Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zyla Life Sciences | a15-7463_38k.htm |
Exhibit 99.1
|
|
Corporate update March 2015 Delivering pain relief with peace of mind NASDAQ: EGLT 1 |
|
|
Forward-Looking Statements 2 Statements included that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties and risks. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: the success of integrating recent acquisitions; our ability to obtain regulatory approval of our product candidates; our ability to successfully commercialize SPRIX and OXAYDO; competitive factors; general market conditions; and other risk factors described in Egalet's filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this presentation whether as a result of new information or future events, except as may be required by law. Please refer to www.oxaydo.com and www.sprix.com for full prescribing information. |
|
|
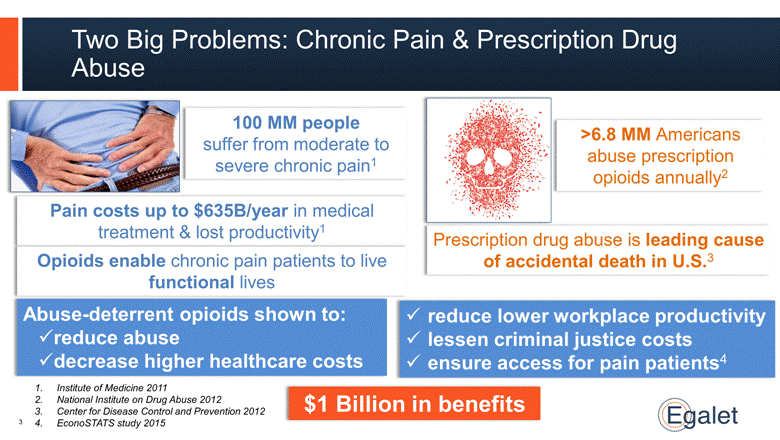
Prescription drug abuse is leading cause of accidental death in U.S.3 Two Big Problems: Chronic Pain & Prescription Drug Abuse >6.8 MM Americans abuse prescription opioids annually2 100 MM people suffer from moderate to severe chronic pain1 Pain costs up to $635B/year in medical treatment & lost productivity1 Opioids enable chronic pain patients to live functional lives Abuse-deterrent opioids shown to: reduce abuse decrease higher healthcare costs Institute of Medicine 2011 National Institute on Drug Abuse 2012 Center for Disease Control and Prevention 2012 EconoSTATS study 2015 reduce lower workplace productivity lessen criminal justice costs ensure access for pain patients4 $1 Billion in benefits 3 |
|
|
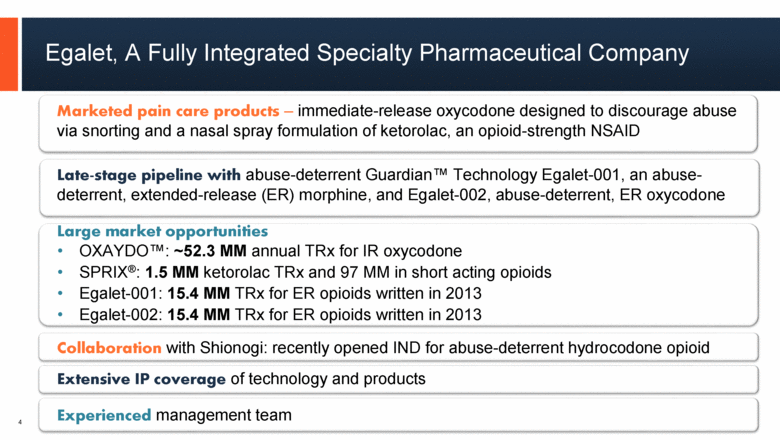
4 Egalet, A Fully Integrated Specialty Pharmaceutical Company 4 Marketed pain care products – immediate-release oxycodone designed to discourage abuse via snorting and a nasal spray formulation of ketorolac, an opioid-strength NSAID Late-stage pipeline with abuse-deterrent Guardian™ Technology Egalet-001, an abuse-deterrent, extended-release (ER) morphine, and Egalet-002, abuse-deterrent, ER oxycodone Large market opportunities OXAYDO™: ~52.3 MM annual TRx for IR oxycodone SPRIX®: 1.5 MM ketorolac TRx and 97 MM in short acting opioids Egalet-001: 15.4 MM TRx for ER opioids written in 2013 Egalet-002: 15.4 MM TRx for ER opioids written in 2013 Collaboration with Shionogi: recently opened IND for abuse-deterrent hydrocodone opioid Extensive IP coverage of technology and products Experienced management team |
