Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a15-15217_18k.htm |
Exhibit 99.1
|
|
July, 2015 Better Cancer Monitoring with Cell-Free DNA |
|
|
Forward-Looking Statements Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. 2 Copyright ©2015 Trovagene, Inc. Confidential |
|
|
Our Goal To Improve Treatment Outcomes with 3 Non-invasive Cancer Monitoring Trovagene’s technology non-invasively detects and quantitates circulating tumor DNA in urine and plasma for improved disease management Copyright ©2015 Trovagene, Inc. Confidential |
|
|
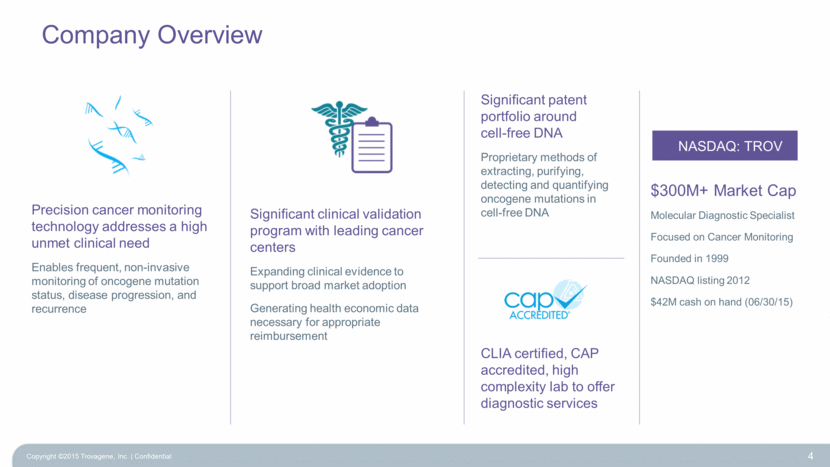
Company Overview 4 Significant clinical validation program with leading cancer centers Expanding clinical evidence to support broad market adoption Generating health economic data necessary for appropriate reimbursement CLIA certified, CAP accredited, high complexity lab to offer diagnostic services Precision cancer monitoring technology addresses a high unmet clinical need Enables frequent, non-invasive monitoring of oncogene mutation status, disease progression, and recurrence Significant patent portfolio around cell-free DNA Proprietary methods of extracting, purifying, detecting and quantifying oncogene mutations in cell-free DNA NASDAQ: TROV $300M+ Market Cap Molecular Diagnostic Specialist Focused on Cancer Monitoring Founded in 1999 NASDAQ listing 2012 $42M cash on hand (06/30/15) Copyright ©2015 Trovagene, Inc. Confidential |
|
|
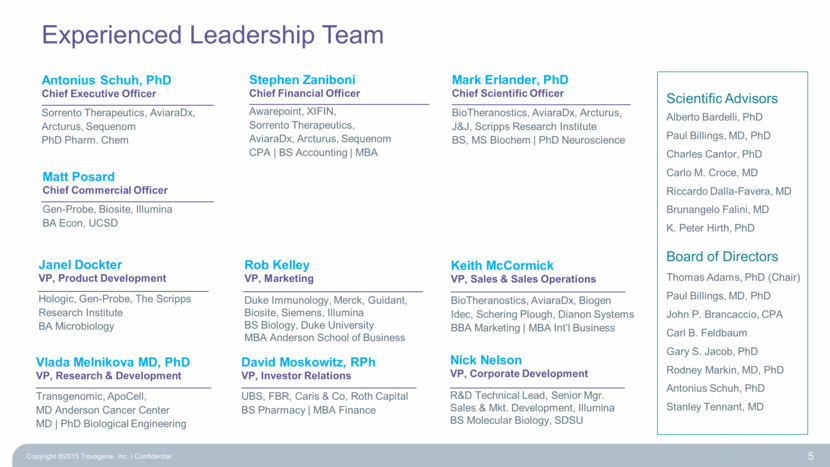
Experienced Leadership Team 5 Mark Erlander, PhD Chief Scientific Officer BioTheranostics, AviaraDx, Arcturus, J&J, Scripps Research Institute BS, MS Biochem PhD Neuroscience Antonius Schuh, PhD Chief Executive Officer Sorrento Therapeutics, AviaraDx, Arcturus, Sequenom PhD Pharm. Chem Stephen Zaniboni Chief Financial Officer Awarepoint, XIFIN, Sorrento Therapeutics, AviaraDx, Arcturus, Sequenom CPA BS Accounting MBA Scientific Advisors Alberto Bardelli, PhD Paul Billings, MD, PhD Charles Cantor, PhD Carlo M. Croce, MD Riccardo Dalla-Favera, MD Brunangelo Falini, MD K. Peter Hirth, PhD Board of Directors Thomas Adams, PhD (Chair) Paul Billings, MD, PhD John P. Brancaccio, CPA Carl B. Feldbaum Gary S. Jacob, PhD Rodney Markin, MD, PhD Antonius Schuh, PhD Stanley Tennant, MD Matt Posard Chief Commercial Officer Gen-Probe, Biosite, Illumina BA Econ, UCSD Vlada Melnikova MD, PhD VP, Research & Development Transgenomic, ApoCell, MD Anderson Cancer Center MD PhD Biological Engineering Keith McCormick VP, Sales & Sales Operations BioTheranostics, AviaraDx, Biogen Idec, Schering Plough, Dianon Systems BBA Marketing MBA Int’l Business Janel Dockter VP, Product Development Hologic, Gen-Probe, The Scripps Research Institute BA Microbiology David Moskowitz, RPh VP, Investor Relations UBS, FBR, Caris & Co, Roth Capital BS Pharmacy MBA Finance Rob Kelley VP, Marketing Duke Immunology, Merck, Guidant, Biosite, Siemens, Illumina BS Biology, Duke University MBA Anderson School of Business Nick Nelson VP, Corporate Development R&D Technical Lead, Senior Mgr. Sales & Mkt. Development, Illumina BS Molecular Biology, SDSU Copyright ©2015 Trovagene, Inc. Confidential |
|
|
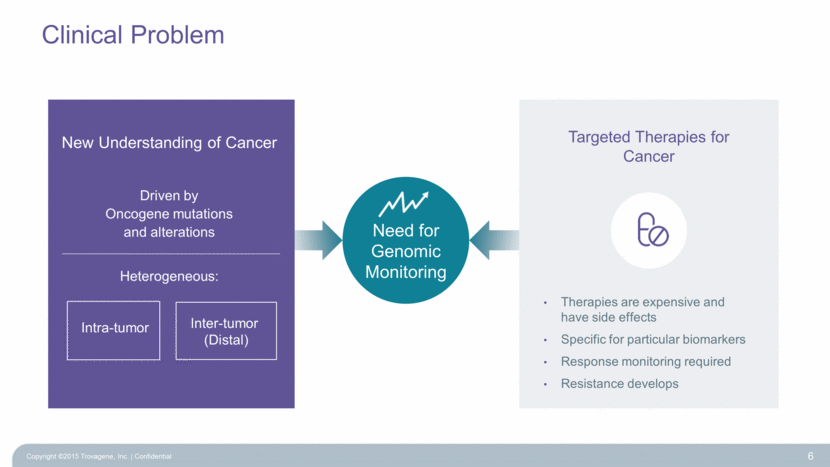
Targeted Therapies for Cancer Therapies are expensive and have side effects Specific for particular biomarkers Response monitoring required Resistance develops Clinical Problem 6 Driven by Oncogene mutations and alterations Heterogeneous: Inter-tumor (Distal) New Understanding of Cancer Intra-tumor Need for Genomic Monitoring Copyright ©2015 Trovagene, Inc. Confidential |
|
|
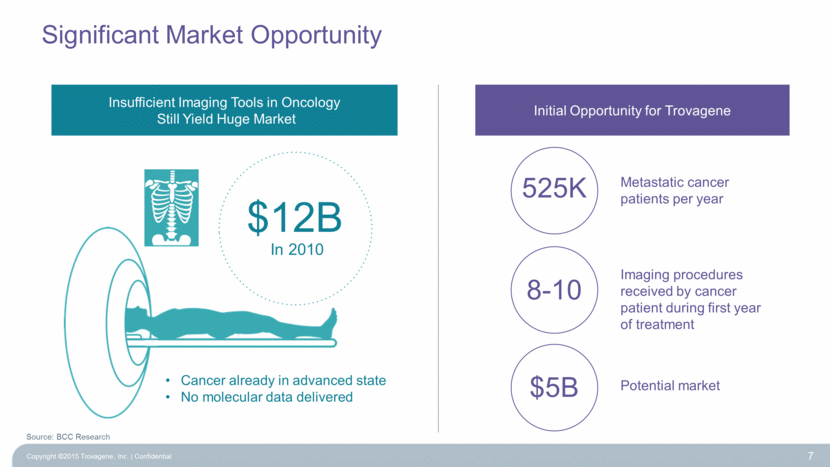
