Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - BIOCRYST PHARMACEUTICALS INC | Financial_Report.xls |
| EX-31.2 - EXHIBIT 31.2 - BIOCRYST PHARMACEUTICALS INC | exh_312.htm |
| EX-32.1 - EXHIBIT 32.1 - BIOCRYST PHARMACEUTICALS INC | exh_321.htm |
| EX-32.2 - EXHIBIT 32.2 - BIOCRYST PHARMACEUTICALS INC | exh_322.htm |
| 10-Q - FORM 10-Q - BIOCRYST PHARMACEUTICALS INC | f10q_050815.htm |
| EX-31.1 - EXHIBIT 31.1 - BIOCRYST PHARMACEUTICALS INC | exh_311.htm |
Exhibit 10.2
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
AWARD/CONTRACT
|
1. THIS CONTRACT IS A RATED ORDER
UNDER DPAS (15 CFR 350)
|
RATING
N/A
|
PAGE
1
|
OF PAGES
55
|
|||
|
2. CONTRACT (Proc. Inst. Ident.) NO.
HHSO100201500007C
|
3. EFFECTIVE DATE
March 31, 2015
|
4. REQUISITION/PURCHASE REQUEST/PROJECT NO.
OS150001
|
|||||
|
5. ISSUED BY
|
CODE
|
6. ADMINISTERED BY (If other than Item 6)
|
CODE
|
||||
|
Office of Acquisitions Management, Contracts, and Grants
(AMCG)
330 Independence Ave., S.W. Room G640
Washington, D.C. 20201
|
See Block 5.
|
||||||
|
7. NAME AND ADDRESS OF CONTRACTOR (No. street, county, state and ZIP Code)
BioCryst Pharmaceuticals
Nottingham Hall
4505 Emperor Blvd., Suite 200
Durham, NC 27703
|
8. DELIVERY
See Schedule.
|
||||||
|
CAGE: 4GBX7
|
9/ DISCOUNT FOR PROMPT PAYMENT
N/A
|
||||||
|
10. SUBMIT INVOICES
|
ITEM
|
||||||
|
CODE DUNS No. 618194609
|
FACILITY CODE
|
ADDRESS SHOWN IN: F.3
|
See Section G.
|
||||
|
11. SHIP TO/MARK FOR
|
CODE
|
N/A
|
12. PAYMENT WILL BE MADE BY
|
CODE
|
N/A | ||
|
See Block 5
|
See Block 5
|
||||||
| o | o | ||||||
|
13. AUTHORITY FOR USING OTHER FULL AND OPEN COMPETITION: N/A
|
14. ACCOUNTING AND APPROPRIATION DATA
|
||||||
|
10 U.S.C. 2304(c)( )
|
41 U.S.C. 253(c)( ) |
Object Class - 25103
CAN# - 1990500
|
|||||
|
15A. ITEM NO.
|
15B. SUPPLIES/SERVICES
|
15C. UNIT PRICE
|
15D. AMOUNT
|
15E. UNIT PRICE
|
15F. AMOUNT
|
||
|
Title: BCX4430 NDA Enabling CMC and Non-Clinical Toxicology Studies
|
(See Schedule)
|
(See Schedule) |
(See Schedule)
|
(See Schedule)
|
|||
|
15G. TOTAL AMOUNT OF CONTRACT
|
› |
$12,133,606
|
|||||
| 16. TABLE OF CONTENTS | |||||||
| (ü) | SEC. | DESCRIPTION | PAGE(S) | (ü) | SEC. | DESCRIPTION | PAGE(S) |
|
PART I - THE SCHEDULE
|
PART II - CONTRACT CLAUSES
|
||||||
| x | A |
SOLICITATION/CONTRACT FORM
|
01 | x | I | CONTRACT CLAUSES | 47 |
| x | B |
SUPPLIES OR SERVICES AND PRICE/COST
|
03 |
PART III - LIST OF DOCUMENTS, EXHIBITS AND OTHER ATTACH.
|
|||
| x | C |
DESCRIPTION / SPECS / WORK STATEMENT
|
10 | x | J | LIST OF ATTACHMENTS | 54 |
| x | D |
PACKAGING AND MARKING
|
11 |
PART IV - REPRESENTATIONS AND INSTRUCTIONS
|
|||
| x | E |
INSPECTION AND ACCEPTANCE
|
11 | x | K | REPRESENTATIONS, CERTIFICATIONS AND OTHER STATEMENTS OF OFFERORS | 55 |
| x | F |
DELIVERIES OR PERFORMANCE
|
12 | ||||
| x | G |
CONTRACT ADMINISTRATION DATA
|
22 | o | |||
| x | H |
SPECIAL CONTRACT REQUIREMENTS
|
29 | o | |||
|
CONTRACTING OFFICER WILL COMPLETE ITEM 17 OR 18 AS APPLICABLE
|
||||||
|
17.X CONTRACTOR’S NEGOTIATED AGREEMENT (Contractor is required to sign this document and return 2 copies to issuing office.) Contractor agrees to furnish and deliver all items or perform all the services set forth or otherwise identified above and on any continuation sheets for the consideration stated herein. The rights and obligations of the parties to this contract shall be subject to and governed by the following documents: (a) this award/contract, (b) the solicitation, if any, and (c) such provisions, representations, certifications, and specifications, as are attached or incorporated by reference herein. (Attachments are listed herein.)
|
18.o AWARD (Contractor is not required to sign this document.) Your offer on Solicitation Number __________________, including the additions or changes made by you which additions or changes are set forth in full above, is hereby accepted as to the items listed above and on any continuation sheets. This award consummates the contract which consists of the following documents: (a) the Government’s solicitation and your offer, and (b) this award/contract. No further contractual document is necessary.
|
|||||
|
19A. NAME AND TITLE OF SIGNER (Type or print)
|
20A. NAME OF CONTRACTING OFFICER
|
|||||
| Jon P. Stonehouse |
Thomas P. Hastings
|
|||||
|
19B. NAME OF CONTRACTOR
|
19C. DATE SIGNED
|
20B. UNITED STATES OF AMERICA
|
20C. DATE SIGNED
|
|||
| BY | ||||||
|
(Signature of person authorized to sign)
|
(Signature of person authorized to sign)
|
|||||
Page 1
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
Contents
|
||
|
PART I – THE SCHEDULE
|
||
|
SECTION B – SUPPLIES OR SERVICES AND PRICES/COSTS
|
3
|
|
|
SECTION C - DESCRIPTION/SPECIFICATIONS/WORK STATEMENT
|
10
|
|
|
SECTION D – PACKAGING, MARKING AND SHIPPING
|
11
|
|
|
SECTION E – INSPECTION AND ACCEPTANCE
|
11
|
|
|
SECTION F – DELIVERIES OR PERFORMANCE
|
12
|
|
|
SECTION G - CONTRACT ADMINISTRATION DATA
|
22
|
|
|
SECTION H - SPECIAL CONTRACT REQUIREMENTS
|
29
|
|
|
PART II - CONTRACT CLAUSES
|
||
|
SECTION I - CONTRACT CLAUSES
|
47
|
|
|
PART III - LIST OF DOCUMENTS, EXHIBITS AND OTHER ATTACHMENTS
|
||
|
SECTION J - LIST OF ATTACHMENTS
|
54
|
|
|
PART IV - REPRESENTATIONS AND INSTRUCTIONS
|
||
|
SECTION K - REPRESENTATIONS AND CERTIFICATIONS
|
55
|
|
Page 2
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
PART I – THE SCHEDULE
SECTION B – SUPPLIES OR SERVICES AND PRICES/COSTS
ARTICLE B.1. BRIEF DESCRIPTION OF SUPPLIES OR SERVICES
There are currently no medical countermeasures (MCMs) available for the prophylaxis or treatment of infection with Ebola virus, a high priority agent for the PHEMCE Implementation Plan.
BioCryst’s proposal focuses on BCX4430, a novel small molecule nucleoside with broad spectrum antiviral activity being developed for diseases caused by RNA pathogens. BCX4430, an inhibitor of viral RNA – dependent RNA polymerase (RdRp), is the lead compound in BioCryst’s broad spectrum antiviral program to meet the need for an effective and broad-spectrum parenteral direct- acting antiviral medical countermeasure (MCM). The proposed activities take into account the Ebola virus disease (EVD) outbreak in West Africa while advancing the development of BCX4430 toward an NDA filing. The tasks include manufacturing process development activities, manufacturing of Drug substance (DS) and drug product (DP) and clinical trial material, and the nonclinical development activities to advance the intra-muscular and intravenous formulation
through NDA-enabling toxicology studies including * * * toxicology and * * * toxicology in the * * *
The Advanced Research and Development effort will progress in specific stages that cover the base work segment and the four (4) option work segments. Work performed during the base segment and in the four (4) option segments each constitutes an independent, non-severable discrete work segment that cannot be subdivided for separate performance. Work specified in each work segment is , and each are necessary to support the development of BCX 4430 as an MCM. Each of the non-severable, discreet work segments contains multiple activities that when reviewed in total shall satisfy a defined end-product for each segment. The Government has determined that it has a Bona Fide Need for each non-severable discrete work segment. That need will be met upon the completion of the defined task(s) listed in the Work Breakdown Statement (WBS) in the Statement of Work (SoW) for each work segment (See Section J- Attachment 1), the completion of the Milestones in the Contract and submission of the deliverables required in the Contract (See Section J–Attachment 2.). Each work segment provides independent merit and value to the Government. Each work segment will be fully funded from an appropriation source that is current at the time the contract is awarded (Base Work Segment) and at the time the Government exercises each option.
ARTICLE B.2. BASE PERIOD (March 31, 2015 through September 30, 2016)
|
a.
|
The total estimated cost of the base period of the contract excluding fee is
|
$ * * * .
|
b.
|
The total fixed fee for the base period of performance is $ * * *
|
|
c.
|
The fixed fee for the base period of performance (CLIN 0001) and any exercised cost- reimbursement contract options shall be paid at a rate equal to * * * % of actual costs incurred per invoicing period, with the balance of fee payable upon successful completion of all work under each CLIN, subject to the following limitations:
|
|
·
|
The government shall withhold the payment of a portion of the fee to protect the government’s interest as set forth in Federal Acquisition Regulation (FAR) 52.216-8, Fixed Fee (June 2011). The government shall withhold 15 percent of the total fixed fee or $100,000, whichever is less, until after government review and acceptance of the Final Technical Progress Report.
|
Page 3
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
d.
|
The total estimated cost of the base period of the contract, CLIN 0001, represented by the sum of the total estimated cost plus fixed fee is $12,133,606. The government will not be responsible for any Contractor-incurred costs that exceed this amount unless a modification to the contract is signed by the Contracting Officer which expressly increases this amount.
|
|
e.
|
The Contractor shall maintain records of all contract costs and such records shall be subject to FAR 52.215-2 (Oct 2010), Audit and Records-Negotiation, and Health and Human Services Acquisition Regulation (HHSAR) 352.242-74, Final Decisions on Audit Findings, incorporated by reference into this contract in SECTION I.
|
|
CLIN
|
Estimated Period of Performance
|
Supplies/Services
|
Total Estimated Cost
|
Fixed Fee
|
Total Estimated Cost Plus Fixed Fee
|
|||
|
0001
|
03/31/2015 –
09/30/2016
|
Manufacture of Clinical Trial Material and Process Improvements
|
* * *
|
* * *
|
$12,133,606
|
ARTICLE B.3. OPTION PRICES
|
|
Pursuant to FAR 52.217-9, Option to Extend the Term of the Contract (Mar 2000), set forth in full in ARTICLE I.3 of this contract, the government may, by unilateral contract modification, require the Contractor to perform discrete portions of additional work as specified in the Statement of Work.
Unless the government exercises one or more optional CLINs, the contract consists only of the base work specified in the Statement of Work as defined in SECTIONS C and F, with estimated costs set forth in ARTICLE B.2 of the contract.
|
|
CLIN
|
Option
|
Estimated Period of Performa nce
|
Supplies/ Services
|
Total Estimated Cost
|
Fixed Fee
|
Total Estimated Cost Plus Fixed Fee
|
|
0002
|
1
|
*** |
Commercial Scale-up and NDA
Registration batches
|
$ ***
|
$ ***
|
$ ***
|
|
0003
|
2
|
*** |
Nonclinical NDA-
enabling Toxicology IM
|
$ ***
|
$ ***
|
$ ***
|
|
0004
|
3
|
*** |
In Vitro Experiments
|
$ ***
|
$ ***
|
$ ***
|
|
0005
|
4
|
*** |
Nonclinical NDA-
enabling Toxicology IV
|
$ ***
|
$ ***
|
$ ***
|
Page 4
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE B.4. LIMITATIONS APPLICABLE TO DIRECT COSTS
|
a.
|
Items Unallowable Unless Otherwise Provided
|
Notwithstanding the clause FAR 52.216-7, Allowable Cost and Payment, incorporated in this contract, unless authorized in writing by the Contracting Officer in the form of a Contracting Officer Authorization (COA), the costs of the following items or activities shall be unallowable as direct costs:
|
1.
|
Acquisition, by purchase or lease, of any interest in real property;
|
|
2.
|
Special rearrangement or alteration of facilities;
|
|
3.
|
Purchase or lease of any item of general purpose office furniture or office equipment regardless of dollar value. (General purpose equipment is defined as any items of personal property which are usable for purposes other than research, such as office equipment and furnishings, pocket calculators, etc.);
|
|
4.
|
Travel to attend general scientific meetings, subject to limitation under Article B.4.b.1;
|
|
5.
|
Foreign travel;
|
|
6.
|
Subcontractor and/or Consultant costs;
|
|
7.
|
Patient care costs;
|
|
8.
|
Accountable government property (defined as both real and personal property with an acquisition cost of $1,000 or more and a life expectancy of more than two years) and “sensitive items” regardless of acquisition value (Section J, Attachment 6).
|
|
9.
|
Printing Costs (as defined in the government Printing and Binding Regulations).
|
|
10.
|
Light Refreshment and Meal Expenditures are not authorized.
|
|
11.
|
Costs for meeting room or conference space used for face to face meetings with United States government (USG) staff in the performance of this contract at Government or Contractor facilities are not authorized.
|
|
b.
|
Travel Costs
|
|
1.
|
Total expenditures for all travel (transportation, lodging, subsistence, and incidental expenses) incurred by the Prime Contractor in direct performance of this contract during the base period shall not exceed $40,000 without the prior written approval of the Contracting Officer. Cost must be consistent with FAR 52.247-63 – Preference for U.S. - Flag Air Carriers.
|
|
2.
|
The Contactor shall invoice and be reimbursed for all travel costs in accordance with F A R 31. 20 5 - 4 6, T r a v el C os t s a nd GSA Per Diem Rates (www.gsa.gov/perdiem).
|
Page 5
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
3.
|
Requests for foreign travel must be submitted at least four weeks in advance and shall contain the following:
|
(i) meeting(s) and place(s) to be visited, with costs and dates;
(ii) names(s) and title(s) of Contractor personnel to travel and their functions in the contract project;
(iii) contract purpose to be served by the travel;
(iv) how travel of Contractor personnel will benefit and contribute to accomplishing the contract project, or will otherwise justify the expenditure of AMCG contract funds;
(v) how such advantages justify the costs for travel and absence from the project of more than one person if such are suggested; and
(vi) what additional functions may be performed by the travelers to accomplish other purpose of the contact and thus further benefit the project.
ARTICLE B.5. ADVANCE UNDERSTANDINGS
a. Subcontracts
Prior written consent from the Contracting Officer in the form of a Contracting Officer Authorization (COA) is required for any subcontract that:
Is of the cost-reimbursement type or Time-and-Materials (T&M);
Is Fixed-Price and exceeds $150,000 or 5% of the total estimated cost of the Contract, whichever value is greater.
The Contracting Officer shall request appropriate supporting documentation in order to review and determine authorization, pursuant with FAR Clause 52.244-2, Subcontracts (Alternate I). After receiving written consent of the subcontract by the Contracting Officer, a copy of the signed, executed subcontract and consulting agreement shall be provided to the Contracting Officer within ten (10) calendar days.
|
|
Note: Consulting services are treated as subcontracts and subject to the ‘consent to subcontract’ provisions set forth in this Article.
|
b. * * * as major subcontractor under the Contract
The services under the Contract will include certain chemical manufacturing services. It is anticipated that, a large portion of the chemical manufacturing services will be subcontracted to * * * by the Contractor. Accordingly, * * * is expected to be a material subcontractor under the Contract and COA approval is hereby granted.
|
c.
|
Services Performed under Contract with NIAID
|
Certain development services have been funded under by HHS/NIAID under contract number HHS27220130017C (the “NIAID Contract”). The focus of the NIAID Contract were certain IND enabling studies as well as certain contract manufacturing transfer and scale up services and certain Phase 1 studies. The parties recognize that certain work under this Contract is not expected to begin until certain related services (“Related Services”) are completed under the NIAID Contract. Such services include but are not limited to the technology transfer and scale up chemical manufacturing services at * * * The parties agree that if for any reason the Related Services are delayed or unable to be completed under the NIAID Contract, Contractor shall be relieved of its obligations to continue performance under this Contract.
Page 6
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
d.
|
Security
|
A Security Plan is required for this effort. A security waiver may be requested. In the event a security waiver cannot successfully be attained, the Government will notify the Contractor who will subsequently be required to deliver a security plan to the Government, conforming with the following paragraphs.
The work to be performed under this contract will involve access to sensitive Biomedical Advanced Research and Development Authority [BARDA] program information. Upon contract award, the Program Protection Officer (PPO) will request submission of and review the Draft Security Plan in detail and submit comments within ten (10) business days to the CO to be forwarded to the Contractor. The Contractor shall review the Draft Security Plan comments, and if changes are required, submit a Final Security Plan to the U.S. Government within thirty (30) calendar days after receipt of the Program Protection Officer’s (PPO) comments. The Final Security Plan shall include a timeline for compliance of all the required security measures. Upon completion of initiating all security measures, the Contractor shall supply to the CO and Contracting Officer’s Representative (COR) a letter certifying compliance to the elements outlined in the Final Security Plan. The execution of the work under this contract shall be in accordance with the approved Final Security Plan. As outlined above, the content of the Final Security Plan shall be considered as part of the Contractor’s Technical Proposal. The Contractor shall ensure that the storage, generation, transmission or exchanging of BARDA sensitive information has the appropriate security controls in place. At a minimum, the Final Security Plan shall address the following items:
Personnel Security Policies and Procedures including, but not limited to: Recruitment of new employees; Interview process; Personnel background checks; Suitability/adjudication policy; Access determination; Rules of behavior/conduct; Termination procedures; Non-disclosure agreements.
Physical Security Policies and Procedures including but not limited to: Internal/external access control; Identification/badge requirements; Facility visitor access; Parking areas and access; Barriers/perimeter fencing; Shipping, receiving and transport (on and off- site); Security lighting; Restricted areas; Signage; Intrusion detection systems; Closed circuit television; Other control measures.
|
|
Information Security Policies and Procedures including but not limited to: Identification of sensitive information; Access control/determination; Secured storage infrastructure; Document control; Retention/destruction requirements.
|
Information Technology Security Policies and Procedures including but not limited to: Intrusion detection and prevention systems; firewalls, Encryption systems; Identification of sensitive information/media; Passwords; Removable media; Laptop policy; Media access control/determination; Secure storage; System document control; System backup; System disaster recovery.
Security Reporting Requirement - Violations of established security protocols shall be reported to the CO and COR within 24 hours of the contractor’s discovery of any compromise, intrusion, loss or interference of its security processes and procedures. The Contractor shall ensure that all software components that are not required for the operation and maintenance of the database/control system have been removed and/or disabled. The Contractor shall provide to the CO and the COR information appropriate to Information and Information Technology software and service updates and/or workarounds to mitigate all vulnerabilities associated with the data and shall maintain the required level of system security.
Page 7
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
The Contractor will investigate violations to determine the cause, extent, loss or compromise of sensitive program information, and corrective actions taken to prevent future violations. The CO in coordination with BARDA will determine the severity of the violation. Any contractual actions resulting from the violation will be determined by the Contracting Officer.
|
e.
|
Confidential Treatment of Sensitive Information
|
The Contractor shall, to the extent permitted by law, guarantee strict confidentiality of sensitive/confidential information/data that is provided by the USG during the performance of the contract. The USG has determined that certain information/data that the Contractor will be provided during the performance of the contract is of a sensitive nature.
Disclosure of confidential/sensitive information/data, in whole or in part, by the Contractor can only be made after the Contractor receives prior written approval from the Contracting Officer. Whenever the Contractor is uncertain with regard to the proper handling of information/data under the contract, the Contractor shall obtain a written determination from the Contracting Officer.
Notwithstanding the foregoing, such information/data shall not be deemed of a sensitive or confidential nature with respect to the Contractor for purposes of this contract if such information/data: (a) was already known to the Contractor other than by prior disclosure by the USG or discovered through work under a prior USG contract; (b) was generally available or known, or was otherwise part of the public domain, at the time of its disclosure to the Contractor; (c) became generally available or known, or otherwise became part of the public domain, after its disclosure to, or, with respect to the information/data by, the Contractor through no fault of the Contractor; (d) was disclosed to the Contractor, other than under an obligation of confidentiality or non-use, by a third party who had no obligation to the USG that controls such information/data not to disclose such information/data to others; or (e) was independently discovered or developed by the Contractor, as evidenced by its written records, without the use of information/data belonging to the USG.
The Contractor may disclose information/data of a sensitive nature provided by the USG to the extent that such disclosure is: (a) made in response to a valid order of a court of competent jurisdiction (b) otherwise required by law or regulation, (c) made by the Contractor to the Regulatory Authorities as required in connection with any filing, application or request for Regulatory Approval; provided, however, that reasonable measures shall be taken to assure confidential treatment of such information/data.
|
f.
|
Sharing of contract deliverables within United States Government (USG)
|
In an effort to build a robust medical countermeasure pipeline through increased collaboration, BARDA may share technical deliverables with USG entities responsible for Medical Countermeasure Development. In accordance with recommendations from the Public Health Emergency Medical Countermeasure Enterprise Review, agreements established in the Integrated Portfolio Advisory Committee (PAC) Charter, and agreements between BARDA and the Department of Defense and the National Institutes of Health, BARDA may share technical deliverables and data created in the performance of this contract with colleagues within the Integrated Portfolio. This advance understanding does not authorize BARDA to share financial information outside of the United States Government. The Contractor is advised to review the terms of FAR 52.227-14, Rights in Data – General, regarding the government’s rights to deliverables submitted during
Page 8
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
performance as well as the government’s rights to data contained within those deliverables.
|
|
g.
|
Approval of Protocols
|
The Contractor shall submit all protocols as referenced under this Contract to the COR f o r review and approval. The Government requires no fewer than eight (8) business days to perform a review. The Contractor shall take this review time into account and submit protocols as early as possible to avoid delays. The Government’s comments and feedback shall be addressed prior to approval. The COR will review and provide approval of protocols.
|
h.
|
Rights in Data
|
The contract will incorporate the Alternate II to FAR Clause 52.227-14, Rights in Data—general, pursuant to FAR Clause 52.227-14 (g)(3). In the event that the U.S. Government requires the delivery of pre-existing privately funded data, BioCryst will identify that specific pre-existing privately funded data and that data will be marked with the limited rights notice specified under FAR Clause 52.227-14 (g)(3)(a).
Page 9
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE C.1. STATEMENT OF WORK
Independently and not as an agent of the government, the Contractor shall furnish all the necessary services, qualified personnel, material, equipment, and facilities not otherwise provided by the government as needed to perform the Statement of Work dated March 27, 2015, set forth in SECTION J - List of Attachments, attached hereto and made a part of the contract.
ARTICLE C.2. REPORTING REQUIREMENTS
Refer to ARTICLE F.2 for specific instructions regarding Reporting Requirements.
ARTICLE C.3. EARNED VALUE MANAGEMENT SYSTEM (EVMS) IMPLEMENTATION REQUIREMENTS
The Contractor and BARDA agree that the EVMS implementation requirements that are contained in the contract are limited to the implementation requirements outlined by the 7 Principles of Earned Value Management Tier 3 System Implementation Intent Guide contained in the Attachments (Section J.) of the contract. The total amount of this contract reflects the use of the 7 Principles of EVMS Implementation.
Refer to Article F.2. for specifics on EVMS deliverables.
ARTICLE C.4. PROJECT MEETING CONFERENCE CALLS
|
|
A conference call between the Contracting Officer’s Representative and designees and the Contractor’s Project Leader/delegate and designees shall occur bi-weekly or as otherwise mutually agreed upon by the USG and the Contractor or determined by the Contracting Officer. During this call the Contractor’s Project Leader/delegate and designees will discuss the activities since the last call, any problems that have arisen and the activities planned until the next call takes place. The Contractor’s Project Leader/delegate may choose to include other key personnel on the conference call to give detailed updates on specific projects or this may be requested by the Contracting Officer’s Representative. Electronic copy of conference call meeting minutes/summaries shall be provided via e-mail to the CO, COR, and uploaded in e-room by the Contractor within five (5) business days after the conference call is held.
|
ARTICLE C.5. OTHER PROJECT MEETINGS
|
a.
|
Kickoff Meeting
|
The Contractor and USG shall conduct a kickoff meeting within 30 calendar days after contract award. Contractor shall provide an itinerary/agenda no later than 5 business days before meeting. Minutes from the kickoff meeting must be provided within 10 business days of the event.
|
b.
|
Quarterly and Ad-Hoc Meetings
|
The contractor shall participate in Project Meetings to coordinate the performance of the contract, as requested by the Contracting Officer’s Representative. These meetings may include face-to-face meetings with AMCG and BARDA in Washington, D.C. and at work sites of the Contractor and subcontractors. Such meetings may include, but are not limited to, meetings of the Contractor to discuss study designs, site visits to the Contractor’s facilities, and meetings with the Contractor and HHS officials to discuss the technical, regulatory, and ethical aspects of the program. Subject to the data rights provisions in this contract, the Contractor will provide data, reports, and presentations to groups of outside experts and USG personnel as required by the Contracting Officer’s Representative in order to facilitate review of contract activities. Contractor shall provide itinerary/agenda at least 5 business days in advance of face-to-face meeting.
Page 10
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
c.
|
Face-to-Face Project Review Meetings
|
The contractor shall, at a time to be determined later, present a comprehensive review of contract progress to date in a face-to-face meeting in Washington, DC. The contractor will be responsible for updating BARDA program on technical progress under the Statement of Work.
Presentation must be delivered seven (7) business days prior to the scheduled meeting.
All deliverables required under this contract shall be packaged, marked and shipped in accordance with USG specifications. At a minimum, all deliverables shall be marked with the contract number and Contractor name. The Contractor shall guarantee that all required materials shall be delivered in immediate usable and acceptable condition
|
|
Unless otherwise specified by the Contracting Officer, delivery of reports to be furnished to the USG under this contract (including invoices) shall be delivered to AMCG and BARDA electronically along with a concurrent email notification to the Contracting Officer, Contract Specialist, and COR (as defined in SECTION F.3. ELECTRONIC SUBMISSION) summarizing the electronic delivery.
|
ARTICLE E.1. FAR 52.252-2, CLAUSES INCORPORATED BY REFERENCE (FEBRUARY 1998)
|
|
This contract incorporates the following clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available. Also, the full text of a clause may be accessed electronically at these addresses: https://www.acquisition.gov/FAR/ . HHSAR Clauses at: http://www.hhs.gov/policies/hhsar/subpart352.html.
|
| FAR Clause | Title and Date | |
| 52.246-9 | Inspection of Research and Development (Short Form) (Apr 1984) |
For the purpose of this SECTION E, the designated Contracting Officer’s Representative (COR) is the authorized representative of the Contracting Officer. The COR will assist in resolving technical issues that arise during performance. The COR however is not authorized to change any contract terms or authorize any changes in the Statement of Work or modify or extend the period of performance, or authorize reimbursement of any costs incurred during performance.
