Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a16-1392_28k.htm |
Exhibit 99.1
'trovagene DNA November 2015 Better Cancer Monitoring with Cell-Free

Forward-Looking Statements Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. 2 Copyright ©2015 Trovagene, Inc. | Confidential

Our Goal Improve Treatment Outcomes with Noninvasive Cancer Monitoring Trovagene’s technology noninvasively detects and quantitates circulating tumor DNA in urine and plasma for improved disease management 3

NASDAQ: TROV Company Overview Founded in 1999 NASDAQ listing in 2012 Technology feasibility established 2013 Clinical feasibility established 2014 Marketing launch initiated 2015 Cash: $74M as of Sept 30, 2015 Proprietary methods of extracting, purifying, detecting and quantifying oncogene mutations in cell-free DNA Clinical collaborations with leading cancer centers and pharmaceutical partners Trovagene’s noninvasive liquid biopsy assays are used to detect and monitor oncogene mutation status, response to therapy, disease progression, minimal residual disease and recurrence CLIA certified, CAP accredited, high complexity lab to offer diagnostic services 4 Copyright ©2015 Trovagene, Inc. | Confidential

Experienced Leadership Team Antonius Schuh, PhD Chief Executive Officer Stephen Zaniboni Chief Financial Officer Mark Erlander, PhD Chief Scientific Officer Sorrento Therapeutics, AviaraDx, Arcturus, Sequenom PhD Pharmaceutical Chemistry, University of Bonn Awarepoint, XIFIN, Sorrento Therapeutics, AviaraDx, Arcturus, Sequenom CPA, BS Accounting Boston University, MBA Boston College BioTheranostics, AviaraDx, Arcturus, J&J, Scripps Research Institute BS UCDavis, MS Biochemstry Iowa State University, PhD Neuroscience UCLA Matt Posard Chief Commercial Officer Karsten Schmidt, PhD Senior VP, Technology Operations Illumina Inc., Biosite Dx, Gen-Probe BA Quantitative Economics and Decision Sciences, UCSD Sequenom, Inc., Sanofi-Aventis Deutschland GmbH (formerly: Rhône-Poulenc Rorer), Fisons plc PhD The University of Bonn 5 Copyright ©2015 Trovagene, Inc. | Confidential

Strong Scientific Scientific Advisors Advisory Board and Board Board of Directors of Directors Thomas Adams, PhD Chairman Paul R. Billings, MD, PhD Director, Member Executive Compensation and Nominating Committees Gary Jacob, PhD Director, Member Executive Compensation Committee Rodney S. Markin, MD, PhD Director, Member Executive Compensation, Nominating and Audit Committees Antonius Schuh, PhD Director, Chief Executive Officer 6 Copyright ©2015 Trovagene, Inc. | Confidential Peter Hirth, PhD Co-founder and Retired Chief Executive Officer of Plexxikon Riccardo Dalla-Favera, MD Director, Institute for Cancer Genetics and Herbert Irving Comprehensive Cancer Center, Columbia University Brunangelo Falini, MD Director, Institute Hematology, University of Perugia Charles Cantor, PhD Founder and Retired Chief Scientific Officer Sequenom Carlo M. Croce, MD Chairman, Department of Molecular Virology, Immunology and Medical Genetics at Ohio State University, and Director of the Institute of Genetics at the Ohio State University Comprehensive Cancer Center John P. Brancaccio, CPA Director, Chair Audit Committee Stanley Tennant, MD Director, Chair Executive Compensation Committee and Member Audit Committee Alberto Bardelli, PhD Department of Oncology, Torino Medical School, Institute for Cancer Research Paul Billings, MD, PhD Chief Medical Officer, Omica
