Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Adhera Therapeutics, Inc. | t1501433_8k.htm |
Exhibit 99.1

Nucleic Acid Therapeutics and Rare Diseases 2015 BIO International Convention 16 June 2015

Forward Looking Statement This document contains forward-looking statements about Marina Biotech, Inc. (the “Company”) that are based on current management expectations. These statements reflect the Company’s current views with respect to uncertain future events and are based on imprecise estimates and assumptions and are subject to risk and uncertainties. Given these uncertainties, investors should not place undue reliance on the forward-looking statements contained in or made in connection with this document. The Company’s actual results, performance or achievements could differ materially from those contemplated, expressed or implied by these forward-looking statements for a variety of reasons. The Company undertakes no duty or obligation to update or revise forward-looking statements as a result of new information, future events or changes in the Company’s expectations. Factors that could cause actual results to differ materially from those contained in the forward-looking statements in this document include, but are not limited to: (i) the Company’s ability to obtain additional and substantial funding when required and on acceptable terms; (ii) the Company’s ability to attract and/or maintain research, development, commercialization and manufacturing partners; (iii) the ability of the Company and/or a partner to successfully complete product research and development, including pre-clinical and clinical studies and commercialization; (iv) the ability of the Company and/or a partner to obtain required governmental approvals; (v) the ability of the Company and/or a partner to develop and commercialize products that can compete favorably with those of its competitors; (vi) the timing of costs and expenses related to the R&D programs of the Company and/or its partners; (vii) the timing and recognition of revenue from milestone payments and other sources not related to product sales; (viii) the Company’s ability to obtain suitable facilities in which to conduct its planned business operations on acceptable terms and on a timely basis; (ix) the Company’s ability to attract and retain qualified officers, employees and consultants on a timely basis; and (x) costs associated with any product liability claims, patent prosecution, patent infringement lawsuits and other lawsuits. Additional factors that could cause actual results to differ materially from those projected or suggested in any forward-looking statements are contained in the Company’s most recent filings with the Securities and Exchange Commission (the “SEC”), including the sections entitled “Risk Factors” and “Forward-Looking Statements” contained in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2014. You may access the Company’s filings with the SEC for free by visiting the SEC’s website at http://www.sec.gov.
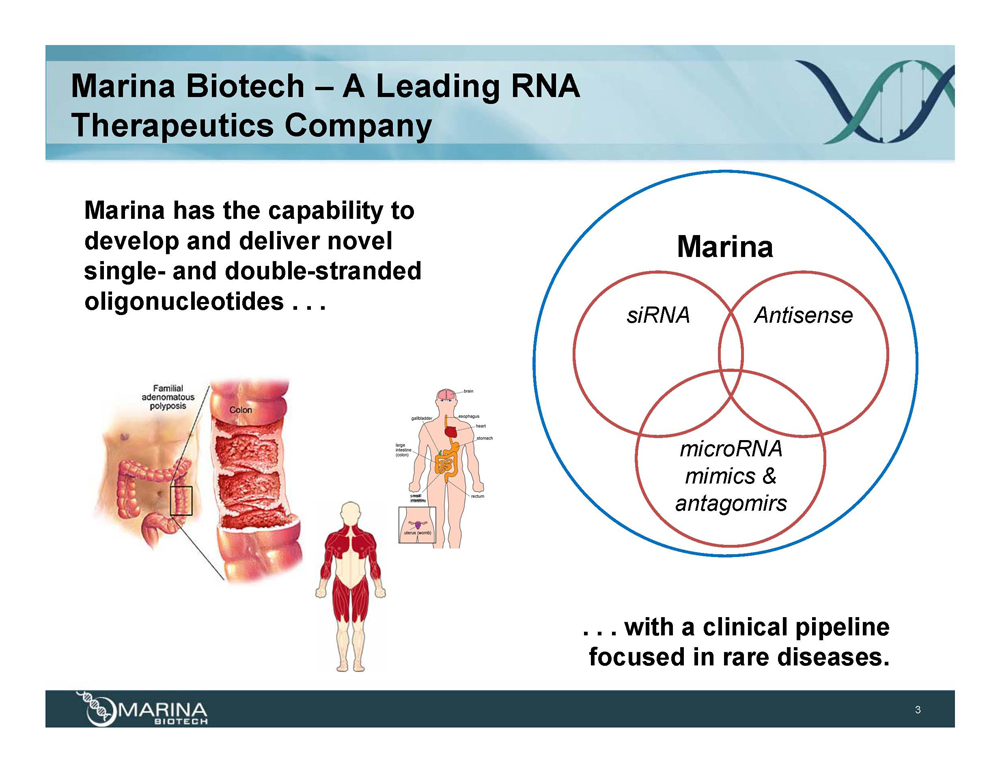
Marina Biotech – A Leading RNA Therapeutics Company Marina has the capability to develop and deliver novel single- and double-stranded oligonucleotides . . . Marina siRNA Antisense microRNA mimics & antagomirs . . . with a clinical pipeline focused in rare diseases.