|
|
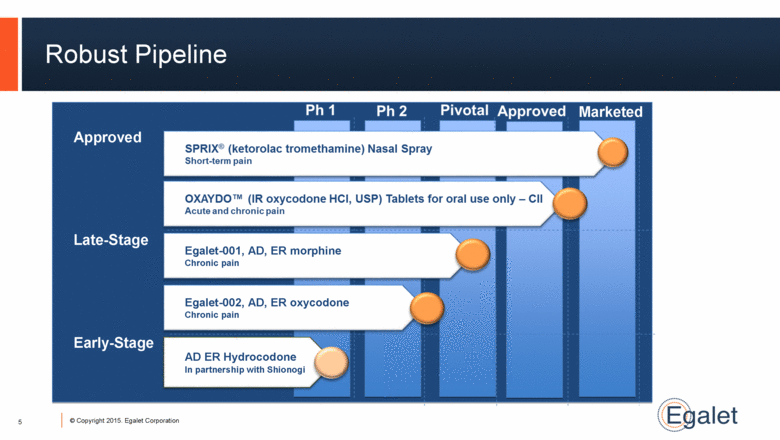
Robust Pipeline 5 Approved Egalet-001, AD, ER morphine Chronic pain Egalet-002, AD, ER oxycodone Chronic pain Late-Stage Early-Stage Marketed AD ER Hydrocodone In partnership with Shionogi |
|
|
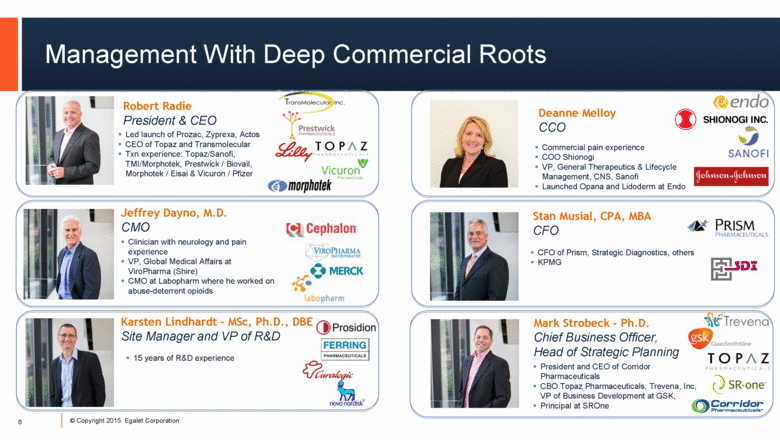
Management With Deep Commercial Roots 6 Robert Radie President & CEO Led launch of Prozac, Zyprexa, Actos CEO of Topaz and Transmolecular Txn experience: Topaz/Sanofi, TMI/Morphotek, Prestwick / Biovail, Morphotek / Eisai & Vicuron / Pfizer Jeffrey Dayno, M.D. CMO Clinician with neurology and pain experience VP, Global Medical Affairs at ViroPharma (Shire) CMO at Labopharm where he worked on abuse-deterrent opioids Karsten Lindhardt – MSc, Ph.D., DBE Site Manager and VP of R&D 15 years of R&D experience Deanne Melloy CCO Commercial pain experience COO Shionogi VP, General Therapeutics & Lifecycle Management, CNS, Sanofi Launched Opana and Lidoderm at Endo Stan Musial, CPA, MBA CFO CFO of Prism, Strategic Diagnostics, others KPMG Mark Strobeck - Ph.D. Chief Business Officer, Head of Strategic Planning President and CEO of Corridor Pharmaceuticals CBO Topaz Pharmaceuticals, Trevena, Inc, VP of Business Development at GSK, Principal at SROne |
|
|
Management With Deep Commercial Roots 7 Tim Cochran Vice president sales and market access Ryan Daufenbach Executive director marketing Donna Zarycranski Executive director medical affairs Robert Radie President & CEO Led launch of Prozac, Zyprexa, Actos CEO of Topaz and Transmolecular Txn experience: Topaz/Sanofi, TMI/Morphotek, Prestwick / Biovail, Morphotek / Eisai & Vicuron / Pfizer Deanne Melloy CCO Commercial pain experience COO Shionogi VP, General Therapeutics & Lifecycle Management, CNS, Sanofi Launched Opana and Lidoderm at Endo Jeffrey Dayno, M.D. CMO Clinician with neurology and pain experience VP, Global Medical Affairs at ViroPharma (Shire) CMO at Labopharm where he worked on abuse-deterrent opioids |
