Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Nexvet Biopharma plc | nvet-ex231_973.htm |
| EX-10.7 - EX-10.7 - Nexvet Biopharma plc | nvet-ex107_816.htm |
| EX-10.10 - EX-10.10 - Nexvet Biopharma plc | nvet-ex1010_784.htm |
| EX-31.2 - EX-31.2 - Nexvet Biopharma plc | nvet-ex312_201506307.htm |
| EX-31.1 - EX-31.1 - Nexvet Biopharma plc | nvet-ex311_201506306.htm |
| EX-32.1 - EX-32.1 - Nexvet Biopharma plc | nvet-ex321_201506308.htm |
| EX-32.2 - EX-32.2 - Nexvet Biopharma plc | nvet-ex322_201506309.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2015
OR
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission File Number 001-36828
Nexvet Biopharma
public limited company
(Exact name of Registrant as specified in its Charter)
|
Ireland |
98-1205017 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer |
|
National Institute for Bioprocessing Research and Training Foster’s Avenue, Mount Merrion Blackrock, Co. Dublin, Ireland |
|
|
(Address of principal executive offices) |
|
Registrant’s telephone number, including area code: +353 (1) 901 0339
Securities registered pursuant to Section 12(b) of the Act: Ordinary shares, nominal value $0.125 per share; ordinary shares traded on The NASDAQ Stock Market
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES o NO x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES o NO x
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES x NO o
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). YES x NO o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definition of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
|
o |
|
Accelerated filer |
|
o |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
x (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
o |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES o NO x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the ordinary shares on The NASDAQ Stock Market on August 31, 2015, was $51,073,868.
The number of Registrant’s ordinary shares outstanding as of August 31, 2015 was 11,451,540.
Portions of the Registrant’s Definitive Proxy Statement relating to the Annual Meeting of Shareholders, scheduled to be held on November 19, 2015, are incorporated by reference into Part III of this Report.
|
|
|
Page |
|
|
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS |
|
|
|
|
|
|
|
|
|
PART I |
|
|
|
|
Item 1. |
|
3 |
|
|
Item 1A. |
|
19 |
|
|
Item 1B. |
|
43 |
|
|
Item 2. |
|
43 |
|
|
Item 3. |
|
43 |
|
|
Item 4. |
|
43 |
|
|
|
|
|
|
|
PART II |
|
|
|
|
Item 5. |
|
44 |
|
|
Item 6. |
|
46 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
48 |
|
Item 7A. |
|
60 |
|
|
Item 8. |
|
60 |
|
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
|
60 |
|
Item 9A. |
|
60 |
|
|
Item 9B. |
|
60 |
|
|
|
|
|
|
|
PART III |
|
|
|
|
Item 10. |
|
61 |
|
|
Item 11. |
|
61 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
61 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
|
61 |
|
Item 14. |
|
61 |
|
|
|
|
|
|
|
PART IV |
|
|
|
|
Item 15. |
|
62 |
|
Unless otherwise indicated or the context otherwise requires, references to “we,” “us,” “our,” “Nexvet,” “Nexvet Biopharma plc” or the “Company” refer to Nexvet Biopharma public limited company and its consolidated subsidiaries.
Unless otherwise indicated or the context otherwise requires, all dollar amounts presented in this Annual Report on Form 10-K, or this Report, are in U.S. dollars ($). This report translates certain Australian dollar amounts to U.S. dollars at the exchange rates for A$ into US$. For assets and liabilities, the exchange rate at the balance sheet date is used. For revenue and expenses and gains and losses, a weighted-average exchange rate for the period is used to translate those elements. For transactions effected in Australian dollars, the exchange rate on the date of the transaction is used. Information not in U.S. dollars is identified by “€” for Euro-denominated amounts and “A$” for Australian dollar-denominated amounts.
PETization, PETisation, Nexplora, Nexvet, our logo and our other registered or common law trademarks, trade names or service marks appearing in this Report are owned by us. Other trademarks, trade names or service marks appearing in this Report are the property of their respective owners. Solely for convenience, our trademarks and tradenames referred to in this Report appear without the ® or ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks and tradenames.
i
SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS
This Report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements consist of all statements other than statements of historical fact, including statements regarding our future results of operations and financial position, ability to reach a statistically significant endpoint at the primary assessment of the NV‑01 pivotal safety and efficacy study, results of the current, new or any future NV‑01 study, future expenditures relating to NV‑01, the time for completion of any of our studies, business strategy, prospective products, ability to obtain product approvals, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. The words “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “might,” “objective,” “plan,” “potential,” “predict,” “project,” “position,” “seek,” “should,” “target,” “will,” “would,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate, and management’s beliefs and assumptions are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors.
Factors that could cause actual results to differ materially from our expectations expressed in this report include those summarized under Risk Factors elsewhere in this report. Given these risks and uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our management’s beliefs and assumptions only as of the date of this report. Except as required by law, we do not intend, and undertake no obligation, to revise or update these forward-looking statements or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
2
We are a clinical-stage biopharmaceutical company focused on transforming the therapeutic market for companion animals by developing and commercializing novel, species-specific biologics based on human biologics. Biologics are therapeutic proteins derived from biological sources. As a class, biologics have transformed human medicine in recent decades and represent some of the top-selling therapies on the market today. Our proprietary platform, which we refer to as “PETization,” is an algorithmic approach that enables us to rapidly create monoclonal antibodies that are designed to be recognized as “self” or “native” by an animal’s immune system, a property we refer to as “100% species-specificity.” PETization is also designed to build upon the safety and efficacy data from clinically tested human therapies to create new therapies for companion animals, thereby reducing clinical risk and development cost.
Biologics generally include monoclonal antibodies, or mAbs, which are targeted antibodies derived from identical, or clonal, cells; and fusion proteins, which are proteins created by joining two or more genes coded for separate proteins. Our first product candidate, NV‑01, is a mAb that targets and inhibits the function of nerve growth factor, or NGF, for the control of pain associated with osteoarthritis in dogs. NGF is a protein involved in neural signaling, including pain signals. NGF inhibitors seek to interrupt those signals to reduce pain. We expect data from our NV‑01 pivotal safety and efficacy study and an additional pilot field safety and efficacy study by the end of 2015. This latter study was initiated following receipt of a sample size reassessment of the pivotal safety and efficacy study in March 2015 and will assess various doses and dosing regimens of NV‑01. This study is expected to provide valuable information that may assist the design of a future pivotal study.
Our second product candidate, NV‑02, is a mAb that is an NGF inhibitor for the control of pain associated with degenerative joint disease, including osteoarthritis, in cats. We announced positive top-line results from our proof-of-concept efficacy study and our pilot safety study of NV‑02 in June 2015. We anticipate results from a pilot field safety and efficacy study of NV‑02 in the first quarter of 2016. Our third product candidate, NV‑08, is a fusion protein that is a tumor necrosis factor, or TNF, inhibitor for the treatment of chronic inflammatory diseases, including atopic dermatitis, in dogs. TNF is a protein that causes inflammation, and TNF inhibitors suppress this inflammation. If our proof-of-concept safety and efficacy studies for NV‑08 are successful, we plan to progress this product into formal development. In addition, primarily using PETization, we are seeking to advance one new product candidate into development per year.
Veterinary care is one of the fastest growing industries in the overall U.S. companion animal market and reached approximately $15.0 billion in 2014. We are targeting the companion animal therapeutics segment of the veterinary care industry. This segment is currently dominated by synthetic chemical drugs commonly referred to as “small molecule” drugs. The size and growth of the veterinary care industry reflects many factors, including higher rates of companion animal ownership, improved quality of veterinary care, and the increasingly important role of companion animals in our lives, who are often considered members of our families. We believe these factors, together with the introduction of our product candidates with their favorable safety and compliance profiles, will increase overall demand for companion animal therapeutics.
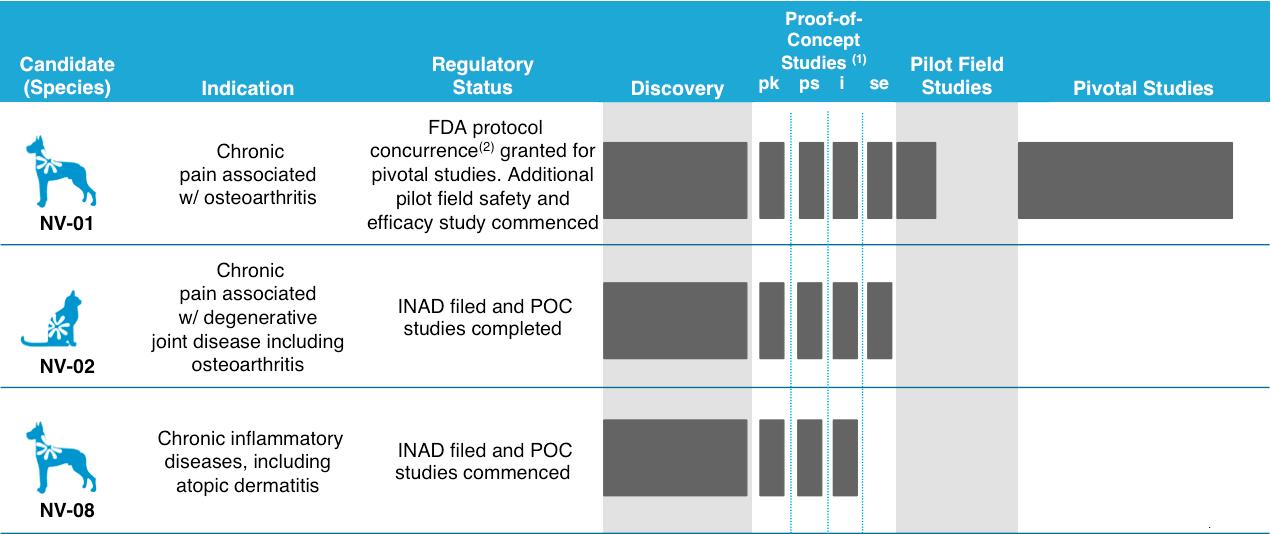
Product Pipeline
We have identified three lead product candidates, NV‑01, NV‑02 and NV‑08. We submitted an investigational new animal drug application, or INAD, with the U.S. Food and Drug Administration, or the FDA, for NV‑01 in 2012, for NV‑02 in 2013 and for NV‑08 in 2014. In addition, we are identifying further candidates for development in a number of indications.
3
|
(1) |
Our proof-of-concept, or POC, studies include four sequential stages: pharmacokinetics (P), preliminary safety (PS), immunogenicity (I) and safety and efficacy (SE). |
|
(2) |
FDA protocol concurrence means the Center for Veterinary Medicine within the FDA fundamentally agrees with the design, execution and analyses proposed in the protocol and there is a commitment that it will not later alter its perspective on these issues unless public or animal health concerns appear that were not recognized at the time of the protocol assessment. We have obtained protocol concurrences for our pivotal safety and efficacy study for NV‑01. |
Development of companion animal therapeutics is typically faster and less expensive than human drug development. It requires fewer clinical trials, fewer subjects and less pre-clinical work and can be conducted directly in the target species. According to Pharmaceutical Commerce, companion animal therapeutics can obtain U.S. regulatory approval in under six years. We expect the costs to complete the development and manufacturing scale-up of each of our three lead product candidates to be approximately $13.0 to $15.0 million per candidate. In contrast, receipt of regulatory approval for human therapeutics may take 12 to 13 years and development can cost hundreds of millions of dollars per drug.
NV‑01 (Area: chronic canine pain)
Overview
NV‑01 is a 100% species-specific mAb targeting NGF, discovered through PETization and developed as a long-acting analgesic for chronic pain, specifically pain associated with osteoarthritis, in dogs. We have commenced a pivotal safety and efficacy study for NV‑01, for which we obtained protocol concurrence from the FDA, and we have also commenced an additional pilot field safety and efficacy study assessing various doses and dosing regimens of NV‑01. We expect data from our NV‑01 pivotal safety and efficacy study and our additional pilot field safety and efficacy study by the end of 2015.
Market Opportunity and Current Treatment Limitations
According to the 2015-2016 American Pet Products Association National Pet Owners Survey and the European Pet Food Industry Federation’s Facts and Figures 2012, there are approximately 78 million pet dogs in the United States and approximately 61 million pet dogs in Europe. Pain in dogs can be a result of chronic diseases, such as osteoarthritis, or produced via trauma, surgery, cancer or infection. Osteoarthritis is an age-associated degenerative condition affecting the joint tissues and, with the exception of joint replacement surgery, there are no curative treatments. The incidence of osteoarthritis in dogs has increased by 38% since 2007 according to a 2012 Banfield Pet Hospital study.
The market for canine pain management products has been growing over the last 15 years. In the United States alone, sales of such products increased from less than $10 million in 1995 to nearly $272 million in 2014, following the introduction in 1996 of Rimadyl, a non-steroidal anti-inflammatory drug, or NSAID. In 2013, Rimadyl sales accounted for approximately $90 million of this market. The U.S. canine pain market is currently dominated by several NSAIDs, which together accounted for over 83% of canine pain medication sales in 2013.
4
NSAIDs account for the largest number of adverse drug events reported to the FDA, including vomiting, gastrointestinal bleeding, diarrhea, and liver and kidney toxicities. We believe that the limitations of NSAIDs create a significant commercial opportunity for the development of a novel, long-acting treatment for chronic pain that has comparable efficacy to NSAIDs without their adverse events. This view is supported by the proprietary report we commissioned from GfK in December 2013, which revealed that of the 390 veterinarians surveyed in the United States, United Kingdom, France and Germany, between 93% and 97% reported they would use a product such as NV‑01 for the treatment of chronic pain in dogs.
Our Solution
NV‑01 is a PETized mAb that inhibits the activity of NGF in dogs. NGF is elevated in the joints of dogs with osteoarthritis. NGF is produced by nerve cells; chondrocytes, which are cells that produce and maintain cartilage; and a variety of inflammatory and immune cells. It acts on pain-sensing nerve fibers to increase their excitability and increase the sprouting of new nerve fibers into inflamed tissues. mAbs targeting NGF have been extensively studied in humans through to Phase 3 clinical studies and have been shown to be effective in managing osteoarthritic pain in patients.
NV‑01 has been found to be safe and well tolerated following intravenous infusion in dogs and has displayed a prolonged elimination half-life, or the time it takes to lose half of its effect, after a single injection. NV‑01 has been shown to reduce pain scores in a canine model of acute inflammatory pain with efficacy comparable to meloxicam, a standard NSAID used in canine osteoarthritis, and repeated administration has not induced any significant neutralizing antibody response, indicating the canine immune system did not identify NV‑01 as a “foreign” protein, an important step in successfully designing a biologic therapy for repeated administration.
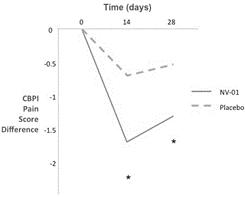
In a proof-of-concept study, NV‑01 was given to nine dogs with chronic lameness due to osteoarthritis and was shown to reduce canine brief pain inventory, or CBPI, pain scores, with a duration of action of at least four weeks. NV‑01 was well tolerated in all of the dogs. CBPI is an owner assessment tool for measuring pain severity and pain interference (with activity) that is recognized by the FDA. This data was published in the peer-reviewed American Journal of Veterinary Research and is illustrated in the figures below. Asterisks indicate timepoints where there was a statistically significant reduction (p<0.05) in pain severity or pain interference score. P-value is a standard measure of statistical significance. It is the probability that study results are due to random fluctuations and not the effect of NV‑01. A low P-value means the study results have a low probability of being due to chance and a high probability of being due to NV‑01’s effects.
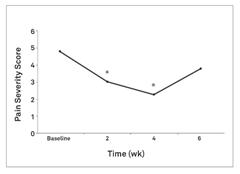
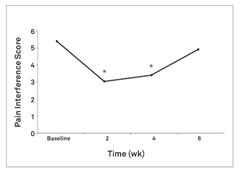
Figure 1: Summary data from a study of NV‑01 in nine dogs with osteoarthritis using CBPI assessment.
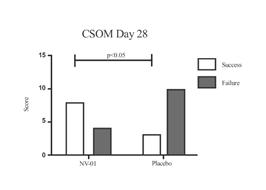
A study of NV‑01 in 26 dogs with naturally-occurring osteoarthritis yielded further positive results, summarized in Figure 2 below. This study used both CBPI and another owner assessment tool, client specific outcome measures, or CSOM. The CSOM measures activities that have become difficult or have changed for the dog as a result of osteoarthritis. For the CSOM assessment, precise activities that have changed in dogs as a result of osteoarthritis are defined and re-evaluated at each assessment. The relative ability to perform such activities is assigned a numerical score at each observation.
5
Figure 2: Summary data from a blinded, placebo-controlled study of intravenously administered NV‑01 in 26 dogs with osteoarthritis using both CBPI and CSOM assessment.
The CBPI assessment demonstrated significantly improved pain severity scores (indicated by asterisks) for the group treated with NV‑01 on both days 14 and 28, as compared to no statistically significant changes for the placebo group (p<0.05). The CSOM assessment shows significantly more dogs improved their pain scores and had results deemed a “success” rather than a “failure” at the day 28 timepoint following treatment with NV‑01, as compared to the placebo group (using a standard measure of statistical significance, P<0.05). “Success” was determined where each CSOM score for a dog improved or stayed the same and there was a total positive score change of two or more over the measured activities. This data was published in the peer-reviewed journal BMC Veterinary Research in April 2015.
The data we have generated in proof-of-concept studies in osteoarthritic dogs was supportive of the decision to move NV‑01 forward into full development.
NV‑01 Status
We have completed the production of a stable, high-expressing clonal cell line at a prior third-party contract manufacturer. Material from manufactured clonal cells, from which biologics are created, is being used in our pivotal clinical safety and efficacy study and our additional pilot field safety and efficacy study and is intended to be used later for commercial supply after successful manufacturing scale-up and process validation. Pilot batch scale-up has shown that NV‑01 is expressed at high concentrations from a clonal cell line, and is stable in liquid formulations.
We commenced our pivotal safety and efficacy study in October 2014. In March 2015, we received results from the planned sample size reassessment of the study. This statistical analysis indicated that, in order for the study to achieve a high probability of a statistically significant primary endpoint, it would have been necessary to substantially increase the size of the study. We then assessed the impact of time, cost and related logistical issues regarding the feasibility of substantially increasing the number of dogs in the study, or initiating additional studies, in consultation with both the FDA’s Center for Veterinary Medicine and Virbac, our collaborator and distributor outside of the United States and Canada. We concluded that we should continue the current study to completion without a change in study size, while also commencing a new placebo-controlled multi-site pilot field safety and efficacy study to assess various doses and dosing regimens of NV‑01. The pivotal study remains blinded to preserve the integrity of its data. The new pilot field safety and efficacy study is expected to enroll approximately 150 dogs, to cost approximately $1.0 million, and to provide valuable information that may assist the design of a future pivotal study. The target completion date of this new study is, like the ongoing NV‑01 pivotal study, the fourth quarter of 2015.
NV‑01 Pivotal Safety and Efficacy Study Design
The NV‑01 pivotal safety and efficacy study has enrolled over 250 dogs with naturally-occurring osteoarthritis. The study is taking place at veterinary clinics in various locations in the United States and the EU. The dogs have been randomized into treatment groups with a 2:1 ratio of NV‑01-treated to placebo-treated study subjects. Each dog receives three treatments at 28-day intervals. Efficacy is being measured using two different owner-administered assessment tools, the CSOM and CBPI, together with veterinarian assessments of pain and lameness. A comparison of change in CSOM score between enrollment and day 28 is the primary endpoint for the efficacy evaluation. CSOM assessments on other days, CBPI assessments and veterinarian pain and lameness assessments are secondary endpoints. A sample size reassessment was performed by an independent statistician during the study, when 133 dogs had reached the primary end-point, to check the assumptions made in the original sample size calculations. The statistician looked at the emerging difference between the two treatment groups to evaluate whether the study was adequately powered to deliver a definitive outcome on the primary end-point. This process was designed to confirm the validity of the planned sample size, recommend expanding the sample size or confirm that a futility criterion was met (indicating that the study would be unable to achieve its objective).
6
The study is double-blinded, meaning participants and veterinarians will be unaware of which treatment group will be receiving NV‑01 or a placebo. CSOM, CBPI and veterinarian assessments are being measured at enrollment and on days 14, 28, 56 and 84.
NV‑02 (Area: chronic feline pain)
Overview
NV‑02 is a 100% species-specific mAb targeting NGF, discovered through PETization and developed as a long-acting analgesic for chronic pain, specifically for the pain associated with degenerative joint disease, including osteoarthritis, in cats. We have initiated regulatory preparations with the FDA and the European Medicines Agency, or EMA; have completed proof-of-concept pharmacokinetic, preliminary safety, and immunogenicity studies; and in June 2015 announced positive top-line results from a proof‑of‑concept efficacy study and a pilot safety study.
Market Opportunity and Current Treatment Limitations
According to the 2015-2016 American Pet Products Association National Pet Owners Survey and the European Pet Food Industry Federation’s Facts and Figures 2012, there are approximately 85.8 million pet cats in the United States and approximately 66.5 million pet cats in Europe.
Clinical studies indicate a majority of cats display radiographic evidence of degenerative joint disease, the progression of which is associated with increasing pain and disability. However, cats’ sensitivity to NSAID toxicity has meant that in the United States, no NSAID is approved for use in cats for more than three days.
The unmet medical need for safer pain therapeutics that can be administered to cats is supported by a proprietary report we commissioned from GfK in December 2013, which revealed that, of the 390 veterinarians surveyed in the United States, United Kingdom, France and Germany, between 86% and 98% would use a product such as NV‑02 for the treatment of chronic pain in cats.
Our Solution
NV‑02 has been found to be safe and well tolerated when administered subcutaneously or intravenously and at high doses, and to have a prolonged elimination half-life after a single injection. Repeated administration has not induced any significant neutralizing antibody response in a previous study of four cats, indicating the feline immune system did not identify NV‑02 as a ‘foreign’ protein. In another previous study, NV‑02 was also shown to reduce lameness scores in an acute feline model of inflammatory pain, with efficacy comparable to meloxicam, a standard NSAID used in acute feline pain management for up to three days.
In June 2015, we announced positive top-line results from the proof-of-concept efficacy study for NV‑02 in cats with degenerative joint disease. Additionally, we received positive data from a high-dose pilot safety study of NV‑02 in cats, which was conducted by an independent contract research organization, or CRO.
The efficacy endpoints of the proof-of-concept study were measured using:
|
|
· |
Collar-mounted accelerometer data, which measures overall patient activity levels; and |
|
|
· |
Owner-assessments (for example, CSOM), which measure changes in certain patient behaviors based on owner observations. |
These measurements were performed over nine weeks after administration in both the treatment and placebo groups. In both the proof-of-concept and safety studies, the feline subjects were dosed with a single subcutaneous injection of NV‑02.
The top-line outcomes from the two studies are:
|
|
· |
A statistically significant improvement in activity was measured with accelerometer data, when compared to placebo from weeks two to five following administration (p<0.05) in the proof-of-concept study (figure 3). |
|
|
· |
A statistically significant clinical improvement from baseline was observed in CSOM scores taken three weeks after treatment, when compared with placebo (p<0.05) in the proof-of-concept study. As shown in figure 4, median change in CSOM score at this timepoint for cats treated with NV‑02 was 5.5, while the placebo group demonstrated a median change in CSOM score of 2. |
|
|
· |
No adverse events were observed at the doses tested in the proof-of-concept study, nor in the separate safety study where cats were treated with NV‑02 at more than 10 times the highest dose used in the proof-of-concept study. |
7
Our data to-date suggests that NV‑02 has a safety and efficacy profile that warrants continued development.
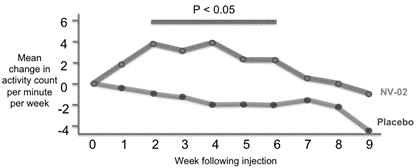
Figure 3: NV‑02 proof-of-concept efficacy study actimetry data summary
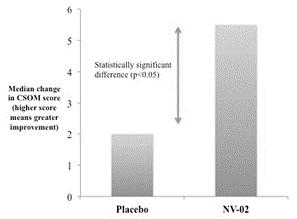
Figure 4: NV‑02 proof-of-concept efficacy study: median CSOM change at three weeks post-treatment
NV‑02 Status
We have generated stable, high-expressing clonal cell lines producing NV‑02 and are undertaking stability, purification and formulation studies. As described above, in June 2015, we announced positive top-line results from our proof-of-concept efficacy study of NV‑02 in 32 cats with degenerative joint disease. This study demonstrated statistically significant improvements on two measures: CSOM scores 3 weeks after treatment, and general activity as measured by collar-mounted accelerometers in weeks 2‑5 following treatment. Additionally, we received positive data from a separate high-dose pilot safety study of NV‑02 in cats.
We plan to shortly commence a placebo‐controlled, blinded, multi-site pilot field safety and efficacy study targeting enrollment of 90 cats with naturally occurring osteoarthritis. Feline participants will be assessed using a variety of methods for assessing pain and mobility in companion animals. The key outcomes will be a comparison between NV-02 dosage groups and a placebo group, over a period of two months. We anticipate results from this study in the first quarter of 2016.
NV‑08 (Area: chronic canine inflammation)
Overview
NV‑08 is a proprietary fusion protein that is a TNF inhibitor for canine inflammatory diseases. We identified a proprietary variant of the TNF receptor in dogs, and, using fusion protein technology, we created NV‑08. NV‑08, a canine biologic analogous to Enbrel, a leading human fusion protein, has been selected for further development for the treatment of chronic inflammatory diseases, including atopic dermatitis, a major skin condition in dogs. We have demonstrated in a pre-clinical study that NV‑08 has improved potency compared to Humira, a leading human mAb. We have commenced proof-of-concept studies, which we expect to complete by the end of the first quarter of 2016. If our proof-of-concept safety and efficacy studies for NV‑08 are successful, we plan to progress this product into formal development.
8
Market Opportunity and Current Treatment Limitations
Chronic inflammatory diseases affect a number of organ systems in dogs and cats, similar to the effects seen in humans. Examples include atopic dermatitis, arthritis, inflammatory bowel disease, type 1 diabetes and cardiovascular disease. Atopic dermatitis is a common condition in dogs of all ages, estimated to affect approximately 10% of all dogs. Dogs with atopic dermatitis display skin lesions with itching that often lead to further damage and infection which exacerbates their symptoms.
Atopic dermatitis is currently treated with various approaches, including dietary supplements, topical shampoos, emollients and ointments, anti-histamines, NSAIDs and immunosuppressant drugs such as oral steroids and Atopica. Although potent immunosuppressant drugs, like Atopica, can be effective in controlling the clinical signs of itch, they can also have serious side effects and can be expensive. Apoquel, a Janus kinase-inhibitor introduced by Zoetis in 2014, has demonstrated efficacy in controlling itch, but it carries several label warnings. The U.S. label states it is not for use in dogs less than 12 months old or those with serious infections, and it may increase susceptibility to infections and tumors. Its chronic use in the field (post-launch) has not yet been fully evaluated.
We believe that a treatment possessing similar efficacy to Atopica and Apoquel, but with improved safety and convenience of administration, would be well received by veterinarians.
Our Solution
NV‑08 is a fusion protein that links the part of a canine antibody that binds to receptors to activate the immune system with the extracellular domains of the canine TNF receptor. NV‑08 acts in an analogous manner to the human therapeutic fusion protein, Enbrel, to bind and inhibit the activity of the important inflammatory mediator TNF‑α. We have identified a novel TNF receptor variant in dogs, which is the portion of NV‑08 that binds TNF with high affinity.
There is published evidence to demonstrate the involvement of TNF in canine atopic dermatitis and to support further development for this indication. For example, third-party studies have shown TNF messenger ribonucleic acid, or mRNA, which carries genetic information, to be elevated in lesional canine atopic dermatitis as has TNF protein expression. In a small study in humans with moderate-severe atopic dermatitis, administration of a TNF inhibitor mAb demonstrated a significant improvement in clinical symptoms.
We have demonstrated that NV‑08 can neutralize canine TNF in cell culture systems with potency equivalent to the human TNF inhibitor drug Enbrel (a fusion protein) and significantly greater than the human TNF inhibitor drug Humira (a mAb). Repeated injection did not induce a neutralizing immune response in a study of four dogs, indicating the canine immune system did not identify NV‑08 as a “foreign” protein.
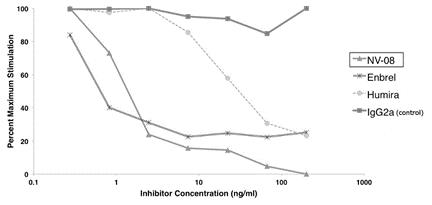
Figure 5: Summary of potency comparison between NV‑08 and leading human anti-TNF drugs
NV‑08 Status
We filed an INAD with the FDA in December 2014. We expect to complete preliminary proof-of-concept safety and efficacy studies by the first quarter of 2016. If these are successful and achieve their endpoints, we plan to progress NV‑08 into formal development, which would include the manufacture of a clonal cell line and additional studies to support FDA and EMA approval.
9
Identification of new product candidates
We have established an internal research and drug discovery team with processes to identify and prioritize our target mAbs to be PETized. Preferred features of donor mAbs that we consider for PETization include:
|
|
· |
the donor mAb has demonstrated safety and efficacy in human clinical trials; |
|
|
· |
the donor mAb retains its function, binding to the same target in the companion animal species; |
|
|
· |
the target has a comparable mechanism of action in companion animals as in humans; and |
|
|
· |
the disease in the target species that the mAb will target is of commercial significance. |
Selected donor mAbs are PETized. The PETized mAb is manufactured at small scale and its biological properties are confirmed in appropriate biological test systems, including initial proof-of-concept and safety and efficacy studies to develop an initial target product profile. The target product profile is tested with clinical key opinion leaders or through surveys of veterinarians. We also meet with U.S. regulators to discuss regulatory pathway and determine jurisdiction.
In addition to current and potential mAb product candidates, we are evaluating biologic candidates of other therapeutic classes for development in other indications. These evaluations are of both internally developed candidates and others owned by third parties, upon which we have based a research and development collaboration. This is in line with our strategy to identify biologics with therapeutic value in other species, and offer strategic partnerships to develop (and PETize, in the case of mAb candidates) these candidates for use in companion animals.
Our PETization Platform
Our PETization platform is a proprietary algorithmic approach that has demonstrated a reduction in the time and cost typically associated with the development of mAbs using conventional interspecies conversion methods. By applying our algorithms to analyze large data sets from our proprietary complementary deoxyribonucleic acid, or cDNA, library, PETization is designed to determine the minimal number of changes required in the mAb framework region, and generate a 100% species-specific mAb that preserves the attraction between a biologic and its target, a property known as affinity. We have used PETization to successfully convert human and rodent mAbs into 100% species-specific canine, feline and equine mAbs, thereby leveraging their safety and efficacy profile for our companion animal therapies in development.
An antibody is generally made up of two short, or “light,” chains and two longer, or “heavy,” chains of amino acids arranged in a specific sequence. At one end of the chains are the complementarity-determining regions, or CDRs, which allow the antibody to bind to its target. Between the CDRs are framework regions, which are typically species-specific, that determine the immune response caused by the antibody. If these sequences of amino acids in the framework regions do not match ones that occur naturally in the target animal, the antibody could induce a significant negative immune response, or immunogenicity. These non-matching sequences must therefore be identified and adjusted to create an antibody that retains therapeutic benefits while matching the target animal’s naturally-occurring sequences. Minimizing the changes to these sequences makes the converted antibody more likely to be recognized as “self” or “native” by the target’s immune system.
Conventional interspecies conversion methods graft the desired CDRs from a donor mAb into a single antibody sequence from the target species. Donor mAb sequences are then reintroduced in attempts to restore affinity, a process called “affinity maturation.” By contrast, our PETization algorithm compares the sequences of the heavy and light chains of a donor mAb to a database of known heavy and light chain sequences from the target veterinary species. The algorithm determines the minimal number of changes at each position in the amino acid sequences required to convert donor mAb heavy and light chain sequences into mAb sequences containing only amino acids identified within the target species, which reduces the risk of rejection due to immunogenicity. PETization is thus designed to avoid the need for the conventional method of affinity maturation, which based on our experience can require six to 18 months to complete, depending on the project, and which can increase the foreign sequence content, thereby increasing the risk of immunogenicity.
Primarily using PETization, we are seeking to advance one new product candidate into development per year. Our internal research and drug discovery team is studying mAbs that bind to canine, feline and equine targets relevant to pain, inflammation, cancer and other chronic conditions. We have also completed a survey of specialist veterinarians in the United States and the European Union, or the EU, to identify key areas of unmet medical need where mAbs could have a significant impact. The results of this survey are guiding our product development priorities.
10
We believe our PETization platform offers the following important advantages over other approaches to the design, discovery and development of mAbs in the veterinary care industry:
|
|
· |
Rapid creation of new products. PETization is designed to substantially reduce the time involved in the discovery process for new mAbs with high affinity, when compared to conventional discovery techniques. |
|
|
· |
Cost efficiencies in production. mAbs with higher affinity require less active pharmaceutical ingredient to achieve a therapeutic dose, leading to lower cost of goods. |
|
|
· |
Efficient development pathway. Harnessing existing donor mAb manufacturing, safety and clinical efficacy data can significantly reduce costs, time-to-market and regulatory and clinical risk. |
|
|
· |
Scalability across species. PETization enables us to rapidly identify new product candidates for many indications across multiple species. |
|
|
· |
Proprietary cDNA approach to mAb identification. Our proprietary cDNA library of mAb sequences allows us to use the natural variations found in mAbs to generate novel, species-specific mAbs. |
We believe that our PETization platform will create differentiated, high-value companion animal therapies with better health outcomes through the following characteristics:
|
|
· |
Efficacy. PETized mAbs are designed to retain the efficacy of the donor mAbs. |
|
|
· |
Safety. PETized mAbs match the structure of the target species more successfully than conventional approaches, thereby reducing the risk of immunogenicity. |
|
|
· |
Ease of compliance. PETized mAbs are designed to be injected every four to six weeks, as compared to small molecule treatments, which can require daily or more frequent injections or oral dosing. |
We believe that these product characteristics align favorably with veterinarian preferences and will contribute to the widespread market adoption of PETized mAbs. We may also develop biologics outside of our PETization platform, such as NV‑08.
Companion Animal Therapeutic Market Overview
The U.S. companion animal market, which includes veterinary care, food, supplies and over-the-counter medications, live animal purchases and services such as grooming and boarding, is estimated to exceed $60.0 billion in sales in 2015. Veterinary care, which includes sales of companion animal therapeutics, parasiticides and vaccines and other medical expenses for veterinarian visits, is one of the fastest growing industries in the U.S. companion animal market. We are targeting the companion animal therapeutics segment of the veterinary care industry. We estimate that in 2013 consumers spent $2.3 billion on companion animal therapeutics. This segment is currently dominated by small molecule drugs. We believe only one mAb for veterinary use has received a full license from the United States Department of Agriculture, or USDA, and there are currently no mAbs for the management of pain or inflammation in companion animals fully approved for marketing in the United States or the European Union, or EU. Biologics, including mAbs, have grown to be the largest class of therapeutics within the top ten best-selling drugs for humans. We believe that mAbs will drive a similar trend in the companion animal therapeutics market.
