Attached files
| file | filename |
|---|---|
| 8-K - 8-K - XENOPORT INC | d805111d8k.htm |
 XenoPort, Inc.
Investor Presentation
October 2014
©
Copyright 2014 XenoPort, Inc. All rights reserved.
NASDAQ:XNPT
Exhibit 99.1 |
 These
slides
and
the
accompanying
oral
presentation
by
XenoPort,
Inc.
contain forward-looking statements that involve risks and uncertainties,
including statements relating to the commercial opportunity and value
proposition for HORIZANT; potential future sales and commercialization
activity for HORIZANT and REGNITE; the planned development of
HORIZANT with the NIAAA for AUD; the planned development of AP by
Reckitt;
the
XP23829
clinical
development
program,
including
the
initiation
or
conduct of current or potential future clinical trials and regulatory submissions
and the timing thereof; expected patent coverage; and the therapeutic and
commercial potential of XP23829. XenoPort can give no assurance with
respect to these statements, and we assume no obligation to update
them.
For
detailed
information
about
the
risks
and
uncertainties
that
could
cause actual results to differ materially from those implied by,
or anticipated in,
these forward-looking statements, please refer to the Risk Factors section of
our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014
and filed with the SEC.
October 2014
Investor Slide Presentation
2
Safe Harbor Language |
 A
commercial-stage biopharmaceutical company with an internally discovered
pipeline focused on CNS and dermatology indications Growing revenues from
HORIZANT sales from 2 FDA-approved indications. New indication
(alcohol use disorder) will enter potential registration trial in 1H 2015 in
collaboration with NIAAA Differentiated MMF prodrug (XP23829) currently in
Phase 2 trial for psoriasis with results expected in 3Q 2015. Plans to
enter Phase 3 in psoriasis and/or relapsing forms of MS in 2016
Preparing to enter Phase 3 development in advanced Parkinson’s
disease (XP21279) and Phase 2 in alcohol use disorder (arbaclofen
placarbil)
October 2014
Investor Slide Presentation
3
XenoPort Overview |
 October 2014
Investor Slide Presentation
4
GABAPENTIN ENACARBIL
Restless
Legs
Syndrome
(RLS)
–
U.S.
Restless
Legs
Syndrome
–
Japan
Postherpetic
Neuralgia
(PHN)
–
U.S.
Alcohol
Use
Disorder
(AUD)–
U.S.
XP23829
Psoriasis
Relapsing Forms of MS
ARBACLOFEN PLACARBIL (AP)
AUD
XP21279
Advanced Parkinson’s Disease(
(Pending resources),
)
PHASE 1
PHASE 2
PHASE 3
NDA FILED
MARKETED
PRECLINICAL
PARTNER
XenoPort Pipeline |
 HORIZANT net sales
increased 66% in 2Q vs.
1Q 2014 to $4.9 million
$124.9 million of cash,
cash equivalents and short-
term investments at
6/30/14
$5.0 million in non-dilutive
cash in 3Q 2014
associated with AP
licensing agreement
No debt
5
Investor Slide Presentation
October 2014
Financials |
  |
 Approved for moderate-to-severe primary RLS in adults in
April 2011
Approved for the management of PHN in adults in June 2012
XenoPort promotional efforts began in June 2013
6 Orange Book listed patents with expiry dates from 2022 -
2029
•
Patent term extension requested for composition-of-matter patent from 2022
to 2025
October 2014
Investor Slide Presentation
7
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation
and dizziness, and in PHN patients: somnolence, dizziness and headache.
HORIZANT: XenoPort’s First
Commercial Product |
 Drug class: alpha-2-delta ligand (gabapentin, pregabalin)
Actively transported prodrug of gabapentin
•
Addresses pharmacokinetic deficiencies of gabapentin
Only approved extended-release alpha-2-delta product
Not interchangeable with other gabapentin products
October 2014
Investor Slide Presentation
8
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation
and dizziness, and in PHN patients: somnolence, dizziness and headache.
