Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CANTEL MEDICAL LLC | a15-10332_18k.htm |
Exhibit 99.1
|
|
FORWARD LOOKING STATEMENT This presentation contains forward-looking statements. All forward-looking statements involve risks and uncertainties, including, without limitation, the risks detailed in the Company’s filings and reports with the Securities and Exchange Commission. Such statements are only predictions, and actual events or results may differ materially from those projected. NYSE:CMN April 2015 / Investor Presentation |
|
|
2 $524 Cantel Medical is a NYSE leader in Infection Prevention & Control (IP&C) MILLION1 1 Sales for the last twelve months ended January 31, 2015. |
|
|
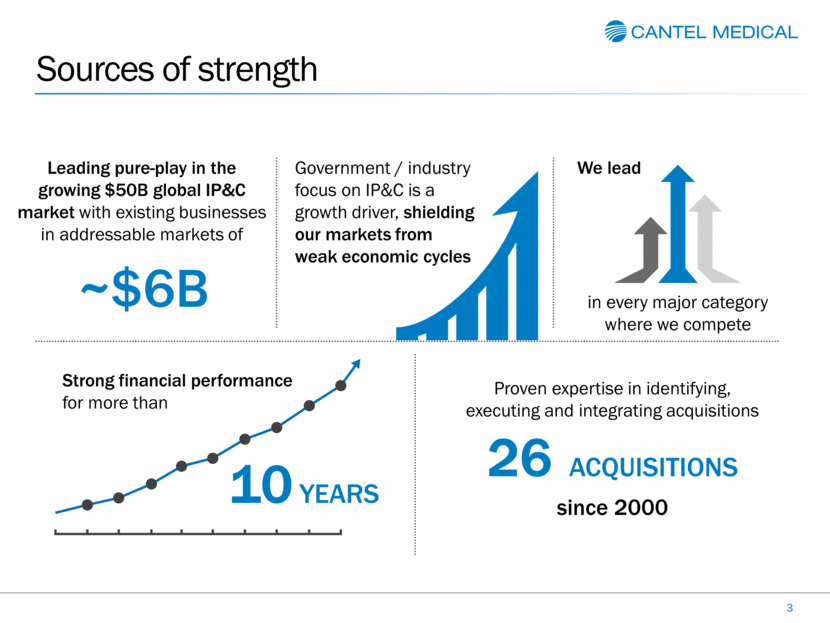
~$6B Sources of strength 3 Leading pure-play in the growing $50B global IP&C market with existing businesses in addressable markets of Government / industry focus on IP&C is a growth driver, shielding our markets from weak economic cycles We lead Strong financial performance for more than Proven expertise in identifying, executing and integrating acquisitions in every major category where we compete 10 YEARS 26 ACQUISITIONS since 2000 |
|
|
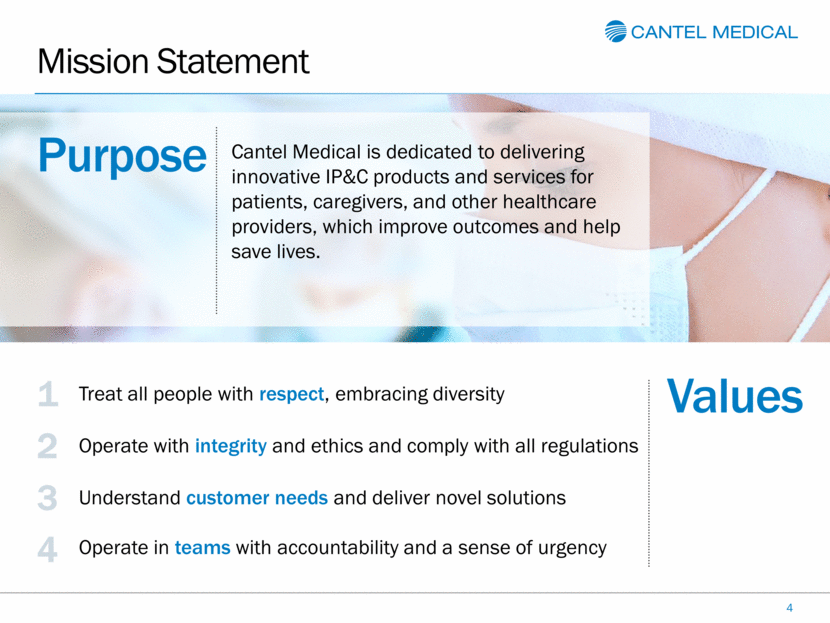
Mission Statement 4 Cantel Medical is dedicated to delivering innovative IP&C products and services for patients, caregivers, and other healthcare providers, which improve outcomes and help save lives. 3 Understand customer needs and deliver novel solutions 4 Operate in teams with accountability and a sense of urgency 1 Treat all people with respect, embracing diversity 2 Operate with integrity and ethics and comply with all regulations Values Purpose |
|
|
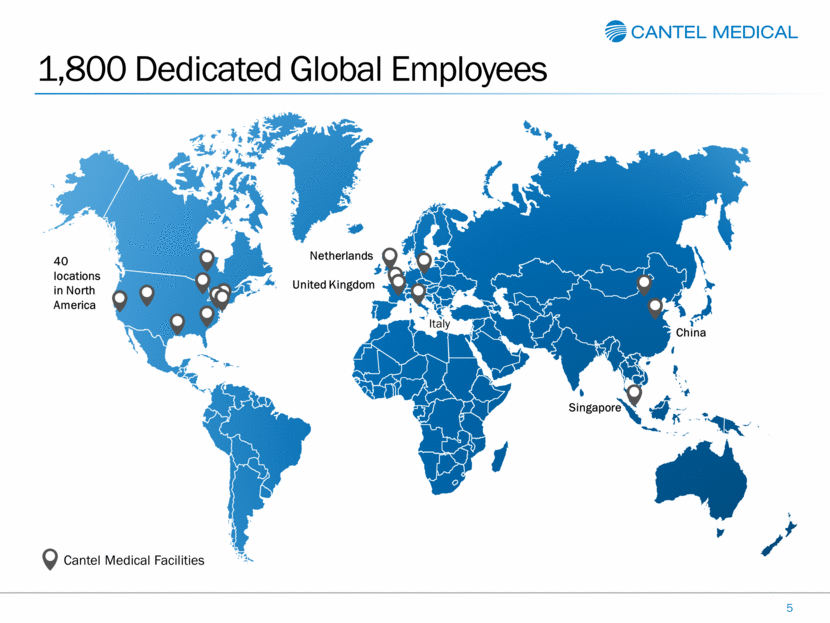
1,800 Dedicated Global Employees 5 Cantel Medical Facilities 40 locations in North America Netherlands Singapore China United Kingdom Italy Italy |
|
|
Infection Prevention & Control Markets 6 Over 80 million procedures annually growing at an estimated 5% CAGR2 Colorectal cancer is a leading global cause of death 2 MILLION US HEALTHCARE ASSOCIATED INFECTIONS (HAIs) PER YEAR COST THE HEALTHCARE SYSTEM $33 BILLION ANNUALLY1 Endoscopy ESRD patient population of 448,000 growing at 3-4% annually3 De novo clinic build growing at 5% annually3 Total of 6,284 dialysis clinics in the US3 Water Purification – Dialysis Medical Water Healthcare Disposables Approximately 150,000 dentists in the US 3.6 million dental visits per year in the US4 1 Klevens, R. Monina, et. al. (2002) “Estimating Healthcare-Associated Infections and Deaths in U.S. Hospitals”, CDC Public Health Reports March-April 2007, Volume 122 2 2010 US Report on Endoscopy, Millennium Research Group; 2014 European Markets for Gastrointestinal Endoscopic Devices, iData Research; 2012 China GI Census, Chinese Society of Digestive Endoscopy 3 2014 US Renal Data System, http://www.usrds.org/adr.aspx 4 2010 American Dental Association, Surveys of Dental Practice, http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/10_sdpc.ashx |