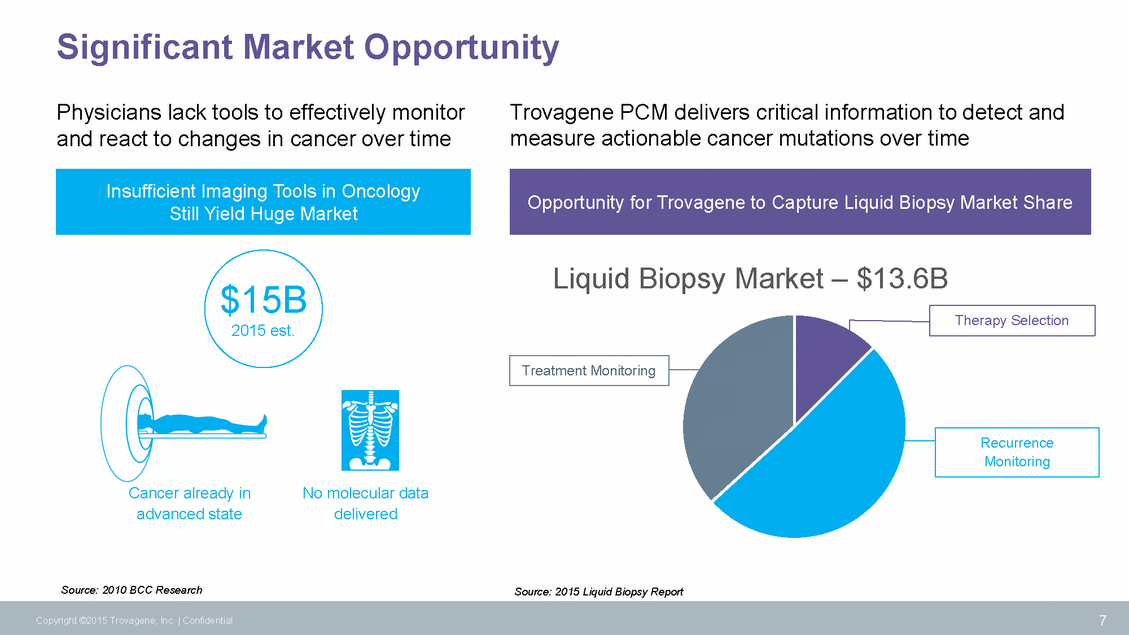
Significant Market Opportunity Physicians lack tools to effectively monitor and react to changes in cancer over time Trovagene PCM delivers critical information to detect and measure actionable cancer mutations over time Liquid Biopsy Market – $13.6B $15B 2015 est. Cancer already in advanced state No molecular data delivered Source: 2010 BCC Research Source: 2015 Liquid Biopsy Report 7 Copyright ©2015 Trovagene, Inc. | Confidential Recurrence Monitoring Treatment Monitoring Therapy Selection Opportunity for Trovagene to Capture Liquid Biopsy Market Share Insufficient Imaging Tools in Oncology Still Yield Huge Market
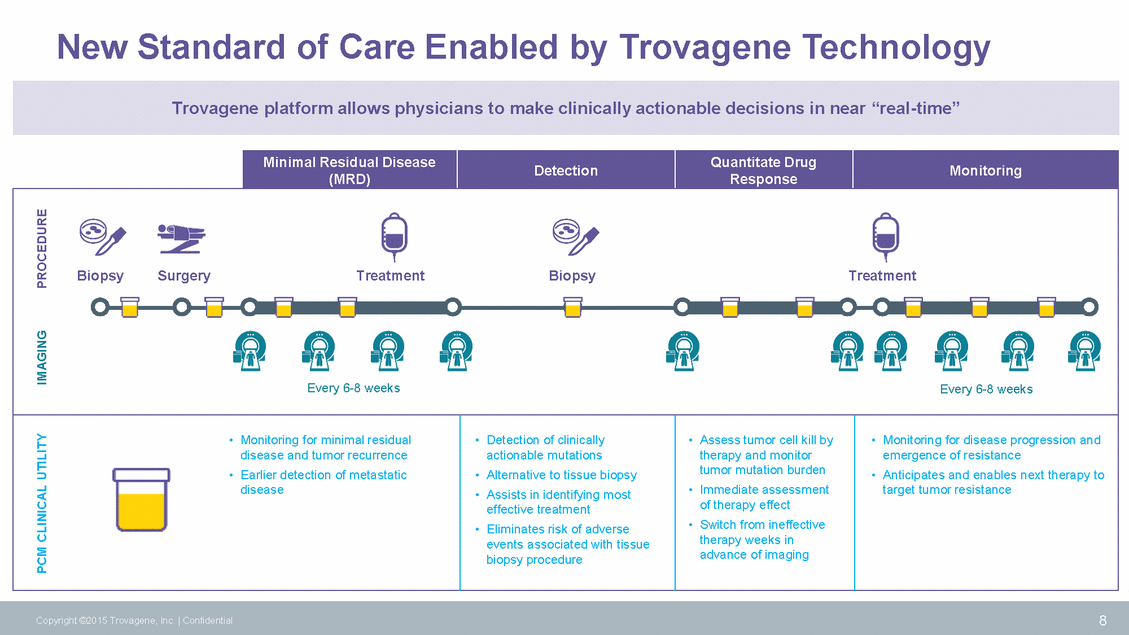
New Standard of Care Enabled by Trovagene Technology therapy weeks in 8 Copyright ©2015 Trovagene, Inc. | Confidential PCM CLINICAL UTILITY IMAGING PROCEDURE Minimal Residual Disease (MRD) Detection Quantitate Drug Response Monitoring BiopsySurgeryTreatmentBiopsyTreatment Every 6-8 weeksEvery 6-8 weeks • Monitoring for minimal residual disease and tumor recurrence • Earlier detection of metastatic disease • Detection of clinically actionable mutations • Alternative to tissue biopsy • Assists in identifying most effective treatment • Eliminates risk of adverse events associated with tissue biopsy procedure • Assess tumor cell kill by therapy and monitor tumor mutation burden • Immediate assessment of therapy effect • Switch from ineffective advance of imaging • Monitoring for disease progression and emergence of resistance • Anticipates and enables next therapy to target tumor resistance Trovagene platform allows physicians to make clinically actionable decisions in near “real-time”
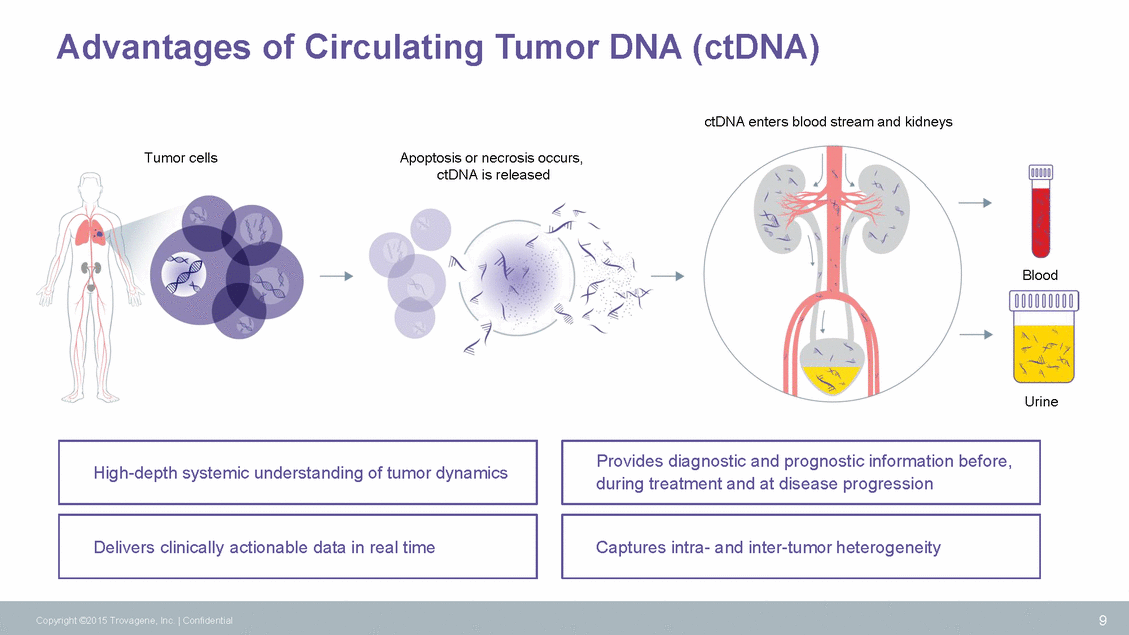
Advantages of Circulating Tumor DNA (ctDNA) ctDNA enters blood stream and kidneys Tumor cells Apoptosis or necrosis occurs, ctDNA is released Blood Urine during treatment and at disease progression 9 Copyright ©2015 Trovagene, Inc. | Confidential Captures intra-and inter-tumor heterogeneity Delivers clinically actionable data in real time Provides diagnostic and prognostic information before, High-depth systemic understanding of tumor dynamics
Trovagene Precision Cancer Monitoring (PCMSM ) Platform Quantification 10 Copyright ©2015 Trovagene, Inc. | Confidential Integrates with NGS and digital PCR platforms LDT and CLIA compliant Compatible for tech transfer agreements Enables monitoring over time ~300,000 genome equivalents of cfDNA/200 mL of urine 100-1000 fold enrichment Detection and Mutant Allele Enrichment Collection, Extraction and Isolation of ctDNA Ultra-Sensitive Detection and Quantitative Monitoring
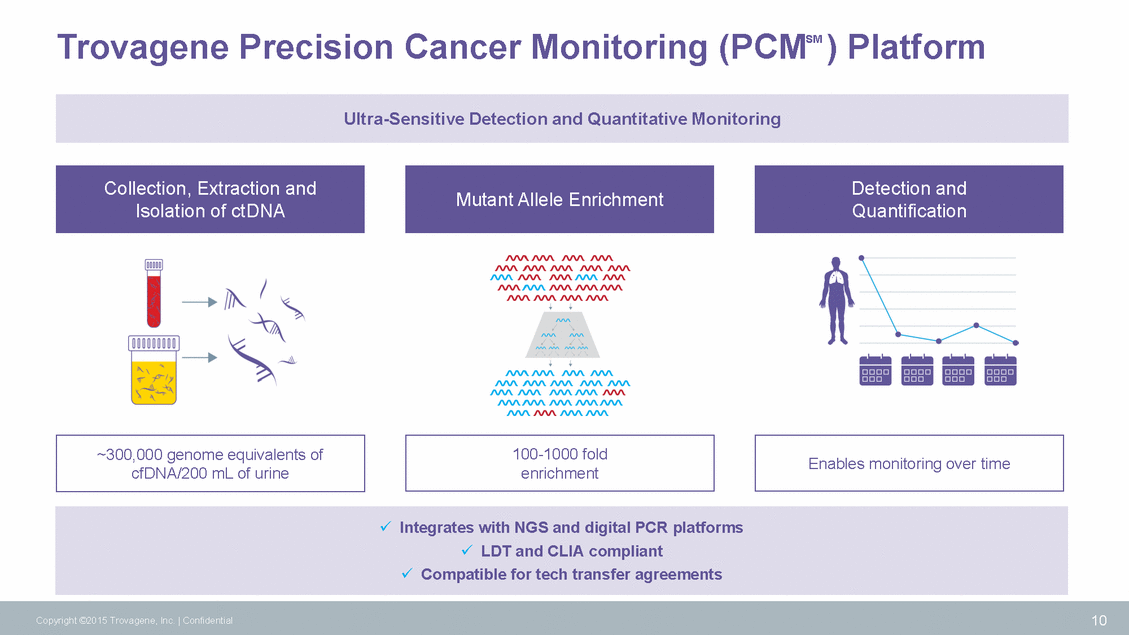