Significant Market Opportunity 7 $12B In 2010 Insufficient Imaging Tools in Oncology Still Yield Huge Market Initial Opportunity for Trovagene 525K Metastatic cancer patients per year 8-10 Imaging procedures received by cancer patient during first year of treatment $5B Potential market Cancer already in advanced state No molecular data delivered Source: BCC Research Copyright ©2015 Trovagene, Inc. Confidential |
|
|
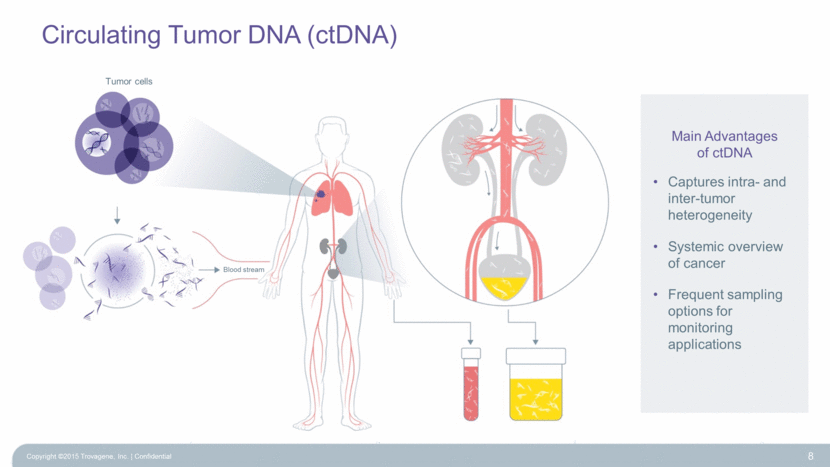
Circulating Tumor DNA (ctDNA) 8 Main Advantages of ctDNA Captures intra- and inter-tumor heterogeneity Systemic overview of cancer Frequent sampling options for monitoring applications Copyright ©2015 Trovagene, Inc. Confidential Tumor cells Blood stream |
|
|
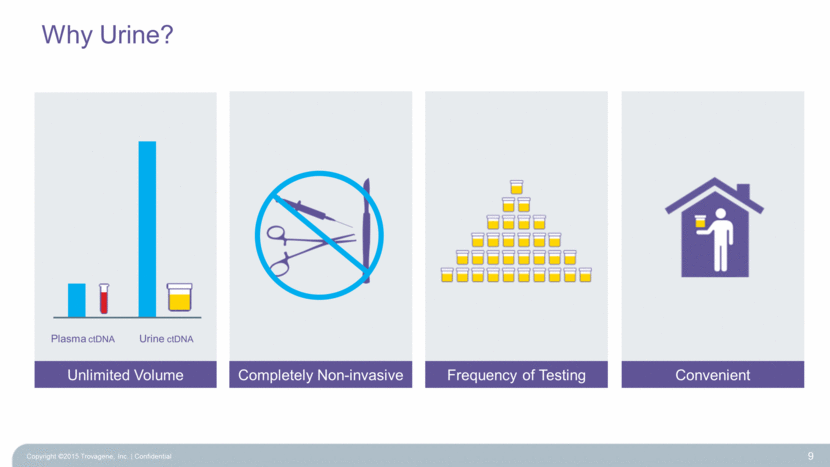
Why Urine? 9 Unlimited Volume Completely Non-invasive Frequency of Testing Convenient Urine ctDNA Plasma ctDNA Copyright ©2015 Trovagene, Inc. Confidential |
|
|
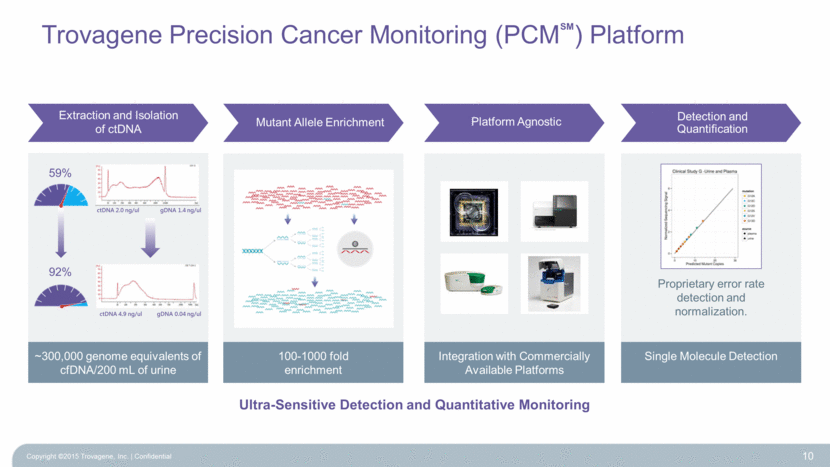
Trovagene Precision Cancer Monitoring (PCM ) Platform 10 Ultra-Sensitive Detection and Quantitative Monitoring 100-1000 fold enrichment ~300,000 genome equivalents of cfDNA/200 mL of urine Integration with Commercially Available Platforms Single Molecule Detection ctDNA 4.9 ng/ul gDNA 0.04 ng/ul ctDNA 2.0 ng/ul gDNA 1.4 ng/ul Extraction and Isolation of ctDNA Mutant Allele Enrichment Platform Agnostic Detection and Quantification 59% 92% SM Proprietary error rate detection and normalization. Copyright ©2015 Trovagene, Inc. Confidential |
|
|
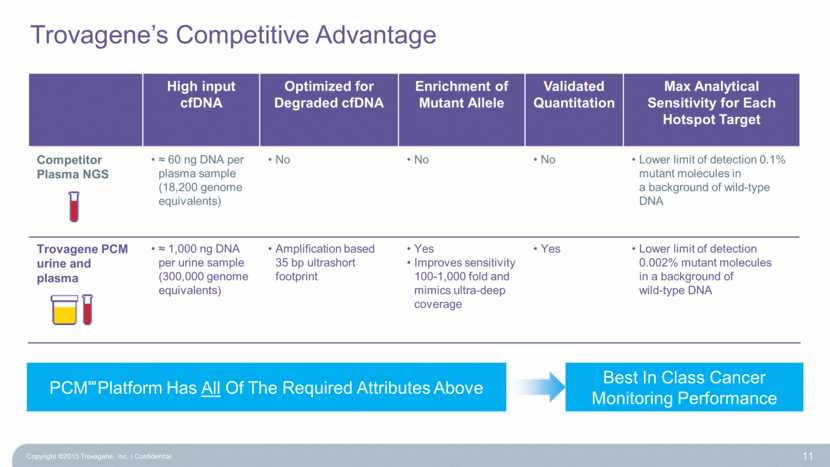
Trovagene’s Competitive Advantage Copyright ©2015 Trovagene, Inc. Confidential 11 PCM Platform Has All Of The Required Attributes Above Best In Class Cancer Monitoring Performance SM High input cfDNA Optimized for Degraded cfDNA Enrichment of Mutant Allele Validated Quantitation Max Analytical Sensitivity for Each Hotspot Target Competitor Plasma NGS 60 ng DNA per plasma sample (18,200 genome equivalents) No No No Lower limit of detection 0.1% mutant molecules in a background of wild-type DNA Trovagene PCM urine and plasma 1,000 ng DNA per urine sample (300,000 genome equivalents) Amplification based 35 bp ultrashort footprint Yes Improves sensitivity 100-1,000 fold and mimics ultra-deep coverage Yes Lower limit of detection 0.002% mutant molecules in a background of wild-type DNA |
|
|
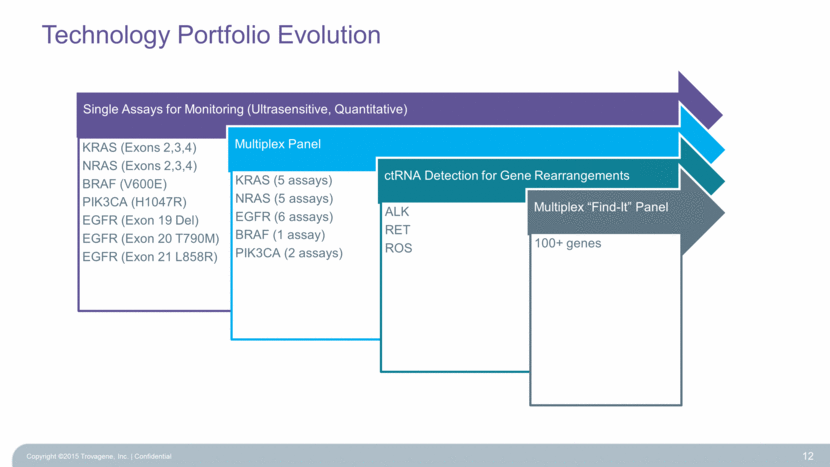
Technology Portfolio Evolution Copyright ©2015 Trovagene, Inc. Confidential 12 Single Assays for Monitoring (Ultrasensitive, Quantitative) KRAS (Exons 2,3,4) NRAS (Exons 2,3,4) BRAF (V600E) PIK3CA (H1047R) EGFR (Exon 19 Del) EGFR (Exon 20 T790M) EGFR (Exon 21 L858R) Multiplex Panel KRAS (5 assays) NRAS (5 assays) EGFR (6 assays) BRAF (1 assay) PIK3CA (2 assays) ctRNA Detection for Gene Rearrangements ALK RET ROS Multiplex “Find-It” Panel 100+ genes |
|
|
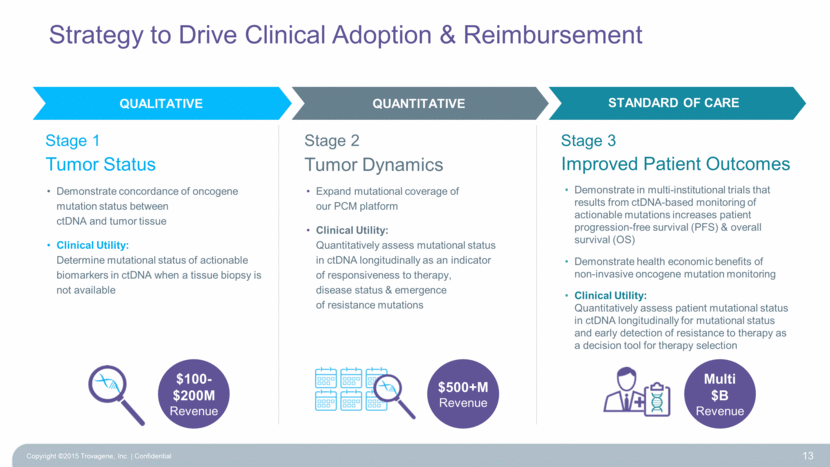
Strategy to Drive Clinical Adoption & Reimbursement Copyright ©2015 Trovagene, Inc. Confidential 13 Demonstrate concordance of oncogene mutation status between ctDNA and tumor tissue Clinical Utility: Determine mutational status of actionable biomarkers in ctDNA when a tissue biopsy is not available $100-$200M Revenue Expand mutational coverage of our PCM platform Clinical Utility: Quantitatively assess mutational status in ctDNA longitudinally as an indicator of responsiveness to therapy, disease status & emergence of resistance mutations Demonstrate in multi-institutional trials that results from ctDNA-based monitoring of actionable mutations increases patient progression-free survival (PFS) & overall survival (OS) Demonstrate health economic benefits of non-invasive oncogene mutation monitoring Clinical Utility: Quantitatively assess patient mutational status in ctDNA longitudinally for mutational status and early detection of resistance to therapy as a decision tool for therapy selection $500+M Revenue Multi $B Revenue QUALITATIVE QUANTITATIVE STANDARD OF CARE Tumor Status Stage 1 Tumor Dynamics Stage 2 Improved Patient Outcomes Stage 3 |
