Attached files
| file | filename |
|---|---|
| EX-10.1 - EX-10.1 - Zyla Life Sciences | a15-1448_2ex10d1.htm |
| EX-99.1 - EX-99.1 - Zyla Life Sciences | a15-1448_2ex99d1.htm |
| 8-K - 8-K - Zyla Life Sciences | a15-1448_28k.htm |
Exhibit 99.2
|
|
Corporate update January 2015 Delivering pain relief with peace of mind NASDAQ: EGLT 1 |
|
|
Forward-looking statement 2 Statements included that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties and risks. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: the success of integrating recent acquisitions; our ability to obtain regulatory approval of our product candidates; our ability to successfully commercialize SPRIX and OXAYDO, competitive factors; general market conditions; and other risk factors described in Egalet's filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this press release whether as a result of new information or future events, except as may be required by law. |
|
|
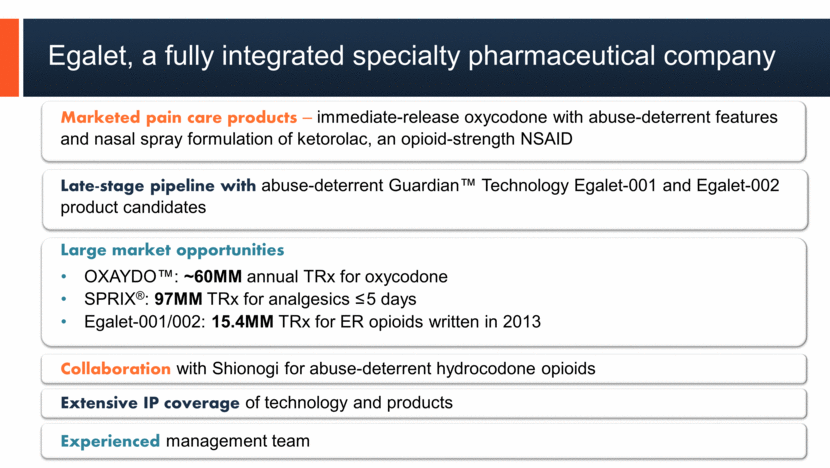
3 Egalet, a fully integrated specialty pharmaceutical company 3 Marketed pain care products – immediate-release oxycodone with abuse-deterrent features and nasal spray formulation of ketorolac, an opioid-strength NSAID Late-stage pipeline with abuse-deterrent Guardian™ Technology Egalet-001 and Egalet-002 product candidates Large market opportunities OXAYDO™: ~60MM annual TRx for oxycodone SPRIX®: 97MM TRx for analgesics <5 days Egalet-001/002: 15.4MM TRx for ER opioids written in 2013 Collaboration with Shionogi for abuse-deterrent hydrocodone opioids Extensive IP coverage of technology and products Experienced management team |