Historically, most companion animal therapeutics have been in areas overlapping with livestock health, notably vaccines, parasiticides and anti-infectives. Similar to human therapeutics, and given the safety and efficacy profile of biologics, we see opportunities for biologic therapies for companion animals in the areas of pain, inflammation, oncology, dermatology, allergy and gastrointestinal disease. We expect the market for companion animal biologic therapies to increase primarily due to:
|
|
· |
Higher standards and better care. Because owners are increasingly regarding their companion animals as important members of their families, owners are demanding better care and treatment options for their companion animals. In a 2011 survey by Kelton Research, 81% of respondents considered their dogs to be true family members. According to the American Pet Products Association, approximately 78% of U.S. dog owners treated their dogs with medications in 2010, as compared to 50% in 1998. In a 2010 poll by Associated Press, 35% of companion animal owners indicated they were willing to spend $2,000 to treat their companion animal for a serious medical condition. |
|
|
· |
Aging companion animal population. Companion animals are living longer, leading to increased incidence of diseases commonly associated with aging, such as arthritis, diabetes, tumors and kidney disease. According to a 2013 report by Banfield Pet Hospital, the average lifespan of dogs increased from 10.5 years in 2002 to 11 years in 2012, and the average lifespan of cats increased from 11 years in 2002 to 12 years in 2012. |
We believe these favorable demographics create a significant opportunity for companion animal biologic therapies.
11
Limitations of Current Standard of Care and the Promise of Biologics
Despite the growing market for veterinary care and favorable market dynamics, there are few treatment options approved for use in companion animals relative to the diversity of available human therapeutics. Current approved therapeutics for the management of many conditions, including pain, inflammation and cancer, are predominantly small molecule drugs.
Small molecules are typically formulated as oral dose forms that owners must administer to their companion animals, often daily or more frequently. There are concerns regarding the use of small molecule therapeutics in some areas of companion animal medicine. For example, many NSAIDs commonly prescribed for pain and inflammatory diseases have black box warnings in their labelling of potentially serious side effects that limit their chronic use in dogs and largely preclude their chronic use in cats.
We believe that small molecule products as a class present significant limitations in the following areas:
|
|
· |
Toxicity. Many small molecule therapies carry the risk of increased toxicity due to off-target effects. Furthermore, some small molecule therapies can result in toxicity and bleeding and, in extreme cases, death for certain breeds of dogs. For example, NSAID therapy can result in gastrointestinal toxicity in humans and animals, but in dogs it can also result in renal and hepatic toxicity. Treatment in cats is further complicated given their livers are unable to process NSAIDs effectively, further complicated by renal toxicity. For this reason, substantially all NSAIDs used in cats in the United States are approved for use for no more than three consecutive days. |
|
|
· |
Administration challenges. Because many small molecule therapies typically suffer from a short half-life (time in the blood), they typically require daily or more frequent injections or oral dosing. In addition, because most small molecule products are administered orally, it is difficult to ensure the product is ingested and absorbed. As a result, daily or more frequent injections or oral dosing of small molecule products provides challenges to owners that increase the risk of poor compliance with the treatment regimen. |
In recent years, particularly in inflammation and cancer, human drug development has increasingly focused on biologics, such as mAbs, which generally offer safety and efficacy profiles that make them attractive alternatives to small molecule drugs for many indications. Seven of the top ten best-selling human drugs in 2014 were biologics, including Humira, Enbrel and Remicade for inflammatory diseases, Rituxan for oncology and inflammatory disease, Avastin and Herceptin for oncology and the insulin analog Lantus, which collectively amounted to worldwide sales in 2014 of approximately $60.0 billion (or approximately 73% of the top ten drugs’ revenue). We believe this illustrates a significant opportunity for us to develop first-in-class therapeutics for the unmet medical needs of companion animals utilizing our proprietary PETization platform.
Our Strategy
We strive to be at the forefront of companion animal therapeutic innovation by developing and commercializing a portfolio of biologics for companion animals. To achieve this goal, we intend to:
|
|
· |
Leverage our proprietary PETization platform and experience to develop multiple companion animal therapeutics. Our proprietary PETization platform enables the rapid translation of mAbs between species, positioning us to identify and develop multiple companion animal therapeutics. In addition, we have assembled a team of professionals that is highly experienced in the development and commercialization of companion animal and human therapeutics for global markets. We believe our platform and experience will enable us to develop and commercialize effective biologics for companion animals. |
|
|
· |
Focus on common conditions impacting the quality of life of companion animals to make a positive impact on their health. Improving available therapies and addressing the unmet medical needs of dogs and cats will allow us to make an immediate positive impact on their health. For both dogs and cats, we intend to conduct our pivotal safety and efficacy studies in order to obtain regulatory approval for safer, more effective products than those that are currently available, in areas such as pain, inflammation and cancer. In addition, due to the toxicity of currently available treatments in cats particularly for pain management, we believe biologics can provide safe and effective treatment alternatives for a variety of chronic diseases for which there are presently limited options. |
|
|
· |
Commercialize our lead product candidates with a direct sales force and distributors in the United States and through strategic alliances in international markets. If our lead product candidates are approved for commercialization, we intend to recruit senior executives with animal health sales and marketing expertise, and through an energetic sales force we intend to train veterinarians to understand the science and benefits of biologics. By adding complementary distributor relationships in the United States and strategic alliances in the EU and elsewhere, such as our existing relationship with Virbac, we believe we can optimize our penetration of the veterinary markets. |
12
|
|
· |
Collaborate with leaders in human and veterinary biologics to bring to market the next generation of companion animal therapeutics. We believe that universities, as well as pharmaceutical and animal health companies, present an opportunity to leverage our PETization platform. Through collaborations, we intend to design, develop, manufacture and bring to market the next generation of mAbs and complementary technologies. |
In order to execute our strategy we have assembled a management team and board of directors who have held senior positions in leading biopharmaceutical and animal health companies and who have extensive experience in the discovery, development and commercialization of therapeutics.
Process Development, Manufacturing and Distribution
We currently have no significant internal capability to manufacture the formulated product candidates for use in our registrational studies or commercial supplies of any of our product candidates that may be approved, and we are primarily dependent upon third-party manufacturers for such supplies. We have contracted with Fujifilm Diosynth Biotechnologies UK Ltd, or Fujifilm, to provide future clinical-scale biomanufacturing services for NV‑01 and NV‑02. Our contract with Fujifilm also provides the opportunity for Fujifilm to become the commercial manufacturer of these product candidates. In light of requirements imposed by the Brazilian regulatory authority (ANVISA) regarding segregation of human and veterinary manufacturing areas, we were unable to agree on acceptable terms for commercial supply with our prior manufacturer. As a part of our ongoing evaluation of manufacturing providers, we intend to continue to evaluate alternatives in the near term for active pharmaceutical ingredient and finished drug product for commercial supply to mitigate risks.
We depend on third parties to supply all of our required raw materials and finished products for our pre-clinical, proof-of-concept, pilot and pivotal safety and efficacy studies and commercial supply. We believe that the raw materials that we require to manufacture our lead product candidates are readily available commodities commonly used in the biopharmaceutical industry.
We currently do not have a direct sales organization. In order to commercialize any of our current or future product candidates, we must build our marketing, sales, distribution, managerial and other non-technical capabilities or make or maintain arrangements with third parties to perform these services, and we may not be successful in doing so. We expect to establish a small direct sales organization in the United States and to utilize distributors to commercialize our lead product candidates. In jurisdictions outside of the United States, we intend to utilize companies with an established commercial presence to market our lead product candidates in those jurisdictions, but we may be unable to enter into or maintain such arrangements on acceptable terms, if at all.
We entered into a master collaboration, supply and distribution agreement, or the Master Agreement, and a specific distribution agreement, or SDA, for NV‑01 with Virbac in November 2014. Pursuant to the Master Agreement, we appointed Virbac as our sole and exclusive distributor of NV‑01 and any other products for which we enter into an SDA with Virbac in the veterinary field worldwide except for the United States and Canada. We refer collectively to the applicable area as the “Territory.” After a specified period, we will have the right to distribute and sell the product ourselves in the Territory. If we do so, we have agreed to make a lump sum payment to Virbac and Virbac shall become a non-exclusive distributor for such product in the Territory.
Pursuant to the Master Agreement, Virbac must provide clinical, regulatory, marketing and sales input via a joint steering committee, advise us in the drafting of regulatory submissions in the countries within the Territory, sell our products, meet or exceed minimum annual net sales obligations for each product, manage local complaints and transfer drug safety data, not apply for the registration of any trademark that is the same as or similar to any of our trademarks and launch the product within a specified time period after marketing authorization. We have agreed to develop the products in accordance with a development plan agreed with Virbac and to use commercially reasonable efforts to obtain and maintain market authorization of the products, all at our own cost. We have agreed to arrange manufacture and supply of the product to Virbac on a cost-plus basis. Virbac must pay to us certain milestone payments for our research and development work for our products, as well as fees based on Virbac’s net sales of the product reduced by specified amounts intended to reflect certain costs of goods and costs of selling, each as provided in the SDA for the product.
Pursuant to the Master Agreement, Virbac may not distribute any competing product in a country where a marketing authorization has been obtained for a period of five years from Virbac’s first commercial arm’s length bona fide sale of one of our products in certain European countries specified in the Master Agreement. Virbac has the first option right to negotiate an SDA with us for our future products in the Territory. If we receive a bona fide proposal from a third party for the assignment of the registration dossier (containing information necessary for obtaining a marketing authorization) for any products covered by the Master Agreement, we have agreed to notify Virbac, which will have the right to make an offer to purchase the registration dossier.
13
The Master Agreement (and any SDA) has an initial term of ten years from the date of Virbac’s first commercial arm’s length bona fide sale of a product in certain European countries specified in the Master Agreement and, unless otherwise agreed by the parties, will automatically renew for successive periods of two years each. Each party may terminate the Master Agreement for the other party’s uncured material breach or insolvency. Virbac may terminate the Master Agreement if we do not obtain in a timely fashion a marketing authorization for NV‑01 in Europe, there is a material failure to comply with the target product profiles for NV‑01, there is a material deviation from a development plan for NV‑01 or the distribution of all products covered by the agreement is prevented in certain important countries specified in the Master Agreement as a result of a potential infringement of a third party’s intellectual property rights. Each party may terminate the applicable SDA if the commercial margin for the product falls below a certain threshold. Virbac may terminate an SDA if the distribution of the applicable product is prevented in certain important countries specified in the Master Agreement as a result of a potential infringement of a third party’s intellectual property rights, or there is a material deviation from a development plan.
The SDA for NV‑01 specifies the conditions under which Virbac will place orders to us for the provision of NV‑01 in accordance with a target product profile, the marketing authorization in Europe, the product specifications and specific terms of Virbac’s distributions of NV‑01. These terms include milestone payments aggregating to $1.0 million for our receipt of our first marketing authorization in Europe for NV‑01 and Virbac’s first commercial arm’s length bona fide sale of one of NV‑01 in certain European countries specified in the Master Agreement. The SDA also provides that we will receive a fixed percentage between 45-55% of the commercial margin from sales of NV‑01, which is calculated based on the net sales of NV‑01 reduced by specified amounts intended to reflect certain costs of goods and costs of selling, as provided in the SDA.
Competition
The companion animal therapeutics segment of the veterinary care industry is highly competitive and characterized by rapid technological change. Key competitive factors in our industry include, among others, the ability to successfully advance the development of a lead product candidate through pivotal safety and efficacy studies; the timing and scope of regulatory approvals, if ever achieved, average selling price of competing products and animal therapeutic products in general, the availability of raw materials, contract manufacturing and manufacturing capacity, manufacturing costs, establishing and maintaining intellectual property and patent rights and their protection, and sales and marketing capabilities.
We believe our main competitors are animal health companies that are developing products for use in companion animals, such as Aratana Therapeutics, Inc., Kindred Biosciences, Inc. and Zoetis, Inc. In addition, there are a number of large biopharmaceutical companies with animal health divisions, such as Bayer AG; Boehringer Ingelheim GmbH; Eli Lilly and Company (Elanco division); Merck & Co., Inc.; and Sanofi S.A. (Merial division). If approved, we expect NV‑01 and NV‑02 will face competition from Deramaxx, marketed by Elanco; Metacam, marketed by Boehringer Ingelheim; Previcox, marketed by Merial; and Rimadyl, marketed by Zoetis, as well as from generic Meloxicam and Carprofen and other pain-treating products. We believe that Aratana and Kindred are developing, and that other companies may develop, similar products as well. In addition, private-label products may compete with our lead product candidates. If companion animal therapeutics customers increase their use of new or existing private-label products, our operating results and financial condition could be adversely affected.
We are a clinical-stage biopharmaceutical company with a limited operating history and many of our competitors have substantially more resources than we do, including financial, technical and sales resources. In addition, many of our competitors have more experience than we have in the development, manufacture, regulation and worldwide commercialization of companion animal therapeutics. We are also competing with academic institutions, governmental agencies and private organizations that are conducting research in the field of companion animal therapeutics. Our competition will be determined in part by the potential indications for which our lead product candidates are developed and ultimately approved by the regulatory authorities. Additionally, the timing of market introduction of some of our future products or of competitors’ products may be an important competitive factor. Accordingly, we expect the speed with which we can develop our lead product candidates, complete pivotal safety and efficacy studies and approval processes, and supply commercial quantities to market to be important competitive factors.
Research and Development
Research and development expense consists primarily of employee compensation for internal research personnel, fees for regulatory, professional and other consultants and third party development costs. Research and development expense was $9.8 million, $5.6 million and $2.7 million for fiscal years 2015, 2014 and 2013, respectively.
14
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, novel discoveries, product development technologies and other know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. We seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trademarks, trade secrets, know-how, confidentiality and other agreements and continuing technological innovation to develop and maintain our proprietary position.
We generally pursue composition and therapeutic use patents for the product candidates we develop and intend to commercialize. We have also pursued patents with respect to our proprietary PETization platform.
We have filed patent applications with respect to 10 patent families with claims directed to our PETization platform, our NV‑01, NV‑02 and NV‑08 product candidate compositions, several of our pipeline product candidate compositions and therapeutic uses of such product candidate compositions. We have filed approximately 90 patents and pending patent applications, around the world, focusing on countries in our expected key markets, including Australia, Brazil, Canada, China, Europe, Hong Kong, India, Japan, Malaysia, New Zealand, Russia, Singapore, South Korea and the United States. We have eight pending patent applications in the United States. We have six registered patents (five in New Zealand and one in the United Kingdom) in our portfolio.
Patents extend for varying periods depending on the date of the patent filing or grant and the patent term provided by law in the countries where patents are obtained. Our patents, or the patents we may receive pursuant to our pending patent applications, relating to NV-01, NV-02, NV-08 and our PETization platform expire, or are expected to expire, in 2032 and 2033. We have submitted a number of other patent applications with claims that relate to other indications we may pursue in the future.
Government Regulation
The development and testing, regulatory approval, import, distribution, marketing, sale and post-marketing oversight of veterinary care products are each governed by the laws and regulations of each country in which we intend to sell our lead product candidates. To comply with these regulatory requirements, we have established processes and resources to provide oversight of the development, regulatory approval and launch of our lead product candidates and their maintenance in the market.
Requirements for Approval of Veterinary Medicinal Products Worldwide
We operate as a global company and, therefore, are developing our lead product candidates with a global regulatory strategy. We intend to initially market our lead product candidates in the United States and the EU.
As a condition for the regulatory approval for sale of veterinary medicinal products, regulatory agencies worldwide generally require that a product to be used for animals be demonstrated to:
|
|
· |
be safe for the intended use in the intended species; |
|
|
· |
have substantial evidence of effectiveness for the intended use; |
|
|
· |
have a defined manufacturing process that ensures that the product can be made with high quality and consistency; and |
|
|
· |
be safe for humans handling the product and for the environment. |
Safety
To determine that a new veterinary medicine is safe for use, the regulatory authorities require developers to provide data from one or more target animal safety studies generated in the species of interest in a laboratory environment tested at doses higher than the intended label dose, over a period of time determined by the intended length of dosing of the product. In the case of the FDA, a review of the design of the safety study and the study protocol can be completed prior to initiation of the study to help assure that the data generated will meet FDA requirements. Scientific advice can be also obtained from the EMA.
These studies are conducted under rigorous quality processes, including good laboratory practices, to assure integrity of the data. They are designed to clearly define a safety margin, identify any potential safety concerns and establish a safe dose for the product. In addition, safety data from pivotal studies conducted under good clinical practice standards are evaluated to further define the safety profile of the product in the target population.
15
Furthermore, because safety and efficacy studies must conform to the Veterinary International Conference on Harmonization guidelines, which are established under an international program aimed at harmonizing technical requirements for veterinary product registration, they can be utilized by regulatory bodies in the United States, the EU, Japan, Canada, New Zealand and Australia, as well as other regulatory bodies worldwide. This initiative assists in reducing the number of animals used to determine if a veterinary product is safe and efficacious.
Efficacy
A range of efficacy studies varying in size and design may be conducted throughout the course of a product candidate’s development. Early proof-of-concept studies may be conducted in the species of interest in a laboratory environment to establish efficacy and the dose range for each product. Data regarding drug absorption via different routes of administration and the relationship of the dose to efficacy are studied. When an effective dose is established, a study to test the product in “real world” or “field” conditions is designed via a documented protocol, prior to beginning the study. Studies that may be used to support regulatory applications for marketing approval are termed “registrational” or “pivotal.”
Non-registrational or “pilot” field studies may also be conducted to further develop the evidence base supporting a product candidate and optimize its dosing regimen among other variables. They typically precede a pivotal study and help to optimize the design of the latter. They may also be used post-registration for studies in new indications. Large pilot field studies may enroll 100 – 150 animals, approaching a pivotal study in size.
A pivotal field safety and efficacy study must be conducted with the formulation of the product that is intended to be commercialized and is a multi-site, randomized, controlled study, often with a placebo control. To reduce bias in the study, individuals doing the assessment are not told whether the subject is in the group receiving the treatment being tested or the placebo group. The number of subjects enrolled in such studies is determined by statistical procedures using pilot studies as a guide. In both the United States and the EU, the number of subjects enrolled in a pivotal safety and efficacy study is required to be at least 100 to 150 animal subjects treated with the test product. In many cases, numbers of animals enrolled will exceed these numbers.
A pivotal study may be designed with clinical sites in both the United States as well as the EU, and other jurisdictions, and this single study may satisfy regulatory requirements in several jurisdictions. In the case of the FDA, the pivotal efficacy study protocol can be submitted for review and concurrence prior to study initiation, to help assure that the data generated will meet requirements. Scientific advice can be also obtained from the EMA before conducting the study.
Chemistry, Manufacturing and Controls
To assure that the product can be manufactured consistently, regulatory agencies require developers to provide documentation of the process by which the active pharmaceutical ingredient is made and the controls applicable to that process that assure the active pharmaceutical ingredient and the formulation of the final commercial product meet certain criteria, including quality, purity, and stability. After a product is approved, developers are required to communicate with the regulatory bodies any changes in the procedures or manufacturing site. Both the active pharmaceutical ingredient and the commercial formulations are required to be manufactured at facilities that engage in pharmaceutical good manufacturing practices.
Environmental and Human Safety
U.S. law requires sponsors to provide an environmental impact statement for products given at the home of the animal’s owner or in a veterinary clinic or hospital. If products might result in some type of environmental exposure or release, the environmental impact must be assessed. For approval in the EU, a risk assessment for potential human exposure is required.
Labeling
The intended label for the product, and also any information regarding additional research that has been conducted with the product, must be submitted to the FDA and other regulatory bodies for review. In addition, advertising and promotion of veterinary care products is controlled by regulations in many countries. These rules generally restrict advertising and promotion to those claims and uses that have been reviewed and approved by the applicable agency.
United States
Three federal regulatory agencies regulate the health aspects of veterinary care products in the United States: the FDA, the USDA, and the Environmental Protection Agency, or EPA.
The Center for Veterinary Medicine at the FDA regulates animal pharmaceuticals and certain biopharmaceuticals under the Federal Food, Drug and Cosmetic Act. The Animal and Plant Health Inspection Service at the Center for Veterinary Biologics at the USDA regulates veterinary vaccines and certain biologics pursuant to the Virus-Serum-Toxin Act. The EPA Office of Pesticide Programs regulates veterinary pesticides under the Federal Insecticide, Fungicide and Rodenticide Act.
16
Our current lead product candidates are animal pharmaceuticals, biopharmaceuticals or biologics for the treatment of pain associated with osteoarthritis, which are regulated by the FDA, and the USDA has advised us that it expects to regulate certain other potential product candidates currently in research. Manufacturers of veterinary pharmaceuticals and biologics must demonstrate that their products are safe, effective, and produced by a consistent method of manufacture. While their procedures vary, both the USDA and the FDA conduct post-approval monitoring of products, require submission of reports of any product quality defects, adverse events or unexpected results and may conduct regulatory inspections from time to time.
Regulatory Process at the FDA
Under the Federal Food, Drug, and Cosmetic Act, a new animal drug may not be sold into interstate commerce unless it is the subject of an approved new animal drug application, or NADA, the subject of an administrative NADA, there is a conditional licensure or the drug is included in the FDA’s index of legally-marketed unapproved new animal drugs for minor species.
To begin the development process for products in the United States, an INAD submission is made to the FDA. Pre-development meetings may be held with the FDA to reach general agreement on the plans for providing the data necessary to fulfill requirements for a NADA. Testing submitted in support of an application to the FDA generally must be conducted in accordance with good laboratory practices, and certain research conducted at research facilities will be subject to additional requirements of the Animal Welfare Act, which affords oversight of animal study subjects. During development, pivotal protocols may be submitted to the FDA for review and concurrence prior to conducting the required studies. Data on manufacturing, safety and efficacy (major technical sections) must be submitted to the FDA for review in the NADA, with review conducted according to timelines specified in the Animal Drug User Fee Act.
Once the data have been submitted and the review completed for each technical section—safety, efficacy and chemistry, manufacturing and controls, or CMC—the FDA issues a technical section complete letter pertaining to each section upon successful review. When complete letters have been received for the major technical sections, a draft of the Freedom of Information Summary is compiled by the sponsor, and the proposed product labeling, and all other relevant information, is submitted to FDA for review. An administrative NADA submission is the final step after complete letters are received for all technical sections of a NADA.
After approval, reports of any and all adverse events must be submitted on a regular basis to the FDA and other ongoing recordkeeping and compliance requirements will apply, including those relating to manufacturing, advertising, labeling and any changes to the product.
Regulatory Process at the USDA
Under federal law, including the Virus-Serum-Toxin Act, producers of veterinary biologics in the United States must have a U.S. Veterinary Biologics Establishment License and a U.S. Veterinary Biological Product License for each product. To qualify for an establishment license, an applicant also must qualify for at least one product license. For those entities or individuals wishing to market imported veterinary biological products in the United States, a U.S. Veterinary Biological Product Permit is required.
To begin the development process for veterinary biological products regulated by the USDA, an Application for U.S. Veterinary Biological Product License is filed with the USDA. Meetings may be held with the USDA to reach general agreement on the plans for providing the data necessary to fulfill requirements for an approval, and a licensing plan, including pivotal study protocols, may be submitted to the USDA for review and comment prior to initiating work that will be used to support product licensure. During development, data on manufacturing, including purity and potency, safety and efficacy must be submitted to the USDA for review. Once all data have been submitted and reviewed, the USDA issues its decision. Unlike the FDA, there are no timelines specified by law for the USDA’s review. The USDA also offers conditional licensure. Conditional licensure is available in order to meet an emergency condition, limited market, local situation or special circumstance and, when granted, allows a manufacturer to develop full efficacy data while marketing a product for which it has demonstrated purity, safety and a reasonable expectation of efficacy.
After licensure, manufacturers must contemporaneously record and maintain information related to adverse events of which they become aware (which may be reviewed upon inspection), and manufacturers are obligated to inform the USDA immediately when they are aware of problems with the purity, potency, safety, efficacy, preparation, testing or distribution of a product. Manufacturers are responsible for maintaining the compliance of the product post-licensure.
17
European Union and the EMA Regulatory Process
The EMA regulates the scientific evaluation of medicines developed by pharmaceutical companies for use in the EU. The EMA’s veterinary review section is distinct from the review section for human medicines. To perform these evaluations the EMA utilizes a specific scientific committee, the Committee for Medicinal Products for Veterinary Use. The EMA considers applications submitted by companies for the marketing authorization of veterinary medicinal products and evaluates whether or not the medicines meet the necessary quality, safety and efficacy requirements. Assessments conducted by the EMA are based on scientific criteria and are intended to ensure that veterinary medicines reaching the marketplace have a positive risk-benefit balance in favor of the animal population for which they are intended. Based on the EMA’s recommendation, a centralized marketing authorization is granted by the EMA, which allows the product to be marketed in all of the EU states, together with Norway, Lichtenstein and Iceland. The EMA is also responsible for various post-authorization and maintenance activities, including the assessment of modifications or extensions to an existing marketing authorization.
To obtain a marketing authorization from the EMA, an application for marketing authorization must be submitted. This application is the EMA’s equivalent of the FDA’s NADA and includes data from studies showing the quality, safety and efficacy of the product. The EMA reviews and evaluates the data. For each application, a rapporteur and co-rapporteur are appointed from the members of the EMA. Their role is to lead the scientific evaluation and prepare the assessment report. The rapporteur can utilize experts to assist in performing the assessment. The assessment report is critiqued by the co-rapporteur and other members of the EMA before the EMA makes its determination. The final opinion of the EMA is generally given within 210 days of the submission of an application, but the EMA makes the final decision on the approval of products. In general, the requirements for regulatory approval of a veterinary medicinal product in the EU are similar to those in the United States, requiring demonstrated evidence of purity, safety, efficacy and consistency of manufacturing processes.
Rest of World
Other countries around the world have their own regulatory requirements for approving and marketing veterinary medicinal products. In Japan, before granting marketing authorization, the Ministry of Agriculture, Forestry and Fisheries examines each veterinary medicinal product by categories including name, ingredient, composition, manufacturing methods, administration and dose, indications or effects and adverse reactions on the basis of data submitted by the applicant. In Australia, the Australian Pesticides and Veterinary Medicines Authority is the government authority for the registration of all agricultural and veterinary products, and it assesses applications from manufacturers of veterinary pharmaceuticals and related products.
Many country-specific laws and regulations include requirements for labeling, safety, efficacy and manufacturers’ quality control procedures designed to assure the consistency of the products, as well as company records and reports. With the exception of those countries that have well-developed veterinary product regulation, the regulatory agencies of many other countries typically refer to the regulatory authorities in the United States and the EU and other international animal health entities, including the World Organisation for Animal Health and the Codex Alimentarius Commission, in establishing standards and regulations for veterinary pharmaceuticals and vaccines.
Employees
As of June 30, 2015, we had 28 full-time employees. Of these employees, 18 were in research and development, including clinical and regulatory functions, and ten were in general and administrative functions. We have never had a work stoppage and our employees are not represented by labor unions or covered by collective bargaining agreements.
Information about Segments and Geographic Revenue
Information about segments and geographic revenue is set forth in Note 2 of the Notes to Consolidated Financial Statements under Item 8 of this Report. In addition, financial information regarding our operations, assets and liabilities, including our total revenue and net loss for the years ended June 30, 2015 and 2014 and our total assets as of June 30, 2015 and 2014, is included in our Consolidated Financial Statements under Item 8 of this Report.
18
Nexvet Australia Pty Ltd, formerly known as Nexvet Biopharma Pty Ltd, or Nexvet Australia, was incorporated in Australia in February 2010. In September 2014, Nexvet Biopharma Limited, a newly-formed Irish private company, became the parent company of Nexvet Australia and its subsidiaries pursuant to a transaction in which all of the holders of ordinary shares, preference shares, restricted share units and options and warrants to purchase ordinary shares of Nexvet Australia exchanged their holdings for equivalent ordinary shares, preference shares, restricted share units or options or warrants to purchase ordinary shares, as applicable, of Nexvet Biopharma Limited. We refer to this transaction as the “Irish Exchange.” Nexvet Biopharma Limited then re-registered as an Irish public limited company in September 2014. We refer to this re-registration as the “Re-registration” and we refer to the Irish Exchange and the Re-registration collectively as the “Irish Reorganization.” Nexvet Biopharma plc became the parent company of Nexvet Australia pursuant to the Irish Reorganization, and for financial reporting purposes the historical consolidated financial statements of Nexvet Australia became the historical consolidated financial statements of Nexvet Biopharma plc and its subsidiaries as a continuation of the predecessor.
Our principal executive offices are located at National Institute for Bioprocessing Research and Training, or NIBRT, Foster’s Avenue, Mount Merrion, Blackrock, Co. Dublin, Ireland, and our telephone number is +353 1 901 0339. Our internet address is www.nexvet.com. Information contained on, or accessible through, our website is not a part of, and is not incorporated into, this Report and references to our website in this Report are inactive textual references only.
You should carefully consider the risks described below, as well as the other information in this Report, including our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our ordinary shares could decline, and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may have similar adverse effects on us.
Risks Related to Our Business
We have a limited operating history, are not profitable and may never become profitable.
We are a clinical-stage biopharmaceutical company focused on the therapeutics segment of the companion animal market, with a limited operating history. Since our formation in February 2010, our operations have been limited to the identification of product candidates and to the research and development of our lead product candidates, which primarily target pain associated with osteoarthritis in dogs (NV‑01), pain associated with degenerative joint disease, including osteoarthritis, in cats (NV‑02) and chronic inflammatory diseases, including atopic dermatitis, in dogs (NV‑08). As a result, we have limited historical operations upon which you can evaluate our business and prospects, and we have not yet demonstrated an ability to successfully overcome the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in companion animal therapeutics. We do not have any products approved for sale, have not generated any revenue from product sales since our inception and do not expect to generate any revenue from product sales in the near future. We have incurred significant net losses since our inception. We incurred net losses of $11.9 million, $6.7 million, and $3.2 million for fiscal years 2015, 2014 and 2013, respectively, and as of June 30, 2015, we had an accumulated deficit of $23.7 million. This accumulated deficit has resulted principally from costs incurred in connection with research and development of our product candidates and general and administrative costs associated with our operations.
We expect to continue to incur net losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates and begin commercialization activities in anticipation of regulatory approval. We will not be able to commercialize any product candidates until we successfully complete the required studies and they are approved by applicable regulatory authorities. In the United States, these authorities include the Center for Veterinary Medicine within the FDA and the Center for Veterinary Biologics within the Animal and Plant Health Inspection Service within the U.S. Department of Agriculture, or the USDA. In Europe, these authorities include the European Medicines Agency, or the EMA. We refer to the FDA, the USDA and the EMA collectively as the “Regulatory Authorities.” Even if we succeed in developing and commercializing one or more product candidates, we expect to continue to incur net losses for the foreseeable future, and we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. If we fail to achieve or maintain profitability, it would adversely affect the value of our ordinary shares.
19
We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development, product portfolio expansion, other operations or commercialization efforts.
Completing the development and obtaining regulatory approval of our product candidates will require substantial funds. We believe our cash balance of $52.0 million as of June 30, 2015 will be sufficient to fund our anticipated level of operations for at least the next 12 months. Our operating plan may change as a result of many factors currently unknown to us, and we may seek additional funds sooner than planned through public or private equity or debt financings or other sources such as strategic collaborations. We have no current agreements or arrangements with respect to any such financings or collaborations, and any such financings or collaborations may result in dilution to our shareholders, the imposition of debt covenants and repayment obligations or other restrictions that may adversely affect our business or the value of our ordinary shares. Even if we believe we have sufficient funds on hand for our current or planned future business and operations, we may seek from time to time to raise additional capital based upon favorable market conditions or strategic considerations such as potential acquisitions.
Since our inception, nearly all of our resources have been dedicated to the research and development of our lead product candidates. We believe that we will continue to expend substantial resources for the foreseeable future for the development of our lead product candidates and any future product candidates we may choose to pursue. Because the outcome of our development activities is uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development of any of our current or future product candidates and, if approved, successfully commercialize them.
Our future capital requirements will depend on many factors, including:
|
|
· |
the scope, progress, results and costs of researching and developing our current or future product candidates, including conducting proof-of-concept, pilot and pivotal safety and efficacy studies; |
|
|
· |
the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future product candidates; |
|
|
· |
the number and characteristics of the product candidates we pursue; |
|
|
· |
whether we acquire or license any other companies, assets, intellectual property or technologies in the future; |
|
|
· |
the cost of commercialization activities, if any of our current or future product candidates are approved for sale, including marketing, sales and distribution costs; |
|
|
· |
the cost of manufacturing our current and future product candidates and any approved products we successfully commercialize; |
|
|
· |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
|
|
· |
the expenses needed to attract and retain skilled personnel; |
|
|
· |
the costs associated with being a public company; and |
|
|
· |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, if any arise, including litigation costs and the outcome of such litigation. |
In addition, we may also have unanticipated costs. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate one or more of our product development, product portfolio expansion, other operations or commercialization efforts.
Our success depends largely upon our ability to advance our product candidates through the various stages of development, especially through pivotal safety and efficacy studies. If we are unable to successfully advance or develop our product candidates, our business will be harmed.
We currently have no products approved for commercial distribution and are focused primarily on the development of our lead product candidates. None of our product candidates have completed pivotal safety and efficacy studies and their commercial viability will depend on the success of these studies, receipt of regulatory approvals and the viability of manufacturing processes. For our first lead product candidate, NV‑01, we commenced our pivotal safety and efficacy study in October 2014, and commenced an additional pilot field safety and efficacy study in April 2015. We expect data from both these studies by the end of 2015. For our second lead product candidate, NV‑02, we completed our final proof-of-concept study, for efficacy, in mid-2015. We anticipate results from a pilot field safety and efficacy study of NV‑02 in the first quarter of 2016. For NV‑08, we expect to complete a proof-of-concept study, for efficacy, by the end of the first quarter of 2016.
20
The success of our business ultimately depends upon our ability to advance the development of our product candidates from proof-of-concept studies through pilot and pivotal safety and efficacy studies in a manner that meets extensive regulatory requirements, establish facilities capable of consistently manufacturing them in accordance with strict specifications and regulations, obtain approval for their sale by the Regulatory Authorities, and ultimately have our product candidates successfully commercialized by us or a strategic partner or licensee. The results of our ongoing research and development activities, including pivotal safety and efficacy studies, may not support or justify the continued development of our product candidates, and we may not receive approval from the Regulatory Authorities to advance the development of our product candidates. Failure to advance the development of one or more of our product candidates may have an adverse effect on our business.
Our product candidates must satisfy regulatory standards of purity, safety, potency and efficacy, and standards related to manufacturing, before we can advance or complete their development or they can be approved for sale. To satisfy these standards, we must engage in expensive and sometimes lengthy proof-of-concept, pilot and pivotal safety and efficacy studies and develop acceptable and cost-effective manufacturing processes. Despite these efforts, our product candidates may not:
|
|
· |
offer therapeutic or other medical benefits over existing drugs or other product candidates in development to treat the same animal population; |
|
|
· |
be proven to be safe and effective in pivotal safety and efficacy studies; |
|
|
· |
have the desired effects; |
|
|
· |
be free from undesirable or unexpected effects; |
|
|
· |
meet applicable regulatory standards (for example, be shown to be pure, safe, potent and effective); |
|
|
· |
be capable of being formulated and manufactured in commercially suitable quantities and at an acceptable cost; or |
|
|
· |
be successfully commercialized by us or by our collaborators. |
Even if we demonstrate favorable results in proof-of-concept or pilot studies for our lead product candidates, the results of pivotal safety and efficacy studies may not be sufficient to support the continued development of these product candidates. The development of companion animal therapeutics can be subject to significant delays, setbacks and failures in all stages of development, including pivotal safety and efficacy studies, even after achieving promising results in proof-of-concept studies.
Accordingly, results from completed proof-of-concept or pilot studies of our product candidates may not be predictive of the results we may obtain in pivotal safety and efficacy studies. Furthermore, even if the data collected from proof-of-concept studies, pilot and pivotal safety and efficacy studies involving any of our product candidates demonstrate a satisfactory safety and efficacy profile, such results may not be sufficient to obtain regulatory approval from the Regulatory Authorities, which is required to market and sell a product.
The discovery, development and manufacturing of biologics involves relatively novel technologies and an expensive and lengthy process with uncertain outcomes.
