Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - XOMA Corp | Financial_Report.xls |
| EX-99.1 - EXHIBIT 99.1 - XOMA Corp | ex99_1.htm |
| EX-31.2 - EXHIBIT 31.2 - XOMA Corp | ex31_2.htm |
| EX-23.1 - EXHIBIT 23.1 - XOMA Corp | ex23_1.htm |
| EX-31.1 - EXHIBIT 31.1 - XOMA Corp | ex31_1.htm |
| EX-32.1 - EXHIBIT 32.1 - XOMA Corp | ex32_1.htm |
| EX-10.71 - EXHIBIT 10.71 - XOMA Corp | ex10_71.htm |
| EX-10.72 - EXHIBIT 10.72 - XOMA Corp | ex10_72.htm |
| EX-10.73 - EXHIBIT 10.73 - XOMA Corp | ex10_73.htm |
| EX-10.74 - EXHIBIT 10.74 - XOMA Corp | ex10_74.htm |
| EX-21.1 - EXHIBIT 21.1 - XOMA Corp | ex21_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 0-14710
XOMA Corporation
(Exact name of registrant as specified in its charter)
|
Delaware
|
52-2154066
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
2910 Seventh Street, Berkeley,
California 94710
|
(510) 204-7200
|
|
|
(Address of principal executive offices, including zip code)
|
(Telephone number)
|
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $0.0075 par value Preferred Stock Purchase Rights
|
The NASDAQ Stock Market, LLC
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large Accelerated Filer ☐ Accelerated Filer ☒ Non-Accelerated filer ☐ Smaller reporting company ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act of 1934). Yes ☐ No ☒
The aggregate market value of voting common equity held by non-affiliates of the registrant is $487,653,571 as of June 30, 2014.
Number of shares of Common Stock outstanding as of March 9, 2015: 116,185,969
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the Company’s Proxy Statement for the Company’s 2015 Annual General Meeting of Stockholders are incorporated by reference into Part III of this Report.
XOMA Corporation
2014 FORM 10-K ANNUAL REPORT
|
PART I
|
||
|
Item 1.
|
1
|
|
|
Item 1A.
|
20
|
|
|
Item 1B.
|
38
|
|
|
Item 2.
|
38
|
|
|
Item 3.
|
38
|
|
|
Item 4.
|
38
|
|
|
38
|
||
|
PART II
|
||
|
Item 5.
|
39
|
|
|
Item 6.
|
41
|
|
|
Item 7.
|
42
|
|
|
Item 7A.
|
60
|
|
|
Item 8.
|
61
|
|
|
Item 9.
|
61
|
|
|
Item 9A.
|
61
|
|
|
Item 9B.
|
62
|
|
|
PART III
|
||
|
Item 10.
|
64
|
|
|
Item 11.
|
64
|
|
|
Item 12.
|
64
|
|
|
Item 13.
|
64
|
|
|
Item 14.
|
64
|
|
|
PART IV
|
||
|
Item 15.
|
65
|
|
|
66
|
||
|
F-1
|
||
|
i
|
||
This annual report on Form 10-K includes trademarks, service marks and trade names owned by us or others. “XOMA,” the XOMA logo and all other XOMA product and service names are registered or unregistered trademarks of XOMA Corporation or a subsidiary of XOMA Corporation in the United States and in other selected countries. EYEGUARD is a service mark of a subsidiary of XOMA Corporation in the United States. All other trademarks, service marks and trade names included or incorporated by reference in this annual report are the property of their respective owners.
PART I
Certain statements contained herein related to the anticipated size of clinical trials, the anticipated timing of initiation of clinical trials, the expected availability of clinical trial results, the sufficiency of our cash resources, the estimated costs of clinical trials and the amounts of certain revenues and certain costs in comparison to prior years, or that otherwise relate to future periods, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The words “believe,” “may,” “estimate,” “continue,” “could,” “anticipate,” “assume,” “intend,” “expect,” “predict,” “potential” “should,” “would,” and similar expressions are intended to identify forward-looking statements. These statements are based on assumptions that may not prove accurate. Actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. Among other things: our product candidates are still being developed, and we will require substantial funds to continue development which may not be available; we have sustained losses in the past and we expect to sustain losses in the future; we are substantially dependent on Les Laboratoires Servier (“Servier”) for the development and commercialization of gevokizumab and for other aspects of our business; we have received negative results from certain of our clinical trials, and we face uncertain results of other clinical trials of our product candidates; if our therapeutic product candidates do not receive regulatory approval, neither our third-party collaborators, our contract manufacturers nor we will be able to manufacture and market them; we may not obtain orphan drug exclusivity or we may not receive the full benefit of orphan drug exclusivity even if we obtain such exclusivity; even once approved, a product may be subject to additional testing or significant marketing restrictions, its approval may be withdrawn or it may be voluntarily taken off the market; we may not be successful in commercializing our products, which could also affect our development efforts; we are subject to various state and federal healthcare related laws and regulations that may impact the commercialization of our product candidates and could subject us to significant fines and penalties; and certain of our technologies are in-licensed from third parties, so our capabilities using them are restricted and subject to additional risks. These and other risks, including those related to current economic and financial market conditions, are contained principally in Item 1, Business; Item 1A, Risk Factors; Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations; and other sections of this Annual Report on Form 10-K. Factors that could cause or contribute to these differences include those discussed in Item 1A, Risk Factors, as well as those discussed elsewhere in this Annual Report on Form 10-K.
Forward-looking statements are inherently uncertain and you should not place undue reliance on these statements, which speak only as of the date that they were made. These cautionary statements should be considered in connection with any written or oral forward-looking statements that we may issue in the future. We do not undertake any obligation to release publicly any revisions to these forward-looking statements after completion of the filing of this Annual Report on Form 10-K to reflect later events or circumstances or to reflect the occurrence of unanticipated events.
Overview
XOMA Corporation (“XOMA”), a Delaware corporation, discovers and develops innovative antibody-based therapeutics. Several of our antibodies have unique properties due to their interaction at allosteric sites on specific protein rather than the orthosteric, or active sites. The compounds are designed to either enhance or diminish the protein’s activity as desired. We believe allosteric-modulating antibodies may be more selective or offer a safety advantage in certain disease indications when compared to more traditional modes of action.
Our lead product candidate, gevokizumab, is a proprietary potent, humanized allosteric-modulating monoclonal antibody that binds to the inflammatory cytokine interleukin-1 beta (“IL-1 beta”). We believe that by targeting IL-1 beta, gevokizumab has the potential to address the underlying inflammatory causes of a wide range of diseases that have been identified as having unmet medical needs.
Together with our development partner, Servier, a leading independent French pharmaceutical research company, we initiated three pivotal clinical trials evaluating gevokizumab for the treatment of non-infectious intermediate, posterior or pan-uveitis (“NIU”) and Behçet’s disease uveitis. We are responsible for all of the clinical study sites in the United States, and Servier is responsible for all of the clinical study sites outside of the United States. These studies are known as the EYEGUARD™ program, which includes EYEGUARD-A (patients with active NIU), EYEGUARD-B (patients with Behçet’s disease uveitis outside of the United States), and EYEGUARD-C (patients with a history of NIU currently controlled with systemic treatment).
Our strategy is to pursue Behçet’s disease uveitis as our first indication for gevokizumab in the United States. Upon the successful completion of Servier’s EYEGUARD-B study, we intend to meet with the U.S. Food and Drug Administration (“FDA” or “the Agency”) to review the Phase 3 EYEGUARD-B data together with the data from the two Behçet’s disease uveitis Phase 2 studies conducted independently by XOMA and Servier. We believe the seriousness of this disease and the small patient population warrant consideration for approval based upon positive data from a single pivotal study. There is significant precedence for regulatory approval based upon a single study for indications of similar seriousness and patient populations. Should EYEGUARD-B demonstrate that patients with Behçet’s disease uveitis who receive gevokizumab took longer to exacerbate than the placebo-treated patients during the tapering of administered steroids, we believe we will be in position to begin the Biologics License Application (“BLA”) submission process.
In September 2014, we opened the EYEGUARD-US supplemental gevokizumab clinical study of Behçet’s disease uveitis to patients in the United States. The supplemental EYEGUARD-US study may be used in one of several ways. It may not be required for the initial BLA submission, in which case it would merely provide further information related to U.S. physicians’ and patients’ experiences with gevokizumab. It may be required for the FDA’s review of our submission for informational purposes without being considered a pivotal study. In this case, the study would be unmasked at a predetermined time when we are in a position to submit the BLA. Finally, it may be required as a second pivotal study in order for the FDA to accept our submission. We’ve designed the EYEGUARD-US study in a manner intended to fulfill whatever directive we are given by the Agency and respond as quickly as possible.
In addition to the NIU clinical trials, we are studying gevokizumab in pyoderma gangrenosum (“PG”), a rare ulcerative skin disease that is a specific indication under the umbrella of diseases known as neutrophilic dermatoses. Patients experience painful expanding skin ulcers that have a significant impact on their quality of life. Approximately 50 to 70 percent of the PG patient population have an underlying systemic condition, while the remainder is idiopathic (unknown cause). The most prevalent underlying conditions are ulcerative colitis and Crohn’s disease. The prognosis for PG is linked directly to the patient’s response to therapy for the underlying disease. Physicians currently treat patients with systemic therapies that are approved for the underlying disease and with topical therapies applied directly to the ulcers, yet published literature suggests that, on average, current therapies can take six months to stop the ulcers from expanding and over eleven months to heal. The U.S. Department of Health and Human Services’ National Institutes of Health’s Office of Rare Disease Research lists PG as occurring in about one per 100,000 people. Claims data compiled over the past three years indicate the number of diagnosed PG patients in the U.S. ranges between 11,000 and 14,000 annually.
Based upon what we believe are compelling data from our pilot study in patients with PG, we initiated a Phase 3 clinical program. Final comments from the FDA were received in the third quarter of 2014, and we initiated the first Phase 3 study in October 2014. The Phase 3 PG program includes two double-blind, placebo-controlled clinical studies, each of which is designed to enroll 58 patients with active PG to receive gevokizumab 60 mg or placebo dosed subcutaneously once monthly, in addition to their current treatment regimen of low-dose corticosteroids and/or immunosuppressants. The primary endpoint is the complete closure of the PG target ulcer determined at Day 126 with confirmation of complete closure a minimum of two weeks later on or after Day 140.
Published literature indicates approximately 50% of patients will experience a recurrence within two to three years. To follow the patients enrolled in our pilot study, we designed an extension study that allows the pilot study patients the opportunity to receive further treatment if they experience new ulcers and allows us to capture information on how gevokizumab performs with successive treatments. Four of the six patients from our pilot study entered the extension study; three of whom were fully healed during the initial study, and one patient who had an ulcer that was fully healed at Day 56 but reopened after an injury. To date, three of the four patients enrolled in the extension study have received additional gevokizumab therapy for PG. One patient recurred at 7.5 months and upon receiving additional gevokizumab therapy obtained 100% ulcer closure prior to Day 56. One patient recurred at 7 months and after additional gevokizumab therapy obtained 100% ulcer closure by Day 84. The patient who had initially healed but whose ulcer reopened after an injury continued receiving therapy and obtained 100% ulcer closure. One patient has not received additional gevokizumab therapy as the condition has not recurred for more than one year following initial gevokizumab treatment. All four patients continue to be enrolled in the extension study and will be followed for up to 92 weeks.
We also have an active gevokizumab Proof-of-Concept (“POC”) development program to identify other illness for late-stage development. Two studies are being conducted in collaboration with the U.S. National Institutes of Health (“NIH”). The National Eye Institute (“NEI”) is conducting a gevokizumab study in patients with non-infectious anterior scleritis. The North Shore-Long Island Jewish Health System in collaboration with the National Institute on Deafness and Other Communication Disorders (“NIDCD”) is conducting a gevokizumab clinical study in patients with inflammatory autoimmune inner ear disease (AIED).
Previously, we conducted POC trials in moderate-to-severe inflammatory acne and in erosive osteoarthritis of the hand (“EOA”). We have decided not to further pursue the acne indication based on our commercial analysis. The EOA results led to our decision not to pursue Phase 3 testing in the broad EOA population, although we continue to review the data to determine if there is a subgroup of the EOA population that could benefit from gevokizumab therapy.
Gevokizumab has been generally well tolerated across all of our clinical studies. In both the acne and EOA studies, there were no drug-related serious adverse events reported. The most common adverse events were headache, pain, arthralgia, urinary tract infections, upper respiratory tract infections and pneumonia, and they were comparable between gevokizumab and placebo.
Separately, Servier instituted its own active development program for gevokizumab beyond the NIU and Behçet’s disease uveitis Phase 3 program. In 2012, Servier initiated a Phase 2 gevokizumab study in patients with acute coronary syndrome, a cardiovascular disease within the cardiometabolic field where it has world-wide rights. In 2013, Servier began testing gevokizumab in a variety of POC studies, including polymyositis/dermatomyositis, Schnitzler syndrome, and giant cell arteritis. Servier has indicated these are the first studies in an extensive multi-indication exploratory program it expects to conduct.
Our proprietary pipeline includes classes of allosteric modulating antibodies that activate, sensitize or deactivate the insulin receptor in vivo, which we have named XOMA Metabolic or XMet. Insulin is the primary hormone for lowering blood glucose levels. Abnormal increases in insulin secretion can lead to profound hypoglycemia (low blood sugar), a state that may result in significant morbidities, including cerebral damage and epilepsy. In some instances, profound hypoglycemia can result in fatality. There are three programs in the XMet portfolio, XMetD, which is designed to deactivate the insulin receptor, XMetA, which is designed to activate the insulin receptor, and XMetS, which is designed to sensitize the insulin receptor when in an insulin resistant state. These programs are highly novel as the antibodies bind to different sites on the insulin receptor than currently marketed drugs. This portfolio of antibodies represents potential new therapeutic approaches to the treatment of several rare diseases that have insulin involvement and diabetes.
The lead compound from our XMetD program, XOMA 358, is a fully human monoclonal allosteric modulating antibody that binds to insulin receptors and attenuates insulin action. It is designed to negatively modulate the insulin receptor and its downstream signaling capabilities. We launched clinical development activities for XOMA 358 in October 2014, with the first patient dosed in our Phase 1 safety and tolerability study. The Phase 1 study was successful, and we intend to investigate this compound as a novel treatment for non-drug-induced, endogenous hyperinsulinemic hypoglycemia (low blood glucose caused by excessive insulin produced by the body). A therapy that safely and effectively mitigates insulin-induced hypoglycemia has the potential to address a significant unmet therapeutic need for certain rare medical conditions associated with hyperinsulinism.
We intend to retain full ownership of XOMA 358 and the compounds in the XMetD program, as they align with our focus on developing products for diseases with significant unmet medical need that are treated by the specialist prescriber. We intend to out-license the insulin receptor-activating drug candidate(s) for development in diabetes to a pharmaceutical company with expertise in developing and commercializing compounds for Types 1 and 2 diabetes.
We have developed these and other antibodies using some or all of our ADAPT™ antibody discovery and development platform, our ModulX™ technologies for generating allosterically modulating antibodies, and our OptimX™ technologies for optimizing biophysical properties of antibodies, including affinity, immunogenicity, stability and manufacturability.
Our biodefense initiatives include XOMA 3AB, a biodefense anti-botulism product candidate comprised of a combination of three antibodies. XOMA 3AB is directed against botulinum toxin serotype A and has been developed through funding from the National Institute of Allergy and Infectious Diseases (“NIAID”), a part of the NIH. A Phase 1 XOMA 3AB trial was completed with no product-related serious adverse events. Should the government choose to acquire XOMA 3AB or other biodefense products in the future, we expect to be able to produce these antibodies through an outside manufacturer.
We also have developed antibody product candidates with premier pharmaceutical companies including Novartis AG (“Novartis”) and Takeda Pharmaceutical Company Limited (“Takeda”).
Corporate Strategy
We are committed to establishing XOMA as a commercial organization in the United States. Our commercialization strategy is to market products in the United States through our own focused sales teams calling on the specialist prescriber. For indications that will require clinical studies that are prohibitively large or the targeted patient populations are not treated by the specialist provider, we will seek a development and commercialization partner. For gevokizumab, we select our clinical development indications based upon data that supports IL-1 beta’s role in the disease state and upon data indicating the affected patients are treated by a specialized physician base. Additionally, we may seek to expand our pipeline by developing additional proprietary products and technologies and by entering into additional licensing and collaborative arrangements with pharmaceutical and biotechnology companies. The principal elements of our corporate strategy are to:
| · | Complete pivotal clinical development for gevokizumab, our lead product candidate, in Behçet’s disease uveitis, a rare ocular indication. Upon the receipt of successful primary endpoint data from the EYEGUARD-B study in patients with Behçet’s disease uveitis, we will seek guidance from the FDA to determine the requirements necessary to support a BLA in this rare indication. We believe positive EYEGUARD-B results, combined with the results obtained by Servier and our independent Phase 2 studies, will be compelling and warrant the submission of a BLA for Behçet’s disease uveitis. The FDA may require us to conduct a confirmatory study in patients with Behçet’s disease uveitis to support a BLA filing in this indication; therefore we initiated the EYEGUARD-US study. Depending upon the FDA’s feedback regarding what data is required to support a BLA filing for Behçet’s disease uveitis, we hope to be able to file the application shortly after our pre-BLA meeting. This strategy was designed to accelerate our path to commercialization. |
| · | Complete Phase 3 clinical development for gevokizumab in NIU. With Servier, we launched the global gevokizumab Phase 3 clinical development program, named EYEGUARD™, in 2012. The global program includes two Phase 3 trials in active and controlled NIU (EYEGUARD-A and EYEGUARD-C, respectively) and a Phase 3 trial outside the United States in patients who suffer from Behçet’s disease uveitis (EYEGUARD-B). The EYEGUARD-A study defines active NIU as a vitreous haze score of equal to or greater than two on the Standardization of Uveitis Nomenclature (“SUN”)/NEI scale. The vitreous is a normally transparent gel that fills the eyeball behind the lens, and vitreous haze is the clouding of that gel. The EYEGUARD-C study is designed to determine if physicians can reduce or eliminate corticosteroid use from NIU patients without causing their disease to flare, or exacerbate. EYEGUARD-A and -C are ongoing and continue to enroll patients. The EYEGUARD-B study is also designed to determine if physicians can reduce or eliminate corticosteroid use in Behçet’s disease patients without causing an acute exacerbation of their uveitis. In addition to establishing efficacy, we believe these trials have been designed to provide data necessary to meet the minimum FDA requirements for demonstrating safety for ophthalmic indications: at least 300 patients must be treated for at least six months and 100 patients for one year at the to-be-marketed dose. |
EYEGUARD-A and -C require 300 patients to be enrolled in each study. The pace of enrollment in both studies has been slower than both Servier and we had anticipated. We increased the number of study sites in the U.S. to 72 for EYEGUARD-A, 57 of which are open and 77 for EYEGUARD-C, of which 53 are open. We have implemented and continue to implement a variety of activities to accelerate patient enrollment. We are seeing slow but steady progress in enrolling patients in the United States, particularly in EYEGUARD-C. As of March 1, 2015, Servier has obtained regulatory approval for EYEGUARD-A and EYEGUARD-C in 20 of its targeted 22 territories and 19 of its targeted 23 territories, respectively. These countries represent 52 of the planned 72 and 53 of the planned 77 clinical sites in the Servier territory for EYEGUARD-A and -C, respectively. Opening the study sites in Servier’s territories is crucial to getting the EYEGUARD-A and -C studies completed. Based upon the pace of enrollment in EYEGUARD-C, we expect to complete enrollment in 2015. The primary endpoint readout will be achieved six months after achieving full enrollment. For EYEGUARD-A, enrollment needs to increase outside of the United States, and the primary endpoint readout will be achieved two months after achieving full enrollment. The pace of enrollment in EYEGUARD-A or-C will need to accelerate from its present pace for us to have data from either study in 2015. We believe we need positive results from any two of these three studies in order to file a BLA in NIU with the FDA.
|
●
|
Advance a Phase 3 program in PG, a rare skin disease classified under the broader indication of neutrophilic dermatoses. In late 2013, we launched a pilot study to determine gevokizumab’s ability to treat acute inflammatory PG, one of several rare skin diseases classified under the broader cluster of neutrophilic dermatoses. We designed the study to enroll as many as four patients to receive gevokizumab 60mg, dosed once monthly for three months. After this cohort completed one dose, we reviewed the data and elected not to proceed to a higher dosing regimen, as the patients were responding to gevokizumab. Three patients showed improvement in ulcer size by Day 28. One patient had total resolution of the ulcer by Day 84; a second patient had 93% improvement in ulcer size by Day 56. Two additional patients were enrolled in the pilot program. Today, four of the six patients in our pilot study are enrolled in an extension study, and three have received additional therapy with gevokizumab, with similar results. One patient has not exacerbated after the initial gevokizumab treatment over one year ago. In November 2014, we launched the first of two Phase 3 clinical trials and are in the process of completing the regulatory filings and clinical site identification to initiate the second study.
|
| · | Continue to assess gevokizumab’s ability to treat a variety of diseases. In April 2013, NEI, a division of NIH, opened its noninfectious, active, anterior scleritis trial for patient enrollment. The open-label single-arm Phase 1/2 study is designed to assess the safety and potential efficacy of gevokizumab in up to 10 patients experiencing non-infectious, active, anterior scleritis, which is the inflammation of the sclera (the fibrous white membrane surrounding the eyeball excluding the cornea). |
In August 2013, we announced a single-center clinical trial in ten patients with AIED, which falls under the umbrella of sensorineural hearing loss. Patients with AIED usually experience multiple episodes of rapid hearing loss either concurrently or sequentially in both ears. This study is being run by the Feinstein Institute for Medical Research, Hearing & Speech Center at North Shore-Long Island Jewish Health System in collaboration with, and with funding from, the NIDCD and the NIH.
| · | Establish commercial-scale manufacturing for gevokizumab. In August 2012, Servier and we announced an agreement with Boehringer Ingelheim to transfer XOMA's technology and processes for the validation of our technology and processes in preparation for the commercial manufacture of gevokizumab. Boehringer Ingelheim has completed Good Manufacturing Practices (“GMP”) runs with successful biological comparability, including all process validation batches of the XOMA processes. Boehringer Ingelheim is making preparations for the production of gevokizumab commercial batches at its facility in Biberach, Germany. |
| · | Advance our proprietary pipeline candidates and generate revenues from our proprietary technologies. We continue to develop assets in our proprietary pipeline, primarily focusing on the development of allosteric modulating monoclonal antibodies. Our most advanced program, which targets the insulin receptor, has generated three new classes of fully human monoclonal antibodies known as Selective Insulin Receptor Modulators (“SIRMs”). These allosteric modulating antibodies activate (“XMetA”), sensitize (“XMetS”) or deactivate/antagonize (“XMetD”) the insulin receptor in vivo. XMetA and XMetS represent the potential for distinct, new therapeutic approaches for the treatment of patients with diabetes. Separate studies of XMetA and XMetS have demonstrated reduced fasting blood glucose levels and improved glucose tolerance in animal models of diabetes. We intend to out-license the insulin receptor-activating drug candidate(s) for development in diabetes. |
In the case of XMetD, we plan to develop this portfolio of compounds internally, as they have the potential to treat several rare, life-threatening or severely debilitating diseases: hyperinsulinemic hypoglycemia in post-gastric bypass surgery patients, congenital hyperinsulinism (“CHI”), and insulinomas. The most advanced of these compounds is XOMA 358, a fully human, allosteric modulating monoclonal antibody engineered to deactivate the insulin receptor. In preclinical models, XOMA 358 has emulated the glucose lowering seen in patients with insulinomas, a beta cell tumor that over secretes insulin, and with CHI, a hereditary disease resulting in lack of insulin regulation and profound hypoglycemia that can result in seizures and brain damage. These models demonstrated XOMA 358 was capable of restoring fasting blood glucose to normal levels. We launched clinical development for XOMA 358 in October 2014 and successfully completed a Phase 1 clinical study of XOMA 358. We have presented the results at the Endocrine Society’s Annual Meeting, ENDO 2015, which show XOMA 358’s ability to deactivate the insulin receptor and its downward signaling. XOMA 358 is being evaluated for the treatment of non-drug-induced, endogenous hyperinsulinemic hypoglycemia (low blood glucose caused by excessive insulin produced endogenously). We ultimately hope to pursue the compound for use in patients with endogenous hypoglycemia.
| · | Complete current biodefense contracts. To date, we have been awarded four contracts totaling approximately $120 million from NIAID to support development of XOMA 3AB and several additional product candidates for the treatment of botulism poisoning with botulinum toxin serotypes A, B and E, as well as C and D. |
NIAID has completed a Phase 1 trial of XOMA 3AB, a novel formulation of three antibodies designed to prevent and treat botulism poisoning from serotype A. This double-blind, dose-escalation study in 24 healthy volunteers was designed to assess the safety and tolerability and determine the pharmacokinetic profile of XOMA 3AB. This trial did not observe any drug product-related serious adverse events. The results of this trial strongly support our platform approach for the remaining serotype-directed anti-toxins. Under NIAID funding XOMA has also produced XOMA 3B drug product (directed against Botulinum Toxin Serotype B (“BoNT”/B) and XOMA 3E drug product (directed against BoNT/E). Pre- Investigational New Drug (“IND”) work has been completed for these products and Phase 1 studies will be scheduled by NIAID. Under additional funding, XOMA is developing an anti-BoNT/C/D combination antibody product. This product will advance separately to IND and clinical studies.
Proprietary Products
As part of our strategy, we are focusing our technology and resources on advancing our emerging proprietary pipeline. Below is a summary of our proprietary products:
|
●
|
Gevokizumab is a proprietary potent humanized monoclonal antibody with unique allosteric modulating properties that has the potential to treat patients with a wide variety of inflammatory diseases. Gevokizumab binds strongly to IL-1 beta, a pro-inflammatory cytokine involved in NIU and Behçet’s disease uveitis, PG, active non-infectious anterior scleritis, AIED, cardiovascular disease, diseases under the neutrophilic dermatoses designation, Schnitzler syndrome and other diseases. By binding to IL-1 beta, gevokizumab modulates the activation of the IL-1 receptor, thereby preventing the cellular signaling events that produce inflammation. Based on its binding properties, specificity for IL-1 beta and its half-life (the time it takes for the amount administered to be reduced by one-half) in the body, gevokizumab may provide convenient dosing of once per month.
|
In December 2010, we entered into an agreement with Servier to jointly develop and commercialize gevokizumab in multiple indications. Under the terms of that agreement, Servier has worldwide rights to gevokizumab for cardiovascular disease and diabetes indications (cardiometabolic field) and rights outside the United States and Japan to all other indications. We retain development and commercialization rights in the United States and Japan to all indications except cardiovascular disease and diabetes, yet we have the option to reacquire rights to these indications from Servier in these territories. In 2012, Servier initiated a gevokizumab Phase 2 study in patients with acute coronary syndrome, a cardiovascular disease. In 2013, Servier also began testing gevokizumab in a variety of proof-of-concept studies, including polymyositis/dermatomyositis, Schnitzler syndrome, and giant cell arteritis.
|
●
|
XMet: XOMA Metabolic Activating, Sensitizing and Deactivating/Antagonizing Antibodies. Insulin receptor-activating antibodies, such as XMetA, are designed to provide long-acting reduction of hyperglycemia in Type 2 diabetic patients, potentially reducing the advancement to a number of insulin injections needed to control their blood glucose levels. Insulin receptor-sensitizing antibodies, such as XMetS, are being evaluated to reduce insulin resistance and could enable diabetic patients to use their own insulin more effectively to control blood glucose levels. Insulin receptor-deactivating/antagonizing antibodies, such as XOMA 358, are in development to treat several diseases that result from the over-production or abnormal regulation to insulin. There are three rare disease indications that may benefit from XOMA 358 that are of greatest interest to us: CHI, post-meal hypoglycemia in post-gastric bypass surgery patients and possibly certain insulinomas. XOMA 358 has successfully completed Phase 1 testing, and we are exploring clinical trial designs to further advance XOMA 358.
|
Studies performed with XMetA have demonstrated that it reduced hyperglycemia in rodents and larger animals both acutely and over 6-week dosing periods. As significant reduction in Hemoglobin A1c (“HbA1c”) levels – a standard clinical and regulatory measure for glucose metabolism – were also observed, the potential product profile is encouraging for development in Type 2 diabetes.
|
●
|
XOMA’s antibotulinum toxin program has multiple antibody candidates that are in development. Botulinum toxins Types A, B, C, D, and E cause paralysis and are potential bioterrorism threats. XOMA 3AB is a multi-antibody product designed to neutralize the most potent of the botulinum toxins, Type A. XOMA 3E is a multi-antibody product designed to neutralize another of the most prevalent of the botulinum toxins, Type E. XOMA CD is a multi-antibody product directed to BoNT/C and BoNT/D, and is our first divalent product directed simultaneously against two serotypes of botulinum toxin, Types C and D. The antibodies are designed to bind to each toxin, neutralize them, and enhance the clearance of the toxin from the body. The use of multiple antibodies increases the likelihood of clearing the harmful toxins by providing specific protection against each toxin type. In contrast to existing agents that treat botulism, XOMA uses advanced human monoclonal antibody technologies in an effort to achieve superior safety, potency and efficacy and avoid life-threatening immune reactions associated with animal-derived products. NIAID has completed a Phase 1 trial of XOMA 3AB with no product-related serious adverse events.
|
|
●
|
XOMA 629 is a topical anti-bacterial formulation of a peptide derived from bactericidal/permeability-increasing protein (“BPI”), an integral part of the protective human immune system. In 2012, XOMA entered into a license agreement with Margaux Biologics, Inc. (“Margaux”), under which XOMA transferred its rights, title, and interest in BPI. As consideration for the transferred assets and licenses, Margaux issued shares of its common stock to XOMA, representing an amount of capital stock equal to 7% of the then outstanding capital stock of Margaux. Under the terms of this agreement, we may receive milestone payments aggregating up to $5.6 million and low- to mid-single-digit royalties on future sales of products subject to this license.
|
|
●
|
Preclinical Product Pipeline: We are pursuing additional opportunities to further broaden our preclinical product pipeline, including internal discovery programs.
|
Partnership Products
Historically, we have provided research and development collaboration services for world-class organizations, including Novartis and Takeda, in pursuit of new antibody products. In more recent years, we have evolved our business focus from a service provider model to a proprietary product development model. However, we will continue to capitalize on partnered product arrangements as opportunities arise. Below is a list of such partnerships:
| · | Therapeutic Antibodies with Takeda: Since 2006, Takeda has been a collaboration partner for therapeutic monoclonal antibody discovery and development against multiple targets selected by them. In February 2009, we expanded our existing collaboration to provide Takeda with access to multiple antibody technologies, including a suite of research and development technologies and integrated information and data management systems. We may receive potential milestones and royalties on sales of antibody products in the future. |
| · | Therapeutic Antibodies with Novartis In November 2008, we restructured our product development collaboration with Novartis, which was entered into in 2004 with Novartis (then Chiron Corporation). Under the restructured agreement, Novartis received control over the two ongoing programs relating to CD40 and prolactin receptor. Control of the prolactin receptor antibody program was returned to us in 2014. We may, in the future, receive milestones and/or double-digit royalty rates for the CD40 antibody program and options to develop or receive royalties from four additional programs. |
Technologies
We have a unique set of antibody discovery, optimization and development technologies, including:
| · | ADAPT™ (Antibody Discovery Advanced Platform Technologies): proprietary phage display libraries integrated with yeast and mammalian display to enable antibody discovery; |
| · | ModulX™: technology that enables positive and negative modulation of biological pathways using allosterically modulating antibodies; and |
| · | OptimX™: technologies used for optimizing biophysical properties of antibodies, including affinity, immunogenicity, stability and manufacturability. |
Technology Licenses
Below is a summary of certain proprietary technologies owned by us and available for licensing to other companies:
| · | Antibody Discovery Technologies: We use human antibody phage display libraries, integrated with yeast and mammalian display, which we call ADAPT™ Integrated Display, in our discovery of therapeutic candidates. We offer access to this platform, including novel phage libraries developed internally, as part of our collaboration business. We believe access to ADAPT™ Integrated Display offers a number of benefits to us and our collaboration partners because it enables us to combine the diversity of phage libraries with accelerated discovery due to rapid immunoglobulin (“IgG”) reformatting and Fluorescence-Activated Cell Sorting (“FACS”) based screening using yeast and mammalian display. This increases the probability of technical and business success in finding rare and unique functional antibodies directed to targets of interest. |
| · | ModulX™ technology: ModulX™ technology allows modulation of biological pathways using monoclonal antibodies and offers insights into regulation of signaling pathways, homeostatic control, and disease biology. Using ModulX™, XOMA is generating product candidates with novel mechanisms of action that specifically alter the kinetics of interaction between molecular constituents (e.g. receptor-ligand). ModulX™ technology enables expanded target and therapeutic options and offers a unique approach in the treatment of disease. |
| · | OptimX™ technologies: |
Human Engineering™ (“HE™”): HE™ is a proprietary humanization technology that allows modification of non-human monoclonal antibodies to reduce or eliminate detectable immunogenicity and make them suitable for medical purposes in humans. The technology uses a unique method developed by us, based on analysis of the conserved structure-function relationships among antibodies. The method defines which residues in a non-human variable region are candidates to be modified. The result is an HE™ antibody with preserved antigen binding, structure and function that has eliminated or greatly reduced immunogenicity. HE™ technology was used in development of gevokizumab and is used in the development of certain other antibody products.
Targeted Affinity Enhancement™ (“TAE™”): TAE™ is a proprietary technology involving the assessment and guided substitution of amino acids in antibody variable regions, enabling efficient optimization of antibody binding affinity and selectivity modulation. TAE™ generates a comprehensive map of the effects of amino acid mutations in the CDR region likely to impact binding. The technology is utilized by XOMA scientists and has been licensed to a number of our collaborators.
| · | Flexible Manufacturing: This patented technology relates to a flexible arrangement of mobile clean rooms (“MCRs”) within a manufacturing facility, with each MCR providing a portable, self-contained environment that allows for drug development. The facility design allows MCRs to connect easily and quickly to a central supply of utilities such as air, water, and electricity. This unique arrangement facilitates flexible manufacturing and eliminates change-over downtime. This translates into significantly reduced capital expenditures, production costs, and maintenance costs while offering meaningful time advantages over conventional manufacturing facilities. When MCRs are not in use, they can be easily moved to cleaning/refurbishing areas and prepared MCRs can be "plugged in" for manufacturing. The flexible manufacturing system can be applied to fields as diverse as pharmaceuticals, biologics, and electronics. |
Financial and Legal Arrangements of Product Collaborations, Licensing and Other Arrangements
Collaboration and Licensing Agreements
Servier -- Gevokizumab
We have entered into a license and collaboration agreement with Servier to jointly develop and commercialize gevokizumab in multiple indications that provided us with a non-refundable upfront payment of $15 million, which we received in January 2011. Under the terms of the agreement, Servier has worldwide rights to cardiovascular disease and diabetes indications (cardiometabolic field) and rights outside the United States and Japan to all other indications, including NIU, Behçet’s disease uveitis and other inflammatory and oncology indications. XOMA retains development and commercialization rights in the United States and Japan for all indications other than cardiovascular disease and diabetes. XOMA has an option to reacquire rights to cardiovascular disease and diabetes indications from Servier in the United States and Japan (the “Cardiometabolic Indications Option”). If we exercise the Cardiometabolic Indications Option, we will be required to pay Servier an option fee and partially reimburse their incurred development expenses. Each party has the right in certain circumstances to pursue development in indications not specified in the agreement, and in such event, the other party will have certain options to participate in such development, including reimbursement of a portion of the developing party’s expenses.
Under this agreement, Servier will fund all activities to advance the global clinical development and future commercialization of gevokizumab in cardiovascular-related diseases and diabetes. Also, Servier funded the first $50 million of gevokizumab global clinical development and Chemistry, Manufacturing and Controls (“CMC”) expenses and continues to fund 50% of further expenses related to the NIU and Behçet’s disease uveitis indications.
In addition, under the agreement, we are eligible to receive a combination of Euro- and U.S. Dollar (“USD”)-denominated, development and sales milestones for multiple indications aggregating to a potential maximum of approximately $433 million when converted using the December 31, 2014, Euro to USD exchange rate (the “12/31/14 Exchange Rate of 1.216”), if XOMA reacquires cardiovascular and/or diabetes rights for use in the United States and Japan. If XOMA does not reacquire these rights, then the milestone payments aggregate to a potential maximum of approximately $770 million converted using the December 31, 2014 Exchange Rate of 1.216. Servier’s obligation to pay development and commercialization milestones will continue for so long as Servier is developing or selling products under the agreement.
We are eligible to receive royalties on gevokizumab sales from sales outside of the United States and Japan, and from global sales in cardiometabolic indications, which are tiered based on sales levels and range from a mid-single-digit to up to a mid-teens percentage rate. Our right to royalties with respect to a particular product and country will continue for so long as such product is sold in such country.
The collaboration is carried out and managed by committees mutually established by XOMA and Servier. In general, in the event of any disputes, each party has decision-making authority over matters relating to its areas of responsibility and territory, but neither party has unilateral decision-making rights if the decision would have a material adverse impact on the other party’s rights in its territory. The agreement contains customary termination rights relating to matters such as material breach by either party, safety issues and patents. Servier also has a unilateral right to terminate the agreement on a country-by-country basis or in its entirety on six months’ notice.
We also entered into a loan agreement with Servier (the “Servier Loan Agreement”) that provided for an advance of up to €15.0 million. The loan was fully funded in January 2011, with the proceeds converting to approximately $19.5 million at the date of funding. The loan is secured by an interest in XOMA’s intellectual property rights to all gevokizumab indications worldwide, excluding certain rights in the United States and Japan. Interest is calculated at a floating rate based on a Euro Inter-Bank Offered Rate (“EURIBOR”) and is subject to a cap. The interest rate is reset semi-annually in January and July of each year. The interest rate for the initial interest period was 3.22% and was reset semi-annually ranging from 2.31% to 3.83%. Interest for the six-month period from mid-January 2015 through mid-July 2015 was reset to 2.16%. Interest is payable semi-annually; however, the Servier Loan Agreement provided for a deferral of interest payments over a period specified in the agreement. During the deferral period, accrued interest was added to the outstanding principal amount for the purpose of interest calculation for the next six-month interest period. On the repayment commencement date, all unpaid and accrued interest shall be paid to Servier, and thereafter, all accrued and unpaid interest shall be due and payable at the end of each six-month period. In January 2015, we paid $0.2 million in accrued interest to Servier.
On January 9, 2015, Servier and we entered into Amendment No. 2 (“Loan Amendment”) to the Servier Loan Agreement. The Loan Agreement was initially entered into on December 30, 2010 and subsequently amended by a Consent, Transfer, Assumption and Amendment Agreement entered into as of August 12, 2013. The Loan Amendment modifies the maturity date of the loan from January 13, 2016, to three tranches due on January 15, 2016, January 15, 2017 and January 15, 2018; however, after a specified period prior to final maturity, the loan is to be repaid (i) at Servier's option, by applying up to a significant percentage of any milestone or royalty payments owed by Servier under our collaboration agreement and (ii) using a significant percentage of any upfront, milestone or royalty payments we receive from any third-party collaboration or development partner for rights to gevokizumab in the United States and/or Japan. In addition, the loan becomes immediately due and payable upon certain customary events of default. At December 31, 2014, the outstanding principal balance under this loan was $18.2 million using the December 31, 2014 Exchange Rate of 1.216. Refer to Management’s Discussion and Analysis of Financial Condition and Results of Operations for further information regarding the Servier Loan Agreement.
NIAID
In September 2008, we were awarded a third NIAID contract for $64.8 million under Contract No. HHSN272200800028C (“NIAID 3”) to continue development of our anti-botulinum antibody product candidates, including XOMA 3AB and additional product candidates directed against the B and E toxin serotypes. As part of the contract, we have developed, evaluated and produced the clinical supplies to support an IND filing with the FDA for XOMA 3AB. A Phase 1 trial was completed on XOMA 3AB, with no product-related serious adverse events. Subsequently, XOMA manufactured XOMA 3B and XOMA 3E, which are currently on stability and are in the process of IND preparation.
In October 2011, we announced we had been awarded a fourth NIAID contract for up to $28.0 million over five years under Contract No. HHSN 272201100031C (“NIAID 4”) to develop broad-spectrum antitoxins for the treatment of human botulism poisoning directed against the C and D toxin serotypes. XOMA has identified and produced the product antibodies and is in the process of preparing for the execution of non-clinical work and filling of the current Good Manufacturing Practices (“cGMP”) drug product.
Takeda
In November 2006, we entered into a fully funded collaboration agreement with Takeda for therapeutic monoclonal antibody discovery and development activities under which we agreed to discover and optimize therapeutic antibodies against multiple targets selected by Takeda. Takeda agreed to make up-front, annual maintenance and milestone payments to us, fund our research and development and manufacturing activities for preclinical and early clinical studies and pay royalties on sales of products resulting from the collaboration. Takeda is responsible for clinical trials and commercialization of drugs after an IND submission and is granted the right to manufacture once a product enters into Phase 2 clinical trials. We have completed a technology transfer and do not expect to perform any further research and development services under this program. From 2011 through 2014, we received milestone payments relating to one currently active program.
Under the terms of this agreement, we may receive milestone payments aggregating up to $19.0 million relating to one undisclosed product candidate and low single-digit royalties on future sales of all products subject to this license. In addition, in the event Takeda develops additional qualifying product candidates under the terms of our agreement, we would be eligible for milestone payments aggregating up to $20.75 million for each such qualifying product candidate. Our right to milestone payments expires on the later of the receipt of payment from Takeda of the last amount to be paid under the agreement or the cessation by Takeda of all research and development activities with respect to all program antibodies, collaboration targets and/or collaboration products. Our right to royalties expires on the later of 13.5 years from the first commercial sale of each royalty-bearing discovery product or the expiration of the last-to-expire licensed patent.
In February 2009, we expanded our existing collaboration to provide Takeda with access to multiple antibody technologies, including a suite of research and development technologies and integrated information and data management systems. We may receive milestones of up to $3.25 million per discovery product candidate and low single-digit royalties on future sales of all antibody products subject to this license. Our right to milestone payments expires on the later of the receipt of payment from Takeda of the last amount to be paid under the agreement or the cessation by Takeda of all research and development activities with respect to all program antibodies, collaboration targets and/or collaboration products. Our right to royalties expires on the later of 10 years from the first commercial sale of such royalty-bearing discovery product or the expiration of the last-to-expire licensed patent.
Novartis
In November 2008, we restructured our product development collaboration with Novartis. Under the restructured agreement, Novartis made a payment to us of $6.2 million in cash and reduced our existing debt by $7.5 million; agreed to fund all future research and development expenses; agreed to pay potential milestones of up to $14.0 million and royalty rates ranging from low-double-digit to high-teen percentage rates for certain antibody products binding to CD40 or prolactin receptor antibody programs; and has provided us with options to develop or receive royalties on four additional programs. In exchange, Novartis received control over the CD40 and prolactin receptor antibody programs, as well as the right to expand the development of these programs into additional indications outside of oncology. Novartis has initiated clinical studies to test CFZ533, an anti-CD40 antibody arising from its collaboration with XOMA, in de novo renal transplantation and in Primary Sjögren's Syndrome. Novartis has returned control of the prolactin receptor antibody program to us and we are evaluating options for its continued development. In 2013, we received a $7.0 million milestone relating to one currently active program. Our right to milestone payments expires at such time as no collaboration product or former collaboration product is being developed or commercialized anywhere in the world and no royalty payments on these products are due. Our right to royalty payments expires on the later of the expiration of any licensed patent covering each product or 20 years from the launch of each product.
