Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PDS Biotechnology Corp | s001074x1_8k.htm |
Exhibit 99.1

October 2015

Disclaimer This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make.

We are a clinical-stage biotechnology company focused on the discovery, development, and commercialization of novel, hospital-based therapies capable of transforming treatment paradigms in the management of acute, life-threatening conditions. Our lead product candidate, EG-1962, is in development to fundamentally improve patient outcomes after Aneurysmal Subarachnoid Hemorrhage (aSAH).

Key Investment Highlights Lead Candidate EG-1962 is Phase 3-ready; could replace standard of care in aSAHTargeted and sustained release of standard of care nimodipine NEWTON Phase 1/2 data shows dramatic 2x improvement in favorable outcomes Superior safety profileGranted Orphan Designation in US and EU; De-risked (505(b)(2)) regulatory pathwayAttractive commercial opportunity for EG-1962 in high unmet need600,000 aSAH patients WW; 35,000 patients in US annuallyConcentrated market: requires only small, highly focused sales force Compelling pharmacoeconomic benefitWholly owned - Worldwide rightsProprietary PRECISA™ Technology: Platform to develop future productsEG-1964 for cSDH and earlier stage discovery efforts ongoingStrong Financial PositionCash balance at June 30, 2015 was $58.5 million Raised approximately $92.5 million in IPO, which closed October 6, 2015
Edge’s Experienced Leadership Team Chairman of Scientific Advisory Board Robert Langer, PhD David H. Koch Institute Professor at MIT Biomedical Engineering Chairman of the Board of Directors Sol Barer, PhD Executive Management Team Brian A. Leuthner President & CEO, Co-founder, Director R. Loch Macdonald, MD, PhD Chief Scientific Officer, Co-founder, Director Herbert Faleck, DO Chief Medical Officer Andrew J. Einhorn Chief Financial Officer Keenan Endowed Chair of Neurosurgery 20+ yearsInvestment Banking Former CEO and Chairman Pediatric Neurologist
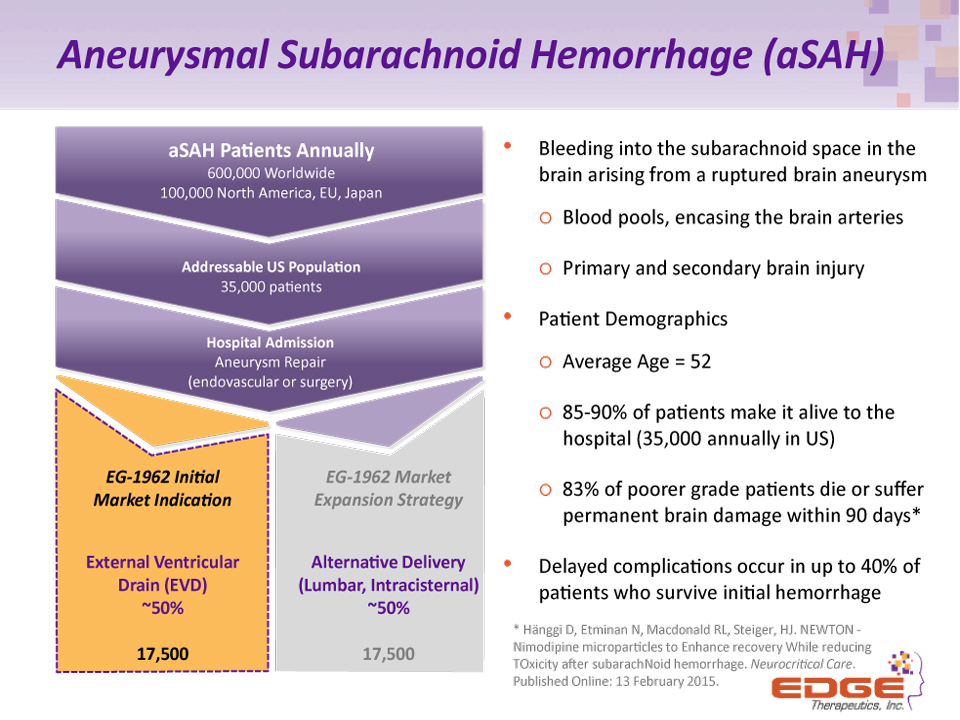
Aneurysmal Subarachnoid Hemorrhage (aSAH) Bleeding into the subarachnoid space in the brain arising from a ruptured brain aneurysmBlood pools, encasing the brain arteriesPrimary and secondary brain injuryPatient DemographicsAverage Age = 5285-90% of patients make it alive to the hospital (35,000 annually in US)83% of poorer grade patients die or suffer permanent brain damage within 90 days*Delayed complications occur in up to 40% of patients who survive initial hemorrhage aSAH Patients Annually600,000 Worldwide100,000 North America, EU, Japan EG-1962 Market Expansion StrategyAlternative Delivery(Lumbar, Intracisternal)~50%17,500 EG-1962 Initial Market IndicationExternal Ventricular Drain (EVD)~50%17,500 Addressable US Population35,000 patients Hospital AdmissionAneurysm Repair(endovascular or surgery) * Hänggi D, Etminan N, Macdonald RL, Steiger, HJ. NEWTON - Nimodipine microparticles to Enhance recovery While reducing TOxicity after subarachNoid hemorrhage. Neurocritical Care. Published Online: 13 February 2015.
