Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - IMMUNE PHARMACEUTICALS INC | v404241_8k.htm |

Investor Presentation March 2015

Forward - Looking Statement This presentation and any oral statements made with respect to the information contained in this presentation contain forward - looking statements that involve risks and uncertainties regarding the operations and future results of Immune Pharmaceuticals, Inc . You are urged to consider statements that include the words “may,” “will,” “would,” “could,” “should,” “believes,” “estimates,” “projects,” “potential,” “expects,” “plans,” “anticipates,” “intends,” “continues,” “forecast,” “designed,” “goal” or the negative of those words or other comparable words to be uncertain and forward - looking . Such forward - looking statements include statements that express plans, anticipation, intent, contingency, goals, targets, future development and are otherwise not statements of historical fact . These statements are based on our current expectations and are subject to risks and uncertainties that could cause actual results or developments to be materially different from historical results or from any future results expressed or implied by such forward - looking statements . Factors that may cause actual results or developments to differ materially include, but not limited to : the risks associated with the adequacy of our existing cash resources and our ability to continue as a going concern ; the risks associated with our ability to continue to meet our obligations under our existing debt agreements ; the risk that clinical trials for bertilimumab or AmiKet ™ will not be successful ; the risk that bertilimumab , AmiKet ™ or compounds arising from our NanomAb ® program will not receive regulatory approval or achieve significant commercial success ; the risk that we will not be able to find a partner to help conduct the Phase III trials for AmiKet ™ on attractive terms, on a timely basis or at all ; the risk that our other product candidates that appeared promising in early research and clinical trials do not demonstrate safety and/or efficacy in larger - scale or later - stage clinical trials ; the risk that we will not obtain approval to market any of our product candidates ; the risks associated with dependence upon key personnel ; the risks associated with reliance on collaborative partners and others for further clinical trials, development, manufacturing and commercialization of our product candidates ; the cost, delays and uncertainties associated with our scientific research, product development, clinical trials and regulatory approval process ; our history of operating losses since our inception ; the highly competitive nature of our business ; risks associated with litigation ; risks associated with our ability to protect our intellectual property ; risks associate with our ability to raise additional capital ; and our liquidity . These factors and other material risks are more fully discussed in our periodic reports, including our reports on Forms 8 - K, 10 - Q and 10 - K and other filings with the U . S . Securities and Exchange Commission . You are urged to carefully review and consider the disclosures found in our filings which are available at www . sec . gov or at www . immunepharmaceuticals . com . You are cautioned not to place undue reliance on any forward - looking statements, any of which could turn out to be wrong due to inaccurate assumptions, unknown risks or uncertainties or other risk factors . We expressly disclaim any obligation to publicly update any forward looking statements contained herein, whether as a result of new information, future events or otherwise, except as required by law . 2


Immune Pharmaceuticals (NASDAQ:IMNP) Corporate Overview Leadership Team in New York City • NASDAQ listed company (IMNP) • First in Class Phase 2 Biologic candidate, Bertilimumab, for Auto - Immune Diseases ▪ Targeted Personalized Antibody Therapeutic with Companion Diagnostic opportunity ▪ Focus on Bullous Pemphigoid (BP), an Orphan indication, Ulcerative Colitis and NASH • Phase 3 ready product, AmiKet, for Neuropathic Pain with Orphan Drug designation in Post Herpetic Neuralgia (PHN) Partnering process on track for completion Q 2 2015 . • Platform technology, NanomAbs, enabling pipeline expansion in cancer therapeutics 4
▪ Personalized Medicine based on patient selection and targeted therapy ▪ Bertilimumab : First in Class fully H uman mAb originally developed by CAT (now part of AZ ) to improve the lives of patients with Severe Inflammatory and/ or Auto - Immune Diseases due to Eotaxin - 1 over expression including : ▪ Bullous Pemphigoid, an Orphan Dermatological Indication ▪ Ulcerative Colitis and Crohn’s Disease ▪ Non Alcoholic Steato - Hepatitis (NASH) ▪ Severe Asthma ▪ NanomAbs (Antibody Nanoparticle Conjugates) for the targeted delivery of cytotoxic drugs to cancer cells with pre - treatment tumor imaging ▪ Anti - HER 2 NanomAbs, ▪ Anti - EGFR NanomAbs, ▪ Anti - H - ferritin NanomAbs 5 Our Goal Is to Transform Targeted Immunotherapy and Improve The Lives of Patients With Auto - immune Diseases And Cancer