|
|
Mission: bring peace of mind to patients and healthcare providers Building a pain franchise OXAYDO, first and only immediate-release oxycodone designed to discourage abuse associated with snorting SPRIX, an opioid-strength NSAID for short-term pain Guardian™ Technology pipeline including Egalet-001 and Egalet-002 Focus on ~5,700 high decile healthcare providers prescribing pain medications Establishes commercial presence to ensure successful launch of Guardian Technology abuse-deterrent products Focused Approach on HCPs Prescribing Pain Medications 8 |
|
|
5 Region Business Directors P&L owners 50 - 60 Territory Managers Top volume markets based on short acting opioids, oxycodone IR and branded ER-LA opioids Differential deployment based on market potential and access Performance-based incentives Building a Best in Class Dedicated Sales Organization 9 |
|
|
Selecting to Achieve Excellent Results 1-3 years of sales experience Not a traditional pharma sales team Proven account management skills 10 |
|
|
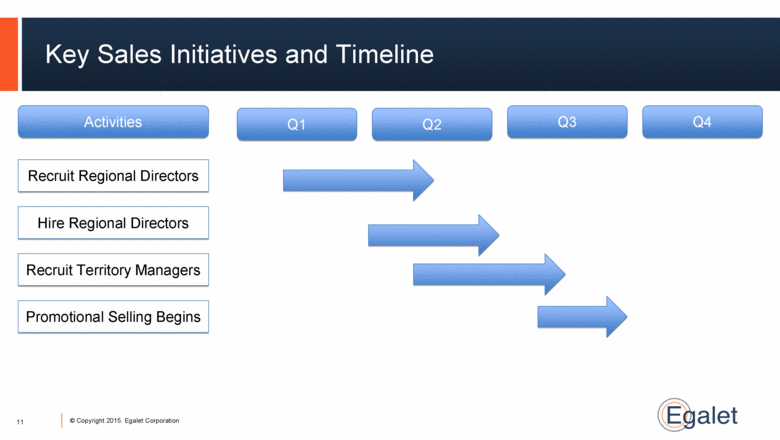
Key Sales Initiatives and Timeline Q1 Q2 Q3 Q4 Hire Regional Directors Recruit Territory Managers Promotional Selling Begins Recruit Regional Directors Activities 11 |
|
|
SPRIX Marketing Plan 12 |
|
|
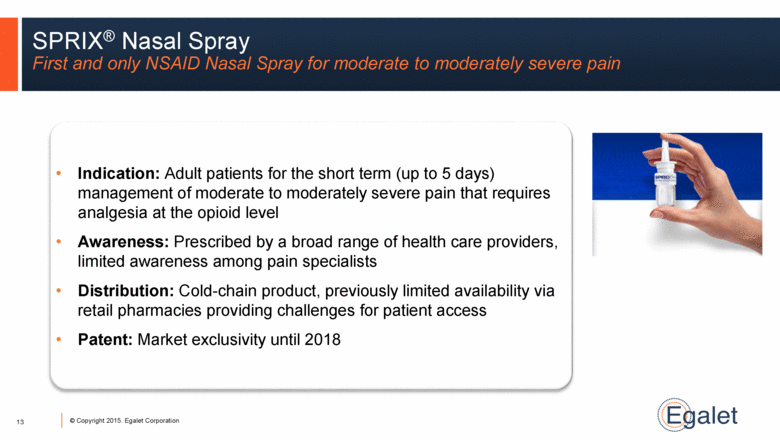
SPRIX® Nasal Spray First and only NSAID Nasal Spray for moderate to moderately severe pain Indication: Adult patients for the short term (up to 5 days) management of moderate to moderately severe pain that requires analgesia at the opioid level Awareness: Prescribed by a broad range of health care providers, limited awareness among pain specialists Distribution: Cold-chain product, previously limited availability via retail pharmacies providing challenges for patient access Patent: Market exclusivity until 2018 13 |
|
|
SPRIX® Market Opportunity 14 1.5 MM Ketorolac TRxs 97 MM TRxs Short-acting Analgesics Growth opportunity based on comfort with API Grow with efficacy profile: opioid-level analgesia |
|
|
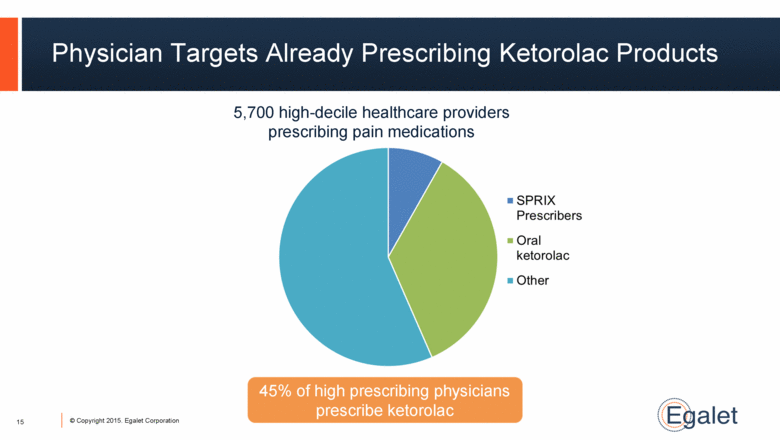