In connection with the collaboration between XOMA and Novartis (then Chiron Corporation), a secured note agreement was executed in May 2005. The note agreement is secured by our interest in the collaboration and is due and payable in full in June 2015. At December 31, 2014, the outstanding principal balance under this note agreement totaled $13.4 million and was included in our current portion of interest bearing obligations in our consolidated balance sheet for the period ended December 31, 2014. Pursuant to the terms of the arrangement as restructured in November 2008, we will not make any additional borrowings on the Novartis note. Pursuant to our obligations under the note agreement, in January 2014, we made a payment equal to 25 percent of a $7.0 million milestone received, or $1.75 million, toward our outstanding debt obligation to Novartis.
Pfizer
In August 2007, we entered into a license agreement with Pfizer Inc. (“Pfizer”) for non-exclusive, worldwide rights for XOMA’s patented bacterial cell expression technology for research, development and manufacturing of antibody products. Under the terms of the agreement, we received a license fee payment of $30 million in 2007.
From 2011 through 2014, we have received milestone payments, and we also may be eligible for additional milestone payments aggregating up to $17.9 million and low single-digit royalties on future sales of all products subject to this license. In addition, we may receive potential milestone payments aggregating up to $1.7 million for each additional qualifying product candidate. Our right to milestone payments expires on the later of the expiration of the last-to-expire licensed patent or the tenth anniversary of the effective date. Our right to royalties expires upon the expiration of the last-to-expire licensed patent. We expect to recognize revenue on milestones when and if they are achieved and on royalties when and if the underlying sales occur.
In August 2005, we entered into a license agreement with Wyeth (subsequently acquired by Pfizer) for non-exclusive, worldwide rights for certain of XOMA’s patented bacterial cell expression technology for vaccine manufacturing. Under the terms of this agreement, we received a milestone payment in January 2015 relating to TRUMENBA®, a meningococcal group B vaccine marketed by Pfizer. We anticipate receiving a fraction of a percentage of sales of TRUMENBA as royalties. Our right to royalties expires on a country-by-country basis upon the later of the expiration of the last-to-expire licensed patent or 10 years from the first commercial sale of TRUMENBA.
Symplmed
In July 2013, we transferred U.S. development and commercialization rights of the perindopril franchise, and sublicensed the ACEON® U.S. marketing rights to Symplmed Pharmaceuticals, LLC (“Symplmed”). Under the terms of the arrangement, we received a minority equity position in Symplmed and up to double-digit royalties on sales of the first fixed-dose combination containing perindopril arginine and amlodipine besylate, if it is approved by the FDA. We recorded the minority equity position in other assets on our consolidated balance sheets. In July 2014, the ACEON new drug application (“NDA”) was transferred to Symplmed. Pursuant to the terms of the transfer, Symplmed will pay us single-digit royalties on sales of ACEON and for the year ended December 31, 2014, royalties received were de minimis.
On January 26, 2015, Symplmed announced that the FDA approved PRESTALIA® (perindopril arginine and amlodipine) tablets, licensed from Servier, for the treatment of hypertension. In July 2013, we transferred the development and commercialization rights of Prestalia to Symplmed. Pursuant to the transfer agreement with Symplmed, we will receive up to double-digit in royalties on sales of PRESTALIA. Refer to Management’s Discussion and Analysis of Financial Condition and Results of Operations for further information regarding Symplmed Pharmaceuticals.
Tamus
In October 2014, we entered into a license agreement with Texas A&M University (“Tamus”) for non-exclusive, worldwide rights for XOMA’s innovative design of a manufacturing facility. The patented technology relates to a flexible arrangement of MCRs within the manufacturing facility with each MCR providing a portable, self-contained environment that allows for drug development.
Financing Agreements
Outstanding Warrants
In March 2012, we issued warrants in connection with an underwritten public offering, which entitle the holders to purchase up to an aggregate of 14,834,577 shares of XOMA common stock at an exercise price equal to $1.76 per share. The warrants are exercisable immediately and will expire on March 6, 2017. As of December 31, 2014, 12,109,418 of these warrants were outstanding.
In September 2012, we issued warrants in connection with an amendment to an existing debt financing, which entitle the holder to purchase up to an aggregate of 39,346 unregistered shares of XOMA common stock at an exercise price equal to $3.54 per share. The warrants are exercisable immediately and will expire on September 27, 2017. As of December 31, 2014, all of these warrants were outstanding.
In December 2014, we issued warrants in connection with a registered direct offering, which entitle the holders to purchase up to an aggregate of 8,097,165 shares of XOMA common stock at an exercise price equal to $7.90 per share. The warrants are exercisable immediately and will expire on December 7, 2016. As of December 31, 2014, all of these warrants were outstanding.
In February 2015, we entered into a new debt financing with Hercules Technology III, L.P., as lender, an affiliate of Hercules Technology Growth Capital, Inc., as agent (collectively, “Hercules”). In connection with this debt financing, we issued a warrant which entitles the holder to purchase up to an aggregate of 181,268 shares of XOMA common stock at an exercise price equal to $3.31 per share. The warrant is exercisable immediately and will expire on February 27, 2020. As of March 9, 2015, all of these warrants were outstanding. Refer to Management’s Discussion and Analysis of Financial Condition and Results of Operations for further information regarding the Hercules Loan Agreement.
General Electric Capital Corporation Term Loan
In December 2011, we entered into a loan agreement (the “GECC Loan Agreement”) with General Electric Capital Corporation (“GECC”), under which GECC agreed to make a term loan in an aggregate principal amount of $10.0 million (the “Term Loan”) to us, and upon execution of the GECC Loan Agreement, GECC funded the Term Loan. As security for our obligations under the GECC Loan Agreement, we granted a security interest in substantially all of our existing and after-acquired assets, excluding its intellectual property assets (such as those relating to our gevokizumab and anti-botulism products). The Term Loan accrued interest at a fixed rate of 11.71% per annum and was to be repaid over a period of 42 consecutive equal monthly installments of principal and accrued interest and was due and payable in full on June 15, 2015. We incurred debt issuance costs of approximately $1.3 million in connection with the Term Loan and were required to pay a final payment fee equal to $500,000 on the maturity date, or such earlier date as the Term Loan is paid in full. The debt issuance costs and final payment fee were being amortized and accreted, respectively, to interest expense over the term of the Term Loan using the effective interest method.
In connection with the GECC Loan Agreement, we issued to GECC unregistered warrants that entitle GECC to purchase up to an aggregate of 263,158 unregistered shares of XOMA common stock at an exercise price equal to $1.14 per share. These warrants are exercisable immediately and have a five-year term. We allocated the aggregate proceeds of the GECC Term Loan between the warrants and the debt obligation based on their relative fair values. The fair value of the warrants issued to GECC was determined using the Black-Scholes Model. The warrants’ fair value of $0.2 million was recorded as a discount to the debt obligation and was being amortized over the term of the loan using the effective interest method.
In September 2012, we entered into an amendment to the GECC Loan Agreement providing for an additional term loan in the amount of $4.6 million, increasing the term loan obligation to $12.5 million (the “Amended Term Loan”) and providing for an interest-only monthly repayment period following the effective date of the amendment through March 1, 2013, at a stated interest rate of 10.9% per annum. Thereafter, we were obligated to make monthly principal payments of $347,222, plus accrued interest, over a 27-month period commencing on April 1, 2013, and through June 15, 2015, at which time the remaining outstanding principal amount of $3.1 million, plus accrued interest, was due. We incurred debt issuance costs of approximately $0.2 million and were required to make a final payment fee in the amount of $875,000 on the date upon which the outstanding principal amount was required to be repaid in full. This final payment fee replaced the original final payment fee of $500,000. The debt issuance costs and final payment fee were being amortized and accreted, respectively, to interest expense over the term of the Amended Term Loan using the effective interest method.
In connection with the amendment, on September 27, 2012, we issued to GECC unregistered stock purchase warrants, which entitle GECC to purchase up to an aggregate of 39,346 shares of XOMA common stock at an exercise price equal to $3.54 per share. These warrants are exercisable immediately and have a five-year term. The warrants’ fair value of $0.1 million was recorded as a discount to the debt obligation and was being amortized over the term of the loan using the effective interest method. The warrants are classified in permanent equity on the consolidated balance sheets.
The Amended Term Loan did not change the remaining terms of the GECC Loan Agreement. The GECC Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including restrictions on the ability to incur indebtedness, grant liens, make investments, dispose of assets, enter into transactions with affiliates and amend existing material agreements, in each case subject to various exceptions. In addition, the GECC Loan Agreement contains customary events of default that entitle GECC to cause any or all of the indebtedness under the GECC Loan Agreement to become immediately due and payable. The events of default include any event of default under a material agreement or certain other indebtedness.
We had the option to prepay the Amended Term Loan voluntarily in full, but not in part, and any voluntary and certain mandatory prepayments are subject to a prepayment premium of 3% in the first year after the effective date of the amendment to the GECC Loan Agreement, 2% in the second year and 1% thereafter, with certain exceptions. We also were required to pay the $875,000 final payment fee in connection with any voluntary or mandatory prepayment. On the effective date of the amendment to the GECC Loan Agreement, we paid an accrued final payment fee in the amount of $0.2 million relating to the original final payment fee of $500,000. At December 31, 2014, the outstanding principal balance under the Amended Term Loan was $5.2 million and was included in our current portion of interest bearing obligations in our consolidated balance sheet for the period ended December 31, 2014.
On February 27, 2015, XOMA entered into the Hercules Loan Agreement, under which we borrowed $20.0 million. We used a portion of the proceeds received under the Hercules Loan Agreement to repay GECC’s outstanding balance and interest of $5.5 million.
Hercules Loan and Security Agreement
In February 2015, we entered into a Loan and Security Agreement with Hercules, (the “Hercules Loan Agreement”) under which we borrowed $20.0 million. We used a portion of the proceeds received under the Hercules Loan Agreement to repay the outstanding GECC Loan Agreement balance, and we plan to use the remaining proceeds for general corporate purposes.
The interest rate under the Hercules Loan Agreement will be calculated at a rate equal to the greater of either (i) 9.40% plus the prime rate as reported from time to time in The Wall Street Journal minus 7.25%, and (ii) 9.40%. Payments under the Hercules Loan Agreement are interest only until one month prior to the Amortization Date, defined as July 1, 2016 (which will be extended to October 1, 2016, if we achieve certain clinical milestones on or before July 1, 2016). The interest only period will be followed by equal monthly payments of principal and interest amortized over a 30 month schedule through the scheduled maturity date of September 1, 2018 (the “Hercules Loan Maturity Date”). The entire principal balance, including a balloon payment of principal, as applicable, will be due and payable on the Hercules Loan Maturity Date. In addition, a final payment equal to $1,150,000 will be due on the Hercules Loan Maturity Date, or such earlier date specified in the Hercules Loan Agreement. Our obligations under the Hercules Loan Agreement are secured by a security interest in substantially all of our assets, other than our intellectual property.
If we prepay the loan prior to the Hercules Loan Maturity Date, we will pay Hercules a prepayment charge, based on a prepayment fee equal to 3.00% of the amount prepaid, if the prepayment occurs in any of the first 12 months following the closing date, 2.00% of the amount prepaid, if the prepayment occurs after 12 months from the closing date but prior to 24 months from the closing date, and 1.00% of the amount prepaid if the prepayment occurs after 24 months from the closing date.
The Hercules Loan Agreement includes customary affirmative and restrictive covenants, but does not include any financial maintenance covenants, and also includes standard events of default, including payment defaults. Upon the occurrence of an event of default, a default interest rate of an additional 5% may be applied to the outstanding loan balances, and Hercules may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Hercules Loan Agreement.
In connection with the Hercules Loan Agreement, we issued a warrant to Hercules that is exercisable for an aggregate of up to 181,268 shares of XOMA common stock at an exercise price of $3.31 per share (the “Hercules Warrant”). The Hercules Warrant may be exercised on a cashless basis and is exercisable for a term beginning on the date of issuance and ending on the earlier to occur of five years from the date of issuance or the consummation of certain acquisitions of XOMA as set forth in the Hercules Warrant. The number of shares for which the Hercules Warrant is exercisable and the associated exercise price are subject to certain proportional adjustments as set forth in the Hercules Warrant.
Research and Development
Our research and development expenses currently include costs of personnel, supplies, facilities and equipment, consultants, third-party costs and other expenses related to preclinical and clinical testing. In 2014, our research and development expenses were $80.7 million, compared with $74.9 million in 2013 and $68.5 million in 2012.
Our research and development activities can be divided into those related to our internal projects and those related to collaborative and contract arrangements, which are reimbursed by our customers. In 2014, research and development expenses relating to internal projects were $51.3 million, compared with $47.5 million in 2013 and $30.5 million in 2012. In 2014, research and development expenses related to collaborative and contract arrangements were $29.4 million, compared with $27.4 million in 2013 and $37.9 million in 2012. Refer to Management’s Discussion and Analysis of Financial Condition and Results of Operations- Research and Development Expenses for further information regarding our research and development expenses.
Competition
The biotechnology and pharmaceutical industries are subject to continuous and substantial technological change. Competition in antibody-based technologies is intense and is expected to increase as new technologies emerge and established biotechnology firms and large chemical and pharmaceutical companies continue to advance in the field. A number of these large pharmaceutical and chemical companies have enhanced their capabilities by entering into arrangements with or acquiring biotechnology companies or entering into business combinations with other large pharmaceutical companies. Many of these companies have significantly greater financial resources, larger research and development and marketing staffs and larger production facilities than ours. Moreover, certain of these companies have extensive experience in undertaking preclinical testing and human clinical trials. These factors may enable other companies to develop products and processes competitive with or superior to ours. In addition, a significant amount of research in biotechnology is being carried out in universities and other non-profit research organizations. These entities are becoming increasingly interested in the commercial value of their work and may become more aggressive in seeking patent protection and licensing arrangements. Furthermore, many companies and universities tend not to announce or disclose important discoveries or development programs until their patent position is secure or, for other reasons, later. As a result, we may not be able to track development of competitive products, particularly at the early stages. There can be no assurance that developments by others will not render our products or technologies obsolete or uncompetitive.
Without limiting the foregoing, we are aware of the following competitors for the products and candidates shown in the table below. This table is not intended to be representative of all existing competitors in the market:
|
Product/Candidate
|
Competitors
|
|
Gevokizumab
|
AbbVie Inc.
Biovitrum AB
Allergan
Novartis AG
pSivida
Regeneron Pharmaceuticals, Inc.
Santen Pharmaceutical Co., Ltd.
|
|
XOMA 3AB
|
Emergent BioSolutions, Inc.
|
Government Regulation
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, pre-market approval, manufacture, marketing, import, export and distribution of biopharmaceutical products. These agencies and other regulatory agencies regulate research and development activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, storage, recordkeeping, advertising and promotion of products and product candidates. Failure to comply with applicable FDA or other regulatory requirements may result in Warning Letters, civil or criminal penalties, suspension or delays in clinical development, recall or seizure of products, partial or total suspension of production or withdrawal of a product from the market. The development and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. We must obtain approval of our product candidates from the FDA before we can begin marketing them in the United States. Similar approvals are also required in other countries.
Product development and approval within this regulatory framework is uncertain, can take many years and requires the expenditure of substantial resources. The nature and extent of the governmental review process for our product candidates will vary, depending on the regulatory categorization of particular product candidates and various other factors.
The necessary steps before a new biopharmaceutical product may be sold in the United States ordinarily include:
| ● | preclinical in vitro and in vivo tests, which must comply with Good Laboratory Practices (“GLP”); |
| ● | submission to the FDA of an IND which must become effective before clinical trials may commence, and which must be updated annually with a report on development; |
| ● | completion of adequate and well controlled human clinical trials to establish the safety and efficacy of the product candidate for its intended use; |
| ● | submission to the FDA of a BLA, which must often be accompanied by payment of a substantial user fee; |
| ● | FDA pre-approval inspection of manufacturing facilities for current Good Manufacturing Practices, or GMP, compliance and FDA inspection of select clinical trial sites for Good Clinical Practice (“GCP”), compliance; and |
| ● | FDA review and approval of the BLA and product prescribing information prior to any commercial sale. |
The results of preclinical tests (which include laboratory evaluation as well as preclinical GLP studies to evaluate toxicity) for a particular product candidate, together with related manufacturing information and analytical data, are submitted as part of an IND to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. IND submissions may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board (“IRB”), for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP regulations and regulations for informed consent and privacy of individually identifiable information.
Clinical trials generally are conducted in three sequential phases that may overlap or in some instances, be skipped. In Phase 1, the initial introduction of the product into humans, the product is tested to assess safety, metabolism, pharmacokinetics and pharmacological actions associated with increasing doses. Phase 2 usually involves trials in a limited patient population to evaluate the efficacy of the potential product for specific, targeted indications, determine dosage tolerance and optimum dosage and further identify possible adverse reactions and safety risks. Phase 3 and pivotal trials are undertaken to evaluate further clinical efficacy and safety often in comparison to standard therapies within a broader patient population, generally at geographically dispersed clinical sites. Phase 4, or post-marketing, trials may be required as a condition of commercial approval by the FDA and may also be voluntarily initiated by us or our collaborators. Phase 1, Phase 2 or Phase 3 testing may not be completed within any specific period of time, if at all, with respect to any of our product candidates. Similarly, suggestions of safety, tolerability or efficacy in earlier-stage trials do not necessarily predict findings of safety and effectiveness in subsequent trials. Furthermore, the FDA, an IRB, or we may suspend a clinical trial at any time for various reasons, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical trials are subject to central registration and results reporting requirements, such as on www.clinicaltrials.gov.
The results of preclinical studies, pharmaceutical development and clinical trials, together with information on a product’s chemistry, manufacturing, and controls, are submitted to the FDA in the form of a BLA, for approval of the manufacture, marketing and commercial shipment of the biopharmaceutical product. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaborators interpret data. For all compounds seeking their first marketing approval, the FDA convenes an Advisory Committee of external advisors to answer questions regarding the compound’s approvability and what labeling the compound should receive based upon its NDA or BLA. Approved compounds seeking to expand their label may also be the subject of an Advisory Committee depending upon the indication. The FDA also may convene an Advisory Committee of external advisors to answer questions regarding the approvability and labeling of an application. The FDA is not obligated to follow the Advisory Committee’s recommendation. The submission of a BLA is required to be accompanied by a substantial user fee, with few exceptions or waivers. The user fee is administered under the Prescription Drug User Fee Act, which sets goals for the timeliness of the FDA’s review. A standard review period is twelve months from submission of the application, while priority review is eight months from submission of the application. The testing and approval process is likely to require substantial time, effort and resources, and there can be no assurance that any approval will be granted on a timely basis, if at all. The FDA may deny review of an application by refusing to file the application or not approve an application by issuance of a complete response letter if applicable regulatory criteria are not satisfied, require additional testing or information, or require risk management programs and post-market testing and surveillance to monitor the safety or efficacy of the product. Approval may occur with significant Risk Evaluation and Mitigation Strategies, or REMS, which limit the clinical use in the prescribing information, distribution or promotion of a product. Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market.
Orphan drugs are those intended for use in rare diseases or conditions. As a result of the high cost of development and the low return on investment for rare diseases, governments provide regulatory and commercial incentives for the development of drugs for small disease populations. In the United States, the term ‘‘rare disease or condition’’ means any disease or condition that affects fewer than 200,000 people in the United States. Applications for U.S. orphan drug status are evaluated and granted by the Office of Orphan Products Development (“OOPD”) of the FDA and must be requested before submitting a BLA. In the United States, orphan drugs are subject to the standard regulatory process for marketing approval but are exempt from the payment of user fees for licensure, may receive market exclusivity for a period of seven years and some tax benefits, and are eligible for OOPD grants. If a product with orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means the FDA may not approve any other applications to market the same drug or biological product for the same indication, except in very limited circumstances, for seven years. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug or biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity.
Products manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including manufacture, labeling, advertising, distribution, advertising, promotion, recordkeeping, annual product quality review and reporting requirements. Adverse event experience with the product must be reported to the FDA in a timely fashion and pharmacovigilance programs to proactively look for these adverse events are mandated by the FDA. Manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Following such inspections, the FDA may issue notices on Form 483 and Warning Letters that could cause us to modify certain activities. A Form 483 notice, if issued at the conclusion of an FDA inspection, can list conditions the FDA investigators believe may have violated cGMP or other FDA regulations or guidance. Failure to adequately and promptly correct the observations(s) can result in further regulatory enforcement action. In addition to Form 483 notices and Warning Letters, failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. We cannot be certain that we or our present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory requirements. If we or our present or future third-party manufacturers or suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, not approve our products, and require us to recall a product from distribution or withdraw approval of the BLA for that product. Failure to comply with ongoing regulatory obligations can result in delay of approval or Warning Letters, product seizures, criminal penalties, and withdrawal of approved products, among other enforcement remedies.
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. These regulations include standards and restrictions for direct-to-consumer advertising, industry-sponsored scientific and educational activities, promotional activities involving the internet, and off-label promotion. While physicians may prescribe for off-label uses, manufacturers may only promote for the approved indications and in accordance with the provisions of the approved label. The FDA has very broad enforcement authority under the Federal Food, Drug, and Cosmetic Act, and failure to abide by these regulations can result in penalties, including the issuance of a warning letter directing entities to correct deviations from FDA standards, and state and federal civil and criminal investigations and prosecutions.
Federal and state healthcare laws, including fraud and abuse and health information privacy and security laws, are also applicable to our business. We could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected. The laws that may affect our ability to operate include: the federal Anti-Kickback Statute, which prohibits soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent; and the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters and was amended by the Health Information Technology and Clinical Health Act (“HITECH”), and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. State law equivalents of each of the above federal laws exist, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
International Regulation
In addition to regulations in the United States, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of any future products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials or market the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Patents and Trade Secrets
Patent and trade secret protection are important to our business and our future will depend in part on our ability to obtain patents, maintain trade secret protection and operate without infringing on the proprietary rights of others. As a result of our ongoing activities, we hold and have filed applications for a number of patents in the United States and internationally to protect our products and important processes. We also have obtained or have the right to obtain exclusive licenses to certain patents and applications filed by others. However, the patent position of biotechnology companies generally is highly uncertain and consistent policy regarding the breadth of allowed claims has not emerged from the actions of the U.S. Patent and Trademark Office (“Patent Office”) with respect to biotechnology patents. Accordingly, no assurance can be given that our patents will afford protection against competitors with similar technologies or others will not obtain patents claiming aspects similar to those covered by our patent applications.
We have established a portfolio of patents in the United States, Europe and certain other countries for our gevokizumab program. U.S. Patent Nos. 7,531,166 (which expires in 2027) and 7,582,742 cover gevokizumab and other antibodies and antibody fragments with similar binding properties for IL-1 beta, as well as nucleic acids, expression vectors and production cell lines for the manufacture of such antibodies and antibody fragments. U.S. Patent Nos. 7,744,865, 7,744,866 and 7,943,121 relate to additional IL-1 beta binding antibodies and binding fragments. U.S. Patent Nos. 7,695,718, 8,101,166, 8,586,036, 8,545,846 and 8,377,429 relate to methods of treating Type 2 diabetes or Type 2 diabetes-induced diseases or conditions with high affinity antibodies and antibody fragments that bind to IL-1 beta, including gevokizumab. U.S. Patent No. 8,637,029 relates to methods of treating gout with certain doses of IL-1 beta binding antibodies or binding fragments. U.S. Patent No. 7,695,717 relates to methods of treating certain IL-1 related inflammatory diseases, including rheumatoid arthritis and osteoarthritis, with gevokizumab and other antibodies and antibody fragments with similar binding properties for IL-1 beta. U.S. Patent No. 7,829,093 relates to methods of treating diabetes mellitus (“Type 1”) with gevokizumab or other IL-1 beta antibodies and fragments having similar binding properties. U.S. Patent No. 7,829,094 relates to methods of treating certain cancers with gevokizumab or other IL-1 beta antibodies and fragments having similar binding properties, with the cancer being selected from multiple myeloma, acute myelogenous leukemia and chronic myelogenous leukemia. U.S. Patent No. 7,988,968 relates to methods of treating certain IL-1 beta related coronary conditions, including myocardial infarction, with gevokizumab or other IL-1 beta antibodies and fragments having similar binding properties. U.S. Patent No. 8,377,442 relates to methods of treating certain IL-1 beta related conditions, including inflammatory eye disease or uveitis, with gevokizumab or other IL-1 beta antibodies and fragments having similar binding properties. U.S. Patent No. 8,551,487 relates to methods of treating refractory uveitis with IL-1 beta binding antibodies and binding fragments. Also, patents have been granted by the European Patent Office and certain other countries for gevokizumab, as well as nucleic acids, expression vectors and production cell lines for the manufacture of gevokizumab.
On January 6, 2015 we were awarded U.S. Patent No. 8,926,976 covering insulin receptor-activating antibodies having the functional properties of the lead antibody in our XMetA program. Patent applications covering our other XMet programs are pending in the U.S. and certain other countries.
We have exclusively in-licensed a portfolio of patents and applications covering anti-botulinum toxin antibodies from the Regents of the University of California. These include U.S. Patent Nos. 7,700,738, 7,999,079, and 8,263,747 covering certain XOMA 3AB antibodies, the longest of which expire in 2026, and U.S. Patent No. 8,598,321 covering a XOMA 3B antibody. In September 2014, we were awarded U.S. Patent No. 8,821,879 covering stable pharmaceutical formulations of certain anti-botulinum toxin antibodies.
We have established a portfolio of patents related to our bacterial expression technology, including claims to methods for expression and secretion of recombinant proteins from bacteria, including immunoglobulin gene products, and improved methods and cells for expression of recombinant protein products. U.S. Patent No. 5,618,920 relates to secretable immunoglobulin chains, DNA encoding the chains and methods for their recombinant production. U.S. Patent Nos. 5,693,493, 5,698,417 and 6,204,023 relate to methods for recombinant production/secretion of functional immunoglobulin molecules. U.S. Patent Nos. 7,094,579, 7,396,661, 7,972,811, 7,977,068 and 8,476,040 relate to particular eukaryotic signal sequences and their use in methods for prokaryotic expression of polypeptides and for preparing polypeptide display libraries. U.S. Patent Nos. 8,546,307 and 8,546,308 relate to novel triple tag sequences, phage display antibody libraries with such sequences, and methods of screening the libraries. U.S. Patent No. 6,803,210 relates to improved bacterial host cells that are deficient in one or more of the active transport systems for an inducer of an inducible promoter, such as arabinose for an araB promoter, and methods for the use of such cells for the production of recombinant proteins. Most of the more important licensed European patents in this portfolio expired in July 2008 or earlier. The last of the more important licensed United States patents in this portfolio expired in December 2014. We have granted more than 60 licenses to biotechnology and pharmaceutical companies to use the Company’s patented and proprietary technologies relating to bacterial expression of recombinant pharmaceutical products. The last-to-expire patent licensed under the majority of these license agreements is Canadian patent 1,341,235, which is expected to expire in May 2018.
We also have established a portfolio of patents related to our mammalian expression technology, including U.S. Patent Nos. 7,192,737, 7,993,915 and 7,794,976, which relate to methods of producing recombinant proteins using particular vectors, including expression vectors comprising multiple copies of a transcription unit encoding a polypeptide separated by at least one selective marker gene.
We have established a portfolio of patents related to our Human Engineering™ technology, including U.S. Patent No. 5,766,886, directed to methods of modifying antibody variable domains to reduce immunogenicity. We believe our patented Human Engineering™ technology provides an attractive alternative to other humanization technologies.
In November 2013, we were awarded U.S. Patent No. 8,584,349, entitled "Flexible Manufacturing System." This patent is directed to a flexible system of movable manufacturing bays, adapted to easily and quickly connect to a central supply of utilities such as air, water, and electricity. This unique arrangement facilitates flexible design and eliminates change-over downtime, which translates into significantly reduced capital expenditures, production costs, and maintenance costs. The flexible manufacturing system can be applied to fields as diverse as pharmaceuticals, biologics, and electronics. In October 2014 we announced that the Texas A&M University System agreed to a non-exclusive license to this technology.
If certain patents issued to others are upheld or if certain patent applications filed by others issue and are upheld, we may require certain licenses from others in order to develop and commercialize certain potential products incorporating our technology. There can be no assurance that such licenses, if required, will be available on acceptable terms.
Where appropriate, we also rely on trade secrets to protect aspects of our technology. However, trade secrets are difficult to protect. We protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants and collaborators. These parties may breach these agreements, and we may not have adequate remedies for any breach. Our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that we or our consultants or collaborators use intellectual property owned by others, we may have disputes with our collaborators or consultants or other third parties as to the rights in related or resulting know-how and inventions.
Financial Information about Geographic Areas
We believe, because the pharmaceutical industry is global in nature, international activities will be a significant part of our future business activities, and when and if we are able to generate income, a portion of that income may be derived from product sales and other activities outside the United States. As one of our immediate strategic goals is to establish XOMA as a commercial organization in the United States, we will rely upon other companies to market our product outside of the United States for the foreseeable future. Our decision to retain the U.S. commercial rights to our product candidates while licensing the rights to our product candidates outside the United States, or to license our product candidates globally to one or more partners, depends upon a number of factors, including the primary indication and size of the potential patient population, the size of the clinical trials required to obtain marketing approval in the United States and globally, and the size of the sales force required to sell the product.
We have determined that we operate in one business segment as we only report operating results on an aggregate basis to the chief operating decision maker of the XOMA. Our property and equipment is held primarily in the United States.
Financial information regarding the geographic areas in which we operate and segment information is included in Note 12 to the December 31, 2014, Financial Statements: Concentration of Risk, Segment and Geographic Information.
Concentration of Risk
In 2014, NIAID and Servier accounted for 51 percent and 28 percent, respectively, of our total revenue. Servier, NIAID and Novartis accounted for 43 percent, 26 percent, and 20 percent respectively, of our total revenue in 2013. Servier and NIAID accounted for 47 percent and 33 percent, respectively, in 2012. At December 31, 2014, NIAID and Servier accounted for 44 percent and 34 percent, respectively, of the accounts receivable balance. Servier and NIAID accounted for 13 percent and 73 percent, respectively, of our total accounts receivable balance in 2013 and 58 percent and 35 percent, respectively, at the same period of 2012. None of these parties represent a related party to XOMA and the loss of one or more of these customers could have a material effect on our business and financial condition.
Organization
We were incorporated in Delaware in 1981 and became a Bermuda-exempted company in December 1998. Effective December 31, 2011, we changed our jurisdiction of incorporation from Bermuda to Delaware and changed our name from XOMA Ltd. to XOMA Corporation. When referring to a time or period before December 31, 1998, or when the context so requires, the terms “Company” and “XOMA” refer to XOMA Corporation, a Delaware corporation, and when referring to a time or period after December 31, 1998, and before December 31, 2011, such terms refer to XOMA Ltd., a Bermuda company.
Employees
As of March 9, 2015, we employed 183 full-time employees at our facilities, principally in Berkeley, California, none of whom are unionized. Our employees primarily are engaged in clinical, process development, research and product development, and in executive, business development, finance and administrative positions. We consider our employee relations to be excellent.
Available Information
For information on XOMA’s investment prospects and risks, please contact Investor Relations and Corporate Communications at (510) 204-7200 or by sending an e-mail message to investorrelations@xoma.com. Our principal executive offices are located at 2910 Seventh Street, Berkeley, California 94710, U.S.A. Our telephone number is (510) 204-7200.
The following information can be found on our website at http://www.xoma.com or can be obtained free of charge by contacting our Investor Relations Department:
|
●
|
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act will be available as soon as reasonably practicable after such material is electronically filed or otherwise furnished to the SEC. All reports we file with the SEC also can be obtained free of charge via EDGAR through the SEC’s website at http://www.sec.gov.
|
|
●
|
Our policies related to corporate governance, including our Code of Ethics applying to our directors, officers and employees (including our principal executive officer and principal financial and accounting officer) that we have adopted to meet the requirements set forth in the rules and regulations of the SEC and its corporate governance principles, are available.
|
|
●
|
The charters of the Audit, Compensation and Nominating & Governance Committees of our Board of Directors are available.
|
We intend to satisfy the applicable disclosure requirements regarding amendments to, or waivers from, provisions of our Code of Ethics by posting such information on our website.
The following risk factors and other information included in this annual report should be carefully considered. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us also may impair our business operations. If any of the following risks occur, our business, financial condition, operating results and cash flows could be materially adversely affected.
Because our product candidates are still being developed, we will require substantial funds to continue; we cannot be certain that funds will be available, and if they are not available, we may be forced to delay, reduce, or eliminate our product development programs or to take actions that could adversely affect your investment and may not be able to continue operations.
We will need to commit substantial funds to continue development of our product candidates, and we may not be able to obtain sufficient funds on acceptable terms, or at all. If we raise additional funds by issuing equity securities, our stockholders will experience dilution. Any debt financing or additional equity that we raise may contain terms that are not favorable to our stockholders or us. If we raise additional funds through collaboration and licensing arrangements with third parties, we may be required to relinquish some rights to our technologies or our product candidates, grant licenses on terms that are not favorable to us or enter into a collaboration arrangement for a product candidate at an earlier stage of development or for a lesser amount than we might otherwise choose.
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may:
| · | terminate or delay clinical trials for one or more of our product candidates; reduce or eliminate certain product development efforts or commercialization efforts; |
| · | further reduce our headcount and capital or operating expenditures; or |
| · | curtail our spending on protecting our intellectual property. |
We finance our operations primarily through our multiple revenue streams resulting from discovery and development collaborations, biodefense contracts, the licensing of our antibody technologies, debt and through sales of our common stock.
Based on our cash and cash equivalents of $78.4 million at December 31, 2014, anticipated spending levels, anticipated cash inflows from collaborations, biodefense contracts and licensing transactions, funding availability included under our loan agreements, the proceeds from our equity offerings and other sources of funding that we believe to be available, we anticipate that we will be required to seek additional equity or debt financing or to increase the level or collaborative revenue to fund operations through at least December 31, 2015. Any significant revenue shortfalls, increases in planned spending on development programs, more rapid progress of development programs than anticipated, or the initiation of new clinical trials, as well as the unavailability of anticipated sources of funding, could shorten this period or otherwise have a material adverse impact on our ability to finance our continued operations. Progress or setbacks by potentially competing products also may affect our ability to raise new funding on acceptable terms.
We do not know when or whether:
| · | operations will generate meaningful funds; |
| · | additional agreements for product development funding can be reached; |
| · | strategic alliances can be negotiated; or |
| · | adequate additional financing will be available for us to finance our own development on acceptable terms, or at all. |
If adequate funds are not available, we will be required to delay, reduce the scope of, or eliminate one or more of our product development programs and further reduce personnel-related costs.
We have sustained losses in the past, and we expect to sustain losses in the future.
We have been and are developing numerous product candidates, and as a result have experienced significant losses. As of December 31, 2014, we had an accumulated deficit of $1.1 billion.
For the year ended December 31, 2014, we had a net loss of approximately $38.3 million, or $0.36 per share of common stock, basic and $0.67 per share of common stock, diluted. For the year ended December 31, 2013, we had a net loss of approximately $124.1 million, or $1.43 per share of common stock (basic and diluted).
Our ability to achieve profitability is dependent in large part on the success of our development programs, obtaining regulatory approval for our product candidates and licensing certain of our preclinical compounds, all of which are uncertain. Our product candidates are still being developed, and we do not know whether we will ever achieve sustained profitability or whether cash flow from future operations will be sufficient to meet our needs.
We are substantially dependent on Servier for the development and commercialization of gevokizumab and for other aspects of our business, and if we are unable to maintain our relationship with Servier, or Servier does not perform under its agreements with us, our business would be harmed significantly.
We have a number of agreements with Servier that are material to the conduct of our business, including:
| · | In December 2010, we entered into a license and collaboration agreement with Servier, to jointly develop and commercialize gevokizumab in multiple indications. Under the terms of the agreement, Servier has worldwide rights to cardiovascular disease and diabetes indications and rights outside the United States and Japan to all other indications, including Behçet’s disease uveitis and other inflammatory and oncology indications. In late 2011, we announced Servier agreed to include the NIU Phase 3 trials under the terms of the collaboration agreement for Behçet’s disease uveitis. We retain development and commercialization rights for NIU and other inflammatory disease and oncology indications in the United States and Japan and have an option to reacquire rights to cardiovascular disease and diabetes indications from Servier in these territories. Should we exercise this option, we will be required to pay an option fee to Servier and partially reimburse a specified portion of Servier’s incurred development expenses. The agreement contains mutual customary termination rights relating to matters such as material breach by either party. Servier may terminate for safety issues, and we may terminate the agreement, with respect to a particular country or the European Patent Organization (“EPO”) member states, for any challenge to our patent rights in that country or any EPO member state, respectively, by Servier. Servier also has a unilateral right to terminate the agreement for the European Union (“EU”) or for non-EU countries, on a country-by-country basis, or in its entirety, in each case with six months’ notice. |
| · | In December 2010, we entered into a loan agreement with Servier (the “Servier Loan Agreement”), which provides for an advance of up to €15.0 million and was funded fully in January 2011 with the proceeds converting to approximately $19.5 million at the January 13, 2011, Euro-to-U.S.-dollar exchange rate of 1.3020. This loan is secured by an interest in our intellectual property rights to all gevokizumab indications worldwide, excluding the United States and Japan. The loan has a final maturity date in 2016; however, after a specified period prior to final maturity, the loan is required to be repaid (1) at Servier’s option, by applying up to a significant percentage of any milestone or royalty payments owed by Servier under our collaboration agreement and (2) using a significant percentage of any upfront, milestone or royalty payments we receive from any third-party collaboration or development partner for rights to gevokizumab in the United States and/or Japan. In addition, the loan becomes immediately due and payable upon certain customary events of default. At December 31, 2014, the €15.0 million outstanding principal balance under this Servier Loan Agreement would have equaled approximately $18.2 million using the December 31, 2014 Euro-to-U.S.-dollar exchange rate of 1.216. |
On January 9, 2015, Servier and we entered into Amendment No. 2 (“Loan Amendment”). The Servier Loan Agreement was initially entered into on December 30, 2010 and subsequently amended by a Consent, Transfer, Assumption and Amendment Agreement entered into as of August 12, 2013. The Loan Amendment modifies the maturity date of the loan from January 13, 2016 to three tranches due on January 15, 2016, January 15, 2017 and January 15, 2018 and provides that principal shall be repaid as follows: €3.0 million to be repaid on January 13, 2016, €5.0 million to be repaid on January 15, 2017 and €7.0 million to be repaid on January 15, 2018. All other terms of the Loan Agreement remain unchanged, including the interest rate calculations, EURIBOR+2% and the formula for resetting the interest rate on the 15th of January and 15th of July every six months.
On January 9, 2015, Servier and we entered into an Amendment No. 2 to the Collaboration Agreement Under the Collaboration Agreement we were eligible to receive up to approximately $433 million in the aggregate in milestone payments, most of which were denominated in Euros, if we re-acquire cardiovascular and/or diabetes rights for use in the United States, and approximately $770 million in aggregate milestone payments if we do not re-acquire those rights. Under the Collaboration Amendment, we would be eligible to receive up to $415 million in the aggregate in milestone payments in the event we re-acquire the cardiovascular and/or diabetes rights for use in the United States and approximately $752 million if we do not re-acquire those rights. The milestone reductions are related to a very low prevalence indication of which Servier would not have pursued development had these payments been required. All other terms of the Collaboration Agreement remain unchanged.
Because Servier is an independent third party, it may be subject to different risks than we are and has significant discretion in, and different criteria for, determining the efforts and resources it will apply related to its agreements with us. Even though we have a collaborative relationship with Servier, our relationship could deteriorate or other circumstances may prevent our relationship with Servier from resulting in successful development of marketable products. If we are not able to maintain our working relationship with Servier, or if Servier does not perform under its agreements with us, our ability to develop and commercialize gevokizumab would be materially and adversely affected.
If our therapeutic product candidates do not receive regulatory approval, neither our third-party collaborators nor we will be able to market them.
Our product candidates (including gevokizumab, XMetA, XMetS, XOMA 358 and XOMA 3AB) cannot be manufactured and marketed in the United States or any other countries without required regulatory approvals. The U.S. government and governments of other countries extensively regulate many aspects of our product candidates, including:
| · | clinical development and testing; |
| · | manufacturing; |
| · | labeling; |
| · | storage; |
| · | record keeping; |
| · | promotion and marketing; and |
| · | importing and exporting. |
In the United States, the FDA regulates pharmaceutical products under the Federal Food, Drug, and Cosmetic Act and other laws, including, in the case of biologics, the Public Health Service Act. At the present time, we believe many of our product candidates (including gevokizumab, XMetA, XMetD, XMetS and XOMA 3AB) will be regulated by the FDA as biologics. Initiation of clinical trials requires approval by health authorities. Clinical trials involve the administration of the investigational new drug to healthy volunteers or to patients under the supervision of a qualified principal investigator. Clinical trials must be conducted in accordance with FDA and International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practices and the European Clinical Trials Directive under protocols that detail the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Other national, foreign and local regulations also may apply. The developer of the drug must provide information relating to the characterization and controls of the product before administration to the patients participating in the clinical trials. This requires developing approved assays of the product to test before administration to the patient and during the conduct of the trial. In addition, developers of pharmaceutical products must provide periodic data regarding clinical trials to the FDA and other health authorities, and these health authorities may issue a clinical hold upon a trial if they do not believe, or cannot confirm, that the trial can be conducted without unreasonable risk to the trial participants. We cannot assure you that U.S. and foreign health authorities will not issue a clinical hold with respect to any of our clinical trials in the future.
The results of the preclinical studies and clinical testing, together with chemistry, manufacturing and controls information, are submitted to the FDA and other health authorities in the form of an NDA for a drug, and in the form of a Biologic License Application (“BLA”) for a biological product, requesting approval to commence commercial sales. In responding to an NDA or BLA, the FDA or foreign health authorities may grant marketing approvals, request additional information or further research, or deny the application if it determines the application does not satisfy its regulatory approval criteria. Regulatory approval of an NDA, BLA, or supplement never is guaranteed, the approval process can take several years, is extremely expensive and can vary substantially based upon the type, complexity, and novelty of the products involved, as well as the target indications. FDA regulations and policies permit applicants to request accelerated or priority review pathways for products intended to treat certain serious or life-threatening illnesses in certain circumstances. If granted by the FDA, these review pathways can provide a shortened timeline to commercialize the product, although the shortened review timeline is often accompanied with additional post-market requirements. Although we may pursue the FDA’s accelerated or priority review programs, we cannot guarantee the FDA will permit us to utilize these pathways or the FDA’s review of our application will not be delayed. Moreover, even if the FDA agrees to an accelerated or priority review of any of our applications, we ultimately may not be able to obtain approval of our application in a timely fashion or at all. The FDA and foreign health authorities have substantial discretion in the drug and biologics approval processes. Despite the time and expense incurred, failure can occur at any stage, and we could encounter problems that cause us to abandon clinical trials or to repeat or perform additional preclinical, clinical or manufacturing-related studies.
Changes in the regulatory approval policy during the development period, changes in, or the enactment of additional regulations or statutes, or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. State regulations may also affect our proposed products.
The FDA and other regulatory agencies have substantial discretion in both the product approval process and manufacturing facility approval process, and as a result of this discretion and uncertainties about outcomes of testing, we cannot predict at what point, or whether, the FDA or other regulatory agencies will be satisfied with our or our collaborators’ submissions or whether the FDA or other regulatory agencies will raise questions that may be material and delay or preclude product approval or manufacturing facility approval. In light of this discretion and the complexities of the scientific, medical and regulatory environment, our interpretation or understanding of the FDA’s or other regulatory agencies’ requirements, guidelines or expectations may prove incorrect, which also could delay further or increase the cost of the approval process. As we accumulate additional clinical data, we will submit it to the FDA and other regulatory agencies, as appropriate, and such data may have a material impact on the approval process.