Many biologics have been approved for use in humans, but apart from vaccines, relatively few recombinant proteins or antibodies have been approved for use in animals. There are unique risks and uncertainties associated with biologics, the discovery, development and manufacturing of which are subject to regulations that are complex and extensive. In addition, we develop biologics for companion animals using our proprietary PETization platform, which is a new platform that has not resulted in any commercialized products. Identification, optimization and manufacturing of biologics, including companion animal therapeutics, is a relatively new field in which unanticipated difficulties or challenges could arise. For example, there are no established practices or regulatory standards for pre-launch batch size manufacturing scale-up of a companion animal biological product candidate to commercial levels, which may cause our estimated manufacturing costs to materially increase due to unforeseen requirements from regulators or our third party manufacturer. Success in preliminary safety, proof-of-concept, pilot, field and prior target animal studies or pivotal studies, or success in studies of products similar to our product candidates but conducted in humans, does not ensure that our target animal studies or pivotal studies will be successful, and the results of development efforts by other parties may not be indicative of the results of our target animal studies, pivotal studies and other development efforts. We may be unable to identify biologics suitable for development or to achieve the potency and stability required for use in target animals. In particular, canine, feline and equine antibodies represent novel types of product candidates that may be difficult to identify through PETization or develop successfully.
21
Development of biologics, including companion animal therapeutics, is expensive and can take many years to complete, its outcome is inherently uncertain, and our development activities may not be successful. To gain approval to market an animal therapeutic for a particular species of animal, we must incur substantial expense for, and devote significant time to, pivotal safety and efficacy studies and provide the Regulatory Authorities with data that adequately demonstrate the safety and efficacy of that product in the target animal for the intended indication applied for in the new animal drug application, or NADA, product license or other regulatory filing. We rely on contract research organizations, or CROs, and other third parties to ensure the proper and timely conduct of our studies and development efforts, but we have limited influence over their actual performance. Pivotal safety and efficacy studies require adequate supplies of material and sufficient target animal enrollment. Delays in target animal enrollment can result in increased costs and longer development times that threaten the ability to complete the study. Pivotal safety and efficacy studies can be delayed or discontinued for a variety of reasons, including delays in or failure to:
|
|
· |
reach agreement on acceptable terms with study sites, which can be subject to extensive negotiation and may vary significantly among different sites; |
|
|
· |
complete pivotal safety and efficacy studies due to deviations from study protocol or the occurrence of adverse events; |
|
|
· |
address any safety concerns that arise during the course of testing; |
|
|
· |
address any conflicts with new or existing laws or regulations; |
|
|
· |
add new study sites; or |
|
|
· |
manufacture sufficient quantities of formulated biologics of adequate quality for use in studies. |
Even if we successfully complete pivotal safety and efficacy studies for our product candidates, we might not file the required regulatory submissions in a timely manner and may not receive regulatory approval for the product candidate. As a result, our product candidates may not successfully progress further through the drug development process or result in a commercially viable product.
In addition, manufacturing biologics, especially in large quantities, is complex and may require the use of technologies that we may need to develop ourselves or in conjunction with third-party collaborators. Small changes in the manufacturing process can have a significant impact on product quality, consistency and yield. Such manufacturing requires facilities specifically designed and validated for this purpose and sophisticated quality assurance and quality control procedures. Biologics are also usually costly to manufacture. Manufacturing biologics may be more technically challenging, time-consuming and expensive than we anticipate or subject to other regulatory uncertainties. For example, human nerve growth factor inhibitor product candidates were previously subject to classwide partial clinical holds imposed by the FDA in recent years due to the emergence of safety signals in humans, including rapidly progressive osteoarthritis, as a result of various factors including co-administration with non-steroidal, anti-inflammatory drugs. Classwide adverse effects may be seen in companion animals with the PETized product candidates we seek to manufacture and result in clinical holds or protocol revisions or otherwise cause delays or increased costs. We may be unable to manufacture biologics at full commercial scale and at an economical cost, if at all.
The results of our proof-of-concept or pilot studies for our product candidates may not be predictive of the results in any future pivotal safety and efficacy studies, and we may not be able to obtain any regulatory approvals.
The results of our proof-of-concept or pilot studies, other initial development activities and any previous studies in animals or conducted in humans may not be predictive of future results of our pivotal safety and efficacy studies. Proof-of-concept studies involve relatively small numbers of animals compared to pivotal studies. For example, our proof-of-concept study completed for the efficacy of NV‑01 involved 26 dogs, while our pivotal safety and efficacy study being conducted for NV‑01 has enrolled at least 250 dogs. In March 2015, we received the results of a sample size reassessment for this study, which indicated that, in order to have a high probability of a statistically significant endpoint at the day 28 primary assessment, it would be necessary to increase the current size of the study. Following this sample size reassessment, we assessed the impact of time, cost and related logistical issues regarding the feasibility of substantially increasing the number of dogs in the current study, or initiating additional studies. This assessment included consulting with both the United States Food and Drug Administration’s Center for Veterinary Medicine and Virbac S.A., our sole and exclusive distributor of NV‑01 and any other products for which we enter into a specific distribution agreement with Virbac in the veterinary field worldwide except for the United States and Canada. We concluded that we should continue the current study to completion without a change in study size, while also commencing a new placebo-controlled multi-site pilot field safety and efficacy study to assess various doses and dosing regimens of NV‑01. This new study is expected to enroll approximately 150 dogs and to cost approximately $1.0 million, although it could result in additional costs. We expect this study to be completed in the fourth quarter of 2015, but unforeseen circumstances may delay its completion. While the purpose of this new study is to obtain information that may assist the design of a future, additional pivotal study for NV‑01, if needed, it may not result in our obtaining such information. We may not receive results in any NV‑01 pivotal safety and efficacy study that are sufficient to obtain regulatory approval.
22
Failure can occur at any time during the conduct of our studies and other development activities. Even if our pivotal safety and efficacy studies and other development activities are completed as planned, they may not yield adequate results or be sufficient to obtain regulatory approval for any of our product candidates. Even if we obtain regulatory approval, we may not be able to successfully commercialize any particular product candidate. Because our product candidates are developed for a particular species, our ability to leverage our experience from the development of our lead product candidates into product candidates for other species will be limited.
We may be unable to obtain regulatory approval for our existing or future product candidates under applicable regulatory requirements. The denial or delay of any such approval would delay commercialization efforts and adversely impact our potential to generate revenue, our business and our results of operations.
Our product candidates are in various stages of development, and our business currently depends entirely on their successful development, regulatory approval and commercialization. We currently have no products approved for sale, and we may never obtain regulatory approval to commercialize any of our current or future product candidates. The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of animal therapeutic products are subject to extensive regulation by the FDA (which regulates the manufacturing and distribution of animal drugs and pharmaceuticals), the USDA (which regulates the manufacturing and distribution of veterinary biological products to prevent, diagnose, and treat animal diseases), the EMA and other regulatory authorities, and regulations differ for each Regulatory Authority and from country to country.
Any delay or failure in obtaining applicable regulatory approval from any Regulatory Authority for the intended indications of our product candidates would delay or prevent commercialization of such product candidates and would adversely impact our business and prospects. Even if an approved product reaches market, circumstances could result in the need to withdraw a product from the market.
In order to market any product outside of the United States, including in the European Economic Area, or EEA (which is composed of 27 of the 28 member states of the EU, plus Norway, Iceland and Liechtenstein), and many other foreign jurisdictions, separate regulatory approvals are required. For example, in the EEA, companion animal therapeutics can only be commercialized after obtaining a marketing authorization. Before granting a marketing authorization, the EMA or the competent national authorities of the member states of the EEA assess the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
The approval procedures vary among countries and can involve additional studies and testing, and the time required to obtain approval may differ from that required to obtain approval from the Regulatory Authorities. Animal studies conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA or the USDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or in the United States. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining approval in the United States. We may not be able to file for regulatory approvals or to do so on a timely basis and, even if we do file them, we may not receive necessary approvals to commercialize our lead product candidates in any market.
If any of our product candidates are approved but do not gain meaningful market acceptance, we are not likely to generate significant revenue.
Even if we obtain approvals from the Regulatory Authorities, our current or future product candidates may not achieve market acceptance among veterinarians and animal owners, and may not be commercially successful. Market acceptance of any of our current or future product candidates for which we receive approval depends on a number of factors, including:
|
|
· |
the safety of our lead product candidates and the prevalence and severity of adverse side effects as demonstrated in our pivotal safety and efficacy studies; |
|
|
· |
the indications for which our lead product candidates are approved; |
|
|
· |
the acceptance by veterinarians and animal owners of the product as a safe and effective treatment; |
|
|
· |
the proper training regarding, and administration of, our lead product candidates by veterinarians; |
|
|
· |
the relative convenience and ease of administration of our lead product candidates; |
|
|
· |
the potential and perceived advantages of our product candidates over alternative treatments; |
|
|
· |
the cost of treatment in relation to alternative treatments and willingness to pay for our lead product candidates, if approved, on the part of veterinarians and animal owners; |
23
|
|
· |
the willingness of animal owners to pay for our lead product candidates, relative to other discretionary items, especially during economically challenging times; |
|
|
· |
the effectiveness of our sales and marketing efforts and those of our collaborators and distributors; and |
|
|
· |
the willingness of veterinarians to prescribe or administer our lead product candidates to the intended target animal population. |
The market for therapeutic veterinary biologics such as our product candidates is very limited at present and will require education and outreach efforts to establish a therapeutic biologics market among veterinarian practices. If our lead product candidates do not achieve meaningful market acceptance, or if the market for our lead product candidates proves to be smaller than anticipated, we may never generate significant revenue.
The commercial potential of a product candidate in development is difficult to predict. The market for our product candidates is uncertain and may be smaller than we anticipate, which could significantly and negatively impact our revenue, results of operations and financial condition.
It is difficult to estimate the commercial potential of any of our product candidates because of the emerging nature of the companion animal therapeutics segment of the veterinary care industry. This segment continues to evolve, and it is difficult to predict the market potential for what we believe to be the unmet medical needs of companion animals. This market potential will depend on important factors such as safety and efficacy compared to other available treatments, changing standards of care, preferences of veterinarians, the willingness of animal owners to pay for such products, and the availability of competitive alternatives that may emerge either during the product development process or after commercial introduction. In addition, our efforts to influence veterinarian preferences by educating them about the benefits of biologics compared to currently available treatments may not be successful. If the demand for our product candidates is less than we anticipate due to one or more of these or other factors, it could negatively impact our business, financial condition and results of operations. Further, the willingness of animal owners to pay for our product candidates, if approved, may be less than we anticipate and may be negatively affected by overall economic conditions.
Our product candidates, if approved, will face competition, and our failure to effectively compete may prevent us from achieving significant market penetration.
The companion animal therapeutics segment of the veterinary care industry is highly competitive and characterized by rapid technological change. Key competitive factors in our segment include, among others, the ability to successfully advance the development of a product candidate through pivotal safety and efficacy studies, the timing and scope of regulatory approvals, if ever achieved, average selling price of competing products and animal therapeutic products in general, the availability of raw materials, contract manufacturing and manufacturing capacity, manufacturing costs, establishing and maintaining intellectual property and patent rights and their protection, and sales and marketing capabilities.
We believe our main competitors are animal health companies that are developing products for use in companion animals, such as Aratana Therapeutics, Inc., Kindred Biosciences, Inc. and Zoetis, Inc. In addition, there are a number of large biopharmaceutical companies with animal health divisions, such as Bayer AG; Boehringer Ingelheim GmbH; Eli Lilly and Company (Elanco division); Merck & Co., Inc.; and Sanofi S.A. (Merial division). If approved, we expect NV‑01 and NV‑02 will face competition from Deramaxx, marketed by Elanco; Metacam, marketed by Boehringer Ingelheim; Previcox, marketed by Merial; and Rimadyl, marketed by Zoetis, as well as from generic Meloxicam and Carprofen and other pain-treating products. We believe that Aratana and Kindred are developing, and that other companies may develop, similar products as well. In addition, private-label products may compete with our lead product candidates. If companion animal therapeutic customers increase their use of new or existing private-label products, our operating results and financial condition could be adversely affected.
We are a clinical-stage biopharmaceutical company with a limited operating history and many of our competitors have substantially more resources than we do, including financial, technical and sales resources. In addition, many of our competitors have more experience than we have in the development, manufacture, regulation and worldwide commercialization of companion animal therapeutics. We are also competing with academic institutions, governmental agencies and private organizations that are conducting research in the field of companion animal therapeutics. Our competition will be determined in part by the potential indications for which our lead product candidates are developed and ultimately approved by the Regulatory Authorities. Additionally, the timing of market introduction of some of our future products or of competitors’ products may be an important competitive factor. Accordingly, we expect the speed with which we can develop our product candidates, complete pivotal safety and efficacy studies and approval processes, and supply commercial quantities to market to be important competitive factors.
24
If we are unable to attract or retain key employees, advisors or consultants, we may be unable to successfully develop our product candidates in a timely manner, if at all, or otherwise manage our business effectively.
As of June 30 2015, we had 28 full-time employees, and we anticipate the need to increase the size of our organization, which will require us to hire, train, retain, manage and motivate current and additional employees, consultants or advisors with experience in a number of disciplines, including research and development, sales, finance, and manufacturing. Our success depends in part on our ability to attract and retain highly qualified key management, personnel and directors to develop, implement and execute our business strategy and operations, and oversee the activities of our consultants and vendors, as well as academic and corporate advisors or consultants that assist us in this regard. We are currently highly dependent upon the efforts of our management team to accomplish this, specifically including Mark Heffernan, our co-founder and Chief Executive Officer, David Gearing, our co-founder and Chief Scientific Officer, and Damian Lismore, our Chief Financial Officer. We do not have any “key man” insurance.
Although we have successfully attracted and retained key personnel in the past, we may not be able to continue to do so in the future on acceptable terms, if at all. In addition, competition for qualified personnel in the companion animal therapeutics segment of the veterinary care industry is intense, because there are a limited number of individuals who are trained or experienced in the field. We may also face difficulty in expanding and enhancing our operational, financial and management systems as we grow. If we lose any key employees, or are unable to attract or retain qualified key personnel, directors, advisors or consultants, the development of our product candidates could be significantly delayed or discontinued.
We rely on third-party manufacturers for supplies of our lead product candidates, and we intend to rely on third-party manufacturers for commercial quantities of any of our product candidates that may be approved.
We currently have no significant internal capability to manufacture the formulated product candidates for use in our registrational studies or commercial supplies of any of our product candidates that may be approved, and we are primarily dependent upon third-party manufacturers for such supplies. We currently have a relationship with only one contract manufacturer, Fujifilm, for the potential manufacturing of our lead product candidates for clinical testing purposes and commercial supply of drug substance. Fujifilm may terminate the agreements we have entered into with them if the services cannot be completed due to technical or scientific reasons, subject to certain conditions. If Fujifilm does not perform as agreed or terminates our agreements, we may be required to replace them as our manufacturer, and we may be unable to do so on a timely basis, on similar terms or at all.
We also expect to rely upon third parties to produce materials required for the commercial production of our product candidates if we succeed in obtaining the necessary regulatory approvals. We may be unable to identify and reach agreement with a third-party manufacturer for our product candidates in a timely manner on commercially reasonable terms, or at all. Any delay in our ability to identify and contract with these third-party manufacturers on commercially reasonable terms, or at all, would have an adverse impact upon our current product development activities and future commercialization efforts.
The facilities used by Fujifilm or other third-party manufacturers we engage to manufacture the active pharmaceutical ingredients and formulated biologics will be subject to inspections by the Regulatory Authorities that will be conducted after we submit our NADA to and obtain approval by the FDA, or during a potential USDA licensing process for product candidates or the EMA approval process. Among other things, these inspections may consider whether Fujifilm or other third-party manufacturers are following strict procedures associated with pharmaceutical manufacturing operations. Our third-party manufacturers are also expected by us and by other companies for which they manufacture supplies to produce such supplies in conformity to specifications and regulatory requirements and to maintain quality control and quality assurance practices and not to employ disqualified personnel. If our third-party manufacturers’ facilities or quality control and quality assurance practices do not comply with regulatory requirements or our expectations, we may need to find alternative manufacturing facilities, which would adversely impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved. For example, the Brazilian regulatory authority (ANVISA) imposes certain requirements regarding segregation of human and veterinary manufacturing areas. In light of these requirements, we were unable to agree on acceptable terms for commercial supply with our prior manufacturer. Minor deviations in our manufacturing processes, such as temperature excursions or improper package sealing, could result in delays, inventory shortages, unanticipated costs, product recalls, product liability or regulatory action. In addition, a number of factors could cause production interruptions, including:
|
|
· |
equipment malfunctions; |
|
|
· |
shortages of materials; |
|
|
· |
changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, shipping distributions or physical limitations; |
|
|
· |
labor problems; |
|
|
· |
natural disasters or power outages; |
25
|
|
· |
the outbreak of any highly contagious diseases near our production sites. |
These interruptions could result in launch delays, inventory shortages, recalls, unanticipated costs or otherwise adversely affect our operating results.
In accordance with good manufacturing practice, changing manufacturers may require the re-validation of manufacturing processes and procedures and may require further studies to show comparability between the materials produced by different manufacturers. Changing our current or future third-party manufacturers may be difficult, if possible for us, and could be costly, which could result in our inability to manufacture our product candidates for an extended period of time and therefore a delay in the development or marketing of our product candidates. Further, in order to maintain our development timelines in the event of a change in our third-party manufacturer, we may incur higher costs to manufacture our product candidates.
We depend on third-party suppliers for key raw materials to be used in our manufacturing processes, and the loss of these third party suppliers on their inability to supply us with adequate raw materials could harm our business.
We currently rely on third-party suppliers for the raw materials required for the production of our product candidates. Our dependence on these third-party suppliers and the challenges we may face in obtaining adequate supplies of raw materials involve several risks, including limited control over pricing, availability, quality and delivery schedules. Our suppliers may not provide us with quantities of these raw materials that we require or satisfy our anticipated specifications and quality requirements. Any supply interruption in limited or sole-sourced raw materials could materially harm our ability to manufacture our product candidates until a new source of supply, if any, could be identified and qualified. Although we believe there are currently several other suppliers of these raw materials, we may be unable to find a sufficient alternative supply channel in a reasonable time or on commercially reasonable terms. Any performance failure on the part of our suppliers could delay the development and potential commercialization of our product candidates, including limiting supplies necessary for clinical trials and regulatory approvals, which would harm our business.
Our business involves environmental, health and safety risks.
Our business involves the controlled use of hazardous materials and chemicals and is subject to numerous environmental, health and safety laws and regulations and to periodic inspections for possible violations of these laws and regulations. Under certain of those laws and regulations, we could be liable for any contamination at our properties or third party waste disposal sites. In addition to significant remediation costs, contamination can give rise to third party claims for fines, penalties, natural resource damages, personal injury and damage (including property damage). The costs of compliance with environmental, health and safety laws and regulations are significant. Any violations, even if inadvertent or accidental, of current or future environmental, health or safety laws or regulations, the cost of compliance with any resulting order or find and any liability imposed in connection with any contamination for which we may be responsible could adversely affect our business, financial condition, cash flows and results of operations.
We currently rely on third parties to conduct all of our pivotal safety and efficacy studies and certain other development efforts. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize our current or future product candidates.
We currently do not conduct our pivotal safety and efficacy studies, and we rely on CROs to conduct these studies. The third parties with whom we contract for the execution of our studies play a significant role in the conduct of these studies and the subsequent collection and analysis of data. However, these third parties are not our employees, and except for contractual duties and obligations, we have limited ability to control the amount or timing of resources that they devote to our programs. Although we rely on these third parties to conduct our studies, we remain responsible for ensuring that each of our studies is conducted in accordance with the development plan and protocol. Moreover, the Regulatory Authorities require us to comply with good laboratory practices and good clinical practices for conducting, monitoring, recording and reporting the results of our studies to ensure that the data and results are scientifically credible and accurate.
26
In addition, the execution of pivotal safety and efficacy studies and the subsequent compilation and analysis of the data produced requires coordination among various parties. In order for these functions to be carried out effectively and efficiently, it is imperative that these parties communicate and coordinate with one another. Moreover, these third parties may also have relationships with other commercial entities, some of which may compete with us. Many of our agreements with these third parties may be terminated by these third parties upon as little as 30 days’ prior written notice of a material breach by us that is not cured within 30 days. Many of these agreements may also be terminated by such third parties under certain other circumstances, including our insolvency or our failure to comply with applicable laws. In general, these agreements require such third parties to reasonably cooperate with us at our expense for an orderly winding down of services of such third parties under the agreements. If the third parties conducting our pivotal safety and efficacy studies do not perform their contractual duties or obligations, experience work stoppages, do not meet expected deadlines, terminate their agreements with us or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our development protocols or good clinical practices, or for any other reason, we may need to enter into new arrangements with alternative third parties, which could be difficult and costly, and our pivotal safety and efficacy studies may be extended, delayed or terminated or may need to be repeated. If any of the foregoing were to occur, the regulatory approval for and commercialization of the product candidate being tested in such studies may be delayed or require us to utilize additional resources.
If we do not establish strategic collaborations, we may not be able to bring our products to market and generate revenue.
Our drug research and development programs and potential commercialization of our product candidates will require substantial additional cash to fund expenses. Our strategy includes potentially collaborating with other pharmaceutical and biotechnology companies to assist us in furthering development and potential commercialization of some of our product candidates, in some or all geographies. It may be difficult to enter into one or more of such collaborations in the future. We face significant competition in seeking appropriate collaborators and these collaborations are complex and time-consuming to negotiate and document. We may not be able to negotiate collaborations on acceptable terms, or at all, in which case we may have to curtail the development of a particular drug candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we will need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to bring our product candidates to market and generate product revenue.
We currently have no direct sales organization. If we are unable to establish sales capabilities on our own, or maintain or establish new distribution relationships with third parties, we may not be able to market and sell our current or future product candidates, if approved, or generate product revenue.
We currently do not have a direct sales organization. In order to commercialize any of our current or future product candidates, we must build our marketing, sales, distribution, managerial and other non-technical capabilities or make or maintain arrangements with third parties to perform these services, and we may not be successful in doing so. We expect to establish a small direct sales organization in the United States and to utilize distributors, which may be expensive and time-consuming. In jurisdictions outside of the United States, we intend to utilize companies with an established commercial presence to market our lead product candidates in those jurisdictions, but we may be unable to enter into or maintain such arrangements on acceptable terms, if at all.
We entered into a master collaboration, supply and distribution agreement, and a specific distribution agreement for NV‑01, with Virbac S.A., or Virbac, an animal health pharmaceutical company, in November 2014. Pursuant to these agreements, we appointed Virbac as our sole and exclusive distributor of NV‑01 and any other products for which we enter into a specific distribution agreement with Virbac in the veterinary field worldwide except for the United States and Canada (and for NV-08, also except for Japan, South Korea, Taiwan, the Philippines, Malaysia, Singapore, the People’s Republic of China, Indonesia, Thailand, India, Vietnam and Myanmar, because of pre-existing distribution rights). Virbac must provide clinical, regulatory, marketing and sales input via a joint steering committee, advise us in the drafting of regulatory submissions in the countries within the applicable territory, sell our products, meet or exceed minimum annual net sales obligations for each product, manage local complaints and transfer drug safety data, not apply for the registration of any trademark that is the same as or similar to any of our trademarks and launch the product within a specified time period after marketing authorization. Virbac may terminate these agreements if, among other things, we do not timely obtain a marketing authorization for NV‑01 in Europe, there is a material failure to comply with the target product profiles for NV‑01, there is a material deviation from a development plan for NV‑01 or the distribution of all products covered by these agreements is prevented in certain important countries specified in these agreements as a result of a potential infringement of a third party’s intellectual property rights. If Virbac does not perform as agreed or terminates our agreements, we may be required to replace Virbac, and we may be unable to do so on a timely basis, on similar terms or at all.
27
We have no prior experience in the marketing, sale and distribution of companion animal therapeutics, and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and motivate qualified individuals, generate sufficient sales leads, and effectively manage a sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. If we are not successful in commercializing any of our current or future product candidates, either on our own or through one or more distributors, we may never generate significant revenue and may continue to incur significant losses, which would adversely affect our financial condition and results of operations.
Consolidation of our customers could negatively affect the pricing of any of our approved products.
Veterinarians will be our primary customers for any approved products. In recent years, there has been a trend towards the consolidation of veterinary clinics and animal hospitals. If this trend continues, these large clinics and hospitals could attempt to leverage their buying power to obtain favorable pricing from us or standardize the products they offer within their clinics or hospitals. Any resulting decrease in prices could have an adverse effect on our operating results and financial condition.
Our revenue, expenses and results of operations may be subject to significant fluctuations, which will make it difficult to compare our operating results from period to period.
We expect that our operating results will vary significantly from quarter-to-quarter and year-to-year as a result of the initiation and success or failure of proof-of-concept, pilot or pivotal safety and efficacy studies, the timing of the formulation and manufacture of our product candidates or other development related factors. In addition, we recognized research and development incentive income of $3.5 million, $2.3 million and $1.1 million, respectively, for fiscal years 2015, 2014 and 2013 under the AusIndustry research and development tax incentive program. This incentive, which constituted our largest source of income in fiscal years 2015, 2014 and 2013, may not continue in future years if we no longer meet the eligibility requirements for the tax incentive or if the program is modified or terminated. Accordingly, our revenue, expense and results of operations for any period may not be comparable to the revenue, expense or results of operations for any other period.
Risks Related to Intellectual Property
Our commercial success will depend, in part, on obtaining and maintaining patent protection for our lead product candidates.
We currently have four granted patents (all in New Zealand) but do not have any other issued patents for our lead product candidates. However, we have filed patent applications covering various aspects of our lead product candidates. Our patent applications may never result in the issuance of patents, or patents issued to us may be dominated by the patents of third parties, including, for example, patents issued to analogous human drug, drug targets or biological compositions and their usages. Furthermore, even if they are unchallenged by third parties, our patents, if issued, may not adequately protect our intellectual property or prevent others from designing around their claims. In order to commercialize our lead product candidates in one or more species, we could be required to enter into third-party licenses or, if a license is not available on terms that we consider reasonable, we could be required to cease development or commercialization of one or more of our lead product candidates. Thus, if we cannot obtain ownership of issued patents covering our lead product candidates, our business and prospects would be adversely affected.
It is possible that no patents will issue to us that cover our lead product candidates, or that we will have little to no commercial protection against competing products. In such cases, we would then rely solely on other forms of exclusivity, such as regulatory exclusivity provided by the Federal Food, Drug and Cosmetic Act, if available, which may provide less protection to our competitive position.
Our commercial success depends upon our ability to develop, manufacture, market and sell our lead product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. In the future, it may become necessary for us to use the patented or proprietary technology of a third party to commercialize our own technology or products, in which case we would be required to obtain a license from such third party. A third party may hold intellectual property, including patent rights, that are important or necessary to the development of our lead product candidates. A license to such intellectual property may not be available on commercially reasonable terms, if at all. If we obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies. Should we obtain a license, circumstances may arise where we are not able meet our obligations under the license, which could result in termination of the license agreement and impair our ability to develop or market our lead product candidates.
28
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any patents that issue. In September 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation, and switch the U.S. patent system from a “first-to-invent” system to a “first-to-file” system. Under a “first-to-file” system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The U.S. Patent and Trademark Office, or the USPTO, recently developed new regulations and procedures regarding aspects of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, in particular the first-to-file provisions, only became effective in March 2013. The full effect of these changes are currently unclear as the USPTO has not yet adopted all pertinent final rules and regulations, and the courts have yet to address all of the new provisions. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any patents that issue, all of which could have an adverse effect on our business and financial condition.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce patents that we might obtain in the future.
If a third party claims we are infringing on its intellectual property rights, we could incur significant expenses, or be prevented from further developing or commercializing our lead product candidates.
Our success will also depend on our ability to operate without infringing the patents and other proprietary intellectual property rights of third parties. This is generally referred to as having the “freedom to operate.” We work with our patent attorneys to review third party patents and patent applications that may affect our freedom to operate, and, when we think necessary, we take action to seek to limit or challenge claims made by patents or patent applications of third parties that may adversely affect our freedom to operate. However, the biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. The defense and prosecution of intellectual property claims, interference proceedings and related legal and administrative proceedings, both in the United States and internationally, involve complex legal and factual questions. As a result, such proceedings are lengthy, costly and time-consuming, and their outcome is highly uncertain. We may become involved in protracted and expensive litigation in order to determine the enforceability, scope and validity of the proprietary rights of others, or to determine whether we have the freedom to operate with respect to the intellectual property rights of others.
Third parties may assert that we are employing their proprietary technology without authorization. For example, as a result of searching patent literature in support of patent protection and otherwise evaluating the patent landscape, we are aware of third party patents or patent applications in territories where we intend to commercialize our anti-nerve growth factor products, which contain granted claims, pending claims or claims for which patent offices have issued a notice of intention to grant with coverage that could potentially be asserted with respect to NV‑01 and NV‑02. While we do not believe such assertions would be valid or enforceable or otherwise materially adversely affect commercialization of NV‑01 and NV‑02, we may be incorrect in this belief. If any third party patent were held by a court of competent jurisdiction to cover aspects of any of our product candidates or any method of use of any of our product candidates, the holder of any such patent may be able to block our ability to develop and commercialize such product candidate unless we obtain a license under the applicable patent or limit or modify the formulation or use, or until such patent expires or is finally determined to be invalid or unenforceable. If a license to a third-party patent is needed, such a license may not be available on commercially reasonable terms or at all. Any successful assertion by a third party that one of our product candidates infringes one or more issued patents could materially affect our commercialization of that product candidate if such product candidate is approved.
29
There may be patents already issued, of which we are unaware, that might be infringed by the commercialization of one or more of our product candidates. Moreover, it is also possible that patents may exist that we are aware of, but that we do not believe are relevant to our current or future product candidates, which could nevertheless be found to block our freedom to market these product candidates. Patent applications are, in most cases, maintained in secrecy until approximately 18 months after the patent application is filed. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Therefore, patent applications relating to product candidates similar to ours may have already been filed by others without our knowledge. In the event that a third party has also filed a patent application covering our lead product candidates or other claims, we may have to participate in an adversarial proceeding, such as an interference or derivation proceeding in the USPTO or similar proceedings in other countries, to determine the priority of invention. In the event an infringement claim is brought against us, we may be required to pay substantial legal fees and other expenses to defend such a claim and, if we are unsuccessful in defending the claim, we may be prevented from pursuing the development and commercialization of a lead product candidate and may be subject to injunctions or awards for damages.
In the future, the USPTO or a foreign patent office may grant patent rights to our lead product candidates or other claims to third parties. Subject to the issuance of these future patents, the claims of which will be unknown until issued, we may need to obtain a license or sublicense to these rights in order to have the appropriate freedom to further develop or commercialize them.
Any required licenses may not be available to us on acceptable terms, if at all. If we need to obtain such licenses or sublicenses, but are unable to do so, we could encounter delays in the development of our lead product candidates, or be prevented from developing, manufacturing and commercializing our lead product candidates at all. If it is determined that we have infringed an issued patent and do not have the freedom to operate, we could be subject to injunctions, and compelled to pay significant damages, including punitive damages. In cases where we have in-licensed intellectual property, our failure to comply with the terms and conditions of such agreements could harm our business.
It is becoming common for third parties to challenge patent claims on any successfully developed product candidate or approved drug. Third-party preissuance submission of prior art to the USPTO, or post-grant opposition, derivation, reexamination, inter partes review or interference proceedings, or other preissuance or post-grant proceedings in the United States or other jurisdictions provoked by third parties may be necessary to determine the priority of inventions with respect to our patent applications or future issued patents. An unfavorable outcome could require us to cease using the related technology and commercializing our lead product candidates, or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all. Even if we obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies. Even if we successfully defend such litigation or proceeding, we may incur substantial costs and it may distract our management and other employees. We could be found liable for monetary damages if we are found to have willfully infringed a patent.
If we or our collaborators become involved in any patent litigation or other legal proceedings, we could incur substantial expense, and the efforts of our technical and management personnel could be significantly diverted. A negative outcome of such litigation or proceedings may expose us to the loss of our proprietary position or to significant liabilities, or require us to seek licenses that may not be available from third parties on commercially acceptable terms, if at all. We may be restricted or prevented from developing, manufacturing and selling our lead product candidates in the event of an adverse determination in a judicial or an administrative proceeding, or if we fail to obtain necessary licenses.
If our efforts to protect the proprietary nature of the intellectual property related to any of our current or future product candidates are not adequate, we may not be able to compete effectively in our market.
We intend to rely upon a combination of patents, trade secret protection, confidentiality and license agreements to protect the intellectual property related to our current product candidates and our development programs. Patents containing composition-of-matter claims on the active ingredients in pharmaceutical products, including companion animal therapeutics, are generally considered to be the strongest form of intellectual property protection, as such claims provide protection without regard to any particular method of use or manufacture. Patents containing claims directed to methods-of-use protect the use of a product for the specified methods. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. While our pending patent families contain pending composition-of-matter and method-of-use claims, our patents, if allowed, may not contain both types of claims.
The strength of patents in the field of companion animal therapeutics involves complex legal and scientific questions and can be uncertain. The patent applications that we own or license may fail to result in issued patents in the United States or in other foreign countries. Even if the patents do successfully issue, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. We also rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or for which we have not filed patent applications, processes for which patents are difficult to enforce and other elements of our product development processes that involve proprietary know-how, information or technology that is not covered by patents.
30
We require all of our employees to assign their inventions to us, and we endeavor to execute confidentiality agreements with all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology. However, we may not have executed such agreements with all parties who may have helped to develop our intellectual property or had access to our proprietary information, and our agreements may be breached.
Our trade secrets and other confidential proprietary information may be disclosed or competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
We may be involved in lawsuits to protect or enforce any future patents issued to us, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe any patents that may issue to us in the future, or the patents of our licensors that are licensed to us. To counter infringement or unauthorized use of any patents we may obtain, we may be required to file infringement claims, which can be expensive and time-consuming to litigate. In addition, if we or one of our future collaborators were to initiate legal proceedings against a third party to enforce a patent covering our current lead product candidates, or one of our future products, the defendant could counterclaim that the patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace grounds for a validity challenge and could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar claims before the USPTO, even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. There may be invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of any future patent protection on our current or future product candidates. Such a loss of patent protection could harm our business.
Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be unsuccessful, it could have an adverse effect on the price of our ordinary shares. Finally, we may not be able to prevent, alone or with the support of our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States.
We have filed trademark applications for our company name in the United States and certain other countries, and we have filed trademark applications for certain proprietary technology in the United States; however, registration is not yet complete for these filings and failure to finally secure these registrations could adversely affect our business.
We have current registered trademarks for our company name (NEXVET) in Australia and New Zealand and a registered trademark for the word PETisation in Australia. We also have registered trademarks for the word PETization (and similar derivations) in Australia and New Zealand. Several trademarks for our company name and the word PETization (and similar derivations) are still going through the application process in the United States, the EU and eight other foreign jurisdictions. These applications may not result in registered trademarks. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. Moreover, any name we propose to use with our lead product candidates in the United States must be approved by the Regulatory Authorities, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names, and have in the past objected (and may in the future object) to applicants’ proposed product names. If the Regulatory Authorities object to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the Regulatory Authorities.
31
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates throughout the world could be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. These products may compete with our lead product candidates in jurisdictions where we do not have any issued or licensed patents and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and may not adequately protect our intellectual property.
We rely on trade secrets to protect our technology, especially where we do not believe patent protection is obtainable, or prior to us filing patent applications on inventions we may make from time to time. However, trade secrets are difficult to protect. In order to protect our proprietary technology and processes, we also rely in part on agreements that contain confidentiality and intellectual property assignment provisions with our corporate and academic partners, employees, consultants, outside scientific collaborators and sponsored researchers and other advisors.