|
|
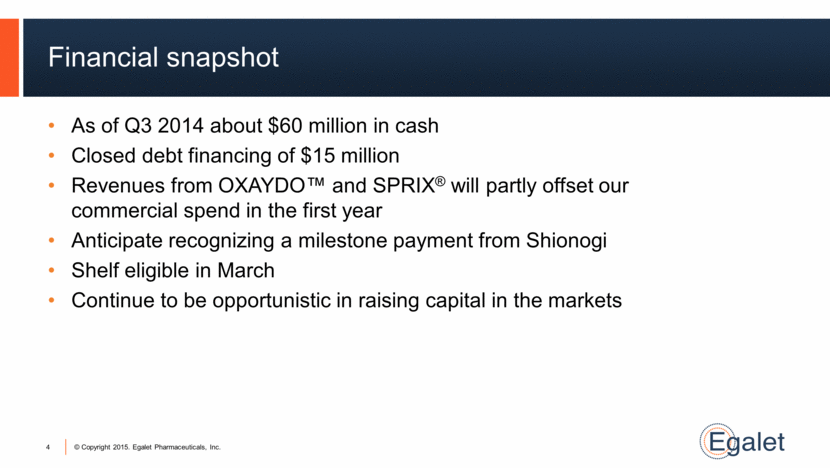
Financial snapshot As of Q3 2014 about $60 million in cash Closed debt financing of $15 million Revenues from OXAYDO™ and SPRIX® will partly offset our commercial spend in the first year Anticipate recognizing a milestone payment from Shionogi Shelf eligible in March Continue to be opportunistic in raising capital in the markets 4 |
|
|
Commercial transformation 5 EGALET-001 EGALET-002 EGALET-002 OXAYDO™ |
|
|
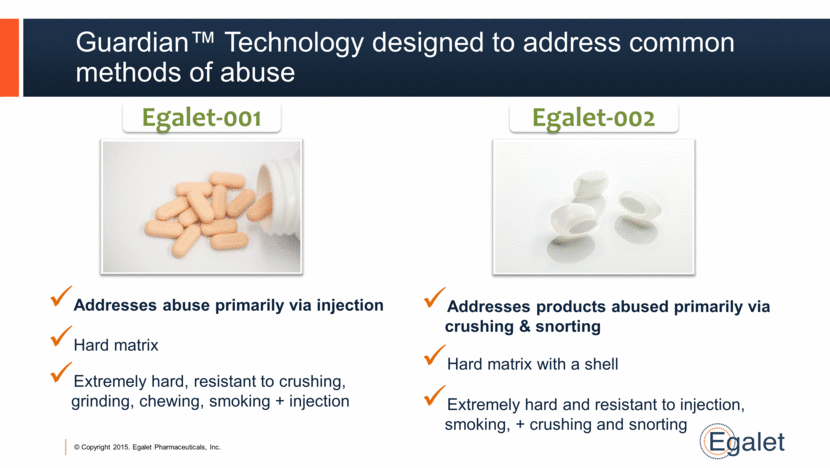
Guardian™ Technology designed to address common methods of abuse Extremely hard, resistant to crushing, grinding, chewing, smoking + injection Egalet-001 Egalet-002 Hard matrix Addresses abuse primarily via injection Extremely hard and resistant to injection, smoking, + crushing and snorting Hard matrix with a shell Addresses products abused primarily via crushing & snorting |
|
|
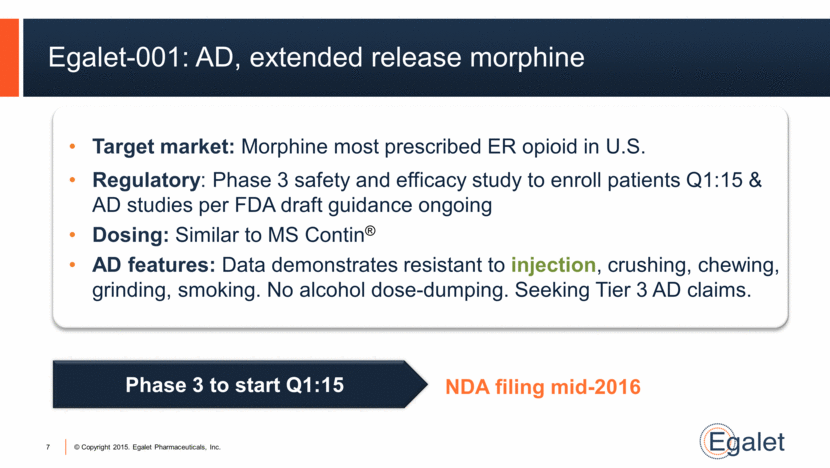
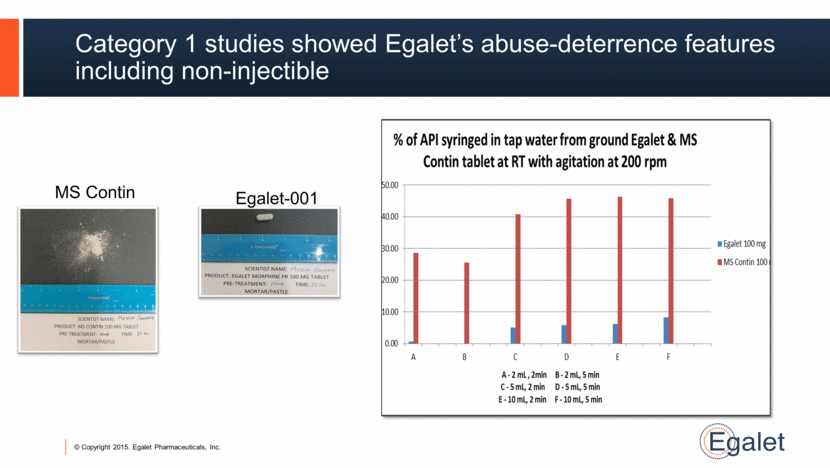
Egalet-001: AD, extended release morphine 7 Target market: Morphine most prescribed ER opioid in U.S. Regulatory: Phase 3 safety and efficacy study to enroll patients Q1:15 & AD studies per FDA draft guidance ongoing Dosing: Similar to MS Contin® AD features: Data demonstrates resistant to injection, crushing, chewing, grinding, smoking. No alcohol dose-dumping. Seeking Tier 3 AD claims. Phase 3 to start Q1:15 NDA filing mid-2016 |
|
|
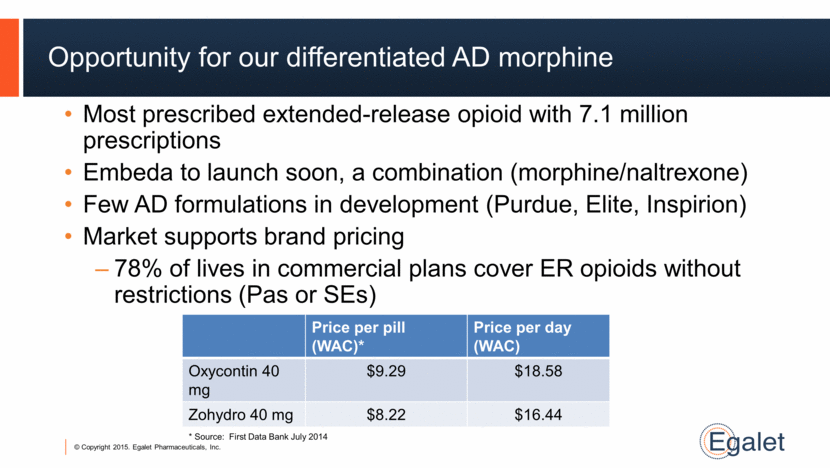
