Attached files
| file | filename |
|---|---|
| EX-32.1 - CERTIFICATION OF CHIEF EXECUTIVE OFFICER - Kintara Therapeutics, Inc. | f10k2015ex32i_delmarpharma.htm |
| EX-31.1 - CERTIFICATION OF CHIEF EXECUTIVE OFFICER - Kintara Therapeutics, Inc. | f10k2015ex31i_delmarpharma.htm |
| EX-32.2 - CERTIFICATION OF CHIEF FINANCIAL OFFICER - Kintara Therapeutics, Inc. | f10k2015ex32ii_delmarpharma.htm |
| EX-31.2 - CERTIFICATION OF CHIEF FINANCIAL OFFICER - Kintara Therapeutics, Inc. | f10k2015ex31ii_delmarpharma.htm |
| EX-10.21 - AUDIT COMMITTEE CHARTER - Kintara Therapeutics, Inc. | f10k2015ex10xxi_delmarpharma.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D. C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the year ended June 30, 2015
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from
Commission file number 000-54801
DelMar Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 99-0360497 | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
Suite 720-999 West Broadway
Vancouver, British Columbia, Canada V5Z 1K5
(Address of principal executive offices)
(604) 629-5989
(Issuer's telephone number)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.001
Indicate by check mark whether the registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act. o Yes þ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes þ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. þ Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). þ Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K . þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| Non-accelerated filer | ☐ | Smaller reporting company | R |
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Exchange Act) ☐ Yes þ No
As of December 31, 2014, the aggregate market value of the issued and outstanding common stock held by non-affiliates of the registrant, based upon the closing price of our common stock of $0.81 was approximately $22.8 million. For purposes of the above statement only, all directors, executive officers and 10% shareholders are assumed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for any other purpose.
Number of shares of common stock outstanding as of August 26, 2015 was 39,477,556.
DOCUMENTS INCORPORATED BY REFERENCE – None
FORM 10-K
FOR THE FISCL YEAR ENDED JUNE 30, 2015
TABLE OF CONTENTS
| 2 |
Background
We are a clinical stage drug development company with a focus on the treatment of cancer. Our mission is to benefit patients and create shareholder value by rapidly developing and commercializing anti-cancer therapies in orphan cancer indications where patients have failed or are unlikely to respond to modern therapy. In October 2011 we initiated clinical trials with our drug candidate, VAL-083, in the United States as a potential new treatment for glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer. We have presented interim data from our clinical trial at peer reviewed scientific meetings demonstrating that VAL-083 can shrink or halt the growth of tumors in some brain cancer patients who have failed other approved treatments. Currently, there is no approved therapy for these patients.
In addition to our clinical development activities in the United States, we have obtained certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. In October 2012, we announced that we had entered into a collaboration agreement with the only manufacturer presently licensed by the China Food and Drug Administration (“CFDA”) to produce the product for the China market. This agreement potentially positions us to generate revenue through product sales or royalties for its approved indications in China while we seek global approval in new indications.
We have filed a broad portfolio of patent applications to protect our intellectual property. Our patent applications claim compositions and methods related to the use of VAL-083 and related compounds as well as methods of synthesis and quality controls for the manufacturing process of VAL-083. In addition, VAL-083 has been granted orphan drug status by the United States Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”). We believe that our portfolio of intellectual property rights provides a defensible market position for the commercialization of VAL-083.
Our drug discovery research focuses on identifying well-validated clinical and commercial-stage compounds and establishing a scientific rationale for development in modern orphan drug indications. Through our relationship with Valent Technologies, LLC (“Valent”), a company owned by Dr. Dennis Brown, our Chief Scientific Officer, we are able to utilize Valent’s proprietary ChemEstate bioinformatics tools which are used to screen and identify potential candidates. Promising candidates are further researched through our network of consultants and contract research organizations. This approach allows us to rapidly identify and advance potential drug candidates without significant investment in “wet lab” infrastructure. Based on this strategy, we acquired the initial VAL-083 intellectual property and prototype drug product from Valent and have identified additional drug candidates that we may have the opportunity to license or acquire in the future.
We also believe the experience of our clinical development team will position us to acquire or license additional product candidates to establish a pipeline of product opportunities. We plan to seek marketing partnerships to supplement our own commercialization efforts and potentially generate future royalty revenue. We have secured four non-refundable financial contributions from the National Research Council of Canada for total financial contributions of approximately CDN $420,000 to date.
Recent Highlights
We continued to make progress with our drug development programs:
| ● | We reported progress in our Phase I/II clinical trial with VAL-083 in refractory GBM at peer-reviewed cancer meetings including the Society for Neuro-Oncology (“SNO”), the American Association of Cancer Research (“AACR”) and the American Society of Clinical Oncology (“ASCO”); |
| ● | We completed the Phase I portion of the clinical trial and confirmed an optimized dosing regimen for advancement to an expanded Phase II clinical trial. The optimized dosing regimen delivers substantially higher doses compared to previous clinical trials conducted by the National Cancer Institutes (“NCI”) in the United States. We believe that such higher doses may enhance the potential of VAL-083 to impact a patient’s tumor and as well as to improve patient outcomes; |
| 3 |
| ● | We
reported the observation of a promising dose-response trend in the Phase I portion of the clinical trial. Patients receiving a
dose ≥30mg/m2 had a median survival of 9 months vs. 5 months at doses up to 5mg/m2; |
| ● | We reported additional non-clinical data supporting the favorable differentiation of VAL-083 vs. standard of care in the treatment of GBM, non-small cell lung cancer (“NSCLC”) and other solid tumors. We believe these data support the potential of VAL-083 to address the modern unmet medical needs in the treatment of a range of cancers, especially where other therapies have failed or are predicted to give sub-optimal outcomes; and |
| ● | We announced that the Mayo Clinic Cancer Center in Rochester, Minnesota and the Sarah Cannon Cancer Research Center at HealthOne, Denver, Colorado have had been added as clinical trial sites for our ongoing, multicenter Phase I/II clinical trial study of VAL-083 in patients with refractory GBM. |
We accessed additional capital to support our drug development and research programs:
| ● | A non-dilutive investment of $1,404,177 was obtained through the exercise of Investor Warrants during the fiscal year ending June 30, 2015; |
| ● | Increased grant and other non-repayable funding contributions of $155,635 was obtained in June, 2014; and |
| ● | Subsequent to the end of the fiscal year, we completed a registered financial offering for gross proceeds of $2.6 million. |
We continued to strengthen our intellectual property portfolio. DelMar Pharmaceuticals now holds five issued US patents and two issued international patents. In addition, we have filed eleven patent applications across eight patent families.
Our stock was approved for trading on the OTCQX. We believe this was an important step forward to building liquidity for our shareholders as part of our overall mission to deliver long-term shareholder value.
VAL-083
Our product candidate,VAL-083, represents a “first-in-class” small molecule chemotherapeutic which means that the molecular structure of VAL-083 is not an analogue or derivative of other small molecule chemotherapeutics approved for the treatment of cancer. VAL-083 was originally discovered in the 1960’s and has been assessed in 42 Phase 1 and Phase 2 clinical trials sponsored by the NCI in the United States as a treatment against various cancers including lung, brain, cervical, ovarian tumors and leukemia. Published pre-clinical and clinical data suggest that VAL-083 may be active against a range of tumor types. VAL-083 is approved as a cancer chemotherapeutic in China for the treatment of CML and lung cancer. VAL-083 has not been approved for any indications outside of China.
Upon obtaining regulatory approval, we intend to commercialize VAL-083 for the treatment of orphan and other cancer indications where patients have failed other therapies or have limited medical options. Orphan diseases are defined in the United States under the Rare Disease Act of 2002 as “any disease or condition that affects fewer than 200,000 persons in the United States”. The Orphan Drug Act of 1983 is a federal law that provides financial and other incentives including a period of market exclusivity to encourage the development of new treatments for orphan diseases. In February 2012, we announced that VAL-083 has been granted protection under the Orphan Drug Act by the FDA for the treatment of glioma, including GBM. In January 2013, the EMA also granted orphan drug protection to VAL-083 for the treatment of glioma.
We research the mechanism of action of potential product candidates to determine the clinical indications best suited for therapy and seek to rapidly advance them into human clinical trials and toward commercialization. The mechanism of action of VAL-083 is understood to be a bi-functional alkylating agent. Alkylating agents are a commonly used class of chemotherapy drugs. They work by binding to DNA and interfering with normal processes within the cancer cell, which prevents the cell from making the proteins needed to grow and survive. After exposure to alkylating agents, the cancer cell becomes dysfunctional and dies. There are a number of alkylating agents on the market that are used by physicians to treat different types of cancer.
Based on published research and our own data, the cytotoxic functional groups and the mechanism of action of VAL-083 are understood to be functionally different from alkylating agents commonly used in the treatment of cancer. VAL-083 has previously demonstrated activity in cell-lines that are resistant to other types of chemotherapy. No evidence of cross-resistance has been reported in published clinical studies. Therefore, we believe that VAL-083 may be effective in treating tumors that have failed or become resistant to other chemotherapies.
We have presented new research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies.
| 4 |
VAL-083 readily crosses the blood brain barrier (“BBB”) where it maintains a long half-life in comparison to the plasma. Published pre-clinical and clinical research demonstrates that VAL-083 is selective for brain tumor tissue.
The main dose-limiting toxicity (“DLT”) related to the administration of VAL-083 in previous NCI-sponsored clinical studies was myelosuppression. Myelosuppression is the decrease in cells responsible for providing immunity, carrying oxygen, and those responsible for normal blood clotting. Myelosuppression is a common side effect of chemotherapy. There is no evidence of lung, liver or kidney toxicity even with prolonged treatment by VAL-083. Commercial data from the Chinese market where the drug has been approved for more than 15 years supports the safety findings of the NCI studies.
We note that the DLT of VAL-083 at the NCI was established prior to the development of various types of medications and other forms of therapy now available for management of myelosuppressive side effects. We believe this offers the potential of increasing the dose of VAL-083 in the modern patient population thereby providing a potential opportunity to improve the drugs already established efficacy profile.
Background on GBM
Worldwide, there are an estimated 240,000 new cases of brain and central nervous system (“CNS”) tumors each year. Gliomas are a type of CNS tumor that arises from glial cells in the brain or spine. Glial cells are the cells surrounding nerves. Their primary function is to provide support and protection for neurons in the CNS.
GBM, also known as Grade IV astrocytoma, is the most common and the most lethal form of glioma. According to the World Health Organization, GBM occurs with an incidence of 3.17 per 100,000 person-years. Approximately 15,000 new cases of GBM are expected to be diagnosed in the United States during 2015.
GBM progresses quickly and patients deteriorate rapidly. Common symptoms include headaches, seizures, nausea, weakness, paralysis and personality or cognitive changes such as loss of speech or difficulty in thinking clearly.
The majority of GBM patients do not survive for more than two years following diagnosis, and the median survival in newly diagnosed patients with best available treatments is 14.6 months.
Standard treatment following diagnosis includes surgical resection to remove as much of the tumor as possible (debulking) followed by radiotherapy with concomitant and adjuvant chemotherapy with Temodar® (temozolomide, “TMZ”). Nearly all patients diagnosed with GBM will relapse following first-line treatment, with a 1-year survival rate of approximately 25% following failure of front-line therapy, with average 5-year survival rate less than 3%.
Avastin® (bevacizumab - an anti-VEGF antibody) is approved as a single agent for patients with recurrent GBM following prior therapy as an alternative to corticosteroids to relieve disease symptoms in the US, Canada, Australia and Japan. Avastin® carries a “black-box warning” related to severe, sometimes fatal, side effects related to gastrointestinal perforations, wound healing complications and hemorrhage. There are no data demonstrating an improvement in disease-related symptoms or increased survival in refractory GBM with Avastin®.
TMZ and the nitrosoureas, including carmustine, lomustine, and nimustine, are alkylating agents that readily cross the BBB and are used in the treatment of CNS cancers, including GBM. Alkylating agents are among the oldest type of cancer chemotherapies in use today. Alkylating agents bind to DNA to cause damage to cancer cells. Their anti-tumor mechanism is via alkylation of DNA resulting in base-pair mismatch or strand-mediated cross links between base pairs. The DNA damage caused by alkylating agents mimics naturally occurring errors, resulting in apoptosis and tumor cell death.
The primary anti-cancer mechanism of TMZ and the nitrosoureas is to attack the tumor’s DNA via alkylation of the O6 position of the DNA base residue, guanine. TMZ treatment causes DNA damage mainly by methylation at the O6 position of guanine resulting in guanine-thymine base pair mismatches during replication. Nitrosoureas mediate their cytotoxic effect by ethylation at the O6 position of guanine which produces a cross-link to cytosine residues resulting in double-strand DNA breaks during mitosis.
A majority of GBM patients’ tumors are resistant to TMZ or nitrosourea therapy due to high expression of a naturally occurring enzyme called O6-DNA methylguanine methyl-transferase (“MGMT”) enzyme which repairs O6-guanine lesions. MGMT repair in turn inhibits the activity of TMZ and nitrosoureas and allows a patients’ GBM tumor to continue to grow in spite of treatment.
| 5 |
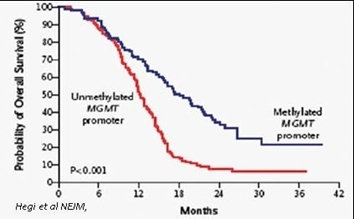
Consistent with the importance of its repair activity, high expression of MGMT is strongly correlated with poor patient outcomes. Several clinical studies have established that MGMT is an important prognostic indicator of response to TMZ and patient survival.
Probability of GBM Patient Survival Correlated to Expression of MGMT Enzyme
(Unmethylated promoter = High MGMT Expression and Significantly Shorter Survival)
VAL-083 in GBM
VAL-083 is an alkylating agent which readily crosses the BBB. Its primary cytotoxic mechanism, epoxide derived DNA cross-links at the N7 position of guanine, is distinct from TMZ or the nitrosoureas.
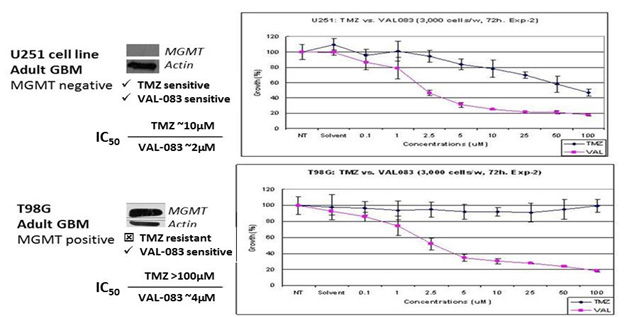
Our research demonstrates that VAL-083’s N7 targeting mechanism retains cytotoxic activity independent of MGMT expression in vitro. We have presented new research at peer-reviewed scientific meetings demonstrating that VAL-083 is active in patient-derived tumor cell lines and cancer stem cells that are resistant to other chemotherapies. Of particular importance is resistance to Temodar® due to activity of the repair enzyme known as MGMT, which results in chemoresistance in many GBM patients. At AACR in 2012, we presented data demonstrating that VAL-083 is active independent of MGMT resistance in laboratory studies. VAL-083 has more potent activity against brain tumor cells in comparison to TMZ and overcome resistance associated with MGMT suggesting the potential to surpass the current standard-of-care in the treatment of GBM.
A Summary of Our Data Demonstrating that VAL-083’s Anti-Tumor Mechanism is Distinct from, and
can Overcome, MGMT-Related Chemoresistance in the Treatment of GBM
| 6 |
VAL-083 has been assessed in multiple historical NCI-sponsored clinical studies as chemotherapy in the treatment of newly diagnosed and recurrent brain tumors and other cancers. In general, tumor regression in brain cancer was achieved following therapy in greater than 40% of patients treated and stabilization was achieved in an additional 20% to 30%. In published clinical studies VAL-083 has previously been shown to have a statistically significant impact on median survival in high grade glioma brain tumors when combined with radiation versus radiation alone with results similar or superior to other chemotherapies approved for use in GBM.
A Summary of Published Data adapted from Separate Sources Comparing the Efficacy of VAL-083 and
Other Therapies in the Treatment of GBM
| Comparative Therapy | Median Survival Benefit | |||||
| Chemotherapy | Radiation (XRT) | Radiation + Chemotherapy | vs. XRT alone | |||
| Temodar | 12.1 months |
58 weeks (14.6 months) |
2.5 months | |||
| VAL-083 | 8.8 months |
67 weeks (16.8 months) |
8.0 months | |||
| Lomustine | 52 weeks | |||||
| Carmustine | 40-50 weeks | |||||
| Semustine | 35 weeks | |||||
| Avastin | n.a. | |||||
Additional support for the differentiated profile of VAL-083 and TMZ comes from the results of studies with GBM cancer stem cells (“CSCs”). GBM CSCs display strong resistance to TMZ, even where MGMT expression is low. However, our data demonstrates that GBM CSCs are susceptible to VAL-083 independent of MGMT expression.
Based on historical data and our own research, we believe that VAL-083 has the potential to offer physicians and patients a new paradigm in the treatment of GBM that will address significant unmet medical needs. In addition, the profile of VAL-083 offers the potential of additive or synergistic benefit as a future combination therapy with existing chemotherapeutic agents or novel vaccines or immunotherapy approaches currently under investigation.
Interim Phase I/II Results in Refractory GBM
We filed an investigational new drug (“IND”) application with the FDA and initiated human clinical trials with VAL-083 as a potential treatment for GBM in 2011. Details of the study, including enrollment estimates, are available at http://www.clinicaltrials.gov/ct2/show/NCT01478178?term=VAL-083&rank=1)
Our clinical trial is a Phase I/II an open-label, single arm dose-escalation study designed to evaluate the safety, tolerability, pharmacokinetics and anti-cancer activity of VAL-083 in patients with GBM. To be eligible for our clinical trial, patients must have been previously treated for GBM with surgery and/or radiation, if appropriate, and must have failed both bevacizumab (Avastin®) and temozolomide (Temodar®), unless either or both are contra-indicated.
Response to treatment with VAL-083 is measured prior to each treatment cycle. An initial phase of the study involves dose escalation cohorts until a maximum tolerated dose (“MTD”) is established in the context of modern care. The goal of our Phase I/II clinical trial is to determine a modernized dosing regimen for advancement into a registration directed clinical trial.
In August 2013, we received a notice of allowance from the FDA enabling the Company to implement a revised dose-escalation scheme in our Phase I/II clinical trial. The revised dosing regimen was allowed by the FDA following an extensive safety review of patients treated prior to that date. In comparison to the original dose-escalation scheme, the revised plan enabled us to skip two interim doses and reach higher doses than originally contemplated.
We have presented interim data from our Phase I/II clinical trial at peer-reviewed scientific meetings including most recently at the annual meetings of ASCO in June 2015, AACR in April 2015 and SNO in November 2014. We anticipate presenting additional data at upcoming scientific meetings during 2015 and 2016.
Copies of our scientific poster presentations are available on our website.
| 7 |
In summary, at doses tested to date, our interim clinical data is as follows:
Enrollment and dosing
We confirmed that 30 GBM patients were enrolled across 8 dose cohorts ranging from 1.5 to 50 mg/m2/d. DLT consisting of thrombocytopenia (low platelet counts) was observed at 50 mg/m2/d. The low point of platelet counts (nadir) occurred around day 20 and generally DLT-related symptoms resolved rapidly and spontaneously without concomitant treatment.
Efficacy results
We reported that the progression free survival following treatment with VAL-083 was short (1.2 - 1.4 months) as expected since patients were not re-resected. However, preliminary analysis shows favorable increasing dose-dependent median survival after only two cycles of treatment with VAL-083: Median overall survival (“OS”) of 9 months for patients enrolled in cohorts 6 & 7 (VAL-083 dose ≥30 mg/m2/day) vs. 5 months for patients enrolled in cohorts 1 - 3 (VAL-083 doses up to 5mg/m2/day).
Safety and Tolerability
We confirmed that no drug-related severe adverse events were reported and myelosuppression was mild at doses ≤40mg/ m2/d. One of three GBM patients in cohort 7 (40mg/m2) and one of three GBM patients in cohort 6 (30 mg/m2) exhibited stable disease after one or two cycles of treatment. In earlier cohorts, we reported that two patients exhibited a response (stable disease or partial response) with a maximum response of 84 weeks and improved clinical signs prior to discontinuing due to adverse events unrelated to the study.
Expansion Phase
We confirmed that we had begun enrollment in the Phase II expansion cohort of our Phase I/II clinical trial. The expansion cohort will enroll up to 14 patients at the proposed therapeutic dose of 40 mg/m2/day. A small expansion cohort (n=3) at an interim 45mg/m2/day dose will also be studied, and the expansion cohort may be continued at this higher dose if safety data warrants.
Doses Achieved
We confirmed that we achieved doses of VAL-083 that are substantially higher than were utilized in the original published NCI-sponsored clinical trials. A summary of doses completed in our dose-escalation phase of our clinical trial in comparison to the NCI’s historical regimen is as follows:
|
Dosing Regimen & Study |
Single Dose |
Acute Regimen (single cycle) |
Comparative Cumulative Dose (@ 35 days) |
Dose Intensity (dose per week) |
||||||||||
|
NCI GBM historical regimen (Eagan etal) daily x 5 q 5wks (cycle = 35 days) |
25 mg/m2 | x5 days = | 125 mg/m2 | 125 mg/m2 | 25mg/m2/wk | |||||||||
| DelMar VAL-083 regimen daily x 3 q 3wks (cycle = 21 days) |
30 mg/m2 40 mg/m2 50 mg/m2 |
x3 days = |
90 mg/m2 120 mg/m2 150 mg/m2 |
180 mg/m2 240 mg/m 2 300 mg/m 2 |
30mg/m2/wk 40mg/m2/wk 50mg/m2/wk |
|||||||||
| Daily x 5 q 5wks refers to a dosing regimen of once per day for five consecutive days every five weeks (35 day cycle); while daily x 3 q 3wks refers to a dosing regimen of once per day for three consecutive days every three weeks (21 day cycle). |
| 8 |
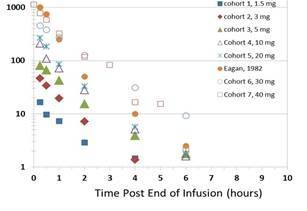
Pharmacokinetics
We reported that observed pharmacokinetics are linear and consistent with previous published data suggesting that concentrations of VAL-083 at a dose of 40mg/m2 achieve tissue levels in the central nervous system that have shown to be effective against glioma cell lines in vitro.
 |
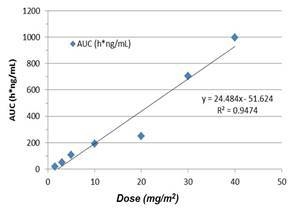 | |
| Observed pharmacokinetics measured by plasma concentration over time in escalating dose cohorts compared to historically literature (Eagan 1982) | Relationship between plasma exposure measured by area under the curve (AUC) vs. dose of VAL-083) |
| The estimated tissue concentration in brain tumor tissue was calculated based on observed concentration of VAL-083 in the plasma and historical observations from the literature |
| Dose
and Dosing Day of Each Cycle | Plasma Cmax | Estimated
Maximum Tumor Concentration in Brain b | IC50
in GBM Cell Lines d | |||||||||||
| Current Trial | (g/mL) a | (g/g tissue) | µM c | µM | ||||||||||
| 40mg/m2 Day-1 | 0.781 | 0.344 | 2.36 | |||||||||||
| 40mg/m2 Day-2 | 0.781 | 0.503 | 3.45 |
~2 - 4 | ||||||||||
| 40mg/m2 Day-3 | 0.781 | 0.563 | 3.86 | |||||||||||
|
a PK was conducted only on Day 1, given the short t-1/2 of ~1h Cmax is assumed to be same for day 2 & 3*Volume of 1 g tissue assumed to be 1 mL b Percent of plasma drug concentration in brain tumor = 44%, Eckhardt, 1977 c Half-life of drug in human brain tumor tissue = 20h, Eckhardt, 1977 d IC50 range for low MGMT (U251 and SF188) and high MGMT (T98G) GBM cells treated with VAL-083 in vitro |
Patient History
We confirmed that GBM patients enrolled in our Phase I/II clinical trial failed prior treatment with standard front-line (temozolomide plus radiation) and 92% also failed Avastin®. In addition, 77% percent of GBM patients enrolled had also failed one or more courses of additional salvage therapy beyond temozolomide and Avastin® prior to treatment with VAL-083. Patients were not re-resected prior to treatment with VAL-083 and therefore had a growing refractory GBM tumor at the time of enrollment in our clinical trial and were considered salvage patients with an expected poor prognosis.
| 9 |
We also reported all patients in our current trial whose tumors were characterized exhibited high expression of MGMT, suggesting that these patients would be expected to have a poor prognosis and further highlighting the promising dose-dependent survival trend observed in the Phase I dose escalation portion of our clinical trial.
Based on these interim results, we believe that our modernized dosing regimen takes advantage of improved side-effect management and new knowledge of the pharmacokinetic, toxicity profile and anti-cancer mechanism of VAL-083. Our strategy to “hit the tumor harder more often” has allowed us to achieve higher levels of drug at the tumor-site, which we believe will result in significant and meaningful clinical benefit for GBM patients who have failed both temozolomide and Avastin® and increased survival via slowed tumor growth or tumor regression. These patients currently have no viable treatment options.
Observed survival in the dose escalation phase of our Phase I/II clinical trial in comparison to
historical outcomes for GBM patients following Avastin® failure as described in the scientific literature

While these data are interim in nature and based on a small number of patients, we believe they support the further development of VAL-083. We anticipate presenting additional data, including data from the Phase II expansion cohort of our current clinical trial at scientific meetings in the second half of 2015. The purpose of the 14 patient Phase II expansion is to gather further safety data at our chosen therapeutic dose and to further clarify median overall survival in this patient population.
We are currently conducting our clinical trial at five centers: the Mayo Clinic in Rochester, Minnesota (“Mayo”), the Brain Tumor Center at University of California, San Francisco (“UCSF”), the Sarah Cannon Cancer Research Center (“SCRI”) in Nashville, Tennessee, Denver, Colorado, and the SCRI affiliate site at the Florida Cancer Specialist Research Institute in Sarasota, Florida. We plan to add additional clinical sites in order to accelerate enrollment as the trial progresses.
Patients being enrolled in our current Phase I/II clinical trial have a growing brain tumor that has failed to respond to any other approved treatment. The correlation between tumor progression and impending death in this patient population is well-documented. Therefore we believe that our interim results demonstrating that VAL-083 can either stabilize disease progression by slowing or halting tumor growth or by shrinking the tumor is expected to result in longer patient survival and improved quality of life.
We plan to continue our clinical trials with VAL-083 as a potential treatment for GBM patients who have failed other therapies. Currently, there is no approved therapy for these patients. The goal of our current Phase I/II clinical trial is to establish a modernized dosing regimen for advancement into registration directed trials in the United States as a potential new therapy for the treatment of refractory GBM.
In accordance with the protocol that has been filed with the FDA we have initiated enrollment of an expanded Phase II cohort at a dose deemed to be at or below the MTD. We will enroll approximately 14 additional GBM patients to obtain additional safety and activity data at the dose proposed for advancement into registration-directed Phase II/III clinical trials.
We anticipate that the Phase II/III registration-directed trial will be an open-label trial with overall survival as the primary endpoint. We plan to request a guidance meeting with the FDA to discuss our proposed Phase II/III registration trial design. The dose chosen, size, design and timing of initiation of the registration-directed clinical trial will depend on review of the data from the Phase II expansion phase of our current study and discussions with the FDA and our clinical advisors. Based on our current enrollment and timelines, we believe it is possible that we will initiate Phase II/III registration-directed studies within the next 9 - 12 months. We will provide a formal update, including any adjustment to our projected timelines based on our discussions with the FDA and our clinical advisors.
| 10 |
Based on historical development of other products in GBM, we believe that we may be able to obtain FDA approval to commercialize VAL-083 to treat patients who have failed other therapies from an open-label Phase II/III registration-directed clinical trial, which will save significant costs of a large randomized Phase III clinical trial. We also believe that the FDA may grant Breakthrough Therapy, Fast Track, Accelerated Approval and/or Priority Review status to VAL-083, which will enable us to begin filing for commercial approval during the clinical trial process. Breakthrough Therapy, Fast Track, Accelerated Approval and Priority Review are approaches established by the FDA that are intended to make therapeutically important drugs available at an earlier time. (See “Government Regulation and Product Approval”).
Data from our planned registration-directed Phase II/III trial will form the basis of our application for FDA approval. Our overall goal remains to complete registration-directed clinical trial with VAL-083 and to seek FDA approval as a new therapy for refractory glioblastoma in the timeliest manner possible. Based on our current financial resources, initiation of the registration-directed trial will require additional funding to support the expanded clinical operations necessary to conduct and manage the study.
We also believe that VAL-083 may be a potentially superior alternative to currently approved chemotherapies used in the treatment of newly diagnosed GBM patients. Subject to the availability of financial resources, we plan to investigate VAL-083 in clinical trials for newly diagnosed GBM patients whose tumors exhibit molecular features suggesting that they are unlikely to respond to currently available chemotherapies.
In February 2012, VAL-083 was granted protection under the Orphan Drug Act by the FDA for the treatment of glioma. In January 2013, the European Union also granted orphan drug protection to VAL-083. Orphan drugs generally follow the same regulatory development path as any other pharmaceutical product. However, incentives such as scientific advice and reduction or waiver of registration fees and access to specialized grant funding may be available to support and accelerate development of orphan drug candidates. In addition, we may sell VAL-083 as a treatment for glioma without competition for seven years in the U.S. and for ten years in the EU following market approval, due to the orphan drug protection afforded - meaning that the neither the FDA nor the EU regulatory authority will approve a medicinal product containing a similar active substance for the same indication during that time.
As part of our ASCO presentation on June 1, 2013, we also announced that we plan to split our current Phase I/II clinical trial protocol into two separate studies: one focusing solely on refractory GBM and the other focusing on secondary brain cancers caused by other tumors that have spread to the brain. Due to prior chemotherapy and radiation therapy, patients with secondary brain tumors are likely more prone to myelosuppression and may have a different toxicity and MTD than patients with GBM. We believe the strategy of splitting the trial into two separate studies will enable us to focus on accelerating the development of VAL-083 as a potential new treatment for GBM while appropriately exploring the potential of the drug to treat patients with solid tumors that have spread to the brain. In the future, we may develop a separate protocol for the continued exploration of VAL-083 in patients with secondary brain cancer caused by a solid tumor spreading to the brain.
