internal research and discovery efforts focused in oncology, neurology and other areas. A summary of our commercial product and clinical stage pipeline is provided below:
|
Target/Drug Compound |
|
Indication |
|
Status |
|
|
|
|
|
|
|
XTANDI |
|
metastatic CRPC |
|
Commercial |
|
Enzalutamide |
|
non-metastatic CRPC |
|
Phase 3 |
|
Enzalutamide |
|
non-metastatic HSPC (rising PSA) |
|
Phase 3 |
|
Enzalutamide |
|
metastatic HSPC |
|
Phase 3 (initiating) |
|
Enzalutamide |
|
hepatocellular carcinoma |
|
Phase 2 |
|
Enzalutamide |
|
AR+ TNBC |
|
Phase 2 |
|
Enzalutamide |
|
ER/PR+ & HER2 normal breast cancer |
|
Phase 2 |
|
Enzalutamide |
|
AR+ HER2 amplified breast cancer |
|
Phase 2 |
|
|
|
|
|
|
|
Talazoparib (MDV3800) |
|
gBRCA mutated advanced HER2 normal breast cancer |
|
Phase 3 |
|
|
|
|
|
|
|
Pidilizumab (MDV9300) |
|
Relapsed or refractory diffuse large B-cell lymphoma |
|
Phase 2 (potentially pivotal) |
|
|
|
|
|
|
|
MDV4463 |
|
Nonalcoholic steatohepatitis |
|
Phase 1 |
Additional information regarding XTANDI and our development programs for enzalutamide, MDV3800, MDV9300 and other research and drug discovery programs is included below.
OUR ENZALUTAMIDE PROGRAM
Summary of Our Enzalutamide Program
We and our collaboration partner Astellas have a number of clinical trials for which enrollment is ongoing or completed, including those described in the sections that follow.
2
According to the American Cancer Society, prostate cancer is the most commonly diagnosed cancer in men in the United States, other than skin cancer. The American Cancer Society estimates that approximately 221,000 new cases of prostate cancer were diagnosed, and approximately 28,000 men died of prostate cancer, in the United States alone during 2015. Prostate cancer is thus the second-leading cause of cancer death in men in the United States, after lung cancer. According to the American Cancer Society, about 1 in 7 men will be diagnosed with prostate cancer during his lifetime and about 1 in 38 men will die of prostate cancer.
Prostate cancer is generally seen as a slowly-progressing disease. According to the American Cancer Society, when including all stages of disease, the relative 15-year survival rate is 94%. In the United States, prostate cancer patients are typically managed clinically by two different physician specialties—urologists and oncologists. Urologists typically manage prostate cancer patients for the large majority of a patient’s disease course, starting with the initial diagnosis and continuing for many years until the patient develops castration-resistant disease and symptoms. Once the patient develops symptoms, such as pain from metastases of his prostate cancer to his bone, he is typically referred to an oncologist to manage the final stages of his disease, which typically represent the last 9 to 13 months of the patient’s life.
Prostate cancer is frequently diagnosed at a stage where it is believed to be confined to the prostate gland and its immediate surroundings—i.e., it has not yet spread to other areas of the body. Prostate cancer detected at this stage generally is treated either with prostatectomy (surgical removal of the prostate gland) or with radiation. For most men, these procedures are successful in curing the disease. However, for some men, these procedures are not curative and their prostate cancer continues to spread. This disease progression is typically detected by rising levels of serum prostate-specific antigen, or PSA, a marker of tumor burden in prostate cancer. Men whose disease continues to progress following surgery or radiation are considered to have advanced prostate cancer.
Because the male sex hormone testosterone is the primary fuel of prostate cancer growth, first-line therapy for advanced prostate cancer typically entails treatment with a class of hormonal drugs known as luteinizing-hormone releasing hormone analogs, or LHRH analogs, which reduce testosterone to castrate levels—i.e., the levels that would be achieved following surgical castration. Patients treated with LHRH analogs typically remain on those drugs for the remainder of their lives to keep testosterone levels suppressed to castrate levels. Patients typically respond to LHRH analog treatment, as evidenced by reduced PSA levels. Eventually, however, virtually all advanced prostate cancers will become resistant to LHRH analogs, and the patient’s PSA level will begin to rise. Once a patient progresses beyond hormone sensitive prostate cancer, or HSPC, while being treated with an LHRH analog, he is said to have castration-resistant prostate cancer, or CRPC. The term “castration-resistant” refers to the fact that these patients have had their serum testosterone reduced to castrate levels, and yet their prostate cancers continue to progress.
Once a patient has developed CRPC, the typical second line therapy is a class of hormonal drugs known as anti-androgens, which block the ability of testosterone to bind its receptor, the androgen receptor. Bicalutamide, widely available in generic form, is the most commonly used anti-androgen drug. Like LHRH analogs, bicalutamide typically suppresses tumor growth for a certain period of time, as evidenced by a declining PSA level. However, virtually all prostate cancers become resistant to bicalutamide as well. In addition, once prostate cancer becomes resistant to bicalutamide, the effect of that drug may convert from suppressing tumor growth to fueling tumor growth. For this reason, bicalutamide treatment is typically discontinued once patients begin to progress on the drug.
The next line of treatment for CRPC patients who have failed treatment on both LHRH analogs and anti-androgen drugs is frequently the chemotherapy drug Taxotere® (docetaxel), which has been shown in clinical studies to prolong survival. However, docetaxel is an infused cytotoxic chemotherapy, and thus entails an increased risk of serious adverse effects, including fluid retention, liver toxicity, low white blood cell counts, and death. Prior to 2010, there were no treatments approved by the U.S. Food and Drug Administration, or FDA, for CRPC patients whose prostate cancer had progressed following treatment with docetaxel. These patients typically had only palliative care, or pain reducing, options available to them. Since 2010, four new agents in addition to XTANDI have been approved by the FDA for the treatment of patients with mCRPC: Jevtana® (cabazitaxel); Provenge® (sipuleucel-T); Zytiga® (abiraterone acetate) plus prednisone; and Xofigo® (radium RA-223 dichloride); and others are currently being studied.
Based on the above treatment algorithm, advanced prostate cancer patients are generally subdivided into various sub-populations that regulatory agencies typically consider to be distinct patient populations. Because they view them as distinct, regulatory agencies typically require separate clinical studies in each patient population to grant marketing approval covering that patient population. These sub-populations are defined primarily by two factors: (a) the prior treatments the patient has undergone; and (b) whether the patient has metastatic disease. The sub-populations are the following:
|
|
• |
Patients with HSPC. Patients in this sub-population have progressive prostate cancer, are not castrate, and have not yet been treated with an LHRH analog drug, an anti-androgen drug or any other hormonal drug for progressive disease. Hormone-sensitive prostate cancer patients may have metastatic or non-metastatic disease. |
3
Our Prostate Cancer Clinical Trials
|
|
• |
TERRAIN – The Phase 2 TERRAIN trial was initiated in March 2011 and completed enrollment in July 2013. The trial evaluated enzalutamide head-to-head versus bicalutamide, the leading marketed anti-androgen drug, in 375 pre-chemotherapy mCRPC patients. The randomized, double-blind trial was conducted primarily in Europe. The primary endpoint of the trial was progression-free survival, or PFS, defined as time from randomization to centrally determined radiographic progression, skeletal-related event, initiation of new anti-neoplastic therapy or death, whichever occurred first. In January 2015, we and Astellas reported top-line results from the Phase 2 TERRAIN trial. The trial achieved its primary endpoint demonstrating a statistically significant increase in PFS for patients with mCRPC for enzalutamide compared to bicalutamide (Hazard Ratio = 0.44; 95% Confidence Interval (CI): 0.34, 0.57; p < 0.0001). Median PFS was 15.7 months in the enzalutamide group compared to 5.8 months in the bicalutamide group. The median time on treatment in the TERRAIN trial was 11.7 months in the enzalutamide group versus 5.8 months in the bicalutamide group. Serious adverse events were reported in 31.1% of enzalutamide-treated patients and 23.3% of bicalutamide-treated patients. Grade 3 or higher cardiac adverse events were reported in 5.5% of enzalutamide-treated patients versus 2.1% of bicalutamide-treated patients. Two seizures were reported in the enzalutamide group and one in the bicalutamide group. Results from the TERRAIN trial were published in an online issue of Lancet Oncology in January 2016. We have submitted the results of the TERRAIN trial to regulatory agencies for potential inclusion in the relevant clinical trials sections of the XTANDI label within the current metastatic CRPC indication. The Prescription Drug User Fee Act, or PDUFA, goal date for a decision by the FDA is October 22, 2016. |
|
|
• |
STRIVE – The Phase 2 STRIVE trial was initiated in August 2012 and completed enrollment in March 2014. The trial evaluated enzalutamide head-to-head versus bicalutamide, the leading marketed anti-androgen drug, in 396 pre-chemotherapy CRPC patients. The randomized, double-blind trial enrolled both metastatic and non-metastatic patients and was conducted in the United States. The primary endpoint of the trial was PFS defined as time from randomization to radiographic (bone or soft tissue) progression, PSA progression (defined by Prostate Cancer Working Group 2 criteria), or death due to any cause, whichever occurs first. In April 2015, we and Astellas reported top-line results from the Phase 2 STRIVE trial. The trial achieved its primary endpoint demonstrating a statistically significant prolongation of PFS for patients with CRPC for enzalutamide compared with bicalutamide (Hazard Ratio = 0.24; 95% CI: 0.18, 0.32; p < 0.0001). Median PFS was 19.4 months in the enzalutamide group compared with 5.7 months in the bicalutamide group. The median time on treatment in the STRIVE trial was 14.7 months in the enzalutamide group versus 8.4 months in the bicalutamide group. Serious adverse events were reported in 29.4% of enzalutamide-treated patients and 28.3% of bicalutamide-treated patients. Grade 3 or higher cardiac adverse events were reported in 5.1% of enzalutamide-treated patients versus 4.0% of bicalutamide-treated patients. One seizure was reported in the trial in the enzalutamide-treated group and none in the bicalutamide-treated group. Results from the STRIVE trial were published in an online issue of Journal of Clinical Oncology in January 2016. We have submitted the results of STRIVE to regulatory agencies for potential inclusion in the relevant clinical trials sections of the XTANDI label within the current metastatic CRPC indication. The PDUFA goal date for a decision by the FDA is October 22, 2016. |
|
|
• |
PROSPER – The first patient was enrolled in the Phase 3 PROSPER trial in December 2013. The trial is evaluating the safety and efficacy of enzalutamide in patients with non-metastatic CRPC. The PROSPER trial is intended to enroll a high-risk subgroup of patients with prostate cancer who are progressing despite androgen deprivation therapy, but who are asymptomatic with no prior or present evidence of metastatic disease. The Phase 3 randomized, double-blind, placebo-controlled, multi-national trial is designed to enroll approximately 1,560 patients with non-metastatic CRPC. The primary endpoint of the trial is metastasis-free survival. |
|
|
• |
EMBARK – The first patient was enrolled in the Phase 3 EMBARK trial in January 2015. The trial is intended to evaluate the efficacy and safety of enzalutamide alone or in combination with androgen deprivation therapy compared with androgen deprivation therapy alone in patients with high-risk, hormone-sensitive, non-metastatic prostate cancer that has biochemically recurred (rising PSA level) following definitive local therapy with radical prostatectomy and/or radiation. The purpose of the trial is to help determine if enzalutamide can delay or prevent the development of metastatic prostate cancer in high-risk men with a rapidly rising PSA. The trial is designed to enroll approximately 1,860 patients. The primary endpoint of the trial is metastasis-free survival. |
4
|
|
prednisone as compared to abiraterone acetate and prednisone alone in patients with chemotherapy-naive CRPC whose disease has progressed following enzalutamide therapy. The purpose of the trial is to help determine the potential clinical benefit of continued enzalutamide treatment after progression on enzalutamide therapy. The global randomized, double-blind, placebo-controlled trial has enrolled 509 chemotherapy-naive patients with mCRPC. The primary endpoint of the trial is PFS defined as time from randomization to radiographic progression, unequivocal clinical progression, or death, whichever occurs first. Top-line results from PLATO may be available as early as the second half of 2016. |
|
|
• |
M1 HSPC – In January 2016, Astellas initiated start-up activities for a Phase 3 trial in patients with metastatic hormone-sensitive prostate cancer, or mHSPC. The trial is intended to evaluate the efficacy and safety of enzalutamide in combination with androgen deprivation therapy compared with androgen deprivation therapy alone in approximately 1,100 patients. The primary endpoint of the trial is overall survival. |
|
|
• |
AFFIRM – Our FDA approval in post-chemotherapy mCRPC was based on the results of the AFFIRM trial, a randomized, double-blind Phase 3 trial evaluating XTANDI (160 mg once daily) as compared to placebo in 1,199 post-chemotherapy mCRPC patients. The primary endpoint of the AFFIRM trial was overall survival. Data from the AFFIRM trial were first reported in November 2011 and were published in The New England Journal of Medicine in August 2012. The AFFIRM trial also led to the initial marketing approvals of XTANDI by regulatory authorities in Europe, Japan and numerous other countries worldwide. The open-label extension of this trial is ongoing. |
|
|
• |
PREVAIL – Our FDA approval in pre-chemotherapy mCRPC was based on the results of the PREVAIL trial, a randomized, double-blind, placebo-controlled Phase 3 trial, evaluating enzalutamide (160 mg once daily) as compared to placebo in 1,717 patients with pre-chemotherapy mCRPC. The co-primary endpoints were radiographic PFS and overall survival. Positive results from the PREVAIL trial were first reported in October 2013 and were published in The New England Journal of Medicine in June 2014. The PREVAIL trial also led to expanded marketing approvals by regulatory authorities in Europe, Japan, and numerous other countries worldwide. In July 2015, the FDA approved a label update for XTANDI based on an updated overall survival analysis of the Phase 3 PREVAIL trial. This analysis was conducted when 784 deaths were observed and found an overall survival benefit with a 23% reduction in risk of death (Hazard ratio = 0.77; 95% CI: 0.67, 0.88) and a 4-month improvement in median survival with enzalutamide (35.3 months [95% CI: 32.2, not yet reached]) over placebo (31.3 months [95% CI: 28.8, 34.2]). The open-label extension of this trial is ongoing. |
Breast Cancer
According to the American Cancer Society, breast cancer is the most common cancer in women in the United States, other than skin cancer. The American Cancer Society estimates that approximately 234,000 new cases of breast cancer were diagnosed in women, and approximately 41,000 women died of breast cancer, in the United States alone during 2015. Breast cancer is the second-leading cause of cancer death in women in the United States, after lung cancer. According to the American Cancer Society, the chance that breast cancer will be responsible for a woman’s death is about 1 in 36. Death rates from breast cancer have been declining since about 1989, with larger decreases in women younger than 50. These decreases are believed to be the result of earlier detection through screening and increased awareness, as well as improved treatment.
Systemic treatment of recurrent/advanced breast cancer has been shown, in some situations, to prolong survival and/or quality of life, but it is not curative. Therefore treatments associated with minimal toxicity are preferred. There are many chemotherapies approved for use in breast cancer. Most are given as single agents as there is no compelling evidence that combination regimens are superior to sequential single agents once the disease has recurred. The length of treatment depends on whether the cancer shrinks, how much it shrinks, and how well the treatment is tolerated. A central component of the treatment of breast cancer is full knowledge of its biologic features, which provides information that may predict a patient’s response to therapy. These factors are determined by examination of excised tissue and routinely provided in a written pathology report and are based, in part, on three targeted receptors in breast cancer cells – the estrogen receptor, or ER, progesterone receptor, or PgR, and human epidermal growth factor receptor 2, or HER2. Androgen receptor expression has been observed in the majority of ER/PgR+ (>65%) and HER2 amplified (>50%) breast cancer and in up to one-third of triple negative breast cancer.
The estrogen and progesterone receptors are in the nuclear steroid receptor family and their expression is observed in two-thirds of all breast cancer. There are five commonly used agents approved for use in patients whose tumor express one or both of these receptors: tamoxifen, fulvestrant, exemestane, letrozole and anastrozole. The use of minimally toxic endocrine therapies is preferred to the use of cytotoxic therapy whenever reasonable. Everolimus and palbociclib have recently been approved in combination with exemestane and with letrozole or fulvestrant, respectively, for the treatment of this subtype of disease that is advanced.
Overexpression of the growth-promoting protein known as HER2 is found in about 20% of breast cancer and, in the absence of HER2 targeted therapy, is associated with a worse prognosis. A number of drugs have been developed that target HER2: trastuzumab, Ado-trastuzumab emtansine and pertuzumab, which are monoclonal antibodies that are administered intravenously, and lapatinib which is administered orally. Breast cancer that is HER2+ may, or may not, also express ER or PgR.
5
Triple negative breast cancer, or TNBC, is a type of breast cancer that is so named by the observation that it lacks expression of the three targeted receptors in breast cancer, namely ER, PgR and HER2. It is estimated that approximately 20% of women with metastatic breast cancer are in this category. Triple negative breast cancer has no recognized target and standard therapy is cytotoxic chemotherapy.
Our Breast Cancer Clinical Trials
In April 2012, we and Astellas expanded the clinical development of enzalutamide to include breast cancer. We currently have three Phase 2 clinical trials evaluating enzalutamide in three subsets of breast cancer:
|
|
• |
AR+, TNBC – This Phase 2 open-label, single arm, multicenter clinical trial evaluated enzalutamide as a single agent for the treatment of advanced androgen receptor positive, or AR+, TNBC. 118 women with advanced TNBC were enrolled in this Simon 2 stage study at sites in the United States, Canada, and Europe. The objective of the study was to evaluate the safety and clinical benefit of enzalutamide, 160 mg/day orally, for advanced TNBC and to identify a biomarker to better select those women more likely to respond to enzalutamide. The primary endpoint of the trial was the clinical benefit rate at 16 weeks, or CBR16, defined as the proportion of evaluable patients with a complete response, or CR, partial response, or PR, or stable disease for at least 16 weeks. Secondary endpoints of the trial included clinical benefit rate at 24 weeks, or CBR24, and PFS, defined as time from the date of first dose of study drug until documented disease progression or death due to any cause, overall survival, and safety. |
The primary endpoint analysis was pre-specified to be conducted in the evaluable patient population, defined as all patients who had at least one follow-up tumor assessment after starting enzalutamide and whose TNBC had at least 10% of the tumor cells stain positive for AR using a central immunohistochemistry, or IHC, assay. The evaluable population was pre-specified based on the hypothesis that patients whose tumor tissue expressed higher levels of AR protein may be more likely to receive benefit from enzalutamide than those whose tumors had less than 10% cells with AR expression. Of the 118 patients enrolled into the study, 75 were evaluable. Analyses for clinical benefit and safety were also conducted in all 118 patients enrolled.
The study met its primary endpoint, achieving a CBR16 of 35% (95% CI: 24, 46), including six CRs or PRs (8%) in the evaluable population based on results as of March 25, 2015. The secondary endpoint of CBR24 was 29% (95% CI: 20, 41), and the median PFS on enzalutamide therapy was 14.7 weeks (95% CI: 8.1, 19.3) in the evaluable population. Using the total intent-to-treat, or ITT, population, CBR16 was achieved in 25% (95% CI: 17, 33) including seven CRs or PRs (6%), CBR24 was achieved in 20% (95% CI: 14, 29), and the median PFS was 12.6 weeks (95% CI: 8.1, 15.7).
Nearly half of the enrolled patients (47%) tested positive for a novel gene expression profile. Of these 56 diagnostic positive women with advanced TNBC, 39% achieved CBR16 (95% CI: 27, 53) and 36% achieved CBR24 (95% CI: 24, 49). Of the 62 women who were diagnostic negative for the novel gene expression profile, 11% achieved a CBR16 (95% CI: 5, 21) and 6% achieved CBR24 (95% CI: 2, 16). Median PFS in the diagnostic positive group was 16.1 weeks (95% CI: 13.3, 27.4) compared with 8.1 weeks in the diagnostic negative group (95% CI: 7.4, 12.6). Over half (n=62) of the ITT patients enrolled received enzalutamide as their first- or second-line treatment and median PFS on enzalutamide in diagnostic positive patients was 40.4 weeks (95% CI: 16.1, not yet reached) whereas it was 9 weeks (95% CI: 7.3, 15.7) in the diagnostic negative group.
An exploratory analysis of overall survival data collected as of September 15, 2015 demonstrated that patients treated with enzalutamide whose tumors tested positive according to the novel diagnostic assay experienced a 13.8-month longer median overall survival duration compared to those patients whose tumors tested negative for the novel gene expression profile. Median overall survival in the diagnostic positive group treated with enzalutamide was 21.3 months (95% CI: 12.9, 21.3) compared with 7.5 (95% CI: 4.8, 11.2) months for the diagnostic negative group treated with enzalutamide.
Multicovariate analyses demonstrated that only diagnostic status (positive) and number of prior lines of therapy (0-1) were statistically significantly and independently associated with both improved PFS and overall survival outcomes. Additional variables included in the multi-covariate analyses were age (≥65 vs. < 65 years), disease free interval between primary and metastatic disease (≥12 months vs. <12 months), AR IHC (≥10% vs. <10%).
The most common (reported in ≥10% of patients) adverse events reported as related to enzalutamide treatment in the ITT population were fatigue (35%), nausea (26%), decreased appetite (13%), diarrhea (10%), and hot flush (10%).
Additionally, we entered into a collaboration agreement with NanoString Technologies, Inc. and our partner Astellas, in January 2016, to translate our gene expression profile into a companion diagnostic assay using NanoString’s nCounter® Dx Analysis System for potential use as a companion diagnostic test for enzalutamide for TNBC.
6
|
|
• |
AR+ HER2 Amplified – In August 2014, Astellas initiated a Simon 2 stage Phase 2 clinical trial evaluating the safety and efficacy of adding enzalutamide to trastuzumab in approximately 80 patients with advanced breast cancer that is AR+ and HER2 amplified. Patients must also have previously received and progressed on trastuzumab. The primary endpoint of the trial is clinical benefit rate defined as complete response or partial response or stable disease for at least 24 weeks. The trial met the criteria to proceed to stage 2 which required a minimum of three evaluable patients in the first 21 evaluable patients to achieve CBR24. Like the TNBC clinical trial, the evaluable population consists of patients with at least one follow-up tumor assessment after starting enzalutamide and whose breast cancer has AR protein expression by central IHC testing in at least 10% of tumor cells. |
Hepatocellular Carcinoma
The American Cancer Society estimates that approximately 36,000 new cases of liver cancer were diagnosed in the United States alone during 2015, approximately three-fourths of which were hepatocellular carcinoma, or HCC. Liver cancer incidence rates are about three times higher in men than in women, and have doubled in each sex over the past two decades. Common symptoms, which do not usually appear until the cancer is advanced, include abdominal pain and/or swelling, weight loss, weakness, loss of appetite, jaundice and fever. Enlargement of the liver is the most common physical sign. The most important risk factors for liver cancer are obesity, diabetes, alcoholic liver disease, chronic infection with hepatitis B virus and/or hepatitis C virus, and tobacco smoking. Early stage liver cancer can sometimes be treated successfully with partial hepatectomy (surgical removal of the liver); however, only a limited number of patients have sufficient healthy liver tissue for this to be an option. Liver transplantation may be an option for individuals with small tumors who are not candidates for partial hepatectomy. Other treatment options include ablation (techniques used to destroy liver tumors without removing them) or embolization (a procedure that injects substances to try to block or reduce the blood flow to cancer cells). Fewer treatment options exist for patients diagnosed at an advanced stage. Sorafenib is a targeted drug approved for the treatment of HCC when it cannot be treated with surgery.
In January 2016, Astellas enrolled the first patient into a Phase 2 trial evaluating enzalutamide in hepatocellular carcinoma. The trial will assess approximately 144 patients with advanced hepatocellular carcinoma that have failed sorafenib or other anti-vascular endothelial growth factor therapies. The primary endpoint of the trial is overall survival.
The Astellas Collaboration Agreement
We have a collaboration agreement with Astellas, or the Astellas Collaboration Agreement, pursuant to which we are collaborating to develop and commercialize XTANDI globally for all indications, dosages, and formulations of enzalutamide. We licensed the intellectual property rights covering XTANDI from the Regents of the University of California, or UCLA or Regents, pursuant to a license agreement described in the section below titled, “License Agreement with UCLA.” Under the Astellas Collaboration Agreement, Astellas paid us a non-refundable, upfront cash payment of $110.0 million in 2009. As of December 31, 2015, we have earned all development and sales milestone payments under the Astellas Collaboration Agreement, totaling $655.0 million, including $245.0 million, $307.0 million, and $45.0 million in the years ended December 31, 2015, 2014, and 2013, respectively.
In the United States, decisions are generally made by consensus, pre-tax profits and losses are shared equally, and, subject to certain exceptions, development and commercialization costs are also shared equally. Outside the United States, decisions are generally made by Astellas and all development and commercialization costs are borne by Astellas. Astellas retains all ex-U.S. profits and losses, and pays us a tiered royalty ranging from the low teens to the low twenties as a percentage of aggregate net sales of XTANDI outside the United States, or ex-U.S. XTANDI net sales.
The Astellas Collaboration Agreement establishes several joint committees consisting of representatives from both parties that operate by consensus to oversee the collaboration. In the event that a joint committee is unable to reach consensus on a particular issue, then, depending on the issue, a dispute may be decided at the joint committee level by the party with the final decision on the issue or escalated to senior management of the parties. If a dispute is escalated to senior management and no consensus is reached, then the dispute may be decided by the party to whom the contract grants final decision on such issue. Other issues can only be decided by consensus of the parties, and unless and until the parties’ representatives reach agreement on such issue, no decision on such issue will be made, and the status quo will be maintained.
7
Unless terminated earlier by us or Astellas pursuant to the terms thereof, the Astellas Collaboration Agreement will remain in effect: (a) in the United States, until such time as Astellas notifies us that Astellas has permanently stopped selling products covered by the Astellas Collaboration Agreement in the United States; and (b) in each other country of the world, on a country-by-country basis, until such time as (i) products covered by the Astellas Collaboration Agreement cease to be protected by patents or regulatory exclusivity in such country and (ii) commercial sales of generic equivalent products have commenced in such country.
We and Astellas are each permitted to terminate the Astellas Collaboration Agreement for an uncured material breach by the other party or for the insolvency of the other party. Astellas has the right to terminate the Astellas Collaboration Agreement unilaterally by advance written notice to us. Following any termination of the Astellas Collaboration Agreement in its entirety, all rights to develop and commercialize XTANDI will revert to us, and Astellas will grant a license to us to enable us to continue such development and commercialization. In addition, except in the case of termination by Astellas for our uncured material breach, Astellas will supply XTANDI to us during a specified transition period.
The Astellas Collaboration Agreement further provides for a standstill period during which Astellas and its Affiliates, as defined in the Astellas Collaboration Agreement, agreed to certain restrictive covenants, including that they would not, directly or indirectly (subject to certain exceptions), unless invited to do so by us, acquire (a) all or substantially all of our consolidated assets or (b) beneficial ownership of more than five percent of any voting securities of us or any subsidiary or Affiliate of us. The standstill period will expire in September 2016.
Additional information about the Astellas Collaboration Agreement, including geographic financial information, is included in Note 3, “Collaboration Agreement,” to our audited consolidated financial statements included elsewhere in this Annual Report.
License Agreement with UCLA
Under an August 2005 license agreement with UCLA, our subsidiary Medivation Prostate Therapeutics, Inc. holds an exclusive worldwide license under several UCLA patents and patent applications covering XTANDI and related compounds. Under the Astellas Collaboration Agreement, we granted Astellas a sublicense under the patent rights licensed to us by UCLA.
We are required to pay UCLA (a) an annual maintenance fee, (b) $2.8 million in aggregate milestone payments upon achievement of certain development and regulatory milestone events with respect to XTANDI (all of which has been paid as of December 31, 2015), (c) ten percent of all Sublicensing Income, as defined in the agreement, which we earn under the Astellas Collaboration Agreement, and (d) a four percent royalty on global net sales of XTANDI, as defined. Under the terms of the Astellas Collaboration Agreement, we share this royalty obligation equally with Astellas with respect to sales in the United States, and Astellas is responsible for this entire royalty obligation with respect to sales outside of the United States. We are currently involved in litigation with UCLA regarding this agreement. For more information about this litigation, see “Legal Proceedings” in Item 3 of Part I below.
UCLA may terminate the agreement if we do not meet a general obligation to diligently proceed with the development, manufacturing and sale of licensed products, or if we commit any other uncured material breach of the agreement. We may terminate the agreement at any time upon advance written notice to UCLA. If neither party terminates the agreement early, the agreement will continue in force until the expiration of the last-to-expire patent on a country-by-country basis.
MDV3800 (TALAZOPARIB) PROGRAM
In the fourth quarter of 2015, we acquired all worldwide rights to talazoparib (which we refer to as MDV3800) from BioMarin Pharmaceutical Inc., or BioMarin. Upon closing of the transaction, we paid BioMarin an upfront cash payment of $410.0 million. We also assumed certain costs for ongoing clinical trials of MDV3800, and commitments under certain agreements previously entered into or assumed by BioMarin and assigned to us. BioMarin is eligible to receive up to an additional $160.0 million upon the achievement of defined regulatory and sales-based milestones, and mid-single digit royalties on net sales of products that contain MDV3800 during the royalty term specified in the asset purchase agreement. We further entered into a transition services agreement at the closing of the transaction to facilitate the transition of research and development activities relating to MDV3800 from BioMarin to us, including responsibility for the ongoing clinical studies.
MDV3800 is an orally available poly-ADP ribose polymerase, or PARP, inhibitor currently in a Phase 3 clinical trial for the treatment of patients with gBRCA mutated breast cancer. The Phase 3 EMBRACA trial is an open-label, 2:1 randomized, parallel, two-arm study of MDV3800 as compared to the protocol-specified physicians’ choice of chemotherapy (capecitabine, eribulin, gemcitabine or vinorelbine) in gBRCA-mutated locally advanced and/or metastatic breast cancer patients who have received prior chemotherapy for their metastatic disease. The study is enrolling up to 430 patients. The primary objective of the study is to compare PFS of patients treated with MDV3800 as a monotherapy relative to those treated with protocol-specified physicians’ choice. We anticipate fully enrolling this trial by the end of 2016 with top-line results as early as the first half of 2017. We are targeting a number of other indications in which to investigate MDV3800, including breast cancer (beyond gBRCA mutations), prostate cancer, small cell lung cancer, and ovarian cancer.
8
PARP is a family of proteins involved in a wide range of cellular functions including deoxyribonucleic acid, or DNA, transcription, DNA damage response, genomic stability maintenance, cell cycle regulation, and cell death. One well-studied area of PARP activity relates to DNA repair. Since DNA is the vehicle by which fundamental information is passed on when a cell divides, it is critical to the viability of cells and human health that DNA damage can be repaired. If a cell’s DNA damage repair system is impaired, the cell will die.
DNA can be damaged as a result of environmental exposure or agents, or through errors introduced during replication. Cells have a number of different mechanisms to repair damaged DNA. PARPs aid in repair by binding to single-strand breaks in DNA and recruiting additional repair proteins to the site of damage, a process called base excision repair. PARP inhibitors are thought to block this activity, thus preventing the repair of DNA. PARP inhibitors exert a cytotoxic effect by two mechanisms: (a) catalytic inhibition of PARP enzyme activity and (b) PARP trapping.
A single-stranded DNA break signals PARP protein to attach to DNA, a critical step in DNA repair. Inhibition of PARP’s catalytic activity impairs the recruitment of additional DNA repair proteins critical for base excision repair and single stranded breaks can become double stranded breaks which require homologous recombination for repair. Cells harboring mutations or deficiencies in homologous recombination (e.g., cells with BRCA1/2 mutations), are particularly susceptible to the cytotoxic effects of PARP inhibitors as these cells tend to accumulate double strand breaks that are inaccurately repaired by alternative pathways.
Another potentially important mechanism of action to MDV3800 is the phenomenon called PARP trapping. The attachment and release of PARP molecules to DNA is facilitated by the reduction of nicotinamide adenine dinucleotide to its reduced form. MDV3800 traps the PARP molecule on the DNA creating a large protein moiety that interferes with the cell’s ability to replicate its DNA thereby causing cell death.
DNA damaging therapies such as radiation and chemotherapy (alkylating agents like temozolomide) modify DNA and initiate PARP-dependent repair processes including base excision repair. Single strand breaks resulting from these therapies create additional sites for PARP to be trapped by MDV3800 thereby further increasing its cytotoxicity. In 2015 in the U.S. alone, approximately 500,000 patients received DNA damaging radiation therapy, while more than 500,000 received DNA damaging chemotherapy. As single-stranded breaks are potential sites for PARP trapping, MDV3800 in combination with these DNA damaging agents may have broad activity beyond tumors harboring BRCA1/2 mutations which represent only a small portion of the total oncology market.
MDV9300 (PIDILIZUMAB) PROGRAM
In the fourth quarter of 2014, we licensed exclusive worldwide rights to MDV9300, an antibody with immune-mediated anti-tumor effects, for all potential indications, from Israel-based CureTech. Under the license agreement, we are responsible for all development, regulatory, manufacturing, and commercialization activities for MDV9300.
MDV9300 was not generated against a single purified recombinant protein but, rather, was generated against a lymphoblast cell line called DAUDI. Although the molecule was originally understood to work primarily via PD-1 (Programmed Death-1) binding, our work as well as that of others has shown other potential activity as well. In the year since we acquired rights to the molecule, we conducted testing and characterization work to better identify the binding target and, in late December 2015, we concluded that MDV9300 does not bind PD-1. Clinical and preclinical data continue to indicate that MDV9300 administration is associated with enhanced maturation and survival of T lymphocytes, which may improve adaptive immunity, as well as activation of natural killer cells, which may improve innate immunity that facilitates anti-tumor activity. Such broad effects on both sides of the immune response are not widely reported with checkpoint inhibitors and may serve to differentiate MDV9300 from other agents in the competitive PD-1 landscape.
Prior to concluding non-binding of PD-1, in the fourth quarter of 2015, we initiated a Phase 2 clinical trial evaluating MDV9300 in patients with relapsed or refractory diffuse large B-cell lymphoma, or DLBCL. The open-label trial is expected to enroll approximately 180 patients with an incomplete response following salvage therapy or autologous stem cell transplantation for relapsed or refractory CD20+ diffuse large B cell lymphoma, transformed indolent lymphoma or primary mediastinal B-cell lymphoma. Patients will be assessed in two parallel cohorts of approximately 90 patients each. One cohort will enroll patients who have received an autologous stem cell transplant, and the second cohort will enroll patients who have received salvage chemotherapy, but are transplant ineligible. MDV9300 will be administered at a dose of 200 mg by IV infusion. The primary endpoint of the trial is best overall response rate.
In early January 2016, we promptly advised FDA of our conclusion that MDV9300 does not bind PD-1, and the agency placed the IND on partial clinical hold and requested that we revise our characterization of MDV9300 in the related investigator brochure, protocols and informed consent documents. The partial clinical hold was not related to concerns regarding the safety of MDV9300. Under the hold, we may not enroll patients into the Phase 2 DLBCL trial until such documents are revised. We since submitted the revised documents in early February 2016 to the agency, and the FDA has 30 days thereafter to notify us if the partial hold is lifted.
9
Although the Phase 2 clinical trial was initiated in December 2015, no patients have been enrolled to date. Under the partial clinical hold, patients who are currently receiving MDV9300 through investigator-sponsored trials of MDV9300 that cross-reference our IND may continue on treatment, but we must inform those investigators to update their protocols and informed consent documents to state that MDV9300 is not an anti-PD-1 antibody.
In addition, we intend to submit an amendment to the Chemistry, Manufacturing and Controls, or CMC, section of our IND for MDV9300 to provide for larger manufacturing lot sizes to better support our planned clinical activities with MDV9300. We plan to submit the CMC amendment in the first half of 2016 and we intend that patients in our Phase 2 DLBCL trial will be treated with MDV9300 manufactured in accordance with the amended IND. This decision will also contribute to a later timeline for completion of the Phase 2 DLBCL trial.
We continue to deepen our understanding of the properties of MDV9300, which we believe may differentiate it from the other immuno-oncology agents in development. Based on clinical and preclinical data, including data from Dr. Benson presented at the American Society of Hematology, we plan to develop MDV9300 in other hematologic malignancies such as multiple myeloma.
RESEARCH AND DRUG DISCOVERY PROGRAMS
An important element of our corporate strategy is to diversify beyond XTANDI to build a portfolio of multiple approved products. To further this strategy, we are investing in a number of research and drug discovery programs, from the discovery stage through early clinical studies. In the discovery stage, we identify disease states of interest and therapeutic approaches that we believe may be safe and effective in treating a disease state. We conduct preclinical studies designed to identify one or more development candidates. A development candidate is a specific product candidate that embodies the therapeutic approach of interest and which we believe may possess the properties necessary to successfully complete the subsequent preclinical and clinical testing required to support an application for marketing approval. Once we identify a development candidate, we conduct any additional preclinical testing required to support an application for regulatory approval to begin human testing. Once such approval is granted, we may conduct Phase 1 trials where the development candidate is introduced into healthy human subjects or patients with the target indication and tested for safety, dosage tolerance, absorption, metabolism, distribution, and excretion, and, if possible, early evidence on effectiveness. Our current internal research and drug discovery programs are focused in oncology, neurology and other areas.
From our research and drug discovery efforts, we have developed a novel small molecule inhibitor of the sterol regulatory element-binding protein, or SREBP, pathway which we refer to as MDV4463. SREBPs are master regulators of cholesterol and lipid biosynthesis. SREBP modulates the expression of multiple metabolic proteins, including but not limited to PCSK9 and HMG-CoA reductase, the target of all statins. In some preclinical studies, we have demonstrated that MDV4463 lowers triglycerides, cholesterol, glucose, insulin and weight in animals. MDV4463 also reduced lipids in the liver of animal models of nonalcoholic steatohepatitis, or NASH. MDV4463 is currently in a Phase 1 trial in healthy volunteers.
Our research and drug discovery activities are conducted largely on an outsourced basis at facilities owned and operated by independent third party contractors, with oversight and monitoring by our employees. In 2015, we constructed certain limited internal research, development and pharmaceutical operations capabilities in laboratory space that is currently leased in San Francisco, California.
INTELLECTUAL PROPERTY
Our success is dependent in part on our ability to protect the products we develop, acquire, or license and we rely on patent protection as well as trade secrets.
We have an exclusive license to multiple issued patents and pending patent applications covering XTANDI, related compounds and uses thereof, including issued composition of matter patents covering XTANDI in the United States, Europe, and Japan. The terms of these issued XTANDI composition of matter patents have a base expiry in 2027 in the United States and in 2026 in Europe and Japan. Supplemental Protection Certificates, or SPCs, and patent term extensions are pending or have been granted in Europe and Japan. Certain of the issued U.S. patents are listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. U.S. federal law provides that, for a period of five years following approval of a drug containing a new molecular entity, no abbreviated new drug application for a generic version of such drug can be submitted unless the submission contains a Paragraph IV certification stating that the patents listed in the Orange Book are invalid or will not be infringed by the generic version of the drug, in which case the submission may be made four years following the original product approval. XTANDI was approved by the FDA on August 31, 2012 and was a new molecular entity at the time of its approval. Companies seeking approval to market a generic version of a reference drug may also seek to challenge the validity of the patents covering such reference drug by filing petitions for inter partes review with the U.S. Patent and Trademark Office. For more information regarding the review and approval process of generic drugs, see the section below titled “Government Regulation—Abbreviated New Drug Applications and Section 505(b)(2) New Drug Applications.”
10
We have an exclusive license to multiple issued patents and pending patent applications covering MDV9300, including the antibody and methods of use of MDV9300. In addition, we expect to rely on the 12 years of data exclusivity available for biologic products. For more information regarding the review and approval of biosimilars, see the section below titled “Government Regulation—Biologics Price Competition and Innovation Act.”
Our acquisition of worldwide rights to MDV3800 included issued patents, pending patent applications and know-how covering MDV3800’s composition of matter as well as methods of use and methods of manufacturing MDV3800. As we continue to develop MDV3800, MDV9300 and product candidates discovered from our own research and drug discovery programs, we will actively seek patent protection for such inventions. However, the actual protection afforded by a patent, which can vary from country to country, depends on the type of patent, the scope of its coverage and the availability of legal remedies in the country. Other pharmaceutical and biotechnology companies, universities and research institutions may have filed patent applications or may have been granted patents that cover technologies similar to the technologies owned or licensed to us. Because patent applications are confidential for a period of time, we may not know if we were the first to invent or the first to file an application covering an invention that is the subject of our patent applications.
We rely upon trade secret protection for our confidential and proprietary information. It is our policy to require our employees and consultants, outside collaborators, and other advisors who receive confidential information from us to execute confidentiality agreements upon the commencement of employment, consulting or other relationships with us. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
XTANDI® is a registered trademark of Astellas Pharma, Inc. and is referred to as XTANDI throughout this Annual Report.
COMPETITION
The pharmaceutical and biopharmaceutical industries are highly competitive and require an ongoing, extensive search for technological innovation. These industries are characterized by rapidly evolving technologies, intense competition and a strong emphasis on proprietary products. They also require, among other things, the ability to effectively discover, develop, and obtain regulatory approval for products, as well as the ability to effectively commercialize, market, and promote approved products. Once a product is approved, it must compete successfully against other commercial products primarily on efficacy, safety, tolerability, acceptance by physicians, ease of patient compliance, patent protection, ease of use, price, insurance, and other reimbursement coverage, distribution, and marketing. Many of our competitors are large pharmaceutical companies with considerably greater financial resources, human resources and experience than us.
XTANDI
XTANDI faces and will continue to face intense competition as we market it in its approved indications, develop it across the prostate cancer treatment paradigm and seek additional marketing approvals in new indications. Our competition will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential products or of competitors’ products may be an important competitive factor.
Many products currently compete or are expected to compete with XTANDI for the treatment of advanced prostate cancer. Products approved for advanced prostate cancer include Casodex® (bicalutamide), which is widely available in generic form and its generic pricing constitutes a competitive advantage relative to any branded drugs that are, or may later be, approved to treat pre-chemotherapy or non-metastatic CRPC or HSPC; Jevtana® (cabazitaxel); Provenge® (sipuleucel-T); Zytiga® (abiraterone acetate) plus prednisone; and Xofigo® (radium RA-223 dichloride). Drugs still in development for advanced prostate cancer include apalutamide (formerly JNJ-56021927, or JNJ-927, and ARN-509) by Johnson & Johnson’s subsidiary Aragon Pharmaceuticals, or Aragon; ODM-201, which is being developed by Bayer and Orion Corporation; and galeterone (formerly TOK-001) which is being developed by Tokai. For information about certain pending litigation between us and Aragon, among other parties, concerning apalutamide, see “Legal Proceedings” in Item 3 of Part I below.
11
Enzalutamide for the Treatment of Breast Cancer
There are many products available or currently in development for patients with breast cancer and which could compete with enzalutamide for the treatment of advanced breast cancer, including the following:
|
|
• |
Bicalutamide – Bicalutamide is approved for prostate cancer and is being studied in several Phase 2 studies in androgen receptor-positive breast cancer. Positive proof-of-concept with enzalutamide or other drugs targeting androgen receptor signaling may encourage spontaneous use of bicalutamide in breast cancer. |
|
|
• |
IbranceTM (palbociclib) – Pfizer’s kinase inhibitor targeting CDK4/6 is approved in combination with letrozole or with fulvestrant for estrogen receptor-positive, HER2-negative, advanced breast cancer. |
|
|
• |
Pembrolizumab – Merck’s monoclonal antibody targeting PD-1 is being studied in a randomized Phase 3 study comparing the effects of pembrolizumab against investigator’s choice chemotherapy in patients with metastatic triple-negative breast cancer who have received 1-2 prior therapies for metastatic disease. |
|
|
• |
Atezolizumab – Roche’s monoclonal antibody targeting PD-L1 is being studied in a randomized Phase 3 study comparing the effects of atezolizumab in combination with nab-paclitaxel against nab-paclitaxel in patients with previously untreated, metastatic, triple-negative breast cancer. |
|
|
• |
Enobosarm – GTx Therapeutics’ investigational androgen receptor modulator is being studied in a Phase 2 open-label trial to assess preliminary efficacy in advanced triple-negative breast cancer patients whose tumors are deemed positive for androgen receptor expression. |
MDV3800
MDV3800 faces competition from other PARP inhibitors, including:
|
|
• |
Lynparza™ (olaparib) – AstraZeneca’s product is approved for patients with deleterious or suspected deleterious gBRCA mutated advanced ovarian cancer. Olaparib is being studied in a Phase 3 trial comparing the effects of olaparib against investigator’s choice chemotherapy in metastatic, HER2-negative breast cancer patients with deleterious germline BRCA1/2 mutations, and in Phase 2 studies for several other tumor types. |
|
|
• |
Rucaparib – Rucaparib is an investigational PARP inhibitor being developed by Clovis Oncology. Rucaparib is currently in several clinical studies, including a randomized, double-blind pivotal Phase 3 study in woman with platinum-sensitive, relapsed, high grade serous or endometrioid ovarian fallopian tube, or primary peritoneal cancer comparing the effects of rucaparib against placebo to evaluate whether rucaparib given as a maintenance therapy to platinum-sensitive patients can extend the period of time for which the disease is controlled after a positive outcome with platinum-based chemotherapy. In April 2015, the FDA granted Breakthrough Therapy designation for rucaparib as monotherapy treatment of advanced ovarian cancer in patients who have received at least two lines of prior platinum-containing therapy, with BRCA-mutated tumors, inclusive of both germline BRCA and somatic BRCA. |
|
|
• |
Veliparib - Veliparib is an investigational PARP inhibitor being developed by AbbVie. It is currently in Phase 3 clinical trials for the treatment of HER2-negative, germline BRCA1/2-mutated breast cancer and non-small cell lung cancer, and Phase 2 studies for a variety of other cancers. |
|
|
• |
Niraparib – Niraparib is an investigational PARP inhibitor being developed by Tesaro. It is currently in Phase 3 clinical trials for ovarian cancer and BRCA+ breast cancer. |
MDV9300
The development and commercialization of MDV9300 will face strong competition from other immuno-oncology agents which are currently approved and on the market for certain indications and being developed for additional indications by larger companies with substantial resources and relatively more experience developing, manufacturing, and commercializing biologic molecules. The immuno-oncology field is competitively crowded with more than 100 drug therapy agents, including biologic molecules, vaccines, modified T-cells, and adjuvants, in development for various tumor types and patient populations by larger more experienced companies than ours, such as Bristol Myers Squibb, Roche, AstraZeneca, Pfizer and Merck. Specifically in DLBCL, MDV9300’s development and commercialization may face competition from Bristol Myers Squibb’s monoclonal antibody nivolumab (targeting PD-1), currently being studied in a potentially registrational Phase 2 study in relapsed/refractory DLBCL patients.
12
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, safety, storage, tracking, recordkeeping, approval, labeling, pricing, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. A new drug must be approved by the FDA through the new drug application, or NDA, process and a new biologic must be approved by the FDA through the biologics license application, or BLA, process before it can be legally marketed in the United States.
Depending on the circumstances, violations of federal and state healthcare laws, including those described below, can result in penalties, including but not limited to criminal, civil and/or administrative penalties, damages, fines, civil monetary penalties, disgorgement, individual imprisonment, the curtailment or restructuring of operations, and exclusion from participation in federal healthcare programs, among others.
FDA Approval Process
In the United States, the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act, or FDCA, and in the case of biologics, also under the Public Health Service Act, or PHSA, and implementing regulations. Failure to comply with applicable U.S. law and regulations at any time during the product development process, approval process, or after approval, may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil penalties, and criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us. The process required by the FDA before a drug or biologic may be marketed in the United States generally involves the following:
|
|
• |
completion of preclinical laboratory tests, animal studies and formulation studies according to current Good Laboratory Practices or other applicable regulations; |
|
|
• |
submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin; |
|
|
• |
performance of adequate and well-controlled human clinical trials according to current Good Clinical Practices, or cGCP, to establish the safety and efficacy of the proposed drug for its intended use; |
|
|
• |
submission to the FDA of an NDA or BLA after completion of all pivotal clinical trials; |
|
|
• |
satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the active pharmaceutical ingredient, or API, and finished drug product is manufactured and tested to assess compliance with current Good Manufacturing Practices, or cGMP; and |
|
|
• |
FDA review and approval of the NDA or BLA prior to any commercial marketing or sale of the drug in the United States. |
Before clinical testing can begin in humans, a sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor will also include a protocol, detailing, among other things, the objectives of the first phase of the clinical trials, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated, if applicable.