Intellectual Property Patent Families Patent Term US provisional app filed US provisional app filed US, EU, Canada US, EU, JP, China, Australia, Canada, India 11 Copyright ©2015 Trovagene, Inc. | Confidential Concentrating Urine Family 2035 51-110 bp TrNA Family 2034 Small Footprint Family 2034 US provisional app filed Monitoring Disease Family 2034 US provisional app filed 20-50 bp TrNA Family 2029 US & EU Anion Exchange Purification Family 2027 Viral and Pathogen TrNA Families 2026 TrNA Patent Family 2018 US & EU
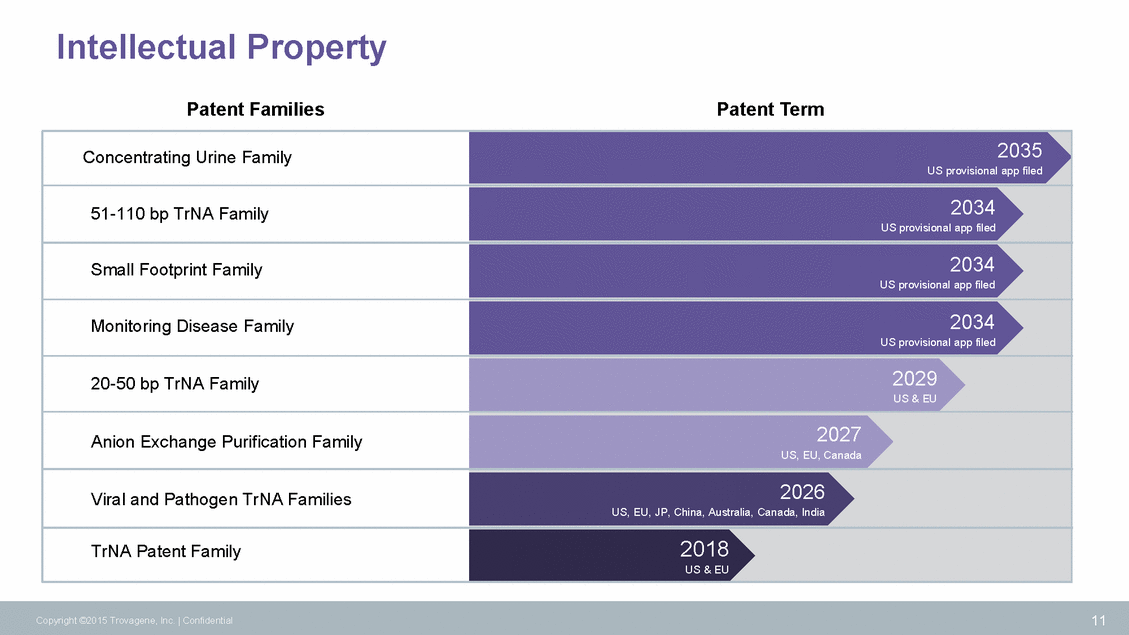
Technology Portfolio Evolution 12 Copyright ©2015 Trovagene, Inc. | Confidential Feasibility Analysis In Development Commercially Available Single Assays for MonitoringKRAS (Exon 2) (Ultrasensitive, Quantitative) BRAF (V600E) EGFR (Exon 19 Del) EGFR (Exon 20 T790M) EGFR (Exon 21 L858R) Multi-gene PanelKRAS (5 assays) NRAS (5 assays) EGFR (6 assays) BRAF (1 assays) PIK3CA (2 assays) Find-It Follow-It Gene RearrangementsALK RET ROS
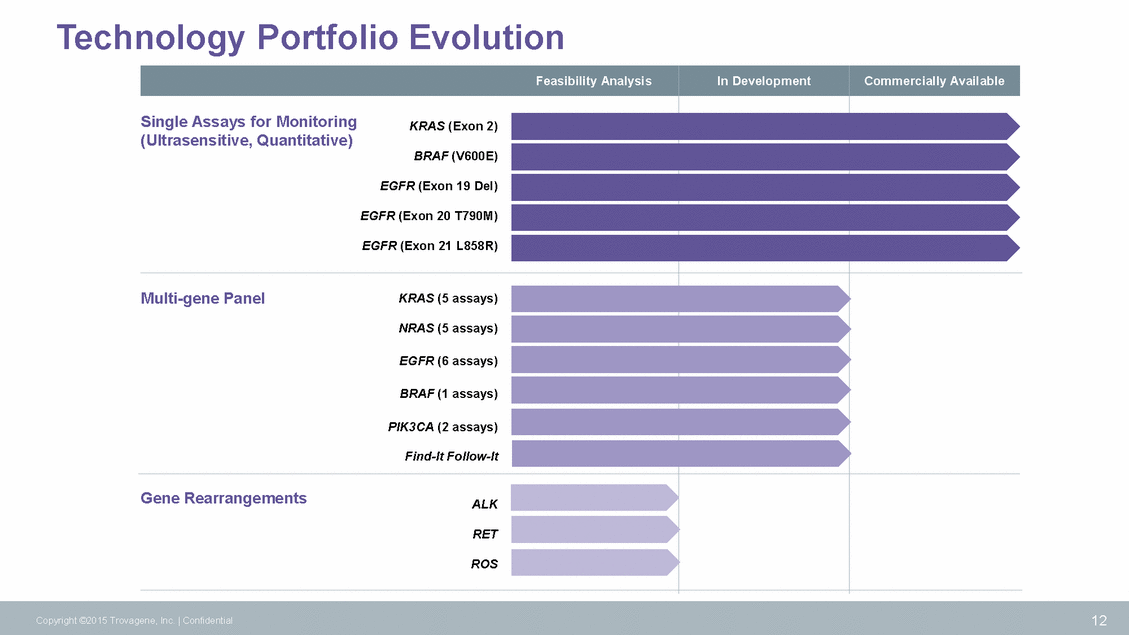
Distribution and Service Options CLIA Lab Pharma Services Cloud Exchange Tech Transfer 13 Copyright ©2015 Trovagene, Inc. | Confidential Democratized Centralized
Trovagene Research Institute (TRI) World Class Team & Technology to Enhance Our Clinical Expertise and Commercial Reach University of Torino Technology and intellectual property transferred to TRI Leverage relationships with key opinion leaders and pharmaceutical companies Conduct clinical and translational studies in Europe World leader in cancer genomic research and translational science Accomplished translational research team Center of Excellence in Europe Established network with pharmaceutical and life science companies Alberto Bardelli, PhD Copyright ©2015 Trovagene, Inc. | Confidential Collaboration with European Subsidiary of Trovagene Scientific Chair Goal: Accelerate adoption of Trovagene's Precision Cancer Monitoring platform in translational research and clinical applications
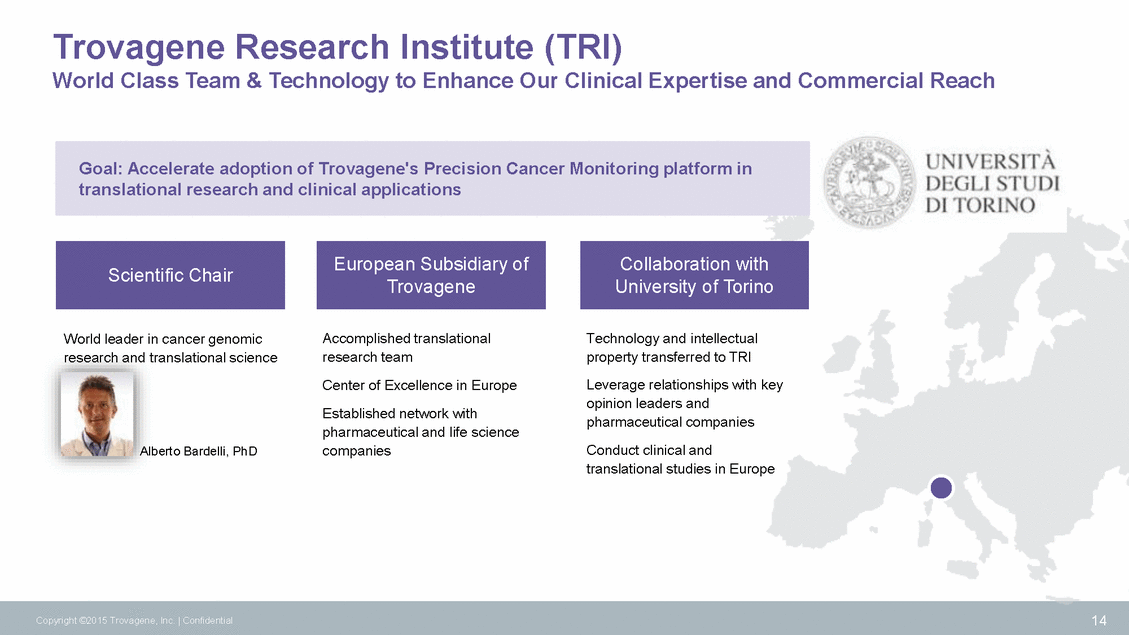
Strategy to Drive Clinical Adoption and Reimbursement STANDARD OF CARE QUALITATIVE QUANTITATIVE Stage 2 Tumor Dynamics Stage 3 Improve Patient Outcomes Stage 1 Tumor Status • Demonstrate concordance of oncogene mutation status between ctDNA and tumor tissue • Expand mutational coverage of the PCM platform • Demonstrate, in multi-institutional trials, that results from ctDNA-based monitoring of actionable mutations increases patient progression-free survival (PFS) and overall survival (OS) • Clinical Utility: Quantitatively assess mutational status in ctDNA longitudinally as an indicator of responsiveness to therapy, disease status and emergence of resistance mutations • Clinical Utility: Determine mutational status of actionable biomarkers in ctDNA when a tissue biopsy is not available • Demonstrate health economic benefits of noninvasive oncogene mutation monitoring • Clinical Utility: Quantitatively assess patient mutational status in ctDNA longitudinally for mutational status and early detection of resistance to therapy as a decision tool for therapy selection 15 Copyright ©2015 Trovagene, Inc. | Confidential