Most prescribed extended-release opioid with 7.1 million prescriptions Embeda to launch soon, a combination (morphine/naltrexone) Few AD formulations in development (Purdue, Elite, Inspirion) Market supports brand pricing 78% of lives in commercial plans cover ER opioids without restrictions (Pas or SEs) Opportunity for our differentiated AD morphine Price per pill (WAC)* Price per day (WAC) Oxycontin 40 mg $9.29 $18.58 Zohydro 40 mg $8.22 $16.44 * Source: First Data Bank July 2014 |
|
|
Category 1 studies showed Egalet’s abuse-deterrence features including non-injectible MS Contin Egalet-001 |
|
|
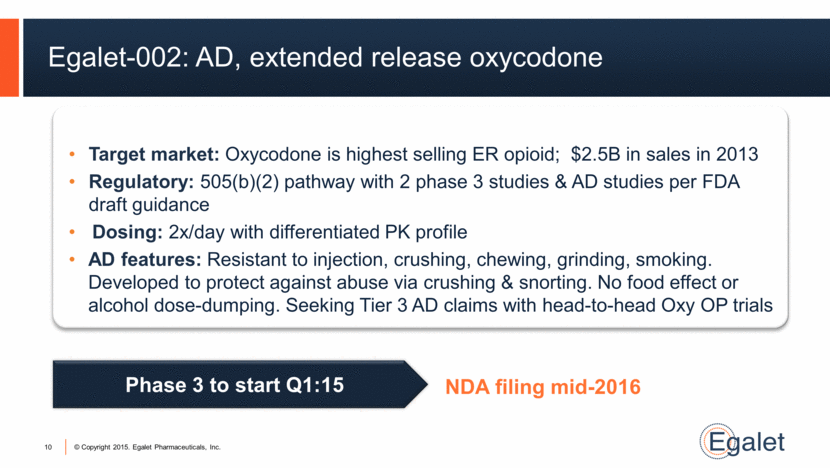
Egalet-002: AD, extended release oxycodone 10 Target market: Oxycodone is highest selling ER opioid; $2.5B in sales in 2013 Regulatory: 505(b)(2) pathway with 2 phase 3 studies & AD studies per FDA draft guidance Dosing: 2x/day with differentiated PK profile AD features: Resistant to injection, crushing, chewing, grinding, smoking. Developed to protect against abuse via crushing & snorting. No food effect or alcohol dose-dumping. Seeking Tier 3 AD claims with head-to-head Oxy OP trials Phase 3 to start Q1:15 NDA filing mid-2016 |
|
|
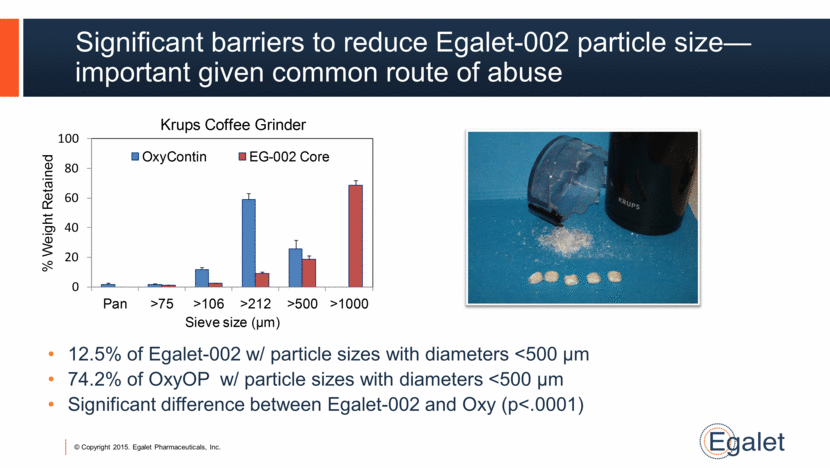
Significant barriers to reduce Egalet-002 particle size—important given common route of abuse 12.5% of Egalet-002 w/ particle sizes with diameters <500 µm 74.2% of OxyOP w/ particle sizes with diameters <500 µm Significant difference between Egalet-002 and Oxy (p<.0001) 0 20 40 60 80 100 Pan >75 >106 >212 >500 >1000 % Weight Retained Sieve size (µm) Krups Coffee Grinder OxyContin EG - 002 Core |