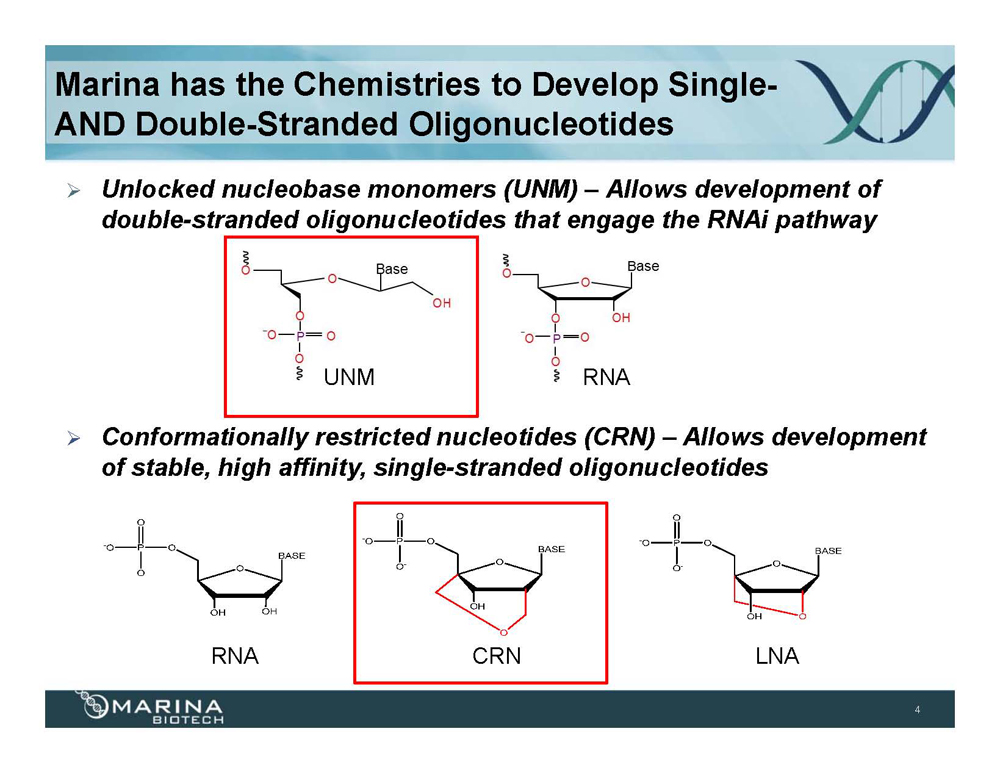
Marina has the Chemistries to Develop Single- AND Double-Stranded Oligonucleotides Unlocked nucleobase monomers (UNM) – Allows development of double-stranded oligonucleotides that engage the RNAi pathway Conformationally restricted nucleotides (CRN) – Allows development of stable, high affinity, single-stranded oligonucleotides RNA CRN LNA
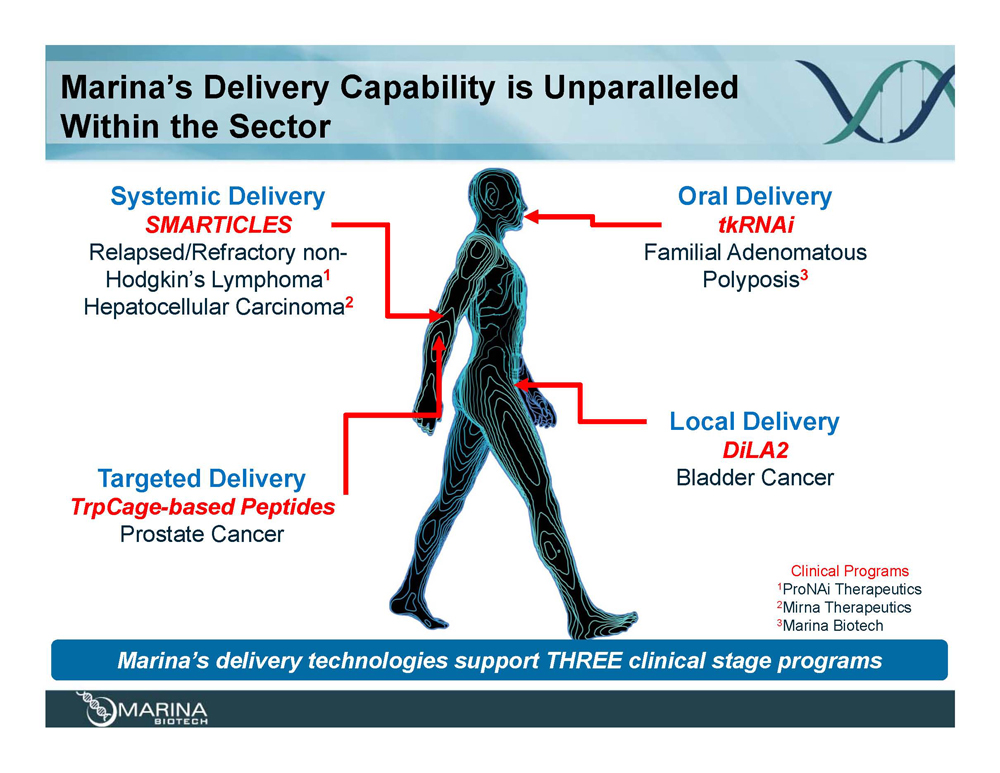
Marina’s Delivery Capability is Unparalleled Within the Sector Systemic Delivery SMARTICLES Relapsed/Refractory non-Hodgkin’s Lymphoma1 Hepatocellular Carcinoma2 Targeted Delivery TrpCage-based Peptides Prostate Cancer Oral Delivery tkRNAi Familial Adenomatous Polyposis3 Local Delivery DiLA2 Bladder Cancer Clinical Programs 1ProNAi Therapeutics 2Mirna Therapeutics 3Marina Biotech Marina’s delivery technologies support THREE clinical stage programs
Most Versatile Delivery System in Clinical Development – SMARTICLES® Effective delivery of single-stranded and double-stranded nucleic acids ProNAi Therapeutics - PNT2258 (24mer single-stranded DNA decoy) Mirna Therapeutics - MRX34 (19-21mer double-stranded microRNA mimic) Delivery to cell nucleus (PNT2258) and cell cytoplasm (MRX34) Delivery outside the liver, i.e. tumors, immune cells Interim Phase II data as reported by ProNAi: 11 of 13 patients achieved clinical benefit, with ongoing Progression Free Survival (PFS) extending to 18 months and beyond Durable and clinically meaningful complete and partial responses in patients with aggressive disease – Richter’s and Burkitt’s-like DLBCL Interim Phase I data as reported by Mirna: Manageable safety profile May have potential activity in HCC, SCLC, NET, CRC and lymphoma Molecular analysis of white blood cells showed a dose-dependent repression of miR-34 targets Safe and well tolerated – over 100 patients dosed to date Most widely adopted clinical stage nucleic acid delivery technology

Only Orally Available RNAi Delivery Technology in Clinical Development - tkRNAi ™ Non-pathogenic bacteria engineered to produce, deliver and release interfering RNA mediators (shRNA) to targeted tissue Efficient delivery to the epithelium of the gastrointestinal tract via oral administration Safe and well tolerated: Daily dosing of up to 109 cfu (colony forming units) in patients for 30 days with no serious adverse events Well tolerated with daily dosing of up to 1011 cfu in non-human primates for 270 days Clinical data indicates the potential for exposure throughout entire intestinal tract; providing opportunities to treat GI inflammatory diseases Ability to modify delivery vehicle to accommodate emerging therapeutic approaches such as a CRISPR/Cas9-based modality
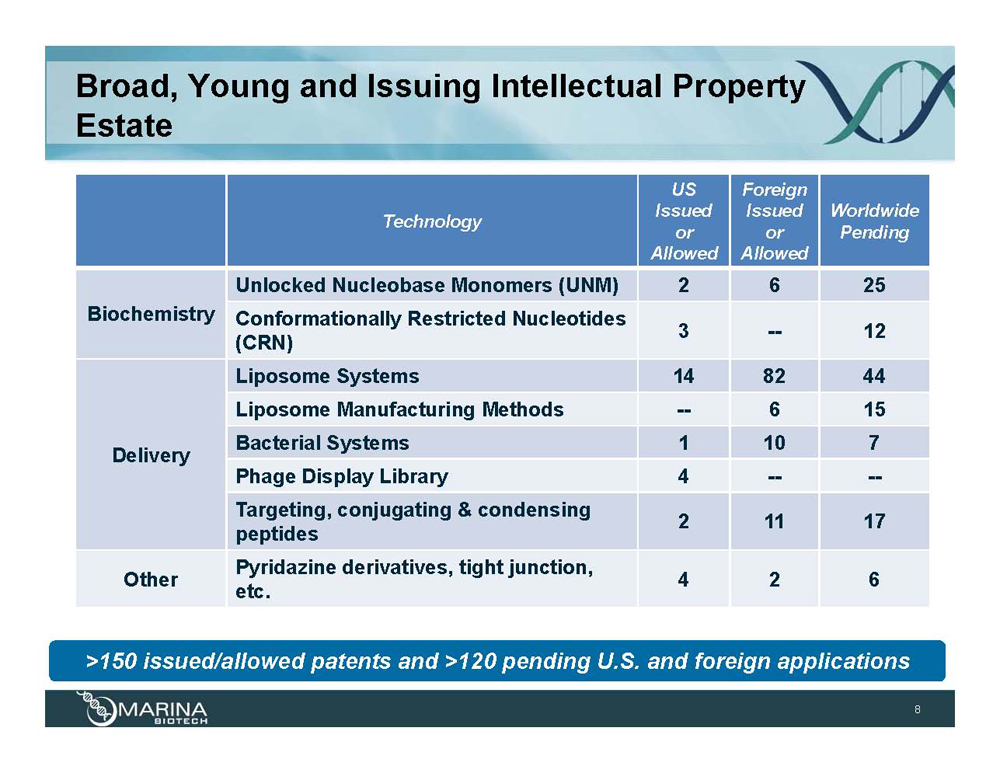
Broad, Young and Issuing Intellectual Property Estate Technology US Issued or Allowed Foreign Issued or Allowed Worldwide Pending Biochemistry Unlocked Nucleobase Monomers (UNM) 2 6 25 Conformationally Restricted Nucleotides (CRN) 3 -- 12 Delivery Liposome Systems 14 82 44 Liposome Manufacturing Methods -- 6 15 Bacterial Systems 1 10 7 Phage Display Library 4 -- -- Targeting, conjugating & condensing peptides 2 11 17 Other Pyridazine derivatives, tight junction, etc. 4 2 6 >150 issued/allowed patents and >120 pending U.S. and foreign applications