Given that regulatory review is an interactive and continuous process, we maintain a policy of limiting announcements and comments upon the specific details of regulatory review of our product candidates, subject to our obligations under the securities laws, until definitive action is taken.
We have received negative results from certain of our clinical trials, and we face uncertain results of other clinical trials of our product candidates.
Drug development has inherent risk, and we are required to demonstrate through adequate and well-controlled clinical trials that our product candidates are effective, with a favorable benefit-risk profile for use in their target profiles before we can seek regulatory approvals for their commercial use. It is possible we may never receive regulatory approval for any of our product candidates. Even if a product candidate receives regulatory approval, the resulting product may not gain market acceptance among physicians, patients, healthcare payors and the medical community. In March 2011, we announced our 421-patient Phase 2b trial of gevokizumab in Type 2 diabetes did not achieve the primary endpoint of reduction in hemoglobin A1c (“HbA1c”) after six monthly treatments with gevokizumab compared to placebo. In June 2011, we announced top-line trial results from our six-month 74-patient Phase 2a trial of gevokizumab in Type 2 diabetes, and there were no differences in glycemic control between the drug and placebo groups as measured by HbA1c levels. In March 2014, we reported that despite early positive results in our gevokizumab proof-of-concept study in patients with erosive osteoarthritis of the hand (“EOA”) and elevated C-reactive protein, the top-line data at Day 168 in that study, as well as data at Day 84 in patients with EOA and non-elevated CRP, were not positive.
Many of our product candidates, including gevokizumab, XMetA, XMetS, XOMA 358 and XOMA 3AB, require significant additional research and development, extensive preclinical studies and clinical trials and regulatory approval prior to any commercial sales. This process is lengthy and expensive, often taking a number of years. As clinical results frequently are susceptible to varying interpretations that may delay, limit or prevent regulatory approvals, the length of time necessary to complete clinical trials and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly. As a result, it is uncertain whether:
| · | our future filings will be delayed; |
| · | our preclinical and clinical studies will be successful; |
| · | we will be successful in generating viable product candidates to targets; |
| · | we will be able to provide necessary additional data; |
| · | results of future clinical trials will justify further development; or |
| · | we ultimately will achieve regulatory approval for any of these product candidates. |
The timing of the commencement, continuation and completion of clinical trials may be subject to significant delays relating to various causes, including completion of preclinical testing and earlier-stage clinical trials in a timely manner, engaging contract research organizations and other service providers, scheduling conflicts with participating clinicians and clinical institutions, difficulties in identifying and enrolling patients who meet trial eligibility criteria and shortages of available drug supply. Patient enrollment is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the existence of competing clinical trials and the availability of alternative or new treatments. Regardless of the initial size or relative complexity of a clinical trial, the costs of such trial may be higher than expected due to increases in duration or size of the trial, changes in the protocol pursuant to which the trial is being conducted, additional or special requirements of one or more of the healthcare centers where the trial is being conducted, or changes in the regulatory requirements applicable to the trial or in the standards or guidelines for approval of the product candidate being tested or for other unforeseen reasons. In addition, we conduct clinical trials in foreign countries, which may subject us to further delays and expenses as a result of increased drug shipment costs, additional regulatory requirements and the engagement of foreign clinical research organizations, as well as expose us to risks associated with foreign currency transactions insofar as we might desire to use U.S. Dollars to make contract payments denominated in the foreign currency where the trial is being conducted.
All of our product candidates are prone to the risks of failure inherent in drug development. Preclinical studies may not yield results that satisfactorily support the filing of an Investigational New Drug application (“IND”) (or a foreign equivalent) with respect to our product candidates. Even if these applications would be or have been filed with respect to our product candidates, the results of preclinical studies do not necessarily predict the results of clinical trials. Similarly, early stage clinical trials in healthy volunteers do not predict the results of later-stage clinical trials, including the safety and efficacy profiles of any particular product candidates. In addition, there can be no assurance the design of our clinical trials is focused on appropriate indications, patient populations, dosing regimens or other variables that will result in obtaining the desired efficacy data to support regulatory approval to commercialize the drug. Moreover, FDA officials or foreign regulatory agency officials may question the integrity of our data or otherwise subject our clinical trials to additional scrutiny when the clinical trials are conducted by principal investigators who serve, or previously served, as scientific advisors or consultants to us and receive cash compensation in connection with such services. Preclinical and clinical data can also be interpreted in different ways. Accordingly, FDA officials or officials from foreign regulatory authorities could interpret the data differently than we or our collaboration or development partners do, which could delay, limit or prevent regulatory approval.
Administering any of our products or potential products may produce undesirable side effects, also known as adverse effects. Toxicities and adverse effects that we have observed in preclinical studies for some compounds in a particular research and development program may occur in preclinical studies or clinical trials of other compounds from the same program. Such toxicities or adverse effects could delay or prevent the filing of an IND (or a foreign equivalent) with respect to such products or potential products or cause us to cease clinical trials with respect to any drug candidate. In clinical trials, administering any of our products or product candidates to humans may produce adverse effects. These adverse effects could interrupt, delay or halt clinical trials of our products and product candidates and could result in the FDA or other regulatory authorities denying approval of our products or product candidates for any or all targeted indications. The FDA, other regulatory authorities, our collaboration or development partners or we may suspend or terminate clinical trials at any time. Even if one or more of our product candidates were approved for sale, the occurrence of even a limited number of toxicities or adverse effects when used in large populations may cause the FDA or other regulatory authorities to impose restrictions on, or stop, the further marketing of such drugs. Indications of potential adverse effects or toxicities that may occur in clinical trials and that we believe are not significant during the course of such clinical trials may actually turn out later to constitute serious adverse effects or toxicities when a drug has been used in large populations or for extended periods of time. Any failure or significant delay in completing preclinical studies or clinical trials for our product candidates, or in receiving and maintaining regulatory approval for the sale of any drugs resulting from our product candidates, may severely harm our reputation and business.
We rely on third parties to provide services in connection with our product candidate development and manufacturing programs. The inadequate performance by or loss of any of these service providers could affect our product candidate development.
Several third parties provide services in connection with our preclinical and clinical development programs, including in vitro and in vivo studies, assay and reagent development, immunohistochemistry, toxicology, pharmacokinetics, clinical trial support, manufacturing and other outsourced activities. If these service providers do not adequately perform the services for which we have contracted or cease to continue operations and we are not able to find a replacement provider quickly or we lose information or items associated with our product candidates, our development programs may be delayed.
We may not obtain orphan drug exclusivity, or we may not receive the full benefit of orphan drug exclusivity even if we obtain such exclusivity.
The FDA has awarded orphan drug status to gevokizumab for the treatment of non-infectious, intermediate, posterior or pan uveitis, chronic non-infectious anterior uveitis, pyoderma gangrenosum and Behçet’s uveitis. Under the Orphan Drug Act, the first company to receive FDA approval for another drug for the designated orphan drug indication will obtain seven years of marketing exclusivity, during which time the FDA may not approve another company’s application for the same orphan indication unless the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. Even though we have obtained orphan drug designation for certain indications for gevokizumab and even if we obtain orphan drug designation for our future product candidates or other indications, due to the uncertainties associated with developing pharmaceutical products, we may not be the first to obtain marketing approval for any particular orphan indication, or we may not obtain approval for an indication for which we have obtained orphan drug designation. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not protect the product effectively from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for another condition if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. Orphan drug designation neither shortens the development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process.
Even after FDA approval, a product may be subject to additional testing or significant marketing restrictions, its approval may be withdrawn or it may be removed voluntarily from the market.
Even if we receive regulatory approval for our product candidates, we will be subject to ongoing regulatory oversight and review by the FDA and other regulatory entities. The FDA, the European Medicines Agency (“EMA”) or another regulatory agency may impose, as a condition of the approval, ongoing requirements for post-approval studies or post-approval obligations, including additional research and development and clinical trials, and the FDA, EMA or other regulatory agency subsequently may withdraw approval based on these additional trials.
Even for approved products, the FDA, EMA or other regulatory agency may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product. In addition, the labeling, packaging, adverse event reporting, storage, advertising, promotion and record-keeping for our products are subject to extensive regulatory requirements.
Furthermore, a marketing approval of a product may be withdrawn by the FDA, the EMA or another regulatory agency or such a product may be withdrawn voluntarily by the company marketing it based, for example, on subsequently arising safety concerns. The FDA, EMA and other agencies also may impose various civil or criminal sanctions for failure to comply with regulatory requirements, including withdrawal of product approval.
We may issue additional equity securities and thereby materially and adversely affect the price of our common stock.
We are authorized to issue, without stockholder approval, 1,000,000 shares of preferred stock, of which none were issued and outstanding as of March 9, 2015, which may give other stockholders dividend, conversion, voting, and liquidation rights, among other rights, which may be superior to the rights of holders of our common stock. In addition, we are authorized to issue, generally without stockholder approval, up to 277,333,332 shares of common stock, of which 116,185,969 were issued and outstanding as of March 9, 2015. If we issue additional equity securities, the price of our common stock may be materially and adversely affected.
As part of our fundraising efforts, we offer securities through underwritten public offerings from time to time. In 2013, we completed two such offerings, one in August 2013 where we sold 8,736,187 shares of our common stock at a public offering price of $3.62 per share and the other in December 2013, where we sold 10,925,000 shares of our common stock at a public offering price of $5.25 per share. In 2014, we completed a registered direct offering where we sold 8,097,165 shares of our common stock at an offering price of $4.94 per share.
In addition, funding from collaboration partners and others has in the past and may in the future involve issuance by us of our common stock. We cannot be certain how the purchase price of such shares, the relevant market price or premium, if any, will be determined or when such determinations will be made.
Any issuance by us of equity securities, whether through an underwritten public offering, an at the market offering, a private placement, in connection with a collaboration or otherwise could result in dilution in the value of our issued and outstanding shares, and a decrease in the trading price of our common stock.
Our share price may be volatile and there may not be an active trading market for our common stock.
There can be no assurance the market price of our common stock will not decline below its present market price or there will be an active trading market for our common stock. The market prices of biotechnology companies have been and are likely to continue to be highly volatile. Fluctuations in our operating results and general market conditions for biotechnology stocks could have a significant impact on the volatility of our common stock price. We have experienced significant volatility in the price of our common stock. From January 1, 2014, through March 9, 2015, the share price of our common stock has ranged from a high of $9.57 to a low of $3.22. Factors contributing to such volatility include, but are not limited to:
| · | results of preclinical studies and clinical trials; |
| · | information relating to the safety or efficacy of products or product candidates; |
| · | developments regarding regulatory filings; |
| · | announcements of new collaborations; |
| · | failure to enter into collaborations; |
| · | developments in existing collaborations; |
| · | our funding requirements and the terms of our financing arrangements; |
| · | technological innovations or new indications for our therapeutic products and product candidates; |
| · | introduction of new products or technologies by us or our competitors; |
| · | sales and estimated or forecasted sales of products for which we receive royalties, if any; |
| · | government regulations; |
| · | developments in patent or other proprietary rights; |
| · | the number of shares issued and outstanding; |
| · | the number of shares trading on an average trading day; |
| · | announcements regarding other participants in the biotechnology and pharmaceutical industries; and |
| · | market speculation regarding any of the foregoing. |
As a public company in the United States, we are subject to the Sarbanes-Oxley Act. We have determined our disclosure controls and procedures and our internal control over financial reporting are effective. We can provide no assurance that we will, at all times, in the future be able to report that our internal controls over financial reporting are effective.
Companies that file reports with the Securities and Exchange Commission, or the SEC, including us, are subject to the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Section 404 requires management to establish and maintain a system of internal control over financial reporting, and annual reports on Form 10-K filed under the Securities Exchange Act of 1934, as amended, or the Exchange Act, must contain a report from management assessing the effectiveness of our internal control over financial reporting. Ensuring we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a time-consuming effort that needs to be re-evaluated frequently. Failure on our part to have effective internal financial and accounting controls would cause our financial reporting to be unreliable, could have a material adverse effect on our business, operating results, and financial condition, and could cause the trading price of our common stock to fall.
We disclosed in our Quarterly Reports on Form 10-Q/A and 10-Q for the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014, that we had identified a material weakness in our internal control over financial reporting such that our disclosure controls and procedures related to the calculation and disclosure of diluted earnings (loss) per share as it applies to our March 2012 warrants were not effective. During 2014, we implemented improvements in our internal controls over financial reporting to address the material weakness described above, including performing a more effective quarterly review of our diluted earnings (loss) per share calculation. Our remediation efforts, including the testing of these controls continued throughout 2014. This material weakness was considered remediated in the fourth quarter of 2014, once these controls were shown to be operational for a sufficient period of time to allow management to conclude that these controls were operating effectively.
We are subject to various state and federal healthcare related laws and regulations that may impact the commercialization of our product candidates or could subject us to significant fines and penalties.
Our operations may be directly or indirectly subject to various state and federal healthcare laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act and state and federal privacy and security laws. These laws may impact, among other things, the commercial operations for any of our product candidates that may be approved for commercial sale.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, penalties, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs.
The federal False Claims Act prohibits persons from knowingly filing, or causing to be filed, a false claim to, or the knowing use of false statements to obtain payment from the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers”, may share in any amounts paid by the entity to the government in fines or settlement. The filing of qui tam actions has caused a number of pharmaceutical, medical device and other healthcare companies to have to defend a False Claims Act action. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. Various states also have enacted laws modeled after the federal False Claims Act.
The Federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including private payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. HIPAA, as amended by the Health Information Technology and Clinical Health Act (“HITECH”), and its implementing regulations, also impose certain requirements relating to the privacy, security and transmission of individually identifiable health information. We take our obligation to maintain our compliance with these various laws and regulations seriously.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, (collectively, “PPACA”), among other things, imposed new requirements on manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare & Medicaid Services (“CMS”), information related to payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by physicians (as defined above) and their immediate family members and payments or other “transfers of value” to such physician owners and their immediate family members. Manufacturers were required to begin data collection on August 1, 2013, and were required to report such data to the government by March 31, 2014, and will be by the 90th calendar day of each year thereafter. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (or up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission.
Many states also have adopted laws similar to each of the federal laws described above, some of which apply to healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs. In addition, some states have laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources, and to report information related to payments and other transfers of value to physicians and other healthcare providers; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Because of the breadth of these laws, it is possible that some of our business activities could be subject to challenge under one or more of such laws. The PPACA also make several important changes to the federal Anti-Kickback Statute, false claims laws, and health care fraud statute by weakening the intent requirement under the anti-kickback and health care fraud statutes that may make it easier for the government, or whistleblowers to charge such fraud and abuse violations. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes.
If we are found to be in violation of any of the laws and regulations described above or other applicable state and federal healthcare laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare reimbursement programs and the curtailment or restructuring of our operations, any of which could have a material adverse effect on our business and results of operations.
Certain of our technologies are in-licensed from third parties, so our capabilities using them are restricted and subject to additional risks.
We license technologies from third parties. These technologies include but are not limited to phage display technologies licensed to us in connection with our bacterial cell expression technology licensing program and antibody products. However, our use of these technologies is limited by certain contractual provisions in the licenses relating to them, and although we have obtained numerous licenses, intellectual property rights in the area of phage display are particularly complex. If the owners of the patent rights underlying the technologies that we license do not properly maintain or enforce those patents, our competitive position and business prospects could be harmed. Our success will depend in part on the ability of our licensors to obtain, maintain and enforce our in-licensed intellectual property. Our licensors may not be successful in prosecuting the patent applications to which we have licenses, or our licensors may fail to maintain existing patents. They may determine not to pursue litigation against other companies that are infringing these patents, or they may pursue such litigation less aggressively than we would. Our licensors also may seek to terminate our license, which could cause us to lose the right to use the licensed intellectual property and adversely affect our ability to commercialize our technologies, products or services.
We do not know whether there will be, or will continue to be, a viable market for the products in which we have an ownership or royalty interest.
Even if products in which we have an interest receive approval in the future, they may not be accepted in the marketplace. In addition, we or our collaborators or licensees may experience difficulties in launching new products, many of which are novel and based on technologies that are unfamiliar to the healthcare community. We have no assurance healthcare providers and patients will accept such products, if developed. For example, physicians and/or patients may not accept a product for a particular indication because it has been biologically derived (and not discovered and developed by more traditional means) or if no biologically derived products are currently in widespread use in that indication. Similarly, physicians may not accept a product if they believe other products to be more effective or more cost effective or are more comfortable prescribing other products.
Safety concerns also may arise in the course of on-going clinical trials or patient treatment as a result of adverse events or reactions. For example, in February 2009, the EMA announced it had recommended suspension of the marketing authorization of RAPTIVA in the EU, and EMD Serono Inc., the company that marketed RAPTIVA in Canada (“EMD Serono”) announced that in consultation with Health Canada, the Canadian health authority (“Health Canada”), it would suspend marketing of RAPTIVA in Canada. In March 2009, Merck Serono Australia Pty Ltd, the company that marketed RAPTIVA in Australia (“Merck Serono Australia”), following a recommendation from the Therapeutic Goods Administration, the Australian health authority (“TGA”), announced it was withdrawing RAPTIVA from the Australian market. In the second quarter of 2009, Genentech announced and carried out a phased voluntary withdrawal of RAPTIVA from the U.S. market, based on the association of RAPTIVA with an increased risk of PML, and sales of the product ceased.
Furthermore, government agencies, as well as private organizations involved in healthcare, from time to time publish guidelines or recommendations to healthcare providers and patients. Such guidelines or recommendations can be very influential and may adversely affect product usage directly (for example, by recommending a decreased dosage of a product in conjunction with a concomitant therapy or a government entity withdrawing its recommendation to screen blood donations for certain viruses) or indirectly (for example, by recommending a competitive product over our product). Consequently, we do not know if physicians or patients will adopt or use our products for their approved indications.
Even approved and marketed products are subject to risks relating to changes in the market for such products. Introduction or increased availability of generic versions of products can alter the market acceptance of branded products. In addition, unforeseen safety issues may arise at any time, regardless of the length of time a product has been on the market.
In addition to our agreements with Servier, our agreements with other third parties, many of which are significant to our business, expose us to numerous risks.
Our financial resources and our marketing experience and expertise are limited. Consequently, our ability to develop products successfully depends, to a large extent, upon securing the financial resources and/or marketing capabilities of third parties other than Servier. For example:
| · | In March 2004, we announced we had agreed to collaborate with Chiron Corporation (now Novartis) for the development and commercialization of antibody products for the treatment of cancer. In April 2005, we announced the initiation of clinical testing of the first product candidate out of the collaboration, HCD122, an anti-CD40 antibody, in patients with advanced chronic lymphocytic leukemia. In October 2005, we announced the initiation of the second clinical trial of HCD122 in patients with multiple myeloma. In November 2008, we announced the restructuring of this product development collaboration, which involved six development programs including CD40 and prolactin receptor antibody programs. In exchange for cash and debt reduction on our existing loan facility with Novartis, Novartis received control over the CD40 and prolactin receptor antibody programs, as well as the right to expand the development of these programs into additional indications outside of oncology. Novartis has initiated clinical studies to test CFZ533, an anti-CD40 antibody arising from its collaboration with XOMA, in de novo renal transplantation and in Primary Sjögren's Syndrome. Novartis has returned control of the prolactin receptor antibody program to us, and we are evaluating options for its continued development. |
| · | In March 2005, we entered into a contract with the National Institute of Allergy and Infectious Diseases (“NIAID”) to produce three monoclonal antibodies designed to protect U.S. citizens against the harmful effects of botulinum neurotoxin used in bioterrorism. In July 2006, we entered into an additional contract with NIAID for the development of an appropriate formulation for human administration of these three antibodies in a single injection. In September 2008, we announced we had been awarded an additional contract with NIAID to support our on-going development of drug candidates toward clinical trials in the treatment of botulism poisoning. In October 2011, we announced we had been awarded an additional contract with NIAID to develop broad-spectrum antitoxins for the treatment of human botulism poisoning. |
| · | We have licensed our bacterial cell expression technology, a set of enabling technologies used to discover and screen, as well as develop and manufacture, recombinant antibodies and other proteins for commercial purposes, to over 60 companies. As of March 9, 2015, we were aware of three products manufactured using this technology that have received FDA approval: Genentech’s LUCENTIS® (ranibizumab injection) for treatment of neovascular wet age-related macular degeneration, Macular Edema Following Vein Occulsion, Diabetic Macular Edema, and Diabetic Retinopathy in patients with Diabetic Macular Edema; UCB’s CIMZIA® (certolizumab pegol) for treatment of Crohn’s disease and rheumatoid arthritis; and Pfizer’s TRUMENBA®, a meningococcal group B vaccine. In the third quarter of 2009, we sold our LUCENTIS royalty interest to Genentech. In the third quarter of 2010, we sold our CIMZIA royalty interest. We anticipate receiving a fraction of a percentage royalty on sales of TRUMENBA. |
| · | In August 2012, Servier and we announced an agreement with Boehringer Ingelheim to transfer XOMA's technology and processes for the validation of our technology and processes in preparation for the commercial manufacture of gevokizumab. Boehringer Ingelheim has completed GMP runs with successful biological comparability, including all process validation batches of the XOMA processes. Boehringer Ingelheim is making preparations for the production of gevokizumab commercial batches at its facility in Biberach, Germany. |
Because our collaborators, licensees, suppliers and contractors are independent third parties, they may be subject to different risks than we are and have significant discretion in, and different criteria for, determining the efforts and resources they will apply related to their agreements with us. If these collaborators, licensees, suppliers and contractors do not successfully perform the functions for which they are responsible, we may not have the capabilities, resources or rights to do so on our own.
We do not know whether we, our collaborators or licensees will successfully develop and market any of the products that are or may become the subject of any of our collaboration or licensing arrangements. In some cases these arrangements provide for funding solely by our collaborators or licensees, and in other cases, all of the funding for certain projects and a significant portion of the funding for other projects is to be provided by our collaborator or licensee, and we provide the balance of the funding. Even when we have a collaborative relationship, other circumstances may prevent it from resulting in successful development of marketable products. In addition, third-party arrangements such as ours also increase uncertainties in the related decision-making processes and resulting progress under the arrangements, as we and our collaborators or licensees may reach different conclusions, or support different paths forward, based on the same information, particularly when large amounts of technical data are involved. Furthermore, our contracts with NIAID contain numerous standard terms and conditions provided for in the applicable Federal acquisition regulations and customary in many government contracts, some of which could allow the U.S. government to exercise certain rights under the technology developed under these contracts. Uncertainty exists as to whether we will be able to comply with these terms and conditions in a timely manner, if at all. In addition, we are uncertain as to the extent of NIAID’s demands and the flexibility that will be granted to us in meeting those demands. Under our contract with NIAID, we invoice using NIH provisional rates, and these are subject to future audits at the discretion of NIAID’s contracting office. These audits can result in an adjustment to revenue previously reported, which potentially could be significant.
Although we continue to evaluate additional strategic alliances and potential partnerships, we do not know whether or when any such alliances or partnerships will be entered into.
Products and technologies of other companies may render some or all of our products and product candidates noncompetitive or obsolete.
Developments by others may render our products, product candidates, or technologies obsolete or uncompetitive. Technologies developed and utilized by the biotechnology and pharmaceutical industries are changing continuously and substantially. Competition in antibody-based technologies is intense and is expected to increase in the future as a number of established biotechnology firms and large chemical and pharmaceutical companies advance in these fields. Many of these competitors may be able to develop products and processes competitive with or superior to our own for many reasons, including that they may have:
| · | significantly greater financial resources; |
| · | larger research and development and marketing staffs; |
| · | larger production facilities; |
| · | entered into arrangements with, or acquired, biotechnology companies to enhance their capabilities; or |
| · | extensive experience in preclinical testing and human clinical trials. |
These factors may enable others to develop products and processes competitive with or superior to our own or those of our collaborators. In addition, a significant amount of research in biotechnology is being carried out in universities and other non-profit research organizations. These entities are becoming increasingly interested in the commercial value of their work and may become more aggressive in seeking patent protection and licensing arrangements. Furthermore, many companies and universities tend not to announce or disclose important discoveries or development programs until their patent position is secure or, for other reasons, later; as a result, we may not be able to track development of competitive products, particularly at the early stages. Positive or negative developments in connection with a potentially competing product may have an adverse impact on our ability to raise additional funding on acceptable terms. For example, if another product is perceived to have a competitive advantage, or another product’s failure is perceived to increase the likelihood that our product will fail, then investors may choose not to invest in us on terms we would accept or at all.
The examples below pertain to competitive events in the market that we review quarterly yet are not intended to be representative of all existing competitive events.
Gevokizumab
We, in collaboration with Servier, are developing gevokizumab, a potent monoclonal antibody with unique allosteric modulating properties that binds strongly to interleukin-1 beta (IL-1 beta), a pro-inflammatory cytokine. In binding to IL-1 beta, gevokizumab inhibits the activation of the IL-1 receptor, thereby modulating the cellular signaling events that produce inflammation. Certain other companies are developing products based on the same or similar therapeutic targets as gevokizumab. The efficacy and safety profile of gevokizumab relative to these potential competitors is unknown.
| · | Novartis markets and is developing ILARIS® (canakinumab, ACZ885), a fully human monoclonal antibody that selectively binds to and neutralizes IL-1 beta. Since 2009, canakinumab has been approved in over 50 countries for the treatment of children and adults suffering from Cryopyrin-Associated Periodic Syndrome (“CAPS”). The product is indicated in the U.S. for the treatment of CAPS in patients over four years of age, including familial cold auto-inflammatory syndrome (“FCAS”) and Muckle-Wells syndrome (“MWS”), as well as for active systemic juvenile idiopathic arthritis (“SJIA”) in patients aged two years and older. In the EU, canakinumab is indicated for the treatment of FCAS, MWS, neonatal-onset multisystem inflammatory disease (“NOMID”)/ chronic infantile neurological cutaneous articular syndrome (“CINCA syndrome”), severe forms of FCAS/familial cold urticarial (“FCU”) presenting with signs and symptoms beyond cold-induced urticaria skin rash, for the symptomatic treatment of adults with frequent gouty arthritis attacks, and for SJIA in patients aged two years and above who have responded inadequately to previous therapy with non-steroidal anti-inflammatory drugs and systemic corticosteroids. In Japan, canakinumab is indicated for the treatment of CAPS and associated autoinflammatory symptoms, including FCAS, MWS and NOMID. Novartis also is pursuing other diseases in which IL-1 beta may play a prominent role, such as: systemic secondary prevention of cardiovascular events; hereditary periodic fever (familial Mediterranean fever (“FMF”)); chronic obstructive pulmonary disorder (“COPD”); osteoarthritis; urticarial vasculitis; tumor necrosis factor receptor-associated periodic syndrome (“TRAPS”); xerophthalmia; Schnitzler syndrome; polymyalgia rheumatica; hyperimmunoglobulinemia D (hyper-IgD) and periodic fever syndrome (“HIDS”); and abdominal aortic aneurysm (“AAA”). |
| · | Regeneron markets and is developing ARCALYSTt® (rilonacept), an interleukin-1 blocker currently indicated in the U.S. for the treatment of CAPS, including FCAS and MWS in adults and children 12 and older. Rilonacept is also approved, but not marketed, in the EU for the same patient population. |
| · | In 2008, Swedish Orphan Biovitrum obtained from Amgen the global exclusive rights to Kineret® (anakinra) for rheumatoid arthritis as currently indicated in its label. In November 2009, the agreement regarding Swedish Orphan Biovitrum’s Kineret license was expanded to include certain orphan indications. Kineret is an IL-1 receptor antagonist (IL-1ra) that has been evaluated in multiple IL- 1-mediated diseases, including indications we are considering for gevokizumab. In addition to other on-going studies, a proof-of concept clinical trial investigating Kineret in patients with a certain type of myocardial infarction, or heart attack, has been completed in the United Kingdom. In January 2013, Biovitrum obtained FDA approval for NOMID, a severe form of CAPS. In November 2013, Kineret was approved by the European Commission for the treatment of CAPS. Shanghai CP Guojian Pharmaceutical is developing an injectable formulation of recombinant human IL-1Ra, presumed to be a follow-on biologic version of anakinra, for the potential treatment of rheumatoid arthritis. In February 2010, an NDA was filed with the China Food and Drug Administration (“SFDA”); in January 2012, supplemental materials were required by the SFDA to conclude the review. |
| · | The following companies have completed or are conducting or planning Phase 3 clinical trials of the following products for the treatment of noninfectious intermediate, posterior or pan-uveitis: AbbVie - HUMIRA® (adalimumab); Novartis - Myfortic® (mycophenalate sodium); Santen Pharmaceutical Co., Ltd. - Opsiria® (intravitreal sirolimus); pSivida Corp. - Fluocinolone Acetonide Intravitreal; and Allergan - Ozurdex® (dexamethasone). |
In May 2014, AbbVie announced the FDA had granted HUMIRA® (adalimumab) orphan drug designation for the treatment of noninfectious intermediate, posterior, or pan-uveitis, or chronic non-infectious anterior uveitis.
In April 2014, Santen announced SAKURA 1, the first of two Global Phase 3 studies in patients with non-infectious posterior segment uveitis, met its primary endpoint.
XOMA 3AB
We also are developing XOMA 3AB, a combination, or cocktail, of antibodies designed to neutralize the most potent of botulinum toxins. Other companies are developing other products targeting botulism poisoning, and these products may prove more effective than XOMA 3AB. We are aware:
| · | Emergent Biosolutions Inc. has a contract with the U.S. Department of Health & Human Services, expected to be worth $423.0 million, to manufacture and supply an equine heptavalent botulism anti-toxin. In March 2013, the product was approved by the FDA. |
Manufacturing risks and inefficiencies may adversely affect our ability to manufacture products for ourselves or others.
To the extent we continue to provide manufacturing services for our own benefit or to third parties, we are subject to manufacturing risks. Additionally, unanticipated fluctuations in customer requirements may lead to manufacturing inefficiencies, which if significant could lead to an impairment on our long-lived assets or restructuring activities. We must utilize our manufacturing operations in compliance with regulatory requirements, in sufficient quantities and on a timely basis, while maintaining acceptable product quality and manufacturing costs. Additional resources and changes in our manufacturing processes may be required for each new product, product modification or customer or to meet changing regulatory or third-party requirements, and this work may not be completed successfully or efficiently.
Manufacturing and quality problems may arise in the future to the extent we continue to perform these manufacturing activities for our own benefit or for third parties. Consequently, our development goals or milestones may not be achieved in a timely manner or at a commercially reasonable cost, or at all. In addition, to the extent we continue to make investments to improve our manufacturing operations, our efforts may not yield the improvements that we expect.
Failure of our products to meet current Good Manufacturing Practices standards may subject us to delays in regulatory approval and penalties for noncompliance.
Our contract manufacturers are required to produce our clinical product candidates under current Good Manufacturing Practices (“cGMP”) to meet acceptable standards for use in our clinical trials and for commercial sale, as applicable. If such standards change, the ability of contract manufacturers to produce our product candidates on the schedule we require for our clinical trials or to meet commercial requirements may be affected. In addition, contract manufacturers may not perform their obligations under their agreements with us or may discontinue their business before the time required by us to successfully produce clinical and commercial supplies of our product candidates.
We and our contract manufacturers are subject to pre-approval inspections and periodic unannounced inspections by the FDA and corresponding state and foreign authorities to ensure strict compliance with cGMP and other applicable government regulations and corresponding foreign standards. We do not have control over a third-party manufacturer’s compliance with these regulations and standards. Any difficulties or delays in our contractors’ manufacturing and supply of our product candidates or any failure of our contractors to maintain compliance with the applicable regulations and standards could increase our costs, cause us to lose revenue, make us postpone or cancel clinical trials, prevent or delay regulatory approval by the FDA and corresponding state and foreign authorities, prevent the import and/or export of our product candidates, or cause any of our product candidates that may be approved for commercial sale to be recalled or withdrawn.
Because many of the companies with which we do business also are in the biotechnology sector, the volatility of that sector can affect us indirectly as well as directly.
As a biotechnology company that collaborates with other biotechnology companies, the same factors that affect us directly also can adversely impact us indirectly by affecting the ability of our collaborators, partners and others with whom we do business to meet their obligations to us and reduce our ability to realize the value of the consideration provided to us by these other companies.
For example, in connection with our licensing transactions, we have in the past and may in the future agree to accept equity securities of the licensee in payment of license fees. The future value of these or any other shares we receive is subject both to market risks affecting our ability to realize the value of these shares and more generally to the business and other risks to which the issuer of these shares may be subject.
As we do more business internationally, we will be subject to additional political, economic and regulatory uncertainties.
We may not be able to operate successfully in any foreign market. We believe that because the pharmaceutical industry is global in nature, international activities will be a significant part of our future business activities and when and if we are able to generate income, a substantial portion of that income will be derived from product sales and other activities outside the United States. Foreign regulatory agencies often establish standards different from those in the United States, and an inability to obtain foreign regulatory approvals on a timely basis could put us at a competitive disadvantage or make it uneconomical to proceed with a product or product candidate’s development. International sales may be limited or disrupted by:
| · | imposition of government controls; |
| · | export license requirements; |
| · | political or economic instability; |
| · | trade restrictions; |
| · | changes in tariffs; |
| · | restrictions on repatriating profits; |
| · | exchange rate fluctuations; and |
| · | withholding and other taxation. |
We are subject to foreign currency exchange rate risks.
We are subject to foreign currency exchange rate risks because substantially all of our revenues and operating expenses are paid in U.S. Dollars, but we incur certain expenses, as well as interest and principal obligations with respect to our loan from Servier in Euros. To the extent the U.S. Dollar declines in value against the Euro, the effective cost of servicing our Euro-denominated debt will be higher. Changes in the exchange rate result in foreign currency gains or losses. Although we have managed some of our exposure to changes in foreign currency exchange rates by entering into foreign exchange option contracts, there can be no assurance foreign currency fluctuations will not have a material adverse effect on our business, financial condition, liquidity or results of operations. In addition, our foreign exchange option contracts are re-valued at each financial reporting period, which also may result in gains or losses from time to time.
If we and our partners are unable to protect our intellectual property, in particular our patent protection for our principal products, product candidates and processes, and prevent its use of the covered subject matter by third parties, our ability to compete in the market will be harmed, and we may not realize our profit potential.
We rely on patent protection, as well as a combination of copyright, trade secret, and trademark laws to protect our proprietary technology and prevent others from duplicating our products or product candidates. However, these means may afford only limited protection and may not:
| · | prevent our competitors from duplicating our products; |
| · | prevent our competitors from gaining access to our proprietary information and technology; or |
| · | permit us to gain or maintain a competitive advantage. |
Because of the length of time and the expense associated with bringing new products to the marketplace, we and our collaboration and development partners hold and are in the process of applying for a number of patents in the United States and abroad to protect our product candidates and important processes and also have obtained or have the right to obtain exclusive licenses to certain patents and applications filed by others. However, the mere issuance of a patent is not conclusive as to its validity or its enforceability. The U.S. Federal Courts, the U.S. Patent & Trademark Office or equivalent national courts or patent offices elsewhere may invalidate our patents or find them unenforceable. In addition, the laws of foreign countries may not protect our intellectual property rights effectively or to the same extent as the laws of the United States. If our intellectual property rights are not protected adequately, we may not be able to commercialize our technologies, products, or services, and our competitors could commercialize our technologies, which could result in a decrease in our sales and market share that would harm our business and operating results. Specifically, the patent position of biotechnology companies generally is highly uncertain and involves complex legal and factual questions. The legal standards governing the validity of biotechnology patents are in transition, and current defenses as to issued biotechnology patents may not be adequate in the future. Accordingly, there is uncertainty as to:
| · | whether any pending or future patent applications held by us will result in an issued patent, or that if patents are issued to us, that such patents will provide meaningful protection against competitors or competitive technologies; |
| · | whether competitors will be able to design around our patents or develop and obtain patent protection for technologies, designs or methods that are more effective than those covered by our patents and patent applications; or |
| · | the extent to which our product candidates could infringe on the intellectual property rights of others, which may lead to costly litigation, result in the payment of substantial damages or royalties, and/or prevent us from using technology that is essential to our business. |
We have established a portfolio of patents, both United States and foreign, related to our bacterial cell expression technology, including claims to novel promoter sequences, secretion signal sequences, compositions and methods for expression and secretion of recombinant proteins from bacteria, including immunoglobulin gene products. Most of the more important licensed European patents in our bacterial cell expression patent portfolio expired in July 2008 or earlier. The last of the more important licensed United States patents in our bacterial cell expression (“BCE”) patent portfolio expired in December 2014. The last-to-expire patent licensed under the majority of our BCE license agreements is Canadian patent 1,341,235, which is expected to expire in May 2018.
If certain patents issued to others are upheld or if certain patent applications filed by others issue and are upheld, we may require licenses from others to develop and commercialize certain potential products incorporating our technology or we may become involved in litigation to determine the proprietary rights of others. These licenses, if required, may not be available on acceptable terms, and any such litigation may be costly and may have other adverse effects on our business, such as inhibiting our ability to compete in the marketplace and absorbing significant management time.
Due to the uncertainties regarding biotechnology patents, we also have relied and will continue to rely upon trade secrets, know-how and continuing technological advancement to develop and maintain our competitive position. All of our employees have signed confidentiality agreements under which they have agreed not to use or disclose any of our proprietary information. Research and development contracts and relationships between us and our scientific consultants and potential customers provide access to aspects of our know-how that are protected generally under confidentiality agreements. These confidentiality agreements may be breached or may not be enforced by a court. To the extent proprietary information is divulged to competitors or to the public generally, such disclosure may affect our ability to develop or commercialize our products adversely by giving others a competitive advantage or by undermining our patent position.
Litigation regarding intellectual property can be costly and expose us to risks of counterclaims against us.
We may be required to engage in litigation or other proceedings to protect our intellectual property. The cost to us of this litigation, even if resolved in our favor, could be substantial. Such litigation also could divert management’s attention and resources. In addition, if this litigation is resolved against us, our patents may be declared invalid, and we could be held liable for significant damages. In addition, we may be subject to a claim that we are infringing another party’s patent. If such claim is resolved against us, we or our collaborators may be enjoined from developing, manufacturing, selling or importing products, processes or services unless we obtain a license from the other party.
Such license may not be available on reasonable terms, thus preventing us from using these products, processes or services and adversely affecting our revenue.
We may be unable to price our products effectively or obtain adequate reimbursement for sales of our products, which would prevent our products from becoming profitable.
If we or our third-party collaborators or licensees succeed in bringing our product candidates to the market, they may not be considered cost effective, and reimbursement to the patient may not be available or may not be sufficient to allow us to sell our products on a competitive basis. In both the United States and elsewhere, sales of medical products and treatments are dependent, in part, on the availability of reimbursement to the patient from third-party payors, such as government and private insurance plans. Third-party payors are increasingly challenging the prices charged for pharmaceutical products and services. Our business is affected by the efforts of government and third-party payors to contain or reduce the cost of healthcare through various means. In the United States, there have been and will continue to be a number of federal and state proposals to implement government controls on pricing.
In addition, the emphasis on managed care in the United States has increased and will continue to increase the pressure on the pricing of pharmaceutical products. We cannot predict whether any legislative or regulatory proposals will be adopted or the effect these proposals or managed care efforts may have on our business.
Healthcare reform measures and other statutory or regulatory changes could adversely affect our business.
In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could impact our business. In March 2010, the U.S. Congress enacted and President Obama signed into law the PPACA, which includes a number of healthcare reform provisions that are expected to significantly impact the pharmaceutical industry. The PPACA, among other things, imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs”; increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%; requires collection of rebates for drugs paid by Medicaid managed care organizations; addresses new methodologies by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, and for drugs that are line extension products; and requires manufacturers to participate in a coverage gap discount program, under which they must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D. While the law may increase the number of patients who have insurance coverage for our products or product candidates, its cost containment measures also could adversely affect coverage and reimbursement for our existing or potential products; however, the full effects of this law cannot be known until these provisions are implemented and the relevant Federal and state agencies issue applicable regulations or guidance.
Other legislative changes have been proposed and adopted since the PPACA was enacted. On August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve its targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reductions to several government programs. These reductions include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect on April 1, 2013, and are scheduled to remain in effect until 2024. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012 (“ATRA”), which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers. We expect additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products once approved or additional pricing pressures, a decrease in the share price of our common stock, limit our ability to raise capital or to obtain strategic collaborations or licenses or successfully commercialize our products.
The pharmaceutical and biotechnology industries are subject to extensive regulation, and from time to time, legislative bodies and governmental agencies consider changes to such regulations that could have significant impact on industry participants. For example, in light of certain highly publicized safety issues regarding certain drugs that had received marketing approval, the U.S. Congress has considered various proposals regarding drug safety, including some that would require additional safety studies and monitoring and could make drug development more costly. We are unable to predict what additional legislation or regulation, if any, relating to safety or other aspects of drug development may be enacted in the future or what effect such legislation or regulation would have on our business.
We are exposed to an increased risk of product liability claims.
The testing, marketing and sales of medical products entails an inherent risk of allegations of product liability. In the past, we were party to product liability claims filed against Genentech Inc. and, even though Genentech agreed to indemnify us in connection with these matters and these matters have been settled, there can be no assurance other products liability lawsuits will not result in liability to us or that our insurance or contractual arrangements will provide us with adequate protection against such liabilities. In the event of one or more large, unforeseen awards of damages against us, our product liability insurance may not provide adequate coverage. A significant product liability claim for which we were not covered by insurance or indemnified by a third party would have to be paid from cash or other assets, which could have an adverse effect on our business and the value of our common stock. To the extent we have sufficient insurance coverage, such a claim would result in higher subsequent insurance rates. In addition, product liability claims can have various other ramifications, including loss of future sales opportunities, increased costs associated with replacing products, a negative impact on our goodwill and reputation, and divert our management’s attention from our business, each of which could also adversely affect our business and operating results.
The loss of key personnel, including our Chief Executive Officer, could delay or prevent achieving our objectives.
Our research, product development and business efforts could be affected adversely by the loss of one or more key members of our scientific or management staff, particularly our executive officers: John Varian, our Chief Executive Officer; Patrick J. Scannon, M.D., Ph.D., our Executive Vice President and Chief Scientific Officer; Paul D. Rubin, M.D., our Senior Vice President, Research and Development and Chief Medical Officer; and Tom Klein, our Vice President and Chief Commercial Officer. We currently do not have key person insurance on any of our employees.
Our ability to use our net operating loss carry-forwards and other tax attributes will be substantially limited by Section 382 of the U.S. Internal Revenue Code.
Section 382 of the U.S. Internal Revenue Code of 1986, as amended, generally limits the ability of a corporation that undergoes an “ownership change” to utilize its net operating loss carry-forwards (“NOLs”) and certain other tax attributes against any taxable income in taxable periods after the ownership change. The amount of taxable income in each taxable year after the ownership change that may be offset by pre-change NOLs and certain other pre-change tax attributes is generally equal to the product of (a) the fair market value of the corporation’s outstanding shares (or, in the case of a foreign corporation, the fair market value of items treated as connected with the conduct of a trade or business in the United States) immediately prior to the ownership change and (b) the long-term tax exempt rate (i.e., a rate of interest established by the U.S. Internal Revenue Service (“IRS”) that fluctuates from month to month). In general, an “ownership change” occurs whenever the percentage of the shares of a corporation owned, directly or indirectly, by “5-percent shareholders” (within the meaning of Section 382 of the Internal Revenue Code) increases by more than 50 percentage points over the lowest percentage of the shares of such corporation owned, directly or indirectly, by such “5-percent shareholders” at any time over the preceding three years.