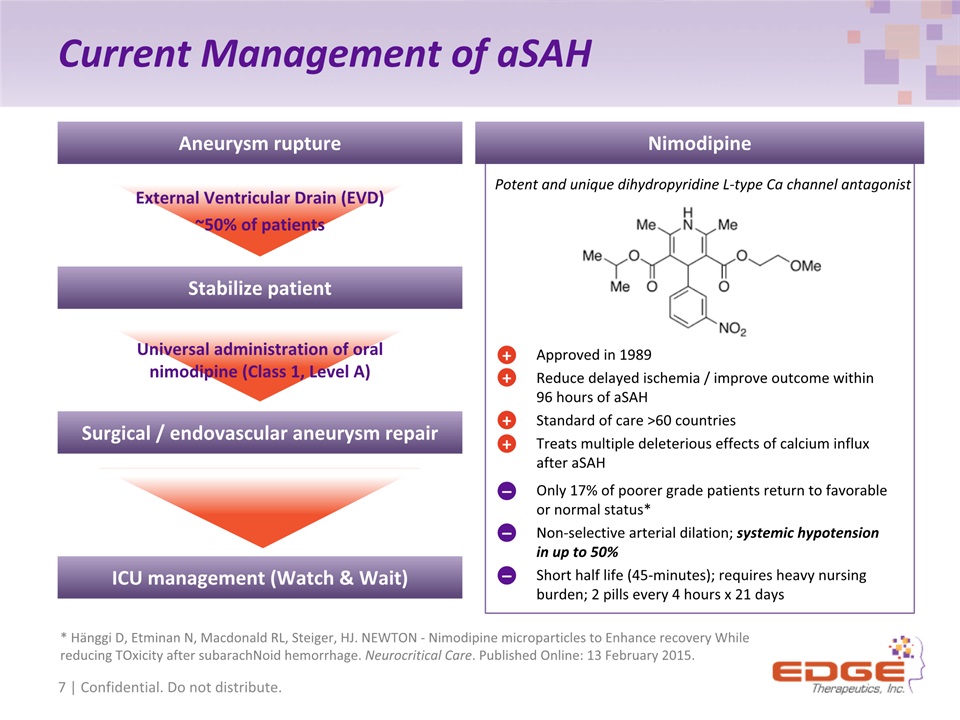
Current Management of aSAH Approved in 1989Reduce delayed ischemia / improve outcome within 96 hours of aSAHStandard of care >60 countries Treats multiple deleterious effects of calcium influx after aSAH Potent and unique dihydropyridine L-type Ca channel antagonist + + + + Only 17% of poorer grade patients return to favorable or normal status* Non-selective arterial dilation; systemic hypotension in up to 50% Short half life (45-minutes); requires heavy nursing burden; 2 pills every 4 hours x 21 days – – – Nimodipine External Ventricular Drain (EVD) ~50% of patients Universal administration of oral nimodipine (Class 1, Level A) Aneurysm rupture Stabilize patient Surgical / endovascular aneurysm repair ICU management (Watch & Wait) 7 | Confidential. Do not distribute. * Hänggi D, Etminan N, Macdonald RL, Steiger, HJ. NEWTON - Nimodipine microparticles to Enhance recovery While reducing TOxicity after subarachNoid hemorrhage. Neurocritical Care. Published Online: 13 February 2015.

EG-1962 Differentiation and Existing Data
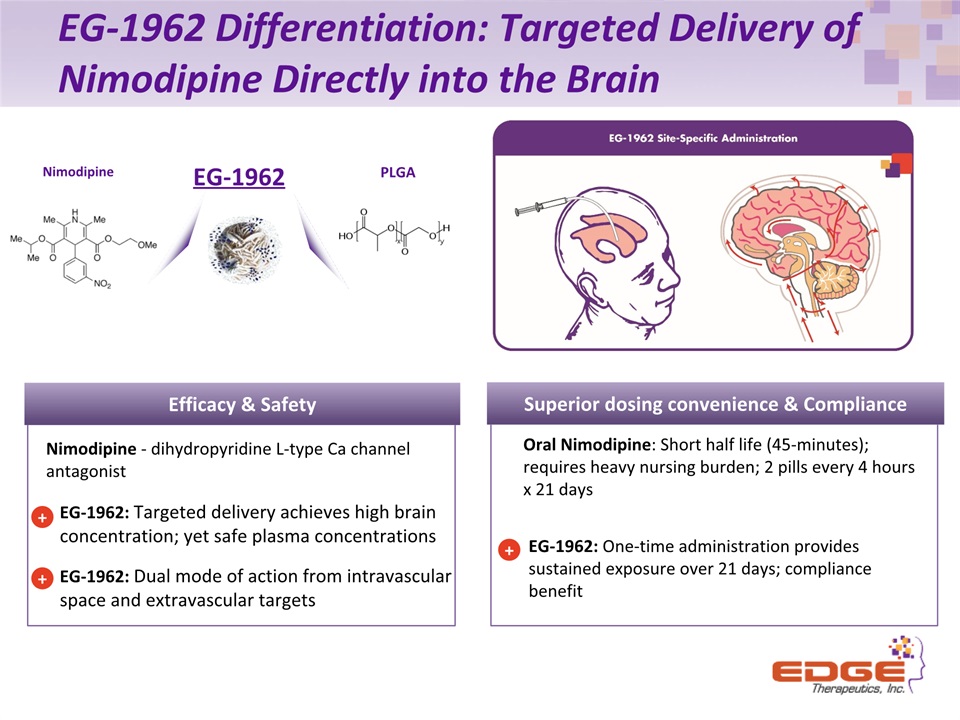
