Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a15-20183_18k.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a15-20183_1ex99d3.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a15-20183_1ex99d1.htm |
Exhibit 99.2
|
|
Clinically Important Deterioration in Patients With COPD Using Umeclidinium/Vilanterol, Tiotropium or Placebo: Pooled Data |
|
Poster No. PA1001
Maleki-Yazdi MR(1), Singh D(2), Anzueto A(3), Tombs L(4), Naya I(5), Harris S(6), Iqbal A(6)
(1) Division of Respiratory Medicine Women’s College Hospital University of Toronto, ON, Canada;(2)Medicines Evaluation Unit, University of Manchester, University Hospital of South Manchester Foundation Trust, Manchester, UK; (3)South Texas Veterans Health Care System Audie L. Murphy Hospital, and University of Texas Health Science Center, San Antonio, TX, USA; (4)Precise Approach LTD, Contingent worker who is working on assignment at GSK; (5)GSK, Respiratory Medicines Development Centre, Stockley Park, Middlesex, UK; (6) § Former employee of GSK, Research Triangle Park, NC, USA
Aims
· The long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) combination, umeclidinium and vilanterol (UMEC/VI) is an approved maintenance treatment for chronic obstructive pulmonary disease (COPD) in the EU, the US, Canada and several other countries.(1)-(3)
· UMEC/VI treatment results in greater improvements in lung function and health status compared with the LAMA tiotropium (TIO) in patients with moderate-to-severe COPD.(4),(5)
· Early treatment in maintenance-naïve (MN) patients may improve lung function and health status.(6),(7) The ability of early treatment to reduce the risk of deteriorations in lung function, and health status in patients with COPD is not well characterised.
· Deterioration in patients with COPD can be measured using an exploratory composite endpoint (clinically important deterioration [CID]) which assesses recognised deteriorations in disease control including the incidence of COPD exacerbations, and clinically relevant deteriorations in lung function and health status from baseline.
· The objective of this analysis was to investigate the efficacy of UMEC/VI in preventing CIDs in patients with moderate-to-severe COPD in both intent-to-treat (ITT) and MN populations.
Methods
Analysis Plan
· This was a post hoc analysis of data from four Phase III multicentre, randomised, blinded, parallel-group, 24-week trials comparing once-daily inhaled UMEC/VI 62.5/25 mcg (delivering 55 mcg and 22 mcg, respectively) versus TIO 18 mcg (delivering 10 mcg; (ZEP117115 [NCT01777334](5), DB2113360 [NCT01316900](4); DB2113374 [NCT01316913](4), data pooled into a single analysis) or placebo (PBO; DB2113373 [NCT01313650](8)).
· The DB2113373 trial also included UMEC 62.5 mcg and VI 25 mcg treatment groups. DB2113360 and DB2113374 included VI 25 mcg and UMEC/VI 125/25 mcg (delivering 113 mcg and 22 mcg), treatment groups (data not presented in this poster).
· Key inclusion criteria included a diagnosis of symptomatic COPD, modified Medical Research Council (mMRC) Dyspnea Scale score >2, post-bronchodilator forced expiratory volume in 1 second (FEV1) <70% predicted and FEV1 forced vital capacity <0.70.
· The analyses below were performed on the ITT population and a subgroup of MN patients (defined as no maintenance therapy for >30 days before screening).
· The primary endpoint in all studies was change from baseline in trough FEV1 on Day 169, defined as the mean of the FEV1 values obtained 23 h and 24 h after dosing on Day 168.
· This post hoc analysis also examined the time to first CID, defined as a decrease of >100 mL(9) from baseline in trough FEV1; or deterioration in health-related quality of life defined as >4 unit increase(10) from baseline in St George’s Respiratory Questionnaire (SGRQ) total score; or the occurrence of an on-treatment moderate-to-severe COPD exacerbation (defined as a worsening of COPD symptoms requiring the use of any additional treatment other than study drug or rescue salbutamol and an emergency department visit or hospitalisation).
· Lung function was determined at 7 visits post randomisation and SGRQ at 3 trial visits (4, 12, and 24 weeks).
Results
Patient Demographics
· In the pooled analysis of ZEP117115, DB2113374 and DB2113360 trials, the ITT population (randomised and receiving at least one dose of study medication), treated with UMEC/VI or TIO comprised 1,747 patients, and 533 (31%) were included in the MN population.
· In the DB2113373 trial, the ITT population treated with UMEC/VI or PBO comprised 693 patients and 257 (37%) were included in the MN population.