HORIZANT
Different by Design |
 Over 5 million U.S. adults
suffer from moderate-to-severe
primary RLS
Widespread use of dopamine
agonists
Growing awareness of issues
related to dopamine agonist
use in treatment of RLS
•
New treatment guidelines
October 2014
Investor Slide Presentation
9
Sources: RLS Prevelance-NINDs, NIH, Sleep Medicine, Volume 14, No. 7 ,
2013, Mayo Clinic Proceedings, Volume 88, No. 9, 2013,
Sleep, Vol. 35, No. 8, 2012
Moderate-to-Severe Primary RLS
Market Opportunity in U.S. |
 First and only non-dopamine agonist approved for the treatment of
moderate-to- severe primary RLS in adults
Proven effective in relieving RLS symptoms (clinical trial data)
Convenient once-a-day dosing
No titration required
No evidence of augmentation, rebound or impulse control disorders*
Recommended as a first-line treatment in recently published treatment
guidelines October 2014
*In two
12-week clinical trials, patients taking HORIZANT showed no evidence of symptom augmentation.
The duration of these trials may not have been sufficient to adequately assess
symptom augmentation. Investor Slide Presentation
10
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation and
dizziness, and in PHN patients: somnolence, dizziness and headache.
HORIZANT
Attributes for RLS |
  Results from damage that
occurs to the peripheral nerve
fibers during a shingles
outbreak
Pain associated with PHN can
be very intense
About 200,000 patients suffer
from PHN in the U.S.
Clear unmet medical need
•
~30% of patients receive
>50%
reduction in PHN pain with gabapentin,
the most widely used agent to treat
PHN
October 2014
Sources: Decision Resources, Inc. 2010, Neurontin Product Label
Investor Slide Presentation
11
Postherpetic Neuralgia (PHN) |
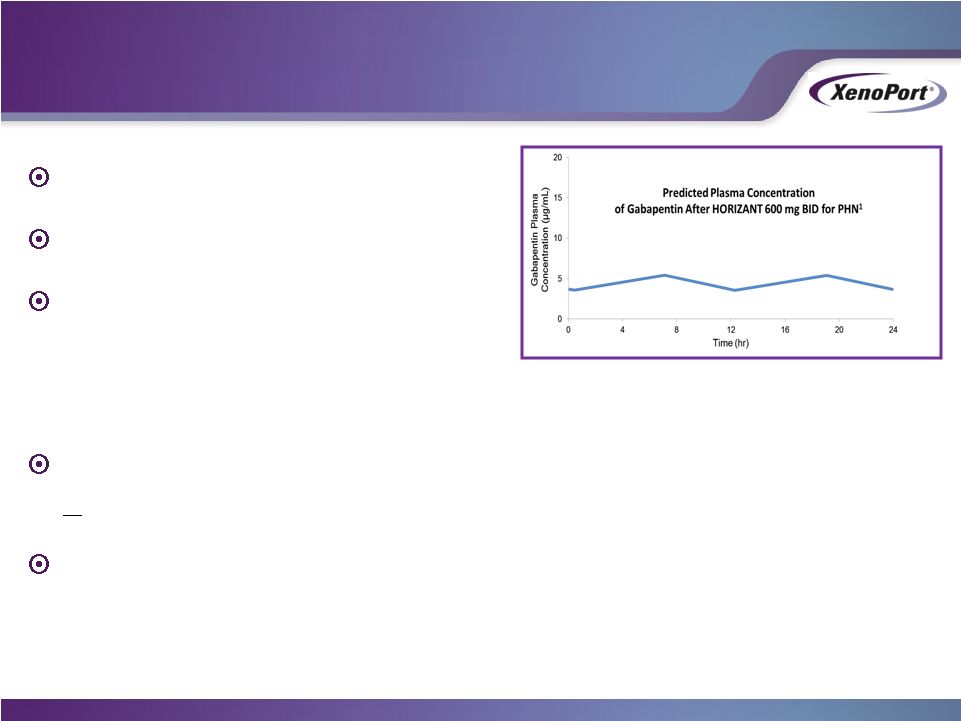 Simple 4-day titration
Efficacy as early as one week
Pharmacokinetic differentiation
•
High bioavailability (75%)
•
Sustained 24-hour gabapentin blood levels
Pivotal trial showed 42% of PHN patients experienced
>50% reduction in pain intensity score from baseline
Pain relief over 24 hours
October 2014
Please review the full prescribing and safety information for HORIZANT. The most
common adverse reactions of HORIZANT in RLS patients: somnolence/sedation
and dizziness, and in PHN patients: somnolence, dizziness and headache.