Clinical trials involve the administration of the investigational drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (a) in compliance with federal regulations; (b) in compliance with cGCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (c) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, or Ethics Committee, or EC, for approval. An IRB/EC may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB/EC’s requirements, or may impose other conditions.
Human clinical trials are typically conducted in three sequential phases, but the phases may overlap or be combined:
Phase 1: The product candidate is initially introduced into healthy human subjects or patients with the target indication and tested for safety, dosage tolerance, absorption, metabolism, distribution, and excretion, and, if possible, early evidence on effectiveness.
13
Phase 2: The product candidate is administered to a limited patient population to identify possible adverse side effects and safety risks, to preliminarily evaluate the efficacy of the product candidate for specific target indication and to determine dosage tolerance and optimal dosage.
Phase 3: When Phase 2 evaluations demonstrate that a dosage range of the drug is effective and has an acceptable safety profile, Phase 3 clinical trials are undertaken to confirm the clinical efficacy from Phase 2 and to further test for safety in an expanded population at geographically dispersed clinical trial sites.
In the United States, the results of product development, preclinical studies and clinical trials must be submitted to the FDA for review and approval prior to marketing and commercial distribution of the product candidate. The NDA (for a drug) or BLA (for a biologic) must include a substantial amount of data and other information concerning safety and effectiveness (for a drug) and safety, purity and potency (for a biologic) of the compound from laboratory, animal and clinical testing, as well as data and information on manufacturing, product stability, and proposed product labeling.
Once an NDA or BLA is submitted for FDA approval, the FDA will accept the NDA or BLA for filing if deemed complete, thereby triggering substantive review of the application. The FDA can refuse to file any NDA or BLA that it deems incomplete or not properly reviewable. The FDA has 60 days from its receipt of an NDA or BLA to determine whether the application will be accepted for filing. NDAs or BLAs that are accepted for review will receive either standard or priority review. Priority review can be applied to drugs that the FDA determines offer major advances in treatment, or provide a treatment in which no adequate therapy exists. For biologics, priority review is further limited only for drugs intended to treat a serious or life-threatening disease relative to the currently approved products. The FDA has established performance goals for the review of NDAs and BLAs: six months for priority applications and ten months for regular applications, with two additional months added to each period for new molecular entities. However, the FDA is not legally required to complete its review within these periods and these performance goals may change over time. Moreover, the outcome of the review, even if generally favorable, typically is not an actual approval but a “complete response” letter that describes additional work that must be done before the application can be approved. The FDA’s review of an application may involve review and recommendations by an independent FDA advisory committee. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
If the FDA approves a product, it will limit the approved therapeutic uses for the product as described in the product labeling, may require that contraindications or warning statements be included in the product labeling, may require that additional studies or clinical trials be conducted following approval as a condition of the approval, may impose restrictions and conditions on product distribution, prescribing or dispensing in the form of a risk evaluation and mitigation strategy, or REMS, or may otherwise limit the scope of any approval. Under REMS, the FDA may impose significant restrictions on distribution and use of a marketed product, may require the distribution of medication guides to patients and/or healthcare professionals or patient communication plans, and may impose a timetable for submission of assessments of the effectiveness of REMS. Once issued, the FDA may withdraw a product approval if compliance with regulatory standards is not maintained, including failure to conduct and submit post-marketing studies, or if problems are identified following initial marketing. The FDA may also impose REMS after product approval, or require labeling changes. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the agency has the power to prevent or limit further marketing of a product based on the results of the post-marketing programs.
The process of obtaining regulatory approvals and subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Satisfaction of the above FDA requirements or similar requirements of state, local, and foreign regulatory agencies typically takes several years or more and the actual time required may vary substantially, based on the type, complexity and novelty of the product candidate. Government regulation may delay or prevent marketing of potential products for a considerable period of time or permanently and impose costly procedures upon a company’s activities. Even if a product candidate receives regulatory approval, the approval may be significantly limited to specific indications. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product, labeling changes or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain and maintain, regulatory approvals would harm our business.
Marketing our product candidates abroad will require similar regulatory approvals and is subject to similar risks. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
14
Abbreviated New Drug Applications and Section 505(b)(2) New Drug Applications
Once an NDA is approved, the product covered thereby becomes a listed drug. The NDA, in turn, can be relied upon by potential competitors in support of approval of an abbreviated new drug application, or ANDA, or 505(b)(2) NDA. The FDA may approve an ANDA if the product is the same in important respects as the listed drug or if the FDA has declared it suitable for an ANDA submission. In these situations, applicants must submit studies showing that the product is bioequivalent to the listed drug, meaning that the rate and extent of absorption of the drug does not show a significant difference from the rate and extent of absorption of the listed drug. ANDA applicants are not required to conduct or submit results of preclinical or clinical tests to prove the safety or effectiveness of their drug product, other that the requirement for bioequivalence testing. Conducting bioequivalence testing is generally less time-consuming and costly than conducting preclinical and clinical trials necessary to support an NDA. Drugs approved via ANDAs on the basis that they are the “same” as a listed drug are commonly referred to as “generic equivalents” to the listed drug, and can often be and are substituted by pharmacists under prescriptions written for the original listed drug.
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent with claims that cover the applicant’s product or method of using the product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. An ANDA applicant relying on a listed drug is required to certify to the FDA concerning any patents for the listed drug product in the FDA’s Orange Book. Specifically, the applicant must certify with respect to each patent that: (1) the required patent information has not been filed; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (4) the listed patent is invalid, unenforceable, or will not be infringed by the new product.
A certification that the proposed generic product will not infringe the already approved product’s listed patents, or that such patents are invalid or unenforceable, is called a Paragraph IV certification. If the ANDA applicant does not challenge the listed patents or indicate that it is not seeking approval of a patented method of use, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA also will not be approved until any applicable non-patent exclusivity period, such as exclusivity for obtaining approval of a new chemical entity, for the referenced product has expired, unless the exclusivity period protects an indication or other aspect of labeling that can be “carved out” of the labeling for the proposed generic product. Federal law provides a period of five years following approval of a drug containing no previously approved active moiety during which ANDAs for generic versions of those drugs cannot be submitted unless the submission contains a Paragraph IV challenge to a listed patent, in which case the submission may be made four years following the original product approval. U.S. federal law provides for a period of three years of exclusivity during which the FDA cannot grant effective approval of an ANDA if a listed drug contains a previously approved active moiety but FDA requires as a condition of approval new clinical trials conducted by or for the sponsor. This three-year exclusivity period often protects changes to a previously approved product, such as a new dosage form, route of administration, combination, or indication.
Most drug products obtain FDA marketing approval pursuant to an NDA or an ANDA. A third alternative is a special type of NDA, commonly referred to as a Section 505(b)(2) NDA, which enables the applicant to rely, in part, on the FDA’s previous approval of a similar product, or published literature, in support of its application. Section 505(b)(2) NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. If the Section 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the labeled indications for which the referenced product has been approved, as well as for any new indication(s) sought by the Section 505(b)(2) applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would be required to do so. As a result, approval of a Section 505(b)(2) NDA can be prevented until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
15
Biologics Price Competition and Innovation Act
Under the Biologics Price Competition and Innovation Act, or BPCIA, enacted in the United States in 2010, the FDA has the authority to approve biosimilar and interchangeable versions of previously-approved biological products through an abbreviated pathway following periods of data and marketing exclusivity. Biosimilarity sufficient to reference a prior FDA-approved product requires that there be no differences in condition of use, route of administration, dosage form, and strength, and no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency. In order to meet the standards of interchangeability, a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical results as the reference product, and for a product that is administered more than once, that the risk of switching between the reference product and the biosimilar product is not greater than the risk of maintaining the patient on the reference product. Such biosimilars would reference biological products approved in the United States. The law establishes a period of 12 years of data exclusivity for referenced products, which protects the data in the original BLA by prohibiting sponsors of biosimilars from gaining FDA approval based in part on reference to data in the original BLA. Should we obtain product approvals under BLAs, those products could be reference products for such abbreviated BLAs.
Post-Approval Requirements
After a drug or biologic has been approved by the FDA for marketing, the FDA may require that certain post-approval requirements be satisfied, including the conduct of additional clinical studies or post-marketing testing, known as Phase 4 testing, REMS, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product and requires routine pharmacovigilance (post-marketing adverse event monitoring). We and Astellas are required to conduct an open-label safety study of XTANDI in patients with known risk factor(s) for seizure and to report the results of that study to the FDA in 2019. In addition, holders of an approved NDA or BLA are required to (i) report certain adverse reactions to the FDA and maintain pharmacovigilance programs to proactively look for these adverse events; (ii) comply with certain requirements concerning advertising and promotional labeling for their products; and (iii) continue to have quality control and manufacturing procedures conform to cGMPs after approval. If post-approval conditions are not satisfied, the FDA may withdraw its approval of the drug or biologic.
The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing facilities, including assessment of ongoing compliance with cGMPs. Accordingly, manufacturers must continue to expend considerable time, money and effort in the areas of production and quality control to maintain compliance with cGMP. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered. In addition, prescription drug manufacturers in the United States must comply with applicable provisions of the Drug Supply Chain Security Act and provide and receive product tracing information, maintain appropriate licenses, ensure they only work with properly licensed entities, and have procedures in place to identify and properly handle suspect and illegitimate products.
In the event of certain changes to an approved product, such as adding new indications, making certain manufacturing changes, or making certain additional labeling claims, such changes are subject to further FDA review and approval. Approval for additional indications typically requires additional clinical studies to be conducted and submitted through either a new or supplemental NDA.
Expedited Review and Approval
The FDA has various programs, including Fast Track, Breakthrough Therapy Designation, priority review, and accelerated approval, which are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval on the basis of surrogate endpoints. Even if a drug qualifies for one or more of these programs, the FDA may later decide that the drug no longer meets the conditions for qualification or that the time period for FDA review or approval will not be shortened. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious diseases and fill an unmet medical need. The request may be made at the time of IND submission and generally no later than the pre-BLA or pre-NDA meeting. The FDA will respond within 60 calendar days of receipt of the request. Breakthrough Therapy Designation is available for drugs that are intended to treat a serious condition where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies. The request may be made at the time of IND submission and generally no later than end-of-phase 2 meeting. The FDA will respond within 60 calendar days of receipt of the request. Breakthrough Therapy Designation conveys all of the Fast Track program features along with more intensive FDA guidance and interaction and eligibility for rolling review and priority review. Priority review, which is requested at the time of BLA or NDA submission, is designed to give drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months as compared to a standard review time of ten months. Although Fast Track, Breakthrough Therapy Designation and priority review do not affect the standards for approval, the FDA will attempt to expedite review of the application. Accelerated approval provides an earlier approval of drugs to treat serious diseases, and that fill an unmet medical need based on a surrogate endpoint, which is a laboratory measurement or physical sign used as an indirect or substitute measurement representing a clinically meaningful outcome.
16
Discussions with the FDA about the feasibility of an accelerated approval typically begin early in the development of the drug in order to identify, among other things, an appropriate endpoint. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials to confirm the appropriateness of the surrogate marker trial.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use will be disclosed publicly by the FDA; the posting will also indicate whether a drug is no longer designated as an orphan drug. More than one product candidate may receive an orphan drug designation for the same indication. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to seven years of orphan product exclusivity. During the seven-year exclusivity period, the FDA may not approve any other applications to market a drug or biological product containing the same active moiety for the same disease, except in very limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. A product is clinically superior if it is safer, more effective or makes a major contribution to patient care. Thus, orphan drug exclusivity could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA and we are not able to show the clinical superiority of our drug or if our product candidate’s indication is determined to be contained within the competitor’s product orphan indication. In addition, the FDA will not recognize orphan drug exclusivity if a sponsor fails to demonstrate upon approval that the drug is clinically superior to a previously approved drug containing the same active moiety for the same orphan condition, regardless of whether or not the approved drug was designated an orphan drug or had orphan drug exclusivity.
Data Privacy and Security Regulations
We are also subject to laws and regulations regarding the privacy and security of health-related and other personal information. The Health Insurance Portability and Accountability Act, or HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, imposes certain federal requirements relating to the privacy, security and transmission of individually identifiable health information. We are also subject to other state and federal privacy and security laws that are not specific to the healthcare industry, such as the Federal Trade Commission’s enforcement of Section 5 of the Federal Trade Commission Act and state security breach notification laws. The EU Data Protection Directive, as implemented into national laws by the EU member states, imposes strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting. Failing to comply with these laws could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
Advertising and Promotion
Any products distributed by us pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including advertising and promotion restrictions. The FDA’s rules for advertising and promotion require, among other things, that we do not promote our products for unapproved uses and that our promotion be fairly balanced and adequately substantiated by clinical studies. The FDA closely regulates the post-approval marketing and promotion of drugs, including but not limited to, standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. The FDA also enforces the requirements of the Prescription Drug Marketing Act, or PDMA, which, among other things, imposes various requirements in connection with the distribution of product samples to physicians.
Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new or supplemental NDA or BLA before the change can be implemented. An NDA or BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing supplements as it does in reviewing NDAs and BLAs.
17
Anti-Kickback Statutes, Sunshine Act, and Federal False Claims Act
Our business activities, including, but not limited to, research, sales, marketing, promotion, distribution, medical education and other activities are subject to regulation by numerous regulatory and enforcement authorities in the United States and abroad. Government officials have focused their enforcement efforts on marketing of health care services and products, among other activities, and recently have brought cases against companies, and certain sales, marketing, and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
The Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal health care program such as Medicare or Medicaid. The definition of “remuneration” has been broadly interpreted to include anything of value, including for example gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payment of cash and waivers of payment. Liability under the Anti-Kickback Statute may be established without a person or entity having actual knowledge of the statute or specific intent to violate it. Penalties include criminal penalties and civil sanctions such fines, imprisonment, and possible exclusion from Medicare, Medicaid and other federal health care programs.
Many states also have statutes or regulations similar to the Anti-Kickback Statute. Some of these state provisions apply to referral of patients for health care items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Another development affecting the health care industry is the increased use of the Federal False Claims Act, or FFCA, and, in particular, action brought pursuant to the FFCA’s “whistleblower” or “qui tam” provisions. The FFCA imposes liability on any person or entity who, among other things, knowingly presents or causes to be presented a false or fraudulent claim for payment by the federal government, including a federal health care program, such as Medicare and Medicaid. The qui tam provisions of the FFCA allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share a proportion of any monetary recovery. In recent years, the number of suits brought against health care providers and entities by private individuals has increased dramatically. In addition, various states now have enacted false claims laws analogous to the FFCA, although many of these state laws apply where a claim is submitted to any third-party payor, including private insurance companies, and not merely a federal health care program.
When a person or entity is determined to have violated the FFCA, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties. Liability arises primarily when an entity knowingly submits or causes another to submit a false claim for reimbursement to the federal government. The federal government has used the FFCA to assert liability on, among other things, the basis of inadequate care, kickbacks, and other improper referrals, and the improper use of Medicare numbers when detailing the provider of services, in addition to the more predictable allegations as to misrepresentations with respect to the services rendered. In addition, the federal government has prosecuted companies under the FFCA in connection with off-label promotion of products. We are unable to predict whether we will be subject to actions under the FFCA or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly impact our financial performance.
Additionally, the civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
HIPAA created new federal criminal statutes that prohibit, among other actions, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, liability may be established without a person or entity having actual knowledge of the statute or specific intent to violate it.
We and our business activities are also subject to the federal Physician Payments Sunshine Act which requires certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report information related to certain payments or other transfers of value provided to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. The reported information is made publicly available by the Centers for Medicare and Medicaid Services on a searchable website. We are current on all required submissions of information related to the federal Physician Payments Sunshine Act.
18
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, including state laws that are similar to the aforementioned federal laws, we may be subject to penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the imposition of a Corporate Integrity Agreement or similar government oversight program or curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act of 1977, as amended, or FCPA, prohibits U.S. companies and their representatives from offering, processing, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad. In many countries, the health care professionals we regularly interact with may meet the definition of a foreign government official for the purposes of the FCPA. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect their transactions and to devise and maintain an adequate system of internal accounting controls. Our international activities create the risk of unauthorized payments or offers of payments by our employees, consultants and agents, even though they may not always be subject to our control. We discourage these practices by our employees, consultants, and agents. However, our existing safeguards may prove to be less than effective, and our employees, consultants and agents may engage in conduct for which we might be held responsible.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of XTANDI and any future products of ours are dependent in significant part on the availability of coverage and adequate reimbursement from third-party payors, including federal and state programs such as Medicare and Medicaid, and private organizations such as commercial health insurance and managed care companies. Astellas is responsible for negotiating favorable coverage determinations and adequate reimbursement rates with third-party payors for XTANDI.
Although there are currently no direct government price controls over private sector purchases in the United States, federal legislation requires pharmaceutical manufacturers to pay prescribed rebates on certain drugs to enable them to be eligible for reimbursement under certain public healthcare programs such as Medicaid. Various states have adopted further mechanisms under Medicaid and other programs that seek to control drug prices, including by disfavoring certain higher priced drugs and by seeking supplemental rebates from manufacturers. Managed care has also increased downward pressure on the prices of pharmaceutical products.
Third-party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit. Third-party payors may provide coverage but place stringent limitations on such coverage, such as requiring alternative treatments to be tried first. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substituting generic equivalents for branded prescription drugs. Adoption of such controls and measures and tightening of existing policies could limit payments for our products and could adversely affect our business.
Different pricing and reimbursement schemes exist in other countries. For example, in the European Union, some governments influence the price of pharmaceutical products through reference pricing approaches to pharmaceutical reimbursement for national healthcare systems that fund a large part of the cost of such products to consumers. The approach taken varies from member state to member state. Some jurisdictions operate positive and/or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits and may limit or restrict reimbursement. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products, as exemplified by the National Institute for Health and Care Excellence, or NICE, in the United Kingdom which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries cross-border imports from low-priced markets (parallel imports) exert commercial pressure on pricing within a country.
19
In addition to regulation in the United States, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of products. Whether or not we obtain FDA approval for a drug candidate, we must obtain approval by the comparable regulatory authorities of foreign countries and economic areas, such as the European Union, before we can commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Other Applicable Laws
We are also subject to various other federal, state and local laws, including laws regarding working conditions and the storage, transportation, or discharge of items that may be considered hazardous substances, hazardous waste, or environmental contaminants. We monitor our compliance with laws on an ongoing basis.
As a publicly-traded company, we are also subject to significant regulations and laws, including the Sarbanes-Oxley Act of 2002. Since its enactment, we have developed and instituted a corporate compliance program based on what we believe are current best practices and we continue to update the program in response to newly implemented or changing regulatory requirements.
MANUFACTURING
Pursuant to the Astellas Collaboration Agreement, Astellas has global manufacturing responsibility for XTANDI as currently formulated. Astellas intends to fulfill clinical and commercial requirements of XTANDI largely through third-party contract manufacturers.
We are a party to agreements with contract manufacturers for clinical supply of drug substances and drug products for our MDV3800 and MDV9300 programs. These agreements are managed by professionals within our organization who have expertise in pharmaceutical and biologic manufacturing, product development, regulatory compliance, quality assurance, and supply chain management. We may further expand our capabilities.
We currently do not have specific plans to imminently develop our own manufacturing operations.
OUR FINANCIAL HISTORY
We had net income of $244.7 million for the year ended December 31, 2015. Our 2015 results benefited from $245.0 million of milestones earned from Astellas as a result of the achievement of defined sales milestone events. As of December 31, 2015, we have earned all development and sales milestone payments under the Astellas Collaboration Agreement. In 2014, we earned sales and development milestones in excess of $300.0 million under the agreement. We have a history of net losses since our inception through 2013, and we have only now achieved two sequential years of profitability.
A significant portion of our operating expense is related to research and development, and we intend to maintain our strong commitment to research and development. Our research and development activities currently include activities related to enzalutamide, MDV3800, MDV9300 and early stage programs. For the years ended December 31, 2015, 2014, and 2013, we recorded $232.1 million, $189.6 million, and $119.0 million, respectively, of research and development expenses representing 44%, 44%, and 40% of total operating expenses for the years ended December 31, 2015, 2014, and 2013, respectively.
We anticipate that we will incur higher operating costs in the foreseeable future in order to continue to commercialize XTANDI in the U.S. market, to conduct clinical and preclinical studies of enzalutamide, MDV3800, MDV9300 and early-stage programs, to carry out potential business development activities, and to provide expanded facilities to support our growth. We have funded our operations in prior years primarily through public offerings of our common stock, the issuance of 2.625% convertible senior notes due April 1, 2017, or the Convertible Notes (which we redeemed in July 2015 and are no longer outstanding). More recently, we funded our cash requirements from collaboration revenue associated with the global growth of XTANDI and, to a lesser extent, from short-term borrowings under our Revolving Credit Facility. The portion of collaboration revenue derived from sales and development milestones earned from Astellas exceeded $550.0 million in the two calendar years just completed.
EMPLOYEES
At December 31, 2015, we had 628 full-time employees, none of whom are represented by labor unions or covered by collective bargaining agreements. We consider our relationships with our employees to be good.
20
We are a corporation formed in Delaware in October 1995, under our former name, Orion Acquisition Corp. II. We, together with our subsidiaries, operate in one business segment. All of our revenue is currently derived from our collaboration agreement with Astellas. Our long-lived assets are primarily located in the United States, except for certain de minimus laboratory equipment. Information regarding our collaboration agreement revenue, profit or loss, and total assets is included in our audited consolidated financial statements in Part II, Item 8 of this Annual Report.
AVAILABLE INFORMATION
We file or furnish electronically with the Securities and Exchange Commission, or SEC, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. The SEC maintains a website that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov. You may also read and copy any of our materials filed with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information regarding the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330.
We make available on or through our website at www.medivation.com, free of charge, copies of these reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information found on, or that can be accessed through, our website is not incorporated by reference into this Annual Report.
Our business faces significant risks, some of which are set forth below to enable readers to assess, and be appropriately apprised of, many of the risks and uncertainties applicable to the forward-looking statements made in this Annual Report. You should carefully consider these risk factors as each of these risks could adversely affect our business, operating results, cash flows and financial condition. If any of the events or circumstances described in the following risk factors actually occurs, our business may suffer, the trading price of our common stock could decline and our financial condition or results of operations could be harmed. Given these risks and uncertainties, you are cautioned not to place undue reliance on forward-looking statements. These risks should be read in conjunction with the other information set forth in this Annual Report. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not currently known to us, or that we currently believe to be immaterial, may also adversely affect our business.
Risks Related to XTANDI® (enzalutamide) capsules
We and Astellas may not be able to further commercialize XTANDI in the United States, and may fail to continue to generate significant revenue from the sale of XTANDI in the United States. XTANDI may fail to obtain regulatory approval and reimbursement, to be successfully commercialized and to generate significant revenue outside the United States.
We only have one commercial product, XTANDI® (enzalutamide) capsules, or XTANDI, which is approved in the United States to treat men with metastatic castration-resistant prostate cancer, or mCRPC. However, the further commercialization of XTANDI in the United States for the treatment of mCRPC and any other patient populations for which XTANDI is being developed and may subsequently be approved, may not be successful for a number of reasons, including:
|
|
• |
we and our collaboration partner, Astellas Pharma, Inc., or Astellas, may not be able to establish or demonstrate in the medical community the safety and efficacy of XTANDI and its potential advantages over, and side effects compared to, competing therapeutics and products currently in clinical development for each applicable patient population; |
|
|
• |
our limited experience in marketing XTANDI to urologists; |
|
|
• |
reimbursement and coverage policies of government and private payors such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators; |
|
|
• |
the price of XTANDI as compared to alternative treatment options; |
|
|
• |
changes or increases in regulatory restrictions; |
|
|
• |
changes to the label for XTANDI that further restrict how we and Astellas market XTANDI, including as a result of routine pharmacovigilance activities and/or data collected from the safety study in patients with known risk factor(s) for seizure that the FDA required us to undertake as a post-marketing requirement or from any other ongoing or future studies; |
|
|
• |
we and Astellas may not have adequate financial or other resources to successfully commercialize XTANDI; and |
|
|
• |
we and Astellas may not be able to obtain adequate commercial supplies of XTANDI to meet demand or at an acceptable cost. |
21
If the further commercialization of XTANDI in the United States is unsuccessful, our ability to generate revenue from product sales and maintain profitability would be adversely affected and our business could be severely negatively impacted.
XTANDI has received marketing approval in the European Union (or Europe, or EU) for the treatment of patients with mCRPC and in Japan for the treatment of patients with castration-resistant prostate cancer, and numerous other countries worldwide for the treatment of patients with mCRPC. Additional marketing applications for the mCRPC indication are under review in numerous other countries. Unless we and Astellas can obtain additional regulatory approval and reimbursement of XTANDI outside the United States, Japan and the EU, Astellas’ ability to successfully commercialize XTANDI further and our ability to generate additional revenue from XTANDI worldwide, could be significantly limited.
XTANDI may fail to effectively compete commercially with other approved products and other products in development.
There are a number of currently marketed therapies for advanced prostate cancer that compete directly with XTANDI. In addition, several companies are currently developing products or are expected to be marketing products in the near future that compete or may compete directly with XTANDI in its currently approved indication for mCRPC and any other indication for which enzalutamide may be subsequently approved. Our current and future competitors are generally large pharmaceutical companies with considerably greater financial, human, and development and commercialization resources and experiences than ours, including Johnson & Johnson, Sanofi, and Bayer Pharma AG. Some competitive drugs already have acquired substantial shares in these markets or are generic which may make it more difficult for us to compete successfully in these markets notwithstanding any positive results that we may generate from our current or potential future clinical trials for enzalutamide. Also, intense competition from products and compounds in development could impact our ability to successfully conduct upstream clinical trials, as trials may become more difficult to enroll, or complete successfully, as patients may have more treatment options with demonstrated efficacy and safety. Factors upon which XTANDI would have to compete successfully include efficacy, safety, price and cost effectiveness. We cannot guarantee that we and Astellas will be able to compete successfully in the context of any of these factors.
Pricing pressure from third parties and price competition could substantially impact Astellas’ or our ability to generate revenue from XTANDI and, therefore, negatively impact our business.
The realized price of XTANDI could be subject to pricing pressure from aggressive competitive pricing activity and managed care organizations and institutional purchasers, who use cost considerations to restrict the sale of preferred drugs that their physicians may prescribe. To the extent that payors believe similar lower-priced, branded or generic competitor products may be suitable alternatives for patients, we and our partner Astellas may consider reducing the price of XTANDI or be subject to formulary restrictions, which could result in a loss of sales revenue and/or market share. Additionally, XTANDI currently competes against products and could compete in the future with products marketed by some of the world’s largest and most experienced pharmaceutical companies, such as Johnson & Johnson, who have more resources and greater flexibility to engage in aggressive price competition in order to gain revenues and market share. It is uncertain whether we and Astellas could successfully compete with such competition and our failure to compete or a decision to reduce the price of XTANDI in order to compete could severely impact our business.
Moreover, non-profit groups have recently petitioned the U.S. federal government to exercise their “march-in” rights under the Bayh-Dole Act due to the difference in price of XTANDI in the U.S. versus abroad since certain patents which cover XTANDI were allegedly developed with federal funding. While the government has yet to exercise its march-in rights in response to pricing pressures or shortage problems, it may do so in the future which would significantly negatively impact our ability to generate revenue from XTANDI.
Competition from other approved products, including those that mechanistically operate similarly to XTANDI, could negatively impact the expected use or duration of therapy of XTANDI, and impact our ability to generate revenue.
We are competing and will continue to compete against drugs that operate similarly to XTANDI. If XTANDI is unable to successfully compete for a position in the prostate cancer treatment paradigm ahead of drugs like Zytiga and/or potentially apalutamide (formerly JNJ-56021927, or JNJ-927, and ARN-509), which are now being investigated in Phase 3 clinical studies in earlier stage prostate cancer, sales of XTANDI may be negatively impacted. In addition, the availability of multiple other approved agents to treat the same patients being treated with XTANDI could cause treating physicians to switch patients off of XTANDI and onto competing therapies more quickly than would otherwise be the case, which could also negatively impact XTANDI net sales.
Competition from generic products could potentially harm our business and result in a decrease in our revenue.
Like other branded pharmaceutical companies, we face competition from generic products that could potentially harm our business and result in a decrease in our revenue. Such competition may arise from the loss or expiration of intellectual property rights on enzalutamide or a competitor product, or the approval by the FDA of a generic of our product or a competitor product, such as Zytiga, any of which could adversely affect our business by putting downward pressure on the price and market share of XTANDI. Generic products for the treatment of prostate cancer that have already been approved by the FDA, e.g., Casodex, are generally sold at a lower price than branded drugs. Furthermore, Abbreviated New Drug Applications (ANDAs) requesting approval
22
for generic versions of Zytiga were submitted to the FDA on April 28, 2015. See the risk factor below entitled, “Risks Related to the Pharmaceutical Industry, Including the Activities of Medivation, Inc.— If the FDA or other applicable regulatory authorities approve generic products that compete with any of our products or product candidates, the sales of our products or product candidates may be adversely affected,” for additional information regarding general risks related to generic and biosimilar competition in our industry.
We have recently increased our promotional efforts and focus in the United States to include urologists as well as oncologists. If we are not successful in marketing XTANDI to these physician audiences, the commercial potential of XTANDI may not be realized.
We have recently increased promotional efforts in the United States to include urologists as well as oncologists as a result of the expanded label of XTANDI in the United States to treat mCRPC patients who have not yet received chemotherapy (September 2014). Our efforts include expanding and dividing our sales force into urology- and oncology-focused teams. Failure to successfully commercialize XTANDI to urologists and oncologists would have a negative impact on our business and future prospects.
We are dependent upon our collaborative relationship with Astellas to further develop, fund, manufacture and commercialize XTANDI, and if such relationship is unsuccessful, or if Astellas terminates our collaboration agreement with them, it could negatively impact our ability to conduct our business and generate revenue from XTANDI.
Under our collaboration agreement with Astellas, Astellas is responsible for developing, seeking regulatory approval for, and commercializing XTANDI outside the United States and is responsible globally for all manufacture of product for both clinical and commercial purposes. We and Astellas are jointly responsible for commercializing XTANDI in the United States. We and Astellas share equally the costs (subject to certain exceptions), profits and losses arising from development and commercialization of XTANDI in the United States. For clinical trials useful both in the United States and in Europe or Japan, we are responsible for one-third of the total costs and Astellas is responsible for the remaining two-thirds. We are subject to a number of risks associated with our dependence on our collaborative relationship with Astellas, including:
|
|
• |
Astellas’ right to terminate the collaboration agreement with us on limited notice for convenience (subject to certain limitations), or for other reasons specified in the collaboration agreement; |
|
|
• |
the need for us to identify and secure on commercially reasonable terms the services of third parties to perform key activities currently performed by Astellas in the event that Astellas were to terminate its collaboration with us, including development and commercialization activities outside of the United States and manufacturing activities globally; |
|
|
• |
adverse decisions by Astellas regarding the amount and timing of resource expenditures for the commercialization of XTANDI; |
|
|
• |
adverse decisions by Astellas regarding whether or not to initiate claims or litigation against third parties for infringement of proprietary rights protecting XTANDI or to determine the scope and validity of proprietary rights covering XTANDI or proprietary rights held by competitors; |
|
|
• |
failure by Astellas to negotiate favorable coverage determinations and adequate reimbursement rates with third-party payors; |
|
|
• |
decisions by Astellas to prioritize other of its present or future products more highly than XTANDI for either development and/or commercial purposes; |
|
|
• |
possible disagreements with Astellas as to the timing, nature and extent of our development plans, including clinical trials or regulatory approval strategy, which if we disagree could significantly delay or halt development of XTANDI; and |
|
|
• |
the financial returns to us, if any, under our collaboration agreement with Astellas, depend in large part on the generation of product sales, and if Astellas fails to perform or satisfy its obligations to us, the development or commercialization of XTANDI would be delayed or may not occur and our business and prospects could be materially and adversely affected. |
Due to these factors and other possible disagreements with Astellas, we may be delayed or prevented from further developing, manufacturing or commercializing XTANDI or we may become involved in litigation or arbitration, which would be time consuming and expensive. For example, we understand that Astellas has not made a final decision on the phase 3 clinical trial evaluating enzalutamide in triple negative breast cancer.
If Astellas were to terminate our collaborative relationship unilaterally, we would need to undertake development and commercialization activities for XTANDI solely at our own expense and/or seek one or more other partners for some or all of these activities, worldwide. If we pursued these activities on our own, it would significantly increase our capital and infrastructure requirements, might limit the indications we are able to pursue for XTANDI, and could prevent us from effectively commercializing XTANDI. If we sought to find one or more other pharmaceutical company partners for some or all of these activities, we may not be successful in such efforts, or they may result in collaborations that have us expending greater funds and efforts than our current relationship with Astellas.
23
We are dependent on third-party manufacturers for commercial supply of XTANDI and for clinical trial materials and if we fail to receive such adequate supplies, global sales of XTANDI could be limited and clinical trials could be delayed.
We require adequate supplies of enzalutamide for commercial supply of XTANDI, and for use in clinical trials. Under our collaboration agreement, Astellas has the responsibility to manufacture commercial supplies of XTANDI for all markets and provide material for clinical studies. Astellas fulfills its manufacturing and supply obligations largely through third-party contract manufacturers. Consequently, we are, and expect to remain, dependent on Astellas and its contract manufacturers for commercial and clinical trial materials. If Astellas cannot provide the materials on a timely basis due to, for example, raw materials availability, quality issues or failure of the contracting facilities to perform, it could result in decreased sales or put at risk ongoing clinical studies. If Astellas or its contract manufacturers do not perform, we may be forced to incur additional expenses, delays, or both, to arrange or take responsibility for contract manufacturers to manufacture or package XTANDI or enzalutamide on our behalf, as we do not have any internal manufacturing or packaging capabilities.
We also rely on our own third-party vendors for clinical supplies. If clinical supplies cannot be provided on a timely basis it could put at risk our sponsored clinical studies with XTANDI or enzalutamide.
In some instances, we and Astellas are dependent on third-party suppliers of raw materials, intermediates or finished goods of commercial supplies of XTANDI and clinical trial materials. If any of these suppliers or subcontractors fails to meet our or Astellas’ needs, we may not have readily available alternatives. If we or Astellas experience a material supplier or subcontractor inability to supply, our ability to satisfactorily and timely complete our clinical trial or delivery obligations could be negatively impacted which could result in reduced sales, termination of contracts and damages to our relationships with clinical trial sites and the medical community. We could also incur additional costs to address and resolve such an issue. Any of these events could have a negative impact on our results of operations and financial condition.
We are dependent on Astellas to distribute and sell XTANDI, and if Astellas fails to adequately perform these activities, our business would be negatively impacted.
Under our collaboration agreement with Astellas, we and Astellas have the right to jointly promote XTANDI to customers in the United States. However, Astellas has the sole right to distribute and sell XTANDI to customers in the United States and the sole right to promote, distribute and sell XTANDI to customers outside the United States. We are thus partially dependent on Astellas to successfully promote XTANDI in the United States, and solely dependent on Astellas to successfully distribute and sell XTANDI in the United States and to promote, distribute and sell XTANDI outside of the United States. In the United States, we depend on customer support from specialty pharmaceutical distributors and wholesalers in Astellas’ network. Astellas has contracted with a limited number of specialty pharmaceutical distributors and wholesalers to deliver XTANDI to end users. The use of specialty pharmacies and wholesalers requires significant coordination with Astellas’ sales and marketing, medical affairs, regulatory affairs, legal and finance organizations and involves risks, including but not limited to risks that these specialty pharmacies and wholesalers will:
|
|
• |
not provide Astellas accurate or timely information regarding their inventories, patient- or account-level data or safety complaints regarding XTANDI; |
|
|
• |
not effectively sell or support XTANDI; |
|
|
• |
not devote the resources necessary to sell XTANDI in the volumes and within the timeframes that we expect; or |
|
|
• |
cease operations. |
We generally do not have control over the resource or degree of effort that any of the specialty pharmacies and distributors may devote to XTANDI, and if their performance is substandard, this will adversely affect sales of XTANDI. If Astellas’ network of specialty pharmacies and distributors fails to adequately perform, it could negatively impact sales of XTANDI, which would negatively impact our business, results of our operations, cash flows and liquidity.
XTANDI may not be commercially successful if not widely covered and appropriately reimbursed by third-party payors, and we are dependent upon Astellas for the execution of third-party payor access and reimbursement strategies for XTANDI.
Our ability to successfully commercialize XTANDI for its approved indications depends, in part, on the extent to which coverage and adequate reimbursement for XTANDI is available from government and health administration authorities, private health insurers, managed care programs and other third-party payors, both in the United States and globally.
24
In addition, even if third-party payors ultimately elect to cover and reimburse XTANDI, most payors will not reimburse 100% of the cost, but rather require patients to pay a portion of the cost through a co-payment. Thus, even if reimbursement is available, the percentage of drug cost required to be borne by patients may make use of XTANDI financially difficult or impossible for certain patients, which would have a negative impact on sales of XTANDI. For example, in the United States there exists a coverage gap, or “donut hole”, in the Medicare Part D coverage for prescription medications for participants, which renews annually each January 1st. While in the donut hole, Medicare Part D participants, including many patients in XTANDI’s approved indication, may have to pay out-of-pocket a substantial portion of their prescription drug costs, which may discourage physicians from prescribing or patients from accessing XTANDI. It is increasingly difficult to obtain coverage and adequate reimbursement levels from third-party payors, and we may be unable to achieve these objectives. Moreover, our commercial prospects would be further weakened if payors approve coverage for XTANDI only as second- or later-line treatments, or if they place XTANDI in tiers requiring unacceptably high patient co-payments. Since launch, several third-party payors have included in their coverage criteria use of XTANDI after patient treatment on Zytiga plus prednisone or if there is a contra-indication to prednisone or Zytiga. These coverage situations may persist even with expanded indications for XTANDI. Because XTANDI works via a similar molecular signaling pathway as Zytiga does, patients who have already failed treatment with Zytiga may not have as strong a response on XTANDI as would patients who are Zytiga-naïve. Failure to overturn coverage decisions or stop additional coverage decisions could materially harm our (or our partner’s) ability to successfully market XTANDI in the United States. Achieving coverage and acceptable reimbursement levels typically involves negotiating with individual payors and is a time-consuming and costly process. Therefore, obtaining acceptable coverage and reimbursement from one payor does not guarantee similar acceptable coverage or reimbursement from another payor. Additionally, even if favorable coverage and adequate reimbursement levels are achieved, the payor may change its decision in the future. We are dependent upon Astellas globally for the achievement of such coverage and acceptable reimbursement, and for negotiation with individual payors.
We and Astellas are required to undertake certain studies to comply with post-marketing requirements or commitments in the EU and the United States, which could result in adverse modifications to XTANDI’s existing labeling, and risk XTANDI’s ability to obtain additional regulatory approvals for additional patient populations.
In the EU, we and Astellas are required to collect efficacy data on mCRPC patients previously treated with Zytiga to determine XTANDI’s efficacy response in such patients, which we do not expect to be as good as in patients naïve to Zytiga, and anticipate that these results may be available as early as 2016. In the United States, we and Astellas are required to conduct an open-label safety study of XTANDI in patients with known risk factor(s) for seizure and to report the results of that study to the FDA in 2019. If the results of this study reveal unacceptable safety risks, this could result in decreased commercial utilization of XTANDI for mCRPC patients in the United States and in the EU, failure to obtain approval in other indications, and modifications to the existing label for XTANDI, including potentially a boxed warning, or additional clinical testing. Any one or more of these outcomes would seriously harm our business. Additionally, we could receive additional post-marketing requirements as we seek approval of XTANDI in additional patient populations. Failure to conduct the post-marketing requirements or commitments in a timely manner may result in withdrawal of approval for XTANDI and substantial civil and/or criminal penalties.
If significant patient safety issues were to arise for XTANDI or our other product candidates, our future sales may be reduced, adversely affecting our results of operations.
The data supporting the marketing approvals for our products and forming the basis for the safety warnings in our product labels were obtained in controlled clinical trials of limited duration and, in some cases, from post-approval use. As our products are used over longer periods of time by many patients with underlying health problems, taking numerous other medicines, we expect to continue to find new issues such as safety, resistance or drug interactions of XTANDI or in other products, which may require us to provide additional warnings or contraindications on our labels or narrow our approved indications, each of which could reduce the market acceptance of these products. For example, in 2015, based upon routine pharmacovigilance review and signal detection, we and Astellas submitted a label change to the FDA after identifying two post-marketing reports of posterior reversible encephalopathy syndrome, or PRES, in patients receiving XTANDI. PRES is a neurological disorder which can present with rapidly evolving symptoms including seizure, headache, lethargy, confusion, blindness, and other visual and neurological disturbances, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably magnetic resonance imaging, or MRI. Because PRES was reported voluntarily from a post-marketing population of uncertain size, it is not possible to reliably estimate the frequency or establish a causal relationship to XTANDI. Discontinue XTANDI in patients who develop PRES.
Regulatory authorities have been moving towards more active and transparent pharmacovigilance and are making greater amounts of stand-alone safety information directly available to the public through websites and other means, e.g., periodic safety update report summaries, risk management plan summaries and various adverse event data. Safety information, without the appropriate context and expertise, may be misinterpreted and lead to misperception or legal action which may potentially cause our product sales or stock price to decline.
Further, if serious safety, resistance or drug interaction issues arise with XTANDI, product sales could be limited or halted by us or by regulatory authorities and our results of operations would be adversely affected.
25
XTANDI and any other product candidates that may receive regulatory approval in the future will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense as well as significant penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products and product candidates.
We are required to monitor the safety and efficacy of XTANDI and any other products candidates that are approved by the FDA. In addition, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for any approved products, such as XTANDI, will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration and listing, as well as continued compliance with current good clinical practices, or cGCP, for any clinical trials that we conduct post-approval. We and our contract manufacturers will be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with current good manufacturing practices, or cGMP. We must also comply with requirements concerning advertising and promotion for XTANDI and any other product candidates for which we obtain marketing approval in the future. Promotional communications with respect to prescription drugs, including biologics, are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labeling. Thus, we will not be able to promote XTANDI or any other products candidates for which we might obtain approval in the future for indications or uses for which they are not approved. Later discovery of previously unknown problems with XTANDI or any other product candidate for which we might obtain approval in the future, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
|
• |
restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials; |
|
|
• |
restrictions on such products’ manufacturing processes; |
|
|
• |
restrictions on the marketing of a product; |
|
|
• |
restrictions on product distribution; |
|
|
• |
requirements to conduct post-marketing clinical trials; |
|
|
• |
untitled or warning letters; |
|
|
• |
withdrawal of the products from the market; |
|
|
• |
refusal to approve pending applications or supplements to approved applications that we submit; |
|
|
• |
recall of products; |
|
|
• |
fines, restitution or disgorgement of profits or revenue; |
|
|
• |
suspension or withdrawal of regulatory approvals; |
|
|
• |
refusal to permit the import or export of our products; |
|
|
• |
product seizure; |
|
|
• |
injunctions; |
|
|
• |
imposition of civil penalties; or |
|
|
• |
criminal prosecution. |
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could affect the marketing of XTANDI or prevent, limit or delay regulatory approval of our other product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not sustain profitability.
If we are unable to successfully develop enzalutamide for breast cancer with a suitable diagnostic, we may not be able to obtain regulatory approval for enzalutamide for breast cancer or realize the full potential for enzalutamide.
We may initiate a Phase 3 clinical trial evaluating enzalutamide in triple negative breast cancer, or TNBC. A key element of our strategy to develop enzalutamide in TNBC is the use of a companion diagnostic to identify patients with TNBC appropriate for treatment with enzalutamide. Patients whose tumors test positive for a gene expression profile may be more likely to respond to treatment with enzalutamide; patients whose tumors test negative may not be responsive to treatment with enzalutamide. Both regulatory approval and, if approval is obtained, successful commercialization of enzalutamide in TNBC will, therefore, likely depend on our successful development of such a companion diagnostic.
26
We and Astellas have entered into a collaboration with NanoString Technologies, Inc. to translate our gene expression signature algorithm into a companion diagnostic assay using NanoString’s nCounter Dx Analysis System. However, we may encounter difficulties in developing enzalutamide for TNBC and/or developing the companion diagnostic for clinical, regulatory and/or commercial reasons including issues related to analytical validation, reproducibility or clinical validation.
Companion diagnostics are subject to regulation by the FDA as a medical device and require separate regulatory clearance prior to commercialization. Companion diagnostics are also subject to regulation by foreign regulatory authorities and such regulations may change or new regulations may be enacted during our development of the companion diagnostic. We may be unable to alter our development program in time to comply with the revised or new regulations, or we may only be able to do so by expending greater funds and efforts, and any delay or failure to obtain regulatory approval could delay or prevent the approval of enzalutamide for TNBC.
Risks Related to our License and Manufacturing and Supply Agreement with CureTech, Ltd.
The clinical molecule MDV9300 we licensed from CureTech is a biologic molecule, and we do not have long-standing experience or expertise in the development, manufacture, or commercialization of biologic molecules.
Under the license agreement, we are responsible for all development, manufacturing, and commercialization activities for MDV9300 for all indications, including in oncology. We have limited history, experience, or expertise in the development, manufacturing and commercialization, including regulatory interactions, commercial manufacturing, and distribution of biologic molecules, like MDV9300.
The successful development, testing, manufacturing and commercialization of biologics involves a long, expensive, and uncertain process. There are unique risks and uncertainties with biologics, including:
|
|
• |
The development, manufacturing and marketing of biologics are subject to regulation by the FDA, the European Medicines Agency or EMA, and other regulatory bodies. These regulations are often more complex and extensive than the regulations applicable to other pharmaceutical products; |
|
|
• |
Manufacturing biologics, especially in large quantities, is often complex and may require the use of innovative technologies to handle living cells. Each lot of an approved biologic must undergo thorough testing for identity, strength, quality, purity and potency. Manufacturing biologics requires facilities specifically designed for and validated for this purpose, and sophisticated quality assurance and quality control procedures are necessary. Slight deviations anywhere in the manufacturing process, including filling, labeling, packaging, storage and shipping and quality control and testing, may result in lot failures, product recalls or spoilage. When changes are made to the manufacturing process, we may be required to provide preclinical and clinical data showing the comparable identity, strength, quality, purity or potency of the products before and after such changes. For MDV9300, we plan to implement changes in manufacturing site and to improve the manufacturing process to provide for larger manufacturing lot sizes, as well as the critical analytical assays. Comparability data will be required to support these changes; and |
|
|
• |
The use of biologically derived ingredients can lead to allegations of harm, including infections or allergic reactions, or closure of product facilities due to possible contamination. Any of these events could result in substantial costs. |
We are currently dependent on CureTech to produce clinical supply of MDV9300 that meets global regulatory standards and CureTech is dependent on its suppliers for sufficient quantities of raw materials, starting materials, and supplies. If CureTech fails to manufacture clinical supply of MDV9300 in sufficient quantities or fails to source raw or starting materials or supplies in sufficient quantities, our ability to conduct clinical trials could be further delayed or otherwise negatively impacted.
Our failure to successfully develop, manufacture, or commercialize MDV9300 could significantly impact our ability to generate value from the License Agreement with CureTech.
27
The IND for MDV9300 was recently placed on partial clinical hold by the FDA, and we now intend to modify the production process for MDV9300, resulting in a delay of our Phase 2 clinical trial of MDV9300 in DLBCL as well as potential subsequent trials.
In early January 2016, we advised the FDA of our conclusion that MDV9300 does not bind PD-1, and the agency placed the IND on partial clinical hold and requested that we revise our characterization of MDV9300 in the related investigator brochure, protocols and informed consent documents. While we submitted the revised documents in early February 2016, and the FDA has 30 days thereafter to notify us if the partial clinical hold is lifted, the FDA may request additional information in response to our submission or may determine that our response did not satisfactorily address their request. In addition, we intend to submit an amendment to our IND for MDV9300 to modify the production process to better support our planned clinical activities. We plan to submit the IND amendment in the first half of 2016 and intend that patients in our Phase 2 DLBCL trial will be treated with MDV9300 manufactured in accordance with the amended IND. Since we cannot enroll patients in the Phase 2 DLBCL trial until the partial clinical hold is lifted and do not intend to proceed with the trial until MDV9300 manufactured in accordance with the amended IND is available, there will be a delay in the resumption of our planned clinical trial of MDV9300 for DLBCL. These circumstances could result in increased cost and could delay, prevent or limit our ability to generate value from and realize the commercial potential of MDV9300.