· Overall, patient demographics and characteristics in the UMEC/VI, TIO and PBO groups were generally similar in both the ITT (Table 1) and MN populations (data not shown), though the MN population of Study DB2113373 had a mean baseline FEV1 approximately 100 mL higher in the UMEC/VI group compared with the PBO group.
Table 1. Summary of Patient Demographics and Baseline Characteristics (ITT Population)
|
|
|
ZEP117115, DB2113374, DB2113360 |
|
DB2113373 |
| ||||
|
|
|
UMEC/VI |
|
TIO |
|
UMEC/VI |
|
PBO |
|
|
Age, years |
|
63.0 (8.58) |
|
63.4 (8.70) |
|
63.1 (8.71) |
|
62.2 (9.04) |
|
|
Male, n (%) |
|
596 (68) |
|
594 (68) |
|
305 (74) |
|
195 (70) |
|
|
Current smoker at screening(a), n (%) |
|
457 (52) |
|
439 (51) |
|
203 (49) |
|
150 (54) |
|
|
Smoking pack-years(b) mean (%) |
|
45.1 (25.57) |
|
46.1 (27.01) |
|
46.5 (25.80) |
|
47.2 (27.21) |
|
|
Baseline FEV1, L |
|
1.24 (0.50) |
|
1.24 (0.49) |
|
1.28 (0.56) |
|
1.20 (0.47) |
|
|
GOLD stage, n (%) |
|
|
|
|
|
|
|
|
|
|
II |
|
393 (45) |
|
385 (44) |
|
201 (49) |
|
119 (43) |
|
|
III |
|
372 (42) |
|
375 (43) |
|
166 (40) |
|
133 (48) |
|
|
IV |
|
111 (13) |
|
106 (12) |
|
45 (11) |
|
28 (10) |
|
|
ICS use at screening(c), n (%) |
|
|
|
|
|
|
|
|
|
|
Yes |
|
443 (50) |
|
445 (51) |
|
212 (51) |
|
137 (49) |
|
|
Maintenance naïve at baseline(d) n (%) |
|
275 (31) |
|
258 (30) |
|
152 (37) |
|
105 (38) |
|
|
SGRQ total score at baseline(e) |
|
49.28 (17.68) |
|
49.19 (17.05) |
|
48.58 (18.24) |
|
51.28 (18.12) |
|
|
Exacerbation history(f), n (%) |
|
|
|
|
|
|
|
|
|
|
Required corticosteroid and/or antibiotic (without hospitalisation) |
|
193 (22) |
|
215 (25) |
|
99 (24) |
|
78 (28) |
|
|
Required hospitalisation |
|
73 (8) |
|
80 (9) |
|
39 (9) |
|
31 (11) |
|
All data shown are mean (standard deviation) unless otherwise specified; GOLD, Global initiative for chronic Obstructive Lung Disease; ICS, inhaled corticosteroid.
(a)Patient reclassified as current smoker if smoked within 6 months; (b)smoking pack-years = (number of cigarettes smoked per day/20) x number of years smoked; (c)ICS use was defined as those patients who were currently taking ICS medications at the Screening Visit; (d)defined as no maintenance therapy for >30 days before screening; (e)DB2113373: UMEC/VI, n=403; PBO, n=274; (f)patients experiencing >1 exacerbation during the 12 months prior to screening.
Trough FEV1 at Day 169
· UMEC/VI provided a statistically significant improvement in trough FEV1 of 95 mL in the ITT and 146 mL in the MN populations at Day 169 versus TIO (both p<0.001).
· Similarly UMEC/VI provided an improvement in trough FEV1 of 167 mL in the ITT and 156 mL in the MN populations at Day 169 versus PBO (both p<0.001; Table 2).
Table 2. Change from Baseline In Trough FEV1 on Day 169
|
|
|
ZEP117115, DB2113374, DB2113360 |
|
DB2113373 |
| ||||
|
|
|
UMEC/VI |
|
TIO |
|
UMEC/VI |
|
PBO |
|
|
ITT population, n(a) |
|
738 |
|
736 |
|
330 |
|
201 |
|
|
LS mean change, mL (SE) |
|
211 (8.7) |
|
116 (8.7) |
|
171 (12.6) |
|
4 (15.8) |
|
|
UMEC/VI vs TIO or PBO, difference (95% CI)(b) |
|
95 (71, 118)* |
|
167 (128, 207)* |
| ||||
|
MN population, n(a) |
|
230 |
|
216 |
|
120 |
|
74 |
|
|
LS mean change, mL (SE) |
|
252 (15.6) |
|
107 (16.2) |
|
152 (22.0) |
|
-4 (27.5) |
|
|
UMEC/VI vs TIO or PBO, difference (95% CI)(b) |
|
146 (102, 189)* |
|
156 (87, 225)* |
| ||||
(a)Number of patients with analysable data at the current time point; (b)analysis performed using a repeated measures model with covariates of study, baseline FEV1 (the mean of the two FEV1 assessments made 30 min and 5 min pre-dose on Day 1), treatment, smoking status, centre group, Day, Day by baseline and Day by treatment interactions.
