Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NephroGenex, Inc. | a15-18852_18k.htm |
Exhibit 99.1
|
|
Corporate Presentation September 2015 |
|
|
Forward-Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting kidney diseases, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2014 filed with the Securities and Exchange Commission on March 24, 2015 and the “Risk Factors” sections of our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2015 and June 30, 2015 filed with the Securities and Exchange Commission on May 13, 2015 and August 12, 2015, respectively. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law. You should read carefully our Forward-Looking Statements and the factors described in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2014 filed with the Securities and Exchange Commission on March 24, 2015 and the “Risk Factors” sections of our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2015 and June 30, 2015 filed with the Securities and Exchange Commission on May 13, 2015 and August 12, 2015, respectively, to better understand the risks and uncertainties inherent in our business. 2 |
|
|
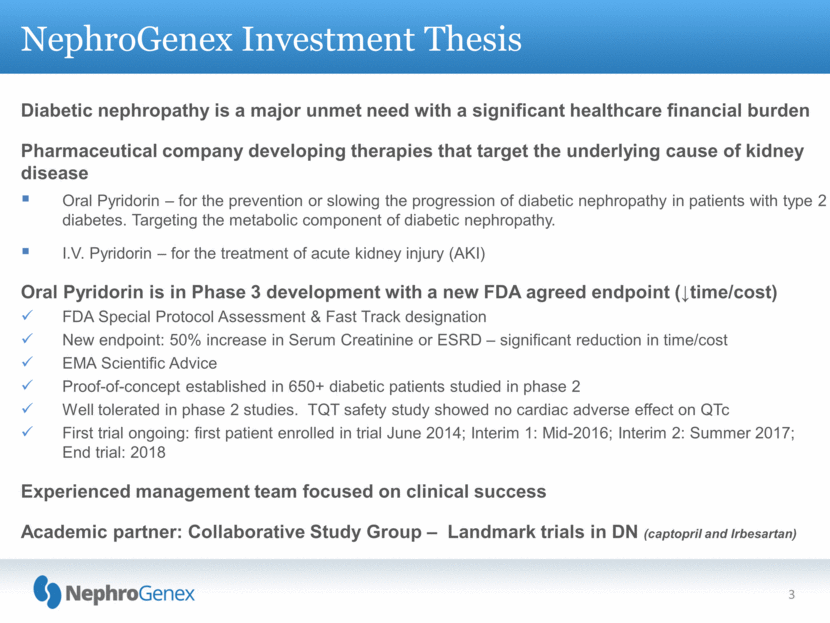
NephroGenex Investment Thesis 3 Diabetic nephropathy is a major unmet need with a significant healthcare financial burden Pharmaceutical company developing therapies that target the underlying cause of kidney disease Oral Pyridorin – for the prevention or slowing the progression of diabetic nephropathy in patients with type 2 diabetes. Targeting the metabolic component of diabetic nephropathy. I.V. Pyridorin – for the treatment of acute kidney injury (AKI) Oral Pyridorin is in Phase 3 development with a new FDA agreed endpoint (time/cost) FDA Special Protocol Assessment & Fast Track designation New endpoint: 50% increase in Serum Creatinine or ESRD – significant reduction in time/cost EMA Scientific Advice Proof-of-concept established in 650+ diabetic patients studied in phase 2 Well tolerated in phase 2 studies. TQT safety study showed no cardiac adverse effect on QTc First trial ongoing: first patient enrolled in trial June 2014; Interim 1: Mid-2016; Interim 2: Summer 2017; End trial: 2018 Experienced management team focused on clinical success Academic partner: Collaborative Study Group – Landmark trials in DN (captopril and Irbesartan) |
|
|
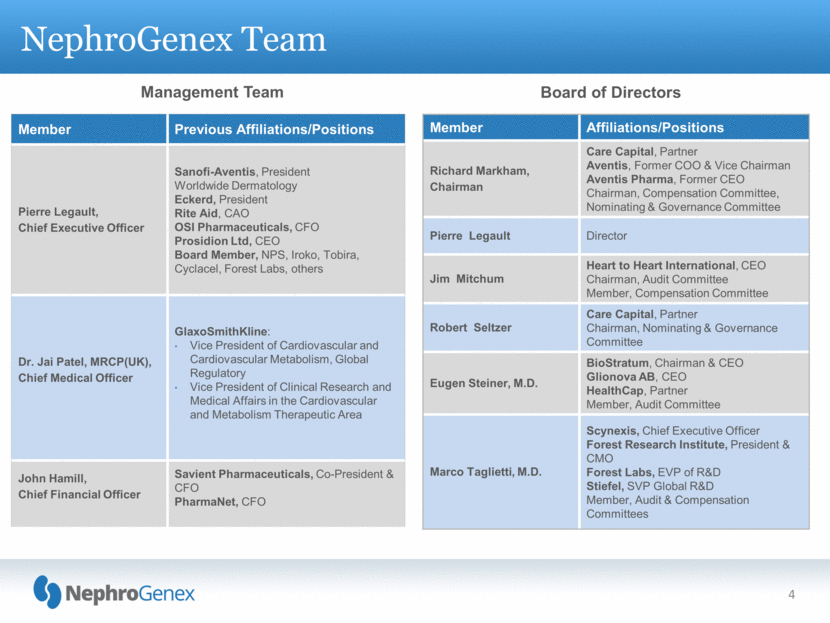