VAL-083 in Lung Cancer
Lung cancer is a leading cause of cancer-related mortality around the world and effective treatment for lung cancer remains a significant global unmet need despite advances in therapy. In general, prognosis for lung cancer patients remains poor, with 5-year relative survival less than 14% among males and less than 18% among females in most countries. Globally, the market for lung cancer treatment may exceed $7 billion by 2019 according to a report published by Transparency Market research.
Non-small cell lung cancer (“NSCLC”) is the most common type of lung cancer. There are three common forms of NSCLC: adenocarcinomas are often found in an outer area of the lung; squamous cell carcinomas are usually found in the center of the lung next to an air tube (bronchus); and large cell carcinomas, which can occur in any part of the lung and tend to grow and spread faster than adenocarcinoma. NSCLC accounts for 85% of all lung cancer cases in the United States and approximately 90% of lung cancer cases diagnosed in China.
Smoking is the most important risk factor in the development of lung cancer. According to the World Cancer Report (2008), 21% of cancer deaths are related to smoking, especially lung cancer. Additionally, high levels of air pollution have been implicated as significant causes of lung cancer. Incidence of lung cancer in the United States is approximately 59 per 100,000 with the majority (52:100,000) being NSCLC.
According to The Nationwide Nutrition and Health Survey (2002), China has the world’s largest smoking population, with a smoking rate of 24.0% on average (50.2% for men and 2.8% for women), and a total number of 350 million smokers. The World Health Organization reports that the incidence of lung cancer in China is 34 per 100,000 population. However, some estimates are much higher exceeding 120 per 100,000 population for males aged 55-60 in urban areas.
| 11 |
According to a survey conducted by the Chinese Ministry of Health and the Ministry of Science and Technology, smoking, poor diet, water pollution and environmental problems have caused the nation's cancer death rate to rise 80 percent in the past 30 years and cancer is now accountable for 25 percent of all urban deaths and 21 percent of all rural deaths. Based on these trends, the World Health Organization projects that the incidence of lung cancer in China is expected to exceed one million (1,000,000) new cases per year by 2025.
The activity of VAL-083 against solid tumors, including lung cancer, has been established in both pre-clinical and human clinical trials conducted by the NCI. VAL-083 has been approved by the CFDA for the treatment of lung cancer. However, sales of VAL-083 in China have been limited by a lack of modern data, poor distribution, and preference for targeted therapies such as tyrosine kinase inhibitors (“TKIs”) in the modern era.
The current standard of care for newly diagnosed NSCLC is platinum-based combination therapy or TKI therapy for patients whose cancer exhibits epidermal growth factor receptor (“EGFR”) mutations. Patients exhibiting EGFR mutations have shown an initial response rate to TKIs which exceeds the response rate for conventional chemotherapy. However, TKI resistance has emerged as an important unmet medical need.
We believe VAL-083’s unique bi-functional alkylating mechanism of action could make it a valuable drug of choice in NSCLC patients who are or become resistant to TKI therapy. In addition, VAL-083 readily crosses the blood brain barrier suggesting that it may be possible for VAL-083 to treat patients whose lung cancer has spread to the brain.
Based on these beliefs, we have acquired certain commercial rights to VAL-083 in China where it is approved for the treatment of lung cancer. We have begun to establish a strong scientific and clinical rationale to support the development of VAL-083 as a potential treatment for NSCLC in the modern era.
We plan to work with leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in NSCLC patients who are resistant to TKIs. We believe this strategy will result in sales growth for VAL-083 in China and generate future revenue for the Company through sales and marketing partnerships as well as position VAL-083 for global development in lung cancer.
In April 2014 at AACR we announced results of a pre-clinical study designed to evaluate the activity of VAL-083 in in vivo models of drug-resistant NSCLC in comparison to cisplatin. In an established murine xenograft model of NSCLC, the activity of VAL-083 was compared to standard platinum-based therapy with cisplatin against human NSCLC cell lines A549 (TKI-sensitive) and H1975 (TKI-resistant). In the study, VAL-083 demonstrated superior efficacy and safety in the treatment of TKI-susceptible (A549) tumors and in TKI-resistant (H1975) tumors.
| ● | Treatment of TKI-sensitive (A549) NSCLC with 3 mg/kg of VAL-083 resulted in tumor growth delay of 26 days compared to untreated controls. Cisplatin (5 mg/kg) resulted in tumor growth delay of just four days. In addition, mean tumor volume on day 68 was significantly reduced in animals treated with 3 mg/kg VAL-083 (p=0.001) compared to untreated controls. |
| ● | Treatment of TKI-resistant (H1975) NSCLC with 4 mg/kg of VAL-083 resulted in a statistically significant reduction in tumor volume (p=0.01) versus untreated control after 27 days. In the same model, treatment with 5 mg/kg of cisplatin failed to achieve statistically significant reduction in tumor volume (p=0.23) versus untreated control after 27 days. Longer-term safety assessments are ongoing in this model. |
In April 2015, we presented new non-clinical data at the AACR annual meeting. These data demonstrated that VAL-083’s mechanism is distinct from platinum-based chemotherapy, the current standard of care for NSCLC. VAL-083 retains its high level of anti-cancer activity in p53 mutated NSCLC cell lines compared to cisplatin or oxaliplatin.
The p53 gene plays a central role in the protection of the human body from cancer and is responsible for initiating the process of programmed cell death, or apoptosis, which directs a cell to commit suicide if it becomes damaged or cancerous. The p53 pathway is also integral to the activity of many chemotherapy drugs. p53 is frequently mutated in NSCLC and p53 mutations are highly correlated with resistance to chemotherapy and poor patient outcomes in NSCLC.
In addition, we demonstrated that the combination of VAL-083 with either cisplatin or oxaliplatin demonstrated a superadditive (synergistic) effect against NSCLC cell lines, including those resistant to TKI therapy in vitro.
In October 2014, we presented non-clinical data at the AACR New Horizon’s in Cancer Research Meeting. These data also support superior activity of VAL-083 compared to standard platinum-based treatment in both TKI-sensitive and TKI-resistant tumor models. Further, our data demonstrate that VAL-083 may have a synergistic effect in combination with cisplatin. These data suggest the potential of VAL-083 to be used in combination with platinum-based chemotherapy and to address modern unmet medical needs in the treatment of TKI-resistant NSCLC, especially where platinum-based therapy has already failed or is predicted to give sub-optimal outcomes.
| 12 |
These results may have immediate implications in the treatment of NSCLC in China, where VAL-083 is approved for as a chemotherapy for the treatment of lung cancer. The data also support exploring future clinical development of VAL-083 as a lung cancer therapy in the rest of the world thereby providing DelMar with a potential opportunity to expand our clinical development focus beyond glioblastoma.
As a next step in the investigation of VAL-083 as a potential treatment for NSCLC, we have developed a protocol for a post-market clinical study to be conducted by a leading cancer clinician in the context of the current approval in China.
We plan to conduct this trial in collaboration with Guangxi Wuzhou Pharmaceutical Group Co. Ltd. (Guangxi Wuzhou Pharma). Under the terms of our collaboration agreement with Guangxi Wuzhou Pharma, we are responsible for establishing protocols for and conducting clinical trials and Guangxi Wuzhou Pharma is responsible for the costs associated with clinical trials conducted in China. Our goal is to initiate this clinical trial during 2015, with the aim to develop new data to support product growth in China and to establish clinical proof of concept to expand our drug development efforts with VAL-083.
Conducting this clinical trial in China under our collaboration agreement with Guangxi Wuzhou Pharma will allow us to enhance the potential value of VAL-083 without significantly increasing our own planned cash expenditures. We also believe that these new data will support the potential to establish global partnerships and collaborations with larger pharmaceutical companies who have the resources and commercial infrastructure to effectively develop and commercialize VAL-083 as a treatment for NSCLC on a world-wide basis.
VAL-083 in Leukemia and Hematologic Cancers
The NCI studied VAL-083 extensively in laboratory and animal models of hematological malignancies (blood cancers). VAL-083 has been approved for the treatment of chronic myeloid leukemia, or CML, in China.
CML, also known as chronic myeloid leukemia is a cancer of the white blood cells. The incidence of CML in the United States is approximately two per 100,000 of population.
CML is characterized by three progressive phases: chronic, aggressive and blast, each corresponding with poorer prognosis. Approximately 85% of patients with CML are in the chronic phase at the time of diagnosis. Chronic phase patients are usually asymptomatic or have only mild symptoms such as fatigue or no symptoms at all. The duration of chronic phase is variable and depends on how early the disease was diagnosed as well as type of treatment. Without treatment, CML progresses to an accelerated phase and eventually to blast crisis. Blast crisis is the final phase in the evolution of CML and behaves like an acute leukemia with rapid progression and short expected survival.
While VAL-083 maintains labeling for CML in China, use of the drug in the modern era has been limited by a preference for targeted therapies such as TKIs.
TKIs have become the standard of care for CML and certain types of lung cancer. TKI therapy has resulted in vastly improved outcomes. However, patients often develop resistance to TKI therapy. Recent evidence proposes unique mechanisms of resistance in patients of East Asian descent who experience significantly inferior responses to TKIs.
We believe that data from NCI-sponsored studies and commercial evidence from the Chinese market support that there exists a substantive clinical benefit of VAL-083 in CML. We also believe that the unique mechanism of action of VAL-083, in combination with newly developed data positions the drug as a valuable therapy for patients who have failed other treatments, including TKIs. This represents a significant clinical and commercial opportunity for large subsets of patient populations in the existing-approved China market as well as for global development in CML.
Based on these beliefs, we have acquired certain commercial rights to VAL-083 in China, where it is approved for the treatment of CML and lung cancer. We have also developed new non-clinical data demonstrating that VAL-083 is active against TKI-resistant CML.
We have begun to establish a network of leading oncologists to develop new clinical and non-clinical data which will demonstrate the clinical utility of VAL-083 in CML patients who are resistant to TKIs. We believe this strategy may result in sales growth for VAL-083 in China and has the potential to generate revenue for the Company through sales and marketing partnerships as well as position VAL-083 for global development in CML.
| 13 |
In addition to CML and subject to availability of funds, we plan to investigate VAL-083 as a potential treatment for other types of blood cancer. Acute Myeloid Leukemia (“AML”) and Acute Lymphoblastic Leukemia (“ALL”) are of particular interest based on published data and lack of effective therapeutic options. We have initiated preliminary discussions with leading cancer centers regarding the development of a clinical strategy for the development of VAL-083 in other types of blood cancer.
Additional Indications
In historical studies sponsored by the NCI in the United States, VAL-083 exhibited clinical activity against a range of tumor types including central nervous system tumors, solid tumors and hematologic malignancies. We have established new non-clinical data supporting the activity of VAL-083 in different types of cancer that are resistant to modern targeted therapies and we believe that the unique cytotoxic mechanism of VAL-083 may provide benefit to patients in a range of indications. We intend to continue to research these opportunities, and if appropriate, expand our clinical development efforts to include additional indications.
VAL-083 Target Markets
We are targeting cancer indications which we believe represent market opportunities in the hundreds of millions of dollars in North America and potentially in the billions of dollars worldwide. The pharmaceutical industry, in general, is a highly profitable, highly innovative industry. According to a report published by Statistic, the global pharmaceutical industry generated nearly one trillion dollars in revenue during 2013. According to published reports, global pharmaceutical sales are highly stratified by region, with North America, the European Union and Japan accounting for 55% of global pharmaceutical sales in 2009. However, the most rapid growth in the sector is from developing countries, particularly China.
|
Glioblastoma Multiforme: Newly diagnosed patients suffering from GBM are initially treated through invasive brain surgery, although disease progression following surgical resection is nearly 100%. Temozolomide (Temodar®) in combination with radiation is the front-line therapy for GBM following surgery. Temodar® currently generates more than $950 million annually in global revenues even though most patients fail to gain long-term therapeutic benefits. Approximately 60% of GBM patients treated with Temodar® experience tumor progression within one year.
Bevacizumab (Avastin®) has been approved for the treatment of GBM in patients failing Temodar®. In clinical studies, only about 20% of patients failing Temodar® respond to Avastin® therapy. In spite of these low efficacy results, treatment of GBM in North America alone is projected to add $200 million annually to the revenues of Avastin® with projected growth in GBM to $650 million by 2016.
Approximately 48% of patients who are diagnosed with GBM will fail both front-line therapy and Avastin®. Based on disease incidence, we believe the market for treating GBM patients the post-Avastin® failure exceeds $200 million annually in North America. Subject to successfully completing clinical trials and obtaining approval by the FDA and other applicable regulatory agencies globally, we also believe that VAL-083 could potentially generate sales in excess of $1 billion worldwide as a potential front-line therapy for GBM. |
|
| Lung Cancer: The potential of VAL-083 in the treatment of NSLSC has been established in both human clinical trials conducted by the NCI and by the drug’s commercial approval in China. Lung cancer is the most common cancer in the world with 1.8 million cases in 2012, representing 13% of all cancers according to a report published by the World Cancer Research Fund International. Lung cancer has a higher mortality rate than the next top three cancers combined and it is responsible for 1.6 million deaths annually, representing 19% of all cancer deaths. NSCLC represents approximately 90% of newly diagnosed lung cancers. A report published by Transparency Market Research states that the global NSCLC drug market will increase from $4.3 billion in 2009 to $6.9 billion in 2019 and the market is growing with a CAGR of 4.84% during 2009 to 2019. |
| 14 |
| Leukemia: The potential of VAL-083 in the treatment of CML has been established in both human clinical trials conducted by the NCI and by the drug’s commercial approval in China. The Tyrosine Kinase Inhibitor Gleevec® is currently used as front-line therapy in the treatment of CML achieved global revenue in excess of $4.7 billion annually in 2012. We believe that VAL-083 has potential to capture a portion of the CML market through demonstration of activity in TKI-resistant CML patients. We also believe that VAL-083 may offer significant commercial opportunities through the treatment of other types of blood cancer such as AML or ALL. |
VAL-083 Manufacturing
VAL-083 is currently manufactured in accordance with CFDA and Chinese Pharmacopoeia guidelines to ensure drug quality control, drug use safety, and drug efficacy. Approval by the FDA will require VAL-083 and other products developed by us to be manufactured in accordance with United States Pharmacopeia (“USP”) in accordance with Good Manufacturing Practices (“cGMP”) regulations. cGMP provides for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the cGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations.
We have established an exclusive purchasing relationship with a Chinese manufacturer that has enabled us to obtain drug product for human clinical trials in the United States and certain commercial rights in China. The Chinese manufacturer has established a commercial-scale manufacturing process based on the North American process originally developed for the NCI.
Ensuring a viable long-term supply of the VAL-083 drug product suitable for registration and commercialization in North America and Europe will require investment in improved manufacturing and quality controls. We will seek to build upon our expertise and our intellectual property related to the existing manufacturing processes for VAL-083 in collaboration with the current manufacturer to allow compliance with cGMP. In addition, we have identified third party contract manufacturers with the capabilities to establish the processes, procedures and quality systems necessary to meet U.S., Canadian, E.U. and other international cGMP manufacturing requirements. Such requirements include strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories.
Patents and Proprietary Rights
Our success will depend in part on our ability to protect our existing product candidate and the products we acquire or license by obtaining and maintaining a strong proprietary position. To develop and maintain our position, we intend to continue relying upon patent protection, orphan drug status, Hatch-Waxman exclusivity, trade secrets, know-how, continuing technological innovations and licensing opportunities.
We have filed patent applications covering VAL-083 where we have claimed the use of, and improvements related to VAL-083 and other novel aspects of our proposed treatment regimen, manufacturing process improvements and the formulation and composition of the active pharmaceutical ingredient and finished dosage form of VAL-083 products. We are prosecuting our patent applications in the United States and in international jurisdictions which we deem important for the potential commercial success of VAL-083.
Our patents and patent applications can be summarized in eight series as follows:
| ● | Series I is generally directed to synthesis of VAL-083. |
| Patent or Patent Application No. | Title | Expiry | ||||
| United States Patent No. 8,563,758 | Method Of Synthesis Of Substituted Hexitols Such As Dianhydrogalactitol | 2031 | ||||
| United States Patent No. 8,921,585 | Method Of Synthesis Of Substituted Hexitols Such As Dianhydrogalactitol | 2031 | ||||
United States Patent Application Serial No. 14/072,603 Notice of Allowance Received 1/22/15 | Method Of Synthesis Of Substituted Hexitols Such As Dianhydrogalactitol | 2031 | ||||
| United States Patent Application Serial No. 14/550,131 | Method Of Synthesis Of Substituted Hexitols Such As Dianhydrogalactitol | 2031 | ||||
| Mexican Patent No. 323310 | Method Of Synthesis Of Substituted Hexitols Such As Dianhydrogalactitol | 2031 | ||||
| PCT Patent Application Serial No. PCT/US2011/048032 | Method Of Synthesis Of Substituted Hexitols Such As Dianhydrogalactitol National phase applications have published in countries including: Australia, Canada, Chile, China, European Union, Japan, Singapore and South Korea | |||||
| Additional Applications in Series I Not Yet Published |
| 15 |
| ● | Series II is generally directed to use of VAL-083 to treat a range of diseases and conditions, including but not limited to malignancies. |
| Patent or Patent Application No. | Title | Expiry | ||||
United States Patent Application Serial No. 13/817,096 Notice of Allowance Received 2/25/15 | Compositions And Methods To Improve The Therapeutic Benefit Of Suboptimally Administered Chemical Compounds Including Substituted Hexitols Such As Dianhydrogalactitol And Diacetyldianhydrogalactitol | 2031 | ||||
| PCT Patent Application Serial No. PCT/US2011/048031 | Compositions And Methods To Improve The Therapeutic Benefit Of Suboptimally Administered Chemical Compounds Including Substituted Hexitols Such As Dianhydrogalactitol And Diacetyldianhydrogalactitol National phase applications have published in countries including: Australia, Canada, Chile, China, European Union, Japan, Mexico, Singapore and South Korea | |||||
| Additional Applications in Series II Not Yet Published |
| ● | Series III is generally directed to analytical methods for VAL-083. |
| Patent or Patent Application No. | Title | Expiry | ||||
| United States Patent Application Serial No. 13/933,844 | Improved Analytical Methods For Analyzing And Determining Impurities In Dianhydrogalactitol | 2032 | ||||
| United States Patent No. 9,029,164 | Improved Analytical Methods For Analyzing And Determining Impurities In Dianhydrogalactitol | 2032 | ||||
| PCT Patent Application Serial No. PCT/IB2013/000793 | Improved Analytical Methods For Analyzing And Determining Impurities In Dianhydrogalactitol National phase applications have published in countries including: Australia, Canada, China, European Union, Japan and South Korea | |||||
| Additional Applications in Series III Not Yet Published |
| ● | Series IV is generally directed to the use of VAL-083 to treat GBM or medulloblastoma. |
| Patent or Patent Application No. | Title | Expiry | ||||
| United States Patent Application Serial No. 14/373,552 | Use Of Substituted Hexitols Including Dianhydrogalactitol And Analogs To Treat Neoplastic Disease And Cancer Stem Cells Including Glioblastoma Multiforme And Medulloblastoma | 2033 | ||||
| United States Patent Application Serial No. 14/245,738 | Use Of Substituted Hexitols Including Dianhydrogalactitol And Analogs To Treat Neoplastic Disease And Cancer Stem Cells Including Glioblastoma Multiforme And Medulloblastoma | 2033 | ||||
| PCT Patent Application Serial No. PCT/US2013/022505 | Use Of Substituted Hexitols Including Dianhydrogalactitol And Analogs To Treat Neoplastic Disease And Cancer Stem Cells Including Glioblastoma Multiforme And Medulloblastoma National phase applications have published in countries including: Australia, Canada, China, European Union, Japan, and South Korea | |||||
| Additional Applications in Series IV Not Yet Published |
| 16 |
| ● | Series V is generally directed to the veterinary use of VAL-083. |
| Patent or Patent Application No. | Title | Expiry | ||||
| United States Patent Application Serial No. 14/400,271 | Veterinary Use Of Dianhydrogalactitol, Diacetyldianhydrogalactitol, And Dibromodulcitol To Treat Malignancies | 2033 | ||||
| PCT Patent Application Serial No. PCT/US2013/039549 | Veterinary Use Of Dianhydrogalactitol, Diacetyldianhydrogalactitol, And Dibromodulcitol To Treat Malignancies | |||||
| Additional Applications in Series V Not Yet Published |
| ● | Series VI is generally directed to the use of VAL-083 to treat tyrosine-kinase-inhibitor-resistant malignancies. |
| Patent or Patent Application No. | Title | Expiry | ||||
| United States Patent Application Serial No. 14/409,909 | Methods For Treating Tyrosine-Kinase-Inhibitor-Resistant Malignancies In Patients With Genetic Polymorphisms Or Ahi1 Dysregulations Or Mutations Employing Dianhydrogalactitol, Diacetyldianhydrogalactitol, Dibromodulcitol, Or Analogs Or Derivatives Thereof | 2033 | ||||
| PCT Patent Application Serial No. PCT/US2013/047320 | Methods For Treating Tyrosine-Kinase-Inhibitor-Resistant Malignancies In Patients With Genetic Polymorphisms Or Ahi1 Dysregulations Or Mutations Employing Dianhydrogalactitol, Diacetyldianhydrogalactitol, Dibromodulcitol, Or Analogs Or Derivatives Thereof National phase applications have published in countries including: Australia, Canada and Israel | |||||
| Additional Applications in Series VI Not Yet Published |
| ● | Series VII is generally directed to the use of VAL-083 to treat recurrent malignant glioma and progressive secondary brain tumor. |
| Patent or Patent Application No. | Title | Expiry | ||||
| PCT Application Serial No. PCT/US2014/040461 | Use Of Dianhydrogalactitol And Analogs And Derivatives Thereof To Treat Recurrent Malignant Glioma Or Progressive Secondary Brain Tumor |
| ● | Series VIII is generally directed to the use of VAL-083 to treat non-small-cell lung cancer. |
| Patent or Patent Application No. | Title | Expiry | ||||
| Two provisional U.S. patent applications have been filed. No patent application in Series VIII has been published |
One of the inventors listed in one of our Series VIII provisional applications is an employee of the University of California, San Francisco. If a patent issues from that provisional application with a claim that the University of California employee conceived of, in whole or in part, than the Regents of the University of California will share ownership of any such patent with us. Our research agreements with the University of California address this issue by providing the Company with an exclusive option, for a limited period of time, to negotiate a royalty-bearing exclusive license for commercialization of the invention covered by that patent.
| 17 |
In addition to patent protection, we may also seek orphan drug status whenever it is available. If a product which has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the U.S. and Canada, and 10 years in the E.U. Orphan drug designation does not prevent competitors from developing or marketing different drugs for the same indication or the same drug for a different clinical indication.
In February 2012, we announced that the FDA has granted orphan drug status to VAL-083. In January 2013, the EMA also granted orphan drug protection to VAL-083 for the treatment of glioma.
Under the Hatch-Waxman Amendments, newly approved drugs and indications benefit from a statutory period of non-patent marketing exclusivity. These amendments provide five-year data exclusivity to the first applicant to gain approval of an NDA for a new chemical entity, meaning that the FDA has not previously approved any other new drug containing the same active ingredient. The Hatch-Waxman Amendments prohibit the submission of an abbreviated new drug application, also known as an ANDA or generic drug application, during the five-year exclusive period if no patent is listed. If there is a patent listed and the ANDA applicant certifies that the NDA holder’s listed patent for the product is invalid or will not be infringed, the ANDA can be submitted four years after NDA approval. Protection under the Hatch-Waxman Amendments will not prevent the filing or approval of another full NDA; however, the applicant would be required to conduct its own pre-clinical studies and adequate and well-controlled clinical trials to demonstrate safety and effectiveness. The Hatch-Waxman Amendments also provide three years of data exclusivity for the approval of NDAs with new clinical trials for previously approved drugs and supplemental NDAs, for example, for new indications, dosages or strengths of an existing drug, if new clinical investigations were conducted by or on behalf of the sponsor and were essential to the approval of the application. This three-year exclusivity covers only the new changes associated with the supplemental NDA and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient. We intend to rely on the Hatch-Waxman Amendments for five years of data exclusivity for VAL-083.
We also rely on trade secret protection for our confidential and proprietary information. We believe that the substantial costs and resources required to develop technological innovations, such as the manufacturing processes associated with VAL-083, will help us to protect the competitive advantage of our product candidate.
The protection of intellectual property rights in China (where our clinical product candidate, VAL-083, is manufactured pursuant to a collaboration agreement with the only manufacturer presently licensed by the CFDA to produce the product for the China market, and where VAL-03 is approved for the treatment of CML and lung cancer) is relatively weak compared to the United States, which may negatively affect our ability to generate revenue from VAL-083 in China.
Our policy is to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual shall be our exclusive property.
Government Regulation and Product Approval
Regulation by governmental authorities in the U.S. and other countries is a significant factor, affecting the cost and time of our research and product development activities, and will be a significant factor in the manufacture and marketing of any approved products. All of our products require regulatory approval by governmental agencies prior to commercialization. In particular, our products are subject to rigorous pre-clinical and clinical testing and other approval requirements by the FDA and similar regulatory authorities in other countries. Various statutes and regulations also govern or influence the manufacturing, safety, reporting, labeling, transport and storage, record keeping and marketing of our products. The lengthy process of seeking these approvals, and the subsequent compliance with applicable statutes and regulations, require the expenditure of substantial resources. Any failure by us to obtain, or any delay in obtaining, the necessary regulatory approvals could harm our business.
The regulatory requirements relating to the testing, manufacturing and marketing of our products may change from time to time and this may impact our ability to conduct clinical trials and the ability of independent investigators to conduct their own research with support from us.
The clinical development, manufacturing and marketing of our products are subject to regulation by various authorities in the U.S., the E.U. and other countries, including, in the U.S., the FDA, in Canada, Health Canada, and, in the E.U., the EMA. The Federal Food, Drug, and Cosmetic Act, the Public Health Service Act in the U.S. and numerous directives, regulations, local laws and guidelines in Canada and the E.U. govern the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of our products. Product development and approval within these regulatory frameworks takes a number of years and involves the expenditure of substantial resources.
| 18 |
Regulatory approval will be required in all the major markets in which we seek to develop our products. At a minimum, approval requires the generation and evaluation of data relating to the quality, safety, and efficacy of an investigational product for its proposed use. The specific types of data required and the regulations relating to this data will differ depending on the territory, the drug involved, the proposed indication and the stage of development.
In general, new chemical entities are tested in animals until adequate evidence of safety is established to support the proposed clinical study protocol designs. Clinical trials for new products are typically conducted in three sequential phases that may overlap. In Phase I, the initial introduction of the pharmaceutical into either healthy human volunteers or patients with the disease (20 to 50 subjects), the emphasis is on testing for safety (adverse effects), dosage tolerance, metabolism, distribution, excretion and clinical pharmacology. Phase II involves studies in a limited patient population (50 to 200 patients) to determine the initial efficacy of the pharmaceutical for specific targeted indications, to determine dosage tolerance and optimal dosage and to identify possible adverse side effects and safety risks. Once a compound shows preliminary evidence of some effectiveness and is found to have an acceptable safety profile in Phase II evaluations, Phase III trials are undertaken to more fully evaluate clinical outcomes in a larger patient population in adequate and well-controlled studies designed to yield statistically sufficient clinical data to demonstrate efficacy and safety.
In the U.S., specific pre-clinical data, manufacturing and chemical data, as described above, need to be submitted to the FDA as part of an IND application, which, unless the FDA objects, will become effective 30 days following receipt by the FDA. Phase I studies in human volunteers may commence only after the application becomes effective. Prior regulatory approval for human healthy volunteer studies is also required in member states of the E.U. Currently, in each member state of the E.U., following successful completion of Phase I studies, data are submitted in summarized format to the applicable regulatory authority in the member state in respect of applications for the conduct of later Phase II studies. The regulatory authorities in the E.U. typically have between one and three months in which to raise any objections to the proposed study, and they often have the right to extend this review period at their discretion. In the U.S., following completion of Phase I studies, further submissions to regulatory authorities are necessary in relation to Phase II and III studies to update the existing IND. Authorities may require additional data before allowing the studies to commence and could demand that the studies be discontinued at any time if there are significant safety issues. In addition to the regulatory review, a study involving human subjects has to be approved by an independent body. The exact composition and responsibilities of this body will differ from country to country. In the U.S., for example, each study will be conducted under the auspices of an independent institutional review board at each institution at which the study is conducted. This board considers among other things, the design of the study, ethical factors, the privacy of protected health information as defined under the Health Insurance Portability and Accountability Act, the safety of the human subjects and the possible liability risk for the institution. Equivalent rules to protect subjects’ rights and welfare apply in each member state of the E.U. where one or more independent ethics committees, which typically operate similarly to an institutional review board, will review the ethics of conducting the proposed research. Other regulatory authorities around the rest of the world have slightly differing requirements involving both the execution of clinical trials and the import/export of pharmaceutical products. It is our responsibility to ensure we conduct our business in accordance with the regulations of each relevant territory.
By leveraging existing pre-clinical and clinical data, we are seeking build upon an existing pre-clinical and clinical safety and efficacy database to accelerate our research. In addition, our focus on end-stage population which has no current treatment options, commercialization may be achieved in an accelerated manner. Approval by the FDA in this category generally has been based on objective response rates and duration of responses rather than demonstration of survival benefit. As a result, trials of drugs to treat end-stage refractory cancer indications have historically involved fewer patients and generally have been faster to complete than trials of drugs for other indications. We are aware that the FDA and other similar agencies are regularly reviewing the use of objective endpoints for commercial approval and that policy changes may impact the size of trials required for approval, timelines and expenditures significantly.
In order to gain marketing approval we must submit a dossier to the relevant authority for review, which is known in the U.S. as an NDA and in the E.U. as a marketing authorization application, or MAA. The format is usually specific and laid out by each authority, although in general it will include information on the quality of the chemistry, manufacturing and pharmaceutical aspects of the product as well as the non-clinical and clinical data. Once the submitted NDA is accepted for filing by the FDA, it undertakes the review process that takes 10 months, unless an expedited priority review is granted which takes six months to complete. Approval can take several months to several years, if multiple 10-month review cycles are needed before final approval is obtained, if at all.
