Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OPKO HEALTH, INC. | d741430d8k.htm |
| EX-99.3 - EX-99.3 - OPKO HEALTH, INC. | d741430dex993.htm |
| EX-99.2 - EX-99.2 - OPKO HEALTH, INC. | d741430dex992.htm |
 Update on Phase 3 Development
of
Rayaldee
™
Capsules
June 12, 2014
Exhibit 99.1 |
 2 |
 To
Improve People’s Lives by Treating and Preventing the Clinical
Consequences of Vitamin D Insufficiency and Secondary
Hyperparathyroidism
Mission Statement (for Renal Division)
3 |
 Vitamin D
Insufficiency
Obesity
Bone Diseases
Infection
Diabetes
Critical Illness
Cancer
Psoriasis
Skeletal-related
Events (SRE’s)
CKD
Cystic Fibrosis
Diseases Associated with Vitamin D Insufficiency
4
SHPT
Autism
SHPT = Secondary Hyperparathyroidism |
 5
Vitamin D
25 hydroxy-
vitamin D
1,25 dihydroxy-
vitamin D
Liver Enzyme
Kidney Enzyme
Ergocalciferol
Cholecalciferol
Limited and unreliable efficacy
What is Vitamin D Insufficiency?
Vitamin
D
insufficiency
is
serum
25-hydroxyvitamin
D
3
below
30
ng/mL |
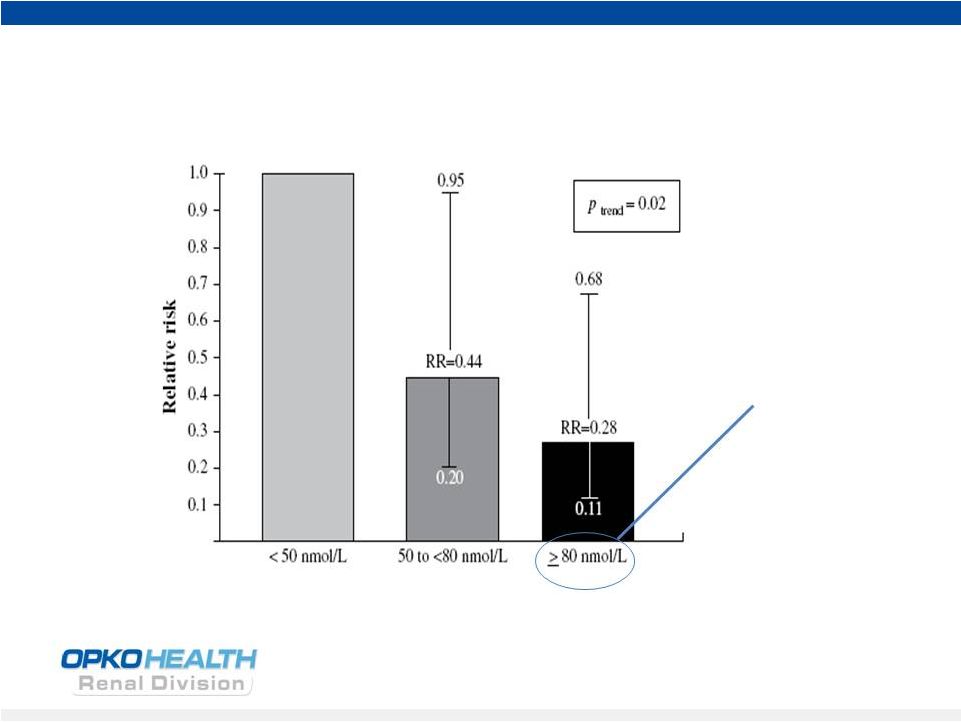
 Vitamin D Insufficiency: Increases Mortality in
Breast Cancer
6
Mohr et al 2014 Anticancer Res |
 7
Garland CF et al. Ann Epidemiol 2009
Vitamin D Insufficiency: Increases Mortality in Colon
Cancer
~32
ng/mL |
 Vitamin D Insufficiency: Increases Mortality in CKD
8
Ravani et al Kidney Int 2008 |
 9
Rayaldee (CTAP101) –
A Late-Stage Investigational Drug
Product Overview
•
Modified-release
(MR)
oral
formulation
of
25D
3
*
addresses significant unmet market need
•
Safe and effective treatment for elevated PTH
(SHPT) associated with low 25D levels in Stages
3-4 CKD
•
Achieves more reliable increases in serum 25D
and reductions in plasma PTH than nutritional
vitamin D
•
Lower risk of side effects compared to active
1,25D
**
products
•
Preserves protective renal feedback mechanism
•
Additional potential for new indications including
institutionalized elderly, osteoporosis & cancer.
Clinical Status
•
SPA agreement with FDA in 3Q 2012
•
Phase 3 trials initiated 3Q 2012
•
Patient recruitment completed in 4Q 2013
•
Clinical development guided by “all-star”
Scientific Advisory Board
•
NDA filing expected in 1H 2015
Intellectual Property
•
Formulation and method of use patents allowed
•
Rayaldee US patents issued, protected through
2028
•
Additional global patents allowed or pending
** 1,25-Dihydroxyvitamin D or
Calcitriol *
25-Hydroxyvitamin
D
or
Calcifediol
3 |
 Market Opportunity: Chronic Kidney Disease (U.S.)