NephroGenex Team Member Previous Affiliations/Positions Pierre Legault, Chief Executive Officer Sanofi-Aventis, President Worldwide Dermatology Eckerd, President Rite Aid, CAO OSI Pharmaceuticals, CFO Prosidion Ltd, CEO Board Member, NPS, Iroko, Tobira, Cyclacel, Forest Labs, others Dr. Jai Patel, MRCP(UK), Chief Medical Officer GlaxoSmithKline: Vice President of Cardiovascular and Cardiovascular Metabolism, Global Regulatory Vice President of Clinical Research and Medical Affairs in the Cardiovascular and Metabolism Therapeutic Area John Hamill, Chief Financial Officer Savient Pharmaceuticals, Co-President & CFO PharmaNet, CFO Management Team Board of Directors Member Affiliations/Positions Richard Markham, Chairman Care Capital, Partner Aventis, Former COO & Vice Chairman Aventis Pharma, Former CEO Chairman, Compensation Committee, Nominating & Governance Committee Pierre Legault Director Jim Mitchum Heart to Heart International, CEO Chairman, Audit Committee Member, Compensation Committee Robert Seltzer Care Capital, Partner Chairman, Nominating & Governance Committee Eugen Steiner, M.D. BioStratum, Chairman & CEO Glionova AB, CEO HealthCap, Partner Member, Audit Committee Marco Taglietti, M.D. Scynexis, Chief Executive Officer Forest Research Institute, President & CMO Forest Labs, EVP of R&D Stiefel, SVP Global R&D Member, Audit & Compensation Committees 4 |
|
|
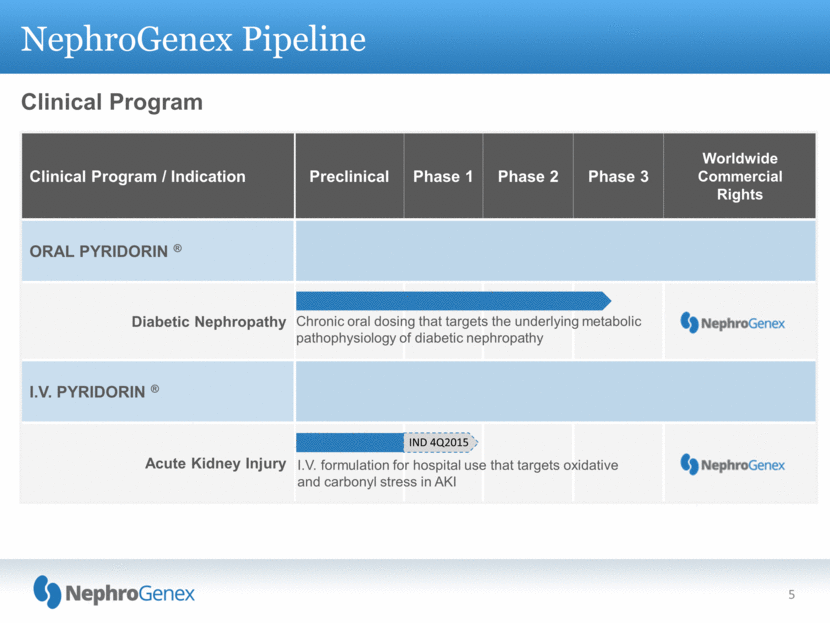
NephroGenex Pipeline Clinical Program Clinical Program / Indication Preclinical Phase 1 Phase 2 Phase 3 Worldwide Commercial Rights ORAL PYRIDORIN ® Diabetic Nephropathy I.V. PYRIDORIN ® Acute Kidney Injury Chronic oral dosing that targets the underlying metabolic pathophysiology of diabetic nephropathy I.V. formulation for hospital use that targets oxidative and carbonyl stress in AKI 5 IND 4Q2015 |
|
|
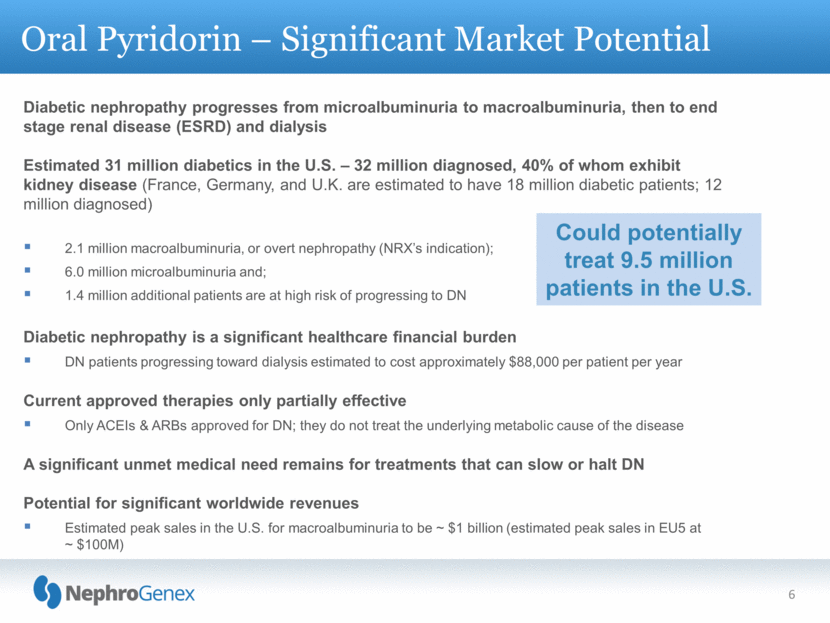
Oral Pyridorin – Significant Market Potential 6 Diabetic nephropathy progresses from microalbuminuria to macroalbuminuria, then to end stage renal disease (ESRD) and dialysis Estimated 31 million diabetics in the U.S. – 32 million diagnosed, 40% of whom exhibit kidney disease (France, Germany, and U.K. are estimated to have 18 million diabetic patients; 12 million diagnosed) 2.1 million macroalbuminuria, or overt nephropathy (NRX’s indication); 6.0 million microalbuminuria and; 1.4 million additional patients are at high risk of progressing to DN Diabetic nephropathy is a significant healthcare financial burden DN patients progressing toward dialysis estimated to cost approximately $88,000 per patient per year Current approved therapies only partially effective Only ACEIs & ARBs approved for DN; they do not treat the underlying metabolic cause of the disease A significant unmet medical need remains for treatments that can slow or halt DN Potential for significant worldwide revenues Estimated peak sales in the U.S. for macroalbuminuria to be ~ $1 billion (estimated peak sales in EU5 at ~ $100M) Could potentially treat 9.5 million patients in the U.S. |
|
|
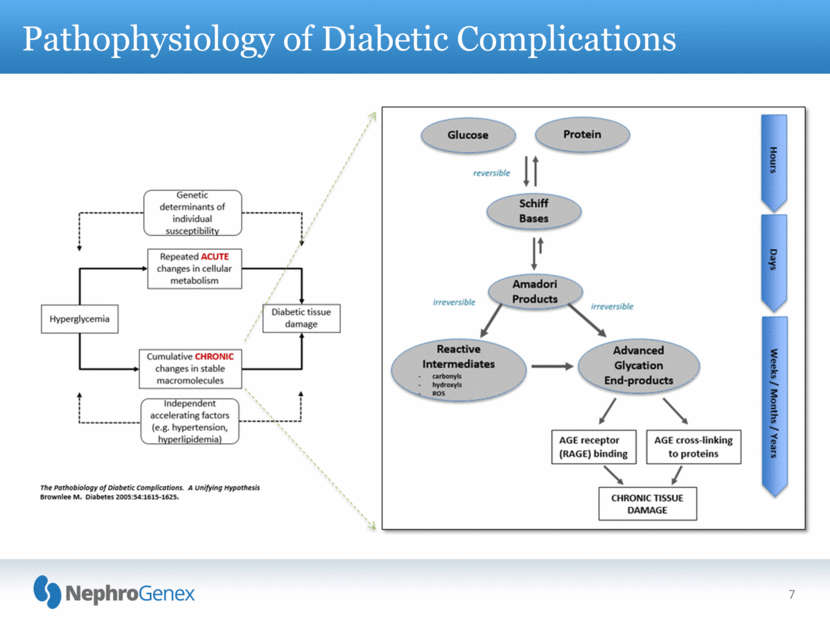
7 Pathophysiology of Diabetic Complications |