Based on an analysis under Section 382 of the Internal Revenue Code (which subjects the amount of pre-change NOLs and certain other pre-change tax attributes that can be utilized to an annual limitation), we experienced ownership changes in 2009 and 2012, which substantially limit the future use of our pre-change NOLs and certain other pre-change tax attributes per year. As of December 31, 2014, we have excluded the NOLs and R&D credits that will expire as a result of the annual limitations. To the extent that we do not utilize its carry-forwards within the applicable statutory carry-forward periods, either because of Section 382 limitations or the lack of sufficient taxable income, the carry-forwards will also expire unused.
Because we are a relatively small biopharmaceutical company with limited resources, we may not be able to attract and retain qualified personnel.
Our success in developing marketable products and achieving a competitive position will depend, in part, on our ability to attract and retain qualified scientific and management personnel, particularly in areas requiring specific technical, scientific or medical expertise. We had approximately 183 employees as of March 9, 2015. We may require additional experienced executive, accounting, research and development, legal, administrative and other personnel from time to time in the future. There is intense competition for the services of these personnel, especially in California. Moreover, we expect that the high cost of living in the San Francisco Bay Area, where our headquarters and manufacturing facilities are located, may impair our ability to attract and retain employees in the future. If we do not succeed in attracting new personnel and retaining and motivating existing personnel, our operations may suffer and we may be unable to implement our current initiatives or grow effectively.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our current and any future collaborators, licensees, suppliers, contractors and consultants are vulnerable to damage from cyber−attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. We could experience failures in our information systems and computer servers, which could be the result of a cyber−attack and could result in an interruption of our normal business operations and require substantial expenditure of financial and administrative resources to remedy. System failures, accidents or security breaches can cause interruptions in our operations and can result in a material disruption of our development programs, commercialization activities and other business operations. The loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Similarly, we rely on third parties to supply components for and manufacture our product and product candidates, conduct clinical trials of our product candidates, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of gevokizumab or any of our other product candidates could be delayed or otherwise adversely affected.
Data breaches and cyber-attacks could compromise our intellectual property or other sensitive information and cause significant damage to our business and reputation.
In the ordinary course of our business, we maintain sensitive data on our networks, including our intellectual property and proprietary or confidential business information relating to our business and that of our customers and business partners. The secure maintenance of this information is critical to our business and reputation. We believe companies have been increasingly subject to a wide variety of security incidents, cyber-attacks and other attempts to gain unauthorized access. These threats can come from a variety of sources, all ranging in sophistication from an individual hacker to a state-sponsored attack. Cyber threats may be generic, or they may be custom-crafted against our information systems. Over the past year, cyber-attacks have become more prevalent and much harder to detect and defend against. Our network and storage applications may be subject to unauthorized access by hackers or breached due to operator error, malfeasance or other system disruptions. It is often difficult to anticipate or immediately detect such incidents and the damage caused by such incidents. These data breaches and any unauthorized access or disclosure of our information or intellectual property could compromise our intellectual property and expose sensitive business information. A data security breach could also lead to public exposure of personal information of our clinical trial patients, customers and others. Cyber-attacks could cause us to incur significant remediation costs, result in product development delays, disrupt key business operations and divert attention of management and key information technology resources. These incidents could also subject us to liability, expose us to significant expense and cause significant harm to our reputation and business.
Calamities, power shortages or power interruptions at our Berkeley headquarters and manufacturing facility could disrupt our business and adversely affect our operations.
Our principal operations are located in Northern California, including our corporate headquarters and manufacturing facility in Berkeley, California. This location is in an area of seismic activity near active earthquake faults. Any earthquake, terrorist attack, fire, power shortage or other calamity affecting our facilities may disrupt our business and could have material adverse effect on our business and results of operations.
We have a significant stockholder, which may limit other stockholders’ ability to influence corporate matters and may give rise to conflicts of interest.
Entities controlled by Felix J. Baker and Julian C. Baker beneficially own approximately 18.0% of our outstanding common stock as of March 9, 2015, which includes warrants to purchase approximately 7.6 million shares of XOMA’s common stock at an exercise price of $1.76 per share. On July 19, 2012, our Board of Directors elected Kelvin Neu, M.D., to serve on our Board of Directors. Dr. Neu is a Managing Director at Baker Bros. Advisors, LLC, an entity controlled by Felix J. Baker and Julian C. Baker. Accordingly, these entities may exert significant influence over us and any action requiring the approval of the holders of our stock, including the election of directors and approval of significant corporate transactions. In addition, on June 12, 2014, we entered into a registration rights agreement with entities affiliated with Felix J. Baker and Julian C. Baker, pursuant to which we subsequently filed a registration statement to register for resale the shares of our common stock (including shares issuable upon the exercise of warrants) held by these entities. Furthermore, conflicts of interest could arise in the future between us, on the one hand, and these entities, on the other hand, concerning potential competitive business activities, business opportunities, the issuance of additional securities and other matters.
Our organizational documents contain provisions that may prevent transactions that could be beneficial to our stockholders and may insulate our management from removal.
Our charter and by-laws:
| · | require certain procedures to be followed and time periods to be met for any stockholder to propose matters to be considered at annual meetings of stockholders, including nominating directors for election at those meetings; and |
| · | authorize our Board of Directors to issue up to 1,000,000 shares of preferred stock without stockholder approval and to set the rights, preferences and other designations, including voting rights, of those shares as the Board of Directors may determine. |
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”), that may prohibit large stockholders, in particular those owning 15% or more of our outstanding common stock, from merging or combining with us.
These provisions of our organizational documents and the DGCL, alone or in combination with each other, may discourage transactions involving actual or potential changes of control, including transactions that otherwise could involve payment of a premium over prevailing market prices to holders of common stock, could limit the ability of stockholders to approve transactions that they may deem to be in their best interests, and could make it considerably more difficult for a potential acquirer to replace management.
None.
Our corporate headquarters and development and manufacturing facilities are located in Berkeley and Emeryville, California. We currently lease three buildings and space in a fourth building, for which we had a sublease tenant under contract through May 2014. These buildings house our research and development laboratories, manufacturing facilities and office space. A separate pilot scale manufacturing facility is owned by us. Our building leases expire in the period from 2021 to 2023, and total minimum lease payments due from January 2015 until expiration of the leases are $28.9 million. We have the option to renew our lease agreements for periods ranging from three to ten years.
None.
Not applicable.
Our executive officers and their respective ages, as of December 31, 2014, and positions are as follows:
|
Name
|
Age
|
Title
|
||
|
John Varian
|
55
|
Chief Executive Officer
|
||
|
Patrick J. Scannon, M.D., Ph.D.
|
67
|
Executive Vice President and Chief Scientific Officer
|
||
|
Paul D. Rubin, M.D.
|
61
|
Senior Vice President, Research and Development and Chief Medical Officer
|
||
|
Fred Kurland
|
64
|
Vice President, Finance, Chief Financial Officer, and Secretary
|
||
|
Tom Klein
|
53
|
Vice President, Chief Commercial Officer
|
The Board of Directors elects all officers annually. There is no family relationship between or among any of the officers or directors.
Business Experience
Mr. Varian was appointed Chief Executive Officer of XOMA in January 2012 after serving as Interim Chief Executive Officer since August 31, 2011. He has served as a XOMA director since December 2008. He was Chief Operating Officer of Aryx Therapeutics from December 2003 through August 2011 and was its Chief Financial Officer from April 2006 through March 2011. Previously, Mr. Varian was Chief Financial Officer of Genset S.A., until the company's sale to Serono S.A. in 2002. From October 1998 to April 2000, Mr. Varian served as Senior Vice President, Finance and Administration of Elan Pharmaceuticals, Inc., joining the company as part of its acquisition of Neurex Corporation. Prior to the acquisition, he served as Neurex Corporation's Chief Financial Officer from June 1997 until October 1998. From 1991 until 1997, Mr. Varian served as the Vice President Finance and Chief Financial Officer of Anergen Inc. Mr. Varian was an Audit Principal / Senior Manager at Ernst & Young from 1987 until 1991 where he focused on life sciences. He is a founding member of the Bay Area Bioscience Center and a former chairman of the Association of Bioscience Financial Officers International Conference. Mr. Varian received a B.B.A. degree from Western Michigan University. Currently, Mr. Varian serves as a member of the Board of Directors of Versartis, Inc., a publicly trade biopharmaceutical company.
Dr. Scannon is one of our founders and has served as a Director since our formation. Dr. Scannon became Executive Vice President and Chief Scientific Officer in February 2011. In January 2014, Dr. Scannon’s employment agreement was amended to change his status from full- to part-time, continuing to serve in his previous roles a Director and Executive Vice President and Chief Scientific Officer. Previously he was our Executive Vice President and Chief Medical Officer beginning in March 2009 and served as Executive Vice President and Chief Biotechnology Officer from May 2006 until March 2009, Chief Scientific and Medical Officer from March 1993 until May 2006, Vice Chairman, Scientific and Medical Affairs from April 1992 to March 1993 and our President from our formation until April 1992. From 2007 until 2012, Dr. Scannon served on the National Biodefense Science Board, reporting to the Secretary for the Department of Health and Human Services. In 2007, he also became a member of the Board of Directors for Pain Therapeutics, Inc., a biopharmaceutical company. He has served on the Defense Sciences Research Council for the Defense Advanced Research Projects Agency (DARPA) and on the Threat Reduction Advisory Committee for the Department of Defense. From 1979 until 1981, Dr. Scannon was a clinical research scientist at the Letterman Army Institute of Research in San Francisco. A Board-certified internist, Dr. Scannon holds a Ph.D. in organic chemistry from the University of California, Berkeley and an M.D. from the Medical College of Georgia.
Dr. Rubin is our Senior Vice President, Clinical Development and Chief Medical Officer. Dr. Rubin joined the Company in June 2011. Prior to joining XOMA, Dr. Rubin was Chief Medical Officer at Funxional Therapeutics Ltd. He was Chief Executive Officer of Resolvyx Pharmaceuticals, Inc. from 2007 to 2009 and President and Chief Executive Officer of Critical Therapeutics, Inc. from 2002 to 2007. From 1996 to 2002, Dr. Rubin served as Senior Vice President, Development, and later as Executive Vice President, Research & Development at Sepracor. He was responsible for the successful development of all of Sepracor's internally developed approved products including Xopenex®, Lunesta®, Xopenex HFA® and Brovana®. From 1993 to 1996, Dr. Rubin held senior level positions at Glaxo-Wellcome Pharmaceuticals, most recently as Vice President of Worldwide Clinical Pharmacology and Early Clinical Development. During his tenure with Abbott from 1987 to 1993, Dr. Rubin served as Vice President, Immunology and Endocrinology, where he successfully advanced from discovery to approval zilueton, the first 5-lipoxygenase inhibitor for the treatment of asthma. Dr. Rubin received a BA from Occidental College and his M.D. from Rush Medical College. He completed his training in internal medicine at the University of Wisconsin.
Mr. Kurland is our Vice President, Finance, Chief Financial Officer, and Secretary. He joined XOMA in December 2008. Mr. Kurland is responsible for directing the Company’s financial strategy, accounting, financial planning and investor relations functions. He has more than 30 years of experience in biotechnology and pharmaceutical companies including Aviron/MedImmune, Protein Design Labs and Syntex/Roche. Prior to joining XOMA, Mr. Kurland served as Chief Financial Officer of Bayhill Therapeutics, Inc., Corcept Therapeutics Incorporated and Genitope Corporation. From 1998 to 2002, Mr. Kurland served as Senior Vice President and Chief Financial Officer of Aviron, acquired by MedImmune in 2001 and developer of FluMist. From 1996 to 1998, he was Vice President and Chief Financial Officer of Protein Design Labs, Inc., an antibody design company, and from 1995 to 1996, he served as Vice President and Chief Financial Officer of Applied Immune Sciences, Inc. Mr. Kurland also held a number of financial management positions at Syntex Corporation, a pharmaceutical company acquired by Roche, including Vice President and Controller between 1991 and 1995. He received his J.D. and M.B.A. degrees from the University of Chicago and his B.S. degree from Lehigh University.
Mr. Klein is our Vice President, Chief Commercial Officer. He joined XOMA in 2013 from Genentech, where he was Vice President, Business Unit Head, Virology and Specialty Care from 2009-2012. He joined Genentech from Roche, where he was the Vice President and Franchise Head for the Virology Sale and Marketing. From 2002 to 2006, he was the Vice President, Primary Care Sales overseeing a variety of brands including Tamiflu®, Boniva® and Rocephin®. Mr. Klein also held a number of senior commercial positions in both specialty and primary care therapeutic areas. Prior to his 12 years with Roche, Mr. Klein spent 11 years with Westwood-Squibb/Bristol Myers-Squibb in several sales and product management roles. He has an MBA, Management from Temple University and a BA, Marketing, from Pennsylvania State University.
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market for Registrant’s Common Equity
Our common stock trades on The NASDAQ Global Market under the symbol “XOMA.” All references to numbers of shares of common stock and per-share information in this Annual Report have been adjusted retroactively to reflect the our reverse stock split effective August of 2010. The following table sets forth the quarterly range of high and low reported sale prices of our common stock on The NASDAQ Global Market for the periods indicated:
|
|
Price Range
|
|||||||
|
|
High
|
Low
|
||||||
|
|
||||||||
|
2014
|
||||||||
|
First Quarter
|
$
|
9.57
|
$
|
4.77
|
||||
|
Second Quarter
|
$
|
5.54
|
$
|
3.42
|
||||
|
Third Quarter
|
$
|
4.95
|
$
|
3.66
|
||||
|
Fourth Quarter
|
$
|
5.95
|
$
|
3.50
|
||||
|
2013
|
||||||||
|
First Quarter
|
$
|
3.67
|
$
|
2.43
|
||||
|
Second Quarter
|
$
|
4.40
|
$
|
3.02
|
||||
|
Third Quarter
|
$
|
5.53
|
$
|
3.61
|
||||
|
Fourth Quarter
|
$
|
7.45
|
$
|
3.67
|
||||
On March 9, 2015, there were 809 stockholders of record of our common stock, one of which was Cede & Co., a nominee for Depository Trust Company (“DTC”). All of the shares of our common stock held by brokerage firms, banks and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC and are therefore considered to be held of record by Cede & Co. as one stockholder.
Dividend Policy
We have not paid dividends on our common stock. We currently intend to retain any earnings for use in the development and expansion of our business. We, therefore, do not anticipate paying cash dividends on our common stock in the foreseeable future. In addition, our loan agreement with Hercules generally restricts the declaration and payment of cash dividends.
Performance Graph
The following graph compares the five-year cumulative total stockholder return for XOMA common stock with the comparable cumulative return of certain indices. The graph assumes $100 invested on the same date in each of the indices. Returns of the company are not indicative of future performance.
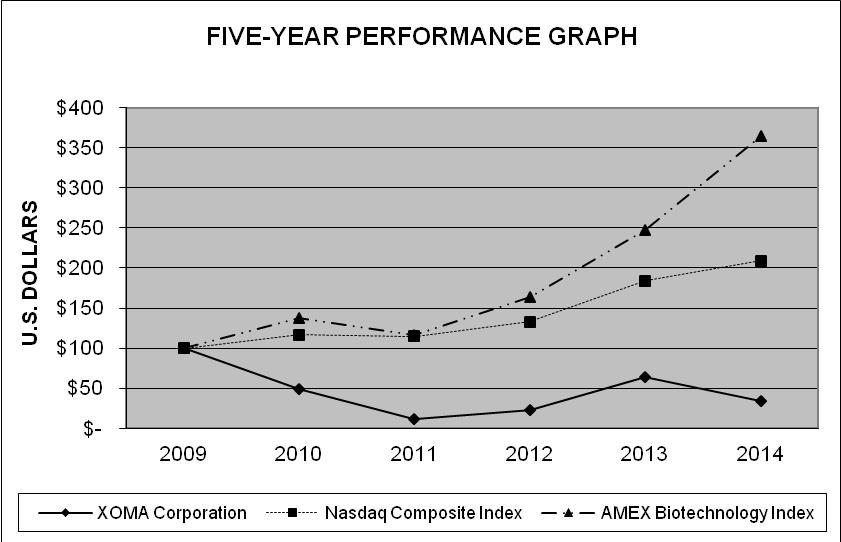
This Section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of XOMA Corporation under the Securities Act, or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
As of
December 31, |
XOMA
Corporation
|
Nasdaq
Composite
Index
|
AMEX
Biotechnology
Index
|
|||||||||
|
2009
|
$
|
100.00
|
$
|
100.00
|
$
|
100.00
|
||||||
|
2010
|
$
|
48.86
|
$
|
116.91
|
$
|
137.73
|
||||||
|
2011
|
$
|
10.95
|
$
|
114.81
|
$
|
115.85
|
||||||
|
2012
|
$
|
22.86
|
$
|
133.07
|
$
|
164.21
|
||||||
|
2013
|
$
|
64.10
|
$
|
184.06
|
$
|
247.36
|
||||||
|
2014
|
$
|
34.19
|
$
|
208.71
|
$
|
365.04
|
||||||
The following table contains our selected financial information including consolidated statement of operations and consolidated balance sheet data for the years 2010 through 2014. The selected financial information has been derived from our audited consolidated financial statements. The selected financial information should be read in conjunction with Item 8: Financial Statements and Supplementary Data and Item 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations included in this Annual Report. The data set forth below is not necessarily indicative of the results of future operations.
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
|
(In thousands, except per share amounts)
|
|||||||||||||||||||
|
Consolidated Statement of Operations Data
|
||||||||||||||||||||
|
Total revenues (1)
|
$
|
18,866
|
$
|
35,451
|
$
|
33,782
|
$
|
58,196
|
$
|
33,641
|
||||||||||
|
Total operating costs and expenses
|
100,614
|
93,328
|
85,332
|
92,151
|
100,663
|
|||||||||||||||
|
Restructuring costs
|
84
|
328
|
5,074
|
-
|
82
|
|||||||||||||||
|
Loss from operations
|
(81,832
|
)
|
(58,205
|
)
|
(56,624
|
)
|
(33,955
|
)
|
(67,104
|
)
|
||||||||||
|
Other income (expense), net (2)
|
43,531
|
(65,867
|
)
|
(14,515
|
)
|
1,227
|
(1,625
|
)
|
||||||||||||
|
Net loss before taxes
|
(38,301
|
)
|
(124,072
|
)
|
(71,139
|
)
|
(32,728
|
)
|
(68,729
|
)
|
||||||||||
|
Income tax benefit (expense), net
|
-
|
14
|
74
|
(15
|
)
|
(27
|
)
|
|||||||||||||
|
Net loss
|
$
|
(38,301
|
)
|
$
|
(124,058
|
)
|
$
|
(71,065
|
)
|
$
|
(32,743
|
)
|
$
|
(68,756
|
)
|
|||||
|
Basic net loss per share of common stock
|
$
|
(0.36
|
)
|
$
|
(1.43
|
)
|
$
|
(1.10
|
)
|
$
|
(1.04
|
)
|
$
|
(3.69
|
)
|
|||||
|
Diluted net loss per share of common stock
|
$
|
(0.67
|
)
|
$
|
(1.43
|
)
|
$
|
(1.10
|
)
|
$
|
(1.04
|
)
|
$
|
(3.69
|
)
|
|||||
|
December 31,
|
||||||||||||||||||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
||||||||||||||||
|
(In thousands)
|
||||||||||||||||||||
|
Balance Sheet Data
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
78,445
|
$
|
101,659
|
$
|
45,345
|
$
|
48,344
|
$
|
37,304
|
||||||||||
|
Short-term investments
|
$
|
-
|
$
|
19,990
|
$
|
39,987
|
$
|
-
|
$
|
-
|
||||||||||
|
Current assets
|
$
|
83,842
|
$
|
127,060
|
$
|
95,837
|
$
|
62,695
|
$
|
58,880
|
||||||||||
|
Working capital
|
$
|
47,367
|
$
|
97,415
|
$
|
72,004
|
$
|
42,064
|
$
|
23,352
|
||||||||||
|
Total assets
|
$
|
89,631
|
$
|
134,782
|
$
|
105,676
|
$
|
78,036
|
$
|
74,252
|
||||||||||
|
Current liabilities
|
$
|
36,475
|
$
|
29,645
|
$
|
23,833
|
$
|
20,631
|
$
|
35,528
|
||||||||||
|
Long-term liabilities (3)
|
$
|
50,057
|
$
|
109,124
|
$
|
60,376
|
$
|
42,394
|
$
|
15,133
|
||||||||||
|
Redeemable convertible preferred stock, at par value
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
1
|
||||||||||
|
Accumulated deficit
|
$
|
(1,119,477
|
)
|
$
|
(1,081,176
|
)
|
$
|
(957,118
|
)
|
$
|
(886,053
|
)
|
$
|
(853,310
|
)
|
|||||
|
Total stockholders' equity (deficit)
|
$
|
3,099
|
$
|
(3,987
|
)
|
$
|
21,467
|
$
|
15,011
|
$
|
23,591
|
|||||||||
We have paid no dividends in the past five years.
| (1) | 2010 includes a non-recurring fee of $4.0 million related to the sale of our CIMZIA® royalty interest to an undisclosed buyer. |
| (2) | 2014, 2013 and 2012 include $39.5 million, ($59.9) million and ($9.5) million, respectively, related to the revaluation of contingent warrant liabilities issued in connection with an equity financing in March 2012. 2010 includes a loss associated with the $4.5 million paid in the first quarter of 2010 to the holders of warrants issued in June 2009, upon modification of the terms. |
| (3) | 2014, 2013 and 2012 include $26.7 million, $68.7 million and $15.0 million, respectively, related to contingent warrant liabilities in connection with an equity financing in March 2012. The balance in 2014, 2013, 2012, and 2011 includes a €15.0 million loan from Servier, which had a principal balance equal to approximately $18.2 million, $20.6 million, $19.8 million, and $19.4 million as of December 31, 2014, 2013, 2012, and 2011, respectively, and a term loan from GECC, which had a principal balance equal to $5.2 million, $9.4 million, $12.5 million, and $10.0 million as of December 31, 2014, 2013, 2012, and 2011, respectively. |
Overview
XOMA Corporation (“XOMA”), a Delaware corporation, discovers and develops innovative antibody-based therapeutics. Several of our antibodies have unique properties due to their interaction at allosteric sites on specific protein rather than the orthosteric, or active sites. The compounds are designed to either enhance or diminish the protein’s activity as desired. We believe allosteric-modulating antibodies may be more selective or offer a safety advantage in certain disease indications when compared to more traditional modes of action.
Our lead product candidate, gevokizumab, is a proprietary potent, humanized allosteric-modulating monoclonal antibody that binds to the inflammatory cytokine interleukin-1 beta (“IL-1 beta”). We believe that by targeting IL-1 beta, gevokizumab has the potential to address the underlying inflammatory causes of a wide range of diseases that have been identified as having unmet medical needs.
Together with our development partner, Servier, a leading independent French pharmaceutical research company, we initiated three pivotal clinical trials evaluating gevokizumab for the treatment of non-infectious intermediate, posterior or pan-uveitis (“NIU”) and Behçet’s disease uveitis. We are responsible for all of the clinical study sites in the United States, and Servier is responsible for all of the clinical study sites outside of the United States. These studies are known as the EYEGUARD™ program, which includes EYEGUARD-A (patients with active NIU), EYEGUARD-B (patients with Behçet’s disease uveitis outside of the United States), EYEGUARD-C (patients with a history of NIU currently controlled with systemic treatment).
Our strategy is to pursue Behçet’s disease uveitis as our first indication for gevokizumab in the United States. Upon the successful completion of Servier’s EYEGUARD-B study, we intend to meet with the U.S. Food and Drug Administration (“FDA” or “the Agency”) to review the Phase 3 EYEGUARD-B data together with the data from the two Behçet’s disease uveitis Phase 2 studies conducted independently by XOMA and Servier. We believe the seriousness of this disease and the small patient population warrant consideration for approval based upon positive data from a single pivotal study. There is significant precedence for regulatory approval based upon a single study for indications of similar seriousness and patient populations. Should EYEGUARD-B demonstrate that patients with Behçet’s disease uveitis who receive gevokizumab took longer to exacerbate than the placebo-treated patients during the tapering of administered steroids, we believe we will be in position to begin the Biologics License Application (“BLA”) submission process.
In September 2014, we opened the EYEGUARD-US supplemental gevokizumab clinical study of Behçet’s disease uveitis patients in the United States. The supplemental EYEGUARD-US study may be used in one of several ways. It may not be required for the initial BLA submission so that it merely provides further information related to U.S. physicians’ and patients’ experiences with gevokizumab. It may be required for the FDA’s review of our submission but for informational purposes without being considered a pivotal study. In this case, the study would be unmasked at a predetermined time when we are in a position to submit the BLA. Finally, it may be required as a second pivotal study in order for the FDA to accept our submission. We’ve designed the EYEGUARD-US study in a manner intended to fulfill whatever directive we are given by the Agency and respond as quickly as possible.
In addition to the NIU clinical trials, we are studying gevokizumab in pyoderma gangrenosum (“PG”), a rare ulcerative skin disease that is a specific indication under the umbrella of diseases known as neutrophilic dermatoses. Patients experience painful expanding skin ulcers that have a significant impact on their quality of life. Approximately 50 to 70 percent of the PG patient population have an underlying systemic condition, while the remainder is idiopathic (unknown cause). The most prevalent underlying conditions are ulcerative colitis and Crohn’s disease. The prognosis for PG is linked directly to the patient’s response to therapy for the underlying disease. Physicians currently treat patients with systemic therapies that are approved for the underlying disease and with topical therapies applied directly to the ulcers, yet published literature suggests that, on average, current therapies can take six months to stop the ulcers from expanding and over eleven months to heal. The U.S. Department of Health and Human Services’ National Institutes of Health’s Office of Rare Disease Research lists PG as occurring in about one per 100,000 people. Claims data compiled over the past three years indicate the number of diagnosed PG patients in the U.S. ranges between 11,000 and 14,000 annually.
Based upon what we believe are compelling data from our pilot study in patients with PG, we initiated a Phase 3 clinical program. Final comments from the FDA were received in the third quarter of 2014, and we initiated the first Phase 3 study in October 2014. The Phase 3 PG program includes two double-blind, placebo-controlled clinical studies, each of which is designed to enroll 58 patients with active PG to receive gevokizumab 60 mg or placebo dosed subcutaneously once monthly, in addition to their current treatment regimen of low-dose corticosteroids and/or immunosuppressants. The primary endpoint is the complete closure of the PG target ulcer determined at Day 126 with confirmation of complete closure a minimum of two weeks later on or after Day 140.
Published literature indicates approximately 50% of patients will experience a recurrence within two to three years. To follow the patients enrolled in our pilot study, we designed an extension study that allows the pilot study patients the opportunity to receive further treatment if they experience new ulcers and allows us to capture information on how gevokizumab performs with successive treatments. Four of the six patients from our pilot study entered the extension study; three of whom were fully healed during the initial study, and one patient who had an ulcer which was fully healed at Day 56 but reopened after an injury. To date, three of the four patients enrolled in the extension study have received additional gevokizumab therapy for PG. One patient recurred at 7.5 months and upon receiving additional gevokizumab therapy obtained 100% ulcer closure prior to Day 56. One patient recurred at 7 months and after additional gevokizumab therapy obtained 100% ulcer closure by Day 84. The patient who had initially healed but whose ulcer reopened after an injury continued receiving therapy and obtained 100% ulcer closure. One patient has not received additional gevokizumab therapy as the condition has not recurred more than one year following initial gevokizumab treatment. All four patients continue to be enrolled in the extension study and will be followed for up to 92 weeks.
We also have an active gevokizumab Proof-of-Concept (“POC”) development program to identify other illness for late-stage development. Two studies are being conducted in collaboration with the U.S. National Institutes of Health (“NIH”). The National Eye Institute (“NEI”) is conducting a gevokizumab study in patients with non-infectious anterior scleritis. The North Shore-Long Island Jewish Health System in collaboration with the National Institute on Deafness and Other Communication Disorders (“NIDCD”) are conducting a gevokizumab clinical study in patients with inflammatory AIED.
Previously, we conducted POC trials in moderate-to-severe inflammatory acne and in erosive osteoarthritis of the hand (“EOA”). We have decided not to further pursue the acne indication based on our commercial analysis. The EOA results led to our decision not to pursue Phase 3 testing in the broad EOA population, although we continue to review the data to determine if there is a subgroup of the EOA population that could benefit from gevokizumab therapy.
Gevokizumab has been generally well tolerated across all of our clinical studies. In both the acne and EOA studies, there were no drug-related serious adverse events reported. The most common adverse events were headache, pain, arthralgia, urinary tract infections, upper respiratory tract infections and pneumonia, and they were comparable between gevokizumab and placebo.
Separately, Servier instituted its own active development program for gevokizumab beyond the NIU and Behçet’s disease uveitis Phase 3 program. In 2012, Servier initiated a Phase 2 gevokizumab study in patients with acute coronary syndrome, a cardiovascular disease within the cardiometabolic field where it has world-wide rights. In 2013, Servier began testing gevokizumab in a variety of POC studies, including polymyositis/dermatomyositis, Schnitzler syndrome, and giant cell arteritis. Servier has indicated these are the first studies in an extensive multi-indication exploratory program it expects to conduct.
Our proprietary pipeline includes classes of allosteric modulating antibodies that activate, sensitize or deactivate the insulin receptor in vivo, which we have named XOMA Metabolic or XMet. Insulin is the primary hormone for lowering blood glucose levels. Abnormal increases in insulin secretion can lead to profound hypoglycemia (low blood sugar), a state that may result in significant morbidities, including cerebral damage and epilepsy. In some instances, profound hypoglycemia can result in fatality. There are three programs in the XMet portfolio, XMetD, which is designed to deactivate the insulin receptor, XMetA, which is designed to activate the insulin receptor, and XMetS, which is designed to sensitize the insulin receptor when in an insulin resistant state. These programs are highly novel as the antibodies bind to different sites on the insulin receptor than currently marketed drugs. This portfolio of antibodies represents potential new therapeutic approaches to the treatment of several rare diseases that have insulin involvement and diabetes.
The lead compound from our XMetD program, XOMA 358, is a fully human monoclonal allosteric modulating antibody that binds to insulin receptors and attenuates insulin action. It is designed to negatively modulate the insulin receptor and its downstream signaling capabilities. We launched clinical development activities for XOMA 358 in October 2014, with the first patient dosed in our Phase 1 safety and tolerability study. The Phase 1 study was successful, and we intend to investigate this compound as a novel treatment for non-drug-induced, endogenous hyperinsulinemic hypoglycemia (low blood glucose caused by excessive insulin produced by the body). A therapy that safely and effectively mitigates insulin-induced hypoglycemia has the potential to address a significant unmet therapeutic need for certain rare medical conditions associated with hyperinsulinism.
We intend to retain full ownership of XOMA 358 and the compounds in the XMetD program, as they align with our focus on developing products for diseases with significant unmet medical need that are treated by the specialist prescriber. We intend to out-license the insulin receptor-activating drug candidate(s) for development in diabetes to a pharmaceutical company with expertise in developing and commercializing compounds for Types 1 and 2 diabetes.
We have developed these and other antibodies using some or all of our ADAPT™ antibody discovery and development platform, our ModulX™ technologies for generating allosterically modulating antibodies, and our OptimX™ technologies for optimizing biophysical properties of antibodies, including affinity, immunogenicity, stability and manufacturability.
Our biodefense initiatives include XOMA 3AB, a biodefense anti-botulism product candidate comprised of a combination of three antibodies. XOMA 3AB is directed against botulinum toxin serotype A and has been developed through funding from the National Institute of Allergy and Infectious Diseases (“NIAID”), a part of the NIH. A Phase 1 XOMA 3AB trial was completed with no product-related serious adverse events. Should the government choose to acquire XOMA 3AB or other biodefense products in the future, we expect to be able to produce these antibodies through an outside manufacturer.
We also have developed antibody product candidates with premier pharmaceutical companies including Novartis AG (“Novartis”) and Takeda Pharmaceutical Company Limited (“Takeda”).
Significant Developments in 2014
Gevokizumab
| · | In February 2014, we announced gevokizumab has been granted Orphan Drug Designation by the FDA for the treatment of Pyoderma Gangrenosum. |
| · | In March 2014, we reported that despite early positive results in the first of two Phase 2 programs in patients with EOA, the top-line data at Day 168 in that study, as well as data at Day 84 in the second study, were not positive. These results led to our decision not to pursue Phase 3 testing in the broad EOA population. We continue to review the data to determine if there is a subgroup of the EOA population that could benefit from gevokizumab therapy. |
| · | In April 2014, we finalized our plans for our gevokizumab Phase 3 program in PG and submitted the protocols to the FDA for any further comments. Final comments from the FDA were received in the third quarter of 2014. The Phase 3 program will include two double-blind, placebo-controlled clinical studies, each of which is designed to enroll 58 patients with active PG. The primary endpoint is the complete closure of the PG target ulcer determined at Day 126 and confirmation of complete closure a minimum of two weeks later at Day 140. |
| · | In September 2014, we announced we opened the EYEGUARD-US supplemental clinical study to patients at study sites located in the United States. The objective of this trial is to assess the efficacy and safety of gevokizumab in treating Behçet's disease uveitis. Upon the successful completion of SERVIER’s EYEGUARD-B study, we intend to meet with the FDA to review the Phase 3 EYEGUARD-B data together with the data from the two Behçet’s disease uveitis Phase 2 studies conducted by XOMA and Servier. Should EYEGUARD-B successfully demonstrate that Behçet’s disease uveitis patients receiving gevokizumab took longer to exacerbate than the placebo-treated patients during the tapering of administered steroids, we believe we will be in position to begin the BLA submission process. The supplemental EYEGUARD-US study may be used in one of several ways. It may not be required for the initial BLA submission so that it merely provides further information as to U.S. physicians and patients’ experiences with gevokizumab. It may be required for the FDA’s review of our submission but for informational purposes without being considered a pivotal study. In this case, the study would be unmasked at a predetermined time when we are in a position to submit the BLA. Finally, it may be required by the FDA as a second pivotal study of U.S. Behçet’s disease uveitis patients in order for the Agency to accept our submission. We’ve designed the EYEGUARD-US study to fulfill whatever directive we are given by the FDA. We are prepared to respond as quickly as possible to any of the anticipated outcomes from our pre-BLA meeting. |
| · | In October 2014, we announced we have initiated dosing in our Phase 1 study exploring the safety and tolerability of single intravenous dose (“IV”) of XOMA 358, the lead compound from the our XMetD program, in healthy volunteers. The study also will explore the biologic effects of ascending single IV doses of XOMA 358 on glucose and insulin levels, as well as insulin sensitivity. |
| · | In November 2014, we announced the pivotal Phase 3 gevokizumab study in patients with active PG, is open for patient enrollment. The objective of the study is to assess the efficacy and safety of gevokizumab in treating the active ulcers caused by this rare and debilitating disease. The Phase 3 randomized, placebo-controlled study will enroll 58 patients with active PG to receive gevokizumab 60 mg or placebo dosed subcutaneously once monthly, in addition to their current treatment regimen of low-dose corticosteroids and/or immunosuppressants. |
Licensing
| · | In July 2014, we completed the transfer of our U.S. marketing rights in ACEON to Symplmed and are no longer selling this drug product. |
| · | In September 2014, we granted a non-exclusive license for our innovative design of a manufacturing facility to the Texas A&M University System. The patented technology relates to a flexible arrangement of MCRs within the manufacturing facility, with each MCR providing a portable, self-contained environment for product manufacture. The flexible manufacturing facility design allows MCRs to connect easily and quickly to a central supply of utilities such as air, water, and electricity. The unique arrangement facilitates flexible design and eliminates change-over downtime. This translates into significantly reduced capital expenditures, production costs, and maintenance costs, while offering meaningful time advantages over conventional manufacturing facilities. When MCRs are not in use, they can be easily moved to cleaning/refurbishing areas and prepared MCRs can be "plugged in" for manufacturing. The A&M System will use MCRs for certain government programs at The National Center for Therapeutics Manufacturing (“NCTM”) facility, a multidisciplinary workforce education institution and biopharmaceutical manufacturing center, located at Texas A&M University in College Station, Texas. |
Financing
| · | In December 2014, we completed a registered direct offering of 8,097,165 shares of our common stock for gross proceeds of $40.0 million to select institutional investors, before deducting underwriting discounts and commissions an estimated offering expenses totaling approximately $2.3 million. In connection with the offering, we issued two year warrants to purchase up to an aggregate of 8,097,165 shares of our common stock at an exercise price of $7.90 per share. |
| · | In February 2015, we entered into a Loan and Security Agreement with Hercules in which we borrowed $20.0 million. We used a portion of the proceeds received under the Hercules Loan Agreement to repay existing indebtedness, and we plan to use the remaining proceeds for general corporate purposes. In connection with the Hercules Loan Agreement, we issued a warrant to Hercules which is exercisable for an aggregate of up to 181,268 shares of XOMA common stock at an exercise price of $3.31 per share. |
Critical Accounting Estimates
The accompanying discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements and the related disclosures, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts in our consolidated financial statements and accompanying notes. On an ongoing basis, we evaluate our estimates, assumptions and judgments described below that have the greatest potential impact on our consolidated financial statements, including those related to revenue recognition, research and development activities warrant liabilities and stock-based compensation. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Accounting assumptions and estimates are inherently uncertain and actual results may differ materially from these estimates under different assumptions or conditions.
The consolidated financial statements include the accounts of XOMA and its wholly-owned subsidiaries. All significant intercompany accounts and transactions among the entities have been eliminated.
While our significant accounting policies are more fully described in Note 2 to the Consolidated Financial Statements, we believe the following policies to be the most critical to an understanding of our financial condition and results of operations because they require us to make estimates, assumptions and judgments about matters that are inherently uncertain.
Revenue Recognition
License and Collaborative Fees
Revenue from non-refundable license, technology access or other payments under license and collaborative agreements where we have a continuing obligation to perform is recognized as revenue over the estimated period of the continuing performance obligation. We estimate the performance period at the inception of the arrangement and re-evaluate it each reporting period. Management makes its best estimate of the period over which it expects to fulfill the performance obligations, which may include clinical development activities. Given the uncertainties of research and development collaborations, significant judgment is required to determine the duration of the performance period. This re-evaluation may shorten or lengthen the period over which the remaining revenue is recognized. Changes to these estimates are recorded on a prospective basis. Cost reimbursement revenue under collaborative agreements is recognized as the related research and development costs are incurred, as provided for under the terms of these agreements.
Our license and collaboration agreements with certain third parties also provide for contingent payments to be paid to us based solely upon the performance of the partner. For such contingent payments we recognize the payments as revenue upon completion of the milestone event, once confirmation is received from the third party, provided that collection is reasonably assured and the other revenue recognition criteria have been satisfied.
Contract Revenue
Contract revenue for research and development involves our providing research and development and manufacturing services to collaborative partners, biodefense contractors or others. Revenue for certain contracts is accounted for by a proportional performance, or output-based, method where performance is based on estimated progress toward elements defined in the contract. The amount of contract revenue and related costs recognized in each accounting period are based on estimates of the proportional performance during the period. Adjustments to estimates based on actual performance are recognized on a prospective basis and do not result in reversal of revenue should the estimate to complete be extended.
In addition, revenue related to certain research and development contracts is billed based on actual hours incurred by XOMA related to the contract, multiplied by full-time equivalent (“FTE”) rates plus a mark-up. The FTE rates are developed based on our best estimates of labor, materials and overhead costs. For certain contracts, such as our government contracts, the FTE rates are agreed upon at the beginning of the contract and are subject to review or audit by the contracting party at any time. Under our contracts with NIAID, a part of the NIH, we bill using NIH provisional rates and thus are subject to future audits at the discretion of NIAID’s contracting office. These audits can result in adjustments to previously reported revenue.
In 2011, the NIH conducted an audit of our actual data under two contracts for the period from January 1, 2007, through December 31, 2009, and developed final billing rates for this period. As a result, we retroactively applied these NIH rates to the invoices from this period, which resulted in an increase in revenue of $3.1 million from the NIH, excluding $0.9 million billed to the NIH in 2010 as a result of a comparison of 2009 calculated costs incurred and costs billed to the government under provisional rates. Final rates were settled for one contract resulting in the recognition of revenue of $2.0 million in 2012. The remaining deferred revenue in connection with the 2011 NIH rate audit will be recognized upon negotiation with and approval by NIH. In 2014, upon completion of a NIAID review of hours and external expenses for the period spanning from 2008 to 2013, XOMA agreed to exclude certain hours and external expense resulting in a $1.8 million adjustment, which reduced deferred revenue and accounts receivable.
Upfront fees are recognized ratably over the expected benefit period under the arrangement. Given the uncertainties of research and development collaborations, significant judgment is required to determine the duration of the arrangement.
Research and Development Expenses
We expense research and development costs as incurred. Research and development expenses consist of direct costs such as salaries and related personnel costs, and material and supply costs, and research-related allocated overhead costs, such as facilities costs. In addition, research and development expenses include costs related to clinical trials. From time to time, research and development expenses may include up-front fees and milestones paid to collaborative partners for the purchase of rights to in-process research and development. Such amounts are expensed as incurred.
Our accrual for clinical trials is based on estimates of the services received and efforts expended pursuant to contracts with clinical trial centers and clinical research organizations. Payments under the contracts depend on factors such as the achievement of certain events, successful enrollment of patients, and completion of portions of the clinical trial or similar conditions. We may terminate these contracts upon written notice and we are generally only liable for actual effort expended by the organizations to the date of termination, although in certain instances we may be further responsible for termination fees and penalties. We make estimates of our accrued expenses as of each balance sheet date based on the facts and circumstances known to us at that time. Expenses resulting from clinical trials are recorded when incurred based, in part, on estimates as to the status of the various trials. There have been no material adjustments to our prior period accrued estimates for clinical trial activities through December 31, 2014.
Stock-based Compensation
Stock-based compensation expense for stock options and other stock awards is estimated at the grant date based on the award’s fair value-based measurement and is recognized on a straight-line basis over the award’s vesting period, assuming appropriate forfeiture rates. The valuation of stock-based compensation awards is determined at the date of grant using the Black-Scholes option pricing model (the “Black-Scholes Model”). This model requires highly complex and subjective inputs, such as the expected term of the option, expected volatility, and risk-free interest rate. Further, the forfeiture rate also impacts the amount of aggregate compensation. These inputs are subjective and generally require significant analysis and judgment to develop. Our current estimate of volatility is based on the historical volatility of our stock price. To the extent volatility in our stock price increases in the future, our estimates of the fair value of options granted in the future could increase, thereby increasing stock-based compensation cost recognized in future periods. To establish an estimate of expected term, we consider the vesting period and contractual period of the award and our historical experience of stock option exercises, post-vesting cancellations and volatility. To establish an estimate of forfeiture rate, we consider our historical experience of option forfeitures and terminations. The risk-free rate is based on the yield available on United States Treasury zero-coupon issues. We review our valuation assumptions quarterly and, as a result, we likely will change our valuation assumptions used to value stock-based awards granted in future periods. Stock-based compensation expense is recognized ratably over the requisite service period. In the future, as additional empirical evidence regarding these input estimates becomes available, we may change or refine our approach of deriving these input estimates. These changes could impact our fair value-based measurement of stock options granted in the future. Changes in the fair value-based measurement of stock awards could materially impact our operating results.
Warrants
We have issued warrants to purchase shares of our common stock in connection with financing activities. We account for some of these warrants as a liability at fair value and others as equity at fair value. The fair value of the outstanding warrants is estimated using the Black-Scholes Model. The Black-Scholes Model requires inputs, such as the expected term of the warrants, expected volatility and risk-free interest rate. These inputs are subjective and require significant analysis and judgment to develop. For the estimate of the expected term, we use the full remaining contractual term of the warrant. We base our estimate of expected volatility on our historical stock price volatility. The assumptions associated with contingent warrant liabilities are reviewed each reporting period and changes in the estimated fair value of these contingent warrant liabilities are recognized in other income (expense).