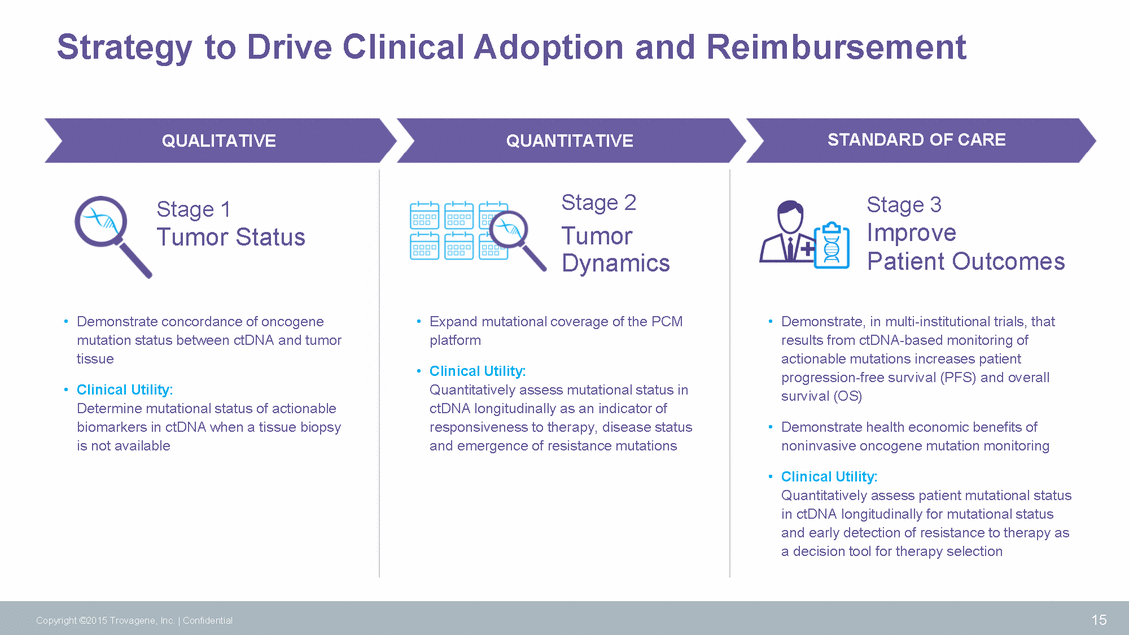
Histiocytic Disorders: ECD/LCH Standard of Care ECD/LCH First Line Treatment Tissue Biopsy Imaging (every 2-3 months) 16 Copyright ©2015 Trovagene, Inc. | Confidential STUDIES TROVAGENE CLINICAL YTILITY MSKCC, MDACC, NIH (30 patients) Detection BRAF V600E Monitoring Therapeutic Response BRAF V600E ECD/LCH ALL STAGES •Molecular diagnosis of BRAF mutation – patients are placed on targeted therapy •Re-staging of cancer
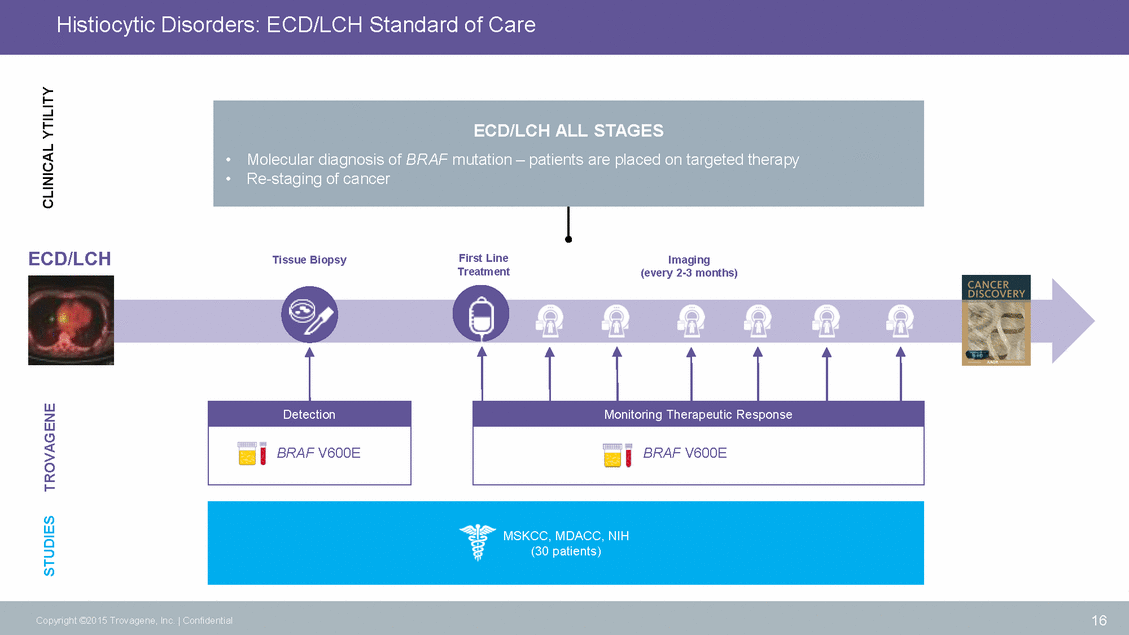
Detection Urinary ctDNA Outperforms Tissue Biopsies1 100% concordance between tissue, urine and plasma in treatment naive patients. 1Hyman et al., Cancer Discov. 2015 Jan;5(1):64-71 17 Copyright ©2015 Trovagene, Inc. | Confidential Tissue Biopsies 70% BRAF mutational status obtained n=15Mutant Tissue BRAF V600E genotype Indeterminate Genotypen=9BRAFV600E BRAFn=6 Wildtype Urine 100% BRAF mutational status obtained Urinary ctDNA BRAF V600E genotype n=16 BRAFV600E BRAFn=14Mutant Wildtype
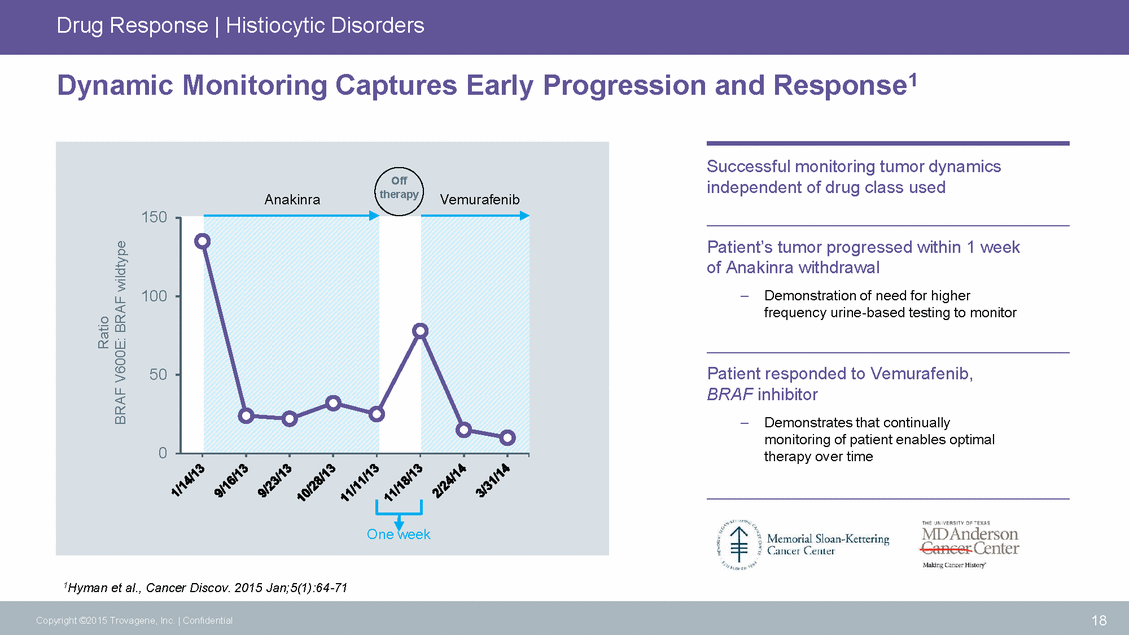
Drug Response | Histiocytic Disorders Early Progression and Response1 Dynamic Monitoring Captures Successful monitoring tumor dynamics independent of drug class used Patient’s tumor progressed within 1 week of Anakinra withdrawal –Demonstration of need for higher frequency urine-based testing to monitor Patient responded to Vemurafenib, BRAF inhibitor – Demonstrates that continually monitoring of patient enables optimal therapy over time 1Hyman et al., Cancer Discov. 2015 Jan;5(1):64-71 18 Copyright ©2015 Trovagene, Inc. | Confidential Ratio BRAF V600E: BRAF wildtype Off AnakinratherapyVemurafenib 150 100 50 0 One week
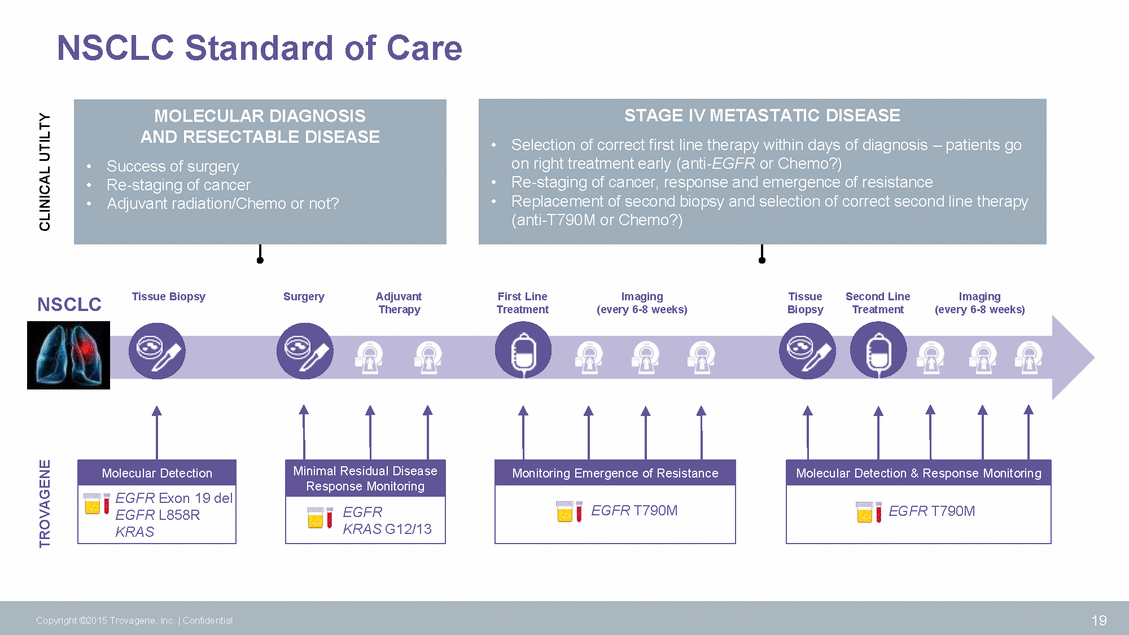
NSCLC Standard of Care Tissue Biopsy Surgery Adjuvant Therapy First Line Treatment Imaging (every 6-8 weeks) Tissue Biopsy Second Line Treatment Imaging (every 6-8 weeks) NSCLC Response Monitoring KRAS G12/13 19 Copyright ©2015 Trovagene, Inc. | Confidential TROVAGENE CLINICAL UTILTY Minimal Residual Disease EGFR Molecular Detection EGFR Exon 19 del EGFR L858R KRAS Monitoring Emergence of Resistance EGFR T790M Molecular Detection & Response Monitoring EGFR T790M STAGE IV METASTATIC DISEASE •Selection of correct first line therapy within days of diagnosis – patients go on right treatment early (anti-EGFR or Chemo?) •Re-staging of cancer, response and emergence of resistance •Replacement of second biopsy and selection of correct second line therapy (anti-T790M or Chemo?) MOLECULAR DIAGNOSIS AND RESECTABLE DISEASE •Success of surgery •Re-staging of cancer •Adjuvant radiation/Chemo or not?
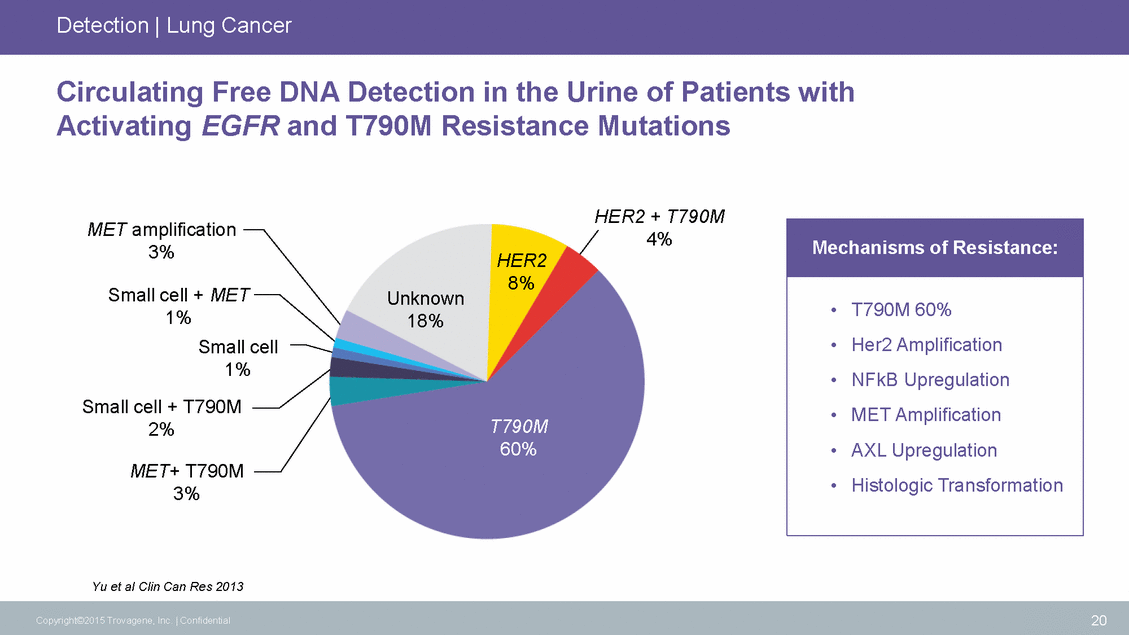
Detection | Lung Cancer Circulating Free DNA Detection in the Urine of Patients with Activating EGFR and T790M Resistance Mutations HER2 + T790M 4% MET amplification 3% HER2 8% Small cell + MET 1% Unknown 18% Small cell 1% Small cell + T790M 2% T790M 60% MET+ T790M 3% Yu et al Clin Can Res 2013 20 Copyright©2015 Trovagene, Inc. | Confidential Mechanisms of Resistance: • T790M 60% • Her2 Amplification • NFkB Upregulation • MET Amplification • AXL Upregulation • Histologic Transformation