|
|
Market supports branded pricing Oxycodone was highest selling ER opioid in 2013 Two FDA approved AD products (Targiniq and OxyOP) AD products in development (Collegium, ALO-02) Pfizer returned Remoxy to Pain Therapeutics Market opportunity for abuse-deterrent oxycodone |
|
|
Accelerates transformation into a commercial company w/revenues two years ahead of plan Revenues help offset commercial expenses Establishes commercial presence to ensure successful launch of Guardian™ Technology abuse-deterrent products Continue to develop relationships with KOLs, pain care specialists, and other health care providers, as well as payers and patient advocacy groups Creates pain franchise with complementary products Adds immediate-release oxycodone with abuse-deterrent features and an opioid-strength NSAID for short-term pain Products complement the goal of bringing peace of mind to patients and physicians Uses primarily non-dilutive source of capital to fund transactions Covers purchase prices Product acquisition and license benefits 13 |
|
|
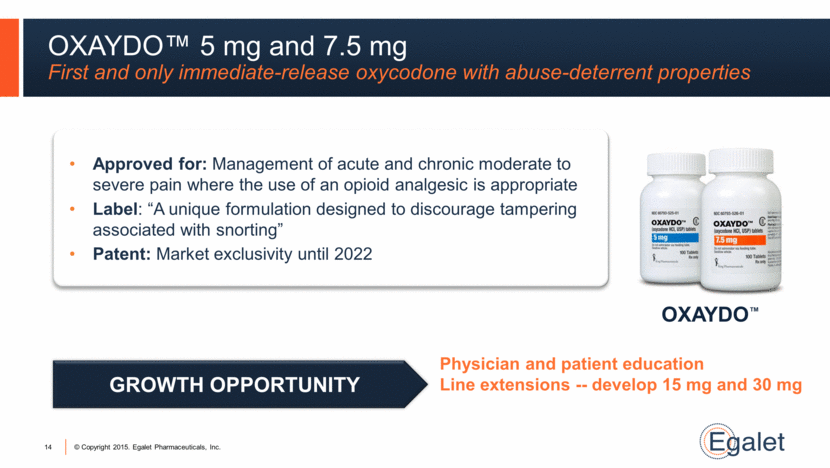
OXAYDO™ OXAYDO™ 5 mg and 7.5 mg First and only immediate-release oxycodone with abuse-deterrent properties 14 Approved for: Management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate Label: “A unique formulation designed to discourage tampering associated with snorting” Patent: Market exclusivity until 2022 Physician and patient education Line extensions -- develop 15 mg and 30 mg GROWTH OPPORTUNITY |
|