|
|
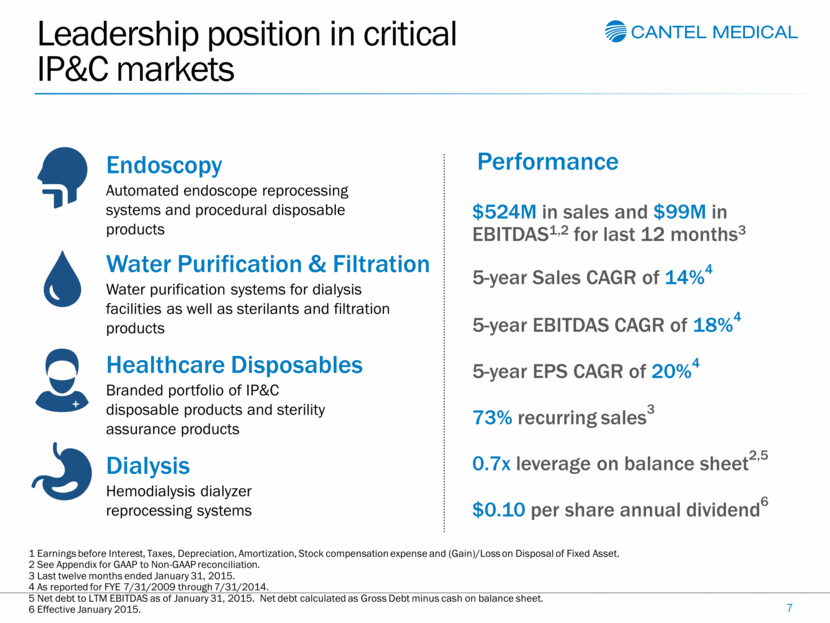
Leadership position in critical IP&C markets 7 Endoscopy Automated endoscope reprocessing systems and procedural disposable products Water Purification & Filtration Water purification systems for dialysis facilities as well as sterilants and filtration products Healthcare Disposables Branded portfolio of IP&C disposable products and sterility assurance products Dialysis Hemodialysis dialyzer reprocessing systems Performance $524M in sales and $99M in EBITDAS1,2 for last 12 months3 5-year Sales CAGR of 14%4 5-year EBITDAS CAGR of 18%4 5-year EPS CAGR of 20%4 73% recurring sales3 0.7x leverage on balance sheet2,5 $0.10 per share annual dividend6 1 Earnings before Interest, Taxes, Depreciation, Amortization, Stock compensation expense and (Gain)/Loss on Disposal of Fixed Asset. 2 See Appendix for GAAP to Non-GAAP reconciliation. 3 Last twelve months ended January 31, 2015. 4 As reported for FYE 7/31/2009 through 7/31/2014. 5 Net debt to LTM EBITDAS as of January 31, 2015. Net debt calculated as Gross Debt minus cash on balance sheet. 6 Effective January 2015. |
|
|
Three Synergistic Segments 8 Endoscopy Water Purification & Filtration Healthcare Disposables Endoscopy Reprocessing & Procedure Room Products Medical & Life Science Water Purification & Filters Dental, Hospital, Sterility Assurance Chemistries, Manufacturing, Regulatory, R&D, HR, International Infrastructure, Supply Chain |
|
|
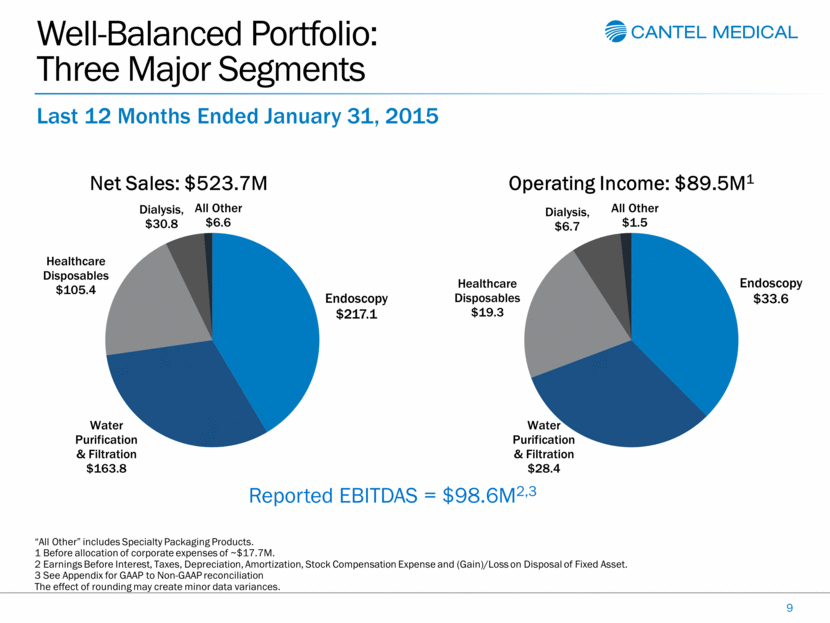
Well-Balanced Portfolio: Three Major Segments 9 Last 12 Months Ended January 31, 2015 Reported EBITDAS = $98.6M2,3 “All Other” includes Specialty Packaging Products. 1 Before allocation of corporate expenses of ~$17.7M. 2 Earnings Before Interest, Taxes, Depreciation, Amortization, Stock Compensation Expense and (Gain)/Loss on Disposal of Fixed Asset. 3 See Appendix for GAAP to Non-GAAP reconciliation The effect of rounding may create minor data variances. Endoscopy $217.1 Water Purification & Filtration $163.8 Healthcare Disposables $105.4 Dialysis , $30.8 All Other $6.6 Net Sales: $523.7M Endoscopy $33.6 Water Purification & Filtration $28.4 Healthcare Disposables $19.3 Dialysis , $6.7 All Other $1.5 Operating Income: $ 89.5M 1 |
|
|
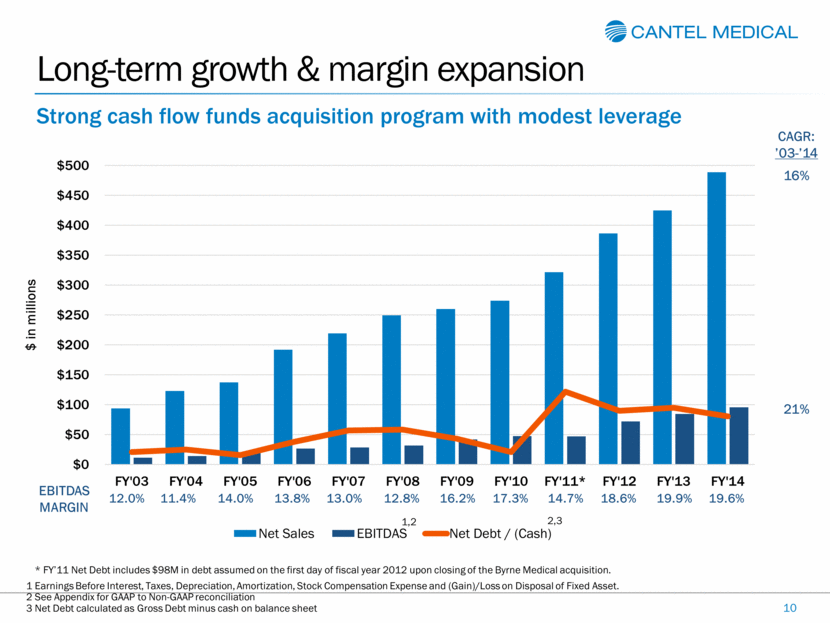
Long-term growth & margin expansion 10 Strong cash flow funds acquisition program with modest leverage 12.0% 11.4% 14.0% 13.8% 13.0% 12.8% 16.2% 17.3% 14.7% 18.6% 19.9% 19.6% EBITDAS MARGIN CAGR: ’03-’14 16% 21% * FY’11 Net Debt includes $98M in debt assumed on the first day of fiscal year 2012 upon closing of the Byrne Medical acquisition. 1 Earnings Before Interest, Taxes, Depreciation, Amortization, Stock Compensation Expense and (Gain)/Loss on Disposal of Fixed Asset. 2 See Appendix for GAAP to Non-GAAP reconciliation 3 Net Debt calculated as Gross Debt minus cash on balance sheet $0 $50 $100 $150 $200 $250 $300 $350 $400 $450 $500 FY'03 FY'04 FY'05 FY'06 FY'07 FY'08 FY'09 FY'10 FY'11* FY'12 FY'13 FY'14 $ in millions Net Sales EBITDAS Net Debt / (Cash) 1,2 2,3 |