|
|
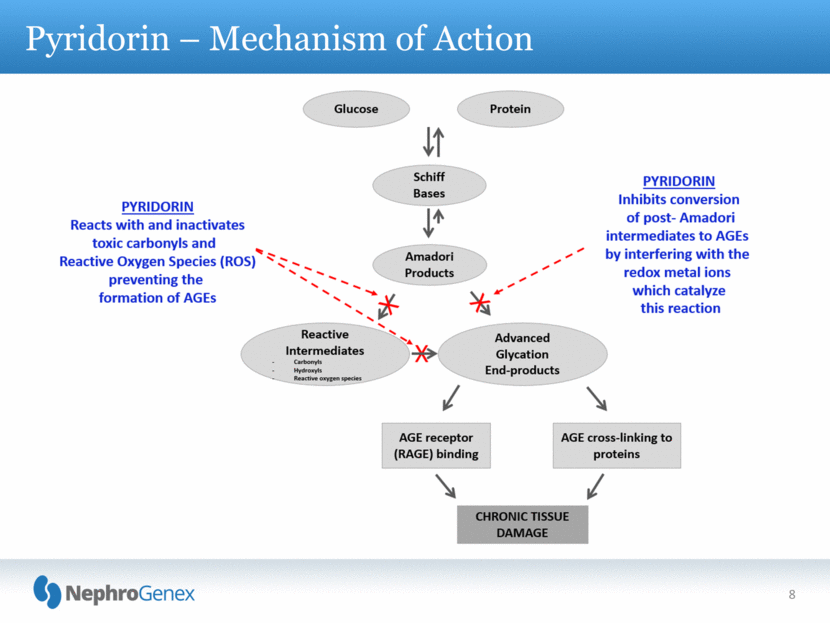
8 Pyridorin – Mechanism of Action |
|
|
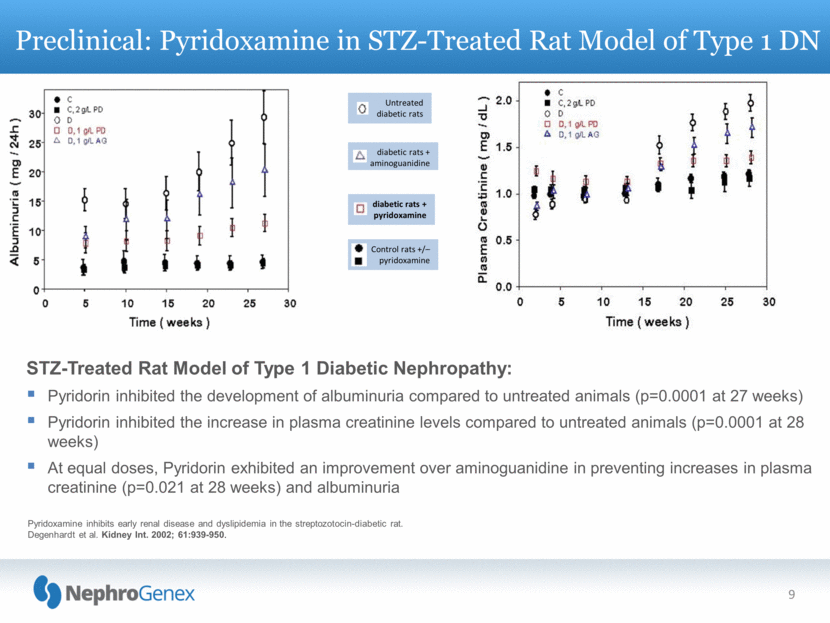
9 Preclinical: Pyridoxamine in STZ-Treated Rat Model of Type 1 DN Control rats +/– pyridoxamine Untreated diabetic rats diabetic rats + aminoguanidine diabetic rats + pyridoxamine Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Degenhardt et al. Kidney Int. 2002; 61:939-950. STZ-Treated Rat Model of Type 1 Diabetic Nephropathy: Pyridorin inhibited the development of albuminuria compared to untreated animals (p=0.0001 at 27 weeks) Pyridorin inhibited the increase in plasma creatinine levels compared to untreated animals (p=0.0001 at 28 weeks) At equal doses, Pyridorin exhibited an improvement over aminoguanidine in preventing increases in plasma creatinine (p=0.021 at 28 weeks) and albuminuria |
|
|
10 Pyridorin Development Program Target Indication: Prevention or slowing the progression of diabetic nephropathy with type 2 diabetes Program: Two identical, double-blind Phase 3 studies of 600 patients each Regulatory Agreements: FDA Special Protocol Assessment New endpoint of 50% increase in serum creatinine (decreased time/cost) Fast Track designation EMA Scientific Advice Collaborative Study Group – experience with Landmark DN Trials: Captopril study in diabetic nephropathy Irbesartan in diabetic nephropathy trial (IDNT) |
|
|
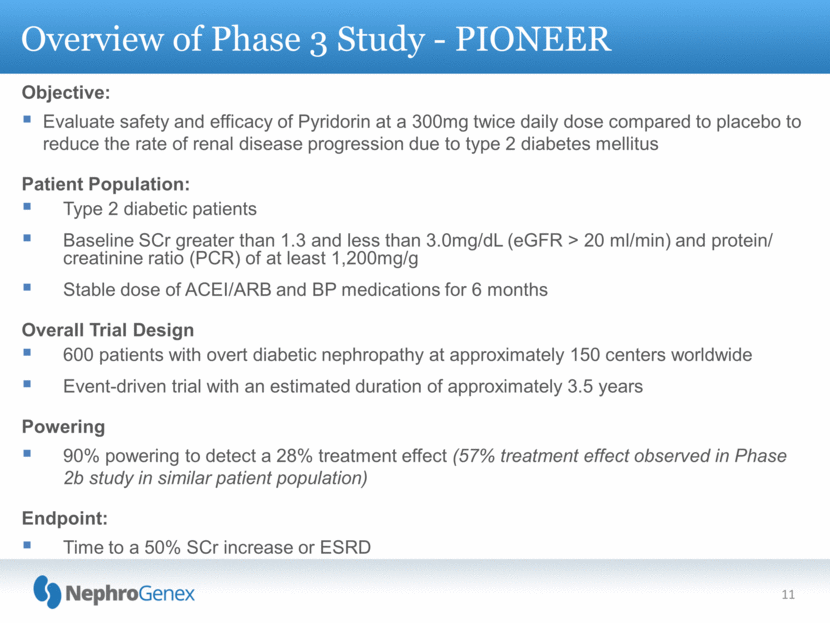
Objective: Evaluate safety and efficacy of Pyridorin at a 300mg twice daily dose compared to placebo to reduce the rate of renal disease progression due to type 2 diabetes mellitus Patient Population: Type 2 diabetic patients Baseline SCr greater than 1.3 and less than 3.0mg/dL (eGFR > 20 ml/min) and protein/ creatinine ratio (PCR) of at least 1,200mg/g Stable dose of ACEI/ARB and BP medications for 6 months Overall Trial Design 600 patients with overt diabetic nephropathy at approximately 150 centers worldwide Event-driven trial with an estimated duration of approximately 3.5 years Powering 90% powering to detect a 28% treatment effect (57% treatment effect observed in Phase 2b study in similar patient population) Endpoint: Time to a 50% SCr increase or ESRD Overview of Phase 3 Study - PIONEER 11 |
|
|
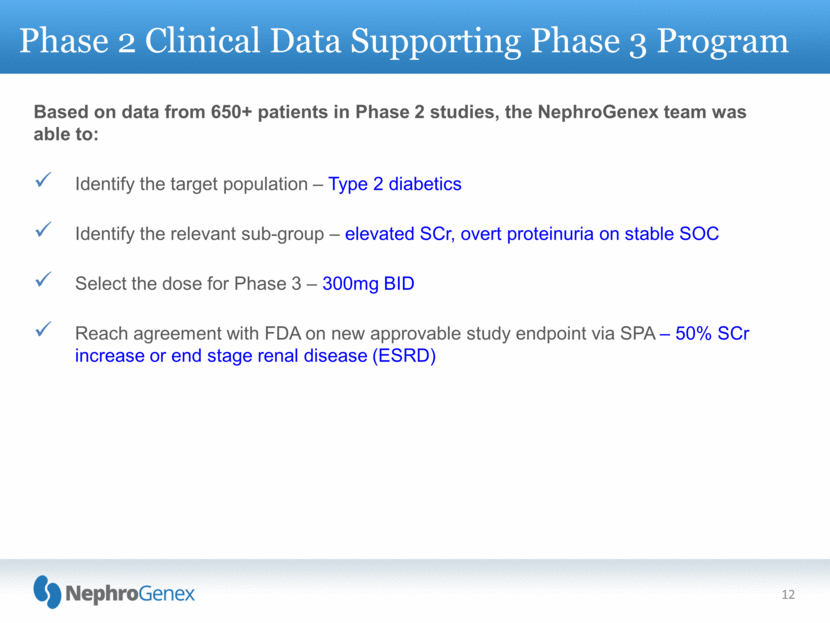
12 Phase 2 Clinical Data Supporting Phase 3 Program Based on data from 650+ patients in Phase 2 studies, the NephroGenex team was able to: Identify the target population – Type 2 diabetics Identify the relevant sub-group – elevated SCr, overt proteinuria on stable SOC Select the dose for Phase 3 – 300mg BID Reach agreement with FDA on new approvable study endpoint via SPA – 50% SCr increase or end stage renal disease (ESRD) |
|
|
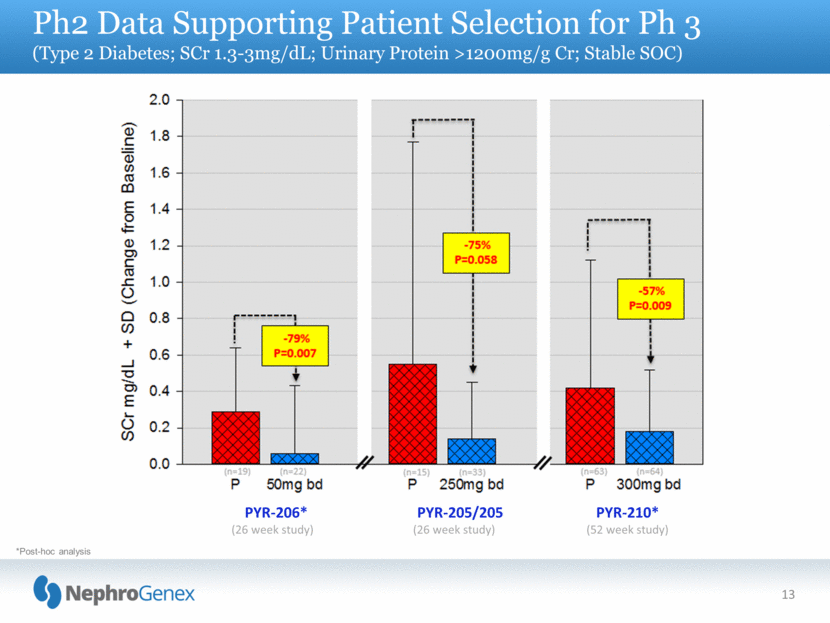