The approval process can be affected by a number of factors. The NDA may be approvable requiring additional pre-clinical, manufacturing data or clinical trials which may be requested at the end of the 10 month NDA review cycle, thereby delaying marketing approval until the additional data are submitted and may involve substantial unbudgeted costs. The regulatory authorities usually will conduct an inspection of relevant manufacturing facilities, and review manufacturing procedures, operating systems and personnel qualifications. In addition to obtaining approval for each product, in many cases each drug manufacturing facility must be approved. Further inspections may occur over the life of the product. An inspection of the clinical investigation sites by a competent authority may be required as part of the regulatory approval procedure. As a condition of marketing approval, the regulatory agency may require post-marketing surveillance to monitor for adverse effects or other additional studies as deemed appropriate. After approval for the initial indication, further clinical studies are usually necessary to gain approval for any additional indications. The terms of any approval, including labeling content, may be more restrictive than expected and could affect the marketability of a product.
| 19 |
The FDA offers a number of regulatory mechanisms that provide expedited or accelerated approval procedures for selected drugs in the indications on which we are focusing our efforts. These include accelerated approval under Subpart H of the agency’s NDA approval regulations, fast track drug development procedures and priority review. At this time, we have not determined whether any of these approval procedures will apply to our current drug candidate.
The U.S., E.U. and other jurisdictions may grant orphan drug designation to drugs intended to treat a “rare disease or condition,” which, in the U.S., is generally a disease or condition that affects no more than 200,000 individuals. In the E.U., orphan drug designation can be granted if: the disease is life threatening or chronically debilitating and affects no more than 50 in 100,000 persons in the E.U.; without incentive it is unlikely that the drug would generate sufficient return to justify the necessary investment; and no satisfactory method of treatment for the condition exists or, if it does, the new drug will provide a significant benefit to those affected by the condition. If a product that has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the U.S. and 10 years in the E.U. Orphan drug designation does not prevent competitors from developing or marketing different drugs for the same indication or the same drug for different indications. Orphan drug designation must be requested before submitting an NDA or MAA. After orphan drug designation is granted, the identity of the therapeutic agent and its potential orphan use are publicly disclosed. Orphan drug designation does not convey an advantage in, or shorten the duration of, the review and approval process. However, this designation provides an exemption from marketing and authorization (NDA) fees.
We are also subject to numerous environmental and safety laws and regulations, including those governing the use and disposal of hazardous materials. The cost of compliance with and any violation of these regulations could have a material adverse effect on our business and results of operations. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by state and federal regulations, accidental contamination or injury from these materials may occur. Compliance with laws and regulations relating to the protection of the environment has not had a material effect on our capital expenditures or our competitive position. However, we are not able to predict the extent of government regulation, and the cost and effect thereof on our competitive position, which might result from any legislative or administrative action pertaining to environmental or safety matters.
Competition
The development and commercialization of new drugs is highly competitive and we may face competition from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions worldwide.
Various products currently are marketed for the treatment of GBM and other cancers that we may target with our product candidate and a number of companies are developing new treatments. Companies also developing products for GBM include but are not limited to Celgene Corp., Celldex Therapeutics, Northwest Biotherapeutics, Inc., Immunocellular Therapeutics Ltd., and many major pharmaceutical companies. Our success will be based in part on our ability to build and actively manage a portfolio of drugs that addresses unmet medical needs and create value in patient therapy.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than products that we may develop. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
We expect that our ability to compete effectively will depend upon our ability to:
| ● | successfully and rapidly complete adequate and well-controlled clinical trials that demonstrate statistically significant safety and efficacy and to obtain all requisite regulatory approvals in a cost-effective manner; |
| 20 |
| ● | maintain a proprietary position for our manufacturing processes and other technology; |
| ● | produce our products in accordance with United States FDA and international regulatory guidelines; |
| ● | attract and retain key personnel; and |
| ● | build an adequate sales and marketing infrastructure for any approved products. |
Failure to do one or more of these activities could have an adverse effect on our business, financial condition or results of operations.
Corporate History
DelMar Pharmaceuticals, Inc. (the “Company”) is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. Prior to the Reverse Acquisition (discussed below), Berry did not have any significant assets or operations. On January 21, 2013, the Company changed its name to DelMar Pharmaceuticals, Inc.
On January 25, 2013 (the “Closing Date”), the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with DelMar (BC), 0959454 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Callco”), 0959456 B.C. Ltd., a British Columbia corporation and a wholly-owned subsidiary of the Company (“Exchangeco”), and securityholders of DelMar (BC). Pursuant to the Exchange Agreement, (i) the Company issued 4,340,417 shares of common stock (the “Parent Shares”) to the shareholders of DelMar (BC) who are United States residents (the “U.S. Holders”) in exchange for the transfer to Exchangeco of all 4,340,417 outstanding common shares of DelMar (BC) held by the U.S. Holders, (ii) the shareholders of DelMar (BC) who are Canadian residents (the “Canadian Holders”) received, in exchange for the transfer to Exchangeco of all 8,729,583 outstanding common shares of DelMar (BC) held by the Canadian Holders, 8,729,583 exchangeable shares (the “Exchangeable Shares”) of Exchangeco, and (iii) outstanding warrants to purchase 3,360,000 common shares of DelMar (BC) and outstanding options to purchase 1,020,000 common shares of DelMar (BC) were deemed to be amended such that, rather than entitling the holder to acquire common shares of DelMar (BC), such options and warrants (as amended, the “Exchange Agreement Warrants”) will entitle the holders to acquire shares of common stock of the Company. The Canadian Holders will be entitled to require Exchangeco to redeem (or, at the option of the Company or Callco, to have the Company or Callco purchase) the Exchangeable Shares, and upon such redemption or purchase to receive an equal number of shares of common stock of the Company.
Effective on the Closing Date, pursuant to the Exchange Agreement, DelMar (BC) became (indirectly through Exchangeco) a wholly-owned subsidiary of the Company. The acquisition of DelMar (BC) is treated as a reverse acquisition (the “Reverse Acquisition”), and the business of DelMar (BC) became the business of the Company. At the time of the Reverse Acquisition, Berry was not engaged in any active business.
Research and Development
During the year ended June 30, 2015, we incurred $2,555,754 on research and development.
Employees
We have four full-time employees and retain the services of approximately 15 persons on an independent contractor/consultant and contract-employment basis. As such, we currently operate in a “virtual” corporate structure in order to minimize fixed personnel costs. Over time, we plan to establish a base of full time employees and corporate infrastructure.
An investment in the Company’s common stock involves a high degree of risk. In determining whether to purchase the Company’s common stock, an investor should carefully consider all of the material risks described below, together with the other information contained in this report before making a decision to purchase the Company’s securities. An investor should only purchase the Company’s securities if he or she can afford to suffer the loss of his or her entire investment.
Risks Related to Our Business
Our independent registered auditors have expressed substantial doubt about our ability to continue as a going concern.
Our audited financial statements for the fiscal year ended June 30, 2015, include an explanatory paragraph that such financial statements were prepared assuming that we would continue as a going concern. As discussed in Note 1 to the consolidated financial statements for the year ended June 30, 2015, included with this report, because we have not begun to generate revenues and do not have the prospect of generating revenues in the near future, have reported a net loss of $4,796,030 and negative cash flow from operations of $3,853,069 for the year ended June 30, 2015, and have an accumulated deficit of $23,465,711 as of June 30, 2015, there is substantial doubt about our ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. If we are unable to continue as a going concern, shareholders may lose their entire investments.
| 21 |
We have a limited operating history and a history of operating losses, and expect to incur significant additional operating losses.
We are an early stage company and there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. We expect to incur substantial additional net expenses over the next several years as our research, development and commercial activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things, successful completion of the preclinical and clinical development of our product candidate; obtaining necessary regulatory approvals from the U.S. Food and Drug Administration (“FDA”) and international regulatory agencies; successful manufacturing, sales and marketing arrangements; and raising sufficient funds to finance our activities. If we are unsuccessful at some or all of these undertakings, our business, prospects and results of operations may be materially adversely affected.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings and/or license and development agreements with collaboration partners. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, then-existing stockholders’ interests may be materially diluted, and the terms of such securities could include liquidation or other preferences that adversely their rights as common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specified actions, such as incurring additional debt, making capital expenditures or declaring dividends. In addition, debt financing would result in fixed payment obligations.
If we raise funds through collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We are an early-stage company with an unproven business strategy and may never achieve commercialization of our candidate products or profitability.
We are at an early stage of development and commercialization of our technologies and product candidate. We have not yet begun to market any products and, accordingly, have not begun or generate revenues from the commercialization of our product. Our product will require significant additional clinical testing and investment prior to commercialization. A commitment of substantial resources by ourselves and, potentially, our partners to conduct time-consuming research and clinical trials will be required if we are to complete the development of our product candidate. There can be no assurance that our product candidate will meet applicable regulatory standards, obtain required regulatory approvals, be capable of being produced in commercial quantities at reasonable costs or be successfully marketed. Our product candidate is not expected to be commercially available for several years, if at all.
We are currently focused on the development of a single product candidate.
Our product development efforts are currently focused on a single product, VAL-083, for which we are researching multiple indications. If VAL-083 fails to achieve clinical endpoints or exhibits unanticipated toxicity or if a superior product is developed by a competitor, our prospects for obtaining regulatory approval and commercialization may be negatively impacted. In the long term, we hope to establish a pipeline of product candidates, and we have identified additional product candidates that we may be able to acquire or license in the future. However, at this time we do not have any formal agreements granting us any rights to such additional product candidates.
Even if we are able to commercialize any product candidate that we develop, the product may become subject to unfavorable pricing regulations, third party payor reimbursement practices or healthcare reform initiatives that could harm our business.
The commercial success of our product candidate will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidate will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities (such as Medicare and Medicaid), private health coverage insurers and other third party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidate. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish and maintain pricing sufficient to realize a meaningful return on our investment.
| 22 |
There is significant uncertainty related to third party payor coverage and reimbursement of newly approved drugs. Marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some non-U.S. markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay commercial launch of the product, possibly for lengthy time periods, which may negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval.
Our ability to commercialize VAL-083 or any other product candidate will depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third party payors, such as private health insurers and health maintenance organizations, decide which medications they will cover and establish reimbursement levels. The healthcare industry is acutely focused on cost containment, both in the United States and elsewhere. Government authorities and third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability to sell our product candidate profitably. These payors may not view our products, if any, as cost-effective, and coverage and reimbursement may not be available to our customers, or may not be sufficient to allow our products, if any, to be marketed on a competitive basis. Cost-control initiatives could cause us to decrease the price we might establish for products, which could result in lower than anticipated product revenues. If the prices for our products, if any, decrease or if governmental and other third party payors do not provide adequate coverage or reimbursement, our prospects for revenue and profitability will suffer.
There may also be delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the indications for which the drug is approved by the FDA or comparable non-U.S. regulatory authorities. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Reimbursement rates may vary, by way of example, according to the use of the drug and the clinical setting in which it is used. Reimbursement rates may also be based on reimbursement levels already set for lower cost drugs or may be incorporated into existing payments for other services.
In addition, increasingly, third party payors are requiring higher levels of evidence of the benefits and clinical outcomes of new technologies and are challenging the prices charged. We cannot be sure that coverage will be available for any product candidate that we, or they, commercialize and, if available, that the reimbursement rates will be adequate. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. An inability to promptly obtain coverage and adequate payment rates from both government-funded and private payors for any our product candidates for which we obtain marketing approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
We are dependent on obtaining certain patents and protecting our proprietary rights.
Our success will depend, in part, on our ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of third parties or having third parties circumvent our rights. We have filed and are actively pursuing patent applications for our products. The patent positions of biotechnology, biopharmaceutical and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions. Thus, there can be no assurance that any of our patent applications will result in the issuance of patents, that we will develop additional proprietary products that are patentable, that any patents issued to us or those that already have been issued will provide us with any competitive advantages or will not be challenged by any third parties, that the patents of others will not impede our ability to do business or that third parties will not be able to circumvent our patents. Furthermore, there can be no assurance that others will not independently develop similar products, duplicate any of our products not under patent protection, or, if patents are issued to us, design around the patented products we developed or will develop.
We may be required to obtain licenses from third parties to avoid infringing patents or other proprietary rights. No assurance can be given that any licenses required under any such patents or proprietary rights would be made available, if at all, on terms we find acceptable. If we do not obtain such licenses, we could encounter delays in the introduction of products or could find that the development, manufacture or sale of products requiring such licenses could be prohibited.
A number of pharmaceutical, biopharmaceutical and biotechnology companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to or affect our business. Some of these technologies, applications or patents may conflict with our technologies or patent applications. Such conflict could limit the scope of the patents, if any, that we may be able to obtain or result in the denial of our patent applications. In addition, if patents that cover our activities are issued to other companies, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost or be able to develop or obtain alternative technology. If we do not obtain such licenses, we could encounter delays in the introduction of products, or could find that the development, manufacture or sale of products requiring such licenses could be prohibited. In addition, we could incur substantial costs in defending ourselves in suits brought against us on patents it might infringe or in filing suits against others to have such patents declared invalid.
| 23 |
Patent applications in the U.S. are maintained in secrecy and not published if either: i) the application is a provisional application or, ii) the application is filed and we request no publication, and certify that the invention disclosed “has not and will not” be the subject of a published foreign application. Otherwise, U.S. applications or foreign counterparts, if any, publish 18 months after the priority application has been filed. Since publication of discoveries in the scientific or patent literature often lag behind actual discoveries, we cannot be certain that we or any licensor were the first creator of inventions covered by pending patent applications or that we or such licensor was the first to file patent applications for such inventions. Moreover, we might have to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention, which could result in substantial cost to us, even if the eventual outcome were favorable to us. There can be no assurance that our patents, if issued, would be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe such patents.
Moreover, we may be subject to third party preissuance submissions of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third party patent rights.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us, or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate, or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
In addition, the protection of intellectual property rights in China (where our clinical product candidate, VAL-083, is manufactured pursuant to a collaboration agreement with the only manufacturer presently licensed by the China Food and Drug Administration to produce the product for the China market, and where VAL-083 is approved for the treatment of CML and lung cancer) is relatively weak compared to the United States, which may negatively affect our ability to generate royalty revenue from sales of VAL-083 in China.
Much of our know-how and technology may not be patentable. To protect our rights, we require employees, consultants, advisors and collaborators to enter into confidentiality agreements. There can be no assurance, however, that these agreements will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Further, our business may be adversely affected by competitors who independently develop competing technologies, especially if we obtain no, or only narrow, patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. The Leahy-Smith Act includes provisions that affect the way patent applications are prosecuted and affect patent litigation. The United States Patent and Trademark Office, or PTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act. However, many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
| 24 |
We may be unable to protect our patents and proprietary rights.
Our future success will depend to a significant extent on our ability to:
| ● | obtain and keep patent protection for our products and technologies on an international basis; |
| ● | enforce our patents to prevent others from using our inventions; |
| ● | maintain and prevent others from using our trade secrets; and |
| ● | operate and commercialize products without infringing on the patents or proprietary rights of others. |
We can provide no assurance that our patent rights will afford any competitive advantages and these rights may be challenged or circumvented by third parties. Further, patents may not be issued on any of our pending patent applications in the U.S. or abroad. Because of the extensive time required for development, testing and regulatory review of a product candidate, it is possible that before a product candidate can be commercialized, any related patent may expire, or remain in existence for only a short period following commercialization, reducing or eliminating any advantage of the patent.
If we sue others for infringing our patents, a court may determine that such patents are invalid or unenforceable. Even if the validity of our patent rights is upheld by a court, a court may not prevent the alleged infringement of our patent rights on the grounds that such activity is not covered by our patent claims.
In addition, third parties may sue us for infringing their patents. In the event of a successful claim of infringement against us, we may be required to:
| ● | defend litigation or administrative proceedings; |
| ● | pay substantial damages; |
| ● | stop using our technologies and methods; |
| ● | stop certain research and development efforts; |
| ● | develop non-infringing products or methods; and |
| ● | obtain one or more licenses from third parties. |
If required, we can provide no assurance that we will be able to obtain such licenses on acceptable terms, or at all. If we are sued for infringement, we could encounter substantial delays in development, manufacture and commercialization of our product candidates. Any litigation, whether to enforce our patent rights or to defend against allegations that we infringe third party rights, will be costly, time consuming, and may distract management from other important tasks.
As is commonplace in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. To the extent our employees are involved in research areas which are similar to those areas in which they were involved at their former employers, we may be subject to claims that such employees and/or we have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of the former employers. Litigation may be necessary to defend against such claims, which could result in substantial costs and be a distraction to management and which may have a material adverse effect on us, even if we are successful in defending such claims.
We are subject to various government regulations.
The manufacture and sale of human therapeutic and diagnostic products in the U.S., Canada and foreign jurisdictions are governed by a variety of statutes and regulations. These laws require approval of manufacturing facilities, controlled research and testing of products and government review and approval of a submission containing manufacturing, preclinical and clinical data in order to obtain marketing approval based on establishing the safety and efficacy of the product for each use sought, including adherence to current cGMP during production and storage, and control of marketing activities, including advertising and labeling.
The product we are currently developing will require significant development, preclinical and clinical testing and investment of substantial funds prior to its commercialization. The process of obtaining required approvals can be costly and time-consuming, and there can be no assurance that future products will be successfully developed and will prove to be safe and effective in clinical trials or receive applicable regulatory approvals. Markets other than the U.S. and Canada have similar restrictions. Potential investors and shareholders should be aware of the risks, problems, delays, expenses and difficulties which we may encounter in view of the extensive regulatory environment which controls our business.
| 25 |
If we are unable to keep up with rapid technological changes in our field or compete effectively, we will be unable to operate profitably.
We are engaged in a rapidly changing field. Other products and therapies that will compete directly with the products that we are seeking to develop and market currently exist or are being developed. Competition from fully integrated pharmaceutical companies and more established biotechnology companies is intense and is expected to increase. Most of these companies have significantly greater financial resources and expertise in discovery and development, manufacturing, preclinical and clinical testing, obtaining regulatory approvals and marketing than us. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biopharmaceutical or biotechnology companies. Many of these competitors have significant products that have been approved or are in development and operate large, well-funded discovery and development programs. Academic institutions, governmental agencies and other public and private research organizations also conduct research, seek patent protection and establish collaborative arrangements for therapeutic products and clinical development and marketing. These companies and institutions compete with us in recruiting and retaining highly qualified scientific and management personnel. In addition to the above factors, we will face competition based on product efficacy and safety, the timing and scope of regulatory approvals, availability of supply, marketing and sales capability, reimbursement coverage, price and patent position. There is no assurance that our competitors will not develop more effective or more affordable products, or achieve earlier patent protection or product commercialization, than our own.
Other companies may succeed in developing products earlier than ourselves, obtaining Health Canada, European Medicines Agency (“EMA”) and FDA approvals for such products more rapidly than we will, or in developing products that are more effective than products we propose to develop. While we will seek to expand our technological capabilities in order to remain competitive, there can be no assurance that research and development by others will not render our technology or products obsolete or non-competitive or result in treatments or cures superior to any therapy we develop, or that any therapy we develop will be preferred to any existing or newly developed technologies.
We may request priority review for our product candidate in the future. The FDA may not grant priority review for our product candidate. Moreover, even if the FDA designated such product for priority review, that designation may not lead to a faster regulatory review or approval process and, in any event, would not assure FDA approval.
We may be eligible for priority review designation for our product candidate if the FDA determines such product candidate offers major advances in treatment or provides a treatment where no adequate therapy exists. A priority review designation means that the goal for the FDA to review an application is six months, rather than the standard review period of ten months. The FDA has broad discretion with respect to whether or not to grant priority review status to a product candidate, so even if we believe a particular product candidate is eligible for such designation or status, the FDA may decide not to grant it. Thus, while the FDA has granted priority review to other oncology disease products, our product candidate, should we determine to seek priority review, may not receive similar designation. Moreover, even if our product candidate is designated for priority review, such a designation does not necessarily mean a faster regulatory review process or necessarily confer any advantage with respect to approval compared to conventional FDA procedures. Receiving priority review from the FDA does not guarantee approval within an accelerated timeline or thereafter.
We believe we may in some instances be able to secure approval from the FDA or comparable non-U.S. regulatory authorities to use accelerated development pathways. If we are unable to obtain such approval, we may be required to conduct additional preclinical studies or clinical trials beyond those that we contemplate, which could increase the expense of obtaining, and delay the receipt of, necessary marketing approvals.
We anticipate that we may seek an accelerated approval pathway for our product candidate. Under the accelerated approval provisions in the Federal Food, Drug, and Cosmetic Act, or FDCA, and the FDA’s implementing regulations, the FDA may grant accelerated approval to a product designed to treat a serious or life-threatening condition that provides meaningful therapeutic benefit over available therapies upon a determination that the product has an effect on a surrogate endpoint or intermediate clinical endpoint that is reasonably likely to predict clinical benefit. The FDA considers a clinical benefit to be a positive therapeutic effect that is clinically meaningful in the context of a given disease, such as irreversible morbidity or mortality. For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign, or other measure that is thought to predict clinical benefit, but is not itself a measure of clinical benefit. An intermediate clinical endpoint is a clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit. The accelerated approval pathway may be used in cases in which the advantage of a new drug over available therapy may not be a direct therapeutic advantage, but is a clinically important improvement from a patient and public health perspective. If granted, accelerated approval is usually contingent on the sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the drug’s clinical benefit. If such post-approval studies fail to confirm the drug’s clinical benefit, the FDA may withdraw its approval of the drug.
| 26 |
Prior to seeking such accelerated approval, we will seek feedback from the FDA and will otherwise evaluate our ability to seek and receive such accelerated approval. There can also be no assurance that after our evaluation of the feedback and other factors we will decide to pursue or submit a Biologics License Application, or BLA, or a New Drug Application, or NDA, for accelerated approval or any other form of expedited development, review or approval. Similarly, there can be no assurance that after subsequent FDA feedback that we will continue to pursue or apply for accelerated approval or any other form of expedited development, review or approval, even if we initially decide to do so. Furthermore, if we decide to submit an application for accelerated approval or under another expedited regulatory designation (e.g., breakthrough therapy designation), there can be no assurance that such submission or application will be accepted or that any expedited development, review or approval will be granted on a timely basis, or at all. The FDA or other non-U.S. authorities could also require us to conduct further studies prior to considering our application or granting approval of any type. A failure to obtain accelerated approval or any other form of expedited development, review or approval for any of our product candidates that we determine to seek accelerated approval for would result in a longer time period to commercialization of such product candidate, could increase the cost of development of such product candidate and could harm our competitive position in the marketplace.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete the development and commercialization of our product candidate.
Our only current product candidate is in clinical development and the risk of failure of our product candidate is high. It is impossible to predict when or if our product candidate will prove effective or safe in humans or will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidate in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The clinical development of our product candidate is susceptible to the risk of failure inherent at any stage of drug development, including failure to demonstrate efficacy in a clinical trial or across a broad population of patients, the occurrence of severe or medically or commercially unacceptable adverse events, failure to comply with protocols or applicable regulatory requirements and determination by the FDA or any comparable non-U.S. regulatory authority that a drug product is not safe or effective for its intended uses. It is possible that even if our product candidate has a beneficial effect, that effect will not be detected during clinical evaluation as a result of one or more of a variety of factors, including the size, duration, design, measurements, conduct or analysis of our clinical trials. Conversely, as a result of the same factors, our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any. Similarly, in our clinical trials we may fail to detect toxicity of or intolerability caused by our product candidate, or mistakenly believe that our product candidate is toxic or not well tolerated when that is not in fact the case.
The outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in earlier development, and we cannot be certain that we will not face additional setbacks.
The design of a clinical trial can determine whether its results will support approval of a product; however, flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced or completed. We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support marketing approval. In addition, preclinical and clinical data are often susceptible to varying interpretations and analyses. Many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval for the product candidates. Even if we believe that the results of clinical trials for our product candidate warrant marketing approval, the FDA or comparable non-U.S. regulatory authorities may disagree and may not grant marketing approval of our product candidate.
In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the clinical trial protocols and the rate of dropout among clinical trial participants. Any Phase 2, Phase 3 or other clinical trials that we may conduct may not demonstrate the efficacy and safety necessary to obtain regulatory approval to market our product candidate.
We have conducted, and may in the future conduct, clinical trials for certain of our product candidate at sites outside the United States, and the FDA may not accept data from trials conducted in such locations.
We have conducted and may in the future choose to conduct one or more of our clinical trials outside the United States. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to certain conditions imposed by the FDA. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with ethical principles. The trial population must also adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deems clinically meaningful. Generally, the patient population for any clinical trials conducted outside of the United States must be representative of the population for whom we intend to seek approval in the United States. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will be dependent upon its determination that the trials also complied with all applicable U.S. laws and regulations. There can be no assurance that the FDA will accept data from trials conducted outside of the United States. If the FDA does not accept the data from any of our clinical trials that we determine to conduct outside the United States, it would likely result in the need for additional trials, which would be costly and time-consuming and delay or permanently halt our development of the product candidate.
| 27 |
In addition, the conduct of clinical trials outside the United States could have a significant impact on us. Risks inherent in conducting international clinical trials include:
| ● | foreign regulatory requirements that could restrict or limit our ability to conduct our clinical trials; |
| ● | administrative burdens of conducting clinical trials under multiple foreign regulatory schema; |
| ● | foreign exchange fluctuations; and |
| ● | diminished protection of intellectual property in some countries. |
If clinical trials of our product candidate fail to demonstrate safety and efficacy to the satisfaction of the FDA and comparable non-U.S. regulators, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidate.
We are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing approval from the FDA. Comparable non-U.S. regulatory authorities, such as the EMA, impose similar restrictions. We may never receive such approvals. We must complete extensive preclinical development and clinical trials to demonstrate the safety and efficacy of our product candidate in humans before we will be able to obtain these approvals.
Clinical testing is expensive, difficult to design and implement, can take many years to complete and is inherently uncertain as to outcome. We have not previously submitted a BLA or an NDA to the FDA or similar drug approval filings to comparable non-U.S. regulatory authorities for any product candidate.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us and impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. In addition, if (1) we are required to conduct additional clinical trials or other testing of our product candidate beyond the trials and testing that we contemplate, (2) we are unable to successfully complete clinical trials of our product candidate or other testing, (3) the results of these trials or tests are unfavorable, uncertain or are only modestly favorable, or (4) there are unacceptable safety concerns associated with our product candidate, we, in addition to incurring additional costs, may:
| ● | be delayed in obtaining marketing approval for our product candidates; |
| ● | not obtain marketing approval at all; |
| ● | obtain approval for indications or patient populations that are not as broad as we intended or desired; |
| ● | obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings; |
| ● | be subject to additional post-marketing testing or other requirements; or |
| ● | be required to remove the product from the market after obtaining marketing approval. |
| 28 |
If we experience any of a number of possible unforeseen events in connection with clinical trials of our product candidate, potential marketing approval or commercialization of our product candidate could be delayed or prevented.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent marketing approval of our product candidate, including:
| ● | clinical trials of our product candidate may produce unfavorable or inconclusive results; |
| ● | we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
| ● | the number of patients required for clinical trials of our product candidate may be larger than we anticipate, patient enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| ● | data safety monitoring committees may recommend suspension, termination or a clinical hold for various reasons, including concerns about patient safety; |
| ● | regulators or IRBs may suspend or terminate the trial or impose a clinical hold for various reasons, including noncompliance with regulatory requirements or concerns about patient safety; |
| ● | patients with serious, life-threatening diseases included in our clinical trials may die or suffer other adverse medical events for reasons that may not be related to our product candidate; |
| ● | participating patients may be subject to unacceptable health risks; |
| ● | patients may not complete clinical trials due to safety issues, side effects, or other reasons; |
| ● | changes in regulatory requirements and guidance may occur, which require us to amend clinical trial protocols to reflect these changes; |
| ● | our third party contractors, including those manufacturing our product candidate or components or ingredients thereof or conducting clinical trials on our behalf, may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner or at all; |
| ● | regulators or institutional review boards, or IRBs may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| ● | we may experience delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| ● | patients who enroll in a clinical trial may misrepresent their eligibility to do so or may otherwise not comply with the clinical trial protocol, resulting in the need to drop the patients from the clinical trial, increase the needed enrollment size for the clinical trial or extend the clinical trial’s duration; |
| ● | we may have to suspend or terminate clinical trials of our product candidate for various reasons, including a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of a product candidate; |
| ● | regulators or IRBs may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or their respective standards of conduct, a finding that the participants are being exposed to unacceptable health risks, undesirable side effects or other unexpected characteristics of the product candidate or findings of undesirable effects caused by a chemically or mechanistically similar drug or drug candidate; |
| ● | the FDA or comparable non-U.S. regulatory authorities may disagree with our clinical trial design or our interpretation of data from preclinical studies and clinical trials; |
| ● | the FDA or comparable non-U.S. regulatory authorities may fail to approve or subsequently find fault with the manufacturing processes or facilities of third party manufacturers with which we enter into agreements for clinical and commercial supplies; |
| ● | the supply or quality of raw materials or manufactured product candidate or other materials necessary to conduct clinical trials of our product candidate may be insufficient, inadequate, delayed, or not available at an acceptable cost, or we may experience interruptions in supply; and |
| ● | the approval policies or regulations of the FDA or comparable non-U.S. regulatory authorities may significantly change in a manner rendering our clinical data insufficient to obtain marketing approval. |
| 29 |
Product development costs for us will increase if we experience delays in testing or pursuing marketing approvals and we may be required to obtain additional funds to complete clinical trials and prepare for possible commercialization of our product candidate. We do not know whether any preclinical tests or clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Significant preclinical or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidate or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our product candidate and may harm our business and results of operations. In addition, many of the factors that cause, or lead to, clinical trial delays may ultimately lead to the denial of marketing approval of our product candidate.
If we experience delays or difficulties in the enrollment of patients in clinical trials, we may not achieve our clinical development on our anticipated timeline, or at all, and our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for VAL-083 or any other product candidate if we are unable to locate and enroll a sufficient number of eligible patients to participate in clinical trials. Patient enrollment is a significant factor in the timing of clinical trials, and is affected by many factors, including:
| ● | the size and nature of the patient population; |
| ● | the severity of the disease under investigation; |
| ● | the proximity of patients to clinical sites; |
| ● | the eligibility criteria for the trial; |
| ● | the design of the clinical trial; |
| ● | efforts to facilitate timely enrollment; |
| ● | competing clinical trials; and |
| ● | clinicians’ and patients’ perceptions as to the potential advantages and risks of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. |
Our inability to enroll a sufficient number of patients for our clinical trials could result in significant delays or may require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our product candidate, delay or halt the development of and approval processes for our product candidate and jeopardize our ability to achieve our clinical development timeline and goals, including the dates by which we will commence, complete and receive results from clinical trials. Enrollment delays may also delay or jeopardize our ability to commence sales and generate revenues from our product candidate. Any of the foregoing could cause the value of the Company to decline and limit our ability to obtain additional financing, if needed.