Investor Slide Presentation
12
1. Adapted from Lal R, et al. J Clin Pharmacol. 2013;53(1):29-40
HORIZANT
Attributes for PHN |
 Initial Plan Upon Return of
Product Rights
•
Establish responsiveness
quickly and efficiently
•
Focus on specialists and high
prescribing PCPs of RLS and
PHN drugs
•
Personal promotion and
marketing efforts focused in ~ 40
territories
XenoPort Initial Plan: Sales specialists called
on <10% of the potential market
October 2014
Investor Slide Presentation
13
Initial Plan
Current Plan
•
Expanded to additional ~25 new territories
•
Trained and in field
HORIZANT Commercialization |
 October 2014
Investor Slide Presentation
14
Source: SHA, PHAST 2014
Previous Partner’s
Sales Team of ~300-500
XenoPort
Sales Team of ~40
Approved Dose: RLS = 1 tablet per day; PHN = 2 tablets per day
Currently >85% of prescribed
tablets from XenoPort territories
XenoPort Launch
HORIZANT
Dispensed Tablets per Month |
 Source: SHA, PHAST 2014
XenoPort Launch &
First Promotion of
PHN Indication
Investor Slide Presentation
October 2014
15
XenoPort Launch &
First Promotion of
PHN Indication
Prescriptions by Specialty
% Prescriptions 1200 mg/day*
*Source: Healthcare Analytics, a Symphony Health Solutions Company
The information attributed to Source Healthcare Analytics herein
is provided as is, and Source Healthcare Analytics, LLC
makes no representation and/or warranty of any kind, including but not limited to
the accuracy and/or completeness of such information. Source
Healthcare Analytics is credited as a source of certain data only. The attribution of Source Healthcare
Analytics as the source of such data shall not be construed as an endorsement
by Source Healthcare Analytics of the views, opinions or findings
expressed, shared or otherwise published herein. HORIZANT
Prescribers and Daily Dose |
 Randomized, double-blind, placebo-controlled trial of the safety and
efficacy of HORIZANT in patients who have AUD
XenoPort to supply clinical trial material
NIAAA will conduct and pay all other expenses associated with proposed clinical
trial •
Six-month treatment duration; Enrolling approximately 350 patients; expect
initiation in 1H 2015 XenoPort and NIAAA to meet with FDA to discuss
possibility of utilizing the results of this trial as the basis for a
potential supplemental new drug application (sNDA) submission for HORIZANT
16
October 2014
Investor Slide Presentation
Clinical Trial Agreement
“Scientists at XenoPort designed gabapentin enacarbil extended-release
tablets to address certain limitations
of
drug
levels
in
the
body,
which
may
make
it
a
more
attractive
treatment
option
for
people
with
AUD.”
-
NIAAA press release 9/9/14
XenoPort and NIAAA
Clinical Trial Agreement |
    XP23829 for Potential
Treatment of Psoriasis
and/or Relapsing
Forms of MS |
 FUMADERM
mixture of dimethylfumarate (DMF) and
monoethyl fumarate salts
•
Approved in 1990s and widely used for the treatment of psoriasis
in Germany
TECFIDERA (dimethylfumarate)
•
Approved in March 2013 in the U.S. for the treatment of relapsing forms of
MS •
Approved in February 2014 in the EU for relapsing-remitting MS
•
2Q 2014 TECFIDERA revenues were $700 million ($585 million in U.S.; $115
million in sales outside the U.S.)
XP23829 has a novel chemical structure that produces
monomethylfumarate (MMF), the same active metabolite as
dimethylfumarate
October 2014
Investor Slide Presentation
18
Background:
Fumaric Acid Ester Products |
 October 2014
Investor Slide Presentation
19
XP23829
DMF
MMF
MMF
Promoiety
Methanol
+
+
Esterases
XP23829 and DMF produce the same active metabolite (MMF) in the body
DMF and XP23829 are
Prodrugs of MMF |
 Lower incidence and/or less severe GI side effects and
flushing
•
Improved compliance; fewer treatment failures
Onset and/or magnitude of efficacy
•
Earlier onset of immunomodulation
Dosing frequency
•
QD rather than BID (TECFIDERA) or TID (FUMADERM)*
Indication
•
TECFIDERA and FUMADERM not approved for psoriasis in the U.S.
October 2014
Investor Slide Presentation
20
*QD: once daily; BID: twice daily; TID: three times daily.