13 Ph2 Data Supporting Patient Selection for Ph 3 (Type 2 Diabetes; SCr 1.3-3mg/dL; Urinary Protein >1200mg/g Cr; Stable SOC) *Post-hoc analysis PYR-206* (26 week study) PYR-205/205 (26 week study) PYR-210* (52 week study) |
|
|
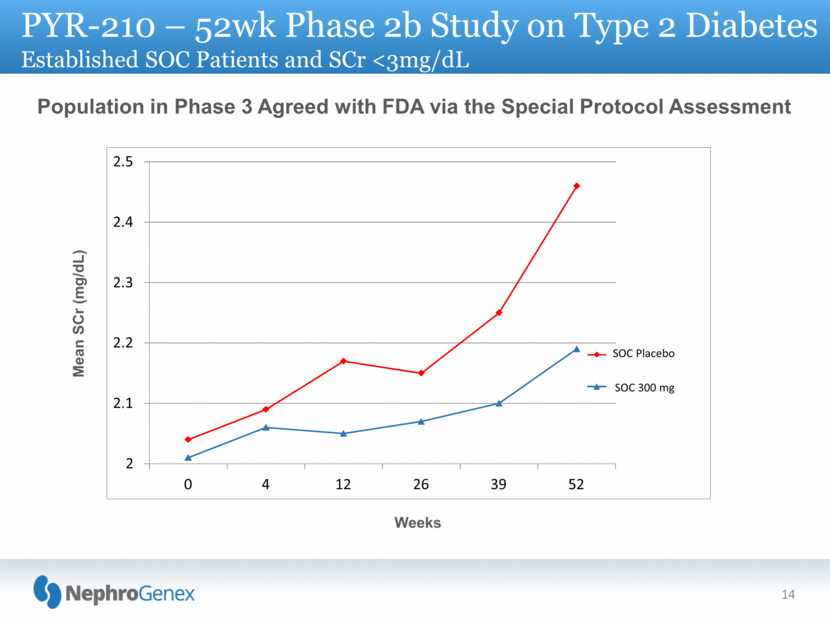
2 2.1 2.2 2.3 2.4 2.5 0 4 12 26 39 52 SOC Placebo SOC 300 mg 14 Population in Phase 3 Agreed with FDA via the Special Protocol Assessment Weeks Mean SCr (mg/dL) PYR-210 – 52wk Phase 2b Study on Type 2 Diabetes Established SOC Patients and SCr <3mg/dL |
|
|
PIONEER Phase 3 Interim Analysis 15 DSMB: Monitoring unblinded safety information on an ongoing basis Interim Analysis 1* (mid-2016): Independent biostatistician will undertake an interim analysis when at least 150 patients have completed a minimum of 12 months on treatment Interim Analysis 2* (summer-2017): Independent biostatistician will undertake an interim analysis when approximately 50% of the primary endpoint events of 50% increase in SCr or ESRD have been accrued Interim Analysis Output: The study is on track to reach the primary endpoint The study will need additional events than originally planned to reach the primary endpoint The study is unlikely to reach the primary endpoint and results should be examined to generate new hypothesis for investigation * Timing and design to be finalized & agreed with FDA |
|
|
Pyridorin was well tolerated in Phase 1 & 2 trials with a safety profile comparable to placebo: No maximum dose tolerability found in animals and healthy humans No significant differences between groups in SAEs or mortality No differences between groups for laboratory parameters (hematology, chemistry, HbA1c) No adverse effect on the QTc interval at therapeutic and supra-therapeutic doses No clinically meaningful differences between groups in the AE profile with the possible exception of a numerical increase in diarrhea and constipation with the higher dose of 300mg BID NephroGenex Phase 1 & 2 Safety Trials 16 |
|
|
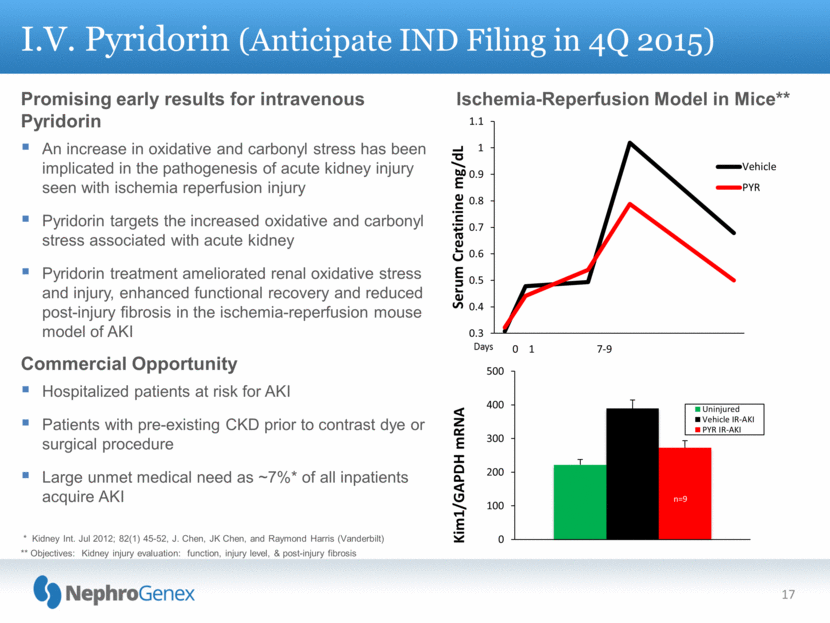
Promising early results for intravenous Pyridorin An increase in oxidative and carbonyl stress has been implicated in the pathogenesis of acute kidney injury seen with ischemia reperfusion injury Pyridorin targets the increased oxidative and carbonyl stress associated with acute kidney Pyridorin treatment ameliorated renal oxidative stress and injury, enhanced functional recovery and reduced post-injury fibrosis in the ischemia-reperfusion mouse model of AKI Commercial Opportunity Hospitalized patients at risk for AKI Patients with pre-existing CKD prior to contrast dye or surgical procedure Large unmet medical need as ~7%* of all inpatients acquire AKI I.V. Pyridorin (Anticipate IND Filing in 4Q 2015) * Kidney Int. Jul 2012; 82(1) 45-52, J. Chen, JK Chen, and Raymond Harris (Vanderbilt) ** Objectives: Kidney injury evaluation: function, injury level, & post-injury fibrosis Ischemia-Reperfusion Model in Mice** 17 0 100 200 300 400 500 Kim1/GAPDH mRNA Uninjured Vehicle IR-AKI PYR IR-AKI n=9 n=10 n=10 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 1.1 Serum Creatinine mg/dL Vehicle PYR 0 1 7 - 9 |