5,700 high-decile healthcare providers prescribing pain medications 45% of high prescribing physicians prescribe ketorolac Physician Targets Already Prescribing Ketorolac Products 15 |
|
|
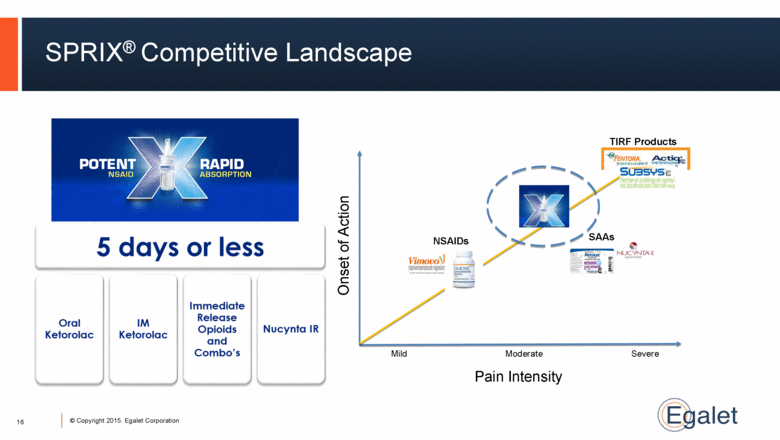
16 SPRIX® Competitive Landscape Pain Intensity Onset of Action TIRF Products SAAs Mild Moderate Severe NSAIDs 16 |
|
|
Maintain SPRIX utilization among current prescribers and reengage former prescribers Position SPRIX among pain healthcare providers for acute pain Strategy to Ensure Commercial Success of SPRIX® Ensure access for patients 3 17 2 1 |
|
|
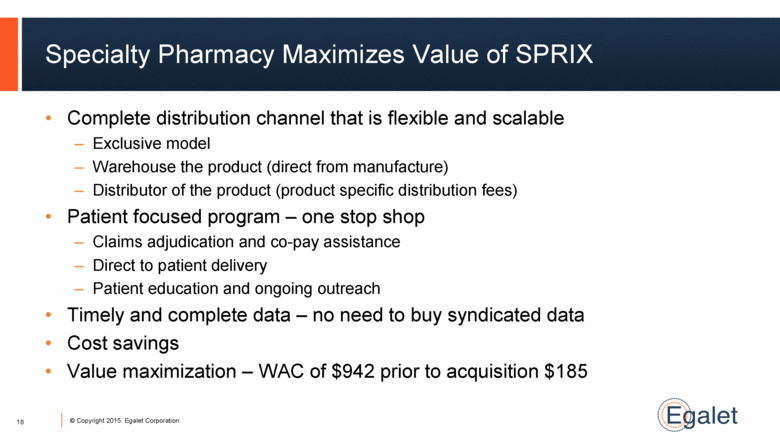
Specialty Pharmacy Maximizes Value of SPRIX Complete distribution channel that is flexible and scalable Exclusive model Warehouse the product (direct from manufacture) Distributor of the product (product specific distribution fees) Patient focused program – one stop shop Claims adjudication and co-pay assistance Direct to patient delivery Patient education and ongoing outreach Timely and complete data – no need to buy syndicated data Cost savings Value maximization – WAC of $942 prior to acquisition $185 18 |
|
|
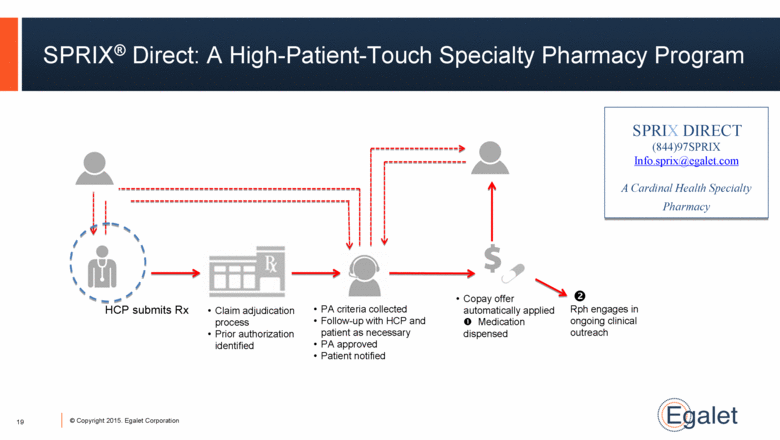
SPRIX® Direct: A High-Patient-Touch Specialty Pharmacy Program SPRIX DIRECT (844)97SPRIX Info.sprix@egalet.com A Cardinal Health Specialty Pharmacy HCP submits Rx Claim adjudication process Prior authorization identified PA criteria collected Follow-up with HCP and patient as necessary PA approved Patient notified Rph engages in ongoing clinical outreach Copay offer automatically applied Medication dispensed 19 |
|
|
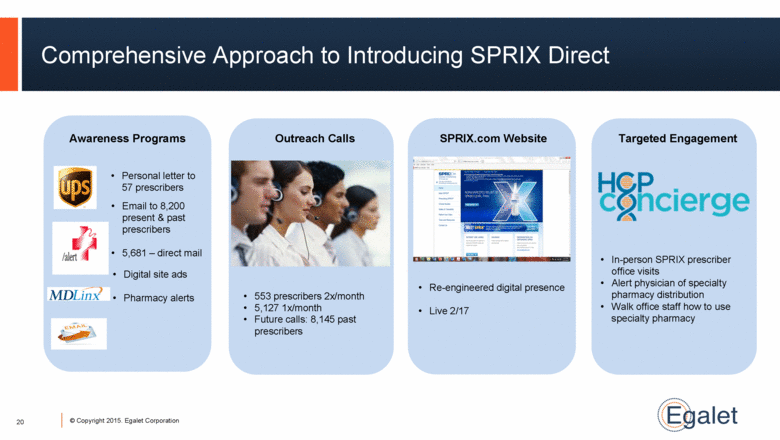
Comprehensive Approach to Introducing SPRIX Direct SPRIX.com Website Awareness Programs Personal letter to 57 prescribers Email to 8,200 present & past prescribers 5,681 – direct mail Digital site ads Pharmacy alerts Re-engineered digital presence Live 2/17 In-person SPRIX prescriber office visits Alert physician of specialty pharmacy distribution Walk office staff how to use specialty pharmacy 20 |
|
|
OXAYDO Commercial Launch Plan 21 |
|
|
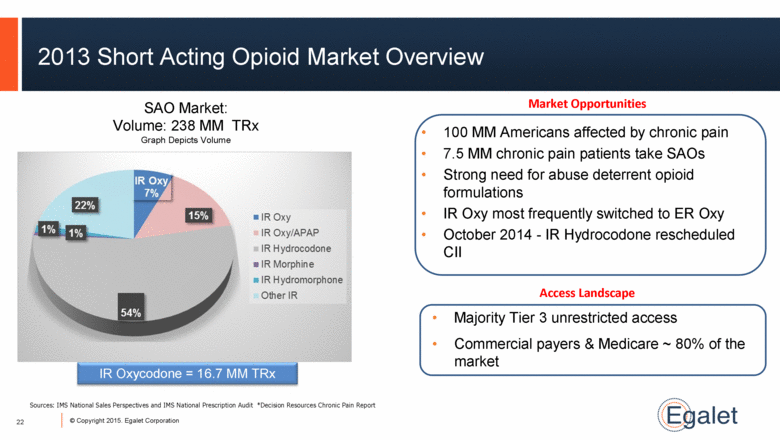