|
|
Sustainable growth strategy 11 Market Expansion Strategic Acquisitions New Products CONTINUOUS IMPROVEMENT |
|
|
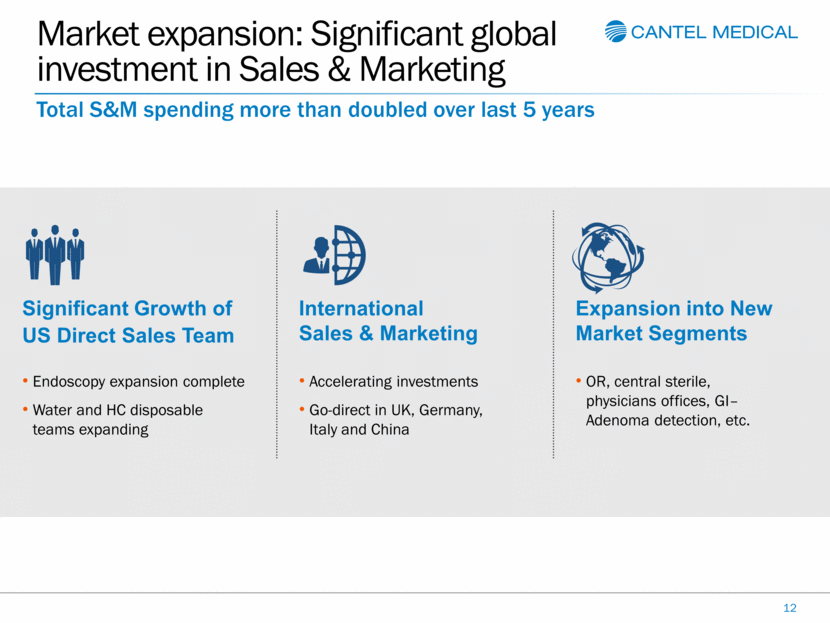
Market expansion: Significant global investment in Sales & Marketing 12 Total S&M spending more than doubled over last 5 years Endoscopy expansion complete Water and HC disposable teams expanding Significant Growth of US Direct Sales Team Accelerating investments Go-direct in UK, Germany, Italy and China International Sales & Marketing OR, central sterile, physicians offices, GI–Adenoma detection, etc. Expansion into New Market Segments |
|
|
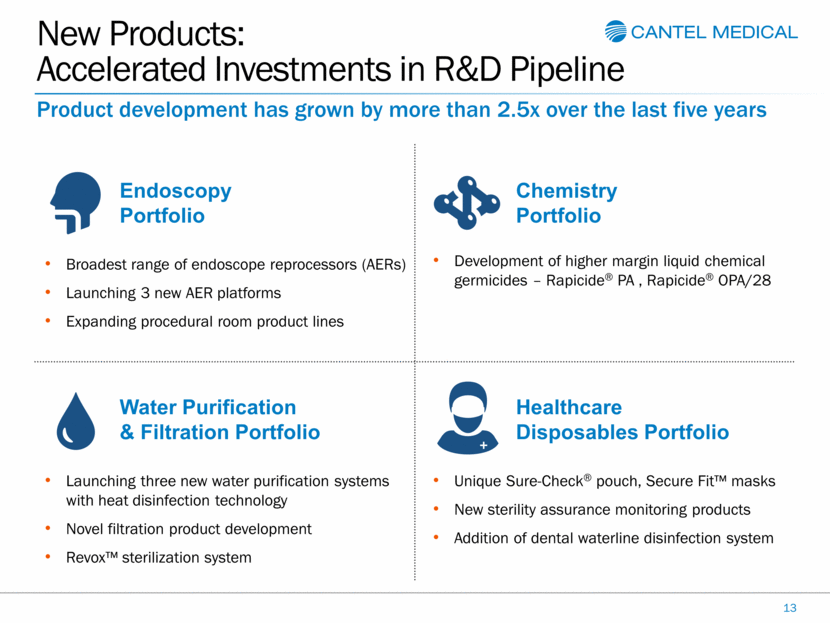
New Products: Accelerated Investments in R&D Pipeline 13 Product development has grown by more than 2.5x over the last five years Broadest range of endoscope reprocessors (AERs) Launching 3 new AER platforms Expanding procedural room product lines Endoscopy Portfolio Development of higher margin liquid chemical germicides – Rapicide® PA , Rapicide® OPA/28 Chemistry Portfolio Launching three new water purification systems with heat disinfection technology Novel filtration product development Revox™ sterilization system Water Purification & Filtration Portfolio Unique Sure-Check® pouch, Secure Fit™ masks New sterility assurance monitoring products Addition of dental waterline disinfection system Healthcare Disposables Portfolio |
|
|
Strategic acquisitions: Investing in all core segments & international capabilities 14 Proven ability to integrate, leverage & grow acquired businesses Byrne Medical, PuriCore (UK), IMS (Italy) New products, new markets, manufacturing expansion & international presence Endoscopy ConFirm Monitoring, SPS Medical, DentaPure Created leading sterility assurance monitoring platform and now dental waterline disinfection Healthcare Disposables Portfolio PuriCore (UK) becomes Cantel Medical (UK) IMS (Italy) becomes Cantel Medical (Italy) International Gambro, Siemens, Pure Water Solutions New technology, customer relationships & geographic footprint Water Purification & Filtration |
|
|
Long Term Growth Drivers: Endoscopy 15 Colorectal cancer is a leading global cause of death, driving worldwide screening and treatment efforts Improved adenoma detection rates will accelerate colonoscopy procedures LARGE AND GROWING GLOBAL ADDRESSABLE MARKET TOTALING OVER $4 BILLION Single-shot AERs drive continued high-margin chemistry growth Dedicated US Sales and Service team driving full circle solutions a key differentiator New product development including 3 new AER platforms and acquisition opportunities Recent European acquisitions provide great expansion opportunities Go-direct international implementations in the UK, Italy, Germany and China |