EG-1962 Differentiation: Targeted Delivery of Nimodipine Directly into the Brain EG-1962: One-time administration provides sustained exposure over 21 days; compliance benefit + Superior dosing convenience & Compliance Nimodipine PLGA EG-1962 Oral Nimodipine: Short half life (45-minutes); requires heavy nursing burden; 2 pills every 4 hours x 21 days EG-1962: Targeted delivery achieves high brain concentration; yet safe plasma concentrationsEG-1962: Dual mode of action from intravascular space and extravascular targets Efficacy & Safety + Nimodipine - dihydropyridine L-type Ca channel antagonist +
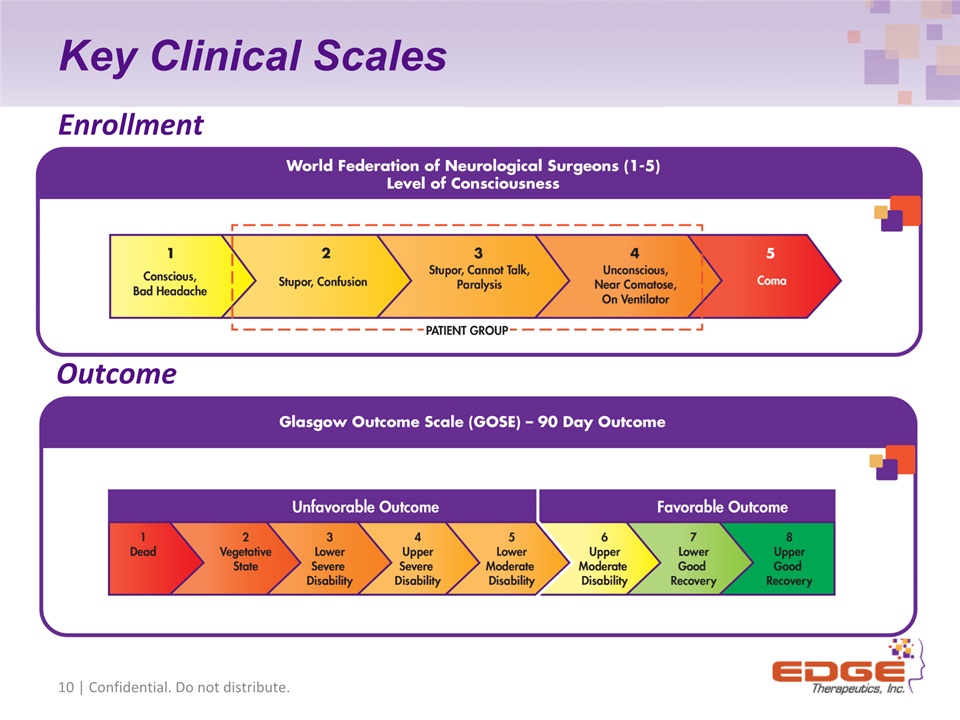
Key Clinical Scales 10 | Confidential. Do not distribute. Enrollment Outcome
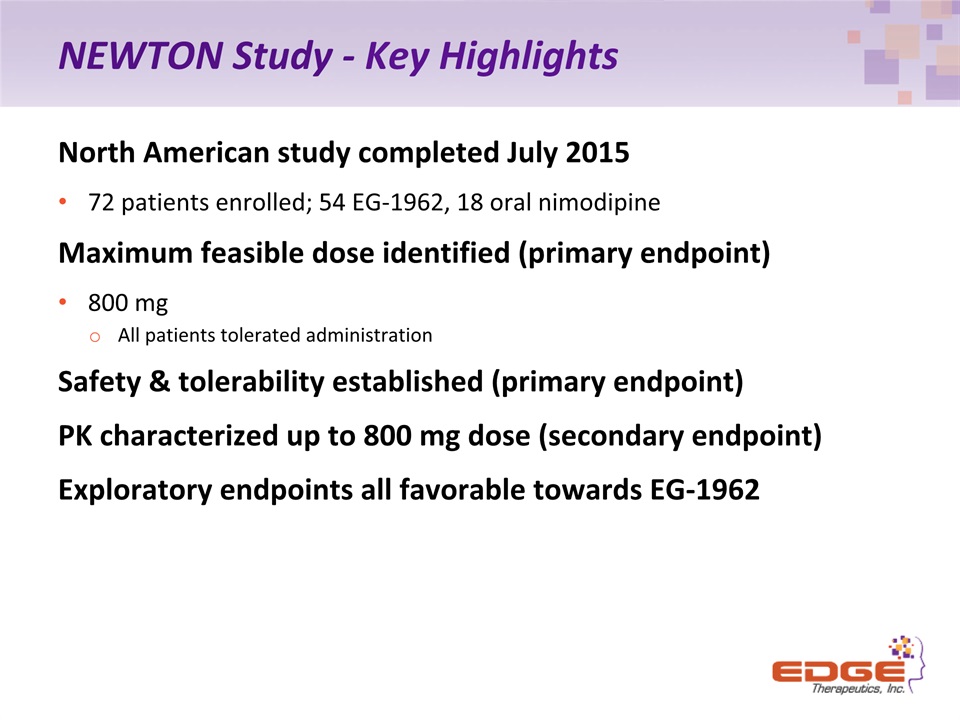
NEWTON Study - Key Highlights North American study completed July 201572 patients enrolled; 54 EG-1962, 18 oral nimodipineMaximum feasible dose identified (primary endpoint)800 mg All patients tolerated administration Safety & tolerability established (primary endpoint)PK characterized up to 800 mg dose (secondary endpoint)Exploratory endpoints all favorable towards EG-1962
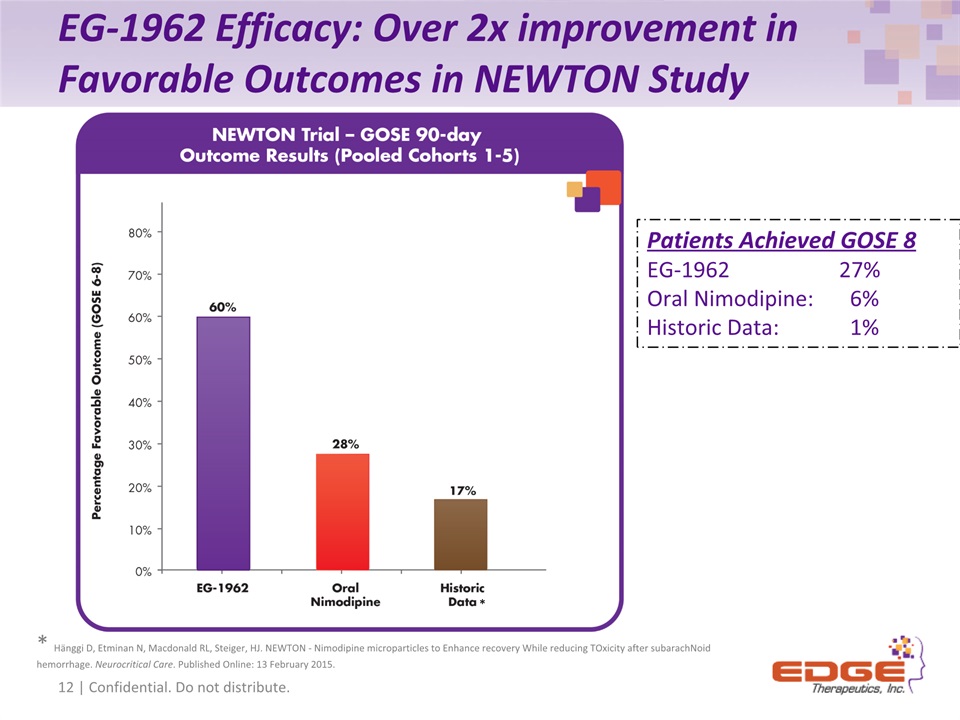
EG-1962 Efficacy: Over 2x improvement in Favorable Outcomes in NEWTON Study 17 of 44 12 of 17 12 | Confidential. Do not distribute. * Hänggi D, Etminan N, Macdonald RL, Steiger, HJ. NEWTON - Nimodipine microparticles to Enhance