Positive results in previous clinical trials of VAL-083 may not be replicated in future clinical trials, which could result in development delays or a failure to obtain marketing approval.
Positive results in previous clinical studies of VAL-083 may not be predictive of similar results in future clinical trials. Also, interim results during a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in early-stage development. Accordingly, the results from the completed preclinical studies and clinical trials for VAL-083 may not be predictive of the results we may obtain in later stage trials. Our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain FDA or EMA, or other regulatory agency, approval for their products.
FDA approval of VAL-083 may be denied.
There can be no assurance that the FDA will ultimately approve our NDA. The FDA may deny approval of VAL-083 for many reasons, including:
| ● | we may be unable to demonstrate to the satisfaction of the FDA that VAL-083 is safe and effective for its intended uses; |
| 30 |
| ● | the FDA may disagree with our interpretation of data from the clinical trials; |
| ● | we may be unable to demonstrate that any clinical or other benefits of VAL-083 outweigh any safety or other perceived risks; or |
| ● | we may not be able to successfully address any other issues raised by the FDA. |
| ● | If VAL-083 fails to receive FDA approval, our business and prospects will be materially adversely impacted. |
We expect to rely on orphan drug status to develop and commercialize our product candidate, but our orphan drug designations may not confer marketing exclusivity or other expected commercial benefits as anticipated.
Market exclusivity afforded by orphan drug designation is generally offered as an incentive to drug developers to invest in developing and commercializing products for unique diseases that impact a limited number of patients. The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. Qualification to maintain orphan drug status is generally monitored by the regulatory authorities during the orphan drug exclusivity period, currently seven years from the date of approval in the United States.
We have been granted orphan drug designation in the United States for GBM and we expect to rely on orphan drug exclusivity for our product candidate. It is possible that with the approval of VAL-083 in the United States, that the incidence and prevalence numbers for GBM could change. Should the incidence and prevalence of GBM patients materially increase, it is possible that the orphan drug designation, and related market exclusivity, in the United States could be lost. Further, while we have been granted this orphan designation, the FDA can still approve different drugs for use in treating the same indication or disease, which would create a more competitive market for us and our revenues will be diminished.
Further, for our product candidate, it is possible that another company also holding orphan drug designation for the same product candidate will receive marketing approval for the same indication before we do. If that were to happen, our applications for that indication may not be approved until the competing company’s period of exclusivity expires. Even if we are the first to obtain marketing authorization for an orphan drug indication, there are circumstances under which a competing product may be approved for the same indication during the seven-year period of marketing exclusivity, such as if the later product is shown to be clinically superior to the orphan product, or if the later product is deemed a different product than ours. Further, the seven-year marketing exclusivity would not prevent competitors from obtaining approval of the same product candidate as ours for indications other than those in which we have been granted orphan drug designation, or for the use of other types of products in the same indications as our orphan product.
If the market opportunities for our product candidate are smaller than we believe they are, our revenues may be adversely affected and our business may suffer. Because the target patient populations of our product candidate are small, we must be able to successfully identify patients and capture a significant market share to achieve and maintain profitability.
We focus our research and product development on treatments for orphan cancer indications. Our projections of both the number of people who have failed other therapies or have limited medical options, are based on estimates. These estimates may prove to be incorrect and new studies may change the estimated incidence or prevalence. The number of patients in the United States, Europe and elsewhere may turn out to be lower than expected or may not be otherwise amenable to treatment with our products, or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business. Additionally, because our target patient populations are small, we will be required to capture a significant market share to achieve and maintain profitability.
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidate.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering any product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidates for any or all targeted indications. Ultimately, some or all of our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects or even death as a result of participating in our clinical trials.
| 31 |
We may not receive regulatory approvals for our product candidate or there may be a delay in obtaining such approvals.
Our product and our ongoing development activities are subject to regulation by regulatory authorities in the countries in which we or our collaborators and distributors wish to test, manufacture or market our products. For instance, the FDA will regulate the product in the U.S. and equivalent authorities, such as the EMA, will regulate in Europe. Regulatory approval by these authorities will be subject to the evaluation of data relating to the quality, efficacy and safety of the product for its proposed use, and there can be no assurance that the regulatory authorities will find our data sufficient to support product approval of VAL-083.
The time required to obtain regulatory approval varies between countries. In the U.S., for products without “Fast Track” status, it can take up to eighteen (18) months after submission of an application for product approval to receive the FDA's decision. Even with Fast Track status, FDA review and decision can take up to twelve (12) months. At present, we do not have Fast Track status for our clinical product candidate, VAL-083.
Different regulators may impose their own requirements and may refuse to grant, or may require additional data before granting, an approval, notwithstanding that regulatory approval may have been granted by other regulators. Regulatory approval may be delayed, limited or denied for a number of reasons, including insufficient clinical data, the product not meeting safety or efficacy requirements or any relevant manufacturing processes or facilities not meeting applicable requirements as well as case load at the regulatory agency at the time.
We may fail to comply with regulatory requirements.
Our success will be dependent upon our ability, and our collaborative partners’ abilities, to maintain compliance with regulatory requirements, including cGMP, and safety reporting obligations. The failure to comply with applicable regulatory requirements can result in, among other things, fines, injunctions, civil penalties, total or partial suspension of regulatory approvals, refusal to approve pending applications, recalls or seizures of products, operating and production restrictions and criminal prosecutions.
Even if our product candidate receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third party payors and others in the medical community necessary for commercial success and the market opportunity for the product candidate may be smaller than we estimate.
We have never commercialized a product. Even if VAL-083 or any other product candidate is approved by the appropriate regulatory authorities for marketing and sale, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third party payors and others in the medical community. For example, physicians are often reluctant to switch their patients from existing therapies even when new and potentially more effective or convenient treatments enter the market. Further, patients often acclimate to the therapy that they are currently taking and do not want to switch unless their physicians recommend switching products or they are required to switch therapies due to lack of reimbursement for existing therapies.
Efforts to educate the medical community and third party payors on the benefits of our product candidate may require significant resources and may not be successful. If our product candidate is approved but does not achieve an adequate level of market acceptance, we may not generate significant revenues and we may not become profitable. The degree of market acceptance of VAL-083 or any other product candidate, if approved for commercial sale, will depend on a number of factors, including:
| ● | the efficacy and safety of the product; |
| ● | the potential advantages of the product compared to alternative treatments; |
| ● | the prevalence and severity of any side effects; |
| ● | the clinical indications for which the product is approved; |
| ● | whether the product is designated under physician treatment guidelines as a first-line therapy or as a second- or third-line therapy; |
| ● | limitations or warnings, including distribution or use restrictions, contained in the product’s approved labeling; |
| ● | our ability to offer the product for sale at competitive prices; |
| ● | our ability to establish and maintain pricing sufficient to realize a meaningful return on our investment; |
| ● | the product’s convenience and ease of administration compared to alternative treatments; |
| 32 |
| ● | the willingness of the target patient population to try, and of physicians to prescribe, the product; |
| ● | the strength of sales, marketing and distribution support; |
| ● | the approval of other new products for the same indications; |
| ● | changes in the standard of care for the targeted indications for the product; |
| ● | the timing of market introduction of our approved products as well as competitive products and other therapies; |
| ● | availability and amount of reimbursement from government payors, managed care plans and other third party payors; |
| ● | adverse publicity about the product or favorable publicity about competitive products; and |
| ● | potential product liability claims. |
The potential market opportunities for our product candidate are difficult to estimate precisely. Our estimates of the potential market opportunities are predicated on many assumptions, including industry knowledge and publications, third party research reports and other surveys. While we believe that our internal assumptions are reasonable, these assumptions involve the exercise of significant judgment on the part of our management, are inherently uncertain and the reasonableness of these assumptions has not been assessed by an independent source. If any of the assumptions proves to be inaccurate, the actual markets for our product candidate could be smaller than our estimates of the potential market opportunities.
If our product candidate receives marketing approval and we, or others, later discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, our ability to market the drug could be compromised.
Clinical trials of our product candidate are conducted in carefully defined subsets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, or alternatively fail to identify undesirable side effects. If, following approval of our product candidate, we, or others, discover that the drug is less effective than previously believed or causes undesirable side effects that were not previously identified, any of the following adverse events could occur:
| ● | regulatory authorities may withdraw their approval of the drug or seize the drug; |
| ● | we may be required to recall the drug or change the way the drug is administered; |
| ● | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular drug; |
| ● | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| ● | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| ● | we may be required to create a Medication Guide outlining the risks of the previously unidentified side effects for distribution to patients; |
| ● | we could be sued and held liable for harm caused to patients; |
| ● | the drug may become less competitive; and |
| ● | our reputation may suffer. |
Any of these events could have a material and adverse effect on our operations and business and could adversely impact our stock price.
| 33 |
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, qualification testing, post-approval clinical data, labeling and promotional activities for such product, will be subject to continual and additional requirements of the FDA and other regulatory authorities.
These requirements include submissions of safety and other post-marketing information, reports, registration and listing requirements, good manufacturing practices, or GMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, and recordkeeping. Even if marketing approval of our product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of pharmaceutical products to ensure such products are marketed only for the approved indications and in accordance with the provisions of the approved labeling.
In addition, later discovery of previously unknown problems with our products, manufacturing processes, or failure to comply with regulatory requirements, may lead to various adverse results, including:
| ● | restrictions on such products, manufacturers or manufacturing processes; |
| ● | restrictions on the labeling or marketing of a product; |
| ● | restrictions on product distribution or use; |
| ● | requirements to conduct post-marketing clinical trials; |
| ● | requirements to institute a risk evaluation mitigation strategy, or REMS, to monitor safety of the product post-approval; |
| ● | warning letters issued by the FDA or other regulatory authorities; |
| ● | withdrawal of the products from the market; |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; |
| ● | recall of products, fines, restitution or disgorgement of profits or revenue; |
| ● | suspension, revocation or withdrawal of marketing approvals; |
| ● | refusal to permit the import or export of our products; and |
| ● | injunctions or the imposition of civil or criminal penalties. |
If we are unable to establish sales, marketing and distribution capabilities or enter into acceptable sales, marketing and distribution arrangements with third parties, we may not be successful in commercializing any product candidates that we develop, if and when those product candidates are approved.
We do not have a sales, marketing or distribution infrastructure and have limited experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization, outsource these functions to third parties, or license our product candidates to others. If approved, we may seek to license VAL-083 to a large pharmaceutical company with greater resources and experience than us. We may not be able license the VAL-083 on reasonable terms, if at all. The development of sales, marketing and distribution capabilities will require substantial resources, will be time-consuming and could delay any product launch. We expect that we will commence the development of these capabilities prior to receiving approval of our product candidate. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing and distribution capabilities is delayed or does not occur for any reason, we could have prematurely or unnecessarily incurred these commercialization costs. Such a delay may be costly, and our investment could be lost if we cannot retain or reposition our sales and marketing personnel. In addition, we may not be able to hire or retain a sales force in the United States that is sufficient in size or has adequate expertise in the medical markets that we plan to target. If we are unable to establish or retain a sales force and marketing and distribution capabilities, our operating results may be adversely affected. If a potential partner has development or commercialization expertise that we believe is particularly relevant to our product candidate, then we may seek to collaborate with that potential partner even if we believe we could otherwise develop and commercialize the product independently.
We expect to seek one or more strategic partners for commercialization of our product candidate outside the United States. As a result of entering into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues may be lower, perhaps substantially lower, than if we were to directly market and sell products in those markets. Furthermore, we may be unsuccessful in entering into the necessary arrangements with third parties or may be unable to do so on terms that are favorable to us. In addition, we may have little or no control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively.
| 34 |
If we do not establish sales and marketing capabilities, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidate.
We face substantial competition from other pharmaceutical and biotechnology companies and our operating results may suffer if we fail to compete effectively.
The development and commercialization of new drug products is highly competitive. We expect that we will face significant competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide with respect to VAL-083 and any other of our product candidates that we may seek to develop or commercialize in the future. Specifically, due to the large unmet medical need, global demographics and relatively attractive reimbursement dynamics, the oncology market is fiercely competitive and there are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of product candidates for the treatment of cancer and the immunization of infectious diseases. Our competitors may succeed in developing, acquiring or licensing technologies and drug products that are more effective, have fewer or more tolerable side effects or are less costly than any product candidates that we are currently developing or that we may develop, which could render our product candidates obsolete and noncompetitive.
All of the top ten global pharmaceutical companies and most of the mid-size pharmaceutical companies have a strong research and development and commercial presence in oncology. Smaller companies also focus on oncology, including companies such as ARIAD Pharmaceuticals, Inc., Agios Pharmaceuticals, Inc., BIND Therapeutics, Inc., Clovis Oncology, Inc., Endocyte, Inc., Epizyme, Inc., ImmunoGen, Inc., Incyte Corporation, Infinity Pharmaceuticals, Inc., MacroGenics, Inc., Merrimack Pharmaceuticals, Inc., OncoMed Pharmaceuticals, Inc., Onconova Therapeutics, Inc., Pharmacyclics, Inc., Puma Biotechnology, Inc., Seattle Genetics, Inc. and TESARO, Inc.
Several companies are marketing and developing oncology immunotherapy products. Companies with approved marketed oncology products for GBM are Merck (Temodar® ) and Genentech (Avastin®). Companies with oncology immunotherapy product candidates in clinical development include Northwest Biotherapeutics (DCVax-L), Celldex Therapeutics (Rindopepimut (CDX-110)) and ImmunoCellular Therapeutics (ICT-107).
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other marketing approval for their products before we are able to obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our existing and potential future competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining marketing approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
If we are unable to or delayed in obtaining state regulatory licenses for the distribution of our product, we would not be able to sell our product candidate.
The majority of states require manufacturer and/or wholesaler licenses for the sale and distribution of drugs into that state. The application process is complicated, time consuming and requires dedicated personnel or a third party to oversee and manage. If we are delayed in obtaining these state licenses, or denied the licenses, even with FDA approval, we would not be able to sell or ship product into that state which would adversely affect our sales and revenues.
We rely on key personnel and, if we are unable to retain or motivate key personnel or hire qualified personnel, we may not be able to grow effectively.
We are dependent on certain members of our management, scientific and drug development staff and consultants, the loss of services of one or more of whom could materially adversely affect us.
We currently have four full-time employees, and retain the services of approximately 15 persons on an independent contractor/consultant and contract-employment basis. Our ability to manage growth effectively will require us to continue to implement and improve our management systems and to recruit and train new employees. Although we have done so in the past and expect to do so in the future, there can be no assurance that we will be able to successfully attract and retain skilled and experienced personnel.
| 35 |
Our success depends in large part upon our ability to attract and retain highly qualified personnel. We compete in our hiring efforts with other pharmaceutical and biotechnology companies, as well as universities and nonprofit research organizations, and we may have to pay higher salaries to attract and retain personnel, which would be very costly.
We may be subject to foreign exchange fluctuation.
Our functional and reporting currency is the United States dollar. We maintain bank accounts in United States and Canadian dollars. A portion of our expenditures are in foreign currencies, most notably in Canadian dollars, and therefore we are subject to foreign currency fluctuations, which may, from time to time, impact our financial position and results. We may enter into hedging arrangements under specific circumstances, typically through the use of forward or futures currency contracts, to minimize the impact of increases in the value of the Canadian dollar. In order to minimize our exposure to foreign exchange fluctuations we may hold sufficient Canadian dollars to cover our expected Canadian dollar expenditures.
Product liability lawsuits against us could divert our resources, cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
We face an inherent risk of product liability claims as a result of the clinical testing of our product candidate despite obtaining appropriate informed consents from our clinical trial participants. We will face an even greater risk if we commercially sell any product that we may develop. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidate. Regardless of the merits or eventual outcome, liability claims may result in:
| ● | decreased demand for our product candidate or products that we may develop; |
| ● | injury to our reputation and significant negative media attention; |
| ● | withdrawal of clinical trial participants; |
| ● | significant costs to defend resulting litigation; |
| ● | substantial monetary awards to trial participants or patients; |
| ● | loss of revenue; |
| ● | reduced resources of our management to pursue our business strategy; and |
| ● | the inability to commercialize any products that we may develop. |
Although we maintain general liability insurance, this insurance may not fully cover potential liabilities that we may incur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. We will need to increase our insurance coverage if and when we begin selling any product candidate that receives marketing approval. In addition, insurance coverage is becoming increasingly expensive. If we are unable to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, it could prevent or inhibit the development and commercial production and sale of our product candidate, which could adversely affect our business, financial condition, results of operations and prospects.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct clinical trials for our product candidate. Any failure by a third party to meet its obligations with respect to the clinical development of our product candidate may delay or impair our ability to obtain regulatory approval for our product candidate.
We rely on academic institutions and private oncology centers to conduct and sponsor clinical trials relating to VAL-083. Our reliance on third parties to conduct clinical trials could, depending on the actions of such third parties, jeopardize the validity of the clinical data generated and adversely affect our ability to obtain marketing approval from the FDA or other applicable regulatory authorities.
| 36 |
Such clinical trial arrangements provide us with information rights with respect to the clinical data, including access to and the ability to use and reference the data, including for our own regulatory filings, resulting from the clinical trials. If investigators or institutions breach their obligations with respect to the clinical trials of our product candidate, or if the data proves to be inadequate, then our ability to design and conduct any future clinical trials may be adversely affected.
We rely, and expect to continue to rely, on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We currently rely on third party clinical research organizations, or CROs, to conduct our clinical trials. We expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions and clinical investigators, to conduct our clinical trials. Our agreements with these third parties generally allow the third party to terminate the agreement at any time. If we are required to enter into alternative arrangements because of any such termination the introduction of our product candidates to market could be delayed.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we design our clinical trials and will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as good clinical practices, or GCPs, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidate or commercialization of our products, producing additional losses and depriving us of potential product revenue.
We may seek to enter into collaborations with third parties for the development and commercialization of our product candidate. If we fail to enter into such collaborations, or such collaborations are not successful, we may not be able to capitalize on the market potential of our product candidate.
We may seek third-party collaborators for development and commercialization of our product candidate. Our likely collaborators for any marketing, distribution, development, licensing or broader collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies, non-profit organizations, government agencies, and biotechnology companies. We are currently party to a limited number of such arrangements and have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidate. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidate currently pose, and will continue to pose, the following risks to us:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ● | collaborators may not pursue development and commercialization of our product candidate or may elect not to continue or renew development or commercialization programs based on preclinical or clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| 37 |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidate if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| ● | collaborators with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation; |
| ● | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| ● | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidate or that result in costly litigation or arbitration that diverts management attention and resources; and |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
Collaboration agreements may not lead to development or commercialization of our product candidate in the most efficient manner or at all. If a collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
If we are not able to establish collaborations, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our product candidate will require substantial additional cash to fund expenses. We may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of our product candidate.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of preclinical studies or clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under future license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of our product candidate, reduce or delay its development program, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidate or bring it to market and generate product revenue.
We manufacture our clinical supplies at a single location. Any disruption at this facility could adversely affect our business and results of operations.
We rely on our manufacturing partner, Guangxi Wuzhou Pharmaceuticals (Group) Co. Ltd., for the manufacture of clinical supply of VAL-083. If our partner’s facility were damaged or destroyed, or otherwise subject to disruption, it would require substantial lead-time to replace our clinical supply. In such event, we would be forced to rely entirely on other third-party contract manufacturers for an indefinite period of time. We have established a relationship with a back-up manufacturer, which has produced quantities of the active pharmaceutical ingredient contained in VAL-083. However, at this time no drug product has been manufactured by a third-party back-up manufacturer. Any disruptions or delays by Guangxi Wuzhou Pharmaceuticals or their failure to meet regulatory compliance could impair our ability to develop VAL-083, which would adversely affect our business and results of operations.
| 38 |
We may become subject to liabilities related to risks inherent in working with hazardous materials.
Our discovery and development processes involve the controlled use of hazardous and radioactive materials. We are subject to federal, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by such laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our resources. We are not specifically insured with respect to this liability. Although we believe that we are in compliance in all material respects with applicable environmental laws and regulations and currently do not expect to make material capital expenditures for environmental control facilities in the near-term, there can be no assurance that we will not be required to incur significant costs to comply with environmental laws and regulations in the future, or that our operations, business or assets will not be materially adversely affected by current or future environmental laws or regulations.
Risks Related to Our Common Stock
There is a limited trading market for our common stock, and shareholders may have difficulty trading and obtaining quotations for our common stock.
Our common stock is registered under the Exchange Act and is quoted on the OTCQX. Prior to January 25, 2013, there was no reported trading in our common stock. Since January 25, 2013, there has been limited trading in our common stock. As a result, investors may find it difficult to dispose of, or to obtain accurate quotations of the price of, our securities. This severely limits the liquidity of the common stock, and may adversely affect the market price of our common stock. A limited market may also impair our ability to raise capital by selling shares of capital stock and may impair our ability to acquire other companies or assets by using common stock as consideration.
The market price of our common stock is, and is likely to continue to be, highly volatile and subject to wide fluctuations.
The market price of our common stock is highly volatile and could be subject to wide fluctuations in response to a number of factors that are beyond our control, including:
| ● | variations in our quarterly operating results; |
| ● | announcements that our revenue or income are below analysts’ expectations; |
| ● | general economic slowdowns; |
| ● | sales of large blocks of our common stock; and |
| ● | announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments. |
Because we became public by means of a reverse acquisition, we may not be able to attract, or maintain, the attention of brokerage firms.
Because we became public through a “reverse acquisition”, securities analysts of brokerage firms may not provide or continue to provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any follow-on offerings on behalf of the Company in the future.
Our common stock is subject to the “penny stock” rules of the Securities and Exchange Commission, which may make it more difficult for stockholders to sell our common stock.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
| 39 |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person, and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination, and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of the Company’s common stock if and when such shares are eligible for sale and may cause a decline in the market value of its stock.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for the Company to retain or attract qualified officers and directors, which could adversely affect the management of its business and its ability to obtain or retain listing of its common stock.
The Company may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. The Company may have difficulty attracting and retaining directors with the requisite qualifications. If the Company is unable to attract and retain qualified officers and directors, the management of its business and its ability to obtain or retain listing of our shares of common stock on any stock exchange (assuming the Company elects to seek and are successful in obtaining such listing) could be adversely affected.
Voting power of our shareholders is highly concentrated by insiders.
Our officers and directors control, either directly or indirectly, a substantial portion of our voting securities. Therefore, our management may significantly affect the outcome of all corporate actions and decisions for an indefinite period of time including election of directors, amendment of charter documents and approval of mergers and other significant corporate transactions.
We do not intend to pay dividends for the foreseeable future.
We have paid no dividends on our common stock to date and we do not anticipate paying any dividends to holders of our common stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of the business, we currently anticipate that any earnings will be retained to finance our future expansion and for the implementation of our business plan. Investors should take note of the fact that a lack of a dividend can further affect the market value of our common stock, and could significantly affect the value of any investment in the Company.
Our articles of incorporation allow for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our Board of Directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our Board of Directors has the authority to issue up to 5,000,000 shares of our preferred stock (of which 1 share has been designated Special Voting Preferred Stock and is issued and outstanding, and 278,530 shares have been designated Series A Preferred Stock and are issued and outstanding) without further stockholder approval. As a result, our Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our Board of Directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders. Although we have no present intention to issue any additional shares of preferred stock or to create any additional series of preferred stock, we may issue such shares in the future.
| 40 |
Our issuance of common stock upon exercise of warrants or options or exchange of Exchangeable Shares may depress the price of our common stock.
As of June 30, 2015, we have 35,199,889 shares of common stock issued and outstanding, 4,256,042 shares of common stock issuable upon exchange of the Exchangeable Shares, outstanding warrants to purchase 13,472,870 shares of common stock, and outstanding options to purchase 3,595,000 shares of common stock. The issuance of shares of common stock upon exercise of outstanding warrants or options or exchange of Exchangeable Shares could result in substantial dilution to our stockholders, which may have a negative effect on the price of our common stock.
Additional stock offerings in the future may dilute then-existing shareholders’ percentage ownership of the Company.
Given our plans and expectations that we may need additional capital and personnel, we may need to issue additional shares of common stock or securities convertible or exercisable for shares of common stock, including convertible preferred stock, convertible notes, stock options or warrants. The issuance of additional securities in the future will dilute the percentage ownership of then current stockholders.
FORWARD-LOOKING STATEMENTS
Statements in this Annual Report on Form 10-K may be “forward-looking statements.” Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may, and are likely to, differ materially from what is expressed or forecasted in the forward-looking statements due to numerous factors, including those described above under “Risk Factors,” and under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this report and in other documents which we file with the Securities and Exchange Commission. In addition, such statements could be affected by risks and uncertainties related to our ability to raise any financing which we may require for our operations, competition, government regulations and requirements, pricing and development difficulties, our ability to make acquisitions and successfully integrate those acquisitions with our business, as well as general industry and market conditions and growth rates, and general economic conditions. Any forward-looking statements speak only as of the date on which they are made, and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of this report, except as required under applicable securities laws.
Item 1B. Unresolved Staff Comments.
Not required for a smaller reporting company.
Our corporate headquarters are located at Suite 720-999 West Broadway, Vancouver, British Columbia, Canada. Our clinical operations are managed at 3475 Edison Way, Suite R, Menlo Park, California, 94025. Our current monthly base rent for our corporate headquarters is $3,082 (CDN $3,850) under a one-year lease which will expire in June 2016. In addition, Valent, which is owned by Dr. Dennis Brown, our Chief Scientific Officer, leases facilities in California and we have access to such facilities pursuant to an informal unwritten arrangement with Valent. Our leased premises, academic relationships, and access to the Valent facility are sufficient to meet the immediate needs of our business, research and operations.
There are no legal proceedings to which the Company or any of its property is the subject.
Item 4. Mine Safety Disclosures.
Not applicable.
| 41 |
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock is quoted on the OTCQX, under the symbol “DMPI”. Previously, the Company’s common stock was quoted on the OTCQB. There was no reported trading in our common stock prior to January 25, 2013.
The following table sets forth the range of high and low bid prices of our common stock as reported and summarized on the OTCQB or OTCQX, as applicable, for the periods indicated. These prices are based on inter-dealer bid and asked prices, without markup, markdown, commissions, or adjustments and may not represent actual transactions.
| Calendar Quarter | High Bid | Low Bid | ||||||
| 2013 First Quarter | $ | 2.50 | $ | 1.30 | ||||
| 2013 Second Quarter | $ | 2.48 | $ | 1.55 | ||||
| 2013 Third Quarter | $ | 2.04 | $ | 0.90 | ||||
| 2013 Fourth Quarter | $ | 1.48 | $ | 0.75 | ||||
| 2014 First Quarter | $ | 1.60 | $ | 0.79 | ||||
| 2014 Second Quarter | $ | 1.41 | $ | 0.75 | ||||
| 2014 Third Quarter | $ | 1.03 | $ | 0.62 | ||||
| 2014 Fourth Quarter | $ | 1.02 | $ | 0.73 | ||||
| 2015 First Quarter | $ | 0.99 | $ | 0.64 | ||||
| 2015 Second Quarter | $ | 0.90 | $ | 0.64 | ||||
As of July 1, 2015, there were approximately 189 holders of record of the Company’s common stock.
Dividends
The Company has never declared or paid any cash dividends on its common stock. The Company currently intends to retain future earnings, if any, to finance the expansion of its business. As a result, the Company does not anticipate paying any cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth the aggregate information of our equity compensation plans in effect as of June 30, 2015:
| Plan | Number
of | Weighted-average | Number of securities | |||||||||
| Equity compensation plans approved by security holders | - | - | - | |||||||||
| Equity compensation plans not approved by security holders – Amended and Restated 2003 Employee Stock Option Plan | 3,595,000 | 0.94 | 644,285 | |||||||||
| Totals | 3,595,000 | 644,285 | ||||||||||
Sales of Unregistered Securities
During the year ended June 30, 2015, the Company issued 187,000 shares of common stock for services.
In connection with the foregoing, the Company relied upon the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended, for transactions not involving a public offering.
Issuer Purchases of Equity Securities
None.
| 42 |
Item 6. Selected Financial Data.
Not required for a smaller reporting company.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS FOR THE SIX MONTHS ENDED JUNE 30, 2014
This Management’s Discussion and Analysis (“MD&A”) contains “forward-looking statements”, which represent our projections, estimates, expectations or beliefs concerning, among other things, financial items that relate to management’s future plans or objectives or to our future economic and financial performance. In some cases, you can identify these statements by terminology such as “may”, “should”, “plans”, “believe”, “will”, “anticipate”, “estimate”, “expect” “project”, or “intend”, including their opposites or similar phrases or expressions. You should be aware that these statements are projections or estimates as to future events and are subject to a number of factors that may tend to influence the accuracy of the statements. These forward-looking statements should not be regarded as a representation by the Company or any other person that the events or plans of the Company will be achieved. You should not unduly rely on these forward-looking statements, which speak only as of the date of this report. Except as may be required under applicable securities laws, we undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this report or to reflect the occurrence of unanticipated events.
You should review the factors and risks we describe under “Risk Factors” elsewhere in this report on Form 10-K and in the Company’s other filings with the Securities and Exchange Commission, available at www.sec.gov. Actual results may differ materially from any forward-looking statement.
Overview
DelMar Pharmaceuticals, Inc. (the “Company”) is a clinical stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with our product candidate, VAL-083, as a potential new treatment for glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. In order to accelerate our development timelines and reduce technical risk, we leverage existing clinical and commercial data from a wide range of sources. We plan to seek marketing partnerships in China and elsewhere in order to supplement our own commercialization efforts and potentially generate future royalty revenue.