If we are unable to determine the binding target for MDV9300, we may fail to successfully develop MDV9300 for DLBCL, multiple myeloma or any other indication, and we may not be able to obtain regulatory approval or realize the full potential for MDV9300.
Although the molecule was originally understood to work primarily via PD-1 (Programmed Death-1) binding, our work as well as that of others has shown other potential activity as well. In the year since we acquired rights to the molecule, we conducted testing and characterization work to better identify the binding target and, in late December 2015, we concluded that MDV9300 does not bind PD-1. While we intend to continue work to identify the binding target and thereafter develop a target-specific release assay, these activities are time consuming, expensive and there is no assurance that we will be successful since the binding mechanism is currently unknown. Furthermore, there can be no assurance that we will not experience additional development issues in the future which may cause further delays or unanticipated costs, or that we will be able to solve such development issues. Our failure to identify the binding target for MDV9300 or to develop a target-specific release assay would significantly impact our ability to obtain regulatory approval and could materially harm our business.
The development and commercialization of MDV9300 may face strong competition from other immuno-oncology agents that have already received marketing approval and are being developed for additional indications by larger companies with substantial resources and relatively more experience developing, manufacturing, and commercializing biologic molecules.
The immuno-oncology field is competitively crowded with more than 100 immuno-oncology agents, including biologic molecules, vaccines, modified T-cells, and adjuvants, currently approved and on the market or in development for various tumor types and patient populations by larger more experienced companies than ours, such as Bristol Myers Squibb, Roche, AstraZeneca, Pfizer and Merck. This competitive environment could compromise our ability to develop MDV9300 by limiting the availability of clinical trial investigators, sites, and/or appropriate clinical patients, which could slow, delay or limit the progress of MDV9300’s development. Even if we are able to successfully develop MDV9300 and obtain regulatory approval for it in an oncology indication, the competition from already approved immuno-oncology molecules and other agents in the same or similar oncology indications could significantly limit our ability to generate revenue from MDV9300. While we have some experience developing and in certain aspects of the commercialization of small molecule products, we may be required to hire additional qualified employees with experience developing, manufacturing, and commercializing, including regulatory interactions, commercial manufacturing, and distributing biological molecules, like MDV9300. Many of our competitors are large, multinational pharmaceutical and biotechnology companies with considerably more resources and experience with biological molecules than us.
Risks Related to Our Future Product Development Candidates
Our business strategy depends on our ability to identify and acquire additional product candidates. We may never acquire or identify such product candidates for reasons that may not be in our control, or are otherwise unforeseen or unforeseeable to us.
A key component of our business strategy is to diversify our product development risk by identifying and acquiring new product opportunities for development. However, we may not be able to identify promising new technologies. In addition, the competition to acquire promising biomedical technologies is fierce, and many of our competitors are large, multinational pharmaceutical, biotechnology and medical device companies with considerably more financial, development and commercialization resources and experience than we have. Thus, even if we succeed in identifying promising technologies, we may not be able to compete against our larger competitors with considerably more financial resources or we may not be able to acquire rights to them on acceptable terms or at all. If we are unable to identify and acquire new technologies, we will be unable to diversify our product risk. We believe that any such failure would have a significant negative impact on our prospects.
28
Pharmaceutical and biological product candidates require extensive, time-consuming and expensive preclinical and clinical testing to establish safety and efficacy, and to obtain regulatory approval. If we are unable to successfully develop and test our product candidates, we will not be successful.
The research and development of pharmaceuticals and biological products is an extremely risky industry. Only a small percentage of product candidates that enter the development process ever receive regulatory approval. The process of conducting the preclinical and clinical testing required to establish safety and efficacy and obtain regulatory approval is expensive and uncertain and takes many years. If we are unable to complete preclinical or clinical trials of current or future product candidates, due to safety concerns with a product candidate, or if the results of these trials are not satisfactory to convince regulatory authorities of their safety or efficacy, we will not be able to obtain regulatory approval for commercialization. We cannot be certain if any of our product candidates will be approved by regulatory authorities. Furthermore, even if we are able to obtain regulatory approvals for any of our product candidates, those approvals may be for indications that are not as broad as desired or may contain other limitations that would adversely affect our ability to generate revenue from sales of those products. If this occurs, our business would be materially harmed and our ability to generate revenue would be severely impaired.
Enrollment and retention of patients in clinical trials of enzalutamide and other product candidates are expensive and time-consuming processes, could be made more difficult or rendered impossible by competing treatments or clinical trials of competing drugs in the same or other indications, and could result in significant delays, cost overruns, or both, in our product development activities, or in the failure of such activities.
We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical trials, and even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. Patient enrollment and retention in clinical trials depends on many factors, including the size of the patient population, the nature of the trial protocol, the existing body of safety and efficacy data with respect to the study drug, the number and nature of competing treatments and ongoing clinical trials of competing drugs for the same indication, the proximity of patients to clinical sites and the eligibility criteria for the study. Furthermore, any negative results we may report in clinical trials of enzalutamide or any potential future product candidates may make it difficult or impossible to recruit and retain patients in other clinical studies of that same product candidate. Delays or failures in planned patient enrollment and/or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop enzalutamide or any product candidates, or could render further development impossible.
Our reliance on third parties for the operation of our business may result in material delays, cost overruns and/or quality deficiencies in our development programs.
We currently rely on third parties to perform key product development tasks, such as laboratories to conduct preclinical testing, clinical research organizations to help us manage our clinical studies, investigators and investigative sites who conduct the clinical trials, and contract manufacturing organizations to manufacture our product candidates at appropriate scale for preclinical and clinical trials. In addition, we currently rely on Astellas and its third party vendors to supply commercial quantities of XTANDI. To manage our business successfully, we will need to identify, engage and properly manage qualified third parties that will perform these development and manufacturing activities. For example, we need to monitor the activities of our vendors closely to ensure that they are performing their tasks correctly, on time, on budget and in compliance with strictly enforced regulatory standards. We need to monitor investigators who participate in our clinical trials to ensure that they are conducting the trial in compliance with the protocol, good clinical practices and all applicable laws. Our ability to identify and retain key vendors, investigators and investigative sites with the requisite knowledge is critical to our business and the failure to do so could negatively impact our business. Because all of our key vendors perform services for other clients in addition to us, we also need to ensure that they are appropriately prioritizing our projects. If we fail to manage our key vendors well, we could incur material delays, cost overruns or quality deficiencies in our development and commercialization programs, as well as other material disruptions to our business.
Patient safety issues may arise from investigator-sponsored research with our product candidates, which may adversely affect our development program for such product candidates.
We are committed to improving patient care through research that advances the medical and scientific knowledge of XTANDI and our product candidates. In support of this commitment, we, in collaboration with our partner Astellas, have established an investigator-sponsored research program as a mechanism for independent researchers to seek support for preclinical and clinical research studies with enzalutamide. We also provide support for investigator-sponsored research with MDV3800 and MDV9300. However, because we are not the sponsor of investigator-sponsored research studies, we cannot control how such studies are conducted. If new patient safety issues arise from use of our product candidates in these investigator-sponsored research studies, our development programs may be adversely affected which could impact our business and the results of our operations.
29
Risks Related to the Operation of our Business
We may incur substantial costs in the foreseeable future as we continue our development and commercialization activities and may not maintain or increase profitability on a quarterly or annual basis.
We have incurred significant costs principally from funding our research and development activities, from general and administrative expenses and from our XTANDI commercialization activities. We may incur substantial costs in the foreseeable future as we continue to finance the commercialization of XTANDI in the U.S. market, preclinical and clinical studies of enzalutamide, MDV3800, MDV9300 and early stage programs, potential business development activities, and our corporate overhead costs, which could impact our ability to maintain or increase profitability on a quarterly or annual basis. Our ability to generate revenue sufficient to offset these costs in order to maintain or increase profitability on a quarterly or annual basis is dependent on our ability, alone or with collaboration partners, to successfully commercialize products for which we receive marketing approval.
Our operating results are unpredictable and may fluctuate. If our operating results are below the expectations of securities analysts or investors, the market value of our common stock could decline.
Our operating results are difficult to predict and will likely fluctuate from quarter to quarter and year to year. Due to the competitive market for mCRPC therapies, XTANDI net sales will be difficult to predict from period to period. As a result, you should not rely on XTANDI net sales results in any period as being indicative of future performance and sales of XTANDI may be below the expectation of securities analysts or investors in the future. Additionally, you should not place undue reliance on the forward-looking statements about expectations for future XTANDI net sales from our partner Astellas, as we may not agree with such statements, or from us, as XTANDI net sales results are difficult to predict. We believe that our quarterly and annual results of operations may be affected by a variety of factors, including:
|
|
• |
the price and level of demand for XTANDI; |
|
|
• |
the extent to which coverage and reimbursement for XTANDI is available from government and health administration authorities, private health insurers, managed care programs and other third-party payors; |
|
|
• |
the timing, cost and level of investment in our and Astellas’ sales and marketing efforts to support XTANDI sales; |
|
|
• |
the timing, cost and level of investment in our research and development activities involving XTANDI and our product candidates; |
|
|
• |
the cost of manufacturing XTANDI, and the amount of legally mandated discounts to government entities, other discounts and rebates, product returns and other gross-to-net deductions; |
|
|
• |
the risk/benefit profile, cost and reimbursement of existing and potential future branded and generic drugs which compete with XTANDI; |
|
|
• |
the timeliness and accuracy of financial information we receive from Astellas regarding XTANDI net sales globally, and shared U.S. development and commercialization costs for XTANDI incurred by Astellas, including the accuracy of the estimates Astellas uses in calculating any such financial information including for gross-to-net revenue adjustments; |
|
|
• |
inventory levels at pharmaceutical wholesalers and distributors; |
|
|
• |
expenditures that we will or may incur to acquire or develop additional technologies, product candidates and products; and |
|
|
• |
the impact of fluctuations in foreign currency exchange rates. |
In addition, we measure compensation cost for stock-based awards made to employees at the grant date of the award, based on the fair value of the award, and recognize the cost as a non-cash operating expense over the employee’s requisite service period. As the variables that we use as a basis for valuing these awards change over time, including our underlying stock price and stock price volatility, the magnitude of the non-cash expense that we must recognize may vary significantly.
We are required to evaluate the fair value of intangible assets, including in-process research and development, or IPR&D, for impairment at least annually or more frequently if impairment indicators exist. These assets are initially measured at fair value and therefore any change to our assumptions used in the valuations could potentially lead to impairment. Some of the more common potential risks leading to impairment include competition, earlier than expected loss of exclusivity, pricing pressures, adverse regulatory changes or clinical trial results, delay or failure to obtain regulatory approval and additional development costs, inability to achieve expected synergies, higher operating costs, changes in tax laws and other macro-economic changes. If any of these or similar issues arise during development, we may need to re-evaluate our IPR&D and incur a non-cash impairment charge, adversely affecting our consolidated results of operations.
30
Additionally, each reporting period, we revalue contingent consideration obligations associated with business acquisitions to their fair value and record increases in fair value as non-cash contingent consideration expense and decreases in fair value as non-cash contingent consideration income. Changes in contingent consideration result from changes in assumptions regarding the probability of successful achievement of related milestones, the estimated timing in which the milestones may be achieved and the discount rates used to estimate the fair value of the liability. Contingent consideration may change significantly as our development programs progress, revenue estimates evolve and additional data is obtained, impacting our estimates.
For these and other reasons, it is difficult for us to accurately forecast future profits or losses. As a result, it is possible that in some quarters our operating results could be below the expectations of securities analysts or investors.
Sales fluctuations of XTANDI as a result of inventory levels at Astellas’ customers (such as pharmaceutical wholesalers and distributors) may cause our revenue to fluctuate, which could adversely affect our financial results and the market value of our common stock.
The pharmaceutical wholesalers and distributors with whom Astellas has entered into inventory management agreements make estimates to determine end user demand and may not always be effective in matching their inventory levels to actual end user demand. As a result, changes in inventory levels held by those wholesalers and distributors can cause our operating results to fluctuate unexpectedly if sales of XTANDI to these wholesalers do not match end user demand. Adverse changes in economic conditions or other factors may cause wholesalers and distributors to reduce their inventories of XTANDI. As inventory of XTANDI in the distribution channel fluctuates from quarter to quarter, we may see corresponding fluctuations in reported collaboration revenue from XTANDI net sales.
A significant and growing amount of the collaboration revenue we generate is in currency other than U.S. dollars, primarily the Euro and the Japanese yen, thereby exposing us to foreign currency exchange rate risk.
A significant and growing amount of our collaboration revenue is generated from royalties on ex-U.S. net sales of XTANDI. The royalties we receive from Astellas on net sales of XTANDI outside of the United States are calculated by converting the respective countries’ XTANDI net sales in local currency to U.S. dollars for payment to us on a quarterly basis. A strengthening of the U.S. dollar relative to current currency exchange rates would result in lower collaboration revenue related to ex-U.S. XTANDI net sales than otherwise would have been reported by us and paid to us.
In 2016, we intend to hedge a portion of our exposure to fluctuating currency rates for the Euro and the Japanese yen in order to mitigate unexpected short-term volatility of such rates. Depending on the size of the exposures and the relative movements of exchange rates, if we fail to effectively hedge our exposures, we could experience adverse effects on our reported financial results.
If we fail to attract and keep senior management personnel, we may be unable to successfully develop our product candidates, identify and acquire promising new technologies, conduct our clinical trials, and commercialize our products, and our business could be harmed.
Our future success depends upon the continued services of our executive officers and our ability to attract, retain, and motivate highly qualified management to oversee our business. None of our executive officers is bound by an employment agreement for any specific term, and they may terminate their employment at any time. We are particularly dependent on the continued services of David Hung, M.D., our president and chief executive officer and a member of our board of directors. Dr. Hung identified enzalutamide for acquisition and has primary responsibility for identifying and evaluating other potential product candidates. We believe that Dr. Hung’s services in this capacity would be difficult to replace.
Although we have not historically experienced unique difficulties attracting and retaining qualified employees, we could experience such problems in the future. For example, competition for qualified personnel in the biotechnology and pharmaceutical fields is intense, especially in the San Francisco Bay Area. The loss of any of our executive officers or a significant turnover in our senior management could impair the successful commercialization of XTANDI, delay or prevent continued development activities for enzalutamide, MDV3800, MDV9300 and other product candidates, delay or prevent the transfer and integration of MDV3800 into our business and adversely affect or preclude the identification and acquisition of new product candidates, any of which events could harm our business.
In addition, we will need to hire additional personnel as we expand our clinical development and commercial activities. We may not be able to attract and retain qualified personnel on acceptable terms.
31
We may encounter difficulty managing our growth and expanding our operations successfully.
Our business has experienced significant growth, which we expect will continue as we expand our commercialization efforts for XTANDI, seek to advance our product candidates through clinical trials, and integrate the acquisition of MDV3800 and other potential business development activities. We expect that we will need to expand our development, regulatory, manufacturing, and commercial capabilities in concert with contracting with third parties to provide certain of these activities for us. To that end, we must be able to hire, train and integrate additional personnel effectively in order to manage our commercialization and development activities.
We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. If we are not able to effectively manage the expansion of our operations, it may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. In addition, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Thus, our future financial performance and our ability to develop and commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively.
We will need to expand our organization to successfully commercialize our un-partnered product candidates outside of the United States in some degree, and we may experience difficulties, which could disrupt our operations and harm our business.
We have no experience marketing or selling outside of the United States. To successfully commercialize MDV3800, MDV9300, or any other products that emerge from our development programs, we will need to build or otherwise secure these capabilities from third parties. Developing a commercial infrastructure outside the United States is a significant undertaking that requires substantial financial and managerial resources. We may need to establish internal marketing and sales capabilities for several major ex-U.S. geographies to reach the commercial potential of MDV3800, MDV9300, or any other products that result from our development programs. Such expansion would require attracting, retaining and motivating the necessary skilled personnel and, in addition, it may require significant capital and operating expenditures. As a result, such activity may divert human and financial resources from other projects, such as the development or acquisition of additional product candidates. If we encounter unexpected or unforeseen delays in establishing a commercial infrastructure outside the United States, for example, it may negatively impact our ability to launch a commercial product or it may increase the cost and number of resources required for successful commercialization. In addition, it may negatively impact the future financial performance of our business and our ability to generate sufficient revenue to sustain our business. In the future, as a result of these financial and execution risks of expanding geographically ourselves, we may choose to partner with others who have existing capability to commercialize these products.
Our significant level of debt and lease obligations could adversely affect our financial condition. In addition, we may not have sufficient funds to service our debt and lease obligations when payments are due.
At December 31, 2015, we had $75.0 million outstanding under our Revolving Credit Facility and approximately $54.9 million of minimum lease commitments. We may incur additional indebtedness to meet future financing needs, including for example in connection with business development activities and for other general corporate purposes. Substantial indebtedness could have significant effects on our business, results of operations and financial condition. For example, it could:
|
|
• |
make it more difficult for us to satisfy our financial obligations, including with respect to our debt and lease obligations; |
|
|
• |
increase our vulnerability to general adverse economic, industry and competitive conditions; |
|
|
• |
reduce the availability of our cash resources to fund our operations because we will be required to dedicate a substantial portion of our cash resources to the payment of principal and interest on our indebtedness and lease payments; |
|
|
• |
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
|
|
• |
place us at a competitive disadvantage compared to our competitors that are less highly leveraged and that, therefore, may be able to take advantage of opportunities that our leverage prevents us from exploring; and |
|
|
• |
limit our ability to obtain financing. |
Each of these factors may have a material and adverse effect on our financial condition and viability.
32
With the acquisition of new product candidates in recent periods, we may need additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all, which would force us to delay, scale back or eliminate some or all of our currently-planned development programs and other operations, to restructure or refinance our indebtedness, or to determine to do any combination of the foregoing. Raising additional capital may subject us to unfavorable terms or conditions, may cause dilution to our existing stockholders, or may restrict our operations or require us to relinquish rights to our product candidates and technologies.
We require significant capital to fund our operations. We have historically funded our operations primarily through public offerings of our common stock, the issuance of the Convertible Notes (which we redeemed in July 2015 and are no longer outstanding), and from the upfront, milestone, and cost-sharing payments under agreements with our current and former collaboration partners, from collaboration revenue related to XTANDI net sales and, more recently and to a lesser extent, from short-term borrowings under a Revolving Credit Facility. At December 31, 2015, we had cash and cash equivalents totaling $225.9 million available to fund our operations. We expect to devote substantial financial resources to our future operations. Based on our current expectations, we believe our current capital resources, amounts available to us under our Revolving Credit Facility, and projected operating and other cash flows will be sufficient to fund our currently planned operations for at least the next twelve months. This estimate is based on a number of assumptions that may prove to be wrong, including assumptions regarding the amount and timing of net sales of XTANDI, potential XTANDI approvals in new markets and for other indications, and potential receipt of profit sharing and royalty payments under our Astellas Collaboration Agreement, causing us to exhaust our available cash, cash equivalents, and short-term investments sooner than anticipated. For example, we may be required or choose to seek additional funds for the commercialization of XTANDI in the United States, to expand our preclinical and clinical development activities for enzalutamide, MDV3800, MDV9300 and other existing or potential future product candidates, to fund business acquisitions, investments or to in-license technologies, particularly if we face challenges or delays in connection with our clinical trials, and to maintain minimum cash balances that we deem reasonable and prudent. Our ability to raise additional funds on acceptable terms will be dependent on the climate of worldwide capital markets, which could be challenging for companies such as ours.
We may be constrained by our obligations under the Revolving Credit Facility to operate our business to its full potential.
The terms of our Revolving Credit Facility contain customary representations and warranties and customary affirmative and negative covenants, including, among other things, restrictions on indebtedness, liens, investments, mergers, dispositions, prepayment of other indebtedness and dividends and other distributions. Furthermore, under the terms of the Revolving Credit Facility, we are required to comply with a maximum senior secured net leverage ratio and minimum interest coverage ratio covenants. These terms may restrict our ability to operate our business in the manner we deem most effective or desirable, and may restrict our ability to fund our operations through new public offerings of our common stock or strengthen our candidate development pipeline through acquisitions or in-licenses which cause us to exceed our maximum senior secured net leverage ratio.
Failure to comply with the representations and warranties or affirmative and negative covenants could constitute an event of default which, if continued beyond the cure period, would allow the Administrative Agent (as defined in the Revolving Credit Facility), at the request of or with the consent of the Lenders holding a majority of the loans and commitments under the facility, to terminate the commitments of the Lenders to make further loans and declare all the obligations of the Loan Parties under the Revolving Credit Facility to be immediately due and payable, either of which would harm our business.
We may have additional tax liabilities.
We are subject to income taxes in various jurisdictions. Significant judgment is required in determining our provision for income taxes and other tax liabilities. Our effective income tax rate in the future is subject to volatility and could be adversely affected by a number of factors, including interpretations of existing tax laws, changes in tax laws and rates, future levels of research and development expenditures, changes in the mix of earnings in countries with differing statutory tax rates in which we may conduct business, changes in the valuation of deferred tax assets and liabilities, state income taxes, the tax impact of stock-based compensation, changes in estimates of prior years’ items, tax costs for acquisition-related items, changes in accounting standards, non-deductible officers’ compensation, limitations on the utilization of net operating losses and tax credits due to changes in ownership, and overall levels of income before taxes. The impact of our income tax provision resulting from these items may be significant and could have a negative impact on our net income.
We are also subject to non-income based taxes, such as payroll, sales, use, net worth, property, and goods and services taxes in the United States. We may have additional exposure to non-income based tax liabilities.
We are regularly subject to audits by tax authorities in the jurisdictions in which we conduct business. Although we believe our tax positions are reasonable, the final outcome of tax audits and related litigation could be materially different than that reflected in our historical income tax provisions and accruals, and we could be subject to assessments of additional taxes and/or substantial fines or penalties. The resolution of any audits or litigation could have an adverse effect on our financial position and results of operations.
33
We and our subsidiaries are engaged in a number of intercompany transactions. Although we believe that these transactions reflect arm’s length terms and that proper transfer pricing documentation is in place, which should be respected for tax purposes, the transfer prices and terms and conditions of such transactions may be scrutinized by tax authorities, which could result in additional tax and/or penalties becoming due.
Intellectual property protection for our product candidates is crucial to our business, and is subject to a significant degree of legal risk, particularly in the life sciences industry.
The success of our business will depend in part on our ability to obtain and maintain intellectual property protection, primarily patent protection for the XTANDI product and any potential future product candidates, as well as successfully asserting and defending these patents against third-party challenges. We and/or our collaborators will only be able to protect the XTANDI product and our potential future product candidates from unauthorized commercialization by third parties to the extent that valid and enforceable patents or trade secrets cover them. Furthermore, future protection of our proprietary rights is uncertain because legal means may afford only limited protection and may not adequately protect our rights or permit us or our potential future collaborators to gain or keep our competitive advantage.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Further, changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property rights. Accordingly, we cannot predict the breadth of claims that may be granted or enforced for our patents or for third-party patents that we have licensed. For example:
|
|
• |
we or our licensors might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
|
|
• |
we or our licensors might not have been the first to file patent applications for these inventions; |
|
|
• |
others may independently develop similar or alternative technologies or duplicate any of our technologies; |
|
|
• |
it is possible that none of our pending patent applications or the pending patent applications of our licensors will result in issued patents; |
|
|
• |
our issued patents and future issued patents, or those of our licensors, may not provide a basis for protecting commercially viable products, may not provide us with any competitive advantages, or may be challenged by third parties and invalidated or rendered unenforceable; and |
|
|
• |
we may not develop additional proprietary technologies or product candidates that are patentable. |
Further, even if we can obtain protection for and defend the intellectual property position of the XTANDI product or any potential future product candidates, we or any of our potential future collaborators still may not be able to exclude competitors from developing or marketing competing drugs. Should this occur, we and our potential future collaborators may not generate any revenues or profits from the XTANDI product or any potential future product candidates or our revenue or profits would be significantly decreased.
We could become subject to litigation or other challenges regarding intellectual property rights, which could divert management attention, cause us to incur significant costs, prevent us from selling or using the challenged technology and/or subject us to competition by lower priced generic products.
During the course of obtaining patent protection, we may receive rejections from national patent offices or be subject to pre-grant opposition proceedings instituted by third parties. Even after a patent is granted and issued by the national patent office, many countries allow post-grant opposition proceedings. In the U.S., these post-grant opposition proceedings include inter partes review whereby a third party can seek to challenge and invalidate an issued patent in an administrative proceeding before the U.S. Patent and Trademark Office, rather than through litigation in court, which is often lengthier and costlier. Our patents and patent applications, or those of our licensors, could face other challenges, such as interference proceedings, opposition proceedings, re-examination proceedings, derivation proceedings and pre-grant submissions. Any such challenge, if successful, could result in the invalidation of, or in a narrowing of the scope of, any of our patents and patent applications subject to the challenge. Any such challenges, regardless of their success, would likely be time-consuming and expensive to defend and resolve and would divert our management’s time and attention.
Generic and other pharmaceutical manufacturers are and have been very aggressive in challenging the validity of patents held by proprietary pharmaceutical companies, especially if these patents are commercially significant. Pursuant to the provisions of the Hatch-Waxman Act, the XTANDI patents listed in the FDA “Orange Book” could be challenged by generic manufacturers as early as four years from the initial approval of the XTANDI product in the United States, which occurred on August 31, 2012. Companies seeking approval to market a generic version of a reference drug may also seek to challenge the validity of patents listed in the Orange Book or other patents covering such reference drug by filing petitions for inter partes review with the U.S. Patent and Trademark Office. If a generic pharmaceutical company or other third party were able to successfully invalidate any of our present or future
34
patents, the XTANDI product and any potential future product candidates that may ultimately receive marketing approval could face additional competition from lower-priced generic products that would result in significant price and revenue erosion and have a significantly negative impact on the commercial viability of the affected product candidate(s).
In addition, we may be a party to litigation to protect our intellectual property or to defend our activities in response to alleged infringement of a third party’s intellectual property. These claims and any resulting lawsuit, if successful, could subject us to significant liability for damages and invalidation, or a narrowing of the scope, of our proprietary rights. These lawsuits, regardless of their success, would likely be time-consuming and expensive to litigate and resolve and would divert management time and attention. Any potential intellectual property litigation also could force us or our potential licensees to do one or more of the following:
|
|
• |
discontinue our products that use or are covered by the challenged intellectual property; or |
|
|
• |
obtain from the owner of the allegedly infringed intellectual property right a license to sell or use the relevant technology, which license may not be available on reasonable terms, or at all. |
If we or our potential licensees are forced to take any of these actions, our business may be seriously harmed. Although we carry general liability insurance, our insurance does not cover potential claims of this type.
We may in the future initiate claims or litigation against third parties for infringement to protect our proprietary rights or to determine the scope and validity of our proprietary rights or the proprietary rights of competitors. These claims could result in costly litigation and the diversion of our technical and management personnel and we may not prevail in making these claims.
We rely on license agreements for certain aspects of our product candidates and our technology, and failure to meet our obligations under those agreements could severely negatively impact our business, and ability to generate revenue.
We have entered into agreements with third-party commercial and academic institutions to license intellectual property rights and technology. For example, we have a license agreement with UCLA pursuant to which we were granted exclusive worldwide rights to certain UCLA patents related to XTANDI and a family of related compounds. Some of these license agreements, including our license agreement with UCLA, contain diligence and milestone-based termination provisions, in which case our failure to meet any agreed upon diligence requirements or milestones may allow the licensor to terminate the agreement. If our licensors terminate our license agreements or if we are unable to maintain the exclusivity of our exclusive license agreements, we may be unable to continue to develop and commercialize XTANDI or any potential future product candidates based on licensed intellectual property rights and technology.
In the future, we may need to obtain additional licenses of third-party technology that may not be available to us or are available only on commercially unreasonable terms, and which may cause us to operate our business in a more costly or otherwise adverse manner that was not anticipated.
From time to time we may be required to license technology from additional third parties to further develop XTANDI and any future product candidates. Should we be required to obtain licenses to any third-party technology, including any such patents based on biological activities or required to manufacture our product candidates, such licenses may not be available to us on commercially reasonable terms, or at all. The inability to obtain any third-party license required to develop any of our product candidates could cause us to abandon any related development efforts, which could seriously harm our business and operations.
We may become involved in disputes with Astellas, NanoString or any potential future collaborators over intellectual property ownership, and publications by our research collaborators and scientific advisors could impair our ability to obtain patent protection or protect our proprietary information, which, in either case, could have a significant impact on our business.
Inventions discovered under research, material transfer or other such collaboration agreements, including the Astellas Collaboration Agreement or NanoString collaboration agreement, may become jointly owned by us and the other party(ies) to such agreements in some cases and the exclusive property of one of the parties in other cases. Under some circumstances, it may be difficult to determine who owns a particular invention, or whether it is jointly owned, and disputes could arise regarding ownership of those inventions. These disputes could be costly and time consuming and an unfavorable outcome could have a significant adverse effect on our business if we were not able to protect or license rights to these inventions. In addition, our research collaborators and scientific advisors generally have contractual rights to publish our data and other proprietary information, subject to our prior review. Publications by our research collaborators and scientific advisors containing such information, either with our permission or in contravention of the terms of their agreements with us, may impair our ability to obtain patent protection or protect our proprietary information, which could significantly harm our business.
Trade secrets may not provide adequate protection for our business and technology.
We also rely on trade secrets to protect our technology, especially where we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we use reasonable efforts to protect our trade secrets, our or any
35
potential collaborators’ employees, consultants, contractors or scientific and other advisors may unintentionally or willfully disclose our information to competitors. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, our enforcement efforts would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, if our competitors independently develop equivalent knowledge, methods or know-how, it will be more difficult or impossible for us to enforce our rights and our business could be harmed.
Significant disruptions of information technology systems or breaches of data security could adversely affect our business.
Our business is increasingly dependent on critical, complex and interdependent information technology systems to record and maintain financial, clinical trial, safety, laboratory and other corporate records. The size and complexity of our computerized information systems make them vulnerable to breakdown, malicious intrusion, catastrophic events, computer viruses, and human error. We have experienced at least one successful intrusion into our computer systems, and although it did not have a material adverse effect on our operations, there can be no assurance of a similar result in the future as cyberattacks are increasing in their frequency, sophistication and intensity.
We have developed systems and processes that are designed to protect our information and prevent data loss and other security breaches, including systems and processes designed to reduce the impact of a security breach; however, such measures cannot provide absolute security, and we have taken and are continually taking additional security measures to protect against any future intrusion, including employee training. Moreover, the prevalent use of mobile devices that access our systems and confidential information and our relationships with third party vendors, who may or could have access to our confidential information, increases the risk of data security breaches.
A data or security breach of our systems, or that of our third party vendors, such as third party clinical research organizations, third party clinical manufacturing organization, clinical trial sites, and administrators of our corporate employee benefit plans or of our current and potential future collaboration partners, could lead to public exposure of personal information of our employees, clinical trial patients, customers and other business partners, modification of or prevent us from accessing our clinical trial databases, or the loss of trade secrets or other intellectual property.
If we or our third party vendors or business partners experience significant data security breaches, we could suffer harm to our reputation, be compelled to comply with federal and/or state breach notification laws and foreign law equivalents, be subjected to liability under laws and regulations that protect personal data, be subjected to mandatory corrective action or government enforcement action, need to verify the correctness of clinical trial database contents, or be exposed to litigation claims. In addition, because these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. Prolonged disruption would divert management’s attention and could result in a delay to the development of our product candidate.
While we do carry some insurance for these types of claims, our insurance may not be sufficient in type or amount.
Risks generally associated with our company-wide implementation of an enterprise resource planning (ERP) system may adversely affect our business and results of operations or the effectiveness of our internal controls over financial reporting.
We commenced implementation of a company-wide ERP system to upgrade certain existing business, operational, and financial processes. ERP implementations are typically complex and time-consuming projects that may require substantial time and resources to complete. Our results of operations could be adversely affected if we experience delays or cost overruns during the ERP implementation process, or if the ERP system or associated process changes do not give rise to the benefits that we expect.
Risks Related to the Pharmaceutical Industry, Including the Activities of Medivation, Inc.
Our industry is highly regulated by the FDA and comparable foreign regulatory agencies. We must comply with extensive, strictly enforced regulatory requirements to develop, obtain, and maintain marketing approval for any of our product candidates.
Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information for each therapeutic indication to establish the product candidate’s safety and efficacy for its intended use. It takes years to complete the testing of a new drug, biologic or medical device and development delays and/or failure can occur at any stage of testing. Any of our present and future clinical trials may be delayed, halted or approval of any of our products may be delayed or may not be obtained due to any of the following:
|
|
• |
any preclinical test or clinical trial may fail to produce safety and efficacy results satisfactory to the FDA or foreign regulatory authorities; |
|
|
• |
preclinical and clinical data can be interpreted in different ways, which could delay, limit or prevent regulatory approval; |
36
|
|
• |
the FDA or foreign regulatory authorities can place a clinical hold on a trial if, among other reasons, it finds that patients enrolled in the trial are or would be exposed to an unreasonable and significant risk of illness or injury; |
|
|
• |
the facilities that we utilize, or the processes or facilities of third party vendors, including without limitation the contract manufacturers who will be manufacturing drug substance and drug product for us or any potential collaborators, may not complete successful inspections by the FDA or foreign regulatory authorities; and |
|
|
• |
we may encounter delays or rejections based on changes in FDA policies or the policies of foreign regulatory authorities during the period in which we develop a product candidate or the period required for review of any final regulatory approval before we are able to market any product candidate. |
In addition, information generated during the clinical trial process is susceptible to varying interpretations that could delay, limit, or prevent regulatory approval at any stage of the approval process. Moreover, early positive preclinical or clinical trial results may not be replicated in later clinical trials. Failure to demonstrate adequately the quality, safety and efficacy of any of our product candidates would delay or prevent regulatory approval of the applicable product candidate. There can be no assurance that if clinical trials are completed, either we or our collaborative partners will submit applications for required authorizations to manufacture or market potential products or that any such application will be reviewed and approved by appropriate regulatory authorities in a timely manner, if at all.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse and false claims laws and regulations. Prosecutions under such laws have increased in recent years and we may become subject to such litigation. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Commercialization of drugs and biologics that receive FDA approval are subject, directly or indirectly, to various state and federal fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and federal False Claims Act and the state law equivalents of such laws. These laws may impact, among other things, our sales, marketing, and education programs.
The federal Anti-Kickback Statute prohibits persons and entities from knowingly and willingly soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. The Anti-Kickback Statute is broad, and despite a series of narrow regulatory safe harbors and statutory exceptions, prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Penalties for violations of the federal Anti-Kickback Statute include, among other things, criminal and administrative penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Many states have adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, including private insurance programs.
The federal False Claims Act prohibits persons and entities from knowingly filing, or causing to be filed, a false claim, or the knowing use of false statements, to obtain payment from the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government, and such individuals, commonly known as “whistleblowers,” may share in a proportion of any amounts paid by the entity to the government in fines or settlement. The frequency of filing qui tam actions has increased significantly in recent years, causing greater numbers of biotechnology and pharmaceutical companies to have to defend False Claims Act actions. When it is determined that an entity has violated the False Claims Act, the entity may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. Various states have also enacted laws modeled after the federal False Claims Act. Although we have developed, implemented, and continue to improve a program for compliance with all federal and state laws, we cannot guarantee that our compliance program will be sufficient or effective, that our employees will comply with our policies and that our employees will notify us of any violation of our policies, that we will have the ability to take appropriate and timely corrective action in response to any such violation, or that we will make decisions and take actions that will necessarily limit or avoid liability for whistleblower claims that individuals, such as employees or former employees, may bring against us or that governmental authorities may prosecute against us based on information provided by individuals. If one or more individuals bring a whistleblower claim against us or if a governmental authority prosecutes a claim against us on the basis of information provided by one or more individuals, and if we are found liable and a fine and/or an injunction and/or a Corporate Integrity Agreement or similar government oversight program is imposed on us or we agree to pay a fine and/or accept an injunction and/or a Corporate Integrity Agreement or similar government oversight program in settlement of the claim, they could have a material adverse effect on our financial condition and impair or prevent us from conducting our business. In addition, the costs and fees associated with defending a whistleblower claim would be significant.
37
We may also be subject to federal criminal healthcare fraud statutes that were created by the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH. The HIPAA health care fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment, and/or exclusion from government sponsored programs. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
We, and our business activities, are also subject to the federal Physician Payments Sunshine Act, which is within the Patient Protection and Affordable Care Act, as amended by the Health Care Education and Reconciliation Act, (collectively, “PPACA”). The federal Physician Payments Sunshine Act, and its implementing regulations, require certain manufacturers of drugs, devices, biological, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program (with certain exceptions) to report annually information related to certain payments or other transfers of value provided to physicians and teaching hospitals and other healthcare providers. In addition, there has been a recent trend of increased federal and state regulation on payments made to physicians. Certain states mandate implementation of commercial compliance programs, impose restrictions on drug manufacturer marketing practices, and/or the tracking and reporting of gifts, compensation and remuneration to physicians.
We are unable to predict whether we could be subject to actions under any of these or other fraud and abuse laws, or the impact of such actions. If we are found to be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare reimbursement programs and the curtailment or restructuring of our operations, any of which could have a material adverse effect on our business and results of operations.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act and other worldwide anti-bribery laws.
We are subject to the U.S. Foreign Corrupt Practices Act, or FCPA, which generally prohibits companies and their intermediaries from making payments to non-U.S. government officials for purpose of obtaining or retaining business or securing any other improper advantage. We are also subject to similar anti-bribery laws in the jurisdictions in which we operate. Failure to comply with the FCPA or related laws governing the conduct of business with foreign government entities could disrupt our business and lead to severe criminal and civil penalties, including criminal and civil fines, denial of government reimbursement for our products and exclusion from participation in government healthcare programs. Other remedial measures could include further changes or enhancements to our procedures, policies, and controls and potential personnel changes and/or disciplinary actions, any of which could have a material adverse impact on our business, financial condition, results of operations and liquidity. We could also be affected by any allegation that we, or any third party vendors that we rely on to perform key product development tasks, violated such laws.
If the FDA or other applicable regulatory authorities approve generic products that compete with any of our products or product candidates, the sales of our products or product candidates may be adversely affected.
Once an NDA is approved, the product covered thereby becomes a “listed drug” which, in turn can be relied upon by potential competitors in support of an approval of an ANDA or 505(b)(2) application. U.S. laws and other applicable policies provide incentives to manufacturers to create modified, non-infringing versions of a drug to facilitate the approval of an ANDA or other application for a generic substitute. These manufacturers might only be required to conduct a relatively inexpensive study to show that their product has the same active ingredient(s), dosage form, strength, route of administration, and conditions of use, or labeling, as our product or product candidate and that the generic product is bioequivalent to ours, meaning it is absorbed in the body at the same rate and to the same extent as our product or product candidate. These generic equivalents, which must meet the same quality standards as branded pharmaceuticals, would be significantly less costly than ours to bring to market and companies that produce generic equivalents are generally able to offer their products at lower prices. Thus, after the introduction of a generic competitor, a significant percentage of sales of any branded product is typically lost to the generic product. Accordingly, competition from generic equivalents to our products or product candidates would materially adversely impact our revenues, profitability and cash flows and substantially limit our ability to obtain a return on the investments that we have made in our product candidates.
To the extent that we receive regulatory approval to market biological products in the future, we will face competition from biosimilar products. A growing number of companies have announced their intention to develop biosimilar products, some of which could potentially compete with our product candidates. Because of the abbreviated pathway for approval of biosimilars in the United State and abroad, we may in the future face greater competition from biosimilar products. This additional competition could have a material adverse effect on our business and results of operations.
38
Healthcare reform initiatives and other third party cost-containment pressures could cause us to sell our products at lower prices, resulting in decreased revenues.
The United States and some foreign jurisdictions have enacted or are considering enacting a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to profitably sell XTANDI and other product candidates should they receive marketing approval. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
In March 2010, the PPACA became law in the United States. PPACA substantially changed and will continue to change the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. The provisions of PPACA most relevant to the pharmaceutical industry include:
|
|
• |
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain governmental health care programs, not including orphan drug sales; |
|
|
• |
an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; |
|
|
• |
Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D; |
|
|
• |
extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
|
|
• |
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals with income at or below 133% of the Federal Poverty Level beginning in 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
|
|
• |
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
|
|
• |
a new requirement to report certain financial arrangements with physicians and teaching hospitals, as defined in PPACA and its implementing regulations, including reporting any payment or “transfers of value” made or distributed to physicians and teaching hospitals, and reporting any ownership and investment interests held by physicians and their immediate family members and applicable group purchasing organizations; |
|
|
• |
expansion of health care fraud and abuse laws, including the federal False Claims Act and Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
|
|
• |
a licensure framework for follow-on biologic products; and |
|
|
• |
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria or maximum monthly out-of-pocket costs for patients, either of which could put additional downward pressure on the price that we receive for any approved product, and could seriously harm our business. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. If we fail to successfully secure and maintain adequate coverage and reimbursement of our products or are significantly delayed in doing so, we will have difficulty achieving market acceptance of our products and expected revenue and profitability which would have a material adverse effect on our business, results of operations and financial condition. In addition, we will face competition from other approved drugs against which we compete, and the marketers of such other drugs are likely to be significantly larger than us and therefore enjoy significantly more negotiating leverage with respect to the individual payors than we may have.
39
We could experience adverse outcomes under existing litigation or become subject to product liability or other litigation, which could harm our ability to efficiently and effectively conduct our business, and, if successful, could materially and adversely harm our business and financial condition as a result of the costs of liabilities that may be imposed thereby.
Our business exposes us to the risk of product liability claims that is inherent in the development, manufacturing, distribution and sale of pharmaceutical products. If XTANDI, MDV3800, MDV9300 or any other potential future product candidate harms people, or is alleged to be harmful, we may be subject to costly and damaging product liability claims brought against us by clinical trial participants, consumers, health care providers, corporate partners or others. We have product liability insurance covering commercial sales of XTANDI and our ongoing clinical trials. However, the amount of insurance we maintain may not be adequate to cover all liabilities that we may incur. If we are unable to obtain insurance at an acceptable cost or otherwise protect against potential product liability claims, we may be exposed to significant litigation costs and liabilities, which may materially and adversely affect our business and financial position. If we are sued for injuries allegedly caused by XTANDI, MDV3800, MDV9300 or any other of our current or future product candidates, our litigation costs and liability could exceed our total assets and our ability to pay. Regardless of merit or eventual outcome, liability claims may result in:
|
|
• |
decreased demand for XTANDI and any potential future product candidate that we may develop; |
|
|
• |
injury to our reputation; |
|
|
• |
withdrawal of clinical trial participants; |
|
|
• |
significant costs to defend the related litigation; |
|
|
• |
substantial monetary awards to trial participants or patients; |
|
|
• |
loss of revenue; and |
|
|
• |
the inability to commercialize any other products that we may develop. |
In addition, we may from time to time become involved in various lawsuits and legal proceedings which arise in the ordinary course of our business, such as our litigation with the Regents of the University of California, or Regents. See Part II, Item 1, “Legal Proceedings” for additional information on this litigation. Any litigation to which we are subject could require significant involvement of our senior management and may divert management’s attention from our business and operations. Litigation costs or an adverse result in any existing litigation, including our litigation with the Regents for which a decision by the appellate court on the appeal is due within 90 days of the oral argument which occurred on February 23, 2016, or any new litigation that may arise from time to time may adversely impact our operating results or financial condition.
Risks Related to Business Acquisitions, Licenses, Investments and Strategic Alliances
We may not be able to successfully integrate MDV3800, MDV9300 or any other potential future product candidates we may acquire or we may otherwise fail to realize their full potential.
A key component of our business strategy is to diversify our product development risk by identifying and acquiring new product opportunities for development such as our recent acquisition of MDV3800 from BioMarin and our earlier license of MDV9300 from CureTech. However, we cannot ensure that we will be able to manage the risks associated with the transfer of development, regulatory and manufacturing activities for the ongoing clinical trials of MDV3800 from BioMarin to us or manage the risks associated with integrating MDV3800 and MDV9300 into our existing business and infrastructure. We may encounter unexpected difficulties during the transfer, integration or further development of MDV3800 or MDV9300, any of which may cause us to expend greater funds and efforts or may slow, delay or limit the progress of MDV3800’s or MDV9300’s development. Unexpected difficulties may further be disruptive to our ongoing development efforts, put a strain on our existing personnel, infrastructure and business and divert management’s time and attention.
Other companies, such as AstraZeneca, Clovis Oncology, AbbVie, and Tesaro are developing PARP inhibitors for various oncology indications, some of which have already been approved and on the market and others which are further along in development than MDV3800, both of which could limit our development opportunities for MDV3800. The competitive environment could further compromise our ability to successfully enroll the ongoing clinical trial by limiting the availability of clinical trial investigators, sites and/or patients which could slow, delay or limit the progress of MDV3800’s development.
As a result of these or other problems and risks, we may never receive regulatory approval for MDV3800 or MDV9300, we may not realize the full potential or we may never generate significant value or revenues from these assets.
40
Business acquisitions, licenses, investments and strategic alliances could disrupt our operations and may expose us to increased costs and unexpected liabilities.
We may acquire or make investments in other companies, enter into other strategic relationships, or in-license technologies, such as our acquisition of MDV3800 and license of MDV9300. To do so, we may use cash, issue equity that could dilute our current stockholders, or incur debt or assume indebtedness. These transactions involve numerous risks, including but not limited to:
|
|
• |
difficulty integrating acquired technologies, products, operations, and personnel with our existing business; |
|
|
• |
diversion of management’s attention in connection with both negotiating the acquisition or license and integrating the business; |
|
|
• |
strain on managerial and operational resources; |
|
|
• |
difficulty implementing and maintaining effective internal control over financial reporting at businesses that we acquire, particularly if they are not located near our existing operations; |
|
|
• |
exposure to unforeseen liabilities of acquired companies or companies in which we invest; |
|
|
• |
potential costly and time-consuming litigation, including stockholder lawsuits; |
|
|
• |
potential issuance of securities to equity holders of the company being acquired which may have a dilutive effect on our stockholders; |
|
|
• |
the need to incur additional debt or use our existing cash balances; and |
|
|
• |
the requirement to record potentially significant additional future non-cash operating costs for the amortization of intangible assets and potential future impairment or write-down of intangible assets and/or goodwill as well as potentially significant non-cash adjustments to contingent consideration liabilities which would be recorded within operating expenses. |
As a result of these or other problems and risks, businesses we acquire or invest in may not produce the revenues, earnings or business synergies that we anticipated, acquired or licensed technologies may not result in regulatory approvals, and acquired or licensed commercial products may not perform as expected. As a result, we may incur higher costs and realize lower revenues than we had anticipated. We cannot assure you that any acquisitions or investments we have made or may make in the future will be successfully identified and completed or that, if completed, the acquired business, licenses, investments, products, or technologies will generate sufficient revenue to offset the negative costs or other negative effects on our business. Failure to manage effectively our growth through acquisition or in-licensing transactions could adversely affect our growth prospects, business, results of operations, financial condition, and cash flow.
Funding may not be available for us to make acquisitions, investments, strategic alliances or in-license technologies in order to grow our business.
We have made and anticipate that we may continue to engage in strategic transactions to grow our business through acquisitions, investments, strategic alliances or in-licensing of technologies. In particular, we may choose to partner in certain geographies to commercialize our successful product candidates. Our growth plans rely, in part, on the successful identification and completion of these various strategic transactions. At any particular time, we may need to raise substantial additional cash or issue additional equity to finance such transactions, in addition to any amounts available from operations or under our Revolving Credit Facility. There is no assurance that we will be able to secure additional funding on acceptable terms, or at all, or obtain stockholder approvals necessary to issue additional equity to finance such transactions. If we are unsuccessful in obtaining financing, our business would be adversely affected.
Our consolidated financial statements may be impacted in future periods based on the accuracy of our valuations of our acquired businesses and other agreements.
Certain of our asset acquisition or license agreements may be accounted for as a business combination. Accounting for business combinations may involve complex and subjective valuations of the assets and liabilities recorded, and in some instances contingent consideration, which is recorded in our consolidated financial statements pursuant to the standards applicable for business combinations in accordance with accounting principles generally accepted in the United States. Differences between the inputs and assumptions used in the valuations and actual results experienced at a later date could have a material effect on our consolidated financial statements in future periods.
41
We have substantial intangible assets and goodwill as a result of the acquisition of MDV3800 from BioMarin and the license of MDV9300 from CureTech. A significant impairment or write-down of intangible assets and/or goodwill would have a material adverse effect on our consolidated financial statements.