|
|
No composition of matter claim since this molecule already exists in the human body in microscopic quantities U.S. Method of use (including patient population and dosage) patent December 2028 (with anticipated patent term extension) Method of manufacture patent February 2025 NCE - 5 year exclusivity Europe In most EU countries method of use (including patient population and dosage) patent June 2029 (with anticipated 5 year patent term extension) NCE - 10 year exclusivity Canada Method of use (including patient population and dosage) patent June 2024 NCE - 8 year exclusivity Japan NCE - up to 8 year exclusivity No future royalties, one minimal $200K milestone at approval 18 Exclusivity Patents and New Chemical Entity (NCE) |
|
|
NephroGenex Milestones 19 Achieved Expected Feb-2014 Completion of Initial Public Offering Mar-2014 Secured CRO June-2014 Enrolled first patient in PIONEER pivotal Phase 3 trial Nov-2014 Publication on new endpoint Nov-2014 Poster presentations at American Society of Nephrology Dec-2014 Successful completion of TQT cardiac safety study Jan-2015 Secured EMA support of study design & new SCr increase endpoint 2015 I.V. PYR AKI Preclinical results (3Q 2015) IND filing for I.V. PYR AKI (4Q 2015) 2016 Completion of PIONEER Phase 3 recruitment (1H 2016) PIONEER 1st interim analysis (Mid-2016) 2017 PIONEER 2nd interim analysis (Summer 2017) |
|
|
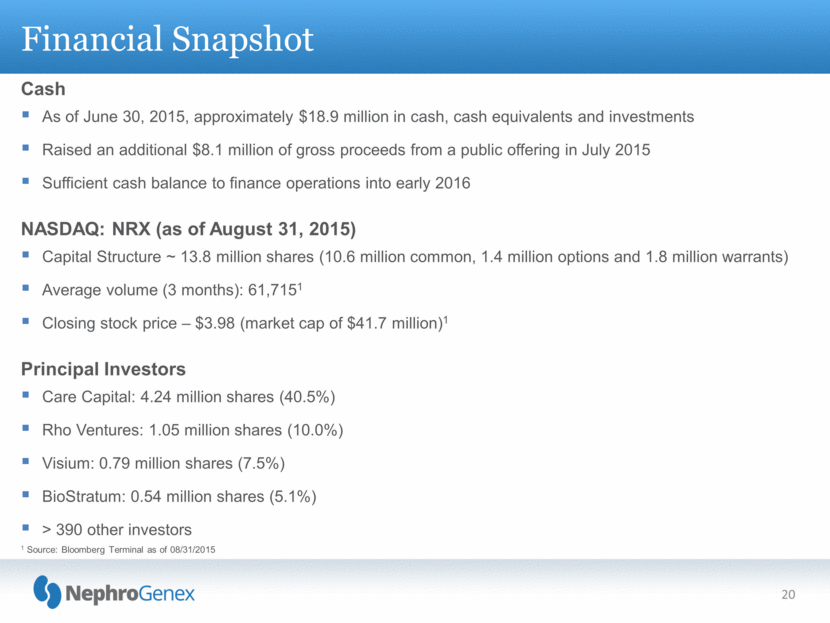
Financial Snapshot Cash As of June 30, 2015, approximately $18.9 million in cash, cash equivalents and investments Raised an additional $8.1 million of gross proceeds from a public offering in July 2015 Sufficient cash balance to finance operations into early 2016 NASDAQ: NRX (as of August 31, 2015) Capital Structure ~ 13.8 million shares (10.6 million common, 1.4 million options and 1.8 million warrants) Average volume (3 months): 61,7151 Closing stock price – $3.98 (market cap of $41.7 million)1 Principal Investors Care Capital: 4.24 million shares (40.5%) Rho Ventures: 1.05 million shares (10.0%) Visium: 0.79 million shares (7.5%) BioStratum: 0.54 million shares (5.1%) > 390 other investors 20 1 Source: Bloomberg Terminal as of 08/31/2015 |
|
|
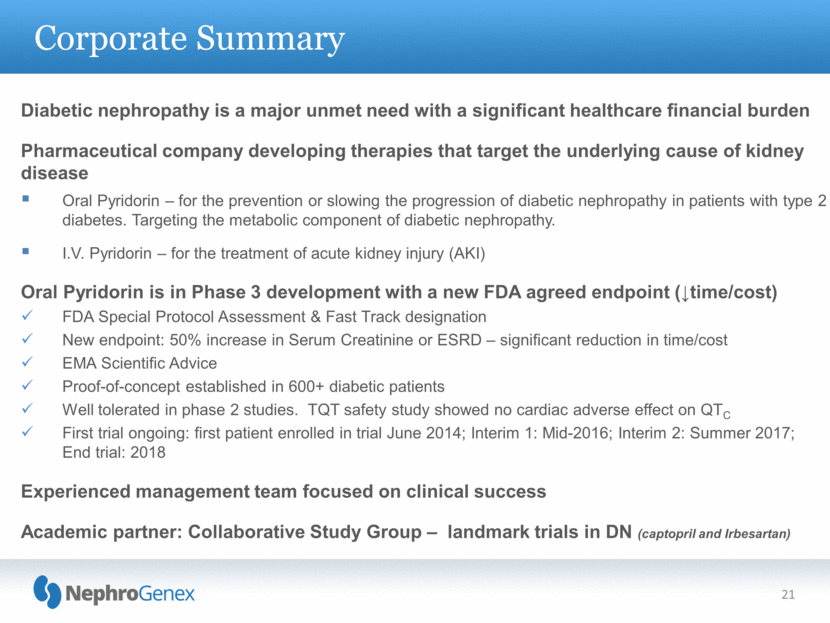