2013 Short Acting Opioid Market Overview Market Opportunities 100 MM Americans affected by chronic pain 7.5 MM chronic pain patients take SAOs Strong need for abuse deterrent opioid formulations IR Oxy most frequently switched to ER Oxy October 2014 - IR Hydrocodone rescheduled CII Access Landscape Majority Tier 3 unrestricted access Commercial payers & Medicare ~ 80% of the market IR Oxycodone = 16.7 MM TRx SAO Market: Volume: 238 MM TRx Graph Depicts Volume Sources: IMS National Sales Perspectives and IMS National Prescription Audit *Decision Resources Chronic Pain Report 22 |
|
|
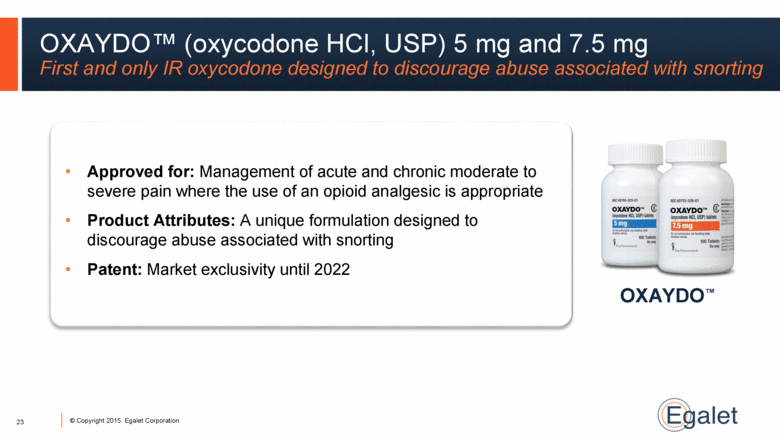
OXAYDO™ OXAYDO™ (oxycodone HCl, USP) 5 mg and 7.5 mg First and only IR oxycodone designed to discourage abuse associated with snorting 23 Approved for: Management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate Product Attributes: A unique formulation designed to discourage abuse associated with snorting Patent: Market exclusivity until 2022 |
|
|
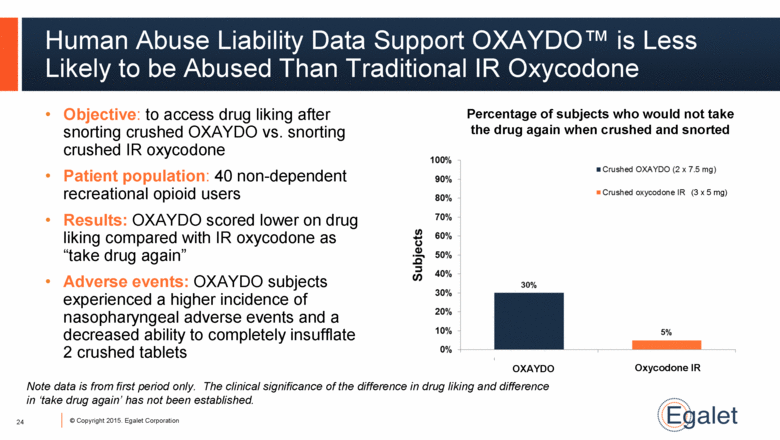
Human Abuse Liability Data Support OXAYDO™ is Less Likely to be Abused Than Traditional IR Oxycodone Objective: to access drug liking after snorting crushed OXAYDO vs. snorting crushed IR oxycodone Patient population: 40 non-dependent recreational opioid users Results: OXAYDO scored lower on drug liking compared with IR oxycodone as “take drug again” Adverse events: OXAYDO subjects experienced a higher incidence of nasopharyngeal adverse events and a decreased ability to completely insufflate 2 crushed tablets 24 Percentage of subjects who would not take the drug again when crushed and snorted OXAYDO Oxycodone IR (3 x 5 mg) Note data is from first period only. The clinical significance of the difference in drug liking and difference in ‘take drug again’ has not been established. |
|
|
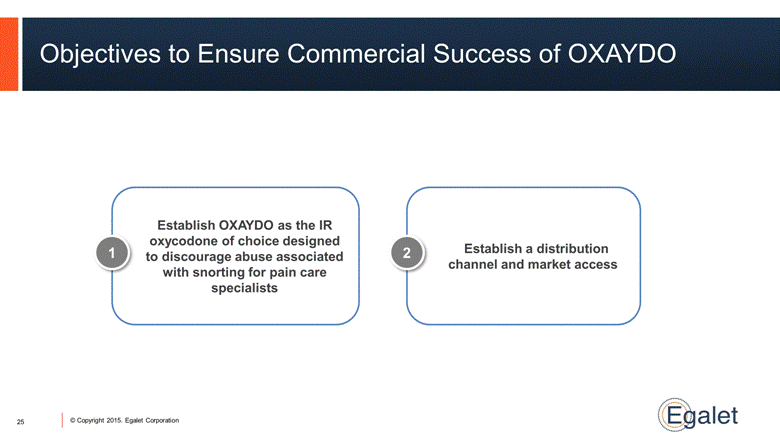
Establish OXAYDO as the IR oxycodone of choice designed to discourage abuse associated with snorting for pain care specialists Establish a distribution channel and market access Objectives to Ensure Commercial Success of OXAYDO 25 1 2 |
|
|
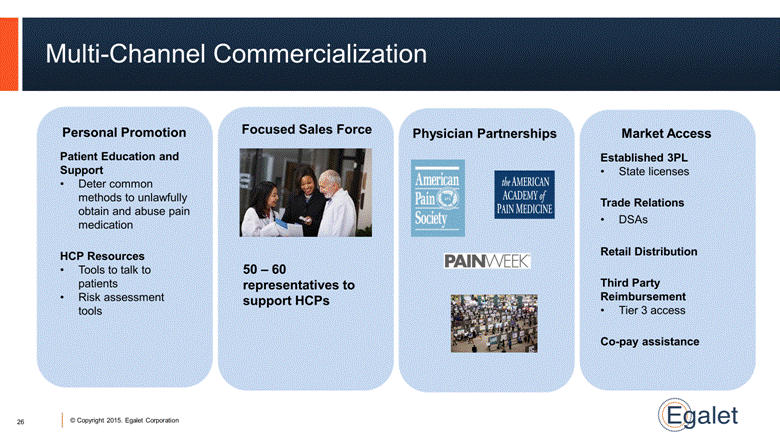