Potential XP23829 Advantages and
Areas of Differentiation |
 Completed preclinical PK and safety studies including 13-week
toxicology studies in 3 animal species. Studies included DMF
comparison arms
•
Demonstrated less skin and GI irritation compared to DMF
•
No adverse findings that were not observed with DMF
Demonstrated efficacy in animal models of MS and psoriasis
Completed three Phase 1 trials establishing human PK, metabolites and
disposition and comparison of PK to TECFIDERA
•
XP23829 produced total MMF exposure in blood similar to TECFIDERA
Demonstrated known pharmacodynamic effects on immune blood cells
with once-a-day dosing in humans
Selected novel delayed and extended-release formulation for Phase 2/3
October 2014
Investor Slide Presentation
21
XenoPort: A Leader in Development of
2
nd
Generation MMF Prodrugs |
 October 2014
Investor Slide Presentation
22
•
pH-independent delayed-release
mechanism to reduce gastric irritation and
food effects on pharmacokinetics
•
Releases XP23829 over 8-10 hours
avoiding high local concentrations in
upper small intestine, lower Cmax and
extended blood levels of MMF
Differentiation from TECFIDERA/FUMADERM
In Vitro Dissolution
XP23829 in a Novel Formulation to
Potentially Provide Differentiation |
 XP23829 Intellectual Property
October 2014
Investor Slide Presentation
23
•
Three issued U.S. patents (US8148414, US8778991 and US8785443)
including claim covering composition-of-matter of XP23829 (expiration
date 2029)
•
17 filed patent application families with pending claims including:
•
Specific MMF prodrug compositions and their uses
•
Crystalline forms of XP23829
•
Oral dosage forms of MMF prodrugs*
•
Methods of treatment with MMF prodrugs*
•
Methods of selecting and/or administering MMF prodrugs* to reduce
side effects
•
Applications filed broadly in major pharmaceutical markets
* “MMF prodrug”
may include XP23829, DMF and certain other MMF prodrug molecules
|
 Conducting Phase 2 psoriasis study to assess the effect of dose and dosing
regimen on efficacy, tolerability, safety and immune cell modulation of
XP23829 •
Trial initiated June 2014
•
Top-line results expected in 3Q 2015
•
Optimal dose(s) expected to translate to relapsing forms of MS, based on
TECFIDERA precedent
Preclinical studies and manufacturing scale-up are underway to allow Phase 3
study in 2016
Clear precedent for psoriasis Phase 3 program
•
Two Phase 3 trials, at least one demonstrating maintenance of efficacy at one
year Discussions ongoing with FDA on potential abbreviated Phase 3 program
for relapsing form of MS
24
October 2014
Investor Slide Presentation
XP23829
Current Development Plan |
 Study Design
Randomized, double-blind, multicenter, parallel group, placebo-controlled,
dose-finding efficacy and safety study in subjects with
moderate-to-severe chronic plaque-type psoriasis Number of
Sites ~35 sites in United States
Number of Subjects
~200 randomized 1:1:1:1
Primary Endpoint
The percent change in Psoriasis Area and Severity Index (PASI) score from Baseline
(12 weeks)
October 2014
Investor Slide Presentation
25
400 mg BID
Placebo
800 mg QD
400 mg QD
Screening/
Washout
Week -4
Week 0
Week 3
Week 12
Week 16
Post-Treatment
Follow-up
Titration
Maintenance Phase
XP23829 Phase 2 Clinical Trial in
Psoriasis Patients |
 October 2014
Investor Slide Presentation
26
Phase 2 Psoriasis Study
Biogen Press Release 2004
Langner, J Am Acad Dermatol 2005
Week 12
Phase 2 Relapsing Forms of MS Study
Kappos, Lancet 2008
Similar Dose Response for TECFIDERA in
Psoriasis & Relapsing Forms of MS |
 October 2014
Investor Slide Presentation
27
•
Worldwide psoriasis prevalence rates vary from 0.6%
to 4.8%
Epidemiology
•
In the U.S., it is estimated that direct costs on health
care approach ~$7B
•
Psoriasis affects the daily lives of patients; studies
estimate 60% of psoriasis patients missed an average of
26 days of work per year due to their illness
Economic Burden
Sources: UpToDate, BioMedTracker, National Psoriasis Foundation,
Horn EJ, et al 2007 J Am Acad Dermatol. 57:963-71;
Fowler et al-
2008 J of Am Acad Derm 3 Clin Exp Rheumatol 2002; 20 (Suppl. 28): S27-S33
Psoriasis is Prevalent, Reduces Quality of Life and
is an Economic Burden to Health Systems
+
In the U.S., estimates of psoriasis prevalence
range from 2.5-3.5%
+
Epidemiological studies of prevalence in the
EU range from 1-3%
+
Moderate and severe psoriasis represent 35%
and 12% of all patients, respectively |
 October 2014
Investor Slide Presentation
28
* Assumes no arthritic involvement
Sources: Back Bay Physician Interviews, DataMonitor 2013 Treatment Flow and Physician
Quantitative Survey U.S.