Corporate History
We are a Nevada corporation formed on June 24, 2009 under the name Berry Only, Inc. Prior to a reverse acquisition undertaken on January 25, 2013 (see note 1 to the June 30, 2015 consolidated financial statements) Berry did not have any significant assets or operations. The Company is the parent company of Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), a British Columbia, Canada corporation incorporated on April 6, 2010, that is focused on the development of drugs for the treatment of cancer. The Company is also the parent company to 0959454 B.C. Ltd., a British Columbia corporation (“Callco”), and 0959456 B.C. Ltd., a British Columbia corporation (“Exchangeco”). Callco and Exchangeco were formed to facilitate the reverse acquisition.
Pursuant to the reverse acquisition, the Company acquired (either directly or indirectly (through Exchangeco)) all of the issued and outstanding shares of DelMar (BC) on January 25, 2013. As a result of the shareholders of DelMar (BC) owning a controlling interest in the Company subsequent to the reverse acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is Berry. Therefore, the historic financial statements of DelMar (BC) are presented as the comparative balances for the periods prior to the reverse acquisition.
| 43 |
References to the Company, “we”, “us”, and “our” refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco.
We acquired intellectual property and prototype drug product related to our drug candidate, VAL-083, from Valent Technologies LLC (“Valent”) in September 2010 and initiated clinical trials in 2011.
Change in Fiscal Year End
On July 21, 2014, the Board of Directors of the Company approved a change in the Company’s fiscal year end from December 31 to June 30. As a result of this change, the Company has prepared consolidated financial statements for the years ended June 30, 2015 and 2014. References to any of the Company’s 2013 or earlier fiscal years mean the fiscal year ending December 31 of that calendar year.
Outstanding Securities
As of August 26, 2015, we have 39,477,556 shares of common stock issued and outstanding, 4,256,042 shares of common stock issuable upon exchange of the Exchangeable Shares (the Exchangeable shares are recognized as issued and outstanding for financial statement purposes), outstanding warrants to purchase 17,866,882 shares of common stock, and outstanding common stock options to purchase 3,595,000 shares of common stock. All Exchangeable shares, warrants, and options are convertible or exercisable into one share of common stock.
Derivative Liability
The Company has issued common stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated statement of operations and comprehensive loss.
CA$0.50 Unit Warrants
During the years ended December 31, 2012 and 2011 the Company issued a total of 5,410,000 units for services, settlement of accounts payable, and cash proceeds for an aggregate of $2,671,923 (CA$2,705,000). The proceeds from the issuance of 3,000,000 of these units were held in escrow pursuant to an exclusive option investment agreement with a strategic investor. Subsequently, the Company elected to allow the option to expire and the related units were cancelled and the funds returned from escrow to the subscriber in order for the Company to retain control over certain intellectual property and commercial rights.
During the year ended June 30, 2014, 241,000 of these warrants were exercised for no additional consideration for 241,000 shares of common stock with $259,315 of the derivative liability being reclassified to equity. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded. On January 25, 2014, the remaining 5,169,000 of these warrants expired.
| 44 |
Investor Warrants
In connection with the reverse acquisition, on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013, the Company entered into and closed a series of subscription agreements with accredited investors, pursuant to which the Company issued an aggregate of 13,125,002 units at a purchase price of $0.80 per unit, for aggregate gross proceeds of $10,500,000 (the “Private Offering”). Each unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. The Investor Warrants are redeemable by the Company at a price of $0.001 per Investor Warrant at any time subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.60 per share with an average trading volume of 50,000 shares per day and (ii) the underlying shares of common stock are registered.
Investor warrant exercises
On June 6, 2014, pursuant to an Election to Exercise Warrants agreement, the Company reduced the Investor Warrant exercise price from $0.80 to $0.65 per share for warrants to purchase 3,652,211 shares of the Company’s common stock. In accordance with the agreements, the holders of the Investor Warrants exercised the Investor Warrants for cash at the foregoing reduced exercise price. The Company received net proceeds of $2,255,240 after paying a 5% warrant agent fee of $118,697. As a result, $984,484 of the derivative liability has been reclassified to equity.
In addition, during the year ended June 30, 2014, 277,313 warrants were exercised at $0.80 per share for 277,313 shares of common stock. The Company received proceeds of $221,850 from the exercise. As a result, $126,064 of the derivative liability has been reclassified to equity.
Tender offer – Investor Warrant exercise price reduction
On June 9, 2014, as amended on June 26, 2014, July 10, 2014, and July 29, 2014, the Company filed a tender offer statement with the Securities and Exchange Commission with respect to the Investor Warrants to provide the holders thereof with the opportunity to amend and exercise their warrants, upon the terms and subject to the conditions set forth in the Company’s tender offer statement. Pursuant to the tender offer, the Company offered to amend Investor Warrants to purchase an aggregate of 9,195,478 shares of common stock (the “Offer to Amend and Exercise”). There was no minimum participation requirement with respect to the Offer to Amend and Exercise.
Pursuant to the Offer to Amend and Exercise, the Investor Warrants subject to the tender offer were amended (the “Amended Warrants”) to: (i) reduce the exercise price of the Investor Warrants from $0.80 per share to $0.65 per share of common stock in cash, (ii) shorten the exercise period of the Investor Warrants so that they expire concurrently with the expiration of the Offer to Amend and Exercise at 5:00 p.m. (Pacific Time) on August 8, 2014, as may be extended by the Company in its sole discretion (“Expiration Date”), (iii) delete the price-based anti-dilution provisions contained in the Investor Warrants, (iv) restrict the ability of the holder of shares issuable upon exercise of the Amended Warrants to sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of such shares without the prior written consent of the Company for a period of time twenty (20) days after the Expiration Date (the “ Lock-Up Period ”); and (v) provide that a holder, acting alone or with others, will agree not to effect any purchases or sales of any securities of the Company in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period.
Upon the expiry of the Offer to Amend and Exercise on August 8, 2014, 762,227 Amended Warrants were exercised for net proceeds of $470,676 after payment by the Company of a 5% warrant agent fee of $24,772. As a result, 8,433,251 Investor Warrants remained outstanding under their original terms subsequent to the tender offer.
| 45 |
In addition to the price reduction tender offer, during the year ended June 30, 2015, 1,223,847 Investor Warrants were exercised at $0.65 per share for 1,223,847 shares of common stock. The Company received proceeds of $795,501 from these exercises.
As a result of all of the Investor Warrants exercised for cash at $0.65 per warrant, including the tender offer relating to the price reduction to $0.65, a total of $391,422 of the derivative liability has been reclassified to equity. All Investor Warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded.
Investor Warrant exchange
On December 31, 2014, the Company issued 414,889 shares of common stock in exchange for 1,244,666 Investor Warrants. The Investor Warrants that have been exchanged were revalued at their exchange date and then a reclassification to equity was recorded. The reclassification to equity upon the exchange was $305,112. The Company recognized a loss of $92,843 at the time of the exchange.
Tender offer warrant exchange
On January 8, 2015, the Company filed a tender offer statement with the Securities and Exchange Commission, and on January 23, 2015, the Company filed an amendment thereto, with respect to certain Investor Warrants to purchase common stock of the Company. The tender offer provided the holders of the Investor Warrants with the opportunity to receive one share of common stock for every three Investor Warrants tendered. The tender offer was available to all 5,964,738 Investor Warrants outstanding on January 8, 2015. To participate in the tender offer the Investor Warrant holders were required to deliver completed exchange documents to the Company, prior to the expiration of the tender offer, which was 5:00 p.m. (Pacific Time) on February 9, 2015.
The tender offer expired on February 9, 2015. A total of 1,591,875 Investor Warrants were exchanged for 530,625 shares of common stock. The Investor Warrants that have been exchanged were revalued at their exchange date and then a reclassification to equity was recorded. The reclassification to equity upon the exchange was $423,723. The Company recognized a loss of $156,219 at the time of the exchange.
The remaining 4,372,863 Investor Warrants outstanding at June 30, 2015 have been re-valued at June 30, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 76.6%, risk free rate – 1.14% and a term of approximately 2.5 years.
All 4,372,863 Investor Warrants outstanding at June 30, 2015 have an exercise price of $0.80 at June 30, 2015. However, subsequent to June 30, 2015, the Company issued shares of common stock at $0.60 per share. As a result of the Investor Warrants being subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions, the 4,372,863 Investor Warrants outstanding at June 30, 2015 now have an exercise price of $0.786.
Dividend warrants
In connection with the reverse acquisition, warrants that Berry issued pursuant to a warrant dividend became warrants of the Company (the “Dividend Warrants”). The Dividend Warrants are exercisable at $1.25 per share until January 24, 2018. The Dividend Warrants will only be exercisable at such times as the underlying shares of common stock are registered. The Dividend Warrants will be redeemable by the Company at a price of $0.001 per Dividend Warrant at any time commencing 18 months following the date of issuance subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $2.50 per share and (ii) the underlying shares of common stock are registered. Subject to the conditions set forth therein, the Dividend Warrants may be redeemed by the Company upon not less than sixty (60) days nor more than ninety (90) days prior written notice.
| 46 |
On October 31, 2014, the Company and all of its Dividend Warrant holders entered into amendments to the Dividend Warrants such that the Company’s redemption rights and certain provisions of the Dividend Warrant agreements relating to potential cash settlement of the Dividend Warrants were removed. The Dividend Warrants were revalued to the date of the amendment on October 31, 2014 which resulted in a reclassification to equity of $975,278.
Warrants issued for services
During the year ended December 31, 2013, the Company issued 300,000 warrants for services. The warrants were issued on September 12, 2013 and are exercisable on a cashless basis at an exercise price of $1.76 for five years. The Company has recognized $124,020 in expense in the consolidated statement of operations.
The warrants have been measured at fair value at their issue date of June 30, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 76.7%, risk free rate - 1.31% and a term of approximately 3.25 years.
The Company’s derivative liability is summarized as follows:
June 30, | June 30, 2014 $ | |||||||
| Opening balance | 3,329,367 | 12,986,827 | ||||||
| Change in fair value of warrants | (179,170 | ) | (8,300,438 | ) | ||||
| Change in fair value due to change in warrant terms | (23,658 | ) | (111,179 | ) | ||||
| Reclassification to equity upon amendment of warrants | (975,278 | ) | - | |||||
| Warrants issued for services | - | 124,020 | ||||||
| Reclassification to equity upon exchange of warrants | (728,835 | ) | - | |||||
| Reclassification to equity upon exercise of warrants | (391,422 | ) | (1,369,863 | ) | ||||
| Closing balance | 1,031,004 | 3,329,367 | ||||||
Selected Annual Information
The financial information reported herein has been prepared in accordance with accounting principles generally accepted in the United States. The Company’s functional currency at June 30, 2015 is the USD. The following table represents selected financial information for the Company as of June 30, 2015 and June 30, 2014.
| 47 |
Selected Balance Sheet Data
June 30, 2015 $ | June 30, 2014 $ | |||||||
| Cash and cash equivalents | 1,754,433 | 4,759,711 | ||||||
| Working capital | 1,722,336 | 4,704,044 | ||||||
| Total assets | 2,575,421 | 5,003,910 | ||||||
| Derivative liability | 1,031,004 | 3,329,367 | ||||||
| Total stockholders’ equity | 511,887 | 880,479 | ||||||
Selected Statement of Operations Data
For the years ended:
| June 30, | June 30, | |||||||
| 2015 | 2014 | |||||||
| $ | $ | |||||||
| Research and development | 2,555,754 | 2,119,217 | ||||||
| General and administrative | 2,168,899 | 3,134,409 | ||||||
| Change in fair value of derivative liability | (179,170 | ) | (8,300,438 | ) | ||||
| Change in fair value of derivative liability due to change in warrant terms | (23,658 | ) | (111,179 | ) | ||||
| Loss on exchange of warrants | 249,062 | - | ||||||
| Foreign exchange loss | 23,415 | 22,581 | ||||||
| Interest expense | 2,091 | 8,140 | ||||||
| Interest income | (363 | ) | (2,078 | ) | ||||
| Net and comprehensive loss (income) | 4,796,030 | (3,129,348 | ) | |||||
| Basic weighted average number of shares outstanding | 38,067,516 | 31,969,595 | ||||||
| Basic loss (income) per share | 0.13 | (0.10 | ) | |||||
| Diluted weighted average number of shares outstanding | 38,067,516 | 39,090,331 | ||||||
| Diluted loss (income) per share | 0.13 | 0.00 | ||||||
Expenses net of share-based compensation expense
The following table discloses research and development, and general and administrative expenses net of share-based payment expenses.
For the years ended:
June 30, | June 30, | |||||||
| Research and development | 2,555,754 | 2,119,217 | ||||||
| Share-based compensation expense included in research and development | (36,284 | ) | (404,177 | ) | ||||
| Research and development net of share-based compensation | 2,519,470 | 1,715,040 | ||||||
| General and administrative | 2,168,899 | 3,134,409 | ||||||
| Share-based compensation expense included in general and administrative | (331,803 | ) | (1,239,787 | ) | ||||
| General and administrative net of share-based compensation | 1,837,096 | 1,894,622 | ||||||
| 48 |
Results of Operations
Comparison of the years ended June 30, 2015 and June 30, 2014
| Years Ended | ||||||||||||||||
June 30, | June 30, | Change | Change | |||||||||||||
| Research and development | 2,555,754 | 2,119,217 | 436,537 | 21 | ||||||||||||
| General and administrative | 2,168,899 | 3,134,409 | (965,510 | ) | (31 | ) | ||||||||||
| Change in fair value of derivative liability | (179,170 | ) | (8,300,438 | ) | 8,121,268 | (98 | ) | |||||||||
| Change in fair value of derivative liability due to change in warrant terms | (23,658 | ) | (111,179 | ) | 87,521 | (79 | ) | |||||||||
| Loss on exchange of warrants | 249,062 | - | 249,062 | - | ||||||||||||
| Foreign exchange loss | 23,415 | 22,581 | 834 | 4 | ||||||||||||
| Interest expense | 2,091 | 8,140 | (6,049 | ) | (74 | ) | ||||||||||
| Interest income | (363 | ) | (2,078 | ) | 1,715 | (83 | ) | |||||||||
| Net and comprehensive loss (income) | 4,796,030 | (3,129,348 | ) | 7,925,378 | ||||||||||||
Research and Development
Research and development expenses increased to $2,555,754 for the year ended June 30, 2015 from $2,119,217 for the year ended June 30, 2014. The increase was largely attributable to an increase in clinical development and intellectual property costs partially offset by a decrease in share-based compensation expense. Share-based compensation expense included in research and development for the year ended June 30, 2015 totalled $36,284 compared to $404,177 for the year ended June 30, 2014. In relation to research and development expenses during the year ended June 30, 2015 the Company incurred share-based compensation expense relating to stock option expense only. During the year ended June 30, 2014 the Company incurred expenses for stock options and the issuance of shares for services. The decrease in stock option expense in the current year was largely due to a decrease in the Company’s share price between 2015 and 2014.
Excluding the impact of share-based compensation expense, research and development expenses increased to $2,519,470 for the year ended June 30, 2015 from $1,715,040 for the year ended June 30, 2014.
Clinical development costs have increased due to higher support costs related to regulatory activities, drug manufacturing and clinical set up costs as the Company prepares for the fourteen patient expansion portion of its Phase I/II clinical study, the registration trial, and for activities relating to the preparation of protocols for the lung cancer and GBM studies in China. Intellectual property costs have increased in the year ended June 30, 2015 compared to the year ended June 30, 2014 as the Company has been active in both submitting patent applications and advancing its previously filed patents.
General and Administrative
General and administrative expenses were $2,168,899 for the year ended June 30, 2015 compared to $3,134,409 for the year ended June 30, 2014. The decrease was partially attributable to a decrease in share-based compensation expense to $331,803 in the year ended June 30, 2015 from $1,239,787 for the year ended June 30, 2014. In relation to general and administrative expenses during the year ended June 30, 2015, the Company incurred share-based compensation expense related to stock options and shares issued for services while during the year ended June 30, 2014 the Company incurred share-based compensation expense relating to stock options, and for shares and warrants issued for services. The decrease in stock option expense in the current period was largely due to a decrease in the Company’s share price in the current year compared to the corresponding period in 2014.
| 49 |
Excluding the impact of share-based compensation expense, general and administrative expenses remained relatively consistent decreasing slightly to $1,837,096 during the year ended June 30, 2015 from $1,894,622 for the year ended June 30, 2014. The principal reasons for the small decrease were lower professional fees partially offset by higher personnel, and facilities, office, and sundry costs. Professional fees were lower during the year ended June 30, 2015 compared to the year ended June 30, 2014 due to lower business development and investor relations costs. Personnel costs increased due to higher management fees and benefits in the current year compared to the corresponding period in 2014. Facilities, office, and sundry costs increased for the year ended June 30, 2015 compared to the year ended June 30, 2014 largely due an increase in promotion and press releases, and filing and related fees. The filings fees related to the Company listing its common stock on the OTCQX.
Change in fair value of derivative liability
Based on the terms of certain warrants issued by the Company, the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value every reporting period with gains or losses on the changes in fair value recorded in the consolidated statement of loss and comprehensive loss. The balances recognized during the years ended June 30, 2015 and 2014 were primarily due to changes in the Company’s common stock price between the date the warrants were last valued and due to changes in assumptions used in the valuation model.
The Company recognized a gain of $179,170 during the year ended June 30, 2015 and a gain of $8,300,438 during the year ended June 30, 2014 from the revaluation of the derivative liability. In addition, as result of amending the Investor Warrants and Dividend Warrants during the period ended June 30, 2015, the Company also recognized a gain of $23,658. All warrants that have been exercised or amended were revalued at their respective exercise or amendment dates and then the reclassification to equity was recorded. Also, during the year ended June 30, 2015, the Company exchanged certain Investor Warrants for shares of common stock resulting in the recognition of a loss of $249,062 on the exchange.
Changes in the Company’s common stock price and assumptions used in the valuation model can result in significant volatility in the Company’s reported net loss due to its impact on the fair value of the derivative liability. As a result of revaluation gains and losses, the Company expects that its reported net income or loss will continue to fluctuate significantly.
Foreign Exchange Gain
The Company’s functional currency at June 30, 2015 is the USD but the Company incurs a portion of its expenses in CDN. The foreign exchange gains and losses are reported in other loss (income) in the consolidated statement of loss and comprehensive loss.
The Company recognized a foreign exchange loss of $23,415 for the year ended June 30, 2015 compared to a loss of $22,581 for the year ended June 30, 2014. The change was due to changes in the exchange rate between the CDN and the USD and to varying levels of CDN cash and accounts payable.
| 50 |
Interest Expense
Pursuant to a loan agreement dated February 3, 2011, the Company received a loan from Valent in the amount of $250,000 for the purchase of the prototype drug product. The loan was payable on demand, unsecured and bore interest at 3% per year. Effective September 30, 2014 the loan balance, including accumulated interest to September 30, 2014, was exchanged for 278,530 shares of the Company’s Series A Preferred Stock. The Series A Preferred Stock pays dividends at the rate of 3% per year, payable quarterly in arrears.
For the year ended June 30, 2015, the Company has recognized $6,267 related to the dividend payable to Valent and $2,091 related to interest from July 1, 2014 to September 30, 2014 when the loan was exchanged for preferred shares. The dividend has been recorded as a direct increase in accumulated deficit and the $2,091 has been recognized as interest expense. For the year ended June 30, 2014 the Company accrued $8,140 in interest on the loan payable with Valent.
Related Parties
During the year ended June 30, 2015
Effective September 30, 2014, the Company entered into and closed an agreement with Valent to exchange its loan with Valent for 278,530 shares of preferred stock of the Company.
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $505,000 in compensation expense for the year ended June 30, 2015.
Included in accounts payable at June 30, 2015 is an aggregate amount of $90,820 owed to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
The Company recognized $119,417 in directors’ fees during the year ended June 30, 2015.
During the year ended June 30, 2014
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $407,196 in compensation expense for the year ended June 30, 2014.
The Company recognized $77,833 in directors’ fees during the year ended June 30, 2014.
Liquidity and Capital Resources
Year ended June 30, 2015 compared to the year ended June 30, 2014
June 30, | June 30, | Change $ | Change % | |||||||||||||
| Cash used in operating activities | (3,853,069 | ) | (4,004,031 | ) | 150,962 | (4 | ) | |||||||||
| Cash flows from financing activities | 847,791 | 2,480,750 | (1,632,959 | ) | (66 | ) | ||||||||||
| 51 |
Operating Activities
Net cash used in operating activities decreased to $3,853,069 for the year ended June 30, 2015 from $4,004,031 for the year ended June 30, 2014. During the year ended June 30, 2015 the Company reported a loss of $4,796,030 compared to an income of $3,129,348 for the year ended June 30, 2014. However, included in the net income in 2014 was a gain of $8,300,438 attributable to changes in the fair value of the derivative liability. During the year ended June 30, 2015, the Company recognized a gain of $179,170 from changes in the fair value of the derivative liability. Excluding the impact of changes in the fair value of the derivative liability, non-cash items relating to accrued interest, gains from amending the terms of certain warrants, losses from the exchange of warrants, and stock-based compensation totaled $595,582 for the year ended June 30, 2015. Non-cash items relating to accrued interest, gains from amending the terms of certain warrants, warrants issued for services, and share-based compensation totaled $1,540,925 for the year ended June 30, 2014. The most significant change in non-cash working capital for the year ended June 30, 2015 was due to an increase in accounts payable and accrued liabilities of $517,359. In the year ended June 30, 2014 the most significant items were due to reductions in related party payables and accounts payable and accrued liabilities of $181,502 and $186,321, respectively.
Financing Activities
The Company received net proceeds of $1,404,177 from the exercise of warrants during the year ended June 30, 2015. The Company also incurred deferred costs of $550,119 relating to the financing the Company completed subsequent to June 30, 2015. In addition, the Company recognized $6,267 in dividends on the Series A Preferred Stock issued to Valent. During the year ended June 30, 2014 the Company received net proceeds of $2,480,750 from the exercise of warrants.
Operating Capital and Capital Expenditure Requirements
Going concern
(See note 1 to the consolidated financial statements)
The financial statements have been prepared on a going concern basis which assumes that the Company will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities in the normal course of business.
For the year ended June 30, 2015, the Company reported a loss of $4,796,030, negative cash flow from operations of $3,853,069 (2014 - $4,004,031) and an accumulated deficit of $23,465,711 at that date. As at June 30, 2015, the Company has cash and cash equivalents on hand of $1,754,433 and a working capital balance of $1,722,336. The Company has not begun to generate revenues from its product candidate and the Company does not have the prospect of achieving revenues in the near future. The Company will require additional funding to maintain its research and development projects and for general operations. These circumstances indicate the existence of a material uncertainty that casts substantial doubt as to the ability of the Company to meet its obligations as they come due.
Consequently, management is pursuing various financing alternatives to fund the Company’s operations so it can continue as a going concern. Subsequent to June 30, 2015 the Company received net proceeds of approximately $1.9 million from a registered-direct offering of its securities (see note 12 to the consolidated financial statements). Management plans to secure the necessary additional financing through the issue of new equity and/or the entering into of strategic partnership arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
The financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
| 52 |
Our future funding requirements will depend on many factors, including but not limited to:
| ● | the rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities; |
| ● | the costs associated with establishing manufacturing and commercialization capabilities; |
| ● | the costs of acquiring or investing in businesses, product candidates and technologies; |
| ● | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| ● | the costs and timing of seeking and obtaining FDA and other regulatory approvals; |
| ● | the effect of competing technological and market developments; and |
| ● | the economic and other terms and timing of any collaboration, licensing or other arrangements into which we may enter. |
Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs primarily through public or private equity offerings, debt financings or strategic collaborations. Although we are not reliant on institutional credit finance and therefore not subject to debt covenant compliance requirements or potential withdrawal of credit by banks, the current economic climate has also impacted the availability of funds and activity in equity markets. We do not know whether additional funding will be available on acceptable terms, or at all. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or make changes to our operating plan. In addition, we may have to seek a partner for one or more of our product candidate programs at an earlier stage of development, which would lower the economic value of those programs to us.
Critical Accounting Policies
The preparation of financial statements, in conformity with generally accepted accounting principles in the United States, requires companies to establish accounting policies and to make estimates that affect both the amount and timing of the recording of assets, liabilities, revenues and expenses. Some of these estimates require judgments about matters that are inherently uncertain and therefore actual results may differ from those estimates.
A detailed presentation of all of the Company’s significant accounting policies and the estimates derived therefrom is included in note 3 to the Company’s consolidated financial statements. While all of the significant accounting policies are important to the Company’s consolidated financial statements, the following accounting policies and the estimates derived therefrom have been identified as being critical:
| ● | Shares for services | |
| ● | Stock options | |
| ● | Derivative liability |
Shares for services
Periodically, the Company has issued equity instruments for services provided by employees and nonemployees. The equity instruments are valued at the fair value of the instrument granted.
| 53 |
Stock options
The Company accounts for these awards under ASC 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of the Company’s share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on the Company’s historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based compensation expense. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies warrants in its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. As quoted prices for the derivative liability are not available, the Company uses a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants require considerable judgment. Any change in the estimates used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
Off-Balance Sheet Arrangements
We do not have any off balance sheet arrangements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not required for a smaller reporting company.
| 54 |
DelMar Pharmaceuticals, Inc.
Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| F-1 |

September 3, 2015
Independent Auditor’s Report
To the Shareholders of DelMar Pharmaceuticals, Inc.
We have audited the accompanying consolidated financial statements of DelMar Pharmaceuticals, Inc. and its subsidiaries, which comprise the consolidated balance sheets as at June 30, 2015 and June 30, 2014 and the consolidated statements of operations and comprehensive loss, changes in stockholders’ equity (deficiency) and consolidated cash flows for the years then ended, and the related notes, which comprise a summary of significant accounting policies and other explanatory information.
Management’s responsibility for the consolidated financial statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with accounting principles generally accepted in the United States of America and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement. Canadian generally accepted auditing standards also require that we comply with ethical requirements.
An audit involves performing procedures to obtain audit evidence, on a test basis, about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. We were not engaged to perform an audit of the company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting principles and policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
PricewaterhouseCoopers LLP
PricewaterhouseCoopers
Place, 250 Howe Street, Suite 700, Vancouver, British Columbia, Canada V6C 3S7
T: +1 604 806 7000, F: +1 604 806 7806, www.pwc.com/ca
“PwC” refers to PricewaterhouseCoopers LLP, an Ontario limited liability partnership.
| F-2 |

Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of DelMar Pharmaceuticals, Inc. and its subsidiaries as at June 30,2015 and June 30,2014 and the result of their operations and their cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America.
Emphasis of matter
The accompanying consolidate financial statements have been prepared assuming that DelMar Pharmaceuticals, Inc. will continue as a going concern. As discussed in note 1 to the consolidated financial statements, there is substantial doubt about DelMar Pharmaceuticals, Inc.’s ability to continue as a going concern. Management’s plans in regards to these matters are described in note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Yours very truly,
PricewaterhouseCoopers LLP
Chartered
Professional Accountants
| F-3 |
DelMar Pharmaceuticals, Inc.
Consolidated Balance Sheet
(in US dollars unless otherwise noted)
Note | June 30, 2015 $ | June 30, 2014 $ | ||||||||||
| Assets | ||||||||||||
| Current assets | ||||||||||||
| Cash and cash equivalents | 1,754,433 | 4,759,711 | ||||||||||
| Taxes and other receivables | 5 | 25,831 | 9,572 | |||||||||
| Prepaid expenses | 245,038 | 234,627 | ||||||||||
| Deferred costs | 12 | 550,119 | - | |||||||||
| 2,575,421 | 5,003,910 | |||||||||||
| Liabilities | ||||||||||||
| Current liabilities | ||||||||||||
| Accounts payable and accrued liabilities | 762,265 | 244,906 | ||||||||||
| Related party payables | 8 | 90,820 | 54,960 | |||||||||
| 853,085 | 299,866 | |||||||||||
| Loan payable to Valent | 4 | - | 276,439 | |||||||||
| Stock option liability | 7 | 179,445 | 217,759 | |||||||||
| Derivative liability | 6 | 1,031,004 | 3,329,367 | |||||||||
| 2,063,534 | 4,123,431 | |||||||||||
| Stockholders’ Equity | ||||||||||||
| Preferred stock | ||||||||||||
| Authorized | ||||||||||||
| 5,000,000 shares, $0.001 par value | ||||||||||||
| Issued and outstanding | ||||||||||||
| 278,530 Series A shares at June 30, 2015 (June 30, 2014 – none) | 4 | 278,530 | - | |||||||||
| 1 special voting share at June 30, 2015 (June 30, 2014 – 1) | - | - | ||||||||||
| Common stock | ||||||||||||
| Authorized | ||||||||||||
| 200,000,000 shares, $0.001 par value | ||||||||||||
| 39,455,931 issued at June 30, 2015 (June 20, 2014 – 35,992,343) | 7 | 39,456 | 35,992 | |||||||||
| Additional paid-in capital | 7 | 17,500,008 | 13,286,278 | |||||||||
| Warrants | 7 | 6,138,426 | 6,200,445 | |||||||||
| Accumulated deficit | (23,465,711 | ) | (18,663,414 | ) | ||||||||
| Accumulated other comprehensive income | 21,178 | 21,178 | ||||||||||
| 511,887 | 880,479 | |||||||||||
| 2,575,421 | 5,003,910 | |||||||||||
Going concern, nature of operations, and corporate history (note 1) | ||||||||||||
| Commitments and contingencies (note 10) | ||||||||||||
| Subsequent events (note 12) |
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
DelMar Pharmaceuticals, Inc.
Consolidated Statement of Operations and Comprehensive Loss
(in US dollars unless otherwise noted)
Note | Year ended June 30, 2015 $ | Year ended June 30, 2014 $ | ||||||||||
| Expenses | ||||||||||||
| Research and development | 2,555,754 | 2,119,217 | ||||||||||
| General and administrative | 2,168,899 | 3,134,409 | ||||||||||
| 4,724,653 | 5,253,626 | |||||||||||
| Other loss (income) | ||||||||||||
| Change in fair value of derivative liability | 6 | (179,170 | ) | (8,300,438 | ) | |||||||
| Change in fair value of derivative liability due to change in warrant terms | 6 | (23,658 | ) | (111,179 | ) | |||||||
| Loss on exchange of warrants | 6 | 249,062 | - | |||||||||
| Foreign exchange loss | 23,415 | 22,581 | ||||||||||
| Interest expense | 4 | 2,091 | 8,140 | |||||||||
| Interest income | (363 | ) | (2,078 | ) | ||||||||
| 71,377 | (8,382,974 | ) | ||||||||||
| Net and comprehensive loss (income) for the year | 4,796,030 | (3,129,348 | ) | |||||||||
| Basic loss (income) per share | 0.13 | (0.10 | ) | |||||||||
| Diluted loss (income) per share | 0.13 | 0.00 | ||||||||||
| Basic weighted average number of shares | 38,067,516 | 31,969,595 | ||||||||||
| Diluted weighted average number of shares | 38,067,516 | 39,090,331 |
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
DelMar Pharmaceuticals, Inc.