Multi-Channel Commercialization Physician Partnerships Focused Sales Force Personal Promotion Market Access Patient Education and Support Deter common methods to unlawfully obtain and abuse pain medication HCP Resources Tools to talk to patients Risk assessment tools 50 – 60 representatives to support HCPs Established 3PL State licenses Trade Relations DSAs Retail Distribution Third Party Reimbursement Tier 3 access Co-pay assistance 26 |
|
|
KOL Engagement 27 Continue to build and strengthen relationships with KOLs in the pain community Develop advocacy for Egalet and our Guardian™ Technology amongst HCPs and Patient advocacy groups Engage around scientific congresses Establish advisory boards: Scientific Advisory Board focused on future applications of our Guardian Technology Clinical/Regulatory Advisory Board dealing with clinical/regulatory plans and strategy Commercial Advisory Board to seek input from clinicians on marketing plans and other commercial strategy |
|
|
Seasoned medical affairs professionals hired and on board Design and implementation of a Medical Information Call Center ahead of plan – to ‘go live’ early May Medical information response for both HCPs and patients Initial intake of adverse events and product complaints (as required by the FDA) Serve as central point of intake for all external customers (via 1-800 #) Establishment of a pharmacovigilance system in collaboration with a PVG vendor Full capabilities for postmarketing AE reporting (as required by the FDA) by end of May Serve as the internal product and disease content experts to the Egalet organization Assist with content development and product and disease training for sales force 28 Building Medical Affairs Capabilities |
|
|
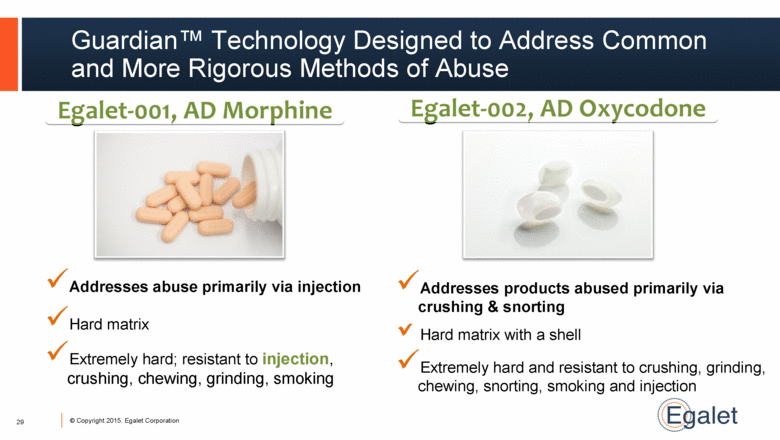
Guardian™ Technology Designed to Address Common and More Rigorous Methods of Abuse Extremely hard; resistant to injection, crushing, chewing, grinding, smoking Egalet-001, AD Morphine Egalet-002, AD Oxycodone Hard matrix Addresses abuse primarily via injection Extremely hard and resistant to crushing, grinding, chewing, snorting, smoking and injection Hard matrix with a shell Addresses products abused primarily via crushing & snorting 29 |
|
|