Inspection and acceptance of the materials services and documentation called for herein shall be accomplished by the Contracting Officer or a duly authorized representative.
Office of Acquisition Management, Contracts, and Grants (AMCG) Office of the Assistant Secretary for Preparedness and Response
|
|
U.S. Department of Health and Human Services 330 Independence Avenue, S.W., Room G640 Washington, D.C. 20201
|
Page 11
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
a.
|
Site Visits and Inspections
|
At the discretion of the USG and independent of activities conducted by the Contractor, with 48 hours’ notice to the contractor, the USG reserves the right to conduct site visits and inspections on an as needed basis, including collection of product samples and intermediates held at the location of the contractor, or subcontractor. All costs reasonably incurred by the Contractor and subcontractor for such visit and/or inspection shall be allowable costs subject to the Allowable cost requirements in FAR Subpart 31.2. The Contractor shall coordinate these visits and shall have the opportunity to accompany the USG on any such visits. Under time-sensitive or critical situations, the USG reserves the right to suspend the 48 hour notice to the Contractor. The areas included under the site visit could include, but are not limited to: security, regulatory and quality systems, manufacturing processes and cGMP/GLP/GCP compliance.
If the USG, Contractor, or other party identifies any issues during an audit, the Contractor shall capture the issues, identify potential solutions, and provide a report to the USG for review and acceptance.
|
·
|
If issues are identified during the audit, the Contractor shall submit a report to the CO and COR within five (5) business days detailing the finding and corrective action(s) of the audit.
|
|
·
|
COR and CO will review the report and provide a response to the Contractor within ten (10) business days.
|
|
·
|
Once corrective action is completed, the Contractor will provide a final report to the CO and COR.
|
ARTICLE F.1. PERIOD OF PERFORMANCE
Base Period
|
|
Under CLIN 0001, the estimated period of performance for the base performance segment of this contract shall be from March 31, 2015 through September 30, 2016 (18 months).
|
Option CLINs
|
CLIN
|
Option
|
Estimated Period of Performance
|
Supplies/Services
|
|
0002
|
1
|
* * * |
Commercial Scale-up and NDA Registration batches
|
|
0003
|
2
|
* * * |
Nonclinical NDA-enabling Toxicology IM
|
|
0004
|
3
|
* * * |
In Vitro Experiments
|
|
0004
|
4
|
* * * |
Nonclinical NDA-enabling Toxicology IV
|
NOTE: Base period and all option periods (if exercised in accordance with FAR clause FAR clause 52.217-09,
Option to Extend the Term of the Contract (Mar 2000), shall not exceed * * * months.
ARTICLE F.2. DELIVERABLES
Successful performance of the final contract shall be deemed to occur upon completion of performance of the work set forth in the Statement of Work dated March 27, 2015, set forth in SECTION J - List of Attachments of this contract and upon delivery and acceptance, as required by the Statement of Work, by the Contracting
Page 12
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
Officer, of each of the deliverables described in SECTION C, SECTION F, and SECTION J, Attachment 2, “Contract WBS Milestones and Related Deliverables”.
|
|
|
All deliverables and reporting documents listed within this section shall be delivered electronically (as defined in SECTION F.3. ELECTRONIC SUBMISSION) to the CO, CS, and the COR unless otherwise specified by the Contracting Officer.
|
|
a.
|
Summary of Contract Deliverables
|
Unless otherwise specified by the Contracting Officer, the deliverables identified in this SECTION F shall also be delivered electronically to the designated eRoom along with a concurrent email notification sent to the Contracting Officer, Contract Specialist, COR, and Alternate COR stating delivery has been made.
All paper/hardcopy documents/reports submitted under this contract shall be printed or copied, double- sided, on at least 30 percent post-consumer fiber paper, whenever practicable, in accordance with FAR 4.302(b). Hard copies of deliverables and reports furnished to the USG under the resultant Contract (including invoices) shall be addressed as follows:
|
|
HHS/ASPR/AMCG
ATTN: Thomas P. Hastings, Contracting Officer 330 Independence Avenue, S.W., Room G640
Washington, DC 20201
* * *
|
|
|
HHS/ASPR/BARDA
ATTN: Kimberly Sciarretta, Contracting Officer’s Representative 330 Independence Avenue, S.W., Room G640
Washington, DC 20201
* * *
|
Page 13
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
Technical Reports
|
|||
|
Item
|
Deliverable
|
Description
|
Deliverable Schedule
|
|
1
|
Once Monthly Teleconference and Meeting Minutes
|
The Contractor shall prepare minutes of all “Project Meetings and “Project Meeting Conference Calls” as defined in Article C. of this contract. In preparation for the monthly calls, briefing materials, including the agenda and documents and information to be discussed will be prepared as needed.
|
Contractor shall provide teleconference agenda and related materials twenty-four (24) hours in advance of the call.
Contractor provides meeting minutes to COR within five (5) business days of the meeting. COR reviews, comments, and approves minutes within 15 business days of receipt.
|
|
2
|
Draft Security Plan
|
Draft Security Plan as detailed in Article B.5.d.
|
Within 10th calendar days following the effective date of the contract
|
|
3
|
Monthly Technical Progress Report and Invoice
|
Monthly Progress report shall address the progress occurring over the corresponding period of time. See below, ARTICLE F.2.(b), “Detailed Description of Select Contract Deliverables,” for detailed instructions. Additionally, submission of the Monthly Technical Progress Report will contain the invoice for actual costs incurred during the previous month that work was performed under the contract. The costs incurred in the invoice will be justified in a summary report contained within the Monthly Technical Progress Report.
|
The 15th calendar day of each month following the first full month of the contract award. The Monthly Progress Report will not be required in months when an Annual or Final Technical Progress Report is due.
|
|
4
|
Annual Progress Report
|
Annual Progress report shall address the progress occurring over the corresponding period of time. See below, Article F.2.(b), “Detailed Description of Select Contract Deliverables,” for detailed instruction.
|
The 15th calendar day of the month following the end of each 12- month performance period. The Monthly Progress Report will not be required in months when an Annual Progress report is due
|
|
5
|
In-Process Review (GO/NO GO
Decision Gate) Presentation
|
In preparation for the IPR, the Contractor shall prepare a presentation demonstrating the technical progress made towards completion of the tasks under each work segment. The presentation shall demonstrate the status or completion of the milestones and deliverables as specified under Section F.
|
The presentation must be submitted to the CO/COR thirty
(30) business days prior to the IPR IPR for BARDA review and comment. Subsequently, a revised/final presentation will be required ten (10) business days prior to the IPR.
The CO will provide a written response within ten (10) business days on the decision to exercise or not exercise an option.
|
Page 14
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
6
|
Earned Value Management Report
|
As described in Article C.3.
|
The 15th calendar day of each month following the first full month of the contract award.
|
||
|
7
|
Draft Final Technical Progress Report
|
A draft Final Report containing a summation of the work performed under each task and subtask and the results obtained for the entire contract Period of Performance (PoP). The draft report shall be duly marked as “Draft.” BARDA will provide comments that the Contractor shall incorporate into the Final Technical Progress Report.
|
Forty-five (45) calendar days before the completion date of the contract.
|
||
|
8
|
Final Technical Progress Report
|
A Final Report containing a summation of the work performed and the results obtained for the entire contract Period of Performance (PoP).
|
Thirty (30) calendar days after the technical period of performance.
|
||
|
9
|
Summary of Salient Results
|
Contractor shall submit, with the Final Report, a summary (not to exceed 200 words) of salient results achieved during the performance of the contract.
|
On or before the expiration date of the contract.
|
||
|
10
|
Deviation Notification. Changes to Execution of Planned Tasks and Mitigation Strategy
|
In order to process for changing tasks, including activities associated with task content, cost and schedule per IMP/Gantt baseline, the Contractor
s h al l notify the Government of significant changes, justification, and rationale for proposed alternative in writing. Cost reallocation and reconciliation of the budget should be included. Contractor shall provide a high-level management strategy for risk mitigation and update the Risk Management Plan
|
Notice due within 1 week after discovery or need for changes to product development plan per Gantt identified.
Contractor shall revise the IMP/Gantt within thirty (30) calendar days, update monthly s part of the Monthly Progress Report, and update the Risk Management Plan.
Contractor must address, in writing, all concerns raised by BARDA and re-submit a IMP/GANTT that reflects or addresses BARDA’s concerns.
|
||
|
11
|
Development Report
|
Final Reports detailing the parameters and capacity of upstream and downstream conditions.
|
Upon successful completion.
|
||
|
Other Technical Reports
|
|||||
|
Item
|
Deliverable
|
Deliverable Schedule
|
|||
|
12
|
Audit Reports
|
Within fifteen (15) calendar days of the audit
|
|||
|
13
|
FDA/Regulatory Agency
Correspond. & Meeting Summaries
|
Within five (5) business days of each meeting for Contractor’s minutes and upon receipt of minutes from FDA/regulatory agency.
|
|||
Page 15
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
14
|
FDA/Regulatory Agency Submissions
|
BARDA shall provide comment within five (5) business days after receipt. BARDA reserves the right to request more than 5 business days for review of any regulatory submission that is of significant length.
The Contractor shall inform BARDA of the anticipated submission length so BARDA can make a determination if more than 5 business days will be needed to complete its review of the document.
|
|
15
|
Supplemental Technical Documents
|
Upon request. Contractor shall provide CO and COR with deliverables from the following contract funded activities: Process Development Reports; Stability Assay Reports; Assay Qualification Plan/Report; Assay Validation Plan/Report; Assay Technology Transfer Report; Batch Records; Contractor/
Subcontractor Standard Operating Procedures (SOPs); Master Production Records; Certificate of Analysis; Clinical Studies Data or Reports. The CO and COR reserve the right to request within the PoP a nonproprietary technical document for distribution within the USG.
Contractor shall provide technical document within 5 business days of CO or COR request.
Contractor can request additional time on an as-needed basis.
*If corrective action is recommended, the Contractor must address, in writing, concerns raised by BARDA.
|
|
16
|
Invention Report
Annual Utilization Report
|
Due on or before the 30th of the month following each 12-month period of performance.
|
|
17
|
Final Invention Report
|
Due on or before the completion date of the contract.
|
|
18
|
Kickoff Meeting
|
Within thirty (30) calendar days of contract award.
|
|
b.
|
Detailed Description of Select Contract Deliverables
|
|
1.
|
Monthly Progress Report
|
This report shall include a description of the activities during the reporting period, and the activities planned for the ensuing reporting period. The first reporting period consists of the first full month of performance plus any fractional part of the initial month. Thereafter, the reporting period shall consist of each calendar month.
|
|
The Contractor shall submit a Monthly Progress Report on or before the 15th calendar day following the last day of each reporting period and shall include the following:
|
A cover page that includes the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission;
Page 16
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
SECTION I - An introduction covering the purpose and scope of the contract effort; SECTION II – PROGRESS
|
SECTION II Part A: OVERALL PROGRESS - A description of overall progress;
SECTION II Part B: MANAGEMENT AND ADMINISTRATIVE UPDATE - A
description of all meetings, conference calls, etc. that have taken place during the reporting period. Include progress on administration and management issues (e.g. evaluating and managing subcontractor performance and personnel changes);
SECTION II Part C: TECHNICAL PROGRESS - For each activity related to the Gantt chart, document the results of work completed and costs incurred during the period covered in relation to proposed progress, effort and budget. The report shall be in sufficient detail to explain comprehensively the results achieved. The description shall include pertinent data and/or graphs in sufficient detail to explain any significant results achieved and preliminary conclusions resulting from analysis and scientific evaluation of data accumulated to date under the contract. Include progress or status updates for all SOW tasks in each of the monthly technical progress reports for which there is activity ongoing in that SOW task area(s) as well as data for completed studies in any SOW task. The report shall also include a description of problems encountered and proposed corrective action; differences between planned and actual progress, why the differences have occurred and what corrective actions are planned; preliminary conclusions resulting from analysis and scientific evaluation of data accumulated to date under the project.
|
|
SECTION II Part D: PROPOSED WORK - A summary of work proposed for the next reporting period and preprints/reprints of papers and abstracts, and a current/updated Gantt chart.
|
SECTION II Part E: Outstanding Issues/Anticipated Areas of Concern - a list of any existing contractual concerns that impact the technical scope of work, schedule, or cost, as well as a list of potential or anticipated areas of concern that may be encountered in the future months.
|
|
A Monthly Progress Report will not be required in the same month that the Annual or Final Technical Progress Reports are submitted.
|
|
2.
|
Annual Progress Reporting Requirement
|
This report shall include a summation of the activities during the reporting period, and the activities planned for the ensuing reporting period. The first reporting period consists of the first full year of performance plus any fractional part of the initial year. Thereafter, the reporting period shall consist of each calendar year.
The Contractor shall submit an Annual Progress Report on or before the 15th calendar day following the last day of each reporting period and shall include the following:
A cover page that includes the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission;
|
|
SECTION I-EXECUTIVE SUMMARY - A brief overview of the work completed and major accomplishments achieved during the reporting period.
|
SECTION II-PROGRESS
Page 17
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
SECTION II Part A: OVERALL PROGRESS - A description of overall progress highlighting the
significant accomplishments in the past year;
SECTION II Part B: MANAGEMENT AND ADMINISTRATIVE UPDATE - A description of all meetings, conference calls, etc. that have taken place during the reporting period. Include progress on administration and management issues (e.g. evaluating and managing subcontractor performance and personnel changes);
SECTION II Part C: TECHNICAL PROGRESS - For each activity, document the results of work completed and cost incurred during the period covered in relation to proposed progress, effort and budget. The report shall be in sufficient detail to explain comprehensively the results achieved. The description shall include pertinent data and/or graphs in sufficient detail to explain any significant results achieved and preliminary conclusions resulting from analysis and scientific evaluation of data accumulated to date under the contract. The report shall include a description of problems encountered and proposed corrective action; differences between planned and actual progress, why the differences have occurred and what corrective actions are planned; preliminary conclusions resulting from analysis and scientific evaluation of data accumulated to date under the project. The report should summarize progress made under each SOW task.
|
|
SECTION II Part D: PROPOSED WORK - A summary of work proposed for the next reporting period; and preprints/reprints of papers, abstracts and a current Gantt chart.
|
A Monthly and Annual Progress Report will not be required for the period when the Final Technical Progress Report is due and a Monthly Progress Report will not be required in the same month that the Annual Progress Report is submitted.
Draft Final Technical Progress Report and Final Technical Progress Report
These reports are to include a summation of the work performed and results obtained for the entire contract period of performance, detailing accomplishments for each task. This report shall be in sufficient detail to describe comprehensively the results achieved. The Draft Final Report and Final Report shall be submitted in accordance with the DELIVERIES Article in SECTION F of the contract. The Draft Final Technical Progress Report shall be submitted forty-five (45) calendar days before completion date of the contract and the Final Technical Progress Report shall be submitted 30 Calendar days post technical period of performance. The report shall conform to the following format:
Cover page to include the contract number, contract title, performance period covered, Contractor's name and address, telephone number, fax number, e- mail address and submission date;
SECTION I: EXECUTIVE SUMMARY - Summarize the purpose and scope of the contract effort including a summary of the major accomplishments relative to the specific activities set forth in the Statement of Work.;
SECTION II: RESULTS - A detailed description of the work performed related to the Gantt chart, the results obtained, and the impact of the results on the scientific and/or public health community, including a listing of all manuscripts (published and in preparation) and abstracts presented during the entire period of performance, and a summary of all inventions.
Draft Final Technical Progress Report: The Contractor is required to submit the Draft Final Technical Progress Report to the Contracting Officer's Representative and Contracting Officer. This report is due forty-five (45) calendar days before the completion date of the contract. The Contracting Officer's Representative and Contracting Officer will review the Draft Final
Page 18
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
Technical Progress Report and provide the Contractor with comments within fifteen (15) calendar days after receipt.
|
Final Technical Progress Report: The contractor shall incorporate all BARDA comments into the Final Technical Progress Report. The Contractor will deliver the final version of the Final Technical Progress Report 30 Calendar days post technical period of performance.
|
3.
|
Summary of Salient Results
|
On or before the expiration of the contract the Contractor shall submit, with the Final Technical Progress Report, a summary (not to exceed 200 words) of salient results achieved during the performance of the contract.
|
4.
|
Audit Reports
|
Within fifteen (15) calendar days of an audit related to conformance to FDA regulations and guidance, including adherence to GLP, GMP, GCP guidelines, the Contractor shall provide copies of the audit report (so long as received from the FDA) and a plan for addressing areas of nonconformance to FDA regulations and guidelines for GLP, GMP, or GCP guidelines as identified in the final audit report.
|
5.
|
Copies of FDA/Regulatory Agency Correspondence and Meeting Summaries
|
|
·
|
Within five business days of any formal meeting with the FDA or other regulatory agency, the contractor shall forward the initial draft minutes to BARDA. The contractor shall forward final draft minutes when available.
|
|
·
|
Within five business days of any informal meeting with the FDA or other regulatory agency, the contractor shall forward the final draft minutes to BARDA.
|
|
·
|
The contractor shall forward the dates and times of any meeting with the FDA and other regulatory agencies to BARDA and make arrangements for appropriate BARDA staff to attend the meetings.
|
|
·
|
The contractor shall provide BARDA the opportunity to review and comment upon any documents to be submitted to the FDA or other regulatory agency. The contractor shall provide BARDA with five (5) business days in which to review and provide comments back to the contractor prior to the contractor’s submission to the FDA.
|
|
·
|
The contractor shall forward Standard Operating Procedures (SOPs) upon request from Project Officer/Contracting Officer.
|
|
·
|
The contractor shall provide upon request animal study and/or other technology packages developed under this contract. Packages shall include complete protocols and critical reagents for animal models developed and/or improved with contract funding.
|
|
·
|
The contractor shall provide upon request raw data and/or specific analysis of data generated with USG funds.
|
|
6.
|
Other Reports/Deliverables
|
|
·
|
Government Rights in Data and Inventions
|
Technology packages developed under the contract that include complete protocols and critical reagents developed and/or improved with contract funding must be submitted at the request of the Contracting Officer’s Representative.
Page 19
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
·
|
Institutional Biosafety Approval
|
The Contractor shall provide documentation of materials submitted for Institutional Biosafety Committee Review and documentation of approval of experiments at the request of the Contracting Officer’s Representative.
|
·
|
Experimental Protocols
|
The Contractor shall submit all study/experiment/test plans, designs, and protocols.
|
7.
|
Data
|
The Contractor shall provide data and/or specific analysis of data generated with contract funding at the request of the Contracting Officer’s Representative.
Earned Value Management (EVM) Deliverables
|
i.
|
Earned Value Management (EVM) / Contract Performance Report (CPR)
|
Contractor will provide a monthly CPR at an agreed upon reporting level using WBS and Variance Analysis report formats agreed upon by ASPR after EVM is implemented. The supplemental monthly Control Account Plan (CAP) report shall contain, at the work package level, time phased budget (budgeted cost of work scheduled), earned value (budgeted cost of work performed), and actual costs of work performed as captured in Contractor’s EVM systems. The Contractor shall provide a rationale in the package of its use of % complete as EVMS methodology, or identity if any other EVMS methodology is being used.
|
·
|
Contractor shall provide EVM/CPR as part of the Monthly Progress Report (this requirement begins only as set forth in the Contract Milestones & Related Deliverables table)
|
|
·
|
Contractor shall provide top level or key changes in baseline cost as a result of anticipated cost savings or risks
|
|
·
|
In accordance with FAR 52.215-2, Audit and Records-Negotiation (Oct 2010), the USG may request, on a monthly or ad hoc basis that the Contractor provide raw data at a reporting level or lower level as ASPR deems necessary.
|
|
·
|
Contractor must address, in writing, all concerns raised by the USG.
|
|
·
|
Reporting will commence after the EVM system has been implemented but no later than six (6) months after start of base period.
|
|
ii.
|
Integrated Master Plan (IMP)
|
|
|
The Contractor shall provide an IMP including WBS, critical path milestones, and Earned Value Management Plan
|
|
·
|
Contractor shall provide the draft IMP within 180 days of contract award with final due 8 months after award and updated monthly as part of the Monthly Progress Report
|
|
·
|
Contractor must address, in writing, all concerns raised by the USG.
|
|
iii.
|
Performance Measurement Baseline Review (PMBR)
|
PMBR Report shall address each of the items listed below and be cross- referenced to the IMP, WBS, SOW, and Risk Management Plan.
Page 20
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
·
|
Contractor provides baseline proposal
|
|
·
|
Responsibility Assignment Matrix
|
|
·
|
A description of the work scope through control account Work Authorization Documents and/or WBS Dictionary down to the agreed upon control account level.
|
|
·
|
Template for work packages
|
|
·
|
Integrated Master Schedule (IMS) with the inclusion of agreed major milestones and control account plans for all control accounts
|
|
·
|
Baseline revision documentation and program log(s) risk management plan
|
|
·
|
PMBR is due within one year of contract award
|
|
·
|
Contractor shall provide baseline proposal .ppt briefing 10 business days prior to meeting
|
|
·
|
Contractor provides agenda to COR 2 business days in advance of meeting
|
|
·
|
COR approves (with CO concurrence) and distributes agenda
|
|
·
|
COR approves (with CO concurrence) all meeting material
|
|
·
|
Contactor provides minutes with 2 business days of the meeting
|
|
·
|
COR reviews and approves (with CO concurrence) minutes
|
|
·
|
ASPR will review documentation and provide written comments and questions to Contractor
|
|
·
|
Contractor shall address BARDA’s comments and resubmit PMBR report for BARDA approval within 10 business days.
|
|
iv.
|
Risk Management Plan
|
The Contractor shall provide a Risk Management Plan that outlines the impacts of each risk in relation to the cost, schedule, and performance objectives. The plan shall include risk mitigation strategies. Each risk mitigation strategy will capture how the corrective action will reduce impacts on cost, schedule and performance.
|
§
|
Due within 180 days of contract award
|
|
§
|
Contractor provides updated Risk Management Plan in Monthly Progress Report
|
|
§
|
ASPR shall provide Contractor with a written list of concerns in response plan submitted
|
|
§
|
Contractor must address, in writing, all concerns raised by ASPR within 20 business days of Contractor’s receipt of ASPR’s concerns.
|
|
v.
|
Requirement for Notification of Deviation and Mitigation Strategy
|
Process for changing IMS activities associated with cost and schedule as baselined at the PMBR. Contractor shall notify ASPR of significant changes to the IMS defined as increases in cost above 10% for Go/No Go Milestones or schedule slippage of more than 180 days, which would require an extension to the period of performance. Contractor shall provide a high level management strategy for risk mitigation. Notice due within one (1) business day after discovery.
ARTICLE F.3. ELECTRONIC SUBMISSION
For electronic delivery, the Contractor shall upload documents to the appropriate folder on https://eroom.bardatools.hhs.gov/eRoom (“eRoom”) which is the designated USG file sharing system. The USG shall provide two contractor representatives authorized log in access to the file share program. Each representative must complete a mandatory training provided by the USG prior to gaining user access. A notification email should be sent to the CO and COR upon electronic delivery of any documents.
Page 21
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE F.4. SUBJECT INVENTION REPORTING REQUIREMENT
All reports and documentation required by FAR Clause 52.227-11, Patent Rights-Ownership by the Contractor, including, but not limited to, the invention disclosure report, the confirmatory license, and the government support certification, one copy of an annual utilization report, and a copy of the final invention statement, shall be submitted to the Contracting Officer. A final invention statement (see FAR 27.303 (b)(2)(ii)) shall be submitted to the Contracting Officer on the expiration date of the contract.
|
|
Reports and documentation submitted to the Contracting Officer shall be sent to the address set forth in SECTION G – CONTRACT ADMINISTRATION DATA.
|
If no invention is disclosed or no activity has occurred on a previously disclosed invention during the applicable reporting period, a negative report shall be submitted to the Contracting Officer at the address listed above.
ARTICLE G.1. CONTRACTING OFFICER
|
|
The following Contracting Officer (CO) will represent the USG for the purpose of this contract:
|
|
|
Thomas P. Hastings
Contracting Officer
DHHS/OS/ASPR/AMCG
330 Independence Avenue, S.W. Room G644 Washington, D.C. 20201
(571) 221-2978
* * *
|
|
1)
|
The Contracting Officer (CO) is the only individual who can legally commit the USG to the expenditure of public funds. No person other than the Contracting Officer can make any changes to the terms, conditions, general provisions, or other stipulations of this contract.
|
|
2)
|
The Contracting Officer is the only person with the authority to act as agent of the USG under this contract. Only the Contracting Officer has authority to (1) direct or negotiate any changes in the statement of work; (2) modify or extend the period of performance; (3) change the delivery schedule; (4) authorize reimbursement to the Contractor of any costs incurred during the performance of this contract; (5) otherwise change any terms and conditions of this contract.
|
|
3)
|
No information other than that which may be contained in an authorized modification to this contract, duly issued by the Contracting Officer, which may be received from any person employed by the US government, other otherwise, shall be considered grounds for deviation from any stipulation of this contract.
|
|
4)
|
The USG may unilaterally change the CO or CS designation.
|
Page 22
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
ARTICLE G.2. CONTRACTING OFFICER'S REPRESENTATIVE (COR) and ALTERNATE CONTRACTING OFFICER'S REPRESENTATIVE (COR)
|
|
|
The following COR and Alternate COR will represent the government for the purpose of this contract:
|
|
|
COR:
Kimberly Sciarretta
Biomedical Advanced Research and Development Authority (BARDA) Office of the
Assistant Secretary for Preparedness and Response Department of Health and Human Services
|
|
|
Email:* * *
* * *
|
|
Alternate COR:
Chia-Wei Tsai
Biomedical Advanced Research and Development Authority (BARDA) Office of the
Assistant Secretary for Preparedness and Response Department of Health and Human Services
* * *
* * *
|
|
|
Mailing Address:
330 Independence Avenue, SW G644 Washington, D.C. 20201 The COR is responsible for:
|
The COR is responsible for:
|
1)
|
Recommending to the Contracting Officer changes in requirements;
|
|
2)
|
Assisting the Contracting Officer in interpreting the statement of work and any other technical performance requirements;
|
|
3)
|
Performing technical evaluation as required;
|
|
4)
|
Performing technical inspections and acceptances required by this contract; and
|
|
5)
|
Assisting in the resolution of technical problems encountered during performance. The USG may unilaterally change the COR designation.
|
ARTICLE G.3. KEY PERSONNEL
The key personnel specified in this contract are considered to be essential to work performance. At least 30 days prior to diverting any of the specified individuals to other programs or contracts (or as soon as possible, if an individual must be replaced, for example, as a result of leaving the employ of the Contractor), the Contractor shall notify the Contracting Officer and shall submit comprehensive justification for the diversion or replacement request (including proposed substitutions for key personnel) to permit evaluation by the USG of the impact on performance under this contract. The Contractor shall not divert or otherwise replace any key personnel without the written consent of the Contracting Officer. The USG may modify the contract to add or delete key personnel at the request of the Contractor or USG.