CI, confidence interval; LS, least squares; SE, standard error; *p<0.001.
The Proportion of Patients With a Composite CID
· The proportion of patients with a first composite CID was lower in the ITT population with UMEC/VI versus TIO (41% vs 56%) or PBO (44% vs 75%), respectively (Figure 1A, B).
· The proportion of patients with a first composite CID was lower in the MN population with UMEC/VI versus TIO (41% vs 55%) or PBO (49% vs 72%), respectively (Figure 1C, D).
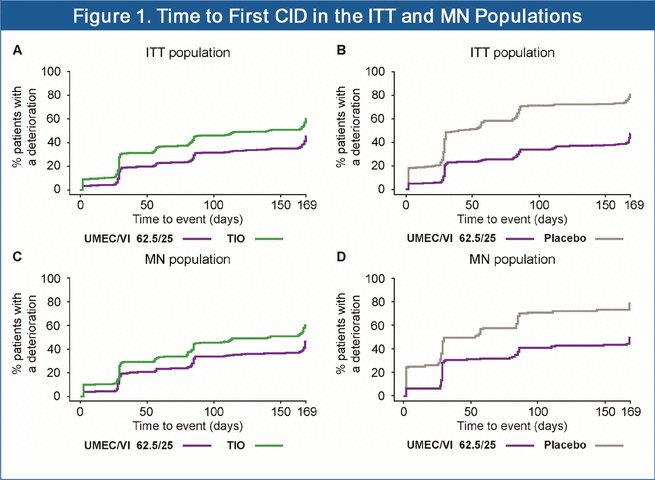
Risk of a First Composite CID
· In both the ITT and MN populations, the risk of a first composite CID was significantly decreased with UMEC/VI versus TIO or PBO (Table 3 and 4, hazard ratios).
· UMEC/VI significantly reduced the risk of deteriorations in lung function and SGRQ score in the ITT population versus TIO (Table 3).
· UMEC/VI significantly reduced the risk of all types of CID in the ITT population versus PBO (Table 3).
· Similar results were found in the MN population with UMEC/VI versus TIO or PBO, with the exception that SGRQ deterioration was not significantly reduced with UMEC/VI versus PBO or TIO (Table 4).
Table 3. Summary of the Risk of a First CID (ITT Population)
|
|
|
ZEP117115, DB2113374, DB2113360 |
|
DB2113373 |
| ||||
|
Deterioration Criteria |
|
UMEC/VI |
|
TIO |
|
UMEC/VI |
|
PBO |
|
|
Composite (any event), n (%) |
|
362 (41) |
|
486 (56) |
|
182 (44) |
|
209 (75) |
|
|
HR (95% CI)(a) |
|
0.62 (0.54, 0.71)* |
|
0.37 (0.30, 0.45)* |
| ||||
|
>100 mL decrease in trough FEV1 |
|
159 (18) |
|
308 (35) |
|
81 (20) |
|
147 (53) |
|
|
HR (95% CI)(a) |
|
0.44 (0.36, 0.53)* |
|
0.26 (0.20, 0.34)* |
| ||||
|
>4 unit SGRQ total score increase |
|
208 (24) |
|
236 (27) |
|
112 (27) |
|
110 (39) |
|
|
HR (95% CI)(a) |
|
0.83 (0.69, 1.00)† |
|
0.58 (0.44, 0.75)* |
| ||||
|
Moderate-to-severe COPD exacerbation |
|
56 (6) |
|
54 (6) |
|
27 (7) |
|
35 (13) |
|
|
HR (95% CI)(a) |
|
1.02 (0.70, 1.48) |
|
0.48 (0.29, 0.79)‡ |
| ||||
*p<0.001; †p=0.045; ‡p=0.004; (a)Hazard ratios (HRs) and CIs are presented for UMEC/VI vs TIO or UMEC/VI vs PBO and derived using a Cox proportional hazards model with covariates of treatment, study, smoking status at screening and geographical region.