These agreements may not effectively prevent disclosure of confidential information or result in the effective assignment to us of intellectual property and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches of the agreements. In addition, others may independently discover our trade secrets and proprietary information, and in such case we could not assert any trade secret rights against such party. Enforcing a claim that a third party illegally obtained and is using our trade secrets is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Obtaining and maintaining, if obtained, our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO, the European Patent Office and various other foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case, which would have an adverse effect on our business.
Risks Related to Government Regulation
The regulatory approval process is uncertain, requires us to utilize significant resources and may prevent us from obtaining approvals for the commercialization of some or all of our lead product candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of companion animal therapeutics are subject to extensive regulation by the Regulatory Authorities, and other regulatory authorities around the world whose regulations differ from country to country. We are not permitted to market any of our current or future product candidates in the United States until we successfully complete our product development and testing, demonstrate that we meet the standards for regulatory approval and receive approval of a NADA from the FDA, a product license from the USDA or foreign approval, as applicable. We have not submitted an application or received regulatory approval for any of our lead product candidates in any jurisdiction. Obtaining approval of a NADA from the FDA or a product license from the USDA is an uncertain process that requires us to utilize significant resources.
A Regulatory Authority may delay, limit or deny approval of any of our lead product candidates for many reasons, including if:
|
|
· |
our proof-of-concept, pilot or pivotal safety and efficacy studies are found to have failed to comply with applicable regulatory standards, causing regulators to deem the data unreliable; |
|
|
· |
we are unable to demonstrate to the satisfaction of the Regulatory Authority that the product candidate is safe and effective for the requested indication and meets all other regulatory standards; |
|
|
· |
the Regulatory Authority disagrees with our interpretation of data from our pivotal safety and efficacy studies and other development efforts; |
|
|
· |
we are unable to demonstrate that the product candidate’s benefits outweigh any safety or other perceived risks; |
|
|
· |
the Regulatory Authority requires additional studies; |
32
|
|
· |
the Regulatory Authority does not approve of the formulation, the labeling or the specifications of the product candidate; |
|
|
· |
the Regulatory Authority fails to approve our manufacturing processes or facilities, or the manufacturing processes or facilities of third-party manufacturers with which we contract; and |
|
|
· |
the approval policies or regulations of the Regulatory Authority significantly change in a manner rendering the data from our studies insufficient for approval. |
Each Regulatory Authority employs different regulatory standards, so we may require multiple manufacturing processes and facilities for the same product candidate or any approved product. In addition, failure to comply with the requirements of the Regulatory Authorities may subject us to administrative or judicially imposed sanctions, including: warning letters, civil and criminal penalties, injunctions, withdrawal of approved products from the market, product seizure or detention, product recalls, total or partial suspension of production, and refusal to approve pending NADAs or product licenses or supplements to approved NADAs or product licenses.
Regulatory approval of a NADA or supplemental NADA, or of a product license, is not guaranteed, and the approval process requires us to utilize significant resources, may take several years and is subject to the substantial discretion of the Regulatory Authorities. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat studies, or perform additional studies. If any of our current or future product candidates fails to demonstrate safety and efficacy in our studies, or for any other reason does not gain regulatory approval, our business and results of operations will be materially and adversely harmed.
Our ability to market our lead product candidates, if approved, will be limited to use for the treatment of the indications for which they are approved, and if we want to expand the indications for which we may market our lead product candidates, we will need to obtain additional regulatory approvals, which may not be granted.
We expect to seek FDA approval in the United States for our lead product candidates for pain associated with osteoarthritis in dogs (NV‑01), pain associated with degenerative joint disease, including osteoarthritis, in cats (NV‑02) and chronic inflammatory diseases, including atopic dermatitis, in dogs (NV‑08). If our lead product candidates are approved, we may only market or advertise them for the treatment of indications for which they are approved, which could limit their adoption by veterinarians and companion animal owners. We may attempt to develop, promote and commercialize new treatment indications and protocols for our lead product candidates in the future, but we may not receive the approvals required to do so on a timely basis or at all. In addition, we would be required to conduct additional pivotal safety and efficacy studies to support our applications, which would utilize additional resources. Our lead product candidates are species-specific and cannot be used in species other than those for which they are being developed or are approved. If we do not obtain additional regulatory approvals, our ability to expand our business will be limited.
Even if we receive regulatory approval for any of our current or future product candidates, we will be subject to ongoing and expensive regulatory obligations, including continued review, labeling and manufacturing requirements and other restrictions. Failure to comply with these regulatory obligations or the occurrence of unanticipated problems with our current or future product candidates could result in significant penalties.
If the Regulatory Authorities approve any of our current or future product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, establishment registration, and product listing, as well as continued compliance with good manufacturing practice as well as good laboratory practices and good clinical practices for any studies that we conduct post-approval.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes and quality assurance, or failure to comply with regulatory requirements, may result in, among other things:
|
|
· |
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary product recalls; |
|
|
· |
fines, warning letters or holds on pivotal safety and efficacy studies; |
|
|
· |
refusal by the Regulatory Authorities to approve pending applications or supplements to approved applications filed by us or our strategic collaborators, or suspension or revocation of product license approvals; |
|
|
· |
product seizure or detention, or refusal to permit the import or export of products; |
|
|
· |
injunctions or the imposition of civil or criminal penalties; and |
|
|
· |
lawsuits from animal owners. |
33
The Regulatory Authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our lead product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any regulatory approval that we may have obtained, and we may not achieve or sustain profitability, which would adversely affect our business.
Post-approval monitoring of products approved by the FDA is required by law, with reports to be provided to the FDA’s Surveillance and Compliance group. Reports of product quality defects, adverse events or unexpected results must be produced in accordance with the law. The USDA obtains information about adverse events via voluntary spontaneous reports and other surveillance activities. In the past, the USDA has proposed more stringent post-approval monitoring and reporting procedures for license holders, and it could do so in the future.
If approved, any of our current or future products may cause or contribute to adverse events that we are required to report to the Regulatory Authorities and, if we fail to do so, we could be subject to sanctions.
If we are successful in commercializing any of our current or future product candidates, the Regulatory Authorities will require that we report certain information about adverse events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe.
We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our lead product candidates. If we fail to comply with our reporting obligations, the Regulatory Authorities could take action including criminal prosecution, the imposition of civil monetary penalties, seizure of our lead product candidates or delay in approval or clearance of future products.
The misuse or extralabel use of our lead product candidates may harm our reputation or result in financial or other damages.
Our lead product candidates, if approved, will be limited for use under specific circumstances for the treatment of certain diseases and conditions in specific species. There may be increased risk of product liability if veterinarians, livestock producers, companion animal owners or others attempt extralabel use of our lead product candidates, including the use of our lead product candidates in species (including humans) for which they have not been approved. Furthermore, the use of our lead product candidates for indications other than those indications for which our lead product candidates have been approved may not be effective, which could harm our reputation and lead to an increased risk of litigation. If we are deemed by a governmental or regulatory agency to have engaged in the promotion of any of our lead product candidates for extralabel use, such agency could request that we modify our training or promotional materials and practices and we could be subject to significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry. Any of these events could materially adversely affect our operating results and financial condition.
Legislative or regulatory reforms with respect to companion animal therapeutics may make it more difficult and costly for us to obtain regulatory clearance or approval of any of our current or future product candidates and to produce, market, and distribute our lead product candidates after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress or the EU that could significantly change the statutory provisions governing the testing, regulatory clearance or approval, manufacture, and marketing of regulated products. In addition, the Regulatory Authorities’ regulations and guidance are often revised or reinterpreted by the regulators in ways that may significantly affect our business and our lead product candidates. Similar changes in laws or regulations can occur in other countries. Any new regulations or revisions or reinterpretations of existing regulations in the United States or in other countries may impose additional costs or lengthen review times of any of our current or future product candidates.
We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
|
|
· |
changes to regulatory criteria for approval; |
|
|
· |
changes to manufacturing methods; |
|
|
· |
recall, replacement, or discontinuance of certain products; |
|
|
· |
additional record keeping; and |
|
|
· |
additional reporting requirements. |
34
Each of these would likely entail substantial time and costs and could harm our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition and results of operations.
Our research and development relies on evaluations in animals, which may become subject to bans or additional regulations.
As a biopharmaceutical company with a focus on companion animal therapeutics, the evaluation of our existing and new products in animals is required to obtain marketing authorization for our lead product candidates. Animal testing in certain industries has been the subject of controversy and adverse publicity.
Some organizations and individuals have attempted to ban animal testing or encourage the adoption of additional regulations applicable to animal testing. To the extent that the activities of such organizations and individuals are successful, our research and development, and by extension our operating results and financial condition, could be adversely affected. In addition, negative publicity about us or our industry could harm our reputation.
If our use of hazardous materials results in contamination or injury, we could suffer significant reputational or financial loss.
Our research activities involve the controlled use of certain hazardous chemical and biological materials from time to time. Notwithstanding the various regulations controlling the use and disposal of these materials, as well as the safety procedures we undertake, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of an accident or environmental discharge or exposure, we may be held liable for any resulting damages, which may negatively impact our operations, our financial resources or our ability to recruit new staff.
Future federal and state legislation may expose us to product liability claims.
Under current federal and state laws, companion animals are generally considered to be personal property of their owners and, as such, owners’ recovery for product liability claims involving their companion animals may be limited to the replacement value of the companion animals. Companion animal owners and their advocates, however, have filed lawsuits from time to time seeking non-economic damages such as pain and suffering and emotional distress for harm to their companion animal based on theories applicable to personal injuries to humans. If new legislation is passed to allow recovery for such non-economic damages, or if precedents are set allowing for such recovery, we could be exposed to increased product liability claims that could result in substantial losses to us if successful.
Although we maintain product liability insurance and revise our coverage from time to time, we currently have a per-claim deductible of $5,000 on our global products liability policy, which may make the insurance of limited utility in many companion animal claims where the companion animal is valued at replacement value. Furthermore, the policy is subject to per-year policy limits of $20.0 million per year for non-U.S. jurisdiction claims and $5.0 million per year for U.S. jurisdiction claims, as well as other terms and conditions. Accordingly, it is possible that our insurance will not cover future product liability claims against us.
Risks Related to Taxation
We are a multinational organization faced with complex tax issues in many jurisdictions, and we could be obligated to pay additional taxes in various jurisdictions.
We are an Irish-registered corporation and we currently have subsidiaries in Australia, Ireland, the United States and the United Kingdom. We have historically operated primarily in Australia, and we have recently commenced operations in Ireland and the United States. Depending on our ability to develop and commercialize our lead product candidates, we may have additional operations around the world. As a multinational organization, we may be subject to taxation in several different jurisdictions with complex tax laws, the application of which can be uncertain. The amount of taxes we pay in these jurisdictions could increase substantially as a result of changes in applicable tax law, including increased tax rates, new tax laws or revised interpretations of existing tax laws and precedents. Such changes could have an adverse effect on our liquidity and results of operations. In addition, the tax authorities in these jurisdictions could review our tax positions and disagree with the approaches taken or our tax returns submitted and seek to impose additional tax, interest and penalties on us. In addition, the tax authorities in those jurisdictions could claim that we have additional tax, withholding tax or other tax filing requirements or could assert that certain benefits of tax treaties are not available to us or our subsidiaries. Furthermore, the tax authorities in one or more jurisdictions in which we do not currently believe we have a taxable presence could assert that we do have a taxable presence in that jurisdiction and on that basis assert that we have tax payment, withholding tax or other tax filing requirements in that jurisdiction. Any such claim or assertion could impact us and our results of operations.
35
Changes in our effective tax rate may reduce our net income in future periods.
We are organized as an Irish company, in part, to improve our ability to maintain a competitive worldwide effective corporate tax rate. Our effective tax rate, however, depends on the tax policies of the jurisdictions in which we operate. In general, under current Irish tax legislation, a company is regarded as resident for tax purposes in Ireland if the company is “centrally managed and controlled” in Ireland, or, in certain circumstances, if the company is incorporated in Ireland. We expect that our company will be centrally managed and controlled from Ireland and accordingly will be treated as an Irish tax resident. However, as a multinational organization, it is possible that we may not be regarded as being centrally managed and controlled in Ireland and instead be treated as a tax resident in another jurisdiction. If such a situation were to arise, it could adversely impact our tax position and our effective tax rate.
Trading income of an Irish resident company is generally taxable at the Irish corporation tax rate of 12.5%. Non-trading income of an Irish resident company, such as interest income, rental income or other passive income, is taxable at a rate of 25%. It is possible that in the future, whether as a result of a change in law in any jurisdiction or the practice of any relevant tax authority or as a result of any change in our business, we could become, or be regarded as having become, tax resident in a jurisdiction other than Ireland. Should we cease to be an Irish tax resident, we may be subject to a charge to Irish capital gains tax on any gain inherent in our assets. Our actual effective tax rate may vary from our expectation and that variance may be material. Additionally, the tax laws of Ireland, the United States, Australia and other jurisdictions could change in the future, and such changes could cause a material change in our effective tax rate. In addition, the changes currently proposed by the Organisation for Economic Co-operation and Development, or the OECD, and their action plan on Base Erosion and Profit Shifting could adversely impact our tax position and our business operations.
A number of factors may increase our future effective tax rates, including:
|
|
· |
the jurisdictions in which profits are determined to be earned and taxed; |
|
|
· |
the resolution of issues arising from any tax audits with various tax authorities; |
|
|
· |
changes in the valuation of our deferred tax assets and liabilities; |
|
|
· |
increases in expenses not deductible for tax purposes, including transaction costs and impairments of goodwill in connection with any acquisitions; |
|
|
· |
changes in available tax credits, including the research and development tax credit in Australia; |
|
|
· |
changes in the taxation of share-based compensation; |
|
|
· |
changes in tax laws or the interpretation of such tax laws, and changes in generally accepted accounting principles; and |
|
|
· |
challenges to the transfer pricing policies related to our structure. |
Our tax position could be adversely impacted by changes in tax rates, tax laws, tax practice, tax treaties or tax regulations or changes in the interpretation of such laws, treaties or regulations by the tax authorities in Ireland, the United States, Australia and other jurisdictions as well as being affected by certain changes currently proposed by the OECD. Such changes may be become more likely as a result of recent economic trends in the jurisdictions in which we operate, particularly if such trends continue. For example, a change in the Irish government’s stated policy of not increasing business taxation could cause a material and adverse change in our worldwide effective tax rate and we may have to take action, at potentially significant expense, to seek to mitigate the effect of such changes. In addition, any amendments to the current double taxation treaties between Ireland and other jurisdictions, including Australia and the United States, could subject us to increased taxation or irrecoverable withholding tax.
Failure to manage the risks associated with such changes, or misinterpretation of the laws relating to taxation, could result in costly audits, interest, penalties and reputational damage, which could adversely affect our business, results of our operations and our financial condition. In the normal course of business, we are currently open to inspection by the tax authorities in Ireland, Australia, the United States and the United Kingdom. We are not currently the subject of any ongoing tax audits.
36
Our ability to use existing tax loss carry forwards to reduce future tax payments may be limited if we experience a change in ownership, or if taxable income does not reach sufficient levels.
Our ability to use our net operating losses is subject to limitations and reassessment due to ownership changes that have occurred or that may occur in the future under the laws of the jurisdictions in which we have net operating losses, which currently include Australia. In addition, use of net operating losses is restricted to income in those jurisdictions. For example, Nexvet Australia’s tax loss carry forwards comprised $7.2 million and $4.0 million of our tax loss carry forwards as of June 30, 2015 and 2014, respectively. Given the change of ownership that occurred in September 2014 to Nexvet Australia, the Australian tax authorities could argue that there has been a change in the underlying business in Australia, which may result in a substantial portion of these tax losses never being recoverable. In June 2015, a change was passed in the relevant small business tax rate applicable to Nexvet Australia from 30% to 28.5%, which will apply from July 1, 2015. Carry forward tax losses have been stated at the applicable 28.5% rate as of June 30, 2015. Depending on the actual amount of any limitation on our ability to use our tax loss carry forwards, a significant portion of our future taxable income could be taxable.
Additionally, tax law limitations may result in our net operating losses expiring before we have the ability to use them. In addition, financing and acquisition transactions that we may enter into in the future could significantly limit or eliminate our ability to realize any value from our net operating losses.
Taxing authorities could reallocate our taxable income among our subsidiaries, which could increase our overall tax liability.
Our parent company is based in Ireland and we currently have subsidiaries in Australia, Ireland, the United States and the United Kingdom. If we succeed in growing our business, we expect to conduct increased operations through our subsidiaries in various tax jurisdictions pursuant to transfer pricing arrangements between our parent company and subsidiaries. If two or more affiliated companies are located in different countries, the tax laws or regulations of each country generally will require that transfer prices be the same as those between unrelated companies dealing at arms’ length and that appropriate documentation is maintained to support the transfer prices. While we believe that we operate in compliance with applicable transfer pricing laws and intend to continue to do so, our transfer pricing procedures are not binding on applicable tax authorities.
If tax authorities in any of these countries were to successfully challenge our transfer prices as not reflecting arms’ length transactions, they could require us to adjust our transfer prices and thereby reallocate our income to reflect these revised transfer prices, which could result in a higher tax liability to us. In addition, if the country from which the income is reallocated does not agree with the reallocation, both countries could tax the same income, resulting in double taxation. If tax authorities were to allocate income to a higher tax jurisdiction, subject our income to double taxation or assess interest and penalties, it would increase our consolidated tax liability, which could adversely affect our financial condition, results of operations and cash flows.
We believe that we may be a passive foreign investment company for U.S. federal income tax purposes, which could subject U.S. Holders to adverse U.S. federal income tax consequences.
We believe that we may be a passive foreign investment company, or a PFIC, for U.S. federal income tax purposes in our current taxable year and we may be a PFIC in other taxable years. A foreign corporation like us generally will be a PFIC if either at least (i) 75% of its gross income is “passive income” or (ii) 50% of the gross value of its assets is attributable to assets that produce, or are held for the production of, passive income. We refer to the passive income test as the “PFIC Income Test” and the asset test as the “PFIC Asset Test.” The proceeds from our initial public offering are a passive asset under these rules and may cause us to meet the PFIC Asset Test for our taxable year that includes our initial public offering (and in later years if we are not deploying the cash at a rate that would allow us to avoid meeting the PFIC Asset Test in such later years). If we are a PFIC in any taxable year in which you hold shares and you are a “U.S. Holder,” we always will be a PFIC with respect to your share ownership (subject to the QEF election discussed immediately below) unless you make an election to “purge” PFIC status as of the beginning of the first taxable year that we are not a PFIC (a year in which do not meet the PFIC Income Test or the PFIC Asset Test) or the first taxable year that you make a QEF election, if such election is made after the first year in which you held our ordinary shares and in which we are a PFIC, all as discussed further below. If we are a PFIC and you are a U.S. Holder and do not make a Qualified Electing Fund election, or QEF election, with respect to us or a mark-to-market election with respect to our ordinary shares, you will be subject to adverse tax consequences, including deferred tax and interest charges with respect to certain distributions on our ordinary shares, any gain realized on a disposition of our ordinary shares and certain other events. The effect of these adverse tax consequences could be materially adverse to you.
37
If you are a U.S. Holder and make a valid, timely QEF election for us, you will not be subject to those adverse tax consequences, but could recognize taxable income in a taxable year with respect to our ordinary shares in excess of any distributions that we make to you in that year, thus giving rise to so-called “phantom income” and to a potential out-of-pocket tax liability. If we are a PFIC with respect to any tax year, we will provide information to all electing shareholders needed to comply with the QEF election in time for each electing shareholder to make and maintain a timely QEF election, taking into account available extensions. If you are a U.S. Holder and make a valid, timely mark-to-market election with respect to our ordinary shares, you will recognize as ordinary income or loss in each year that we are a PFIC an amount equal to the difference between your basis in our ordinary shares and the fair market value of the ordinary shares, thus also possibly giving rise to phantom income and a potential out-of-pocket tax liability. Ordinary loss generally is recognized only to the extent of net mark-to-market gains previously included in income. If one or more of our subsidiaries is a PFIC, U.S. Holders will also need to make the QEF election with respect to each such subsidiary in order to avoid the adverse tax consequences described above. We intend to provide on a timely basis all information necessary for U.S. Holders to make the QEF election with respect to any of our subsidiaries that may be classified as a PFIC in any tax year. U.S. Holders should also be aware that the mark-to-market election generally will not be available with respect to any of our subsidiaries that is a PFIC, rendering such election less beneficial to U.S. Holders than the QEF election. We intend to determine on an annual basis whether we will be a PFIC with respect to any taxable year. As noted above, if we are a PFIC in any taxable year in which you own shares, you do not make a timely QEF election and you are a U.S. Holder, we will remain a PFIC with respect to your share ownership unless you make a “purging election.” The resulting liability from such an election could be substantial.
If the U.S. Internal Revenue Service determines that we are not a PFIC, and you previously paid taxes pursuant to a QEF election or a mark-to-market election, you may pay more taxes than you legally owe.
If the U.S. Internal Revenue Service makes a determination that we are not a PFIC and you previously paid taxes pursuant to a QEF election or mark-to-market election, then you may have paid more taxes than you legally owed due to such election. If you do not, or are not able to, file a refund claim before the expiration of the applicable statute of limitations, you will not be able to claim a refund for those taxes.
Risks Related to Ownership of Our Ordinary Shares
The market price of our ordinary shares has been and may remain volatile, and your investment in us could suffer a decline in value.
The trading price of our ordinary shares has been and could continue fluctuating widely in response to various factors, some of which are beyond our control. These factors include those discussed elsewhere in this “Risk Factors” section of this Report and others, such as:
|
|
· |
results from, and any delays in, our current and future proof-of-concept and pivotal safety and efficacy studies; |
|
|
· |
announcements of regulatory approval or disapproval of any of our current or future product candidates; |
|
|
· |
delays in the commercialization of our current or future product candidates; |
|
|
· |
manufacturing and supply issues related to our development programs and commercialization of our current or future product candidates; |
|
|
· |
quarterly variations in our results of operations or those of our competitors; |
|
|
· |
changes in our earnings estimates or recommendations by securities analysts; |
|
|
· |
announcements by us or our competitors of new product candidates, significant contracts, commercial relationships, acquisitions or capital commitments; |
|
|
· |
announcements relating to future development or license agreements including termination of such agreements; |
|
|
· |
adverse developments with respect to our intellectual property rights or those of our principal collaborators; |
|
|
· |
commencement of litigation involving us or our competitors; |
|
|
· |
changes in our board of directors or management; |
|
|
· |
new legislation in the United States relating to the prescription, sale, distribution or pricing of companion animal therapeutics; |
|
|
· |
product liability claims, other litigation or public concern about the safety of our lead product candidates or future products; |
|
|
· |
market conditions in the companion animal market in general, or in the companion animal therapeutics segment in particular, including performance of our competitors; and |
|
|
· |
general economic conditions in the United States and abroad. |
38
In addition, the stock market in general, or the market for equity securities in our industry or industries related to our industry, may experience extreme volatility unrelated to our operating performance. These broad market fluctuations may adversely affect the trading price or liquidity of our ordinary shares. Any sudden decline in the market price of our ordinary shares could trigger securities class-action lawsuits against us. If any of our shareholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the time and attention of our management would be diverted from our business and operations. We also could be subject to damages claims if we are found to be at fault in connection with a decline in our share price.
If securities or industry analysts do not continue to publish research or reports about our company, or if they issue adverse or misleading opinions regarding us or our ordinary shares, our share price and trading volume could decline.
We currently have limited research coverage by securities and industry analysts, and if coverage is not maintained or initiated from time to time, the market price for our ordinary shares may be adversely affected. Our share price also may decline if any analyst who covers us issues an adverse or misleading opinion regarding us, our business model, our intellectual property or our share performance, or if our pivotal safety and efficacy studies and operating results fail to meet analysts’ expectations. If one or more analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline and possibly adversely affect our ability to engage in future financings.
Our principal shareholders and management own a significant percentage of our ordinary shares and will be able to exert significant control over matters subject to shareholder approval.
Based on shares outstanding as of June 30, 2015, our executive officers, directors, holders of five percent or more of our ordinary shares and their respective affiliates beneficially owned in the aggregate approximately 70.9% of our outstanding ordinary shares. As a result of their share ownership, these shareholders may have the ability to influence our management and policies and will be able to significantly affect the outcome of matters requiring shareholder approval such as elections of directors, amendments of our organizational documents or approvals of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our ordinary shares that you may feel are in your best interest as one of our shareholders.
We will have broad discretion regarding use of the net proceeds from our initial public offering, and we may use them in ways that do not enhance our operating results or the market price of our ordinary shares.
Our management has broad discretion regarding the use of the net proceeds from our initial public offering, and we could spend the net proceeds in ways our shareholders may not agree with or that do not yield a favorable return, if at all. We intend to use the net proceeds from our initial public offering to complete the development and manufacturing scale-up of each of our lead product candidates, to establish our sales force infrastructure in the United States for any approved products and for anticipated promotional and launch costs. We also intend to fund research to develop our pipeline of other product candidates. We intend to use the remainder of the net proceeds from our initial public offering, together with our other cash on hand, for working capital and other general corporate purposes. We may also use a portion of the net proceeds to acquire or in-license additional product candidates or complementary assets or businesses; however, we currently have no agreements, commitments or understandings to complete any such transaction. Further, our actual use of these proceeds may differ substantially from our current intentions. If we do not invest or apply the proceeds from our initial public offering or our other available cash in ways that improve our operating results or our prospects, our share price could decline.
If we raise additional capital in the future, your level of ownership in us could be diluted or require us to relinquish rights.
Any issuance of securities we may undertake in the future to raise additional capital could cause the price of our ordinary shares to decline, or require us to issue shares at a price that is lower than that paid by holders of our ordinary shares in the past, which would result in those newly issued shares being dilutive.
Further, if we obtain funds through a debt financing or through the issuance of debt or preference securities, these securities would likely have rights senior to your rights as an ordinary shareholder, which could impair the value of our ordinary shares. Any debt financing we enter into may include covenants that limit our flexibility in conducting our business. We also could be required to seek funds through arrangements with collaborators or others, which might require us to relinquish valuable rights to our intellectual property or product candidates that we would have otherwise retained.
Your rights as a shareholder will be governed by Irish law and differ from the rights of shareholders under U.S. law.
We are a public limited company under the laws of Ireland. Therefore, the rights of holders of ordinary shares are governed by Irish law and by our memorandum and articles of association, or our Articles. These rights differ from the typical rights of shareholders in U.S. corporations. In certain cases, facts that, under U.S. law, would entitle a shareholder in a U.S. corporation to claim damages may not give rise to a cause of action under Irish law entitling a shareholder in an Irish company to claim damages. For example, the rights of shareholders to bring proceedings against us or against our directors or officers in relation to public statements are more limited under Irish law than under the civil liability provisions of the U.S. securities laws.
39
You may have difficulties enforcing, in actions brought in courts in jurisdictions located outside the United States, judgments obtained in the U.S. courts under the U.S. securities laws. In particular, if you sought to bring proceedings in Ireland based on U.S. securities laws, the Irish court might consider:
|
|
· |
that it did not have jurisdiction; |
|
|
· |
that it was not the appropriate forum for such proceedings; |
|
|
· |
that, applying Irish conflict of laws rules, U.S. law (including U.S. securities laws) did not apply to the relationship between you and us or our directors and officers; or |
|
|
· |
that the U.S. securities laws were of a penal nature or violated Irish public policy and should not be enforced by the Irish court. |
You should also be aware that Irish law does not allow for any form of legal proceedings directly equivalent to the class action available in the United States.
You may have difficulty in effecting service of process within the United States or enforcing judgments obtained in the United States.
We and several members of our senior management and board of directors are residents of countries other than the United States. As a result, it may not be possible for you to:
|
|
· |
effect service of process within the United States upon us, certain members of our senior management and board of directors and certain experts we retain; or |
|
|
· |
obtain discovery of relevant documents or the testimony of witnesses. |
There is no system of reciprocal enforcement in Ireland of judgments obtained in the U.S. courts. Accordingly, a U.S. judgment against any of those persons or us may only be enforced in Ireland by the commencement of a new action before the Irish court based on the judgment of the U.S. court. Summary judgment against any of those persons or us, as the case may be, may be granted by the Irish court without requiring the issues in the U.S. litigation to be reopened on the basis that those matters have already been decided by the U.S. court provided that the Irish court is satisfied that:
|
|
· |
the U.S. judgment is for a definite sum of money; |
|
|
· |
the U.S. judgment is not directly or indirectly for the payment of taxes or other charges of a like nature or a fine or other penalty (for example, punitive or exemplary damages); |
|
|
· |
the U.S. judgment is final and conclusive; |
|
|
· |
the Irish proceedings were commenced within the relevant limitation period; |
|
|
· |
the U.S. judgment is provided by a court of competent jurisdiction (as determined by Irish law); |
|
|
· |
the U.S. judgment remains valid and enforceable in the court in which it was obtained; and |
|
|
· |
the U.S. judgment is not obtained by fraud, did not violate Irish public policy, is not in breach of natural justice and is not irreconcilable with an earlier foreign judgment. |
As a newly public company, we incur significant additional costs, and our management devotes substantial time and attention to our public reporting obligations.
As a publicly-traded company, we incur significant additional legal, accounting and other expenses. In addition, new and changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated and to be promulgated thereunder, as well as under the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and the rules and regulations of the U.S. Securities and Exchange Commission, or the SEC, and The NASDAQ Stock Market have created uncertainty for public companies and increased our costs and the time that our board of directors and management must devote to complying with these rules and regulations. We expect these rules and regulations to increase our legal and financial compliance costs substantially and lead to diversion of management time and attention from revenue-generating activities.
40
We are an “emerging growth company,” and the reduced disclosure requirements applicable to “emerging growth companies” may make our ordinary shares less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act, and, therefore, we may take advantage of reduced disclosure and regulatory requirements that are otherwise generally applicable to public companies, including presenting only two years of audited financial statements and related financial disclosure, not being required to have our internal control over financial reporting audited by our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and any golden parachute payments. We may take advantage of these reduced disclosure and regulatory requirements until we are no longer an “emerging growth company.” We may remain an “emerging growth company” until as late as June 30, 2020 (the fiscal year-end following the fifth anniversary of the completion of our initial public offering), although we may cease to be an “emerging growth company” earlier under certain circumstances, including if the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of any December 31, in which case we would cease to be an “emerging growth company” as of the following June 30, or if our gross revenue exceeds $1 billion in any fiscal year. In addition, the JOBS Act provides that an emerging growth company can delay adopting new or revised accounting standards until those standards apply to private companies. We have irrevocably elected not to avail ourselves of this delayed adoption of new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as public companies that are not emerging growth companies.
The exact implications of the JOBS Act are still subject to interpretations and guidance by the SEC and other regulatory agencies, and we may not be able to take advantage of all of the benefits of the JOBS Act. In addition, investors may find our ordinary shares less attractive if we rely on the exemptions and relief granted by the JOBS Act. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and our share price may decline or become more volatile.
Our prior audit firm previously advised us that we lacked proper internal controls to develop reliable financial statements. If we are not able to develop and maintain effective internal control over financial reporting we may be unable to accurately and timely report our financial results and our business could be harmed.
Our prior auditor previously audited our financial statements in accordance with generally accepted auditing standards in Australia, or Australian GAAS, for fiscal years 2012 and 2013, which were prepared in accordance with international financial reporting standards generally accepted in Australia, or Australian IFRS. Our prior auditor did not previously conduct any audits for any period in accordance with auditing standards generally accepted in the United States or the standards of the Public Company Accounting Oversight Board of our financial statements in conformity with accounting principles generally accepted in the United States, or U.S. GAAP, or international financial reporting standards as issued by the International Accounting Standards Board.
Our prior auditor advised us that, through October 2013, the date of its report, there was a lack of (i) proper segregation of duties and (ii) competent and qualified financial reporting personnel for the development of policies and procedures relating to the financial reporting function and the preparation of reliable financial statements prepared in accordance with Australian IFRS.
Although we were not subject to Public Company Accounting Oversight Board requirements at the time, we believe that the conditions identified by our prior auditor constituted material weaknesses in our internal control over financial reporting during that period. A material weakness is defined under the standards issued by the Public Company Accounting Oversight Board as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our financial statements may not be prevented or detected and corrected on a timely basis.
As of June 30, 2013, we only had six employees and primarily relied upon a third-party consultant to perform bookkeeping services and compile our financial statements. Subsequent to October 2013, we retained Damian Lismore, our Chief Financial Officer, as well as two other personnel within our financial reporting and accounting group, who collectively have experience in U.S. GAAP and the preparation of public company financial statements. Following Mr. Lismore’s retention we have implemented procedures to segregate duties within our financial reporting and accounting group; however, as a small company we are unable to completely segregate duties and must rely upon other controls. In addition, we have adopted accounting policies and procedures relating to the financial reporting function and we plan to further develop and formalize our financial reporting and accounting policies and procedures as we grow. As a result of these actions, we believe that we have remediated the above material weaknesses.
41
Although no material weaknesses were identified in connection with the preparation of our financial statements for fiscal year 2015, neither we nor our current independent registered public accounting firm conducted an evaluation of the operating effectiveness of our internal control over financial reporting as of June 30, 2015. We will be required to evaluate our internal control over financial reporting as required by Section 404 of the Sarbanes-Oxley Act for fiscal year 2016. In addition, under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until we are no longer an “emerging growth company” or if our market capitalization remains below $75 million. The rules governing the standards that must be met for our management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation.
We will continue the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404, and we may not be able to complete our evaluation or testing in a timely fashion. As we prepare for compliance with Section 404 of the Sarbanes-Oxley Act by implementing the necessary procedures and practices related to internal control over financial reporting, we may identify deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance. In addition, we may encounter problems or delays in completing the implementation of any requested improvements and receiving a favorable attestation in connection with the attestation provided by our independent registered public accounting firm. If we fail to achieve and maintain an effective internal control environment our business and share price could be harmed and our ability to report our financial results accurately and in a timely manner could be impaired.
We have never paid cash dividends, do not anticipate paying any cash dividends and our ability to pay dividends, or repurchase or redeem our ordinary shares, is limited by law.
We have never declared or paid cash dividends on our ordinary shares and do not anticipate paying any dividends on our ordinary shares in the foreseeable future. Any determination to pay dividends in the future will at the sole discretion of our board of directors after considering our financial condition, results of operations, capital requirements, contractual restrictions, general business conditions and other factors our board of directors deems relevant, and subject to compliance with applicable laws (including the Irish Companies Act, which requires Irish companies to have distributable reserves available for distribution equal to or greater than the amount of the proposed dividend). Accordingly, the only opportunity to achieve a return on your investment in our company is expected to be if the market price of our ordinary shares appreciates and you sell your ordinary shares at a profit. The price of our ordinary shares prevailing in the market may not exceed the price that you pay.
A future transfer of your ordinary shares, other than one effected by means of the transfer of book-entry interests in DTC, may be subject to Irish stamp duty.
The rate of stamp duty (when applicable) on the transfer of shares in an Irish-incorporated company is 1% of the price paid, or the market value of the shares acquired, whichever is greater. Payment of Irish stamp duty is generally a legal obligation of the transferee. We expect that most of our ordinary shares will be traded through Depositary Trust Company, or DTC, or through brokers who hold such shares on behalf of customers through DTC. As such, the transfer of ordinary shares should be exempt from Irish stamp duty based on established practice of the Irish Revenue Commissioners. We have received confirmation from the Irish Revenue Commissioners that a transfer of our ordinary shares held through DTC and transferred by means of a book-entry interest would be exempt from Irish stamp duty. We have also received confirmation from the Irish Revenue Commissioners that certain transfers of our ordinary shares into (or out of) DTC would be exempt from Irish stamp duty. However, if you hold your ordinary shares directly of record, rather than beneficially through DTC (or through a broker that holds your ordinary shares through DTC), any transfer of your ordinary shares may be subject to Irish stamp duty. The potential for stamp duty to arise could adversely affect the price and liquidity of our ordinary shares. In addition, the terms of our eligibility agreement with DTC require us to provide certain indemnities relating to Irish stamp duty to third parties. If liability were to arise as a result of the indemnities provided under the terms of the eligibility agreement, we may face unexpected costs that could adversely impact our results of operations.