Clinical Stage Portfolio With Multiple Shots on Goal Resources Prioritized For Bertilimumab Phase II BP And UC Trials PROGRAMS Preclinical Phase 1 Phase 2 Phase 3 I NFLAMMATION Bertilimumab Bullous Pemphigoid IBD (Crohn’s and Colitis) Severe Asthma NASH A NTIBODY NANOPARTICLE CONJUGATES NanomAbs® (programs: anti - HER2, anti - EGFR, anti - ferritin) PARTNERING OPPORTUNITIES P AIN THERAPEUTICS AmiKet™ O NCOLOGY T HERAPEUTICS Azixa® Crolibulin™ 6

Highly Experienced Leadership Team Management Daniel Teper, PharmD, MBA Founder & CEO and Director ; Novartis, GSK, Sanofi, Bionest Gad Berdugo, Msc, MBA EVP and Chief Financial Officer ; Abbott, Baxter, Lazard, Tegris Paul Nadler, MD, PhD EVP, R&D and Chief Medical Officer; National Cancer Institute, Roche, Novartis, Protein Design Labs, Alexion, OSI Board of Directors Daniel Kazado, MBA Chairman of the Board Cameron Durrant, MD, MBA Lead Independent Director and Head of Compensation; Merck, GSK, Pharmacia (Pfizer), J&J Elliot Maza JD, CPA Head of the Audit Committee; Ernst & Young, Goldman Sachs, J.P. Morgan , Sullivan & Cromwell Rene Lerer, MD Head of Governance and Nominations; Prudential, Magellan Health, Florida Blue Cross 7

Eotaxin - 1 Is Upregulated In Patients With Inflammatory Diseases in Multiple Organs 9 Bullous Pemphigoid Crohn’s Disease/Ulcerative Colitis Non Alcoholic Steato - hepatitis(NASH ). Severe Asthma

Bertilimumab may reach $ 1.1 B* in US/EU sales in Bullous Pemphigoid Prevalence (# of patients) US/EU Market size currently estimated at 60,000 patients, expected to reach 90,000 by 2025 due to population aging and increased diagnosis Initial target price for bertilimumab in Bullous Pemphigoid projected to be $ 45,000 /year/patient, aligned with IBD Opportunity in Asian Market projected as over $ 500 MM Source: Chardan Capital Markets Research, Initiating Coverage March 9,2015 10 * non Risk - adjusted Forecast based on 28 % peak penetration and price aligned with IBD - 10,000 20,000 30,000 40,000 50,000 60,000 70,000 80,000 90,000 100,000 2010A 2015E 2020E 2025E US/EU

Eosinophils are Predominant in the BP Inflammatory Process Bertilimumab Neutralizes Eotaxin - 1 , a Key Regulator of Eosinophils 11 Significant unmet need for targeted therapy with improved safety profile SOURCE: Dr. Neil Kormin, Case Western

Bertilimumab Targets Bullous Pemphigoid, an Autoimmune Dermatological Orphan Disease 12 SOURCE: IPPF - Internatioal Pemphigus Pemphigoid Foundation - database, P/P Patient Registry, and independent study Rare autoimmune skin blistering Disease Aging orphan disease Estimated population of 60,000 in the USA and Europe, more frequent after 65 Years old Absence of treatment leads in 1 to 5 years towards several complications including infection, potentially leading to a life - threatening sepsis Treatment requires high daily dose of oral steroids and high potency topical steroids Severe side effects related to steroids such as diabetes, high blood pressure, psychosis, osteoporosis, increasing mortality by 2 Other limited and toxic steroid sparing options: immuno - suppressants, IVIgG, Rituxan Recent positive clinical data with Anti IgEmAb (Xolair) Need for Safer, Better, Targeted Treatments

Bertilimumab: Positive Preclinical Efficacy In Dermal Inflammation Model (A) total cell and (B) eosinophil recruitment to the perivascular dermis Bertilimumab efficacy was evaluated in monkey inflammatory dermis Study included 16 cynomolgus monkeys, assigned to 3 different treatment groups Bertilimumab significantly suppressed the strong inflammatory responses in monkey dermis. 13 Source: data on file