|
|
Long Term Growth Drivers: Water Purification & Filtration 16 End-Stage Renal Disease patient demographics drive de novo clinic build in the US Improved IP&C standards for dialysis patients, labor savings to facility operators Upgrade opportunity: 6,000+ US clinics today, majority use old technology Market adoption of heat-based water disinfection technology New opportunities in filtration and chemical sterilants Launch of new Revox™ sterilization system New product development, international growth and acquisitions US ESRD patient population growing at 3-4% annually1 De novo clinic build increasing 5% annually1 1 2014 US Renal Data System, http://www.usrds.org/adr.aspx |
|
|
Long Term Growth Drivers: Healthcare Disposables 17 Sterility assurance category adds high-growth, higher-margin platform and call point in hospital channel Rapicide® OPA/28 launched in the United States, now launching in international markets DentaPure® Waterline Disinfection System adds new growth category Innovative high-margin face masks/pandemic preparedness International growth opportunities New product development and acquisition opportunities LEADING BRANDED PLAYER IN THE US DENTAL MARKET Approximately 150,000 dentists in the US 3.6 million patient dental visits annually1 1 2010 American Dental Association, Surveys of Dental Practice, http://www.ada.org/~/media/ADA/Science%20and%20Research/HPI/Files/10_sdpc.ashx |
|
|
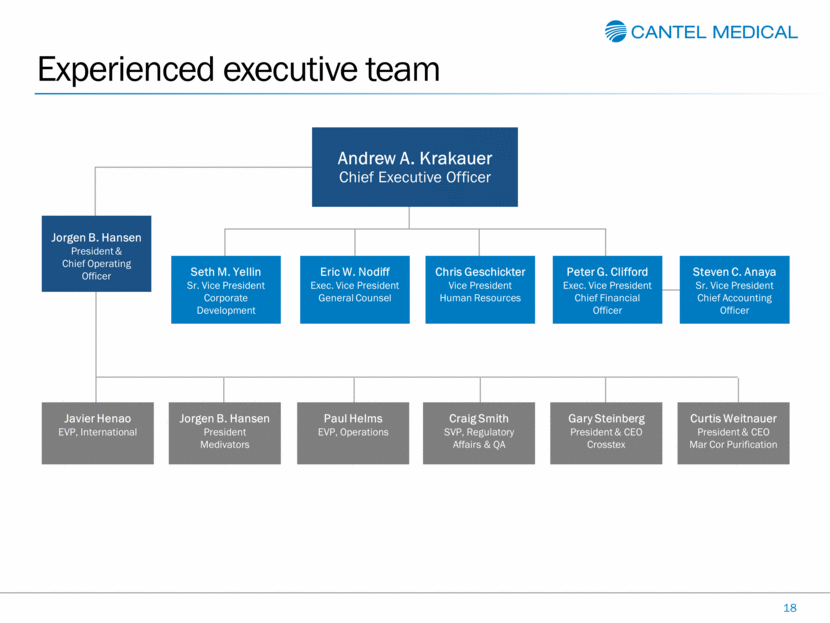
Experienced executive team 18 Jorgen B. Hansen President & Chief Operating Officer Andrew A. Krakauer Chief Executive Officer Peter G. Clifford Exec. Vice President Chief Financial Officer Eric W. Nodiff Exec. Vice President General Counsel Steven C. Anaya Sr. Vice President Chief Accounting Officer Chris Geschickter Vice President Human Resources Gary Steinberg President & CEO Crosstex Curtis Weitnauer President & CEO Mar Cor Purification Jorgen B. Hansen President Medivators Javier Henao EVP, International Paul Helms EVP, Operations Craig Smith SVP, Regulatory Affairs & QA Seth M. Yellin Sr. Vice President Corporate Development |
|
|
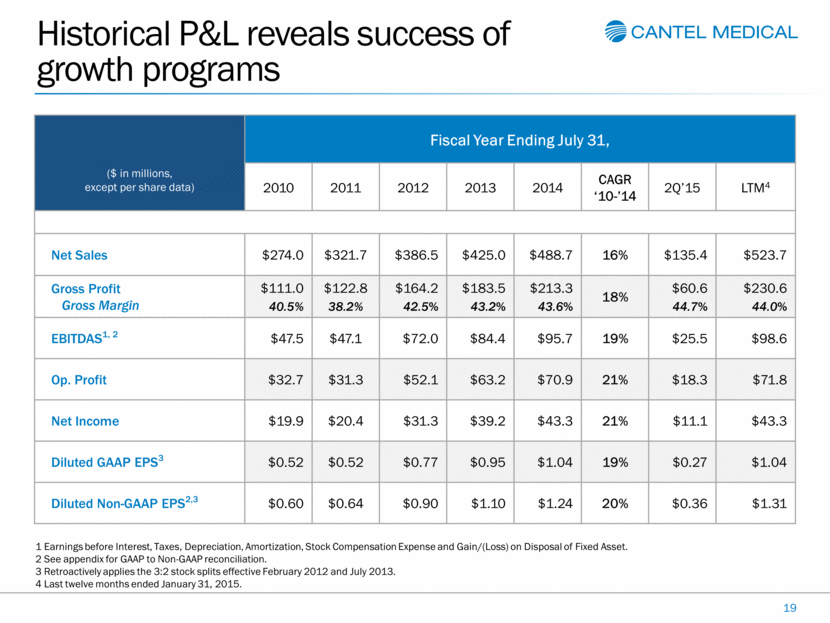
Historical P&L reveals success of growth programs 19 ($ in millions, except per share data) Fiscal Year Ending July 31, 2010 2011 2012 2013 2014 CAGR ‘10-’14 2Q’15 LTM4 Net Sales $274.0 $321.7 $386.5 $425.0 $488.7 16% $135.4 $523.7 Gross Profit Gross Margin $111.0 40.5% $122.8 38.2% $164.2 42.5% $183.5 43.2% $213.3 43.6% 18% $60.6 44.7% $230.6 44.0% EBITDAS1, 2 $47.5 $47.1 $72.0 $84.4 $95.7 19% $25.5 $98.6 Op. Profit $32.7 $31.3 $52.1 $63.2 $70.9 21% $18.3 $71.8 Net Income $19.9 $20.4 $31.3 $39.2 $43.3 21% $11.1 $43.3 Diluted GAAP EPS3 $0.52 $0.52 $0.77 $0.95 $1.04 19% $0.27 $1.04 Diluted Non-GAAP EPS2,3 $0.60 $0.64 $0.90 $1.10 $1.24 20% $0.36 $1.31 1 Earnings before Interest, Taxes, Depreciation, Amortization, Stock Compensation Expense and Gain/(Loss) on Disposal of Fixed Asset. 