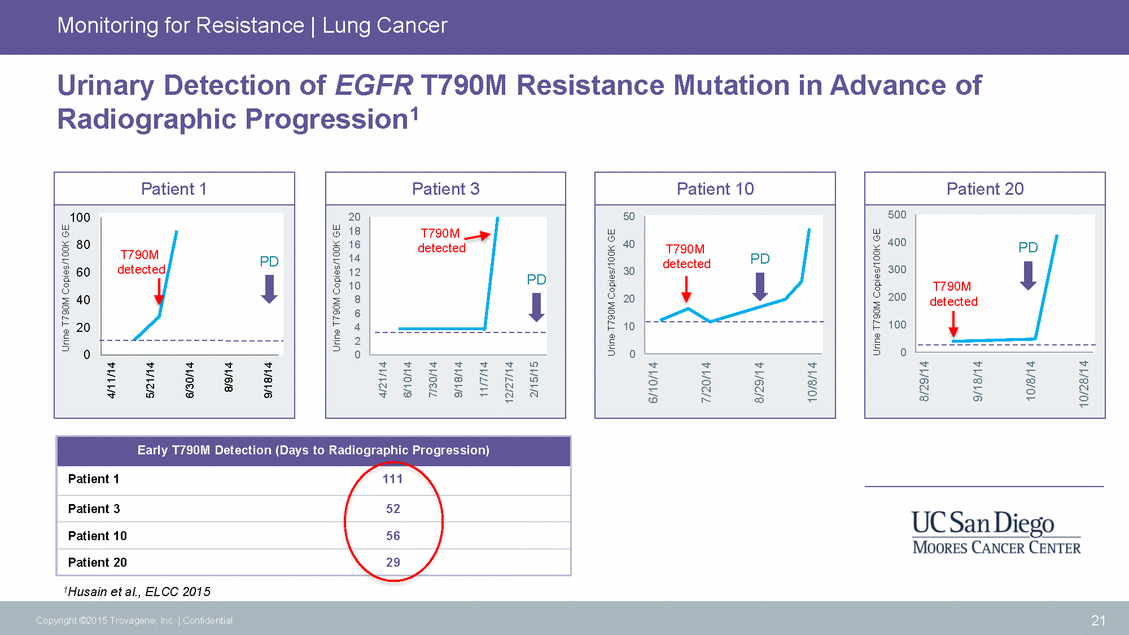
Monitoring for Resistance | Lung Cancer Urinary Detection of EGFR T790M Radiographic Progression1 Resistance Mutation in Advance of 10 1Husain et al., ELCC 2015 21 Copyright ©2015 Trovagene, Inc. | Confidential Urine T790M Copies/100K GE 4/11/14 5/21/14 6/30/14 8/9/14 9/18/14 Urine T790M Copies/100K GE 4/21/14 6/10/14 7/30/14 9/18/14 11/7/14 12/27/14 2/15/15 Urine T790M Copies/100K GE 6/10/14 7/20/14 8/29/14 10/8/14 Urine T790M Copies/100K GE 8/29/14 9/18/14 10/8/14 10/28/14 Early T790M Detection (Days to Radiographic Progression) Patient 1111 Patient 352 Patient 1056 Patient 2029 Patient 1 100 80 60detected 40 20 0 T790MPD Patient 3 20 18 16 14 12 8 6 4 2 0 T790M detected PD Patient 10 50 40 30 20 10 0 T790M detectedPD Patient 20 500 400 300 200 100 0 PD T790M detected
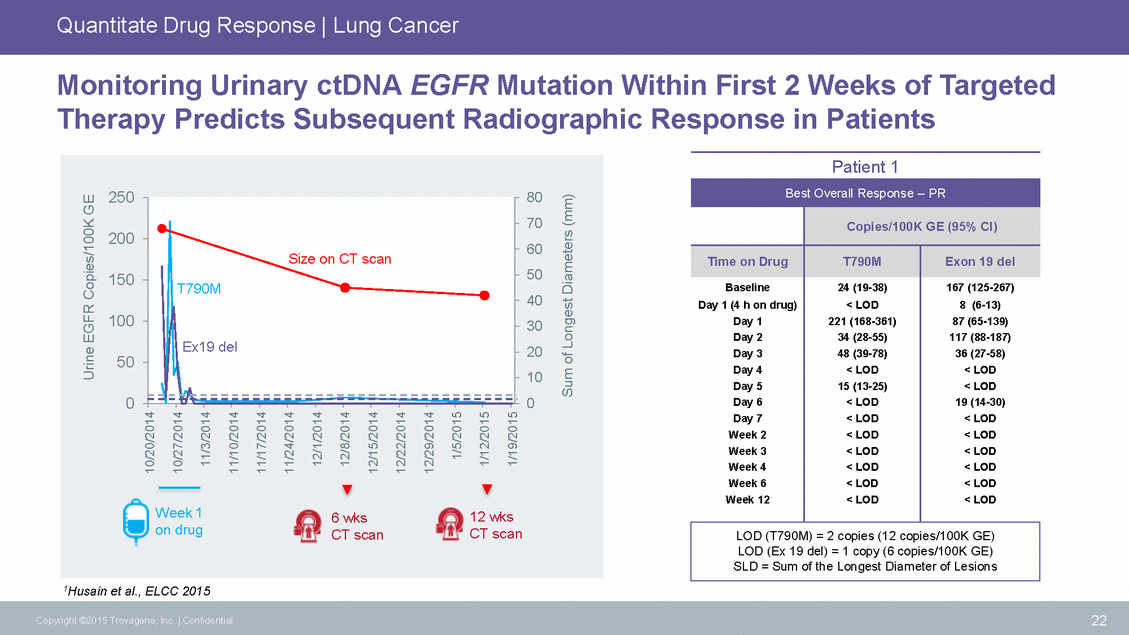
Quantitate Drug Response | Lung Cancer Monitoring Urinary ctDNA EGFR Mutation Within First 2 Weeks of Targeted Therapy Predicts Subsequent Radiographic Response in Patients Patient 1 60 8 (6-13) 117 (88-187) < LOD 1Husain et al., ELCC 2015 22 Copyright ©2015 Trovagene, Inc. | Confidential Urine EGFR Copies/100K GE 10/20/2014 10/27/2014 11/3/2014 11/10/2014 11/17/2014 11/24/2014 12/1/2014 12/8/2014 12/15/2014 12/22/2014 12/29/2014 1/5/2015 1/12/2015 1/19/2015 Sum of Longest Diameters (mm) Best Overall Response – PR Copies/100K GE (95% CI) Time on Drug T790M Exon 19 del Baseline Day 1 (4 h on drug) Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Week 2 Week 3 Week 4 Week 6 Week 12 24 (19-38) < LOD 221 (168-361) 34 (28-55) 48 (39-78) < LOD 15 (13-25) < LOD < LOD < LOD < LOD < LOD < LOD < LOD 167 (125-267) 87 (65-139) 36 (27-58) < LOD 19 (14-30) < LOD < LOD < LOD < LOD < LOD < LOD LOD (T790M) = 2 copies (12 copies/100K GE) LOD (Ex 19 del) = 1 copy (6 copies/100K GE) SLD = Sum of the Longest Diameter of Lesions 25080 70 200 15050 40 10030 20 50 10 00 Week 16 wks12 wks on drugCT scanCT scan Size on CT scan T790M Ex19 del
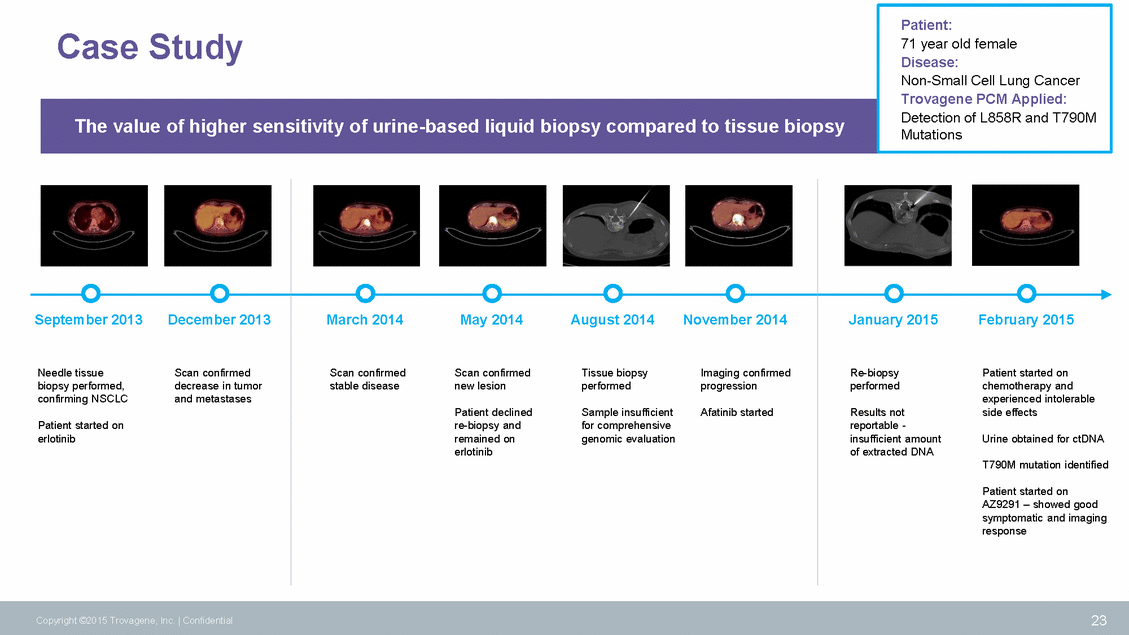
71 year old female September 2013 December 2013 March 2014 May 2014 August 2014 November 2014 January 2015 February 2015 Needle tissue biopsy performed, confirming NSCLC Scan confirmed decrease in tumor and metastases Scan confirmed stable disease Scan confirmed new lesion Tissue biopsy performed Imaging confirmed progression Re-biopsy performed Patient started on chemotherapy and experienced intolerable side effects Patient declined re-biopsy and remained on erlotinib Sample insufficient for comprehensive genomic evaluation Afatinib started Results not reportable - insufficient amount of extracted DNA Patient started on erlotinib Urine obtained for ctDNA T790M mutation identified Patient started on AZ9291 – showed good symptomatic and imaging response 23 Copyright ©2015 Trovagene, Inc. | Confidential Case Study Patient: Disease: Non-Small Cell Lung Cancer Trovagene PCM Applied: Detection of L858R and T790M Mutations The value of higher sensitivity of urine-based liquid biopsy compared to tissue biopsy