|
|
30+ Clinical Studies to Validate & Integrate our PCM Platform Copyright ©2015 Trovagene, Inc. Confidential 14 Disease Ongoing Pending Protocol in Dev/Approval Under Evaluation Market Size U.S. (# of pts) Lung Cancer (NSCLC) 7 0 – 399,000 Colorectal Cancer 3 3 – 1,154,000 Pancreatic Cancer 4 – 1 42,000 Melanoma 2 2 – 922.000 ECD/LCH 2 1 – 5,000 Brain Cancer 1 1 – 142,000 Prostate Cancer – 1 – 2,618,000 All-Comers, Metastatic Cancers 2 – – 525,000 HPV 4 – 3 Total # of Studies 20 12 5 SM |
|
|
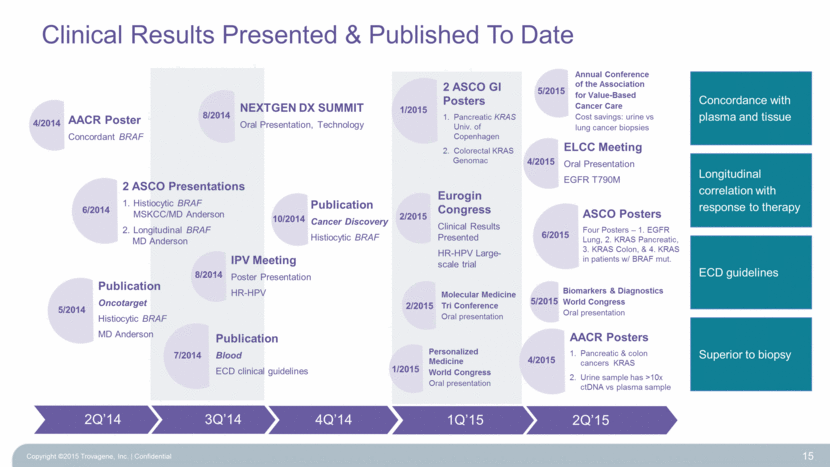
Clinical Results Presented & Published To Date Copyright ©2015 Trovagene, Inc. Confidential 15 2 ASCO Presentations Histiocytic BRAF MSKCC/MD Anderson Longitudinal BRAF MD Anderson AACR Poster Concordant BRAF NEXTGEN DX SUMMIT Oral Presentation, Technology IPV Meeting Poster Presentation HR-HPV Publication Cancer Discovery Histiocytic BRAF 2Q’14 3Q’14 4/2014 6/2014 8/2014 8/2014 Publication Oncotarget Histiocytic BRAF MD Anderson 5/2014 Publication Blood ECD clinical guidelines 7/2014 10/2014 4Q’14 1Q’15 2Q’15 Eurogin Congress Clinical Results Presented HR-HPV Large-scale trial 2/2015 ELCC Meeting Oral Presentation EGFR T790M 4/2015 2 ASCO GI Posters Pancreatic KRAS Univ. of Copenhagen Colorectal KRAS Genomac 1/2015 AACR Posters Pancreatic & colon cancers KRAS Urine sample has >10x ctDNA vs plasma sample 4/2015 Personalized Medicine World Congress Oral presentation 1/2015 Molecular Medicine Tri Conference Oral presentation 2/2015 Annual Conference of the Association for Value-Based Cancer Care Cost savings: urine vs lung cancer biopsies 5/2015 Biomarkers & Diagnostics World Congress Oral presentation 5/2015 Concordance with plasma and tissue Longitudinal correlation with response to therapy ECD guidelines Superior to biopsy ASCO Posters Four Posters – 1. EGFR Lung, 2. KRAS Pancreatic, 3. KRAS Colon, & 4. KRAS in patients w/ BRAF mut. 6/2015 |
|
|
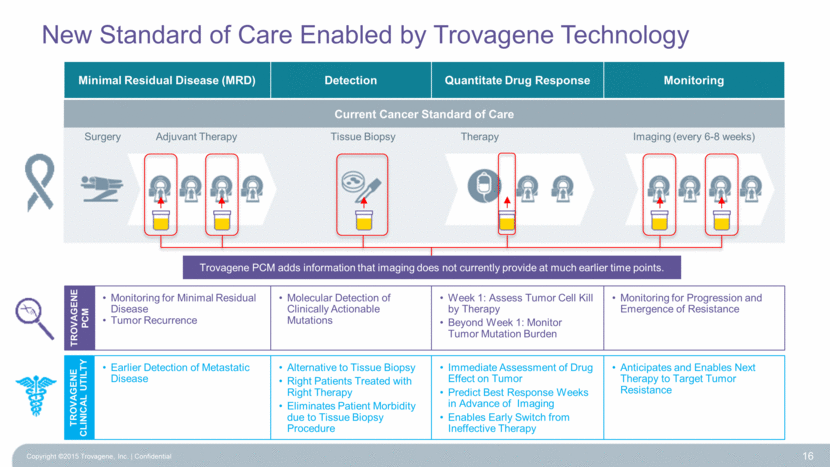
New Standard of Care Enabled by Trovagene Technology Copyright ©2015 Trovagene, Inc. Confidential 16 Detection Minimal Residual Disease (MRD) Quantitate Drug Response Monitoring Tissue Biopsy Imaging (every 6-8 weeks) Surgery Adjuvant Therapy Therapy Monitoring for Progression and Emergence of Resistance Molecular Detection of Clinically Actionable Mutations Monitoring for Minimal Residual Disease Tumor Recurrence Week 1: Assess Tumor Cell Kill by Therapy Beyond Week 1: Monitor Tumor Mutation Burden TROVAGENE CLINICAL UTILTY TROVAGENE PCM Earlier Detection of Metastatic Disease Alternative to Tissue Biopsy Right Patients Treated with Right Therapy Eliminates Patient Morbidity due to Tissue Biopsy Procedure Anticipates and Enables Next Therapy to Target Tumor Resistance Immediate Assessment of Drug Effect on Tumor Predict Best Response Weeks in Advance of Imaging Enables Early Switch from Ineffective Therapy Current Cancer Standard of Care Trovagene PCM adds information that imaging does not currently provide at much earlier time points. |
|
|
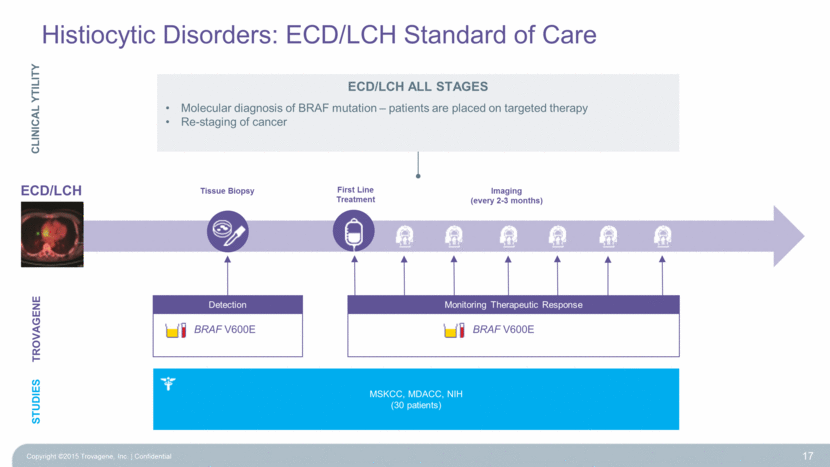
ECD/LCH Tissue Biopsy First Line Treatment Imaging (every 2-3 months) Histiocytic Disorders: ECD/LCH Standard of Care Copyright ©2015 Trovagene, Inc. Confidential 17 Monitoring Therapeutic Response BRAF V600E Detection BRAF V600E TROVAGENE ECD/LCH ALL STAGES Molecular diagnosis of BRAF mutation – patients are placed on targeted therapy Re-staging of cancer CLINICAL YTILITY MSKCC, MDACC, NIH (30 patients) STUDIES |
|
|
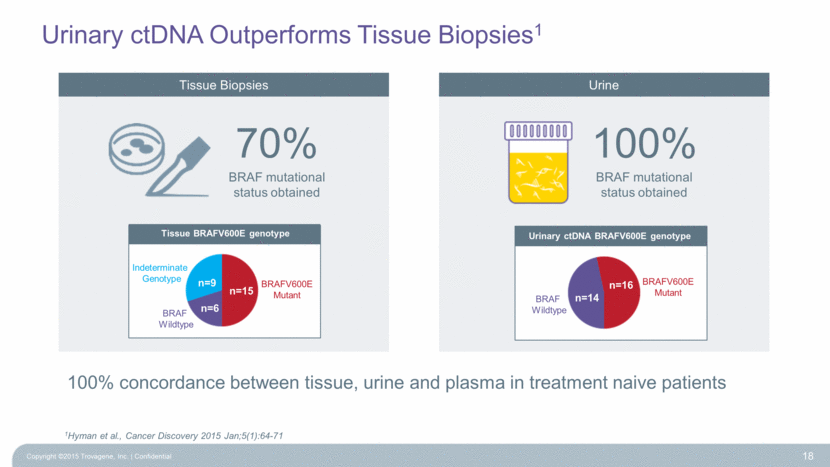