Results of Operations
Revenue
Total revenues for the years ended December 31, 2014, 2013, and 2012, were as follows (in thousands):
|
Year ended December 31,
|
2013-2014
|
2012-2013
|
||||||||||||||||||
|
2014
|
2013
|
2012
|
Change
|
Change
|
||||||||||||||||
|
License and collaborative fees
|
$
|
5,683
|
$
|
11,028
|
$
|
5,727
|
$
|
(5,345
|
)
|
$
|
5,301
|
|||||||||
|
Contract and other revenue
|
13,183
|
24,423
|
28,055
|
(11,240
|
)
|
(3,632
|
)
|
|||||||||||||
|
Total revenues
|
$
|
18,866
|
$
|
35,451
|
$
|
33,782
|
$
|
(16,585
|
)
|
$
|
1,669
|
|||||||||
License and Collaborative Fees
License and collaborative fee revenue includes fees and milestone payments related to the out-licensing of our products and technologies. The primary components of license and collaboration fee revenue in 2014 were $3.0 million in milestone payments relating to various out-licensing arrangements, including $1.9 million in revenue recognized related to the loan agreement with Servier and $0.8 million in upfront fees and annual maintenance fees relating to various out-licensing arrangements.
The primary components of license and collaboration fee revenue in 2013 were $8.6 million in milestone payments relating to various out-licensing arrangements, including a $7.0 million milestone payment from Novartis, $1.6 million in revenue recognized related to the loan agreement with Servier, and $0.8 million in upfront fees and annual maintenance fees relating to various out-licensing arrangements.
The primary components of license and collaboration fee revenue in 2012 were $3.3 million in upfront fees and annual maintenance fees relating to various out-licensing arrangements, $1.4 million in revenue recognized related to the loan agreement with Servier, and $1.0 million recognized for six milestone payments.
Contract and Other Revenue
Contract and other revenues include agreements where we provide contracted research and development services to our contract and collaboration partners, including Servier and NIAID. Contract and other revenues also include net product sales and royalties. The following table shows the activity in contract and other revenue for the years ended December 31, 2014, 2013, and 2012 (in thousands):
|
Year ended December 31,
|
2013-2014 | 2012-2013 | ||||||||||||||||||
|
2014
|
2013
|
2012
|
Change
|
Change
|
||||||||||||||||
|
Servier
|
$
|
3,523
|
$
|
13,568
|
$
|
14,529
|
$
|
(10,045
|
)
|
$
|
(961
|
)
|
||||||||
|
NIAID
|
9,565
|
9,098
|
11,191
|
467
|
(2,093
|
)
|
||||||||||||||
|
Other
|
95
|
1,757
|
2,335
|
(1,662
|
)
|
(578
|
)
|
|||||||||||||
|
Total revenues
|
$
|
13,183
|
$
|
24,423
|
$
|
28,055
|
$
|
(11,240
|
)
|
$
|
(3,632
|
)
|
||||||||
The 2014 decrease in contract and other revenue, as compared to 2013, was primarily due to a decrease of $6.3 million in reimbursement due to our collaboration with Servier meeting the initial $50.0 million cap of fully reimbursable NIU costs in third quarter of 2013. Servier and XOMA will each pay 50% of the remaining NIU clinical development and CMC costs. Also contributing to the decrease were a decrease of $3.9 million for the partial funding of fixed dose combination of perindopril arginine and amlodipine besylate (“FDC1”) Phase 3 trial received from Servier in 2013 for which there was no equivalent payment received in 2014, a decrease of $0.8 million received from ACEON sales and a decrease of $0.7 million in manufacturing activities for Allergan. The decrease in contracts and other revenue was partially offset by a $0.5 million increase in NIAID related revenue.
The 2013 decrease in contract and other revenue, as compared to 2012, was primarily due to the 2012 recognition of $2.0 million in revenue related to an adjustment to previously reported revenue from NIAID resulting from an audit by NIAID’s contracting office. Also contributing to the decrease were decreases of $1.4 million in CMC activity and $0.6 million in gevokizumab clinical development activity under our collaboration with Servier, partially offset by a $0.9 million increase in partial funding received from Servier for the FDC1 Phase 3 trial.
We expect revenue to increase in 2015 compared to 2014 levels based on anticipated licensing activities.
The following table shows the activity in deferred revenue for the years ended December 31, 2014, 2013, and 2012 (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Beginning deferred revenue
|
$
|
6,323
|
$
|
9,724
|
$
|
13,234
|
||||||
|
Revenue deferred
|
798
|
1,478
|
5,881
|
|||||||||
|
Revenue recognized
|
(2,246
|
)
|
(4,879
|
)
|
(9,391
|
)
|
||||||
|
Deferred revenue adjustment
|
(1,847
|
)
|
-
|
-
|
||||||||
|
Ending deferred revenue
|
$
|
3,028
|
$
|
6,323
|
$
|
9,724
|
||||||
We defer revenue until all requirements under our revenue recognition policy are met. In 2014, we deferred revenue from contracts including Servier and NIAID. In 2013 and 2012, we deferred revenue from contracts including Servier, NIAID and Takeda.
In 2014, we recorded an adjustment to reduce deferred revenue by $1.8 million as a result of a final settlement with NIAID upon the completion of their review of billed hours and external expenses.
We expect a significant portion of the $3.0 million in deferred revenue as of December 31, 2014 to be recognized in 2015 with the remainder to be earned during 2016. Future amounts may be affected by additional consideration received, if any, under existing or any future licensing or other collaborative arrangements as well as changes in the estimated period of obligation or services to be provided under the arrangements.
Research and Development Expenses
Biopharmaceutical development includes a series of steps, including in vitro and in vivo preclinical testing, and Phase 1, 2 and 3 clinical studies in humans. Each of these steps is typically more expensive than the previous step, but actual timing and the cost to us depends on the product being tested, the nature of the potential disease indication and the terms of any collaborative or development arrangements with other companies or entities. After successful conclusion of all of these steps, regulatory filings for approval to market the products must be completed, including approval of manufacturing processes and facilities for the product. Our research and development expenses currently include costs of personnel, supplies, facilities and equipment, consultants, other third-party costs and expenses related to preclinical and clinical testing.
Research and development expenses were $80.7 million in 2014, compared with $74.9 million in 2013 and $68.5 million in 2012. The increase of $5.8 million in 2014, compared to 2013, was primarily due to higher clinical trial-related cost, salaries and related personnel costs and outside consulting services, partially offset by decrease in external manufacturing activities. External manufacturing activity, internal proprietary project cost and salaries and related personnel costs increased in 2013, as compared to 2012, however, this increase was offset by decreases in FDC clinical trial costs and internal facility costs.
Salaries and related personnel costs are a significant component of research and development expenses. We recorded $31.8 million in research and development salaries and employee-related expenses in 2014, compared with $27.0 million in 2013 and $25.9 million in 2012. Included in these expenses for 2014 were $23.4 million for salaries and benefits, $2.8 million for bonus expense and $5.6 million for stock-based compensation. The increase in salaries and personnel costs in 2014, as compared to 2013, was primarily due to an increase in salaries and benefits of $1.6 million resulting from increased headcount and an increase of $3.2 million in stock-based compensation.
Included in these expenses for 2013 were $21.7 million for salaries and benefits, $2.9 million for bonus expense and $2.4 million for stock-based compensation. The increase in 2013, as compared to 2012, was primarily due to an increase in salaries and benefits of $0.9 million resulting from increased headcount.
Our research and development activities can be divided into earlier-stage programs and later-stage programs. Earlier-stage programs include molecular biology, process development, pilot-scale production and preclinical testing. Also included in earlier-stage programs are costs related to excess manufacturing capacity. We expect excess manufacturing capacity to continue to decrease in 2015 compared to 2014 due to our streamlining objective implemented in 2012 to utilize a contract manufacturing organization. Later-stage programs include clinical testing, regulatory affairs and manufacturing clinical supplies. The costs associated with these programs approximate the following (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Earlier stage programs(1)
|
$
|
28,327
|
$
|
40,840
|
$
|
33,170
|
||||||
|
Later stage programs(1)
|
52,421
|
34,011
|
35,297
|
|||||||||
|
Total
|
$
|
80,748
|
$
|
74,851
|
$
|
68,467
|
||||||
| (1) | Certain research and development segment reclassifications have been made to previously reported amounts to conform to the current year's presentation. |
Our research and development activities also can be divided into those related to our internal projects and those projects related to collaborative and contract arrangements. The costs related to internal projects versus collaborative and contract arrangements approximate the following (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Internal projects(1)
|
$
|
51,281
|
$
|
47,489
|
$
|
30,531
|
||||||
|
Collaborative and contract arrangements(1)
|
29,467
|
27,362
|
37,936
|
|||||||||
|
Total
|
$
|
80,748
|
$
|
74,851
|
$
|
68,467
|
||||||
| (1) | Certain research and development segment reclassifications have been made to previously reported amounts to conform to the current year's presentation. |
In 2014, the gevokizumab program, for which we incurred the largest amount of expense, accounted for more than 40% but less than 50% of our total research and development expenses. A second development program, XMet, accounted for more than 10% but less than 20% of our total research and development expenses and a third development program, NIAID, accounted for more than 10% but less than 20% of our total research and development expenses.
In 2013, the gevokizumab program, for which we incurred the largest amount of expense, accounted for more than 40% but less than 50% of our total research and development expenses. XMet, accounted for more than 20% but less than 30% of our total research and development expenses. NIAID accounted for more than 10% but less than 20% of our total research and development expenses.
In 2012, the gevokizumab program, for which we incurred the largest amount of expense, accounted for more than 40% but less than 50% of our total research and development expenses. NIAID accounted for more than 20% but less than 30% of our total research and development expenses and XMet accounted for more than 10% but less than 20% of our total research and development expenses.
We expect our research and development spending in 2015 will be comparable primarily due to our ongoing global Phase 3 clinical program for gevokizumab for the NIU indication under our license and collaboration agreement with Servier, our ongoing gevokizumab Phase 2 proof-of-concept program, our PG indications and the continued development of our XMet program.
Future research and development spending also may be impacted by potential new licensing or collaboration arrangements, as well as the termination of existing agreements. Beyond this, the scope and magnitude of future research and development expenses are difficult to predict at this time.
Selling, General and Administrative Expenses
Selling, general and administrative expenses include salaries and related personnel costs, facilities cost and professional fees. In 2014, selling, general and administrative expenses were $19.9 million compared with $18.5 million in 2013 and $16.9 million in 2012. The increase in selling, general and administrative expenses in 2014 as compared with 2013 was primarily due to increases in salaries and related personnel costs of $1.1 million, and an increase of $2.5 million in stock-based compensation, partially offset by a decrease in professional service costs of $1.7 million.
The increase in selling, general and administrative expenses in 2013 as compared with 2012 primarily was due to increases in consulting services of $1.3 million, primarily reflecting investments in market research activities made during 2013, and salaries and related personnel costs of $0.5 million, partially offset by a decrease in profession service costs of $0.6 million.
We expect selling, general and administrative expenses in 2015 to be comparable to 2014 levels.
Restructuring Charges
In January 2012, we implemented a streamlining of operations, which resulted in a restructuring plan designed to sharpen our focus on value-creating opportunities led by gevokizumab and its unique antibody discovery and development capabilities. The restructuring plan included a reduction of XOMA’s personnel by 84 positions, or 34%. These staff reductions resulted primarily from our decisions to utilize a contract manufacturing organization for Phase 3 and commercial antibody production and to eliminate internal research functions that are non-differentiating or that can be obtained cost effectively by contract service providers.
In connection with the streamlining of operations, we incurred restructuring charges in 2012 of $2.0 million related to severance, other termination benefits and outplacement services, $2.2 million related to the impairment and accelerated depreciation of various assets and leasehold improvements, and $0.7 million related to moving and other facility costs. In 2014 and 2013, we incurred $0.1 million and $0.3 million, respectively in restructuring charges related to facility costs.
Other Income (Expense)
Interest Expense
Interest expense and amortization of debt issuance costs and discounts are shown below for the years ended December 31, 2014, 2013, and 2012 (in thousands):
|
Year ended December 31,
|
2013-2014 | 2012-2013 | ||||||||||||||||||
|
2014
|
2013
|
2012
|
Change
|
Change
|
||||||||||||||||
|
Interest expense
|
||||||||||||||||||||
|
Servier loan
|
$
|
2,330
|
$
|
2,152
|
$
|
2,097
|
$
|
178
|
$
|
55
|
||||||||||
|
GECC term loan
|
1,638
|
2,064
|
1,850
|
(426
|
)
|
214
|
||||||||||||||
|
Novartis note
|
312
|
362
|
397
|
(50
|
)
|
(35
|
)
|
|||||||||||||
|
Other
|
23
|
53
|
43
|
(30
|
)
|
10
|
||||||||||||||
|
Total interest expense
|
$
|
4,303
|
$
|
4,631
|
$
|
4,387
|
$
|
(328
|
)
|
$
|
244
|
|||||||||
The decrease in interest expense in 2014 as compared to 2013 was due primarily to a decrease in the principal balance of GECC term loan.
The increase interest expense in 2013 as compared to 2012 was due primarily to an increase in the principal balance of the GECC term loan, which was amended in September 2012.
Other Income (Expense)
Other expense primarily consisted of unrealized (losses) gains. The following table shows the activity in other expense for the years ended December 31, 2014, 2013, and 2012 (in thousands):
|
Year ended December 31,
|
2013-2014 | 2012-2013 | ||||||||||||||||||
|
2014
|
2013
|
2012
|
Change
|
Change
|
||||||||||||||||
|
Other income (expense)
|
||||||||||||||||||||
|
Unrealized foreign exchange gain (loss) (1)
|
$
|
2,447
|
$
|
(442
|
)
|
$
|
(329
|
)
|
$
|
2,889
|
$
|
(113
|
)
|
|||||||
|
Realized foreign exchange gain (loss)
|
-
|
(90
|
)
|
6
|
90
|
(96
|
)
|
|||||||||||||
|
Unrealized loss on foreign exchange options
|
(355
|
)
|
(127
|
)
|
(714
|
)
|
(228
|
)
|
587
|
|||||||||||
|
Other
|
(31
|
)
|
462
|
81
|
(493
|
)
|
381
|
|||||||||||||
|
Total other income (expense)
|
$
|
2,061
|
$
|
(197
|
)
|
$
|
(956
|
)
|
$
|
2,258
|
$
|
759
|
||||||||
|
(1)
|
Unrealized foreign exchange gain (loss) for the years ended December 31, 2014, 2013, and 2012 primarily relates to the re-measurement of the €15 million Servier loan.
|
Revaluation of Contingent Warrant Liabilities
In December 2014, in connection with a registered direct offering, we issued two-year warrants to purchase 8,097,165 shares of XOMA’s common stock at an exercise price of $7.90 per share. These warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate us to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Option Pricing Model (the “Black-Scholes Model”) on the date of such change in control. Due to these provisions, we account for the warrants issued in December 2014 as a liability at fair value. In addition, the estimated liability related to the warrants is revalued at each reporting period until the earlier of the exercise of the warrants, at which time the liability will be reclassified to stockholders’ equity, or expiration of the warrants. As of December 8, 2014, the date of issuance of the warrants, the fair value of the liability was estimated to be approximately $10.3 million using the Black-Scholes Model. We revalued the warrant liability at December 31, 2014, and recorded a $5.1 million decline in the fair value as a gain in the revaluation of contingent warrant liabilities in the consolidated statements of comprehensive loss. The decrease in liability is due primarily to the decrease in the market price of XOMA’s common stock at December 31, 2014. As of December 31, 2014, all of the warrants were outstanding and had a fair value of $5.2 million.
In March 2012, in connection with an underwritten offering, we issued five-year warrants to purchase 14,834,577 shares of XOMA’s common stock at an exercise price of $1.76 per share. These warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate us to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, we account for the warrants issued in March 2012 as a liability at fair value. In addition, the estimated liability related to the warrants is revalued at each reporting period until the earlier of the exercise of the warrants, at which time the liability will be reclassified to stockholders' equity, or expiration of the warrants. At December 31, 2013, 12,562,682 of these warrants were outstanding and had a fair value of $68.7 million. We revalued the warrant liability at December 31, 2014 using the Black-Scholes Model and recorded a $39.5 million decline in the fair value as a gain in the revaluation of contingent warrant liabilities in the accompanying consolidated statements of comprehensive loss. In 2014, we also reclassified $2.5 million from contingent warrant liabilities to equity on our consolidated balance sheet due to the exercise of warrants. As of December 31, 2014, 12,109,418 of these warrants were outstanding and had a fair value of $26.7 million.
In February 2010, in connection with an underwritten offering, we issued five-year warrants to purchase 1,260,000 shares of XOMA’s common stock at an exercise price of $10.50 per share. The warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate us to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, we account for the warrants as liabilities at fair value. As of December 31, 2013, all of the warrants were outstanding and had a fair value of $1.0 million, using the Black-Scholes Model. We revalued the warrant liability at December 31, 2014 using the Black-Scholes Model and recorded a $1.0 million decrease in the fair value as a gain in the revaluation of contingent warrant liabilities in the accompanying consolidated statements of comprehensive loss. As of December 31, 2014, all of the warrants were outstanding and the fair value was de minimis. The decrease in liability is due primarily to the decrease in the market price of XOMA’s common stock at December 31, 2014.
In June 2009, we issued warrants to certain institutional investors as part of a registered direct offering. The warrants represent the right to acquire an aggregate of up to 347,826 shares of XOMA’s common stock over a five-year period beginning December 11, 2009 at an exercise price of $19.50 per share. The warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate us to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, we account for the warrants as liabilities at fair value. At December 31, 2013 all of the warrants were outstanding and had a fair value of $0.1 million using the Black-Scholes Model. As of December 31, 2014, the warrants had expired unexercised.
The following table provides a summary of the changes in fair value of contingent warrant liabilities for the years ended December 31, 2014, 2013, and 2012 (in thousands):
|
Warrant
Liabilities
|
||||
|
Balance at December 31, 2011
|
$
|
379
|
||
|
Initial fair value of warrants issued in March 2012
|
6,390
|
|||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
(940
|
)
|
||
|
Net increase in fair value of contingent warrant liabilities upon revaluation
|
9,172
|
|||
|
Balance at December 31, 2012
|
15,001
|
|||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
(6,171
|
)
|
||
|
Net increase in fair value of contingent warrant liabilities upon revaluation
|
61,039
|
|||
|
Balance at December 31, 2013
|
69,869
|
|||
|
Initial fair value of warrants issued in December 2014 warrant
|
10,258
|
|||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
(2,526
|
)
|
||
|
Net decrease in fair value of contingent warrant liabilities upon revaluation
|
(45,773
|
)
|
||
|
Balance at December 31, 2014
|
$
|
31,828
|
||
Income Taxes
There was no income tax expense for the years ended December 31, 2014, 2013 and 2012. The income tax benefit in 2013 and 2012 relates to federal refundable credits.
Accounting Standards Codification Topic 740, Income Taxes ("ASC 740") provides for the recognition of deferred tax assets if realization of such assets is more likely than not. Based upon the weight of available evidence, which includes our historical operating performance and carry-back potential, we have determined that total deferred tax assets should be fully offset by a valuation allowance.
We have recorded cumulative gross deferred tax assets of $190.1 million and $160.1 million at December 31, 2014 and 2013, respectively, principally attributable to the timing of the deduction of certain expenses associated with certain research and development expenses, net operating loss and other carry-forwards. We also recorded corresponding valuation allowances of $190.1 million and $160.1 million at December 31, 2014 and 2013, respectively, to offset these deferred tax assets, as management cannot predict with reasonable certainty that the deferred tax assets to which the valuation allowances relate will be realized.
As of December 31, 2014, we had federal net operating loss carry-forwards (“NOLs”) of approximately $292.3 million and state net operating loss carry-forwards of approximately $132.3 million to offset future taxable income. We also had federal research and development tax credit carry-forwards of approximately $1.5 million and state research and development tax credit carry-forwards of approximately $18.1 million.
Based on an analysis under Section 382 of the Internal Revenue Code (which subjects the amount of pre-change NOLs and certain other pre-change tax attributes that can be utilized to an annual limitation), we experienced ownership changes in 2009 and 2012 which substantially limit the future use of our pre-change NOLs and certain other pre-change tax attributes per year. We have excluded the NOLs and R&D credits that will expire as a result of the annual limitations in the deferred tax assets as of December 31, 2014. To the extent that we do not utilize our carry-forwards within the applicable statutory carry-forward periods, either because of Section 382 limitations or the lack of sufficient taxable income, the carry-forwards will expire unused.
We do not expect the unrecognized tax benefits to change significantly over the next twelve months. We will recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. As of December 31, 2014, we have not accrued interest or penalties related to uncertain tax positions.
Liquidity and Capital Resources
The following table summarizes our cash, cash equivalents and short-term investments, our working capital and our cash flow activities as of the end of, and for each of, the periods presented (in thousands):
|
December 31,
|
2013-2014
|
|||||||||||
|
2014
|
2013
|
Change
|
||||||||||
|
Cash and cash equivalents
|
$
|
78,445
|
$
|
101,659
|
$
|
(23,214
|
)
|
|||||
|
Short-term investments
|
$
|
-
|
$
|
19,990
|
$
|
(19,990
|
)
|
|||||
|
Working Capital
|
$
|
47,367
|
$
|
97,415
|
$
|
(50,048
|
)
|
|||||
|
Year ended December 31,
|
2013-2014
|
2012-2013
|
||||||||||||||||||
|
2014
|
2013
|
2012
|
Change
|
Change
|
||||||||||||||||
|
Net cash used in operating activities
|
$
|
(78,282
|
)
|
$
|
(45,915
|
)
|
$
|
(40,765
|
)
|
$
|
(32,367
|
)
|
$
|
(5,150
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
19,675
|
18,840
|
(42,016
|
)
|
835
|
60,856
|
||||||||||||||
|
Net cash provided by financing activities
|
35,560
|
83,389
|
79,782
|
(47,829
|
)
|
3,607
|
||||||||||||||
|
Effect of exchange rate changes on cash
|
(167
|
)
|
-
|
-
|
(167
|
)
|
-
|
|||||||||||||
|
Net (decrease) increase in cash and cash equivalents
|
$
|
(23,214
|
)
|
$
|
56,314
|
$
|
(2,999
|
)
|
$
|
(79,528
|
)
|
$
|
59,313
|
|||||||
Working Capital
The decrease in working capital in 2014 as compared to 2013 was primarily due to XOMA’s gevokizumab clinical development programs and preclinical development activities related to the XMet platform. Also, contributing to the decrease was the reclassification from long-term to short-term liabilities of $16.3 million of principal payment related to our loans with GECC and Novartis, which are due to mature in June 2015. The increase is partially offset by the completion of an equity offering in 2014 contributing to a net $37.7 million increase in cash and cash equivalents.
On February 27, 2015, XOMA entered into the Hercules Loan Agreement, under which we borrowed $20.0 million. We used a portion of the proceeds received under the Hercules Loan Agreement to repay GECC’s outstanding balance and interest of $5.5 million. Refer to the Subsequent Events section below for further information regarding the Hercules Loan Agreement.
Cash Used in Operating Activities
The increase in net cash used in operating activities in 2014 as compared to 2013 was primarily due an increase in research and development spending primarily related to gevokizumab clinical development programs and an increase in salaries and related personnel expenses primarily related to an increase in headcount.
The increase in net cash used in operating activities in 2013 as compared to 2012 was primarily due to an increase in research and development spending relating to external manufacturing costs and internal proprietary projects.
We expect net cash used in operating activities in 2015 to decrease compared to 2014 levels due to projected spending levels and anticipated licensing activities.
Cash Used in Investing Activities
Cash provided by investing activities for the year ended December 31, 2014, consisted of $20.0 million in proceeds from maturities of investments, partially offset by $0.3 million in purchases of fixed assets. Cash provided by investing activities for the year ended December 31, 2013, consisted of $40.0 million in proceeds from maturities of short-term investments, partially offset by purchases of short-term investments of $20.0 million and fixed asset purchases of $1.2 million. Cash used in investing activities for the year ended December 31, 2012, consisted of purchases of short-term investments of $57.0 million and fixed asset purchases of $2.5 million, partially offset by $17.0 million in proceeds from maturities of short-term investments and $0.5 million in proceeds from the sale of fixed assets.
Cash Provided by Financing Activities
Net cash provided by financing activities for the year ended December 31, 2014, was primarily related to net proceeds received from the issuance of common stock of $37.7 million, net of offering expenses, from the December 2014 registered direct offering, and $3.7 million from employee stock purchases. These net proceeds were partially offset by $5.9 million of principal payments on our loans with GECC and Novartis. Refer to the Subsequent Events section below for information regarding debt financing activity in February 2015.
Net cash provided by financing activities for the year ended December 31, 2013, was primarily related to net proceeds received from the issuance of common stock of $29.4 million from the August 2013 public offering, $53.6 million from the December 2013 public offering, $2.2 million of net proceeds from the exercise of warrants, and $1.4 million of net proceeds received from employee stock purchases. These net proceeds were partially offset by $3.1 million of principal payments on our loan with GECC.
Net cash provided by financing activities for the year ended December 31, 2012, was primarily related to net proceeds received from the issuance of common stock of $77.5 million, including net proceeds of $36.2 million from the March 2012 underwritten public offering, net proceeds of $37.0 million from the October 2012 underwritten public offering, net proceeds of $3.2 million received from the issuance of common stock under the 2011 ATM Agreement, net proceeds of $1.0 million from the exercise of warrants issued as part of the March 2012 underwritten public offering, and net proceeds of $0.2 million from the exercise of outstanding options. Also contributing to net cash provided by financing activities was net loan proceeds of $4.4 million received from GECC, partially offset by $2.1 million principal payments on our loan with GECC.
Registered Direct Offerings
In June of 2009, we entered into a definitive agreement with certain institutional investors to sell 695,652 units, with each unit consisting of one share of our common stock and a warrant to purchase 0.50 of a share of our common stock, for gross proceeds of approximately $12.0 million, before deducting placement agent fees and estimated offering expenses of $0.8 million, in a second registered direct offering. The investor purchased the units at a price of $17.25 per unit. The warrants, which represent the right to acquire an aggregate of up to 347,826 shares of common stock, are exercisable at any time on or prior to December 10, 2014 at an exercise price of $19.50 per share. As of December 31, 2014 these warrants were expired.
On December 8, 2014, we completed a registered direct offering of 8,097,165 shares of our common stock, and accompanying warrants to purchase up to 8,097,165 shares of our common stock at a public offering price of $4.94 per share to certain institutional investors. Total gross proceeds from the offering were approximately $40.0 million, before deducting underwriting discounts and commissions and offering expenses totaling approximately $2.3 million. The warrants are exercisable immediately and have a two-year term and an exercise price of $7.90 per share. As of December 31, 2014, all of these warrants were outstanding.
Underwritten Offerings
In February 2010, we completed an underwritten offering of 2.8 million units, with each unit consisting of one share of our common stock and a warrant to purchase 0.45 of a share of our common stock, for gross proceeds of approximately $21.0 million, before deducting underwriting discounts and commissions and estimated offering expenses of $1.7 million. The warrants, which represent the right to acquire an aggregate of up to 1.26 million shares of our common stock, are exercisable beginning six months and one day after issuance and have a five-year term and an exercise price of $10.50 per share. As of December 31, 2014, all of these warrants were outstanding.
On March 9, 2012, we completed an underwritten public offering of 29,669,154 shares of our common stock, and accompanying warrants to purchase one half of a share of common stock for each share purchased, at a public offering price of $1.32 per share. Total gross proceeds from the offering were approximately $39.2 million, before deducting underwriting discounts and commissions and offering expenses totaling approximately $3.0 million. The warrants, which represent the right to acquire an aggregate of up to 14,834,577 shares of common stock, are exercisable immediately and have a five-year term and an exercise price of $1.76 per share. As of December 31, 2014, 12,109,418 of these warrants were outstanding.
On October 29, 2012, we completed an underwritten public offering of 13,333,333 shares of our common stock, at a public offering price of $3.00 per share. Total gross proceeds from the offering were approximately $40.0 million, before deducting underwriting discounts and commissions and offering expenses totaling approximately $3.0 million.
On August 23, 2013, we completed an underwritten public offering of 8,736,187 shares of our common stock, including 1,139,502 shares of our common stock that were issued upon the exercise of the underwriters’ 30-day over-allotment option, at a public offering price of $3.62 per share. Total gross proceeds from the offering were approximately $31.6 million, before deducting underwriting discounts and commissions and estimated offering expenses totaling approximately $2.2 million.
On December 18, 2013, we completed an underwritten public offering of 10,925,000 shares of our common stock, including 1,425,000 shares of our common stock that were issued upon the exercise of the underwriters’ 30-day over-allotment option, at a public offering price of $5.25 per share. Total gross proceeds from the offering were approximately $57.4 million, before deducting underwriting discounts and commissions and estimated offering expenses totaling approximately $3.8 million.
ATM Agreements
On February 4, 2011, we entered into an At Market Issuance Sales Agreement (the “2011 ATM Agreement”), with McNicoll, Lewis & Vlak LLC (now known as MLV & Co. LLC, “MLV”), under which we may sell shares of our common stock from time to time through MLV, as our agent for the offer and sale of the shares, in an aggregate amount not to exceed the amount that can be sold under our registration statement on Form S-3 (File No. 333-172197) filed with the SEC on February 11, 2011, and amended on March 10, 2011, June 3, 2011, and January 3, 2012, which was most recently declared effective by the SEC on January 17, 2012. MLV may sell the shares by any method permitted by law deemed to be an “at the market” offering as defined in Rule 415 of the Securities Act, including without limitation sales made directly on The NASDAQ Global Market, on any other existing trading market for our common stock or to or through a market maker. MLV also may sell the shares in privately negotiated transactions, subject to our prior approval. We will pay MLV a commission equal to 3% of the gross proceeds of the sales price of all shares sold through it as sales agent under the 2011 ATM Agreement. From the inception of the 2011 ATM Agreement through December 31, 2013, we sold a total of 7,572,327 shares of common stock under this agreement for aggregate gross proceeds of $14.6 million. No shares of common stock have been sold under this agreement since February 3, 2012. Total offering expenses incurred related to sales under the 2011 ATM Agreement from inception to December 31, 2013, were $0.5 million. The registration statement under which the 2011 ATM was entered expired in June of 2014, and as of December 31, 2014, the 2011 ATM agreement is expired.
Servier Loan
In December 2010, we entered into a loan agreement with Servier (the “Servier Loan Agreement”), which provided for an advance of up to €15.0 million. The loan was fully funded in January 2011, with the proceeds converting to approximately $19.5 million at the date of funding. The loan is secured by an interest in XOMA’s intellectual property rights to all gevokizumab indications worldwide, excluding certain rights in the U.S. and Japan. Interest is calculated at a floating rate based on a Euro Inter-Bank Offered Rate (“EURIBOR”) and is subject to a cap. The interest rate is reset semi-annually in January and July of each year. The interest rate for the initial interest period was 3.22% and was reset semi-annually ranging from 2.31% to 3.83%. Interest for the six-month period from mid-January 2015 through mid-July 2015 was reset to 2.16%. Interest is payable semi-annually; however, the Servier Loan Agreement provides for a deferral of interest payments over a period specified in the agreement. During the deferral period, accrued interest will be added to the outstanding principal amount for the purpose of interest calculation for the next six-month interest period. On the repayment commencement date, all unpaid and accrued interest shall be paid to Servier, and thereafter, all accrued and unpaid interest shall be due and payable at the end of each six-month period. In January 2015, we paid $0.2 million in accrued interest to Servier. The loan matures in 2016; however, after a specified period prior to final maturity, the loan is to be repaid (i) at Servier's option, by applying up to a significant percentage of any milestone or royalty payments owed by Servier under our collaboration agreement and (ii) using a significant percentage of any upfront, milestone or royalty payments we receive from any third-party collaboration or development partner for rights to gevokizumab in the U.S. and/or Japan. In addition, the loan becomes immediately due and payable upon certain customary events of default. At December 31, 2014, the outstanding principal balance under this loan was $18.2 million using the December 31, 2014 Euro to U.S. Dollar exchange rate of 1.216. On January 9, 2015, Servier and we entered into Amendment No. 2 (“Loan Amendment”), to the Servier Loan Agreement initially entered into on December 30, 2010, and subsequently amended by a Consent, Transfer, Assumption and Amendment Agreement entered into as of August 12, 2013. Refer to the Subsequent Events section below for further information regarding Amendment No. 2 to the Servier Loan Agreement.
GECC Term Loan
In December 2011, we entered into a loan agreement (the “GECC Loan Agreement”) with GECC, under which GECC agreed to make a term loan in an aggregate principal amount of $10 million (the “Term Loan”) to us, and upon execution of the GECC Loan Agreement, GECC funded the Term Loan. As security for our obligations under the GECC Loan Agreement, we granted a security interest in substantially all of our existing and after-acquired assets, excluding our intellectual property assets (such as those relating to our gevokizumab and anti-botulism products). The Term Loan accrued interest at a fixed rate of 11.71% per annum and was to be repaid over a period of 42 consecutive equal monthly installments of principal and accrued interest and was due and payable in full on June 15, 2015. We incurred debt issuance costs of approximately $1.3 million in connection with the Term Loan and were required to pay a final payment fee equal to $500,000 on the maturity date, or such earlier date as the Term Loan is paid in full.
In connection with the GECC Loan Agreement, we issued to GECC unregistered warrants that entitle GECC to purchase up to an aggregate of 263,158 unregistered shares of XOMA common stock at an exercise price equal to $1.14 per share. These warrants were exercisable immediately upon issuance and have a five-year term.
In September 2012, we entered into an amendment to the GECC Loan Agreement providing for an additional term loan in the amount of $4.6 million, increasing the term loan obligation to $12.5 million (the “Amended Term Loan”) and providing for an interest-only monthly repayment period following the effective date of the amendment through March 1, 2013, at a stated interest rate of 10.9% per annum. Thereafter, we are obligated to make monthly principal payments of $347,222, plus accrued interest, over a 27-month period commencing on April 1, 2013, and through June 15, 2015, at which time the remaining outstanding principal amount of $3.1 million, plus accrued interest, is due. We incurred debt issuance costs of approximately $0.2 million and are required to make a final payment fee in the amount of $875,000 on the date upon which the outstanding principal amount is required to be repaid in full. This final payment fee replaced the original final payment fee of $500,000.
In connection with the amendment, on September 27, 2012, we issued to GECC unregistered stock purchase warrants, which entitle GECC to purchase up to an aggregate of 39,346 shares of XOMA common stock at an exercise price equal to $3.54 per share. These warrants were exercisable immediately upon issuance and have a five-year term.
At December 31, 2014, the outstanding principal balance under the Amended Term Loan was $5.2 million. On February 27, 2015, XOMA entered into the Hercules Loan Agreement, under which we borrowed $20.0 million. We used a portion of the proceeds to repay GECC’s outstanding balance and interest of $5.5 million. Refer to the Subsequent Event section below for further information regarding the Hercules Loan Agreement.
Proceeds received during the years 2014, 2013, and 2012 are being used to continue development of our gevokizumab and XMet product candidate and for other working capital and general corporate purposes.
* * *
We have incurred operating losses since inception and have an accumulated deficit of $1.1 billion at December 31, 2014. Management expects operating losses and negative cash flows to continue for the foreseeable future. As of December 31, 2014, we had $78.4 million in cash and cash equivalents, which is available to fund future operations. Taking into account the repayment of our outstanding debt classified within current liabilities on our Consolidated Balance Sheet as of December 31, 2014, we anticipate that we will be required to seek additional equity or debt financing or to increase the level of collaborative revenue to fund operations through at least December 31, 2015. If we are unable to achieve the level of revenues from licensing, development and collaboration agreements and the level of government funding and external financing during 2015, as contemplated in our operating plan, we have plans to implement certain cost cutting actions commencing early in the fourth quarter of 2015 to reduce our working capital requirements. Consistent with the actions we have taken in the past, we will prioritize necessary and appropriate steps to enable the continued operations of the business and preservation of the value of our assets beyond the next twelve months, including but not limited to actions, such as reduced personnel-related costs, additional curtailment of our development activities and other discretionary expenditures that are within our control. These reductions in expenditures, if required, may have an adverse impact on our ability to achieve certain of our planned objectives during 2015. In addition to seeking equity or debt financing, we may seek to access additional capital to support future operations through licensing, partnering or other strategic collaborative arrangements. It is unclear if or when any such transactions will occur, on satisfactory terms or at all.
Our ability to raise additional capital in the equity and debt markets, should we choose to do so, is dependent on a number of factors, including, but not limited to, the market demand for our common stock, which itself is subject to a number of pharmaceutical development and business risks and uncertainties, as well as the uncertainty that we would be able to raise such additional capital at a price or on terms that are favorable to us.
Commitments and Contingencies
Schedule of Contractual Obligations
Payments by period due under contractual obligations at December 31, 2014, are as follows (in thousands):
|
Contractual Obligations
|
Total
|
Less than 1
year
|
1 to 3 years
|
3 to 5 years
|
More than 5
years
|
|||||||||||||||
|
Operating leases(1)
|
$
|
28,923
|
$
|
3,428
|
$
|
7,167
|
$
|
7,594
|
$
|
10,733
|
||||||||||
|
Debt Obligations(2)
|
||||||||||||||||||||
|
Principal
|
36,797
|
18,565
|
18,232
|
-
|
-
|
|||||||||||||||
|
Interest
|
1,926
|
1,711
|
215
|
-
|
-
|
|||||||||||||||
|
Total
|
$
|
67,646
|
$
|
23,704
|
$
|
25,614
|
$
|
7,594
|
$
|
10,733
|
||||||||||
(1) See Note 11: Commitment and Contingencies to the accompanying consolidated financial statements for further discussion.
(2) See Item 7A: Quantitative and Qualitative Disclosures about Market Risk and Note 7: Long-Term Debt and Other Arrangements to the accompanying consolidated financial statements for further discussion of our debt obligation. Refer to Management’s Discussion and Analysis of Financial Condition and Results of Operations for further information regarding the Hercules Loan Agreement.
We lease administrative, research facilities and office equipment under operating leases expiring on various dates through April 2023. These leases require us to pay taxes, insurance, maintenance and minimum lease payments. In addition to the above, we have committed to make potential future “milestone” payments to third parties as part of licensing and development programs. Payments under these agreements become due and payable only upon the achievement of certain developmental, regulatory and/or commercial milestones. Because it is uncertain if and when these milestones will be achieved, such contingencies, aggregating up to $77.3 million (assuming one product per contract meets all milestones) have not been recorded on our consolidated balance sheet as of December 31, 2014. We are also obligated to pay royalties, ranging generally from 0.5% to 5% of the selling price of the licensed component and up to 40% of any sublicense fees to various universities and other research institutions based on future sales or licensing of products that incorporate certain products and technologies developed by those institutions. We are unable to determine precisely when and if our payment obligations under the agreements will become due as these obligations are based on future events, the achievement of which is subject to a significant number of risks and uncertainties.
Although operations are influenced by general economic conditions, we do not believe inflation had a material impact on financial results for the periods presented. We believe that we are not dependent on materials or other resources that would be significantly impacted by inflation or changing economic conditions in the foreseeable future.
Off Balance Sheet Arrangements
We do not have any off balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated by the SEC.
Subsequent Events
On January 9, 2015, Servier and we entered into Amendment No. 2 (“Loan Amendment”), to the Servier Loan Agreement initially entered into on December 30, 2010, and subsequently amended by a Consent, Transfer, Assumption and Amendment Agreement entered into as of August 12, 2013. The Loan Amendment modifies the maturity date of the loan from January 13, 2016 to three tranches due on January 15, 2016, January 15, 2017 and January 15, 2018 and provides that principal shall be repaid as follows: €3.0 million to be repaid on January 15, 2016, €5.0 million to be repaid on January 15, 2017 and €7.0 million to be repaid on January 15, 2018. All other terms of the Loan Agreement remain unchanged, including the interest rate calculations, EURIBOR+2% and the formula for resetting the interest rate on the 15th of January and July each year.
On January 9, 2015, Servier and we entered into Amendment No. 2 to the Collaboration Agreement. Under the Collaboration Agreement, we are eligible to receive up to approximately $433 million in the aggregate in milestone payments, most of which were denominated in Euros, if XOMA re-acquires cardiovascular and/or diabetes rights for use in the United States, and approximately $770 million in aggregate milestone payments if we do not re-acquire those rights. Under the Collaboration Amendment, we would be eligible to receive up to $415 million in the aggregate in milestone payments in the event we re-acquire the cardiovascular and/or diabetes rights for use in the United States and approximately $752 million if we do not re-acquire those rights. The milestone reductions are related to a very low prevalence indication of which Servier would not have pursued development had these payments been required. All other terms of the Collaboration Agreement remain unchanged.
On January 26, 2015, Symplmed Pharmaceuticals announced that the FDA approved PRESTALIA® (perindopril arginine and amlodipine) tablets, licensed from Servier, for the treatment of hypertension. In July 2013, we transferred the development and commercialization rights of Prestalia to Symplmed. Pursuant to the transfer agreement with Symplmed, we will receive up to double-digit in royalties on sales of PRESTALIA.
On February 26, 2015, Fred Kurland, Vice President, Finance, Chief Financial Officer and Secretary of XOMA, who serves as the Company’s principal financial and accounting officer, notified the Board of Directors of XOMA of his retirement from XOMA, effective as of April 3, 2015. Mr. Kurland qualifies as “retirement eligible” under our equity incentive plan and will, therefore, be eligible to hold the awards of options and restricted stock units for the remainder of the life of each award granted to him by us. On February 26, 2015, XOMA’s Board of Directors appointed Thomas Burns, currently Vice President, Finance of the Company as our Chief Financial Officer, effective immediately upon Mr. Kurland’s retirement from XOMA. As Chief Financial Officer, Mr. Burns will serve as the XOMA’s principal financial and accounting officer.
On February 27, 2015, Hercules and we entered into a Loan and Security Agreement (the “Hercules Loan Agreement”), under which we borrowed $20.0 million. We used a portion of the proceeds under the Hercules Loan Agreement to repay GECC’s outstanding principle balance and interest of $5.5 million and we plan to use the remaining proceeds for general corporate purposes. The interest rate will be calculated at a rate equal to the greater of either (i) 9.40% plus the prime rate as reported from time to time in The Wall Street Journal minus 7.25%, and (ii) 9.40%. Payments under the Hercules Loan Agreement are interest only until one month prior to the Amortization Date, defined as July 1, 2016 (which will be extended to October 1, 2016, if we achieve certain clinical milestones on or before July 1, 2016). The interest only period will be followed by equal monthly payments of principal and interest amortized over a 30-month schedule through the scheduled maturity date of September 1, 2018 (the “Loan Maturity Date”). The entire principal balance, including a balloon payment of principal, as applicable, will be due and payable on the Loan Maturity Date. In addition, a final payment equal to $1,150,000 will be due on the Loan Maturity Date, or such earlier date specified in the Hercules Loan Agreement. Our obligations under the Hercules Loan Agreement are secured by a security interest in substantially all of our assets, other than our intellectual property. If we prepay the loan prior to the Loan Maturity Date, we will pay Hercules a prepayment charge based on a prepayment fee equal to 3.00% of the amount prepaid, if the prepayment occurs in any of the first 12 months following the Closing Date, 2.00% of the amount prepaid if the prepayment occurs after 12 months from the Closing Date but prior to 24 months from the Closing Date, and 1.00% of the amount prepaid if the prepayment occurs after 24 months from the Closing Date. The Hercules Loan Agreement includes customary affirmative and restrictive covenants, but does not include any financial maintenance covenants, and also includes standard events of default, including payment defaults. Upon the occurrence of an event of default, a default interest rate of an additional 5% may be applied to the outstanding loan balances, and Hercules may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Hercules Loan Agreement.