Consolidated Statement of Changes in Stockholders' Equity (Deficiency)
(in US dollars unless otherwise noted)
Number of shares | Common stock $ | Additional paid-in capital $ | Accumulated other comprehensive income $ | Preferred $ | Warrants $ | Accumulated deficit $ | Stockholders' equity (deficiency) $ | |||||||||||||||||||||||||
| Balance – June 30, 2013 | 31,150,009 | 31,150 | 7,585,810 | 21,178 | - | 6,441,700 | (21,792,762 | ) | (7,712,924 | ) | ||||||||||||||||||||||
| Exercise of placement agent warrants (note 7) | 123,810 | 124 | 239,476 | - | - | (239,600 | ) | - | - | |||||||||||||||||||||||
| Exercise of CA$0.50 unit warrants (note 7) | 241,000 | 241 | 259,074 | - | - | - | - | 259,315 | ||||||||||||||||||||||||
| Exercise of Investor Warrants net of cash issue costs (note 7) | 3,929,524 | 3,929 | 2,473,161 | - | - | - | - | 2,477,090 | ||||||||||||||||||||||||
| Reclassification of derivative liability to equity upon exercise of Investor Warrants (note 6) | - | - | 1,110,548 | - | - | - | - | 1,110,548 | ||||||||||||||||||||||||
| Exercise of CA$0,50 broker warrants (note 7) | 8,000 | 8 | 4,751 | - | - | (1,099 | ) | - | 3,660 | |||||||||||||||||||||||
| Expiry of broker warrants (note 7) | - | - | 556 | - | - | (556 | ) | - | - | |||||||||||||||||||||||
| Shares issued for services (note 7) | 540,000 | 540 | 632,960 | - | - | - | - | 633,500 | ||||||||||||||||||||||||
| Stock-based compensation (note 7) | - | - | 979,942 | - | - | - | - | 979,942 | ||||||||||||||||||||||||
| Income for the period | - | - | - | - | - | - | 3,129,348 | 3,129,348 | ||||||||||||||||||||||||
| Balance - June 30, 2014 | 35,992,343 | 35,992 | 13,286,278 | 21,178 | - | 6,200,445 | (18,663,414 | ) | 880,479 | |||||||||||||||||||||||
| Exercise of Investor Warrants net of cash issue costs (note 7) | 1,986,074 | 1,986 | 1,264,191 | - | - | - | - | 1,266,177 | ||||||||||||||||||||||||
| Reclassification of derivative liability to equity upon exercise of Investor Warrants (note 6) | - | - | 391,422 | - | - | - | - | 391,422 | ||||||||||||||||||||||||
| Shares issued upon the exchange of warrants (note 6) | 945,514 | 946 | 976,951 | - | - | - | - | 977,897 | ||||||||||||||||||||||||
| Reclassification of derivative liability to equity upon amendment of warrant terms (note 6) | - | - | 975,278 | - | - | - | - | 975,278 | ||||||||||||||||||||||||
| Reclassification of stock option liability (note 7) | - | - | 38,038 | - | - | - | - | 38,038 | ||||||||||||||||||||||||
| Exercise of CA $0.50 broker warrants (note 7 ) | 345,000 | 345 | 187,034 | - | - | (49,379 | ) | - | 138,000 | |||||||||||||||||||||||
| Expiration of broker warrants (note 7) | - | - | 12,640 | - | - | (12,640 | ) | - | - | |||||||||||||||||||||||
| Issuance of Series A preferred stock (note 4) | - | - | - | - | 278,530 | - | - | 278,530 | ||||||||||||||||||||||||
| Shares issued for services (note 7) | 187,000 | 187 | 181,000 | - | - | - | - | 181,187 | ||||||||||||||||||||||||
| Stock-based compensation (note 7) | - | - | 187,176 | - | - | - | - | 187,176 | ||||||||||||||||||||||||
| Series A preferred stock dividend (note 4) | - | - | - | - | - | - | (6,267 | ) | (6,267 | ) | ||||||||||||||||||||||
| Loss for the period | - | - | - | - | - | - | (4,796,030 | ) | (4,796,030 | ) | ||||||||||||||||||||||
| Balance - June 30, 2015 | 39,455,931 | 39,456 | 17,500,008 | 21,178 | 278,530 | 6,138,426 | (23,465,711 | ) | 511,887 | |||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
DelMar Pharmaceuticals, Inc.
Consolidated Statement of Cash Flows
(in US dollars unless otherwise noted)
Year ended June 30, 2015 $ | Year ended June 30, 2014 $ | |||||||
| Cash flows from operating activities | ||||||||
| (Loss) income for the period | (4,796,030 | ) | 3,129,348 | |||||
| Items not affecting cash | ||||||||
| Accrued interest | 2,091 | 8,140 | ||||||
| Change in fair value of derivative liability | (179,170 | ) | (8,300,438 | ) | ||||
| Change in fair value of derivative liability due change in warrant terms | (23,658 | ) | (111,179 | ) | ||||
| Loss on exchange of warrants | 249,062 | - | ||||||
| Warrants issued for services | - | 124,020 | ||||||
| Stock-based compensation | 368,087 | 1,519,944 | ||||||
| (4,379,618 | ) | (3,630,165 | ) | |||||
| Changes in non-cash working capital | ||||||||
| Taxes and other receivables | (16,259 | ) | 7,322 | |||||
| Prepaid expenses | (10,411 | ) | (13,365 | ) | ||||
| Accounts payable and accrued liabilities | 517,359 | (186,321 | ) | |||||
| Related party payables | 35,860 | (181,502 | ) | |||||
| 526,549 | (373,866 | ) | ||||||
| (3,853,069 | ) | (4,004,031 | ) | |||||
| Cash flows from financing activities | ||||||||
| Net proceeds from the exercise of warrants | 1,404,177 | 2,480,750 | ||||||
| Deferred costs | (550,119 | ) | - | |||||
| Series A preferred stock dividend | (6,267 | ) | - | |||||
| 847,791 | 2,480,750 | |||||||
| Increase (decrease) in cash and cash equivalents | (3,005,278 | ) | (1,523,281 | ) | ||||
| Cash and cash equivalents - beginning of year | 4,759,711 | 6,282,992 | ||||||
| Cash and cash equivalents - end of year | 1,754,433 | 4,759,711 | ||||||
| Supplementary information | ||||||||
| Issuance of preferred shares for the settlement of the loan payable with Valent (note 4) | 278,530 | - | ||||||
| Reclassification of derivative liability to equity upon the exercise of Investor Warrants (note 6) | 391,422 | 1,110,548 | ||||||
| Reclassification of derivative liability to equity upon the exchange of Investor Warrants (note 6) | 728,835 | - | ||||||
| Reclassification of derivative liability to equity upon the amendment of Dividend Warrants (note 6) | 975,278 | - | ||||||
| Reclassification of stock option liability to equity upon the forfeiture of stock options (note 6) | 38,038 | - | ||||||
| Exercise of CA$0.50 warrants for no additional consideration (note 6) | - | 259,315 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-7 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 1 | Going concern, nature of operations, and corporate history |
Going concern
These
financial statements have been prepared on a going concern basis which assumes that DelMar Pharmaceuticals, Inc. (the “Company”)
will continue its operations for the foreseeable future and contemplates the realization of assets and the settlement of liabilities
in the normal course of business.
For the year ended June 30, 2015, the Company reported a loss of $4,796,030, negative cash
flow from operations of $3,853,069 (2014 - $4,004,031) and an accumulated deficit of $23,465,711 at that date. As at June 30,
2015, the Company has cash and cash equivalents on hand of $1,754,433 and a working capital balance of $1,722,336. The Company
has not begun to generate revenues from its product candidate and the Company does not have the prospect of achieving revenues
in the near future. The Company will require additional funding to maintain its research and development projects and for general
operations. These circumstances indicate the existence of a material uncertainty that casts substantial doubt as to the ability
of the Company to meet its obligations as they come due.
Consequently, management is pursuing various financing alternatives to fund the Company’s
operations so it can continue as a going concern. Subsequent to June 30, 2015 the Company received net proceeds of approximately
$1.9 million from a registered-direct offering of common stock and common stock purchase warrants (note 12). Management plans
to secure the necessary additional financing through the issue of new equity and/or the entering into of strategic partnership
arrangements. Nevertheless, there is no assurance that these initiatives will be successful.
These financial statements do not give effect to any adjustments to the amounts and classification of assets and liabilities that may be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
Nature of operations
The Company is a clinical stage drug development company with a focus on the treatment of cancer. We are conducting clinical trials in the United States with our product candidate, VAL-083, as a potential new treatment for glioblastoma multiforme (“GBM”), the most common and aggressive form of brain cancer. We have also acquired certain exclusive commercial rights to VAL-083 in China where it is approved as a chemotherapy for the treatment of chronic myelogenous leukemia (“CML”) and lung cancer. In order to accelerate our development timelines and reduce technical risk, we leverage existing clinical and commercial data from a wide range of sources. We plan to seek marketing partnerships in China in order to potentially generate future royalty revenue.
The address of the Company’s administrative offices is Suite 720 - 999 West Broadway, Vancouver, British Columbia, V5Z 1K5 with clinical operations located at 3485 Edison Way, Suite R, Menlo Park, California, 94025.
Corporate history
The Company is a Nevada corporation formed on June 24, 2009 under the name Berry Only Inc. On January 25, 2013 (the “Closing Date”), the Company entered into and closed an exchange agreement (the “Exchange Agreement”), with Del Mar Pharmaceuticals (BC) Ltd. (“DelMar (BC)”), 0959454 B.C. Ltd. (“Callco”), and 0959456 B.C. Ltd. (“Exchangeco”) and the security holders of DelMar (BC).
| F-8 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Upon completion of the Exchange Agreement, DelMar (BC) became a wholly-owned subsidiary of the Company (the “Reverse Acquisition”). As a result of the shareholders of DelMar (BC) having a controlling interest in the Company subsequent to the Reverse Acquisition, for accounting purposes the transaction is a capital transaction with DelMar (BC) being the accounting acquirer even though the legal acquirer is Berry. No goodwill was recorded with respect to the transaction as it did not constitute a business combination. For accounting purposes, the transaction is reflected as a recapitalization of DelMar (BC) and consideration for the Reverse Acquisition was deemed to be the fair value of the shares that were issued by DelMar (BC) to acquire the net liabilities of Berry on January 25, 2013. Therefore, the historic financial statements of DelMar (BC) are presented as the comparative balances for the periods prior to the Reverse
Acquisition.
DelMar Pharmaceuticals, Inc. is the parent company of DelMar (BC), a British Columbia, Canada corporation incorporated on April 6, 2010, which is a clinical stage company with a focus on the development of drugs for the treatment of cancer. The Company is also the parent company to Callco and Exchangeco which are British Columbia, Canada corporations. Callco and Exchangeco were formed to facilitate the Reverse Acquisition.
References to the Company refer to the Company and its wholly-owned subsidiaries, DelMar (BC), Callco and Exchangeco.
| F-9 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 2 | Change in fiscal year end |
On July 21, 2014, the Board of Directors of the Company approved a change in the Company’s fiscal year end from December 31 to June 30. As a result of this change, the Company has prepared consolidated financial statements for the years ended June 30, 2015 and 2014. References to any of the Company’s 2013 or earlier fiscal years mean the fiscal year ending December 31 of that calendar year.
| 3 | Significant accounting policies |
Basis of presentation
The financial statements of the Company have been prepared in accordance with United States generally accepted accounting principles (“US GAAP”) and are presented in United States dollars. The Company’s functional currency is the United States dollar.
The principal accounting policies applied in the preparation of these consolidated financial statements are set out below and have been consistently applied to all periods presented.
Consolidation
The consolidated financial statements include the accounts of Del Mar (BC), Callco, and Exchangeco as of and for the period ended June 30, 2015. Inter-company balances and transactions have been eliminated on consolidation.
Use of estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions about future events that affect the reported amounts of assets, liabilities, expenses, contingent assets and contingent liabilities as at the end or during the reporting period. Actual results could significantly differ from those estimates. Significant areas requiring management to make estimates include the derivative liability and the valuation of equity instruments issued for services. Further details of the nature of these assumptions and conditions may be found in the relevant notes to these consolidated financial statements.
Cash and cash equivalents
Cash and cash equivalents consist of cash on deposit and highly liquid short-term interest bearing securities with maturities at the date of purchase of three months or less. Cash and cash equivalents are held at recognized Canadian and United States financial institutions. Interest earned is recognized in the consolidated statement of operations and comprehensive loss.
Foreign currency translation
The functional currency of the Company at June 30, 2015 is the United States dollar. Transactions that are denominated in a foreign currency are re-measured into the functional currency at the current exchange rate on the date of the transaction. Any foreign-currency denominated monetary assets and liabilities are subsequently re-measured at current exchange rates, with gains or losses recognized as foreign exchange losses or gains in the consolidated statement of operations and comprehensive loss. Non-monetary assets and liabilities are translated at historical exchange rates. Expenses are translated at average exchange rates during the period. Exchange gains and losses are included in consolidated statement of operations and comprehensive loss for the period.
| F-10 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Current and future income taxes
The Company follows the liability method of accounting for income taxes. Under this method, current income taxes are recognized for the estimated income taxes payable for the current period. Income taxes are accounted for using the asset and liability method of accounting. Future income taxes are recognized for the future income tax consequences attributable to differences between the carrying values of assets and liabilities and their respective income tax bases and for loss carry-forwards. Future income tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the periods in which temporary differences are expected to be recovered or settled. The effect on future income tax assets and liabilities of a change in tax laws or rates is included in earnings in the period that includes the enactment date. When realization of future income tax assets does not meet the more-likely-than-not criterion for recognition, a valuation allowance is provided.
Financial instruments
The Company has financial instruments that are measured at fair value. To determine the fair value, we use the fair value hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use to value an asset or liability and are developed based on market data obtained from independent sources. Unobservable inputs are inputs based on assumptions about the factors market participants would use to value an asset or liability. The three levels of inputs that may be used to measure fair value are as follows:
| ● | Level one - inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| ● | Level two - inputs are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly such as interest rates, foreign exchange rates, and yield curves that are observable at commonly quoted intervals; and |
| ● | Level three - unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy.
The Company’s financial instruments consist of cash and cash equivalents, taxes and other receivables, accounts payable and accrued liabilities, related party payables and derivative liability. The carrying values of cash and cash equivalents, taxes and other receivables, accounts payable and accrued liabilities and related party payables approximate their fair values due to the immediate or short-term maturity of these financial instruments.
| F-11 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Derivative liability
The Company accounts for certain warrants under the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock, on the understanding that in compliance with applicable securities laws, the warrants require the issuance of securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. The Company classifies these warrants on its balance sheet as a derivative liability which is fair valued at each reporting period subsequent to the initial issuance. The Company has used a simulated probability valuation model to value the warrants. Determining the appropriate fair-value model and calculating the fair value of warrants requires considerable judgment. Any change in the estimates (specifically probabilities) used may cause the value to be higher or lower than that reported. The estimated volatility of the Company’s common stock at the date of issuance, and at each subsequent reporting period, is based on the historical volatility of similar life sciences companies. The risk-free interest rate is based on rates published by the government for bonds with a maturity similar to the expected remaining life of the warrants at the valuation date. The expected life of the warrants is assumed to be equivalent to their remaining contractual term.
| a) | Fair value of derivative liability |
The derivative is not
traded in an active market and the fair value is determined using valuation techniques. The Company uses judgment to select a
variety of methods to make assumptions that are based on specific management plans and market conditions at the end of each reporting
period. The Company uses a fair value estimate to determine the fair value of the derivative liability. The carrying value of
the derivative liability would be higher or lower as management estimates around specific probabilities change. The estimates
may be significantly different from those recorded in the consolidated financial statements because of the use of judgment and
the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market. All changes
in the fair value are recorded in the consolidated statement of operations and comprehensive loss each reporting period. This
is considered to be a Level 2 financial instrument.
The Company has the following liabilities under the fair value hierarchy:
| June 30, 2015 | ||||||||||||
| Liability | Level 1 | Level 2 | Level 3 | |||||||||
| Derivative liability | - | 1,031,004 | - | |||||||||
| June 30, 2014 | ||||||||||||
| Liability | Level 1 | Level 2 | Level 3 | |||||||||
| Derivative liability | - | 3,329,367 | - | |||||||||
| F-12 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Intangible assets
Expenditures associated with the filing, or maintenance of patents, licensing or technology agreements are expensed as incurred. Costs previously recognized as an expense are not recognized as an asset in subsequent periods.
Once the technology has achieved commercialization, patent costs will be deferred and amortized over the remaining life of the related patent.
Research and development costs (including clinical trial expenses)
Research and development expenses include payroll, employee benefits, stock-based compensation expense, and other headcount-related expenses associated with product research and development. Research and development expenses also include third-party development and clinical trial expenses noted bel0w. Such costs related to product research and development are included in research and development expense until the point that technological feasibility is reached, which for our drug candidate, is generally shortly before the drug is approved by the relevant food and drug administration. Once technological feasibility is reached, such costs are capitalized and amortized to cost of revenue over the estimated life of the product.
Clinical trial expenses are a component of research and development costs and include fees paid to contract research organizations, investigators and other service providers who conduct specific research for product development activities on behalf of the Company. The amount of clinical trial expenses recognized in a period related to service agreements is based on estimates of the work performed on an accrual basis. These estimates are based on patient enrollment, services provided and goods delivered, contractual terms and experience with similar contracts. The Company monitors these factors to the extent possible and adjusts our estimates accordingly. Prepaid expenses or accrued liabilities are adjusted if payments to service providers differ from estimates of the amount of service completed in a given period.
Research and development costs are expensed in the period incurred. At June 30, 2015 and 2014 all research and development costs have been expensed.
Shares for services
The Company has issued equity instruments for services provided by employees and non-employees. The equity instruments are valued at the fair value of the instrument granted (see notes 6 and 7 for assumptions).
Stock options
The Company accounts for these awards under Accounting Standards Codification (“ASC”) 718, “Compensation - Stock Compensation” (“ASC 718”). ASC 718 requires measurement of compensation cost for all stock-based awards at fair value on the date of grant and recognition of compensation over the requisite service period for awards expected to vest. Compensation expense for unvested options to non-employees is revalued at each period end and is being amortized over the vesting period of the options. The determination of grant-date fair value for stock option awards is estimated using the Black-Scholes model, which includes variables such as the expected volatility of the Company’s share price, the anticipated exercise behavior of its grantee, interest rates, and dividend yields. These variables are projected based on the Company’s historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight-line attribution method. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from current estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including type of awards granted, employee class, and historical experience. Actual results and future estimates may differ substantially from current estimates.
| F-13 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Comprehensive income
In accordance with ASC 220, “Comprehensive Income” (“ASC 220”) all components of comprehensive income, including net loss, are reported in the financial statements in the period in which they are recognized. Comprehensive income is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net loss and other comprehensive (income) loss, including foreign currency translation adjustments, are reported, net of any related tax effect, to arrive at comprehensive income. No taxes were recorded on items of other comprehensive income.
Loss per share
Income or loss per share is calculated based on the weighted average number of common shares outstanding. For the year ended June 30, 2015 diluted loss per share does not differ from basic loss per share since the effect of the Company’s warrants and stock options are anti-dilutive. At June 30, 2015, potential common shares of 17,067,870 (2014 – 21,919,699) related to outstanding warrants and stock options were excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive.
For the year ended June 30, 2014 diluted income per share has also been presented. Diluted income per share is calculated using the treasury stock method which uses the weighted average number of common shares outstanding during the period and also includes the dilutive effect of potentially issuable common shares from outstanding stock options and warrants.
Segment information
The Company identifies its operating segments based on business activities, management responsibility and geographical location. The Company operates within a single operating segment being the research and development of cancer indications, and operates in one geographic area, being North America. All of the Company’s assets are located in either Canada or the United States.
Recent accounting pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
| F-14 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Accounting Standards Update (“ASU”) 2014-15 - Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern
The objective of the guidance is to require management to explicitly assess an entity's ability to continue as a going concern, and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management will assess if there is substantial doubt about an entity's ability to continue as a going concern within one year after the issuance date of an entity’s financial statements. The new standard defines substantial doubt and provides examples of indicators thereof. The definition of substantial doubt incorporates a likelihood threshold of "probable" similar to the current use of that term in U.S. GAAP for loss contingencies. The new standard will be effective for all entities in the first annual period ending after December 15, 2016 (December 31, 2016 for calendar year-end entities). Earlier application is permitted. The Company is currently assessing this standard for its impact on future reporting periods.
ASU 2014-10 Topic 915, Development Stage Entities
The objective of the guidance is to reduce cost and complexity in the financial reporting system by eliminating inception-to-date information from the financial statements of development stage entities. The new standard eliminates the concept of a development stage entity (“DSE”) from US GAAP. Therefore, the current incremental reporting requirements for a DSE, including inception-to-date information, will no longer apply. This standard is effective for annual reporting periods beginning after December 15, 2014. The Company has elected to early adopt this guidance effective with its June 30, 2014 consolidated financial statements.
ASU 3013-05 Topic 830, Accounting for Cumulative Translation Adjustments
The standard amends cumulative translation adjustment derecognition guidance in particular when (i) an entity ceases to have a controlling financial interest in certain subsidiaries or groups of assets within a foreign entity, or (ii) there is a loss of a controlling financial interest in a foreign entity or a step acquisition involving an equity method investment that is a foreign entity. This is effective for public entities for years, and interim periods within those years, beginning after December 15, 2013.
ASU 2013-11 Topic 740, Accounting for Cumulative Translation Adjustments
The standard amends guidance on financial statement presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. This is effective for public entities for years, and interim periods within those years, beginning after December 15, 2013.
| F-15 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 4 | Valent Technologies LLC agreements |
On September 12, 2010 the Company entered into a Patent Assignment Agreement (the “Assignment Agreement”) with Valent to acquire patents and the prototype drug product related to VAL-083. In accordance with the Assignment Agreement the Company paid $250,000 to acquire the prototype drug product. In addition, under certain circumstances Valent agreed to assign, convey and transfer to the Company all its right, title and interest in and to the patents in exchange for share purchase warrants. The Company will then be responsible for the further development and commercialization of VAL-083. Valent retained an option to reacquire certain intellectual property until a Financing Transaction is completed by the Company. Under the Assignment Agreement, a ‘Financing Transaction’ is defined as a cumulative equity or debt financing(s), or a merger, acquisition, amalgamation, reverse takeover or other combination, or any combination of the foregoing, cumulatively totaling at least $2,000,000. In accordance with the terms of the Assignment Agreement, Valent is entitled to receive a future royalty on revenues derived from the development and commercialization of VAL-083. In the event that the Company terminates the agreement, the Company may be entitled to receive royalties from Valent’s subsequent development of VAL-083 depending on the development milestones the Company has achieved prior to the termination of the Assignment Agreement.
On January 25, 2013, in connection with the Company’s Reverse Acquisition (note 1), the Company issued to Valent 1,150,000 shares of common stock, in exchange for Valent reducing certain future royalties under the Assignment Agreement.
Pursuant to a loan agreement dated February 3, 2011, the Company received a loan from Valent of $250,000 for the purchase of the prototype drug product. The loan was unsecured and bears interest at 3.00% per year. The loan was originally due on demand but was converted to a five year term loan due, along with all accrued interest, on June 30, 2019. As a result, the Company has presented the full loan and accrued interest balance as a non-current liability at June 30, 2014.
Pursuant to the Assignment Agreement with Valent, the Company agreed to issue warrants to Valent under certain circumstances. The financing completed by the Company that closed in February 2012 constituted a Financing Transaction under the terms of the Assignment Agreement and resulted in the Company issuing 500,000 warrants to Valent on February 1, 2012 at an exercise price of CA$0.50 per warrant (note 7). In exchange for the warrants Valent has assigned all of its right, title and interest in and to the patents for VAL-083 to the Company. The fair value of the contingent warrants of $89,432 has been recognized as an expense and a corresponding increase to additional paid-in capital in prior periods.
On September 30, 2014, the Company entered into an exchange agreement (the “Valent Exchange Agreement”) with Valent. Pursuant to the Valent Exchange Agreement, Valent exchanged its loan payable in the outstanding amount of $278,530 (including aggregate accrued interest to September 30, 2014 of $28,530), issued to Valent by DelMar (BC), for 278,530 shares of the Company’s Series A Preferred Stock.
Effective September 30, 2014, the Company filed a Certificate of Designation of Series A Preferred Stock (the “Series A Certificate of Designation”) with the Secretary of State of Nevada. Pursuant to the Series A Certificate of Designation, the Company designated 278,530 shares of preferred stock as Series A Preferred Stock. The shares of Series A Preferred Stock have a stated value of $1.00 per share (the “Stated Value”) and are not convertible into common stock. The holder of the Series A Preferred Stock will be entitled to dividends at the rate of 3% of the Stated Value per year, payable quarterly in arrears. Upon any liquidation of the Company, the holder of the Series A Preferred Stock will be entitled to be paid, out of any assets of the Company available for distribution to stockholders, the Stated Value of the shares of Series A Preferred Stock held by such holder, plus any accrued but unpaid dividends thereon, prior to any payments being made with respect to the common stock.
| F-16 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
For the year ended June 30, 2015, the Company recorded $6,267 related to the dividend payable to Valent and $2,091 related to interest from July 1, 2014 to September 30, 2014 when the loan was exchanged for preferred stock. The dividend of $6,267 has been recorded as a direct increase in accumulated deficit while the $2,091 has been recorded as interest expense. For the year ended June 30, 2014 the Company accrued $8,140 in interest expense on its loan payable with Valent.
One of the Company’s officers and directors is a principal of Valent and as result Valent is a related party to the Company (note 8).
| 5 | Taxes and other receivables |
June 30, 2015 $ | June 30, 2014 $ | |||||||
| Government grants | 9,820 | 562 | ||||||
| Other receivables | 16,011 | 9,010 | ||||||
| 25,831 | 9,572 |
On June 15, 2014, the Company was granted a non-repayable financial contribution from the National Research Council of Canada Industrial Research Assistance Program (“IRAP”). The Company will be reimbursed for certain research and development costs to a maximum of $155,635 (CA$194,398) in the period from June 15, 2014 through June 15, 2017.
The total amount credited in the statement of operations for all IRAP grants for the year ended June 30, 2015 was $62,943 (2014 - $562).
| F-17 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 6 | Derivative liability |
The Company has issued common stock purchase warrants. Based on the terms of certain of these warrants the Company determined that the warrants were a derivative liability which is recognized at fair value at the date of the transaction and re-measured at fair value each reporting period with the changes in fair value recorded in the consolidated statement of operations and comprehensive loss.
CA$0.50 Unit Warrants
During the years ended December 31, 2012 and 2011 the Company issued a total of 5,410,000 units for services, settlement of accounts payable, and cash proceeds for an aggregate of $2,671,923 (CA$2,705,000). The proceeds from the issuance of 3,000,000 of these units were held in escrow pursuant to an exclusive option investment agreement with a strategic investor. Subsequently, the Company elected to allow the option to expire and the related units were cancelled and the funds returned from escrow to the subscriber in order for the Company to retain control over certain intellectual property and commercial rights.
During the year ended June 30, 2014, 241,000 of these warrants were exercised for no additional consideration for 241,000 shares of common stock with $259,315 of the derivative liability being reclassified to equity. The warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded. On January 25, 2014, the remaining 2,169,000 of these warrants expired.
Investor Warrants
In connection with the Reverse Acquisition (note 1), on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013, the Company entered into and closed a series of subscription agreements with accredited investors (the “Investors”), pursuant to which the Company issued an aggregate of 13,125,002 units at a purchase price of $0.80 per unit, for aggregate gross proceeds of $10,500,000 (the “Private Offering”). Each unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.80. The exercise price of the Investor Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. The Investor Warrants are redeemable by the Company at a price of $0.001 per Investor Warrant at any time subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.60 per share with an average trading volume of 50,000 shares per day and (ii) the underlying shares of common stock are registered.
Investor warrant exercises
On June 6, 2014, pursuant to an Election to Exercise Warrants agreement, the Company reduced the Investor Warrant exercise price from $0.80 to $0.65 per share for warrants to purchase 3,652,211 shares of the Company’s common stock. In accordance with the agreements, the holders of the Investor Warrants exercised the Investor Warrants for cash at the foregoing reduced exercise price. The Company received net proceeds of $2,255,240 after paying a 5% warrant agent fee of $118,697. As a result, $984,484 of the derivative liability has been reclassified to equity.
| F-18 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
In addition, during the year ended June 30, 2014, 277,313 warrants were exercised at $0.80 per warrant for 277,313 shares of common stock. The Company received proceeds of $221,850 from the exercise. As a result, $126,064 of the derivative liability has been reclassified to equity.
Tender offer – Investor Warrant exercise price reduction
On June 9, 2014, as amended on June 26, 2014, July 10, 2014, and July 29, 2014, the Company filed a tender offer statement with the Securities and Exchange Commission with respect to certain Investor Warrants to provide the holders thereof with the opportunity to amend and exercise their warrants, upon the terms and subject to the conditions set forth in the Company’s tender offer statement. Pursuant to the tender offer, the Company offered to amend Investor Warrants to purchase an aggregate of 9,195,478 shares of common stock (the “Offer to Amend and Exercise”). There was no minimum participation requirement with respect to the Offer to Amend and Exercise.