Urinary ctDNA Outperforms Tissue Biopsies1 Copyright ©2015 Trovagene, Inc. Confidential 18 1Hyman et al., Cancer Discovery 2015 Jan;5(1):64-71 Urinary ctDNA BRAFV600E genotype Tissue BRAFV600E genotype Indeterminate Genotype BRAFV600E Mutant BRAF Wildtype n=9 n=6 n=15 BRAFV600E Mutant BRAF Wildtype n=14 n=16 100% concordance between tissue, urine and plasma in treatment naive patients 70% BRAF mutational status obtained 100% BRAF mutational status obtained Tissue Biopsies Urine |
|
|
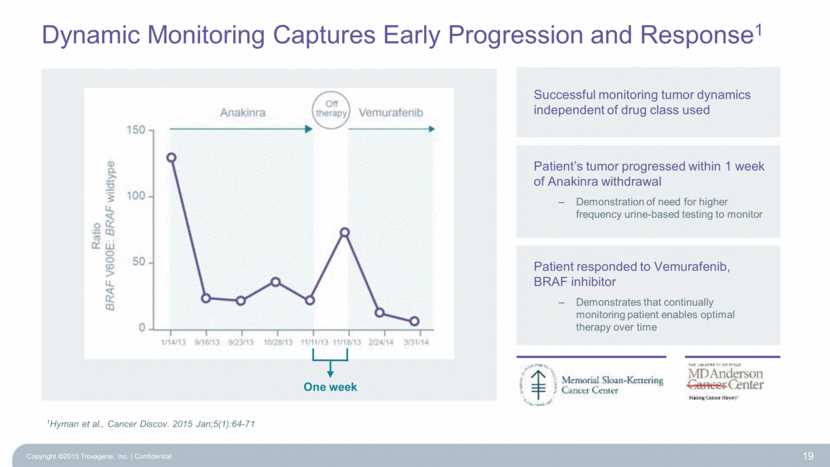
Dynamic Monitoring Captures Early Progression and Response1 One week Successful monitoring tumor dynamics independent of drug class used Patient’s tumor progressed within 1 week of Anakinra withdrawal Demonstration of need for higher frequency urine-based testing to monitor Patient responded to Vemurafenib, BRAF inhibitor Demonstrates that continually monitoring patient enables optimal therapy over time 1Hyman et al., Cancer Discov. 2015 Jan;5(1):64-71 19 Copyright ©2015 Trovagene, Inc. Confidential |
|
|
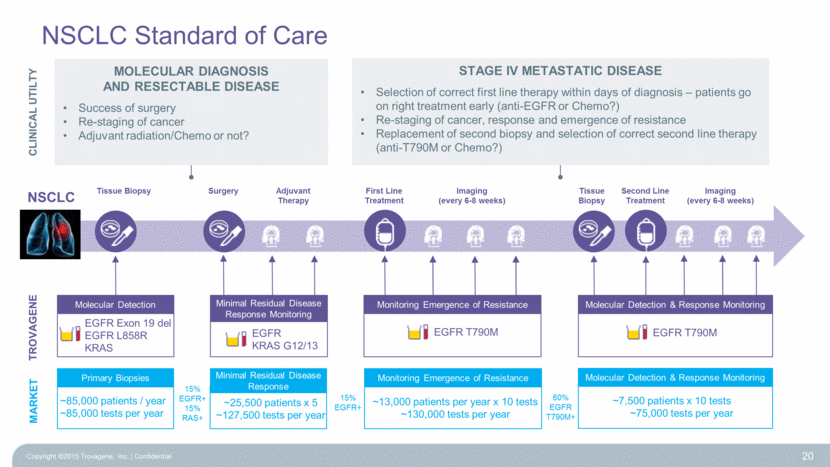
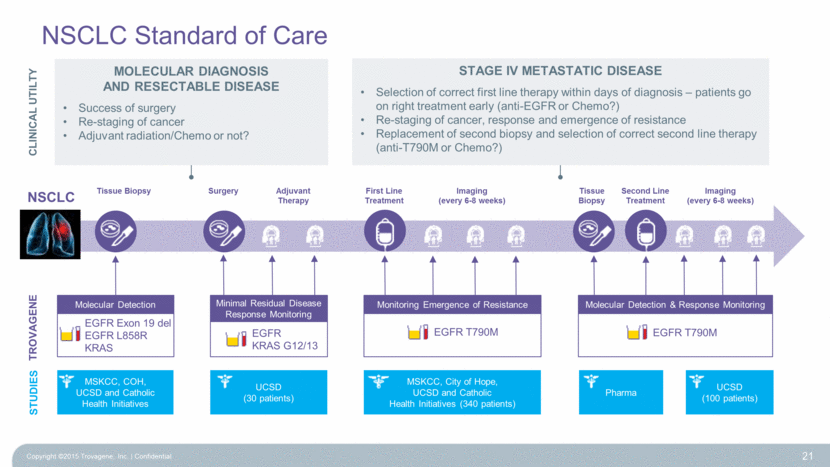
Molecular Detection & Response Monitoring EGFR T790M Monitoring Emergence of Resistance EGFR T790M NSCLC Standard of Care TROVAGENE NSCLC Tissue Biopsy First Line Treatment Tissue Biopsy Second Line Treatment Imaging (every 6-8 weeks) Surgery Imaging (every 6-8 weeks) Adjuvant Therapy Primary Biopsies ~85,000 patients / year ~85,000 tests per year MARKET 15% EGFR+ Monitoring Emergence of Resistance ~13,000 patients per year x 10 tests ~130,000 tests per year 60% EGFR T790M+ Molecular Detection & Response Monitoring ~7,500 patients x 10 tests ~75,000 tests per year Minimal Residual Disease Response ~25,500 patients x 5 ~127,500 tests per year 15% EGFR+ 15% RAS+ 20 Molecular Detection EGFR Exon 19 del EGFR L858R KRAS EGFR KRAS G12/13 Minimal Residual Disease Response Monitoring CLINICAL UTILTY Molecular DIAGNOSIS and RESECTABLE DISEASE Success of surgery Re-staging of cancer Adjuvant radiation/Chemo or not? Stage IV Metastatic DISEASE Selection of correct first line therapy within days of diagnosis – patients go on right treatment early (anti-EGFR or Chemo?) Re-staging of cancer, response and emergence of resistance Replacement of second biopsy and selection of correct second line therapy (anti-T790M or Chemo?) Copyright ©2015 Trovagene, Inc. Confidential |
|
|
NSCLC Standard of Care Copyright ©2015 Trovagene, Inc. Confidential 21 Molecular Detection & Response Monitoring EGFR T790M Monitoring Emergence of Resistance EGFR T790M Molecular Detection EGFR Exon 19 del EGFR L858R KRAS TROVAGENE NSCLC Tissue Biopsy First Line Treatment Second Line Treatment Imaging (every 6-8 weeks) Surgery Imaging (every 6-8 weeks) EGFR KRAS G12/13 Minimal Residual Disease Response Monitoring CLINICAL UTILTY Adjuvant Therapy STUDIES MSKCC, COH, UCSD and Catholic Health Initiatives UCSD (30 patients) Pharma UCSD (100 patients) MSKCC, City of Hope, UCSD and Catholic Health Initiatives (340 patients) Molecular DIAGNOSIS and RESECTABLE DISEASE Success of surgery Re-staging of cancer Adjuvant radiation/Chemo or not? Stage IV Metastatic DISEASE Selection of correct first line therapy within days of diagnosis – patients go on right treatment early (anti-EGFR or Chemo?) Re-staging of cancer, response and emergence of resistance Replacement of second biopsy and selection of correct second line therapy (anti-T790M or Chemo?) Tissue Biopsy |
|
|
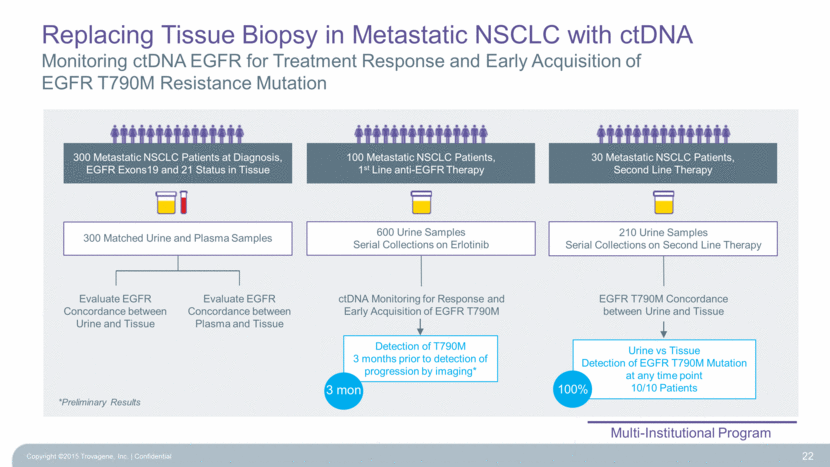
Replacing Tissue Biopsy in Metastatic NSCLC with ctDNA Monitoring ctDNA EGFR for Treatment Response and Early Acquisition of EGFR T790M Resistance Mutation 22 30 Metastatic NSCLC Patients, Second Line Therapy 300 Metastatic NSCLC Patients at Diagnosis, EGFR Exons19 and 21 Status in Tissue ctDNA Monitoring for Response and Early Acquisition of EGFR T790M 210 Urine Samples Serial Collections on Second Line Therapy 100 Metastatic NSCLC Patients, 1st Line anti-EGFR Therapy 600 Urine Samples Serial Collections on Erlotinib Evaluate EGFR Concordance between Urine and Tissue Evaluate EGFR Concordance between Plasma and Tissue 300 Matched Urine and Plasma Samples Multi-Institutional Program EGFR T790M Concordance between Urine and Tissue Urine vs Tissue Detection of EGFR T790M Mutation at any time point 10/10 Patients 100% Detection of T790M 3 months prior to detection of progression by imaging* 3 mon *Preliminary Results Copyright ©2015 Trovagene, Inc. Confidential |