In connection with the Hercules Loan Agreement, we issued a warrant to Hercules which is exercisable in whole or in part for an aggregate of up to 181,268 shares of common stock with an exercise price of $3.31 per share (the “Warrant”). The Warrant may be exercised on a cashless basis and is exercisable for a term beginning on the date of issuance and ending on the earlier to occur of five years from the date of issuance or the consummation of certain acquisitions of us as set forth in the Warrant. The number of shares for which the Warrant is exercisable and the associated exercise price are subject to certain proportional adjustments as set forth in the Warrant.
Interest Rate Risk
Our exposure to market rate risk for changes in interest rates relates primarily to our investment portfolio and our loan facilities. By policy, we make our investments in high-quality debt securities, limit the amount of credit exposure to any one non-U.S. Treasury issuer, and limit duration by restricting the term of the instrument. We generally hold investments to maturity, with a weighted average portfolio period of less than twelve months. However, if the need arose to liquidate such securities before maturity, we may experience losses on liquidation.
We hold interest-bearing instruments that are classified as cash, cash equivalents and short-term investments. Fluctuations in interest rates can affect the principal values and yields of fixed income investments. If interest rates in the general economy were to rise rapidly in a short period of time, our fixed income investments could lose value.
The following table presents the amounts and related weighted average interest rates of our cash, cash equivalents, and short-term investments at December 31, 2014 and 2013 (in thousands, except interest rate):
|
|
Maturity
|
Carrying
Amount
(in thousands) |
Fair Value
(in thousands) |
Weighted
Average Interest Rate |
||||||||||||
|
December 31, 2014
|
||||||||||||||||
|
Cash and cash equivalents
|
Daily to 90 days
|
$
|
78,445
|
$
|
78,445
|
0.07
|
%
|
|||||||||
|
December 31, 2013
|
||||||||||||||||
|
Cash, cash equivalents, and short-term investments
|
Daily to 90 days
|
$
|
121,649
|
$
|
121,649
|
0.08
|
%
|
|||||||||
As of December 31, 2014, we have an outstanding principal balance on our note with Novartis of $13.4 million, which is due in 2015. The interest rate on this note is charged at a rate of USD six-month London Interbank Offered Rate (“LIBOR”) plus 2%, which was 2.35% at December 31, 2014. No further borrowing is available under this note.
As of December 31, 2014, we have an outstanding principal balance on our loan with Servier of €15.0 million, which converts to approximately $18.2 million at December 31, 2014. The interest rate on this loan is charged at a floating rate based on a Euro Inter-Bank Offered Rate (“EURIBOR”) and subject to a cap. The interest rate for the initial interest period was 3.22% and was reset semi-annually ranging from 2.31% to 3.83%. Interest for the six-month period from mid-January 2015 through mid-July 2015 was reset to 2.16%. No further borrowing is available under this loan.
As of December 31, 2014, we have an outstanding principal balance on our loan with GECC of $5.2 million, which is to be repaid with monthly principal payments of $347,222, plus accrued interest, over a 27-month period commencing on April 1, 2013, and through June 15, 2015, at which time the remaining outstanding principal amount of $3.1 million, plus accrued interest, is due. The loan accrues interest at a fixed rate of 10.90% per annum. No further borrowing is available under this loan.
The variable interest rate related to our long-term debt instruments is based on LIBOR for our Novartis note and EURIBOR for our Servier loan. We estimate a hypothetical 100 basis point change in interest rates could increase or decrease our interest expense by approximately $0.3 million on an annualized basis. Our loan with GECC is not subject to interest rate risk as it accrues interest at a fixed rate.
Foreign Currency Risk
We hold debt, incur expenses, and may be owed milestones denominated in foreign currencies. The amount of debt owed, expenses incurred, or milestones owed to us will be impacted by fluctuations in these foreign currencies. When the U.S. Dollar weakens against foreign currencies, the U.S. Dollar value of the foreign-currency denominated debt, expense, and milestones increases, and when the U.S. Dollar strengthens against these currencies, the U.S. dollar value of the foreign-currency denominated debt, expense, and milestones decreases. Consequently, changes in exchange rates will affect the amount we are required to repay on our €15.0 million loan from Servier and may affect our results of operations. We estimate that a hypothetical 0.01 change in the Euro to USD exchange rate could increase or decrease our unrealized gains or losses by approximately $0.2 million.
Our loan from Servier was fully funded in January 2011, with the proceeds converting to approximately $19.5 million using the January 13, 2011 Euro-to-U.S.-Dollar exchange rate of 1.3020. At December 31, 2014, the €15.0 million outstanding principal balance under the Servier Loan Agreement would have equaled approximately $18.2 million using the December 31, 2014 Euro-to-USD exchange rate of 1.216. In May 2011, in order to manage our foreign currency exposure relating to our principal and interest payments on our loan from Servier, we entered into two foreign exchange option contracts to buy €1.5 million and €15.0 million in January 2014 and January 2016, respectively. Upfront premiums paid on these foreign exchange option contracts totaled $1.5 million, as of December 31, 2014, one option contract had expired. The remaining foreign exchange option contract had a fair value of $6,000 at December 31, 2014. Our use of derivative financial instruments represents risk management; we do not enter into derivative financial contracts for trading purposes.
The following consolidated financial statements of the registrant, related notes and report of independent registered public accounting firm are set forth beginning on page F-1 of this report.
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets
|
F-3
|
|
Consolidated Statements of Comprehensive Loss
|
F-4
|
|
Consolidated Statements of Stockholders' Equity (Deficit)
|
F-5
|
|
Consolidated Statements of Cash Flows
|
F-6
|
|
Notes to the Consolidated Financial Statements
|
F-7
|
Not applicable.
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Vice President, Finance, Chief Financial Officer and Secretary, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15the promulgated under the Securities Exchange Act of 1934, as amended, as of the end of the period covered by this report. Our disclosure controls and procedures are intended to ensure that the information we are required to disclose in the reports that we file or submit under the Securities Exchange Act of 1934 is (i) recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and (ii) accumulated and communicated to our management, including the Chief Executive Officer and Vice President, Finance, Chief Financial Officer and Secretary, as the principal executive and financial officers, respectively, to allow timely decisions regarding required disclosures. Based on this evaluation, our Chief Executive Officer and our Vice President, Finance, Chief Financial Officer and Secretary concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report.
Management’s Report on Internal Control over Financial Reporting
Management, including our Chief Executive Officer and our Vice President, Finance, Chief Financial Officer and Secretary, is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Exchange Act Rules 13a-159f). The Company’s internal control system was designed to provide reasonable assurance to the Company’s management and board of directors regarding the preparation and fair presentation of published financial statements in accordance with accounting principles generally accepted in the United States.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2014. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework (2013 Framework). Based on our assessment we believe that, as of December 31, 2014, our internal control over financial reporting is effective based on those criteria.
The Company’s internal control over financial reporting as of December 31, 2014, has been audited by Ernst & Young, LLP, independent registered public accounting firm who also audited the Company’s consolidated financial statements. Ernst & Young’s report on the Company’s internal control over financial reporting follows.
Changes in Internal Control over Financial Reporting
As disclosed in our Quarterly Reports on Form 10-Q/A and 10-Q for the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014, we identified a material weakness in our internal control over financial reporting such that our disclosure controls and procedures related to the calculation and disclosure of diluted earnings (loss) per share as it applies to our March 2012 warrants were not effective. During 2014, we implemented improvements in our internal controls over financial reporting to address the material weakness described above, including performing a more effective quarterly review of our diluted earnings (loss) per share calculation. Our remediation efforts, including the testing of these controls continued throughout 2014. This material weakness was considered remediated in the fourth quarter of 2014, once these controls were shown to be operational for a sufficient period of time to allow management to conclude that these controls were operating effectively.
Except as noted in the preceding paragraph, there were no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information |
None.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of XOMA Corporation:
We have audited XOMA Corporation’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) (the COSO criteria). XOMA Corporation’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, XOMA Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of XOMA Corporation as of December 31, 2014 and 2013 and the related consolidated statements of comprehensive loss, stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2014, and our report dated March 11, 2015 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Francisco, California
March 11, 2015
PART III
Certain information regarding our executive officers required by this Item is set forth as a Supplementary Item at the end of Part I of this Form 10-K (pursuant to Instruction 3 to Item 401(b) of Regulation S-K). Other information required by this Item will be included in the Company’s proxy statement for the 2014 Annual General Meeting of Stockholders (“2015 Proxy Statement”), under the sections labeled “Item 1—Election of Directors” and “Compliance with Section 16(a) of the Securities Exchange Act of 1934”, and is incorporated herein by reference. The 2015 Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year to which this report relates.
Code of Ethics
The Company’s Code of Ethics applies to all employees, officers and directors including the Chief Executive Officer (principal executive officer) and the Vice President, Finance, Chief Financial Officer and Secretary (principal financial and principal accounting officer) and is posted on the Company’s website at www.xoma.com. We intend to satisfy the applicable disclosure requirements regarding amendments to, or waivers from, provisions of our Code of Ethics by posting such information on our website.
Information required by this Item will be included in the sections labeled “Compensation of Executive Officers”, “Summary Compensation Table”, “Grants of Plan-Based Awards”, “Outstanding Equity Awards as of December 31, 2014”, “Option Exercises and Shares Vested”, “Pension Benefits”, “Non-Qualified Deferred Compensation” and “Compensation of Directors” appearing in our 2015 Proxy Statement, and is incorporated herein by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Information required by this Item will be included in the sections labeled “Stock Ownership” and “Equity Compensation Plan Information” appearing in our 2015 Proxy Statement, and is incorporated herein by reference.
Information required by this Item will be included in the section labeled “Transactions with Related Persons” appearing in our 2015 Proxy Statement, and is incorporated herein by reference.
Information required by this Item will be included in the section labeled “Item 2—Appointment of Independent Registered Public Accounting Firm” appearing in our 2015 Proxy Statement, and is incorporated herein by reference.
PART IV
| (a) | The following documents are included as part of this Annual Report on Form 10-K: |
| (1) | Financial Statements: |
All financial statements of the registrant referred to in Item 8 of this Report on Form 10-K.
| (2) | Financial Statement Schedules: |
All financial statements schedules have been omitted because the required information is included in the consolidated financial statements or the notes thereto or is not applicable or required.
| (3) | Exhibits: |
The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on this 11th day of March 2015.
|
XOMA CORPORATION
|
||
|
By:
|
/s/ JOHN VARIAN
|
|
|
John Varian
Chief Executive Officer and Director
|
||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints John Varian and Fred Kurland, and each of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the SEC, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ John Varian
|
|
Chief Executive Officer (Principal Executive Officer) and Director
|
|
March 11, 2015
|
|
(John Varian)
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Fred Kurland
|
|
Vice President, Finance, Chief Financial Officer and Secretary
|
|
March 11, 2015
|
|
(Fred Kurland)
|
|
(Principal Financial and Principal Accounting Officer) |
|
|
|
|
|
|
|
|
|
/s/ Patrick J. Scannon
|
|
Executive Vice President and Chief Scientific Officer and Director
|
|
March 11, 2015
|
|
(Patrick J. Scannon)
|
|
|
|
|
|
|
|
|
|
|
|
/s/ W. Denman Van Ness
|
|
Chairman of the Board of Directors
|
|
March 11, 2015
|
|
(W. Denman Van Ness)
|
|
|
|
|
|
|
|
|
|
|
|
/s/ William K. Bowes, Jr.
|
|
Director
|
|
March 11, 2015
|
|
(William K. Bowes, Jr.)
|
|
|
|
|
|
|
|
|
|
|
|
|
Director
|
|
March 11, 2015
|
|
|
(Peter Barton Hutt)
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Joseph M. Limber
|
|
Director
|
|
March 11, 2015
|
|
(Joseph M. Limber)
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Kelvin M. Neu
|
|
Director
|
|
March 11, 2015
|
|
(Kelvin M. Neu)
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Timothy P. Walbert
|
|
Director
|
|
March 11, 2015
|
|
(Timothy P. Walbert)
|
|
|
|
|
|
|
|
|
|
|
|
|
Director
|
|
March 11, 2015
|
|
|
(Jack L. Wyszomierski)
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets
|
F-3
|
|
Consolidated Statements of Comprehensive Loss
|
F-4
|
|
Consolidated Statements of Stockholders' Equity (Deficit)
|
F-5
|
|
Consolidated Statements of Cash Flows
|
F-6
|
|
Notes to the Consolidated Financial Statements
|
F-7
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of XOMA Corporation:
We have audited the accompanying consolidated balance sheets of XOMA Corporation as of December 31, 2014 and 2013, and the related consolidated statements of comprehensive loss, stockholders' equity (deficit), and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of XOMA Corporation’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of XOMA Corporation at December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), XOMA Corporation’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) and our report dated March 11, 2015 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Francisco, California
March 11, 2015
XOMA Corporation
CONSOLIDATED BALANCE SHEETS
(in thousands, except share data)
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
78,445
|
$
|
101,659
|
||||
|
Short-term investments
|
-
|
19,990
|
||||||
|
Trade and other receivables, net
|
3,309
|
3,781
|
||||||
|
Prepaid expenses and other current assets
|
2,088
|
1,630
|
||||||
|
Total current assets
|
83,842
|
127,060
|
||||||
|
Property and equipment, net
|
5,120
|
6,456
|
||||||
|
Other assets
|
669
|
1,266
|
||||||
|
Total assets
|
$
|
89,631
|
$
|
134,782
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
5,990
|
$
|
9,616
|
||||
|
Accrued and other liabilities
|
9,892
|
9,934
|
||||||
|
Deferred revenue – current
|
1,089
|
2,218
|
||||||
|
Interest bearing obligations – current
|
19,247
|
5,835
|
||||||
|
Accrued interest on interest bearing obligations – current
|
257
|
2,042
|
||||||
|
Total current liabilities
|
36,475
|
29,645
|
||||||
|
Deferred revenue – long-term
|
1,939
|
4,105
|
||||||
|
Interest bearing obligations – long-term
|
16,290
|
35,150
|
||||||
|
Contingent warrant liabilities
|
31,828
|
69,869
|
||||||
|
Total liabilities
|
86,532
|
138,769
|
||||||
|
Commitments and contingencies (Note 11)
|
||||||||
|
Stockholders’ equity (deficit):
|
||||||||
|
Preferred stock, $0.05 par value, 1,000,000 shares authorized, 0 issued and outstanding
|
-
|
-
|
||||||
|
Common stock, $0.0075 par value, 277,333,332 and 138,666,666 shares authorized at December 31, 2014 and 2013, respectively, 115,892,450 and 105,386,216 shares issued and outstanding at December 31, 2014 and 2013, respectively
|
869
|
787
|
||||||
|
Additional paid-in capital
|
1,121,707
|
1,076,403
|
||||||
|
Accumulated comprehensive loss
|
-
|
(1
|
)
|
|||||
|
Accumulated deficit
|
(1,119,477
|
)
|
(1,081,176
|
)
|
||||
|
Total stockholders’ equity (deficit)
|
3,099
|
(3,987
|
)
|
|||||
|
Total liabilities and stockholders’ equity (deficit)
|
$
|
89,631
|
$
|
134,782
|
||||
The accompanying notes are an integral part of these consolidated financial statements.
XOMA Corporation
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands, except per share amounts)
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Revenues:
|
||||||||||||
|
License and collaborative fees
|
$
|
5,683
|
$
|
11,028
|
$
|
5,727
|
||||||
|
Contract and other
|
13,183
|
24,423
|
28,055
|
|||||||||
|
Total revenues
|
18,866
|
35,451
|
33,782
|
|||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
80,748
|
74,851
|
68,467
|
|||||||||
|
Selling, general and administrative
|
19,866
|
18,477
|
16,865
|
|||||||||
|
Restructuring
|
84
|
328
|
5,074
|
|||||||||
|
Total operating expenses
|
100,698
|
93,656
|
90,406
|
|||||||||
|
Loss from operations
|
(81,832
|
)
|
(58,205
|
)
|
(56,624
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Interest expense
|
(4,303
|
)
|
(4,631
|
)
|
(4,387
|
)
|
||||||
|
Other income (expense), net
|
2,061
|
(197
|
)
|
(956
|
)
|
|||||||
|
Revaluation of contingent warrant liabilities
|
45,773
|
(61,039
|
)
|
(9,172
|
)
|
|||||||
|
Net loss before taxes
|
(38,301
|
)
|
(124,072
|
)
|
(71,139
|
)
|
||||||
|
Benefit from income taxes
|
-
|
14
|
74
|
|||||||||
|
Net loss
|
$
|
(38,301
|
)
|
$
|
(124,058
|
)
|
$
|
(71,065
|
)
|
|||
|
Basic net loss per share of common stock
|
$
|
(0.36
|
)
|
$
|
(1.43
|
)
|
$
|
(1.10
|
)
|
|||
|
Diluted net loss per share of common stock
|
$
|
(0.67
|
)
|
$
|
(1.43
|
)
|
$
|
(1.10
|
)
|
|||
|
Shares used in computing basic net loss per share of common stock
|
107,435
|
86,938
|
64,629
|
|||||||||
|
Shares used in computing diluted net loss per share of common stock
|
115,333
|
86,938
|
64,629
|
|||||||||
|
Other comprehensive loss:
|
||||||||||||
|
Net loss
|
$
|
(38,301
|
)
|
$
|
(124,058
|
)
|
$
|
(71,065
|
)
|
|||
|
Net unrealized gain (loss) on available-for-sale securities
|
1
|
(9
|
)
|
8
|
||||||||
|
Comprehensive loss
|
$
|
(38,300
|
)
|
$
|
(124,067
|
)
|
$
|
(71,057
|
)
|
|||
The accompanying notes are an integral part of these consolidated financial statements.
XOMA Corporation
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands)
|
Common Stock
|
Paid-In
|
Accumulated Comprehensive
|
Accumulated
|
Total Stockholders'
(Deficit) Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Income
|
Deficit
|
||||||||||||||||||||
|
Balance, December 31, 2011
|
35,107
|
$
|
263
|
$
|
900,801
|
$
|
─
|
$
|
(886,053
|
)
|
$
|
15,011
|
||||||||||||
|
Exercise of stock options, contributions to 401(k) and incentive plans
|
1,089
|
8
|
1,323
|
─
|
─
|
1,331
|
||||||||||||||||||
|
Release of restricted stock units
|
397
|
─
|
─
|
─
|
─
|
─
|
||||||||||||||||||
|
Stock-based compensation expense
|
─
|
─
|
4,284
|
─
|
─
|
4,284
|
||||||||||||||||||
|
Sale of shares of common stock
|
45,288
|
340
|
75,960
|
─
|
─
|
76,300
|
||||||||||||||||||
|
Issuance of warrants
|
─
|
─
|
(6,335
|
)
|
─
|
─
|
(6,335
|
)
|
||||||||||||||||
|
Exercise of warrants
|
566
|
4
|
1,929
|
─
|
─
|
1,933
|
||||||||||||||||||
|
Net loss
|
─
|
─
|
─
|
─
|
(71,065
|
)
|
(71,065
|
)
|
||||||||||||||||
|
Other comprehensive income
|
─
|
─
|
─
|
8
|
─
|
8
|
||||||||||||||||||
|
Balance, December 31, 2012
|
82,447
|
615
|
977,962
|
8
|
(957,118
|
)
|
21,467
|
|||||||||||||||||
|
Exercise of stock options, contributions to 401(k) and incentive plans
|
933
|
7
|
2,213
|
─
|
─
|
2,220
|
||||||||||||||||||
|
Release of restricted stock units
|
801
|
6
|
(6
|
)
|
─
|
─
|
─
|
|||||||||||||||||
|
Stock-based compensation expense
|
─
|
─
|
5,099
|
─
|
─
|
5,099
|
||||||||||||||||||
|
Sale of shares of common stock
|
19,661
|
147
|
82,799
|
─
|
─
|
82,946
|
||||||||||||||||||
|
Exercise of warrants
|
1,544
|
12
|
8,336
|
─
|
─
|
8,348
|
||||||||||||||||||
|
Net loss
|
─
|
─
|
─
|
─
|
(124,058
|
)
|
(124,058
|
)
|
||||||||||||||||
|
Other comprehensive loss
|
─
|
─
|
─
|
(9
|
)
|
─
|
(9
|
)
|
||||||||||||||||
|
Balance, December 31, 2013
|
105,386
|
787
|
1,076,403
|
(1
|
)
|
(1,081,176
|
)
|
(3,987
|
)
|
|||||||||||||||
|
Exercise of stock options, contributions to 401(k) and incentive plans
|
1,065
|
11
|
4,515
|
─
|
─
|
4,526
|
||||||||||||||||||
|
Release of restricted stock units
|
981
|
7
|
(7
|
)
|
─
|
─
|
─
|
|||||||||||||||||
|
Stock-based compensation expense
|
─
|
─
|
10,772
|
─
|
─
|
10,772
|
||||||||||||||||||
|
Sale of shares of common stock
|
8,097
|
61
|
37,725
|
─
|
─
|
37,786
|
||||||||||||||||||
|
Issuance of warrants
|
(10,258
|
)
|
(10,258
|
)
|
||||||||||||||||||||
|
Exercise of warrants
|
363
|
3
|
2,557
|
─
|
─
|
2,560
|
||||||||||||||||||
|
Net loss
|
─
|
─
|
─
|
─
|
(38,301
|
)
|
(38,301
|
)
|
||||||||||||||||
|
Other comprehensive income
|
─
|
─
|
─
|
1
|
─
|
1
|
||||||||||||||||||
|
Balance, December 31, 2014
|
115,892
|
$
|
869
|
$
|
1,121,707
|
$
|
-
|
$
|
(1,119,477
|
)
|
$
|
3,099
|
||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
XOMA Corporation
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$
|
(38,301
|
)
|
$
|
(124,058
|
)
|
$
|
(71,065
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation
|
1,856
|
2,575
|
4,124
|
|||||||||
|
Common stock contribution to 401(k)
|
870
|
828
|
1,134
|
|||||||||
|
Stock-based compensation expense
|
10,772
|
5,099
|
4,284
|
|||||||||
|
Accrued interest on interest bearing obligations
|
(1,444
|
)
|
2,284
|
1,186
|
||||||||
|
Revaluation of contingent warrant liabilities
|
(45,773
|
)
|
61,039
|
9,172
|
||||||||
|
Restructuring charge related to long-lived assets
|
-
|
-
|
2,460
|
|||||||||
|
Amortization of debt discount, final payment fee on debt, and debt issuance costs
|
2,707
|
2,470
|
1,958
|
|||||||||
|
Loss on sale and retirement of property and equipment
|
-
|
281
|
29
|
|||||||||
|
Unrealized loss on foreign currency exchange
|
(2,280
|
)
|
662
|
295
|
||||||||
|
Unrealized loss on foreign exchange options
|
355
|
127
|
714
|
|||||||||
|
Other non-cash adjustments
|
(9
|
)
|
(20
|
)
|
(11
|
)
|
||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Trade and other receivables, net
|
472
|
4,486
|
4,064
|
|||||||||
|
Prepaid expenses and other assets
|
(662
|
)
|
481
|
(158
|
)
|
|||||||
|
Accounts payable and accrued liabilities
|
(3,774
|
)
|
2,901
|
4,485
|
||||||||
|
Deferred revenue
|
(2,983
|
)
|
(3,399
|
)
|
(3,511
|
)
|
||||||
|
Other liabilities
|
(88
|
)
|
(1,671
|
)
|
75
|
|||||||
|
Net cash used in operating activities
|
(78,282
|
)
|
(45,915
|
)
|
(40,765
|
)
|
||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchase of investments
|
-
|
(19,991
|
)
|
(56,970
|
)
|
|||||||
|
Proceeds from maturities of investments
|
20,000
|
40,000
|
17,000
|
|||||||||
|
Purchase of property and equipment
|
(325
|
)
|
(1,169
|
)
|
(2,509
|
)
|
||||||
|
Proceeds from sale of property and equipment
|
-
|
-
|
463
|
|||||||||
|
Net cash provided by (used in) investing activities
|
19,675
|
18,840
|
(42,016
|
)
|
||||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Proceeds from issuance of common stock, net of issuance costs
|
41,442
|
84,338
|
76,498
|
|||||||||
|
Proceeds from exercise of warrants
|
35
|
2,176
|
993
|
|||||||||
|
Proceeds from issuance of long-term debt, net of issuance costs
|
-
|
-
|
4,434
|
|||||||||
|
Principal payments of debt
|
(5,917
|
)
|
(3,125
|
)
|
(2,143
|
)
|
||||||
|
Net cash provided by financing activities
|
35,560
|
83,389
|
79,782
|
|||||||||
|
Effect of exchange rate changes on cash
|
(167
|
)
|
-
|
-
|
||||||||
|
Net (decrease) increase in cash and cash equivalents
|
(23,214
|
)
|
56,314
|
(2,999
|
)
|
|||||||
|
Cash and cash equivalents at the beginning of the year
|
101,659
|
45,345
|
48,344
|
|||||||||
|
Cash and cash equivalents at the end of the year
|
$
|
78,445
|
$
|
101,659
|
$
|
45,345
|
||||||
|
Supplemental Cash Flow Information:
|
||||||||||||
|
Cash paid during the year for:
|
||||||||||||
|
Interest
|
$
|
3,009
|
$
|
1,262
|
$
|
1,035
|
||||||
|
Non-cash investing and financing activities:
|
||||||||||||
|
Issuance of warrants
|
$
|
10,258
|
$
|
-
|
$
|
6,390
|
||||||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
$
|
(2,526
|
)
|
$
|
(6,171
|
)
|
$
|
(940
|
)
|
|||
|
Interest added to principal balances on long-term debt
|
$
|
313
|
$
|
935
|
$
|
1,160
|
||||||
|
Investment in Symplmed Pharmaceuticals, LLC
|
$
|
-
|
$
|
171
|
$
|
-
|
||||||
|
Discount on long-term debt
|
$
|
-
|
$
|
-
|
$
|
(55
|
)
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Description of Business |
XOMA Corporation (“XOMA” or the “Company”), a Delaware corporation combines a portfolio of late-stage clinical programs and research activities to develop innovative therapeutic antibodies for which it intends to commercialize. XOMA focuses its scientific research on allosteric modulation, which offers opportunities for new classes of therapeutic antibodies to treat a wide range of human diseases. XOMA is developing its lead product candidate gevokizumab (IL-1 beta modulating antibody) with Les Laboratoires Servier (“Servier”) through a global Phase 3 clinical development program and ongoing proof-of-concept studies in other IL-1-mediated diseases. XOMA’s scientific research also has produced the XMet platform, which consists of three classes of preclinical antibodies, including selective insulin receptor modulators that could offer new approaches in the treatment of diabetes. The Company’s products are presently in various stages of development and most are subject to regulatory approval before they can be commercially launched.
Liquidity and Management Plans
The Company has incurred operating losses since its inception and had an accumulated deficit of $1.1 billion at December 31, 2014. Management expects operating losses and negative cash flows to continue for the foreseeable future. As of December 31, 2014, the Company had $78.4 million in cash and cash equivalents, which is available to fund future operations. Taking into account the repayment of its outstanding debt classified within current liabilities on the Company’s Consolidated Balance Sheet as of December 31, 2014, the Company anticipates that it will be required to seek additional equity or debt financing or to increase the level of collaborative revenues to fund its operations through December 31, 2015. If the Company is unable to achieve the level of revenues from licensing, development and collaboration agreement and the level of government funding and external financing during 2015, as contemplated in its operating plan, the Company has plans to implement certain cost cutting actions commencing early in the fourth quarter of 2015 to reduce its working capital requirements. Consistent with the actions the Company has taken in the past, it will prioritize necessary and appropriate steps to enable the continued operations of the business and preservation of the value of its assets beyond the next twelve months, including but not limited to actions such as reduced personnel-related costs, additional curtailment of the Company’s development activities and other discretionary expenditures that are within the Company’s control. These reductions in expenditures, if required, may have an adverse impact on the Company’s ability to achieve certain of its planned objectives during 2015. In addition to seeking equity or debt financing, the Company may seek to access additional capital to support future operations through licensing, partnering or other strategic collaborative arrangements. It is unclear if or when any such transactions will occur, on satisfactory terms or at all. The Company’s ability to raise additional capital in the equity and debt markets, should the Company choose to do so, is dependent on a number of factors, including, but not limited to, the market demand for the Company’s common stock, which itself is subject to a number of pharmaceutical development and business risks and uncertainties, as well as the uncertainty that the Company would be able to raise such additional capital at a price or on terms that are favorable to the Company.
| 2. | Basis of Presentation and Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions among the entities have been eliminated from consolidated financial statements.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosures. On an on-going basis, management evaluates its estimates including, but not limited to, those related to contingent warrant liabilities, revenue recognition, research and development expense, long-lived assets, derivative instruments and stock-based compensation. The Company bases its estimates on historical experience and on various other market-specific and other relevant assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ significantly from these estimates, such as the Company’s billing under government contracts and the Company’s accrual for clinical trial expenses. Under the Company’s contracts with the National Institute of Allergy and Infectious Diseases (“NIAID”), a part of the National Institutes of Health (“NIH”), the Company bills using NIH provisional rates and thus are subject to future audits at the discretion of NIAID’s contracting office. These audits can result in an adjustment to revenue previously reported which potentially could be significant. The Company’s accrual for clinical trials is based on estimates of the services received and efforts expended pursuant to contracts with clinical trial centers and clinical research organizations. Payments under the contracts depend on factors such as the achievement of certain events, successful enrollment of patients, and completion of portions of the clinical trial or similar conditions.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Correction of an Immaterial Error
During the fourth quarter of 2014, we identified an immaterial error in our interim consolidated financial statements primarily pertaining to the three month period ended September 30, 2014 driven by certain stock-based compensation expense recorded in the period. We corrected the immaterial error in the fourth quarter of 2014, resulting in a decrease to operating expenses and net loss by $1.6 million and a decrease to basic and diluted loss per share of $0.01 and $0.02, respectively, for the three months ended December 31, 2014. The error does not affect results from operations for the year ended December 31, 2014. Based on management's evaluation of the materiality of the error from a qualitative and quantitative perspective as required by authoritative guidance, we concluded that correcting the error had no material impact on any of the Company's previously issued interim financial statements, would be immaterial to the fourth quarter results for 2014 and had no effect on the trend of financial results.
Reclassifications
Certain reclassifications of prior period amounts have been made to the financial statements and accompanying notes to conform to the current period presentation. Prior period presentations of net product sales and royalty revenue have been reclassified into contract and other revenue because the net product sales and royalty revenue were not material for all periods presented. These reclassifications had no impact on the Company’s previously reported net loss or cash flows.
Revenue Recognition
Revenue is recognized when the four basic criteria of revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. The determination of criteria (2) is based on management’s judgments regarding whether a continuing performance obligation exists. The determination of criteria (3) and (4) are based on management’s judgments regarding the nature of the fee charged for products or services delivered and the collectability of those fees. Allowances are established for estimated uncollectible amounts, if any.
The Company recognizes revenue from its license and collaboration arrangements, contract services, product sales and royalties. Revenue arrangements with multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered items. Each deliverable in the arrangement is evaluated to determine whether it meets the criteria to be accounted for as a separate unit of accounting or whether it should be combined with other deliverables. In order to account for the multiple-element arrangements, the Company identifies the deliverables included within the arrangement and evaluates which deliverables represent separate units of accounting. Analyzing the arrangement to identify deliverables requires the use of judgment, and each deliverable may be an obligation to deliver services, a right or license to use an asset, or another performance obligation. The consideration received is allocated among the separate units based on their respective fair values and the applicable revenue recognition criteria are applied to each of the separate units. Advance payments received in excess of amounts earned are classified as deferred revenue until earned.
License and Collaborative Fees
Revenue from non-refundable license, technology access or other payments under license and collaborative agreements where the Company has a continuing obligation to perform is recognized as revenue over the estimated period of the continuing performance obligation. The Company estimates the performance period at the inception of the arrangement and reevaluates it each reporting period. Management makes its best estimate of the period over which it expects to fulfill the performance obligations, which may include clinical development activities. Given the uncertainties of research and development collaborations, significant judgment is required to determine the duration of the performance period. This reevaluation may shorten or lengthen the period over which the remaining revenue is recognized. Changes to these estimates are recorded on a prospective basis. Cost reimbursement revenue under collaborative agreements is recognized as the related research and development costs are incurred, as provided for under the terms of these agreements.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
License and collaboration agreements with certain third parties also provide for contingent payments to be paid to XOMA based solely upon the performance of the partner. For such contingent payments revenue is recognized upon completion of the milestone event, once confirmation is received from the third party, provided that collection is reasonably assured and the other revenue recognition criteria have been satisfied. Milestone payments that are not substantive or that require a continuing performance obligation on the part of the Company are recognized over the expected period of the continuing performance obligation. Amounts received in advance are recorded as deferred revenue until the related milestone is completed.
Contract Revenue
Contract revenue for research and development involves the Company providing research and development and manufacturing services to collaborative partners, biodefense contractors or others. Revenue for certain contracts is accounted for by a proportional performance, or output-based, method where performance is based on estimated progress toward elements defined in the contract. The amount of contract revenue and related costs recognized in each accounting period are based on management’s estimates of the proportional performance during the period. Adjustments to estimates based on actual performance are recognized on a prospective basis and do not result in reversal of revenue should the estimate to complete be extended. In 2014, the Company had a $1.8 million adjustment to decrease previously invoiced balances from the NIAID contract. Refer to Note 4 Collaborative, Licensing and Other Arrangements.
Up-front fees are recognized in the same manner as the final deliverable, which is generally ratably over the period of the continuing performance obligation. Given the uncertainties of research and development collaborations, significant judgment is required to determine the duration of the arrangement.
Royalty Revenue
Royalty revenue and royalty receivables are recorded in the periods these royalty amounts are earned, and collection is reasonably assured. The royalty revenue and receivables recorded in these instances are based upon communication with collaborative partners or licensees, historical information and forecasted sales trends.
Research and Development Expenses
The Company expenses research and development costs as incurred. Research and development expenses consist of direct costs such as salaries and related personnel costs, and material and supply costs, and research-related allocated overhead costs, such as facilities costs. In addition, research and development expenses include costs related to clinical trials. From time to time, research and development expenses may include up-front fees and milestones paid to collaborative partners for the purchase of rights to in-process research and development. Such amounts are expensed as incurred.
The Company’s accrual for clinical trials is based on estimates of the services received and efforts expended pursuant to contracts with clinical trial centers and clinical research organizations. The Company may terminate these contracts upon written notice and are generally only liable for actual effort expended by the organizations to the date of termination, although in certain instances the Company may be further responsible for termination fees and penalties. The Company makes estimates of its accrued expenses as of each balance sheet date based on the facts and circumstances known to the Company at that time. Expenses resulting from clinical trials are recorded when incurred based, in part on estimates as to the status of the various trials. In 2014, the Company changed its methodology of accrual for the per-patient component of clinical trial expense from straight-line over the patient treatment period to scheduled costs as projected by the contract research organization. The change resulted in a $0.2 million adjustment to the Company’s accrued estimates for clinical trial activities from inception of the trials through December 31, 2014.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Cash and Cash Equivalents and Short-term Investments
The Company considers all highly liquid debt instruments with maturities of three months or less at the time the Company acquires them and that can be liquidated without prior notice or penalty to be cash equivalents.
Short-term investments include debt securities classified as available-for-sale. Available-for-sale securities are carried at fair value, with unrealized gains and losses, net of tax, if any, reported in other comprehensive income (loss). The estimate of fair value is based on publicly available market information. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are also included in other income (expense). The Company reviews its instruments for other-than-temporary impairment whenever the value of the instrument is less than the amortized cost. The cost of investments sold is based on the specific identification method. Interest and dividends on securities classified as available-for-sale are included in other income (expense).
Property and Equipment and Long-Lived Assets
Property and equipment is stated at cost less depreciation. Equipment depreciation is calculated using the straight-line method over the estimated useful lives of the assets (three to seven years). Leasehold improvements, buildings and building improvements are depreciated using the straight-line method over the shorter of the lease terms or the useful lives (one to fifteen years).
The Company reviews the carrying values and depreciation lives of its long-lived assets whenever events or changes in business circumstances or planned use of long-lived assets indicate that the asset may not be recoverable. An impairment loss is recognized when the estimated future net cash flows expected to result from the use of an asset is less than its carrying amount. Long-lived assets include property and equipment and building and leasehold improvements.
Warrants
The Company has issued warrants to purchase shares of its common stock in connection with financing activities. The Company accounts for some of these warrants as a liability at fair value and others as equity at fair value. The fair value of the outstanding warrants is estimated using the Black-Scholes Option Pricing Model (the “Black-Scholes Model”). The Black-Scholes Model requires inputs such as the expected term of the warrants, expected volatility and risk-free interest rate. These inputs are subjective and require significant analysis and judgment to develop. For the estimate of the expected term, the Company uses the full remaining contractual term of the warrant. In 2013, the Company changed its expected volatility assumption in the Black-Scholes Model from a volatility implied from warrants issued by XOMA in recent private placement transactions to a volatility based on historical stock price volatility observed on XOMA’s underlying stock. A historical stock price volatility rate was determined to be a more precise indicator for the fair value calculation of the Company’s warrants due to time elapsed since these warrants were granted. The assumptions associated with contingent warrant liabilities are reviewed each reporting period and changes in the estimated fair value of these contingent warrant liabilities are recognized in revaluation of contingent warrant liabilities within the Consolidated Statements of Comprehensive Loss.
Income Taxes
The Company accounts for uncertain tax positions in accordance with Accounting Standards Codification Topic 740, Income Taxes ("ASC 740"). The application of income tax law and regulations are inherently complex.
Accounting standards provide for the recognition of deferred tax assets if realization of such assets is more likely than not. The Company assessed the likelihood that deferred tax assets will be recovered as deductions from future taxable income. The Company has provided a full valuation allowance on its deferred tax assets at December 31, 2014 and 2013 because it believes it is more likely than not that the deferred tax assets will not be realized as of December 31, 2014, and 2013.
Based upon the weight of available evidence, which includes the Company’s historical operating performance and carry-back potential, the Company has determined that total deferred tax assets should be fully offset by a valuation allowance.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Net Loss per Share of Common Stock
Basic net loss per share of common stock is based on the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share of common stock is based on the weighted average number of shares outstanding during the period, adjusted to include the assumed conversion of certain stock options, restricted stock units (“RSUs”), and warrants for common stock. The calculation of diluted loss per share requires that, to the extent the average market price of the underlying shares for the reporting period exceeds the exercise price of the warrants and the presumed exercise of such securities are dilutive to loss per share for the period, adjustments to net income or net loss used in the calculation are required to remove the change in fair value of the warrants for the period. Likewise, adjustments to the denominator are required to reflect the related dilutive shares.
Potentially dilutive securities are excluded from the calculation of loss per share if their inclusion is anti-dilutive. The following table shows the total outstanding securities considered anti-dilutive and therefore excluded from the computation of diluted net loss per share (in thousands):
|
December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Options for common stock
|
6,666
|
7,087
|
5,603
|
|||||||||
|
Warrants for common stock
|
2,073
|
15,839
|
13,840
|
|||||||||
|
Total
|
8,739
|
22,926
|
19,443
|
|||||||||
For the year ended December 31, 2014, the following is a reconciliation of the numerators and denominators of the basic and diluted net loss per share of common stock (in thousands):
|
December 31,
|
||||
|
2014
|
||||
|
Numerator
|
||||
|
Net loss before taxes
|
||||
|
Basic
|
$
|
(38,301
|
)
|
|
|
Adjustment for revaluation of contingent warrant liabilities
|
(39,512
|
)
|
||
|
Diluted
|
$
|
(77,813
|
)
|
|
|
Denominator
|
||||
|
Weighted average shares outstanding used for basic net loss per share
|
107,435
|
|||
|
Effect of dilutive warrants
|
7,898
|
|||
|
Weighted average shares outstanding for dilutive net loss per share
|
115,333
|
|||
For the years ended December 31, 2013 and 2012, all potentially dilutive securities outstanding were considered anti-dilutive, and therefore the calculations of basic and diluted net loss per share were the same.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued guidance codified in Accounting Standards Codification (“ASC”) 606, Revenue Recognition — Revenue from Contracts with Customers, which amends the guidance in former ASC 605, Revenue Recognition. The standard’s core principle is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The standard is effective for public entities for annual and interim periods beginning after December 15, 2016. Early adoption is not permitted. The Company is currently evaluating the impact of the provisions of ASC 606.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In August 2014, the FASB issued ASU No. 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. This ASU introduces an explicit requirement for management to assess if there is substantial doubt about an entity’s ability to continue as a going concern, and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management must assess if there is substantial doubt about an entity’s ability to continue as a going concern within one year after the issuance date. Disclosures are required if conditions give rise to substantial doubt. ASU 2014-15 is effective for all entities in the first annual period ending after December 15, 2016. The Company is currently assessing the potential effects of this ASU on the consolidated financial statements.
In November 2014, the FASB issued ASU No. 2014-16, Determining whether the Host Contract in a Hybrid Instrument issued in the form of a share is more akin to debt or to equity. This ASU introduces a requirement for management to separate an embedded derivative feature from the host contract and account for the feature as a derivative according to Subtopic 815-10 on derivatives and hedging if certain criteria are met. That is, management should determine the nature of the host contract by considering the economic characteristics and risks of the entire hybrid financial instrument, including the embedded derivative feature that is being evaluated for separate accounting from the host contract. ASU 2014-16 is effective date for public entities for annual and interim report beginning after December 15, 2015. Early adoption in an interim period, is permitted. The Company is currently evaluating the potential effects of this ASU on the consolidated financial statements.
| 3. | Consolidated Financial Statement Detail |
Cash and Cash Equivalents
At December 31, 2014, cash equivalents consisted of demand deposits of $10.8 million and money market funds of $67.6 million with maturities of less than 90 days at the date of purchase. At December 31, 2013, cash equivalents consisted of demand deposits of $18.9 million and money market funds of $82.8 million with maturities of less than 90 days at the date of purchase.
Short-term Investments
At December 31, 2014, there were no short term investments. At December 31, 2013, short-term investments consisted of U.S. treasury securities of $20.0 million, with maturities of greater than 90 days and less than one year from the date of purchase.
Foreign Exchange Options
The Company holds debt and may incur revenue and expenses denominated in foreign currencies, which exposes it to market risk associated with foreign currency exchange rate fluctuations between the U.S. dollar and the Euro. The Company is required in the future to make principal and accrued interest payments in Euros on its €15.0 million loan from Servier (See Note 7: Long-Term Debt and Other Arrangements). In order to manage its foreign currency exposure related to these payments, in May 2011, the Company entered into two foreign exchange option contracts to buy €1.5 million and €15.0 million in January 2014 and January 2016, respectively. By having these option contracts in place, the Company’s foreign exchange rate risk is reduced if the U.S. dollar weakens against the Euro. However, if the U.S. dollar strengthens against the Euro, the Company is not required to exercise these options, but will not receive any refund on premiums paid.
Upfront premiums paid on these foreign exchange option contracts totaled $1.5 million. The fair values of these option contracts are revalued at each reporting period and are estimated based on pricing models using readily observable inputs from actively quoted markets. The fair values of these option contracts are included in other assets on the consolidated balance sheet and changes in fair value on these contracts are included in other income (expense) on the consolidated statements of comprehensive loss.
As of December 31, 2014, one option contract had expired. The remaining foreign exchange option was revalued at December 31, 2014 and the fair value was de minimis. As of December 31, 2013, the fair value was $0.4 million. The Company recognized losses of $0.4 million, $0.1 million, and $0.7 million related to the revaluation for the years ended December 31, 2014, 2013, and 2012, respectively.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Receivables
Accounts receivable are stated at their net realizable value. Specific allowances are recorded for known troubled accounts or based on other available information. Accounts receivable are written off after all reasonable means to collect the full amount have been exhausted.