Anti-takeover provisions in our Articles and under Irish law could make an acquisition of us more difficult, limit attempts by our shareholders to replace or remove our current directors and management team, and limit the market price of our ordinary shares.
Our Articles contain provisions that may delay or prevent a change of control, discourage bids at a premium over the market price of our ordinary shares, and adversely affect the market price of our ordinary shares and the voting and other rights of the holders of our ordinary shares. These provisions will include:
|
|
· |
dividing our board of directors into two classes, with each class serving a staggered two-year term; |
|
|
· |
permitting our board of directors to issue additional preference shares, with such rights, preferences and privileges as they may designate; and |
|
|
· |
establishing an advance notice procedure for shareholder proposals to be brought before an annual meeting, including proposed nominations of persons for election to our board of directors. |
42
These provisions would apply even if the offer may be considered beneficial by some shareholders. In addition, these provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management team by making it more difficult for shareholders to replace members of our board of directors, which is responsible for appointing the members of our management.
Irish law differs from the laws in effect in the United States with respect to defending unwanted takeover proposals and may give our board of directors less ability to control negotiations with hostile offerors.
We are subject to the Irish Takeover Rules. Under the Irish Takeover Rules, our board of directors is not permitted to take any action that might frustrate an offer for our ordinary shares once our board of directors has received an approach that may lead to an offer or has reason to believe that such an offer is or may be imminent, subject to certain exceptions. Potentially frustrating actions such as (i) the issue of shares, options, restricted share units or convertible securities, (ii) material acquisitions or disposals, (iii) entering into contracts other than in the ordinary course of business or (iv) any action, other than seeking alternative offers, which may result in frustration of an offer, are prohibited during the course of an offer or at any earlier time during which our board of directors has reason to believe an offer is or may be imminent. These provisions may give our board of directors less ability to control negotiations with hostile offerors than would be the case for a corporation incorporated in a jurisdiction of the United States.
Item 1B. Unresolved Staff Comments.
None
Our corporate headquarters are located at NIBRT, Foster’s Avenue, Mount Merrion, Blackrock, Co. Dublin, Ireland, where we occupy approximately 80 square meters of office space pursuant to a license that expires in January 2017. We lease approximately 274 square meters of office space in Melbourne, Australia pursuant to a lease that expires in January 2019. We also occupy approximately 130 square meters of office space and laboratory facility at Monash University, Melbourne, Australia on a monthly facility access and use agreement, and we are in the process of negotiating to relocate to larger laboratory facilities. We do not own any real property. We intend to enter into a lease for office space in San Francisco, California at a future point. We believe the facilities we lease and intend to lease will be adequate to support our operations for the foreseeable future. Refer to Note 14 to our Consolidated Financial Statements appearing elsewhere in this Report for further information.
We are not currently subject to any material pending legal proceedings.
Item 4. Mine Safety Disclosures.
Not applicable
43
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our ordinary shares have been trading on the NASDAQ Global Market under the symbol “NVET” since our initial public offering on February 4, 2015. Prior to this date, there was no public market for our ordinary shares. As a result, we have only set forth below quarterly information with respect to the high and low prices for our ordinary shares since our initial public offering.
On August 31, 2015, the closing price per share for our ordinary shares as reported on the NASDAQ Global Market was $4.46 per share. The following table sets for the high and low intraday sale prices per share of our ordinary shares for the periods indicated as reported by the NASDAQ Global Market.
|
|
High |
|
Low |
||||
|
2015 |
|
|
|
||||
|
Fourth Quarter |
$ |
9.45 |
|
|
$ |
4.40 |
|
|
Third Quarter (beginning February 4, 2015) |
10.57 |
|
|
7.08 |
|
||
Dividend Policy
We have never declared or paid any cash dividends on our ordinary shares and do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, contractual restrictions, general business conditions and other factors our board of directors deems relevant, and subject to compliance with applicable laws (including the Irish Companies Act, which requires Irish companies to have profits available for distribution equal to or greater than the amount of the proposed dividend). We may require distributions from our subsidiary companies, in order to pay dividends, which may subject us to dividend income tax.
Holders of Record
As of June 30, 2015, we had 11,406,916 ordinary shares outstanding held by approximately 73 shareholders of record. The actual number of shareholders is greater than this number of record holders, and includes shareholders who are beneficial owners, but whose shares are held in “street” name by brokers and other nominees. This number of holders or record also does not include shareholders whose shares may be held in trust by other entities.
Securities Authorized for Issuance under Equity Compensation Plans
The information called for by this item is incorporated by reference to our Proxy Statement for the Annual Meeting of Shareholders to be held in 2015. See Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management.”
Sales of Unregistered Equity Securities
1. Through August 2014, Nexvet Australia issued share options to purchase 215,799 ordinary shares at a zero exercise price and restricted share units to acquire 29,214 ordinary shares at a zero conversion price to then-existing employees, directors and consultants under the 2013 Long Term Incentive Plan, or our 2013 Plan, without any general solicitation or advertising. In August 2014, Nexvet Australia, the share option holders and the restricted share unit holders agreed to revise the exercise or conversion price, as applicable, to the nominal value per ordinary share.
2. Through August 2014, Nexvet Australia issued an aggregate of 19,115 ordinary shares, without any general solicitation or advertising, to then-existing employees, directors and consultants upon the exercise of share options issued under our 2013 Plan at a zero exercise price, as permitted by Australian law, for aggregate cash consideration of zero.
3. In August 2014, we issued one ordinary share at a price per share of the nominal value of $0.10 per ordinary share (which nominal value became $0.125 per ordinary share in connection with the four-for-five share consolidation in November 2014) in connection with the formation of Nexvet Biopharma Limited, without any general solicitation or advertising.
44
4. In September 2014, pursuant to share exchange agreements, we allotted 1,142,858 ordinary shares, 1,737,132 Series A investment preference shares, or SIRPS Preference Shares, and 4,200,006 Series B convertible preference shares, or Series B Preference Shares, to shareholders of Nexvet Australia in exchange for 100% of the issued and outstanding capital shares of Nexvet Australia. In addition, in connection with the share exchange agreements, we issued warrants to purchase 1,574,998 of our ordinary shares with an exercise price of $8.625 per ordinary share and warrants to purchase 192,000 of our ordinary shares with an exercise price of $7.50 per ordinary share in exchange for the equivalent warrants issued by Nexvet Australia. In connection with the share exchange agreements, we also issued share options to purchase 470,479 of our ordinary shares with an exercise price of the nominal value of $0.10 per ordinary share and restricted share units to acquire 29,215 of our ordinary shares with a conversion price of the nominal value of $0.10 per ordinary share under our 2013 Plan in exchange for the equivalent share options and restricted share units issued by Nexvet Australia. Such exercise or conversion price, as applicable, was revised to the nominal value of $0.125 per ordinary share in connection with the four-for-five share consolidation in November 2014.
5. In August 2014, Nexvet Australia repurchased 145,069 ordinary shares subject to limited recourse loans from then-existing employees, directors and consultants. As consideration for such repurchase, Nexvet Australia forgave the remaining loan amounts, aggregating to $0.9 million, and, in September 2014, we issued share options to purchase 145,069 ordinary shares at an exercise price of $6.35 per ordinary share to such employees, directors and consultants, without any general solicitation or advertising.
6. From September to October 2014, we issued share options to purchase an aggregate of 158,592 ordinary shares at an exercise price of the nominal value of $0.10 per ordinary share and restricted share units to acquire 37,667 ordinary shares at a conversion price of the nominal value of $0.10 per ordinary share to then-existing employees and directors under our 2013 Plan, without any general solicitation or advertising. Such exercise or conversion price, as applicable, was revised to the nominal value of $0.125 per ordinary share in connection with the four-for-five share consolidation in November 2014.
7. In November 2014, we issued one ordinary share, two SIRPS Preference Shares and three Series B Preference Shares, each at a price per share of $12.00, in connection with the four-for-five share consolidation, without any general solicitation or advertising.
8. In October and November 2014, we issued an aggregate of 16,009 ordinary shares, without any general solicitation or advertising, to then-existing employees, directors and consultants upon the conversion of restricted share units issued under our 2013 Plan at a conversion price per share of $0.125, for aggregate consideration of $2,001.
9. In November 2014, we issued an aggregate of 73,825 ordinary shares, without any general solicitation or advertising, to then-existing employees, directors and consultants upon the exercise of share options issued under our 2013 Plan at an exercise price per share of $0.125, for aggregate consideration of $9,228.
10. In January 2015, we issued an aggregate of 3,700 ordinary shares, without any general solicitation or advertising, to then-existing directors upon the conversion of restricted share units issued under our 2013 Plan at a conversion price per share of $0.125, for aggregate consideration of $462.50.
11. In January 2015, we issued 380 ordinary shares, without any general solicitation or advertising, to a director in lieu of a cash payment of his director fees.
The offers and sales of securities described in paragraphs 1 through 11 were not registered under the Securities Act in reliance on, and in compliance with, Regulation S as issuances to non-U.S. investors outside the United States, Section 4(a)(2) of the Securities Act or Rule 506 of Regulation D promulgated thereunder. All persons acquiring these securities were either accredited investors (as defined in Rule 501 of Regulation D) or non-U.S. persons (as defined in Rule 902 of Regulation S).
The offers and sales of the securities described in paragraphs 1, 2, 6 and 8 through 11 were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 as transactions under compensatory benefit plans and contracts relating to compensation.
Share Performance Graph
The information in this section shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or incorporated by reference into any of our other filings under the Exchange Act or the Securities Act, except to the extent we specifically incorporate it by reference into such other filings.
The following chart compares the cumulative total return on our ordinary shares with that of the Nasdaq Composite Index and the Nasdaq Biotechnology Index. The chart assumes $100 was invested at the close of market on February 5, 2015, in our ordinary shares and these indexes, and assumes the reinvestment of any dividends. The share price performance on the following graph is not necessarily indicative of future share price performance.
45
|
Company/Index |
Base Period |
2/28/2015 |
|
3/31/2015 |
|
4/30/2015 |
|
5/31/2015 |
|
6/30/2015 |
|||||||
|
Nexvet Biopharma plc |
$ |
100.00 |
|
$ |
100.30 |
|
$ |
80.30 |
|
$ |
86.50 |
|
$ |
45.55 |
|
$ |
49.70 |
|
Nasdaq Composite Index |
100.00 |
|
104.16 |
|
102.85 |
|
103.69 |
|
106.40 |
|
104.65 |
||||||
|
Nasdaq Biotechnology Index |
100.00 |
|
105.41 |
|
107.43 |
|
104.48 |
|
114.13 |
|
115.42 |
||||||
Use of Proceeds
On February 4, 2015, our registration statement on Form S–1 (File No. 333-201309) was declared effective by the SEC for our initial public offering, pursuant to which we sold an aggregate of 4.2 million ordinary shares (including the underwriters’ partial exercise of their overallotment option) at a price to the public of $10.00 per share. Merrill Lynch, Pierce, Fenner & Smith Incorporated, Cowen and Company, LLC, Piper Jaffray & Co. and JMP Securities LLC acted as underwriters. Following the sale of the securities registered in the registration statement, the offering terminated. On February 10, 2015 and March 11, 2015, we closed the sale of such shares, resulting in aggregate gross proceeds to us of $41.8 million and net proceeds to us of $38.0 million, after deducting the underwriting discount of $2.9 million and estimated offering expenses payable by us of $0.9 million. As of June 30, 2015, we have not used any of the proceeds from our initial public offering and have not made any payments to our directors, officers or persons owning ten percent or more of our ordinary shares or to their associates, or to our affiliates. There has been no material change in the planned use of proceeds from our initial public offering as described in our final prospectus filed with the SEC on February 5, 2015 pursuant to Rule 424(b).
Issuer Purchases of Equity Securities
None.
Item 6. Selected Financial Data.
We derived the selected consolidated statements of operations data for fiscal years 2015, 2014 and 2013 and the balance sheet data as of June 30, 2015 and 2014. You should read the following selected consolidated financial data in conjunction with the section of this Report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our audited consolidated financial statements and related notes included elsewhere in this Report. Our historical results are not necessarily indicative of the results to be expected in the future.
46
Nexvet Biopharma plc became the parent company of Nexvet Australia pursuant to the Irish Reorganization, and for financial reporting purposes the historical consolidated financial statements of Nexvet Australia became the historical consolidated financial statements of Nexvet Biopharma plc and its subsidiaries as a continuation of the predecessor.
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except share and per share amounts) |
|
|||||||||
|
Consolidated Statements of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
License and collaboration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
244 |
|
|
Other |
|
|
25 |
|
|
|
13 |
|
|
|
85 |
|
|
Total revenue |
|
|
25 |
|
|
|
13 |
|
|
|
329 |
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
9,845 |
|
|
|
5,617 |
|
|
|
2,722 |
|
|
General and administrative |
|
|
10,191 |
|
|
|
4,426 |
|
|
|
2,103 |
|
|
Total operating expenses |
|
|
20,036 |
|
|
|
10,043 |
|
|
|
4,825 |
|
|
Loss from operations |
|
|
(20,011 |
) |
|
|
(10,030 |
) |
|
|
(4,496 |
) |
|
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development incentive income |
|
|
3,532 |
|
|
|
2,337 |
|
|
|
1,135 |
|
|
Government grant income |
|
|
403 |
|
|
|
1,317 |
|
|
|
108 |
|
|
Exchange gain (loss) |
|
|
4,151 |
|
|
|
(375 |
) |
|
|
— |
|
|
Interest income |
|
|
68 |
|
|
|
41 |
|
|
|
12 |
|
|
Net loss |
|
$ |
(11,857 |
) |
|
$ |
(6,710 |
) |
|
$ |
(3,241 |
) |
|
Net loss per share attributable to ordinary shareholders, basic and diluted (1) |
|
$ |
(2.27 |
) |
|
$ |
(6.70 |
) |
|
$ |
(3.62 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average ordinary shares outstanding, basic and diluted (1) |
|
|
5,214,957 |
|
|
|
1,000,872 |
|
|
|
894,794 |
|
|
(1) |
See Notes 2 and 11 to our consolidated financial statements included elsewhere in this Report for a description of the method used to compute basic and diluted net loss per share attributable to ordinary shareholders. |
|
|
|
June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|||||
|
Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
52,033 |
|
|
$ |
30,041 |
|
|
Total assets |
|
|
56,672 |
|
|
|
33,604 |
|
|
Total liabilities |
|
|
3,094 |
|
|
|
8,735 |
|
|
Convertible preference shares |
|
|
— |
|
|
|
33,826 |
|
|
Total shareholders’ equity (deficit) |
|
|
53,578 |
|
|
|
(8,957 |
) |
47
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes included elsewhere in this Report. The information contained in this discussion and analysis and set forth elsewhere in this Report includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the section of this Report titled “Risk Factors,” our actual results could differ materially from the results described in or implied by the forward-looking statements.
Overview
We are a clinical-stage biopharmaceutical company focused on transforming the therapeutic market for companion animals by developing and commercializing novel, species-specific biologics based on human biologics. Biologics are therapeutic proteins derived from biological sources. As a class, biologics, which include mAbs and fusion proteins, have transformed human medicine in recent decades and represent some of the top-selling therapies on the market today. Our proprietary platform, which we refer to as “PETization,” is an algorithmic approach that enables us to rapidly create mAbs that are designed to be recognized as “self” or “native” by an animal’s immune system, a property we refer to as “100% species-specificity.” PETization is also designed to build upon the safety and efficacy data from clinically tested human therapies to create new therapies for companion animals, thereby reducing clinical risk and development cost.
Through PETization and other discovery research, we have developed three lead product candidates. NV‑01 is a mAb that targets and inhibits the function of NGF for the control of pain associated with osteoarthritis in dogs. We expect data from our NV‑01 pivotal safety and efficacy study and an additional pilot field safety and efficacy study by the end of 2015. This latter study was initiated following receipt of a sample size reassessment of the pivotal safety and efficacy study in March 2015 and will assess various doses and dosing regimens of NV‑01. This study is expected to provide valuable information that may assist the design of a future pivotal study.
Our second product candidate, NV‑02, is a mAb that is an NGF inhibitor for the control of pain associated with degenerative joint disease, including osteoarthritis, in cats. We announced positive top-line results from our proof-of-concept efficacy study and our pilot safety study of NV‑02 in June 2015. We anticipate results from a pilot field safety and efficacy study of NV‑02 in the first quarter of 2016. Our third product candidate, NV‑08, is a fusion protein that is a TNF inhibitor for the treatment of chronic inflammatory diseases, including atopic dermatitis, in dogs. If our proof-of-concept safety and efficacy studies for NV‑08 are successful, we plan to progress this product candidate into formal development. In addition, primarily using PETization, we are seeking to advance one new product candidate into development per year.
Since our inception, we have been developing our PETization platform, completing proof-of-concept studies for NV-01, NV-02, NV-08 and other product candidates, establishing clonal cell lines, conducting market surveys regarding our lead product candidates and preparing for and conducting pivotal safety and efficacy studies. We also have focused on securing intellectual property, recruiting management and key employees and fundraising activities. We do not have any products approved for sale, have not generated any revenue from product sales since our inception. We have incurred significant net losses since our inception, including $11.9 million, $6.7 million, and $3.2 million for fiscal years 2015, 2014 and 2013, respectively, and as of June 30, 2015 we had an accumulated deficit of $23.7 million. This accumulated deficit has resulted principally from costs incurred in connection with research and development of our product candidates and general and administrative costs associated with our operations.
We will require additional capital until we can generate revenue in excess of operating expenses. We may seek such funding through public or private equity, debt financing or other sources, such as corporate collaborations and licensing arrangements. We may not be able to obtain financing on acceptable terms, or at all. The sale of additional equity would result in additional dilution to our shareholders, and the terms of any financing may adversely affect the rights of our shareholders. The incurrence of any debt financing could result in debt service obligations and the instruments governing such debt could provide for operating and financing covenants that would restrict our operations. If we are unable to obtain funding, we could be forced to delay, reduce or eliminate our research and development programs or commercialization efforts, which could adversely affect our business prospects.
In February 2015, we closed our initial public offering of 4.0 million ordinary shares at a price to the public of $10.00 per share. In March 2015, the underwriters partially exercised their over-allotment option and purchased an additional 0.2 million shares. Following the sales of these securities, we received aggregate gross proceeds of $41.8 million and net proceeds of $38.0 million, after deducting the underwriting discount of $2.9 million and estimated offering expenses of $0.9 million payable by us. Upon the initial closing, all preference shares were converted to ordinary shares. Our ordinary shares are listed on the Nasdaq Global Market under the symbol “NVET.”
48
In March 2015, we received the results of a sample size reassessment for our NV‑01 pivotal safety and efficacy study. The results indicated that, in order to have a high probability of a statistically significant endpoint at the day 28 primary assessment, it would be necessary to increase the current size of the study. The results also indicated that placebo was not superior to NV‑01 with regard to treatment success and that it was not necessary to increase the number of NV‑01 treated dogs to potentially over 1,000 participants. We assessed the impact of time, cost and related logistical issues regarding the feasibility of substantially increasing the number of dogs in the current study, or initiating additional studies. In April 2015, we concluded that we should continue the current study to completion without a change in study size, while also commencing a new placebo-controlled multi-site pilot field safety and efficacy study to assess various doses and dosing regimens of NV‑01. This new study is expected to enroll approximately 150 dogs, to cost approximately $1.0 million, to be completed in the fourth quarter of 2015 and to provide valuable information that may assist the design of a future pivotal study.
In June 2015, we announced positive top-line results from our proof-of-concept efficacy study and our pilot safety study of NV‑02 in 32 cats. We plan to shortly commence a placebo-controlled, blinded, multi-site pilot field safety and efficacy study targeting enrollment of 90 cats with naturally occurring osteoarthritis. We anticipate results from this pilot study in the first quarter of 2016.
Share Consolidations and Irish Reorganization
In August 2014, Nexvet Australia Pty Ltd, or Nexvet Australia, completed a one-for-four share consolidation pursuant to which each holder of ordinary shares received one ordinary share for every four ordinary shares held by such holder, and each holder of preference shares received one preference share for every four preference shares held by such holder. The number of ordinary shares that may be acquired upon exercise of options or warrants or upon conversion of restricted share units was similarly reduced on a one-for-four basis, with a proportionate adjustment to the exercise or conversion price, as applicable. Because Irish law requires the payment to an issuer of at least the nominal value per share in order to acquire such shares from the issuer, any options or restricted share units with a zero exercise or conversion price became exercisable or convertible, as applicable, at the nominal value per ordinary share in August 2014 in anticipation of the Irish Exchange. This nominal value became $0.10 per ordinary share in September 2014 in connection with the Irish Exchange and was revised to $0.125 per ordinary share in connection with the four-for-five share consolidation in November 2014.
In September 2014, Nexvet Australia completed the Irish Exchange, pursuant to which (i) Nexvet Biopharma Limited became the parent company of Nexvet Australia and its subsidiaries and (ii) holders of ordinary shares, preference shares, restricted share units and options and warrants to purchase ordinary shares of Nexvet Australia exchanged their holdings for equivalent ordinary shares, preference shares, restricted share units or options or warrants to purchase ordinary shares, as applicable, of Nexvet Biopharma Limited. We refer to this transaction as the “Irish Exchange.” Nexvet Biopharma Limited then re-registered as an Irish public limited company in September 2014. We refer to this re-registration and the Irish Exchange collectively as the “Irish Reorganization.” Nexvet Biopharma plc became the parent company of Nexvet Australia pursuant to the Irish Reorganization, and for financial reporting purposes the historical consolidated financial statements of Nexvet Australia became the historical consolidated financial statements of Nexvet Biopharma plc and its subsidiaries as a continuation of the predecessor.
In November 2014, we completed a four-for-five share consolidation pursuant to which each holder of ordinary shares received four ordinary shares for every five ordinary shares held by such holder, and each holder of preference shares received four preference shares for every five preference shares held by such holder. The number of ordinary shares that may be acquired upon exercise of options or warrants or upon conversion of restricted share units was similarly reduced on a four-for-five basis, with a proportionate adjustment to the exercise or conversion price, as applicable.
Basis of Presentation
Revenue
License and collaboration revenue in fiscal year 2013 consisted of an upfront payment under a license and collaboration agreement. Upon execution of the agreement, we were entitled to the upfront payment without any further performance obligations.
Operating Expenses
The majority of our operating expenses have been research and development activities related to our lead product candidates and general and administrative costs associated with our business.
49
Research and Development Expense
Research and development costs are expensed as incurred and consist primarily of (i) payroll and related expense for all employees engaged in scientific research and development functions, including wages, related benefits and share-based compensation, (ii) fees for regulatory, professional and other consultants and (iii) development costs, including costs of drug discovery, safety, proof-of-concept, pilot and pivotal safety and efficacy studies, development of biological materials, and service providers. We are currently pursuing our NV‑01, NV‑02 and NV‑08 lead product candidates and typically use our employee and infrastructure resources across multiple development programs. We allocate outsourced development costs by lead product candidates for billing and research and development incentive income purposes, but we do not allocate personnel or other internal costs related to development to specific product candidates.
We expect research and development expense to increase significantly for the foreseeable future as we continue to increase our headcount, commence and conduct pivotal safety and efficacy studies and further develop our lead product candidates. We expect research and development expense associated with NV‑01 to be approximately $14.0 million over the next three years, which includes $1.0 million for our new pilot field safety and efficacy study, $4.0 million for the cost of our pivotal safety and efficacy study and $9.0 million for chemistry, manufacturing and controls studies, stability studies, regulatory compliance and other miscellaneous costs principally using third parties. Our development of NV‑02 and NV‑08 is not as advanced as our development of NV‑01. However, assuming development costs similar to those for NV‑01, we estimate research and development expense for the development of each of NV‑02 and NV‑08 to be approximately $13.0 to $15.0 million.
Drug development is inherently unpredictable and the nature, specific timing and estimated costs of the efforts that will be necessary to complete the development of our lead product candidates are subject to numerous factors. For example, the nature, timing and amount of research and development expense incurred will depend largely upon the outcomes of current and future pivotal safety and efficacy studies for our lead product candidates as well as the related regulatory requirements, manufacturing costs and other costs associated with the development of our lead product candidates. Factors that can influence the duration, cost and timing of our pivotal safety and efficacy studies and development of our lead product candidates include:
|
|
· |
the scope, rate of progress and expense of our ongoing, as well as any additional, pivotal safety and efficacy studies and other research and development activities; |
|
|
· |
results of future pivotal safety and efficacy studies; |
|
|
· |
potential changes in government regulation; and |
|
|
· |
the timing and receipt of any regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a lead product candidate could mean a significant change in the costs and timing associated with the development of that product candidate.
General and Administrative Expense
General and administrative expense consists primarily of non-research and development-related payroll and related expense for employees, consultants and directors, including wages, related benefits and share-based compensation. General and administrative expense also includes professional and consulting fees for legal, accounting, tax services and other general business services, as well other expenses such as travel, rent and facilities costs. We expect general and administrative expense to increase as we operate as a public company and continue to build our corporate infrastructure and prepare to commercialize and market our products.
Other Income (Expense)
Research and Development Incentive Income
We are eligible under the AusIndustry research and development tax incentive program to obtain a cash amount from the Australian Taxation Office. The tax incentive is available to us on the basis of specific criteria with which we must comply. Although the research and development assistance is administered through the Australian Taxation Office, we have accounted for the tax incentive outside the scope of Accounting Standards Codification, or ASC, Topic 740, Income Taxes, as an income tax benefit since we meet the applicable requirements to participate in the program and the incentive is not linked to our income tax liability and can be realized regardless of whether we have generated taxable income. Research and development incentive income is recognized when the research and development activities have been undertaken and we have completed our assessment of whether such activities meet the relevant qualifying criteria. We intend to continue conducting research and development in Australia, and we expect to therefore remain eligible for this incentive.
50
We recognize government grant income at the fair value of the grant when it is received and all substantive conditions have been satisfied. We do not expect that future Australian government grant income will be available under our existing grant award.
Exchange Gain (Loss)
Exchange gain (loss) consists primarily of gains or losses due to foreign exchange translation, primarily reflecting changes in Australian and U.S. foreign exchange rates. Under accounting principles generally accepted in the United States, or U.S. GAAP, these gains and losses relate to a translation of U.S. dollar-denominated bank accounts into Nexvet Australia’s Australian dollar functional currency and represent a non-cash item.
Interest Income
We earn interest on the cash balances held with financial institutions and recognize interest when earned on an accruals basis over time.
Results of Operations
Results of operations for fiscal years 2015, 2014 and 2013 were as follows:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
License and collaboration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
244 |
|
|
Other |
|
|
25 |
|
|
|
13 |
|
|
|
85 |
|
|
Total revenue |
|
|
25 |
|
|
|
13 |
|
|
|
329 |
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
9,845 |
|
|
|
5,617 |
|
|
|
2,722 |
|
|
General and administrative |
|
|
10,191 |
|
|
|
4,426 |
|
|
|
2,103 |
|
|
Total operating expenses |
|
|
20,036 |
|
|
|
10,043 |
|
|
|
4,825 |
|
|
Loss from operations |
|
|
(20,011 |
) |
|
|
(10,030 |
) |
|
|
(4,496 |
) |
|
Other income and expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development incentive income |
|
|
3,532 |
|
|
|
2,337 |
|
|
|
1,135 |
|
|
Government grant income |
|
|
403 |
|
|
|
1,317 |
|
|
|
108 |
|
|
Exchange gain (loss) |
|
|
4,151 |
|
|
|
(375 |
) |
|
|
— |
|
|
Interest income |
|
|
68 |
|
|
|
41 |
|
|
|
12 |
|
|
Net loss |
|
$ |
(11,857 |
) |
|
|
(6,710 |
) |
|
$ |
(3,241 |
) |
Foreign Currency
Our functional currency is U.S dollars, and the functional currency for most subsidiaries is their local currency. Foreign currency transactions are translated into the functional currency using the current exchange rate at the date of the transaction. At year end, monetary items denominated in a foreign currency are translated into the functional currency of the relevant entity using the year end spot rate. The exchange gain of $4.2 million in fiscal year 2015 and exchange loss of $0.4 million in fiscal year 2014 primarily relate to the translation of Nexvet Australia’s U.S. dollar-denominated bank accounts to its Australian dollar functional currency.
In preparing our consolidated financial statements, the financial statements of the subsidiaries are translated at year-end exchange rates as to assets and liabilities and weighted-average rates as to revenue and expenses. The resulting translation adjustments are recognized in other comprehensive income (loss), or OCI. The non-cash translation adjustment in accumulated OCI was a loss of $4.9 million in fiscal year 2015 and a gain of $0.3 million in fiscal year 2014. These adjustments primarily relate to the translation of U.S. dollar-denominated bank accounts within Nexvet Australia’s balance sheet to the U.S dollar presentation currency of the consolidated balance sheet.
Although the two foreign exchange-related items both relate to our U.S. dollar-denominated bank accounts, which in substance offset each other, under U.S. GAAP, there is no offset of these two items within the consolidated statements of operations and comprehensive loss. Accordingly, in addition to U.S. GAAP measures, management uses net loss excluding exchange gain (loss), which is a non-GAAP financial measure, to evaluate the business as management believes that it is a better reflection of the underlying business performance.
51
Net loss excluding exchange gain (loss) for the fiscal year ended June 30, 2015, 2014 and 2013, and a reconciliation to net loss, is presented below:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Net loss as reported |
|
$ |
(11,857 |
) |
|
$ |
(6,710 |
) |
|
$ |
(3,241 |
) |
|
Exchange gain (loss) |
|
|
4,151 |
|
|
|
(375) |
|
|
|
— |
|
|
Net loss excluding exchange gain (loss) |
|
$ |
(16,008 |
) |
|
$ |
(6,335 |
) |
|
$ |
(3,241 |
) |
Comparison of Fiscal Year 2015 to Fiscal Year 2014
Other Revenue
Other revenue in fiscal years 2015 and 2014 of $25,000 and $13,000, respectively, was attributable to development services.
Research and Development Expense
Research and development expense for fiscal years 2015 and 2014 was as follows:
|
|
|
Year Ended June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Payroll and related |
|
$ |
3,158 |
|
|
$ |
1,162 |
|
|
Consulting |
|
|
340 |
|
|
|
249 |
|
|
Development costs |
|
|
6,347 |
|
|
|
4,206 |
|
|
Total |
|
$ |
9,845 |
|
|
$ |
5,617 |
|
Research and development expense increased in fiscal year 2015 by $4.2 million to $9.8 million, or 75.2%, from $5.6 million in fiscal year 2014. The increase was primarily attributable to a $2.0 million increase in payroll and related costs and $2.1 million increase in development costs. Payroll and related costs increased as we continued to build our research and development team with headcount in this division increasing from 13 employees at June 30, 2014 to 18 employees at June 30, 2015, and share-based compensation increasing by $0.7 million. Development costs increased as we advanced the development of our lead product candidates, including NV‑01’s pivotal safety and efficacy study and additional pilot field safety and efficacy study and NV‑02’s proof-of-concept studies.
In fiscal year 2015, outsourced development costs for NV‑01, NV‑02, NV‑08 and general research were $3.8 million, $1.4 million, $0.3 million and $0.8 million, respectively, compared to $1.7 million, $1.2 million, $0.5 million and $0.8 million in fiscal year 2014.
General and Administrative Expense
General and administrative expense for fiscal years 2015 and 2014 was as follows:
|
|
|
Year Ended June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Payroll and related |
|
$ |
3,736 |
|
|
$ |
1,498 |
|
|
Consulting and legal fees |
|
|
4,403 |
|
|
|
1,753 |
|
|
Other costs |
|
|
2,052 |
|
|
|
1,175 |
|
|
Total |
|
$ |
10,191 |
|
|
$ |
4,426 |
|
General and administrative expense increased in fiscal year 2015 by $5.8 million to $10.2 million, or 130%, from $4.4 million in fiscal year 2014. The increase was primarily attributable to a $2.2 million increase in payroll and related costs, $2.6 million in consulting and legal fees and $0.9 million of other costs. Within the overall increase in fiscal year 2015, initial public offering related costs were $2.3 million, including $0.5 million associated with the Irish Reorganization, and share-based compensation costs were $1.3 million.
52
The increase in payroll and related costs reflect the increased remuneration of our key management and directors to align with the remuneration of executives and directors of other publicly listed companies and include share-based compensation of $1.3 million (compared to $0.2 million in fiscal year 2014) mainly due to awards issued to employees and directors associated with the completion of our initial public offering. The increase in consulting and legal costs was mainly due to fees paid to legal advisors and auditors in connection with our initial public offering. The increase in other costs was mainly due to $0.3 million of travel costs, $0.3 million of printing costs and $0.2 million of regulatory costs mostly associated with our initial public offering.
Research and Development Incentive Income
Research and development incentive income increased by $1.2 million, from $2.3 million in fiscal year 2014 to $3.5 million in fiscal year 2015, primarily due to our increase in qualifying expenditures in fiscal year 2015.
At the current enacted rate of 45%, the research and development incentive is $3.6 million in fiscal year 2015. Given the proposed reduction in the legislative rate to 43.5%, we have made an allowance of $0.1 million on the grounds of official notification that the new rate will be retrospectively applied if enacted.
Government Grant Income
In fiscal years 2015 and 2014, we recognized $0.4 million and $1.3 million, respectively, in income from an Australian government grant.
Comparison of Fiscal Year 2014 to Fiscal Year 2013
Revenue
Revenue in fiscal year 2013 was $0.3 million, which was primarily attributable to an upfront payment pursuant to a license and collaboration agreement of $0.2 million and $0.1 million in other revenue from an insurance refund.
Research and Development Expense
Research and development expense for fiscal years 2014 and 2013 was as follows:
|
|
|
Year Ended June 30, |
|
|||||
|
|
|
2014 |
|
|
2013 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Payroll and related |
|
$ |
1,162 |
|
|
$ |
642 |
|
|
Consulting |
|
|
249 |
|
|
|
262 |
|
|
Development costs |
|
|
4,206 |
|
|
|
1,818 |
|
|
Total |
|
$ |
5,617 |
|
|
$ |
2,722 |
|
Research and development expense increased in fiscal year 2014 by $2.9 million to $5.6 million, or 107%, from $2.7 million in fiscal year 2013. The increase was primarily attributable to a $0.5 million increase in payroll and related costs and a $2.4 million increase in development costs. The increase in payroll and related costs was mainly due to building our research and development team. The increase in development costs was mainly due to advancing the development of our lead product candidates, including NV‑01’s clonal cell development work, initiation of our pivotal safety and efficacy study and for NV‑02’s proof-of-concept study.
In each of fiscal years 2014 and 2013, outsourced development costs were $1.7 million and $1.4 million, respectively, for NV‑01, $1.2 million and $0.1 million, respectively, for NV‑02 and $0.5 million and $0.1 million, respectively, for NV‑08.