We are required to perform an annual, or in certain situations a more frequent, assessment of intangible assets and goodwill for possible impairment for accounting purposes. We recorded significant intangible assets and goodwill as a result of the CureTech and BioMarin transactions during the years ended December 31, 2014 and 2015, respectively. At December 31, 2015, we had intangible assets and goodwill of approximately $662.9 million, or approximately 46% of our total assets. During the fourth quarter of 2015, we recorded a $30.0 million impairment charge related to our IPR&D asset for MDV9300. If our development activities with respect to IPR&D for acquired product candidates are not successful in future periods, we may be required to incur further non-cash impairment charges that could materially adversely affect our consolidated results of operations or our recorded assets and liabilities.
Risks Related to Ownership of Our Common Stock
Our stock price has been and may continue to be volatile and our stockholders’ investment in our common stock could decline in value.
The market prices for our securities and those of other life sciences companies have been highly volatile and often unrelated or disproportionate to the operating performance of those companies, and may continue to be highly volatile in the future. There has been particular volatility in the market prices of securities of life sciences companies because of problems or successes in a given market segment or because investor interest has shifted to other segments. These broad market and industry factors may cause the market price of our common stock to decline, regardless of our operating performance. We have no control over this volatility and can only focus our efforts on our own operations, and even these may be affected due to the state of the capital markets.
The following factors, in addition to other risk factors described herein, may have a significant impact on the market price of our common stock:
|
|
• |
comments made by securities analysts, including changes in their recommendations; |
|
|
• |
selling by existing stockholders and short-sellers; |
|
|
• |
sales of our common stock by our directors, officers or significant stockholders, including sales effected pursuant to predetermined trading plans adopted under the safe-harbor afforded by Rule 10b5-1; |
|
|
• |
negative opinions that are misleading and/or inaccurate regarding our business, management or future prospects published by certain market participants intent on putting downward pressure on the price of our common stock; |
|
|
• |
lack of volume of stock trading leading to low liquidity; |
|
|
• |
announcements by us of financing transactions and/or future sales of equity or debt securities; |
|
|
• |
the recruitment or departure of key management personnel; |
|
|
• |
our ability to meet the expectations of investors and securities analysts related to collaboration revenue generated from net sales of XTANDI, including the timing and amount thereof, and other financial metrics; |
|
|
• |
changes in our financial guidance or financial estimates by any securities analysts who might cover our company, or our failure to meet our financial guidance or estimates made by securities analysts; |
|
|
• |
variations in our quarterly financial results or those of companies that are perceived to be similar to us; |
|
|
• |
new products, product candidates or uses for existing products or technologies introduced or announced by our competitors and the timing of these introductions and announcements; |
|
|
• |
the progress and results of preclinical studies and clinical trials of our product candidates conducted by us, Astellas or any future collaborative partners or licensees, if any, and any delays in enrolling a sufficient number of patients to complete clinical trials of our product candidates; |
|
|
• |
developments concerning our collaboration with Astellas or any future collaborations; |
|
|
• |
our dependence on third parties, including Astellas, clinical manufacturing organizations and clinical research organizations, clinical trial sponsors and clinical investigators; |
|
|
• |
legislation or regulatory developments in the United States or other countries affecting XTANDI or product candidates, including those of our competitors, including the passage of laws, rules or regulations relating to healthcare and reimbursement or the public announcement of inquiries relating to these subjects; |
|
|
• |
developments or disputes concerning patent applications, issued patents or other proprietary rights; |
|
|
• |
the receipt or failure to receive funding necessary to conduct our business; |
|
|
• |
changes in the market valuations of other companies in our industry; |
42
|
|
• |
general economic, industry and market conditions and other factors that may be unrelated to our operating performance, including market conditions in the pharmaceutical and biotechnology sectors. |
These factors and fluctuations, as well as political and other market conditions, may adversely affect the market price of our common stock. Securities-related class action litigation is often brought against a company following periods of volatility in the market price of its securities. Securities-related litigation, whether with or without merit, could result in substantial costs and divert management’s attention and financial resources, which could harm our business and financial condition, as well as the market price of our common stock. Additionally, volatility or lack of positive performance in our stock price may adversely affect our ability to retain or recruit key employees, all of whom have been or will be granted equity awards as a part of their compensation.
If our operating results are below the expectations of securities analysts, the market price of our common stock could decline.
Various securities analysts follow our financial results and issue reports on us. These reports include information about our historical financial results, our projected development expenses, as well as the analysts’ estimates of our future performance. The analysts’ estimates are based upon their own opinions and are often different from our estimates or expectations. We expect our projected development expenses to increase as we advance our product pipeline and grow our infrastructure. While management believes these expenses are an investment in the Company’s long-term prospects, such expenses could substantially reduce our near-term earnings below the expectations of security analysts. If our operating results are below their expectations, the market value of our common stock could decline, perhaps substantially.
We do not intend to pay cash dividends on our common stock for the foreseeable future.
We do not expect for the foreseeable future to pay cash dividends on our common stock. Any future determination to pay cash dividends on or repurchase shares of our common stock will be at the discretion of our board of directors and will depend upon, among other factors, our success in completing sales or partnerships of our programs, our results of operations, financial condition, capital requirements, contractual restrictions (such as our Revolving Credit Facility) and applicable law.
Provisions of our corporate charter documents, our stockholder rights plan and Delaware law could make it more difficult for a third party to acquire us, even if the offer may be considered beneficial by our stockholders.
Provisions of the Delaware General Corporation Law could discourage potential acquisition proposals and could delay, deter or prevent a change in control. The anti-takeover provisions of the Delaware General Corporation Law impose various impediments to the ability of a third party to acquire control of us, even if a change in control would be beneficial to our existing stockholders. Specifically, Section 203 of the Delaware General Corporation Law, unless its application has been waived, provides certain default anti-takeover protections in connection with transactions between us and an “interested stockholder.” Generally, Section 203 prohibits stockholders who, alone or together with their affiliates and associates, own more than 15% of the subject company from engaging in certain business combinations for a period of three years following the date that the stockholder became an interested stockholder of such subject company without approval of the board or the vote of two-thirds of the shares held by the independent stockholders. Our board of directors has also adopted a stockholder rights plan, or “poison pill,” which would significantly dilute the ownership of a hostile acquirer. Additionally, provisions of our amended and restated certificate of incorporation and bylaws could deter, delay or prevent a third party from acquiring us, even if doing so would benefit our stockholders, including without limitation, the authority of the board of directors to issue, without stockholder approval, preferred stock with such terms as the board of directors may determine.
We may issue additional shares of our common stock or instruments convertible into shares of our common stock, which could cause our stock price to fall and cause dilution to existing stockholders. In addition, a sale of a substantial number of shares of our common stock in the public market could cause the market price of our common stock to decline significantly.
We may from time to time issue additional shares of common stock or other instruments convertible into, or exchangeable or exercisable for, shares of our common stock, including in connection with potential in-licensing and acquisition transactions. The issuance of additional shares of our common stock would dilute the ownership interests of existing holders of our common stock.
The issuance of a substantial number of shares of our common stock, the sale of a substantial number of shares of our common stock that were previously restricted from sale in the public market, or the perception that these issuances or sales might occur, could depress the market price of our common stock. As a result, investors may not be able to sell their shares of our securities at a price equal to or above the price they paid to acquire them.
Furthermore, the issuance of additional shares of our common stock, or the perception that such issuances might occur, could impair our ability to raise capital through the sale of additional equity securities.
43
We rely on Astellas to timely deliver important financial information relating to net sales of XTANDI. In the event that this information is inaccurate, incomplete, or not timely, we may not be able to timely or fully meet our financial reporting obligations as required by the SEC.
Under the Astellas Collaboration Agreement, Astellas has exclusive control over the flow of information relating to net sales of XTANDI that we are dependent upon to meet our SEC reporting obligations. Astellas is required under the Astellas Collaboration Agreement to provide us with timely and accurate financial data related to net sales of XTANDI so that we may meet our reporting obligations under federal securities laws. In the event that Astellas fails to provide us with timely and accurate information, we may incur significant liability with respect to federal securities laws, our internal controls and procedures under the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, may be inadequate, and we could be required to record adjustments in future periods, any of which could adversely affect the market price of our common stock and subject us to securities litigation.
To the extent that we create any joint ventures or have any variable interest entities for which we are required to consolidate, we would need to rely on those entities to timely deliver important financial information to us. In the event that the financial information is inaccurate, incomplete, or not timely, we would not be able to meet our financial reporting obligations as required by the SEC.
While we currently do not have any joint ventures or variable interest entities that we are required to consolidate, to the extent we create such joint ventures or variable interest entities for which we would be required to consolidate and the financial statements of such entities are not prepared by us, we will not have direct control over their financial statement preparation. As a result, we will, for our financial reporting, depend on what these entities report to us, which could result in us adding monitoring and audit processes, which could increase the difficulty of implementing and maintaining adequate controls over our financial processes and reporting in the future. This may be particularly true when such entities do not have sophisticated financial accounting processes in place, or where we are entering into new relationships at a rapid pace, straining our integration capacity. Additionally, if we do not receive the information from the joint venture or variable interest entity on a timely basis, this could cause delays in our external reporting obligations as required by the SEC.
Changes in, or interpretations of, accounting principles could have a significant impact on our financial position and results of operations.
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP. These principles are subject to interpretation by the SEC and various other bodies formed to interpret and create appropriate accounting principles. A change in these principles can have a significant effect on our reported results and may even retroactively affect previously reported transactions.
For example, the Financial Accounting Standards Board, or FASB, is currently working together with the International Accounting Standards Board, or IASB, on several projects to further align accounting principles and facilitate more comparable financial reporting between companies who are required to follow U.S. GAAP under SEC regulations and those who are required to follow International Financial Reporting Standards outside of the United States. These efforts by the FASB and the IASB may result in different accounting principles under U.S. GAAP that may result in materially different financial results for us in areas including, but not limited to, principles for recognizing revenue and lease accounting.
Failure to maintain effective internal control over financial reporting in accordance with the Sarbanes-Oxley Act could have a material adverse effect on our stock price.
Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC require an annual management assessment of the effectiveness of our internal control over financial reporting and a report by our independent registered public accounting firm attesting to the effectiveness of our internal control over financial reporting at the end of the fiscal year. If we fail to maintain the adequacy of our internal control over financial reporting, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective control over financial reporting in accordance with the Sarbanes-Oxley Act and the related rules and regulations of the SEC. If we cannot in the future favorably assess, or our independent registered public accounting firm is unable to provide an unqualified attestation report on, the effectiveness of our internal control over financial reporting, investor confidence in the reliability of our financial reports may be adversely affected, which could have a material adverse effect on our stock price.
None.
44
We currently do not own any properties. Our leased properties are as follows:
|
Approximate Square Feet |
|
Location |
|
Purpose |
|
Term |
|
|
|
|
|
|
|
|
|
143,000 |
|
San Francisco, California |
|
Corporate headquarters |
|
Expiration June 2019 |
|
|
|
|
|
|
|
|
|
44,000 |
|
San Francisco, California |
|
Office and |
|
Expiration August 2024 |
|
|
|
|
|
|
|
|
|
15,000 |
|
Oakbrook Terrace, Illinois |
|
Former commercial |
|
Expiration December |
We believe our properties are adequately maintained and suitable for their intended use. As we continue to expand our operations, we may need to lease additional or alternate facilities.
We are involved in legal proceedings, investigations, and claims in the ordinary course of our business, including the matters described below.
In May 2011, we filed a lawsuit in San Francisco Superior Court against the Regents of the University of California, and one of its professors, alleging breach of contract and fraud claims, among others. Our allegations in this lawsuit include that we have exclusive commercial rights to apalutamide, an investigational drug originally known as ARN-509 (previously also referred to as JNJ-56021927, or JNJ-927), which is currently being developed by Aragon Pharmaceuticals, or Aragon. In August 2013, Johnson & Johnson and Aragon completed a transaction in which Johnson & Johnson acquired all apalutamide assets owned by Aragon. We sought remedies including a declaration that we are the proper licensee of apalutamide, contractual remedies conferring to us exclusive patent license rights regarding apalutamide, and other equitable and monetary relief. On August 7, 2012, the Regents filed a cross-complaint against us seeking declaratory relief that the Regents are entitled to ten percent of any sales milestone payments under the Astellas Collaboration Agreement.
On December 20, 2012, and January 25, 2013, the Court granted summary judgment motions filed by defendants Regents and Aragon, resulting in dismissal of all claims against Regents and Aragon, but denied such motions filed by the remaining Regents professor. On April 15, 2013, we filed a Notice of Appeal seeking appeal of the judgment in favor of Aragon, which is now wholly-owned by Johnson & Johnson. The bench trial of the Regent’s cross-complaint against us was conducted in July 2013, and on January 15, 2014, the Court entered a judgment in the cross-complaint in favor of Regents. We appealed this judgment on February 13, 2014 along with the December 2012 summary judgment order in favor of Regents. The jury trial of our breach of contract and fraud claims against the remaining Regents professor was conducted in October and November 2013. On November 15, 2013, the jury rendered a verdict in the case, finding in our favor on one of the breach of contract claims, and in favor of the Regents professor on the fraud claims. The Company appealed the resulting judgment on the fraud claims. All appeals from this matter were consolidated, and oral arguments were heard on February 23, 2016. A decision of the appellate court is required to be rendered within 90 days of the oral argument.
On April 11, 2014, the Regents filed a complaint against us in which UCLA alleges that the “Operating Profits” we have received (and will continue to receive) from Astellas, as a result of the Astellas Collaboration Agreement, constitute Sublicensing Income under the license agreement between us and the Regents and that we and our subsidiary, Medivation Prostate Therapeutics, Inc., have failed to pay the Regents ten percent of such Operating Profits. We deny the Regents’ allegations and are vigorously defending the litigation. We are currently awaiting a trial date to be set by the Courts.
While we believe we have meritorious positions with respect to the claims above and intend to advance our positions in these lawsuits vigorously, including on appeal, the process of resolving matters through litigation or other means is inherently uncertain, and it is not possible to predict the ultimate resolution of any such proceeding. The actual costs of defending our position may be significant, and we may not prevail.
None.
45
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information
Our common stock, par value $0.01, is listed on the NASDAQ Global Select Market under the symbol “MDVN.” The following table sets forth on a per share basis the high and low intraday sales prices of our common stock as reported by the NASDAQ Global Select Market:
|
|
|
High (1) |
|
|
Low (1) |
|
||
|
2015: |
|
|
|
|
|
|
|
|
|
Quarter ended March 31, 2015 |
|
$ |
70.79 |
|
|
$ |
48.80 |
|
|
Quarter ended June 30, 2015 |
|
$ |
67.67 |
|
|
$ |
54.93 |
|
|
Quarter ended September 30, 2015 |
|
$ |
58.08 |
|
|
$ |
38.08 |
|
|
Quarter ended December 31, 2015 |
|
$ |
48.77 |
|
|
$ |
37.63 |
|
|
2014: |
|
|
|
|
|
|
|
|
|
Quarter ended March 31, 2014 |
|
$ |
44.10 |
|
|
$ |
30.31 |
|
|
Quarter ended June 30, 2014 |
|
$ |
39.18 |
|
|
$ |
27.19 |
|
|
Quarter ended September 30, 2014 |
|
$ |
51.94 |
|
|
$ |
34.56 |
|
|
Quarter ended December 31, 2014 |
|
$ |
58.62 |
|
|
$ |
43.24 |
|
|
(1) |
All sales prices have been retroactively adjusted to reflect our September 15, 2015, two-for-one forward stock split effected through a stock dividend. |
Stockholders
As of the close of business on February 16, 2016, there were 18 stockholders of record of our common stock. The number of stockholders of record is based upon the actual number of stockholders registered at such date and does not include holders of shares in “street names” or persons, partnerships, associates, or corporations, or other entities identified in security listings maintained by depositories.
Dividends
We have never paid our stockholders cash dividends and we do not anticipate paying any cash dividends in the foreseeable future as we intend to retain all of our cash for use in our business. Any future determination to pay dividends will be at the discretion of our Board of Directors, and will depend on a number of factors, including but not limited to any contractual restrictions on paying dividends, outstanding indebtedness, earnings, capital requirements, financial condition and future prospects, and applicable Delaware law. Our Revolving Credit Facility imposes contractual restrictions on us with respect to paying cash dividends.
46
We have presented below the cumulative total return to our stockholders during the period from December 31, 2010 through December 31, 2015, in comparison to the cumulative total returns of the NASDAQ Composite Index and the NASDAQ Biotechnology Index. All values assume a $100 initial investment and the reinvestment of the full amount of all dividends and are calculated as of the last stock trading day of each year. The comparisons are based on historical data and are not indicative of, nor intended to forecast, the future performance of our common stock.
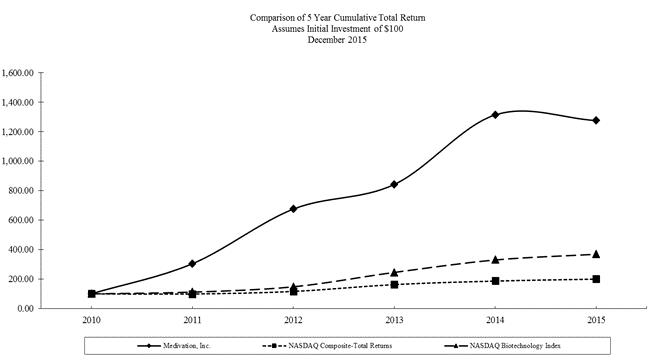
|
|
|
2010 |
|
|
2011 |
|
|
2012 |
|
|
2013 |
|
|
2014 |
|
|
2015 |
|
||||||
|
Medivation, Inc. |
|
$ |
100.00 |
|
|
$ |
304.22 |
|
|
$ |
674.93 |
|
|
$ |
841.95 |
|
|
$ |
1,314.25 |
|
|
$ |
1,275.46 |
|
|
NASDAQ Composite Index |
|
$ |
100.00 |
|
|
$ |
99.17 |
|
|
$ |
116.48 |
|
|
$ |
163.21 |
|
|
$ |
187.27 |
|
|
$ |
200.31 |
|
|
NASDAQ Biotechnology Index |
|
$ |
100.00 |
|
|
$ |
112.07 |
|
|
$ |
148.26 |
|
|
$ |
246.14 |
|
|
$ |
330.83 |
|
|
$ |
369.75 |
|
The information under “Performance Graph” is not deemed to be “soliciting material” or “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference in any filing of Medivation under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K and irrespective of any general incorporation language in those filings.
Recent Sales of Unregistered Securities
None.
Repurchases of Equity Securities
None.
47
The following is a summary of our historical consolidated financial data for the years ended and on the dates indicated below. The historical consolidated financial data for the years ended December 31, 2015, 2014 and 2013 and as of December 31, 2015 and 2014 have been derived from our audited consolidated financial statements included in this Annual Report on Form 10-K, or Annual Report. The historical financial data for the years ended December 31, 2012 and 2011 and as of December 31, 2013, 2012 and 2011 have been derived from our audited consolidated financial statements not included in this Annual Report. The information below is not necessarily indicative of results of future operations, and should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the consolidated financial statements and related notes thereto included in this Annual Report to fully understand factors that may affect the comparability of the information presented below.
|
|
|
Years Ended December 31, |
|
|||||||||||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
2012 |
|
|
2011 |
|
|||||
|
|
|
(in thousands, except per share data) |
|
|||||||||||||||||
|
Consolidated Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration revenue |
|
$ |
943,258 |
|
|
$ |
710,487 |
|
|
$ |
272,942 |
|
|
$ |
181,696 |
|
|
$ |
60,389 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
232,100 |
|
|
|
189,570 |
|
|
|
118,952 |
|
|
|
95,628 |
|
|
|
73,432 |
|
|
Selling, general and administrative expenses |
|
|
296,545 |
|
|
|
239,071 |
|
|
|
176,231 |
|
|
|
112,282 |
|
|
|
29,887 |
|
|
Total operating expenses |
|
|
528,645 |
|
|
|
428,641 |
|
|
|
295,183 |
|
|
|
207,910 |
|
|
|
103,319 |
|
|
Income (loss) from operations |
|
|
414,613 |
|
|
|
281,846 |
|
|
|
(22,241 |
) |
|
|
(26,214 |
) |
|
|
(42,930 |
) |
|
Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss on extinguishment of convertible debt |
|
|
(21,087 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Interest expense |
|
|
(12,483 |
) |
|
|
(21,690 |
) |
|
|
(20,249 |
) |
|
|
(14,985 |
) |
|
|
— |
|
|
Other, net |
|
|
275 |
|
|
|
38 |
|
|
|
(8 |
) |
|
|
(51 |
) |
|
|
(242 |
) |
|
Total other income (expense), net |
|
|
(33,295 |
) |
|
|
(21,652 |
) |
|
|
(20,257 |
) |
|
|
(15,036 |
) |
|
|
(242 |
) |
|
Income (loss) before income tax benefit (expense) |
|
|
381,318 |
|
|
|
260,194 |
|
|
|
(42,498 |
) |
|
|
(41,250 |
) |
|
|
(43,172 |
) |
|
Income tax benefit (expense) |
|
|
(136,593 |
) |
|
|
16,258 |
|
|
|
(115 |
) |
|
|
(7 |
) |
|
|
4,331 |
|
|
Net income (loss) |
|
$ |
244,725 |
|
|
$ |
276,452 |
|
|
$ |
(42,613 |
) |
|
$ |
(41,257 |
) |
|
$ |
(38,841 |
) |
|
Basic net income (loss) per common share(1) |
|
$ |
1.53 |
|
|
$ |
1.80 |
|
|
$ |
(0.28 |
) |
|
$ |
(0.28 |
) |
|
$ |
(0.28 |
) |
|
Diluted net income (loss) per common share(1) |
|
$ |
1.47 |
|
|
$ |
1.71 |
|
|
$ |
(0.28 |
) |
|
$ |
(0.28 |
) |
|
$ |
(0.28 |
) |
|
Weighted-average common shares used in the calculation of basic net income (loss) per common share(1) |
|
|
160,345 |
|
|
|
153,859 |
|
|
|
150,331 |
|
|
|
146,949 |
|
|
|
139,842 |
|
|
Weighted-average common shares used in the calculation of diluted net income (loss) per common share(1) |
|
|
169,324 |
|
|
|
170,001 |
|
|
|
150,331 |
|
|
|
146,949 |
|
|
|
139,842 |
|
|
|
|
December 31, |
|
|||||||||||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
2012 |
|
|
2011 |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and short-term investments |
|
$ |
225,853 |
|
|
$ |
502,677 |
|
|
$ |
228,788 |
|
|
$ |
296,240 |
|
|
$ |
145,132 |
|
|
Working capital |
|
$ |
368,115 |
|
|
$ |
602,216 |
|
|
$ |
258,290 |
|
|
$ |
259,330 |
|
|
$ |
78,555 |
|
|
Total assets |
|
$ |
1,431,591 |
|
|
$ |
911,619 |
|
|
$ |
392,650 |
|
|
$ |
371,866 |
|
|
$ |
175,117 |
|
|
Convertible Notes, net of unamortized discount |
|
|
— |
|
|
$ |
222,140 |
|
|
$ |
208,414 |
|
|
$ |
196,007 |
|
|
|
— |
|
|
Retained earnings (accumulated deficit) |
|
$ |
186,199 |
|
|
$ |
(57,709 |
) |
|
$ |
(334,161 |
) |
|
$ |
(291,548 |
) |
|
$ |
(250,291 |
) |
|
Total stockholders’ equity |
|
$ |
872,679 |
|
|
$ |
449,299 |
|
|
$ |
76,947 |
|
|
$ |
73,645 |
|
|
$ |
1,321 |
|
|
(1) |
All share and per share amounts have been retroactively adjusted to reflect our September 15, 2015, two-for-one forward stock split effected through a stock dividend, which resulted in the issuance of approximately 81.7 million shares of our common stock, and our September 21, 2012, two-for-one forward stock split, which resulted in the increase of approximately 37.0 million shares of our outstanding common stock. |
48
The following discussion and analysis should be read in conjunction with our audited consolidated financial statements and notes thereto for the year ended December 31, 2015, included elsewhere in this Annual Report on Form 10-K, or Annual Report. The following discussion and analysis contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. We intend that these forward-looking statements be subject to the safe harbors created by those provisions. Please see the section entitled “Forward-Looking Statements” at the beginning of this Annual Report for important information you should know regarding these forward-looking statements and how to identify them, including cautionary language regarding undue reliance on these forward-looking statements. We disclaim any intention or obligation to update, supplement or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
OVERVIEW
We are a biopharmaceutical company focused on the development and commercialization of medically innovative therapies to treat serious diseases for which there are limited treatment options. We have one commercial product, XTANDI® (enzalutamide) capsules, or XTANDI, through our collaboration with Astellas Pharma, Inc., or Astellas. XTANDI has received marketing approval in the United States, Europe and numerous other countries worldwide for the treatment of patients with metastatic castration-resistant prostate cancer, or mCRPC, and in Japan for the treatment of patients with castration-resistant prostate cancer, or CRPC. We and Astellas are also conducting investigational studies of enzalutamide in prostate cancer, advanced breast cancer, and hepatocellular carcinoma. Under our collaboration agreement with Astellas, we share equally with Astellas all profits (losses) related to U.S. net sales of XTANDI. We also receive royalties ranging from the low teens to the low twenties as a percentage of ex-U.S. XTANDI net sales. The collaboration also involved certain milestone payments from Astellas to us upon the achievement of defined development, regulatory and sales events, all of which have been achieved as of December 31, 2015.
We seek to become a more fully-integrated biopharmaceutical company through the continued commercialization of XTANDI, the acquisition or in-license and development and commercialization of other product opportunities, and through the advancement of our own proprietary research and development programs. We expect that our future growth may come from both internal research efforts and third-party business development activities. In the fourth quarter of 2015, we acquired all worldwide rights to talazoparib (which we refer to as MDV3800), an orally available poly-ADP ribose polymerase, or PARP, inhibitor, from BioMarin Pharmaceutical Inc., or BioMarin. MDV3800 is currently in a Phase 3 clinical trial for the treatment of patients with germline BRCA, or gBRCA, mutated advanced breast cancer (i.e., advanced breast cancer in patients whose BRCA genes contain germline mutations). We are targeting a number of other indications in which to investigate MDV3800, including breast cancer (beyond gBRCA mutations), prostate cancer, small cell lung cancer, and ovarian cancer. In the fourth quarter of 2014, we licensed exclusive worldwide rights to pidilizumab (which we refer to as MDV9300), an antibody with immune-mediated anti-tumor effects for all potential indications from CureTech, Ltd., or CureTech. Under the license agreement, we are responsible for all development, regulatory, manufacturing, and commercialization activities for MDV9300. We initiated a Phase 2 clinical trial evaluating MDV9300 in patients with relapsed or refractory diffuse large B-cell lymphoma in the fourth quarter of 2015, which is on partial clinical hold pending our revision of certain investigator brochure, protocols and informed consent documents. We submitted the revised documents to the FDA in early February 2016 and the FDA has 30 days thereafter to notify us if the partial hold is lifted. We also plan to develop MDV9300 in other hematologic malignancies such as multiple myeloma.
In addition to the above activities, we have various internal research and discovery efforts focused in oncology, neurology and other areas. Additional information regarding our business is included in Part I, Item 1, “Business,” included elsewhere in this Annual Report. Business and financial highlights for the year ended December 31, 2015 are included below in the sections entitled, “2015 Clinical and Business Highlights” and “2015 Financial Highlights,” respectively.
OUTLOOK 2016
In 2016, our key objectives are to increase the commercial potential of XTANDI and advance our pipeline.
The largest opportunity for XTANDI lies in earlier stage prostate cancer, which is primarily treated by urologists. In late 2015, we expanded our sales force and created separate urology- and oncology-focused teams. This, along with the potential inclusion of data from STRIVE and TERRAIN into the relevant clinical trials section of our XTANDI label within the current mCRPC indication, which may occur as early as the second half of 2016, may help us capture the next wave of growth for XTANDI. We are sensitive, however, to conditions that may affect XTANDI’s growth, such as pricing pressure from third party payors and advocacy groups to reduce healthcare costs in the U.S. and abroad.
49
From a research and development perspective, we plan to continue to invest in our ongoing clinical studies to cover the continuum of prostate cancer care, advance our development of enzalutamide for the treatment of advanced breast cancer, fully enroll our gBRCA mutated advanced breast cancer clinical trial for MDV3800, advance MDV9300 in DLBCL and prepare for other hematologic indications such as multiple myeloma, and further the development and growth of our internal pipeline. Furthermore, we also plan to initiate start-up activities for a number of phase 2 and potentially pivotal clinical trials to develop MDV3800 beyond gBRCA mutations. As a result, we expect that our research and development and selling, general and administrative expenses will notably increase above the levels recorded in 2015.
2015 CLINICAL AND BUSINESS HIGHLIGHTS
The following summarizes our recent clinical and business highlights:
|
|
• |
In December 2015, we expanded our XTANDI U.S. sales force and created separate urology- and oncology-focused teams. We believe this structure will afford us greater ability to reach and detail the respective physician audiences more effectively. |
|
|
• |
During 2015, we presented updated data from our Phase 2 study evaluating the investigational use of enzalutamide as a single agent for the treatment of advanced androgen receptor, or AR, positive, triple-negative breast cancer, or TNBC. An exploratory analysis of overall survival data collected as of September 15, 2015 demonstrated that patients treated with enzalutamide whose tumors tested positive according to a novel diagnostic assay experienced a 13.8-month longer median overall survival duration compared to those patients whose tumor tested negative for the novel gene expression profile. Median overall survival in the diagnostic positive group treated with enzalutamide was 21.3 months (95% CI: 12.9, 21.3) compared with 7.5 (95% CI: 4.8, 11.2) months for the diagnostic negative group treated with enzalutamide. Multicovariate analyses demonstrated that only diagnostic status (positive) and number of prior lines of therapy (0-1) were statistically significantly and independently associated with both improved PFS and overall survival outcomes. Additional variables included in the multi-covariate analyses were age (≥65 vs. < 65 years), disease free interval between primary and metastatic disease (≥12 months vs. <12 months), AR immunohistochemistry (≥10% vs. <10%). The most common (reported in ³10% of patients) adverse events reported as related to enzalutamide treatment were fatigue (34%), nausea (25%), decreased appetite (13%), diarrhea (10%), and hot flush (10%). |
Additionally, we entered into a collaboration agreement with NanoString Technologies, Inc. and our partner Astellas, in January 2016, to translate our gene expression profile into a companion diagnostic assay using NanoString’s nCounter® Dx Analysis System for potential use as a companion diagnostic test for enzalutamide for TNBC.
|
|
• |
In October 2015, we completed an acquisition of all rights to MDV3800 from BioMarin pursuant to an asset purchase agreement. The acquired MDV3800 assets include all patents, data, know-how, third-party agreements, regulatory materials and pre-commercial inventories. We also assumed certain costs for ongoing clinical trials of MDV3800, and commitments under certain agreements previously entered into or assumed by BioMarin and assigned to us. In connection with the transaction, we paid BioMarin an upfront cash payment of $410.0 million in the fourth quarter of 2015. BioMarin is eligible to receive up to an additional $160.0 million upon the achievement of defined regulatory and sales-based milestones and mid-single digit royalties on net sales of products that contain MDV3800 during the royalty term specified in the agreement. The parties entered into a transition services agreement at the closing of the transaction to facilitate the transition of the research and development activities relating to MDV3800 from BioMarin to us, including responsibility for the ongoing clinical studies. |
|
|
• |
In September 2015, we entered into a Credit Agreement, or the Original Credit Agreement, with JPMorgan Chase Bank, N.A., which provided for (i) a one-year $75.0 million revolving loan facility and (ii) an uncommitted accordion facility subject to the satisfaction of certain conditions. We executed a borrowing of $75.0 million under this facility in September 2015. In October 2015, we entered into an amendment and restatement of the Original Credit Agreement, or the Credit Agreement, which provides for (i) a five-year $300.0 million revolving loan facility, or the Revolving Credit Facility, and (ii) an uncommitted accordion facility subject to the satisfaction of certain conditions. In October 2015, we borrowed $75.0 million under the Revolving Credit Facility, which was used to repay the $75.0 million outstanding at September 30, 2015 under the Original Credit Agreement. In January 2016, we repaid the $75.0 million outstanding under the Revolving Credit Facility. Additional information is included in Note 10, “Debt,” to our consolidated financial statements included elsewhere in this Annual Report. |
|
|
• |
In July 2015, the U.S. Food and Drug Administration, or FDA, approved a label update for XTANDI based on an updated overall survival analysis of the Phase 3 PREVAIL trial. This analysis was conducted when 784 deaths were observed and found an overall survival benefit with a 23% reduction in risk of death (Hazard ratio = 0.77; 95% CI: 0.67, 0.88) and a 4-month improvement in median survival with enzalutamide (35.3 months [95% CI: 32.2, not yet reached]) over placebo (31.3 months [95% CI: 28.8, 34.2]). |
50
|
|
• |
In January 2015, we and Astellas reported top-line results from the Phase 2 TERRAIN trial. The trial achieved its primary endpoint demonstrating a statistically significant increase in PFS, defined as time from randomization to centrally determined radiographic progression, skeletal-related event, initiation of new anti-neoplastic therapy or death, whichever occurred first, for patients with metastatic CRPC for enzalutamide compared to bicalutamide (Hazard Ratio = 0.44; 95% CI: 0.34, 0.57; p < 0.0001). Median PFS was 15.7 months in the enzalutamide group compared to 5.8 months in the bicalutamide group. The median time on treatment in the TERRAIN trial was 11.7 months in the enzalutamide group versus 5.8 months in the bicalutamide group. Serious adverse events were reported in 31.1% of enzalutamide-treated patients and 23.3% of bicalutamide-treated patients. Grade 3 or higher cardiac adverse events were reported in 5.5% of enzalutamide-treated patients versus 2.1% of bicalutamide-treated patients. Two seizures were reported in the enzalutamide group and one in the bicalutamide group. |
2015 FINANCIAL HIGHLIGHTS
|
|
• |
Net sales of XTANDI as reported by Astellas and collaboration revenue related to net sales of XTANDI are summarized in the table below (dollars in millions): |
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Worldwide net sales of XTANDI (as reported by Astellas) |
|
$ |
1,908 |
|
|
$ |
1,061 |
|
|
Increase year-over-year |
|
$ |
847 |
|
|
|
|
|
|
Percentage change |
|
|
80 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. XTANDI net sales (as reported by Astellas) |
|
$ |
1,151 |
|
|
$ |
680 |
|
|
Increase year-over-year |
|
$ |
471 |
|
|
|
|
|
|
Percentage change |
|
|
69 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ex-U.S. XTANDI net sales (as reported by Astellas) |
|
$ |
757 |
|
|
$ |
381 |
|
|
Increase year-over-year |
|
$ |
376 |
|
|
|
|
|
|
Percentage change |
|
|
99 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration revenue |
|
$ |
943 |
|
|
$ |
710 |
|
|
Increase year-over-year |
|
$ |
233 |
|
|
|
|
|
|
Percentage change |
|
|
33 |
% |
|
|
|
|
|
|
• |
Milestone payments earned under the Astellas Collaboration Agreement were $245.0 million for the year ended December 31, 2015. During the third quarter of 2015, we earned a $70.0 million sales milestone payment triggered by the achievement of $1.2 billion in worldwide XTANDI net sales, as reported by Astellas. During the fourth quarter of 2015, we earned a $175.0 million sales milestone payment triggered by the achievement of $1.6 billion in worldwide XTANDI net sales, as reported by Astellas. As of December 31, 2015, we have earned all development and sales milestone payments under the Astellas Collaboration Agreement. |
|
|
• |
Total operating expenses for the year ended December 31, 2015 were $528.6 million, an increase of $100.0 million, or 23%, from the year ended December 31, 2014. Operating expenses included non-cash stock-based compensation expense of $54.9 million and $45.1 million for the years ended December 31, 2015 and 2014, respectively. Operating expenses for the year ended December 31, 2015 also included non-cash expense of $30.0 million resulting from an impairment charge related to our intangible assets. |
51
|
|
in this Annual Report. At December 31, 2015, the collaboration receivable from Astellas was $391.6 million, all of which was received in the first quarter of 2016. |
CHANGES TO CAPITAL STRUCTURE
|
|
• |
On June 15, 2015, we filed a Certificate of Amendment to our Amended and Restated Certificate of Incorporation, as amended, effecting an increase in the total number of authorized shares of our capital stock from 171,000,000 to 341,000,000 and an increase in the total number of shares of authorized shares of our common stock from 170,000,000 to 340,000,000. |
|
|
• |
On September 15, 2015, we effected a two-for-one forward stock split of our common stock in the form of a stock dividend. Stockholders of record as of August 13, 2015, received one additional share of our common stock, par value $0.01, for each share they held as of the record date. We issued approximately 81.7 million shares of our common stock as a result of the stock dividend. The par value of our common stock remained unchanged at $0.01 per share. |
|
|
• |
During the year ended December 31, 2015, we settled $258.8 million aggregate principal amount of our 2.625% convertible senior notes due April 1, 2017, or the Convertible Notes, through a combination of $259.9 million in cash and 5,638,576 shares of our common stock. We recorded a non-cash loss on extinguishment of the Convertible Notes of $21.1 million for the year ended December 31, 2015. Upon settlement, the Convertible Notes were no longer outstanding, interest ceased to accrue thereon, and all rights of the holders of the Convertible Notes ceased to exist. |
CRITICAL ACCOUNTING POLICIES AND THE USE OF ESTIMATES
The preparation of our consolidated financial statements and related footnotes requires us to make estimates, assumptions and judgments in certain circumstances that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We have based our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We have discussed the development, selection and disclosure of these estimates with the Audit Committee of our Board of Directors. Actual results could differ materially from these estimates under different assumptions or conditions.
An accounting policy is considered to be critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time the estimate is made, and if different estimates that reasonably could have been used, or changes in the accounting estimates that are reasonably likely to occur periodically, could materially impact the consolidated financial statements. We believe that certain of our accounting policies are critical because they are the most important to the preparation of our consolidated financial statements. These policies require our most subjective and complex judgments, often requiring the use of estimates about the effects of matters that are inherently uncertain. Once adopted, we consistently apply our critical accounting policies.
A detailed description of our significant accounting policies is included in the footnotes to our audited consolidated financial statements included elsewhere in this Annual Report. A subsection of these accounting policies identified by management as “Critical Accounting Policies” are included below.
Collaboration Agreements
Estimated Performance Periods
Our collaboration agreements contain multiple elements and deliverables. We evaluated the facts and circumstances of the Astellas Collaboration Agreement and concluded that we had multiple deliverables, including deliverables relating to grants of technology licenses, and performance of manufacturing, regulatory and clinical development activities in the United States. Under the Astellas Collaboration Agreement, the period in which we performed our deliverables began in the fourth quarter of 2009 and concluded during the third quarter of 2015 upon completion of our remaining performance obligations. We also concluded that our deliverables under the Astellas collaboration should be accounted for as a single unit.
Estimation of the performance periods of our deliverables requires the use of our management’s judgment. Significant factors considered in management’s evaluation of the estimated performance periods include, but are not limited to, our experience, along with our collaboration partner’s experience, in conducting manufacturing, clinical development and regulatory activities. We review the estimated duration of our performance period under our collaboration agreements on a quarterly basis and make any appropriate adjustments on a prospective basis.
Collaboration Agreement Payments
We account for the various payment flows under the Astellas Collaboration Agreement in a consistent manner, as follows:
Upfront Payments. We received a non-refundable, upfront cash payment of $110.0 million pursuant to the Astellas Collaboration Agreement. We recognized the payment as collaboration revenue on a straight-line basis over the applicable estimated
52
performance period. As of December 31, 2015, there was no deferred revenue related to payments received under the Astellas Collaboration Agreement.
Milestone Payments. We have been eligible to receive milestone payments under the Astellas Collaboration Agreement based on achievement of specified development, regulatory and commercial events. We evaluated the nature of the events triggering these contingent payments, and concluded that these events fall into two categories: (a) events which involve the performance of our obligations under the Astellas Collaboration Agreement, and (b) events which do not involve the performance of our obligations under the Astellas Collaboration Agreement.
The former category of milestone payments consists of those triggered by development and regulatory activities in the United States and by the acceptance for review of marketing applications in Europe and Japan. We concluded that each of these payments, with one exception, constitute substantive milestone payments. This conclusion was based primarily on the facts that (i) each triggering event represented a specific outcome that could be achieved only through successful performance by us of one or more of our deliverables, (ii) achievement of each triggering event was subject to inherent risk and uncertainty and would result in additional payments becoming due to us, (iii) each of the milestone payments was non-refundable, (iv) substantial effort was required to complete each milestone, (v) the amount of each milestone payment was reasonable in relation to the value created in achieving the milestone, (vi) a substantial amount of time was expected to pass between the upfront payment and the potential milestone payments, and (vii) the milestone payments related solely to past performance. Based on the foregoing, we recognized any revenue from these milestone payments in the period in which the underlying triggering event occurs. The one exception is the milestone payment for initiation of the Phase 3 PREVAIL trial, an event which we deemed to be reasonably assured at the inception of the Astellas collaboration. This milestone payment was triggered in the third quarter of 2010, and we recognized it as collaboration revenue on a straight-line basis over the estimated remaining performance period of the Astellas Collaboration Agreement.
The latter category of milestone payments consist of those triggered by potential marketing approvals in Europe and Japan, and commercial activities globally, all of which were areas in which we had no pertinent contractual responsibilities under the Astellas Collaboration Agreement. We concluded that these payments constitute contingent revenues and thus recognized them as revenue in the period in which the contingency was met.
As of December 31, 2015, we have earned all development and sales milestone payments under the Astellas Collaboration Agreement.
Royalties and Profit (Loss) Sharing Payments. Under the Astellas Collaboration Agreement, we share equally profit (losses) on sales of products in the United States and we are eligible to receive royalties on sales of products outside the United States. We consider these payments to be contingent revenues, and we recognize them as revenue in the period in which the applicable contingency is resolved.
Cost-Sharing Payments. Under the Astellas Collaboration Agreement, we and Astellas share certain development and commercialization costs in the United States, including cost of goods sold and the royalty on net sales payable to The Regents of the University of California, or UCLA (or the Regents), under our license agreement with UCLA. We and Astellas make quarterly cost-sharing payments to one another in amounts necessary to ensure that each party bears its contractual share of the overall shared U.S. development and commercialization costs incurred. Our policy is to account for cost-sharing payments to our collaboration partners as increases in expense in our consolidated statements of operations, while cost-sharing payments by our collaboration partners to us are accounted for as reductions in expense. Cost-sharing payments related to development activities and commercialization activities are recorded in research and development expenses, or R&D expenses, and selling, general and administrative expenses, or SG&A expenses, respectively.
Reliance on Third-Party Information. Under the Astellas Collaboration Agreement, Astellas records all XTANDI sales globally and has operational responsibility for certain development and commercialization activities in the United States for which we share costs. Thus, Astellas has control over certain XTANDI-related financial information needed to prepare our financial statements and related disclosures, including information regarding gross sales, net sales, gross-to-net sales deductions, including estimates of potential future product returns, and shared U.S. development and commercialization costs incurred by Astellas. We are dependent on Astellas to provide us with such information in a timely and accurate manner for use in preparing our consolidated financial statements and related disclosures. Certain of this information provided by Astellas is subject to estimates, including estimates used in determining gross-to-net revenue deductions such as payor mix, discounts (including legally mandated discounts to government entities), returns, chargebacks, rebates, and participation levels in patient assistance programs, and estimates regarding accrued development and commercialization costs incurred by Astellas. Under the Astellas Collaboration Agreement, the deductions from gross sales used to derive net sales of XTANDI are determined in a manner consistent with GAAP, consistently applied. Should Astellas fail to provide us with any such financial information in a timely manner, or should any such financial information provided by Astellas, or any of the estimates upon which such financial information was based, prove to be inaccurate, we could be required to record adjustments in future periods.
53
Research and Development Expense and Accruals
R&D expenses include personnel and facility-related expenses, outside contracted services including clinical trial costs, manufacturing and process development costs, research costs, upfront and development milestone payments under license agreements, and other consulting services. Research and development costs are expensed as incurred unless there is an alternative future use in other research and development projects. Non-refundable advance payment for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when payment has been made. In instances where we enter into agreements with third parties to provide research and development services to us, costs are expensed as services are performed. Amounts due under such arrangements may be either fixed fee or fee for service, and may include upfront payments, monthly payments, and payments upon the completion of milestones or receipt of deliverables.
Our accruals for clinical trials and other research and development activities are based on estimates of the services received and efforts expended pursuant to contracts with numerous clinical trial centers, contract research organizations, and clinical manufacturing organizations. In the normal course of business we contract with third parties to perform various research and development activities in the on-going development of our product candidates, including without limitation, third-party clinical trial centers and contract research organizations that perform and administer our clinical trials on our behalf and clinical manufacturing organizations that manufacture clinical trial materials. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under these agreements depend on factors such as the achievement of certain events, the successful enrollment of patients, and the completion of portions of the clinical trial or similar conditions. The objective of our accrual policy is to match the recording of expenses in our consolidated financial statements to the actual services received and efforts expended. As such, expense accruals related to clinical trials and other research and development activities are recognized based on our estimate of the degree of completion of the event or events specified in the specific agreement.
Our accrual estimates are dependent upon the timeliness and accuracy of data provided by third parties regarding the status and cost of studies, and may not match the actual services performed by these organizations. During the course of a clinical trial, we adjust our rate of clinical trial expense recognition if actual results differ from our estimates. We make estimates of our accrued clinical trial expense as of each balance sheet date based on facts and circumstances known at that time. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and result in us reporting amounts that are too high or too low for any particular period. This could result in adjustment to our R&D expense in future periods. We have had no significant adjustments to previously recorded amounts.
Stock-Based Compensation
We have outstanding stock options, restricted stock units, and stock appreciation rights pursuant to our Amended and Restated 2004 Equity Incentive Award Plan, or the Medivation Equity Incentive Plan, and eligible employees may purchase shares pursuant to the Medivation, Inc. 2013 Employee Stock Purchase Plan, or ESPP.
Stock-based compensation expense associated with stock options is based on the estimated grant date fair value using the Black-Scholes valuation model, which requires the use of subjective assumptions related to the expected stock price volatility, option term, risk-free interest rate and dividend yield. We recognize compensation expense over the vesting period of the awards that are ultimately expected to vest.
Stock-based compensation expense associated with restricted stock units is based on the fair value of our common stock on the grant date, which equals the closing market price of our common stock on the grant date. For restricted stock units, we recognize compensation expense over the vesting period of the awards that are ultimately expected to vest.
The fair value of stock-settled and cash-settled stock appreciation rights is initially measured on the grant date using the Black-Scholes valuation model, which requires the use of subjective assumptions related to the expected stock price volatility, term, risk-free interest rate and dividend yield. Similar to stock options, compensation expense for stock-settled stock appreciation rights is recognized over the vesting period of the awards that are ultimately expected to vest based on the grant-date fair value. Cash-settled stock appreciation rights are liability-classified awards for which compensation expense and the associated liability are remeasured at each reporting date through the date of settlement based on the portion of the requisite service period rendered. Upon the conversion of cash-settled stock appreciation rights to stock-settled stock appreciation rights, the awards are remeasured using the then-current Black-Scholes assumptions and the remeasured liability is reclassified to additional paid-in capital.
Equity awards to consultants are typically remeasured at fair value at each reporting date until the awards vest.
We account for the ESPP as a compensatory plan. The fair value of each purchase under the ESPP is estimated on the date of the beginning of the offering period using the Black-Scholes valuation model, which requires the use of subjective assumptions related to the expected stock price volatility, term, risk-free interest rate and dividend yield. We recognize compensation expense over the vesting period of the awards that are ultimately expected to vest.
54
Determining an estimate of the fair value of equity awards using the Black-Scholes valuation model requires the use of highly subjective assumptions related to expected stock price volatility, term, risk-free interest rate and dividend yield. The Black-Scholes valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable; characteristics not present in the our stock options, stock appreciation rights, or ESPP shares. If the model permitted consideration of the unique characteristics of employee stock options, stock appreciation rights, and ESPP shares, the resulting estimate of fair value of the stock options, stock appreciation rights, and ESPP shares could be different. In addition, if we had made different assumptions and estimates for use in the Black-Scholes valuation model, the amount of recognized and to be recognized stock-based compensation expense could have been different. Please refer to the footnotes to the audited consolidated financial statements included elsewhere in this Annual Report for further information about the assumptions made by us and the impact of such assumptions.
We apply a forfeiture rate when determining stock-based compensation expense to account for an estimate of the granted awards not expected to vest. If actual forfeitures differ from the expected rate, we may be required to make additional adjustments to compensation expense in future periods.