recovery While reducing TOxicity after subarachNoid hemorrhage. Neurocritical Care. Published Online: 13 February 2015. * Patients Achieved GOSE 8EG-1962 27%Oral Nimodipine: 6%Historic Data: 1%
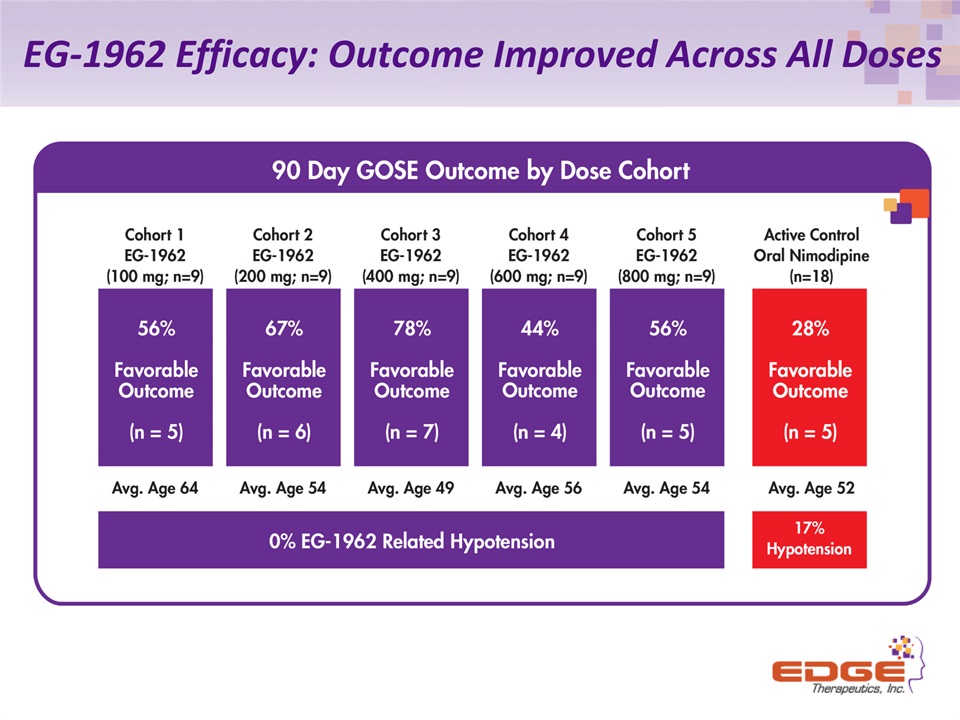
EG-1962 Efficacy: Outcome Improved Across All Doses
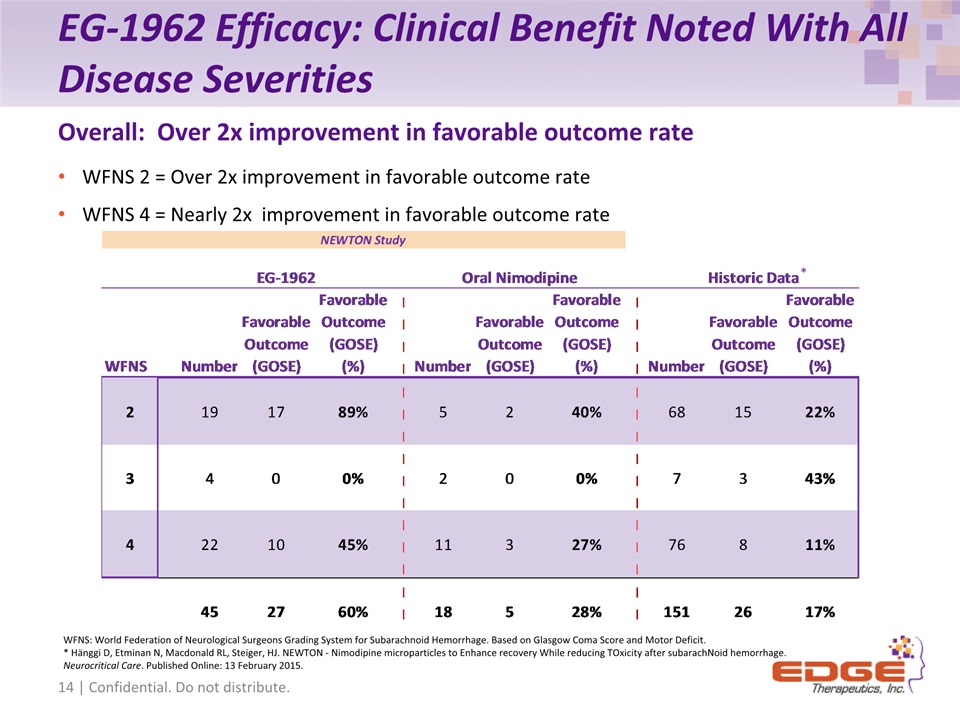
EG-1962 Efficacy: Clinical Benefit Noted With All Disease Severities WFNS 2 = Over 2x improvement in favorable outcome rateWFNS 4 = Nearly 2x improvement in favorable outcome rate Overall: Over 2x improvement in favorable outcome rate WFNS: World Federation of Neurological Surgeons Grading System for Subarachnoid Hemorrhage. Based on Glasgow Coma Score and Motor Deficit.* Hänggi D, Etminan N, Macdonald RL, Steiger, HJ. NEWTON - Nimodipine microparticles to Enhance recovery While reducing TOxicity after subarachNoid hemorrhage. Neurocritical Care. Published Online: 13 February 2015. 14 | Confidential. Do not distribute. *
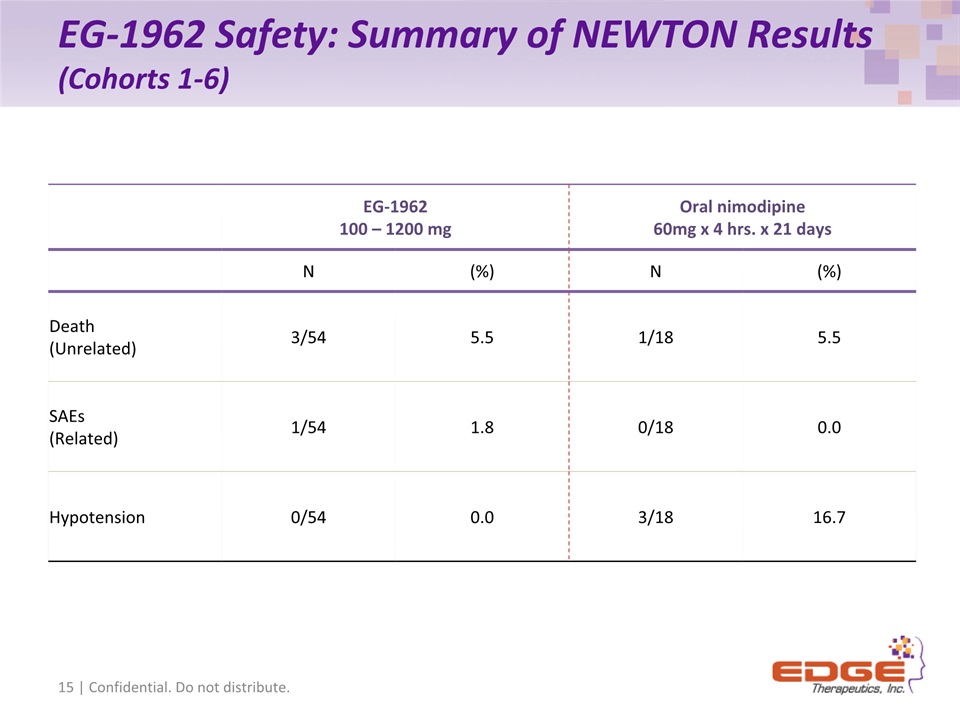
EG-1962 Safety: Summary of NEWTON Results (Cohorts 1-6) 15 | Confidential. Do not distribute. EG-1962100 – 1200 mg Oral nimodipine60mg x 4 hrs. x 21 days N (%) N (%) Death(Unrelated) 3/54 5.5 1/18 5.5 SAEs (Related) 1/54 1.8 0/18 0.0 Hypotension 0/54 0.0 3/18 16.7