2 See appendix for GAAP to Non-GAAP reconciliation. 3 Retroactively applies the 3:2 stock splits effective February 2012 and July 2013. 4 Last twelve months ended January 31, 2015. |
|
|
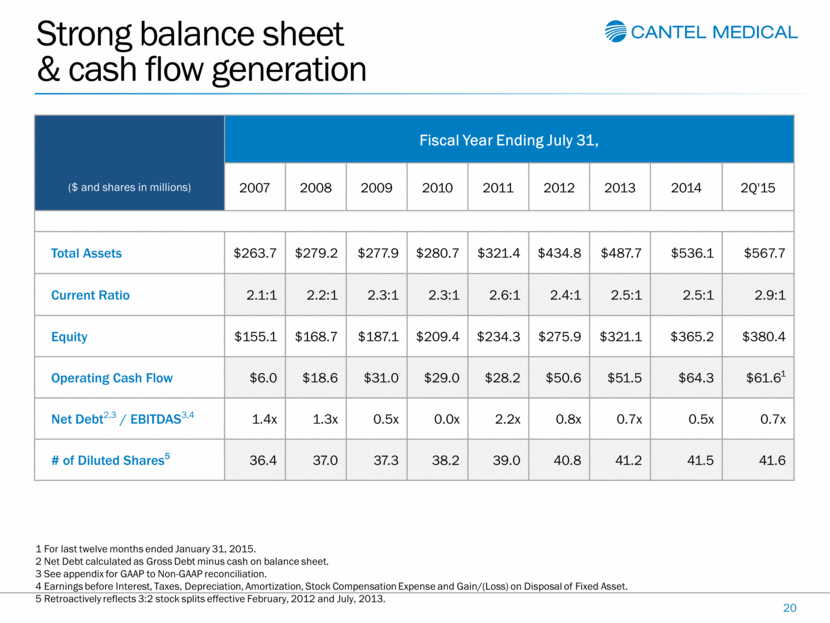
Strong balance sheet & cash flow generation 20 ($ and shares in millions) Fiscal Year Ending July 31, 2007 2008 2009 2010 2011 2012 2013 2014 2Q'15 Total Assets $263.7 $279.2 $277.9 $280.7 $321.4 $434.8 $487.7 $536.1 $567.7 Current Ratio 2.1:1 2.2:1 2.3:1 2.3:1 2.6:1 2.4:1 2.5:1 2.5:1 2.9:1 Equity $155.1 $168.7 $187.1 $209.4 $234.3 $275.9 $321.1 $365.2 $380.4 Operating Cash Flow $6.0 $18.6 $31.0 $29.0 $28.2 $50.6 $51.5 $64.3 $61.61 Net Debt2,3 / EBITDAS3,4 1.4x 1.3x 0.5x 0.0x 2.2x 0.8x 0.7x 0.5x 0.7x # of Diluted Shares5 36.4 37.0 37.3 38.2 39.0 40.8 41.2 41.5 41.6 1 For last twelve months ended January 31, 2015. 2 Net Debt calculated as Gross Debt minus cash on balance sheet. 3 See appendix for GAAP to Non-GAAP reconciliation. 4 Earnings before Interest, Taxes, Depreciation, Amortization, Stock Compensation Expense and Gain/(Loss) on Disposal of Fixed Asset. 5 Retroactively reflects 3:2 stock splits effective February, 2012 and July, 2013. |
|
|
5-Yr Strategic Plan: Double Sales & EPS from FY13 to FY18 Successfully completed year 1 of plan Continued investments in high-growth, high-margin product lines Focus on channel expansion including international and hospital markets Execute proven acquisition program to accelerate global growth Accelerate focus on operational excellence Investment in operational capabilities Ongoing investment in people 21 |
|
|
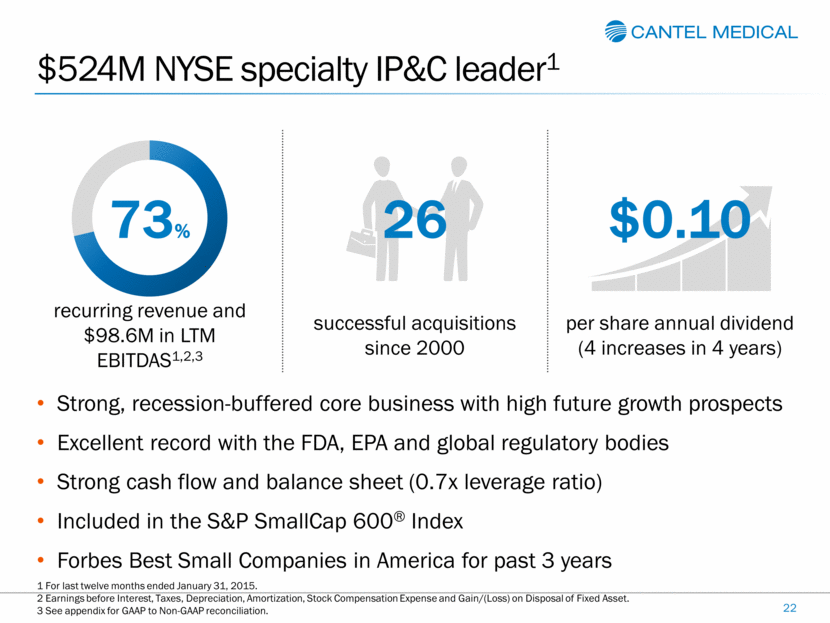
$524M NYSE specialty IP&C leader1 Strong, recession-buffered core business with high future growth prospects Excellent record with the FDA, EPA and global regulatory bodies Strong cash flow and balance sheet (0.7x leverage ratio) Included in the S&P SmallCap 600® Index Forbes Best Small Companies in America for past 3 years 73% 26 $0.10 recurring revenue and $98.6M in LTM EBITDAS1,2,3 successful acquisitions since 2000 per share annual dividend (4 increases in 4 years) 22 1 For last twelve months ended January 31, 2015. 2 Earnings before Interest, Taxes, Depreciation, Amortization, Stock Compensation Expense and Gain/(Loss) on Disposal of Fixed Asset. 3 See appendix for GAAP to Non-GAAP reconciliation. |
|
|
appendix |
|
|
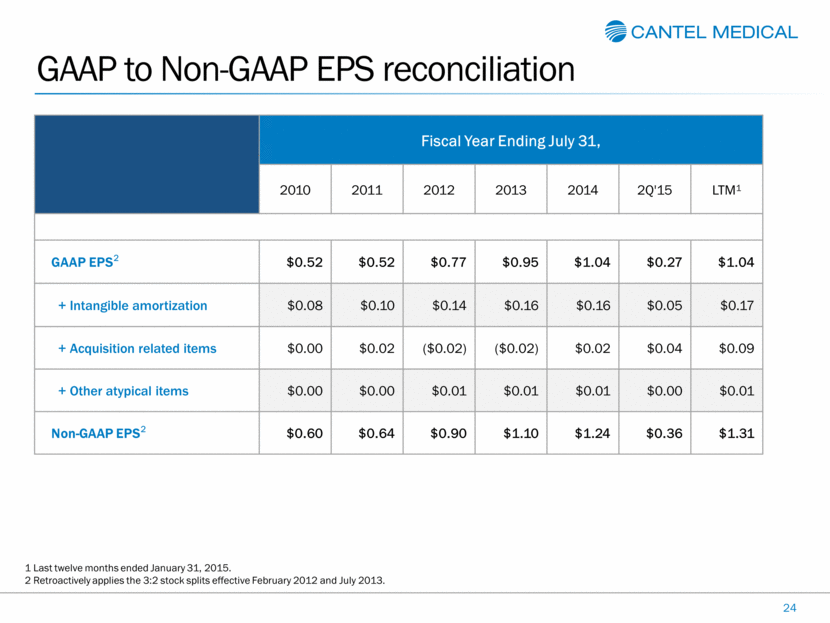