General and Administrative Expense
General and administrative expense for fiscal years 2014 and 2013 was as follows:
|
|
|
Year Ended June 30, |
|
|||||
|
|
|
2014 |
|
|
2013 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Payroll and related |
|
$ |
1,498 |
|
|
$ |
687 |
|
|
Consulting and legal fees |
|
|
1,753 |
|
|
|
911 |
|
|
Development costs |
|
|
1,175 |
|
|
|
505 |
|
|
Total |
|
$ |
4,426 |
|
|
$ |
2,103 |
|
53
General and administrative expense increased in fiscal year 2014 by $2.3 million to $4.4 million, or 110%, from $2.1 million in fiscal year 2013. Payroll and related costs increased $0.8 million mainly due to building our general and administrative team, including $0.2 million of share-based payment expense. Consulting and legal expenses increased $0.8 million. Other costs increased $0.7 million mainly due to increases in travel and property lease costs.
Research and Development Incentive Income
Research and development incentive income increased by $1.2 million, from $1.1 million in fiscal year 2013 to $2.3 million in fiscal year 2014, due to our increase in qualifying expenditures in fiscal year 2014.
Government Grant Income
In fiscal year 2013, we were awarded an Australian government grant of $1.5 million, of which $1.3 million was recognized in fiscal year 2014 and $0.1 million was recognized in fiscal year 2013.
Liquidity and Capital Resources
We have incurred losses and negative cash flows from operations since our inception. As of June 30, 2015, we had an accumulated deficit of $23.7 million. From our inception until our initial public offering, we raised aggregate gross proceeds of $41.4 million from the sale of preference shares, ordinary shares, convertible notes and warrants. In February and March 2015, we raised a further aggregate gross proceeds of $41.8 million and aggregate net proceeds of $38.0 million, after deducting the underwriting discount of $2.9 million and estimated offering expenses of $0.9 million payable by us, from the sale of ordinary shares in our initial public offering. We believe our cash balance of $52.0 million as of June 30, 2015, will be sufficient to fund our anticipated level of operations for at least the next 12 months. For the foreseeable future, we expect to continue to incur losses, which will increase from historical levels as we expand our product development activities, seek regulatory approvals for our lead product candidates and begin commercialization activities in anticipation of regulatory approval.
However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other sources, such as strategic collaborations. Such financing may result in dilution to shareholders, imposition of debt covenants and repayment obligations or other restrictions that may affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plan.
Our future capital requirements will depend on many factors, including:
|
|
· |
the scope, progress, results and costs of researching and developing our current or future product candidates, including conducting proof-of-concept, pilot and pivotal safety and efficacy studies; |
|
|
· |
the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future product candidates; |
|
|
· |
the number and characteristics of the product candidates we pursue; |
|
|
· |
whether we acquire or license any other companies, assets, intellectual property or technologies in the future; |
|
|
· |
the cost of commercialization activities, if any of our current or future product candidates are approved for sale, including marketing, sales and distribution costs; |
|
|
· |
the cost of manufacturing our current and future product candidates and any approved products we successfully commercialize; |
|
|
· |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
|
|
· |
the expenses needed to attract and retain skilled personnel; |
|
|
· |
the costs associated with being a public company; and |
|
|
· |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, if any arise, including litigation costs and the outcome of such litigation. |
54
The following table shows a summary of our cash flows for the periods set forth below:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Net cash used in operating activities |
|
$ |
(10,852 |
) |
|
$ |
(5,944 |
) |
|
$ |
(2,962 |
) |
|
Net cash used in investing activities |
|
|
(313 |
) |
|
|
(514 |
) |
|
|
(54 |
) |
|
Net cash provided by financing activities |
|
|
37,970 |
|
|
|
35,701 |
|
|
|
3,392 |
|
|
Effect of exchange rate changes on cash |
|
|
(4,813 |
) |
|
|
315 |
|
|
|
88 |
|
Net Cash Used in Operating Activities
For fiscal year 2015, net cash used in operating activities was $10.9 million. Net cash used in operating activities was primarily attributable to our net loss of $11.9 million and changes in our operating assets and liabilities of $1.3 million, offset by non-cash share-based compensation expense of $2.1 million and $0.2 million relating to non-cash depreciation and amortization.
For fiscal year 2014, net cash used in operating activities was $5.9 million. Net cash used in operating activities was primarily attributable to our net loss of $6.7 million, offset by non-cash share-based compensation expense of $0.3 million, $0.1 million relating to non-cash depreciation and amortization and changes in our operating assets and liabilities of $0.4 million.
For fiscal year 2013, net cash used in operating activities was $3.0 million. Net cash used in operating activities was primarily attributable to our net loss of $3.2 million and changes in our operating assets and liabilities of $0.5 million, partially offset by non-cash share-based compensation expense of $0.7 million.
Net Cash Used in Investing Activities
For fiscal year 2015, net cash used in investing activities was $0.3 million, which was primarily attributable to purchases of property, plant and equipment.
For fiscal year 2014, net cash used in investing activities was $0.5 million, which was primarily attributable to purchases of property, plant and equipment.
For fiscal year 2013, net cash used in investing activities was $0.1 million, which was solely attributable to purchases of property, plant and equipment.
Net Cash Provided by Financing Activities
For fiscal year 2015, net cash provided by financing activities was $38.0 million. Net cash provided by financing activities was primarily attributable to gross proceeds of $41.8 million from our initial public offering, offset by issuance costs of $3.8 million.
For fiscal year 2014, net cash provided by financing activities was $35.7 million. Net cash provided by financing activities was primarily attributable to gross proceeds of $36.4 million from the sale of our preference shares, offset by issuance costs of
$1.3 million, of which $0.6 million was non-cash issuance costs in the form of warrants to advisors.
For fiscal year 2013, net cash provided by financing activities was $3.4 million. Net cash provided by financing activities was attributable to gross proceeds of $3.6 million from sale of our preference shares, offset by repayment of $0.2 million of notes to a related party.
55
Contractual Obligations and Commitments
The following table summarizes our principal contractual obligations and commitments, which are related to property leases and supplier purchase obligations, at June 30, 2015.
|
June 30, 2015 |
|
Total |
|
|
Less than 1 Year |
|
|
1 to 3 Years |
|
|
3 to 5 Years |
|
|
After 5 Years |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
Operating leases (1) |
|
$ |
352 |
|
|
$ |
109 |
|
|
$ |
190 |
|
|
$ |
53 |
|
|
$ |
— |
|
|
Purchase obligations (2) |
|
|
680 |
|
|
|
680 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
1,032 |
|
|
$ |
789 |
|
|
$ |
190 |
|
|
$ |
53 |
|
|
$ |
— |
|
|
(1) |
Represents future minimum lease payments under our non-cancelable operating lease, including our Melbourne and Ireland facilities. |
|
(2) |
Represents future payments pursuant to contracts with suppliers of goods and services to support our product development. We enter into agreements in the normal course of business with CROs for pivotal safety and efficacy studies and with vendors for pre-clinical research studies and other services and products for operating purposes which are cancelable at any time by us, generally upon 30 days prior written notice. These payments are not included in this table of contractual obligations. |
Off-Balance Sheet Arrangements
Since inception, we have not engaged in the use of any off-balance sheet arrangements, such as structured finance entities, special purpose entities or variable interest entities.
Critical Accounting Policies and Significant Judgments and Estimates
Our consolidated financial statements have been prepared in accordance with U.S. GAAP. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, and revenue, costs and expenses and related disclosures during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements included elsewhere in this Report, we believe that the estimates and assumptions involved in the following accounting policies may have the greatest potential impact on our consolidated financial statements.
Research and Development Accruals
As part of the process of preparing our consolidated financial statements, we are required to estimate accrued research and development expenses. Examples of estimated accrued expenses include fees paid to vendors and clinical sites in connection with our pivotal safety and efficacy studies, to CROs in connection with our proof-of-concept or pivotal safety and efficacy studies and to contract manufacturers in connection with the production of our lead product candidates and formulated biologics.
We review new and open contracts and communicate with applicable internal and vendor personnel to identify services that have been performed on our behalf and estimate the level of service performed and the associated costs incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost for accrued expenses. The majority of our service providers invoice us monthly in arrears for services performed or as milestones are achieved in relation to our contract manufacturers. We make estimates of our accrued expenses as of each balance sheet date.
We base our accrued expenses related to proof-of-concept and pivotal safety and efficacy studies on our estimates of the services received and efforts expended pursuant to contracts with vendors, our internal resources, and payments to clinical sites based on animal enrollments. The financial terms of the vendor agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of animals and the completion of development milestones. We estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the related expense accrual accordingly on a prospective basis. If we do not identify costs that have been incurred or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. To date, we have not made any material adjustments to our estimates of accrued research and development expenses or the level of services performed in any reporting period presented.
56
We recognize share-based compensation expense based on the grant date fair value of the award over the period during which an employee is required to provide service in exchange for the award. Where performance conditions are attached to the awards, compensation expense is recognized in the period in which it becomes probable that the performance target will be achieved, net of estimate of pre-vesting forfeitures over the requisite service period. The probability of vesting is reassessed at each reporting period for awards with performance conditions and compensation expense is adjusted based on its probability assessment.
Determining the fair value of share-based awards at the grant date requires judgment, and our estimates of the fair value of our ordinary shares prior to the completion of our initial public offering was highly complex and subjective.
We use the binomial option-pricing model to determine the fair value of share options. This determination is affected by our estimated fair value per ordinary share as well as assumptions regarding a number of other complex and subjective variables. These variables include the fair value of our ordinary shares, the expected term of the share option, our volatility over that expected term, expected dividend yield and risk-free interest rates. Restricted share units are valued at the fair value of the underlying ordinary shares as of the date of grant. See Note 13 to our consolidated financial statements included elsewhere in this Report for further details.
In addition to these assumptions, we estimate forfeitures based upon our historical experience. At each period end, we review the estimated forfeiture rate and make changes as factors affecting the forfeiture rate calculations and assumptions change.
If any assumptions used in the binomial option-pricing model change significantly, share-based compensation for future awards may differ materially compared with the awards granted previously. For fiscal years 2015, 2014 and 2013, share-based compensation expense was $2.1 million, $0.3 million, and $0.7 million, respectively. We had an aggregate of $2.8 million of unrecognized share-based compensation expense as of June 30, 2015, which we expect to recognize over an estimated period of 4 years for awards outstanding.
As permitted by Australian law, the board of directors of Nexvet Australia historically granted share options and restricted share units with an exercise or conversion price of zero. Because Irish law requires the payment to an issuer of at least the nominal value of shares in order to acquire such shares from the issuer, any options or restricted share units with a zero exercise or conversion price became exercisable or convertible, as applicable, at the nominal value per ordinary share in August 2014 in anticipation of the Irish Exchange. This nominal value became $0.10 per ordinary share in September 2014 in connection with the Irish Exchange and was revised to $0.125 per ordinary share in connection with the four-for-five share consolidation in November 2014. Contemporaneously with these awards and based upon information available at the time of grant, the board of directors, with the assistance of management, also determined the fair value of the shares underlying these awards for financial reporting purposes. The intention has been that all awards granted have been ascribed a value per ordinary share for financial reporting purposes equal to the fair value per ordinary share underlying those awards on the date of grant. Given the absence of a public trading market for our ordinary shares, and in accordance with the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, our board of directors has exercised reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of the fair value of our ordinary shares at each grant date prior to our initial public offering. These factors included:
|
|
· |
contemporaneous third-party valuations; |
|
|
· |
current business conditions and projections; |
|
|
· |
risks inherent to the development of our research and development programs, including the status of pivotal safety and efficacy studies for our lead product candidates; |
|
|
· |
our financial condition, including cash on hand; |
|
|
· |
our need for future financing to fund our research and development efforts and the commercialization of our lead product candidates, including the likelihood of achieving further funding or an initial public offering; |
|
|
· |
the composition of, and changes to, our management team and board of directors; |
|
|
· |
the rights and preferences of our preference shares relative to our ordinary shares; |
|
|
· |
the lack of marketability of our ordinary shares; and |
|
|
· |
external market and economic conditions and other trends and conditions affecting the pharmaceutical, veterinary care and biotechnology industries. |
The aggregate intrinsic value of share-based awards as of June 30, 2015 was $2.4 million, of which $0.2 million related to vested share-based awards and $2.2 million related to unvested share-based awards.
57
We record certain assets and liabilities at fair value in accordance with the provisions of ASC Topic 820, Fair Value Measurements. As defined in the guidance, fair value, defined as an exit price, represents the amount that would be received to sell an asset or pay to transfer a liability in an orderly transaction between market participants. As a result, fair value is a market-based approach that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering these assumptions, the guidance defines a three-tier value hierarchy that prioritizes the inputs used in the valuation methodologies in measuring fair value.
At June 30, 2014, our liabilities primarily consisted of warrants that were issued to investors and financial advisors in connection with private placements of our securities in May 2014. The warrants permit the holders to purchase ordinary shares at exercise prices of $8.625 and $7.50 per share on or before May 2019. Because the warrants may be net exercised and are exercisable in U.S. dollars, and the functional currency of Nexvet Australia, the original issuer of the warrants, is Australian dollars, they were classified as a liability as of June 30, 2014 and were reclassified as shareholders’ equity in September 2014 following the Irish Reorganization (in which the original warrants were exchanged for warrants issued by Nexvet Biopharma plc) and the change in our functional currency to U.S. dollars.
Warrants recorded as liabilities ($5.4 million as of June 30, 2014) are valued at fair value using the binomial option-pricing model. The expected term used in the model has been adjusted, based on management’s best estimate, for the effects of non-transferability, exercise restrictions and behavioral considerations. At each balance sheet date, the outstanding warrants are revalued to their current fair value, with the difference in fair value recorded in the consolidated statements of operations and comprehensive loss.
Jumpstart Our Business Startups Act
The JOBS Act provides that an “emerging growth company” can delay adopting new or revised accounting standards until those standards apply to private companies. We have irrevocably elected not to avail ourselves of this delayed adoption of new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as public companies that are not emerging growth companies.
Income Taxes
We have historically filed income tax returns in Australia and the United States and in the future also expect to file tax returns in Ireland.
As of June 30, 2015, we had total deferred tax assets of $2.2 million. Our management has evaluated the factors bearing upon the ability to realize our deferred tax assets, which are comprised largely of tax loss carry forwards including Australian income tax losses of $7.2 million. Our management concluded that a full valuation allowance was necessary to offset our net deferred tax assets due to our lack of taxable income prospects for the foreseeable future. In June 2015, a change was passed in the relevant small business tax rate applicable to Nexvet Australia from 30% to 28.5%, which will apply from July 1, 2015. Deferred tax balances have been stated at the applicable 28.5% at June 30, 2015.
Recently Adopted Accounting Pronouncements
We have early adopted the provisions of Accounting Standards Update, or ASU, No. 2014-10, Elimination of Certain Financial Requirements, Including an Amendment to Variable Interest Entities Guidance Topic in Topic 810, Consolidation, starting in fiscal year 2014. In June 2014, the Financial Accounting Standards Board, or FASB, issued guidance removing the definition of a development stage entity from the Master Glossary of the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. This guidance also eliminates an exception provided to development stage entities in ASC Topic 810, Consolidation, for determining whether an entity is a variable interest entity on the basis of the amount of the investment equity that is at risk. On adoption, we were not required to present or disclose any information required by ASC Topic 915, Development Stage Entities.
58
Recently Issued Accounting Pronouncements
In May 2014, the FASB issued ASU, No. 2014-09, Revenue from Contracts with Customers. This guidance requires an entity to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This guidance also requires an entity to disclose sufficient information to enable users of financial statements to understand the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. Qualitative and quantitative information is required about:
|
|
· |
Contracts with customers—including revenue and impairments recognized, disaggregation of revenue and information about contract balances and performance obligations (including the transaction price allocated to the remaining performance obligations). |
|
|
· |
Significant judgments and changes in judgments—determining the timing of satisfaction of performance obligations (over time or at a point in time), and determining the transaction price and amounts allocated to performance obligations. |
|
|
· |
Certain assets—assets recognized from the costs to obtain or fulfill a contract. |
In July 2015, the FASB delayed the effective date of this guidance. As a result, this guidance will be effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period. Earlier application is permitted only as of annual reporting periods beginning after December 15, 2016, including interim reporting periods within that reporting period. We are currently evaluating the impact that this guidance will have on our consolidated results of operations, financial position and cash flows.
In June 2014, the FASB issued ASU 2014-12, Compensation—Stock Compensation. This guidance requires that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply the existing guidance in ASC Topic 718, Compensation—Stock Compensation, as it relates to awards with performance conditions that affect vesting to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation expense should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation expense attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation expense should be recognized prospectively over the remaining requisite service period. The total amount of compensation expense recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. This guidance will be effective for annual reporting periods beginning after December 15, 2015. We are currently evaluating the impact that this guidance will have on our consolidated results of operations, financial position and cash flows.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40). This guidance defines management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and to provide related footnote disclosures. Under the guidance, management is required to evaluate, for each annual and interim reporting period, whether it is probable that the entity will not be able to meet its obligations as they become due within one year after the date that the financial statements are issued or are available to be issued. When management identifies substantial doubt about the entity’s ability to continue as a going concern, additional disclosures are required. This guidance will be effective for annual reporting periods beginning after December 15, 2016. We do not expect the adoption of this guidance to have a material impact on our consolidated results of operations, financial position and cash flows.
In February 2015, the FASB issued ASU 2015-02, Consolidation. This guidance amends existing consolidation guidance in which a reporting entity might be required to consolidate another legal entity in situations in which the reporting entity’s contractual rights do not give it the ability to act primarily on its own behalf, the reporting entity does not hold a majority of the legal entity’s voting rights, or the reporting entity is not exposed to a majority of the legal entity’s economic benefits or obligations. The guidance:
|
|
· |
modifies the evaluations of whether limited partnerships and similar legal entities are variable interest entities or voting interest entities; |
|
|
· |
eliminates the presumption that a general partner should consolidate a limited partner; |
|
|
· |
affects the consolidation analysis of reporting entities that are involved with variable interest entities, particularly those that have fee arrangements and related party relationships; and |
|
|
· |
provides a scope exception from consolidation guidance for reporting entities with interests in certain investment funds. |
The guidance is effective for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2015. We do not expect the adoption of this guidance to have a material impact on our consolidated results of operations, financial position or cash flows.
59
In April 2015, the FASB issued ASU 2015-03, Interest—Imputation of Interest. This guidance simplifies the presentation of debt issuance costs by requiring that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of the debt liability, consistent with debt discounts. The guidance will be effective for annual reporting periods beginning after December 15, 2015, and interim periods within fiscal years beginning after December 15, 2016. We do not expect the adoption of this guidance to have a material impact on our consolidated results of operations, financial position or cash flows.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Our cash as of June 30, 2015 was deposited with several large commercial banks located in the United States and Australia. Our primary exposure to market risk for our cash is interest income sensitivity, which is affected by changes in the general level of U.S. and, to a lesser extent, Australian interest rates. However, because our cash is held in bank accounts, a sudden change in the interest rates associated with our cash balances would not be expected to have a material impact on our financial condition or results of operations.
We do not have any major foreign currency or derivative financial instruments. Cash is predominantly held in U.S. dollars. We have operations in different economic environments that use the local currency as the functional currency. This exposes us to currency exchange fluctuations.
Item 8. Financial Statements and Supplementary Data.
The financial statements required by this item are set forth beginning at page F-1 of this Report and are incorporated herein by reference.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of disclosure controls and procedures.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15(d)-15(e)), as of the end of the period covered by this Report. Based upon the evaluation, the Chief Executive Officer and Chief Financial Officer concluded that, as of the end of such period, the disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed in the reports we file and submit under the Exchange Act, is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Management’s Report on Internal Control Over Financial Reporting
This Report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our independent registered public accounting firm due to a transition period established by the rules of the SEC for newly public companies.
Changes in Internal Control Over Financial Reporting
There have been no significant changes in our internal control over financial reporting during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
60
Item 10. Directors, Executive Officers and Corporate Governance.
The information called for by this item is incorporated by reference to our Proxy Statement for the Annual Meeting of Shareholders to be filed with the SEC within 120 days of the end of the financial year covered by this Annual Report.
Item 11. Executive Compensation.
The information called for by this item is incorporated by reference to our Proxy Statement for the Annual Meeting of Shareholders to be filed with the SEC within 120 days of the end of the financial year covered by this Annual Report.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information called for by this item is incorporated by reference to our Proxy Statement for the Annual Meeting of Shareholders to be filed with the SEC within 120 days of the end of the financial year covered by this Annual Report.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information called for by this item is incorporated by reference to our Proxy Statement for the Annual Meeting of Shareholders to be filed with the SEC within 120 days of the end of the financial year covered by this Annual Report.
Item 14. Principal Accounting Fees and Services.
The information called for by this item is incorporated by reference to our Proxy Statement for the Annual Meeting of Shareholders to be filed with the SEC within 120 days of the end of the financial year covered by this Annual Report.
61
Item 15. Exhibits, Financial Statement Schedules.
The financial statement schedules and exhibits filed as part of this Report are as follows:
(a)(1) Financial Statements
Reference is made to the consolidated financial statements included in Item 8 of Part II of this Report.
(a)(2) Financial Statement Schedules
Financial statement schedules have been omitted because the required information is not present, or not present in amounts sufficient to require submission of the schedules, or because the required information is provided in the financial statements or notes thereto.
(a)(3) Exhibits
The exhibits required to be filed as part of this Report are listed in the Exhibit Index attached hereto and are incorporated herein by reference.
62
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
Nexvet Biopharma public limited company |
||
|
|
|
|
||
|
Date: September 3, 2015 |
|
By: |
|
/s/ Mark Heffernan |
|
|
|
|
|
Mark Heffernan, Ph.D. |
|
|
|
|
|
Chief Executive Officer and Director |
|
|
|
|
|
|
|
Date: September 3, 2015 |
|
By: |
|
/s/ Damian Lismore |
|
|
|
|
|
Damian Lismore |
|
|
|
|
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
|
|
|
|
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Mark Heffernan, Ph.D. and Damian Lismore and both or any one of them, such person’s true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for such person and in such person’s name, place and stead, in any and all capacities, to sign any and all amendments hereto, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any substitute(s) thereof, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
||
|
/s/ Mark Heffernan Mark Heffernan, Ph.D. |
|
Chief Executive Officer and Director (Principal Executive Officer) |
|
September 3, 2015 |
|
|
|
|
||
|
/s/ Damian Lismore Damian Lismore |
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|
September 3, 2015 |
|
|
|
|
||
|
/s/ George Gunn |
|
Chairman of the Board |
|
September 3, 2015 |
|
George Gunn |
|
|
|
|
|
|
|
|
||
|
/s/ Chris Brown Chris Brown
|
|
Director |
|
September 3, 2015 |
|
/s/ Ashraf Hanna Ashraf Hanna, Ph.D., M.D. |
|
Director |
|
September 3, 2015 |
|
|
|
|
||
|
/s/ Cormac Kilty Cormac Kilty, Ph.D. |
|
Director |
|
September 3, 2015 |
|
|
|
|
||
|
/s/ Joseph McCracken Joseph McCracken, DVM
|
|
Director |
|
September 3, 2015 |
|
/s/ Rajiv Patel |
|
Director |
|
September 3, 2015 |
|
Rajiv Patel |
|
|
|
|
|
|
|
|
|
|
|
/s/ John Payne |
|
Director |
|
September 3, 2015 |
|
John Payne |
|
|
|
|
63
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
F-2 |
|
|
|
|
|
|
|
F-3 |
|
|
|
|
|
|
|
F-4 |
|
|
|
|
|
|
|
F-5 |
|
|
|
|
|
|
Consolidated Statements of Cash Flows for the Years Ended June 30, 2015, 2014 and 2013 |
|
F-6 |
|
|
|
|
|
|
F-7 |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Nexvet Biopharma public limited company,
We have audited the accompanying consolidated balance sheets of Nexvet Biopharma public limited company and its subsidiaries as of June 30, 2015 and June 30, 2014, and the related consolidated statements of operations and comprehensive loss, of changes in convertible preference shares and shareholders’ equity (deficit), and of cash flows for each of the three years ended June 30, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Nexvet Biopharma public limited company and its subsidiaries as of June 30, 2015 and June 30, 2014, and the results of their operations and their cash flows for each of the three years ended June 30, 2015 in conformity with accounting principles generally accepted in the United States of America.
/s/ PricewaterhouseCoopers
Melbourne, Australia
September 3, 2015
F-2
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
|
|
|
As of June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands, except share and per share amounts) |
|
|||||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
52,033 |
|
|
$ |
30,041 |
|
|
Other income receivable |
|
|
3,301 |
|
|
|
2,404 |
|
|
Prepaid expenses and other |
|
|
607 |
|
|
|
643 |
|
|
Total current assets |
|
|
55,941 |
|
|
|
33,088 |
|
|
Prepaid expenses |
|
|
163 |
|
|
|
— |
|
|
Total noncurrent assets |
|
|
163 |
|
|
|
— |
|
|
Property, plant and equipment, net |
|
|
549 |
|
|
|
514 |
|
|
Intangible assets, net |
|
|
19 |
|
|
|
2 |
|
|
Total assets |
|
$ |
56,672 |
|
|
$ |
33,604 |
|
|
Liabilities, Convertible Preference Shares and Shareholders’ Equity |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
658 |
|
|
$ |
870 |
|
|
Accrued expenses |
|
|
2,352 |
|
|
|
2,299 |
|
|
Deferred lease incentive |
|
|
23 |
|
|
|
28 |
|
|
Total current liabilities |
|
|
3,033 |
|
|
|
3,197 |
|
|
Deferred lease incentive |
|
|
61 |
|
|
|
103 |
|
|
Warrants |
|
|
— |
|
|
|
5,435 |
|
|
Total noncurrent liabilities |
|
|
61 |
|
|
|
5,538 |
|
|
Total liabilities |
|
$ |
3,094 |
|
|
$ |
8,735 |
|
|
Commitments and contingencies (Note 14) |
|
|
|
|
|
|
|
|
|
Convertible preference shares |
|
|
|
|
|
|
|
|
|
SIRPS, $0.125 nominal value per share, zero and 4,000,000 shares authorized as of June 30, 2015 and 2014, respectively—zero and 1,737,132 shares issued and outstanding as of June 30, 2015 and 2014, respectively |
|
$ |
— |
|
|
$ |
8,177 |
|
|
Series B, $0.125 nominal value per share, zero and 8,000,000 shares authorized as of June 30, 2015 and 2014, respectively—zero and 4,200,006 shares issued and outstanding as of June 30, 2015 and 2014, respectively |
|
|
— |
|
|
|
25,649 |
|
|
Total convertible preference shares |
|
$ |
— |
|
|
$ |
33,826 |
|
|
Shareholders’ equity (deficit) |
|
|
|
|
|
|
|
|
|
Ordinary shares, $0.125 nominal value per share, 100,000,000 and 20,000,000 shares authorized as of June 30, 2015 and 2014, respectively—11,406,916 and 1,268,810 shares issued and outstanding as of June 30, 2015 and 2014, respectively |
|
$ |
1,426 |
|
|
$ |
126 |
|
|
Euro deferred shares, €100 nominal value per share, 400 and zero shares authorized as of June 30, 2015 and 2014, respectively—400 and zero shares issued and outstanding as of June 30, 2015 and 2014, respectively |
|
|
13 |
|
|
|
— |
|
|
Additional paid-in capital |
|
|
80,275 |
|
|
|
2,342 |
|
|
Accumulated other comprehensive (loss) income |
|
|
(4,481 |
) |
|
|
373 |
|
|
Accumulated deficit |
|
|
(23,655 |
) |
|
|
(11,798 |
) |
|
Total shareholders’ equity (deficit) |
|
|
53,578 |
|
|
|
(8,957 |
) |
|
Total liabilities, convertible preference shares and shareholders’ equity |
|
$ |
56,672 |
|
|
$ |
33,604 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-3
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Consolidated Statements of Operations and Comprehensive Loss
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands, except share and per share amounts) |
|
|||||||||
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
License and collaboration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
244 |
|
|
Other |
|
|
25 |
|
|
|
13 |
|
|
|
85 |
|
|
Total revenue |
|
|
25 |
|
|
|
13 |
|
|
|
329 |
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
9,845 |
|
|
|
5,617 |
|
|
|
2,722 |
|
|
General and administrative |
|
|
10,191 |
|
|
|
4,426 |
|
|
|
2,103 |
|
|
Total operating expenses |
|
|
20,036 |
|
|
|
10,043 |
|
|
|
4,825 |
|
|
Loss from operations |
|
|
(20,011 |
) |
|
|
(10,030 |
) |
|
|
(4,496 |
) |
|
Other Income (Expense) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development incentive income |
|
|
3,532 |
|
|
|
2,337 |
|
|
|
1,135 |
|
|
Government grant income |
|
|
403 |
|
|
|
1,317 |
|
|
|
108 |
|
|
Exchange gain (loss) |
|
|
4,151 |
|
|
|
(375 |
) |
|
|
— |
|
|
Interest income |
|
|
68 |
|
|
|
41 |
|
|
|
12 |
|
|
Net loss |
|
$ |
(11,857 |
) |
|
$ |
(6,710 |
) |
|
$ |
(3,241 |
) |
|
Net loss per share attributable to ordinary shareholders, basic and diluted |
|
$ |
(2.27 |
) |
|
$ |
(6.70 |
) |
|
$ |
(3.62 |
) |
|
Weighted-average ordinary shares outstanding, basic and diluted |
|
|
5,214,957 |
|
|
|
1,000,872 |
|
|
|
894,794 |
|
|
Comprehensive Loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(11,857 |
) |
|
$ |
(6,710 |
) |
|
$ |
(3,241 |
) |
|
Net change in foreign currency translation adjustments |
|
|
(4,854 |
) |
|
|
302 |
|
|
|
58 |
|
|
Total comprehensive loss |
|
$ |
(16,711 |
) |
|
$ |
(6,408 |
) |
|
$ |
(3,183 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Consolidated Statements of Changes in Convertible Preference Shares and Shareholders’ Equity (Deficit)
(in thousands, except share amounts)
|
|
|
Convertible Preference Shares |
|
|
|
Ordinary Shares |
|
|
Ordinary Shares Subject to Limited Recourse Loans |
|
|
Euro Deferred Shares |
|
|
Convertible Preference Shares |
|
|
Additional Paid-in Capital |
|
|
Accumulated Other Comprehensive Income (Loss) |
|
|
Accumulated Deficit |
|
|
Total Shareholders' (Deficit) Equity |
|
|||||||||||||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Other |
|
|
Share Premium |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Balance as of July 1, 2012 |
|
|
— |
|
|
$ |
— |
|
|
|
|
200,000 |
|
|
$ |
25 |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(15 |
) |
|
$ |
13 |
|
|
$ |
(1,847 |
) |
|
$ |
(1,824 |
) |
|
Subdivision of shares |
|
|
— |
|
|
|
— |
|
|
|
|
265,455 |
|
|
|
33 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(33 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
534,545 |
|
|
|
66 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,396 |
|
|
|
— |
|
|
|
— |
|
|
|
1,462 |
|
|
Issuance of SIRPS convertible preference shares, zero issuance costs |
|
|
498,199 |
|
|
|
2,093 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of ordinary shares subject to limited recourse loans |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
264,386 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of SIRPS convertible preference shares, zero issuance costs |
|
|
293,918 |
|
|
|
1,504 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
631 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
631 |
|
|
Exchange difference on translation of foreign operations |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
58 |
|
|
|
— |
|
|
|
58 |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,241 |
) |
|
|
(3,241 |
) |
|
Balance as of June 30, 2013 |
|
|
792,117 |
|
|
$ |
3,597 |
|
|
|
|
1,000,000 |
|
|
$ |
124 |
|
|
|
264,386 |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
631 |
|
|
$ |
1,348 |
|
|
$ |
71 |
|
|
$ |
(5,088 |
) |
|
$ |
(2,914 |
) |
|
Issuance of ordinary shares— payment of limited recourse loan |
|
|
— |
|
|
$ |
— |
|
|
|
|
9,706 |
|
|
$ |
1 |
|
|
|
(9,706 |
) |
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
36 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
37 |
|
|
Issuance of ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
|
4,424 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
21 |
|
|
|
— |
|
|
|
— |
|
|
|
22 |
|
|
Issuance of SIRPS convertible preference shares, net of issuance costs of $294 |
|
|
945,015 |
|
|
|
4,580 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of Series B convertible preference shares, net of issuance costs of $1,021 |
|
|
4,200,006 |
|
|
|
25,649 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
306 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
306 |
|
|
Exchange difference on translation of foreign operations |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
302 |
|
|
|
— |
|
|
|
302 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6,710 |
) |
|
|
(6,710 |
) |
|
Balance as of June 30, 2014 |
|
|
5,937,138 |
|
|
$ |
33,826 |
|
|
|
|
1,014,130 |
|
|
$ |
126 |
|
|
|
254,680 |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
937 |
|
|
$ |
1,405 |
|
|
$ |
373 |
|
|
$ |
(11,798 |
) |
|
$ |
(8,957 |
) |
|
Issuance of ordinary shares and Euro deferred shares |
|
|
— |
|
|
$ |
— |
|
|
|
|
2 |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
400 |
|
|
$ |
13 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
13 |
|
|
Ordinary shares no longer subject to limited recourse loan |
|
|
— |
|
|
|
— |
|
|
|
|
109,611 |
|
|
|
14 |
|
|
|
(109,611 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(14 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share repurchase |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
(145,069 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of share warrants |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,435 |
|
|
|
— |
|
|
|
— |
|
|
|
5,435 |
|
|
Issuance of ordinary shares— conversion of share-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
126,410 |
|
|
|
16 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(645 |
) |
|
|
642 |
|
|
|
— |
|
|
|
— |
|
|
|
13 |
|
|
Issuance of ordinary shares, net of issuance costs of $3,830 |
|
|
6 |
|
|
|
|
|
|
|
|
4,176,903 |
|
|
|
522 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
37,416 |
|
|
|
— |
|
|
|
— |
|
|
|
37,938 |
|
|
Adjustment for shares issued in connection with share consolidation |
|
|
16 |
|
|
|
— |
|
|
|
|
9 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share bonus award |
|
|
|
|
|
|
|
|
|
|
|
42,691 |
|
|
|
6 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
(304 |
) |
|
|
304 |
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
Reclassification into shareholders’ equity |
|
|
(5,937,160 |
) |
|
|
(33,826 |
) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,937,160 |
|
|
|
33,826 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
33,826 |
|
|
Reclassification to ordinary shares |
|
|
|
|
|
|
|
|
|
|
|
5,937,160 |
|
|
|
742 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5,937,160 |
) |
|
|
(33,826 |
) |
|
|
— |
|
|
|
33,084 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Share-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,081 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,081 |
|
|
Exchange difference on translation of foreign operations |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4 |
) |
|
|
(62 |
) |
|
|
(4,854 |
) |
|
|
— |
|
|
|
(4,920 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(11,857 |
) |
|
|
(11,857 |
) |
|
Balance as of June 30, 2015 |
|
|
— |
|
|
$ |
— |
|
|
|
|
11,406,916 |
|
|
$ |
1,426 |
|
|
|
— |
|
|
$ |
— |
|
|
|
400 |
|
|
$ |
13 |
|
|
|
|
|
|
$ |
— |
|
|
$ |
2,065 |
|
|
$ |
78,210 |
|
|
$ |
(4,481 |
) |
|
$ |
(23,655 |
) |
|
$ |
53,578 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-5
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Consolidated Statements of Cash Flows
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Cash Flows from Operating Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(11,857 |
) |
|
$ |
(6,710 |
) |
|
$ |
(3,241 |
) |
|
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Share-based compensation expense |
|
|
2,081 |
|
|
|
298 |
|
|
|
710 |
|
|
Depreciation and amortization expense |
|
|
154 |
|
|
|
56 |
|
|
|
11 |
|
|
Other |
|
|
— |
|
|
|
22 |
|
|
|
— |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income receivable |
|
|
(897 |
) |
|
|
(1,338 |
) |
|
|
(679 |
) |
|
Prepaid expenses and other |
|
|
(127 |
) |
|
|
(250 |
) |
|
|
(377 |
) |
|
Accounts payable, accrued expenses and deferred lease incentive |
|
|
(206 |
) |
|
|
1,978 |
|
|
|
614 |
|
|
Net cash used in operating activities |
|
|
(10,852 |
) |
|
|
(5,944 |
) |
|
|
(2,962 |
) |
|
Cash Flows from Investing Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property, plant and equipment |
|
|
(294 |
) |
|
|
(512 |
) |
|
|
(54 |
) |
|
Purchase of intangible assets |
|
|
(19 |
) |
|
|
(2 |
) |
|
|
— |
|
|
Net cash used in investing activities |
|
|
(313 |
) |
|
|
(514 |
) |
|
|
(54 |
) |
|
Cash Flows from Financing Activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net proceeds from issuance of ordinary shares |
|
|
37,970 |
|
|
|
37 |
|
|
|
— |
|
|
Net proceeds from issuance of convertible preference shares and warrants |
|
|
— |
|
|
|
35,664 |
|
|
|
3,597 |
|
|
Repayments of note payable to related party |
|
|
— |
|
|
|
— |
|
|
|
(205 |
) |
|
Net cash provided by financing activities |
|
|
37,970 |
|
|
|
35,701 |
|
|
|
3,392 |
|
|
Effect of exchange rate changes on cash |
|
|
(4,813 |
) |
|
|
315 |
|
|
|
88 |
|
|
Net increase in cash |
|
|
21,992 |
|
|
|
29,558 |
|
|
|
464 |
|
|
Cash at beginning of year |
|
|
30,041 |
|
|
|
483 |
|
|
|
19 |
|
|
Cash at end of year |
|
$ |
52,033 |
|
|
$ |
30,041 |
|
|
$ |
483 |
|
|
Supplemental Disclosure |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest during period |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
27 |
|
|
Conversion of note payable into ordinary shares |
|
|
— |
|
|
|
— |
|
|
|
1,368 |
|
|
Offering costs in connection with issuance of ordinary shares, convertible preference shares and warrants recorded in equity |
|
|
3,830 |
|
|
|
1,315 |
|
|
|
— |
|
|
Issuance of share options for accrued consulting services |
|
|
— |
|
|
|
22 |
|
|
|
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-6
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements
Note 1. Organization and Description of Business
Nexvet Biopharma public limited company and its subsidiaries (the “Company”) is a clinical-stage biopharmaceutical company focused on transforming the therapeutic market for companion animals by developing and commercializing novel, species-specific biologics based on human biologics. Biologics are therapeutic proteins derived from biological sources. As a class, biologics have transformed human medicine in recent decades and represent some of the top-selling therapies on the market today. The Company’s proprietary platform, which it refers to as “PETization,” is an algorithmic approach that enables the Company to rapidly create monoclonal antibodies (“mAbs”) that are designed to be recognized as “self” or “native” by an animal’s immune system, a property referred to as “100% species-specificity.” PETization is also designed to build upon the safety and efficacy data from clinically tested human therapies to create new therapies for companion animals, thereby reducing clinical risk and development cost.