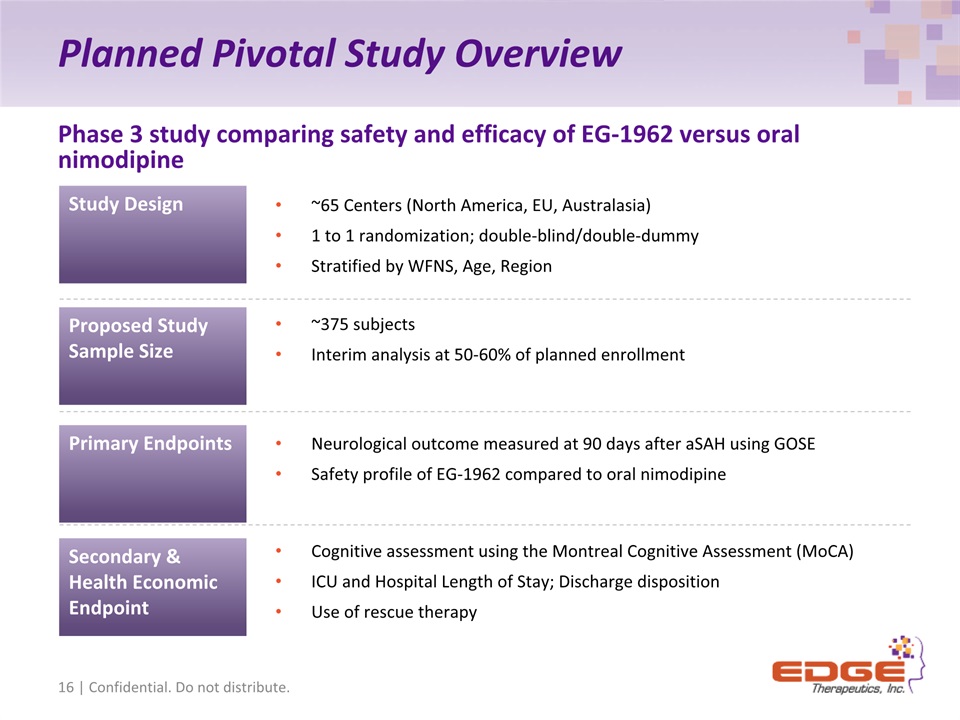
Planned Pivotal Study Overview Phase 3 study comparing safety and efficacy of EG-1962 versus oral nimodipine Primary Endpoints Secondary & Health Economic Endpoint Proposed Study Sample Size Neurological outcome measured at 90 days after aSAH using GOSESafety profile of EG-1962 compared to oral nimodipine Cognitive assessment using the Montreal Cognitive Assessment (MoCA)ICU and Hospital Length of Stay; Discharge dispositionUse of rescue therapy ~375 subjectsInterim analysis at 50-60% of planned enrollment 16 | Confidential. Do not distribute. ~65 Centers (North America, EU, Australasia)1 to 1 randomization; double-blind/double-dummyStratified by WFNS, Age, Region Study Design
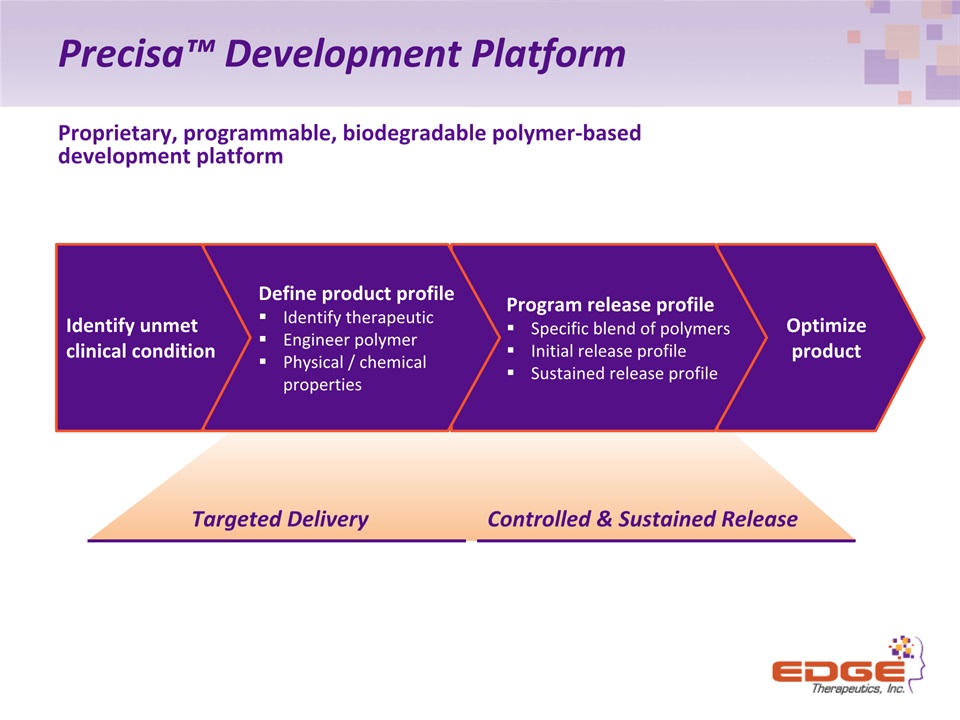
Precisa™ Development Platform Proprietary, programmable, biodegradable polymer-baseddevelopment platform Optimize product Program release profileSpecific blend of polymers Initial release profileSustained release profile Define product profileIdentify therapeuticEngineer polymerPhysical / chemical properties Identify unmetclinical condition Targeted Delivery Controlled & Sustained Release
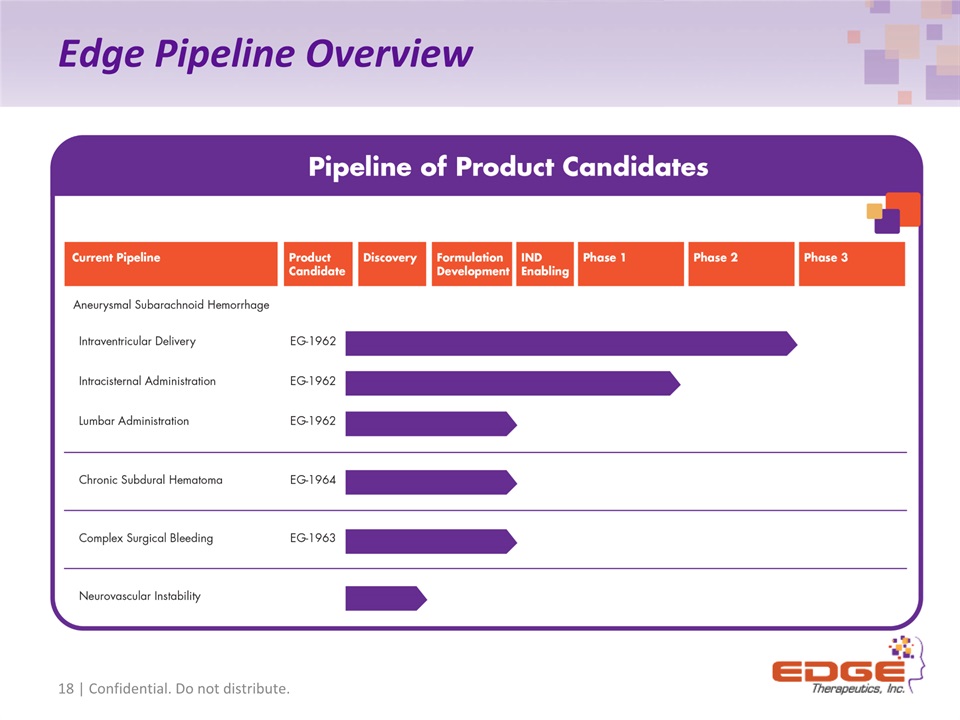
Edge Pipeline Overview 18 | Confidential. Do not distribute.