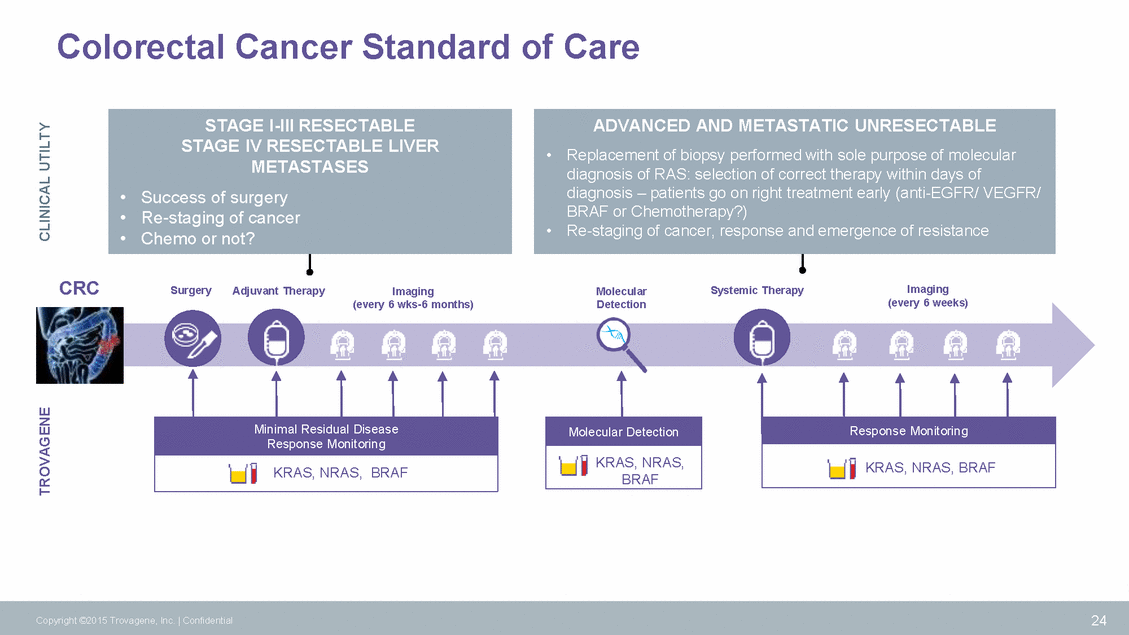
Colorectal Cancer Standard of Care diagnosis of RAS: selection of correct therapy within days of BRAF or Chemotherapy?) CRC Imaging (every 6 weeks) Surgery Adjuvant Therapy Systemic Therapy Imaging (every 6 wks-6 months) Molecular Detection 24 Copyright ©2015 Trovagene, Inc. | Confidential TROVAGENE CLINICAL UTILTY Minimal Residual Disease Response Monitoring KRAS, NRAS, BRAF Response Monitoring KRAS, NRAS, BRAF Molecular Detection KRAS, NRAS, BRAF ADVANCED AND METASTATIC UNRESECTABLE •Replacement of biopsy performed with sole purpose of molecular diagnosis – patients go on right treatment early (anti-EGFR/ VEGFR/ •Re-staging of cancer, response and emergence of resistance STAGE I-III RESECTABLE STAGE IV RESECTABLE LIVER METASTASES • Success of surgery • Re-staging of cancer • Chemo or not?
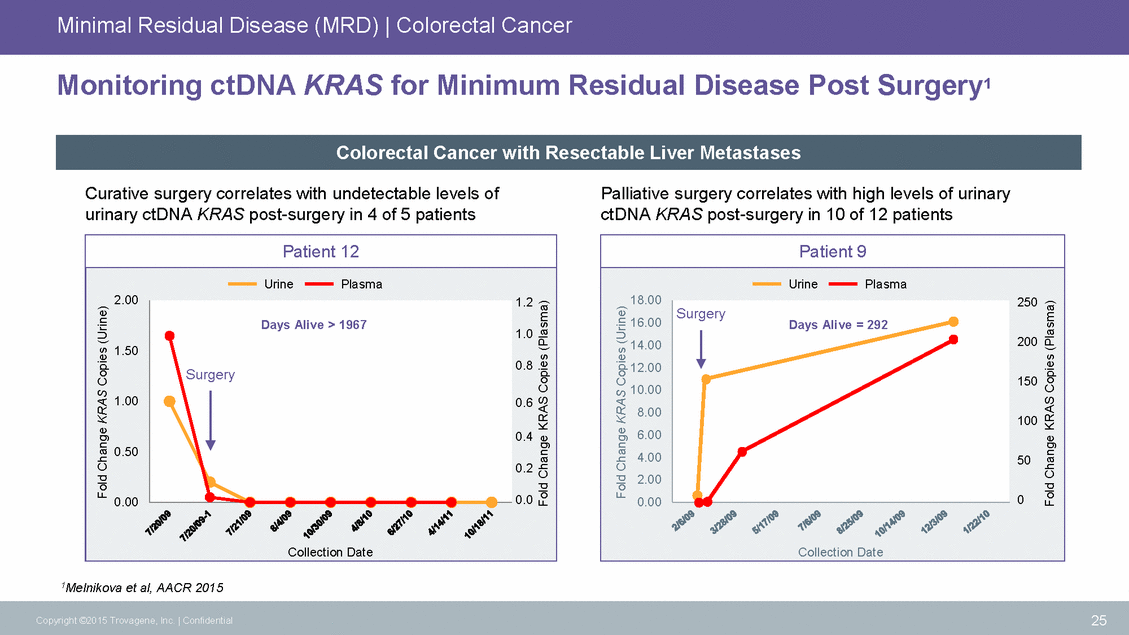
Minimal Residual Disease (MRD) | Colorectal Cancer Monitoring ctDNA KRAS for Minimum Residual Disease Post Surgery1 Curative surgery correlates with undetectable levels of urinary ctDNA KRAS post-surgery in 4 of 5 patients Palliative surgery correlates with high levels of urinary ctDNA KRAS post-surgery in 10 of 12 patients 1.50 10.00 0.2 1Melnikova et al, AACR 2015 25 Copyright ©2015 Trovagene, Inc. | Confidential Fold Change KRAS Copies (Urine) Fold Change KRAS Copies (Plasma) Fold Change KRAS Copies (Urine) Fold Change KRAS Copies (Plasma) Patient 12 UrinePlasma 2.001.2 1.0 0.8 1.000.6 0.4 0.50 0.000.0 Collection Date Days Alive > 1967 Surgery Patient 9 UrinePlasma 18.00250 16.00 14.00200 12.00 150 8.00100 6.00 4.0050 2.00 0.000 Collection Date Surgery Days Alive = 292 Colorectal Cancer with Resectable Liver Metastases
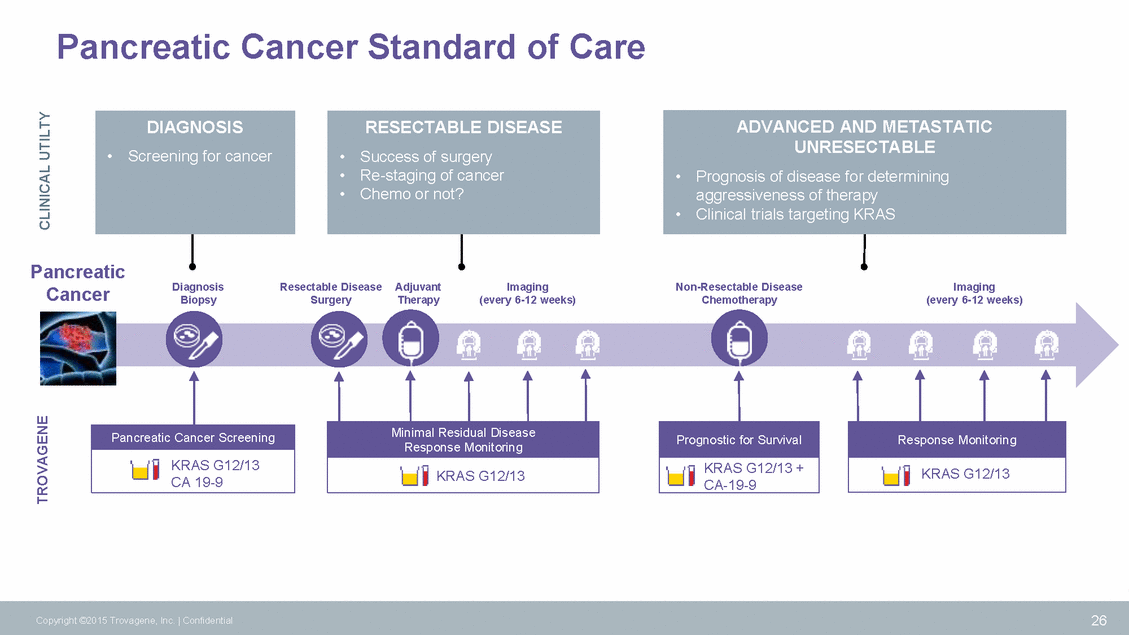
Pancreatic Cancer Standard of Care Pancreatic Cancer Diagnosis Biopsy Resectable Disease Surgery Adjuvant Therapy Imaging (every 6-12 weeks) Non-Resectable Disease Chemotherapy Imaging (every 6-12 weeks) Response Monitoring Response Monitoring 26 Copyright ©2015 Trovagene, Inc. | Confidential TROVAGENE CLINICAL UTILTY KRAS G12/13 Pancreatic Cancer Screening KRAS G12/13 CA 19-9 Minimal Residual Disease KRAS G12/13 Prognostic for Survival KRAS G12/13 + CA-19-9 ADVANCED AND METASTATIC UNRESECTABLE •Prognosis of disease for determining aggressiveness of therapy •Clinical trials targeting KRAS RESECTABLE DISEASE •Success of surgery •Re-staging of cancer •Chemo or not? DIAGNOSIS •Screening for cancer
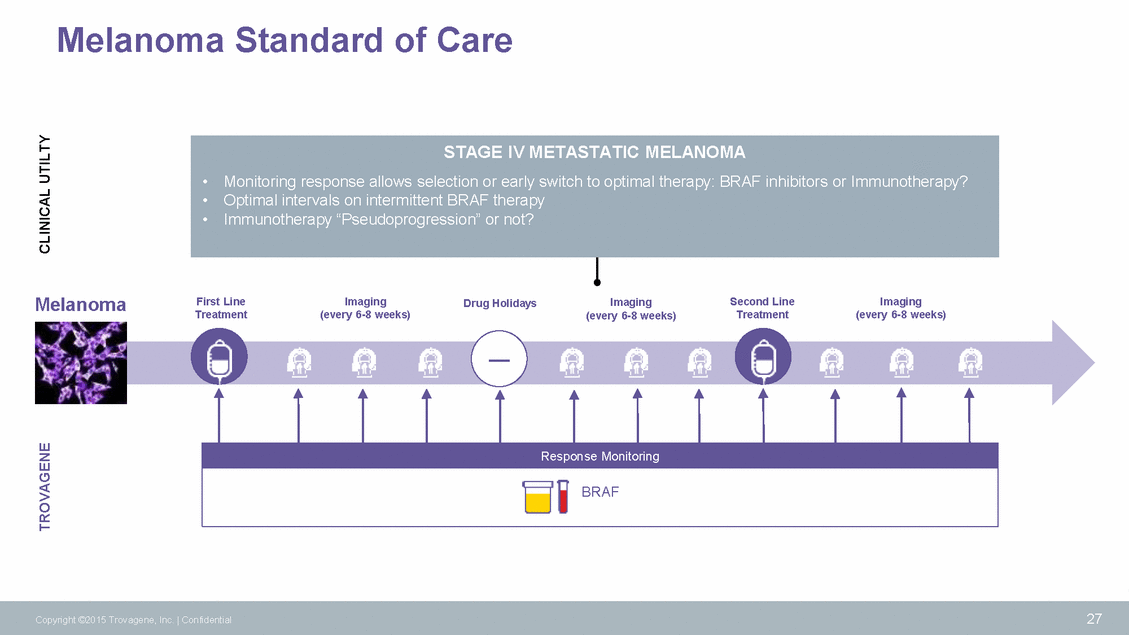
Melanoma Standard of Care Melanoma First Line Treatment Imaging (every 6-8 weeks) Second Line Treatment Imaging (every 6-8 weeks) Drug Holidays Imaging (every 6-8 weeks) — 27 Copyright ©2015 Trovagene, Inc. | Confidential TROVAGENE CLINICAL UTILTY Response Monitoring BRAF STAGE IV METASTATIC MELANOMA •Monitoring response allows selection or early switch to optimal therapy: BRAF inhibitors or Immunotherapy? •Optimal intervals on intermittent BRAF therapy •Immunotherapy “Pseudoprogression” or not?