The following individuals are considered to be essential to the work being performed hereunder:
|
Name
|
Title
|
|
William Sheridan
|
SVP and Chief Medical Officer and Principal Investigator
|
|
Ray Taylor
|
VP Project Management and Project Team Leader
|
|
Elliott Berger
|
SVP Regulatory Affairs
|
Page 23
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE G.4. CONTRACT FINANCIAL REPORT
|
a.
|
Financial reports on the attached Financial Report of Individual Project/Contract shall be submitted by the Contractor to the CO with a copy to the COR in accordance with the instructions for completing this form, which accompany the form, in an original and one electronic copy, not later than the 30th business day after the close of the reporting period. The line entries for subdivisions of work and elements of cost (expenditure categories), which shall be reported within the total contract, are discussed in paragraph e., below. Subsequent changes and/or additions in the line entries shall be made in writing.
|
|
b.
|
Unless otherwise stated in the instructions for completing this form, all columns A through J, shall be completed for each report submitted.
|
|
c.
|
The first financial report shall cover the period consisting of the first full three calendar months following the date of the contract, in addition to any fractional part of the initial month. Thereafter, reports will be on a quarterly basis.
|
|
d.
|
The Contracting Officer may require the Contractor to submit detailed support for costs contained in one or more interim financial reports. This clause does not supersede the record retention requirements in FAR Part 4.7.
|
|
e.
|
The listing of expenditure categories to be reported is incorporated as a part of this contract and can be found under SECTION J Attachment 4 entitled, "Financial Report of Individual Project/Contract,".
|
|
f.
|
The USG may unilaterally revise the “Financial Report of Individual Project/Contract” to reflect the allotment of additional funds.
|
ARTICLE G.5. INVOICE/FINANCING REQUEST AND CONTRACT FINANCIAL REPORTING
Include Program Support Center (PSC) in Receipt of Invoices:
Documents shall be delivered electronically to the Contracting Officer (CO), the Contracting Specialist (CS), the Contracting Officer’s Representative (COR) and PSC. Unless otherwise specified by the Contracting Officer all deliverables and reports furnished to the Government under the resultant contract (including invoices) shall be addressed as follows:
|
Thomas P. Hastings Contracting Officer HHS/ASPR/AMCG
330 Independence Ave., S.W., Room G640
Washington, DC 20201 Email:* * *
|
Kimberly Sciarretta Contracting Officer Representative HHS/ASPR/BARDA
330 Independence Ave., S.W., Room G640 Washington, DC 20201 Email:* * *
|
* * *
|
|
a.
|
Contractor invoices/financial reports shall conform to the form, format, and content requirements of the instructions for Invoice/Financing requests and Contract Financial Reporting.
|
|
b.
|
Monthly invoices must include the cumulative total expenses to date, adjusted (as applicable) to show any amounts suspended by the USG.
|
|
c.
|
The Contractor agrees to immediately notify the CO in writing if there is an anticipated overrun (any amount) or unexpended balance (greater than 10 percent) of the estimated costs for the base period or any option period(s) (See estimated costs under Articles B.2) and the reasons for the variance. These requirements are in addition to the specified requirements of FAR Clause 52.232-20, Limitation of Cost
|
Page 24
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
that is incorporated by reference under Article I.1 which states;
Limitation of Cost (Apr 1984)
|
|
(a)
|
The parties estimate that performance of this contract, exclusive of any fee, will not cost the Government more than (1) the estimated cost specified in the Schedule or, (2) if this is a cost-sharing contract, the Government’s share of the estimated cost specified in the Schedule. The Contractor agrees to use its best efforts to perform the work specified in the Schedule and all obligations under this contract within the estimated cost, which, if this is a cost-sharing contract, includes both the Government’s and the Contractor’s share of the cost.
|
|
(b)
|
The Contractor shall notify the Contracting Officer in writing whenever it has reason to believe that—
|
|
(1)
|
The costs the Contractor expects to incur under this contract in the next 60 days, when added to all costs previously incurred, will exceed 75 percent of the estimated cost specified in the Schedule; or
|
|
(2)
|
The total cost for the performance of this contract, exclusive of any fee, will be either greater or substantially less than had been previously estimated.
|
|
(c)
|
As part of the notification, the Contractor shall provide the Contracting Officer a revised estimate of the total cost of performing this contract.
|
|
(d)
|
Except as required by other provisions of this contract, specifically citing and stated to be an exception to this clause—
|
|
(1)
|
The Government is not obligated to reimburse the Contractor for costs incurred in excess of (i) the estimated cost specified in the Schedule or, (ii) if this is a cost-sharing contract, the estimated cost to the Government specified in the Schedule; and |
|
(2)
|
The Contractor is not obligated to continue performance under this contract (including actions under the Termination clause of this contract) or otherwise incur costs in excess of the estimated cost specified in the Schedule, until the Contracting Officer (i) notifies the Contractor in writing that the estimated cost has been increased and (ii) provides a revised estimated total cost of performing this contract. If this is a cost-sharing contract, the increase shall be allocated in accordance with the formula specified in the Schedule.
|
|
(e)
|
No notice, communication, or representation in any form other than that specified in paragraph (d)(2) of this clause, or from any person other than the Contracting Officer, shall affect this contract’s estimated cost to the Government. In the absence of the specified notice, the Government is not obligated to reimburse the Contractor for any costs in excess of the estimated cost or, if this is a cost- sharing contract, for any costs in excess of the estimated cost to the Government specified in the Schedule, whether those excess costs were incurred during the course of the contract or as a result of termination. |
|
(f)
|
If the estimated cost specified in the Schedule is increased, any costs the Contractor incurs before the increase that are in excess of the previously estimated cost shall be allowable to the same extent as if incurred afterward, unless the Contracting Officer issues a termination or other notice directing that the increase is solely to cover termination or other specified expenses.
|
Page 25
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
(g)
|
Change orders shall not be considered an authorization to exceed the estimated cost to the Government specified in the Schedule, unless they contain a statement increasing the estimated cost.
|
|
(h)
|
If this contract is terminated or the estimated cost is not increased, the Government and the Contractor shall negotiate an equitable distribution of all property produced or purchased under the contract, based upon the share of costs incurred by each.
|
|
d.
|
The Contractor shall submit an electronic copy of the payment request to the approving official instead of a paper copy. The payment request shall be transmitted as an attachment via e-mail to the address listed above in one of the following formats: MSWord, MS Excel, or Adobe Portable Document Format (PDF). Only one payment request shall be submitted per e-mail and the subject line of the e-mail shall include the Contractor's name, contract number, and unique invoice number.
|
|
e.
|
An electronic copy of the payment request shall be uploaded into the designated eRoom (as defined in SECTION F.3 ELECTRONIC SUBMISSION) and an e-mail notification of the upload will be provided to the CO and COR.
|
|
f.
|
All invoice submissions shall be in accordance with FAR Clause 52.232-25, Prompt Payment (Oct 2008).
|
|
g.
|
Invoices - Cost and Personnel Reporting, and Variances from the Negotiated Budget
|
The Contractor agrees to provide a detailed breakdown on invoices of the following cost categories:
|
a.
|
Direct Labor - List individuals by name, title/position, hourly/annual rate, level of effort (actual hours or % of effort), and amount claimed.
|
|
b.
|
Fringe Benefits - Cite rate and amount
|
|
c.
|
Overhead - Cite rate and amount
|
|
d.
|
Materials & Supplies - Include detailed breakdown when total amount is over $1,000.
|
|
e.
|
Travel - Identify travelers, dates, destination, purpose of trip, and total breaking out amounts for transportation (plane, car etc), lodging, M&IE. Cite COA, if appropriate. List separately, domestic travel, general scientific meeting travel, and foreign travel.
|
|
f.
|
Consultant Fees - Identify individuals, amounts and activities. Cite appropriate COA
|
|
g.
|
Subcontracts - Attach subcontractor invoice(s). Cite appropriate COA
|
|
h.
|
Equipment - Cite authorization and amount. Cite appropriate COA
|
|
i.
|
Other Direct Costs - Include detailed breakdown when total amount is over $1,000.
|
|
j.
|
G&A - Cite rate and amount.
|
|
k.
|
Total Cost
|
|
l.
|
Fee
|
|
m.
|
Total Cost Plus Fixed Fee
|
Monthly invoices must include the cumulative total expenses to date, adjusted (as applicable) to show any amounts suspended by the USG. Nothing in this section discharges the contractor’s responsibility to comply with any applicable FAR Parts 30 or 31 clauses’ relating to cost reimbursement subcontracts. In order to verify allowability, further breakdown of costs may be requested at the USG’s discretion. The Contractor shall subcontract with Firm Fixed Price Contracts to the maximum extent practicable.
Additional instructions and an invoice template are provided in Attachment 3, Invoice/Financing Request Instructions and Contract Financial Reporting Instructions for Cost-Reimbursement Type Contracts. All invoices must be signed by a representative of the contractor authorized to certify listed charges are accurate and comply with government regulations. Invoices should be submitted electronically (in accordance with ARTICLE F.4., (ELECTRONIC SUBMISSION) and in hard copy with original signature.
Page 26
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE G.6. INDIRECT COST RATES
|
|
The following contractor established provisional billing rates are incorporated into the contract, and will be utilized for billing purposes during both the base and contract option periods pending the establishment of final indirect cost rates for each fiscal year or until revised by the contracting officer in accordance with the provisions of FAR 42.705-1. See FAR Clause 52.216-7.
|
|
BioCryst Pharmaceuticals
|
||||
|
Rate Type
|
Rate | Allocation Base | ||
|
Fringe Benefits
|
* * * % | * * * | ||
|
Overhead
|
* * * % | * * * | ||
|
G&A
|
* * * % | * * * | ||
Use of the above provisional rates does not change any cost ceilings, contract obligations, or specific allowance or disallowance provided for in the contract.
Contractor must notify the contracting officer promptly for an adjustment of the provisional rates if it becomes evident that the rates would cause substantial overpayment or underpayment of indirect expenses to BioCryst Pharmaceuticals.
The final billing rates for each fiscal year will be based on the incurred cost submission subject to Government audit determination. Indirect costs rate proposals must be submitted to the cognizant agency’s Contracting Officer within 6 months subsequent to each of the contractor’s fiscal year ends. (See also FAR Clause 52.216-7(d) (2) incorporated herein). Copies of the indirect cost submission for each fiscal year must also be submitted to the AMCG contracting officer, and the AMCG auditor identified as follows:
Director, Acquisition Program Support
Office of Acquisition Management, Contracts and Grants (AMCG)
Office of the Assistant Secretary for Preparedness and Response (ASPR) US Department of Health and Human Services (DHHS)
300 Independence Avenue, SW, Room G644 Washington, DC 20201
ARTICLE G.7. REIMBURSEMENT OF COST
|
1)
|
The USG shall reimburse the Contractor those costs determined by the Contracting Officer to be allowable (hereinafter referred to as allowable cost) in accordance with FAR 52.216-7, Allowable Cost and Payment and FAR Subpart 31.2. Examples of allowable costs include, but are not limited to, the following:
|
|
a)
|
All direct materials and supplies that are used in the performing of the work provided for under the contract, including those purchased for subcontracts and purchase orders.
|
|
b)
|
All direct labor, including supervisory, that is properly chargeable directly to the contract, plus fringe benefits.
|
|
c)
|
All other items of cost budgeted for and accepted in the negotiation of this basic contract or modifications thereto.
|
|
d)
|
Travel costs including per diem or actual subsistence for personnel while in an actual travel status in direct performance of the work and services required under this contract subject to the restrictions under Article B.4. b. and the following:
|
Page 27
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
i.
|
Air travel shall be by the most direct route using “air coach” or “air tourist” (less than first class) unless it is clearly unreasonable or impractical (e.g., not available for reasons other than avoidable delay in making reservations, would require circuitous routing or entail additional expense offsetting the savings on fare, or would not make necessary connections) and must comply with the Fly America Act (49 U.S.C. 40118).
|
|
ii.
|
Rail travel shall be by the most direct route, first class with lower berth or nearest equivalent.
|
|
iii.
|
Costs incurred for lodging, meals, and incidental expenses shall be considered reasonable and allowable to the extent that they do not exceed on a daily basis the per diem rates set forth in the Federal Travel Regulation (FTR).
|
|
iv.
|
Travel via privately owned automobile shall be reimbursed at not more than the current General Services Administration (GSA) FTR established mileage rate.
|
ARTICLE G.8. POST AWARD EVALUATION OF CONTRACTOR PERFORMANCE
|
1.
|
Contractor Performance Evaluations
|
Interim and final evaluations of Contractor performance will be prepared on this contract in accordance with FAR Subpart 42.1502. The final performance evaluation will be prepared at the time of completion of work. In addition to the final evaluation, an interim evaluation shall be submitted at least once during the contract period of performance. The interim evaluation is expected to be submitted on December 23, 2015.
Interim and final evaluations will be provided to the Contractor as soon as practicable after completion of the evaluation. The Contractor will be permitted thirty days to review the document and to submit additional information or a rebutting statement. If agreement cannot be reached between the parties, the matter will be referred to an individual one level above the Contracting Officer whose decision will be final.
Copies of the evaluations, Contractor responses, and review comments, if any, will be retained as part of the contract file, and may be used to support future award decisions.
|
2.
|
Electronic Access to Contractor Performance Evaluations
|
The USG website for Contractor Performance Assessment Reporting System (CPARS) is http://www.cpars.gov. Through this website Contractors may access evaluations through a secure website for review and comment by completing the online registration form. The registration process requires the Contractor to identify an individual that will serve as a primary contact and who will be authorized access to the evaluation for review and comment. In addition, the Contractor will be required to identify an alternate contact that will be responsible for notifying the cognizant contracting official in the event the primary contact is unavailable to process the evaluation within the required 30-day time frame.
ARTICLE G.9. CONTRACT COMMUNICATIONS/CORRESPONDENCE
The Contractor shall identify all correspondence, reports, and other data pertinent to this contract by imprinting the contract number HHSO100201500007C from Page 1 of the contract.
Page 28
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE G.10. OVERTIME COMPENSATION
No overtime (premium) compensation is authorized under this contract. Billing of actual hours should be limited to total working hours in a month.
The Contractor, depending upon the nature of the work, is responsible for following the provisions below in conducting its own work under this Contract. The Contractor also is responsible for incorporating these provisions into any subcontract awarded, if applicable to the specific nature of the work in the subcontract. Accordingly, those provisions shall be flowed- down as applicable.
ARTICLE H.1 CLINICAL AND NON-CLINICAL TERMS OF AWARD
BARDA has a responsibility to obtain documentation concerning mechanisms and procedures that are in place to protect the safety of participants and animals in BARDA funded clinical trials and non-clinical studies. Therefore, the Contractor shall develop a protocol for each clinical trial and non-clinical study funded under this contract and submit all such protocols and protocol amendments to the Contracting Officer’s Representative (COR) for evaluation and comment. Approval by the COR is required before work under a protocol may begin. The COR comments will be forwarded to the Contractor within ten (10) business days. The Contractor must address, in writing, all concerns (e.g. study design, safety, regulatory, ethical, and conflict of interest) noted by the COR.
If the draft protocols are to be submitted to the FDA, BARDA review shall occur before submission, pursuant to the terms set forth by ARTICLE F.2 of this contract. The Contractor shall consider revising their protocols to address BARDA’s concerns and recommendations prior to FDA submission. The Contractor must provide BARDA with a copy of FDA submissions, within the time frame set forth by ARTICLE F.2 of this contract.
Execution of clinical and non-clinical studies requires written authorization from the government. The USG will provide written authorization to the Contractor upon either 1) receiving documentation in which all COR comments have been satisfactorily addressed; or 2) receiving documentation that the FDA has reviewed and commented on the protocol.
The government shall have rights to all protocols, data resulting from execution of these protocols, and final reports funded by BARDA under this contract, as set forth in PART II of this contract and defined in the FAR. The government reserves the right to request that the Contractor provide any contract deliverable in a non-proprietary form to ensure the government has the ability to review and distribute the deliverables as the government deems necessary.
|
|
Important information regarding performing human subject research is available at http://www3.niaid.nih.gov/healthscience/clinicalstudies/.
|
Any updates to technical reports are to be addressed in the Monthly and Annual Progress Reports. The Contractor shall advise the Contracting Officer’s Representative or designee in writing and via electronic communication in a timely manner of any issues potentially affecting contract performance.
Page 29
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
1.
|
Non-Clinical Terms of Award
|
These Non-Clinical Terms of Award detail an agreement between the Biomedical Advanced Research and Development Authority (BARDA) and the Contractor; they apply to all grants and contracts that involve non-clinical research.
|
a.
|
Safety and Monitoring Issues
|
|
i.
|
PHS Policy on Humane Care and use of Laboratory Animals
|
Before award and then with the annual progress report, the Contractor must submit to BARDA a copy of the current Institutional Animal Care and Use Committees (IACUC) documentation of continuing review and approval and the Office of Laboratory Animal Welfare (OLAW) federal wide assurance number for the institution or site.
If other institutions are involved in the research (e.g., a multicenter trial or study), each institution’s IACUC must review and approve the protocol. They must also provide BARDA initial and annual documentation of continuing review and approval and federal wide assurance number.
The Contractor must ensure that the application, as well as all protocols, are reviewed by the performing institution’s IACUC.
To help ensure the safety of animals used in BARDA-funded studies, the Contractor must provide BARDA copies of documents related to all major changes in the status of ongoing protocols, including the following:
|
·
|
All amendments or changes to the protocol, identified by protocol version number, date, or both and date it is valid.
|
|
·
|
All material changes in IACUC policies and procedures, identified by version number, date, and all required signatories (if applicable).
|
|
·
|
Termination or temporary suspension of the study(ies) for regulatory issues.
|
|
·
|
Termination or temporary suspension of the protocol.
|
|
·
|
Any change that is made in the specific IACUC approval for the indicated study(ies).
|
|
·
|
Any other problems or issues that could affect the scientific integrity of the study(ies), i.e., fraud, misrepresentation, misappropriation of funds, etc.
|
|
|
Contractor must notify BARDA of any of the above changes within five (5) working days from the time the Contractor becomes aware of such changes by email or fax, followed by a letter signed by the institutional business official, detailing notification of the change of status to the local IACUC and a copy of any responses from the IACUC.
If a non-clinical protocol has been reviewed by an institutional biosafety committee (IBC) or the NIH Recombinant DNA Advisory Committee (RAC), the Contractor must provide information about the initial and
|
Page 30
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
ongoing review and approval, if any. See the NIH Guidelines for Research Involving Recombinant DNA Molecules.
|
ii. Non-Clinical Data and Safety Monitoring Requirements
BARDA strongly recommends continued safety monitoring for all non- clinical studies of investigational drugs, devices, or biologics. FDA expects non-clinical studies to include safety in addition to efficacy. The Contractor should consider evaluation of clinical relevant safety markers in the pivotal and non-pivotal, non-clinical studies. In preparation for clinical trials of licensed or not yet licensed products, it is imperative that BARDA- sponsored studies of any type measure the risk and safety parameters that are elicited and provide a safety profile from the studies for future human risk assessment.
A risk is minimal where the probability and magnitude of harm or discomfort anticipated in the proposed research are not greater than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests. For example, the risk of drawing a small amount of blood from a healthy subject for research purposes is no greater than the risk of doing so as part of a routine physical examination (45 CFR 46.102(i)).
BARDA will work with the Contractor on decisions regarding the type and extent of safety data accrual to be employed before the start of efficacy or safety studies.
The Contractor shall inform BARDA of any upcoming site visits and/or audits of CRO facilities funded under this effort. BARDA reserves the right to accompany the Contractor on site visits and/or audits of CRO’s as BARDA deems necessary.
|
b.
|
BARDA Review Process before Non-Clinical study Execution Begins
|
BARDA is under the same policy-driven assurances as NIH in that it has a responsibility to ensure that mechanisms and procedures are in place to protect the safety and welfare of animals used in BARDA-funded non-clinical trials.
|
|
Therefore, before study execution, the Contractor must provide the following (as applicable) for review and comment by BARDA:
|
|
·
|
IACUC approved (signed) non-clinical research protocol identified by version number, date, or both, including details of study design, euthanasia criteria, proposed interventions, and exclusion criteria.
|
|
·
|
For non-pivotal mouse studies, the Contractor will provide an annual animal care and use protocol.
|
|
·
|
Documentation of IACUC approval, including OLAW federal wide number, IACUC registration number, and IACUC name.
|
|
·
|
Contractor should reduce the number of animals required for a study using power of statistics.
|
|
·
|
Plans for the management of side effects, rules for interventions and euthanasia criteria.
|
Page 31
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
·
|
Procedures for assessing and collecting safety data were appropriate.
|
|
·
|
If a study is contracted through Contract Research Organizations (CROs), work orders and service agreements the Contractor shall assure an integrated safety documentation plan is in place for the study site, pharmacy service records on the dosing material to be used and excipients, and laboratory services (including histopathology).
|
|
·
|
Documentation that the Contractor and all required staff responsible for the conduct of the research have received training in the protection and handling of animals, or that the CRO has the required documentation.
|
|
·
|
Purchasing of animals and/or other supplies for non-clinical studies funded in part or in whole by BARDA requires written approval by the Contracting Officer in accordance with the contract. The Contractor must have the ability to return/re-sell animals, at purchase price, to distributor or a third part, in the event that the Contracting Officer Authorization is not granted.
|
|
·
|
Provide justification for whether studies require good laboratory practice (GLP) conditions.
|
|
·
|
Provide justification for whether studies will be classified as non- pivotal or pivotal studies.
|
|
|
Documentation of each of the above items shall be submitted to BARDA for evaluation and comment in conjunction with the protocol. Execution of non- clinical studies requires written authorization from the Contracting Officer in accordance with this section of the contract.
|
|
c.
|
References
|
Public Health Service Policy on Humane Care and Use of Laboratory Animals: http://grants.nih.gov/grants/olaw/InvestigatorsNeed2Know.pdf
|
|
USDA Animal Welfare Act:
|
http://awic.nal.usda.gov/nal_display/index.php?info_center=3&tax_level=3&tax_su bject=182&topic_id=1118&level3_id=6735&level4_id=0&level5_id=0&placement_d e fault=0
Page 32
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
2.
|
Clinical Terms of Award
|
These Clinical Terms of Award detail an agreement between the government and the Contractor; they apply to all grants and contracts that involve clinical research.
|
i.
|
Safety and Monitoring Issues
|
|
a.
|
Institutional Review Board or Independent Ethics Committee Approval
|
Before award and then with the annual progress report, the Contractor must submit to BARDA a copy of the current IRB-or IEC-approved informed consent document, documentation of continuing review and approval and the OHRP federal wide assurance number for the institution or site.
If other institutions are involved in the research (e.g., a multicenter clinical trial or study), each institution’s IRB or IEC must review and approve the protocol. They must also provide BARDA initial and annual documentation of continuing review and approval, including the current approved informed consent document and federal wide number.
The Contractor must ensure that the application as well as all protocols are reviewed by their IRB or IEC.
To help ensure the safety of participants enrolled in BARDA-funded studies, the Contractor must provide BARDA copies of documents related to all major changes in the status of ongoing protocols, including the following:
|
·
|
All amendments or changes to the protocol, identified by protocol version number, date, or both and dates it is valid.
|
|
·
|
All changes in informed consent documents, identified by version number, dates, or both and dates it is valid.
|
|
·
|
Termination or temporary suspension of patient accrual.
|
|
·
|
Termination or temporary suspension of the protocol.
|
|
·
|
Any change in IRB approval.
|
|
·
|
Any other problems or issues that could affect the participants in the studies.
|
The Contractor must notify BARDA through the COR and CO of any of the above changes within five (5) working days by email or fax, followed by a letter signed by the institutional business official, detailing notification of the change of status to the local IRB and a copy of any responses from the IRB or IEC.
If a clinical protocol has been reviewed by an institutional biosafety committee (IBC) or the NIH Recombinant DNA Advisory Committee (RAC), the Contractor must provide information about the initial and ongoing review and approval, if any. See the NIH Guidelines for Research Involving Recombinant DNA Molecules.
Page 33
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
b.
|
Data and Safety Monitoring Requirements
|
BARDA strongly recommends independent safety monitoring for clinical trials of investigational drugs, devices, or biologics; clinical trial of licensed products; and clinical research of any type involving more than minimal risk to volunteers. Independent monitoring can take a variety of forms. Phase III clinical trials must be reviewed by an independent data and safety monitoring board (DSMB); other trials may require DSMB oversight as well. The Contractor shall inform BARDA of any upcoming site visits and/or audits of CRO facilities funded under this effort.
|
|
BARDA reserves the right to accompany the Contractor on site visits and/or audits of CROs as BARDA deems necessary.
|
A risk is minimal where the probability and magnitude of harm or discomfort anticipated in the proposed research and not greater than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests. For examples, the risk of drawing a small amount of blood from a healthy individual for research purposes is no greater than the risk of doing so as part of a routine physical examination (45 CFR 46.102I).
Final decisions regarding the type of monitoring to be used must be made jointly by BARDA and the Contractor before enrollment starts. Discussions with the responsible BARDA Project Officer regarding appropriate safety monitoring and approval of the final monitoring plan by BARDA must occur before patient enrollment begins and may include discussions about the appointment of one of the following.
|
·
|
Independent Safety Monitor – a physician or other appropriate expert who is independent of the study and available in real time to review and recommend appropriate action regarding adverse events and other safety issues.
|
|
·
|
Independent Monitoring Committee (IMC) or Safety Monitoring Committee (SMC) – a small group of independent investigators and biostatisticians who review data from a particular study.
|
|
·
|
Data and Safety Monitoring Board – an independent committee charged with reviewing safety and trial progress and providing advice with respect to study continuation, modification, and termination. The Contractor may be required to use an established BARDA DSMB or to organize an independent DSMB. All phase III clinical trials must be reviewed by a DSMB; other trials may require DSMB oversight as well. Please refer to: NIAID Principles for Use of a Data and Safety Monitoring Board (DSMB) For Oversight of Clinical Trials Policy
|
When a monitor or monitoring board is organized, a description of it, its charter or operating procedures (including a proposed meeting schedule and plan for review of adverse events), and roster and curriculum vitae from all members must be submitted to and approved by BARDA before enrollment starts. The Contractor will also ensure that the monitors and board members report any conflicts of interest and the Contractor will maintain a record of this. The Contractor will share conflict of interest reports with BARDA.
Additionally, the Contractor must submit written summaries of all reviews conducted by the monitoring group to the BARDA within thirty (30) days of reviews or meetings.
Page 34
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ii. BARDA Protocol Review Process Before Patient Enrollment Begins BARDA has a responsibility to ensure that mechanisms and procedures are in place to protect the safety of participants in BARDA-supported clinical trials. Therefore, before patient accrual or participant enrollment, the Contractor must ensure the following (as applicable) are in place at each participating institution, prior to patient accrual or enrollment:
|
·
|
IRB- or IEC-approved clinical research protocol identified by version number, date, or both, including details of study design, proposed interventions, patient eligibility, and exclusion criteria.
|
|
·
|
Documentation of IRB or IEC approval, including OHRP federal wide number, IRB or IEC registration number, and IRB and IEC name.
|
|
·
|
IRB- or IEC- approved informed consent document, identified by version number, date, or both and dates it is valid.
|
|
·
|
Plans for the management of side effects.
|
|
·
|
Procedures for assessing and reporting adverse events.
|
|
·
|
Plans for data and safety monitoring (see above) and monitoring of the clinical study site, pharmacy, and laboratory.
|
|
·
|
Documentation that the Contractor and all study staff responsible for the design or conduct of the research have received training in the protection of human subjects.
|
Documentation to demonstrate that each of the above items are in place shall be submitted to the BARDA) for evaluation and comment in conjunction with the protocol. Execution of clinical studies requires written authorization from BARDA in accordance with this section of this contract.
iii Investigational New drug or Investigational Device Exemption Requirements
Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
|
|
Exceptions must be granted in writing by FDA. If the proposed clinical trial will be performed under an IND or IDE, the Contractor must provide BARDA with the name and institution of the IND or IDE sponsor, the date the IND or IDE was filed with FDA, the FDA IND or IDE number, any written comments from FDA, and the written responses to those comments.
|
Unless FDA notifies Contractor otherwise, The Contractor must wait thirty (30) calendar days from FDA receipt of an initial IND or IDE application before initiating a clinical trial.