|
|
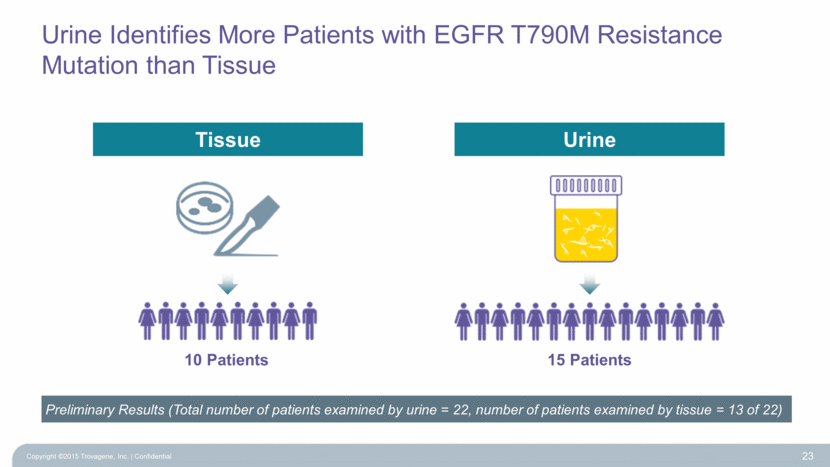
Urine Identifies More Patients with EGFR T790M Resistance Mutation than Tissue Copyright ©2015 Trovagene, Inc. Confidential 23 Preliminary Results (Total number of patients examined by urine = 22, number of patients examined by tissue = 13 of 22) 10 Patients 15 Patients Urine Tissue |
|
|
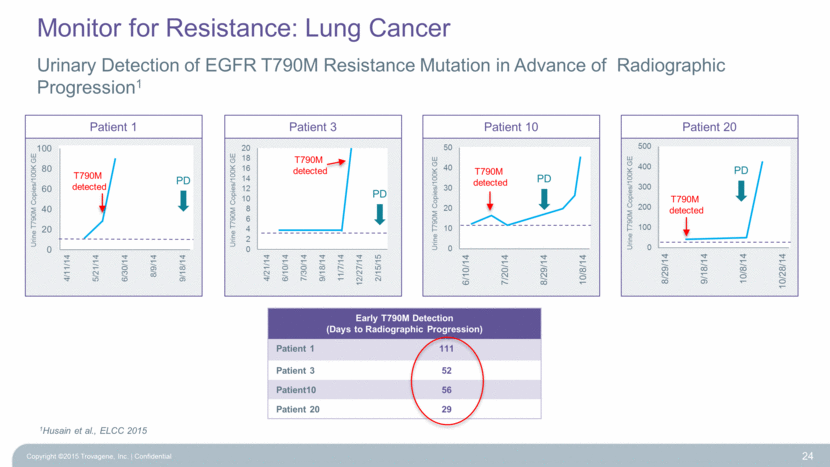
Patient 20 Patient 10 Patient 3 Copyright ©2015 Trovagene, Inc. Confidential 24 1Husain et al., ELCC 2015 Early T790M Detection (Days to Radiographic Progression) Patient 1 111 Patient 3 52 Patient10 56 Patient 20 29 PD PD PD T790M detected T790M detected PD T790M detected T790M detected Urine T790M Copies/100K GE Patient 1 Urine T790M Copies/100K GE Urine T790M Copies/100K GE Urine T790M Copies/100K GE Urinary Detection of EGFR T790M Resistance Mutation in Advance of Radiographic Progression1 Monitor for Resistance: Lung Cancer 0 2 4 6 8 10 12 14 16 18 20 4/21/14 6/10/14 7/30/14 9/18/14 11/7/14 12/27/14 2/15/15 0 20 40 60 80 100 4/11/14 5/21/14 6/30/14 8/9/14 9/18/14 0 10 20 30 40 50 6/10/14 7/20/14 8/29/14 10/8/14 0 100 200 300 400 500 8/29/14 9/18/14 10/8/14 10/28/14 |
|
|
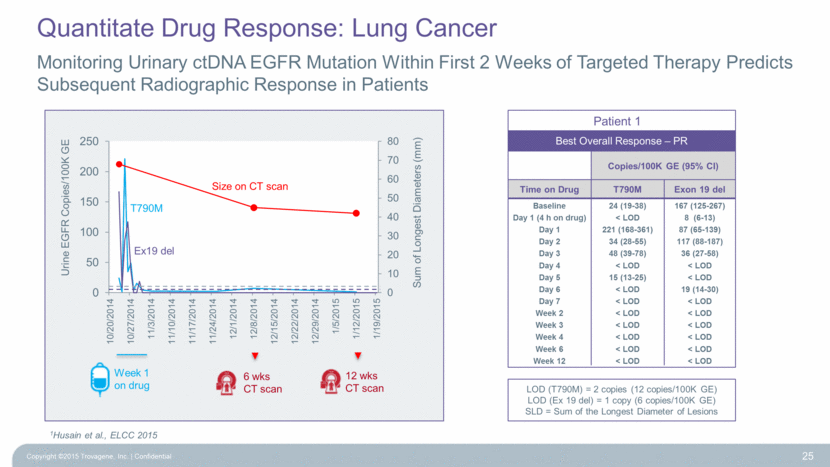
Monitoring Urinary ctDNA EGFR Mutation Within First 2 Weeks of Targeted Therapy Predicts Subsequent Radiographic Response in Patients Copyright ©2015 Trovagene, Inc. Confidential 25 1Husain et al., ELCC 2015 Patient 1 Copies/100K GE (95% CI) Time on Drug T790M Exon 19 del Baseline 24 (19-38) 167 (125-267) Day 1 (4 h on drug) < LOD 8 (6-13) Day 1 221 (168-361) 87 (65-139) Day 2 34 (28-55) 117 (88-187) Day 3 48 (39-78) 36 (27-58) Day 4 < LOD < LOD Day 5 15 (13-25) < LOD Day 6 < LOD 19 (14-30) Day 7 < LOD < LOD Week 2 < LOD < LOD Week 3 < LOD < LOD Week 4 < LOD < LOD Week 6 < LOD < LOD Week 12 < LOD < LOD Best Overall Response – PR LOD (T790M) = 2 copies (12 copies/100K GE) LOD (Ex 19 del) = 1 copy (6 copies/100K GE) SLD = Sum of the Longest Diameter of Lesions T790M Ex19 del Size on CT scan Sum of Longest Diameters (mm) Week 1 on drug 6 wks CT scan 12 wks CT scan Quantitate Drug Response: Lung Cancer 0 10 20 30 40 50 60 70 80 0 50 100 150 200 250 10/20/2014 10/27/2014 11/3/2014 11/10/2014 11/17/2014 11/24/2014 12/1/2014 12/8/2014 12/15/2014 12/22/2014 12/29/2014 1/5/2015 1/12/2015 1/19/2015 Urine EGFR Copies/100K GE |
|
|
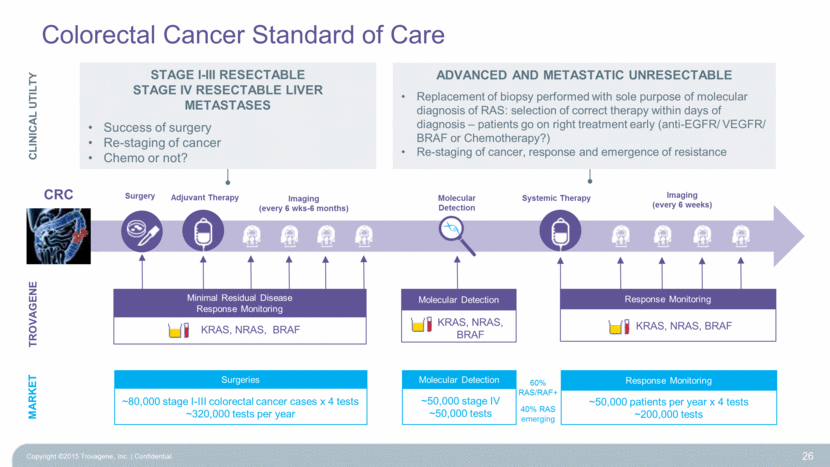
TROVAGENE Adjuvant Therapy Imaging (every 6 weeks) Systemic Therapy Imaging (every 6 wks-6 months) Surgery Molecular Detection Molecular Detection ~50,000 stage IV ~50,000 tests MARKET Surgeries ~50,000 patients per year x 4 tests ~200,000 tests Response Monitoring ~80,000 stage I-III colorectal cancer cases x 4 tests ~320,000 tests per year 60% RAS/RAF+ 40% RAS emerging 26 KRAS, NRAS, BRAF Minimal Residual Disease Response Monitoring Molecular Detection KRAS, NRAS, BRAF KRAS, NRAS, BRAF Response Monitoring CRC CLINICAL UTILTY Stage I-III Resectable Stage IV Resectable Liver Metastases Success of surgery Re-staging of cancer Chemo or not? Advanced and metastatic unresectable Replacement of biopsy performed with sole purpose of molecular diagnosis of RAS: selection of correct therapy within days of diagnosis – patients go on right treatment early (anti-EGFR/ VEGFR/ BRAF or Chemotherapy?) Re-staging of cancer, response and emergence of resistance Colorectal Cancer Standard of Care Copyright ©2015 Trovagene, Inc. Confidential |