•
Orals have 40-50% market share
as
1
st
and
2
nd
line
Germany
•
Orals
have
80-90%
market
share
as
1
st
line
•
FUMADERM
has
highest
market
share
Oral Therapy Market Share for Moderate and Severe
Psoriasis Differs in U.S. and Germany |
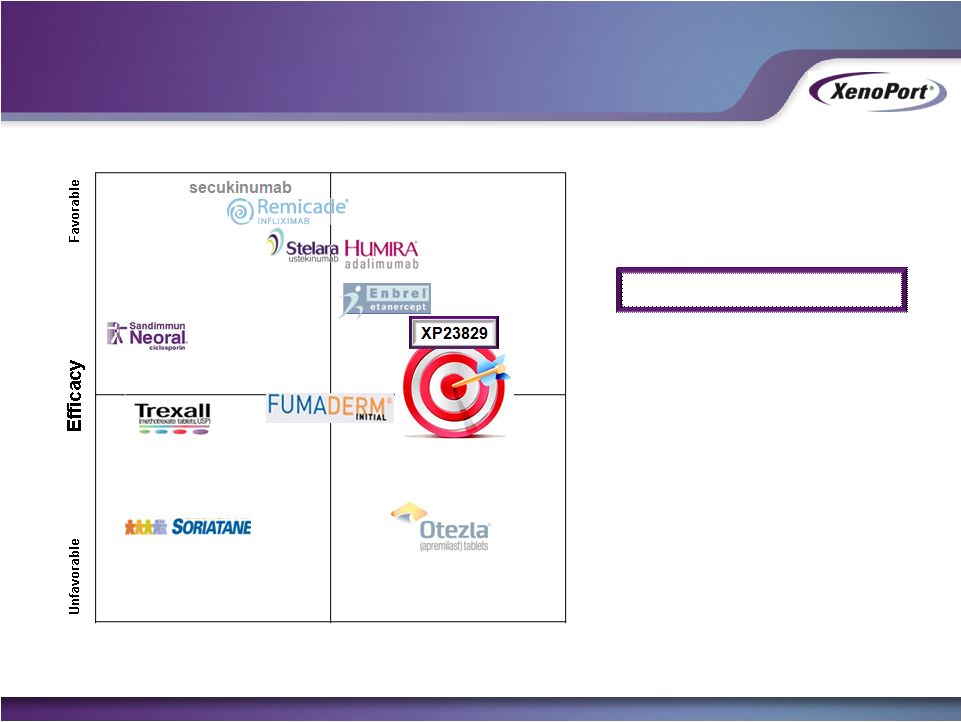 October 2014
Investor Slide Presentation
29
Safety/Tolerability
Unfavorable
Favorable
Qualitative Positioning
Efficacy similar to FUMADERM
(PASI 75 = 40-50%)
with favorable tolerability
and long-term safety
Desired XP23829 Profile
Desired Product Positioning for XP23829 in
Moderate-to-Severe Chronic Plaque-Type
Psoriasis |
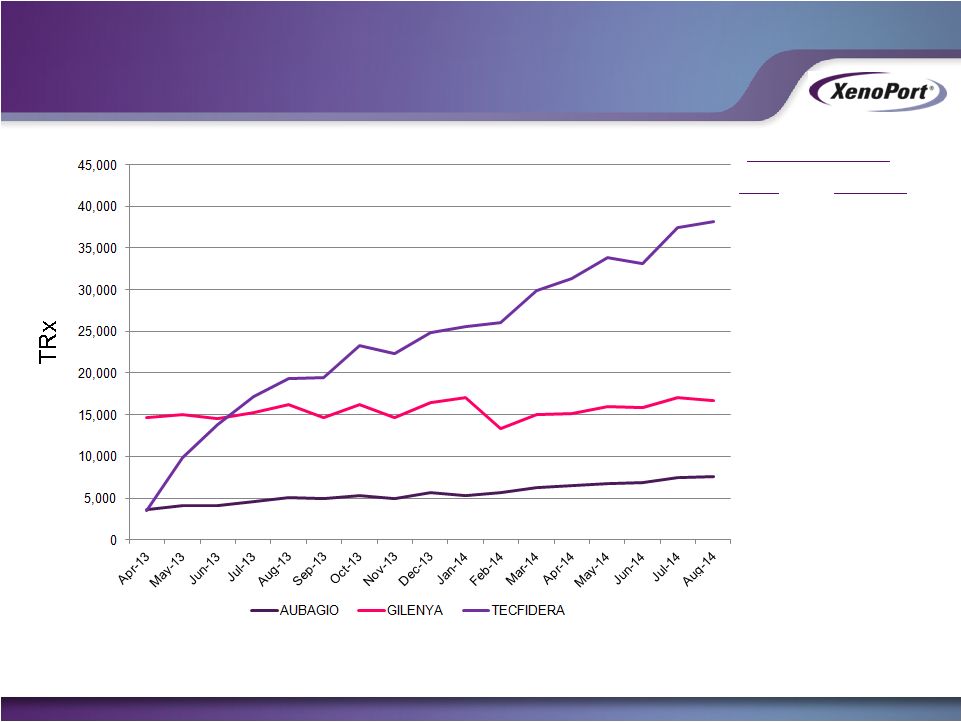 October 2014
Investor Slide Presentation
30
2Q 2014 Sales
U.S.