The Contractor must notify BARDA if the FDA places the study on clinical hold and provide BARDA any written comments from FDA, written responses to the comments, and documentation in writing that the hold has been lifted.
The Contractor must not use grant or contract funds during a clinical hold to fund clinical studies that are on hold. The Contractor must not enter into any new financial obligations related to clinical activities for the clinical trial on clinical hold.
Page 35
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
v.
|
Required Time-Sensitive Notification
|
|
|
Under an IND or IDE, the sponsor must provide FDA safety reports of serious adverse events. Under these Clinical Terms of Award, the Contractor must submit copies to the responsible Contracting Officer’s Representative (COR) as follows:
|
|
ii.
|
Expedited safety report of unexpected or life-threatening experience or death:
|
A copy of any report of unexpected or life-threatening experience or death associated with the use of an IND drug, which must be reported to FDA by telephone or fax as soon as possible but no later than seven (7) days after the IND sponsor’s receipt of the information, must be submitted to the COR within
24 hours of FDA notification.
|
iii.
|
Expedited safety reports of serious and unexpected adverse experiences:
|
A copy of any report of unexpected and serious adverse experience associated with use of an IND drug or any finding from tests in laboratory animals that suggests a significant risk for human subjects, which must be reported in writing to FDA as soon as possible but no later than 15 day after the IND sponsor’s receipt of the information, must be submitted to the COR within 24 hours of FDA notification.
|
iv.
|
IDE reports of unanticipated adverse device effect:
|
A copy of any reports of unanticipated adverse device effect submitted to FDA must be submitted to the COR within 24 hours of FDA notification.
|
v.
|
Expedited safety reports:
|
Sent to the COR concurrently with the report to FDA.
|
vi.
|
Other adverse events documented during the course of the trial should be included in the annual IND or IDE report and reported to BARDA annually.
|
|
|
In case of problems or issues, the Contracting Officer’s Representative will contact the Contractor within ten (10) working days by email or fax, followed within thirty (30) calendar days by an official letter to the Contractor’s Project Manager, with a copy to the institutions’ office of sponsored programs, listing issues and appropriate actions to be discussed.
|
|
vii.
|
Safety reporting for research not performed under an IND or IDE.
|
Final decisions regarding ongoing safety reporting requirements for research not performed under an IND or IDE must be made jointly by the Contracting Officer’s Representative and the Contractor.
ARTICLE H.2. CARE OF LIVE VERTEBRATE ANIMALS, HHSAR 352.270-5 (October 2009)
|
a.
|
Before undertaking performance of any contract involving animal-related activities where the species is regulated by USDA, the Contractor shall register with the Secretary of Agriculture of the United States in accordance with 7 U.S.C. 2136 and 9 CFR sections 2.25 through
|
2.28. The Contractor shall furnish evidence of the registration to the Contracting Officer.
Page 36
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
b.
|
The Contractor shall acquire vertebrate animals used in research from a dealer licensed by the Secretary of Agriculture under 7 U.S.C. 2133 and 9 CFR Sections 2.1-2.11, or from a source that is exempt from licensing under those sections.
|
|
c.
|
The Contractor agrees that the care, use and intended use of any live vertebrate animals in the performance of this contract shall conform with the Public Health Service (PHS) Policy on Humane Care of Use of Laboratory Animals (PHS Policy), the current Animal Welfare Assurance (Assurance), the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC) and the pertinent laws and regulations of the United States Department of Agriculture (see 7 U.S.C. 2131 et seq. and 9 CFR Subchapter A, Parts 1-4). In case of conflict between standards, the more stringent standard shall govern.
|
|
d.
|
If at any time during performance of this contract, the Contracting Officer determines, in consultation with the Office of Laboratory Animal Welfare (OLAW), National Institutes of Health (NIH), that the Contractor is not in compliance with any of the requirements and standards stated in paragraphs (a) through (c) above, the Contracting Officer may immediately suspend, in whole or in part, work and further payments under this contract until the Contractor corrects the noncompliance. Notice of the suspension may be communicated by telephone and confirmed in writing. If the Contractor fails to complete corrective action within the period of time designated in the Contracting Officer's written notice of suspension, the Contracting Officer may, in consultation with OLAW, NIH, terminate this contract in whole or in part, and the Contractor's name may be removed from the list of those contractors with approved Assurances.
|
Note: The Contractor may request registration of its facility and a current listing of licensed dealers from the Regional Office of the Animal and Plant Health Inspection Service (APHIS), USDA, for the region in which its research facility is located. The location of the appropriate APHIS Regional Office, as well as information concerning this program may be obtained by contacting the Animal Care Staff, USDA/APHIS, 4700 River Road, Riverdale, Maryland 20737 (E-mail: ace@aphis.usda.gov;W eb site: (http://www.aphis.usda.gov/animal_welfare).
ARTICLE H.3. ANIMAL WELFARE
All research involving live, vertebrate animals shall be conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. This policy may be accessed at:
http://grants1.nih.gov/grants/olaw/references/phspol.htm
ARTICLE H.4. INFORMATION ON COMPLIANCE WITH ANIMAL CARE REQUIREMENTS
Registration with the U. S. Dept. of Agriculture (USDA) is required to use regulated species of animals for biomedical purposes. USDA is responsible for the enforcement of the Animal Welfare Act (7 U.S.C. 2131 et. seq.), http://www.nal.usda.gov/awic/legislat/awa.htm.
The Public Health Service (PHS) Policy is administered by the Office of Laboratory Animal Welfare (OLAW) http://grants2.nih.gov/grants/olaw/olaw.htm. An essential requirement of the PHS Policy http://grants2.nih.gov/grants/olaw/references/phspol.htm is that every institution using live vertebrate animals must obtain an approved assurance from OLAW before they can receive funding from any component of the U. S. Public Health Service.
The PHS Policy requires that Assured institutions base their programs of animal care and use on the Guide for the Care and Use of Laboratory
Animals http://www.nap.edu/readingroom/books/labrats/ and that they comply with the
Page 37
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
regulations (9 CFR, Subchapter A)http://www.nal.usda.gov/awic/legislat/usdaleg1.htm issued by the U.S. Department of Agriculture (USDA) under the Animal Welfare Act. The Guide may differ from USDA regulations in some respects. Compliance with the USDA regulations is an absolute requirement of this Policy.
The Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) http://www.aaalac.org is a professional organization that inspects and evaluates programs of animal care for institutions at their request. Those that meet the high standards are given the accredited status. As of the 2002 revision of the PHS Policy, the only accrediting body recognized by PHS is the AAALAC. While AAALAC Accreditation is not required to conduct biomedical research, it is highly desirable. AAALAC uses the Guide as their primary evaluation tool. They also use the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. It is published by the Federated of Animal Science
Societies http://www.fass.org.
|
|
ARTICLE H.5. REQUIREMENTS FOR ADEQUATE ASSURANCE OF PROTECTION OF VERTEBRATE ANIMAL SUBJECTS
|
The PHS Policy on Humane Care and Use of Laboratory Animals requires that applicant organizations proposing to use vertebrate animals file a written Animal Welfare Assurance with the Office for Laboratory Animal Welfare (OLAW), establishing appropriate policies and procedures to ensure the humane care and use of live vertebrate animals involved in research activities supported by the PHS. The PHS Policy stipulates that an applicant organization, whether domestic or foreign, bears responsibility for the humane care and use of animals in
PHS- supported research activities. Also, the PHS policy defines “animal” as “any live, vertebrate animal used, or intended for use, in research, research training, experimentation, biological testing or for related purposes.” This Policy implements and supplements the U.S. government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training, and requires that institutions use the Guide for the Care and Use of Laboratory Animals as a basis for developing and implementing an institutional animal care and use program. This Policy does not affect applicable State or local laws or regulations that impose more stringent standards for the care and use of laboratory animals. All institutions are required to comply, as applicable, with the Animal Welfare Act as amended (7 USC 2131 et. seq.) and other Federal statutes and regulations relating to animals. These documents are available from the Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD 20892, (301) 496-7163. See http://grants.nih.gov/grants/olaw/olaw.htm.
No PHS supported work for research involving vertebrate animals will be conducted by an organization, unless that organization is operating in accordance with an approved Animal Welfare Assurance and provides verification that the Institutional Animal Care and Use Committee (IACUC) has reviewed and approved the proposed activity in accordance with the PHS policy. Applications may be referred by the PHS back to the institution for further review in the case of apparent or potential violations of the PHS Policy. No award to an individual will be made unless that individual is affiliated with an assured organization that accepts responsibility for compliance with the PHS Policy. Foreign applicant organizations applying for PHS awards for activities involving vertebrate animals are required to comply with PHS Policy or provide evidence that acceptable standards for the humane care and use of animals will be met. Foreign applicant organizations are not required to submit IACUC approval, but should provide information that is satisfactory to the USG to provide assurances for the humane care of such animals.
ARTICLE H.6. APPROVAL OF REQUIRED ASSURANCE BY OLAW
Under governing regulations, federal funds which are administered by the Department of Health and Human Services, Office of Biomedical Advanced Research and Development Authority (BARDA) shall not be expended by the Contractor for research involving live vertebrate animals,
Page 38
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
nor shall live vertebrate animals be involved in research activities by the Contractor under this award unless a satisfactory assurance of compliance with 7 U.S.C. 2316 and 9 CFR Sections 2.25-2.28 is submitted within 30 days of the date of this award and approved by the Office of Laboratory Animal Welfare (OLAW). Each performance site (if any) must also assure compliance with 7 U.S.C. 2316 and 9 CFR Sections 2.25-2.28 with the following restriction: Only activities which do not directly involve live vertebrate animals (i.e. are clearly severable and independent from those activities that do involve live vertebrate animals) may be conducted by the Contractor or individual performance sites pending OLAW approval of their respective assurance of compliance with 7 U.S.C. 2316 and 9 CFR Sections 2.25-2.28. Additional information regarding OLAW may be obtained via the Internet
at http://grants2.nih.gov/grants/olaw/references/phspol.htm
ARTICLE H.7. REPORTING MATTERS INVOLVING FRAUD, WASTE AND ABUSE
Anyone who becomes aware of the existence or apparent existence of fraud, waste and abuse in BARDA funded programs should report such matters to the HHS Inspector General's Office in writing or on the Inspector General's Hotline. The toll free number is 1-800-HHS-TIPS (1-800- 447-8477). All telephone calls will be handled confidentially. The e-mail address
is Htips@os.dhhs.gov and the mailing address is:
Office of Inspector General
Department of Health and Human Services TIPS HOTLINE
P.O. Box 23489 Washington, D.C. 20026
|
|
ARTICLE H.8. PROHIBITION ON CONTRACTOR INVOLVEMENT WITH TERRORIST ACTIVITIES
|
The Contractor acknowledges that U.S. Executive Orders and Laws, including but not limited to
E.O. 13224 and P.L. 107-56, prohibit transactions with, and the provision of resources and support to, individuals and organizations associated with terrorism. It is the legal responsibility of the Contractor to ensure compliance with these Executive Orders and Laws. This clause must be included in all subcontracts issued under this contract.
ARTICLE H.9. IDENTIFICATION AND DISPOSITION OF DATA
|
|
The Contractor will be required to provide certain data generated under this contract to the Department of Health and Human Services (DHHS). DHHS reserves the right to review any other data determined by DHHS to be relevant to this contract. The contractor shall keep copies of all data required by the Food and Drug Administration (FDA) relevant to this contract for the time specified by the FDA.
|
ARTICLE H.10. EXPORT CONTROL NOTIFICATION
Contractors are responsible for ensuring compliance with all export control laws and regulations that may be applicable to the export of and foreign access to their proposed technologies.
Contractors may consult with the Department of State with any questions regarding the International Traffic in Arms Regulation (ITAR) (22 CRF Parts 120-130) and /or the Department of Commerce regarding the Export Administration Regulations (15 CRF Parts 730-774).
ARTICLE H.11. CONFLICT OF INTEREST
The Contractor represents and warrants that, to the best of the Contractor's knowledge and belief, there are no relevant facts or circumstances which could give rise to an organizational conflict of interest, as defined in FAR 2.101 and Subpart 9.5, or that the Contractor has disclosed all such relevant information. Prior to commencement of any work, the Contractor agrees to notify the Contracting Officer promptly that, to the best of its knowledge and belief, no actual or potential conflict of interest exists or to identify to the Contracting Officer any actual or potential
Page 39
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
conflict of interest the firm may have. In emergency situations, however, work may begin but notification shall be made within five (5) working days. The Contractor agrees that if an actual or potential organizational conflict of interest is identified during performance, the Contractor shall promptly make a full disclosure in writing to the Contracting Officer. This disclosure shall include a description of actions which the Contractor has taken or proposes to take, after consultation with the Contracting Officer, to avoid, mitigate, or neutralize the actual or potential conflict of interest. The Contractor shall continue performance until notified by the Contracting Officer of any contrary action to be taken. Remedies include termination of this contract for convenience, in whole or in part, if the Contracting Officer deems such termination necessary to avoid an organizational conflict of interest. If the Contractor was aware of a potential organizational conflict of interest prior to award or discovered an actual or potential conflict after award and did not disclose it or misrepresented relevant information to the Contracting Officer, the USG may terminate the contract for default, debar the Contractor from USG contracting, or pursue such other remedies as may be permitted by law or this contract.
ARTICLE H.12. INSTITUTIONAL RESPONSIBILITY REGARDING INVESTIGATOR FINANCIAL CONFLICTS OF INTEREST
The Contractor shall comply with the requirements of 45 CFR Part 94, Responsible Prospective Contractors, which promotes objectivity in research by establishing standards to ensure that Investigators (defined as the project director or principal Investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research funded under BARDA contracts, or proposed for such funding, which may include, for example, collaborators or consultants) will not be biased by any Investigator financial conflicts of interest.
If the failure of an Investigator to comply with an Institution's financial conflicts of interest policy or a financial conflict of interest management plan appears to have biased the design, conduct, or reporting of the BARDA-funded research, the Contractor must promptly notify the Contracting Officer of the corrective action taken or to be taken. The Contracting Officer will consider the situation and, as necessary, take appropriate action or refer the matter to the Contractor for further action, which may include directions to the Contractor on how to maintain appropriate objectivity in the BARDA-funded research project.
The Contracting Officer and/or HHS may inquire at any time before, during, or after award into any Investigator disclosure of financial interests, and the Contractor’s review of, and response to, such disclosure, regardless of whether the disclosure resulted in the Contractor’s determination of a financial conflict of interests. The Contracting Officer may require submission of the records or review them on site. On the basis of this review of records or other information that may be available, the Contracting Officer may decide that a particular financial conflict of interest will bias the objectivity of the BARDA-funded research to such an extent that further corrective action is needed or that the Institution has not managed the financial conflict of interest in accordance with 4 5 C F R Part 94. The issuance of a Stop Work Order by the Contracting Officer may be necessary until the matter is resolved.
If the Contracting Officer determines that BARDA-funded clinical research, whose purpose is to evaluate the safety or effectiveness of a drug, medical device, or treatment, has been designed, conducted, or reported by an Investigator with a financial conflict of interest that was not disclosed managed or reported the Contractor shall require the Investigator involved to disclose the financial conflict of interest in each public presentation of the results of the research and to request an addendum to previously published presentations.
ARTICLE H.13. NEEDLE DISTRIBUTION
|
|
The Contractor shall not use contract funds to carry out any program of distributing sterile needles or syringes for the hypodermic injection of any illegal drug.
|
Page 40
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE H.14. RESTRICTION ON ABORTIONS
The Contractor shall not use contract funds for any abortion.
ARTICLE H.15. CONTINUED BAN ON FUNDING OF HUMAN EMBRYO RESEARCH
The Contractor shall not use contract funds for (1) the creation of a human embryo or embryos for research purposes; or (2) research in which a human embryo or embryos are destroyed, discarded, or knowingly subjected to risk of injury or death greater than that allowed for research on fetuses in utero under 45 CFR 46.204(b) and Section 498(b) of the Public Health Service Act (42 U.S.C. 289g(b)). The term "human embryo or embryos" includes any organism, not protected as a human subject under 45 CFR 46 as of the date of the enactment of this Act, that is derived by fertilization, parthenogenesis, cloning, or any other means from one or more human gametes or human diploid cells.
Additionally, in accordance with a March 4, 1997 Presidential Memorandum, Federal funds may not be used for cloning of human beings.
ARTICLE H.16. DISSEMINATION OF FALSE OR DELIBERATELY MISLEADING INFORMATION
|
|
The Contractor shall not use contract funds to disseminate information that is deliberately false or misleading.
|
ARTICLE H.17. CONFIDENTIALITY OF INFORMATION
|
a.
|
Confidential information, as used in this article, means information or data of a personal nature about an individual or proprietary information or data submitted by or pertaining to an institution or organization.
|
|
b.
|
The Contracting Officer and the Contractor may, by mutual consent, identify elsewhere in this contract specific information and/or categories of information which the USG will furnish to the Contractor or that the Contractor is expected to generate which is confidential and providing further that the government is not entitled to unlimited rights to that information pursuant to FAR 52.227-14. Similarly, the Contracting Officer and the Contractor may, by mutual consent, identify such confidential information from time to time during the performance of the contract. Failure to agree will be settled pursuant to the "Disputes" clause.
|
|
c.
|
If it is established elsewhere in this contract that information to be utilized under this contract, or a portion thereof, is subject to the Privacy Act, the Contractor will follow the rules and procedures of disclosure set forth in the Privacy Act of 1974, 5 U.S.C. 552a, and implementing regulations and policies, with respect to systems of records determined to be subject to the Privacy Act.
|
|
d.
|
Confidential information, as defined in paragraph (a) of this article, shall not be disclosed without the prior written consent of the individual, institution, or organization.
|
|
e.
|
Whenever the Contractor is uncertain with regard to the proper handling of material under the contract, or if the material in question is subject to the Privacy Act or is confidential information subject to the provisions of this article, the Contractor should obtain a written determination from the Contracting Officer prior to any release, disclosure, dissemination, or publication.
|
|
f.
|
The provisions of paragraph (d) of this article shall not apply to conflicting or overlapping provisions in other Federal, State or local laws.
|
Page 41
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE H.18. ACCESS TO DOCUMENTATION/DATA
The USG shall have physical and electronic access to all documentation and data generated under this contract, including: all data documenting Contractor performance; all data generated; all communications and correspondence with regulatory agencies and bodies to include all audit observations, inspection reports, milestone completion documents, and all Offeror commitments and responses. Contractor shall provide the USG with an electronic copy of all correspondence with the FDA within 5 business days of receipt. The USG shall acquire unlimited rights to all data funded under this contract in accordance with FAR Subpart 27.4 and FAR Clause 52.227-14.
ARTICLE H.19. EPA ENERGY STAR REQUIREMENTS
In compliance with Executive Order 12845 (requiring Agencies to purchase energy efficient computer equipment), all microcomputers, including personal computers, monitors, and printers that are purchased using USG funds in performance of a contract shall be equipped with or meet the energy efficient low-power standby feature as defined by the EPA Energy Star program unless the equipment always meets EPA Energy Star efficiency levels. The microcomputer, as configured with all components, must be Energy Star compliant.
This low-power feature must already be activated when the computer equipment is delivered to the agency and be of equivalent functionality of similar power managed models. If the equipment will be used on a local area network, the vendor must provide equipment that is fully compatible with the network environment. In addition, the equipment will run commercial off-the-shelf software both before and after recovery from its energy conservation mode.
ARTICLE H.20. ACKNOWLEDGMENT OF FEDERAL FUNDING
Section 507 of P.L. 104-208 mandates that Contractors funded with Federal dollars, in whole or in part, acknowledge Federal funding when issuing statements, press releases, requests for proposals, bid solicitations and other documents. This requirement is in addition to the continuing requirement to provide an acknowledgment of support and disclaimer on any publication reporting the results of a contract funded activity.
|
A.
|
Publication and Publicity
|
No information related to data obtained under this contract shall be released or publicized without providing BARDA with at least thirty (30) days advanced notice and an opportunity to review the proposed release or publication.
|
|
In addition to the requirements set forth in HHSAR Clause 352.227-70, Publications and Publicity incorporated by reference in SECTION I of this contract, Section 507 of P.L. 104-208 mandates that Contractors funded with Federal dollars, in whole or in part, acknowledge Federal funding when issuing statements, press releases, requests for proposals, bid solicitations and other documents. Contractors are required to state:
|
|
(1)
|
the percentage and dollar amounts of the total program or project costs financed with Federal money and;
|
|
(2)
|
the percentage and dollar amount of the total costs financed by nongovernmental sources
|
For purposes of this contract "publication" is defined as an issue of printed material offered for distribution or any communication or oral presentation of information, including any manuscript or scientific meeting abstract. Any publication containing data generated under this contract must be submitted for BARDA review no less than thirty (30) calendar days for manuscripts and fifteen (15) calendar days for abstracts before
Page 42
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
submission for public presentation or publication. Contract support shall be acknowledged in all such publications substantially as follows:
|
|
"This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201500007C.”
|
|
B.
|
Press Releases
|
Misrepresenting contract results or releasing information that is injurious to the integrity of BARDA may be construed as improper conduct. Press releases shall be considered to include the public release of information to any medium, excluding peer-reviewed scientific publications. The contractor shall ensure that the COR has received an advance copy of any press release related to the contract not less than six (6) business days prior to the issuance of the press release.
The Contractor shall acknowledge the support of the Department of Health and Human Service, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, whenever publicizing the work under this contract in any media by including an acknowledgment substantially as follows:
"This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No.
HHSO100201500003C.”
ARTICLE H.21. IN-PROCESS REVIEW
In Process Reviews (IPR) will be conducted at the discretion of the USG to discuss the progression of the milestones. The USG reserves the right to revise the milestones and budget pending the development of the project. Deliverables such as an overall project summary report and/or slides will be required when the IPRs are conducted. The Contractor’s success in completing the required tasks under each work segment must be demonstrated through the Deliverables and Milestones specified under SECTION F. Those deliverables will constitute the basis for the USG’s decision, at its sole discretion, to proceed with the work segment, or institute changes to the work segment, or terminate the work segment.
|
|
IPRs may be scheduled at the discretion of the USG to discuss progression of the contract. The Contractor shall provide a presentation following a prescribed template which will be provided by the USG at least 30 business days prior to the IPR. Subsequently, the contractor will be requested to provide a revised/final presentation to the Contracting Officer at least 10 business days prior to the IPR.
|
ARTICLE H.22. PROHIBITION ON THE USE OF APPROPRIATED FUNDS FOR LOBBYING ACTIVITIES AND HHSAR 352.203-70 ANTI-LOBBYING (March 2012))
The Contractor is hereby notified of the restrictions on the use of Department of Health and Human Service's funding for lobbying of Federal, State and Local legislative bodies.
Section 1352 of Title 10, United Stated Code (Public Law 101-121, effective 12/23/89), among other things, prohibits a recipient (and their subcontractors) of a Federal contract, grant, loan, or cooperative agreement from using appropriated funds (other than profits from a federal contract) to pay any person for influencing or attempting to influence an officer or employee of any agency, a Member of Congress, an officer or employee of Congress, or an employee of a Member of
Page 43
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Congress in connection with any of the following covered Federal actions; the awarding of any Federal contract; the making of any Federal grant; the making of any Federal loan; the entering into of any cooperative agreement; or the modification of any Federal contract, grant, loan, or cooperative agreement. For additional information of prohibitions against lobbying activities, see FAR Subpart 3.8 and FAR Clause 52.203-12.
In addition, as set forth in HHSAR 352.203-70 “Anti-Lobbying” (March 2012)), the current Department of Health and Human Services Appropriations Act provides that no part of any appropriation contained in this Act shall be used, other than for normal and recognized executive- legislative relationships, for publicity or propaganda purposes, for the preparation, distribution, or use of any kit, pamphlet, booklet, publication, radio, television, or video presentation designed to support, or defeat legislation pending before the Congress, or any State or Local legislature except in presentation to the Congress, or any State or Local legislative body itself.
The current Department of Health and Human Services Appropriations Act also provides that no part of any appropriation contained in this Act shall be used to pay the salary or expenses of any contract or grant recipient, or agent acting for such recipient, related to any activity designed to influence legislation or appropriations pending before the Congress, or any State or Local legislature.
ARTICLE H.23. PRIVACY ACT APPLICABILITY
|
1)
|
Notification is hereby given that the Contractor and its employees are subject to criminal penalties for violation of the Privacy Act to the same extent as employees of the USG. The Contractor shall assure that each of its employees knows the prescribed rules of conduct and that each is aware that he or she can be subjected to criminal penalty for violation of the Act.
|
A copy of 45 CFR Part 5b, Privacy Act Regulations, may be obtained at http://www.gpoaccess.gov/cfr/index.html
|
2)
|
The Project Officer is hereby designated as the official who is responsible for monitoring contractor compliance with the Privacy Act.
|
|
3)
|
The Contractor shall follow the Privacy Act guidance as contained in the Privacy Act System of Records number 09-25-0200. This document may be obtained at the following link: http://oma.od.nih.gov/ms/privacy/pa-files/0200.htm
|
ARTICLE H.24. LABORATORY LICENSE REQUIREMENTS
|
|
The Contractor shall comply with all applicable requirements of Section 353 of the Public Health Service Act (Clinical Laboratory Improvement Act as amended) (42 U.S.C. 263a and 42 CFR Part 493). This requirement shall also be included in any subcontract for services under the contract.
|
ARTICLE H.25. QUALITY ASSURANCE (QA) AUDIT REPORTS
|
|
BARDA reserves the right to participate in QA audits. Upon completion of the audit/site visit the Contractor shall provide a report capturing the findings, results and next steps in proceeding with the subcontractor. If action is requested of the subcontractor, detailed concerns for addressing areas of non-conformance to FDA regulations for GLP, GMP, or GCP guidelines, as identified in the audit report, must be provided to BARDA. The Contractor shall provide responses from the subcontractors to address these concerns and plans for corrective action execution.
|
|
·
|
Contractor shall notify CO and COR of upcoming, ongoing, or recent audits/site visits of subcontractors as part of weekly communications
|
|
·
|
Contractor shall notify the COR and CO within 5 business days of report completion.
|
Page 44
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE H.26. BARDA AUDITS
|
|
Contractor shall accommodate periodic or ad hoc site visits by the USG. If the USG, the Contractor, or other parties identifies any issues during an audit, the Contractor shall capture the issues, identify potential solutions, and provide a report to the USG.
|
|
·
|
If issues are identified during the audit, Contractor shall submit a report to the CO and COR detailing the finding and corrective action(s) within 10 business days of the audit.
|
|
·
|
COR and CO will review the report and provide a response to the Contractor with 10 business days.
|
|
·
|
Once corrective action is completed, the Contractor will provide a final report to the CO and COR.
|
ARTICLE H.27. SECURITY REPORTING REQUIREMENT
|
|
Violations of established security protocols shall be reported to the Contracting Officer (CO) and Contracting Officer’s Representative (COR) upon discovery and within 24 hours of any compromise, intrusion, loss or interference of its security processes and procedures. The Contractor shall ensure that all software components that are not required for the operation and maintenance of the database/control system has been removed and/or disabled. The Contractor shall provide to the CO and the COR information appropriate to Information and Information Technology software and service updates and/or workarounds to mitigate all vulnerabilities associated with the data and shall maintain the required level of system security.
|
The Contractor will investigate violations to determine the cause, extent, loss or compromise of sensitive program information, and corrective actions taken to prevent future violations. The Contracting Officer in coordination with BARDA will determine the severity of the violation. Any contractual actions resulting from the violation will be determined by the Contracting Officer.