|
|
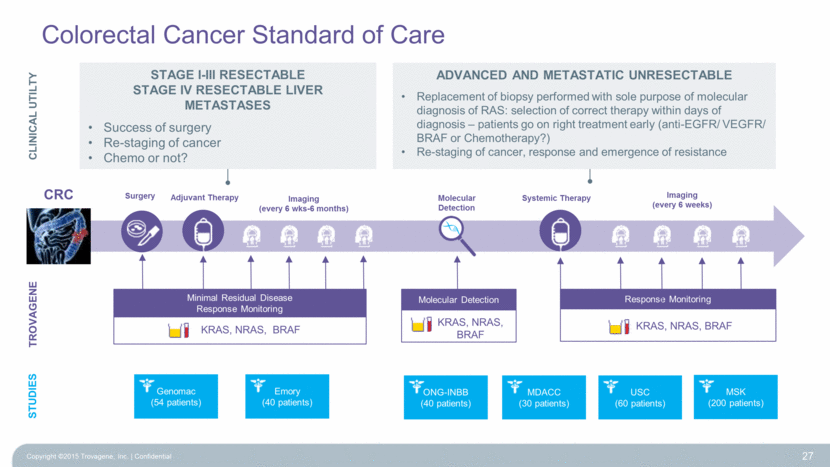
Colorectal Cancer Standard of Care 27 KRAS, NRAS, BRAF Minimal Residual Disease Response Monitoring TROVAGENE Adjuvant Therapy Imaging (every 6 weeks) Systemic Therapy Imaging (every 6 wks-6 months) Molecular Detection KRAS, NRAS, BRAF Surgery KRAS, NRAS, BRAF Response Monitoring Molecular Detection ONG-INBB (40 patients) MDACC (30 patients) USC (60 patients) MSK (200 patients) Genomac (54 patients) Emory (40 patients) STUDIES CLINICAL UTILTY Stage I-III Resectable Stage IV Resectable Liver Metastases Success of surgery Re-staging of cancer Chemo or not? Advanced and metastatic unresectable Replacement of biopsy performed with sole purpose of molecular diagnosis of RAS: selection of correct therapy within days of diagnosis – patients go on right treatment early (anti-EGFR/ VEGFR/ BRAF or Chemotherapy?) Re-staging of cancer, response and emergence of resistance CRC Copyright ©2015 Trovagene, Inc. Confidential |
|
|
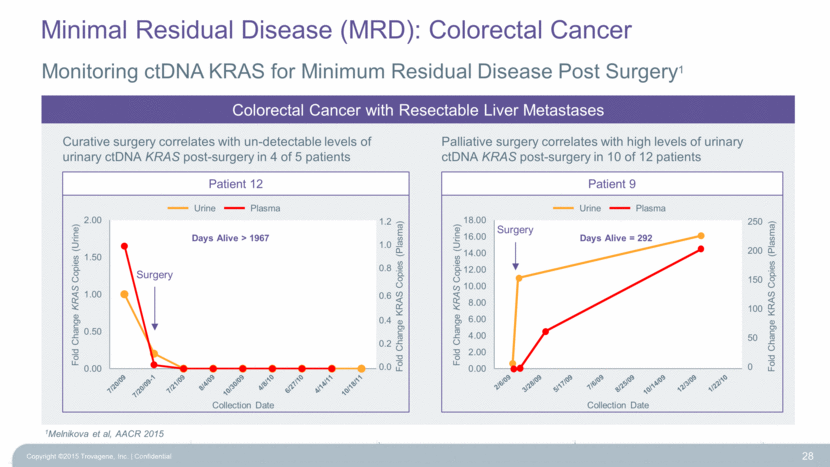
Monitoring ctDNA KRAS for Minimum Residual Disease Post Surgery1 28 1Melnikova et al, AACR 2015 Curative surgery correlates with un-detectable levels of urinary ctDNA KRAS post-surgery in 4 of 5 patients Palliative surgery correlates with high levels of urinary ctDNA KRAS post-surgery in 10 of 12 patients Colorectal Cancer with Resectable Liver Metastases Surgery Days Alive > 1967 Patient 9 Days Alive = 292 Surgery 1.2 1.0 0.8 0.6 0.4 0.2 0.0 Fold Change KRAS Copies (Plasma) 250 200 150 100 50 0 Fold Change KRAS Copies (Plasma) Urine Plasma Urine Plasma Patient 12 Minimal Residual Disease (MRD): Colorectal Cancer Copyright ©2015 Trovagene, Inc. Confidential 0.00 0.50 1.00 1.50 2.00 Fold Change KRAS Copies (Urine) Collection Date 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 Fold Change KRAS Copies (Urine) Collection Date |
|
|
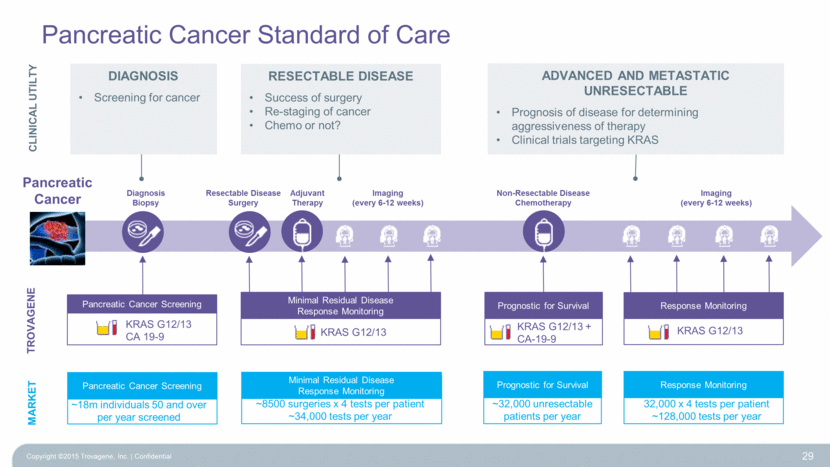
~32,000 unresectable patients per year Pancreatic Cancer Standard of Care 29 TROVAGENE Diagnosis Biopsy Adjuvant Therapy Imaging (every 6-12 weeks) Imaging (every 6-12 weeks) Resectable Disease Surgery Non-Resectable Disease Chemotherapy CLINICAL UTILTY Diagnosis Screening for cancer RESECTABLE DISEASE Success of surgery Re-staging of cancer Chemo or not? Advanced and Metastatic Unresectable Prognosis of disease for determining aggressiveness of therapy Clinical trials targeting KRAS MARKET ~8500 surgeries x 4 tests per patient ~34,000 tests per year 32,000 x 4 tests per patient ~128,000 tests per year ~18m individuals 50 and over per year screened Pancreatic Cancer Screening Minimal Residual Disease Response Monitoring Prognostic for Survival Response Monitoring Prognostic for Survival KRAS G12/13 + CA-19-9 KRAS G12/13 KRAS G12/13 Minimal Residual Disease Response Monitoring Pancreatic Cancer Screening KRAS G12/13 CA 19-9 Response Monitoring Pancreatic Cancer Copyright ©2015 Trovagene, Inc. Confidential |
|
|
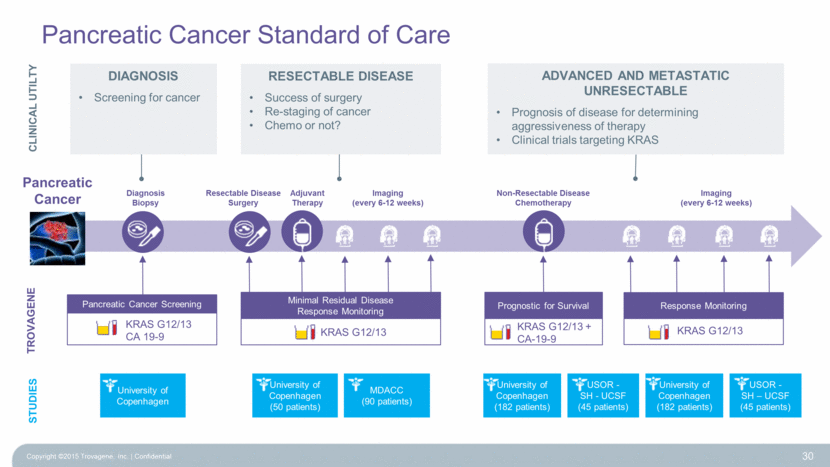
Pancreatic Cancer Standard of Care 30 TROVAGENE Diagnosis Biopsy Adjuvant Therapy Imaging (every 6-12 weeks) Imaging (every 6-12 weeks) Resectable Disease Surgery Non-Resectable Disease Chemotherapy University of Copenhagen University of Copenhagen (50 patients) MDACC (90 patients) University of Copenhagen (182 patients) USOR - SH - UCSF (45 patients) University of Copenhagen (182 patients) USOR - SH – UCSF (45 patients) STUDIES Prognostic for Survival KRAS G12/13 + CA-19-9 KRAS G12/13 KRAS G12/13 Minimal Residual Disease Response Monitoring Pancreatic Cancer Screening KRAS G12/13 CA 19-9 Response Monitoring CLINICAL UTILTY Diagnosis Screening for cancer RESECTABLE DISEASE Success of surgery Re-staging of cancer Chemo or not? Advanced and Metastatic Unresectable Prognosis of disease for determining aggressiveness of therapy Clinical trials targeting KRAS Pancreatic Cancer Copyright ©2015 Trovagene, Inc. Confidential |