Receivables consisted of the following at December 31, 2014 and 2013 (in thousands):
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Trade receivables, net
|
$
|
2,993
|
$
|
3,731
|
||||
|
Other receivables
|
316
|
50
|
||||||
|
Total
|
$
|
3,309
|
$
|
3,781
|
||||
Property and Equipment
Property and equipment consisted of the following at December 31, 2014 and 2013 (in thousands):
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Equipment and furniture
|
$
|
28,638
|
$
|
28,365
|
||||
|
Buildings, leasehold and building improvements
|
9,343
|
9,316
|
||||||
|
Construction-in-progress
|
337
|
225
|
||||||
|
Land
|
310
|
310
|
||||||
|
38,628
|
38,216
|
|||||||
|
Less: Accumulated depreciation and amortization
|
(33,508
|
)
|
(31,760
|
)
|
||||
|
Property and equipment, net
|
$
|
5,120
|
$
|
6,456
|
||||
Depreciation and amortization expense was $1.9 million, $2.9 million and $4.1 million for the years ended December 31, 2014, 2013, and 2012, respectively.
Accrued Liabilities
Accrued liabilities consisted of the following at December 31, 2014 and 2013 (in thousands):
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Incentive compensation
|
$
|
4,295
|
$
|
4,386
|
||||
|
Accrued payroll and other benefits
|
3,061
|
3,009
|
||||||
|
Accrued clinical trial costs
|
1,424
|
878
|
||||||
|
Other
|
1,112
|
1,661
|
||||||
|
Total
|
$
|
9,892
|
$
|
9,934
|
||||
Contingent Warrant Liabilities
In December 2014, in connection with a registered direct offering to select institutional investors, the Company issued two-year warrants to purchase up to an aggregate of 8,097,165 shares of XOMA’s common stock at an exercise price of $7.90 per share. These warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate the Company to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, the Company accounts for the warrants issued in December 2014 as a liability at fair value. In addition, the estimated liability related to the warrants is revalued at each reporting period until the earlier of the exercise of the warrants, at which time the liability will be reclassified to stockholders’ equity, or expiration of the warrants. On December 8, 2014, the date of issuance, the fair value of the warrant liability was estimated to be $10.3 million using the Black-Scholes Model. The Company revalued the warrant liability at December 31, 2014, and recorded a $5.1 million decline in the fair value as a gain in the revaluation of contingent warrant liabilities in the accompanying consolidated statements of comprehensive loss. The decrease in liability is due primarily to the decrease in the market price of XOMA’s common stock at December 31, 2014. As of December 31, 2014, all of the warrants were outstanding and had a fair value of $5.2 million.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In March 2012, in connection with an underwritten offering, the Company issued five-year warrants to purchase 14,834,577 shares of XOMA’s common stock at an exercise price of $1.76 per share. These warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate the Company to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, the Company accounts for the warrants issued in March 2012 as a liability at fair value. In addition, the estimated liability related to the warrants is revalued at each reporting period until the earlier of the exercise of the warrants, at which time the liability will be reclassified to stockholders' equity, or expiration of the warrants. As of December 31, 2013, 12,562,682 of these warrants were outstanding and had a fair value of $68.7 million. The Company revalued the warrant liability at December 31, 2014 using the Black-Scholes Model and recorded the $39.5 million decrease in the fair value of as a gain in the revaluation of contingent warrant liabilities in the accompanying consolidated statements of comprehensive loss. In 2014, the Company reclassified $2.5 million from contingent warrant liabilities to equity on the consolidated balance sheet due to the exercise of these warrants. As of December 31, 2014, 12,109,418 of these warrants were outstanding and had a fair value of $26.7 million. The decrease in liability is due primarily to the decrease in the market price of XOMA’s common stock at December 31, 2014.
In February 2010, in connection with an underwritten offering, the Company issued five-year warrants to purchase 1,260,000 shares of XOMA’s common stock at an exercise price of $10.50 per share. The warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate the Company to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, the Company accounts for the warrants as liabilities at fair value. At December 31, 2013, all of the warrants were outstanding and had a fair value of $1.1 million. The Company revalued the warrant liability at December 31, 2014 using the Black-Scholes Model and recorded the $1.1 million decrease in the fair value as a gain in the revaluation of contingent warrant liabilities in the accompanying consolidated statements of comprehensive loss. As of December 31, 2014, all of the warrants were outstanding and the fair value was de minimis. The decrease in liability is due primarily to the decrease in the market price of XOMA’s common stock at December 31, 2014.
In June 2009, the Company issued warrants to certain institutional investors as part of a registered direct offering. The warrants represent the right to acquire an aggregate of up to 347,826 shares of XOMA’s common stock over a five year period beginning December 11, 2009 at an exercise price of $19.50 per share. The warrants contain provisions that are contingent on the occurrence of a change in control, which could conditionally obligate us to repurchase the warrants for cash in an amount equal to their fair value using the Black-Scholes Model on the date of such change in control. Due to these provisions, the Company accounts for the warrants as liabilities at fair value. At December 31, 2013, all of the warrants were outstanding and had a fair value of $0.1 million. As of December 31, 2014, all of the warrants had expired unexercised.
| 4. | Collaborative, Licensing and Other Arrangements |
Collaborative and Other Agreements
Servier
In December 2010, the Company entered into a license and collaboration agreement with Servier, to jointly develop and commercialize gevokizumab in multiple indications, which provided for a non-refundable upfront payment of $15.0 million that was received by the Company in January 2011. The upfront payment was recognized over the eight month period that the initial group of deliverables were provided to Servier. In addition, the Company received a loan of €15.0 million, which was fully funded in January 2011, with the proceeds converting to $19.5 million at the date of funding. See Note 7: Long-Term Debt and Other Arrangements. Under the terms of the agreement, Servier has worldwide rights to cardiovascular disease and diabetes indications and rights outside the United States and Japan to all other indications, including NIU, Behçet’s disease uveitis and other inflammatory and oncology indications. XOMA retains development and commercialization rights in the United States and Japan for all indications other than cardiovascular disease and diabetes. XOMA has an option to reacquire rights to cardiovascular disease and diabetes indications from Servier in the United States and Japan (the “Cardiometabolic Indications Option”). If the Company exercises the Cardiometabolic Indications Option, the Company will be required to pay Servier an option fee and partially reimburse their incurred development expenses. Each party has the right in certain circumstances to pursue development in indications not specified in the agreement, and in such event, the other party will have the option to participate in such development in certain circumstances, including reimbursement of a portion of the developing party’s expenses.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Under this agreement, Servier will fund all activities to advance the global clinical development and future commercialization of gevokizumab in cardiovascular-related diseases and diabetes. Also, Servier funded the first $50 million of gevokizumab global clinical development and Chemistry, Manufacturing and Controls (“CMC”) expenses and continues to fund 50% of further expenses related to the pan-uveitis (“NIU”) and Behçet’s disease uveitis indications. For the years ended December 31, 2014, 2013, and 2012, the Company recorded revenue of $3.5 million, $13.6 million, and $14.5 million, respectively.
Under the agreement, the Company is eligible to receive a combination of Euro and USD-denominated, development and sales milestones for multiple indications aggregating to a potential maximum of approximately $433 million converted using the December 31, 2014 Euro to U.S. Dollar (“USD”) exchange rate (the “December 31, 2014 Exchange Rate of 1.216”) if XOMA reacquires cardiovascular and/or diabetes rights in the U.S. and Japan. If XOMA does not reacquire these rights, then the milestone payments aggregate to a potential maximum of approximately $770 million converted using the December 31, 2014 Exchange Rate of 1.216. Servier’s obligation to pay development and commercialization milestones will continue for so long as Servier is developing or selling products under the agreement.
The Company is also eligible to receive royalties on gevokizumab sales, which are tiered based on sales levels and range from a mid-single digit to up to a mid-teens percentage rate. The Company’s right to royalties with respect to a particular product and country will continue for so long as such product is sold in such country.
On January 9, 2015, the Company and Servier entered into Amendment No. 2 to the Collaboration Agreement. Refer to Note 13, Subsequent Events for further discussion.
NIAID
In July 2006, the Company was awarded a $16.3 million contract to produce monoclonal antibodies for the treatment of botulism to protect United States citizens against the harmful effects of botulinum neurotoxins used in bioterrorism. The contract work was performed on a cost plus fixed fee basis. The original contract was for a three-year period, however the contract was extended into 2010. The Company recognizing revenue as the services are performed on a proportional performance basis. This work was complete in the third quarter of 2010. In 2011, the NIH conducted an audit of the Company’s actual data for the period from January 1, 2007 through December 31, 2009 and developed final billing rates for this period. As a result, the Company retroactively applied these NIH rates to the invoices from this period resulting in an increase in revenue of $2.0 million from the NIH. Upon settlement, the Company recognized the $2.0 million in revenue in 2012.
In September 2008, the Company announced that it had been awarded a $64.8 million multiple-year contract funded with federal funds from NIAID, a part of the NIH (Contract No. HHSN272200800028C), to continue development of anti-botulinum antibody product candidates. The contract work is being performed on a cost plus fixed fee basis over a three-year period. The Company is recognizing revenue under the arrangement as the services are performed on a proportional performance basis. In 2011, the NIH conducted an audit of the Company’s actual data for period from January 1, 2007 through December 31, 2009 and developed final billing rates for this period. As a result, the Company retroactively applied these NIH rates to the invoices from this period resulting in an increase in revenue of $1.1 million from the NIH, excluding $0.9 million billed to the NIH in 2010 resulting from the Company’s performance of a comparison of 2009 calculated costs incurred and costs billed to the government under provisional rates. In 2014, upon completion of a NIAID review of hours and external expenses, XOMA agreed to exclude certain hours and external expenses resulting in a $1.8 million adjustment to decrease previously invoiced balances. The adjustment was offset by a $1.9 million deferred revenue balance that was recorded in 2012 as a result of a rate adjustment for the period 2007 to 2009. This adjustment reduced accounts receivable and deferred revenue by $1.8 million to reflect the final settlement of the 2008 to 2013 hours and external review. The remaining $0.1 million in deferred revenue in connection with the 2011 NIH rate audit will be recognized upon completion of negotiations with and approval by the NIH. In 2014, the Company recognized revenue of $1.2 million under this contract, compared with $4.4 million in 2013 and $6.6 million in 2012.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In October 2011, the Company announced that NIAID had awarded the Company a new contract under Contract No. HHSN272201100031C for up to $28.0 million over 5 years to develop broad-spectrum antitoxins for the treatment of human botulism poisoning. The contract work is being performed on a cost plus fixed fee basis over the life of the contract and the Company is recognizing revenue under the arrangement as the services are performed on a proportional performance basis. In 2014, the Company recognized revenue of $8.4 million under this contract, compared with $4.7 million in 2013 and $2.5 million in 2012.
Servier – U.S. Perindopril Franchise
On January 17, 2012, the Company announced it had acquired certain U.S. rights to a portfolio of antihypertensive products from Servier. The portfolio includes ACEON® (perindopril erbumine), a currently marketed angiotensin converting enzyme (“ACE”) inhibitor, and three Fixed Dose Combination (“FDC”) product candidates where a form of proprietary perindopril (perindopril arginine) is combined with another active ingredient(s). The Company assumed commercialization activities for ACEON in January 2012. In November 2012, the Company announced that the 837-patient Phase 3 trial for the FDC of perindopril arginine and amlodipine besylate (“FDC1”) met its primary endpoint. Partial funding for the trial was provided by Servier.
In connection with the original agreement, the Company paid a $1.5 million license fee to Servier in the third quarter of 2010. In July 2013, the Company transferred U.S. development and commercialization rights of perindopril franchise and sublicensed the U.S. marketing rights to ACEON, to Symplmed. Under the terms of the arrangement, the Company received a minority equity position in Symplmed and up to double-digit royalties on sales of the first fixed-dose combination containing perindopril arginine and amlodipine besylate, if it is approved by the FDA. The Company recorded the minority equity position in other assets of the Company’s consolidated balance sheets. Symplmed, under a sublicense agreement, assumes U.S. marketing responsibilities for ACEON (perindopril erbumine). Following the ACEON NDA transfer, Symplmed will pay the Company single-digit royalties on sales of ACEON. In July 2014, the U.S. marketing rights to ACEON New Drug Application (“NDA”) was transferred to Symplmed. In 2014, the Company recognized a de minimis amount in royalties.
Takeda
In November 2006, the Company entered into a fully funded collaboration agreement with Takeda for therapeutic monoclonal antibody discovery and development. Under the agreement, Takeda will make up-front, annual maintenance and milestone payments to the Company, fund its research and development and manufacturing activities for preclinical and early clinical studies and pay royalties on sales of products resulting from the collaboration. Takeda will be responsible for clinical trials and commercialization of drugs after an Investigational New Drug Application (“IND”) submission and is granted the right to manufacture once the product enters into Phase 2 clinical trials. During the collaboration, the Company will discover therapeutic antibodies against targets selected by Takeda. The Company will recognize revenue on the up-front and annual payments on a straight-line basis over the expected term of each target antibody discovery, on the research and development and manufacturing services as they are performed on a time and materials basis, on the milestones when they are achieved and on the royalties when the underlying sales occur. In 2014, the Company recognized revenue of $1.6 million under this agreement, compared with $0.1 million in 2013 and $1.2 million in 2012.
Under the terms of this agreement, the Company may receive milestone payments aggregating up to $19.0 million relating to one undisclosed product candidate and low single-digit royalties on future sales of all products subject to this license. In addition, in the event Takeda were to develop additional future qualifying product candidates under the terms of the agreement, the Company would be eligible for milestone payments aggregating up to $20.75 million for each such qualifying product candidate. The Company’s right to milestone payments expires on the later of the receipt of payment from Takeda of the last amount to be paid under the agreement or the cessation of all research and development activities with respect to all program antibodies, collaboration targets and/or collaboration products. The Company’s right to royalties expires on the later of 13.5 years from the first commercial sale of each royalty-bearing discovery product or the expiration of the last-to-expire licensed patent.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In February 2009, the Company expanded its existing collaboration agreement with Takeda to provide Takeda with access to multiple antibody technologies, including a suite of research and development technologies and integrated information and data management systems. The Company may receive milestones of up to $3.25 million per discovery product candidate and low single-digit royalties on future sales of all antibody products subject to this license. The Company’s right to milestone payments expires on the later of the receipt of payment from Takeda of the last amount to be paid under the agreement or the cessation of all research and development activities with respect to all program antibodies, collaboration targets and/or collaboration products. The Company’s right to royalties expires on the later of 10 years from the first commercial sale of such royalty-bearing discovery product, or the expiration of the last-to-expire licensed patent.
Novartis
In November 2008, the Company restructured its product development collaboration with Novartis entered into in 2004 for the development and commercialization of antibody products for the treatment of cancer. Under the restructured agreement, the Company received $6.2 million in cash and $7.5 million in the form of debt reduction on its existing loan facility with Novartis. In addition, the Company may, in the future, receive potential milestones of up to $14.0 million and royalty rates ranging from low-double digit to high-teen percentage rates for two ongoing product programs, HCD122 and LFA 102 and options to develop or receive royalties on additional programs. In exchange, Novartis received control over the HCD122 and LFA 102 programs, as well as the right to expand the development of these programs into additional indications outside of oncology. Novartis has returned control of the prolactin receptor antibody program to the Company and is evaluating options for its continued development. The Company’s right to royalty-style payments expires on the later of the expiration of any licensed patent covering each product or 20 years from the launch of each product that is produced from a cell line provided to Novartis by XOMA. In 2013, the Company received a $7.0 million milestone relating to one currently active program. Pursuant to the obligations under the agreement, in January 2014, the Company made a payment, equal to 25 percent of the milestone received, or $1.75 million, toward its outstanding debt obligation to Novartis. In 2014, no revenue was recognized under the collaboration agreement with Novartis.
A loan facility of up to $50 million was available to the Company to fund up to 75% of its share of development expenses incurred beginning in 2005. Refer to Note 7: Long-Term Debt and Other Arrangements for additional disclosure of the financing arrangement between the Company and Novartis.
Licensing Agreements
XOMA has granted more than 60 licenses to biotechnology and pharmaceutical companies to use the Company’s patented and proprietary technologies relating to bacterial expression of recombinant pharmaceutical products. In exchange, the Company receives license and other fees as well as access to certain of these companies’ antibody display libraries, intellectual property and/or services that complement the Company’s existing development capabilities and support the Company’s own antibody product development pipeline.
Certain of these agreements also provide releases of the licensee companies and their collaborators from claims under the XOMA patents arising from past activities using the companies’ respective technologies to the extent they also used XOMA’s antibody expression technology. Licensees are often also allowed to use XOMA’s technology in combination with their own technology in future collaborations.
Pfizer
In August 2007, the Company entered into a license agreement with Pfizer Inc. (“Pfizer”) for non-exclusive, worldwide rights for XOMA’s patented bacterial cell expression technology for research, development and manufacturing of antibody products. Under the terms of the agreement, the Company received a license fee payment of $30 million in 2007.
From 2011 through 2014, the Company received milestone payments and also may be eligible for additional milestone payments aggregating up to $17.9 million and low single-digit royalties on future sales of all products subject to this license. In addition, the Company may receive potential milestone payments aggregating up to $1.7 million for each additional qualifying product candidate. The Company’s right to milestone payments expires on the later of the expiration of the last-to-expire licensed patent or the tenth anniversary of the effective date. The Company’s right to royalties expires upon the expiration of the last-to-expire licensed patent. The Company will recognize revenue on milestones when they are achieved and on royalties when the underlying sales occur.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 5. | Restructuring Charges |
In January 2012, the Company implemented a streamlining of operations, which resulted in a restructuring plan designed to sharpen its focus on value-creating opportunities led by gevokizumab and its unique antibody discovery and development capabilities. The restructuring plan included a reduction of XOMA’s personnel by 84 positions, or 34%. These staff reductions resulted primarily from the Company’s decisions to utilize a contract manufacturing organization for Phase 3 and commercial antibody production, and to eliminate internal research functions that are non-differentiating or that can be obtained cost effectively by contract service providers.
In 2014, 2013 and 2012, the Company incurred $0.1 million, $0.3 million and $4.9 million, respectively in restructuring charges related to facility costs.
The outstanding restructuring liabilities are included in accrued and other liabilities and on the accompanying consolidated balance sheets and are based upon restructuring charges recognized as of December 31, 2014 and 2013 in connection with the Company’s restructuring plans. As of December 31, 2014 and 2013, the components of these liabilities are shown below (in thousands):
|
Facility
Charges (1)
|
||||
|
Balance at December 31, 2013
|
$
|
21
|
||
|
Restructuring charges
|
84
|
|||
|
Cash payments
|
(128
|
)
|
||
|
Adjustments
|
23
|
|||
|
Balance at December 31, 2014
|
$
|
-
|
||
|
Facility
Charges (1)
|
||||
|
Balance at December 31, 2012
|
$
|
75
|
||
|
Restructuring charges
|
328
|
|||
|
Cash payments
|
(434
|
)
|
||
|
Adjustments
|
52
|
|||
|
Balance at December 31, 2013
|
$
|
21
|
||
| (1) | Includes moving and relocation costs, and lease payments, net of sublease payments. |
| 6. | Available-for-Sale and Fair Value Measurements |
The classification of the Company’s available-for-sale securities consisted of the following (in thousands):
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Money Market funds
|
$
|
67,569
|
$
|
82,759
|
||||
|
U.S. treasury securities
|
-
|
19,989
|
||||||
|
$
|
67,569
|
$
|
102,748
|
|||||
The Company had no unrealized gains or losses associated with its available-for-sale securities as of December 31, 2014. As of December 31, 2013, gross unrealized losses of approximately $1,000 were included in accumulated comprehensive loss on its consolidated balance sheet.
Fair value is defined as the exchange price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company applies Accounting Standards Codification Topic 820, Fair Value Measurement and Disclosures, (“ASC 820”), which establishes a framework for measuring fair value and a fair value hierarchy that prioritizes the inputs used in valuation techniques. The accounting standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
Level 1 – Observable inputs, such as quoted prices in active markets for identical assets or liabilities.
Level 2 – Observable inputs, either directly or indirectly, other than quoted prices in active markets for similar assets or liabilities, that are not active or other inputs that are not observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities; therefore, requiring an entity to develop its own valuation techniques and assumptions.
The following tables set forth the Company’s fair value hierarchy for its financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2014 and 2013 as follows (in thousands):
|
Fair Value Measurements at December 31, 2014 Using
|
||||||||||||||||
|
Quoted Prices in
Active Markets
for Identical
Assets
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
|
||||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market funds (1)
|
$
|
67,569
|
$
|
-
|
$
|
-
|
$
|
67,569
|
||||||||
|
Foreign exchange options
|
-
|
6
|
-
|
6
|
||||||||||||
|
Total
|
$
|
67,569
|
$
|
6
|
$
|
-
|
$
|
67,575
|
||||||||
|
Liabilities:
|
||||||||||||||||
|
Contingent warrant liabilities
|
$
|
-
|
$
|
-
|
$
|
31,828
|
$
|
31,828
|
||||||||
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
Fair Value Measurements at December 31, 2013 Using
|
||||||||||||||||
|
Quoted Prices in
Active Markets
for Identical
Assets
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
|
||||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market funds (1)
|
$
|
82,759
|
$
|
-
|
$
|
-
|
$
|
82,759
|
||||||||
|
U.S. treasury securities
|
19,989
|
-
|
-
|
19,989
|
||||||||||||
|
Foreign exchange options
|
-
|
361
|
-
|
361
|
||||||||||||
|
Total
|
$
|
102,748
|
$
|
361
|
$
|
-
|
$
|
103,109
|
||||||||
|
Liabilities:
|
||||||||||||||||
|
Contingent warrant liabilities
|
$
|
-
|
$
|
-
|
$
|
69,869
|
$
|
69,869
|
||||||||
| (1) | Included in cash and cash equivalents |
There were no transfers between Level 1 and Level 2 during the twelve months ended December 31, 2014.
The fair value of the foreign exchange options at December 31, 2014 and 2013 was determined using readily observable market inputs from actively quoted markets obtained from various third-party data providers. These inputs, such as spot rate, forward rate and volatility have been derived from readily observable market data, meeting the criteria for Level 2 in the fair value hierarchy.
The fair value of the contingent warrant liabilities at December 31, 2014 and 2013 was determined using the Black-Scholes Model, which requires inputs such as the expected term of the warrants, volatility and risk-free interest rate. These inputs are subjective and generally require significant analysis and judgment to develop. In 2013, the Company changed its expected volatility assumption in the Black-Scholes Model from a volatility implied from warrants issued by XOMA in recent private placement transactions to a volatility based on historical stock price volatility observed on XOMA’s underlying stock. A historical stock price volatility rate was determined to be a more precise indicator for the fair value calculation of the Company’s warrants due to time elapsed since these warrants were granted. The Company’s common stock price represents a significant input that impacts sensitivity in the valuation of the warrants.
The fair value of the contingent warrant liabilities was estimated using the following range of assumptions at December 31, 2014 and 2013:
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Expected volatility
|
69.6% - 72.9
|
%
|
66.1% - 86.6
|
%
|
||||
|
Risk-free interest rate
|
0.03% - 0.67
|
%
|
0.10% - 0.80
|
%
|
||||
|
Expected term
|
0.09 - 2.19 years
|
0.90 - 3.20 years
|
||||||
The following table provides a summary of changes in the fair value of the Company’s Level 3 financial liabilities for the years ended December 31, 2014, 2013, and 2012 (in thousands):
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
Warrant
Liabilities
|
||||
|
Balance at December 31, 2011
|
$
|
379
|
||
|
Initial fair value of warrants issued in March 2012
|
6,390
|
|||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
(940
|
)
|
||
|
Net increase in fair value of contingent warrant liabilities upon revaluation
|
9,172
|
|||
|
Balance at December 31, 2012
|
15,001
|
|||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
(6,171
|
)
|
||
|
Net increase in fair value of contingent warrant liabilities upon revaluation
|
61,039
|
|||
|
Balance at December 31, 2013
|
69,869
|
|||
|
Initial fair value of warrants issued in December 2014 warrant
|
10,258
|
|||
|
Reclassification of contingent warrant liability to equity upon exercise of warrants
|
(2,526
|
)
|
||
|
Net decrease in fair value of contingent warrant liabilities upon revaluation
|
(45,773
|
)
|
||
|
Balance at December 31, 2014
|
$
|
31,828
|
||
The fair value of the Company’s outstanding debt is estimated based on market interest rates. The carrying amount and the estimated fair value of the Company’s outstanding debt at December 31, 2014 and 2013 are as follows:
|
December 31, 2014
|
December 31, 2013
|
|||||||||||||||
|
Carrying Amount
|
Fair Value
|
Carrying Amount
|
Fair Value
|
|||||||||||||
|
Outstanding debt
|
$
|
35,537
|
$
|
36,461
|
$
|
40,985
|
$
|
41,813
|
||||||||
| 7. | Loans and Other Arrangements |
Novartis Note
In May 2005, the Company executed a secured note agreement with Novartis (then Chiron Corporation), which is due and payable in full in June 2015. Under the note agreement, the Company borrowed semi-annually to fund up to 75% of the Company’s research and development and commercialization costs under its collaboration arrangement with Novartis, not to exceed $50 million in aggregate principal amount. Interest on the principal amount of the loan accrues at six-month LIBOR plus 2%, which was equal to 2.35% at December 31, 2014, and is payable semi-annually in June and December of each year. Additionally, the interest rate resets in June and December of each year. At the Company’s election, the semi-annual interest payments can be added to the outstanding principal amount, in lieu of a cash payment, as long as the aggregate principal amount does not exceed $50 million. The Company has made this election for all interest payments thus far. Loans under the note agreement are secured by the Company’s interest in its collaboration with Novartis, including any payments owed to it thereunder.
At December 31, 2014 and 2013, the outstanding principal balance under this note agreement was $13.4 million and $14.8 million. Pursuant to the terms of the arrangement as restructured in November 2008, the Company will not make any additional borrowings under the Novartis note. Accrued interest of $0.3 million, $0.4 million and $0.4 million was added to the principal balance of the loan for the years ended December 31, 2014, 2013 and 2012, respectively.
Pursuant to its obligations under the collaboration with Novartis, in January 2014, the Company made a payment, equal to 25 percent of a $7.0 million milestone received, or $1.75 million, toward its outstanding debt obligation to Novartis.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Servier Loan
In December 2010, in connection with the license and collaboration agreement entered into with Servier, the Company executed a loan agreement with Servier (the “Servier Loan Agreement”), which provided for an advance of up to €15.0 million. The loan was fully funded in January 2011, with the proceeds converting to approximately $19.5 million. The loan is secured by an interest in XOMA’s intellectual property rights to all gevokizumab indications worldwide, excluding certain rights in the U.S. and Japan. Interest is calculated at a floating rate based on a Euro Inter-Bank Offered Rate (“EURIBOR”) and subject to a cap. The interest rate is reset semi-annually in January and July of each year. The interest rate for the initial interest period was 3.22% and was reset semi-annually ranging from 2.31% to 3.83%. Interest for the six-month period from mid-January 2015 through mid-July 2015 was reset to 2.16%. Interest is payable semi-annually; however, the Servier Loan Agreement provides for a deferral of interest payments over a period specified in the agreement. During the deferral period, accrued interest will be added to the outstanding principal amount for the purpose of interest calculation for the next six-month interest period. On the repayment commencement date, all unpaid and accrued interest shall be paid to Servier and thereafter, all accrued and unpaid interest shall be due and payable at the end of each six-month period. In January 2015, the Company paid $0.2 million in accrued interest to Servier.
The loan matures in 2016; however, after a specified period prior to final maturity, the loan is to be repaid (i) at Servier's option, by applying up to a significant percentage of any milestone or royalty payments owed by Servier under the Company’s collaboration agreement and (ii) using a significant percentage of any upfront, milestone or royalty payments the Company receives from any third party collaboration or development partner for rights to gevokizumab in the U.S. and/or Japan. In addition, the loan becomes immediately due and payable upon certain customary events of default. At December 31, 2014, the outstanding principal balance under this loan was $18.2 million using the December 31, 2014 Exchange Rate of 1.216. For the year ended December 31, 2014, the Company recorded unrealized foreign exchange gain of $2.4 million, and for the years ended December 2013 and 2012 the Company recorded unrealized foreign exchange losses of $0.8 million and $0.4 million, respectively, related to the re-measurement of the loan.
The loan has a stated interest rate lower than the market rate based on comparable loans held by similar companies, which represents additional value to the Company. The Company recorded this additional value as a discount to the face value of the loan amount, at its fair value of $8.9 million. The fair value of this discount, which was determined using a discounted cash flow model, represents the differential between the stated terms and rates of the loan, and market rates. Based on the association of the loan with the collaboration arrangement, the Company recorded the offset to this discount as deferred revenue.
The loan discount is amortized under the effective interest method over the expected five-year life of the loan. For the years ended December 31, 2014, 2013, and 2012, the Company recorded non-cash interest expense of $1.9 million, $1.6 million, and $1.4 million, respectively, resulting from the amortization of the loan discount. At December 31, 2014 and 2013, the net carrying value of the loan was $16.2 million and $16.5 million, respectively. For the year ended December 31, 2014 the Company recorded unrealized foreign exchange loss of $0.3 million and for the years ended December 2013 and 2012, the Company recorded unrealized foreign exchange gains of $0.2 million and $0.1 million, respectively, related to the re-measurement of the loan discount.
The Company believes that realization of the benefit and the associated deferred revenue is contingent on the loan remaining outstanding over the five-year contractual term of the loan. If the Company were to stop providing service under the collaboration arrangement and the arrangement is terminated, the maturity date of the loan would be accelerated and a portion of measured benefit would not be realized. As the realization of the benefit is contingent, in part, on the provision of future services, the Company is recognizing the deferred revenue over the expected five-year life of the loan. The deferred revenue is amortized under the effective interest method, and for the years ended December 31, 2014, 2013, and 2012, the Company recorded $1.9 million, $1.6 million, and $1.4 million, respectively, of related non-cash revenue.
On January 9, 2015, Servier and the Company entered into Amendment No. 2 (“Loan Amendment”) to the Servier Loan Agreement. Refer to Note 13, Subsequent Events for further discussion.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
General Electric Capital Corporation Term Loan
In December 2011, the Company entered into a loan agreement (the “GECC Loan Agreement”) with General Electric Capital Corporation (“GECC”), under which GECC agreed to make a term loan in an aggregate principal amount of $10 million (the “Term Loan”) to the Company, and upon execution of the GECC Loan Agreement, GECC funded the Term Loan. As security for its obligations under the GECC Loan Agreement, the Company granted a security interest in substantially all of its existing and after-acquired assets, excluding its intellectual property assets (such as those relating to its gevokizumab and anti-botulism products). The Term Loan accrued interest at a fixed rate of 11.71% per annum and was to be repaid over a period of 42 consecutive equal monthly installments of principal and accrued interest and was due and payable in full on June 15, 2015. The Company incurred debt issuance costs of approximately $1.3 million in connection with the Term Loan and was required to pay a final payment fee equal to $500,000 on the maturity date, or such earlier date as the Term Loan is paid in full. The debt issuance costs and final payment fee were being amortized and accreted, respectively, to interest expense over the term of the Term Loan using the effective interest method.
In connection with the GECC Loan Agreement, the Company issued to GECC unregistered warrants that entitle GECC to purchase up to an aggregate of 263,158 unregistered shares of XOMA common stock at an exercise price equal to $1.14 per share. These warrants are exercisable immediately and have a five-year term. The Company allocated the aggregate proceeds of the GECC Term Loan between the warrants and the debt obligation based on their relative fair values. The fair value of the warrants issued to GECC was determined using the Black-Scholes Model. The warrants’ fair value of $0.2 million was recorded as a discount to the debt obligation and was being amortized over the term of the loan using the effective interest method.
In September 2012, the Company entered into an amendment to the GECC Loan Agreement providing for an additional term loan in the amount of $4.6 million, increasing the term loan obligation to $12.5 million (the “Amended Term Loan”) and providing for an interest-only monthly repayment period following the effective date of the amendment through March 1, 2013, at a stated interest rate of 10.9% per annum. Thereafter, the Company is obligated to make monthly principal payments of $347,222, plus accrued interest, over a 27-month period commencing on April 1, 2013, and through June 15, 2015, at which time the remaining outstanding principal amount of $3.1 million, plus accrued interest, is due. The Company incurred debt issuance costs of approximately $0.2 million and are required to make a final payment fee in the amount of $875,000 on the date upon which the outstanding principal amount is required to be repaid in full. This final payment fee replaced the original final payment fee of $500,000. The debt issuance costs and final payment fee are being amortized and accreted, respectively, to interest expense over the term of the Amended Term Loan using the effective interest method.
In connection with the amendment, on September 27, 2012 the Company issued to GECC unregistered stock purchase warrants, which entitle GECC to purchase up to an aggregate of 39,346 shares of XOMA common stock at an exercise price equal to $3.54 per share. These warrants are exercisable immediately and have a five-year term. The warrants’ fair value of $0.1 million was recorded as a discount to the debt obligation and is being amortized over the term of the loan using the effective interest method. The warrants are classified in permanent equity on the consolidated balance sheets.
The Amended Term Loan does not change the remaining terms of the GECC Loan Agreement. The GECC Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including restrictions on the ability to incur indebtedness, grant liens, make investments, dispose of assets, enter into transactions with affiliates and amend existing material agreements, in each case subject to various exceptions. In addition, the GECC Loan Agreement contains customary events of default that entitle GECC to cause any or all of the indebtedness under the GECC Loan Agreement to become immediately due and payable. The events of default include any event of default under a material agreement or certain other indebtedness.
The Company may prepay the Amended Term Loan voluntarily in full, but not in part, and any voluntary and certain mandatory prepayments are subject to a prepayment premium of 3% in the first year after the effective date of the loan amendment, 2% in the second year and 1% thereafter, with certain exceptions. The Company will also be required to pay the $875,000 final payment fee in connection with any voluntary or mandatory prepayment. On the effective date of the loan amendment, the Company paid an accrued final payment fee in the amount of $0.2 million relating to the original final payment fee of $500,000.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
At December 31, 2014 and 2013, the outstanding principal balance under the Amended Term Loan was $5.2 million and $9.4 million, respectively.
Aggregate future principal, final fee payments and discounts of the Company’s total interest bearing obligations - long-term as of December 31, 2014 are as follows (in thousands):
|
Year Ending December 31,
|
Total
|
|||
|
2015
|
$
|
20,276
|
||
|
2016
|
18,447
|
|||
|
38,723
|
||||
|
Less: interest, final payment and discount
|
(3,186
|
)
|
||
|
35,537
|
||||
|
Less: current portion
|
(19,247
|
)
|
||
|
Total
|
$
|
16,290
|
||
On February 27, 2015, the Company entered into a Loan and Security Agreement with Hercules Technology III, L.P., as lender, an affiliate of Hercules Technology Growth Capital, Inc., as agent (collectively, “Hercules”), under which the Company borrowed $20.0 million. The Company used a portion of the proceeds to repay GECC’s outstanding principle balance and interest of $5.5 million. Refer to Note 13, Subsequent Events for further discussion.
Interest Expense
Interest expense and amortization of debt issuance costs and discounts, recorded as other expense in the accompanying consolidated statements of comprehensive loss for the year ended December 31, 2014, 2013 and 2012 are shown below (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Interest expense
|
||||||||||||
|
Servier loan
|
$
|
2,330
|
$
|
2,152
|
$
|
2,097
|
||||||
|
GECC term loan
|
1,638
|
2,064
|
1,850
|
|||||||||
|
Novartis note
|
312
|
362
|
397
|
|||||||||
|
Other
|
23
|
53
|
43
|
|||||||||
|
Total interest expense
|
$
|
4,303
|
$
|
4,631
|
$
|
4,387
|
||||||
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 8. | Income Taxes |
The total income tax benefit consists of the following (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Federal income tax (benefit) provision
|
$
|
-
|
$
|
(14
|
)
|
$
|
(74
|
)
|
||||
|
Total
|
$
|
-
|
$
|
(14
|
)
|
$
|
(74
|
)
|
||||
The Company has significant losses in 2014, 2013 and 2012 and as such there was no income tax expense for the years ended December 31, 2014, 2013 and 2012. The income tax benefit in 2013 and 2012 relate to federal refundable credits.
Reconciliation between the tax provision computed at the federal statutory income tax rate of 34% and the Company’s actual effective income tax rate is as follows:
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Federal tax at statutory rate
|
34
|
%
|
34
|
%
|
34
|
%
|
||||||
|
Warrant valuation
|
40
|
%
|
-17
|
%
|
-4
|
%
|
||||||
|
Permanent items and other
|
-1
|
%
|
0
|
%
|
-1
|
%
|
||||||
|
Valuation allowance
|
-73
|
%
|
-17
|
%
|
-29
|
%
|
||||||
|
Total
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
The significant components of net deferred tax assets as of December 31, 2014 and 2013 were as follows (in millions):
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Capitalized research and development expenses
|
$
|
50.9
|
$
|
49.4
|
||||
|
Net operating loss carryforwards
|
105.0
|
78.4
|
||||||
|
Research and development and other credit carryforwards
|
12.1
|
8.8
|
||||||
|
Other
|
22.1
|
23.5
|
||||||
|
Total deferred tax assets
|
190.1
|
160.1
|
||||||
|
Valuation allowance
|
(190.1
|
)
|
(160.1
|
)
|
||||
|
Net deferred tax assets
|
$
|
-
|
$
|
-
|
||||
The net increase (decrease) in the valuation allowance was $30.0 million, $(74.0) million and $(6.0) million for the years ended December 31, 2014, 2013 and 2012, respectively.
As of December 31, 2014, the Company had federal net operating loss carry-forwards of approximately $292.3 million and state net operating loss carry-forwards of approximately $132.3 million to offset future taxable income. The net operating loss carry-forwards include $5.2 million which relates to stock option deductions that will be recognized through additional paid in capital when utilized. As such, these deductions are not reflected in the Company’s deferred tax assets. No federal net operating loss carry-forward expired in 2014, 2013 and 2012. California net operating losses of $54.3 million, $16.8 million, and $10.4 million expired in the years 2014, 2013 and 2012, respectively.
Accounting standards provide for the recognition of deferred tax assets if realization of such assets is more likely than not. Based upon the weight of available evidence, which includes the Company’s historical operating performance and carry-back potential, the Company has determined that total deferred tax assets should be fully offset by a valuation allowance.
Based on an analysis under Section 382 of the Internal Revenue Code (which subjects the amount of pre-change NOLs and certain other pre-change tax attributes that can be utilized to an annual limitation), the Company experienced ownership changes in 2009 and 2012 which substantially limit the future use of its pre-change Net Operating Losses (“NOLs”) and certain other pre-change tax attributes per year. The Company has excluded the NOLs and R&D credits that will expire as a result of the annual limitations in the deferred tax assets as of December 31, 2014. To the extent that the Company does not utilize its carry-forwards within the applicable statutory carry-forward periods, either because of Section 382 limitations or the lack of sufficient taxable income, the carry-forwards will expire unused.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company files income tax returns in the U.S. federal jurisdiction, State of California, and Ireland. The Internal Revenue Service has completed an audit of the Company's 2009 and 2010 federal income tax returns which resulted in no change. The Company’s federal income tax returns for tax years 2012 and beyond remain subject to examination by the Internal Revenue Service. The Company’s California and Irish income tax returns for tax years 2010 and beyond remain subject to examination by the Franchise Tax Board and Irish Revenue Commissioner. In addition, all of the net operating losses and research and development credit carry-forwards that may be used in future years are still subject to adjustment.
The following table summarizes the Company's activity related to its unrecognized tax benefits (in thousands):
|
2014
|
2013
|
2012
|
||||||||||
|
Balance at January 1
|
$
|
4,274
|
$
|
4,104
|
$
|
-
|
||||||
|
Increase related to current year tax position
|
720
|
164
|
49
|
|||||||||
|
Increase related to prior year tax position
|
509
|
6
|
4,055
|
|||||||||
|
Balance at December 31
|
$
|
5,503
|
$
|
4,274
|
$
|
4,104
|
||||||
As of December 31, 2014, the Company had a total of $4.0 million of net unrecognized tax benefits, none of which would affect the effective tax rate upon realization. The Company currently has a full valuation allowance against its U.S. net deferred tax assets which would impact the timing of the effective tax rate benefit should any of these uncertain tax positions be favorably settled in the future.
The Company does not expect the unrecognized tax benefits to change significantly over the next twelve months. The Company will recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. As of December 31, 2014, the Company has not accrued interest or penalties related to uncertain tax positions.
| 9. | Compensation and Other Benefit Plans |
The Company grants qualified and non-qualified stock options, restricted stock units (“RSUs”), common stock and other stock-based awards under various plans to directors, officers, employees and other individuals. Stock options are granted at exercise prices of not less than the fair market value of the Company’s common stock on the date of grant. Generally, stock options granted to employees fully vest four years from the grant date and expire ten years from the date of the grant or three months from the date of termination of employment (longer in case of death or certain retirements). However, certain options granted to employees vest monthly or immediately, certain options granted to directors vest monthly over one year or three years and certain options may fully vest upon a change of control of the Company or may accelerate based on performance-driven measures. Additionally, the Company has an Amended and Restated Employee Stock Purchase Plan (“ESPP”) that allows employees to purchase Company shares at a purchase price equal to 95% of the closing price on the exercise date.
Employee Stock Purchase Plan
Under the ESPP plan approved by the Company’s stockholders, the Company is authorized to issue up to 233,333 shares of common stock to employees through payroll deductions at a purchase price per share equal to 95% of the closing price of XOMA shares on the exercise date. An employee may elect to have payroll deductions made under the ESPP for the purchase of shares in an amount not to exceed 15% of the employee’s compensation.
In 2014, 2013, and 2012, employees purchased 17,702, 15,262, and 17,054 shares of common stock, respectively, under the ESPP. Net payroll deductions under the ESPP totaled $74,000, $60,000, and $46,000 for 2014, 2013, and 2012, respectively.
Deferred Savings Plan
Under section 401(k) of the Internal Revenue Code of 1986, the Board of Directors adopted, effective June 1, 1987, a tax-qualified deferred compensation plan for employees of the Company. Participants may make contributions which defer up to 50% of their eligible compensation per payroll period, up to a maximum for 2014 of $17,500 (or $23,000 for employees over 50 years of age). The Company may, at its sole discretion, make contributions each plan year, in cash or in shares of the Company’s common stock, in amounts which match up to 50% of the salary deferred by the participants. The expense related to these contributions was $1.0 million, $0.9 million, and $0.8 million for the years ended December 31, 2014, 2013, and 2012, respectively, and 100% was paid in common stock in each year.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Stock Option Plans
Historically, option grants intended as long-term incentive compensation have been made pursuant to the Company’s 1981 Share Option Plan (the “Option Plan”) and Restricted Share Plan (the “Restricted Plan”). In May of 2010, the Compensation Committee and the full Board adopted, and in July of 2010 the Company’s stockholders approved, a new equity-based compensation plan, the 2010 Long Term Incentive and Share Award Plan, which has since been amended and restated as the Amended and Restated 2010 Long Term Incentive and Stock Award Plan (the “Long Term Incentive Plan”). The Long Term Incentive Plan is intended to consolidate the Company’s long-term incentive compensation under a single plan, by replacing the Option Plan, the Restricted Plan and the 1992 Directors Share Option Plan (the “Directors Plan”) going forward, and to provide a more current set of terms pursuant to which to provide this type of compensation. In May 2014, the Company’s stockholders approved an amendment to the Company’s Long Term Incentive Plan to (a) increase the number of shares of common stock issuable over the term of the plan by an additional 5,350,000 to 18,771,206 shares in the aggregate and (b) provide that, for each stock appreciation right, restricted share, restricted stock unit, performance share, performance unit, dividend equivalent or other stock-based award issued, the number of available shares under the plan will be reduced by 1.18 shares.