▪ Over 3 million sufferers g lobally, 50 % showing high eotaxin 1 level ▪ Anti TNF sell over $ 4 B in moderate to severe inflammatory bowels diseases but show limitations with loss of efficacy after 1 year ▪ Entivyo (Takeda) was approved in 2014 as first line biologic and opens the market for more targeted treatment 14 B Source: Global data
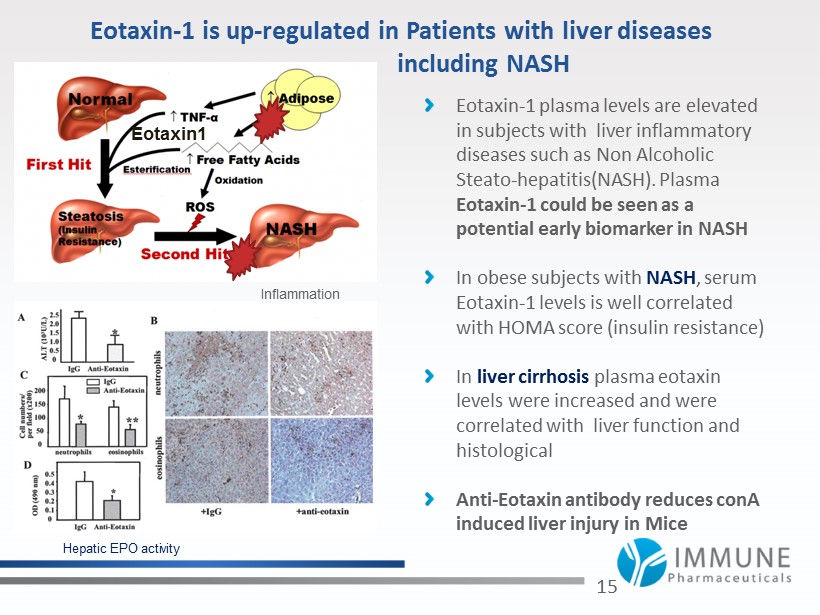
Eotaxin - 1 is up - regulated in Patients with liver diseases including NASH Eotaxin - 1 plasma levels are elevated in subjects with liver inflammatory diseases such as Non Alcoholic Steato - hepatitis (NASH). Plasma Eotaxin - 1 could be seen as a potential early biomarker in NASH In obese subjects with NASH , serum Eotaxin - 1 levels is well correlated with HOMA score (insulin resistance) In liver cirrhosis plasma eotaxin levels were increased and were correlated with liver function and histological Anti - Eotaxin antibody reduces conA induced liver injury in Mice 15 Hepatic EPO activity Eotaxin 1 Inflammation

Recent Publications in Leading Journals Linking Eotaxin - 1 with Inflammatory Liver Disease, Obesity, Cardiovascular Disease, Insulin Resistance and Diabetes Differential serum levels of eosinophilic eotaxins in primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis Eosinophils and type 2 cytokines signaling in macrophages Orchestrate development of functional beige fat Adipose tissue dysfunction signals progression of hepatic steatosis towards non - alcholicsteatohepatitis in C 57 BI/ 6 mice Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis Carotid intima - media thickness is predicted by combined eotaxin levels and severity of hepatic steatosis at Ultrasonography in obese patients with non - alcoholic fatty liver disease Eosinophils in Fat: Pink is the New Brown Crucial role of IL - 4 /STAT 6 in T Cell Mediated Hepatitis: Up - regulating Eotaxins and IL - 5 and Recruiting Leukocytes Eotaxin and obesity 16 2013 2010 2014 2003 2014 2011 2014 2006