*National Kidney Foundation 2002
**US Renal Data Service 2011 Annual Data Report
Sources: Levin, A et al., Kidney International 2007; 71: pp.31-38.
Gonzalez, E et al. Am J Nephrol 2004;24:503-510.
LaClair, R et al. Am J Kidney Dis 2005;45:1026-1033.
The CKD patient population is large and growing as a result of:
•
Obesity
•
Hypertension
•
Diabetes
CKD
Prevalence
rates
are
similarly
high
outside
of
the
U.S.,
and the population base is much larger.
10
% of CKD Patients with:
Stage
Kidney Function
CKD Prevalence
Vitamin D
Insufficiency
(
25D)
SHPT
(
PTH)
Hyperphosphatemia
(
Phosphorus)
3
Moderate impairment
7.6 Million*
70%
56%
37%
4
Severe impairment
0.4 Million*
80%
60%
50%
5
Failure
0.5 Million**
90%
90%
70% |
 Rayaldee raises serum total 25-hydroxyvitamin D (25D) and lowers plasma
iPTH more effectively than any over-the-counter (OTC) or prescription (Rx)
product currently marketed without the risk of hypercalcemia.
Comparison of Vitamin D Therapies for Stage 3-4 CKD
*And generics
**25-hydroxyvitamin D
11
Effect on Blood Levels of:
Drug
Active
Type
25D
**
Ca
iPTH
Rayaldee
Calcifediol
(25-hydroxyvitamin
D3)
Rx
Vitamin D
Cholecalciferol/Ergocalciferol
(vitamin
D3/vitamin D2)
OTC
Drisdol
™*
Ergocalciferol
(vitamin
D2)
Rx
Rocaltrol
™*
Calcitriol
(1
,25-dihydroxyvitamin
D3)
Rx
Hectorol
™
Doxercalciferol
(1
-hydroxyvitamin
D2)
Rx
Zemplar
™*
Paricalcitol
(19-nor-1
,25-dihydroxyvitamin
D2)
Rx |
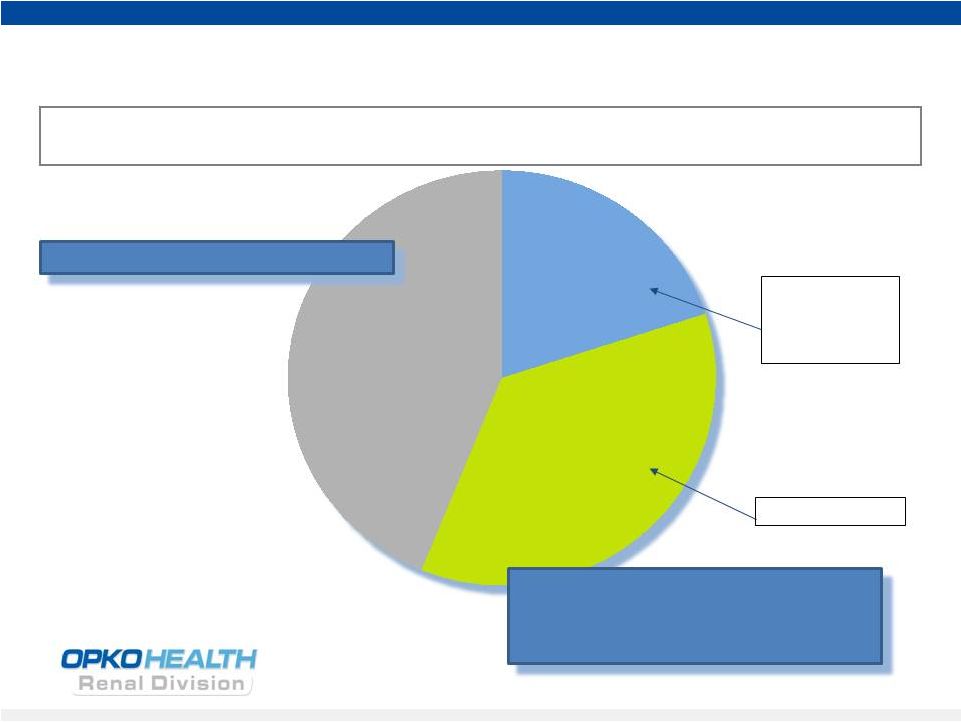 12
Rayaldee (CTAP101) -
Commercial Opportunity
Source: BioTrends Research Group, Inc. December 2010
Untreated
26-44%
Vitamin D
Hormone
20-36%
Nutritional
Vitamin D
36-38%
Untreated
26-44%
Safety concerns;
exacerbates
vitamin D
insufficiency
Efficacy Concerns
–
8 million CKD Stage 3-4 patients in the US
–
4 million patients with low serum 25D and high plasma PTH
Stage 3 & 4 CKD Treatment
Rayaldee is expected to take significant
market share in Stage 3 and 4 CKD
patients
suffering
from
SHPT
–