Corporate Summary 21 Diabetic nephropathy is a major unmet need with a significant healthcare financial burden Pharmaceutical company developing therapies that target the underlying cause of kidney disease Oral Pyridorin – for the prevention or slowing the progression of diabetic nephropathy in patients with type 2 diabetes. Targeting the metabolic component of diabetic nephropathy. I.V. Pyridorin – for the treatment of acute kidney injury (AKI) Oral Pyridorin is in Phase 3 development with a new FDA agreed endpoint (time/cost) FDA Special Protocol Assessment & Fast Track designation New endpoint: 50% increase in Serum Creatinine or ESRD – significant reduction in time/cost EMA Scientific Advice Proof-of-concept established in 600+ diabetic patients Well tolerated in phase 2 studies. TQT safety study showed no cardiac adverse effect on QTC First trial ongoing: first patient enrolled in trial June 2014; Interim 1: Mid-2016; Interim 2: Summer 2017; End trial: 2018 Experienced management team focused on clinical success Academic partner: Collaborative Study Group – landmark trials in DN (captopril and Irbesartan) |
|
|
Appendix 22 |
|
|
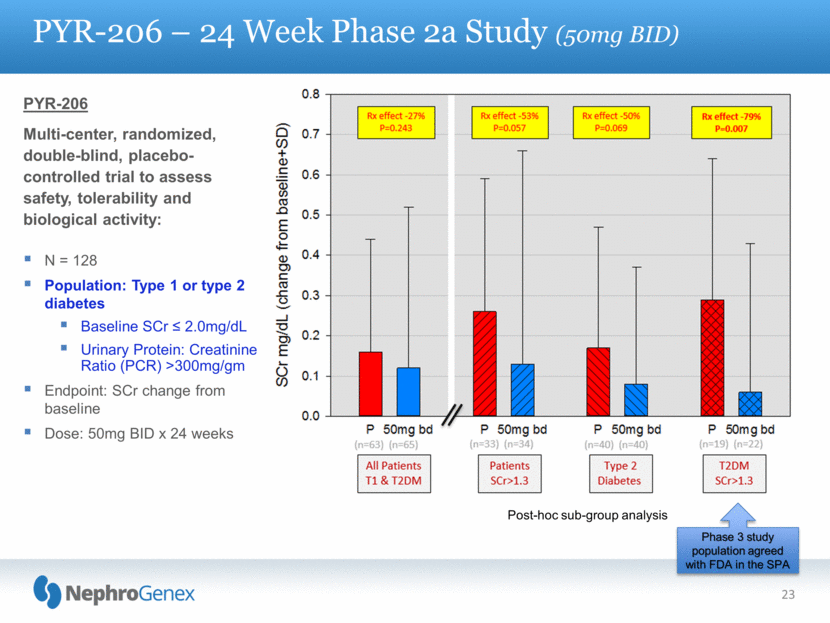
23 PYR-206 – 24 Week Phase 2a Study (50mg BID) PYR-206 Multi-center, randomized, double-blind, placebo-controlled trial to assess safety, tolerability and biological activity: N = 128 Population: Type 1 or type 2 diabetes Baseline SCr < 2.0mg/dL Urinary Protein: Creatinine Ratio (PCR) >300mg/gm Endpoint: SCr change from baseline Dose: 50mg BID x 24 weeks Post-hoc sub-group analysis Phase 3 study population agreed with FDA in the SPA |
|
|
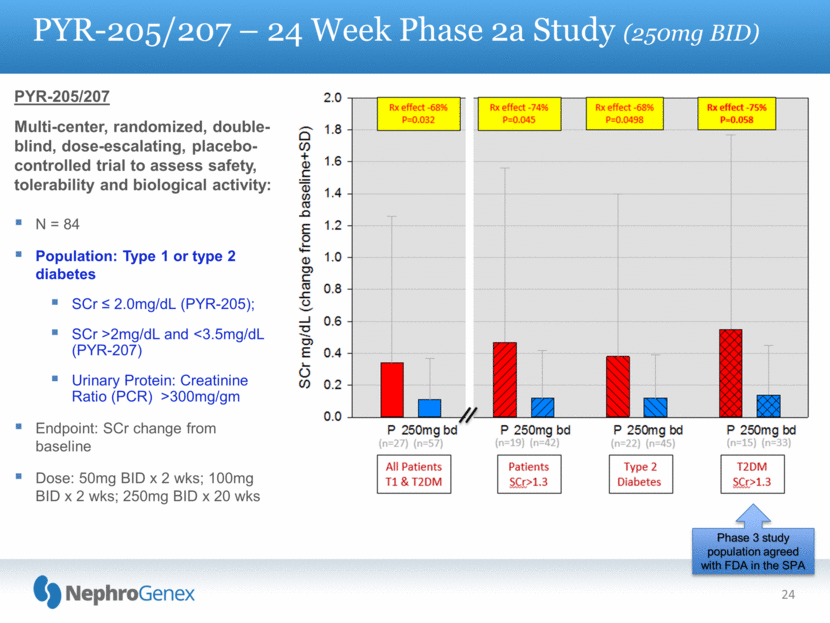
24 PYR-205/207 – 24 Week Phase 2a Study (250mg BID) PYR-205/207 Multi-center, randomized, double-blind, dose-escalating, placebo-controlled trial to assess safety, tolerability and biological activity: N = 84 Population: Type 1 or type 2 diabetes SCr < 2.0mg/dL (PYR-205); SCr >2mg/dL and <3.5mg/dL (PYR-207) Urinary Protein: Creatinine Ratio (PCR) >300mg/gm Endpoint: SCr change from baseline Dose: 50mg BID x 2 wks; 100mg BID x 2 wks; 250mg BID x 20 wks Phase 3 study population agreed with FDA in the SPA |
|
|
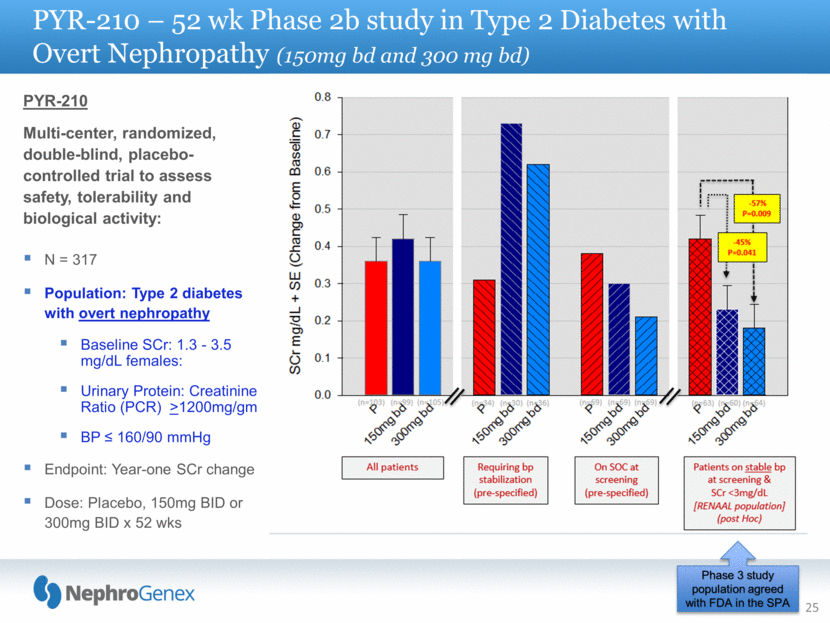
25 PYR-210 – 52 wk Phase 2b study in Type 2 Diabetes with Overt Nephropathy (150mg bd and 300 mg bd) PYR-210 Multi-center, randomized, double-blind, placebo-controlled trial to assess safety, tolerability and biological activity: N = 317 Population: Type 2 diabetes with overt nephropathy Baseline SCr: 1.3 - 3.5 mg/dL females: Urinary Protein: Creatinine Ratio (PCR) >1200mg/gm BP 160/90 mmHg Endpoint: Year-one SCr change Dose: Placebo, 150mg BID or 300mg BID x 52 wks Phase 3 study population agreed with FDA in the SPA |
|
|
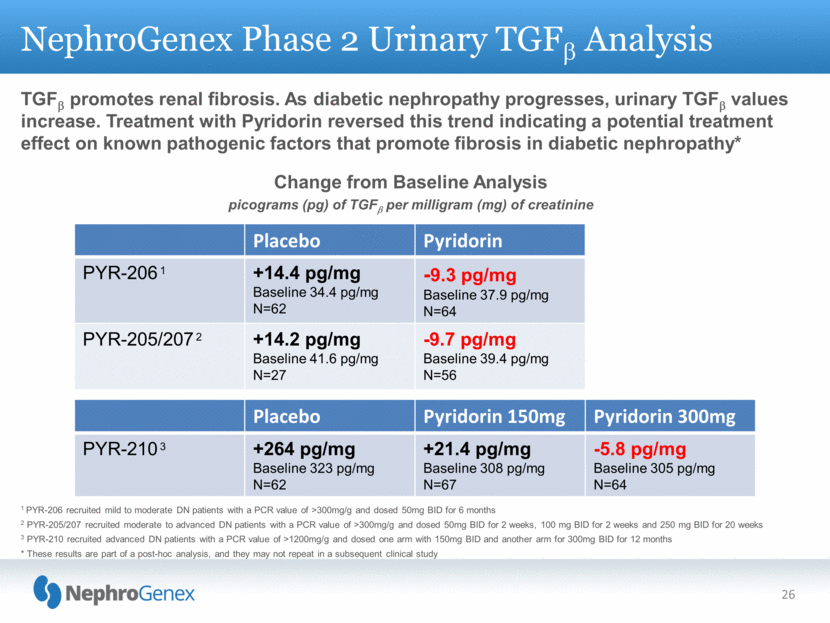