Manufacturing Process Drives Differentiation Proprietary Guardian™ Technology provides AD features First to combine injection molding w/ pharmaceutical production Established and well-characterized process Know-how creates barriers to entry Drives key product attributes 30 |
|
|
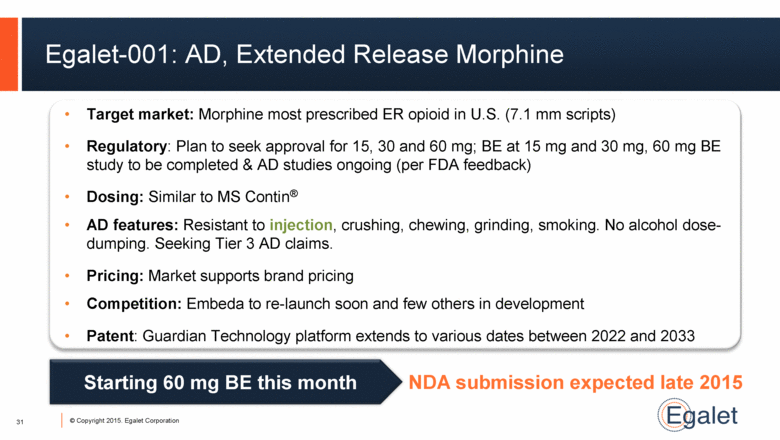
Egalet-001: AD, Extended Release Morphine 31 Target market: Morphine most prescribed ER opioid in U.S. (7.1 mm scripts) Regulatory: Plan to seek approval for 15, 30 and 60 mg; BE at 15 mg and 30 mg, 60 mg BE study to be completed & AD studies ongoing (per FDA feedback) Dosing: Similar to MS Contin® AD features: Resistant to injection, crushing, chewing, grinding, smoking. No alcohol dose-dumping. Seeking Tier 3 AD claims. Pricing: Market supports brand pricing Competition: Embeda to re-launch soon and few others in development Patent: Guardian Technology platform extends to various dates between 2022 and 2033 Starting 60 mg BE this month NDA submission expected late 2015 |
|
|
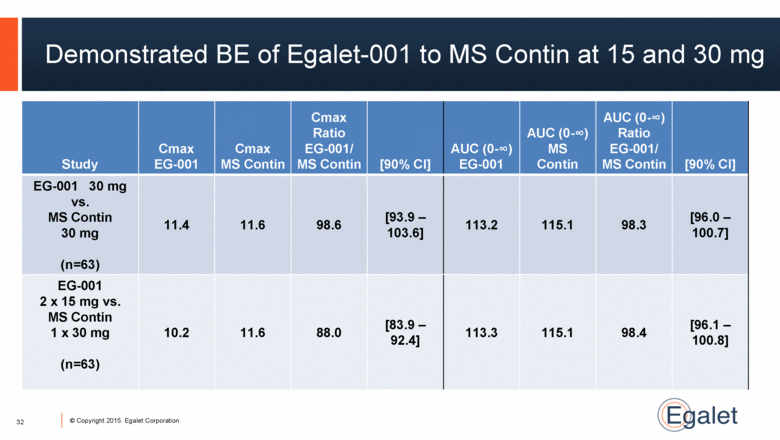
Demonstrated BE of Egalet-001 to MS Contin at 15 and 30 mg 32 Study Cmax EG-001 Cmax MS Contin Cmax Ratio EG-001/ MS Contin [90% CI] AUC (0-∞) EG-001 AUC (0-∞) MS Contin AUC (0-∞) Ratio EG-001/ MS Contin [90% CI] EG-001 30 mg vs. MS Contin 30 mg (n=63) 11.4 11.6 98.6 [93.9 – 103.6] 113.2 115.1 98.3 [96.0 – 100.7] EG-001 2 x 15 mg vs. MS Contin 1 x 30 mg (n=63) 10.2 11.6 88.0 [83.9 – 92.4] 113.3 115.1 98.4 [96.1 – 100.8] |
|
|
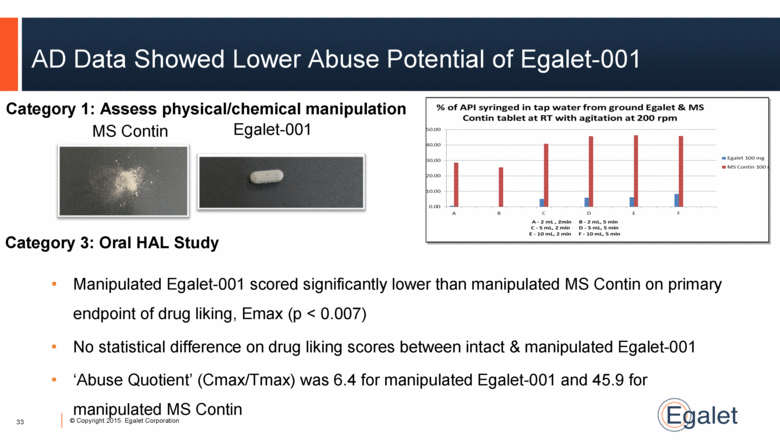
AD Data Showed Lower Abuse Potential of Egalet-001 MS Contin Egalet-001 33 Category 1: Assess physical/chemical manipulation Category 3: Oral HAL Study Manipulated Egalet-001 scored significantly lower than manipulated MS Contin on primary endpoint of drug liking, Emax (p < 0.007) No statistical difference on drug liking scores between intact & manipulated Egalet-001 ‘Abuse Quotient’ (Cmax/Tmax) was 6.4 for manipulated Egalet-001 and 45.9 for manipulated MS Contin |
|
|