Leases
We use a consistent lease period (generally, the initial non-cancellable lease term plus renewal option periods provided for in the lease that can be reasonably assured) when calculating amortization of leasehold improvements and in determining straight-line rent expense and classification of a lease as either an operating lease or capital lease. The lease term and straight-line rent expense commence on the date when we take possession and have the right to control the use of the leased premises. Funds received from the lessor intended to reimburse us for the cost of leasehold improvements are recorded as deferred rent resulting from a lease incentive and are amortized over the lease term as a reduction to rent expense.
Build-to-Suit Lease Accounting
In certain lease arrangements, we are involved in the construction of the building. To the extent we are involved with structural improvements of the construction project or take construction risk prior to the commencement of a lease, Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, 840-40, “Leases – Sale-Leaseback Transactions (Subsection 05-5)”, requires us to be considered the owner for accounting purposes of these types of projects during the construction period. Therefore, we record an asset in property and equipment, net on the consolidated balance sheets, including capitalized interest costs, for the replacement cost of our portion of the pre-existing building plus the amount of estimated structural construction costs incurred by the landlord and us as of the balance sheet date. We record a corresponding build-to-suit lease obligation on our consolidated balance sheets representing the amounts paid by the lessor.
Once construction is complete, we consider the requirements for sale-leaseback accounting treatment, including evaluating whether all risks of ownership have been transferred back to the landlord, as evidenced by a lack of continuing involvement in the leased property. If the arrangement does not qualify for sale-leaseback accounting treatment, the building asset remains on our consolidated balance sheets at its historical cost, and such asset is depreciated over its estimated useful life. We bifurcate our lease payments into a portion allocated to the building, and a portion allocated to the parcel of land on which the building has been built. The portion of the lease payments allocated to the land are treated for accounting purposes as operating lease payments, and therefore recorded as rent expense in the consolidated statements of operations. The portion of the lease payments allocated to the building is further bifurcated into a portion allocated to interest expense and a portion allocated to reduce the build-to-suit lease obligation.
The interest rate used for the build-to-suit lease obligation represents our estimated incremental borrowing rate, adjusted to reduce any built in loss.
The initial recording of these assets and liabilities is classified as non-cash investing and financing items, respectively, for purposes of the consolidated statements of cash flows.
The most significant estimates used by management in accounting for build-to-suit leases and the impact of these estimates are as follows:
|
|
• |
Expected lease term- Our expected lease term includes both contractual lease periods and cancelable option extension periods in which failure to exercise such options would result in an economic penalty. The expected lease term is used in determining the depreciable life of the asset or the straight-line rent recognition period for the portion of the lease payment allocable to the land component. Increasing the expected lease term to include cancelable optional extension periods would generally result in higher interest and depreciation expense for a build-to-suit lease recorded on our consolidated balance sheet. |
55
|
|
sheet with a related build-to-suit lease obligation, the incremental borrowing rate is used in allocating our rental payments between interest expense and a reduction of the outstanding build-to-suit lease obligation. |
|
|
• |
Fair market value of leased asset- The fair market value of a build-to-suit lease property is based on replacement cost of the pre-construction shell and comparable market data. Fair market value is used in determining the amount of the property asset and related build-to-suit lease obligation to be recognized on our consolidated balance sheet for build-to-suit leases. |
Goodwill and Intangible Assets
Assets acquired and liabilities assumed in business combinations are recognized at the date of acquisition at their respective fair values. Any excess of the purchase price over the estimated fair values of the net assets acquired is recognized as goodwill. We determine the fair value of intangible assets, including in-process research and development, or IPR&D, using the multi-period excess earnings model of the “income method.” This method starts with a forecast of net cash flows, risk adjusted for estimated probabilities of technical and regulatory success and adjusted to present value using an appropriate discount rate that reflects the risk associated with the cash flow streams. The valuation process is very complex and requires significant input and judgment using internal and external sources. Although the valuations are required to be finalized within a one-year period, we must consider all and only those facts and evidence available at the acquisition date. The most complex and judgmental matters applicable to the valuation process are summarized below:
|
|
• |
Unit of account—In valuing the IPR&D we consider whether the intangible asset should be valued as a single global asset rather than multiple assets for each jurisdiction or indication after considering the development stage, expected levels of incremental costs to obtain the initial and subsequent regulatory approvals, risks associated with further development, amount and timing of benefits expected to be derived in the future, expected patent lives in various jurisdictions and the intention to promote the assets as a global brand. |
|
|
• |
Estimated useful life—The asset life expected to contribute meaningful cash flows is determined after considering all pertinent matters associated with the asset, including expected regulatory approval dates (if unapproved), exclusivity periods, and other legal, regulatory, or contractual periods as well as the effects of any obsolescence, demand, competition, and other economic factors, including barriers to entry. |
|
|
• |
Probability of Technical and Regulatory Success (“PTS”) rate—PTS rates are determined considering industry averages of the respective program’s development stage and disease indication and adjusted for specific information or data known at the acquisition date. Subsequent clinical results or other internal or external data obtained could alter the PTS rate and materially impact the estimated fair value of the intangible asset in subsequent periods leading to non-cash impairment charges in the consolidated statement of operations. |
|
|
• |
Projections—Future revenues are estimated after considering many factors such as initial market opportunity, pricing, sales trajectories to peak sales levels, competitive environment and product evolution. Future costs and expenses are estimated after considering historical trends, market participant synergies and timing and level of additional development costs to obtain the initial or additional regulatory approvals, maintain or further enhance the product. We generally assume initial positive cash flows to commence shortly after the receipt of expected regulatory approvals which typically may not occur for a number of years. Actual cash flows attributable to the project are likely to be different than those assumed since projections are subjected to multiple factors including trial results and regulatory matters which could materially change the ultimate commercial success of the asset as well as significantly alter the costs to develop the respective asset into commercially viable products. |
|
|
• |
Discount rate— Discount rates are selected after considering the risks inherent in the future cash flows; the assessment of the asset’s life cycle and the competitive trends impacting the asset, including consideration of any technical, legal, regulatory, or economic barriers to entry, as well as expected changes in standards of practices for indications addressed by the asset. |
Goodwill is tested at least annually for impairment or when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable, on an enterprise level by assessing qualitative factors or performing a quantitative analysis in determining whether it is more likely than not that its fair value exceeds the carrying value. Examples of qualitative factors include our share price, our financial performance compared to budgets, long-term financial plans, macroeconomic, industry and market conditions as well as the substantial excess of fair value over the carrying value of net assets.
We test IPR&D for impairment at least annually or more frequently if impairment indicators exist. These assets are initially measured at fair value and therefore any reduction in expectations used in the valuations could potentially lead to impairment. Some of the more common potential risks leading to impairment include competition, earlier than expected loss of exclusivity, pricing pressures, adverse regulatory changes or clinical trial results, delay or failure to obtain regulatory approval and additional development costs, inability to achieve expected synergies, higher operating costs, changes in tax laws and other macro-economic changes. The complexity in estimating the fair value of intangible assets in connection with an impairment test is similar to the initial valuation.
56
During the fourth quarter of 2015, we recorded a $30.0 million impairment charge related to our IPR&D asset for MDV9300 as a result of an extended clinical development timeline and other factors. In the fourth quarter, we concluded our testing that revealed MDV9300 is not an anti-PD-1 antibody and, after advising the FDA of this conclusion in early-January 2016, our IND was placed on partial clinical hold pending revision of all related study documentation characterizing MDV9300 as an anti-PD-1 antibody. While we believe the partial clinical hold does not relate to concerns regarding the safety or previously-observed efficacy of MDV9300, we extended our clinical study timelines which, in turn, gave rise to changes to the significant inputs to our IPR&D valuation including forecasted revenues and probabilities of success. The quantitative assessment resulted in a net impairment charge, which we recorded as a reduction in the IPR&D asset and an increase to R&D expense in the fourth quarter.
Considering the high-risk nature of research and development and the industry’s success rate of bringing developmental compounds to market, IPR&D impairment charges are not uncommon. IPR&D is monitored and assessed each reporting period for possible triggering events that could lead to impairment.
Contingent Consideration
We initially determine the fair value of contingent consideration obligations at the acquisition date using a probability-based income approach utilizing an appropriate discount rate. Each reporting period we revalue the contingent consideration obligations associated with acquisitions to their fair value and record increases in the fair value as non-cash contingent consideration expense and decreases in the fair value as non-cash contingent consideration income in the consolidated statements of operations. Changes in contingent consideration could result from changes in the assumptions regarding probabilities of successful achievement of related milestones, the estimated timing in which the milestones are expected to be achieved and the discount rate used to estimate the fair value of the liability. Contingent consideration may change significantly as our development programs progress, revenue estimates evolve and additional data are obtained, impacting our assumptions. The assumptions used in estimating fair value require significant judgment. The use of different assumptions and judgments could result in a materially different estimate of fair value which may have a material impact on our results from operations and financial position.
In connection with the acquisitions of MDV9300 and MDV3800, we recorded contingent consideration liabilities pertaining to amounts potentially payable to CureTech and BioMarin, respectively. During the year ended December 31, 2015, we recorded fair value adjustments of $2.6 million and $7.7 million as a decrease to R&D expenses and SG&A expenses, respectively, related to the CureTech contingent consideration liability. Since the date of the fourth quarter acquisition of MDV3800, we also recorded nominal fair value adjustments to the related contingent consideration liability. Such adjustments were primarily due to the time value of money.
Convertible Notes
We account for convertible debt instruments that may be settled in cash upon conversion by separating the liability and the equity components of the instruments in a manner that reflects our nonconvertible debt borrowing rate when we recognize interest expense in subsequent periods. The debt component of the Convertible Notes, which excludes the associated equity conversion feature, was recorded at fair value on the issuance date. The equity component, representing the difference between the aggregate principal amount of the Convertible Notes and the fair value of the debt component, was recorded in additional paid-in capital on the consolidated balance sheet. The discount initially excluded from the carrying value of the Convertible Notes due to the bifurcation will be subsequently accreted to the Convertible Notes principal amount through the recognition of non-cash interest expense.
Costs related to the issuance of the Convertible Notes, consisting primarily of investment banking, legal and other professional fees were allocated to the debt and equity components of the Convertible Notes in proportion to the allocation of the principal proceeds. Amounts allocated to the debt component were capitalized and are being amortized as non-cash interest expense using the effective yield method over the five-year contract term of the Convertible Notes. Amounts allocated to the equity component were charged against additional paid-in capital.
Determining the fair value of the debt component of the Convertible Notes requires the use of management estimates and assumptions, including without limitation estimates of the fair value of similar debt instruments that do not include an equity conversion feature. These estimates and assumptions are judgmental in nature and could have a significant impact on our consolidated financial statements, including the carrying value of the Convertible Notes, the associated debt discount, and the amounts of non-cash interest expense reported.
During the year ended December 31, 2015, all of our Convertible Notes were settled.
Litigation
We are party to legal proceedings, investigations, and claims in the ordinary course of business. We record accruals for outstanding legal matters when we believe that it is both probable that a liability has been incurred and the amount of such liability can be reasonably estimated. We evaluate, on a quarterly basis, developments in significant legal matters that could affect the amount of
57
any accrual and developments that would make a loss contingency both probable and reasonably estimable. To the extent new information is obtained and our views on the probable outcomes of claims, suits, assessments, investigations or legal proceedings change, changes in our accrued liabilities would be recorded in the period in which such determination is made. In addition, in accordance with the relevant authoritative guidance, for matters for which the likelihood of material loss is at least reasonably possible, we provide disclosure of the possible loss or range of loss; however, if a reasonable estimate cannot be made, we will provide disclosure to that effect. Gain contingencies, if any, are recorded when they are realized.
Income Taxes
We record a valuation allowance to reduce our deferred tax assets to reflect the net amount that we believe is more likely than not to be realized. Realization of our deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. The valuation allowance requires an assessment of both positive and negative evidence when determining whether it is more likely than not that deferred tax assets are recoverable; such assessment is required on a jurisdiction by jurisdiction basis. Based upon the weight of available evidence at December 31, 2014, we determined that it was more likely than not that a portion of our deferred tax assets would be realizable and consequently we released our valuation allowance against Federal and certain state net deferred tax assets and recorded a discrete tax benefit of $33.4 million during the fourth quarter of 2014. The decision to reverse a portion of the valuation allowance was made after management considered all available evidence, both positive and negative, including but not limited to our historical operating results, income or loss in recent periods, cumulative income in recent years, forecasted earnings, future taxable income, and significant risk and uncertainty related to forecasts. The release of the valuation allowance resulted in the recognition of certain deferred tax assets and a decrease to income tax expense.
Significant judgment is required in evaluating our uncertain income tax positions based on the guidance in ASC 740-10-25, “Accounting for Uncertainty in Income Taxes.” We recognize a tax benefit from an uncertain tax position only if it is more likely than not that the position will be sustained upon examination by tax authorities. The tax benefit recognized in the financial statements on a particular tax position is measured on the largest benefit that is more likely than not to be realized. We evaluate uncertain tax positions on a quarterly basis and adjust the liability for changes in facts and circumstances, such as new regulations or interpretations by the taxing authorities, new information obtained during a tax examination, significant amendment to an existing tax law, or resolution of an examination. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences will impact the income tax provision in the period in which such determination is made. The resolution of our uncertain income tax positions is dependent on uncontrollable factors such as law changes, new case law, and the willingness of the income tax authorities to settle, including the timing thereof and other factors. Although we do not anticipate significant changes to our uncertain income tax positions in the next twelve months, items outside of our control could cause our uncertain income tax positions to change in the future, which would be recorded in our consolidated statements of operations. Interest and/or penalties related to income tax matters are recognized as a component of income tax expense as incurred.
Net Income (Loss) Per Common Share
We apply the guidance in ASC 260, “Earnings per Share,” when calculating net income per common share. The provisions of ASC 260 require that for contracts which provide a company with a choice of settlement methods, the company is to assume that the contract will be settled in shares. That presumption may be overcome if past experience or a stated policy provides for a reasonable basis to believe that it is probable that the contract will be paid partially or wholly in cash.
During the second quarter of 2015, we asserted our intent and ability to settle the outstanding Convertible Notes for a combination of cash and common stock. Under the “cash settlement” method, interest is not added back to the numerator, and only the contingently issuable shares related to the conversion spread are included in the denominator, if dilutive. Under such method, the settlement of the conversion spread has a dilutive effect when the average share price of our common stock during the period exceeds the conversion price. The computation of diluted net income per share for the year ended December 31, 2015 reflects the application of the “if-converted” method for the first quarter of 2015 and the “cash settlement” method for the second and third quarters of 2015 given the demonstrated and asserted redemption of the outstanding debt. We completed the settlement of all of our Convertible Notes during the third quarter of 2015 as described elsewhere in this Annual Report. The calculation of diluted net income per common share for the year ended December 31, 2015 includes approximately 3.9 million contingently issuable shares related to the Convertible Notes.
We used the “if-converted” method to compute the dilutive effect of the Convertible Notes for the calculation of diluted net income per common share for the year ended December 31, 2014. Under the “if-converted” method, interest expense, net of tax, related to the Convertible Notes is added back to net income, and the Convertible Notes are assumed to have been converted into common shares at the beginning of the period in periods in which there would have been a dilutive effect. The calculation of diluted net income per common share for the year ended December 31, 2014 includes approximately 10.1 million contingently issuable shares related to the Convertible Notes.
Additional information regarding net income per common share is included in Note 6, “Net Income (Loss) per Common Share,” to our consolidated financial statements included elsewhere in this Annual Report.
58
Collaboration Revenue
We have a collaboration agreement with Astellas pursuant to which we are collaborating with Astellas to develop and commercialize XTANDI globally. The terms of the collaboration agreement are described in Note 3 “Collaboration Agreement,” to our audited consolidated financial statements included elsewhere in this Annual Report. Collaboration revenue consists of three components: (a) collaboration revenue related to U.S. XTANDI net sales; (b) collaboration revenue related to ex-U.S. XTANDI net sales; and (c) collaboration revenue related to upfront and milestone payments.
Collaboration revenue was as follows (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Collaboration revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Related to U.S. XTANDI net sales |
|
$ |
575,658 |
|
|
$ |
339,902 |
|
|
$ |
196,208 |
|
|
Related to ex-U.S. XTANDI net sales |
|
|
119,778 |
|
|
|
49,476 |
|
|
|
6,338 |
|
|
Related to upfront and milestone payments |
|
|
247,822 |
|
|
|
321,109 |
|
|
|
70,396 |
|
|
Total |
|
$ |
943,258 |
|
|
$ |
710,487 |
|
|
$ |
272,942 |
|
We are required to pay UCLA ten percent of all Sublicensing Income, as defined in our license agreement with UCLA. We are currently involved in litigation with UCLA regarding certain terms of the license agreement and other matters, which are discussed in Part I, Item 3, “Legal Proceedings.”
Collaboration Revenue Related to U.S. XTANDI Net Sales
Collaboration revenue related to U.S. XTANDI net sales was as follows (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
U.S. XTANDI net sales (as reported by Astellas) |
|
$ |
1,151,317 |
|
|
$ |
679,805 |
|
|
$ |
392,415 |
|
|
Shared U.S. development and commercialization costs |
|
|
(375,008 |
) |
|
|
(323,730 |
) |
|
|
(241,106 |
) |
|
Pre-tax U.S. profit |
|
$ |
776,309 |
|
|
$ |
356,075 |
|
|
$ |
151,309 |
|
|
Medivation’s share of pre-tax U.S. profit |
|
$ |
388,154 |
|
|
$ |
178,037 |
|
|
$ |
75,655 |
|
|
Reimbursement of Medivation’s share of shared U.S. costs |
|
|
187,504 |
|
|
|
161,865 |
|
|
|
120,553 |
|
|
Collaboration revenue related to U.S. XTANDI net sales |
|
$ |
575,658 |
|
|
$ |
339,902 |
|
|
$ |
196,208 |
|
U.S. net sales of XTANDI (as reported by Astellas) for the year ended December 31, 2015 were $1.2 billion, an increase of $471.5 million, or 69%, compared with net sales for the prior year. The majority of the increase was due to higher sales volume with a minor increase resulting from changes in price elements. The increase in price elements includes a net favorable adjustment in 2015 of $20.0 million related to changes in prior period estimates of deductions against gross sales.
U.S. net sales of XTANDI (as reported by Astellas) for the year ended December 31, 2014 were $679.8 million, an increase of $287.4 million, or 73%, compared with net sales for the prior year. Approximately 83% of the increase was due to higher sales volume and approximately 17% of the increase was due to changes in price elements. The increase in price elements includes a net favorable adjustment in 2014 of $14.9 million related to changes in prior period estimates of deductions against gross sales.
In the fourth quarters of 2015 and 2014, we experienced cyclical buying patterns of XTANDI in the U.S. by the Astellas distribution channel partners. Specifically, fourth quarter U.S. XTANDI net sales (as reported by Astellas) in 2015 and 2014 included approximately one-half week and one week of additional channel partner inventory, respectively. As experienced a year ago, we anticipate that the fourth quarter 2015 channel partner inventory levels may be reduced slightly in the first quarter of 2016. We also anticipate that the estimated gross-to-net sales discount applied by Astellas in the first quarter of 2016 may be several percentage points higher than that utilized in the fourth quarter of 2015 because of the impact of Medicare Part D coverage and other government programs in the new calendar year.
Collaboration revenue related to U.S. XTANDI net sales for the year ended December 31, 2015 was $575.7 million, an increase of $235.8 million, or 69%, from the prior year. Collaboration revenue related to U.S. XTANDI net sales for the year ended December 31, 2014 was $339.9 million, an increase of $143.7 million, or 73%, from the prior year. The increases in 2015 and 2014 were the result of an increase in our share of pre-tax U.S. profit in the collaboration with Astellas as well as an increase in their reimbursement of our shared U.S. costs.
59
Along with other manufacturers of branded pharmaceutical products, we are subject to various provisions of the Patient Protection and Affordable Care Act of 2010, or PPACA, and other healthcare reform legislation. The stated goals of this legislation include reducing the number of uninsured Americans, improving the quality of healthcare delivery and reducing projected U.S. healthcare costs. The largest component of the deductions from gross sales used in deriving net sales of XTANDI in the United States is legally mandated discounts or rebates to Medicare and other government programs such as Medicaid. Although the full impact to us of all elements of PPACA and other healthcare reform legislation cannot be specifically determined, we estimate that legally mandated discounts or rebates for Medicaid and Medicare Part D programs, including the 23.1% rebate and the Medicare Part D coverage gap provisions, reduced U.S. XTANDI net sales by approximately 2% for the year ended December 31, 2015. These provisions are anticipated to continue to impact U.S. sales of XTANDI to a similar degree in future periods. The financial impact of U.S. healthcare reform legislation to our consolidated financial statements in future periods depends on a number of factors, including the timing of and changes in sales volumes for our products and the number of patients eligible for these government programs. Additional information regarding the impact of the provisions of PPACA and other healthcare reform legislation on us is included in Part I, Item 1A, “Risk Factors.”
Collaboration Revenue Related to Ex-U.S. XTANDI Net Sales
Net sales of XTANDI outside of the United States (as reported by Astellas) were approximately $757.0 million, $381.0 million and $53.0 million for the years ended December 31, 2015, 2014 and 2013, respectively. Collaboration revenue attributable to ex-U.S. XTANDI net sales was $119.8 million, $49.5 million and $6.3 million for the years ended December 31, 2015, 2014 and 2013, respectively. XTANDI was first approved for sale outside of the United States in June 2013.
Net sales of XTANDI outside of the United States (as reported by Astellas) in 2015 increased by approximately 99% compared to net sales in 2014. Changes in foreign currency exchange rates reduced the U.S. dollar equivalent of such 2015 net sales (as reported by Astellas) by approximately 14% compared with 2014.
Collaboration Revenue Related to Upfront and Milestone Payments
Collaboration revenue related to upfront and milestone payments was as follows (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Sales milestones earned |
|
$ |
245,000 |
|
|
$ |
50,000 |
|
|
$ |
25,000 |
|
|
Development milestones earned |
|
|
— |
|
|
|
257,000 |
|
|
|
20,000 |
|
|
Amortization of deferred upfront and development milestones |
|
|
2,822 |
|
|
|
14,109 |
|
|
|
25,396 |
|
|
Total |
|
$ |
247,822 |
|
|
$ |
321,109 |
|
|
$ |
70,396 |
|
Collaboration revenue related to upfront and milestone payments from Astellas for the year ended December 31, 2015 was $247.8 million, a decrease of $73.3 million, or 23%, from $321.1 million for the prior year. The decrease in collaboration revenue related to upfront and milestone payments from Astellas was primarily due to the timing of the recognition of development and sales milestone payments upon the achievement of certain defined milestone events. Additional information regarding the milestones earned during 2015 is included in the section of Management’s Discussion and Analysis of Financial Condition and Results of Operations entitled “2015 Financial Highlights.”
Collaboration revenue related to upfront and milestone payments from Astellas for the year ended December 31, 2014 was $321.1 million, an increase of $250.7 million, or 356% from $70.4 million for the prior year. The increase in collaboration revenue related to upfront and milestone payments from Astellas was due to the timing of the recognition of development and sales milestone payments upon the achievement of certain defined milestone events as well as a change in the estimated remaining performance period under the Astellas Collaboration Agreement.
As of December 31, 2015, we have earned all development and sales milestone payments under the Astellas Collaboration Agreement, totaling $655.0 million, including $245.0 million, $307.0 million, and $45.0 million in the years ended December 31, 2015, 2014, and 2013, respectively.
Research and Development Expenses
Research and development, or R&D, expenses were as follows (dollars in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Research and development expenses |
|
$ |
232,100 |
|
|
$ |
189,570 |
|
|
$ |
118,952 |
|
|
Percentage change |
|
|
22 |
% |
|
|
59 |
% |
|
|
|
|
60
R&D expenses increased by $42.5 million, or 22%, to $232.1 million for the year ended December 31, 2015, from $189.6 million for the prior year. The increase was primarily due to a $27.8 million increase in third-party clinical and preclinical development costs as a result of increased activities, including those related to the acquisition of MDV3800 during the fourth quarter of 2015, a $30.0 million impairment charge related to intangible assets, a $15.4 million increase in personnel costs resulting from higher staffing levels and a $7.0 million increase in facilities and information technology costs, partially offset by $25.7 million of payments to UCLA related to the development milestone payments we earned from Astellas during the year ended December 31, 2014 and a $12.0 million upfront license and research agreement fee to a third party, both of which were not repeated in the year ended December 31, 2015.
R&D expenses increased by $70.6 million, or 59%, to $189.6 million, for the year ended December 31, 2014, from $119.0 million for the prior year. The increase was primarily due to an increase of approximately $27.2 million of third-party clinical and preclinical development costs as a result of increased clinical and preclinical activities, a $23.7 million increase in payments to UCLA related to the development milestone payments we earned from Astellas, a $10.1 million increase in facilities and information technology infrastructure costs, and a $9.6 million increase in personnel costs resulting from higher staffing levels.
We expect that R&D expenses will increase in 2016 as a result of higher personnel costs related to additional headcount and higher third-party clinical and preclinical development costs as a result of increased activities, including those related to clinical manufacturing of MDV9300 and costs to develop certain limited laboratory and pharmaceutical operations capabilities. In addition, with the acquisition of MDV3800 from BioMarin that was completed in October 2015, we are responsible for ongoing clinical trial costs for the compound from that date forward as well as the costs of any new trials we might elect to initiate in the future.
Under the Astellas Collaboration Agreement, we and Astellas share certain development costs in the United States. Development cost-sharing payments from Astellas were $60.8 million, $63.5 million and $46.6 million for the years ended December 31, 2015, 2014 and 2013, respectively. Development cost-sharing payments from Astellas are recorded as reductions in R&D expenses.
We were engaged in four R&D programs during the periods presented: (1) the development of enzalutamide for the treatment of prostate cancer, advanced breast cancer, and hepatocellular carcinoma; (2) the development of MDV3800; (3) the development of MDV9300; and (4) multiple proprietary research and drug discovery projects. R&D costs are identified as either directly allocable to one of our R&D programs or indirect costs, with only direct costs being tracked by specific program. Direct costs consist primarily of clinical, preclinical, and drug discovery costs, cost of supplying drug substance and drug product for use in clinical and preclinical studies, including clinical manufacturing costs, upfront and development milestone payments under license agreements, non-cash fair value adjustments related to contingent purchase consideration, non-cash IPR&D impairment charges, personnel costs, contract research organization fees, and other contracted services pertaining to specific clinical and preclinical studies. Indirect costs consist of corporate overhead costs and other administrative and support costs. The following table summarizes the direct costs attributable to each program and total indirect costs (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Direct costs: |
|
|
|
|
|
|
|
|
|
|
|
|
|
XTANDI (enzalutamide) program (1) |
|
$ |
74,616 |
|
|
$ |
102,669 |
|
|
$ |
73,076 |
|
|
MDV3800 program |
|
|
12,454 |
|
|
|
— |
|
|
|
— |
|
|
MDV9300 program (2) |
|
|
59,099 |
|
|
|
5,949 |
|
|
|
— |
|
|
Early-stage programs |
|
|
59,071 |
|
|
|
61,063 |
|
|
|
36,140 |
|
|
Total direct costs |
|
|
205,240 |
|
|
|
169,681 |
|
|
|
109,216 |
|
|
Indirect costs |
|
|
26,860 |
|
|
|
19,889 |
|
|
|
9,736 |
|
|
Total |
|
$ |
232,100 |
|
|
$ |
189,570 |
|
|
$ |
118,952 |
|
|
(1) |
Direct costs for the XTANDI (enzalutamide) program include $25.7 million and $2.0 million for the years ended December 31, 2014 and 2013, respectively, of payments to UCLA related to the development milestones we earned from Astellas during those periods. |
|
(2) |
Direct costs for the MDV9300 program include $30.0 million for the year ended December 31, 2015 resulting from the partial impairment of the IPR&D intangible asset. |
Our R&D programs may be subject to change from time to time as we evaluate our priorities and available resources.
For a detailed discussion of the risks and uncertainties associated with the timing and cost of completing a product development plan, see Part I, Item 1A, “Risk Factors—Risks Related to Our Future Product Development Candidates” of this Annual Report.
61
Selling, General and Administrative Expenses
Selling, general and administrative, or SG&A, expenses were as follows (dollars in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Selling, general and administrative expenses |
|
$ |
296,545 |
|
|
$ |
239,071 |
|
|
$ |
176,231 |
|
|
Percentage change |
|
|
24 |
% |
|
|
36 |
% |
|
|
|
|
SG&A expenses increased by $57.5 million, or 24%, to $296.5 million for the year ended December 31, 2015, from $239.1 million for the prior year. The increase was primarily due to higher sales, marketing and medical affairs costs, as well as higher administrative and personnel-related costs. We incurred $8.8 million in higher royalty expenses as a result of an increase in net sales of XTANDI. SG&A expenses for the year ended December 31, 2015 included $24.5 million to UCLA associated with sales milestones that we earned from Astellas during 2015, as well as non-cash fair value adjustments of $7.9 million, which were recorded as a reduction to SG&A expenses, for contingent consideration related to our CureTech and BioMarin acquisitions.
SG&A expenses increased by $62.8 million, or 36%, to $239.1 million, for the year ended December 31, 2014, from $176.2 million for the prior year. The increase was primarily due to higher sales and marketing costs as well as certain medical affairs costs and other initiatives associated with launch of XTANDI in the pre-chemotherapy setting in the United States. These included higher collaboration expenses from Astellas and additional internal personnel costs due to higher staffing levels. In addition, we incurred approximately $8.0 million higher royalty expense based on sales and accrued payments to UCLA associated with sales milestones we earned from Astellas, partially offset by a reduction in legal fees.
We expect that SG&A expenses will increase in 2016 as a result of increased personnel costs related to additional headcount, an increase in sales, marketing and medical affairs activities, and higher corporate overhead costs in support of our expanded research, development, and commercial activities.
Under our collaboration with Astellas, we are responsible for fifty percent of the cost of goods sold and the royalty payable to UCLA on U.S. net sales of XTANDI. Our share of these items is included in SG&A expenses in our consolidated statements of operations. As such, those components of our reported SG&A expenses will fluctuate in correlation with net sales of XTANDI in the United States.
On July 28, 2014, the IRS issued final rules and regulations in the U.S. for the Branded Prescription Drug Fee, an annual fee payable to the federal government under PPACA based on an allocation for a given year based on a company’s market share for branded prescription drugs sold to certain government programs in the prior year. The final rules have the effect of accelerating the expense recognition criteria for the fee obligation from the year in which the fee is paid, to the year in which the market share used to allocate the fee is determined. This change requires us and other industry participants to recognize an additional year of Branded Prescription Drug Fee expense in 2014. We share this annual fee equally with Astellas with respect to U.S. XTANDI sales sold through these government programs. As a result, an additional expense of $6.4 million was recognized as commercialization cost-sharing payments to Astellas within SG&A during the year ended December 31, 2014.
Under the Astellas Collaboration Agreement, we and Astellas share certain commercialization costs in the United States. Commercialization cost-sharing payments to Astellas were $37.5 million, $36.1 million and $12.0 million for the years ended December 31, 2015, 2014 and 2013, respectively. Commercialization cost-sharing payments to Astellas are recorded as increases in SG&A expenses.
Other Income (Expense), net
The components of other income (expense), net were as follows (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash loss on extinguishment of Convertible Notes |
|
$ |
(21,087 |
) |
|
$ |
— |
|
|
$ |
— |
|
|
Non-cash amortization of debt discount and issuance costs |
|
|
(8,613 |
) |
|
|
(14,898 |
) |
|
|
(13,456 |
) |
|
Coupon interest expense |
|
|
(3,870 |
) |
|
|
(6,792 |
) |
|
|
(6,793 |
) |
|
Other, net |
|
|
275 |
|
|
|
38 |
|
|
|
(8 |
) |
|
Total |
|
|
(33,295 |
) |
|
|
(21,652 |
) |
|
|
(20,257 |
) |
62
Other income (expense), net consists of coupon interest expense and non-cash interest expense, interest income earned and net gains (losses) on sales of our short-term investments, and the impact of changes in foreign exchange rates on our foreign currency-denominated payables, which were not significant.
Other income (expense), net for the year ended December 31, 2015 also includes non-cash losses on extinguishment of Convertible Notes. Additional information regarding the extinguishment of Convertible Notes is included in Note 10, “Debt,” in the accompanying notes to our consolidated financial statements.
Income Tax (Expense) Benefit
Income tax (expense) benefit and the effective income tax rate were as follows (dollars in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Income tax (expense) benefit |
|
$ |
(136,593 |
) |
|
$ |
16,258 |
|
|
$ |
(115 |
) |
|
Effective income tax rate |
|
|
35.8 |
% |
|
|
(6.2 |
%) |
|
|
(0.3 |
%) |
Income tax expense for the year ended December 31, 2015 was $136.6 million. The provision for income taxes in 2015 was higher than the tax computed at the U.S. federal statutory rate due primarily to state income taxes and non-deductible stock-based compensation, net of Federal research and development credit. Income tax benefit for the year ended December 31, 2014, was $16.3 million. The provision for income taxes for 2014 was lower than the tax computed at the U.S. federal statutory rate due primarily to utilization of net operating loss and tax credit carryforwards and the release of the valuation allowance on a portion of our net deferred tax assets. Income tax expense for the year ended December 31, 2013 was not significant, due to our net loss for this year for which no tax benefit was recognized due to our valuation allowance.
Our effective tax rate was 35.8% for the year ended December 31, 2015. Our effective tax rate was (6.2%) for year ended December 31, 2014. Our effective tax rate for the year ended December 31, 2013 was not significant. The increase in the effective tax rate for the year ended December 31, 2015 as compared to the prior year was primarily due to the release of the valuation allowance against Federal and certain state deferred tax assets during the fourth quarter of 2014.
During the year ended December 31, 2015, we reduced our current Federal and state taxes payable by $100.2 million related to excess tax benefits from stock-based compensation, offsetting additional paid-in capital. In addition, for the year ended December 31, 2015, we recorded a credit to additional paid-in capital of $11.8 million related to certain tax impacts of the extinguishment of Convertible Notes.
We record a valuation allowance to reduce deferred tax assets to reflect the net amount that is more likely than not to be realized. Realization of our deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. Based upon the weight of available evidence at December 31, 2014, we determined that it was more likely than not that a portion of our deferred tax assets would be realizable and consequently we released our valuation allowance against Federal and certain state net deferred tax assets and recorded a discrete tax benefit of $33.4 million during the fourth quarter of 2014. The decision to reverse a portion of the valuation allowance was made after management considered all available evidence, both positive and negative, including but not limited to our historical operating results, income or loss in recent periods, cumulative income in recent years, forecasted earnings, forecasted future taxable income, and significant risk and uncertainty related to forecasts. The release of the valuation allowance resulted in the recognition of certain deferred tax assets and a decrease to income tax expense.
We have fully utilized all of our Federal gross net operating losses and Federal tax credit carryforwards for tax return purposes as of December 31, 2015.
LIQUIDITY AND CAPITAL RESOURCES
Our principal source of liquidity is cash generated from our collaboration agreement with Astellas, which is described elsewhere in this Annual Report.
At December 31, 2015, we had cash and cash equivalents of $225.9 million, compared with $502.7 million at December 31, 2014. At December 31, 2015, our collaboration receivable from Astellas was $391.6 million, compared with $184.7 million at December 31, 2014. In the fourth quarter of 2015, we utilized $410.0 million of our cash balances to pay an upfront fee to BioMarin for the acquisition of MDV3800 as described elsewhere in this Annual Report. Based on our current expectations, we believe our net current assets, amounts that are available under our Revolving Credit Facility, and our projected cash flows will be sufficient to fund our currently planned operations for at least the next twelve months. This estimate is based on a number of assumptions that may prove to be wrong. In addition, we may choose to raise additional funds in the form of equity, debt, or otherwise due to market conditions or strategic considerations even if we believe we have sufficient funds for our current and future operating plans. For example, we may choose to raise additional capital to fund business development activities, and for other general corporate purposes.
63
For a detailed discussion of the risks and uncertainties associated with our sources of liquidity and access to capital, see Part I, Item 1A, “Risk Factors—Risks Related to the Operation of Our Business.”
On October 23, 2015, we entered into an amendment and restatement of our Original Credit Agreements described elsewhere in this Annual Report. The Credit Agreement provides for (i) a five-year $300.0 million Revolving Credit Facility; and (ii) an uncommitted accordion facility subject to the satisfaction of certain conditions (collectively, the “Senior Secured Credit Facility”). The Revolving Credit Facility includes a $50.0 million multicurrency sub-facility, a $20.0 million letter of credit sub-facility, and a $10.0 million swing line loan sub-facility. On October 23, 2015, we borrowed $75.0 million under the Revolving Credit Facility, which was used to repay the $75.0 million outstanding at September 30, 2015 under the Original Credit Agreement. On January 25, 2016, we repaid the $75.0 million outstanding under the Revolving Credit Facility.
Cash Flow Analysis
The following table summarizes our cash flows (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
219,939 |
|
|
$ |
240,599 |
|
|
$ |
(68,209 |
) |
|
Investing activities |
|
|
(430,987 |
) |
|
|
(17,410 |
) |
|
|
216,826 |
|
|
Financing activities |
|
|
(65,776 |
) |
|
|
50,700 |
|
|
|
8,870 |
|
|
Net change in cash and cash equivalents |
|
$ |
(276,824 |
) |
|
$ |
273,889 |
|
|
$ |
157,487 |
|
Operating Activities
Net cash provided by operating activities totaled $219.9 million for 2015, which consisted of our net income of $244.7 million, partially offset by negative changes in our operating assets and liabilities of $24.4 million and non-cash items of $0.4 million. Net cash provided by operating activities was primarily driven by collaboration revenue related to U.S. XTANDI sales, royalties on ex-U.S. XTANDI sales, and milestone payments received from Astellas during 2015, partially offset by cash utilized in operations that arose in the ordinary course of business.
Net cash provided by operating activities totaled $240.6 million for 2014, which consisted of our net income of $276.5 million, partially offset by non-cash items of $1.9 million and negative changes in our operating assets and liabilities of $34.0 million. Net cash provided by operating activities was primarily driven by collaboration revenue related to U.S. XTANDI sales, royalties on ex-U.S. XTANDI sales, and milestone payments received from Astellas during 2014, partially offset by cash utilized in operations that arose in the ordinary course of business.
Net cash used in operating activities totaled $68.2 million for 2013, which consisted of negative changes in our operating assets and liabilities of $54.2 million and our net loss of $42.6 million, partially offset by non-cash items of $28.6 million. Non-cash items consisted primarily of non-cash stock-based compensation expense of $37.1 million, non-cash interest expense on the Convertible Notes of $13.5 million and depreciation expense on property and equipment of $3.4 million, partially offset by non-cash amortization of deferred revenue of $25.4 million. The negative cash flow from changes in operating assets and liabilities of $54.2 million arose in the ordinary course of business.
Investing Activities
Net cash used in investing activities totaled $431.0 million for 2015, which consisted of a $410.0 million payment to BioMarin under the asset purchase agreement, purchases of short-term investments of $90.4 million, capital expenditures of $19.5 million, and an increase in letters of credit collateralized by restricted cash to secure various leases of $1.4 million, partially offset by sales and maturities of short-term investments of $90.2 million.
Net cash used in investing activities totaled $17.4 million for 2014, and consisted primarily of capital expenditures of $10.5 million, a $5.0 million payment to CureTech, Ltd. under the License Agreement and an increase in letters of credit collateralized by restricted cash to secure various leases of $1.9 million.
Net cash provided by investing activities totaled $216.8 million for 2013, consisting of net maturities of short-term investments of $225.0 million, partially offset by $7.5 million of capital expenditures and an increase of $0.7 million in letters of credit collateralized by restricted cash to secure operating leases.
64
Net cash used in financing activities totaled $65.8 million for 2015, and consisted primarily of repayment of Convertible Notes principal and conversion premium of $259.9 million, repayment of our Revolving Credit Facility of $75.0 million and financing transaction costs of $1.7 million, partially offset by proceeds from our Revolving Credit Facility of $150.0 million, excess tax benefits from stock-based compensation of $100.2 million and proceeds from the issuance of common stock under equity incentive and stock purchase plans of $21.5 million.
Net cash provided by financing activities totaled $50.7 million for 2014, and consisted of primarily of $33.9 million of proceeds from the issuance of common stock under the Medivation Equity Incentive Plan and ESPP and $17.0 million of excess tax benefits from stock-based compensation.
Net cash provided by financing activities totaled $8.9 million for 2013 and consisted entirely of proceeds from the issuance of common stock under the Medivation Equity Incentive Plan.
Commitments and Contingencies
At December 31, 2015, our future minimum contractual commitments were as follows (in thousands):
|
|
|
Payment due by Period |
|
|||||||||||||||||
|
|
|
Total |
|
|
Less than 1 Year |
|
|
1-3 Years |
|
|
3-5 Years |
|
|
More than 5 Years |
|
|||||
|
Revolving Credit Facility(1) |
|
$ |
75,000 |
|
|
$ |
75,000 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Lease obligations(2) |
|
|
54,885 |
|
|
|
11,521 |
|
|
|
23,840 |
|
|
|
9,897 |
|
|
|
9,627 |
|
|
Other(3) |
|
|
17,500 |
|
|
|
17,500 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
147,385 |
|
|
$ |
104,021 |
|
|
$ |
23,840 |
|
|
$ |
9,897 |
|
|
$ |
9,627 |
|
|
(1) |
See Note 10, “Debt,” to our audited consolidated financial statements included elsewhere in this Annual Report. We repaid the $75.0 million outstanding under the Revolving Credit Facility on January 25, 2016. |
|
(2) |
See Note 15, “Commitments and Contingencies,” to our audited consolidated financial statements included elsewhere in this Annual Report. |
|
(3) |
The amount represents a payment made to UCLA in the first quarter of 2016. Additional information regarding this payment is included below. |
Potential Obligations Not Included in the Table Above
In addition to the contractual obligations disclosed in the table above, we have other potential obligations for which the timing and the extent of future payments are not known. We have described these potential obligations in the following paragraphs.
License Agreement with UCLA
Under our license agreement with UCLA, we may be required to make various future payments including (a) ten percent of all Sublicensing Income, as defined in the agreement, and (b) a four percent royalty on sales of products falling within the scope of the patent rights licensed from UCLA. Under the terms of our Astellas Collaboration Agreement, we share this royalty obligation with Astellas 50/50 with respect to sales in the United States, and Astellas is responsible for this entire royalty obligation with respect to sales outside of the United States. In ongoing litigation initiated by us, UCLA filed a cross-complaint alleging that we are required to pay it ten percent of the $320.0 million in sales milestone payments that we are eligible to earn under the Astellas Collaboration Agreement because such milestones constitute Sublicensing Income under the Astellas Collaboration Agreement. As of December 31, 2015, we have paid UCLA $14.5 million, representing ten percent of the $145.0 million sales milestone payments we earned from Astellas through September 30, 2015. At December 31, 2015, we have recorded a $17.5 million accrued payment to UCLA, representing ten percent of the $175.0 million sales milestone payment we earned from Astellas during the fourth quarter of 2015. This amount, which is included in the table above, was paid to UCLA during the first quarter of 2016. Other potential payments to UCLA are not included in the contractual obligations table above because they are contingent on future events that may or may not materialize.
On April 11, 2014, the Regents filed a complaint against us in which UCLA alleges that the “Operating Profits” we have received (and will continue to receive) from Astellas, as a result of the Astellas Collaboration Agreement, constitute Sublicensing Income under the license agreement between us and the Regents and that we and our subsidiary, Medivation Prostate Therapeutics, Inc., have failed to pay the Regents ten percent of such Operating Profits. We deny the Regents’ allegations and are vigorously defending the litigation. For more information about this litigation, see Part I, Item 3, “Legal Proceedings.”
65
Commitments Related to Our Collaboration Agreement with Astellas
Under the Astellas Collaboration Agreement, we share with Astellas certain development and commercialization costs. The actual amounts that we pay Astellas or that Astellas pays us will depend on numerous factors, some of which are outside of our control and some of which are contingent upon the success of certain development and commercialization activities. Future development and commercialization payments to Astellas are not included in the table above as the timing and amounts of such payments are not determinable.
Contingent Consideration Liabilities
In accordance with accounting for business combinations guidance, contingent cash payments are recorded as contingent consideration liabilities on our consolidated balance sheets at fair value.
In the fourth quarter of 2015, we completed an acquisition of all rights to MDV3800 from BioMarin pursuant to an Asset Purchase Agreement, or the Agreement. In connection with the Agreement, we recorded contingent consideration pertaining to amounts potentially payable to BioMarin by us. The aggregate remaining, undiscounted amount of contingent consideration that we could be required to pay to BioMarin under the Agreement consists of a total of $160.0 million upon the achievement of defined regulatory and sales-based milestones, and mid-single digit royalties on net sales of products that contain MDV3800 during the royalty term specified in the Agreement.
In the fourth quarter of 2014, we entered into a License Agreement with CureTech, pursuant to which we have licensed exclusive worldwide rights to MDV9300. In connection with the CureTech License Agreement, we recorded contingent consideration pertaining to amounts potentially payable to CureTech by us. The aggregate remaining, undiscounted amount of contingent consideration that we could be required to pay to CureTech under the License Agreement consists of a total of $85.0 million upon attainment of certain development and regulatory milestones, up to $245.0 million upon the achievement of certain annual worldwide net sales thresholds, and tiered royalties ranging from 5% to 11% on annual worldwide net sales. CureTech is also entitled to a $5.0 million milestone payment upon completion of the Manufacturing Technology Transfer discussed below.
Future potential payments to BioMarin and CureTech are not included in the contractual obligations table above because they are all contingent on various future events that may or may not materialize. Additional information regarding each transaction is included in Note 4, “Business Acquisitions,” to our audited consolidated financial statements included elsewhere in this Annual Report.
As of December 31, 2015, the contingent consideration liabilities that we could be required to pay are our only financial liabilities measured and recorded using Level 3 inputs in accordance with accounting guidance for fair value measurements, and represent approximately 48% of our total liabilities. See Note 14, “Fair Value Disclosures,” in the accompanying notes to our consolidated financial statements for additional information.
Manufacturing Services and Supply Agreement with CureTech, Ltd.
Contemporaneous with the execution of our License Agreement with CureTech, we entered into a Manufacturing Services and Supply Agreement, or MSA, with CureTech pursuant to which CureTech will provide clinical trial supply of MDV9300 over a three year period. In accordance with the terms of the MSA, as amended, we paid CureTech upfront and setup fees of $3.0 million during the fourth quarter of 2014, $0.2 million during the second quarter of 2015 and $0.1 million during the fourth quarter of 2015. We are required to pay CureTech a one-time milestone payment of $5.0 million upon the completion of the Manufacturing Technology Transfer, as defined. We are also responsible for providing Manufacturing Funding of up to $19.3 million for clinical trial materials of MDV9300 over a three year period. The Manufacturing Funding is contingent upon the successful achievement of the requirements set forth in the Manufacturing Plan, and any such amounts may be reduced or eliminated by us under the terms of the MSA, of which approximately $9.0 million has been paid through December 31, 2015. Future potential payments to CureTech pursuant to the MSA are not included in the contractual obligations table above because they are contingent upon future events that may or may not materialize and the timing of such payments is not certain. Additional information regarding the Manufacturing Services and Supply Agreement with CureTech is included in Note 15, “Commitments and Contingencies,” to our audited consolidated financial statements included elsewhere in this Annual Report.
66
Development and Manufacturing Services Agreement
During the fourth quarter of 2014, we entered into a Development and Manufacturing Services Agreement with a third-party clinical manufacturing organization. The term of the agreement is for the longer of (i) a period of five (5) years or (ii) through the completion of the Services, as defined. Under the current statement of work under this agreement, as amended, we intend to transfer the current manufacturing process of MDV9300 from CureTech to this third party, further scale up and production of Phase 3 clinical trial material of MDV9300 from this entity’s manufacturing facility. The estimated total consideration under the current statement of work is up to approximately $15.2 million, of which approximately $5.1 million has been paid through December 31, 2015. Future potential payments pursuant to this agreement are not included in the table above because the timing of such payments is not certain. Additional information regarding the Development and Manufacturing Services Agreement is included in Note 15, “Commitments and Contingencies,” to our audited consolidated financial statements included elsewhere in this Annual Report.
