Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OPKO HEALTH, INC. | d741430d8k.htm |
| EX-99.3 - EX-99.3 - OPKO HEALTH, INC. | d741430dex993.htm |
| EX-99.1 - EX-99.1 - OPKO HEALTH, INC. | d741430dex991.htm |
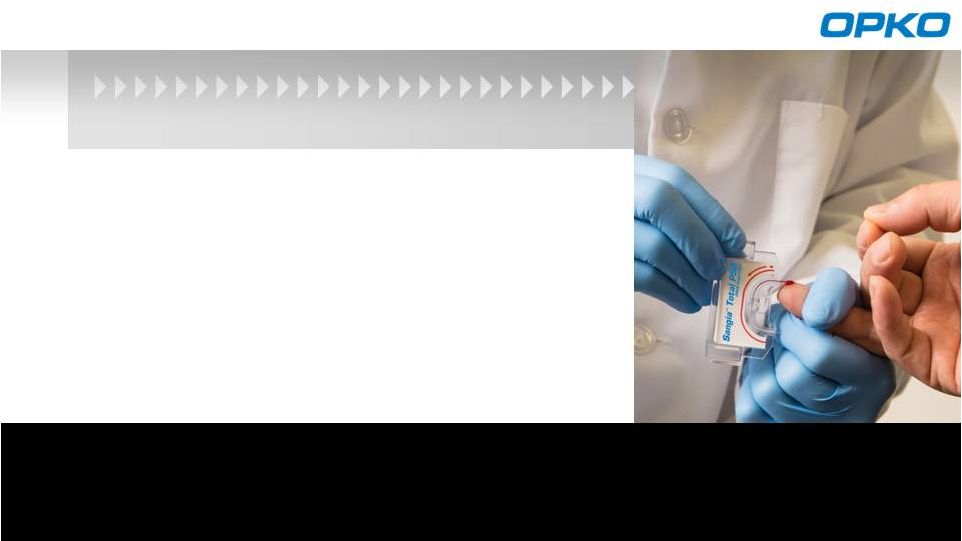
 Lab
quality results in 10 minutes from a finger-stick blood sample
Exhibit 99.2 |
 Time
and discomfort for venous blood
draw
1-3 day delay in lab
results, diagnosis,
and treatment
Maintenance of
certifications
and lab quality
COST
PATIENT
CARE
Challenges of Obtaining Diagnostic Information |
 Real
World Convenience 1-2
mins
10
mins |
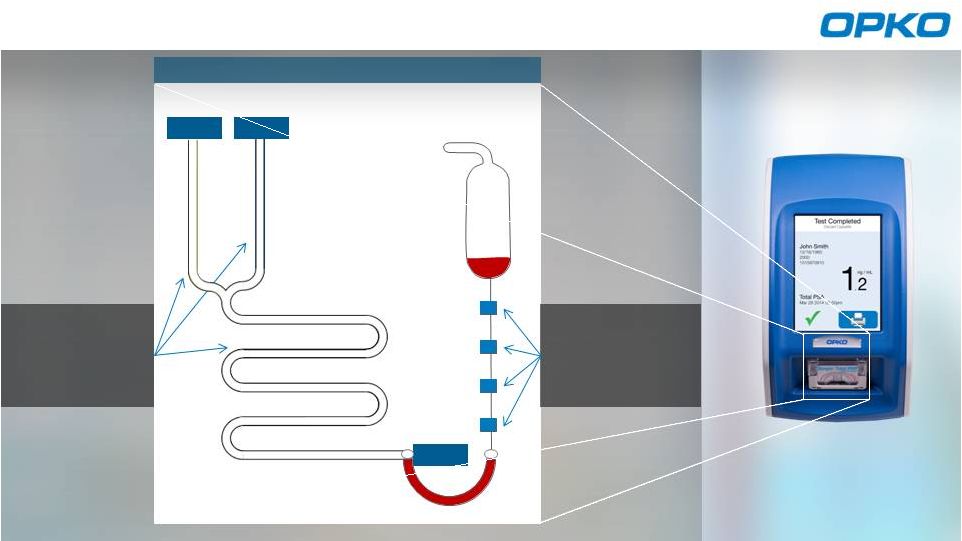 Stable and
contamination
free reagents
Sample collected
Cassette
activated by
vacuum
Analyzes results
Claros Microfluidic Assay Process
Seal
Seal
Seal
Cassette |
 Sangia™
Microfluidic Cassette
CONVENIENT
LAB QUALITY
ACCURACY
Single, self-contained cartridge |
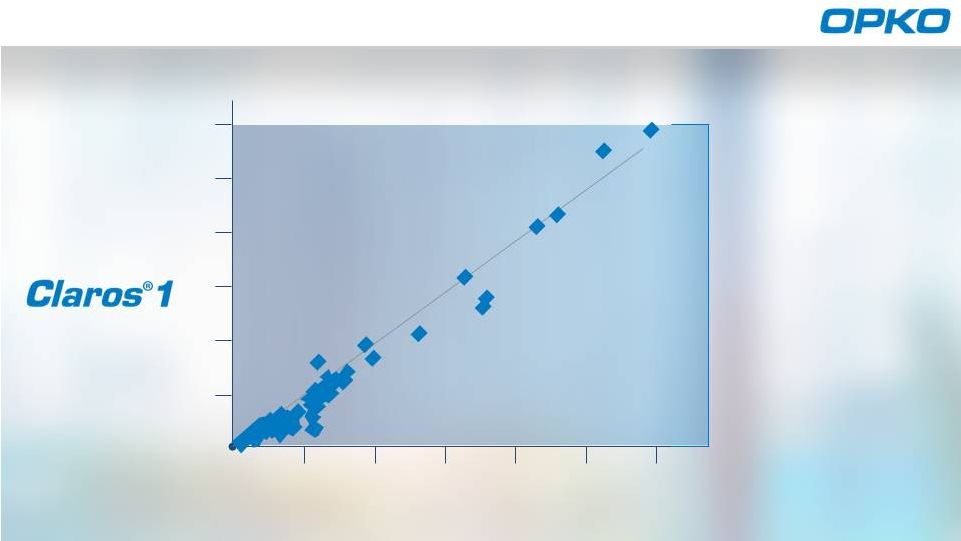 2
4
6
8
10
12
2
4
6
8
10
12
ng/mL
Sangia Total PSA
Roche Cobas central lab system
ng/mL
Elecsys PSA
EXCELLENT
CORRELATION
ensures
LAB QUALITY
RESULTS
IN-OFFICE
R of 0.970
Proven Results
2 |
| Claros 1 Update
•
Formulation of blood collector providing high reliability for results
reporting •
SMA Chemistry providing rapid attachment and low LOQ
•
Testosterone
–
FDA: Pre-submission comments received from FDA
–
On track to file 510(k) in 2014
–
CE Mark: 4Q2014
•
PSA
–
FDA: Pre-submission response expected in August
–
Timing of 510k submission based on longitudinal trial requirements
–
CE Mark Update (Formulation and Chemistry): 4Q2014
•
Vitamin D
–
On track to support launch of Rayaldee 1Q2016 |
 Personal risk for high-grade prostate cancer
…from a blood test |
 The
Issues of PSA Screening |
 |
 |
 |
 |
 Aiding Decision for Prostate Biopsy
Poor accuracy in distinguishing
cancers