30+ Clinical Studies to Validate & Integrate Our PCM Platform Size U.S. 28 Copyright ©2015 Trovagene, Inc. | Confidential Disease Ongoing Pending Protocol in Dev/Approval Under Evaluation Market (# of pts) Lung Cancer (NSCLC) 7 0 — 399,000 Colorectal Cancer 3 3 — 1,154,000 Pancreatic Cancer 4 — 1 42,000 Melanoma 2 2 — 922.000 ECD/LCH 2 1 — 5,000 Brain Cancer 1 1 — 142,000 Prostate Cancer — 1 — 2,618,000 All-Comers, Metastatic Cancers 2 — — 525,000 HPV 4 — 3 Total # of Studies 25 8 4

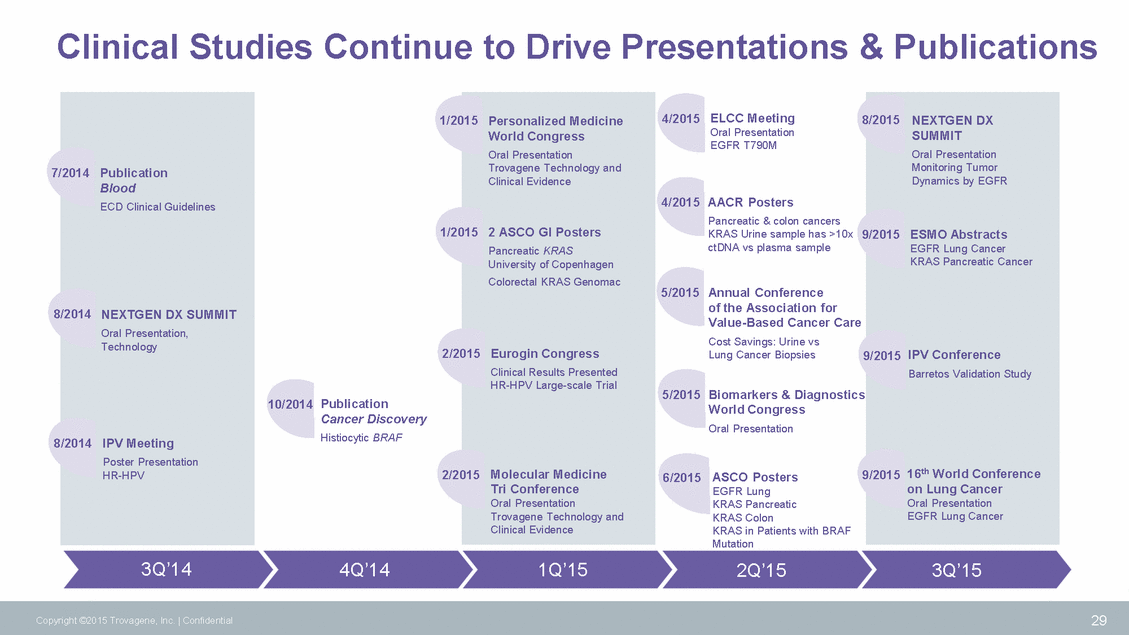
Clinical Studies Continue to Drive Presentations & Publications 4/2015 ELCC Meeting Oral Presentation EGFR T790M 1/2015 Personalized Medicine 8/2015 NEXTGEN DX 7/2014 Publication 4/2015 AACR Posters Pancreatic & colon cancers 1/2015 2 ASCO GI Posters KRAS Urine sample has >10x 9/2015 ESMO Abstracts ctDNA vs plasma sample 5/2015 Annual Conference of the Association for Value-Based Cancer Care Cost Savings: Urine vs Lung Cancer Biopsies 8 2/2015 Eurogin Congress 9/2015 IPV Conference 5/2015 Biomarkers & Diagnostics World Congress Oral Presentation 10/2014 Publication Cancer Discovery Histiocytic BRAF 8 9/2015 16th World Conference 2/2015 Molecular Medicine 6/2015 ASCO Posters EGFR Lung KRAS Pancreatic KRAS Colon KRAS in Patients with BRAF Mutation 2Q’15 3Q’14 4Q’14 1Q’15 3Q’15 29 Copyright ©2015 Trovagene, Inc. | Confidential Blood ECD Clinical Guidelines /2014 NEXTGEN DX SUMMIT Oral Presentation, Technology /2014 IPV Meeting Poster Presentation HR-HPV SUMMIT Oral Presentation Monitoring Tumor Dynamics by EGFR EGFR Lung Cancer KRAS Pancreatic Cancer Barretos Validation Study on Lung Cancer Oral Presentation EGFR Lung Cancer World Congress Oral Presentation Trovagene Technology and Clinical Evidence Pancreatic KRAS University of Copenhagen Colorectal KRAS Genomac Clinical Results Presented HR-HPV Large-scale Trial Tri Conference Oral Presentation Trovagene Technology and Clinical Evidence
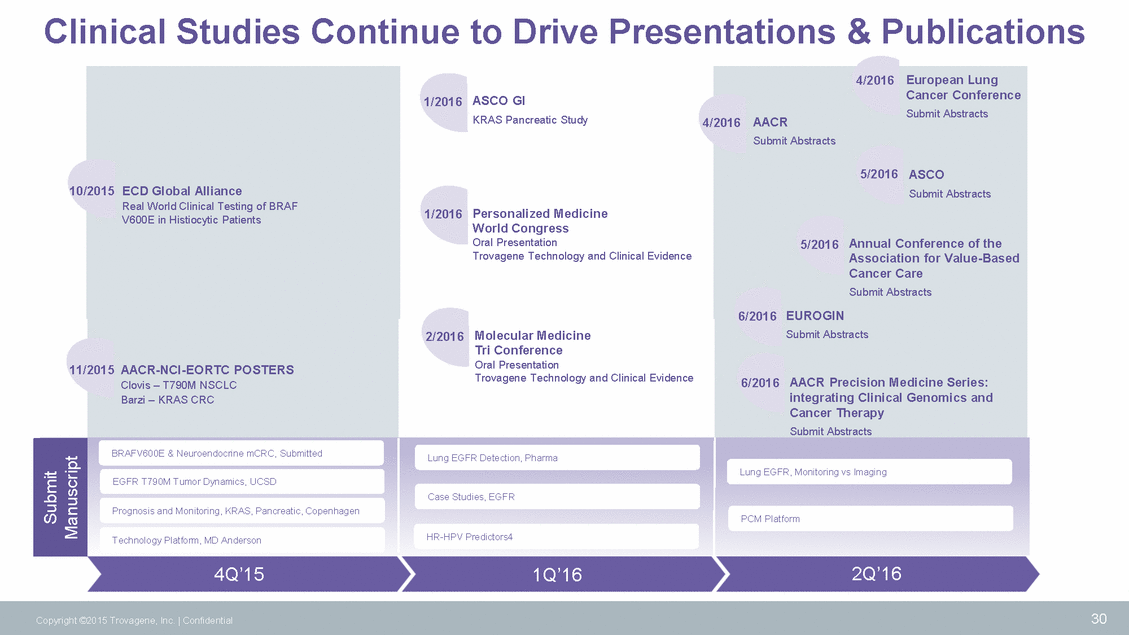
Clinical Studies Continue to Drive Presentations & Publications 1/2016 ASCO GI KRAS Pancreatic Study 4/ Personalized Medicine World Congress Oral Presentation Trovagene Technology and Clinical Evidence 1/2016 Molecular Medicine Tri Conference Oral Presentation Trovagene Technology and Clinical Evidence 2/2016 Lung EGFR Detection, Pharma Case Studies, EGFR HR-HPV Predictors4 2Q’16 4Q’15 1Q’16 30 Copyright ©2015 Trovagene, Inc. | Confidential 10/ 11/ 2015 ECD Global Alliance Real World Clinical Testing of BRAF V600E in Histiocytic Patients 2015 AACR-NCI-EORTC POSTERS Clovis – T790M NSCLC Barzi – KRAS CRC Submit Manuscript BRAFV600E & Neuroendocrine mCRC, Submitted EGFR T790M Tumor Dynamics, UCSD Prognosis and Monitoring, KRAS, Pancreatic, Copenhagen