ARTICLE H.28. RESTRICTION ON EMPLOYMENT OF UNAUTHORIZED ALIEN WORKERS
The Contractor shall not use contract funds to employ workers described in section 274A (h) (3) of the Immigration and National Act, which reads as follows:
“(3) Definition of unauthorized alien – As used in this section, the term ‘unauthorized alien’ with respect to the employment of an alien at a particular time, that the alien is not at that time either (A) an alien lawfully admitted for permanent residence, or (B) authorized to be so employed by this Act or by the Attorney General.”
|
|
ARTICLE H.29. NOTIFICATION OF CRITICAL PROGRAMMATIC CONCERNS, RISKS, OR POTENTIAL RISKS
|
If any action occurs that creates a cause for critical programmatic concern, risk, or potential risk to BARDA or the Contractor and Incident Report shall be delivered to BARDA.
|
·
|
Within 48 hours of activity or incident or within 24 hours for a security related activity or incident, Contractor must notify BARDA.
|
|
·
|
Additional updates due to COR and CO within 48 hours of additional developments.
|
|
·
|
Contractor shall submit within 5 business days a Corrective Action Plan (if deemed necessary by either party) to address any potential issues.
|
|
|
If corrective action is deemed necessary, Contractor must address in writing, its consideration of concerns raised by BARDA within 5 business days.
|
Page 45
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
|
ARTICLE H.30. PUBLIC READINESS AND EMERGENCY PREPARDENESS ACT (“PREP ACT”)
|
Effective December 3, 2014 the Secretary of the Department of Health and Human Services has issued a declaration pursuant to section 319F-3 of the Public Health Service Act (42 U.S.C. 247d- 6d) to provide liability protection for activities related to Ebola Virus Disease Vaccines consistent with the terms of the declaration. GlaxoSmithKline (GSK Code Name GSK3390107A) “Recombinant Replication Deficient Chimpanzee Adenovirus Type 3-Vectored Ebola Zaire Vaccine”, is named in the declaration as a covered countermeasure, and as such the Government shall provide liability protection for activities related to GSK3390107A, consistent with the terms of the declaration.
ARTICLE H.31. PERSON IN PLANT
With seven (7) business days advance notice to the Contractor in writing from the Contracting Officer, the USG may place a person-in-plant in the Contractor’s or subcontractor’s facility, who shall be subject to the Contractor’s or subcontractor’s policies and procedures regarding security and facility access at all times while in the facility.
An article substantially similar to this Person-in-Plant article shall be incorporated into any subcontract for experimental or manufacturing work.
Page 46
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE I.1. FAR 52.252-2, CLAUSES INCORPORATED BY REFERENCE (FEBRUARY 1998)
This contract incorporates the following clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available.
Also, the full text of a clause may be accessed electronically at these addresses: https://www.acquisition.gov/FAR/ . HHSAR Clauses
at: http://www.hhs.gov/policies/hhsar/subpart352.html .
FEDERAL ACQUISIITON REGULATION (48 CFR CHAPTER 1) CLAUSE:
52.242-15, Stop Work Order (August 1989)
Clauses for Cost-Reimbursement Research and Development Contract
|
(1)
|
FEDERAL ACQUISITION REGULATION (FAR) (48 CFR CHAPTER 1) CLAUSES:
|
|
FAR CL AUSE
|
DATE
|
CLAUSE TITLE
|
|
52.202-1
|
Nov 2013
|
Definitions
|
|
52.203-3
|
Apr 1984
|
Gratuities
|
|
52.203-5
|
May 2014
|
Covenant Against Contingent Fees
|
|
52.203-6
|
Sep 2006
|
Restrictions on Subcontractor Sales to the government
|
|
52.203-7
|
May 2014
|
Anti-Kickback Procedures
|
|
52.203-8
|
May 2014
|
Cancellation, Rescission, and Recovery of Funds for Illegal or Improper Activity
|
|
52.203-10
|
May 2014
|
Price or Fee Adjustment for Illegal or Improper Activity
|
|
52.203-12
|
Oct 2010
|
Limitation on Payments to Influence Certain Federal Transactions
|
|
52.203-13
|
Apr 2010
|
Contractor Code of Business Ethics and Conduct
|
|
52.203-17
|
April 2014
|
Contractor Employee Whistleblower Rights and Requirements to Inform Employees of Whistleblower rights
|
|
52.204-4
|
May 2011
|
Printed or Copied Double-Sided on Recycled Paper
|
|
52.204-7
|
Jul 2013
|
System for Award Management
|
|
52.204-10
|
Jul 2013
|
Reporting Executive Compensation and First-Tier Subcontract Awards
|
|
52.204-13
|
Jul 2013
|
System for Award Management Maintenance
|
Page 47
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
52.209-6
|
Aug 2013
|
Protecting the government’s Interests When Subcontracting With Contractors Debarred, Suspended, or Proposed for Debarment
|
|
52.209-9
|
Jul 2013
|
Updates of Publicly Available Information Regarding Responsibility Matters
|
|
52.210-1
|
Apr 2011
|
Market Research
|
|
52.215-2
|
Oct 2010
|
Audit and Records – Negotiation
|
|
52.215-8
|
Oct 1997
|
Order of Precedence - Uniform Contract Format
|
|
52.215-10
|
Aug 2011
|
Price Reduction for Defective Certified Cost or Pricing Data
|
|
52.215-12
|
Oct 2010
|
Subcontractor Certified Cost or Pricing Data
|
|
52.215-15
|
Oct 2010
|
Pension Adjustments and Asset Reversions
|
|
52.215-17
|
Oct 1997
|
Facilities Capital Cost of Money
|
|
52.215-18
|
Jul 2005
|
Reversion or Adjustment of Plans for Post-Retirement Benefits (PRB) other than Pensions
|
|
52.215-19
|
Oct 1997
|
Notification of Ownership Changes
|
|
52.215-21
|
Oct 2010
|
Requirements for Certified Cost or Pricing Data and Data Other Than Certified Cost or Pricing Data – Modifications
|
|
52.215-23
|
Oct 2009
|
Limitations on Pass-Through Charges
|
|
52.216-7
|
Jun 2013
|
Allowable Cost and Payment
|
|
52.216-8
|
Jun 2011
|
Fixed Fee
|
|
52.217-8
|
Nov 1999
|
Option to Extend Services
|
|
52.219-8
|
May 2014
|
Utilization of Small Business Concerns
|
|
52.222-2
|
Jan 2011
|
Payment for Overtime Premiums
|
|
52.222-3
|
Jun 2003
|
Convict Labor
|
|
52.222-21
|
Feb 1999
|
Prohibition of Segregated Facilities
|
|
52.222-26
|
Mar 2007
|
Equal Opportunity
|
|
52.222-35
|
Jul 2014
|
Equal Opportunity for Veterans
|
|
52.222-36
|
Jul 2014
|
Equal Opportunities for Workers with Disabilities
|
|
52.222-37
|
Jul 2014
|
Employment Reports on Veterans
|
|
52.222-40
|
Dec 2010
|
Notification of Employee Rights Under the National Labor Relations Act
|
|
52.222-50
|
Feb 2009
|
Combating Trafficking in Persons
|
|
52.222-54
|
Aug 2013
|
Employment Eligibility Verification
|
|
52.223-6
|
May 2001
|
Drug-Free Workplace
|
|
52.223-18
|
Aug 2011
|
Encouraging Contractor Policies to Ban Text Messaging While Driving
|
Page 48
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
52.224-1
|
April 1984
|
Privacy Act Notification
|
|
52.224-2
|
April 1984
|
Privacy Act
|
|
52.225-13
|
Jun 2008
|
Restrictions on Certain Foreign Purchases
|
|
52.227-1
|
Dec 2007
|
Authorization and Consent, Alternate I
|
|
52.227-2
|
Dec 2007
|
Notice and Assistance Regarding Patent and Copyright Infringement
|
|
52.227-3
|
April 1984
|
Patent Indemnity
|
|
52.227-11
|
May 2014
|
Patent Rights - Ownership by the Contractor (Note: In accordance with FAR 27.303(b)(2), paragraph (e) is modified to include the requirements in FAR 27.303(b)(2)(i) through (iv). The frequency of reporting in (i) is annual.)
|
|
52.227-14
|
May 2014
|
Rights in Data – General
|
|
52.227-16
|
Jun 1987
|
Additional Data Requirements
|
|
52.232-9
|
Apr 1984
|
Limitation on Withholding of Payments
|
|
52.232-17
|
May 2014
|
Interest
|
|
52.232-20
|
Apr 1984
|
Limitation of Cost
|
|
52.232-23
|
May 2014
|
Assignment of Claims
|
|
52.232-25
|
Jun 2013
|
Prompt Payment Alternate I (Feb 2002)
|
|
52.232-33
|
Jul 2013
|
Payment by Electronic Funds Transfer--System for Award Management
|
|
52.233-1
|
Jul 2002
|
Disputes
|
|
52.233-3
|
Aug 1996
|
Protest After Award, Alternate I (June 1985)
|
|
52.233-4
|
Oct 2004
|
Applicable Law for Breach of Contract Claim
|
|
52.242-1
|
Apr 1984
|
Notice of Intent to Disallow Costs
|
|
52.242-3
|
May 2014
|
Penalties for Unallowable Costs
|
|
2.242-4
|
Jan 1997
|
Certification of Final Indirect Costs
|
|
52.242-13
|
Jul 1995
|
Bankruptcy
|
|
52.243-2
|
Aug 1987
|
Changes - Cost Reimbursement, Alternate V (Apr 1984)
|
|
52.244-2
|
Oct 2010
|
Subcontracts, Alternate I (Jun 2007)
|
|
52.244-5
|
Dec 1996
|
Competition in Subcontracting
|
|
52.244-6
|
Oct 2014
|
Subcontracts for Commercial Items
|
|
52.245-1
|
Apr 2012
|
Government Property
|
|
52.245-9
|
Apr 2012
|
Use and Charges
|
|
52.246-9
|
May 2001
|
Inspection of Research and Development (Short Form)
|
Page 49
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
52.246-23
|
Feb 1997
|
Limitation of Liability
|
|
52.246-25
|
Feb 1997
|
Limitation of Liability – Services
|
|
52.247-63
|
Jun 2003
|
Preference for U.S.-Flag Air Carriers
|
|
52.247-67
|
Feb 2006
|
Submission of Transportation Documents for Audit
|
|
52.249-6
|
May 2004
|
Termination (Cost-Reimbursement)
|
|
52-249-14
|
Apr 1984
|
Excusable Delays
|
|
52.251-1
|
Apr 2012
|
Government Supply Sources
|
|
52.253-1
|
Jan 1991
|
Computer Generated Forms
|
|
(2)
|
DEPARTMENT OF HEALTH AND HUMAN SERVICES ACQUISITION REGULATION (HHSAR) (48 CFR CHAPTER 3) CLAUSES:
|
|
HHSAR CLA USE NO.
|
DATE
|
TITLE
|
|
352.201-70
|
Jan 2006
|
Paperwork Reduction Act
|
|
352.202-1
|
Jan 2006
|
Definitions, with Alternate paragraph (h)
|
|
352.203-70
|
Mar 2012
|
Anti-Lobbying
|
|
352.216-70
|
Jan 2006
|
Additional Cost Principles
|
|
352.222-70
|
Jan 2010
|
Contractor Cooperation in Equal Employment Opportunity Investigations
|
|
352.223-70
|
Jan 2006
|
Safety and Health
|
|
352.224-70
|
Jan 2006
|
Privacy Act
|
|
352.227-70
|
Jan 2006
|
Publications and Publicity
|
|
352.228-7
|
Dec 1991
|
Insurance - Liability to Third Persons
|
|
352.231-70
|
Aug 2012
|
Salary Rate Limitation
|
|
352.231-71
|
Jan 2001
|
Pricing of Adjustments
|
|
352.233-71
|
Jan 2006
|
Litigation and Claims
|
|
352.242-70
|
Jan 2006
|
Key Personnel
|
|
352.242-73
|
Jan 2006
|
Withholding of Contract Payments
|
|
352.242-74
|
Apr 1984
|
Final Decisions on Audit Findings
|
|
352.270-4
|
Jan 2006
|
Protection of Human Subjects
|
Page 50
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ARTICLE I.2. ADDITIONAL CONTRACT CLAUSES
|
|
This contract incorporates the following clauses by reference, with the same force and effect, as if they were given in full text. Upon request, the Contracting Officer will make their full text available.
|
|
a.
|
FEDERAL ACQUISITION REGULATION (FAR) (48 CFR CHAPTER 1) CLAUSES
|
ARTICLE I.3. ADDITIONAL FAR CLAUSES INCLUDED IN FULL TEXT
FAR 52.217-9 Option to Extend the Term of the Contract
OPTION TO EXTEND THE TERM OF THE CONTRACT (MAR 2000)
(a) The government may extend the term of this contract by written notice to the Contractor within 15 days of the date the contract expires; provided that the government gives the Contractor a preliminary written notice of its intent to extend at least 60 days before the contract expires. The preliminary notice does not commit the government to an extension.
(b) If the government exercises this option, the extended contract shall be considered to include this option clause.
(c) The total duration of this contract, including base contract and the exercise of any options under this clause, shall not exceed twenty-eight (28) months.
FAR 52.219-1 Small Business Program Representations
SMALL BUSINESS PROGRAM REPRESENTATIONS (OCT 2014)
|
(b)
|
(1) The North American Industry Classification System (NAICS) code for this acquisition is 541711.
(2) The small business size standard is 500 employees.
(3) The small business size standard for a concern which submits an offer in its own name, other than on a construction or service contract, but which proposes to furnish a product which it did not itself manufacture, is 500 employees.
|
|
(c)
|
Representations.
|
|
(1)
|
The offeror represents as part of its offer that it [X] is, [ ] is not a small business concern.
|
|
(2)
|
The offeror represents as part of its offer that it [ ] is, [X] is not, a small disadvantaged business concern as defined in 13 CFR 124.1002.
|
|
(3)
|
The offeror represents as part of its offer that it [ ] is, [X] is not a woman-owned small business concern.
|
Page 51
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
FAR 52.219-28, Post-Award Small Business Program Representation
POST-AWARD SMALL BUSINESS PROGRAM REPRESENTATION (JUL 2013)
(a) Definitions . As used in this clause--
Long-term contract means a contract of more than five years in duration, including options. However, the term does not include contracts that exceed five years in duration because the period of performance has been extended for a cumulative period not to exceed six months under the clause at 52.217-8, Option to Extend Services, or other appropriate authority.
Small business concern means a concern, including its affiliates, which is independently owned and operated, not dominant in the field of operation in which it is bidding on government contracts, and qualified as a small business under the criteria in 13 CFR part 121 and the size standard in paragraph (c) of this clause. Such a concern is "not dominant in its field of operation" when it does not exercise a controlling or major influence on a national basis in a kind of business activity in which a number of business concerns are primarily engaged. In determining whether dominance exists, consideration shall be given to all appropriate factors, including volume of business, number of employees, financial resources, competitive status or position, ownership or control of materials, processes, patents, license agreements, facilities, sales territory, and nature of business activity.
(b) If the Contractor represented that it was a small business concern prior to award of this contract, the Contractor shall represent its size status according to paragraph (e) of this clause or, if applicable, paragraph (g) of this clause, upon the occurrence of any of the following:
(1) Within 30 days after execution of a novation agreement or within 30 days after modification of the contract to include this clause, if the novation agreement was executed prior to inclusion of this clause in the contract.
(2) Within 30 days after a merger or acquisition that does not require a novation or within 30 days after modification of the contract to include this clause, if the merger or acquisition occurred prior to inclusion of this clause in the contract.
(3) For long-term contracts--
(i) Within 60 to 120 days prior to the end of the fifth year of the contract; and
(ii) Within 60 to 120 days prior to the date specified in the contract for exercising any option thereafter.
(c) The Contractor shall represent its size status in accordance with the size standard in effect at the time of this representation that corresponds to the North American Industry Classification System (NAICS) code assigned to this contract. The small business size standard corresponding to this NAICS code can be found
at http://www.sba.gov/contractingopportunities/officials/size/index.html .
(d) The small business size standard for a Contractor providing a product which it does not manufacture itself, for a contract other than a construction or service contract, is 500 employees.
(e) Except as provided in paragraph (g) of this clause, the Contractor shall make the representation required by paragraph (b) of this clause by validating or updating all its representations in the Online Representations and Certifications Application and its data in the Central Contractor Registration, as necessary, to ensure that they reflect the Contractor's current status. The Contractor shall notify the contracting office in writing within the timeframes specified in paragraph (b) of this clause that the data have been validated or updated, and provide the date of the validation or update.
Page 52
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
(f) If the Contractor represented that it was other than a small business concern prior to award of this contract, the Contractor may, but is not required to, take the actions required by paragraphs (e) or (g) of this clause.
(g) If the Contractor does not have representations and certifications in ORCA, or does not have a representation in ORCA for the NAICS code applicable to this contract, the Contractor is required to complete the following representation and submit it to the contracting office, along with the contract number and the date on which the representation was completed:
The Contractor represents that it [] is, [X] is not a small business concern under NAICS Code 541711 assigned to contract number HHSO100201500007C.
|
|
FAR 52.232-40, Providing Accelerated Payment to Small Business Subcontractors
|
PROVIDING ACCELERATED PAYMENT TO SMALL BUSINESS SUBCONTRACTORS (DEC 2013)
(a) Upon receipt of accelerated payments from the government, the contractor shall make accelerated payments to its small business subcontractors under this contract, to the maximum extent practicable and prior to when such payment is otherwise required under the applicable contract or subcontract after receipt of a proper invoice and all other required documentation from the small business subcontractor.
(b) The acceleration of payments under this clause does not provide any new rights under the Prompt Payment Act.
(c) Include the substance of this clause, including this paragraph (c), in all subcontracts with small business concerns, including subcontracts with small business concerns for the acquisition of commercial items.
Page 53
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
The following documents are attached and incorporated in this contract:
|
1.
|
Statement of Work, dated March 27, 2015 (16 pages).
|
|
2.
|
Milestones and Deliverables Chart (4 pages)
|
|
3.
|
Manufacturing Chart (1 page)
|
|
4.
|
High-level Gantt Chart (2 pages)
|
|
5.
|
Invoice/Financing Request Instructions and Contract Financial Reporting Instructions for Cost-Reimbursement Type Contracts (5 pages).
|
|
6.
|
Financial Report of Individual Project/Contract (1 page)
|
|
7.
|
Instructions for Completing Financial Report of Individual Project/Contract (3 pages)
|
|
8.
|
7 Principles of Earned Value Management Tier 2 System Implementation Intent Guide (26 pages)
|
Page 54
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
The following documents are incorporated by reference in this contract:
|
1.
|
Annual Representations and Certifications completed and located at the System for Award Management website (SAM.gov).
|
|
2.
|
Animal Welfare Assurance – No Animal Studies will be conducted at BioCryst facilities. Animal Welfare Assurance Numbers will be procured from any subcontractors.
|
End of Contract No. HHSO100201500007C
Page 55
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ATTACHMENT 1
Statement of Work HHSO100201500007C
March 27, 2015
BCX4430 NDA Enabling CMC and Non-Clinical Toxicology Studies
PREAMBLE
Independently and not as an agent of the Government, the Contractor shall furnish all the necessary services, qualified personnel, material, equipment, and facilities, not otherwise provided by the Government as needed to perform the Statement of Work submitted in response to the BARDA Broad Agency Announcement (BAA) CBRN-BAA-10-100-SOL-00013.
The Government reserves the right to modify the milestones, progress, schedule, budget, or deliverables to add or delete deliverables, process, or schedules if the need arises. Because of the nature of this research and development (R&D) contract and the complexities inherent in this and prior programs, at designated milestones the Government will evaluate whether work should be redirected, removed, or whether schedule or budget adjustments should be made.
OVERALL OBJECTIVES AND SCOPE
The overall objective of this contract is to advance the development of BCX4430, a novel small molecule nucleoside with broad spectrum antiviral activity being developed for diseases caused by RNA pathogens. BCX4430, an inhibitor of viral RNA – dependent RNA polymerase (RdRp), is the lead compound in our broad- spectrum antiviral program to meet the need for a parenteral, direct-acting antiviral medical countermeasure (MCM) having efficacy across multiple viruses. The scope of work for this contract includes preclinical and manufacturing development activities that fall into the following areas: manufacturing of clinical trial material, manufacturing process improvements and development, non-clinical toxicology studies; and all associated regulatory, quality assurance, management, and administrative activities. The proposed activities take into account the Ebola virus disease (EVD) outbreak in West Africa. The R&D effort will contribute toward an NDA filing for BCX4430. Overall, this Statement of Work (SOW) focuses on:
|
·
|
Drug substance (DS) and drug product (DP) manufacturing process development activities that will be conducted at US based facilities, which will result in the ability to consistently produce high quality, cGMP compliant material and deliver drug supply that could be available for deployment as a medical countermeasure during the Ebola crisis and support future clinical and non-clinical studies
|
|
·
|
Nonclinical development activities to advance the intramuscular (IM) and intravenous (IV) formulation through NDA-enabling toxicology including * * * and * * * studies in the * * *
|
Page 56
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
1 BASE: MANUFACTURE OF CLINICAL TRIAL MATERIAL
Duration: 18 months
Drug Substance and Drug Product cGMP manufacturing by US suppliers to support non-clinical and clinical activities. Drug Substance will be produced following an * * * starting with * * *, which will be used as the GMP starting material to produce BCX4430.
The primary objectives will be to
|
·
|
Produce * * * batches of BCX4430 drug substance following * * *.
|
|
·
|
Conduct drug product process improvements that will be focused on validation of analytical methods, stress testing, stability studies, and process design space optimization
|
|
1.1.
|
Procurement of Starting Materials
|
The contractor shall procure the starting materials to produce * * * of the current manufacturing process of BCX4430. The key starting materials to be procured are * * * and * * *.
|
1.1.1.
|
Procurement of Starting Materials to produce * * * batches of * * *
|
The contractor shall procure * * * and * * * for the manufacture of * * * of * * * to support a * * * campaign (WBS 1.4.1 and 1.4.2) and a * * * batch of BCX4430 (Clinical Trial Material Batch * * * WBS1.7.1)
|
1.2.
|
Further Process Improvements of * * *
|
The contractor shall conduct further process improvements that may be identified focused on improving the existing plant-scale processes following generally the same * * * .
|
1.2.1
|
Conduct Process Improvements
|
The contractor shall conduct the process improvements with existing plant-scale processes.
|
1.2.2
|
Determination that Process is Sufficient to move to Commercial Scale up
|
The contractor shall evaluate the processes developed and provide sufficient information through a deliverable that will enable BARDA to determine that the processes are sufficient to move to commercial scale –up activities.
|
1.3.
|
Manufacture of * * * at * * *
|
The contractor shall produce * * * of * * * will be utilized as the starting material for the manufacture of BCX4430 in accordance with cGMP guidance.
|
1.3.1.
|
Manufacture of * * * of * * * (Batch * * * )
|
The contractor shall produce * * * of * * * using the existing process at * * * .
|
1.3.2.
|
Manufacture of * * * of * * * (Batch * * * )
|
The contractor shall produce * * * of * * * using the existing process at * * * .
|
1.4.
|
Manufacturing Campaign of BCX4430
|
The contractor shall produce * * * of BCX4430 at * * * in compliance with cGMP.
Page 57
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
1.4.1.
|
Manufacturing of * * * of cGMP BCX4430 (DS Batch * * * )
|
The contractor shall produce and release * * * of cGMP BCX4430 * * * .
|
1.4.2.
|
Manufacturing of * * * of cGMP BCX4430 (DS Batch * * * )
|
The contractor shall produce and release * * * of cGMP BCX4430 * * * .
|
1.4.3.
|
Prepare a Campaign Summary Report
|
The contractor shall prepare a campaign summary report of the manufacture and release of DS Batches * * * .
|
1.4.4.
|
Drug Substance Stability Studies
|
The contractor shall place samples from DS Batch * * * of BCX4430 on a * * * stability program at * * * and a
* * * accelerated stability study at * * * .
Table 1. BCX4430 Drug Substance Stability Study Sampling Points
|
Test
ID
|
Months
|
|||||||||
|
0
|
1
|
3
|
6
|
9
|
12
|
18
|
24
|
48
|
60
|
|
|
A
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
|
B
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
|
C
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
|
D
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
|
E
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
|
F
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
||
|
G
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
X
|
||
NOTE: BARDA will only cover stability activities for * * * . Table 2. BCX4430 Drug Substance Stability Tests
|
Test ID
|
Test
|
|
A
|
* * *
|
|
B
|
* * *
|
|
C
|
* * *
|
|
D
|
* * *
|
|
E
|
* * *
|
|
F
|
* * *
|
|
G
|
* * *
|
|
1.5.
|
Drug Product Development
|
The contractor shall conduct drug product process improvements that will be focused on validation of analytical methods, stress testing, stability studies, and process design space optimization.
The contractor shall conduct formulation development activities and produce a sterile, parenteral formulation containing * * * of the active compound per unit in compliance with cGMP guidance. Initial development efforts will be focused on delivering a * * * that tolerates terminal sterilization and provides an acceptable stability profile to support a * * * shelf life. Additionally, studies to include: * * * will be conducted to evaluate the feasibility of * * * .
Page 58
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
1.5.1.
|
Stress Conditions Studies
|
The contractor shall conduct a series of experiments under conditions outlined in ICH guidance to evaluate the stability of the drug product made from available drug substance.
|
1.5.2.
|
Design Space Studies
|
The contractor shall conduct studies to evaluate and define the design space of the formulation process.
|
1.5.3.
|
Analytical Method Validation
|
The contractor shall conduct analytical methods validation or qualification as listed in the table below.
Table 3. BCX4430 Drug Product Methods that will be Validated or Qualified
|
Test
|
Method and Objective
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
* * *
|
* * *
|
|
1.5.4.
|
Prepare Process Development Report for DP
|
The contractor shall prepare a process development report summarizing the experiments and results of the studies conducted to define the process and evaluate the formulation.
|
1.5.5.
|
Pre-formulation and Physicochemical Properties Studies
|
The contractor shall conduct studies to determine the physicochemical properties of the drug substance and identify potential formulations and primary packaging for an IM injection based on stability and ability to be produced on manufacturing lines.
|
1.5.6.
|
Feasibility Runs
|
The contractor shall conduct small-scale, nonGMP manufacturing runs of potential formulations and formats.
|
1.5.7.
|
Extractable/Leachable Study
|
The contractor shall conduct studies to determine drug product stability in primary packaging and of syringe types that will be used for delivery.
|
1.5.8.
|
Excipient Compatibility Studies for IV Formulation from IM
|
The contractor shall conduct studies to evaluate the compatibility of the IM formulation added to various standard IV fluids.
|
1.6.
|
Manufacturing of Drug Product to Support Clinical Trials
|
The contractor shall produce * * * batches of approximately * * * of drug product suitable for clinical trial use. The drug product will produced from the * * * cGMP DS Batches * * * (WBS 1.4.1 and 1.4.2)
Page 59
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
1.6.1.
|
Manufacture of Clinical Trial Material (CTM Batch * * * )
|
The contractor shall produce approximately * * * per cGMP for use in clinical trials.
|
1.6.2.
|
Manufacture of Clinical Trial Material (CTM Batch * * * )
|
The contractor shall produce approximately * * * per cGMP for use in clinical trials.
|
1.6.3.
|
Prepare a Campaign Summary Reports
|
The contractor shall prepare a campaign summary report of the manufacture and release of CTM * * * .
|
1.6.4.
|
Drug Product Stability Studies
|
The contractor shall place drug product on stability based on the following conditions:
Table 4. BCX4430 Drug Product Stability Testing Conditions and Sampling
|
Stability Conditions
|
Configuration
|
Test Points
|
|
* * *
|
* * *
|
* * *
|
|
* * *
|
* * *
|
* * *
|
|
* * *
|
* * *
|
* * *
|
NOTE: BARDA will only cover stability activities for * * *
Table 5. BCX4430 Drug Product Stability Tests
|
Test
|
|
* * *
|
|
* * *
|
|
* * *
|
|
* * *
|
|
* * *
|
|
* * *
|
|
* * *
|
|
* * *
|
|
1.6.5.
|
Comparability Study
|
The contractor shall conduct comparability studies evaluating drug substance produced by * * * and * * * , and the subsequently produced drug product, will be conduct per FDA Guidance. These studies will evaluate: in- process checks, impurity profiles, release testing results, and stability profiles at standard and accelerated conditions to ensure the material produced by both manufacturers is substantially comparable.