Biologics generally include mAbs, which are targeted antibodies derived from identical, or clonal, cells; and fusion proteins, which are proteins created by joining two or more genes coded for separate proteins. The Company’s first product candidate, NV‑01, is a mAb that targets and inhibits the function of nerve growth factor (“NGF’) for the control of pain associated with osteoarthritis in dogs. NGF is a protein involved in neural signaling, including pain signals. NGF inhibitors seek to interrupt those signals to reduce pain. The Company expects data from its NV‑01 pivotal safety and efficacy study and an additional pilot field safety and efficacy study by the end of 2015. This latter study was initiated following receipt of a sample size reassessment of the pivotal safety and efficacy study in March 2015 and will assess various doses and dosing regimens of NV‑01. This study is expected to provide valuable information that may assist the design of a future pivotal study.
The Company’s second product candidate, NV‑02, is a mAb that is an NGF inhibitor for the control of pain associated with degenerative joint disease, including osteoarthritis, in cats. The Company announced positive top-line results from its proof-of-concept efficacy study and its pilot safety study of NV‑02 in June 2015. The Company anticipates results from a pilot field safety and efficacy study of NV‑02 in the first quarter of 2016. The Company’s third product candidate, NV‑08, is a fusion protein that is a tumor necrosis factor (“TNF”) inhibitor for the treatment of chronic inflammatory diseases, including atopic dermatitis, in dogs. TNF is a protein that causes inflammation, and TNF inhibitors suppress this inflammation. If its proof-of-concept safety and efficacy studies for NV‑08 are successful, the Company plans to progress this product into formal development. In addition, primarily using PETization, the Company is seeking to advance one new product candidate into development per year.
The Company has experienced losses since its inception and had an accumulated deficit of $23.7 million and $11.8 million as of June 30, 2015 and 2014, respectively. For the foreseeable future, management expects the Company to continue to incur losses and negative cash flows, which will increase significantly from historical levels as the Company expands its development activities, seeks regulatory approvals for its lead product candidates and begins to commercialize any approved products. To date, the Company has been funded primarily through sales of capital shares. Management believes the Company’s cash of $52.0 million as of June 30, 2015 will be sufficient to fund its operations for at least the next 12 months.
The Company will require additional capital until such time as the Company can generate revenue in excess of operating expenses. The Company may seek such funding through public or private equity, debt financing or other sources, such as corporate collaborations and licensing arrangements. The Company may not be able to obtain financing on acceptable terms, or at all. The sale of additional equity would result in additional dilution to the Company’s shareholders, and the terms of any financing may adversely affect the rights of the Company’s shareholders. The incurrence of any debt financing could result in debt service obligations and the instruments governing such debt could provide for operating and financing covenants that would restrict the Company’s operations. If the Company is unable to obtain funding, the Company could be forced to delay, reduce or eliminate its research and development programs or commercialization efforts, which could adversely affect its business prospects.
In August 2014, the Company completed a one-for-four share consolidation. Each holder of ordinary shares and preference shares received one ordinary share or preference share for every four ordinary shares or preference shares held by such holder. The number of ordinary shares that may be acquired upon exercise of options or warrants or upon conversion of restricted share units was similarly reduced on a one-for-four basis, with a proportionate adjustment to the exercise or conversion price, as applicable.
F-7
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
In September 2014, Nexvet Biopharma Limited, a newly-formed Irish private company, became the parent company of Nexvet Australia Pty Ltd, formerly known as Nexvet Biopharma Pty Ltd (“Nexvet Australia”), and its subsidiaries pursuant to a transaction in which all of the holders of ordinary shares, preference shares, restricted share units and options and warrants to purchase ordinary shares of Nexvet Australia exchanged their holdings for equivalent ordinary shares, preference shares, restricted share units or options or warrants to purchase ordinary shares, as applicable, of Nexvet Biopharma Limited. Nexvet Biopharma Limited then re-registered as an Irish public limited company in September 2014 (the “Irish Reorganization”). Nexvet Biopharma public limited company became the parent company of Nexvet Australia pursuant to the Irish Reorganization, and for financial reporting purposes the historical consolidated financial statements of Nexvet Australia became the historical consolidated financial statements of Nexvet Biopharma public limited company and its subsidiaries as a continuation of the predecessor. The capital structure presented is that of Nexvet Biopharma public limited company.
In November 2014, the Company completed a four-for-five share consolidation. Each holder of ordinary shares and preference shares received four ordinary shares or four preference shares for every five ordinary shares or five preference shares held by such holder. The number of ordinary shares that may be acquired upon exercise of options or warrants or upon conversion of restricted share units was similarly reduced on a four-for-five basis, with a proportionate adjustment to the exercise or conversion price, as applicable.
In February 2015, the Company closed its initial public offering of 4.0 million ordinary shares at a price to the public of $10.00 per share. In March 2015, the underwriters partially exercised their over-allotment option and purchased an additional 0.2 million shares. Following the sales of these securities, the Company received aggregate gross proceeds of $41.8 million and aggregate net proceeds of $38.0 million, after deducting the underwriting discount of $2.9 million and estimated offering expenses of $0.9 million payable by the Company. Upon the initial closing, an amended Memorandum and Articles of Association (the “New M&A”) became effective for the Company and all 5,937,160 convertible preference shares then outstanding were converted into 5,937,160 ordinary shares.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements of the Company include the operations of all its wholly-owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Such operations include the Company, Nexvet Australia, NVIP Pty Limited, Nexvet Ireland Limited, Tevxen Limited, Nexvet UK Limited and Nexvet US, Inc. All intercompany balances and transactions have been eliminated in consolidation. The Company’s fiscal year ends on June 30, and references to any fiscal year are to the Company’s year ended June 30 in that year.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions relating to the reported amount of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the period. Significant items subject to such estimates and assumptions include research and development incentive income, research and development accruals, share-based payments, valuation of warrants, options and restricted share units and deferred income taxes. Actual results could differ from those estimates.
Net Loss Per Share
Net loss per share information is determined using the two-class method, which includes the weighted-average number of ordinary shares outstanding during the period and other securities that participate in dividends (a participating security). The Company’s convertible preference shares are participating securities as defined by Accounting Standards Codification (“ASC”) Topic 260-10, Earnings Per Share. Net loss per share disclosures have been revised to give effect to the share consolidations that took place in the reporting period
Under the two-class method, basic net loss per share applicable to ordinary shareholders is computed by dividing the net loss applicable to ordinary shareholders by the weighted-average number of shares of ordinary shares outstanding for the reporting period.
F-8
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Diluted net loss per share gives effect to all potentially dilutive securities, including convertible preference shares and shares issuable upon the exercise or conversion, as applicable, of outstanding warrants, share options and restricted share units, using the treasury shares method. The Company has excluded the effects of all potentially dilutive shares, which include convertible preference shares, warrants to purchase ordinary shares, ordinary share options, restricted share units and the ordinary shares issued subject to limited recourse loans, from the weighted-average number of ordinary shares outstanding as their inclusion in the computation for all periods would be anti-dilutive due to net losses.
Cash
As of June 30, 2015 and 2014, the Company’s cash consisted of cash deposited in a business operating account or in short-term deposit accounts of less than 90 days’ duration.
Concentration of Credit Risk and Other Risks and Uncertainties
The Company receives research and development incentive income and grants from a single source, the Australian government.
The Company’s cash is deposited with several large commercial banks located in the United States and Australia that are federally insured or guaranteed, limiting the amount of credit exposure to any one financial institution. The Company’s cash balances with these financial institutions often exceed the amount insured.
The Company is subject to risks common to companies in the biotechnology industry. The Company’s research and development may not be successfully completed, adequate protection for the Company’s technology may not be obtained, any products developed may not obtain necessary government regulatory approval and any approved products may not be commercially viable. The Company operates in an environment of substantial competition from other animal health companies, some of which have substantially more resources at their disposal. In addition, the Company is dependent upon the services of its employees and consultants, as well as third-party contract research organizations and manufacturers.
Fair Value Measurements
The Company records certain assets and liabilities at fair value in accordance with the provisions of ASC Topic 820, Fair Value Measurements. As defined in the guidance, fair value, defined as an exit price, represents the amount that would be received to sell an asset or pay to transfer a liability in an orderly transaction between market participants. As a result, fair value is a market-based approach that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering these assumptions, the guidance defines a three-tier value hierarchy that prioritizes the inputs used in the valuation methodologies in measuring fair value.
|
|
· |
Level 1—Unadjusted quoted prices in active, accessible markets for identical assets or liabilities. |
|
|
· |
Level 2—Other inputs that are directly or indirectly observable in the marketplace. |
|
|
· |
Level 3—Unobservable inputs that are supported by little or no market activity. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The Company’s material financial instruments include cash, other income receivables, accrued liabilities and warrants. The carrying amounts of these instruments are considered to be representative of their respective fair values because of the short-term nature of those investments. In 2014, the Company determined its warrants were Level 3 liabilities until they were reclassified into equity (see –“Warrants” below).
Other Income Receivable
Other income receivable is recorded at the invoiced amount where available.
F-9
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Nexvet Australia is eligible under the AusIndustry research and tax development tax incentive program to obtain a cash amount from the Australian Taxation Office (“ATO”). The tax incentive is available to Nexvet Australia on the basis of specific criteria with which Nexvet Australia must comply. Specifically, Nexvet Australia must have revenue of less than A$20 million and cannot be controlled by income tax exempt entities. The Company recognized other income for the 2015 fiscal year at 43.5% of qualifying expenditure on the basis of the proposed revisions to the rebate rate announced by the Australian government in 2015 that, if passed as law, are expected to be retrospectively applied to the 2015 fiscal year.
Property, Plant and Equipment
Property, plant and equipment are recorded at acquisition cost, net of accumulated depreciation and impairment. Depreciation on property and equipment is calculated using the straight-line method over the estimated useful lives of the assets. The estimated useful life of machinery and equipment is three to 20 years. Leasehold improvements are amortized on the straight-line method over the shorter of the remaining lease term or estimated useful life of the asset. Upon retirement or sale of an asset, its cost and related accumulated depreciation or accumulated amortization are removed from the property accounts and any gain or loss is included in the results of operations. Maintenance and repairs are expensed as incurred.
Intangible Assets
The Company accounts for intangible assets under ASC 350, Intangibles—Goodwill and Other, which consists of internal use computer software costs. Costs which include acquiring off the shelf software and licenses that are expected to provide future period financial benefits are capitalized to computer software intangibles. No material internal or external costs are incurred in making the software ready for use. Maintenance costs are expensed as incurred. Amortization is calculated on a straight-line basis over periods ranging from one to three years.
Impairment of Long-Lived Assets
The Company reviews its tangible and intangible assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the asset may not be fully recoverable. Recoverability of assets is measured by a comparison of the carrying amount of an asset to the estimated undiscounted cash flows expected to be generated by the asset. If the carrying amount of the asset exceeds its estimated future cash flows, an impairment charge will be recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. There were no impairments of long-lived assets during fiscal years 2015, 2014 or 2013.
Foreign Currency
The Company’s functional currency is U.S dollars, and the functional currency for most subsidiaries is their local currency. Foreign currency transactions are translated into the functional currency using the current exchange rate at the date of the transaction. At year end, monetary items denominated in a foreign currency are translated into the functional currency of the relevant entity using the year end spot rate. The exchange gain of $4.2 million in fiscal year 2015 and exchange loss of $0.4 million in fiscal year 2014 primarily relate to the translation of Nexvet Australia’s U.S. dollar-denominated bank accounts to its Australian dollar functional currency.
In preparing the Company’s consolidated financial statements, the financial statements of the subsidiaries are translated at year-end exchange rates as to assets and liabilities and weighted-average rates as to revenue and expenses. The resulting translation adjustments are recognized in other comprehensive income (loss) (“OCI”). The non-cash translation adjustment in accumulated OCI was a loss of $4.9 million in fiscal year 2015 and a gain of $0.3 million in fiscal year 2014. These adjustments primarily relate to the translation of U.S. dollar-denominated bank accounts within Nexvet Australia’s balance sheet to the U.S dollar presentation currency of the consolidated balance sheet.
Under U.S. GAAP, there is no offset of these two exchange-related items within the consolidated statements of operations and comprehensive loss. Net loss and associated calculations are impacted by this treatment.
F-10
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
As of June 30, 2014, the Company’s liabilities primarily consist of warrants that were issued to investors and financial advisors in connection with private placements of the Company’s securities in May 2014. The warrants permit the holders to purchase ordinary shares at exercise prices of $8.625 and $7.50 per share on or before May 2019. Because the warrants may be net exercised and are exercisable in U.S. dollars, and the functional currency of Nexvet Australia, the original issuer of the warrants, is Australian dollars, they were classified as a liability as of June 30, 2014. The warrants were reclassified as shareholders’ equity in September 2014 following the Irish Reorganization (in which the original warrants were exchanged for warrants issued by the Company) and the change in the Company’s functional currency to U.S. dollars. On reclassification, the warrants were recognized at cost in shareholders’ equity and no longer required to be remearsured at fair value at June 30, 2015.
Warrants recorded as liabilities ($5.4 million as of June 30, 2014) are valued at balance date using the binomial option-pricing model. The expected term used in the model has been adjusted, based on management’s best estimate, for the effects of non-transferability, exercise restrictions and behavioral considerations. At each balance sheet date, the outstanding warrants are revalued to their current fair value, with the difference in fair value recorded in the consolidated statements of operations and comprehensive loss.
Warrants are classified within Level 3 of the fair value hierarchy at June 30, 2014 wherein fair value is estimated using significant unobservable inputs. The significant assumptions used in estimating the fair value of the warrants include the estimated fair value of the underlying shares, exercise price, volatility of the shares underlying the warrant and the expected term of the warrant. The fair value of the underlying ordinary shares was estimated by reference to the price per share paid by investors for the Company’s Series B preference shares in May 2014. The fair values of the warrants were estimated using the following assumptions:
|
|
|
June 30, 2014 |
|
Fair value per ordinary share |
|
$6.35 |
|
Risk free interest rate |
|
1.7% |
|
Expected term (in years) |
|
4 years |
|
Expected volatility |
|
75% |
|
Expected dividend yield |
|
Nil |
There were warrants to purchase 1,766,998 ordinary shares issued during fiscal year 2014. There were no warrants issued during fiscal years 2015 and 2013.
Income Taxes
The Company has historically filed income tax returns in Australia and the United States and in the future also expects to file tax returns in Ireland.
The Company applies ASC Topic 740, Income Taxes, which establishes financial accounting and reporting requirements for the effects of income taxes that result from the Company’s activities during the current and preceding years. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities on their respective tax bases, and operating losses and tax loss carry forwards. Deferred tax assets and liabilities are measured using enacted statutory tax rates expected to apply to taxable income in the jurisdictions and years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date.
When the Company determines that it is more likely than not that some portion or all of the deferred tax assets will not be realized in the future, the deferred tax assets are reduced by a valuation allowance. The valuation allowance is sufficient to reduce the deferred tax assets to the amount that the Company determines is more likely than not to be realized.
The income tax benefit from an uncertain tax position is only recognized if it is more likely than not that the tax position will be sustained on examination by taxing authorities, based on technical merits of the position. The Company evaluates and adjusts these accruals based on changing facts and circumstances. The Company’s policy is to record interest and penalties related to income taxes as part of its income tax provision.
F-11
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
License and Collaboration Agreement Revenue Recognition
Future revenue under a license and collaboration agreement is expected to consist of fees for services, royalties for product sales or payments when specific milestones are met and match underlying activities occurring during the term of the arrangement.
In fiscal year 2013, the Company entered into a license and collaboration agreement with a third party for the research and development of animal health products in Japan. The terms of the agreement include non-refundable signing and license fees, development milestone payments, the potential for manufacturing and supply services and royalties on any product sales derived from the collaboration. The Company analyzed this arrangement to determine whether the deliverables, which included license and performance obligations such as research and steering committee services, can be separated or whether these must be accounted for as a single unit of accounting. The Company recognizes license payments as revenue upon delivery of the license only if the license has stand-alone value and there are no undelivered performance obligations related to the license.
If the license is considered not to have stand-alone value, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations would be recognized as revenue over the estimated period of when the performance obligations are performed. When the Company determines that an arrangement should be accounted for as a single unit of accounting, it determines the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a proportional performance or straight-line method. The Company recognizes revenue using the proportional performance method when the level of effort required to complete its performance obligations under an arrangement can be reasonably estimated, and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as the measurement of performance. If the Company cannot reasonably estimate the level of effort to complete its performance obligations under an arrangement, then revenue under the arrangement would be recognized on a straight-line basis over the period the Company is expected to complete its performance obligations.
The Company’s license and collaboration agreement entitles it to additional payments upon the achievement of performance-based milestones. Milestones that involve substantial effort on the Company’s part are considered “substantive milestones.” A substantive milestone is included in the Company’s revenue model when the milestone is achieved. To date, no milestone payments have been received.
Royalty revenue is recognized upon the sale of the related products, provided the Company has no remaining performance obligations under the arrangement.
Research and Development Expense
Research and development costs are expensed as incurred and consist primarily of (i) payroll and related expense for all employees engaged in scientific research and development functions, including wages, related benefits, and share-based compensation, (ii) fees for regulatory, professional and other consultants and (iii) development costs, including costs of drug discovery, safety, and proof-of-concept, pilot and pivotal safety and efficacy studies, development of biological materials, and service providers. The Company is currently pursuing its NV‑01, NV‑02 and NV‑08 lead product candidates and typically uses its employee and infrastructure resources across multiple development programs. The Company allocates outsourced development costs by lead product candidates but does not allocate personnel or other internal costs related to development to specific product candidates.
General and Administrative Expense
General and administrative expense consists primarily of non-research and development-related payroll and related expense for employees, consultants and directors, including wages, related benefits and share-based compensation. General and administrative expense also includes professional and consulting fees for legal, accounting, tax services and other general business services, as well other expenses such as travel, rent and facilities costs.
F-12
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Nexvet Australia is eligible under the AusIndustry research and development tax incentive program to obtain a cash amount from the ATO. The tax incentive is available to Nexvet Australia on the basis of specific criteria with which Nexvet Australia must comply. Although the tax incentive is administered through the ATO, the Company has accounted for the tax incentive outside the scope of ASC Topic 740, Income Taxes, as an income tax benefit since Nexvet Australia meets the applicable requirements to participate in the program and the incentive is not linked to Nexvet Australia’s income tax liability and can be realized regardless of whether Nexvet Australia has generated taxable income. Research and development incentive income is recognized when the research and development activities have been undertaken and the Company has completed its assessment of whether such activities meet the relevant qualifying criteria.
The Company recognizes government grant income at the fair value of the grant when it is received and all substantive conditions have been satisfied. When the grant relates to an expense item, it is recognized as income over the periods necessary to match the grant on a systematic basis to the costs that it is intended to compensate.
Exchange gain (loss) consists primarily of gains or losses due to foreign exchange translation, primarily reflecting changes in Australian and U.S. foreign exchange rates. Under U.S. GAAP, these gains (losses) relate to a translation of U.S. dollar-denominated bank accounts into Nexvet Australia’s Australian dollar functional currency and represent a non-cash item.
The Company earns interest on the cash balances held with financial institutions and recognizes interest when earned on an accruals basis over time.
Comprehensive Loss
Comprehensive loss is defined as the total change in shareholders’ deficit during the period other than from transactions with shareholders, which for the Company, includes net change in foreign currency translation adjustments.
Share-Based Compensation
The Company’s share-based compensation plan (see Note 12) provides for the grant of share options, restricted share units and other share-based awards. The fair value of share options is determined as of the date of grant using the binomial option-pricing model. This method incorporates the fair value of the Company’s ordinary shares at the date of each grant and various assumptions such as the risk-free interest rate, expected volatility based on the historic volatility of peer companies, expected dividend yield, and expected term of the share option. Restricted share units are valued at the fair value of the underlying ordinary shares as of the date of grant. The Company classifies share-based compensation expense in the statements of operations and comprehensive loss in the same manner in which the award recipient’s payroll costs are classified.
The Company recognizes share-based compensation expense based on the grant date fair value of the entire award over the total period during which an employee is required to provide service in exchange for the award. In accordance with ASC 718, the amount of compensation expense recognized at each balance date is at least equal to the grant date fair value of the vested portion of the award on that date. Where performance conditions are attached to the awards, compensation expense is recognized in the period in which it becomes probable that the performance target will be achieved, net of estimate of pre-vesting forfeitures over the requisite service period. The probability of vesting is reassessed at each reporting period for awards with performance conditions and compensation expense is adjusted based on its probability assessment. Share-based compensation expense is classified in the statements of operation and comprehensive loss in the same manner in which the award recipient’s payroll costs are classified.
Equity instruments issued to non-employees, including consultants, are accounted for in accordance with Financial Accounting Standards Board (“FASB”) guidance. All transactions in which services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date of the fair value of the equity instrument issued is the earlier of the date on which the counterparty’s performance is complete or the date on which it is probable that performance will occur.
For transactions where the fair value of the equity instrument issued to non-employees is the more reliable measurement and a measurement date has not been reached, the fair value is re-measured at each balance sheet date using the binomial option-pricing model. Compensation expense for these share-based awards is recognized over the term of the consulting agreement or until the award is approved and settled.
F-13
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The Company is a clinical-stage biopharmaceutical company focusing on developing therapies for companion animals. At June 30, 2014 all major assets were held in Australia. As of June 30, 2015, $38.0 million of cash raised on completion of the initial public offering has been retained in the United States. All other major assets are held in Australia.
Recently Adopted Accounting Pronouncements
The Company has early adopted the provisions of Accounting Standards Update (“ASU”) No. 2014-10, Elimination of Certain Financial Requirements, Including an Amendment to Variable Interest Entities Guidance Topic in Topic 810, Consolidation, starting in fiscal year 2014. In June 2014, the Financial Accounting Standards Board (“FASB”) issued guidance removing the definition of a development stage entity from the Master Glossary of the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. This guidance also eliminates an exception provided to development stage entities in ASC Topic 810, Consolidation, for determining whether an entity is a variable interest entity on the basis of the amount of the investment equity that is at risk. On adoption, the Company was not required to present or disclose any information required by ASC Topic 915, Development Stage Entities.
Recently Issued Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers. This guidance requires an entity to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This guidance also requires an entity to disclose sufficient information to enable users of financial statements to understand the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. Qualitative and quantitative information is required about:
|
|
· |
Contracts with customers—including revenue and impairments recognized, disaggregation of revenue and information about contract balances and performance obligations (including the transaction price allocated to the remaining performance obligations). |
|
|
· |
Significant judgments and changes in judgments—determining the timing of satisfaction of performance obligations (over time or at a point in time), and determining the transaction price and amounts allocated to performance obligations. |
|
|
· |
Certain assets—assets recognized from the costs to obtain or fulfill a contract. |
In July 2015, the FASB delayed the effective date of this guidance. As a result, this guidance will be effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period. Earlier application is permitted only as of annual reporting periods beginning after December 15, 2016, including interim reporting periods within that reporting period. The Company is currently evaluating the impact that this guidance will have on the Company’s consolidated results of operations, financial position and cash flows.
In June 2014, the FASB issued ASU 2014-12, Compensation—Stock Compensation. This guidance requires that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply the existing guidance in ASC Topic 718, Compensation—Stock Compensation, as it relates to awards with performance conditions that affect vesting to account for such awards. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation expense should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation expense attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation expense should be recognized prospectively over the remaining requisite service period. The total amount of compensation expense recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved. This guidance will be effective for annual reporting periods beginning after December 15, 2015. The Company is currently evaluating the impact that this guidance will have on the Company’s consolidated results of operations, financial position and cash flows.
F-14
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40). This guidance defines management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and to provide related footnote disclosures. Under the guidance, management is required to evaluate, for each annual and interim reporting period, whether it is probable that the entity will not be able to meet its obligations as they become due within one year after the date that the financial statements are issued or are available to be issued. When management identifies substantial doubt about the entity’s ability to continue as a going concern, additional disclosures are required. This guidance will be effective for annual reporting periods beginning after December 15, 2016. The Company does not expect the adoption of this guidance to have a material impact on the Company’s consolidated results of operations, financial position or cash flows.
In February 2015, the FASB issued ASU 2015-02, Consolidation. This guidance amends existing consolidation guidance in which a reporting entity might be required to consolidate another legal entity in situations in which the reporting entity’s contractual rights do not give it the ability to act primarily on its own behalf, the reporting entity does not hold a majority of the legal entity’s voting rights, or the reporting entity is not exposed to a majority of the legal entity’s economic benefits or obligations. The guidance:
|
|
· |
modifies the evaluations of whether limited partnerships and similar legal entities are variable interest entities or voting interest entities; |
|
|
· |
eliminates the presumption that a general partner should consolidate a limited partner; |
|
|
· |
affects the consolidation analysis of reporting entities that are involved with variable interest entities, particularly those that have fee arrangements and related party relationships; and |
|
|
· |
provides a scope exception from consolidation guidance for reporting entities with interests in certain investment funds. |
The guidance is effective for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2015. The Company does not expect the adoption of this guidance to have a material impact on the Company’s consolidated results of operations, financial position or cash flows.
In April 2015, the FASB issued ASU 2015-03, Interest—Imputation of Interest. This guidance simplifies the presentation of debt issuance costs by requiring that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of the debt liability, consistent with debt discounts. The guidance will be effective for annual reporting periods beginning after December 15, 2015, and interim periods within fiscal years beginning after December 15, 2016. The Company does not expect the adoption of this guidance to have a material impact on the Company’s consolidated results of operations, financial position or cash flows.
Note 3. Property, Plant and Equipment
Property, plant and equipment, net consisted of the following as of the dates indicated:
|
|
|
Useful Lives |
|
|
June 30, |
|
||||||
|
|
|
|
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in years) |
|
|
(in thousands) |
|
||||||
|
Computer equipment |
|
3-7 |
|
|
$ |
89 |
|
|
$ |
68 |
|
|
|
Research and development equipment |
|
5-10 |
|
|
|
432 |
|
|
|
242 |
|
|
|
Office equipment |
|
5-20 |
|
|
|
83 |
|
|
|
100 |
|
|
|
Leasehold improvements |
|
|
5 |
|
|
|
133 |
|
|
|
162 |
|
|
Less: Accumulated depreciation and amortization |
|
|
|
|
|
|
(188 |
) |
|
|
(58 |
) |
|
Property, plant and equipment, net |
|
|
|
|
|
$ |
549 |
|
|
$ |
514 |
|
F-15
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
|
|
|
Computer equipment |
|
|
Research and development equipment |
|
|
Office equipment |
|
|
Leasehold improvements |
|
|
Total |
|
|||||
|
Opening balance July 1, 2013 |
|
|
3 |
|
|
|
37 |
|
|
|
7 |
|
|
|
— |
|
|
|
47 |
|
|
Additions |
|
|
65 |
|
|
|
193 |
|
|
|
94 |
|
|
|
160 |
|
|
|
512 |
|
|
Disposals |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Depreciation |
|
|
(11 |
) |
|
|
(31 |
) |
|
|
(5 |
) |
|
|
(9 |
) |
|
|
(56 |
) |
|
Exchange rate adjustment |
|
|
— |
|
|
|
15 |
|
|
|
(4 |
) |
|
|
— |
|
|
|
11 |
|
|
Closing balance June 30, 2014 |
|
|
57 |
|
|
|
214 |
|
|
|
92 |
|
|
|
151 |
|
|
|
514 |
|
|
Additions |
|
|
35 |
|
|
|
258 |
|
|
|
1 |
|
|
|
— |
|
|
|
294 |
|
|
Disposals |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Depreciation |
|
|
(25 |
) |
|
|
(86 |
) |
|
|
(12 |
) |
|
|
(29 |
) |
|
|
(152 |
) |
|
Exchange rate adjustment |
|
|
(11 |
) |
|
|
(52 |
) |
|
|
(19 |
) |
|
|
(25 |
) |
|
|
(107 |
) |
|
Closing balance June 30, 2015 |
|
|
56 |
|
|
|
334 |
|
|
|
62 |
|
|
|
97 |
|
|
|
549 |
|
Note 4. Intangible Assets
Intangible assets, net consisted of the following as of the dates indicated:
|
|
|
Useful Lives |
|
June 30, |
|
|||||
|
|
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in years) |
|
(in thousands) |
|
|||||
|
Computer software |
|
1-3 |
|
$ |
21 |
|
|
$ |
2 |
|
|
Less: Accumulated amortization |
|
|
|
|
(2 |
) |
|
|
— |
|
|
Intangible assets, net |
|
|
|
$ |
19 |
|
|
$ |
2 |
|
Intangible assets will be fully amortized over their useful lives.
Note 5. Accrued Expenses
Accrued expenses consisted of the following as of the dates indicated:
|
|
|
June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Accrued payroll and related expenses |
|
$ |
1,027 |
|
|
$ |
502 |
|
|
Accrued professional fees |
|
|
361 |
|
|
|
355 |
|
|
Accrued research and development costs |
|
|
964 |
|
|
|
1,442 |
|
|
Accrued expenses |
|
$ |
2,352 |
|
|
$ |
2,299 |
|
Note 6. Deferred Lease Incentive
Deferred lease incentive consisted of the following as of the dates indicated:
|
|
|
June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Current |
|
$ |
23 |
|
|
$ |
28 |
|
|
Non-Current |
|
$ |
61 |
|
|
$ |
103 |
|
In June 2014, a build-out incentive was negotiated with the landlord to reimburse the Company for the build-out of the Company’s Melbourne, Australia office space. The Company expects to offset such amounts within rental expense over the lease term.
F-16
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Note 7. Warrants
|
|
|
June 30, |
|
|
June 30, |
|
||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Warrants |
|
$ |
— |
|
|
$ |
5,435 |
|
In May 2014, the Company issued warrants to purchase 1,574,998 ordinary shares to purchasers of its Series B preference shares with an exercise price of $8.625 per share. In addition, the Company issued warrants to purchase 192,000 ordinary shares to financial advisors with an exercise price of $7.50 per share.
All warrants have a five year contractual life, are exercisable in U.S. dollars and were revalued to fair value at June 30, 2014 using the appropriate exchange rate. The warrants were reclassified to shareholders’ equity in September 2014 following the Irish Reorganization and the change in the Company’s functional currency to U.S. dollars.
Note 8. Convertible Preference Shares
Prior to February 2015, the Company had issued Series A investment preference shares (“SIRPS Preference Shares”) and Series B convertible preference shares (“Series B Preference Shares”) (collectively, the “Preference Shares”). The Company’s Memorandum and Articles of Association as of June 30, 2014 authorized 12,000,000 Preference Shares with a nominal value of $0.125 per Preference Share.
Preference Shares consisted of the following as of June 30, 2014:
|
June 30, 2014 |
|
Preference Shares Issued and Outstanding |
|
|
Liquidation Preference |
|
|
Carrying Value |
|
|||
|
|
|
|
|
|
|
(in thousands) |
|
|||||
|
SIRPS Preference Shares |
|
|
1,737,132 |
|
|
$ |
16,353 |
|
|
$ |
8,177 |
|
|
Series B Preference Shares |
|
|
4,200,006 |
|
|
|
51,298 |
|
|
|
25,649 |
|
|
|
|
|
5,937,138 |
|
|
$ |
67,651 |
|
|
$ |
33,826 |
|
Prior to August 2014, upon certain insolvency and other events, the holders of the Preference Shares could require their redemption. Therefore, the Preference Shares were classified outside of shareholders’ equity (deficit) in accordance with authoritative guidance for the classification and measurement of potentially redeemable securities as of June 30, 2014. In August 2014, these redemption features were eliminated, and, in November 2014, 16 additional convertible preference shares were issued in connection with the second share consolidation. Following changes to the redemption features of the Preference Shares in August 2014, the Preference Shares were reclassified into shareholders’ equity (deficit).
In February 2015, on completion of the Company’s initial public offering, each preference share automatically converted into one ordinary share.
Note 9. Ordinary Shares
In February 2015, upon the initial closing of the Company’s initial public offering, the New Memorandum and Articles (“M&A”) became effective for the Company. The New M&A amended the Company’s authorized capital to 400 Euro deferred shares with a nominal value of €100 each, 100,000,000 ordinary shares with a nominal value of US$0.125 each and 10,000,000 undesignated preferred shares with a nominal value of US$0.01 each. As of June 30, 2014, the Company’s authorized capital was 20,000,000 ordinary shares and 12,000,000 Preference Shares, each with a $0.125 nominal value per share.
As of June 30, 2015 and 2014, there were 11,406,916, and 1,268,810 ordinary shares outstanding, respectively, which included zero and 254,680 ordinary shares subject to limited recourse loans, respectively.
In the Company’s initial public offering, an aggregate of 4,176,903 ordinary shares were issued. Upon the initial closing of the Company’s initial public offering, all 5,937,160 Preference Shares then outstanding were converted into 5,937,160 ordinary shares.