PSA SCREENING
BIOPSY CANDIDATES |
 Peter Scardino
(MSKCC, New York)
Andrew Vickers
(MSKCC, New York)
Hans Lilja
(MSKCC/Malmö/Oxford)
Kim Pettersson
(Univ. of Turku, Finland)
International team of researchers
Rigorous Clinical Research
Monique Roobol
Netherlands
Angel Cronin
USA
Caroline Savage
USA
Fritz Schröder
Netherlands
Mari Peltola
Finland
Charlotte Becker
Sweden
Sigrid Carlsson
USA/Sweden
Gunnar Aus
Sweden
Carl-Gustav Pihl
Sweden
Amine Benchikh
France
Alexandra Mashino
USA
Arnauld Villers
France
Chris Bangma
Netherlands
Ewout Steyerberg
Netherlands
Theo van der Kwast
Netherlands
Daniel Sjoberg
USA
Jonas Hugosson
Sweden
Timo Lövgren
(Univ. of Turku, Finland)
Amit Gupta
USA
3
decades of
biomarker research
Over
10,000
men studied
9
cohorts in
peer-reviewed
publications |
 Results of each
biomarker
Digital rectal exam (DRE)
Prior biopsy
Age of patient
7 Elements of the 4Kscore Test
46 years
53 years
65 years
68 years
Total PSA
Free PSA
Intact PSA
hK2
Kallikrein blood tests |
 37%
Negative or
Gleason
score
6
20%
Gleason
score
7
77%
Negative or
Gleason
score
6
23%
Gleason
score
7
9% cut off level
Number of
patients
1000
800
600
400
200
Current US practice
0
Replication of Performance in a US Cohort
26 Urology Centers
1,012 Patients
Prospective, Blinded
Clinical study sites
43%
Biopsies
avoided |
 Decrease Unnecessary Biopsies
Proven Results
Accurate Probability for
High-grade Cancer |
| 4Kscore Test US Commercial Activities
•
Post AUA follow-ups with LUGPA, PI Sites, etc.
•
Test seeding program with select, regional urologists
•
Launch web and social media platform
•
Complete build out of a blood draw site network
•
Publication of US Clinical Study (in preparation)
•
Establish a US Speakers Bureau for local advisory board
meetings
•
Initiate Reimbursement process with private insurance,
CMS |