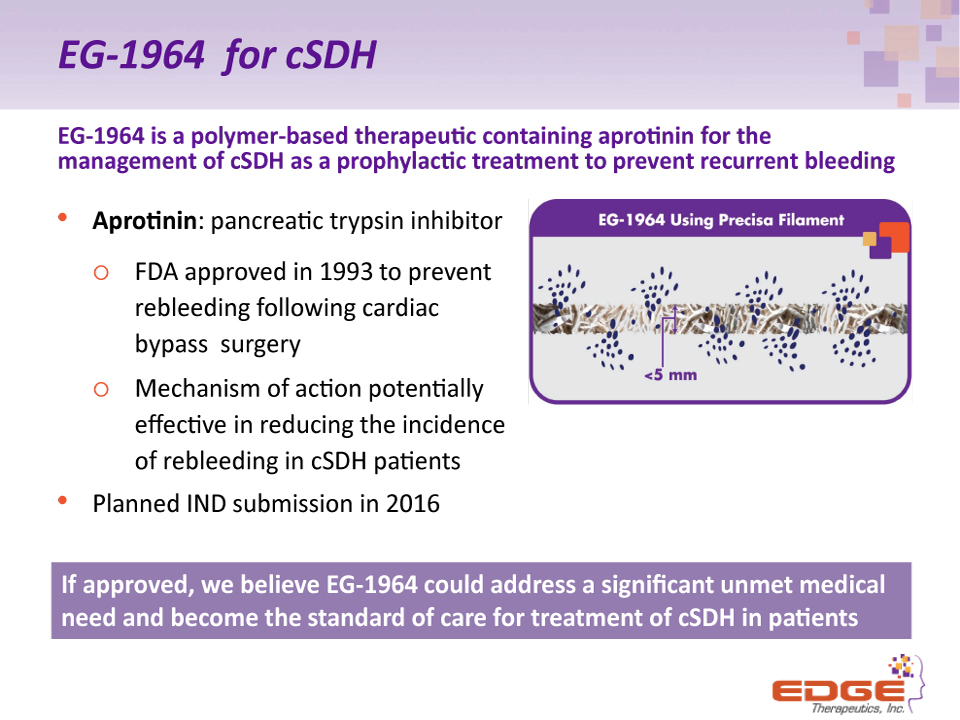
EG-1964 for cSDH EG-1964 is a polymer-based therapeutic containing aprotinin for the management of cSDH as a prophylactic treatment to prevent recurrent bleeding Aprotinin: pancreatic trypsin inhibitor FDA approved in 1993 to prevent rebleeding following cardiac bypass surgeryMechanism of action potentially effective in reducing the incidence of rebleeding in cSDH patientsPlanned IND submission in 2016 If approved, we believe EG-1964 could address a significant unmet medical need and become the standard of care for treatment of cSDH in patients (2)
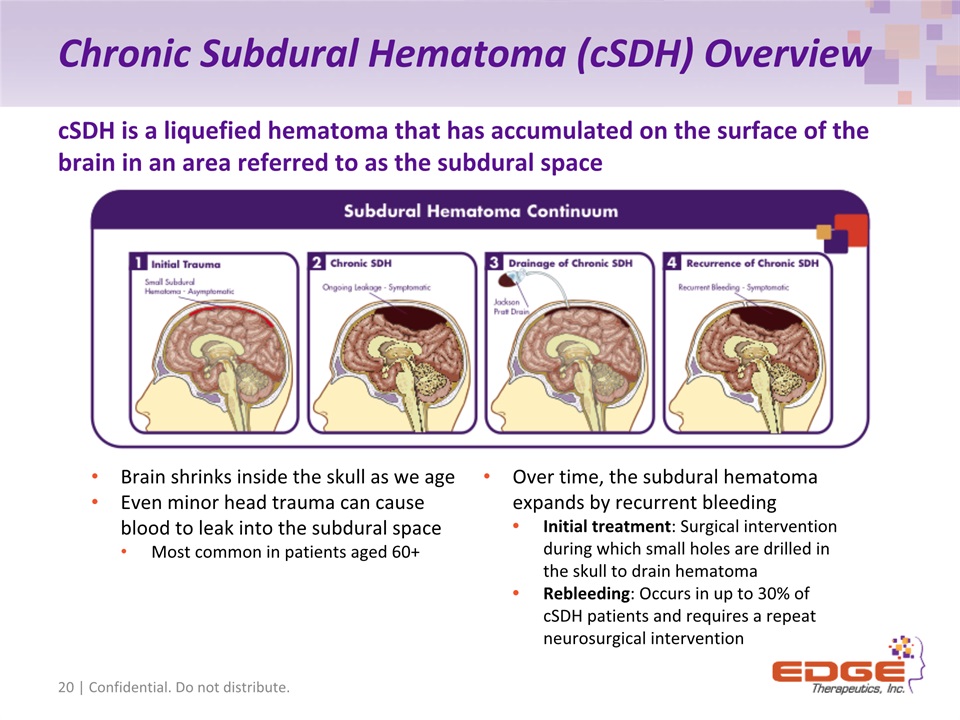
Chronic Subdural Hematoma (cSDH) Overview 20 | Confidential. Do not distribute. cSDH is a liquefied hematoma that has accumulated on the surface of the brain in an area referred to as the subdural space Brain shrinks inside the skull as we ageEven minor head trauma can cause blood to leak into the subdural spaceMost common in patients aged 60+ Over time, the subdural hematoma expands by recurrent bleedingInitial treatment: Surgical intervention during which small holes are drilled in the skull to drain hematomaRebleeding: Occurs in up to 30% of cSDH patients and requires a repeat neurosurgical intervention

Commercial Opportunity 21 | Confidential. Do not distribute.