GAAP to Non-GAAP EPS reconciliation 24 Fiscal Year Ending July 31, 2010 2011 2012 2013 2014 2Q'15 LTM1 GAAP EPS2 $0.52 $0.52 $0.77 $0.95 $1.04 $0.27 $1.04 + Intangible amortization $0.08 $0.10 $0.14 $0.16 $0.16 $0.05 $0.17 + Acquisition related items $0.00 $0.02 ($0.02) ($0.02) $0.02 $0.04 $0.09 + Other atypical items $0.00 $0.00 $0.01 $0.01 $0.01 $0.00 $0.01 Non-GAAP EPS2 $0.60 $0.64 $0.90 $1.10 $1.24 $0.36 $1.31 1 Last twelve months ended January 31, 2015. 2 Retroactively applies the 3:2 stock splits effective February 2012 and July 2013. |
|
|
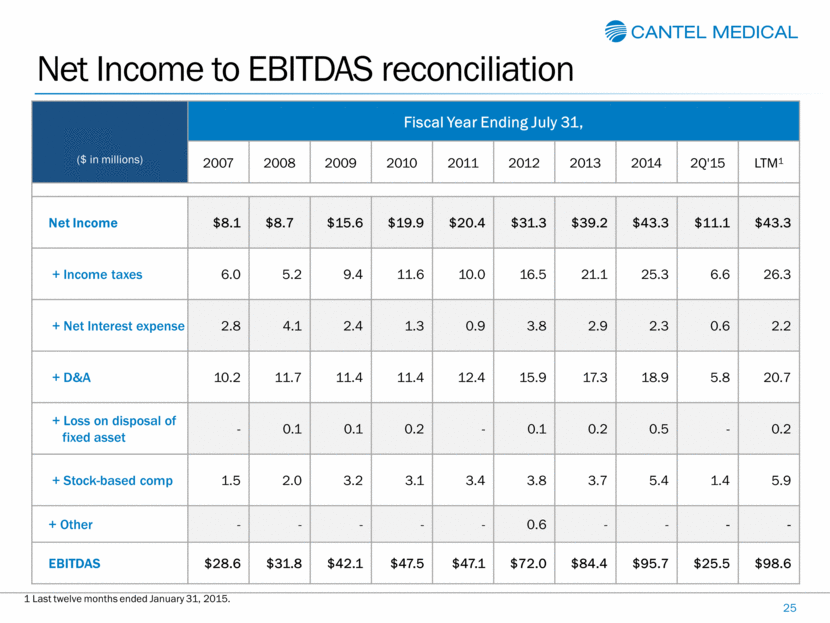
Net Income to EBITDAS reconciliation 25 ($ in millions) Fiscal Year Ending July 31, 2007 2008 2009 2010 2011 2012 2013 2014 2Q'15 LTM1 Net Income $8.1 $8.7 $15.6 $19.9 $20.4 $31.3 $39.2 $43.3 $11.1 $43.3 + Income taxes 6.0 5.2 9.4 11.6 10.0 16.5 21.1 25.3 6.6 26.3 + Net Interest expense 2.8 4.1 2.4 1.3 0.9 3.8 2.9 2.3 0.6 2.2 + D&A 10.2 11.7 11.4 11.4 12.4 15.9 17.3 18.9 5.8 20.7 + Loss on disposal of fixed asset - 0.1 0.1 0.2 - 0.1 0.2 0.5 - 0.2 + Stock-based comp 1.5 2.0 3.2 3.1 3.4 3.8 3.7 5.4 1.4 5.9 + Other - - - - - 0.6 - - - - EBITDAS $28.6 $31.8 $42.1 $47.5 $47.1 $72.0 $84.4 $95.7 $25.5 $98.6 1 Last twelve months ended January 31, 2015. |
|
|
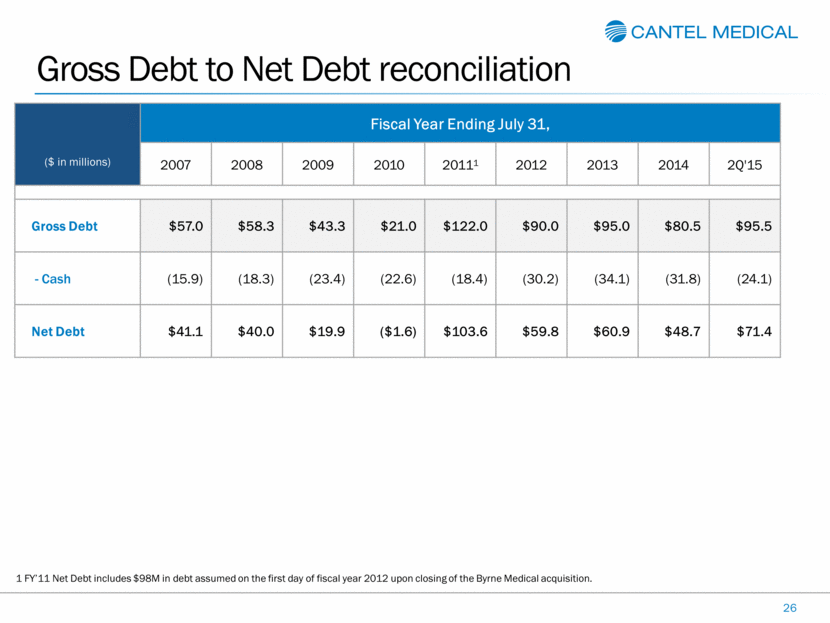
Gross Debt to Net Debt reconciliation 26 ($ in millions) Fiscal Year Ending July 31, 2007 2008 2009 2010 20111 2012 2013 2014 2Q'15 Gross Debt $57.0 $58.3 $43.3 $21.0 $122.0 $90.0 $95.0 $80.5 $95.5 - Cash (15.9) (18.3) (23.4) (22.6) (18.4) (30.2) (34.1) (31.8) (24.1) Net Debt $41.1 $40.0 $19.9 ($1.6) $103.6 $59.8 $60.9 $48.7 $71.4 1 FY’11 Net Debt includes $98M in debt assumed on the first day of fiscal year 2012 upon closing of the Byrne Medical acquisition. |
|
|
GAAP to Non-GAAP EPS disclosure 27 Non-GAAP financial measures contained herein supplement information previously reported in filings on Form 10-Q and Form 10-K as well as in presentations by Company management to investors, analysts and others. The information below will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or incorporated by reference in any filing under the Securities Act of 1933, as amended. Non-GAAP Financial Measures In evaluating our operating performance, we supplement the reporting of our financial information determined under accounting principles generally accepted in the United States (“GAAP”) with certain internally driven non-GAAP financial measures, namely (a) adjusted diluted earnings per share (“EPS”), (b) income before interest, taxes, depreciation, amortization and stock-based compensation expense ("EBITDAS") and (c) net debt. These non-GAAP financial measures are indicators of the Company’s performance that is not required by, or presented in accordance with, GAAP. They are presented with the intent of providing greater transparency to financial information used by us in our financial analysis and operational decision-making. We believe that these