Table 4. Summary of the Risk of a First CID (MN population)
|
|
|
ZEP117115, DB2113374, DB2113360 |
|
DB2113373 |
| ||||
|
Deterioration criteria |
|
UMEC/VI |
|
TIO |
|
UMEC/VI |
|
PBO |
|
|
Composite (any event), n (%) |
|
114 (41) |
|
141 (55) |
|
74 (49) |
|
76 (72) |
|
|
HR (95% CI)(a) |
|
0.66 (0.51, 0.85)* |
|
0.45 (0.33, 0.62)* |
| ||||
|
>100 mL decrease in trough FEV1 |
|
53 (19) |
|
93 (36) |
|
34 (22) |
|
60 (57) |
|
|
HR (95% CI)(a) |
|
0.44 (0.32, 0.62)* |
|
0.26 (0.17, 0.40)* |
| ||||
|
>4 unit SGRQ total score increase |
|
66 (24) |
|
69 (27) |
|
53 (35) |
|
33 (31) |
|
|
HR (95% CI)(a) |
|
0.92 (0.65, 1.29) |
|
1.07 (0.69, 1.65) |
| ||||
|
Moderate-to-severe COPD exacerbation |
|
13 (5) |
|
9 (3) |
|
6 (4) |
|
12 (11) |
|
|
HR (95% CI)(a) |
|
1.33 (0.57, 3.13) |
|
0.32 (0.12, 0.85)† |
| ||||
*p<0.001; †p=0.023; (a)HRs and CIs are presented for UMEC/VI vs TIO or UMEC/VI vs PBO and derived using a Cox proportional hazards model with covariates of treatment, study, smoking status at screening and geographical region.
Safety
· In the ITT population, headache (UMEC/VI [9%] vs TIO [6%]; UMEC/VI [8%] vs PBO [9%]) and nasopharyngitis (UMEC/VI [7%] vs TIO [7%]; UMEC/VI [9%] vs PBO [6%]) were the most commonly reported adverse events (AEs).
· Similar results were seen in the MN population.
Conclusions
· In this post hoc analysis in patients with moderate-to-severe COPD, UMEC/VI treatment resulted in similar improvements in trough FEV1 from baseline versus TIO or PBO in both the ITT and MN populations.
· UMEC/VI treatment also reduced the risk of a first composite CID to a similar extent in both the ITT and MN populations versus TIO or PBO.
· The incidence of AEs was similar between treatment groups in the ITT and MN populations.
· These findings may provide a rationale for the use of dual bronchodilator therapy in MN populations.
References
1. GSK ANORO™ ELLIPTA® summary of product characteristics. http://www.medicines.org.uk/emc/medicine/28949#INDICATIONS [accessed June 2015]; 2. GSK ANORO™ ELLIPTA® prescribing information, https://www.gsksource.com/gskprm/htdocs/documents/ANORO-ELLIPTA-PI-MG.PDF [accessed June 2015]; 3. Blair HA, Deeks ED. Drugs 2015; 75: 61-74; 4. Decramer M, et al. Lancet Respir Med 2014; 2: 472-86; 5. Maleki-Yazdi MR, et al. Respir Med 2014;108: 1752-60; 6. Decramer M, Cooper CB. Thorax 2010, 65: 837-41; 7. Troosters T, et al. NPJ Prim Care Respir Med 2014; 24: 14003; 8. Donohue JF, et al Respir Med 2013, 107: 1538-46; 9. Donohue JF. COPD 2005; 2: 111-24; 10. Jones PWF. COPD 2005; 2: 75-9.
Acknowledgements
· The presenting author (MRM-Y) declares the following real or perceived conflicts of interest: acted as a consultant for Almirall, AstraZeneca, Boehringer Ingelheim, Forest Laboratories, GSK, Novartis, Merck, Ono Pharmaceuticals and Pfizer. DS declares the following real or perceived conflicts of interest: has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GSK, Glenmark, Johnson & Johnson, Merck, NAPP, Novartis, Pfizer, Skyepharma, Takeda, Teva, Theravance and Verona. AA acted as a consultant for AstraZeneca, Boehringer Ingelheim, Forest Laboratories, GSK, Novartis and Pfizer. IN and SH are employees of GSK and hold stocks/shares in GSK. LT is a contingent worker at GSK. §AI was an employee of GSK at the time the analysis was completed and the abstract submitted and holds GSK stock/shares.
· This study was funded by GSK (GSK analysis #202065 and #202066, using data from four clinical trials: NCT01316900, DB2113360; NCT01316913, DB2113374; NCT01777334, ZEP117115; DB2113373; NCT01313650).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Alex Lowe, PhD, at Fishawack Indicia, UK, and was funded by GSK.
|
|
Presented at the Annual Congress of the European Respiratory Society (ERS), Amsterdam, The Netherlands, 26–30 September, 2015 |
|