1 PYR-206 recruited mild to moderate DN patients with a PCR value of >300mg/g and dosed 50mg BID for 6 months 2 PYR-205/207 recruited moderate to advanced DN patients with a PCR value of >300mg/g and dosed 50mg BID for 2 weeks, 100 mg BID for 2 weeks and 250 mg BID for 20 weeks 3 PYR-210 recruited advanced DN patients with a PCR value of >1200mg/g and dosed one arm with 150mg BID and another arm for 300mg BID for 12 months * These results are part of a post-hoc analysis, and they may not repeat in a subsequent clinical study NephroGenex Phase 2 Urinary TGFb Analysis TGFb promotes renal fibrosis. As diabetic nephropathy progresses, urinary TGFb values increase. Treatment with Pyridorin reversed this trend indicating a potential treatment effect on known pathogenic factors that promote fibrosis in diabetic nephropathy* 26 Placebo Pyridorin PYR-206 1 +14.4 pg/mg Baseline 34.4 pg/mg N=62 -9.3 pg/mg Baseline 37.9 pg/mg N=64 PYR-205/207 2 +14.2 pg/mg Baseline 41.6 pg/mg N=27 -9.7 pg/mg Baseline 39.4 pg/mg N=56 Placebo Pyridorin 150mg Pyridorin 300mg PYR-210 3 +264 pg/mg Baseline 323 pg/mg N=62 +21.4 pg/mg Baseline 308 pg/mg N=67 -5.8 pg/mg Baseline 305 pg/mg N=64 Change from Baseline Analysis picograms (pg) of TGFb per milligram (mg) of creatinine |
|
|
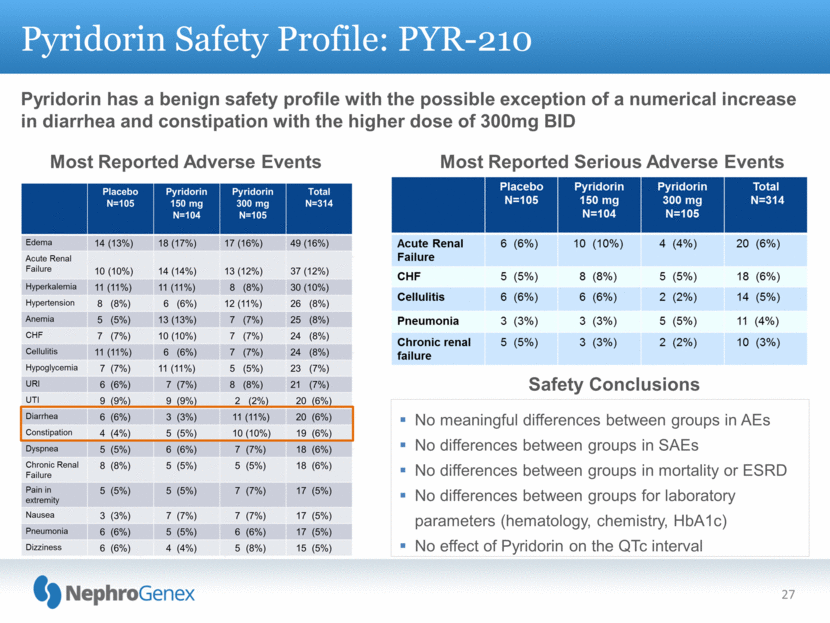
Pyridorin has a benign safety profile with the possible exception of a numerical increase in diarrhea and constipation with the higher dose of 300mg BID Pyridorin Safety Profile: PYR-210 27 Most Reported Adverse Events Most Reported Serious Adverse Events No meaningful differences between groups in AEs No differences between groups in SAEs No differences between groups in mortality or ESRD No differences between groups for laboratory parameters (hematology, chemistry, HbA1c) No effect of Pyridorin on the QTc interval Safety Conclusions |
|
|
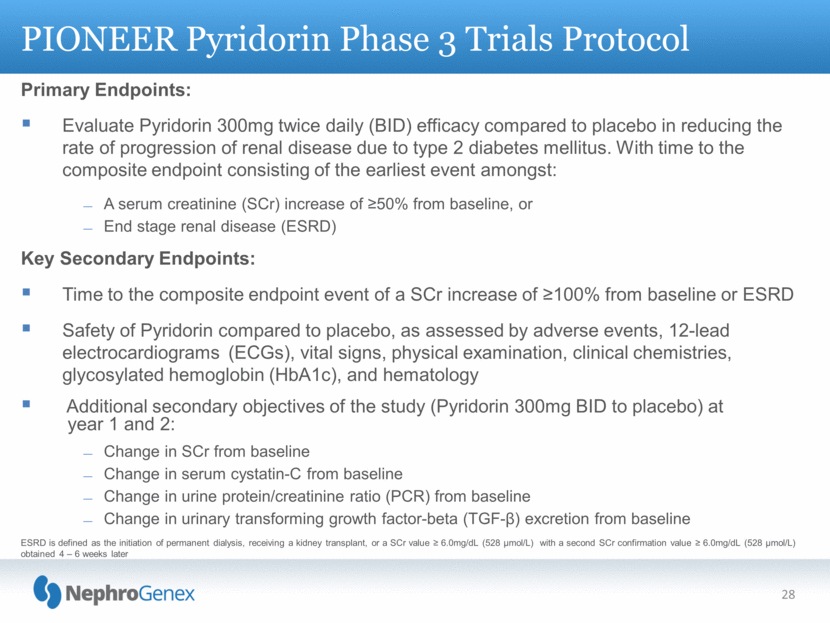
Primary Endpoints: Evaluate Pyridorin 300mg twice daily (BID) efficacy compared to placebo in reducing the rate of progression of renal disease due to type 2 diabetes mellitus. With time to the composite endpoint consisting of the earliest event amongst: A serum creatinine (SCr) increase of >50% from baseline, or End stage renal disease (ESRD) Key Secondary Endpoints: Time to the composite endpoint event of a SCr increase of 100% from baseline or ESRD Safety of Pyridorin compared to placebo, as assessed by adverse events, 12-lead electrocardiograms (ECGs), vital signs, physical examination, clinical chemistries, glycosylated hemoglobin (HbA1c), and hematology Additional secondary objectives of the study (Pyridorin 300mg BID to placebo) at year 1 and 2: Change in SCr from baseline Change in serum cystatin-C from baseline Change in urine protein/creatinine ratio (PCR) from baseline Change in urinary transforming growth factor-beta (TGF-) excretion from baseline PIONEER Pyridorin Phase 3 Trials Protocol 28 ESRD is defined as the initiation of permanent dialysis, receiving a kidney transplant, or a SCr value 6.0mg/dL (528 mol/L) with a second SCr confirmation value 6.0mg/dL (528 mol/L) obtained 4 – 6 weeks later |