non-GAAP measures provide meaningful information to assist investors, shareholders and other readers of our Condensed Consolidated Financial Statements in making comparisons to our historical operating results, analyzing the underlying performance of our results of operations and assessing our financial strength. These non-GAAP financial measures are not intended to be, and should not be, considered separately from, or as an alternative to, the most directly comparable GAAP financial measures. (a) EPS We define adjusted diluted EPS as diluted EPS adjusted to exclude amortization, acquisition related items, significant reorganization and restructuring charges, major tax events and other significant items management deems atypical or non-operating in nature. Intangible Amortization Amortization expense, which is excluded from adjusted diluted EPS for all periods presented below, is a non-cash expense related to intangibles that were primarily the result of business acquisitions. Our history of acquiring businesses has resulted in significant increases in amortization of intangible assets that reduced the Company’s net income. The removal of amortization from our overall operating performance helps in assessing our cash generated from operations including our return on invested capital, which we believe is an important analysis for measuring our ability to generate cash and invest in our continued growth. |
|
|
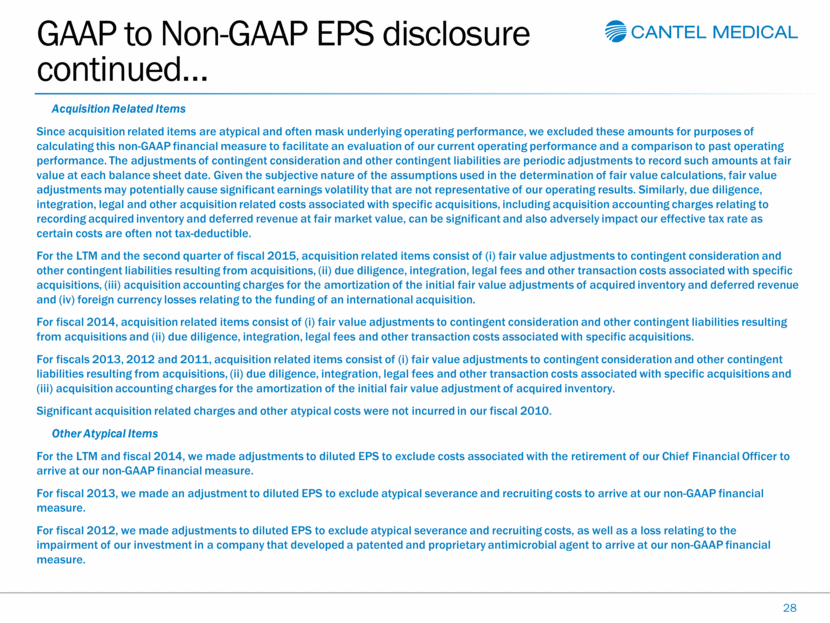
GAAP to Non-GAAP EPS disclosure continued 28 Acquisition Related Items Since acquisition related items are atypical and often mask underlying operating performance, we excluded these amounts for purposes of calculating this non-GAAP financial measure to facilitate an evaluation of our current operating performance and a comparison to past operating performance. The adjustments of contingent consideration and other contingent liabilities are periodic adjustments to record such amounts at fair value at each balance sheet date. Given the subjective nature of the assumptions used in the determination of fair value calculations, fair value adjustments may potentially cause significant earnings volatility that are not representative of our operating results. Similarly, due diligence, integration, legal and other acquisition related costs associated with specific acquisitions, including acquisition accounting charges relating to recording acquired inventory and deferred revenue at fair market value, can be significant and also adversely impact our effective tax rate as certain costs are often not tax-deductible. For the LTM and the second quarter of fiscal 2015, acquisition related items consist of (i) fair value adjustments to contingent consideration and other contingent liabilities resulting from acquisitions, (ii) due diligence, integration, legal fees and other