Page 60
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
1.7.
|
Manufacture of Additional Supply
|
That contractor shall produce * * * of BCX4430 based on * * * facility and subsequently produce * * * of drug product at * * * suitable for clinical trial use.
|
1.7.1.
|
Manufacturing of * * * cGMP BCX4430 (DS Batch * * * )
|
The contractor shall produce * * * of cGMP BCX4430 from * * * .
|
1.7.2.
|
Prepare a Campaign Summary Report
|
The contractor shall prepare a campaign summary report of the manufacture and release of * * * .
|
1.7.3.
|
Manufacturing of approximately * * * (CTM Batch * * * )
|
The contractor shall produce approximately * * * of cGMP BCX4430 drug product using drug substance from the * * * cGMP BCX4430 (DS Batch * * * , WBS 1.7.1).
|
1.7.4.
|
Prepare a Campaign Summary Report
|
The contractor shall prepare a campaign summary report of the manufacture and release of CTM Batches * * *
|
1.7.5.
|
Stability Studies for DS and DP
|
The contractor shall conduct drug substance and drug product stabilities for the DS and DP manufactured in from the * * * of cGMP BCX4430 drug substance based on * * * (WBS 1.6).
NOTE: BARDA will only cover stability activities for * * *
|
1.7.6.
|
Comparability Studies
|
The contractor shall conduct comparability studies evaluating drug substance produced by * * * and both process at * * * , and the subsequently produced drug product, will be conduct per FDA Guidance. These studies will evaluate: in-process checks, impurity profiles, release testing results, and stability profiles at standard and accelerated conditions to ensure the material produced by both manufacturers and both * * * process are substantially comparable.
Page 61
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
2
|
OPTION 1: COMMERCIAL SCALE UP AND NDA REGISTRATION BATCHES
|
Duration: * * *
Go/No Go Criteria to Initiate: WBS 1.2.2 BARDA approval of process developed
Through optimization of manufacturing processes, BARDA will evaluate and determine what process should be sufficient to initiate commercial scale up activities in this Option. This Option will add value to the project through conducting manufacturing regulatory activities that will be needed for future product approval with the FDA.
Decision Criteria:
· * * *
· * * *
The objective is to produce DS registration batches from the qualified process, * * * . This will be determined upon the conclusion of the DS development effort being undertaken by BioCryst and funded by NIAID that is scheduled to conclude in December 2015. Additionally during this stage, the DP manufacturing process would be finalized for the commercial presentation and registration batches produced.
|
2.1.
|
Procurement
|
The contractor shall procure the * * * starting materials needed to produce BCX4430.
|
2.1.1.
|
Procurement of * * *
|
The contractor shall qualify a vendor(s) to produce the * * * and procure enough material to support the manufacture of * * * batches of BCX4430 drug substance.
|
2.1.2.
|
Procurement of * * *
|
The contractor shall qualify a vendor(s) to produce the * * * and procure enough material to support the manufacture of * * * batches of BCX4430 drug substance.
|
2.2.
|
Drug Substance Process Scale-up
|
The contractor shall conduct process development work targeting a * * * that can be scaled to plant equipment. This work will include: development of a * * * through lab scale studies, process hazard evaluation including RC-1 and digital scanning calorimetry, lab scale qualification runs, pilot plant scale-up technical batches, necessary modifications to analytical methods based on the * * * , and plant-scale registration runs.
|
2.2.1
|
Further Process Improvements of Final Route
|
The contractor shall conduct further process improvements that may be identified focused on improving the * *
|
*
|
to be scaled up. The * * * will be based either * * * .
|
|
2.2.2
|
Process Hazard Evaluation
|
The contractor shall conduct process hazard evaluation studies needed for the scale-up of the optimized process into the plant.
|
2.2.3
|
Scale-up Technical Run
|
The contractor shall conduct a nonGMP manufacturing run at plant-scale to ensure the safety and output of the optimized process.
Page 62
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
2.2.4
|
Analytical Method Development and Qualification
|
The contractor shall modify, add to, revalidate, or requalify the analytical methods.
|
2.2.5
|
Prepare Process Development Report
|
The contractor shall prepare a process development report describing the experiments and results leading to the selection of the optimized manufacturing process
|
2.3
|
Manufacture of cGMP Drug Substance Registration Batches
|
The contractor shall produce * * * cGMP batches of BCX4430 following the * * * at a scale comparable to the estimated commercial scale.
|
2.3.1
|
Manufacture of DS Batch (DS Batch * * * )
|
The contractor shall manufacture a batch of cGMP BCX4430 at plant scale.
|
2.3.2
|
Manufacture of DS Batch (DS Batch * * * )
|
The contractor shall manufacture a batch of cGMP BCX4430 at plant scale.
|
2.3.3
|
Manufacture of DS Batch (DS Batch * * * )
|
The contractor shall manufacture a batch of cGMP BCX4430 at plant scale.
|
2.3.4
|
Prepare Campaign Summary Report
|
The contractor shall prepare a report summarizing the conduct, observations, and results of the manufacturing of DS Batches * * * .
|
2.4
|
Drug Product Registration Batches
|
The contractor shall produce * * * drug product lots at * * * that will be used as the NDA registration batches.
|
2.4.1
|
Manufacture of DP Registration (DPR Batch * * * )
|
The contractor shall manufacture a NDA registration batch of cGMP BCX4430 drug product at a scale at least
|
*
|
* * the estimated commercial scale.
|
|
2.4.2
|
Manufacture of DP Registration (DPR Batch * * * )
|
The contractor shall manufacture a NDA registration batch of cGMP BCX4430 drug product at a scale at least
|
*
|
* * the estimated commercial scale.
|
|
2.4.3
|
Manufacture of DP Registration (DPR Batch * * * )
|
The contractor shall manufacture a NDA registration batch of cGMP BCX4430 drug product at a scale at least
|
*
|
* * the estimated commercial scale.
|
|
2.4.4
|
Prepare campaign summary report
|
The contractor shall prepare a report summarizing the conduct, observations, and results of the manufacturing of DPR Batches * * * .
Page 63
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
2.5
|
Stability studies for DS and DP
|
The contractor shall conduct drug substance and drug product stabilities as described in Table 2 and Table 3 .
NOTE: BARDA will only cover stability activities for * * *
|
2.6
|
Comparability study
|
The contractor shall conduct comparability studies evaluating drug substance produced for clinical trials in the base period versus the NDA registration batches manufactured via * * * in Option 1, and the subsequently produced drug product, will be conduct per FDA Guidance. These studies will evaluate: in-process checks, impurity profiles, release testing results, and stability profiles at standard and accelerated conditions to ensure the material produced by both processes is substantially comparable.
Page 64
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
3
|
OPTION 2: NONCLINICAL NDA-ENABLING TOXICOLOGY - IM
|
Duration: * * *
Go/No Go Criteria to Initiate: WBS 1.4.1 Completion of Manufacture cGMP BCX4430 ( * * * campaign DS Batch * * * )
Through completion of manufacturing of this Drug substance Batch * * * , then there will be material to conduct further regulatory activities such as the IM non-clinical NDA-enabling toxicology studies in this option. This Option will add value to the project through conducting additional non-clinical activities that will support a potential future NDA.
Decision Criterion:
· * * *
· * * *
The contractor shall perform nonclinical GLP studies of IM BCX4430 to characterize * * *
|
3.1.
|
GLP * * * Toxicology – IM
|
The contractor shall for each toxicology study develop the protocol, select and qualify the vendor, conduct in life and recovery phases and analyze study data resulting in a final study report.
Studies include:
|
3.1.1.
|
Conduct GLP * * * IM toxicology study - * * *
|
|
3.1.2.
|
Conduct GLP * * * IM general toxicology study - * * *
|
|
3.2.
|
* * * toxicology - IM
|
The contractor shall for each * * * study segment develop the protocol, select and qualify the vendor, conduct in life and recovery phases and analyze study data resulting in a final study report.
Studies include:
|
3.2.1.
|
Conduct * * * assessment in * * *
|
|
3.2.2.
|
Conduct * * * Dose Range Finding Studies in the * * *
|
|
3.2.3.
|
Conduct Definitive * * * toxicology in the * * *
|
|
3.2.4.
|
Conduct * * * toxicology * * *
|
|
3.3.
|
Nonclinical ADME - IM
|
The contractor shall procure radiolabeled BCX4430. In addition for each ADME study, the contractor shall develop the protocol, select and qualify the vendor, conduct the study and analyze study data resulting in a final study report. Studies include:
Page 65
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
3.3.1.
|
Conduct Radiolabeled ADME study - * * *
|
|
3.3.2.
|
Conduct Radiolabeled ADME – * * *
|
A listing of the proposed studies for the nonclinical NDA-enabling toxicology studies for the IM formulation is provided.
Table 6 Nonclinical NDA-enabling Toxicology Studies for an IM formulation
|
Study #
|
Description
|
Objective(s)
|
Species (N)
|
|
GLP * * * IM general toxicology
|
* * *
|
* * *
|
|
|
GLP * * * IM general toxicology
|
* * *
|
* * *
|
|
|
* * * assessment
|
* * *
|
* * *
|
|
|
* * * Dose Range Finding
|
* * *
|
* * *
|
|
|
* * * Dose Range Finding
|
* * *
|
* * *
|
|
|
Definitive * * *
toxicology
|
* * *
|
* * *
|
|
|
Definitive * * *
toxicology
|
* * *
|
* * *
|
|
|
* * * toxicology
|
* * *
|
* * *
|
|
|
Radiolabeled ADME
|
Determine the absorption, distribution, metabolism and excretion of the test article following IM dosing
|
* * *
|
|
|
Radiolabeled ADME
|
Determine the absorption, metabolism and excretion of the test article following IM dosing
|
* * *
|
Page 66
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
4
|
OPTION 3: IN VITRO EXPERIMENTS – IV
|
Duration: * * *
Go/No Go to Initiate: WBS 1.5.8 Completion of Excipient compatibility studies for IV formulation Through completion of IV formulation studies, it will be determined what excipients are appropriate for IV
formulation to then continue with toxicology studies of the IV formulation under this Option. This Option will add value to determine if there is any identified toxicology in in vitro assays before moving to animal studies in Option 4.
Decision Criterion:
· * * *
4.1. * * * – IV
The contractor shall develop the protocol, select and qualify the vendor, conduct the experiments and analyze the data resulting in a study report.
4.2. Conduct * * * Test – IV
The contractor shall develop the protocol, select and qualify the vendor, conduct the experiment and analyze the data resulting in a study report.
A listing of the proposed experiments for the in vitro experiments to be conducted in advance of the nonclinical NDA-enabling toxicology studies for the IV formulation is provided Table 7 .
Table 7 In vitro Experiments - IV
|
Study #
|
Description
|
Objective(s)
|
Species (N)
|
|
* * *
|
* * *
|
* * *
|
|
|
* * *
|
* * *
|
* * *
|
4.3 Study Report on all in vitro assays
The contractor shall submit a summarized study report with data and conclusions from all in vitro experiments conducted in table 7 to determine whether there is any toxicology before advancing into non-clinical NDA enabling toxicology studies (Option 5).
Page 67
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
5
|
OPTION 4: NONCLINICAL NDA-ENABLING TOXICOLOGY - IV
|
Duration: * * *
Go/No Go Criteria to Initiate: WBS 4.3 Study Report on all in vitro assays
Through completion of the IV in vitro toxicology studies with the IV formulation conducted in Option 3 and summarized in WBS 4.3, it will be determined if the IV formulation is safe to move into non-clinical toxicology animal studies in this Option. This Option will add value to the project through conducting additional non- clinical activities that will support a potential future NDA.
Decision Criterion:
· * * *
· * * *
|
5.1.
|
GLP * * * Toxicology – IV
|
The contractor shall for each toxicology study develop the protocol, select and qualify the vendor, conduct in life and recovery phases and analyze study data resulting in a final study report. Studies include:
|
5.1.1.
|
Conduct GLP * * * IV general toxicology study - * * *
|
|
5.1.2.
|
Conduct GLP * * * IV toxicology study - * * *
|
|
5.2.
|
* * * toxicology - IV
|
The contractor shall for each *** study segment develop the protocol, select and qualify the vendor, conduct in life and recovery phases and analyze study data resulting in a final study report. Studies include:
|
5.2.1.
|
Conduct * * * assessment in * * *
|
|
5.2.2.
|
Conduct * * * Dose Range Finding Studies in the * * *
|
|
5.2.3.
|
Conduct Definitive * * * toxicology in the * * *
|
|
5.2.4.
|
Conduct * * * Developmental toxicology * * *
|
A listing of the proposed studies for the nonclinical NDA-enabling toxicology studies for the IV formulation is provided in Table 8
Table 8. Nonclinical NDA-enabling Toxicology Studies for an IV Formulation
|
Study #
|
Description
|
Objective(s)
|
Species (N)
|
|
GLP * * * IV general toxicology
|
* * *
|
* * *
|
|
|
GLP * * * IV general toxicology
|
* * *
|
* * *
|
|
|
* * * assessment
|
* * *
|
* * *
|
|
|
* * * Dose Range Finding
|
* * *
|
* * *
|
|
|
* * * Dose Range Finding
|
* * *
|
* * *
|
Page 68
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
Definitive * * *
toxicology
|
* * *
|
* * *
|
|
|
Definitive * * *
toxicology
|
* * *
|
* * *
|
|
|
* * * Developmental toxicology
|
* * *
|
* * *
|
Page 69
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
6
|
PROGRAM MANAGEMENT
|
The contractor shall provide all expertise needed for the implementation of the activities to be performed under this contract, including: research, manufacturing, regulatory, clinical, statistical analyses, management and administrative activities.
|
6.1.
|
Technical and Project Management Support
|
The contractor shall appoint a Principal Investigator (PI) who will be responsible for all aspects of project performance and communication with the BARDA.
The contractor shall provide project management that will ensure day-to-day monitoring and tracking of progress and timelines, the coordination of project activities and costs incurred.
The contractor shall provide all managerial and administrative functions necessary for overall planning, monitoring, and implementing activities for the completion of the strategic product development plan.
The contractor shall provide for all necessary legal affairs required to ensure the timely acquisition of all proprietary rights, including intellectual property rights and all materials needed to perform the project, as well as reporting to the Government all inventions made in the performance of the project.
|
6.2.
|
Subcontractor Management
|
The contractor shall provide for tracking, coordination and oversight of subcontractor efforts and manage communications with subcontractors.
|
6.3.
|
Risk Management
|
The contractor shall identify project risks, develop risk management strategies and implement mitigation actions.
|
6.4.
|
Earned Value Management (EVM)
|
The contractor shall provide EVM information.
|
6.5.
|
Project Communications
|
The contractor shall provide for project communications including communications with BARDA and external experts.
The contractor shall provide planning and steps required for the conduct of contract review meetings.
Page 70
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
7
|
REGULATORY
|
The contractor shall ensure adherence to FDA regulations and guidance, including requirements for the conduct of animal studies and assays under GLP, the manufacturing of the therapeutic product under cGMP, and the conduct of clinical trials under GCP standards.
|
7.1.
|
Regulatory Authority Interactions
|
The contractor shall prepare and submit documentation and correspondence to regulatory authorities as required. The contractor shall request and conduct meetings with regulatory authorities to ensure the development program is conducted in accordance with regulatory guidelines and expectations.
|
7.2.
|
Quality Assurance
|
The contractor shall maintain quality assurance documentation. The contractor shall arrange for audits of subcontractor facilities to ensure all planned procedures comply with the FDA regulations and guidance that are required to meet GLP, cGMP and GCP standards. In addition, the contractor shall ensure that all contractor and/or subcontractor records and staff are available for site visits or audits.
|
7.3.
|
Expert Collaborations
|
The contractor shall collaborate with experts in the field in the design of experiments and studies that support the advancement of the development program.
Page 71
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ATTACHMENT 2
MILESTONE AND DELIVERABLES CHART MARCH 27, 2015
HHSO100201500007C
|
WBS
|
Milestone
|
Deliverable
|
Success Criteria
|
Timing
|
Go/No-Go for initiation
|
|
|
CLIN 0001 - MANUFACTURE OF CLINICAL TRIAL MATERIAL
|
||||||
|
1.2.1
|
Process Improvements Report
|
Report on Process
Development
|
Process Developed
|
* * *
|
||
|
1.2.2
|
Determination of Sufficient Process for Commercial Scale up
|
Evaluation report
|
BARDA
approval of developed process
|
* * *
|
N/A
|
|
|
1.3.2
|
Manufacture * * * (Batch * * *)
|
* * *
|
Acceptable quality and yield
|
* * *
|
N/A
|
|
|
1.4.1
|
Manufacture cGMP BCX4430 (* * *
campaign DS Batch *
* *)
|
BCX4430 DS
CofA,
|
Acceptable quality and yield
|
* * *
|
N/A
|
|
|
1.4.2
|
Manufacture cGMP BCX4430 (* * *
campaign DS Batch *
* *)
|
BCX4430 DS
CofA,
|
Acceptable quality and yield
|
* * *
|
N/A
|
|
|
1.4.3
|
Prepare a Campaign Summary Reports
|
Campaign Reports (DS Batches * * *)
|
Completion of DS Campaigns
|
* * *
|
N/A
|
|
|
1.4.4
|
Drug substance stability study
|
Initial Report on stability activities
|
Stability data
|
* * *
|
||
|
1.5
|
Drug Product Development
|
DP Process Development
Report (WBS 1.5.4)
Pre-formulation and
Physicochemical Report (WBS1.5.5)
Extractable/Leachable Report (WBS 1.5.7)
|
Completion of Studies
|
* * *
|
N/A
|
|
|
1.5.8
|
Excipient Compatibility Report for IV Formulation
|
Compatibility Report
|
IV formulation completed
|
* * *
|
||
|
1.6.1
|
Manufacture cGMP DP (CTM Batch * * *)
|
BCX4430 DP
CofA,
|
Acceptable quality and yield
|
* * *
|
Accepted GMP DS
|
|
|
1.6.2
|
Manufacture cGMP DP (CTM Batch * * *)
|
BCX4430 DP
CofA,
|
Acceptable quality and yield
|
* * *
|
Accepted GMP DS
|
|
|
1.6.3
|
Prepare a Campaign Summary Reports
|
Campaign Reports (CTM Batches * * *)
|
Completion of DP Campaigns
|
* * *
|
N/A
|
|
Page 72
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
1.6.4
|
Drug Product stability study
|
Initial Report on stability activities
|
Stability Data
|
* * *
|
||
|
1.6.5
|
Comparability Study
|
Comparability Protocol and Report
|
Completion of DS and DP Campaigns
|
* * *
|
||
|
1.7.1
|
Manufacture cGMP BCX4340 (* * *
campaign DS Batch *
* * )
|
BCX4430 DS
CofA
|
Acceptable DS process
|
* * *
|
N/A
|
|
|
1.7.2
|
Prepare a Campaign Summary Report
|
Campaign Reports (DS Batch* * *)
|
Completion of DS Campaign
|
* * *
|
N/A
|
|
|
1.7.2
|
Manufacture cGMP DP (CTM Batch * * *)
|
BCX4430 DP
CofA,
|
Acceptable quality and yield
|
* * *
|
Accepted GMP DS
|
|
|
1.7.4
|
Prepare a Campaign Summary Report
|
Campaign Report (CTM Batches * * *)
|
Completion of DS Campaigns
|
* * *
|
N/A
|
|
|
1.7.5
|
Drug Substance and Drug Product stability study
|
Initial report on stability activities
|
Stability Data
|
* * *
|
Manufacture of 1.7.1 drug substance and 1.7.2 drug product
|
|
|
1.7.6
|
Comparability Study
|
Comparability Protocol and Report
|
Comparable DS and DP profiles
|
* * *
|
N/A
|
|
|
CLIN 0002 – COMMERCIAL SCALE UP AND NDA REGISTRATION BATCHES
Go/No Go Criteria to Initiate: WBS 1.2.2 BARDA approval of process developed
|
||||||
|
2.2
|
Drug Substance Process Scale-up
|
Process Development Report (WBS 2.2.4)
|
Selection of the optimized manufacturing process
|
* * *
|
* * * process
|
|
|
2.3.1
|
Manufacture BCX4340 DS (DS Registration Batch * * *)
|
BCX4430 Registration DS
CofA
|
Acceptable quality and yield
|
* * *
|
* * * process
|
|
|
2.3.2
|
Manufacture BCX4340 DS (DS Registration Batch * * *)
|
BCX4430 Registration DS
CofA,
|
Acceptable quality and yield
|
* * *
|
* * * process
|
|
|
2.3.3
|
Manufacture BCX4340 DS (DS Registration Batch * * *)
|
BCX4430 Registration DS
CofA,
|
Acceptable quality and yield
|
* * *
|
* * * process
|
|
|
2.3.4
|
Prepare a Campaign Summary Report
|
Campaign Reports (DS Batches * * *)
|
Completion of DS Campaign
|
* * *
|
N/A
|
|
|
2.4.1
|
Manufacture BCX4430 DP (DP Registration Batch * * *)
|
BCX4430 DP
CofA,
|
Acceptable quality and yield
|
* * *
|
Accepted GMP DS
|
|
|
2.4.2
|
Manufacture BCX4430 DP (DP Registration Batch * * *)
|
BCX4430 DP
CofA,
|
Acceptable quality and yield
|
* * *
|
Accepted GMP DS
|
|
Page 73
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
2.4.3
|
Manufacture BCX4430 DP (DP Registration Batch * * *)
|
BCX4430 DP
CofA,
|
Acceptable quality and yield
|
* * *
|
Accepted GMP DS
|
|
2.4.4
|
Prepare a Campaign Summary Report
|
Campaign Report (CTM Registration Batches * *
*)
|
Completion of DS Campaigns
|
* * *
|
N/A
|
|
2.5
|
Drug substance and Drug Product stability study
|
Report on stability activities
|
Stability Data
|
* * *
|
|
|
2.6
|
Comparability Study
|
Comparability Protocol and Report
|
Comparable DS and DP profiles
|
* * *
|
Accepted GMP DS
|
|
CLIN 0003 – NONCLINICAL NDA-ENABLING TOXICOLOGY - IM
Go/No Go Criteria to Initiate: WBS 1.4.1 Completion of Manufacture cGMP BCX4430 (* * *campaign DS Batch *
* *)
|
|||||
|
3.1.1
|
Complete GLP * * * IM Tox Study - * * *
|
Study Report
|
Established NOAEL
|
* * *
|
Drug Substance confirming to release criteria
|
|
3.1.2
|
Complete GLP * * * IM Tox Study - * * *
|
Study Report
|
Established NOAEL
|
* * *
|
Drug Substance confirming to release criteria
|
|
3.2.1
|
Conduct * * * assessment in * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
3.2.2
|
Conduct * * * Dose Range Finding Studies in the * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
3.2.3
|
Conduct Definitive * * * toxicology in the * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
3.2.4
|
Conduct * * * toxicology * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
3.3.1
|
Conduct Radiolabeled ADME study - * * *
|
Study Report
|
Characterize drug disposition
|
* * *
|
Acceptable Radiolabel Material
|
|
3.3.2
|
Conduct Radiolabeled ADME – * * *
|
Study Report
|
Characterize drug disposition
|
* * *
|
Acceptable Radiolabel Material
|
|
CLIN 0004 – IN VITRO EXPERIMENTS – IV
Go/No Go to Initiate: WBS 1.5.8 Completion of Excipient compatibility studies for IV formulation
|
|||||
|
4.1.
|
Conduct * * * Test – IV
|
Study Report
|
No effect on *
* *
|
* * *
|
IV
formulation WBS 1.5.8
|
|
4.2.
|
Conduct * * * Test – IV
|
Study Report
|
No effect on *
* *
|
* * *
|
N/A
|
|
4.3
|
* * * IV experiments
|
Study report on all * *
*assays with recommendation to proceed CLIN0005
|
No toxicology *
* *
|
* * *
|
|
|
CLIN 0005 – NONCLINICAL NDA-ENABLING TOXICOLOGY – IV
Go/No Go to Initiate: WBS 4.3 Completion of * * * IV toxicology studies
|
|||||
Page 74
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
5.1.1
|
Complete GLP * * *IV Tox Study - * * *
|
Study Report
|
Established NOAEL
|
* * *
|
Drug Substance confirming to release criteria
|
|
5.1.2
|
Complete GLP * * * IV Tox Study - * * *
|
Study Report
|
Established NOAEL
|
* * *
|
Drug Substance confirming to release criteria
|
|
5.2.1
|
Conduct * * * assessment in * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
5.2.2
|
Conduct * * * Dose Range Finding Studies in the * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
5.2.3
|
Conduct Definitive * * * toxicology in the * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
|
5.2.4
|
Conduct * * * toxicology * * *
|
Study Report
|
No significant findings
|
* * *
|
N/A
|
Page 75
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ATTACHMENT 3
BCX4430 MANUFACTURING SUMMARY MARCH 2 , 2015
|
Starting Material
|
DS
Campaign
|
CMO
|
DS Process Desc
|
DS
Timing
|
DP
Campaign
|
DP
Timing
|
Use
|
Funding
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|||
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
||
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
||
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
||
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
* * *
|
||
|
* * *
|
||||||||
Page 76
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ATTACHMENT 4
HIGH-LEVEL GANTT CHART MARCH 27, 2015
* * *
All activities covered under this BioCryst contract will provide merit and value to the Government by ensuring that funding supported under the Base period results in progress towards several activities to ensure moving the BioCryst BCX4430 compound through the development pipeline for future FDA approval. This activities include manufacturing clinical trial material to be used in clinical trials as an urgent need to the Ebola epidemic in west Africa (Base), commercial scale up and manufacturing of registration batches, a regulatory approval requirement (Option 1), non-clinical toxicology studies of the IM formulation to support NDA regulatory requirements (Option 2) and non-clinical toxicology studies of the IV formulation first in vitro (Option 3) and then in animals (Option 4) to support NDA regulatory requirements. BioCryst’s ability to complete three critical GO/NO GO contract milestones in the Base period will determine whether to exercise any follow-on option phases to continue supporting development of the compound.