Pursuant to the Offer to Amend and Exercise, the Investor Warrants subject to the tender offer were amended (the “Amended Warrants”) to: (i) reduce the exercise price of the Investor Warrants from $0.80 per share to $0.65 per share of common stock in cash, (ii) shorten the exercise period of the Investor Warrants so that they expire concurrently with the expiration of the Offer to Amend and Exercise at 5:00 p.m. (Pacific Time) on August 8, 2014, as may be extended by the Company in its sole discretion (“Expiration Date”), (iii) delete the price-based anti-dilution provisions contained in the Investor Warrants, (iv) restrict the ability of the holder of shares issuable upon exercise of the Amended Warrants to sell, make any short sale of, loan, grant any option for the purchase of, or otherwise dispose of any of such shares without the prior written consent of the Company for a period of time twenty (20) days after the Expiration Date (the “ Lock-Up Period ”); and (v) provide that a holder, acting alone or with others, will agree not to effect any purchases or sales of any securities of the Company in any “short sales” as defined in Rule 200 promulgated under Regulation SHO under the Exchange Act, or any type of direct and indirect stock pledges, forward sale contracts, options, puts, calls, short sales, swaps, “put equivalent positions” (as defined in Rule 16a-1(h) under the Exchange Act) or similar arrangements, or sales or other transactions through non-U.S. broker dealers or foreign regulated brokers through the expiration of the Lock-Up Period.
Upon the expiry of the Offer to Amend and Exercise on August 8, 2014, 762,227 Amended Warrants were exercised for net proceeds of $470,676 after payment by the Company of a 5% warrant agent fee of $24,772. As a result, 8,433,251 Investor Warrants remained outstanding under their original terms subsequent to the tender offer.
In addition to the price reduction tender offer, during the year ended June 30, 2015, 1,223,847 Investor Warrants were exercised at $0.65 per share for 1,223,847 shares of common stock. The Company received proceeds of $795,501 from these exercises.
As a result of all of the Investor Warrants exercised for cash at $0.65 per warrant, including the tender offer relating to the price reduction to $0.65, a total of $391,422 of the derivative liability has been reclassified to equity. All Investor Warrants that have been exercised were revalued at their exercise date and then the reclassification to equity was recorded.
| F-19 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Investor Warrant exchange
On December 31, 2014, the Company issued 414,889 shares of common stock in exchange for 1,244,666 Investor Warrants. The Investor Warrants that have been exchanged were revalued at their exchange date and then a reclassification to equity was recorded. The reclassification to equity upon the exchange was $305,112. The Company recognized a loss of $92,843 at the time of the exchange.
Tender offer warrant exchange
On January 8, 2015, the Company filed a tender offer statement with the Securities and Exchange Commission, and on January 23, 2015, the Company filed an amendment thereto, with respect to certain Investor Warrants to purchase common stock of the Company. The tender offer provided the holders of the Investor Warrants with the opportunity to receive one share of common stock for every three Investor Warrants tendered. The tender offer was available to all 5,964,738 Investor Warrants outstanding on January 8, 2015. To participate in the tender offer the Investor Warrant holders were required to deliver completed exchange documents to the Company, prior to the expiration of the tender offer, which was 5:00 p.m. (Pacific Time) on February 9, 2015.
The tender offer expired on February 9, 2015. A total of 1,591,875 Investor Warrants were exchanged for 530,625 shares of common stock. The Investor Warrants that have been exchanged were revalued at their exchange date and then a reclassification to equity was recorded. The reclassification to equity upon the exchange was $423,723. The Company recognized a loss of $156,219 at the time of the exchange.
The remaining 4,372,863 Investor Warrants outstanding at June 30, 2015 have been re-valued at June 30, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 76.6%, risk free rate – 1.14% and a term of approximately 2.5 years.
All 4,372,863 Investor Warrants outstanding at June 30, 2015 have an exercise price of $0.80 at June 30, 2015. However, subsequent to June 30, 2015, the Company issued shares of common stock at $0.60 per share (note 12). As a result of the Investor Warrants being subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions, the 4,372,863 Investor Warrants outstanding at June 30, 2015 now have an exercise price of $0.786.
Dividend warrants
In connection with the Reverse Acquisition (note 1), warrants that Berry issued pursuant a warrant dividend became warrants of the Company (the “Dividend Warrants”). The Dividend Warrants are exercisable at $1.25 per share until January 24, 2018. The Dividend Warrants will only be exercisable at such times as the underlying shares of common stock are registered. The Dividend Warrants will be redeemable by the Company at a price of $0.001 per Dividend Warrant at any time commencing 18 months following the date of issuance subject to the conditions that (i) the Company’s common stock has traded for twenty (20) consecutive trading days with a closing price of at least $2.50 per share and (ii) the underlying shares of common stock are registered. Subject to the conditions set forth therein, the Dividend Warrants may be redeemed by the Company upon not less than ninety (60) days nor more than ninety (90) days prior written notice.
| F-20 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
On October 31, 2014, the Company and all of its Dividend Warrant holders entered into amendments to the Dividend Warrants such that the Company’s redemption rights and certain provisions of the Dividend Warrant agreements relating to potential cash settlement of the Dividend Warrants were removed. The Dividend Warrants were revalued to the date of the amendment on October 31, 2014 which resulted in a reclassification to equity of $975,278.
Warrants issued for services
During the year ended December 31, 2013, the Company issued 300,000 warrants for services. The warrants were issued on September 12, 2013 and are exercisable on a cashless basis at an exercise price of $1.76 for five years. The Company has recognized $124,020 in expense in the consolidated statement of operations.
The warrants have been measured at fair value at their issue date of June 30, 2015 using a simulated probability valuation model using the following assumptions: dividend rate - 0%, volatility – 76.7%, risk free rate - 1.31% and a term of approximately 3.25 years.
The Company’s derivative liability is summarized as follows:
| June 30, 2015 $ | June 30, 2014 $ | |||||||
| Opening balance | 3,329,367 | 12,986,827 | ||||||
| Change in fair value of warrants | (179,170 | ) | (8,300,438 | ) | ||||
| Change in fair value due to change in warrant terms | (23,658 | ) | (111,179 | ) | ||||
| Reclassification to equity upon amendment of warrants | (975,278 | ) | - | |||||
| Warrants issued for services | - | 124,020 | ||||||
| Reclassification to equity upon exchange of warrants | (728,835 | ) | - | |||||
| Reclassification to equity upon exercise of warrants | (391,422 | ) | (1,369,863 | ) | ||||
| Closing balance | 1,031,004 | 3,329,367 | ||||||
| 7 | Stockholders’ equity (deficiency) |
Preferred stock
Authorized
5,000,000 preferred shares, $0.001 par value
Issued and outstanding
Special voting shares – at June 30, 2015 and 2014 – 1
Series A shares – at June 30, 2015 – 278,530 (June 30, 2014 – none)
| F-21 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
Effective September 30, 2014 pursuant to the Valent Exchange Agreement (note 4), the Company filed the Series A Certificate of Designation with the Secretary of State of Nevada. Pursuant to the Series A Certificate of Designation, the Company designated 278,530 shares of preferred stock as Series A Preferred Stock. The shares of Series A Preferred Stock have a stated value of $1.00 per share (the “Stated Value”) and are not convertible into common stock. The holder of the Series A Preferred Stock will be entitled dividends at the rate of 3% of the Stated Value per year, payable quarterly in arrears. Upon any liquidation of the Company, the holder of the Series A Preferred Stock will be entitled to be paid, out of any assets of the Company available for distribution to stockholders, the Stated Value of the shares of Series A Preferred Stock held by such holder, plus any accrued but unpaid dividends thereon, prior to any payments being made with respect to the common stock.
In connection with the Exchange Agreement (note 1), on the Closing Date, the Company, Callco, Exchangeco and Computershare Trust Company of Canada (the “Trustee”) entered into a voting and exchange trust agreement (the “Trust Agreement”). Pursuant to the Trust Agreement, Company issued one share of Special Voting Preferred Stock (the “Special Voting Share”) to the Trustee, and the parties created a trust for the Trustee to hold the Special Voting Share for the benefit of the holders of the shares of Exchangeco acquired as part of the Reverse Acquisition (the “Exchangeable Shares”) (other than the Company and any affiliated companies) (the “Beneficiaries”). Pursuant to the Trust Agreement, the Beneficiaries will have voting rights in the Company equivalent to what they would have had they received shares of common stock in the same amount as the Exchangeable Shares held by the Beneficiaries.
In connection with the Exchange Agreement and the Trust Agreement, on January 17, 2013, the Company filed a certificate of designation of Special Voting Preferred Stock (the “Special Voting Certificate of Designation”) with the Secretary of State of Nevada. Pursuant to the Special Voting Certificate of Designation, one share of the Company’s blank check preferred stock was designated as Special Voting Preferred Stock. The Special Voting Preferred Stock votes as a single class with the common stock and is entitled to a number of votes equal to the number of Exchangeable Shares of Exchangeco outstanding as of the applicable record date (i) that are not owned by the Company or any affiliated companies and (ii) as to which the holder has received voting instructions from the holders of such Exchangeable Shares in accordance with the Trust Agreement.
The Special Voting Preferred Stock is not entitled to receive any dividends or to receive any assets of the Company upon any liquidation, and is not convertible into common stock of the Company.
The voting rights of the Special Voting Preferred Stock will terminate pursuant to and in accordance with the Trust Agreement. The Special Voting Preferred Stock will be automatically cancelled at such time as the share of Special Voting Preferred Stock has no votes attached to it.
Common stock
Authorized
200,000,000 common shares, $0.001 par value
Issued and outstanding at June 30, 2015 – 39,455,931 (2014 – 35,992,343). The issued and outstanding common shares at June 30, 2015 include 4,256,042 (2014 - 7,044,583) shares of common stock on an as-exchanged basis with respect to the Exchangeable Shares (note 1).
| F-22 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| a) | Shared issued for services |
During the year ended June 30, 2015, the Company issued 187,000 (June 30, 2014 – 540,000) shares of common stock for services resulting in the recognition of $181,187 (June 30, 2014 - $633,500) in expense.
The total shares for services expense for the year ended June 30, 2015 of $181,187 (June 30, 2014 - $633,500) in addition to the stock option expense for the period of $186,900 (June 30, 2014 - $886,444) and warrants issued for services of $nil (2014 - $124,020) results in a total share-based payment expense of $368,087 for the year ended June 30, 2015 (June 30, 2014 - $1,643,964). This total expense has been recognized as to $36,284 (June 30, 2014 - $404,177) and $331,803 (June 30 2014 - $1,239,787) for research and development, and general and administrative respectively for the year ended June 30, 2015.
Stock options
On February 1, 2012, the Company’s Board of Directors approved its stock option plan (the “Plan”). Under the Plan the number of common shares that will be reserved for issuance to officers, directors, employees and consultants under the Plan will not exceed 7.5% of the share capital of the Company on a fully diluted basis. The requisite service period of the options ranges from six months to three years and also has a range of six months to three years contractual term.
In the event of the sale of 66 2/3% of the equity securities of the Company where equity securities include shares, warrants, stock options, and any convertible securities of the Company, any options not yet granted under the Plan shall be deemed granted to the principal founders of the Company on a pro-rata basis in accordance with their ownership of the Company on a fully-diluted basis immediately prior to the closing of such a sale.
The following table sets forth the options outstanding under the Plan as of June 30, 2015:
Number of stock options outstanding | Weighted average exercise price | |||||||||
| Balance – June 30, 2013 | 1,140,000 | 0.78 | ||||||||
| Granted | 2,100,000 | 1.06 | ||||||||
| Forfeited | (52,786 | ) | 0.87 | |||||||
| Balance – June 30, 2014 | 3,187,214 | 0.96 | ||||||||
| Granted | 600,000 | 0.88 | ||||||||
| Cancelled | (120,000 | ) | 1.05 | |||||||
| Forfeited | (72,214 | ) | 0.53 | |||||||
| Balance - June 30, 2015 | 3,595,000 | 0.94 |
| F-23 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
The following table summarizes stock options currently outstanding and exercisable at June 30, 2015:
Exercise
$ | Number Outstanding at June 30, 2015 | Weighted average remaining contractual life (years) | Weighted average exercise price $ | Number exercisable at June 30, 2015 | Exercise price $ | |||||||||||||||||
| 0.40 | 825,000 | 6,63 | 0.40 | 825,000 | 0.40 | |||||||||||||||||
| 0.74 | 180,000 | 9.60 | 0.74 | 56,389 | 0.74 | |||||||||||||||||
| 0.80 | 120,000 | 9.75 | 0.80 | 30,000 | 0.80 | |||||||||||||||||
| 1.00 | 300,000 | 4.25 | 1.00 | 50,000 | 1.00 | |||||||||||||||||
| 1.05 | 1,870,000 | 8.13 | 1.05 | 1,611,250 | 1.05 | |||||||||||||||||
| 1.54 | 180,000 | 7.75 | 1.54 | 180,000 | 1.54 | |||||||||||||||||
| 2.30 | 120,000 | 7.92 | 2.30 | 120,000 | 2.30 | |||||||||||||||||
| 3,595,000 | 0.94 | 2,872,639 | 0.94 |
Included in the number of stock options outstanding are 825,000 stock options granted at an exercise price of CA$ $0.50. The exercise prices for these stock options shown in the above table have been converted to $0.40 US$ using the period ending closing exchange rate. Certain stock options have been granted to non-employees and will be revalued at each reporting date until they have fully vested.
The stock options have been valued using a Black-Scholes pricing model using the following assumptions:
June 30, 2015 | June 30, 2014 | |||||||
| Dividend rate | 0 | % | 0 | % | ||||
| Volatility | 67% to 85% | 73% to 76% | ||||||
| Risk-free rate | 1.00% to 1.25% | 1.25 | % | |||||
| Term - years | 0.5 to 2.5 | 0.5 to 2.5 |
The Company has recognized the following amounts as stock-based compensation expense for the periods noted:
| Years ended June 30, | ||||||||
| 2015 $ | 2014 $ | |||||||
| Research and development | 36,284 | 358,177 | ||||||
| General and administrative | 150,616 | 528,267 | ||||||
| 186,900 | 886,444 | |||||||
Of the total stock option expense of $186,900 (2014 - $886,443) for the year ended June 30, 2015, $225,214 (2014 - $979,942) has been recognized as additional paid in capital and $38,314 (2014 – a reduction of $93,498) has been recognized as reduction to stock option liability. The aggregate intrinsic value of stock options outstanding at June 30, 2015 was $203,528 (2014 - $372,454) and the aggregate intrinsic value of stock options exercisable at June 30, 2015 was also $203,528 (2014 - $336,853). As of June 30, 2015 there was $57,335 in unrecognized compensation expense that will be recognized over the next year. No stock options granted under the Plan have been exercised to June 30 2015. Upon the exercise of stock options new shares will be issued.
| F-24 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
A summary of status of the Company’s unvested stock options as of June 30, 2015 under all plans is presented below:
Number of options | Weighted average exercise price $ | Weighted average grant date fair value $ | ||||||||||||
| Unvested at June 30, 2013 | 420,083 | 1.06 | 0.61 | |||||||||||
| Granted | 2,100,000 | 1.06 | 0.58 | |||||||||||
| Vested | (1,731,616 | ) | 1.06 | 0.58 | ||||||||||
| Forfeited | (52,786 | ) | 0.87 | 0.49 | ||||||||||
| Unvested at June 30, 2014 | 735,681 | 0.98 | 0.54 | |||||||||||
| Granted | 600,000 | 0.88 | 0.32 | |||||||||||
| Vested | (421,106 | ) | 0.94 | 0.48 | ||||||||||
| Forfeited | (72,214 | ) | 0.53 | 0.36 | ||||||||||
| Cancelled | (120,000 | ) | 1.05 | 0.57 | ||||||||||
| Unvested at June 30, 2015 | 722,361 | 0.95 | 0.41 |
The aggregate intrinsic value of unvested stock options at June 30, 2015 was $0 (2014 - $35,601). The unvested stock options have a remaining weighted average contractual term of 7.24 years.
Warrants
Number of warrants | Amount $ | |||||||
| Balance – June 30, 2013 | 6,200,000 | 6,441,700 | ||||||
| Warrants exercised on a cashless basis (i) | (200,000 | ) | (239,600 | ) | ||||
| Expiry of broker warrants (ii) | (5,000 | ) | (556 | ) | ||||
| Exercise of broker warrants (iii) | (8,000 | ) | (1,099 | ) | ||||
| Balance – June 30, 2014 | 5,987,000 | 6,200,445 | ||||||
| Expiry of broker warrants (ii) | (92,000 | ) | (12,640 | ) | ||||
| Exercise of broker warrants (iv) | (345,000 | ) | (49,379 | ) | ||||
| Balance - June 30, 2015 | 5,550,000 | 6,138,426 | ||||||
| F-25 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| i) | During the year ended June 30, 2014, 200,000 warrants issued for placement agent services (the “Placement Agent Warrants”) were exercised on a cashless basis for 123,810 shares of common stock. |
| ii) | The Company has issued broker warrants as finder’s fees in relation to the issuance of certain securities. All of the warrants were issued on March 1, 2012 and have an exercise price of CA$0.50 per warrant. Of the total, 5,000 expired during the year ended June 30, 2014 and 92,000 expired during the year ended June 30, 2015. |
| iii) | During the year ended June 30, 2014, 8,000 broker warrants were exercised for proceeds of $3,660 (CA$4,000). |
| iv) | The Company has issued 345,000 warrants for investor relations services. The warrants were issued on February 1, 2012 and vested in 12 equal installments over a 12-month period commencing on March 1, 2012. The warrants have an exercise price of CA$0.50 per warrant. The 345,000 warrants were exercised during the year ended June 30, 2015 for cash proceeds of $138,000 (CA$ 172,500). |
Certain of the Company’s warrants have been recognized as a derivative liability (note 6).
The following table summarizes all of the Company’s outstanding warrants as of June 30, 2015:
| Description | Number | |||
| Issued for patents (i) | 500,000 | |||
| Investor Warrants (ii) | 4,372,863 | |||
| Dividend Warrants (iii) | 3,250,007 | |||
| Placement Agent (iv) | 5,050,000 | |||
| Issued for services (v) | 300,000 | |||
| Closing balance - June 30, 2015 | 13,472,870 |
| i) | The Company issued 500,000 warrants to Valent (note 4). The warrants have an exercise price of CA$0.50 per warrant and expire February 1, 2017. |
| ii) | The Investor Warrants were issued as part of the Company’s $0.80 unit offering. They were issued in tranches on January 25, 2013, January 31, 2013, February 8, 2013, February 21, 2013, February 28, 2013, March 1, 2013, and March 6, 2013 respectively. Of the initial number issued of 13,125,002, 277,313 have been exercised at $0.80, 5,638,285 have been exercised at $0.65, and 2,836,541 have been exchanged on a three for one basis for 945,514 shares of common stock. As a result of the Company issuing common shares at $0.60 per share subsequent to June 30, 2015, the exercise price of all remaining 4,372,863 Investor Warrants has been reduced to $0.786 per Investor Warrant (note 12). |
| F-26 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| iii) | The Dividend Warrants are exercisable at $1.25 per share until January 24, 2018. |
| iv) | The Placement Agent Warrants are exercisable at $0.80 per share until March 6, 2018 but can be exercised on a cashless basis. The Placement Agent Warrants were all issued on March 6, 2013. The exercise price of the Placement Agent Warrants is subject to adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions. As a result of the Company issuing common shares at $0.60 per share subsequent to June 30, 2015, the exercise price of all remaining 5,050,000 Placement Investor Warrants has been reduced to $0.786 per share (note 12). |
| v) | The warrants are exercisable on a cashless basis at a price of $1.76 per share until September 12, 2018. |
| 8 | Related party transactions |
During the year ended June 30, 2015
Effective September 30, 2014, the Company entered into and closed an agreement with Valent to exchange its loan with Valent for 278,530 shares of preferred stock of the Company (note 4).
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $505,000 in compensation expense for the year ended June 30, 2015.
Included in accounts payable at June 30, 2015 is an aggregate amount of $90,820 owed to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
The Company recognized $119,417 in directors’ fees during the year ended June 30, 2015.
During the year ended June 30, 2014
Pursuant to consulting agreements with the Company’s officers the Company recognized a total of $425,845 in compensation expense for the year ended June 30, 2014.
The Company recognized $77,833 in directors’ fees during the year ended June 30, 2014.
| F-27 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 9 | Current and future income taxes |
The Company has the following non-capital losses available to reduce taxable income of future years:
| Expiry date | $ | |||||
| 2029 | 65,450 | |||||
| 2030 | 1,043,923 | |||||
| 2031 | 1,132,631 | |||||
| 2033 | 3,913,138 | |||||
| 2034 | 5,417,455 | |||||
| 2035 | 2,843,797 |
Significant components of the Company’s future tax assets are shown below:
June 30, 2015 $ | June 30, 2014 $ | |||||||
| Non-capital losses carried forward | 4,342,685 | 2,686,530 | ||||||
| Financing costs | 10,565 | 7,737 | ||||||
| Scientific research and development | 183,913 | 144,235 | ||||||
| 4,537,163 | 2,838,502 | |||||||
| Valuation allowance | (4,537,163 | ) | (2,838,502 | ) | ||||
| Net future tax assets | - | - |
The income tax benefit of these tax attributes has not been recorded in these consolidated financial statements because of the uncertainty of their recovery.
The Company’s effective income tax rate differs from the statutory income tax rate of 34% (2014 - 34%).
The differences arise from the following items:
June 30, 2015 $ | June 30, 2014 $ | |||||||
| Tax recovery at statutory income tax rates | (1,630,650 | ) | (989,430 | ) | ||||
| Permanent differences | (49,820 | ) | 110,113 | |||||
| Effect of rate differentials between jurisdictions | 327,485 | 149,219 | ||||||
| Other | (4,783 | ) | (8,713 | ) | ||||
| Change in valuation allowance | 1,357,768 | 738,811 | ||||||
| - | - |
| F-28 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 10 | Commitments and contingencies |
Office lease
The Company currently rents its offices pursuant to a one-year lease that commenced on May 27, 2015 at a rate of $3,082 (CA$3,850) per month. During the year ended June 30, 2015, the Company recorded $29,429 as rent expense (2014 - $23,850).
| 11 | Financial risk management |
Market risk
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or valuation of its financial instruments.
The Company is exposed to financial risk related to fluctuation of foreign exchange rates. Foreign currency risk is limited to the portion of the Company’s business transactions denominated in currencies other than the United States dollar, primarily general and administrative expenses incurred in Canadian dollars. The Company believes that the results of operations, financial position and cash flows would be affected by a sudden change in foreign exchange rates, but would not impair or enhance its ability to pay its Canadian dollar accounts payable. The Company manages foreign exchange risk by converting its US$ to CA$ as needed. The Company maintains the majority of its cash in US$. As at June 30, 2015, Canadian dollar denominated accounts payable and accrued liabilities exposure in US$ totaled $217,423.
| a) | Foreign exchange risk |
Foreign exchange risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. If foreign exchange rates were to fluctuate within +/-10% of the closing rate at year-end, the maximum exposure is $21,742.
Balances in foreign currencies at June 30, 2015 and 2014 are as follows:
June 30, 2015 balances CA$ | June 30, 2014 balances CA$ | |||||||
| Trade payables | 201,169 | 136,825 | ||||||
| Cash | 88,205 | 65,513 |
| b) | Interest rate risk |
The Company is subject to interest rate risk on its cash and cash equivalents and believes that the results of operations, financial position and cash flows would not be significantly affected by a sudden change in market interest rates relative to the investment interest rates due to the short-term nature of the investments. As at June 30, 2015, cash and cash equivalents held in Canadian dollar accounts or short-term investments were $70,618. The Company’s cash balance currently earns interest at standard bank rates. If interest rates were to fluctuate within +/-10% of the closing rate at year end the impact of the Company’s interest bearing accounts will be not be significant.
| F-29 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
The only financial instruments that expose the Company to interest rate risk are its cash and cash equivalents.
Liquidity risk
Liquidity risk is the risk that the Company will encounter difficulty in raising funds to meet cash flow requirements associated with financial instruments. The Company continues to manage its liquidity risk based on the outflows experienced for the period ended June 30, 2015 and is undertaking efforts to conserve cash resources wherever possible. The maximum exposure of the Company’s liquidity risk is $853,084 at June 30, 2015 (note 1).
Credit risk
Credit risk arises from cash and cash equivalents, deposits with banks and financial institutions, as well as outstanding receivables. The Company limits its exposure to credit risk, with respect to cash and cash equivalents, by placing them with high quality credit financial institutions. The Company’s cash equivalents consist primarily of operating funds with commercial banks. Of the amounts with financial institutions on deposit, the following table summarizes the amounts at risk should the financial institutions with which the deposits are held cease trading:
The maximum exposure of the Company’s credit risk is $25,831 at June 30, 2015.
Cash and cash equivalents $ | Insured amount $ | Non-insured amount $ | ||||||||
| 1,754,433 | 70,618 | 1,683,815 |
Concentration of credit risk
Financial instruments that subject the Company to credit risk consist primarily of cash and cash equivalents. The Company places its cash and cash equivalents in accredited financial institutions and therefore the Company’s management believes these funds are subject to minimal credit risk. The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements
| F-30 |
DelMar Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
June 30, 2015
(in US dollars unless otherwise noted)
| 12 | Subsequent events |
Issuance of common shares and warrants
On July 15, 2015 the Company’s Registration Statement on Form S-1 relating to a public offering by the Company of common stock and common stock purchase warrants (the “Offering”) was declared effective by the Securities and Exchange Commission. Pursuant to the Offering, the Company issued 4,277,667 shares of common stock at $0.60 per share and 4,277,667 warrants (the “Offering Warrants”) to purchase shares of common stock at $0.001 per warrant for total gross proceeds of $2,566,660. The Offering Warrants are exercisable at $0.75 per share for a period of five years until they expire on July 31, 2020.
The Company engaged certain placement agents
for the sale of a portion of the shares and Offering Warrants. Under the Company’s engagement agreements with these placement
agents, the Company agreed to pay up to a 7% cash commission and issue warrants to purchase shares of common stock (the “Agent
Warrants”) up to the number of shares of our common stock equal to 5% of the aggregate number of shares sold in the Offering
by such placement agents. Pursuant to the placement agent agreements the Company paid a total cash commission of $80,575 and issued
56,345 Agent Warrants. The Agent Warrants are exercisable at a per share price equal to $0.75 during the five-year period commencing
six months from the effective date of the Offering, which period shall not extend further than five years from the effective date
of the Offering. Therefore, all Agent Warrants expire on July 15, 2020.
In addition to the cash commission of $80,575 the Company also incurred
additional issue and closing costs of approximately $565,000 (including costs deferred at June 30, 2015 of $550,119) resulting
in net cash proceeds of approximately $1,921,085.
The exercise price of the Investor Warrants (note 6) issued by the Company during the period ended June 30, 2013 is subject to
adjustment in the event that the Company sells common stock at a price lower than the exercise price, subject to certain exceptions.
As a result of the Offering, the exercise price of the 4,372,863 Investor Warrants currently outstanding, was reduced to $0.786
per share.
Warrants for services
Subsequent to June 30, 2015, the Company issued 60,000 warrants to purchase
common stock for services. These warrants vest in tranches of 20,000 warrants each on November 30, 2015, December 31, 2015, and
January 31, 2016 and are exercisable commencing January 1, 2016 at $0.75 until they expire on July 15, 2020.
Stock options
Subsequent to June 30, 2015, the Company cancelled 50,000 stock options
exercisable at $1.05.
| F-31 |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act are recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file under the Exchange Act is accumulated and communicated to our management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure.
As of the end of the period covered by this annual report, we conducted an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of our disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) of the Exchange Act). Based upon this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures are effective to ensure that information required to be disclosed by the Company in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and also are effective in ensuring that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure.
Management's Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting of the Company. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate.
Management, with the participation of our Chief Executive Officer and Chief Financial Officer, have evaluated the effectiveness of our internal control over financial reporting as of June 30, 2015 based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control — Integrated Framework (2013 framework). Based on this evaluation, management concluded that, as of June 30, 2015, our internal control over financial reporting is effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles.
This annual report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s independent registered public accounting firm pursuant to rules of the SEC that permit the Company to provide only management’s report in this annual report.
Changes in internal controls
There have been no changes in our internal control over financial reporting that occurred during the quarter ended June 30, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
| 55 |
Item 10. Directors, Executive Officers and Corporate Governance.
Below are the names and certain information regarding the Company’s executive officers and directors.
| Name | Age | Position | ||
| Jeffrey Bacha | 47 | President, Chief Executive Officer and Director | ||
| Dennis Brown | 65 | Chief Scientific Officer and Director | ||
| Scott Praill | 49 | Chief Financial Officer | ||
| John K. Bell | 67 | Director | ||
| Lynda Cranston | 67 | Director | ||
| William Garner | 49 | Director | ||
| Erich Mohr | 60 | Director | ||
| Robert J. Toth, Jr. | 52 | Director |
Jeffrey Bacha, BSc, MBA has been Chief Executive Officer and President of the Company since January 25, 2013, and director of the Company since February 11, 2013. Mr. Bacha is one of our founders and has been President, Chief Executive Officer and director of DelMar (BC) since inception. Mr. Bacha is a seasoned executive leader with nearly twenty years of life sciences experience in the areas of operations, strategy and finance. His background includes successful public and private company building from both a start-up and turn around perspective; establishing and leading thriving management and technical teams; and raising capital in both the public and private markets. From July 2006 to August 2009, Mr. Bacha was Executive Vice President Corporate Affairs and Chief Operating Officer at Clera, Inc. From March 2005 to July 2006 Mr. Bacha was Consultant and held various positions at Clera Inc., Urigen Holdings Inc. and XBiotech, Inc. From 1999 through 2004, Mr. Bacha served as President & CEO of Inimex Pharmaceuticals, a venture-capital funded drug discovery and development company and is a former Senior Manager and Director of KPMG Health Ventures. Mr. Bacha holds an MBA from the Goizueta Business School at Emory University and a degree in BioPhysics from the University of California, San Diego. Mr. Bacha’s experience as one of our founders and Chief Executive Officer qualifies him to serve on our Board of Directors.
Dr. Dennis M. Brown, PhD, has been Chief Scientific Officer of the Company since January 25, 2013 and director of the Company since February 11, 2013. Dr. Brown is one of our founders and has served as Chief Scientific Officer and director of DelMar (BC) since inception. Dr. Brown has more than thirty years of drug discovery and development experience. He has served as Chairman of Mountain View Pharmaceutical's board of directors since 2000 and is the President of Valent. In 1999 he founded ChemGenex Therapeutics, which merged with a publicly traded Australian company in 2004 to become ChemGenex Pharmaceuticals (ASX: CXS/NASDAQ: CXSP), of which he served as President and a Director until 2009. He was previously a co-founder of Matrix Pharmaceutical, Inc., where he served as Vice President (VP) of Scientific Affairs from 1985-1995 and as VP, Discovery Research, from 1995-1999. He also previously served as an Assistant Professor of Radiology at Harvard University Medical School and as a Research Associate in Radiology at Stanford University Medical School. He received his B.A. in Biology and Chemistry (1971), M.S. in Cell Biology (1975) and Ph.D. in Radiation and Cancer Biology (1979), all from New York University. Dr. Brown is an inventor of about 34 issued U.S. patents and applications, many with foreign counterparts. Dr. Brown’s scientific knowledge and experience qualifies him to serve on our Board of Directors.