|
|
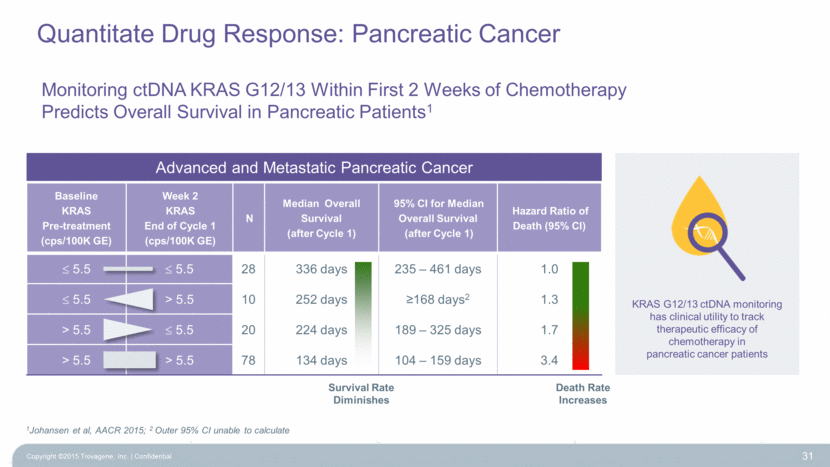
Baseline KRAS Pre-treatment (cps/100K GE) Week 2 KRAS End of Cycle 1 (cps/100K GE) N Median Overall Survival (after Cycle 1) 95% CI for Median Overall Survival (after Cycle 1) Hazard Ratio of Death (95% CI) 5.5 5.5 28 336 days 235 – 461 days 1.0 5.5 > 5.5 10 252 days >168 days2 1.3 > 5.5 5.5 20 224 days 189 – 325 days 1.7 > 5.5 > 5.5 78 134 days 104 – 159 days 3.4 Monitoring ctDNA KRAS G12/13 Within First 2 Weeks of Chemotherapy Predicts Overall Survival in Pancreatic Patients1 31 1Johansen et al, AACR 2015; 2 Outer 95% CI unable to calculate KRAS G12/13 ctDNA monitoring has clinical utility to track therapeutic efficacy of chemotherapy in pancreatic cancer patients Advanced and Metastatic Pancreatic Cancer Survival Rate Diminishes Death Rate Increases Quantitate Drug Response: Pancreatic Cancer Copyright ©2015 Trovagene, Inc. Confidential |
|
|
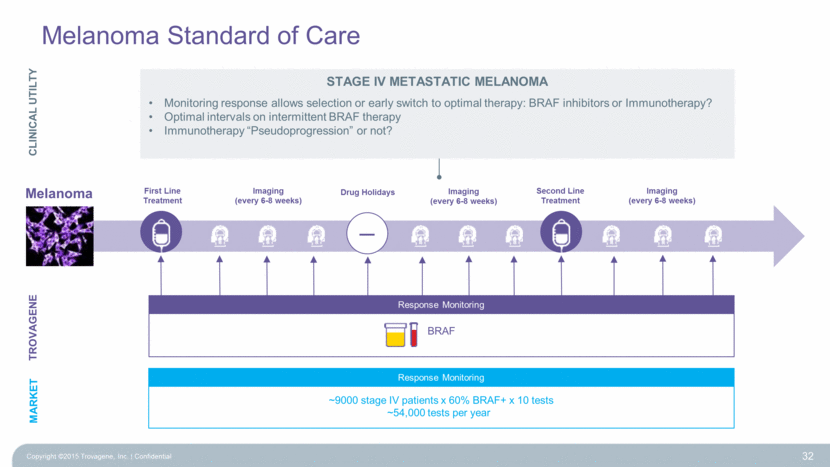
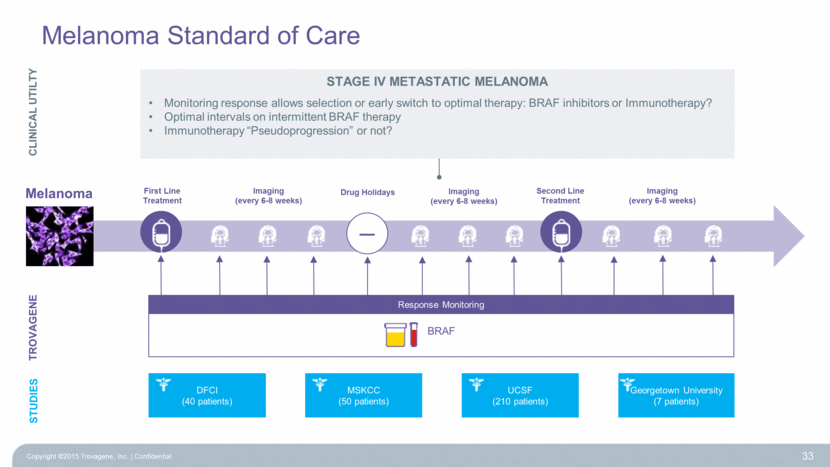
Melanoma Melanoma Standard of Care TROVAGENE First Line Treatment Second Line Treatment Imaging (every 6-8 weeks) Imaging (every 6-8 weeks) Response Monitoring BRAF MARKET Response Monitoring ~9000 stage IV patients x 60% BRAF+ x 10 tests ~54,000 tests per year – Drug Holidays Imaging (every 6-8 weeks) 32 CLINICAL UTILTY Stage IV Metastatic MELANOMA Monitoring response allows selection or early switch to optimal therapy: BRAF inhibitors or Immunotherapy? Optimal intervals on intermittent BRAF therapy Immunotherapy “Pseudoprogression” or not? Copyright ©2015 Trovagene, Inc. Confidential |
|
|
Melanoma Standard of Care 33 TROVAGENE First Line Treatment Second Line Treatment Imaging (every 6-8 weeks) Imaging (every 6-8 weeks) STUDIES Response Monitoring BRAF – Drug Holidays Imaging (every 6-8 weeks) Georgetown University (7 patients) UCSF (210 patients) MSKCC (50 patients) DFCI (40 patients) CLINICAL UTILTY Stage IV Metastatic MELANOMA Monitoring response allows selection or early switch to optimal therapy: BRAF inhibitors or Immunotherapy? Optimal intervals on intermittent BRAF therapy Immunotherapy “Pseudoprogression” or not? Melanoma Copyright ©2015 Trovagene, Inc. Confidential |
|
|
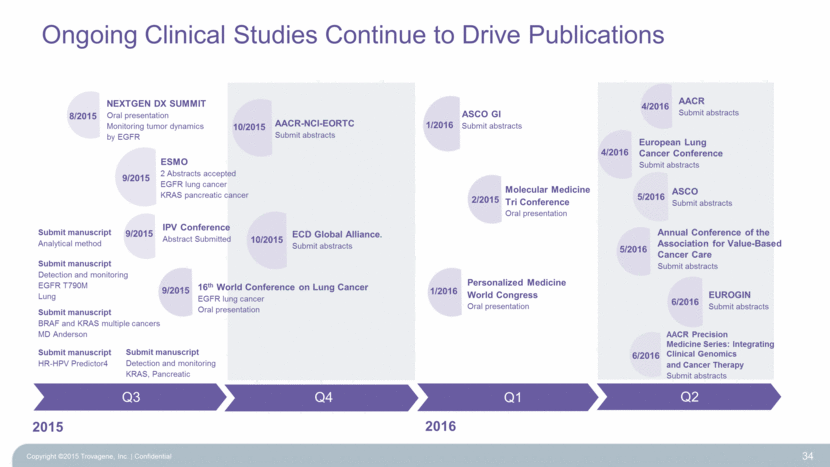
Ongoing Clinical Studies Continue to Drive Publications 34 2015 AACR-NCI-EORTC Submit abstracts ECD Global Alliance. Submit abstracts Q4 Q1 Q2 10/2015 Submit manuscript BRAF and KRAS multiple cancers MD Anderson IPV Conference Abstract Submitted 8/2015 9/2015 NEXTGEN DX SUMMIT Oral presentation Monitoring tumor dynamics by EGFR 16th World Conference on Lung Cancer EGFR lung cancer Oral presentation 9/2015 ESMO 2 Abstracts accepted EGFR lung cancer KRAS pancreatic cancer 9/2015 Q3 2016 Submit manuscript Detection and monitoring EGFR T790M Lung EUROGIN Submit abstracts 6/2016 ASCO GI Submit abstracts 1/2016 Personalized Medicine World Congress Oral presentation 1/2016 Molecular Medicine Tri Conference Oral presentation 2/2015 10/2015 AACR Submit abstracts 4/2016 European Lung Cancer Conference Submit abstracts 4/2016 AACR Precision Medicine Series: Integrating Clinical Genomics and Cancer Therapy Submit abstracts 6/2016 Annual Conference of the Association for Value-Based Cancer Care Submit abstracts 5/2016 ASCO Submit abstracts 5/2016 Submit manuscript HR-HPV Predictor4 Submit manuscript Detection and monitoring KRAS, Pancreatic Submit manuscript Analytical method Copyright ©2015 Trovagene, Inc. Confidential |
|
|
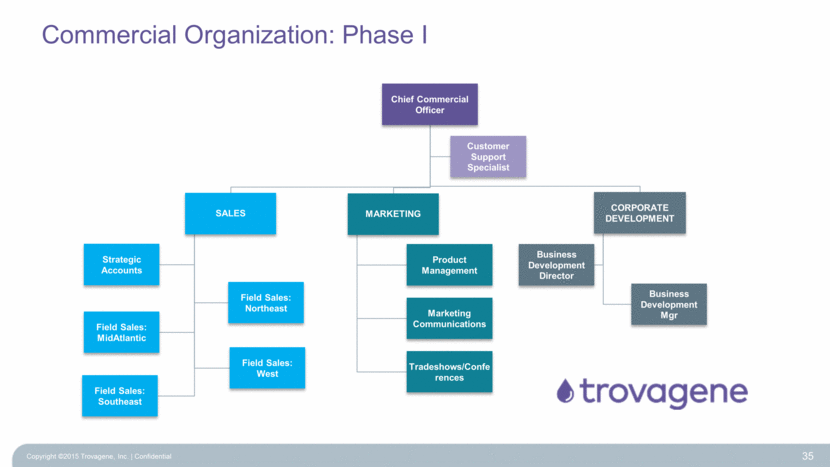