Egalet-002: AD, Extended Release Oxycodone 34 Target market: Oxycodone is highest selling ER opioid; $2.5B in sales in 2013 Regulatory: 505(b)(2) pathway with two phase 3 studies & AD studies per FDA draft guidance Dosing: 2x/day with differentiated PK profile AD features: Resistant to injection, crushing, snorting, chewing, grinding, smoking. Developed to protect against abuse especially via crushing & snorting. Seeking Tier 3 AD claims; AD studies being conducted head-to-head vs. Oxy OP Pricing Market supports brand pricing Competition OxyContin, Targiniq withdrawn, products in development Patent: Guardian Technology platform extends to various dates between 2022 and 2033 Phase 3 to start Q2:15 NDA submission expected second half 2016 |
|
|
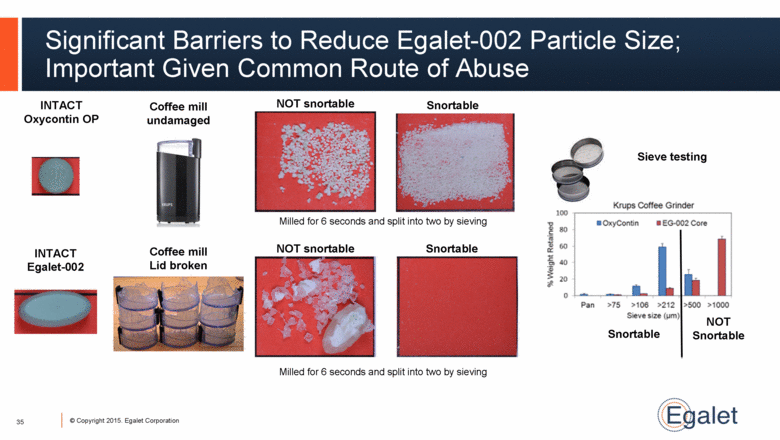
Significant Barriers to Reduce Egalet-002 Particle Size; Important Given Common Route of Abuse 35 INTACT Oxycontin OP INTACT Egalet-002 Coffee mill undamaged Sieve testing Snortable NOT Snortable Snortable NOT snortable NOT snortable Snortable Coffee mill Lid broken Milled for 6 seconds and split into two by sieving Milled for 6 seconds and split into two by sieving |
|
|
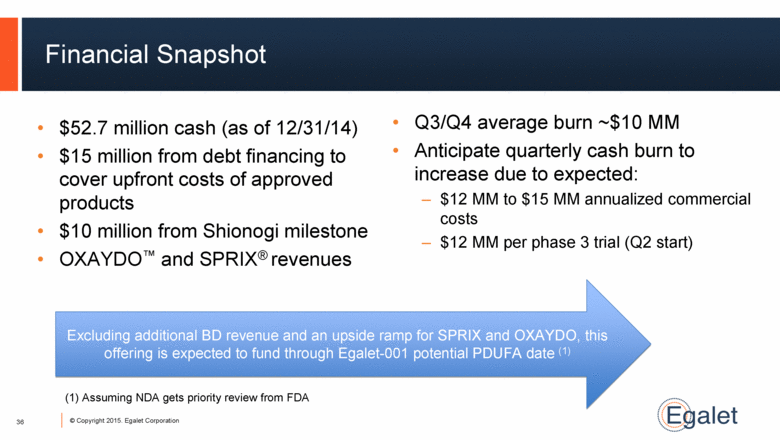
Financial Snapshot Q3/Q4 average burn ~$10 MM Anticipate quarterly cash burn to increase due to expected: $12 MM to $15 MM annualized commercial costs $12 MM per phase 3 trial (Q2 start) 36 $52.7 million cash (as of 12/31/14) $15 million from debt financing to cover upfront costs of approved products $10 million from Shionogi milestone OXAYDO™ and SPRIX® revenues Excluding additional BD revenue and an upside ramp for SPRIX and OXAYDO, this offering is expected to fund through Egalet-001 potential PDUFA date (1) (1) Assuming NDA gets priority review from FDA |
|
|
2015 Q1:15 Initiate pivotal 60 mg BE study Egalet-001 Q1:15 Complete Category 3 oral HAL AD study Egalet-001 Q1:15 Initiate promotional activities SPRIX® Q1:15 Commercial update SPRIX & OXAYDO™ Q2:15 Initiate pivotal Phase 3 safety and efficacy studies Egalet-002 Q2:15 Complete Category 3 intranasal abuse deterrent study Egalet-001 Q3:15 Complete Category 3 oral HAL abuse-deterrent study Egalet-002 Q3:15 Promotional sales launch SPRIX & OXAYDO Q3:15 Complete 60 mg pivotal BE study Egalet-001 Q4:15 Submit NDA Egalet-001 2016 H2:16 Complete two pivotal Phase 3 studies Egalet-002 Mid-16 H2:16 Submit NDA Egalet-002 ‘15 Will be Busy with Commercial and Development Milestones 37 |
|
|
Delivering pain relief with peace of mind 38 |