Scott Praill, CPA, BSc. has been Chief Financial Officer of the Company since January 29, 2013 and previously served as a consultant to DelMar (BC). Since 2004, Mr. Praill has been an independent consultant providing accounting and administrative services to companies in the resource industry. Mr. Praill served as CFO of Strata Oil & Gas, Inc. from June 2007 to September 2008. From November 1999 to October 2003 Mr. Praill was Director of Finance at Inflazyme Pharmaceuticals Inc. Mr. Praill completed his articling at Price Waterhouse (now PricewaterhouseCoopers LLP) and obtained his Chartered Professional Accountant designation in 1996. Mr. Praill obtained his Certified Public Accountant (Illinois) designation in 2001. Mr. Praill received a Financial Management Diploma (Honors), from British Columbia Institute of Technology in 1993, and a Bachelor of Science from Simon Fraser University in 1989.
John K. Bell, FCPA, FCA, ICD.D has served as a director of the Company since February 11, 2013. John K. Bell is Chairman of Onbelay Capital Inc, a Canadian based private equity company with principal investments in Telematics and auto parts manufacturing (for past 5 years). Prior to that, from 1996 to 2005, Mr. Bell was CEO and owner of Polymer Technologies Inc., an automotive parts manufacturer. Prior to that, from 1977 to 1995, Mr. Bell was founder and owner of Shred-Tech Limited a global manufacturer and supplier of industrial shredders and mobile document shredders. Mr. Bell served as interim CEO and director of ATS Automation Tooling Systems (TSX-ATA) in 2007. Mr. Bell is a director of Strongco Corporation (TSX-SQP), Tweed Marijuana Inc.(TSX-V-TWD), and the Royal Canadian Mint (TSX-MNT). Mr. Bell is the past National secretary and board member of The Crohns and Colitis Foundation of Canada. Mr. Bell is also the past Chairman of Waterloo Regional Police, Cambridge Memorial Hospital, Canada’s Technology Triangle accelerator network and The Region of Waterloo prosperity counsel. Mr. Bell is a graduate of Western University Ivey School of Business, a Fellow of the institute of Chartered Accountants of Ontario, a graduate of the Institute of Directors Program of Canada and the owner’s president program at Harvard and International marketing program at Oxford. Mr. Bell’s financial and executive business experience qualifies him to serve on our Board of Directors.
| 56 |
Lynda Cranston BScN, MScN, ICD.D has served as a director of the Company since February 5, 2015 and serves as the Chair of our Governance and Compensation Committee. Mrs. Cranston recently retired from healthcare where she had been a CEO for over 20 years. Her last appointment prior to her retirement was as the first CEO of the Provincial Health Services Authority (2002 to 2013). Prior to this appointment Mrs. Cranston had been the first CEO of the Canadian Blood Services in Ottawa, ON (1998-2001). Before moving to Ottawa, Mrs. Cranston, as the CEO of BC Women's Hospital and Healthcare Centre had merged the organization with the BC Children's Hospital and the Sunny Hill Health Centre for Children to become the Children's and Women's Healthcare Centre of BC. Following the merger Mrs. Cranston became the first CEO. Mrs. Cranston also sits on the national board of the Gastrointestinal Society as its chair. In 2013, Mrs. Cranston was identified as a member of Diversity 50 by the Canadian Board Diversity Council as being one of Canada's most board ready candidates. Mrs. Cranston was awarded the Board Chair Award of Excellence by the HealthCare Leaders' Association of British Columbia in 2008. In 2007, she was inducted into Canada's Most Powerful Women Top 100 Hall of Fame after having been identified in '04,'05 & '06 as one of Canada's Most Powerful Women Top 100. Mrs. Cranston is a recipient of the YWCA Women of Distinction Award, the 125th Anniversary of the Confederation of Canada Commemorative Medal for community contributions, and the Queen's Golden Jubilee Medal for contribution to Canada and community. Ms. Cranston’s healthcare industry and executive knowledge and experience qualify her to serve on our Board of Directors.
Dr. William Garner, MD, MPH has served as a director of the Company since February 11, 2013. Dr. Garner is one of our founders and has served as a director of DelMar (BC) since inception. Dr. Garner is an experienced entrepreneur and investor. He is founder and managing director of EGB Advisors, LLC ("EGB"), a pharmaceutical commercialization boutique. Through this entity, Dr. Garner has worked on a number of pharmaceutical business transactions and has raised financing for several drug development companies including Update Pharma, Inc. where he is currently Executive Chairman. Other EGB companies include Urigen Pharmaceuticals, Inc., and Inverseon, Inc., which is developing a novel therapy for smoking cessation, asthma and other pulmonary diseases. In 2012, he merged Inverseon with another company to form Invion Ltd. (ASX:IVX), serving as CEO until May of 2013. He also served as President and Chief Executive Officer of Urigen Pharmaceuticals, Inc. (URGP.PK) from December 2005 to December 2010 where he moved a procedure-based drug from a university license to a phase II multi-center clinical trial which achieved statistical significance on all end points in Painful Bladder Syndrome/Interstitial Cystitis. Before this, Dr. Garner worked in medical affairs at Hoffmann LaRoche in oncology. Prior to Roche, Dr. Garner was in the venture capital department at Paramount Capital Investments in New York City. He serves on the board of ImmunoGenetix in Kansas City. Dr. Garner has a Master of Public Health from Harvard and received his M.D. degree from New York Medical College. Dr. Garner did residency training in Anatomic Pathology at Columbia-Presbyterian and is currently a licensed physician in the State of New York. Dr. Garner’s medical and scientific knowledge and experience qualifies him to serve on our Board of Directors.
Dr. Erich Mohr, Ph.D., R. Psych., has served as a director of the Company since March 31, 2015. Dr. Mohr has nearly two decades of biotechnology experience in executive leadership roles as co-founder, chief scientific officer, chief executive officer and board member. Dr. Mohr has overseen and participated in dozens of clinical development programs and regulatory advisory panels. He is currently Chairman, Chief Executive Officer and Founder of MedGenesis Therapeutix Inc., a privately-held biopharmaceutical company committed to developing and commercializing innovative therapeutics to provide life-enhancing treatments to patients with serious neurologic diseases. Formerly, he was Chairman and Chief Executive Officer of CroMedica Global Inc., which merged with PRA International in 2002 to form one of the major contract research organizations in the world. In addition to his industry experience, Dr. Mohr has over 30 years of experience in experimental therapeutics of CNS disorders including eight years at the University of Ottawa, ultimately as a Professor of Medicine (Neurology) and Psychology. Dr. Mohr is the author of over 150 publications, books, book chapters and abstracts. Currently, he is the Chair of the Board of Governors of the University of Victoria, British Columbia, having previously served as a member and as Vice Chair. He earned his Masters of Science and Ph.D. in Neuropsychology at the University of Victoria, British Columbia, and his Bachelors of Arts in Psychology and dual Bachelors of Science in Chemistry and Biology from the University of the Pacific in Stockton, California. Dr. Mohr’s scientific and business executive knowledge and experience qualify him to serve on our Board of Directors.
Robert J. Toth, Jr., MBA has served as a director of the Company since August 20, 2013. Since 2005, Mr. Toth has primarily been managing his personal investment portfolio. From 2004-2005, Mr. Toth served as a consulting analyst to Narragansett Asset Management, a New York-based healthcare-focused hedge fund, where he advised the firm on biotechnology investments. From 2001-2003, he was the Senior Portfolio Manager for San Francisco-based EGM Capital’s Medical Technology hedge fund, where he was responsible for managing and maintaining a dedicated medical technology portfolio. Mr. Toth began his Wall Street career in 1996 as an Equity Research Associate for Vector Securities International, a healthcare-focused brokerage firm. From 1997–1999 he served as Senior Biotechnology Analyst. He joined Prudential Securities as Senior Vice President and Biotechnology Analyst where he served from 1999-2001 following Prudential’s acquisition of Vector. His responsibilities included the analysis of commercial, clinical and scientific fundamentals of oncology- and genomics-based biotechnology companies on behalf of institutional investors. Mr. Toth was named to the Wall Street Journal’s Allstar List for stock picking in 1999. Mr. Toth received an MBA from the University of Washington and Bachelor of Science degrees in Biological Sciences and Biochemistry from California Polytechnic State University, San Luis Obispo. Mr. Toth’s financial and biotechnology industry knowledge and experience quality him to serve on our Board of Directors.
| 57 |
The Company’s executive officers are not full-time employees, but are engaged by us on an independent contractor or contract-employment basis. Mr. Bacha and Mr. Praill each devote 100% of their business time to us, and Dr. Brown devotes approximately 80% of his business time to us. See “Executive Compensation”.
Officers and Directors are elected annually by the Board of Directors and serve at the discretion of the Board of Directors. The Directors are elected to serve one year terms.
Involvement in Certain Legal Proceedings
To our knowledge, our directors and executive officers have not been involved in any of the following events during the past ten years:
| 1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; | |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); | |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; | |
| 4. | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; | |
| 5. | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or | |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Board Committees
The Board of Directors has formed an Audit Committee, which currently consists of John K. Bell, Chair, and Robert Toth, both of whom are independent (as that term is defined under the Nasdaq Marketplace Rules) and financially literate (as such qualification is interpreted by the Board of Directors in its business judgment). The Board of Directors intends to expand the Audit Committee at such time as the Board of Directors has additional independent members. We are relying upon the exemption in section 6.1 of Canadian National Instrument 52-110 – Audit Committees from Parts 3 and 5 thereof.
The Board of Directors has also recently formed a Corporate Governance and Compensation Committee which consists of Lynda Cranston, Chair, John K. Bell, Erich Mohr, and Robert Toth. The Corporate Governance and Compensation Committee assists the Board of Directors in fulfilling its oversight responsibilities relating to (i) corporate governance practices and policies and (ii) compensation matters, including compensation of the directors and senior management of the Company and the administration of compensation plans of the Company.
| 58 |
Nomination of Directors
The Board of Directors assesses potential candidates to fill perceived needs on the Board of Directors for required skills, expertise, independence and other factors.
Orientation and Continuing Education
New members of the Board of Directors are provided with sufficient information to ensure that they are familiarized with the Company and the policies and mandates of the Board of Directors. Members of the Board of Directors are encouraged to communicate with management, legal counsel and, where applicable, auditors and technical consultants of the Company to keep themselves current with industry developments and applicable legal, accounting and regulatory changes.
Board Leadership Structure and Role in Risk Oversight
Mr. Bacha serves as Chairman and Chief Executive Officer. Due to the small size and early stage of the Company, we believe it is currently most effective to have the Chairman and Chief Executive Officer positions combined.
Our Board of Directors is primarily responsible for overseeing our risk management processes. The Board of Directors receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding the Company’s assessment of risks. The Board of Directors focuses on the most significant risks facing the Company and the Company’s general risk management strategy, and also ensures that risks undertaken by the Company are consistent with the board’s appetite for risk. While the Board of Directors oversees the Company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing the Company and that our board leadership structure supports this approach.
Code of Ethics
We have not adopted a Code of Business Conduct and Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions because of the small number of persons involved in the management of the Company. The Board of Directors is committed to a high standard of corporate governance practices and, through its oversight role, encourages and promotes a culture of ethical business conduct.
Assessments
The Board of Directors assesses, on an ongoing basis, its overall performance and that of its committees in order to determine whether they are performing effectively. The Board of Directors also assesses, on an ongoing basis, the effectiveness and contribution of each director of the Company, having regard to the competencies and skills each director is expected to bring to the Board of Directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers and persons who own more than 10% of the issued and outstanding shares of our common stock to file reports of initial ownership of common stock and other equity securities and subsequent changes in that ownership with the SEC. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file. To our knowledge, during the fiscal year ended June 30, 2015, all Section 16(a) filing requirements applicable to our officers, directors and greater than 10% beneficial owners were complied with, except that a Form 4 for William Garner was filed late, resulting in one transaction not being reported on a timely basis.
Item 11. Executive Compensation.
The Board of Directors has formed a Corporate Governance and Compensation Committee which is undertaking a review of management compensation. The Corporate Governance and Compensation Committee will be responsible for approving management compensation on a go forward basis. The Company seeks to provide competitive compensation arrangements that attract and retain key talent necessary to achieve the business objectives of the Company.
| 59 |
The following table sets forth all compensation paid in respect of the Company’s principal executive officers and those individuals who received compensation in excess of $100,000 per year for the years ended June 30, 2015 and 2014.
| Name and Principal Position | Period | Salary (US$) | Option Awards (US$) | Total (US$) | ||||||||||||
| Jeffrey Bacha CEO | Year Ended June 30, 2015 | 180,000 | - | 180,000 | ||||||||||||
| Year Ended June 30, 2014 | 140,976 | 199,850 (1) | 340,826 | |||||||||||||
| Dennis Brown Chief Scientific Officer | Year Ended June 30, 2015 | 150,000 | - | 150,000 | ||||||||||||
| Year Ended June 30, 2014 | 120,000 | 199,850 (1) | 319,850 | |||||||||||||
| Scott Praill, Chief Financial Officer | Year Ended June 30, 2015 | 175,000 | - | 175,000 | ||||||||||||
| Year Ended June 30, 2014 | 117,480 | 199,850 (2) | 317,330 | |||||||||||||
(1) Represents the grant date fair value of 350,000 options with an exercise price of $1.05 granted on August 15, 2013. The options vested over a 12 month period and expire 10 years from the date of grant.
(2) Represents the grant date fair value of 350,000 options with an exercise price of $1.05 issued on August 15, 2013. The options vest over a 36 month period and expire 10 years from the date of grant.
The previous consulting agreements between DelMar (BC) and each of Mr. Bacha and Dr. Brown expired on December 31, 2012. Until July 1, 2014 we continued to compensate Mr. Bacha and Dr. Brown at the rates set forth in their respective consulting agreements (CDN $12,000 per month and $10,00 per month respectively) except that commencing January 1, 2014 we began paying compensation in USD rather than CDN. Commencing July 1, 2014, the Company began to compensate Mr. Bacha USD $15,000 on a monthly basis and Dr. Brown at USD $12,500 on a monthly basis. The Company recently completed consulting agreements with Mr. Bacha and Dr. Brown that are retroactive to January 1, 2015. The terms of the new agreements are substantially the same as the previously expired agreements except that they reflect the current compensation noted above.
Mr. Bacha and Dr. Brown have continued to provide services to us as Chief Executive Officer and Chief Scientific Officer, respectively. Mr. Bacha devotes 100% of his business time to us and Dr. Brown devotes approximately 80% of his business time to us. The consulting agreements between DelMar (BC) and Mr. Bacha and Dr. Brown, respectively, do not specify the amount of time Mr. Bacha and Dr. Brown are required to devote to us, but did require that Mr. Bacha and Dr. Brown each provide us with the full benefit of their respective knowledge, expertise and ingenuity, and prohibit Mr. Bacha and Dr. Brown from engaging in any business, enterprise or activity contrary to or that would detract from our business.
We were party to a consulting agreement, dated February 1, 2013, with Scott Praill, our Chief Financial Officer. Pursuant to the consulting agreement, we agreed to pay Mr. Praill a fee of CDN$10,000 per month and a one-time fee of CDN $30,000 for services rendered to that date. The consulting agreement did not specify the amount of time Mr. Praill is required to devote to us, but did require that Mr. Praill provide us with the full benefit of his knowledge, expertise and ingenuity, and prohibited Mr. Praill from engaging in any business, enterprise or activity contrary to or that would detract from our business. The consulting agreement expired on December 31, 2013. Mr. Praill devotes 100% of his business time to us. Since the expiration of the consulting agreement, we have continued to compensate Mr. Praill under the terms of the original agreement except that commencing January 1, 2014 we began paying compensation in USD rather than CDN. Commencing July 1, 2014, the Company began to compensate Mr. Praill USD $12,500 on a monthly basis. Mr. Praill continues to serve as our Chief Financial Officer. The Company recently completed a consulting agreement with Mr. Praill that is retroactive to January 1, 2015. The terms of the new agreements are substantially the same as the previously expired agreements except that it reflects the current compensation noted above.
As a result of the Company establishing a Corporate Governance and Compensation Committee, the Company anticipates entering into employment agreements with Mr. Bacha, Mr. Praill, and Dr. Brown in the near future. The contemplated employment agreements will replace the existing consulting agreements.
| 60 |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth outstanding equity awards to our named executive officers as of June 30, 2015.
| Option awards | ||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) Exercisable | Number of securities underlying unexercised options (#) unexercisable | Equity incentive plan awards: number of securities underlying unexercised unearned options (#) | Option exercise price (US$) | Option expiration date | |||||||||||||
| Jeffrey Bacha (1) | 150,000 | - | - | 0.40 | Feb 1, 2022 | |||||||||||||
| 350,000 | - | - | 1.05 | Aug 15, 2023 | ||||||||||||||
| Dennis Brown (1) | 150,000 | - | - | 0.40 | Feb 1, 2022 | |||||||||||||
| 350,000 | - | - | 1.05 | Aug 15, 2023 | ||||||||||||||
| Scott Praill (1) | 50,000 | - | - | 0.40 | Feb 1, 2022 | |||||||||||||
| 218,750 | 131,250 | - | 1.05 | Aug 15, 2023 | ||||||||||||||
(1) Actual exercise price is CDN $0.50. Price disclosed is U.S. dollar equivalent as of June 30, 2015.
Director Compensation
Director compensation is intended to provide an appropriate level of remuneration considering the responsibilities, time requirements and accountability of the Directors.
The following table sets forth director compensation for the fiscal year ended June 30, 2015 (excluding compensation to the Company’s executive officers set forth in the summary compensation table above) paid by the Company.
| Fees Earned | Nonqualified | |||||||||||||||||||||||||||
| or | Non-Equity | Deferred | ||||||||||||||||||||||||||
| Paid in | Stock | Option | Incentive Plan | Compensation | All Other | |||||||||||||||||||||||
| Cash | Awards | Awards | Compensation | Earnings | Compensation | Total | ||||||||||||||||||||||
| Name | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||
| William Garner | 29,000 | - | - | - | - | - | 29,000 | |||||||||||||||||||||
| John K. Bell | 37,000 | - | - | - | - | - | 37,000 | |||||||||||||||||||||
| Robert J, Toth, Jr. | 31,500 | - | - | - | - | - | 31,500 | |||||||||||||||||||||
| Lynda Cranston | 14,167 | - | - | - | - | - | 14,167 | |||||||||||||||||||||
| Erich Mohr | 7,750 | - | - | - | - | - | 7,750 | |||||||||||||||||||||
Risk Management
The Company does not believe risks arising from its compensation policies and practices for its employees are reasonably likely to have a material adverse effect on the Company.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth certain information, as of August 26, 2015, with respect to the beneficial ownership of the outstanding common stock by (i) any holder of more than five (5%) percent; (ii) each of the Company’s executive officers and directors; and (iii) the Company’s directors and executive officers as a group. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned.
| 61 |
| Name of Beneficial Owner (1) | Common Stock Beneficially Owned | Percentage of Common Stock (2) | ||||||
| Directors and Officers: | ||||||||
| Jeffrey Bacha | 4,182,027 | (3) | 9.6 | % | ||||
| Dennis Brown | 4,122,542 | (4) | 10.2 | % | ||||
| William Garner | 2,701,873 | (5) | 6.8 | % | ||||
| John K. Bell | 407,000 | (6) | 1.0 | % | ||||
| Scott Praill | 610,000 | (7) | 1.5 | % | ||||
| Robert J. Toth, Jr. | 163,500 | (8) | * | |||||
| Lynda Cranston | 100,000 | (9) | * | |||||
| Erich Mohr | 80,000 | (9) | * | |||||
| All officers and directors as a group (8 persons) | 12,366,942 | 27.3 | % | |||||
| Beneficial owners of more than 5%: | ||||||||
| Valent Technologies LLC | 2,150,000 | (10) | 5.4 | % | ||||
| Howard K. Fuguet (11) | 2,500,000 | 6.3 | % | |||||
| 62 |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Certain Relationships and Related Transactions
On September 12, 2010, DelMar (BC) entered into a Patent Assignment Agreement (the “Assignment”) with Valent Technologies LLC pursuant to which Valent assigned to DelMar (BC) its rights to patent applications and the prototype drug product related to VAL-083. In accordance with the Assignment the consideration paid by DelMar (BC) was $250,000 to acquire the prototype drug product. In accordance with the terms of the Assignment, Valent is entitled to receive a future royalty (in the single digits) on certain revenues derived from the development and commercialization of VAL-083. In the event that DelMar (BC) terminates the agreement, DelMar (BC) may be entitled to receive royalties from Valent’s subsequent development of VAL-083 depending on the development milestones DelMar (BC) has achieved prior to the termination of the Assignment. The Assignment has a term (on a country-by-country basis), of the later of ten years or until patent rights covered by the Assignment no longer exist, subject to earlier termination in the event DelMar (BC) breaches its payment obligations and fails to remedy such breach within 60 days, or if either party materially beaches any of its obligations and does not cure such breach within 30 days after receipt of notice thereof.
On January 25, 2013, the Company issued to Valent 1,150,000 shares of common stock, in exchange for Valent agreeing to reduce certain royalties payable to it under the Assignment.
Pursuant to a loan agreement dated February 3, 2011, between DelMar (BC) and Valent, Valent loaned DelMar $250,000 for the purchase of the prototype drug product under the Assignment. The loan is unsecured, bears interest at 3% per year, and is payable on demand. Effective September 30, 2014, the Company entered into and closed an agreement with Valent to exchange its loan, including accrued interest to September 30, 2014, with Valent for 278,530 shares of preferred stock of the Company. The preferred stock has an annual 3% dividend.
In addition, under the terms of the Assignment, DelMar (BC) issued to Valent warrants to acquire 500,000 common shares at an exercise price of CDN $0.50 per upon the completion of the financing transaction that closed in February 2012.
On April 30, 2012, DelMar (BC) issued 500,000 common shares in partial settlement of accounts payable in the amount of CDN $250,000 (U.S. $253,050) owed to Valent.
Valent, which is owned by Dr. Dennis Brown, our Chief Scientific Officer, leases facilities in California and we have access to such facilities pursuant to an informal unwritten arrangement with Valent.
Included in accounts payable at June 30, 2015 is an aggregate amount of $90,820 owed to the Company’s officers and directors for fees and expenses. The Company pays related party payables incurred for fees and expenses under normal commercial terms.
Director Independence
William J. Garner, John K. Bell, Robert J. Toth, Jr., Lynda Cranston and Erich Mohr are independent as that term is defined under the Nasdaq Marketplace Rules.
Item 14. Principal Accounting Fees and Services.
The following is a summary of fees for professional services rendered by PricewaterhouseCoopers LLP (“PWC”), our registered independent public accounting firm for the years ended June 30, 2015 and 2014:
| Year Ended June 30, 2015 | Year Ended June 30, | |||||||
| Audit Fees | $ | 115,000 | $ | 115,000 | ||||
| Audit Related Fees | $ | 90,000 | $ | 54,500 | ||||
| Tax Fees | $ | - | $ | - | ||||
| All other fees | $ | - | $ | - | ||||
| Total Fees | $ | 205,000 | $ | 169,500 | ||||
Audit fees. Audit fees represent fees for professional services performed by PWC for the audit of our annual financial statements and the review of our quarterly financial statements, as well as services that are normally provided in connection with statutory and regulatory filings or engagements.
Audit-related fees. Audit-related fees represent fees for assurance and related services performed by PWC that are reasonably related to the performance of the audit or review of our financial statements.
Tax Fees. PWC did not perform any tax compliance services for us during the years ended June 30, 2015 or 2014.
All other fees. PWC did not receive any other audit fees for the years ended June 30, 2015 or 2014.
| 63 |
| 1.1 | Form of Placement Agent Agreement (11) | |
| 2.1 | Exchange Agreement, dated January 25, 2013, among the Company, Exchangeco, Callco, DelMar (BC) and securityholders of DelMar (BC) (1) | |
| 3.1 | Articles of Incorporation of the Company (2) | |
| 3.2 | Articles of Merger of the Company (3) | |
| 3.3 | Certificate of Designation of Special Voting Preferred Stock of the Company (3) | |
| 3.4 | Bylaws of the Company (2) | |
| 3.5 | Amendment to Bylaws of the Company (4) | |
| 3.6 | Certificate of Designation of Series A Preferred Stock (7) | |
| 4.1 | Form of Warrant (12) | |
| 10.1 | Intercompany Funding Agreement, dated January 25, 2013, between the Company and Exchangeco (1) | |
| 10.2 | Support Agreement, dated January 25, 2013, among the Company, Exchangeco and Callco (1) | |
| 10.3 | Voting and Exchange Trust Agreement, dated January 25, 2013, among the Company, Callco, Exchangeco, and the Trustee (1) | |
| 10.4 | Form of Subscription Agreement (1) | |
| 10.5 | Form of Registration Rights Agreement (1) | |
| 10.6 | Form of Investor Warrant (1) | |
| 10.7 | Form of Dividend Warrant (1) | |
| 10.8† | Memorandum of Understanding and Collaboration Agreement between Guangxi Wuzhou Pharmaceutical (Group) Co. Ltd. and DelMar (BC) (1) | |
| 10.9† | Patent Assignment Agreement, dated September 12, 2010, between DelMar (BC) and Valent (5) | |
| 10.10 | Amendment, dated January 21, 2013, to Patent Assignment Agreement, dated September 12, 2010, between DelMar (BC) and Valent (5) | |
| 10.11 | Loan Agreement, dated February 3, 2011, between DelMar (BC) and Valent (5) | |
| 10.12 | Form of Election to Exercise Warrants (6) | |
| 10.13 | Form of Investor Warrant Amendment (8) | |
| 10.14 | Form of Dividend Warrant Amendment (8) | |
| 10.15 | Form of Exchange Agreement (9) | |
| 10.16 | Consulting Agreement, effective January 1, 2015 between DelMar (BC) and Jeffrey Bacha (12) | |
| 10.17 | Consulting Agreement, effective January 1, 2015 between DelMar (BC) and Dennis Brown (12) | |
| 10.18 | Consulting Agreement, effective January 1, 2015 between DelMar (BC) and Scott Praill (12) | |
| 10.19 | Form of Subscription Agreement (United States) (13) | |
| 10.20 | Form of Subscription Agreement (Canada) (13) | |
| 10.21 | Audit Committee Charter * | |
| 16 | Letter from John Kinross-Kennedy (1) | |
| 21 | Subsidiaries (10) | |
| 31.1 | Certification of principal executive officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 * | |
| 31.2 | Certification of principal financial officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 * | |
| 32.1 | Certification of principal executive officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| 32.2 | Certification of principal financial officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 * | |
| EX-101.INS | XBRL Instance Document * | |
| EX-101.SCH | XBRL Taxonomy Extension Schema Document * |
| 64 |
| EX-101.CAL | XBRL Taxonomy Extension Calculation Linkbase * | |
| EX-101.DEF | XBRL Taxonomy Extension Definition Linkbase * | |
| EX-101.LAB | XBRL Taxonomy Extension Labels Linkbase * | |
| EX-101.PRE | XBRL Taxonomy Extension Presentation Linkbase * |
* Filed herewith
| (1) | Filed as exhibit to 8-K filed on January 31, 2013 and incorporated herein by reference. |
| (2) | Filed as an exhibit to S-1 filed August 17, 2010 and incorporated herein by reference. |
| (3) | Filed as an exhibit to 8-K filed January 23, 2013 and incorporated herein by reference. |
| (4) | Filed as an exhibit to 8-K filed February 14, 2013 and incorporated herein by reference. |
| (5) | Filed as exhibit to 8-K/A filed on March 14, 2103 and incorporated herein by reference. |
| (6) | Filed as an exhibit to 8-K filed June 9, 2014 and incorporated herein by reference. |
| (7) | Filed as an exhibit to 8-K filed October 3, 2014 and incorporated herein by reference. |
| (8) | Filed as an exhibit to 8-K filed November 6, 2014 and incorporated herein by reference. |
| (9) | Filed as an exhibit to 8-K filed January 7, 2015 and incorporated herein. |
| (10) | Filed as an exhibit to S-1 (Registration No. 333-189337) filed June 14, 2013 and incorporated herein by reference. |
| (11) | Filed as an exhibit to S-1/A (Registration No. 333-203357) filed July 15, 2015 and incorporated herein by reference. |
| (12) | Filed as an exhibit to S-1 (Registration No. 333-203357) filed April 4, 2015 and incorporated herein by reference. |
| (13) | Filed as an exhibit to S-1/A (Registration No. 333-203357) filed July 9, 2015 and incorporated herein by reference. |
| 65 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| DELMAR PHARMACEUTICALS, INC. | ||
| Dated: September 3, 2015 | By: | /s/ Jeffrey Bacha |
| Name: Jeffrey Bacha | ||
| Title:
Chief Executive Officer (principal executive officer) | ||
| Dated: September 3, 2015 | By: | /s/ Scott Praill |
| Name: Scott Praill | ||
| Title:
Chief Financial Officer (principal financial and accounting officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| SIGNATURE | TITLE | DATE | ||
| /s/ Jeffrey Bacha | Chief Executive Officer, Director | September 3, 2015 | ||
| Jeffrey Bacha | (principal executive officer) | |||
| /s/ Scott Praill | Chief Financial Officer | September 3, 2015 | ||
| Scott Praill | (principal financial and accounting officer) | |||
| /s/ Dennis Brown | Director | September 3, 2015 | ||
| Dennis Brown | ||||
| /s/ William Garner | Director | September 3, 2015 | ||
| William Garner | ||||
| /s/ John K. Bell | Director | September 3, 2015 | ||
| John K. Bell | ||||
| /s/ Robert J. Toth, Jr. | Director | September 3, 2015 | ||
| Robert J. Toth | ||||
| /s/ Lynda Cranston | Director | September 3, 2015 | ||
| Lynda Cranston | ||||
| /s/ Erich Mohr | Director | September 3, 2015 | ||
| Erich Mohr |
66