Commercial Organization: Phase I Copyright ©2015 Trovagene, Inc. Confidential 35 Chief Commercial Officer SALES Strategic Accounts Field Sales: Northeast Field Sales: MidAtlantic Field Sales: Southeast Field Sales: West MARKETING Product Management Marketing Communications Tradeshows/Conferences CORPORATE DEVELOPMENT Business Development Director Business Development Mgr Customer Support Specialist |
|
|
Copyright ©2015 Trovagene, Inc. Confidential 36 External Awareness |
|
|
Creating Physician Awareness & Demand Copyright ©2015 Trovagene, Inc. Confidential 37 Allows physicians hands on experience with their patients Assess benefits of: Urine as a sample Quantitation Ultra sensitivity Choice of 3 applications: Diagnostic in lieu of a tissue biopsy result Baseline & monitor Early therapeutic response Trovagene’s Clinical Experience Program (CEP) |
|
|
Early Adopters INSERT PHOTO MONTAGE Copyright ©2015 Trovagene, Inc. Confidential 38 |
|
|
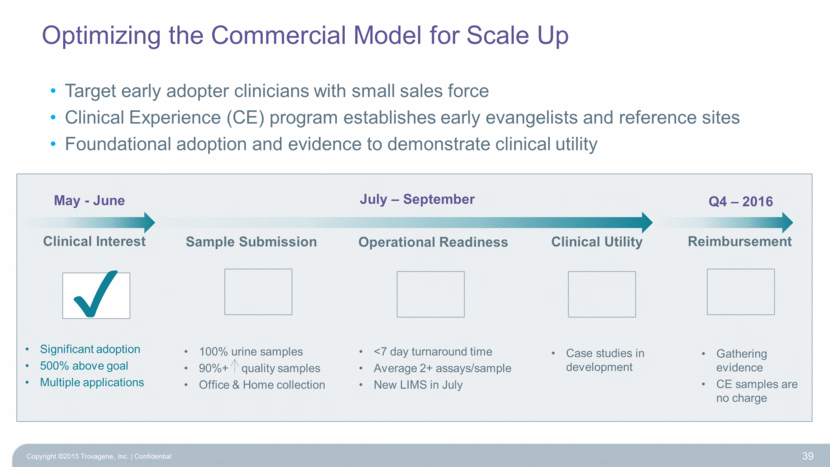
Optimizing the Commercial Model for Scale Up Clinical Interest Copyright ©2015 Trovagene, Inc. Confidential 39 Sample Submission Operational Readiness Clinical Utility Reimbursement Significant adoption 500% above goal Multiple applications 100% urine samples 90%+ quality samples Office & Home collection <7 day turnaround time Average 2+ assays/sample New LIMS in July Target early adopter clinicians with small sales force Clinical Experience (CE) program establishes early evangelists and reference sites Foundational adoption and evidence to demonstrate clinical utility Case studies in development Gathering evidence CE samples are no charge May - June July – September Q4 – 2016 |
|
|
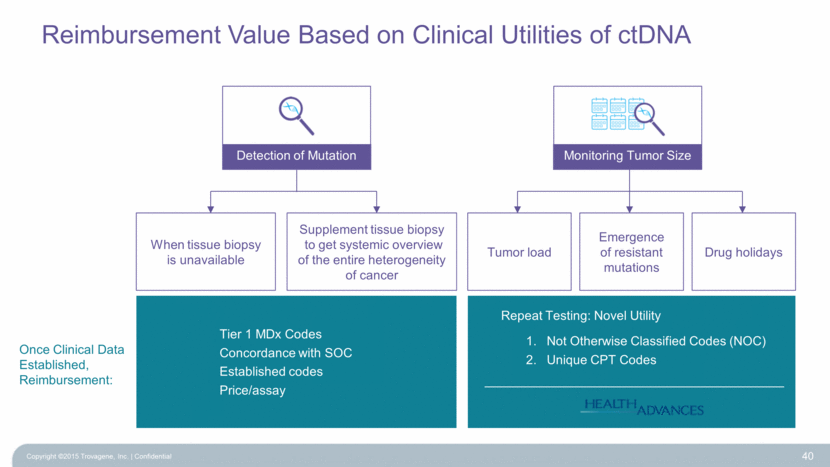
Reimbursement Value Based on Clinical Utilities of ctDNA Copyright ©2015 Trovagene, Inc. Confidential 40 Detection of Mutation Monitoring Tumor Size When tissue biopsy is unavailable Tumor load Emergence of resistant mutations Drug holidays Tier 1 MDx Codes Concordance with SOC Established codes Price/assay Repeat Testing: Novel Utility Not Otherwise Classified Codes (NOC) Unique CPT Codes Once Clinical Data Established, Reimbursement: Supplement tissue biopsy to get systemic overview of the entire heterogeneity of cancer |
|
|
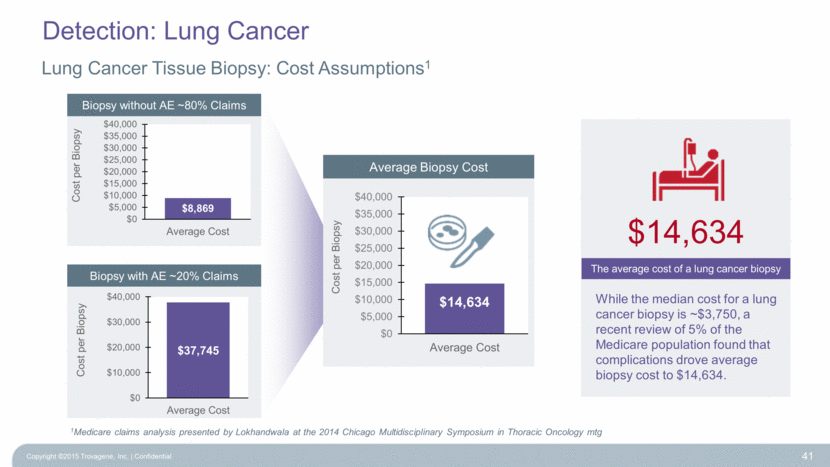
Lung Cancer Tissue Biopsy: Cost Assumptions1 Copyright ©2015 Trovagene, Inc. Confidential 41 While the median cost for a lung cancer biopsy is ~$3,750, a recent review of 5% of the Medicare population found that complications drove average biopsy cost to $14,634. 1Medicare claims analysis presented by Lokhandwala at the 2014 Chicago Multidisciplinary Symposium in Thoracic Oncology mtg Average Biopsy Cost Biopsy without AE ~80% Claims Biopsy with AE ~20% Claims $14,634 The average cost of a lung cancer biopsy Detection: Lung Cancer $14,634 $0 $5,000 $10,000 $15,000 $20,000 $25,000 $30,000 $35,000 $40,000 Average Cost Cost per Biopsy $37,745 $0 $10,000 $20,000 $30,000 $40,000 Average Cost Cost per Biopsy $8,869 $0 $5,000 $10,000 $15,000 $20,000 $25,000 $30,000 $35,000 $40,000 Average Cost Cost per Biopsy |
|
|
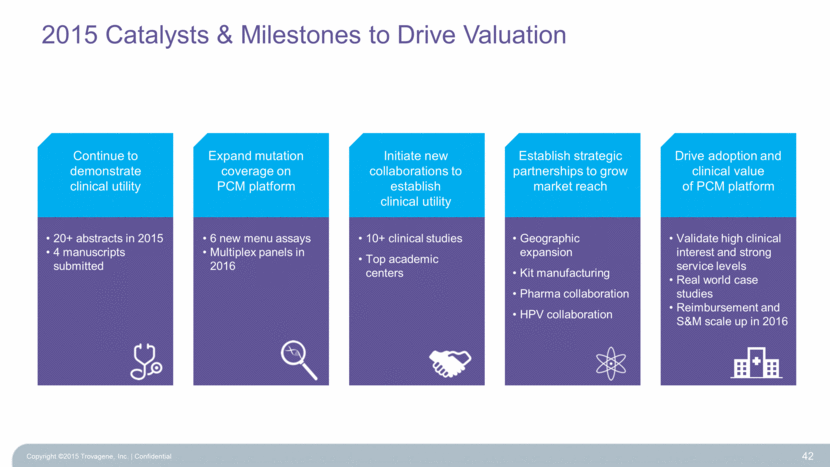
2015 Catalysts & Milestones to Drive Valuation Copyright ©2015 Trovagene, Inc. Confidential 42 Continue to demonstrate clinical utility Expand mutation coverage on PCM platform Initiate new collaborations to establish clinical utility Establish strategic partnerships to grow market reach Drive adoption and clinical value of PCM platform 20+ abstracts in 2015 4 manuscripts submitted 6 new menu assays Multiplex panels in 2016 10+ clinical studies Top academic centers Geographic expansion Kit manufacturing Pharma collaboration HPV collaboration Validate high clinical interest and strong service levels Real world case studies Reimbursement and S&M scale up in 2016 |
|
|
For further information Please contact: Stephen Zaniboni, CFO szaniboni@trovagene.com Antonius Schuh, CEO aschuh@trovagene.com |