Clinical Trials
As of December 31, 2015, we have several on-going studies in various clinical trial stages. Under agreements with various clinical research organizations and clinical trial sites, we incur expenses related to clinical trials of enzalutamide, MDV3800, MDV9300, and potential other clinical candidates. The timing and amounts of these disbursements are contingent upon the achievement of certain milestones, patient enrollment and services rendered or as expenses are incurred by the contract research organizations, contract manufacturing organizations, or clinical trial sites. Therefore, we cannot estimate the potential timing and amount of these payments and they have been excluded from the table above. Although our material contracts with contract research organizations and contract manufacturing organizations are cancellable, we have generally not cancelled such contracts.
Unrecognized Tax Benefits
At December 31, 2015, we had $14.1 million of gross unrecognized tax benefits, excluding interest, of which approximately $7.7 million represents the amount of unrecognized tax benefits that would impact the effective tax rate, if recognized. The ultimate resolution of our uncertain income tax positions is dependent on uncontrollable factors such as law changes, new case law, and the willingness of the income tax authorities to settle, including the timing thereof, and other factors. Although we do not anticipate significant changes to our uncertain income tax positions in the next twelve months, items outside of our control could cause our uncertain income tax positions to change in the future. Such amounts have been included on our audited consolidated balance sheet at December 31, 2015, but have not been included in the table above.
OFF-BALANCE SHEET ARRANGEMENTS
We are involved in a variable interest entity, or VIE, that performs contract research for us. We have not consolidated this entity because we do not have the power to direct the activities that most significantly impact the VIE’s economic performance and, thus, we are not considered the primary beneficiary of the VIE.
Market risk is the exposure to loss resulting from changes in interest rates, foreign currency exchange rates, commodity prices and equity prices.
Interest Rate Risk
Our investment policy emphasizes the safety and preservation of principal while maximizing the income we receive from our investments without assuming significant risk of loss. Specifically, our investment objectives in order of priority are (1) the safety and preservation of principal, (2) maintain liquidity sufficient to meet the requirements of our operations and strategic initiatives, and (3) deliver competitive after-tax returns relative to stated objectives and market conditions. The securities permitted under our investment policy may be subject to market risk related to changes in interest rates and other market factors. We manage our sensitivity to these risks by investing in short term, investment grade marketable securities. We currently do not use derivative financial instruments to hedge market risk exposures related to the investment portfolio.
Our cash equivalents are exposed to the impact of interest rate changes and our interest income fluctuates as interest rates change. Due to the short-term, highly liquid nature of our cash equivalents, we do not believe that we are subject to any material market risk exposure related to interest rates.
Foreign Currency Exchange Risk
Under the Astellas Collaboration Agreement, Astellas records all ex-U.S. XTANDI sales and pays us a tiered royalty ranging from the low teens to the low twenties on ex-U.S. XTANDI sales. The royalties we receive from Astellas on net sales of XTANDI outside of the United States are calculated by converting the respective countries’ XTANDI net sales in local currency to U.S. dollars. The royalties are paid to us in U.S. dollars on a quarterly basis. To date, a significant portion of ex-U.S. sales of XTANDI have been
67
generated in the European Union and in Japan. Therefore, the royalties we receive from Astellas related to ex-U.S. sales of XTANDI are dependent on the value of the U.S. dollar versus the Euro and Japanese Yen. A strengthening of the U.S. dollar compared to current exchange rates would likely result in lower collaboration revenue related to ex-U.S. XTANDI sales. We conduct certain R&D activities outside of the United States, with expenses incurred in various currencies, including the Euro and Japanese Yen. These expenses partially offset the currency exposure related to our collaboration revenue from royalties. In 2016, we intend to hedge a portion of these net exposures to manage the volatility of foreign exchange risk. Foreign exchange gains and losses recorded in our audited consolidated statements of operations for the years ended December 31, 2015, 2014, and 2013, were not significant.
Equity Price Risk
The market price and volatility of our common stock impacts our non-cash stock-based compensation expense. Further information is included in Note 11, “Stockholders Equity,” to our audited consolidated financial statements included elsewhere in this Annual Report.
All information required by this item is included in the financial statements which follow the signature page of this Annual Report on Form 10-K and is incorporated into this item by reference.
None.
Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as such term is defined in Rule 13a-15(e) under the Exchange Act, that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms and that such information is communicated to our management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer), as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that disclosure controls and procedures, no matter how well designed and operated, can provide only reasonable, but not absolute, assurance that the objectives of the disclosure controls and procedures are met. Our disclosure controls and procedures have been designed to meet the reasonable assurance standards. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
As required by Rule 13a-15(b) or Rule 15d-15(b) of the Exchange Act, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer), of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2015. Based on the foregoing, our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer) concluded that our disclosure controls and procedures were effective as of December 31, 2015 at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f) and 15d-15(f). Management, with the participation of our principal executive officer and principal financial officer, has conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework set forth in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework set forth in Internal Control—Integrated Framework (2013), our management concluded that our internal control over financial reporting was effective as of December 31, 2015.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of our internal control over financial reporting as of December 31, 2015, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears elsewhere herein.
68
Changes in Internal Control Over Financial Reporting
There were no changes in internal control over financial reporting during the quarter ended December 31, 2015, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
69
The information required by Part III is omitted from this Annual Report on Form 10-K since we intend to file our definitive Proxy Statement for our 2016 Annual Meeting of Stockholders, pursuant to Regulation 14A of the Exchange Act, not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K, and certain information to be included in the Proxy Statement is incorporated herein by reference.
Information required by this item regarding directors and director nominees, executive officers, the board of directors and its committees, and certain corporate governance matters is incorporated by reference to the information set forth under the captions “Election of Directors,” “Information Regarding the Board of Directors and Corporate Governance” and “Executive Officers” in our Proxy Statement for the 2016 Annual Meeting of Stockholders. Information required by this item regarding compliance with Section 16(a) of the Exchange Act is incorporated by reference to the information set forth under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
We have adopted a written code of business conduct and ethics that applies to our principal executive officer, principal financial officer, principal accounting officer, or persons serving similar functions. The code of business conduct and ethics is available on our corporate website at www.medivation.com. If we make any substantive amendments to our code of business conduct and ethics or grant to any of our directors or executive officers any waiver, including any implicit waiver, from a provision of our code of business conduct and ethics, we will disclose the nature of the waiver or amendment on our website or in a Current Report on Form 8-K.
Information required by this item regarding executive compensation is incorporated by reference to the information set forth under the captions “Executive Compensation,” “Director Compensation” and “Information Regarding the Board of Directors and Corporate Governance” in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Information required by this item regarding security ownership of certain beneficial owners and management is incorporated by reference to the information set forth under the caption “Security Ownership of Certain Beneficial Owners and Management” in our Proxy Statement for the 2016 Annual Meeting of Stockholders. Information required by this item regarding securities authorized for issuance under our equity compensation plans is incorporated by reference to the information set forth under the caption “Equity Compensation Plan Information” in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
Information required by this item regarding certain relationships and related transactions is incorporated by reference to the information set forth under the caption “Transactions with Related Persons” in our Proxy Statement for the 2016 Annual Meeting of Stockholders. Information required by this item regarding director independence is incorporated by reference to the information set forth under the caption “Information Regarding the Board of Directors and Corporate Governance” in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
Information required by this item regarding principal accounting fees and services is incorporated by reference to the information set forth under the caption “Ratification of Selection of Independent Registered Public Accounting Firm” in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
70
(a) The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements. Our audited consolidated financial statements and the Report of Independent Registered Public Accounting Firm, are included herein on the pages indicated:
2. Financial Statement Schedules: None.
3. Exhibits: See the Exhibit List which follows the financial statements attached following the signature page of this Annual Report on Form 10-K, which is incorporated here by reference.
71
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
MEDIVATION, INC. |
|
|
|
|
|
|
/S/ RICHARD A. BIERLY |
|
|
Richard A. Bierly Chief Financial Officer (Principal Financial Officer) |
Dated: February 26, 2016
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Richard A. Bierly and Andrew K. W. Powell, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments to this Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
|
/S/ David T. Hung, M.D. |
|
President, Chief Executive Officer and |
|
February 26, 2016 |
|
David T. Hung, M.D. |
|
Director (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/S/ Richard A. Bierly |
|
Chief Financial Officer |
|
February 26, 2016 |
|
Richard A. Bierly |
|
(Principal Financial Officer) |
|
|
|
|
|
|
|
|
|
/S/ Tseli Lily Yang |
|
Vice President, Finance and Accounting |
|
February 26, 2016 |
|
Tseli Lily Yang |
|
(Principal Accounting Officer) |
|
|
|
|
|
|
|
|
|
/S/ Kim D. Blickenstaff |
|
Director |
|
February 26, 2016 |
|
Kim D. Blickenstaff |
|
|
|
|
|
|
|
|
|
|
|
/S/ Kathryn E. Falberg |
|
Director |
|
February 26, 2016 |
|
Kathryn E. Falberg |
|
|
|
|
|
|
|
|
|
|
|
/S/ Michael L. King |
|
Director |
|
February 26, 2016 |
|
Michael L. King |
|
|
|
|
|
|
|
|
|
|
|
/S/ C. Patrick Machado |
|
Director |
|
February 26, 2016 |
|
C. Patrick Machado |
|
|
|
|
|
|
|
|
|
|
|
/S/ Dawn Svoronos |
|
Director |
|
February 26, 2016 |
|
Dawn Svoronos |
|
|
|
|
|
|
|
|
|
|
|
/S/ W. Anthony Vernon |
|
Director |
|
February 26, 2016 |
|
W. Anthony Vernon |
|
|
|
|
|
|
|
|
|
|
|
/S/ Wendy L. Yarno |
|
Director |
|
February 26, 2016 |
|
Wendy L. Yarno |
|
|
|
|
72
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Medivation, Inc.:
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of Medivation, Inc. and its subsidiaries at December 31, 2015 and December 31, 2014, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting, appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it classifies deferred taxes in 2015 due to the adoption of Accounting Standards Update 2015-17, Balance Sheet Classification of Deferred Taxes.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
San Jose, California
February 26, 2016
73
(in thousands, except share and per share data)
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
225,853 |
|
|
$ |
502,677 |
|
|
Receivable from collaboration partner |
|
|
391,558 |
|
|
|
184,737 |
|
|
Deferred income tax assets |
|
|
— |
|
|
|
21,987 |
|
|
Prepaid expenses and other current assets |
|
|
15,877 |
|
|
|
12,264 |
|
|
Restricted cash |
|
|
930 |
|
|
|
203 |
|
|
Total current assets |
|
|
634,218 |
|
|
|
721,868 |
|
|
Property and equipment, net |
|
|
58,142 |
|
|
|
41,161 |
|
|
Intangible assets |
|
|
644,299 |
|
|
|
101,000 |
|
|
Deferred income tax assets, non-current |
|
|
57,011 |
|
|
|
15,176 |
|
|
Restricted cash, net of current |
|
|
12,206 |
|
|
|
11,562 |
|
|
Goodwill |
|
|
18,643 |
|
|
|
10,000 |
|
|
Other non-current assets |
|
|
7,072 |
|
|
|
10,852 |
|
|
Total assets |
|
$ |
1,431,591 |
|
|
$ |
911,619 |
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable, accrued expenses and other current liabilities |
|
$ |
186,203 |
|
|
$ |
106,128 |
|
|
Borrowings under Revolving Credit Facility |
|
|
75,000 |
|
|
|
— |
|
|
Contingent consideration |
|
|
4,900 |
|
|
|
10,000 |
|
|
Deferred revenue |
|
|
— |
|
|
|
2,822 |
|
|
Current portion of build-to-suit lease obligation |
|
|
— |
|
|
|
698 |
|
|
Current portion of Convertible Notes, net of unamortized discount of $— and $1 at December 31, 2015 and 2014, respectively |
|
|
— |
|
|
|
4 |
|
|
Total current liabilities |
|
|
266,103 |
|
|
|
119,652 |
|
|
Convertible Notes, net of unamortized discount of $— and $36,598 at December 31, 2015 and 2014, respectively |
|
|
— |
|
|
|
222,140 |
|
|
Contingent consideration |
|
|
262,368 |
|
|
|
96,000 |
|
|
Build-to-suit lease obligation, excluding current portion |
|
|
17,406 |
|
|
|
18,711 |
|
|
Other non-current liabilities |
|
|
13,035 |
|
|
|
5,817 |
|
|
Total liabilities |
|
|
558,912 |
|
|
|
462,320 |
|
|
Commitments and contingencies (Note 15) |
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.01 par value per share; 1,000,000 shares authorized; no shares issued and outstanding |
|
|
— |
|
|
|
— |
|
|
Common stock, $0.01 par value per share; 340,000,000 shares authorized; 163,905,342 and 156,234,454 shares issued and outstanding at December 31, 2015 and 2014, respectively |
|
|
1,639 |
|
|
|
1,562 |
|
|
Additional paid-in capital |
|
|
684,841 |
|
|
|
505,446 |
|
|
Retained earnings (accumulated deficit) |
|
|
186,199 |
|
|
|
(57,709 |
) |
|
Total stockholders’ equity |
|
|
872,679 |
|
|
|
449,299 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
1,431,591 |
|
|
$ |
911,619 |
|
SEE ACCOMPANYING NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
74
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
|
|
|
Years ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Collaboration revenue |
|
$ |
943,258 |
|
|
$ |
710,487 |
|
|
$ |
272,942 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
232,100 |
|
|
|
189,570 |
|
|
|
118,952 |
|
|
Selling, general and administrative expenses |
|
|
296,545 |
|
|
|
239,071 |
|
|
|
176,231 |
|
|
Total operating expenses |
|
|
528,645 |
|
|
|
428,641 |
|
|
|
295,183 |
|
|
Income (loss) from operations |
|
|
414,613 |
|
|
|
281,846 |
|
|
|
(22,241 |
) |
|
Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss on extinguishment of Convertible Notes |
|
|
(21,087 |
) |
|
|
— |
|
|
|
— |
|
|
Interest expense |
|
|
(12,483 |
) |
|
|
(21,690 |
) |
|
|
(20,249 |
) |
|
Other, net |
|
|
275 |
|
|
|
38 |
|
|
|
(8 |
) |
|
Total other income (expense), net |
|
|
(33,295 |
) |
|
|
(21,652 |
) |
|
|
(20,257 |
) |
|
Income (loss) before income tax (expense) benefit |
|
|
381,318 |
|
|
|
260,194 |
|
|
|
(42,498 |
) |
|
Income tax (expense) benefit |
|
|
(136,593 |
) |
|
|
16,258 |
|
|
|
(115 |
) |
|
Net income (loss) |
|
$ |
244,725 |
|
|
$ |
276,452 |
|
|
$ |
(42,613 |
) |
|
Basic net income (loss) per common share |
|
$ |
1.53 |
|
|
$ |
1.80 |
|
|
$ |
(0.28 |
) |
|
Diluted net income (loss) per common share |
|
$ |
1.47 |
|
|
$ |
1.71 |
|
|
$ |
(0.28 |
) |
|
Weighted-average common shares used in the calculation of basic net income (loss) per common share |
|
|
160,345 |
|
|
|
153,859 |
|
|
|
150,331 |
|
|
Weighted-average common shares used in the calculation of diluted net income (loss) per common share |
|
|
169,324 |
|
|
|
170,001 |
|
|
|
150,331 |
|
SEE ACCOMPANYING NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
75
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(in thousands)
|
|
|
Years ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Net income (loss) |
|
$ |
244,725 |
|
|
$ |
276,452 |
|
|
$ |
(42,613 |
) |
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in unrealized loss on available-for-sale securities, net |
|
|
(10 |
) |
|
|
— |
|
|
|
(33 |
) |
|
Amounts reclassified into earnings related to investments |
|
|
10 |
|
|
|
— |
|
|
|
— |
|
|
Other comprehensive income (loss), net |
|
|
— |
|
|
|
— |
|
|
|
(33 |
) |
|
Comprehensive income (loss) |
|
$ |
244,725 |
|
|
$ |
276,452 |
|
|
$ |
(42,646 |
) |
SEE ACCOMPANYING NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
76
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
|
|
Years ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
244,725 |
|
|
$ |
276,452 |
|
|
$ |
(42,613 |
) |
|
Adjustments for non-cash operating items: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
54,862 |
|
|
|
45,134 |
|
|
|
37,078 |
|
|
Impairment of intangible assets |
|
|
30,000 |
|
|
|
— |
|
|
|
— |
|
|
Loss on extinguishment of Convertible Notes |
|
|
21,087 |
|
|
|
— |
|
|
|
— |
|
|
Amortization of debt discount and debt issuance costs |
|
|
8,613 |
|
|
|
14,898 |
|
|
|
13,456 |
|
|
Depreciation on property and equipment |
|
|
7,023 |
|
|
|
5,239 |
|
|
|
3,449 |
|
|
Excess tax benefits from stock-based compensation |
|
|
(100,243 |
) |
|
|
(16,965 |
) |
|
|
— |
|
|
Changes in deferred income taxes |
|
|
(8,270 |
) |
|
|
(3,827 |
) |
|
|
— |
|
|
Change in fair value of contingent purchase consideration |
|
|
(10,674 |
) |
|
|
— |
|
|
|
— |
|
|
Amortization of deferred revenue |
|
|
(2,822 |
) |
|
|
(14,109 |
) |
|
|
(25,396 |
) |
|
Release of valuation allowance for deferred income taxes |
|
|
— |
|
|
|
(33,403 |
) |
|
|
— |
|
|
Other non-cash items |
|
|
52 |
|
|
|
1,124 |
|
|
|
(10 |
) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Receivable from collaboration partners |
|
|
(206,821 |
) |
|
|
(77,527 |
) |
|
|
(71,752 |
) |
|
Prepaid expenses and other current assets |
|
|
(4,556 |
) |
|
|
301 |
|
|
|
(3,953 |
) |
|
Other non-current assets |
|
|
3,279 |
|
|
|
(401 |
) |
|
|
(7,353 |
) |
|
Accounts payable, accrued expenses and other current liabilities |
|
|
145,997 |
|
|
|
41,965 |
|
|
|
27,852 |
|
|
Current taxes payable |
|
|
30,459 |
|
|
|
1,970 |
|
|
|
37 |
|
|
Other non-current liabilities |
|
|
7,228 |
|
|
|
(252 |
) |
|
|
996 |
|
|
Net cash provided by (used) in operating activities |
|
|
219,939 |
|
|
|
240,599 |
|
|
|
(68,209 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments to BioMarin Pharmaceutical Inc. under Asset Purchase Agreement |
|
|
(410,000 |
) |
|
|
— |
|
|
|
— |
|
|
Payments to CureTech, Ltd. under License Agreement |
|
|
— |
|
|
|
(5,000 |
) |
|
|
— |
|
|
Purchases of short-term investments |
|
|
(90,381 |
) |
|
|
— |
|
|
|
(144,926 |
) |
|
Sales or maturities of short-term investments |
|
|
90,224 |
|
|
|
— |
|
|
|
370,000 |
|
|
Purchases of property and equipment |
|
|
(19,459 |
) |
|
|
(10,544 |
) |
|
|
(7,535 |
) |
|
Change in restricted cash |
|
|
(1,371 |
) |
|
|
(1,866 |
) |
|
|
(713 |
) |
|
Net cash (used in) provided by investing activities |
|
|
(430,987 |
) |
|
|
(17,410 |
) |
|
|
216,826 |
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Principal repayment of Convertible Notes |
|
|
(258,742 |
) |
|
|
(12 |
) |
|
|
— |
|
|
Cash settlement of Convertible Notes conversion premium |
|
|
(1,126 |
) |
|
|
— |
|
|
|
— |
|
|
Proceeds from borrowings under Revolving Credit Facility |
|
|
150,000 |
|
|
|
— |
|
|
|
— |
|
|
Principal repayment of borrowings under Revolving Credit Facility |
|
|
(75,000 |
) |
|
|
— |
|
|
|
— |
|
|
Financing transaction costs |
|
|
(1,715 |
) |
|
|
— |
|
|
|
— |
|
|
Excess tax benefits from stock-based compensation |
|
|
100,243 |
|
|
|
16,965 |
|
|
|
— |
|
|
Proceeds from issuance of common stock under equity incentive and stock purchase plans |
|
|
21,459 |
|
|
|
33,882 |
|
|
|
8,870 |
|
|
Reduction of build-to-suit lease obligation |
|
|
(895 |
) |
|
|
(135 |
) |
|
|
— |
|
|
Net cash (used in) provided by financing activities |
|
|
(65,776 |
) |
|
|
50,700 |
|
|
|
8,870 |
|
|
Net (decrease) increase in cash and cash equivalents |
|
|
(276,824 |
) |
|
|
273,889 |
|
|
|
157,487 |
|
|
Cash and cash equivalents at beginning of year |
|
|
502,677 |
|
|
|
228,788 |
|
|
|
71,301 |
|
|
Cash and cash equivalents at end of year |
|
$ |
225,853 |
|
|
$ |
502,677 |
|
|
$ |
228,788 |
|
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash payments (refunds) for: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest |
|
$ |
3,577 |
|
|
$ |
6,792 |
|
|
$ |
6,792 |
|
|
Income taxes, net of refunds |
|
$ |
2,122 |
|
|
$ |
1,525 |
|
|
$ |
(127 |
) |
|
Non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Reacquisition of Convertible Notes equity component upon conversion |
|
$ |
324,177 |
|
|
|
— |
|
|
|
— |
|
|
Fair value of common stock issued for conversion of Convertible Notes |
|
$ |
312,990 |
|
|
|
— |
|
|
|
— |
|
|
Derecognition of build-to-suit lease asset |
|
$ |
3,241 |
|
|
|
— |
|
|
|
— |
|
|
Derecognition of build-to-suit lease obligations |
|
$ |
3,176 |
|
|
|
— |
|
|
|
— |
|
|
Property and equipment expenditures incurred but not yet paid |
|
$ |
5,828 |
|
|
$ |
242 |
|
|
$ |
99 |
|
|
Interest capitalized during construction period for build-to-suit lease transactions |
|
$ |
2,024 |
|
|
$ |
1,459 |
|
|
|
— |
|
|
Accrued interest payable forfeited upon conversion of Convertible Notes |
|
$ |
1,686 |
|
|
|
— |
|
|
|
— |
|
|
Amounts capitalized under build-to-suit lease transactions |
|
$ |
44 |
|
|
$ |
18,085 |
|
|
|
— |
|
SEE ACCOMPANYING NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
77
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except share amounts)
|
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated Other Comprehensive |
|
|
Retained Earnings (Accumulated |
|
|
Total Stockholders’ |
|
|||||||||
|
|
|
Shares (1) |
|
|
Amount (1) |
|
|
Capital (1) |
|
|
Income (Loss) |
|
|
Deficit) |
|
|
Equity |
|
||||||
|
Balances at January 1, 2013 |
|
|
149,549,878 |
|
|
$ |
1,495 |
|
|
$ |
363,665 |
|
|
$ |
33 |
|
|
$ |
(291,548 |
) |
|
$ |
73,645 |
|
|
Common stock issued under equity incentive plan |
|
|
2,005,218 |
|
|
|
20 |
|
|
|
8,850 |
|
|
|
— |
|
|
|
— |
|
|
|
8,870 |
|
|
Common stock issued for warrant exercises, net of shares withheld for exercise price |
|
|
50,944 |
|
|
|
1 |
|
|
|
(1 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
37,078 |
|
|
|
— |
|
|
|
— |
|
|
|
37,078 |
|
|
Change in comprehensive income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(33 |
) |
|
|
— |
|
|
|
(33 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(42,613 |
) |
|
|
(42,613 |
) |
|
Balances at December 31, 2013 |
|
|
151,606,040 |
|
|
|
1,516 |
|
|
|
409,592 |
|
|
|
— |
|
|
|
(334,161 |
) |
|
|
76,947 |
|
|
Common stock issued under equity incentive and employee stock purchase plans |
|
|
4,628,414 |
|
|
|
46 |
|
|
|
33,836 |
|
|
|
— |
|
|
|
— |
|
|
|
33,882 |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
45,134 |
|
|
|
— |
|
|
|
— |
|
|
|
45,134 |
|
|
Excess tax benefits from stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
16,965 |
|
|
|
— |
|
|
|
— |
|
|
|
16,965 |
|
|
Tax shortfalls from stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
(77 |
) |
|
|
— |
|
|
|
— |
|
|
|
(77 |
) |
|
Repayment of Convertible Notes |
|
|
— |
|
|
|
— |
|
|
|
(4 |
) |
|
|
— |
|
|
|
— |
|
|
|
(4 |
) |
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
276,452 |
|
|
|
276,452 |
|
|
Balances at December 31, 2014 |
|
|
156,234,454 |
|
|
|
1,562 |
|
|
|
505,446 |
|
|
|
— |
|
|
|
(57,709 |
) |
|
|
449,299 |
|
|
Common stock issued under equity incentive and employee stock purchase plans |
|
|
2,032,312 |
|
|
|
21 |
|
|
|
21,438 |
|
|
|
— |
|
|
|
— |
|
|
|
21,459 |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
54,862 |
|
|
|
— |
|
|
|
— |
|
|
|
54,862 |
|
|
Excess tax benefits from stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
100,243 |
|
|
|
— |
|
|
|
— |
|
|
|
100,243 |
|
|
Tax shortfalls from stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
(259 |
) |
|
|
— |
|
|
|
— |
|
|
|
(259 |
) |
|
Common stock issued for conversion of Convertible Notes |
|
|
5,638,576 |
|
|
|
56 |
|
|
|
(11,239 |
) |
|
|
— |
|
|
|
— |
|
|
|
(11,183 |
) |
|
Tax impact of extinguishment of Convertible Notes |
|
|
— |
|
|
|
— |
|
|
|
11,847 |
|
|
|
— |
|
|
|
— |
|
|
|
11,847 |
|
|
Reclassification of accrued interest on Convertible Notes upon conversion |
|
|
— |
|
|
|
— |
|
|
|
1,686 |
|
|
|
— |
|
|
|
— |
|
|
|
1,686 |
|
|
Stock dividend |
|
|
— |
|
|
|
— |
|
|
|
817 |
|
|
|
— |
|
|
|
(817 |
) |
|
|
— |
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
244,725 |
|
|
|
244,725 |
|
|
Balances at December 31, 2015 |
|
|
163,905,342 |
|
|
$ |
1,639 |
|
|
$ |
684,841 |
|
|
$ |
— |
|
|
$ |
186,199 |
|
|
$ |
872,679 |
|
|
(1) |
All share, par, and additional paid-in capital amounts have been retroactively adjusted to reflect the Company’s September 15, 2015, two-for-one forward stock split effected through a stock dividend. This stock split resulted in the issuance of approximately 81.7 million shares of the Company’s common stock. |
SEE ACCOMPANYING NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
78
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2015
NOTE 1. DESCRIPTION OF BUSINESS
Medivation, Inc. (the “Company” or “Medivation”) is a biopharmaceutical company focused on the development and commercialization of medically innovative therapies to treat serious diseases for which there are limited treatment options. It has one commercial product, XTANDI® (enzalutamide) capsules, or XTANDI, through the Company’s collaboration with Astellas Pharma, Inc., or Astellas. XTANDI has received marketing approval in the United States, Europe and numerous other countries worldwide for the treatment of patients with metastatic castration-resistant prostate cancer, or mCRPC, and in Japan for the treatment of patients with castration-resistant prostate cancer, or CRPC. The Company and Astellas are also conducting investigational studies of enzalutamide in prostate cancer, advanced breast cancer, and hepatocellular carcinoma. Under the Company’s collaboration agreement with Astellas, it shares equally with Astellas all profits (losses) related to U.S. net sales of XTANDI. The Company also receives royalties ranging from the low teens to the low twenties as a percentage of ex-U.S. XTANDI net sales. The collaboration also involved certain milestone payments from Astellas to the Company upon the achievement of defined development, regulatory and sales events, all of which have been achieved as of December 31, 2015.
The Company seeks to become a more fully-integrated biopharmaceutical company through the continued commercialization of XTANDI, the acquisition or in-license and development and commercialization of other product opportunities, and through the advancement of its own proprietary research and development programs. The Company expects that its future growth may come from both internal research efforts and third-party business development activities. In the fourth quarter of 2015, the Company acquired all worldwide rights to talazoparib (which is referred to as MDV3800), an orally available poly-ADP ribose polymerase, or PARP, inhibitor from BioMarin Pharmaceutical Inc., or BioMarin. MDV3800 is currently in a Phase 3 clinical trial for the treatment of patients with germline BRCA, or gBRCA, mutated advanced breast cancer (i.e., advanced breast cancer in patients whose BRCA genes contain germline mutations). The Company is targeting a number of other indications in which to investigate MDV3800, including breast cancer (beyond gBRCA mutations), prostate cancer, small cell lung cancer, and ovarian cancer. In the fourth quarter of 2014, the Company licensed exclusive worldwide rights to pidilizumab (which is referred to as MDV9300), an antibody with immune-mediated anti-tumor effects for all potential indications from CureTech, Ltd., or CureTech. Under the license agreement, the Company is responsible for all development, regulatory, manufacturing, and commercialization activities for MDV9300. The Company initiated a Phase 2 clinical trial evaluating MDV9300 in patients with relapsed or refractory diffuse large B-cell lymphoma in the fourth quarter of 2015, which is on partial clinical hold pending its revision of certain investigator brochure, protocols and informed consent documents. The Company submitted the revised documents to the FDA in early February 2016 and the FDA has 30 days thereafter to notify the Company if the partial hold is lifted. The Company also plans to develop MDV9300 in other hematologic malignancies such as multiple myeloma.
In addition to the above activities, the Company has various internal research and discovery efforts focused in oncology, neurology and other areas.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP, and include the accounts of the Company and its subsidiaries. All intercompany transactions and balances have been eliminated in consolidation. The Company operates in one business segment.
All tabular disclosures of dollar and share amounts are presented in thousands unless otherwise indicated. All per share amounts are presented at their actual amounts. The number of shares issuable under the Amended and Restated 2004 Equity Incentive Award Plan, or the Medivation Equity Incentive Plan, and the Medivation, Inc. 2013 Employee Stock Purchase Plan, or ESPP, disclosed in Note 11, “Stockholders’ Equity,” are presented at their actual amounts unless otherwise indicated. Amounts presented herein may not calculate or sum precisely due to rounding.
Certain prior period amounts have been reclassified to conform to the current year presentation. There was no effect on net income (loss) or stockholders’ equity related to these reclassifications.
(b) Use of Estimates
The preparation of consolidated financial statements in accordance with U.S. GAAP requires that management make estimates and assumptions in certain circumstances that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on assumptions believed to be reasonable under the circumstances.
79
Although management believes that these estimates are reasonable, actual future results could differ materially from those estimates. In addition, had different estimates and assumptions been used, the consolidated financial statements could have differed materially from what is presented.
Significant estimates and assumptions used by management principally relate to revenue recognition, including reliance on third-party information, estimating the performance periods of the Company’s deliverables under collaboration agreements, and estimating the various deductions from gross sales to calculate net sales of XTANDI. Additionally, significant estimates and assumptions used by management include those related to contingent purchase consideration, intangible assets, goodwill, the Convertible Notes, determining whether the Company is the primary beneficiary of any variable interest entities, leases, taxes, research and development and other accruals, share-based compensation, and the calculation of diluted net income per common share.
(c) Capital Structure
On June 15, 2015, the Company filed a Certificate of Amendment to its Amended and Restated Certificate of Incorporation, as amended, effecting an increase in the total number of authorized shares of capital stock of the Company from 171,000,000 to 341,000,000 and an increase in the total number of authorized shares of common stock of the Company from 170,000,000 to 340,000,000.
On September 15, 2015, the Company effected a two-for-one forward stock split of its common stock in the form of a stock dividend. Stockholders of record as of August 13, 2015 received one additional share of the Company’s common stock, par value $0.01, for each share they held as of the record date. The Company issued approximately 81.7 million shares of its common stock as a result of the stock dividend. The par value of the Company’s common stock remained unchanged at $0.01 per share.
During the year ended December 31, 2015, the Company settled $258.8 million aggregate principal amount of its 2.625% convertible senior notes due April 1, 2017, or the Convertible Notes, through a combination of $259.9 million in cash and 5,638,576 shares of its common stock. Upon settlement, the Convertible Notes were no longer outstanding, interest ceased to accrue thereon, and all rights of the holders of the Convertible Notes ceased to exist.
Information regarding shares of common stock (except par value per share), par, additional paid-in capital, and net income (loss) per common share for all periods presented has been retroactively adjusted to reflect the effects of the stock split. The number of shares of the Company’s common stock issuable upon exercise of outstanding stock options and stock appreciation rights and vesting of other stock-based awards was proportionally increased, and the exercise price per share thereof, as applicable, was proportionally decreased, in accordance with the terms of the Medivation Equity Incentive Plan, as amended, and the ESPP.
(d) Collaboration Agreement Payments
The Company accounts for the various payment flows under its collaboration agreement with Astellas, or the Astellas Collaboration Agreement, as follows:
Estimated Performance Periods
The Astellas Collaboration Agreement contains multiple elements and deliverables, and required evaluation pursuant to Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, 605-25, “Revenue Recognition—Multiple-Element Arrangements.” The Company concluded that it had multiple deliverables under the Astellas Collaboration Agreement, including deliverables relating to grants of technology licenses, and performance of manufacturing, regulatory and clinical development activities in the United States. The period in which the Company performed its deliverables began in the fourth quarter of 2009 and concluded in the third quarter of 2015 upon substantial completion of the Company’s remaining performance obligations. The Company also concluded that its deliverables under the Astellas Collaboration Agreement should be accounted for as a single unit under ASC 605-25.
Estimation of the performance periods of the Company’s deliverables requires the use of management’s judgment. Significant factors considered in management’s evaluation of the estimated performance periods include, but are not limited to, the Company’s experience, along with its collaboration partners’ experience, in conducting manufacturing, clinical development and regulatory activities. The Company reviews the estimated duration of its performance periods under its collaboration agreements on a quarterly basis and makes any appropriate adjustments on a prospective basis.
Upfront Payments
The Company received a non-refundable, upfront cash payment of $110.0 million under the Astellas Collaboration Agreement. The Company recognized the payment as collaboration revenue on a straight-line basis over the applicable estimated performance period. As of December 31, 2015, there was no deferred revenue related to payments received under the Astellas Collaboration Agreement.
80
The Company has been eligible to receive milestone payments under the Astellas Collaboration Agreement based on achievement of specified development, regulatory and commercial events. Management evaluated the nature of the events triggering these contingent payments, and concluded that these events fall into two categories: (a) events which involve the performance of the Company’s obligations under the Astellas Collaboration Agreement, and (b) events which do not involve the performance of the Company’s obligations under the Astellas Collaboration Agreement.
The former category of milestone payments consist of those triggered by development and regulatory activities in the United States and by the acceptance for review of marketing applications in Europe and Japan. Management concluded that each of these payments, with one exception, constitute substantive milestone payments. This conclusion was based primarily on the facts that (i) each triggering event represented a specific outcome that could be achieved only through successful performance by the Company of one or more of its deliverables, (ii) achievement of each triggering event was subject to inherent risk and uncertainty and would result in additional payments becoming due to the Company, (iii) each of the milestone payments was non-refundable, (iv) substantial effort was required to complete each milestone, (v) the amount of each milestone payment was reasonable in relation to the value created in achieving the milestone, (vi) a substantial amount of time was expected to pass between the upfront payment and the potential milestone payments, and (vii) the milestone payments related solely to past performance. Based on the foregoing, the Company recognized any revenue from these milestone payments in the period in which the underlying triggering event occurs. The one exception is the milestone payment for initiation of the Phase 3 PREVAIL trial, an event which management deemed to be reasonably assured at the inception of the Astellas collaboration. This milestone payment was triggered in the third quarter of 2010, and the Company recognized it as collaboration revenue on a straight-line basis over the estimated remaining performance period of the Astellas Collaboration Agreement.
The latter category of milestone payments consist of those triggered by potential marketing approvals in Europe and Japan, and commercial activities globally, all of which were areas in which the Company had no pertinent contractual responsibilities under the Astellas Collaboration Agreement. Management concluded that these payments constitute contingent revenues and thus recognized them as revenue in the period in which the contingency was met.
As of December 31, 2015, the Company has earned all development and sales milestone payments under the Astellas Collaboration Agreement.
Royalties and Profit (Loss) Sharing Payments
Under the Astellas Collaboration Agreement, the Company shares equally profits (losses) on sales of products in the United States and receives royalties on sales of products outside the United States. The Company recognizes revenue from these events based on the revenue recognition criteria set forth in ASC 605-10-25-1, “Revenue Recognition.” Based on those criteria, the Company considers these payments to be contingent revenues, and recognizes them as revenue in the period in which the applicable contingency is resolved.
Cost-Sharing Payments
Under the Astellas Collaboration Agreement, the Company and Astellas share certain development and commercialization costs in the United States, including cost of goods sold and the royalty on net sales payable to The Regents of the University of California, or UCLA, under the Company’s license agreement with UCLA. The Company and Astellas make quarterly cost-sharing payments to one another in amounts necessary to ensure that each party bears its contractual share of the overall shared U.S. development and commercialization costs incurred. The Company’s policy is to account for cost-sharing payments to its collaboration partners as increases in expense in its consolidated statements of operations, while cost-sharing payments from its collaboration partners to the Company are accounted for as reductions in expense. Cost-sharing payments related to development activities and commercialization activities are recorded in research and development expenses, or R&D expenses, and selling, general and administrative expenses, or SG&A expenses, respectively.
Reliance on Third-Party Information
Under the Astellas Collaboration Agreement, Astellas records all XTANDI sales globally and has operational responsibility for certain development and commercialization activities in the United States for which the Company shares costs. Thus, Astellas has control over certain XTANDI-related financial information needed to prepare the Company’s financial statements and related disclosures, including information regarding gross sales, net sales, gross-to-net sales deductions, including estimates of potential future product returns, and shared U.S. development and commercialization costs incurred by Astellas. The Company is dependent on Astellas to provide it with such information in a timely and accurate manner for use in preparing the Company’s consolidated financial statements and disclosures. Certain of this information provided by Astellas is subject to estimates, including estimates used in determining gross-to-net revenue deductions such as payor mix, discounts (including legally mandated discounts to government entities), returns, chargebacks, rebates, and participation levels in patient assistance programs, and estimates regarding accrued development and commercialization costs incurred by Astellas. Under the Astellas Collaboration Agreement, the deductions from
81
gross sales used to derive net sales of XTANDI are determined in a manner consistent with GAAP, consistently applied. Should Astellas fail to provide the Company with any such financial information in a timely manner, or should any such financial information provided by Astellas, or any of the estimates upon which such financial information was based, prove to be inaccurate, the Company could be required to record adjustments in future periods.
(e) Research and Development Expenses and Accruals
R&D expenses are charged to expense as incurred unless there is an alternative future use in other research and development projects or otherwise. R&D expenses are comprised of costs incurred in performing research and development activities, including personnel-related costs, share-based compensation, and facilities-related overhead, outside contracted services including clinical trial costs, manufacturing and process development costs for both clinical and preclinical materials, research costs, upfront and development milestone payments under license agreements and other consulting services. Non-refundable advance payment for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made. In instances where the Company enters into agreements with third parties to provide research and development services, costs are expensed as services are performed. Amounts due under such arrangements may be either fixed fee or fee for service, and may include upfront payments, monthly payments, and payments upon the completion of milestones or receipt of deliverables.
The Company’s accruals for clinical trials and other research and development activities are based on estimates of the services received and efforts expended pursuant to contracts with numerous clinical trial centers, contract research organizations and clinical manufacturing organizations. In the normal course of business the Company contracts with third parties to perform various research and development activities in the on-going development of its product candidates, including, without limitation, third-party clinical trial centers and contract research organizations that perform and administer the Company’s clinical trials on its behalf and clinical manufacturing organizations that manufacture clinical trial materials. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under these agreements depend on factors such as the achievement of certain events, the successful enrollment of patients, and the completion of portions of the clinical trial or similar conditions. The objective of the Company’s accrual policy is to match the recording of expenses in its consolidated financial statements to the actual services received and efforts expended. As such, expense accruals related to clinical trials and other research and development activities are recognized based on the Company’s estimate of the degree of completion of the event or events specified in the specific agreement.
The Company’s accrual estimates are dependent upon the timeliness and accuracy of data provided by third parties regarding the status and cost of studies, and may not match the actual services performed by the organizations. During the course of a clinical trial, the Company adjusts its rate of clinical trial expense recognition if actual results differ from its estimates. The Company makes estimates of its accrued clinical trial expenses as of each balance sheet date based on facts and circumstances known at that time. Although the Company does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in the Company reporting amounts that are too high or too low for any particular period. This could result in adjustment to the Company’s R&D expense in future periods. The Company has had no significant adjustments to previously recorded research and development amounts.
(f) Promotional and Advertising Costs
Promotional and advertising costs are classified as SG&A expenses and are expensed as incurred. Promotional and advertising expenses consist primarily of the costs of designing, producing and distributing materials promoting the Company and its products, including its corporate website. Under the Astellas Collaboration Agreement, the Company and its collaboration partners share certain commercialization costs, including certain promotional and advertising costs, in the United States. See Note 3, “Collaboration Agreement,” for additional information regarding cost-sharing with its collaboration partners.
(g) Stock-Based Compensation
The Company has outstanding stock options, restricted stock units, and stock appreciation rights pursuant to the terms of the Medivation Equity Incentive Plan, and eligible employees may purchase shares pursuant to the ESPP. The Company accounts for stock-based compensation awards to employees and directors and ESPP shares in accordance with ASC 718, “Stock Compensation,” and stock-based compensation awards to consultants in accordance with ASC 505-50, “Equity-Based Payments to Non-Employees.”
Stock-based compensation expense associated with stock options is based on the estimated grant date fair value using the Black-Scholes valuation model, which requires the use of subjective assumptions related to the expected stock price volatility, option term, risk-free interest rate and dividend yield. The Company recognizes compensation expense over the vesting period of the awards that are ultimately expected to vest.
82
Stock-based compensation expense associated with restricted stock units is based on the fair value of the Company’s common stock on the grant date, which equals the closing market price of the Company’s common stock on the grant date. For restricted stock units, the Company recognizes compensation expense over the vesting period of the awards that are ultimately expected to vest.
The fair value of stock-settled and cash-settled stock appreciation rights is initially measured on the grant date using the Black-Scholes valuation model, which requires the use of subjective assumptions related to the expected stock price volatility, term, risk-free interest rate and dividend yield. Similar to stock options, compensation expense for stock-settled stock appreciation rights is recognized over the vesting period of the awards that are ultimately expected to vest based on the grant-date fair value. Cash-settled stock appreciation rights are liability-classified awards for which compensation expense and the liability are remeasured at each reporting date through the date of settlement based on the portion of the requisite service period rendered. Upon the conversion of cash-settled stock appreciation rights to stock-settled stock appreciation rights, the awards are remeasured using the then-current Black-Scholes assumptions and the remeasured liability is reclassified to additional paid-in capital.
The Company accounts for the ESPP as a compensatory plan. The fair value of each purchase under the Company’s ESPP is estimated on the date of the beginning of the offering period using the Black-Scholes valuation model, which requires the use of subjective assumptions related to the expected stock price volatility, term, risk-free interest rate and dividend yield. The Company recognizes compensation expense over the vesting period of the awards that are ultimately expected to vest.
Equity awards to consultants are typically remeasured at fair value at each reporting date until the awards vest.
The Company applies a forfeiture rate when determining stock-based compensation expense to account for an estimate of the granted awards not expected to vest. If actual forfeitures differ from the expected rate, the Company may be required to make additional adjustments to compensation expense in future periods.
The Black-Scholes valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable, characteristics not present in the Company’s stock options, stock appreciation rights, or ESPP shares. If the model permitted consideration of the unique characteristics of employee stock options, stock appreciation rights, and ESPP shares, the resulting estimate of fair value of the stock options, stock appreciation rights, and ESPP shares could be different. In addition, if the Company had made different assumptions and estimates for use in the Black-Scholes valuation model, the amount of recognized and to be recognized stock-based compensation expense could have been different.
(h) Cash and Cash Equivalents
Cash and cash equivalents are stated at cost, which approximates fair market value. Cash and cash equivalents consist of cash on deposit with banks, money market funds, and all highly liquid investments with a remaining maturity of three months or less at the time of purchase.
(i) Short-Term Investments
The Company considers all highly liquid investments with a remaining maturity at the time of acquisition of more than three months but no longer than 12 months to be short-term investments. The Company classifies its short-term investments as available-for-sale securities and reports them at fair value with related unrealized gains and losses included as a component of stockholders’ equity. The amortized cost of debt securities in this category is adjusted for amortization of premiums and accretion of discounts to maturity, which is included in other income (expense), net, on the consolidated statements of operations. Realized gains and losses and declines in value judged to be other-than-temporary, if any, on available-for-sale securities are included in other income (expense), net. The cost of securities sold is based on the specific identification method. Interest and dividends on securities classified as available-for-sale are included in other income (expense), net.
For the year ended December 31, 2015, total realized gains and losses on sales of available-for-sale securities were not material. There were no realized gains or losses from sales of available-for-sale securities for the years ended December 31, 2014 and 2013.
(j) Concentration of Credit Risk
The Company is subject to credit risk from its portfolio of cash, cash equivalents and short-term investments. The goals of the Company’s investment policy, in order of priority, are (1) the safety and preservation of principal, (2) maintain liquidity sufficient to meet the requirements of the Company’s operations and strategic initiatives, and (3) deliver competitive after-tax returns relative to stated objectives and market conditions. The Company’s investment policy limits investments by credit rating, maturity, industry group, investment type and issuer, except for securities issued by the U.S. government. Given this investment policy, the Company does not believe its exposure to credit risk with respect to the issuers of the securities in which it invests is material. The Company’s cash and cash equivalents are primarily invested in deposits and money market accounts with one major financial institution in the United States. Deposits in this financial institution may exceed the amount of insurance provided on such deposits.
83
Restricted cash represents certificates of deposit held in the Company’s name with a major financial institution to secure the Company’s contingent obligations under irrevocable letters of credit issued to certain of its lessors.
(l) Property and Equipment
Property and equipment are recorded at cost, less accumulated depreciation and amortization. Repairs and maintenance costs are expensed in the period incurred. Property and equipment is generally depreciated on a straight-line basis over the estimated useful lives of the assets as follows:
|
Description |
|
Estimated Useful Life |
|
Furniture and fixtures |
|
3-5 years |
|
Computer equipment and software |
|
3-5 years |
|
Laboratory equipment |
|
5 years |
Leasehold improvements are amortized over their estimated useful life or the related lease term, whichever is shorter.
(m) Leases
At the inception of a lease, the Company evaluates the lease agreement to determine whether the lease is an operating, capital or build-to-suit lease using the criteria in ASC 840, “Leases.”
Certain lease agreements also require the Company to make additional payments for taxes, insurance, and other operating expenses incurred during the lease period, which are expensed as incurred.
Operating Leases
For operating leases, the Company recognizes rent expense on a straight-line basis over the lease term and records the difference between cash rent payments and the recognition of rent expense as a deferred liability. Where lease agreements contain rent escalation clauses, rent abatements and/or concessions, such as rent holidays and tenant improvement allowances, the Company applies them in the determination of straight-line expense over the lease term.
Capital Leases
Capital leases are recorded as an asset within property and equipment, net and as an obligation at an amount equal to the present value of the minimum lease payments during the lease term. The asset is generally amortized over its estimated useful life or the related lease term, whichever is shorter. Lease payments under capital leases are recognized as a reduction of the capital lease obligation and interest expense. The Company currently does not have any capital leases.
Build-to-Suit Leases
In certain lease arrangements, the Company is involved in the construction of the building. To the extent the Company is involved with the structural improvements of the construction project or takes construction risk prior to the commencement of a lease, ASC 840-40, “Leases – Sale-Leaseback Transactions (Subsection 05-5),” requires the Company to be considered the owner for accounting purposes of these types of projects during the construction period. Therefore, the Company records an asset in property and equipment, net on the consolidated balance sheets, including capitalized interest costs, for the replacement cost of the Company’s portion of the pre-existing building plus the amount of estimated structural construction costs incurred by the landlord and the Company as of the balance sheet date. The Company records a corresponding build-to-suit lease obligation on its consolidated balance sheets representing the amounts paid by the lessor.
Once construction is complete, the Company considers the requirements for sale-leaseback accounting treatment, including evaluating whether all risks of ownership have been transferred back to the landlord, as evidenced by a lack of continuing involvement in the leased property. If the arrangement does not qualify for sale-leaseback accounting treatment, the building asset remains on the Company’s consolidated balance sheets at its historical cost, and such asset is depreciated over its estimated useful life. The Company bifurcates its lease payments into a portion allocated to the building and a portion allocated to the parcel of land on which the building has been built. The portion of the lease payments allocated to the land is treated for accounting purposes as operating lease payments, and therefore is recorded as rent expense in the consolidated statements of operations. The portion of the lease payments allocated to the building is further bifurcated into a portion allocated to interest expense and a portion allocated to reduce the build-to-suit lease obligation. The interest rate used for the build-to-suit lease obligation represents the Company’s estimated incremental borrowing rate at inception of the lease, adjusted to reduce any built in loss. The initial recording of these assets and liabilities is classified as non-cash investing and financing items, respectively, for purposes of the consolidated statements of cash flows.