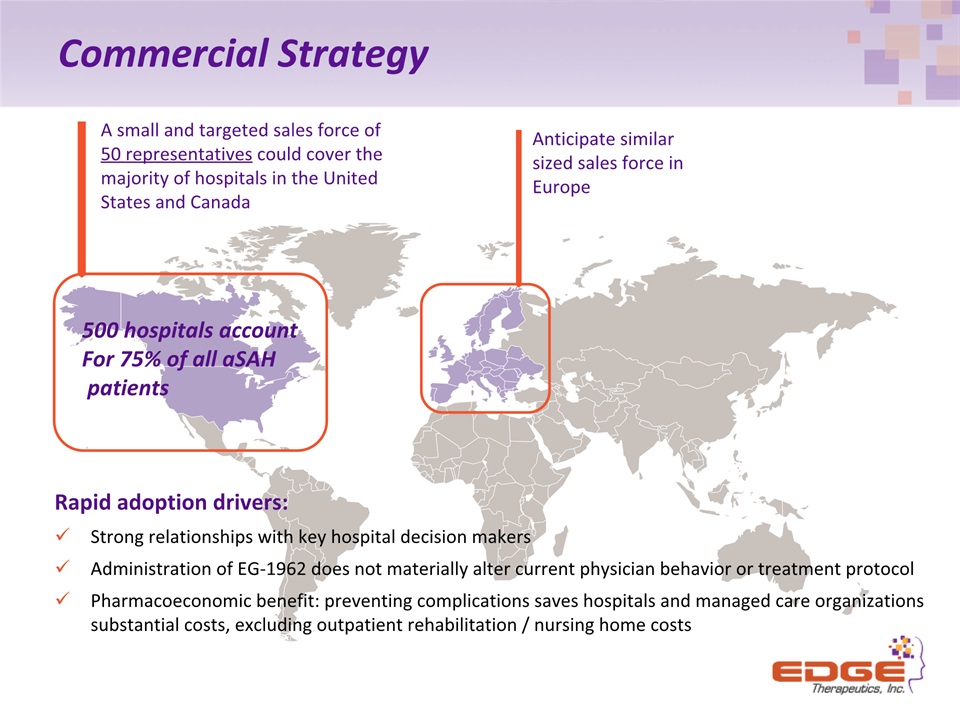
Commercial Strategy A small and targeted sales force of 50 representatives could cover the majority of hospitals in the United States and Canada Anticipate similar sized sales force in Europe 500 hospitals accountFor 75% of all aSAH patients Rapid adoption drivers: Strong relationships with key hospital decision makersAdministration of EG-1962 does not materially alter current physician behavior or treatment protocolPharmacoeconomic benefit: preventing complications saves hospitals and managed care organizations substantial costs, excluding outpatient rehabilitation / nursing home costs
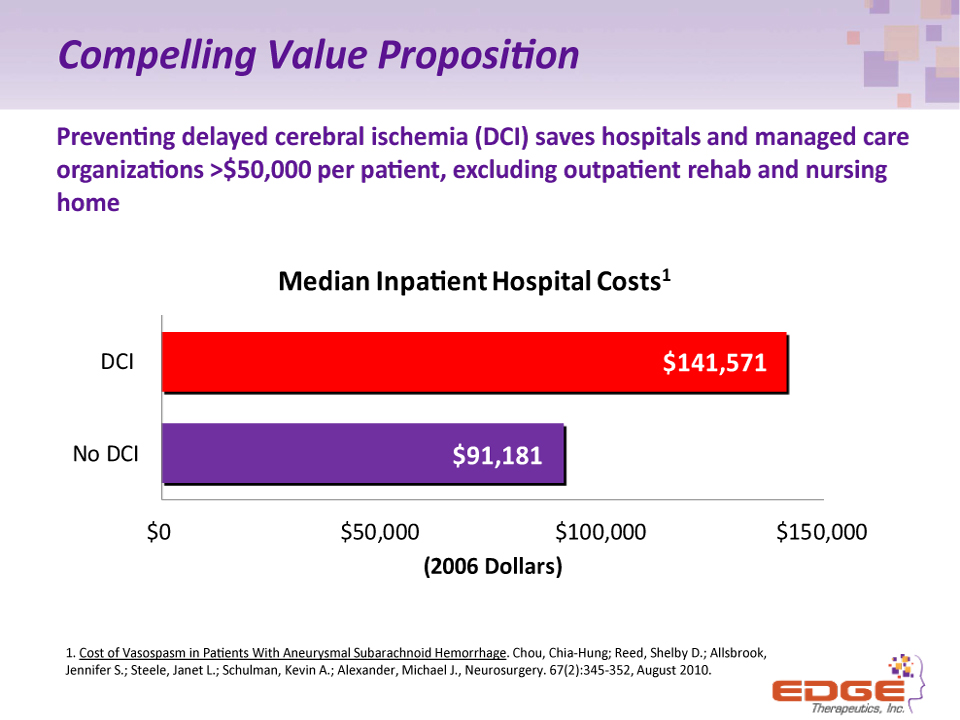
Compelling Value Proposition Preventing delayed cerebral ischemia (DCI) saves hospitals and managed care organizations >$50,000 per patient, excluding outpatient rehab and nursing home 1. Cost of Vasospasm in Patients With Aneurysmal Subarachnoid Hemorrhage. Chou, Chia-Hung; Reed, Shelby D.; Allsbrook, Jennifer S.; Steele, Janet L.; Schulman, Kevin A.; Alexander, Michael J., Neurosurgery. 67(2):345-352, August 2010.