|
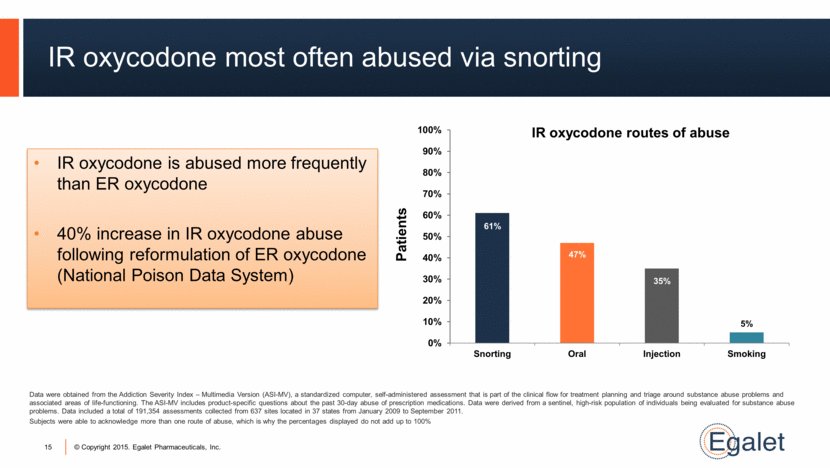
15 IR oxycodone most often abused via snorting IR oxycodone is abused more frequently than ER oxycodone 40% increase in IR oxycodone abuse following reformulation of ER oxycodone (National Poison Data System) Data were obtained from the Addiction Severity Index – Multimedia Version (ASI-MV), a standardized computer, self-administered assessment that is part of the clinical flow for treatment planning and triage around substance abuse problems and associated areas of Iife-functioning. The ASI-MV includes product-specific questions about the past 30-day abuse of prescription medications. Data were derived from a sentinel, high-risk population of individuals being evaluated for substance abuse problems. Data included a total of 191,354 assessments collected from 637 sites located in 37 states from January 2009 to September 2011. Subjects were able to acknowledge more than one route of abuse, which is why the percentages displayed do not add up to 100% IR oxycodone routes of abuse 61% 47% 35% 5% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Snorting Oral Injection Smoking Patients |
|
|
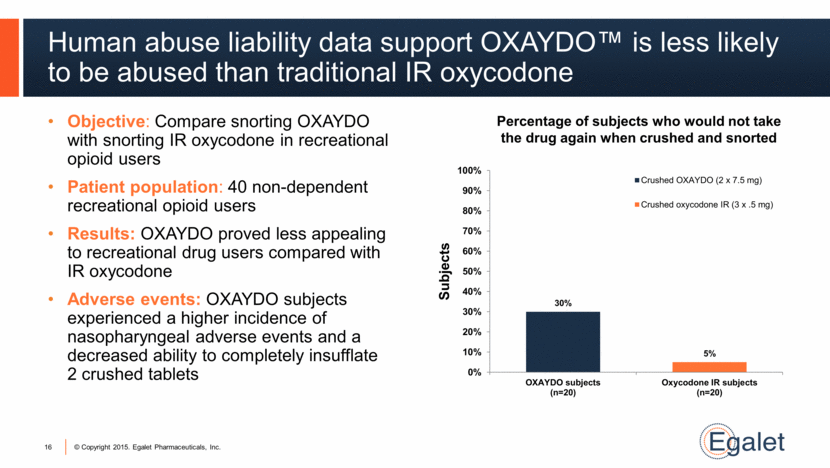
Human abuse liability data support OXAYDO™ is less likely to be abused than traditional IR oxycodone Objective: Compare snorting OXAYDO with snorting IR oxycodone in recreational opioid users Patient population: 40 non-dependent recreational opioid users Results: OXAYDO proved less appealing to recreational drug users compared with IR oxycodone Adverse events: OXAYDO subjects experienced a higher incidence of nasopharyngeal adverse events and a decreased ability to completely insufflate 2 crushed tablets 16 Percentage of subjects who would not take the drug again when crushed and snorted 30% 5% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% OXAYDO subjects (n=20) Oxycodone IR subjects (n=20) Subjects Crushed OXAYDO (2 x 7.5 mg) Crushed oxycodone IR (3 x .5 mg) |
|
|
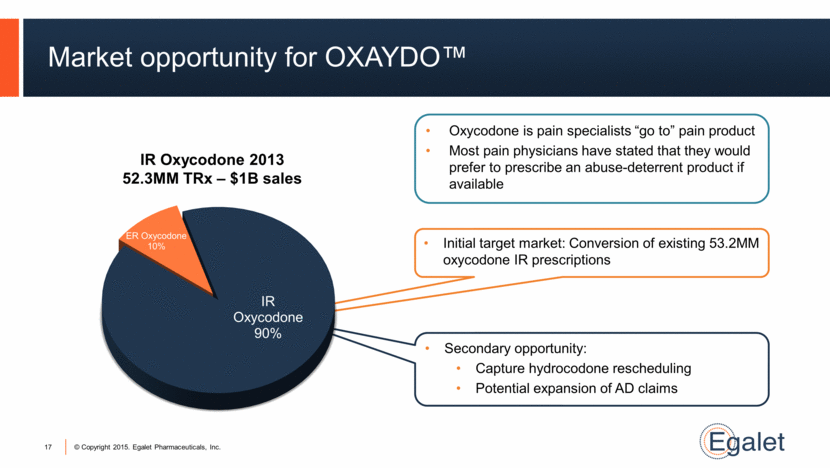
Market opportunity for OXAYDO™ 17 IR Oxycodone 2013 52.3MM TRx – $1B sales Secondary opportunity: Capture hydrocodone rescheduling Potential expansion of AD claims Initial target market: Conversion of existing 53.2MM oxycodone IR prescriptions Oxycodone is pain specialists “go to” pain product Most pain physicians have stated that they would prefer to prescribe an abuse-deterrent product if available IR Oxycodone 90% ER Oxycodone 10% |
|
|
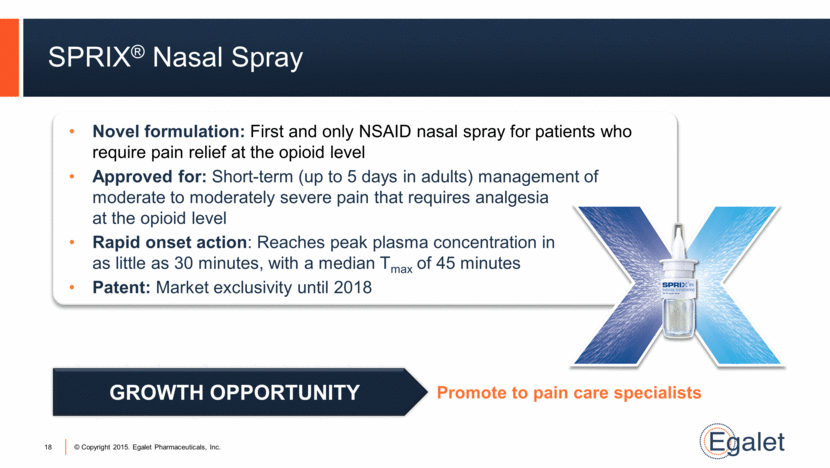
SPRIX® Nasal Spray 18 Novel formulation: First and only NSAID nasal spray for patients who require pain relief at the opioid level Approved for: Short-term (up to 5 days in adults) management of moderate to moderately severe pain that requires analgesia at the opioid level Rapid onset action: Reaches peak plasma concentration in as little as 30 minutes, with a median Tmax of 45 minutes Patent: Market exclusivity until 2018 Promote to pain care specialists GROWTH OPPORTUNITY |
|
|
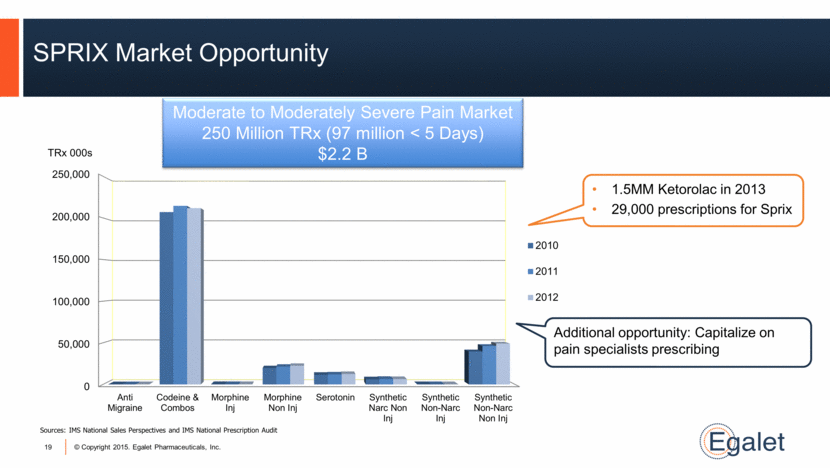