F-17
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Note 10. Fair Value Measurement
Assets and liabilities carried at fair value on a recurring basis as of June 30, 2015 and 2014, including financial instruments which the Company accounts for under the fair value option, are summarized in the following tables.
|
June 30, 2015 |
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Assets/ Liabilities at Fair Value |
|
||||
|
|
|
(in thousands) |
|
|||||||||||||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
52,033 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
52,033 |
|
|
June 30, 2014 |
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Assets/ Liabilities at Fair Value |
|
||||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
30,041 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
30,041 |
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
5,435 |
|
|
$ |
5,435 |
|
During fiscal years 2015 and 2014, there were no transfers between the levels.
The following table presents a reconciliation of all assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3), including net realized and unrealized gains (losses) included in earnings and accumulated OCI.
|
Warrants (in thousands) |
|
|
|
|
|
Balance as of June 30, 2013 |
|
|
— |
|
|
Issuances |
|
|
5,435 |
|
|
Balance as of June 30, 2014 |
|
|
5,435 |
|
|
Reclassification into shareholders’ equity |
|
|
(5,435 |
) |
|
Balance June 30, 2015 |
|
$ |
— |
|
The following table present information about significant unobservable inputs related to the Company’s material categories of Level 3 assets and liabilities as of June 30, 2014.
|
Financial Instruments |
|
Fair Value |
|
|
Valuation Technique |
|
Significant Unobservable Input |
|
Range of Inputs |
|
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
Warrants |
|
$ |
5,435 |
|
|
Binomial option-pricing model |
|
Volatility |
|
70-80% |
|
|
|
|
|
|
|
|
|
Expected term |
|
4 years |
The warrants were reclassified as shareholders’ equity in September 2014 following the Irish Reorganization and the change in the Company’s functional currency to U.S. dollars.
Sensitivity of Fair Value Measures to Changes in Unobservable Inputs
The volatility assumption is representative of the level of uncertainty expected in the movements of the Company’s share price over the expected term of the warrant. A significant increase in volatility would increase the fair value of the warrants while a significant decrease in volatility would decrease the fair value of the warrants.
The highest value of a warrant instrument is attained if it is assumed to be held to expiry. Due to risk aversion, wealth diversification and the lack of marketability, warrant holders occasionally exercise prior to expiry. Based on the market value approach adopted for the valuation of the warrants, it is assumed that the warrants will be held on average for four years. An increase in the expected term of the warrants by one year would increase the fair value of the warrants by $0.5 million, or 10%, while similarly a decrease by one year would decrease the fair value of the warrants by $0.7 million, or 12%.
F-18
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Note 11. Net Loss Per Share and Pro Forma Net Loss Per Share
The calculation of net loss per participating securities (“EPS”) for fiscal years 2015 and 2014 is presented below.
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands, except share and per share amounts) |
|
|||||||||
|
Net loss |
|
$ |
(11,857 |
) |
|
$ |
(6,710 |
) |
|
$ |
(3,241 |
) |
|
Weighted-average ordinary shares issued and outstanding—basic and diluted |
|
|
5,214,957 |
|
|
|
1,000,872 |
|
|
|
894,794 |
|
|
Net loss per ordinary share—basic and diluted |
|
$ |
(2.27 |
) |
|
$ |
(6.70 |
) |
|
$ |
(3.62 |
) |
The following ordinary share equivalents were excluded from the calculation of diluted net loss per share for the periods ended on the dates indicated because including them would have an anti-dilutive effect:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Preference shares |
|
|
— |
|
|
|
5,937,138 |
|
|
|
792,117 |
|
|
Share-based awards |
|
|
834,303 |
|
|
|
245,020 |
|
|
|
— |
|
|
Warrants |
|
|
1,766,998 |
|
|
|
1,766,998 |
|
|
|
— |
|
|
Shares subject to limited recourse loans (see Note 12) |
|
|
— |
|
|
|
254,680 |
|
|
|
264,386 |
|
|
Total |
|
|
2,601,301 |
|
|
|
8,203,836 |
|
|
|
1,056,503 |
|
Basic and diluted net loss per share is computed using the weighted-average number of ordinary shares outstanding after giving effect to the conversion of all convertible preference shares into ordinary shares as if such conversion had occurred at the beginning of the period presented, or the date of original issuance, if later.
Note 12. Share-Based Awards
As permitted by Australian law, the Company’s board of directors has historically granted share options and restricted share units with an exercise or conversion price, as applicable, of zero to recipients in Australia. Contemporaneously with these awards and based upon information available at the time of grant, the Company’s board of directors, with the assistance of management, also determined the fair value of the shares underlying these share options for financial reporting purposes. To determine the best estimate of the fair value of the Company’s ordinary shares at each grant date, the Company’s board of directors considered numerous factors, including contemporaneous third-party valuations, current business conditions and projections, risks inherent to the development of the Company’s research and development programs, including the status of pivotal safety and efficacy studies for its lead product candidates, the Company’s financial condition, the Company’s need for future financing to fund its research and development efforts and the commercialization of its lead product candidates, and other relevant factors.
2012 Employee Share Option Plan
In August 2012, the Company’s board of directors adopted the Company’s Employee Share Option Plan (the “2012 Plan”). Pursuant to the 2012 Plan, the Company issued 264,386 ordinary shares at $4.20 per share to employees (including executive officers), consultants and each member of the Company’s board of directors who could purchase such ordinary shares with an interest-free, limited recourse loan payable to the Company. These limited recourse loans were not collateralized and were not recourse to the assets of the borrower, except to the extent of the shares issued. Because the loans were the sole consideration for the shares issued, the Company accounted for these arrangements as share options since the substance is similar to the grant of an option, with a deemed exercise price equal to the loan amount. The fair value of the notional share options was expensed in fiscal year 2013 when vested with a corresponding credit to additional paid-in capital.
F-19
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
The limited recourse loans were repayable within 30 days of the termination of service to the Company of the employee, director or consultant. Failure to pay back the loan within that time frame would have resulted in relinquishing of those shares by the shareholder. The balance of loans outstanding as of June 30, 2015 and 2014 was $zero and $1.0 million, respectively. As discussed in Note 2, the Company does not recognize a separate receivable for limited recourse loans.
The 2012 Plan is no longer in use. Between June 30, 2014 and September 30, 2014, all of the limited recourse loans were repaid in cash or satisfied by the repurchase by the Company of certain ordinary shares issued subject to such loans at a purchase price of $6.35 per ordinary share. The Company issued to each former holder of such ordinary shares an option to purchase a number of ordinary shares equal to the number of ordinary shares repurchased with an exercise price of $6.35 per ordinary share. The new options expire in February 2018, consistent with the original repayment date of the loan. With respect to Dr. Heffernan, his $0.3 million loan amount was satisfied with a repurchase by the Company of 52,040 ordinary shares held by him and the grant to him of an option to purchase 52,040 ordinary shares. With respect to Dr. Gearing, his $0.3million loan amount was satisfied with a repurchase by the Company of 46,372 ordinary shares held by him and the grant to him of an option to purchase 46,372 ordinary shares. As a result of the repurchases, all of the limited recourse loans were repaid in the Company’s first fiscal quarter of 2015.
2013 Long Term Incentive Plan
In September 2013, the Company’s board of directors approved a long-term incentive plan for its employees (including executive officers), directors and consultants pursuant to which in November 2013 the Company issued share options to purchase 215,799 ordinary shares and restricted share units to acquire 29,214 ordinary shares to employees, directors and consultants. The underlying ordinary shares had a fair value of $5.15 per share, but the awards had an exercise or conversion price, as applicable, of zero, as permitted under Australian law. Because Irish law requires the payment to an issuer of at least the nominal value of shares in order to acquire such shares from the issuer, any options or restricted share units with a zero exercise or conversion price became exercisable or convertible, as applicable, at their nominal value in August 2014 in anticipation of the Irish Exchange. This nominal value became $0.10 per ordinary share in September 2014 in connection with the Irish Exchange and was revised to $0.125 per ordinary share in connection with the four-for-five share consolidation in November 2014. In September 2014, the Company also issued share options to purchase 16,800 ordinary shares and restricted share units to acquire 21,240 ordinary shares to employees, directors and consultants. The underlying ordinary shares had a fair value of $6.35 per ordinary share, but the awards had an exercise or conversion price of the nominal value of $0.10 per ordinary share, which nominal value became $0.125 per ordinary share in connection with the four-for five share consolidation in November 2014. Except for share options and restricted share units held by directors (which vested in November 2014), share options and restricted share units held by employees and consultants vest in three equal tranches in November 2014, November 2015 and November 2016. The Company revised this plan in September 2014 and refers to this plan as its “2013 Plan.”
In November 2014, the Company awarded employees 141,792 share options and restricted share units to acquire 16,427 ordinary shares. The underlying ordinary shares had a grant date fair value of $9.37. The share options and restricted share units have an exercise price and conversion price of $0.125 per ordinary share. The awards are subject to a specified performance condition and service period and they vest in tranches over one to three years.
The 2013 Plan was terminated in connection with the Company’s initial public offering. The 2013 Plan will continue to govern outstanding awards granted thereunder. Appropriate adjustments will be made in the number of authorized ordinary shares and other numerical limits in the 2013 Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a share split or other change in the Company’s capital structure.
Prior to its termination, the 2013 Plan was administered by the Company’s board of directors. Subject to the provisions of the 2013 Plan, the board of directors determined, in its discretion, the persons to whom, and the times at which, awards were granted, as well as the size, terms and conditions of each award, under the 2013 Plan. All awards are evidenced by a written agreement between the Company and the holder of the award. The board of directors has the authority to construe and interpret the terms of the 2013 Plan and awards granted under the 2013 Plan.
F-20
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
In the event of a change of control as described in the 2013 Plan, the acquiring or successor entity may assume or continue all or any awards outstanding under the 2013 Plan or substitute substantially equivalent awards. Any awards which are not assumed or continued in connection with a change of control or are not exercised or settled prior to the change of control will terminate effective as of the time of the change of control. The board of directors may provide for the acceleration of vesting of any or all outstanding awards upon such terms and to such extent as it determines, except that the vesting of all awards held by members of the board of directors who are not employees will automatically be accelerated in full. The 2013 Plan also authorizes the board of directors, in its discretion and without the consent of any participant, to cancel each or any outstanding award denominated in ordinary shares upon a change of control in exchange for a payment to the participant with respect to each share subject to the cancelled award of an amount equal to the excess of the consideration to be paid per ordinary share in the change of control transaction over the exercise price per ordinary share, if any, under the award.
2015 Equity Incentive Plan
In September 2014, the Company’s board of directors adopted, and in November 2014 the Company’s shareholders approved, the 2015 Equity Incentive Plan (“2015 Plan”). The 2015 Plan was amended by the board of directors in January 2015 and became effective on the date immediately prior to the date of the prospectus for initial public offering. The 2015 Plan is intended to provide incentives that will assist the Company to attract, retain, and motivate employees, including officers, consultants and directors. The Company may provide these incentives through the grant of share options, restricted share units, performance shares and units and other cash-based or share-based awards.
A total of 1,280,000 of the Company’s ordinary shares were initially authorized and reserved for issuance under the 2015 Plan. This reserve has or will automatically increase on July 1 of each year through 2024 by an amount equal to the lesser of:
|
|
· |
Four percent of the number of the Company’s ordinary shares issued and outstanding on the immediately preceding June 30; and |
|
|
· |
An amount determined by the Company’s board of directors. |
The ordinary shares available under the 2015 Plan will not be reduced by awards settled in cash, but will be reduced by ordinary shares withheld to satisfy tax withholding obligations with respect to ordinary share options (but not other types of awards). The gross number of ordinary shares issued upon the exercise of options exercised by means of a net exercise or by tender of previously-owned ordinary shares will be deducted from the ordinary shares available under the 2015 Plan. Notwithstanding the foregoing, and subject to adjustment as described below, the maximum aggregate number of ordinary shares that may be subject to issuance at any given time under the 2015 Plan in connection with outstanding awards shall not exceed a number equal to ten percent of the Company’s total issued and outstanding ordinary shares (calculated on a non-diluted basis).
Appropriate adjustments will be made in the number of authorized ordinary shares and other numerical limits in the 2015 Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a share split or other change in the Company’s capital structure. Ordinary shares subject to awards which expire or are cancelled or forfeited will again become available for issuance under the 2015 Plan.
In May 2015, 360,000 share options and 20,280 restricted share units to acquire ordinary shares were awarded to employees and directors under the 2015 Plan with a grant date fair value of $5.93 and $6.99, respectively. The share option and restricted share units have an exercise price of $15.00 and a conversion price of $0.125 per ordinary share, respectively. Except for 2,280 restricted share units held by directors (which vest immediately), the awards vest in tranches over one to five years.
In June 2015, bonus share awards of an aggregate 42,691 ordinary shares were issued to employees subject to the payment of $0.125 per ordinary share. The underlying ordinary share had a grant date fair value of $7.115 per ordinary share.
Share Awards to Consultants or Advisors for Services Provided
There were no share awards to consultants or advisors issued in fiscal year 2015.
In November 2013, the Company entered into an agreement with a financial advisor for the provision of certain financial services. Pursuant to the agreement, the financial advisor elected to receive payment of fees in ordinary shares. In November 2013, the Company issued 8,849 SIRPS Preference Shares at $5.18 per share to the financial advisor.
F-21
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
In December 2012, the Company entered into an agreement with Robert Gearing under which the Company agreed to pay to him a commission for arranging certain research and development services. The Company issued Mr. Gearing 6,250 ordinary shares in fiscal year 2013 and 4,424 ordinary shares in fiscal year 2014. The ordinary shares had an aggregate value of $48,000 and the Company recognized share-based compensation expense of $26,000 and $22,000 in fiscal years 2013 and 2014, respectively.
Note 13. Valuation of Share Awards
The fair value of each share option is estimated on the date of grant using the binomial option-pricing model. The Company was a private company until February 2015 and lacked company-specific historical and implied volatility information. Therefore, it has estimated its expected share volatility based on the historical volatility of its publicly traded peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded share price. The expected term of the Company’s share options has been determined utilizing the “simplified” method as the Company has insufficient historical experience for share options overall, rendering existing historical experience irrelevant to expectations for current grants. The risk-free interest rate is determined by reference to the appropriate reserve bank yield in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future. The fair value of the underlying ordinary shares considered the price per share paid by investors in the Company’s private financings, including the Series B Preference Shares in May 2014. The fair value of the share options was estimated using the following assumptions:
|
|
|
Year Ended June 30, |
||
|
|
|
2015 |
|
2014 |
|
Fair value per ordinary share |
|
$5.00-$15.00 |
|
$5.15 |
|
Risk free interest rate |
|
1.7% |
|
4.0% |
|
Expected term (in years) |
|
3-6 years |
|
5-10 years |
|
Expected volatility |
|
80% |
|
80% |
|
Expected dividend yield |
|
zero |
|
zero |
F-22
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Since completion of the Company’s initial public offering, the fair value of the underlying ordinary shares was based on the price quoted on NASDAQ Global Market at date of grant.
The following table summarizes share option activity for fiscal years 2014 and 2015:
|
|
|
Shares Issuable Under Options |
|
|
|
Weighted- Average Exercise Price |
|
|
Weighted- Average Remaining Contractual Term (in years) |
|
|
Aggregate Intrinsic Value (in thousands) |
|
||||
|
Outstanding as of July 1, 2013 |
|
|
264,386 |
|
|
|
$ |
4.20 |
|
|
|
5 |
|
|
$ |
708 |
|
|
Granted |
|
|
215,799 |
|
|
|
|
0.125 |
|
|
|
4.75 |
|
|
|
1,115 |
|
|
Exercised |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Ordinary shares no longer subject to limited recourse loans |
|
(9,706 |
) |
(1) |
|
|
4.20 |
|
|
|
5 |
|
|
|
(26 |
) |
|
|
Expired or forfeited |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Outstanding as of June 30, 2014 |
|
|
470,479 |
|
|
|
$ |
2.33 |
|
|
|
5 |
|
|
$ |
1,797 |
|
|
Granted |
|
|
663,661 |
|
|
|
$ |
9.55 |
|
|
|
5 |
|
|
$ |
2,355 |
|
|
Exercised |
|
|
(96,540 |
) |
|
|
|
0.125 |
|
|
|
5 |
|
|
|
(501 |
) |
|
Ordinary shares no longer subject to limited recourse loans |
|
|
(109,611 |
) |
(1) |
|
|
4.20 |
|
|
|
5 |
|
|
|
(460 |
) |
|
Repurchased |
|
|
(145,069 |
) |
(1) |
|
|
6.35 |
|
|
|
5 |
|
|
|
(222 |
) |
|
Expired or forfeited |
|
|
(6,289 |
) |
|
|
|
0.125 |
|
|
|
— |
|
|
|
(45 |
) |
|
Outstanding as of June 30, 2015 |
|
|
776,631 |
|
|
|
$ |
8.18 |
|
|
|
5 |
|
|
$ |
2,924 |
|
|
Options vested and expected to vest, as of June 30, 2013 |
|
|
— |
|
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Options vested and expected to vest, as of June 30, 2014 |
|
|
6,195 |
|
|
|
$ |
0.125 |
|
|
|
|
|
|
|
|
|
|
Options vested and expected to vest, as of June 30, 2015 |
|
|
159,937 |
|
|
|
$ |
5.77 |
|
|
|
|
|
|
|
|
|
(1)Reflects ordinary shares issued subject to limited recourse loans. See Note 12.
F-23
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
In addition to the share options described above, the Company has granted restricted share units to its directors, employees and consultants. Restricted share units are valued at the fair value of the underlying ordinary shares as of the date of grant. The fair value of the ordinary shares issuable upon conversion of restricted share units considered the price per share paid by investors in the Company’s private financings, including the SIRPS Preference Shares in July 2013. The ordinary shares subject to the restricted share units are generally issued when they vest. The table below presents the Company’s restricted share unit activity for fiscal years 2015 and 2014:
|
|
|
Number of Restricted Share Units |
|
|
Weighted Average Grant Date Fair Value |
|
|
Weighted Average Remaining Contractual Term (in years) |
|
|
Aggregate Intrinsic Value (in thousands) |
|
||||
|
Outstanding as of July 1, 2013 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
Granted |
|
|
29,214 |
|
|
|
5.15 |
|
|
|
2.4 |
|
|
|
151 |
|
|
Converted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Forfeited |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Outstanding as of June 30, 2014 |
|
|
29,214 |
|
|
$ |
5.15 |
|
|
|
2.4 |
|
|
$ |
151 |
|
|
Granted |
|
|
57,947 |
|
|
|
7.83 |
|
|
2.1 |
|
|
|
453 |
|
|
|
Converted |
|
|
(29,489 |
) |
|
|
5.85 |
|
|
|
— |
|
|
|
(172 |
) |
|
Forfeited |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Outstanding as of June 30, 2015 |
|
|
57,672 |
|
|
$ |
7.48 |
|
|
|
2.1 |
|
|
$ |
432 |
|
|
Converted and expected to convert, as of June 30, 2013 |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Converted and expected to convert, as of June 30, 2014 |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Converted and expected to convert, as of June 30, 2015 |
|
|
4,400 |
|
|
$ |
9.19 |
|
|
|
|
|
|
|
|
|
Share-Based Compensation
The Company recognizes share-based compensation expense for only the portion of awards that are expected to vest. In developing a forfeiture rate estimate, the Company has considered its historical experience to estimate pre-vesting forfeitures for service-based awards. The impact of a forfeiture rate adjustment will be recognized in full in the period of adjustment, and if the actual forfeiture rate is materially different from the Company’s estimate, the Company may be required to record adjustments to share-based compensation expense in future periods.
The Company recorded share-based compensation expense related to share options and restricted share units for the fiscal years 2015, 2014 and 2013 as follows:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Research and development |
|
$ |
756 |
|
|
$ |
74 |
|
|
$ |
298 |
|
|
General and administrative |
|
|
1,325 |
|
|
|
224 |
|
|
|
412 |
|
|
Total |
|
$ |
2,081 |
|
|
$ |
298 |
|
|
$ |
710 |
|
The Company had an aggregate of $3.1 million and $0.9 million, respectively, of unrecognized share-based compensation expense for share options and restricted share units outstanding as of June 30, 2015 and 2014, which is expected to be recognized over an estimated period of 4.0 years and 2.3 years, respectively, for share options and restricted share units.
F-24
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Note 14. Commitments and Contingencies
Indemnities and Guarantees
The Company has made certain indemnities and guarantees, under which it may be required to make payments to a guaranteed or indemnified party, in relation to certain transactions. The Company indemnifies its officers and directors to the maximum extent permitted under the laws of Ireland. The duration of these indemnities and guarantees varies and, in certain cases, is indefinite. These indemnities and guarantees do not provide for any limitation of the maximum potential future payments the Company could be obligated to make. Historically, the Company has not been obligated to make any payments for these obligations and no liabilities have been recorded for these indemnities and guarantees in the accompanying balance sheets.
Operating Lease
The Company entered into a lease for its office in Melbourne, Australia commencing December 2013 for a period of five years. As of June 30, 2015 and June 30, 2014 commitments totaled $0.4 million and $0.5 million, respectively. Rent expense was $0.2 million, $0.1 million and $22,000 for fiscal years 2015, 2014 and 2013, respectively. Included in rent expense is a build-out incentive of $26,000, $11,000 and zero for fiscal years 2015, 2014 and 2013, respectively. A portion of the incentive paid by the landlord is to be repaid by the Company if the lease is early terminated, determined by the unexpired term of the lease over the original 60-month lease term. There are no escalation clauses in the lease agreement.
In connection with the development of animal biopharmaceuticals the Company has open contracts with suppliers for goods and services of $0.7 million and $1.7 million as of June 30, 2015 and 2014, respectively.
As of June 30, 2015 and 2014, future payments under these non-cancellable operating leases and non-cancellable purchase obligations were as follows:
|
|
|
Total |
|
|
Less than 1 Year |
|
|
1-3 Years |
|
|
3-5 Years |
|
|
After 5 Years |
|
|||||
|
June 30, 2015 |
|
(in thousands) |
|
|||||||||||||||||
|
Operating leases |
|
$ |
352 |
|
|
$ |
109 |
|
|
$ |
190 |
|
|
$ |
53 |
|
|
$ |
— |
|
|
Purchase obligations |
|
|
680 |
|
|
|
680 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
1,032 |
|
|
$ |
789 |
|
|
$ |
190 |
|
|
$ |
53 |
|
|
$ |
— |
|
|
|
|
Total |
|
|
Less than 1 Year |
|
|
1-3 Years |
|
|
3-5 Years |
|
|
After 5 Years |
|
|||||
|
|
|
|
|
|||||||||||||||||
|
June 30, 2014 |
|
(in thousands) |
|
|||||||||||||||||
|
Operating leases |
|
$ |
475 |
|
|
$ |
97 |
|
|
$ |
205 |
|
|
$ |
173 |
|
|
$ |
— |
|
|
Purchase obligations |
|
|
1,670 |
|
|
|
1,670 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
2,145 |
|
|
$ |
1,767 |
|
|
$ |
205 |
|
|
$ |
173 |
|
|
$ |
— |
|
F-25
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Note 15. Income Taxes
There is no provision for income taxes because the Company has historically incurred operating losses and maintains a full valuation allowance against its net deferred tax assets. The reported amount of income tax expense differs from the amount that would result from applying domestic federal statutory tax rates to pretax losses primarily because of changes in valuation allowance. In all periods presented, all revenue was earned in Australia.
A reconciliation of the statutory income tax rate to the Company’s effective income tax rate is as follows:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Statutory income tax rate |
|
$ |
1,482 |
|
|
$ |
1,988 |
|
|
$ |
972 |
|
|
Adjustment for foreign tax rates |
|
|
1,913 |
|
|
|
— |
|
|
|
— |
|
|
Non-assessable income |
|
|
1,722 |
|
|
|
703 |
|
|
|
(415 |
) |
|
Non-deductible expenses |
|
|
(3,669 |
) |
|
|
(1,687 |
) |
|
|
(281 |
) |
|
Change in deferred tax asset valuation allowance |
|
|
(1,344 |
) |
|
|
(1,004 |
) |
|
|
(276 |
) |
|
Change in tax rates |
|
|
(104 |
) |
|
|
— |
|
|
|
— |
|
|
Effective income tax rate |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
Non-assessable income and non-deductible expenses represent the gross amounts assessed under the research and development incentive program.
Net deferred tax assets as of June 30, 2015 and 2014 consisted of the following:
|
|
|
June 30, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Net operation loss carryforward |
|
$ |
2,578 |
|
|
$ |
1,234 |
|
|
Accrued expenses and deferred lease incentive |
|
|
218 |
|
|
|
503 |
|
|
Exchange (gain)/loss |
|
|
(591 |
) |
|
|
— |
|
|
Net deferred tax assets |
|
|
2,205 |
|
|
|
1,737 |
|
|
Valuation allowance |
|
|
(2,205 |
) |
|
|
(1,737 |
) |
|
Net deferred tax assets, net of valuation allowance |
|
$ |
— |
|
|
$ |
— |
|
As of June 30, 2015 and 2014, the Company had total deferred tax assets of $2.2 million and $1.7 million, respectively. Management has evaluated the factors bearing upon the reliability of its deferred tax assets, which consist principally of tax loss carry forwards for Australian income tax purposes of $7.2 million and $4.0 million, respectively. Management concluded that uncertainty of realizing any tax benefits as of June 30, 2015 and 2014, a full valuation allowance was necessary to offset its net deferred tax assets due to the Company’s lack of taxable income prospects for the foreseeable future.
While preparing its financial statements for the year ended June 30, 2015, management identified an adjustment to the tax disclosure in its previously issued annual financial statements for the years ended June 30, 2014 and 2013 and interim financial statements for the quarter ended September 30, 2014. The Company did not properly present tax loss carry forward amounts and related total deferred tax assets and valuation allowance amounts in its Income Taxes footnote. The Company deemed the revision of the disclosure as not material to the June 30, 2014 and June 30, 2013 annual financial statements or September 30, 2014 interim financial statements.
F-26
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
The Company has revised the June 30, 2014 amounts included in this filing, to properly report tax loss carry forwards, deferred tax assets, and the valuation allowance amounts and associated annual movement amounts for the fiscal year then ended. The Company has also revised the June 30, 2013 amounts included in this filing, to properly report the valuation allowance amount and associated annual movement amount for the fiscal year then ended. As a result of the revision, comparative deferred tax assets relating to tax loss carry forwards have been decreased by $2.9 million, $0.8 million and $4.5 million with a corresponding change in valuation allowance for the years ended June 30, 2014 and June 30, 2013 and quarter ended September 30, 2104, respectively. As disclosed in the notes to the financial statements, the Company has historically incurred operating losses and maintains a full valuation allowance against its net deferred tax assets. Therefore, there are no changes to the amounts recorded in the balance sheets, statements of operations and comprehensive loss and statements of cash flows.
Utilization of tax loss carry forwards is subject to potential limitation as a consequence of the Australian loss recoupment rules and future ownership changes, future capital raisings or ongoing changes in the Company’s business. Given the change of ownership that occurred in September 2014 (see Note 1), the Australian tax authorities could argue that there has been a change in the Company’s underlying business in Australia, which may result in these tax losses never being recoverable. Should this occur, future Australian taxable profits would be taxed at the full corporate rate, which is currently 28.5% (30% in 2014). Depending on the actual amount of any limitation on the Company’s ability to use its tax loss carry forwards, a significant portion of its future taxable income could be taxable.
Changes in the valuation allowance for deferred tax assets during fiscal years 2015, 2014 and 2013 were as follows:
|
|
|
June 30, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Valuation allowance as of beginning of year |
|
$ |
1,737 |
|
|
$ |
479 |
|
|
$ |
204 |
|
|
Increases recorded to income tax provision |
|
|
468 |
|
|
|
1,258 |
|
|
|
275 |
|
|
Valuation allowance as of end of year |
|
$ |
2,205 |
|
|
$ |
1,737 |
|
|
$ |
479 |
|
The Company has not recorded any amounts for unrecognized tax benefits as of June 30, 2015 or 2014. The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal and state jurisdictions, where applicable. There are currently no pending income tax examinations. The Company’s tax years are still open under statute from 2011 to the present. The Company’s policy is to record interest and penalties related to income taxes as part of its income tax provision.
Note 16. Related Party Transaction
Dr. Paul Wood is a former director of the Company. In May 2013, the Company entered into a consultancy agreement with Dr. Wood for consulting services. The Company recorded expense of $23,000, $23,000 and $8,000 in fiscal years 2015, 2014 and 2013, respectively, related to this agreement.
Peter Howard is a former director of the Company, In July 2011, the Company entered into a consultancy agreement with Mr. Howard for financial advisory services. The Company recorded expense of $28,000 and $30,000 in fiscal years 2015 and 2014, respectively, related to this agreement.
Ridge Biotechnology Consulting, LLC is owned and operated by Dr. Robert Gearing, the brother of David Gearing, a co-founder of the Company and its Chief Scientific Officer. In October 2010, the Company entered into a consulting agreement with Ridge Biotechnology Consulting, LLC for the provision of services to the Company. The agreement was superseded by agreements entered into in April 2011 and April 2012, and a new consulting agreement with Ridge Biotechnology Consulting, LLC was entered into in January 2014. The Company recorded general and administrative expense of $0.2 million , $0.1 million and $51,000 in fiscal years 2015, 2014 and 2013, respectively, related to these agreements. As of June 30, 2015 and 2014, there was $13,000 and $19,000 payable to Ridge Biotechnology Consulting, LLC, respectively. Dr. Robert Gearing was also a party to a 2012 agreement with the Company pursuant to which the Company issued Dr. Gearing 4,4240 ordinary shares in fiscal year 2014 and 6,250 ordinary shares in fiscal year 2013, with an aggregate value of $48,000, for arranging certain research and development services.
F-27
NEXVET BIOPHARMA PUBLIC LIMITED COMPANY
Notes to Consolidated Financial Statements — Continued
Dr. Andrew Gearing is a former director, a co-founder of the Company and a brother of David Gearing, a co-founder of the Company and its Chief Scientific Officer. Dr. Andrew Gearing serves on the board of directors of Biocomm Square Pty Ltd. In August 2010 and August 2013, the Company entered into consulting agreements with Biocomm Square Pty Ltd for research and development support services. These agreements were superseded by a new consulting agreement in December 2013, which was amended in April 2014. In addition, the Company entered into an agreement with Biocomm Square Pty Ltd in November 2011 for assistance in obtaining partnering arrangements with Japanese entities. The Company recorded research and development expense of $0.2 million, $0.2 million and $0.1 million in fiscal years 2015, 2014 and 2013, respectively, related to these agreements. As of June 30, 2015 and 2014, there was $17,000 and $23,000 payable to Biocomm Square Pty Ltd, respectively.
David Gearing, a co-founder of the Company and its Chief Scientific Officer, loaned the Company $0.2 million in May 2012, accruing interest at 15% per annum. In February 2013, the Company repaid the loan together with $21,000 in accrued interest.
Note 17. Subsequent Events
There were no material subsequent events occurring after June 30, 2015 requiring disclosure.
Note 18. Selected Quarterly Financial Data (Unaudited)
The following table presents selected unaudited quarterly financial information for the years ended June 30, 2015 and 2014. The results for any quarter are not necessarily indicative of future quarterly results and, accordingly, period to period comparisons should not be relied upon as an indication of future performance.
|
|
|
For the Quarter Ended |
|
|||||||||||||
|
|
|
June 30, |
|
|
March 31, |
|
|
December 31, |
|
|
September 30, |
|
||||
|
2015 |
|
(in thousands, except share and per share amounts) |
|
|||||||||||||
|
Operating expenses |
|
$ |
(4,904 |
) |
|
$ |
(5,109 |
) |
|
$ |
(4,666 |
) |
|
$ |
(5,357 |
) |
|
Net loss attributable to ordinary shareholders |
|
$ |
(4,479 |
) |
|
$ |
(2,668 |
) |
|
$ |
(2,413 |
) |
|
$ |
(2,297 |
) |
|
Net loss per share attributable to ordinary shareholders—basic and diluted |
|
$ |
(0.39 |
) |
|
$ |
(0.36 |
) |
|
$ |
(2.01 |
) |
|
$ |
(2.13 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
$ |
(3,878 |
) |
|
$ |
(2,693 |
) |
|
$ |
(1,664 |
) |
|
$ |
(1,808 |
) |
|
Net loss attributable to ordinary shareholders |
|
$ |
(3,231 |
) |
|
$ |
(1,470 |
) |
|
$ |
(993 |
) |
|
$ |
(1,016 |
) |
|
Net loss per share attributable to ordinary shareholders—basic and diluted |
|
$ |
(3.22 |
) |
|
$ |
(1.47 |
) |
|
$ |
(0.99 |
) |
|
$ |
(1.02 |
) |
F-28
|
|
|
|
|
Incorporated by Reference |
|
Filed/Furnished Herewith |
|||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit Filing Date |
|
||
|
3.1 |
|
Memorandum and Articles of Association. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
4.1 |
|
Form of Share Certificate. |
|
S-1 |
|
333-201309 |
|
1/26/15 |
|
|
|
|
4.2 |
|
Form of Warrant to Purchase Ordinary Shares. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.1† |
|
Master Collaboration, Supply and Distribution Agreement, dated November 24, 2014, by and between Nexvet Ireland Limited and Virbac S.A. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.2† |
|
Specific Distribution Agreement for NV‑01 Product, dated November 24, 2014, by and between Nexvet Ireland Limited and Virbac S.A. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.3+ |
|
Employment Agreement, dated December 22, 2014, by and between the Registrant and Mark Heffernan, Ph.D. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.4+ |
|
Employment Agreement, dated December 22, 2014, by and between the Registrant and David Gearing, Ph.D. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.5+ |
|
Employment Agreement, dated December 22, 2014, by and between the Registrant and Damian Lismore. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.6+ |
|
2013 Long Term Incentive Plan. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.7+ |
|
2015 Equity Incentive Plan, as amended. |
|
|
|
|
|
|
|
X |
|
|
10.8 |
|
Form of Deed of Indemnity, Insurance and Access entered into or to be entered into by directors and officers. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
10.9 |
|
Registration Rights Agreement, dated January 22, 2015, by and between the Registrant and the parties listed on Schedule A thereto. |
|
S-1 |
|
333-201309 |
|
1/26/15 |
|
|
|
|
10.10+ |
|
Employment Agreement, dated April 29, 2015, by and between the Registrant and Juergen Horn, Ph.D. |
|
|
|
|
|
|
|
X |
|
|
21.1 |
|
List of subsidiaries of the Registrant. |
|
S-1 |
|
333-201309 |
|
12/30/14 |
|
|
|
|
23.1 |
|
Consent of PricewaterhouseCoopers, independent registered public accounting firm. |
|
|
|
|
|
|
|
X |
|
|
24.1 |
|
Power of Attorney (included in the signature page). |
|
|
|
|
|
|
|
X |
|
|
31.1 |
|
Certification of Principal Executive Officer, pursuant to Rule 13a‑14(a)/15d‑14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
X |
|
|
31.2 |
|
Certification of Principal Financial Officer, pursuant to Rule 13a‑14(a)/15d‑14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
X |
|
|
32.1* |
|
Certification of Chief Executive Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
X |
|
|
32.2* |
|
Certification of Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
X |
|
|
101.INS |
|
XBRL Instance Document. |
|
|
|
|
|
|
|
X |
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
|
|
X |
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
|
|
X |
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
|
|
|
X |
|
|
101.LAB |
|
XBRL Taxonomy Extension Labels Linkbase Document. |
|
|
|
|
|
|
|
X |
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
|
|
X |
|
|
|
|
||||||||||
|
+ |
Indicates management compensation plan |
||||||||||
|
† |
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 under the Securities Exchange Act of 1934, as amended |
||||||||||
|
* |
This certification is deemed not filed for purpose of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act. |
||||||||||
|
|
|
|