The Long Term Incentive Plan grants stock options, RSUs, and other stock-based awards to eligible employees, consultants and directors. No further grants or awards will be made under the Option Plan, the Restricted Share Plan or the Directors Plan. Shares underlying options previously issued under the Option Plan, the Restricted Share Plan or the Directors Plan that are currently outstanding will, upon forfeiture, cancellation, surrender or other termination, become available under the Long Term Incentive Plan. Stock-based awards granted under the Long Term Incentive Plan may be exercised when vested and generally expire ten years from the date of the grant or three to six months from the date of termination of employment (longer in case of death or certain retirements). Vesting periods vary based on awards granted, however, certain stock-based awards may vest immediately or may accelerate based on performance-driven measures.
As of December 31, 2014, the Company had 6,221,101 shares available for grant under the stock option plans. As of December 31, 2014, options and RSUs covering 10,005,649 shares of common stock were outstanding under the stock option plans.
Stock Options
In 2014, the Board of Directors of the Company approved grants under the Company’s Amended and Restated 2010 Long Term Incentive Plan for an aggregate of 1,891,989 stock options to certain employees and directors of the Company. The stock options vest monthly over four years for employees and one year for directors.
In 2013, the Board of Directors of the Company approved grants under the Company’s Amended and Restated 2010 Long Term Incentive Plan for an aggregate of 1,168,203 stock options to certain employees and the directors of the Company. The stock options vest monthly over four years for employees and one year for directors.
In 2012, the Board of Directors of the Company approved grants under the Company’s Amended and Restated 2010 Long Term Incentive Plan for an aggregate of 2,351,445 stock options to certain employees and the directors of the Company. The stock options vest monthly over four years for employees and one year for directors.
Stock options held by employees who qualify for retirement age (defined as employees that are a minimum of 55 years of age and the sum of their age plus years of full-time employment with the Company exceeds 70 years) vest immediately.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Stock Option Plans Summary
The following table summarizes the Company’s stock option activity:
|
2014
|
2013
|
2012
|
||||||||||||||||||||||
|
Number of
Shares
|
Weighted
Average
Exercise
Price
|
Number of
Shares
|
Weighted
Average
Exercise
Price
|
Number of
Shares
|
Weighted
Average
Exercise
Price
|
|||||||||||||||||||
|
Outstanding at beginning of year
|
7,216,041
|
$
|
8.42
|
6,788,383
|
$
|
8.99
|
5,053,435
|
$
|
12.55
|
|||||||||||||||
|
Granted
|
1,891,989
|
6.69
|
1,168,203
|
3.13
|
2,351,445
|
2.59
|
||||||||||||||||||
|
Exercised
|
(915,911
|
)
|
3.91
|
(589,355
|
)
|
2.26
|
(90,252
|
)
|
1.68
|
|||||||||||||||
|
Forfeited, expired or cancelled
|
(489,810
|
)
|
14.36
|
(151,190
|
)
|
17.46
|
(526,245
|
)
|
15.84
|
|||||||||||||||
|
Outstanding at end of year
|
7,702,309
|
8.15
|
7,216,041
|
8.42
|
6,788,383
|
8.99
|
||||||||||||||||||
|
Exercisable at end of year
|
4,908,925
|
9.98
|
4,814,926
|
11.14
|
4,276,834
|
12.42
|
||||||||||||||||||
|
Weighted average grant date fair value
|
$
|
4.49
|
$
|
2.27
|
$
|
1.89
|
||||||||||||||||||
The aggregate intrinsic value of stock options exercised in 2014, 2013, and 2012 was $2.9 million, $1.7 million, and $0.1 million, respectively.
As of December 31, 2014, there were 7,418,259 stock options vested and expected to vest with a weighted average exercise price per share of $8.27, aggregate intrinsic value of $3.1 million, and a weighted average remaining contractual term of 6.7 years. As of December 31, 2014, there were 4,908,925 stock options exercisable with an aggregate intrinsic value of $2.3 million and a weighted average remaining contractual term of 5.7 years.
As of December 31, 2014, $6.0 million of total unrecognized compensation expense related to stock options is expected to be recognized over a weighted average period of 2.4 years.
Restricted Stock Units
In 2014, the Board of Directors of the Company approved grants under the Amended and Restated 2010 Long Term Incentive Plan for an aggregate of 1,506,194 RSUs to certain employees and directors of the Company. The RSUs vest annually over three years in equal increments.
In 2013, the Board of Directors of the Company approved grants under the Amended and Restated 2010 Long Term Incentive Plan for an aggregate of 958,385 RSUs to certain employees and directors of the Company. The RSUs vest annually over three years in equal increments.
In 2012, the Board of Directors of the Company approved grants under the Amended and Restated 2010 Long Term Incentive Plan for an aggregate of 1,292,923 RSUs to certain employees and directors of the Company. The RSUs vest annually over three years in equal increments.
RSUs held by employees who qualify for retirement age (defined as employees that are a minimum of 55 years of age and the sum of their age plus years of full-time employment with the Company exceeds 70 years) vest immediately.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Unvested RSU activity for the year ended December 31, 2014 is summarized below:
|
Number of
Shares
|
Weighted
Average Grant
Date Fair Value
|
|||||||
|
Unvested awards at December 31, 2013
|
1,738,037
|
$
|
2.73
|
|||||
|
Granted
|
1,506,194
|
7.03
|
||||||
|
Vested
|
(1,099,701
|
)
|
3.51
|
|||||
|
Forfeited
|
(190,651
|
)
|
4.22
|
|||||
|
Unvested awards at December 31, 2014
|
1,953,879
|
5.46
|
||||||
The total grant-date fair value of RSUs that vested during the year ended December 31, 2014 was $3.9 million. As of December 31, 2014, $5.9 million of total unrecognized compensation expense related to employee RSUs was expected to vest over a weighted average period of 1.7 years.
Stock-based Compensation Expense
The Company recognizes compensation expense for all stock-based payment awards made to the Company’s employees, consultants and directors that are expected to vest based on estimated fair values. The valuation of stock option awards is determined at the date of grant using the Black-Scholes option pricing model. This model requires inputs such as the expected term of the option, expected volatility and risk-free interest rate. To establish an estimate of expected term, the Company considers the vesting period and contractual period of the award and its historical experience of stock option exercises, post-vesting cancellations and volatility. The estimate of expected volatility is based on the Company’s historical volatility. The risk-free rate is based on the yield available on United States Treasury zero-coupon issues corresponding to the expected term of the award. To establish an estimate of forfeiture rate, the Company considers its historical experience of option forfeitures and terminations.
The fair value of stock option awards was estimated using the Black-Scholes model with the following weighted average assumptions for the years ended December 31, 2014, 2013, and 2012:
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Expected volatility
|
92
|
%
|
92
|
%
|
92
|
%
|
||||||
|
Risk-free interest rate
|
1.72
|
%
|
0.89
|
%
|
0.82
|
%
|
||||||
|
Expected term
|
5.6 years
|
5.6 years
|
5.6 years
|
|||||||||
The valuation of RSUs is determined at the date of grant using the closing stock price. The forfeiture rate impacts the amount of aggregate compensation for both stock options and RSUs. To establish an estimate of forfeiture rate, the Company used an independent third party to consider the Company’s historical experience of option forfeitures and terminations.
The following table shows total stock-based compensation expense included in the accompanying consolidated statements of comprehensive loss for the years ended December 31, 2014, 2013, and 2012 (in thousands):
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Research and development
|
$
|
5,557
|
$
|
2,358
|
$
|
2,391
|
||||||
|
Selling, general and administrative
|
5,215
|
2,741
|
1,893
|
|||||||||
|
Total stock-based compensation expense
|
$
|
10,772
|
$
|
5,099
|
$
|
4,284
|
||||||
There was no capitalized stock-based compensation cost as of December 31, 2014, 2013 and 2012, and there were no recognized tax benefits related to the Company’s stock-based compensation expense during the years ended December 31, 2014, 2013 and 2012.
|
10.
|
Capital Stock
|
In May 2014, the Company’s stockholders approved an amendment to the Company’s Certificate of Incorporation to increase the number of authorized shares of the Company’s common stock, par value $0.0075 per share, by an additional 138,666,666 to 277,333,332 shares.
Registered Direct Offerings
In June of 2009, the Company entered into a definitive agreement with certain institutional investors to sell 695,652 units, with each unit consisting of one share of the Company’s common stock and a warrant to purchase 0.50 of a share of common stock, for gross proceeds of approximately $12.0 million, before deducting placement agent fees and estimated offering expenses of $0.8 million, in a second registered direct offering. The investor purchased the units at a price of $17.25 per unit. The warrants, which represent the right to acquire an aggregate of up to 347,826 shares of common stock, are exercisable at any time on or prior to December 10, 2014 at an exercise price of $19.50 per share. As of December 31, 2014 these warrants have expired unexercised.
On December 8, 2014, the Company completed a registered direct offering of 8,097,165 shares of its common stock, and accompanying warrants to purchase one share of common stock for each share purchased at an offering price of $4.94 per share to certain institutional investors. Total gross proceeds from the offering were approximately $40.0 million before deducting underwriting discounts, commissions and estimated offering expenses totaling approximately $2.3 million. The warrants, which represent the right to acquire up to an aggregate of 8,097,165 shares of common stock, are exercisable immediately, have a two-year term and an exercise price of $7.90 per share. As of December 31, 2014 all of these warrants were outstanding.
Underwritten Offerings
In February of 2010, the Company completed an underwritten offering of 2.8 million units, with each unit consisting of one share of the Company’s common stock and a warrant to purchase 0.45 of a share of common stock, for gross proceeds of approximately $21 million. As of December 31, 2014 all of these warrants were outstanding.
On March 9, 2012, the Company completed an underwritten public offering of 29,669,154 shares of its common stock, and accompanying warrants to purchase one half of a share of common stock for each share purchased, at a public offering price of $1.32 per share. Total gross proceeds from the offering were approximately $39.2 million, before deducting underwriting discounts and commissions and offering expenses totaling approximately $3.0 million. The warrants, which represent the right to acquire an aggregate of up to 14,834,577 shares of common stock, are immediately exercisable and have a five-year term and an exercise price of $1.76 per share. As of December 31, 2014, 12,109,418 of these warrants were outstanding.
On October 29, 2012, the Company completed an underwritten public offering of 13,333,333 shares of its common stock, at a public offering price of $3.00 per share. Total gross proceeds from the offering were approximately $40.0 million, before deducting underwriting discounts and commissions and offering expenses totaling approximately $3.0 million.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On August 23, 2013, the Company completed an underwritten public offering of 8,736,187 shares of its common stock, including 1,139,502 shares of its common stock that were issued upon the exercise of the underwriters’ 30-day over-allotment option, at a public offering price of $3.62 per share. Total gross proceeds from the offering were approximately $31.6 million, before deducting underwriting discounts and commissions and estimated offering expenses totaling approximately $2.2 million.
On December 18, 2013, the Company completed an underwritten public offering of 10,925,000 shares of its common stock, including 1,425,000 shares of its common stock that were issued upon the exercise of the underwriters’ 30-day over-allotment option, at a public offering price of $5.25 per share. Total gross proceeds from the offering were approximately $57.4 million, before deducting underwriting discounts and commissions and estimated offering expenses totaling approximately $3.8 million.
ATM Agreements
On February 4, 2011, the Company entered into an At Market Issuance Sales Agreement (the “2011 ATM Agreement”), with McNicoll, Lewis & Vlak LLC (now known as MLV & Co. LLC, “MLV”), under which it may sell shares of its common stock from time to time through the MLV, as the agent for the offer and sale of the shares, in an aggregate amount not to exceed the amount that can be sold under the Company’s registration statement on Form S-3 (File No. 333-172197) filed with the SEC on February 11, 2011 and amended on March 10, 2011, June 3, 2011 and January 3, 2012, which was most recently declared effective by the SEC on January 17, 2012. MLV may sell the shares by any method permitted by law deemed to be an “at the market” offering as defined in Rule 415 of the Securities Act, including without limitation sales made directly on The NASDAQ Global Market, on any other existing trading market for the Company’s common stock or to or through a market maker. MLV also may sell the shares in privately negotiated transactions, subject to the Company’s prior approval. The Company will pay MLV a commission equal to 3% of the gross proceeds of the sales price of all shares sold through it as sales agent under the 2011 ATM Agreement. From the inception of the 2011 ATM Agreement through December 31, 2013, the Company sold a total of 7,572,327 shares of common stock under this agreement for aggregate gross proceeds of $14.6 million. No shares of common stock have been sold under this agreement since February 3, 2012. Total offering expenses incurred related to sales under the 2011 ATM Agreement from inception to December 31, 2013, were $0.5 million. As of December 31, 2014, the 2011 ATM Agreement expired.
| 11. | Commitments and Contingencies |
Collaborative Agreements, Royalties and Milestone Payments
The Company is obligated to pay royalties, ranging from 0.5% to 5% of the selling price of the licensed component and up to 40% of any sublicense fees to various universities and other research institutions based on future sales or licensing of products that incorporate certain products and technologies developed by those institutions.
In addition, the Company has committed to make potential future “milestone” payments to third parties as part of licensing and development programs. Payments under these agreements become due and payable only upon the achievement of certain developmental, regulatory and/or commercial milestones. Because it is uncertain if and when these milestones will be achieved, such contingencies, aggregating up to $77.3 million (assuming one product per contract meets all milestones events) have not been recorded on the accompanying consolidated balance sheet. The Company is unable to determine precisely when and if payment obligations under the agreements will become due as these obligations are based on milestone events, the achievement of which is subject to a significant number of risks and uncertainties.
Leases
As of December 31, 2014, the Company leased administrative, research facilities, and office equipment under operating leases expiring on various dates through April 2023. These leases require the Company to pay taxes, insurance, maintenance and minimum lease payments.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company estimates future minimum lease payments, excluding sub-lease income as of December 31, 2014 to be (in thousands):
|
Operating
Leases
|
||||
|
2015
|
$
|
3,428
|
||
|
2016
|
3,531
|
|||
|
2017
|
3,637
|
|||
|
2018
|
3,742
|
|||
|
2019
|
3,852
|
|||
|
Thereafter
|
10,733
|
|||
|
Minimum lease payments
|
$
|
28,923
|
||
Total rental expense, including other costs required under the Company’s leases, was approximately $3.5 million, $3.5 million and $4.5 million for the years ended December 31, 2014, 2013, and 2012, respectively. Rental expense based on leases allowing for escalated rent payments are recognized on a straight-line basis. At the expiration of the lease, the Company is required to restore certain of its leased property to certain conditions in place at the time of lease inception. The Company believes these costs will not be material to its operations.
In 2012, the Company vacated and subleased two of its leased facilities, which housed its large scale manufacturing operations and associated quality functions. The Company incurred $0.1 million in restructuring charges during 2014 in connection with a portion of lease payments not offset by sublease income for these buildings. The Company does not expect to incur restructuring charges during 2015 in connection with these lease payments.
As a result of the restructuring in the second quarter of 2009, the Company vacated one of its leased buildings. Effective December 2010, the Company entered into a sublease agreement for this building through May of 2014. For the year ended December 31, 2014, the Company recognized $49,000 in sublease income under this agreement.
| 12. | Concentration of Risk, Segment and Geographic Information |
Concentration of Risk
Cash equivalents, short-term investments, and receivables are financial instruments, which potentially subject the Company to concentrations of credit risk, as well as liquidity risk for certain cash equivalents such as money market funds. The Company has not encountered such issues during 2014. The Company’s policy is to investments with high credit quality and liquidity to limit the amount of credit exposure. The Company currently maintains a portfolio of cash equivalents and have not experienced any losses.
The Company has not experienced any significant credit losses and does not generally require collateral on receivables. For the year ended December 31, 2014, two customers represented 51% and 28% of total revenue, respectively, and as of December 31, 2014, and three customers represented 44%, 34% and 12% of the accounts receivable balance, respectively.
For the year ended December 31, 2013, three customers represented 43%, 26%, and 20% of total revenue, respectively, and as of December 31, 2013, and two customers represented 73% and 13% of the accounts receivable balance respectively.
Receivables are recorded at invoice value. The Company reviews their exposure to accounts receivable, including the requirement for allowances based on management’s judgment. The Company has not historically experienced any significant losses. The Company does not currently require collateral for any of its accounts receivable.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Segment Information
The Company has determined that it operates in one business segment as it only reports operating results on an aggregate basis to the chief operating decision maker of the Company. The Company’s property and equipment is held primarily in the United States.
Geographic Information
Revenue attributed to the following geographic regions for each of the three years ended December 31, 2014, 2013 and 2012 was as follows (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
United States
|
$
|
11,756
|
$
|
19,955
|
$
|
14,134
|
||||||
|
Europe
|
5,510
|
15,396
|
18,454
|
|||||||||
|
Asia Pacific
|
1,600
|
100
|
1,194
|
|||||||||
|
Total
|
$
|
18,866
|
$
|
35,451
|
$
|
33,782
|
||||||
| 13. | Subsequent Events |
On January 9, 2015, Servier and the Company entered into Amendment No. 2 (“Loan Amendment”) to the Servier Loan Agreement initially entered into on December 30, 2010 and subsequently amended by a Consent, Transfer, Assumption and Amendment Agreement entered into as of August 12, 2013. The Loan Amendment modifies the maturity date of the loan from January 13, 2016 to three tranches due on January 15, 2016, January 15, 2017 and January 15, 2018 and provides that principal shall be repaid as follows: €3.0 million to be repaid on January 15, 2016, €5.0 million to be repaid on January 15, 2017, and €7.0 million to be repaid on January 15, 2018. All other terms of the Loan Agreement remain unchanged, including the interest rate calculations, EURIBOR+2% and the formula for resetting the interest rate on the 15th of January and July every six months.
On January 9, 2015, Servier and the Company entered into Amendment No. 2 to the Collaboration Agreement. Under the Collaboration Agreement the Company was eligible to receive up to approximately $433 million in the aggregate in milestone payments, most of which were denominated in Euros, if the Company re-acquires cardiovascular and/or diabetes rights for use in the United States, and approximately $770 million in aggregate milestone payments if the Company does not re-acquire those rights. Under the Collaboration Amendment, the Company would be eligible to receive up to $415 million in the aggregate in milestone payments in the event the Company re-acquires the cardiovascular and/or diabetes rights for use in the United States and approximately $752 million if the Company does not re-acquire those rights. The milestone reductions are related to a very low prevalence indication of which Servier would not have pursued development had these payments been required. All other terms of the Collaboration Agreement remain unchanged.
On January 26, 2015, Symplmed Pharmaceuticals announced the FDA approved PRESTALIA® (perindopril arginine and amlodipine) tablets, licensed from Servier, for the treatment of hypertension. In July 2013, the Company transferred the development and commercialization rights of Prestalia to Symplmed. Pursuant to the transfer agreement with Symplmed, the Company will receive up to double-digit royalties on sales of PRESTALIA.
On February 27, 2015, Hercules and the Company, entered into a Loan and Security Agreement (the “Hercules Loan Agreement”), under which the Company borrowed $20.0 million. The Company used a portion of the proceeds under the Hercules Loan Agreement to repay GECC’s outstanding principle balance and interest of $5.5 million and plans to use the remaining proceeds for general corporate purposes. The interest rate will be calculated at a rate equal to the greater of either (i) 9.40% plus the prime rate as reported from time to time in The Wall Street Journal minus 7.25%, and (ii) 9.40%. Payments under the Hercules Loan Agreement are interest only until one month prior to the Amortization Date, defined as July 1, 2016 (which will be extended to October 1, 2016, if the Borrower achieves certain clinical milestones on or before July 1, 2016). The interest only period will be followed by equal monthly payments of principal and interest amortized over a 30 month schedule through the scheduled maturity date of September 1, 2018 (the “Loan Maturity Date”). The entire principal balance, including a balloon payment of principal, as applicable, will be due and payable on the Loan Maturity Date. In addition, a final payment equal to $1,150,000 will be due on the Loan Maturity Date, or such earlier date specified in the Hercules Loan Agreement. The Company’s obligations under the Hercules Loan Agreement are secured by a security interest in substantially all of its assets, other than its intellectual property. If the Company prepays the loan prior to the Loan Maturity Date, it will pay Hercules a prepayment charge, based on a prepayment fee equal to 3.00% of the amount prepaid, if the prepayment occurs in any of the first 12 months following the Closing Date, 2.00% of the amount prepaid, if the prepayment occurs after 12 months from the Closing Date but prior to 24 months from the Closing Date, and 1.00% of the amount prepaid if the prepayment occurs after 24 months from the Closing Date. The Hercules Loan Agreement includes customary affirmative and restrictive covenants, but does not include any financial maintenance covenants, and also includes standard events of default, including payment defaults. Upon the occurrence of an event of default, a default interest rate of an additional 5% may be applied to the outstanding loan balances, and Hercules may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Hercules Loan Agreement.
XOMA Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In connection with the Hercules Loan Agreement, the Company issued a warrant to Hercules which is exercisable in whole or in part for up to an aggregate of 181,268 shares of common stock with an exercise price of $3.31 per share (the “Warrant”). The Warrant may be exercised on a cashless basis and is exercisable for a term beginning on the date of issuance and ending on the earlier to occur of five years from the date of issuance or the consummation of certain acquisitions of the Company as set forth in the Warrant. The number of shares for which the Warrant is exercisable and the associated exercise price are subject to certain proportional adjustments as set forth in the Warrant.
|
14.
|
Quarterly Financial Information (unaudited)
|
The following is a summary of the quarterly results of operations for the years ended December 31, 2014 and 2013:
|
Consolidated Statements of Operations
|
||||||||||||||||
|
Quarter Ended
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
(In thousands, except per share amounts)
|
||||||||||||||||
|
2014
|
||||||||||||||||
|
Total revenues
|
$
|
3,410
|
$
|
5,973
|
$
|
5,136
|
$
|
4,347
|
||||||||
|
Total operating costs and expenses (1)
|
(26,884
|
)
|
(24,750
|
)
|
(25,589
|
)
|
(23,475
|
)
|
||||||||
|
Other income (expense), net (2)
|
18,787
|
6,880
|
6,054
|
11,810
|
||||||||||||
|
Income tax benefit
|
-
|
-
|
-
|
-
|
||||||||||||
|
Net loss
|
$
|
(4,687
|
)
|
$
|
(11,897
|
)
|
$
|
(14,399
|
)
|
$
|
(7,318
|
)
|
||||
|
Basice net loss per share of common stock
|
$
|
(0.04
|
)
|
$
|
(0.11
|
)
|
$
|
(0.13
|
)
|
$
|
(0.07
|
)
|
||||
|
Diluted net loss per share of common stock
|
$
|
(0.21
|
)
|
$
|
(0.17
|
)
|
$
|
(0.17
|
)
|
$
|
(0.12
|
)
|
||||
|
2013
|
||||||||||||||||
|
Total revenues
|
$
|
9,453
|
$
|
7,151
|
$
|
6,312
|
$
|
12,535
|
||||||||
|
Total operating costs and expenses
|
(20,777
|
)
|
(21,230
|
)
|
(23,535
|
)
|
(28,114
|
)
|
||||||||
|
Other (expense) income, net (2)
|
(13,563
|
)
|
(3,169
|
)
|
(12,416
|
)
|
(36,719
|
)
|
||||||||
|
Income tax benefit (expense)
|
-
|
-
|
15
|
(1
|
)
|
|||||||||||
|
Net loss
|
$
|
(24,887
|
)
|
$
|
(17,248
|
)
|
$
|
(29,624
|
)
|
$
|
(52,299
|
)
|
||||
|
Basic and diluted net loss per share of common stock
|
$
|
(0.30
|
)
|
$
|
(0.21
|
)
|
$
|
(0.34
|
)
|
$
|
(0.55
|
)
|
||||
| (1) | In 2014, the Company corrected an immaterial error driven by certain stock-based compensation expense in the fourth quarter of 2014, resulting in a decrease to operating expenses and net loss by $1.6 million and a decrease to basic and diluted loss per share of $0.01 and $0.02, respectively, for the three months ended December 31, 2014. Refer to Note 2, Basis of Presentation and Significant Accounting Policies - Correction of an Immaterial Error |
| (2) | Fluctuations in 2014 and 2013 primarily relate to (losses) gains on the revaluation of the contingent warrant liabilities. |
|
Incorporation By Reference
|
|||||
|
Exhibit
Number |
Exhibit Description
|
Form
|
SEC File No.
|
Exhibit
|
Filing Date
|
|
3.1
|
Certificate of Incorporation of XOMA Corporation
|
8-K
|
000-14710
|
3.1
|
01/03/2012
|
|
3.2A
|
Certificate of Amendment of Certificate of Incorporation of XOMA Corporation
|
8-K
|
000-14710
|
3.1
|
05/31/2012
|
|
3.2B
|
Certificate of Amendment of Certificate of Incorporation of XOMA Corporation
|
8-K
|
000-14710
|
3.1
|
05/28/2014
|
|
3.3
|
By-laws of XOMA Corporation
|
8-K
|
000-14710
|
3.2
|
01/03/2012
|
|
4.1
|
Reference is made to Exhibits 3.1, 3.2 and 3.3
|
||||
|
4.2
|
Specimen of Common Stock Certificate
|
8-K
|
000-14710
|
4.1
|
01/03/2012
|
|
4.3
|
Form of Certificate of Designations of Series A Preferred Stock
|
8-K
|
000-14710
|
3.1
|
01/03/2012
|
|
4.4
|
Form of Amended and Restated Warrant (June 2009 Warrants)
|
8-K
|
000-14710
|
10.6
|
02/02/2010
|
|
4.5
|
Form of Warrant (February 2010 Warrants)
|
8-K
|
000-14710
|
10.2
|
02/02/2010
|
|
4.6
|
Form of Warrant (December 2011 Warrants)
|
10-K
|
000-14710
|
4.9
|
03/14/2012
|
|
4.7
|
Form of Warrant (March 2012 Warrants)
|
8-K
|
000-14710
|
4.1
|
03/07/2012
|
|
4.8
|
Form of Warrant (September 2012 Warrants)
|
8-K
|
000-14710
|
4.10
|
10/03/2012
|
|
4.9
|
Registration Rights Agreement, dated June 12, 2014, by and among XOMA Corporation, 667, L.P., Baker Brothers Life Sciences, L.P., and 14159, L.P.
|
8-K
|
000-14710
|
4.1
|
06/12/2014
|
|
4.10
|
Form of Warrants (December 2014 Warrants)
|
8-K
|
000-14710
|
4.1
|
12/09/2014
|
|
10.1*
|
1981 Share Option Plan as amended and restated
|
S-8
|
333-171429
|
10.1
|
12/27/2010
|
|
10.2*
|
Form of Share Option Agreement for 1981 Share Option Plan
|
10-K
|
000-14710
|
10.1A
|
03/11/2008
|
|
10.3*
|
Restricted Share Plan as amended and restated
|
S-8
|
333-171429
|
10.1
|
12/27/2010
|
|
10.4*
|
Form of Share Option Agreement for Restricted Share Plan
|
10-K
|
000-14710
|
10.2A
|
03/11/2008
|
|
10.5*
|
2007 CEO Share Option Plan
|
8-K
|
000-14710
|
10.7
|
08/07/2007
|
|
10.6*
|
1992 Directors Share Option Plan as amended and restated
|
S-8
|
333-171429
|
10.1
|
12/27/2010
|
|
10.7*
|
Form of Share Option Agreement for 1992 Directors Share Option Plan (initial grants)
|
10-K
|
000-14710
|
10.3A
|
03/11/2008
|
|
10.8*
|
Form of Share Option Agreement for 1992 Directors Share Option Plan (subsequent grants)
|
10-K
|
000-14710
|
10.3B
|
03/11/2008
|
|
10.9*
|
2002 Director Share Option Plan
|
S-8
|
333-151416
|
10.10
|
06/04/2008
|
|
10.10*
|
XOMA Corporation Amended and Restated 2010 Long Term Incentive and Stock Award Plan
|
S-8
|
000-14710
|
99.1
|
09/12/2014
|
|
10.11*
|
Form of Stock Option Agreement for Amended and Restated 2010 Long Term Incentive and Stock Award Plan
|
10-K
|
000-14710
|
10.6A
|
03/14/2012
|
|
10.12*
|
Form of Restricted Stock Unit Agreement for Amended and Restated 2010 Long Term Incentive and Stock Award Plan
|
10-K
|
000-14710
|
10.6B
|
03/14/2012
|
|
10.13*
|
Management Incentive Compensation Plan as amended and restated
|
8-K
|
000-14710
|
10.3
|
11/06/2007
|
|
10.14*
|
CEO Incentive Compensation Plan
|
10-K
|
000-14710
|
10.4A
|
03/11/2008
|
|
10.15*
|
Amendment No. 1 to CEO Incentive Compensation Plan
|
10-K
|
000-14710
|
10.7B
|
03/14/2012
|
|
10.16*
|
Bonus Compensation Plan
|
10-K
|
000-14710
|
10.4B
|
03/11/2008
|
|
10.17*
|
Amended and Restated 1998 Employee Stock Purchase Plan
|
POS AM
|
333-174730
|
10.2
|
01/03/2012
|
|
10.18
|
Form of Amended and Restated Indemnification Agreement for Officers
|
10-K
|
000-14710
|
10.6
|
03/08/2007
|
|
10.19
|
Form of Amended and Restated Indemnification Agreement for Employee Directors
|
10-K
|
000-14710
|
10.7
|
03/08/2007
|
|
10.20
|
Form of Amended and Restated Indemnification Agreement for Non-employee Directors
|
10-K
|
000-14710
|
10.8
|
03/08/2007
|
|
10.21*
|
Employment Agreement entered into between XOMA (US) LLC and Fred Kurland, dated as of December 29, 2008
|
10-K/A
|
000-14710
|
10.7B
|
12/27/2010
|
|
10.22*
|
Amended and Restated Employment Agreement entered into between XOMA (US) LLC and Charles C. Wells, dated as of December 30, 2008
|
10-K/A
|
000-14710
|
10.7D
|
12/27/2010
|
|
10.23
|
Officer Employment Agreement dated March 19, 2013 between XOMA Corporation and Paul Rubin
|
10-K
|
000-14710 | 10.23 |
03/12/2014
|
|
10.24*
|
Employment Agreement effective as of January 4, 2012 between XOMA (US) LLC and John Varian
|
10-K
|
000-14710
|
10.10G
|
03/14/2012
|
|
10.25
|
Officer Employment Agreement dated March 10, 2014 between XOMA Corporation and Pat Scannon
|
10-K |
000-14710
|
10.25 | 03/12/2014 |
|
10.26*
|
Form of Change of Control Severance Agreement entered into between XOMA Ltd. and certain of its executives
|
10-K
|
000-14710
|
10.12
|
03/10/2011
|
|
10.27*
|
Change of Control Agreement entered into between XOMA Ltd. and John Varian, dated January 4, 2012
|
10-K
|
000-14710
|
10.12A
|
03/14/2012
|
|
10.28
|
Retention Benefit Agreement entered into between XOMA Corporation and John Varian, dated March 11, 2014
|
10-K
|
000-14710
|
10.28 | 03/12/2014 |
|
10.29
|
Lease of premises at 804 Heinz Street, Berkeley, California dated February 13, 2013
|
10-K
|
000-14710
|
10.29 | 03/12/2014 |
|
10.30
|
Lease of premises at 2910 Seventh Street, Berkeley, California dated February 13, 2013
|
10-K
|
000-14710
|
10.30 | 03/12/2014 |
|
10.31
|
First amendment to lease of premises at 2910 Seventh Street, Berkeley, California dated February 22, 2013
|
10-K
|
000-14710
|
10.31 | 03/12/2014 |
|
10.32
|
Lease of premises at 5860 and 5864 Hollis Street, Emeryville, California dated February 13, 2013
|
10-K
|
000-14710
|
10.32 | 03/12/2014 |
|
10.33
|
Lease of premises at 2850 Seventh Street, Second Floor, Berkeley, California dated as of December 28, 2001 (with addendum and guaranty)
|
10-K
|
000-14710
|
10.20
|
04/01/2002
|
|
10.34†
|
Second Amended and Restated Collaboration Agreement dated January 12, 2005, by and between XOMA (US) LLC and Genentech, Inc.
|
10-K
|
000-14710
|
10.26C
|
03/15/2005
|
|
10.35†
|
Agreement related to LUCENTIS® License Agreement and RAPTIVA® Collaboration Agreement dated September 9, 2009, by and between XOMA (Bermuda) Ltd., XOMA (US) LLC and Genentech, Inc.
|
10-Q
|
000-14710
|
10.18A
|
11/09/2009
|
|
10.36†
|
License Agreement by and between XOMA Ireland Limited and MorphoSys AG, dated as of February 1, 2002
|
10-K
|
000-14710
|
10.43
|
02/01/2002
|
|
10.37A†
|
License Agreement, dated as of December 29, 2003, by and between Diversa Corporation (n/k/a BP Biofuels Advanced Technology Inc.) and XOMA Ireland Limited
|
8-K/A
|
000-14710
|
2
|
03/19/2004
|
|
10.37B
|
First Amendment, dated October 28, 2014, to the License Agreement between XOMA (US) LLC (assigned to it by XOMA Ireland Limited) and BP Biofuels Advanced Technology Inc. (previously Diversa Corporation, previously Verenium Corporation).
|
10-Q
|
000-14710
|
10.3
|
11/06/2014
|
|
10.38†
|
GSSM License Agreement, effective as of May 2, 2008, by and between Verenium Corporation (n/k/a BP Biofuels Advanced Technology Inc.) and XOMA Ireland Limited
|
10-K
|
000-14710
|
10.25A
|
03/10/2011
|
|
10.39†
|
Secured Note Agreement, dated as of May 26, 2005, by and between Chiron Corporation and XOMA (US) LLC
|
10-Q
|
000-14710
|
10.3
|
08/08/2005
|
|
10.40†
|
Amended and Restated Research, Development and Commercialization Agreement, executed November 7, 2008, by and between Novartis Vaccines and Diagnostics, Inc. (formerly Chiron Corporation) and XOMA (US) LLC
|
10-K
|
000-14710
|
10.24C
|
03/11/2009
|
|
10.41†
|
Amendment No. 1 to Amended and Restated Research, Development and Commercialization Agreement, effective as of April 30, 2010, by and between Novartis Vaccines and Diagnostics, Inc. and XOMA (US) LLC
|
10-K
|
000-14710
|
10.25B
|
03/14/2012
|
|
10.42
|
Manufacturing and Technology Transfer Agreement, executed December 16, 2008, by and between Novartis Vaccines and Diagnostics, Inc. (formerly Chiron Corporation) and XOMA (US) LLC
|
10-K
|
000-14710
|
10.24D
|
03/11/2009
|
|
10.43
|
Agreement dated March 8, 2005, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases
|
10-K
|
000-14710
|
10.53
|
03/15/2005
|
|
10.44
|
Agreement dated July 28, 2006, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases
|
10-K
|
000-14710
|
10.60
|
08/09/2006
|
|
10.45†
|
Agreement dated September 15, 2008, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases
|
10-Q
|
000-14710
|
10.39
|
11/10/2008
|
|
10.46
|
Second Amendment to Agreement dated September 15, 2008, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases
|
10-Q
|
000-14710
|
10.24C
|
11/04/2010
|
|
10.47
|
Agreement dated September 30, 2011, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases
|
S-4
|
000-14710
|
10.28D
|
10/04/2011
|
|
10.48†
|
Collaboration Agreement, dated as of November 1, 2006, between Takeda Pharmaceutical Company Limited and XOMA (US) LLC
|
10-K
|
000-14710
|
10.46
|
03/08/2007
|
|
10.49
|
First Amendment to Collaboration Agreement, effective as of February 28, 2007, between Takeda Pharmaceutical Company Limited and XOMA (US) LLC
|
10-Q/A
|
000-14710
|
10.48
|
03/05/2010
|
|
10.50
|
Second Amendment to Collaboration Agreement, effective as of February 9, 2009, among Takeda Pharmaceutical Company Limited and XOMA (US) LLC
|
10-K
|
000-14710
|
10.31B
|
03/11/2009
|
|
10.51†
|
License Agreement, effective as of August 27, 2007, by and between Pfizer Inc. and XOMA Ireland Limited
|
8-K
|
000-14710
|
2
|
09/13/2007
|
|
10.52
|
Common Share Purchase Agreement, dated as of July 23, 2010, by and between XOMA Ltd. and Azimuth Opportunity Ltd.
|
8-K
|
000-14710
|
10.1
|
07/23/2010
|
|
10.53
|
Securities Purchase Agreement dated June 5, 2009, between XOMA Ltd. and the investors named therein
|
8-K
|
000-14710
|
10.1
|
06/10/2009
|
|
10.54
|
Engagement Letter dated June 4, 2009
|
8-K
|
000-14710
|
10.3
|
06/10/2009
|
|
10.55†
|
Discovery Collaboration Agreement dated September 9, 2009, by and between XOMA Development Corporation and Arana Therapeutics Limited
|
10-Q/A
|
000-14710
|
10.35
|
03/05/2010
|
|
10.56
|
Amendment to At Market Issuance Sales Agreement dated December 31, 2011, between XOMA Corporation and McNicoll, Lewis & Vlak LLC
|
POS AM
|
333-172197
|
1.2
|
01/03/2012
|
|
10.57
|
Form of Warrant Amendment Agreement dated February 2, 2010 (June 2009 Warrants)
|
8-K
|
000-14710
|
10.3
|
02/02/2010
|
|
10.58†
|
Royalty Purchase Agreement, dated as of August 12, 2010, by and among XOMA CDRA LLC, XOMA (US) LLC, XOMA Ltd. and the buyer named therein
|
10-Q/A
|
000-14710
|
10.38
|
04/13/2011
|
|
10.59†
|
Collaboration and License Agreement dated as of December 30, 2010, by and between XOMA Ireland Limited, Les Laboratoires Servier and Institut de Recherches Servier
|
10-K
|
000-14710
|
10.42
|
03/10/2011
|
|
10.60†
|
Amended and Restated Collaboration and License Agreement dated as of February 14, 2012, by and between XOMA Ireland Limited, Les Laboratoires Servier and Institut de Recherches Servier
|
10-K
|
000-14710
|
10.41A
|
03/14/2012
|
|
10.61†
|
Loan Agreement dated as of December 30, 2010, by and between XOMA Ireland Limited and Les Laboratoires Servier
|
10-K/A
|
000-14710
|
10.42A
|
05/26/2011
|
|
10.62
|
Foreign Exchange and Options Master Agreement (FEOMA) dated as of May 16, 2011, between Royal Bank of Canada and XOMA Ltd., with letter agreement dated May 17, 2011
|
10-Q
|
000-14710
|
10.1
|
08/04/2011
|
|
10.63†
|
Loan Agreement dated as of December 30, 2011, among XOMA (US) LLC, as Borrower, XOMA Ltd., as Parent, each other loan party from time to time party thereto, General Electric Capital Corporation, as Agent, and each other lender from time to time party thereto
|
10-K
|
000-14710
|
10.43
|
03/14/2012
|
|
10.64†
|
Guaranty, Pledge and Security Agreement dated as of December 30, 2011, among XOMA (US) LLC, each other guarantor from time to time party thereto and General Electric Capital Corporation, as Agent
|
10-K
|
000-14710
|
10.43A
|
03/14/2012
|
|
10.65†
|
Amended and Restated License and Commercialization Agreement effective as of January 11, 2012, by and between Les Laboratoires Servier and XOMA Ireland Limited
|
10-K
|
000-14710
|
10.44
|
03/14/2012
|
|
10.66†
|
Amended and Restated Trademark License Agreement entered into as of January 11, 2012, between Biofarma and XOMA Ireland Limited
|
10-K
|
000-14710
|
10.44A
|
03/14/2012
|
|
10.67†
|
Master Services Agreement dated as of November 9, 2009, between Medpace, Inc. and XOMA (US) LLC
|
10-K
|
000-14710
|
10.45
|
03/14/2012
|
|
10.68†
|
Amendment No. 1 to Master Services Agreement dated as of October 4, 2011, between Medpace, Inc. and XOMA (US) LLC
|
10-K
|
000-14710
|
10.45A
|
03/14/2012
|
|
10.69
|
First Amendment to Loan Agreement, by and between General Electric Capital Corporation, the Company as guarantor, XOMA (US) LLC as borrower, and certain other wholly-owned subsidiaries of the Company, dated September 27, 2012
|
8-K
|
000-14710
|
10.46
|
10/03/2012
|
|
10.70
|
Second Amendment to Loan Agreement, by and between General Electric Capital Corporation, the Company as guarantor, XOMA (US) LLC as borrower, and certain other wholly-owned subsidiaries of the Company, dated August 12, 2013
|
10-Q
|
000-14710
|
10.1
|
11/06/2014
|
|
10.70
|
Third Amendment to Loan Agreement, by and between General Electric Capital Corporation, the Company as guarantor, XOMA (US) LLC as borrower, and certain other wholly-owned subsidiaries of the Company, dated August 22, 2014 and effective as of August 18, 2014
|
10-Q
|
000-14710
|
10.2
|
11/06/2014
|
|
Amendment No. 2, effective January 9, 2015, to the Loan Agreement, effective December 30, 2010, by and among XOMA (US) LLC, Les Laboratoires Servier and Institut de Recherches Servier
|
|||||
|
Amendment No. 2, effective January 9, 2015, to the Amended and Restated Collaboration and License Agreement, effective February 14, 2012, by and among XOMA (US) LLC, Les Laboratoires Servier and Institut de Recherches Servier
|
|||||
|
Amendment No. 1, effective November 4, 2014, to the Amended and Restated Collaboration and License Agreement, effective February 14, 2012, by and among XOMA (US) LLC (assigned from XOMA Ireland Limited), Les Laboratoires Servier and Institut de Recherches Servier
|
|||||
|
Amendment No. 1 (Consent, Transfer, Assumption and Amendment), effective August 12, 2013, to the Loan Agreement, effective December 30, 2010, by and among XOMA (US) LLC, Les Laboratoires Servier and Institut de Recherches Servier
|
|||||
|
10.75
|
Reference is made to Exhibit 4.9
|
||||
|
Subsidiaries of the Company
|
|||||
|
Consent of Independent Registered Public Accounting Firm
|
|||||
|
24.1+
|
Power of Attorney (included on the signature pages hereto)
|
||||
|
Certification of Chief Executive Officer, as required by Rule 13a-14(a) or Rule 15d-14(a)
|
|||||
|
Certification of Chief Financial Officer, as required by Rule 13a-14(a) or Rule 15d-14(a)
|
|
Certification of Chief Executive Officer and Chief Financial Officer, as required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350)(1)
|
|||||
|
Press Release dated March 11, 2014
|
|||||
|
101.INS+
|
XBRL Instance Document
|
||||
|
101.SCH+
|
XBRL Taxonomy Extension Schema Document
|
||||
|
101.CAL+
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
||||
|
101.DEF+
|
XBRL Taxonomy Extension Definition Linkbase Document
|
||||
|
101.LAB+
|
XBRL Taxonomy Extension Labels Linkbase Document
|
||||
|
101.PRE+
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
†
|
Confidential treatment has been granted with respect to certain portions of this exhibit. This exhibit omits the information subject to this confidentiality request. Omitted portions have been filed separately with the SEC.
|
|
*
|
Indicates a management contract or compensation plan or arrangement.
|
|
+
|
Filed herewith
|
|
(1)
|
This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of the Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.
|