a potential
$12 billion revenue opportunity
Low serum 25D and elevated plasma PTH are prevalent in CKD Stage 3-4 patients |
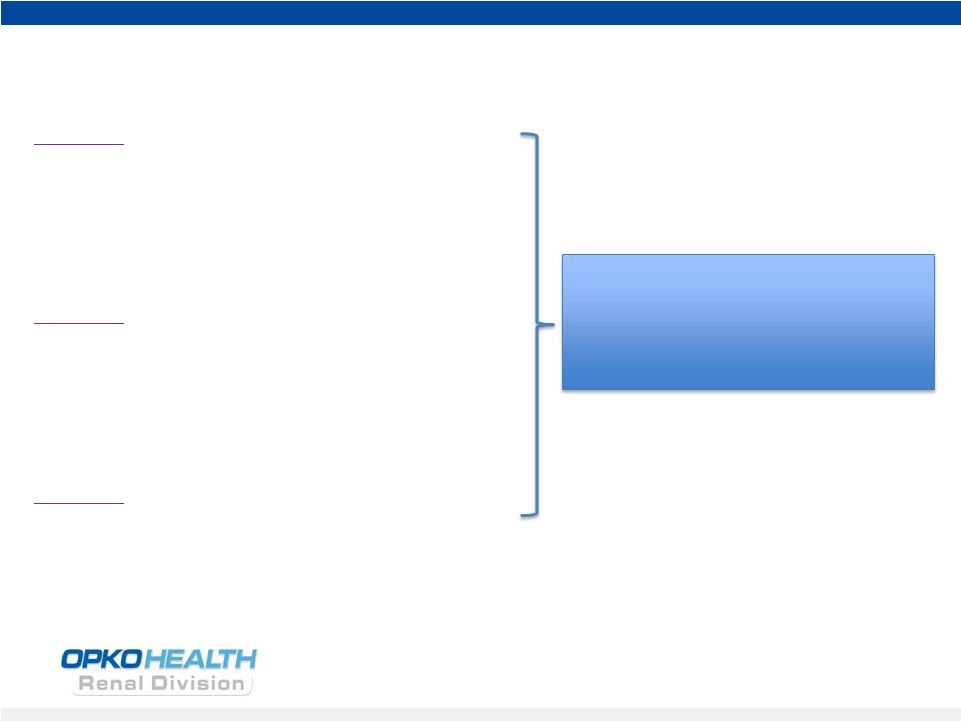
 13
Vitamin D
25 hydroxy-
vitamin D
1,25 dihydroxy -
vitamin D
Inactivated
Vitamin D
Liver Enzyme
Kidney Enzyme
CYP24 Enzyme
Parathyroid
Hormone (iPTH)
Feedback Loop
Target Tissues
(Parathyroid gland)
Ergocalciferol
Cholecalciferol
Rayaldee
TM
Capsules
Calcitriol
Paricalcitol
Doxercalciferol
Limited and unreliable efficacy
to raise 25D
and lower PTH
Risk of side effects limits dose
and efficacy
Rayaldee Capsules: Positioning vs. Competitive Products |
 Rayaldee: Phase 3 and NDA Timelines
2012
Q1
Q2
Q3
Q4
2013
Q1
Q2
Q3
Q4
2014
Q1
Q2
Q3
Q4
NDA
Filing
CL-3002
Data
CL-3001 trial
SPA
Open Label Extension (OLE) study
Q1
Q2
Q3
2015
Q4
NDA
Safety
Update
NDA
Approval
TG Mouse carcinogenicity study
Dose ranging studies
SPA
CL-3001
Data
Stability studies on 3 registration lots
Final
Report
Final
Report
Publicly announced timelines:
•
Top-line data from CL-3001 & CL-3002:
mid-2014
•
NDA filing: H1 2015
CL-3002 trial
14 |
 15
CL-3001 & CL-3002 Combined Site Map
= Sites for CL-3001 (n=45 )
= Sites for CL-3002 (n=45) |
 16
Status
Update:
Phase
3
Trials
(as
of
June
5,
2014)
CL-3001:
•
637subjects entered screening
•
213 subjects were enrolled (~210 needed)
•
Enrollment is 100% complete
•
109 subjects had stage 3 CKD (target = ~105)
•
104 subjects had stage 4 CKD (target = ~105)
CL-3002:
•
582 subjects entered screening
•
216 subjects were enrolled (~210 needed)
•
Enrollment is 100% complete
•
113 subjects had stage 3 CKD (target = ~105)
•
103 subjects had stage 4 CKD (target = ~105)
CL-3003:
•
255 subjects have been enrolled
•
Rollover failure rate from pivotal trials is 15.8% (budget = 20%)
•
91 subjects have completed the 6-month treatment period and exited
•
303 subjects have completed
•
62 subjects are still enrolled
•
64 premature terminations (15%) |
 Thanks!
17 |