84
Business combinations are accounted for under the acquisition method of accounting. The purchase price, including the fair value of any contingent consideration, is allocated between tangible and intangible assets acquired and liabilities assumed from the acquired business based on their estimated fair values, with the residual of the purchase price recorded as goodwill. Transaction costs are expensed as incurred.
Contingent Consideration
The Company determines the fair value of contingent consideration payable at the acquisition date using a probability-based income approach utilizing an appropriate discount rate. Each reporting period thereafter, the Company re-measures the contingent consideration and records increases or decreases in their fair value as non-cash adjustments in the consolidated statements of operations. Changes in the fair value of contingent consideration payable can result from adjustments to the estimated probability and assumed timing of achieving the underlying milestones, as well as from changes to the discount rates and periods.
In-Process Research and Development
In-process research and development, or IPR&D, represents the fair value assigned to incomplete research projects that the Company acquires through business combinations which, at the time of acquisition, have not reached technological feasibility. The amounts are capitalized and accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each project, the Company will make a determination as to the then useful life of the intangible asset, generally determined by the period in which the substantial majority of the cash flows are expected to be generated, and begin amortization over that period. The Company tests IPR&D for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the IPR&D is less than its carrying amount. If the Company concludes it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the IPR&D with its carrying value is performed. If the fair value is less than the carrying amount, a non-cash impairment change is recognized in the consolidated statements of operations.
Goodwill
Goodwill represents the excess of the consideration transferred over the fair value of net assets of businesses acquired. Goodwill is assigned to reporting units and tested at least annually for impairment or when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable, on an enterprise level by assessing qualitative factors or performing a quantitative analysis in determining whether it is more likely than not that its fair value exceeds the carrying value. Examples of qualitative factors include the Company’s share price, its financial performance compared to budgets, long-term financial plans, macroeconomic, industry and market conditions as well as the substantial excess of fair value over the carrying value of net assets. If the carrying value of goodwill exceeds its implied fair value, the excess is recorded as a non-cash impairment charge in the consolidated statement of operations.
(o) Convertible Notes
The debt and equity components of the Company’s Convertible Notes were bifurcated and accounted for separately based on the authoritative guidance in ASC 470-20, “Debt with Conversion and Other Options.” The debt component of the Convertible Notes, which excluded the associated equity conversion feature, was recorded at fair value on the issuance date. The equity component, representing the difference between the aggregate principal amount of the Convertible Notes and the fair value of the debt component, was recorded in additional paid-in capital on the consolidated balance sheet. The discounted carrying value of the Convertible Notes resulting from the bifurcation was subsequently accreted to its principal amount through the recognition of non-cash interest expense.
Costs related to the issuance of the Convertible Notes, consisting primarily of investment banking, legal and other professional fees were allocated to the debt and equity components of the Company’s Convertible Notes in proportion to the allocation of the principal. Amounts allocated to the debt component were capitalized and amortized as non-cash interest expense using the effective yield method over the five-year contract term of the Convertible Notes. Amounts allocated to the equity component were recorded against additional paid-in capital.
During the year ended December 31, 2015, all of the Company’s Convertible Notes were settled.
(p) Fair Value of Financial Instruments
The estimated fair value of the Company’s cash equivalents is based on quoted market prices. The estimated fair value of contingent consideration is determined utilizing a model that considers the probability of achieving each milestone and an appropriate discount rate. The estimated fair value of the Company’s Convertible Notes was determined using recent trading prices of the Convertible Notes. Other financial instruments, including bank deposits, receivable from collaboration partner, accounts payable,
85
accrued expenses, borrowings under the Revolving Credit Facility, and other current liabilities are carried at cost, which the Company believes approximates fair value because of the short-term maturities of these instruments.
(q) Litigation
The Company is party to legal proceedings, investigations, and claims in the ordinary course of its business. The Company records accruals for outstanding legal matters when it believes that it is both probable that a liability has been incurred and the amount of such liability can be reasonably estimated. The Company evaluates, on a quarterly basis, developments in significant legal matters that could affect the amount of any accrual and developments that would make a loss contingency both probable and reasonably estimable. To the extent new information is obtained and the Company’s views on the probable outcomes of claims, suits, assessments, investigations or legal proceedings change, changes in the Company’s accrued liabilities would be recorded in the period in which such determination is made. In addition, in accordance with the relevant authoritative guidance, for matters for which the likelihood of material loss is at least reasonably possible, the Company provides disclosure of the possible loss or range of loss; however, if a reasonable estimate cannot be made, the Company will provide disclosure to that effect. Gain contingencies, if any, are recorded when they are realized.
(r) Income Taxes
The Company accounts for income taxes using an asset and liability approach in accordance with the guidance provided by ASC 740-10, “Accounting for Income Taxes.” ASC 740-10 requires the recognition of taxes payable or refundable for the current year and deferred tax assets and liabilities for the future tax consequences of events that have been recognized in the consolidated financial statements or tax returns. The measurement of current and deferred tax assets and liabilities is based on provisions of the enacted tax law; the effects of future changes in tax laws or rates are not anticipated. The measurement of deferred tax assets is reduced, if necessary, by the amount of any tax benefits that, based on available evidence, is not expected to be realized.
The Company records a valuation allowance to reduce its deferred tax assets to reflect the net amount that it believes is more likely than not to be realized. Realization of the deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. Based upon the weight of available evidence at December 31, 2014, the Company determined that it was more likely than not that a portion of its deferred tax assets would be realizable and consequently released the valuation allowance against Federal and certain state net deferred tax assets and recorded a discrete tax benefit of $33.4 million during the fourth quarter of 2014. The decision to reverse a portion of the valuation allowance was made after management considered all available evidence, both positive and negative, including but not limited to the Company’s historical operating results, income or loss in recent periods, cumulative income in recent years, forecasted earnings, forecasted future taxable income, and significant risk and uncertainty related to forecasts. The release of the valuation allowance resulted in the recognition of certain deferred tax assets and a decrease to income tax expense.
Significant judgment in required in evaluating the Company’s uncertain income tax positions based on the guidance in ASC 740-10-25, “Accounting for Uncertainty in Income Taxes.” The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not that the position will be sustained upon examination by tax authorities. The tax benefit recognized in the financial statements on a particular tax position is measured on the largest benefit that is more likely than not to be realized. The Company evaluates uncertain tax positions on a quarterly basis and adjusts the liability for changes in facts and circumstances, such as new regulations or interpretations by the taxing authorities, new information obtained during a tax examination, significant amendment to an existing tax law, or resolution of an examination. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences will impact the income tax provision in the period in which such determination is made. The resolution of the Company’s uncertain income tax positions is dependent on uncontrollable factors such as law changes, new case law, and the willingness of the income tax authorities to settle, including the timing thereof and other factors. Although The Company does not anticipate significant changes to its uncertain income tax positions in the next twelve months, items outside of the Company’s control could cause the uncertain income tax positions to change in the future, which would be recorded in the consolidated statements of operations. Interest and/or penalties related to income tax matters are recognized as a component of income tax expense as incurred.
(s) New Accounting Pronouncements
In November 2015, the FASB issued Accounting Standards Update, or ASU, 2015-17, “Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes.” ASU 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. The amended guidance is effective for fiscal years beginning after December 15, 2016, including interim periods within that reporting period, and may be applied either prospectively to all deferred tax liabilities and assets or retrospectively to all periods presented. Earlier application is permitted for all entities as of the beginning of an interim or annual reporting period. The Company adopted the amended guidance prospectively as of October 1, 2015. Prior periods were not retrospectively adjusted. The adoption of ASU 2015-17 resulted in a reclassification of current deferred tax assets to non-current on the Company’s consolidated balance sheet as of December 31, 2015. See Note 13, “Income Taxes,” for additional information regarding the adoption of ASU 2015-17.
86
In September 2015, the FASB issued ASU 2015-16, “Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments.” ASU 2015-16 requires that the acquirer recognize adjustments to provisional amounts recognized in a business combination that are identified during the measurement period in the reporting period in which the adjustment amounts are determined and record, in the same period’s financial statements, the effect on earnings of changes in depreciation, amortization, or other income effects, if any, as a result of the change to the provisional amounts, calculated as if the accounting had been completed at the acquisition date. The amended guidance eliminates the requirement to retrospectively account for adjustments made to provisional amounts during the measurement period. The amended guidance is effective for fiscal years beginning after December 15, 2015, including interim periods within that reporting period, and should be applied prospectively to provisional amounts that occur after the effective date. The Company does not currently expect that the adoption of ASU 2015-16 will have a material impact on its consolidated financial statements and related disclosures.
In August 2015, the FASB issued ASU 2015-15, “Interest – Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line of Credit Arrangements.” The amended guidance, which became effective immediately upon issuance, clarifies that entities are permitted to defer and present such debt issuance costs as an asset to be amortized ratably over the term of the line of credit arrangement, regardless of whether there are any outstanding borrowings on the line of credit arrangement. The adoption of ASU 2015-15 did not have a material impact on the Company’s consolidated financial statements and related disclosures.
In April 2015, the FASB issued ASU 2015-03, “Interest – Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs,” which requires that debt issuance costs related to a recognized debt liability be presented on the balance sheet as a direct deduction from the carrying amount of the debt liability, consistent with debt discounts. The ASU requires retrospective adoption and is effective for fiscal years beginning after December 15, 2015, including interim periods within that reporting period. Early adoption is permitted. The Company does not currently expect that the adoption of ASU 2015-03 will have a material impact on its consolidated financial statements and related disclosures.
In February 2015, the FASB issued ASU 2015-02, “Consolidation (Topic 820): Amendments to the Consolidation Analysis.” The amended guidance provides a revised consolidation model for all reporting entities to use in evaluating whether they should consolidate certain legal entities. All legal entities will be subject to reevaluation under this revised consolidation model. The revised consolidation model, among other things, (i) modifies the evaluation of whether limited partnerships and similar legal entities are voting interest entities, (ii) eliminates the presumption that a general partner should consolidate a limited partnership, and (iii) modifies the consolidation analysis of reporting entities that are involved with voting interest entities through fee arrangements and related party relationships. The amended guidance is effective for fiscal years beginning after December 15, 2015, including interim periods within that reporting period. The Company does not currently expect that the adoption of ASU 2015-02 will have a material impact on its consolidated financial statements and related disclosures.
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers” (Topic 606), a comprehensive new revenue recognition standard that will supersede the existing revenue recognition guidance. The new accounting guidance creates a framework by which an entity will allocate the transaction price to separate performance obligations and recognize revenue when (or as) each performance obligation is satisfied. Under the new standard, entities will be required to use judgment and make estimates, including identifying performance obligations in a contract, estimating the amount of variable consideration to include in the transaction price, allocating the transaction price to each separate performance obligation and determining when an entity satisfies its performance obligations. The standard allows for either “full retrospective” adoption, meaning that the standard is applied to all of the periods presented with a cumulative catch-up as of the earliest period presented, or “modified retrospective” adoption, meaning the standard is applied only to the most current period presented in the financial statements with a cumulative catch-up as of the current period. In August 2015, the effective date of the new revenue standard was delayed by one year to December 15, 2017 for annual reporting periods beginning after that date. The FASB also agreed to permit early adoption of the standard, but not before the original effective date of December 15, 2016. The Company has not yet selected a transition method and is currently evaluating the effect that the updated standard will have on its consolidated financial statements and related disclosures.
(t) Out-of-Period Adjustment
In the first quarter of 2013, the Company recorded an out-of-period correcting adjustment that increased operating expenses and net loss by $3.6 million for the three months ended March 31, 2013. Management concluded that the adjustment is not material to the full year 2013 results or any previously reported financial statements.
NOTE 3. COLLABORATION AGREEMENT
(a) Collaboration Agreement with Astellas
In October 2009, the Company entered into the Astellas Collaboration Agreement, pursuant to which it is collaborating with Astellas to develop and commercialize XTANDI globally for all indications, dosages and formulations of enzalutamide. Under the agreement, decision making and economic participation differs between the U.S. market and the ex-U.S. market. In the United States,
87
decisions are generally made by consensus, pre-tax profits and losses are shared equally, and, subject to certain exceptions, development and commercialization costs (including cost of goods sold and the royalty on net sales payable to UCLA under the Company’s license agreement with UCLA) are also shared equally. The primary exceptions to equal cost sharing in the U.S. market are that each party is responsible for its own commercial full-time equivalent, or FTE, costs, and that development costs supporting marketing approvals in both the United States and either Europe or Japan are borne one-third by the Company and two-thirds by Astellas. The Company and Astellas are co-promoting XTANDI in the U.S. market, with each company providing half of the sales and medical affairs effort in support of the product. Both the Company and Astellas are entitled to receive a fee for each qualifying detail made by its respective sales representatives. Outside the United States, decisions are generally made by Astellas and all development and commercialization costs (including cost of goods sold and the royalty on net sales payable to UCLA) are borne by Astellas. Astellas retains all ex-U.S. profits and losses, and pays the Company a tiered royalty ranging from the low teens to the low twenties as a percentage of the aggregate net sales of XTANDI outside the United States, or ex-U.S. XTANDI net sales. Astellas has sole responsibility for promoting XTANDI outside the United States and for recording all XTANDI net sales both inside and outside the United States. Both the Company and Astellas have agreed not to commercialize certain other products having a similar mechanism of action (as defined by the Astellas Collaboration Agreement) as XTANDI for the treatment of prostate cancer for a specified time period, subject to certain exceptions.
Under the Astellas Collaboration Agreement, Astellas paid the Company a non-refundable, upfront cash payment of $110.0 million in the fourth quarter of 2009. The Company was also eligible to receive up to $335.0 million in development milestone payments and up to $320.0 million in sales milestone payments. As of December 31, 2015, the Company has earned all development and sales milestone payments under the Astellas Collaboration Agreement.
The Company and Astellas are each permitted to terminate the Astellas Collaboration Agreement for an uncured material breach by the other party or for the insolvency of the other party. Astellas has a right to terminate the Astellas Collaboration Agreement unilaterally by advance written notice to the Company. Following any termination of the Astellas Collaboration Agreement in its entirety, all rights to develop and commercialize XTANDI will revert to the Company, and Astellas will grant a license to the Company to enable it to continue such development and commercialization. In addition, except in the case of a termination by Astellas for the Company’s material breach, Astellas will supply XTANDI to the Company during a specified transition period.
Unless terminated earlier by the Company or Astellas pursuant to the terms thereof, the Astellas Collaboration Agreement will remain in effect: (a) in the United States, until such time as Astellas notifies the Company that Astellas has permanently stopped selling products covered by the Astellas Collaboration Agreement in the United States; and (b) in each other country of the world, on a country-by-country basis, until such time as (i) products covered by the Astellas Collaboration Agreement cease to be protected by patents or regulatory exclusivity in such country and (ii) commercial sales of generic equivalent products have commenced in such country.
(b) Collaboration Revenue
Collaboration revenue was as follows:
|
|
|
Years Ended December 31, |
|
|||||||||||
|
|
|
2015 |
|
|
2014 |
|
|
|
|
2013 |
|
|||
|
Collaboration revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Related to U.S. XTANDI net sales |
|
$ |
575,658 |
|
|
$ |
339,902 |
|
|
|
|
$ |
196,208 |
|
|
Related to ex-U.S. XTANDI net sales |
|
|
119,778 |
|
|
|
49,476 |
|
|
|
|
|
6,338 |
|
|
Related to upfront and milestone payments |
|
|
247,822 |
|
|
|
321,109 |
|
|
|
|
|
70,396 |
|
|
Total |
|
$ |
943,258 |
|
|
$ |
710,487 |
|
|
|
|
$ |
272,942 |
|
The Company is required to pay UCLA ten percent of all Sublicensing Income, as defined in its license agreement with UCLA. The Company is currently involved in litigation with UCLA regarding certain terms of the license agreement and other matters, which are discussed in Note 15, “Commitments and Contingencies.”
Collaboration Revenue Related to U.S. XTANDI Net Sales
Under the Astellas Collaboration Agreement, Astellas records all U.S. XTANDI net sales. The Company and Astellas share equally all pre-tax profits and losses from U.S. XTANDI net sales. Subject to certain exceptions, the Company and Astellas also share equally all XTANDI development and commercialization costs attributable to the U.S. market, including cost of goods sold and the royalty on net sales payable to UCLA under the Company’s license agreement with UCLA. The primary exceptions to the 50/50 cost sharing are that each party is responsible for its own commercial FTE costs and that development costs supporting marketing approvals in both the United States and either Europe or Japan are borne one-third by the Company and two-thirds by Astellas. The Company recognizes collaboration revenue related to U.S. XTANDI net sales in the period in which such sales occur. Collaboration revenue related to U.S. XTANDI net sales consists of the Company’s share of pre-tax profits and losses from U.S. XTANDI net sales,
88
plus reimbursement of the Company’s share of reimbursable U.S. development and commercialization costs. The Company’s collaboration revenue related to U.S. XTANDI net sales in any given period is equal to 50% of U.S. XTANDI net sales as reported by Astellas for the applicable period.
Collaboration revenue related to U.S. XTANDI net sales was as follows:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
U.S. XTANDI net sales (as reported by Astellas) |
|
$ |
1,151,317 |
|
|
$ |
679,805 |
|
|
$ |
392,415 |
|
|
Shared U.S. development and commercialization costs |
|
|
(375,008 |
) |
|
|
(323,730 |
) |
|
|
(241,106 |
) |
|
Pre-tax U.S. profit |
|
$ |
776,309 |
|
|
$ |
356,075 |
|
|
$ |
151,309 |
|
|
Medivation’s share of pre-tax U.S. profit |
|
$ |
388,154 |
|
|
$ |
178,037 |
|
|
$ |
75,655 |
|
|
Reimbursement of Medivation’s share of shared U.S. costs |
|
|
187,504 |
|
|
|
161,865 |
|
|
|
120,553 |
|
|
Collaboration revenue related to U.S. XTANDI net sales |
|
$ |
575,658 |
|
|
$ |
339,902 |
|
|
$ |
196,208 |
|
Collaboration Revenue Related to Ex-U.S. XTANDI Net Sales
Under the Astellas Collaboration Agreement, Astellas records all ex-U.S. XTANDI net sales. Astellas is responsible for all development and commercialization costs for XTANDI outside the United States, including cost of goods sold and the royalty on net sales payable to UCLA under the Company’s license agreement with UCLA, and pays the Company a tiered royalty ranging from the low teens to the low twenties on net ex-U.S. XTANDI net sales. The Company recognizes collaboration revenue related to ex-U.S. XTANDI net sales in the period in which such sales occur. Collaboration revenue related to ex-U.S. XTANDI net sales consists of royalties from Astellas on those sales.
Collaboration Revenue Related to Upfront and Milestone Payments
Collaboration revenue related to upfront and milestone payments from Astellas was as follows:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Sales milestones earned |
|
$ |
245,000 |
|
|
$ |
50,000 |
|
|
$ |
25,000 |
|
|
Development milestones earned |
|
|
— |
|
|
|
257,000 |
|
|
|
20,000 |
|
|
Amortization of deferred upfront and development milestones |
|
|
2,822 |
|
|
|
14,109 |
|
|
|
25,396 |
|
|
Total |
|
$ |
247,822 |
|
|
$ |
321,109 |
|
|
$ |
70,396 |
|
As of December 31, 2015, the Company has earned all development and sales milestone payments under the Astellas Collaboration Agreement, including $245.0 million, $307.0 million, and $45.0 million in the years ended December 31, 2015, 2014, and 2013, respectively.
Deferred revenue related to payments received under the Astellas Collaboration Agreement was $2.8 million at December 31, 2014. There was no deferred revenue related to payments received under the Astellas Collaboration Agreement at December 31, 2015.
(c) Cost-Sharing Payments
Under the Astellas Collaboration Agreement, the Company and Astellas share certain development and commercialization costs (including cost of goods sold and the royalty on net sales payable to UCLA under the Company’s license agreement with UCLA) in the United States. Development cost-sharing payments from Astellas were $60.8 million, $63.5 million and $46.6 million for the years ended December 31, 2015, 2014 and 2013, respectively. Commercialization cost-sharing payments to Astellas were $37.5 million, $36.1 million and $12.0 million for the years ended December 31, 2015, 2014 and 2013, respectively. Development cost-sharing payments from Astellas are recorded as reductions in R&D expenses. Commercialization cost-sharing payments to Astellas are recorded as increases in SG&A expenses.
(d) Collaboration Receivable
At December 31, 2015 and 2014, collaboration receivable from Astellas was $391.6 million and $184.7 million, respectively. The amounts receivable at December 31, 2015 and 2014 were received in the first quarter of 2016 and 2015, respectively.
89
(a) Acquisition of MDV3800 from BioMarin
In the fourth quarter of 2015, the Company completed an acquisition of all rights to MDV3800, a PARP inhibitor, from BioMarin pursuant to an Asset Purchase Agreement. The acquired MDV3800 assets include all patents, data, know-how, third-party agreements, regulatory materials and pre-commercial inventories. The Company also assumed certain costs for ongoing clinical trials of MDV3800, and commitments under certain agreements previously entered into or assumed by BioMarin and assigned to the Company. The parties entered into a Transition Services Agreement at the closing of the transaction to facilitate the transition of the research and development activities relating to MDV3800 from BioMarin to the Company, including responsibility for the ongoing clinical studies.
The Company has concluded that the acquisition of MDV3800 from BioMarin is an acquisition of a business and will account for it in accordance with ASC 805-10, “Business Combinations.” The acquisition was completed on October 6, 2015, and results of operations related to MDV3800 since that date have been included in the consolidated statement of operations and were not significant.
In connection with the acquisition, the Company paid BioMarin an upfront cash payment of $410.0 million during the fourth quarter of 2015. In addition, BioMarin is entitled to contingent payments totaling up to $160.0 million upon the achievement of defined regulatory and sales-based milestones, and mid-single digit royalties on net sales of products that contain MDV3800 during the royalty term specified in the Asset Purchase Agreement. The acquisition-date fair value of the contingent consideration payments totaled $171.9 million and was estimated by applying a probability-based income model using a discounted cash flow analysis for contingent regulatory and royalty payments and a Monte Carlo simulation model for the contingent sales milestone payments. The estimation was based on significant inputs that are not observable in the market, referred to as Level 3 inputs, as described in more detail in Note 14, “Fair Value Disclosures.”
The following table presents the final allocation of the purchase consideration for the MDV3800 acquisition, including the contingent consideration payable, based on fair value:
|
Purchase consideration: |
|
|
|
|
|
Cash |
|
$ |
410,000 |
|
|
Acquisition-date fair value of contingent consideration |
|
|
171,942 |
|
|
Total purchase consideration |
|
$ |
581,942 |
|
|
Allocation of the purchase consideration: |
|
|
|
|
|
Assets: |
|
|
|
|
|
Identifiable intangible assets- IPR&D |
|
$ |
573,299 |
|
|
Net identifiable assets acquired |
|
|
573,299 |
|
|
Goodwill |
|
|
8,643 |
|
|
Net assets acquired |
|
$ |
581,942 |
|
Identifiable intangible assets totaled $573.3 million and consist entirely of IPR&D for MDV3800. As of the valuation date, the Company determined that MDV3800 was the only R&D project with substance, such that the project had undergone conceptual stages, and research, development, and preproduction had been started for the project. As such, no other intangible assets were identified in the transaction other than MDV3800 as separate and apart from goodwill. The Company utilized the multi-period excess earnings model of the “income method” to determine the fair value of the IPR&D as of the acquisition date. The excess of the consideration over the fair values assigned to the net assets acquired was $8.6 million, which represents the amount of goodwill resulting from the acquisition. The Company believes that the goodwill primarily represents benefits to it, such as the potential to diversify its product portfolio in the area of oncology, that do not qualify for separate recognition as acquired intangible assets. The amount of goodwill that is expected to be deductible for income tax purposes is $8.6 million. The Company recorded the goodwill on its consolidated balance sheet as of the acquisition date.
90
Pro forma Financial Information
The following unaudited pro forma financial information presents the combined results of operations of Medivation and MDV3800 as if the acquisition of MDV3800 had been completed on January 1, 2014, with adjustments to give effect to pro forma events that are directly attributable to the acquisition. The unaudited pro forma results do not reflect any operating efficiencies or potential cost savings which may result from the consolidation of the operations of Medivation and MDV3800. Accordingly, these unaudited pro forma results are presented for informational purposes only and are not necessarily indicative of what the actual results of operations of the combined company would have been if the acquisition had occurred at the beginning of the period presented, nor are they indicative of future results of operations:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Total revenues |
|
$ |
943,258 |
|
|
$ |
710,487 |
|
|
Net income |
|
$ |
201,161 |
|
|
$ |
230,271 |
|
(b) Acquisition of MDV9300 from CureTech
In the fourth quarter of 2014, the Company entered into a License Agreement with Israel-based CureTech, pursuant to which it licensed exclusive worldwide rights to CureTech’s late-stage clinical molecule MDV9300, an antibody with immune-mediated anti-tumor effects. Under the License Agreement, the Company is responsible for all development, regulatory, manufacturing, and commercialization activities for MDV9300 for all indications, including oncology. The Company has concluded that the in-license transaction is an acquisition of a business and will account for it in accordance with ASC 805-10, “Business Combinations.”
In connection with the acquisition, the Company made upfront cash payments to CureTech totaling $5.0 million during the fourth quarter of 2014. In addition, CureTech is entitled to contingent payments totaling up to $85.0 million upon attainment of certain development and regulatory milestones, up to $245.0 million upon the achievement of certain annual worldwide net sales thresholds, and tiered royalties ranging from 5% to 11% on annual worldwide net sales. CureTech is also entitled to a $5.0 million milestone payment upon completion of the Manufacturing Technology Transfer as described in Note 15, “Commitments and Contingencies.” The acquisition-date fair value of the contingent consideration payments totaled $106.0 million and was estimated by applying a probability-based income approach using an appropriate discount rate. The estimation was based on significant inputs that are not observable in the market, referred to as Level 3 inputs, as described in more detail in Note 14, “Fair Value Disclosures.”
The following table presents the final allocation of the purchase consideration for the MDV9300 acquisition, including the contingent consideration payable, based on fair value:
|
Purchase consideration: |
|
|
|
|
|
Cash |
|
$ |
5,000 |
|
|
Acquisition-date fair value of contingent consideration |
|
|
106,000 |
|
|
Total purchase consideration |
|
$ |
111,000 |
|
|
Allocation of the purchase consideration: |
|
|
|
|
|
Assets: |
|
|
|
|
|
Identifiable intangible assets- IPR&D |
|
$ |
101,000 |
|
|
Net identifiable assets acquired |
|
|
101,000 |
|
|
Goodwill |
|
|
10,000 |
|
|
Net assets acquired |
|
$ |
111,000 |
|
Identifiable intangible assets totaled $101.0 million and consist entirely of IPR&D for MDV9300. As of the valuation date, the Company determined that MDV9300 was the only R&D project with substance, such that the project had undergone conceptual stages, and research, development, and preproduction had been started for the project. As such, no other intangible assets were identified in the transaction other than MDV9300 as separate and apart from goodwill. The Company utilized the multi-period excess earnings model of the “income method” to determine the fair value of the IPR&D as of the acquisition date. The excess of the consideration over the fair values assigned to the net assets acquired was $10.0 million, which represents the amount of goodwill resulting from the acquisition. The Company believes that the goodwill primarily represents benefits to it, such as the potential to diversify its product portfolio in the area of oncology, that do not qualify for separate recognition as acquired intangible assets. The amount of goodwill that is expected to be deductible for income tax purposes is $10.0 million. The Company recorded the goodwill on its consolidated balance sheet as of the acquisition date.
Pro forma Financial Information
The following unaudited pro forma financial information presents the combined results of operations of Medivation and MDV9300 as if the acquisition of MDV9300 had been completed on January 1, 2013, with adjustments to give effect to pro forma
91
events that are directly attributable to the acquisition. The unaudited pro forma results do not reflect any operating efficiencies or potential cost savings which may result from the consolidation of the operations of Medivation and MDV9300. Accordingly, these unaudited pro forma results are presented for informational purposes only and are not necessarily indicative of what the actual results of operations of the combined company would have been if the acquisition had occurred at the beginning of the period presented, nor are they indicative of future results of operations:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2014 |
|
|
2013 |
|
||
|
Total revenues |
|
$ |
710,487 |
|
|
$ |
272,942 |
|
|
Net income (loss) |
|
$ |
274,626 |
|
|
$ |
(45,685 |
) |
NOTE 5. INTANGIBLE ASSETS AND GOODWILL
Intangible assets consist of IPR&D acquired from business acquisitions. The Company accounts for IPR&D as indefinite-lived intangible assets until regulatory approval or discontinuation of the related R&D efforts. Upon obtaining regulatory approval, the Company reclassifies the IPR&D as a definite-lived intangible asset and determines the economic life for amortization purposes. The Company assesses the impairment of indefinite-lived intangible assets and goodwill on an annual basis or more frequently whenever events or changes in circumstances may indicate that the carrying value might not be recoverable.
During the fourth quarter of 2015, the Company recorded a $30.0 million impairment charge related to its IPR&D asset for MDV9300 as a result of an extended clinical development timeline and other factors. In the fourth quarter, the Company concluded its testing that revealed MDV9300 is not an anti-PD-1 antibody and, after advising the FDA of this conclusion in early-January 2016, its IND was placed on partial clinical hold pending revision of all related study documentation characterizing MDV9300 as an anti-PD-1 antibody. While the Company believes the partial clinical hold does not relate to concerns regarding the safety or previously-observed efficacy of MDV9300, the Company extended its clinical study timelines which, in turn, gave rise to changes to the significant inputs to its IPR&D valuation including forecasted revenues and probabilities of success. The quantitative assessment resulted in a net impairment charge, which the Company recorded as a reduction in the IPR&D asset and an increase to R&D expense in the fourth quarter of 2015.
The following table summarizes the Company’s indefinite-lived intangible assets:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Indefinite-lived intangible asset – MDV3800 |
|
$ |
573,299 |
|
|
|
— |
|
|
Indefinite-lived intangible asset – MDV9300 |
|
|
71,000 |
|
|
|
101,000 |
|
|
Total |
|
$ |
644,299 |
|
|
$ |
101,000 |
|
The following table summarizes the changes in the carrying amount of goodwill:
|
|
Year Ended December 31, 2015 |
|
|
|
Goodwill: |
|
|
|
|
Balance at beginning of period |
$ |
10,000 |
|
|
Goodwill at acquisition date – MDV3800 |
|
8,643 |
|
|
Balance at end of period |
$ |
18,643 |
|
NOTE 6. NET INCOME (LOSS) PER COMMON SHARE
The computation of basic net income (loss) per common share is based on the weighted-average number of common shares outstanding during each period. The computation of diluted net income (loss) per common share is based on the weighted-average number of common shares outstanding during the period plus, when their effect is dilutive, incremental shares consisting of shares subject to stock options, restricted stock units, stock appreciation rights, ESPP shares, warrants, and shares issuable upon conversion of convertible debt.
92
During the second quarter of 2015, the Company asserted its intent and ability to settle the outstanding Convertible Notes for a combination of cash and common stock. Under the “cash settlement” method, interest is not added back to the numerator, and only the contingently issuable shares related to the conversion spread are included in the denominator, if dilutive. Under such method, the settlement of the conversion spread has a dilutive effect when the average share price of the Company’s common stock during the period exceeds the conversion price. The computation of diluted net income per share for the year ended December 31, 2015 reflects the application of the “if-converted” method for the first quarter of 2015 and the “cash settlement” method for the second and third quarters of 2015 given the demonstrated and asserted redemption of the outstanding debt. The Company completed the settlement of all of its Convertible Notes during the third quarter of 2015 as described in Note 10, “Debt.” The calculation of diluted net income per common share for the year ended December 31, 2015 includes approximately 3.9 million contingently issuable shares related to the Convertible Notes.
The Company used the “if-converted” method to compute the dilutive effect of the Convertible Notes for the calculation of diluted net income per common share for the year ended December 31, 2014. Under the “if-converted” method, interest expense, net of tax, related to the Convertible Notes is added back to net income, and the Convertible Notes are assumed to have been converted into common shares at the beginning of the period in periods in which there would have been a dilutive effect. The calculation of diluted net income per common share for the year ended December 31, 2014 includes approximately 10.1 million contingently issuable shares related to the Convertible Notes.
In periods in which the Company reports a net loss, all common stock equivalents are deemed anti-dilutive such that basic net loss per common share and diluted net loss per common share are equal.
For the years ended December 31, 2015 and 2014, employee stock-based awards to purchase approximately 1.4 million and 1.3 million shares of the Company’s common stock, respectively, were excluded from the computation of diluted net income per common share because their effect would have been anti-dilutive. For the year ended December 31, 2013, approximately 25.1 million potentially dilutive common shares were excluded from the diluted net loss per common share computation because such securities had an anti-dilutive effect on net loss per common share due to the Company’s net loss for that year.
The following table reconciles the numerator and denominator used to calculate diluted net income (loss) per common share:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
244,725 |
|
|
$ |
276,452 |
|
|
$ |
(42,613 |
) |
|
Interest expense on convertible notes, net of tax |
|
|
3,629 |
|
|
|
14,030 |
|
|
|
— |
|
|
Numerator for diluted net income (loss) per common share calculation |
|
$ |
248,354 |
|
|
$ |
290,482 |
|
|
$ |
(42,613 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares, basic |
|
|
160,345 |
|
|
|
153,859 |
|
|
|
150,331 |
|
|
Dilutive effect of common stock equivalents |
|
|
8,979 |
|
|
|
16,142 |
|
|
|
— |
|
|
Weighted-average common shares, diluted |
|
|
169,324 |
|
|
|
170,001 |
|
|
|
150,331 |
|
NOTE 7. BUILD-TO-SUIT LEASE OBLIGATION
In the fourth quarter of 2013, the Company entered into a property lease for approximately 52,000 square feet of space located in San Francisco, California. In the second quarter of 2015, the Company entered into an amended lease agreement to reduce the amount of leased space at this property to approximately 44,000 square feet. The lease agreement expires in August 2024, and the Company has an option to extend the lease term for up to an additional five years.
The Company is deemed, for accounting purposes only, to be the owner of the entire project including the building shell, even though it is not the legal owner. In connection with the Company’s accounting for this transaction, the Company capitalized $14.5 million as a build-to-suit property within property and equipment, net, and recognized a corresponding build-to-suit lease obligation for the same amount. The Company has also recognized, as an additional build-to-suit lease property and obligation, structural tenant improvements totaling $3.6 million for amounts paid by the landlord and $3.5 million for capitalized interest during the construction period through December 31, 2015.
As a result of the amended agreement, the Company surrendered a portion of the property totaling approximately 8,000 square feet to the lessor. The Company has no continuing involvement with the portion of the property surrendered to the lessor. Accordingly, the Company derecognized a portion of the build-to-suit asset totaling $3.2 million and a portion of the build-to-suit lease obligation totaling $3.2 million during 2015 related to the portion of the property that was surrendered to the lessor.
93
A portion of the monthly lease payment is allocated to land rent and recorded as an operating lease expense and the non-interest portion of the amortized lease payments to the landlord related to the rent of the building is applied to reduce the build-to-suit lease obligation. At December 31, 2015, the build-to-suit lease obligation totaling $17.4 million was classified as a non-current liability on the consolidated balance sheet. Expected reductions (increases) in the build-to-suit lease obligation are as follows:
|
Years Ending December 31, |
|
Build-To-Suit Lease Obligation |
|
|
|
2016 |
|
$ |
(8 |
) |
|
2017 |
|
|
58 |
|
|
2018 |
|
|
130 |
|
|
2019 |
|
|
208 |
|
|
2020 |
|
|
292 |
|
|
2021 and thereafter |
|
|
16,726 |
|
|
Total |
|
$ |
17,406 |
|
The amounts included in the table above represent the reductions (increases) in the build-to-suit lease obligation included on the Company’s consolidated balance sheet at December 31, 2015 related to each of the periods presented. The amount in the terminal period includes the amount to derecognize the build-to-suit lease obligation at the end of the lease term. The expected reductions (increases) in the build-to-suit lease obligation presented in the table above are impacted by the timing of the completion of the construction project. Actual expected lease payments under the build-to-suit lease obligation are included in Note 15, “Commitments and Contingencies.”
NOTE 8. PROPERTY AND EQUIPMENT, NET
Property and equipment, net, consisted of the following:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Build-to-suit property |
|
$ |
18,371 |
|
|
$ |
19,544 |
|
|
Leasehold improvements |
|
|
19,074 |
|
|
|
15,051 |
|
|
Computer equipment and software |
|
|
16,083 |
|
|
|
9,499 |
|
|
Furniture and fixtures |
|
|
5,714 |
|
|
|
4,667 |
|
|
Construction in progress |
|
|
14,440 |
|
|
|
1,360 |
|
|
Laboratory equipment |
|
|
748 |
|
|
|
735 |
|
|
|
|
|
74,430 |
|
|
|
50,856 |
|
|
Less: Accumulated depreciation |
|
|
(16,288 |
) |
|
|
(9,695 |
) |
|
Total |
|
$ |
58,142 |
|
|
$ |
41,161 |
|
NOTE 9. ACCOUNTS PAYABLE, ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accounts payable, accrued expenses and other current liabilities consisted of the following:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Clinical and preclinical |
|
$ |
48,975 |
|
|
$ |
31,069 |
|
|
Taxes payable |
|
|
32,565 |
|
|
|
2,106 |
|
|
Payroll and payroll-related |
|
|
29,215 |
|
|
|
33,272 |
|
|
Accounts payable |
|
|
22,696 |
|
|
|
10,492 |
|
|
Royalties payable |
|
|
20,665 |
|
|
|
13,582 |
|
|
Other payable to licensor |
|
|
17,500 |
|
|
|
5,000 |
|
|
Accrued professional services and other current liabilities |
|
|
14,282 |
|
|
|
8,909 |
|
|
Interest payable |
|
|
305 |
|
|
|
1,698 |
|
|
Total |
|
$ |
186,203 |
|
|
$ |
106,128 |
|
Accounts payable represents short-term liabilities for which the Company has received and processed a vendor invoice prior to the end of the reporting period. Accrued expenses and other current liabilities represent, among other things, compensation and related benefits to employees, royalties due to licensors of technologies, interest payable related to the Company’s Convertible Notes and Revolving Credit Facility, estimated amounts due to third-party vendors for services rendered prior to the end of the reporting period, invoices received from third-party vendors that have not yet been processed, taxes payable, and other accrued items.
94
NOTE 10. DEBT
(a) Revolving Credit Facility
On September 4, 2015, the Company, as borrower, entered into a credit agreement (the “Original Credit Agreement”) with JPMorgan Chase Bank, N.A., as administrative agent, and the lenders from time to time party thereto providing for (i) a one-year $75.0 million revolving loan facility and (ii) an uncommitted accordion facility subject to the satisfaction of certain conditions. The revolving loan facility included a $20.0 million multicurrency sub-facility, a $10.0 million letter of credit sub-facility and a $100,000 swing line loan sub-facility. On September 17, 2015, the Company executed a borrowing of $75.0 million under this revolving credit facility. The interest rate for this borrowing was 2.3125% and was applied on an actual/360 day basis.
On October 23, 2015, the Company entered into an amendment and restatement of the Original Credit Agreement (the “Credit Agreement”) with JPMorgan Chase Bank, N.A., as administrative agent, and the lenders from time to time party thereto (the “Lenders”), providing for (i) a five-year $300 million revolving loan facility (the “Revolving Credit Facility”); and (ii) an uncommitted accordion facility subject to the satisfaction of certain conditions (collectively, the “Senior Secured Credit Facility”). The Revolving Credit Facility includes a $50 million multicurrency sub-facility, a $20 million letter of credit sub-facility and a $10 million swing line loan sub-facility.
Loans under the Revolving Credit Facility bear interest, at the Company’s option, at a rate equal to either (a) the LIBOR rate, plus an applicable margin ranging from 1.75% to 2.50% per annum, based upon the secured leverage ratio (as defined in the Credit Agreement) or (b) the prime lending rate, plus an applicable margin ranging from 0.75% to 1.50% per annum, based upon the senior secured net leverage ratio (as defined in the Credit Agreement). On October 23, 2015, the Company borrowed $75.0 million under the Revolving Credit Facility, which was used to repay the $75.0 million outstanding at September 30, 2015 under the Original Credit Agreement. The interest rate for this borrowing was 2.1250% and was applied on an actual/360 day basis. On January 25, 2016, the Company repaid the $75.0 million outstanding under the Revolving Credit Facility.
The obligations under the Credit Agreement and any swap obligations and banking services obligations owing to a lender (or an affiliate of a lender) thereunder are and will be guaranteed by the Company and each of the Company’s existing and subsequently acquired or organized direct and indirect domestic subsidiaries (other than certain immaterial domestic subsidiaries, certain Domestic Foreign Holding Companies, and certain domestic subsidiaries whose equity interests are owned directly or indirectly by certain foreign subsidiaries) (collectively, the “Loan Parties”). The obligations under the Credit Agreement and any such swap and banking services obligations are secured, subject to customary permitted liens and other agreed upon exceptions, by a perfected security interest in (i) all tangible and intangible assets of the Loan Parties, except for certain customary excluded assets, and (ii) all of the capital stock owned by the Loan Parties thereunder (limited, in the case of the stock of certain non-U.S. subsidiaries of the Company and Domestic Foreign Holding Companies, to 65% of the capital stock of such subsidiaries).
The Credit Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among other things, restrictions on indebtedness, liens, investments, mergers, dispositions, prepayment of other indebtedness and dividends and other distributions. Under the terms of the Credit Agreement, the Company is required to comply with a maximum senior secured net leverage ratio and minimum interest coverage ratio covenants. At December 31, 2015, the Company was in compliance with these covenants.
In accordance with ASU 2015-15, the Company deferred $1.7 million of debt issuance costs associated with the Revolving Credit Facility, including underwriting, legal and accounting fees, and will amortize this amount ratably over the five-year access period of the facility. Amortization of the debt issuance costs will be recorded as non-cash interest expense on the consolidated statement of operations.
(b) Convertible Notes Due 2017
On March 19, 2012, the Company issued $258.8 million aggregate principal amount of the Convertible Notes. The Company was required to pay interest semi-annually in arrears on April 1 and October 1 of each year, at a rate of 2.625% per annum. The Convertible Notes were convertible upon the occurrence of certain conditions into shares of the Company’s common stock at a conversion rate of 39.0344 shares of common stock per $1,000 principal amount of the Convertible Notes, equivalent to a conversion price of approximately $25.62 per share of common stock. The Convertible Notes were scheduled to mature on April 1, 2017, unless earlier converted, redeemed or repurchased in accordance with their terms. Upon a conversion of the Convertible Notes, the Company was required to pay or deliver, as the case may be, cash, shares of the Company’s common stock, or a combination of both, at the Company’s election.
The debt and equity components of the Convertible Notes were bifurcated and accounted for separately based on the authoritative accounting guidance in ASC 470-20. The $258.8 million aggregate principal amount of Convertible Notes was bifurcated between the debt component ($187.1 million) and the equity component ($71.7 million). The amount allocated to the debt component was estimated based on the fair value of similar debt instruments that did not include an equity conversion feature. The Convertible
95
Notes were recorded at an initial carrying value of $187.1 million, net of $71.7 million in debt discount, which was being accreted to the carrying value of the Convertible Notes as non-cash interest expense over the life of the Convertible Notes.
During the year ended December 31, 2015, the Company settled $258.8 million aggregate principal amount of the Convertible Notes through a combination of $259.9 million in cash and 5,638,576 shares of its common stock. The Company recorded a non-cash loss on extinguishment of the Convertible Notes of $21.1 million for the year ended December 31, 2015, which was included in other income (expense), net, on the consolidated statements of operations. Forfeited accrued interest payable of $1.7 million was reclassified to additional paid-in capital during the year ended December 31, 2015. Upon settlement, the Convertible Notes were no longer outstanding, interest ceased to accrue thereon, and all rights of the holders of the Convertible Notes ceased to exist.
NOTE 11. STOCKHOLDERS’ EQUITY
(a) Stock Purchase Rights
All shares of the Company’s common stock, if issued prior to the termination by the Company of its rights agreement, dated as of December 4, 2006, include stock purchase rights. The rights are exercisable only if a person or group acquires twenty percent or more of the Company’s common stock or announces a tender or exchange offer which would result in ownership of twenty percent or more of the Company’s common stock. Following the acquisition of twenty percent or more of the Company’s common stock, the holders of the rights, other than the acquiring person or group, may purchase Medivation common stock at half of its fair market value. In the event of a merger or other acquisition of the Company, the holders of the rights, other than the acquiring person or group, may purchase shares of the acquiring entity at half of their fair market value. The rights were not exercisable at December 31, 2015.
(b) Medivation Equity Incentive Plan
The Medivation Equity Incentive Plan, which is stockholder-approved, provides for the issuance of options and other stock-based awards, including restricted stock units and stock appreciation rights. The vesting of all outstanding awards under the Medivation Equity Incentive Plan will accelerate, and all such awards will become immediately exercisable, upon a “change of control” of Medivation, as defined in the Medivation Equity Incentive Plan.
On June 16, 2015, the Company’s stockholders approved an amendment and restatement of the Medivation Equity Incentive Plan to increase the aggregate number of shares of common stock authorized for issuance under the Medivation Equity Incentive Plan from 21,150,000 to 23,850,000. As a result of the two-for-one stock split effected through a stock dividend described in Note 2, “Summary of Significant Accounting Policies,” the number of shares of common stock authorized for issuance under the Medivation Equity Incentive Plan was increased to 47,700,000 effective on September 15, 2015. As a result of the stock dividend, the number of shares of the Company’s common stock issuable upon exercise of outstanding stock options and vesting of other stock-based awards was proportionally increased, and the exercise price per share as applicable for options and stock appreciation rights was proportionally decreased, in accordance with the terms of the Medivation Equity Incentive Plan.
As of December 31, 2015, approximately 8.7 million shares were available for issuance under the Medivation Equity Incentive Plan.
Stock Options
The terms of stock options granted under the Medivation Equity Incentive Plan cannot exceed ten years. Stock options generally have an exercise price equal to the fair market value of the Company’s common stock on the grant date, and generally vest over a period of four years, except for annual stock option grants to non-employee directors, which vest over a period of one year.
The following table summarizes stock option activity for the year ended December 31, 2015, as adjusted to reflect the two-for-one forward stock split effected through a stock dividend on September 15, 2015:
|
|
|
Number of Options |
|
|
Weighted- Average Exercise Price |
|
|
Weighted- Average Remaining Contractual Term (in years) |
|
|
Aggregate Intrinsic Value(1) (in millions) |
|
||||
|
Outstanding at December 31, 2014 |
|
|
10,135,914 |
|
|
$ |
16.76 |
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
1,822,724 |
|
|
$ |
52.66 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(1,446,047 |
) |
|
$ |
11.24 |
|
|
|
|
|
|
|
|
|
|
Forfeited/expired |
|
|
(404,422 |
) |
|
$ |
41.20 |
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2015 |
|
|
10,108,169 |
|
|
$ |
23.05 |
|
|
|
6.01 |
|
|
$ |
266.4 |
|
|
Vested and exercisable at December 31, 2015 |
|
|
7,043,757 |
|
|
$ |
13.88 |
|
|
|
4.84 |
|
|
$ |
242.9 |
|
96
|
(1) |
The aggregate intrinsic value is calculated as the pre-tax difference between the weighted-average exercise price of the underlying awards and the closing price per share of $48.34 of the Company’s common stock on December 31, 2015. The calculation excludes any awards with an exercise price higher than the closing price of the Company’s common stock on December 31, 2015. |
Additional information regarding stock options is set forth below (in thousands, except per share data):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Intrinsic value of options exercised |
|
$ |
63,550 |
|
|
$ |
145,842 |
|
|
$ |
35,155 |
|
|
Grant-date fair value of options vested |
|
$ |
26,542 |
|
|
$ |
38,147 |
|
|
$ |
22,278 |
|
|
Weighted-average grant-date fair value per share of options granted (split-adjusted) |
|
$ |
25.08 |
|
|
$ |
21.68 |
|
|
$ |
15.20 |
|
Restricted Stock Units
A restricted stock unit is an agreement to issue shares of the Company’s common stock at the time of vesting. Restricted stock units generally vest in three equal installments on approximately the first, second and third anniversaries of the grant date, except for annual restricted stock unit grants to non-employee directors, which vest on approximately the first anniversary of the grant date.