Technology Platform, MD Anderson 4/2016 European Lung Cancer Conference 2016 AACRSubmit Abstracts Submit Abstracts 5/2016 ASCO Submit Abstracts 5/2016 Annual Conference of the Association for Value-Based Cancer Care Submit Abstracts 6/2016 EUROGIN Submit Abstracts 6/2016 AACR Precision Medicine Series: integrating Clinical Genomics and Cancer Therapy Submit Abstracts Lung EGFR, Monitoring vs Imaging PCM Platform
Creating Physician Awareness & Demand The Trovagene Clinical Experience Program (CEP) offers physicians hands-on experience with their patients biopsy result 31 Copyright ©2015 Trovagene, Inc. | Confidential Physician Access Benefits Choice of 3 Applications Urine as a sample Diagnostic in lieu of a tissue Quantitation Baseline & monitor Ultra sensitivity Early therapeutic response
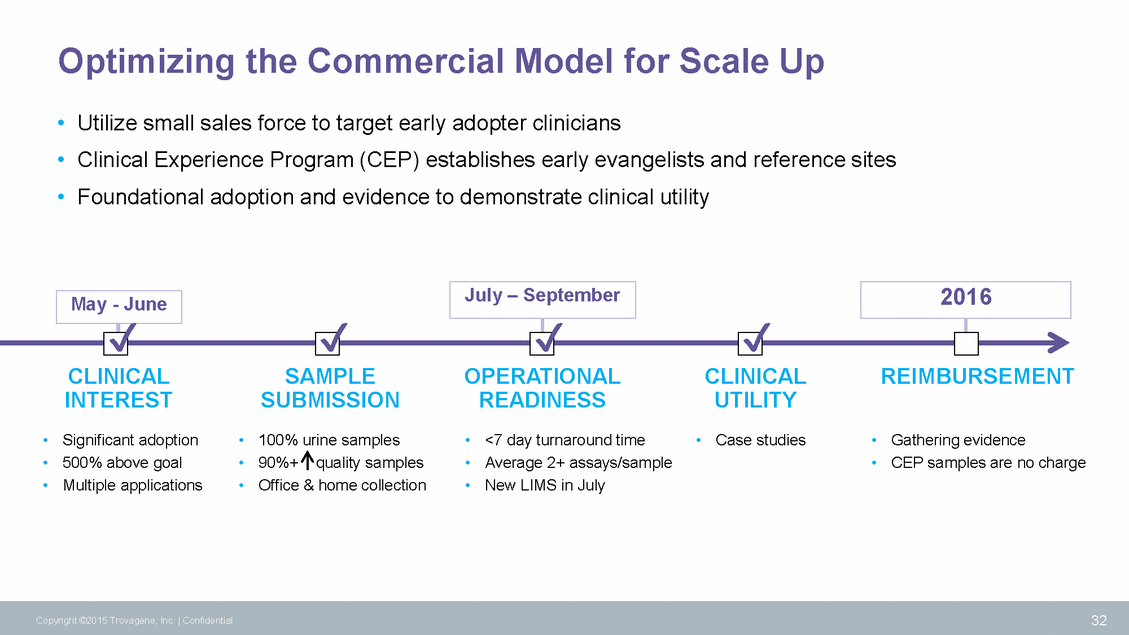
Optimizing the Commercial Model for Scale Up • • • Utilize small sales force to target early adopter clinicians Clinical Experience Program (CEP) establishes early evangelists and reference sites Foundational adoption and evidence to demonstrate clinical utility CLINICAL INTEREST Significant adoption 500% above goal Multiple applications SAMPLE SUBMISSION 100% urine samples 90%+quality samples Office & home collection OPERATIONAL READINESS CLINICAL UTILITY • Case studies REIMBURSEMENT • • • • • • • • • <7 day turnaround time Average 2+ assays/sample New LIMS in July • Gathering evidence • CEP samples are no charge 32 Copyright ©2015 Trovagene, Inc. | Confidential May - June 2016 July – September
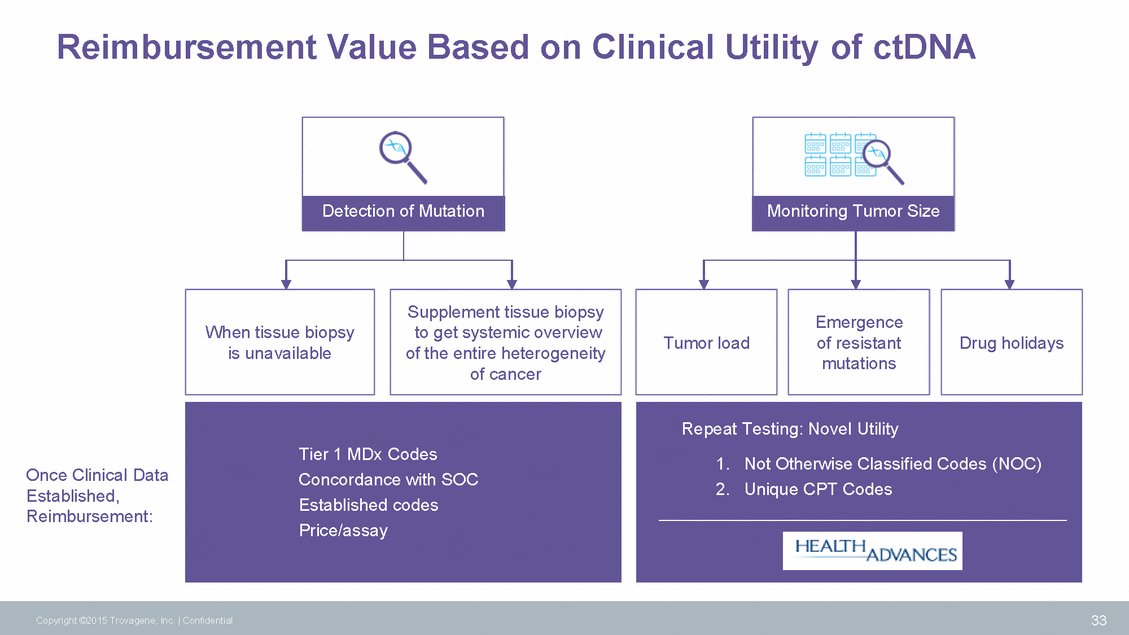
Reimbursement Value Based on Clinical Utility of ctDNA Detection of Mutation Monitoring Tumor Size Supplement tissue biopsy to get systemic overview of the entire heterogeneity of cancer Emergence of resistant mutations When tissue biopsy is unavailable Tumor load Drug holidays Once Clinical Data Established, Reimbursement: 33 Copyright ©2015 Trovagene, Inc. | Confidential Repeat Testing: Novel Utility 1. Not Otherwise Classified Codes (NOC) 2. Unique CPT Codes Tier 1 MDx Codes Concordance with SOC Established codes Price/assay
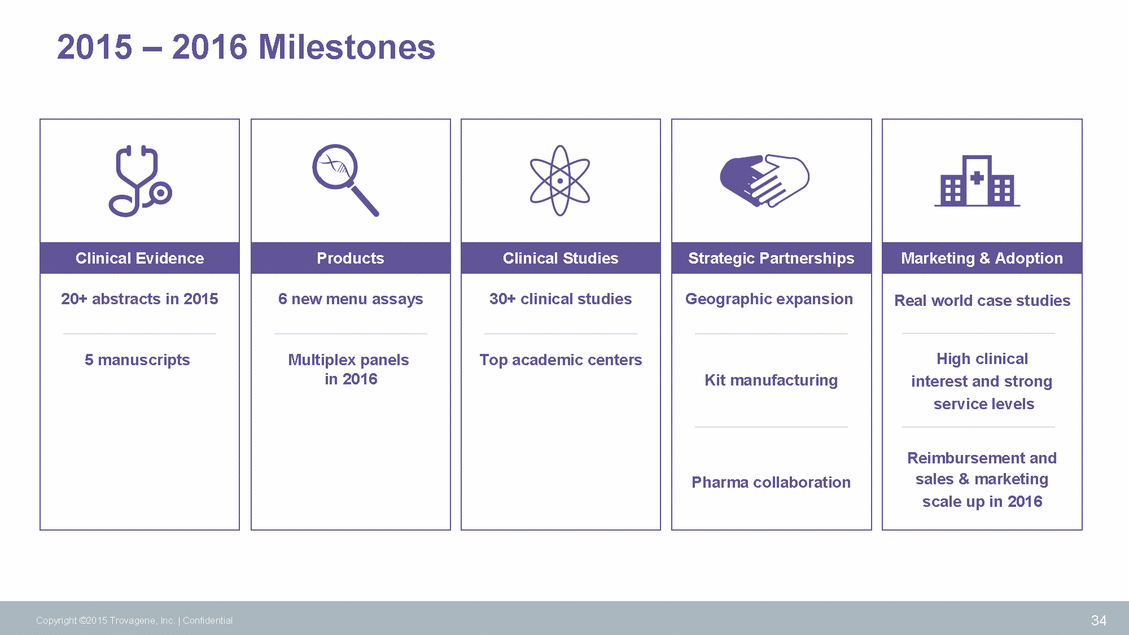
2015 – 2016 Milestones 34 Copyright ©2015 Trovagene, Inc. | Confidential Marketing & Adoption Real world case studies High clinical interest and strong service levels Reimbursement and sales & marketing scale up in 2016 Strategic Partnerships Geographic expansion Kit manufacturing Pharma collaboration Clinical Studies 30+ clinical studies Top academic centers Products 6 new menu assays Multiplex panels in 2016 Clinical Evidence 20+ abstracts in 2015 5 manuscripts
For further information Please contact: Stephen Zaniboni, CFO szaniboni@trovagene.com