Partner of Choice for Rare Disease Therapeutic R&D
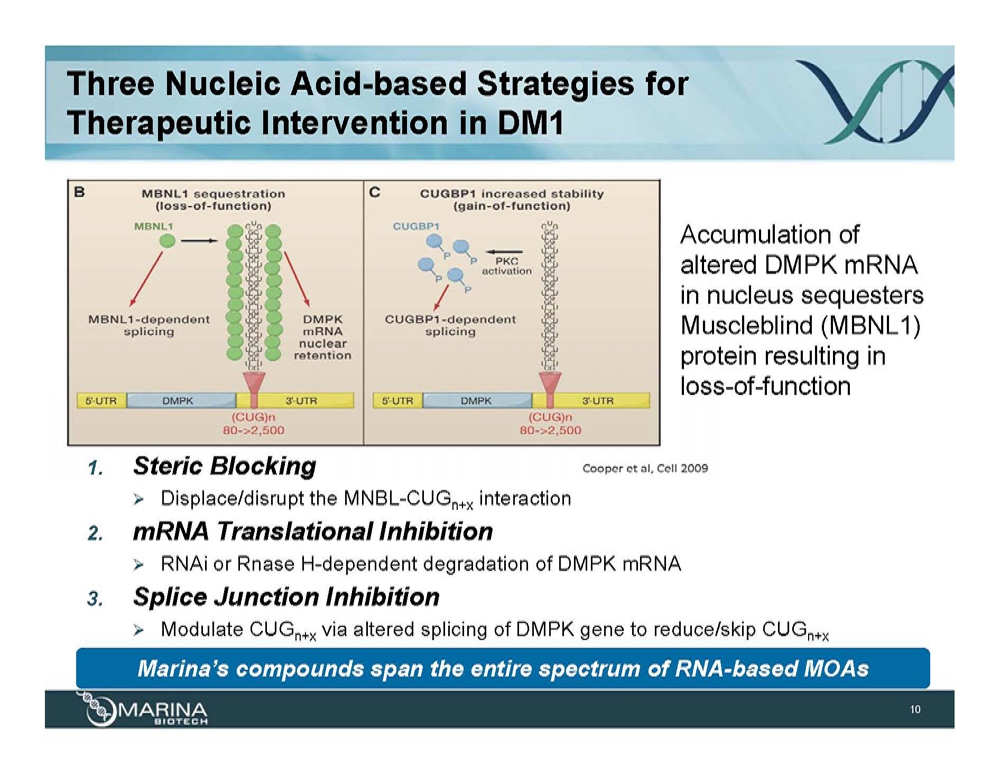
Three Nucleic Acid-based Strategies for Therapeutic Intervention in DM1 Accumulation of altered DMPK mRNA in nucleus sequesters Muscleblind (MBNL1) protein resulting in loss-of-function 1. Steric Blocking Displace/disrupt the MNBL-CUGn+x interaction 2. mRNA Translational Inhibition RNAi or Rnase H-dependent degradation of DMPK mRNA 3. Splice Junction Inhibition Modulate CUGn+x via altered splicing of DMPK gene to reduce/skip CUGn+x Marina’s compounds span the entire spectrum of RNA-based MOAs
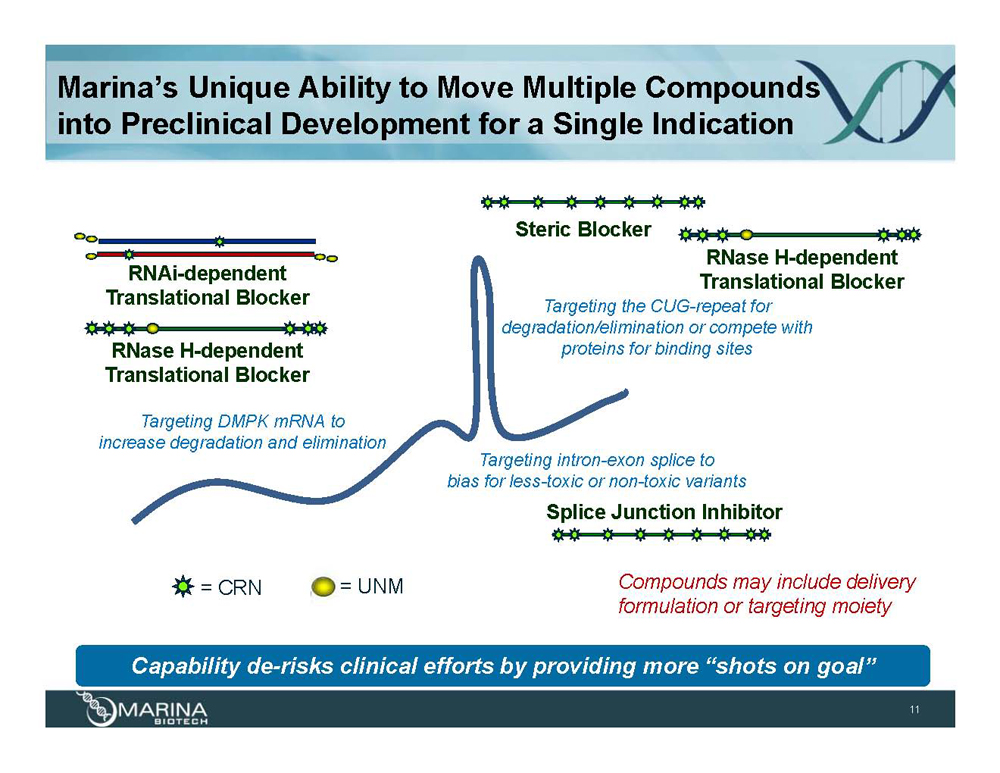
Marina’s Unique Ability to Move Multiple Compounds into Preclinical Development for a Single Indication RNAi-dependent Translational Blocker RNase H-dependent Translational Blocker Targeting DMPK mRNA to increase degradation and elimination Steric Blocker RNase H-dependent Translational Blocker Targeting the CUG-repeat for degradation/elimination or compete with proteins for binding sites Targeting intron-exon splice to bias for less-toxic or non-toxic variants Splice Junction Inhibitor Compounds may include delivery formulation or targeting moiety = CRN = UNM Capability de-risks clinical efforts by providing more “shots on goal”

Partnering Opportunity

Marina’s Near Term Partnering Focus Rare Diseases Collaboration/licensing of current pipeline Out-licensing of CEQ508 in Europe, Asia and ROW territories Preclinical collaboration in Duchenne muscular dystrophy Fast-follower approach with IND potential within 24 months: Amyotrophic lateral sclerosis, coagulation disorders, cystic fibrosis, Friedreich’s ataxia, sickle cell, spinal muscular atrophy Oncology Broad therapeutic drug discovery and development collaboration: Demonstrated delivery and activity, in humans, of single-and double-stranded oligonucleotides targeting solid tumors Pre-IND program in bladder cancer Proprietary cell-targeting peptide with demonstrated in vitro specificity to prostate cancer cells Emerging Therapeutic Approaches Licensing of delivery technologies for emerging modalities such as CRISPR/Cas9, RNA-based vaccines, mRNA and other nucleic acid cargos

Thank You