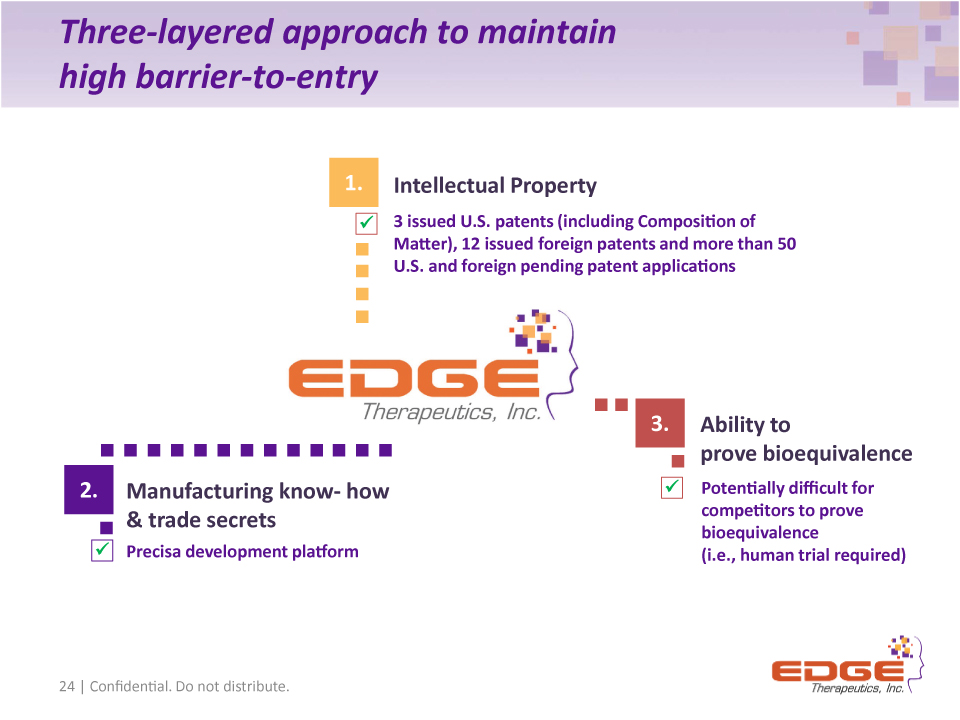
Three-layered approach to maintain high barrier-to-entry 24 | Confidential. Do not distribute. 3 issued U.S. patents (including Composition of Matter), 12 issued foreign patents and more than 50 U.S. and foreign pending patent applications Potentially difficult for competitors to prove bioequivalence (i.e., human trial required) Intellectual Property Ability to prove bioequivalence Manufacturing know- how & trade secrets 1. 2. 3. Precisa development platform
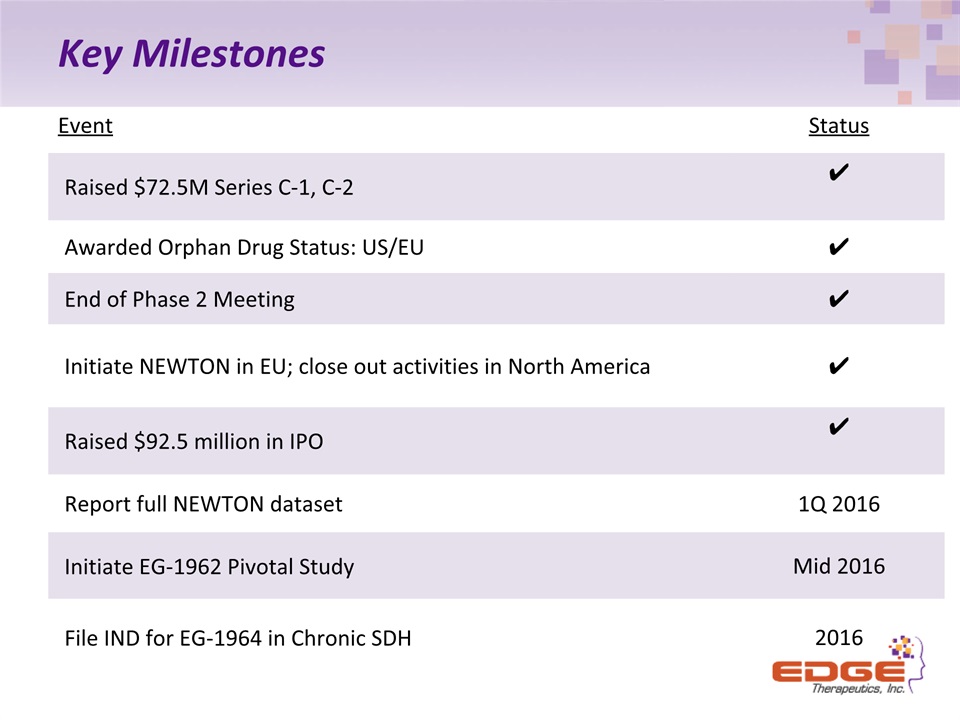
Key Milestones Event Status Raised $72.5M Series C-1, C-2 ✔ Awarded Orphan Drug Status: US/EU ✔ End of Phase 2 Meeting ✔ Initiate NEWTON in EU; close out activities in North America ✔ Raised $92.5 million in IPO ✔ Report full NEWTON dataset 1Q 2016 Initiate EG-1962 Pivotal Study Mid 2016 File IND for EG-1964 in Chronic SDH 2016
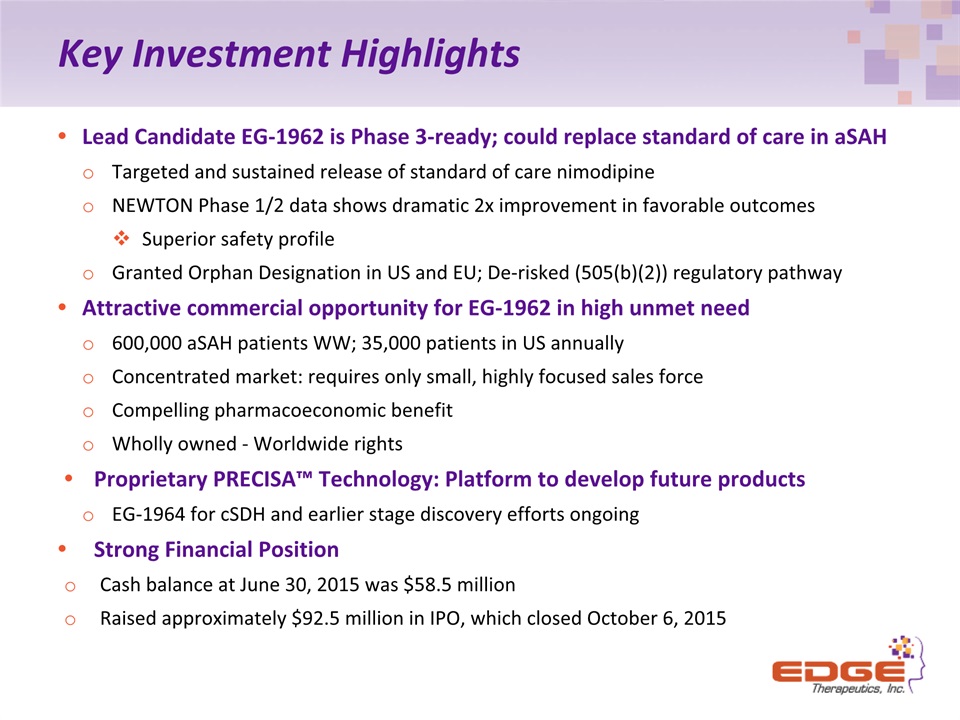
Key Investment Highlights Lead Candidate EG-1962 is Phase 3-ready; could replace standard of care in aSAHTargeted and sustained release of standard of care nimodipine NEWTON Phase 1/2 data shows dramatic 2x improvement in favorable outcomes Superior safety profileGranted Orphan Designation in US and EU; De-risked (505(b)(2)) regulatory pathwayAttractive commercial opportunity for EG-1962 in high unmet need600,000 aSAH patients WW; 35,000 patients in US annuallyConcentrated market: requires only small, highly focused sales force Compelling pharmacoeconomic benefitWholly owned - Worldwide rightsProprietary PRECISA™ Technology: Platform to develop future productsEG-1964 for cSDH and earlier stage discovery efforts ongoingStrong Financial PositionCash balance at June 30, 2015 was $58.5 million Raised approximately $92.5 million in IPO, which closed October 6, 2015