transaction costs associated with specific acquisitions, (iii) acquisition accounting charges for the amortization of the initial fair value adjustments of acquired inventory and deferred revenue and (iv) foreign currency losses relating to the funding of an international acquisition. For fiscal 2014, acquisition related items consist of (i) fair value adjustments to contingent consideration and other contingent liabilities resulting from acquisitions and (ii) due diligence, integration, legal fees and other transaction costs associated with specific acquisitions. For fiscals 2013, 2012 and 2011, acquisition related items consist of (i) fair value adjustments to contingent consideration and other contingent liabilities resulting from acquisitions, (ii) due diligence, integration, legal fees and other transaction costs associated with specific acquisitions and (iii) acquisition accounting charges for the amortization of the initial fair value adjustment of acquired inventory. Significant acquisition related charges and other atypical costs were not incurred in our fiscal 2010. Other Atypical Items For the LTM and fiscal 2014, we made adjustments to diluted EPS to exclude costs associated with the retirement of our Chief Financial Officer to arrive at our non-GAAP financial measure. For fiscal 2013, we made an adjustment to diluted EPS to exclude atypical severance and recruiting costs to arrive at our non-GAAP financial measure. For fiscal 2012, we made adjustments to diluted EPS to exclude atypical severance and recruiting costs, as well as a loss relating to the impairment of our investment in a company that developed a patented and proprietary antimicrobial agent to arrive at our non-GAAP financial measure. |
|
|
GAAP to Non-GAAP EPS disclosure continued 29 (b) EBITDAS The Company believes EBITDAS is an important valuation measurement for management and investors given the increasing effect that non-cash charges, such as stock-based compensation, amortization related to acquisitions and depreciation of capital equipment, has on the Company's net income. In particular, acquisitions have historically resulted in significant increases in amortization of intangible assets that reduce the Company's net income. Additionally, the Company regards EBITDAS as a useful measure of operating performance and cash flow before the effect of interest expense and complements operating income, net income and other GAAP financial performance measures. (c ) Net Debt We define net debt as long-term debt less cash and cash equivalents. Each of the components of net debt appears in our Consolidated Balance Sheets. We believe that the presentation of net debt provides useful information to investors because we review net debt as part of our management of our overall liquidity, financial flexibility, capital structure and leverage. |