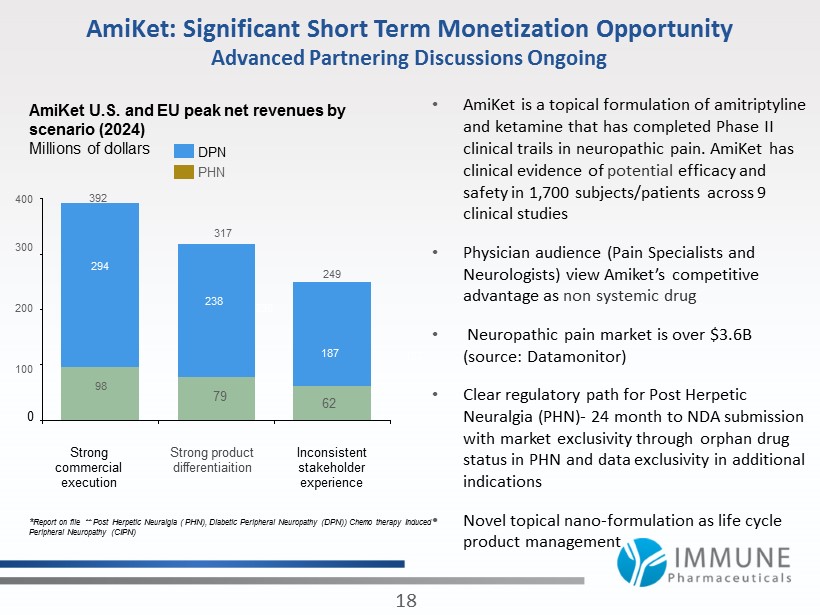
AmiKet: Significant Short Term Monetization Opportunity Advanced Partnering Discussions Ongoing • AmiKet is a topical formulation of amitriptyline and ketamine that has completed Phase II clinical trails in neuropathic pain. AmiKet has clinical evidence of potential efficacy and safety in 1,700 subjects/patients across 9 clinical studies • Physician audience (Pain Specialists and Neurologists) view Amiket’s competitive advantage as non systemic drug • Neuropathic pain market is over $ 3.6 B (source: Datamonitor) • Clear regulatory path for Post Herpetic Neuralgia (PHN) - 24 month to NDA submission with market exclusivity through orphan drug status in PHN and data exclusivity in additional indications • Novel topical nano - formulation as life cycle product management 18 238 392 98 294 200 400 300 0 100 Inconsistent stakeholder experience Strong commercial execution Strong product differentiaition 317 238 249 62 187 * Report on file ** Post Herpetic Neuralgia ( PHN), Diabetic Peripheral Neuropathy (DPN)) Chemo therapy Induced Peripheral Neuropathy (CIPN) AmiKet U.S. and EU peak net revenues by scenario ( 2024 ) Millions of dollars 79 PHN DPN 187

AmiKet Non - Inferior to Gabapentin HD in Post Herpetic Neuralgia (PHN) 0 1 2 3 4 5 6 7 Placebo n=76 Amiket n=135 Gabapentin n=138 Baseline End of Week 4 Mean Pain Score p = 0.044 p = 0.84

Physicians Express Strong Demand For a Highly Effective Topical Drug Source: Interviews The main advantage of AmiKet is that it is not a systemic drug, and would prescribe it as a monotherapy for many of my patients … ” - EU Pain Specialist, Ambroise Pare Hospital ( France) “… Since a topical treatment would be safer than systemic treatments, I am willing to give AMIKET a shot. In general, I am not as worried about topical formulations, as there is little systemic absorption …” - U.S. PCP, Private Practice (NJ ) “… Some patients don’t tolerate patches, something that you smear would be more acceptable to those people …” - U.S. Pain Specialist, Baylor College of Medicine (TX ) “… Some patients have difficulty using patches since they have issues covering the whole spot of pain with the patch …” - U.S. Neurologist, Brigham & Women’s Hospital (MA) I typically use gabapentin first, but if topical solutions had better efficacy, I would consider them as first line treatments …” - U.S. Neurologist, Brigham & Women’s Hospital ( MA ) 20


Existing and Novel mAb targets with strong internalization properties HER 2 , EGFR, H - Ferritin, Others High Payload: Up to 20,000 molecules per nanoparticle Versatility of drugs to be delivered , not limited to toxins , Triple Targeting : Tumor Targeting, Cellular Targeting, Molecular Targeting Tailored Pharmacokinetics through formulation of polymeric nanoparticles Pre - Treatment Target Imaging TUMOR TISSUE TARGETING TUMOR CELL TARGETING MOLECULAR TARGETING NanomAbs: ADC 2.0 May Optimize s the Efficacy/ Safety Ratio and May Improve the Resistance Profile of Chemotherapeutics Through Targeted Drug Delivery Multiple Development Partnerships expected in 2015

2015 Milestones / Stock Catalysts Quarter 2 Quarter 3 Quarter 4 Partnering of AMIKET Q 2 2015 Initiation of Amiket NP development Q 2 2015 First Patient in BP Phase II Study with Bertilimumab Q 2 2015 Orphan Drug designation for Bertilimumab in BP Q 3 2015 First Patient in UC phase II study with Bertilimumab Q 3 2015 Pre - clinical Data availability for Bertilimumab in NASH Q 3 2015 Planned Initiation of Bertilimumab NASH clinical development program Q 4 2015 Initial Results of Bertilimumab Bullous Pemphigoid (BP) phase II study Q 4 2015 NanomAb Partnerships Q 3 / Q 4 2015 Initiation of Amiket PHN phase III Q 4 2015