|
|
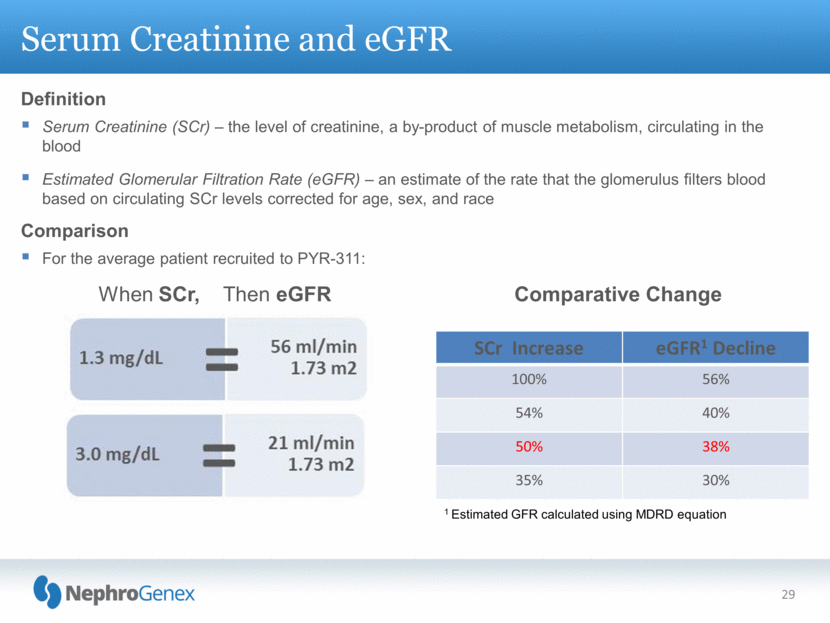
Definition Serum Creatinine (SCr) – the level of creatinine, a by-product of muscle metabolism, circulating in the blood Estimated Glomerular Filtration Rate (eGFR) – an estimate of the rate that the glomerulus filters blood based on circulating SCr levels corrected for age, sex, and race Comparison For the average patient recruited to PYR-311: Serum Creatinine and eGFR 29 When SCr, Then eGFR Comparative Change 1 Estimated GFR calculated using MDRD equation SCr Increase eGFR1 Decline 100% 56% 54% 40% 50% 38% 35% 30% |
|
|
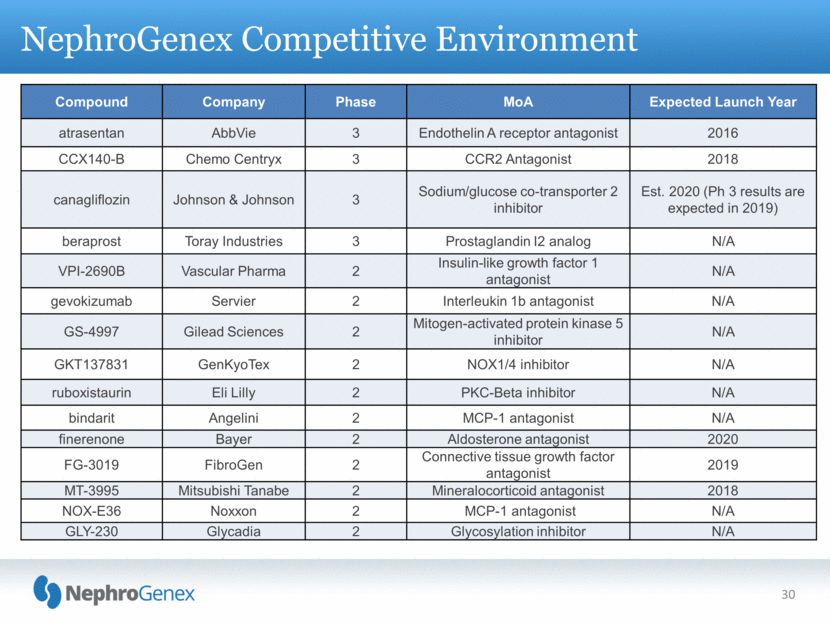
NephroGenex Competitive Environment 30 Compound Company Phase MoA Expected Launch Year atrasentan AbbVie 3 Endothelin A receptor antagonist 2016 CCX140-B Chemo Centryx 3 CCR2 Antagonist 2018 canagliflozin Johnson & Johnson 3 Sodium/glucose co-transporter 2 inhibitor Est. 2020 (Ph 3 results are expected in 2019) beraprost Toray Industries 3 Prostaglandin I2 analog N/A VPI-2690B Vascular Pharma 2 Insulin-like growth factor 1 antagonist N/A gevokizumab Servier 2 Interleukin 1b antagonist N/A GS-4997 Gilead Sciences 2 Mitogen-activated protein kinase 5 inhibitor N/A GKT137831 GenKyoTex 2 NOX1/4 inhibitor N/A ruboxistaurin Eli Lilly 2 PKC-Beta inhibitor N/A bindarit Angelini 2 MCP-1 antagonist N/A finerenone Bayer 2 Aldosterone antagonist 2020 FG-3019 FibroGen 2 Connective tissue growth factor antagonist 2019 MT-3995 Mitsubishi Tanabe 2 Mineralocorticoid antagonist 2018 NOX-E36 Noxxon 2 MCP-1 antagonist N/A GLY-230 Glycadia 2 Glycosylation inhibitor N/A |
|
|
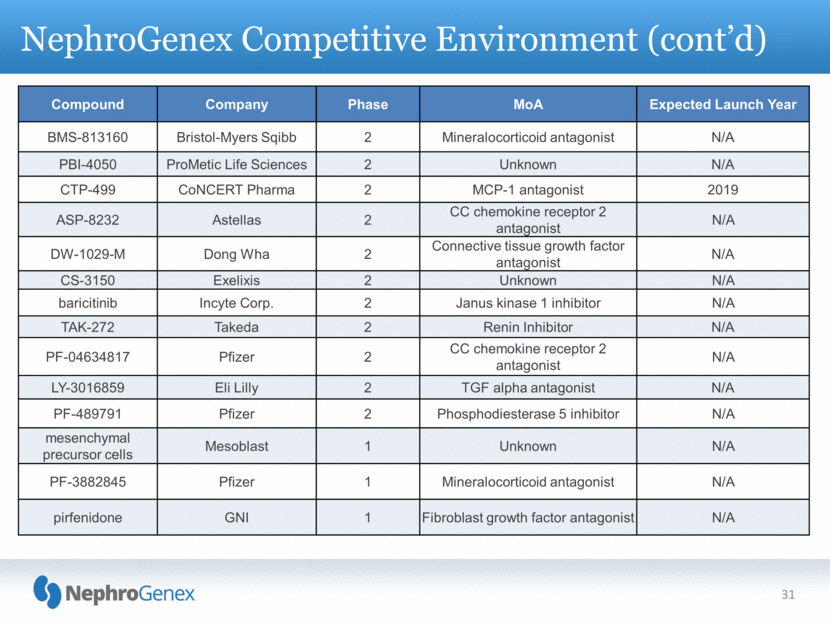
NephroGenex Competitive Environment (cont’d) 31 Compound Company Phase MoA Expected Launch Year BMS-813160 Bristol-Myers Sqibb 2 Mineralocorticoid antagonist N/A PBI-4050 ProMetic Life Sciences 2 Unknown N/A CTP-499 CoNCERT Pharma 2 MCP-1 antagonist 2019 ASP-8232 Astellas 2 CC chemokine receptor 2 antagonist N/A DW-1029-M Dong Wha 2 Connective tissue growth factor antagonist N/A CS-3150 Exelixis 2 Unknown N/A baricitinib Incyte Corp. 2 Janus kinase 1 inhibitor N/A TAK-272 Takeda 2 Renin Inhibitor N/A PF-04634817 Pfizer 2 CC chemokine receptor 2 antagonist N/A LY-3016859 Eli Lilly 2 TGF alpha antagonist N/A PF-489791 Pfizer 2 Phosphodiesterase 5 inhibitor N/A mesenchymal precursor cells Mesoblast 1 Unknown N/A PF-3882845 Pfizer 1 Mineralocorticoid antagonist N/A pirfenidone GNI 1 Fibroblast growth factor antagonist N/A |