Ex-U.S.
$585M
$115M
$91M
$27M
$284M
$322M
Source: Symphony Health Solutions, PHAST Prescription Monthly, April 2013
– August 2014
1% market share is ~$100M in annual U.S. product sales at current prices
Oral Treatments for Relapsing
Forms of MS |
    FUTURE
OPPORTUNITIES
XP21279 for Parkinson’s Disease
AP for Alcohol Use Disorder |
       October 2014
Investor Slide Presentation
32
C
avg
C
avg
Threshold for
“off-time”
SINEMET
SINEMET
Dyskinesia
Threshold
XP21279
XP21279
A dose of XP21279
resulting in a higher Cavg
concentration could
reduce “off”
time without
increasing dyskinesias
High peak-trough ratios of
levodopa concentrations
result from SINEMET’s
inherent PK properties
Therapeutic
Window
XP21279: Designed to Keep Levodopa Exposure
in the Target Therapeutic Window |
 Achieved levodopa levels with XP21279/Carbidopa tablet
within the target therapeutic window
Minimal Fluctuation in Levodopa Blood Levels
October 2014
Investor Slide Presentation
33
XP21279 Phase 2 Study in
Advanced Parkinson’s Patients |
 A
single successful pivotal 12-week Phase 3 study comparing optimized
doses of XP21279 to SINEMET could support approval for treatment
of
patients with advanced Parkinson’s disease with motor fluctuations
•
Data comparing to SINEMET would be in product label
Gained agreement on long-term safety database size and duration of
exposures
A single successful 3-month placebo-controlled study could support
approval of XP21279 in patients with early Parkinson’s disease
•
Based on 505(b)(2) cross-reference to SINEMET
Program ready to enter Phase 3 development with further funding
October 2014
Investor Slide Presentation
34
FDA Agreements For Potential
XP21279 Phase 3 Program |
 Exclusive world-wide rights granted to Reckitt Benckiser Pharmaceuticals
•
Proven leader in commercialization of addiction treatments, e.g., SUBOXONE
Planned initial development focus: AUD
$20 million up-front plus $5 million for material transfer
Up to $70 million in development and regulatory milestones
Up to $50 million in commercial milestones
Tiered double-digit royalty payments up to mid-teens on a percentage basis
on potential future net sales in the U.S.
High single-digit royalty payments on potential future net sales outside
the U.S.
October 2014
Investor Slide Presentation
35
AP Agreement with Reckitt Benckiser
Pharmaceuticals |
 HORIZANT
•
Growing net sales in U.S. and royalties in Japan
•
Additional ex-U.S. partnership(s)
•
FDA agreement of AUD development plan
•
Initiation of AUD pivotal study in 1H 2015
XP23829
•
Top-line results of Phase 2 psoriasis study expected in 3Q 2015
•
Completion of non-clinical studies to support Phase 3 in 2016
•
FDA agreement on Phase 3 plan for psoriasis and/or relapsing forms of MS
XP21279
•
Partner and/or initiate Phase 3 development activities as resources permit
AP
•
Expected initiation of Phase 2 study in AUD by partner Reckitt Benckiser
Pharmaceuticals
October 2014
Investor Slide Presentation
36
XenoPort Anticipated Milestones |
 ©
Copyright 2014 XenoPort, Inc. All rights reserved.
NASDAQ:XNPT
*******************
*******************
*******************
*******************
******************* |