There are 4 decision points which will trigger advancement to Options.
|
1.
|
Trigger 1 (for start of Option 1): Further optimization processes for manufacturing will be developed under the WBS 1.2.1 and then BioCryst will evaluate the processes developed and provide sufficient information through deliverable WBS 1.2.2 that will enable BARDA to determine that the processes are sufficient to move to commercial scale –up activities (milestone WBS1.2.2, July 2015), and then Option 1 can begin. In Option 1, the process developed in WBS 1.2 and other processes being developed to manufacture the BCX4430 compound will be evaluated, such that BioCryst can select a process of most value to the Government to generate * * * drug substance registration batches that will then be used to make * * * drug product registration batches at a scale at least * * * the estimated commercial scale which is a regulatory requirement for qualifying a GMP process for manufacturing.
|
|
2.
|
Trigger 2 (for start of Option 3): In preparation for manufacturing clinical trial (drug product)
|
Page 77
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
material from the drug substance manufactured in the Base, the contractor will conduct formulation activities for both Intramuscular (IM) and intravenous (IV) administration. These formulations will then be applied to the generation of drug product (clinical trial material) in the remainder of the base. However, once an IV formulation is determined as compatible (WBS 1.5.8, July 2015) then preliminary toxicology studies in vitro of this formulation can be initiated in Option 3 (in Vitro experiments- IV) before moving in to animal toxicology studies (Option 4).
|
3.
|
Trigger 3 (for start of Option 2): In order to conduct further regulatory activities to support NDA activities, toxicology experiments will need to be conducted with the IM formulation in
|
animals. However, drug product is needed for these studies. As soon as some of the drug substance material is manufactured in the base period (WBS 1.4.1,January 2016), then some of this material can be utilized for conducting these non-clinical animal toxicology studies in Option 2, a NDA regulatory requirement.
|
4.
|
Trigger 4 (for start of Option 4): In order to proceed with conducting further regulatory activities to support NDA activities for the IV formulation, toxicology experiments will need to be conducted in animals. However, this can only proceed if the preliminary toxicology studies performed in vitro, as evaluated in the in vitro study report (WBS 4.3, March 2016), demonstrate that the IV formulation is safe.
|
Page 78
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ATTACHMENT 5
INVOICE/FINANCING REQUEST AND CONTRACT FINANCIAL
REPORTING INSTRUCTIONS FOR COST-REIMBURSEMENT TYPE CONTRACTS
Format: Payment requests shall be submitted on the Contractor’s self-generated form in the manner and format prescribed herein and as illustrated in the Sample Invoice/Financing Request. Standard Form 1034, Public Voucher for Purchases and Services Other Than Personal, may be used in lieu of the Contractor’s self-generated form provided it contains all of the information shown on the Sample Invoice/Financing Request. DO NOT include a cover letter with the payment request.
Number of Copies: Payment requests shall be submitted in the quantity specified in the Invoice Submission Instructions in Section B of the Contract.
Frequency: Payment requests should not be submitted more frequently than once every two weeks in accordance with the Allowable Cost and Payment Clause incorporated into this contract unless otherwise instructed by the Contract Officer. Small business concerns may submit invoices/financing requests more frequently than every two weeks when authorized by the Contracting Officer.
Cost Incurrence Period: Costs incurred must be within the contract performance period or covered by previously established pre contract cost provisions.
Billing of Costs Incurred: If billed costs include (1) costs of a prior billing period, but not previously billed, or (2) costs incurred during the contract period and claimed after the contract period has expired, the Contractor shall cite the amount(s) and month(s) in which it incurred such costs.
Contractor's Fiscal Year: Payment requests shall be prepared in such a manner that the Government can identify costs claimed with the Contractor's fiscal year.
Currency: All contracts are expressed in United States dollars. When the Government pays in a currency other than United States dollars, billings shall be expressed, and payment by the Government shall be made, in that other currency at amounts coincident with actual costs incurred. Currency fluctuations may not be a basis of gain or loss to the Contractor. Notwithstanding the above, the total of all invoices paid under this contract may not exceed the United States dollars authorized.
Costs Requiring Prior Approval: Costs requiring the Contracting Officer's approval, which are not set forth in an Advance Understanding in the contract, shall be identified and reference the Contracting Officer's Authorization (COA) Number. In addition, the Contractor shall show any cost set forth in an Advance Understanding as a separate line item on the payment request.
Invoice/Financing Request Identification: Each payment request shall be identified as either:
|
(a)
|
Interim Invoice/Contract Financing Request: These are interim payment requests submitted during the contract performance period.
|
|
(b)
|
Completion Invoice: The completion invoice shall be submitted promptly upon completion of the work, but no later than one year from the contract completion date, or within 120 days after settlement of the final indirect cost rates covering the year in which the contract is physically complete (whichever date is later). The Contractor shall submit the completion invoice when all costs have been assigned to the contract and it completes all performance provisions.
|
|
(c)
|
Final Invoice: A final invoice may be required after the amounts owed have been settled between the Government and the Contractor (e.g., resolution of all suspensions and audit exceptions).
|
Preparation and Itemization of the Invoice/Financing Request: The Contractor shall furnish the information set forth in the instructions below. The instructions are keyed to the entries on the Sample Invoice/Financing Request.
|
(a)
|
Designated Billing Office Name and Address: Enter the designated billing office name and address, as identified in the Invoice Submission Instructions in Section B and F of the Contract Schedule.
|
|
(b)
|
Contractor's Name, Address, Point of Contact, VIN, and DUNS or DUNS+4 Number: Show the Contractor's name and address exactly as they appear in the contract, along with the name, title, phone number, and e-mail address of the person to notify in the event of an improper invoice or, in the case of payment by method other than Electronic Funds
|
Page 79
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Transfer, to whom payment is to be sent. Provide the Contractor’s Vendor Identification Number (VIN), and Data Universal Numbering System (DUNS) number or DUNS+4. The DUNS number must identify the Contractor’s name and address exactly as stated on the face page of the contract. When an approved assignment has been made by the Contractor, or a different payee has been designated, provide the same information for the payee as is required for the Contractor (i.e., name, address, point of contact, VIN, and DUNS).
|
(c)
|
Invoice/Financing Request Number: Insert the appropriate serial number of the payment request.
|
|
(d)
|
Date Invoice/Financing Request Prepared: Insert the date the payment request is prepared.
|
|
(e)
|
Contract Number and Order Number (if applicable): Insert the contract number and order number (if applicable).
|
|
(f)
|
Effective Date: Insert the effective date of the contract or if billing under an order, the effective date of the order.
|
|
(g)
|
Total Estimated Cost of Contract/Order: Insert the total estimated cost of the contract, exclusive of fixed-fee. If billing under an order, insert the total estimated cost of the order, exclusive of fixed-fee.
|
|
(h)
|
Total Fixed-Fee: Insert the total fixed-fee (where applicable).
|
|
(i)
|
Two-Way/Three-Way Match: Identify payment to be made using a three-way match.
|
|
(j)
|
Office of Acquisitions: Insert the name of the Office of Acquisitions, as identified in Section G of the Contract Schedule.
|
|
(k)
|
Central Point of Distribution: Insert the Central Point of Distribution, as identified in the Invoice Submission Instructions in Section G of the Contract Schedule.
|
|
(l)
|
Billing Period: Insert the beginning and ending dates (month, day, and year) of the period in which costs were incurred and for which reimbursement is claimed.
|
|
(m)
|
Amount Billed - Current Period: Insert the amount claimed for the current billing period by major cost element, including any adjustments and fixed-fee. If the Contract Schedule contains separately priced line items, identify the contract line item(s) on the payment request and include a separate breakdown (by major cost element) for each line item.
|
|
(n)
|
Amount Billed - Cumulative: Insert the cumulative amounts claimed by major cost element, including any adjustments and fixed-fee. If the Contract Schedule contains separately priced line items, identify the contract line item(s) on the payment request and include a separate breakdown (by major cost element) for each line item.
|
|
(o)
|
Direct Costs: Insert the major cost elements. For each element, consider the application of the paragraph entitled "Costs Requiring Prior Approval" on page 1 of these instructions.
|
|
(1)
|
|
|
(2)
|
Direct Labor: Include salaries and wages paid (or accrued) for direct performance of the contract.
|
For Level of Effort contracts only, the Contractor shall provide the following information on a separate sheet of paper attached to the payment request:
- hours or percentage of effort and cost by labor category (as specified in the Level of Effort Article in Section F of the contract) for the current billing period, and
- hours or percentage of effort and cost by labor category from contract inception through the current billing period. (NOTE: The Contracting Officer may require the Contractor to provide additional breakdown for direct labor, such as position title, employee name, and salary or hourly rate.)
|
(3)
|
Fringe Benefits: List any fringe benefits applicable to direct labor and billed as a direct cost. Do not include in this category fringe benefits that are included in indirect costs.
|
|
(4)
|
Accountable Personal Property: Include permanent research equipment and general purpose equipment having a unit acquisition cost of $1,000 or more, with a life expectancy of more than two years, and sensitive property regardless of cost (see the HHS Contractor's Guide for Control of Government Property). Show permanent research equipment separate from general purpose equipment.
|
Page 80
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
On a separate sheet of paper attached to the payment request, list each item for which reimbursement is requested. An asterisk (*) shall precede the item if the equipment is below the $1,000 approval level. Include reference to the following (as applicable):
|
-
|
item number for the specific piece of equipment listed in the Property Schedule, and
|
|
-
|
COA number, if the equipment is not covered by the Property Schedule.
|
The Contracting Officer may require the Contractor to provide further itemization of property having specific limitations set forth in the contract.
|
(5)
|
Materials and Supplies: Include equipment with unit costs of less than $1,000 or an expected service life of two years or less, and consumable material and supplies regardless of amount.
|
|
(6)
|
Premium Pay: List remuneration in excess of the basic hourly rate.
|
|
(7)
|
Consultant Fee: List fees paid to consultants. Identify consultant by name or category as set forth in the contract or COA, as well as the effort (i.e., number of hours, days, etc.) and rate billed.
|
|
(8)
|
Travel: Include domestic and foreign travel. Foreign travel is travel outside of Canada, the United States and its territories and possessions. However, for an organization located outside Canada, the United States and its territories and possessions, foreign travel means travel outside that country. Foreign travel must be billed separately from domestic travel.
|
|
(9)
|
Subcontract Costs: List subcontractor(s) by name and amount billed. Cite applicable COA or notification.
|
|
(10)
|
Other: List all other direct costs in total unless exceeding $1,000 in amount. If over $1,000, list cost elements and dollar amounts separately. If the contract contains restrictions on any cost element, that cost element must be listed separately.
|
|
(p)
|
Cost of Money (COM): Cite the COM factor and base in effect during the time the cost was incurred and for which reimbursement is claimed.
|
|
(q)
|
Indirect Costs: Identify the indirect cost base (IDC), indirect cost rate, and amount billed for each indirect cost category.
|
|
(r)
|
Fixed-Fee: Cite the formula or method of computation for fixed-fee, if applicable. The fixed-fee must be claimed as provided for by the contract.
|
|
(s)
|
Total Amounts Claimed: Insert the total amounts claimed for the current and cumulative periods.
|
|
(t)
|
Adjustments: Include amounts conceded by the Contractor, outstanding suspensions, and/or disapprovals subject to appeal.
|
|
(u)
|
Grand Totals
|
|
(v)
|
Certification of Salary Rate Limitation: If required by the contract (see Invoice Submission Instructions in Section G of the Contract Schedule), the Contractor shall include the following certification at the bottom of the payment request:
|
“I hereby certify that the salaries billed in this payment request are in compliance with the Salary Rate Limitation Provisions in Section H of the contract.”
|
(w)
|
Signature
|
|
|
The Contracting Officer may require the Contractor to submit detailed support for costs claimed on one or more interim payment requests.
|
FINANCIAL REPORTING INSTRUCTIONS:
These instructions are keyed to the Columns on the sample invoice/financing request.
Column A - Expenditure Category: Enter the expenditure categories required by the contract.
Page 81
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Column B - Cumulative Percentage of Effort/Hrs. - Negotiated: Enter the percentage of effort or number of hours agreed to for each employee or labor category listed in Column A.
Column C - Cumulative Percentage of Effort/Hrs. - Actual: Enter the percentage of effort or number of hours worked by each employee or labor category listed in Column A.
Column D - Amount Billed - Current: Enter amounts billed during the current period.
Column E - Amount Billed - Cumulative: Enter the cumulative amounts to date.
Column F - Cost at Completion: Enter data only when the Contractor estimates that a particular expenditure category will vary from the amount negotiated. Realistic estimates are essential.
Column G - Contract Amount: Enter the costs agreed to for all expenditure categories listed in Column A.
Column H - Variance (Over or Under): Show the difference between the estimated costs at completion (Column F) and negotiated costs (Column G) when entries have been made in Column F. This column need not be filled in when Column F is blank. When a line item varies by plus or minus 10 percent, i.e., the percentage arrived at by dividing Column F by Column G, an explanation of the variance should be submitted. In the case of an overrun (net negative variance), this submission shall not be deemed as notice under the Limitation of Cost (Funds) Clause of the contract.
Modifications: Any modification in the amount negotiated for an item since the preceding report should be listed in the appropriate cost category.
Expenditures Not Negotiated: An expenditure for an item for which no amount was negotiated (e.g., at the discretion of the Contractor in performance of its contract) should be listed in the appropriate cost category and all columns filled in, except for
G. Column H will of course show a 100 percent variance and will be explained along with those identified under H above.
Page 82
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
SAMPLE INVOICE/FINANCING REQUEST AND CONTRACT FINANCIAL REPORT
|
(a)
(b)
|
Designated Billing Office Name and Address:
DHHS/OS/ASPR/BARDA
Attn: Contracting Officer
330 Independence Ave., S.W. Room G644
Washington, D.C. 20201
Contractor’s Name, Address, Point of Contact, VIN, and DUNS or DUNS+4 Number:
ABC CORPORATION
100 Main Street Anywhere, USA Zip Code
Name, Title, Phone Number, and E-mail Address of person to notify in the event of an improper invoice or, in the case of payment by method other than Electronic Funds Transfer, to whom payment is to be sent.
VIN:
DUNS or DUNS+4:
|
|
(c) Invoice/Financing Request No.:
(d) Date Invoice Prepared:
(e) Contract No. and Order No. (if applicable):
(f) Effective Date:
(g) Total Estimated Cost of Contract/Order:
(h) Total Fixed-Fee (if applicable):
[ ] Two-Way Match:
[ ] Three-Way Match:
(i) Office of Acquisitions:
(j) Central Point of Distribution:
|
||||||
|
(l) This invoice/financing request represents reimbursable costs for the period from to
|
|||||||||
|
■
Expenditure Category*
A
|
Cumulative Percentage of Effort/Hrs.
|
Amount Billed
|
Cost at
Completion
F
|
Contract
Amount
G
|
Variance
H
|
||||
|
Negotiated
B
|
Actual
C
|
(m)
Current
D
|
(n)
Cumulative
E
|
||||||
|
(o) Direct Costs:
|
|||||||||
|
(1) Direct Labor
|
|||||||||
|
(2) Fringe Benefits
|
|||||||||
|
(3) Accountable Property
|
|||||||||
|
(4) Materials & Supplies
|
|||||||||
|
(5) Premium Pay
|
|||||||||
|
(6) Consultant Fees
|
|||||||||
|
(7) Travel
|
|||||||||
|
(8) Subcontracts
|
|||||||||
|
(9) Other
|
|||||||||
|
Total Direct Costs
|
|||||||||
|
(p) Cost of Money
|
|||||||||
|
(q) Indirect Costs
|
|||||||||
|
(r) Fixed Fee
|
|||||||||
|
(s) Total Amount Claimed
|
|||||||||
|
(t) Adjustments
|
|||||||||
|
(u) Grand Totals
|
|||||||||
|
I certify that all payments are for appropriate purposes and in accordance with the contract.
(Name of Official) (Title)
* Attach details as specified in the contract
|
|||||||||
Page 83
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
|
FINANCIAL REPORT OF INDIVIDUAL PROJECT/CONTRACT
Note: Complete this Form in Accordance with Accompanying Instructions.
|
Project Task:
|
Contract No.:
|
Date of Report:
|
0990-0134
0990-0131
|
|||||||||
|
Reporting Period:
|
Contractor Name and Address:
|
||||||||||||
|
Expenditure Category
|
Percentage of Effort/Hours
|
Cumulative
Incurred Cost
at End of Prior
Period
|
Incurred Cost--
Current
Period
|
Cumulative
Cost to Date
(D + E)
|
Estimated
Cost to
Complete
|
Estimated Cost at Completion
(F + G)
|
Negotiated Contract Amount
|
Variance (Over or Under) (I - H)
|
|||||
|
Negotiated
|
Actual
|
||||||||||||
|
A
|
B
|
C
|
D
|
E
|
F
|
G
|
H
|
I
|
J
|
||||
Page 84
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
ATTACHMENT 7
INSTRUCTIONS FOR COMPLETING
"FINANCIAL REPORT OF INDIVIDUAL PROJECT/CONTRACT"
GENERAL INFORMATION
|
|
Purpose. This Quarterly Financial Report is designed to: (1) provide a management tool for use by be BARDA in monitoring the application of financial and personnel resources to the BARDA contracts; (2) provide contractors with financial and personnel management data which is usable in their management processes; (3) promptly indicate potential areas of contract underruns or overruns by making possible comparisons of actual performance and projections with prior estimates on individual elements of cost and personnel; and (4) obtain contractor's analyses of cause and effect of significant variations between actual and prior estimates of financial and personnel performance.
|
REPORTING REQUIREMENTS
Scope. The specific cost and personnel elements to be reported shall be established by mutual agreement prior to award. The Government may require the contractor to provide detailed documentation to support any element(s) on one or more financial reports.
Number of Copies and Mailing Address. An original and two (2) copies of the report(s) shall be sent to the contracting officer at the address shown on the face page of the contract, no later than 30 working days after the end of the period reported. However, the contract may provide for one of the copies to be sent directly to the Contracting Officer’s Technical Representative.
REPORTING STATISTICS
A modification which extends the period of performance of an existing contract will not require reporting on a separate quarterly report, except where it is determined by the contracting officer that separate reporting is necessary. Furthermore, when incrementally funded contracts are involved, each separate allotment is not considered a separate contract entity (only a funding action). Therefore, the statistics under incrementally funded contracts should be reported cumulatively from the inception of the contract through completion.
Definitions and Instructions for Completing the Quarterly Report. For the purpose of establishing expenditure categories in Column A, the following definitions and instructions will be utilized. Each contract will specify the categories to be reported.
|
(1)
|
Key Personnel. Include key personnel regardless of annual salary rates. All such individuals should be listed by names and job titles on a separate line including those whose salary is not directly charged to the contract but whose effort is directly associated with the contract. The listing must be kept up to date.
|
|
(2)
|
Personnel--Other. List as one amount unless otherwise required by the contract.
|
|
(3)
|
Fringe Benefits. Include allowances and services provided by the contractor to employees as compensation in addition to regular salaries and wages. If a fringe benefit rate(s) has been established, identify the base, rate, and amount billed for each category. If a rate has not been established, the various fringe benefit costs may be required to be shown separately. Fringe benefits which are included in the indirect cost rate should not be shown here.
|
|
(4)
|
Accountable Personal Property. Include nonexpendable personal property with an acquisition cost of $1,000 or more and with an expected useful life of two or more years, and sensitive items regardless of cost. Form HHS 565, "Report of Accountable Property," must accompany the contractor's public voucher (SF 1034/SF 1035) or this report if not previously submitted. See "Contractor's Guide for Control of Government Property."
|
|
(5)
|
Supplies. Include the cost of supplies and material and equipment charged directly to the contract, but excludes the cost of nonexpendable equipment as defined in (4) above.
|
|
(6)
|
Inpatient Care. Include costs associated with a subject while occupying a bed in a patient care setting. It normally includes both routine and ancillary costs.
|
|
(7)
|
Outpatient Care. Include costs associated with a subject while not occupying a bed. It normally includes ancillary costs only.
|
|
(8)
|
Travel. Include all direct costs of travel, including transportation, subsistence and miscellaneous expenses. Travel for staff and consultants shall be shown separately. Identify foreign and domestic travel separately. If required by the contract, the following information shall be submitted: (i) Name of traveler and purpose of trip; (ii)
|
Page 85
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Place of departure, destination and return, including time and dates; and (iii) Total cost of trip.
|
(9)
|
Consultant Fee. Include fees paid to consultant(s). Identify each consultant with effort expended, billing rate, and amount billed.
|
|
(10)
|
Premium Pay. Include the amount of salaries and wages over and above the basic rate of pay.
|
|
(11)
|
Subcontracts. List each subcontract by name and amount billed.
|
|
(12)
|
Other Costs. Include any expenditure categories for which the Government does not require individual line item reporting. It may include some of the above categories.
|
|
(13)
|
Overhead/Indirect Costs. Identify the cost base, indirect cost rate, and amount billed for each indirect cost category.
|
|
(14)
|
General and Administrative Expense. Cite the rate and the base. In the case of nonprofit organizations, this item will usually be included in the indirect cost.
|
|
(15)
|
Fee. Cite the fee earned, if any.
|
|
(16)
|
Total Costs to the Government.
|
PREPARATION INSTRUCTIONS
These instructions are keyed to the Columns on the Quarterly Report.
Column A--Expenditure Category. Enter the expenditure categories required by the contract.
Column B--Percentage of Effort/Hours Negotiated. Enter the percentage of effort or number of hours agreed to during contract negotiations for each labor category listed in Column A.
Column C--Percentage of Effort/Hours-Actual. Enter the cumulative percentage of effort or number of hours worked by each employee or group of employees listed in Column A.
Column D--Cumulative Incurred Cost at End of Prior Period. Enter the cumulative incurred costs up to the end of the prior reporting period. This column will be blank at the time of the submission of the initial report.
Column E--Incurred Cost-Current Period. Enter the costs which were incurred during the current period.
Column F--Cumulative Incurred Cost to Date. Enter the combined total of Columns D and E.
Column G--Estimated Cost to Complete. Make entries only when the contractor estimates that a particular expenditure category will vary from the amount negotiated. Realistic estimates are essential.
Column H--Estimated Costs at Completion. Complete only if an entry is made in Column G.
Column I--Negotiated Contract Amount. Enter in this column the costs agreed to during contract negotiations for all expenditure categories listed in Column A.
Column J--Variance (Over or Under). Complete only if an entry is made in Column H. When entries have been made in Column H, this column should show the difference between the estimated costs at completion (Column H) and negotiated costs (Column I). When a line item varies by plus or minus 10 percent, i.e., the percentage arrived at by dividing Column J by Column I, an explanation of the variance should be submitted. In the case of an overrun (net negative variance), this submission shall not be deemed as notice under the Limitation of Cost (Funds) Clause of the contract.
Modifications. List any modification in the amount negotiated for an item since the preceding report in the appropriate cost category.
Page 86
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Expenditures Not Negotiated. List any expenditure for an item for which no amount was negotiated (e.g., at the discretion of the contractor in performance of its contract) in the appropriate cost category and complete all columns except for I. Column J will of course show a 100 percent variance and will be explained along with those identified under J above.
Page 87
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Department of Health & Human Services HHS
7 Principles of Earned Value Management
Tier 3
System Implementation Intent Guide
01 October 2011

Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
TABLE OF CONTENTS
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
OVERVIEW
Earned Value Management (EVM) is a program management tool, technique, and discipline that facilitates systematic planning for and monitoring of, high value, complex projects. It integrates a project’s scope of work with the related budget and schedule to permit detailed assessment of overall performance during the life of the project.
Several government-wide guidance documents govern the definition and use of EVM systems. Guidelines outlining the qualities and characteristics of an EVM system are set forth in the American National Standards Institute/Electronic Industries Alliance (ANSI/EIA) Standard-748 (most current version). More detailed and specific guidance and direction is contained in OMB Circular A-11, Preparation, Submission and Execution of the Budget, specifically in Part 7 of that Circular A-11, Planning, Budgeting, Acquisition, and Management of Capital Assets, and its supplement, the Capital Programming Guide. Based on this collective OMB guidance, EVMS is intended to be used on those parts of acquisitions that will involve developmental effort. This would include not only those acquisitions designated by the agency as major systems but also those acquisitions that include significant developmental, modification, or upgrade during the operational or steady-state phase of a program.
The FAR rule on EVMS became effective on July 5, 2006. Its purpose is to implement EVMS policy in accordance with OMB Circular A-11. Because the new FAR coverage applies throughout the executive branch and to agencies with disparate definitions of and processes and procedures for major systems acquisitions, the FAR Council decided against a “one-size-fits all” approach and left several significant aspects of the detailed implementation up to the discretion of each covered agency.
The FAR and Health and Human Services Acquisition Regulations (HHSAR) language for EVMS will be utilized for all construction or Information Technology (IT) projects. Since most of the acquisitions at the Biomedical Advanced Research and Development Agency (BARDA) are unique in that most acquisitions are not Information Technology projects or construction projects, BARDA is developing EVM language that incorporates the 7 Principles of Earned Value Management. These principles allow flexibility to an EVM system structure but still meet the spirit of the ANSI/EIA Standard-748. It also incorporates discipline in implementation and operations and also provides the same reporting data outlined by OMB.
|
2.
|
|
3.
|
1
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
6.
|
2
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
BARDA will be implementing a tiered approach to EVM based on the type of acquisition, size of the acquisition and the technical readiness level. There are three tiers and they are as follows:
For all construction contracts and IT contracts the ANSI/EIA-748 Standard for Earned Value Management Systems will apply and all relevant FAR/HHSAR clauses pertaining to EVMS will be incorporated in the contract. The National Defense Industrial Association (NDIA) Program Management Systems Committee (PMSC) ANSI/EIA-748 Standard for Earned Value Management Systems Intent Guide should be used as guidance.
TIER 2
For countermeasure research and development contracts that have a total acquisition costs greater than or equal to $25 million and have a Technical Readiness Level (TRL) of less than 7 will apply EVM principles for tracking cost, schedule and technical performance that comply with the 7 Principles of EVM Implementation.
TIER 3
For countermeasure research and development contracts that have total acquisition costs less than $25 million but greater than $10 million will apply EVM principles for tracking cost, schedule and technical performance that are consistent with the 7 Principles of EVM Implementation.
This Guide is an explanation of the intent of what is expected for a Tier 2 system implementation of the 7 Principles of EVM.
3
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
In a performance measurement system implementation the Statement of Work (SOW) should reflect all work that is to be performed. In a 7 Principles implementation a Work Breakdown Structure (WBS) shall be developed to include all elements of the SOW. The level of the WBS may not be as detailed as in a Tier 1 implementation. It would be developed at a higher level, such as level three or four, however, the government may expand specific technical legs to lower than level four and it may retract some non-technical legs to higher than 3. It is beneficial and required to develop a WBS dictionary that explains what work is going to be performed in each WBS in detail. This will ensure that the contractor has identified all work scope and left no major work undefined. It is recommended that the work packages descriptions are clear and detailed so that there is an understanding of the work that is to be performed in the work packages. For the 7 Principles implementation programs it would be acceptable for the WBS Dictionary be expanded to include information that would normally be kept on a Work Authorization Document, such as charge numbers associated with the work, period of performance, the manager who is responsible for the work, and budget associated with the WBS. The additional “WAD info” would only be added to the lowest level (i.e. level 3 or 4) of the WBS. The roll up level WBS would only include scope. By doing this documentation is limited to one document instead of two.
By developing a WBS and a WBS Dictionary/Work Authorization Document the work scope has been defined but the documentation is greatly reduced and the costs associated with developing and updating the documentation is reduced. The intent of the combination document is not to reduce the level of information provided to the government but to reduce the amount of documents that need to be produced. An example of a WBS dictionary and Work Authorization document and what is expected on the document(s) is provided.
In a Tier 3 implementation it is not necessary to provide a WBS Dictionary or a Work Authorization Document but it is important to develop a WBS and define a scope of work for each level of the WBS at the reporting level (usually level 3 or 2).
In a 7 Principles Tier 2 implementation it is recommended that the work be broken into finite pieces in the schedule tool. It is recommended to plan the work by the lowest level WBS. The lowest level WBS (level 3 or 4) should be the control account and the activities would act as the work packages. Most of the normal functions accomplished when scheduling will be required on a 7 Principles Tier 3 implementation. These normal functions include, network scheduling, horizontal and vertical traceability, forecasting schedule start and completion dates, and running critical path analysis. As part of vertical traceability it is expected that all contract milestones will be listed on the schedule.