The following table summarizes restricted stock unit activity for the year ended December 31, 2015, as adjusted to reflect the two-for-one forward stock split effected through a stock dividend on September 15, 2015:
|
|
|
Number of Shares |
|
|
Weighted- Average Grant-Date Fair Value |
|
||
|
Unvested at December 31, 2014 |
|
|
967,160 |
|
|
$ |
38.10 |
|
|
Granted |
|
|
670,244 |
|
|
$ |
52.34 |
|
|
Vested |
|
|
(404,906 |
) |
|
$ |
34.92 |
|
|
Forfeited |
|
|
(143,452 |
) |
|
$ |
45.45 |
|
|
Unvested at December 31, 2015 |
|
|
1,089,046 |
|
|
$ |
47.08 |
|
The total fair value of restricted stock units that vested during the years ended December 31, 2015, 2014, and 2013 was $14.1 million, $7.0 million, and $11.8 million, respectively.
Stock Appreciation Rights
Stock appreciation rights give the holder the right, upon exercise, to receive the difference between the market price per share of the Company’s common stock at the time of exercise and the exercise price of the stock appreciation right. The exercise price of the stock appreciation right is equal to the market price of the Company’s common stock at the date of the grant.
The following table summarizes stock appreciation rights activity for the year ended December 31, 2015, as adjusted to reflect the two-for-one forward stock split effected through a stock dividend on September 15, 2015:
|
|
|
Number of Rights |
|
|
Weighted- Average Exercise Price |
|
|
Weighted- Average Remaining Contractual Term (in years) |
|
|
Aggregate Intrinsic Value(1) (in millions) |
|
||||
|
Outstanding at December 31, 2014 |
|
|
1,376,456 |
|
|
$ |
12.03 |
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
(61,172 |
) |
|
$ |
11.60 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(17,156 |
) |
|
$ |
11.60 |
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2015 |
|
|
1,298,128 |
|
|
$ |
12.05 |
|
|
|
5.96 |
|
|
$ |
47.1 |
|
|
Vested and exercisable at December 31, 2015 |
|
|
1,290,720 |
|
|
$ |
12.05 |
|
|
|
5.96 |
|
|
$ |
46.8 |
|
|
(1) |
The aggregate intrinsic value is calculated as the pre-tax difference between the weighted-average exercise price of the underlying awards and the closing price per share of $48.34 of the Company’s common stock on December 31, 2015. The calculation excludes any awards with an exercise price higher than the closing price of the Company’s common stock on December 31, 2015. |
97
Additional information regarding stock appreciation rights is set forth below:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Intrinsic value of stock appreciation rights exercised |
|
$ |
3,034 |
|
|
$ |
7,024 |
|
|
$ |
1,090 |
|
|
Fair value of stock appreciation rights vested (based on remeasurement-date fair value) |
|
$ |
5,730 |
|
|
$ |
6,834 |
|
|
$ |
9,955 |
|
No stock appreciation rights were granted during the years ended December 31, 2015, 2014 and 2013.
(c) Medivation Employee Stock Purchase Plan
The ESPP, which is stockholder approved, permits eligible employees to purchase shares of the Company’s common stock through payroll deductions at the lower of 85% of the fair market value of the common stock at the beginning or end of a purchase period. Eligible employee purchases are limited on an annual basis to $25,000 in accordance with Section 423 of the Internal Revenue Code. As a result of the stock dividend described in Note 2, “Summary of Significant Accounting Policies,” the number of shares of common stock authorized for issuance under the ESPP increased from 3,000,000 shares to 6,000,000 shares effective September 15, 2015. As of December 31, 2015, approximately 97,662 shares are reserved for issuance under the current purchase period and 312,086 shares have been issued. At December 31, 2015, total employee withholdings for ESPP shares of $1.7 million were recorded in “accounts payable, accrued expenses and other current liabilities” on the consolidated balance sheet.
(d) Stock-Based Compensation
The Company estimates the fair value of stock options, stock appreciation rights, and ESPP shares using the Black-Scholes valuation model. The Company estimates expected volatility based on the historical price volatility of its common stock and implied volatility of its common stock inherent in the market price of publicly traded options in its common stock. The Company estimates the expected term of stock options and stock appreciation rights based on actual exercise experience and an assumption that unexercised options will remain outstanding for a period equal to the midpoint between the date the option vests in full and the contractual option termination date. The Company estimates the expected term of ESPP shares based on the duration of the applicable purchase period. The risk-free interest rate is based on the U.S. Treasury yield for a term consistent with the expected term of the awards at the time of grant. The Company uses a dividend yield of zero as it has no history or expectation of paying cash dividends on its common stock.
The Black-Scholes assumptions used to estimate the fair value of stock options granted were as follows:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Expected volatility |
|
50-64% |
|
|
60-65% |
|
|
64-75% |
|
|||
|
Expected term (in years) |
|
4.98-7.17 |
|
|
5.0-5.5 |
|
|
5.2-5.5 |
|
|||
|
Risk-free interest rate |
|
1.33-1.77% |
|
|
1.56-1.79% |
|
|
0.73-1.64% |
|
|||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
No significant stock options were granted to consultants during the periods presented above.
The Black-Scholes assumptions used to estimate the fair value of shares issued under the ESPP on the commencement date of the offering period were as follows:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Expected volatility |
|
37-48% |
|
|
43-53% |
|
|
|
45 |
% |
||
|
Expected term (in years) |
|
0.5 |
|
|
0.5 |
|
|
0.5 |
|
|||
|
Risk-free interest rate |
|
0.08-0.12% |
|
|
0.04-0.06% |
|
|
|
0.04 |
% |
||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Stock-based compensation expense was as follows:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Stock-based compensation expense recognized as: |
|
|
|
|
|
|
|
|
|
|
|
|
|
R&D expenses |
|
$ |
24,368 |
|
|
$ |
17,913 |
|
|
$ |
16,503 |
|
|
SG&A expenses |
|
|
30,494 |
|
|
|
27,221 |
|
|
|
20,575 |
|
|
Total |
|
$ |
54,862 |
|
|
$ |
45,134 |
|
|
$ |
37,078 |
|
98
Unrecognized stock-based compensation expense was approximately $87.2 million at December 31, 2015, and is expected to be recognized over a weighted-average period of approximately 2.5 years.
(e) Warrants
At December 31, 2015, an aggregate of 40,000 warrants to purchase shares of Medivation common stock at an exercise price of $3.47 per share were outstanding. These outstanding warrants expire in 2017. During the year ended December 31, 2013, an aggregate of 51,616 warrants to purchase shares of Medivation common stock at an exercise price of $0.39 per share were exercised.
NOTE 12. RETIREMENT PLAN
The Company has a defined contribution savings plan, which qualifies as a deferred salary arrangement under Section 401(k) of the Internal Revenue Code, or IRC. The 401(k) Plan is for the benefit of all participating employees and permits voluntary contributions by employees up to 100% of their annual pre-tax compensation limited by the Internal Revenue Service, or the IRS, imposed maximum contribution. The Company matches 100% of the first 3% of employee contributions and 50% of the next 2% of employee contributions. The Company’s contributions and the employee contributions are fully vested when contributed. The plan assets are held in trust and invested as directed by the plan participants. Employer matching contributions to the plan were $3.5 million, $2.7 million, and $1.9 million for the years ended December 31, 2015, 2014, and 2013, respectively.
NOTE 13. INCOME TAXES
The Company’s income before income tax (expense) benefit was $381.3 million and $260.2 million for the years ended December 31, 2015 and 2014, respectively. The Company’s loss before income tax expense was $42.5 million for the year ended December 31, 2013. Since inception, the Company has only generated pre-tax income (losses) in the United States and has not generated any pre-tax income (losses) outside the United States. Income tax (expense) benefit for the periods presented consisted of the following:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Current: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
(140,987 |
) |
|
$ |
(19,476 |
) |
|
$ |
(7 |
) |
|
State |
|
|
(3,875 |
) |
|
|
(1,496 |
) |
|
|
(108 |
) |
|
Total current |
|
|
(144,862 |
) |
|
|
(20,972 |
) |
|
|
(115 |
) |
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
8,179 |
|
|
|
36,917 |
|
|
|
— |
|
|
State |
|
|
90 |
|
|
|
313 |
|
|
|
— |
|
|
Total deferred |
|
|
8,269 |
|
|
|
37,230 |
|
|
|
— |
|
|
Total income tax (expense) benefit |
|
$ |
(136,593 |
) |
|
$ |
16,258 |
|
|
$ |
(115 |
) |
During 2015 and 2014, the Company reduced its current Federal and state taxes payable by $100.2 million and $17.0 million, respectively, related to excess tax benefits from stock-based compensation, offsetting additional paid-in capital. In addition, for the year ended December 31, 2015, the Company recorded a credit to additional paid-in capital of $11.8 million related to certain tax impacts of the extinguishment of Convertible Notes. The Company had unrecorded state excess stock-based compensation tax benefits of $3.4 million (tax-effected) as of December 31, 2015. These amounts will be credited to additional paid-in capital when such amounts reduce cash taxes payable.
A reconciliation of the statutory Federal income tax rate of 35% to the Company’s effective income tax rates is as follows:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Federal tax provision at statutory rate |
|
|
35.0 |
% |
|
|
35.0 |
% |
|
|
35.0 |
% |
|
State taxes (net of Federal benefit) |
|
|
0.6 |
% |
|
|
0.4 |
% |
|
|
1.4 |
% |
|
Stock-based compensation |
|
|
0.1 |
% |
|
|
0.1 |
% |
|
|
(0.2 |
%) |
|
Non-deductible officer compensation |
|
|
0.2 |
% |
|
|
0.1 |
% |
|
|
(1.8 |
%) |
|
Change in valuation allowance |
|
|
— |
|
|
|
(40.5 |
%) |
|
|
(52.1 |
%) |
|
Research and development credits |
|
|
(0.2 |
%) |
|
|
(1.5 |
%) |
|
|
17.1 |
% |
|
Other |
|
|
0.1 |
% |
|
|
0.2 |
% |
|
|
0.3 |
% |
|
Effective income tax rate |
|
|
35.8 |
% |
|
|
(6.2 |
%) |
|
|
(0.3 |
%) |
99
The Company recorded income tax expense of $136.6 million for the year ended December 31, 2015. The Company recorded an income tax benefit of $16.3 million for the year ended December 31, 2014. For the year ended December 31, 2015, the provision for income taxes was higher than the tax computed at the U.S. Federal statutory rate primarily due to state income taxes and non-deductible stock-based compensation, net of Federal research and development credit. For the year ended December 31, 2014, the provision for income taxes was lower than the tax computed at the U.S. Federal statutory rate due primarily to utilization of net operating loss and tax credit carryforwards and the release of the valuation allowance on a portion of the Company’s net deferred tax assets. The income tax expense for the year ended December 31, 2013 was not significant. The increase in the effective tax rate for the year ended December 31, 2015 as compared to the year ended December 31, 2014 was primarily due to the release of the valuation allowance against federal and certain state deferred tax assets during the fourth quarter of 2014.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the tax basis of assets and liabilities. Significant components of the Company’s deferred tax assets for Federal and state income taxes are follows:
|
|
|
December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Deferred revenue |
|
$ |
— |
|
|
$ |
1,005 |
|
|
Net operating loss carryforward |
|
|
8,257 |
|
|
|
9,100 |
|
|
Stock-based compensation |
|
|
35,482 |
|
|
|
26,000 |
|
|
Tax credits |
|
|
8,076 |
|
|
|
12,098 |
|
|
Intangible assets |
|
|
9,833 |
|
|
|
5,259 |
|
|
Accruals and reserves |
|
|
19,156 |
|
|
|
15,247 |
|
|
Total deferred tax assets |
|
|
80,804 |
|
|
|
68,709 |
|
|
Less: valuation allowance |
|
|
(16,827 |
) |
|
|
(16,023 |
) |
|
Total net deferred tax assets |
|
|
63,977 |
|
|
|
52,686 |
|
|
Deferred tax liabilities |
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
(3,168 |
) |
|
|
(2,741 |
) |
|
Convertible Notes |
|
|
— |
|
|
|
(12,792 |
) |
|
Contingent consideration |
|
|
(3,798 |
) |
|
|
— |
|
|
Total deferred tax liabilities |
|
|
(6,966 |
) |
|
|
(15,533 |
) |
|
Net deferred tax assets |
|
$ |
57,011 |
|
|
$ |
37,153 |
|
|
Recorded as: |
|
|
|
|
|
|
|
|
|
Net current deferred tax assets |
|
$ |
— |
|
|
$ |
21,987 |
|
|
Net non-current deferred tax assets |
|
|
57,011 |
|
|
|
15,176 |
|
|
Net non-current deferred tax liabilities (included in “Other non-current liabilities”) |
|
|
— |
|
|
|
(10 |
) |
|
Net deferred tax assets |
|
$ |
57,011 |
|
|
$ |
37,153 |
|
As of December 31, 2015, all deferred tax assets and liabilities are classified as non-current on the Company’s balance sheet in accordance with the early adoption of ASU 2015-17. See additional discussion in Note 2, “Summary of Significant Accounting Policies”.
As of December 31, 2015, the Company had no Federal net operating loss carryforwards for tax return purposes as the Company fully utilized all of its tax attributes during 2015. Also, as of December 31, 2015, the Company had state net operating loss carryforwards for tax return purposes of approximately $216.3 million, which will expire at various dates between the years 2017 and 2033, if not utilized.
The Company has fully utilized all of its Federal research and development credit and Orphan Drug credit carryforwards and alternative minimum tax credits as of December 31, 2015 for tax return purposes. In addition, the Company has California research and development credit carryforwards for tax purposes of approximately $16.6 million as of December 31, 2015. The California research credits do not expire. On December 18, 2015, the Consolidated Appropriations Act of 2016 was signed, permanently renewing the Federal research and development tax credit retroactive to January 1, 2015. ASC 740-10-45-15, “Income Taxes,” requires that the effects of a change in tax laws or rates be recognized in the period that includes the enactment date; consequently, the Company recognized the benefit of the Federal research and development credit during the fourth quarter of 2015.
Federal and state tax laws impose substantial restrictions on the utilization of net operating loss and credit carryforwards in the event of an “ownership change” for tax purposes, as defined in IRC Section 382. The Company completed Section 382 studies through September 30, 2015, and concluded that ownership changes occurred in 2004, 2007 and 2010. The ownership changes did not
100
result in a reduction of its net operating loss or in its research and development credits expiring unused. If additional ownership changes occur, the utilization of net operating loss and credit carryforwards could be significantly reduced.
The valuation allowance increased by $0.8 million in 2015, decreased by $104.8 million in 2014 and increased by $22.1 million in 2013.
The Company records a valuation allowance to reduce deferred tax assets to reflect the net amount that is more likely than not to be realized. Based upon the weight of available evidence at December 31, 2014, the Company determined that it was more likely than not that a portion of its deferred tax assets would be realizable and consequently released the valuation allowance against Federal and certain state net deferred tax assets and recorded a discrete tax benefit of $33.4 million during the fourth quarter of 2014. The decision to reverse a portion of the valuation allowance was made after management considered all available evidence, both positive and negative, including but not limited to the historical operating results, income or loss in recent periods, cumulative income in recent years, forecasted earnings, forecasted future taxable income, and significant risk and uncertainty related to forecasts. The release of the valuation allowance resulted in the recognition of certain deferred net tax assets and a decrease to income tax expense.
The following table summarizes activity related to the Company’s gross unrecognized tax positions:
|
|
|
December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Balance as of beginning of year |
|
$ |
12,367 |
|
|
$ |
5,955 |
|
|
$ |
4,602 |
|
|
Additions based on tax positions related to the current year |
|
|
2,898 |
|
|
|
6,439 |
|
|
|
702 |
|
|
Additions based on tax position related to prior year |
|
|
135 |
|
|
|
— |
|
|
|
660 |
|
|
Decreases based on tax positions related to prior year |
|
|
(1,324 |
) |
|
|
(27 |
) |
|
|
(9 |
) |
|
Balance as of end of year |
|
$ |
14,076 |
|
|
$ |
12,367 |
|
|
$ |
5,955 |
|
Approximately $7.7 million of the total gross unrecognized tax benefits at December 31, 2015, if recognized, would affect the effective tax rate. The Company does not anticipate a material change in unrecognized tax benefits during the next twelve months.
Interest and/or penalties related to income tax matters are recognized as a component of income tax expense as incurred. The interest expense related to uncertain tax positions in the income tax expense line of the Company’s consolidated statements of operations was not significant during 2015, 2014, and 2013. Interest related to income tax matters accrued as of December 31, 2015 and 2014 was not significant.
As a result of the Company’s net operating loss and tax credit carryforwards, all of its tax years are subject to Federal and state examination. The Company’s Federal income tax returns were audited by the Internal Revenue Service for tax years 2008 and 2012 and resulted in no material adjustments. The Company’s 2009 and 2010 California income tax returns are currently under audit by the California tax authorities. The Company believes that it has adequately provided for any reasonable foreseeable outcomes related to its Federal and California income tax returns.
The future effective tax rate is subject to volatility and may be materially impacted by various internal and external factors. These factors may include, but are not limited to, the amount of income tax benefits and charges from: interpretations of existing tax laws; changes in tax laws and rates; future levels of research and development expenditures; changes in the mix of earnings in countries with differing statutory tax rates in which the Company may conduct business; changes in the valuation of deferred tax assets and liabilities; state income taxes; the tax impact of stock-based compensation; accounting for uncertain tax positions; closure of statute of limitations or settlement of tax audits; changes in estimates of prior years’ items; tax costs for acquisition-related items; changes in accounting standards; non-deductible officers’ compensation; limitations on the utilization of net operating losses and tax credits due to changes in ownership; and overall levels of income before taxes.
NOTE 14. FAIR VALUE DISCLOSURES
The Company follows ASC 820-10, “Fair Value Measurements and Disclosures,” which among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, a three-tier fair value hierarchy has been established, which prioritizes the inputs used in measuring fair value as follows:
|
|
· |
Level 1—Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date. |
|
|
· |
Level 2—Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life. |
101
The following table presents the Company’s financial assets and liabilities that are measured at fair value on a recurring basis:
|
|
|
|
|
|
|
Fair value measurements using: |
|
|||||||||
|
|
|
Fair Value |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
4,900 |
|
|
|
— |
|
|
|
— |
|
|
$ |
4,900 |
|
|
Long-term liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
262,368 |
|
|
|
— |
|
|
|
— |
|
|
$ |
262,368 |
|
|
December 31, 2014: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
189,031 |
|
|
$ |
189,031 |
|
|
|
— |
|
|
|
— |
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
10,000 |
|
|
|
— |
|
|
|
— |
|
|
$ |
10,000 |
|
|
Long-term liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
96,000 |
|
|
|
— |
|
|
|
— |
|
|
$ |
96,000 |
|
The Company classifies money market funds, which are based on quoted market prices in active markets with no valuation adjustments, as Level 1 assets within the valuation hierarchy.
The Company estimates the fair values of Level 2 assets by taking into consideration valuations obtained from third-party pricing sources. These pricing sources utilize industry standard valuation models, including both income and market-based approaches, for which all significant inputs are observable, either directly, or indirectly, to estimate fair value. These inputs include market pricing based on real-time trade data for the same or similar assets, issuer credit spreads, benchmark yields, and other observable inputs. The Company validates the prices provided by its third-party pricing sources by understanding the models used, obtaining market values from other pricing sources, and/or analyzing pricing data in certain instances.
In connection with the acquisitions of MDV9300 and MDV3800, the Company recorded contingent consideration liabilities pertaining to amounts potentially payable to CureTech and BioMarin, respectively. The fair value of contingent consideration is considered a Level 3 liability and was estimated utilizing a model with key assumptions that included estimated revenues or completion of certain development, regulatory and sales milestone targets during the earn-out period, volatility, and estimated discount rates corresponding to the periods of expected payments. The estimated fair value of the contingent consideration liability is measured at each reporting date based on significant inputs not observable in the market. The Company assesses these estimates on an ongoing basis as additional data impacting the assumptions is obtained. Changes in the estimated fair value of contingent consideration are reflected as non-cash adjustments to operating expenses in the consolidated statements of operations.
During the year ended December 31, 2015, the Company recorded fair value adjustments of $2.6 million and $7.7 million as a decrease to R&D expenses and SG&A expenses, respectively, related to the CureTech contingent consideration liability. Since the date of the fourth quarter acquisition of MDV3800, the Company also recorded nominal fair value adjustments to the related contingent consideration liability. Such adjustments were primarily due to the time value of money.
The aggregate remaining, undiscounted amount of contingent consideration that the Company could potentially be required to pay to CureTech and BioMarin under each respective transaction is included in Note 4, “Business Acquisitions.”
Contingent consideration may change significantly as development progresses and additional data is obtained that will affect the Company’s assumptions regarding probabilities of successful achievement of related milestones used to estimate the fair value of the liability and the timing in which they are expected to be achieved. Updates to these assumptions could have a significant impact on our results of operations. For example, a significant increase in the probability of achieving a milestone would result in a significantly higher fair value measurement, while a significant increase in the expected timing of achieving the milestone would result in a significantly lower fair value measurement. Considerable judgment is required to interpret the market data used to develop the assumptions. The estimates of fair value may not be indicative of amounts that could be realized in a current market exchange. Accordingly, the use of different market assumptions and/or different valuation techniques could result in materially different fair value estimates.
102
There were no transfers between Level 1 and Level 2 financial instruments during the year ended December 31, 2015. The following table includes a roll-forward of the fair value of Level 3 financial instruments for the year ended December 31, 2015:
|
|
|
Year Ended December 31, 2015 |
|
|
|
Contingent consideration (current and long-term): |
|
|
|
|
|
Balance at beginning of period |
|
$ |
106,000 |
|
|
Amounts acquired or issued |
|
|
171,942 |
|
|
Net change in fair value |
|
|
(10,674 |
) |
|
Settlements |
|
|
— |
|
|
Transfers in and/or out of Level 3 |
|
|
— |
|
|
Balance at end of period |
|
$ |
267,268 |
|
The following table presents the total balance of the Company’s other financial instruments that are not measured at fair value on a recurring basis.
|
|
|
|
|
|
|
Fair value measurements using: |
|
|||||||||
|
|
|
Total Balance |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank deposits (included in “Cash and cash equivalents”) |
|
$ |
225,853 |
|
|
$ |
225,853 |
|
|
|
— |
|
|
|
— |
|
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Borrowings under Revolving Credit Facility |
|
$ |
75,000 |
|
|
$ |
75,000 |
|
|
|
— |
|
|
|
— |
|
|
December 31, 2014: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank deposits (included in “Cash and cash equivalents”) |
|
$ |
313,646 |
|
|
$ |
313,646 |
|
|
|
— |
|
|
|
— |
|
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Notes |
|
$ |
359,219 |
|
|
|
— |
|
|
$ |
359,219 |
|
|
|
— |
|
Due to their short-term maturities, the Company believes that the fair value of its bank deposits, receivable from collaboration partner, accounts payable and accrued expenses, short-term borrowings under the Revolving Credit Facility, and other current assets and liabilities approximate their carrying value.
The estimated fair value of the Company’s Convertible Notes, including the equity component, was $496.8 million at December 31, 2014 and was determined using recent trading prices of the Convertible Notes. The fair value of the Convertible Notes included in the table above at December 31, 2014 represents only the liability component of the Convertible Notes, because the equity component is included in stockholders’ equity on the consolidated balance sheet. The Company settled its remaining Convertible Notes in the third quarter of 2015 as discussed further in Note 10, “Debt.”
NOTE 15. COMMITMENTS AND CONTINGENCIES
(a) Leases
The Company leases approximately 158,000 square feet of office space, including 143,000 square feet of office space at its corporate headquarters, pursuant to operating lease agreements expiring at various dates through December 2019. The Company has the option to extend the lease term of its corporate headquarters for an additional five years.
Future operating lease obligations as of December 31, 2015, are as follows:
|
Years Ending December 31, |
|
Operating Leases |
|
|
|
2016 |
|
$ |
9,343 |
|
|
2017 |
|
|
9,542 |
|
|
2018 |
|
|
9,743 |
|
|
2019 |
|
|
5,065 |
|
|
2020 |
|
|
— |
|
|
2021 and thereafter |
|
|
— |
|
|
Total |
|
$ |
33,693 |
|
103
The Company is considered the “accounting owner” for a build-to-suit property and has recorded a build-to-suit lease obligation on its consolidated balance sheets. Additional information regarding the build-to-suit lease obligation is included in Note 7, “Build-To-Suit Lease Obligation.”
Expected future lease payments under the build-to-suit lease as of December 31, 2015 are as follows:
|
Years Ending December 31, |
|
Expected Cash Payments Under Build-To-Suit Lease Obligation |
|
|
|
2016 |
|
$ |
2,178 |
|
|
2017 |
|
|
2,244 |
|
|
2018 |
|
|
2,311 |
|
|
2019 |
|
|
2,380 |
|
|
2020 |
|
|
2,452 |
|
|
2021 and thereafter |
|
|
9,627 |
|
|
Total minimum lease payments |
|
$ |
21,192 |
|
Rent expense for the years ended December 31, 2015, 2014, and 2013 was $10.3 million, $8.1 million, and $5.2 million, respectively. In addition to the amounts included in the tables above, certain lease agreements also require the Company to make additional payments during the lease term for taxes, insurance, and other operating expenses.
(b) License Agreement with UCLA
Under an August 2005 license agreement with UCLA, the Company’s subsidiary Medivation Prostate Therapeutics, Inc., or MPT, holds an exclusive worldwide license under several UCLA patents and patent applications covering XTANDI and related compounds. Under the Astellas Collaboration Agreement, the Company granted Astellas a sublicense under the patent rights licensed to it by UCLA.
The Company is required to pay UCLA (a) an annual maintenance fee, (b) $2.8 million in aggregate milestone payments upon achievement of certain development and regulatory milestone events with respect to XTANDI (all of which has been paid as of December 31, 2015), (c) ten percent of all Sublicensing Income, as defined in the agreement, which the Company earns under the Astellas Collaboration Agreement, and (d) a four percent royalty on global net sales of XTANDI, as defined. Under the terms of the Astellas Collaboration Agreement, the Company shares this royalty obligation equally with Astellas with respect to sales in the United States, and Astellas is responsible for this entire royalty obligation with respect to sales outside of the United States. The Company is currently involved in litigation with UCLA, which are discussed in the section titled “Litigation” below.
UCLA may terminate the agreement if the Company does not meet a general obligation to diligently proceed with the development, manufacturing and sale of licensed products, or if it commits any other uncured material breach of the agreement. The Company may terminate the agreement at any time upon advance written notice to UCLA. If neither party terminates the agreement early, the agreement will continue in force until the expiration of the last-to-expire patent on a country-by-country basis.
(c) Clinical Manufacturing Agreements
Manufacturing Services and Supply Agreements
Contemporaneous with the execution of the License Agreement with CureTech, the Company entered into a Manufacturing Services and Supply Agreement, or MSA, with CureTech pursuant to which CureTech will provide clinical trial supply of MDV9300 over a three-year period. In accordance with the terms of the MSA, as amended, the Company paid CureTech upfront and setup fees of $3.0 million during the fourth quarter of 2014, $0.2 million during the second quarter of 2015 and $0.1 million during the third quarter of 2015. The Company is required to pay CureTech a one-time milestone payment of $5.0 million upon the completion of the Manufacturing Technology Transfer, as defined. In accordance with the terms of the MSA, the Company is also responsible for providing Manufacturing Funding totaling up to $19.3 million for clinical trial materials of MDV9300 over the three-year term of the MSA, of which $9.0 million has been paid through December 31, 2015. The Manufacturing Funding is contingent upon the successful achievement of the requirements set forth in the Manufacturing Plan, and any such amounts may be reduced or eliminated by the Company under the terms of the MSA.
104
Development and Manufacturing Services Agreement
During the fourth quarter of 2014, the Company entered into a Development and Manufacturing Services Agreement with a third party clinical manufacturing organization. The term of the agreement is for the longer of (i) a period of five (5) years or (ii) through the completion of the Services, as defined. Under the current statement of work under this agreement, as amended, the Company intends to transfer the current manufacturing process of MDV9300 from CureTech to this third party, further scale up and production of Phase 3 clinical trial material of MDV9300 from this entity’s manufacturing facility. The estimated total consideration payable under the current statement of work, as amended, is approximately $15.2 million, of which approximately $5.1 million has been paid through December 31, 2015.
(f) Litigation
The Company is party to legal proceedings, investigations, and claims in the ordinary course of its business, including the matters described below. The Company records accruals for outstanding legal matters when it believes that it is both probable that a liability has been incurred and the amount of such liability can be reasonably estimated. The Company evaluates, on a quarterly basis, developments in significant legal matters that could affect the amount of any accrual and developments that would make a loss contingency both probable and reasonably estimable. To the extent new information is obtained and the Company’s views on the probable outcomes of claims, suits, assessments, investigations or legal proceedings change, changes in the Company’s accrued liabilities would be recorded in the period in which such determination is made. In addition, in accordance with the relevant authoritative guidance, for matters for which the likelihood of material loss is at least reasonably possible, the Company provides disclosure of the possible loss or range of loss; however, if a reasonable estimate cannot be made, the Company will provide disclosure to that effect. Gain contingencies, if any, are recorded when they are realized.
In May 2011, the Company filed a lawsuit in San Francisco Superior Court against the Regents of the University of California, or the Regents, and one of its professors, alleging breach of contract and fraud claims, among others. The Company’s allegations in this lawsuit include that it has exclusive commercial rights to apalutamide, an investigational drug originally known as ARN-509 (previously also referred to as JNJ-56021927, or JNJ-927), which is currently being developed by Aragon Pharmaceuticals, or Aragon. In August 2013, Johnson & Johnson and Aragon completed a transaction in which Johnson & Johnson acquired all apalutamide assets owned by Aragon. The Company sought remedies including a declaration that it is the proper licensee of apalutamide, contractual remedies conferring to it exclusive patent license rights regarding apalutamide, and other equitable and monetary relief. On August 7, 2012, the Regents filed a cross-complaint against the Company seeking declaratory relief that the Regents are entitled to ten percent of any sales milestone payments under the Astellas Collaboration Agreement.
On December 20, 2012, and January 25, 2013, the Court granted summary judgment motions filed by defendants Regents and Aragon, resulting in dismissal of all claims against Regents and Aragon, but denied such motions filed by the remaining Regents professor. On April 15, 2013, the Company filed a Notice of Appeal seeking appeal of the judgment in favor of Aragon, which is now wholly-owned by Johnson & Johnson. The bench trial of the Regent’s cross-complaint against the Company was conducted in July 2013, and on January 15, 2014, the Court entered a judgment in the cross-complaint in favor of Regents. The Company appealed this judgment on February 13, 2014 along with the December 2012 summary judgment order in favor of Regents. The jury trial of the Company’s breach of contract and fraud claims against the remaining Regents professor was conducted in October and November 2013. On November 15, 2013, the jury rendered a verdict in the case, finding in favor of Medivation on one of the breach of contract claims, and in favor of the Regents professor on the fraud claims. The Company appealed the resulting judgment on the fraud claims. All appeals from this matter were consolidated, and oral arguments were heard on February 23, 2016. A decision of the appellate court is required to be rendered within 90 days of the oral argument.
On April 11, 2014, the Regents filed a complaint against the Company in which UCLA alleges that the “Operating Profits” Medivation has received (and will continue to receive) from Astellas, as a result of the Astellas Collaboration Agreement, constitute Sublicensing Income under the license agreement between Medivation and the Regents and that Medivation and its subsidiary, MPT, have failed to pay the Regents ten percent of such Operating Profits. The Company denies the Regents’ allegations and is vigorously defending the litigation. The Company is currently awaiting a trial date to be set by the Courts.
While the Company believes it has meritorious positions with respect to the claims above and intends to advance its positions in these lawsuits vigorously, including on appeal, the process of resolving matters through litigation or other means is inherently uncertain, and it is not possible to predict the ultimate resolution of any such proceeding. The actual costs of defending the Company’s position may be significant, and the Company may not prevail.
105
NOTE 16. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
The following table presents the unaudited quarterly results of operations of the Company for the years ended December 31, 2015 and 2014, respectively. The unaudited financial information is prepared on the same basis as the audited consolidated financial statements. The Company’s operating results for any quarter are not necessarily indicative of results for any future quarters or for a full year. All share and per share amounts have been retroactively adjusted to reflect the Company’s September 15, 2015, two-for-one forward stock split effected through a stock dividend.
|
|
|
Quarters Ended |
|
|||||||||||||
|
|
|
March 31, |
|
|
June 30, |
|
|
September 30, |
|
|
December 31, |
|
||||
|
2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration revenue |
|
$ |
129,188 |
|
|
$ |
175,657 |
|
|
$ |
260,665 |
|
|
$ |
377,748 |
|
|
Operating expenses |
|
$ |
(128,615 |
) |
|
$ |
(122,002 |
) |
|
$ |
(121,680 |
) |
|
$ |
(156,348 |
) |
|
Income from operations |
|
$ |
573 |
|
|
$ |
53,655 |
|
|
$ |
138,985 |
|
|
$ |
221,400 |
|
|
Net income (loss) |
|
$ |
(3,118 |
) |
|
$ |
25,826 |
|
|
$ |
79,510 |
|
|
$ |
142,507 |
|
|
Basic net income (loss) per common share |
|
$ |
(0.02 |
) |
|
$ |
0.16 |
|
|
$ |
0.49 |
|
|
$ |
0.87 |
|
|
Diluted net income (loss) per common share |
|
$ |
(0.02 |
) |
|
$ |
0.15 |
|
|
$ |
0.47 |
|
|
$ |
0.85 |
|
|
Weighted-average common shares used in the calculation of basic net income (loss) per common share |
|
|
156,637 |
|
|
|
158,505 |
|
|
|
162,390 |
|
|
|
163,746 |
|
|
Weighted-average common shares used in the calculation of diluted net income (loss) per common share |
|
|
156,637 |
|
|
|
168,690 |
|
|
|
168,070 |
|
|
|
168,368 |
|
|
2014: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration revenue |
|
$ |
87,189 |
|
|
$ |
148,090 |
|
|
$ |
200,478 |
|
|
$ |
274,730 |
|
|
Operating expenses |
|
$ |
(95,654 |
) |
|
$ |
(93,139 |
) |
|
$ |
(108,595 |
) |
|
$ |
(131,253 |
) |
|
Income (loss) from operations |
|
$ |
(8,465 |
) |
|
$ |
54,951 |
|
|
$ |
91,883 |
|
|
$ |
143,477 |
|
|
Net income (loss) |
|
$ |
(13,665 |
) |
|
$ |
47,919 |
|
|
$ |
77,993 |
|
|
$ |
164,205 |
|
|
Basic net income (loss) per common share |
|
$ |
(0.09 |
) |
|
$ |
0.31 |
|
|
$ |
0.51 |
|
|
$ |
1.06 |
|
|
Diluted net income (loss) per common share |
|
$ |
(0.09 |
) |
|
$ |
0.30 |
|
|
$ |
0.48 |
|
|
$ |
0.98 |
|
|
Weighted-average common shares used in the calculation of basic net income (loss) per common share |
|
|
152,489 |
|
|
|
153,153 |
|
|
|
154,112 |
|
|
|
155,644 |
|
|
Weighted-average common shares used in the calculation of diluted net income (loss) per common share |
|
|
152,489 |
|
|
|
160,983 |
|
|
|
162,446 |
|
|
|
171,513 |
|
Net income (loss) per common share amounts for the 2015 and 2014 quarters and full years have been computed separately. Accordingly, quarterly amounts may not add to the annual amounts because of differences in the weighted average shares outstanding during each quarter due to the effect of potentially dilutive securities only in the periods in which such effect would be dilutive.
106
|
|
|
|
|
|
|
|
Incorporated By Reference |
|
|
|||
|
Exhibit |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.1** |
|
Asset Purchase Agreement, dated August 21, 2015, by and between Medivation, Inc. and BioMarin Pharmaceutical, Inc. |
|
8-K |
|
001-32836 |
|
2.1 |
|
10/07/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2** |
|
License Agreement between Medivation, Inc. and CureTech Ltd., dated as of October 23, 2014 |
|
8-K |
|
001-32836 |
|
2.1 |
|
11/06/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
Restated Certificate of Incorporation. |
|
8-K |
|
001-32836 |
|
3.4 |
|
10/17/2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
Certificate of Amendment to Amended and Restated Certificate of Designation of Series C Junior Participating Preferred Stock of Medivation, Inc. |
|
8-K |
|
001-32836 |
|
3.1 |
|
2/13/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.3 |
|
Certificate of Amendment of Restated Certificate of Incorporation |
|
8-K |
|
001-32836 |
|
3.1 |
|
6/19/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.4 |
|
Amended and Restated Bylaws of Medivation, Inc. |
|
8-K |
|
001-32836 |
|
3.2 |
|
2/13/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
Common Stock Certificate. |
|
10-Q |
|
001-32836 |
|
4.1 |
|
5/9/2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.2 |
|
Rights Agreement, dated as of December 4, 2006, between Medivation, Inc. and American Stock Transfer & Trust Company, as Rights Agent, which includes the form of Certificate of Designations of the Series C Junior Participating Preferred Stock of Medivation, Inc. as Exhibit A, the form of Right Certificate as Exhibit B and the Summary of Rights to Purchase Preferred Shares as Exhibit C. |
|
8-K |
|
001-32836 |
|
4.1 |
|
12/4/2006 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1* |
|
Amended and Restated 2004 Equity Incentive Award Plan. |
|
DEF14A |
|
001-32836 |
|
Annex A |
|
4/30/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2* |
|
Form of Stock Option Agreement for Early Exercisable Options under the Amended and Restated 2004 Equity Incentive Award Plan. |
|
10-KSB |
|
000-20837 |
|
10.7(c) |
|
2/11/2005 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3* |
|
Form of Stock Option Agreement under the 2004 Equity Incentive Award Plan (for use from July 1, 2004 through October 6, 2013). |
|
10-KSB |
|
000-20837 |
|
10.7(b) |
|
2/11/2005 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4* |
|
Form of Stock Option Grant Notice and Non-Qualified Stock Option Agreement under the Amended and Restated 2004 Equity Incentive Award Plan (for use after October 6, 2013). |
|
10-K |
|
001-32836 |
|
10.39 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5* |
|
Form of Restricted Stock Unit Grant Notice and Agreement under the 2004 Amended and Restated Equity Incentive Award Plan (for use from December 10, 2010 through October 6, 2013). |
|
10-K |
|
001-32836 |
|
10.22 |
|
3/16/2011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6* |
|
Form of Restricted Stock Unit Grant Notice and Agreement under the Amended and Restated 2004 Equity Incentive Plan (for use from October 7, 2013 through December 11, 2014). |
|
10-K |
|
001-32836 |
|
10.40 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7* |
|
Form of Restricted Stock Unit Grant Notice and Agreement under the Amended and Restated 2004 Equity Incentive Award Plan (for use after December 11, 2014 through December 31, 2015). |
|
10-K |
|
001-32836 |
|
10.41 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
107
|
|
|
|
|
|
|
|
Incorporated By Reference |
|
|
|||
|
Exhibit |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Form of Restricted Stock Unit Grant Notice and Agreement under the Amended and Restated 2004 Equity Incentive Award Plan (for use after January 1, 2016). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9* |
|
Form of Restricted Stock Grant Notice and Agreement for Non-Employee Directors under the Amended and Restated 2004 Equity Incentive Plan and Joint Escrow Instructions (for use after January 1, 2016). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10* |
|
The Medivation, Inc. Employee Stock Purchase Plan. |
|
8-K |
|
001-32836 |
|
10.1 |
|
7/1/2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.11** |
|
The Exclusive License Agreement, by and among Medivation, Inc., the Regents of the University of California, and Medivation Prostate Therapeutics, Inc., dated as of August 12, 2005. |
|
10-Q |
|
001-32836 |
|
10.1 |
|
11/12/2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12 |
|
First Amendment to Exclusive License Agreement by and among Medivation, Inc., the Regents of the University of California, and Medivation Prostate Therapeutics, Inc., dated as of November 4, 2005. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.13 |
|
Second Amendment to Exclusive License Agreement by and among Medivation, Inc., the Regents of the University of California, and Medivation Prostate Therapeutics, Inc., dated as of May 8, 2006. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.14 |
|
Third Amendment to Exclusive License Agreement by and among Medivation, Inc., the Regents of the University of California, and Medivation Prostate Therapeutics, Inc., dated as of June 12, 2006. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15 |
|
Fourth Amendment to Exclusive License Agreement by and among Medivation, Inc., the Regents of the University of California, and Medivation Prostate Therapeutics, Inc., dated as of July 17, 2007. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.16 |
|
Fifth Amendment to Exclusive License Agreement by and among Medivation, Inc., the Regents of the University of California, and Medivation Prostate Therapeutics, Inc., dated as of October 21, 2009. |
|
|
|
|
|
|
|
|
|
X |
|
10.17** |
|
Collaboration Agreement, dated as of October 26, 2009, by and between Medivation, Inc. and Astellas US LLC. |
|
10-K/A |
|
001-32836 |
|
10.15 |
|
3/24/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.18** |
|
Amendment No. 1 to Collaboration Agreement, dated January 1, 2010, by and among Medivation, Inc., Astellas Pharma Inc. and Astellas US LLC. |
|
10-Q |
|
001-32836 |
|
10.1 |
|
8/9/2011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.19** |
|
Amendment No. 2 to Collaboration Agreement, dated May 13, 2011, by and among Medivation, Inc., Astellas Pharma Inc. and Astellas US LLC. |
|
10-Q/A |
|
001-32836 |
|
10.2 |
|
3/24/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.20 |
|
Amendment No. 3 to Collaboration Agreement, dated April 1, 2013, by and among Medivation, Inc., Astellas Pharma Inc., and Astellas US LLC. |
|
10-K |
|
001-32836 |
|
10.15 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.21 |
|
Amendment No. 4 to Collaboration Agreement, dated October 29, 2013, by and among Medivation, Inc., Astellas Pharma Inc., and Astellas US LLC. |
|
10-K |
|
001-32836 |
|
10.16 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.22 |
|
Amendment No. 5 to Collaboration Agreement, dated May 6, 2014, by and among Medivation, Inc., Astellas Pharma Inc., and Astellas US LLC. |
|
10-K |
|
001-32836 |
|
10.17 |
|
2/25/2015 |
|
|
108
|
|
|
|
|
|
|
|
Incorporated By Reference |
|
|
|||
|
Exhibit |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.23** |
|
Amendment No. 6 to Collaboration Agreement dated January 6, 2016, by and among Medivation, Inc., Astellas Pharma Inc., and Astellas US LLC. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.24 |
|
Office Lease, dated as of December 28, 2011, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-K |
|
001-32836 |
|
10.27 |
|
2/29/2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.25 |
|
First Amendment to Office Lease, dated as of December 28, 2011, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-K |
|
001-32836 |
|
10.32 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.26 |
|
Second Amendment to Lease, dated as of July 6, 2012, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
11/9/2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.27 |
|
Third Amendment to Lease, dated as of September 27, 2012, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-Q |
|
001-32836 |
|
10.3 |
|
11/9/2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.28 |
|
Fourth Amendment to Lease, dated as of June 26, 2013, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
8/8/2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.29 |
|
Fifth Amendment to Lease, dated as of February 12, 2014, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
8/7/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.30 |
|
Sixth Amendment to Lease, dated as of January 12, 2015, by and between Knickerbocker Properties, Inc. XXXIII and Medivation, Inc. |
|
10-Q |
|
001-32836 |
|
10.3 |
|
5/7/2015 |
|
|
|
10.31 |
|
Credit Agreement, dated as of September 4, 2015, among Medivation, Inc., the lenders party thereto and JPMorgan Chase Bank, N.A., as Administrative Agent. |
|
8-K |
|
001-32836 |
|
10.1 |
|
9/9/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.32 |
|
Amended and Restated Credit Agreement, dated as of October 23, 2015, by and among Medivation, Inc., the lenders party thereto and JPMorgan Chase Bank, N.A., as Administrative Agent |
|
8-K |
|
001-32836 |
|
10.1 |
|
10/26/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.33 |
|
Amendment No. 1 dated as of November 13, 2015 to Amended and Restated Credit Agreement, dated as of October 23, 2015, by and among Medivation, Inc., the lenders party thereto and JPMorgan Chase Bank, N.A., as Administrative Agent |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.34* |
|
Change of Control Severance Benefits Agreement, dated as of February 2, 2009, between Medivation, Inc. and David Hung, M.D. |
|
10-K |
|
001-32836 |
|
10.11 |
|
3/16/2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.35* |
|
Form of Medivation, Inc. Change of Control Severance Benefits Agreement. |
|
10-K |
|
001-32836 |
|
10.13 |
|
3/16/2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.36* |
|
Form of Indemnification Agreement for directors and officers. |
|
10-K |
|
001-32836 |
|
10.25 |
|
2/29/2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.37 |
|
Compensation Information for Non-Employee Directors. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
5/7/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38* |
|
The Medivation, Inc. 2013 Cash Performance Incentive Plan. |
|
8-K |
|
001-32836 |
|
10.3 |
|
7/1/2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.39* |
|
2014 Bonuses, 2015 Base Salaries and Equity Awards for Certain Executive Officers. |
|
8-K |
|
001-32836 |
|
Item 5.02 |
|
2/13/2015 |
|
|
109
|
|
|
|
|
|
|
|
Incorporated By Reference |
|
|
|||
|
Exhibit |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.40* |
|
2015 Bonuses, 2016 Base Salaries and Bonus Plan, and Equity Awards for Certain Executive Officers. |
|
8-K |
|
001-32836 |
|
Item 5.02 |
|
1/11/2015; 2/23/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.41* |
|
Offer Letter, dated February 28, 2014, by and between Medivation, Inc. and Rick Bierly, as amended March 3, 2014. |
|
10-Q |
|
001-32836 |
|
10.3 |
|
5/8/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.42* |
|
Consulting Agreement, dated July 14, 2014, by and between Medivation, Inc. and Dawn Svoronos. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
11/6/2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.43* |
|
Amendment to Consulting Agreement, dated October 14, 2014, by and between Medivation, Inc., and Dawn Svoronos. |
|
10-K |
|
001-32836 |
|
10.29 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.44* |
|
Offer Letter, dated November 10, 2014 by and between Medivation Field Solutions, Inc. and Joseph Lobacki. |
|
10-K |
|
001-32836 |
|
10.27 |
|
2/25/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.45* |
|
Offer Letter, dated May 13, 2015, by and between Medivation, Inc. and Andrew Powell. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
8/6/2015 |
|
|
|
10.46* |
|
Separation Agreement between Medivation, Inc. and Jennifer J. Rhodes, dated August 3, 2015. |
|
10-Q |
|
001-32836 |
|
10.2 |
|
11/6/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.47* |
|
Offer Letter between Medivation, Inc. and Thomas Templeman, Ph.D., dated July 1, 2015. |
|
10-Q |
|
001-32836 |
|
10.3 |
|
11/6/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.48* |
|
Offer Letter between Medivation, Inc. and Mohammad Hirmand, M.D., dated September 30, 2015. |
|
10-Q |
|
001-32836 |
|
10.4 |
|
11/6/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.49* |
|
Separation Agreement between Medivation, Inc. and Lynn Seely, M.D., dated September 23, 2015 |
|
10-Q |
|
001-32836 |
|
10.5 |
|
11/6/2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.50* |
|
Offer Letter, dated January 6, 2016, by and between Medivation, Inc. and Marion McCourt |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.51* |
|
Form of Performance Share Unit Grant Notice and Agreement under the Amended and Restated 2004 Equity Incentive Award Plan (for use from February 18, 2016) |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21.1 |
|
Subsidiaries of Medivation, Inc. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.1 |
|
Consent of Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24.1 |
|
Power of Attorney (contained on signature page). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
Certification pursuant to Rule 13a-14(a)/15d-14(a). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
Certification pursuant to Rule 13a-14(a)/15d-14(a). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1† |
|
Certifications of Chief Executive Officer and Chief Financial Officer. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
|
XBRL Instance Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Labels Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
110
|
|
|
|
|
|
|
|
Incorporated By Reference |
|
|
|||
|
Exhibit |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
* |
Indicates management contract or compensatory plan or arrangement. |
|
** |
Confidential treatment has been granted with respect to certain portions of this exhibit. |
|
† |
The certifications attached as Exhibit 32.1 accompanying this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Medivation, Inc., under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing. |
111