SPRIX Market Opportunity Moderate to Moderately Severe Pain Market 250 Million TRx (97 million < 5 Days) $2.2 B Sources: IMS National Sales Perspectives and IMS National Prescription Audit 19 TRx 000s 1.5MM Ketorolac in 2013 29,000 prescriptions for Sprix Additional opportunity: Capitalize on pain specialists prescribing |
|
|
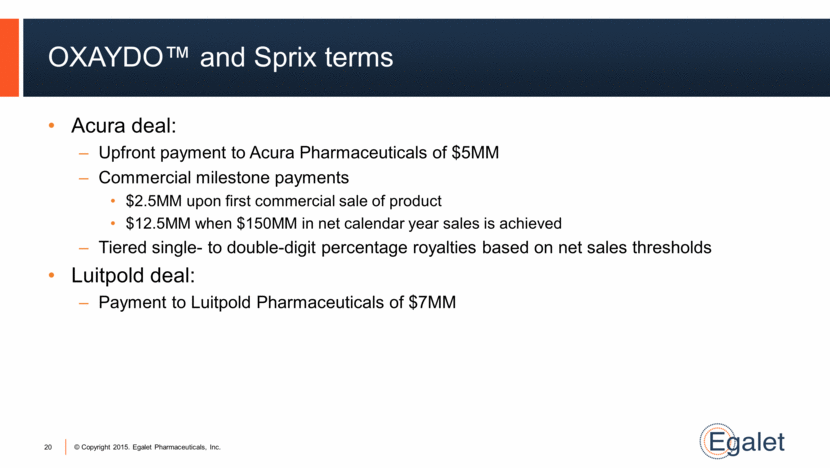
OXAYDO™ and Sprix terms Acura deal: Upfront payment to Acura Pharmaceuticals of $5MM Commercial milestone payments $2.5MM upon first commercial sale of product $12.5MM when $150MM in net calendar year sales is achieved Tiered single- to double-digit percentage royalties based on net sales thresholds Luitpold deal: Payment to Luitpold Pharmaceuticals of $7MM 20 |
|
|
Small specialty sales force can target pain care specialists 50-60 sales professionals Pain management specialists/KOLs High-prescribing HCPs Partner PCPs + |
|
|
Expanding Egalet’s target markets 22 EGALET-001 EGALET-002 EGALET-002 OXAYDO™ |
|
|
2015 Q1:15 Initiate pivotal Phase 3 safety and efficacy study Egalet-001 Q1:15 Initiate promotional activities SPRIX® Q1:15 Commercial update SPRIX & OXAYDO™ Q1:15 Initiate pivotal Phase 3 safety and efficacy studies Egalet-002 Q3:15 Product launch OXAYDO H1:15 Complete Category 3 abuse deterrent study Egalet-001 Mid-15 Complete Category 3 abuse-deterrent study Egalet-002 2016 H1:16 Complete two pivotal Phase 3 studies Egalet-002 H1:16 Complete pivotal Phase 3 study Egalet-001 Mid-16 Submit NDA Egalet-002 Mid-16 Submit NDA Egalet-001 ‘15 will be busy with commercial and development milestones |
|
|
Delivering pain relief with peace of mind 24 |