WBS number
Work package number
4
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
Duration
Baseline Start and Finish Dates
Actual Start and Finish Dates
Forecast Start and Finish Dates
Predecessor/Successors
Activity Percent Complete
All the work scheduled at the lowest level WBS should be identified by a single responsible manager. This manager, known as a Control Account Manager should be identified in the schedule tool and/or in a cost tool. In a 7 Principles implementation, only individuals at the lowest level WBS need be identified and there is no requirement for the costs to roll up by organization, although if it is not cost intensive or tool restricted then developing the OBS is recommended. In many cases, BARDA will provide the top three levels of the WBS for the contractor to use.
This principle integrates the work scope, the schedule and the budget into a performance measurement baseline. Since we discussed work scope and schedule the focus of this principle is the incorporation of the budget in a time-phased manner. The budget must be integrated with the scope of work and the schedule into a Performance Measurement Baseline (PMB). The budget is made up of both direct and indirect dollars. An accepted way of incorporating the budget and integrating with the scope and schedule is to resource load the Microsoft Project (or other scheduling tool) schedule. This is done by loading the individual people and their loaded rate into the tool. This budget data will be input at the work package level with a rate that includes the indirect costs. The budget will have to have the capability to be rolled up to the control account level and will need to be reported in a way that provides the responsible manager (Control Account Manager) with information needed to manage the program. Resource loading of the schedule is not the only way to incorporate the budget. As long as the budget in the budget/EV tool is linked to the schedule activities and it is flexible to change when schedule baseline dates change, then loading the budget in the Budget/EV tool is an acceptable way to integrate the cost and schedule baselines. The budget information will be displayed on the time- phased Control Account Plan reports. These reports should have the flexibility to report the dollars both in total dollars, as well as, direct and indirect broken out separately. Also the report is generally required as a deliverable on most contracts and must have the capability to include earned value or Budgeted Cost of Work Performed (BCWP) and actual costs or Actual Costs of Work Performed (ACWP).
Budgeting of subcontractor effort will vary depending on whether or not the subcontractor is a cost plus or fixed price subcontract. If it is cost plus then the expectation is that there will be monthly billing of costs from the subcontractor to the prime contractor and therefore budget must be planned in accordance with the work completed and billed. If it is fixed price then the budget should be planned with work execution or milestones completed and budget should only be planned in those months where work is expected to be completed.
5
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
It is recommended that management reserve and undistributed budget be utilized in the budgeting process. Undistributed budget is budget that has not yet been distributed to a control account and it requires additional time to plan the work and distribute the budget to a control account. It is a temporary holding account and budget should only stay in Undistributed Budget for one or two months. If the work scope is easily identified to all the control accounts then the use of Undistributed Budget may not be necessary.
Management Reserve is budget that is set aside, normally by the Program Manager, to be used to budget future but currently unknown tasks. It is associated with risk issues and is to be used to mitigate risk. It is not part of the Performance Measurement Baseline and it should not be used for out of scope work and to cover overruns.
A properly controlled PMB is crucial to effective program management. The timely and accurate incorporation of contractual changes ensures that the information generated from the execution of the baseline plan provides an accurate picture of progress and facilitates correct management actions and decisions. The accurate and timely incorporation of authorized and negotiated changes into the PMB ensures that valid performance measurement information is generated for the new scope being executed. Near term new scope effort should be planned and have budget in control accounts. Far term new scope effort that cannot be reasonably planned in the near term can either be put in planning packages in the control account or left in Undistributed Budget if the control account has not been identified. The timely and accurate incorporation of authorized and negotiated changes into the PMB ensures that valid performance measurement information is generated for the new scope being executed. Budget revisions are made when work is added to the contract and are traceable from authorized contract target costs to the control account budgets or from management reserve. Management reserve may be used for future work when additional in- scope work has been identified.
Retroactive changes to the baseline may mask variance trends and prevent the use of performance data to project estimates of cost and schedule at completion. Controlling retroactive adjustments, which should only be made in the current period, if possible, is imperative because they could arbitrarily eliminate existing cost and schedule variances.
The use of program budget logs should be used to track and log all budget changes. The ability to track budget values for both the internal and external changes will help in the maintenance of the performance measurement baseline from program start to completion. Contractor is expected to utilize baseline change documentation facilitating the change. It should provide the rationale/justification, approval process, work scope additions or deletions, dollars, changes to schedules, estimate at completion, etc. It should also include contractual change documents for external changes, such as a contract modification, letter to proceed, not to exceed letter, change order, etc., that transmit and authorize the change or addition to work, budget, and schedule. Other documents that should change if a change of scope has been authorized is: Statement of Work, WBS (changes if applicable); WBS Dictionary (additions or deletions to scope); work authorization documents authorizing new scope, schedule and budget; schedules.
6
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
Some of the new acquisitions at BARDA will be required to be compliant with the Cost Accounting Standards. For Tier 3 implementation contractors must utilize a work order/job order/task code charge number structure that uniquely identifies costs at the control account level, which may be as high as the reporting level of the WBS. This will allow for accumulation and summarization of costs to higher levels of the work breakdown structure. Actual costs are accumulated in the formal accounting system in a manner consistent with the way the related work is planned and budgeted. Actual costs reported in the performance reports agrees with the costs recorded in the accounting system or can be explained as timing differences. The contractor will have to be able to incorporate and reconcile to the accounting system actual costs on their Contract Performance Reports (CPR) to the customer.
Depending on the amount of material and subcontractors on the program, it may be necessary for reporting purposes, to include accruals, or estimated actuals, for these costs. Since material and subcontractor invoices are not paid and recorded in the accounting system for up to several months after the work has been planned, performance data will be skewed. Accruing or estimating actual costs based on receipt (for material) and expended hours for subcontractors will alleviate this issue. The use of accrual/estimated actuals should be reviewed on a case by case basis depending on the size of program, the amount of material or subcontractor budget and costs. If the material and subcontract effort on the project is minimal (represents less than 5% of the project budget) then the time and effort needed to manage the accruals would outweigh the benefit of having the costs accrued since the performance data would only be minimally affected. Although actual costs are generally reported to the USG in total dollars the system must be able to differentiate and report direct costs and indirect costs if requested.
If the subcontractor has a fixed price contract the prime contractor, then the prime contractor must report actual costs in accordance with the work that is accomplished. This is acheived by recording the actual costs equal to the work that was performed in the EVM system and on the CPR. If the subcontractor is a cost plus contract its imperative the costs the prime reports is in accordance with the costs incurred in that month. This is necessary to ensure that the data reported is not skewed. With this premise, fixed price subcontractors cost variances should not exist or be reported on the CPR whereas the cost reported for cost plus subcontractors should be based on what was incurred and not what has been invoiced to date, which may be months behind.
|
|
In order to meet this Principle, the scheduling of the scope of work in work packages or activities need to incorporate measurable units or milestones in order to objectively assess accomplishments or obtain what we call “earned value”. These units or milestones are given a value based on labor resources needed to accomplish the work (which becomes the Budgeted Cost of Work Scheduled or BCWS). When they are accomplished (known as Budgeted Cost of Work Performed or BCWP) they receive the value associated with the budget which measures progress.
|
7
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
|
Schedule status to measure progress needs to be on at least on a monthly basis although it is preferred on a bi-weekly basis. As part of the status process progress dates, such as actual start/complete and forecast start/complete need to be updated.
|
|
|
Since Microsoft Project seems to be the schedule tool of choice by most contractors, there are four types of earned value methodologies utilized by Microsoft Project of which two assess progress by the completion of milestones and they are the 50/50 and 0/100 methodologies. In both cases, progress is reported for completion milestones and in the 50/50 methodology fifty percent of the value of the work package/activity is credited for starting the work. The other two earned value methodologies are assessed percent complete (also know as Supervisor’s Estimate) and level of effort (LOE). All four methodologies are legitimate earn value measurement techniques.
|
|
|
Additional earned value methodologies, such as the weighted milestone methodology and percent complete with milestone gates may be utilized. The weighted milestone method allows value to be earned based on the resource value in each month, which eliminates artificial schedule variances.
|
|
|
For subcontractors that have a fixed price contract with the prime contractor, the expectation is that there will be no cost variance. The ACWP reported on the CPR will equal the BCWP earned, regardless of the payment schedule with subcontractor.
|
The purpose of this principle is to ensure that the earned value data is analyzed by the contractor and reported to the customer. The 7 Principles programs should be able to calculate the cost variance (BCWP minus Actual Cost of Work Performed (ACWP) and the schedule variance (BCWP minus BCWS) at least on a cumulative basis. It is recommended that variances be calculated on a current month basis also. The EVM system should also provide both monthly and cumulative Cost Performance Index (BCWP divided by ACWP) and Schedule Performance Index (BCWP divided by the BCWS). This data should be provided at the control account level and at the roll up levels and it needs to be in a format for Control Account Managers and program management to be able to utilize in managing the work.
|
|
It is also recommended that the To-Complete Performance Index (TCPI) be included in the Control Account Manager performance report. The TCPI is a valuable index that calculates the cost performance the control account needs to perform at in order to complete the work within the current reported EAC. When the TCPI is compared against the cumulative CPI it gives a good indication whether or not the current EAC is reasonable. For example, if a cumulative CPI is .85 and the TCPI calculates to equal 1.15 that is the performance factor that work would need to perform at in order to meet the current EAC. If the cumulative CPI is .85 then it can be determined that the current EAC might not be reasonable. It allows management and Project Controls the opportunity to question the Control Account Manager as to the validity of the current EAC. As a rule in thumb if the deviation between the CPI and the TCPI is greater than .2 then the CAM should reassess the control account EAC.
|
|
|
These reports, which should be provided monthly, should also include the current Budget at Completion (BAC) and the current Estimate at Completion (EAC). In addition, it would be a
|
8
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
plus if the CAM could see a report with their time-phased spread of hours and dollars for their budget plan (BCWS), work accomplished (BCWP) and actual costs (ACWP).
|
|
For all variances that exceed the contractual variance threshold will include a description of what caused the variance, impact to the control account and the program, and a corrective action.
|
Principle 6b: Prepare an Estimate at Completion Based on Performance to Date and Work to be Performed
|
|
Providing an updated EAC is a prime concern of the customer and the contractor. Therefore a robust EAC process should be in place whether the program is ANSI compliant or not.
|
|
|
Based on the performance to date the Estimates at Completion can be updated on a monthly basis by the Control Account Manager in the scheduling tool during the status process or in the cost/EVM tool at the end of the month’s process prior to submittal of the EVM report. The EAC is an element of the performance measurement system that needs to accurately reflect the contractor’s best estimate of what it will cost to complete the project.
|
|
|
Program management should be able to validate control account manager’s EACs by looking at performance indices, such as the To-Complete Performance Index, as well as independent statistical EACs.
|
|
|
One of the key areas that concerns government Program Management Offices (PMO) is the level of importance that contractor’s place on EVM as a management tool. During a site visit, such as conducting an Integrated Baseline Review, the PMO gauges what the interest, knowledge, and most importantly, the usage of the performance measurement data in managing the program. They want to know that the managers on the program, including the program manager, have received some earned value training. The level of involvement and use of the EVM data to manage their schedule, cost and technical issues is ascertained by questions. The PMO can also tell by how robust the EACs are and if the variance narratives are being written with impacts to the program and corrective actions being monitored by the contractor. It is important that the contractor’s management team, including the Program Manager, utilize the data from the performance measurement system as a management tool. They should be knowledgeable and understand the data. They should know what is causing the variances and ensure that the variance narratives are written properly and answer what the issues, impacts and corrective actions are. They should be able to demonstrate that they use the information to assist them in the management decision process.
|
9
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
The following appendices provide further support in understanding the meaning and intent of properly implementing the 7 Principles of EVM.
Appendix 2 is supplemental guidance on EVM implementation. It provides some guidelines on what is expected in the implementation, required documents needed for the Performance Measurement Baseline Review, expected EVM implementation costs, EVM engines functionality needs, explains what is expected in the monthly EVM facilitation, discusses what EVM consultants need to know, and what the expected costs of EVM to BARDA.
Appendix 3 are examples of some of the EVM documents that are needed in an EVM system. There are three documents and they mostly apply to Tier 2 EVM implementations. These documents are samples and are not a reflection of the specific way the document must look. It’s included to provide contractors with an understanding of the type of information that is expected on these forms.
|
Actual Cost of Work Performed (ACWP)
|
The costs actually applied and recorded in accomplishing the work performed within a specified period.
|
|
Actual Direct Cost
|
Those costs identified specifically with a contract, based upon the contractor's cost identification and accumulation system as accepted by the cognizant DCAA representatives. (See Direct Costs).
|
|
Advance Agreement (AA)
|
An agreement between the contractor and the Contract Administration Office concerning the application of an approved earned value management system to contracts within the affected facility.
|
|
Authorized Work
|
That effort which has been authorized and is on contract, or that for which authorized contract costs have not been agreed to but for which written authorization has been received.
|
10
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
Baseline
|
(See Performance Measurement Baseline).
|
|
Budget at Completion (BAC)
|
The sum of all budgets (BCWS) allocated to the contract. Synonymous with the term Performance Measurement Baseline.
|
|
Budgeted Cost for Work Performed (BCWP)
|
The sum of the budgets for completed Work Packages and completed portions of open Work Packages, plus the appropriate portion of the budgets for level of effort and apportioned effort (Also see Earned Value).
|
|
Budgeted Cost for Work Scheduled (BCWP)
|
The sum of the budgets for completed Work Packages, planning packages, etc., scheduled to be accomplished (including in-process Work Packages), plus the amount of level of effort and apportioned effort scheduled to be accomplished within a given time period.
|
|
Change Order (CO)
|
A formal authorization by the Procuring Contracting Officer for a change of scope to an existing contract
|
|
Contract Modification
|
A written and binding authorization to proceed created after change proposal negotiations.
|
|
Contract Budget Base (CBB)
|
The negotiated contract cost plus the estimated cost of authorized unpriced work, where:
(1) Negotiated Contract Cost is that cost on which contractual agreement has been reached. For an incentive contract, it is the definitized contract target cost plus/minus the value of changes which have been priced and incorporated into the contract through contract change order or supplemental agreement. For fixed-fee contracts, it is the negotiated estimated cost. Changes to the estimated cost will consist only of the formal contract modifications or change orders or change in the contract statement of work, not for cost growth, and
|
11
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
| (2) Estimated cost of authorized, unpriced work is the estimated cost (excluding fee or profit) for that work for which written authorization has been received, but for which definitized contract prices have not been incorporated into the contract through supplemental agreement. | |
|
Control Account
|
A management control point at which actual costs can be accumulated and compared to budgeted cost for work performed. A control account is a natural control point for cost/schedule planning and control since it represents the work assigned to one responsible organizational element on one contract work breakdown structure (CWBS) element.
|
|
Control Account Manager (CAM)
|
A member of a functional organization responsible for task performance detailed in a Control Account and for managing the resources authorized to accomplish the tasks.
|
|
Control Account Plan (CAP) Report
|
A CAP report is a timephased report which reflects all the work and effort to be performed in a control account. The CAP report will reflect the hours and dollars by element of cost (labor, subcontract, ODC, etc) and may also include milestone information.
|
|
Contract Performance Report (CPR)
|
The monthly report submitted to the customer showing the current, cumulative and at completion status, the performance measurement baseline, manpower loading, and a narrative explanation of significant program variances.
|
|
Contract Target Cost
|
The dollar value (excluding fee or profit) negotiated in the original contract plus the cumulative cost (excluding fee or profit) applicable to all definitized changes to the contract. It consists of the estimated cost negotiated for a cost plus fixed fee contract and the definitized target cost for an incentive contract. The contract target cost does not include the value of authorized/un-negotiated work, and is thus equal to the contract budget base only when all authorized work has been negotiated/definitized.
|
12
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
Cost Performance Index (CPI)
|
An efficiency rating reflecting a project's budget performance - either over or under. Measured as a ratio of the budgeted value of work accomplished versus the actual costs expended for a given project time period. The formula for CPI is BCWP/ACWP.
|
|
Discrete Effort
|
Program effort that has a measurable output, product or service.
|
|
Direct Costs
|
Those costs (labor, material, etc.) that can be reasonably and consistently related directly to service performed on a unit of work, and are charged directly to the contract, without distribution to an overhead unit.
|
|
Earned Value
|
See Budgeted Cost for Work Performed (BCWP)
|
|
Earned Value Management System (EVMS)
|
A project management system utilized for measuring project progress in an objective manner. Combines measurements of scope, schedule, and cost in a single integrated system.
|
|
Estimate at Completion (EAC)
|
A value (expressed in dollars and/or hours) developed to represent a realistic appraisal of the final cost of tasks when accomplished. It’s the sum of direct & indirect costs to date plus the estimate of costs for all authorized Work remaining. The EAC = ACWP + the Estimate-to-Complete.
|
|
Estimate to Completion (ETC)
|
A value (expressed in dollar and/or hours) developed to represent a realistic appraisal of the cost of the work still required to be accomplished in completing a task.
|
|
Indirect Costs
|
Represents those costs, because they are incurred for common or joint objectives, are not readily subject to
|
13
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
treatment as direct costs. (See overhead).
|
|
|
Integrated Baseline Review (IBR)
|
An Integrated Baseline Review (IBR) also known as Performance Measurement Baseline Review (PMBR) is a formal review led by the Government Program Manager and Technical Support Staff. An IBR is conducted jointly with the Government and their Contractor counterparts.
The purpose of an IBR is to: verify the technical content of the Performance Measurement Baseline (PMB); assess the accuracy of the related resources (budgets) and schedules; identify potential risks.
|
|
Integrated Master Plan (IMP)
|
The overall program plan including the work definition, technical approach, performance criteria, and completion criteria.
|
|
Integrated Master Schedule (IMS)
|
The IMS expands the IMP to the work planning level. It defines the tasks, their durations, milestones, milestone dates which relate to the IMP completion criteria, and interdependencies required to complete the program. The IMP and IMS are used to track and execute the program.
|
|
Integrated Product Team (IPT)
|
A grouping of project personnel along project objective lines rather than along organizational lines. Integrated Product Teams are work teams that represent a transition from a functional organization structure to a multi- functional project objective arrangement.
|
|
Internal Replanning
|
Replanning actions performed by the program for remaining effort within the recognized total allocated budget.
|
|
Level of Effort (LOE)
|
Work that does not result in a final product, e. g., liaison, coordination, follow-up, or other support activities, and which cannot be effectively associated with a definable end
|
14
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
product process result. It is measured only in terms of resources actually consumed within a given time period.
|
|
|
Management Reserve (MR)
|
An amount of the total Contract Budget Base (CBB) withheld for management control purposes rather than designated for the accomplishment of a specific task or set of tasks. It is not a part of the Performance Measurement Baseline.
|
|
Negotiated Contract Target Cost
|
The estimated cost negotiated in a Cost Plus Award Fee (CPAF), Cost Plus Fixed Fee (CPFF), Cost Plus Incentive Fee (CPIF) or Fixed Price Incentive Fee (FPIF) contract.
|
|
Original Budget
|
The budget established at, or near, the time the contract was signed, based on the negotiated contract cost.
|
|
Overhead
|
Indirect labor and material, supplies and services costs and other charges, which cannot be consistently identified with individual programs.
|
|
Other Direct Costs
|
A group of accounting elements which can be isolated to specific tasks, other than labor and material. Included in ODC are such items as travel, computer time, and services
|
|
Performance Measurement Baseline (PMB)
|
The time-phased budget plan against which contract performance is measured. It is formed by the budgets assigned to scheduled Control Accounts and the allocation of overhead costs. For future effort, not planned to the Control Account level, the performance measurement baseline also includes budgets assigned to higher level WBS elements, and undistributed budgets. It equals the total assigned budget less management reserve.
|
|
Performing Organization
|
A defined unit within the program organization structure, which applies the resources to performs the authorized scope
|
15
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
|
of work.
|
|
|
Planning Package
|
A logical aggregation of far term work within a Control Account that can be identified and budgeted but not yet defined into Work Packages.
|
|
Reprogramming
|
Replanning of the effort remaining in the contract, resulting in a new budget allocation which exceeds the contract budget base. The resulting baseline is called an Over Target Baseline (OTB).
|
|
Responsible Organization
|
A defined unit within program’s organization structure that is assigned responsibility for accomplishing specific tasks.
|
|
Risk Register
|
Is a tool commonly used in project planning and organizational risk assessments. It is often referred to as a Risk Log. It is used for identifying, analyzing and managing risks.
|
|
Schedule Performance Index (SPI)
|
An efficiency rating reflecting how quickly or slowly project work is progressing. Measured as a ratio of work accomplished versus work planned for a given period of time. The formula for SPI is BCWP/BCWS.
|
|
Significant Variances
|
Those differences between planned and actual cost and schedule performance which require further review, analysis, or action. Appropriate thresholds are established as to the magnitude of variances which will require variance analysis.
|
|
Statistical Estimate at Completion
|
Is a single point estimate that can be quickly prepared and used to test the reasonableness of the current cost estimates and budget and to indicate when a comprehensive EAC should be prepared
|
16
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
17
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
| the identity of the CAM. It must be approved by Intermediate Manager, and be agreed to by the Control Account Manager. | |
|
Work Breakdown Structure (WBS)
|
A product-oriented, family-tree composed of hardware, software, services, data and facilities which results from system engineering efforts. A work breakdown structure displays and defines the product(s) to be developed and/ or produced and relates the elements of work to be accomplished to each other and to the end product.
(1) Program WBS. The work breakdown structure that covers the acquisition of a specific defense material item and is related to contractual effort. A program work breakdown structure includes all applicable elements consisting of at least the first three levels of the work breakdown structure and extended by the program manager and /or contractor(s). A program work breakdown structure has uniform element terminology, definition, and placement in the family tree structure.
(2) Contract WBS (CWBS) The complete WBS for a contract, developed and used by a contractor within the guidelines of MIL-Handbook 881 (latest revision) or NASA WBS Handbook (insert reference) or other customer guidelines and according to the contract work statement. It includes the approved work breakdown structure for reporting purposes and its discretionary extension to the lower levels by the contractor, in accordance with MIL- Handbook 881 and the contract work statement. It includes all the elements for the products (hardware, software, data, or services) which are the responsibility of the contractor.
|
|
Work Packages
|
Detailed short-span jobs, or material items, identified by the contractor for accomplishing work required to complete the contract. A Work Package has the following characteristics.
|
18
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
19
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
Implementation of a 7 Principles of EVM system should be less expensive than if there was an ANSI/EIA-748. There is no need for the system to have to go through an EVM compliance review, plus the level of documentation should be streamlined.
|
·
|
|
|
Implementation costs should range $75K-$125K
|
|
|
Implementation costs should range ($50K - $100K)
|
Depending on the size of the contract would predicate the level of functionality that would be needed. For Tier 2 contracts a larger, more robust EVM engine would be needed. For the Tier 3 small contracts MS Project or the MSP wrap-around would probably suffice although the more robust EVM engines can be used also.
It is recommended that one of the larger and flexible EVM engines be utilized. The tool should have the flexibility to be able to download data from MS Project and be able to upload or input budget data to provide time-phased budget information down to the work package level. It should be able to incorporate the companies Organization Breakdown Structure. It should be able
20
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
to maintain baseline, actual costs, forecast and performance periodic data. It should be able to forecast Estimate to Complete with the ability to set up different rate tables if necessary. It should have the capability to use all earned value methodologies. It should be able to print many types of EVM reports that can provide information to the Control Account Managers (CAM) and Program Managers (PM), as well as, the Contract Performance Report (CPR) and the Control Account Plans (CAP) that are contract deliverables.
For Tier 3 projects, a company can certainly utilize an EVM engine as listed above or a less robust, less expensive EVM engine that provides the CPR and timephased S/P/A report. It may also use the Microsoft Project wrap-around tools of which there are several on the market. These tools also will provide the CPR and timephased S/P/A report for contract deliverable purposes.
Depending on the size of contract, a contractor should have an EVM/cost analyst and schedule analyst for a Tier 2 contract and one combined cost/schedule analyst for a Tier 3 contract. The costs for a schedule analyst on a yearly basis for an employee hire should be equal to or less than $125K. For a cost analyst it should be equal to or less than $110K. If a company is bringing in a contractor to provide staff implementation the costs should be up to $125/hr for a schedule analyst and $110/hr for an EVM/cost analyst.
There may be the need to bring in consultants to help set up your EVM system and perhaps provide EVM staff augmentation to provide the monthly facilitation. Make sure that you shop around and get several quotes. Also make sure that the consultants understand the statement of work pertaining to the BARDA EVM requirements. Most EVM consultants are used to working with companies that have a requirement to implement an ANSI/748 compliant EVM system per the DoD requirements and it is important that they have an understanding of what is required in a 7 Principles EVM implementation so that they don’t propose much more complex EVM system than is needed. Please be advised that the government will only accept reasonable costs associated with implementing a 7 Principles of EVM system.
21
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
BARDA is working diligently to keep the costs of EVM implementation and facilitation at a reasonable level. Since the goal at BARDA is to provide an integrated, systematic approach to the development and purchase of the necessary vaccines, drugs, therapies, and diagnostic tools for public health medical emergencies, it is imperative that the funds for product development are used for that such purpose. BARDA expects the costs for implementation and facilitation of EVM to range 1%-2% of development budget. This is ratified by the white paper by Dr. Christenson titled “The Costs and Benefits of the Earned Value Management Process”.
22
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
WBS 1.4.1.x Cardiac (QTc) Safety
Description
Study Title: “A Phase 1 study to assess the cardiovascular safety of intravenous (IV) Panaceomycin in volunteers” (Thorough QT Study)
|
|
We will conduct a thorough evaluation of the cardiac effect of Panaceomycin Injection via a randomized, double-blind crossover study. A total of 100 participants (18-22 per arm) will randomize to one of five study arms to receive in a double-blind fashion a single IV infusion of either Panaceomycin Injection 10 mg/kg, Panaceomycin Injection at a supra-therapeutic dose, ciprofloxacin (positive control), or placebo. 12-Lead digital ECGs will be collected in triplicate via Holter monitor from each participant during dosing. Seven days after dosing, participants will be re-randomized to receive another treatment. ECGs will be collected and analyzed. A full statistical analysis and expert ECG report will be generated. Serum PK samples will also be collected at ECG collection time points and analyzed to confirm exposure.
|
23
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
Pursuant to 17 CFR 20.24b-2, confidential information has been omitted in places marked " * * * " and has been filed
separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application with the Commission.
7 Principles of EVM Tier 2 System Implementation Intent Guide
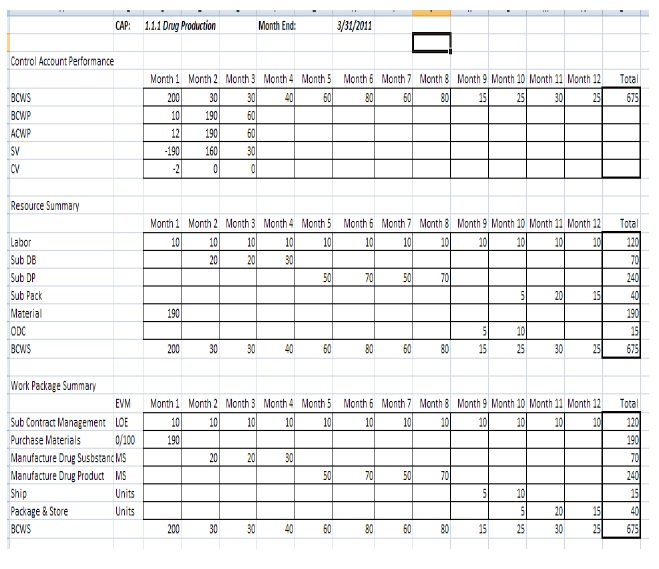
24
